User login
What’s the most likely cause of this man’s severe headaches?
He reports that these started 3 days ago. His headache is worse when he stands, and resolves when he lies down. Valsalva maneuver makes the headache much worse. The headaches are present in the occipital region. He also has noticed the onset of tinnitus. A physical exam reveals that his blood pressure is 110/70 mm Hg, his pulse is 60 beats per minute, and his temperature is 36.4° C. His standing BP is 105/60 mm Hg and standing pulse is 66 bpm. Both his neurologic exam and noncontrast head CT scan are normal.
Which of the following is the most likely diagnosis?
A) Subarachnoid hemorrhage
B) POTS (Postural orthostatic tachycardia syndrome)
C) Hypnic headache
D) Spontaneous intracranial hypotension (SIH)
E) Acoustic neuroma
The most likely cause for this patient’s headaches given his set of symptoms is spontaneous intracranial hypotension. Orthostatic headaches are common with POTS, but the absence of tachycardia with standing makes this diagnosis unlikely.
Spontaneous intracranial hypotension has symptoms that we are all familiar with in the post–lumbar puncture patient. In patients with post-LP headache, the positional nature makes it easy to diagnose. Patients who have had a lumbar puncture have a clear reason they have a cerebrospinal fluid (CSF) leak, leading to intracranial hypotension. Those with SIH do not.
Related research
Schievink summarized a lot of useful information in a review of patients with spontaneous intracranial hypotension.1 The incidence is about 5/100,000, with the most common age around 40 years old. The most common symptom is orthostatic headache. The headache usually occurs within 15 minutes upon standing, and many patients have the onset of headache rapidly upon standing.
Usually the headache improves with lying down, and it is often brought on with Valsalva maneuver. Many patients report headaches that are worse in the second half of the day.
Orthostatic headache occurs in almost all patients with spontaneous intracranial hypotension, but in one series it occurred only in 77% of patients with SIH.2 The patients who did not have typical headaches are more likely to have auditory symptoms such as tinnitus and muffled hearing.3
When you suspect SIH, appropriate workup is to start with brain MR imaging with contrast. Krantz and colleagues found dural enhancement was present in 83% of cases of SIH, venous distention sign in 75%, and brain sagging in 61%.4
About 10% of patients with SIH have normal brain imaging, so if the clinical features strongly suggest the diagnosis, moving on to spinal imaging with CT myelography or spinal MR are appropriate next steps.5
The causes of SIH are meningeal diverticula (usually in the thoracic or upper lumbar regions), ventral dural tears (usually from osteophytes), and cerebrospinal fluid–venous fistulas. Treatment of SIH has traditionally included a conservative approach of bed rest, oral hydration, and caffeine. The effectiveness of this is unknown, and, in one small series, 61% had headache symptoms at 6 months.6
Epidural blood patches are likely more rapidly effective than conservative therapy. In one study comparing the two treatments, Chung and colleagues found that 77% of the patients who received an epidural blood patch had complete headache relief at 4 weeks, compared with 40% of those who received conservative measures (P < .05).7
Clinical pearls
- Strongly consider SIH in patients with positional headache.
- Brain MR should be the first diagnostic test.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-96.
2. Mea E et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164-65.
3. Krantz PG et al. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948-59.
4. Krantz PG et. al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016;37:1374-8.
5. Krantz PG et al. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019;29:581-94.
6. Kong D-S et. al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91-6.
7. Chung SJ et al. Short- and long-term outcomes of spontaneous CSF hypovolemia. Eur Neurol. 2005;54:63-7.
He reports that these started 3 days ago. His headache is worse when he stands, and resolves when he lies down. Valsalva maneuver makes the headache much worse. The headaches are present in the occipital region. He also has noticed the onset of tinnitus. A physical exam reveals that his blood pressure is 110/70 mm Hg, his pulse is 60 beats per minute, and his temperature is 36.4° C. His standing BP is 105/60 mm Hg and standing pulse is 66 bpm. Both his neurologic exam and noncontrast head CT scan are normal.
Which of the following is the most likely diagnosis?
A) Subarachnoid hemorrhage
B) POTS (Postural orthostatic tachycardia syndrome)
C) Hypnic headache
D) Spontaneous intracranial hypotension (SIH)
E) Acoustic neuroma
The most likely cause for this patient’s headaches given his set of symptoms is spontaneous intracranial hypotension. Orthostatic headaches are common with POTS, but the absence of tachycardia with standing makes this diagnosis unlikely.
Spontaneous intracranial hypotension has symptoms that we are all familiar with in the post–lumbar puncture patient. In patients with post-LP headache, the positional nature makes it easy to diagnose. Patients who have had a lumbar puncture have a clear reason they have a cerebrospinal fluid (CSF) leak, leading to intracranial hypotension. Those with SIH do not.
Related research
Schievink summarized a lot of useful information in a review of patients with spontaneous intracranial hypotension.1 The incidence is about 5/100,000, with the most common age around 40 years old. The most common symptom is orthostatic headache. The headache usually occurs within 15 minutes upon standing, and many patients have the onset of headache rapidly upon standing.
Usually the headache improves with lying down, and it is often brought on with Valsalva maneuver. Many patients report headaches that are worse in the second half of the day.
Orthostatic headache occurs in almost all patients with spontaneous intracranial hypotension, but in one series it occurred only in 77% of patients with SIH.2 The patients who did not have typical headaches are more likely to have auditory symptoms such as tinnitus and muffled hearing.3
When you suspect SIH, appropriate workup is to start with brain MR imaging with contrast. Krantz and colleagues found dural enhancement was present in 83% of cases of SIH, venous distention sign in 75%, and brain sagging in 61%.4
About 10% of patients with SIH have normal brain imaging, so if the clinical features strongly suggest the diagnosis, moving on to spinal imaging with CT myelography or spinal MR are appropriate next steps.5
The causes of SIH are meningeal diverticula (usually in the thoracic or upper lumbar regions), ventral dural tears (usually from osteophytes), and cerebrospinal fluid–venous fistulas. Treatment of SIH has traditionally included a conservative approach of bed rest, oral hydration, and caffeine. The effectiveness of this is unknown, and, in one small series, 61% had headache symptoms at 6 months.6
Epidural blood patches are likely more rapidly effective than conservative therapy. In one study comparing the two treatments, Chung and colleagues found that 77% of the patients who received an epidural blood patch had complete headache relief at 4 weeks, compared with 40% of those who received conservative measures (P < .05).7
Clinical pearls
- Strongly consider SIH in patients with positional headache.
- Brain MR should be the first diagnostic test.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-96.
2. Mea E et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164-65.
3. Krantz PG et al. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948-59.
4. Krantz PG et. al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016;37:1374-8.
5. Krantz PG et al. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019;29:581-94.
6. Kong D-S et. al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91-6.
7. Chung SJ et al. Short- and long-term outcomes of spontaneous CSF hypovolemia. Eur Neurol. 2005;54:63-7.
He reports that these started 3 days ago. His headache is worse when he stands, and resolves when he lies down. Valsalva maneuver makes the headache much worse. The headaches are present in the occipital region. He also has noticed the onset of tinnitus. A physical exam reveals that his blood pressure is 110/70 mm Hg, his pulse is 60 beats per minute, and his temperature is 36.4° C. His standing BP is 105/60 mm Hg and standing pulse is 66 bpm. Both his neurologic exam and noncontrast head CT scan are normal.
Which of the following is the most likely diagnosis?
A) Subarachnoid hemorrhage
B) POTS (Postural orthostatic tachycardia syndrome)
C) Hypnic headache
D) Spontaneous intracranial hypotension (SIH)
E) Acoustic neuroma
The most likely cause for this patient’s headaches given his set of symptoms is spontaneous intracranial hypotension. Orthostatic headaches are common with POTS, but the absence of tachycardia with standing makes this diagnosis unlikely.
Spontaneous intracranial hypotension has symptoms that we are all familiar with in the post–lumbar puncture patient. In patients with post-LP headache, the positional nature makes it easy to diagnose. Patients who have had a lumbar puncture have a clear reason they have a cerebrospinal fluid (CSF) leak, leading to intracranial hypotension. Those with SIH do not.
Related research
Schievink summarized a lot of useful information in a review of patients with spontaneous intracranial hypotension.1 The incidence is about 5/100,000, with the most common age around 40 years old. The most common symptom is orthostatic headache. The headache usually occurs within 15 minutes upon standing, and many patients have the onset of headache rapidly upon standing.
Usually the headache improves with lying down, and it is often brought on with Valsalva maneuver. Many patients report headaches that are worse in the second half of the day.
Orthostatic headache occurs in almost all patients with spontaneous intracranial hypotension, but in one series it occurred only in 77% of patients with SIH.2 The patients who did not have typical headaches are more likely to have auditory symptoms such as tinnitus and muffled hearing.3
When you suspect SIH, appropriate workup is to start with brain MR imaging with contrast. Krantz and colleagues found dural enhancement was present in 83% of cases of SIH, venous distention sign in 75%, and brain sagging in 61%.4
About 10% of patients with SIH have normal brain imaging, so if the clinical features strongly suggest the diagnosis, moving on to spinal imaging with CT myelography or spinal MR are appropriate next steps.5
The causes of SIH are meningeal diverticula (usually in the thoracic or upper lumbar regions), ventral dural tears (usually from osteophytes), and cerebrospinal fluid–venous fistulas. Treatment of SIH has traditionally included a conservative approach of bed rest, oral hydration, and caffeine. The effectiveness of this is unknown, and, in one small series, 61% had headache symptoms at 6 months.6
Epidural blood patches are likely more rapidly effective than conservative therapy. In one study comparing the two treatments, Chung and colleagues found that 77% of the patients who received an epidural blood patch had complete headache relief at 4 weeks, compared with 40% of those who received conservative measures (P < .05).7
Clinical pearls
- Strongly consider SIH in patients with positional headache.
- Brain MR should be the first diagnostic test.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-96.
2. Mea E et al. Headache attributed to spontaneous intracranial hypotension. Neurol Sci. 2008;29:164-65.
3. Krantz PG et al. Spontaneous Intracranial Hypotension: 10 Myths and Misperceptions. Headache. 2018;58:948-59.
4. Krantz PG et. al. Imaging signs in spontaneous intracranial hypotension: prevalence and relationship to CSF pressure. AJNR Am J Neuroradiol. 2016;37:1374-8.
5. Krantz PG et al. Spontaneous intracranial hypotension: Pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. 2019;29:581-94.
6. Kong D-S et. al. Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery. 2005;57:91-6.
7. Chung SJ et al. Short- and long-term outcomes of spontaneous CSF hypovolemia. Eur Neurol. 2005;54:63-7.
The work after work
Across the country, taxes unite us. Not that we all share the same, rather that we all have to do them. It was recently tax weekend in our house: The Saturday and Sunday that cap off weeks of hunting and gathering faded receipts and sorting through reams of credit card bills to find all the dollars we spent on work. The task is more tedious than all the Wednesdays of taking out trash bins combined, and equally as exciting. But wait, that’s not all.
This weekend I’ve been chatting with bots from a solar company trying to solve our drop in energy production and sat on terminal hold with apparently one person who answers the phone for Amazon. There’s also an homeowner’s association meeting to prepare for and research to be done on ceiling fans.
“Life admin” is a crisp phrase coined by Elizabeth Emens, JD, PhD, that captures the never-ending to-do list that comes with running a household. An accomplished law professor at Columbia University, New York, Dr. Emens noticed the negative impact this life admin has on our quality of life. Reading her book, “Life Admin: How I Learned to Do Less, Do Better, and Live More” (New York: HarperOne, 2019), your eyes widen as she magically makes salient all this hidden work that is stealing our time. Life admin, kidmin, mom and dadmin, just rattling them off feels like donning x-ray glasses allowing us to see how much work we do outside of our work. As doctors, I would add “family house calls,” as a contributing factor: Random family and friends who want to talk for a minute about their knee replacement or what drug the ICU should give Uncle Larry who is fighting COVID. (I only know ivermectin, but it would only help if he just had scabies).
By all accounts, the amount of life admin is growing insidiously, worsened by the great pandemic. There are events to plan and reply to, more DIY customer service to fix your own problems, more work to find a VRBO for a weekend getaway at the beach. (There are none on the entire coast of California this summer, so I just saved you time there. You’re welcome.)
There is no good time to do this work and combined with the heavy burden of our responsibilities as physicians, it can feel like fuel feeding the burnout fire.
Dr. Emens has some top tips to help. First up, know your admin type. Are you a super doer, reluctant doer, admin denier, or admin avoider? I’m mostly in the avoider quadrant, dropping into reluctant doer when consequences loom. Next, choose strategies that fit you. Instead of avoiding, there are some things I might deflect. For example, When your aunt in Peoria asks where she can get a COVID test, you can use LMGTFY.com to generate a link that will show them how to use Google to help with their question. Dr. Emens is joking, but the point rang true. We can lighten the load a bit if we delegate or push back the excessive or undue requests. For some tasks, we’d be better off paying someone to take it over. Last tip here, try doing life admin with a partner, be it spouse, friend, or colleague. This is particularly useful when your partner is a super doer, as mine is. Not only can they make the work lighter, but also less dreary.
We physicians are focused on fixing physician burnout. Maybe we should also be looking at what happens in the “second shift” at home. Tax season is over, but will be back soon.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Across the country, taxes unite us. Not that we all share the same, rather that we all have to do them. It was recently tax weekend in our house: The Saturday and Sunday that cap off weeks of hunting and gathering faded receipts and sorting through reams of credit card bills to find all the dollars we spent on work. The task is more tedious than all the Wednesdays of taking out trash bins combined, and equally as exciting. But wait, that’s not all.
This weekend I’ve been chatting with bots from a solar company trying to solve our drop in energy production and sat on terminal hold with apparently one person who answers the phone for Amazon. There’s also an homeowner’s association meeting to prepare for and research to be done on ceiling fans.
“Life admin” is a crisp phrase coined by Elizabeth Emens, JD, PhD, that captures the never-ending to-do list that comes with running a household. An accomplished law professor at Columbia University, New York, Dr. Emens noticed the negative impact this life admin has on our quality of life. Reading her book, “Life Admin: How I Learned to Do Less, Do Better, and Live More” (New York: HarperOne, 2019), your eyes widen as she magically makes salient all this hidden work that is stealing our time. Life admin, kidmin, mom and dadmin, just rattling them off feels like donning x-ray glasses allowing us to see how much work we do outside of our work. As doctors, I would add “family house calls,” as a contributing factor: Random family and friends who want to talk for a minute about their knee replacement or what drug the ICU should give Uncle Larry who is fighting COVID. (I only know ivermectin, but it would only help if he just had scabies).
By all accounts, the amount of life admin is growing insidiously, worsened by the great pandemic. There are events to plan and reply to, more DIY customer service to fix your own problems, more work to find a VRBO for a weekend getaway at the beach. (There are none on the entire coast of California this summer, so I just saved you time there. You’re welcome.)
There is no good time to do this work and combined with the heavy burden of our responsibilities as physicians, it can feel like fuel feeding the burnout fire.
Dr. Emens has some top tips to help. First up, know your admin type. Are you a super doer, reluctant doer, admin denier, or admin avoider? I’m mostly in the avoider quadrant, dropping into reluctant doer when consequences loom. Next, choose strategies that fit you. Instead of avoiding, there are some things I might deflect. For example, When your aunt in Peoria asks where she can get a COVID test, you can use LMGTFY.com to generate a link that will show them how to use Google to help with their question. Dr. Emens is joking, but the point rang true. We can lighten the load a bit if we delegate or push back the excessive or undue requests. For some tasks, we’d be better off paying someone to take it over. Last tip here, try doing life admin with a partner, be it spouse, friend, or colleague. This is particularly useful when your partner is a super doer, as mine is. Not only can they make the work lighter, but also less dreary.
We physicians are focused on fixing physician burnout. Maybe we should also be looking at what happens in the “second shift” at home. Tax season is over, but will be back soon.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Across the country, taxes unite us. Not that we all share the same, rather that we all have to do them. It was recently tax weekend in our house: The Saturday and Sunday that cap off weeks of hunting and gathering faded receipts and sorting through reams of credit card bills to find all the dollars we spent on work. The task is more tedious than all the Wednesdays of taking out trash bins combined, and equally as exciting. But wait, that’s not all.
This weekend I’ve been chatting with bots from a solar company trying to solve our drop in energy production and sat on terminal hold with apparently one person who answers the phone for Amazon. There’s also an homeowner’s association meeting to prepare for and research to be done on ceiling fans.
“Life admin” is a crisp phrase coined by Elizabeth Emens, JD, PhD, that captures the never-ending to-do list that comes with running a household. An accomplished law professor at Columbia University, New York, Dr. Emens noticed the negative impact this life admin has on our quality of life. Reading her book, “Life Admin: How I Learned to Do Less, Do Better, and Live More” (New York: HarperOne, 2019), your eyes widen as she magically makes salient all this hidden work that is stealing our time. Life admin, kidmin, mom and dadmin, just rattling them off feels like donning x-ray glasses allowing us to see how much work we do outside of our work. As doctors, I would add “family house calls,” as a contributing factor: Random family and friends who want to talk for a minute about their knee replacement or what drug the ICU should give Uncle Larry who is fighting COVID. (I only know ivermectin, but it would only help if he just had scabies).
By all accounts, the amount of life admin is growing insidiously, worsened by the great pandemic. There are events to plan and reply to, more DIY customer service to fix your own problems, more work to find a VRBO for a weekend getaway at the beach. (There are none on the entire coast of California this summer, so I just saved you time there. You’re welcome.)
There is no good time to do this work and combined with the heavy burden of our responsibilities as physicians, it can feel like fuel feeding the burnout fire.
Dr. Emens has some top tips to help. First up, know your admin type. Are you a super doer, reluctant doer, admin denier, or admin avoider? I’m mostly in the avoider quadrant, dropping into reluctant doer when consequences loom. Next, choose strategies that fit you. Instead of avoiding, there are some things I might deflect. For example, When your aunt in Peoria asks where she can get a COVID test, you can use LMGTFY.com to generate a link that will show them how to use Google to help with their question. Dr. Emens is joking, but the point rang true. We can lighten the load a bit if we delegate or push back the excessive or undue requests. For some tasks, we’d be better off paying someone to take it over. Last tip here, try doing life admin with a partner, be it spouse, friend, or colleague. This is particularly useful when your partner is a super doer, as mine is. Not only can they make the work lighter, but also less dreary.
We physicians are focused on fixing physician burnout. Maybe we should also be looking at what happens in the “second shift” at home. Tax season is over, but will be back soon.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
The unseen benefit of an MRI
Mrs. Smith came in for neck pain.
This isn’t a new issue, her last flare was 4 or 5 years ago. I’d done an MRI back then, which just showed reassuringly typical arthritic changes, and she did great with a few sessions of physical therapy.
She’d woke one morning a few months ago with a stiff and aching neck, similar to how this started last time. A couple weeks of rest and NSAIDs hadn’t helped. There were no radiating symptoms and her exam was the same as it’s been since I met her back in 2010.
I wrote her an order for physical therapy and found the address and phone number of the place she’d gone to for it a few years ago. She looked at my order, then set it on my desk and said “Doctor, I’d really like an MRI.”
I went back to her chart and reread my note for her last flare of neck pain. Identical symptoms, identical exam. I pulled up the previous MRI report and went over it. Then I explained to her that there really was no indication for an MRI at this point. I suggested we go ahead with physical therapy, and if that didn’t help I would then re-check the study.
She wasn’t going to budge. A friend of hers had recently needed urgent surgery for a cervical myelopathy and was in rehab. Mrs. Smith’s husband’s health was getting worse, and if her neck had something seriously wrong she wouldn’t be able to take care of him if it went unchecked.
So I backed down and ordered a cervical spine MRI, which was pretty much unchanged from the previous MRI. After it came back she was willing to do therapy.
I’m sure some out there will accuse me, the doctor, of letting the patient call the shots. To some degree you’re correct. But it’s not like the request was insanely unreasonable. Obviously, there were other factors going on, too. She was scared and needed reassurance that there wasn’t anything therapy wouldn’t help and that she would be able to keep caring for her ailing husband during his cancer treatments.
There are doctors out there with a more paternalistic view of patient care than mine. They’re probably thinking I should have taken a hardball approach of “you don’t need an MRI. You can do therapy, or you can find another doctor.” But that’s not me. I can’t do that to a nice older lady, especially one who’s been coming to me for various ailments over the last 12 years.
Not only that, but such an approach seemed doomed to fail in this case. It might have gotten her to go to therapy, but I suspect she wouldn’t have gotten better. Her fears about a serious neck issue would increase over time, until she (or the therapist) finally called, said she wasn’t getting better, and could I order an MRI now?
In that way, maybe the MRI helped guarantee that she’d have a good response to therapy.
I can’t say that what I did was the right thing. But it was right for Mrs. Smith.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Smith came in for neck pain.
This isn’t a new issue, her last flare was 4 or 5 years ago. I’d done an MRI back then, which just showed reassuringly typical arthritic changes, and she did great with a few sessions of physical therapy.
She’d woke one morning a few months ago with a stiff and aching neck, similar to how this started last time. A couple weeks of rest and NSAIDs hadn’t helped. There were no radiating symptoms and her exam was the same as it’s been since I met her back in 2010.
I wrote her an order for physical therapy and found the address and phone number of the place she’d gone to for it a few years ago. She looked at my order, then set it on my desk and said “Doctor, I’d really like an MRI.”
I went back to her chart and reread my note for her last flare of neck pain. Identical symptoms, identical exam. I pulled up the previous MRI report and went over it. Then I explained to her that there really was no indication for an MRI at this point. I suggested we go ahead with physical therapy, and if that didn’t help I would then re-check the study.
She wasn’t going to budge. A friend of hers had recently needed urgent surgery for a cervical myelopathy and was in rehab. Mrs. Smith’s husband’s health was getting worse, and if her neck had something seriously wrong she wouldn’t be able to take care of him if it went unchecked.
So I backed down and ordered a cervical spine MRI, which was pretty much unchanged from the previous MRI. After it came back she was willing to do therapy.
I’m sure some out there will accuse me, the doctor, of letting the patient call the shots. To some degree you’re correct. But it’s not like the request was insanely unreasonable. Obviously, there were other factors going on, too. She was scared and needed reassurance that there wasn’t anything therapy wouldn’t help and that she would be able to keep caring for her ailing husband during his cancer treatments.
There are doctors out there with a more paternalistic view of patient care than mine. They’re probably thinking I should have taken a hardball approach of “you don’t need an MRI. You can do therapy, or you can find another doctor.” But that’s not me. I can’t do that to a nice older lady, especially one who’s been coming to me for various ailments over the last 12 years.
Not only that, but such an approach seemed doomed to fail in this case. It might have gotten her to go to therapy, but I suspect she wouldn’t have gotten better. Her fears about a serious neck issue would increase over time, until she (or the therapist) finally called, said she wasn’t getting better, and could I order an MRI now?
In that way, maybe the MRI helped guarantee that she’d have a good response to therapy.
I can’t say that what I did was the right thing. But it was right for Mrs. Smith.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Smith came in for neck pain.
This isn’t a new issue, her last flare was 4 or 5 years ago. I’d done an MRI back then, which just showed reassuringly typical arthritic changes, and she did great with a few sessions of physical therapy.
She’d woke one morning a few months ago with a stiff and aching neck, similar to how this started last time. A couple weeks of rest and NSAIDs hadn’t helped. There were no radiating symptoms and her exam was the same as it’s been since I met her back in 2010.
I wrote her an order for physical therapy and found the address and phone number of the place she’d gone to for it a few years ago. She looked at my order, then set it on my desk and said “Doctor, I’d really like an MRI.”
I went back to her chart and reread my note for her last flare of neck pain. Identical symptoms, identical exam. I pulled up the previous MRI report and went over it. Then I explained to her that there really was no indication for an MRI at this point. I suggested we go ahead with physical therapy, and if that didn’t help I would then re-check the study.
She wasn’t going to budge. A friend of hers had recently needed urgent surgery for a cervical myelopathy and was in rehab. Mrs. Smith’s husband’s health was getting worse, and if her neck had something seriously wrong she wouldn’t be able to take care of him if it went unchecked.
So I backed down and ordered a cervical spine MRI, which was pretty much unchanged from the previous MRI. After it came back she was willing to do therapy.
I’m sure some out there will accuse me, the doctor, of letting the patient call the shots. To some degree you’re correct. But it’s not like the request was insanely unreasonable. Obviously, there were other factors going on, too. She was scared and needed reassurance that there wasn’t anything therapy wouldn’t help and that she would be able to keep caring for her ailing husband during his cancer treatments.
There are doctors out there with a more paternalistic view of patient care than mine. They’re probably thinking I should have taken a hardball approach of “you don’t need an MRI. You can do therapy, or you can find another doctor.” But that’s not me. I can’t do that to a nice older lady, especially one who’s been coming to me for various ailments over the last 12 years.
Not only that, but such an approach seemed doomed to fail in this case. It might have gotten her to go to therapy, but I suspect she wouldn’t have gotten better. Her fears about a serious neck issue would increase over time, until she (or the therapist) finally called, said she wasn’t getting better, and could I order an MRI now?
In that way, maybe the MRI helped guarantee that she’d have a good response to therapy.
I can’t say that what I did was the right thing. But it was right for Mrs. Smith.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Surgery shows no survival, morbidity benefit for mild hyperparathyroidism
Patients who receive parathyroidectomy for mild primary hyperparathyroidism show no benefits in survival or morbidity, including fractures, cancer, or cardiovascular outcomes over more than 10 years, compared with those not receiving the surgery, results from a randomized, prospective trial show.
“In contrast to existing data showing increased mortality and cardiovascular morbidity in mild primary hyperparathyroidism, we did not find any treatment effect of parathyroidectomy on these important clinical endpoints,” report the authors of the study, published in the Annals of Internal Medicine.
Reason to evaluate and revise current recommendations?
With mild primary hyperparathyroidism becoming the predominant form of hyperparathyroidism, the results suggest rethinking the current recommendations for the condition, the study authors note.
“Over the years, more active management of mild primary hyperparathyroidism has been recommended, with a widening of criteria for parathyroidectomy,” they write.
“With the low number of kidney stones (n = 5) and no effect of parathyroidectomy on fractures, there may be a need to evaluate and potentially revise the current recommendations.”
The authors of an accompanying editorial agree that “the [results] provide a strong rationale for nonoperative management of patients with mild primary hyperparathyroidism.”
“The findings suggest that most patients can be managed nonoperatively, with monitoring of serum calcium levels every 1 to 2 years or if symptoms occur,” write the editorial authors, Mark J. Bolland, PhD, and Andrew Grey, MD, of the department of medicine, University of Auckland, New Zealand.
Although parathyroidectomy is recommended for the treatment in patients with hyperparathyroidism with severe hypercalcemia or overt symptoms, there has been debate on the long-term benefits of surgery among those with milder cases.
Most previous studies that have shown benefits, such as reductions in the risk of fracture with parathyroidectomy, have importantly not distinguished between mild and more severe primary hyperparathyroidism, the authors note.
No significant differences in mortality between surgery, nonsurgery groups
For the Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) trial, first author Mikkel Pretorius, MD, Oslo University Hospital and Faculty of Medicine, University of Oslo, and colleagues enrolled 191 patients between 1998 and 2005 in Sweden, Norway, and Denmark, who were aged 50-80 years and had mild primary hyperparathyroidism, defined as serum calcium levels of 10.42-11.22 mg/dL.
Participants were randomized to receive surgery (n = 95) or nonoperative observation without intervention (n = 96).
After a 10-year follow-up, 129 patients had completed the final visit. The overall death rate was 7.6%, and, with eight deaths in the surgery group and seven in the nonsurgery group, there were no significant differences between groups in terms of mortality (HR, 1.17; P = .76).
During an extended observation period that lasted until 2018, mortality rates increased by 23%, but with a relatively even distribution of 24 deaths in the surgery group and 20 among those with no surgery.
Chronic hypercalcemia related to primary hyperparathyroidism has been debated as being associated with an increased risk of cardiovascular disease or cancer, however, “the absolute numbers for these and the other disease-specific causes of death were nearly identical between groups,” the authors write, with 17 deaths from cardiovascular disease, eight from cancer, and eight from cerebrovascular disease.
In terms of morbidity, including cardiovascular events, cerebrovascular events, cancer, peripheral fractures, and renal stones, there were 101 events overall, with 52 in the parathyroidectomy group and 49 in the nonsurgery group, which again, was not a significant difference.
Sixteen vertebral fractures occurred overall in 14 patients, which were evenly split at seven patients in each group.
The authors note that “the incidence of peripheral fractures for women in our study was around 2,900 per 100,000 person-years, in the same range as for 70-year-old women in a study in Gothenburg, Sweden (about 2,600 per 100,000 person-years).”
There were no between-group differences in terms of time to death or first morbidity event for any of the prespecified events.
Of the 96 patients originally assigned to the nonsurgery group, 17 (18%) had surgery during follow-up, including three for serious hypercalcemia, three by their own choice, two for decreasing bone density, one for kidney stones, and the others for unclear or unrelated reasons.
Study limitations include that only 26 men (13 in each group) were included, and only 16 completed the study. “The external validity for men based on this study is therefore limited,” the authors note.
And although most people with primary hyperparathyroidism are adults, the older age of participants suggests the results should not be generalized to younger patients with benign parathyroid tumors.
The editorialists note that age should be one of the few factors that may, indeed, suggest appropriate candidates for parathyroidectomy.
“Younger patients (aged < 50 years) may have more aggressive disease,” they explain.
In addition, “patients with serum calcium levels above 3 mmol/L (> 12 mg/dL) are at greater risk for symptomatic hypercalcemia, and patients with a recent history of kidney stones may have fewer future stones after surgical cure.”
“Yet, such patients are a small minority of those with primary hyperparathyroidism,” they note.
The study authors underscore that “our data add evidence to guide the decisionmaking process in deliberative dialogue between clinicians and patients.”
The study received funding from Swedish government grants, the Norwegian Research Council, and the South-Eastern Norway Regional Health Authority.
A version of this article first appeared on Medscape.com.
Patients who receive parathyroidectomy for mild primary hyperparathyroidism show no benefits in survival or morbidity, including fractures, cancer, or cardiovascular outcomes over more than 10 years, compared with those not receiving the surgery, results from a randomized, prospective trial show.
“In contrast to existing data showing increased mortality and cardiovascular morbidity in mild primary hyperparathyroidism, we did not find any treatment effect of parathyroidectomy on these important clinical endpoints,” report the authors of the study, published in the Annals of Internal Medicine.
Reason to evaluate and revise current recommendations?
With mild primary hyperparathyroidism becoming the predominant form of hyperparathyroidism, the results suggest rethinking the current recommendations for the condition, the study authors note.
“Over the years, more active management of mild primary hyperparathyroidism has been recommended, with a widening of criteria for parathyroidectomy,” they write.
“With the low number of kidney stones (n = 5) and no effect of parathyroidectomy on fractures, there may be a need to evaluate and potentially revise the current recommendations.”
The authors of an accompanying editorial agree that “the [results] provide a strong rationale for nonoperative management of patients with mild primary hyperparathyroidism.”
“The findings suggest that most patients can be managed nonoperatively, with monitoring of serum calcium levels every 1 to 2 years or if symptoms occur,” write the editorial authors, Mark J. Bolland, PhD, and Andrew Grey, MD, of the department of medicine, University of Auckland, New Zealand.
Although parathyroidectomy is recommended for the treatment in patients with hyperparathyroidism with severe hypercalcemia or overt symptoms, there has been debate on the long-term benefits of surgery among those with milder cases.
Most previous studies that have shown benefits, such as reductions in the risk of fracture with parathyroidectomy, have importantly not distinguished between mild and more severe primary hyperparathyroidism, the authors note.
No significant differences in mortality between surgery, nonsurgery groups
For the Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) trial, first author Mikkel Pretorius, MD, Oslo University Hospital and Faculty of Medicine, University of Oslo, and colleagues enrolled 191 patients between 1998 and 2005 in Sweden, Norway, and Denmark, who were aged 50-80 years and had mild primary hyperparathyroidism, defined as serum calcium levels of 10.42-11.22 mg/dL.
Participants were randomized to receive surgery (n = 95) or nonoperative observation without intervention (n = 96).
After a 10-year follow-up, 129 patients had completed the final visit. The overall death rate was 7.6%, and, with eight deaths in the surgery group and seven in the nonsurgery group, there were no significant differences between groups in terms of mortality (HR, 1.17; P = .76).
During an extended observation period that lasted until 2018, mortality rates increased by 23%, but with a relatively even distribution of 24 deaths in the surgery group and 20 among those with no surgery.
Chronic hypercalcemia related to primary hyperparathyroidism has been debated as being associated with an increased risk of cardiovascular disease or cancer, however, “the absolute numbers for these and the other disease-specific causes of death were nearly identical between groups,” the authors write, with 17 deaths from cardiovascular disease, eight from cancer, and eight from cerebrovascular disease.
In terms of morbidity, including cardiovascular events, cerebrovascular events, cancer, peripheral fractures, and renal stones, there were 101 events overall, with 52 in the parathyroidectomy group and 49 in the nonsurgery group, which again, was not a significant difference.
Sixteen vertebral fractures occurred overall in 14 patients, which were evenly split at seven patients in each group.
The authors note that “the incidence of peripheral fractures for women in our study was around 2,900 per 100,000 person-years, in the same range as for 70-year-old women in a study in Gothenburg, Sweden (about 2,600 per 100,000 person-years).”
There were no between-group differences in terms of time to death or first morbidity event for any of the prespecified events.
Of the 96 patients originally assigned to the nonsurgery group, 17 (18%) had surgery during follow-up, including three for serious hypercalcemia, three by their own choice, two for decreasing bone density, one for kidney stones, and the others for unclear or unrelated reasons.
Study limitations include that only 26 men (13 in each group) were included, and only 16 completed the study. “The external validity for men based on this study is therefore limited,” the authors note.
And although most people with primary hyperparathyroidism are adults, the older age of participants suggests the results should not be generalized to younger patients with benign parathyroid tumors.
The editorialists note that age should be one of the few factors that may, indeed, suggest appropriate candidates for parathyroidectomy.
“Younger patients (aged < 50 years) may have more aggressive disease,” they explain.
In addition, “patients with serum calcium levels above 3 mmol/L (> 12 mg/dL) are at greater risk for symptomatic hypercalcemia, and patients with a recent history of kidney stones may have fewer future stones after surgical cure.”
“Yet, such patients are a small minority of those with primary hyperparathyroidism,” they note.
The study authors underscore that “our data add evidence to guide the decisionmaking process in deliberative dialogue between clinicians and patients.”
The study received funding from Swedish government grants, the Norwegian Research Council, and the South-Eastern Norway Regional Health Authority.
A version of this article first appeared on Medscape.com.
Patients who receive parathyroidectomy for mild primary hyperparathyroidism show no benefits in survival or morbidity, including fractures, cancer, or cardiovascular outcomes over more than 10 years, compared with those not receiving the surgery, results from a randomized, prospective trial show.
“In contrast to existing data showing increased mortality and cardiovascular morbidity in mild primary hyperparathyroidism, we did not find any treatment effect of parathyroidectomy on these important clinical endpoints,” report the authors of the study, published in the Annals of Internal Medicine.
Reason to evaluate and revise current recommendations?
With mild primary hyperparathyroidism becoming the predominant form of hyperparathyroidism, the results suggest rethinking the current recommendations for the condition, the study authors note.
“Over the years, more active management of mild primary hyperparathyroidism has been recommended, with a widening of criteria for parathyroidectomy,” they write.
“With the low number of kidney stones (n = 5) and no effect of parathyroidectomy on fractures, there may be a need to evaluate and potentially revise the current recommendations.”
The authors of an accompanying editorial agree that “the [results] provide a strong rationale for nonoperative management of patients with mild primary hyperparathyroidism.”
“The findings suggest that most patients can be managed nonoperatively, with monitoring of serum calcium levels every 1 to 2 years or if symptoms occur,” write the editorial authors, Mark J. Bolland, PhD, and Andrew Grey, MD, of the department of medicine, University of Auckland, New Zealand.
Although parathyroidectomy is recommended for the treatment in patients with hyperparathyroidism with severe hypercalcemia or overt symptoms, there has been debate on the long-term benefits of surgery among those with milder cases.
Most previous studies that have shown benefits, such as reductions in the risk of fracture with parathyroidectomy, have importantly not distinguished between mild and more severe primary hyperparathyroidism, the authors note.
No significant differences in mortality between surgery, nonsurgery groups
For the Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) trial, first author Mikkel Pretorius, MD, Oslo University Hospital and Faculty of Medicine, University of Oslo, and colleagues enrolled 191 patients between 1998 and 2005 in Sweden, Norway, and Denmark, who were aged 50-80 years and had mild primary hyperparathyroidism, defined as serum calcium levels of 10.42-11.22 mg/dL.
Participants were randomized to receive surgery (n = 95) or nonoperative observation without intervention (n = 96).
After a 10-year follow-up, 129 patients had completed the final visit. The overall death rate was 7.6%, and, with eight deaths in the surgery group and seven in the nonsurgery group, there were no significant differences between groups in terms of mortality (HR, 1.17; P = .76).
During an extended observation period that lasted until 2018, mortality rates increased by 23%, but with a relatively even distribution of 24 deaths in the surgery group and 20 among those with no surgery.
Chronic hypercalcemia related to primary hyperparathyroidism has been debated as being associated with an increased risk of cardiovascular disease or cancer, however, “the absolute numbers for these and the other disease-specific causes of death were nearly identical between groups,” the authors write, with 17 deaths from cardiovascular disease, eight from cancer, and eight from cerebrovascular disease.
In terms of morbidity, including cardiovascular events, cerebrovascular events, cancer, peripheral fractures, and renal stones, there were 101 events overall, with 52 in the parathyroidectomy group and 49 in the nonsurgery group, which again, was not a significant difference.
Sixteen vertebral fractures occurred overall in 14 patients, which were evenly split at seven patients in each group.
The authors note that “the incidence of peripheral fractures for women in our study was around 2,900 per 100,000 person-years, in the same range as for 70-year-old women in a study in Gothenburg, Sweden (about 2,600 per 100,000 person-years).”
There were no between-group differences in terms of time to death or first morbidity event for any of the prespecified events.
Of the 96 patients originally assigned to the nonsurgery group, 17 (18%) had surgery during follow-up, including three for serious hypercalcemia, three by their own choice, two for decreasing bone density, one for kidney stones, and the others for unclear or unrelated reasons.
Study limitations include that only 26 men (13 in each group) were included, and only 16 completed the study. “The external validity for men based on this study is therefore limited,” the authors note.
And although most people with primary hyperparathyroidism are adults, the older age of participants suggests the results should not be generalized to younger patients with benign parathyroid tumors.
The editorialists note that age should be one of the few factors that may, indeed, suggest appropriate candidates for parathyroidectomy.
“Younger patients (aged < 50 years) may have more aggressive disease,” they explain.
In addition, “patients with serum calcium levels above 3 mmol/L (> 12 mg/dL) are at greater risk for symptomatic hypercalcemia, and patients with a recent history of kidney stones may have fewer future stones after surgical cure.”
“Yet, such patients are a small minority of those with primary hyperparathyroidism,” they note.
The study authors underscore that “our data add evidence to guide the decisionmaking process in deliberative dialogue between clinicians and patients.”
The study received funding from Swedish government grants, the Norwegian Research Council, and the South-Eastern Norway Regional Health Authority.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
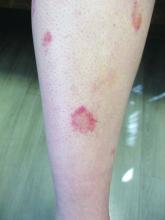
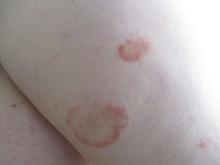
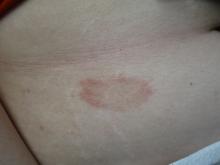
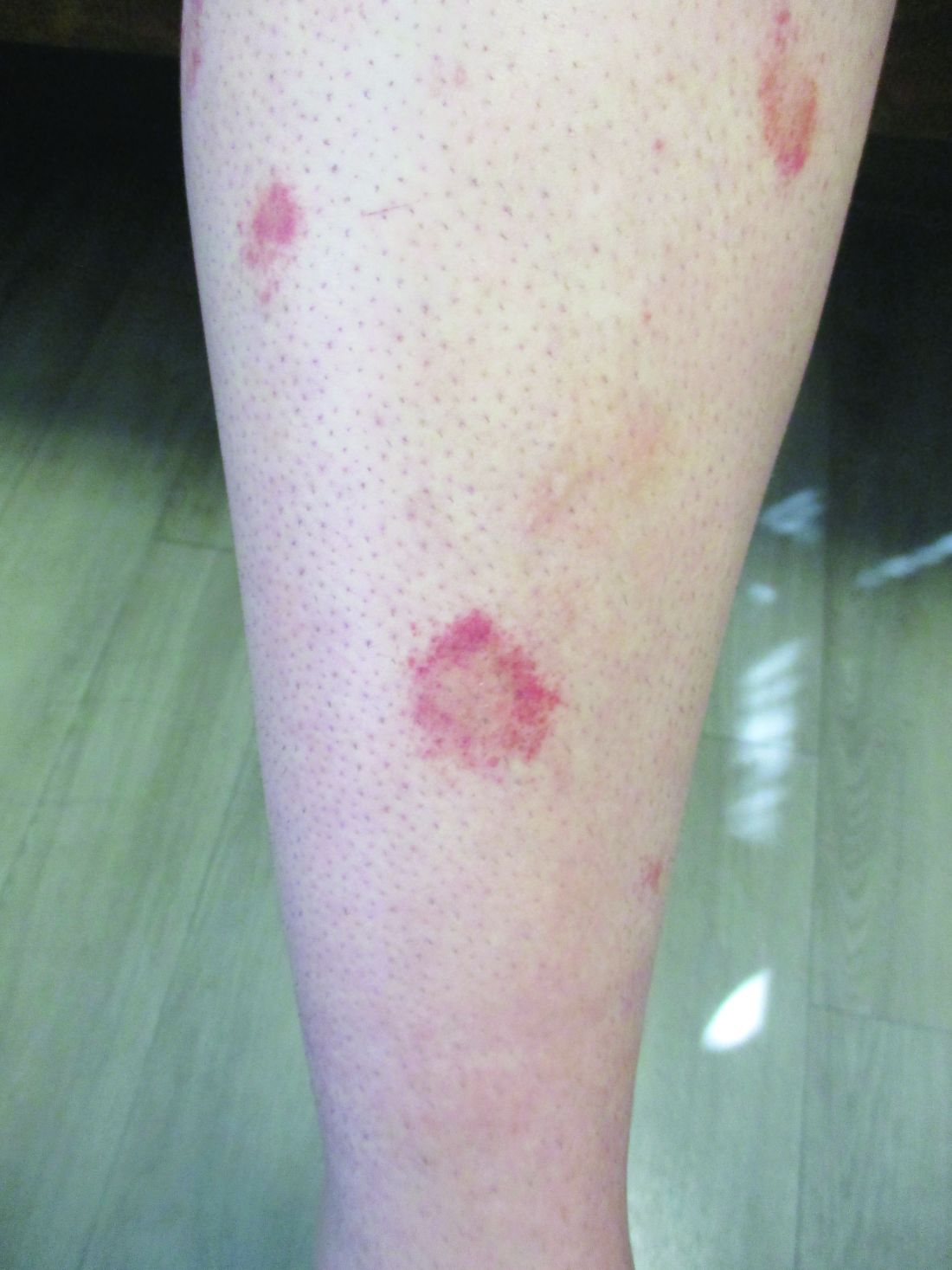
24-year-old female presents with a 3-month history of nonpruritic rash
, typically characterized by symmetrical, nonblanching, purpuric, telangiectatic, and atrophic patches with a predilection for the lower extremities and buttocks.
Plaques are usually 1-3 cm in diameter and annular with punctate telangiectasias and cayenne pepper petechiae in the border. The annular patches may form concentric rings. It is most commonly seen in children and young females.
The etiology of Majocchi’s disease is largely unknown and idiopathic.
Triggers are not always detected but may be associated with viral infections, chronic comorbidities, and medications. Levofloxacin and isotretinoin have been described in as reports as causing PATM. Other medications reported to cause PPD include sedatives, stimulants, antibiotics, NSAIDS, and cardiovascular drugs.
Diagnosis of PATM is clinical and histopathologic. Direct immunofluorescence (DIF) may show fibrinogen, IgM, and/or C3 deposition in superficial dermal vessels. Histopathologic findings show lymphocytic infiltrate involving the superficial small vessels, extravasated red blood cells, and hemosiderin-laden macrophages.
There is no consensus regarding treatment with variable responses to proposed treatment based on reports and case studies. The first line of treatment is topical corticosteroids and compression hose. Additional treatments, including narrowband UVB phototherapy (NBUVB), griseofulvin, pentoxifylline, cyclosporine, colchicine, rutoside with ascorbic acid, and methotrexate, have been used with varying success.
In this patient, a punch biopsy was performed, which revealed lymphocytes and extravasated erythrocytes and siderophages in the dermis. She was treated with topical steroids with improvement. She started NBUVB, a short course of griseofulvin, and vitamin C supplements.
This case and the photos were photo submitted by Ms. Xu, of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology. Dr. Donna Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Garcez A et al. An Bras Dermatol. Sep-Oct 2020;95(5):664-6. doi: 10.1016/j.abd.2020.02.007.
2. Asadbeigi S, Momtahen S. Pigmented purpuric dermatosis. PathologyOutlines.com website.
3. Martínez P et al. Actas Dermosifiliogr (Engl Ed). 2020 Apr;111(3):196-204. doi: 10.1016/j.ad.2019.02.013.
4. Hoesly FJ et al. Int J Dermatol. 2009 Oct;48(10):1129-33. doi: 10.1111/j.1365-4632.2009.04160.x.
, typically characterized by symmetrical, nonblanching, purpuric, telangiectatic, and atrophic patches with a predilection for the lower extremities and buttocks.
Plaques are usually 1-3 cm in diameter and annular with punctate telangiectasias and cayenne pepper petechiae in the border. The annular patches may form concentric rings. It is most commonly seen in children and young females.
The etiology of Majocchi’s disease is largely unknown and idiopathic.
Triggers are not always detected but may be associated with viral infections, chronic comorbidities, and medications. Levofloxacin and isotretinoin have been described in as reports as causing PATM. Other medications reported to cause PPD include sedatives, stimulants, antibiotics, NSAIDS, and cardiovascular drugs.
Diagnosis of PATM is clinical and histopathologic. Direct immunofluorescence (DIF) may show fibrinogen, IgM, and/or C3 deposition in superficial dermal vessels. Histopathologic findings show lymphocytic infiltrate involving the superficial small vessels, extravasated red blood cells, and hemosiderin-laden macrophages.
There is no consensus regarding treatment with variable responses to proposed treatment based on reports and case studies. The first line of treatment is topical corticosteroids and compression hose. Additional treatments, including narrowband UVB phototherapy (NBUVB), griseofulvin, pentoxifylline, cyclosporine, colchicine, rutoside with ascorbic acid, and methotrexate, have been used with varying success.
In this patient, a punch biopsy was performed, which revealed lymphocytes and extravasated erythrocytes and siderophages in the dermis. She was treated with topical steroids with improvement. She started NBUVB, a short course of griseofulvin, and vitamin C supplements.
This case and the photos were photo submitted by Ms. Xu, of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology. Dr. Donna Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Garcez A et al. An Bras Dermatol. Sep-Oct 2020;95(5):664-6. doi: 10.1016/j.abd.2020.02.007.
2. Asadbeigi S, Momtahen S. Pigmented purpuric dermatosis. PathologyOutlines.com website.
3. Martínez P et al. Actas Dermosifiliogr (Engl Ed). 2020 Apr;111(3):196-204. doi: 10.1016/j.ad.2019.02.013.
4. Hoesly FJ et al. Int J Dermatol. 2009 Oct;48(10):1129-33. doi: 10.1111/j.1365-4632.2009.04160.x.
, typically characterized by symmetrical, nonblanching, purpuric, telangiectatic, and atrophic patches with a predilection for the lower extremities and buttocks.
Plaques are usually 1-3 cm in diameter and annular with punctate telangiectasias and cayenne pepper petechiae in the border. The annular patches may form concentric rings. It is most commonly seen in children and young females.
The etiology of Majocchi’s disease is largely unknown and idiopathic.
Triggers are not always detected but may be associated with viral infections, chronic comorbidities, and medications. Levofloxacin and isotretinoin have been described in as reports as causing PATM. Other medications reported to cause PPD include sedatives, stimulants, antibiotics, NSAIDS, and cardiovascular drugs.
Diagnosis of PATM is clinical and histopathologic. Direct immunofluorescence (DIF) may show fibrinogen, IgM, and/or C3 deposition in superficial dermal vessels. Histopathologic findings show lymphocytic infiltrate involving the superficial small vessels, extravasated red blood cells, and hemosiderin-laden macrophages.
There is no consensus regarding treatment with variable responses to proposed treatment based on reports and case studies. The first line of treatment is topical corticosteroids and compression hose. Additional treatments, including narrowband UVB phototherapy (NBUVB), griseofulvin, pentoxifylline, cyclosporine, colchicine, rutoside with ascorbic acid, and methotrexate, have been used with varying success.
In this patient, a punch biopsy was performed, which revealed lymphocytes and extravasated erythrocytes and siderophages in the dermis. She was treated with topical steroids with improvement. She started NBUVB, a short course of griseofulvin, and vitamin C supplements.
This case and the photos were photo submitted by Ms. Xu, of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology. Dr. Donna Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Garcez A et al. An Bras Dermatol. Sep-Oct 2020;95(5):664-6. doi: 10.1016/j.abd.2020.02.007.
2. Asadbeigi S, Momtahen S. Pigmented purpuric dermatosis. PathologyOutlines.com website.
3. Martínez P et al. Actas Dermosifiliogr (Engl Ed). 2020 Apr;111(3):196-204. doi: 10.1016/j.ad.2019.02.013.
4. Hoesly FJ et al. Int J Dermatol. 2009 Oct;48(10):1129-33. doi: 10.1111/j.1365-4632.2009.04160.x.
ILD progression, not diagnosis, triggers palliative care
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most health care providers are comfortable recommending palliative care (PC) for their patients with interstitial lung disease (ILD), but most do so at the time of disease progression, rather than diagnosis, as indicated on survey data from 128 clinicians.
ILD is associated with a high mortality rate and profound symptoms that contribute to poor quality of life, Rebecca A. Gersen, MD, of Johns Hopkins University, Baltimore, and colleagues wrote.
“Nevertheless, there is often a lack of preparedness for death by both patients and providers, contributing to increased distress,” they said. Clinician perspectives on the use of PC for ILD patients have not been well studied, although PC is not limited to end-of-life care and is recommended for ILD patients by professional organizations, including the American Thoracic Society. “PC is successful in improving breathlessness in chronic lung disease and can increase survival.”
In a study published in the journal CHEST®, the researchers surveyed health care providers at 68 Pulmonary Fibrosis Foundation centers across the United States. The survey was sent and collected by email and a restricted social media platform. A total of 128 providers from 34 states completed the survey between October 2020 and January 2021. Of these, 61% were physicians, and 67% identified as White.
Overall, 95% of the respondents agreed or strongly agreed that addressing advance directives is important, but only 66% agreed or strongly agreed that they themselves addressed advance directives in the outpatient ILD clinic setting. A greater number (91%) agreed or strongly agreed that they had a high level of comfort in discussing prognosis, while 88% agreed or strongly agreed that they felt comfortable assessing a patient’s readiness for and acceptance of PC. Approximately two-thirds (67%) agreed or strongly agreed that they use PC services for ILD patients. There were no significant differences in responses from clinicians who had more than 10 years of experience and those who had less.
Of the providers who referred patients to PC, 54% did so at objective disease progression, and 80% did so at objective and/or symptomatic progress; 2% referred patients to PC at initial ILD diagnosis.
Lack of resources
Health care providers who reported that they rarely referred patients to palliative care were significantly more likely to cite a lack of local PC options (P < .01). Those who rarely referred patients for PC also were significantly less likely to feel comfortable discussing prognoses or advance directives in the ILD clinic (P = .03 and P = .02, respectively).
Among the 23% of responders who reported that they rarely referred patients, 66% said they did not have PC at their institution.
“In addition to understanding and addressing barriers to care, educational resources may be key to improving PC delivery to the ILD population,” the researchers wrote.
The study findings were limited by several factors, including voluntary participation, lack of a validated questionnaire, and use of self-reports, which may not reflect physicians’ actual practice, the researchers noted. Other limitations include the use of U.S. data only, which may not generalize to countries with different health care models.
However, the results were strengthened by the use of data from providers at a range of institutions across the United States and by the high overall survey response rate, the researchers said.
“While ILD providers reassuringly demonstrate knowledge and interest in PC involvement, no current system exists to facilitate and monitor response to referral,” they noted. “Future research is desperately needed to address barriers to the provision of PC in order to enhance access to a critical service in the management and care of patients with ILD.”
The study was supported by the National Heart, Lung, and Blood Institute. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL CHEST®
Inadequate pain relief in OA, high opioid use before TKA
Inadequate pain relief was recorded in 68.8% of a sample of people with hip or knee OA who participated in the population-based EpiReumaPt study, researchers reported at the OARSI 2022 World Congress.
“This can be explained by a lack of effectiveness of current management strategies, low uptake of recommended interventions by health care professionals, and also by low adherence by patients to medication and lifestyle interventions,” said Daniela Sofia Albino Costa, MSc, a PhD student at NOVA University Lisbon.
In addition to looking at the prevalence of inadequate pain relief – defined as a score of 5 or higher on the Numeric Pain Rating Scale (NPRS) – the study she presented at the congress, which was sponsored by the Osteoarthritis Research Society International, looked at the predictors for inadequate pain control.
It was found that being female, obesity, and having multimorbidity doubled the risk of inadequate versus adequate pain control, with respective odds ratios of 2.32 (P < .001), 2.26 (P = .006), and 2.07 (P = .001). Overweight was also associated with an increased odds ratio for poor pain control (OR, 1.84; P = .0035).
“We found that patients with inadequate pain relief also have a low performance on activities of daily living and a low quality of life,” Ms. Costa said.
Nearly one-third (29%) of patients in the inadequate pain relief group (n = 765) took medication, versus 15% of patients in the adequate pain relief group (n = 270). This was mostly NSAIDs, but also included analgesics and antipyretics, and in a few cases (4.8% vs. 1.3%), simple opioids.
“We know that current care is not concordant with recommendations,” said Ms. Costa, noting that medication being used as first-line treatment and core nonpharmacologic interventions are being offered to less than half of patients who are eligible.
In addition, the rate for total joint replacement has increased globally, and pain is an important predictor for this.
“So, we need to evaluate pain control and current management offered to people with hip or knee arthritis to identify to identify areas for improvement,” Ms. Costa said.
High rates of prescription opioid use before TKA
In a separate study also presented at the congress, Daniel Rhon, DPT, DSc, director of musculoskeletal research in primary care at Brooke Army Medical Center in San Antonio, gave a worrying glimpse of high rates of opioid use in the 4 years before total knee arthroplasty (TKA).
Using data from the U.S. Military Health System, the records of all individuals who had a knee replacement procedure between January 2017 and December 2018 were studied, to identify and characterize the use of prescription opioids.
Of the 46,362 individuals, 52.9% had prior opioid use, despite the fact that “opioids are not recommended for the management of knee OA,” said Dr. Rhon.
He also reported that as many as 40% of those who had at least one prescription for opioids had received a high-potency drug, such as fentanyl or oxycodone. The mean age of participants overall was 65 years, with a higher mean for those receiving opioids than those who did not (68 vs. 61.5 years). Data on sex and ethnicity were not available in time for presentation at the congress.
“Most of these individuals are getting these opioid prescriptions probably within 6 months, which maybe aligns with escalation of pain and maybe the decision to have that knee replacement,” Dr. Rhon said. Individuals that used opioids filled their most recent prescription a median of 146 days before TKA to surgery, with a mean of 317 days.
“You can’t always link the reason for the opioid prescription, that’s not really clear in the database,” he admitted; however, an analysis was performed to check if other surgeries had been performed that may have warranted the opioid treatment. The results revealed that very few of the opioid users (4%-7%) had undergone another type of surgical procedure.
“So, we feel a little bit better, that these findings weren’t for other surgical procedures,” said Dr. Rhon. He added that future qualitative research was needed to understand why health care professionals were prescribing opioids, and why patients felt like they needed them.
“That’s bad,” Haxby Abbott, PhD, DPT, a research professor at the University of Otago, Dunedin, New Zealand, commented on Twitter.
Dr. Abbott, who was not involved in the study, added: “We’ve done a similar study of the whole NZ population [currently under review] – similar to Australia and not nearly as bad as you found. That needs urgent attention.”
Sharp rise in opioid use 2 years before TKA
Lower rates of opioid use before TKA were seen in two European cohorts, at 43% in England and 33% in Sweden, as reported by Clara Hellberg, PhD, MD, of Lund (Sweden) University. However, rates had increased over a 10-year study period from a respective 23% and 16%, with a sharp increase in use in the 2 years before knee replacement.
The analysis was based on 49,043 patients from the English national database Clinical Practice Research Datalink, and 5,955 patients from the Swedish Skåne Healthcare register who had undergone total knee replacement between 2015 and 2019 and were matched by age, sex and general practice to individuals not undergoing knee replacement.
The prevalence ratio for using opioids over a 10-year period increased from 1.6 to 2.7 in England, and from 1.6 to 2.6 in Sweden.
“While the overall prevalence of opioid use was higher in England, the majority of both cases and controls were using weak opioids,” Dr. Hellberg said.
“Codeine was classified as a weak opioid, whereas morphine was classified as a strong opioid,” she added.
In contrast, the proportion of people using strong opioids in Sweden was greater than in England, she said.
The high opioid use found in the study highlights “the need for better opioid stewardship, and the availability of acceptable, effective alternatives,” Dr. Hellberg and associates concluded in their abstract.
The study presented by Ms. Costa was funded by the Portuguese national funding agency for science, research and technology and by an independent research grant from Pfizer. Dr. Rhon acknowledged grant funding from the National Institutes of Health and the U.S. Department of Defense. Dr. Hellberg had no conflicts of interest to disclose.
Inadequate pain relief was recorded in 68.8% of a sample of people with hip or knee OA who participated in the population-based EpiReumaPt study, researchers reported at the OARSI 2022 World Congress.
“This can be explained by a lack of effectiveness of current management strategies, low uptake of recommended interventions by health care professionals, and also by low adherence by patients to medication and lifestyle interventions,” said Daniela Sofia Albino Costa, MSc, a PhD student at NOVA University Lisbon.
In addition to looking at the prevalence of inadequate pain relief – defined as a score of 5 or higher on the Numeric Pain Rating Scale (NPRS) – the study she presented at the congress, which was sponsored by the Osteoarthritis Research Society International, looked at the predictors for inadequate pain control.
It was found that being female, obesity, and having multimorbidity doubled the risk of inadequate versus adequate pain control, with respective odds ratios of 2.32 (P < .001), 2.26 (P = .006), and 2.07 (P = .001). Overweight was also associated with an increased odds ratio for poor pain control (OR, 1.84; P = .0035).
“We found that patients with inadequate pain relief also have a low performance on activities of daily living and a low quality of life,” Ms. Costa said.
Nearly one-third (29%) of patients in the inadequate pain relief group (n = 765) took medication, versus 15% of patients in the adequate pain relief group (n = 270). This was mostly NSAIDs, but also included analgesics and antipyretics, and in a few cases (4.8% vs. 1.3%), simple opioids.
“We know that current care is not concordant with recommendations,” said Ms. Costa, noting that medication being used as first-line treatment and core nonpharmacologic interventions are being offered to less than half of patients who are eligible.
In addition, the rate for total joint replacement has increased globally, and pain is an important predictor for this.
“So, we need to evaluate pain control and current management offered to people with hip or knee arthritis to identify to identify areas for improvement,” Ms. Costa said.
High rates of prescription opioid use before TKA
In a separate study also presented at the congress, Daniel Rhon, DPT, DSc, director of musculoskeletal research in primary care at Brooke Army Medical Center in San Antonio, gave a worrying glimpse of high rates of opioid use in the 4 years before total knee arthroplasty (TKA).
Using data from the U.S. Military Health System, the records of all individuals who had a knee replacement procedure between January 2017 and December 2018 were studied, to identify and characterize the use of prescription opioids.
Of the 46,362 individuals, 52.9% had prior opioid use, despite the fact that “opioids are not recommended for the management of knee OA,” said Dr. Rhon.
He also reported that as many as 40% of those who had at least one prescription for opioids had received a high-potency drug, such as fentanyl or oxycodone. The mean age of participants overall was 65 years, with a higher mean for those receiving opioids than those who did not (68 vs. 61.5 years). Data on sex and ethnicity were not available in time for presentation at the congress.
“Most of these individuals are getting these opioid prescriptions probably within 6 months, which maybe aligns with escalation of pain and maybe the decision to have that knee replacement,” Dr. Rhon said. Individuals that used opioids filled their most recent prescription a median of 146 days before TKA to surgery, with a mean of 317 days.
“You can’t always link the reason for the opioid prescription, that’s not really clear in the database,” he admitted; however, an analysis was performed to check if other surgeries had been performed that may have warranted the opioid treatment. The results revealed that very few of the opioid users (4%-7%) had undergone another type of surgical procedure.
“So, we feel a little bit better, that these findings weren’t for other surgical procedures,” said Dr. Rhon. He added that future qualitative research was needed to understand why health care professionals were prescribing opioids, and why patients felt like they needed them.
“That’s bad,” Haxby Abbott, PhD, DPT, a research professor at the University of Otago, Dunedin, New Zealand, commented on Twitter.
Dr. Abbott, who was not involved in the study, added: “We’ve done a similar study of the whole NZ population [currently under review] – similar to Australia and not nearly as bad as you found. That needs urgent attention.”
Sharp rise in opioid use 2 years before TKA
Lower rates of opioid use before TKA were seen in two European cohorts, at 43% in England and 33% in Sweden, as reported by Clara Hellberg, PhD, MD, of Lund (Sweden) University. However, rates had increased over a 10-year study period from a respective 23% and 16%, with a sharp increase in use in the 2 years before knee replacement.
The analysis was based on 49,043 patients from the English national database Clinical Practice Research Datalink, and 5,955 patients from the Swedish Skåne Healthcare register who had undergone total knee replacement between 2015 and 2019 and were matched by age, sex and general practice to individuals not undergoing knee replacement.
The prevalence ratio for using opioids over a 10-year period increased from 1.6 to 2.7 in England, and from 1.6 to 2.6 in Sweden.
“While the overall prevalence of opioid use was higher in England, the majority of both cases and controls were using weak opioids,” Dr. Hellberg said.
“Codeine was classified as a weak opioid, whereas morphine was classified as a strong opioid,” she added.
In contrast, the proportion of people using strong opioids in Sweden was greater than in England, she said.
The high opioid use found in the study highlights “the need for better opioid stewardship, and the availability of acceptable, effective alternatives,” Dr. Hellberg and associates concluded in their abstract.
The study presented by Ms. Costa was funded by the Portuguese national funding agency for science, research and technology and by an independent research grant from Pfizer. Dr. Rhon acknowledged grant funding from the National Institutes of Health and the U.S. Department of Defense. Dr. Hellberg had no conflicts of interest to disclose.
Inadequate pain relief was recorded in 68.8% of a sample of people with hip or knee OA who participated in the population-based EpiReumaPt study, researchers reported at the OARSI 2022 World Congress.
“This can be explained by a lack of effectiveness of current management strategies, low uptake of recommended interventions by health care professionals, and also by low adherence by patients to medication and lifestyle interventions,” said Daniela Sofia Albino Costa, MSc, a PhD student at NOVA University Lisbon.
In addition to looking at the prevalence of inadequate pain relief – defined as a score of 5 or higher on the Numeric Pain Rating Scale (NPRS) – the study she presented at the congress, which was sponsored by the Osteoarthritis Research Society International, looked at the predictors for inadequate pain control.
It was found that being female, obesity, and having multimorbidity doubled the risk of inadequate versus adequate pain control, with respective odds ratios of 2.32 (P < .001), 2.26 (P = .006), and 2.07 (P = .001). Overweight was also associated with an increased odds ratio for poor pain control (OR, 1.84; P = .0035).
“We found that patients with inadequate pain relief also have a low performance on activities of daily living and a low quality of life,” Ms. Costa said.
Nearly one-third (29%) of patients in the inadequate pain relief group (n = 765) took medication, versus 15% of patients in the adequate pain relief group (n = 270). This was mostly NSAIDs, but also included analgesics and antipyretics, and in a few cases (4.8% vs. 1.3%), simple opioids.
“We know that current care is not concordant with recommendations,” said Ms. Costa, noting that medication being used as first-line treatment and core nonpharmacologic interventions are being offered to less than half of patients who are eligible.
In addition, the rate for total joint replacement has increased globally, and pain is an important predictor for this.
“So, we need to evaluate pain control and current management offered to people with hip or knee arthritis to identify to identify areas for improvement,” Ms. Costa said.
High rates of prescription opioid use before TKA
In a separate study also presented at the congress, Daniel Rhon, DPT, DSc, director of musculoskeletal research in primary care at Brooke Army Medical Center in San Antonio, gave a worrying glimpse of high rates of opioid use in the 4 years before total knee arthroplasty (TKA).
Using data from the U.S. Military Health System, the records of all individuals who had a knee replacement procedure between January 2017 and December 2018 were studied, to identify and characterize the use of prescription opioids.
Of the 46,362 individuals, 52.9% had prior opioid use, despite the fact that “opioids are not recommended for the management of knee OA,” said Dr. Rhon.
He also reported that as many as 40% of those who had at least one prescription for opioids had received a high-potency drug, such as fentanyl or oxycodone. The mean age of participants overall was 65 years, with a higher mean for those receiving opioids than those who did not (68 vs. 61.5 years). Data on sex and ethnicity were not available in time for presentation at the congress.
“Most of these individuals are getting these opioid prescriptions probably within 6 months, which maybe aligns with escalation of pain and maybe the decision to have that knee replacement,” Dr. Rhon said. Individuals that used opioids filled their most recent prescription a median of 146 days before TKA to surgery, with a mean of 317 days.
“You can’t always link the reason for the opioid prescription, that’s not really clear in the database,” he admitted; however, an analysis was performed to check if other surgeries had been performed that may have warranted the opioid treatment. The results revealed that very few of the opioid users (4%-7%) had undergone another type of surgical procedure.
“So, we feel a little bit better, that these findings weren’t for other surgical procedures,” said Dr. Rhon. He added that future qualitative research was needed to understand why health care professionals were prescribing opioids, and why patients felt like they needed them.
“That’s bad,” Haxby Abbott, PhD, DPT, a research professor at the University of Otago, Dunedin, New Zealand, commented on Twitter.
Dr. Abbott, who was not involved in the study, added: “We’ve done a similar study of the whole NZ population [currently under review] – similar to Australia and not nearly as bad as you found. That needs urgent attention.”
Sharp rise in opioid use 2 years before TKA
Lower rates of opioid use before TKA were seen in two European cohorts, at 43% in England and 33% in Sweden, as reported by Clara Hellberg, PhD, MD, of Lund (Sweden) University. However, rates had increased over a 10-year study period from a respective 23% and 16%, with a sharp increase in use in the 2 years before knee replacement.
The analysis was based on 49,043 patients from the English national database Clinical Practice Research Datalink, and 5,955 patients from the Swedish Skåne Healthcare register who had undergone total knee replacement between 2015 and 2019 and were matched by age, sex and general practice to individuals not undergoing knee replacement.
The prevalence ratio for using opioids over a 10-year period increased from 1.6 to 2.7 in England, and from 1.6 to 2.6 in Sweden.
“While the overall prevalence of opioid use was higher in England, the majority of both cases and controls were using weak opioids,” Dr. Hellberg said.
“Codeine was classified as a weak opioid, whereas morphine was classified as a strong opioid,” she added.
In contrast, the proportion of people using strong opioids in Sweden was greater than in England, she said.
The high opioid use found in the study highlights “the need for better opioid stewardship, and the availability of acceptable, effective alternatives,” Dr. Hellberg and associates concluded in their abstract.
The study presented by Ms. Costa was funded by the Portuguese national funding agency for science, research and technology and by an independent research grant from Pfizer. Dr. Rhon acknowledged grant funding from the National Institutes of Health and the U.S. Department of Defense. Dr. Hellberg had no conflicts of interest to disclose.
FROM OARSI 2022
Women in rheumatology: A look back, a look forward
Jean Liew, MD, recalls the long list of women mentors who have guided her career in rheumatology.
It started during her residency, when Jennifer Barton, MD, at Oregon Health & Science University, Portland, exposed her to new ways of conducting clinical research on patient outcomes.
In fellowship, she met Lianne Gensler, MD, a leader in axial spondyloarthritis, at the annual meeting of the American College of Rheumatology. Through Dr. Gensler’s mentorship and sponsorship, she was introduced to Maureen Dubreuil, MD, at Boston University, whose research focuses on pharmacoepidemiologic approaches using large databases.
Dr. Liew currently practices rheumatology under the leadership of Tuhina Neogi, MD, a world-renowned expert in osteoarthritis and gout. “She’s my research mentor,” Dr. Liew, an assistant professor of medicine at Boston University, said in an interview.
Her academic timeline reflects the powerful network and influence of women rheumatologists, who represent half of the adult rheumatology workforce in the United States. “In the research arena, many experts are women and they serve as role models and mentors to many,” Dr. Liew said.
But there’s more work to do, she and others acknowledged.
Rheumatology faces ongoing workforce shortages while struggling with a gender gap that’s closing but not as quickly as many women rheumatologists would like to see.
The gap persists, despite overall gains in the field of medicine, Vaneet Sandhu, MD, a rheumatologist with Loma Linda (Calif.) University, said in an interview. Women have exceeded men as enrollees in medical colleges, reported the Association of American Medical Colleges. And yet, “our colleagues reported last year that, in academic rheumatology, women are less likely to be full or associate professors than men,” she said.
The odds of being a fellowship program director or division director is similar in both males and females. “So, we’ve had some gains, but there’s always room for more,” Dr. Sandhu said.
Too few physicians
The next 10 years forecasts a dearth in American physicians.
AAMC projects a shortage of 124,000 doctors in the United States by 2034. Following on a similar trajectory, the ACR in 2015 anticipated a 25% drop in the supply of rheumatology clinical providers by 2030, with demand exceeding supply by more than 4,100 clinical employees.
The ACR’s workforce study projected that more women would come into rheumatology, noted Marcy Bolster, MD, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston. Women make up at least 50% of the workforce and 66% of fellows If these numbers hold, “we’ll definitely see an increase in the percent of women in the workforce” moving forward, Dr. Bolster said in an interview.
Women have helped the shortage to a great extent, said Nilanjana Bose, MD, a rheumatologist at Lonestar Rheumatology, Houston.
The work-life balance that rheumatology offers, combined with its focus on the cognitive part of internal medicine, explains why the field has attracted so many women. Rheumatology provides flexible work options. Women “get to teach or do rounds in the hospital or have a private practice where you’re mostly outpatient with some hospital work,” Dr. Bose said in an interview.
With anticipated shortages looming over the next decade, the profession needs to be cognizant of the different demands women face in their careers and how it can accommodate the workforce to meet the needs of its providers and maintain access for patients, Dr. Bolster said.
There are many innovative ways to match the demand for access. One thought is to create shared positions. Instead of employing four full-time physicians and one person part time, have two people who are working part time, Dr. Bolster suggested. “It is also important to not only expand our workforce with advanced practice providers, but to ensure their retention in the rheumatology workforce, to improve access to care for those with rheumatic diseases.”
Increasing the number of residency positions is another step toward addressing the shortage, Dr. Sandhu offered.
Women rheumatologists should make their voices heard by contacting members of Congress to support legislation that advocates for workforce shortage solutions, “in addition to generally supporting women’s rights and growth in the workplace,” she said.
The gender divide continues
Rosalind Ramsey-Goldman, MD, DrPH, remembers being the only woman in a group of five during her fellowship in the mid-1980s. Few women role models existed within the ACR, especially those in academic careers. “Now, most fellowships have more than 50% women, reflecting the number of women going to medical school,” said Dr. Ramsey-Goldman, Gallagher Research Professor in Rheumatology at Northwestern University and Northwestern Medicine, Chicago.
As more women enter the profession, women rheumatologists in academic rheumatology have started to outpace men in recent years. Some research suggests they’ve made headway in gaining leadership spots at institutions.
One recent paper, a cross-sectional national study of more than 6,100 rheumatologists, found that women had similar odds of attaining fellowship program or division director positions as men. As directors of training programs, women in rheumatology “instill this collaborative and growth mindset that encourages learners to self-reflect and work as a team,” Dr. Sandhu said.
Women bring a different perspective to training, and how curriculum works, Dr. Bose said. Studies have shown that women tend to be more empathic. They ask more questions. “That’s not to say men aren’t good. Women just have an inborn ability for connecting,” and this perspective helps to enrich the educational experience for trainees.
Women who lead training programs are also attuned to realities that female trainees confront, such as dealing with the challenges of achieving the best possible education while also raising a family, noted Graciela S. Alarcón, MD, MPH, who holds emeritus positions at the University of Alabama at Birmingham and the Universidad Peruana Cayetano Heredia in Lima, Peru.
“These program directors cultivate the ability to relate to women trainees in a very personal manner, supporting them in their efforts to achieve a balance between their training demands and their family/personal responsibilities,” she said.
Other research suggests the gender gap hasn’t gone away. Women continue to have lower odds of holding a higher-level professorship, receiving a federal grant, or speaking at academic conferences. They are also less likely to serve as first authors on rheumatology guidelines or recommendations.
Some studies suggest that women see fewer patients and earn less than their male counterparts. At peak difference, men can earn up to $100,000+ more than women. “My own impression is that it takes more efforts for women to reach the same level of recognition than men, and although overt discrimination is rare nowadays, subtle discrimination still occurs,” according to Dr. Alarcón.
Over a lifetime, female physicians can expect to earn less than their male counterparts, with clear implications for different retirement income levels, she said.
Fixing a leaky academic pipeline
The reality is the academic pipeline, and especially the physician-scientist pipeline, “continues to be leaky,” Dr. Liew said. “We know that caregivers to young children have larger barriers to surmount in academics and in research, and that there is a gender disparity present.” The toll of academic medicine on early career women who are parents is especially pronounced. While the pandemic has intensified this problem, it was around pre-COVID, she added.
Women who start in academia as academic clinicians or clinician researchers aren’t always able to meet their goals for promotion within the appropriate time frame. This is because of inequities in the system and lack of support related to maternity leave, childcare, and other issues. As a result, they leave academia and go into private practice or industry, Dr. Liew said.
The ACR in its 2015 survey projected that more women would be seeking part-time positions.
The good news is many academic institutions are taking a more equitable view about different career paths, offering equal parental leave to both men and women, Dr. Bolster noted. “It is essential that workforce planning encompasses the changing responsibilities within families and account for more parental leave by both men and women.” If certain projections come true, with 50% of the profession retiring between 2015 and 2030, combined with more men and women working part time, “it is requisite that workforce strategies plan for this.”
When Dr. Ramsey-Goldman was a trainee and junior faculty, there were no formal maternity leave policies.
Now, this benefit is available, she said. In another critical change, the ACR has made childcare services and a lactation room available for young mothers during its annual meeting. “Virtual meetings afford further ways to interact with colleagues,” she added.
Whether women choose to stay in academia or go into clinical practice is a very personal decision. “But it is also fair that, in some programs, training directors and faculty members can encourage trainees toward academia and its fascinating research possibilities,” Dr. Alarcón offered.
Making gains in research
Women are increasingly driving groundbreaking rheumatology research at all levels, Dr. Sandhu said. “And women empower women. Not infrequently, our female leaders, veterans in rheumatology research, seek younger female rheumatologists to help them grow in their niches. This has been one of the most beautiful things of the sisterhood in rheumatology that I have been blessed to be part of.”
In pediatric rheumatology, young female researchers are leading global research efforts. Some standouts include Kate Webb, MD, a pediatric rheumatologist in Cape Town, South Africa, and scientist who has worked on multisystem inflammatory syndrome during the pandemic. Sheila Angeles-Han, MD, who works on uveitis in juvenile idiopathic arthritis, had a role in recent ACR guidelines. Laura Lewandowski, MD, has also contributed to global rheumatology efforts, especially in low- and middle-income countries, Dr. Liew said.
The 2021 ACR annual meeting highlighted the research efforts of women rheumatologists from around the world. A global rheumatology summit at the meeting featured many women voices, including Dzifa Dey, MD, from Ghana, who received the ACR Distinguished International Rheumatology Professional Award. Ashira Blazer, MD, and Irene Blanco, MD, have spearheaded the ACR’s diversity, equity, and inclusion initiatives.
Women researchers have many opportunities to study rheumatologic diseases that disproportionately affect women, Dr. Alarcón said.
Lupus, for example, affects women in a much higher proportion than men (90% vs. 10%). This may be an attractive target for the best and brightest among future women researchers, Dr. Alarcón suggested. “It is a fact that publications related to lupus in leading internal medicine and rheumatology journals often include women either as first or senior authors. In that context, it can be said that several advances in the study of lupus worldwide can be attributed to women.”
This applies to disparities in social determinants of health that account for extremely complex outcomes in lupus among women of color, compared with White women, in addition to the costs associated with the disease and its impact on morbidity, mortality, and quality of life.
Women rheumatologists have advanced the work in reproductive management of rheumatic diseases, including a recent ACR-endorsed publication that provides formal guidance on managing reproductive health in women with rheumatic disease, Dr. Sandhu said. “One thing is clear: Without women, the work on reproductive diseases in rheumatology to date would not likely be where it is.”
Dr. Ramsey-Goldman added that “this critical work will not only set the stage for clinical care of both women and men regarding their reproductive health but will also inform education strategies for trainees and future research activities, and help direct policy regarding access to care, medication development, and costs of treatment.”
Obtaining grant funding to support salaries and researcher endeavors remains a challenge, Dr. Liew said. “It takes working evenings, weekends, and holidays to meet those goals within a set time frame. So you can see why a female faculty member with children might be disadvantaged, compared to a male counterpart without children.”
Competition for grant funding remains fierce as budgets become tighter, she added.
“We will lose a lot more brilliant and compassionate rheumatologists (clinicians, physician-scientists, and scientists alike) if we do not think of ways to make things more equitable or do not acknowledge the privileges that support some to continued career successes and leave others behind,” Dr. Liew said.
Women who choose a research field should seek out mentor and financial support that will allow them enough protected time to balance out research with other clinical activities, such as teaching and patient care, Dr. Alarcón said.
Training directors, mentors, and faculty should prioritize the needs of current and future women researchers, she said. “The guidance provided to young female trainees toward a successful research career is a formidable challenge that may provide, in turn, enormous satisfaction. There are established avenues to seek funding as new investigators.”
Progress in diversity
Rheumatology as a field is attracting more candidates and all races and genders, Dr. Bose said. “I think in the coming years we will see more and more women from minorities being incorporated into the rheumatology workforce.”
Others would like to see further improvements in diversity and attracting women from historically excluded backgrounds. Patients will benefit from rheumatologists who are able to connect with them through shared languages, cultures, and other life experiences, Dr. Liew said. “It is imperative that we work on recruitment, mentorship, and retention in this regard.”
While the representation of women of color is still inadequate, there has been some progress, Dr. Sandhu said. The number of female Hispanic, Latinx, and Black or African American graduates from medical school has seen a steady rise since 2017. And, AAMC has established task forces such as the Women of Color Initiative to identify strategies for furthering the careers of women of color in academic medicine.
“There’s still a lot of room to grow. I am, however, proud to say we will finally have a woman of color as the president of ACR in 2023,” said Dr. Sandhu, referring to Deborah Dyett Desir, MD.
Dr. Desir discussed the importance of diversifying the ACR in a recent interview.
All rheumatologists know that there is a place for them in the ACR, she stressed. “The demographics of our membership should reflect that of our population.”
As growth in diverse representation occurs, so will recruitment, retention, and a greater awareness and distribution of knowledge and means to address implicit biases and microaggressions, Dr. Sandhu said. “We will see a greater quality of health care, where patients may feel more connected to someone they can identify with.”
Looking ahead
Dr. Alarcón expects women to continue to play a major role in rheumatology, not just in research, education, and patient care but in leadership of academic societies and professional organizations.
“Women in rheumatology have come a long way – a piece of history that I have been fortunate to witness from my beginnings in the early 1970s. We have, I think, paved the way for the next generations of leaders in our beloved specialty field.”
Dr. Bolster is a member of the ACR board of directors and board liaison of the ACR Workforce Solutions Committee. Dr. Ramsey-Goldman has been a GlaxoSmithKline consultant for lupus studies, a consultant and site investigator with Exagen Diagnostics for lupus biomarker studies, and a site investigator for Xencor and Horizon Pharma lupus trials. Dr. Sandhu serves on the ACR’s Committee on Rheumatology Training and Workforce Issues.
Related article
Pioneer days of rheumatology: One veteran looks back
Patricia Woo, CBE, FMedSci, FRCP, has seen it all.
As a member of the British Rheumatology Society and fellow of the Royal College of Physicians, she presented the case for and obtained official training approval for pediatric rheumatology in the 1990s. She also set the wheels in motion to form the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology European Society.
Now 74, Dr. Woo remembers the discrimination she faced in the 1970s. “I was told I couldn’t become an investigator or consultant if I were to marry or have children.” Around the same time, she found out a male clinician researcher didn’t want to work with her, not because of her qualifications, but because she was a woman.
That wouldn’t happen now with all the antidiscrimination laws in place, noted Dr. Woo, an emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. Looking at the advances made by women in rheumatology, “there’s a major difference between 3 decades ago and today. If anyone discriminates today, they are called out.”
As the founding president of the Paediatric Rheumatology European Society, Dr. Woo is one of many early trailblazers who weathered many changes and made gains in the profession.
It’s important to recognize the work of Barbara Ansell, MD, the founder of pediatric rheumatology in the Canadian Red Cross Memorial Hospital, said Dr. Woo. Back in the 1960s, this wasn’t even a subspecialty. “Sick kids in general were taken either to pediatricians who didn’t know much about undescribed rheumatological conditions, and rheumatologists who didn’t know or have facilities for pediatric care.”
Dr. Ansell started this work, and Dr. Woo took over when she retired. With her colleagues, she set up a syllabus for pediatric rheumatology to formalize training for all junior doctors. This established a model of multidisciplinary clinical care and research. “Over the years, more women doctors have been attracted to pediatric rheumatology and have done well,” she said.
The rise of female leaders in rheumatology over the past few decades has been exponential, she continued. Women have become presidents of rheumatologic societies. Some established themselves as leaders in specific disciplines.
Carol Black, MD, from the United Kingdom is renowned for her international collaborative work in scleroderma research and clinical care. Patience White, MD in Washington, D.C., started research on the process of transitioning from childhood to adolescent to adult clinical care, a discipline that now has a strong international presence, Dr. Woo said.
The European Alliance of Associations for Rheumatology, which created a task force on gender equity in academic rheumatology, is evolving, she continued. The Academy of Medical Sciences in the United Kingdom also has active gender equality and mentoring programs, including a program to boost the careers of all researchers.
It’s also much easier now for women to become lead authors on papers since many are heads of lab or clinical services, Dr. Woo continued. “I don’t think there’s much discrimination if you’re a good clinician, and/or a good scientist. If women do their work well, they get the appropriate acknowledgment.”
Jean Liew, MD, recalls the long list of women mentors who have guided her career in rheumatology.
It started during her residency, when Jennifer Barton, MD, at Oregon Health & Science University, Portland, exposed her to new ways of conducting clinical research on patient outcomes.
In fellowship, she met Lianne Gensler, MD, a leader in axial spondyloarthritis, at the annual meeting of the American College of Rheumatology. Through Dr. Gensler’s mentorship and sponsorship, she was introduced to Maureen Dubreuil, MD, at Boston University, whose research focuses on pharmacoepidemiologic approaches using large databases.
Dr. Liew currently practices rheumatology under the leadership of Tuhina Neogi, MD, a world-renowned expert in osteoarthritis and gout. “She’s my research mentor,” Dr. Liew, an assistant professor of medicine at Boston University, said in an interview.
Her academic timeline reflects the powerful network and influence of women rheumatologists, who represent half of the adult rheumatology workforce in the United States. “In the research arena, many experts are women and they serve as role models and mentors to many,” Dr. Liew said.
But there’s more work to do, she and others acknowledged.
Rheumatology faces ongoing workforce shortages while struggling with a gender gap that’s closing but not as quickly as many women rheumatologists would like to see.
The gap persists, despite overall gains in the field of medicine, Vaneet Sandhu, MD, a rheumatologist with Loma Linda (Calif.) University, said in an interview. Women have exceeded men as enrollees in medical colleges, reported the Association of American Medical Colleges. And yet, “our colleagues reported last year that, in academic rheumatology, women are less likely to be full or associate professors than men,” she said.
The odds of being a fellowship program director or division director is similar in both males and females. “So, we’ve had some gains, but there’s always room for more,” Dr. Sandhu said.
Too few physicians
The next 10 years forecasts a dearth in American physicians.
AAMC projects a shortage of 124,000 doctors in the United States by 2034. Following on a similar trajectory, the ACR in 2015 anticipated a 25% drop in the supply of rheumatology clinical providers by 2030, with demand exceeding supply by more than 4,100 clinical employees.
The ACR’s workforce study projected that more women would come into rheumatology, noted Marcy Bolster, MD, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston. Women make up at least 50% of the workforce and 66% of fellows If these numbers hold, “we’ll definitely see an increase in the percent of women in the workforce” moving forward, Dr. Bolster said in an interview.
Women have helped the shortage to a great extent, said Nilanjana Bose, MD, a rheumatologist at Lonestar Rheumatology, Houston.
The work-life balance that rheumatology offers, combined with its focus on the cognitive part of internal medicine, explains why the field has attracted so many women. Rheumatology provides flexible work options. Women “get to teach or do rounds in the hospital or have a private practice where you’re mostly outpatient with some hospital work,” Dr. Bose said in an interview.
With anticipated shortages looming over the next decade, the profession needs to be cognizant of the different demands women face in their careers and how it can accommodate the workforce to meet the needs of its providers and maintain access for patients, Dr. Bolster said.
There are many innovative ways to match the demand for access. One thought is to create shared positions. Instead of employing four full-time physicians and one person part time, have two people who are working part time, Dr. Bolster suggested. “It is also important to not only expand our workforce with advanced practice providers, but to ensure their retention in the rheumatology workforce, to improve access to care for those with rheumatic diseases.”
Increasing the number of residency positions is another step toward addressing the shortage, Dr. Sandhu offered.
Women rheumatologists should make their voices heard by contacting members of Congress to support legislation that advocates for workforce shortage solutions, “in addition to generally supporting women’s rights and growth in the workplace,” she said.
The gender divide continues
Rosalind Ramsey-Goldman, MD, DrPH, remembers being the only woman in a group of five during her fellowship in the mid-1980s. Few women role models existed within the ACR, especially those in academic careers. “Now, most fellowships have more than 50% women, reflecting the number of women going to medical school,” said Dr. Ramsey-Goldman, Gallagher Research Professor in Rheumatology at Northwestern University and Northwestern Medicine, Chicago.
As more women enter the profession, women rheumatologists in academic rheumatology have started to outpace men in recent years. Some research suggests they’ve made headway in gaining leadership spots at institutions.
One recent paper, a cross-sectional national study of more than 6,100 rheumatologists, found that women had similar odds of attaining fellowship program or division director positions as men. As directors of training programs, women in rheumatology “instill this collaborative and growth mindset that encourages learners to self-reflect and work as a team,” Dr. Sandhu said.
Women bring a different perspective to training, and how curriculum works, Dr. Bose said. Studies have shown that women tend to be more empathic. They ask more questions. “That’s not to say men aren’t good. Women just have an inborn ability for connecting,” and this perspective helps to enrich the educational experience for trainees.
Women who lead training programs are also attuned to realities that female trainees confront, such as dealing with the challenges of achieving the best possible education while also raising a family, noted Graciela S. Alarcón, MD, MPH, who holds emeritus positions at the University of Alabama at Birmingham and the Universidad Peruana Cayetano Heredia in Lima, Peru.
“These program directors cultivate the ability to relate to women trainees in a very personal manner, supporting them in their efforts to achieve a balance between their training demands and their family/personal responsibilities,” she said.
Other research suggests the gender gap hasn’t gone away. Women continue to have lower odds of holding a higher-level professorship, receiving a federal grant, or speaking at academic conferences. They are also less likely to serve as first authors on rheumatology guidelines or recommendations.
Some studies suggest that women see fewer patients and earn less than their male counterparts. At peak difference, men can earn up to $100,000+ more than women. “My own impression is that it takes more efforts for women to reach the same level of recognition than men, and although overt discrimination is rare nowadays, subtle discrimination still occurs,” according to Dr. Alarcón.
Over a lifetime, female physicians can expect to earn less than their male counterparts, with clear implications for different retirement income levels, she said.
Fixing a leaky academic pipeline
The reality is the academic pipeline, and especially the physician-scientist pipeline, “continues to be leaky,” Dr. Liew said. “We know that caregivers to young children have larger barriers to surmount in academics and in research, and that there is a gender disparity present.” The toll of academic medicine on early career women who are parents is especially pronounced. While the pandemic has intensified this problem, it was around pre-COVID, she added.
Women who start in academia as academic clinicians or clinician researchers aren’t always able to meet their goals for promotion within the appropriate time frame. This is because of inequities in the system and lack of support related to maternity leave, childcare, and other issues. As a result, they leave academia and go into private practice or industry, Dr. Liew said.
The ACR in its 2015 survey projected that more women would be seeking part-time positions.
The good news is many academic institutions are taking a more equitable view about different career paths, offering equal parental leave to both men and women, Dr. Bolster noted. “It is essential that workforce planning encompasses the changing responsibilities within families and account for more parental leave by both men and women.” If certain projections come true, with 50% of the profession retiring between 2015 and 2030, combined with more men and women working part time, “it is requisite that workforce strategies plan for this.”
When Dr. Ramsey-Goldman was a trainee and junior faculty, there were no formal maternity leave policies.
Now, this benefit is available, she said. In another critical change, the ACR has made childcare services and a lactation room available for young mothers during its annual meeting. “Virtual meetings afford further ways to interact with colleagues,” she added.
Whether women choose to stay in academia or go into clinical practice is a very personal decision. “But it is also fair that, in some programs, training directors and faculty members can encourage trainees toward academia and its fascinating research possibilities,” Dr. Alarcón offered.
Making gains in research
Women are increasingly driving groundbreaking rheumatology research at all levels, Dr. Sandhu said. “And women empower women. Not infrequently, our female leaders, veterans in rheumatology research, seek younger female rheumatologists to help them grow in their niches. This has been one of the most beautiful things of the sisterhood in rheumatology that I have been blessed to be part of.”
In pediatric rheumatology, young female researchers are leading global research efforts. Some standouts include Kate Webb, MD, a pediatric rheumatologist in Cape Town, South Africa, and scientist who has worked on multisystem inflammatory syndrome during the pandemic. Sheila Angeles-Han, MD, who works on uveitis in juvenile idiopathic arthritis, had a role in recent ACR guidelines. Laura Lewandowski, MD, has also contributed to global rheumatology efforts, especially in low- and middle-income countries, Dr. Liew said.
The 2021 ACR annual meeting highlighted the research efforts of women rheumatologists from around the world. A global rheumatology summit at the meeting featured many women voices, including Dzifa Dey, MD, from Ghana, who received the ACR Distinguished International Rheumatology Professional Award. Ashira Blazer, MD, and Irene Blanco, MD, have spearheaded the ACR’s diversity, equity, and inclusion initiatives.
Women researchers have many opportunities to study rheumatologic diseases that disproportionately affect women, Dr. Alarcón said.
Lupus, for example, affects women in a much higher proportion than men (90% vs. 10%). This may be an attractive target for the best and brightest among future women researchers, Dr. Alarcón suggested. “It is a fact that publications related to lupus in leading internal medicine and rheumatology journals often include women either as first or senior authors. In that context, it can be said that several advances in the study of lupus worldwide can be attributed to women.”
This applies to disparities in social determinants of health that account for extremely complex outcomes in lupus among women of color, compared with White women, in addition to the costs associated with the disease and its impact on morbidity, mortality, and quality of life.
Women rheumatologists have advanced the work in reproductive management of rheumatic diseases, including a recent ACR-endorsed publication that provides formal guidance on managing reproductive health in women with rheumatic disease, Dr. Sandhu said. “One thing is clear: Without women, the work on reproductive diseases in rheumatology to date would not likely be where it is.”
Dr. Ramsey-Goldman added that “this critical work will not only set the stage for clinical care of both women and men regarding their reproductive health but will also inform education strategies for trainees and future research activities, and help direct policy regarding access to care, medication development, and costs of treatment.”
Obtaining grant funding to support salaries and researcher endeavors remains a challenge, Dr. Liew said. “It takes working evenings, weekends, and holidays to meet those goals within a set time frame. So you can see why a female faculty member with children might be disadvantaged, compared to a male counterpart without children.”
Competition for grant funding remains fierce as budgets become tighter, she added.
“We will lose a lot more brilliant and compassionate rheumatologists (clinicians, physician-scientists, and scientists alike) if we do not think of ways to make things more equitable or do not acknowledge the privileges that support some to continued career successes and leave others behind,” Dr. Liew said.
Women who choose a research field should seek out mentor and financial support that will allow them enough protected time to balance out research with other clinical activities, such as teaching and patient care, Dr. Alarcón said.
Training directors, mentors, and faculty should prioritize the needs of current and future women researchers, she said. “The guidance provided to young female trainees toward a successful research career is a formidable challenge that may provide, in turn, enormous satisfaction. There are established avenues to seek funding as new investigators.”
Progress in diversity
Rheumatology as a field is attracting more candidates and all races and genders, Dr. Bose said. “I think in the coming years we will see more and more women from minorities being incorporated into the rheumatology workforce.”
Others would like to see further improvements in diversity and attracting women from historically excluded backgrounds. Patients will benefit from rheumatologists who are able to connect with them through shared languages, cultures, and other life experiences, Dr. Liew said. “It is imperative that we work on recruitment, mentorship, and retention in this regard.”
While the representation of women of color is still inadequate, there has been some progress, Dr. Sandhu said. The number of female Hispanic, Latinx, and Black or African American graduates from medical school has seen a steady rise since 2017. And, AAMC has established task forces such as the Women of Color Initiative to identify strategies for furthering the careers of women of color in academic medicine.
“There’s still a lot of room to grow. I am, however, proud to say we will finally have a woman of color as the president of ACR in 2023,” said Dr. Sandhu, referring to Deborah Dyett Desir, MD.
Dr. Desir discussed the importance of diversifying the ACR in a recent interview.
All rheumatologists know that there is a place for them in the ACR, she stressed. “The demographics of our membership should reflect that of our population.”
As growth in diverse representation occurs, so will recruitment, retention, and a greater awareness and distribution of knowledge and means to address implicit biases and microaggressions, Dr. Sandhu said. “We will see a greater quality of health care, where patients may feel more connected to someone they can identify with.”
Looking ahead
Dr. Alarcón expects women to continue to play a major role in rheumatology, not just in research, education, and patient care but in leadership of academic societies and professional organizations.
“Women in rheumatology have come a long way – a piece of history that I have been fortunate to witness from my beginnings in the early 1970s. We have, I think, paved the way for the next generations of leaders in our beloved specialty field.”
Dr. Bolster is a member of the ACR board of directors and board liaison of the ACR Workforce Solutions Committee. Dr. Ramsey-Goldman has been a GlaxoSmithKline consultant for lupus studies, a consultant and site investigator with Exagen Diagnostics for lupus biomarker studies, and a site investigator for Xencor and Horizon Pharma lupus trials. Dr. Sandhu serves on the ACR’s Committee on Rheumatology Training and Workforce Issues.
Related article
Pioneer days of rheumatology: One veteran looks back
Patricia Woo, CBE, FMedSci, FRCP, has seen it all.
As a member of the British Rheumatology Society and fellow of the Royal College of Physicians, she presented the case for and obtained official training approval for pediatric rheumatology in the 1990s. She also set the wheels in motion to form the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology European Society.
Now 74, Dr. Woo remembers the discrimination she faced in the 1970s. “I was told I couldn’t become an investigator or consultant if I were to marry or have children.” Around the same time, she found out a male clinician researcher didn’t want to work with her, not because of her qualifications, but because she was a woman.
That wouldn’t happen now with all the antidiscrimination laws in place, noted Dr. Woo, an emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. Looking at the advances made by women in rheumatology, “there’s a major difference between 3 decades ago and today. If anyone discriminates today, they are called out.”
As the founding president of the Paediatric Rheumatology European Society, Dr. Woo is one of many early trailblazers who weathered many changes and made gains in the profession.
It’s important to recognize the work of Barbara Ansell, MD, the founder of pediatric rheumatology in the Canadian Red Cross Memorial Hospital, said Dr. Woo. Back in the 1960s, this wasn’t even a subspecialty. “Sick kids in general were taken either to pediatricians who didn’t know much about undescribed rheumatological conditions, and rheumatologists who didn’t know or have facilities for pediatric care.”
Dr. Ansell started this work, and Dr. Woo took over when she retired. With her colleagues, she set up a syllabus for pediatric rheumatology to formalize training for all junior doctors. This established a model of multidisciplinary clinical care and research. “Over the years, more women doctors have been attracted to pediatric rheumatology and have done well,” she said.
The rise of female leaders in rheumatology over the past few decades has been exponential, she continued. Women have become presidents of rheumatologic societies. Some established themselves as leaders in specific disciplines.
Carol Black, MD, from the United Kingdom is renowned for her international collaborative work in scleroderma research and clinical care. Patience White, MD in Washington, D.C., started research on the process of transitioning from childhood to adolescent to adult clinical care, a discipline that now has a strong international presence, Dr. Woo said.
The European Alliance of Associations for Rheumatology, which created a task force on gender equity in academic rheumatology, is evolving, she continued. The Academy of Medical Sciences in the United Kingdom also has active gender equality and mentoring programs, including a program to boost the careers of all researchers.
It’s also much easier now for women to become lead authors on papers since many are heads of lab or clinical services, Dr. Woo continued. “I don’t think there’s much discrimination if you’re a good clinician, and/or a good scientist. If women do their work well, they get the appropriate acknowledgment.”
Jean Liew, MD, recalls the long list of women mentors who have guided her career in rheumatology.
It started during her residency, when Jennifer Barton, MD, at Oregon Health & Science University, Portland, exposed her to new ways of conducting clinical research on patient outcomes.
In fellowship, she met Lianne Gensler, MD, a leader in axial spondyloarthritis, at the annual meeting of the American College of Rheumatology. Through Dr. Gensler’s mentorship and sponsorship, she was introduced to Maureen Dubreuil, MD, at Boston University, whose research focuses on pharmacoepidemiologic approaches using large databases.
Dr. Liew currently practices rheumatology under the leadership of Tuhina Neogi, MD, a world-renowned expert in osteoarthritis and gout. “She’s my research mentor,” Dr. Liew, an assistant professor of medicine at Boston University, said in an interview.
Her academic timeline reflects the powerful network and influence of women rheumatologists, who represent half of the adult rheumatology workforce in the United States. “In the research arena, many experts are women and they serve as role models and mentors to many,” Dr. Liew said.
But there’s more work to do, she and others acknowledged.
Rheumatology faces ongoing workforce shortages while struggling with a gender gap that’s closing but not as quickly as many women rheumatologists would like to see.
The gap persists, despite overall gains in the field of medicine, Vaneet Sandhu, MD, a rheumatologist with Loma Linda (Calif.) University, said in an interview. Women have exceeded men as enrollees in medical colleges, reported the Association of American Medical Colleges. And yet, “our colleagues reported last year that, in academic rheumatology, women are less likely to be full or associate professors than men,” she said.
The odds of being a fellowship program director or division director is similar in both males and females. “So, we’ve had some gains, but there’s always room for more,” Dr. Sandhu said.
Too few physicians
The next 10 years forecasts a dearth in American physicians.
AAMC projects a shortage of 124,000 doctors in the United States by 2034. Following on a similar trajectory, the ACR in 2015 anticipated a 25% drop in the supply of rheumatology clinical providers by 2030, with demand exceeding supply by more than 4,100 clinical employees.
The ACR’s workforce study projected that more women would come into rheumatology, noted Marcy Bolster, MD, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston. Women make up at least 50% of the workforce and 66% of fellows If these numbers hold, “we’ll definitely see an increase in the percent of women in the workforce” moving forward, Dr. Bolster said in an interview.
Women have helped the shortage to a great extent, said Nilanjana Bose, MD, a rheumatologist at Lonestar Rheumatology, Houston.
The work-life balance that rheumatology offers, combined with its focus on the cognitive part of internal medicine, explains why the field has attracted so many women. Rheumatology provides flexible work options. Women “get to teach or do rounds in the hospital or have a private practice where you’re mostly outpatient with some hospital work,” Dr. Bose said in an interview.
With anticipated shortages looming over the next decade, the profession needs to be cognizant of the different demands women face in their careers and how it can accommodate the workforce to meet the needs of its providers and maintain access for patients, Dr. Bolster said.
There are many innovative ways to match the demand for access. One thought is to create shared positions. Instead of employing four full-time physicians and one person part time, have two people who are working part time, Dr. Bolster suggested. “It is also important to not only expand our workforce with advanced practice providers, but to ensure their retention in the rheumatology workforce, to improve access to care for those with rheumatic diseases.”
Increasing the number of residency positions is another step toward addressing the shortage, Dr. Sandhu offered.
Women rheumatologists should make their voices heard by contacting members of Congress to support legislation that advocates for workforce shortage solutions, “in addition to generally supporting women’s rights and growth in the workplace,” she said.
The gender divide continues
Rosalind Ramsey-Goldman, MD, DrPH, remembers being the only woman in a group of five during her fellowship in the mid-1980s. Few women role models existed within the ACR, especially those in academic careers. “Now, most fellowships have more than 50% women, reflecting the number of women going to medical school,” said Dr. Ramsey-Goldman, Gallagher Research Professor in Rheumatology at Northwestern University and Northwestern Medicine, Chicago.
As more women enter the profession, women rheumatologists in academic rheumatology have started to outpace men in recent years. Some research suggests they’ve made headway in gaining leadership spots at institutions.
One recent paper, a cross-sectional national study of more than 6,100 rheumatologists, found that women had similar odds of attaining fellowship program or division director positions as men. As directors of training programs, women in rheumatology “instill this collaborative and growth mindset that encourages learners to self-reflect and work as a team,” Dr. Sandhu said.
Women bring a different perspective to training, and how curriculum works, Dr. Bose said. Studies have shown that women tend to be more empathic. They ask more questions. “That’s not to say men aren’t good. Women just have an inborn ability for connecting,” and this perspective helps to enrich the educational experience for trainees.
Women who lead training programs are also attuned to realities that female trainees confront, such as dealing with the challenges of achieving the best possible education while also raising a family, noted Graciela S. Alarcón, MD, MPH, who holds emeritus positions at the University of Alabama at Birmingham and the Universidad Peruana Cayetano Heredia in Lima, Peru.
“These program directors cultivate the ability to relate to women trainees in a very personal manner, supporting them in their efforts to achieve a balance between their training demands and their family/personal responsibilities,” she said.
Other research suggests the gender gap hasn’t gone away. Women continue to have lower odds of holding a higher-level professorship, receiving a federal grant, or speaking at academic conferences. They are also less likely to serve as first authors on rheumatology guidelines or recommendations.
Some studies suggest that women see fewer patients and earn less than their male counterparts. At peak difference, men can earn up to $100,000+ more than women. “My own impression is that it takes more efforts for women to reach the same level of recognition than men, and although overt discrimination is rare nowadays, subtle discrimination still occurs,” according to Dr. Alarcón.
Over a lifetime, female physicians can expect to earn less than their male counterparts, with clear implications for different retirement income levels, she said.
Fixing a leaky academic pipeline
The reality is the academic pipeline, and especially the physician-scientist pipeline, “continues to be leaky,” Dr. Liew said. “We know that caregivers to young children have larger barriers to surmount in academics and in research, and that there is a gender disparity present.” The toll of academic medicine on early career women who are parents is especially pronounced. While the pandemic has intensified this problem, it was around pre-COVID, she added.
Women who start in academia as academic clinicians or clinician researchers aren’t always able to meet their goals for promotion within the appropriate time frame. This is because of inequities in the system and lack of support related to maternity leave, childcare, and other issues. As a result, they leave academia and go into private practice or industry, Dr. Liew said.
The ACR in its 2015 survey projected that more women would be seeking part-time positions.
The good news is many academic institutions are taking a more equitable view about different career paths, offering equal parental leave to both men and women, Dr. Bolster noted. “It is essential that workforce planning encompasses the changing responsibilities within families and account for more parental leave by both men and women.” If certain projections come true, with 50% of the profession retiring between 2015 and 2030, combined with more men and women working part time, “it is requisite that workforce strategies plan for this.”
When Dr. Ramsey-Goldman was a trainee and junior faculty, there were no formal maternity leave policies.
Now, this benefit is available, she said. In another critical change, the ACR has made childcare services and a lactation room available for young mothers during its annual meeting. “Virtual meetings afford further ways to interact with colleagues,” she added.
Whether women choose to stay in academia or go into clinical practice is a very personal decision. “But it is also fair that, in some programs, training directors and faculty members can encourage trainees toward academia and its fascinating research possibilities,” Dr. Alarcón offered.
Making gains in research
Women are increasingly driving groundbreaking rheumatology research at all levels, Dr. Sandhu said. “And women empower women. Not infrequently, our female leaders, veterans in rheumatology research, seek younger female rheumatologists to help them grow in their niches. This has been one of the most beautiful things of the sisterhood in rheumatology that I have been blessed to be part of.”
In pediatric rheumatology, young female researchers are leading global research efforts. Some standouts include Kate Webb, MD, a pediatric rheumatologist in Cape Town, South Africa, and scientist who has worked on multisystem inflammatory syndrome during the pandemic. Sheila Angeles-Han, MD, who works on uveitis in juvenile idiopathic arthritis, had a role in recent ACR guidelines. Laura Lewandowski, MD, has also contributed to global rheumatology efforts, especially in low- and middle-income countries, Dr. Liew said.
The 2021 ACR annual meeting highlighted the research efforts of women rheumatologists from around the world. A global rheumatology summit at the meeting featured many women voices, including Dzifa Dey, MD, from Ghana, who received the ACR Distinguished International Rheumatology Professional Award. Ashira Blazer, MD, and Irene Blanco, MD, have spearheaded the ACR’s diversity, equity, and inclusion initiatives.
Women researchers have many opportunities to study rheumatologic diseases that disproportionately affect women, Dr. Alarcón said.
Lupus, for example, affects women in a much higher proportion than men (90% vs. 10%). This may be an attractive target for the best and brightest among future women researchers, Dr. Alarcón suggested. “It is a fact that publications related to lupus in leading internal medicine and rheumatology journals often include women either as first or senior authors. In that context, it can be said that several advances in the study of lupus worldwide can be attributed to women.”
This applies to disparities in social determinants of health that account for extremely complex outcomes in lupus among women of color, compared with White women, in addition to the costs associated with the disease and its impact on morbidity, mortality, and quality of life.
Women rheumatologists have advanced the work in reproductive management of rheumatic diseases, including a recent ACR-endorsed publication that provides formal guidance on managing reproductive health in women with rheumatic disease, Dr. Sandhu said. “One thing is clear: Without women, the work on reproductive diseases in rheumatology to date would not likely be where it is.”
Dr. Ramsey-Goldman added that “this critical work will not only set the stage for clinical care of both women and men regarding their reproductive health but will also inform education strategies for trainees and future research activities, and help direct policy regarding access to care, medication development, and costs of treatment.”
Obtaining grant funding to support salaries and researcher endeavors remains a challenge, Dr. Liew said. “It takes working evenings, weekends, and holidays to meet those goals within a set time frame. So you can see why a female faculty member with children might be disadvantaged, compared to a male counterpart without children.”
Competition for grant funding remains fierce as budgets become tighter, she added.
“We will lose a lot more brilliant and compassionate rheumatologists (clinicians, physician-scientists, and scientists alike) if we do not think of ways to make things more equitable or do not acknowledge the privileges that support some to continued career successes and leave others behind,” Dr. Liew said.
Women who choose a research field should seek out mentor and financial support that will allow them enough protected time to balance out research with other clinical activities, such as teaching and patient care, Dr. Alarcón said.
Training directors, mentors, and faculty should prioritize the needs of current and future women researchers, she said. “The guidance provided to young female trainees toward a successful research career is a formidable challenge that may provide, in turn, enormous satisfaction. There are established avenues to seek funding as new investigators.”
Progress in diversity
Rheumatology as a field is attracting more candidates and all races and genders, Dr. Bose said. “I think in the coming years we will see more and more women from minorities being incorporated into the rheumatology workforce.”
Others would like to see further improvements in diversity and attracting women from historically excluded backgrounds. Patients will benefit from rheumatologists who are able to connect with them through shared languages, cultures, and other life experiences, Dr. Liew said. “It is imperative that we work on recruitment, mentorship, and retention in this regard.”
While the representation of women of color is still inadequate, there has been some progress, Dr. Sandhu said. The number of female Hispanic, Latinx, and Black or African American graduates from medical school has seen a steady rise since 2017. And, AAMC has established task forces such as the Women of Color Initiative to identify strategies for furthering the careers of women of color in academic medicine.
“There’s still a lot of room to grow. I am, however, proud to say we will finally have a woman of color as the president of ACR in 2023,” said Dr. Sandhu, referring to Deborah Dyett Desir, MD.
Dr. Desir discussed the importance of diversifying the ACR in a recent interview.
All rheumatologists know that there is a place for them in the ACR, she stressed. “The demographics of our membership should reflect that of our population.”
As growth in diverse representation occurs, so will recruitment, retention, and a greater awareness and distribution of knowledge and means to address implicit biases and microaggressions, Dr. Sandhu said. “We will see a greater quality of health care, where patients may feel more connected to someone they can identify with.”
Looking ahead
Dr. Alarcón expects women to continue to play a major role in rheumatology, not just in research, education, and patient care but in leadership of academic societies and professional organizations.
“Women in rheumatology have come a long way – a piece of history that I have been fortunate to witness from my beginnings in the early 1970s. We have, I think, paved the way for the next generations of leaders in our beloved specialty field.”
Dr. Bolster is a member of the ACR board of directors and board liaison of the ACR Workforce Solutions Committee. Dr. Ramsey-Goldman has been a GlaxoSmithKline consultant for lupus studies, a consultant and site investigator with Exagen Diagnostics for lupus biomarker studies, and a site investigator for Xencor and Horizon Pharma lupus trials. Dr. Sandhu serves on the ACR’s Committee on Rheumatology Training and Workforce Issues.
Related article
Pioneer days of rheumatology: One veteran looks back
Patricia Woo, CBE, FMedSci, FRCP, has seen it all.
As a member of the British Rheumatology Society and fellow of the Royal College of Physicians, she presented the case for and obtained official training approval for pediatric rheumatology in the 1990s. She also set the wheels in motion to form the Paediatric Rheumatology International Trials Organisation and the Paediatric Rheumatology European Society.
Now 74, Dr. Woo remembers the discrimination she faced in the 1970s. “I was told I couldn’t become an investigator or consultant if I were to marry or have children.” Around the same time, she found out a male clinician researcher didn’t want to work with her, not because of her qualifications, but because she was a woman.
That wouldn’t happen now with all the antidiscrimination laws in place, noted Dr. Woo, an emeritus professor of pediatric rheumatology and previous head of the Centre for Paediatric and Adolescent Rheumatology at UCL, London. Looking at the advances made by women in rheumatology, “there’s a major difference between 3 decades ago and today. If anyone discriminates today, they are called out.”
As the founding president of the Paediatric Rheumatology European Society, Dr. Woo is one of many early trailblazers who weathered many changes and made gains in the profession.
It’s important to recognize the work of Barbara Ansell, MD, the founder of pediatric rheumatology in the Canadian Red Cross Memorial Hospital, said Dr. Woo. Back in the 1960s, this wasn’t even a subspecialty. “Sick kids in general were taken either to pediatricians who didn’t know much about undescribed rheumatological conditions, and rheumatologists who didn’t know or have facilities for pediatric care.”
Dr. Ansell started this work, and Dr. Woo took over when she retired. With her colleagues, she set up a syllabus for pediatric rheumatology to formalize training for all junior doctors. This established a model of multidisciplinary clinical care and research. “Over the years, more women doctors have been attracted to pediatric rheumatology and have done well,” she said.
The rise of female leaders in rheumatology over the past few decades has been exponential, she continued. Women have become presidents of rheumatologic societies. Some established themselves as leaders in specific disciplines.
Carol Black, MD, from the United Kingdom is renowned for her international collaborative work in scleroderma research and clinical care. Patience White, MD in Washington, D.C., started research on the process of transitioning from childhood to adolescent to adult clinical care, a discipline that now has a strong international presence, Dr. Woo said.
The European Alliance of Associations for Rheumatology, which created a task force on gender equity in academic rheumatology, is evolving, she continued. The Academy of Medical Sciences in the United Kingdom also has active gender equality and mentoring programs, including a program to boost the careers of all researchers.
It’s also much easier now for women to become lead authors on papers since many are heads of lab or clinical services, Dr. Woo continued. “I don’t think there’s much discrimination if you’re a good clinician, and/or a good scientist. If women do their work well, they get the appropriate acknowledgment.”
Med school to pay $1.2 million to students in refunds and debt cancellation in FTC settlement
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Although it disputed the allegations, The complaint referenced the school’s medical license exam test pass rate and residency matches along with violations of rules that protect consumers, including those dealing with credit contracts.
The school, based in the Caribbean with operations in Illinois, agreed to pay $1.2 million toward refunds and debt cancellation for students harmed by the marketing in the past 5 years.
“While we strongly disagree with the FTC’s approach to this matter, we did not want a lengthy legal process to distract from our mission of providing a quality medical education at an affordable cost,” Kaushik Guha, executive vice president of the parent of the school, Human Resources Development Services, said in a YouTube statement posted on the school’s website.
“Saint James lured students by lying about their chances of success,” Samuel Levine, director of the FTC’s Bureau of Consumer Protection, said in a press release. The settlement agreement was with HRDS, which bills itself as providing students from “non-traditional backgrounds the opportunity to pursue a medical degree and practice in the U.S. or Canada,” according to the school’s statement.
The complaint alleges that, since at least April 2018, the school, HRDS, and its operator Mr. Guha has lured students using “phony claims about the standardized test pass rate and students’ residency or job prospects. They lured consumers with false guarantees of student success at passing a critical medical school standardized test, the United States Medical Licensing Examination Step 1 Exam.”
For example, a brochure distributed at open houses claimed a first-time Step 1 pass rate of about 96.8%. The brochure further claimed: “Saint James is the first and only medical school to offer a USMLE Step 1 Pass Guarantee,” according to the FTC complaint.
The FTC said the USMLE rate is lower than touted and lower than reported by other U.S. and Canadian medical schools. “Since 2017, only 35% of Saint James students who have completed the necessary coursework to take the USMLE Step 1 exam passed the test.”
The school also misrepresented the residency match rate as “the same” as American medical schools, according to the complaint. For example, the school instructed telemarketers to tell consumers that the match rate for the school’s students was 85%-90%. The school stated on its website that the residency match rate for Saint James students was 83%. “In fact, the match rate for SJSM students is lower than touted and lower than that reported by U.S. medical schools. Since 2018, defendants’ average match rate has been 63%.”
The FTC also claims the school used illegal credit contracts when marketing financing for tuition and living expenses for students. “The financing contracts contained language attempting to waive consumers’ rights under federal law and omit legally mandated disclosures.”
Saint James’ tuition ranges from about $6,650 to $9,859 per trimester, depending on campus and course study, the complaint states. Between 2016 and 2020, about 1,300 students were enrolled each year in Saint James’ schools. Students who attended the schools between 2016 and 2022 are eligible for a refund under the settlement.
Saint James is required to notify consumers whose debts are being canceled through Delta Financial Solutions, Saint James’ financing partner. The debt will also be deleted from consumers’ credit reports.
“We have chosen to settle with the FTC over its allegations that disclosures on our website and in Delta’s loan agreements were insufficient,” Mr. Guha stated on the school website. “However, we have added additional language and clarifications any time the USMLE pass rate and placement rates are mentioned.”
He said he hopes the school will be “an industry leader for transparency and accountability” and that the school’s “efforts will lead to lasting change throughout the for-profit educational industry.”
Mr. Guha added that more than 600 of the school’s alumni are serving as doctors, including many “working to bridge the health equity gap in underserved areas in North America.”
The FTC has been cracking down on deceptive practices by for-profit institutions. In October, the FTC put 70 for-profit colleges on notice that it would investigate false promises the schools make about their graduates’ job prospects, expected earnings, and other educational outcomes and would levy significant financial penalties against violators. Saint James was not on that list, which included several of the largest for-profit universities in the nation, including Capella University, DeVry University, Strayer University, and Walden University.
A version of this article first appeared on Medscape.com.
Real-world data suggest coprescribing PDE5 inhibitors and nitrates may be safe
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
The authors of the new research specifically examined how frequently phosphodiesterase type 5 (PDE5) inhibitors, such as Viagra, were prescribed. The U.S. Food and Drug Administration and the European Medicines Agency have warned that these drugs for erectile dysfunction are contraindicated for use with nitrates because of concerns about cardiovascular risks.
“Small, randomized, pharmacologic studies have reported an amplified decrease in blood pressure during controlled coexposure with nitrates and [phosphodiesterase type 5 inhibitors], both in healthy participants and in participants with IHD,” wrote lead author Anders Holt, MD, of Copenhagen University Hospital–Herlev and Gentofte and colleagues, in Annals of Internal Medicine. “Potentially, this increases the risk for vascular ischemic events including myocardial infarction and stroke.”
But there is a scarcity of real-world data showing that using both types of drugs together increase these risks, the researchers noted.
To address this knowledge gap, Dr. Holt and colleagues conducted a retrospective study involving 249,541 Danish men with IHD. In this overall population, from 2000 to 2018, prescriptions for PDE5 inhibitors increased 10-fold, from 3.1 to 30.9 prescriptions per 100 persons per year. Within a subgroup of 42,073 patients continuously prescribed oral organic nitrates, PDE5-inhibitor prescriptions jumped twice that magnitude, from 0.9 to 19.7 prescriptions per 100 persons per year.
Despite this surge in coprescribing, the investigators did not observe a significant increase in either of two composite measures of cardiovascular adverse events. The first composite included ischemic stroke, shock, cardiac arrest, myocardial infarction, or acute coronary arteriography (odds ratio, 0.58; 95% confidence interval, 0.28-1.13). The second composite included drug-related adverse events, angina pectoris, or syncope (OR, 0.73; CI, 0.40-1.32).
Lead author speculates on reasons for findings
“I propose several explanations [for these findings],” Dr. Holt said in an interview, “but I want to emphasize that our study does not contain any data to back it up. It is just speculation. First, the observed drop in blood pressure may not cause a condition for which patients seek a hospital. A drop in blood pressure has been shown in pharmacologic trials, but it might not translate to a real-life risk for cardiovascular outcomes. Second, patients could be well informed and adherent to guidance that the prescribing physician has provided. For example, patients are aware of the recommended pause in nitrate treatment before PDE5-inhibitor use and follow these recommendations. Third, nitrates are often taken in the morning, and with the careful assumption that most PDE5-inhibitor activities take place in the evening, the nitrates could be metabolized to a degree such that the synergistic interaction is negligible.”
Dr. Holt went on to suggest a novel clinical approach based on the new findings.
“Coadministration should still be contraindicated due to the proven drop in blood pressure,” he said. “However, perhaps physicians can allow for coprescription if patients are adequately informed.”
A qualitative study is needed to determine how patients and physicians discuss coprescription, including avoidance of coadministration, Dr. Holt added.
Findings call for a reassessment of whether the contraindication is warranted
Robert A. Kloner, MD, PhD, chief science officer at the Huntington Medical Research Institutes in Pasadena, Calif., and professor of medicine at University of Southern California, Los Angeles, previously conducted research exploring drug interactions with PDE5 inhibitors, and in 2018, coauthored a literature review that concluded that PDE5 inhibitors and nitrates are contraindicated.
But now, considering these new findings, Dr. Kloner is offering a fresh perspective.
“This study is reassuring,” Dr. Kloner said in an interview. “I think that it’s time to reassess whether there should be an absolute contraindication, or this should be more of like a warning.”
He noted that in controlled studies, like the ones he previously conducted, PDE5 inhibitors and nitrates were administered “very close to each other, on purpose,” yet this probably doesn’t reflect typical practice, in which clinicians can guide usage based on durations of drug metabolism.
“I think that physicians might be more comfortable now prescribing the drugs at the same time, but then telling patients that they shouldn’t take the two drugs simultaneously; they should wait and take the nitrate 24 hours after the last Viagra, or the nitrate 48 hours after the last Cialis,” Dr. Kloner said. “I suspect that that is happening. I suspect also the fact that people would be more likely to take the nitrate in the morning and the PDE5 inhibitor at night probably also contributes to the safety findings.”
Dr. Kloner noted that blood pressures vary throughout the day based on circadian rhythm, and that the body can adapt to some fluctuations without negative effects.
There could still be some people who experience a drop in blood pressure and get sick from it from the two drugs interacting, but that’s probably not that common, he said.
The study was supported by several grants. The investigators disclosed relationships with Merck, BMS, Bayer, and others. Dr. Kloner consults for Sanofi.
FROM ANNALS OF INTERNAL MEDICINE