User login
T2D: Dapagliflozin consistently reduces CV and kidney disease risk irrespective of background therapy
Key clinical point: Dapagliflozin consistently reduced the risk for cardiovascular (CV) death or hospitalization for heart failure (HHF) and kidney disease progression irrespective of the background CV medication in patients with type 2 diabetes (T2D) with a consistent safety profile.
Major finding: Dapagliflozin vs placebo led to a consistent reduction in the composite of CV death/HHF, HHF alone, and kidney-specific outcomes irrespective of the background CV medications (Pinteraction > .05), with patients not using diuretics showing better kidney specific outcomes (Pinteraction = .003). Serious adverse events were not significantly different between the dapagliflozin and placebo groups.
Study details: Findings are from a prespecified secondary analysis of the DECLARE-TIMI 58 trial including 17,160 patients with T2D and either atherosclerotic disease or multiple CV risk factors.
Disclosures: The DECLAR-TIMI 58 trial was supported by AstraZeneca. One author reported being an employee and shareholder of AstraZeneca. Some authors reported receiving research funding or support, honoraria, personal fees, or consulting or speaker fees, or serving as advisory board members for various sources, including AstraZeneca.
Source: Oyama K et al. Efficacy and safety of dapagliflozin according to background use of cardiovascular medications in patients with type 2 diabetes: A prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2022 (Jul 20). Doi: 10.1001/jamacardio.2022.2006
Key clinical point: Dapagliflozin consistently reduced the risk for cardiovascular (CV) death or hospitalization for heart failure (HHF) and kidney disease progression irrespective of the background CV medication in patients with type 2 diabetes (T2D) with a consistent safety profile.
Major finding: Dapagliflozin vs placebo led to a consistent reduction in the composite of CV death/HHF, HHF alone, and kidney-specific outcomes irrespective of the background CV medications (Pinteraction > .05), with patients not using diuretics showing better kidney specific outcomes (Pinteraction = .003). Serious adverse events were not significantly different between the dapagliflozin and placebo groups.
Study details: Findings are from a prespecified secondary analysis of the DECLARE-TIMI 58 trial including 17,160 patients with T2D and either atherosclerotic disease or multiple CV risk factors.
Disclosures: The DECLAR-TIMI 58 trial was supported by AstraZeneca. One author reported being an employee and shareholder of AstraZeneca. Some authors reported receiving research funding or support, honoraria, personal fees, or consulting or speaker fees, or serving as advisory board members for various sources, including AstraZeneca.
Source: Oyama K et al. Efficacy and safety of dapagliflozin according to background use of cardiovascular medications in patients with type 2 diabetes: A prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2022 (Jul 20). Doi: 10.1001/jamacardio.2022.2006
Key clinical point: Dapagliflozin consistently reduced the risk for cardiovascular (CV) death or hospitalization for heart failure (HHF) and kidney disease progression irrespective of the background CV medication in patients with type 2 diabetes (T2D) with a consistent safety profile.
Major finding: Dapagliflozin vs placebo led to a consistent reduction in the composite of CV death/HHF, HHF alone, and kidney-specific outcomes irrespective of the background CV medications (Pinteraction > .05), with patients not using diuretics showing better kidney specific outcomes (Pinteraction = .003). Serious adverse events were not significantly different between the dapagliflozin and placebo groups.
Study details: Findings are from a prespecified secondary analysis of the DECLARE-TIMI 58 trial including 17,160 patients with T2D and either atherosclerotic disease or multiple CV risk factors.
Disclosures: The DECLAR-TIMI 58 trial was supported by AstraZeneca. One author reported being an employee and shareholder of AstraZeneca. Some authors reported receiving research funding or support, honoraria, personal fees, or consulting or speaker fees, or serving as advisory board members for various sources, including AstraZeneca.
Source: Oyama K et al. Efficacy and safety of dapagliflozin according to background use of cardiovascular medications in patients with type 2 diabetes: A prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2022 (Jul 20). Doi: 10.1001/jamacardio.2022.2006
Comparative efficacy and safety of Gla-300 and Deg-100 in T2D
Key clinical point: Initiation of 300 units/mL insulin glargine (Gla-300) or 100 units/mL degludec (Deg-100) was associated with similar improvements in glycemic control, no weight gain, and low rates of hypoglycemia in insulin-naive patients with type 2 diabetes (T2D).
Major finding: After 6 months, both Gla-300 and Deg-100 led to a significant and similar reduction in glycated hemoglobin (between group difference [Δ] −0.01%; P = .49) and fasting blood glucose (Δ −2.09 mg/dL; P = .74) levels, with no significant changes in body weight in both treatment groups. Overall, the incidence of hypoglycemia was low, with no severe episodes reported.
Study details: This was a retrospective study including insulin-naive patients with T2D who initiated Gla-300 and were propensity matched with those who initiated Deg-100 (n = 357).
Disclosures: This study was funded by Sanofi S.r.l., Milan, Italy. Some authors declared receiving lecture fees, consulting fees, research funding, or speaking honoraria from various sources, including Sanofi. M Larosa declared being an employee and holding stock in Sanofi.
Source: Fadini GP et al. Comparative effectiveness and safety of glargine 300 U/mL versus degludec 100 U/mL in insulin-naïve patients with type 2 diabetes. A multicenter retrospective real-world study (RESTORE-2 NAIVE STUDY). Acta Diabetol. 2022 (Jul 21). Doi: 10.1007/s00592-022-01925-9
Key clinical point: Initiation of 300 units/mL insulin glargine (Gla-300) or 100 units/mL degludec (Deg-100) was associated with similar improvements in glycemic control, no weight gain, and low rates of hypoglycemia in insulin-naive patients with type 2 diabetes (T2D).
Major finding: After 6 months, both Gla-300 and Deg-100 led to a significant and similar reduction in glycated hemoglobin (between group difference [Δ] −0.01%; P = .49) and fasting blood glucose (Δ −2.09 mg/dL; P = .74) levels, with no significant changes in body weight in both treatment groups. Overall, the incidence of hypoglycemia was low, with no severe episodes reported.
Study details: This was a retrospective study including insulin-naive patients with T2D who initiated Gla-300 and were propensity matched with those who initiated Deg-100 (n = 357).
Disclosures: This study was funded by Sanofi S.r.l., Milan, Italy. Some authors declared receiving lecture fees, consulting fees, research funding, or speaking honoraria from various sources, including Sanofi. M Larosa declared being an employee and holding stock in Sanofi.
Source: Fadini GP et al. Comparative effectiveness and safety of glargine 300 U/mL versus degludec 100 U/mL in insulin-naïve patients with type 2 diabetes. A multicenter retrospective real-world study (RESTORE-2 NAIVE STUDY). Acta Diabetol. 2022 (Jul 21). Doi: 10.1007/s00592-022-01925-9
Key clinical point: Initiation of 300 units/mL insulin glargine (Gla-300) or 100 units/mL degludec (Deg-100) was associated with similar improvements in glycemic control, no weight gain, and low rates of hypoglycemia in insulin-naive patients with type 2 diabetes (T2D).
Major finding: After 6 months, both Gla-300 and Deg-100 led to a significant and similar reduction in glycated hemoglobin (between group difference [Δ] −0.01%; P = .49) and fasting blood glucose (Δ −2.09 mg/dL; P = .74) levels, with no significant changes in body weight in both treatment groups. Overall, the incidence of hypoglycemia was low, with no severe episodes reported.
Study details: This was a retrospective study including insulin-naive patients with T2D who initiated Gla-300 and were propensity matched with those who initiated Deg-100 (n = 357).
Disclosures: This study was funded by Sanofi S.r.l., Milan, Italy. Some authors declared receiving lecture fees, consulting fees, research funding, or speaking honoraria from various sources, including Sanofi. M Larosa declared being an employee and holding stock in Sanofi.
Source: Fadini GP et al. Comparative effectiveness and safety of glargine 300 U/mL versus degludec 100 U/mL in insulin-naïve patients with type 2 diabetes. A multicenter retrospective real-world study (RESTORE-2 NAIVE STUDY). Acta Diabetol. 2022 (Jul 21). Doi: 10.1007/s00592-022-01925-9
T2D: No evidence to suggest increased fracture risk with DPP-4i, GLP-1 RA, and SGLT-2i
Key clinical point: Dipeptidyl peptidase-4 inhibitors (DPP-4i), glucagon-like peptide-1 receptor agonists (GLP-1 RA), and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) did not increase the risk for fracture in patients with type 2 diabetes (T2D) compared with other antidiabetic agents or placebo.
Major finding: DPP-4i was not associated with a higher risk for total fracture compared with insulin (odds ratio [OR] 0.86; 95% CI 0.39-1.90), metformin (OR 1.41; 95% CI 0.48-4.19), sulfonylureas (OR 0.77; 95% CI 0.50-1.20), thiazolidinediones (OR 0.82; 95% CI 0.27-2.44), α-glucosidase inhibitor (OR 4.92; 95% CI 0.23-103.83), and placebo (OR 1.04; 95% CI 0.84-1.29), with findings being similar for GLP-1 RA and SGLT-2i.
Study details: The data come from a systematic review and network meta-analysis of 177 randomized controlled trials including 165,081 patients with T2D.
Disclosures: This study was supported by the National Natural Science Foundation, China, and others. The authors declared no conflicts of interest.
Source: Chai S et al. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis combining 177 randomized controlled trials with a median follow-up of 26 weeks. Front Pharmacol. 2022;13:825417 (Jul 1). Doi: 10.3389/fphar.2022.825417
Key clinical point: Dipeptidyl peptidase-4 inhibitors (DPP-4i), glucagon-like peptide-1 receptor agonists (GLP-1 RA), and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) did not increase the risk for fracture in patients with type 2 diabetes (T2D) compared with other antidiabetic agents or placebo.
Major finding: DPP-4i was not associated with a higher risk for total fracture compared with insulin (odds ratio [OR] 0.86; 95% CI 0.39-1.90), metformin (OR 1.41; 95% CI 0.48-4.19), sulfonylureas (OR 0.77; 95% CI 0.50-1.20), thiazolidinediones (OR 0.82; 95% CI 0.27-2.44), α-glucosidase inhibitor (OR 4.92; 95% CI 0.23-103.83), and placebo (OR 1.04; 95% CI 0.84-1.29), with findings being similar for GLP-1 RA and SGLT-2i.
Study details: The data come from a systematic review and network meta-analysis of 177 randomized controlled trials including 165,081 patients with T2D.
Disclosures: This study was supported by the National Natural Science Foundation, China, and others. The authors declared no conflicts of interest.
Source: Chai S et al. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis combining 177 randomized controlled trials with a median follow-up of 26 weeks. Front Pharmacol. 2022;13:825417 (Jul 1). Doi: 10.3389/fphar.2022.825417
Key clinical point: Dipeptidyl peptidase-4 inhibitors (DPP-4i), glucagon-like peptide-1 receptor agonists (GLP-1 RA), and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) did not increase the risk for fracture in patients with type 2 diabetes (T2D) compared with other antidiabetic agents or placebo.
Major finding: DPP-4i was not associated with a higher risk for total fracture compared with insulin (odds ratio [OR] 0.86; 95% CI 0.39-1.90), metformin (OR 1.41; 95% CI 0.48-4.19), sulfonylureas (OR 0.77; 95% CI 0.50-1.20), thiazolidinediones (OR 0.82; 95% CI 0.27-2.44), α-glucosidase inhibitor (OR 4.92; 95% CI 0.23-103.83), and placebo (OR 1.04; 95% CI 0.84-1.29), with findings being similar for GLP-1 RA and SGLT-2i.
Study details: The data come from a systematic review and network meta-analysis of 177 randomized controlled trials including 165,081 patients with T2D.
Disclosures: This study was supported by the National Natural Science Foundation, China, and others. The authors declared no conflicts of interest.
Source: Chai S et al. Risk of fracture with dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis combining 177 randomized controlled trials with a median follow-up of 26 weeks. Front Pharmacol. 2022;13:825417 (Jul 1). Doi: 10.3389/fphar.2022.825417
DPP-4 inhibitor but not GLP-1 RA raises risk for acute liver injury in T2D
Key clinical point: Dipeptidyl peptidase 4 inhibitors (DPP-4i), but not glucagon-like peptide 1 receptor agonists (GLP-1 RA), significantly increased the risk for acute liver injury in patients with type 2 diabetes (T2D) compared with sodium-glucose cotransporter-2 inhibitors (SGLT-2i).
Major finding: Compared with SGLT-2i, DPP-4i (hazard ratio [HR] 1.53; 95% CI 1.02-2.30), but not GLP-1 RA (HR 1.11; 95% CI 0.57-2.16), were associated with a higher risk for acute liver injury; however, the risk was significantly higher in women receiving DPP-4i (HR 3.22; 95% CI 1.67-6.21) and GLP-1 RA (HR 3.23; 95% CI 1.44-7.25).
Study details: Findings are from a population-based study including 2 new-user, active-comparator cohorts; the first cohort included 106,310 and 27,277 new users of DPP-4i and SGLT-2i, respectively, and the second cohort included 9470 and 26,936 new users of GLP-1 RA and SGLT-2i, respectively.
Disclosures: This study was funded by a Canadian Institutes of Health Research Foundation Scheme grant. L Azoulay declared receiving consulting fees from Janssen Pharmaceuticals and Pfizer outside this work.
Source: Pradhan R et al. Incretin-based drugs and the risk of acute liver injury among patients with type 2 diabetes. Diabetes Care. 2022 (Jul 22). Doi: 10.2337/dc22-0712
Key clinical point: Dipeptidyl peptidase 4 inhibitors (DPP-4i), but not glucagon-like peptide 1 receptor agonists (GLP-1 RA), significantly increased the risk for acute liver injury in patients with type 2 diabetes (T2D) compared with sodium-glucose cotransporter-2 inhibitors (SGLT-2i).
Major finding: Compared with SGLT-2i, DPP-4i (hazard ratio [HR] 1.53; 95% CI 1.02-2.30), but not GLP-1 RA (HR 1.11; 95% CI 0.57-2.16), were associated with a higher risk for acute liver injury; however, the risk was significantly higher in women receiving DPP-4i (HR 3.22; 95% CI 1.67-6.21) and GLP-1 RA (HR 3.23; 95% CI 1.44-7.25).
Study details: Findings are from a population-based study including 2 new-user, active-comparator cohorts; the first cohort included 106,310 and 27,277 new users of DPP-4i and SGLT-2i, respectively, and the second cohort included 9470 and 26,936 new users of GLP-1 RA and SGLT-2i, respectively.
Disclosures: This study was funded by a Canadian Institutes of Health Research Foundation Scheme grant. L Azoulay declared receiving consulting fees from Janssen Pharmaceuticals and Pfizer outside this work.
Source: Pradhan R et al. Incretin-based drugs and the risk of acute liver injury among patients with type 2 diabetes. Diabetes Care. 2022 (Jul 22). Doi: 10.2337/dc22-0712
Key clinical point: Dipeptidyl peptidase 4 inhibitors (DPP-4i), but not glucagon-like peptide 1 receptor agonists (GLP-1 RA), significantly increased the risk for acute liver injury in patients with type 2 diabetes (T2D) compared with sodium-glucose cotransporter-2 inhibitors (SGLT-2i).
Major finding: Compared with SGLT-2i, DPP-4i (hazard ratio [HR] 1.53; 95% CI 1.02-2.30), but not GLP-1 RA (HR 1.11; 95% CI 0.57-2.16), were associated with a higher risk for acute liver injury; however, the risk was significantly higher in women receiving DPP-4i (HR 3.22; 95% CI 1.67-6.21) and GLP-1 RA (HR 3.23; 95% CI 1.44-7.25).
Study details: Findings are from a population-based study including 2 new-user, active-comparator cohorts; the first cohort included 106,310 and 27,277 new users of DPP-4i and SGLT-2i, respectively, and the second cohort included 9470 and 26,936 new users of GLP-1 RA and SGLT-2i, respectively.
Disclosures: This study was funded by a Canadian Institutes of Health Research Foundation Scheme grant. L Azoulay declared receiving consulting fees from Janssen Pharmaceuticals and Pfizer outside this work.
Source: Pradhan R et al. Incretin-based drugs and the risk of acute liver injury among patients with type 2 diabetes. Diabetes Care. 2022 (Jul 22). Doi: 10.2337/dc22-0712
Exenatide as a new treatment option for youth with T2D
Key clinical point: Once-weekly exenatide was superior to placebo in improving glycemic control and was well tolerated in youth with type 2 diabetes (T2D) who were suboptimally controlled with current treatments. It had a safety profile similar to that in adults.
Major finding: At 24 weeks, the least squares mean change in the glycated hemoglobin level in the exenatide vs placebo group was −0.36% vs 0.49%, respectively, with a between-group difference of −0.85% (P = .012) showing the superiority of exenatide over placebo. Adverse events were reported by 61.0% and 73.9% of participants in the exenatide and placebo groups, respectively.
Study details: Findings are from a multicenter, parallel-group, phase 3 study including 72 patients with T2D suboptimally controlled with current treatments who were randomly assigned to receive once-weekly exenatide (n = 49) or placebo (n = 23).
Disclosures: This study was funded by AstraZeneca. N Shehadeh and O Doehring declared receiving support from AstraZeneca. The other authors declared being employees or holding stocks in AstraZeneca.
Source: Tamborlane WV et al. Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care. 2022;45(8):1833–1840 (Jul 26). Doi: 10.2337/dc21-2275
Key clinical point: Once-weekly exenatide was superior to placebo in improving glycemic control and was well tolerated in youth with type 2 diabetes (T2D) who were suboptimally controlled with current treatments. It had a safety profile similar to that in adults.
Major finding: At 24 weeks, the least squares mean change in the glycated hemoglobin level in the exenatide vs placebo group was −0.36% vs 0.49%, respectively, with a between-group difference of −0.85% (P = .012) showing the superiority of exenatide over placebo. Adverse events were reported by 61.0% and 73.9% of participants in the exenatide and placebo groups, respectively.
Study details: Findings are from a multicenter, parallel-group, phase 3 study including 72 patients with T2D suboptimally controlled with current treatments who were randomly assigned to receive once-weekly exenatide (n = 49) or placebo (n = 23).
Disclosures: This study was funded by AstraZeneca. N Shehadeh and O Doehring declared receiving support from AstraZeneca. The other authors declared being employees or holding stocks in AstraZeneca.
Source: Tamborlane WV et al. Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care. 2022;45(8):1833–1840 (Jul 26). Doi: 10.2337/dc21-2275
Key clinical point: Once-weekly exenatide was superior to placebo in improving glycemic control and was well tolerated in youth with type 2 diabetes (T2D) who were suboptimally controlled with current treatments. It had a safety profile similar to that in adults.
Major finding: At 24 weeks, the least squares mean change in the glycated hemoglobin level in the exenatide vs placebo group was −0.36% vs 0.49%, respectively, with a between-group difference of −0.85% (P = .012) showing the superiority of exenatide over placebo. Adverse events were reported by 61.0% and 73.9% of participants in the exenatide and placebo groups, respectively.
Study details: Findings are from a multicenter, parallel-group, phase 3 study including 72 patients with T2D suboptimally controlled with current treatments who were randomly assigned to receive once-weekly exenatide (n = 49) or placebo (n = 23).
Disclosures: This study was funded by AstraZeneca. N Shehadeh and O Doehring declared receiving support from AstraZeneca. The other authors declared being employees or holding stocks in AstraZeneca.
Source: Tamborlane WV et al. Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care. 2022;45(8):1833–1840 (Jul 26). Doi: 10.2337/dc21-2275
The Ethical Implications of Dermatology Residents Treating Attending Physicians
Residents are confronted daily with situations in clinic that require a foundation in medical ethics to assist in decision-making. Attending physicians require health care services and at times may seek care from resident physicians. If the attending physician has direct oversight over the resident, however, the ethics of the resident treating them need to be addressed. Although patients have autonomy to choose whoever they want as a physician, nonmaleficence dictates that the resident may forego treatment due to concerns for providing suboptimal care; however, this same attending may be treated under specific circumstances. This column explores the ethical implications of both situations.
The Ethical Dilemma of Treating an Attending
Imagine this scenario: You are in your resident general dermatology clinic seeing patients with an attending overseeing your clinical decisions following each encounter. You look on your schedule and see that the next patient is one of your pediatric dermatology attendings for a total-body skin examination (TBSE). You have never treated a physician that oversees you, and you ponder whether you should perform the examination or fetch your attending to perform the encounter alone.
This conundrum then brings other questions to mind: Would changing the reason for the appointment (ie, an acute problem vs a TBSE) alter your decision as to whether or not you would treat this attending? Would the situation be different if this was an attending in a different department?
Ethics Curriculum for Residents
Medical providers face ethical dilemmas daily, and dermatologists and dermatology residents are not excluded. Dermatoethics can provide a framework for the best approach to this hypothetical situation. To equip residents with resources on ethics and a cognitive framework to approach similar situations, the American Board of Dermatology has created an ethics curriculum for residents to learn over their 3 years of training.1
One study that analyzed the ethical themes portrayed in essays by fourth-year medical students showed that the most common themes included autonomy, social justice, nonmaleficence, beneficence, honesty, and respect.2 These themes must be considered in different permutations throughout ethical conundrums.
In the situation of an attending physician who supervises a resident in another clinic voluntarily attending the resident clinic, the physician is aware of the resident’s skills and qualifications and knows that supervision is being provided by an attending physician, which allows informed consent to be made, as a study by Unruh et al3 shows. The patient’s autonomy allows them to choose their treating provider.
However, there are several reasons why the resident may be hesitant to enter the room. One concern may be that during a TBSE the provider usually examines the patient’s genitals, rectum, and breasts.4 Because the resident knows the individual personally, the patient and/or the provider may be uncomfortable checking these areas, leaving a portion of the examination unperformed. This neglect may harm the patient (eg, a genital melanoma is missed), violating the tenant of nonmaleficence.
The effect of the medical hierarchy also should be considered. The de facto hierarchy of attendings supervising residents, interns, and medical students, with each group having some oversight over the next, can have positive effects on education and appropriate patient management but also can prove to be detrimental to the patient and provider in some circumstances. Studies have shown that residents may be less willing to disagree with their superior’s opinions for fear of negative reactions and harmful effects on their future careers.5-7 The hierarchy of medicine also can affect a resident’s moral judgement by intimidating the practitioner to perform tasks or make diagnoses they may not wish to make.5,6,8,9 For example, the resident may send a prescription for a medication that the attending requested despite no clear indication of need. This mingling of patient and supervisor roles can result in a resident treating their attending physician inconsistently with their standard of care.
Navigating the Ethics of Treating Family Members
The American Medical Association Code of Medical Ethics Opinions on Patient-Physician Relationships highlights treating family members as an important ethical topic. Although most residents and attendings are not biologically related, a familial-style relationship exists in many dermatology programs between attendings and residents due to the close-knit nature of dermatology programs. Diagnostic and treatment accuracy may be diminished by the discomfort or disbelief that a condition could affect someone the resident cares about.10
The American Medical Association also states that a physician can treat family members in an emergency situation or for short-term minor problems. If these 2 exceptions were to be extrapolated to apply to situations involving residents and attendings in addition to family, there would be situations where a dermatology resident could ethically treat their attending physician.10 If the attending physician was worried about a problem that was deemed potentially life-threatening, such as a rapidly progressive bullous eruption concerning for Stevens-Johnson syndrome following the initiation of a new medication, and they wanted an urgent evaluation and biopsy, an ethicist could argue that urgent treatment is medically indicated as deferring treatment could have negative consequences on the patient’s health. In addition, if the attending found a splinter in their finger following yardwork and needed assistance in removal, this also could be treated by their resident, as it is minimally invasive and has a finite conclusion.
Treating Nonsupervisory Attendings
In the case of performing a TBSE on an attending from another specialty, it would be acceptable and less ethically ambiguous if no close personal relationship existed between the two practitioners, as this patient would have no direct oversight over the resident physician.
Final Thoughts
Each situation that residents face may carry ethical implications with perspectives from the patient, provider, and bystanders. The above scenarios highlight specific instances that a dermatology resident may face and provide insight into how they may approach the situations. At the same time, it is important to remember that every situation is different and requires a unique approach. Fortunately,physicians—specifically dermatologists—are provided many resources to help navigate challenging scenarios.
Acknowledgments—The author thanks Jane M. Grant-Kels, MD (Farmington, Connecticut), for reviewing this paper and providing feedback to improve its content, as well as Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and article.
- Dermatoethics. American Board of Dermatology website. Accessed August 9, 2022. https://www.abderm.org/residents-and-fellows/dermatoethics
- House JB, Theyyunni N, Barnosky AR, et al. Understanding ethical dilemmas in the emergency department: views from medical students’ essays. J Emerg Med. 2015;48:492-498.
- Unruh KP, Dhulipala SC, Holt GE. Patient understanding of the role of the orthopedic resident. J Surg Educ. 2013;70:345-349.
- Grandhi R, Grant-Kels JM. Naked and vulnerable: the ethics of chaperoning full-body skin examinations. J Am Acad Dermatol. 2017;76:1221-1223.
- Salehi PP, Jacobs D, Suhail-Sindhu T, et al. Consequences of medical hierarchy on medical students, residents, and medical education in otolaryngology. Otolaryngol Head Neck Surg. 2020;163:906-914.
- Lomis KD, Carpenter RO, Miller BM. Moral distress in the third year of medical school: a descriptive review of student case reflections. Am J Surg. 2009;197:107-112.
- Troughton R, Mariano V, Campbell A, et al. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019;8:116.
- Chiu PP, Hilliard RI, Azzie G, et al. Experience of moral distress among pediatric surgery trainees. J Pediatr Surg. 2008;43:986-993.
- Martinez W, Lo B. Medical students’ experiences with medical errors: an analysis of medical student essays. Med Educ. 2008;42:733-741.
- Chapter 1. opinions on patient-physician relationships. American Medical Association website. Accessed on August 9, 2022. https://www.ama-assn.org/system/files/code-of-medical-ethics-chapter-1.pdf
Residents are confronted daily with situations in clinic that require a foundation in medical ethics to assist in decision-making. Attending physicians require health care services and at times may seek care from resident physicians. If the attending physician has direct oversight over the resident, however, the ethics of the resident treating them need to be addressed. Although patients have autonomy to choose whoever they want as a physician, nonmaleficence dictates that the resident may forego treatment due to concerns for providing suboptimal care; however, this same attending may be treated under specific circumstances. This column explores the ethical implications of both situations.
The Ethical Dilemma of Treating an Attending
Imagine this scenario: You are in your resident general dermatology clinic seeing patients with an attending overseeing your clinical decisions following each encounter. You look on your schedule and see that the next patient is one of your pediatric dermatology attendings for a total-body skin examination (TBSE). You have never treated a physician that oversees you, and you ponder whether you should perform the examination or fetch your attending to perform the encounter alone.
This conundrum then brings other questions to mind: Would changing the reason for the appointment (ie, an acute problem vs a TBSE) alter your decision as to whether or not you would treat this attending? Would the situation be different if this was an attending in a different department?
Ethics Curriculum for Residents
Medical providers face ethical dilemmas daily, and dermatologists and dermatology residents are not excluded. Dermatoethics can provide a framework for the best approach to this hypothetical situation. To equip residents with resources on ethics and a cognitive framework to approach similar situations, the American Board of Dermatology has created an ethics curriculum for residents to learn over their 3 years of training.1
One study that analyzed the ethical themes portrayed in essays by fourth-year medical students showed that the most common themes included autonomy, social justice, nonmaleficence, beneficence, honesty, and respect.2 These themes must be considered in different permutations throughout ethical conundrums.
In the situation of an attending physician who supervises a resident in another clinic voluntarily attending the resident clinic, the physician is aware of the resident’s skills and qualifications and knows that supervision is being provided by an attending physician, which allows informed consent to be made, as a study by Unruh et al3 shows. The patient’s autonomy allows them to choose their treating provider.
However, there are several reasons why the resident may be hesitant to enter the room. One concern may be that during a TBSE the provider usually examines the patient’s genitals, rectum, and breasts.4 Because the resident knows the individual personally, the patient and/or the provider may be uncomfortable checking these areas, leaving a portion of the examination unperformed. This neglect may harm the patient (eg, a genital melanoma is missed), violating the tenant of nonmaleficence.
The effect of the medical hierarchy also should be considered. The de facto hierarchy of attendings supervising residents, interns, and medical students, with each group having some oversight over the next, can have positive effects on education and appropriate patient management but also can prove to be detrimental to the patient and provider in some circumstances. Studies have shown that residents may be less willing to disagree with their superior’s opinions for fear of negative reactions and harmful effects on their future careers.5-7 The hierarchy of medicine also can affect a resident’s moral judgement by intimidating the practitioner to perform tasks or make diagnoses they may not wish to make.5,6,8,9 For example, the resident may send a prescription for a medication that the attending requested despite no clear indication of need. This mingling of patient and supervisor roles can result in a resident treating their attending physician inconsistently with their standard of care.
Navigating the Ethics of Treating Family Members
The American Medical Association Code of Medical Ethics Opinions on Patient-Physician Relationships highlights treating family members as an important ethical topic. Although most residents and attendings are not biologically related, a familial-style relationship exists in many dermatology programs between attendings and residents due to the close-knit nature of dermatology programs. Diagnostic and treatment accuracy may be diminished by the discomfort or disbelief that a condition could affect someone the resident cares about.10
The American Medical Association also states that a physician can treat family members in an emergency situation or for short-term minor problems. If these 2 exceptions were to be extrapolated to apply to situations involving residents and attendings in addition to family, there would be situations where a dermatology resident could ethically treat their attending physician.10 If the attending physician was worried about a problem that was deemed potentially life-threatening, such as a rapidly progressive bullous eruption concerning for Stevens-Johnson syndrome following the initiation of a new medication, and they wanted an urgent evaluation and biopsy, an ethicist could argue that urgent treatment is medically indicated as deferring treatment could have negative consequences on the patient’s health. In addition, if the attending found a splinter in their finger following yardwork and needed assistance in removal, this also could be treated by their resident, as it is minimally invasive and has a finite conclusion.
Treating Nonsupervisory Attendings
In the case of performing a TBSE on an attending from another specialty, it would be acceptable and less ethically ambiguous if no close personal relationship existed between the two practitioners, as this patient would have no direct oversight over the resident physician.
Final Thoughts
Each situation that residents face may carry ethical implications with perspectives from the patient, provider, and bystanders. The above scenarios highlight specific instances that a dermatology resident may face and provide insight into how they may approach the situations. At the same time, it is important to remember that every situation is different and requires a unique approach. Fortunately,physicians—specifically dermatologists—are provided many resources to help navigate challenging scenarios.
Acknowledgments—The author thanks Jane M. Grant-Kels, MD (Farmington, Connecticut), for reviewing this paper and providing feedback to improve its content, as well as Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and article.
Residents are confronted daily with situations in clinic that require a foundation in medical ethics to assist in decision-making. Attending physicians require health care services and at times may seek care from resident physicians. If the attending physician has direct oversight over the resident, however, the ethics of the resident treating them need to be addressed. Although patients have autonomy to choose whoever they want as a physician, nonmaleficence dictates that the resident may forego treatment due to concerns for providing suboptimal care; however, this same attending may be treated under specific circumstances. This column explores the ethical implications of both situations.
The Ethical Dilemma of Treating an Attending
Imagine this scenario: You are in your resident general dermatology clinic seeing patients with an attending overseeing your clinical decisions following each encounter. You look on your schedule and see that the next patient is one of your pediatric dermatology attendings for a total-body skin examination (TBSE). You have never treated a physician that oversees you, and you ponder whether you should perform the examination or fetch your attending to perform the encounter alone.
This conundrum then brings other questions to mind: Would changing the reason for the appointment (ie, an acute problem vs a TBSE) alter your decision as to whether or not you would treat this attending? Would the situation be different if this was an attending in a different department?
Ethics Curriculum for Residents
Medical providers face ethical dilemmas daily, and dermatologists and dermatology residents are not excluded. Dermatoethics can provide a framework for the best approach to this hypothetical situation. To equip residents with resources on ethics and a cognitive framework to approach similar situations, the American Board of Dermatology has created an ethics curriculum for residents to learn over their 3 years of training.1
One study that analyzed the ethical themes portrayed in essays by fourth-year medical students showed that the most common themes included autonomy, social justice, nonmaleficence, beneficence, honesty, and respect.2 These themes must be considered in different permutations throughout ethical conundrums.
In the situation of an attending physician who supervises a resident in another clinic voluntarily attending the resident clinic, the physician is aware of the resident’s skills and qualifications and knows that supervision is being provided by an attending physician, which allows informed consent to be made, as a study by Unruh et al3 shows. The patient’s autonomy allows them to choose their treating provider.
However, there are several reasons why the resident may be hesitant to enter the room. One concern may be that during a TBSE the provider usually examines the patient’s genitals, rectum, and breasts.4 Because the resident knows the individual personally, the patient and/or the provider may be uncomfortable checking these areas, leaving a portion of the examination unperformed. This neglect may harm the patient (eg, a genital melanoma is missed), violating the tenant of nonmaleficence.
The effect of the medical hierarchy also should be considered. The de facto hierarchy of attendings supervising residents, interns, and medical students, with each group having some oversight over the next, can have positive effects on education and appropriate patient management but also can prove to be detrimental to the patient and provider in some circumstances. Studies have shown that residents may be less willing to disagree with their superior’s opinions for fear of negative reactions and harmful effects on their future careers.5-7 The hierarchy of medicine also can affect a resident’s moral judgement by intimidating the practitioner to perform tasks or make diagnoses they may not wish to make.5,6,8,9 For example, the resident may send a prescription for a medication that the attending requested despite no clear indication of need. This mingling of patient and supervisor roles can result in a resident treating their attending physician inconsistently with their standard of care.
Navigating the Ethics of Treating Family Members
The American Medical Association Code of Medical Ethics Opinions on Patient-Physician Relationships highlights treating family members as an important ethical topic. Although most residents and attendings are not biologically related, a familial-style relationship exists in many dermatology programs between attendings and residents due to the close-knit nature of dermatology programs. Diagnostic and treatment accuracy may be diminished by the discomfort or disbelief that a condition could affect someone the resident cares about.10
The American Medical Association also states that a physician can treat family members in an emergency situation or for short-term minor problems. If these 2 exceptions were to be extrapolated to apply to situations involving residents and attendings in addition to family, there would be situations where a dermatology resident could ethically treat their attending physician.10 If the attending physician was worried about a problem that was deemed potentially life-threatening, such as a rapidly progressive bullous eruption concerning for Stevens-Johnson syndrome following the initiation of a new medication, and they wanted an urgent evaluation and biopsy, an ethicist could argue that urgent treatment is medically indicated as deferring treatment could have negative consequences on the patient’s health. In addition, if the attending found a splinter in their finger following yardwork and needed assistance in removal, this also could be treated by their resident, as it is minimally invasive and has a finite conclusion.
Treating Nonsupervisory Attendings
In the case of performing a TBSE on an attending from another specialty, it would be acceptable and less ethically ambiguous if no close personal relationship existed between the two practitioners, as this patient would have no direct oversight over the resident physician.
Final Thoughts
Each situation that residents face may carry ethical implications with perspectives from the patient, provider, and bystanders. The above scenarios highlight specific instances that a dermatology resident may face and provide insight into how they may approach the situations. At the same time, it is important to remember that every situation is different and requires a unique approach. Fortunately,physicians—specifically dermatologists—are provided many resources to help navigate challenging scenarios.
Acknowledgments—The author thanks Jane M. Grant-Kels, MD (Farmington, Connecticut), for reviewing this paper and providing feedback to improve its content, as well as Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and article.
- Dermatoethics. American Board of Dermatology website. Accessed August 9, 2022. https://www.abderm.org/residents-and-fellows/dermatoethics
- House JB, Theyyunni N, Barnosky AR, et al. Understanding ethical dilemmas in the emergency department: views from medical students’ essays. J Emerg Med. 2015;48:492-498.
- Unruh KP, Dhulipala SC, Holt GE. Patient understanding of the role of the orthopedic resident. J Surg Educ. 2013;70:345-349.
- Grandhi R, Grant-Kels JM. Naked and vulnerable: the ethics of chaperoning full-body skin examinations. J Am Acad Dermatol. 2017;76:1221-1223.
- Salehi PP, Jacobs D, Suhail-Sindhu T, et al. Consequences of medical hierarchy on medical students, residents, and medical education in otolaryngology. Otolaryngol Head Neck Surg. 2020;163:906-914.
- Lomis KD, Carpenter RO, Miller BM. Moral distress in the third year of medical school: a descriptive review of student case reflections. Am J Surg. 2009;197:107-112.
- Troughton R, Mariano V, Campbell A, et al. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019;8:116.
- Chiu PP, Hilliard RI, Azzie G, et al. Experience of moral distress among pediatric surgery trainees. J Pediatr Surg. 2008;43:986-993.
- Martinez W, Lo B. Medical students’ experiences with medical errors: an analysis of medical student essays. Med Educ. 2008;42:733-741.
- Chapter 1. opinions on patient-physician relationships. American Medical Association website. Accessed on August 9, 2022. https://www.ama-assn.org/system/files/code-of-medical-ethics-chapter-1.pdf
- Dermatoethics. American Board of Dermatology website. Accessed August 9, 2022. https://www.abderm.org/residents-and-fellows/dermatoethics
- House JB, Theyyunni N, Barnosky AR, et al. Understanding ethical dilemmas in the emergency department: views from medical students’ essays. J Emerg Med. 2015;48:492-498.
- Unruh KP, Dhulipala SC, Holt GE. Patient understanding of the role of the orthopedic resident. J Surg Educ. 2013;70:345-349.
- Grandhi R, Grant-Kels JM. Naked and vulnerable: the ethics of chaperoning full-body skin examinations. J Am Acad Dermatol. 2017;76:1221-1223.
- Salehi PP, Jacobs D, Suhail-Sindhu T, et al. Consequences of medical hierarchy on medical students, residents, and medical education in otolaryngology. Otolaryngol Head Neck Surg. 2020;163:906-914.
- Lomis KD, Carpenter RO, Miller BM. Moral distress in the third year of medical school: a descriptive review of student case reflections. Am J Surg. 2009;197:107-112.
- Troughton R, Mariano V, Campbell A, et al. Understanding determinants of infection control practices in surgery: the role of shared ownership and team hierarchy. Antimicrob Resist Infect Control. 2019;8:116.
- Chiu PP, Hilliard RI, Azzie G, et al. Experience of moral distress among pediatric surgery trainees. J Pediatr Surg. 2008;43:986-993.
- Martinez W, Lo B. Medical students’ experiences with medical errors: an analysis of medical student essays. Med Educ. 2008;42:733-741.
- Chapter 1. opinions on patient-physician relationships. American Medical Association website. Accessed on August 9, 2022. https://www.ama-assn.org/system/files/code-of-medical-ethics-chapter-1.pdf
Resident Pearls
- Dermatology residents should not perform total-body skin examinations on or provide long-term care to attending physicians that directly oversee them.
- Residents should only provide care to their attending physicians if the attending’s life is in imminent danger from delay of treatment or if it is a self-limited, minor problem.
FDA authorizes updated COVID boosters to target newest variants
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
Scattered Flesh-Colored Papules in a Linear Array in the Setting of Diffuse Skin Thickening
The Diagnosis: Scleromyxedema
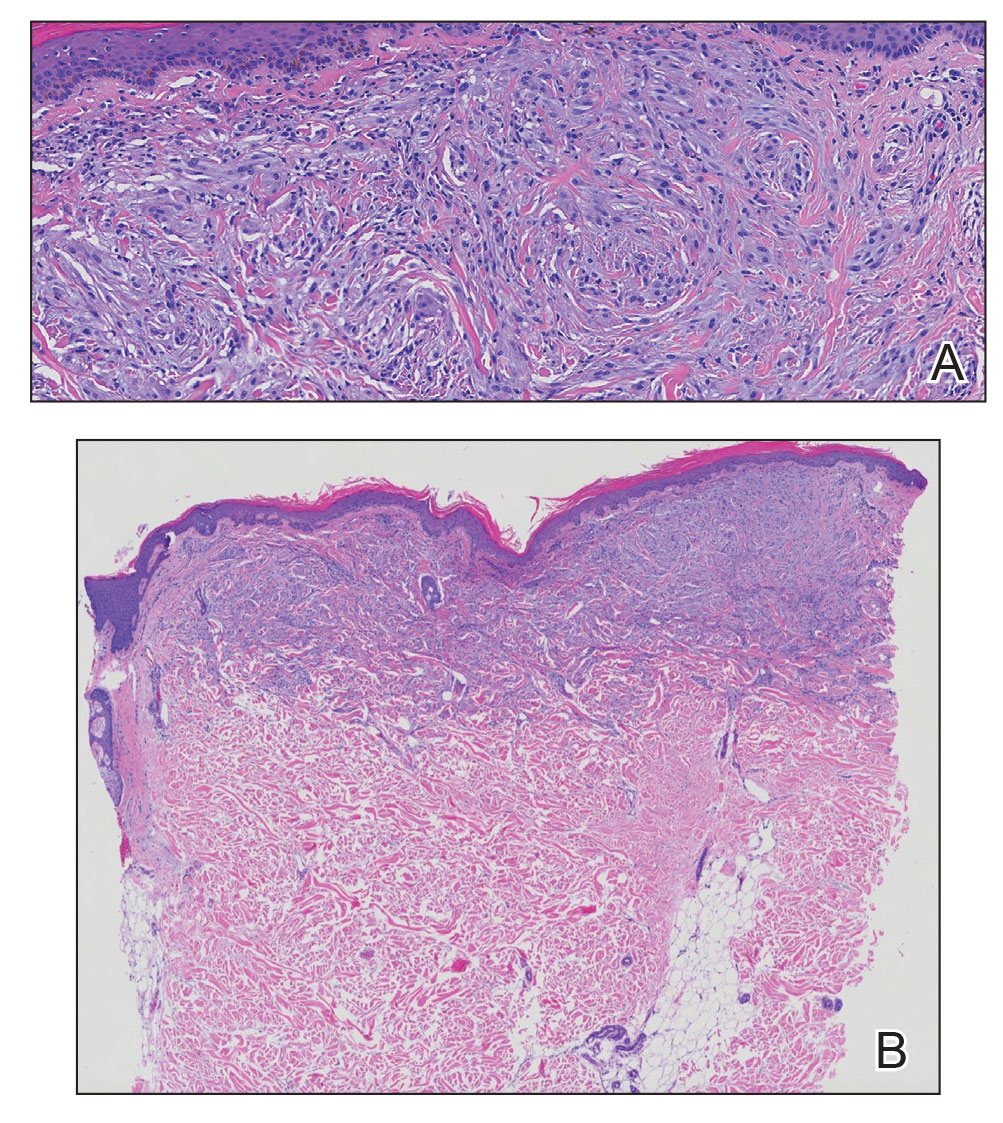
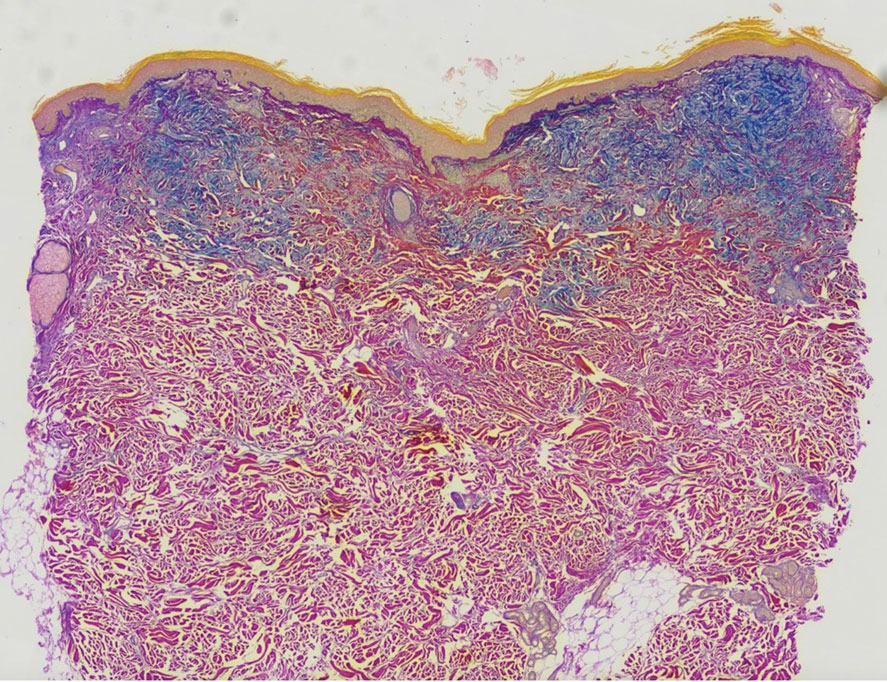
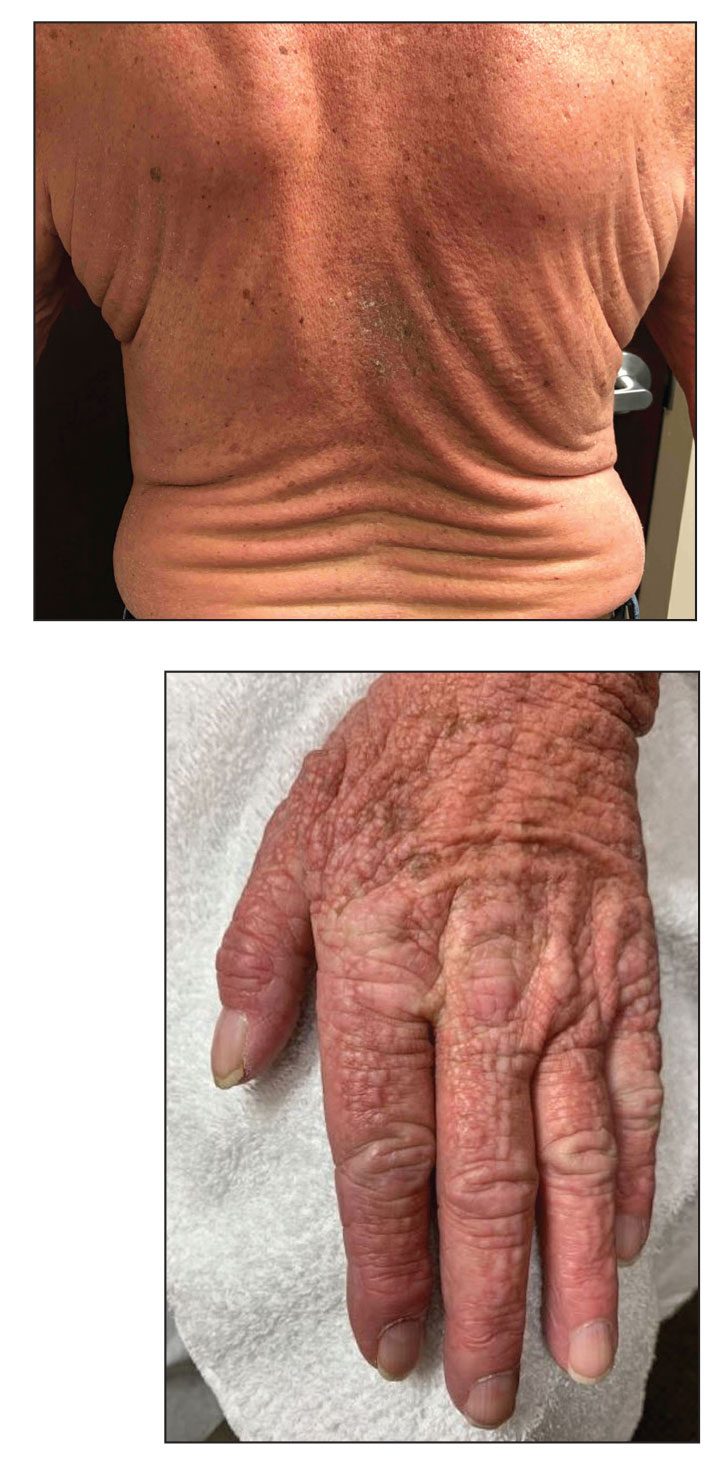
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.
Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2
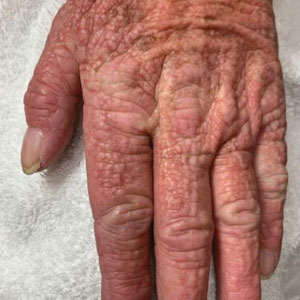
The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
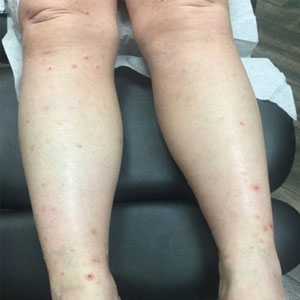
A 76-year-old man presented to our clinic with diffusely thickened and tightened skin that worsened over the course of 1 year, as well as numerous scattered small, firm, flesh-colored papules arranged in a linear pattern over the face, ears, neck, chest, abdomen, arms, hands, and knees. His symptoms progressed to include substantial skin thickening initially over the thighs followed by the arms, chest, back (top), and face. He developed confluent cobblestonelike plaques over the elbows and hands (bottom) and eventually developed decreased oral aperture limiting oral intake as well as decreased range of motion in the hands. The patient had a deep furrowed appearance of the brow accompanied by discrete, scattered, flesh-colored papules on the forehead and behind the ears. Deep furrows also were present on the back. When the proximal interphalangeal joints of the hands were extended, elevated rings with central depression were seen instead of horizontal folds.
Men at higher risk than are women for many cancers: Why?
Men have a significantly increased risk of developing 11 different cancers, and the risk is three times greater for men for certain cancers, including those of the esophagus, larynx, gastric cardia, and bladder.
But why?
“There are differences in cancer incidence that are not explained by environmental exposures alone,” said lead author Sarah S. Jackson, PhD, of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“This suggests that there are intrinsic biological differences between men and women that affect susceptibility to cancer,” she added in a statement.
The study was published online in the journal Cancer.
“Understanding the sex-related biologic mechanisms that lead to the male predominance of cancer at shared anatomic sites could have important implications for etiology and prevention,” the researchers suggested.
In an interview, Dr. Jackson said that the results “do not support changes to existing cancer prevention protocol” to address the disparities in cancer rates between men and women.
“More research is needed before any recommendations can be made,” she told this news organization. “For example, we need more research on the female immune response. If we can discover the mechanisms by which females have an immune advantage, we may be able to develop therapeutics to bolster the immune system to prevent and treat cancer.
“We also should start reporting our findings on cancer incidence, screening, and survival by sex to ensure that we are not missing important sex-specific associations.”
Comprehensive analyses
The researchers “should be applauded” for their “thorough and comprehensive analyses,” said the authors of an accompanying editorial, Jingqin R. Luo, PhD, and Graham A. Colditz, MD, DrPH, both from Washington University in St. Louis.
This study “has furthered our understanding on sex disparities in cancer, particularly in terms of the contributions of risk factors.”
However, as it included a largely elderly population and omitted comorbidities such as hypertension, hypercholesterolemia, and cardiovascular disease, the study has some “pertinent” limitations, they said.
The contribution of risk factors to sex disparities is “likely by means of complex interactions,” and the editorialists wondered if the statistical modeling used in the study was “over-stringent.” Other aspects that need to be considered include race as well as socioeconomic determinants of health, they suggested.
Nevertheless, they pointed out that sex disparities have been “observed in nearly every aspect of the cancer continuum,” and a “multifaceted approach” is needed to address them.
“Strategically including sex as a biologic variable should be enforced along the whole cancer continuum, from risk prediction and cancer primary prevention, cancer screening, and secondary prevention to cancer treatment and patient management,” Dr. Luo and Dr. Colditz concluded.
Details of the analysis
In their paper, Dr. Jackson and colleagues pointed out that the lifetime probability of developing cancer is “approximately equal” in men and women, at 40% vs. 39%.
However, the burden of cancer at shared anatomic sites is “significantly higher” in men, with the relative risk more than twofold higher than in women.
Some previous studies have pointed to differences in smoking, alcohol use, diet, access to and use of health care, and cancer screening between men and women, to explain the sex disparity, the researchers noted, but few have used individual-level data.
They therefore examined records from the prospective National Institutes of Health–AARP Diet and Health Study. This was launched in 1995 with a baseline questionnaire sent to 3.5 million members of AARP aged 50-71 years and living in six U.S. states. At the time, 617,119 returned the baseline questionnaire (a 17.6% response rate).
The current study focused on 334,905 participants who also completed a follow-up questionnaire between 1996 and 1997, which included more detailed information on diet and other lifestyle factors.
After excluding those who had already had a cancer diagnosis, self-reported poor health, extremely high or low caloric intake, or conflicting gender information, the researchers focused on 294,100 individuals (58% men, 42% women, median age 63.5 years).
After more than a decade of follow-up (mean of 11.5 person-years for men and 12.4 person-years for women), the team found 26,693 incident cancers at 21 shared anatomical cancer sites. Of those, 17,951 were in men and 8,742 in women.
The five most common cancers were nearly the same: the top three were lung, colon, and skin cancer in both men and women, and the fifth most common was kidney cancer in both. No. 4 for men was bladder cancer and for women it was pancreatic cancer.
After adjusting for demographic, lifestyle, and dietary covariates, the researchers found that the cancers with the highest male-to-female hazard ratios were esophageal adenocarcinoma, at 10.80, larynx cancer, at 3.53, gastric cardia cancer, at 3.49, and bladder cancer, at 3.33.
In contrast, men had a reduced risk of thyroid cancer, at a hazard ratio versus women of 0.55, and gallbladder cancer, at a hazard ratio of 0.33.
The team said that, overall, the increased relative risk among men was retained after adjustment for covariates for 11 cancers, but the relationship was no longer significant for many others, including lung, pancreas, small intestine, colon, oral cavity, esophagus-squamous cell carcinoma, and other head and neck cancers.
Cox proportional hazards regression modeling using the Peters-Belson method indicated that sex differences in risk factors explained at least some of the observed differences between men and women for seven cancer sites.
These were lung, colon, rectum, other biliary tract, skin, bladder, and esophageal adenocarcinoma, with 11.2% of the variance explained by risk factor differences for esophageal adenocarcinoma, rising to 49.4% for lung cancer.
There were no significant interactions between cancer rates at any of the anatomic sites and alcohol use, smoking status, body mass index, and age group.
Dr. Jackson told this news organization that sex differences in cancer outcomes “represents a very promising area of research” and the researchers “absolutely want to examine these associations further.”
“The dataset we used consists largely of non-Hispanic White adults. We’d like to see if the same sex bias is present in other ethnic groups, which would provide more evidence for a biological basis for these differences.
“We’d also like to explore the contribution of sex hormones and genetics to cancer incidence in future research,” Dr. Jackson added.
The study was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. Morgan A. Marks, PhD, performed this work as a postdoctoral fellow at the division of cancer epidemiology and genetics, National Cancer Institute. Dr. Marks reports relationships with Merck outside the submitted work.
The editorial was supported in part by a National Cancer Institute Cancer Center Support Grant. Dr. Luo reports grants from the National Institutes of Health outside the submitted work. Dr. Colditz reports grants from the Breast Cancer Research Foundation and the National Cancer Institute outside the submitted work.
No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Men have a significantly increased risk of developing 11 different cancers, and the risk is three times greater for men for certain cancers, including those of the esophagus, larynx, gastric cardia, and bladder.
But why?
“There are differences in cancer incidence that are not explained by environmental exposures alone,” said lead author Sarah S. Jackson, PhD, of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“This suggests that there are intrinsic biological differences between men and women that affect susceptibility to cancer,” she added in a statement.
The study was published online in the journal Cancer.
“Understanding the sex-related biologic mechanisms that lead to the male predominance of cancer at shared anatomic sites could have important implications for etiology and prevention,” the researchers suggested.
In an interview, Dr. Jackson said that the results “do not support changes to existing cancer prevention protocol” to address the disparities in cancer rates between men and women.
“More research is needed before any recommendations can be made,” she told this news organization. “For example, we need more research on the female immune response. If we can discover the mechanisms by which females have an immune advantage, we may be able to develop therapeutics to bolster the immune system to prevent and treat cancer.
“We also should start reporting our findings on cancer incidence, screening, and survival by sex to ensure that we are not missing important sex-specific associations.”
Comprehensive analyses
The researchers “should be applauded” for their “thorough and comprehensive analyses,” said the authors of an accompanying editorial, Jingqin R. Luo, PhD, and Graham A. Colditz, MD, DrPH, both from Washington University in St. Louis.
This study “has furthered our understanding on sex disparities in cancer, particularly in terms of the contributions of risk factors.”
However, as it included a largely elderly population and omitted comorbidities such as hypertension, hypercholesterolemia, and cardiovascular disease, the study has some “pertinent” limitations, they said.
The contribution of risk factors to sex disparities is “likely by means of complex interactions,” and the editorialists wondered if the statistical modeling used in the study was “over-stringent.” Other aspects that need to be considered include race as well as socioeconomic determinants of health, they suggested.
Nevertheless, they pointed out that sex disparities have been “observed in nearly every aspect of the cancer continuum,” and a “multifaceted approach” is needed to address them.
“Strategically including sex as a biologic variable should be enforced along the whole cancer continuum, from risk prediction and cancer primary prevention, cancer screening, and secondary prevention to cancer treatment and patient management,” Dr. Luo and Dr. Colditz concluded.
Details of the analysis
In their paper, Dr. Jackson and colleagues pointed out that the lifetime probability of developing cancer is “approximately equal” in men and women, at 40% vs. 39%.
However, the burden of cancer at shared anatomic sites is “significantly higher” in men, with the relative risk more than twofold higher than in women.
Some previous studies have pointed to differences in smoking, alcohol use, diet, access to and use of health care, and cancer screening between men and women, to explain the sex disparity, the researchers noted, but few have used individual-level data.
They therefore examined records from the prospective National Institutes of Health–AARP Diet and Health Study. This was launched in 1995 with a baseline questionnaire sent to 3.5 million members of AARP aged 50-71 years and living in six U.S. states. At the time, 617,119 returned the baseline questionnaire (a 17.6% response rate).
The current study focused on 334,905 participants who also completed a follow-up questionnaire between 1996 and 1997, which included more detailed information on diet and other lifestyle factors.
After excluding those who had already had a cancer diagnosis, self-reported poor health, extremely high or low caloric intake, or conflicting gender information, the researchers focused on 294,100 individuals (58% men, 42% women, median age 63.5 years).
After more than a decade of follow-up (mean of 11.5 person-years for men and 12.4 person-years for women), the team found 26,693 incident cancers at 21 shared anatomical cancer sites. Of those, 17,951 were in men and 8,742 in women.
The five most common cancers were nearly the same: the top three were lung, colon, and skin cancer in both men and women, and the fifth most common was kidney cancer in both. No. 4 for men was bladder cancer and for women it was pancreatic cancer.
After adjusting for demographic, lifestyle, and dietary covariates, the researchers found that the cancers with the highest male-to-female hazard ratios were esophageal adenocarcinoma, at 10.80, larynx cancer, at 3.53, gastric cardia cancer, at 3.49, and bladder cancer, at 3.33.
In contrast, men had a reduced risk of thyroid cancer, at a hazard ratio versus women of 0.55, and gallbladder cancer, at a hazard ratio of 0.33.
The team said that, overall, the increased relative risk among men was retained after adjustment for covariates for 11 cancers, but the relationship was no longer significant for many others, including lung, pancreas, small intestine, colon, oral cavity, esophagus-squamous cell carcinoma, and other head and neck cancers.
Cox proportional hazards regression modeling using the Peters-Belson method indicated that sex differences in risk factors explained at least some of the observed differences between men and women for seven cancer sites.
These were lung, colon, rectum, other biliary tract, skin, bladder, and esophageal adenocarcinoma, with 11.2% of the variance explained by risk factor differences for esophageal adenocarcinoma, rising to 49.4% for lung cancer.
There were no significant interactions between cancer rates at any of the anatomic sites and alcohol use, smoking status, body mass index, and age group.
Dr. Jackson told this news organization that sex differences in cancer outcomes “represents a very promising area of research” and the researchers “absolutely want to examine these associations further.”
“The dataset we used consists largely of non-Hispanic White adults. We’d like to see if the same sex bias is present in other ethnic groups, which would provide more evidence for a biological basis for these differences.
“We’d also like to explore the contribution of sex hormones and genetics to cancer incidence in future research,” Dr. Jackson added.
The study was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. Morgan A. Marks, PhD, performed this work as a postdoctoral fellow at the division of cancer epidemiology and genetics, National Cancer Institute. Dr. Marks reports relationships with Merck outside the submitted work.
The editorial was supported in part by a National Cancer Institute Cancer Center Support Grant. Dr. Luo reports grants from the National Institutes of Health outside the submitted work. Dr. Colditz reports grants from the Breast Cancer Research Foundation and the National Cancer Institute outside the submitted work.
No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Men have a significantly increased risk of developing 11 different cancers, and the risk is three times greater for men for certain cancers, including those of the esophagus, larynx, gastric cardia, and bladder.
But why?
“There are differences in cancer incidence that are not explained by environmental exposures alone,” said lead author Sarah S. Jackson, PhD, of the division of cancer epidemiology and genetics at the National Cancer Institute in Bethesda, Md.
“This suggests that there are intrinsic biological differences between men and women that affect susceptibility to cancer,” she added in a statement.
The study was published online in the journal Cancer.
“Understanding the sex-related biologic mechanisms that lead to the male predominance of cancer at shared anatomic sites could have important implications for etiology and prevention,” the researchers suggested.
In an interview, Dr. Jackson said that the results “do not support changes to existing cancer prevention protocol” to address the disparities in cancer rates between men and women.
“More research is needed before any recommendations can be made,” she told this news organization. “For example, we need more research on the female immune response. If we can discover the mechanisms by which females have an immune advantage, we may be able to develop therapeutics to bolster the immune system to prevent and treat cancer.
“We also should start reporting our findings on cancer incidence, screening, and survival by sex to ensure that we are not missing important sex-specific associations.”
Comprehensive analyses
The researchers “should be applauded” for their “thorough and comprehensive analyses,” said the authors of an accompanying editorial, Jingqin R. Luo, PhD, and Graham A. Colditz, MD, DrPH, both from Washington University in St. Louis.
This study “has furthered our understanding on sex disparities in cancer, particularly in terms of the contributions of risk factors.”
However, as it included a largely elderly population and omitted comorbidities such as hypertension, hypercholesterolemia, and cardiovascular disease, the study has some “pertinent” limitations, they said.
The contribution of risk factors to sex disparities is “likely by means of complex interactions,” and the editorialists wondered if the statistical modeling used in the study was “over-stringent.” Other aspects that need to be considered include race as well as socioeconomic determinants of health, they suggested.
Nevertheless, they pointed out that sex disparities have been “observed in nearly every aspect of the cancer continuum,” and a “multifaceted approach” is needed to address them.
“Strategically including sex as a biologic variable should be enforced along the whole cancer continuum, from risk prediction and cancer primary prevention, cancer screening, and secondary prevention to cancer treatment and patient management,” Dr. Luo and Dr. Colditz concluded.
Details of the analysis
In their paper, Dr. Jackson and colleagues pointed out that the lifetime probability of developing cancer is “approximately equal” in men and women, at 40% vs. 39%.
However, the burden of cancer at shared anatomic sites is “significantly higher” in men, with the relative risk more than twofold higher than in women.
Some previous studies have pointed to differences in smoking, alcohol use, diet, access to and use of health care, and cancer screening between men and women, to explain the sex disparity, the researchers noted, but few have used individual-level data.
They therefore examined records from the prospective National Institutes of Health–AARP Diet and Health Study. This was launched in 1995 with a baseline questionnaire sent to 3.5 million members of AARP aged 50-71 years and living in six U.S. states. At the time, 617,119 returned the baseline questionnaire (a 17.6% response rate).
The current study focused on 334,905 participants who also completed a follow-up questionnaire between 1996 and 1997, which included more detailed information on diet and other lifestyle factors.
After excluding those who had already had a cancer diagnosis, self-reported poor health, extremely high or low caloric intake, or conflicting gender information, the researchers focused on 294,100 individuals (58% men, 42% women, median age 63.5 years).
After more than a decade of follow-up (mean of 11.5 person-years for men and 12.4 person-years for women), the team found 26,693 incident cancers at 21 shared anatomical cancer sites. Of those, 17,951 were in men and 8,742 in women.
The five most common cancers were nearly the same: the top three were lung, colon, and skin cancer in both men and women, and the fifth most common was kidney cancer in both. No. 4 for men was bladder cancer and for women it was pancreatic cancer.
After adjusting for demographic, lifestyle, and dietary covariates, the researchers found that the cancers with the highest male-to-female hazard ratios were esophageal adenocarcinoma, at 10.80, larynx cancer, at 3.53, gastric cardia cancer, at 3.49, and bladder cancer, at 3.33.
In contrast, men had a reduced risk of thyroid cancer, at a hazard ratio versus women of 0.55, and gallbladder cancer, at a hazard ratio of 0.33.
The team said that, overall, the increased relative risk among men was retained after adjustment for covariates for 11 cancers, but the relationship was no longer significant for many others, including lung, pancreas, small intestine, colon, oral cavity, esophagus-squamous cell carcinoma, and other head and neck cancers.
Cox proportional hazards regression modeling using the Peters-Belson method indicated that sex differences in risk factors explained at least some of the observed differences between men and women for seven cancer sites.
These were lung, colon, rectum, other biliary tract, skin, bladder, and esophageal adenocarcinoma, with 11.2% of the variance explained by risk factor differences for esophageal adenocarcinoma, rising to 49.4% for lung cancer.
There were no significant interactions between cancer rates at any of the anatomic sites and alcohol use, smoking status, body mass index, and age group.
Dr. Jackson told this news organization that sex differences in cancer outcomes “represents a very promising area of research” and the researchers “absolutely want to examine these associations further.”
“The dataset we used consists largely of non-Hispanic White adults. We’d like to see if the same sex bias is present in other ethnic groups, which would provide more evidence for a biological basis for these differences.
“We’d also like to explore the contribution of sex hormones and genetics to cancer incidence in future research,” Dr. Jackson added.
The study was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. Morgan A. Marks, PhD, performed this work as a postdoctoral fellow at the division of cancer epidemiology and genetics, National Cancer Institute. Dr. Marks reports relationships with Merck outside the submitted work.
The editorial was supported in part by a National Cancer Institute Cancer Center Support Grant. Dr. Luo reports grants from the National Institutes of Health outside the submitted work. Dr. Colditz reports grants from the Breast Cancer Research Foundation and the National Cancer Institute outside the submitted work.
No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Angiolymphoid Hyperplasia with Eosinophilia in a Patient With Coccidioidomycosis
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.
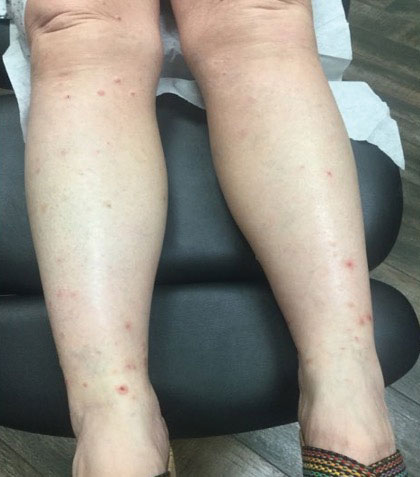
Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.
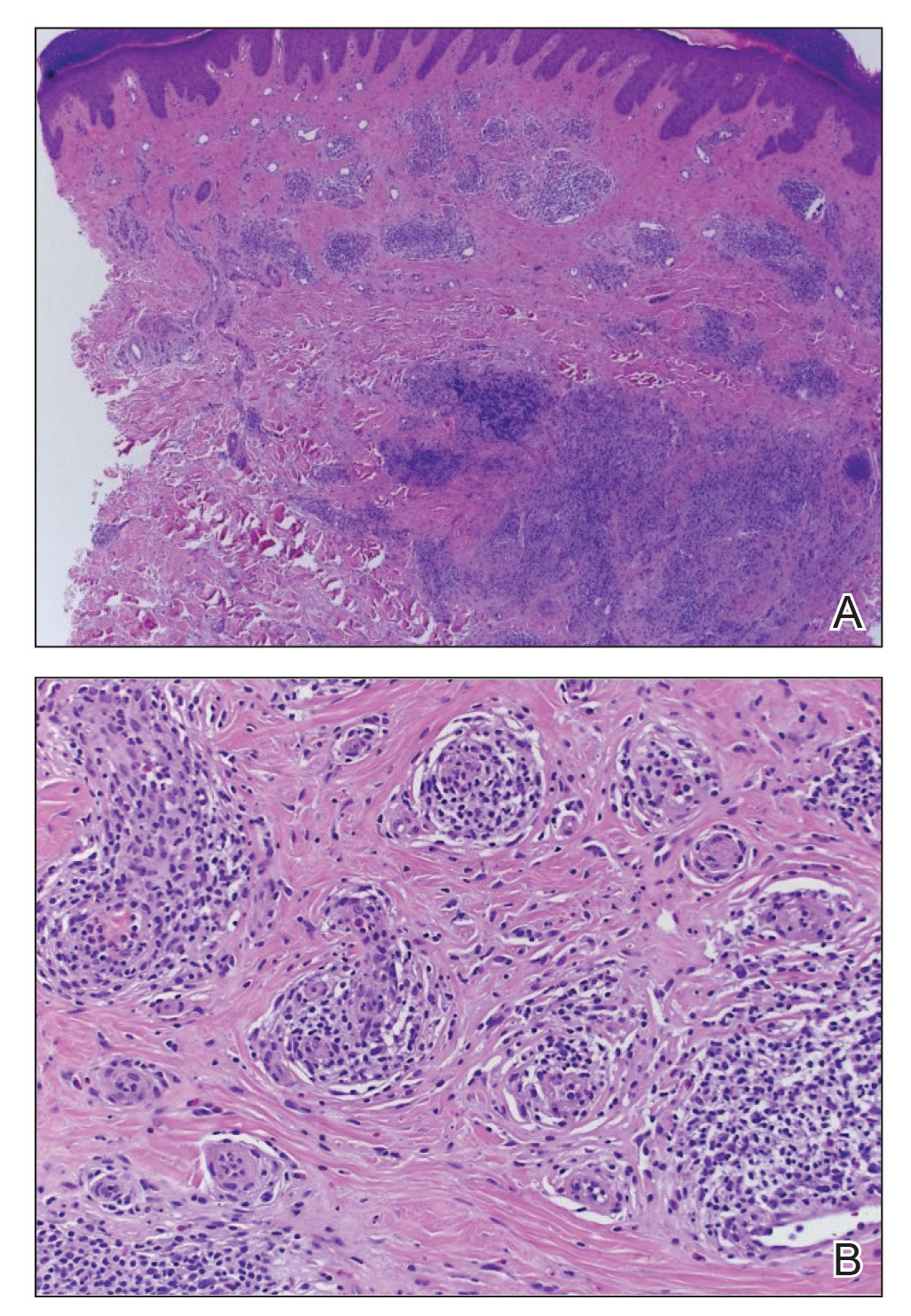
The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.
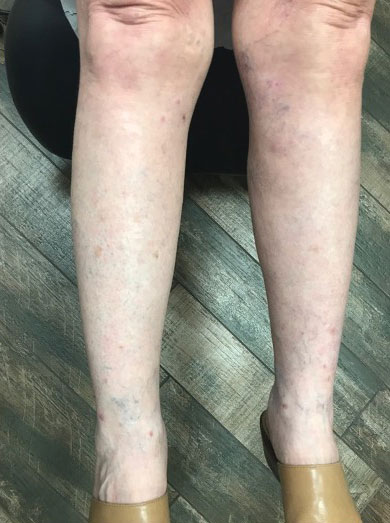
Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.

Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.

The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.

Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare nodular unencapsulated mass that is characterized by benign anomalous vascular hyperplasia of epithelioidlike endothelial cells attached to dilated blood vessels. The mass is surrounded by lymphocytes and eosinophils that can present clinically as papules, plaques, or nodules.1 The etiology of ALHE is unknown; it is hypothesized that it is a vascular neoplasm or a lymphoproliferative disorder.
Coccidioidomycosis (CM) is a prevalent deep fungal infection endemic to the southwestern United States caused by Coccidioides immitis and Coccidioides posadasii. Infection can occur from direct inoculation through abrasions or direct trauma but usually occurs through the inhalation of spores and can result in a reactive rash (eg, Sweet syndrome, erythema nodosum, interstitial granulomatous dermatitis).2 Coccidioidomycosis also can result in respiratory pneumonia and dissemination from pulmonary infection of the skin. As such, it is important to distinguish CM and its immunologically mediated eruptions for accurate diagnosis and treatment.
We report a novel case of ALHE as a reactive dermatologic presentation in a patient with CM.
Case Report
A 72-year-old woman presented to the dermatology clinic with itchy papules and plaques on the arms and legs of 17 years’ duration. Her medical history included coronary artery disease and hypercholesterolemia as well as a remote history of cutaneous marginal zone B-cell lymphoma of the nose, which was confirmed by histology and treated more than 10 years prior and has remained in remission for 6 years. Her current medications included aspirin, atorvastatin, lisinopril, and metoprolol succinate.
Our patient first presented to our dermatology clinic for itchy nodules and papules on the legs and arms. The patient previously had been seen by another dermatologist 2 months prior for the same condition. At that time, biopsies of the lesions were reported as prurigo nodules. Physical examination at the current presentation revealed round, pink to flesh-colored, raised papules and plaques scattered on the arms and legs (Figure 1). The differential diagnosis included lymphomatoid papulosis, cutaneous B-cell lymphoma, pseudolymphoma, cutaneous CM, and papular mucinosis.

Four-mm punch biopsies of the right proximal pretibial region and left knee region were taken and sent for histologic analysis, direct immunofluorescence testing, and tissue culture. Testing for atypical mycobacteria and deep fungal infection was negative; bacterial cultures and sensitivity testing were negative. Direct immunofluorescence testing was negative. Microscopic examination of material from the right proximal pretibial region showed widely dilated, variously shaped, large blood vessels in a multinodular pattern; the vessels also were surrounded by an inflammatory cell infiltrate containing eosinophils. Histologic findings were consistent with ALHE.
Subsequent biopsies were completed 2 weeks and 1 month from the initial presentation. Both histology reports—from 2 different histopathology laboratories—were consistent with ALHE (Figure 2). Additional work-up during the patient’s initial visit to our clinic for the rash included CM serologic testing, which demonstrated IgM and IgG antibodies. Subsequently, chest radiography revealed a 2.2×2.3-cm mass in the right lower lobe of the lung. Follow-up computed tomography 1 month later confirmed the nodule in the same area to be 2.3×2.1×1.8 cm.

The patient was referred to pulmonology and was treated for pulmonary CM with oral fluconazole 200 mg twice daily for 4 months. Initial treatment also included clobetasol cream 0.05% applied twice daily, which did not produce marked improvement in pruritus. Narrowband UVB phototherapy was attempted, but the patient could not complete the course because of travel time to the office; however, the patient’s ALHE improved considerably with the fluconazole treatment for pulmonary CM.
Oral doxycycline 100 mg twice daily was added to the fluconazole 2 months after her initial visit to our office, which kept the ALHE at bay and helped with the pruritus (Figure 3). Pulmonology and primary care comanaged the pulmonary CM with oral fluconazole 200 mg twice daily. Repeat serologic testing for CM was negative for IgG and IgM after 14 months since the initial visit to the office.

Comment
Pulmonary CM infection has varying dermatologic manifestations. A PubMed search of articles indexed for MEDLINE using the terms ALHE and coccidioidomycosis yielded no case reports; in fact, there have been few reported cases of ALHE at all. Notable conditions associated with ALHE include membranous nephropathy and arteriovenous malformations treated with corticosteroids and surgery, respectively.3,4 Our case is a rare presentation of CM infection manifesting with ALHE. Following treatment and remission for our patient’s CM infection, the ALHE lesion decreased in size.
Standard treatment of uncomplicated CM involves azole antifungals, typically oral fluconazole or itraconazole 400 to 600 mg/d. In more severe cases (eg, immunocompromised patients) amphotericin B can be used.5 Our patient was treated with oral fluconazole 200 mg twice daily for 4 months.
In the literature, treatment via surgical excision, steroid injection, pulsed-dye laser therapy, and radiotherapy also has been described.6-8 Antibiotics including clindamycin, doxycycline, and amoxicillin-clavulanate also have been shown to be effective.9
In our patient, ALHE improved when oral doxycycline 100 mg twice daily was added to the oral fluconazole. In fact, after 4 months of treatment, the CM infection and ALHE lesions both improved to a point at which the lesions were not visible. When those lesions recurred 15 months later, they responded with another course of doxycycline and fluconazole.
Upon recurrence, the patient was asked to have her care transferred to her pulmonologist, who then managed the fluconazole regimen. During the pulmonologist’s workup, no peripheral eosinophilia was found. This is important because eosinophils can be a marker for CM infection; in this case, however, the ALHE lesion was a reactive process to the infection. Classically known to play a reactive role in fungal infection, these white blood cells demonstrate reactivity to the environmental fungus Alternaria alternata by contact-dependent killing, utilizing β2 integrins and CD11b to recognize and adhere to β-glucan. Eosinophils react through contact-dependent killing, releasing cytotoxic granule proteins and proinflammatory mediators, and have been documented to occur in CM and Paracoccidioides brasiliensis infection, in which they deposit major basic protein on the organism.10 Most pertinent to our case with ALHE and CM is the ability of eosinophils to communicate with other immune cells. Eosinophils play a role in the active inflammation of CM through cytokine signaling, which may propagate formation of ALHE.
The function of eosinophils in ALHE is poorly understood; it is unclear whether they act as a primary driver of pathogenesis or are simply indicators of secondary infiltration or infection. Our review of the current literature suggests that eosinophils are unnecessary for progression of ALHE but might be involved at its onset. As reported, even monoclonal antibody therapy (eg, mepolizumab and benralizumab) that effectively depletes eosinophil levels by negating IL-5 signaling do not slow progression of ALHE.11 Symptomatic changes are modest at best (ie, simply softening the ALHE nodules).
Our patient had no peripheral eosinophilia, suggesting that the onset of ALHE might not be caused by eosinophilia but a different inflammatory process—in this patient, by CM. Because peripheral eosinophilia was not seen in our patient, the presence of eosinophils in the ALHE lesion likely is unnecessary for its onset or progression but is a secondary process that exacerbates the lesion. The pathogenesis is unknown but could be directed toward lymphocytes and plasma cells, with eosinophils as part of the dynamic process.11
Conclusion
Because reports of an association between CM and ALHE are limited, our case is distinguished by a unique clinical presentation of ALHE. When a patient is given a diagnosis of ALHE, it therefore is important to consider exposure to CM as a cause, especially in patients who reside in or travel to a region where CM is endemic.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14. doi:10.1111/j.1365-2133.1969.tb15914.x
- DiCaudo D. Coccidioidomycosis. Semin Cutan Med Surg. 2014;33:140-145. doi:10.12788/j.sder.0111
- Onishi Y, Ohara K. Angiolymphoid hyperplasia with eosinophilia associated with arteriovenous malformation: a clinicopathological correlation with angiography and serial estimation of serum levels of renin, eosinophil cationic protein and interleukin 5. Br J Dermatol. 1999;140:1153-1156. doi:10.1046/j.1365-2133.1999.02880.x
- Matsumoto A, Matsui I, Namba T, et al. VEGF-A links angiolymphoid hyperplasia with eosinophilia (ALHE) to THSD7A membranous nephropathy: a report of 2 cases. Am J Kidney Dis. 2019;73:880-885. doi:10.1053/j.ajkd.2018.10.009
- Bercovitch RS, Catanzaro A, Schwartz BS, et al. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. doi:10.1093/cid/cir410
- Youssef A, Hasan AR, Youssef Y, et al. Angiolymphoid hyperplasia with eosinophilia: a case report. J Med Case Rep. 2018;12:89. doi:10.1186/s13256-018-1599-x
- Abrahamson TG, Davis DA. Angiolymphoid hyperplasia witheosinophilia responsive to pulsed dye laser. J Am Acad Dermatol. 2003;49(2 suppl case reports):S195-S196. doi:10.1067/mjd.2003.314
- Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: when a traditional therapy is still up-to-date. Case Rep Dermatol. 2011;3:64-67. doi:10.1159/000323182
- Cleveland E. Atypical presentation of angiolymphomatous hyperplasia with eosinophilia. J Am Acad Dermatol. 2018;79(3 suppl 1):AB53. doi:10.1016/j.jaad.2018.05.249
- Ravin KA, Loy M. The eosinophil in infection. Clin Rev Allergy Immunol. 2015;50:214-227. doi:10.1007/s12016-015-8525-4
- Grünewald M, Stölzl D, Wehkamp U, et al. Role of eosinophils in angiolymphoid hyperplasia with eosinophilia. JAMA Dermatol. 2021;157:1241-1243. doi:10.1001/jamadermatol.2021.2732
Practice Points
- Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare entity of unknown etiology.
- There is an association between ALHE and coccidioidomycosis (CM). Patients who present with ALHE and reside in a CM-endemic region should be examined for CM.