User login
Infectious disease pop quiz: Clinical challenge #23 for the ObGyn
What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Continue to the answer...
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci).
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Continue to the answer...
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci).
What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Continue to the answer...
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci).
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Weight gain may exacerbate structural damage in knee OA
researchers reported at the OARSI 2022 World Congress.
Using data from the Osteoarthritis Initiative (OAI), researchers from the University of California found that a greater than 5% increase in body weight over 4 years was associated with a 29% increased risk for medial joint space narrowing (JSN), compared with controls (P = .038). There was also a 34% increased risk for developing frequent knee pain (P = .009)
Conversely, weight loss appeared to offer some protection from structural damage in knee OA, Gabby B. Joseph, PhD, a specialist in radiology and biomedical imaging, said at the congress, sponsored by the Osteoarthritis Research Society International.
Indeed, individuals who had achieved a weight loss of more than 5% at 4-year follow up were less likely to have a worsened Kellgren and Lawrence (KL) grade than those whose body weight remained the same (odds ratio, 0.69, P = .009).
Weight loss was also associated with a higher change of experiencing resolution in knee pain over 12 months, with an OR of 1.40 (P = .019).
Importance of weight change in OA
“We know that weight loss has beneficial effects on knee OA symptoms, such as pain relief and improvement in physical function,” commented Xingzhong Jin, PhD, an NHMRC Early Career Fellow at the Centre for Big Data Research in Health at the University of New South Wales, Sydney.
“But what is unclear is whether weight loss could slow down structural degradation in the joint in the long run,” he said in an interview. “These findings mean that weight control is clearly very important for knee OA, in terms of improving symptoms as well as preventing structural progression.”
He added: “The evidence on hip OA is less clear. As most of the knowledge in this space was generated from people with knee OA, this work is an important contribution to knowledge around the care of people with hip OA.”
Why look at weight change effects in OA?
“Obesity is a modifiable risk factor for osteoarthritis,” Dr. Joseph said at the start of her virtual presentation. Indeed, patients with obesity are more than twice as likely to develop knee OA than their normal weight counterparts.
Although there have been various studies looking at weight loss and weight gain in OA, most have focused on weight loss rather than gain, and OA in the knee rather than the hip, she explained.
The aim of the present study, therefore, was to take a closer look at the possible effect of both weight gain and weight loss in people with hip or knee OA in terms of radiographic outcomes (KL grade change, medial JSN), symptomatic outcomes (knee pain and resolution at 12 months), and the need for joint replacement.
“The clinical implications are to develop targeted long-term strategies for site-specific informed recommendations to prevent joint degeneration,” Dr. Joseph said.
Using data on nearly 3,000 individuals from the OAI, Dr Joseph and collaborators classified people with OA into one of three groups: those with at least a 5% gain in weight, (n = 714), those with no (–3% to 3%) change in weight (n = 1,553), and those with at least a 5% loss in weight over a 4-year period.
The results, which were published in Arthritis Care & Research, also revealed no differences in the rate of total hip or knee arthroplasties between the groups, and no differences between the weight gain and weight loss groups and controls in term of hip radiographic or symptomatic changes.
“Why are there differing effects of weight change in the knee versus the hip? This could be multifactorial, but there could be a few things going on,” said Dr. Joseph. “First, the joint structure is clearly different between the knee and the hip. The knee is a hinge joint. The hip is a ball and socket joint malalignment could affect these in different ways.”
Additionally, “the knee also has thicker cartilage, the hip has thinner cartilage again, and the loading patterns may be different in these joints.”
There were also differences in the rate of progression between the knee and the hip, “this was especially noticeable for the radiographic progression,” Dr. Joseph said, with rates being higher in the knee.
Noting that the study is limited by its retrospective design, Dr. Joseph concluded: “We don’t know why these people lost or gained weight. So, this would be something that would be more apparent in a prospective study.
“Also, there were no MRI outcomes, as MRI imaging was not available in the hip in the OAI, but clearly morphology T1 and T2 would be useful to assess as outcomes here as well.”
The OAI is a public-private partnership funded by the National Institutes of Health and initial support from Merck, Novartis, GlaxoSmithKline and Pfizer. Dr. Joseph and Dr. Jin reported having no conflicts of interest to disclose.
researchers reported at the OARSI 2022 World Congress.
Using data from the Osteoarthritis Initiative (OAI), researchers from the University of California found that a greater than 5% increase in body weight over 4 years was associated with a 29% increased risk for medial joint space narrowing (JSN), compared with controls (P = .038). There was also a 34% increased risk for developing frequent knee pain (P = .009)
Conversely, weight loss appeared to offer some protection from structural damage in knee OA, Gabby B. Joseph, PhD, a specialist in radiology and biomedical imaging, said at the congress, sponsored by the Osteoarthritis Research Society International.
Indeed, individuals who had achieved a weight loss of more than 5% at 4-year follow up were less likely to have a worsened Kellgren and Lawrence (KL) grade than those whose body weight remained the same (odds ratio, 0.69, P = .009).
Weight loss was also associated with a higher change of experiencing resolution in knee pain over 12 months, with an OR of 1.40 (P = .019).
Importance of weight change in OA
“We know that weight loss has beneficial effects on knee OA symptoms, such as pain relief and improvement in physical function,” commented Xingzhong Jin, PhD, an NHMRC Early Career Fellow at the Centre for Big Data Research in Health at the University of New South Wales, Sydney.
“But what is unclear is whether weight loss could slow down structural degradation in the joint in the long run,” he said in an interview. “These findings mean that weight control is clearly very important for knee OA, in terms of improving symptoms as well as preventing structural progression.”
He added: “The evidence on hip OA is less clear. As most of the knowledge in this space was generated from people with knee OA, this work is an important contribution to knowledge around the care of people with hip OA.”
Why look at weight change effects in OA?
“Obesity is a modifiable risk factor for osteoarthritis,” Dr. Joseph said at the start of her virtual presentation. Indeed, patients with obesity are more than twice as likely to develop knee OA than their normal weight counterparts.
Although there have been various studies looking at weight loss and weight gain in OA, most have focused on weight loss rather than gain, and OA in the knee rather than the hip, she explained.
The aim of the present study, therefore, was to take a closer look at the possible effect of both weight gain and weight loss in people with hip or knee OA in terms of radiographic outcomes (KL grade change, medial JSN), symptomatic outcomes (knee pain and resolution at 12 months), and the need for joint replacement.
“The clinical implications are to develop targeted long-term strategies for site-specific informed recommendations to prevent joint degeneration,” Dr. Joseph said.
Using data on nearly 3,000 individuals from the OAI, Dr Joseph and collaborators classified people with OA into one of three groups: those with at least a 5% gain in weight, (n = 714), those with no (–3% to 3%) change in weight (n = 1,553), and those with at least a 5% loss in weight over a 4-year period.
The results, which were published in Arthritis Care & Research, also revealed no differences in the rate of total hip or knee arthroplasties between the groups, and no differences between the weight gain and weight loss groups and controls in term of hip radiographic or symptomatic changes.
“Why are there differing effects of weight change in the knee versus the hip? This could be multifactorial, but there could be a few things going on,” said Dr. Joseph. “First, the joint structure is clearly different between the knee and the hip. The knee is a hinge joint. The hip is a ball and socket joint malalignment could affect these in different ways.”
Additionally, “the knee also has thicker cartilage, the hip has thinner cartilage again, and the loading patterns may be different in these joints.”
There were also differences in the rate of progression between the knee and the hip, “this was especially noticeable for the radiographic progression,” Dr. Joseph said, with rates being higher in the knee.
Noting that the study is limited by its retrospective design, Dr. Joseph concluded: “We don’t know why these people lost or gained weight. So, this would be something that would be more apparent in a prospective study.
“Also, there were no MRI outcomes, as MRI imaging was not available in the hip in the OAI, but clearly morphology T1 and T2 would be useful to assess as outcomes here as well.”
The OAI is a public-private partnership funded by the National Institutes of Health and initial support from Merck, Novartis, GlaxoSmithKline and Pfizer. Dr. Joseph and Dr. Jin reported having no conflicts of interest to disclose.
researchers reported at the OARSI 2022 World Congress.
Using data from the Osteoarthritis Initiative (OAI), researchers from the University of California found that a greater than 5% increase in body weight over 4 years was associated with a 29% increased risk for medial joint space narrowing (JSN), compared with controls (P = .038). There was also a 34% increased risk for developing frequent knee pain (P = .009)
Conversely, weight loss appeared to offer some protection from structural damage in knee OA, Gabby B. Joseph, PhD, a specialist in radiology and biomedical imaging, said at the congress, sponsored by the Osteoarthritis Research Society International.
Indeed, individuals who had achieved a weight loss of more than 5% at 4-year follow up were less likely to have a worsened Kellgren and Lawrence (KL) grade than those whose body weight remained the same (odds ratio, 0.69, P = .009).
Weight loss was also associated with a higher change of experiencing resolution in knee pain over 12 months, with an OR of 1.40 (P = .019).
Importance of weight change in OA
“We know that weight loss has beneficial effects on knee OA symptoms, such as pain relief and improvement in physical function,” commented Xingzhong Jin, PhD, an NHMRC Early Career Fellow at the Centre for Big Data Research in Health at the University of New South Wales, Sydney.
“But what is unclear is whether weight loss could slow down structural degradation in the joint in the long run,” he said in an interview. “These findings mean that weight control is clearly very important for knee OA, in terms of improving symptoms as well as preventing structural progression.”
He added: “The evidence on hip OA is less clear. As most of the knowledge in this space was generated from people with knee OA, this work is an important contribution to knowledge around the care of people with hip OA.”
Why look at weight change effects in OA?
“Obesity is a modifiable risk factor for osteoarthritis,” Dr. Joseph said at the start of her virtual presentation. Indeed, patients with obesity are more than twice as likely to develop knee OA than their normal weight counterparts.
Although there have been various studies looking at weight loss and weight gain in OA, most have focused on weight loss rather than gain, and OA in the knee rather than the hip, she explained.
The aim of the present study, therefore, was to take a closer look at the possible effect of both weight gain and weight loss in people with hip or knee OA in terms of radiographic outcomes (KL grade change, medial JSN), symptomatic outcomes (knee pain and resolution at 12 months), and the need for joint replacement.
“The clinical implications are to develop targeted long-term strategies for site-specific informed recommendations to prevent joint degeneration,” Dr. Joseph said.
Using data on nearly 3,000 individuals from the OAI, Dr Joseph and collaborators classified people with OA into one of three groups: those with at least a 5% gain in weight, (n = 714), those with no (–3% to 3%) change in weight (n = 1,553), and those with at least a 5% loss in weight over a 4-year period.
The results, which were published in Arthritis Care & Research, also revealed no differences in the rate of total hip or knee arthroplasties between the groups, and no differences between the weight gain and weight loss groups and controls in term of hip radiographic or symptomatic changes.
“Why are there differing effects of weight change in the knee versus the hip? This could be multifactorial, but there could be a few things going on,” said Dr. Joseph. “First, the joint structure is clearly different between the knee and the hip. The knee is a hinge joint. The hip is a ball and socket joint malalignment could affect these in different ways.”
Additionally, “the knee also has thicker cartilage, the hip has thinner cartilage again, and the loading patterns may be different in these joints.”
There were also differences in the rate of progression between the knee and the hip, “this was especially noticeable for the radiographic progression,” Dr. Joseph said, with rates being higher in the knee.
Noting that the study is limited by its retrospective design, Dr. Joseph concluded: “We don’t know why these people lost or gained weight. So, this would be something that would be more apparent in a prospective study.
“Also, there were no MRI outcomes, as MRI imaging was not available in the hip in the OAI, but clearly morphology T1 and T2 would be useful to assess as outcomes here as well.”
The OAI is a public-private partnership funded by the National Institutes of Health and initial support from Merck, Novartis, GlaxoSmithKline and Pfizer. Dr. Joseph and Dr. Jin reported having no conflicts of interest to disclose.
FROM OARSI 2022
FDA recommends switch to partially or fully disposable duodenoscope models
Health care facilities and providers should complete the transition to fully disposable duodenoscopes and those with disposable components, the U.S. Food and Drug Administration announced this week after an analysis of postmarket surveillance studies was completed.
The FDA’s directive updates its April 2020 recommendations on the subject. It cites concerns about cleaning fixed endcap duodenoscopes and the increasing availability of models that eliminate the need for reprocessing.
The announcement highlighted the potential for a dramatic difference in between-patient contamination risk, reducing it “by half or more as compared to reusable, or fixed endcaps.”
“Interim results from one duodenoscope model with a removable component show a contamination rate of just 0.5%, as compared to older duodenoscope models which had contamination rates as high as 6%,” the FDA writes.
Duodenoscopes are used in more than 500,000 procedures each year in the United States and are key in assessing and treating diseases and conditions of the pancreas and bile ducts.
Upgrade to new models to decrease infections
Manufacturers no longer market fixed endcap models in the United States, but some health care facilities continue to use them. The FDA recommends that all fixed endcap models be replaced.
The FDA says some manufacturers are offering replacement programs to upgrade to a model with a disposable component at no cost.
Two fully disposable models and five with disposable components have been cleared by the FDA. (One model is no longer marketed and thus not listed here.)
Fully Disposable:
Ambu Innovation GmbH, Duodenoscope model aScope Duodeno
Boston Scientific Corporation, EXALT Model D Single-Use Duodenoscope
Disposable Components:
Fujifilm Corporation, Duodenoscope model ED-580XT
Olympus Medical Systems, Evis Exera III Duodenovideoscope Olympus TJF-Q190V
Pentax Medical, Duodenoscope model ED34-i10T2
Pentax Medical, Duodenoscope model ED32-i10
Additionally, the failure to correctly reprocess a duodenoscope could result in tissue or fluid from one patient transferring to a subsequent patient.
“In rare cases, this can lead to patient-to-patient disease transmission,” the FDA says.
Postmarket surveillance studies
In 2015, the FDA ordered three manufacturers of reusable devices (Fujifilm, Olympus, and Pentax) to conduct postmarket surveillance studies to determine contamination rates after reprocessing.
In 2019, the FDA also ordered postmarket surveillance studies to the makers of duodenoscopes with disposable endcaps to verify that the new designs reduce the contamination rate.
The final results of the fixed endcap design indicate that contamination rates were as high as 6.6% with high-concern organisms after contamination. High-concern organisms are those more often associated with disease, such as E coli and Pseudomonas contamination.
“As a result, Pentax and Olympus are withdrawing their fixed endcap duodenoscopes from the market, and Fujifilm has completed withdrawal of its fixed endcap duodenoscope,” the FDA writes.
Studies are not yet complete for duodenoscopes with removable components. As of August 12, 2021, the Fujifilm ED-580XT duodenoscope with a removable cap had 57% of the samples required. Interim results indicate that no samples tested positive for enough low-concern organisms to indicate a reprocessing failure, and only 0.5% tested positive for high-concern organisms.
In addition to the contamination risk sampling, each manufacturer was ordered to do postmarket surveillance studies to evaluate whether staff could understand and follow the manufacturer’s reprocessing instructions in real-world health care settings.
According to the FDA, the results showed that users frequently had difficulty understanding and following the manufacturers’ instructions and were not able to successfully complete reprocessing with the older models.
However, the newer models had high user success rates for understanding instructions and correctly performing reprocessing tasks, the FDA says.
A version of this article first appeared on Medscape.com.
AGA supports FDA’s continued efforts to reduce the risk of disease transmission by duodenoscopes. Through the AGA Center for GI Innovation and Technology, AGA continues to support innovation in medical technology. To get up to date on past challenges with scope infections and future directions, check out AGA’s Innovation in Duodenoscope Design program, consisting of articles, webinars, and podcasts with leading experts in this space.
Health care facilities and providers should complete the transition to fully disposable duodenoscopes and those with disposable components, the U.S. Food and Drug Administration announced this week after an analysis of postmarket surveillance studies was completed.
The FDA’s directive updates its April 2020 recommendations on the subject. It cites concerns about cleaning fixed endcap duodenoscopes and the increasing availability of models that eliminate the need for reprocessing.
The announcement highlighted the potential for a dramatic difference in between-patient contamination risk, reducing it “by half or more as compared to reusable, or fixed endcaps.”
“Interim results from one duodenoscope model with a removable component show a contamination rate of just 0.5%, as compared to older duodenoscope models which had contamination rates as high as 6%,” the FDA writes.
Duodenoscopes are used in more than 500,000 procedures each year in the United States and are key in assessing and treating diseases and conditions of the pancreas and bile ducts.
Upgrade to new models to decrease infections
Manufacturers no longer market fixed endcap models in the United States, but some health care facilities continue to use them. The FDA recommends that all fixed endcap models be replaced.
The FDA says some manufacturers are offering replacement programs to upgrade to a model with a disposable component at no cost.
Two fully disposable models and five with disposable components have been cleared by the FDA. (One model is no longer marketed and thus not listed here.)
Fully Disposable:
Ambu Innovation GmbH, Duodenoscope model aScope Duodeno
Boston Scientific Corporation, EXALT Model D Single-Use Duodenoscope
Disposable Components:
Fujifilm Corporation, Duodenoscope model ED-580XT
Olympus Medical Systems, Evis Exera III Duodenovideoscope Olympus TJF-Q190V
Pentax Medical, Duodenoscope model ED34-i10T2
Pentax Medical, Duodenoscope model ED32-i10
Additionally, the failure to correctly reprocess a duodenoscope could result in tissue or fluid from one patient transferring to a subsequent patient.
“In rare cases, this can lead to patient-to-patient disease transmission,” the FDA says.
Postmarket surveillance studies
In 2015, the FDA ordered three manufacturers of reusable devices (Fujifilm, Olympus, and Pentax) to conduct postmarket surveillance studies to determine contamination rates after reprocessing.
In 2019, the FDA also ordered postmarket surveillance studies to the makers of duodenoscopes with disposable endcaps to verify that the new designs reduce the contamination rate.
The final results of the fixed endcap design indicate that contamination rates were as high as 6.6% with high-concern organisms after contamination. High-concern organisms are those more often associated with disease, such as E coli and Pseudomonas contamination.
“As a result, Pentax and Olympus are withdrawing their fixed endcap duodenoscopes from the market, and Fujifilm has completed withdrawal of its fixed endcap duodenoscope,” the FDA writes.
Studies are not yet complete for duodenoscopes with removable components. As of August 12, 2021, the Fujifilm ED-580XT duodenoscope with a removable cap had 57% of the samples required. Interim results indicate that no samples tested positive for enough low-concern organisms to indicate a reprocessing failure, and only 0.5% tested positive for high-concern organisms.
In addition to the contamination risk sampling, each manufacturer was ordered to do postmarket surveillance studies to evaluate whether staff could understand and follow the manufacturer’s reprocessing instructions in real-world health care settings.
According to the FDA, the results showed that users frequently had difficulty understanding and following the manufacturers’ instructions and were not able to successfully complete reprocessing with the older models.
However, the newer models had high user success rates for understanding instructions and correctly performing reprocessing tasks, the FDA says.
A version of this article first appeared on Medscape.com.
AGA supports FDA’s continued efforts to reduce the risk of disease transmission by duodenoscopes. Through the AGA Center for GI Innovation and Technology, AGA continues to support innovation in medical technology. To get up to date on past challenges with scope infections and future directions, check out AGA’s Innovation in Duodenoscope Design program, consisting of articles, webinars, and podcasts with leading experts in this space.
Health care facilities and providers should complete the transition to fully disposable duodenoscopes and those with disposable components, the U.S. Food and Drug Administration announced this week after an analysis of postmarket surveillance studies was completed.
The FDA’s directive updates its April 2020 recommendations on the subject. It cites concerns about cleaning fixed endcap duodenoscopes and the increasing availability of models that eliminate the need for reprocessing.
The announcement highlighted the potential for a dramatic difference in between-patient contamination risk, reducing it “by half or more as compared to reusable, or fixed endcaps.”
“Interim results from one duodenoscope model with a removable component show a contamination rate of just 0.5%, as compared to older duodenoscope models which had contamination rates as high as 6%,” the FDA writes.
Duodenoscopes are used in more than 500,000 procedures each year in the United States and are key in assessing and treating diseases and conditions of the pancreas and bile ducts.
Upgrade to new models to decrease infections
Manufacturers no longer market fixed endcap models in the United States, but some health care facilities continue to use them. The FDA recommends that all fixed endcap models be replaced.
The FDA says some manufacturers are offering replacement programs to upgrade to a model with a disposable component at no cost.
Two fully disposable models and five with disposable components have been cleared by the FDA. (One model is no longer marketed and thus not listed here.)
Fully Disposable:
Ambu Innovation GmbH, Duodenoscope model aScope Duodeno
Boston Scientific Corporation, EXALT Model D Single-Use Duodenoscope
Disposable Components:
Fujifilm Corporation, Duodenoscope model ED-580XT
Olympus Medical Systems, Evis Exera III Duodenovideoscope Olympus TJF-Q190V
Pentax Medical, Duodenoscope model ED34-i10T2
Pentax Medical, Duodenoscope model ED32-i10
Additionally, the failure to correctly reprocess a duodenoscope could result in tissue or fluid from one patient transferring to a subsequent patient.
“In rare cases, this can lead to patient-to-patient disease transmission,” the FDA says.
Postmarket surveillance studies
In 2015, the FDA ordered three manufacturers of reusable devices (Fujifilm, Olympus, and Pentax) to conduct postmarket surveillance studies to determine contamination rates after reprocessing.
In 2019, the FDA also ordered postmarket surveillance studies to the makers of duodenoscopes with disposable endcaps to verify that the new designs reduce the contamination rate.
The final results of the fixed endcap design indicate that contamination rates were as high as 6.6% with high-concern organisms after contamination. High-concern organisms are those more often associated with disease, such as E coli and Pseudomonas contamination.
“As a result, Pentax and Olympus are withdrawing their fixed endcap duodenoscopes from the market, and Fujifilm has completed withdrawal of its fixed endcap duodenoscope,” the FDA writes.
Studies are not yet complete for duodenoscopes with removable components. As of August 12, 2021, the Fujifilm ED-580XT duodenoscope with a removable cap had 57% of the samples required. Interim results indicate that no samples tested positive for enough low-concern organisms to indicate a reprocessing failure, and only 0.5% tested positive for high-concern organisms.
In addition to the contamination risk sampling, each manufacturer was ordered to do postmarket surveillance studies to evaluate whether staff could understand and follow the manufacturer’s reprocessing instructions in real-world health care settings.
According to the FDA, the results showed that users frequently had difficulty understanding and following the manufacturers’ instructions and were not able to successfully complete reprocessing with the older models.
However, the newer models had high user success rates for understanding instructions and correctly performing reprocessing tasks, the FDA says.
A version of this article first appeared on Medscape.com.
AGA supports FDA’s continued efforts to reduce the risk of disease transmission by duodenoscopes. Through the AGA Center for GI Innovation and Technology, AGA continues to support innovation in medical technology. To get up to date on past challenges with scope infections and future directions, check out AGA’s Innovation in Duodenoscope Design program, consisting of articles, webinars, and podcasts with leading experts in this space.
Unraveling primary ovarian insufficiency
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years
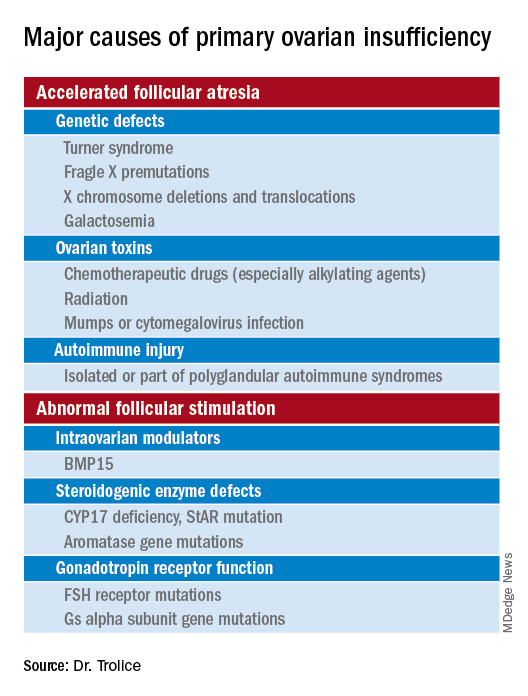
Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Assay-guided chemo in recurrent glioma linked to longer survival
New research suggests that chemotherapy treatments for recurrent high-grade gliomas indicated by an assay-guided tool called ChemoID can boost median survival, compared with physician choice.
The randomized, phase 3 trial results were presented at the annual meeting of the American Association for Cancer Research.
Over a median follow-up of 9 months, median overall survival in the ChemoID group was 12.5 months (95% confidence interval, 10.2-14.7), compared with 9 months (95% CI, 4.2-13.8) in the group whose treatments were chosen by physicians (P = .010).
“While the prognosis is very dismal, we’re still providing a 3.5-month benefit in the guided arm versus physician choice,” said study coauthor Jagan Valluri, PhD, professor of cellular biology and integrative medicine at Marshall University, Huntington, W. Va.
As Dr. Valluri noted, patients with recurrent high-grade gliomas typically have failed radiation and are left with poor prognoses. Fewer than one in four patients respond to chemotherapy at this point, he said, and the response is inconsistent from patient to patient.
“We developed ChemoID since cancer is very unique,” he said, “and any kind of chemotherapy should be tailored to each individual patient on a case-by-case basis.”
The ChemoID tool, a proprietary assay, tests the response of patient cells to various chemotherapy treatments. A test costs $3,500, and some insurers cover it, Dr. Valluri said.
For the new study, researchers randomly assigned 50 patients with grade III/IV recurrent glioma to be treated with chemotherapy chosen by physicians or chemotherapy recommended by the ChemoID tool.
Risk of death in the ChemoID group was lower than in the physician-guided group (hazard ratio, 0.44; 95% CI, 0.24-0.81; P = .008), and median progression-free survival was higher in the ChemoID group (10.1 months vs. 3.5 months; 95% CI, 4.8-15.4 vs. 1.9-5.1; HR, 0.25; 95% CI, 0.14-0.44; P < .001).
“We want the treating physician to have actionable tools in front of them before they treat the patient,” Dr. Valluri said. “We want this assay to become mainstream and part of the standard care workup.”
The study is funded by Cordgenics, where Dr. Valluri serves as chief operating officer.
New research suggests that chemotherapy treatments for recurrent high-grade gliomas indicated by an assay-guided tool called ChemoID can boost median survival, compared with physician choice.
The randomized, phase 3 trial results were presented at the annual meeting of the American Association for Cancer Research.
Over a median follow-up of 9 months, median overall survival in the ChemoID group was 12.5 months (95% confidence interval, 10.2-14.7), compared with 9 months (95% CI, 4.2-13.8) in the group whose treatments were chosen by physicians (P = .010).
“While the prognosis is very dismal, we’re still providing a 3.5-month benefit in the guided arm versus physician choice,” said study coauthor Jagan Valluri, PhD, professor of cellular biology and integrative medicine at Marshall University, Huntington, W. Va.
As Dr. Valluri noted, patients with recurrent high-grade gliomas typically have failed radiation and are left with poor prognoses. Fewer than one in four patients respond to chemotherapy at this point, he said, and the response is inconsistent from patient to patient.
“We developed ChemoID since cancer is very unique,” he said, “and any kind of chemotherapy should be tailored to each individual patient on a case-by-case basis.”
The ChemoID tool, a proprietary assay, tests the response of patient cells to various chemotherapy treatments. A test costs $3,500, and some insurers cover it, Dr. Valluri said.
For the new study, researchers randomly assigned 50 patients with grade III/IV recurrent glioma to be treated with chemotherapy chosen by physicians or chemotherapy recommended by the ChemoID tool.
Risk of death in the ChemoID group was lower than in the physician-guided group (hazard ratio, 0.44; 95% CI, 0.24-0.81; P = .008), and median progression-free survival was higher in the ChemoID group (10.1 months vs. 3.5 months; 95% CI, 4.8-15.4 vs. 1.9-5.1; HR, 0.25; 95% CI, 0.14-0.44; P < .001).
“We want the treating physician to have actionable tools in front of them before they treat the patient,” Dr. Valluri said. “We want this assay to become mainstream and part of the standard care workup.”
The study is funded by Cordgenics, where Dr. Valluri serves as chief operating officer.
New research suggests that chemotherapy treatments for recurrent high-grade gliomas indicated by an assay-guided tool called ChemoID can boost median survival, compared with physician choice.
The randomized, phase 3 trial results were presented at the annual meeting of the American Association for Cancer Research.
Over a median follow-up of 9 months, median overall survival in the ChemoID group was 12.5 months (95% confidence interval, 10.2-14.7), compared with 9 months (95% CI, 4.2-13.8) in the group whose treatments were chosen by physicians (P = .010).
“While the prognosis is very dismal, we’re still providing a 3.5-month benefit in the guided arm versus physician choice,” said study coauthor Jagan Valluri, PhD, professor of cellular biology and integrative medicine at Marshall University, Huntington, W. Va.
As Dr. Valluri noted, patients with recurrent high-grade gliomas typically have failed radiation and are left with poor prognoses. Fewer than one in four patients respond to chemotherapy at this point, he said, and the response is inconsistent from patient to patient.
“We developed ChemoID since cancer is very unique,” he said, “and any kind of chemotherapy should be tailored to each individual patient on a case-by-case basis.”
The ChemoID tool, a proprietary assay, tests the response of patient cells to various chemotherapy treatments. A test costs $3,500, and some insurers cover it, Dr. Valluri said.
For the new study, researchers randomly assigned 50 patients with grade III/IV recurrent glioma to be treated with chemotherapy chosen by physicians or chemotherapy recommended by the ChemoID tool.
Risk of death in the ChemoID group was lower than in the physician-guided group (hazard ratio, 0.44; 95% CI, 0.24-0.81; P = .008), and median progression-free survival was higher in the ChemoID group (10.1 months vs. 3.5 months; 95% CI, 4.8-15.4 vs. 1.9-5.1; HR, 0.25; 95% CI, 0.14-0.44; P < .001).
“We want the treating physician to have actionable tools in front of them before they treat the patient,” Dr. Valluri said. “We want this assay to become mainstream and part of the standard care workup.”
The study is funded by Cordgenics, where Dr. Valluri serves as chief operating officer.
FROM AACR 2022
Persistent problem: High C-section rates plague the South
All along, Julia Maeda knew she wanted to have her baby naturally. For her, that meant in a hospital, vaginally, without an epidural for pain relief.
This was her first pregnancy. And although she is a nurse, she was working with cancer patients at the time, not with laboring mothers or babies. “I really didn’t know what I was getting into,” said Ms. Maeda, now 32. “I didn’t do much preparation.”
Her home state of Mississippi has the highest cesarean section rate in the United States – nearly 4 in 10 women who give birth there deliver their babies via C-section. Almost 2 weeks past her due date in 2019, Ms. Maeda became one of them after her doctor came to her bedside while she was in labor.
“‘You’re not in distress, and your baby is not in distress – but we don’t want you to get that way, so we need to think about a C-section,’” she recalled her doctor saying. “I was totally defeated. I just gave in.”
C-sections are sometimes necessary and even lifesaving, but public health experts have long contended that too many performed in the U.S. aren’t. They argue it is major surgery accompanied by significant risk and a high price tag.
Overall, 31.8% of all births in the U.S. were C-sections in 2020, just a slight tick up from 31.7% the year before, according to the latest data from the Centers for Disease Control and Prevention. But that’s close to the peak in 2009, when it was 32.9%. And the rates are far higher in many states, especially across the South.
These high C-section rates have persisted – and in some states, such as Alabama and Kentucky, even grown slightly – despite continual calls to reduce them. And although the pandemic presented new challenges for pregnant women, research suggests that the U.S. C-section rate was unaffected by COVID. Instead, obstetricians and other health experts say the high rate is an intractable problem.
Some states, such as California and New Jersey, have reduced their rates through a variety of strategies, including sharing C-section data with doctors and hospitals. But change has proved difficult elsewhere, especially in the South and in Texas, where women are generally less healthy heading into their pregnancies and maternal and infant health problems are among the highest in the United States.
“We have to restructure how we think about C-sections,” said Veronica Gillispie-Bell, MD, an ob.gyn. who is medical director of the Louisiana Perinatal Quality Collaborative, Kenner, La., a group of 43 birthing hospitals focused on lowering Louisiana’s C-section rate. “It’s a lifesaving technique, but it’s also not without risks.”
She said C-sections, like any operation, create scar tissue, including in the uterus, which may complicate future pregnancies or abdominal surgeries. C-sections also typically lead to an extended hospital stay and recovery period and increase the chance of infection. Babies face risks, too. In rare cases, they can be nicked or cut during an incision.
Although C-sections are sometimes necessary, public health leaders say these surgeries have been overused in many places. Black women, particularly, are more likely to give birth by C-section than any other racial group in the country. Often, hospitals and even regions have wide, unexplained variations in rates.
“If you were delivering in Miami-Dade County, you had a 75% greater chance of having a cesarean than in northern Florida,” said William Sappenfield, MD, an ob.gyn. and epidemiologist at the University of South Florida, Tampa, who has studied the state’s high C-section rate.
Some physicians say their rates are driven by mothers who request the procedure, not by doctors. But Rebekah Gee, MD, an ob.gyn. at Louisiana State University Healthcare Network, New Orleans, and former secretary of the Louisiana Department of Health, said she saw C-section rates go dramatically up at 4 and 5 p.m. – around the time when doctors tend to want to go home.
She led several initiatives to improve birth outcomes in Louisiana, including leveling Medicaid payment rates to hospitals for vaginal deliveries and C-sections. In most places, C-sections are significantly more expensive than vaginal deliveries, making high C-section rates not only a concern for expectant mothers but also for taxpayers.
Medicaid pays for 60% of all births in Louisiana, according to KFF, and about half of all births in most Southern states, compared with 42% nationally. That’s one reason some states – including Louisiana, Tennessee, and Minnesota – have tried to tackle high C-section rates by changing how much Medicaid pays for them. But payment reform alone isn’t enough, Dr. Gee said.
“There was a guy in central Louisiana who was doing more C-sections and early elective deliveries than anyone in the U.S.,” she said. “When you have a culture like that, it’s hard to shift from it.”
Linda Schwimmer, president and CEO of the New Jersey Health Care Quality Institute, said many hospitals and doctors don’t even know their C-section rates. Sharing this data with doctors and hospitals – and making it public – made some providers uncomfortable, she said, but it ultimately worked. New Jersey’s C-section rate among first-time, low-risk mothers dropped from 33.1% in 2013 to 26.7% 6 years later once the state began sharing these data, among other initiatives.
The New Jersey Health Care Quality Institute and other groups like it around the country focus on reducing a subset of C-sections called “nulliparous, term, singleton, vertex” C-sections, or surgeries on first-time, full-term moms giving birth to a single infant who is positioned head-down in the uterus.
NTSV C-sections are important to track because women who have a C-section during their first pregnancy face a 90% chance of having another in subsequent pregnancies. Across the U.S., the rate for these C-sections was 25.9% in 2020 and 25.6% in 2019.
Elliott Main, MD, a maternal-fetal specialist at Stanford (Calif.) University and the medical director of the California Maternal Quality Care Collaborative, coauthored a paper, published in JAMA last year, that outlines interventions the collaborative took that lowered California’s NTSV C-Section rate from 26.0% in 2014 to 22.8% in 2019. Nationally, the rate was unchanged during that period.
Allowing women to labor for longer stretches of time before resorting to surgery is important, he said.
The cervix must be 10 cm dilated before a woman gives birth. The threshold for “active labor” used to be when the cervix was dilated at least 4 cm. In more recent years, though, the onset of active labor has been changed to 5-6 cm.
“People show up at the hospital too early,” said Toni Hill, president of the Mississippi Midwives Alliance. “If you show up to the hospital at 2-3 centimeters, you can be at 2-3 centimeters for weeks. I don’t even consider that labor.”
Too often, she said, women at an early stage of labor end up being induced and deliver via C-section.
“It’s almost like, at this point, C-sections are being handed out like lollipops,” said LA’Patricia Washington, a doula based in Jackson, Miss. Doulas are trained, nonmedical workers who help parents before, during, and after delivery.
Ms. Washington works with a nonprofit group, the Jackson Safer Childbirth Experience, that pays for doulas to help expectant mothers in the region. Some state Medicaid programs, such as New Jersey’s, reimburse for services by doulas because research shows they can reduce C-section rates. California has been trying to roll out the same benefit for its Medicaid members.
In 2020, when Julia Maeda became pregnant again, she paid out-of-pocket for a doula to attend the birth. The experience of having her son via C-section the previous year had been “emotionally and psychologically traumatic,” Ms. Maeda said.
She told her ob.gyn. that she wanted a VBAC, short for “vaginal birth after cesarean.” But, she said, “he just shook his head and said, ‘That’s not a good idea.’”
She had VBAC anyway. Ms. Maeda credits her doula with making it happen.
“Maybe just her presence relayed to the nursing staff that this was something I was serious about,” Ms. Maeda said. “They want you to have your baby during business hours. And biology doesn’t work that way.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
All along, Julia Maeda knew she wanted to have her baby naturally. For her, that meant in a hospital, vaginally, without an epidural for pain relief.
This was her first pregnancy. And although she is a nurse, she was working with cancer patients at the time, not with laboring mothers or babies. “I really didn’t know what I was getting into,” said Ms. Maeda, now 32. “I didn’t do much preparation.”
Her home state of Mississippi has the highest cesarean section rate in the United States – nearly 4 in 10 women who give birth there deliver their babies via C-section. Almost 2 weeks past her due date in 2019, Ms. Maeda became one of them after her doctor came to her bedside while she was in labor.
“‘You’re not in distress, and your baby is not in distress – but we don’t want you to get that way, so we need to think about a C-section,’” she recalled her doctor saying. “I was totally defeated. I just gave in.”
C-sections are sometimes necessary and even lifesaving, but public health experts have long contended that too many performed in the U.S. aren’t. They argue it is major surgery accompanied by significant risk and a high price tag.
Overall, 31.8% of all births in the U.S. were C-sections in 2020, just a slight tick up from 31.7% the year before, according to the latest data from the Centers for Disease Control and Prevention. But that’s close to the peak in 2009, when it was 32.9%. And the rates are far higher in many states, especially across the South.
These high C-section rates have persisted – and in some states, such as Alabama and Kentucky, even grown slightly – despite continual calls to reduce them. And although the pandemic presented new challenges for pregnant women, research suggests that the U.S. C-section rate was unaffected by COVID. Instead, obstetricians and other health experts say the high rate is an intractable problem.
Some states, such as California and New Jersey, have reduced their rates through a variety of strategies, including sharing C-section data with doctors and hospitals. But change has proved difficult elsewhere, especially in the South and in Texas, where women are generally less healthy heading into their pregnancies and maternal and infant health problems are among the highest in the United States.
“We have to restructure how we think about C-sections,” said Veronica Gillispie-Bell, MD, an ob.gyn. who is medical director of the Louisiana Perinatal Quality Collaborative, Kenner, La., a group of 43 birthing hospitals focused on lowering Louisiana’s C-section rate. “It’s a lifesaving technique, but it’s also not without risks.”
She said C-sections, like any operation, create scar tissue, including in the uterus, which may complicate future pregnancies or abdominal surgeries. C-sections also typically lead to an extended hospital stay and recovery period and increase the chance of infection. Babies face risks, too. In rare cases, they can be nicked or cut during an incision.
Although C-sections are sometimes necessary, public health leaders say these surgeries have been overused in many places. Black women, particularly, are more likely to give birth by C-section than any other racial group in the country. Often, hospitals and even regions have wide, unexplained variations in rates.
“If you were delivering in Miami-Dade County, you had a 75% greater chance of having a cesarean than in northern Florida,” said William Sappenfield, MD, an ob.gyn. and epidemiologist at the University of South Florida, Tampa, who has studied the state’s high C-section rate.
Some physicians say their rates are driven by mothers who request the procedure, not by doctors. But Rebekah Gee, MD, an ob.gyn. at Louisiana State University Healthcare Network, New Orleans, and former secretary of the Louisiana Department of Health, said she saw C-section rates go dramatically up at 4 and 5 p.m. – around the time when doctors tend to want to go home.
She led several initiatives to improve birth outcomes in Louisiana, including leveling Medicaid payment rates to hospitals for vaginal deliveries and C-sections. In most places, C-sections are significantly more expensive than vaginal deliveries, making high C-section rates not only a concern for expectant mothers but also for taxpayers.
Medicaid pays for 60% of all births in Louisiana, according to KFF, and about half of all births in most Southern states, compared with 42% nationally. That’s one reason some states – including Louisiana, Tennessee, and Minnesota – have tried to tackle high C-section rates by changing how much Medicaid pays for them. But payment reform alone isn’t enough, Dr. Gee said.
“There was a guy in central Louisiana who was doing more C-sections and early elective deliveries than anyone in the U.S.,” she said. “When you have a culture like that, it’s hard to shift from it.”
Linda Schwimmer, president and CEO of the New Jersey Health Care Quality Institute, said many hospitals and doctors don’t even know their C-section rates. Sharing this data with doctors and hospitals – and making it public – made some providers uncomfortable, she said, but it ultimately worked. New Jersey’s C-section rate among first-time, low-risk mothers dropped from 33.1% in 2013 to 26.7% 6 years later once the state began sharing these data, among other initiatives.
The New Jersey Health Care Quality Institute and other groups like it around the country focus on reducing a subset of C-sections called “nulliparous, term, singleton, vertex” C-sections, or surgeries on first-time, full-term moms giving birth to a single infant who is positioned head-down in the uterus.
NTSV C-sections are important to track because women who have a C-section during their first pregnancy face a 90% chance of having another in subsequent pregnancies. Across the U.S., the rate for these C-sections was 25.9% in 2020 and 25.6% in 2019.
Elliott Main, MD, a maternal-fetal specialist at Stanford (Calif.) University and the medical director of the California Maternal Quality Care Collaborative, coauthored a paper, published in JAMA last year, that outlines interventions the collaborative took that lowered California’s NTSV C-Section rate from 26.0% in 2014 to 22.8% in 2019. Nationally, the rate was unchanged during that period.
Allowing women to labor for longer stretches of time before resorting to surgery is important, he said.
The cervix must be 10 cm dilated before a woman gives birth. The threshold for “active labor” used to be when the cervix was dilated at least 4 cm. In more recent years, though, the onset of active labor has been changed to 5-6 cm.
“People show up at the hospital too early,” said Toni Hill, president of the Mississippi Midwives Alliance. “If you show up to the hospital at 2-3 centimeters, you can be at 2-3 centimeters for weeks. I don’t even consider that labor.”
Too often, she said, women at an early stage of labor end up being induced and deliver via C-section.
“It’s almost like, at this point, C-sections are being handed out like lollipops,” said LA’Patricia Washington, a doula based in Jackson, Miss. Doulas are trained, nonmedical workers who help parents before, during, and after delivery.
Ms. Washington works with a nonprofit group, the Jackson Safer Childbirth Experience, that pays for doulas to help expectant mothers in the region. Some state Medicaid programs, such as New Jersey’s, reimburse for services by doulas because research shows they can reduce C-section rates. California has been trying to roll out the same benefit for its Medicaid members.
In 2020, when Julia Maeda became pregnant again, she paid out-of-pocket for a doula to attend the birth. The experience of having her son via C-section the previous year had been “emotionally and psychologically traumatic,” Ms. Maeda said.
She told her ob.gyn. that she wanted a VBAC, short for “vaginal birth after cesarean.” But, she said, “he just shook his head and said, ‘That’s not a good idea.’”
She had VBAC anyway. Ms. Maeda credits her doula with making it happen.
“Maybe just her presence relayed to the nursing staff that this was something I was serious about,” Ms. Maeda said. “They want you to have your baby during business hours. And biology doesn’t work that way.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
All along, Julia Maeda knew she wanted to have her baby naturally. For her, that meant in a hospital, vaginally, without an epidural for pain relief.
This was her first pregnancy. And although she is a nurse, she was working with cancer patients at the time, not with laboring mothers or babies. “I really didn’t know what I was getting into,” said Ms. Maeda, now 32. “I didn’t do much preparation.”
Her home state of Mississippi has the highest cesarean section rate in the United States – nearly 4 in 10 women who give birth there deliver their babies via C-section. Almost 2 weeks past her due date in 2019, Ms. Maeda became one of them after her doctor came to her bedside while she was in labor.
“‘You’re not in distress, and your baby is not in distress – but we don’t want you to get that way, so we need to think about a C-section,’” she recalled her doctor saying. “I was totally defeated. I just gave in.”
C-sections are sometimes necessary and even lifesaving, but public health experts have long contended that too many performed in the U.S. aren’t. They argue it is major surgery accompanied by significant risk and a high price tag.
Overall, 31.8% of all births in the U.S. were C-sections in 2020, just a slight tick up from 31.7% the year before, according to the latest data from the Centers for Disease Control and Prevention. But that’s close to the peak in 2009, when it was 32.9%. And the rates are far higher in many states, especially across the South.
These high C-section rates have persisted – and in some states, such as Alabama and Kentucky, even grown slightly – despite continual calls to reduce them. And although the pandemic presented new challenges for pregnant women, research suggests that the U.S. C-section rate was unaffected by COVID. Instead, obstetricians and other health experts say the high rate is an intractable problem.
Some states, such as California and New Jersey, have reduced their rates through a variety of strategies, including sharing C-section data with doctors and hospitals. But change has proved difficult elsewhere, especially in the South and in Texas, where women are generally less healthy heading into their pregnancies and maternal and infant health problems are among the highest in the United States.
“We have to restructure how we think about C-sections,” said Veronica Gillispie-Bell, MD, an ob.gyn. who is medical director of the Louisiana Perinatal Quality Collaborative, Kenner, La., a group of 43 birthing hospitals focused on lowering Louisiana’s C-section rate. “It’s a lifesaving technique, but it’s also not without risks.”
She said C-sections, like any operation, create scar tissue, including in the uterus, which may complicate future pregnancies or abdominal surgeries. C-sections also typically lead to an extended hospital stay and recovery period and increase the chance of infection. Babies face risks, too. In rare cases, they can be nicked or cut during an incision.
Although C-sections are sometimes necessary, public health leaders say these surgeries have been overused in many places. Black women, particularly, are more likely to give birth by C-section than any other racial group in the country. Often, hospitals and even regions have wide, unexplained variations in rates.
“If you were delivering in Miami-Dade County, you had a 75% greater chance of having a cesarean than in northern Florida,” said William Sappenfield, MD, an ob.gyn. and epidemiologist at the University of South Florida, Tampa, who has studied the state’s high C-section rate.
Some physicians say their rates are driven by mothers who request the procedure, not by doctors. But Rebekah Gee, MD, an ob.gyn. at Louisiana State University Healthcare Network, New Orleans, and former secretary of the Louisiana Department of Health, said she saw C-section rates go dramatically up at 4 and 5 p.m. – around the time when doctors tend to want to go home.
She led several initiatives to improve birth outcomes in Louisiana, including leveling Medicaid payment rates to hospitals for vaginal deliveries and C-sections. In most places, C-sections are significantly more expensive than vaginal deliveries, making high C-section rates not only a concern for expectant mothers but also for taxpayers.
Medicaid pays for 60% of all births in Louisiana, according to KFF, and about half of all births in most Southern states, compared with 42% nationally. That’s one reason some states – including Louisiana, Tennessee, and Minnesota – have tried to tackle high C-section rates by changing how much Medicaid pays for them. But payment reform alone isn’t enough, Dr. Gee said.
“There was a guy in central Louisiana who was doing more C-sections and early elective deliveries than anyone in the U.S.,” she said. “When you have a culture like that, it’s hard to shift from it.”
Linda Schwimmer, president and CEO of the New Jersey Health Care Quality Institute, said many hospitals and doctors don’t even know their C-section rates. Sharing this data with doctors and hospitals – and making it public – made some providers uncomfortable, she said, but it ultimately worked. New Jersey’s C-section rate among first-time, low-risk mothers dropped from 33.1% in 2013 to 26.7% 6 years later once the state began sharing these data, among other initiatives.
The New Jersey Health Care Quality Institute and other groups like it around the country focus on reducing a subset of C-sections called “nulliparous, term, singleton, vertex” C-sections, or surgeries on first-time, full-term moms giving birth to a single infant who is positioned head-down in the uterus.
NTSV C-sections are important to track because women who have a C-section during their first pregnancy face a 90% chance of having another in subsequent pregnancies. Across the U.S., the rate for these C-sections was 25.9% in 2020 and 25.6% in 2019.
Elliott Main, MD, a maternal-fetal specialist at Stanford (Calif.) University and the medical director of the California Maternal Quality Care Collaborative, coauthored a paper, published in JAMA last year, that outlines interventions the collaborative took that lowered California’s NTSV C-Section rate from 26.0% in 2014 to 22.8% in 2019. Nationally, the rate was unchanged during that period.
Allowing women to labor for longer stretches of time before resorting to surgery is important, he said.
The cervix must be 10 cm dilated before a woman gives birth. The threshold for “active labor” used to be when the cervix was dilated at least 4 cm. In more recent years, though, the onset of active labor has been changed to 5-6 cm.
“People show up at the hospital too early,” said Toni Hill, president of the Mississippi Midwives Alliance. “If you show up to the hospital at 2-3 centimeters, you can be at 2-3 centimeters for weeks. I don’t even consider that labor.”
Too often, she said, women at an early stage of labor end up being induced and deliver via C-section.
“It’s almost like, at this point, C-sections are being handed out like lollipops,” said LA’Patricia Washington, a doula based in Jackson, Miss. Doulas are trained, nonmedical workers who help parents before, during, and after delivery.
Ms. Washington works with a nonprofit group, the Jackson Safer Childbirth Experience, that pays for doulas to help expectant mothers in the region. Some state Medicaid programs, such as New Jersey’s, reimburse for services by doulas because research shows they can reduce C-section rates. California has been trying to roll out the same benefit for its Medicaid members.
In 2020, when Julia Maeda became pregnant again, she paid out-of-pocket for a doula to attend the birth. The experience of having her son via C-section the previous year had been “emotionally and psychologically traumatic,” Ms. Maeda said.
She told her ob.gyn. that she wanted a VBAC, short for “vaginal birth after cesarean.” But, she said, “he just shook his head and said, ‘That’s not a good idea.’”
She had VBAC anyway. Ms. Maeda credits her doula with making it happen.
“Maybe just her presence relayed to the nursing staff that this was something I was serious about,” Ms. Maeda said. “They want you to have your baby during business hours. And biology doesn’t work that way.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Overuse of surveillance in bladder cancer, despite guidelines
(NMIBC), a new study concludes.
These cancers are associated with low rates of recurrence, progression, and bladder cancer–specific death, so current clinical practice guidelines recommend against frequent monitoring and testing.
However, the study authors found that patients with a low grade Ta NMIBC diagnosis underwent a median of three cystoscopies per year, and many also received a median of two imagine scans (CT or MRI) as well as 2-3 urine-based tests.
“These data suggest a need for ongoing efforts to limit overuse of treatment and surveillance, which may in turn mitigate associated increases in the costs of care,” write the authors, led by Kelly K. Bree, MD, from the department of urology, University of Texas MD Anderson Cancer Center, Houston. Bladder cancer has the highest lifetime treatment cost of all malignancies, they point out.
The study was published online in JAMA Network Open.
Higher value and more evidence-based
The impact of increased surveillance of this patient cohort has broad implications for patients and the health care system in general, say experts writing in an accompanying editorial.
“It has been well established that workup for NMIBC can have negative consequences for the physical and psychological health of patients,” note Grayden S. Cook, BS, and Jeffrey M. Howard, MD, PhD, both from University of Texas Southwestern Medical Center, Dallas.
“Many of these patients undergo frequent CT imaging of the urinary tract, which carries a high dose of radiation as well as the potential for financial toxic effects (that is, detrimental consequences to the patient because of health care costs),” they write.
Additionally, patient distress is a factor, as they may experience preprocedural anxiety, physical discomfort during procedures, and worry about disease progression, they point out.
“The impact of these patterns is substantial and may have negative consequences for both patients and the health care system,” they conclude. “Thus, it is imperative to move forward with initiatives that provide higher value and are more evidence-based and patient-centered.”
Study finds frequent surveillance
The American Urological Association (AUA)/Society of Urologic Oncologists (SUO), the European Association of Urology, and the International Bladder Cancer Group have made an effort to de-escalate surveillance and treatment for patients with low-grade Ta disease, while at the same time maintaining appropriate surveillance for high-grade aggressive disease.
However, the new study found that in practice, such patients undergo frequent testing.
The study involved 13,054 patients with low-grade Ta NMIBC. Most of the participants were male (73.5%), with a median age of 76 years, and had no or few comorbidities (71.2%).
Most patients had undergone cystoscopy, and rates increased over time: from 79.3% of patients in 2004 to 81.5% of patients in 2013 (P = .007). Patients underwent a median of 3.0 cystoscopies per year following their diagnosis, and upper-tract imaging was performed in most patients.
The use of kidney ultrasonography also rose from 19% of patients in 2004 to 23.2% in 2013, as did retrograde pyelography (20.9% in 2004 vs. 24.2% in 2013). Conversely, the use of intravenous pyelography declined (from 14.5% in 2004 to 1.7% in 2012), but there was an increase in CT and MRI in all years except 2010 (from 30.4% of patients in 2004 to 47% of patients in 2013; P < .001). The rate of urine-based testing also significantly increased during the study period (from 44.8% in 2004 to 54.9% in 2013; P < .001), with patients undergoing between two to three tests per year.
Adherence to current guidelines remained similar during the study time frame. For example, 55.2% of patients received two cystoscopies per year in 2004-2008, compared with 53.8% in 2009-2013 (P = .11), suggesting that there was an overuse of all surveillance testing modalities.
As for treatment, 17.2% received intravesical immunotherapy with bacillus Calmette-Guérin, 6.1% were treated with intravesical chemotherapy (excluding receipt of a single perioperative dose). Disease recurrence within this cohort was 1.7%, and only 0.4% experienced disease progression.
When looking at the cost, the total median expenditures at 1 year after diagnosis increased by 60% during the study period, from $34,792 in 2004 to $53,986 in 2013. Higher costs were seen among patients who experienced a recurrence versus no recurrence ($76,669 vs. $53,909).
The study was supported by a grant from the U.S. Department of Defense Peer Reviewed Cancer Research Program. Several of the authors have disclosed relationships with industry, as noted in the original article. Editorialists Mr. Cook and Dr. Howard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NMIBC), a new study concludes.
These cancers are associated with low rates of recurrence, progression, and bladder cancer–specific death, so current clinical practice guidelines recommend against frequent monitoring and testing.
However, the study authors found that patients with a low grade Ta NMIBC diagnosis underwent a median of three cystoscopies per year, and many also received a median of two imagine scans (CT or MRI) as well as 2-3 urine-based tests.
“These data suggest a need for ongoing efforts to limit overuse of treatment and surveillance, which may in turn mitigate associated increases in the costs of care,” write the authors, led by Kelly K. Bree, MD, from the department of urology, University of Texas MD Anderson Cancer Center, Houston. Bladder cancer has the highest lifetime treatment cost of all malignancies, they point out.
The study was published online in JAMA Network Open.
Higher value and more evidence-based
The impact of increased surveillance of this patient cohort has broad implications for patients and the health care system in general, say experts writing in an accompanying editorial.
“It has been well established that workup for NMIBC can have negative consequences for the physical and psychological health of patients,” note Grayden S. Cook, BS, and Jeffrey M. Howard, MD, PhD, both from University of Texas Southwestern Medical Center, Dallas.
“Many of these patients undergo frequent CT imaging of the urinary tract, which carries a high dose of radiation as well as the potential for financial toxic effects (that is, detrimental consequences to the patient because of health care costs),” they write.
Additionally, patient distress is a factor, as they may experience preprocedural anxiety, physical discomfort during procedures, and worry about disease progression, they point out.
“The impact of these patterns is substantial and may have negative consequences for both patients and the health care system,” they conclude. “Thus, it is imperative to move forward with initiatives that provide higher value and are more evidence-based and patient-centered.”
Study finds frequent surveillance
The American Urological Association (AUA)/Society of Urologic Oncologists (SUO), the European Association of Urology, and the International Bladder Cancer Group have made an effort to de-escalate surveillance and treatment for patients with low-grade Ta disease, while at the same time maintaining appropriate surveillance for high-grade aggressive disease.
However, the new study found that in practice, such patients undergo frequent testing.
The study involved 13,054 patients with low-grade Ta NMIBC. Most of the participants were male (73.5%), with a median age of 76 years, and had no or few comorbidities (71.2%).
Most patients had undergone cystoscopy, and rates increased over time: from 79.3% of patients in 2004 to 81.5% of patients in 2013 (P = .007). Patients underwent a median of 3.0 cystoscopies per year following their diagnosis, and upper-tract imaging was performed in most patients.
The use of kidney ultrasonography also rose from 19% of patients in 2004 to 23.2% in 2013, as did retrograde pyelography (20.9% in 2004 vs. 24.2% in 2013). Conversely, the use of intravenous pyelography declined (from 14.5% in 2004 to 1.7% in 2012), but there was an increase in CT and MRI in all years except 2010 (from 30.4% of patients in 2004 to 47% of patients in 2013; P < .001). The rate of urine-based testing also significantly increased during the study period (from 44.8% in 2004 to 54.9% in 2013; P < .001), with patients undergoing between two to three tests per year.
Adherence to current guidelines remained similar during the study time frame. For example, 55.2% of patients received two cystoscopies per year in 2004-2008, compared with 53.8% in 2009-2013 (P = .11), suggesting that there was an overuse of all surveillance testing modalities.
As for treatment, 17.2% received intravesical immunotherapy with bacillus Calmette-Guérin, 6.1% were treated with intravesical chemotherapy (excluding receipt of a single perioperative dose). Disease recurrence within this cohort was 1.7%, and only 0.4% experienced disease progression.
When looking at the cost, the total median expenditures at 1 year after diagnosis increased by 60% during the study period, from $34,792 in 2004 to $53,986 in 2013. Higher costs were seen among patients who experienced a recurrence versus no recurrence ($76,669 vs. $53,909).
The study was supported by a grant from the U.S. Department of Defense Peer Reviewed Cancer Research Program. Several of the authors have disclosed relationships with industry, as noted in the original article. Editorialists Mr. Cook and Dr. Howard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NMIBC), a new study concludes.
These cancers are associated with low rates of recurrence, progression, and bladder cancer–specific death, so current clinical practice guidelines recommend against frequent monitoring and testing.
However, the study authors found that patients with a low grade Ta NMIBC diagnosis underwent a median of three cystoscopies per year, and many also received a median of two imagine scans (CT or MRI) as well as 2-3 urine-based tests.
“These data suggest a need for ongoing efforts to limit overuse of treatment and surveillance, which may in turn mitigate associated increases in the costs of care,” write the authors, led by Kelly K. Bree, MD, from the department of urology, University of Texas MD Anderson Cancer Center, Houston. Bladder cancer has the highest lifetime treatment cost of all malignancies, they point out.
The study was published online in JAMA Network Open.
Higher value and more evidence-based
The impact of increased surveillance of this patient cohort has broad implications for patients and the health care system in general, say experts writing in an accompanying editorial.
“It has been well established that workup for NMIBC can have negative consequences for the physical and psychological health of patients,” note Grayden S. Cook, BS, and Jeffrey M. Howard, MD, PhD, both from University of Texas Southwestern Medical Center, Dallas.
“Many of these patients undergo frequent CT imaging of the urinary tract, which carries a high dose of radiation as well as the potential for financial toxic effects (that is, detrimental consequences to the patient because of health care costs),” they write.
Additionally, patient distress is a factor, as they may experience preprocedural anxiety, physical discomfort during procedures, and worry about disease progression, they point out.
“The impact of these patterns is substantial and may have negative consequences for both patients and the health care system,” they conclude. “Thus, it is imperative to move forward with initiatives that provide higher value and are more evidence-based and patient-centered.”
Study finds frequent surveillance
The American Urological Association (AUA)/Society of Urologic Oncologists (SUO), the European Association of Urology, and the International Bladder Cancer Group have made an effort to de-escalate surveillance and treatment for patients with low-grade Ta disease, while at the same time maintaining appropriate surveillance for high-grade aggressive disease.
However, the new study found that in practice, such patients undergo frequent testing.
The study involved 13,054 patients with low-grade Ta NMIBC. Most of the participants were male (73.5%), with a median age of 76 years, and had no or few comorbidities (71.2%).
Most patients had undergone cystoscopy, and rates increased over time: from 79.3% of patients in 2004 to 81.5% of patients in 2013 (P = .007). Patients underwent a median of 3.0 cystoscopies per year following their diagnosis, and upper-tract imaging was performed in most patients.
The use of kidney ultrasonography also rose from 19% of patients in 2004 to 23.2% in 2013, as did retrograde pyelography (20.9% in 2004 vs. 24.2% in 2013). Conversely, the use of intravenous pyelography declined (from 14.5% in 2004 to 1.7% in 2012), but there was an increase in CT and MRI in all years except 2010 (from 30.4% of patients in 2004 to 47% of patients in 2013; P < .001). The rate of urine-based testing also significantly increased during the study period (from 44.8% in 2004 to 54.9% in 2013; P < .001), with patients undergoing between two to three tests per year.
Adherence to current guidelines remained similar during the study time frame. For example, 55.2% of patients received two cystoscopies per year in 2004-2008, compared with 53.8% in 2009-2013 (P = .11), suggesting that there was an overuse of all surveillance testing modalities.
As for treatment, 17.2% received intravesical immunotherapy with bacillus Calmette-Guérin, 6.1% were treated with intravesical chemotherapy (excluding receipt of a single perioperative dose). Disease recurrence within this cohort was 1.7%, and only 0.4% experienced disease progression.
When looking at the cost, the total median expenditures at 1 year after diagnosis increased by 60% during the study period, from $34,792 in 2004 to $53,986 in 2013. Higher costs were seen among patients who experienced a recurrence versus no recurrence ($76,669 vs. $53,909).
The study was supported by a grant from the U.S. Department of Defense Peer Reviewed Cancer Research Program. Several of the authors have disclosed relationships with industry, as noted in the original article. Editorialists Mr. Cook and Dr. Howard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Reducing Attacks and Neuropathic Pain in NMOSD
Patients with neuromyelitis optica spectrum disorder (NMOSD) experience unpredictable episodes of inflammation involving the optic nerve, spine, or both. Debilitating neuropathic pain accompanies the healing process, which lasts anywhere from 2 to 6 months after an attack.
In this ReCAP, Dr Michael Levy, of Harvard Medical School in Boston, Massachusetts, outlines the heavy psychological and economic burdens associated with NMOSD and reports on three new targeted therapies that have been approved to prevent relapse and delay disease progression.
He then looks at current pharmaceutical treatments for NMOSD pain, before reporting promising trial data exploring transcutaneous electrical nerve stimulation as a safe and cost-effective treatment for neuropathic pain.
--
Michael Levy, MD, PhD, Associate Professor, Department of Neurology, Harvard Medical School, Boston, Massachusetts
Michael Levy, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Alexion; Horizon; Genentech; UCB; Sanofi; Quest
Received research grant from: National Institutes of Health; Sanofi; Genentech; Horizon; Alexion
Patients with neuromyelitis optica spectrum disorder (NMOSD) experience unpredictable episodes of inflammation involving the optic nerve, spine, or both. Debilitating neuropathic pain accompanies the healing process, which lasts anywhere from 2 to 6 months after an attack.
In this ReCAP, Dr Michael Levy, of Harvard Medical School in Boston, Massachusetts, outlines the heavy psychological and economic burdens associated with NMOSD and reports on three new targeted therapies that have been approved to prevent relapse and delay disease progression.
He then looks at current pharmaceutical treatments for NMOSD pain, before reporting promising trial data exploring transcutaneous electrical nerve stimulation as a safe and cost-effective treatment for neuropathic pain.
--
Michael Levy, MD, PhD, Associate Professor, Department of Neurology, Harvard Medical School, Boston, Massachusetts
Michael Levy, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Alexion; Horizon; Genentech; UCB; Sanofi; Quest
Received research grant from: National Institutes of Health; Sanofi; Genentech; Horizon; Alexion
Patients with neuromyelitis optica spectrum disorder (NMOSD) experience unpredictable episodes of inflammation involving the optic nerve, spine, or both. Debilitating neuropathic pain accompanies the healing process, which lasts anywhere from 2 to 6 months after an attack.
In this ReCAP, Dr Michael Levy, of Harvard Medical School in Boston, Massachusetts, outlines the heavy psychological and economic burdens associated with NMOSD and reports on three new targeted therapies that have been approved to prevent relapse and delay disease progression.
He then looks at current pharmaceutical treatments for NMOSD pain, before reporting promising trial data exploring transcutaneous electrical nerve stimulation as a safe and cost-effective treatment for neuropathic pain.
--
Michael Levy, MD, PhD, Associate Professor, Department of Neurology, Harvard Medical School, Boston, Massachusetts
Michael Levy, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Alexion; Horizon; Genentech; UCB; Sanofi; Quest
Received research grant from: National Institutes of Health; Sanofi; Genentech; Horizon; Alexion

Managing Heavy Menstrual Bleeding Associated with Fibroids
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Aspirin exposure fails to reduce cardiovascular event risk
The benefits of aspirin use for the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have been questioned in light of data showing neutral outcomes in low-risk patients and concerns about increased bleeding risk and mortality in healthy older adults, wrote Rita Del Pinto, MD, of University of L’Aquila (Italy) and colleagues in JAMA Network Open.
In the study, Dr. Del Pinto and colleagues conducted a post hoc analysis of data from more than 2,500 participants in SPRINT (Systolic Blood Pressure Intervention Trial), a multicenter, randomized trial conducted from 2010 to 2013.
The goal of SPRINT was to compare intensive and standard blood pressure–lowering strategies for hypertension patients. The primary outcome of the current study was risk of a first cardiovascular event, which included adjudicated myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute heart failure, and CVD death.“There has been considerable improvement in the management of cardiovascular risk factors since the first reports on aspirin use for cardiovascular prevention,” Dr. Del Pinto said in an interview.
“As for hypertension, not only have more effective antihypertensive medications become available, but also evidence has recently emerged in support of a downwards redefinition of blood pressure targets during treatment,” she said. “In this context, in an era when great attention is paid to the personalization of treatment, no specific studies had addressed the association of aspirin use as a primary prevention strategy in a cohort of relatively old, high-risk individuals with treated systolic blood pressure steadily below the recommended target,” she added.
The researchers assessed whether aspirin use in addition to standard blood pressure management (a target of less than 140 mm Hg) decreased risk and improved survival.
The study population included 2,664 adult patients; 29.3% were women, and 24.5% were aged 75 years and older. Half of the patients (1,332) received aspirin and 1,332 did not.
In a multivariate analysis, 42 cardiovascular events occurred in the aspirin group, compared with 20 events in those not exposed to aspirin (hazard ratio, 2.30). The findings were consistent in subgroup analyses of younger individuals, current and former smokers, and patients on statins.
An additional subgroup analysis of individuals randomized to standard care or intensive care in the SPRINT study showed no significant difference in primary outcome rates between individuals who received aspirin and those who did not. The rates for aspirin use vs. non–aspirin use were 5.85% vs. 3.60% in the standard treatment group and 4.66% vs. 2.56% in the intensive treatment group.
The study findings were limited by several factors, including the post hoc design, short follow-up period, and lack of data on the initiation of aspirin and bleeding events, the researchers wrote. However, the results suggest that modern management of hypertension may have redefined the potential benefits of aspirin in patients with hypertension, they concluded.
Findings confirm value of preventive care
“The study was conducted as a post-hoc analysis on an experimental cohort, which must be considered when interpreting the results,” Dr. Del Pinto said.
Despite the limitations, the study findings affirm that effective treatment of major cardiovascular risk factors, such as hypertension, with proven drugs is “a mainstay of the primary prevention of ASCVD,” she emphasized.
As for additional research, “Testing our findings in a dedicated setting with sufficiently long follow-up, where aspirin dose and indication, as well as any possible bleeding event, are reported could expand the clinical meaning of our observations,” said Dr. Del Pinto. “Also, the clinical impact of aspirin, even in combination with novel cardiovascular drugs such as direct oral anticoagulants, in populations exposed to combinations of risk factors, deserves further investigation.”
Data support shared decision-making
“While recent evidence has not shown a benefit of aspirin in the primary prevention of ASCVD in several populations, the subpopulation of patients with hypertension as an ASCVD risk factor is also of interest to the clinician,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview. “The lack of benefit of aspirin in this study, despite its limitations, was surprising, and I would be eager to see how the role of aspirin in ASCVD prevention would continue to evolve in conjunction with improvement in other therapies for modification of risk factors.”
“The decision to continue aspirin in this subgroup of patients should warrant a discussion with patients and a reexamination of risks and benefits until further data are available,” Dr. Pal emphasized.
Larger studies with long-term follow-ups would be required to further clarify the role of aspirin in primary prevention of ASCVD in patients with hypertension without diabetes or chronic kidney disease, he added.
Data were supplied courtesy of BioLINCC. The study received no outside funding. The researchers and Dr. Pal had no financial conflicts to disclose.
The benefits of aspirin use for the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have been questioned in light of data showing neutral outcomes in low-risk patients and concerns about increased bleeding risk and mortality in healthy older adults, wrote Rita Del Pinto, MD, of University of L’Aquila (Italy) and colleagues in JAMA Network Open.
In the study, Dr. Del Pinto and colleagues conducted a post hoc analysis of data from more than 2,500 participants in SPRINT (Systolic Blood Pressure Intervention Trial), a multicenter, randomized trial conducted from 2010 to 2013.
The goal of SPRINT was to compare intensive and standard blood pressure–lowering strategies for hypertension patients. The primary outcome of the current study was risk of a first cardiovascular event, which included adjudicated myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute heart failure, and CVD death.“There has been considerable improvement in the management of cardiovascular risk factors since the first reports on aspirin use for cardiovascular prevention,” Dr. Del Pinto said in an interview.
“As for hypertension, not only have more effective antihypertensive medications become available, but also evidence has recently emerged in support of a downwards redefinition of blood pressure targets during treatment,” she said. “In this context, in an era when great attention is paid to the personalization of treatment, no specific studies had addressed the association of aspirin use as a primary prevention strategy in a cohort of relatively old, high-risk individuals with treated systolic blood pressure steadily below the recommended target,” she added.
The researchers assessed whether aspirin use in addition to standard blood pressure management (a target of less than 140 mm Hg) decreased risk and improved survival.
The study population included 2,664 adult patients; 29.3% were women, and 24.5% were aged 75 years and older. Half of the patients (1,332) received aspirin and 1,332 did not.
In a multivariate analysis, 42 cardiovascular events occurred in the aspirin group, compared with 20 events in those not exposed to aspirin (hazard ratio, 2.30). The findings were consistent in subgroup analyses of younger individuals, current and former smokers, and patients on statins.
An additional subgroup analysis of individuals randomized to standard care or intensive care in the SPRINT study showed no significant difference in primary outcome rates between individuals who received aspirin and those who did not. The rates for aspirin use vs. non–aspirin use were 5.85% vs. 3.60% in the standard treatment group and 4.66% vs. 2.56% in the intensive treatment group.
The study findings were limited by several factors, including the post hoc design, short follow-up period, and lack of data on the initiation of aspirin and bleeding events, the researchers wrote. However, the results suggest that modern management of hypertension may have redefined the potential benefits of aspirin in patients with hypertension, they concluded.
Findings confirm value of preventive care
“The study was conducted as a post-hoc analysis on an experimental cohort, which must be considered when interpreting the results,” Dr. Del Pinto said.
Despite the limitations, the study findings affirm that effective treatment of major cardiovascular risk factors, such as hypertension, with proven drugs is “a mainstay of the primary prevention of ASCVD,” she emphasized.
As for additional research, “Testing our findings in a dedicated setting with sufficiently long follow-up, where aspirin dose and indication, as well as any possible bleeding event, are reported could expand the clinical meaning of our observations,” said Dr. Del Pinto. “Also, the clinical impact of aspirin, even in combination with novel cardiovascular drugs such as direct oral anticoagulants, in populations exposed to combinations of risk factors, deserves further investigation.”
Data support shared decision-making
“While recent evidence has not shown a benefit of aspirin in the primary prevention of ASCVD in several populations, the subpopulation of patients with hypertension as an ASCVD risk factor is also of interest to the clinician,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview. “The lack of benefit of aspirin in this study, despite its limitations, was surprising, and I would be eager to see how the role of aspirin in ASCVD prevention would continue to evolve in conjunction with improvement in other therapies for modification of risk factors.”
“The decision to continue aspirin in this subgroup of patients should warrant a discussion with patients and a reexamination of risks and benefits until further data are available,” Dr. Pal emphasized.
Larger studies with long-term follow-ups would be required to further clarify the role of aspirin in primary prevention of ASCVD in patients with hypertension without diabetes or chronic kidney disease, he added.
Data were supplied courtesy of BioLINCC. The study received no outside funding. The researchers and Dr. Pal had no financial conflicts to disclose.
The benefits of aspirin use for the primary prevention of atherosclerotic cardiovascular disease (ASCVD) have been questioned in light of data showing neutral outcomes in low-risk patients and concerns about increased bleeding risk and mortality in healthy older adults, wrote Rita Del Pinto, MD, of University of L’Aquila (Italy) and colleagues in JAMA Network Open.
In the study, Dr. Del Pinto and colleagues conducted a post hoc analysis of data from more than 2,500 participants in SPRINT (Systolic Blood Pressure Intervention Trial), a multicenter, randomized trial conducted from 2010 to 2013.
The goal of SPRINT was to compare intensive and standard blood pressure–lowering strategies for hypertension patients. The primary outcome of the current study was risk of a first cardiovascular event, which included adjudicated myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, acute heart failure, and CVD death.“There has been considerable improvement in the management of cardiovascular risk factors since the first reports on aspirin use for cardiovascular prevention,” Dr. Del Pinto said in an interview.
“As for hypertension, not only have more effective antihypertensive medications become available, but also evidence has recently emerged in support of a downwards redefinition of blood pressure targets during treatment,” she said. “In this context, in an era when great attention is paid to the personalization of treatment, no specific studies had addressed the association of aspirin use as a primary prevention strategy in a cohort of relatively old, high-risk individuals with treated systolic blood pressure steadily below the recommended target,” she added.
The researchers assessed whether aspirin use in addition to standard blood pressure management (a target of less than 140 mm Hg) decreased risk and improved survival.
The study population included 2,664 adult patients; 29.3% were women, and 24.5% were aged 75 years and older. Half of the patients (1,332) received aspirin and 1,332 did not.
In a multivariate analysis, 42 cardiovascular events occurred in the aspirin group, compared with 20 events in those not exposed to aspirin (hazard ratio, 2.30). The findings were consistent in subgroup analyses of younger individuals, current and former smokers, and patients on statins.
An additional subgroup analysis of individuals randomized to standard care or intensive care in the SPRINT study showed no significant difference in primary outcome rates between individuals who received aspirin and those who did not. The rates for aspirin use vs. non–aspirin use were 5.85% vs. 3.60% in the standard treatment group and 4.66% vs. 2.56% in the intensive treatment group.
The study findings were limited by several factors, including the post hoc design, short follow-up period, and lack of data on the initiation of aspirin and bleeding events, the researchers wrote. However, the results suggest that modern management of hypertension may have redefined the potential benefits of aspirin in patients with hypertension, they concluded.
Findings confirm value of preventive care
“The study was conducted as a post-hoc analysis on an experimental cohort, which must be considered when interpreting the results,” Dr. Del Pinto said.
Despite the limitations, the study findings affirm that effective treatment of major cardiovascular risk factors, such as hypertension, with proven drugs is “a mainstay of the primary prevention of ASCVD,” she emphasized.
As for additional research, “Testing our findings in a dedicated setting with sufficiently long follow-up, where aspirin dose and indication, as well as any possible bleeding event, are reported could expand the clinical meaning of our observations,” said Dr. Del Pinto. “Also, the clinical impact of aspirin, even in combination with novel cardiovascular drugs such as direct oral anticoagulants, in populations exposed to combinations of risk factors, deserves further investigation.”
Data support shared decision-making
“While recent evidence has not shown a benefit of aspirin in the primary prevention of ASCVD in several populations, the subpopulation of patients with hypertension as an ASCVD risk factor is also of interest to the clinician,” Suman Pal, MD, of the University of New Mexico, Albuquerque, said in an interview. “The lack of benefit of aspirin in this study, despite its limitations, was surprising, and I would be eager to see how the role of aspirin in ASCVD prevention would continue to evolve in conjunction with improvement in other therapies for modification of risk factors.”
“The decision to continue aspirin in this subgroup of patients should warrant a discussion with patients and a reexamination of risks and benefits until further data are available,” Dr. Pal emphasized.
Larger studies with long-term follow-ups would be required to further clarify the role of aspirin in primary prevention of ASCVD in patients with hypertension without diabetes or chronic kidney disease, he added.
Data were supplied courtesy of BioLINCC. The study received no outside funding. The researchers and Dr. Pal had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN






