User login
Scaly rash

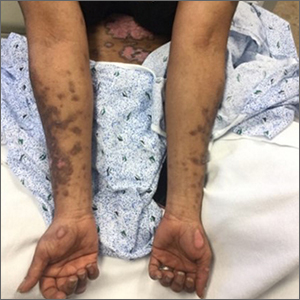
Scaly plaques on sun-exposed skin with hyperpigmentation and dyspigmentation are classic signs of cutaneous lupus erythematosus (CLE). (The dyspigmentation seen in this case signaled that she likely had chronic cutaneous lupus erythematosus [CCLE]—a subtype of CLE.) At the patient’s follow-up primary care visit, her antinuclear antibodies titer was 1:1280 (≥ 1:160 is considered a positive test) and her 24-hour urine protein was 1188 mg (normal levels in adults, < 150 mg/d). In light of the patient’s joint pain, lab findings, and skin manifestations, she was also given a diagnosis of systemic lupus erythematosus (SLE).
Lupus erythematosus has an increased prevalence in women and typically occurs between the ages of 20 to 50 years.1 The incidence and prevalence of this condition is also greater in Black patients. CLE can either occur with SLE or independently. Patients with CLE should be monitored for the development of SLE. A diagnosis of CLE is based mainly on clinical features; biopsy is only indicated if there is a high degree of uncertainty.
Patients with CLE may suffer from a lower quality of life compared to patients with other dermatologic conditions due to the often disfiguring and disabling nature of the condition.1,2 Additionally, Black patients have an even higher chance of developing depressive symptoms associated with CCLE.2
Therapeutic management for CLE involves photoprotection by wearing sun-protective clothing, sunscreen, and limiting sun exposure.1 Initial treatment includes topical or intralesional corticosteroids, or topical calcineurin inhibitors. Systemic therapy is similar to that used for SLE. Oral glucocorticoids, and antimalarial agents are considered first-line systemic therapy.1 Second-line treatment includes methotrexate, mycophenolate mofetil, systemic retinoids, and azathioprine. Other immunosuppressive agents that are less commonly used include clofazimine, cyclophosphamide, and rituximab.1
The patient was treated sequentially with trials of oral azathioprine 50 mg bid, then prednisone 10 mg once daily, and then hydroxychloroquine 400 mg daily, without significant change in her condition. Additionally, topical steroids did not improve the patient’s symptoms. She was subsequently started on rituximab 1000 mg intravenously with a second dose repeated 2 weeks later, and another treatment 6 months after that. One year after her visit to the ED, the patient was experiencing marked improvement in her lesions.
Photo courtesy of Christy Nwankwo BA. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135-146. doi:10.1007/s40257-016-0173-9
2. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol. 2019;20:465-475. doi:10.1007/s40257-019-00429-7

Scaly plaques on sun-exposed skin with hyperpigmentation and dyspigmentation are classic signs of cutaneous lupus erythematosus (CLE). (The dyspigmentation seen in this case signaled that she likely had chronic cutaneous lupus erythematosus [CCLE]—a subtype of CLE.) At the patient’s follow-up primary care visit, her antinuclear antibodies titer was 1:1280 (≥ 1:160 is considered a positive test) and her 24-hour urine protein was 1188 mg (normal levels in adults, < 150 mg/d). In light of the patient’s joint pain, lab findings, and skin manifestations, she was also given a diagnosis of systemic lupus erythematosus (SLE).
Lupus erythematosus has an increased prevalence in women and typically occurs between the ages of 20 to 50 years.1 The incidence and prevalence of this condition is also greater in Black patients. CLE can either occur with SLE or independently. Patients with CLE should be monitored for the development of SLE. A diagnosis of CLE is based mainly on clinical features; biopsy is only indicated if there is a high degree of uncertainty.
Patients with CLE may suffer from a lower quality of life compared to patients with other dermatologic conditions due to the often disfiguring and disabling nature of the condition.1,2 Additionally, Black patients have an even higher chance of developing depressive symptoms associated with CCLE.2
Therapeutic management for CLE involves photoprotection by wearing sun-protective clothing, sunscreen, and limiting sun exposure.1 Initial treatment includes topical or intralesional corticosteroids, or topical calcineurin inhibitors. Systemic therapy is similar to that used for SLE. Oral glucocorticoids, and antimalarial agents are considered first-line systemic therapy.1 Second-line treatment includes methotrexate, mycophenolate mofetil, systemic retinoids, and azathioprine. Other immunosuppressive agents that are less commonly used include clofazimine, cyclophosphamide, and rituximab.1
The patient was treated sequentially with trials of oral azathioprine 50 mg bid, then prednisone 10 mg once daily, and then hydroxychloroquine 400 mg daily, without significant change in her condition. Additionally, topical steroids did not improve the patient’s symptoms. She was subsequently started on rituximab 1000 mg intravenously with a second dose repeated 2 weeks later, and another treatment 6 months after that. One year after her visit to the ED, the patient was experiencing marked improvement in her lesions.
Photo courtesy of Christy Nwankwo BA. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

Scaly plaques on sun-exposed skin with hyperpigmentation and dyspigmentation are classic signs of cutaneous lupus erythematosus (CLE). (The dyspigmentation seen in this case signaled that she likely had chronic cutaneous lupus erythematosus [CCLE]—a subtype of CLE.) At the patient’s follow-up primary care visit, her antinuclear antibodies titer was 1:1280 (≥ 1:160 is considered a positive test) and her 24-hour urine protein was 1188 mg (normal levels in adults, < 150 mg/d). In light of the patient’s joint pain, lab findings, and skin manifestations, she was also given a diagnosis of systemic lupus erythematosus (SLE).
Lupus erythematosus has an increased prevalence in women and typically occurs between the ages of 20 to 50 years.1 The incidence and prevalence of this condition is also greater in Black patients. CLE can either occur with SLE or independently. Patients with CLE should be monitored for the development of SLE. A diagnosis of CLE is based mainly on clinical features; biopsy is only indicated if there is a high degree of uncertainty.
Patients with CLE may suffer from a lower quality of life compared to patients with other dermatologic conditions due to the often disfiguring and disabling nature of the condition.1,2 Additionally, Black patients have an even higher chance of developing depressive symptoms associated with CCLE.2
Therapeutic management for CLE involves photoprotection by wearing sun-protective clothing, sunscreen, and limiting sun exposure.1 Initial treatment includes topical or intralesional corticosteroids, or topical calcineurin inhibitors. Systemic therapy is similar to that used for SLE. Oral glucocorticoids, and antimalarial agents are considered first-line systemic therapy.1 Second-line treatment includes methotrexate, mycophenolate mofetil, systemic retinoids, and azathioprine. Other immunosuppressive agents that are less commonly used include clofazimine, cyclophosphamide, and rituximab.1
The patient was treated sequentially with trials of oral azathioprine 50 mg bid, then prednisone 10 mg once daily, and then hydroxychloroquine 400 mg daily, without significant change in her condition. Additionally, topical steroids did not improve the patient’s symptoms. She was subsequently started on rituximab 1000 mg intravenously with a second dose repeated 2 weeks later, and another treatment 6 months after that. One year after her visit to the ED, the patient was experiencing marked improvement in her lesions.
Photo courtesy of Christy Nwankwo BA. Text courtesy of Christy Nwankwo, BA, University of Missouri-Kansas City School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135-146. doi:10.1007/s40257-016-0173-9
2. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol. 2019;20:465-475. doi:10.1007/s40257-019-00429-7
1. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17:135-146. doi:10.1007/s40257-016-0173-9
2. Hong J, Aspey L, Bao G, et al. Chronic cutaneous lupus erythematosus: depression burden and associated factors. Am J Clin Dermatol. 2019;20:465-475. doi:10.1007/s40257-019-00429-7
The Empire strikes out against one physician’s homemade star fighter
The force is with Ukraine, always
Of all the things we could want from Star Wars, a lightsaber is at the top of the list. And someone is working on that. But second is probably the iconic X-wing. It was used to blow up the Death Star after all: Who wouldn’t want one?
A real-life star fighter may be outside our technological capabilities, but Dr. Akaki Lekiachvili of Atlanta has done the next best thing and constructed a two-thirds scale model to encourage kids to enter the sciences and, with the advent of the war in Ukraine, raise money for medical supplies to assist doctors in the embattled country. Perhaps unsurprisingly, Dr. Lekiachvili, originally from Georgia (the country, former Soviet republic, and previous target of Russian aggression in 2008), takes a dim view toward the invasion of Ukraine: “Russia is like the Evil Empire and Ukraine the Rebel Alliance.”
It’s been a long road finishing the X-Wing, as Dr. Lekiachvili started the project in 2016 and spent $60,000 on it, posting numerous updates on social media over that time, even attracting the attention of Luke Skywalker himself, actor Mark Hamill. Now that he’s done, he’s brought his model out to the public multiple times, delighting kids and adults alike. It can’t fly, but it has an engine and wheels so it can move, the wings can lock into attack position, the thrusters light up, and the voices of Obi-Wan Kenobi and R2-D2 guide children along as they sit in the cockpit.
Dr. Lekiachvili hopes to auction off his creation to a collector and donate the proceeds to Ukrainian charities, and we’re sure he’ll receive far more than the $60,000 he spent building his masterpiece. Now, if you’ll excuse us, we’re off to raid our bank accounts. We have a Death Star to destroy.
I’m a doctor, not a hologram
Telemedicine got a big boost during the early phase of the pandemic when hospitals and medical offices were off limits to anyone without COVID-19, but things have cooled off, telemedically speaking, since then. Well, NASA may have heated them up again. Or maybe it was Starfleet. Hmm, wait a second while we check. … No, it was NASA.
The space agency used the Microsoft Hololens Kinect camera and a personal computer with custom software from Aexa Aerospace to “holoport” NASA flight surgeon Josef Schmid up to the International Space Station, where he had a conversation with European Space Agency astronaut Thomas Pesquet, who wore an augmented reality headset that allowed him to see, hear, and interact with a 3D representation of the earthbound medical provider.
“Holoportation has been in use since at least 2016 by Microsoft, but this is the first use in such an extreme and remote environment such as space,” NASA said in a recent written statement, noting that the extreme house call took place on Oct. 8, 2021.
They seem to be forgetting about Star Trek, but we’ll let them slide on that one. Anyway, NASA didn’t share any details of the medical holoconversation – which may have strained the limits of HIPAA’s portability provisions – but Dr. Schmid described it as “a brand-new way of human exploration, where our human entity is able to travel off the planet. Our physical body is not there, but our human entity absolutely is there.”
Boldly doctoring where no doctor has gone before, you might say. You also might notice from the photo that Dr. Schmid went full Trekkie with a genuine Vulcan salute. Live long and prosper, Dr. Schmid. Live long and prosper.
Add electricity for umami
Salt makes everything taste better. Unfortunately, excess salt can cause problems for our bodies down the line, starting with high blood pressure and continuing on to heart disease and strokes. So how do we enjoy our deliciously salty foods without putting ourselves at risk? One answer may be electricity.
Researchers at Meiji University in Tokyo partnered with food and beverage maker Kirin to develop a set of electric chopsticks to boost the taste of salt in foods without the extra sodium. According to codeveloper and Meiji University professor Homei Miyashita, the device, worn like a watch with a wire attached to one of the chopsticks, “uses a weak electrical current to transmit sodium ions from food, through the chopsticks, to the mouth where they create a sense of saltines,” Reuters said.
In a country like Japan, where a lot of food is made with heavily sodium-based ingredients like miso and soy sauce, the average adult consumes 10 g of salt a day. That’s twice the recommended amount proposed by the World Health Organization. To not sacrifice bland food for better health, this device, which enhances the saltiness of the food consumed by 1.5 times, offers a fairly easy solution to a big public health crisis.
The chopsticks were tested by giving participants reduced-sodium miso soup. They told the researchers that the food was improved in “richness, sweetness, and overall tastiness,” the Guardian said.
Worried about having something electric in your mouth? Don’t worry. Kirin said in a statement that the electricity is very weak and not enough to affect the body.
The chopsticks are still in a prototype stage, but you may be able to get your pair as soon as next year. Until then, maybe be a little mindful of the salt.
Pet poop works in mysterious ways
We usually see it as a burden when our pets poop and pee in the house, but those bodily excretions may be able to tell us something about cancer-causing toxins running rampant in our homes.
Those toxins, known as aromatic amines, can be found in tobacco smoke and dyes used in make-up, textiles, and plastics. “Our findings suggest that pets are coming into contact with aromatic amines that leach from products in their household environment,” lead author Sridhar Chinthakindi, PhD, of NYU Langone Health, said in a statement from the university. “As these substances have been tied to bladder, colorectal, and other forms of cancer, our results may help explain why so many dogs and cats develop such diseases.”
Tobacco smoke was not the main source of the aromatic amines found in the poop and urine, but 70% of dogs and 80% of cats had these chemicals in their waste. The researchers looked for 30 types of aromatic amines plus nicotine in the sample and found 8. The chemical concentrations were much higher in cats than in dogs, possibly because of differences in exposure and metabolism between the two species, they suggested.
“If [pets] are getting exposed to toxins in our homes, then we had better take a closer look at our own exposure,” said senior author Kurunthachalam Kannan, PhD, of NYU Langone.
So the next time your pet poops or pees in the house, don’t get mad. Maybe they’re just trying to help you out by supplying some easy-to-collect samples.
The force is with Ukraine, always
Of all the things we could want from Star Wars, a lightsaber is at the top of the list. And someone is working on that. But second is probably the iconic X-wing. It was used to blow up the Death Star after all: Who wouldn’t want one?
A real-life star fighter may be outside our technological capabilities, but Dr. Akaki Lekiachvili of Atlanta has done the next best thing and constructed a two-thirds scale model to encourage kids to enter the sciences and, with the advent of the war in Ukraine, raise money for medical supplies to assist doctors in the embattled country. Perhaps unsurprisingly, Dr. Lekiachvili, originally from Georgia (the country, former Soviet republic, and previous target of Russian aggression in 2008), takes a dim view toward the invasion of Ukraine: “Russia is like the Evil Empire and Ukraine the Rebel Alliance.”
It’s been a long road finishing the X-Wing, as Dr. Lekiachvili started the project in 2016 and spent $60,000 on it, posting numerous updates on social media over that time, even attracting the attention of Luke Skywalker himself, actor Mark Hamill. Now that he’s done, he’s brought his model out to the public multiple times, delighting kids and adults alike. It can’t fly, but it has an engine and wheels so it can move, the wings can lock into attack position, the thrusters light up, and the voices of Obi-Wan Kenobi and R2-D2 guide children along as they sit in the cockpit.
Dr. Lekiachvili hopes to auction off his creation to a collector and donate the proceeds to Ukrainian charities, and we’re sure he’ll receive far more than the $60,000 he spent building his masterpiece. Now, if you’ll excuse us, we’re off to raid our bank accounts. We have a Death Star to destroy.
I’m a doctor, not a hologram
Telemedicine got a big boost during the early phase of the pandemic when hospitals and medical offices were off limits to anyone without COVID-19, but things have cooled off, telemedically speaking, since then. Well, NASA may have heated them up again. Or maybe it was Starfleet. Hmm, wait a second while we check. … No, it was NASA.
The space agency used the Microsoft Hololens Kinect camera and a personal computer with custom software from Aexa Aerospace to “holoport” NASA flight surgeon Josef Schmid up to the International Space Station, where he had a conversation with European Space Agency astronaut Thomas Pesquet, who wore an augmented reality headset that allowed him to see, hear, and interact with a 3D representation of the earthbound medical provider.
“Holoportation has been in use since at least 2016 by Microsoft, but this is the first use in such an extreme and remote environment such as space,” NASA said in a recent written statement, noting that the extreme house call took place on Oct. 8, 2021.
They seem to be forgetting about Star Trek, but we’ll let them slide on that one. Anyway, NASA didn’t share any details of the medical holoconversation – which may have strained the limits of HIPAA’s portability provisions – but Dr. Schmid described it as “a brand-new way of human exploration, where our human entity is able to travel off the planet. Our physical body is not there, but our human entity absolutely is there.”
Boldly doctoring where no doctor has gone before, you might say. You also might notice from the photo that Dr. Schmid went full Trekkie with a genuine Vulcan salute. Live long and prosper, Dr. Schmid. Live long and prosper.
Add electricity for umami
Salt makes everything taste better. Unfortunately, excess salt can cause problems for our bodies down the line, starting with high blood pressure and continuing on to heart disease and strokes. So how do we enjoy our deliciously salty foods without putting ourselves at risk? One answer may be electricity.
Researchers at Meiji University in Tokyo partnered with food and beverage maker Kirin to develop a set of electric chopsticks to boost the taste of salt in foods without the extra sodium. According to codeveloper and Meiji University professor Homei Miyashita, the device, worn like a watch with a wire attached to one of the chopsticks, “uses a weak electrical current to transmit sodium ions from food, through the chopsticks, to the mouth where they create a sense of saltines,” Reuters said.
In a country like Japan, where a lot of food is made with heavily sodium-based ingredients like miso and soy sauce, the average adult consumes 10 g of salt a day. That’s twice the recommended amount proposed by the World Health Organization. To not sacrifice bland food for better health, this device, which enhances the saltiness of the food consumed by 1.5 times, offers a fairly easy solution to a big public health crisis.
The chopsticks were tested by giving participants reduced-sodium miso soup. They told the researchers that the food was improved in “richness, sweetness, and overall tastiness,” the Guardian said.
Worried about having something electric in your mouth? Don’t worry. Kirin said in a statement that the electricity is very weak and not enough to affect the body.
The chopsticks are still in a prototype stage, but you may be able to get your pair as soon as next year. Until then, maybe be a little mindful of the salt.
Pet poop works in mysterious ways
We usually see it as a burden when our pets poop and pee in the house, but those bodily excretions may be able to tell us something about cancer-causing toxins running rampant in our homes.
Those toxins, known as aromatic amines, can be found in tobacco smoke and dyes used in make-up, textiles, and plastics. “Our findings suggest that pets are coming into contact with aromatic amines that leach from products in their household environment,” lead author Sridhar Chinthakindi, PhD, of NYU Langone Health, said in a statement from the university. “As these substances have been tied to bladder, colorectal, and other forms of cancer, our results may help explain why so many dogs and cats develop such diseases.”
Tobacco smoke was not the main source of the aromatic amines found in the poop and urine, but 70% of dogs and 80% of cats had these chemicals in their waste. The researchers looked for 30 types of aromatic amines plus nicotine in the sample and found 8. The chemical concentrations were much higher in cats than in dogs, possibly because of differences in exposure and metabolism between the two species, they suggested.
“If [pets] are getting exposed to toxins in our homes, then we had better take a closer look at our own exposure,” said senior author Kurunthachalam Kannan, PhD, of NYU Langone.
So the next time your pet poops or pees in the house, don’t get mad. Maybe they’re just trying to help you out by supplying some easy-to-collect samples.
The force is with Ukraine, always
Of all the things we could want from Star Wars, a lightsaber is at the top of the list. And someone is working on that. But second is probably the iconic X-wing. It was used to blow up the Death Star after all: Who wouldn’t want one?
A real-life star fighter may be outside our technological capabilities, but Dr. Akaki Lekiachvili of Atlanta has done the next best thing and constructed a two-thirds scale model to encourage kids to enter the sciences and, with the advent of the war in Ukraine, raise money for medical supplies to assist doctors in the embattled country. Perhaps unsurprisingly, Dr. Lekiachvili, originally from Georgia (the country, former Soviet republic, and previous target of Russian aggression in 2008), takes a dim view toward the invasion of Ukraine: “Russia is like the Evil Empire and Ukraine the Rebel Alliance.”
It’s been a long road finishing the X-Wing, as Dr. Lekiachvili started the project in 2016 and spent $60,000 on it, posting numerous updates on social media over that time, even attracting the attention of Luke Skywalker himself, actor Mark Hamill. Now that he’s done, he’s brought his model out to the public multiple times, delighting kids and adults alike. It can’t fly, but it has an engine and wheels so it can move, the wings can lock into attack position, the thrusters light up, and the voices of Obi-Wan Kenobi and R2-D2 guide children along as they sit in the cockpit.
Dr. Lekiachvili hopes to auction off his creation to a collector and donate the proceeds to Ukrainian charities, and we’re sure he’ll receive far more than the $60,000 he spent building his masterpiece. Now, if you’ll excuse us, we’re off to raid our bank accounts. We have a Death Star to destroy.
I’m a doctor, not a hologram
Telemedicine got a big boost during the early phase of the pandemic when hospitals and medical offices were off limits to anyone without COVID-19, but things have cooled off, telemedically speaking, since then. Well, NASA may have heated them up again. Or maybe it was Starfleet. Hmm, wait a second while we check. … No, it was NASA.
The space agency used the Microsoft Hololens Kinect camera and a personal computer with custom software from Aexa Aerospace to “holoport” NASA flight surgeon Josef Schmid up to the International Space Station, where he had a conversation with European Space Agency astronaut Thomas Pesquet, who wore an augmented reality headset that allowed him to see, hear, and interact with a 3D representation of the earthbound medical provider.
“Holoportation has been in use since at least 2016 by Microsoft, but this is the first use in such an extreme and remote environment such as space,” NASA said in a recent written statement, noting that the extreme house call took place on Oct. 8, 2021.
They seem to be forgetting about Star Trek, but we’ll let them slide on that one. Anyway, NASA didn’t share any details of the medical holoconversation – which may have strained the limits of HIPAA’s portability provisions – but Dr. Schmid described it as “a brand-new way of human exploration, where our human entity is able to travel off the planet. Our physical body is not there, but our human entity absolutely is there.”
Boldly doctoring where no doctor has gone before, you might say. You also might notice from the photo that Dr. Schmid went full Trekkie with a genuine Vulcan salute. Live long and prosper, Dr. Schmid. Live long and prosper.
Add electricity for umami
Salt makes everything taste better. Unfortunately, excess salt can cause problems for our bodies down the line, starting with high blood pressure and continuing on to heart disease and strokes. So how do we enjoy our deliciously salty foods without putting ourselves at risk? One answer may be electricity.
Researchers at Meiji University in Tokyo partnered with food and beverage maker Kirin to develop a set of electric chopsticks to boost the taste of salt in foods without the extra sodium. According to codeveloper and Meiji University professor Homei Miyashita, the device, worn like a watch with a wire attached to one of the chopsticks, “uses a weak electrical current to transmit sodium ions from food, through the chopsticks, to the mouth where they create a sense of saltines,” Reuters said.
In a country like Japan, where a lot of food is made with heavily sodium-based ingredients like miso and soy sauce, the average adult consumes 10 g of salt a day. That’s twice the recommended amount proposed by the World Health Organization. To not sacrifice bland food for better health, this device, which enhances the saltiness of the food consumed by 1.5 times, offers a fairly easy solution to a big public health crisis.
The chopsticks were tested by giving participants reduced-sodium miso soup. They told the researchers that the food was improved in “richness, sweetness, and overall tastiness,” the Guardian said.
Worried about having something electric in your mouth? Don’t worry. Kirin said in a statement that the electricity is very weak and not enough to affect the body.
The chopsticks are still in a prototype stage, but you may be able to get your pair as soon as next year. Until then, maybe be a little mindful of the salt.
Pet poop works in mysterious ways
We usually see it as a burden when our pets poop and pee in the house, but those bodily excretions may be able to tell us something about cancer-causing toxins running rampant in our homes.
Those toxins, known as aromatic amines, can be found in tobacco smoke and dyes used in make-up, textiles, and plastics. “Our findings suggest that pets are coming into contact with aromatic amines that leach from products in their household environment,” lead author Sridhar Chinthakindi, PhD, of NYU Langone Health, said in a statement from the university. “As these substances have been tied to bladder, colorectal, and other forms of cancer, our results may help explain why so many dogs and cats develop such diseases.”
Tobacco smoke was not the main source of the aromatic amines found in the poop and urine, but 70% of dogs and 80% of cats had these chemicals in their waste. The researchers looked for 30 types of aromatic amines plus nicotine in the sample and found 8. The chemical concentrations were much higher in cats than in dogs, possibly because of differences in exposure and metabolism between the two species, they suggested.
“If [pets] are getting exposed to toxins in our homes, then we had better take a closer look at our own exposure,” said senior author Kurunthachalam Kannan, PhD, of NYU Langone.
So the next time your pet poops or pees in the house, don’t get mad. Maybe they’re just trying to help you out by supplying some easy-to-collect samples.
Acid series: Trichloroacetic acid
– yet can be one of the most dangerous treatments in the hands of an untrained user.
TCA, in a clear colorless solution, is available in concentrations up to 100%, and has not been associated with allergic reactions or systemic toxicity. The available concentrations include those used for superficial depth peels (10%-30%), medium depth peels (35%-50%), and deep peels (greater than 50%).
TCA causes coagulation of the cellular membrane of epidermal proteins in the epidermis and, depending on the concentration, the dermis, which results in frosting of the skin. Repair of the epidermal cells induces resurfacing of the skin and neocollagenesis. TCA can be combined with other acids, including glycolic acid (Coleman peel), Jessner solution (Monheit peel), and solid CO2 (Brody peel). It has also been combined with lactic acid, mandelic acid, and salicylic acid in combination peels of various concentrations.
Although there are many studies, case reports, and textbooks related to this topic and the applications, combinations and treatment options for TCA peels, it is important to highlight here how many of these solutions – at high concentrations – are available directly to consumers, med spas, and the general public through online websites, including Amazon and overseas sites. Over the last 15 years, I have seen complications of this acid alone in people who have bought TCA online, related to applications not just on the face but on the body, neck, eyes, vaginal, and anal areas. Pigmentation, erosions, ulcers, and strictures are just some of the possible complications that occur not just with a more concentrated solution, but more often from application errors, aggressive layering of the acid, allowing the acid to sit on the skin too long, and improper tissue prepping and posttreatment skin care.
TCA can be an untamable acid, with little control over the depth of penetration even in the most controlled situations. The inability to be neutralize TCA creates an environment in which the depth of penetration and tissue coagulation is not a precise science. Once applied, the tissue reaction cannot be “stopped” or rapidly reversed making it highly variable in its mechanism. Patients of all skin types have the potential to develop complications as the epidermal and dermal thickness, moisture content, sebum production, and pigmentation are highly varied between individuals.
In my opinion, it is a dangerous product to have on the market – not just for the untrained medical providers using it but for estheticians and the general public who now can buy TCA anywhere.
But with effective training, reliable sourcing and appropriate preparation of the patient’s skin, however, a TCA peel can be a highly effective tool for difficult-to-treat dermatological problems, such as scarring and xanthelasma.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. She has no relevant disclosures. Write to them at [email protected].
– yet can be one of the most dangerous treatments in the hands of an untrained user.
TCA, in a clear colorless solution, is available in concentrations up to 100%, and has not been associated with allergic reactions or systemic toxicity. The available concentrations include those used for superficial depth peels (10%-30%), medium depth peels (35%-50%), and deep peels (greater than 50%).
TCA causes coagulation of the cellular membrane of epidermal proteins in the epidermis and, depending on the concentration, the dermis, which results in frosting of the skin. Repair of the epidermal cells induces resurfacing of the skin and neocollagenesis. TCA can be combined with other acids, including glycolic acid (Coleman peel), Jessner solution (Monheit peel), and solid CO2 (Brody peel). It has also been combined with lactic acid, mandelic acid, and salicylic acid in combination peels of various concentrations.
Although there are many studies, case reports, and textbooks related to this topic and the applications, combinations and treatment options for TCA peels, it is important to highlight here how many of these solutions – at high concentrations – are available directly to consumers, med spas, and the general public through online websites, including Amazon and overseas sites. Over the last 15 years, I have seen complications of this acid alone in people who have bought TCA online, related to applications not just on the face but on the body, neck, eyes, vaginal, and anal areas. Pigmentation, erosions, ulcers, and strictures are just some of the possible complications that occur not just with a more concentrated solution, but more often from application errors, aggressive layering of the acid, allowing the acid to sit on the skin too long, and improper tissue prepping and posttreatment skin care.
TCA can be an untamable acid, with little control over the depth of penetration even in the most controlled situations. The inability to be neutralize TCA creates an environment in which the depth of penetration and tissue coagulation is not a precise science. Once applied, the tissue reaction cannot be “stopped” or rapidly reversed making it highly variable in its mechanism. Patients of all skin types have the potential to develop complications as the epidermal and dermal thickness, moisture content, sebum production, and pigmentation are highly varied between individuals.
In my opinion, it is a dangerous product to have on the market – not just for the untrained medical providers using it but for estheticians and the general public who now can buy TCA anywhere.
But with effective training, reliable sourcing and appropriate preparation of the patient’s skin, however, a TCA peel can be a highly effective tool for difficult-to-treat dermatological problems, such as scarring and xanthelasma.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. She has no relevant disclosures. Write to them at [email protected].
– yet can be one of the most dangerous treatments in the hands of an untrained user.
TCA, in a clear colorless solution, is available in concentrations up to 100%, and has not been associated with allergic reactions or systemic toxicity. The available concentrations include those used for superficial depth peels (10%-30%), medium depth peels (35%-50%), and deep peels (greater than 50%).
TCA causes coagulation of the cellular membrane of epidermal proteins in the epidermis and, depending on the concentration, the dermis, which results in frosting of the skin. Repair of the epidermal cells induces resurfacing of the skin and neocollagenesis. TCA can be combined with other acids, including glycolic acid (Coleman peel), Jessner solution (Monheit peel), and solid CO2 (Brody peel). It has also been combined with lactic acid, mandelic acid, and salicylic acid in combination peels of various concentrations.
Although there are many studies, case reports, and textbooks related to this topic and the applications, combinations and treatment options for TCA peels, it is important to highlight here how many of these solutions – at high concentrations – are available directly to consumers, med spas, and the general public through online websites, including Amazon and overseas sites. Over the last 15 years, I have seen complications of this acid alone in people who have bought TCA online, related to applications not just on the face but on the body, neck, eyes, vaginal, and anal areas. Pigmentation, erosions, ulcers, and strictures are just some of the possible complications that occur not just with a more concentrated solution, but more often from application errors, aggressive layering of the acid, allowing the acid to sit on the skin too long, and improper tissue prepping and posttreatment skin care.
TCA can be an untamable acid, with little control over the depth of penetration even in the most controlled situations. The inability to be neutralize TCA creates an environment in which the depth of penetration and tissue coagulation is not a precise science. Once applied, the tissue reaction cannot be “stopped” or rapidly reversed making it highly variable in its mechanism. Patients of all skin types have the potential to develop complications as the epidermal and dermal thickness, moisture content, sebum production, and pigmentation are highly varied between individuals.
In my opinion, it is a dangerous product to have on the market – not just for the untrained medical providers using it but for estheticians and the general public who now can buy TCA anywhere.
But with effective training, reliable sourcing and appropriate preparation of the patient’s skin, however, a TCA peel can be a highly effective tool for difficult-to-treat dermatological problems, such as scarring and xanthelasma.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. She has no relevant disclosures. Write to them at [email protected].
Study: Fasting plus calorie counting offered no weight-loss benefit over calorie counting alone
Not so fast!
Over the course of a year, study participants who ate only from 8:00 a.m. to 4:00 p.m. did not lose significantly more weight than individuals who ate whenever they wanted, nor did they achieve significantly greater improvements in other obesity-related health measures like body mass index (BMI) or metabolic risk, reported lead author Deying Liu, MD, of Nanfang Hospital, Southern Medical University, Guangzhou, China, and colleagues.
“[Daily fasting] has gained popularity because it is a weight-loss strategy that is simple to follow, which may enhance adherence,” Dr. Liu and colleagues wrote in the New England Journal of Medicine. However, “the long-term efficacy and safety of time-restricted eating as a weight-loss strategy are still uncertain, and the long-term effects on weight loss of time-restricted eating as compared with daily calorie restriction alone have not been fully explored.”
To learn more, Dr. Liu and colleagues recruited 139 adult patients with BMIs between 28 and 45. Individuals with serious medical conditions, such as malignant tumors, diabetes, chronic kidney disease, and others were excluded. Other exclusion criteria included smoking, ongoing participation in a weight-loss program, GI surgery within the prior year, use of medications that impact energy balance and weight, and planned or current pregnancy.
All participants were advised to eat calorie-restricted diets, with ranges of 1,500-1,800 kcal per day for men and 1,200-1,500 kcal per day for women. To determine the added impact of fasting, participants were randomized in a 1:1 ratio into time-restricted (fasting) or non–time-restricted (nonfasting) groups, in which fasting participants ate only during an 8-hour window from 8:00 a.m. to 4:00 p.m., whereas nonfasting participants ate whenever they wanted.
At 6 months and 12 months, participants were re-evaluated for changes in weight, body fat, BMI, blood pressure, lean body mass, and metabolic risk factors, including glucose level, triglycerides, blood pressure, and others.
Caloric intake restriction seems to explain most of beneficial effects
At one-year follow-up, 118 participants (84.9%) remained in the study. Although members of the fasting group lost slightly more weight on average than those in the non-fasting group (mean, 8.0 kg vs. 6.3 kg), the difference between groups was not statistically significant (95% confidence interval, −4.0 to 0.4; P = .11).
Most of the other obesity-related health measures also trended toward favoring the fasting group, but again, none of these improvements was statistically significant. Weight circumference at 1 year, for example, decreased by a mean of 9.4 cm in the fasting group versus 8.8 cm in the nonfasting group, a net difference of 1.8 cm (95% CI, –4.0 to 0.5).
“We found that the two weight-loss regimens that we evaluated had similar success in patients with obesity, regardless of whether they reduced their calorie consumption through time-restricted eating or through calorie restriction alone,” Dr. Liu and colleagues concluded.
Principal investigator Huijie Zhang MD, PhD, professor, chief physician, and deputy director of the department of endocrinology and metabolism at Nafang Hospital, noted that their findings are “consistent with the findings in previous studies.”
“Our data suggest that caloric intake restriction explained most of the beneficial effects of a time-restricted eating regimen,” Dr. Zhang said.
Still, Dr. Zhang called time-restricted eating “a viable and sustainable approach for a person who wants to lose weight.”
More work is needed, Dr. Zhang said, to uncover the impact of fasting in “diverse groups,” including patients with chronic disease like diabetes and cardiovascular disease. Investigators should also conduct studies to compare outcomes between men and women, and evaluate the effects of other fasting durations.
Can trial be applied to a wider population?
According to Blandine Laferrère, MD, PhD, and Satchidananda Panda, PhD, of Columbia University Irving Medical Center, New York, and the Salk Institute for Biological Studies, La Jolla, Calif., respectively, “the results of the trial suggest that calorie restriction combined with time restriction, when delivered with intensive coaching and monitoring, is an approach that is as safe, sustainable, and effective for weight loss as calorie restriction alone.”
Yet Dr. Laferrère and Dr. Panda also expressed skepticism about broader implementation of a similar regime.
“The applicability of this trial to wider populations is debatable,” they wrote in an accompanying editorial. “The short time period for eating at baseline may be specific to the population studied, since investigators outside China have reported longer time windows. The rigorous coaching and monitoring by trial staff also leaves open the question of whether time-restricted eating is easier to adhere to than intentional calorie restriction. Such cost-benefit analyses are important for the assessment of the scalability of a lifestyle intervention.”
Duration is trial’s greatest strength
Kristina Varady, PhD, professor of nutrition in the department of kinesiology and nutrition at the University of Illinois at Chicago, said the “key strength” of the trial was its duration, at 12 months, making it the longest time-restricted eating trial to date”; however, she was critical of the design.
“Quite frankly, I’m surprised this study got into such a high-caliber medical journal,” Dr. Varady said in a written comment. “It doesn’t even have a control group! It goes to show how popular these diets are and how much people want to know about them.”
She also noted that “the study was flawed in that it didn’t really look at the effects of true time-restricted eating.” According to Dr. Varady, combining calorie restriction with time-restricted eating “kind of defeats the purpose” of a time-restricted diet.
“The main benefit of time-restricted eating is that you don’t need to count calories in order to lose weight,” Dr. Varady said, citing two of her own studies from 2018 and 2020. “Just by limiting the eating window to 8 hours per day, people naturally cut out 300-500 calories per day. That’s why people like [time-restricted eating] so much.”
Dr. Varady was also “very surprised” at the adherence data. At 1 year, approximately 85% of the patients were still following the protocol, a notably higher rate than most dietary intervention studies, which typically report adherence rates of 50-60%, she said. The high adherence rate was particularly unexpected because of the 8:00 a.m.–4:00 p.m. eating window, Dr. Varady added, since that meant skipping “the family/social meal every evening over 1 whole year!”
The study was funded by the National Key Research and Development Project and others. The study investigators reported no conflicts of interest. Dr. Varady disclosed author fees from the Hachette Book group for her book “The Every Other Day Diet.”
Not so fast!
Over the course of a year, study participants who ate only from 8:00 a.m. to 4:00 p.m. did not lose significantly more weight than individuals who ate whenever they wanted, nor did they achieve significantly greater improvements in other obesity-related health measures like body mass index (BMI) or metabolic risk, reported lead author Deying Liu, MD, of Nanfang Hospital, Southern Medical University, Guangzhou, China, and colleagues.
“[Daily fasting] has gained popularity because it is a weight-loss strategy that is simple to follow, which may enhance adherence,” Dr. Liu and colleagues wrote in the New England Journal of Medicine. However, “the long-term efficacy and safety of time-restricted eating as a weight-loss strategy are still uncertain, and the long-term effects on weight loss of time-restricted eating as compared with daily calorie restriction alone have not been fully explored.”
To learn more, Dr. Liu and colleagues recruited 139 adult patients with BMIs between 28 and 45. Individuals with serious medical conditions, such as malignant tumors, diabetes, chronic kidney disease, and others were excluded. Other exclusion criteria included smoking, ongoing participation in a weight-loss program, GI surgery within the prior year, use of medications that impact energy balance and weight, and planned or current pregnancy.
All participants were advised to eat calorie-restricted diets, with ranges of 1,500-1,800 kcal per day for men and 1,200-1,500 kcal per day for women. To determine the added impact of fasting, participants were randomized in a 1:1 ratio into time-restricted (fasting) or non–time-restricted (nonfasting) groups, in which fasting participants ate only during an 8-hour window from 8:00 a.m. to 4:00 p.m., whereas nonfasting participants ate whenever they wanted.
At 6 months and 12 months, participants were re-evaluated for changes in weight, body fat, BMI, blood pressure, lean body mass, and metabolic risk factors, including glucose level, triglycerides, blood pressure, and others.
Caloric intake restriction seems to explain most of beneficial effects
At one-year follow-up, 118 participants (84.9%) remained in the study. Although members of the fasting group lost slightly more weight on average than those in the non-fasting group (mean, 8.0 kg vs. 6.3 kg), the difference between groups was not statistically significant (95% confidence interval, −4.0 to 0.4; P = .11).
Most of the other obesity-related health measures also trended toward favoring the fasting group, but again, none of these improvements was statistically significant. Weight circumference at 1 year, for example, decreased by a mean of 9.4 cm in the fasting group versus 8.8 cm in the nonfasting group, a net difference of 1.8 cm (95% CI, –4.0 to 0.5).
“We found that the two weight-loss regimens that we evaluated had similar success in patients with obesity, regardless of whether they reduced their calorie consumption through time-restricted eating or through calorie restriction alone,” Dr. Liu and colleagues concluded.
Principal investigator Huijie Zhang MD, PhD, professor, chief physician, and deputy director of the department of endocrinology and metabolism at Nafang Hospital, noted that their findings are “consistent with the findings in previous studies.”
“Our data suggest that caloric intake restriction explained most of the beneficial effects of a time-restricted eating regimen,” Dr. Zhang said.
Still, Dr. Zhang called time-restricted eating “a viable and sustainable approach for a person who wants to lose weight.”
More work is needed, Dr. Zhang said, to uncover the impact of fasting in “diverse groups,” including patients with chronic disease like diabetes and cardiovascular disease. Investigators should also conduct studies to compare outcomes between men and women, and evaluate the effects of other fasting durations.
Can trial be applied to a wider population?
According to Blandine Laferrère, MD, PhD, and Satchidananda Panda, PhD, of Columbia University Irving Medical Center, New York, and the Salk Institute for Biological Studies, La Jolla, Calif., respectively, “the results of the trial suggest that calorie restriction combined with time restriction, when delivered with intensive coaching and monitoring, is an approach that is as safe, sustainable, and effective for weight loss as calorie restriction alone.”
Yet Dr. Laferrère and Dr. Panda also expressed skepticism about broader implementation of a similar regime.
“The applicability of this trial to wider populations is debatable,” they wrote in an accompanying editorial. “The short time period for eating at baseline may be specific to the population studied, since investigators outside China have reported longer time windows. The rigorous coaching and monitoring by trial staff also leaves open the question of whether time-restricted eating is easier to adhere to than intentional calorie restriction. Such cost-benefit analyses are important for the assessment of the scalability of a lifestyle intervention.”
Duration is trial’s greatest strength
Kristina Varady, PhD, professor of nutrition in the department of kinesiology and nutrition at the University of Illinois at Chicago, said the “key strength” of the trial was its duration, at 12 months, making it the longest time-restricted eating trial to date”; however, she was critical of the design.
“Quite frankly, I’m surprised this study got into such a high-caliber medical journal,” Dr. Varady said in a written comment. “It doesn’t even have a control group! It goes to show how popular these diets are and how much people want to know about them.”
She also noted that “the study was flawed in that it didn’t really look at the effects of true time-restricted eating.” According to Dr. Varady, combining calorie restriction with time-restricted eating “kind of defeats the purpose” of a time-restricted diet.
“The main benefit of time-restricted eating is that you don’t need to count calories in order to lose weight,” Dr. Varady said, citing two of her own studies from 2018 and 2020. “Just by limiting the eating window to 8 hours per day, people naturally cut out 300-500 calories per day. That’s why people like [time-restricted eating] so much.”
Dr. Varady was also “very surprised” at the adherence data. At 1 year, approximately 85% of the patients were still following the protocol, a notably higher rate than most dietary intervention studies, which typically report adherence rates of 50-60%, she said. The high adherence rate was particularly unexpected because of the 8:00 a.m.–4:00 p.m. eating window, Dr. Varady added, since that meant skipping “the family/social meal every evening over 1 whole year!”
The study was funded by the National Key Research and Development Project and others. The study investigators reported no conflicts of interest. Dr. Varady disclosed author fees from the Hachette Book group for her book “The Every Other Day Diet.”
Not so fast!
Over the course of a year, study participants who ate only from 8:00 a.m. to 4:00 p.m. did not lose significantly more weight than individuals who ate whenever they wanted, nor did they achieve significantly greater improvements in other obesity-related health measures like body mass index (BMI) or metabolic risk, reported lead author Deying Liu, MD, of Nanfang Hospital, Southern Medical University, Guangzhou, China, and colleagues.
“[Daily fasting] has gained popularity because it is a weight-loss strategy that is simple to follow, which may enhance adherence,” Dr. Liu and colleagues wrote in the New England Journal of Medicine. However, “the long-term efficacy and safety of time-restricted eating as a weight-loss strategy are still uncertain, and the long-term effects on weight loss of time-restricted eating as compared with daily calorie restriction alone have not been fully explored.”
To learn more, Dr. Liu and colleagues recruited 139 adult patients with BMIs between 28 and 45. Individuals with serious medical conditions, such as malignant tumors, diabetes, chronic kidney disease, and others were excluded. Other exclusion criteria included smoking, ongoing participation in a weight-loss program, GI surgery within the prior year, use of medications that impact energy balance and weight, and planned or current pregnancy.
All participants were advised to eat calorie-restricted diets, with ranges of 1,500-1,800 kcal per day for men and 1,200-1,500 kcal per day for women. To determine the added impact of fasting, participants were randomized in a 1:1 ratio into time-restricted (fasting) or non–time-restricted (nonfasting) groups, in which fasting participants ate only during an 8-hour window from 8:00 a.m. to 4:00 p.m., whereas nonfasting participants ate whenever they wanted.
At 6 months and 12 months, participants were re-evaluated for changes in weight, body fat, BMI, blood pressure, lean body mass, and metabolic risk factors, including glucose level, triglycerides, blood pressure, and others.
Caloric intake restriction seems to explain most of beneficial effects
At one-year follow-up, 118 participants (84.9%) remained in the study. Although members of the fasting group lost slightly more weight on average than those in the non-fasting group (mean, 8.0 kg vs. 6.3 kg), the difference between groups was not statistically significant (95% confidence interval, −4.0 to 0.4; P = .11).
Most of the other obesity-related health measures also trended toward favoring the fasting group, but again, none of these improvements was statistically significant. Weight circumference at 1 year, for example, decreased by a mean of 9.4 cm in the fasting group versus 8.8 cm in the nonfasting group, a net difference of 1.8 cm (95% CI, –4.0 to 0.5).
“We found that the two weight-loss regimens that we evaluated had similar success in patients with obesity, regardless of whether they reduced their calorie consumption through time-restricted eating or through calorie restriction alone,” Dr. Liu and colleagues concluded.
Principal investigator Huijie Zhang MD, PhD, professor, chief physician, and deputy director of the department of endocrinology and metabolism at Nafang Hospital, noted that their findings are “consistent with the findings in previous studies.”
“Our data suggest that caloric intake restriction explained most of the beneficial effects of a time-restricted eating regimen,” Dr. Zhang said.
Still, Dr. Zhang called time-restricted eating “a viable and sustainable approach for a person who wants to lose weight.”
More work is needed, Dr. Zhang said, to uncover the impact of fasting in “diverse groups,” including patients with chronic disease like diabetes and cardiovascular disease. Investigators should also conduct studies to compare outcomes between men and women, and evaluate the effects of other fasting durations.
Can trial be applied to a wider population?
According to Blandine Laferrère, MD, PhD, and Satchidananda Panda, PhD, of Columbia University Irving Medical Center, New York, and the Salk Institute for Biological Studies, La Jolla, Calif., respectively, “the results of the trial suggest that calorie restriction combined with time restriction, when delivered with intensive coaching and monitoring, is an approach that is as safe, sustainable, and effective for weight loss as calorie restriction alone.”
Yet Dr. Laferrère and Dr. Panda also expressed skepticism about broader implementation of a similar regime.
“The applicability of this trial to wider populations is debatable,” they wrote in an accompanying editorial. “The short time period for eating at baseline may be specific to the population studied, since investigators outside China have reported longer time windows. The rigorous coaching and monitoring by trial staff also leaves open the question of whether time-restricted eating is easier to adhere to than intentional calorie restriction. Such cost-benefit analyses are important for the assessment of the scalability of a lifestyle intervention.”
Duration is trial’s greatest strength
Kristina Varady, PhD, professor of nutrition in the department of kinesiology and nutrition at the University of Illinois at Chicago, said the “key strength” of the trial was its duration, at 12 months, making it the longest time-restricted eating trial to date”; however, she was critical of the design.
“Quite frankly, I’m surprised this study got into such a high-caliber medical journal,” Dr. Varady said in a written comment. “It doesn’t even have a control group! It goes to show how popular these diets are and how much people want to know about them.”
She also noted that “the study was flawed in that it didn’t really look at the effects of true time-restricted eating.” According to Dr. Varady, combining calorie restriction with time-restricted eating “kind of defeats the purpose” of a time-restricted diet.
“The main benefit of time-restricted eating is that you don’t need to count calories in order to lose weight,” Dr. Varady said, citing two of her own studies from 2018 and 2020. “Just by limiting the eating window to 8 hours per day, people naturally cut out 300-500 calories per day. That’s why people like [time-restricted eating] so much.”
Dr. Varady was also “very surprised” at the adherence data. At 1 year, approximately 85% of the patients were still following the protocol, a notably higher rate than most dietary intervention studies, which typically report adherence rates of 50-60%, she said. The high adherence rate was particularly unexpected because of the 8:00 a.m.–4:00 p.m. eating window, Dr. Varady added, since that meant skipping “the family/social meal every evening over 1 whole year!”
The study was funded by the National Key Research and Development Project and others. The study investigators reported no conflicts of interest. Dr. Varady disclosed author fees from the Hachette Book group for her book “The Every Other Day Diet.”
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Commentary: Emerging tick-borne pathogen has spread to state of Georgia
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Peripheral muscle fatigue limits post-COVID exercise
Peripheral muscle fatigue was the most common cause of exercise limitation in patients recovered from COVID-19 regardless of disease severity, in a study of nearly 300 individuals.
The source and magnitude of exercise intolerance in post–COVID-19 patients has not been well studied, said Mauricio Milani, MD, of Fitcordis Exercise Medicine Clinic, Brasilia, Brazil, in a presentation at the annual congress of the European Association of Preventive Cardiology.
To assess exercise intolerance, the researchers performed cardiopulmonary exercise testing (CPET) on 144 adults who had recovered from COVID-19 and 144 matched controls who had not had COVID-19. The average age of the participants was 43 years, and 57% were male. COVID-19 was defined as mild, moderate, or severe in 60%, 21%, and 19% of the cases, respectively.
Residual symptoms were present in 41% of cases. CPET was performed at roughly 14 weeks after disease onset.
Among the COVID-19 patients, most of the CPET limitations (92%) were caused by muscle fatigue; cardiovascular limitations were noted in 2%, and pulmonary limitations were noted in 6%.
Data from the post-COVID CPET showed differences in peak oxygen consumption, as well as the first and second ventilatory thresholds (VT1 and VT2) between COVID-19 patients and controls, and with lower values related to higher illness severities, Dr. Milani said. Heart rate also varied according to illness severity, with lower values significantly related to higher illness severities and significant differences between COVID patients and controls.
A total of 42 individuals with COVID-19 had previous CPET data for comparison (27 with mild disease and 15 with moderate or severe disease), Dr. Milani said. In the subgroup with mild disease, the only significant difference in CPET results before and after COVID-19 was peak speed. In the moderate/severe group, the researchers observed higher reductions in peak speed and also reductions in oxygen consumption at peak and thresholds.
However, peak oxygen flows were not different before and after COVID-19 in either the mild or moderate/severe subgroups, Dr. Milani said.
The study findings were limited in part by the relatively small study population; however, the results indicate that peripheral muscle fatigue is the primary etiology in exercise limitation in post–COVID-19 patients.
“Our data suggest that treatment should emphasize comprehensive rehabilitation programs, including aerobic and muscle strengthening components,” Dr. Milani concluded.
COVID challenges remain unclear
“After COVID, patients often display a postviral syndrome with a wide range of symptoms,” Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., in an interview said. “These conditions frequently lead to a sense of tiredness and weakness, pain, difficulty concentrating, and headaches that linger after the viral infection has cleared,” and these symptoms may continue for weeks.
However, this scenario is not unique to COVID-19: “This study confirms the importance of muscle fatigue in recovery,” said Dr. Martinez. “Recovery from viral illness requires hydration, sleep and slow progression return to exercise.” Consequently, Dr. Martinez said he was not surprised by the current study findings.
The take-home message for clinicians is to be aware that COVID-19 can have postviral syndrome, as is common after other infections, Dr. Martinez noted. The findings provide a starting point for discussing concerns with patients and explaining that a slow return to normal with usual care is expected. “Time to recovery will vary by individual,” he said. “Additional research is needed to identify which specific therapies are most important to help reduce time to recovery, and what new therapies could be developed to help facilitate muscle fatigue recovery and reduce time needed to recover.”
The study was supported by CAPES and CNPq. Dr. Milani had no financial conflicts to disclose. Dr. Martinez had no financial conflicts to disclose.
Peripheral muscle fatigue was the most common cause of exercise limitation in patients recovered from COVID-19 regardless of disease severity, in a study of nearly 300 individuals.
The source and magnitude of exercise intolerance in post–COVID-19 patients has not been well studied, said Mauricio Milani, MD, of Fitcordis Exercise Medicine Clinic, Brasilia, Brazil, in a presentation at the annual congress of the European Association of Preventive Cardiology.
To assess exercise intolerance, the researchers performed cardiopulmonary exercise testing (CPET) on 144 adults who had recovered from COVID-19 and 144 matched controls who had not had COVID-19. The average age of the participants was 43 years, and 57% were male. COVID-19 was defined as mild, moderate, or severe in 60%, 21%, and 19% of the cases, respectively.
Residual symptoms were present in 41% of cases. CPET was performed at roughly 14 weeks after disease onset.
Among the COVID-19 patients, most of the CPET limitations (92%) were caused by muscle fatigue; cardiovascular limitations were noted in 2%, and pulmonary limitations were noted in 6%.
Data from the post-COVID CPET showed differences in peak oxygen consumption, as well as the first and second ventilatory thresholds (VT1 and VT2) between COVID-19 patients and controls, and with lower values related to higher illness severities, Dr. Milani said. Heart rate also varied according to illness severity, with lower values significantly related to higher illness severities and significant differences between COVID patients and controls.
A total of 42 individuals with COVID-19 had previous CPET data for comparison (27 with mild disease and 15 with moderate or severe disease), Dr. Milani said. In the subgroup with mild disease, the only significant difference in CPET results before and after COVID-19 was peak speed. In the moderate/severe group, the researchers observed higher reductions in peak speed and also reductions in oxygen consumption at peak and thresholds.
However, peak oxygen flows were not different before and after COVID-19 in either the mild or moderate/severe subgroups, Dr. Milani said.
The study findings were limited in part by the relatively small study population; however, the results indicate that peripheral muscle fatigue is the primary etiology in exercise limitation in post–COVID-19 patients.
“Our data suggest that treatment should emphasize comprehensive rehabilitation programs, including aerobic and muscle strengthening components,” Dr. Milani concluded.
COVID challenges remain unclear
“After COVID, patients often display a postviral syndrome with a wide range of symptoms,” Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., in an interview said. “These conditions frequently lead to a sense of tiredness and weakness, pain, difficulty concentrating, and headaches that linger after the viral infection has cleared,” and these symptoms may continue for weeks.
However, this scenario is not unique to COVID-19: “This study confirms the importance of muscle fatigue in recovery,” said Dr. Martinez. “Recovery from viral illness requires hydration, sleep and slow progression return to exercise.” Consequently, Dr. Martinez said he was not surprised by the current study findings.
The take-home message for clinicians is to be aware that COVID-19 can have postviral syndrome, as is common after other infections, Dr. Martinez noted. The findings provide a starting point for discussing concerns with patients and explaining that a slow return to normal with usual care is expected. “Time to recovery will vary by individual,” he said. “Additional research is needed to identify which specific therapies are most important to help reduce time to recovery, and what new therapies could be developed to help facilitate muscle fatigue recovery and reduce time needed to recover.”
The study was supported by CAPES and CNPq. Dr. Milani had no financial conflicts to disclose. Dr. Martinez had no financial conflicts to disclose.
Peripheral muscle fatigue was the most common cause of exercise limitation in patients recovered from COVID-19 regardless of disease severity, in a study of nearly 300 individuals.
The source and magnitude of exercise intolerance in post–COVID-19 patients has not been well studied, said Mauricio Milani, MD, of Fitcordis Exercise Medicine Clinic, Brasilia, Brazil, in a presentation at the annual congress of the European Association of Preventive Cardiology.
To assess exercise intolerance, the researchers performed cardiopulmonary exercise testing (CPET) on 144 adults who had recovered from COVID-19 and 144 matched controls who had not had COVID-19. The average age of the participants was 43 years, and 57% were male. COVID-19 was defined as mild, moderate, or severe in 60%, 21%, and 19% of the cases, respectively.
Residual symptoms were present in 41% of cases. CPET was performed at roughly 14 weeks after disease onset.
Among the COVID-19 patients, most of the CPET limitations (92%) were caused by muscle fatigue; cardiovascular limitations were noted in 2%, and pulmonary limitations were noted in 6%.
Data from the post-COVID CPET showed differences in peak oxygen consumption, as well as the first and second ventilatory thresholds (VT1 and VT2) between COVID-19 patients and controls, and with lower values related to higher illness severities, Dr. Milani said. Heart rate also varied according to illness severity, with lower values significantly related to higher illness severities and significant differences between COVID patients and controls.
A total of 42 individuals with COVID-19 had previous CPET data for comparison (27 with mild disease and 15 with moderate or severe disease), Dr. Milani said. In the subgroup with mild disease, the only significant difference in CPET results before and after COVID-19 was peak speed. In the moderate/severe group, the researchers observed higher reductions in peak speed and also reductions in oxygen consumption at peak and thresholds.
However, peak oxygen flows were not different before and after COVID-19 in either the mild or moderate/severe subgroups, Dr. Milani said.
The study findings were limited in part by the relatively small study population; however, the results indicate that peripheral muscle fatigue is the primary etiology in exercise limitation in post–COVID-19 patients.
“Our data suggest that treatment should emphasize comprehensive rehabilitation programs, including aerobic and muscle strengthening components,” Dr. Milani concluded.
COVID challenges remain unclear
“After COVID, patients often display a postviral syndrome with a wide range of symptoms,” Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., in an interview said. “These conditions frequently lead to a sense of tiredness and weakness, pain, difficulty concentrating, and headaches that linger after the viral infection has cleared,” and these symptoms may continue for weeks.
However, this scenario is not unique to COVID-19: “This study confirms the importance of muscle fatigue in recovery,” said Dr. Martinez. “Recovery from viral illness requires hydration, sleep and slow progression return to exercise.” Consequently, Dr. Martinez said he was not surprised by the current study findings.
The take-home message for clinicians is to be aware that COVID-19 can have postviral syndrome, as is common after other infections, Dr. Martinez noted. The findings provide a starting point for discussing concerns with patients and explaining that a slow return to normal with usual care is expected. “Time to recovery will vary by individual,” he said. “Additional research is needed to identify which specific therapies are most important to help reduce time to recovery, and what new therapies could be developed to help facilitate muscle fatigue recovery and reduce time needed to recover.”
The study was supported by CAPES and CNPq. Dr. Milani had no financial conflicts to disclose. Dr. Martinez had no financial conflicts to disclose.
FROM ESC PREVENTIVE CARDIOLOGY 2022
FDA warns companies selling OTC skin lighteners
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
The as the active ingredient, and don’t meet the requirements to be sold legally over the counter. The letters were dated April 13.
The 12 products with hydroquinone are “unapproved drugs and are not generally recognized as safe and effective” (abbreviated as GRASE), the FDA said.
Among the side effects associated with hydroquinone products reported to the FDA are skin rashes, facial swelling, and skin discoloration or ochronosis. The discoloration can be permanent, the FDA said. The lighteners are marketed for use on age or dark spots on the skin associated with melasma.
Tri-Luma, a prescription product for the treatment of moderate to severe melasma of the face, is the only FDA-approved drug containing hydroquinone, according to the FDA. It contains 4% hydroquinone and two other ingredients. It is meant to be used under the supervision of a health care professional. Tri-Luma is indicated for up to 8 weeks of treatment for moderate to severe melasma of the face. The OTC products contain up to 2%. (Generic versions of 4% hydroquinone are available by prescription, dermatologists said.)
“Hydroquinone is a very effective medication, and that’s exactly what it is, a medication,” said Lily Talakoub, MD, a dermatologist in McLean, Va., who supports the FDA action. “It’s very effective and very safe to use in the right hands, but when it is overused or used in the wrong situation, it can cause problems.” Those problems often occur, she said, when there is no health care professional overseeing the use of the OTC products, and when people use them over the long term.
The FDA action to ban the OTC products is “very appropriate,” said dermatologist Pooja Sodha, MD, assistant professor and director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington. “We know patients pick this up [an OTC product] and use it without physician oversight.” When patients use the products longer than is appropriate, which is also common, it can worsen the initial skin issue, she said.
The action follows reforms finalized under the CARES Act (Coronavirus Aid, Relief and Economic Security Act), which included not only COVID-19 response efforts but also updated the method in which certain OTC drugs are regulated. Manufacturers of the skin lightening products that don’t have FDA approval had been told to remove the products from the market by September 2020.
The recent letters were sent to a dozen companies still marketing their products without an FDA new drug approval. The agency asked the companies to take prompt action and respond with 15 days, stating what they have done to correct the violations.
The 12 companies are AMBI Enterprises, Clinical Formula, Elements Brands Inc., Genomma Lab USA, Intilight/Dr Thomas Balshi, M&M Beauty and Wellness, Neoteric Cosmetics/Scott’s Liquid Gold, Skin Authority, Skin Pro, Skin PS Brands, True Earth Health Products, and Ultimark Products.
Health care professionals and consumers can report adverse reactions associated with these products to the FDA’s MedWatch Adverse Event Reporting program.
A version of this article first appeared on Medscape.com.
Hormones after cancer: Are they safe?
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
Deprived of sleep, many turn to melatonin despite risks
Can’t sleep? When slumber doesn’t come naturally, some are turning to melatonin, an over-the-counter sleep aid that often is mistaken for a supplement. This powerful hormone plays an important role in human biology, and specialists are questioning whether increasing levels could be doing more harm than good.
And while the health advisory checking the evidence is underway, the academy is recommending that melatonin not be used for insomnia in adults or children.
But what is insomnia, and how is it different from a few bad nights of sleep? Insomnia disturbs sleep at least three times a week for more than 3 months, often causing people to feel tired during the day as well.
Production of melatonin (dubbed the “vampire hormone”) begins at night, when it starts getting dark outside. Melatonin release is scheduled by the small but mighty pineal gland at the back of the head. Melatonin signals to the body that it’s time to sleep. And as the sun rises and light shines, melatonin levels decline again to help the body wake.
Sometimes packaged in gummy bear fruit flavors, melatonin can have an alluring appeal to sleep-deprived parents looking for relief for themselves and their children.
Muhammad Adeel Rishi, MD, vice chair of the public safety committee for the American Academy of Sleep Medicine, said he has a doctor colleague who started taking melatonin to help him during the pandemic when he was having trouble falling asleep at night. His doctor friend started giving the hormone to his own children, who were also having sleep issues.
But Dr. Rishi said there are important reasons to not use melatonin for insomnia until more information is available.
Melatonin affects sleep, but this hormone also influences other functions in the body. “It has an impact on body temperature, blood sugar, and even the tone of blood vessels,” Dr. Rishi said.
And because melatonin is available over the counter in the United States, it hasn’t been approved as a medicine under the Food and Drug Administration. A previous study of melatonin products, for instance, flagged problems with inconsistent doses, which make it hard for people to know exactly how much they are getting and prompted calls for more FDA oversight.
Imprecise doses
While melatonin doses typically range from 1 to 5 milligrams, bottles examined have been off target with much more or less hormone in the product than listed on the label.
Researchers from the University of Guelph (Ont.), tested 30 commercially available formulas and found the melatonin content varied from the ingredients labeled on the bottles by more than 10%. In addition to melatonin, the researchers found other substances in the bottles too: In about a quarter of the products, they also identified serotonin.
Impurities
While melatonin plays a role in setting the body’s biological clock and the sleep and wake cycle, serotonin is also at work. Occurring naturally in our bodies, serotonin is involved in mood and helps with deep REM sleep. But adding serotonin in unknown amounts could be unhealthy.
Dr. Rishi said it can be dangerous to use a product as a medication when doses can be so off and there are unknown byproducts in it.
Serotonin can influence the heart, blood vessels, and brain, so it’s not something Dr. Rishi wants to see people taking without paying attention. People taking medication for mood disorders could be especially affected by the serotonin in their sleep aid, he warns.
For anyone taking melatonin, Dr. Rishi recommended they check the bottle to see whether they are using a product with a USP-verified check mark, which indicates that the product meets the standards of the U.S. Pharmacopeia Convention.
The risk of impurities is a good reason for kids to not be given the hormone, but another worry is whether melatonin interferes with puberty in children – which is also a question researchers at the Children’s Hospital of Eastern Ontario in Ottawa are asking.
Disrupting puberty
While short-term melatonin use is considered safe, the researchers reported, concerns that long-term use might delay children’s sexual maturation require more study. One theory is that nightly melatonin use might interrupt the decline of natural hormone levels and interfere with the start of puberty.
Researchers from the Children’s Hospital of Michigan in Detroit also reported an uptick in accidental ingestion of melatonin in children. Kids got their hands on melatonin and swallowed too many capsules more often than other pill-related mishaps during the pandemic.
Dr. Rishi said more research is needed to assess the safe use of melatonin in children. He points out that the hormone can treat circadian rhythm disorders in adults.
While specialists weigh the benefits and risks of melatonin use and where it is safest to try, Dr. Rishi said the hormone does have a role in medicine.
Melatonin will probably need to be regulated by the FDA as a medication – especially for children – Dr. Rishi pointed out. And what place, if any, it will have for managing chronic insomnia is “a big question mark.”
Results of the investigation by the American Academy of Sleep Medicine will be published on its sleepeducation.org website in a few months.
A version of this article first appeared on WebMD.com.
Can’t sleep? When slumber doesn’t come naturally, some are turning to melatonin, an over-the-counter sleep aid that often is mistaken for a supplement. This powerful hormone plays an important role in human biology, and specialists are questioning whether increasing levels could be doing more harm than good.
And while the health advisory checking the evidence is underway, the academy is recommending that melatonin not be used for insomnia in adults or children.
But what is insomnia, and how is it different from a few bad nights of sleep? Insomnia disturbs sleep at least three times a week for more than 3 months, often causing people to feel tired during the day as well.
Production of melatonin (dubbed the “vampire hormone”) begins at night, when it starts getting dark outside. Melatonin release is scheduled by the small but mighty pineal gland at the back of the head. Melatonin signals to the body that it’s time to sleep. And as the sun rises and light shines, melatonin levels decline again to help the body wake.
Sometimes packaged in gummy bear fruit flavors, melatonin can have an alluring appeal to sleep-deprived parents looking for relief for themselves and their children.
Muhammad Adeel Rishi, MD, vice chair of the public safety committee for the American Academy of Sleep Medicine, said he has a doctor colleague who started taking melatonin to help him during the pandemic when he was having trouble falling asleep at night. His doctor friend started giving the hormone to his own children, who were also having sleep issues.
But Dr. Rishi said there are important reasons to not use melatonin for insomnia until more information is available.
Melatonin affects sleep, but this hormone also influences other functions in the body. “It has an impact on body temperature, blood sugar, and even the tone of blood vessels,” Dr. Rishi said.
And because melatonin is available over the counter in the United States, it hasn’t been approved as a medicine under the Food and Drug Administration. A previous study of melatonin products, for instance, flagged problems with inconsistent doses, which make it hard for people to know exactly how much they are getting and prompted calls for more FDA oversight.
Imprecise doses
While melatonin doses typically range from 1 to 5 milligrams, bottles examined have been off target with much more or less hormone in the product than listed on the label.
Researchers from the University of Guelph (Ont.), tested 30 commercially available formulas and found the melatonin content varied from the ingredients labeled on the bottles by more than 10%. In addition to melatonin, the researchers found other substances in the bottles too: In about a quarter of the products, they also identified serotonin.
Impurities
While melatonin plays a role in setting the body’s biological clock and the sleep and wake cycle, serotonin is also at work. Occurring naturally in our bodies, serotonin is involved in mood and helps with deep REM sleep. But adding serotonin in unknown amounts could be unhealthy.
Dr. Rishi said it can be dangerous to use a product as a medication when doses can be so off and there are unknown byproducts in it.
Serotonin can influence the heart, blood vessels, and brain, so it’s not something Dr. Rishi wants to see people taking without paying attention. People taking medication for mood disorders could be especially affected by the serotonin in their sleep aid, he warns.
For anyone taking melatonin, Dr. Rishi recommended they check the bottle to see whether they are using a product with a USP-verified check mark, which indicates that the product meets the standards of the U.S. Pharmacopeia Convention.
The risk of impurities is a good reason for kids to not be given the hormone, but another worry is whether melatonin interferes with puberty in children – which is also a question researchers at the Children’s Hospital of Eastern Ontario in Ottawa are asking.
Disrupting puberty
While short-term melatonin use is considered safe, the researchers reported, concerns that long-term use might delay children’s sexual maturation require more study. One theory is that nightly melatonin use might interrupt the decline of natural hormone levels and interfere with the start of puberty.
Researchers from the Children’s Hospital of Michigan in Detroit also reported an uptick in accidental ingestion of melatonin in children. Kids got their hands on melatonin and swallowed too many capsules more often than other pill-related mishaps during the pandemic.
Dr. Rishi said more research is needed to assess the safe use of melatonin in children. He points out that the hormone can treat circadian rhythm disorders in adults.
While specialists weigh the benefits and risks of melatonin use and where it is safest to try, Dr. Rishi said the hormone does have a role in medicine.
Melatonin will probably need to be regulated by the FDA as a medication – especially for children – Dr. Rishi pointed out. And what place, if any, it will have for managing chronic insomnia is “a big question mark.”
Results of the investigation by the American Academy of Sleep Medicine will be published on its sleepeducation.org website in a few months.
A version of this article first appeared on WebMD.com.
Can’t sleep? When slumber doesn’t come naturally, some are turning to melatonin, an over-the-counter sleep aid that often is mistaken for a supplement. This powerful hormone plays an important role in human biology, and specialists are questioning whether increasing levels could be doing more harm than good.
And while the health advisory checking the evidence is underway, the academy is recommending that melatonin not be used for insomnia in adults or children.
But what is insomnia, and how is it different from a few bad nights of sleep? Insomnia disturbs sleep at least three times a week for more than 3 months, often causing people to feel tired during the day as well.
Production of melatonin (dubbed the “vampire hormone”) begins at night, when it starts getting dark outside. Melatonin release is scheduled by the small but mighty pineal gland at the back of the head. Melatonin signals to the body that it’s time to sleep. And as the sun rises and light shines, melatonin levels decline again to help the body wake.
Sometimes packaged in gummy bear fruit flavors, melatonin can have an alluring appeal to sleep-deprived parents looking for relief for themselves and their children.
Muhammad Adeel Rishi, MD, vice chair of the public safety committee for the American Academy of Sleep Medicine, said he has a doctor colleague who started taking melatonin to help him during the pandemic when he was having trouble falling asleep at night. His doctor friend started giving the hormone to his own children, who were also having sleep issues.
But Dr. Rishi said there are important reasons to not use melatonin for insomnia until more information is available.
Melatonin affects sleep, but this hormone also influences other functions in the body. “It has an impact on body temperature, blood sugar, and even the tone of blood vessels,” Dr. Rishi said.
And because melatonin is available over the counter in the United States, it hasn’t been approved as a medicine under the Food and Drug Administration. A previous study of melatonin products, for instance, flagged problems with inconsistent doses, which make it hard for people to know exactly how much they are getting and prompted calls for more FDA oversight.
Imprecise doses
While melatonin doses typically range from 1 to 5 milligrams, bottles examined have been off target with much more or less hormone in the product than listed on the label.
Researchers from the University of Guelph (Ont.), tested 30 commercially available formulas and found the melatonin content varied from the ingredients labeled on the bottles by more than 10%. In addition to melatonin, the researchers found other substances in the bottles too: In about a quarter of the products, they also identified serotonin.
Impurities
While melatonin plays a role in setting the body’s biological clock and the sleep and wake cycle, serotonin is also at work. Occurring naturally in our bodies, serotonin is involved in mood and helps with deep REM sleep. But adding serotonin in unknown amounts could be unhealthy.
Dr. Rishi said it can be dangerous to use a product as a medication when doses can be so off and there are unknown byproducts in it.
Serotonin can influence the heart, blood vessels, and brain, so it’s not something Dr. Rishi wants to see people taking without paying attention. People taking medication for mood disorders could be especially affected by the serotonin in their sleep aid, he warns.
For anyone taking melatonin, Dr. Rishi recommended they check the bottle to see whether they are using a product with a USP-verified check mark, which indicates that the product meets the standards of the U.S. Pharmacopeia Convention.
The risk of impurities is a good reason for kids to not be given the hormone, but another worry is whether melatonin interferes with puberty in children – which is also a question researchers at the Children’s Hospital of Eastern Ontario in Ottawa are asking.
Disrupting puberty
While short-term melatonin use is considered safe, the researchers reported, concerns that long-term use might delay children’s sexual maturation require more study. One theory is that nightly melatonin use might interrupt the decline of natural hormone levels and interfere with the start of puberty.
Researchers from the Children’s Hospital of Michigan in Detroit also reported an uptick in accidental ingestion of melatonin in children. Kids got their hands on melatonin and swallowed too many capsules more often than other pill-related mishaps during the pandemic.
Dr. Rishi said more research is needed to assess the safe use of melatonin in children. He points out that the hormone can treat circadian rhythm disorders in adults.
While specialists weigh the benefits and risks of melatonin use and where it is safest to try, Dr. Rishi said the hormone does have a role in medicine.
Melatonin will probably need to be regulated by the FDA as a medication – especially for children – Dr. Rishi pointed out. And what place, if any, it will have for managing chronic insomnia is “a big question mark.”
Results of the investigation by the American Academy of Sleep Medicine will be published on its sleepeducation.org website in a few months.
A version of this article first appeared on WebMD.com.
Fetuses suffer the effects of poverty in the womb
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
Poverty is known to be associated with poor health outcomes throughout life. Now, new research has shown that, from as early as the second trimester of pregnancy, fetuses are already feeling the effects of poverty.
“There is a well-recognized health inequality where quality and duration of life are lower among the most poor. This divide is present both within and between countries,” said Steve Turner, who led the study.
Given the association of poverty and low birth weight, the authors of the new multi-national study, published in the Journal of Epidemiology and Community Health, hypothesized that “individuals from highest household income compared to those with lowest household income will have increased fetal size in the second and third trimester and birth.”
For their study, researchers from the University of Aberdeen gathered details of ante-natal and birth size – second and third trimester fetal ultrasound measurements of estimated fetal weight, biparietal diameter, and femur length, as well as birth measurements of weight, occipitofrontal circumference, and crown heel length – from eight cohorts that included 21,714 individuals from nations including Scotland, England, Saudi Arabia, the U.S., Netherlands, Spain, Norway, Sweden, and France.
They then related these to household income, taking into account other factors, including mother’s age, height, number of other children, and smoking, analyzing the data using cross-sectional two-stage individual patient data analyses and a longitudinal one-stage individual patient data analysis.
Household income closely related to birth size
The authors found that higher household income was associated with larger fetal head size and weight but not length, from the second half of pregnancy, compared with lowest household income. They said that their results argue for “a relationship where household income is closely related to birth size.”
The results showed that, across the countries studied, babies were smaller at birth if they came from a lower income household, and this discrepancy in size was already apparent at 20 weeks gestation.
“This is the first time that size differences have been found at such an early stage of development,” the authors said, “and also the first time it has been compared across continents.”
Professor Turner pointed out that “what this study shows is that the inequality, as seen by reduced size in fetal life, is present long before birth, and this poverty gap widens between twenty weeks gestation and birth.”
He added: “Basically, regardless of whether you live in Saudi, the U.S., or Europe, and accounting for things that might affect fetal growth, if your parents are poor, you will be smaller before birth and at birth compared to if your parents were not poor.”
Increase engagement with pregnant mothers living in poverty
He emphasized how this was problematic, as small size before and after birth puts an individual at “increased risk for many serious illnesses in later life.”
The authors hope that this study will encourage health care providers to recognize the health risks associated with lower income for mothers and their unborn children and to provide more support and guidance to mitigate the risks.
They said, “interventions aimed at softening the impact of poverty on pregnant mothers could reduce incidence of small for gestational age and the associated burden of excessive morbidity and mortality throughout the life course.”
Professor Turner described how the mechanisms that drive this inequity may be explained by pregnant mothers from poor households having difficulty in accessing or engaging with antenatal care.
“We would like to see health care providers around the world strive to increase engagement with pregnant mothers living in poverty,” he said. “This engagement will reward all of society by putting unborn children on a trajectory to longer and healthier lives.”
A version of this article first appeared on Medscape UK.
FROM THE JOURNAL OF EPIDEMIOLOGY AND COMMUNITY HEALTH