User login
Meta-analysis confirms neuroprotective benefit of metformin
Key takeaways
, according to a systematic review and meta-analysis of longitudinal data.
However, the heterogeneity between the available studies and the potential heterogeneity of diagnostic criteria may mean that validation studies are needed.
Why is this important?
Data suggest that metformin, the most commonly prescribed antidiabetic drug, may be neuroprotective, while diabetes is associated with an excess risk of neurodegenerative disease. Results of studies conducted specifically to investigate the benefit of the antidiabetic drug on cognitive prognosis have been unclear. A meta-analysis was published in 2020, but it included cross-sectional and case-control studies. Given the long observation period needed to measure such an outcome, only cohort studies conducted over several years can provide reliable results. This new meta-analysis attempts to circumvent this limitation.
Methods
The meta-analysis was conducted using studies published up to March 2021 that met the inclusion criteria (population-based cohort studies published in English in which the administration of metformin and associated risk of exposure were reported).
Main results
Twelve studies were included in this analysis, of which eight were retrospective and 11 were considered to be of good methodologic quality. In total, 194,792 patients were included.
Pooled data showed that the relative risk associated with onset of neurodegenerative disease was 0.77 (95% CI, 0.67-0.88) for patients with diabetes taking metformin versus those not taking metformin. However, heterogeneity between studies was high (I2; 78.8%; P < .001).
The effect was greater with longer metformin use, with an RR of 0.29 (95% CI, 0.13-0.44) for those who took metformin for 4 years or more. Similarly, the studies conducted in Asian countries versus other locations suggested an added benefit for this population (RR, 0.69; 95% CI, 0.64-0.74).
Sensitivity analyses confirmed these results, and subtype analyses showed no difference according to the nature of the neurodegenerative disease.
A version of this article first appeared on Univadis.
Key takeaways
, according to a systematic review and meta-analysis of longitudinal data.
However, the heterogeneity between the available studies and the potential heterogeneity of diagnostic criteria may mean that validation studies are needed.
Why is this important?
Data suggest that metformin, the most commonly prescribed antidiabetic drug, may be neuroprotective, while diabetes is associated with an excess risk of neurodegenerative disease. Results of studies conducted specifically to investigate the benefit of the antidiabetic drug on cognitive prognosis have been unclear. A meta-analysis was published in 2020, but it included cross-sectional and case-control studies. Given the long observation period needed to measure such an outcome, only cohort studies conducted over several years can provide reliable results. This new meta-analysis attempts to circumvent this limitation.
Methods
The meta-analysis was conducted using studies published up to March 2021 that met the inclusion criteria (population-based cohort studies published in English in which the administration of metformin and associated risk of exposure were reported).
Main results
Twelve studies were included in this analysis, of which eight were retrospective and 11 were considered to be of good methodologic quality. In total, 194,792 patients were included.
Pooled data showed that the relative risk associated with onset of neurodegenerative disease was 0.77 (95% CI, 0.67-0.88) for patients with diabetes taking metformin versus those not taking metformin. However, heterogeneity between studies was high (I2; 78.8%; P < .001).
The effect was greater with longer metformin use, with an RR of 0.29 (95% CI, 0.13-0.44) for those who took metformin for 4 years or more. Similarly, the studies conducted in Asian countries versus other locations suggested an added benefit for this population (RR, 0.69; 95% CI, 0.64-0.74).
Sensitivity analyses confirmed these results, and subtype analyses showed no difference according to the nature of the neurodegenerative disease.
A version of this article first appeared on Univadis.
Key takeaways
, according to a systematic review and meta-analysis of longitudinal data.
However, the heterogeneity between the available studies and the potential heterogeneity of diagnostic criteria may mean that validation studies are needed.
Why is this important?
Data suggest that metformin, the most commonly prescribed antidiabetic drug, may be neuroprotective, while diabetes is associated with an excess risk of neurodegenerative disease. Results of studies conducted specifically to investigate the benefit of the antidiabetic drug on cognitive prognosis have been unclear. A meta-analysis was published in 2020, but it included cross-sectional and case-control studies. Given the long observation period needed to measure such an outcome, only cohort studies conducted over several years can provide reliable results. This new meta-analysis attempts to circumvent this limitation.
Methods
The meta-analysis was conducted using studies published up to March 2021 that met the inclusion criteria (population-based cohort studies published in English in which the administration of metformin and associated risk of exposure were reported).
Main results
Twelve studies were included in this analysis, of which eight were retrospective and 11 were considered to be of good methodologic quality. In total, 194,792 patients were included.
Pooled data showed that the relative risk associated with onset of neurodegenerative disease was 0.77 (95% CI, 0.67-0.88) for patients with diabetes taking metformin versus those not taking metformin. However, heterogeneity between studies was high (I2; 78.8%; P < .001).
The effect was greater with longer metformin use, with an RR of 0.29 (95% CI, 0.13-0.44) for those who took metformin for 4 years or more. Similarly, the studies conducted in Asian countries versus other locations suggested an added benefit for this population (RR, 0.69; 95% CI, 0.64-0.74).
Sensitivity analyses confirmed these results, and subtype analyses showed no difference according to the nature of the neurodegenerative disease.
A version of this article first appeared on Univadis.
Blistering Lesions in a Newborn
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
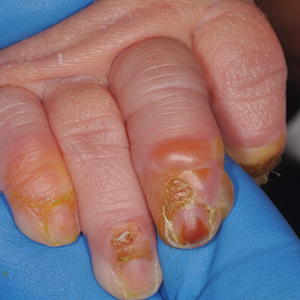
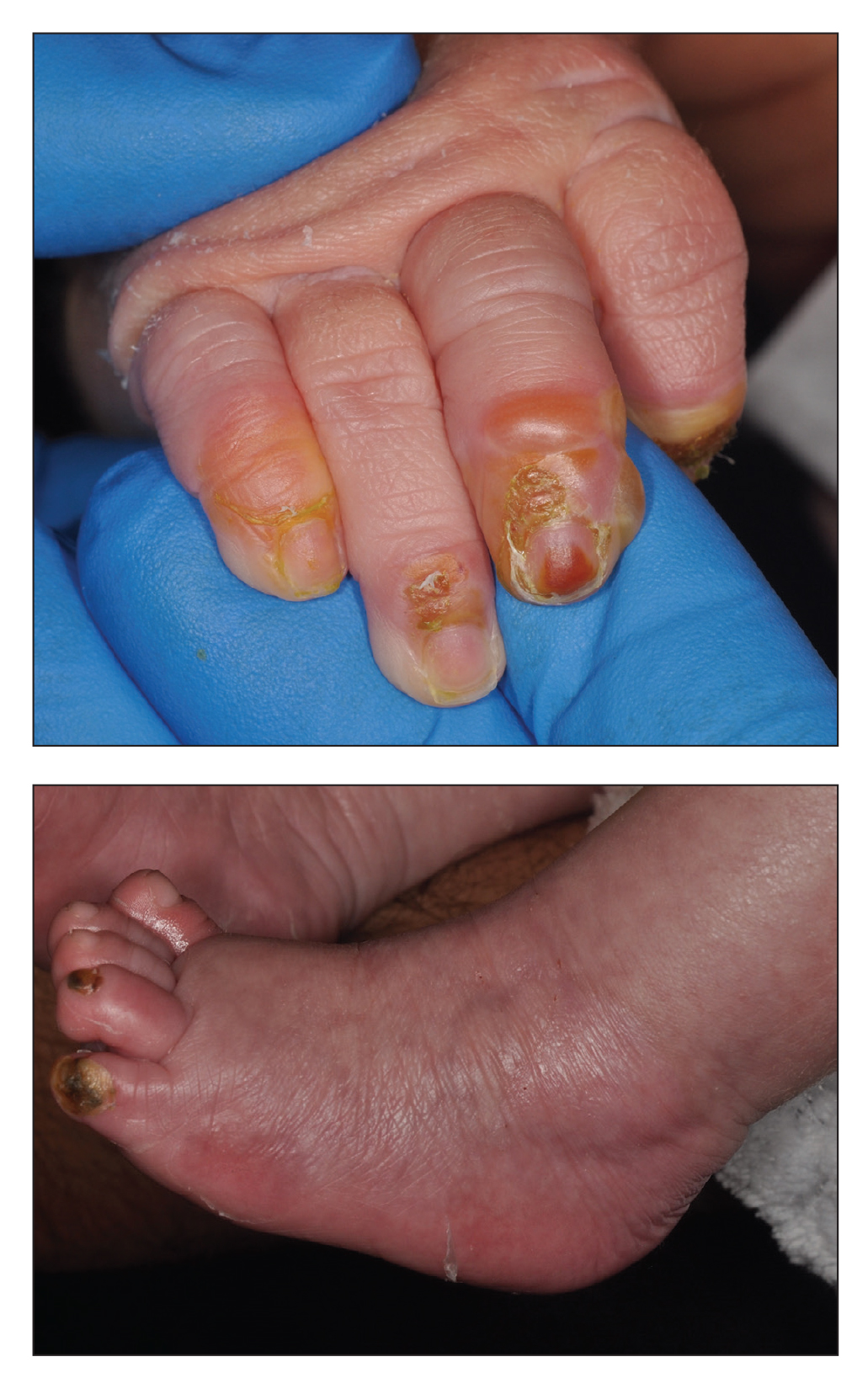
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.
Age and ferritin levels may predict MIS-C severity
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
Bariatric surgery cuts cardiovascular events, even in seniors
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Bariatric surgery can reduce the risk of long-term cardiovascular outcomes in older Medicare beneficiaries with obesity, a large new observational study in which a third of the patients were over age 65 years suggests.
Overall, patients who underwent bariatric surgery had 37% lower all-cause mortality and were significantly less likely to have admissions for new-onset heart failure (64% risk reduction), myocardial infarction (37% risk reduction), and ischemic stroke (29% risk reduction), compared with similar patients who received more conservative treatment, after a median of 4 years of follow-up, report Amgad Mentias, MD, MS, a clinical cardiologist at the Cleveland Clinic Foundation, Ohio, and colleagues.
The results were published in the Journal of the American College of Cardiology.
Previous studies on bariatric surgery outcomes have primarily focused on individuals from select health care networks or medical facilities with restricted coverage in the United States or on patients with diabetes, noted Tiffany M. Powell-Wiley, MD, MPH, of the National Institutes of Health’s National Heart, Lung, and Blood Institute, Bethesda, Maryland, and colleagues in an accompanying editorial.
Moreover, other long-term and observational studies have shown that bariatric surgery can decrease the risk of myocardial infarction, death, and stroke in young and middle-aged patients with obesity, but the evidence is less clear for older patients and those without diabetes, noted Dr. Mentias in a phone interview.
“To date, this is one of the first studies to support bariatric surgery for CVD risk reduction in patients older than 65 years, a population at highest risk for developing heart failure,” the editorial points out.
“We should consider referring patients who qualify for bariatric surgery based on BMI; it really should be considered as a treatment option for patients with class 3 obesity, especially with a body mass index over 40 kg/m2,” Dr. Powell-Wiley told this news organization.
“We know that patients are generally under-referred for bariatric surgery, and this highlights the need to refer patients for bariatric surgery,” she added.
“There should be discussion about expanding insurance coverage to include bariatric surgery for eligible patients,” Dr. Mentias added.
Contemporary cohort of patients
“A lot of the studies showed long-term outcomes outside of the U.S., specifically in Europe,” Dr. Mentias added.
The aim of this study was to evaluate the long-term association between bariatric surgery and risk of adverse cardiovascular outcomes in a contemporary large cohort from the United States.
Older patients (> 65 years) and those without diabetes were looked at as specific subgroups.
The researchers assessed 189,770 patients. There were 94,885 matched patients in each cohort. Mean age was 62.33 years. Female patients comprised 70% of the cohort. The study group had an average BMI of 44.7 kg/m2.
The study cohort was matched 1:1. Participants were either part of a control group with obesity or a group of Medicare beneficiaries who had bariatric surgery between 2013 and 2019. Sex, propensity score matching on 87 clinical variables, age, and BMI were used to match patients.
Myocardial infarction, new-onset heart failure, ischemic stroke, and all-cause mortality were all study outcomes. As a sensitivity analysis, the study team conducted an instrumental variable assessment.
More specifically, the findings showed that bariatric surgery was linked with the following after a median follow-up of 4.0 years:
- Myocardial infarction (hazard ratio, 0.63; 95% confidence interval, 0.59-0.68)
- Stroke (HR, 0.71; 95% CI, 0.65-0.79)
- New-onset heart failure (HR, 0.46; 95% CI, 0.44-0.49)
- Reduced risk of death (9.2 vs. 14.7 per 1000 person-years; HR, 0.63; 95% CI, 0.60-0.66)
Findings for those over the age of 65 were similar – lower risks of all-cause mortality (HR, 0.64), new-onset heart failure (HR, 0.52), myocardial infarction (HR, 0.70), and stroke (HR, 0.76; all P < .001). Similar findings were shown in subgroup analyses in men and women and in patients with and without diabetes.
The study cohort primarily consisted of Medicare patients, which limits the generalizability of the data. Lack of data on medications taken for cardiovascular and weight loss purposes and potential coding errors because the information was gathered from an administrative database were all limitations of the study, the researchers note.
An additional limitation was that residual unmeasured confounders, particularly patient-focused physical, social, and mental support factors, could play a role in whether a patient opted to have bariatric surgery, the study authors note.
“Additional studies are needed to compare cardiovascular outcomes after bariatric surgery with weight loss medications like glucagon-like peptide-1 (GLP-1) analogues,” the researchers add.
This study was partially funded by philanthropic contributions by the Khouri family, Bailey family, and Haslam family to the Cleveland Clinic for co-author Dr. Milind Y. Desai’s research. Dr. Mentias has disclosed no relevant financial relationships. Dr. Powell-Wiley disclosed relationships with the National Institute on Minority Health and Health Disparities and the Division of Intramural Research of the National, Heart, Lung, and Blood Institute of the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
PD-L1 test debated in gastroesophageal cancer immunotherapy
on whether measuring programmed death–ligand 1 (PD-L1) is essential before prescribing checkpoint inhibitors for gastroesophageal cancer.
“In the last couple of years, the incorporation of PD-1 antibodies is really changing our standard of care and international guidelines in this disease,” said Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital, London.
He moderated a debate at the 2022 Gastrointestinal Cancers Symposium on the importance of measuring PD-L1 expression levels before administering immune checkpoint inhibitor therapy.
Tumor cells can use PD-1 signaling to deactivate the response of T cells that would otherwise destroy them, and several new drugs are designed to block that signaling.
Multiple randomized controlled trials have shown the benefit of adding such checkpoint inhibitors to chemotherapy for gastroesophageal cancer and currently, chemotherapy plus a checkpoint inhibitor is standard care, Dr. Chau said.
PD-1–blocking antibodies include pembrolizumab (Keytruda, Merck) for colorectal cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma, among other cancers. And, nivolumab (Opdivo, Bristol-Myers Squibb) approved for renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and esophageal squamous cell carcinoma, among other cancers.
Regulators differ on whether these treatments should be limited to patients whose expression level of PD-L1 reaches a defined threshold. The FDA has not required the measurement of any biomarker before starting therapy with either of these drugs. However, the EMA requires a PD-L1 combined positive score (CPS) of at least 10 for pembrolizumab and at least 5 for nivolumab.
In a poll conducted before the start of the debate, 83% of physician attendees said they favor of the EMA position, while 17% disagreed.
Florian Lordick, MD, PhD, director of the University of Leipzig (Germany) Cancer Center, argued that tests for PD-L1 expression are accurate. About half of patients have CPS of at least 1. And, pathologists are in agreement in interpreting the tests about 97% of the time.
Pivotal decision-making clinical trials
The EMA requires a PD-L1 assay primarily based on its interpretation of the data from KEYNOTE-590 and CheckMate-648.
KEYNOTE-590 included 749 patients with esophageal carcinoma who were randomized to receive either pembrolizumab with standard of care chemotherapy, or placebo and standard of care chemotherapy. Patients receiving pembrolizumab who had PD-L1 CPS scores of 10 or more survived a few months longer on average. But in a post hoc analysis, the investigators found that, in patients with PD-L1 CPS scores less than 10, the difference between the treatment and placebo groups was not statistically significant.
In CheckMate-648, 970 patients with esophageal carcinoma were randomized to receive nivolumab with ipilimumab, nivolumab with chemotherapy, or chemotherapy alone. Investigators used a slightly different measurement of PD-L1 tumor proportion score (TPS), in comparing chemotherapy plus nivolumab to chemotherapy alone for advanced esophageal squamous cell carcinoma.
The median survival time of patients with TPS of at least 1% was 15.4 months in the nivolumab group and 9.2 months in the control group. But among patients with a TPS of less than 1%, the median overall survival was 12.0 months in the nivolumab group and 12.2 in the control group. CPS thresholds of 5 or 1 resulted in similar effects.
Dr. Lordick cited a systematic review of four studies in press at ESMO Open. Hazard ratios for three of the studies favored immunotherapy only in patients with CPS of at least 10.
Deciding which patients to treat matters because these drugs are expensive, he said. A single dose of 240 mg nivolumab costs $7,228.70, and treatment for 1 year can cost $173,488.80 in addition to costs for hospitalization because of immune-related adverse events, labs, imaging, colonoscopy, and other related costs.
“This is a lot of money, isn’t it? It’s the same price the hospital pay for two registered nurses in the U.S., at least when we are talking about the average price. I’m not sure I want to spend this money for a drug that does not work,” Dr. Lordick said.
Aaron Scott, MD, an oncologist with the University of Arizona Cancer Center, Tucson, said that “patients and clinicians want and need options” because there may be other factors that should be considered.
“PD-L1 has shown inconsistent results. And while I agree it is the best that we have for predictive biomarker in the space, it is far from perfect. PD-L1 has not been predictive for response in a variety of settings and trials. In first-line, second-line, third-line trials we have examples where it does not predict response,” he said.
JUPITER06 compared toripalimab or placebo with paclitaxel and cisplatin for patients with esophageal squamous cell carcinoma. Patients who received toripalimab lived longer than patients who received the placebo, but within the toripalimab group, there was no difference in median overall survival between those patients above and those below the threshold of CPS 1.
ORIENT-15 compared sintilimab or placebo with chemotherapy as first-line therapy for patients with esophageal squamous cell carcinoma. Although the treatment group fared better, survival rates were the same whether patients were above or below the CPS 10 threshold.
Dr. Scott cited three other trials in which PD-L1 was not predictive of response to checkpoint inhibitors.
The differences among studies could be attributed to different assays, he said. “Where you biopsy and when you biopsy seems to matter.”
In a second opinion poll conducted after the presentations, the proportion of physician attendees saying PD-L1 was essential before initiating checkpoint inhibitors, dropped to about two-thirds.
Dr. Chau reported financial relationships with Bristol-Myers Squibb, Merck Serono, and other pharmaceutical companies. Dr. Lordick reported financial relationships Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, and other pharmaceutical companies.
on whether measuring programmed death–ligand 1 (PD-L1) is essential before prescribing checkpoint inhibitors for gastroesophageal cancer.
“In the last couple of years, the incorporation of PD-1 antibodies is really changing our standard of care and international guidelines in this disease,” said Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital, London.
He moderated a debate at the 2022 Gastrointestinal Cancers Symposium on the importance of measuring PD-L1 expression levels before administering immune checkpoint inhibitor therapy.
Tumor cells can use PD-1 signaling to deactivate the response of T cells that would otherwise destroy them, and several new drugs are designed to block that signaling.
Multiple randomized controlled trials have shown the benefit of adding such checkpoint inhibitors to chemotherapy for gastroesophageal cancer and currently, chemotherapy plus a checkpoint inhibitor is standard care, Dr. Chau said.
PD-1–blocking antibodies include pembrolizumab (Keytruda, Merck) for colorectal cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma, among other cancers. And, nivolumab (Opdivo, Bristol-Myers Squibb) approved for renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and esophageal squamous cell carcinoma, among other cancers.
Regulators differ on whether these treatments should be limited to patients whose expression level of PD-L1 reaches a defined threshold. The FDA has not required the measurement of any biomarker before starting therapy with either of these drugs. However, the EMA requires a PD-L1 combined positive score (CPS) of at least 10 for pembrolizumab and at least 5 for nivolumab.
In a poll conducted before the start of the debate, 83% of physician attendees said they favor of the EMA position, while 17% disagreed.
Florian Lordick, MD, PhD, director of the University of Leipzig (Germany) Cancer Center, argued that tests for PD-L1 expression are accurate. About half of patients have CPS of at least 1. And, pathologists are in agreement in interpreting the tests about 97% of the time.
Pivotal decision-making clinical trials
The EMA requires a PD-L1 assay primarily based on its interpretation of the data from KEYNOTE-590 and CheckMate-648.
KEYNOTE-590 included 749 patients with esophageal carcinoma who were randomized to receive either pembrolizumab with standard of care chemotherapy, or placebo and standard of care chemotherapy. Patients receiving pembrolizumab who had PD-L1 CPS scores of 10 or more survived a few months longer on average. But in a post hoc analysis, the investigators found that, in patients with PD-L1 CPS scores less than 10, the difference between the treatment and placebo groups was not statistically significant.
In CheckMate-648, 970 patients with esophageal carcinoma were randomized to receive nivolumab with ipilimumab, nivolumab with chemotherapy, or chemotherapy alone. Investigators used a slightly different measurement of PD-L1 tumor proportion score (TPS), in comparing chemotherapy plus nivolumab to chemotherapy alone for advanced esophageal squamous cell carcinoma.
The median survival time of patients with TPS of at least 1% was 15.4 months in the nivolumab group and 9.2 months in the control group. But among patients with a TPS of less than 1%, the median overall survival was 12.0 months in the nivolumab group and 12.2 in the control group. CPS thresholds of 5 or 1 resulted in similar effects.
Dr. Lordick cited a systematic review of four studies in press at ESMO Open. Hazard ratios for three of the studies favored immunotherapy only in patients with CPS of at least 10.
Deciding which patients to treat matters because these drugs are expensive, he said. A single dose of 240 mg nivolumab costs $7,228.70, and treatment for 1 year can cost $173,488.80 in addition to costs for hospitalization because of immune-related adverse events, labs, imaging, colonoscopy, and other related costs.
“This is a lot of money, isn’t it? It’s the same price the hospital pay for two registered nurses in the U.S., at least when we are talking about the average price. I’m not sure I want to spend this money for a drug that does not work,” Dr. Lordick said.
Aaron Scott, MD, an oncologist with the University of Arizona Cancer Center, Tucson, said that “patients and clinicians want and need options” because there may be other factors that should be considered.
“PD-L1 has shown inconsistent results. And while I agree it is the best that we have for predictive biomarker in the space, it is far from perfect. PD-L1 has not been predictive for response in a variety of settings and trials. In first-line, second-line, third-line trials we have examples where it does not predict response,” he said.
JUPITER06 compared toripalimab or placebo with paclitaxel and cisplatin for patients with esophageal squamous cell carcinoma. Patients who received toripalimab lived longer than patients who received the placebo, but within the toripalimab group, there was no difference in median overall survival between those patients above and those below the threshold of CPS 1.
ORIENT-15 compared sintilimab or placebo with chemotherapy as first-line therapy for patients with esophageal squamous cell carcinoma. Although the treatment group fared better, survival rates were the same whether patients were above or below the CPS 10 threshold.
Dr. Scott cited three other trials in which PD-L1 was not predictive of response to checkpoint inhibitors.
The differences among studies could be attributed to different assays, he said. “Where you biopsy and when you biopsy seems to matter.”
In a second opinion poll conducted after the presentations, the proportion of physician attendees saying PD-L1 was essential before initiating checkpoint inhibitors, dropped to about two-thirds.
Dr. Chau reported financial relationships with Bristol-Myers Squibb, Merck Serono, and other pharmaceutical companies. Dr. Lordick reported financial relationships Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, and other pharmaceutical companies.
on whether measuring programmed death–ligand 1 (PD-L1) is essential before prescribing checkpoint inhibitors for gastroesophageal cancer.
“In the last couple of years, the incorporation of PD-1 antibodies is really changing our standard of care and international guidelines in this disease,” said Ian Chau, MD, a consultant medical oncologist at the Royal Marsden Hospital, London.
He moderated a debate at the 2022 Gastrointestinal Cancers Symposium on the importance of measuring PD-L1 expression levels before administering immune checkpoint inhibitor therapy.
Tumor cells can use PD-1 signaling to deactivate the response of T cells that would otherwise destroy them, and several new drugs are designed to block that signaling.
Multiple randomized controlled trials have shown the benefit of adding such checkpoint inhibitors to chemotherapy for gastroesophageal cancer and currently, chemotherapy plus a checkpoint inhibitor is standard care, Dr. Chau said.
PD-1–blocking antibodies include pembrolizumab (Keytruda, Merck) for colorectal cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, and renal cell carcinoma, among other cancers. And, nivolumab (Opdivo, Bristol-Myers Squibb) approved for renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, and esophageal squamous cell carcinoma, among other cancers.
Regulators differ on whether these treatments should be limited to patients whose expression level of PD-L1 reaches a defined threshold. The FDA has not required the measurement of any biomarker before starting therapy with either of these drugs. However, the EMA requires a PD-L1 combined positive score (CPS) of at least 10 for pembrolizumab and at least 5 for nivolumab.
In a poll conducted before the start of the debate, 83% of physician attendees said they favor of the EMA position, while 17% disagreed.
Florian Lordick, MD, PhD, director of the University of Leipzig (Germany) Cancer Center, argued that tests for PD-L1 expression are accurate. About half of patients have CPS of at least 1. And, pathologists are in agreement in interpreting the tests about 97% of the time.
Pivotal decision-making clinical trials
The EMA requires a PD-L1 assay primarily based on its interpretation of the data from KEYNOTE-590 and CheckMate-648.
KEYNOTE-590 included 749 patients with esophageal carcinoma who were randomized to receive either pembrolizumab with standard of care chemotherapy, or placebo and standard of care chemotherapy. Patients receiving pembrolizumab who had PD-L1 CPS scores of 10 or more survived a few months longer on average. But in a post hoc analysis, the investigators found that, in patients with PD-L1 CPS scores less than 10, the difference between the treatment and placebo groups was not statistically significant.
In CheckMate-648, 970 patients with esophageal carcinoma were randomized to receive nivolumab with ipilimumab, nivolumab with chemotherapy, or chemotherapy alone. Investigators used a slightly different measurement of PD-L1 tumor proportion score (TPS), in comparing chemotherapy plus nivolumab to chemotherapy alone for advanced esophageal squamous cell carcinoma.
The median survival time of patients with TPS of at least 1% was 15.4 months in the nivolumab group and 9.2 months in the control group. But among patients with a TPS of less than 1%, the median overall survival was 12.0 months in the nivolumab group and 12.2 in the control group. CPS thresholds of 5 or 1 resulted in similar effects.
Dr. Lordick cited a systematic review of four studies in press at ESMO Open. Hazard ratios for three of the studies favored immunotherapy only in patients with CPS of at least 10.
Deciding which patients to treat matters because these drugs are expensive, he said. A single dose of 240 mg nivolumab costs $7,228.70, and treatment for 1 year can cost $173,488.80 in addition to costs for hospitalization because of immune-related adverse events, labs, imaging, colonoscopy, and other related costs.
“This is a lot of money, isn’t it? It’s the same price the hospital pay for two registered nurses in the U.S., at least when we are talking about the average price. I’m not sure I want to spend this money for a drug that does not work,” Dr. Lordick said.
Aaron Scott, MD, an oncologist with the University of Arizona Cancer Center, Tucson, said that “patients and clinicians want and need options” because there may be other factors that should be considered.
“PD-L1 has shown inconsistent results. And while I agree it is the best that we have for predictive biomarker in the space, it is far from perfect. PD-L1 has not been predictive for response in a variety of settings and trials. In first-line, second-line, third-line trials we have examples where it does not predict response,” he said.
JUPITER06 compared toripalimab or placebo with paclitaxel and cisplatin for patients with esophageal squamous cell carcinoma. Patients who received toripalimab lived longer than patients who received the placebo, but within the toripalimab group, there was no difference in median overall survival between those patients above and those below the threshold of CPS 1.
ORIENT-15 compared sintilimab or placebo with chemotherapy as first-line therapy for patients with esophageal squamous cell carcinoma. Although the treatment group fared better, survival rates were the same whether patients were above or below the CPS 10 threshold.
Dr. Scott cited three other trials in which PD-L1 was not predictive of response to checkpoint inhibitors.
The differences among studies could be attributed to different assays, he said. “Where you biopsy and when you biopsy seems to matter.”
In a second opinion poll conducted after the presentations, the proportion of physician attendees saying PD-L1 was essential before initiating checkpoint inhibitors, dropped to about two-thirds.
Dr. Chau reported financial relationships with Bristol-Myers Squibb, Merck Serono, and other pharmaceutical companies. Dr. Lordick reported financial relationships Bristol-Myers Squibb, Merck Sharp & Dohme, Merck, and other pharmaceutical companies.
FROM ASCO GI 2022
Zanubrutinib shows worth against standard CLL drugs
MANCHESTER, ENGLAND – A new treatment option may soon be available for patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).
Zanubrutinib (Brukinsa), an irreversible, next-generation Bruton tyrosine kinase (BTK) inhibitor, is designed to minimize the off-target cardiovascular toxicities, such as atrial fibrillation and hypertension, seen with the first-generation ibrutinib (Imbruvica).
Zanubrutinib is already approved for use in mantle cell and marginal zone lymphomas and Waldenström’s macroglobulinemia.
Now it has also shown efficacy in CLL.
However, experts question whether the drug will find its place in an increasingly crowded space for the management of CLL.
Data from two phase 3 trials
The new data from two phase 3 clinical trials were presented recently at the British Society for Haematology 62nd annual scientific meeting, held in Manchester, England.
The ALPINE trial compared zanubrutinib with ibrutinib in 415 patients with CLL/SLL and showed that the novel drug was associated with a significant improvement in overall response rate, at 78% versus 63%.
This first interim analysis also showed that there was an increase in progression-free survival (PFS) with zanubrutinib, and crucially, it was associated with a lower atrial fibrillation/flutter rate than ibrutinib.
“These data support that more selective BTK inhibition, with more complete and sustained BTK occupancy, results in improved efficacy and safety outcomes,” said lead author Peter Hillmen, MBChB, FRCP, PhD, St. James’s University Hospital, Leeds, England.
The SEQUOIA study looked at zanubrutinib versus bendamustine plus rituximab in patients with untreated CLL/SLL with a 17p deletion and showed that PFS was improved with zanubrutinib by 58%.
Zanubrutinib was also associated with improved overall response rates and was well tolerated.
The results therefore “support the potential utility of zanubrutinib in the frontline management of patients with previously untreated CLL/SLL,” said lead author Talha Munir, MBBS, also of St. James’s University Hospital.
Improvement over ibrutinib
Ibrutinib, the first BTK inhibitor, “truly revolutionized the way we treat CLL,” commented Renata Walewska, MRCP, PhD, consultant hematologist at the Royal Bournemouth (England) Hospital and chair of the UKCLL Forum.
“But it has got quite a lot of, especially cardiac, problems, with atrial fibrillation and hypertension,” she said in an interview. The problem is that it acts not only as an inhibitor of Bruton kinase, but also affects other kinases, she explained.
Zanubrutinib is “much cleaner,” continued Dr. Walewska, who was lead author of the recently published British Society of Haematology guideline for the treatment of CLL.
However, the drug “is not that groundbreaking,” she commented, as acalabrutinib (Calquence), another next-generation BTK inhibitor, is already available for use in the clinic.
“We’re really lucky in CLL,” Dr. Walewska said, “we’ve got so many new drugs available, and it’s getting quite crowded. Trying to find a place for zanubrutinib is going be tricky.”
Lee Greenberger, PhD, chief scientific officer at the Leukemia & Lymphoma Society, commented that he “gives a lot of credit” to BeiGene, the company behind zanubrutinib, for “taking on these big studies.”
He said that, with the improvements in PFS and reduced atrial fibrillation with the drug, “there will be many clinicians paying attention to this and zanubrutinib could be preferred over conventional options.”
However, he agreed that it will have to compete with acalabrutinib, adding that, beyond BTK inhibitors, there are “a lot of options” for patients with CLL.
“That makes it very difficult for physicians to figure out what is the best type of therapy” to use in these patients, he added.
Dr. Greenberger told this news organization that further studies will need to demonstrate that zanubrutinib is associated with extended survival, which is “just not possible to show” at the moment with the current follow-up period.
He also noted that, in 10 years, ibrutinib will be off-patent, but zanubrutinib will not, at which point the “substantial” cost of the medication, which is a source of “hardship to patients,” will be increasingly relevant.
Study details
The phase 3 ALPINE study involved 415 adults with CLL/SLL refractory to one or more prior systemic therapies and measurable lymphadenopathy on imaging.
They were randomized 1:1 to zanubrutinib or ibrutinib until disease progression or withdrawal from the study.
Most patients had Binet stage A/B or Ann Arbor stage I/II disease, and 7.3% of patients treated with zanubrutinib and 10.1% of those assigned to ibrutinib had received more than three prior lines of therapy.
Over 60% of patients were aged 65 years or older and around 70% were men, with no significant differences between treatment groups.
Patients were randomized 1:1 to zanubrutinib or ibrutinib until disease progression or study withdrawal.
After a median follow-up of 15 months, the overall response rate was significantly higher with zanubrutinib than ibrutinib, at 78.3% versus 62.5% (P = .0006).
Subgroup analysis confirmed that the effect was seen regardless of age, sex, disease stage, number of prior lines of therapy, mutation status, or bulky disease.
Over a median follow-up of 14 months, the investigator-assessed 12-month PFS was 94.9% for zanubrutinib and 84.0% for ibrutinib (P = .0007). Overall survival at 12 months was 97% versus 92.7%, but the difference was not significant (P = .1081).
Patients treated with zanubrutinib experienced more grade 3 or higher adverse events than those given ibrutinib, at 55.9% versus 51.2%, although they had fewer adverse events leading to treatment discontinuation, at 7.8% versus 13.0%.
More importantly, there were fewer cardiac disorders of any grade with zanubrutinib versus ibrutinib, and any-grade atrial fibrillation was significantly less common, at 2.5% versus 10.1% (P = .0014).
Rates of hypertension and hemorrhage were similar between the two treatments, while rates of neutropenia were higher with zanubrutinib versus ibrutinib, at 28.4% versus 21.7%.
The phase 3 SEQUOIA study looked at an earlier stage of disease and included patients with previously untreated CLL/SLL (without 17p depletion) who were unsuitable for treatment with fludarabine, cyclophosphamide, and rituximab.
This trial involved 479 patients randomized to zanubrutinib or bendamustine (days 1 and 2) plus rituximab for six cycles of 28 days each (B+R).
The median age of patients was 70 years, and approximately 80% were at least 65 years old. Just over 60% were men and most (over 70%) were from Europe.
After a median of 26.2 months, independent review committee–assessed PFS was significantly longer with zanubrutinib versus B+R (hazard ratio, 0.42; P < .0001), with an estimated 24-month PFS of 85.5% versus 69.5%.
These results held whether patients were stratified by age, Binet stage, bulky disease, or 11q deletion status, and for patients with an unmutated, but not mutated, immunoglobulin heavy chain gene.
The overall response rate with zanubrutinib was 94.6% versus 85.3% with B+R, and estimated 24-month overall survival was 94.3% versus 94.6%.
Rates of adverse events of any grade were similar between the two treatment groups, although B+R was associated with a higher (grade ≥ 3) adverse event rate, at 79.7%, versus 52.5% for zanubrutinib, and a higher rate of treatment discontinuation because of adverse events, at 13.7% versus 8.3%.
Interestingly, any-grade hypertension was more common with zanubrutinib versus B+R, at 14.2% versus 10.6%, but much lower rates of neutropenia were more common with zanubrutinib, at 15.8% versus 56.8%.
The studies were sponsored by BeiGene. Dr. Hillmen has reported relationships with Janssen, AbbVie, Pharmacyclics, Roche, Gilead, AstraZeneca, SOBI, and BeiGene. Dr. Munir has reported relationships with AbbVie, AstraZeneca, Roche, Alexion, Janssen, MorphoSys, and SOBI. Other authors have also declared numerous relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – A new treatment option may soon be available for patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).
Zanubrutinib (Brukinsa), an irreversible, next-generation Bruton tyrosine kinase (BTK) inhibitor, is designed to minimize the off-target cardiovascular toxicities, such as atrial fibrillation and hypertension, seen with the first-generation ibrutinib (Imbruvica).
Zanubrutinib is already approved for use in mantle cell and marginal zone lymphomas and Waldenström’s macroglobulinemia.
Now it has also shown efficacy in CLL.
However, experts question whether the drug will find its place in an increasingly crowded space for the management of CLL.
Data from two phase 3 trials
The new data from two phase 3 clinical trials were presented recently at the British Society for Haematology 62nd annual scientific meeting, held in Manchester, England.
The ALPINE trial compared zanubrutinib with ibrutinib in 415 patients with CLL/SLL and showed that the novel drug was associated with a significant improvement in overall response rate, at 78% versus 63%.
This first interim analysis also showed that there was an increase in progression-free survival (PFS) with zanubrutinib, and crucially, it was associated with a lower atrial fibrillation/flutter rate than ibrutinib.
“These data support that more selective BTK inhibition, with more complete and sustained BTK occupancy, results in improved efficacy and safety outcomes,” said lead author Peter Hillmen, MBChB, FRCP, PhD, St. James’s University Hospital, Leeds, England.
The SEQUOIA study looked at zanubrutinib versus bendamustine plus rituximab in patients with untreated CLL/SLL with a 17p deletion and showed that PFS was improved with zanubrutinib by 58%.
Zanubrutinib was also associated with improved overall response rates and was well tolerated.
The results therefore “support the potential utility of zanubrutinib in the frontline management of patients with previously untreated CLL/SLL,” said lead author Talha Munir, MBBS, also of St. James’s University Hospital.
Improvement over ibrutinib
Ibrutinib, the first BTK inhibitor, “truly revolutionized the way we treat CLL,” commented Renata Walewska, MRCP, PhD, consultant hematologist at the Royal Bournemouth (England) Hospital and chair of the UKCLL Forum.
“But it has got quite a lot of, especially cardiac, problems, with atrial fibrillation and hypertension,” she said in an interview. The problem is that it acts not only as an inhibitor of Bruton kinase, but also affects other kinases, she explained.
Zanubrutinib is “much cleaner,” continued Dr. Walewska, who was lead author of the recently published British Society of Haematology guideline for the treatment of CLL.
However, the drug “is not that groundbreaking,” she commented, as acalabrutinib (Calquence), another next-generation BTK inhibitor, is already available for use in the clinic.
“We’re really lucky in CLL,” Dr. Walewska said, “we’ve got so many new drugs available, and it’s getting quite crowded. Trying to find a place for zanubrutinib is going be tricky.”
Lee Greenberger, PhD, chief scientific officer at the Leukemia & Lymphoma Society, commented that he “gives a lot of credit” to BeiGene, the company behind zanubrutinib, for “taking on these big studies.”
He said that, with the improvements in PFS and reduced atrial fibrillation with the drug, “there will be many clinicians paying attention to this and zanubrutinib could be preferred over conventional options.”
However, he agreed that it will have to compete with acalabrutinib, adding that, beyond BTK inhibitors, there are “a lot of options” for patients with CLL.
“That makes it very difficult for physicians to figure out what is the best type of therapy” to use in these patients, he added.
Dr. Greenberger told this news organization that further studies will need to demonstrate that zanubrutinib is associated with extended survival, which is “just not possible to show” at the moment with the current follow-up period.
He also noted that, in 10 years, ibrutinib will be off-patent, but zanubrutinib will not, at which point the “substantial” cost of the medication, which is a source of “hardship to patients,” will be increasingly relevant.
Study details
The phase 3 ALPINE study involved 415 adults with CLL/SLL refractory to one or more prior systemic therapies and measurable lymphadenopathy on imaging.
They were randomized 1:1 to zanubrutinib or ibrutinib until disease progression or withdrawal from the study.
Most patients had Binet stage A/B or Ann Arbor stage I/II disease, and 7.3% of patients treated with zanubrutinib and 10.1% of those assigned to ibrutinib had received more than three prior lines of therapy.
Over 60% of patients were aged 65 years or older and around 70% were men, with no significant differences between treatment groups.
Patients were randomized 1:1 to zanubrutinib or ibrutinib until disease progression or study withdrawal.
After a median follow-up of 15 months, the overall response rate was significantly higher with zanubrutinib than ibrutinib, at 78.3% versus 62.5% (P = .0006).
Subgroup analysis confirmed that the effect was seen regardless of age, sex, disease stage, number of prior lines of therapy, mutation status, or bulky disease.
Over a median follow-up of 14 months, the investigator-assessed 12-month PFS was 94.9% for zanubrutinib and 84.0% for ibrutinib (P = .0007). Overall survival at 12 months was 97% versus 92.7%, but the difference was not significant (P = .1081).
Patients treated with zanubrutinib experienced more grade 3 or higher adverse events than those given ibrutinib, at 55.9% versus 51.2%, although they had fewer adverse events leading to treatment discontinuation, at 7.8% versus 13.0%.
More importantly, there were fewer cardiac disorders of any grade with zanubrutinib versus ibrutinib, and any-grade atrial fibrillation was significantly less common, at 2.5% versus 10.1% (P = .0014).
Rates of hypertension and hemorrhage were similar between the two treatments, while rates of neutropenia were higher with zanubrutinib versus ibrutinib, at 28.4% versus 21.7%.
The phase 3 SEQUOIA study looked at an earlier stage of disease and included patients with previously untreated CLL/SLL (without 17p depletion) who were unsuitable for treatment with fludarabine, cyclophosphamide, and rituximab.
This trial involved 479 patients randomized to zanubrutinib or bendamustine (days 1 and 2) plus rituximab for six cycles of 28 days each (B+R).
The median age of patients was 70 years, and approximately 80% were at least 65 years old. Just over 60% were men and most (over 70%) were from Europe.
After a median of 26.2 months, independent review committee–assessed PFS was significantly longer with zanubrutinib versus B+R (hazard ratio, 0.42; P < .0001), with an estimated 24-month PFS of 85.5% versus 69.5%.
These results held whether patients were stratified by age, Binet stage, bulky disease, or 11q deletion status, and for patients with an unmutated, but not mutated, immunoglobulin heavy chain gene.
The overall response rate with zanubrutinib was 94.6% versus 85.3% with B+R, and estimated 24-month overall survival was 94.3% versus 94.6%.
Rates of adverse events of any grade were similar between the two treatment groups, although B+R was associated with a higher (grade ≥ 3) adverse event rate, at 79.7%, versus 52.5% for zanubrutinib, and a higher rate of treatment discontinuation because of adverse events, at 13.7% versus 8.3%.
Interestingly, any-grade hypertension was more common with zanubrutinib versus B+R, at 14.2% versus 10.6%, but much lower rates of neutropenia were more common with zanubrutinib, at 15.8% versus 56.8%.
The studies were sponsored by BeiGene. Dr. Hillmen has reported relationships with Janssen, AbbVie, Pharmacyclics, Roche, Gilead, AstraZeneca, SOBI, and BeiGene. Dr. Munir has reported relationships with AbbVie, AstraZeneca, Roche, Alexion, Janssen, MorphoSys, and SOBI. Other authors have also declared numerous relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – A new treatment option may soon be available for patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL).
Zanubrutinib (Brukinsa), an irreversible, next-generation Bruton tyrosine kinase (BTK) inhibitor, is designed to minimize the off-target cardiovascular toxicities, such as atrial fibrillation and hypertension, seen with the first-generation ibrutinib (Imbruvica).
Zanubrutinib is already approved for use in mantle cell and marginal zone lymphomas and Waldenström’s macroglobulinemia.
Now it has also shown efficacy in CLL.
However, experts question whether the drug will find its place in an increasingly crowded space for the management of CLL.
Data from two phase 3 trials
The new data from two phase 3 clinical trials were presented recently at the British Society for Haematology 62nd annual scientific meeting, held in Manchester, England.
The ALPINE trial compared zanubrutinib with ibrutinib in 415 patients with CLL/SLL and showed that the novel drug was associated with a significant improvement in overall response rate, at 78% versus 63%.
This first interim analysis also showed that there was an increase in progression-free survival (PFS) with zanubrutinib, and crucially, it was associated with a lower atrial fibrillation/flutter rate than ibrutinib.
“These data support that more selective BTK inhibition, with more complete and sustained BTK occupancy, results in improved efficacy and safety outcomes,” said lead author Peter Hillmen, MBChB, FRCP, PhD, St. James’s University Hospital, Leeds, England.
The SEQUOIA study looked at zanubrutinib versus bendamustine plus rituximab in patients with untreated CLL/SLL with a 17p deletion and showed that PFS was improved with zanubrutinib by 58%.
Zanubrutinib was also associated with improved overall response rates and was well tolerated.
The results therefore “support the potential utility of zanubrutinib in the frontline management of patients with previously untreated CLL/SLL,” said lead author Talha Munir, MBBS, also of St. James’s University Hospital.
Improvement over ibrutinib
Ibrutinib, the first BTK inhibitor, “truly revolutionized the way we treat CLL,” commented Renata Walewska, MRCP, PhD, consultant hematologist at the Royal Bournemouth (England) Hospital and chair of the UKCLL Forum.
“But it has got quite a lot of, especially cardiac, problems, with atrial fibrillation and hypertension,” she said in an interview. The problem is that it acts not only as an inhibitor of Bruton kinase, but also affects other kinases, she explained.
Zanubrutinib is “much cleaner,” continued Dr. Walewska, who was lead author of the recently published British Society of Haematology guideline for the treatment of CLL.
However, the drug “is not that groundbreaking,” she commented, as acalabrutinib (Calquence), another next-generation BTK inhibitor, is already available for use in the clinic.
“We’re really lucky in CLL,” Dr. Walewska said, “we’ve got so many new drugs available, and it’s getting quite crowded. Trying to find a place for zanubrutinib is going be tricky.”
Lee Greenberger, PhD, chief scientific officer at the Leukemia & Lymphoma Society, commented that he “gives a lot of credit” to BeiGene, the company behind zanubrutinib, for “taking on these big studies.”
He said that, with the improvements in PFS and reduced atrial fibrillation with the drug, “there will be many clinicians paying attention to this and zanubrutinib could be preferred over conventional options.”
However, he agreed that it will have to compete with acalabrutinib, adding that, beyond BTK inhibitors, there are “a lot of options” for patients with CLL.
“That makes it very difficult for physicians to figure out what is the best type of therapy” to use in these patients, he added.
Dr. Greenberger told this news organization that further studies will need to demonstrate that zanubrutinib is associated with extended survival, which is “just not possible to show” at the moment with the current follow-up period.
He also noted that, in 10 years, ibrutinib will be off-patent, but zanubrutinib will not, at which point the “substantial” cost of the medication, which is a source of “hardship to patients,” will be increasingly relevant.
Study details
The phase 3 ALPINE study involved 415 adults with CLL/SLL refractory to one or more prior systemic therapies and measurable lymphadenopathy on imaging.
They were randomized 1:1 to zanubrutinib or ibrutinib until disease progression or withdrawal from the study.
Most patients had Binet stage A/B or Ann Arbor stage I/II disease, and 7.3% of patients treated with zanubrutinib and 10.1% of those assigned to ibrutinib had received more than three prior lines of therapy.
Over 60% of patients were aged 65 years or older and around 70% were men, with no significant differences between treatment groups.
Patients were randomized 1:1 to zanubrutinib or ibrutinib until disease progression or study withdrawal.
After a median follow-up of 15 months, the overall response rate was significantly higher with zanubrutinib than ibrutinib, at 78.3% versus 62.5% (P = .0006).
Subgroup analysis confirmed that the effect was seen regardless of age, sex, disease stage, number of prior lines of therapy, mutation status, or bulky disease.
Over a median follow-up of 14 months, the investigator-assessed 12-month PFS was 94.9% for zanubrutinib and 84.0% for ibrutinib (P = .0007). Overall survival at 12 months was 97% versus 92.7%, but the difference was not significant (P = .1081).
Patients treated with zanubrutinib experienced more grade 3 or higher adverse events than those given ibrutinib, at 55.9% versus 51.2%, although they had fewer adverse events leading to treatment discontinuation, at 7.8% versus 13.0%.
More importantly, there were fewer cardiac disorders of any grade with zanubrutinib versus ibrutinib, and any-grade atrial fibrillation was significantly less common, at 2.5% versus 10.1% (P = .0014).
Rates of hypertension and hemorrhage were similar between the two treatments, while rates of neutropenia were higher with zanubrutinib versus ibrutinib, at 28.4% versus 21.7%.
The phase 3 SEQUOIA study looked at an earlier stage of disease and included patients with previously untreated CLL/SLL (without 17p depletion) who were unsuitable for treatment with fludarabine, cyclophosphamide, and rituximab.
This trial involved 479 patients randomized to zanubrutinib or bendamustine (days 1 and 2) plus rituximab for six cycles of 28 days each (B+R).
The median age of patients was 70 years, and approximately 80% were at least 65 years old. Just over 60% were men and most (over 70%) were from Europe.
After a median of 26.2 months, independent review committee–assessed PFS was significantly longer with zanubrutinib versus B+R (hazard ratio, 0.42; P < .0001), with an estimated 24-month PFS of 85.5% versus 69.5%.
These results held whether patients were stratified by age, Binet stage, bulky disease, or 11q deletion status, and for patients with an unmutated, but not mutated, immunoglobulin heavy chain gene.
The overall response rate with zanubrutinib was 94.6% versus 85.3% with B+R, and estimated 24-month overall survival was 94.3% versus 94.6%.
Rates of adverse events of any grade were similar between the two treatment groups, although B+R was associated with a higher (grade ≥ 3) adverse event rate, at 79.7%, versus 52.5% for zanubrutinib, and a higher rate of treatment discontinuation because of adverse events, at 13.7% versus 8.3%.
Interestingly, any-grade hypertension was more common with zanubrutinib versus B+R, at 14.2% versus 10.6%, but much lower rates of neutropenia were more common with zanubrutinib, at 15.8% versus 56.8%.
The studies were sponsored by BeiGene. Dr. Hillmen has reported relationships with Janssen, AbbVie, Pharmacyclics, Roche, Gilead, AstraZeneca, SOBI, and BeiGene. Dr. Munir has reported relationships with AbbVie, AstraZeneca, Roche, Alexion, Janssen, MorphoSys, and SOBI. Other authors have also declared numerous relationships.
A version of this article first appeared on Medscape.com.
Cancer diet studies: Veggies get another rave, while red meat’s busted again
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
FROM AACR 2022
‘Time is blood’: Researchers devise shortcut to AHA diagnosis
“A simple algorithm for unexplained bleeding might be helpful to emergency department physicians and other frontline workers to improve recognition of the disease,” said Amar Kelkar, MD, a fellow at the Dana-Farber Cancer Institute, Boston, and corresponding author of “Time is blood: The impact of diagnostic delays on acquired hemophilia A,” a report that appeared in the journal Cureus.
According to Dr. Kelkar, AHA is an autoimmune disease caused by the formation of autoantibodies against factor VIII (FVIII). “Classically, patients present with various forms of bleeding symptoms, including extensive bruising, spontaneous prolonged or persistent bleeding, and blood in the urine. These symptoms are usually accompanied by a prolonged activated partial thromboplastin time (aPTT) test,” he said in an interview. “While this is a rare diagnosis to be seen in primary, critical, or emergency care, it’s a disease that most hematologists should have seen and managed before.”
For the new study, researchers retrospectively tracked patients with AHA at the OSF Healthcare System in Illinois from 2010 to 2017. They focused on six patients (mean age, 79.5; male = 5). Cancer was considered a cause in four cases, and autoimmune disease in one. The sixth case was idiopathic. Five of the six patients died, with all but one death related to bleeding.
The researchers note that they saw more cases than expected (6 per 2.1 million vs. an estimated incidence of 1.48 per 1 million per year), although they attributed this high incidence to the population being made up of older hospitalized patients. In fact, Dr. Kelkar said, researchers believe this is an undercount reflecting diagnostic misses.
The median time to diagnosis was 14 days, the authors report, reflecting other studies that have also shown delays. Pseudo-thrombosis and preexisting anticoagulant therapy likely contribute to the diagnostic delays, they write.
In their new report, the authors developed an algorithm to speed diagnosis.
“The initial step is the identification of a patient with new, unexplained bleeding,” they write. “In the setting of unexplained bleeding, a detailed clinical history, including medication use, along with a thorough physical examination is critical. Prompt primary laboratory testing should include a complete blood count, a metabolic panel including creatinine and bilirubin, and coagulation testing including aPTT and prothrombin time with international normalized ratio (PT/INR). A resulting isolated aPTT elevation will initiate subsequent steps. Early inpatient hematology consultation is recommended.”
The authors add: “An important point to highlight is that we recommend concurrently ordering an aPTT mixing study and a factor VIII activity (FVIII:C) once a prolonged aPTT is confirmed. This may decrease the time to initiate treatment and improve patient outcomes. If the mixing study result is abnormal with low FVIII:C, hemostatic treatment could be initiated with concurrent confirmatory Bethesda assay or anti-FVIII ELISA, preventing further delay in patient recovery and hopefully reducing potential complications. If there is limited availability of specialty testing or prolonged delays in getting test results, such as for FVIII:C, or an inability to confirm a diagnosis at any stage of the algorithm, transferring the patient to a higher level of care with these laboratory and hematology services should be strongly considered.”
The authors also note that “when the diagnostic delay is greater than 1 month, there will be a significant increase in the days that the patient is required to be on hemostatic therapy, compared to diagnosis before 1 month (23.8 ± 13 vs. 7.6 ± 5.7 days, respectively; P = .003).”
The algorithm is meant to be widely available, Dr. Kelkar said. “That is why we targeted an open-source, general medicine journal like Cureus.”
Jerome Teitel, MD, a hematologist with St. Michael’s Hospital in Toronto, said in an interview that the algorithm “might be a useful guide for initial investigation at community hospitals.”
However, he recommended against emphasizing the use of mixing studies. They are “often ambiguous and just delay ordering the definitive tests (FVIII activity and inhibitor assay), which will need to be done regardless,” he said. “The most important message should be that patients with AHA should be referred to, or at least comanaged with, a hematologist who has specific experience and expertise in the field, and who will likely have access to specialized coagulation tests with short turnaround times.”
Another hematologist, George M. Rodgers III, MD, of the University of Utah, Salt Lake City, said in an interview that the algorithm is appropriate for the evaluation of possible AHA. “Patients with the disorder who present with minor bleeding are not evaluated with high priority by physicians,” he said. “Patients with bleeding and a prolonged PTT should be taken very seriously because AHA patients can develop spontaneous fatal bleeding.”
No study funding is reported. The authors, Dr. Teitel, and Dr. Rodgers report no relevant disclosures.
“A simple algorithm for unexplained bleeding might be helpful to emergency department physicians and other frontline workers to improve recognition of the disease,” said Amar Kelkar, MD, a fellow at the Dana-Farber Cancer Institute, Boston, and corresponding author of “Time is blood: The impact of diagnostic delays on acquired hemophilia A,” a report that appeared in the journal Cureus.
According to Dr. Kelkar, AHA is an autoimmune disease caused by the formation of autoantibodies against factor VIII (FVIII). “Classically, patients present with various forms of bleeding symptoms, including extensive bruising, spontaneous prolonged or persistent bleeding, and blood in the urine. These symptoms are usually accompanied by a prolonged activated partial thromboplastin time (aPTT) test,” he said in an interview. “While this is a rare diagnosis to be seen in primary, critical, or emergency care, it’s a disease that most hematologists should have seen and managed before.”
For the new study, researchers retrospectively tracked patients with AHA at the OSF Healthcare System in Illinois from 2010 to 2017. They focused on six patients (mean age, 79.5; male = 5). Cancer was considered a cause in four cases, and autoimmune disease in one. The sixth case was idiopathic. Five of the six patients died, with all but one death related to bleeding.
The researchers note that they saw more cases than expected (6 per 2.1 million vs. an estimated incidence of 1.48 per 1 million per year), although they attributed this high incidence to the population being made up of older hospitalized patients. In fact, Dr. Kelkar said, researchers believe this is an undercount reflecting diagnostic misses.
The median time to diagnosis was 14 days, the authors report, reflecting other studies that have also shown delays. Pseudo-thrombosis and preexisting anticoagulant therapy likely contribute to the diagnostic delays, they write.
In their new report, the authors developed an algorithm to speed diagnosis.
“The initial step is the identification of a patient with new, unexplained bleeding,” they write. “In the setting of unexplained bleeding, a detailed clinical history, including medication use, along with a thorough physical examination is critical. Prompt primary laboratory testing should include a complete blood count, a metabolic panel including creatinine and bilirubin, and coagulation testing including aPTT and prothrombin time with international normalized ratio (PT/INR). A resulting isolated aPTT elevation will initiate subsequent steps. Early inpatient hematology consultation is recommended.”
The authors add: “An important point to highlight is that we recommend concurrently ordering an aPTT mixing study and a factor VIII activity (FVIII:C) once a prolonged aPTT is confirmed. This may decrease the time to initiate treatment and improve patient outcomes. If the mixing study result is abnormal with low FVIII:C, hemostatic treatment could be initiated with concurrent confirmatory Bethesda assay or anti-FVIII ELISA, preventing further delay in patient recovery and hopefully reducing potential complications. If there is limited availability of specialty testing or prolonged delays in getting test results, such as for FVIII:C, or an inability to confirm a diagnosis at any stage of the algorithm, transferring the patient to a higher level of care with these laboratory and hematology services should be strongly considered.”
The authors also note that “when the diagnostic delay is greater than 1 month, there will be a significant increase in the days that the patient is required to be on hemostatic therapy, compared to diagnosis before 1 month (23.8 ± 13 vs. 7.6 ± 5.7 days, respectively; P = .003).”
The algorithm is meant to be widely available, Dr. Kelkar said. “That is why we targeted an open-source, general medicine journal like Cureus.”
Jerome Teitel, MD, a hematologist with St. Michael’s Hospital in Toronto, said in an interview that the algorithm “might be a useful guide for initial investigation at community hospitals.”
However, he recommended against emphasizing the use of mixing studies. They are “often ambiguous and just delay ordering the definitive tests (FVIII activity and inhibitor assay), which will need to be done regardless,” he said. “The most important message should be that patients with AHA should be referred to, or at least comanaged with, a hematologist who has specific experience and expertise in the field, and who will likely have access to specialized coagulation tests with short turnaround times.”
Another hematologist, George M. Rodgers III, MD, of the University of Utah, Salt Lake City, said in an interview that the algorithm is appropriate for the evaluation of possible AHA. “Patients with the disorder who present with minor bleeding are not evaluated with high priority by physicians,” he said. “Patients with bleeding and a prolonged PTT should be taken very seriously because AHA patients can develop spontaneous fatal bleeding.”
No study funding is reported. The authors, Dr. Teitel, and Dr. Rodgers report no relevant disclosures.
“A simple algorithm for unexplained bleeding might be helpful to emergency department physicians and other frontline workers to improve recognition of the disease,” said Amar Kelkar, MD, a fellow at the Dana-Farber Cancer Institute, Boston, and corresponding author of “Time is blood: The impact of diagnostic delays on acquired hemophilia A,” a report that appeared in the journal Cureus.
According to Dr. Kelkar, AHA is an autoimmune disease caused by the formation of autoantibodies against factor VIII (FVIII). “Classically, patients present with various forms of bleeding symptoms, including extensive bruising, spontaneous prolonged or persistent bleeding, and blood in the urine. These symptoms are usually accompanied by a prolonged activated partial thromboplastin time (aPTT) test,” he said in an interview. “While this is a rare diagnosis to be seen in primary, critical, or emergency care, it’s a disease that most hematologists should have seen and managed before.”
For the new study, researchers retrospectively tracked patients with AHA at the OSF Healthcare System in Illinois from 2010 to 2017. They focused on six patients (mean age, 79.5; male = 5). Cancer was considered a cause in four cases, and autoimmune disease in one. The sixth case was idiopathic. Five of the six patients died, with all but one death related to bleeding.
The researchers note that they saw more cases than expected (6 per 2.1 million vs. an estimated incidence of 1.48 per 1 million per year), although they attributed this high incidence to the population being made up of older hospitalized patients. In fact, Dr. Kelkar said, researchers believe this is an undercount reflecting diagnostic misses.
The median time to diagnosis was 14 days, the authors report, reflecting other studies that have also shown delays. Pseudo-thrombosis and preexisting anticoagulant therapy likely contribute to the diagnostic delays, they write.
In their new report, the authors developed an algorithm to speed diagnosis.
“The initial step is the identification of a patient with new, unexplained bleeding,” they write. “In the setting of unexplained bleeding, a detailed clinical history, including medication use, along with a thorough physical examination is critical. Prompt primary laboratory testing should include a complete blood count, a metabolic panel including creatinine and bilirubin, and coagulation testing including aPTT and prothrombin time with international normalized ratio (PT/INR). A resulting isolated aPTT elevation will initiate subsequent steps. Early inpatient hematology consultation is recommended.”
The authors add: “An important point to highlight is that we recommend concurrently ordering an aPTT mixing study and a factor VIII activity (FVIII:C) once a prolonged aPTT is confirmed. This may decrease the time to initiate treatment and improve patient outcomes. If the mixing study result is abnormal with low FVIII:C, hemostatic treatment could be initiated with concurrent confirmatory Bethesda assay or anti-FVIII ELISA, preventing further delay in patient recovery and hopefully reducing potential complications. If there is limited availability of specialty testing or prolonged delays in getting test results, such as for FVIII:C, or an inability to confirm a diagnosis at any stage of the algorithm, transferring the patient to a higher level of care with these laboratory and hematology services should be strongly considered.”
The authors also note that “when the diagnostic delay is greater than 1 month, there will be a significant increase in the days that the patient is required to be on hemostatic therapy, compared to diagnosis before 1 month (23.8 ± 13 vs. 7.6 ± 5.7 days, respectively; P = .003).”
The algorithm is meant to be widely available, Dr. Kelkar said. “That is why we targeted an open-source, general medicine journal like Cureus.”
Jerome Teitel, MD, a hematologist with St. Michael’s Hospital in Toronto, said in an interview that the algorithm “might be a useful guide for initial investigation at community hospitals.”
However, he recommended against emphasizing the use of mixing studies. They are “often ambiguous and just delay ordering the definitive tests (FVIII activity and inhibitor assay), which will need to be done regardless,” he said. “The most important message should be that patients with AHA should be referred to, or at least comanaged with, a hematologist who has specific experience and expertise in the field, and who will likely have access to specialized coagulation tests with short turnaround times.”
Another hematologist, George M. Rodgers III, MD, of the University of Utah, Salt Lake City, said in an interview that the algorithm is appropriate for the evaluation of possible AHA. “Patients with the disorder who present with minor bleeding are not evaluated with high priority by physicians,” he said. “Patients with bleeding and a prolonged PTT should be taken very seriously because AHA patients can develop spontaneous fatal bleeding.”
No study funding is reported. The authors, Dr. Teitel, and Dr. Rodgers report no relevant disclosures.
FROM CUREUS
Judge strikes down Biden mask mandate for planes, transit
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
Fresh data confirm healthy plant foods link to lower diabetes risk
A scientific analysis of metabolites from plant-based-diets – especially those rich in whole grains, fruits, and vegetables – may in the future yield clues as to how such eating patterns lower the risk of type 2 diabetes, finds a new study of more than 8,000 people.
The research looked at healthy, unhealthy, and overall plant-based diets, but only metabolic profiles for the healthy and overall plant-based diets showed an inverse relationship with type 2 diabetes.
A primarily “unhealthy” plant-based diet was one including mainly refined grains (e.g., white bread and pasta), fruit juices, potatoes, sugar-sweetened beverages, and sweets/desserts.
“Individual metabolites from consumption of polyphenol-rich plant foods like fruits, vegetables, coffee, and legumes are all closely linked to healthy plant-based diet and lower risk of diabetes,” lead author Frank Hu, MD, said in a press release.
Dr. Hu, of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and colleagues reported their findings in Diabetologia.
High-throughput profiling of the metabolome
Given that an individual’s metabolic profile reflects their diet, there is a growing trend in nutritional research to use a technique called high-throughput metabolomics to profile biological samples.
The team conducted an analysis of blood plasma samples and dietary intake using food frequency questionnaires of 10,684 participants from three prospective cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study). Participants were predominantly White and middle-aged (mean age 54 years), with a mean body mass index of 25.6 kg/m2.
Metabolite profile scores were generated from the blood samples, taken in the 1980s and 1990s, and matched to any cases of incident type 2 diabetes reported during follow-up, which ended in 2016-2017.
The team looked at three different plant-based diets – by definition, higher in plant foods and lower in animal foods – and further categorized them according to the actual foods consumed, to generate an overall plant diet index (PDI), a healthy PDI, or an unhealthy PDI.
In all, 8,827 participants completed the study, and 270 cases of diabetes were reported.
Multi-metabolite profiles were composed of 55 metabolites for the overall PDI, 93 metabolites for healthy PDI, and 75 metabolites for unhealthy PDI.
The findings are that metabolomics can be harnessed and “the identified metabolic profiles could be used to assess adherence to ... plant-based diets as part of type 2 diabetes prevention ... and provide new insights for future investigation,” the researchers concluded.
One coauthor received research support from the California Walnut Commission and Swiss ReManagement; another reported being a scientific consultant to LayerIV. The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A scientific analysis of metabolites from plant-based-diets – especially those rich in whole grains, fruits, and vegetables – may in the future yield clues as to how such eating patterns lower the risk of type 2 diabetes, finds a new study of more than 8,000 people.
The research looked at healthy, unhealthy, and overall plant-based diets, but only metabolic profiles for the healthy and overall plant-based diets showed an inverse relationship with type 2 diabetes.
A primarily “unhealthy” plant-based diet was one including mainly refined grains (e.g., white bread and pasta), fruit juices, potatoes, sugar-sweetened beverages, and sweets/desserts.
“Individual metabolites from consumption of polyphenol-rich plant foods like fruits, vegetables, coffee, and legumes are all closely linked to healthy plant-based diet and lower risk of diabetes,” lead author Frank Hu, MD, said in a press release.
Dr. Hu, of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and colleagues reported their findings in Diabetologia.
High-throughput profiling of the metabolome
Given that an individual’s metabolic profile reflects their diet, there is a growing trend in nutritional research to use a technique called high-throughput metabolomics to profile biological samples.
The team conducted an analysis of blood plasma samples and dietary intake using food frequency questionnaires of 10,684 participants from three prospective cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study). Participants were predominantly White and middle-aged (mean age 54 years), with a mean body mass index of 25.6 kg/m2.
Metabolite profile scores were generated from the blood samples, taken in the 1980s and 1990s, and matched to any cases of incident type 2 diabetes reported during follow-up, which ended in 2016-2017.
The team looked at three different plant-based diets – by definition, higher in plant foods and lower in animal foods – and further categorized them according to the actual foods consumed, to generate an overall plant diet index (PDI), a healthy PDI, or an unhealthy PDI.
In all, 8,827 participants completed the study, and 270 cases of diabetes were reported.
Multi-metabolite profiles were composed of 55 metabolites for the overall PDI, 93 metabolites for healthy PDI, and 75 metabolites for unhealthy PDI.
The findings are that metabolomics can be harnessed and “the identified metabolic profiles could be used to assess adherence to ... plant-based diets as part of type 2 diabetes prevention ... and provide new insights for future investigation,” the researchers concluded.
One coauthor received research support from the California Walnut Commission and Swiss ReManagement; another reported being a scientific consultant to LayerIV. The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A scientific analysis of metabolites from plant-based-diets – especially those rich in whole grains, fruits, and vegetables – may in the future yield clues as to how such eating patterns lower the risk of type 2 diabetes, finds a new study of more than 8,000 people.
The research looked at healthy, unhealthy, and overall plant-based diets, but only metabolic profiles for the healthy and overall plant-based diets showed an inverse relationship with type 2 diabetes.
A primarily “unhealthy” plant-based diet was one including mainly refined grains (e.g., white bread and pasta), fruit juices, potatoes, sugar-sweetened beverages, and sweets/desserts.
“Individual metabolites from consumption of polyphenol-rich plant foods like fruits, vegetables, coffee, and legumes are all closely linked to healthy plant-based diet and lower risk of diabetes,” lead author Frank Hu, MD, said in a press release.
Dr. Hu, of the department of nutrition at Harvard T.H. Chan School of Public Health, Boston, and colleagues reported their findings in Diabetologia.
High-throughput profiling of the metabolome
Given that an individual’s metabolic profile reflects their diet, there is a growing trend in nutritional research to use a technique called high-throughput metabolomics to profile biological samples.
The team conducted an analysis of blood plasma samples and dietary intake using food frequency questionnaires of 10,684 participants from three prospective cohorts (Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study). Participants were predominantly White and middle-aged (mean age 54 years), with a mean body mass index of 25.6 kg/m2.
Metabolite profile scores were generated from the blood samples, taken in the 1980s and 1990s, and matched to any cases of incident type 2 diabetes reported during follow-up, which ended in 2016-2017.
The team looked at three different plant-based diets – by definition, higher in plant foods and lower in animal foods – and further categorized them according to the actual foods consumed, to generate an overall plant diet index (PDI), a healthy PDI, or an unhealthy PDI.
In all, 8,827 participants completed the study, and 270 cases of diabetes were reported.
Multi-metabolite profiles were composed of 55 metabolites for the overall PDI, 93 metabolites for healthy PDI, and 75 metabolites for unhealthy PDI.
The findings are that metabolomics can be harnessed and “the identified metabolic profiles could be used to assess adherence to ... plant-based diets as part of type 2 diabetes prevention ... and provide new insights for future investigation,” the researchers concluded.
One coauthor received research support from the California Walnut Commission and Swiss ReManagement; another reported being a scientific consultant to LayerIV. The other authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA