User login
Obesity linked to smaller testes and possible infertility
new data suggest.
Testicular volume is a fertility marker directly related to sperm count that has halved in the past 40 years worldwide for unknown reasons. At the same time, childhood obesity has risen dramatically and infertility appears to have risen as well, Rossella Cannarella, MD, of the department of endocrinology and andrology, University of Catania (Italy), said at the annual meeting of the Endocrine Society.
According to recent Italian studies, between 14% and 23% of young men aged 18-19 had testicular hypotrophy. “Worryingly, we don’t know the reason for this hypotrophy. And therefore, they are at risk for future infertility,” Dr. Cannarella said during a press briefing.
Her study, which included a total of 264 male children and adolescents, also linked lower testicular volume to hyperinsulinemia and insulin resistance. “The testis is not quiescent in childhood and is sensitive to the hormone insulin. Obesity and metabolic impairment actually can have an effect and negative impact on Sertoli cell proliferation,” Dr. Cannarella said.
Screen testicular volume at all visits
If other studies confirm these results, she said that pediatricians should begin routinely assessing testicular volume at all visits as is now done with height and weight to identify early deflection of the testicular growth curve.
In addition, “include male infertility as a possible consequence of obesity in counseling of male obese children,” she advised.
Asked to comment, Amin Sedaghat Herati, MD, director of male infertility and men’s health at Johns Hopkins Hospital, and assistant professor of urology at Johns Hopkins Medicine, both in Baltimore, said in an interview: “I think what’s really interesting about this study is the association that they’ve made between testicular volume and obesity.”
But, he noted, “it does not implicate necessarily the development of infertility. It’s an extrapolation. So it’s a step towards the link between obesity and infertility, and it’s an important study to establish the association, but changes in testicular volume and even changes in semen panel don’t necessarily indicate fertility or infertility.”
The findings are “consistent with what we know as far as what obesity can potentially do to the activity of the cells in the testes. The authors are postulating that it’s more the support cells, called Sertoli cells, but I would say it’s probably all of the cells that are being affected by obesity and specifically elevated leptin levels,” Dr. Herati said.
He agrees with the recommendation that pediatricians screen all boys for testicular volume. “I agree it’s a good idea so they don’t miss any cases in which the testes don’t develop the way they should or any other conditions,” Dr. Herati said. “I think in general it’s a good practice, especially in the peripubertal stage, to make sure that kids are on the same growth curve and that they’re meeting their Tanner staging. [Pediatricians] should be looking at the size of the testes and tracking, maybe not at every visit, but at least on an annual basis.”
And, he noted, “I think any study that establishes a link that we can point to when we’re educating patients and parents is important.”
Links found between overweight/obesity, testicular hypotrophy
The study population included 61 male children and adolescents with normal weight, 53 with overweight, and 150 with obesity. Insulin resistance (Homeostatic Model Assessment for Insulin Resistance index ≥ 2.5) was present in 97 participants, 22 had prediabetes, and 3 had type 2 diabetes. Clinical data were collected retrospectively.
Among the boys aged 9-14 years, those with overweight and obesity had significantly lower testicular volume, compared with those of normal weight.
Those who were in Tanner Stage 1 were more likely to have overweight and obesity than those with normal weight, suggesting that “overweight and obese adolescents start puberty later than those of normal weight,” Dr. Cannarella said.
In the 14- to 16-year-old age group, those with insulin resistance had lower testicular volume, compared with those without insulin resistance (HOMA index < 2.5). The number of insulin-resistant adolescents was greater than that of controls in the Tanner stage 2 group.
In both the prepubertal (< 9 years) and pubertal (14-16 years) groups, hyperinsulinemia was associated with lower levels of testicular volume.
Hyperinsulinemia did not influence the timing of puberty onset.
No way to quantify the effect of obesity on fertility just yet
During a press briefing, Dr. Cannarella commented that obesity is likely just one of several factors influencing what appears to be an increase in male infertility over time. “It isn’t of course the only reason, but many factors in our environment have drastically changed, compared to 40 years ago, including the prevalence of heavy metals and endocrine disruptors, and of course, the change in habits and higher prevalence of metabolic disease. All of this has an impact on the proliferation of Sertoli cells in childhood and this may explain the trend toward the decline of sperm concentration and count.”
Longitudinal data are needed to establish cause and effect, she noted. “We need longitudinal studies that link the degrees of testicular volume with the degree of the sperm concentration and count starting from childhood and ending with the adult age. This is the missing link so far.”
Dr. Cannarella has reported no relevant financial relationships. Dr. Herati has reported being an advisor for Dadi, LiNA Medical, and Teleflex.
A version of this article first appeared on Medscape.com.
new data suggest.
Testicular volume is a fertility marker directly related to sperm count that has halved in the past 40 years worldwide for unknown reasons. At the same time, childhood obesity has risen dramatically and infertility appears to have risen as well, Rossella Cannarella, MD, of the department of endocrinology and andrology, University of Catania (Italy), said at the annual meeting of the Endocrine Society.
According to recent Italian studies, between 14% and 23% of young men aged 18-19 had testicular hypotrophy. “Worryingly, we don’t know the reason for this hypotrophy. And therefore, they are at risk for future infertility,” Dr. Cannarella said during a press briefing.
Her study, which included a total of 264 male children and adolescents, also linked lower testicular volume to hyperinsulinemia and insulin resistance. “The testis is not quiescent in childhood and is sensitive to the hormone insulin. Obesity and metabolic impairment actually can have an effect and negative impact on Sertoli cell proliferation,” Dr. Cannarella said.
Screen testicular volume at all visits
If other studies confirm these results, she said that pediatricians should begin routinely assessing testicular volume at all visits as is now done with height and weight to identify early deflection of the testicular growth curve.
In addition, “include male infertility as a possible consequence of obesity in counseling of male obese children,” she advised.
Asked to comment, Amin Sedaghat Herati, MD, director of male infertility and men’s health at Johns Hopkins Hospital, and assistant professor of urology at Johns Hopkins Medicine, both in Baltimore, said in an interview: “I think what’s really interesting about this study is the association that they’ve made between testicular volume and obesity.”
But, he noted, “it does not implicate necessarily the development of infertility. It’s an extrapolation. So it’s a step towards the link between obesity and infertility, and it’s an important study to establish the association, but changes in testicular volume and even changes in semen panel don’t necessarily indicate fertility or infertility.”
The findings are “consistent with what we know as far as what obesity can potentially do to the activity of the cells in the testes. The authors are postulating that it’s more the support cells, called Sertoli cells, but I would say it’s probably all of the cells that are being affected by obesity and specifically elevated leptin levels,” Dr. Herati said.
He agrees with the recommendation that pediatricians screen all boys for testicular volume. “I agree it’s a good idea so they don’t miss any cases in which the testes don’t develop the way they should or any other conditions,” Dr. Herati said. “I think in general it’s a good practice, especially in the peripubertal stage, to make sure that kids are on the same growth curve and that they’re meeting their Tanner staging. [Pediatricians] should be looking at the size of the testes and tracking, maybe not at every visit, but at least on an annual basis.”
And, he noted, “I think any study that establishes a link that we can point to when we’re educating patients and parents is important.”
Links found between overweight/obesity, testicular hypotrophy
The study population included 61 male children and adolescents with normal weight, 53 with overweight, and 150 with obesity. Insulin resistance (Homeostatic Model Assessment for Insulin Resistance index ≥ 2.5) was present in 97 participants, 22 had prediabetes, and 3 had type 2 diabetes. Clinical data were collected retrospectively.
Among the boys aged 9-14 years, those with overweight and obesity had significantly lower testicular volume, compared with those of normal weight.
Those who were in Tanner Stage 1 were more likely to have overweight and obesity than those with normal weight, suggesting that “overweight and obese adolescents start puberty later than those of normal weight,” Dr. Cannarella said.
In the 14- to 16-year-old age group, those with insulin resistance had lower testicular volume, compared with those without insulin resistance (HOMA index < 2.5). The number of insulin-resistant adolescents was greater than that of controls in the Tanner stage 2 group.
In both the prepubertal (< 9 years) and pubertal (14-16 years) groups, hyperinsulinemia was associated with lower levels of testicular volume.
Hyperinsulinemia did not influence the timing of puberty onset.
No way to quantify the effect of obesity on fertility just yet
During a press briefing, Dr. Cannarella commented that obesity is likely just one of several factors influencing what appears to be an increase in male infertility over time. “It isn’t of course the only reason, but many factors in our environment have drastically changed, compared to 40 years ago, including the prevalence of heavy metals and endocrine disruptors, and of course, the change in habits and higher prevalence of metabolic disease. All of this has an impact on the proliferation of Sertoli cells in childhood and this may explain the trend toward the decline of sperm concentration and count.”
Longitudinal data are needed to establish cause and effect, she noted. “We need longitudinal studies that link the degrees of testicular volume with the degree of the sperm concentration and count starting from childhood and ending with the adult age. This is the missing link so far.”
Dr. Cannarella has reported no relevant financial relationships. Dr. Herati has reported being an advisor for Dadi, LiNA Medical, and Teleflex.
A version of this article first appeared on Medscape.com.
new data suggest.
Testicular volume is a fertility marker directly related to sperm count that has halved in the past 40 years worldwide for unknown reasons. At the same time, childhood obesity has risen dramatically and infertility appears to have risen as well, Rossella Cannarella, MD, of the department of endocrinology and andrology, University of Catania (Italy), said at the annual meeting of the Endocrine Society.
According to recent Italian studies, between 14% and 23% of young men aged 18-19 had testicular hypotrophy. “Worryingly, we don’t know the reason for this hypotrophy. And therefore, they are at risk for future infertility,” Dr. Cannarella said during a press briefing.
Her study, which included a total of 264 male children and adolescents, also linked lower testicular volume to hyperinsulinemia and insulin resistance. “The testis is not quiescent in childhood and is sensitive to the hormone insulin. Obesity and metabolic impairment actually can have an effect and negative impact on Sertoli cell proliferation,” Dr. Cannarella said.
Screen testicular volume at all visits
If other studies confirm these results, she said that pediatricians should begin routinely assessing testicular volume at all visits as is now done with height and weight to identify early deflection of the testicular growth curve.
In addition, “include male infertility as a possible consequence of obesity in counseling of male obese children,” she advised.
Asked to comment, Amin Sedaghat Herati, MD, director of male infertility and men’s health at Johns Hopkins Hospital, and assistant professor of urology at Johns Hopkins Medicine, both in Baltimore, said in an interview: “I think what’s really interesting about this study is the association that they’ve made between testicular volume and obesity.”
But, he noted, “it does not implicate necessarily the development of infertility. It’s an extrapolation. So it’s a step towards the link between obesity and infertility, and it’s an important study to establish the association, but changes in testicular volume and even changes in semen panel don’t necessarily indicate fertility or infertility.”
The findings are “consistent with what we know as far as what obesity can potentially do to the activity of the cells in the testes. The authors are postulating that it’s more the support cells, called Sertoli cells, but I would say it’s probably all of the cells that are being affected by obesity and specifically elevated leptin levels,” Dr. Herati said.
He agrees with the recommendation that pediatricians screen all boys for testicular volume. “I agree it’s a good idea so they don’t miss any cases in which the testes don’t develop the way they should or any other conditions,” Dr. Herati said. “I think in general it’s a good practice, especially in the peripubertal stage, to make sure that kids are on the same growth curve and that they’re meeting their Tanner staging. [Pediatricians] should be looking at the size of the testes and tracking, maybe not at every visit, but at least on an annual basis.”
And, he noted, “I think any study that establishes a link that we can point to when we’re educating patients and parents is important.”
Links found between overweight/obesity, testicular hypotrophy
The study population included 61 male children and adolescents with normal weight, 53 with overweight, and 150 with obesity. Insulin resistance (Homeostatic Model Assessment for Insulin Resistance index ≥ 2.5) was present in 97 participants, 22 had prediabetes, and 3 had type 2 diabetes. Clinical data were collected retrospectively.
Among the boys aged 9-14 years, those with overweight and obesity had significantly lower testicular volume, compared with those of normal weight.
Those who were in Tanner Stage 1 were more likely to have overweight and obesity than those with normal weight, suggesting that “overweight and obese adolescents start puberty later than those of normal weight,” Dr. Cannarella said.
In the 14- to 16-year-old age group, those with insulin resistance had lower testicular volume, compared with those without insulin resistance (HOMA index < 2.5). The number of insulin-resistant adolescents was greater than that of controls in the Tanner stage 2 group.
In both the prepubertal (< 9 years) and pubertal (14-16 years) groups, hyperinsulinemia was associated with lower levels of testicular volume.
Hyperinsulinemia did not influence the timing of puberty onset.
No way to quantify the effect of obesity on fertility just yet
During a press briefing, Dr. Cannarella commented that obesity is likely just one of several factors influencing what appears to be an increase in male infertility over time. “It isn’t of course the only reason, but many factors in our environment have drastically changed, compared to 40 years ago, including the prevalence of heavy metals and endocrine disruptors, and of course, the change in habits and higher prevalence of metabolic disease. All of this has an impact on the proliferation of Sertoli cells in childhood and this may explain the trend toward the decline of sperm concentration and count.”
Longitudinal data are needed to establish cause and effect, she noted. “We need longitudinal studies that link the degrees of testicular volume with the degree of the sperm concentration and count starting from childhood and ending with the adult age. This is the missing link so far.”
Dr. Cannarella has reported no relevant financial relationships. Dr. Herati has reported being an advisor for Dadi, LiNA Medical, and Teleflex.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
Key Data on Insulin in Type 2 Diabetes From ADA 2022
Dr George Grunberger, of Wayne State University School of Medicine in Detroit, Michigan, discusses key takeaways on the use of insulin in adults with type 2 diabetes from the 2022 American Diabetes Association Scientific Sessions, held June 3-7 in New Orleans, Louisiana.
Dr Grunberger highlights presentations that address three challenges among insulin users: cost, hypoglycemia, and the high treatment burden of daily injections.
On cost, Dr Grunberger reports on a study of Basaglar, a follow-on biologic approved to reference Lantus insulin. After 1 year, Basaglar demonstrated comparable A1c lowering and adverse events, higher adherence, and lower cost.
Next, on hypoglycemia, Dr Grunberger cites the GRADE trial, which examined adding insulin glargine or the sulfonylurea glimepiride to patients taking metformin. The incidence of severe hypoglycemia proved to be lower in the insulin glargine group.
Finally, Dr Grunberger reported on studies that explored progress in the development of weekly insulin, with an eye toward decreasing treatment burden.
--
George Grunberger, MD, Chairman, Grunberger Diabetes Institute, Bloomfield Hills; Clinical Professor, Department of Internal Medicine and Molecular Medicine & Genetics, Wayne State University School of Medicine, Detroit, Michigan; Professor, Department of Internal Medicine, Oakland University William Beaumont School of Medicine, Rochester, Michigan
George Grunberger, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Nevro; Lifescan
Serve(d) as a speaker or a member of a speakers bureau for: Eli Lilly; Novo Nordisk
Dr George Grunberger, of Wayne State University School of Medicine in Detroit, Michigan, discusses key takeaways on the use of insulin in adults with type 2 diabetes from the 2022 American Diabetes Association Scientific Sessions, held June 3-7 in New Orleans, Louisiana.
Dr Grunberger highlights presentations that address three challenges among insulin users: cost, hypoglycemia, and the high treatment burden of daily injections.
On cost, Dr Grunberger reports on a study of Basaglar, a follow-on biologic approved to reference Lantus insulin. After 1 year, Basaglar demonstrated comparable A1c lowering and adverse events, higher adherence, and lower cost.
Next, on hypoglycemia, Dr Grunberger cites the GRADE trial, which examined adding insulin glargine or the sulfonylurea glimepiride to patients taking metformin. The incidence of severe hypoglycemia proved to be lower in the insulin glargine group.
Finally, Dr Grunberger reported on studies that explored progress in the development of weekly insulin, with an eye toward decreasing treatment burden.
--
George Grunberger, MD, Chairman, Grunberger Diabetes Institute, Bloomfield Hills; Clinical Professor, Department of Internal Medicine and Molecular Medicine & Genetics, Wayne State University School of Medicine, Detroit, Michigan; Professor, Department of Internal Medicine, Oakland University William Beaumont School of Medicine, Rochester, Michigan
George Grunberger, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Nevro; Lifescan
Serve(d) as a speaker or a member of a speakers bureau for: Eli Lilly; Novo Nordisk
Dr George Grunberger, of Wayne State University School of Medicine in Detroit, Michigan, discusses key takeaways on the use of insulin in adults with type 2 diabetes from the 2022 American Diabetes Association Scientific Sessions, held June 3-7 in New Orleans, Louisiana.
Dr Grunberger highlights presentations that address three challenges among insulin users: cost, hypoglycemia, and the high treatment burden of daily injections.
On cost, Dr Grunberger reports on a study of Basaglar, a follow-on biologic approved to reference Lantus insulin. After 1 year, Basaglar demonstrated comparable A1c lowering and adverse events, higher adherence, and lower cost.
Next, on hypoglycemia, Dr Grunberger cites the GRADE trial, which examined adding insulin glargine or the sulfonylurea glimepiride to patients taking metformin. The incidence of severe hypoglycemia proved to be lower in the insulin glargine group.
Finally, Dr Grunberger reported on studies that explored progress in the development of weekly insulin, with an eye toward decreasing treatment burden.
--
George Grunberger, MD, Chairman, Grunberger Diabetes Institute, Bloomfield Hills; Clinical Professor, Department of Internal Medicine and Molecular Medicine & Genetics, Wayne State University School of Medicine, Detroit, Michigan; Professor, Department of Internal Medicine, Oakland University William Beaumont School of Medicine, Rochester, Michigan
George Grunberger, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Nevro; Lifescan
Serve(d) as a speaker or a member of a speakers bureau for: Eli Lilly; Novo Nordisk

Glycemic Control in Type 2 Diabetes From ADA 2022
New guidelines and key studies in glycemic control from the 2022 annual meeting of the American Diabetes Association (ADA) are highlighted by Dr Ildiko Lingvay, from the University of Texas Southwestern Medical Center.
Dr Lingvay opens by reporting on forthcoming guidelines by the ADA in joint partnership with the European Association for the Study of Diabetes (EASD) on the treatment of hyperglycemia in type 2 diabetes. This consensus paper, she says, will endorse a holistic, person-centered approach to managing type 2 diabetes. The finalized paper will be presented at the EASD annual meeting in Stockholm in September 2022. Next, Dr Lingvay discusses the yearly update to the ADA Standards of Medical Care in Diabetes, which include the benefits of finerenone, the benefits of SGLT2 inhibitors on heart failure and renal outcomes, and the endorsement of the updated eGFR calculator, which omits patient race from calculations.
She then looks at the SURMOUNT-1 study, a phase 3 trial investigating tirzepatide, a dual GLP/GLP-1 receptor agonist, for the treatment of obesity. In addition to a demonstration of substantial weight loss, the study indicated that tirzepatide provided impressive glucose-lowering benefit.
Dr Lingvay also examines post hoc data from the STEP 1 and 4 trials showing that semaglutide, compared with placebo, reduced the risk for type 2 diabetes in patients with obesity by approximately 60%, regardless of their baseline glycemic status.
Finally, Dr Lingvay discusses a novel triple GIP, GLP-1, and glucagon receptor agonist called LY3437943, currently in phase 1 trials, which demonstrated promising glycemic and body weight lowering efficacy after a short 12-week trial.
--
Ildiko Lingvay, MD, MPH, Professor, Department of Internal Medicine, Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, Texas
Ildiko Lingvay, MD, MPH, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: NovoNordisk; Sanofi; Eli Lilly; AstraZeneca; Target Pharma; Boehringer Ingelheim; Intercept; Merck; Janssen; Intarcia; Merck
Received research grant from: NovoNordisk; Mylan; Sanofi; Merck; Pfizer; Boehringer Ingelheim
New guidelines and key studies in glycemic control from the 2022 annual meeting of the American Diabetes Association (ADA) are highlighted by Dr Ildiko Lingvay, from the University of Texas Southwestern Medical Center.
Dr Lingvay opens by reporting on forthcoming guidelines by the ADA in joint partnership with the European Association for the Study of Diabetes (EASD) on the treatment of hyperglycemia in type 2 diabetes. This consensus paper, she says, will endorse a holistic, person-centered approach to managing type 2 diabetes. The finalized paper will be presented at the EASD annual meeting in Stockholm in September 2022. Next, Dr Lingvay discusses the yearly update to the ADA Standards of Medical Care in Diabetes, which include the benefits of finerenone, the benefits of SGLT2 inhibitors on heart failure and renal outcomes, and the endorsement of the updated eGFR calculator, which omits patient race from calculations.
She then looks at the SURMOUNT-1 study, a phase 3 trial investigating tirzepatide, a dual GLP/GLP-1 receptor agonist, for the treatment of obesity. In addition to a demonstration of substantial weight loss, the study indicated that tirzepatide provided impressive glucose-lowering benefit.
Dr Lingvay also examines post hoc data from the STEP 1 and 4 trials showing that semaglutide, compared with placebo, reduced the risk for type 2 diabetes in patients with obesity by approximately 60%, regardless of their baseline glycemic status.
Finally, Dr Lingvay discusses a novel triple GIP, GLP-1, and glucagon receptor agonist called LY3437943, currently in phase 1 trials, which demonstrated promising glycemic and body weight lowering efficacy after a short 12-week trial.
--
Ildiko Lingvay, MD, MPH, Professor, Department of Internal Medicine, Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, Texas
Ildiko Lingvay, MD, MPH, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: NovoNordisk; Sanofi; Eli Lilly; AstraZeneca; Target Pharma; Boehringer Ingelheim; Intercept; Merck; Janssen; Intarcia; Merck
Received research grant from: NovoNordisk; Mylan; Sanofi; Merck; Pfizer; Boehringer Ingelheim
New guidelines and key studies in glycemic control from the 2022 annual meeting of the American Diabetes Association (ADA) are highlighted by Dr Ildiko Lingvay, from the University of Texas Southwestern Medical Center.
Dr Lingvay opens by reporting on forthcoming guidelines by the ADA in joint partnership with the European Association for the Study of Diabetes (EASD) on the treatment of hyperglycemia in type 2 diabetes. This consensus paper, she says, will endorse a holistic, person-centered approach to managing type 2 diabetes. The finalized paper will be presented at the EASD annual meeting in Stockholm in September 2022. Next, Dr Lingvay discusses the yearly update to the ADA Standards of Medical Care in Diabetes, which include the benefits of finerenone, the benefits of SGLT2 inhibitors on heart failure and renal outcomes, and the endorsement of the updated eGFR calculator, which omits patient race from calculations.
She then looks at the SURMOUNT-1 study, a phase 3 trial investigating tirzepatide, a dual GLP/GLP-1 receptor agonist, for the treatment of obesity. In addition to a demonstration of substantial weight loss, the study indicated that tirzepatide provided impressive glucose-lowering benefit.
Dr Lingvay also examines post hoc data from the STEP 1 and 4 trials showing that semaglutide, compared with placebo, reduced the risk for type 2 diabetes in patients with obesity by approximately 60%, regardless of their baseline glycemic status.
Finally, Dr Lingvay discusses a novel triple GIP, GLP-1, and glucagon receptor agonist called LY3437943, currently in phase 1 trials, which demonstrated promising glycemic and body weight lowering efficacy after a short 12-week trial.
--
Ildiko Lingvay, MD, MPH, Professor, Department of Internal Medicine, Department of Population and Data Sciences, University of Texas Southwestern Medical Center, Dallas, Texas
Ildiko Lingvay, MD, MPH, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: NovoNordisk; Sanofi; Eli Lilly; AstraZeneca; Target Pharma; Boehringer Ingelheim; Intercept; Merck; Janssen; Intarcia; Merck
Received research grant from: NovoNordisk; Mylan; Sanofi; Merck; Pfizer; Boehringer Ingelheim

Fatty liver disease drives rise in liver cancer deaths
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the meeting sponsored by the European Association for the Study of the Liver.
Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009–2019, the annual percent change of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense (Denmark) Liver Research Centre at SDU and Odense University Hospital, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around 2 billion cases of chronic liver disease globally, he said. He noted that, over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that the overall global prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policy makers is very low. “From my perspective, raising awareness of NAFLD is the No. 1 priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Karg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the meeting sponsored by the European Association for the Study of the Liver.
Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009–2019, the annual percent change of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense (Denmark) Liver Research Centre at SDU and Odense University Hospital, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around 2 billion cases of chronic liver disease globally, he said. He noted that, over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that the overall global prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policy makers is very low. “From my perspective, raising awareness of NAFLD is the No. 1 priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Karg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the meeting sponsored by the European Association for the Study of the Liver.
Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009–2019, the annual percent change of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense (Denmark) Liver Research Centre at SDU and Odense University Hospital, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around 2 billion cases of chronic liver disease globally, he said. He noted that, over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that the overall global prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policy makers is very low. “From my perspective, raising awareness of NAFLD is the No. 1 priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Karg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
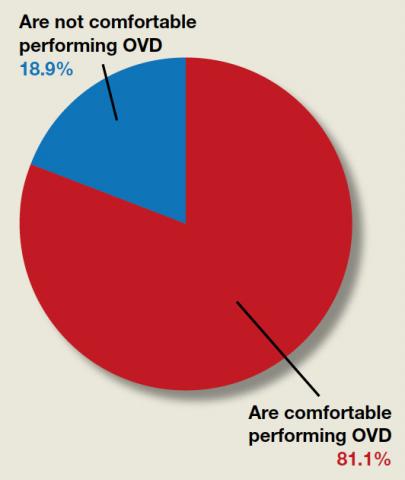
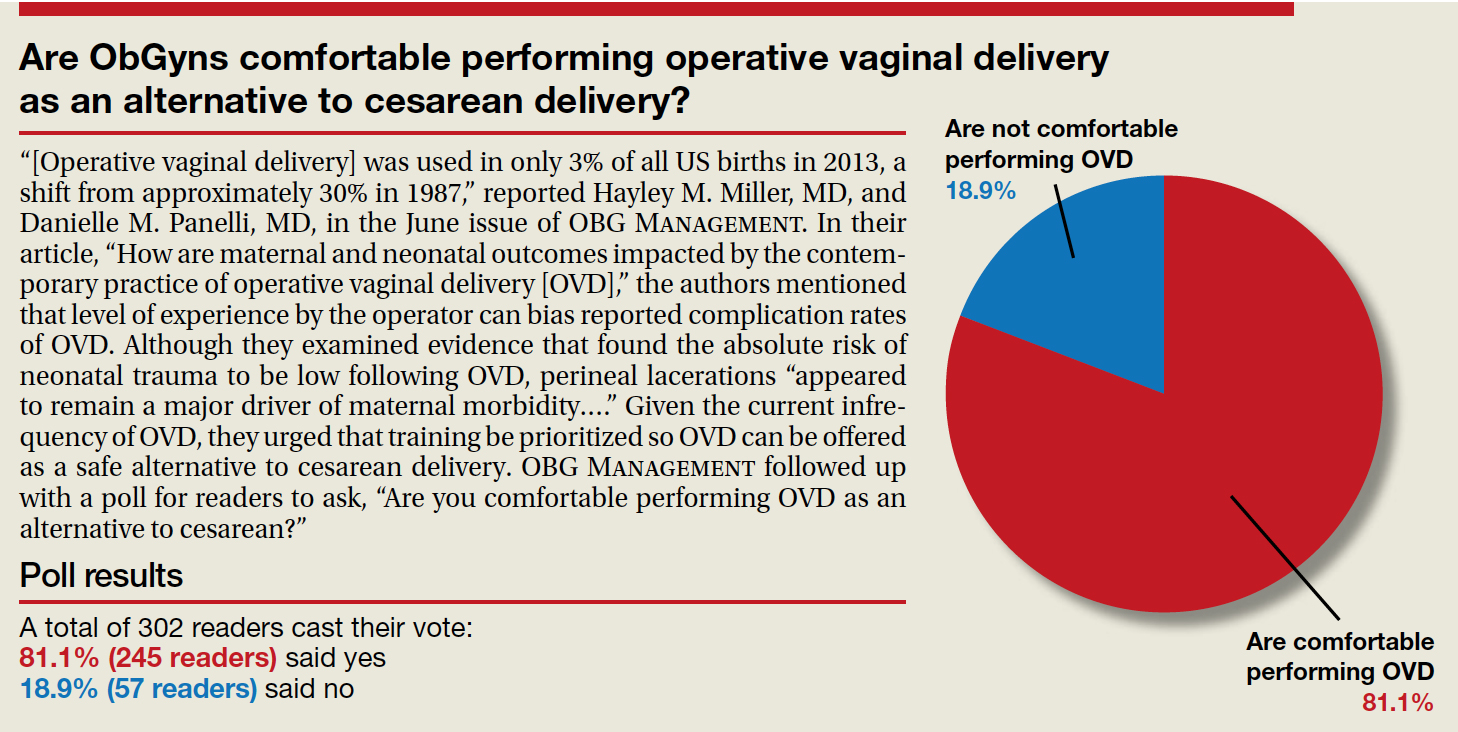
Are ObGyns comfortable performing operative vaginal delivery as an alternative to cesarean delivery?
“[Operative vaginal delivery] was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987,” reported Hayley M. Miller, MD, and Danielle M. Panelli, MD, in the June issue of OBG Management. In their article, “How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery [OVD],” the authors mentioned that level of experience by the operator can bias reported complication rates of OVD. Although they examined evidence that found the absolute risk of neonatal trauma to be low following OVD, perineal lacerations “appeared to remain a major driver of maternal morbidity….” Given the current infrequency of OVD, they urged that training be prioritized so OVD can be offered as a safe alternative to cesarean delivery. OBG Management followed up with a poll for readers to ask, “Are you comfortable performing OVD as an alternative to cesarean?”
A total of 302 readers cast their vote:
81.1% (245 readers) said yes
18.9% (57 readers) said no
“[Operative vaginal delivery] was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987,” reported Hayley M. Miller, MD, and Danielle M. Panelli, MD, in the June issue of OBG Management. In their article, “How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery [OVD],” the authors mentioned that level of experience by the operator can bias reported complication rates of OVD. Although they examined evidence that found the absolute risk of neonatal trauma to be low following OVD, perineal lacerations “appeared to remain a major driver of maternal morbidity….” Given the current infrequency of OVD, they urged that training be prioritized so OVD can be offered as a safe alternative to cesarean delivery. OBG Management followed up with a poll for readers to ask, “Are you comfortable performing OVD as an alternative to cesarean?”
A total of 302 readers cast their vote:
81.1% (245 readers) said yes
18.9% (57 readers) said no

“[Operative vaginal delivery] was used in only 3% of all US births in 2013, a shift from approximately 30% in 1987,” reported Hayley M. Miller, MD, and Danielle M. Panelli, MD, in the June issue of OBG Management. In their article, “How are maternal and neonatal outcomes impacted by the contemporary practice of operative vaginal delivery [OVD],” the authors mentioned that level of experience by the operator can bias reported complication rates of OVD. Although they examined evidence that found the absolute risk of neonatal trauma to be low following OVD, perineal lacerations “appeared to remain a major driver of maternal morbidity….” Given the current infrequency of OVD, they urged that training be prioritized so OVD can be offered as a safe alternative to cesarean delivery. OBG Management followed up with a poll for readers to ask, “Are you comfortable performing OVD as an alternative to cesarean?”
A total of 302 readers cast their vote:
81.1% (245 readers) said yes
18.9% (57 readers) said no

Key Studies on Diabetes Comorbidities From ADA 2022
Dr Anastassios Pittas, Chief of Endocrinology at Tufts Medical Center in Boston, discusses key data from the American Diabetes Association Scientific Sessions 2022 on the management of common comorbidities in diabetes.
Dr Pittas looks first at a prespecified analysis of the SURPASS-4 trial, which highlighted the role of tirzepatide on kidney outcomes in patients with type 2 diabetes.
He then discusses results of the SPLENDID trial in which patients with diabetes who received bariatric surgery for weight loss showed reduced rates of cancer.
Finally, Dr Pittas reports on a study examining the effect of food insecurity and diet quality on maintaining good cholesterol levels in diabetes.
--
Anastassios G. Pittas, MD, Chief, Division of Endocrinology, Diabetes, and Metabolism; Co-director, Diabetes and Lipid Center; Professor of Medicine, Tufts University School of Medicine, Boston, Massachusetts
Anastassios G. Pittas, MD, has disclosed no relevant financial relationships.
Dr Anastassios Pittas, Chief of Endocrinology at Tufts Medical Center in Boston, discusses key data from the American Diabetes Association Scientific Sessions 2022 on the management of common comorbidities in diabetes.
Dr Pittas looks first at a prespecified analysis of the SURPASS-4 trial, which highlighted the role of tirzepatide on kidney outcomes in patients with type 2 diabetes.
He then discusses results of the SPLENDID trial in which patients with diabetes who received bariatric surgery for weight loss showed reduced rates of cancer.
Finally, Dr Pittas reports on a study examining the effect of food insecurity and diet quality on maintaining good cholesterol levels in diabetes.
--
Anastassios G. Pittas, MD, Chief, Division of Endocrinology, Diabetes, and Metabolism; Co-director, Diabetes and Lipid Center; Professor of Medicine, Tufts University School of Medicine, Boston, Massachusetts
Anastassios G. Pittas, MD, has disclosed no relevant financial relationships.
Dr Anastassios Pittas, Chief of Endocrinology at Tufts Medical Center in Boston, discusses key data from the American Diabetes Association Scientific Sessions 2022 on the management of common comorbidities in diabetes.
Dr Pittas looks first at a prespecified analysis of the SURPASS-4 trial, which highlighted the role of tirzepatide on kidney outcomes in patients with type 2 diabetes.
He then discusses results of the SPLENDID trial in which patients with diabetes who received bariatric surgery for weight loss showed reduced rates of cancer.
Finally, Dr Pittas reports on a study examining the effect of food insecurity and diet quality on maintaining good cholesterol levels in diabetes.
--
Anastassios G. Pittas, MD, Chief, Division of Endocrinology, Diabetes, and Metabolism; Co-director, Diabetes and Lipid Center; Professor of Medicine, Tufts University School of Medicine, Boston, Massachusetts
Anastassios G. Pittas, MD, has disclosed no relevant financial relationships.

Racial/ethnic disparities exacerbated maternal death rise during 2020 pandemic.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
U.S. maternal deaths – those during pregnancy or within 42 days of pregnancy – increased substantially by 33.3% after March 2020 corresponding to the COVID-19 pandemic onset, according to new research published in JAMA Network Open.
Data from the National Center for Health Statistics (NCHS) revealed this rise in maternal deaths was higher than the 22% overall excess death estimate associated with the pandemic in 2020.
Increases were highest for Hispanic and non-Hispanic Black women, exacerbating already high rates of disparity in comparison with White women, wrote Marie E. Thoma, PhD, an associate professor at the University of Maryland, College Park, and Eugene R. Declercq, PhD, a professor at Boston University.
The authors noted that this spike in maternal deaths might be caused either by conditions directly related to COVID-19, such as respiratory or viral infections, or by conditions worsened by pandemic-associated health care disruptions including those for diabetes or cardiovascular disease.
The precise causes, however, could not be discerned from the data, the authors noted.
The NCHS reported an 18.4% increase in U.S. maternal mortality from 2019 to 2020. The relative increase was 44.4% among Hispanic, 25.7% among non-Hispanic Black, and 6.1% among non-Hispanic White women.
“The rise in maternal mortality among Hispanic women was unprecedented,” Dr. Thoma said in an interview. Given a 16.8% increase in overall U.S. mortality in 2020, largely attributed to the COVID-19 pandemic, the authors examined the pandemic’s role in [the higher] maternal death rates for 2020.
“Prior to this report, the NCHS released an e-report that there had been a rise in maternal mortality in 2020, but questions remained about the role of the pandemic in this rise that their report hadn’t addressed,” Dr. Thoma said in an interview “So we decided to look at the data further to assess whether the rise coincided with the pandemic and how this differed by race/ethnicity, whether there were changes in the causes of maternal death, and how often COVID-19 was listed as a contributory factor in those deaths.”
A total of 1,588 maternal deaths (18.8 per 100,000 live births) occurred before the pandemic versus 684 deaths (25.1 per 100,000 live births) during the 2020 phase of the pandemic, for a relative increase of 33.3%.
Direct obstetrical causes of death included diabetes, hypertensive and liver disorders, pregnancy-related infections, and obstetrical hemorrhage and embolism. Indirect causes comprised, among others, nonobstetrical infections and diseases of the circulatory and respiratory systems as well as mental and nervous disorders.
Relative increases in direct causes (27.7%) were mostly associated with diabetes (95.9%), hypertensive disorders (39.0%), and other specified pregnancy-related conditions (48.0%).
COVID-19 was commonly listed as a lethal condition along with other viral diseases (16 of 16 deaths and diseases of the respiratory system (11 of 19 deaths).
Late maternal mortality – defined as more than 42 days but less than 1 year after pregnancy – increased by 41%. “This was surprising as we might anticipate risk being higher during pregnancy given that pregnant women may be more susceptible, but we see that this rise was also found among people in the later postpartum period,” Dr. Thoma said.
Absolute and relative changes were highest for Hispanic women (8.9 per 100,000 live births and 74.2%, respectively) and non-Hispanic Black women (16.8 per 100,000 live births and 40.2%). In contrast, non-Hispanic White women saw increases of just 2.9 per 100,000 live births and 17.2%.
“Overall, we found the rise in maternal mortality in 2020 was concentrated after the start of pandemic, particularly for non-Hispanic Black and Hispanic women, and we saw a dramatic rise in respiratory-related conditions,” Dr. Thoma said.
In a comment, Steven Woolf, MD, MPH, director emeritus of the Center on Society and Health at Virginia Commonwealth University, Richmond, said the findings are very consistent with his and others research showing dramatic increases in overall death rates from many causes during the pandemic, with these ranging from COVID-19 leading conditions such as diabetes, cardiovascular and Alzheimer’s disease to less-studied causes such as drug overdoses and alcoholism caused by the stresses of the pandemic. Again, deaths were likely caused by both COVID-19 infections and disruptions in diagnosis and care.
“So a rise in maternal mortality would unfortunately also be expected, and these researchers have shown that,” he said in an interview. In addition, they have confirmed “the pattern of stark health disparities in the Hispanic and Black populations relative to the White. Our group has shown marked decreases in the life expectancies of the Black and Hispanic populations relative to the White population.”
While he might take issue with the study’s research methodology, Dr. Woolf said, “The work is useful partly because we need to work out the best research methods to do this kind of analysis because we really need to understand the effects on maternal mortality.”
He said sorting out the best way to do this type of research will be important for looking at excess deaths and maternal mortality following other events, for example, in the wake of the Supreme Court’s recent decision to reverse Roe v. Wade.
The authors acknowledged certain study limitations, including the large percentage of COVID-19 cases with a nonspecific underlying cause. According to Dr. Thoma and Dr. Declercq, that reflects a maternal death coding problem that needs to be addressed, as well as a partitioning of data. The latter resulted in small numbers for some categories, with rates suppressed for fewer than 16 deaths because of reduced reliability.
“We found that more specific information is often available on death certificates but is lost in the process of coding,” said Dr. Thoma. “We were able to reclassify many of these causes to a more specific cause that we attributed to be the primary cause of death.”
The authors said future studies of maternal death should examine the contribution of the pandemic to racial and ethnic disparities and should identify specific causes of maternal deaths overall and associated with COVID-19.
In earlier research, the authors previously warned of possible misclassifications of maternal deaths.
They found evidence of both underreporting and overreporting of deaths, with possible overreporting predominant, whereas accurate data are essential for measuring the effectiveness of maternal mortality reduction programs.
Dr. Thoma’s group will continue to monitor mortality trends with the release of 2021 data. “We hope we will see improvements in 2021 given greater access to vaccines, treatments, and fewer health care disruptions,” Dr. Thoma said. “It will be important to continue to stress the importance of COVID-19 vaccines for pregnant and postpartum people.”
This study had no external funding. The authors disclosed no competing interests. Dr. Woolf declared no conflicts of interest.
FROM JAMA NETWORK OPEN
Abortion pills over the counter? Experts see major hurdles in widening U.S. access
WASHINGTON (Reuters) – A pill used to terminate early pregnancies is unlikely to become available without a prescription for years, if ever, experts told Reuters, as the conservative-leaning U.S. Supreme Court dramatically curbed abortion rights.
The Supreme Court on June 24 overturned the landmark 1973 Roe v. Wade ruling that recognized the constitutional right to an abortion and legalized it nationwide. The new ruling stung abortion rights advocates and was a momentous victory to Republicans and religious conservatives.
Many U.S. states are expected to severely limit or outright ban abortions following the Supreme Court ruling. President Joe Biden’s administration is considering options to increase access to so-called medication abortions, which can be administered at home.
“Today I am directing the Department of Health & Human Services to take steps to ensure these critical medications are available to the fullest extent possible,” Mr. Biden said in remarks from the White House.
The pill, mifepristone, is used in combination with a second drug called misoprostol to induce an abortion up to 10 weeks into a pregnancy and is heavily restricted – only available through a certified doctor’s prescription. Abortion rights activists have stepped up calls to make it available for anyone to buy at pharmacies without a prescription.
“We will double down and use every lever we have to protect access to abortion care,” Secretary of Health and Human Services Xavier Becerra said in a statement, adding the department was committed to ensuring access to “medication abortion that has been approved by the FDA for over 20 years.”
Neither Mr. Biden nor Mr. Becerra addressed making the pills available over-the-counter, a process that could take years according to medical and regulatory experts interviewed by Reuters. They said drugmakers would need to conduct new studies showing directions on the product’s packaging would enable a consumer to safely use it without professional medical guidance.
The two companies that make the pill for the U.S. market have shown no interest in conducting the research. Should they do so, any Food and Drug Administration approval would become a target for lawsuits from abortion opponents that could delay implementation for years, experts said.
“The hard part that I see is getting the evidence or the agreement that no prescriber is needed at all,” said Susan Wood, a former Assistant Commissioner for Women’s Health at the FDA.
“I personally don’t see it happening in the next couple of years,” said Ms. Wood, now director of George Washington University’s Jacobs Institute of Women’s Health.
The next battle
Access to abortion pills is expected to become the next big battle, as their use is harder to track. The FDA has already relaxed some restrictions, making it easier for certified doctors to prescribe them.
The agency now allows doctors to prescribe mifepristone after a telehealth visit rather than in-person. Patients can receive it by mail, making it easier for women in U.S. states that already restrict its use.
The White House has already considered making abortion pills available online and from pharmacies abroad, with a prescription. However, the import possibility has been curtailed by Congress in broader legislation about drug regulation.
An over-the-counter designation would make it much easier for pregnant women to access the pills in states that seek to restrict their use. For example, they could more easily be mailed to a patient from a friend or supporter in a state where they are not banned.
An FDA spokesperson declined to comment on whether over-the-counter use of abortion pills has been considered. A spokesperson for Danco Laboratories, a manufacturer of mifepristone, said that it does not plan to seek over-the-counter approval. GenBioPro, the second maker of mifepristone for the U.S. market, did not respond to requests for comment.
Are they safe?
Medication abortion involves two drugs, taken over a day or two. The first, mifepristone, blocks the pregnancy-sustaining hormone progesterone. The second, misoprostol, induces uterine contractions.
When taken together, the pills halt the pregnancy and prompt cramping and bleeding to empty the uterus, in a process similar to miscarriage.
Abortion rights activists say the pills have a long track record of being safe and effective, with no risk of overdose or addiction. In several countries, including India and Mexico, women can buy mifepristone and misoprostol without a prescription to induce abortion.
“Medication abortion really does meet all the FDA criteria for an over-the-counter switch,” said Antonia Biggs, associate professor at the University of California, San Francisco’s obstetrics, gynecology and reproductive sciences department.
A recent study by Ms. Biggs and colleagues found that the majority of participants would understand a medication abortion over-the-counter label. Ms. Biggs said she was not in talks with drugmakers over her research.
The Charlotte Lozier Institute and Susan B. Anthony List, which advocate against abortion, have said that the FDA decision to relax restrictions on mifepristone ignored data on complications and put women at risk.
Others point to the decade-long legal fight for over-the-counter Plan B, a form of emergency contraception taken within days of sexual intercourse to prevent a pregnancy. Approval for women 18 and over was granted in 2006 and for use by women of all ages in 2013.
“There was very strong support that you did not need a prescriber,” said Ms. Wood, who resigned from the FDA in 2005 over the delay. “Everybody under the sun agreed except for a small group of people who somehow had an enormous political influence.”
Reuters Health Information © 2022
WASHINGTON (Reuters) – A pill used to terminate early pregnancies is unlikely to become available without a prescription for years, if ever, experts told Reuters, as the conservative-leaning U.S. Supreme Court dramatically curbed abortion rights.
The Supreme Court on June 24 overturned the landmark 1973 Roe v. Wade ruling that recognized the constitutional right to an abortion and legalized it nationwide. The new ruling stung abortion rights advocates and was a momentous victory to Republicans and religious conservatives.
Many U.S. states are expected to severely limit or outright ban abortions following the Supreme Court ruling. President Joe Biden’s administration is considering options to increase access to so-called medication abortions, which can be administered at home.
“Today I am directing the Department of Health & Human Services to take steps to ensure these critical medications are available to the fullest extent possible,” Mr. Biden said in remarks from the White House.
The pill, mifepristone, is used in combination with a second drug called misoprostol to induce an abortion up to 10 weeks into a pregnancy and is heavily restricted – only available through a certified doctor’s prescription. Abortion rights activists have stepped up calls to make it available for anyone to buy at pharmacies without a prescription.
“We will double down and use every lever we have to protect access to abortion care,” Secretary of Health and Human Services Xavier Becerra said in a statement, adding the department was committed to ensuring access to “medication abortion that has been approved by the FDA for over 20 years.”
Neither Mr. Biden nor Mr. Becerra addressed making the pills available over-the-counter, a process that could take years according to medical and regulatory experts interviewed by Reuters. They said drugmakers would need to conduct new studies showing directions on the product’s packaging would enable a consumer to safely use it without professional medical guidance.
The two companies that make the pill for the U.S. market have shown no interest in conducting the research. Should they do so, any Food and Drug Administration approval would become a target for lawsuits from abortion opponents that could delay implementation for years, experts said.
“The hard part that I see is getting the evidence or the agreement that no prescriber is needed at all,” said Susan Wood, a former Assistant Commissioner for Women’s Health at the FDA.
“I personally don’t see it happening in the next couple of years,” said Ms. Wood, now director of George Washington University’s Jacobs Institute of Women’s Health.
The next battle
Access to abortion pills is expected to become the next big battle, as their use is harder to track. The FDA has already relaxed some restrictions, making it easier for certified doctors to prescribe them.
The agency now allows doctors to prescribe mifepristone after a telehealth visit rather than in-person. Patients can receive it by mail, making it easier for women in U.S. states that already restrict its use.
The White House has already considered making abortion pills available online and from pharmacies abroad, with a prescription. However, the import possibility has been curtailed by Congress in broader legislation about drug regulation.
An over-the-counter designation would make it much easier for pregnant women to access the pills in states that seek to restrict their use. For example, they could more easily be mailed to a patient from a friend or supporter in a state where they are not banned.
An FDA spokesperson declined to comment on whether over-the-counter use of abortion pills has been considered. A spokesperson for Danco Laboratories, a manufacturer of mifepristone, said that it does not plan to seek over-the-counter approval. GenBioPro, the second maker of mifepristone for the U.S. market, did not respond to requests for comment.
Are they safe?
Medication abortion involves two drugs, taken over a day or two. The first, mifepristone, blocks the pregnancy-sustaining hormone progesterone. The second, misoprostol, induces uterine contractions.
When taken together, the pills halt the pregnancy and prompt cramping and bleeding to empty the uterus, in a process similar to miscarriage.
Abortion rights activists say the pills have a long track record of being safe and effective, with no risk of overdose or addiction. In several countries, including India and Mexico, women can buy mifepristone and misoprostol without a prescription to induce abortion.
“Medication abortion really does meet all the FDA criteria for an over-the-counter switch,” said Antonia Biggs, associate professor at the University of California, San Francisco’s obstetrics, gynecology and reproductive sciences department.
A recent study by Ms. Biggs and colleagues found that the majority of participants would understand a medication abortion over-the-counter label. Ms. Biggs said she was not in talks with drugmakers over her research.
The Charlotte Lozier Institute and Susan B. Anthony List, which advocate against abortion, have said that the FDA decision to relax restrictions on mifepristone ignored data on complications and put women at risk.
Others point to the decade-long legal fight for over-the-counter Plan B, a form of emergency contraception taken within days of sexual intercourse to prevent a pregnancy. Approval for women 18 and over was granted in 2006 and for use by women of all ages in 2013.
“There was very strong support that you did not need a prescriber,” said Ms. Wood, who resigned from the FDA in 2005 over the delay. “Everybody under the sun agreed except for a small group of people who somehow had an enormous political influence.”
Reuters Health Information © 2022
WASHINGTON (Reuters) – A pill used to terminate early pregnancies is unlikely to become available without a prescription for years, if ever, experts told Reuters, as the conservative-leaning U.S. Supreme Court dramatically curbed abortion rights.
The Supreme Court on June 24 overturned the landmark 1973 Roe v. Wade ruling that recognized the constitutional right to an abortion and legalized it nationwide. The new ruling stung abortion rights advocates and was a momentous victory to Republicans and religious conservatives.
Many U.S. states are expected to severely limit or outright ban abortions following the Supreme Court ruling. President Joe Biden’s administration is considering options to increase access to so-called medication abortions, which can be administered at home.
“Today I am directing the Department of Health & Human Services to take steps to ensure these critical medications are available to the fullest extent possible,” Mr. Biden said in remarks from the White House.
The pill, mifepristone, is used in combination with a second drug called misoprostol to induce an abortion up to 10 weeks into a pregnancy and is heavily restricted – only available through a certified doctor’s prescription. Abortion rights activists have stepped up calls to make it available for anyone to buy at pharmacies without a prescription.
“We will double down and use every lever we have to protect access to abortion care,” Secretary of Health and Human Services Xavier Becerra said in a statement, adding the department was committed to ensuring access to “medication abortion that has been approved by the FDA for over 20 years.”
Neither Mr. Biden nor Mr. Becerra addressed making the pills available over-the-counter, a process that could take years according to medical and regulatory experts interviewed by Reuters. They said drugmakers would need to conduct new studies showing directions on the product’s packaging would enable a consumer to safely use it without professional medical guidance.
The two companies that make the pill for the U.S. market have shown no interest in conducting the research. Should they do so, any Food and Drug Administration approval would become a target for lawsuits from abortion opponents that could delay implementation for years, experts said.
“The hard part that I see is getting the evidence or the agreement that no prescriber is needed at all,” said Susan Wood, a former Assistant Commissioner for Women’s Health at the FDA.
“I personally don’t see it happening in the next couple of years,” said Ms. Wood, now director of George Washington University’s Jacobs Institute of Women’s Health.
The next battle
Access to abortion pills is expected to become the next big battle, as their use is harder to track. The FDA has already relaxed some restrictions, making it easier for certified doctors to prescribe them.
The agency now allows doctors to prescribe mifepristone after a telehealth visit rather than in-person. Patients can receive it by mail, making it easier for women in U.S. states that already restrict its use.
The White House has already considered making abortion pills available online and from pharmacies abroad, with a prescription. However, the import possibility has been curtailed by Congress in broader legislation about drug regulation.
An over-the-counter designation would make it much easier for pregnant women to access the pills in states that seek to restrict their use. For example, they could more easily be mailed to a patient from a friend or supporter in a state where they are not banned.
An FDA spokesperson declined to comment on whether over-the-counter use of abortion pills has been considered. A spokesperson for Danco Laboratories, a manufacturer of mifepristone, said that it does not plan to seek over-the-counter approval. GenBioPro, the second maker of mifepristone for the U.S. market, did not respond to requests for comment.
Are they safe?
Medication abortion involves two drugs, taken over a day or two. The first, mifepristone, blocks the pregnancy-sustaining hormone progesterone. The second, misoprostol, induces uterine contractions.
When taken together, the pills halt the pregnancy and prompt cramping and bleeding to empty the uterus, in a process similar to miscarriage.
Abortion rights activists say the pills have a long track record of being safe and effective, with no risk of overdose or addiction. In several countries, including India and Mexico, women can buy mifepristone and misoprostol without a prescription to induce abortion.
“Medication abortion really does meet all the FDA criteria for an over-the-counter switch,” said Antonia Biggs, associate professor at the University of California, San Francisco’s obstetrics, gynecology and reproductive sciences department.
A recent study by Ms. Biggs and colleagues found that the majority of participants would understand a medication abortion over-the-counter label. Ms. Biggs said she was not in talks with drugmakers over her research.
The Charlotte Lozier Institute and Susan B. Anthony List, which advocate against abortion, have said that the FDA decision to relax restrictions on mifepristone ignored data on complications and put women at risk.
Others point to the decade-long legal fight for over-the-counter Plan B, a form of emergency contraception taken within days of sexual intercourse to prevent a pregnancy. Approval for women 18 and over was granted in 2006 and for use by women of all ages in 2013.
“There was very strong support that you did not need a prescriber,” said Ms. Wood, who resigned from the FDA in 2005 over the delay. “Everybody under the sun agreed except for a small group of people who somehow had an enormous political influence.”
Reuters Health Information © 2022
My picks for best of ASCO 2022
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
CHICAGO – The American Society of Clinical Oncology recently wrapped its annual meeting in Chicago. Here, I highlight some presentations that stood out to me.
A first-line treatment for metastatic colorectal cancer
The plenary session did not disappoint. In abstract LBA1, investigators presented first-line treatment for patients with metastatic colorectal cancer who were randomized to receive mFOLFOX6 with either bevacizumab or panitumumab in RAS wild-type positive patients. This was the phase 3 PARADIGM trial.
The primary outcome for this study was overall survival. It included 823 patients who were randomized 1:1 with a subset analysis of whether the primary tumor was on the left or right side of the colon. At 61 months follow-up, the median overall survival results for left-sided colon cancer was 38 months versus 34 months. It was statistically significant favoring the panitumumab arm. It improved the curable resection rate for patients with left-sided tumors from 11% in the bevacizumab arm to 18% in the panitumumab arm. Interestingly, patients randomized with right-sided tumors showed no difference in overall survival. The investigator, Takayuki Yoshino, MD, PhD, National Cancer Center Hospital East, Kashiwa, Japan, said the study findings support the use of mFOLFOX6 with panitumumab in left-sided RAS wild type as first-line therapy in metastatic colorectal patients.
A possible new standard of care in breast cancer
Shanu Modi, MD, of Memorial Sloan Kettering Cancer Center, New York, received a standing ovation and deserved it. In the phase 3 clinical trial DESTINY-Breast04 (abstract LBA3), she demonstrated that trastuzumab deruxtecan (T-DXd) for patients with metastatic breast cancer who were HER2 low (IHC 1+ or 2+ ISH-), led to a statistically significant and clinically meaningful benefit in both progression free survival and overall survival. In this trial, patients were randomized 2:1 to receive trastuzumab deruxtecan or physician’s choice of chemotherapy. All patients had at least one to two lines of chemotherapy before entering the trial. Hormone-positive patients were allowed if they had already received and failed, or progressed on hormone therapy.
Previously, most patients were treated either with eribulin with some receiving capecitabine, gemcitabine or taxane, or hormone therapy if hormone positive.
The progression-free survival was 10.1 versus 5.4 months in hormone-positive patients, and in all patients (hormone receptor positive or negative), there was a likewise improvement of 9.9 versus 5.1 months progression free survival.
Overall survival was equally impressive. In the hormone receptor–positive patients, the hazard ratio was 0.64 with a 23.9 versus 17.5 month survival. If all patients were included, the HR was again 0.64 with 23.4 versus 16.8 month survival. Even the triple-negative breast cancer patients had a HR of 0.48 with 18.2 versus 8.3 months survival. Adverse events were quite tolerable with some nausea, some decreased white count, and only an interstitial lung disease of grade 2 or less in 12%.
Trastuzumab deruxtecan is a targeted treatment which, in addition to striking its target, also targets other tumor cells that are part of the cancer. The results of this study may lead to a new standard of care of this patient population.
The study by Dr. Modi and colleagues was simultaneously published in the New England Journal of Medicine.
Improving outcomes in multiple myeloma
In abstract LBA4, Paul G. Richardson, MD, of the Dana-Farber Cancer Institute, Boston, asks if autologous stem cell transplant (ASCT) can improve outcomes after induction with an RVD regimen (lenalidomide, bortezomib, and dexamethasone) and lenalidomide (Revlimid) maintenance for newly diagnosed patients with multiple myeloma in the DETERMINATION study.
The take home here was quite interesting. In fact, there is no difference in overall survival if patients get this standard RVD/lenalidomide maintenance induction with or without ASCT. However, the progression free survival was better with ASCT: 46 versus 67 months (improvement of 21 months). However, there were some caveats. There was toxicity and change in quality of life for a while in those patients receiving ASCT as would be expected. Furthermore, the study only allowed 65 years old or younger and ASCT may not be wise for older patients. The discussant made a strong point that African Americans tend to have higher risk disease with different mutations and might also be better served by have ASCT later.
The conclusion was that, given all the new therapies in myeloma for second line and beyond, ASCT should be a discussion with each new patient and not an automatic decision.
This study was simultaneously published in the New England Journal of Medicine.
Adagrasib promising for pretreated patients with NSCLC with KRAS mutation
In patients with advanced or metastatic non–small cell lung cancer (NSCLC), adagrasib was found to be well tolerated and “demonstrates promising efficacy” for patients with the KRAS G12C mutation (KRYSTAL-1, abstract 9002). This was a phase 2 registration trial of 116 patients who were treated with 600 mg of adagrasib twice orally. Patients all had previous chemotherapy or immunotherapy or both. The overall response rate was a surprisingly good 43% (complete response and partial response). Disease control was an incredible 80% if stable disease was included. The duration of response was 8.5 months, progression-free survival was 6.5 months, and overall survival was 12.6 months. Furthermore, 33% of those with brain metastases had a complete response or partial response.
The take-home message is that, since 15% of NSCLC metastatic patients are KRAS mutant G12C, we should be watching for such patients in our biomarker analysis. While we have sotorasib – approved by the Food and Drug Administration for NSCLC – the results of this study suggests we may have another new molecule in the same class.
Neoadjuvant chemotherapy with immunotherapy for NSCLC
It may be time to consider neoadjuvant chemotherapy with immunotherapy, such as nivolumab, for patients with NSCLC in order to achieve the best response possible.
In NADIM II, investigators led by Mariano Provencio-Pulla, MD, of the Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, confirmed the superiority of chemotherapy with immunotherapy for patients with resectable stage IIIA NSCLC. NADIM included patients with resectable stage IIIA/B NSCLC who were randomized 2:1 to receive carboplatin taxol neoadjuvant therapy with or without nivolumab before and after surgery. The pathological complete response rates overall were 36% versus 7%, favoring the nivolumab arm, but even higher pCR rates occurred in patients with PD-L1 over 50%.
In closing, always check MMR, KRAS, BRAF, and HER2. For wild-type left-sided mCRC, consider FOLFOX or FOLFIRI with an anti-EGFR. For KRAS mutant or right-sided colon tumor, consider FOLFOX or FOLFIRI with bevacizumab, followed by maintenance 5FU or capecitabine, with or without bevacizumab.
AT ASCO 2022
Chronic Retiform Purpura of the Abdomen and Thighs: A Fatal Case of Intravascular Large Cell Lymphoma
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).
A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.
The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).

A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.

The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
To the Editor:
Intravascular large cell lymphoma (ILCL) is a rare B-cell lymphoma that is defined by the presence of large neoplastic B cells in the lumen of blood vessels.1 At least 3 variants of ILCL have been described based on case reports and a small case series: classic, cutaneous, and hemophagocytic. The classic variant presents in elderly patients as nonspecific constitutional symptoms (fever or pain, or less frequently weight loss) or as signs of multiorgan failure (most commonly of the central nervous system). Skin involvement, which is present in nearly half of these patients, can take on multiple morphologies, including retiform purpura, ulcerated nodules, or pseudocellulitis. The cutaneous variant typically presents in middle-aged women with normal hematologic studies. Systemic involvement is less common in this variant of disease than the classic variant, which may partly explain why overall survival is superior in this variant. The hemophagocytic variant manifests as intravascular lymphoma accompanied by hemophagocytic syndrome (fever, hepatosplenomegaly, thrombocytopenia, and bone marrow involvement).

A 69-year-old man presented to the emergency department for failure to thrive and nonhealing wounds of 1 year’s duration. His medical history was notable for poorly controlled diabetes mellitus, progressive multifocal ischemic and hemorrhagic cerebral infarcts, and bilateral deep venous thromboses. Physical examination revealed large purpuric to brown plaques in a retiform configuration with central necrotic eschars on the thighs and abdomen (Figure 1). There was no palpable lymphadenopathy. Laboratory tests revealed normocytic anemia with a hemoglobin level of 10.5 g/dL (reference range, 12–18 g/dL), elevated lactate dehydrogenase level of 525 U/L (reference range, 118–242 U/L), elevated erythrocyte sedimentation rate of 73 mm/h (reference range, <20 mm/h), antinuclear antibody (ANA) titer of 1:2560 (reference range, <1:80), and polyclonal hypergammaglobulinemia. The patient’s white blood cell and platelet counts, creatinine level, and liver function tests were within reference range. Cryoglobulins, coagulation studies, and cardiolipin antibodies were negative. Chest and abdominal imaging also were negative. An incisional skin biopsy and skin punch biopsy showed thrombotic coagulopathy and dilated vessels. A bone marrow biopsy revealed a hypercellular marrow but no plasma cell neoplasm. A repeat incisional skin biopsy demonstrated large CD20+ and CD45+ atypical lymphocytes within the small capillaries of the deep dermis and subcutaneous fat (Figure 2), which confirmed ILCL. Too deconditioned to tolerate chemotherapy, the patient opted for palliative care and died 18 months after initial presentation.

The diagnosis of ILCL often is delayed for several reasons.2 Patients can present with a variety of signs and symptoms related to small vessel occlusion that can be misattributed to other conditions.3,4 In our case, the patient’s recurrent infarcts were thought to be due to his poorly controlled diabetes mellitus, which was diagnosed a few weeks prior, and a positive ANA, even though the workup for antiphospholipid syndrome was negative. Interestingly, a positive ANA (without signs or symptoms of lupus or other autoimmune conditions) has been reported in patients with lymphoma.3 A positive antineutrophil cytoplasmic antibody level (without symptoms or other signs of vasculitis) has been reported in patients with ILCL.4,5 Therefore, distractors are common.
Multiple incisional skin biopsies in the absence of clinical findings (ie, random skin biopsy) are moderately sensitive (77.8%) for the diagnosis of ILCL.2 In a study by Matsue et al,2 111 suspected cases of ILCL underwent 3 incisional biopsies of fat-containing areas of the skin, such as the thigh, abdomen, and upper arm. Intravascular large cell lymphoma was confirmed in 26 cases. Seven additional cases were diagnosed as ILCL, 2 by additional skin biopsies (1 by a second round and 1 by a third round) and 5 by internal organ biopsy (4 bone marrow and 1 adrenal gland). The remaining cases ultimately were found to be a diagnostic mimicker of ILCL, including non-ILCL.2 Although random skin biopsies are reasonably sensitive for ILCL, multiple biopsies are needed, and in some cases, sampling of an internal organ may be required to establish the diagnosis of ILCL.
The prognosis of ILCL is poor; the 3-year overall survival rate for classic and cutaneous variants is 22% and 56%, respectively.6 Anthracycline-based chemotherapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), is considered first-line treatment, and the addition of rituximab to the CHOP regimen may improve remission rates and survival.7
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks [published online August 15, 2018]. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Matsue K, Abe Y, Kitadate A, et al. Sensitivity and specificity of incisional random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Blood. 2019;133:1257-1259.
- Altintas A, Cil T, Pasa S, et al. Clinical significance of elevated antinuclear antibody test in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Minerva Med. 2008;99:7-14.
- Shinkawa Y, Hatachi S, Yagita M. Intravascular large B-cell lymphoma with a high titer of proteinase-3-anti-neutrophil cytoplasmic antibody mimicking granulomatosis with polyangiitis. Mod Rheumatol. 2019;29:195-197.
- Sugiyama A, Kobayashi M, Daizo A, et al. Diffuse cerebral vasoconstriction in a intravascular lymphoma patient with a high serum MPO-ANCA level. Intern Med. 2017;56:1715-1718.
- Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant.’ Br J Haematol. 2004;127:173-183.
- Ferreri AJM, Dognini GP, Bairey O, et al; International Extranodal Lyphoma Study Group. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma [published online August 10, 2008]. Br J Haematol. 2008;143:253-257. doi:10.1111/j.1365-2141.2008.07338.x
Practice Points
- Intravascular large cell lymphoma (ILCL) is a life-threatening malignancy that can present with retiform purpura and other symptoms of vascular occlusion.
- The diagnosis of ILCL can be challenging because of the presence of distractors, and multiple biopsies may be required to establish pathology.