User login
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA Volara ventilator warning upgraded to full recall
The Food and Drug Administration has changed the warning about the Volara system to a Class I recall, the most severe level of recall, which is reserved for products that may cause injury or death. At the time of the warning, one injury had been associated with the product; as of June 23, there have been two deaths and one complaint, according to the FDA’s release.
Normally, the Volara system is used for breathing treatments that are administered at home. The medical device company that manufactures it, Baxter International, says the product is designed to help expand the airways and clear mucus for patients who use it. But because of recent product malfunctions, patients are at risk of choking on mucus, developing an infection in their lungs that cuts off their ability to take in oxygen, or in the worst cases, developing brain injury and death.
The risk is especially high because Volara is designed to be used in outpatient settings, not in the hospital under the supervision of a health care professional. It’s supposed to require less supervision than other ventilators. But if there is a problem with the device, or if it’s not connected properly, or if no one is available to assist, people are more likely to be harmed.
People who use the Volara ventilator system at home or people who assist in the use of it should be on alert for these problems. But the FDA advises that patients continue using the therapy if the device has been recommended by a doctor. The device should be used with extra precaution, and patients should be monitored for signs of distress, the release says. Problems while using the device should be reported to the FDA’s Medwatch database.
In addition to these reports, Baxter and its subsidiary company Hillrom say they will update the instructions for the device and will dispatch trainers to make home visits for users. The contact information for the company, as well as additional resources, are listed at the bottom of the release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has changed the warning about the Volara system to a Class I recall, the most severe level of recall, which is reserved for products that may cause injury or death. At the time of the warning, one injury had been associated with the product; as of June 23, there have been two deaths and one complaint, according to the FDA’s release.
Normally, the Volara system is used for breathing treatments that are administered at home. The medical device company that manufactures it, Baxter International, says the product is designed to help expand the airways and clear mucus for patients who use it. But because of recent product malfunctions, patients are at risk of choking on mucus, developing an infection in their lungs that cuts off their ability to take in oxygen, or in the worst cases, developing brain injury and death.
The risk is especially high because Volara is designed to be used in outpatient settings, not in the hospital under the supervision of a health care professional. It’s supposed to require less supervision than other ventilators. But if there is a problem with the device, or if it’s not connected properly, or if no one is available to assist, people are more likely to be harmed.
People who use the Volara ventilator system at home or people who assist in the use of it should be on alert for these problems. But the FDA advises that patients continue using the therapy if the device has been recommended by a doctor. The device should be used with extra precaution, and patients should be monitored for signs of distress, the release says. Problems while using the device should be reported to the FDA’s Medwatch database.
In addition to these reports, Baxter and its subsidiary company Hillrom say they will update the instructions for the device and will dispatch trainers to make home visits for users. The contact information for the company, as well as additional resources, are listed at the bottom of the release.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has changed the warning about the Volara system to a Class I recall, the most severe level of recall, which is reserved for products that may cause injury or death. At the time of the warning, one injury had been associated with the product; as of June 23, there have been two deaths and one complaint, according to the FDA’s release.
Normally, the Volara system is used for breathing treatments that are administered at home. The medical device company that manufactures it, Baxter International, says the product is designed to help expand the airways and clear mucus for patients who use it. But because of recent product malfunctions, patients are at risk of choking on mucus, developing an infection in their lungs that cuts off their ability to take in oxygen, or in the worst cases, developing brain injury and death.
The risk is especially high because Volara is designed to be used in outpatient settings, not in the hospital under the supervision of a health care professional. It’s supposed to require less supervision than other ventilators. But if there is a problem with the device, or if it’s not connected properly, or if no one is available to assist, people are more likely to be harmed.
People who use the Volara ventilator system at home or people who assist in the use of it should be on alert for these problems. But the FDA advises that patients continue using the therapy if the device has been recommended by a doctor. The device should be used with extra precaution, and patients should be monitored for signs of distress, the release says. Problems while using the device should be reported to the FDA’s Medwatch database.
In addition to these reports, Baxter and its subsidiary company Hillrom say they will update the instructions for the device and will dispatch trainers to make home visits for users. The contact information for the company, as well as additional resources, are listed at the bottom of the release.
A version of this article first appeared on Medscape.com.
New data, film highlight islet cell transplantation progress
New data and a new documentary called “The Human Trial” together illuminate the hard work, sacrifice, and slow, iterative progress in the long search for a biological cure for type 1 diabetes.
Opening in select theaters on June 24, the film was written by Los Angeles filmmaker Lisa Hepner, who has type 1 diabetes, and codirected by Ms. Hepner and her husband Guy Mossman, who also filmed it. The couple co-own a film production company.
“The Human Trial” follows the personal journeys of two of the first participants in ViaCyte’s early phase 2 trial of stem cell–derived islet cell transplants, as well as those of the investigators and Ms. Hepner herself, who narrates and appears in the film, interweaving her own experience with type 1 diabetes while acting as a “bridge” between the trial’s participants and scientists. The film spans 7 years of the trial.
The timing of the film’s opening happens to follow presentations at two major medical meetings in early June of more recent islet cell transplantation data from ViaCyte and two other companies, Sernova and Vertex. Each is taking a different practical approach, with the most effective and safe technique yet to be determined.
But all are pursuing the same goal: A biological “cure” for type 1 diabetes with the aim of restoring fully functioning islet cells that can produce insulin and keep blood sugar levels in target range. Ultimately, the hope is to eliminate the need for both exogenous insulin and immunosuppression for all people with type 1 diabetes.
“Cell therapy is an attempt to drastically and substantially change the paradigm of how we actually treat type 1 diabetes,” Manasi S. Jaiman, MD, pediatric endocrinologist and chief medical officer at ViaCyte, said during a presentation at the annual meeting of the Endocrine Society.
Transplantation of cadaver-derived pancreatic islet cells to treat type 1 diabetes dates back more than 20 years to the landmark Edmonton Protocol, with many refinements since. About 1,500 recipients have received them, and roughly a quarter has maintained insulin independence after 10 years, Dr. Jaiman said.
More recently, islets derived from stem cells – either embryonic or autologous – have been used to address the supply and quality problems that arise from cadaveric (dead) donors.
Still, though, the need for lifelong immune suppression means the only current recipients are people with type 1 diabetes for whom the risk of diabetes outweighs that of immune suppression, such as those with hypoglycemic unawareness or extreme glucose swings.
Many research efforts are underway to counter the need for immune suppression by a variety of techniques including cell encapsulation or gene modification.
While the data thus far are encouraging, most of the reports align with what Ms. Hepner says in the film: “We all want stories with a beginning, middle, and end where all the loose pieces fit together. But clinical research is messy and hard. It doesn’t fit into a tidy headline, no matter how much you want it to.”
Companies use different approaches for transplanting islets
At ENDO 2022, Dr. Jaiman presented results for three patients who received pancreatic precursor (PEC-01) cells derived from ViaCyte’s proprietary pluripotent stem cell line. The cells are housed in an open delivery device about the size of a standard bandage to allow direct vascularization and are implanted in a patient’s forearm. An earlier version of the device was used in the two patients in “The Human Trial.”
All three patients experienced improved blood glucose levels with lower daily insulin doses and a rise from undetectable C-peptide to levels above 0.3 ng/mL. Of the three, the best results were seen in a 52-year-old woman with type 1 diabetes for 36 years complicated by hypoglycemic unawareness. At 1-year post transplant, her hemoglobin A1c dropped from 7.4% to 6.9%, and time in range [of ideal blood glucose] from 55% to 94%, plus she had a reduction in daily exogenous insulin use of 70%. However, at 18 months her time-in-range had dropped to about 75%.
“We are watching very closely to see what this means,” Dr. Jaiman said.
Further optimization of the approach is planned. “We’re still waiting on the bulk of the data and analyzing it ... We do realize this is a journey but we’re very excited by where we are,” she enthused.
In February 2022, ViaCyte announced it had teamed up with CRISPR Therapeutics to develop an allogeneic, gene-edited stem cell-derived product designed to produce insulin while at the same time evading the immune system.
Preliminary data from another company, Sernova, using a pouch device were presented at the 2022 annual scientific sessions of the American Diabetes Association by Piotr J. Bachul, MD, of the Transplantation Institute at the University of Chicago.
The Sernova Cell Pouch System containing cadaver islets was successfully transplanted into the abdominal wall of six of seven patients. After waiting a month to allow for vascularization, the cells are then placed into the pouch (as opposed to ViaCyte’s method where they are implanted together). The first three patients achieved islet cell graft function – with positive C-peptide – for up to 1 year, although all also required supplemental transplants into the portal vein to achieve insulin independence.
In May 2022, Sernova announced a partnership with Evotec to develop a product that will combine induced pluripotent stem cell (iPSC)-based beta cells for use with the Cell Pouch System.
Clinical testing is scheduled to begin in 2024, a Sernova representative told this news organization.
And as reported earlier in June, findings from Vertex Pharmaceuticals showed success in two patients who received that company›s investigational allogeneic stem-cell derived islets (VX-880), with the first person completely insulin independent 9 months post transplant.
In contrast to the other two companies, Vertex’s approach is to transplant the cells directly to the hepatic portal vein rather than into a subcutaneous pouch.
“The only space that has ever worked efficiently for islets is the liver because they immediately get blood. ... The subcutaneous space is an interesting place, but the problem is it’s not very well vascularized,” James F. Markmann, MD, PhD, chief of the division of transplant surgery at Massachusetts General Hospital, Boston, who worked on the Vertex trials, told this news organization.
However, the Sernova representative countered: “With the Cell Pouch transplant, not only can surgeons avoid the risks associated with [hepatic] portal vein infusion – including immediate blood-mediated inflammatory reaction, which is known to kill a large proportion of infused islets – but also liver pathologies.”
Furthermore, the cells remaining in the pouch “may be entirely removed from the patient in the event of a subsequently detected cell quality issue,” which isn’t possible with cells delivered into the portal vein.
“I think it will be interesting how it plays out,” Dr. Markmann said, referring to the field as a whole.
‘The Human Trial’ spotlights the real people behind the data
“The Human Trial” ties together the lives of two young adult study participants: a mother named Maren Badger, who qualified for the study because she regularly experienced severe low blood sugar accompanied by seizures, and Greg Romero, a father who has sight-threatening diabetic retinopathy and other complications, as well as financial hardship.
The film chronicles their experiences over 7 years after receiving the transplant. It’s not easy for either of them to undergo all the implantation and explantation procedures as well as cope with the uncertainty as to whether the transplanted cells are working.
At the same time, the researchers’ emotional and sometimes frustrating journey is shown, as are scenes following company executives to Saudi Arabia and Japan in their pursuit of trial funding.
Ms. Hepner herself is featured pursuing the film’s storyline by frequently questioning company executives, in person and virtually, as well as telling her own story.
A visit to the Banting House Historic Site in London, Ontario, with her young son gives Ms. Hepner the opportunity to explain that after Canadian surgeon Frederick Banting discovered insulin, he sold the patent to the University of Toronto for one dollar.
“One hundred years ago, insulin wasn’t a business. It was a medical breakthrough that saved millions of lives. When Banting accepted his Nobel [Prize], he famously said: ‘Insulin doesn’t belong to me, it belongs to the world.’ ... Now, there’s a $245 billion industry designed to manage our disease,” Ms. Hepner says in the film.
But, she adds: “There’s a catch-22: Biotech needs big pharma’s profits to fund clinical trials. Without that support the researchers wouldn’t have gotten this far. Like most relationships, it’s complicated.”
Nonetheless, the film ultimately uplifts. As one company executive says: “Data show the product is producing insulin in patients for the first time. ... This is a big deal. We know now that the cells work.
“We didn’t know that 5 years ago. All the pieces are there, it’s just a matter of completing the puzzle.”
The ViaCyte work presented by Dr. Jaiman received funding from the European Commission Horizon 2020, the California Institute for Regenerative Medicine, and the JDRF. Jaiman is an employee of ViaCyte. The Sernova work was funded by Sernova and JDRF. Dr. Markmann has reported serving on advisory boards for iTolerance, eGenesis, and Qihan Biotech, and being a consultant for Vertex Pharmaceuticals. Ms. Hepner and Mr. Mossman run LA-based Vox Pop Films, a production company specializing in nonfiction content and commercials. “The Human Trial” was made in collaboration with the nonprofit Beyond Type 1.
A version of this article first appeared on Medscape.com.
New data and a new documentary called “The Human Trial” together illuminate the hard work, sacrifice, and slow, iterative progress in the long search for a biological cure for type 1 diabetes.
Opening in select theaters on June 24, the film was written by Los Angeles filmmaker Lisa Hepner, who has type 1 diabetes, and codirected by Ms. Hepner and her husband Guy Mossman, who also filmed it. The couple co-own a film production company.
“The Human Trial” follows the personal journeys of two of the first participants in ViaCyte’s early phase 2 trial of stem cell–derived islet cell transplants, as well as those of the investigators and Ms. Hepner herself, who narrates and appears in the film, interweaving her own experience with type 1 diabetes while acting as a “bridge” between the trial’s participants and scientists. The film spans 7 years of the trial.
The timing of the film’s opening happens to follow presentations at two major medical meetings in early June of more recent islet cell transplantation data from ViaCyte and two other companies, Sernova and Vertex. Each is taking a different practical approach, with the most effective and safe technique yet to be determined.
But all are pursuing the same goal: A biological “cure” for type 1 diabetes with the aim of restoring fully functioning islet cells that can produce insulin and keep blood sugar levels in target range. Ultimately, the hope is to eliminate the need for both exogenous insulin and immunosuppression for all people with type 1 diabetes.
“Cell therapy is an attempt to drastically and substantially change the paradigm of how we actually treat type 1 diabetes,” Manasi S. Jaiman, MD, pediatric endocrinologist and chief medical officer at ViaCyte, said during a presentation at the annual meeting of the Endocrine Society.
Transplantation of cadaver-derived pancreatic islet cells to treat type 1 diabetes dates back more than 20 years to the landmark Edmonton Protocol, with many refinements since. About 1,500 recipients have received them, and roughly a quarter has maintained insulin independence after 10 years, Dr. Jaiman said.
More recently, islets derived from stem cells – either embryonic or autologous – have been used to address the supply and quality problems that arise from cadaveric (dead) donors.
Still, though, the need for lifelong immune suppression means the only current recipients are people with type 1 diabetes for whom the risk of diabetes outweighs that of immune suppression, such as those with hypoglycemic unawareness or extreme glucose swings.
Many research efforts are underway to counter the need for immune suppression by a variety of techniques including cell encapsulation or gene modification.
While the data thus far are encouraging, most of the reports align with what Ms. Hepner says in the film: “We all want stories with a beginning, middle, and end where all the loose pieces fit together. But clinical research is messy and hard. It doesn’t fit into a tidy headline, no matter how much you want it to.”
Companies use different approaches for transplanting islets
At ENDO 2022, Dr. Jaiman presented results for three patients who received pancreatic precursor (PEC-01) cells derived from ViaCyte’s proprietary pluripotent stem cell line. The cells are housed in an open delivery device about the size of a standard bandage to allow direct vascularization and are implanted in a patient’s forearm. An earlier version of the device was used in the two patients in “The Human Trial.”
All three patients experienced improved blood glucose levels with lower daily insulin doses and a rise from undetectable C-peptide to levels above 0.3 ng/mL. Of the three, the best results were seen in a 52-year-old woman with type 1 diabetes for 36 years complicated by hypoglycemic unawareness. At 1-year post transplant, her hemoglobin A1c dropped from 7.4% to 6.9%, and time in range [of ideal blood glucose] from 55% to 94%, plus she had a reduction in daily exogenous insulin use of 70%. However, at 18 months her time-in-range had dropped to about 75%.
“We are watching very closely to see what this means,” Dr. Jaiman said.
Further optimization of the approach is planned. “We’re still waiting on the bulk of the data and analyzing it ... We do realize this is a journey but we’re very excited by where we are,” she enthused.
In February 2022, ViaCyte announced it had teamed up with CRISPR Therapeutics to develop an allogeneic, gene-edited stem cell-derived product designed to produce insulin while at the same time evading the immune system.
Preliminary data from another company, Sernova, using a pouch device were presented at the 2022 annual scientific sessions of the American Diabetes Association by Piotr J. Bachul, MD, of the Transplantation Institute at the University of Chicago.
The Sernova Cell Pouch System containing cadaver islets was successfully transplanted into the abdominal wall of six of seven patients. After waiting a month to allow for vascularization, the cells are then placed into the pouch (as opposed to ViaCyte’s method where they are implanted together). The first three patients achieved islet cell graft function – with positive C-peptide – for up to 1 year, although all also required supplemental transplants into the portal vein to achieve insulin independence.
In May 2022, Sernova announced a partnership with Evotec to develop a product that will combine induced pluripotent stem cell (iPSC)-based beta cells for use with the Cell Pouch System.
Clinical testing is scheduled to begin in 2024, a Sernova representative told this news organization.
And as reported earlier in June, findings from Vertex Pharmaceuticals showed success in two patients who received that company›s investigational allogeneic stem-cell derived islets (VX-880), with the first person completely insulin independent 9 months post transplant.
In contrast to the other two companies, Vertex’s approach is to transplant the cells directly to the hepatic portal vein rather than into a subcutaneous pouch.
“The only space that has ever worked efficiently for islets is the liver because they immediately get blood. ... The subcutaneous space is an interesting place, but the problem is it’s not very well vascularized,” James F. Markmann, MD, PhD, chief of the division of transplant surgery at Massachusetts General Hospital, Boston, who worked on the Vertex trials, told this news organization.
However, the Sernova representative countered: “With the Cell Pouch transplant, not only can surgeons avoid the risks associated with [hepatic] portal vein infusion – including immediate blood-mediated inflammatory reaction, which is known to kill a large proportion of infused islets – but also liver pathologies.”
Furthermore, the cells remaining in the pouch “may be entirely removed from the patient in the event of a subsequently detected cell quality issue,” which isn’t possible with cells delivered into the portal vein.
“I think it will be interesting how it plays out,” Dr. Markmann said, referring to the field as a whole.
‘The Human Trial’ spotlights the real people behind the data
“The Human Trial” ties together the lives of two young adult study participants: a mother named Maren Badger, who qualified for the study because she regularly experienced severe low blood sugar accompanied by seizures, and Greg Romero, a father who has sight-threatening diabetic retinopathy and other complications, as well as financial hardship.
The film chronicles their experiences over 7 years after receiving the transplant. It’s not easy for either of them to undergo all the implantation and explantation procedures as well as cope with the uncertainty as to whether the transplanted cells are working.
At the same time, the researchers’ emotional and sometimes frustrating journey is shown, as are scenes following company executives to Saudi Arabia and Japan in their pursuit of trial funding.
Ms. Hepner herself is featured pursuing the film’s storyline by frequently questioning company executives, in person and virtually, as well as telling her own story.
A visit to the Banting House Historic Site in London, Ontario, with her young son gives Ms. Hepner the opportunity to explain that after Canadian surgeon Frederick Banting discovered insulin, he sold the patent to the University of Toronto for one dollar.
“One hundred years ago, insulin wasn’t a business. It was a medical breakthrough that saved millions of lives. When Banting accepted his Nobel [Prize], he famously said: ‘Insulin doesn’t belong to me, it belongs to the world.’ ... Now, there’s a $245 billion industry designed to manage our disease,” Ms. Hepner says in the film.
But, she adds: “There’s a catch-22: Biotech needs big pharma’s profits to fund clinical trials. Without that support the researchers wouldn’t have gotten this far. Like most relationships, it’s complicated.”
Nonetheless, the film ultimately uplifts. As one company executive says: “Data show the product is producing insulin in patients for the first time. ... This is a big deal. We know now that the cells work.
“We didn’t know that 5 years ago. All the pieces are there, it’s just a matter of completing the puzzle.”
The ViaCyte work presented by Dr. Jaiman received funding from the European Commission Horizon 2020, the California Institute for Regenerative Medicine, and the JDRF. Jaiman is an employee of ViaCyte. The Sernova work was funded by Sernova and JDRF. Dr. Markmann has reported serving on advisory boards for iTolerance, eGenesis, and Qihan Biotech, and being a consultant for Vertex Pharmaceuticals. Ms. Hepner and Mr. Mossman run LA-based Vox Pop Films, a production company specializing in nonfiction content and commercials. “The Human Trial” was made in collaboration with the nonprofit Beyond Type 1.
A version of this article first appeared on Medscape.com.
New data and a new documentary called “The Human Trial” together illuminate the hard work, sacrifice, and slow, iterative progress in the long search for a biological cure for type 1 diabetes.
Opening in select theaters on June 24, the film was written by Los Angeles filmmaker Lisa Hepner, who has type 1 diabetes, and codirected by Ms. Hepner and her husband Guy Mossman, who also filmed it. The couple co-own a film production company.
“The Human Trial” follows the personal journeys of two of the first participants in ViaCyte’s early phase 2 trial of stem cell–derived islet cell transplants, as well as those of the investigators and Ms. Hepner herself, who narrates and appears in the film, interweaving her own experience with type 1 diabetes while acting as a “bridge” between the trial’s participants and scientists. The film spans 7 years of the trial.
The timing of the film’s opening happens to follow presentations at two major medical meetings in early June of more recent islet cell transplantation data from ViaCyte and two other companies, Sernova and Vertex. Each is taking a different practical approach, with the most effective and safe technique yet to be determined.
But all are pursuing the same goal: A biological “cure” for type 1 diabetes with the aim of restoring fully functioning islet cells that can produce insulin and keep blood sugar levels in target range. Ultimately, the hope is to eliminate the need for both exogenous insulin and immunosuppression for all people with type 1 diabetes.
“Cell therapy is an attempt to drastically and substantially change the paradigm of how we actually treat type 1 diabetes,” Manasi S. Jaiman, MD, pediatric endocrinologist and chief medical officer at ViaCyte, said during a presentation at the annual meeting of the Endocrine Society.
Transplantation of cadaver-derived pancreatic islet cells to treat type 1 diabetes dates back more than 20 years to the landmark Edmonton Protocol, with many refinements since. About 1,500 recipients have received them, and roughly a quarter has maintained insulin independence after 10 years, Dr. Jaiman said.
More recently, islets derived from stem cells – either embryonic or autologous – have been used to address the supply and quality problems that arise from cadaveric (dead) donors.
Still, though, the need for lifelong immune suppression means the only current recipients are people with type 1 diabetes for whom the risk of diabetes outweighs that of immune suppression, such as those with hypoglycemic unawareness or extreme glucose swings.
Many research efforts are underway to counter the need for immune suppression by a variety of techniques including cell encapsulation or gene modification.
While the data thus far are encouraging, most of the reports align with what Ms. Hepner says in the film: “We all want stories with a beginning, middle, and end where all the loose pieces fit together. But clinical research is messy and hard. It doesn’t fit into a tidy headline, no matter how much you want it to.”
Companies use different approaches for transplanting islets
At ENDO 2022, Dr. Jaiman presented results for three patients who received pancreatic precursor (PEC-01) cells derived from ViaCyte’s proprietary pluripotent stem cell line. The cells are housed in an open delivery device about the size of a standard bandage to allow direct vascularization and are implanted in a patient’s forearm. An earlier version of the device was used in the two patients in “The Human Trial.”
All three patients experienced improved blood glucose levels with lower daily insulin doses and a rise from undetectable C-peptide to levels above 0.3 ng/mL. Of the three, the best results were seen in a 52-year-old woman with type 1 diabetes for 36 years complicated by hypoglycemic unawareness. At 1-year post transplant, her hemoglobin A1c dropped from 7.4% to 6.9%, and time in range [of ideal blood glucose] from 55% to 94%, plus she had a reduction in daily exogenous insulin use of 70%. However, at 18 months her time-in-range had dropped to about 75%.
“We are watching very closely to see what this means,” Dr. Jaiman said.
Further optimization of the approach is planned. “We’re still waiting on the bulk of the data and analyzing it ... We do realize this is a journey but we’re very excited by where we are,” she enthused.
In February 2022, ViaCyte announced it had teamed up with CRISPR Therapeutics to develop an allogeneic, gene-edited stem cell-derived product designed to produce insulin while at the same time evading the immune system.
Preliminary data from another company, Sernova, using a pouch device were presented at the 2022 annual scientific sessions of the American Diabetes Association by Piotr J. Bachul, MD, of the Transplantation Institute at the University of Chicago.
The Sernova Cell Pouch System containing cadaver islets was successfully transplanted into the abdominal wall of six of seven patients. After waiting a month to allow for vascularization, the cells are then placed into the pouch (as opposed to ViaCyte’s method where they are implanted together). The first three patients achieved islet cell graft function – with positive C-peptide – for up to 1 year, although all also required supplemental transplants into the portal vein to achieve insulin independence.
In May 2022, Sernova announced a partnership with Evotec to develop a product that will combine induced pluripotent stem cell (iPSC)-based beta cells for use with the Cell Pouch System.
Clinical testing is scheduled to begin in 2024, a Sernova representative told this news organization.
And as reported earlier in June, findings from Vertex Pharmaceuticals showed success in two patients who received that company›s investigational allogeneic stem-cell derived islets (VX-880), with the first person completely insulin independent 9 months post transplant.
In contrast to the other two companies, Vertex’s approach is to transplant the cells directly to the hepatic portal vein rather than into a subcutaneous pouch.
“The only space that has ever worked efficiently for islets is the liver because they immediately get blood. ... The subcutaneous space is an interesting place, but the problem is it’s not very well vascularized,” James F. Markmann, MD, PhD, chief of the division of transplant surgery at Massachusetts General Hospital, Boston, who worked on the Vertex trials, told this news organization.
However, the Sernova representative countered: “With the Cell Pouch transplant, not only can surgeons avoid the risks associated with [hepatic] portal vein infusion – including immediate blood-mediated inflammatory reaction, which is known to kill a large proportion of infused islets – but also liver pathologies.”
Furthermore, the cells remaining in the pouch “may be entirely removed from the patient in the event of a subsequently detected cell quality issue,” which isn’t possible with cells delivered into the portal vein.
“I think it will be interesting how it plays out,” Dr. Markmann said, referring to the field as a whole.
‘The Human Trial’ spotlights the real people behind the data
“The Human Trial” ties together the lives of two young adult study participants: a mother named Maren Badger, who qualified for the study because she regularly experienced severe low blood sugar accompanied by seizures, and Greg Romero, a father who has sight-threatening diabetic retinopathy and other complications, as well as financial hardship.
The film chronicles their experiences over 7 years after receiving the transplant. It’s not easy for either of them to undergo all the implantation and explantation procedures as well as cope with the uncertainty as to whether the transplanted cells are working.
At the same time, the researchers’ emotional and sometimes frustrating journey is shown, as are scenes following company executives to Saudi Arabia and Japan in their pursuit of trial funding.
Ms. Hepner herself is featured pursuing the film’s storyline by frequently questioning company executives, in person and virtually, as well as telling her own story.
A visit to the Banting House Historic Site in London, Ontario, with her young son gives Ms. Hepner the opportunity to explain that after Canadian surgeon Frederick Banting discovered insulin, he sold the patent to the University of Toronto for one dollar.
“One hundred years ago, insulin wasn’t a business. It was a medical breakthrough that saved millions of lives. When Banting accepted his Nobel [Prize], he famously said: ‘Insulin doesn’t belong to me, it belongs to the world.’ ... Now, there’s a $245 billion industry designed to manage our disease,” Ms. Hepner says in the film.
But, she adds: “There’s a catch-22: Biotech needs big pharma’s profits to fund clinical trials. Without that support the researchers wouldn’t have gotten this far. Like most relationships, it’s complicated.”
Nonetheless, the film ultimately uplifts. As one company executive says: “Data show the product is producing insulin in patients for the first time. ... This is a big deal. We know now that the cells work.
“We didn’t know that 5 years ago. All the pieces are there, it’s just a matter of completing the puzzle.”
The ViaCyte work presented by Dr. Jaiman received funding from the European Commission Horizon 2020, the California Institute for Regenerative Medicine, and the JDRF. Jaiman is an employee of ViaCyte. The Sernova work was funded by Sernova and JDRF. Dr. Markmann has reported serving on advisory boards for iTolerance, eGenesis, and Qihan Biotech, and being a consultant for Vertex Pharmaceuticals. Ms. Hepner and Mr. Mossman run LA-based Vox Pop Films, a production company specializing in nonfiction content and commercials. “The Human Trial” was made in collaboration with the nonprofit Beyond Type 1.
A version of this article first appeared on Medscape.com.
Are pain meds the only option for chronic pain in cirrhosis?
Pain is common in patients with cirrhosis, and its management presents significant challenges to health care providers, such as worries about GI bleeding, renal injury, falls, and hepatic encephalopathy.
To address those issues, researchers at the University of Michigan, Ann Arbor, authored a review, published in Hepatology, that describes the pain syndromes experienced by patients, as well as pharmaceutical and nonpharmaceutical treatment options.
“I think it’s a very pragmatic approach to a very common problem. Health care providers are concerned about prescribing analgesia for people with cirrhosis for a number of different reasons. One of them is acetaminophen can be toxic to the liver, but generally only in pretty large doses. It’s actually a pretty good option in a dose of less than about 2 g/day because it doesn’t have some of the side effects that other painkillers such as the NSAIDs have. It doesn’t irritate the stomach, and it doesn’t affect kidney function,” said Paul Martin, MD, who was asked to comment on the review. He is chief of digestive health and liver diseases at the University of Miami.
He appreciated the discussion of both pharmacologic and nonpharmacologic interventions, including diet and psychological interventions. “And I think it provides a useful overview of the pharmacological agents we can use in patients with cirrhosis, so I think it’s a very useful contribution to the literature,” said Dr. Martin.
An estimated 40%-79% of cirrhosis patients experience chronic pain, and it can be a key factor in worsening functional status and quality of life. The authors noted that, although recent practice guidance had recommended involving palliative care providers, psychiatry, and physical therapy in for patients with decompensated cirrhosis, this is not always feasible. The authors also pointed out that there are different pain phenotypes in cirrhosis, and these require different management strategies.
They described three mechanistic categories of chronic pain: Nociceptive pain involves tissue damage and inflammation; neuropathic pain results from nerve damage; and nociplastic pain describes situations in which there is no evidence of tissue or nerve damage, but clinical or psychophysical signs suggest changes to nociception.
The different pain types are best assessed using different tools: The 2016 Fibromyalgia Survey Criteria is useful for nociplastic pain, the Neuropathic Pain Questionnaire and painDETECT can be useful for neuropathic pain, and a physical examination can pinpoint nociceptive pain.
When managing chronic pain, the initial patient workup should include a complete evaluation of the location, quality, and severity of pain, along with any functional interference or associated symptoms like fatigue, mood disturbance, or sensory sensitivity. One option is to use a body map to assess how widespread the pain is. Multisite pain is often a signal that it could be nociplastic. Any comorbid psychiatric disorders should be identified and treated.
The first treatment option for any pain should be self-directed, nonpharmacologic interventions. This is because most analgesics are only modestly effective in the treatment of chronic pain, leading to improvement in only about one in three cases, the authors noted. Opioids have poor efficacy against chronic pain, particularly nociplastic pain, which may even be worsened by opioid use.
Although there is evidence that patients are motivated to seek out nonpharmacologic pain treatment, they have reported frustration by a dearth of simple, evidence-based therapies. The authors noted that digital self-management tools for pain have been developed, including their own PainGuide, which focuses on exercise and behavioral interventions for chronic pain. Other nonpharmacologic approaches include diet modification and sleep hygiene. Patients should be allowed to choose the approach that interests them most, with the physician emphasizing the importance of self-directed management.
Pharmacologic therapy may be added to these approaches, but they have limited utility and are associated with adverse effects. For nociceptive pain, topical NSAIDs like diclofenac gel can be used, as can acetaminophen (500 mg every 6 hours, maximum dose of 2 g/day). Opioids can be employed for short-term treatment of acute pain (for example: hydromorphone 1 mg every 6 hours as needed, oxycodone 2.5 mg by mouth every 6-8 hours as needed, or fentanyl patch in select patients). Tricyclic antidepressants may be used for multiple symptoms or neuropathic pain, but with caution. Neuropathic pain, as well as associated depression or fatigue, can be treated with low-dose serotonin and norepinephrine reuptake inhibitors, though there is a small risk of hepatotoxicity. Neuropathic pain, sleep difficulties, or anxiety can be treated with gabapentin at low starting doses (for example, 300 mg/day) or pregabalin (for example, 50 mg twice a day). Lidocaine patches are an option for peripheral neuropathic pain or postherpetic neuralgia, and topical capsaicin may be used for peripheral neuropathic pain.
“Since all pain types can co-occur, interventions to address nociplastic pain may be broadly therapeutic,” the authors concluded. “The treatment of nociplastic pain emphasizes nonpharmacologic management, including self-management techniques addressing mood, cognitions, behaviors, sleep, and environment. Future research should continue to explore methods of pain phenotyping, as well as self-management therapies, including implementation tools.”
The authors and Dr. Martin reported no relevant conflicts of interest.
Pain is common in patients with cirrhosis, and its management presents significant challenges to health care providers, such as worries about GI bleeding, renal injury, falls, and hepatic encephalopathy.
To address those issues, researchers at the University of Michigan, Ann Arbor, authored a review, published in Hepatology, that describes the pain syndromes experienced by patients, as well as pharmaceutical and nonpharmaceutical treatment options.
“I think it’s a very pragmatic approach to a very common problem. Health care providers are concerned about prescribing analgesia for people with cirrhosis for a number of different reasons. One of them is acetaminophen can be toxic to the liver, but generally only in pretty large doses. It’s actually a pretty good option in a dose of less than about 2 g/day because it doesn’t have some of the side effects that other painkillers such as the NSAIDs have. It doesn’t irritate the stomach, and it doesn’t affect kidney function,” said Paul Martin, MD, who was asked to comment on the review. He is chief of digestive health and liver diseases at the University of Miami.
He appreciated the discussion of both pharmacologic and nonpharmacologic interventions, including diet and psychological interventions. “And I think it provides a useful overview of the pharmacological agents we can use in patients with cirrhosis, so I think it’s a very useful contribution to the literature,” said Dr. Martin.
An estimated 40%-79% of cirrhosis patients experience chronic pain, and it can be a key factor in worsening functional status and quality of life. The authors noted that, although recent practice guidance had recommended involving palliative care providers, psychiatry, and physical therapy in for patients with decompensated cirrhosis, this is not always feasible. The authors also pointed out that there are different pain phenotypes in cirrhosis, and these require different management strategies.
They described three mechanistic categories of chronic pain: Nociceptive pain involves tissue damage and inflammation; neuropathic pain results from nerve damage; and nociplastic pain describes situations in which there is no evidence of tissue or nerve damage, but clinical or psychophysical signs suggest changes to nociception.
The different pain types are best assessed using different tools: The 2016 Fibromyalgia Survey Criteria is useful for nociplastic pain, the Neuropathic Pain Questionnaire and painDETECT can be useful for neuropathic pain, and a physical examination can pinpoint nociceptive pain.
When managing chronic pain, the initial patient workup should include a complete evaluation of the location, quality, and severity of pain, along with any functional interference or associated symptoms like fatigue, mood disturbance, or sensory sensitivity. One option is to use a body map to assess how widespread the pain is. Multisite pain is often a signal that it could be nociplastic. Any comorbid psychiatric disorders should be identified and treated.
The first treatment option for any pain should be self-directed, nonpharmacologic interventions. This is because most analgesics are only modestly effective in the treatment of chronic pain, leading to improvement in only about one in three cases, the authors noted. Opioids have poor efficacy against chronic pain, particularly nociplastic pain, which may even be worsened by opioid use.
Although there is evidence that patients are motivated to seek out nonpharmacologic pain treatment, they have reported frustration by a dearth of simple, evidence-based therapies. The authors noted that digital self-management tools for pain have been developed, including their own PainGuide, which focuses on exercise and behavioral interventions for chronic pain. Other nonpharmacologic approaches include diet modification and sleep hygiene. Patients should be allowed to choose the approach that interests them most, with the physician emphasizing the importance of self-directed management.
Pharmacologic therapy may be added to these approaches, but they have limited utility and are associated with adverse effects. For nociceptive pain, topical NSAIDs like diclofenac gel can be used, as can acetaminophen (500 mg every 6 hours, maximum dose of 2 g/day). Opioids can be employed for short-term treatment of acute pain (for example: hydromorphone 1 mg every 6 hours as needed, oxycodone 2.5 mg by mouth every 6-8 hours as needed, or fentanyl patch in select patients). Tricyclic antidepressants may be used for multiple symptoms or neuropathic pain, but with caution. Neuropathic pain, as well as associated depression or fatigue, can be treated with low-dose serotonin and norepinephrine reuptake inhibitors, though there is a small risk of hepatotoxicity. Neuropathic pain, sleep difficulties, or anxiety can be treated with gabapentin at low starting doses (for example, 300 mg/day) or pregabalin (for example, 50 mg twice a day). Lidocaine patches are an option for peripheral neuropathic pain or postherpetic neuralgia, and topical capsaicin may be used for peripheral neuropathic pain.
“Since all pain types can co-occur, interventions to address nociplastic pain may be broadly therapeutic,” the authors concluded. “The treatment of nociplastic pain emphasizes nonpharmacologic management, including self-management techniques addressing mood, cognitions, behaviors, sleep, and environment. Future research should continue to explore methods of pain phenotyping, as well as self-management therapies, including implementation tools.”
The authors and Dr. Martin reported no relevant conflicts of interest.
Pain is common in patients with cirrhosis, and its management presents significant challenges to health care providers, such as worries about GI bleeding, renal injury, falls, and hepatic encephalopathy.
To address those issues, researchers at the University of Michigan, Ann Arbor, authored a review, published in Hepatology, that describes the pain syndromes experienced by patients, as well as pharmaceutical and nonpharmaceutical treatment options.
“I think it’s a very pragmatic approach to a very common problem. Health care providers are concerned about prescribing analgesia for people with cirrhosis for a number of different reasons. One of them is acetaminophen can be toxic to the liver, but generally only in pretty large doses. It’s actually a pretty good option in a dose of less than about 2 g/day because it doesn’t have some of the side effects that other painkillers such as the NSAIDs have. It doesn’t irritate the stomach, and it doesn’t affect kidney function,” said Paul Martin, MD, who was asked to comment on the review. He is chief of digestive health and liver diseases at the University of Miami.
He appreciated the discussion of both pharmacologic and nonpharmacologic interventions, including diet and psychological interventions. “And I think it provides a useful overview of the pharmacological agents we can use in patients with cirrhosis, so I think it’s a very useful contribution to the literature,” said Dr. Martin.
An estimated 40%-79% of cirrhosis patients experience chronic pain, and it can be a key factor in worsening functional status and quality of life. The authors noted that, although recent practice guidance had recommended involving palliative care providers, psychiatry, and physical therapy in for patients with decompensated cirrhosis, this is not always feasible. The authors also pointed out that there are different pain phenotypes in cirrhosis, and these require different management strategies.
They described three mechanistic categories of chronic pain: Nociceptive pain involves tissue damage and inflammation; neuropathic pain results from nerve damage; and nociplastic pain describes situations in which there is no evidence of tissue or nerve damage, but clinical or psychophysical signs suggest changes to nociception.
The different pain types are best assessed using different tools: The 2016 Fibromyalgia Survey Criteria is useful for nociplastic pain, the Neuropathic Pain Questionnaire and painDETECT can be useful for neuropathic pain, and a physical examination can pinpoint nociceptive pain.
When managing chronic pain, the initial patient workup should include a complete evaluation of the location, quality, and severity of pain, along with any functional interference or associated symptoms like fatigue, mood disturbance, or sensory sensitivity. One option is to use a body map to assess how widespread the pain is. Multisite pain is often a signal that it could be nociplastic. Any comorbid psychiatric disorders should be identified and treated.
The first treatment option for any pain should be self-directed, nonpharmacologic interventions. This is because most analgesics are only modestly effective in the treatment of chronic pain, leading to improvement in only about one in three cases, the authors noted. Opioids have poor efficacy against chronic pain, particularly nociplastic pain, which may even be worsened by opioid use.
Although there is evidence that patients are motivated to seek out nonpharmacologic pain treatment, they have reported frustration by a dearth of simple, evidence-based therapies. The authors noted that digital self-management tools for pain have been developed, including their own PainGuide, which focuses on exercise and behavioral interventions for chronic pain. Other nonpharmacologic approaches include diet modification and sleep hygiene. Patients should be allowed to choose the approach that interests them most, with the physician emphasizing the importance of self-directed management.
Pharmacologic therapy may be added to these approaches, but they have limited utility and are associated with adverse effects. For nociceptive pain, topical NSAIDs like diclofenac gel can be used, as can acetaminophen (500 mg every 6 hours, maximum dose of 2 g/day). Opioids can be employed for short-term treatment of acute pain (for example: hydromorphone 1 mg every 6 hours as needed, oxycodone 2.5 mg by mouth every 6-8 hours as needed, or fentanyl patch in select patients). Tricyclic antidepressants may be used for multiple symptoms or neuropathic pain, but with caution. Neuropathic pain, as well as associated depression or fatigue, can be treated with low-dose serotonin and norepinephrine reuptake inhibitors, though there is a small risk of hepatotoxicity. Neuropathic pain, sleep difficulties, or anxiety can be treated with gabapentin at low starting doses (for example, 300 mg/day) or pregabalin (for example, 50 mg twice a day). Lidocaine patches are an option for peripheral neuropathic pain or postherpetic neuralgia, and topical capsaicin may be used for peripheral neuropathic pain.
“Since all pain types can co-occur, interventions to address nociplastic pain may be broadly therapeutic,” the authors concluded. “The treatment of nociplastic pain emphasizes nonpharmacologic management, including self-management techniques addressing mood, cognitions, behaviors, sleep, and environment. Future research should continue to explore methods of pain phenotyping, as well as self-management therapies, including implementation tools.”
The authors and Dr. Martin reported no relevant conflicts of interest.
FROM HEPATOLOGY
Roe v. Wade overturned, ending 50 years of abortion protections
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
According to some estimates, about 25 million women of reproductive age will now live in states that ban or severely restrict abortion. Twenty-six states are “certain or likely” to ban abortion, according to the Guttmacher Institute, which supports abortion rights.
Thirteen states have so-called trigger laws that will ban abortion almost immediately, while nine other states are now likely to try to enforce near-total bans or severe restrictions that have been blocked by courts pending the outcome of the just-issued decision in Dobbs v. Jackson Women’s Health Organization. Four states also have a history or have shown a recent desire to prohibit abortion, according to the Guttmacher Institute.
Doctors and others who provide abortion services, or in some states “aid or abet” an abortion, could be fined thousands of dollars or sent to prison.
The court voted in favor of Mississippi and its 2018 law that outlawed abortion after 15 weeks. Jackson Women’s Health, the state’s sole remaining abortion provider, sued to block the law soon after it passed.
The Supreme Court decision is not a surprise, as the justices indicated they were leaning that way during oral arguments in December. The majority’s thoughts were further revealed when a draft of the opinion was leaked to the news outlet Politico on May 2.
In the final opinion, Justice Samuel Alito, writing for the majority, “It is time to heed the Constitution and return the issue of abortion to the people’s elected representatives.”
The decision strikes down both precedent-setting rulings that established a right to abortion until the point of viability, long considered to be 24 weeks: Roe v. Wade (1973) and Planned Parenthood v. Casey (1992).
Twenty-five medical professional societies – representing OB/GYNs, family medicine doctors, fertility specialists, geneticists, hospitalists, internists, pediatricians, psychiatrists, nurses, nurse practitioners, and midwives – had urged the court to throw out the Mississippi law. And more than 2,500 medical professionals signed on to a petition in June, urging the court to uphold the right to abortion.
The number of abortions has recently increased from what had been a long decline. The Guttmacher Institute estimates there were there were 930,160 abortion procedures in 2020 (compared to 3.6 million births), an 8% increase from 2017. The number does not include self-managed abortions. The organization said the increase was potentially due to expanded Medicaid coverage and reduced access to contraception due to Trump administration policies.
Trigger laws and bans
When trigger laws and new restrictions go into effect, women in the South, Midwest, and Inter-Mountain West will likely have to drive hundreds of miles for an abortion, according to Guttmacher. Women in Louisiana, for instance, would have to drive 660 miles to get to the nearest provider in Illinois.
University of Utah researchers estimated that almost half of women will see a big increase in the distance to abortion care, from a median distance of 39 miles to 113 miles. State bans will disproportionately impact women of color, those living in poverty, and people with less education, they said.
The CDC has reported that Black women are three times more likely to die from a pregnancy-related cause than white women.
Doctors and other abortion providers could face serious penalties. The maximum penalty in Texas is life in prison, and the sentence could be 10 to 15 years in 11 other states, according to an article in the medical journal JAMA by attorneys Rebecca B. Reingold and Lawrence O. Gostin.
“Threats of prosecution undermine clinicians’ ability to provide safe, evidence-based care and to counsel patients honestly, impeding the patient-physician relationship,” they wrote. “Given harsh penalties, physicians may cease treating pregnancy loss, with no clear line between treating miscarriages and abortions.”
In preparing for these attacks on patients and doctors, New York Gov. Kathy Hochul on June 13 signed a bill that immediately protects anyone who has an abortion and medical professionals in the state who provide them from legal retaliation by states that restrict or prohibit abortion.
Even while Roe was still the law, Mississippi had banned most abortions after 20 weeks, and 16 states prohibited abortion after 22 weeks. A Texas ban on abortion after 6 weeks – which also allows private citizens to sue abortion providers – was allowed to stay in place while it was being challenged.
On May 26, Oklahoma Gov. Kevin Stitt signed a bill banning abortion from the moment of conception. Just as in Texas, the Oklahoma law allows what critics have called “bounty hunting” of abortion providers.
Four states have a constitutional amendment declaring that the state constitution does not secure or protect the right to abortion or allow the use of public funds for abortion: Alabama, Louisiana, Tennessee, and West Virginia.
Some states protecting rights
At least 16 states have proactively protected a right to an abortion, according to Guttmacher, while The New York Times reports that Washington, DC, has laws that protect abortion, along with 20 states: Alaska, Colorado, Illinois, Maine, Massachusetts, Minnesota, Nevada, New Hampshire, New Mexico, Rhode Island, California, Connecticut, Delaware, Hawaii, Maryland, New Jersey, New York, Oregon, Vermont, and Washington.
Some of these states are gearing up for a potential influx of patients. Washington Gov. Jay Inslee signed a law that authorizes physician assistants, advanced registered nurse practitioners, and other providers acting within their scope of practice to perform abortions. And the Maryland Legislature overrode a veto by Gov. Larry Hogan of a law that expands who can perform abortions.
Wisconsin Gov. Tony Evers in early June called a special legislative session to repeal the state’s 173-year-old dormant ban on abortion. But the majority Republican legislature vowed to take no action.
B. Jessie Hill, JD, associate dean for academic affairs and a professor at the Case Western Reserve University School of Law, says she expects anti-abortion groups to challenge these protective laws, “by saying that fetuses are persons under the Constitution with a right to life and therefore that the state has to protect them.”
But, she says, “there’s going to be big, big challenges with those lawsuits,” and they will not be “winners off the bat.”
Medication abortions, travel next battle
Some states are also trying to outlaw or severely restrict the use of RU-486, the abortion pill. A Tennessee law that goes into effect in 2023 would ban delivery of pills by mail and require a patient to have two doctor visits – one consultation and one to pick up the pills.
Mississippi has also enacted restrictions including the requirement that women meet with a doctor first – and is being sued by pill maker GenBioPro.
Guttmacher estimates that medication abortion accounted for 39% of all abortions in the U.S. in 2017 and 60% of all abortions that occurred before 10 weeks’ gestation.
Some states have floated the idea of prohibiting anyone from traveling to another state for an abortion.
George Mason University law professor Ilya Somin, JD, has written that such a law would likely violate the Dormant Commerce Clause, “which forbids state regulations that specifically restrict interstate commerce or discriminate against it.”
He also wrote that states lack the authority to regulate activity that takes place beyond their borders and that such bans “are open to challenge because they violate the constitutional right to travel.”
Hill also said a travel ban would be problematic, noting that it might be difficult to prosecute someone for “something you did completely in another state.”
A version of this article first appeared on Medscape.com.
Common endoscopic procedure needs quality improvement
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
One of the most common procedures in gastroenterology – esophagogastroduodenoscopy (EGD) – needs to consistently meet quality measures, but data on interventions to improve them is lacking, according to a recent review.
Researchers, led by Fateh Bazerbachi, MD, with CentraCare, Interventional Endoscopy Program, at St. Cloud (Minn.) Hospital performed a systematic review of the literature to identify which interventions and measures have improved the performance of EGD quality indicators previously identified by the American Society for Gastrointestinal Endoscopy. They also looked for demonstrations of improving compliance with the prioritized indicators. The review appeared in Gastrointestinal Endoscopy.
The authors pointed out that more than 6.1 million EGDs are performed every year in the United States. Although gastroenterologists perform most of them, other providers also perform them, including primary care physicians, surgeons, and sometimes advanced practice providers. Therefore, establishing well-defined quality measures is critical for consistent outcomes.
Daniel C. Buckles, MD, associate professor of gastroenterology, hepatology & motility at the University of Kansas Medical Center in Kansas City, who was not part of the review, said high-quality EGDs are critical for many reasons, including avoiding overuse when results of the procedure are not likely to change a patient’s treatment but add risk to the patient and increase costs to the health care system.
“Lack of training to recognize important GI pathology seen on an EGD and lack of standardized reporting of GI abnormalities using validated classification systems can lead to suboptimal treatment and follow-up for patients,” he noted.
Testing provider adherence to guidelines years after publication helps providers understand what works and can improve outcomes, Dr. Buckles said.
Dr. Buckles said that one of the highlights of the review was that researchers were able to confidently say that use of standardized checklists and frequent auditing had value in improving preprocedural and postprocedural quality indicators.
“The authors also concluded that focused educational interventions might improve endoscopists’ abilities to adhere to standardized Barrett’s esophagus examinations,” he added. “Unfortunately, the authors were not able to find much evidence for interventions that would improve intraprocedural EGD quality indicators.”
The authors pointed to a prospective study that evaluated whether an audit intervention helped in 10,000 consecutive EGDs. They found the audits “improved EGD report quality, such as justification for incompleteness or accurate lesion/segment description, regardless of the endoscopist’s experience documenting the report (specialist vs. trainee).” When audits were used in other studies to evaluate endoscopy overall performance (including EGD and colonoscopy) results showed similar improvement in important endpoints, the authors wrote. Additionally, “use of dictation templates has been demonstrated to improve the completeness of endoscopy report.”
A study led by the European Network for the Investigation of Gastrointestinal Mucosal Alterations found inconsistent compliance with EGD biopsy–sampling guidelines in patients with signs of gastric pathology, even in academic centers. The authors of that study recommended dedicated educational programs to raise awareness of which scenarios warrant gastric sampling during EGD.
The authors of the current study also acknowledge that many sound practices that likely improve quality, for instance time-outs or checklists for administering antibiotics during percutaneous feeding tube placement, are unlikely to be formally studied.
“Nevertheless, such practices should be encouraged and monitored,” they wrote.
“This document synthesizes practices and interventions that may allow for a high-quality upper endoscopy,” they concluded. “Furthermore, the scarcity of strong data to support interventions that can improve important quality indicators in upper-GI endoscopy should be seen as an opportunity.”
Several authors disclosed relationships with commercial interests, such as Boston Scientific, Salix, and Janssen. Dr. Buckles reports no relevant financial relationships.
FROM GASTROINTESTINAL ENDOSCOPY
Plan a gift that offers a better future for GI
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!
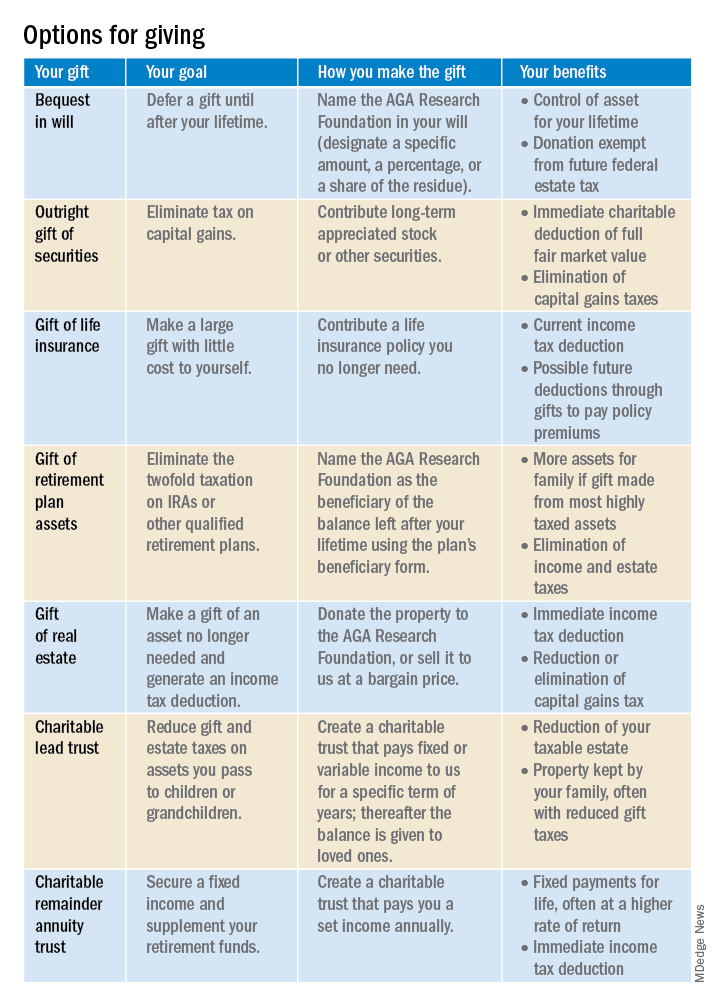
Learn more by visiting http://gastro.planmylegacy.org.
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!

Learn more by visiting http://gastro.planmylegacy.org.
Planned giving provides an opportunity for all who have benefited from digestive disease research to give back to the field in a unique and lasting way.
Your investment in the AGA Research Foundation will enable the foundation to continue our investment in the future of gastroenterological research and innovation. With donations from AGA members, we can provide young researchers with a secure, ongoing stable source of funding that drives advancement in the diagnosis, treatment, and cure of digestive diseases.
If you make a contribution, it will be because you believe in what we do and because you want to help make a difference in the lives of others. But we’d also like to make sure you benefit from making a gift to the AGA Research Foundation.
Your giving options
There are several gift arrangements to choose from. The chart below summarizes the benefits of some of the main types of charitable gifts. Just think of what you want to accomplish with your gift, and there’s probably a way to do it!

Learn more by visiting http://gastro.planmylegacy.org.
Type 2 Diabetes and COVID-19
How much does the risk of new-onset type 2 diabetes (T2D) increase in patients who have had a mild SARS-CoV-2 (COVID-19) infection?
Dr. Jain: We are now finding many associations between COVID-19 and T2D. Recently, there have been studies, especially from the United States and Germany, showing that even after mild COVID-19, the occurrence of new onset T2D is greater than what we thought previously. For instance, we are now seeing that the rate of new-onset T2D in adults who have had mild COVID-19 is about 18 additional adults per 1000 people. These people have about a 46% higher risk of developing T2D compared to those who did not have COVID-19. This is something we must keep in mind moving forward when it comes to screening for diabetes.
How have you navigated through the diagnostic components of T2D and COVID-19?
Dr. Jain: When people were exclusively doing virtual appointments during the COVID-19 lockdowns, there wasn't enough screening for retinopathy, worsening blood pressure, or even basic lab testing, for instance. We could not perform electrocardiography screenings for our patients with diabetes; we had to defer it unless people were symptomatic. This was not the ideal situation, as many people with diabetes may have “silent” coronary artery disease, making screening crucial even in those patients who are not symptomatic. The impact of this is anyone’s guess at this time. Unfortunately, there is no literature that has looked at the impact of deferred screenings during the pandemic.
However, in my own practice, we are now transitioning back to in-person appointments and making sure that all of these screening visits are being conducted in a timely manner so that we can catch the micro- and macrovascular complications of diabetes sooner.
Although data on the long-term impact are not available, what studies have been done regarding the treatment of T2D during the pandemic?
Dr. Jain: There are some observational studies. There was a claims database analysis that looked at patient visits, screening tests, filling of medications, and glycated hemoglobin (A1C) levels in 2020 vs 2019 in the United States. There was no significant difference between the A1C levels and medication fills. I think one reason for this is that we all were able to adapt to the changes required from us, and we were able to incorporate the virtual appointments. That's reassuring. I think we still need some more data to make any definitive assumptions about what the overall care of diabetes has been as we are coming out of the pandemic.
Have you seen any particular characteristics or disparities in those patients who have been impacted by T2D and COVID-19?
Dr. Jain: With the mask mandate, physical distancing requirements, and differing vaccination rates across parts of the world, we have seen disparities in the impact of COVID-19, especially for those that are the most vulnerable. This includes people with multiple comorbidities.
We also know that, even at baseline, patients with T2D are often prone to multiple cardiovascular issues and dialysis requirements. These individuals still have to be extremely cautious. I have seen that those patients who actually need the most attention are still not able to go out and get the care. It is important that we are inclusive in our understanding of the requirements of people at high risk for T2D and other comorbidities. Necessary requirements to reduce infection risk include following physical distancing and masking requirements as appropriate and ensuring timely screening for retinopathy, nephropathy, and coronary artery disease as well as getting foot exams.
We have to give them all the care and access to healthcare that they need. Expert consensus suggests that we ensure optimization of vaccination status, glycemic control, cardiovascular risk, and weight control. We also need to ensure that these high-risk individuals have not slipped through the cracks and have appropriate appointment follow-ups, labs, etc. booked in a timely manner.
What guidelines and standards do you rely upon to ensure that patients are getting the most out of their treatment?
Dr. Jain: COVID-19 aside, we still want to make sure that the management of T2D is not glucose-centric. We now understand that diabetes requires 360-degree care, and that involves not only controlling the blood sugars but also making sure that we are mitigating risk factors for vascular complications. That includes ensuring blood pressure is well controlled and that cholesterol levels are in target range for patients who either have history of heart attacks, strokes, heart failure, kidney disease, or who are at future risk for these events.
We are using medications that can help protect the heart and the kidneys. Considering that T2D and obesity are so closely interlinked, we are using medications that help with overcoming the weight aspect as well. I think that a patient-centric, multidisciplinary approach is the most crucial and the most reliable way of getting that 360-degree comprehensive care for patients with diabetes.
Is there anything else you would like to share with your colleagues or peers?
Dr. Jain: As we are coming out of the pandemic, we still we need to realize that the repercussions of COVID-19 will still stay with us for a long, long time. We do know that COVID-19 infection leads to a tsunami of inflammation in the body. This can have long-lasting effects, including chronic conditions.
It is important that we continue to screen for diabetes given that there is a higher incidence of T2D in those with even mild COVID-19, and especially those at highest risk, such as people with obesity. We need to make sure that we are not missing any pieces of the puzzle there, especially because diabetes is a silent disease. We should not rely on symptoms to determine when we should screen; instead, we must be proactive about screening and management.
- Watson C. Diabetes risk rises after COVID, massive study finds. Nature News. Published March 31, 2022. Accessed June 17, 2022. https://www.nature.com/articles/d41586-022-00912-y
- New-onset type 2 diabetes risk higher with mild COVID-19 vs. other respiratory infections. Healio. Published March 17, 2022. Accessed June 17, 2022. https://www.healio.com/news/endocrinology/20220317/newonset-type-2-diabetes-risk-higher-with-mild-covid19-vs-other-respiratory-infections
- Diabetes is 'facet' of long COVID syndrome. Healio. Published April 1, 2022. Accessed June 17, 2022. https://www.healio.com/news/endocrinology/20220331/diabetes-is-facet-of-long-covid-syndrome
- LeBlanc AG, Jun Gao Y, McRae L, Pelletier C. At-a-glance - twenty years of diabetes surveillance using the Canadian Chronic Disease Surveillance System. Health Promot Chronic Dis Prev Can. 2019;39(11):306-309. doi:10.24095/hpcdp.39.11.03
- Patel SY, McCoy RG, Barnett ML, Shah ND, Mehrotra A. Diabetes care and glycemic control during the COVID-19 pandemic in the United States. JAMA Intern Med. 2021;181(10):1412–1414. doi:10.1001/jamainternmed.2021.3047
- Kiran T, Moonen G, Bhattacharyya OK, et al. Managing type 2 diabetes in primary care during COVID-19. Can Fam Physician. 2020;66(10):745-747.
How much does the risk of new-onset type 2 diabetes (T2D) increase in patients who have had a mild SARS-CoV-2 (COVID-19) infection?
Dr. Jain: We are now finding many associations between COVID-19 and T2D. Recently, there have been studies, especially from the United States and Germany, showing that even after mild COVID-19, the occurrence of new onset T2D is greater than what we thought previously. For instance, we are now seeing that the rate of new-onset T2D in adults who have had mild COVID-19 is about 18 additional adults per 1000 people. These people have about a 46% higher risk of developing T2D compared to those who did not have COVID-19. This is something we must keep in mind moving forward when it comes to screening for diabetes.
How have you navigated through the diagnostic components of T2D and COVID-19?
Dr. Jain: When people were exclusively doing virtual appointments during the COVID-19 lockdowns, there wasn't enough screening for retinopathy, worsening blood pressure, or even basic lab testing, for instance. We could not perform electrocardiography screenings for our patients with diabetes; we had to defer it unless people were symptomatic. This was not the ideal situation, as many people with diabetes may have “silent” coronary artery disease, making screening crucial even in those patients who are not symptomatic. The impact of this is anyone’s guess at this time. Unfortunately, there is no literature that has looked at the impact of deferred screenings during the pandemic.
However, in my own practice, we are now transitioning back to in-person appointments and making sure that all of these screening visits are being conducted in a timely manner so that we can catch the micro- and macrovascular complications of diabetes sooner.
Although data on the long-term impact are not available, what studies have been done regarding the treatment of T2D during the pandemic?
Dr. Jain: There are some observational studies. There was a claims database analysis that looked at patient visits, screening tests, filling of medications, and glycated hemoglobin (A1C) levels in 2020 vs 2019 in the United States. There was no significant difference between the A1C levels and medication fills. I think one reason for this is that we all were able to adapt to the changes required from us, and we were able to incorporate the virtual appointments. That's reassuring. I think we still need some more data to make any definitive assumptions about what the overall care of diabetes has been as we are coming out of the pandemic.
Have you seen any particular characteristics or disparities in those patients who have been impacted by T2D and COVID-19?
Dr. Jain: With the mask mandate, physical distancing requirements, and differing vaccination rates across parts of the world, we have seen disparities in the impact of COVID-19, especially for those that are the most vulnerable. This includes people with multiple comorbidities.
We also know that, even at baseline, patients with T2D are often prone to multiple cardiovascular issues and dialysis requirements. These individuals still have to be extremely cautious. I have seen that those patients who actually need the most attention are still not able to go out and get the care. It is important that we are inclusive in our understanding of the requirements of people at high risk for T2D and other comorbidities. Necessary requirements to reduce infection risk include following physical distancing and masking requirements as appropriate and ensuring timely screening for retinopathy, nephropathy, and coronary artery disease as well as getting foot exams.
We have to give them all the care and access to healthcare that they need. Expert consensus suggests that we ensure optimization of vaccination status, glycemic control, cardiovascular risk, and weight control. We also need to ensure that these high-risk individuals have not slipped through the cracks and have appropriate appointment follow-ups, labs, etc. booked in a timely manner.
What guidelines and standards do you rely upon to ensure that patients are getting the most out of their treatment?
Dr. Jain: COVID-19 aside, we still want to make sure that the management of T2D is not glucose-centric. We now understand that diabetes requires 360-degree care, and that involves not only controlling the blood sugars but also making sure that we are mitigating risk factors for vascular complications. That includes ensuring blood pressure is well controlled and that cholesterol levels are in target range for patients who either have history of heart attacks, strokes, heart failure, kidney disease, or who are at future risk for these events.
We are using medications that can help protect the heart and the kidneys. Considering that T2D and obesity are so closely interlinked, we are using medications that help with overcoming the weight aspect as well. I think that a patient-centric, multidisciplinary approach is the most crucial and the most reliable way of getting that 360-degree comprehensive care for patients with diabetes.
Is there anything else you would like to share with your colleagues or peers?
Dr. Jain: As we are coming out of the pandemic, we still we need to realize that the repercussions of COVID-19 will still stay with us for a long, long time. We do know that COVID-19 infection leads to a tsunami of inflammation in the body. This can have long-lasting effects, including chronic conditions.
It is important that we continue to screen for diabetes given that there is a higher incidence of T2D in those with even mild COVID-19, and especially those at highest risk, such as people with obesity. We need to make sure that we are not missing any pieces of the puzzle there, especially because diabetes is a silent disease. We should not rely on symptoms to determine when we should screen; instead, we must be proactive about screening and management.
How much does the risk of new-onset type 2 diabetes (T2D) increase in patients who have had a mild SARS-CoV-2 (COVID-19) infection?
Dr. Jain: We are now finding many associations between COVID-19 and T2D. Recently, there have been studies, especially from the United States and Germany, showing that even after mild COVID-19, the occurrence of new onset T2D is greater than what we thought previously. For instance, we are now seeing that the rate of new-onset T2D in adults who have had mild COVID-19 is about 18 additional adults per 1000 people. These people have about a 46% higher risk of developing T2D compared to those who did not have COVID-19. This is something we must keep in mind moving forward when it comes to screening for diabetes.
How have you navigated through the diagnostic components of T2D and COVID-19?
Dr. Jain: When people were exclusively doing virtual appointments during the COVID-19 lockdowns, there wasn't enough screening for retinopathy, worsening blood pressure, or even basic lab testing, for instance. We could not perform electrocardiography screenings for our patients with diabetes; we had to defer it unless people were symptomatic. This was not the ideal situation, as many people with diabetes may have “silent” coronary artery disease, making screening crucial even in those patients who are not symptomatic. The impact of this is anyone’s guess at this time. Unfortunately, there is no literature that has looked at the impact of deferred screenings during the pandemic.
However, in my own practice, we are now transitioning back to in-person appointments and making sure that all of these screening visits are being conducted in a timely manner so that we can catch the micro- and macrovascular complications of diabetes sooner.
Although data on the long-term impact are not available, what studies have been done regarding the treatment of T2D during the pandemic?
Dr. Jain: There are some observational studies. There was a claims database analysis that looked at patient visits, screening tests, filling of medications, and glycated hemoglobin (A1C) levels in 2020 vs 2019 in the United States. There was no significant difference between the A1C levels and medication fills. I think one reason for this is that we all were able to adapt to the changes required from us, and we were able to incorporate the virtual appointments. That's reassuring. I think we still need some more data to make any definitive assumptions about what the overall care of diabetes has been as we are coming out of the pandemic.
Have you seen any particular characteristics or disparities in those patients who have been impacted by T2D and COVID-19?
Dr. Jain: With the mask mandate, physical distancing requirements, and differing vaccination rates across parts of the world, we have seen disparities in the impact of COVID-19, especially for those that are the most vulnerable. This includes people with multiple comorbidities.
We also know that, even at baseline, patients with T2D are often prone to multiple cardiovascular issues and dialysis requirements. These individuals still have to be extremely cautious. I have seen that those patients who actually need the most attention are still not able to go out and get the care. It is important that we are inclusive in our understanding of the requirements of people at high risk for T2D and other comorbidities. Necessary requirements to reduce infection risk include following physical distancing and masking requirements as appropriate and ensuring timely screening for retinopathy, nephropathy, and coronary artery disease as well as getting foot exams.
We have to give them all the care and access to healthcare that they need. Expert consensus suggests that we ensure optimization of vaccination status, glycemic control, cardiovascular risk, and weight control. We also need to ensure that these high-risk individuals have not slipped through the cracks and have appropriate appointment follow-ups, labs, etc. booked in a timely manner.
What guidelines and standards do you rely upon to ensure that patients are getting the most out of their treatment?
Dr. Jain: COVID-19 aside, we still want to make sure that the management of T2D is not glucose-centric. We now understand that diabetes requires 360-degree care, and that involves not only controlling the blood sugars but also making sure that we are mitigating risk factors for vascular complications. That includes ensuring blood pressure is well controlled and that cholesterol levels are in target range for patients who either have history of heart attacks, strokes, heart failure, kidney disease, or who are at future risk for these events.
We are using medications that can help protect the heart and the kidneys. Considering that T2D and obesity are so closely interlinked, we are using medications that help with overcoming the weight aspect as well. I think that a patient-centric, multidisciplinary approach is the most crucial and the most reliable way of getting that 360-degree comprehensive care for patients with diabetes.
Is there anything else you would like to share with your colleagues or peers?
Dr. Jain: As we are coming out of the pandemic, we still we need to realize that the repercussions of COVID-19 will still stay with us for a long, long time. We do know that COVID-19 infection leads to a tsunami of inflammation in the body. This can have long-lasting effects, including chronic conditions.
It is important that we continue to screen for diabetes given that there is a higher incidence of T2D in those with even mild COVID-19, and especially those at highest risk, such as people with obesity. We need to make sure that we are not missing any pieces of the puzzle there, especially because diabetes is a silent disease. We should not rely on symptoms to determine when we should screen; instead, we must be proactive about screening and management.
- Watson C. Diabetes risk rises after COVID, massive study finds. Nature News. Published March 31, 2022. Accessed June 17, 2022. https://www.nature.com/articles/d41586-022-00912-y
- New-onset type 2 diabetes risk higher with mild COVID-19 vs. other respiratory infections. Healio. Published March 17, 2022. Accessed June 17, 2022. https://www.healio.com/news/endocrinology/20220317/newonset-type-2-diabetes-risk-higher-with-mild-covid19-vs-other-respiratory-infections
- Diabetes is 'facet' of long COVID syndrome. Healio. Published April 1, 2022. Accessed June 17, 2022. https://www.healio.com/news/endocrinology/20220331/diabetes-is-facet-of-long-covid-syndrome
- LeBlanc AG, Jun Gao Y, McRae L, Pelletier C. At-a-glance - twenty years of diabetes surveillance using the Canadian Chronic Disease Surveillance System. Health Promot Chronic Dis Prev Can. 2019;39(11):306-309. doi:10.24095/hpcdp.39.11.03
- Patel SY, McCoy RG, Barnett ML, Shah ND, Mehrotra A. Diabetes care and glycemic control during the COVID-19 pandemic in the United States. JAMA Intern Med. 2021;181(10):1412–1414. doi:10.1001/jamainternmed.2021.3047
- Kiran T, Moonen G, Bhattacharyya OK, et al. Managing type 2 diabetes in primary care during COVID-19. Can Fam Physician. 2020;66(10):745-747.
- Watson C. Diabetes risk rises after COVID, massive study finds. Nature News. Published March 31, 2022. Accessed June 17, 2022. https://www.nature.com/articles/d41586-022-00912-y
- New-onset type 2 diabetes risk higher with mild COVID-19 vs. other respiratory infections. Healio. Published March 17, 2022. Accessed June 17, 2022. https://www.healio.com/news/endocrinology/20220317/newonset-type-2-diabetes-risk-higher-with-mild-covid19-vs-other-respiratory-infections
- Diabetes is 'facet' of long COVID syndrome. Healio. Published April 1, 2022. Accessed June 17, 2022. https://www.healio.com/news/endocrinology/20220331/diabetes-is-facet-of-long-covid-syndrome
- LeBlanc AG, Jun Gao Y, McRae L, Pelletier C. At-a-glance - twenty years of diabetes surveillance using the Canadian Chronic Disease Surveillance System. Health Promot Chronic Dis Prev Can. 2019;39(11):306-309. doi:10.24095/hpcdp.39.11.03
- Patel SY, McCoy RG, Barnett ML, Shah ND, Mehrotra A. Diabetes care and glycemic control during the COVID-19 pandemic in the United States. JAMA Intern Med. 2021;181(10):1412–1414. doi:10.1001/jamainternmed.2021.3047
- Kiran T, Moonen G, Bhattacharyya OK, et al. Managing type 2 diabetes in primary care during COVID-19. Can Fam Physician. 2020;66(10):745-747.
IUD injury risk rises shortly after women give birth
Women for whom an intrauterine device is inserted from 4 days to 6 weeks after giving birth, as well as those who are breastfeeding, are at higher risk of the contraceptive device puncturing their uterus, new research shows.
The risk of perforation was nearly seven times higher for patients who received an IUD within that window than for those with an IUD who’d never given birth or who were more than a year out from delivery, the researchers found. Health care providers should make patients aware of the heightened risk and should monitor these patients more closely, according to Susan Reed, MD, an ob.gyn. at the University of Washington, Seattle, lead author of the new study.
“I’m a surgeon, and I like to be able to give people good information and good data about risks and benefits for their choices,” Dr. Reed told this news organization. “Uterine perforations related to IUDs are exceedingly rare, and to get good data or known risk factors, you need huge studies. This was the largest study done that really provided accurate information for patients and providers.” The new study, which appears in a recent issue of The Lancet, also found that the risk of uterine perforation was lower if an IUD had been inserted immediately after delivery.
Dr. Reed and colleagues analyzed data from the health records of 326,658 women younger than 50 years for whom an IUD was inserted between 2001 and 2018 at four health care systems. Nearly 30% of these patients received an IUD after giving birth.
The researchers identified a total of 1,008 uterine perforations, for a cumulative incidence at 5 years of 0.6%. The cumulative incidence of uterine perforations was lowest in the group of women who were considered “nonpostpartum”; these women either received an IUD a full year after giving birth or had not given birth during the study period (0.29%; 95% confidence interval, 0.26-0.34).
Women who received an IUD during the 3 days after delivery had a nearly threefold increased risk of an IUD perforation over nonpostpartum women.
In addition, the cumulative incidence of perforation was almost double among breastfeeding women, compared with women who were not breastfeeding. However, Dr. Reed and coauthors noted that breastfeeding is highly beneficial for babies and that the risk of IUD perforation is relatively small.
Among the women who received an IUD following birth, Dr. Reed’s group found that 673 uterine perforations – of which 62% were complete – occurred in breastfeeding individuals, 37% more than for those who did not breastfeed.
Dr. Reed said the study provided some clarity on previous notions that women who’d never given birth were possibly at higher risk for uterine perforation because of smaller uteruses.
“We used to be concerned that women who had never had a pregnancy at all might be at higher risk because their uterus was smaller, the cervix was tighter, and therefore perhaps they might have greater risks,” Dr. Reed said in an interview. “As a clinician and as a provider, it’s pretty exciting to me to be able to tell our younger women who have never had a pregnancy that indeed their risk is lower than anybody else’s.”
The findings help women to make informed decisions, but overall, the benefits of IUDs outweigh the risks, said Monica V. Dragoman, MD, assistant professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York.
“The likelihood of anyone experiencing these types of complications of that population level remains really low,” Dr. Dragoman said.
The findings also provide guidance for providers as to which patients should come in for additional follow-up visits following insertion, Dr. Reed said.
“These are small risks, but it does tell us where we need to consider if there’s a challenging insertion,” Dr. Reed said. “You’re going to look with the ultrasound and make sure the placement looks right. You’re going to give instructions that if the woman has pain or a change in her bleeding pattern, you want to see her back.”
Patients should be aware of the symptoms of uterine perforation – an abrupt change in bleeding pattern and pelvic pain. Perforation correction typically consists of a minimally invasive surgical procedure.
The study was conducted as a result of an order from the Food and Drug Administration to Bayer Pharmaceuticals to evaluate risks of uterine perforation for women who’d received the company’s Mirena IUD. The findings led the company to update the language on the packaging of the device so as to specify the main symptoms of uterine perforations.
The study authors received research funding from Bayer. Multiple authors are employees of Bayer. One study author has in the past received funding from CooperSurgical, Bayer Healthcare Pharmaceutical, and Merck. Bayer was provided the opportunity to review the manuscript before submission, and comments were advisory only.
A version of this article first appeared on Medscape.com.
Women for whom an intrauterine device is inserted from 4 days to 6 weeks after giving birth, as well as those who are breastfeeding, are at higher risk of the contraceptive device puncturing their uterus, new research shows.
The risk of perforation was nearly seven times higher for patients who received an IUD within that window than for those with an IUD who’d never given birth or who were more than a year out from delivery, the researchers found. Health care providers should make patients aware of the heightened risk and should monitor these patients more closely, according to Susan Reed, MD, an ob.gyn. at the University of Washington, Seattle, lead author of the new study.
“I’m a surgeon, and I like to be able to give people good information and good data about risks and benefits for their choices,” Dr. Reed told this news organization. “Uterine perforations related to IUDs are exceedingly rare, and to get good data or known risk factors, you need huge studies. This was the largest study done that really provided accurate information for patients and providers.” The new study, which appears in a recent issue of The Lancet, also found that the risk of uterine perforation was lower if an IUD had been inserted immediately after delivery.
Dr. Reed and colleagues analyzed data from the health records of 326,658 women younger than 50 years for whom an IUD was inserted between 2001 and 2018 at four health care systems. Nearly 30% of these patients received an IUD after giving birth.
The researchers identified a total of 1,008 uterine perforations, for a cumulative incidence at 5 years of 0.6%. The cumulative incidence of uterine perforations was lowest in the group of women who were considered “nonpostpartum”; these women either received an IUD a full year after giving birth or had not given birth during the study period (0.29%; 95% confidence interval, 0.26-0.34).
Women who received an IUD during the 3 days after delivery had a nearly threefold increased risk of an IUD perforation over nonpostpartum women.
In addition, the cumulative incidence of perforation was almost double among breastfeeding women, compared with women who were not breastfeeding. However, Dr. Reed and coauthors noted that breastfeeding is highly beneficial for babies and that the risk of IUD perforation is relatively small.
Among the women who received an IUD following birth, Dr. Reed’s group found that 673 uterine perforations – of which 62% were complete – occurred in breastfeeding individuals, 37% more than for those who did not breastfeed.
Dr. Reed said the study provided some clarity on previous notions that women who’d never given birth were possibly at higher risk for uterine perforation because of smaller uteruses.
“We used to be concerned that women who had never had a pregnancy at all might be at higher risk because their uterus was smaller, the cervix was tighter, and therefore perhaps they might have greater risks,” Dr. Reed said in an interview. “As a clinician and as a provider, it’s pretty exciting to me to be able to tell our younger women who have never had a pregnancy that indeed their risk is lower than anybody else’s.”
The findings help women to make informed decisions, but overall, the benefits of IUDs outweigh the risks, said Monica V. Dragoman, MD, assistant professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York.
“The likelihood of anyone experiencing these types of complications of that population level remains really low,” Dr. Dragoman said.
The findings also provide guidance for providers as to which patients should come in for additional follow-up visits following insertion, Dr. Reed said.
“These are small risks, but it does tell us where we need to consider if there’s a challenging insertion,” Dr. Reed said. “You’re going to look with the ultrasound and make sure the placement looks right. You’re going to give instructions that if the woman has pain or a change in her bleeding pattern, you want to see her back.”
Patients should be aware of the symptoms of uterine perforation – an abrupt change in bleeding pattern and pelvic pain. Perforation correction typically consists of a minimally invasive surgical procedure.
The study was conducted as a result of an order from the Food and Drug Administration to Bayer Pharmaceuticals to evaluate risks of uterine perforation for women who’d received the company’s Mirena IUD. The findings led the company to update the language on the packaging of the device so as to specify the main symptoms of uterine perforations.
The study authors received research funding from Bayer. Multiple authors are employees of Bayer. One study author has in the past received funding from CooperSurgical, Bayer Healthcare Pharmaceutical, and Merck. Bayer was provided the opportunity to review the manuscript before submission, and comments were advisory only.
A version of this article first appeared on Medscape.com.
Women for whom an intrauterine device is inserted from 4 days to 6 weeks after giving birth, as well as those who are breastfeeding, are at higher risk of the contraceptive device puncturing their uterus, new research shows.
The risk of perforation was nearly seven times higher for patients who received an IUD within that window than for those with an IUD who’d never given birth or who were more than a year out from delivery, the researchers found. Health care providers should make patients aware of the heightened risk and should monitor these patients more closely, according to Susan Reed, MD, an ob.gyn. at the University of Washington, Seattle, lead author of the new study.
“I’m a surgeon, and I like to be able to give people good information and good data about risks and benefits for their choices,” Dr. Reed told this news organization. “Uterine perforations related to IUDs are exceedingly rare, and to get good data or known risk factors, you need huge studies. This was the largest study done that really provided accurate information for patients and providers.” The new study, which appears in a recent issue of The Lancet, also found that the risk of uterine perforation was lower if an IUD had been inserted immediately after delivery.
Dr. Reed and colleagues analyzed data from the health records of 326,658 women younger than 50 years for whom an IUD was inserted between 2001 and 2018 at four health care systems. Nearly 30% of these patients received an IUD after giving birth.
The researchers identified a total of 1,008 uterine perforations, for a cumulative incidence at 5 years of 0.6%. The cumulative incidence of uterine perforations was lowest in the group of women who were considered “nonpostpartum”; these women either received an IUD a full year after giving birth or had not given birth during the study period (0.29%; 95% confidence interval, 0.26-0.34).
Women who received an IUD during the 3 days after delivery had a nearly threefold increased risk of an IUD perforation over nonpostpartum women.
In addition, the cumulative incidence of perforation was almost double among breastfeeding women, compared with women who were not breastfeeding. However, Dr. Reed and coauthors noted that breastfeeding is highly beneficial for babies and that the risk of IUD perforation is relatively small.
Among the women who received an IUD following birth, Dr. Reed’s group found that 673 uterine perforations – of which 62% were complete – occurred in breastfeeding individuals, 37% more than for those who did not breastfeed.
Dr. Reed said the study provided some clarity on previous notions that women who’d never given birth were possibly at higher risk for uterine perforation because of smaller uteruses.
“We used to be concerned that women who had never had a pregnancy at all might be at higher risk because their uterus was smaller, the cervix was tighter, and therefore perhaps they might have greater risks,” Dr. Reed said in an interview. “As a clinician and as a provider, it’s pretty exciting to me to be able to tell our younger women who have never had a pregnancy that indeed their risk is lower than anybody else’s.”
The findings help women to make informed decisions, but overall, the benefits of IUDs outweigh the risks, said Monica V. Dragoman, MD, assistant professor of obstetrics, gynecology, and reproductive science at the Icahn School of Medicine at Mount Sinai, New York.
“The likelihood of anyone experiencing these types of complications of that population level remains really low,” Dr. Dragoman said.
The findings also provide guidance for providers as to which patients should come in for additional follow-up visits following insertion, Dr. Reed said.
“These are small risks, but it does tell us where we need to consider if there’s a challenging insertion,” Dr. Reed said. “You’re going to look with the ultrasound and make sure the placement looks right. You’re going to give instructions that if the woman has pain or a change in her bleeding pattern, you want to see her back.”
Patients should be aware of the symptoms of uterine perforation – an abrupt change in bleeding pattern and pelvic pain. Perforation correction typically consists of a minimally invasive surgical procedure.
The study was conducted as a result of an order from the Food and Drug Administration to Bayer Pharmaceuticals to evaluate risks of uterine perforation for women who’d received the company’s Mirena IUD. The findings led the company to update the language on the packaging of the device so as to specify the main symptoms of uterine perforations.
The study authors received research funding from Bayer. Multiple authors are employees of Bayer. One study author has in the past received funding from CooperSurgical, Bayer Healthcare Pharmaceutical, and Merck. Bayer was provided the opportunity to review the manuscript before submission, and comments were advisory only.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
$3 billion in cancer drug waste: Can it be salvaged?
Three billion dollars: It’s enough to finance the annual out-of-pocket costs for 1 in 7 patients with cancer. It would cover almost half of the National Cancer Institute’s annual budget. And it could fund President Biden’s entire Cancer Moonshot program, with more than a billion to spare.
It’s also how much the United States spends on unused cancer drugs each year, some experts estimate.
Drug companies typically sell infused drugs in one or two single-dose vial sizes, but patients don’t come in such neat packages. A patient may need 300 mg of a drug that is only sold as 200 mg vials, which means half of a vial will go to waste.
Although most oncology drugs don’t incur substantial waste, even small volumes can translate to millions of dollars a year.
But can this money be saved or reallocated, if only we delivered drugs more efficiently?
Some experts don’t believe that’s possible.
“Attempts to recoup money for discarded drugs wouldn’t happen in a vacuum,” said Robin Yabroff, PhD, MBA, an epidemiologist and scientific vice president of Health Services Research at the American Cancer Society, who was part of a committee commissioned to evaluate the costs associated with discarded drugs.
The potential catch of any widespread effort to seek repayment or reduce the amount of discarded drugs, Dr. Yabroff and colleagues note, is that manufacturers would “simply increase the price of the vial.”
In other words, attempting to fix one problem may lead to another — essentially a whack-a-mole of cancer costs, which are projected to balloon to $246 billion by 2030.
What this means is without sweeping policies to rein in cancer care costs, oncologists can only do so much. And every little bit counts.
“We are left chipping away at this monster of cancer care costs,” said Adam Binder, MD, a medical oncologist at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Millions spent on “reasonable amount” of waste
Michal Sarfaty, MD, was excited when enfortumab vedotin came on the market to treat advanced urothelial cancer in late 2019.
The cost of the drug, however, tempered her enthusiasm.
Enfortumab vedotin is a “great drug,” said Dr. Sarfaty, an oncologist at the Sheba Medical Center, Ramat Gan, Israel. But it can cost upwards of $500,000 a year for an average-weight man.
Given the expense, Dr. Sarfaty wanted to understand how much of the drug gets thrown away. During a fellowship at Memorial Sloan Kettering (MSK) Cancer Center in New York, Dr. Sarfaty explored the amount of unused enfortumab vedotin among the 64 patients who received the drug in 2020. She, along with a team at MSK, calculated the price tag of that waste and extrapolated those estimates for patients across the country.
Although waste occurred in almost half of administered doses (367 of 793), only a small volume got discarded — 2.9% per dose, on average.
Multiplying unused milligrams by the cost per milligram, Dr. Sarfaty and colleagues estimated that, for each patient, $3,127 of the drug got discarded. When calculated over the year, the cost came to just over $200,000 at MSK, and nearly $15 million when projected across the approved patient population in the United States.
“Ultimately, we did not see a lot of waste with this specific drug,” Dr. Sarfaty said. “Under 2.9% is considered a reasonable amount, below the 3% threshold Peter Bach, MD, and colleagues recommend. But even with this small amount of waste, the cost per patient and to the system remains notable.”
The problem with recouping drug waste
Estimates from the Centers for Medicare & Medicaid Services (CMS), which tracks costs associated with discarded weight-based drugs covered under Medicare Part B, support the notion that small quantities of discarded drugs can still translate to big bucks.
Since 2017, CMS has required healthcare providers to report the volume of drugs discarded from a single-dose vial using a code, known as the JW modifier. The JW modifier means that providers can be reimbursed for the entire vial amount, not just the quantity the patient used.
In 2019, claims data from Medicare Part B showed that 1.85% of discarded rituximab came to $33.3 million. For infliximab, the 1.55% of discarded liquid translated to $15 million, and just 0.36% of discarded pembrolizumab reached $10 million.
However, experts question whether the JW modifier accurately reflects the quantity of drugs discarded.
According to the 2021 report from the National Academies of Sciences, Engineering, and Medicine (NASEM), most physicians don’t use the JW modifier. Among Medicare claims, 16.2% included the JW modifier in 2017 and 16.9% did in 2018.
The rate was significantly lower for private insurance. Of more than 4 million private insurance claims on 77 drugs made in 2017 and 2018, only 3.6% included the JW modifier; 15 of these drugs had no JW claims.
“Although we found that most physicians don’t use the JW modifier, even those who do, don’t use it consistently, even for the same patient,” said Dr. Yabroff, a co-author on the report.
Going a step further, Dr. Yabroff and colleagues argue that even if everyone used the JW modifier as intended, manufacturers would probably increase the price of drugs to compensate for any loss, potentially eliminating savings for payers.
That’s because, in the United States, manufacturers typically base drug prices on a patient and payers’ “willingness to pay for better health,” not on the volume of liquid used. Take a patient who pays $2,000 to receive the dose they need. If that dose is 600 mg but requires using two vials of 400 mg, then “to the patient, the 600-mg dose is worth $2,000, and the remainder has no value whatsoever,” the NASEM authors argue.
The authors parallel this scenario to purchasing a designer coat or dress. If that item requires alterations that remove a section of material, “the customer does not typically get a rebate because all the fabric was not needed,” the NASEM team writes.
But there’s a flaw in this rationale, argues Daniel Goldstein, MD, a medical oncologist at the Rabin Medical Center, Petah Tikva, Israel. A person’s willingness to pay for better health assumes that the price of a drug is based on proper market forces, where a drug’s cost and its effectiveness are in harmony.
“The problem is we’re operating in a broken market where the prices of oncology drugs have no real bearing on their efficacy,” said Dr. Goldstein.
And, as Dr. Bach noted in a 2021 Health Affairs piece, willingness to pay also requires that consumers know what they’re paying and allows them to walk away from an excessively high price.
But neither is a reality.
For one, Dr. Bach explains, companies may lowball the monthly price of a drug. In 2020, GlaxoSmithKline (GSK) announced that its new drug Blenrep would carry a list price of $8,277 per vial, or about $23,900 per month for an average 79 kg (175 lb) patient. That price accounts for two vials of the drug. But, according to Dr. Bach, “what GSK left out is that 44% of U.S. adults weigh more than 80 kg, and above that weight, three vials are needed per dose.” That would raise the average monthly cost to $30,479.
Perhaps more importantly, consumers can’t easily walk away.
“Medicare can’t negotiate prices and is forced to pay what a drug company says,” Dr. Goldstein said. “This is very different to when I buy a coat. If the price is too high, I can walk away.”
Fixed dosing: A solution or a new problem?
Efforts to reduce the financial impact of discarded cancer drugs can blow back on physicians, patients, and payers in other unanticipated ways. Take fixed dosing. Although chemotherapy dosing remains weight-based, many targeted therapies — such as nivolumab and pembrolizumab — recently transitioned to a fixed dosing regimen.
Administering a fixed, instead of weight-based, dose eliminates waste but can create new problems.
“Patients with cancer not only tend to get too high a dose of the drug, but costs go up significantly,” said Dr. Goldstein. In a 2017 analysis, Dr. Goldstein and colleagues compared dosing strategies in patients with metastatic non–small cell lung cancer who received pembrolizumab. The team found that the total annual cost of weight-based dosing was $2.6 billion, whereas the cost of the fixed dosing strategy was $3.44 billion — 24% more. In other words, personalized weight-based dosing would save more than $825 million dollars in the United States each year.
A 2020 analysis based in France found a similar cost increase of 26% for fixed dosing of pembrolizumab as well as nivolumab.
“I’ve argued we should go back to weight-based dosing,” Dr. Goldstein said. “Why should we give a higher dose with the same efficacy when that dose will cost significantly more and has the potential to increase adverse events?”
Does dose rounding work?
Rose DiMarco, PharmD, BCPS, BCOP, keeps a tight watch on patients being treated at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Dr. DiMarco educates patients about their treatment plan, reviews their lab results, and monitors them for side effects and drug interactions.
She also thinks a lot about costs.
“We spend about $100,000 a day on oncology drugs, and we want to make sure we’re not being wasteful,” Dr. DiMarco said in an interview.
One major initiative to curb waste and reduce costs at Jefferson has centered on dose rounding, which calculates whether a specific dose can be altered slightly to conserve vials and prevent waste. According to the Hematology/Oncology Pharmacy Association, a patient can receive up to 10% more or less of a weight-based dose without impacting treatment efficacy.
If, for instance, a patient requires 380 mg, but two vials come to 400 mg, rounding up that dose by approximately 5% means eliminating 20 mg that would go unused. But if that patient requires 420 mg, rounding down about 5% means substantial savings from not opening a new vial.
At Jefferson, Dr. DiMarco and her pharmacy colleagues map out dose ranges for all patients. Anyone who falls inside the 10% may be eligible for dose rounding. Anyone who doesn’t will receive the usual dose.
Although it is a challenge to implement, dose rounding has become standard of care at many cancer centers across the United States and is linked to substantial savings.
A 2018 analysis projected annual savings of $865,000 associated with rounding down eight monoclonal antibodies for patients with metastatic disease at a community cancer center. A more recent analysis from the Mayo Clinic found that dose rounding saved a total of 9,814 drug vials — 4485 of which were cancer drugs and 5329 of which were biologics — and resulted in $7.3 million in savings over 6 months in 2019 — $1.56 million from oncology agents and $5.7 from biologics.
And in a small 2019 analysis, researchers at Jefferson showed dose rounding of one monoclonal antibody saved approximately $30,000 in just 3 months, Dr. DiMarco noted.
“Not only does this process reduce costs and waste, but it also standardizes the preparation of hazardous medications, which can help prevent medication errors,” Dr. DiMarco said.
Nibbling around the edges
Despite estimates that scale into the billions of dollars, “drug wastage is just a small part of overall cancer costs,” Dr. Sarfaty said.
Fumiko Chino, MD, a radiation oncologist at MSK, agrees. “When we talk about affordability and cost, we can nibble around the edges of what’s really important,” Dr. Chino said. “Discarded drugs may cost a lot when you consider them in aggregate, but they are not as important as negotiated drug prices, which could substantially reduce overall costs.”
And until drug prices are addressed on a broader policy level, the cost of cancer care likely won’t improve in a meaningful way.
“But for the patient sitting in front of me, my focus will always be to provide the best care possible,” Dr. Binder said.
A version of this article first appeared on Medscape.com.
Three billion dollars: It’s enough to finance the annual out-of-pocket costs for 1 in 7 patients with cancer. It would cover almost half of the National Cancer Institute’s annual budget. And it could fund President Biden’s entire Cancer Moonshot program, with more than a billion to spare.
It’s also how much the United States spends on unused cancer drugs each year, some experts estimate.
Drug companies typically sell infused drugs in one or two single-dose vial sizes, but patients don’t come in such neat packages. A patient may need 300 mg of a drug that is only sold as 200 mg vials, which means half of a vial will go to waste.
Although most oncology drugs don’t incur substantial waste, even small volumes can translate to millions of dollars a year.
But can this money be saved or reallocated, if only we delivered drugs more efficiently?
Some experts don’t believe that’s possible.
“Attempts to recoup money for discarded drugs wouldn’t happen in a vacuum,” said Robin Yabroff, PhD, MBA, an epidemiologist and scientific vice president of Health Services Research at the American Cancer Society, who was part of a committee commissioned to evaluate the costs associated with discarded drugs.
The potential catch of any widespread effort to seek repayment or reduce the amount of discarded drugs, Dr. Yabroff and colleagues note, is that manufacturers would “simply increase the price of the vial.”
In other words, attempting to fix one problem may lead to another — essentially a whack-a-mole of cancer costs, which are projected to balloon to $246 billion by 2030.
What this means is without sweeping policies to rein in cancer care costs, oncologists can only do so much. And every little bit counts.
“We are left chipping away at this monster of cancer care costs,” said Adam Binder, MD, a medical oncologist at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Millions spent on “reasonable amount” of waste
Michal Sarfaty, MD, was excited when enfortumab vedotin came on the market to treat advanced urothelial cancer in late 2019.
The cost of the drug, however, tempered her enthusiasm.
Enfortumab vedotin is a “great drug,” said Dr. Sarfaty, an oncologist at the Sheba Medical Center, Ramat Gan, Israel. But it can cost upwards of $500,000 a year for an average-weight man.
Given the expense, Dr. Sarfaty wanted to understand how much of the drug gets thrown away. During a fellowship at Memorial Sloan Kettering (MSK) Cancer Center in New York, Dr. Sarfaty explored the amount of unused enfortumab vedotin among the 64 patients who received the drug in 2020. She, along with a team at MSK, calculated the price tag of that waste and extrapolated those estimates for patients across the country.
Although waste occurred in almost half of administered doses (367 of 793), only a small volume got discarded — 2.9% per dose, on average.
Multiplying unused milligrams by the cost per milligram, Dr. Sarfaty and colleagues estimated that, for each patient, $3,127 of the drug got discarded. When calculated over the year, the cost came to just over $200,000 at MSK, and nearly $15 million when projected across the approved patient population in the United States.
“Ultimately, we did not see a lot of waste with this specific drug,” Dr. Sarfaty said. “Under 2.9% is considered a reasonable amount, below the 3% threshold Peter Bach, MD, and colleagues recommend. But even with this small amount of waste, the cost per patient and to the system remains notable.”
The problem with recouping drug waste
Estimates from the Centers for Medicare & Medicaid Services (CMS), which tracks costs associated with discarded weight-based drugs covered under Medicare Part B, support the notion that small quantities of discarded drugs can still translate to big bucks.
Since 2017, CMS has required healthcare providers to report the volume of drugs discarded from a single-dose vial using a code, known as the JW modifier. The JW modifier means that providers can be reimbursed for the entire vial amount, not just the quantity the patient used.
In 2019, claims data from Medicare Part B showed that 1.85% of discarded rituximab came to $33.3 million. For infliximab, the 1.55% of discarded liquid translated to $15 million, and just 0.36% of discarded pembrolizumab reached $10 million.
However, experts question whether the JW modifier accurately reflects the quantity of drugs discarded.
According to the 2021 report from the National Academies of Sciences, Engineering, and Medicine (NASEM), most physicians don’t use the JW modifier. Among Medicare claims, 16.2% included the JW modifier in 2017 and 16.9% did in 2018.
The rate was significantly lower for private insurance. Of more than 4 million private insurance claims on 77 drugs made in 2017 and 2018, only 3.6% included the JW modifier; 15 of these drugs had no JW claims.
“Although we found that most physicians don’t use the JW modifier, even those who do, don’t use it consistently, even for the same patient,” said Dr. Yabroff, a co-author on the report.
Going a step further, Dr. Yabroff and colleagues argue that even if everyone used the JW modifier as intended, manufacturers would probably increase the price of drugs to compensate for any loss, potentially eliminating savings for payers.
That’s because, in the United States, manufacturers typically base drug prices on a patient and payers’ “willingness to pay for better health,” not on the volume of liquid used. Take a patient who pays $2,000 to receive the dose they need. If that dose is 600 mg but requires using two vials of 400 mg, then “to the patient, the 600-mg dose is worth $2,000, and the remainder has no value whatsoever,” the NASEM authors argue.
The authors parallel this scenario to purchasing a designer coat or dress. If that item requires alterations that remove a section of material, “the customer does not typically get a rebate because all the fabric was not needed,” the NASEM team writes.
But there’s a flaw in this rationale, argues Daniel Goldstein, MD, a medical oncologist at the Rabin Medical Center, Petah Tikva, Israel. A person’s willingness to pay for better health assumes that the price of a drug is based on proper market forces, where a drug’s cost and its effectiveness are in harmony.
“The problem is we’re operating in a broken market where the prices of oncology drugs have no real bearing on their efficacy,” said Dr. Goldstein.
And, as Dr. Bach noted in a 2021 Health Affairs piece, willingness to pay also requires that consumers know what they’re paying and allows them to walk away from an excessively high price.
But neither is a reality.
For one, Dr. Bach explains, companies may lowball the monthly price of a drug. In 2020, GlaxoSmithKline (GSK) announced that its new drug Blenrep would carry a list price of $8,277 per vial, or about $23,900 per month for an average 79 kg (175 lb) patient. That price accounts for two vials of the drug. But, according to Dr. Bach, “what GSK left out is that 44% of U.S. adults weigh more than 80 kg, and above that weight, three vials are needed per dose.” That would raise the average monthly cost to $30,479.
Perhaps more importantly, consumers can’t easily walk away.
“Medicare can’t negotiate prices and is forced to pay what a drug company says,” Dr. Goldstein said. “This is very different to when I buy a coat. If the price is too high, I can walk away.”
Fixed dosing: A solution or a new problem?
Efforts to reduce the financial impact of discarded cancer drugs can blow back on physicians, patients, and payers in other unanticipated ways. Take fixed dosing. Although chemotherapy dosing remains weight-based, many targeted therapies — such as nivolumab and pembrolizumab — recently transitioned to a fixed dosing regimen.
Administering a fixed, instead of weight-based, dose eliminates waste but can create new problems.
“Patients with cancer not only tend to get too high a dose of the drug, but costs go up significantly,” said Dr. Goldstein. In a 2017 analysis, Dr. Goldstein and colleagues compared dosing strategies in patients with metastatic non–small cell lung cancer who received pembrolizumab. The team found that the total annual cost of weight-based dosing was $2.6 billion, whereas the cost of the fixed dosing strategy was $3.44 billion — 24% more. In other words, personalized weight-based dosing would save more than $825 million dollars in the United States each year.
A 2020 analysis based in France found a similar cost increase of 26% for fixed dosing of pembrolizumab as well as nivolumab.
“I’ve argued we should go back to weight-based dosing,” Dr. Goldstein said. “Why should we give a higher dose with the same efficacy when that dose will cost significantly more and has the potential to increase adverse events?”
Does dose rounding work?
Rose DiMarco, PharmD, BCPS, BCOP, keeps a tight watch on patients being treated at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Dr. DiMarco educates patients about their treatment plan, reviews their lab results, and monitors them for side effects and drug interactions.
She also thinks a lot about costs.
“We spend about $100,000 a day on oncology drugs, and we want to make sure we’re not being wasteful,” Dr. DiMarco said in an interview.
One major initiative to curb waste and reduce costs at Jefferson has centered on dose rounding, which calculates whether a specific dose can be altered slightly to conserve vials and prevent waste. According to the Hematology/Oncology Pharmacy Association, a patient can receive up to 10% more or less of a weight-based dose without impacting treatment efficacy.
If, for instance, a patient requires 380 mg, but two vials come to 400 mg, rounding up that dose by approximately 5% means eliminating 20 mg that would go unused. But if that patient requires 420 mg, rounding down about 5% means substantial savings from not opening a new vial.
At Jefferson, Dr. DiMarco and her pharmacy colleagues map out dose ranges for all patients. Anyone who falls inside the 10% may be eligible for dose rounding. Anyone who doesn’t will receive the usual dose.
Although it is a challenge to implement, dose rounding has become standard of care at many cancer centers across the United States and is linked to substantial savings.
A 2018 analysis projected annual savings of $865,000 associated with rounding down eight monoclonal antibodies for patients with metastatic disease at a community cancer center. A more recent analysis from the Mayo Clinic found that dose rounding saved a total of 9,814 drug vials — 4485 of which were cancer drugs and 5329 of which were biologics — and resulted in $7.3 million in savings over 6 months in 2019 — $1.56 million from oncology agents and $5.7 from biologics.
And in a small 2019 analysis, researchers at Jefferson showed dose rounding of one monoclonal antibody saved approximately $30,000 in just 3 months, Dr. DiMarco noted.
“Not only does this process reduce costs and waste, but it also standardizes the preparation of hazardous medications, which can help prevent medication errors,” Dr. DiMarco said.
Nibbling around the edges
Despite estimates that scale into the billions of dollars, “drug wastage is just a small part of overall cancer costs,” Dr. Sarfaty said.
Fumiko Chino, MD, a radiation oncologist at MSK, agrees. “When we talk about affordability and cost, we can nibble around the edges of what’s really important,” Dr. Chino said. “Discarded drugs may cost a lot when you consider them in aggregate, but they are not as important as negotiated drug prices, which could substantially reduce overall costs.”
And until drug prices are addressed on a broader policy level, the cost of cancer care likely won’t improve in a meaningful way.
“But for the patient sitting in front of me, my focus will always be to provide the best care possible,” Dr. Binder said.
A version of this article first appeared on Medscape.com.
Three billion dollars: It’s enough to finance the annual out-of-pocket costs for 1 in 7 patients with cancer. It would cover almost half of the National Cancer Institute’s annual budget. And it could fund President Biden’s entire Cancer Moonshot program, with more than a billion to spare.
It’s also how much the United States spends on unused cancer drugs each year, some experts estimate.
Drug companies typically sell infused drugs in one or two single-dose vial sizes, but patients don’t come in such neat packages. A patient may need 300 mg of a drug that is only sold as 200 mg vials, which means half of a vial will go to waste.
Although most oncology drugs don’t incur substantial waste, even small volumes can translate to millions of dollars a year.
But can this money be saved or reallocated, if only we delivered drugs more efficiently?
Some experts don’t believe that’s possible.
“Attempts to recoup money for discarded drugs wouldn’t happen in a vacuum,” said Robin Yabroff, PhD, MBA, an epidemiologist and scientific vice president of Health Services Research at the American Cancer Society, who was part of a committee commissioned to evaluate the costs associated with discarded drugs.
The potential catch of any widespread effort to seek repayment or reduce the amount of discarded drugs, Dr. Yabroff and colleagues note, is that manufacturers would “simply increase the price of the vial.”
In other words, attempting to fix one problem may lead to another — essentially a whack-a-mole of cancer costs, which are projected to balloon to $246 billion by 2030.
What this means is without sweeping policies to rein in cancer care costs, oncologists can only do so much. And every little bit counts.
“We are left chipping away at this monster of cancer care costs,” said Adam Binder, MD, a medical oncologist at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Millions spent on “reasonable amount” of waste
Michal Sarfaty, MD, was excited when enfortumab vedotin came on the market to treat advanced urothelial cancer in late 2019.
The cost of the drug, however, tempered her enthusiasm.
Enfortumab vedotin is a “great drug,” said Dr. Sarfaty, an oncologist at the Sheba Medical Center, Ramat Gan, Israel. But it can cost upwards of $500,000 a year for an average-weight man.
Given the expense, Dr. Sarfaty wanted to understand how much of the drug gets thrown away. During a fellowship at Memorial Sloan Kettering (MSK) Cancer Center in New York, Dr. Sarfaty explored the amount of unused enfortumab vedotin among the 64 patients who received the drug in 2020. She, along with a team at MSK, calculated the price tag of that waste and extrapolated those estimates for patients across the country.
Although waste occurred in almost half of administered doses (367 of 793), only a small volume got discarded — 2.9% per dose, on average.
Multiplying unused milligrams by the cost per milligram, Dr. Sarfaty and colleagues estimated that, for each patient, $3,127 of the drug got discarded. When calculated over the year, the cost came to just over $200,000 at MSK, and nearly $15 million when projected across the approved patient population in the United States.
“Ultimately, we did not see a lot of waste with this specific drug,” Dr. Sarfaty said. “Under 2.9% is considered a reasonable amount, below the 3% threshold Peter Bach, MD, and colleagues recommend. But even with this small amount of waste, the cost per patient and to the system remains notable.”
The problem with recouping drug waste
Estimates from the Centers for Medicare & Medicaid Services (CMS), which tracks costs associated with discarded weight-based drugs covered under Medicare Part B, support the notion that small quantities of discarded drugs can still translate to big bucks.
Since 2017, CMS has required healthcare providers to report the volume of drugs discarded from a single-dose vial using a code, known as the JW modifier. The JW modifier means that providers can be reimbursed for the entire vial amount, not just the quantity the patient used.
In 2019, claims data from Medicare Part B showed that 1.85% of discarded rituximab came to $33.3 million. For infliximab, the 1.55% of discarded liquid translated to $15 million, and just 0.36% of discarded pembrolizumab reached $10 million.
However, experts question whether the JW modifier accurately reflects the quantity of drugs discarded.
According to the 2021 report from the National Academies of Sciences, Engineering, and Medicine (NASEM), most physicians don’t use the JW modifier. Among Medicare claims, 16.2% included the JW modifier in 2017 and 16.9% did in 2018.
The rate was significantly lower for private insurance. Of more than 4 million private insurance claims on 77 drugs made in 2017 and 2018, only 3.6% included the JW modifier; 15 of these drugs had no JW claims.
“Although we found that most physicians don’t use the JW modifier, even those who do, don’t use it consistently, even for the same patient,” said Dr. Yabroff, a co-author on the report.
Going a step further, Dr. Yabroff and colleagues argue that even if everyone used the JW modifier as intended, manufacturers would probably increase the price of drugs to compensate for any loss, potentially eliminating savings for payers.
That’s because, in the United States, manufacturers typically base drug prices on a patient and payers’ “willingness to pay for better health,” not on the volume of liquid used. Take a patient who pays $2,000 to receive the dose they need. If that dose is 600 mg but requires using two vials of 400 mg, then “to the patient, the 600-mg dose is worth $2,000, and the remainder has no value whatsoever,” the NASEM authors argue.
The authors parallel this scenario to purchasing a designer coat or dress. If that item requires alterations that remove a section of material, “the customer does not typically get a rebate because all the fabric was not needed,” the NASEM team writes.
But there’s a flaw in this rationale, argues Daniel Goldstein, MD, a medical oncologist at the Rabin Medical Center, Petah Tikva, Israel. A person’s willingness to pay for better health assumes that the price of a drug is based on proper market forces, where a drug’s cost and its effectiveness are in harmony.
“The problem is we’re operating in a broken market where the prices of oncology drugs have no real bearing on their efficacy,” said Dr. Goldstein.
And, as Dr. Bach noted in a 2021 Health Affairs piece, willingness to pay also requires that consumers know what they’re paying and allows them to walk away from an excessively high price.
But neither is a reality.
For one, Dr. Bach explains, companies may lowball the monthly price of a drug. In 2020, GlaxoSmithKline (GSK) announced that its new drug Blenrep would carry a list price of $8,277 per vial, or about $23,900 per month for an average 79 kg (175 lb) patient. That price accounts for two vials of the drug. But, according to Dr. Bach, “what GSK left out is that 44% of U.S. adults weigh more than 80 kg, and above that weight, three vials are needed per dose.” That would raise the average monthly cost to $30,479.
Perhaps more importantly, consumers can’t easily walk away.
“Medicare can’t negotiate prices and is forced to pay what a drug company says,” Dr. Goldstein said. “This is very different to when I buy a coat. If the price is too high, I can walk away.”
Fixed dosing: A solution or a new problem?
Efforts to reduce the financial impact of discarded cancer drugs can blow back on physicians, patients, and payers in other unanticipated ways. Take fixed dosing. Although chemotherapy dosing remains weight-based, many targeted therapies — such as nivolumab and pembrolizumab — recently transitioned to a fixed dosing regimen.
Administering a fixed, instead of weight-based, dose eliminates waste but can create new problems.
“Patients with cancer not only tend to get too high a dose of the drug, but costs go up significantly,” said Dr. Goldstein. In a 2017 analysis, Dr. Goldstein and colleagues compared dosing strategies in patients with metastatic non–small cell lung cancer who received pembrolizumab. The team found that the total annual cost of weight-based dosing was $2.6 billion, whereas the cost of the fixed dosing strategy was $3.44 billion — 24% more. In other words, personalized weight-based dosing would save more than $825 million dollars in the United States each year.
A 2020 analysis based in France found a similar cost increase of 26% for fixed dosing of pembrolizumab as well as nivolumab.
“I’ve argued we should go back to weight-based dosing,” Dr. Goldstein said. “Why should we give a higher dose with the same efficacy when that dose will cost significantly more and has the potential to increase adverse events?”
Does dose rounding work?
Rose DiMarco, PharmD, BCPS, BCOP, keeps a tight watch on patients being treated at the Sidney Kimmel Cancer Center at Jefferson Health in Philadelphia.
Dr. DiMarco educates patients about their treatment plan, reviews their lab results, and monitors them for side effects and drug interactions.
She also thinks a lot about costs.
“We spend about $100,000 a day on oncology drugs, and we want to make sure we’re not being wasteful,” Dr. DiMarco said in an interview.
One major initiative to curb waste and reduce costs at Jefferson has centered on dose rounding, which calculates whether a specific dose can be altered slightly to conserve vials and prevent waste. According to the Hematology/Oncology Pharmacy Association, a patient can receive up to 10% more or less of a weight-based dose without impacting treatment efficacy.
If, for instance, a patient requires 380 mg, but two vials come to 400 mg, rounding up that dose by approximately 5% means eliminating 20 mg that would go unused. But if that patient requires 420 mg, rounding down about 5% means substantial savings from not opening a new vial.
At Jefferson, Dr. DiMarco and her pharmacy colleagues map out dose ranges for all patients. Anyone who falls inside the 10% may be eligible for dose rounding. Anyone who doesn’t will receive the usual dose.
Although it is a challenge to implement, dose rounding has become standard of care at many cancer centers across the United States and is linked to substantial savings.
A 2018 analysis projected annual savings of $865,000 associated with rounding down eight monoclonal antibodies for patients with metastatic disease at a community cancer center. A more recent analysis from the Mayo Clinic found that dose rounding saved a total of 9,814 drug vials — 4485 of which were cancer drugs and 5329 of which were biologics — and resulted in $7.3 million in savings over 6 months in 2019 — $1.56 million from oncology agents and $5.7 from biologics.
And in a small 2019 analysis, researchers at Jefferson showed dose rounding of one monoclonal antibody saved approximately $30,000 in just 3 months, Dr. DiMarco noted.
“Not only does this process reduce costs and waste, but it also standardizes the preparation of hazardous medications, which can help prevent medication errors,” Dr. DiMarco said.
Nibbling around the edges
Despite estimates that scale into the billions of dollars, “drug wastage is just a small part of overall cancer costs,” Dr. Sarfaty said.
Fumiko Chino, MD, a radiation oncologist at MSK, agrees. “When we talk about affordability and cost, we can nibble around the edges of what’s really important,” Dr. Chino said. “Discarded drugs may cost a lot when you consider them in aggregate, but they are not as important as negotiated drug prices, which could substantially reduce overall costs.”
And until drug prices are addressed on a broader policy level, the cost of cancer care likely won’t improve in a meaningful way.
“But for the patient sitting in front of me, my focus will always be to provide the best care possible,” Dr. Binder said.
A version of this article first appeared on Medscape.com.











