User login
Tumor Necrosis Factor α Inhibitor–Induced Lupuslike Syndrome in a Patient Prescribed Certolizumab Pegol
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.
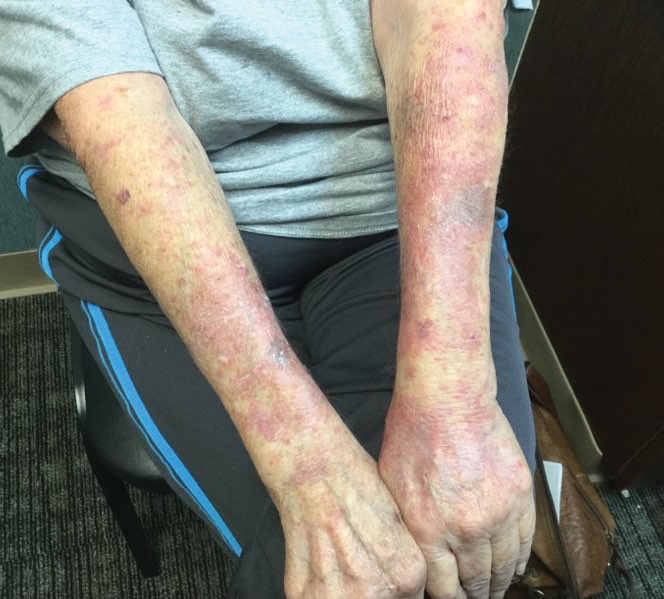
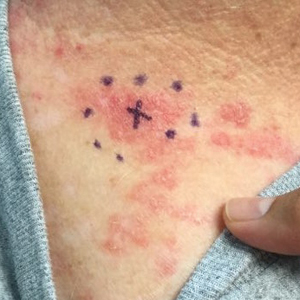
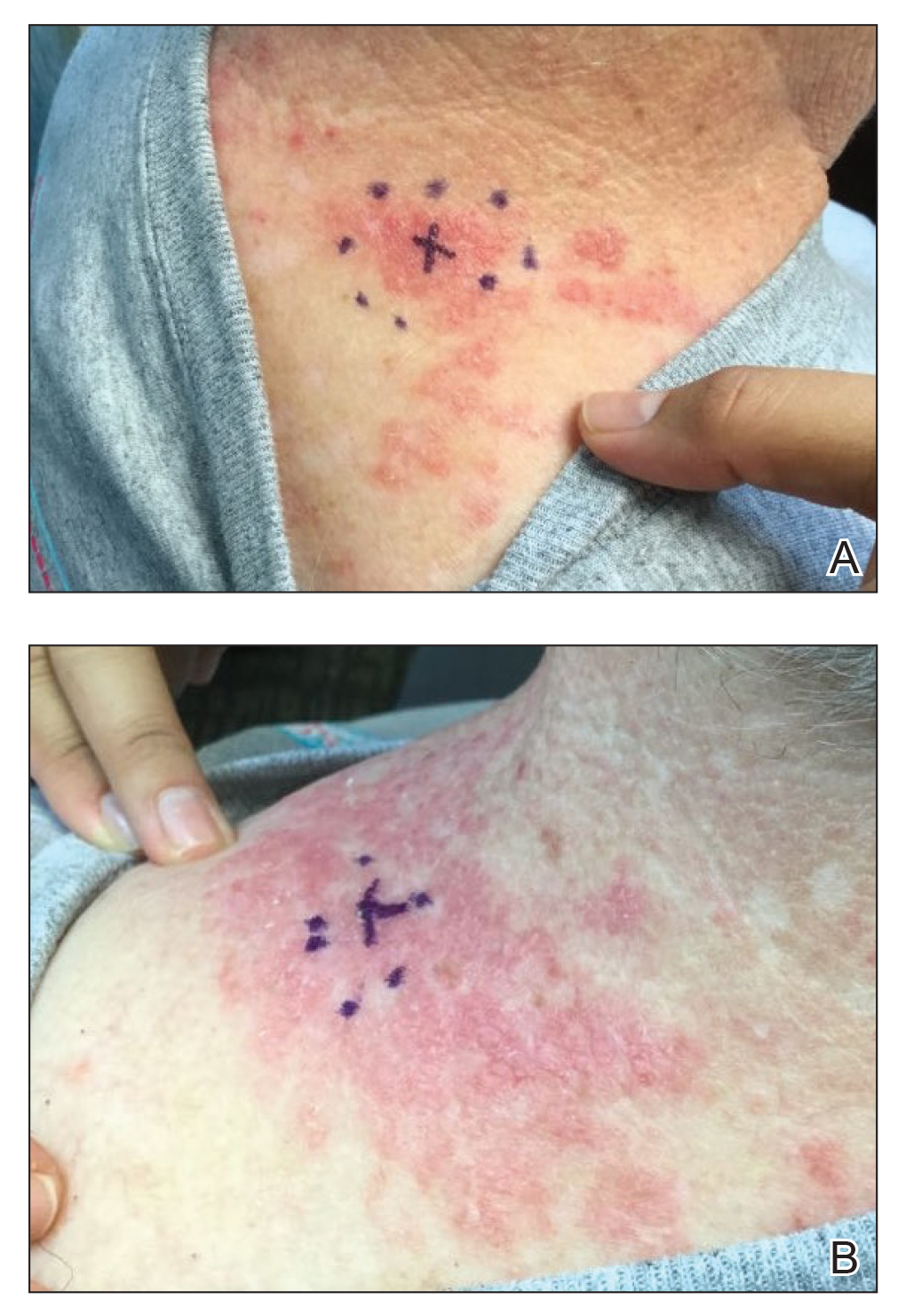
A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.
Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
Practice Points
- Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a form of drug-induced lupus specific to patients on anti–TNF-α therapy.
- The underlying mechanism of disease development is unique compared to other types of drug-induced lupus.
- TAILS most commonly is associated with the use of infliximab and etanercept but also has been reported with adalimumab, golimumab, and certolizumab pegol.
Hard-won medical advances versus miracle cures
I’m not hiding anything.
Occasionally I deal with patients and families who seem to think I have some miracle cure for a condition that I’m not telling them about.
I promise, I don’t work that way. Besides the obvious ethical issues, why would I? What could I possibly gain from doing that?
The trouble is that people are blanketed by news headlines, some reputable and some not, about a research study suggesting a new direction in treatment, or that a new drug in development has promise. Often these stories are forwarded to them by well-meaning relatives and friends, or just show up in their social media feed.
While some of these findings may actually lead somewhere, the vast majority don’t. In my career I’ve seen statins touted as potential treatments for MS and Alzheimer’s disease, and vilified as causes of dementia and peripheral neuropathy, all disproved or (to date) still up in the air.
But nonmedical people don’t understand that. It made the news, so it must mean something. I have no problem trying to explain this to them, but it’s never easy.
It’s even harder to explain to the ones who’ve already purchased a costly over-the-counter placebo for such a condition that they wasted their money.
Far from it.
New discoveries are made, but a lot of times it’s a very slow journey to find the solution. One discovery may not lead to THE answer, but hopefully will get you closer to it.
That generally doesn’t happen overnight. The mathematical problem of Goldbach’s Conjecture has been around since 1742 and still hasn’t been definitively answered.
Medicine isn’t math, either. The people and families dealing with these conditions want answers. I don’t blame them. So do I. Believe me, there would be nothing that would bring me more joy as a doctor than to be able to give someone with a serious diagnosis the comfort that comes with saying it’s also curable.
I never have, and never would, withhold such a thing from a patient. Ever. I just wish some of them would believe me when I say that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not hiding anything.
Occasionally I deal with patients and families who seem to think I have some miracle cure for a condition that I’m not telling them about.
I promise, I don’t work that way. Besides the obvious ethical issues, why would I? What could I possibly gain from doing that?
The trouble is that people are blanketed by news headlines, some reputable and some not, about a research study suggesting a new direction in treatment, or that a new drug in development has promise. Often these stories are forwarded to them by well-meaning relatives and friends, or just show up in their social media feed.
While some of these findings may actually lead somewhere, the vast majority don’t. In my career I’ve seen statins touted as potential treatments for MS and Alzheimer’s disease, and vilified as causes of dementia and peripheral neuropathy, all disproved or (to date) still up in the air.
But nonmedical people don’t understand that. It made the news, so it must mean something. I have no problem trying to explain this to them, but it’s never easy.
It’s even harder to explain to the ones who’ve already purchased a costly over-the-counter placebo for such a condition that they wasted their money.
Far from it.
New discoveries are made, but a lot of times it’s a very slow journey to find the solution. One discovery may not lead to THE answer, but hopefully will get you closer to it.
That generally doesn’t happen overnight. The mathematical problem of Goldbach’s Conjecture has been around since 1742 and still hasn’t been definitively answered.
Medicine isn’t math, either. The people and families dealing with these conditions want answers. I don’t blame them. So do I. Believe me, there would be nothing that would bring me more joy as a doctor than to be able to give someone with a serious diagnosis the comfort that comes with saying it’s also curable.
I never have, and never would, withhold such a thing from a patient. Ever. I just wish some of them would believe me when I say that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not hiding anything.
Occasionally I deal with patients and families who seem to think I have some miracle cure for a condition that I’m not telling them about.
I promise, I don’t work that way. Besides the obvious ethical issues, why would I? What could I possibly gain from doing that?
The trouble is that people are blanketed by news headlines, some reputable and some not, about a research study suggesting a new direction in treatment, or that a new drug in development has promise. Often these stories are forwarded to them by well-meaning relatives and friends, or just show up in their social media feed.
While some of these findings may actually lead somewhere, the vast majority don’t. In my career I’ve seen statins touted as potential treatments for MS and Alzheimer’s disease, and vilified as causes of dementia and peripheral neuropathy, all disproved or (to date) still up in the air.
But nonmedical people don’t understand that. It made the news, so it must mean something. I have no problem trying to explain this to them, but it’s never easy.
It’s even harder to explain to the ones who’ve already purchased a costly over-the-counter placebo for such a condition that they wasted their money.
Far from it.
New discoveries are made, but a lot of times it’s a very slow journey to find the solution. One discovery may not lead to THE answer, but hopefully will get you closer to it.
That generally doesn’t happen overnight. The mathematical problem of Goldbach’s Conjecture has been around since 1742 and still hasn’t been definitively answered.
Medicine isn’t math, either. The people and families dealing with these conditions want answers. I don’t blame them. So do I. Believe me, there would be nothing that would bring me more joy as a doctor than to be able to give someone with a serious diagnosis the comfort that comes with saying it’s also curable.
I never have, and never would, withhold such a thing from a patient. Ever. I just wish some of them would believe me when I say that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Nodules on the Anterior Neck Following Poly-L-lactic Acid Injection
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
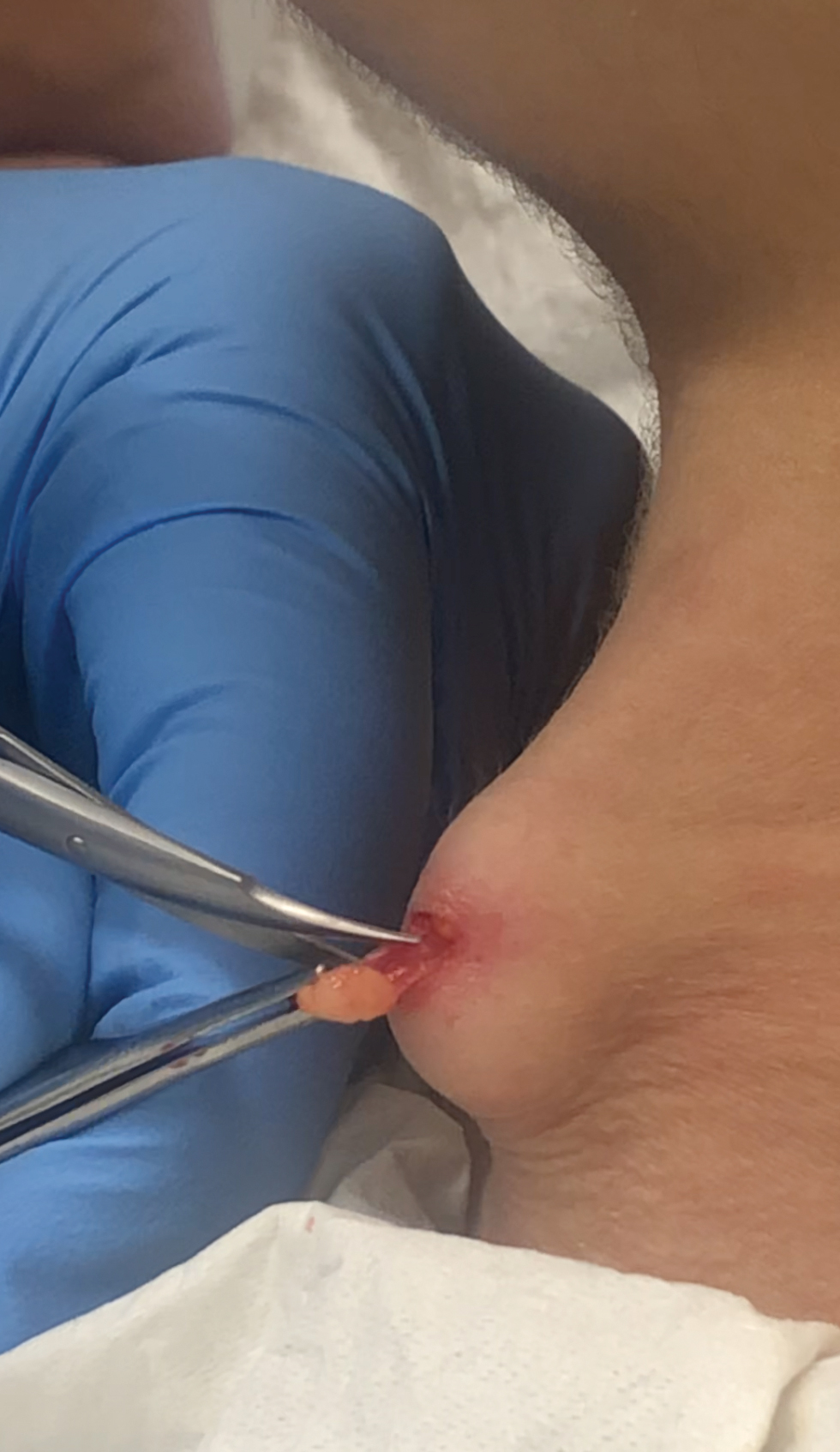
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).
Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Practice Points
- Injecting poly-L-lactic acid (PLLA) into the anterior neck is an off-label procedure and may cause a higher incidence of nodule formation.
- Most nodules from PLLA can be treated with injections of 5-fluorouracil, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals.
- Treatment-resistant nodules may require surgical excision.
Twin study offers new insight into genetics of migraine
DENVER – , even though testosterone is thought to be protective. The findings, presented at the annual meeting of the American Headache Society, also hint at a possible role played by the prenatal environment.
The study marks the first time a large-scale twin dataset has been used to assess sex differences in underlying genetic factors of migraine, lead author Morgan Fitzgerald, a senior research associate at the University of California at San Diego, said in a presentation at the conference. The findings were previously published in Frontiers in Pain Research.
More than genetics
The researchers analyzed data regarding 51,872 participants in the Swedish Twin Registry. According to Dr. Fitzgerald, the database is ideal because it is large and includes both genders.
Per the database, female twins were more likely to have migraines without aura than were male twins (17.6% vs. 5.5%, respectively), reflecting global numbers that suggest 18% of females and 6% of males have migraines each year.
To better understand heritability, the researchers compared identical twins with fraternal twins, and looked for gender-related correlations, Dr. Fitzgerald said.
One analysis suggests that migraine is equally heritable in men and women with a broad sense heritability of 0.45 (95% confidence interval [CI], 0.40-0.50). However, another analysis model provides evidence “that there are differences in the underlying genetic factors contributing to migraine across males and females,” she said.
Unexpectedly, the researchers also found that females with male twins were more likely to have migraines than were those with female twins (odds ratio, 1.51, 95% CI, 1.26-1.81) even though males are less affected by the headaches.
“These results suggest that the prominent sex difference in migraine prevalence is not entirely accounted for by genetic factors, while demonstrating that masculinization of the prenatal environment may increase migraine risk for females,” the authors wrote in the published study. “This effect points to a potential prenatal neuroendocrine factor in the development of migraine.”
Probing the migraine gender gap
Commenting on the research, University of Texas at Dallas neuroscientist and headache researcher Gregory Dussor, PhD, said the new study is “a very unique approach to address the question of nature versus nurture in migraine. It was well designed and used robust statistical modeling.”
As for the findings, “the conclusion that genetics do not explain sex differences in migraine risk by themselves is not surprising given how big of a role hormones in later life are likely to play in the disease and how many factors there are that can influence hormone levels,” he said.
“On the other hand, the surprising part of the findings was that the presence of a male co-twin increases risk of migraine in females. I would have expected to see the opposite, given the lower prevalence of migraine in males and the seemingly protective role that male hormones can play in migraine.”
Overall, the study adds to data implicating environment and hormones in the migraine gender gap, he said. “One thing I wonder from this study is what influence a female co-twin growing up with a male co-twin can have on migraine susceptibility. That female co-twin may end up with a very different set of childhood experiences than if she was with another female co-twin. Twins generally spend an enormous amount of time together and the same sex versus opposite sex experiences are likely to be quite different. This may have an influence on migraine later in life.”
As for the value of the study in terms of diagnosis, treatment, or prevention of migraine, Dr. Dussor said, “it’s possible it could help to identify risk factors for higher migraine susceptibility but it’s far too early to know how this could be used.”
The authors have no disclosures. Dr. Dussor disclosed an NIH-funded grant to study the role of the hormone prolactin in preclinical migraine models.
DENVER – , even though testosterone is thought to be protective. The findings, presented at the annual meeting of the American Headache Society, also hint at a possible role played by the prenatal environment.
The study marks the first time a large-scale twin dataset has been used to assess sex differences in underlying genetic factors of migraine, lead author Morgan Fitzgerald, a senior research associate at the University of California at San Diego, said in a presentation at the conference. The findings were previously published in Frontiers in Pain Research.
More than genetics
The researchers analyzed data regarding 51,872 participants in the Swedish Twin Registry. According to Dr. Fitzgerald, the database is ideal because it is large and includes both genders.
Per the database, female twins were more likely to have migraines without aura than were male twins (17.6% vs. 5.5%, respectively), reflecting global numbers that suggest 18% of females and 6% of males have migraines each year.
To better understand heritability, the researchers compared identical twins with fraternal twins, and looked for gender-related correlations, Dr. Fitzgerald said.
One analysis suggests that migraine is equally heritable in men and women with a broad sense heritability of 0.45 (95% confidence interval [CI], 0.40-0.50). However, another analysis model provides evidence “that there are differences in the underlying genetic factors contributing to migraine across males and females,” she said.
Unexpectedly, the researchers also found that females with male twins were more likely to have migraines than were those with female twins (odds ratio, 1.51, 95% CI, 1.26-1.81) even though males are less affected by the headaches.
“These results suggest that the prominent sex difference in migraine prevalence is not entirely accounted for by genetic factors, while demonstrating that masculinization of the prenatal environment may increase migraine risk for females,” the authors wrote in the published study. “This effect points to a potential prenatal neuroendocrine factor in the development of migraine.”
Probing the migraine gender gap
Commenting on the research, University of Texas at Dallas neuroscientist and headache researcher Gregory Dussor, PhD, said the new study is “a very unique approach to address the question of nature versus nurture in migraine. It was well designed and used robust statistical modeling.”
As for the findings, “the conclusion that genetics do not explain sex differences in migraine risk by themselves is not surprising given how big of a role hormones in later life are likely to play in the disease and how many factors there are that can influence hormone levels,” he said.
“On the other hand, the surprising part of the findings was that the presence of a male co-twin increases risk of migraine in females. I would have expected to see the opposite, given the lower prevalence of migraine in males and the seemingly protective role that male hormones can play in migraine.”
Overall, the study adds to data implicating environment and hormones in the migraine gender gap, he said. “One thing I wonder from this study is what influence a female co-twin growing up with a male co-twin can have on migraine susceptibility. That female co-twin may end up with a very different set of childhood experiences than if she was with another female co-twin. Twins generally spend an enormous amount of time together and the same sex versus opposite sex experiences are likely to be quite different. This may have an influence on migraine later in life.”
As for the value of the study in terms of diagnosis, treatment, or prevention of migraine, Dr. Dussor said, “it’s possible it could help to identify risk factors for higher migraine susceptibility but it’s far too early to know how this could be used.”
The authors have no disclosures. Dr. Dussor disclosed an NIH-funded grant to study the role of the hormone prolactin in preclinical migraine models.
DENVER – , even though testosterone is thought to be protective. The findings, presented at the annual meeting of the American Headache Society, also hint at a possible role played by the prenatal environment.
The study marks the first time a large-scale twin dataset has been used to assess sex differences in underlying genetic factors of migraine, lead author Morgan Fitzgerald, a senior research associate at the University of California at San Diego, said in a presentation at the conference. The findings were previously published in Frontiers in Pain Research.
More than genetics
The researchers analyzed data regarding 51,872 participants in the Swedish Twin Registry. According to Dr. Fitzgerald, the database is ideal because it is large and includes both genders.
Per the database, female twins were more likely to have migraines without aura than were male twins (17.6% vs. 5.5%, respectively), reflecting global numbers that suggest 18% of females and 6% of males have migraines each year.
To better understand heritability, the researchers compared identical twins with fraternal twins, and looked for gender-related correlations, Dr. Fitzgerald said.
One analysis suggests that migraine is equally heritable in men and women with a broad sense heritability of 0.45 (95% confidence interval [CI], 0.40-0.50). However, another analysis model provides evidence “that there are differences in the underlying genetic factors contributing to migraine across males and females,” she said.
Unexpectedly, the researchers also found that females with male twins were more likely to have migraines than were those with female twins (odds ratio, 1.51, 95% CI, 1.26-1.81) even though males are less affected by the headaches.
“These results suggest that the prominent sex difference in migraine prevalence is not entirely accounted for by genetic factors, while demonstrating that masculinization of the prenatal environment may increase migraine risk for females,” the authors wrote in the published study. “This effect points to a potential prenatal neuroendocrine factor in the development of migraine.”
Probing the migraine gender gap
Commenting on the research, University of Texas at Dallas neuroscientist and headache researcher Gregory Dussor, PhD, said the new study is “a very unique approach to address the question of nature versus nurture in migraine. It was well designed and used robust statistical modeling.”
As for the findings, “the conclusion that genetics do not explain sex differences in migraine risk by themselves is not surprising given how big of a role hormones in later life are likely to play in the disease and how many factors there are that can influence hormone levels,” he said.
“On the other hand, the surprising part of the findings was that the presence of a male co-twin increases risk of migraine in females. I would have expected to see the opposite, given the lower prevalence of migraine in males and the seemingly protective role that male hormones can play in migraine.”
Overall, the study adds to data implicating environment and hormones in the migraine gender gap, he said. “One thing I wonder from this study is what influence a female co-twin growing up with a male co-twin can have on migraine susceptibility. That female co-twin may end up with a very different set of childhood experiences than if she was with another female co-twin. Twins generally spend an enormous amount of time together and the same sex versus opposite sex experiences are likely to be quite different. This may have an influence on migraine later in life.”
As for the value of the study in terms of diagnosis, treatment, or prevention of migraine, Dr. Dussor said, “it’s possible it could help to identify risk factors for higher migraine susceptibility but it’s far too early to know how this could be used.”
The authors have no disclosures. Dr. Dussor disclosed an NIH-funded grant to study the role of the hormone prolactin in preclinical migraine models.
AT AHS 2022
Melanoma incidence is up, but death rates are down
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
CHICAGO – , according to a new analysis of the National Cancer Institute SEER database between 1975 and 2019.
“This is very encouraging data and represents the real-world effectiveness of these therapies. The cost of these therapies can be prohibitive for universal treatment access, so the ways to address the accessibility of these treatments and the health care costs need to be supported,” said lead author Navkirat Kaur Kahlon MD, a hematology/oncology fellow at the University of Toledo (Ohio). The study was presented at the annual meeting of the American Society of Clinical Oncology.
According to the American Cancer Society, the 5-year mortality for regional melanoma metastasis is 68%, and 30% for distant metastasis. However, these numbers may underestimate current survival. “People now being diagnosed with melanoma may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least 5 years earlier,” the American Cancer Society wrote.
Other studies have found similar trends. According to Cancer Research UK, 5-year melanoma skin cancer survival approximately doubled, from 46% to 90%, between 1971 and 2010. And, 1-year survival increased from 74% to 96%, but these improvements predated immune checkpoint inhibitors. An analysis of the Canadian Cancer Registry and Canadian Vital Statistics found an increasing incidence of melanoma, but a drop in mortality since 2013. A study of melanoma outcomes in Hungary also found increased incidence, while mortality declined by 16.55% between 2011 and 2019 (P =.013).
“These new drugs, which include immunotherapies and targeted therapies, are effective treatments in the clinical trial data, so the magnitude of drop seen in population mortality was not surprising but very exciting,” Dr. Kahlon said.
The findings are encouraging, but prevention remains the most important strategy. “The utility of sun-protective strategies and policies should be encouraged,” she added.
Cytotoxic chemotherapy has poor efficacy against metastatic melanoma, but novel therapies such as checkpoint inhibitors increased expected survival from months to years. “Given the magnitude of benefit compared to traditional chemotherapy in clinical trials, we decided to see if the real-world population is deriving the same benefit,” Dr. Kahlon said.
The researchers found that the annual percentage change (APC) melanoma mortality rate (MMR) was +1.65% between 1975 and 1988 (P < .01). The APC was 0.01% between 1988 and 2013, which was not statistically significant (P = .85). Between 2013 and 2017, APC was –6.24% (P < .01), and it was –1.56% between 2017 and 2019 (P = .53).
The increase in melanoma mortality between 1975 and 1988 may be due to changes in the way that SEER data was collected. “It is possible that this increase was at least in part due to better capturing of the data. There may also be a contribution of increased mortality due to increased incidence of diagnoses related to increased UV exposure. From the 1920s, increased sun exposure and bronzed skin became fashionable. In the 1940s-1960s, tanning oils and lotions became more popular, and there may have been an increase in UV exposure during that time, which later led to an increase in diagnosis and, without effective therapies, mortality. Further, the use of indoor tanning beds from the 1970s onward may have contributed to increased UV exposure, incidence, and mortality,” she said.
On the other hand, the researchers noted a slowing of mortality reduction between 2017 and 2019. This was not a surprise, Dr. Kahlon said, since by that time most novel therapies were being introduced in the adjuvant setting. “The mortality benefit, if any, from adjuvant treatments is seen over a longer period and may not yet be captured in SEER data. Even the clinical trial data for most of these treatments have not shown an overall survival advantage and require more time for the data to mature. It will be interesting to see how these trends change in the near future,” Dr. Kahlon said.
The study was limited by its retrospective nature. Dr. Kahlon has no relevant financial disclosures.
AT ASCO 2022
Highlights in Early-Stage Non–Small Cell Lung Cancer From ASCO 2022
Highlights in early-stage non–small lung cancer (NSCLC) from the 2022 American Society of Clinical Oncology Annual Meeting are discussed by Dr Jack West of City of Hope Comprehensive Cancer Center in Duarte, California.
He opens with a post hoc analysis of CheckMate 816, which showed that achieving a pathologic complete response with adjuvant nivolumab plus chemotherapy was highly associated with improved event-free survival.
Next, Dr West reports on early results from the NADIM II trial, which confirmed the superiority of neoadjuvant nivolumab plus chemotherapy in resectable stage IIIa NSCLC, as well as the need for careful patient selection.
He moves on to describe the neoSCORE study, which compared two vs three cycles of neoadjuvant sintilimab plus chemotherapy, with the latter achieving a markedly higher major pathologic response rate.
Dr West next examines two interventional studies, starting with an analysis of the SEER database, which showed that addition of radiation therapy to neoadjuvant chemotherapy does not offer a benefit. He ends with a Veterans Affairs study underlining the importance of surgical quality as a predictor of patient outcomes.
--
Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, California; Medical Director, AccessHope, Los Angeles, California
Howard (Jack) West, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; AstraZeneca; Eli Lily; EQRx; Genentech/Roche; Merck; Mirati; Pfizer; Regeneron; Takeda
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Merck
Highlights in early-stage non–small lung cancer (NSCLC) from the 2022 American Society of Clinical Oncology Annual Meeting are discussed by Dr Jack West of City of Hope Comprehensive Cancer Center in Duarte, California.
He opens with a post hoc analysis of CheckMate 816, which showed that achieving a pathologic complete response with adjuvant nivolumab plus chemotherapy was highly associated with improved event-free survival.
Next, Dr West reports on early results from the NADIM II trial, which confirmed the superiority of neoadjuvant nivolumab plus chemotherapy in resectable stage IIIa NSCLC, as well as the need for careful patient selection.
He moves on to describe the neoSCORE study, which compared two vs three cycles of neoadjuvant sintilimab plus chemotherapy, with the latter achieving a markedly higher major pathologic response rate.
Dr West next examines two interventional studies, starting with an analysis of the SEER database, which showed that addition of radiation therapy to neoadjuvant chemotherapy does not offer a benefit. He ends with a Veterans Affairs study underlining the importance of surgical quality as a predictor of patient outcomes.
--
Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, California; Medical Director, AccessHope, Los Angeles, California
Howard (Jack) West, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; AstraZeneca; Eli Lily; EQRx; Genentech/Roche; Merck; Mirati; Pfizer; Regeneron; Takeda
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Merck
Highlights in early-stage non–small lung cancer (NSCLC) from the 2022 American Society of Clinical Oncology Annual Meeting are discussed by Dr Jack West of City of Hope Comprehensive Cancer Center in Duarte, California.
He opens with a post hoc analysis of CheckMate 816, which showed that achieving a pathologic complete response with adjuvant nivolumab plus chemotherapy was highly associated with improved event-free survival.
Next, Dr West reports on early results from the NADIM II trial, which confirmed the superiority of neoadjuvant nivolumab plus chemotherapy in resectable stage IIIa NSCLC, as well as the need for careful patient selection.
He moves on to describe the neoSCORE study, which compared two vs three cycles of neoadjuvant sintilimab plus chemotherapy, with the latter achieving a markedly higher major pathologic response rate.
Dr West next examines two interventional studies, starting with an analysis of the SEER database, which showed that addition of radiation therapy to neoadjuvant chemotherapy does not offer a benefit. He ends with a Veterans Affairs study underlining the importance of surgical quality as a predictor of patient outcomes.
--
Associate Professor, Department of Medical Oncology, City of Hope Comprehensive Cancer Care, Duarte, California; Medical Director, AccessHope, Los Angeles, California
Howard (Jack) West, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; AstraZeneca; Eli Lily; EQRx; Genentech/Roche; Merck; Mirati; Pfizer; Regeneron; Takeda
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Merck

Jury out on low-FODMAP diet for kids
There is scarce evidence to support the use of a FODMAP-lowering diet for children with irritable bowel syndrome (IBS), and there is no evidence to recommend its use for other gastrointestinal (GI) diseases and complaints in children, according to a position paper from the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN).
A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet is increasingly being used to treat children with various GI complaints and disorders.
“Awareness of how and when to use the diet is crucial, as a restrictive diet may impact nutritional adequacy and/or promote distorted eating in vulnerable subjects,” the authors note.
Rut Anne Thomassen, department of pediatric medicine, Oslo University Hospital, and an international team of experts conducted a systematic literature review of the evidence on the safety and efficacy of the low-FODMAP diet in children.
The low-FODMAP diet has not been well studied in children, they report.
From 53 publications and registers that they screened, only seven studies (four randomized clinical trials and three interventions without control group or observational studies) were included in their assessment.
In the seven studies, only 111 children received the low-FODMAP diet, while 85 followed a control diet for comparison (a diet described as healthy, usual, or typical American diet for children).
All of the pediatric studies focused on functional abdominal pain disorders. None addressed nonceliac gluten sensitivity, small-intestinal bacterial overgrowth, or inflammatory bowel disease.
From their review, the authors conclude that, at present, there is “insufficient evidence” to routinely recommend the low-FODMAP diet for the treatment of functional GI disorders, nonceliac gluten sensitivity, inflammatory bowel diseases, or small-intestinal bacterial overgrowth in children.
When the low-FODMAP diet is considered for children, the authors recommend a thorough clinical history, physical examination, and assessment of nutritional status and GI symptoms by a multidisciplinary team.
“Ideally, a standardized questionnaire should be used before and following the start of the diet to assess objectively the effect of the low-FODMAP diet,” the authors advise.
A dietitian should assess the child’s diet to highlight any potential deficiencies, which could be exacerbated by the restrictions of the low-FODMAP diet.
To promote adherence to the diet, potential difficulties, such as how to provide a suitable lunch at school or what to do when the child is staying at a friend’s house, should be addressed.
The authors suggest providing parents with written information about sources of FODMAPs and suitable replacement foods. Offering meal plans can reduce the risk of diet mistakes as well as the risk of offering a diet insufficient in essential nutrients, they say.
‘Useful paper’
“This is a useful paper primarily to outline the paucity of data regarding dietary therapies in children and the importance of doing studies in this population,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, who wasn’t involved in the research, told this news organization.
Samuel Nurko, MD, MPH, director of the Center for Motility and Functional Gastrointestinal Disorders at Boston Children’s Hospital, Massachusetts, noted that some studies have shown that a low-FODMAP diet can be effective in controlling symptoms for both adults and kids.
“The problem in kids is that the trials are very small, and there’s not a lot of them, so the evidence is limited,” said Dr. Nurko, who wasn’t involved in writing the position paper.
That’s not to say that it should not be tried in appropriate cases. “There’s no question that in some patients, taking away the FODMAPs gives them a big improvement in GI symptoms,” Dr. Nurko told this news organization.
“The problem with the low-FODMAP diet is, if you don’t do it right, then you get into trouble with nutritional deficiencies,” he cautioned.
“If you are going to try the low-FODMAP diet, it has to be short, no more than 4-6 weeks, and you need to do a top-down approach. Take FODMAPs out, and then start to reintroduce them. Either kids will respond to the diet, or they won’t. If they don’t, there is no reason to keep them on the diet. It’s a very hard diet to take,” Dr. Nurko said.
No source of funding for the study was disclosed. The authors, Dr. Ananthakrishnan, and Dr. Nurko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is scarce evidence to support the use of a FODMAP-lowering diet for children with irritable bowel syndrome (IBS), and there is no evidence to recommend its use for other gastrointestinal (GI) diseases and complaints in children, according to a position paper from the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN).
A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet is increasingly being used to treat children with various GI complaints and disorders.
“Awareness of how and when to use the diet is crucial, as a restrictive diet may impact nutritional adequacy and/or promote distorted eating in vulnerable subjects,” the authors note.
Rut Anne Thomassen, department of pediatric medicine, Oslo University Hospital, and an international team of experts conducted a systematic literature review of the evidence on the safety and efficacy of the low-FODMAP diet in children.
The low-FODMAP diet has not been well studied in children, they report.
From 53 publications and registers that they screened, only seven studies (four randomized clinical trials and three interventions without control group or observational studies) were included in their assessment.
In the seven studies, only 111 children received the low-FODMAP diet, while 85 followed a control diet for comparison (a diet described as healthy, usual, or typical American diet for children).
All of the pediatric studies focused on functional abdominal pain disorders. None addressed nonceliac gluten sensitivity, small-intestinal bacterial overgrowth, or inflammatory bowel disease.
From their review, the authors conclude that, at present, there is “insufficient evidence” to routinely recommend the low-FODMAP diet for the treatment of functional GI disorders, nonceliac gluten sensitivity, inflammatory bowel diseases, or small-intestinal bacterial overgrowth in children.
When the low-FODMAP diet is considered for children, the authors recommend a thorough clinical history, physical examination, and assessment of nutritional status and GI symptoms by a multidisciplinary team.
“Ideally, a standardized questionnaire should be used before and following the start of the diet to assess objectively the effect of the low-FODMAP diet,” the authors advise.
A dietitian should assess the child’s diet to highlight any potential deficiencies, which could be exacerbated by the restrictions of the low-FODMAP diet.
To promote adherence to the diet, potential difficulties, such as how to provide a suitable lunch at school or what to do when the child is staying at a friend’s house, should be addressed.
The authors suggest providing parents with written information about sources of FODMAPs and suitable replacement foods. Offering meal plans can reduce the risk of diet mistakes as well as the risk of offering a diet insufficient in essential nutrients, they say.
‘Useful paper’
“This is a useful paper primarily to outline the paucity of data regarding dietary therapies in children and the importance of doing studies in this population,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, who wasn’t involved in the research, told this news organization.
Samuel Nurko, MD, MPH, director of the Center for Motility and Functional Gastrointestinal Disorders at Boston Children’s Hospital, Massachusetts, noted that some studies have shown that a low-FODMAP diet can be effective in controlling symptoms for both adults and kids.
“The problem in kids is that the trials are very small, and there’s not a lot of them, so the evidence is limited,” said Dr. Nurko, who wasn’t involved in writing the position paper.
That’s not to say that it should not be tried in appropriate cases. “There’s no question that in some patients, taking away the FODMAPs gives them a big improvement in GI symptoms,” Dr. Nurko told this news organization.
“The problem with the low-FODMAP diet is, if you don’t do it right, then you get into trouble with nutritional deficiencies,” he cautioned.
“If you are going to try the low-FODMAP diet, it has to be short, no more than 4-6 weeks, and you need to do a top-down approach. Take FODMAPs out, and then start to reintroduce them. Either kids will respond to the diet, or they won’t. If they don’t, there is no reason to keep them on the diet. It’s a very hard diet to take,” Dr. Nurko said.
No source of funding for the study was disclosed. The authors, Dr. Ananthakrishnan, and Dr. Nurko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There is scarce evidence to support the use of a FODMAP-lowering diet for children with irritable bowel syndrome (IBS), and there is no evidence to recommend its use for other gastrointestinal (GI) diseases and complaints in children, according to a position paper from the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN).
A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet is increasingly being used to treat children with various GI complaints and disorders.
“Awareness of how and when to use the diet is crucial, as a restrictive diet may impact nutritional adequacy and/or promote distorted eating in vulnerable subjects,” the authors note.
Rut Anne Thomassen, department of pediatric medicine, Oslo University Hospital, and an international team of experts conducted a systematic literature review of the evidence on the safety and efficacy of the low-FODMAP diet in children.
The low-FODMAP diet has not been well studied in children, they report.
From 53 publications and registers that they screened, only seven studies (four randomized clinical trials and three interventions without control group or observational studies) were included in their assessment.
In the seven studies, only 111 children received the low-FODMAP diet, while 85 followed a control diet for comparison (a diet described as healthy, usual, or typical American diet for children).
All of the pediatric studies focused on functional abdominal pain disorders. None addressed nonceliac gluten sensitivity, small-intestinal bacterial overgrowth, or inflammatory bowel disease.
From their review, the authors conclude that, at present, there is “insufficient evidence” to routinely recommend the low-FODMAP diet for the treatment of functional GI disorders, nonceliac gluten sensitivity, inflammatory bowel diseases, or small-intestinal bacterial overgrowth in children.
When the low-FODMAP diet is considered for children, the authors recommend a thorough clinical history, physical examination, and assessment of nutritional status and GI symptoms by a multidisciplinary team.
“Ideally, a standardized questionnaire should be used before and following the start of the diet to assess objectively the effect of the low-FODMAP diet,” the authors advise.
A dietitian should assess the child’s diet to highlight any potential deficiencies, which could be exacerbated by the restrictions of the low-FODMAP diet.
To promote adherence to the diet, potential difficulties, such as how to provide a suitable lunch at school or what to do when the child is staying at a friend’s house, should be addressed.
The authors suggest providing parents with written information about sources of FODMAPs and suitable replacement foods. Offering meal plans can reduce the risk of diet mistakes as well as the risk of offering a diet insufficient in essential nutrients, they say.
‘Useful paper’
“This is a useful paper primarily to outline the paucity of data regarding dietary therapies in children and the importance of doing studies in this population,” Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, who wasn’t involved in the research, told this news organization.
Samuel Nurko, MD, MPH, director of the Center for Motility and Functional Gastrointestinal Disorders at Boston Children’s Hospital, Massachusetts, noted that some studies have shown that a low-FODMAP diet can be effective in controlling symptoms for both adults and kids.
“The problem in kids is that the trials are very small, and there’s not a lot of them, so the evidence is limited,” said Dr. Nurko, who wasn’t involved in writing the position paper.
That’s not to say that it should not be tried in appropriate cases. “There’s no question that in some patients, taking away the FODMAPs gives them a big improvement in GI symptoms,” Dr. Nurko told this news organization.
“The problem with the low-FODMAP diet is, if you don’t do it right, then you get into trouble with nutritional deficiencies,” he cautioned.
“If you are going to try the low-FODMAP diet, it has to be short, no more than 4-6 weeks, and you need to do a top-down approach. Take FODMAPs out, and then start to reintroduce them. Either kids will respond to the diet, or they won’t. If they don’t, there is no reason to keep them on the diet. It’s a very hard diet to take,” Dr. Nurko said.
No source of funding for the study was disclosed. The authors, Dr. Ananthakrishnan, and Dr. Nurko have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
American Academy of Pediatrics recommends adolescent suicide screening
With suicide rates among young people rising in recent years, the American Academy of Pediatrics is now recommending adolescents 12 and up be screened for suicide risk as a part of regular preventive care.
The group recently added the recommendation on screening for suicide risk to its depression screening guidelines. Health care providers are urged to ask their young patients a set of questions to identify thoughts and plans for suicide, WDEF.com reported.
“Number one we need to screen for depression and the presence of depression, and those people will usually have a feeling of depressed mood, hopelessness, helplessness, and/or basically a lack of interest in pleasure or anticipation of happiness,” Timothy Fuller, DO, medical director of behavioral health and pediatrics for the American Academy of Pediatrics, told WDEF.
It’s a myth that talking about suicide makes it more likely a person will attempt suicide, he said.
“One of the biggest things you can do, as well, if you do have a child or teenager that has suicidality or that have depression with serious, significant suicide risk, is to just ask them how they’re doing every day,” Dr. Fuller said, according to WDEF.
The recommendation comes about 6 months after U.S. Surgeon General Vivek Murthy, MD, urged more attention be paid to youth mental health.
“Mental health challenges in children, adolescents, and young adults are real and widespread. Even before the pandemic, an alarming number of young people struggled with feelings of helplessness, depression, and thoughts of suicide – and rates have increased over the past decade,” Dr. Murthy said, according to a news release from the U.S. Department of Health & Human Services.
Between 2007 and 2018, suicide rates among people ages 10-24 in the United States went up by 57%, the department said. Estimates showed over 6,600 suicides among this age group in 2020, it said.
A version of this article first appeared on WebMD.com.
With suicide rates among young people rising in recent years, the American Academy of Pediatrics is now recommending adolescents 12 and up be screened for suicide risk as a part of regular preventive care.
The group recently added the recommendation on screening for suicide risk to its depression screening guidelines. Health care providers are urged to ask their young patients a set of questions to identify thoughts and plans for suicide, WDEF.com reported.
“Number one we need to screen for depression and the presence of depression, and those people will usually have a feeling of depressed mood, hopelessness, helplessness, and/or basically a lack of interest in pleasure or anticipation of happiness,” Timothy Fuller, DO, medical director of behavioral health and pediatrics for the American Academy of Pediatrics, told WDEF.
It’s a myth that talking about suicide makes it more likely a person will attempt suicide, he said.
“One of the biggest things you can do, as well, if you do have a child or teenager that has suicidality or that have depression with serious, significant suicide risk, is to just ask them how they’re doing every day,” Dr. Fuller said, according to WDEF.
The recommendation comes about 6 months after U.S. Surgeon General Vivek Murthy, MD, urged more attention be paid to youth mental health.
“Mental health challenges in children, adolescents, and young adults are real and widespread. Even before the pandemic, an alarming number of young people struggled with feelings of helplessness, depression, and thoughts of suicide – and rates have increased over the past decade,” Dr. Murthy said, according to a news release from the U.S. Department of Health & Human Services.
Between 2007 and 2018, suicide rates among people ages 10-24 in the United States went up by 57%, the department said. Estimates showed over 6,600 suicides among this age group in 2020, it said.
A version of this article first appeared on WebMD.com.
With suicide rates among young people rising in recent years, the American Academy of Pediatrics is now recommending adolescents 12 and up be screened for suicide risk as a part of regular preventive care.
The group recently added the recommendation on screening for suicide risk to its depression screening guidelines. Health care providers are urged to ask their young patients a set of questions to identify thoughts and plans for suicide, WDEF.com reported.
“Number one we need to screen for depression and the presence of depression, and those people will usually have a feeling of depressed mood, hopelessness, helplessness, and/or basically a lack of interest in pleasure or anticipation of happiness,” Timothy Fuller, DO, medical director of behavioral health and pediatrics for the American Academy of Pediatrics, told WDEF.
It’s a myth that talking about suicide makes it more likely a person will attempt suicide, he said.
“One of the biggest things you can do, as well, if you do have a child or teenager that has suicidality or that have depression with serious, significant suicide risk, is to just ask them how they’re doing every day,” Dr. Fuller said, according to WDEF.
The recommendation comes about 6 months after U.S. Surgeon General Vivek Murthy, MD, urged more attention be paid to youth mental health.
“Mental health challenges in children, adolescents, and young adults are real and widespread. Even before the pandemic, an alarming number of young people struggled with feelings of helplessness, depression, and thoughts of suicide – and rates have increased over the past decade,” Dr. Murthy said, according to a news release from the U.S. Department of Health & Human Services.
Between 2007 and 2018, suicide rates among people ages 10-24 in the United States went up by 57%, the department said. Estimates showed over 6,600 suicides among this age group in 2020, it said.
A version of this article first appeared on WebMD.com.
LGBTQ students would get new protections under Biden plan
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
On the 50th anniversary of Title IX’s inception, the Biden administration has proposed changes to the law that would protect transgender students and assault survivors on college and university campuses.
With these changes, the protections provided by Title IX – a civil rights law that prohibits sex-based discrimination in schools that receive federal funding – would now be extended to students who identify as trans. The update would ensure that government-funded schools make proper accommodations for a trans student population, such as allowing students to use bathrooms and other facilities that align with their gender identity, and enforcing the use of students’ correct pronouns.
The revisions also seek to undo amendments made to the law by Betsy DeVos, who was secretary of education during the Trump presidency, which strengthened due process protections for students accused of sexual assault and narrowed the definition of sexual harassment. These rules “weakened protections for survivors of sexual assault and diminished the promise of an education free from discrimination,” the Biden administration said.
“Our proposed changes will allow us to continue that progress and ensure all our nation’s students – no matter where they live, who they are, or whom they love – can learn, grow, and thrive in school,” Education Secretary Miguel Cardona, PhD, said in a news release. “We welcome public comment on these critical regulations so we can further the Biden-Harris Administration’s mission of creating educational environments free from sex discrimination and sexual violence.”
The revisions will go through a long period of public comment before they are set into law. Still, the proposed changes mark a way forward for trans students who are not explicitly protected under Title IX, and they offer solace to assault survivors who may have felt discouraged to come forward and report under Ms. DeVos’s rules.
“The proposed regulations reflect the [Education] Department’s commitment to give full effect to Title IX, ensuring that no person experiences sex discrimination in education, and that school procedures for addressing complaints of sex discrimination, including sexual violence and other forms of sex-based harassment, are clear, effective, and fair to all involved,” said Catherine Lhamon, JD, assistant secretary for the Education Department’s Office Of Civil Rights.
More specific rules about transgender students’ participation in school sports are still to come.
A version of this article first appeared on WebMD.com.
CDC releases new details on mysterious hepatitis in children
A new analysis from the Centers for Disease Control and Prevention provides further details on mysterious cases of pediatric hepatitis identified across the United States. While 45% of patients have tested positive for adenovirus infection, it is likely that these children “represent a heterogenous group of hepatitis etiologies,” the CDC authors wrote.
Of the 296 children diagnosed between Oct. 1, 2021, and June 15, 2022, in the United States, 18 have required liver transplants and 11 have died.
On April 21, 2022, the CDC issued an alert to providers to report pediatric hepatitis cases of unknown etiology in children under 10 after similar cases had been identified in Europe and the United States. While the United Kingdom has found an uptick in cases over the past year, researchers from the CDC published data on June 14 that suggested pediatric hepatitis cases had not increased from 2017 to 2021.
This newest analysis, published Morbidity and Mortality Weekly Report, provides additional demographic data on affected patients and explores possible causes, including previous infection with COVID-19. Investigators had earlier ruled out COVID-19 vaccination as a potential factor in these cases, as most children were unvaccinated or not yet eligible to receive the vaccine. According to the analysis, only five patients had received at least one dose of a COVID-19 vaccine.
The 296 cases included in the analysis occurred in 42 U.S. states and territories, and the median age for patients was 2 years and 2 months. Nearly 60% of patients were male (58.1%) and 40.9% were female. The largest percentage of cases occurred in Hispanic or Latino children (37.8%), followed by non-Hispanic White (32.4%) children. Black patients made up 9.8% of all cases, and 3.7% of affected children were of Asian descent. Vomiting, fatigue, and jaundice were all common symptoms, and about 90% (89.9%) of children required hospitalization..
Of 224 children tested for adenovirus, 44.6% were positive. The analysis also included information on 123 of these hepatitis patients tested for other various pathogens. Nearly 80% (98/123) received a COVID-19 test and just 10.2% were positive. About 26% of patients had previously had COVID-19, and hepatitis onset occurred, on average, 133 days after the reported SARS-CoV-2 infection.
Other viruses detected included rhinovirus/enterovirus (24.5%), rotavirus (14.0%), and acute Epstein-Barr virus (11.4%)
Simultaneous infection with SARS-CoV-2 and adenovirus occurred in three patients.
There was no evidence of viral inclusions in the 36 patients who had pathological evaluation liver biopsies, explants, or autopsied tissue.
The findings suggest that there may be many different causes behind these severe hepatitis cases, and it is estimated that about one-third of hepatitis cases in children do not have a known cause. However, the identification of adenovirus infection in many cases “raises the question whether a new pattern of disease is emerging in this population or if adenovirus might be an underrecognized cause or cofactor in previously indeterminate cases of pediatric hepatitis,” the authors wrote. As the investigation continues, “further clinical data are needed to understand the cause of these cases and to assess the potential association with adenovirus.”
A version of this article first appeared on Medscape.com.
A new analysis from the Centers for Disease Control and Prevention provides further details on mysterious cases of pediatric hepatitis identified across the United States. While 45% of patients have tested positive for adenovirus infection, it is likely that these children “represent a heterogenous group of hepatitis etiologies,” the CDC authors wrote.
Of the 296 children diagnosed between Oct. 1, 2021, and June 15, 2022, in the United States, 18 have required liver transplants and 11 have died.
On April 21, 2022, the CDC issued an alert to providers to report pediatric hepatitis cases of unknown etiology in children under 10 after similar cases had been identified in Europe and the United States. While the United Kingdom has found an uptick in cases over the past year, researchers from the CDC published data on June 14 that suggested pediatric hepatitis cases had not increased from 2017 to 2021.
This newest analysis, published Morbidity and Mortality Weekly Report, provides additional demographic data on affected patients and explores possible causes, including previous infection with COVID-19. Investigators had earlier ruled out COVID-19 vaccination as a potential factor in these cases, as most children were unvaccinated or not yet eligible to receive the vaccine. According to the analysis, only five patients had received at least one dose of a COVID-19 vaccine.
The 296 cases included in the analysis occurred in 42 U.S. states and territories, and the median age for patients was 2 years and 2 months. Nearly 60% of patients were male (58.1%) and 40.9% were female. The largest percentage of cases occurred in Hispanic or Latino children (37.8%), followed by non-Hispanic White (32.4%) children. Black patients made up 9.8% of all cases, and 3.7% of affected children were of Asian descent. Vomiting, fatigue, and jaundice were all common symptoms, and about 90% (89.9%) of children required hospitalization..
Of 224 children tested for adenovirus, 44.6% were positive. The analysis also included information on 123 of these hepatitis patients tested for other various pathogens. Nearly 80% (98/123) received a COVID-19 test and just 10.2% were positive. About 26% of patients had previously had COVID-19, and hepatitis onset occurred, on average, 133 days after the reported SARS-CoV-2 infection.
Other viruses detected included rhinovirus/enterovirus (24.5%), rotavirus (14.0%), and acute Epstein-Barr virus (11.4%)
Simultaneous infection with SARS-CoV-2 and adenovirus occurred in three patients.
There was no evidence of viral inclusions in the 36 patients who had pathological evaluation liver biopsies, explants, or autopsied tissue.
The findings suggest that there may be many different causes behind these severe hepatitis cases, and it is estimated that about one-third of hepatitis cases in children do not have a known cause. However, the identification of adenovirus infection in many cases “raises the question whether a new pattern of disease is emerging in this population or if adenovirus might be an underrecognized cause or cofactor in previously indeterminate cases of pediatric hepatitis,” the authors wrote. As the investigation continues, “further clinical data are needed to understand the cause of these cases and to assess the potential association with adenovirus.”
A version of this article first appeared on Medscape.com.
A new analysis from the Centers for Disease Control and Prevention provides further details on mysterious cases of pediatric hepatitis identified across the United States. While 45% of patients have tested positive for adenovirus infection, it is likely that these children “represent a heterogenous group of hepatitis etiologies,” the CDC authors wrote.
Of the 296 children diagnosed between Oct. 1, 2021, and June 15, 2022, in the United States, 18 have required liver transplants and 11 have died.
On April 21, 2022, the CDC issued an alert to providers to report pediatric hepatitis cases of unknown etiology in children under 10 after similar cases had been identified in Europe and the United States. While the United Kingdom has found an uptick in cases over the past year, researchers from the CDC published data on June 14 that suggested pediatric hepatitis cases had not increased from 2017 to 2021.
This newest analysis, published Morbidity and Mortality Weekly Report, provides additional demographic data on affected patients and explores possible causes, including previous infection with COVID-19. Investigators had earlier ruled out COVID-19 vaccination as a potential factor in these cases, as most children were unvaccinated or not yet eligible to receive the vaccine. According to the analysis, only five patients had received at least one dose of a COVID-19 vaccine.
The 296 cases included in the analysis occurred in 42 U.S. states and territories, and the median age for patients was 2 years and 2 months. Nearly 60% of patients were male (58.1%) and 40.9% were female. The largest percentage of cases occurred in Hispanic or Latino children (37.8%), followed by non-Hispanic White (32.4%) children. Black patients made up 9.8% of all cases, and 3.7% of affected children were of Asian descent. Vomiting, fatigue, and jaundice were all common symptoms, and about 90% (89.9%) of children required hospitalization..
Of 224 children tested for adenovirus, 44.6% were positive. The analysis also included information on 123 of these hepatitis patients tested for other various pathogens. Nearly 80% (98/123) received a COVID-19 test and just 10.2% were positive. About 26% of patients had previously had COVID-19, and hepatitis onset occurred, on average, 133 days after the reported SARS-CoV-2 infection.
Other viruses detected included rhinovirus/enterovirus (24.5%), rotavirus (14.0%), and acute Epstein-Barr virus (11.4%)
Simultaneous infection with SARS-CoV-2 and adenovirus occurred in three patients.
There was no evidence of viral inclusions in the 36 patients who had pathological evaluation liver biopsies, explants, or autopsied tissue.
The findings suggest that there may be many different causes behind these severe hepatitis cases, and it is estimated that about one-third of hepatitis cases in children do not have a known cause. However, the identification of adenovirus infection in many cases “raises the question whether a new pattern of disease is emerging in this population or if adenovirus might be an underrecognized cause or cofactor in previously indeterminate cases of pediatric hepatitis,” the authors wrote. As the investigation continues, “further clinical data are needed to understand the cause of these cases and to assess the potential association with adenovirus.”
A version of this article first appeared on Medscape.com.
FROM THE MMWR