User login
Ancient human teeth revise the history of microbial evolution
The cupboard in Dr. Nicolás Rascovan’s microbial paleogenomics lab at Institut Pasteur in Paris is filled up with cardboard boxes that look as if they were shipped from an office supply store. Yet, instead of pencils and Post-it notes, the boxes are filled with ancient human remains from South America – several-thousand-year-old vertebrae, petrus bones (which protect inner ear structures), and teeth – all neatly packed in plastic bags. It could even, perhaps, rewrite history. “It’s a story of a continent in a closet,” Dr. Rascovan says.
Over the past decade, technologic advances in DNA recovery and sequencing have made it possible for scientists such as Dr. Rascovan, an Argentinian molecular biologist, to analyze ancient specimens relatively quickly and affordably. They’ve been hunting for – and finding – DNA of centuries-old microbes in various archeological samples: from smallpox variola virus and Mycobacterium tuberculosis in mummified tissues, to the Black Death bacteria, Yesinia pestis, in neolithic teeth, to Plasmodium falciparum preserved in historical blood stains.
The ultramodern Parisian offices of the microbial paleogenomics group, a team of five scientists led by Dr. Rascovan, clash with the logo they half-jokingly chose for themselves and plastered all over the lab’s walls: a Jurassic Park–inspired dinosaur baring its giant, ancient teeth, made to look like an image seen under a microscope. Ancient teeth are certainly central to the group’s work, because it’s there where ancient pathogens’ DNA is most likely to be preserved – after death, teeth act like tiny, sealed-up boxes for microbes. “If you have a pathogen that is circulating in the blood, it will sometimes get into the teeth, and when you die, the DNA will stay there,” Dr. Rascovan says.
To process ancient teeth, Dr. Rascovan enters a lab clad head to toe in protective gear. That’s not so much to save himself from potentially deadly disease as to save the samples from contamination, he says. According to Sebastian Duchene Garzon, a microbiologist at the University of Melbourne, “the likelihood of ancient pathogen DNA leading to infections at present is remote, although certainly not impossible, because of how degraded the DNA usually is and because it would still need all the molecular machinery to infect a modern host.”
To process ancient teeth in his lab, Dr. Rascovan starts with a thorough cleaning that involves bleach to remove any modern DNA contamination. Next, he cuts the tooth with a Dremel rotary tool to open it up and get into its pulp – which is not only very durable but also naturally sterile – a perfect place to find ancient pathogens. He then scrapes the pulp into a powder that can be poured into a tube for DNA extraction.
So far, Dr. Rascovan’s biggest breakthrough didn’t come from the teeth he cut up himself, though. It came from analyzing publicly available DNA data from studies of ancient human genomes. When such genomes are sequenced from fossil teeth or bones, scientists pick out the material they need for study of our ancestors’ evolutionary history. However, among the double helixes coding hominid genetic instructions often hide scraps of microbial DNA, which in the past were frequently simply discarded.
Dr. Rascovan downloaded data from published articles on ancient human DNA that had been found in teeth and reanalyzed them, searching for bacteria. One night, when he was alone in his office going through lines and lines of data, he spotted it: DNA of the plague-causing bacteria, Y. pestis. When Dr. Rascovan cross-checked to determine in which samples the bacteria’s DNA was found, his heart raced. “It was not supposed to be there,” he says. He had just discovered the most ancient case of plague in humans – which occurred 4,900 years ago in Sweden.
Scientists used to believe that plague pandemics came to Europe from the Eurasian Steppe. Yet here was the DNA of Y. pestis lodged in the teeth of two farmers, a woman and a man, who died in Scandinavia before the plague’s supposed arrival from the East. Their bodies were buried in an unusually large common grave – of itself a possible indication of an epidemic.
When Dr. Rascovan and his colleagues applied molecular-clock analyses of the phylogenetic tree of the plague bacteria and compared various strains to see which one was the most ancestral, they confirmed that the Swedish strain of Y. pestis, named Gok2, was indeed the oldest – the origin of the Steppe strains rather than its distant cousin. Plague, it seemed, wasn’t brought to Europe during mass migrations from the East. Instead, it might have originated there.
Such work is not simply about rewriting history. By updating our knowledge of ancient pandemics, we can learn how different factors influence each other in fostering outbreaks. For Dr. Rascovan, the Swedish plague story underscores the importance of our lifestyle and environment for the emergence and spread of dangerous pathogens. The Gok2 strain didn’t contain a gene that makes plague particularly virulent, called ymt, yet it might have played an important role in Bronze Age Europe. At that time, mega-settlements of 10,000 to 20,000 people existed in what is now Ukraine, Romania, and Moldova, yet those settlements were frequently burned to the ground and abandoned. According to Dr. Rascovan and his colleagues, that could fit with the plague pandemic story (although this remains very much a hypothesis).
In Mexico, environmental factors might have played an important role in the severity of the 16th century “cocoliztli” epidemic (the word means “pestilence” in a local language), considered one of the most devastating epidemics in New World history. The disease, which caused vomiting, red spots on the skin, and bleeding from various body orifices, didn’t have a known cause. Some hypothesized the bug might have been smallpox, judging by the severity of the outbreak. A 2018 study of a victim’s DNA showed it contained the genome of Salmonella enterica, a bacterium that causes enteric fever – a microbe generally milder than smallpox. The study’s authors argued that specific conditions may have been necessary at the onset of the epidemic for the S. enterica microbe to cause such devastating outcomes. A mix of severe draught, forced relocations of the local population by their Spanish rulers, and new subsistence farming practices all negatively affected hygienic conditions in the local settlements. According to Dr. Rascovan, such research can “place pandemics into their broader context” – with potential lessons for the future.
One of the microbes Dr. Rascovan and his team are hoping to find in the ancient teeth stocked in their lab’s closet is tuberculosis – a pathogen that kills 1.5 million people a year, yet whose evolutionary history remains largely a mystery. The focus of Dr. Rascovan and his colleagues remains on fossils shipped from South America, since we still know very little about microbes that were associated with pre-Columbian populations. South Americans have been isolated from the rest of the world for 20,000 years, making them particularly interesting candidates for the study of emergence, evolution, and spread of pathogens.
Dr. Rascovan believes that ancient microbial genomic data can help scientists better understand antibiotic resistance through comparisons of bacterial evolution before and after the discovery of antibiotics. In general, he says, by studying only current pathogens and the modern outbreaks they cause, we see only a narrow sample of something that is much more diverse and much larger. “We are missing an important part of information. Ancient samples can bring us a perspective,” he says.
A version of this article first appeared on Medscape.com.
The cupboard in Dr. Nicolás Rascovan’s microbial paleogenomics lab at Institut Pasteur in Paris is filled up with cardboard boxes that look as if they were shipped from an office supply store. Yet, instead of pencils and Post-it notes, the boxes are filled with ancient human remains from South America – several-thousand-year-old vertebrae, petrus bones (which protect inner ear structures), and teeth – all neatly packed in plastic bags. It could even, perhaps, rewrite history. “It’s a story of a continent in a closet,” Dr. Rascovan says.
Over the past decade, technologic advances in DNA recovery and sequencing have made it possible for scientists such as Dr. Rascovan, an Argentinian molecular biologist, to analyze ancient specimens relatively quickly and affordably. They’ve been hunting for – and finding – DNA of centuries-old microbes in various archeological samples: from smallpox variola virus and Mycobacterium tuberculosis in mummified tissues, to the Black Death bacteria, Yesinia pestis, in neolithic teeth, to Plasmodium falciparum preserved in historical blood stains.
The ultramodern Parisian offices of the microbial paleogenomics group, a team of five scientists led by Dr. Rascovan, clash with the logo they half-jokingly chose for themselves and plastered all over the lab’s walls: a Jurassic Park–inspired dinosaur baring its giant, ancient teeth, made to look like an image seen under a microscope. Ancient teeth are certainly central to the group’s work, because it’s there where ancient pathogens’ DNA is most likely to be preserved – after death, teeth act like tiny, sealed-up boxes for microbes. “If you have a pathogen that is circulating in the blood, it will sometimes get into the teeth, and when you die, the DNA will stay there,” Dr. Rascovan says.
To process ancient teeth, Dr. Rascovan enters a lab clad head to toe in protective gear. That’s not so much to save himself from potentially deadly disease as to save the samples from contamination, he says. According to Sebastian Duchene Garzon, a microbiologist at the University of Melbourne, “the likelihood of ancient pathogen DNA leading to infections at present is remote, although certainly not impossible, because of how degraded the DNA usually is and because it would still need all the molecular machinery to infect a modern host.”
To process ancient teeth in his lab, Dr. Rascovan starts with a thorough cleaning that involves bleach to remove any modern DNA contamination. Next, he cuts the tooth with a Dremel rotary tool to open it up and get into its pulp – which is not only very durable but also naturally sterile – a perfect place to find ancient pathogens. He then scrapes the pulp into a powder that can be poured into a tube for DNA extraction.
So far, Dr. Rascovan’s biggest breakthrough didn’t come from the teeth he cut up himself, though. It came from analyzing publicly available DNA data from studies of ancient human genomes. When such genomes are sequenced from fossil teeth or bones, scientists pick out the material they need for study of our ancestors’ evolutionary history. However, among the double helixes coding hominid genetic instructions often hide scraps of microbial DNA, which in the past were frequently simply discarded.
Dr. Rascovan downloaded data from published articles on ancient human DNA that had been found in teeth and reanalyzed them, searching for bacteria. One night, when he was alone in his office going through lines and lines of data, he spotted it: DNA of the plague-causing bacteria, Y. pestis. When Dr. Rascovan cross-checked to determine in which samples the bacteria’s DNA was found, his heart raced. “It was not supposed to be there,” he says. He had just discovered the most ancient case of plague in humans – which occurred 4,900 years ago in Sweden.
Scientists used to believe that plague pandemics came to Europe from the Eurasian Steppe. Yet here was the DNA of Y. pestis lodged in the teeth of two farmers, a woman and a man, who died in Scandinavia before the plague’s supposed arrival from the East. Their bodies were buried in an unusually large common grave – of itself a possible indication of an epidemic.
When Dr. Rascovan and his colleagues applied molecular-clock analyses of the phylogenetic tree of the plague bacteria and compared various strains to see which one was the most ancestral, they confirmed that the Swedish strain of Y. pestis, named Gok2, was indeed the oldest – the origin of the Steppe strains rather than its distant cousin. Plague, it seemed, wasn’t brought to Europe during mass migrations from the East. Instead, it might have originated there.
Such work is not simply about rewriting history. By updating our knowledge of ancient pandemics, we can learn how different factors influence each other in fostering outbreaks. For Dr. Rascovan, the Swedish plague story underscores the importance of our lifestyle and environment for the emergence and spread of dangerous pathogens. The Gok2 strain didn’t contain a gene that makes plague particularly virulent, called ymt, yet it might have played an important role in Bronze Age Europe. At that time, mega-settlements of 10,000 to 20,000 people existed in what is now Ukraine, Romania, and Moldova, yet those settlements were frequently burned to the ground and abandoned. According to Dr. Rascovan and his colleagues, that could fit with the plague pandemic story (although this remains very much a hypothesis).
In Mexico, environmental factors might have played an important role in the severity of the 16th century “cocoliztli” epidemic (the word means “pestilence” in a local language), considered one of the most devastating epidemics in New World history. The disease, which caused vomiting, red spots on the skin, and bleeding from various body orifices, didn’t have a known cause. Some hypothesized the bug might have been smallpox, judging by the severity of the outbreak. A 2018 study of a victim’s DNA showed it contained the genome of Salmonella enterica, a bacterium that causes enteric fever – a microbe generally milder than smallpox. The study’s authors argued that specific conditions may have been necessary at the onset of the epidemic for the S. enterica microbe to cause such devastating outcomes. A mix of severe draught, forced relocations of the local population by their Spanish rulers, and new subsistence farming practices all negatively affected hygienic conditions in the local settlements. According to Dr. Rascovan, such research can “place pandemics into their broader context” – with potential lessons for the future.
One of the microbes Dr. Rascovan and his team are hoping to find in the ancient teeth stocked in their lab’s closet is tuberculosis – a pathogen that kills 1.5 million people a year, yet whose evolutionary history remains largely a mystery. The focus of Dr. Rascovan and his colleagues remains on fossils shipped from South America, since we still know very little about microbes that were associated with pre-Columbian populations. South Americans have been isolated from the rest of the world for 20,000 years, making them particularly interesting candidates for the study of emergence, evolution, and spread of pathogens.
Dr. Rascovan believes that ancient microbial genomic data can help scientists better understand antibiotic resistance through comparisons of bacterial evolution before and after the discovery of antibiotics. In general, he says, by studying only current pathogens and the modern outbreaks they cause, we see only a narrow sample of something that is much more diverse and much larger. “We are missing an important part of information. Ancient samples can bring us a perspective,” he says.
A version of this article first appeared on Medscape.com.
The cupboard in Dr. Nicolás Rascovan’s microbial paleogenomics lab at Institut Pasteur in Paris is filled up with cardboard boxes that look as if they were shipped from an office supply store. Yet, instead of pencils and Post-it notes, the boxes are filled with ancient human remains from South America – several-thousand-year-old vertebrae, petrus bones (which protect inner ear structures), and teeth – all neatly packed in plastic bags. It could even, perhaps, rewrite history. “It’s a story of a continent in a closet,” Dr. Rascovan says.
Over the past decade, technologic advances in DNA recovery and sequencing have made it possible for scientists such as Dr. Rascovan, an Argentinian molecular biologist, to analyze ancient specimens relatively quickly and affordably. They’ve been hunting for – and finding – DNA of centuries-old microbes in various archeological samples: from smallpox variola virus and Mycobacterium tuberculosis in mummified tissues, to the Black Death bacteria, Yesinia pestis, in neolithic teeth, to Plasmodium falciparum preserved in historical blood stains.
The ultramodern Parisian offices of the microbial paleogenomics group, a team of five scientists led by Dr. Rascovan, clash with the logo they half-jokingly chose for themselves and plastered all over the lab’s walls: a Jurassic Park–inspired dinosaur baring its giant, ancient teeth, made to look like an image seen under a microscope. Ancient teeth are certainly central to the group’s work, because it’s there where ancient pathogens’ DNA is most likely to be preserved – after death, teeth act like tiny, sealed-up boxes for microbes. “If you have a pathogen that is circulating in the blood, it will sometimes get into the teeth, and when you die, the DNA will stay there,” Dr. Rascovan says.
To process ancient teeth, Dr. Rascovan enters a lab clad head to toe in protective gear. That’s not so much to save himself from potentially deadly disease as to save the samples from contamination, he says. According to Sebastian Duchene Garzon, a microbiologist at the University of Melbourne, “the likelihood of ancient pathogen DNA leading to infections at present is remote, although certainly not impossible, because of how degraded the DNA usually is and because it would still need all the molecular machinery to infect a modern host.”
To process ancient teeth in his lab, Dr. Rascovan starts with a thorough cleaning that involves bleach to remove any modern DNA contamination. Next, he cuts the tooth with a Dremel rotary tool to open it up and get into its pulp – which is not only very durable but also naturally sterile – a perfect place to find ancient pathogens. He then scrapes the pulp into a powder that can be poured into a tube for DNA extraction.
So far, Dr. Rascovan’s biggest breakthrough didn’t come from the teeth he cut up himself, though. It came from analyzing publicly available DNA data from studies of ancient human genomes. When such genomes are sequenced from fossil teeth or bones, scientists pick out the material they need for study of our ancestors’ evolutionary history. However, among the double helixes coding hominid genetic instructions often hide scraps of microbial DNA, which in the past were frequently simply discarded.
Dr. Rascovan downloaded data from published articles on ancient human DNA that had been found in teeth and reanalyzed them, searching for bacteria. One night, when he was alone in his office going through lines and lines of data, he spotted it: DNA of the plague-causing bacteria, Y. pestis. When Dr. Rascovan cross-checked to determine in which samples the bacteria’s DNA was found, his heart raced. “It was not supposed to be there,” he says. He had just discovered the most ancient case of plague in humans – which occurred 4,900 years ago in Sweden.
Scientists used to believe that plague pandemics came to Europe from the Eurasian Steppe. Yet here was the DNA of Y. pestis lodged in the teeth of two farmers, a woman and a man, who died in Scandinavia before the plague’s supposed arrival from the East. Their bodies were buried in an unusually large common grave – of itself a possible indication of an epidemic.
When Dr. Rascovan and his colleagues applied molecular-clock analyses of the phylogenetic tree of the plague bacteria and compared various strains to see which one was the most ancestral, they confirmed that the Swedish strain of Y. pestis, named Gok2, was indeed the oldest – the origin of the Steppe strains rather than its distant cousin. Plague, it seemed, wasn’t brought to Europe during mass migrations from the East. Instead, it might have originated there.
Such work is not simply about rewriting history. By updating our knowledge of ancient pandemics, we can learn how different factors influence each other in fostering outbreaks. For Dr. Rascovan, the Swedish plague story underscores the importance of our lifestyle and environment for the emergence and spread of dangerous pathogens. The Gok2 strain didn’t contain a gene that makes plague particularly virulent, called ymt, yet it might have played an important role in Bronze Age Europe. At that time, mega-settlements of 10,000 to 20,000 people existed in what is now Ukraine, Romania, and Moldova, yet those settlements were frequently burned to the ground and abandoned. According to Dr. Rascovan and his colleagues, that could fit with the plague pandemic story (although this remains very much a hypothesis).
In Mexico, environmental factors might have played an important role in the severity of the 16th century “cocoliztli” epidemic (the word means “pestilence” in a local language), considered one of the most devastating epidemics in New World history. The disease, which caused vomiting, red spots on the skin, and bleeding from various body orifices, didn’t have a known cause. Some hypothesized the bug might have been smallpox, judging by the severity of the outbreak. A 2018 study of a victim’s DNA showed it contained the genome of Salmonella enterica, a bacterium that causes enteric fever – a microbe generally milder than smallpox. The study’s authors argued that specific conditions may have been necessary at the onset of the epidemic for the S. enterica microbe to cause such devastating outcomes. A mix of severe draught, forced relocations of the local population by their Spanish rulers, and new subsistence farming practices all negatively affected hygienic conditions in the local settlements. According to Dr. Rascovan, such research can “place pandemics into their broader context” – with potential lessons for the future.
One of the microbes Dr. Rascovan and his team are hoping to find in the ancient teeth stocked in their lab’s closet is tuberculosis – a pathogen that kills 1.5 million people a year, yet whose evolutionary history remains largely a mystery. The focus of Dr. Rascovan and his colleagues remains on fossils shipped from South America, since we still know very little about microbes that were associated with pre-Columbian populations. South Americans have been isolated from the rest of the world for 20,000 years, making them particularly interesting candidates for the study of emergence, evolution, and spread of pathogens.
Dr. Rascovan believes that ancient microbial genomic data can help scientists better understand antibiotic resistance through comparisons of bacterial evolution before and after the discovery of antibiotics. In general, he says, by studying only current pathogens and the modern outbreaks they cause, we see only a narrow sample of something that is much more diverse and much larger. “We are missing an important part of information. Ancient samples can bring us a perspective,” he says.
A version of this article first appeared on Medscape.com.
Care gaps common after anal sphincter injuries from childbirth
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Postpartum complications may go unrecognized in women who incur anal sphincter injuries during childbirth, a review of electronic medical records at one academic health system suggests.
In the first 3 months after delivery, few patients with an obstetric anal sphincter injury (OASI) had documented pelvic floor problems, compared with higher rates documented in medical literature, the researchers found.
“Lack of identified pelvic floor dysfunction in this population differs from the incidence in previously published data and may reflect lack of identification by obstetric providers,” the researchers reported. The findings “highlight a gap in health care that, when addressed, could significantly improve postpartum quality of life.”
The findings are scheduled to be presented at the annual scientific meeting of the American Urogynecologic Society and International Urogynecological Association.
Anal sphincter injuries occur in about 4.4% of vaginal deliveries and are the most common cause of anal incontinence in women of reproductive age.
For the new study, researchers reviewed records of 287 women who underwent a vaginal birth that resulted in an anal sphincter injury at five Ohio hospitals affiliated with Cleveland Clinic from 2013 to 2015.
Of those who met eligibility criteria, 209 (72.8%) were White, 262 (91.3%) were non-Hispanic, and 249 (86.8%) were aged 20-34 years. Most had an epidural (92%), did not require a blood transfusion (97.9%), did not develop a vaginal hematoma (98.9%), and did not have their injury repaired in an operating room (97.2%), the researchers reported.
Among pelvic floor disorders, urinary incontinence was not reported in 96% of patients, fecal incontinence was not reported in 97.1%, and pelvic organ prolapse was not reported in 99.3%. Most had no recorded complications from their lacerations (87.8%) or postpartum depression (92%), the researchers found.
However, a 2015 study found that, 12 weeks after delivery, women with OASIs commonly reported symptoms of incontinence, with 26% reporting urinary stress incontinence, 21.4% urinary urgency incontinence, 59% anal incontinence, and 15% fecal incontinence.
Depression was also seldom identified despite higher risk of mood disorders among women with OASI, the researchers found.
The team also examined interpregnancy intervals, defined as the time between a woman’s first vaginal delivery and conception of a subsequent pregnancy. Of 178 women for whom data were available, the median interval was 26.4 months (95% confidence interval, 23.7-29.9), similar to the median for births nationally.
Lead researcher Alexandra Nutaitis, DO, a resident in obstetrics and gynecology at Cleveland Clinic Akron General, said in an interview that it’s unclear whether physicians did not inquire about symptoms or didn’t record them. She noted that anal sphincter injuries are a “stigmatized topic.”
Not asked, not told
Carolyn Swenson, MD, an associate professor in urogynecology at the University of Utah, Salt Lake City, said physicians in the study may have relied on patients to bring up their symptoms rather than using questionnaires to screen for problems.
“What we know is that if you don’t ask women about pelvic floor disorders, they often don’t tell you that they are experiencing symptoms,” said Dr. Swenson, who was not involved in the new research.
Dr. Swenson called for validated questionnaires to assess pelvic floor symptoms in postpartum patients.
Regarding interpregnancy intervals, Dr. Nutaitis said she would be surprised if women who experienced an OASI didn’t delay having another child longer than women who did not undergo that physical and psychological trauma – but other factors such as societal pressures may override any reluctance to proceed with another pregnancy.
Dr. Swenson said it’s possible that a subgroup of women who have severe complications, such as those with a fourth-degree tear, might put off having another child. However, more research is needed to find out, she said.
Dr. Nutaitis and Dr. Swenson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AUGS 2022
Eosinophils may predict outcomes in acute COPD exacerbations
High levels of eosinophils had a protective effect for individuals who experienced acute exacerbations of chronic obstructive pulmonary disease, based on data from nearly 1,000 patients.
Several blood biomarkers are under investigation for links to acute exacerbation of chronic obstructive pulmonary disease (AECOPD), which remains one of the top three causes of death worldwide, wrote Riuying Wang, MD, of Third Hospital of Shanxi Medical University, Taiyuan, China, and colleagues.
“Numerous studies have shown the relationship between eosinophilia and clinical outcomes of patients with AECOPD. However, the evidence lacks consensus, and the research thresholds are controversial,” they said.
In a study published in Heart & Lung, the researchers reviewed data from 984 adults with AECOPD over a 3-year follow-up period. The mean age of the patients was 71 years, and 78% were men. The patients’ blood eosinophil levels were grouped into three categories: EOS < 2%, EOS from 2% to < 3%, and 3% or higher. The researchers examined the association between eosinophilia and various comorbidities, treatment, and mortality.
Eosinophilia occurred in 477 cases. The prevalence of eosinophilia in the three groups was 36.48%, 22.87%, and 48.48% respectively, with eosinophilia defined as eosinophil counts of at least 100 cells per microliter, according to the report in Heart & Lung.
An EOS of 2% or higher was associated with significantly fewer cases of complicated pulmonary heart disease and atrial fibrillation than the lower EOS group. Similarly, patients in the EOS group of 2% or higher were less likely to use ventilators and systemic glucocorticoids and those in the EOS less than 2% group had significantly heavier airflow limitation, higher D-dimer, higher burden of infectious inflammation, and higher prevalence of respiratory failure than the other groups.
In addition, significantly fewer deaths occurred during the study period among patients with EOS of 2% or higher, compared with the lower EOS group (P < .01). The findings suggest that “Eosinophils can be used as a prognostic indicator of mortality in AECOPD,” the researchers said.
The researchers also used the area under the curve to examine the predictive value of EOS. The ROC curve showed that the indicators of AUC 0.5 included chest CT imaging, osteoporosis, mental illness, dust exposure, and being a former smoker; however, “the predictive value of EOS by the ROC curve was unstable. Further validation in large samples is needed,” the researchers wrote in their discussion.
The study findings were limited by several factors including the retrospective design and use of data from a single center, the researchers noted. Other limitations included the relatively small sample size and a lack of data on some clinical features and performance metrics, as well as lack of evaluation of chest CT subtypes.
However, the results are consistent with previous studies on infection and antibiotics and reviewed the optimal threshold of AECOPD, the researchers wrote. Based on their findings, “Eosinophils can not only guide clinical treatment but also be used as an index to predict the clinical outcome and prognosis of AECOPD patients,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
High levels of eosinophils had a protective effect for individuals who experienced acute exacerbations of chronic obstructive pulmonary disease, based on data from nearly 1,000 patients.
Several blood biomarkers are under investigation for links to acute exacerbation of chronic obstructive pulmonary disease (AECOPD), which remains one of the top three causes of death worldwide, wrote Riuying Wang, MD, of Third Hospital of Shanxi Medical University, Taiyuan, China, and colleagues.
“Numerous studies have shown the relationship between eosinophilia and clinical outcomes of patients with AECOPD. However, the evidence lacks consensus, and the research thresholds are controversial,” they said.
In a study published in Heart & Lung, the researchers reviewed data from 984 adults with AECOPD over a 3-year follow-up period. The mean age of the patients was 71 years, and 78% were men. The patients’ blood eosinophil levels were grouped into three categories: EOS < 2%, EOS from 2% to < 3%, and 3% or higher. The researchers examined the association between eosinophilia and various comorbidities, treatment, and mortality.
Eosinophilia occurred in 477 cases. The prevalence of eosinophilia in the three groups was 36.48%, 22.87%, and 48.48% respectively, with eosinophilia defined as eosinophil counts of at least 100 cells per microliter, according to the report in Heart & Lung.
An EOS of 2% or higher was associated with significantly fewer cases of complicated pulmonary heart disease and atrial fibrillation than the lower EOS group. Similarly, patients in the EOS group of 2% or higher were less likely to use ventilators and systemic glucocorticoids and those in the EOS less than 2% group had significantly heavier airflow limitation, higher D-dimer, higher burden of infectious inflammation, and higher prevalence of respiratory failure than the other groups.
In addition, significantly fewer deaths occurred during the study period among patients with EOS of 2% or higher, compared with the lower EOS group (P < .01). The findings suggest that “Eosinophils can be used as a prognostic indicator of mortality in AECOPD,” the researchers said.
The researchers also used the area under the curve to examine the predictive value of EOS. The ROC curve showed that the indicators of AUC 0.5 included chest CT imaging, osteoporosis, mental illness, dust exposure, and being a former smoker; however, “the predictive value of EOS by the ROC curve was unstable. Further validation in large samples is needed,” the researchers wrote in their discussion.
The study findings were limited by several factors including the retrospective design and use of data from a single center, the researchers noted. Other limitations included the relatively small sample size and a lack of data on some clinical features and performance metrics, as well as lack of evaluation of chest CT subtypes.
However, the results are consistent with previous studies on infection and antibiotics and reviewed the optimal threshold of AECOPD, the researchers wrote. Based on their findings, “Eosinophils can not only guide clinical treatment but also be used as an index to predict the clinical outcome and prognosis of AECOPD patients,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
High levels of eosinophils had a protective effect for individuals who experienced acute exacerbations of chronic obstructive pulmonary disease, based on data from nearly 1,000 patients.
Several blood biomarkers are under investigation for links to acute exacerbation of chronic obstructive pulmonary disease (AECOPD), which remains one of the top three causes of death worldwide, wrote Riuying Wang, MD, of Third Hospital of Shanxi Medical University, Taiyuan, China, and colleagues.
“Numerous studies have shown the relationship between eosinophilia and clinical outcomes of patients with AECOPD. However, the evidence lacks consensus, and the research thresholds are controversial,” they said.
In a study published in Heart & Lung, the researchers reviewed data from 984 adults with AECOPD over a 3-year follow-up period. The mean age of the patients was 71 years, and 78% were men. The patients’ blood eosinophil levels were grouped into three categories: EOS < 2%, EOS from 2% to < 3%, and 3% or higher. The researchers examined the association between eosinophilia and various comorbidities, treatment, and mortality.
Eosinophilia occurred in 477 cases. The prevalence of eosinophilia in the three groups was 36.48%, 22.87%, and 48.48% respectively, with eosinophilia defined as eosinophil counts of at least 100 cells per microliter, according to the report in Heart & Lung.
An EOS of 2% or higher was associated with significantly fewer cases of complicated pulmonary heart disease and atrial fibrillation than the lower EOS group. Similarly, patients in the EOS group of 2% or higher were less likely to use ventilators and systemic glucocorticoids and those in the EOS less than 2% group had significantly heavier airflow limitation, higher D-dimer, higher burden of infectious inflammation, and higher prevalence of respiratory failure than the other groups.
In addition, significantly fewer deaths occurred during the study period among patients with EOS of 2% or higher, compared with the lower EOS group (P < .01). The findings suggest that “Eosinophils can be used as a prognostic indicator of mortality in AECOPD,” the researchers said.
The researchers also used the area under the curve to examine the predictive value of EOS. The ROC curve showed that the indicators of AUC 0.5 included chest CT imaging, osteoporosis, mental illness, dust exposure, and being a former smoker; however, “the predictive value of EOS by the ROC curve was unstable. Further validation in large samples is needed,” the researchers wrote in their discussion.
The study findings were limited by several factors including the retrospective design and use of data from a single center, the researchers noted. Other limitations included the relatively small sample size and a lack of data on some clinical features and performance metrics, as well as lack of evaluation of chest CT subtypes.
However, the results are consistent with previous studies on infection and antibiotics and reviewed the optimal threshold of AECOPD, the researchers wrote. Based on their findings, “Eosinophils can not only guide clinical treatment but also be used as an index to predict the clinical outcome and prognosis of AECOPD patients,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM HEART & LUNG
Monkeypox: What’s a pediatrician to do?
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Not long ago, a pediatrician working in a local urgent care clinic called me about a teenage girl with a pruritic rash. She described vesicles and pustules located primarily on the face and arms with no surrounding cellulitis or other exam findings.
“She probably has impetigo,” my colleague said. “But I took a travel and exposure history and learned that her grandma had recently returned home from visiting family in the Congo. Do you think I need to worry about monkeypox?”
While most pediatricians in the United States have never seen a case of monkeypox, the virus is not new. An orthopox, it belongs to the same genus that includes smallpox and cowpox viruses. It was discovered in 1958 when two colonies of monkeys kept for research developed pox-like rashes. The earliest human case was reported in 1970 in the Democratic Republic of Congo and now the virus is endemic in some counties in Central and West Africa.
Monkeypox virus is a zoonotic disease – it can spread from animals to people. Rodents and other small mammals – not monkeys – are thought to be the most likely reservoir. The virus typically spreads from person to person through close contact with skin or respiratory secretions or contact with contaminated fomites. Typical infection begins with fever, lymphadenopathy, and flulike symptoms that include headache and malaise. One to four days after the onset of fever, the characteristic rash begins as macular lesions that evolve into papules, then vesicles, and finally pustules. Pustular lesions are deep-seated, well circumscribed, and are usually the same size and in the same stage of development on a given body site. The rash often starts on the face or the mouth, and then moves to the extremities, including the palms and soles. Over time, the lesions umbilicate and ultimately crust over.
On May 20, the Centers for Disease Control and Prevention issued a Health Advisory describing a case of monkeypox in a patient in Massachusetts. A single case normally wouldn’t cause too much alarm. In fact, there were two cases reported in the United States in 2021, both in travelers returning to the United States from Nigeria, a country in which the virus is endemic. No transmissions from these individuals to close contacts were identified.
The Massachusetts case was remarkable for two reasons. It occurred in an individual who had recently returned from a trip to Canada, which is not a country in which the virus is endemic. Additionally, it occurred in the context of a global outbreak of monkey pox that has, to date, disproportionately affected individuals who identify as men who have sex with men. Patients have often lacked the characteristic prodrome and many have had rash localized to the perianal and genital area, with or without symptoms of proctitis (anorectal pain, tenesmus, and bleeding). Clinically, some lesions mimicked sexually transmitted infections that the occur in the anogenital area, including herpes, syphilis, and lymphogranuloma venereum.
As of May 31, 2022, 17 persons in nine states had been diagnosed with presumed monkeypox virus infection. They ranged in age from 28 to 61 years and 16/17 identified as MSM. Fourteen reported international travel in the 3 weeks before developing symptoms. As of June 12, that number had grown to 53, while worldwide the number of confirmed and suspected cases reached 1,584. Up-to-date case counts are available at https://ourworldindata.org/monkeypox.
Back on the phone, my colleague laughed a little nervously. “I guess I’m not really worried about monkeypox in my patient.” She paused and then asked, “This isn’t going to be the next pandemic, is it?”
Public health experts at the Centers for Disease Control and Prevention and the World Health Organization have been reassuring in that regard. Two vaccines are available for the prevention of monkeypox. JYNNEOS is a nonreplicating live viral vaccine licensed as a two-dose series to prevent both monkeypox and smallpox. ACAM 2000 is a live Vaccinia virus preparation licensed to prevent smallpox. These vaccines are effective when given before exposure but are thought to also beneficial when given as postexposure prophylaxis. According to the CDC, vaccination within 4 days of exposure can prevent the development of disease. Vaccination within 14 days of exposure may not prevent the development of disease but may lessen symptoms. Treatment is generally supportive but antiviral therapy could be considered for individuals with severe disease. Tecovirmat is Food and Drug Administration approved for the treatment of smallpox but is available under nonresearch Expanded Access Investigational New Drug (EA-IND) protocol for the treatment of children and adults with severe orthopox infections, including monkeypox.
So, what’s a pediatrician to do? Take a good travel history, as my colleague did, because that is good medicine. At this point in an outbreak though, a lack of travel does not exclude the diagnosis. Perform a thorough exam of skin and mucosal areas. When there are rashes in the genital or perianal area, consider the possibility of monkeypox in addition to typical sexually transmitted infections. Ask about exposure to other persons with similar rashes, as well as close or intimate contact with a persons in a social network experiencing monkeypox infections. This includes MSM who meet partners through an online website, app, or at social events. Monkeypox can also be spread through contact with an animal (dead or alive) that is an African endemic species or use of a product derived from such animals. Public health experts encourage clinicians to be alert for rash illnesses consistent with monkeypox, regardless of a patient’s gender or sexual orientation, history of international travel, or specific risk factors.
Pediatricians see many kids with rashes, and while cases of monkeypox climb daily, the disease is still very rare. Given the media coverage of the outbreak, pediatricians should be prepared for questions from patients and their parents. Clinicians who suspect a case of monkeypox should contact their local or state health department for guidance and the need for testing. Tips for recognizing monkeypox and distinguishing it from more common viral illnesses such as chicken pox are available at www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Biden boosts LGBTQIA+ protections, bans conversion therapy
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
President Joe Biden issued an executive order on June 15 banning conversion therapy and offering other LBGTQIA+ protections as part of White House efforts to advance equality during Pride Month.
“My order will use the full force of the federal government to end inhumane practices of conversion therapy,” President Biden said in a speech before signing the order. “This is the first time the federal government is making a coordinated effort against this dangerous and discredited practice.”
Conversion therapy is any emotional or physical therapy used to “cure” or “repair” a person’s attraction to the same sex, or their gender identity and expression. Providers claim these therapies can make someone heterosexual or “straight.” But there’s no evidence to support this.
Medical and mental health experts have rejected conversion therapy practices as dangerous and discriminatory for decades.
The executive order also addresses:
- The LGBTQIA+ youth mental health crisis, in part by expanding suicide prevention resources for that at-risk population.
- Discrimination within the foster care system against LGBTQIA+ children and parents.
- Discrimination, poverty and isolation challenges faced by LGBTQIA+ seniors.
- Efforts to strengthen federal data collection in this population to counter homelessness, housing insecurity and barriers to health care access.
Enforcement of executive order will rely on legal experts, including the Justice Department.
President Biden’s order comes at a time when multiple states are promoting or passing anti-LGBTQIA+ laws.
“I don’t have to tell you about the ultra-MAGA agenda attacking our freedoms. There are more than 300 discriminatory bills introduced in states across this country,” President Biden said. “In Texas, they are knocking on front doors to investigate parents who are raising transgender children, and in Florida they are going after Mickey Mouse for God’s sake.”
First Lady Jill Biden, PhD, said the order will not solve all problems. “Prejudice and discrimination still lurk. We will not let the progress we fought for slip away. Pride is a celebration of the courage it takes to stand up for what’s right.”
The American Psychiatric Association applauded President Biden’s action. This executive order will “protect the mental health of LGBTQ+ people, particularly children. APA has long condemned the practice of so-called ‘conversion therapy’ and we welcome the federal government’s efforts to raise public awareness about its harms, alongside other practices that will help to end it.”
The goal of the order is to “improve the health, wellbeing, and safety of countless families across the country,” senior White House administration officials said in a June 15 media call. “And they will send a powerful signal from the president of the United States to LGBTQIA+ kids across the country – who may be feeling scared and hopeless – that their president has their back.”
Biden also called on Congress to pass the Equality Act “to enshrine the long overdue civil rights to protect all Americans.”
The event was held in the East Room of the White House at a Pride event attended by Vice President Kamala Harris and her husband, the first lady, Transportation Secretary Pete Buttigieg, and hundreds of LGBTQIA+ leaders.
Guidance on starting transgender treatment
In other LGBTQIA+-related news, an international group focusing on transgender health lowered the minimum ages they recommend for starting hormone therapy or surgery for transgender youth.
The World Professional Association for Transgender Health said that hormones could be started at 14, 2 years earlier than the group’s previous advice. The association also said some surgeries can be performed at age 15 or 17, a year or so earlier than their previous recommendations.
The group acknowledged potential risks but said it is unethical and harmful to withhold early treatment, according to a report from The Associated Press.
Transgender treatment for teens has been a controversial issue, with experts disagreeing about whether teenagers can fully understand the ramifications of such life-altering decisions.
During the White House background media call, senior administration officials pointed to existing policy regarding transgender care. “We’ve already put out guidance through HHS about civil rights protections and making clear that the denial of medical care based on someone’s gender identity is discriminatory and have invited the members of the public to file complaints with the Office of Civil Rights.”
A version of this article first appeared on WebMD.com.
Snoring may lead to a sedentary lifestyle
“People who snore are also likely to have sleep apnea, but those who snore and don’t have sleep apnea are a largely understudied group,” senior author Michael Grandner, PhD, told this news organization.
“We found that even just snoring alone can impact health and well-being,” said Dr. Grandner, director of the sleep and health research program at the University of Arizona, Tucson.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
A viscous cycle
Frequent snoring can signal sleep-disordered breathing, which is associated with a myriad of comorbidities, including increased risk for cardiovascular disease.
Prior studies have shown that sleep-disordered breathing is associated with less physical activity, but few studies have examined this at the population level or in relation to primary snoring.
Dr. Grandner and colleagues evaluated the relationship between snoring frequency and minutes of sedentary activity using 3 years’ worth of data from the National Health and Nutrition Examination Survey. Participants reported snoring frequency and sedentary activity.
After adjusting for sex, age, race, education level, and marital status, adults who were frequent snorers (5+ nights per week) spent about 36 more minutes per day sedentary, compared with peers who reported never snoring.
In addition, those individuals who were determined to be at increased risk of having sleep apnea had about 54 more minutes per day of sedentary time in the adjusted model.
“Snoring is very common, and it doesn’t just affect the nighttime,” said Dr. Grandner.
Snoring can lead to “more tiredness and less energy, which can impact everything from mood to stress to – as we saw – activity level,” he noted.
Commenting on the results for this news organization, Raman Malhotra, MD, of the Washington University Sleep Center in St. Louis, said this study clearly demonstrates how people who snore and people who are at risk for sleep apnea are more sedentary.
This could explain the “vicious cycle” that these patients suffer from, inasmuch as having obesity can lead to sleep apnea, and having sleep apnea can lead to further sedentary lifestyle and weight gain, owing to lack of energy and feeling tired, Dr. Malhotra told this news organization.
“It is important to intervene and treat the sleep disorder to hopefully make people more active,” he added.
The study had no specific funding. Dr. Grandner and Dr. Malhotra disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“People who snore are also likely to have sleep apnea, but those who snore and don’t have sleep apnea are a largely understudied group,” senior author Michael Grandner, PhD, told this news organization.
“We found that even just snoring alone can impact health and well-being,” said Dr. Grandner, director of the sleep and health research program at the University of Arizona, Tucson.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
A viscous cycle
Frequent snoring can signal sleep-disordered breathing, which is associated with a myriad of comorbidities, including increased risk for cardiovascular disease.
Prior studies have shown that sleep-disordered breathing is associated with less physical activity, but few studies have examined this at the population level or in relation to primary snoring.
Dr. Grandner and colleagues evaluated the relationship between snoring frequency and minutes of sedentary activity using 3 years’ worth of data from the National Health and Nutrition Examination Survey. Participants reported snoring frequency and sedentary activity.
After adjusting for sex, age, race, education level, and marital status, adults who were frequent snorers (5+ nights per week) spent about 36 more minutes per day sedentary, compared with peers who reported never snoring.
In addition, those individuals who were determined to be at increased risk of having sleep apnea had about 54 more minutes per day of sedentary time in the adjusted model.
“Snoring is very common, and it doesn’t just affect the nighttime,” said Dr. Grandner.
Snoring can lead to “more tiredness and less energy, which can impact everything from mood to stress to – as we saw – activity level,” he noted.
Commenting on the results for this news organization, Raman Malhotra, MD, of the Washington University Sleep Center in St. Louis, said this study clearly demonstrates how people who snore and people who are at risk for sleep apnea are more sedentary.
This could explain the “vicious cycle” that these patients suffer from, inasmuch as having obesity can lead to sleep apnea, and having sleep apnea can lead to further sedentary lifestyle and weight gain, owing to lack of energy and feeling tired, Dr. Malhotra told this news organization.
“It is important to intervene and treat the sleep disorder to hopefully make people more active,” he added.
The study had no specific funding. Dr. Grandner and Dr. Malhotra disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“People who snore are also likely to have sleep apnea, but those who snore and don’t have sleep apnea are a largely understudied group,” senior author Michael Grandner, PhD, told this news organization.
“We found that even just snoring alone can impact health and well-being,” said Dr. Grandner, director of the sleep and health research program at the University of Arizona, Tucson.
The findings were presented at the annual meeting of the Associated Professional Sleep Societies.
A viscous cycle
Frequent snoring can signal sleep-disordered breathing, which is associated with a myriad of comorbidities, including increased risk for cardiovascular disease.
Prior studies have shown that sleep-disordered breathing is associated with less physical activity, but few studies have examined this at the population level or in relation to primary snoring.
Dr. Grandner and colleagues evaluated the relationship between snoring frequency and minutes of sedentary activity using 3 years’ worth of data from the National Health and Nutrition Examination Survey. Participants reported snoring frequency and sedentary activity.
After adjusting for sex, age, race, education level, and marital status, adults who were frequent snorers (5+ nights per week) spent about 36 more minutes per day sedentary, compared with peers who reported never snoring.
In addition, those individuals who were determined to be at increased risk of having sleep apnea had about 54 more minutes per day of sedentary time in the adjusted model.
“Snoring is very common, and it doesn’t just affect the nighttime,” said Dr. Grandner.
Snoring can lead to “more tiredness and less energy, which can impact everything from mood to stress to – as we saw – activity level,” he noted.
Commenting on the results for this news organization, Raman Malhotra, MD, of the Washington University Sleep Center in St. Louis, said this study clearly demonstrates how people who snore and people who are at risk for sleep apnea are more sedentary.
This could explain the “vicious cycle” that these patients suffer from, inasmuch as having obesity can lead to sleep apnea, and having sleep apnea can lead to further sedentary lifestyle and weight gain, owing to lack of energy and feeling tired, Dr. Malhotra told this news organization.
“It is important to intervene and treat the sleep disorder to hopefully make people more active,” he added.
The study had no specific funding. Dr. Grandner and Dr. Malhotra disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2022
Basal cell carcinoma
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
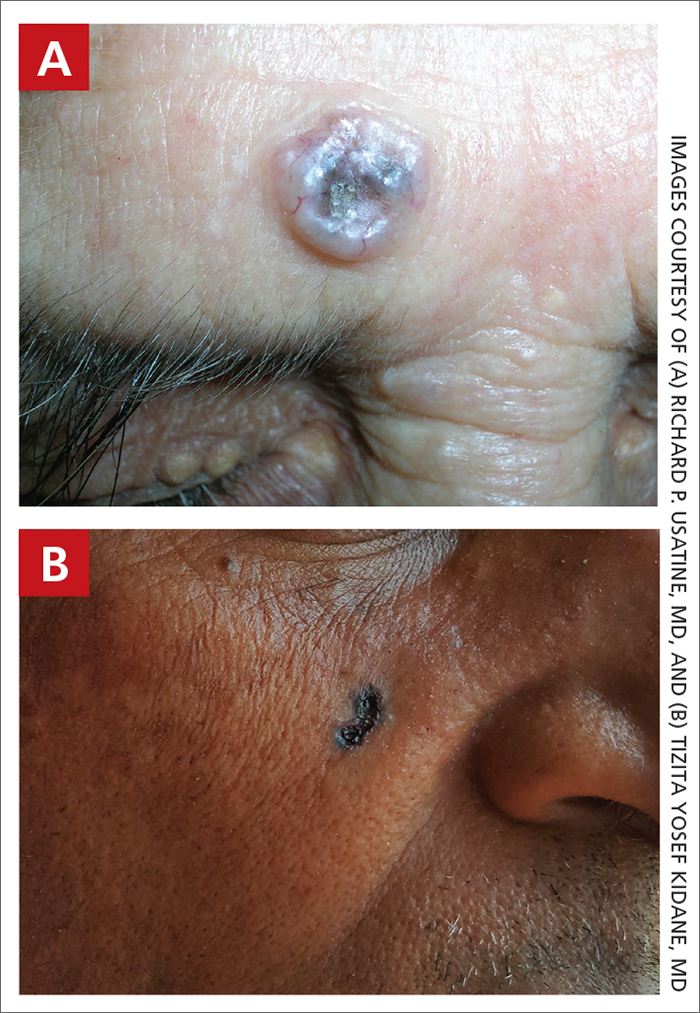
Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.

Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.

Basal cell carcinoma (BCC) is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
BCC is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals. 5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (FIGURE, A) as in non- Hispanic White individuals.7 Any skin cancer can present with ulcerations. So, while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ethnic variability. In my practice in San Antonio (RPU), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (FIGURE, A).
There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes.1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and squamous cell carcinoma found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non-Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (FIGURE, B) should prompt consideration of skin cancer—regardless of skin color.
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
1. Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
2. Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23: 137-151. doi:10.1007/s40257-021-00662-z
3. Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
4. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
5. Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
6. Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/ S0190-9622(81)70067-7
7. Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/ S0190-9622(96)90007-9
8. Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
New law aims to meet crushing need for mental health care professionals
, say state leaders.
Governor J.B. Pritzker (D) signed the legislation, which took effect on June 10.
The law seeks to attract psychologists, social workers, and counselors who have left the workforce within the past 5 years by temporarily ending relicensing requirements, including the need for continuing education credit completion, passing new exams, and fee payments. It also eases the process for those practicing in other states to become licensed in Illinois.
State legislators said there is currently a crushing need for mental health providers, estimating that there are only 14 behavioral health care professionals for every 10,000 Illinois residents. The preamble to the law noted that there will be 8,353 unfilled mental health care jobs in Illinois by 2026.
“We need a mental health care workforce that is robust enough to get people help when they need it – not after months on a waiting list,” Governor Pritzker said in news release. “This legislation invests in mental health infrastructure – and that infrastructure is people,” he added.
Grant pathway
“Being told you have to wait weeks – or months – for care is extremely discouraging,” State Senator Laura Fine (D), a lead sponsor of the legislation, noted in the release.
“We need to support people struggling with mental and behavioral health issues, as well as address difficulties our mental health providers are facing trying to see as many patients as possible,” said Senator Fine.
Marvin Lindsey, CEO of the Community Behavioral Healthcare Association, added that the law would “accelerate the process for out-of-state professionals to obtain their Illinois licensure and [increase] the pipeline and diversity of the behavioral health workforce by implementing a funding mechanism that supports new or existing licensure training of interns.”
The law sets up a grant pathway for community mental health centers, which often serve as training sites. The grants would provide funds to establish or enhance training and supervision of interns and behavioral health providers-in-training seeking to become licensed clinical social workers, licensed clinical professional counselors, or licensed marriage and family therapists.
The money for those grants still has to be appropriated.
The law will also allow patient visits at Specialized Mental Health Rehabilitation Facilities conducted by either a psychiatrist or an advanced practice registered mental health or psychiatric nurse.
Finally, it would establish tax credits for employers who hire individuals in recovery from a substance use disorder or a behavioral disorder. Beginning in January 2023, employers will be eligible for up to $2,000 in credits per employee hired.
A version of this article first appeared on Medscape.com.
, say state leaders.
Governor J.B. Pritzker (D) signed the legislation, which took effect on June 10.
The law seeks to attract psychologists, social workers, and counselors who have left the workforce within the past 5 years by temporarily ending relicensing requirements, including the need for continuing education credit completion, passing new exams, and fee payments. It also eases the process for those practicing in other states to become licensed in Illinois.
State legislators said there is currently a crushing need for mental health providers, estimating that there are only 14 behavioral health care professionals for every 10,000 Illinois residents. The preamble to the law noted that there will be 8,353 unfilled mental health care jobs in Illinois by 2026.
“We need a mental health care workforce that is robust enough to get people help when they need it – not after months on a waiting list,” Governor Pritzker said in news release. “This legislation invests in mental health infrastructure – and that infrastructure is people,” he added.
Grant pathway
“Being told you have to wait weeks – or months – for care is extremely discouraging,” State Senator Laura Fine (D), a lead sponsor of the legislation, noted in the release.
“We need to support people struggling with mental and behavioral health issues, as well as address difficulties our mental health providers are facing trying to see as many patients as possible,” said Senator Fine.
Marvin Lindsey, CEO of the Community Behavioral Healthcare Association, added that the law would “accelerate the process for out-of-state professionals to obtain their Illinois licensure and [increase] the pipeline and diversity of the behavioral health workforce by implementing a funding mechanism that supports new or existing licensure training of interns.”
The law sets up a grant pathway for community mental health centers, which often serve as training sites. The grants would provide funds to establish or enhance training and supervision of interns and behavioral health providers-in-training seeking to become licensed clinical social workers, licensed clinical professional counselors, or licensed marriage and family therapists.
The money for those grants still has to be appropriated.
The law will also allow patient visits at Specialized Mental Health Rehabilitation Facilities conducted by either a psychiatrist or an advanced practice registered mental health or psychiatric nurse.
Finally, it would establish tax credits for employers who hire individuals in recovery from a substance use disorder or a behavioral disorder. Beginning in January 2023, employers will be eligible for up to $2,000 in credits per employee hired.
A version of this article first appeared on Medscape.com.
, say state leaders.
Governor J.B. Pritzker (D) signed the legislation, which took effect on June 10.
The law seeks to attract psychologists, social workers, and counselors who have left the workforce within the past 5 years by temporarily ending relicensing requirements, including the need for continuing education credit completion, passing new exams, and fee payments. It also eases the process for those practicing in other states to become licensed in Illinois.
State legislators said there is currently a crushing need for mental health providers, estimating that there are only 14 behavioral health care professionals for every 10,000 Illinois residents. The preamble to the law noted that there will be 8,353 unfilled mental health care jobs in Illinois by 2026.
“We need a mental health care workforce that is robust enough to get people help when they need it – not after months on a waiting list,” Governor Pritzker said in news release. “This legislation invests in mental health infrastructure – and that infrastructure is people,” he added.
Grant pathway
“Being told you have to wait weeks – or months – for care is extremely discouraging,” State Senator Laura Fine (D), a lead sponsor of the legislation, noted in the release.
“We need to support people struggling with mental and behavioral health issues, as well as address difficulties our mental health providers are facing trying to see as many patients as possible,” said Senator Fine.
Marvin Lindsey, CEO of the Community Behavioral Healthcare Association, added that the law would “accelerate the process for out-of-state professionals to obtain their Illinois licensure and [increase] the pipeline and diversity of the behavioral health workforce by implementing a funding mechanism that supports new or existing licensure training of interns.”
The law sets up a grant pathway for community mental health centers, which often serve as training sites. The grants would provide funds to establish or enhance training and supervision of interns and behavioral health providers-in-training seeking to become licensed clinical social workers, licensed clinical professional counselors, or licensed marriage and family therapists.
The money for those grants still has to be appropriated.
The law will also allow patient visits at Specialized Mental Health Rehabilitation Facilities conducted by either a psychiatrist or an advanced practice registered mental health or psychiatric nurse.
Finally, it would establish tax credits for employers who hire individuals in recovery from a substance use disorder or a behavioral disorder. Beginning in January 2023, employers will be eligible for up to $2,000 in credits per employee hired.
A version of this article first appeared on Medscape.com.
Air pollution tied to ventricular arrhythmias in those with ICDs
Ventricular arrhythmias more commonly occur on days when there are higher levels of air pollution, especially with fine particulate matter (PM), a new study suggests.
The investigators studied the relationship between air pollution and ventricular arrhythmias in Piacenza, Italy by examining 5-year data on patients who received an implantable cardioverter defibrillator (ICD).
They found a significant association between PM2.5 levels and ventricular arrhythmias, especially those treated with direct current shock. Moreover, higher levels of PM2.5 and PM10 were associated with increased risk of all ventricular arrhythmias.
“These data confirm that environmental pollution is not only a climate emergency but also a public health problem,” lead author Alessia Zanni, currently at Maggiore Hospital, Bologna, Italy, and previously at Piacenza Hospital, said in an interview.
“The study suggests that the survival of patients with heart disease is affected not only by pharmacological therapies and advances in cardiology, but also by the air that they breathe,” she said.
The results were presented at European Society of Cardiology Heart Failure 2022.
More ED visits
The World Health Organization estimates around 7 million people die every year from exposure to polluted air, “as 91% of the world’s population lives in areas where air contaminants exceed safety levels,” Dr. Zanni said. Furthermore, “air pollution has been defined as the fourth-highest ranking risk factor for mortality – more important than LDL cholesterol, obesity, physical activity, or alcohol use.”
She noted that Piacenza has “historically been very attentive to the issues of early defibrillation and cardiac arrest.” Her group had previously found a correlation between out-of-hospital cardiac arrests and air pollution in the general population.
Moreover, her group recently observed that ED visits for patients with ICDs “tended to cluster; on some special days, many patients with ICDs had cardiac arrhythmias, and during those days, air pollution levels were particularly high.”
Her group therefore decided to compare the concentration of air pollutants on days when patients suffered from an arrhythmia event versus pollution levels on days without an arrhythmia, she said.
Further piece in a complex puzzle
The researchers studied 146 patients with ICDs between January 2013 and December 2017, assigning exposures (short, mid, and long term) to these patients based on their residential addresses.
They extracted day-by-day urban PM10, PM2.5, CO, NO2, and O3 levels from the Environmental Protection Agency monitoring stations and then, using time-stratified case-crossover analysis methodology, they calculated the association of ventricular arrhythmia onset with 0- to 7-day moving averages of the various air pollutants prior to the event.
Patients had received their ICD to control cardiac dysfunction brought on by previous myocardial infarction (n = 93), genetic or inflammatory conditions (n = 53), secondary prevention after a lethal arrhythmia (n = 67), and primary prevention (n = 79).
Of the 440 ventricular arrhythmias recorded, 322 were treated with antitachycardia pacing, while the remaining 118 were treated with direct current shock.
The researchers found a significant association between PM2.5 levels and ventricular arrhythmia treated with shock, corresponding to a 15% increased risk or every additional 10mg/m3 (P < .019).
They also found that, when PM2.5 concentrations were elevated by 1 mg/m3 for an entire week, compared with average levels, there was a 2.4% higher likelihood of ventricular arrhythmias, regardless of the temperature, and when PM10 was 1 mg/m3 above average for a week, there was a 2.1% increased risk for arrhythmias (odds ratio, 1,024; 95% confidence interval, 1,009-1,040] and OR, 1,021; 95% CI, 1,009-1,033, respectively), Dr. Zanni reported.
“Since the majority of out-of-hospital cardiac arrest causes still remain unclear, our data add a further piece to the complex puzzle of cardiac arrest triggers,” Dr. Zanni commented. “We think that particulate matter can cause acute inflammation of the heart muscle and potentially act as a trigger for lethal cardiac arrhythmias.
“As these toxic particles are emitted from power plants, industries, and cars, we think that cardiovascular research should highlight these new findings to promote green projects among the general population, clarifying the risks to the health of the human being, and we think strategies to prevent air pollutant exposure in high-risk patients [with previous cardiac disease] should be developed,” she added.
Further, “we advise patients at risk, during days with high PM2.5 (> 35 mg/m3) and PM10 (> 50 mg/m3) to use a mask of the N95 type outdoors, to reduce time spent outdoors – particularly in traffic – and to improve home air filtration,” Dr. Zanni said.
Entering the mainstream
In a comment, Joel Kaufman, MD, MPH, professor of internal medicine and environmental health, University of Washington, Seattle, said the study “adds to a fairly substantial literature already on this topic of short-term exposure to air pollution.”
The evidence that air pollutants “can be a trigger of worsening of cardiovascular disease is fairly consistent at this time, and although the effect sizes are small, they are consistent,” said Dr. Kaufman, who was the chair of the writing group for the American Heart Association’s 2020 policy statement, “Guidance to Reduce Cardiovascular Burden of Ambient Air Pollutants.”
“The research into this issue has become clearer during the past 10 years but still is not in the mainstream of most cardiologists’ awareness. They tend to focus more on controlling cholesterol and performing procedures, etc., but there are modifiable risk factors like air pollution that are increasingly recognized as being part of the picture,” said Dr. Kaufman, who was not involved with the current study.
Dr. Zanni added: “It is important that politics work hand in hand with the scientific community in order to win the battle against global warming, which will reduce the number of cardiovascular deaths – the leading cause of death worldwide – as well as environmental integrity.”
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Zanni and coauthors and Dr. Kaufman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ventricular arrhythmias more commonly occur on days when there are higher levels of air pollution, especially with fine particulate matter (PM), a new study suggests.
The investigators studied the relationship between air pollution and ventricular arrhythmias in Piacenza, Italy by examining 5-year data on patients who received an implantable cardioverter defibrillator (ICD).
They found a significant association between PM2.5 levels and ventricular arrhythmias, especially those treated with direct current shock. Moreover, higher levels of PM2.5 and PM10 were associated with increased risk of all ventricular arrhythmias.
“These data confirm that environmental pollution is not only a climate emergency but also a public health problem,” lead author Alessia Zanni, currently at Maggiore Hospital, Bologna, Italy, and previously at Piacenza Hospital, said in an interview.
“The study suggests that the survival of patients with heart disease is affected not only by pharmacological therapies and advances in cardiology, but also by the air that they breathe,” she said.
The results were presented at European Society of Cardiology Heart Failure 2022.
More ED visits
The World Health Organization estimates around 7 million people die every year from exposure to polluted air, “as 91% of the world’s population lives in areas where air contaminants exceed safety levels,” Dr. Zanni said. Furthermore, “air pollution has been defined as the fourth-highest ranking risk factor for mortality – more important than LDL cholesterol, obesity, physical activity, or alcohol use.”
She noted that Piacenza has “historically been very attentive to the issues of early defibrillation and cardiac arrest.” Her group had previously found a correlation between out-of-hospital cardiac arrests and air pollution in the general population.
Moreover, her group recently observed that ED visits for patients with ICDs “tended to cluster; on some special days, many patients with ICDs had cardiac arrhythmias, and during those days, air pollution levels were particularly high.”
Her group therefore decided to compare the concentration of air pollutants on days when patients suffered from an arrhythmia event versus pollution levels on days without an arrhythmia, she said.
Further piece in a complex puzzle
The researchers studied 146 patients with ICDs between January 2013 and December 2017, assigning exposures (short, mid, and long term) to these patients based on their residential addresses.
They extracted day-by-day urban PM10, PM2.5, CO, NO2, and O3 levels from the Environmental Protection Agency monitoring stations and then, using time-stratified case-crossover analysis methodology, they calculated the association of ventricular arrhythmia onset with 0- to 7-day moving averages of the various air pollutants prior to the event.
Patients had received their ICD to control cardiac dysfunction brought on by previous myocardial infarction (n = 93), genetic or inflammatory conditions (n = 53), secondary prevention after a lethal arrhythmia (n = 67), and primary prevention (n = 79).
Of the 440 ventricular arrhythmias recorded, 322 were treated with antitachycardia pacing, while the remaining 118 were treated with direct current shock.
The researchers found a significant association between PM2.5 levels and ventricular arrhythmia treated with shock, corresponding to a 15% increased risk or every additional 10mg/m3 (P < .019).
They also found that, when PM2.5 concentrations were elevated by 1 mg/m3 for an entire week, compared with average levels, there was a 2.4% higher likelihood of ventricular arrhythmias, regardless of the temperature, and when PM10 was 1 mg/m3 above average for a week, there was a 2.1% increased risk for arrhythmias (odds ratio, 1,024; 95% confidence interval, 1,009-1,040] and OR, 1,021; 95% CI, 1,009-1,033, respectively), Dr. Zanni reported.
“Since the majority of out-of-hospital cardiac arrest causes still remain unclear, our data add a further piece to the complex puzzle of cardiac arrest triggers,” Dr. Zanni commented. “We think that particulate matter can cause acute inflammation of the heart muscle and potentially act as a trigger for lethal cardiac arrhythmias.
“As these toxic particles are emitted from power plants, industries, and cars, we think that cardiovascular research should highlight these new findings to promote green projects among the general population, clarifying the risks to the health of the human being, and we think strategies to prevent air pollutant exposure in high-risk patients [with previous cardiac disease] should be developed,” she added.
Further, “we advise patients at risk, during days with high PM2.5 (> 35 mg/m3) and PM10 (> 50 mg/m3) to use a mask of the N95 type outdoors, to reduce time spent outdoors – particularly in traffic – and to improve home air filtration,” Dr. Zanni said.
Entering the mainstream
In a comment, Joel Kaufman, MD, MPH, professor of internal medicine and environmental health, University of Washington, Seattle, said the study “adds to a fairly substantial literature already on this topic of short-term exposure to air pollution.”
The evidence that air pollutants “can be a trigger of worsening of cardiovascular disease is fairly consistent at this time, and although the effect sizes are small, they are consistent,” said Dr. Kaufman, who was the chair of the writing group for the American Heart Association’s 2020 policy statement, “Guidance to Reduce Cardiovascular Burden of Ambient Air Pollutants.”
“The research into this issue has become clearer during the past 10 years but still is not in the mainstream of most cardiologists’ awareness. They tend to focus more on controlling cholesterol and performing procedures, etc., but there are modifiable risk factors like air pollution that are increasingly recognized as being part of the picture,” said Dr. Kaufman, who was not involved with the current study.
Dr. Zanni added: “It is important that politics work hand in hand with the scientific community in order to win the battle against global warming, which will reduce the number of cardiovascular deaths – the leading cause of death worldwide – as well as environmental integrity.”
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Zanni and coauthors and Dr. Kaufman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ventricular arrhythmias more commonly occur on days when there are higher levels of air pollution, especially with fine particulate matter (PM), a new study suggests.
The investigators studied the relationship between air pollution and ventricular arrhythmias in Piacenza, Italy by examining 5-year data on patients who received an implantable cardioverter defibrillator (ICD).
They found a significant association between PM2.5 levels and ventricular arrhythmias, especially those treated with direct current shock. Moreover, higher levels of PM2.5 and PM10 were associated with increased risk of all ventricular arrhythmias.
“These data confirm that environmental pollution is not only a climate emergency but also a public health problem,” lead author Alessia Zanni, currently at Maggiore Hospital, Bologna, Italy, and previously at Piacenza Hospital, said in an interview.
“The study suggests that the survival of patients with heart disease is affected not only by pharmacological therapies and advances in cardiology, but also by the air that they breathe,” she said.
The results were presented at European Society of Cardiology Heart Failure 2022.
More ED visits
The World Health Organization estimates around 7 million people die every year from exposure to polluted air, “as 91% of the world’s population lives in areas where air contaminants exceed safety levels,” Dr. Zanni said. Furthermore, “air pollution has been defined as the fourth-highest ranking risk factor for mortality – more important than LDL cholesterol, obesity, physical activity, or alcohol use.”
She noted that Piacenza has “historically been very attentive to the issues of early defibrillation and cardiac arrest.” Her group had previously found a correlation between out-of-hospital cardiac arrests and air pollution in the general population.
Moreover, her group recently observed that ED visits for patients with ICDs “tended to cluster; on some special days, many patients with ICDs had cardiac arrhythmias, and during those days, air pollution levels were particularly high.”
Her group therefore decided to compare the concentration of air pollutants on days when patients suffered from an arrhythmia event versus pollution levels on days without an arrhythmia, she said.
Further piece in a complex puzzle
The researchers studied 146 patients with ICDs between January 2013 and December 2017, assigning exposures (short, mid, and long term) to these patients based on their residential addresses.
They extracted day-by-day urban PM10, PM2.5, CO, NO2, and O3 levels from the Environmental Protection Agency monitoring stations and then, using time-stratified case-crossover analysis methodology, they calculated the association of ventricular arrhythmia onset with 0- to 7-day moving averages of the various air pollutants prior to the event.
Patients had received their ICD to control cardiac dysfunction brought on by previous myocardial infarction (n = 93), genetic or inflammatory conditions (n = 53), secondary prevention after a lethal arrhythmia (n = 67), and primary prevention (n = 79).
Of the 440 ventricular arrhythmias recorded, 322 were treated with antitachycardia pacing, while the remaining 118 were treated with direct current shock.
The researchers found a significant association between PM2.5 levels and ventricular arrhythmia treated with shock, corresponding to a 15% increased risk or every additional 10mg/m3 (P < .019).
They also found that, when PM2.5 concentrations were elevated by 1 mg/m3 for an entire week, compared with average levels, there was a 2.4% higher likelihood of ventricular arrhythmias, regardless of the temperature, and when PM10 was 1 mg/m3 above average for a week, there was a 2.1% increased risk for arrhythmias (odds ratio, 1,024; 95% confidence interval, 1,009-1,040] and OR, 1,021; 95% CI, 1,009-1,033, respectively), Dr. Zanni reported.
“Since the majority of out-of-hospital cardiac arrest causes still remain unclear, our data add a further piece to the complex puzzle of cardiac arrest triggers,” Dr. Zanni commented. “We think that particulate matter can cause acute inflammation of the heart muscle and potentially act as a trigger for lethal cardiac arrhythmias.
“As these toxic particles are emitted from power plants, industries, and cars, we think that cardiovascular research should highlight these new findings to promote green projects among the general population, clarifying the risks to the health of the human being, and we think strategies to prevent air pollutant exposure in high-risk patients [with previous cardiac disease] should be developed,” she added.
Further, “we advise patients at risk, during days with high PM2.5 (> 35 mg/m3) and PM10 (> 50 mg/m3) to use a mask of the N95 type outdoors, to reduce time spent outdoors – particularly in traffic – and to improve home air filtration,” Dr. Zanni said.
Entering the mainstream
In a comment, Joel Kaufman, MD, MPH, professor of internal medicine and environmental health, University of Washington, Seattle, said the study “adds to a fairly substantial literature already on this topic of short-term exposure to air pollution.”
The evidence that air pollutants “can be a trigger of worsening of cardiovascular disease is fairly consistent at this time, and although the effect sizes are small, they are consistent,” said Dr. Kaufman, who was the chair of the writing group for the American Heart Association’s 2020 policy statement, “Guidance to Reduce Cardiovascular Burden of Ambient Air Pollutants.”
“The research into this issue has become clearer during the past 10 years but still is not in the mainstream of most cardiologists’ awareness. They tend to focus more on controlling cholesterol and performing procedures, etc., but there are modifiable risk factors like air pollution that are increasingly recognized as being part of the picture,” said Dr. Kaufman, who was not involved with the current study.
Dr. Zanni added: “It is important that politics work hand in hand with the scientific community in order to win the battle against global warming, which will reduce the number of cardiovascular deaths – the leading cause of death worldwide – as well as environmental integrity.”
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Zanni and coauthors and Dr. Kaufman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC HEART FAILURE 2022
Hypothyroidism: No more waiting to eat or drink with liquid thyroxine?
ATLANTA -- Liquid formulations of levothyroxine offer the possibility of allowing patients with hypothyroidism to take their medication with meals or coffee and skip the currently recommended 30- to 60-minute waiting period before doing either, new data suggest.
Because food, coffee, and certain medications can interfere with intestinal absorption of levothyroxine (also known as LT4), current guidelines recommend that the drug be taken in a fasting state, typically 30-60 minutes before breakfast. However, compliance may be difficult for some patients.
Now, a potential solution may come from new evidence that liquid levothyroxine formulations that bypass the gastric dissolution phase of absorption may mitigate the interference with food and coffee.
Findings from two bioavailability studies showing no difference in comparisons of Thyquidity (levothyroxine sodium oral solution, Vertice Pharma) with or without waiting periods before consuming coffee or a high-fat meal were presented at the annual meeting of the Endocrine Society (ENDO 2022), by Vertice Pharma Medical Director Kris Washington, PharmD.
And just last month, similar data were published in Thyroid for another levothyroxine oral solution, Tirosint-SOL (IBSA). No difference in pharmacokinetic properties were found with this product with a shorter versus a longer waiting period before consuming a high-fat meal.
Liquid thyroxine may be less affected by food/drink but is expensive
Both products have been approved by the U.S. Food and Drug Administration, but current labeling for both still calls for a 30- to 60-minute waiting period between taking the medication and eating or drinking. Thyquidity is an oral solution of 100 µg/mL levothyroxine sodium that has been shown to be bioequivalent to one of the most popular branded levothyroxine tablets, Synthroid (AbbVie), under fasting conditions. Tirosint-SOL is also an oral solution that comes in 15 different dosage ampules.
“It is important to note that while these findings are exciting and encouraging, we do want you to continue to follow the current FDA-approved label for Thyquidity, recommending that it be taken on an empty stomach 30-60 minutes prior to breakfast and that patients continue to follow all other label instructions,” Dr. Washington said during a press briefing at ENDO 2022.
When asked whether the new data would be submitted to the FDA for a possible amendment to this message, she replied: “We’re still discussing that. We’re exploring all options. ... This is fairly new data. ... It makes sense and certainly solves a lot of the challenges for people who can’t swallow or don’t choose to swallow, or the challenges of splitting or crushing with tablets.”
Asked to comment, Benjamin J. Gigliotti, MD, a clinical thyroidologist at the University of Rochester, New York, told this news organization: “Liquid levothyroxine has the potential to be a clinically useful formulation,” noting that these recent data corroborate prior findings from Europe and elsewhere that liquid levothyroxine is absorbed more rapidly and thus may be less impacted by food or beverages.
However, Dr. Gigliotti also pointed out, “I don’t think malabsorption is a major contributor to suboptimal treatment because if [patients] malabsorb the hormone, we typically just increase their dose a little bit or ask them to take it separately, and that works just fine for most people.”
And the higher cost of the liquid products is a major issue, he noted.
A quick search on GoodRx shows that the lowest price of Tirosint-SOL is $115.52 for a 1 month supply and Thyquidity is $181.04/month. “In the few patients where I tried to obtain Tirosint-SOL, it was not covered by insurance, even with a prior authorization,” Dr. Gigliotti commented.
In contrast, generic levothyroxine tablets are about $4/month, while a common brand name of levothyroxine tablets are $47.81/month.
“Until these liquid formulations are more widely covered by insurance for a reasonable copay, or come down in price compared to generic levothyroxine tablets, most of my patients have voiced that they’d rather deal with the inconveniences of a tablet compared to higher medication cost, especially with rising economic insecurity imposed by the COVID-19 pandemic and recent world events,” Dr. Gigliotti said.
Bioequivalence with shorter versus longer waits before coffee/breakfast
The Thyquidity coffee study was a single-center open-label, randomized, crossover study of 40 healthy adults randomized after a 10-hour overnight fast to 600 µg Thyquidity with water under fasting conditions or to the same dose given 5 minutes prior to drinking an 8-ounce cup of American coffee without milk or sweeteners. After a 40-day washout period, the same participants received the other treatment.
Mean serum thyroxine (T4) concentrations over 48 hours were nearly identical, demonstrating comparable bioavailability. Pharmacokinetics parameters, including area under the curve (AUC) and Cmax, were also comparable for both groups. The geometric least square mean ratios for baseline-adjusted LT4 were 96.0% for Cmax and 94% for AUC. And the corresponding 90% confidence intervals fell within the 80%-125% FDA acceptance range for absence of a food effect on bioavailability, said Dr. Washington when presenting the findings.
There was one adverse event, a decrease in blood glucose level, which was deemed to be mild and unrelated to study treatment. No deaths, serious adverse events, or discontinuations due to adverse events were reported. There were no significant changes in vital signs or on ECG.
In the second Thyquidity study of 38 healthy adults, after a 10-hour fast, the same doses were given 10 or 30 minutes prior to the consumption of a 950-calorie standardized high-fat breakfast.
Again, over 48 hours, mean serum T4 levels were comparable between the two groups. The geometric least squares mean ratios for both AUC and Cmax for baseline-adjusted LT4 were 88.7% and 85.1%, respectively. Again, the corresponding 90% confidence intervals fell within the FDA’s noninterference definition, again demonstrating lack of a food effect on bioavailability, Dr. Washington noted.
Four adverse events were reported in three participants, with three deemed to be possibly related to the medication. All were isolated lab abnormalities without clinical symptoms and deemed to be mild. Three were normal on repeat testing.
There were no deaths or serious adverse events or study discontinuations for adverse events and no significant findings for vital signs or on ECG.
Similar findings for Tirosint-SOL but longer-term studies needed
The recently published Tirosint-SOL study included 36 healthy volunteers randomized to single 600-µg doses of the LT4 oral solution after a 10-hour fast, either 15 or 30 minutes before eating a standardized high-fat, high-calorie meal. Mean serum total thyroxine concentration profiles were similar for both the 15- and 30-minute waits, with similar AUCs.
Geometric mean ratios for AUCs at 48 and 72 hours were 90% and 92%, respectively, and the 90% confidence intervals fell within the 80%-125% FDA boundaries, suggesting similar exposures whether taken 15 or 30 minutes before a meal.
Senior author Francesco S. Celi, MD, chair of the division of endocrinology, diabetes, and metabolism at Virginia Commonwealth University, Richmond, told this news organization: “There is an interest in providing more opportunities for patients and improving adherence to the medication. ... Whatever makes life a bit easier for patients and results in a more predictable response to treatment means down the road there will be fewer visits to the doctor to make adjustments.”
However, he said that in addition to the cost and reimbursement issue, all of these studies have been short term and not conducted in real-life settings.
“Another question is: What happens if the patient goes on low-dose LT4? The studies were conducted on much higher pharmacologic doses. But at least from a safety standpoint, there’s no specific concern.”
Dr. Washington is an employee of Vertice Pharma. Dr. Celi has received unrestricted research grants and worked as a consultant for IBSA. Dr. Gigliotti has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA -- Liquid formulations of levothyroxine offer the possibility of allowing patients with hypothyroidism to take their medication with meals or coffee and skip the currently recommended 30- to 60-minute waiting period before doing either, new data suggest.
Because food, coffee, and certain medications can interfere with intestinal absorption of levothyroxine (also known as LT4), current guidelines recommend that the drug be taken in a fasting state, typically 30-60 minutes before breakfast. However, compliance may be difficult for some patients.
Now, a potential solution may come from new evidence that liquid levothyroxine formulations that bypass the gastric dissolution phase of absorption may mitigate the interference with food and coffee.
Findings from two bioavailability studies showing no difference in comparisons of Thyquidity (levothyroxine sodium oral solution, Vertice Pharma) with or without waiting periods before consuming coffee or a high-fat meal were presented at the annual meeting of the Endocrine Society (ENDO 2022), by Vertice Pharma Medical Director Kris Washington, PharmD.
And just last month, similar data were published in Thyroid for another levothyroxine oral solution, Tirosint-SOL (IBSA). No difference in pharmacokinetic properties were found with this product with a shorter versus a longer waiting period before consuming a high-fat meal.
Liquid thyroxine may be less affected by food/drink but is expensive
Both products have been approved by the U.S. Food and Drug Administration, but current labeling for both still calls for a 30- to 60-minute waiting period between taking the medication and eating or drinking. Thyquidity is an oral solution of 100 µg/mL levothyroxine sodium that has been shown to be bioequivalent to one of the most popular branded levothyroxine tablets, Synthroid (AbbVie), under fasting conditions. Tirosint-SOL is also an oral solution that comes in 15 different dosage ampules.
“It is important to note that while these findings are exciting and encouraging, we do want you to continue to follow the current FDA-approved label for Thyquidity, recommending that it be taken on an empty stomach 30-60 minutes prior to breakfast and that patients continue to follow all other label instructions,” Dr. Washington said during a press briefing at ENDO 2022.
When asked whether the new data would be submitted to the FDA for a possible amendment to this message, she replied: “We’re still discussing that. We’re exploring all options. ... This is fairly new data. ... It makes sense and certainly solves a lot of the challenges for people who can’t swallow or don’t choose to swallow, or the challenges of splitting or crushing with tablets.”
Asked to comment, Benjamin J. Gigliotti, MD, a clinical thyroidologist at the University of Rochester, New York, told this news organization: “Liquid levothyroxine has the potential to be a clinically useful formulation,” noting that these recent data corroborate prior findings from Europe and elsewhere that liquid levothyroxine is absorbed more rapidly and thus may be less impacted by food or beverages.
However, Dr. Gigliotti also pointed out, “I don’t think malabsorption is a major contributor to suboptimal treatment because if [patients] malabsorb the hormone, we typically just increase their dose a little bit or ask them to take it separately, and that works just fine for most people.”
And the higher cost of the liquid products is a major issue, he noted.
A quick search on GoodRx shows that the lowest price of Tirosint-SOL is $115.52 for a 1 month supply and Thyquidity is $181.04/month. “In the few patients where I tried to obtain Tirosint-SOL, it was not covered by insurance, even with a prior authorization,” Dr. Gigliotti commented.
In contrast, generic levothyroxine tablets are about $4/month, while a common brand name of levothyroxine tablets are $47.81/month.
“Until these liquid formulations are more widely covered by insurance for a reasonable copay, or come down in price compared to generic levothyroxine tablets, most of my patients have voiced that they’d rather deal with the inconveniences of a tablet compared to higher medication cost, especially with rising economic insecurity imposed by the COVID-19 pandemic and recent world events,” Dr. Gigliotti said.
Bioequivalence with shorter versus longer waits before coffee/breakfast
The Thyquidity coffee study was a single-center open-label, randomized, crossover study of 40 healthy adults randomized after a 10-hour overnight fast to 600 µg Thyquidity with water under fasting conditions or to the same dose given 5 minutes prior to drinking an 8-ounce cup of American coffee without milk or sweeteners. After a 40-day washout period, the same participants received the other treatment.
Mean serum thyroxine (T4) concentrations over 48 hours were nearly identical, demonstrating comparable bioavailability. Pharmacokinetics parameters, including area under the curve (AUC) and Cmax, were also comparable for both groups. The geometric least square mean ratios for baseline-adjusted LT4 were 96.0% for Cmax and 94% for AUC. And the corresponding 90% confidence intervals fell within the 80%-125% FDA acceptance range for absence of a food effect on bioavailability, said Dr. Washington when presenting the findings.
There was one adverse event, a decrease in blood glucose level, which was deemed to be mild and unrelated to study treatment. No deaths, serious adverse events, or discontinuations due to adverse events were reported. There were no significant changes in vital signs or on ECG.
In the second Thyquidity study of 38 healthy adults, after a 10-hour fast, the same doses were given 10 or 30 minutes prior to the consumption of a 950-calorie standardized high-fat breakfast.
Again, over 48 hours, mean serum T4 levels were comparable between the two groups. The geometric least squares mean ratios for both AUC and Cmax for baseline-adjusted LT4 were 88.7% and 85.1%, respectively. Again, the corresponding 90% confidence intervals fell within the FDA’s noninterference definition, again demonstrating lack of a food effect on bioavailability, Dr. Washington noted.
Four adverse events were reported in three participants, with three deemed to be possibly related to the medication. All were isolated lab abnormalities without clinical symptoms and deemed to be mild. Three were normal on repeat testing.
There were no deaths or serious adverse events or study discontinuations for adverse events and no significant findings for vital signs or on ECG.
Similar findings for Tirosint-SOL but longer-term studies needed
The recently published Tirosint-SOL study included 36 healthy volunteers randomized to single 600-µg doses of the LT4 oral solution after a 10-hour fast, either 15 or 30 minutes before eating a standardized high-fat, high-calorie meal. Mean serum total thyroxine concentration profiles were similar for both the 15- and 30-minute waits, with similar AUCs.
Geometric mean ratios for AUCs at 48 and 72 hours were 90% and 92%, respectively, and the 90% confidence intervals fell within the 80%-125% FDA boundaries, suggesting similar exposures whether taken 15 or 30 minutes before a meal.
Senior author Francesco S. Celi, MD, chair of the division of endocrinology, diabetes, and metabolism at Virginia Commonwealth University, Richmond, told this news organization: “There is an interest in providing more opportunities for patients and improving adherence to the medication. ... Whatever makes life a bit easier for patients and results in a more predictable response to treatment means down the road there will be fewer visits to the doctor to make adjustments.”
However, he said that in addition to the cost and reimbursement issue, all of these studies have been short term and not conducted in real-life settings.
“Another question is: What happens if the patient goes on low-dose LT4? The studies were conducted on much higher pharmacologic doses. But at least from a safety standpoint, there’s no specific concern.”
Dr. Washington is an employee of Vertice Pharma. Dr. Celi has received unrestricted research grants and worked as a consultant for IBSA. Dr. Gigliotti has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA -- Liquid formulations of levothyroxine offer the possibility of allowing patients with hypothyroidism to take their medication with meals or coffee and skip the currently recommended 30- to 60-minute waiting period before doing either, new data suggest.
Because food, coffee, and certain medications can interfere with intestinal absorption of levothyroxine (also known as LT4), current guidelines recommend that the drug be taken in a fasting state, typically 30-60 minutes before breakfast. However, compliance may be difficult for some patients.
Now, a potential solution may come from new evidence that liquid levothyroxine formulations that bypass the gastric dissolution phase of absorption may mitigate the interference with food and coffee.
Findings from two bioavailability studies showing no difference in comparisons of Thyquidity (levothyroxine sodium oral solution, Vertice Pharma) with or without waiting periods before consuming coffee or a high-fat meal were presented at the annual meeting of the Endocrine Society (ENDO 2022), by Vertice Pharma Medical Director Kris Washington, PharmD.
And just last month, similar data were published in Thyroid for another levothyroxine oral solution, Tirosint-SOL (IBSA). No difference in pharmacokinetic properties were found with this product with a shorter versus a longer waiting period before consuming a high-fat meal.
Liquid thyroxine may be less affected by food/drink but is expensive
Both products have been approved by the U.S. Food and Drug Administration, but current labeling for both still calls for a 30- to 60-minute waiting period between taking the medication and eating or drinking. Thyquidity is an oral solution of 100 µg/mL levothyroxine sodium that has been shown to be bioequivalent to one of the most popular branded levothyroxine tablets, Synthroid (AbbVie), under fasting conditions. Tirosint-SOL is also an oral solution that comes in 15 different dosage ampules.
“It is important to note that while these findings are exciting and encouraging, we do want you to continue to follow the current FDA-approved label for Thyquidity, recommending that it be taken on an empty stomach 30-60 minutes prior to breakfast and that patients continue to follow all other label instructions,” Dr. Washington said during a press briefing at ENDO 2022.
When asked whether the new data would be submitted to the FDA for a possible amendment to this message, she replied: “We’re still discussing that. We’re exploring all options. ... This is fairly new data. ... It makes sense and certainly solves a lot of the challenges for people who can’t swallow or don’t choose to swallow, or the challenges of splitting or crushing with tablets.”
Asked to comment, Benjamin J. Gigliotti, MD, a clinical thyroidologist at the University of Rochester, New York, told this news organization: “Liquid levothyroxine has the potential to be a clinically useful formulation,” noting that these recent data corroborate prior findings from Europe and elsewhere that liquid levothyroxine is absorbed more rapidly and thus may be less impacted by food or beverages.
However, Dr. Gigliotti also pointed out, “I don’t think malabsorption is a major contributor to suboptimal treatment because if [patients] malabsorb the hormone, we typically just increase their dose a little bit or ask them to take it separately, and that works just fine for most people.”
And the higher cost of the liquid products is a major issue, he noted.
A quick search on GoodRx shows that the lowest price of Tirosint-SOL is $115.52 for a 1 month supply and Thyquidity is $181.04/month. “In the few patients where I tried to obtain Tirosint-SOL, it was not covered by insurance, even with a prior authorization,” Dr. Gigliotti commented.
In contrast, generic levothyroxine tablets are about $4/month, while a common brand name of levothyroxine tablets are $47.81/month.
“Until these liquid formulations are more widely covered by insurance for a reasonable copay, or come down in price compared to generic levothyroxine tablets, most of my patients have voiced that they’d rather deal with the inconveniences of a tablet compared to higher medication cost, especially with rising economic insecurity imposed by the COVID-19 pandemic and recent world events,” Dr. Gigliotti said.
Bioequivalence with shorter versus longer waits before coffee/breakfast
The Thyquidity coffee study was a single-center open-label, randomized, crossover study of 40 healthy adults randomized after a 10-hour overnight fast to 600 µg Thyquidity with water under fasting conditions or to the same dose given 5 minutes prior to drinking an 8-ounce cup of American coffee without milk or sweeteners. After a 40-day washout period, the same participants received the other treatment.
Mean serum thyroxine (T4) concentrations over 48 hours were nearly identical, demonstrating comparable bioavailability. Pharmacokinetics parameters, including area under the curve (AUC) and Cmax, were also comparable for both groups. The geometric least square mean ratios for baseline-adjusted LT4 were 96.0% for Cmax and 94% for AUC. And the corresponding 90% confidence intervals fell within the 80%-125% FDA acceptance range for absence of a food effect on bioavailability, said Dr. Washington when presenting the findings.
There was one adverse event, a decrease in blood glucose level, which was deemed to be mild and unrelated to study treatment. No deaths, serious adverse events, or discontinuations due to adverse events were reported. There were no significant changes in vital signs or on ECG.
In the second Thyquidity study of 38 healthy adults, after a 10-hour fast, the same doses were given 10 or 30 minutes prior to the consumption of a 950-calorie standardized high-fat breakfast.
Again, over 48 hours, mean serum T4 levels were comparable between the two groups. The geometric least squares mean ratios for both AUC and Cmax for baseline-adjusted LT4 were 88.7% and 85.1%, respectively. Again, the corresponding 90% confidence intervals fell within the FDA’s noninterference definition, again demonstrating lack of a food effect on bioavailability, Dr. Washington noted.
Four adverse events were reported in three participants, with three deemed to be possibly related to the medication. All were isolated lab abnormalities without clinical symptoms and deemed to be mild. Three were normal on repeat testing.
There were no deaths or serious adverse events or study discontinuations for adverse events and no significant findings for vital signs or on ECG.
Similar findings for Tirosint-SOL but longer-term studies needed
The recently published Tirosint-SOL study included 36 healthy volunteers randomized to single 600-µg doses of the LT4 oral solution after a 10-hour fast, either 15 or 30 minutes before eating a standardized high-fat, high-calorie meal. Mean serum total thyroxine concentration profiles were similar for both the 15- and 30-minute waits, with similar AUCs.
Geometric mean ratios for AUCs at 48 and 72 hours were 90% and 92%, respectively, and the 90% confidence intervals fell within the 80%-125% FDA boundaries, suggesting similar exposures whether taken 15 or 30 minutes before a meal.
Senior author Francesco S. Celi, MD, chair of the division of endocrinology, diabetes, and metabolism at Virginia Commonwealth University, Richmond, told this news organization: “There is an interest in providing more opportunities for patients and improving adherence to the medication. ... Whatever makes life a bit easier for patients and results in a more predictable response to treatment means down the road there will be fewer visits to the doctor to make adjustments.”
However, he said that in addition to the cost and reimbursement issue, all of these studies have been short term and not conducted in real-life settings.
“Another question is: What happens if the patient goes on low-dose LT4? The studies were conducted on much higher pharmacologic doses. But at least from a safety standpoint, there’s no specific concern.”
Dr. Washington is an employee of Vertice Pharma. Dr. Celi has received unrestricted research grants and worked as a consultant for IBSA. Dr. Gigliotti has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ENDO 2022