User login
Shortened radiotherapy for endometrial cancer looks safe, questions remain
Postoperative radiotherapy is a mainstay in the treatment of endometrial cancer, but the typical 5-week regimen can be time consuming and expensive. A pilot study found that delivery of approximately the same dose over just two and a half weeks, known as hypofractionation, had good short-term toxicity outcomes.
Nevertheless, shortening the duration of radiotherapy could have benefits, especially in advanced uterine cancer, where chemotherapy is employed against distant metastases. Following surgery, there is a risk of both local recurrence and distant metastasis, complicating the choice of initial treatment. “Chemo can be several months long and radiation is typically several weeks. Therefore a shortened radiation schedule may have potential benefits, especially if there is an opportunity for this to be delivered earlier without delaying or interrupting chemotherapy, for example,” said lead study author Eric Leung, MD, associate professor of radiation oncology at the University of Toronto’s Sunnybrook Health Sciences Centre.
The research was published in JAMA Oncology.
Delivery of hypofractionation is tricky, according to Dr. Leung. “Gynecological cancer patients were treated with hypofractionation radiation to the pelvis which included the vagina, paravaginal tissues and pelvic lymph nodes. With this relatively large pelvic volume with surrounding normal tissues, this requires a highly focused radiation treatment with advanced technology,” said Dr. Leung. The study protocol employed stereotactic technique to deliver 30 Gy in 5 fractions.
Hypofractionation could be beneficial in reduction of travel time and time spent in hospital, as well as reducing financial burden and increasing quality of life. These benefits have taken on a larger role in the context of the COVID-19 pandemic.
Although the findings are encouraging, they are preliminary, according to Vonetta Williams, MD, PhD, who wrote an accompanying editorial. “I would caution that all they’ve done is presented preliminary toxicity data, so we don’t have any proof yet that it is equally effective (compared to standard protocol), and their study cannot answer that at any rate because it was not designed to answer that question,” Dr. Williams said in an interview. She noted that long-term follow-up is needed to measure bowel dysfunction, sexual dysfunction, vaginal stenosis, and other side effects.
It is also uncertain whether hypofractionated doses are actually equivalent to the standard dose. “We know that they’re roughly equivalent, but that is very much a question if they are equivalent in terms of efficacy. I don’t know that I would be confident that they are. That’s probably what would give most radiation oncologists pause, because we don’t have any data to say that it is (equivalent). Although it would be nice to shorten treatment, and I think it would certainly be better for patients, I want to caution that we want to do so once we know what the toxicity and the outcomes really are,” said Dr. Williams.
The researchers enrolled 61 patients with a median age of 66 years. Thirty-nine had endometrioid adenocarcinoma, 15 had serous or clear cell, 3 had carcinosarcoma, and 4 had dedifferentiated disease. Sixteen patients underwent sequential chemotherapy, and 9 underwent additional vault brachytherapy. Over a median follow-up of 9 months, 54% had a worst gastrointestinal side effect of grade 1, while 13% had a worst side effect of grade 2. Among worst genitourinary side effects, 41% had grade 1 and 3% had grade 2. One patient had acute grade 3 diarrhea at fraction 5, but this resolved at follow-up. One patient had diarrhea scores that were both clinically and statistically significantly worse than baseline at fraction 5, and this improved at follow-up.
Patient-reported quality of life outcomes were generally good. Of all measures, only diarrhea was clinically and statistically worse by fraction 5, and improvement was seen at 6 weeks and 3 months. Global health status was consistent throughout treatment and follow-up. There was no change in sexual and vaginal symptoms.
The study authors reported grants, consulting, and personal fees from a variety of pharmaceutical companies. Dr. Williams reported having no disclosures.
Postoperative radiotherapy is a mainstay in the treatment of endometrial cancer, but the typical 5-week regimen can be time consuming and expensive. A pilot study found that delivery of approximately the same dose over just two and a half weeks, known as hypofractionation, had good short-term toxicity outcomes.
Nevertheless, shortening the duration of radiotherapy could have benefits, especially in advanced uterine cancer, where chemotherapy is employed against distant metastases. Following surgery, there is a risk of both local recurrence and distant metastasis, complicating the choice of initial treatment. “Chemo can be several months long and radiation is typically several weeks. Therefore a shortened radiation schedule may have potential benefits, especially if there is an opportunity for this to be delivered earlier without delaying or interrupting chemotherapy, for example,” said lead study author Eric Leung, MD, associate professor of radiation oncology at the University of Toronto’s Sunnybrook Health Sciences Centre.
The research was published in JAMA Oncology.
Delivery of hypofractionation is tricky, according to Dr. Leung. “Gynecological cancer patients were treated with hypofractionation radiation to the pelvis which included the vagina, paravaginal tissues and pelvic lymph nodes. With this relatively large pelvic volume with surrounding normal tissues, this requires a highly focused radiation treatment with advanced technology,” said Dr. Leung. The study protocol employed stereotactic technique to deliver 30 Gy in 5 fractions.
Hypofractionation could be beneficial in reduction of travel time and time spent in hospital, as well as reducing financial burden and increasing quality of life. These benefits have taken on a larger role in the context of the COVID-19 pandemic.
Although the findings are encouraging, they are preliminary, according to Vonetta Williams, MD, PhD, who wrote an accompanying editorial. “I would caution that all they’ve done is presented preliminary toxicity data, so we don’t have any proof yet that it is equally effective (compared to standard protocol), and their study cannot answer that at any rate because it was not designed to answer that question,” Dr. Williams said in an interview. She noted that long-term follow-up is needed to measure bowel dysfunction, sexual dysfunction, vaginal stenosis, and other side effects.
It is also uncertain whether hypofractionated doses are actually equivalent to the standard dose. “We know that they’re roughly equivalent, but that is very much a question if they are equivalent in terms of efficacy. I don’t know that I would be confident that they are. That’s probably what would give most radiation oncologists pause, because we don’t have any data to say that it is (equivalent). Although it would be nice to shorten treatment, and I think it would certainly be better for patients, I want to caution that we want to do so once we know what the toxicity and the outcomes really are,” said Dr. Williams.
The researchers enrolled 61 patients with a median age of 66 years. Thirty-nine had endometrioid adenocarcinoma, 15 had serous or clear cell, 3 had carcinosarcoma, and 4 had dedifferentiated disease. Sixteen patients underwent sequential chemotherapy, and 9 underwent additional vault brachytherapy. Over a median follow-up of 9 months, 54% had a worst gastrointestinal side effect of grade 1, while 13% had a worst side effect of grade 2. Among worst genitourinary side effects, 41% had grade 1 and 3% had grade 2. One patient had acute grade 3 diarrhea at fraction 5, but this resolved at follow-up. One patient had diarrhea scores that were both clinically and statistically significantly worse than baseline at fraction 5, and this improved at follow-up.
Patient-reported quality of life outcomes were generally good. Of all measures, only diarrhea was clinically and statistically worse by fraction 5, and improvement was seen at 6 weeks and 3 months. Global health status was consistent throughout treatment and follow-up. There was no change in sexual and vaginal symptoms.
The study authors reported grants, consulting, and personal fees from a variety of pharmaceutical companies. Dr. Williams reported having no disclosures.
Postoperative radiotherapy is a mainstay in the treatment of endometrial cancer, but the typical 5-week regimen can be time consuming and expensive. A pilot study found that delivery of approximately the same dose over just two and a half weeks, known as hypofractionation, had good short-term toxicity outcomes.
Nevertheless, shortening the duration of radiotherapy could have benefits, especially in advanced uterine cancer, where chemotherapy is employed against distant metastases. Following surgery, there is a risk of both local recurrence and distant metastasis, complicating the choice of initial treatment. “Chemo can be several months long and radiation is typically several weeks. Therefore a shortened radiation schedule may have potential benefits, especially if there is an opportunity for this to be delivered earlier without delaying or interrupting chemotherapy, for example,” said lead study author Eric Leung, MD, associate professor of radiation oncology at the University of Toronto’s Sunnybrook Health Sciences Centre.
The research was published in JAMA Oncology.
Delivery of hypofractionation is tricky, according to Dr. Leung. “Gynecological cancer patients were treated with hypofractionation radiation to the pelvis which included the vagina, paravaginal tissues and pelvic lymph nodes. With this relatively large pelvic volume with surrounding normal tissues, this requires a highly focused radiation treatment with advanced technology,” said Dr. Leung. The study protocol employed stereotactic technique to deliver 30 Gy in 5 fractions.
Hypofractionation could be beneficial in reduction of travel time and time spent in hospital, as well as reducing financial burden and increasing quality of life. These benefits have taken on a larger role in the context of the COVID-19 pandemic.
Although the findings are encouraging, they are preliminary, according to Vonetta Williams, MD, PhD, who wrote an accompanying editorial. “I would caution that all they’ve done is presented preliminary toxicity data, so we don’t have any proof yet that it is equally effective (compared to standard protocol), and their study cannot answer that at any rate because it was not designed to answer that question,” Dr. Williams said in an interview. She noted that long-term follow-up is needed to measure bowel dysfunction, sexual dysfunction, vaginal stenosis, and other side effects.
It is also uncertain whether hypofractionated doses are actually equivalent to the standard dose. “We know that they’re roughly equivalent, but that is very much a question if they are equivalent in terms of efficacy. I don’t know that I would be confident that they are. That’s probably what would give most radiation oncologists pause, because we don’t have any data to say that it is (equivalent). Although it would be nice to shorten treatment, and I think it would certainly be better for patients, I want to caution that we want to do so once we know what the toxicity and the outcomes really are,” said Dr. Williams.
The researchers enrolled 61 patients with a median age of 66 years. Thirty-nine had endometrioid adenocarcinoma, 15 had serous or clear cell, 3 had carcinosarcoma, and 4 had dedifferentiated disease. Sixteen patients underwent sequential chemotherapy, and 9 underwent additional vault brachytherapy. Over a median follow-up of 9 months, 54% had a worst gastrointestinal side effect of grade 1, while 13% had a worst side effect of grade 2. Among worst genitourinary side effects, 41% had grade 1 and 3% had grade 2. One patient had acute grade 3 diarrhea at fraction 5, but this resolved at follow-up. One patient had diarrhea scores that were both clinically and statistically significantly worse than baseline at fraction 5, and this improved at follow-up.
Patient-reported quality of life outcomes were generally good. Of all measures, only diarrhea was clinically and statistically worse by fraction 5, and improvement was seen at 6 weeks and 3 months. Global health status was consistent throughout treatment and follow-up. There was no change in sexual and vaginal symptoms.
The study authors reported grants, consulting, and personal fees from a variety of pharmaceutical companies. Dr. Williams reported having no disclosures.
FROM JAMA ONCOLOGY
Expert shares tips on hair disorders and photoprotection for patients of color
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
PORTLAND, ORE. – , but sometimes their doctors fall short.
“Many times, you may not have race concordant visits with patients of color,” Janiene Luke, MD, said at the annual meeting of the Pacific Dermatologic Association. She referred to a survey of 200 Black women aged 21-83 years, which found that 28% had visited a physician to discuss hair or scalp issues. Of those, 68% felt like their dermatologists did not understand African American hair.
“I recommend trying the best you can to familiarize yourself with various common cultural hair styling methods and practices in patients of color. It’s important to understand what your patients are engaging in and the types of styles they’re using,” said Dr. Luke, associate professor of dermatology at Loma Linda (Calif.) University. “Approach all patients with cultural humility. We know from studies that patients value dermatologists who take time to listen to their concerns, involve them in the decision-making process, and educate them about their conditions,” she added.
National efforts to educate clinicians on treating skin of color have emerged in recent years, including textbooks, CME courses at dermatology conferences, and the American Academy of Dermatology’s Skin of Color Curriculum, which consists of 15-minute modules that can be viewed online.
At the meeting, Dr. Luke, shared her approach to assessing hair and scalp disorders in skin of color. She begins by taking a thorough history, “because not all things that are associated with hair styling will be the reason why your patient comes in,” she said. “Patients of color can have telogen effluvium and seborrheic dermatitis just like anyone else. I ask about the hair styling practices they use. I also ask how often they wash their hair, because sometimes our recommendations for treatment are not realistic based on their current routine.”
Next, she examines the scalp with her hands – which sometimes surprises patients. “I’ve had so many patients come in and say, ‘the dermatologist never touched my scalp,’ or ‘they never even looked at my hair,’ ” said Dr. Luke, who directs the university’s dermatology residency program. She asks patients to remove any hair extensions or weaves prior to the office visit and to remove wigs prior to the exam itself. The lab tests she customarily orders include CBC, TSH, iron, total iron binding capacity, ferritin, vitamin D, and zinc. If there are signs of androgen excess, she may check testosterone, sex hormone binding globulin, and dehydroepiandrosterone sulfate (DHEA-S). She routinely incorporates a dermoscopy-directed biopsy into the evaluation.
Dr. Luke examines the patient from above, the sides, and the back to assess the pattern/distribution of hair loss. A visible scalp at the vertex indicates a 50% reduction in normal hair density. “I’m looking at the hairline, their part width, and the length of their hair,” she said. “I also look at the eyebrows and eyelashes, because these can be involved in alopecia areata, frontal fibrosing alopecia, or congenital hair shaft disorders.”
On closeup examination, she looks for scarring versus non-scarring types of hair loss, and for the presence or absence of follicular ostia. “I also look at hair changes,” she said. “Is the texture of their hair different? Are there signs of breakage or fragility? It’s been noted in studies that breakage can be an early sign of central centrifugal cicatricial alopecia.” (For more tips on examining tightly coiled hair among patients with hair loss in race discordant patient-physician interactions, she recommended a 2021 article in JAMA Dermatology)..
Trichoscopy allows for magnified observation of the hair shafts, hair follicle openings, perifollicular dermis, and blood vessels. Normal trichoscopy findings in skin of color reveal a perifollicular pigment network (honeycomb pattern) and pinpoint white dots that are regularly distributed between follicular units.
Common abnormalities seen on trichoscopy include central centrifugal cicatricial alopecia (CCCA), with one or two hairs emerging together, surrounded by a gray halo; lichen planopilaris/frontal fibrosing alopecia, characterized by hair with peripilar casts and absence of vellus hairs; discoid lupus erythematosus, characterized by keratotic plugs; and traction, characterized by hair casts.
Once a diagnosis is confirmed, Dr. Luke provides other general advice for optimal skin health, including a balanced (whole food) diet to ensure adequate nutrition. “I tend to find a lot of nutrient deficiencies that contribute to and compound their condition,” she said. Other recommendations include avoiding excess tension on the hair, such as hair styles with tight ponytails, buns, braids, and weaves; avoiding or limiting chemical treatments with hair color, relaxers, and permanents; and avoiding or limiting excessive heat styling with blow dryers, flat irons, and curling irons.
Photoprotection misconceptions
At the meeting, Dr. Luke also discussed three misconceptions of photoprotection in skin of color, drawn from an article on the topic published in 2021.
- Myth No. 1: Endogenous melanin provides complete photoprotection for Fitzpatrick skin types IV-V. Many people with skin of color may believe sunscreen is not needed given the melanin already present in their skin, but research has shown that the epidermis of dark skin has an intrinsic sun protection factor (SPF) of 13.4, compared with an SPF of 3.3 in light skin. “That may not provide them with full protection,” Dr. Luke said. “Many dermatologists are not counseling their skin of color patients about photoprotection.”
- Myth No. 2: Individuals with skin of color have negligible risks associated with skin cancer. Skin cancer prevalence in patients with skin of color is significantly lower compared with those with light skin. However, people with skin of color tend to be diagnosed with cancers at a more advanced stage, and cancers associated with a worse prognosis and poorer survival rate. An analysis of ethnic differences among patients with cutaneous melanoma that drew from the Surveillance, Epidemiology, and End Results (SEER) program found that Hispanic individuals (odds ratio [OR], 3.6), Black individuals (OR, 4.2), and Asian individuals (OR, 2.4), were more likely than were White individuals to have stage IV melanoma at the time of presentation. “For melanoma in skin of color, UV radiation does not seem to be a major risk factor, as melanoma tends to occur on palmar/plantar and subungual skin as well as mucous membranes,” Dr. Luke said. “For squamous cell carcinoma in skin of color, lesions are more likely to be present in areas that are not sun exposed. The risk factors for this tend to be chronic wounds, nonhealing ulcers, and people with chronic inflammatory conditions.” For basal cell carcinoma, she added, UV radiation seems to play more of a role and tends to occur in sun-exposed areas in patients with lighter Fitzpatrick skin types. Patients are more likely to present with pigmented BCCs.
- Myth No. 3: Broad-spectrum sunscreens provide photoprotection against all wavelengths of light that cause skin damage. To be labeled “broad-spectrum” the Food and Drug Administration requires that sunscreens have a critical wavelength of 370 nm or below, but Dr. Luke noted that broad-spectrum sunscreens do not necessarily protect against visible light (VL) and UV-A1. Research has demonstrated that VL exposure induces both transient and long-term cutaneous pigmentation in a dose-dependent manner.
“This induces free radicals and reactive oxygen species, leading to a cascade of events including the induction of pro-inflammatory cytokines, matrix metalloproteinases, and melanogenesis,” she said. “More intense and persistent VL-induced pigmentation occurs in subjects with darker skin. However, there is increasing evidence that antioxidants may help to mitigate these negative effects, so we are starting to see the addition of antioxidants into sunscreens.”
Dr. Luke recommends a broad-spectrum sunscreen with an SPF of 30 or higher for skin of color patients. Tinted sunscreens, which contain iron oxide pigments, are recommended for the prevention and treatment of pigmentary disorders in patients with Fitzpatrick skin types IV-VI skin. “What about adding antioxidants to prevent formation of reactive oxygen species?” she asked. “It’s possible but we don’t have a lot of research yet. You also want a sunscreen that’s aesthetically elegant, meaning it doesn’t leave a white cast.”
Dr. Luke reported having no relevant disclosures.
AT PDA 2022
Bias and other barriers to HSCT access
For example, at the June 5 plenary session of the American Society of Clinical Oncology, Paul Richardson, MD, presented results of the DETERMINATION trial. More than 40,000 attendees heard his message that, in patients with newly diagnosed multiple myeloma (MM), up-front high-dose melphalan with autologous hematopoietic stem cell transplant (HSCT) support is associated with a significantly longer median progression-free survival of 67 months, compared with 46 months for patients randomized to delayed transplantation. The 5-year overall survival is similar for both arms.
While I and many of my colleagues in the field of transplantation used this data to strongly encourage MM patients to undergo HSCT as consolidation of their initial remission, others – including many investigators on the DETERMINATION trial – reached a starkly different conclusion. They suggested that delaying transplant was a valid option, since no survival benefit was observed.
Bias, when defined as a prejudice in favor of or against a specific treatment on the part of physicians and patients, has not been carefully studied in the realm of cellular therapies. However, physician and patient perceptions or misperceptions about the value or toxicity of a specific therapy are probably major drivers of whether a patient is referred for and accepts a particular form of treatment. In my specialization, that would mean either a stem cell transplant or other forms of cell therapy.
As with other medical procedures, in my field there are significant disparities in the use of transplantation among patients of different racial, ethnic, and age groups. Rates of both auto- and allo-HSCT are significantly higher for Whites than for African Americans. Hispanic patients have the lowest rates of utilization of auto-HSCT. Patients over the age of 60 have an eightfold risk of nonreferral to an HSCT center. Obviously, these nonreferrals reduce access to HSCT for older patients, particularly if they are seen at nonacademic centers.
One must question whether these disparities are caused by the physicians not believing in the value of transplantation, or simply not understanding its value? Or do they just lack the time to refer patients to a transplant center?
Socioeconomic factors, insurance status, age, and psychosocial characteristics all impact access to HSCT, yet some older patients with fewer economic resources and less insurance coverage still undergo the procedure. Is that because their physicians spent time educating these patients about the potential value of this treatment? Is it because the physicians went the extra mile to get these patients access to HSCT?
Physician preference also plays a significant role in whether a patient receives an allo-HSCT for acute myeloid leukemia and myelodysplastic syndrome. In a large survey of hematologists and oncologists performed by Pidala and colleagues, half of those surveyed agreed with the statement: “I feel the risk (morbidity and mortality) after HSCT is very high.” Most indicated that they “feel outcomes of unrelated donor HCT are much worse than matched sibling HCT.”
More importantly, more than one-third of those surveyed agreed that, “because of the high risks of allogeneic HSCT, I refer only after failure of conventional chemotherapy.” They voiced this opinion despite the fact that mortality rates after HSCT have been reduced significantly. With modern techniques, outcomes of unrelated donors are as good as with sibling donor transplants, and national guidelines strongly recommend that patients get referred before they become refractory to chemotherapy.
What can we do about this problem? Obviously, physician and provider education is important, but primary care physicians and general oncologists are already bombarded daily with new information. Relatively rare conditions like those we treat simply may not get their attention.
Personally, I think one of the most effective ways to overcome bias among physicians would be to target patients through a direct advertising campaign and public service announcements. Only by getting the attention of patients can they be directed to current, accurate information.
This solution could reduce the impact of physician biases or misperceptions and provide patients with greater access to lifesaving cell therapies.
Dr. Giralt is deputy division head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center in New York.
For example, at the June 5 plenary session of the American Society of Clinical Oncology, Paul Richardson, MD, presented results of the DETERMINATION trial. More than 40,000 attendees heard his message that, in patients with newly diagnosed multiple myeloma (MM), up-front high-dose melphalan with autologous hematopoietic stem cell transplant (HSCT) support is associated with a significantly longer median progression-free survival of 67 months, compared with 46 months for patients randomized to delayed transplantation. The 5-year overall survival is similar for both arms.
While I and many of my colleagues in the field of transplantation used this data to strongly encourage MM patients to undergo HSCT as consolidation of their initial remission, others – including many investigators on the DETERMINATION trial – reached a starkly different conclusion. They suggested that delaying transplant was a valid option, since no survival benefit was observed.
Bias, when defined as a prejudice in favor of or against a specific treatment on the part of physicians and patients, has not been carefully studied in the realm of cellular therapies. However, physician and patient perceptions or misperceptions about the value or toxicity of a specific therapy are probably major drivers of whether a patient is referred for and accepts a particular form of treatment. In my specialization, that would mean either a stem cell transplant or other forms of cell therapy.
As with other medical procedures, in my field there are significant disparities in the use of transplantation among patients of different racial, ethnic, and age groups. Rates of both auto- and allo-HSCT are significantly higher for Whites than for African Americans. Hispanic patients have the lowest rates of utilization of auto-HSCT. Patients over the age of 60 have an eightfold risk of nonreferral to an HSCT center. Obviously, these nonreferrals reduce access to HSCT for older patients, particularly if they are seen at nonacademic centers.
One must question whether these disparities are caused by the physicians not believing in the value of transplantation, or simply not understanding its value? Or do they just lack the time to refer patients to a transplant center?
Socioeconomic factors, insurance status, age, and psychosocial characteristics all impact access to HSCT, yet some older patients with fewer economic resources and less insurance coverage still undergo the procedure. Is that because their physicians spent time educating these patients about the potential value of this treatment? Is it because the physicians went the extra mile to get these patients access to HSCT?
Physician preference also plays a significant role in whether a patient receives an allo-HSCT for acute myeloid leukemia and myelodysplastic syndrome. In a large survey of hematologists and oncologists performed by Pidala and colleagues, half of those surveyed agreed with the statement: “I feel the risk (morbidity and mortality) after HSCT is very high.” Most indicated that they “feel outcomes of unrelated donor HCT are much worse than matched sibling HCT.”
More importantly, more than one-third of those surveyed agreed that, “because of the high risks of allogeneic HSCT, I refer only after failure of conventional chemotherapy.” They voiced this opinion despite the fact that mortality rates after HSCT have been reduced significantly. With modern techniques, outcomes of unrelated donors are as good as with sibling donor transplants, and national guidelines strongly recommend that patients get referred before they become refractory to chemotherapy.
What can we do about this problem? Obviously, physician and provider education is important, but primary care physicians and general oncologists are already bombarded daily with new information. Relatively rare conditions like those we treat simply may not get their attention.
Personally, I think one of the most effective ways to overcome bias among physicians would be to target patients through a direct advertising campaign and public service announcements. Only by getting the attention of patients can they be directed to current, accurate information.
This solution could reduce the impact of physician biases or misperceptions and provide patients with greater access to lifesaving cell therapies.
Dr. Giralt is deputy division head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center in New York.
For example, at the June 5 plenary session of the American Society of Clinical Oncology, Paul Richardson, MD, presented results of the DETERMINATION trial. More than 40,000 attendees heard his message that, in patients with newly diagnosed multiple myeloma (MM), up-front high-dose melphalan with autologous hematopoietic stem cell transplant (HSCT) support is associated with a significantly longer median progression-free survival of 67 months, compared with 46 months for patients randomized to delayed transplantation. The 5-year overall survival is similar for both arms.
While I and many of my colleagues in the field of transplantation used this data to strongly encourage MM patients to undergo HSCT as consolidation of their initial remission, others – including many investigators on the DETERMINATION trial – reached a starkly different conclusion. They suggested that delaying transplant was a valid option, since no survival benefit was observed.
Bias, when defined as a prejudice in favor of or against a specific treatment on the part of physicians and patients, has not been carefully studied in the realm of cellular therapies. However, physician and patient perceptions or misperceptions about the value or toxicity of a specific therapy are probably major drivers of whether a patient is referred for and accepts a particular form of treatment. In my specialization, that would mean either a stem cell transplant or other forms of cell therapy.
As with other medical procedures, in my field there are significant disparities in the use of transplantation among patients of different racial, ethnic, and age groups. Rates of both auto- and allo-HSCT are significantly higher for Whites than for African Americans. Hispanic patients have the lowest rates of utilization of auto-HSCT. Patients over the age of 60 have an eightfold risk of nonreferral to an HSCT center. Obviously, these nonreferrals reduce access to HSCT for older patients, particularly if they are seen at nonacademic centers.
One must question whether these disparities are caused by the physicians not believing in the value of transplantation, or simply not understanding its value? Or do they just lack the time to refer patients to a transplant center?
Socioeconomic factors, insurance status, age, and psychosocial characteristics all impact access to HSCT, yet some older patients with fewer economic resources and less insurance coverage still undergo the procedure. Is that because their physicians spent time educating these patients about the potential value of this treatment? Is it because the physicians went the extra mile to get these patients access to HSCT?
Physician preference also plays a significant role in whether a patient receives an allo-HSCT for acute myeloid leukemia and myelodysplastic syndrome. In a large survey of hematologists and oncologists performed by Pidala and colleagues, half of those surveyed agreed with the statement: “I feel the risk (morbidity and mortality) after HSCT is very high.” Most indicated that they “feel outcomes of unrelated donor HCT are much worse than matched sibling HCT.”
More importantly, more than one-third of those surveyed agreed that, “because of the high risks of allogeneic HSCT, I refer only after failure of conventional chemotherapy.” They voiced this opinion despite the fact that mortality rates after HSCT have been reduced significantly. With modern techniques, outcomes of unrelated donors are as good as with sibling donor transplants, and national guidelines strongly recommend that patients get referred before they become refractory to chemotherapy.
What can we do about this problem? Obviously, physician and provider education is important, but primary care physicians and general oncologists are already bombarded daily with new information. Relatively rare conditions like those we treat simply may not get their attention.
Personally, I think one of the most effective ways to overcome bias among physicians would be to target patients through a direct advertising campaign and public service announcements. Only by getting the attention of patients can they be directed to current, accurate information.
This solution could reduce the impact of physician biases or misperceptions and provide patients with greater access to lifesaving cell therapies.
Dr. Giralt is deputy division head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center in New York.
Real medical news: Many teens trust fake medical news
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
The kids aren’t alright (at identifying fake news online)
If there’s one thing today’s teenagers are good at, it’s the Internet. What with their TokTiks, Fortnights, and memes whose lifespans are measured in milliseconds, it’s only natural that a contingent of people who have never known a world where the Internet wasn’t omnipresent would be highly skilled at navigating the dense, labyrinthine virtual world and the many falsehoods contained within.
Ladies and gentlemen, we’ve been duped, bamboozled, and smeckledorfed. New research from Slovakia suggests the opposite, in fact: Teenagers are just as bad as the rest of us, if not worse, at distinguishing between fake and real online health messaging.
For the study, 300 teenagers aged 16-19 years old were shown a group of messages about the health-promoting effects of fruits and vegetables; these messages were either false, true and neutral, or true with some sort of editing (a clickbait title or grammar mistakes) to mask their trustworthiness. Just under half of the subjects identified and trusted the true neutral messages over fake messages, while 41% couldn’t tell the difference and 11% trusted the fake messages more. In addition, they couldn’t tell the difference between fake and true messages when the content seemed plausible.
In a bit of good news, teenagers were just as likely to trust the edited true messages as the true neutral ones, except in instances when the edited message had a clickbait title. They were much less likely to trust those.
Based on their subjects’ rather poor performance, the study authors suggested teenagers go through health literacy and media literacy training, as well as develop their analytical and scientific reasoning. The LOTME staff rather suspects the study authors have never met a teenager. The only thing teenagers are going to get out of health literacy training is fodder for memes to put up on Myspace. Myspace is still a thing, right? We’re not old, we swear.
Can a computer help deliver babies?
Delivering babies can be a complicated business. Most doctors and midwives rely on their years of experience and training to make certain decisions for mothers in labor, but an artificial intelligence (AI) algorithm could make the entire process easier and safer.
Researchers from the Mayo Clinic recently reported that using an AI to analyze women’s labor patterns was very successful in determining whether a vaginal or cesarean delivery was appropriate.
They examined over 700 factors and over 66,000 deliveries from the National Institute of Child Health and Human Development’s multicenter Consortium on Safe Labor database to produce a risk-prediction model that may “provide an alternative to conventional labor charts and promote individualization of clinical decisions using baseline and labor characteristics of each patient,” they said in a written statement from the clinic.
It is hoped that the AI will reduce the risk of possible complications and the costs associated with maternal mortality. The AI also could be a significant tool for doctors and midwives in rural areas to determine when a patient needs to be moved to a location with a higher level of care.
“We believe the algorithm will work in real time, meaning every input of new data during an expectant woman’s labor automatically recalculates the risk of adverse outcome,” said senior author Abimbola Famuyide, MD, of the Mayo Clinic.
If it all works out, many lives and dollars could be saved, thanks to science.
Democracy, meet COVID-19
Everywhere you look, it seems, someone is trying to keep someone else from doing something: Don’t carry a gun. Don’t get an abortion. Don’t drive so fast. Don’t inhale that whipped cream. Don’t get a vaccine. Don’t put that in your mouth.
One of the biggies these days is voting rights. Some people are trying to prevent other people from voting. But why? Well, turns out that turnout can be bad for your health … at least during a worldwide pandemic event.
The evidence for that claim comes from researchers who examined the Italian national constitutional referendum conducted in September 2020 along with elections for assembly representatives in 7 of the country’s 20 regions and for mayors in about 12% of municipalities. The combination mattered: Voter turnout was higher in the municipalities that voted for both the referendum and local elections (69%), compared with municipalities voting only for the referendum (47%), the investigators reported in the Journal of Economic Behavior & Organization.
Also occurring in September of 2020 was, as we mentioned, a worldwide pandemic event. You may have heard about it.
The investigators considered the differences in election turnout between the various municipalities and compared them with new weekly COVID-19 infections at the municipality level. “Our model shows that something as fundamental as casting a vote can come at a cost,” investigator Giuseppe Moscelli, PhD, of the University of Surrey (England) said in a written statement.
What was the cost? Each 1% increase in turnout, they found, amounted to an average 1.1% increase in COVID infections after the elections.
See? More people voting means more COVID, which is bad. Which brings us to today’s lesson in people preventing other people from doing something. Don’t let COVID win. Stay in your house and never come out. And get that smeckledorf out of your mouth. You don’t know where it’s been.
Exercise may counteract genetics for gestational diabetes
Women giving birth for the first time have significantly higher odds of developing gestational diabetes if they have a high polygenic risk score (PRS) and low physical activity, new data suggest.
Researchers, led by Kymberleigh A. Pagel, PhD, with the department of computer science, Indiana University, Bloomington, concluded that physical activity early in pregnancy is associated with reduced risk of gestational diabetes and may help women who are at high risk because of genetic predisposition, age, family history of diabetes, and body mass index.
The researchers included 3,533 women in the analysis (average age, 28.6 years) which was a subcohort of a larger study. They found that physical activity’s association with lower gestational diabetes risk “was particularly significant in individuals who were genetically predisposed to diabetes through PRS or family history,” the authors wrote.
Women with high PRS and low level of physical activity had three times the odds of developing gestational diabetes (odds ratio, 3.4; 95% confidence interval, 2.3-5.3).
Those with high PRS and moderate to high activity levels in early pregnancy (metabolic equivalents of task [METs] of at least 450) had gestational diabetes risk similar to that of the general population, according to the researchers.
The findings were published in JAMA Network Open.
Maisa Feghali, MD, a maternal-fetal specialist at the University of Pittsburgh Medical Center, who was not part of the study, said in an interview she found the link of physical activity and compensation for high predisposition to gestational diabetes most interesting.
“That’s interesting because a lot of studies that have looked at prevention of gestational diabetes either through limited weight gain or through some form of counseling on physical activity have not really shown any benefit,” she noted. “It might just be it’s not just one size fits all and it may be that physical activity is mostly beneficial in those with a high predisposition.”
Research in this area is particularly important as 7% of pregnancies in the United States each year are affected by gestational diabetes and the risk for developing type 2 diabetes “has doubled in the past decade among patients with GD [gestational diabetes],” the authors wrote.
Researchers looked at risks for gestational diabetes in high-risk subgroups, including women who had a body mass index of more than 25 kg/m2 or were at least 35 years old. In that group, women who were either in the in the top 25th percentile for PRS or had low physical activity (METs less than 450) had from 25% to 75% greater risk of developing gestational diabetes.
The findings are consistent with previous research and suggest exercise interventions may be important in improving pregnancy outcomes, the authors wrote.
Christina Han, MD, division director for maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, pointed out several limitations of the study, however.
One of the biggest limitations, she said, was that “they excluded two-thirds of the original study. Essentially, they took only Caucasian [White] patients, which is about one-third of the study.” Additionally, the cohort was made up of people who had never had babies.
“Lots of our gestational diabetes patients are not first-time moms, so this makes the generalizability of the study very limited,” Dr. Han said.
She added that none of the sites where the study was conducted were in the South or Northwest, which also adds questions about generalizability.
Dr. Feghali and Dr. Han reported no relevant financial relationships.
Women giving birth for the first time have significantly higher odds of developing gestational diabetes if they have a high polygenic risk score (PRS) and low physical activity, new data suggest.
Researchers, led by Kymberleigh A. Pagel, PhD, with the department of computer science, Indiana University, Bloomington, concluded that physical activity early in pregnancy is associated with reduced risk of gestational diabetes and may help women who are at high risk because of genetic predisposition, age, family history of diabetes, and body mass index.
The researchers included 3,533 women in the analysis (average age, 28.6 years) which was a subcohort of a larger study. They found that physical activity’s association with lower gestational diabetes risk “was particularly significant in individuals who were genetically predisposed to diabetes through PRS or family history,” the authors wrote.
Women with high PRS and low level of physical activity had three times the odds of developing gestational diabetes (odds ratio, 3.4; 95% confidence interval, 2.3-5.3).
Those with high PRS and moderate to high activity levels in early pregnancy (metabolic equivalents of task [METs] of at least 450) had gestational diabetes risk similar to that of the general population, according to the researchers.
The findings were published in JAMA Network Open.
Maisa Feghali, MD, a maternal-fetal specialist at the University of Pittsburgh Medical Center, who was not part of the study, said in an interview she found the link of physical activity and compensation for high predisposition to gestational diabetes most interesting.
“That’s interesting because a lot of studies that have looked at prevention of gestational diabetes either through limited weight gain or through some form of counseling on physical activity have not really shown any benefit,” she noted. “It might just be it’s not just one size fits all and it may be that physical activity is mostly beneficial in those with a high predisposition.”
Research in this area is particularly important as 7% of pregnancies in the United States each year are affected by gestational diabetes and the risk for developing type 2 diabetes “has doubled in the past decade among patients with GD [gestational diabetes],” the authors wrote.
Researchers looked at risks for gestational diabetes in high-risk subgroups, including women who had a body mass index of more than 25 kg/m2 or were at least 35 years old. In that group, women who were either in the in the top 25th percentile for PRS or had low physical activity (METs less than 450) had from 25% to 75% greater risk of developing gestational diabetes.
The findings are consistent with previous research and suggest exercise interventions may be important in improving pregnancy outcomes, the authors wrote.
Christina Han, MD, division director for maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, pointed out several limitations of the study, however.
One of the biggest limitations, she said, was that “they excluded two-thirds of the original study. Essentially, they took only Caucasian [White] patients, which is about one-third of the study.” Additionally, the cohort was made up of people who had never had babies.
“Lots of our gestational diabetes patients are not first-time moms, so this makes the generalizability of the study very limited,” Dr. Han said.
She added that none of the sites where the study was conducted were in the South or Northwest, which also adds questions about generalizability.
Dr. Feghali and Dr. Han reported no relevant financial relationships.
Women giving birth for the first time have significantly higher odds of developing gestational diabetes if they have a high polygenic risk score (PRS) and low physical activity, new data suggest.
Researchers, led by Kymberleigh A. Pagel, PhD, with the department of computer science, Indiana University, Bloomington, concluded that physical activity early in pregnancy is associated with reduced risk of gestational diabetes and may help women who are at high risk because of genetic predisposition, age, family history of diabetes, and body mass index.
The researchers included 3,533 women in the analysis (average age, 28.6 years) which was a subcohort of a larger study. They found that physical activity’s association with lower gestational diabetes risk “was particularly significant in individuals who were genetically predisposed to diabetes through PRS or family history,” the authors wrote.
Women with high PRS and low level of physical activity had three times the odds of developing gestational diabetes (odds ratio, 3.4; 95% confidence interval, 2.3-5.3).
Those with high PRS and moderate to high activity levels in early pregnancy (metabolic equivalents of task [METs] of at least 450) had gestational diabetes risk similar to that of the general population, according to the researchers.
The findings were published in JAMA Network Open.
Maisa Feghali, MD, a maternal-fetal specialist at the University of Pittsburgh Medical Center, who was not part of the study, said in an interview she found the link of physical activity and compensation for high predisposition to gestational diabetes most interesting.
“That’s interesting because a lot of studies that have looked at prevention of gestational diabetes either through limited weight gain or through some form of counseling on physical activity have not really shown any benefit,” she noted. “It might just be it’s not just one size fits all and it may be that physical activity is mostly beneficial in those with a high predisposition.”
Research in this area is particularly important as 7% of pregnancies in the United States each year are affected by gestational diabetes and the risk for developing type 2 diabetes “has doubled in the past decade among patients with GD [gestational diabetes],” the authors wrote.
Researchers looked at risks for gestational diabetes in high-risk subgroups, including women who had a body mass index of more than 25 kg/m2 or were at least 35 years old. In that group, women who were either in the in the top 25th percentile for PRS or had low physical activity (METs less than 450) had from 25% to 75% greater risk of developing gestational diabetes.
The findings are consistent with previous research and suggest exercise interventions may be important in improving pregnancy outcomes, the authors wrote.
Christina Han, MD, division director for maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, pointed out several limitations of the study, however.
One of the biggest limitations, she said, was that “they excluded two-thirds of the original study. Essentially, they took only Caucasian [White] patients, which is about one-third of the study.” Additionally, the cohort was made up of people who had never had babies.
“Lots of our gestational diabetes patients are not first-time moms, so this makes the generalizability of the study very limited,” Dr. Han said.
She added that none of the sites where the study was conducted were in the South or Northwest, which also adds questions about generalizability.
Dr. Feghali and Dr. Han reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
Living-donor liver transplants linked with substantial survival benefit
Living-donor liver transplant recipients gained an additional 13-17 years of life, compared with patients who remained on the wait list, according to a retrospective case-control study.
The data suggest that the life-years gained are comparable to or greater than those conferred by either other lifesaving procedures or liver transplant from a deceased donor, wrote the researchers, led by Whitney Jackson, MD, assistant professor of gastroenterology and medical director of living-donor liver transplantation at the University of Colorado Anschutz Medical Campus.
“Despite the acceptance of living-donor liver transplant as a lifesaving procedure for end-stage liver disease, it remains underused in the United States,” the authors wrote in JAMA Surgery. “This study’s findings challenge current perceptions regarding when the survival benefit of a living-donor transplant occurs.”
Dr. Jackson and colleagues conducted a retrospective, secondary analysis of the Scientific Registry of Transplant Recipients database for 119,275 U.S. liver transplant candidates and recipients from January 2012 to September 2021. They assessed the survival benefit, life-years saved, and the Model for End-Stage Liver Disease incorporating sodium levels (MELD-Na) score at which the survival benefit was obtained, compared with those who remained on the wait list.
The research team included 116,455 liver transplant candidates who were 18 and older and assigned to the wait list, as well as 2,820 patients who received a living-donor liver transplant. Patients listed for retransplant or multiorgan transplant were excluded, as were those with prior kidney or liver transplants.
The mean age of the study participants was 55 years, and 63% were men. Overall, 70.2% were White, 15.8% were Hispanic or Latinx, 8.2% were Black or African American, 4.3% were Asian, 0.9% were American Indian or Alaska Native, and 0.2% were Native Hawaiian or Pacific Islander. The most common etiologies were alcoholic cirrhosis (23.8%) and nonalcoholic steatohepatitis (15.9%).
Compared with patients on the wait list, recipients of a living-donor liver transplant were younger, more often women, more educated, and more often White. A greater proportion of transplant recipients had a primary etiology of nonalcoholic steatohepatitis (19.8%) and cholestatic liver disease (24.1%). At wait list placement, one-third of candidates had a MELD-Na score of 14 or higher.
The research team found a significant survival benefit for patients receiving a living-donor liver transplant based on mortality risk and survival scores. The survival benefit was significant at a MELD-Na score as low as 11, with a 34% decrease(95% confidence interval [CI], 17.4%-52.0%) in mortality compared with the wait list. In addition, mortality risk models confirmed a survival benefit for patients with a MELD-Na score of 11 or higher at 1 year after transplant (adjusted hazard ratio, 0.64; 95% CI, 0.47-0.88; P = .006). At a MELD-Na score of 14-16, mortality decreased by about 50% (aHR, 0.47; 95% CI, 0.34-0.66; P < .001).
The probability of death from a living-donor liver transplant for patients with very low MELD-Na scores (between 6 and 10) was greater than that for patients on the wait list for the first 259 days, at which point the risk of death for both groups was equal. At 471 days, the probability of survival in both groups was equal. As the MELD-Na score increased, both the time to equal risk of death and the time to equal survival decreased, demonstrating that the survival benefit occurs much earlier for patients with a higher MELD-Na score.
Analysis of life-years from transplant showed living-donor transplant recipients gained 13-17 life-years compared to those who didn’t receive one.
“Living-donor liver transplantation is a valuable yet underutilized strategy to address the significant organ shortage and long waiting times on the transplant list in the U.S.,” said Renu Dhanasekaran, MD, PhD, assistant professor of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Dhanasekaran, who wasn’t involved with this study, also welcomed the finding that living-donor liver transplantation can benefit patients with low MELD-Na scores, even below the expected cutoff at 15. According to the study authors, previous research had suggested benefit would be seen only at MELD-Na 15 and above.
“In my practice, I have several patients whose symptoms are out of proportion to their MELD score, and data like this will convince them and their potential donors to avail a transplant at an earlier stage,” she said.
The findings challenge the current paradigm around the timing of referral for a liver transplant and may have ramifications for allocation policies for deceased donors, the study authors wrote. The data can also help to contextualize risk-benefit discussions for donors and recipients.
“Donating a part of one’s liver to save a patient suffering from end-stage liver disease is an incredible act of selfless love,” Dr. Dhanasekaran said. “I hope strong positive data from studies like this one encourage more donors, patients, and transplant centers to expand the use of [living-donor liver transplant].”
The authors reported no grant support or funding sources for this study. One author disclosed being married to the current chair of the United Network for Organ Sharing’s Liver and Intestinal Organ Transplantation Committee. No other conflicts of interest were reported. Dr. Dhanasekaran reported no relevant disclosures.
Living-donor liver transplant recipients gained an additional 13-17 years of life, compared with patients who remained on the wait list, according to a retrospective case-control study.
The data suggest that the life-years gained are comparable to or greater than those conferred by either other lifesaving procedures or liver transplant from a deceased donor, wrote the researchers, led by Whitney Jackson, MD, assistant professor of gastroenterology and medical director of living-donor liver transplantation at the University of Colorado Anschutz Medical Campus.
“Despite the acceptance of living-donor liver transplant as a lifesaving procedure for end-stage liver disease, it remains underused in the United States,” the authors wrote in JAMA Surgery. “This study’s findings challenge current perceptions regarding when the survival benefit of a living-donor transplant occurs.”
Dr. Jackson and colleagues conducted a retrospective, secondary analysis of the Scientific Registry of Transplant Recipients database for 119,275 U.S. liver transplant candidates and recipients from January 2012 to September 2021. They assessed the survival benefit, life-years saved, and the Model for End-Stage Liver Disease incorporating sodium levels (MELD-Na) score at which the survival benefit was obtained, compared with those who remained on the wait list.
The research team included 116,455 liver transplant candidates who were 18 and older and assigned to the wait list, as well as 2,820 patients who received a living-donor liver transplant. Patients listed for retransplant or multiorgan transplant were excluded, as were those with prior kidney or liver transplants.
The mean age of the study participants was 55 years, and 63% were men. Overall, 70.2% were White, 15.8% were Hispanic or Latinx, 8.2% were Black or African American, 4.3% were Asian, 0.9% were American Indian or Alaska Native, and 0.2% were Native Hawaiian or Pacific Islander. The most common etiologies were alcoholic cirrhosis (23.8%) and nonalcoholic steatohepatitis (15.9%).
Compared with patients on the wait list, recipients of a living-donor liver transplant were younger, more often women, more educated, and more often White. A greater proportion of transplant recipients had a primary etiology of nonalcoholic steatohepatitis (19.8%) and cholestatic liver disease (24.1%). At wait list placement, one-third of candidates had a MELD-Na score of 14 or higher.
The research team found a significant survival benefit for patients receiving a living-donor liver transplant based on mortality risk and survival scores. The survival benefit was significant at a MELD-Na score as low as 11, with a 34% decrease(95% confidence interval [CI], 17.4%-52.0%) in mortality compared with the wait list. In addition, mortality risk models confirmed a survival benefit for patients with a MELD-Na score of 11 or higher at 1 year after transplant (adjusted hazard ratio, 0.64; 95% CI, 0.47-0.88; P = .006). At a MELD-Na score of 14-16, mortality decreased by about 50% (aHR, 0.47; 95% CI, 0.34-0.66; P < .001).
The probability of death from a living-donor liver transplant for patients with very low MELD-Na scores (between 6 and 10) was greater than that for patients on the wait list for the first 259 days, at which point the risk of death for both groups was equal. At 471 days, the probability of survival in both groups was equal. As the MELD-Na score increased, both the time to equal risk of death and the time to equal survival decreased, demonstrating that the survival benefit occurs much earlier for patients with a higher MELD-Na score.
Analysis of life-years from transplant showed living-donor transplant recipients gained 13-17 life-years compared to those who didn’t receive one.
“Living-donor liver transplantation is a valuable yet underutilized strategy to address the significant organ shortage and long waiting times on the transplant list in the U.S.,” said Renu Dhanasekaran, MD, PhD, assistant professor of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Dhanasekaran, who wasn’t involved with this study, also welcomed the finding that living-donor liver transplantation can benefit patients with low MELD-Na scores, even below the expected cutoff at 15. According to the study authors, previous research had suggested benefit would be seen only at MELD-Na 15 and above.
“In my practice, I have several patients whose symptoms are out of proportion to their MELD score, and data like this will convince them and their potential donors to avail a transplant at an earlier stage,” she said.
The findings challenge the current paradigm around the timing of referral for a liver transplant and may have ramifications for allocation policies for deceased donors, the study authors wrote. The data can also help to contextualize risk-benefit discussions for donors and recipients.
“Donating a part of one’s liver to save a patient suffering from end-stage liver disease is an incredible act of selfless love,” Dr. Dhanasekaran said. “I hope strong positive data from studies like this one encourage more donors, patients, and transplant centers to expand the use of [living-donor liver transplant].”
The authors reported no grant support or funding sources for this study. One author disclosed being married to the current chair of the United Network for Organ Sharing’s Liver and Intestinal Organ Transplantation Committee. No other conflicts of interest were reported. Dr. Dhanasekaran reported no relevant disclosures.
Living-donor liver transplant recipients gained an additional 13-17 years of life, compared with patients who remained on the wait list, according to a retrospective case-control study.
The data suggest that the life-years gained are comparable to or greater than those conferred by either other lifesaving procedures or liver transplant from a deceased donor, wrote the researchers, led by Whitney Jackson, MD, assistant professor of gastroenterology and medical director of living-donor liver transplantation at the University of Colorado Anschutz Medical Campus.
“Despite the acceptance of living-donor liver transplant as a lifesaving procedure for end-stage liver disease, it remains underused in the United States,” the authors wrote in JAMA Surgery. “This study’s findings challenge current perceptions regarding when the survival benefit of a living-donor transplant occurs.”
Dr. Jackson and colleagues conducted a retrospective, secondary analysis of the Scientific Registry of Transplant Recipients database for 119,275 U.S. liver transplant candidates and recipients from January 2012 to September 2021. They assessed the survival benefit, life-years saved, and the Model for End-Stage Liver Disease incorporating sodium levels (MELD-Na) score at which the survival benefit was obtained, compared with those who remained on the wait list.
The research team included 116,455 liver transplant candidates who were 18 and older and assigned to the wait list, as well as 2,820 patients who received a living-donor liver transplant. Patients listed for retransplant or multiorgan transplant were excluded, as were those with prior kidney or liver transplants.
The mean age of the study participants was 55 years, and 63% were men. Overall, 70.2% were White, 15.8% were Hispanic or Latinx, 8.2% were Black or African American, 4.3% were Asian, 0.9% were American Indian or Alaska Native, and 0.2% were Native Hawaiian or Pacific Islander. The most common etiologies were alcoholic cirrhosis (23.8%) and nonalcoholic steatohepatitis (15.9%).
Compared with patients on the wait list, recipients of a living-donor liver transplant were younger, more often women, more educated, and more often White. A greater proportion of transplant recipients had a primary etiology of nonalcoholic steatohepatitis (19.8%) and cholestatic liver disease (24.1%). At wait list placement, one-third of candidates had a MELD-Na score of 14 or higher.
The research team found a significant survival benefit for patients receiving a living-donor liver transplant based on mortality risk and survival scores. The survival benefit was significant at a MELD-Na score as low as 11, with a 34% decrease(95% confidence interval [CI], 17.4%-52.0%) in mortality compared with the wait list. In addition, mortality risk models confirmed a survival benefit for patients with a MELD-Na score of 11 or higher at 1 year after transplant (adjusted hazard ratio, 0.64; 95% CI, 0.47-0.88; P = .006). At a MELD-Na score of 14-16, mortality decreased by about 50% (aHR, 0.47; 95% CI, 0.34-0.66; P < .001).
The probability of death from a living-donor liver transplant for patients with very low MELD-Na scores (between 6 and 10) was greater than that for patients on the wait list for the first 259 days, at which point the risk of death for both groups was equal. At 471 days, the probability of survival in both groups was equal. As the MELD-Na score increased, both the time to equal risk of death and the time to equal survival decreased, demonstrating that the survival benefit occurs much earlier for patients with a higher MELD-Na score.
Analysis of life-years from transplant showed living-donor transplant recipients gained 13-17 life-years compared to those who didn’t receive one.
“Living-donor liver transplantation is a valuable yet underutilized strategy to address the significant organ shortage and long waiting times on the transplant list in the U.S.,” said Renu Dhanasekaran, MD, PhD, assistant professor of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Dhanasekaran, who wasn’t involved with this study, also welcomed the finding that living-donor liver transplantation can benefit patients with low MELD-Na scores, even below the expected cutoff at 15. According to the study authors, previous research had suggested benefit would be seen only at MELD-Na 15 and above.
“In my practice, I have several patients whose symptoms are out of proportion to their MELD score, and data like this will convince them and their potential donors to avail a transplant at an earlier stage,” she said.
The findings challenge the current paradigm around the timing of referral for a liver transplant and may have ramifications for allocation policies for deceased donors, the study authors wrote. The data can also help to contextualize risk-benefit discussions for donors and recipients.
“Donating a part of one’s liver to save a patient suffering from end-stage liver disease is an incredible act of selfless love,” Dr. Dhanasekaran said. “I hope strong positive data from studies like this one encourage more donors, patients, and transplant centers to expand the use of [living-donor liver transplant].”
The authors reported no grant support or funding sources for this study. One author disclosed being married to the current chair of the United Network for Organ Sharing’s Liver and Intestinal Organ Transplantation Committee. No other conflicts of interest were reported. Dr. Dhanasekaran reported no relevant disclosures.
FROM JAMA SURGERY
Borderline personality disorder raises relapse risk for MDD patients after ECT
ECT has demonstrated effectiveness for treatment of unipolar and bipolar major depression, but relapses within 6 months are frequent, and potential factors affecting relapse have not been well studied, wrote Matthieu Hein, MD, PhD, of Erasme Hospital, Université Libre de Bruxelles, and colleagues.
Borderline personality disorder (BPD) is a common comorbidity among individuals with major depressive disorder, and previous research suggests a possible negative effect of BPD on ECT response in MDD patients, they wrote.
In a study published in Psychiatry Research, the researchers recruited 68 females and 41 males aged 18 years and older with diagnosed MDD who had partial or complete response to ECT after receiving treatment at a single center. Approximately two-thirds of the patients were aged 50 years and older, and 22 met criteria for BPD. The ECT consisted of three sessions per week; the total number of sessions ranged from 6 to 18.
The primary outcome was relapse at 6 months after ECT treatment. Relapse was defined as a score of 16 or higher on the Hamilton Depression Rating Scale in combination with a mean absolute increase of at least 10 points from the psychiatric interview at the end of the ECT.
Relapse rates at 6 months were 37.6% for the study population overall, but significantly higher for those with BPD, compared with those without BPD (72.7% vs. 28.7%; P < .001).
In a multivariate analysis, adjusting for age, gender, and mood stabilizer use after ECT, relapse was approximately four times more likely among individuals with BPD, compared with those without (hazard ratio, 4.14). No significant association appeared between increased relapse and other comorbid personality disorders, anxiety disorders, alcohol or substance use disorders, or hospitalization during the ECT treatment period.
Potential reasons for the increased relapse risk among individuals with MDD and BPD include the younger age of the individuals with BPD, which has been shown to increase MDD relapse risk; the direct negative impact of BPD on mental functioning; and the documented tendency to poor treatment adherence, the researchers wrote in their discussion.
“Given these different elements, it seems important to screen more systematically for BPD in major depressed individuals treated with ECT in order to allow the implementation of more effective prevention strategies for relapse within 6 months in this particular subpopulation,” they emphasized.
“The demonstration of this higher risk of relapse within 6 months associated with BPD in major depressed individuals treated with ECT could open new therapeutic perspectives to allow better maintenance of euthymia in this particular subpopulation,” they added.
The study findings were limited by several factors including the retrospective design and the focus on only BPD, which may not generalize to other personality disorders, the researchers noted.
However, the results support data from previous studies and highlight the need for more systematic BPD screening in MDD patients to prevent relapse after ECT, they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
ECT has demonstrated effectiveness for treatment of unipolar and bipolar major depression, but relapses within 6 months are frequent, and potential factors affecting relapse have not been well studied, wrote Matthieu Hein, MD, PhD, of Erasme Hospital, Université Libre de Bruxelles, and colleagues.
Borderline personality disorder (BPD) is a common comorbidity among individuals with major depressive disorder, and previous research suggests a possible negative effect of BPD on ECT response in MDD patients, they wrote.
In a study published in Psychiatry Research, the researchers recruited 68 females and 41 males aged 18 years and older with diagnosed MDD who had partial or complete response to ECT after receiving treatment at a single center. Approximately two-thirds of the patients were aged 50 years and older, and 22 met criteria for BPD. The ECT consisted of three sessions per week; the total number of sessions ranged from 6 to 18.
The primary outcome was relapse at 6 months after ECT treatment. Relapse was defined as a score of 16 or higher on the Hamilton Depression Rating Scale in combination with a mean absolute increase of at least 10 points from the psychiatric interview at the end of the ECT.
Relapse rates at 6 months were 37.6% for the study population overall, but significantly higher for those with BPD, compared with those without BPD (72.7% vs. 28.7%; P < .001).
In a multivariate analysis, adjusting for age, gender, and mood stabilizer use after ECT, relapse was approximately four times more likely among individuals with BPD, compared with those without (hazard ratio, 4.14). No significant association appeared between increased relapse and other comorbid personality disorders, anxiety disorders, alcohol or substance use disorders, or hospitalization during the ECT treatment period.
Potential reasons for the increased relapse risk among individuals with MDD and BPD include the younger age of the individuals with BPD, which has been shown to increase MDD relapse risk; the direct negative impact of BPD on mental functioning; and the documented tendency to poor treatment adherence, the researchers wrote in their discussion.
“Given these different elements, it seems important to screen more systematically for BPD in major depressed individuals treated with ECT in order to allow the implementation of more effective prevention strategies for relapse within 6 months in this particular subpopulation,” they emphasized.
“The demonstration of this higher risk of relapse within 6 months associated with BPD in major depressed individuals treated with ECT could open new therapeutic perspectives to allow better maintenance of euthymia in this particular subpopulation,” they added.
The study findings were limited by several factors including the retrospective design and the focus on only BPD, which may not generalize to other personality disorders, the researchers noted.
However, the results support data from previous studies and highlight the need for more systematic BPD screening in MDD patients to prevent relapse after ECT, they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
ECT has demonstrated effectiveness for treatment of unipolar and bipolar major depression, but relapses within 6 months are frequent, and potential factors affecting relapse have not been well studied, wrote Matthieu Hein, MD, PhD, of Erasme Hospital, Université Libre de Bruxelles, and colleagues.
Borderline personality disorder (BPD) is a common comorbidity among individuals with major depressive disorder, and previous research suggests a possible negative effect of BPD on ECT response in MDD patients, they wrote.
In a study published in Psychiatry Research, the researchers recruited 68 females and 41 males aged 18 years and older with diagnosed MDD who had partial or complete response to ECT after receiving treatment at a single center. Approximately two-thirds of the patients were aged 50 years and older, and 22 met criteria for BPD. The ECT consisted of three sessions per week; the total number of sessions ranged from 6 to 18.
The primary outcome was relapse at 6 months after ECT treatment. Relapse was defined as a score of 16 or higher on the Hamilton Depression Rating Scale in combination with a mean absolute increase of at least 10 points from the psychiatric interview at the end of the ECT.
Relapse rates at 6 months were 37.6% for the study population overall, but significantly higher for those with BPD, compared with those without BPD (72.7% vs. 28.7%; P < .001).
In a multivariate analysis, adjusting for age, gender, and mood stabilizer use after ECT, relapse was approximately four times more likely among individuals with BPD, compared with those without (hazard ratio, 4.14). No significant association appeared between increased relapse and other comorbid personality disorders, anxiety disorders, alcohol or substance use disorders, or hospitalization during the ECT treatment period.
Potential reasons for the increased relapse risk among individuals with MDD and BPD include the younger age of the individuals with BPD, which has been shown to increase MDD relapse risk; the direct negative impact of BPD on mental functioning; and the documented tendency to poor treatment adherence, the researchers wrote in their discussion.
“Given these different elements, it seems important to screen more systematically for BPD in major depressed individuals treated with ECT in order to allow the implementation of more effective prevention strategies for relapse within 6 months in this particular subpopulation,” they emphasized.
“The demonstration of this higher risk of relapse within 6 months associated with BPD in major depressed individuals treated with ECT could open new therapeutic perspectives to allow better maintenance of euthymia in this particular subpopulation,” they added.
The study findings were limited by several factors including the retrospective design and the focus on only BPD, which may not generalize to other personality disorders, the researchers noted.
However, the results support data from previous studies and highlight the need for more systematic BPD screening in MDD patients to prevent relapse after ECT, they said.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
Neuropsychiatric symptoms after stroke
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.
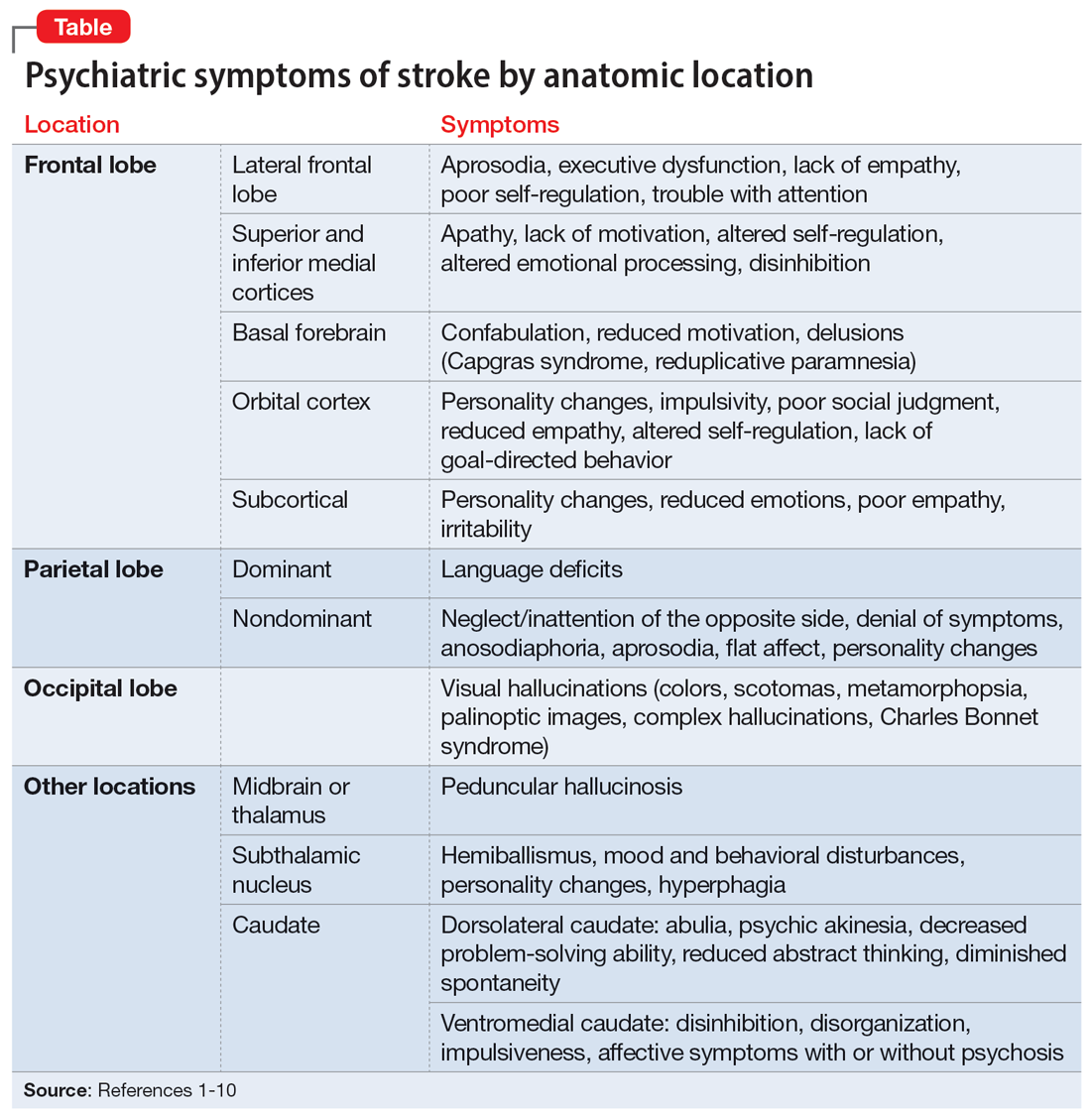
Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
Many patients experience neuropsychiatric symptoms following stroke. There is tremendous variation in the type, severity, and timeline of these symptoms, which have the potential to significantly impact patients’ quality of life. Some symptoms occur as a direct result of ischemic injury to brain structures regulating behavior, executive function, perception, or affect. Other symptoms occur indirectly due to the patient’s often-difficult experiences with the health care system, disrupted routines, or altered poststroke functional abilities. Psychiatric symptoms are not as easily recognized as classic stroke symptoms (such as hemiparesis) and are frequently overlooked, especially in the acute phase. However, these symptoms can negatively influence patients’ interpersonal relationships, rehabilitation, and employment.
Patients and families may not realize certain symptoms are stroke-related and may not discuss them with their clinicians. It is important to ask about and recognize psychiatric symptoms in patients who have experienced a stroke so you can provide optimal education and treatment. In this article, we review the types of psychiatric symptoms associated with strokes in specific brain regions (Table1-10). We also describe symptoms that do not appear directly related to the anatomical structures affected by the infarct, including delirium, psychosis, depression, anxiety, and posttraumatic stress.

Symptoms associated with stroke in specific regions
Frontal lobe strokes
The frontal lobes are the largest lobes in the brain, and damage to areas within these lobes can cause behavioral and personality changes. Lesions in the lateral frontal cortex can cause aprosodia (difficulty expressing or comprehending variations in tone of voice), which can lead to communication errors. Lateral frontal cortex injury can cause executive dysfunction and a lack of empathy1 as well as trouble with attention, planning, and self-regulation that may affect daily functioning. Strokes affecting the superior and inferior mesial cortices may result in apathy, lack of motivation, altered self-regulation, altered emotional processing, and disinhibition. Patients who experience a basal forebrain stroke may exhibit confabulation, reduced motivation, and delusions such as Capgras syndrome (the belief that a person or place has been replaced by an exact copy) and reduplicative paramnesia (the belief that a place has been either moved, duplicated, or exists in 2 places simultaneously). Strokes involving the orbital cortex can be associated with personality changes, impulsivity, poor social judgment, reduced empathy, altered self-regulation, lack of goal-directed behavior, and environmental dependency.
Some strokes may occur primarily in the subcortical white matter within the frontal lobes. Symptoms may be due to a single stroke with sudden onset, or due to repeated ischemic events that accumulate over time, as seen with microvascular disease. In the case of microvascular disease, the onset of symptoms may be insidious and the course progressive. Infarcts in the subcortical area can also cause personality changes (though typically more subtle when compared to orbitofrontal strokes), reduced emotions, poor empathy, and irritability.1 Patients may lack insight into some of or all these symptoms following a frontal lobe infarct, which makes it critical to gather collateral information from the patient’s friends or family.
Parietal lobe strokes
Symptomatology from parietal strokes depends on whether the stroke affects the dominant or nondominant hemisphere. Dominant parietal lesions cause language deficits, and psychiatric symptoms may be difficult to elucidate due to the patient’s inability to communicate.2 On the other hand, patients with nondominant parietal stroke may have neglect of, or inattention to, the opposite (typically left) side.3 This often manifests as a reluctance to use the affected limb or limbs, in some cases despite a lack of true weakness or motor dysfunction. In addition, patients may also have visual and/or tactile inattention towards the affected side, despite a lack of gross visual or sensory impairment.2 In rare cases, a patient’s stroke may be misdiagnosed as a functional disorder due to the perceived unwillingness to use a neurologically intact limb. In severe cases, patients may not recognize an affected extremity as their own. Patients are also frequently unaware of deficits affecting their nondominant side and may argue with those attempting to explain their deficit. Anosodiaphoria—an abnormal lack of concern regarding their deficits—may also be observed. Additionally, aprosodia, flat affect, and personality changes may result from strokes affecting the nondominant hemisphere, which can impact the patient’s relationships and social functioning.3
Occipital lobe strokes
While negative or loss-of-function symptomatology is one of the hallmarks of stroke, occipital lobe infarcts can pose an exception. Although vision loss is the most common symptom with occipital lobe strokes, some patients experience visual hallucinations that may occur acutely or subacutely. In the acute phase, patients may report hallucinations of varied description,4 including poorly formed areas of color, scotomas, metamorphopsia (visual distortion in which straight lines appear curved), more complex and formed hallucinations and/or palinoptic images (images or brief scenes that continue to be perceived after looking away). These hallucinations, often referred to as release phenomena or release hallucinations, are thought to result from disinhibition of the visual cortex, which then fires spontaneously.
Hallucinations are associated with either infarction or hemorrhage in the posterior cerebral artery territory. In some cases, the hallucinations may take on a formed, complex appearance, and Charles Bonnet syndrome (visual hallucinations in the setting of vision loss, with insight into the hallucinations) has been identified in a small portion of patients.5
Continue to: The duration of these...
The duration of these hallucinations varies. Some patients describe very short periods of the disturbance, lasting minutes to hours and corresponding with the onset of their stroke. Others experience prolonged hallucinations, which frequently evolve into formed, complex images, lasting from days to months.6 In the setting of cortical stroke, patients may be at risk for seizures, which could manifest as visual hallucinations. It is essential to ensure that epileptic causes of hallucinations have been ruled out, because seizures may require treatment and other precautions.
Other stroke locations
Strokes in other locations also can result in psychiatric or behavioral symptoms. Acute stroke in the subcortical midbrain or thalamus may result in peduncular hallucinosis, a syndrome of vivid visual hallucinations.7 The midbrain (most commonly the reticular formation) is usually affected; however, certain lesions of the thalamus may also cause peduncular hallucinosis. This phenomenon is theorized to be due to an increase in serotonin activity relative to acetylcholine and is often accompanied by drowsiness.
The subthalamic nucleus is most frequently associated with disordered movement such as hemiballismus, but also causes disturbances in mood and behavior, including hyperphagia and personality changes.8 Irritability, aggressiveness, disinhibition, anxiety, and obscene speech may also be seen with lesions of the subthalamic nucleus.
Finally, the caudate nucleus may cause alterations in executive functioning and behavior.9 A stroke in the dorsolateral caudate may cause abulia and psychic akinesia, decreased problem-solving ability, reduced abstract thinking, and/or diminished spontaneity, whereas an infarct in the ventromedial region of the nucleus may cause disinhibition, disorganization, impulsiveness, and, in severe cases, affective symptoms with psychosis.10 Strokes in any of these areas are at risk for being misdiagnosed because patients may not have a hemiparesis, and isolated positive or psychiatric symptoms may not be recognized as stroke.
Symptoms not related to stroke location
Delirium and psychosis
Following a stroke, a patient may exhibit neuropsychiatric symptoms that do not appear to relate directly to the anatomical structures affected by the infarct. In the acute phase, factors such as older age and medical complications (including infection, metabolic derangement, and lack of sleep due to frequent neurologic checks) create a high risk of delirium.11 Differentiating delirium from alterations in mental status due to seizure, cerebral edema, or other medical complications is essential, and delirium precautions should be exercised to the greatest extent possible. Other neuropsychiatric symptoms may manifest following hospitalization.
Continue to: Poststroke psychosis...
Poststroke psychosis often presents subacutely. Among these patients, the most common psychosis is delusion disorder, followed by schizophrenia-like psychosis and mood disorder with psychotic features.12 Some evidence suggests antipsychotics may be highly effective for many of these patients.12 Poststroke psychosis does appear to correlate somewhat with nondominant hemisphere lesions, including the frontal lobe, parietal lobe, temporal lobe, and/or caudate nucleus. Because high mortality and poor functional outcomes have been associated with poststroke psychosis, early intervention is essential.
Depression
Depression is a common problem following stroke, affecting approximately 35% of stroke patients.13 In addition to impairing quality of life, depression negatively impacts rehabilitation and increases caregiver burden. There is significant variability regarding risk factors that increases the likelihood of poststroke depression; however, psychiatric history, dysphagia, and poor social support consistently correlate with a higher risk.14,15 Characteristics of a patient’s stroke, such as lesion volume and the ability to perform activities of daily living, are also risk factors. Identifying depression among patients who recently had a stroke is sometimes difficult due to a plethora of confounding factors. Patients may not communicate well due to aphasia, while strokes in other locations may result in an altered affect. Depending on the stroke location, patients may also suffer anosognosia (a lack of awareness of their deficits), which may impair their ability to learn and use adaptive strategies and equipment. An additional confounder is the significant overlap between depressive symptoms and those seen in the setting of a major medical event or hospitalization (decreased appetite, fatigue, etc). The prevalence of depression peaks approximately 3 to 6 months after stroke, with symptoms lasting 9 to 12 months on average, although many patients experience symptoms significantly longer.14 Because symptoms can begin within hours to days following a stroke, it is essential that both hospital and outpatient clinicians assess for depression when indicated. Patients with poststroke depression should receive prompt treatment because appropriate treatment correlates with improved rehabilitation, and most patients respond well to antidepressants.16 Early treatment reduces mortality and improves compliance with secondary stroke prevention measures, including pharmacotherapy.17
Anxiety and posttraumatic stress
Anxiety and anxiety-related disorders are additional potential complications following stroke that significantly influence patient outcomes and well-being. The abrupt, unexpected onset of stroke is often frightening to patients and families. The potential for life-altering deficits as well as intense, often invasive, interactions with the health care system does little to assuage patients’ fear. Stroke patients must contend with a change in neurologic function while processing their difficult experiences, and may develop profound fear of a recurrent stroke. As many as 22% of patients have an anxiety disorder 3 months after they have a stroke.18 Phobic disorder is the most prevalent subtype, followed by generalized anxiety disorder. Younger age and previous anxiety or depression place patients at greater risk of developing poststroke anxiety. Patients suffering from poststroke anxiety have a reduced quality of life, are more dependent, and show restricted participation in rehabilitation, all of which culminate in poorer outcomes.
Many patients describe their experiences surrounding their stroke as traumatic, and posttraumatic stress disorder (PTSD) is increasingly acknowledged as a potential complication for patients with recent stroke.19 PTSD profoundly impacts patient quality of life. Interestingly, most patients who develop poststroke PTSD do not have a history of other psychiatric illness, and it is difficult to predict who may develop PTSD. Relatively little is known regarding optimal treatment strategies for poststroke PTSD, or the efficacy of pharmacotherapy and psychotherapeutic strategies to treat it.
Goals: Improve recovery and quality of life
Neuropsychiatric symptoms are common following a stroke and may manifest in a variety of ways. While some symptoms are a direct consequence of injury to a specific brain region, other symptoms may be a response to loss of independence, disability, experience with the medical system, or fear of recurrent stroke. The onset of psychiatric symptoms can be acute, beginning during hospitalization, or delayed. Understanding the association of psychiatric symptoms with the anatomical location of stroke may assist clinicians in identifying such symptoms. This knowledge informs conversations with patients and their caregivers, who may benefit from understanding that such symptoms are common after stroke. Furthermore, identifying psychiatric complications following stroke may affect rehabilitation. Additional investigation is necessary to find more effective treatment modalities and improve early intervention.
Continue to: Bottom Line
Bottom Line
Neuropsychiatric symptoms are frequently overlooked in patients with recent stroke. These symptoms include delirium, psychosis, depression, anxiety, and posttraumatic stress disorder, and can be the direct result of injury to neuroanatomical structures or a consequence of the patient’s experience. Prompt treatment can maximize stroke recovery and quality of life.
Related Resources
- Zhang S, Xu M, Liu ZJ, et al. Neuropsychiatric issues after stroke: clinical significance and therapeutic implications. World J Psychiatry. 2020;10(6):125-138. doi:10.5498/wjp. v10.i6.125
- Saha G, Chakraborty K, Pattojoshi A. Management of psychiatric disorders in patients with stroke and traumatic brain injury. Indian J Psychiatry. 2022;64(Suppl 2): S344-S354.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
1. Eslinger PJ, Reichwein RK. Frontal lobe stroke syndromes. In: Caplan LR, van Gijn J, eds. Stroke Syndromes. 3rd ed. Cambridge University Press; 2012:232-241.
2. Critchley M, Russell WR, Zangwill OL. Discussion on parietal lobe syndromes. Proc R Soc Med. 1951;44(4):337-346.
3. Hier DB, Mondlock J, Caplan LR. Behavioral abnormalities after right hemisphere stroke. Neurology. 1983;33(3):337-344.
4. Brust JC, Behrens MM. “Release hallucinations” as the major symptom of posterior cerebral artery occlusion: a report of 2 cases. Ann Neurol. 1977;2(5):432-436.
5. Kumral E, Uluakay A, Donmez A. Complex visual hallucinations following stroke: epileptic origin or a deafferentiation phenomenon? Austin J Cerebrovasc Dis & Stroke. 2014;1(1):1005.
6. Lee JS, Ko KH, Oh JH, et al. Charles Bonnet syndrome after occipital infarction. J Neurosonol Neuroimag. 2018;10(2):154-157.
7. Young JB. Peduncular hallucinosis. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Elsevier; 2014:848.
8. Etemadifar M, Abtahi SH, Abtahi SM, et al. Hemiballismus, hyperphagia, and behavioral changes following subthalamic infarct. Case Rep Med. 2012;2012:768580. doi:10.1155/2012/768580
9. Kumral E, Evyapan D, Balkir K. Acute caudate vascular lesions. Stroke. 1999;30(1):100-108.
10. Wang PY. Neurobehavioral changes following caudate infarct: a case report with literature review. Zhonghua Yi Xue Za Zhi (Taipei). 1991;47(3):199-203.
11. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-33.
12. Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89(8):879-885.
13. Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris). 2008;164(10):837-840.
14. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253-264.
15. Pritchard KT, Hreha KP, Hong I. Dysphagia associated with risk of depressive symptoms among stroke survivors after discharge from a cluster of inpatient rehabilitation facilities. Swallowing Rehabil. 2020;3(1):33-44.
16. Wiart L, Petit H, Joseph PA, et al. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke. 2000;31(8):1829-1832.
17. Jorge RE, Robinson RG, Arndt S, et al. Mortality and poststroke depression: a placebo-controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823-1829.
18. Chun HY, Whiteley WN, Dennis MS, et al. Anxiety after stroke: the importance of subtyping. Stroke. 2018;49(3):556-564.
19. Garton AL, Sisti JA, Gupta VP, et al. Poststroke post-traumatic stress disorder: a review. Stroke. 2017;48(2):507-512.
Laboratory monitoring for patients on buprenorphine: 10 questions
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
The opioid use disorder (OUD) epidemic is a major public health crisis in the United States.1 Naltrexone, methadone, and buprenorphine are first-line therapies for OUD and have high success rates.2 While studies have shown that naltrexone is effective, patients must achieve opioid detoxification and maintain 7 to 10 days of total abstinence to avoid a precipitated opioid withdrawal before it can be prescribed.3 Methadone does not require detoxification or a period of complete abstinence, but must be prescribed in special clinics and requires daily observed dosing for the first 90 days,4 though these requirements have been relaxed during the COVID-19 pandemic. In contrast, buprenorphine (with or without naloxone) can be used in office-based settings, which significantly improves the accessibility and availability of treatment for patients with OUD. Clinician knowledge and comfort prescribing buprenorphine are limiting factors to treatment.5 Increasing the number of clinicians proficient with buprenorphine management can improve access to effective treatment and recovery services, which is critical for patients with OUD.
Multiple resources are available for clinicians to learn how to prescribe buprenorphine, but clear guidance on laboratory testing for patients receiving buprenorphine is limited. To safely and effectively prescribe buprenorphine, clinicians need to understand its pharmacology (Box 16-9) and how laboratory testing influences treatment. In an effort to increase clinician knowledge of and proficiency with buprenorphine, this article answers 10 common questions about laboratory monitoring of patients receiving this medication.
Box 1
For patients with opioid use disorder, buprenorphine is indicated for opioid detoxification and maintenance. Oral formulations of buprenorphine (including tablets and buccal films) have long durations of action, and when dosed daily can prevent opioid withdrawal for at least 48 hours.6 The recommended formulation is a combination of buprenorphine and naloxone, because this formulation is associated with a lower risk of misuse and diversion compared to formulations containing only buprenorphine.7 However, buprenorphine alone can be effective in patients who experience adverse effects from or are unable to tolerate the combination buprenorphine/naloxone formulation.7 Despite the addition of naloxone, buprenorphine prescriptions may still be misused and diverted, so close monitoring is necessary.
Buprenorphine is metabolized by the cytochrome P450 system (CYP) (primarily CYP3A4) to its active metabolite, norbuprenorphine, both of which are primarily excreted in feces.8 However, small quantities of buprenorphine and norbuprenorphine are excreted in the urine,9 which makes urine specimen the best choice to monitor buprenorphine use for therapeutic purposes.
1. Why is laboratory monitoring important?
Proper laboratory monitoring discourages illicit substance use, encourages medication adherence, and influences treatment modifications. Patient self-reporting on medication compliance may be inaccurate or unreliable.10 Patients who relapse or use other illicit substances may also be reluctant to disclose their substance use.11
On the other hand, laboratory tests are objective markers of treatment outcome and adherence, and can verify a patient’s self-report.12 When used appropriately, laboratory monitoring can be therapeutic. It holds patients accountable, especially when used in conjunction with contingency management or other behavioral therapies.13 Laboratory monitoring is the most reliable method of determining if patients are abstaining from opioids and other illicit substances, or if the treatment plan requires revision.
2. Which tests should I order?
When initiating or maintaining a patient on buprenorphine, order a general urine drug screen (UDS), urine opioid screen (availability varies by institution), urine creatinine levels, urine buprenorphine/norbuprenorphine/naloxone/creatinine levels, urine alcohol metabolite levels, and a urine general toxicology test. It is also recommended to obtain a comprehensive metabolic panel (CMP) before starting buprenorphine,14,15 and to monitor CMP values at least once annually following treatment. Patients with a history of IV drug use or other high-risk factors should also be screened for hepatitis B, hepatitis C, and HIV.14,15
A general UDS can determine if opiates, amphetamines, cocaine, marijuana, or other common illicit substances are present to identify additional substance use. The proficiency of a general UDS may vary depending on the panels used at the respective institution. Some clinics use point-of-care UDS as part of their clinical management; these tests are inexpensive and provide immediate results.16 A basic UDS typically does not detect synthetic opioids due to the specificity of conventional immunoassays. As a result, specific tests for opioids such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, and methadone should also be considered, depending on their availability. Though buprenorphine treatment may trigger a positive opiate or other opioid screen,17 buprenorphine adherence should be confirmed using several urine tests, including creatinine, buprenorphine, norbuprenorphine, and naloxone urine levels.
In addition to screening for illicit substances and buprenorphine adherence, it is important to also screen for alcohol. Alcohol use disorder (AUD) is highly comorbid with OUD,18 and is associated with worse OUD treatment outcomes.19 Alcohol use may also affect liver function necessary for buprenorphine metabolism,8 so urine alcohol metabolites such as ethyl glucuronide and ethyl sulfate, serum transaminases, and gamma-glutamyl transferase should also be obtained.
Continue to: How frequently should patients be tested?
3. How frequently should patients be tested?
As part of the initial assessment, it is recommended to order CMP, UDS, and urine general toxicology.14 If indicated, specific laboratory tests such as specific opioid and alcohol metabolites screens can be ordered. After starting buprenorphine, the frequency of monitoring urine laboratory tests—including UDS, general drug toxicology, buprenorphine/norbuprenorphine/naloxone/creatinine, and alcohol and its metabolites—depends on a variety of factors, including a patient’s treatment response and stability as well as availability and cost of the tests. Ultimately, the frequency of laboratory monitoring should be determined on a patient-by-patient basis and clinicians should use their judgment.
The American Society of Addiction Medicine suggests testing more frequently earlier in the course of treatment (eg, weekly or biweekly), then spacing it out over time (eg, monthly or quarterly) as the patient’s recovery progresses.14,15 To conserve resources and reduce spending, some clinicians and guidelines recommend random monitoring as opposed to monitoring at every follow-up visit (eg, once out of every 3 to 5 visits, on average), which allows for longer intervals between testing while ensuring consistency with medication and abstinence from illicit substances.15,16 We suggest screening every 2 weeks for the first month, then spacing out to monthly and quarterly as patients demonstrate stability, with random screening as indicated. Monitoring of liver function should be done at least once annually.
4. How should urine buprenorphine and other results be interpreted?
There are several issues to consider when interpreting laboratory results. The clinician needs to know what to expect in the sample, and what approximate levels should be detected. To check treatment adherence, laboratory data should include stable urine buprenorphine and norbuprenorphine levels and negative urine screening for other illicit substances.14,15 While urine buprenorphine and norbuprenorphine levels have great interindividual variability due to genetic differences in hepatic metabolism, unusually high levels of buprenorphine (≥700 ng/mL) without norbuprenorphine suggests “urine spiking,” where patients put buprenorphine directly into their urine sample.20,21 Abnormally low or undetectable levels raise concern for medication nonadherence or diversion.
Though urine buprenorphine levels do not reliably correlate with dose, because there is typically not much intraindividual variability, patients should have relatively stable levels on each screen once a maintenance dose has been established.22 Furthermore, the buprenorphine-to-norbuprenorphine ratio (ie, “the metabolic ratio”) typically ranges from 1:2 to 1:4 across all individuals,20,21,23 regardless of dose or metabolic rate. Urine naloxone levels, which typically are included in commercial urine buprenorphine laboratory panels, also may aid in identifying tampered urine specimens when buprenorphine-to-norbuprenorphine ratios are abnormal or inconsistent with an individual’s prior ratio. Naloxone is typically (but not always) poorly absorbed and minimally detected in urine specimens.20 A high level of naloxone coupled with unusually high buprenorphine levels, particularly in the absence of norbuprenorphine in the urine, may indicate urine spiking.20,21,23
Urine creatinine is used to establish the reliability of the specimen. When urine creatinine concentration is <20 mg/dL, the concentration of most substances typically falls to subthreshold levels of detection.24 If a UDS is negative and the urine has a creatinine concentration <20 mg/dL, the patient should provide a new sample, because the urine was likely too diluted to detect any substances.
Continue to: The presence of alcohol...
The presence of alcohol metabolites can alert the clinician to recent alcohol use and possible AUD, which should be assessed and treated if indicated.
Liver enzymes should be normal or unchanged with short- and long-term buprenorphine use when taken as prescribed.25,26 However, acute liver injury may occur if patients inject buprenorphine intravenously, especially in those with underlying hepatitis C.25
5. What can cause a false negative result on UDS?
Laboratory monitoring may occasionally yield false negative drug screens. For urine buprenorphine levels, false negatives may occur in patients who are “rapid metabolizers,” infrequent or as-needed usage of the medication, patient mix-up, or laboratory error.27 For other substances, a false negative result may occur if the patient used the substance(s) outside the window of detection. The most common causes of false negative results, however, are overly diluted urine samples (eg, due to rapid water ingestion), or the use of an inappropriate test to measure a specific opioid or substance.27
Many laboratories use conventional immunoassays with morphine antibodies that react with various opioid substrates to determine the presence of a specific opioid. Some opioids—particularly synthetics such as oxycodone, hydrocodone, hydromorphone, oxymorphone, fentanyl, buprenorphine, and methadone—have poor cross-reactivity with the morphine antibody due to their distinct chemical structures, so standard immunoassays used to detect opioids may result in a false negative result.28 In such situations, a discussion with a clinical pathologist familiar with the laboratory detection method can help ensure proper testing. Additional tests for specific opioids should be ordered to more specifically target substances prone to false negative results.27
6. What can cause a false positive result on UDS?
The cross-reactivity of the morphine substrate may also result in a false positive result.28 Other over-the-counter (OTC) or prescription medications that have cross-reactivity with the morphine antibody include dextromethorphan, verapamil, quinine, fluoroquinolones, and rifampin, which can normally be found in urine 2 to 3 days after consumption.17,27 Poppy seeds have long been known to result in positive opiate screens on urine testing, particularly when laboratories use lower cutoff values (eg, 300 ng/mL), so advise patients to avoid consuming poppy seeds.29
Continue to: For other drugs of abuse...
For other drugs of abuse, false positives are typically caused by cross-reactivity with other prescription or OTC medications. Numerous substances cross-react with amphetamines and produce false positive results on amphetamine immunoassays, including amantadine, bupropion, ephedrine, labetalol, phentermine, pseudoephedrine, ranitidine, selegiline, and trazodone.27 Sertraline and efavirenz are known to produce false positive results on benzodiazepine UDS, and ibuprofen, naproxen, and efavirenz can produce false positive results for cannabinoids.27
7. How do I communicate the results to patients?
Effectively communicating test results to patients is just as important as the results themselves. A trusting, therapeutic alliance between patient and clinician is highly predictive of successful treatment,30 and how the clinician communicates affects the strength of this collaboration. A principle of addiction treatment is the use of neutral language when discussing laboratory results.31,32 To avoid unintentional shaming or moral judgment, use words such as “positive” or “negative” rather than stigmatizing terms such as “clean” or “dirty.”33
Additionally, make it clear that laboratory findings are not used to punish patients, but rather to improve treatment.34 Reassuring the patient that a positive screen will not result in withdrawal of care encourages a working relationship.14 All patients who receive buprenorphine treatment should be informed that collecting a UDS is the standard of care used to monitor their progress. You might want to compare using UDS in patients with OUD to monitoring HbA1c levels in patients with diabetes as an example to demonstrate how laboratory values inform treatment.35,36
Before reporting the results, a helpful strategy to maintain the therapeutic alliance in the face of a positive UDS is to ask the patient what they expect their UDS to show. When the patient has been reassured that treatment will not be withdrawn due to a positive result, they may be more likely to fully disclose substance use. This allows them the opportunity to self-disclose rather than be “called out” by the clinician.35
8. What happens when a patient tests positive for drugs of abuse?
If a patient tests positive for opioids or other drugs of abuse, convey this information to them, ideally by asking them what they expect to see on laboratory findings. Patients may have “slip ups” or relapses, or use certain prescription medications for medical reasons with the intention of establishing abstinence. It is essential to convey laboratory findings in a nonjudgmental tone while maintaining a supportive stance with clear boundaries.
Continue to: Though addiction specialists...
Though addiction specialists often advise complete abstinence from all substances, including alcohol, cannabis, and tobacco, the harm-reduction model emphasizes “meeting patients where they are” in terms of continued substance use.37 If a patient can reduce their substance use or abstain from some substances while continuing others, these accomplishments should be acknowledged.
For patients who continue to test positive for illicit substances (>3 instances) without a clear explanation, schedule an appointment to re-educate them about buprenorphine treatment and reassess the patient’s treatment goals. Consider changing the current treatment plan, such as by having more frequent follow-ups, increasing the dose of the buprenorphine for patients whose cravings are not sufficiently suppressed, switching to another medication such as methadone or naltrexone, or referring the patient to a higher level of care, such as intensive outpatient or residential treatment.
9. What should I do if the results indicate abnormal levels of buprenorphine, norbuprenorphine, and naloxone?
When urine buprenorphine, norbuprenorphine, or naloxone levels appear low or the results indicate a likely “spiking,” clarify whether the sample tampering is due to poor adherence or diversion. Similar to dealing with a positive result for substances of abuse, ask the patient what they expect to find in their urine, and discuss the results in a nonjudgmental manner. Patients who admit to difficulty following their medication regimen may require additional psychoeducation and motivational interviewing to identify and address barriers. Strategies to improve adherence include setting an alarm, involving the family, using a pillbox, or simplifying the regimen.38 A long-acting injectable form of buprenorphine is also available.
If you suspect diversion, refer to your clinic’s policy and use other clinical management skills, such as increasing the frequency of visits, random pill counts, and supervised medication administration in the clinic.39 If diversion occurs repetitively and the patient is not appropriate for or benefiting from buprenorphine treatment, it may make sense to terminate treatment and consider other treatment options (such as methadone or residential treatment).39
10. What should I do if a patient disagrees with laboratory findings?
It is common for patients to disagree with laboratory results. Maintaining an attitude of neutrality and allowing the patient to speak and provide explanations is necessary to ensure they feel heard. Explanations patients frequently provide include passive exposure (“I was around someone who was using it”) or accidental ingestion, when a patient reports taking a medication they were not aware was a substance of concern. In a calm and nonjudgmental manner, provide education on what leads to a positive drug screen, including the possibility of false positive findings.
Continue to: Because a screening test...
Because a screening test has high sensitivity and low specificity, false positives may occur.17,27 Therefore, when a result is in dispute, the use of a high-specificity confirmatory test is often needed (many laboratories have reflex confirmatory testing). However, in the case of diluted urine (urine creatinine concentrations <20 mg/dL), patients should be told the findings are physiologically implausible, and a new urine sample should be obtained.24
Goals of laboratory monitoring
Laboratory monitoring, including UDS and urine buprenorphine levels, is a mainstay of treatment for patients with OUD. The increased use of telehealth has affected how laboratory testing is conducted (Box 240,41). The goal of laboratory testing is to influence treatment and improve patient outcomes. Clinical data such as clinician assessment, patient self-reporting, and collateral information provide essential details for patient management. However, laboratory monitoring is often the most reliable and objective source by which to influence treatment.
Box 2
While delivering therapy via telehealth has been shown to decrease the stigma that surrounds treatment, reduce no-show rates, increase retention in care, improve treatment access for patients who have difficulty commuting, and allow for continuity of outpatient treatment during the COVID-19 pandemic, there are also challenges.40,41 Inducing patients on buprenorphine via telehealth, as well as managing complex treatment cases or repeated failed urine drug screen tests, can be especially challenging. However, treatment standards should be followed as much as possible, and laboratory monitoring as clinically indicated should still be used to improve treatment outcomes.
If needed, patients may be directed to community labs for urine screening and should have results sent to their clinicians prior to the telehealth visit. Complex treatment cases (eg, repeat positive opioid screens, or negative urine buprenorphine screens with comorbid psychiatric conditions) should be handled on an individual basis and in-person appointments may be needed. Video assessment is always preferable to telephone. For patients who are unable to use video and have difficulty maintaining negative drug screens, an in-person visit should be requested.
An increased understanding of recommended laboratory monitoring practices may improve your comfort with OUD treatment and motivate more clinicians to offer buprenorphine, a life-saving and disease-modifying treatment for OUD. Doing so would increase access to OUD treatment for patients to reduce the individual and public health risks associated with untreated OUD.
Bottom Line
Laboratory monitoring, particularly urine drug screens and urine buprenorphine levels, is the most reliable source of information in the treatment of patients with opioid use disorder (OUD). An increased understanding of monitoring practices may improve a clinician’s willingness to offer buprenorphine as an option for therapy and their ability to properly treat patients with OUD.
Related Resources
- Li X, Moore S, Olson C. Urine drug tests: how to make the most of them. Current Psychiatry. 2019;18(8):10-18,20.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49. doi:10.12788/cp.0244
Drug Brand Names
Amantadine • Gocovri
Buprenorphine • Subutex, Sublocade
Bupropion • Wellbutrin, Zyban
Efavirenz • Sustiva
Fentanyl • Actiq
Hydrocodone • Hysingla
Hydromorphone • Dilaudid
Methadone • Methadose
Naloxone • Evzio
Naltrexone • Vivitrol
Oxycodone • Oxycontin
Oxymorphone • Opana
Phentermine • Ionamin
Quinine • Qualaquin
Ranitidine • Zantac
Rifampin • Rifadin
Selegiline • Eldepryl
Sertraline • Zoloft
Trazodone • Oleptro
Verapamil • Verelan
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
1. Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. HHS Publication PEP19-5068, NSDUH Series H-54. May 2019. https://www.samhsa.gov/data/
2. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. doi:10.1056/NEJMp1402780
3. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/S0140-6736(17)32812-X
4. Sharma A, Kelly SM, Mitchell SG, et al. Update on barriers to pharmacotherapy for opioid use disorders. Curr Psychiatry Rep. 2017;19(6):35. doi:10.1007/s11920-017-0783-9
5. DeFlavio JR, Rolin SA, Nordstrom BR, et al. Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health. 2015;15:3019. doi:10.22605/rrh3019
6. Kuhlman JJ Jr, Lalani S, Magluiolo J Jr, et al. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
7. Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949-958. doi:10.1056/NEJMoa022164
8. Brown SM, Holtzman M, Kim T, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115(6):1251-1260. doi:10.1097/ALN.0b013e318238fea0
9. Cone EJ, Gorodetzky CW, Yousefnejad D, et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos. 1984;12(5):577-581.
10. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470-482. doi:10.1007/s13142-015-0315-2
11. Del Boca FK, Noll JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction. 2000;95 Suppl 3:S347-S360. doi:10.1080/09652140020004278
12. Preston KL, Silverman K, Schuster CR, et al. Comparison of self-reported drug use with quantitative and qualitative urinalysis for assessment of drug use in treatment studies. NIDA Res Monogr. 1997;167:130-145.
13. Knezevic NN, Khan OM, Beiranvand A, et al. Repeated quantitative urine toxicology analysis may improve chronic pain patient compliance with opioid therapy. Pain Physician. 2017;20(2S):S135-S145. doi:10.36076/ppj.2017.s145
14. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
15. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl 1):1-91. doi:10.1097/ADM.0000000000000633
16. McDonell MG, Graves MC, West II, et al. Utility of point-of-care urine drug tests in the treatment of primary care patients with drug use disorders. J Addict Med. 2016;10(3):196-201. doi:10.1097/ADM.0000000000000220
17. Algren DA, Christian MR. Buyer beware: pitfalls in toxicology laboratory testing. Mo Med. 2015;112(3):206-210.
18. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123. doi:10.1016/j.jsat.2010.05.008
19. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61. doi:10.1016/j.drugalcdep.2009.09.007
20. Warrington JS, Warrington GS, Francis-Fath S, et al. Urinary buprenorphine, norbuprenorphine and naloxone concentrations and ratios: review and potential clinical implications. J Addict Med. 2020;14(6):e344-e349. doi:10.1097/ADM.0000000000000676
21. Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46-51. doi:10.1016/j.drugalcdep.2017.07.040
22. Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single- and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38(2):358-369. doi:10.1016/j.clinthera.2015.12.016
23. Suzuki J, Zinser J, Issa M, et al. Quantitative testing of buprenorphine and norbuprenorphine to identify urine sample spiking during office-based opioid treatment. Subst Abus. 2017;38(4):504-507. doi:10.1080/08897077.2017.1356796
24. Gowans EM, Fraser CG. Biological variation of serum and urine creatinine and creatinine clearance: ramifications for interpretation of results and patient care. Ann Clin Biochem. 1988;25( Pt 3):259-263. doi:10.1177/000456328802500312
25. Saxon AJ, Ling W, Hillhouse M, et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug Alcohol Depend. 2013;128(1-2):71-76. doi:10.1016/j.drugalcdep.2012.08.002
26. Fareed A, Eilender P, Ketchen B, et al. Factors affecting noncompliance with buprenorphine maintenance treatment. J Addict Med. 2014;8(5):345-350. doi:10.1097/ADM.0000000000000057
27. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. doi:10.4065/83.1.66
28. Keary CJ, Wang Y, Moran JR, et al. Toxicologic testing for opiates: understanding false-positive and false-negative test results. Prim Care Companion CNS Disord. 2012;14(4).PCC.12f01371 doi:10.4088/PCC.12f01371
29. Zebelman AM, Troyer BL, Randall GL, et al. Detection of morphine and codeine following consumption of poppy seeds. J Anal Toxicol. 1987;11(3):131-132. doi:10.1093/jat/11.3.131
30. Meier PS, Barrowclough C, Donmall MC. The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction. 2005;100(3):304-316. doi:10.1111/j.1360-0443.2004.00935.x
31. Kelly JF, Saitz R, Wakeman S. Language, substance use disorders, and policy: the need to reach consensus on an “addiction-ary.” Alcohol Treat Q. 2016;34(1):116-123. doi:10.1080/07347324.2016.1113103
32. Broyles LM, Binswanger IA, Jenkins JA, et al. Confronting inadvertent stigma and pejorative language in addiction scholarship: a recognition and response. Subst Abus. 2014;35(3):217-221. doi:10.1080/08897077.2014.930372
33. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128(1):8-9. doi:10.1016/j.amjmed.2014.07.043
34. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11(3):163-173. doi:10.1097/ADM.0000000000000323
35. Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169(9):628-635. doi:10.7326/M18-1652
36. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 Suppl):S76-S82.
37. Klein A. Harm reduction works: evidence and inclusion in drug policy and advocacy. Health Care Anal. 2020;28(4):404-414. doi:10.1007/s10728-020-00406-w
38. Patel MX, David AS. Medication adherence: predictive factors and enhancement strategies. Psychiatry. 2007;6(9):357-361. doi:10.1016/j.mppsy.2007.06.003
39. Lofwall MR, Walsh SL. A review of buprenorphine diversion and misuse: the current evidence base and experiences from around the world. J Addict Med. 2014;8(5):315-326. doi:10.1097/ADM.0000000000000045
40. Wang L, Weiss J, Ryan EB, et al. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J Subst Abuse Treat. 2021;124:108272. doi:10.1016/ j.jsat.2020.108272
41. Harris MTH, Lambert AM, Maschke AD, et al. “No home to take methadone to”: experiences with addiction services during the COVID-19 pandemic among survivors of opioid overdose in Boston. J Subst Abuse Treat. 2022;135:108655. doi:10.1016/j.jsat.2021.108655
From neuroplasticity to psychoplasticity: Psilocybin may reverse personality disorders and political fanaticism
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
One of psychiatry’s long-standing dogmas is that personality disorders are enduring, unchangeable, and not amenable to treatment with potent psychotropics or intensive psychotherapy. I propose that this dogma may soon be shattered.
Several other dogmas in psychiatry have been demolished over the past several decades:
- that “insanity” is completely irreversible and requires lifetime institutionalization. The serendipitous discovery of chlorpromazine1 annihilated this centuries-old dogma
- that chronic, severe, refractory depression (with ongoing suicidal urges) that fails to improve with pharmacotherapy or electroconvulsive therapy (ECT) is hopeless and untreatable, until ketamine not only pulverized this dogma, but did it with lightning speed, dazzling us all2
- that dissociative agents such as ketamine are dangerous and condemnable drugs of abuse, until the therapeutic effect of ketamine slayed that dragon3
- that ECT “fries” the brain (as malevolently propagated by antipsychiatry cults), which was completely disproven by neuroimaging studies that show the hippocampus (which shrinks during depression) actually grows by >10% after a few ECT sessions4
- that psychotherapy is not a “real” treatment because talking cannot reverse a psychiatric brain disorder, until studies showed significant neuroplasticity with psychotherapy and decrease in inflammatory biomarkers with cognitive-behavioral therapy (CBT)5
- that persons with refractory hallucinations and delusions are doomed to a life of disability, until clozapine torpedoed that pessimistic dogma6
- that hallucinogens/psychedelics are dangerous and should be banned, until a jarring paradigm shift occurred with the discovery of psilocybin’s transformative effects, and the remarkable therapeutic effects of its mystical trips.7
Psilocybin’s therapeutic effects
Psilocybin has already proved to have a strong and lasting effect on depression and promises to have therapeutic benefits for patients with substance use disorders, posttraumatic stress disorder (PTSD), and anxiety.8 In addition, when the multiple psychological and neurobiological effects of psilocybin (and of other psychedelics) are examined, I see a very promising path to amelioration of severe personality disorders such as psychopathy, antisocial behavior, and narcissism. The mechanism(s) of action of psilocybin on the human brain are drastically different from any man-made psychotropic agent. As a psychiatric neuroscientist, I envision the neurologic impact of psilocybin to be conducive to a complete transformation of a patient’s view of themself, other people, and the meaning of life. It is reminiscent of religious conversion.
The psychological effects of psilocybin in humans have been described as follows:
- emotional breakthrough9
- increased psychological flexibility,10,11 a very cortical effect
- mystical experience,12 which results in sudden and significant changes in behavior and perception and includes the following dimensions: sacredness, noetic quality, deeply felt positive mood, ineffability, paradoxicality, and transcendence of time and space13
- oceanic boundlessness, feeling “one with the universe”14
- universal interconnectedness, insightfulness, blissful state, spiritual experience14
- ego dissolution,15 with loss of one’s personal identity
- increased neuroplasticity16
- changes in cognition and increase in insight.17
The neurobiological effects of psilocybin are mediated by serotonin 5HT2A agonism and include the following18:
- reduction in the activity of the medial prefrontal cortex, which regulates memory, attention, inhibitory control, and habit
- a decrease in the connectivity between the medial prefrontal cortex and the posterior cingulate cortex, which regulates memory and emotions
- reducing the default mode network, which is active during rest, stimulating internal thoughts and reminiscing about previous feelings and events, sometimes including ruminations. Psilocybin reverses those processes to thinking about others, not just the self, and becoming more open-minded about the world and other people. This can be therapeutic for depression, which is often associated with negative ruminations but also with entrenched habits (addictive behaviors), anxiety, PTSD, and obsessive-compulsive disorders
- increased global functional connectivity among various brain networks, leading to stronger functional integration of behavior
- collapse of major cortical oscillatory rhythms such as alpha and others that perpetuate “prior” beliefs
- extensive neuroplasticity and recalibration of thought processes and decomposition of pathological beliefs, referred to as REBUS (relaxed beliefs under psychedelics).
The bottom line is psilocybin and other psychedelics can dramatically alter, reshape, and relax rigid beliefs and personality traits by decreasing “neuroticism” and increasing “extraversion,” insightfulness, openness, and possibly conscientiousness.19 Although no studies of psychedelics in psychopathic, antisocial, or narcissistic personality disorders have been conducted, it is very reasonable to speculate that psilocybin may reverse traits of these disorders such as callousness, lack of empathy, and pathological self-centeredness.
Going further, a preliminary report suggests psilocybin can modify political views by decreasing authoritarianism and increasing libertarianism.20,21 In the current political zeitgeist, could psychedelics such as psilocybin reduce or even eliminate political extremism and visceral hatred on all sides? It would be remarkable research to carry out to heal a politically divided populace.The dogma of untreatable personality disorders or hopelessly entrenched political extremism is on the chopping block, and psychedelics offer hope to splinter those beliefs by concurrently remodeling brain tissue (neuroplasticity) and rectifying the mindset (psychoplasticity).
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.
1. Delay J, Deniker P. Neuroleptic effects of chlorpromazine in therapeutics of neuropsychiatry. J Clin Exp Psychopathol. 1955;16(2):104-112.
2. Walsh Z, Mollaahmetoglu OM, Rootman, J, et al. Ketamine for the treatment of mental health and substance use disorders: comprehensive systematic review. BJPsych Open. 2021;8(1):e19. doi:10.1192/bjo.2021.1061
3. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
4. Ayers B, Leaver A, Woods RP, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016;79(4):282-292.
5. Cao B, Li R, Ding L, Xu J, et al. Does cognitive behaviour therapy affect peripheral inflammation of depression? A protocol for the systematic review and meta-analysis. BMJ Open. 2021;11(12):e048162. doi:10.1136/bmjopen-2020-048162
6. Wagner E, Siafis S, Fernando P, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11(1):487.
7. Daws RE, Timmermann C, Giribaldi B, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28(4):844-851.
8. Pearson C, Siegel J, Gold JA. Psilocybin-assisted psychotherapy for depression: emerging research on a psychedelic compound with a rich history. J Neurol Sci. 2022;434:120096. doi:10.1016/j.jns.2021.120096
9. Roseman L, Haijen E, Idialu-Ikato K, et al. Emotional breakthrough and psychedelics: validation of the Emotional Breakthrough Inventory. J Psychopharmacol. 2019;33(9):1076-1087.
10. Davis AK, Barrett FS, Griffiths RR. Psychological flexibility mediates the relations between acute psychedelic effects and subjective decreases in depression and anxiety. J Contextual Behav Sci. 2020;15:39-45.
11. Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther. 2006;44(1):1-25.
12. Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol. 2016;30(12):1165-1180.
13. Stace WT. Mysticism and Philosophy. Macmillan Pub Ltd; 1960:37.
14. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393-430.
15. Nour MM, Evans L, Nutt D, et al. Ego-dissolution and psychedelics: validation of the Ego-Dissolution Inventory (EDI). Front Hum Neurosci. 2016;10:269. doi:10.3389/fnhum.2016.00269
16. Olson DE. The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacol Transl Sci. 2020;4(2):563-567.
17. Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (Berl). 2018;235(2):399-408.
18. Carhart-Harris RL. How do psychedelics work? Curr Opin Psychiatry. 2019;32(1):16-21.
19. Erritzoe D, Roseman L, Nour MM, et al. Effects of psilocybin therapy on personality structure. Acta Psychiatr Scand. 2018;138(5):368-378.
20. Lyons T, Carhart-Harris RL. Increased nature relatedness and decreased authoritarian political views after psilocybin for treatment-resistant depression. J Psychopharmacol. 2018;32(7):811-819.
21. Nour MM, Evans L, Carhart-Harris RL. Psychedelics, personality and political perspectives. J Psychoactive Drugs. 2017;49(3):182-191.

















