User login
Defining six asthma subtypes may promote personalized treatment
Six subtypes of asthma that may facilitate personalized treatment were identified and confirmed in a large database review of approximately 50,000 patients, according to a recent study.
Previous studies of asthma subtypes have involved age of disease onset, the presence of allergies, and level of eosinophilic inflammation, and have been limited by factors including small sample size and lack of formal validation, Elsie M.F. Horne, MD, of the Asthma UK Centre for Applied Research, Edinburgh, and colleagues wrote.
In a study published in the International Journal of Medical Informatics, the researchers used data from two databases in the United Kingdom: the Optimum Patient Care Research Database (OPCRD) and the Secure Anonymised Information Linkage Database (SAIL). Each dataset included 50,000 randomly selected nonoverlapping adult asthma patients.
The researchers identified 45 categorical features from primary care electronic health records. The features included those directly linked to asthma, such as medications; and features indirectly linked to asthma, such as comorbidities.
The subtypes were defined by the clinically applicable features of level of inhaled corticosteroid use, level of health care use, and the presence of comorbidities, using multiple correspondence analysis and k-means cluster analysis.
The six asthma subtypes were identified in the OPCRD study population as follows: low inhaled corticosteroid use and low health care utilization (30%); low to medium ICS use (36%); low to medium ICS use and comorbidities (12%); varied ICS use and comorbid chronic obstructive pulmonary disease (4%); high ICS use (10%); and very high ICS use (7%).
The researchers replicated the subtypes with 91%-92% accuracy in an internal dataset and 84%-86% accuracy in an external dataset. “These subtypes generalized well at two future time points, and in an additional EHR database from a different U.K. nation (the SAIL Databank),” they wrote in their discussion.
The findings were limited by several factors including the retrospective design, the possible inclusion of people without asthma because of the cohort selection criteria, and the possible biases associated with the use of EHRs; however, the results were strengthened by the large dataset and the additional validations, the researchers noted.
“Using these subtypes to summarize asthma populations could help with management and resource planning at the practice level, and could be useful for understanding regional differences in the asthma population,” they noted. For example, key clinical implications for individuals in a low health care utilization subtype could include being flagged for barriers to care and misdiagnoses, while those in a high health care utilization subtype could be considered for reassessment of medication and other options.
The study received no outside funding. Lead author Dr. Horne had no financial conflicts to disclose.
Six subtypes of asthma that may facilitate personalized treatment were identified and confirmed in a large database review of approximately 50,000 patients, according to a recent study.
Previous studies of asthma subtypes have involved age of disease onset, the presence of allergies, and level of eosinophilic inflammation, and have been limited by factors including small sample size and lack of formal validation, Elsie M.F. Horne, MD, of the Asthma UK Centre for Applied Research, Edinburgh, and colleagues wrote.
In a study published in the International Journal of Medical Informatics, the researchers used data from two databases in the United Kingdom: the Optimum Patient Care Research Database (OPCRD) and the Secure Anonymised Information Linkage Database (SAIL). Each dataset included 50,000 randomly selected nonoverlapping adult asthma patients.
The researchers identified 45 categorical features from primary care electronic health records. The features included those directly linked to asthma, such as medications; and features indirectly linked to asthma, such as comorbidities.
The subtypes were defined by the clinically applicable features of level of inhaled corticosteroid use, level of health care use, and the presence of comorbidities, using multiple correspondence analysis and k-means cluster analysis.
The six asthma subtypes were identified in the OPCRD study population as follows: low inhaled corticosteroid use and low health care utilization (30%); low to medium ICS use (36%); low to medium ICS use and comorbidities (12%); varied ICS use and comorbid chronic obstructive pulmonary disease (4%); high ICS use (10%); and very high ICS use (7%).
The researchers replicated the subtypes with 91%-92% accuracy in an internal dataset and 84%-86% accuracy in an external dataset. “These subtypes generalized well at two future time points, and in an additional EHR database from a different U.K. nation (the SAIL Databank),” they wrote in their discussion.
The findings were limited by several factors including the retrospective design, the possible inclusion of people without asthma because of the cohort selection criteria, and the possible biases associated with the use of EHRs; however, the results were strengthened by the large dataset and the additional validations, the researchers noted.
“Using these subtypes to summarize asthma populations could help with management and resource planning at the practice level, and could be useful for understanding regional differences in the asthma population,” they noted. For example, key clinical implications for individuals in a low health care utilization subtype could include being flagged for barriers to care and misdiagnoses, while those in a high health care utilization subtype could be considered for reassessment of medication and other options.
The study received no outside funding. Lead author Dr. Horne had no financial conflicts to disclose.
Six subtypes of asthma that may facilitate personalized treatment were identified and confirmed in a large database review of approximately 50,000 patients, according to a recent study.
Previous studies of asthma subtypes have involved age of disease onset, the presence of allergies, and level of eosinophilic inflammation, and have been limited by factors including small sample size and lack of formal validation, Elsie M.F. Horne, MD, of the Asthma UK Centre for Applied Research, Edinburgh, and colleagues wrote.
In a study published in the International Journal of Medical Informatics, the researchers used data from two databases in the United Kingdom: the Optimum Patient Care Research Database (OPCRD) and the Secure Anonymised Information Linkage Database (SAIL). Each dataset included 50,000 randomly selected nonoverlapping adult asthma patients.
The researchers identified 45 categorical features from primary care electronic health records. The features included those directly linked to asthma, such as medications; and features indirectly linked to asthma, such as comorbidities.
The subtypes were defined by the clinically applicable features of level of inhaled corticosteroid use, level of health care use, and the presence of comorbidities, using multiple correspondence analysis and k-means cluster analysis.
The six asthma subtypes were identified in the OPCRD study population as follows: low inhaled corticosteroid use and low health care utilization (30%); low to medium ICS use (36%); low to medium ICS use and comorbidities (12%); varied ICS use and comorbid chronic obstructive pulmonary disease (4%); high ICS use (10%); and very high ICS use (7%).
The researchers replicated the subtypes with 91%-92% accuracy in an internal dataset and 84%-86% accuracy in an external dataset. “These subtypes generalized well at two future time points, and in an additional EHR database from a different U.K. nation (the SAIL Databank),” they wrote in their discussion.
The findings were limited by several factors including the retrospective design, the possible inclusion of people without asthma because of the cohort selection criteria, and the possible biases associated with the use of EHRs; however, the results were strengthened by the large dataset and the additional validations, the researchers noted.
“Using these subtypes to summarize asthma populations could help with management and resource planning at the practice level, and could be useful for understanding regional differences in the asthma population,” they noted. For example, key clinical implications for individuals in a low health care utilization subtype could include being flagged for barriers to care and misdiagnoses, while those in a high health care utilization subtype could be considered for reassessment of medication and other options.
The study received no outside funding. Lead author Dr. Horne had no financial conflicts to disclose.
FROM THE INTERNATIONAL JOURNAL OF MEDICAL INFORMATICS
CHEST President shares inside look at priorities, plans for 2023
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Genomics data reveal promising PSC therapeutic target
, according to a study published in Gastro Hep Advances.
PSC is very rare, with an incidence of 0-1.3 cases per 100,000 people per year. Because up to 80% of patients with PSC also have inflammatory bowel disease (IBD), a link along the gut-liver axis is suspected. So far, scientists have not understood the causes of PSC, the main complications of which include biliary cirrhosis, bacterial cholangitis, and cholangiocarcinoma.
No treatment is currently available for PSC, but the findings of this genomics study suggest several targets that may be worth pursuing, particularly the gene NR0B2.
“The therapeutic targeting of NR0B2 may potentiate that of FXR [farnesoid X receptor] and enable action on early events of the disease and prevent its progression,” wrote Christophe Desterke, PhD, of the Paul-Brousse Hospital, the French National Institute of Health and Medical Research, and the University of Paris-Saclay in Villejuif, France, and his associates.
The researchers used an algorithmic tool to mine the MEDLINE/PubMed/NCBI database using the three key symptoms of PSC – biliary fibrosis, biliary inflammation, and biliary stasis – as their keywords. This approach allowed them to discover the genes and potential pathways related to PSC in published research text or in clinical, animal, and cellular models.
The researchers initially found 525 genes linked to PSC and then compared them to RNA data from liver biopsies taken from patients with liver disease from various causes. This process led to a ranking of the 10 best markers of PSC, based on the data-mining method and the genes’ association with one or more of the three PSC symptoms.
At the top of the list is NR1H4, also called FXR, which ranks most highly with biliary fibrosis and biliary stasis. NR1H4 is already a clear target for cholestatic and fatty liver diseases, the authors noted. The other genes, in descending order of relevance, are: ABCB4, ABCB11, TGFB1, IFNL3, PNPLA3, IL6, TLR4, GPBAR1, and IL17A. In addition, complications of PSC were significantly associated with upregulation of TNFRS12A, SOX9, ANXA2, MMP7, and LCN2.
Separately, investigation of the 525 initially identified genes in mouse models of PSC revealed that NR0B2 is also a key player in the pathogenesis of PSC.
"NR0B2 was upregulated in PSC livers independent of gender, age, and body mass index,” the authors reported. “Importantly, it was not dependent on the severity of PSC in the prognostic cohort, suggesting that this may be an early event during the disease.”
The researchers also found a possible pathway explaining the autoimmunity of PSC – the involvement of CD274, also known as the PDL1 immune checkpoint. The authors noted that the PDL1 inhibitor pembrolizumab has previously been reported as a cause of sclerosing cholangitis.
Further, the researchers discovered overexpression of FOXP3 in the livers of patients with PSC. Because FOXP3 determines what T-cell subtypes look like, the finding suggests that an “imbalance between Foxp3þ regulatory T cells and Th17 cells may be involved in IBD and PSC,” they wrote.
Also of note was the overexpression of SOX9 in the livers of patients with PSC whose profiles suggested the worst clinical prognoses.
Finally, the researchers identified three genes as potentially involved in development of cholangiocarcinoma: GSTA3, ID2 (which is overexpressed in biliary tract cancer), and especially TMEM45A, a protein in cells’ Golgi apparatus that is already known to be involved in the development of several other cancers.
The research was funded by the French National Institute of Health and Medical Research. The authors reported no conflicts of interest.
Primary sclerosing cholangitis (PSC) is a bile duct disease with few therapeutic options other than liver transplant, and thus its prognosis remains grim. Additionally, the factors that cause the disease are not well understood. Identifying the pathways and genes involved in PSC pathogenesis could help in the development of potential therapeutic targets.
In this report Desterke et al. mined public data sets to identify and define a PSC-specific network. Of the top genes in this list, NR0B2 stood out as a potential player in pathogenesis because of its involvement in regulating bile acid metabolism. The authors showed that upregulation of NR0B2 occurs early in the disease process and in patient tissues is independent of variables such as gender and sex. Interestingly, the authors showed that this upregulation occurs primarily in cholangiocytes, the cells lining the bile duct. Higher expression of NR0B2 results in reprogramming that alters the metabolic function of these cells and predisposes them to malignancy.
This study, which is the first to look at omics data for PSC, highlights the involvement of genes and pathways that were previously unrecognized in disease pathogenesis. By using data derived from human PSC liver biopsies and animal models of PSC, the authors were able to validate their findings across species, which strengthened their conclusions. This approach also showed that NR0B2 deregulation occurs primarily in cholangiocytes, suggesting that future therapies should be targeted to this cell type. These important findings will improve our understanding of this rare but clinically significant disease.
Kari Nejak-Bowen, PhD, MBA, is associate professor, department of pathology, University of Pittsburgh School of Medicine. She has no relevant conflicts of interest.
Primary sclerosing cholangitis (PSC) is a bile duct disease with few therapeutic options other than liver transplant, and thus its prognosis remains grim. Additionally, the factors that cause the disease are not well understood. Identifying the pathways and genes involved in PSC pathogenesis could help in the development of potential therapeutic targets.
In this report Desterke et al. mined public data sets to identify and define a PSC-specific network. Of the top genes in this list, NR0B2 stood out as a potential player in pathogenesis because of its involvement in regulating bile acid metabolism. The authors showed that upregulation of NR0B2 occurs early in the disease process and in patient tissues is independent of variables such as gender and sex. Interestingly, the authors showed that this upregulation occurs primarily in cholangiocytes, the cells lining the bile duct. Higher expression of NR0B2 results in reprogramming that alters the metabolic function of these cells and predisposes them to malignancy.
This study, which is the first to look at omics data for PSC, highlights the involvement of genes and pathways that were previously unrecognized in disease pathogenesis. By using data derived from human PSC liver biopsies and animal models of PSC, the authors were able to validate their findings across species, which strengthened their conclusions. This approach also showed that NR0B2 deregulation occurs primarily in cholangiocytes, suggesting that future therapies should be targeted to this cell type. These important findings will improve our understanding of this rare but clinically significant disease.
Kari Nejak-Bowen, PhD, MBA, is associate professor, department of pathology, University of Pittsburgh School of Medicine. She has no relevant conflicts of interest.
Primary sclerosing cholangitis (PSC) is a bile duct disease with few therapeutic options other than liver transplant, and thus its prognosis remains grim. Additionally, the factors that cause the disease are not well understood. Identifying the pathways and genes involved in PSC pathogenesis could help in the development of potential therapeutic targets.
In this report Desterke et al. mined public data sets to identify and define a PSC-specific network. Of the top genes in this list, NR0B2 stood out as a potential player in pathogenesis because of its involvement in regulating bile acid metabolism. The authors showed that upregulation of NR0B2 occurs early in the disease process and in patient tissues is independent of variables such as gender and sex. Interestingly, the authors showed that this upregulation occurs primarily in cholangiocytes, the cells lining the bile duct. Higher expression of NR0B2 results in reprogramming that alters the metabolic function of these cells and predisposes them to malignancy.
This study, which is the first to look at omics data for PSC, highlights the involvement of genes and pathways that were previously unrecognized in disease pathogenesis. By using data derived from human PSC liver biopsies and animal models of PSC, the authors were able to validate their findings across species, which strengthened their conclusions. This approach also showed that NR0B2 deregulation occurs primarily in cholangiocytes, suggesting that future therapies should be targeted to this cell type. These important findings will improve our understanding of this rare but clinically significant disease.
Kari Nejak-Bowen, PhD, MBA, is associate professor, department of pathology, University of Pittsburgh School of Medicine. She has no relevant conflicts of interest.
, according to a study published in Gastro Hep Advances.
PSC is very rare, with an incidence of 0-1.3 cases per 100,000 people per year. Because up to 80% of patients with PSC also have inflammatory bowel disease (IBD), a link along the gut-liver axis is suspected. So far, scientists have not understood the causes of PSC, the main complications of which include biliary cirrhosis, bacterial cholangitis, and cholangiocarcinoma.
No treatment is currently available for PSC, but the findings of this genomics study suggest several targets that may be worth pursuing, particularly the gene NR0B2.
“The therapeutic targeting of NR0B2 may potentiate that of FXR [farnesoid X receptor] and enable action on early events of the disease and prevent its progression,” wrote Christophe Desterke, PhD, of the Paul-Brousse Hospital, the French National Institute of Health and Medical Research, and the University of Paris-Saclay in Villejuif, France, and his associates.
The researchers used an algorithmic tool to mine the MEDLINE/PubMed/NCBI database using the three key symptoms of PSC – biliary fibrosis, biliary inflammation, and biliary stasis – as their keywords. This approach allowed them to discover the genes and potential pathways related to PSC in published research text or in clinical, animal, and cellular models.
The researchers initially found 525 genes linked to PSC and then compared them to RNA data from liver biopsies taken from patients with liver disease from various causes. This process led to a ranking of the 10 best markers of PSC, based on the data-mining method and the genes’ association with one or more of the three PSC symptoms.
At the top of the list is NR1H4, also called FXR, which ranks most highly with biliary fibrosis and biliary stasis. NR1H4 is already a clear target for cholestatic and fatty liver diseases, the authors noted. The other genes, in descending order of relevance, are: ABCB4, ABCB11, TGFB1, IFNL3, PNPLA3, IL6, TLR4, GPBAR1, and IL17A. In addition, complications of PSC were significantly associated with upregulation of TNFRS12A, SOX9, ANXA2, MMP7, and LCN2.
Separately, investigation of the 525 initially identified genes in mouse models of PSC revealed that NR0B2 is also a key player in the pathogenesis of PSC.
"NR0B2 was upregulated in PSC livers independent of gender, age, and body mass index,” the authors reported. “Importantly, it was not dependent on the severity of PSC in the prognostic cohort, suggesting that this may be an early event during the disease.”
The researchers also found a possible pathway explaining the autoimmunity of PSC – the involvement of CD274, also known as the PDL1 immune checkpoint. The authors noted that the PDL1 inhibitor pembrolizumab has previously been reported as a cause of sclerosing cholangitis.
Further, the researchers discovered overexpression of FOXP3 in the livers of patients with PSC. Because FOXP3 determines what T-cell subtypes look like, the finding suggests that an “imbalance between Foxp3þ regulatory T cells and Th17 cells may be involved in IBD and PSC,” they wrote.
Also of note was the overexpression of SOX9 in the livers of patients with PSC whose profiles suggested the worst clinical prognoses.
Finally, the researchers identified three genes as potentially involved in development of cholangiocarcinoma: GSTA3, ID2 (which is overexpressed in biliary tract cancer), and especially TMEM45A, a protein in cells’ Golgi apparatus that is already known to be involved in the development of several other cancers.
The research was funded by the French National Institute of Health and Medical Research. The authors reported no conflicts of interest.
, according to a study published in Gastro Hep Advances.
PSC is very rare, with an incidence of 0-1.3 cases per 100,000 people per year. Because up to 80% of patients with PSC also have inflammatory bowel disease (IBD), a link along the gut-liver axis is suspected. So far, scientists have not understood the causes of PSC, the main complications of which include biliary cirrhosis, bacterial cholangitis, and cholangiocarcinoma.
No treatment is currently available for PSC, but the findings of this genomics study suggest several targets that may be worth pursuing, particularly the gene NR0B2.
“The therapeutic targeting of NR0B2 may potentiate that of FXR [farnesoid X receptor] and enable action on early events of the disease and prevent its progression,” wrote Christophe Desterke, PhD, of the Paul-Brousse Hospital, the French National Institute of Health and Medical Research, and the University of Paris-Saclay in Villejuif, France, and his associates.
The researchers used an algorithmic tool to mine the MEDLINE/PubMed/NCBI database using the three key symptoms of PSC – biliary fibrosis, biliary inflammation, and biliary stasis – as their keywords. This approach allowed them to discover the genes and potential pathways related to PSC in published research text or in clinical, animal, and cellular models.
The researchers initially found 525 genes linked to PSC and then compared them to RNA data from liver biopsies taken from patients with liver disease from various causes. This process led to a ranking of the 10 best markers of PSC, based on the data-mining method and the genes’ association with one or more of the three PSC symptoms.
At the top of the list is NR1H4, also called FXR, which ranks most highly with biliary fibrosis and biliary stasis. NR1H4 is already a clear target for cholestatic and fatty liver diseases, the authors noted. The other genes, in descending order of relevance, are: ABCB4, ABCB11, TGFB1, IFNL3, PNPLA3, IL6, TLR4, GPBAR1, and IL17A. In addition, complications of PSC were significantly associated with upregulation of TNFRS12A, SOX9, ANXA2, MMP7, and LCN2.
Separately, investigation of the 525 initially identified genes in mouse models of PSC revealed that NR0B2 is also a key player in the pathogenesis of PSC.
"NR0B2 was upregulated in PSC livers independent of gender, age, and body mass index,” the authors reported. “Importantly, it was not dependent on the severity of PSC in the prognostic cohort, suggesting that this may be an early event during the disease.”
The researchers also found a possible pathway explaining the autoimmunity of PSC – the involvement of CD274, also known as the PDL1 immune checkpoint. The authors noted that the PDL1 inhibitor pembrolizumab has previously been reported as a cause of sclerosing cholangitis.
Further, the researchers discovered overexpression of FOXP3 in the livers of patients with PSC. Because FOXP3 determines what T-cell subtypes look like, the finding suggests that an “imbalance between Foxp3þ regulatory T cells and Th17 cells may be involved in IBD and PSC,” they wrote.
Also of note was the overexpression of SOX9 in the livers of patients with PSC whose profiles suggested the worst clinical prognoses.
Finally, the researchers identified three genes as potentially involved in development of cholangiocarcinoma: GSTA3, ID2 (which is overexpressed in biliary tract cancer), and especially TMEM45A, a protein in cells’ Golgi apparatus that is already known to be involved in the development of several other cancers.
The research was funded by the French National Institute of Health and Medical Research. The authors reported no conflicts of interest.
FROM GASTRO HEP ADVANCES
Scar overgrowth
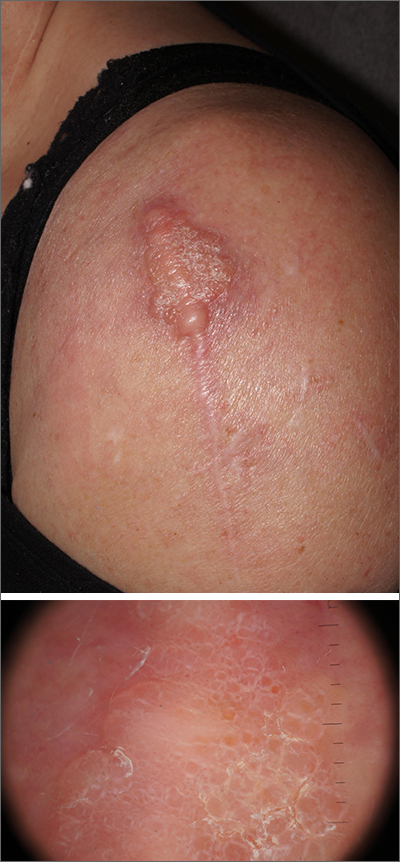
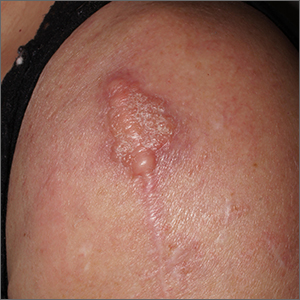
Dermatopathology was consistent with a diagnosis of cutaneous myxoma (CM). There are very few dermoscopic descriptions of CM in the literature, so diagnostic features are not established. However, the absence of more diagnostic features of basal cell carcinoma or squamous cell carcinoma (SCC) increases the likelihood of a rare diagnosis, such as CM.
CMs are rare benign neoplasms that manifest most commonly in young adults as small (< 1 cm) flesh-colored to blue papules on the head, neck, and trunk. The size of this particular CM was an outlier. CMs may be associated with Carney Complex (CNC), a rare inherited syndrome that has been linked to multiple endocrine neoplasias—namely, pituitary adenomas, testicular Sertoli cell tumors, thyroid tumors, and cardiac atrial myxomas.1 Additionally, in CNC, lentigines and multiple blue nevi develop on the skin and mucosal surfaces.
The differential diagnosis for a large, pink to flesh-colored nodule of this size includes benign histiocytoma, SCC, CM, and dermatofibrosarcoma protuberans. Benign histiocytomas and SCCs are much more common than CM. Clinical features only hint at the correct diagnosis, which must be made histologically.
Patients with CMs benefit from ongoing dermatology surveillance to monitor for the development of atypical nevi or new CMs. In this case, a wide excision with generous margins was planned with plastic surgery. (CMs have been reported to recur after surgery, which is why wide margins are essential.)
Additionally, 2 factors prompted an echocardiogram: the association between CMs and possible cardiac tumors and the patient’s need to undergo future orthopedic surgery under general anesthesia. No cardiac tumors were visible on echocardiogram. Thyroid imaging and genetic evaluation were planned but not completed.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-18. doi: 10.1111/cup.12749.

Dermatopathology was consistent with a diagnosis of cutaneous myxoma (CM). There are very few dermoscopic descriptions of CM in the literature, so diagnostic features are not established. However, the absence of more diagnostic features of basal cell carcinoma or squamous cell carcinoma (SCC) increases the likelihood of a rare diagnosis, such as CM.
CMs are rare benign neoplasms that manifest most commonly in young adults as small (< 1 cm) flesh-colored to blue papules on the head, neck, and trunk. The size of this particular CM was an outlier. CMs may be associated with Carney Complex (CNC), a rare inherited syndrome that has been linked to multiple endocrine neoplasias—namely, pituitary adenomas, testicular Sertoli cell tumors, thyroid tumors, and cardiac atrial myxomas.1 Additionally, in CNC, lentigines and multiple blue nevi develop on the skin and mucosal surfaces.
The differential diagnosis for a large, pink to flesh-colored nodule of this size includes benign histiocytoma, SCC, CM, and dermatofibrosarcoma protuberans. Benign histiocytomas and SCCs are much more common than CM. Clinical features only hint at the correct diagnosis, which must be made histologically.
Patients with CMs benefit from ongoing dermatology surveillance to monitor for the development of atypical nevi or new CMs. In this case, a wide excision with generous margins was planned with plastic surgery. (CMs have been reported to recur after surgery, which is why wide margins are essential.)
Additionally, 2 factors prompted an echocardiogram: the association between CMs and possible cardiac tumors and the patient’s need to undergo future orthopedic surgery under general anesthesia. No cardiac tumors were visible on echocardiogram. Thyroid imaging and genetic evaluation were planned but not completed.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermatopathology was consistent with a diagnosis of cutaneous myxoma (CM). There are very few dermoscopic descriptions of CM in the literature, so diagnostic features are not established. However, the absence of more diagnostic features of basal cell carcinoma or squamous cell carcinoma (SCC) increases the likelihood of a rare diagnosis, such as CM.
CMs are rare benign neoplasms that manifest most commonly in young adults as small (< 1 cm) flesh-colored to blue papules on the head, neck, and trunk. The size of this particular CM was an outlier. CMs may be associated with Carney Complex (CNC), a rare inherited syndrome that has been linked to multiple endocrine neoplasias—namely, pituitary adenomas, testicular Sertoli cell tumors, thyroid tumors, and cardiac atrial myxomas.1 Additionally, in CNC, lentigines and multiple blue nevi develop on the skin and mucosal surfaces.
The differential diagnosis for a large, pink to flesh-colored nodule of this size includes benign histiocytoma, SCC, CM, and dermatofibrosarcoma protuberans. Benign histiocytomas and SCCs are much more common than CM. Clinical features only hint at the correct diagnosis, which must be made histologically.
Patients with CMs benefit from ongoing dermatology surveillance to monitor for the development of atypical nevi or new CMs. In this case, a wide excision with generous margins was planned with plastic surgery. (CMs have been reported to recur after surgery, which is why wide margins are essential.)
Additionally, 2 factors prompted an echocardiogram: the association between CMs and possible cardiac tumors and the patient’s need to undergo future orthopedic surgery under general anesthesia. No cardiac tumors were visible on echocardiogram. Thyroid imaging and genetic evaluation were planned but not completed.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-18. doi: 10.1111/cup.12749.
1. Zou Y, Billings SD. Myxoid cutaneous tumors: a review. J Cutan Pathol. 2016;43:903-18. doi: 10.1111/cup.12749.
CHEST 2022 award winners More award winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
New osteoporosis guideline says start with a bisphosphonate
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
This is the first update for 5 years since the previous guidance was published in 2017.
It strongly recommends initial therapy with bisphosphonates for postmenopausal women with osteoporosis, as well as men with osteoporosis, among other recommendations.
However, the author of an accompanying editorial, Susan M. Ott, MD, says: “The decision to start a bisphosphonate is actually not that easy.”
She also queries some of the other recommendations in the guidance.
Her editorial, along with the guideline by Amir Qaseem, MD, PhD, MPH, and colleagues, and systematic review by Chelsea Ayers, MPH, and colleagues, were published in the Annals of Internal Medicine.
Ryan D. Mire, MD, MACP, president of the ACP, gave a brief overview of the new guidance in a video.
Systematic review
The ACP commissioned a review of the evidence because it says new data have emerged on the efficacy of newer medications for osteoporosis and low bone mass, as well as treatment comparisons, and treatment in men.
The review authors identified 34 randomized controlled trials (in 100 publications) and 36 observational studies, which evaluated the following pharmacologic interventions:
- Antiresorptive drugs: four bisphosphonates (alendronate, ibandronate, risedronate, zoledronate) and a RANK ligand inhibitor (denosumab).
- Anabolic drugs: an analog of human parathyroid hormone (PTH)–related protein (abaloparatide), recombinant human PTH (teriparatide), and a sclerostin inhibitor (romosozumab).
- Estrogen agonists: selective estrogen receptor modulators (bazedoxifene, raloxifene).
The authors focused on effectiveness and harms of active drugs compared with placebo or bisphosphonates.
Major changes from 2017 guidelines, some questions
“Though there are many nuanced changes in this [2023 guideline] version, perhaps the major change is the explicit hierarchy of pharmacologic recommendations: bisphosphonates first, then denosumab,” Thomas G. Cooney, MD, senior author of the clinical guideline, explained in an interview.
“Bisphosphonates had the most favorable balance among benefits, harms, patient values and preferences, and cost among the examined drugs in postmenopausal females with primary osteoporosis,” Dr. Cooney, professor of medicine, Oregon Health & Science University, Portland, noted, as is stated in the guideline.
“Denosumab also had a favorable long-term net benefit, but bisphosphonates are much cheaper than other pharmacologic treatments and available in generic formulations,” the document states.
The new guideline suggests use of denosumab as second-line pharmacotherapy in adults who have contraindications to or experience adverse effects with bisphosphonates.
The choice among bisphosphonates (alendronate, risedronate, zoledronic acid) would be based on a patient-centered discussion between physician and patient, addressing costs (often related to insurance), delivery-mode preferences (oral versus intravenous), and “values,” which includes the patient’s priorities, concerns, and expectations regarding their health care, Dr. Cooney explained.
Another update in the new guideline is, “We also clarify the specific, albeit more limited, role of sclerostin inhibitors and recombinant PTH ‘to reduce the risk of fractures only in females with primary osteoporosis with very high-risk of fracture’,” Dr. Cooney noted.
In addition, the guideline now states, “treatment to reduce the risk of fractures in males rather than limiting it to ‘vertebral fracture’ in men,” as in the 2017 guideline.
It also explicitly includes denosumab as second-line therapy for men, Dr. Cooney noted, but as in 2017, the strength of evidence in men remains low.
“Finally, we also clarified that in females over the age of 65 with low bone mass or osteopenia that an individualized approach be taken to treatment (similar to last guideline), but if treatment is initiated, that a bisphosphonate be used (new content),” he said.
The use of estrogen, treatment duration, drug discontinuation, and serial bone mineral density monitoring were not addressed in this guideline, but will likely be evaluated within 2 to 3 years.
‘Osteoporosis treatment: Not easy’ – editorial
In her editorial, Dr. Ott writes: “The data about bisphosphonates may seem overwhelmingly positive, leading to strong recommendations for their use to treat osteoporosis, but the decision to start a bisphosphonate is actually not that easy.”
“A strong recommendation should be given only when future studies are unlikely to change it,” continues Dr. Ott, professor of medicine, University of Washington, Seattle.
“Yet, data already suggest that, in patients with serious osteoporosis, treatment should start with anabolic medications because previous treatment with either bisphosphonates or denosumab will prevent the anabolic response of newer medications.”
“Starting with bisphosphonate will change the bone so it will not respond to the newer medicines, and then a patient will lose the chance for getting the best improvement,” Dr. Ott clarified in an email to this news organization.
But, in fact, the new guidance does suggest that, to reduce the risk of fractures in females with primary osteoporosis at very high risk of fracture, one should consider use of the sclerostin inhibitor romosozumab (moderate-certainty evidence) or recombinant human parathyroid hormone (teriparatide) (low-certainty evidence) followed by a bisphosphonate (conditional recommendation).
Dr. Ott said: “If the [fracture] risk is high, then we should start with an anabolic medication for 1-2 years. If the risk is medium, then use a bisphosphonate for up to 5 years, and then stop and monitor the patient for signs that the medicine is wearing off,” based on blood and urine tests.
‘We need medicines that will stop bone aging’
Osteopenia is defined by an arbitrary bone density measurement, Dr. Ott explained. “About half of women over 65 will have osteopenia, and by age 85 there are hardly any ‘normal’ women left.”
“We need medicines that will stop bone aging, which might sound impossible, but we should still try,” she continued.
“In the meantime, while waiting on new discoveries,” Dr. Ott said, “I would not use bisphosphonates in patients who did not already have a fracture or whose bone density T-score was better than –2.5 because, in the major study, alendronate did not prevent fractures in this group.”
Many people are worried about bisphosphonates because of problems with the jaw or femur. These are real, but they are very rare during the first 5 years of treatment, Dr. Ott noted. Then the risk starts to rise, up to more than 1 in 1,000 after 8 years. So people can get the benefits of these drugs with very low risk for 5 years.
“An immediate [guideline] update is necessary to address the severity of bone loss and the high risk for vertebral fractures after discontinuation of denosumab,” Dr. Ott urged.
“I don’t agree with using denosumab for osteoporosis as a second-line treatment,” she said. “I would use it only in patients who have cancer or unusually high bone resorption. You have to get a dose strictly every 6 months, and if you need to stop, it is recommended to treat with bisphosphonates. Denosumab is a poor choice for somebody who does not want to take a bisphosphonate. Many patients and even too many doctors do not realize how serious it can be to skip a dose.”
“I also think that men could be treated with anabolic medications,” Dr. Ott said. “Clinical trials show they respond the same as women. Many men have osteoporosis as a consequence of low testosterone, and then they can usually be treated with testosterone. Osteoporosis in men is a serious problem that is too often ignored – almost reverse discrimination.”
It is also unfortunate that the review and recommendations do not address estrogen, one of the most effective medications to prevent osteoporotic fractures, according to Dr. Ott.
Clinical considerations in addition to drug types
The new guideline also advises:
- Clinicians treating adults with osteoporosis should encourage adherence to recommended treatments and healthy lifestyle habits, including exercise, and counseling to evaluate and prevent falls.
- All adults with osteopenia or osteoporosis should have adequate calcium and vitamin D intake, as part of fracture prevention.
- Clinicians should assess baseline fracture risk based on bone density, fracture history, fracture risk factors, and response to prior osteoporosis treatments.
- Current evidence suggests that more than 3-5 years of bisphosphonate therapy reduces risk for new vertebral but not other fractures; however, it also increases risk for long-term harms. Therefore, clinicians should consider stopping bisphosphonate treatment after 5 years unless the patient has a strong indication for treatment continuation.
- The decision for a bisphosphonate holiday (temporary discontinuation) and its duration should be based on baseline fracture risk, medication half-life in bone, and benefits and harms.
- Women treated with an anabolic agent who discontinue it should be offered an antiresorptive agent to preserve gains and because of serious risk for rebound and multiple vertebral fractures.
- Adults older than 65 years with osteoporosis may be at increased risk for falls or other adverse events because of drug interactions.
- Transgender persons have variable risk for low bone mass.
The review and guideline were funded by the ACP. Dr. Ott has reported no relevant disclosures. Relevant financial disclosures for other authors are listed with the guideline and review.
A version of this article first appeared on Medscape.com.
FROM THE ANNALS OF INTERNAL MEDICINE
Surgeon’s license suspension spotlights hazards, ethics of live-streaming surgeries
potentially endangering patients. The surgeon has a large social media following.
In November, the State Medical Board of Ohio temporarily suspended the license of Katherine Roxanne Grawe, MD, who practices in the wealthy Columbus suburb of Powell.
Among other accusations of misconduct, the board stated that “during some videos/live-streams you engage in dialogue to respond to viewers’ online questions while the surgical procedure remains actively ongoing.”
One patient needed emergency treatment following liposuction and was diagnosed with a perforated bowel and serious bacterial infection.
“Despite liposuction being a blind surgery that requires awareness of the tip of the cannula to avoid injury, your attention to the camera meant at those moments you were not looking at the patient or palpating the location of the tip of the cannula,” the medical board said.
Neither Dr. Grawe nor her attorney responded to requests for comment.
Dr. Grawe, known as “Dr. Roxy,” has a popular TikTok account – now set to private – with 841,600 followers and 14.6 million likes. She has another 123,000 followers on her Instagram account, also now private.
The Columbus Dispatch reported that Dr. Grawe had previously been warned to protect patient privacy on social media. The board has yet to make a final decision regarding her license.
According to Columbus TV station WSYX, she said in a TikTok video, “We show our surgeries every single day on Snapchat. Patients get to decide if they want to be part of it. And if you do, you can watch your own surgery.”
The TV station quoted former patients who described surgical complications. One said: “I went to her because, I thought, from all of her social media that she uplifted women. That she helped women empower themselves. But she didn’t.”
Dallas plastic surgeon Rod J. Rohrich, MD, who has written about social-media best practices and has 430,000 followers on Instagram, said in an interview that many surgeons have been reprimanded by state medical boards for being distracted by social media during procedures.
“It is best not to do live-streaming unless it is an educational event to demonstrate techniques and technology with full informed consent of the patient. It should be a very well-rehearsed event for education,” he said.
Nurses also have been disciplined for inappropriate posts on social media. In December 2022, an Atlanta hospital announced that four nurses were no longer on the job after they appeared in a TikTok video in scrubs and revealed their “icks” regarding obstetric care.
“My ick is when you ask me how much the baby weighs,” one worker said in the video, “and it’s still ... in your hands.”
Plastic surgeon Christian J. Vercler, MD, of the University of Michigan, Ann Arbor, who’s studied social-media guidelines for surgeons, said in an interview that plastic surgery content on TikTok has “blown up” in recent years.
“Five years or so ago, it was Snapchat where I saw a lot of inappropriate things posted by surgeons,” Dr. Vercler said in an interview. “That may still be happening on Snapchat, but I actually don’t ever use that platform anymore, and neither do my trainees.”
Dr. Vercler cautioned colleagues to consider their motivations for live-streaming surgery and to think about whether they can fully focus on the patient.
“There are many potential distractions in the OR. We get pages, phone calls, nurses asking us questions, anesthesiologists trying to talk to us. Social media is just one more thing competing for the surgeon’s attention,” he said. “Every surgeon should strive to eliminate unnecessary or unavoidable distractions, so the question becomes, ‘who is best being served by me focusing my attention on recording this operation on someone’s phone so we can post it on social media? Is it the patient?’ ”
Dr. Vercler added, “There are many, many plastic surgeons using social media as the powerful platform that it is to build their brands, to connect with potential patients, and to educate the public about what they do. I believe that most are doing this in a way that is respectful to patients and doesn’t exploit patients for the surgeon’s benefit.
“Unfortunately,” he concluded, “there are some who do see patients as merely instruments by which they can achieve fame, notoriety, and wealth.”
Dr. Rohrich and Dr. Vercler disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
potentially endangering patients. The surgeon has a large social media following.
In November, the State Medical Board of Ohio temporarily suspended the license of Katherine Roxanne Grawe, MD, who practices in the wealthy Columbus suburb of Powell.
Among other accusations of misconduct, the board stated that “during some videos/live-streams you engage in dialogue to respond to viewers’ online questions while the surgical procedure remains actively ongoing.”
One patient needed emergency treatment following liposuction and was diagnosed with a perforated bowel and serious bacterial infection.
“Despite liposuction being a blind surgery that requires awareness of the tip of the cannula to avoid injury, your attention to the camera meant at those moments you were not looking at the patient or palpating the location of the tip of the cannula,” the medical board said.
Neither Dr. Grawe nor her attorney responded to requests for comment.
Dr. Grawe, known as “Dr. Roxy,” has a popular TikTok account – now set to private – with 841,600 followers and 14.6 million likes. She has another 123,000 followers on her Instagram account, also now private.
The Columbus Dispatch reported that Dr. Grawe had previously been warned to protect patient privacy on social media. The board has yet to make a final decision regarding her license.
According to Columbus TV station WSYX, she said in a TikTok video, “We show our surgeries every single day on Snapchat. Patients get to decide if they want to be part of it. And if you do, you can watch your own surgery.”
The TV station quoted former patients who described surgical complications. One said: “I went to her because, I thought, from all of her social media that she uplifted women. That she helped women empower themselves. But she didn’t.”
Dallas plastic surgeon Rod J. Rohrich, MD, who has written about social-media best practices and has 430,000 followers on Instagram, said in an interview that many surgeons have been reprimanded by state medical boards for being distracted by social media during procedures.
“It is best not to do live-streaming unless it is an educational event to demonstrate techniques and technology with full informed consent of the patient. It should be a very well-rehearsed event for education,” he said.
Nurses also have been disciplined for inappropriate posts on social media. In December 2022, an Atlanta hospital announced that four nurses were no longer on the job after they appeared in a TikTok video in scrubs and revealed their “icks” regarding obstetric care.
“My ick is when you ask me how much the baby weighs,” one worker said in the video, “and it’s still ... in your hands.”
Plastic surgeon Christian J. Vercler, MD, of the University of Michigan, Ann Arbor, who’s studied social-media guidelines for surgeons, said in an interview that plastic surgery content on TikTok has “blown up” in recent years.
“Five years or so ago, it was Snapchat where I saw a lot of inappropriate things posted by surgeons,” Dr. Vercler said in an interview. “That may still be happening on Snapchat, but I actually don’t ever use that platform anymore, and neither do my trainees.”
Dr. Vercler cautioned colleagues to consider their motivations for live-streaming surgery and to think about whether they can fully focus on the patient.
“There are many potential distractions in the OR. We get pages, phone calls, nurses asking us questions, anesthesiologists trying to talk to us. Social media is just one more thing competing for the surgeon’s attention,” he said. “Every surgeon should strive to eliminate unnecessary or unavoidable distractions, so the question becomes, ‘who is best being served by me focusing my attention on recording this operation on someone’s phone so we can post it on social media? Is it the patient?’ ”
Dr. Vercler added, “There are many, many plastic surgeons using social media as the powerful platform that it is to build their brands, to connect with potential patients, and to educate the public about what they do. I believe that most are doing this in a way that is respectful to patients and doesn’t exploit patients for the surgeon’s benefit.
“Unfortunately,” he concluded, “there are some who do see patients as merely instruments by which they can achieve fame, notoriety, and wealth.”
Dr. Rohrich and Dr. Vercler disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
potentially endangering patients. The surgeon has a large social media following.
In November, the State Medical Board of Ohio temporarily suspended the license of Katherine Roxanne Grawe, MD, who practices in the wealthy Columbus suburb of Powell.
Among other accusations of misconduct, the board stated that “during some videos/live-streams you engage in dialogue to respond to viewers’ online questions while the surgical procedure remains actively ongoing.”
One patient needed emergency treatment following liposuction and was diagnosed with a perforated bowel and serious bacterial infection.
“Despite liposuction being a blind surgery that requires awareness of the tip of the cannula to avoid injury, your attention to the camera meant at those moments you were not looking at the patient or palpating the location of the tip of the cannula,” the medical board said.
Neither Dr. Grawe nor her attorney responded to requests for comment.
Dr. Grawe, known as “Dr. Roxy,” has a popular TikTok account – now set to private – with 841,600 followers and 14.6 million likes. She has another 123,000 followers on her Instagram account, also now private.
The Columbus Dispatch reported that Dr. Grawe had previously been warned to protect patient privacy on social media. The board has yet to make a final decision regarding her license.
According to Columbus TV station WSYX, she said in a TikTok video, “We show our surgeries every single day on Snapchat. Patients get to decide if they want to be part of it. And if you do, you can watch your own surgery.”
The TV station quoted former patients who described surgical complications. One said: “I went to her because, I thought, from all of her social media that she uplifted women. That she helped women empower themselves. But she didn’t.”
Dallas plastic surgeon Rod J. Rohrich, MD, who has written about social-media best practices and has 430,000 followers on Instagram, said in an interview that many surgeons have been reprimanded by state medical boards for being distracted by social media during procedures.
“It is best not to do live-streaming unless it is an educational event to demonstrate techniques and technology with full informed consent of the patient. It should be a very well-rehearsed event for education,” he said.
Nurses also have been disciplined for inappropriate posts on social media. In December 2022, an Atlanta hospital announced that four nurses were no longer on the job after they appeared in a TikTok video in scrubs and revealed their “icks” regarding obstetric care.
“My ick is when you ask me how much the baby weighs,” one worker said in the video, “and it’s still ... in your hands.”
Plastic surgeon Christian J. Vercler, MD, of the University of Michigan, Ann Arbor, who’s studied social-media guidelines for surgeons, said in an interview that plastic surgery content on TikTok has “blown up” in recent years.
“Five years or so ago, it was Snapchat where I saw a lot of inappropriate things posted by surgeons,” Dr. Vercler said in an interview. “That may still be happening on Snapchat, but I actually don’t ever use that platform anymore, and neither do my trainees.”
Dr. Vercler cautioned colleagues to consider their motivations for live-streaming surgery and to think about whether they can fully focus on the patient.
“There are many potential distractions in the OR. We get pages, phone calls, nurses asking us questions, anesthesiologists trying to talk to us. Social media is just one more thing competing for the surgeon’s attention,” he said. “Every surgeon should strive to eliminate unnecessary or unavoidable distractions, so the question becomes, ‘who is best being served by me focusing my attention on recording this operation on someone’s phone so we can post it on social media? Is it the patient?’ ”
Dr. Vercler added, “There are many, many plastic surgeons using social media as the powerful platform that it is to build their brands, to connect with potential patients, and to educate the public about what they do. I believe that most are doing this in a way that is respectful to patients and doesn’t exploit patients for the surgeon’s benefit.
“Unfortunately,” he concluded, “there are some who do see patients as merely instruments by which they can achieve fame, notoriety, and wealth.”
Dr. Rohrich and Dr. Vercler disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Best diets in 2023: Mediterranean diet wins again
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
After all, weight loss usually lands one of the top spots on New Year’s resolution surveys.
And just in time, there’s guidance to pick the best plan, as U.S. News & World Report’s annual rankings of the best diet plans were released on Jan. 3.
Once again, the Mediterranean diet, which emphasizes fruits, vegetables, olive oil, and fish, got the top spot, as best diet overall. It’s the sixth consecutive year for that win. But many other diets got top marks as well.
In 2023, U.S. News, with the help of more than 30 nutritionists, doctors, and epidemiologists, ranked 24 diets in several categories to help people find a plan that meets their goals, whether it’s finding the best weight loss diet, easiest one to follow, or plans for other goals, such as managing diabetes or heart disease. Two new categories were added: Best Diets for Bone & Joint Health and Best Family-Friendly Diets.
In previous years, the publication ranked 40 diets. Even if a diet is no longer ranked, its profile with detailed information remains on the site.
“Each year we ask ourselves what we can do better or differently next time,” said Gretel Schueller, managing editor of health for U.S. News. When the publication got feedback from their experts this year, they had requests to consider sustainability of diets and whether they meet a busy family’s needs, in addition to considering many other factors.
This year’s report ranks plans in 11 categories.
The winners and the categories:
Best diets overall
After the Mediterranean diet, two others tied for second place:
- DASH (Dietary Approaches to Stop Hypertension) diet, which fights high blood pressure and emphasizes fruits, vegetables, whole grains, lean protein, and low-fat dairy.
- Flexitarian diet, which focuses on fruits, vegetables, and other healthy foods but also allows occasional meat.
Best weight-loss diets
WW, formerly known as Weight Watchers, got first place. The plan emphasizes not only weight loss but healthier eating and regular activity. The Points program, which assigns specific points to foods, with a daily Points budget, is more personalized than in the past.
- DASH got second place.
- Mayo Clinic Diet and TLC diet tied for third place. The Mayo Clinic Diet focuses on fruits, vegetables, and whole grains. It helps people improve their eating habits. The TLC diet (Therapeutic Lifestyle Changes) focuses on vegetables, fruit, lean protein, and reducing cholesterol levels.
Best fast weight-loss diets
The keto diet got first place. It’s a high-fat, low-carb diet that aims to achieve weight loss through fat burning. Four others tied for second place:
- Atkins, a diet created by the cardiologist Robert Atkins, which begins with very few carbs and then recommends progressively eating more until the weight loss goal is achieved
- Nutrisystem, a commercial program that includes prepackaged meals and focuses on high-protein, lower-glycemic foods to stabilize blood sugar levels
- Optavia, a plan focused on low-carb, low-calorie foods and including fortified meal replacements
- SlimFast Diet, a plan of shakes, smoothies, and meal bars to replace two of three meals a day
Best diets for healthy eating
- Mediterranean
- DASH
- Flexitarian
Best heart-healthy diets
- DASH
- Mediterranean
- Flexitarian and Ornish tied for third. The Ornish Diet focuses on plant-based and whole foods and limiting animal products. It recommends daily exercise and stress reduction.
Best diets for diabetes
- DASH
- Mediterranean
- Flexitarian
Best diets for bone and joint health
DASH and Mediterranean are in a first-place tie, followed by the flexitarian diet.
Best family-friendly diets
This category has a three-way tie: the flexitarian, Mediterranean, and TLC diets.
Best plant-based diets
Mediterranean was first, then flexitarian and the MIND diet. The MIND diet combines the DASH and Mediterranean diets and focuses on “brain-healthy” foods.
Easiest diets to follow
Flexitarian and TLC tied for first, followed by a tie between DASH and Mediterranean.
Best diet programs (formerly called commercial plans)
- WW
- There was a tie for second place between Jenny Craig and Noom, the latter of which focuses on low-calorie foods, with personalized calorie ranges and coaching to help meet goals.
Methodology
A variety of factors were considered, such as whether a diet includes all food groups, how easy it is to follow, whether it can be customized to meet cultural and personal preferences, and if it has a realistic timeline for weight loss.
Response from diet plans
Representatives from two plans that received mixed reviews in the rankings responded.
Jenny Craig was ranked second for best diet program but much lower for family friendly, landing at 22nd place of 24.
“Our program is designed to address the needs of the individual through personalized experiences,” Jenny Craig CEO Mandy Dowson said. “We have many families that participate in our program together but are still evaluated separately to determine appropriate individual goals.”
Its high ranking for best diet program reflects feedback from satisfied members, she said. Among advances will be the new Jenny Fresh program, a line of entrées prepared fresh and delivered to customers’ doors.
Atkins got second place for best fast weight loss but ranked near the bottom for best overall, best weight loss, diabetes, healthy eating, and heart health. In response, Colette Heimowitz, vice president of nutrition and education for Simply Good Foods, which makes Atkins’s food products, said that low-carb eating approaches are a viable option for anyone today.
“There are more than 130 independent, peer-reviewed published studies that show the efficacy and safety of low-carb eating,” she said. “The studies have been conducted for several decades and counting.”
Expert perspective
Samantha Cassetty, a registered dietitian, nutritionist, and wellness expert in New York and author of Sugar Shock, reviewed the report for this news organization. She was not involved in the rankings.
“I think what this shows you is, the best diet overall is also the best for various conditions,” she said. For instance, the Mediterranean, the No. 1 overall, also got high ranking for diabetes, heart health, and bone and joint health.
For consumers trying to lose weight: “If you see fast weight loss, that should be a red flag. A healthy diet for weight loss is one you can sustain,” she said.
She’s not a fan of the programs with prepackaged foods. “It takes the guesswork out, but the portion sizes tend to be unsatisfying. They don’t teach you how to deal with some of the challenges [such as realizing an ‘ideal’ portion size].”
How to use the report
Ms. Schueller’s advice: “Recognize that no diet fits everyone.” When considering which plan to choose, she suggests thinking long-term.
“Whatever we choose has to work in the long run,” she said.
Consumers should consider expenses, meal prep time, and whether the diet fits their lifestyle.
Ideally, she said, the best diet “teaches you smart food preparation and how to make healthy choices, allows the flexibility to be social and eat with groups, whether family or friends.”
Before choosing a diet to follow, consult a medical professional for input on the decision, U.S. News cautioned.
A version of this article first appeared on Medscape.com.
CHEST Challenge returned to the stage in the Music City
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
Proximal ADR could become important new quality metric
Measurement of the proximal adenoma detection rate may be an important new quality metric for screening colonoscopy, propose researchers in a study that found proportionately more adenomas detected in the right colon with increasing patient age.
As patients age, in fact, the rate of increase of proximal adenomas is far greater than for distal adenomas in both men and women and in all races, wrote Lawrence Kosinski, MD, founder and chief medical officer of Sonar MD in Chicago, and colleagues.
Adenoma detection rate (ADR), the proportion of screening colonoscopies performed by a physician that detect at least one histologically confirmed colorectal adenoma or adenocarcinoma, has become an accepted quality metric because of the association of high ADR with lower rates of postcolonoscopy colorectal cancer (CRC). ADR varies widely among endoscopists, however, which could be related to differences in adenoma detection in different parts of the colon.
the authors wrote. “These differences could be clinically important if CRC occurs after colonoscopy.” The study was published in Techniques and Innovations in Gastrointestinal Endoscopy .
Dr. Kosinski and colleagues analyzed retrospective claims data from all colonoscopies performed between 2016-2018 submitted to the Health Care Service Corporation, which is the exclusive Blue Cross Blue Shield licensee for Illinois, Texas, Oklahoma, New Mexico, and Montana. All 50 states were represented in the patient population, though Illinois and Texas accounted for 66% of the cases.
The research team limited the study group to include patients who underwent a screening colonoscopy, representing 30.9% of the total population. They further refined the data to include only screening colonoscopies performed by the 710 endoscopists with at least 100 screenings during the study period, representing 34.5% of the total patients. They also excluded 10,685 cases with family history because the high-risk patients could alter the results.
Using ICD-10 codes, the researchers identified the polyp detection locations and then calculated the ADR for the entire colon (T-ADR) and both the proximal (P-ADR) and distal (D-ADR) colon to determine differences in the ratio of P-ADR versus D-ADR by age, sex, and race. They were unable to determine whether the polyps were adenomas or sessile serrated lesions, so the ADR calculations include both.
The 182,296 screening colonoscopies included 93,164 women (51%) and 89,132 men (49%). About 79% of patients were aged 50-64 years, and 5.8% were under age 50. The dataset preceded the U.S. Preventive Services Task Force recommendation to initiate screening at age 45.
Overall, T-ADR was consistent with accepted norms in both men (25.99%) and women (19.72%). Compared with women, men had a 4.5% higher prevalence of proximal adenomas and a 2.5% higher prevalence of distal adenomas at any age. The small cohort of Native Americans (296 patients) had a numerically higher T-ADR, P-ADR, and D-ADR than other groups.
By age, T-ADR increased significantly with advancing age, from 0.13 in patients under age 40 to 0.39 in ages 70 and older. The increase was driven by a sharp rise in P-ADR, particularly after age 60. There was a relatively small increase in D-ADR after ages 45-49.
Notably, the P-ADR/D-ADR ratio increased from 1.2 in patients under age 40 to 2.65 in ages 75 and older in both men and women.
Since the experience of the endoscopist affects ADR, the research team also calculated the ADR data by the number of total colonoscopies by endoscopist per decile. T-ADR, P-ADR, and D-ADR were associated directly in a linear relationship with the number of total colonoscopies performed. The slope of the P-ADR trendline was 2.3 times higher than the slope of the D-ADR trendline, indicating a higher volume of procedures directly related to higher polyp detection – specifically the P-ADR.
“Our data demonstrate that it is feasible to measure P-ADR in clinical practice,” the authors wrote. “We propose that P-ADR be considered a quality metric for colonoscopy.”
In addition, because of considerable variation in ADR based on age and sex, calculated ADR should be normalized by the age and sex of the specific patient profile of each endoscopist so relevant benchmarks can be established based on practice demographics, they wrote. For example, an endoscopist with a practice that includes predominantly younger women would have a different benchmark than a colleague with an older male population.
“With appropriate use of gender and age adjustments to ADR, endoscopists in need of further education and mentoring can be identified,” they wrote.
The authors declared no funding for the study. One author reported advisory roles for several medical companies, and the remaining authors disclosed no conflicts.
“What gets measured gets managed” is a common mantra in quality improvement. Adenoma detection rate (ADR) is currently one measure of a “quality” colonoscopy and a metric that is studied to determine means for improvement. ADR is an imperfect measure as it does not necessarily reflect true risk of a postcolonoscopy cancer in all parts of the colon since many post colonoscopy cancers are found in the proximal colon. To try to better understand potential differences in polyps found in different segments of the colon, and to determine if this was a metric that could be measured, Kosinski and colleagues studied a large claims database to understand the potential difference in ADR in the proximal versus distal colon.
Sunanda Kane, MD, MSPH, is professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn. Dr. Kane has no relevant conflicts of interest.
“What gets measured gets managed” is a common mantra in quality improvement. Adenoma detection rate (ADR) is currently one measure of a “quality” colonoscopy and a metric that is studied to determine means for improvement. ADR is an imperfect measure as it does not necessarily reflect true risk of a postcolonoscopy cancer in all parts of the colon since many post colonoscopy cancers are found in the proximal colon. To try to better understand potential differences in polyps found in different segments of the colon, and to determine if this was a metric that could be measured, Kosinski and colleagues studied a large claims database to understand the potential difference in ADR in the proximal versus distal colon.
Sunanda Kane, MD, MSPH, is professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn. Dr. Kane has no relevant conflicts of interest.
“What gets measured gets managed” is a common mantra in quality improvement. Adenoma detection rate (ADR) is currently one measure of a “quality” colonoscopy and a metric that is studied to determine means for improvement. ADR is an imperfect measure as it does not necessarily reflect true risk of a postcolonoscopy cancer in all parts of the colon since many post colonoscopy cancers are found in the proximal colon. To try to better understand potential differences in polyps found in different segments of the colon, and to determine if this was a metric that could be measured, Kosinski and colleagues studied a large claims database to understand the potential difference in ADR in the proximal versus distal colon.
Sunanda Kane, MD, MSPH, is professor of medicine in the division of gastroenterology and hepatology at the Mayo Clinic, Rochester, Minn. Dr. Kane has no relevant conflicts of interest.
Measurement of the proximal adenoma detection rate may be an important new quality metric for screening colonoscopy, propose researchers in a study that found proportionately more adenomas detected in the right colon with increasing patient age.
As patients age, in fact, the rate of increase of proximal adenomas is far greater than for distal adenomas in both men and women and in all races, wrote Lawrence Kosinski, MD, founder and chief medical officer of Sonar MD in Chicago, and colleagues.
Adenoma detection rate (ADR), the proportion of screening colonoscopies performed by a physician that detect at least one histologically confirmed colorectal adenoma or adenocarcinoma, has become an accepted quality metric because of the association of high ADR with lower rates of postcolonoscopy colorectal cancer (CRC). ADR varies widely among endoscopists, however, which could be related to differences in adenoma detection in different parts of the colon.
the authors wrote. “These differences could be clinically important if CRC occurs after colonoscopy.” The study was published in Techniques and Innovations in Gastrointestinal Endoscopy .
Dr. Kosinski and colleagues analyzed retrospective claims data from all colonoscopies performed between 2016-2018 submitted to the Health Care Service Corporation, which is the exclusive Blue Cross Blue Shield licensee for Illinois, Texas, Oklahoma, New Mexico, and Montana. All 50 states were represented in the patient population, though Illinois and Texas accounted for 66% of the cases.
The research team limited the study group to include patients who underwent a screening colonoscopy, representing 30.9% of the total population. They further refined the data to include only screening colonoscopies performed by the 710 endoscopists with at least 100 screenings during the study period, representing 34.5% of the total patients. They also excluded 10,685 cases with family history because the high-risk patients could alter the results.
Using ICD-10 codes, the researchers identified the polyp detection locations and then calculated the ADR for the entire colon (T-ADR) and both the proximal (P-ADR) and distal (D-ADR) colon to determine differences in the ratio of P-ADR versus D-ADR by age, sex, and race. They were unable to determine whether the polyps were adenomas or sessile serrated lesions, so the ADR calculations include both.
The 182,296 screening colonoscopies included 93,164 women (51%) and 89,132 men (49%). About 79% of patients were aged 50-64 years, and 5.8% were under age 50. The dataset preceded the U.S. Preventive Services Task Force recommendation to initiate screening at age 45.
Overall, T-ADR was consistent with accepted norms in both men (25.99%) and women (19.72%). Compared with women, men had a 4.5% higher prevalence of proximal adenomas and a 2.5% higher prevalence of distal adenomas at any age. The small cohort of Native Americans (296 patients) had a numerically higher T-ADR, P-ADR, and D-ADR than other groups.
By age, T-ADR increased significantly with advancing age, from 0.13 in patients under age 40 to 0.39 in ages 70 and older. The increase was driven by a sharp rise in P-ADR, particularly after age 60. There was a relatively small increase in D-ADR after ages 45-49.
Notably, the P-ADR/D-ADR ratio increased from 1.2 in patients under age 40 to 2.65 in ages 75 and older in both men and women.
Since the experience of the endoscopist affects ADR, the research team also calculated the ADR data by the number of total colonoscopies by endoscopist per decile. T-ADR, P-ADR, and D-ADR were associated directly in a linear relationship with the number of total colonoscopies performed. The slope of the P-ADR trendline was 2.3 times higher than the slope of the D-ADR trendline, indicating a higher volume of procedures directly related to higher polyp detection – specifically the P-ADR.
“Our data demonstrate that it is feasible to measure P-ADR in clinical practice,” the authors wrote. “We propose that P-ADR be considered a quality metric for colonoscopy.”
In addition, because of considerable variation in ADR based on age and sex, calculated ADR should be normalized by the age and sex of the specific patient profile of each endoscopist so relevant benchmarks can be established based on practice demographics, they wrote. For example, an endoscopist with a practice that includes predominantly younger women would have a different benchmark than a colleague with an older male population.
“With appropriate use of gender and age adjustments to ADR, endoscopists in need of further education and mentoring can be identified,” they wrote.
The authors declared no funding for the study. One author reported advisory roles for several medical companies, and the remaining authors disclosed no conflicts.
Measurement of the proximal adenoma detection rate may be an important new quality metric for screening colonoscopy, propose researchers in a study that found proportionately more adenomas detected in the right colon with increasing patient age.
As patients age, in fact, the rate of increase of proximal adenomas is far greater than for distal adenomas in both men and women and in all races, wrote Lawrence Kosinski, MD, founder and chief medical officer of Sonar MD in Chicago, and colleagues.
Adenoma detection rate (ADR), the proportion of screening colonoscopies performed by a physician that detect at least one histologically confirmed colorectal adenoma or adenocarcinoma, has become an accepted quality metric because of the association of high ADR with lower rates of postcolonoscopy colorectal cancer (CRC). ADR varies widely among endoscopists, however, which could be related to differences in adenoma detection in different parts of the colon.
the authors wrote. “These differences could be clinically important if CRC occurs after colonoscopy.” The study was published in Techniques and Innovations in Gastrointestinal Endoscopy .
Dr. Kosinski and colleagues analyzed retrospective claims data from all colonoscopies performed between 2016-2018 submitted to the Health Care Service Corporation, which is the exclusive Blue Cross Blue Shield licensee for Illinois, Texas, Oklahoma, New Mexico, and Montana. All 50 states were represented in the patient population, though Illinois and Texas accounted for 66% of the cases.
The research team limited the study group to include patients who underwent a screening colonoscopy, representing 30.9% of the total population. They further refined the data to include only screening colonoscopies performed by the 710 endoscopists with at least 100 screenings during the study period, representing 34.5% of the total patients. They also excluded 10,685 cases with family history because the high-risk patients could alter the results.
Using ICD-10 codes, the researchers identified the polyp detection locations and then calculated the ADR for the entire colon (T-ADR) and both the proximal (P-ADR) and distal (D-ADR) colon to determine differences in the ratio of P-ADR versus D-ADR by age, sex, and race. They were unable to determine whether the polyps were adenomas or sessile serrated lesions, so the ADR calculations include both.
The 182,296 screening colonoscopies included 93,164 women (51%) and 89,132 men (49%). About 79% of patients were aged 50-64 years, and 5.8% were under age 50. The dataset preceded the U.S. Preventive Services Task Force recommendation to initiate screening at age 45.
Overall, T-ADR was consistent with accepted norms in both men (25.99%) and women (19.72%). Compared with women, men had a 4.5% higher prevalence of proximal adenomas and a 2.5% higher prevalence of distal adenomas at any age. The small cohort of Native Americans (296 patients) had a numerically higher T-ADR, P-ADR, and D-ADR than other groups.
By age, T-ADR increased significantly with advancing age, from 0.13 in patients under age 40 to 0.39 in ages 70 and older. The increase was driven by a sharp rise in P-ADR, particularly after age 60. There was a relatively small increase in D-ADR after ages 45-49.
Notably, the P-ADR/D-ADR ratio increased from 1.2 in patients under age 40 to 2.65 in ages 75 and older in both men and women.
Since the experience of the endoscopist affects ADR, the research team also calculated the ADR data by the number of total colonoscopies by endoscopist per decile. T-ADR, P-ADR, and D-ADR were associated directly in a linear relationship with the number of total colonoscopies performed. The slope of the P-ADR trendline was 2.3 times higher than the slope of the D-ADR trendline, indicating a higher volume of procedures directly related to higher polyp detection – specifically the P-ADR.
“Our data demonstrate that it is feasible to measure P-ADR in clinical practice,” the authors wrote. “We propose that P-ADR be considered a quality metric for colonoscopy.”
In addition, because of considerable variation in ADR based on age and sex, calculated ADR should be normalized by the age and sex of the specific patient profile of each endoscopist so relevant benchmarks can be established based on practice demographics, they wrote. For example, an endoscopist with a practice that includes predominantly younger women would have a different benchmark than a colleague with an older male population.
“With appropriate use of gender and age adjustments to ADR, endoscopists in need of further education and mentoring can be identified,” they wrote.
The authors declared no funding for the study. One author reported advisory roles for several medical companies, and the remaining authors disclosed no conflicts.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY