User login
AVAHO 2022: The Importance of Self-care
Bernadette Heron, PharmD, provides a glance at activities planned for the approaching AVAHO 2022 annual meeting and announces with enthusiasm this year's theme: "Your Best Version: Self-care in Cancer Care."
Bringing self-care into the theater of clinically relevant issues is central to optimizing the care of patients with cancer, Dr Heron suggests. To this purpose, the structured sessions and interventions to be presented at AVAHO 2022 include a deep dive into burnout among practitioners as well as everyday clinical care issues.
Bernadette Heron, PharmD, provides a glance at activities planned for the approaching AVAHO 2022 annual meeting and announces with enthusiasm this year's theme: "Your Best Version: Self-care in Cancer Care."
Bringing self-care into the theater of clinically relevant issues is central to optimizing the care of patients with cancer, Dr Heron suggests. To this purpose, the structured sessions and interventions to be presented at AVAHO 2022 include a deep dive into burnout among practitioners as well as everyday clinical care issues.
Bernadette Heron, PharmD, provides a glance at activities planned for the approaching AVAHO 2022 annual meeting and announces with enthusiasm this year's theme: "Your Best Version: Self-care in Cancer Care."
Bringing self-care into the theater of clinically relevant issues is central to optimizing the care of patients with cancer, Dr Heron suggests. To this purpose, the structured sessions and interventions to be presented at AVAHO 2022 include a deep dive into burnout among practitioners as well as everyday clinical care issues.
AVAHO 2022: Bringing Provider Well-Being Into Focus
Nick Burwick, MD, summarizes a series of topics to be addressed at the AVAHO 2022 annual meeting, including provider well-being, patient experience, multidisciplinary reflective care, and precision oncology.
In addition to the exciting presentations and sessions, Dr Burwick invokes the more informal aspects of AVAHO 2022, such as meeting colleagues and friends and taking the time to appreciate the host city of San Diego. Above all, Dr Burwick underscores this year’s theme of self-care and resilience in the cancer care setting.
Nick Burwick, MD, summarizes a series of topics to be addressed at the AVAHO 2022 annual meeting, including provider well-being, patient experience, multidisciplinary reflective care, and precision oncology.
In addition to the exciting presentations and sessions, Dr Burwick invokes the more informal aspects of AVAHO 2022, such as meeting colleagues and friends and taking the time to appreciate the host city of San Diego. Above all, Dr Burwick underscores this year’s theme of self-care and resilience in the cancer care setting.
Nick Burwick, MD, summarizes a series of topics to be addressed at the AVAHO 2022 annual meeting, including provider well-being, patient experience, multidisciplinary reflective care, and precision oncology.
In addition to the exciting presentations and sessions, Dr Burwick invokes the more informal aspects of AVAHO 2022, such as meeting colleagues and friends and taking the time to appreciate the host city of San Diego. Above all, Dr Burwick underscores this year’s theme of self-care and resilience in the cancer care setting.
MS Researchers Wonder Aloud: Is Remyelination Possible?
The 3 “Rs” of multiple sclerosis (MS)—repair, remyelinate, and restore—spell out the goals of patients and physicians alike. MS is an incurable, immune-mediated, neurodegenerative disease of the central nervous system (CNS), and is thought to develop from unexplained autoimmune attacks directed at myelin (the covering on neurons) and glial cells, or “oligodendrocytes.” Neurodegeneration is evident early in the disease process and is characterized by mitochondrial dysfunction, energy failure, and neuronal and glial death.
While most new and investigational therapies aim to address immune dysfunction, a new idea—
one not involving immune dysregulation—is being explored in various studies: Are there agents, outside of traditional MS therapies, able to help with remyelination?
Mitochondria, oxidative stress, and MS
Neurons, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) are particularly sensitive to oxidative stress. In MS, chronic inflammation and autoimmunity are key drivers of oxidative stress and secondary mitochondrial dysfunction.
Mitochondrial dysfunction is particularly relevant for neurodegeneration in MS. The observed dysfunction includes mitochondrial DNA damage, deficiency in mitochondrial DNA repair, reduced levels of antioxidants, and increased free radicals. Furthermore, the structure and number of mitochondria temporarily increase to accommodate the increased energy needs. Despite the attempted adaptation, energy failure ultimately occurs, resulting in a mismatch between energy needs or consumption and energy production. Neuroinflammation and the imbalance between energy consumption and generation create a vicious, continuous cycle that is characteristic in progressive MS. The energy failure is then associated with neuronal death, Wallerian degeneration, and subsequent accumulation of neurologic disability.
Current therapeutic landscape
While the therapeutic landscape for MS continues to evolve, the approved 20-plus therapies are primarily directed at the immune system. The overall goal is to modulate immune dysregulation and decrease inflammation. Current therapies may be able to control this macroscopic inflammatory activity.
However, current treatments only show modest effects on disease progression, and do not help to repair neurons, remyelinate axons, or restore function that was impaired due to disease progression. Some US Food and Drug Administration (FDA)–approved therapies are thought to modulate mitochondrial functions. For example, the class of fumarates (eg, dimethyl fumarate, diroximel fumarate, monomethyl fumarate) activates the nuclear factor erythroid 2 -related factor 2 (Nrf2) pathway in treated MS patients. However, it is unclear whether activation of the Nrf2 pathway is involved in the therapeutic effects of fumarates. A recent study challenged the importance of the Nrf2 pathway as a therapeutic target for fumarates. It showed that in an MS animal model, the effects of fumarates on disease control were similar between Nrf2 knock-out mice and the wild type, suggesting that fumarates' therapeutic effects are independent of the Nrf2 pathway. Furthermore, fumarates failed to show benefits in progressive forms of MS both clinically and on a biomarker level.
Metformin, the mitochondria, and neurodegeneration
Metformin (1,1-dimethylbiguanide) is an oral medication used primarily as first-line treatment for type 2 diabetes. However, due to its pharmacologic properties, mitochondrial effects, and the ability to cross the blood-brain barrier, scientists have shown recent interest in studying metformin in neurodegenerative diseases, including MS. Some of the potential benefits of metformin in neurodegenerative diseases include reduction of oxidative stress and countering mitochondrial dysfunction. It is known that metformin inhibits mitochondrial complex 1. Also, several studies have shown a positive effect of metformin on the reduction of oxidative stress and mitochondrial DNA regulation. Therefore, could metformin help combat mitochondrial dysfunction in MS or rejuvenate certain elements within the CNS in people with neurodegenerative diseases, including MS?
Oligodendrocytes and remyelination
Oligodendrocytes are cells responsible for myelinating axons within the CNS. Those cells originate from progenitors called OPCs. Interestingly, in humans, OPCs can mature into oligodendrocytes throughout their lifecycle, although to a much lesser extent in adults compared with children. However, therapeutic efforts to facilitate OPC maturation in vivo in MS lesions have failed thus far. Examples include high-dose biotin, the anti-LINGO-1 opicinumab, and the anticancer, retinoid-analog drug bexarotene.
So, what is behind these unfortunate failures? Some molecules (eg, biotin, opicinumab) failed to meet their clinical endpoints in randomized clinical trials, while others had severe toxicity that halted further clinical testing (eg, bexarotene). On the other hand, some molecules (eg, clemastine fumarate), showed a modest yet promising effect on biomarkers in small clinical trials.
A discussion on molecule failures
What could explain the failure of molecules with such promising preclinical findings? One could argue that clinical trial designs may have been insufficient to detect small remyelinating effects. One could also argue that the maturation of OPCs into oligodendrocytes is too complex to facilitate using 1 molecule that may be an inhibitor of maturation or to activate/augment a facilitator of the maturation process. There are too many natural inhibitors and facilitators of OPC maturation, and an approach with combination therapy might have a better chance at achieving a favorable therapeutic effect.
Another piece of the complexity of OPC maturation is the recent discovery that, in humans, nonhuman primates, and other mammals, aged OPCs do not have the same capacity to mature into oligodendrocytes as young OPCs. There might be some clinical support here, as children with MS have more ability to recover from MS attacks than their adult counterparts. Also, the older the individual with MS is, the less likely they are to recover from MS attacks and the more likely they are to show signs of disease progression compared with their younger counterparts.
Theoretically, age-related recovery from clinical attacks may be partially explained by complications due to OPC aging. To this point, can we rejuvenate OPCs and restore their ability to mature into oligodendrocytes? Can metformin be the medicine that does so?
Interestingly, scientists could restore the ability of older OPCs to mature into oligodendrocytes, at least in the rodent model, through calorie restriction (eg, intermittent fasting) or by mimicking this state using metformin.
Metformin and the 3 “Rs”
One idea is to use metformin to create a biochemical state that allows OPCs to regain their ability to mature into oligodendrocytes in adult or aging individuals with MS. If that is achieved, other molecules may augment OPC' maturation or inhibit OPC maturation-inhibitors and become successful in promoting remyelination. A phase 2 clinical trial in the United Kingdom that is currently recruiting participants intends to investigate a combination of metformin and clemastine fumarate in 50 patients with relapsing-remitting MS. The goal is to learn whether metformin plus clemastine allows for therapeutic remyelination. In addition, a Canadian study is investigating metformin in children with MS. Two other studies are currently recruiting to study metformin in relapsing MS (Egypt) and progressive MS (United States).
Although testing metformin as a treatment for MS is still in the early stages, the scientific rationale is valid and supported by compelling preclinical evidence. Ongoing clinical trials will likely provide preliminary results on whether metformin will advance in clinical testing and provide clinically meaningful improvements for people living with MS.
If metformin is, in fact, a conditioning agent for use in remyelinating therapies, future clinical trials could be designed to administer metformin to rejuvenate OPCs before the administration of any molecule combination designed to facilitate OPC maturation. However, these trials will need to address an important issue: dosage. In type 2 diabetes, the typical daily dose is between 500 and 3000 mg per day. But in tests on rodents – which weigh about 10 grams – to rejuvenate OPCs, the doses of metformin were very high: 200 to 300 mg/kg. Given the body weight of humans and to avoid drug toxicity, the resulting smaller doses of metformin will take time to exert their potential therapeutic effect.
Should future research be successful in developing combination molecular therapies with diverse and synergistic therapeutic targets, then the 3 “Rs” in MS will allow for a fourth “R” to effectively succeed: repair, remyelinate, restore, and rehabilitate.
The 3 “Rs” of multiple sclerosis (MS)—repair, remyelinate, and restore—spell out the goals of patients and physicians alike. MS is an incurable, immune-mediated, neurodegenerative disease of the central nervous system (CNS), and is thought to develop from unexplained autoimmune attacks directed at myelin (the covering on neurons) and glial cells, or “oligodendrocytes.” Neurodegeneration is evident early in the disease process and is characterized by mitochondrial dysfunction, energy failure, and neuronal and glial death.
While most new and investigational therapies aim to address immune dysfunction, a new idea—
one not involving immune dysregulation—is being explored in various studies: Are there agents, outside of traditional MS therapies, able to help with remyelination?
Mitochondria, oxidative stress, and MS
Neurons, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) are particularly sensitive to oxidative stress. In MS, chronic inflammation and autoimmunity are key drivers of oxidative stress and secondary mitochondrial dysfunction.
Mitochondrial dysfunction is particularly relevant for neurodegeneration in MS. The observed dysfunction includes mitochondrial DNA damage, deficiency in mitochondrial DNA repair, reduced levels of antioxidants, and increased free radicals. Furthermore, the structure and number of mitochondria temporarily increase to accommodate the increased energy needs. Despite the attempted adaptation, energy failure ultimately occurs, resulting in a mismatch between energy needs or consumption and energy production. Neuroinflammation and the imbalance between energy consumption and generation create a vicious, continuous cycle that is characteristic in progressive MS. The energy failure is then associated with neuronal death, Wallerian degeneration, and subsequent accumulation of neurologic disability.
Current therapeutic landscape
While the therapeutic landscape for MS continues to evolve, the approved 20-plus therapies are primarily directed at the immune system. The overall goal is to modulate immune dysregulation and decrease inflammation. Current therapies may be able to control this macroscopic inflammatory activity.
However, current treatments only show modest effects on disease progression, and do not help to repair neurons, remyelinate axons, or restore function that was impaired due to disease progression. Some US Food and Drug Administration (FDA)–approved therapies are thought to modulate mitochondrial functions. For example, the class of fumarates (eg, dimethyl fumarate, diroximel fumarate, monomethyl fumarate) activates the nuclear factor erythroid 2 -related factor 2 (Nrf2) pathway in treated MS patients. However, it is unclear whether activation of the Nrf2 pathway is involved in the therapeutic effects of fumarates. A recent study challenged the importance of the Nrf2 pathway as a therapeutic target for fumarates. It showed that in an MS animal model, the effects of fumarates on disease control were similar between Nrf2 knock-out mice and the wild type, suggesting that fumarates' therapeutic effects are independent of the Nrf2 pathway. Furthermore, fumarates failed to show benefits in progressive forms of MS both clinically and on a biomarker level.
Metformin, the mitochondria, and neurodegeneration
Metformin (1,1-dimethylbiguanide) is an oral medication used primarily as first-line treatment for type 2 diabetes. However, due to its pharmacologic properties, mitochondrial effects, and the ability to cross the blood-brain barrier, scientists have shown recent interest in studying metformin in neurodegenerative diseases, including MS. Some of the potential benefits of metformin in neurodegenerative diseases include reduction of oxidative stress and countering mitochondrial dysfunction. It is known that metformin inhibits mitochondrial complex 1. Also, several studies have shown a positive effect of metformin on the reduction of oxidative stress and mitochondrial DNA regulation. Therefore, could metformin help combat mitochondrial dysfunction in MS or rejuvenate certain elements within the CNS in people with neurodegenerative diseases, including MS?
Oligodendrocytes and remyelination
Oligodendrocytes are cells responsible for myelinating axons within the CNS. Those cells originate from progenitors called OPCs. Interestingly, in humans, OPCs can mature into oligodendrocytes throughout their lifecycle, although to a much lesser extent in adults compared with children. However, therapeutic efforts to facilitate OPC maturation in vivo in MS lesions have failed thus far. Examples include high-dose biotin, the anti-LINGO-1 opicinumab, and the anticancer, retinoid-analog drug bexarotene.
So, what is behind these unfortunate failures? Some molecules (eg, biotin, opicinumab) failed to meet their clinical endpoints in randomized clinical trials, while others had severe toxicity that halted further clinical testing (eg, bexarotene). On the other hand, some molecules (eg, clemastine fumarate), showed a modest yet promising effect on biomarkers in small clinical trials.
A discussion on molecule failures
What could explain the failure of molecules with such promising preclinical findings? One could argue that clinical trial designs may have been insufficient to detect small remyelinating effects. One could also argue that the maturation of OPCs into oligodendrocytes is too complex to facilitate using 1 molecule that may be an inhibitor of maturation or to activate/augment a facilitator of the maturation process. There are too many natural inhibitors and facilitators of OPC maturation, and an approach with combination therapy might have a better chance at achieving a favorable therapeutic effect.
Another piece of the complexity of OPC maturation is the recent discovery that, in humans, nonhuman primates, and other mammals, aged OPCs do not have the same capacity to mature into oligodendrocytes as young OPCs. There might be some clinical support here, as children with MS have more ability to recover from MS attacks than their adult counterparts. Also, the older the individual with MS is, the less likely they are to recover from MS attacks and the more likely they are to show signs of disease progression compared with their younger counterparts.
Theoretically, age-related recovery from clinical attacks may be partially explained by complications due to OPC aging. To this point, can we rejuvenate OPCs and restore their ability to mature into oligodendrocytes? Can metformin be the medicine that does so?
Interestingly, scientists could restore the ability of older OPCs to mature into oligodendrocytes, at least in the rodent model, through calorie restriction (eg, intermittent fasting) or by mimicking this state using metformin.
Metformin and the 3 “Rs”
One idea is to use metformin to create a biochemical state that allows OPCs to regain their ability to mature into oligodendrocytes in adult or aging individuals with MS. If that is achieved, other molecules may augment OPC' maturation or inhibit OPC maturation-inhibitors and become successful in promoting remyelination. A phase 2 clinical trial in the United Kingdom that is currently recruiting participants intends to investigate a combination of metformin and clemastine fumarate in 50 patients with relapsing-remitting MS. The goal is to learn whether metformin plus clemastine allows for therapeutic remyelination. In addition, a Canadian study is investigating metformin in children with MS. Two other studies are currently recruiting to study metformin in relapsing MS (Egypt) and progressive MS (United States).
Although testing metformin as a treatment for MS is still in the early stages, the scientific rationale is valid and supported by compelling preclinical evidence. Ongoing clinical trials will likely provide preliminary results on whether metformin will advance in clinical testing and provide clinically meaningful improvements for people living with MS.
If metformin is, in fact, a conditioning agent for use in remyelinating therapies, future clinical trials could be designed to administer metformin to rejuvenate OPCs before the administration of any molecule combination designed to facilitate OPC maturation. However, these trials will need to address an important issue: dosage. In type 2 diabetes, the typical daily dose is between 500 and 3000 mg per day. But in tests on rodents – which weigh about 10 grams – to rejuvenate OPCs, the doses of metformin were very high: 200 to 300 mg/kg. Given the body weight of humans and to avoid drug toxicity, the resulting smaller doses of metformin will take time to exert their potential therapeutic effect.
Should future research be successful in developing combination molecular therapies with diverse and synergistic therapeutic targets, then the 3 “Rs” in MS will allow for a fourth “R” to effectively succeed: repair, remyelinate, restore, and rehabilitate.
The 3 “Rs” of multiple sclerosis (MS)—repair, remyelinate, and restore—spell out the goals of patients and physicians alike. MS is an incurable, immune-mediated, neurodegenerative disease of the central nervous system (CNS), and is thought to develop from unexplained autoimmune attacks directed at myelin (the covering on neurons) and glial cells, or “oligodendrocytes.” Neurodegeneration is evident early in the disease process and is characterized by mitochondrial dysfunction, energy failure, and neuronal and glial death.
While most new and investigational therapies aim to address immune dysfunction, a new idea—
one not involving immune dysregulation—is being explored in various studies: Are there agents, outside of traditional MS therapies, able to help with remyelination?
Mitochondria, oxidative stress, and MS
Neurons, oligodendrocytes, and oligodendrocyte precursor cells (OPCs) are particularly sensitive to oxidative stress. In MS, chronic inflammation and autoimmunity are key drivers of oxidative stress and secondary mitochondrial dysfunction.
Mitochondrial dysfunction is particularly relevant for neurodegeneration in MS. The observed dysfunction includes mitochondrial DNA damage, deficiency in mitochondrial DNA repair, reduced levels of antioxidants, and increased free radicals. Furthermore, the structure and number of mitochondria temporarily increase to accommodate the increased energy needs. Despite the attempted adaptation, energy failure ultimately occurs, resulting in a mismatch between energy needs or consumption and energy production. Neuroinflammation and the imbalance between energy consumption and generation create a vicious, continuous cycle that is characteristic in progressive MS. The energy failure is then associated with neuronal death, Wallerian degeneration, and subsequent accumulation of neurologic disability.
Current therapeutic landscape
While the therapeutic landscape for MS continues to evolve, the approved 20-plus therapies are primarily directed at the immune system. The overall goal is to modulate immune dysregulation and decrease inflammation. Current therapies may be able to control this macroscopic inflammatory activity.
However, current treatments only show modest effects on disease progression, and do not help to repair neurons, remyelinate axons, or restore function that was impaired due to disease progression. Some US Food and Drug Administration (FDA)–approved therapies are thought to modulate mitochondrial functions. For example, the class of fumarates (eg, dimethyl fumarate, diroximel fumarate, monomethyl fumarate) activates the nuclear factor erythroid 2 -related factor 2 (Nrf2) pathway in treated MS patients. However, it is unclear whether activation of the Nrf2 pathway is involved in the therapeutic effects of fumarates. A recent study challenged the importance of the Nrf2 pathway as a therapeutic target for fumarates. It showed that in an MS animal model, the effects of fumarates on disease control were similar between Nrf2 knock-out mice and the wild type, suggesting that fumarates' therapeutic effects are independent of the Nrf2 pathway. Furthermore, fumarates failed to show benefits in progressive forms of MS both clinically and on a biomarker level.
Metformin, the mitochondria, and neurodegeneration
Metformin (1,1-dimethylbiguanide) is an oral medication used primarily as first-line treatment for type 2 diabetes. However, due to its pharmacologic properties, mitochondrial effects, and the ability to cross the blood-brain barrier, scientists have shown recent interest in studying metformin in neurodegenerative diseases, including MS. Some of the potential benefits of metformin in neurodegenerative diseases include reduction of oxidative stress and countering mitochondrial dysfunction. It is known that metformin inhibits mitochondrial complex 1. Also, several studies have shown a positive effect of metformin on the reduction of oxidative stress and mitochondrial DNA regulation. Therefore, could metformin help combat mitochondrial dysfunction in MS or rejuvenate certain elements within the CNS in people with neurodegenerative diseases, including MS?
Oligodendrocytes and remyelination
Oligodendrocytes are cells responsible for myelinating axons within the CNS. Those cells originate from progenitors called OPCs. Interestingly, in humans, OPCs can mature into oligodendrocytes throughout their lifecycle, although to a much lesser extent in adults compared with children. However, therapeutic efforts to facilitate OPC maturation in vivo in MS lesions have failed thus far. Examples include high-dose biotin, the anti-LINGO-1 opicinumab, and the anticancer, retinoid-analog drug bexarotene.
So, what is behind these unfortunate failures? Some molecules (eg, biotin, opicinumab) failed to meet their clinical endpoints in randomized clinical trials, while others had severe toxicity that halted further clinical testing (eg, bexarotene). On the other hand, some molecules (eg, clemastine fumarate), showed a modest yet promising effect on biomarkers in small clinical trials.
A discussion on molecule failures
What could explain the failure of molecules with such promising preclinical findings? One could argue that clinical trial designs may have been insufficient to detect small remyelinating effects. One could also argue that the maturation of OPCs into oligodendrocytes is too complex to facilitate using 1 molecule that may be an inhibitor of maturation or to activate/augment a facilitator of the maturation process. There are too many natural inhibitors and facilitators of OPC maturation, and an approach with combination therapy might have a better chance at achieving a favorable therapeutic effect.
Another piece of the complexity of OPC maturation is the recent discovery that, in humans, nonhuman primates, and other mammals, aged OPCs do not have the same capacity to mature into oligodendrocytes as young OPCs. There might be some clinical support here, as children with MS have more ability to recover from MS attacks than their adult counterparts. Also, the older the individual with MS is, the less likely they are to recover from MS attacks and the more likely they are to show signs of disease progression compared with their younger counterparts.
Theoretically, age-related recovery from clinical attacks may be partially explained by complications due to OPC aging. To this point, can we rejuvenate OPCs and restore their ability to mature into oligodendrocytes? Can metformin be the medicine that does so?
Interestingly, scientists could restore the ability of older OPCs to mature into oligodendrocytes, at least in the rodent model, through calorie restriction (eg, intermittent fasting) or by mimicking this state using metformin.
Metformin and the 3 “Rs”
One idea is to use metformin to create a biochemical state that allows OPCs to regain their ability to mature into oligodendrocytes in adult or aging individuals with MS. If that is achieved, other molecules may augment OPC' maturation or inhibit OPC maturation-inhibitors and become successful in promoting remyelination. A phase 2 clinical trial in the United Kingdom that is currently recruiting participants intends to investigate a combination of metformin and clemastine fumarate in 50 patients with relapsing-remitting MS. The goal is to learn whether metformin plus clemastine allows for therapeutic remyelination. In addition, a Canadian study is investigating metformin in children with MS. Two other studies are currently recruiting to study metformin in relapsing MS (Egypt) and progressive MS (United States).
Although testing metformin as a treatment for MS is still in the early stages, the scientific rationale is valid and supported by compelling preclinical evidence. Ongoing clinical trials will likely provide preliminary results on whether metformin will advance in clinical testing and provide clinically meaningful improvements for people living with MS.
If metformin is, in fact, a conditioning agent for use in remyelinating therapies, future clinical trials could be designed to administer metformin to rejuvenate OPCs before the administration of any molecule combination designed to facilitate OPC maturation. However, these trials will need to address an important issue: dosage. In type 2 diabetes, the typical daily dose is between 500 and 3000 mg per day. But in tests on rodents – which weigh about 10 grams – to rejuvenate OPCs, the doses of metformin were very high: 200 to 300 mg/kg. Given the body weight of humans and to avoid drug toxicity, the resulting smaller doses of metformin will take time to exert their potential therapeutic effect.
Should future research be successful in developing combination molecular therapies with diverse and synergistic therapeutic targets, then the 3 “Rs” in MS will allow for a fourth “R” to effectively succeed: repair, remyelinate, restore, and rehabilitate.
Understanding GERD phenotypes
Approximately 30% of U.S. adults experience troublesome reflux symptoms of heartburn, regurgitation and noncardiac chest pain. Because the mechanisms driving symptoms vary across patients, phenotyping patients via a step-wise diagnostic framework effectively guides personalized management in GERD.
For instance, PPI trials are appropriate when esophageal symptoms are present, whereas up-front reflux monitoring rather than empiric PPI trials are recommended for evaluation of isolated extra-esophageal symptoms. All patients undergoing evaluation for GERD should receive counseling on weight management and lifestyle modifications as well as the brain-gut axis relationship. In the common scenario of inadequate symptom response to PPIs, upper GI endoscopy is recommended to assess for erosive reflux disease (which confirms a diagnosis of GERD) as well as the anti-reflux barrier integrity. For instance, the presence of a large hiatal hernia and/or grade III/IV gastro-esophageal flap valve may point to mechanical gastro-esophageal reflux as a driver of symptoms and lower the threshold for surgical referral. In the absence of erosive reflux disease the next recommended step is ambulatory reflux monitoring off PPI therapy, either as prolonged wireless telemetry (which can be done concurrently with index endoscopy as long as PPI was discontinued > 7 days) or 24-hour transnasal pH-impedance catheter-based testing. Studies suggest that 96-hour monitoring is optimal for diagnostic accuracy and to guide therapeutic strategies.
Patients without evidence of GERD on endoscopy or ambulatory reflux monitoring likely have a functional esophageal disorder for which therapy hinges on pharmacologic neuromodulation or behavioral interventions as well as PPI cessation.
Alternatively, management for GERD (erosive or nonerosive) aims to optimize lifestyle, PPI therapy and the individualized use of adjunctive therapy, which include H2-receptor antagonists, alginate antacids, GABA agonists, neuromodulation and/or behavioral interventions. Surgical or endoscopic antireflux interventions are also an option for refractory GERD. Prior to intervention, achalasia must be excluded (typically with esophageal manometry), and confirmation of PPI refractory GERD on pH-impedance monitoring on PPI is of value, particularly when the phenotype is unclear. Again, the choice of antireflux intervention (e.g., laparoscopic fundoplication, magnetic sphincter augmentation, transoral incisionless fundoplication, Roux-en-Y gastric bypass) should be individualized to the patient’s anatomy, physiology, and clinical profile.
A multitude of treatment options are available to manage GERD, including behavioral interventions, lifestyle modifications, pharmacotherapy, and endoscopic/surgical interventions. However, not every treatment strategy is appropriate for every patient. Data gathered from the step-down diagnostic approach, which starts with clinical presentation, then endoscopy, then reflux monitoring, then esophageal physiologic testing, helps determine the GERD phenotype and effectively guide therapy.
Dr. Yadlapati is associate professor of clinical medicine, and medical director, UCSD Center for Esophageal Diseases; director, GI Motility Lab, division of gastroenterology, University of California San Diego, La Jolla, Calif. She disclosed ties with Medtronic, Phathom Pharmaceuticals, StatLinkMD, Medscape, Ironwood Pharmaceuticals, and RJS Mediagnostix. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Approximately 30% of U.S. adults experience troublesome reflux symptoms of heartburn, regurgitation and noncardiac chest pain. Because the mechanisms driving symptoms vary across patients, phenotyping patients via a step-wise diagnostic framework effectively guides personalized management in GERD.
For instance, PPI trials are appropriate when esophageal symptoms are present, whereas up-front reflux monitoring rather than empiric PPI trials are recommended for evaluation of isolated extra-esophageal symptoms. All patients undergoing evaluation for GERD should receive counseling on weight management and lifestyle modifications as well as the brain-gut axis relationship. In the common scenario of inadequate symptom response to PPIs, upper GI endoscopy is recommended to assess for erosive reflux disease (which confirms a diagnosis of GERD) as well as the anti-reflux barrier integrity. For instance, the presence of a large hiatal hernia and/or grade III/IV gastro-esophageal flap valve may point to mechanical gastro-esophageal reflux as a driver of symptoms and lower the threshold for surgical referral. In the absence of erosive reflux disease the next recommended step is ambulatory reflux monitoring off PPI therapy, either as prolonged wireless telemetry (which can be done concurrently with index endoscopy as long as PPI was discontinued > 7 days) or 24-hour transnasal pH-impedance catheter-based testing. Studies suggest that 96-hour monitoring is optimal for diagnostic accuracy and to guide therapeutic strategies.
Patients without evidence of GERD on endoscopy or ambulatory reflux monitoring likely have a functional esophageal disorder for which therapy hinges on pharmacologic neuromodulation or behavioral interventions as well as PPI cessation.
Alternatively, management for GERD (erosive or nonerosive) aims to optimize lifestyle, PPI therapy and the individualized use of adjunctive therapy, which include H2-receptor antagonists, alginate antacids, GABA agonists, neuromodulation and/or behavioral interventions. Surgical or endoscopic antireflux interventions are also an option for refractory GERD. Prior to intervention, achalasia must be excluded (typically with esophageal manometry), and confirmation of PPI refractory GERD on pH-impedance monitoring on PPI is of value, particularly when the phenotype is unclear. Again, the choice of antireflux intervention (e.g., laparoscopic fundoplication, magnetic sphincter augmentation, transoral incisionless fundoplication, Roux-en-Y gastric bypass) should be individualized to the patient’s anatomy, physiology, and clinical profile.
A multitude of treatment options are available to manage GERD, including behavioral interventions, lifestyle modifications, pharmacotherapy, and endoscopic/surgical interventions. However, not every treatment strategy is appropriate for every patient. Data gathered from the step-down diagnostic approach, which starts with clinical presentation, then endoscopy, then reflux monitoring, then esophageal physiologic testing, helps determine the GERD phenotype and effectively guide therapy.
Dr. Yadlapati is associate professor of clinical medicine, and medical director, UCSD Center for Esophageal Diseases; director, GI Motility Lab, division of gastroenterology, University of California San Diego, La Jolla, Calif. She disclosed ties with Medtronic, Phathom Pharmaceuticals, StatLinkMD, Medscape, Ironwood Pharmaceuticals, and RJS Mediagnostix. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Approximately 30% of U.S. adults experience troublesome reflux symptoms of heartburn, regurgitation and noncardiac chest pain. Because the mechanisms driving symptoms vary across patients, phenotyping patients via a step-wise diagnostic framework effectively guides personalized management in GERD.
For instance, PPI trials are appropriate when esophageal symptoms are present, whereas up-front reflux monitoring rather than empiric PPI trials are recommended for evaluation of isolated extra-esophageal symptoms. All patients undergoing evaluation for GERD should receive counseling on weight management and lifestyle modifications as well as the brain-gut axis relationship. In the common scenario of inadequate symptom response to PPIs, upper GI endoscopy is recommended to assess for erosive reflux disease (which confirms a diagnosis of GERD) as well as the anti-reflux barrier integrity. For instance, the presence of a large hiatal hernia and/or grade III/IV gastro-esophageal flap valve may point to mechanical gastro-esophageal reflux as a driver of symptoms and lower the threshold for surgical referral. In the absence of erosive reflux disease the next recommended step is ambulatory reflux monitoring off PPI therapy, either as prolonged wireless telemetry (which can be done concurrently with index endoscopy as long as PPI was discontinued > 7 days) or 24-hour transnasal pH-impedance catheter-based testing. Studies suggest that 96-hour monitoring is optimal for diagnostic accuracy and to guide therapeutic strategies.
Patients without evidence of GERD on endoscopy or ambulatory reflux monitoring likely have a functional esophageal disorder for which therapy hinges on pharmacologic neuromodulation or behavioral interventions as well as PPI cessation.
Alternatively, management for GERD (erosive or nonerosive) aims to optimize lifestyle, PPI therapy and the individualized use of adjunctive therapy, which include H2-receptor antagonists, alginate antacids, GABA agonists, neuromodulation and/or behavioral interventions. Surgical or endoscopic antireflux interventions are also an option for refractory GERD. Prior to intervention, achalasia must be excluded (typically with esophageal manometry), and confirmation of PPI refractory GERD on pH-impedance monitoring on PPI is of value, particularly when the phenotype is unclear. Again, the choice of antireflux intervention (e.g., laparoscopic fundoplication, magnetic sphincter augmentation, transoral incisionless fundoplication, Roux-en-Y gastric bypass) should be individualized to the patient’s anatomy, physiology, and clinical profile.
A multitude of treatment options are available to manage GERD, including behavioral interventions, lifestyle modifications, pharmacotherapy, and endoscopic/surgical interventions. However, not every treatment strategy is appropriate for every patient. Data gathered from the step-down diagnostic approach, which starts with clinical presentation, then endoscopy, then reflux monitoring, then esophageal physiologic testing, helps determine the GERD phenotype and effectively guide therapy.
Dr. Yadlapati is associate professor of clinical medicine, and medical director, UCSD Center for Esophageal Diseases; director, GI Motility Lab, division of gastroenterology, University of California San Diego, La Jolla, Calif. She disclosed ties with Medtronic, Phathom Pharmaceuticals, StatLinkMD, Medscape, Ironwood Pharmaceuticals, and RJS Mediagnostix. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
AT DDW 2022
EUS-guided gallbladder drainage for acute cholecystitis
Percutaneous transhepatic gallbladder drainage (PT-GBD) is the most common, nonoperative method for gallbladder decompression in patients unfit for cholecystectomy. However, drain-related complications (20%-75%), including tube changes, dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use. Endoscopic transpapillary gallbladder drainage (ET-GBD) and now, endoscopic ultrasound–guided gallbladder drainage (EUS-GBD), have emerged as options.
ET-GBD is performed at endoscopic retrograde cholangiopancreatography (ERCP) by cannulating the cystic duct, allowing placement of a pigtail plastic stent into the gallbladder. However, obstructing pathology (stone, stricture, metal stent or mass) may result in lower technical and clinical success when compared with EUS-GBD (84% vs. 98% and 91% vs. 97%, respectively). Furthermore, it does not allow for treatment of gallstones, and may require stent exchanges.
EUS-GBD involves placing a stent from the duodenum/stomach into the gallbladder under EUS guidance. Initial use of pigtail plastic stents and biliary self-expandable metal stents were not ideal, because of their risk of leakage, longer length (contralateral wall injury, occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations because of their short length and large flanges, and their large diameters (up to 20 mm) aid passage of gallstones or cholecystoscopy. Several case series and comparative trials have been published on EUS-GBD including a randomized prospective trial of EUS-GBD vs. PT-GBD demonstrating its superiority. Adverse events are uncommon and include misdeployments, bleeding, perforation, bile leaks, occlusion (commonly with food, prompting some endoscopists to place pigtails stents through the LAMS and avoiding the stomach as a target), and migration.
EUS-GBD should be avoided in patients who have a perforated gallbladder, have large volume ascites, or are too sick to tolerate anesthesia. Although there are patients who have subsequently undergone cholecystectomy post EUS-GBD, a discussion with one’s surgeon must be had prior to choosing this approach over ET-GBD.
In conclusion, determining the ideal method for endoscopic GBD in high-surgical-risk patients requires consideration of comorbidities, anatomy (GB position, cystic duct characteristics), presence of ascites, future surgical candidacy, and local expertise. ET-GBD should be prioritized for patients requiring ERCP for alternative reasons, large volume ascites, and as a bridge to cholecystectomy. Conversely, EUS-GBD is preferred with indwelling metal biliary stents covering the cystic duct and/or high-volume cholelithiasis. LAMS can be left long term; however, in patients willing to undergo an additional procedure, exchanging the LAMS for plastic stents can be undertaken at 4-6 weeks. Ultimately, more randomized and prospective data are needed to compare ET- and EUS-GBD outcomes, including a formal cost analysis.
Dr. Irani is with Virginia Mason Medical Center, Seattle. He reports being a consultant for Boston Scientific and Gore, as well as remittance to his clinic. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Percutaneous transhepatic gallbladder drainage (PT-GBD) is the most common, nonoperative method for gallbladder decompression in patients unfit for cholecystectomy. However, drain-related complications (20%-75%), including tube changes, dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use. Endoscopic transpapillary gallbladder drainage (ET-GBD) and now, endoscopic ultrasound–guided gallbladder drainage (EUS-GBD), have emerged as options.
ET-GBD is performed at endoscopic retrograde cholangiopancreatography (ERCP) by cannulating the cystic duct, allowing placement of a pigtail plastic stent into the gallbladder. However, obstructing pathology (stone, stricture, metal stent or mass) may result in lower technical and clinical success when compared with EUS-GBD (84% vs. 98% and 91% vs. 97%, respectively). Furthermore, it does not allow for treatment of gallstones, and may require stent exchanges.
EUS-GBD involves placing a stent from the duodenum/stomach into the gallbladder under EUS guidance. Initial use of pigtail plastic stents and biliary self-expandable metal stents were not ideal, because of their risk of leakage, longer length (contralateral wall injury, occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations because of their short length and large flanges, and their large diameters (up to 20 mm) aid passage of gallstones or cholecystoscopy. Several case series and comparative trials have been published on EUS-GBD including a randomized prospective trial of EUS-GBD vs. PT-GBD demonstrating its superiority. Adverse events are uncommon and include misdeployments, bleeding, perforation, bile leaks, occlusion (commonly with food, prompting some endoscopists to place pigtails stents through the LAMS and avoiding the stomach as a target), and migration.
EUS-GBD should be avoided in patients who have a perforated gallbladder, have large volume ascites, or are too sick to tolerate anesthesia. Although there are patients who have subsequently undergone cholecystectomy post EUS-GBD, a discussion with one’s surgeon must be had prior to choosing this approach over ET-GBD.
In conclusion, determining the ideal method for endoscopic GBD in high-surgical-risk patients requires consideration of comorbidities, anatomy (GB position, cystic duct characteristics), presence of ascites, future surgical candidacy, and local expertise. ET-GBD should be prioritized for patients requiring ERCP for alternative reasons, large volume ascites, and as a bridge to cholecystectomy. Conversely, EUS-GBD is preferred with indwelling metal biliary stents covering the cystic duct and/or high-volume cholelithiasis. LAMS can be left long term; however, in patients willing to undergo an additional procedure, exchanging the LAMS for plastic stents can be undertaken at 4-6 weeks. Ultimately, more randomized and prospective data are needed to compare ET- and EUS-GBD outcomes, including a formal cost analysis.
Dr. Irani is with Virginia Mason Medical Center, Seattle. He reports being a consultant for Boston Scientific and Gore, as well as remittance to his clinic. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Percutaneous transhepatic gallbladder drainage (PT-GBD) is the most common, nonoperative method for gallbladder decompression in patients unfit for cholecystectomy. However, drain-related complications (20%-75%), including tube changes, dyscosmesis, discomfort, and recurrent cholecystitis (up to 15%), limit its long-term use. Endoscopic transpapillary gallbladder drainage (ET-GBD) and now, endoscopic ultrasound–guided gallbladder drainage (EUS-GBD), have emerged as options.
ET-GBD is performed at endoscopic retrograde cholangiopancreatography (ERCP) by cannulating the cystic duct, allowing placement of a pigtail plastic stent into the gallbladder. However, obstructing pathology (stone, stricture, metal stent or mass) may result in lower technical and clinical success when compared with EUS-GBD (84% vs. 98% and 91% vs. 97%, respectively). Furthermore, it does not allow for treatment of gallstones, and may require stent exchanges.
EUS-GBD involves placing a stent from the duodenum/stomach into the gallbladder under EUS guidance. Initial use of pigtail plastic stents and biliary self-expandable metal stents were not ideal, because of their risk of leakage, longer length (contralateral wall injury, occlusions), and migration (lack of flanges). Lumen-apposing metal stents (LAMS) overcame these limitations because of their short length and large flanges, and their large diameters (up to 20 mm) aid passage of gallstones or cholecystoscopy. Several case series and comparative trials have been published on EUS-GBD including a randomized prospective trial of EUS-GBD vs. PT-GBD demonstrating its superiority. Adverse events are uncommon and include misdeployments, bleeding, perforation, bile leaks, occlusion (commonly with food, prompting some endoscopists to place pigtails stents through the LAMS and avoiding the stomach as a target), and migration.
EUS-GBD should be avoided in patients who have a perforated gallbladder, have large volume ascites, or are too sick to tolerate anesthesia. Although there are patients who have subsequently undergone cholecystectomy post EUS-GBD, a discussion with one’s surgeon must be had prior to choosing this approach over ET-GBD.
In conclusion, determining the ideal method for endoscopic GBD in high-surgical-risk patients requires consideration of comorbidities, anatomy (GB position, cystic duct characteristics), presence of ascites, future surgical candidacy, and local expertise. ET-GBD should be prioritized for patients requiring ERCP for alternative reasons, large volume ascites, and as a bridge to cholecystectomy. Conversely, EUS-GBD is preferred with indwelling metal biliary stents covering the cystic duct and/or high-volume cholelithiasis. LAMS can be left long term; however, in patients willing to undergo an additional procedure, exchanging the LAMS for plastic stents can be undertaken at 4-6 weeks. Ultimately, more randomized and prospective data are needed to compare ET- and EUS-GBD outcomes, including a formal cost analysis.
Dr. Irani is with Virginia Mason Medical Center, Seattle. He reports being a consultant for Boston Scientific and Gore, as well as remittance to his clinic. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
AT DDW 2022
An approach to germline genetic testing in your practice
Traditionally, a hereditary colorectal cancer syndrome (HCCS) was suspected in individuals with an obvious personal and/or family cancer phenotype informed by a three-generation family cancer history. Family history is still required to inform cancer risk. Documentation of age at cancer diagnosis, age of relatives’ deaths, and key intestinal and extraintestinal features of a HCCS (for example, macrocephaly, café au lait spots, polyp number, size, and histology) are requisite. Historically, Sanger sequencing was used to determine the presence of a suspected single pathogenic germline variant (PGV). If no PGV was detected, another PGV would be sought. This old “single gene/single syndrome” testing was expensive, time consuming, and inefficient, and has been supplanted by multigene cancer panel testing (MGPT). MGPT-driven low-cost, high-throughput testing has widespread insurance coverage in eligible patients. Since considerable clinical phenotypic overlap exists between HCCSs, casting a broader net for determining PGV, compared with a more limited approach, allows for greater identification of carriers of PGV as well as variants of uncertain significance.
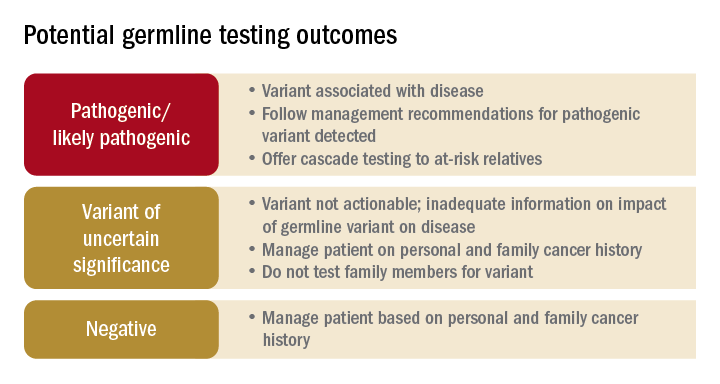
The frequency of PGV detection by MGPT in individuals with CRC is dependent on age at diagnosis and presence of DNA mismatch repair (MMR) deficiency in the tumor. According to one review, PGVs on MGPT are detected in approximately 10% and 34% of individuals aged more than 50 and more than 35 years, respectively.1 Pearlman and colleagues performed MGPT in 450 patients with CRC less than 50 years.2 PGV were found in 8% and 83.3% of cases with MMR-proficient and -deficient tumors, respectively. Overall, 33.3% of patients did not meet genetic testing criteria for the gene in which a PGV was detected, raising the impetus to consider MGPT in all patients with CRC. The Collaborative Group of the Americas on Inherited Gastrointestinal Cancer and National Comprehensive Cancer Network provide guidance on who warrants PGV testing.3,4
Germline testing outcomes and general approaches to patient management are provided in the graphic. HCCS are common and MGPT has broadened the identification of carriers of PGVs. In spite of advances in genetic testing technology, family history remains crucial to deploying risk-mitigation measures, regardless of the results of genetic testing.
Dr. Burke is in the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. She disclosed ties to Janssen Pharma, Emtora Biosciences, Freenome, SLA Pharma, and Ambry Genetics. Dr. Burke is a member of the U.S. Multi-Society Task Force on Colorectal Cancer, National Comprehensive Cancer Network Guideline on Genetic/Familial High-Risk Assessment: Colorectal. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
References
1. Stoffel E and Murphy CC. Gastroenterology. 2020 Jan;158(2):341-353.
2. Pearlman R et al. JAMA Oncol. 2017 Apr 1;3(4):464-471.
3. Heald B et al. Fam Cancer. 2020 Jul;19(3):223-239.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal Version 1.2022. 2022 Jun 8.
Traditionally, a hereditary colorectal cancer syndrome (HCCS) was suspected in individuals with an obvious personal and/or family cancer phenotype informed by a three-generation family cancer history. Family history is still required to inform cancer risk. Documentation of age at cancer diagnosis, age of relatives’ deaths, and key intestinal and extraintestinal features of a HCCS (for example, macrocephaly, café au lait spots, polyp number, size, and histology) are requisite. Historically, Sanger sequencing was used to determine the presence of a suspected single pathogenic germline variant (PGV). If no PGV was detected, another PGV would be sought. This old “single gene/single syndrome” testing was expensive, time consuming, and inefficient, and has been supplanted by multigene cancer panel testing (MGPT). MGPT-driven low-cost, high-throughput testing has widespread insurance coverage in eligible patients. Since considerable clinical phenotypic overlap exists between HCCSs, casting a broader net for determining PGV, compared with a more limited approach, allows for greater identification of carriers of PGV as well as variants of uncertain significance.

The frequency of PGV detection by MGPT in individuals with CRC is dependent on age at diagnosis and presence of DNA mismatch repair (MMR) deficiency in the tumor. According to one review, PGVs on MGPT are detected in approximately 10% and 34% of individuals aged more than 50 and more than 35 years, respectively.1 Pearlman and colleagues performed MGPT in 450 patients with CRC less than 50 years.2 PGV were found in 8% and 83.3% of cases with MMR-proficient and -deficient tumors, respectively. Overall, 33.3% of patients did not meet genetic testing criteria for the gene in which a PGV was detected, raising the impetus to consider MGPT in all patients with CRC. The Collaborative Group of the Americas on Inherited Gastrointestinal Cancer and National Comprehensive Cancer Network provide guidance on who warrants PGV testing.3,4
Germline testing outcomes and general approaches to patient management are provided in the graphic. HCCS are common and MGPT has broadened the identification of carriers of PGVs. In spite of advances in genetic testing technology, family history remains crucial to deploying risk-mitigation measures, regardless of the results of genetic testing.
Dr. Burke is in the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. She disclosed ties to Janssen Pharma, Emtora Biosciences, Freenome, SLA Pharma, and Ambry Genetics. Dr. Burke is a member of the U.S. Multi-Society Task Force on Colorectal Cancer, National Comprehensive Cancer Network Guideline on Genetic/Familial High-Risk Assessment: Colorectal. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
References
1. Stoffel E and Murphy CC. Gastroenterology. 2020 Jan;158(2):341-353.
2. Pearlman R et al. JAMA Oncol. 2017 Apr 1;3(4):464-471.
3. Heald B et al. Fam Cancer. 2020 Jul;19(3):223-239.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal Version 1.2022. 2022 Jun 8.
Traditionally, a hereditary colorectal cancer syndrome (HCCS) was suspected in individuals with an obvious personal and/or family cancer phenotype informed by a three-generation family cancer history. Family history is still required to inform cancer risk. Documentation of age at cancer diagnosis, age of relatives’ deaths, and key intestinal and extraintestinal features of a HCCS (for example, macrocephaly, café au lait spots, polyp number, size, and histology) are requisite. Historically, Sanger sequencing was used to determine the presence of a suspected single pathogenic germline variant (PGV). If no PGV was detected, another PGV would be sought. This old “single gene/single syndrome” testing was expensive, time consuming, and inefficient, and has been supplanted by multigene cancer panel testing (MGPT). MGPT-driven low-cost, high-throughput testing has widespread insurance coverage in eligible patients. Since considerable clinical phenotypic overlap exists between HCCSs, casting a broader net for determining PGV, compared with a more limited approach, allows for greater identification of carriers of PGV as well as variants of uncertain significance.

The frequency of PGV detection by MGPT in individuals with CRC is dependent on age at diagnosis and presence of DNA mismatch repair (MMR) deficiency in the tumor. According to one review, PGVs on MGPT are detected in approximately 10% and 34% of individuals aged more than 50 and more than 35 years, respectively.1 Pearlman and colleagues performed MGPT in 450 patients with CRC less than 50 years.2 PGV were found in 8% and 83.3% of cases with MMR-proficient and -deficient tumors, respectively. Overall, 33.3% of patients did not meet genetic testing criteria for the gene in which a PGV was detected, raising the impetus to consider MGPT in all patients with CRC. The Collaborative Group of the Americas on Inherited Gastrointestinal Cancer and National Comprehensive Cancer Network provide guidance on who warrants PGV testing.3,4
Germline testing outcomes and general approaches to patient management are provided in the graphic. HCCS are common and MGPT has broadened the identification of carriers of PGVs. In spite of advances in genetic testing technology, family history remains crucial to deploying risk-mitigation measures, regardless of the results of genetic testing.
Dr. Burke is in the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. She disclosed ties to Janssen Pharma, Emtora Biosciences, Freenome, SLA Pharma, and Ambry Genetics. Dr. Burke is a member of the U.S. Multi-Society Task Force on Colorectal Cancer, National Comprehensive Cancer Network Guideline on Genetic/Familial High-Risk Assessment: Colorectal. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
References
1. Stoffel E and Murphy CC. Gastroenterology. 2020 Jan;158(2):341-353.
2. Pearlman R et al. JAMA Oncol. 2017 Apr 1;3(4):464-471.
3. Heald B et al. Fam Cancer. 2020 Jul;19(3):223-239.
4. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Colorectal Version 1.2022. 2022 Jun 8.
AT DDW 2022
What is new in cirrhosis management? From frailty to palliative care
There is a rich science around the management of the cirrhotic liver itself – for example, pragmatic prognostic markers such as MELDNa, data-driven strategies to prevent variceal bleeding, and well-utilized algorithms to manage ascites.
But what is new in cirrhosis management is an emerging science around the management of the person living with cirrhosis – a science that seeks to understand how these individuals function in their day-to-day lives, how they feel, and how they can best prepare for their future. What is so exciting is that the field is moving beyond simply understanding those complex aspects of the patient, which is important in and of itself, toward developing practical tools to help clinicians assess their patients’ symptoms and strategies to help improve their patients’ lived experience. Although terms such as “frailty,” “palliative care,” and “advance care planning” are not new in cirrhosis per se, they are now recognized as distinct patient-centered constructs that are highly relevant to the management of patients with cirrhosis. Furthermore, these constructs have been codified through two recent guidance statements sponsored by the American Association for the Study of Liver Diseases.1,2 Pragmatic tools are emerging to facilitate the integration of these patient-centered constructs into routine clinical practice, tools such as the Liver Frailty Index, the Edmonton Symptom Assessment System adapted for patients with cirrhosis, and structured frameworks for guiding goals-of-care discussions. The incorporation of these tools allows for new management strategies directed toward improving the patient’s experience such as timely initiation of nutrition and activity-based interventions, algorithms for pharmacologic and nonpharmacologic strategies for symptom management, and online/video-guided approaches to articulating one’s goals of care.
So, what is new in cirrhosis management is that we are moving beyond managing the cirrhotic liver itself to considering how cirrhosis and its complications impact the patient as a whole. In doing so, we are turning the art of hepatology care into science that can be applied systematically at the bedside for every patient, with the goal of improving care for all patients living with cirrhosis.
Dr. Lai holds the Endowed Professorship of Liver Health and Transplantation at the University of California, San Francisco. She reports having no conflicts of interest. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
References
1. Lai JC et al. Hepatology. 2021 Sep;74(3):1611-44.
2. Rogal S et al. Hepatology. 2022 Feb 1. doi: 10.1002/hep.32378.
There is a rich science around the management of the cirrhotic liver itself – for example, pragmatic prognostic markers such as MELDNa, data-driven strategies to prevent variceal bleeding, and well-utilized algorithms to manage ascites.
But what is new in cirrhosis management is an emerging science around the management of the person living with cirrhosis – a science that seeks to understand how these individuals function in their day-to-day lives, how they feel, and how they can best prepare for their future. What is so exciting is that the field is moving beyond simply understanding those complex aspects of the patient, which is important in and of itself, toward developing practical tools to help clinicians assess their patients’ symptoms and strategies to help improve their patients’ lived experience. Although terms such as “frailty,” “palliative care,” and “advance care planning” are not new in cirrhosis per se, they are now recognized as distinct patient-centered constructs that are highly relevant to the management of patients with cirrhosis. Furthermore, these constructs have been codified through two recent guidance statements sponsored by the American Association for the Study of Liver Diseases.1,2 Pragmatic tools are emerging to facilitate the integration of these patient-centered constructs into routine clinical practice, tools such as the Liver Frailty Index, the Edmonton Symptom Assessment System adapted for patients with cirrhosis, and structured frameworks for guiding goals-of-care discussions. The incorporation of these tools allows for new management strategies directed toward improving the patient’s experience such as timely initiation of nutrition and activity-based interventions, algorithms for pharmacologic and nonpharmacologic strategies for symptom management, and online/video-guided approaches to articulating one’s goals of care.
So, what is new in cirrhosis management is that we are moving beyond managing the cirrhotic liver itself to considering how cirrhosis and its complications impact the patient as a whole. In doing so, we are turning the art of hepatology care into science that can be applied systematically at the bedside for every patient, with the goal of improving care for all patients living with cirrhosis.
Dr. Lai holds the Endowed Professorship of Liver Health and Transplantation at the University of California, San Francisco. She reports having no conflicts of interest. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
References
1. Lai JC et al. Hepatology. 2021 Sep;74(3):1611-44.
2. Rogal S et al. Hepatology. 2022 Feb 1. doi: 10.1002/hep.32378.
There is a rich science around the management of the cirrhotic liver itself – for example, pragmatic prognostic markers such as MELDNa, data-driven strategies to prevent variceal bleeding, and well-utilized algorithms to manage ascites.
But what is new in cirrhosis management is an emerging science around the management of the person living with cirrhosis – a science that seeks to understand how these individuals function in their day-to-day lives, how they feel, and how they can best prepare for their future. What is so exciting is that the field is moving beyond simply understanding those complex aspects of the patient, which is important in and of itself, toward developing practical tools to help clinicians assess their patients’ symptoms and strategies to help improve their patients’ lived experience. Although terms such as “frailty,” “palliative care,” and “advance care planning” are not new in cirrhosis per se, they are now recognized as distinct patient-centered constructs that are highly relevant to the management of patients with cirrhosis. Furthermore, these constructs have been codified through two recent guidance statements sponsored by the American Association for the Study of Liver Diseases.1,2 Pragmatic tools are emerging to facilitate the integration of these patient-centered constructs into routine clinical practice, tools such as the Liver Frailty Index, the Edmonton Symptom Assessment System adapted for patients with cirrhosis, and structured frameworks for guiding goals-of-care discussions. The incorporation of these tools allows for new management strategies directed toward improving the patient’s experience such as timely initiation of nutrition and activity-based interventions, algorithms for pharmacologic and nonpharmacologic strategies for symptom management, and online/video-guided approaches to articulating one’s goals of care.
So, what is new in cirrhosis management is that we are moving beyond managing the cirrhotic liver itself to considering how cirrhosis and its complications impact the patient as a whole. In doing so, we are turning the art of hepatology care into science that can be applied systematically at the bedside for every patient, with the goal of improving care for all patients living with cirrhosis.
Dr. Lai holds the Endowed Professorship of Liver Health and Transplantation at the University of California, San Francisco. She reports having no conflicts of interest. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
References
1. Lai JC et al. Hepatology. 2021 Sep;74(3):1611-44.
2. Rogal S et al. Hepatology. 2022 Feb 1. doi: 10.1002/hep.32378.
AT DDW 2022
Barrett’s esophagus: Key new concepts
Barrett’s esophagus (BE) is the only known precursor of esophageal adenocarcinoma (EAC). The rationale for early detection of BE rests on the premise that, after the diagnosis of BE, patients can be placed under endoscopic surveillance to detect prevalent and incident dysplasia and EAC. Randomized controlled trials have demonstrated that endoscopic eradication therapy (EET) of low-grade dysplasia (LGD) and high-grade dysplasia (HGD) can reduce progression to EAC. Guidelines support endoscopic screening for BE in those with multiple (three or more) risk factors.
However, endoscopy is expensive, invasive, and not widely utilized (less than 10% of those eligible are screened). Most patients with BE are unaware of their diagnosis and hence not under surveillance. Nonendoscopic techniques of BE detection – swallowed cell collection devices providing rich esophageal cytology specimens combined with biomarkers – are being developed. Case-control studies have shown promising accuracy and a recent UK pragmatic primary care study showed the ability of this technology to increase BE detection safely.
Detection of dysplasia in endoscopic surveillance is critical and the neoplasia detection rate (NDR) has been recently proposed as a quality marker. The NDR is the ratio of HGD+EAC detected to all patients with BE undergoing their first surveillance endoscopy. A recent systematic review and meta-analysis showed an inverse association between NDR and postendoscopy BE neoplasia. Additional and prospective studies are required to further correlate NDR values to clinically relevant outcomes similar to the association between adenoma detection rate and postcolonoscopy colorectal cancer.
Detection of dysplasia with endoscopic surveillance is challenging because of sampling error inherent in the Seattle protocol. A recent technology, Wide Area Transepithelial Sampling–3D (WATS), combines the concept of increased sampling of the BE mucosa by using a stiff endoscopic brush followed by use of artificial intelligence neural network enabled selection of abnormal cells, which are presented to a pathologist. This technology has been shown to increase dysplasia and HGD detection, compared to endoscopic surveillance, in a systematic review and meta-analysis. However, WATS is negative in a substantial proportion of cases in which endoscopic Seattle protocol reveals dysplasia. In addition, only limited data are available on the natural history of WATS LGD or HGD. Confirmation of WATS-only dysplasia (LGD, HGD, or EAC) by endoscopic histology is also recommended before the institution of EET. Finally, assessment of progression risk in those with BE is critical to enable more personalized follow up recommendations. Clinical risk scores integrating age, sex, smoking history, and LGD have been proposed and validated. A recent tissue systems pathology test has been shown in multiple case-control studies to identify a subset of BE patients who are at higher risk of progression, independent of LGD. This test is highly specific but only modestly sensitive in identifying progressors.
Dr. Iyer is professor of medicine, director of the Esophageal Interest Group, and codirector of the Advanced Esophageal Fellowship at the Mayo Clinic College of Medicine and Science, Rochester, Minn. He reports relationships with Exact Sciences, Pentax Medical, and others. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Barrett’s esophagus (BE) is the only known precursor of esophageal adenocarcinoma (EAC). The rationale for early detection of BE rests on the premise that, after the diagnosis of BE, patients can be placed under endoscopic surveillance to detect prevalent and incident dysplasia and EAC. Randomized controlled trials have demonstrated that endoscopic eradication therapy (EET) of low-grade dysplasia (LGD) and high-grade dysplasia (HGD) can reduce progression to EAC. Guidelines support endoscopic screening for BE in those with multiple (three or more) risk factors.
However, endoscopy is expensive, invasive, and not widely utilized (less than 10% of those eligible are screened). Most patients with BE are unaware of their diagnosis and hence not under surveillance. Nonendoscopic techniques of BE detection – swallowed cell collection devices providing rich esophageal cytology specimens combined with biomarkers – are being developed. Case-control studies have shown promising accuracy and a recent UK pragmatic primary care study showed the ability of this technology to increase BE detection safely.
Detection of dysplasia in endoscopic surveillance is critical and the neoplasia detection rate (NDR) has been recently proposed as a quality marker. The NDR is the ratio of HGD+EAC detected to all patients with BE undergoing their first surveillance endoscopy. A recent systematic review and meta-analysis showed an inverse association between NDR and postendoscopy BE neoplasia. Additional and prospective studies are required to further correlate NDR values to clinically relevant outcomes similar to the association between adenoma detection rate and postcolonoscopy colorectal cancer.
Detection of dysplasia with endoscopic surveillance is challenging because of sampling error inherent in the Seattle protocol. A recent technology, Wide Area Transepithelial Sampling–3D (WATS), combines the concept of increased sampling of the BE mucosa by using a stiff endoscopic brush followed by use of artificial intelligence neural network enabled selection of abnormal cells, which are presented to a pathologist. This technology has been shown to increase dysplasia and HGD detection, compared to endoscopic surveillance, in a systematic review and meta-analysis. However, WATS is negative in a substantial proportion of cases in which endoscopic Seattle protocol reveals dysplasia. In addition, only limited data are available on the natural history of WATS LGD or HGD. Confirmation of WATS-only dysplasia (LGD, HGD, or EAC) by endoscopic histology is also recommended before the institution of EET. Finally, assessment of progression risk in those with BE is critical to enable more personalized follow up recommendations. Clinical risk scores integrating age, sex, smoking history, and LGD have been proposed and validated. A recent tissue systems pathology test has been shown in multiple case-control studies to identify a subset of BE patients who are at higher risk of progression, independent of LGD. This test is highly specific but only modestly sensitive in identifying progressors.
Dr. Iyer is professor of medicine, director of the Esophageal Interest Group, and codirector of the Advanced Esophageal Fellowship at the Mayo Clinic College of Medicine and Science, Rochester, Minn. He reports relationships with Exact Sciences, Pentax Medical, and others. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
Barrett’s esophagus (BE) is the only known precursor of esophageal adenocarcinoma (EAC). The rationale for early detection of BE rests on the premise that, after the diagnosis of BE, patients can be placed under endoscopic surveillance to detect prevalent and incident dysplasia and EAC. Randomized controlled trials have demonstrated that endoscopic eradication therapy (EET) of low-grade dysplasia (LGD) and high-grade dysplasia (HGD) can reduce progression to EAC. Guidelines support endoscopic screening for BE in those with multiple (three or more) risk factors.
However, endoscopy is expensive, invasive, and not widely utilized (less than 10% of those eligible are screened). Most patients with BE are unaware of their diagnosis and hence not under surveillance. Nonendoscopic techniques of BE detection – swallowed cell collection devices providing rich esophageal cytology specimens combined with biomarkers – are being developed. Case-control studies have shown promising accuracy and a recent UK pragmatic primary care study showed the ability of this technology to increase BE detection safely.
Detection of dysplasia in endoscopic surveillance is critical and the neoplasia detection rate (NDR) has been recently proposed as a quality marker. The NDR is the ratio of HGD+EAC detected to all patients with BE undergoing their first surveillance endoscopy. A recent systematic review and meta-analysis showed an inverse association between NDR and postendoscopy BE neoplasia. Additional and prospective studies are required to further correlate NDR values to clinically relevant outcomes similar to the association between adenoma detection rate and postcolonoscopy colorectal cancer.
Detection of dysplasia with endoscopic surveillance is challenging because of sampling error inherent in the Seattle protocol. A recent technology, Wide Area Transepithelial Sampling–3D (WATS), combines the concept of increased sampling of the BE mucosa by using a stiff endoscopic brush followed by use of artificial intelligence neural network enabled selection of abnormal cells, which are presented to a pathologist. This technology has been shown to increase dysplasia and HGD detection, compared to endoscopic surveillance, in a systematic review and meta-analysis. However, WATS is negative in a substantial proportion of cases in which endoscopic Seattle protocol reveals dysplasia. In addition, only limited data are available on the natural history of WATS LGD or HGD. Confirmation of WATS-only dysplasia (LGD, HGD, or EAC) by endoscopic histology is also recommended before the institution of EET. Finally, assessment of progression risk in those with BE is critical to enable more personalized follow up recommendations. Clinical risk scores integrating age, sex, smoking history, and LGD have been proposed and validated. A recent tissue systems pathology test has been shown in multiple case-control studies to identify a subset of BE patients who are at higher risk of progression, independent of LGD. This test is highly specific but only modestly sensitive in identifying progressors.
Dr. Iyer is professor of medicine, director of the Esophageal Interest Group, and codirector of the Advanced Esophageal Fellowship at the Mayo Clinic College of Medicine and Science, Rochester, Minn. He reports relationships with Exact Sciences, Pentax Medical, and others. These remarks were made during one of the AGA Postgraduate Course sessions held at DDW 2022.
AT DDW 2022
Memorial and honorary gifts: A special tribute
Honor a family member, friend, or colleague while supporting the work of our mission through a gift to the AGA Research Foundation. Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit. The AGA Research Awards program recruits, retains, and supports the most promising investigators in gastroenterology and hepatology.
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help support researchers working toward developing new treatments and diagnostics for patients with GI conditions. Your gift will assist in fostering a new pipeline of scientists – the next generation of leaders in GI. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly, a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
Conclusion
Your gift directly supports talented young researchers working to advance our understanding of digestive diseases. Make a tax-deductible donation to help spur innovation. Donate today at www.gastro.org/donateonline.
Honor a family member, friend, or colleague while supporting the work of our mission through a gift to the AGA Research Foundation. Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit. The AGA Research Awards program recruits, retains, and supports the most promising investigators in gastroenterology and hepatology.
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help support researchers working toward developing new treatments and diagnostics for patients with GI conditions. Your gift will assist in fostering a new pipeline of scientists – the next generation of leaders in GI. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly, a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
Conclusion
Your gift directly supports talented young researchers working to advance our understanding of digestive diseases. Make a tax-deductible donation to help spur innovation. Donate today at www.gastro.org/donateonline.
Honor a family member, friend, or colleague while supporting the work of our mission through a gift to the AGA Research Foundation. Your gift will honor a loved one or yourself and support the AGA Research Awards Program, while giving you a tax benefit. The AGA Research Awards program recruits, retains, and supports the most promising investigators in gastroenterology and hepatology.
- Giving now or later. Any charitable gift can be made in honor or memory of someone.
- A gift today. An outright gift will help support researchers working toward developing new treatments and diagnostics for patients with GI conditions. Your gift will assist in fostering a new pipeline of scientists – the next generation of leaders in GI. The financial benefits include an income tax deduction and possible elimination of capital gains tax.
- A gift through your will or living trust. You can include a bequest in your will or living trust stating that a specific asset, certain dollar amount, or more commonly, a percentage of your estate will pass to the AGA Research Foundation at your death in honor of your loved one.
Conclusion
Your gift directly supports talented young researchers working to advance our understanding of digestive diseases. Make a tax-deductible donation to help spur innovation. Donate today at www.gastro.org/donateonline.
CMS releases proposed payment rule
On July 15, for calendar year 2023.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have identified the following top three takeaways:
Slight increase in ASC payments – The proposed ASC conversion factor increases 2.7% to $51.315 for ASCs that meet quality reporting requirements.
Slight increase in facility fees payments – Hospitals that meet quality reporting requirements also receive a 2.7% proposed increase, which translates to $86.785 – a stark difference from the ASC payment.
18% cuts to some motility and G-tube codes – Hospital outpatient facility payments for motility codes 91117 and 91122 and G-tube codes 43761-43763 could decrease by 18% because of proposed changes to their Ambulatory Payment Classification (APC) family.
On July 15, for calendar year 2023.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have identified the following top three takeaways:
Slight increase in ASC payments – The proposed ASC conversion factor increases 2.7% to $51.315 for ASCs that meet quality reporting requirements.
Slight increase in facility fees payments – Hospitals that meet quality reporting requirements also receive a 2.7% proposed increase, which translates to $86.785 – a stark difference from the ASC payment.
18% cuts to some motility and G-tube codes – Hospital outpatient facility payments for motility codes 91117 and 91122 and G-tube codes 43761-43763 could decrease by 18% because of proposed changes to their Ambulatory Payment Classification (APC) family.
On July 15, for calendar year 2023.
AGA, along with the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy, have identified the following top three takeaways:
Slight increase in ASC payments – The proposed ASC conversion factor increases 2.7% to $51.315 for ASCs that meet quality reporting requirements.
Slight increase in facility fees payments – Hospitals that meet quality reporting requirements also receive a 2.7% proposed increase, which translates to $86.785 – a stark difference from the ASC payment.
18% cuts to some motility and G-tube codes – Hospital outpatient facility payments for motility codes 91117 and 91122 and G-tube codes 43761-43763 could decrease by 18% because of proposed changes to their Ambulatory Payment Classification (APC) family.


















