User login
How do you live with COVID? One doctor’s personal experience
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
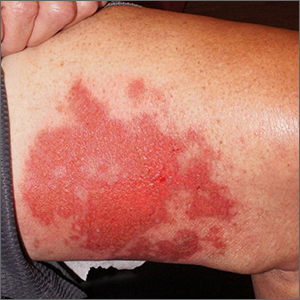
Leg rash
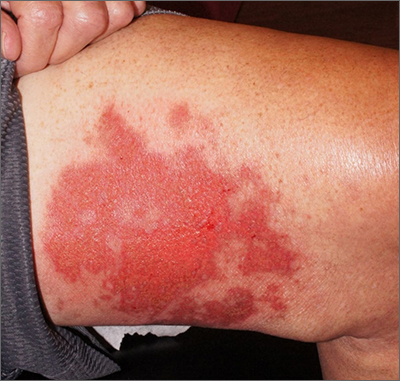
Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5

Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Punch biopsies for standard pathology and direct immunofluorescence were performed and ruled out vesiculobullous disease. Further conversation with the patient revealed that this was a phototoxic drug eruption that resulted from a medication mix-up. The patient had intended to treat an eczema flare with a topical steroid but had inadvertently applied 5-fluorouracil (5-FU), which he had left over from a previous bout of actinic keratosis. While selective to precancerous cells with rapid DNA replication, 5-FU can trigger a significant photodermatitis when applied to heavily sun-exposed skin.
Phototoxic skin reactions can be an adverse result of multiple systemic and topical therapies. Common systemic examples include amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, isotretinoin, nalidixic acid, naproxen, piroxicam, tetracycline, thioridazine, vemurafenib, and voriconazole.1 Topical examples include retinoids, levulinic acid, and 5-FU. Treatment requires that the patient stop the offending medication and use photoprotection. The patient followed this protocol and his erosions resolved over the course of a few weeks.
This case demonstrates that topical therapies, like systemic medications, can have chemical names that are confusing to patients. Further complicating matters can be the practice of folding metal tubes of cream over their life of use, thus obscuring the label.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5
1. Blakely KM, Drucker AM, Rosen CF. Drug-induced photosensitivity-an update: culprit drugs, prevention, and management. Drug Saf. 2019;42:827-847. doi: 10.1007/s40264-019-00806-5
Ublituximab bests teriflunomide in head-to-head clinical trials
Study shows ublituximab’s superiority over teriflunomide in suppressing MS relapses and MRI lesions.
Patients with relapsing multiple sclerosis (MS) treated with intravenous ublituximab had fewer relapses and brain lesions compared with those treated with oral teriflunomide, although both therapies resulted in similar rates of worsening disability, according to results of the two identical phase 3 ULTIMATE I and II trials.
“In these two 96-week trials involving participants with MS, annualized relapse rates were lower with intravenous ublituximab than with oral teriflunomide. Ublituximab was associated with infusion-related reactions. Larger and longer trials are required to determine the efficacy and safety of ublituximab in patients with relapsing MS, including comparison with other disease-modifying treatments such as existing anti-CD20 monoclonal antibodies,” noted lead author Lawrence Steinman, MD, professor of neurology and neurological sciences, pediatrics, and genetics at Stanford (Calif.) University, and colleagues.
The results, published in the New England Journal of Medicine, pave the way for ublituximab’s approval as the third high-efficacy anti-CD20 monoclonal antibody to treat relapsing forms of MS, predicted Patricia Coyle, MD, director of the MS Comprehensive Care Center, and professor of neurology, at Stony Brook (N.Y.) Neurosciences Institute, who was not involved in the research. Ublituximab will “widen the anti-CD20 monoclonal choices for MS, and should directly compete with ocrelizumab and ofatumumab,” she said.
Two trials
The double-blind, double-dummy ULTIMATE I and II trials enrolled 549 and 545 participants respectively, with a median follow-up of 95 weeks. Subjects, aged between 18 and 55 years, were randomized to receive either oral placebo and intravenous ublituximab (150 mg on day 1, followed by 450 mg on day 15 and at weeks 24, 48, and 72), or oral teriflunomide (14 mg once daily) and intravenous placebo. The primary endpoint was the annualized relapse rate, defined as the number of confirmed MS relapses per participant-year, with a range of secondary end points including number of lesions on magnetic resonance imaging (MRI) by 96 weeks, and worsening of disability confirmed at 12 weeks.
Prevention and management of infusion-related reactions was with oral antihistamine and dexamethasone, administered 30 to 60 minutes before each intravenous dose of ublituximab or placebo, as well as reductions in infusion flow rates and discretionary acetaminophen.
Results for the primary endpoint in ULTIMATE I showed the adjusted annualized relapse rate over a period of 96 weeks was 0.08 in the ublituximab group and 0.19 in the teriflunomide group (rate ratio, 0.41; P < .001). Corresponding rates for ULTIMATE II were 0.09 and 0.18 (rate ratio, 0.51; P = .002).
The mean number of lesions in both ublituximab arms of the trials was 0.02 and 0.01 compared with 0.49 and 0.25 in the teriflunomide arms (rate ratios 0.03 and 0.04 respectively; P < .001 for both).
Similar disability worsening in both groups
A pooled analysis of the two trials showed worsening disability in 5.2% of the ublituximab group, and 5.9% of the teriflunomide group (hazard ratio, 0.84; P = 0.51). “In both trials, teriflunomide was associated with a numerically lower rate of worsening of disability than that reported in previous studies with this drug, but no conclusions can be drawn from these comparisons,” noted the authors.
Infusion-related reactions occurred in 47.7% of the participants in the ublituximab group, consisting mainly of mild to moderate pyrexia, headache, chills, and influenza-like illness. “The reactions may have been related to cytokine release from immune cells (B and NK cells) on interaction of the Fc antibody domain with Fc gamma receptors on effector cells,” they suggested.
Although no opportunistic infections occurred, a higher frequency of infections, including serious infections, was observed with ublituximab (5.0%) than with teriflunomide (2.9%).
While the ULTIMATE trials showed no difference between ublituximab and teriflunomide in confirmed worsening of disability, only a small percentage of participants in either arm showed deterioration, Dr. Coyle remarked. “In a relatively short trial (96 weeks), in a relapsing population on active treatment, this result was not surprising … If the study was bigger, or longer it would increase the chances of seeing a progressive slow worsening component to affect the EDSS [Expanded Disability Status Scale],” she added.
Equivalent efficacy
Ultimately, “it appears likely” that ublituximab is “equivalent in efficacy” to the earlier anti-CD20 agents ocrelizumab and ofatumumab, Dr. Coyle said. While all three agents target B-cells, “ublituximab targets a novel CD20 binding site, and is bioengineered to have a particularly potent antibody dependent cell cytotoxicity lysis mechanism,” she added. “It has been touted to ultimately allow a short infusion of 1 hour.”
Although the serious infection rate is slightly higher with ublituximab (5.0% vs. 2.5% for ofatumumab, and 1.3% for ocrelizumab), “it is still low,” and infusion-related reactions are also higher with ublituximab, she added (47.7% vs. 20.2% and 34.3%, respectively). She suggested factors that might influence which treatment is chosen for a given patient might include cost, convenience, whether it is more or less likely to cause low IgG, interference with vaccination, or influence on cancer or COVID risk.
The trials were supported by TG Therapeutics.
Dr. Coyle has received consulting fees from Accordant, Biogen, Bristol Myers Squibb, Celgene, Genentech/Roche, GlaxoSmithKline, Horizon, Janssen, Novartis, Sanofi Genzyme, and Viela Bio and grant funding from Actelion, Alkermes, Bristol Myers Squibb, CorEvitas LLD, Genentech/Roche, Sanofi Genzyme, MedDay, NINDS, and Novartis.
Study shows ublituximab’s superiority over teriflunomide in suppressing MS relapses and MRI lesions.
Study shows ublituximab’s superiority over teriflunomide in suppressing MS relapses and MRI lesions.
Patients with relapsing multiple sclerosis (MS) treated with intravenous ublituximab had fewer relapses and brain lesions compared with those treated with oral teriflunomide, although both therapies resulted in similar rates of worsening disability, according to results of the two identical phase 3 ULTIMATE I and II trials.
“In these two 96-week trials involving participants with MS, annualized relapse rates were lower with intravenous ublituximab than with oral teriflunomide. Ublituximab was associated with infusion-related reactions. Larger and longer trials are required to determine the efficacy and safety of ublituximab in patients with relapsing MS, including comparison with other disease-modifying treatments such as existing anti-CD20 monoclonal antibodies,” noted lead author Lawrence Steinman, MD, professor of neurology and neurological sciences, pediatrics, and genetics at Stanford (Calif.) University, and colleagues.
The results, published in the New England Journal of Medicine, pave the way for ublituximab’s approval as the third high-efficacy anti-CD20 monoclonal antibody to treat relapsing forms of MS, predicted Patricia Coyle, MD, director of the MS Comprehensive Care Center, and professor of neurology, at Stony Brook (N.Y.) Neurosciences Institute, who was not involved in the research. Ublituximab will “widen the anti-CD20 monoclonal choices for MS, and should directly compete with ocrelizumab and ofatumumab,” she said.
Two trials
The double-blind, double-dummy ULTIMATE I and II trials enrolled 549 and 545 participants respectively, with a median follow-up of 95 weeks. Subjects, aged between 18 and 55 years, were randomized to receive either oral placebo and intravenous ublituximab (150 mg on day 1, followed by 450 mg on day 15 and at weeks 24, 48, and 72), or oral teriflunomide (14 mg once daily) and intravenous placebo. The primary endpoint was the annualized relapse rate, defined as the number of confirmed MS relapses per participant-year, with a range of secondary end points including number of lesions on magnetic resonance imaging (MRI) by 96 weeks, and worsening of disability confirmed at 12 weeks.
Prevention and management of infusion-related reactions was with oral antihistamine and dexamethasone, administered 30 to 60 minutes before each intravenous dose of ublituximab or placebo, as well as reductions in infusion flow rates and discretionary acetaminophen.
Results for the primary endpoint in ULTIMATE I showed the adjusted annualized relapse rate over a period of 96 weeks was 0.08 in the ublituximab group and 0.19 in the teriflunomide group (rate ratio, 0.41; P < .001). Corresponding rates for ULTIMATE II were 0.09 and 0.18 (rate ratio, 0.51; P = .002).
The mean number of lesions in both ublituximab arms of the trials was 0.02 and 0.01 compared with 0.49 and 0.25 in the teriflunomide arms (rate ratios 0.03 and 0.04 respectively; P < .001 for both).
Similar disability worsening in both groups
A pooled analysis of the two trials showed worsening disability in 5.2% of the ublituximab group, and 5.9% of the teriflunomide group (hazard ratio, 0.84; P = 0.51). “In both trials, teriflunomide was associated with a numerically lower rate of worsening of disability than that reported in previous studies with this drug, but no conclusions can be drawn from these comparisons,” noted the authors.
Infusion-related reactions occurred in 47.7% of the participants in the ublituximab group, consisting mainly of mild to moderate pyrexia, headache, chills, and influenza-like illness. “The reactions may have been related to cytokine release from immune cells (B and NK cells) on interaction of the Fc antibody domain with Fc gamma receptors on effector cells,” they suggested.
Although no opportunistic infections occurred, a higher frequency of infections, including serious infections, was observed with ublituximab (5.0%) than with teriflunomide (2.9%).
While the ULTIMATE trials showed no difference between ublituximab and teriflunomide in confirmed worsening of disability, only a small percentage of participants in either arm showed deterioration, Dr. Coyle remarked. “In a relatively short trial (96 weeks), in a relapsing population on active treatment, this result was not surprising … If the study was bigger, or longer it would increase the chances of seeing a progressive slow worsening component to affect the EDSS [Expanded Disability Status Scale],” she added.
Equivalent efficacy
Ultimately, “it appears likely” that ublituximab is “equivalent in efficacy” to the earlier anti-CD20 agents ocrelizumab and ofatumumab, Dr. Coyle said. While all three agents target B-cells, “ublituximab targets a novel CD20 binding site, and is bioengineered to have a particularly potent antibody dependent cell cytotoxicity lysis mechanism,” she added. “It has been touted to ultimately allow a short infusion of 1 hour.”
Although the serious infection rate is slightly higher with ublituximab (5.0% vs. 2.5% for ofatumumab, and 1.3% for ocrelizumab), “it is still low,” and infusion-related reactions are also higher with ublituximab, she added (47.7% vs. 20.2% and 34.3%, respectively). She suggested factors that might influence which treatment is chosen for a given patient might include cost, convenience, whether it is more or less likely to cause low IgG, interference with vaccination, or influence on cancer or COVID risk.
The trials were supported by TG Therapeutics.
Dr. Coyle has received consulting fees from Accordant, Biogen, Bristol Myers Squibb, Celgene, Genentech/Roche, GlaxoSmithKline, Horizon, Janssen, Novartis, Sanofi Genzyme, and Viela Bio and grant funding from Actelion, Alkermes, Bristol Myers Squibb, CorEvitas LLD, Genentech/Roche, Sanofi Genzyme, MedDay, NINDS, and Novartis.
Patients with relapsing multiple sclerosis (MS) treated with intravenous ublituximab had fewer relapses and brain lesions compared with those treated with oral teriflunomide, although both therapies resulted in similar rates of worsening disability, according to results of the two identical phase 3 ULTIMATE I and II trials.
“In these two 96-week trials involving participants with MS, annualized relapse rates were lower with intravenous ublituximab than with oral teriflunomide. Ublituximab was associated with infusion-related reactions. Larger and longer trials are required to determine the efficacy and safety of ublituximab in patients with relapsing MS, including comparison with other disease-modifying treatments such as existing anti-CD20 monoclonal antibodies,” noted lead author Lawrence Steinman, MD, professor of neurology and neurological sciences, pediatrics, and genetics at Stanford (Calif.) University, and colleagues.
The results, published in the New England Journal of Medicine, pave the way for ublituximab’s approval as the third high-efficacy anti-CD20 monoclonal antibody to treat relapsing forms of MS, predicted Patricia Coyle, MD, director of the MS Comprehensive Care Center, and professor of neurology, at Stony Brook (N.Y.) Neurosciences Institute, who was not involved in the research. Ublituximab will “widen the anti-CD20 monoclonal choices for MS, and should directly compete with ocrelizumab and ofatumumab,” she said.
Two trials
The double-blind, double-dummy ULTIMATE I and II trials enrolled 549 and 545 participants respectively, with a median follow-up of 95 weeks. Subjects, aged between 18 and 55 years, were randomized to receive either oral placebo and intravenous ublituximab (150 mg on day 1, followed by 450 mg on day 15 and at weeks 24, 48, and 72), or oral teriflunomide (14 mg once daily) and intravenous placebo. The primary endpoint was the annualized relapse rate, defined as the number of confirmed MS relapses per participant-year, with a range of secondary end points including number of lesions on magnetic resonance imaging (MRI) by 96 weeks, and worsening of disability confirmed at 12 weeks.
Prevention and management of infusion-related reactions was with oral antihistamine and dexamethasone, administered 30 to 60 minutes before each intravenous dose of ublituximab or placebo, as well as reductions in infusion flow rates and discretionary acetaminophen.
Results for the primary endpoint in ULTIMATE I showed the adjusted annualized relapse rate over a period of 96 weeks was 0.08 in the ublituximab group and 0.19 in the teriflunomide group (rate ratio, 0.41; P < .001). Corresponding rates for ULTIMATE II were 0.09 and 0.18 (rate ratio, 0.51; P = .002).
The mean number of lesions in both ublituximab arms of the trials was 0.02 and 0.01 compared with 0.49 and 0.25 in the teriflunomide arms (rate ratios 0.03 and 0.04 respectively; P < .001 for both).
Similar disability worsening in both groups
A pooled analysis of the two trials showed worsening disability in 5.2% of the ublituximab group, and 5.9% of the teriflunomide group (hazard ratio, 0.84; P = 0.51). “In both trials, teriflunomide was associated with a numerically lower rate of worsening of disability than that reported in previous studies with this drug, but no conclusions can be drawn from these comparisons,” noted the authors.
Infusion-related reactions occurred in 47.7% of the participants in the ublituximab group, consisting mainly of mild to moderate pyrexia, headache, chills, and influenza-like illness. “The reactions may have been related to cytokine release from immune cells (B and NK cells) on interaction of the Fc antibody domain with Fc gamma receptors on effector cells,” they suggested.
Although no opportunistic infections occurred, a higher frequency of infections, including serious infections, was observed with ublituximab (5.0%) than with teriflunomide (2.9%).
While the ULTIMATE trials showed no difference between ublituximab and teriflunomide in confirmed worsening of disability, only a small percentage of participants in either arm showed deterioration, Dr. Coyle remarked. “In a relatively short trial (96 weeks), in a relapsing population on active treatment, this result was not surprising … If the study was bigger, or longer it would increase the chances of seeing a progressive slow worsening component to affect the EDSS [Expanded Disability Status Scale],” she added.
Equivalent efficacy
Ultimately, “it appears likely” that ublituximab is “equivalent in efficacy” to the earlier anti-CD20 agents ocrelizumab and ofatumumab, Dr. Coyle said. While all three agents target B-cells, “ublituximab targets a novel CD20 binding site, and is bioengineered to have a particularly potent antibody dependent cell cytotoxicity lysis mechanism,” she added. “It has been touted to ultimately allow a short infusion of 1 hour.”
Although the serious infection rate is slightly higher with ublituximab (5.0% vs. 2.5% for ofatumumab, and 1.3% for ocrelizumab), “it is still low,” and infusion-related reactions are also higher with ublituximab, she added (47.7% vs. 20.2% and 34.3%, respectively). She suggested factors that might influence which treatment is chosen for a given patient might include cost, convenience, whether it is more or less likely to cause low IgG, interference with vaccination, or influence on cancer or COVID risk.
The trials were supported by TG Therapeutics.
Dr. Coyle has received consulting fees from Accordant, Biogen, Bristol Myers Squibb, Celgene, Genentech/Roche, GlaxoSmithKline, Horizon, Janssen, Novartis, Sanofi Genzyme, and Viela Bio and grant funding from Actelion, Alkermes, Bristol Myers Squibb, CorEvitas LLD, Genentech/Roche, Sanofi Genzyme, MedDay, NINDS, and Novartis.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Chlorophyll water can trigger pseudoporphyria, expert warns
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
PORTLAND, ORE. – If a child presents with pseudoporphyria – a bullous photodermatosis with the clinical and histological features of porphyria cutanea tarda (PCT) but with normal porphyrins – chlorophyll water could be the culprit.
Commercially available, green pigment–infused chlorophyll water is marketed with claims that it supports cancer prevention and digestive health, facilitates weight loss, and improves skin complexion. “It also absorbs light, so lo and behold, if your patient is photosensitive, they might get pseudoporphyria,” Robert Sidbury, MD, MPH, chief of the division of dermatology at Seattle Children’s Hospital, said at the annual meeting of the Pacific Dermatologic Association.
This was one of the clinical pearls he shared during his presentation.
Dr. Sidbury added that the risk of photosensitivity increases in children who are taking other medications such as doxycycline, methotrexate, or even naproxen. At least two cases of pseudoporphyria following self-medication with chlorophyll have been described in the dermatology literature.
Is it SSSS or SJS?
Another clinical pearl that Dr. Sidbury shared at the meeting related to staphylococcal scalded skin syndrome (SSSS), which causes reddening and blistering of the skin that makes it appear scalded or burned. To rule out Stevens-Johnson Syndrome (SJS) in a child who presents with such skin manifestations, he routinely performs the unscientific lollipop test, which he learned from Bernard A. “Buddy” Cohen, MD, professor of dermatology and pediatrics at Johns Hopkins University, Baltimore.
“If they eat it, it’s Staph scalded skin,” said Dr. Sidbury, who is also professor of pediatrics at the University of Washington, Seattle. “If they don’t, it’s likely SJS. It’s not the most specific test, but it’s easy to do, because there’s no mucous membrane involvement in Staph scalded skin.”
In a poster presented during the 2022 annual meeting of the Society for Pediatric Dermatology, Sarah Cipriano, MD, MPH, and colleagues at the University of Utah, Salt Lake City, retrospectively study 85 patients aged younger than 18 years diagnosed with SSSS between Jan. 1, 2010, and Aug. 21, 2021. They found that ancillary blood cultures and CSF cultures did not improve diagnostic precision in SSSS patients.
“They don’t add anything unless there’s an indication beyond the Staph scalded skin,” said Dr. Sidbury, who was not involved in the study. “The researchers also found that clindamycin does not improve outcomes in these patients, so avoid using it.” Instead, a first-generation cephalosporin is indicated, and an alternate diagnosis should be considered if the patient does not improve within 48 hours.
Dr. Sidbury disclosed that he has conducted research for Regeneron, Galderma, and UCB. He is also an adviser for Leo Pharmaceuticals and a speaker for Biersdorf.
AT PDA 2022
Kicking the can
Medicare, like any other business (regardless of how you want to view it, it’s as much a business as any other insurance company), is dependent on cash flow. Money comes in from young people and their employers through withholding and taxes, and goes back out again in payments to doctors, hospitals, and all the others who bill Medicare for services and supplies in providing health care.
Unlike other businesses, it’s hampered by regulations and competing interests that affect its viability and capacity to adapt to changing markets and circumstances.
For a while, estimates were that Medicare would run out of cash in 2026, but with a stronger-then-expected COVID recovery, it’s been pushed back all the way to ... 2028.
Yeah.
The trouble here is that nobody wants to fix the system to keep it from happening. It’s easier to blame the other side for losing the game than it is to work together to win it. This isn’t a Republican or Democrat issue. Both of them are the problem.
Pushing it back 2 years doesn’t keep it from happening, though it does give more time to find a solution. But that’s only if you have people willing to do so.
Currently politicians favor a strategy of kicking the can down the road for the next congress to deal with. But we’re running out of road to kick it down, and the odds of the next generation of politicians being reasonable, functioning, adults seem to get lower each year.
Can you run your practice like that? In such a way that you know that in a few years your expenses will outweigh your income? And just figure that at some point you’ll get it figured out before your creditors come knocking?
Me neither.
If you were like me, or any other small business owner, you’d sit down and figure out what changes are needed so you’ll still have a viable business down the road.
Of course, that’s part of the issue. The people making these decisions for Medicare don’t have a vested interest in it. If it fails, they have other jobs and income sources to move on to, not to mention some pension-funded health insurance plan. It’s not their problem.
But for the patients, doctors, and other health care professionals who will be depending on it in 6 years, it is a problem, and a serious one.
Hopefully someone will listen before then.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Medicare, like any other business (regardless of how you want to view it, it’s as much a business as any other insurance company), is dependent on cash flow. Money comes in from young people and their employers through withholding and taxes, and goes back out again in payments to doctors, hospitals, and all the others who bill Medicare for services and supplies in providing health care.
Unlike other businesses, it’s hampered by regulations and competing interests that affect its viability and capacity to adapt to changing markets and circumstances.
For a while, estimates were that Medicare would run out of cash in 2026, but with a stronger-then-expected COVID recovery, it’s been pushed back all the way to ... 2028.
Yeah.
The trouble here is that nobody wants to fix the system to keep it from happening. It’s easier to blame the other side for losing the game than it is to work together to win it. This isn’t a Republican or Democrat issue. Both of them are the problem.
Pushing it back 2 years doesn’t keep it from happening, though it does give more time to find a solution. But that’s only if you have people willing to do so.
Currently politicians favor a strategy of kicking the can down the road for the next congress to deal with. But we’re running out of road to kick it down, and the odds of the next generation of politicians being reasonable, functioning, adults seem to get lower each year.
Can you run your practice like that? In such a way that you know that in a few years your expenses will outweigh your income? And just figure that at some point you’ll get it figured out before your creditors come knocking?
Me neither.
If you were like me, or any other small business owner, you’d sit down and figure out what changes are needed so you’ll still have a viable business down the road.
Of course, that’s part of the issue. The people making these decisions for Medicare don’t have a vested interest in it. If it fails, they have other jobs and income sources to move on to, not to mention some pension-funded health insurance plan. It’s not their problem.
But for the patients, doctors, and other health care professionals who will be depending on it in 6 years, it is a problem, and a serious one.
Hopefully someone will listen before then.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Medicare, like any other business (regardless of how you want to view it, it’s as much a business as any other insurance company), is dependent on cash flow. Money comes in from young people and their employers through withholding and taxes, and goes back out again in payments to doctors, hospitals, and all the others who bill Medicare for services and supplies in providing health care.
Unlike other businesses, it’s hampered by regulations and competing interests that affect its viability and capacity to adapt to changing markets and circumstances.
For a while, estimates were that Medicare would run out of cash in 2026, but with a stronger-then-expected COVID recovery, it’s been pushed back all the way to ... 2028.
Yeah.
The trouble here is that nobody wants to fix the system to keep it from happening. It’s easier to blame the other side for losing the game than it is to work together to win it. This isn’t a Republican or Democrat issue. Both of them are the problem.
Pushing it back 2 years doesn’t keep it from happening, though it does give more time to find a solution. But that’s only if you have people willing to do so.
Currently politicians favor a strategy of kicking the can down the road for the next congress to deal with. But we’re running out of road to kick it down, and the odds of the next generation of politicians being reasonable, functioning, adults seem to get lower each year.
Can you run your practice like that? In such a way that you know that in a few years your expenses will outweigh your income? And just figure that at some point you’ll get it figured out before your creditors come knocking?
Me neither.
If you were like me, or any other small business owner, you’d sit down and figure out what changes are needed so you’ll still have a viable business down the road.
Of course, that’s part of the issue. The people making these decisions for Medicare don’t have a vested interest in it. If it fails, they have other jobs and income sources to move on to, not to mention some pension-funded health insurance plan. It’s not their problem.
But for the patients, doctors, and other health care professionals who will be depending on it in 6 years, it is a problem, and a serious one.
Hopefully someone will listen before then.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
VTE risk not elevated in AD patients on JAK inhibitors: Study
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
, according to a new systemic review and meta-analysis, published online in JAMA Dermatology.
“These findings may provide a reference for clinicians in prescribing JAK inhibitors for patients with AD,” Tai-Li Chen, MD, of Taipei (Taiwan) Veterans General Hospital, Taipei, and colleagues wrote in the study.
The results shed some welcome light on treatment for this dermatologic population, for whom enthusiasm about JAK inhibitors was dampened by the addition of a boxed warning to the labels of JAK inhibitors last year, required by the Food and Drug Administration. The warning, which describes an increased risk of “serious heart-related events such as heart attack or stroke, cancer, blood clots, and death” was triggered by results of the ORAL Surveillance study of patients with rheumatoid arthritis (RA) treated with tofacitinib.
The boxed warning is also included in the labels of topical ruxolitinib, a JAK inhibitor approved by the FDA for mild to moderate AD in 2021, and in the labels of two oral JAK inhibitors, upadacitinib and abrocitinib, approved by the FDA for treating moderate to severe AD in January 2022.
Despite the new findings, some dermatologists are still urging caution.
“All the JAK inhibitor trials are short term. I still think the precautionary principle applies and we need to counsel on the risks of JAKs,” tweeted Aaron Drucker, MD, a dermatologist at Women’s College Hospital, and associate professor at the University of Toronto. “It is great to have these as options for our patients. But we need to be aware of the risks associated with this class of medications, counsel patients about them when we are informing them of the risks and benefits of treatment options, and wait for more data specific to this population to make even more informed decisions,” he told this news organization.
The meta-analysis examined both the risk of incident VTE in untreated patients with AD compared with non-AD patients, as well as the risk of VTE in AD patients treated with JAK inhibitors compared with those on either placebo or dupilumab. Four JAK inhibitors were studied: abrocitinib, baricitinib (under FDA review for AD), upadacitinib, and SHR0302 (in clinical trials).
Two studies (458,206 participants) found the overall incidence rate of VTE for patients with AD was 0.23 events per 100 patient-years. The risk was did not differ from that in non-AD patients (pooled hazard ratio [HR], 0.95; 95% confidence interval [CI], 0.62-1.45).
Another 15 studies included 8,787 participants with AD and found no significant differences in the rates of VTE in AD patients treated with JAK inhibitors (0.05%) versus those treated with placebo or dupilumab (0.03%). However “with the increasing applications of JAK inhibitors in AD, more clinical data are needed to identify patients at high risk for VTE,” noted the authors.
“We need more, long-term data,” agreed Dr. Drucker, adding that a major issue is the short-term nature of AD trials to date (generally up to 16 weeks), which “don’t provide adequate reassurance.” He said although the FDA’s boxed warning was prompted by a trial in RA patients treated with tofacitinib (a less selective JAK inhibitor than those approved by the FDA for AD), and the same risks have not been demonstrated specifically for the JAK inhibitors used for a patients with AD, he still remains cautious.
While agreeing on the need for more long-term data, Andrew Blauvelt, MD, MBA, president of Oregon Medical Research Center, Portland, said that the new findings should “provide reassurance” to dermatologists and are “consonant with recent published meta-analyses reporting no increased VTE risk in patients with psoriasis, RA, or inflammatory bowel disease treated with JAK inhibitors” in Arthritis & Rheumatology, and Mayo Clinic Proceedings.
In an interview, Dr. Blauvelt said that safety profiles emerging for the newer JAK inhibitors, which block JAK 1/2, have been overshadowed by the older RA data for tofacitinib – which is a JAK 1/3 inhibitor, “despite emerging long-term, monotherapy, clinical study data for dermatologic diseases showing no or rare risks of developing severe adverse events outlined in the boxed warnings.”
Both Dr. Blauvelt and Dr. Drucker pointed out that people with RA tend to have more comorbidities than those with AD that would predispose them to adverse events. In fact, “approximately 75% of patients in the ORAL Surveillance study were also on concomitant methotrexate and/or prednisone, which can greatly confound safety results,” said Dr. Blauvelt.
The study authors did not report any disclosures. No funding source for the study was provided. Dr. Drucker has no relevant disclosures. Dr. Blauvelt has been a clinical study investigator in trials for AD treatments, including JAK inhibitors; his disclosures include serving as a speaker, scientific adviser, and/or clinical study investigator for multiple companies including AbbVie, Arcutis, Bristol-Myers Squibb, Pfizer, Incyte, Regeneron, Sanofi Genzyme, and UCB Pharma.
FROM JAMA DERMATOLOGY
In America, cancer patients endure debt on top of disease
RAPID CITY, S.D. – Jeni Rae Peters would make promises to herself as she lay awake nights after being diagnosed with breast cancer two years ago.
“My kids had lost so much,” said Ms. Peters, a single mom and mental health counselor. She had just adopted two girls and was fostering four other children. “I swore I wouldn’t force them to have yet another parent.”
Multiple surgeries, radiation, and chemotherapy controlled the cancer. But, despite having insurance, Ms. Peters was left with more than $30,000 of debt, threats from bill collectors, and more anxious nights thinking of her kids. “Do I pull them out of day care? Do I stop their schooling and tutoring? Do I not help them with college?” Ms. Peters asked herself. “My doctor saved my life, but my medical bills are stealing from my children’s lives.”
Cancer kills about 600,000 people in the United States every year, making it a leading cause of death. Many more survive it, because of breakthroughs in medicines and therapies.
That’s forced patients and their families to make gut-wrenching sacrifices even as they confront a grave illness, according to a KHN-NPR investigation of America’s sprawling medical debt problem. The project shows few suffer more than those with cancer.
About two-thirds of adults with health care debt who’ve had cancer themselves or in their family have cut spending on food, clothing, or other household basics, a poll conducted by KFF for this project found. About one in four have declared bankruptcy or lost their home to eviction or foreclosure.
Other research shows that patients from minority groups are more likely to experience financial hardships caused by cancer than White patients, reinforcing racial disparities that shadow the U.S. health care system.
“It’s crippling,” said Dr. Veena Shankaran, MD, an oncologist at the University of Washington, Seattle, who began studying the financial impact of cancer after seeing patients ruined by medical bills. “Even if someone survives the cancer, they often can’t shake the debt.”
Dr. Shankaran found that cancer patients were 71% more likely than Americans without the disease to have bills in collections, face tax liens and mortgage foreclosure, or experience other financial setbacks. Analyzing bankruptcy records and cancer registries in Washington state, Dr. Shankaran and other researchers also discovered that cancer patients were 2½ times as likely to declare bankruptcy as those without the disease. And those who went bankrupt were likelier to die than cancer patients who did not.
Oncologists have a name for this: “financial toxicity,” a term that echoes the intractable vomiting, life-threatening infections, and other noxious effects of chemotherapy.
“Sometimes,” Dr. Shankaran said, “it’s tough to think about what the system puts patients through.”
Cancer diagnosis upends family
At the three-bedroom home in Rapid City that Ms. Peters shares with her children and a friend, there isn’t time most days to dwell on these worries. There are ice skating lessons and driving tests and countless meals to prepare. Teenagers drift in and out, chattering about homework and tattoos and driving.
The smallest children congregate at a small kitchen table under a wall decorated with seven old telephones. (As Ms. Peters tells it, the red one is a hotline to Santa, a green one to the Grinch, and a space shuttle–shaped phone connects to astronauts orbiting the Earth.)
Ms. Peters, 44, presides cheerfully over the chaos, directing her children with snide asides and expressions of love. She watches proudly as one teenage daughter helps another with math in the living room. Later she dances with a 5-year-old to Queen under a disco ball in the entry hall.
Ms. Peters, who sports tattoos and earlier this year dyed her hair purple, never planned to have a family. In her late 30s, she wanted to do more for her adopted community, so she took in foster children, many of whom come from the nearby Pine Ridge Indian Reservation. One of her daughters had been homeless.
“Foster kids are amazing humans,” she said. “I joke I’m the most reluctant parent of the most amazing children that have ever existed. And I get to help raise these little people to be healthy and safe.”
In spring 2020, the secure world Ms. Peters had carefully tended was shattered. As the COVID pandemic spread across the country, she was diagnosed with stage 2 breast cancer.
Within weeks, she had an intravenous port inserted into her chest. Surgeons removed both her breasts, then her ovaries after tests showed she was at risk of ovarian cancer as well.
Cancer treatment today often entails a costly, debilitating march of procedures, infusions, and radiation sessions that can exhaust patients physically and emotionally. It was scary, Ms. Peters said. But she rallied her children. “We talked a lot about how they had all lost siblings or parents or other relatives,” she said. “All I had to do was lose my boobs.”
Much harder, she said, were the endless and perplexing medical bills.
There were bills from the anesthesiologists who attended her surgeries, from the hospital, and from a surgery center. For a while, the hospital stopped sending bills. Then in April, Ms. Peters got a call one morning from a bill collector saying she owed $13,000. In total, Ms. Peters estimates her medical debts now exceed $30,000.
High costs, despite insurance
Debts of that size aren’t unusual. Nationwide, about one in five indebted adults who have had cancer or have a family member who’s been sick say they owe $10,000 or more, according to the KFF poll. Those dealing with cancer are also more likely than others with health care debt to owe large sums and to say they don’t expect to ever pay them off.
This debt has been fueled in part by the advent of lifesaving therapies that also come with eye-popping price tags. The National Cancer Institute calculated the average cost of medical care and drugs tops $42,000 in the year following a cancer diagnosis. Some treatments can exceed $1 million.
Usually, most costs are covered. But patients are increasingly on the hook for large bills because of deductibles and other health plan cost sharing. The average leukemia patient with private health insurance, for example, can expect to pay more than $5,100 in the year after diagnosis, according to an analysis by the consulting firm Milliman. Even Medicare can leave seniors with huge bills. The average blood cancer patient covered by fee-for-service Medicare can expect to pay more than $17,000 out-of-pocket in the year following diagnosis, Milliman found.
Additionally, ongoing surgeries, tests, and medications can make patients pay large out-of-pocket costs year after year. Physicians and patient advocates say this cost sharing -- originally billed as a way to encourage patients to shop for care -- is devastating. “The problem is that model doesn’t work very well with cancer,” said David Eagle, MD, an oncologist at New York Cancer & Blood Specialists.
More broadly, the KHN-NPR investigation found that about 100 million people in the United States are now in debt from medical or dental bills. Poor health is among the most powerful predictors of debt, with this debt concentrated in parts of the country with the highest levels of illness.
According to the KFF poll, 6 in 10 adults with a chronic disease such as cancer, diabetes, or heart disease or with a close family member who is sick have had some kind of health care debt in the past 5 years. The poll was designed to capture not just bills patients haven’t paid, but also other borrowing used to pay for health care, such as credit cards, payment plans, and loans from friends and family.
For her part, Ms. Peters has had seven surgeries since 2020. Through it all, she had health insurance through her employers. Ms. Peters said she knew she had to keep working or would lose coverage and face even bigger bills. Like most plans, however, hers have required she pay thousands of dollars out of pocket.
Within weeks of her diagnosis, the bills rolled in. Then collectors started calling. One call came as Ms. Peters was lying in the recovery room after her double mastectomy. “I was kind of delirious, and I thought it was my kids,” she said. “It was someone asking me to pay a medical bill.”
Ms. Peters faced more bills when she switched jobs later that year and her insurance changed. The deductible and cap on her out-of-pocket costs reset.
In 2021, the deductible and out-of-pocket limit reset again, as they do every year for most health plans. So when Ms. Peters slipped on the ice and broke her wrist – a fracture likely made worse by chemotherapy that weakened her bones – she was charged thousands more.
This year has brought more surgeries and yet more bills, as her deductible and out-of-pocket limit reset again.
“I don’t even know anymore how much I owe,” Ms. Peters said. “Sometimes it feels like people just send me random bills. I don’t even know what they’re for.”
Making sacrifices
Before getting sick, Ms. Peters was earning about $60,000 a year. It was enough to provide for her children, she said, supplemented with a stipend she receives for foster care.
The family budget was always tight. Ms. Peters and her kids don’t take extravagant vacations. Ms. Peters doesn’t own her home and has next to no savings. Now, she said, they are living at the edge. “I keep praying there is a shoe fairy,” she said, joking about the demands of so many growing feet in her home.
Ms. Peters took on extra work to pay some of the bills. Five days a week, she works back-to-back shifts at both a mental health crisis center and a clinic where she counsels teenagers, some of whom are suicidal. In 2021, three friends on the East Coast paid off some of the debt.
But Ms. Peters’ credit score has tumbled below 600. And the bills pile high on the microwave in her kitchen. “I’m middle class,” she said. “Could I make payments on some of these? Yes, I suppose I could.”
That would require trade-offs. She could drop car insurance for her teenage daughter, who just got her license. Canceling ice skating for another daughter would yield an extra $60 a month. But Ms. Peters is reluctant. “Do you know what it feels like to be a foster kid and get a gold medal in ice skating? Do you know what kind of citizen they could become if they know they’re special?” she said. “There seems to be a myth that you can pay for it all. You can’t.”
Many cancer patients face difficult choices.
About 4 in 10 with debt have taken money out of a retirement, college, or other long-term savings account, the KFF poll found; about 3 in 10 have moved in with family or friends or made another change in their living situation.
Kashyap Patel, MD, chief executive of Carolina Blood and Cancer Care Associates, said the South Carolina practice has found patients turning to food banks and other charities to get by. One patient was living in his car. Dr. Patel estimated that half the patients need some kind of financial aid. Even then, many end up in debt.
The Leukemia & Lymphoma Society, which typically helps blood cancer patients navigate health insurance and find food, housing, and other nonmedical assistance, is hearing from more patients simply seeking cash to pay off debt, said Nikki Yuill, who oversees the group’s call center. “People tell us they won’t get follow-up care because they can’t take on more debt,” Ms. Yuill said, recalling one man who refused to call an ambulance even though he couldn’t get to the hospital. “It breaks your heart.”
Academic research has revealed widespread self-rationing by patients. For example, while nearly one in five people taking oral chemotherapy abandon treatment, about half stop when out-of-pocket costs exceed $2,000, according to a 2017 analysis.
Robin Yabroff, PhD, MBA, an epidemiologist at the American Cancer Society, said more research must be done to understand the lasting effects of medical debt on cancer survivors and their families. “What does it mean for a family if they have to liquidate savings or drain college funds or sell their home?” Dr. Yabroff said. “We just don’t know yet.”
As Ms. Peters put away bags of groceries in her kitchen, she conceded she doesn’t know what will happen to her family. Like many patients, she worries about how she’ll pay for tests and follow-up care if the cancer reappears.
She is still wading through collection notices in the mail and fielding calls from debt collectors. Ms. Peters told one that she was prepared to go to court and ask the judge to decide which of her children should be cut off from after-school activities to pay off the debts.
She asked another debt collector whether he had kids. “He told me that it had been my choice to get the surgery,” Ms. Peters recalled. “And I said: ‘Yeah, I guess I chose not to be dead.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
RAPID CITY, S.D. – Jeni Rae Peters would make promises to herself as she lay awake nights after being diagnosed with breast cancer two years ago.
“My kids had lost so much,” said Ms. Peters, a single mom and mental health counselor. She had just adopted two girls and was fostering four other children. “I swore I wouldn’t force them to have yet another parent.”
Multiple surgeries, radiation, and chemotherapy controlled the cancer. But, despite having insurance, Ms. Peters was left with more than $30,000 of debt, threats from bill collectors, and more anxious nights thinking of her kids. “Do I pull them out of day care? Do I stop their schooling and tutoring? Do I not help them with college?” Ms. Peters asked herself. “My doctor saved my life, but my medical bills are stealing from my children’s lives.”
Cancer kills about 600,000 people in the United States every year, making it a leading cause of death. Many more survive it, because of breakthroughs in medicines and therapies.
That’s forced patients and their families to make gut-wrenching sacrifices even as they confront a grave illness, according to a KHN-NPR investigation of America’s sprawling medical debt problem. The project shows few suffer more than those with cancer.
About two-thirds of adults with health care debt who’ve had cancer themselves or in their family have cut spending on food, clothing, or other household basics, a poll conducted by KFF for this project found. About one in four have declared bankruptcy or lost their home to eviction or foreclosure.
Other research shows that patients from minority groups are more likely to experience financial hardships caused by cancer than White patients, reinforcing racial disparities that shadow the U.S. health care system.
“It’s crippling,” said Dr. Veena Shankaran, MD, an oncologist at the University of Washington, Seattle, who began studying the financial impact of cancer after seeing patients ruined by medical bills. “Even if someone survives the cancer, they often can’t shake the debt.”
Dr. Shankaran found that cancer patients were 71% more likely than Americans without the disease to have bills in collections, face tax liens and mortgage foreclosure, or experience other financial setbacks. Analyzing bankruptcy records and cancer registries in Washington state, Dr. Shankaran and other researchers also discovered that cancer patients were 2½ times as likely to declare bankruptcy as those without the disease. And those who went bankrupt were likelier to die than cancer patients who did not.
Oncologists have a name for this: “financial toxicity,” a term that echoes the intractable vomiting, life-threatening infections, and other noxious effects of chemotherapy.
“Sometimes,” Dr. Shankaran said, “it’s tough to think about what the system puts patients through.”
Cancer diagnosis upends family
At the three-bedroom home in Rapid City that Ms. Peters shares with her children and a friend, there isn’t time most days to dwell on these worries. There are ice skating lessons and driving tests and countless meals to prepare. Teenagers drift in and out, chattering about homework and tattoos and driving.
The smallest children congregate at a small kitchen table under a wall decorated with seven old telephones. (As Ms. Peters tells it, the red one is a hotline to Santa, a green one to the Grinch, and a space shuttle–shaped phone connects to astronauts orbiting the Earth.)
Ms. Peters, 44, presides cheerfully over the chaos, directing her children with snide asides and expressions of love. She watches proudly as one teenage daughter helps another with math in the living room. Later she dances with a 5-year-old to Queen under a disco ball in the entry hall.
Ms. Peters, who sports tattoos and earlier this year dyed her hair purple, never planned to have a family. In her late 30s, she wanted to do more for her adopted community, so she took in foster children, many of whom come from the nearby Pine Ridge Indian Reservation. One of her daughters had been homeless.
“Foster kids are amazing humans,” she said. “I joke I’m the most reluctant parent of the most amazing children that have ever existed. And I get to help raise these little people to be healthy and safe.”
In spring 2020, the secure world Ms. Peters had carefully tended was shattered. As the COVID pandemic spread across the country, she was diagnosed with stage 2 breast cancer.
Within weeks, she had an intravenous port inserted into her chest. Surgeons removed both her breasts, then her ovaries after tests showed she was at risk of ovarian cancer as well.
Cancer treatment today often entails a costly, debilitating march of procedures, infusions, and radiation sessions that can exhaust patients physically and emotionally. It was scary, Ms. Peters said. But she rallied her children. “We talked a lot about how they had all lost siblings or parents or other relatives,” she said. “All I had to do was lose my boobs.”
Much harder, she said, were the endless and perplexing medical bills.
There were bills from the anesthesiologists who attended her surgeries, from the hospital, and from a surgery center. For a while, the hospital stopped sending bills. Then in April, Ms. Peters got a call one morning from a bill collector saying she owed $13,000. In total, Ms. Peters estimates her medical debts now exceed $30,000.
High costs, despite insurance
Debts of that size aren’t unusual. Nationwide, about one in five indebted adults who have had cancer or have a family member who’s been sick say they owe $10,000 or more, according to the KFF poll. Those dealing with cancer are also more likely than others with health care debt to owe large sums and to say they don’t expect to ever pay them off.
This debt has been fueled in part by the advent of lifesaving therapies that also come with eye-popping price tags. The National Cancer Institute calculated the average cost of medical care and drugs tops $42,000 in the year following a cancer diagnosis. Some treatments can exceed $1 million.
Usually, most costs are covered. But patients are increasingly on the hook for large bills because of deductibles and other health plan cost sharing. The average leukemia patient with private health insurance, for example, can expect to pay more than $5,100 in the year after diagnosis, according to an analysis by the consulting firm Milliman. Even Medicare can leave seniors with huge bills. The average blood cancer patient covered by fee-for-service Medicare can expect to pay more than $17,000 out-of-pocket in the year following diagnosis, Milliman found.
Additionally, ongoing surgeries, tests, and medications can make patients pay large out-of-pocket costs year after year. Physicians and patient advocates say this cost sharing -- originally billed as a way to encourage patients to shop for care -- is devastating. “The problem is that model doesn’t work very well with cancer,” said David Eagle, MD, an oncologist at New York Cancer & Blood Specialists.
More broadly, the KHN-NPR investigation found that about 100 million people in the United States are now in debt from medical or dental bills. Poor health is among the most powerful predictors of debt, with this debt concentrated in parts of the country with the highest levels of illness.
According to the KFF poll, 6 in 10 adults with a chronic disease such as cancer, diabetes, or heart disease or with a close family member who is sick have had some kind of health care debt in the past 5 years. The poll was designed to capture not just bills patients haven’t paid, but also other borrowing used to pay for health care, such as credit cards, payment plans, and loans from friends and family.
For her part, Ms. Peters has had seven surgeries since 2020. Through it all, she had health insurance through her employers. Ms. Peters said she knew she had to keep working or would lose coverage and face even bigger bills. Like most plans, however, hers have required she pay thousands of dollars out of pocket.
Within weeks of her diagnosis, the bills rolled in. Then collectors started calling. One call came as Ms. Peters was lying in the recovery room after her double mastectomy. “I was kind of delirious, and I thought it was my kids,” she said. “It was someone asking me to pay a medical bill.”
Ms. Peters faced more bills when she switched jobs later that year and her insurance changed. The deductible and cap on her out-of-pocket costs reset.
In 2021, the deductible and out-of-pocket limit reset again, as they do every year for most health plans. So when Ms. Peters slipped on the ice and broke her wrist – a fracture likely made worse by chemotherapy that weakened her bones – she was charged thousands more.
This year has brought more surgeries and yet more bills, as her deductible and out-of-pocket limit reset again.
“I don’t even know anymore how much I owe,” Ms. Peters said. “Sometimes it feels like people just send me random bills. I don’t even know what they’re for.”
Making sacrifices
Before getting sick, Ms. Peters was earning about $60,000 a year. It was enough to provide for her children, she said, supplemented with a stipend she receives for foster care.
The family budget was always tight. Ms. Peters and her kids don’t take extravagant vacations. Ms. Peters doesn’t own her home and has next to no savings. Now, she said, they are living at the edge. “I keep praying there is a shoe fairy,” she said, joking about the demands of so many growing feet in her home.
Ms. Peters took on extra work to pay some of the bills. Five days a week, she works back-to-back shifts at both a mental health crisis center and a clinic where she counsels teenagers, some of whom are suicidal. In 2021, three friends on the East Coast paid off some of the debt.
But Ms. Peters’ credit score has tumbled below 600. And the bills pile high on the microwave in her kitchen. “I’m middle class,” she said. “Could I make payments on some of these? Yes, I suppose I could.”
That would require trade-offs. She could drop car insurance for her teenage daughter, who just got her license. Canceling ice skating for another daughter would yield an extra $60 a month. But Ms. Peters is reluctant. “Do you know what it feels like to be a foster kid and get a gold medal in ice skating? Do you know what kind of citizen they could become if they know they’re special?” she said. “There seems to be a myth that you can pay for it all. You can’t.”
Many cancer patients face difficult choices.
About 4 in 10 with debt have taken money out of a retirement, college, or other long-term savings account, the KFF poll found; about 3 in 10 have moved in with family or friends or made another change in their living situation.
Kashyap Patel, MD, chief executive of Carolina Blood and Cancer Care Associates, said the South Carolina practice has found patients turning to food banks and other charities to get by. One patient was living in his car. Dr. Patel estimated that half the patients need some kind of financial aid. Even then, many end up in debt.
The Leukemia & Lymphoma Society, which typically helps blood cancer patients navigate health insurance and find food, housing, and other nonmedical assistance, is hearing from more patients simply seeking cash to pay off debt, said Nikki Yuill, who oversees the group’s call center. “People tell us they won’t get follow-up care because they can’t take on more debt,” Ms. Yuill said, recalling one man who refused to call an ambulance even though he couldn’t get to the hospital. “It breaks your heart.”
Academic research has revealed widespread self-rationing by patients. For example, while nearly one in five people taking oral chemotherapy abandon treatment, about half stop when out-of-pocket costs exceed $2,000, according to a 2017 analysis.
Robin Yabroff, PhD, MBA, an epidemiologist at the American Cancer Society, said more research must be done to understand the lasting effects of medical debt on cancer survivors and their families. “What does it mean for a family if they have to liquidate savings or drain college funds or sell their home?” Dr. Yabroff said. “We just don’t know yet.”
As Ms. Peters put away bags of groceries in her kitchen, she conceded she doesn’t know what will happen to her family. Like many patients, she worries about how she’ll pay for tests and follow-up care if the cancer reappears.
She is still wading through collection notices in the mail and fielding calls from debt collectors. Ms. Peters told one that she was prepared to go to court and ask the judge to decide which of her children should be cut off from after-school activities to pay off the debts.
She asked another debt collector whether he had kids. “He told me that it had been my choice to get the surgery,” Ms. Peters recalled. “And I said: ‘Yeah, I guess I chose not to be dead.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
RAPID CITY, S.D. – Jeni Rae Peters would make promises to herself as she lay awake nights after being diagnosed with breast cancer two years ago.
“My kids had lost so much,” said Ms. Peters, a single mom and mental health counselor. She had just adopted two girls and was fostering four other children. “I swore I wouldn’t force them to have yet another parent.”
Multiple surgeries, radiation, and chemotherapy controlled the cancer. But, despite having insurance, Ms. Peters was left with more than $30,000 of debt, threats from bill collectors, and more anxious nights thinking of her kids. “Do I pull them out of day care? Do I stop their schooling and tutoring? Do I not help them with college?” Ms. Peters asked herself. “My doctor saved my life, but my medical bills are stealing from my children’s lives.”
Cancer kills about 600,000 people in the United States every year, making it a leading cause of death. Many more survive it, because of breakthroughs in medicines and therapies.
That’s forced patients and their families to make gut-wrenching sacrifices even as they confront a grave illness, according to a KHN-NPR investigation of America’s sprawling medical debt problem. The project shows few suffer more than those with cancer.
About two-thirds of adults with health care debt who’ve had cancer themselves or in their family have cut spending on food, clothing, or other household basics, a poll conducted by KFF for this project found. About one in four have declared bankruptcy or lost their home to eviction or foreclosure.
Other research shows that patients from minority groups are more likely to experience financial hardships caused by cancer than White patients, reinforcing racial disparities that shadow the U.S. health care system.
“It’s crippling,” said Dr. Veena Shankaran, MD, an oncologist at the University of Washington, Seattle, who began studying the financial impact of cancer after seeing patients ruined by medical bills. “Even if someone survives the cancer, they often can’t shake the debt.”
Dr. Shankaran found that cancer patients were 71% more likely than Americans without the disease to have bills in collections, face tax liens and mortgage foreclosure, or experience other financial setbacks. Analyzing bankruptcy records and cancer registries in Washington state, Dr. Shankaran and other researchers also discovered that cancer patients were 2½ times as likely to declare bankruptcy as those without the disease. And those who went bankrupt were likelier to die than cancer patients who did not.
Oncologists have a name for this: “financial toxicity,” a term that echoes the intractable vomiting, life-threatening infections, and other noxious effects of chemotherapy.
“Sometimes,” Dr. Shankaran said, “it’s tough to think about what the system puts patients through.”
Cancer diagnosis upends family
At the three-bedroom home in Rapid City that Ms. Peters shares with her children and a friend, there isn’t time most days to dwell on these worries. There are ice skating lessons and driving tests and countless meals to prepare. Teenagers drift in and out, chattering about homework and tattoos and driving.
The smallest children congregate at a small kitchen table under a wall decorated with seven old telephones. (As Ms. Peters tells it, the red one is a hotline to Santa, a green one to the Grinch, and a space shuttle–shaped phone connects to astronauts orbiting the Earth.)
Ms. Peters, 44, presides cheerfully over the chaos, directing her children with snide asides and expressions of love. She watches proudly as one teenage daughter helps another with math in the living room. Later she dances with a 5-year-old to Queen under a disco ball in the entry hall.
Ms. Peters, who sports tattoos and earlier this year dyed her hair purple, never planned to have a family. In her late 30s, she wanted to do more for her adopted community, so she took in foster children, many of whom come from the nearby Pine Ridge Indian Reservation. One of her daughters had been homeless.
“Foster kids are amazing humans,” she said. “I joke I’m the most reluctant parent of the most amazing children that have ever existed. And I get to help raise these little people to be healthy and safe.”
In spring 2020, the secure world Ms. Peters had carefully tended was shattered. As the COVID pandemic spread across the country, she was diagnosed with stage 2 breast cancer.
Within weeks, she had an intravenous port inserted into her chest. Surgeons removed both her breasts, then her ovaries after tests showed she was at risk of ovarian cancer as well.
Cancer treatment today often entails a costly, debilitating march of procedures, infusions, and radiation sessions that can exhaust patients physically and emotionally. It was scary, Ms. Peters said. But she rallied her children. “We talked a lot about how they had all lost siblings or parents or other relatives,” she said. “All I had to do was lose my boobs.”
Much harder, she said, were the endless and perplexing medical bills.
There were bills from the anesthesiologists who attended her surgeries, from the hospital, and from a surgery center. For a while, the hospital stopped sending bills. Then in April, Ms. Peters got a call one morning from a bill collector saying she owed $13,000. In total, Ms. Peters estimates her medical debts now exceed $30,000.
High costs, despite insurance
Debts of that size aren’t unusual. Nationwide, about one in five indebted adults who have had cancer or have a family member who’s been sick say they owe $10,000 or more, according to the KFF poll. Those dealing with cancer are also more likely than others with health care debt to owe large sums and to say they don’t expect to ever pay them off.
This debt has been fueled in part by the advent of lifesaving therapies that also come with eye-popping price tags. The National Cancer Institute calculated the average cost of medical care and drugs tops $42,000 in the year following a cancer diagnosis. Some treatments can exceed $1 million.
Usually, most costs are covered. But patients are increasingly on the hook for large bills because of deductibles and other health plan cost sharing. The average leukemia patient with private health insurance, for example, can expect to pay more than $5,100 in the year after diagnosis, according to an analysis by the consulting firm Milliman. Even Medicare can leave seniors with huge bills. The average blood cancer patient covered by fee-for-service Medicare can expect to pay more than $17,000 out-of-pocket in the year following diagnosis, Milliman found.
Additionally, ongoing surgeries, tests, and medications can make patients pay large out-of-pocket costs year after year. Physicians and patient advocates say this cost sharing -- originally billed as a way to encourage patients to shop for care -- is devastating. “The problem is that model doesn’t work very well with cancer,” said David Eagle, MD, an oncologist at New York Cancer & Blood Specialists.
More broadly, the KHN-NPR investigation found that about 100 million people in the United States are now in debt from medical or dental bills. Poor health is among the most powerful predictors of debt, with this debt concentrated in parts of the country with the highest levels of illness.
According to the KFF poll, 6 in 10 adults with a chronic disease such as cancer, diabetes, or heart disease or with a close family member who is sick have had some kind of health care debt in the past 5 years. The poll was designed to capture not just bills patients haven’t paid, but also other borrowing used to pay for health care, such as credit cards, payment plans, and loans from friends and family.
For her part, Ms. Peters has had seven surgeries since 2020. Through it all, she had health insurance through her employers. Ms. Peters said she knew she had to keep working or would lose coverage and face even bigger bills. Like most plans, however, hers have required she pay thousands of dollars out of pocket.
Within weeks of her diagnosis, the bills rolled in. Then collectors started calling. One call came as Ms. Peters was lying in the recovery room after her double mastectomy. “I was kind of delirious, and I thought it was my kids,” she said. “It was someone asking me to pay a medical bill.”
Ms. Peters faced more bills when she switched jobs later that year and her insurance changed. The deductible and cap on her out-of-pocket costs reset.
In 2021, the deductible and out-of-pocket limit reset again, as they do every year for most health plans. So when Ms. Peters slipped on the ice and broke her wrist – a fracture likely made worse by chemotherapy that weakened her bones – she was charged thousands more.
This year has brought more surgeries and yet more bills, as her deductible and out-of-pocket limit reset again.
“I don’t even know anymore how much I owe,” Ms. Peters said. “Sometimes it feels like people just send me random bills. I don’t even know what they’re for.”
Making sacrifices
Before getting sick, Ms. Peters was earning about $60,000 a year. It was enough to provide for her children, she said, supplemented with a stipend she receives for foster care.
The family budget was always tight. Ms. Peters and her kids don’t take extravagant vacations. Ms. Peters doesn’t own her home and has next to no savings. Now, she said, they are living at the edge. “I keep praying there is a shoe fairy,” she said, joking about the demands of so many growing feet in her home.
Ms. Peters took on extra work to pay some of the bills. Five days a week, she works back-to-back shifts at both a mental health crisis center and a clinic where she counsels teenagers, some of whom are suicidal. In 2021, three friends on the East Coast paid off some of the debt.
But Ms. Peters’ credit score has tumbled below 600. And the bills pile high on the microwave in her kitchen. “I’m middle class,” she said. “Could I make payments on some of these? Yes, I suppose I could.”
That would require trade-offs. She could drop car insurance for her teenage daughter, who just got her license. Canceling ice skating for another daughter would yield an extra $60 a month. But Ms. Peters is reluctant. “Do you know what it feels like to be a foster kid and get a gold medal in ice skating? Do you know what kind of citizen they could become if they know they’re special?” she said. “There seems to be a myth that you can pay for it all. You can’t.”
Many cancer patients face difficult choices.
About 4 in 10 with debt have taken money out of a retirement, college, or other long-term savings account, the KFF poll found; about 3 in 10 have moved in with family or friends or made another change in their living situation.
Kashyap Patel, MD, chief executive of Carolina Blood and Cancer Care Associates, said the South Carolina practice has found patients turning to food banks and other charities to get by. One patient was living in his car. Dr. Patel estimated that half the patients need some kind of financial aid. Even then, many end up in debt.
The Leukemia & Lymphoma Society, which typically helps blood cancer patients navigate health insurance and find food, housing, and other nonmedical assistance, is hearing from more patients simply seeking cash to pay off debt, said Nikki Yuill, who oversees the group’s call center. “People tell us they won’t get follow-up care because they can’t take on more debt,” Ms. Yuill said, recalling one man who refused to call an ambulance even though he couldn’t get to the hospital. “It breaks your heart.”
Academic research has revealed widespread self-rationing by patients. For example, while nearly one in five people taking oral chemotherapy abandon treatment, about half stop when out-of-pocket costs exceed $2,000, according to a 2017 analysis.
Robin Yabroff, PhD, MBA, an epidemiologist at the American Cancer Society, said more research must be done to understand the lasting effects of medical debt on cancer survivors and their families. “What does it mean for a family if they have to liquidate savings or drain college funds or sell their home?” Dr. Yabroff said. “We just don’t know yet.”
As Ms. Peters put away bags of groceries in her kitchen, she conceded she doesn’t know what will happen to her family. Like many patients, she worries about how she’ll pay for tests and follow-up care if the cancer reappears.
She is still wading through collection notices in the mail and fielding calls from debt collectors. Ms. Peters told one that she was prepared to go to court and ask the judge to decide which of her children should be cut off from after-school activities to pay off the debts.
She asked another debt collector whether he had kids. “He told me that it had been my choice to get the surgery,” Ms. Peters recalled. “And I said: ‘Yeah, I guess I chose not to be dead.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Oncologists often misinterpret posttreatment HNSCC scan details
Patient outcomes could be threatened because of misinterpretation by oncologic surgeons of free-form posttreatment radiological reports in head and neck squamous cell carcinoma (HNSCC), a new study finds.
“Clinician perception of patient response from the post-RT [radiation treament] PET/CT free-form report is unreliable and does not consistently reflect the radiologist’s intended meaning, which was strongly associated with survival,” researchers wrote in a study published Aug. 18 in JAMA Otolaryngology-Head & Neck Surgery. They found “minimal agreement between clinicians’ consensus perspective on the patient’s response status derived from free-form imaging reports and the criterion standard response category assigned by a nuclear medicine specialist after PET/CT image review.”
According to radiation oncologist Ryan T. Hughes, MD, and colleagues at Wake Forest University, Winston-Salem, N.C., it’s common for patients with HNSCC to get PET, CT, or PET/CT imaging following treatment in order to assess how patients responded. Accurate communication about the results is essential to determining next steps, they write.
However, they write, “to our knowledge there is no universally accepted standardized method for communicating results,” such as whether there’s been a complete or partial response. Discrepancies between a radiological posttreatment report and an oncologist’s perception of the findings “may contribute to unnecessary patient care complexities, including elevated patient anxiety, unnecessary follow-up testing/procedures, and failure to recognize and adequately treat residual, recurrent, or progressive disease,” the researchers write.
For the new study, the authors tracked 171 patients (26.3% women, median age 61 years, ethnicity not provided), mainly (87%) with stage III-IV disease. Most (89%) received concurrent chemotherapy, and 30% received radiotherapy following operations.
Four oncologists reviewed free-form radiologic reports and determined whether the patient had a complete, indeterminate or partial response, or progressive disease. “Next, the group conferred to assign a consensus clinician MDS [modified Deauville score] and associated response category to assess the percentage of agreement with the criterion standard nuclear medicine physician MDS response derived from PET/CT image review.”
The researchers found that “interrater reliability of clinician-perceived post-RT PET/CT response was moderate [k = 0.680; 95% confidence interval, 0.638-0.721], and there was minimal reliability and low rate of agreement between clinician perception and radiologist-intended PET/CT response [63.7%; k = 0.365; 95% CI, 0.251-0.478).”
The clinicians were more likely to perceive patients as having an indeterminate response (28.1%), compared with the radiologists (9.3%). “There were 16 instances of significant discordance: 7 patients for whom the clinician perception MDS was 1 to 2 and nuclear medicine MDS 3 to 4, and 9 patients for whom the clinician perception MDS was 3 to 4 and nuclear medicine MDS 1 to 2.”
Due to statistical limitations, the researchers were unable to link the MDS scores to prognoses. The researchers suggest it’s time to further standardize the assessment of posttreatment responses to therapy. They add that “the decision to use a standardized interpretation and reporting system rather than free-form reporting is more important than the specific system selected.”
As for next steps, the researchers report that “prospective studies of post-RT PET/CT standardized reporting among patients with HNSCC are warranted, and a prospective implementation study of this workflow is planned at our institution.”
The study was funded by the National Center for Advancing Translational Sciences and National Institutes of Health. The authors had no disclosures.
Patient outcomes could be threatened because of misinterpretation by oncologic surgeons of free-form posttreatment radiological reports in head and neck squamous cell carcinoma (HNSCC), a new study finds.
“Clinician perception of patient response from the post-RT [radiation treament] PET/CT free-form report is unreliable and does not consistently reflect the radiologist’s intended meaning, which was strongly associated with survival,” researchers wrote in a study published Aug. 18 in JAMA Otolaryngology-Head & Neck Surgery. They found “minimal agreement between clinicians’ consensus perspective on the patient’s response status derived from free-form imaging reports and the criterion standard response category assigned by a nuclear medicine specialist after PET/CT image review.”
According to radiation oncologist Ryan T. Hughes, MD, and colleagues at Wake Forest University, Winston-Salem, N.C., it’s common for patients with HNSCC to get PET, CT, or PET/CT imaging following treatment in order to assess how patients responded. Accurate communication about the results is essential to determining next steps, they write.
However, they write, “to our knowledge there is no universally accepted standardized method for communicating results,” such as whether there’s been a complete or partial response. Discrepancies between a radiological posttreatment report and an oncologist’s perception of the findings “may contribute to unnecessary patient care complexities, including elevated patient anxiety, unnecessary follow-up testing/procedures, and failure to recognize and adequately treat residual, recurrent, or progressive disease,” the researchers write.
For the new study, the authors tracked 171 patients (26.3% women, median age 61 years, ethnicity not provided), mainly (87%) with stage III-IV disease. Most (89%) received concurrent chemotherapy, and 30% received radiotherapy following operations.
Four oncologists reviewed free-form radiologic reports and determined whether the patient had a complete, indeterminate or partial response, or progressive disease. “Next, the group conferred to assign a consensus clinician MDS [modified Deauville score] and associated response category to assess the percentage of agreement with the criterion standard nuclear medicine physician MDS response derived from PET/CT image review.”
The researchers found that “interrater reliability of clinician-perceived post-RT PET/CT response was moderate [k = 0.680; 95% confidence interval, 0.638-0.721], and there was minimal reliability and low rate of agreement between clinician perception and radiologist-intended PET/CT response [63.7%; k = 0.365; 95% CI, 0.251-0.478).”
The clinicians were more likely to perceive patients as having an indeterminate response (28.1%), compared with the radiologists (9.3%). “There were 16 instances of significant discordance: 7 patients for whom the clinician perception MDS was 1 to 2 and nuclear medicine MDS 3 to 4, and 9 patients for whom the clinician perception MDS was 3 to 4 and nuclear medicine MDS 1 to 2.”
Due to statistical limitations, the researchers were unable to link the MDS scores to prognoses. The researchers suggest it’s time to further standardize the assessment of posttreatment responses to therapy. They add that “the decision to use a standardized interpretation and reporting system rather than free-form reporting is more important than the specific system selected.”
As for next steps, the researchers report that “prospective studies of post-RT PET/CT standardized reporting among patients with HNSCC are warranted, and a prospective implementation study of this workflow is planned at our institution.”
The study was funded by the National Center for Advancing Translational Sciences and National Institutes of Health. The authors had no disclosures.
Patient outcomes could be threatened because of misinterpretation by oncologic surgeons of free-form posttreatment radiological reports in head and neck squamous cell carcinoma (HNSCC), a new study finds.
“Clinician perception of patient response from the post-RT [radiation treament] PET/CT free-form report is unreliable and does not consistently reflect the radiologist’s intended meaning, which was strongly associated with survival,” researchers wrote in a study published Aug. 18 in JAMA Otolaryngology-Head & Neck Surgery. They found “minimal agreement between clinicians’ consensus perspective on the patient’s response status derived from free-form imaging reports and the criterion standard response category assigned by a nuclear medicine specialist after PET/CT image review.”
According to radiation oncologist Ryan T. Hughes, MD, and colleagues at Wake Forest University, Winston-Salem, N.C., it’s common for patients with HNSCC to get PET, CT, or PET/CT imaging following treatment in order to assess how patients responded. Accurate communication about the results is essential to determining next steps, they write.
However, they write, “to our knowledge there is no universally accepted standardized method for communicating results,” such as whether there’s been a complete or partial response. Discrepancies between a radiological posttreatment report and an oncologist’s perception of the findings “may contribute to unnecessary patient care complexities, including elevated patient anxiety, unnecessary follow-up testing/procedures, and failure to recognize and adequately treat residual, recurrent, or progressive disease,” the researchers write.
For the new study, the authors tracked 171 patients (26.3% women, median age 61 years, ethnicity not provided), mainly (87%) with stage III-IV disease. Most (89%) received concurrent chemotherapy, and 30% received radiotherapy following operations.
Four oncologists reviewed free-form radiologic reports and determined whether the patient had a complete, indeterminate or partial response, or progressive disease. “Next, the group conferred to assign a consensus clinician MDS [modified Deauville score] and associated response category to assess the percentage of agreement with the criterion standard nuclear medicine physician MDS response derived from PET/CT image review.”
The researchers found that “interrater reliability of clinician-perceived post-RT PET/CT response was moderate [k = 0.680; 95% confidence interval, 0.638-0.721], and there was minimal reliability and low rate of agreement between clinician perception and radiologist-intended PET/CT response [63.7%; k = 0.365; 95% CI, 0.251-0.478).”
The clinicians were more likely to perceive patients as having an indeterminate response (28.1%), compared with the radiologists (9.3%). “There were 16 instances of significant discordance: 7 patients for whom the clinician perception MDS was 1 to 2 and nuclear medicine MDS 3 to 4, and 9 patients for whom the clinician perception MDS was 3 to 4 and nuclear medicine MDS 1 to 2.”
Due to statistical limitations, the researchers were unable to link the MDS scores to prognoses. The researchers suggest it’s time to further standardize the assessment of posttreatment responses to therapy. They add that “the decision to use a standardized interpretation and reporting system rather than free-form reporting is more important than the specific system selected.”
As for next steps, the researchers report that “prospective studies of post-RT PET/CT standardized reporting among patients with HNSCC are warranted, and a prospective implementation study of this workflow is planned at our institution.”
The study was funded by the National Center for Advancing Translational Sciences and National Institutes of Health. The authors had no disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Increased risk of dyspareunia following cesarean section
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
Stable, long-term opioid therapy safer than tapering?
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.
The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.
The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.
The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN