User login
FDA considers regulating CBD products
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
The products can have drug-like effects on the body and contain CBD (cannabidiol) and THC (tetrahydrocannabinol). Both CBD and THC can be derived from hemp, which was legalized by Congress in 2018.
“Given what we know about the safety of CBD so far, it raises concerns for FDA about whether these existing regulatory pathways for food and dietary supplements are appropriate for this substance,” FDA Principal Deputy Commissioner Janet Woodcock, MD, told The Wall Street Journal.
A 2021 FDA report valued the CBD market at $4.6 billion and projected it to quadruple by 2026. The only FDA-approved CBD product is an oil called Epidiolex, which can be prescribed for the seizure-associated disease epilepsy. Research on CBD to treat other diseases is ongoing.
Food, beverage, and beauty products containing CBD are sold in stores and online in many forms, including oils, vaporized liquids, and oil-based capsules, but “research supporting the drug’s benefits is still limited,” the Mayo Clinic said.
Recently, investigations have found that many CBD products also contain THC, which can be derived from legal hemp in a form that is referred to as Delta 8 and produces a psychoactive high. The CDC warned in 2022 that people “mistook” THC products for CBD products, which are often sold at the same stores, and experienced “adverse events.”
The Centers for Disease Control and Prevention and FDA warn that much is unknown about CBD and delta-8 products. The CDC says known CBD risks include liver damage; interference with other drugs you are taking, which may lead to injury or serious side effects; drowsiness or sleepiness; diarrhea or changes in appetite; changes in mood, such as crankiness; potential negative effects on fetuses during pregnancy or on babies during breastfeeding; or unintentional poisoning of children when mistaking THC products for CBD products or due to containing other ingredients such as THC or pesticides.
“I don’t think that we can have the perfect be the enemy of the good when we’re looking at such a vast market that is so available and utilized,” Norman Birenbaum, a senior FDA adviser who is working on the regulatory issue, told the Journal. “You’ve got a widely unregulated market.”
A version of this article first appeared on WebMD.com.
Fast facts about gifts in a will and planned giving
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans ensure your support for our mission to fund young investigators continues even after your lifetime.
#1. Wills are not for older adults only.
Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
#2. Planned gifts are not complicated or confusing.
They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
#3. Planned gifts are not for the wealthy only.
Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2023, consider including a gift to the AGA Research Foundation in your will. You will help support researchers and help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your future plans? Visit our website at https://gastro.planmylegacy.org or contact us at [email protected].
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans ensure your support for our mission to fund young investigators continues even after your lifetime.
#1. Wills are not for older adults only.
Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
#2. Planned gifts are not complicated or confusing.
They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
#3. Planned gifts are not for the wealthy only.
Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2023, consider including a gift to the AGA Research Foundation in your will. You will help support researchers and help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your future plans? Visit our website at https://gastro.planmylegacy.org or contact us at [email protected].
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans ensure your support for our mission to fund young investigators continues even after your lifetime.
#1. Wills are not for older adults only.
Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
#2. Planned gifts are not complicated or confusing.
They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
#3. Planned gifts are not for the wealthy only.
Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2023, consider including a gift to the AGA Research Foundation in your will. You will help support researchers and help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your future plans? Visit our website at https://gastro.planmylegacy.org or contact us at [email protected].
Genomic features may explain sex differences in HBV-associated HCC
In findings that point to a potential treatment strategy, researchers in China have discovered how two risk factors – male hormones and aflatoxin – may drive hepatocellular carcinoma (HCC). The liver cancer genetics and biology differ between men and women and help explain why aflatoxin exposure increases the risk of HCC in hepatitis B virus (HBV)–infected patients, particularly in men.
The researchers found evidence that androgen signaling increased aflatoxin metabolism and genotoxicity, reduced DNA repair capabilities, and quelled antitumor immunity, Chungui Xu, PhD, with the State Key Lab of Molecular Oncology at the National Cancer Center at Peking Union Medical College in Beijing, and colleagues wrote. The study was published in Cellular and Molecular Gastroenterology and Hepatology.
“Androgen signaling in the context of genotoxic stress repressed DNA damage repair,” the authors wrote. “The alteration caused more nuclear DNA leakage into cytosol to activate the cGAS-STING pathway, which increased T-cell infiltration into tumor mass and improved anti–programmed cell death protein 1 [PD-1] immunotherapy in HCCs.”
In the study, the researchers conducted genomic analyses of HCC tumor samples from people with HBV who were exposed to aflatoxin in Qidong, China, an area that until recently had some of the highest liver cancer rates in the world. In subsequent experiments in cell lines and mice, the team investigated how the genetic alterations and transcription dysfunctions reflected the combined carcinogenic effects of aflatoxin and HPV.
Dr. Xu and colleagues performed whole-genome, whole-exome, and RNA sequencing on tumor and matched nonneoplastic liver tissues from 101 HBV-related HCC patients (47 men and 54 women). The patients had received primary hepatectomy without systemic treatment or radiation therapy and were followed for 5 years. Aflatoxin exposure was confirmed by recording aflatoxin M1 in their urine 3-18 years before HCC diagnosis. For comparison, the research team analyzed 113 HBV-related HCC samples without aflatoxin exposure from the Cancer Genome Atlas database. They also looked at 181 Chinese HCC samples from the International Cancer Genome Consortium that had no record of aflatoxin exposure. They found no sex differences in mutation patterns for previously identified HCC driver genes, but the tumor mutation burden was higher in the Qidong set.
In the Qidong samples, the research team identified 71 genes with significantly different mutation frequencies by sex. Among those, 62 genes were associated more frequently with men, and 9 genes were associated with women. None of the genes have been reported previously as HCC drivers, although some have been found previously in other cancers, such as melanoma, lung cancer, and thyroid adenocarcinoma.
From whole-genome sequencing of 88 samples, the research team detected HBV integration in 37 samples and identified 110 breakpoints. No difference in HBV breakpoint numbers was detected between the sexes, though there were differences in somatic mutation profiles and in HBV integration, and only men had HBV breakpoints binding to androgen receptors.
From RNA sequencing of 87 samples, the research team identified 3,070 significantly differentially expressed genes between men and women. The transcription levels of estrogen receptor 1 and 2 were similar between the sexes, but men expressed higher androgen receptor levels.
The researchers then analyzed the variation in gene expression between the male and female gene sets to understand HCC transcriptional dysfunction. The samples from men showed different biological capabilities, with several signaling pathways related to HCC development and progression that were up-regulated. The male samples also showed repression of specific antitumor immunity.
Men’s HCC tumor samples expressed higher levels of aflatoxin metabolism-related genes, such as AHR and CYP1A1, but lower levels of GSTM1 genes.
Turning to cell lines, the researchers used HBV-positive HepG2.2.15 cells and PLC/PRF/5 cells to test sex hormones in the regulation of AHR and CYP1A1 and how their interactions affected aflatoxin B1 cytotoxicity. After aflatoxin treatment, the addition of testosterone to the cultures significantly enhanced the transcription levels of AHR and CYP1A1. The aflatoxin dose needed to cause cell death was reduced by half in the presence of testosterone.
DNA damage from aflatoxin activates DNA repair mechanisms, so the research team analyzed different repair pathways. In the male tumor samples, the most down-regulated pathway was NHEJ. The male samples expressed significantly lower levels of NHEJ factors than did the female samples, including XRCC4, MRE11, ATM, HRCC5, and NBN.
In cell lines, the researchers tested the effects of androgen alone and with aflatoxin on the regulation of NHEJ factors. The transcriptional levels of XRCC4, LIG4, and MRE11 were reduced significantly in cells treated with both aflatoxin and testosterone, compared with those treated with aflatoxin alone. Notably, the addition of 17beta-estradiol estrogen partially reversed the reduction of XRCC4 and MRE11 expression.
The tumor samples from men also showed different gene signatures of immune responses and inflammation from the samples from women. The genes related to interferon I signaling and response were up-regulated significantly in male samples but not in female samples. In addition, the samples from men showed repression of antigen-specific antitumor immunity. The research team detected significantly increased CD8+T-cell infiltration in tumor tissues of men but not women, as well as higher transcriptional levels of PD-1 and CTLA-4, which are two immune checkpoint proteins on T cells that keep them from attacking the tumor. The data indicate that androgen signaling in established HBV-related HCCs contribute to the development of an immunosuppressive microenvironment, the authors wrote, which could render the tumor sensitive to anti–PD-1 immunotherapy.
In mice, the researchers examined the impact of a favorable androgen pathway on anti–PD-1 treatment effects against hepatoma. They administered tamoxifen to block ER signaling in syngeneic tumor-bearing mice. In both male and female mice, tamoxifen enhanced the anti–PD-1 effects to eradicate the tumor quickly. They also administered flutamide to tumor-bearing mice to block the androgen pathway and found no significant difference in tumor growth in female mice, but in male mice, tumors grew faster in the flutamide-treated mice.
“Therapeutics that favor androgen signaling and/or blocking estrogen signaling may provide a new strategy to improve the efficacy of immune checkpoint inhibitors against HCC in combination with radiotherapy or chemotherapy that induced DNA damage,” the authors wrote. “The adjuvant effects of tamoxifen for favorable androgen signaling to boost the anti–PD-1 effect in HCC patients needs future study in a prospective HCC cohort.”
The study was supported by the National Natural Science Foundation Fund of China, Innovation Fund for Medical Sciences of Chinese Academy of Medical Sciences, State Key Project for Infectious Diseases, and Peking Union Medical College. The authors disclosed no conflicts.
To read an editorial that accompanied this study in Cellular and Molecular Gastroenterology and Hepatology, go to https://www.cmghjournal.org/article/S2352-345X(22)00234-X/fulltext.
In findings that point to a potential treatment strategy, researchers in China have discovered how two risk factors – male hormones and aflatoxin – may drive hepatocellular carcinoma (HCC). The liver cancer genetics and biology differ between men and women and help explain why aflatoxin exposure increases the risk of HCC in hepatitis B virus (HBV)–infected patients, particularly in men.
The researchers found evidence that androgen signaling increased aflatoxin metabolism and genotoxicity, reduced DNA repair capabilities, and quelled antitumor immunity, Chungui Xu, PhD, with the State Key Lab of Molecular Oncology at the National Cancer Center at Peking Union Medical College in Beijing, and colleagues wrote. The study was published in Cellular and Molecular Gastroenterology and Hepatology.
“Androgen signaling in the context of genotoxic stress repressed DNA damage repair,” the authors wrote. “The alteration caused more nuclear DNA leakage into cytosol to activate the cGAS-STING pathway, which increased T-cell infiltration into tumor mass and improved anti–programmed cell death protein 1 [PD-1] immunotherapy in HCCs.”
In the study, the researchers conducted genomic analyses of HCC tumor samples from people with HBV who were exposed to aflatoxin in Qidong, China, an area that until recently had some of the highest liver cancer rates in the world. In subsequent experiments in cell lines and mice, the team investigated how the genetic alterations and transcription dysfunctions reflected the combined carcinogenic effects of aflatoxin and HPV.
Dr. Xu and colleagues performed whole-genome, whole-exome, and RNA sequencing on tumor and matched nonneoplastic liver tissues from 101 HBV-related HCC patients (47 men and 54 women). The patients had received primary hepatectomy without systemic treatment or radiation therapy and were followed for 5 years. Aflatoxin exposure was confirmed by recording aflatoxin M1 in their urine 3-18 years before HCC diagnosis. For comparison, the research team analyzed 113 HBV-related HCC samples without aflatoxin exposure from the Cancer Genome Atlas database. They also looked at 181 Chinese HCC samples from the International Cancer Genome Consortium that had no record of aflatoxin exposure. They found no sex differences in mutation patterns for previously identified HCC driver genes, but the tumor mutation burden was higher in the Qidong set.
In the Qidong samples, the research team identified 71 genes with significantly different mutation frequencies by sex. Among those, 62 genes were associated more frequently with men, and 9 genes were associated with women. None of the genes have been reported previously as HCC drivers, although some have been found previously in other cancers, such as melanoma, lung cancer, and thyroid adenocarcinoma.
From whole-genome sequencing of 88 samples, the research team detected HBV integration in 37 samples and identified 110 breakpoints. No difference in HBV breakpoint numbers was detected between the sexes, though there were differences in somatic mutation profiles and in HBV integration, and only men had HBV breakpoints binding to androgen receptors.
From RNA sequencing of 87 samples, the research team identified 3,070 significantly differentially expressed genes between men and women. The transcription levels of estrogen receptor 1 and 2 were similar between the sexes, but men expressed higher androgen receptor levels.
The researchers then analyzed the variation in gene expression between the male and female gene sets to understand HCC transcriptional dysfunction. The samples from men showed different biological capabilities, with several signaling pathways related to HCC development and progression that were up-regulated. The male samples also showed repression of specific antitumor immunity.
Men’s HCC tumor samples expressed higher levels of aflatoxin metabolism-related genes, such as AHR and CYP1A1, but lower levels of GSTM1 genes.
Turning to cell lines, the researchers used HBV-positive HepG2.2.15 cells and PLC/PRF/5 cells to test sex hormones in the regulation of AHR and CYP1A1 and how their interactions affected aflatoxin B1 cytotoxicity. After aflatoxin treatment, the addition of testosterone to the cultures significantly enhanced the transcription levels of AHR and CYP1A1. The aflatoxin dose needed to cause cell death was reduced by half in the presence of testosterone.
DNA damage from aflatoxin activates DNA repair mechanisms, so the research team analyzed different repair pathways. In the male tumor samples, the most down-regulated pathway was NHEJ. The male samples expressed significantly lower levels of NHEJ factors than did the female samples, including XRCC4, MRE11, ATM, HRCC5, and NBN.
In cell lines, the researchers tested the effects of androgen alone and with aflatoxin on the regulation of NHEJ factors. The transcriptional levels of XRCC4, LIG4, and MRE11 were reduced significantly in cells treated with both aflatoxin and testosterone, compared with those treated with aflatoxin alone. Notably, the addition of 17beta-estradiol estrogen partially reversed the reduction of XRCC4 and MRE11 expression.
The tumor samples from men also showed different gene signatures of immune responses and inflammation from the samples from women. The genes related to interferon I signaling and response were up-regulated significantly in male samples but not in female samples. In addition, the samples from men showed repression of antigen-specific antitumor immunity. The research team detected significantly increased CD8+T-cell infiltration in tumor tissues of men but not women, as well as higher transcriptional levels of PD-1 and CTLA-4, which are two immune checkpoint proteins on T cells that keep them from attacking the tumor. The data indicate that androgen signaling in established HBV-related HCCs contribute to the development of an immunosuppressive microenvironment, the authors wrote, which could render the tumor sensitive to anti–PD-1 immunotherapy.
In mice, the researchers examined the impact of a favorable androgen pathway on anti–PD-1 treatment effects against hepatoma. They administered tamoxifen to block ER signaling in syngeneic tumor-bearing mice. In both male and female mice, tamoxifen enhanced the anti–PD-1 effects to eradicate the tumor quickly. They also administered flutamide to tumor-bearing mice to block the androgen pathway and found no significant difference in tumor growth in female mice, but in male mice, tumors grew faster in the flutamide-treated mice.
“Therapeutics that favor androgen signaling and/or blocking estrogen signaling may provide a new strategy to improve the efficacy of immune checkpoint inhibitors against HCC in combination with radiotherapy or chemotherapy that induced DNA damage,” the authors wrote. “The adjuvant effects of tamoxifen for favorable androgen signaling to boost the anti–PD-1 effect in HCC patients needs future study in a prospective HCC cohort.”
The study was supported by the National Natural Science Foundation Fund of China, Innovation Fund for Medical Sciences of Chinese Academy of Medical Sciences, State Key Project for Infectious Diseases, and Peking Union Medical College. The authors disclosed no conflicts.
To read an editorial that accompanied this study in Cellular and Molecular Gastroenterology and Hepatology, go to https://www.cmghjournal.org/article/S2352-345X(22)00234-X/fulltext.
In findings that point to a potential treatment strategy, researchers in China have discovered how two risk factors – male hormones and aflatoxin – may drive hepatocellular carcinoma (HCC). The liver cancer genetics and biology differ between men and women and help explain why aflatoxin exposure increases the risk of HCC in hepatitis B virus (HBV)–infected patients, particularly in men.
The researchers found evidence that androgen signaling increased aflatoxin metabolism and genotoxicity, reduced DNA repair capabilities, and quelled antitumor immunity, Chungui Xu, PhD, with the State Key Lab of Molecular Oncology at the National Cancer Center at Peking Union Medical College in Beijing, and colleagues wrote. The study was published in Cellular and Molecular Gastroenterology and Hepatology.
“Androgen signaling in the context of genotoxic stress repressed DNA damage repair,” the authors wrote. “The alteration caused more nuclear DNA leakage into cytosol to activate the cGAS-STING pathway, which increased T-cell infiltration into tumor mass and improved anti–programmed cell death protein 1 [PD-1] immunotherapy in HCCs.”
In the study, the researchers conducted genomic analyses of HCC tumor samples from people with HBV who were exposed to aflatoxin in Qidong, China, an area that until recently had some of the highest liver cancer rates in the world. In subsequent experiments in cell lines and mice, the team investigated how the genetic alterations and transcription dysfunctions reflected the combined carcinogenic effects of aflatoxin and HPV.
Dr. Xu and colleagues performed whole-genome, whole-exome, and RNA sequencing on tumor and matched nonneoplastic liver tissues from 101 HBV-related HCC patients (47 men and 54 women). The patients had received primary hepatectomy without systemic treatment or radiation therapy and were followed for 5 years. Aflatoxin exposure was confirmed by recording aflatoxin M1 in their urine 3-18 years before HCC diagnosis. For comparison, the research team analyzed 113 HBV-related HCC samples without aflatoxin exposure from the Cancer Genome Atlas database. They also looked at 181 Chinese HCC samples from the International Cancer Genome Consortium that had no record of aflatoxin exposure. They found no sex differences in mutation patterns for previously identified HCC driver genes, but the tumor mutation burden was higher in the Qidong set.
In the Qidong samples, the research team identified 71 genes with significantly different mutation frequencies by sex. Among those, 62 genes were associated more frequently with men, and 9 genes were associated with women. None of the genes have been reported previously as HCC drivers, although some have been found previously in other cancers, such as melanoma, lung cancer, and thyroid adenocarcinoma.
From whole-genome sequencing of 88 samples, the research team detected HBV integration in 37 samples and identified 110 breakpoints. No difference in HBV breakpoint numbers was detected between the sexes, though there were differences in somatic mutation profiles and in HBV integration, and only men had HBV breakpoints binding to androgen receptors.
From RNA sequencing of 87 samples, the research team identified 3,070 significantly differentially expressed genes between men and women. The transcription levels of estrogen receptor 1 and 2 were similar between the sexes, but men expressed higher androgen receptor levels.
The researchers then analyzed the variation in gene expression between the male and female gene sets to understand HCC transcriptional dysfunction. The samples from men showed different biological capabilities, with several signaling pathways related to HCC development and progression that were up-regulated. The male samples also showed repression of specific antitumor immunity.
Men’s HCC tumor samples expressed higher levels of aflatoxin metabolism-related genes, such as AHR and CYP1A1, but lower levels of GSTM1 genes.
Turning to cell lines, the researchers used HBV-positive HepG2.2.15 cells and PLC/PRF/5 cells to test sex hormones in the regulation of AHR and CYP1A1 and how their interactions affected aflatoxin B1 cytotoxicity. After aflatoxin treatment, the addition of testosterone to the cultures significantly enhanced the transcription levels of AHR and CYP1A1. The aflatoxin dose needed to cause cell death was reduced by half in the presence of testosterone.
DNA damage from aflatoxin activates DNA repair mechanisms, so the research team analyzed different repair pathways. In the male tumor samples, the most down-regulated pathway was NHEJ. The male samples expressed significantly lower levels of NHEJ factors than did the female samples, including XRCC4, MRE11, ATM, HRCC5, and NBN.
In cell lines, the researchers tested the effects of androgen alone and with aflatoxin on the regulation of NHEJ factors. The transcriptional levels of XRCC4, LIG4, and MRE11 were reduced significantly in cells treated with both aflatoxin and testosterone, compared with those treated with aflatoxin alone. Notably, the addition of 17beta-estradiol estrogen partially reversed the reduction of XRCC4 and MRE11 expression.
The tumor samples from men also showed different gene signatures of immune responses and inflammation from the samples from women. The genes related to interferon I signaling and response were up-regulated significantly in male samples but not in female samples. In addition, the samples from men showed repression of antigen-specific antitumor immunity. The research team detected significantly increased CD8+T-cell infiltration in tumor tissues of men but not women, as well as higher transcriptional levels of PD-1 and CTLA-4, which are two immune checkpoint proteins on T cells that keep them from attacking the tumor. The data indicate that androgen signaling in established HBV-related HCCs contribute to the development of an immunosuppressive microenvironment, the authors wrote, which could render the tumor sensitive to anti–PD-1 immunotherapy.
In mice, the researchers examined the impact of a favorable androgen pathway on anti–PD-1 treatment effects against hepatoma. They administered tamoxifen to block ER signaling in syngeneic tumor-bearing mice. In both male and female mice, tamoxifen enhanced the anti–PD-1 effects to eradicate the tumor quickly. They also administered flutamide to tumor-bearing mice to block the androgen pathway and found no significant difference in tumor growth in female mice, but in male mice, tumors grew faster in the flutamide-treated mice.
“Therapeutics that favor androgen signaling and/or blocking estrogen signaling may provide a new strategy to improve the efficacy of immune checkpoint inhibitors against HCC in combination with radiotherapy or chemotherapy that induced DNA damage,” the authors wrote. “The adjuvant effects of tamoxifen for favorable androgen signaling to boost the anti–PD-1 effect in HCC patients needs future study in a prospective HCC cohort.”
The study was supported by the National Natural Science Foundation Fund of China, Innovation Fund for Medical Sciences of Chinese Academy of Medical Sciences, State Key Project for Infectious Diseases, and Peking Union Medical College. The authors disclosed no conflicts.
To read an editorial that accompanied this study in Cellular and Molecular Gastroenterology and Hepatology, go to https://www.cmghjournal.org/article/S2352-345X(22)00234-X/fulltext.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
The latest on ERS/ATS lung function interpretation guidelines and bronchodilator testing
The European Respiratory Society (ERS) and the American Thoracic Society (ATS) just published an updated technical standard on lung function interpretation. It’s impossible to review in its entirety without more space, so I’ll settle for covering what the authors say about bronchodilator testing. But before I do that, it’s worth reviewing what we think we know about having a patient perform spirometry, inhale a bronchodilator, and then repeat it. This is colloquially referred to as pre- and postbronchodilator testing.
Administering a bronchodilator and measuring changes in lung function seems simple and intuitive. It is biologically plausible that improvement would predict treatment response. It should allow for phenotyping airway diseases and quantifying exacerbation risk. It is easy to perform and can be done in the clinic. But in practice it falls short of its purpose, in part because of technical factors but also because it doesn’t really have a purpose.
The last interpretative strategies document from the ERS/ATS was published in 2005. Reading it many years ago, I was struck by the contrast between our reliance on bronchodilator response and its lack of standardization. It seemed that there was none. After making statements like, “There is no consensus on what constitutes reversibility in subjects with airflow obstruction” and “There is no consensus on how a bronchodilator response should be expressed, the variables to be used, and finally, the kind, dose, and inhalation mode of bronchodilator agent,” the 2005 ERS/ATS authors suggest using the criteria most clinicians are familiar with: A change of 12% and 200 cc in FEV1 or FVC marks a “significant” bronchodilator response. Four puffs of albuterol (100 mcg each for a total of 400 mcg) with a 15- to 20-minute wait before repeat spirometry is also suggested.
The 2005 iteration acknowledges that a significant bronchodilator response isn’t a very accurate predictor of, well, anything. It doesn’t reliably differentiate COPD from asthma and it’s never been as sensitive as bronchoprovocation testing for diagnosing airway reactivity. The absence of a significant bronchodilator response does not preclude a 2-month trial of the same medicine used to test for response. Given these problems with standardization and accuracy, I was left wondering why anyone bothers ordering the test at all.
In my own practice, I continued to order, conduct, and interpret bronchodilator response according to the suggestions made by the ERS/ATS in 2005 when trying to diagnose asthma. I recognized that a nonsignificant response meant nothing, but bronchodilator response testing was easier to obtain than bronchoprovocation at my hospital. It was a matter of convenience for me and the patient. According to the Global Initiative for Asthma (GINA) Guidelines, a significant bronchodilator response conducted and interpreted as recommended by the ERS/ATS 2005 standard provides objective confirmation of asthma in the presence of characteristic clinical symptoms.
The headline from the ERS/ATS 2022 Technical Standard is that the 12% and 200-cc criteria suggested in 2005 are being retired. Why? Well, much of the variability in the 2005 criteria is explained by height, age, sex, and baseline lung function. These factors obscure change related to intrinsic airway abnormalities. Instead, the authors suggest using a threshold change in the predicted values of FEV1 and FVC to determine a significant response. Because predicted values incorporate age, height, and sex, the impact from these variables is minimized. Using a percent predicted (PPD) threshold will also minimize the effect from the inverse relationship between measured values and bronchodilator response.
A 10% change in the PPD value for either FEV1 or FVC constitutes a significant bronchodilator response. Ten percent was chosen because it represents the statistically defined upper limit of normal response; and a greater than 8% change in bronchodilator response is associated with mortality, implying that values above this threshold connote disease. The technical standard seems to be on solid ground here; the rationale is mathematically appropriate and evidence based. The new definition will certainly improve precision.
There’s really no progress on accuracy, though. There are no comments on the protocol to be followed or clinical indications. The reader is referred to the ERS/ATS 2019 technical statement on standardization of spirometry. The statement on standardization is short on details, too, and refers the reader to an online supplement for a suggested protocol. The suggested protocol is identical to that suggested in 2005.
In summary, not a lot is different in the world of bronchodilator response testing. The definition is different now, and though it’s likely to be more precise, we still don’t know enough about accuracy. It’s nice to know that the new criteria will predict mortality, but in clinical practice we don’t use the test for that purpose. The 2022 technical standard acknowledges this and other limitations in a “future directions” paragraph. Perhaps we’ll know more when the next iteration is published.
Dr. Holley is a professor of medicine at Uniformed Services University of the Health Sciences. Bethesda, Md., and a pulmonary medicine/critical care physician at MedStar Washington Hospital Center, Washington. He reported conflicts of interest with Metapharm and the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
The European Respiratory Society (ERS) and the American Thoracic Society (ATS) just published an updated technical standard on lung function interpretation. It’s impossible to review in its entirety without more space, so I’ll settle for covering what the authors say about bronchodilator testing. But before I do that, it’s worth reviewing what we think we know about having a patient perform spirometry, inhale a bronchodilator, and then repeat it. This is colloquially referred to as pre- and postbronchodilator testing.
Administering a bronchodilator and measuring changes in lung function seems simple and intuitive. It is biologically plausible that improvement would predict treatment response. It should allow for phenotyping airway diseases and quantifying exacerbation risk. It is easy to perform and can be done in the clinic. But in practice it falls short of its purpose, in part because of technical factors but also because it doesn’t really have a purpose.
The last interpretative strategies document from the ERS/ATS was published in 2005. Reading it many years ago, I was struck by the contrast between our reliance on bronchodilator response and its lack of standardization. It seemed that there was none. After making statements like, “There is no consensus on what constitutes reversibility in subjects with airflow obstruction” and “There is no consensus on how a bronchodilator response should be expressed, the variables to be used, and finally, the kind, dose, and inhalation mode of bronchodilator agent,” the 2005 ERS/ATS authors suggest using the criteria most clinicians are familiar with: A change of 12% and 200 cc in FEV1 or FVC marks a “significant” bronchodilator response. Four puffs of albuterol (100 mcg each for a total of 400 mcg) with a 15- to 20-minute wait before repeat spirometry is also suggested.
The 2005 iteration acknowledges that a significant bronchodilator response isn’t a very accurate predictor of, well, anything. It doesn’t reliably differentiate COPD from asthma and it’s never been as sensitive as bronchoprovocation testing for diagnosing airway reactivity. The absence of a significant bronchodilator response does not preclude a 2-month trial of the same medicine used to test for response. Given these problems with standardization and accuracy, I was left wondering why anyone bothers ordering the test at all.
In my own practice, I continued to order, conduct, and interpret bronchodilator response according to the suggestions made by the ERS/ATS in 2005 when trying to diagnose asthma. I recognized that a nonsignificant response meant nothing, but bronchodilator response testing was easier to obtain than bronchoprovocation at my hospital. It was a matter of convenience for me and the patient. According to the Global Initiative for Asthma (GINA) Guidelines, a significant bronchodilator response conducted and interpreted as recommended by the ERS/ATS 2005 standard provides objective confirmation of asthma in the presence of characteristic clinical symptoms.
The headline from the ERS/ATS 2022 Technical Standard is that the 12% and 200-cc criteria suggested in 2005 are being retired. Why? Well, much of the variability in the 2005 criteria is explained by height, age, sex, and baseline lung function. These factors obscure change related to intrinsic airway abnormalities. Instead, the authors suggest using a threshold change in the predicted values of FEV1 and FVC to determine a significant response. Because predicted values incorporate age, height, and sex, the impact from these variables is minimized. Using a percent predicted (PPD) threshold will also minimize the effect from the inverse relationship between measured values and bronchodilator response.
A 10% change in the PPD value for either FEV1 or FVC constitutes a significant bronchodilator response. Ten percent was chosen because it represents the statistically defined upper limit of normal response; and a greater than 8% change in bronchodilator response is associated with mortality, implying that values above this threshold connote disease. The technical standard seems to be on solid ground here; the rationale is mathematically appropriate and evidence based. The new definition will certainly improve precision.
There’s really no progress on accuracy, though. There are no comments on the protocol to be followed or clinical indications. The reader is referred to the ERS/ATS 2019 technical statement on standardization of spirometry. The statement on standardization is short on details, too, and refers the reader to an online supplement for a suggested protocol. The suggested protocol is identical to that suggested in 2005.
In summary, not a lot is different in the world of bronchodilator response testing. The definition is different now, and though it’s likely to be more precise, we still don’t know enough about accuracy. It’s nice to know that the new criteria will predict mortality, but in clinical practice we don’t use the test for that purpose. The 2022 technical standard acknowledges this and other limitations in a “future directions” paragraph. Perhaps we’ll know more when the next iteration is published.
Dr. Holley is a professor of medicine at Uniformed Services University of the Health Sciences. Bethesda, Md., and a pulmonary medicine/critical care physician at MedStar Washington Hospital Center, Washington. He reported conflicts of interest with Metapharm and the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
The European Respiratory Society (ERS) and the American Thoracic Society (ATS) just published an updated technical standard on lung function interpretation. It’s impossible to review in its entirety without more space, so I’ll settle for covering what the authors say about bronchodilator testing. But before I do that, it’s worth reviewing what we think we know about having a patient perform spirometry, inhale a bronchodilator, and then repeat it. This is colloquially referred to as pre- and postbronchodilator testing.
Administering a bronchodilator and measuring changes in lung function seems simple and intuitive. It is biologically plausible that improvement would predict treatment response. It should allow for phenotyping airway diseases and quantifying exacerbation risk. It is easy to perform and can be done in the clinic. But in practice it falls short of its purpose, in part because of technical factors but also because it doesn’t really have a purpose.
The last interpretative strategies document from the ERS/ATS was published in 2005. Reading it many years ago, I was struck by the contrast between our reliance on bronchodilator response and its lack of standardization. It seemed that there was none. After making statements like, “There is no consensus on what constitutes reversibility in subjects with airflow obstruction” and “There is no consensus on how a bronchodilator response should be expressed, the variables to be used, and finally, the kind, dose, and inhalation mode of bronchodilator agent,” the 2005 ERS/ATS authors suggest using the criteria most clinicians are familiar with: A change of 12% and 200 cc in FEV1 or FVC marks a “significant” bronchodilator response. Four puffs of albuterol (100 mcg each for a total of 400 mcg) with a 15- to 20-minute wait before repeat spirometry is also suggested.
The 2005 iteration acknowledges that a significant bronchodilator response isn’t a very accurate predictor of, well, anything. It doesn’t reliably differentiate COPD from asthma and it’s never been as sensitive as bronchoprovocation testing for diagnosing airway reactivity. The absence of a significant bronchodilator response does not preclude a 2-month trial of the same medicine used to test for response. Given these problems with standardization and accuracy, I was left wondering why anyone bothers ordering the test at all.
In my own practice, I continued to order, conduct, and interpret bronchodilator response according to the suggestions made by the ERS/ATS in 2005 when trying to diagnose asthma. I recognized that a nonsignificant response meant nothing, but bronchodilator response testing was easier to obtain than bronchoprovocation at my hospital. It was a matter of convenience for me and the patient. According to the Global Initiative for Asthma (GINA) Guidelines, a significant bronchodilator response conducted and interpreted as recommended by the ERS/ATS 2005 standard provides objective confirmation of asthma in the presence of characteristic clinical symptoms.
The headline from the ERS/ATS 2022 Technical Standard is that the 12% and 200-cc criteria suggested in 2005 are being retired. Why? Well, much of the variability in the 2005 criteria is explained by height, age, sex, and baseline lung function. These factors obscure change related to intrinsic airway abnormalities. Instead, the authors suggest using a threshold change in the predicted values of FEV1 and FVC to determine a significant response. Because predicted values incorporate age, height, and sex, the impact from these variables is minimized. Using a percent predicted (PPD) threshold will also minimize the effect from the inverse relationship between measured values and bronchodilator response.
A 10% change in the PPD value for either FEV1 or FVC constitutes a significant bronchodilator response. Ten percent was chosen because it represents the statistically defined upper limit of normal response; and a greater than 8% change in bronchodilator response is associated with mortality, implying that values above this threshold connote disease. The technical standard seems to be on solid ground here; the rationale is mathematically appropriate and evidence based. The new definition will certainly improve precision.
There’s really no progress on accuracy, though. There are no comments on the protocol to be followed or clinical indications. The reader is referred to the ERS/ATS 2019 technical statement on standardization of spirometry. The statement on standardization is short on details, too, and refers the reader to an online supplement for a suggested protocol. The suggested protocol is identical to that suggested in 2005.
In summary, not a lot is different in the world of bronchodilator response testing. The definition is different now, and though it’s likely to be more precise, we still don’t know enough about accuracy. It’s nice to know that the new criteria will predict mortality, but in clinical practice we don’t use the test for that purpose. The 2022 technical standard acknowledges this and other limitations in a “future directions” paragraph. Perhaps we’ll know more when the next iteration is published.
Dr. Holley is a professor of medicine at Uniformed Services University of the Health Sciences. Bethesda, Md., and a pulmonary medicine/critical care physician at MedStar Washington Hospital Center, Washington. He reported conflicts of interest with Metapharm and the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
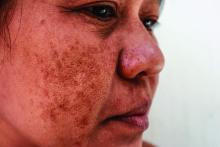
Long-term maintenance required in melasma patients
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
AT MOAS 2022
Why it’s important to offer cosmeceuticals in a cosmetic practice
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2022
Children and COVID: New cases fell as the old year ended
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.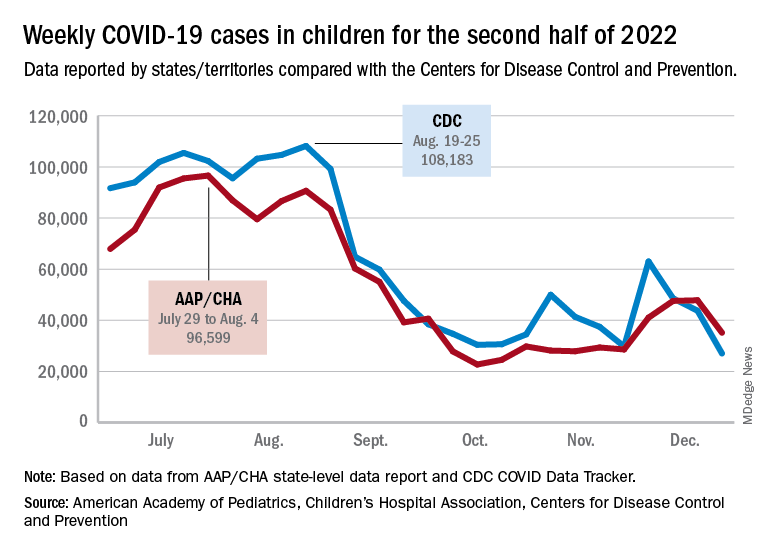
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
The end of 2022 saw a drop in new COVID-19 cases in children, even as rates of emergency department visits continued upward trends that began in late October.
New cases for the week of Dec. 23-29 fell for the first time since late November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP/CHA analysis of publicly available state data differs somewhat from figures reported by the Centers for Disease Control and Prevention, which has new cases for the latest available week, Dec.18-24, at just over 27,000 after 3 straight weeks of declines from a count of almost 63,000 for the week ending Nov. 26. The CDC, however, updates previously reported data on a regular basis, so that 27,000 is likely to increase in the coming weeks.
The CDC line on the graph also shows a peak for the week of Oct. 30 to Nov. 5 when new cases reached almost 50,000, compared with almost 30,000 reported for the week of Oct. 28 to Nov. 3 by the AAP and CHA in their report of state-level data. The AAP and CHA put the total number of child COVID cases since the start of the pandemic at 15.2 million as of Dec. 29, while the CDC reports 16.2 million cases as of Dec. 28.
There have been 1,975 deaths from COVID-19 in children aged 0-17 years, according to the CDC, which amounts to just over 0.2% of all COVID deaths for which age group data were available.
CDC data on emergency department visits involving diagnosed COVID-19 have been rising since late October. In children aged 0-11 years, for example, COVID was involved in 1.0% of ED visits (7-day average) as late as Nov. 4, but by Dec. 27 that rate was 2.6%. Children aged 12-15 years went from 0.6% on Oct. 28 to 1.5% on Dec. 27, while 16- to 17-year-olds had ED visit rates of 0.6% on Oct. 19 and 1.7% on Dec. 27, the CDC said on its COVID Data Tracker.
New hospital admissions with diagnosed COVID, which had been following the same upward trend as ED visits since late October, halted that rise in children aged 0-17 years and have gone no higher than 0.29 per 100,000 population since Dec. 9, the CDC data show.
Antiepileptic drugs tied to increased Parkinson’s disease risk
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.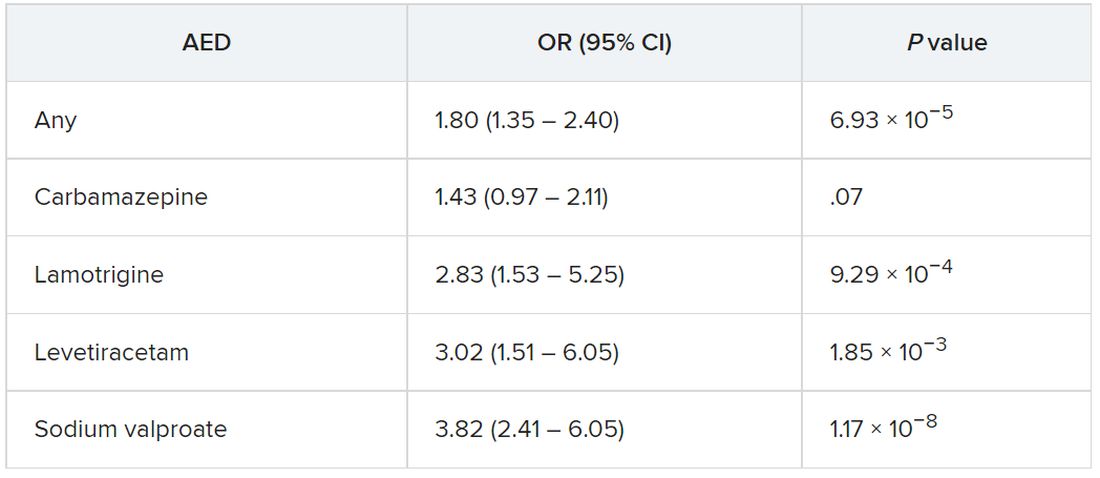
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Drawing on data from the UK Biobank, investigators compared more than 1,400 individuals diagnosed with Parkinson’s disease with matched control persons and found a considerably higher risk of developing Parkinson’s disease among those who had taken AEDs in comparison with those who had not. There was a trend linking a greater number of AED prescriptions and multiple AEDs associated with a greater risk for Parkinson’s disease.
“We observed an association between the most commonly prescribed antiepileptic drugs in the U.K. and Parkinson’s disease using data from UK Biobank,” said senior author Alastair Noyce, PhD, professor of neurology and neuroepidemiology and honorary consultant neurologist, Queen Mary University of London.
“This is the first time that a comprehensive study of the link between AEDs and Parkinson’s disease has been undertaken,” said Dr. Noyce.
He added that the findings have no immediate clinical implications, “but further research is definitely needed, [as] this is an interesting observation made in a research setting.”
The study was published online in JAMA Neurology.
Plausible, but unclear link
Recent observational studies have found a “temporal association” between epilepsy and incident Parkinson’s disease, but the mechanism underlying this association is “unclear,” the authors wrote.
It is “plausible” that AEDs “may account for some or all of the apparent association between epilepsy and Parkinson’s disease” and that movement disorders are potential side effects of AEDs, but the association between AEDs and Parkinson’s disease has “not been well studied,” so it remains “unclear” whether AEDs play a role in the association.
“We have previously reported an association between epilepsy and Parkinson’s disease in several different datasets. Here, we wanted to see if it could be explained by an association with the drugs used to treat epilepsy rather than epilepsy per se,” Dr. Noyce explained.
Are AEDs the culprit?
The researchers used data from the UK Biobank, a longitudinal cohort study with more than 500,000 participants, as well as linked primary care medication data to conduct a nested case-control study to investigate this potential association. Participants ranged in age from 40 to 69 years and were recruited between 2006 and 2010.
The researchers compared 1,433 individuals diagnosed with Parkinson’s disease with 8,598 control persons who were matched in a 6:1 ratio for age, sex, race, ethnicity, and socioeconomic status (median [interquartile range] age, 71 [65-75] years; 60.9% men; 97.5% White).
Of those with Parkinson’s disease, 4.3% had been prescribed an AED prior to the date of their being diagnosed with Parkinson’s disease, compared with 2.5% in the control group; 4.4% had been diagnosed with epilepsy, compared with 1% of the control persons.
The strongest evidence was for the association between lamotrigine, levetiracetam, and sodium valproate and Parkinson’s disease. There was “weaker evidence” for carbamazepine, although all the AEDs were associated with a higher risk of Parkinson’s disease.
The odds of incident Parkinson’s disease were higher among those who were prescribed one or more AEDs and among individuals who were issued a higher number of prescriptions, the authors reported.
It is possible that it is the epilepsy itself that is associated with the risk of Parkinson’s disease, rather than the drugs, and that “likely explains part of the association we are seeing,” said Dr. Noyce.
“The bottom line is that more research into the links between epilepsy – and drugs used to treat epilepsy – and Parkinson’s disease is needed,” he said.
Moreover, “only with time will we work out whether the findings hold any real clinical relevance,” he added.
Alternative explanations
Commenting on the research, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, said, “It has been established in prior research that there is an association between epilepsy and Parkinson’s disease.” The current study “shows that having had a prescription written for one of four antiepileptic medications was associated with subsequently receiving a diagnosis of Parkinson’s disease.”
Although one possible conclusion is that the AEDs themselves increase the risk of developing Parkinson’s disease, “there seem to be other alternative explanations as to why a person who had been prescribed AEDs has an increased risk of receiving a diagnosis of Parkinson’s disease,” said Dr. Gilbert, an associate professor of neurology at Bellevue Hospital Center, New York, who was not involved with the current study.
For example, pre-motor changes in the brain of persons with Parkinson’s disease “may increase the risk of requiring an AED by potentially increasing the risk of having a seizure,” and “changes in the brain caused by the seizures for which AEDs are prescribed may increase the risk of Parkinson’s disease.”
Moreover, psychiatric changes related to Parkinson’s disease may have led to the prescription for AEDs, because at least two of the AEDs are also prescribed for mood stabilization, Dr. Gilbert suggested.
“An unanswered question that the paper acknowledges is, what about people who receive AEDs for reasons other than seizures? Do they also have an increased risk of Parkinson’s disease? This would be an interesting population to focus on because it would remove the link between AEDs and seizure and focus on the association between AEDs and Parkinson’s disease,” Dr. Gilbert said.
She emphasized that people who take AEDs for seizures “should not jump to the conclusion that they must come off these medications so as not to increase their risk of developing Parkinson’s disease.” She noted that having seizures “can be dangerous – injuries can occur during a seizure, and if a seizure can’t be stopped or a number occur in rapid succession, brain injury may result.”
For these reasons, people with “a tendency to have seizures need to protect themselves with AEDs” and “should certainly reach out to their neurologists with any questions,” Dr. Gilbert said.
The Preventive Neurology Unit is funded by Barts Charity. The Apocrita High Performance Cluster facility, supported by Queen Mary University London Research–IT Services, was used for this research. Dr. Noyce has received grants from Barts Charity, Parkinson’s UK, Cure Parkinson’s, the Michael J. Fox Foundation, Innovate UK, Solvemed, and Alchemab and personal fees from AstraZeneca, AbbVie, Zambon, BIAL, uMedeor, Alchemab, Britannia, and Charco Neurotech outside the submitted work. The other authors’ disclosures are listed on the original article. Dr. Gilbert reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Dupilumab effective for eosinophilic esophagitis up to 52 weeks
Few side effects, other than injection pain, emerged in the year-long phase 3 trial that convinced the Food and Drug Administration to approve the drug for EoE in May, said Evan S. Dellon, MD, a gastroenterologist at the University of North Carolina at Chapel Hill.
The FDA approved dupilumab for the treatment of EoE in people 12 years and older who weigh at least 40 kg (about 88 pounds). The study included patients who had failed to benefit from an 8-week course of high-dose proton pump inhibitor (PPI) therapy, and most also hadn’t had relief from topical steroids.
Because dupilumab is a systemic biologic, such patients are the most likely candidates for the medication, Dr. Dellon said.
Dr. Dellon and colleagues published the study results in the New England Journal of Medicine.
A chronic, progressive, type 2 inflammatory disease of the esophagus, EoE can make it difficult to swallow and cause abdominal and chest pain or vomiting. Specialized diets, topical steroids, and PPIs can help manage EoE for many patients, but these therapies don’t always work, diets are difficult to follow, and topical steroids and PPIs can be difficult to take or cause side effects.
A fully human monoclonal antibody developed by Regeneron Pharmaceuticals and Sanofi, dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, central drivers of type 2 inflammation in EoE. The FDA has approved dupilumab for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps.
Three-part trial
For the three-part study conducted in Australia, Canada, Europe, and the United States, participants received 300 mg dupilumab by subcutaneous injection.
In part A, investigators randomly assigned 42 patients to weekly doses of dupilumab and 39 to weekly placebo injections for 24 weeks.
In part B, they randomly divided patients into three groups: Eighty received dupilumab weekly; 81 received dupilumab every 2 weeks; and 79 received a placebo weekly, for 24 weeks.
In part C, the intervention and placebo groups from part A received dupilumab weekly for another 28 weeks. For the two intervention groups in part B, treatment remained the same, while the part B placebo group was evenly split to receive dupilumab weekly or every 2 weeks for 28 weeks.
Over half of patients in remission
In part A, 60% of patients receiving weekly dupilumab achieved histologic remission (defined as no more than six eosinophils per high-power field), compared with 5% of patients receiving placebo, a significant difference (P < .001).
In part B, 59% of patients receiving weekly dupilumab, 60% of those receiving biweekly dupilumab, and 6% of those receiving placebo achieved histologic remission. The difference between those receiving weekly dupilumab or placebo was significant (P < .001).
Symptoms improved with weekly dupilumab, as measured via the Dysphagia Symptom Questionnaire score, which can range from 0 to 84, with higher values indicating more frequent or more severe dysphagia. The mean score at baseline was 33.6 in part A and 36.7 in part B. Scores in patients receiving weekly dupilumab decreased by 12.3 in part A and 9.9 in part B (both P < .001). However, among part B patients receiving biweekly dupilumab, the mean score dropped by only 0.5, which was not significant.
Dupilumab reached a higher serum concentration with the weekly than the biweekly regimen, which may explain the improved symptoms with more frequent dosing, Dr. Dellon said.
Only nine patients experienced serious adverse events during the part A or B treatment period (seven who received weekly dupilumab, one who received biweekly dupilumab, and one who received placebo) and just one patient during the part C treatment period who received placebo in part A and weekly dupilumab in part C. None of these events, except one, were related to the regimen, according to investigators.
Patients who received weekly dupilumab in part A and continued to part C maintained similar treatment effects to week 52, Dr. Dellon said. Although the data on part B patients who continued into part C were not included in the published paper, they were similar, he said.
“The people who responded to the first 24 weeks maintain that response for up to 52 weeks, and there was even a gain of response for some measures,” he said.
Patients, on average, also experienced improvements as measured by endoscopic healing, histological severity scores, and even gene expression, Dr. Dellon said.
‘Welcome addition’
Dupilumab is “a welcome addition to what we do,” said Philip Katz, MD, a professor of medicine in the division of gastroenterology at Weill Cornell Medicine in New York. The publication of this “pivotal” trial provides reassurance about its safety and effectiveness for EoE, he added.
“Dupilumab, or Dupixent, is going to be used in my practice for people who are refractory to both PPIs and topical steroids,” Dr. Katz, who was not involved in the research, said in an interview.
However, it will take practice to know how best to use the drug, and its high cost may make some payers reluctant to cover it, Dr. Katz added.
The study was funded by Sanofi and Regeneron. Dr. Dellon has reported financial relationships with multiple pharmaceutical companies, including Regeneron and Sanofi US. Dr. Katz has reported financial relationships with Phantom Pharmaceuticals, AstraZeneca, and Braintree Laboratories.
A version of this article first appeared on Medscape.com.
Few side effects, other than injection pain, emerged in the year-long phase 3 trial that convinced the Food and Drug Administration to approve the drug for EoE in May, said Evan S. Dellon, MD, a gastroenterologist at the University of North Carolina at Chapel Hill.
The FDA approved dupilumab for the treatment of EoE in people 12 years and older who weigh at least 40 kg (about 88 pounds). The study included patients who had failed to benefit from an 8-week course of high-dose proton pump inhibitor (PPI) therapy, and most also hadn’t had relief from topical steroids.
Because dupilumab is a systemic biologic, such patients are the most likely candidates for the medication, Dr. Dellon said.
Dr. Dellon and colleagues published the study results in the New England Journal of Medicine.
A chronic, progressive, type 2 inflammatory disease of the esophagus, EoE can make it difficult to swallow and cause abdominal and chest pain or vomiting. Specialized diets, topical steroids, and PPIs can help manage EoE for many patients, but these therapies don’t always work, diets are difficult to follow, and topical steroids and PPIs can be difficult to take or cause side effects.
A fully human monoclonal antibody developed by Regeneron Pharmaceuticals and Sanofi, dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, central drivers of type 2 inflammation in EoE. The FDA has approved dupilumab for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps.
Three-part trial
For the three-part study conducted in Australia, Canada, Europe, and the United States, participants received 300 mg dupilumab by subcutaneous injection.
In part A, investigators randomly assigned 42 patients to weekly doses of dupilumab and 39 to weekly placebo injections for 24 weeks.
In part B, they randomly divided patients into three groups: Eighty received dupilumab weekly; 81 received dupilumab every 2 weeks; and 79 received a placebo weekly, for 24 weeks.
In part C, the intervention and placebo groups from part A received dupilumab weekly for another 28 weeks. For the two intervention groups in part B, treatment remained the same, while the part B placebo group was evenly split to receive dupilumab weekly or every 2 weeks for 28 weeks.
Over half of patients in remission
In part A, 60% of patients receiving weekly dupilumab achieved histologic remission (defined as no more than six eosinophils per high-power field), compared with 5% of patients receiving placebo, a significant difference (P < .001).
In part B, 59% of patients receiving weekly dupilumab, 60% of those receiving biweekly dupilumab, and 6% of those receiving placebo achieved histologic remission. The difference between those receiving weekly dupilumab or placebo was significant (P < .001).
Symptoms improved with weekly dupilumab, as measured via the Dysphagia Symptom Questionnaire score, which can range from 0 to 84, with higher values indicating more frequent or more severe dysphagia. The mean score at baseline was 33.6 in part A and 36.7 in part B. Scores in patients receiving weekly dupilumab decreased by 12.3 in part A and 9.9 in part B (both P < .001). However, among part B patients receiving biweekly dupilumab, the mean score dropped by only 0.5, which was not significant.
Dupilumab reached a higher serum concentration with the weekly than the biweekly regimen, which may explain the improved symptoms with more frequent dosing, Dr. Dellon said.
Only nine patients experienced serious adverse events during the part A or B treatment period (seven who received weekly dupilumab, one who received biweekly dupilumab, and one who received placebo) and just one patient during the part C treatment period who received placebo in part A and weekly dupilumab in part C. None of these events, except one, were related to the regimen, according to investigators.
Patients who received weekly dupilumab in part A and continued to part C maintained similar treatment effects to week 52, Dr. Dellon said. Although the data on part B patients who continued into part C were not included in the published paper, they were similar, he said.
“The people who responded to the first 24 weeks maintain that response for up to 52 weeks, and there was even a gain of response for some measures,” he said.
Patients, on average, also experienced improvements as measured by endoscopic healing, histological severity scores, and even gene expression, Dr. Dellon said.
‘Welcome addition’
Dupilumab is “a welcome addition to what we do,” said Philip Katz, MD, a professor of medicine in the division of gastroenterology at Weill Cornell Medicine in New York. The publication of this “pivotal” trial provides reassurance about its safety and effectiveness for EoE, he added.
“Dupilumab, or Dupixent, is going to be used in my practice for people who are refractory to both PPIs and topical steroids,” Dr. Katz, who was not involved in the research, said in an interview.
However, it will take practice to know how best to use the drug, and its high cost may make some payers reluctant to cover it, Dr. Katz added.
The study was funded by Sanofi and Regeneron. Dr. Dellon has reported financial relationships with multiple pharmaceutical companies, including Regeneron and Sanofi US. Dr. Katz has reported financial relationships with Phantom Pharmaceuticals, AstraZeneca, and Braintree Laboratories.
A version of this article first appeared on Medscape.com.
Few side effects, other than injection pain, emerged in the year-long phase 3 trial that convinced the Food and Drug Administration to approve the drug for EoE in May, said Evan S. Dellon, MD, a gastroenterologist at the University of North Carolina at Chapel Hill.
The FDA approved dupilumab for the treatment of EoE in people 12 years and older who weigh at least 40 kg (about 88 pounds). The study included patients who had failed to benefit from an 8-week course of high-dose proton pump inhibitor (PPI) therapy, and most also hadn’t had relief from topical steroids.
Because dupilumab is a systemic biologic, such patients are the most likely candidates for the medication, Dr. Dellon said.
Dr. Dellon and colleagues published the study results in the New England Journal of Medicine.
A chronic, progressive, type 2 inflammatory disease of the esophagus, EoE can make it difficult to swallow and cause abdominal and chest pain or vomiting. Specialized diets, topical steroids, and PPIs can help manage EoE for many patients, but these therapies don’t always work, diets are difficult to follow, and topical steroids and PPIs can be difficult to take or cause side effects.
A fully human monoclonal antibody developed by Regeneron Pharmaceuticals and Sanofi, dupilumab blocks the shared receptor component for interleukin-4 and interleukin-13, central drivers of type 2 inflammation in EoE. The FDA has approved dupilumab for atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps.
Three-part trial
For the three-part study conducted in Australia, Canada, Europe, and the United States, participants received 300 mg dupilumab by subcutaneous injection.
In part A, investigators randomly assigned 42 patients to weekly doses of dupilumab and 39 to weekly placebo injections for 24 weeks.
In part B, they randomly divided patients into three groups: Eighty received dupilumab weekly; 81 received dupilumab every 2 weeks; and 79 received a placebo weekly, for 24 weeks.
In part C, the intervention and placebo groups from part A received dupilumab weekly for another 28 weeks. For the two intervention groups in part B, treatment remained the same, while the part B placebo group was evenly split to receive dupilumab weekly or every 2 weeks for 28 weeks.
Over half of patients in remission
In part A, 60% of patients receiving weekly dupilumab achieved histologic remission (defined as no more than six eosinophils per high-power field), compared with 5% of patients receiving placebo, a significant difference (P < .001).
In part B, 59% of patients receiving weekly dupilumab, 60% of those receiving biweekly dupilumab, and 6% of those receiving placebo achieved histologic remission. The difference between those receiving weekly dupilumab or placebo was significant (P < .001).
Symptoms improved with weekly dupilumab, as measured via the Dysphagia Symptom Questionnaire score, which can range from 0 to 84, with higher values indicating more frequent or more severe dysphagia. The mean score at baseline was 33.6 in part A and 36.7 in part B. Scores in patients receiving weekly dupilumab decreased by 12.3 in part A and 9.9 in part B (both P < .001). However, among part B patients receiving biweekly dupilumab, the mean score dropped by only 0.5, which was not significant.
Dupilumab reached a higher serum concentration with the weekly than the biweekly regimen, which may explain the improved symptoms with more frequent dosing, Dr. Dellon said.
Only nine patients experienced serious adverse events during the part A or B treatment period (seven who received weekly dupilumab, one who received biweekly dupilumab, and one who received placebo) and just one patient during the part C treatment period who received placebo in part A and weekly dupilumab in part C. None of these events, except one, were related to the regimen, according to investigators.
Patients who received weekly dupilumab in part A and continued to part C maintained similar treatment effects to week 52, Dr. Dellon said. Although the data on part B patients who continued into part C were not included in the published paper, they were similar, he said.
“The people who responded to the first 24 weeks maintain that response for up to 52 weeks, and there was even a gain of response for some measures,” he said.
Patients, on average, also experienced improvements as measured by endoscopic healing, histological severity scores, and even gene expression, Dr. Dellon said.
‘Welcome addition’
Dupilumab is “a welcome addition to what we do,” said Philip Katz, MD, a professor of medicine in the division of gastroenterology at Weill Cornell Medicine in New York. The publication of this “pivotal” trial provides reassurance about its safety and effectiveness for EoE, he added.
“Dupilumab, or Dupixent, is going to be used in my practice for people who are refractory to both PPIs and topical steroids,” Dr. Katz, who was not involved in the research, said in an interview.
However, it will take practice to know how best to use the drug, and its high cost may make some payers reluctant to cover it, Dr. Katz added.
The study was funded by Sanofi and Regeneron. Dr. Dellon has reported financial relationships with multiple pharmaceutical companies, including Regeneron and Sanofi US. Dr. Katz has reported financial relationships with Phantom Pharmaceuticals, AstraZeneca, and Braintree Laboratories.
A version of this article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE