User login
Compulsively checking social media linked with altered brain patterns in teens
Teens who compulsively checked social media networks showed different development patterns in parts of the brain that involve reward and punishment than did those who didn’t check their platforms as often, new research suggests.
Results were published online in JAMA Pediatrics.
Researchers, led by Maria T. Maza, of the department of psychology and neuroscience at University of North Carolina at Chapel Hill, included 169 6th- and 7th-grade students recruited from three public middle schools in rural North Carolina in a 3-year longitudinal cohort.
Participants reported how frequently they checked Facebook, Instagram, and Snapchat. Answers were grouped into eight score groups depending on their per-day check times: less than 1; 1; 2-3; 4-5; 6-10; 11-15; 16-20; or more than 20 times. Those groups were then broken into three categories: low (nonhabitual); moderate; and high (habitual).
Imaging shows reactions
Researchers used functional magnetic resonance imaging (fMRI) to see how different areas of the brain react when participants looked at a series of indicators, such as happy and angry faces, which mimic social media rewards, punishments, or neutral feedback.
The research team focused on adolescents, for whom social media participation and neural sensitivity to social feedback from peers are high.
They found that participants who frequently checked social media showed distinct brain patterns when anticipating social feedback compared with those who had moderate or low use, “suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.”
The affected regions of the brain included the networks that respond to motivation and cognitive control.
However, the study was not able to determine whether the differences are a good or bad thing.
“While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment,” the authors wrote.
Chicken-and-egg questions
David Rettew, MD, a child and adolescent psychiatrist at the Oregon Health & Science University in Portland, who was not part of this research, said in an interview that it’s not clear from this study which came first – different brain development in the teens prior to this study that caused compulsive checking, or checking behaviors that caused different brain development. The authors acknowledge this is a limitation of the study.
“Hopefully, someday researchers will look at some of these brain activation patterns before kids have been exposed to social media to help us sort some of these questions out,” Dr. Rettew said.
“It wasn’t as though the groups looked the same at baseline and then diverged as they used more and more social media,” Dr. Rettew said. “It looked like there were some baseline differences that could be traced back maybe years before the study even started.”
People hear “divergent brain development” associated with social media and naturally get alarmed, he acknowledged.
“I get that, but the study isn’t really equipped to tell us what should be happening in the brain and what changes may have implications for other parts of an adolescent’s life,” Dr. Rettew said, “In the end, what we have is an association between heavy social media use and certain brain activation patterns which is cool to see and measure.”
He agrees with the authors, however, that overuse of social media is concerning and studying its effects is important.
Seventy-eight percent of early adolescents check every hour
According to the paper, 78% of 13- to 17-year-olds report checking their devices at least every hour and 46% check “almost constantly.”
“Regardless of which brain regions light up when looking at various emoji responses to their Instagram post, I think it is valid already to have some concerns about youth who can’t stay off their phone for more than 10 minutes,” Dr. Rettew said. “Technology is here to stay, but how we can learn to use it rather than have it use us is probably the more pressing question at this point.”
One coauthor reports grants from the National Institute on Drug Abuse (NIDA) during the conduct of the study and grants from NIDA and the National Science Foundation outside the submitted work; a coauthor reports grants from the Winston Family Foundation; and a coauthor reports a grant from NIDA and funds from the Winston Family Foundation – both during the conduct of the study. No other disclosures were reported. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Teens who compulsively checked social media networks showed different development patterns in parts of the brain that involve reward and punishment than did those who didn’t check their platforms as often, new research suggests.
Results were published online in JAMA Pediatrics.
Researchers, led by Maria T. Maza, of the department of psychology and neuroscience at University of North Carolina at Chapel Hill, included 169 6th- and 7th-grade students recruited from three public middle schools in rural North Carolina in a 3-year longitudinal cohort.
Participants reported how frequently they checked Facebook, Instagram, and Snapchat. Answers were grouped into eight score groups depending on their per-day check times: less than 1; 1; 2-3; 4-5; 6-10; 11-15; 16-20; or more than 20 times. Those groups were then broken into three categories: low (nonhabitual); moderate; and high (habitual).
Imaging shows reactions
Researchers used functional magnetic resonance imaging (fMRI) to see how different areas of the brain react when participants looked at a series of indicators, such as happy and angry faces, which mimic social media rewards, punishments, or neutral feedback.
The research team focused on adolescents, for whom social media participation and neural sensitivity to social feedback from peers are high.
They found that participants who frequently checked social media showed distinct brain patterns when anticipating social feedback compared with those who had moderate or low use, “suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.”
The affected regions of the brain included the networks that respond to motivation and cognitive control.
However, the study was not able to determine whether the differences are a good or bad thing.
“While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment,” the authors wrote.
Chicken-and-egg questions
David Rettew, MD, a child and adolescent psychiatrist at the Oregon Health & Science University in Portland, who was not part of this research, said in an interview that it’s not clear from this study which came first – different brain development in the teens prior to this study that caused compulsive checking, or checking behaviors that caused different brain development. The authors acknowledge this is a limitation of the study.
“Hopefully, someday researchers will look at some of these brain activation patterns before kids have been exposed to social media to help us sort some of these questions out,” Dr. Rettew said.
“It wasn’t as though the groups looked the same at baseline and then diverged as they used more and more social media,” Dr. Rettew said. “It looked like there were some baseline differences that could be traced back maybe years before the study even started.”
People hear “divergent brain development” associated with social media and naturally get alarmed, he acknowledged.
“I get that, but the study isn’t really equipped to tell us what should be happening in the brain and what changes may have implications for other parts of an adolescent’s life,” Dr. Rettew said, “In the end, what we have is an association between heavy social media use and certain brain activation patterns which is cool to see and measure.”
He agrees with the authors, however, that overuse of social media is concerning and studying its effects is important.
Seventy-eight percent of early adolescents check every hour
According to the paper, 78% of 13- to 17-year-olds report checking their devices at least every hour and 46% check “almost constantly.”
“Regardless of which brain regions light up when looking at various emoji responses to their Instagram post, I think it is valid already to have some concerns about youth who can’t stay off their phone for more than 10 minutes,” Dr. Rettew said. “Technology is here to stay, but how we can learn to use it rather than have it use us is probably the more pressing question at this point.”
One coauthor reports grants from the National Institute on Drug Abuse (NIDA) during the conduct of the study and grants from NIDA and the National Science Foundation outside the submitted work; a coauthor reports grants from the Winston Family Foundation; and a coauthor reports a grant from NIDA and funds from the Winston Family Foundation – both during the conduct of the study. No other disclosures were reported. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
Teens who compulsively checked social media networks showed different development patterns in parts of the brain that involve reward and punishment than did those who didn’t check their platforms as often, new research suggests.
Results were published online in JAMA Pediatrics.
Researchers, led by Maria T. Maza, of the department of psychology and neuroscience at University of North Carolina at Chapel Hill, included 169 6th- and 7th-grade students recruited from three public middle schools in rural North Carolina in a 3-year longitudinal cohort.
Participants reported how frequently they checked Facebook, Instagram, and Snapchat. Answers were grouped into eight score groups depending on their per-day check times: less than 1; 1; 2-3; 4-5; 6-10; 11-15; 16-20; or more than 20 times. Those groups were then broken into three categories: low (nonhabitual); moderate; and high (habitual).
Imaging shows reactions
Researchers used functional magnetic resonance imaging (fMRI) to see how different areas of the brain react when participants looked at a series of indicators, such as happy and angry faces, which mimic social media rewards, punishments, or neutral feedback.
The research team focused on adolescents, for whom social media participation and neural sensitivity to social feedback from peers are high.
They found that participants who frequently checked social media showed distinct brain patterns when anticipating social feedback compared with those who had moderate or low use, “suggesting that habitual social media checking early in adolescence is associated with divergent brain development over time.”
The affected regions of the brain included the networks that respond to motivation and cognitive control.
However, the study was not able to determine whether the differences are a good or bad thing.
“While for some individuals with habitual checking behaviors, an initial hyposensitivity to potential social rewards and punishments followed by hypersensitivity may contribute to checking behaviors on social media becoming compulsive and problematic, for others, this change in sensitivity may reflect an adaptive behavior that allows them to better navigate their increasingly digital environment,” the authors wrote.
Chicken-and-egg questions
David Rettew, MD, a child and adolescent psychiatrist at the Oregon Health & Science University in Portland, who was not part of this research, said in an interview that it’s not clear from this study which came first – different brain development in the teens prior to this study that caused compulsive checking, or checking behaviors that caused different brain development. The authors acknowledge this is a limitation of the study.
“Hopefully, someday researchers will look at some of these brain activation patterns before kids have been exposed to social media to help us sort some of these questions out,” Dr. Rettew said.
“It wasn’t as though the groups looked the same at baseline and then diverged as they used more and more social media,” Dr. Rettew said. “It looked like there were some baseline differences that could be traced back maybe years before the study even started.”
People hear “divergent brain development” associated with social media and naturally get alarmed, he acknowledged.
“I get that, but the study isn’t really equipped to tell us what should be happening in the brain and what changes may have implications for other parts of an adolescent’s life,” Dr. Rettew said, “In the end, what we have is an association between heavy social media use and certain brain activation patterns which is cool to see and measure.”
He agrees with the authors, however, that overuse of social media is concerning and studying its effects is important.
Seventy-eight percent of early adolescents check every hour
According to the paper, 78% of 13- to 17-year-olds report checking their devices at least every hour and 46% check “almost constantly.”
“Regardless of which brain regions light up when looking at various emoji responses to their Instagram post, I think it is valid already to have some concerns about youth who can’t stay off their phone for more than 10 minutes,” Dr. Rettew said. “Technology is here to stay, but how we can learn to use it rather than have it use us is probably the more pressing question at this point.”
One coauthor reports grants from the National Institute on Drug Abuse (NIDA) during the conduct of the study and grants from NIDA and the National Science Foundation outside the submitted work; a coauthor reports grants from the Winston Family Foundation; and a coauthor reports a grant from NIDA and funds from the Winston Family Foundation – both during the conduct of the study. No other disclosures were reported. Dr. Rettew is author of the book, “Parenting Made Complicated: What Science Really Knows about the Greatest Debates of Early Childhood.”
FROM JAMA PEDIATRICS
Science reveals link between gut health and exercise motivation
Researchers at the University of Pennsylvania, Philadelphia recently explored this topic when they wanted to find out why some lab mice seem to love their exercise wheel, while others mostly ignore it.
To start, the researchers used a machine-learning algorithm to look for biological traits that could explain the differences in activity levels among mice. And what they found surprised them: Genetics seemed to have little to do with it, but differences in gut bacteria appeared to matter more. A handful of studies backed that up: Thriving gut microbiomes have been linked with optimal muscle function in mice.
Sure enough, when the researchers dosed mice with broad-spectrum antibiotics, killing off their gut bacteria, the distance the rodents were able to run dropped by half. But off the antibiotics, the mice mostly regained their previous performance levels.
The findings, published in the journal Nature, suggest that the gut microbiome may help regulate the desire to exercise.
If confirmed in humans, this hypothesis could help explain why so many Americans fail to get the recommended amount of physical activity. Some may blame lack of time, energy, or interest. But perhaps the reason could come down to the trillions of microbes living in their gut.
This line of research could also lead to microbiome-based ways to get sedentary people off the couch or optimize athletic performance.
But how could one’s microbiome impact the motivation to move? To find the answer, the researchers zeroed in on the brain.
The gut-brain connection
After treating the mice with antibiotics, the researchers sequenced RNA in the rodents’ striatum (the part of the brain responsible for motivation). They found reduced gene expression in the cells’ dopamine receptors – which release the neurochemical dopamine, making one feel like they’ve accomplished something good. In other words: Mice treated with antibiotics were getting less of a dopamine hit after their run.
“Only when we started focusing on the brain did we understand that the microbiome’s effect on exercise capacity was mediated by the central and peripheral nervous systems,” said study author Christoph Thaiss, PhD, a microbiologist at the University of Pennsylvania. “This realization completely changed the trajectory of the project.”
To find out how, exactly, bacteria in the colon were signaling the brain, the researchers performed a series of experiments over several years. They identified two types of bacteria, Eubacterium rectale and Coprococcus eutactus. These strains produce compounds called fatty acid amides that interact with endocannabinoid receptors in the gut.
Those endocannabinoid receptors signal the brain to cut back its production of monoamine oxidase, the compound that breaks down dopamine. With less of this dopamine-clearing compound in the brain, more dopamine could build up after a long run, making the mice feel good and eager to hit the exercise wheel again soon.
This gut-brain pathway “may have evolved to couple the initiation of prolonged physical activity to the nutritional status of the gastrointestinal tract,” Dr. Thaiss said. Gut bacteria monitor what’s in your colon and tell your brain whether you have enough food to fuel a workout.
The colon, or gut, hosts trillions of microbes with potentially hundreds of different bacteria strains. These strains are determined by the food we eat and the environment we occupy.
“The genetic impact on the microbiome is rather minor,” Dr. Thaiss said, “but lifestyle factors strongly impact the composition of the gut microbiome.”
He hopes to develop nutritional interventions to encourage the growth of the motivating types of bacteria, the kind that make a person want to go for a 5-mile run.
What’s next?
Moving forward, the researchers need to find out whether the gut affects motivation in humans, too. To do that, they’re analyzing the gut microbiomes of people with varying levels of exercise motivation.
“With enough samples, we could potentially correlate species of microbiota that exist in exercise-motivated individuals,” said study coauthor Nicholas Betley, PhD, a biologist at the University of Pennsylvania.
Variations in the gut microbiome could help explain the “runner’s high” that some people have in a long-distance race. The research could also help promote weight training or sports participation.
“Imagine if a sports team could optimally motivate the athletes on the team to exercise,” said Dr. Betley. The lab is investigating the microbiome’s impact on high-intensity interval training.
Signals from the gut to the brain could be affecting body processes in other ways too, the researchers speculated.
“There are so many possibilities for how these signals may change physiology and impact health,” Dr. Betley said. “A new set of studies may well establish a whole new branch of exercise physiology.”
A version of this article first appeared on WebMD.com.
Researchers at the University of Pennsylvania, Philadelphia recently explored this topic when they wanted to find out why some lab mice seem to love their exercise wheel, while others mostly ignore it.
To start, the researchers used a machine-learning algorithm to look for biological traits that could explain the differences in activity levels among mice. And what they found surprised them: Genetics seemed to have little to do with it, but differences in gut bacteria appeared to matter more. A handful of studies backed that up: Thriving gut microbiomes have been linked with optimal muscle function in mice.
Sure enough, when the researchers dosed mice with broad-spectrum antibiotics, killing off their gut bacteria, the distance the rodents were able to run dropped by half. But off the antibiotics, the mice mostly regained their previous performance levels.
The findings, published in the journal Nature, suggest that the gut microbiome may help regulate the desire to exercise.
If confirmed in humans, this hypothesis could help explain why so many Americans fail to get the recommended amount of physical activity. Some may blame lack of time, energy, or interest. But perhaps the reason could come down to the trillions of microbes living in their gut.
This line of research could also lead to microbiome-based ways to get sedentary people off the couch or optimize athletic performance.
But how could one’s microbiome impact the motivation to move? To find the answer, the researchers zeroed in on the brain.
The gut-brain connection
After treating the mice with antibiotics, the researchers sequenced RNA in the rodents’ striatum (the part of the brain responsible for motivation). They found reduced gene expression in the cells’ dopamine receptors – which release the neurochemical dopamine, making one feel like they’ve accomplished something good. In other words: Mice treated with antibiotics were getting less of a dopamine hit after their run.
“Only when we started focusing on the brain did we understand that the microbiome’s effect on exercise capacity was mediated by the central and peripheral nervous systems,” said study author Christoph Thaiss, PhD, a microbiologist at the University of Pennsylvania. “This realization completely changed the trajectory of the project.”
To find out how, exactly, bacteria in the colon were signaling the brain, the researchers performed a series of experiments over several years. They identified two types of bacteria, Eubacterium rectale and Coprococcus eutactus. These strains produce compounds called fatty acid amides that interact with endocannabinoid receptors in the gut.
Those endocannabinoid receptors signal the brain to cut back its production of monoamine oxidase, the compound that breaks down dopamine. With less of this dopamine-clearing compound in the brain, more dopamine could build up after a long run, making the mice feel good and eager to hit the exercise wheel again soon.
This gut-brain pathway “may have evolved to couple the initiation of prolonged physical activity to the nutritional status of the gastrointestinal tract,” Dr. Thaiss said. Gut bacteria monitor what’s in your colon and tell your brain whether you have enough food to fuel a workout.
The colon, or gut, hosts trillions of microbes with potentially hundreds of different bacteria strains. These strains are determined by the food we eat and the environment we occupy.
“The genetic impact on the microbiome is rather minor,” Dr. Thaiss said, “but lifestyle factors strongly impact the composition of the gut microbiome.”
He hopes to develop nutritional interventions to encourage the growth of the motivating types of bacteria, the kind that make a person want to go for a 5-mile run.
What’s next?
Moving forward, the researchers need to find out whether the gut affects motivation in humans, too. To do that, they’re analyzing the gut microbiomes of people with varying levels of exercise motivation.
“With enough samples, we could potentially correlate species of microbiota that exist in exercise-motivated individuals,” said study coauthor Nicholas Betley, PhD, a biologist at the University of Pennsylvania.
Variations in the gut microbiome could help explain the “runner’s high” that some people have in a long-distance race. The research could also help promote weight training or sports participation.
“Imagine if a sports team could optimally motivate the athletes on the team to exercise,” said Dr. Betley. The lab is investigating the microbiome’s impact on high-intensity interval training.
Signals from the gut to the brain could be affecting body processes in other ways too, the researchers speculated.
“There are so many possibilities for how these signals may change physiology and impact health,” Dr. Betley said. “A new set of studies may well establish a whole new branch of exercise physiology.”
A version of this article first appeared on WebMD.com.
Researchers at the University of Pennsylvania, Philadelphia recently explored this topic when they wanted to find out why some lab mice seem to love their exercise wheel, while others mostly ignore it.
To start, the researchers used a machine-learning algorithm to look for biological traits that could explain the differences in activity levels among mice. And what they found surprised them: Genetics seemed to have little to do with it, but differences in gut bacteria appeared to matter more. A handful of studies backed that up: Thriving gut microbiomes have been linked with optimal muscle function in mice.
Sure enough, when the researchers dosed mice with broad-spectrum antibiotics, killing off their gut bacteria, the distance the rodents were able to run dropped by half. But off the antibiotics, the mice mostly regained their previous performance levels.
The findings, published in the journal Nature, suggest that the gut microbiome may help regulate the desire to exercise.
If confirmed in humans, this hypothesis could help explain why so many Americans fail to get the recommended amount of physical activity. Some may blame lack of time, energy, or interest. But perhaps the reason could come down to the trillions of microbes living in their gut.
This line of research could also lead to microbiome-based ways to get sedentary people off the couch or optimize athletic performance.
But how could one’s microbiome impact the motivation to move? To find the answer, the researchers zeroed in on the brain.
The gut-brain connection
After treating the mice with antibiotics, the researchers sequenced RNA in the rodents’ striatum (the part of the brain responsible for motivation). They found reduced gene expression in the cells’ dopamine receptors – which release the neurochemical dopamine, making one feel like they’ve accomplished something good. In other words: Mice treated with antibiotics were getting less of a dopamine hit after their run.
“Only when we started focusing on the brain did we understand that the microbiome’s effect on exercise capacity was mediated by the central and peripheral nervous systems,” said study author Christoph Thaiss, PhD, a microbiologist at the University of Pennsylvania. “This realization completely changed the trajectory of the project.”
To find out how, exactly, bacteria in the colon were signaling the brain, the researchers performed a series of experiments over several years. They identified two types of bacteria, Eubacterium rectale and Coprococcus eutactus. These strains produce compounds called fatty acid amides that interact with endocannabinoid receptors in the gut.
Those endocannabinoid receptors signal the brain to cut back its production of monoamine oxidase, the compound that breaks down dopamine. With less of this dopamine-clearing compound in the brain, more dopamine could build up after a long run, making the mice feel good and eager to hit the exercise wheel again soon.
This gut-brain pathway “may have evolved to couple the initiation of prolonged physical activity to the nutritional status of the gastrointestinal tract,” Dr. Thaiss said. Gut bacteria monitor what’s in your colon and tell your brain whether you have enough food to fuel a workout.
The colon, or gut, hosts trillions of microbes with potentially hundreds of different bacteria strains. These strains are determined by the food we eat and the environment we occupy.
“The genetic impact on the microbiome is rather minor,” Dr. Thaiss said, “but lifestyle factors strongly impact the composition of the gut microbiome.”
He hopes to develop nutritional interventions to encourage the growth of the motivating types of bacteria, the kind that make a person want to go for a 5-mile run.
What’s next?
Moving forward, the researchers need to find out whether the gut affects motivation in humans, too. To do that, they’re analyzing the gut microbiomes of people with varying levels of exercise motivation.
“With enough samples, we could potentially correlate species of microbiota that exist in exercise-motivated individuals,” said study coauthor Nicholas Betley, PhD, a biologist at the University of Pennsylvania.
Variations in the gut microbiome could help explain the “runner’s high” that some people have in a long-distance race. The research could also help promote weight training or sports participation.
“Imagine if a sports team could optimally motivate the athletes on the team to exercise,” said Dr. Betley. The lab is investigating the microbiome’s impact on high-intensity interval training.
Signals from the gut to the brain could be affecting body processes in other ways too, the researchers speculated.
“There are so many possibilities for how these signals may change physiology and impact health,” Dr. Betley said. “A new set of studies may well establish a whole new branch of exercise physiology.”
A version of this article first appeared on WebMD.com.
FROM NATURE
Why I decided to get an MBA after becoming a private practice gastroenterologist
It was my dream from an early age to become a physician. Even as a child I was fascinated by medical procedures and interventions. As I pursued my medical degree, I became increasingly interested in a career where I could integrate patient care and the latest innovations in technology.
Training in gastroenterology has provided me an exciting mix of patient care and procedures, with medical devices and technologies that are constantly evolving. As I began my career, I joined Dayton Gastroenterology, a private practice affiliated with GI fellowship at Wright State University, Fairborn, Ohio, because the practice provided an opportunity to care for patients, train GI fellows, and provide employment opportunities to the community I serve.
After spending so many years to become an expert in medicine and then training in gastroenterology, it might have seemed daunting to go back to school to get an education in another field. But we all know the medical environment is constantly changing – in the last decade dramatically so, in technology as well as in how groups are organizing themselves in response to health care consolidation and other external forces.
The importance of understanding the business of health care
Consolidation in health care has increasingly impacted private practices, with more primary care and specialty physicians being employed by hospitals. In some areas of the country, this has affected the flow of patient referrals to independent GI practices, and these practices must now adapt to continue serving their communities. This is being amplified by the increasing demands for patient services coupled with staffing issues and reimbursement cuts.
These challenges have resulted in some smaller practices joining local hospitals systems. Others have come together to form larger groups or managed services organizations (MSO), and some have partnered with private equity firms to compete in response to these market forces.
During our training and education in medical school, we aren’t taught how to run a successful practice. We aren’t taught how to bring together different industry partners, collaborators, and payers or how to build patient-centric practice models. But sometimes the best method of learning is by doing, and my experiences during the merger of Dayton Gastroenterology with One GI, a physician-focused MSO with practices in six states, was invaluable.
That merger process taught me a lot about how companies are valued, the nuances in determining deal flow, networking, human capital, and everything else involved in how a transaction takes place. I developed a greater understanding about how to develop and build successful large practices, with improved employee satisfaction, company culture, and great patient experience.
Developing a positive practice culture
It was during the process of partnering with One GI and during the pandemic that I decided to pursue my desire to get a formal business education, and I’m glad I did. The executive MBA program at the Kellogg School of Management at Northwestern University allowed me to gain an in-depth understanding of various aspects of business, finance, accounting, marketing, leadership, governance, organizational transformation, negotiations, and so much more, all while continuing to work full time as a gastroenterologist in private practice.
We met for classes in-person each month over the course of four days. There were also live and recorded virtual sessions in between each monthly class. The program was rigorous, but worth it. Connecting with leaders from different industries and learning from exceptional professors alongside these professionals was an invaluable experience.
Two of the most vital things I learned were the importance of team building and development of a company culture that will sustain an organization over the long term. I learned management strategies to empower employees, governance best practices, and how to align the interests of internal and external stakeholders.
Anyone can buy a practice, and anyone can merge their practice into a larger entity, but it is critical to understand the components of a successful integration. Culture eats strategy for breakfast. You can have the best minds, develop the best processes, but if there is not a strong culture with the alignment of physicians, staff, and practice management, even the best strategies can easily fail.
What to look for in joining a practice
As physicians, we all want to be the best at what we do. It’s important to be intentional about what you value and how you would like to shape your career. When considering which practice you might join, there are several things to consider, such as the location, medical needs of the community, and services offered by the practice. Equally important is understanding how the practice is managed.
Does the practice promote growth opportunities for its physicians and staff? Are there governance structures and processes in place to empower and retain talented staff? What values does the practice portray? Is there a buy-in or buy-out when becoming a partner in the practice, and are there equity opportunities? These are just some of many questions early-career physicians should ask.
My MBA helped me become a better leader
A physician understands the needs of delivering exceptional medical care, the challenges involved, and the resources required. Increasing the depth and breadth of our knowledge is power. Good people make good organizations, but great people make great organizations. Those of us who are on the front lines are the best advocates for our patients and other frontline workers. We can become powerful advocates and leaders when we better understand how business trends and other external forces affect our ability to care for the patients in the future.
Pursuing a business education provides a strong foundation for physician leaders who have strong analytical intuition and focus on patient-centric practice models. If you are considering a career in private practice and are interested in practice management or growing a practice, an MBA or similar educational programs will provide an understanding of finance, accounting, and other business-related fields that can enable physicians to become agile and empathic leaders.
Dr. Appalaneni is a practicing gastroenterologist at Dayton Gastroenterology in Ohio. She is Executive Vice President of Clinical Innovation at One GI, a physician-led managed services organization. Dr. Appalaneni has no conflicts to declare.
It was my dream from an early age to become a physician. Even as a child I was fascinated by medical procedures and interventions. As I pursued my medical degree, I became increasingly interested in a career where I could integrate patient care and the latest innovations in technology.
Training in gastroenterology has provided me an exciting mix of patient care and procedures, with medical devices and technologies that are constantly evolving. As I began my career, I joined Dayton Gastroenterology, a private practice affiliated with GI fellowship at Wright State University, Fairborn, Ohio, because the practice provided an opportunity to care for patients, train GI fellows, and provide employment opportunities to the community I serve.
After spending so many years to become an expert in medicine and then training in gastroenterology, it might have seemed daunting to go back to school to get an education in another field. But we all know the medical environment is constantly changing – in the last decade dramatically so, in technology as well as in how groups are organizing themselves in response to health care consolidation and other external forces.
The importance of understanding the business of health care
Consolidation in health care has increasingly impacted private practices, with more primary care and specialty physicians being employed by hospitals. In some areas of the country, this has affected the flow of patient referrals to independent GI practices, and these practices must now adapt to continue serving their communities. This is being amplified by the increasing demands for patient services coupled with staffing issues and reimbursement cuts.
These challenges have resulted in some smaller practices joining local hospitals systems. Others have come together to form larger groups or managed services organizations (MSO), and some have partnered with private equity firms to compete in response to these market forces.
During our training and education in medical school, we aren’t taught how to run a successful practice. We aren’t taught how to bring together different industry partners, collaborators, and payers or how to build patient-centric practice models. But sometimes the best method of learning is by doing, and my experiences during the merger of Dayton Gastroenterology with One GI, a physician-focused MSO with practices in six states, was invaluable.
That merger process taught me a lot about how companies are valued, the nuances in determining deal flow, networking, human capital, and everything else involved in how a transaction takes place. I developed a greater understanding about how to develop and build successful large practices, with improved employee satisfaction, company culture, and great patient experience.
Developing a positive practice culture
It was during the process of partnering with One GI and during the pandemic that I decided to pursue my desire to get a formal business education, and I’m glad I did. The executive MBA program at the Kellogg School of Management at Northwestern University allowed me to gain an in-depth understanding of various aspects of business, finance, accounting, marketing, leadership, governance, organizational transformation, negotiations, and so much more, all while continuing to work full time as a gastroenterologist in private practice.
We met for classes in-person each month over the course of four days. There were also live and recorded virtual sessions in between each monthly class. The program was rigorous, but worth it. Connecting with leaders from different industries and learning from exceptional professors alongside these professionals was an invaluable experience.
Two of the most vital things I learned were the importance of team building and development of a company culture that will sustain an organization over the long term. I learned management strategies to empower employees, governance best practices, and how to align the interests of internal and external stakeholders.
Anyone can buy a practice, and anyone can merge their practice into a larger entity, but it is critical to understand the components of a successful integration. Culture eats strategy for breakfast. You can have the best minds, develop the best processes, but if there is not a strong culture with the alignment of physicians, staff, and practice management, even the best strategies can easily fail.
What to look for in joining a practice
As physicians, we all want to be the best at what we do. It’s important to be intentional about what you value and how you would like to shape your career. When considering which practice you might join, there are several things to consider, such as the location, medical needs of the community, and services offered by the practice. Equally important is understanding how the practice is managed.
Does the practice promote growth opportunities for its physicians and staff? Are there governance structures and processes in place to empower and retain talented staff? What values does the practice portray? Is there a buy-in or buy-out when becoming a partner in the practice, and are there equity opportunities? These are just some of many questions early-career physicians should ask.
My MBA helped me become a better leader
A physician understands the needs of delivering exceptional medical care, the challenges involved, and the resources required. Increasing the depth and breadth of our knowledge is power. Good people make good organizations, but great people make great organizations. Those of us who are on the front lines are the best advocates for our patients and other frontline workers. We can become powerful advocates and leaders when we better understand how business trends and other external forces affect our ability to care for the patients in the future.
Pursuing a business education provides a strong foundation for physician leaders who have strong analytical intuition and focus on patient-centric practice models. If you are considering a career in private practice and are interested in practice management or growing a practice, an MBA or similar educational programs will provide an understanding of finance, accounting, and other business-related fields that can enable physicians to become agile and empathic leaders.
Dr. Appalaneni is a practicing gastroenterologist at Dayton Gastroenterology in Ohio. She is Executive Vice President of Clinical Innovation at One GI, a physician-led managed services organization. Dr. Appalaneni has no conflicts to declare.
It was my dream from an early age to become a physician. Even as a child I was fascinated by medical procedures and interventions. As I pursued my medical degree, I became increasingly interested in a career where I could integrate patient care and the latest innovations in technology.
Training in gastroenterology has provided me an exciting mix of patient care and procedures, with medical devices and technologies that are constantly evolving. As I began my career, I joined Dayton Gastroenterology, a private practice affiliated with GI fellowship at Wright State University, Fairborn, Ohio, because the practice provided an opportunity to care for patients, train GI fellows, and provide employment opportunities to the community I serve.
After spending so many years to become an expert in medicine and then training in gastroenterology, it might have seemed daunting to go back to school to get an education in another field. But we all know the medical environment is constantly changing – in the last decade dramatically so, in technology as well as in how groups are organizing themselves in response to health care consolidation and other external forces.
The importance of understanding the business of health care
Consolidation in health care has increasingly impacted private practices, with more primary care and specialty physicians being employed by hospitals. In some areas of the country, this has affected the flow of patient referrals to independent GI practices, and these practices must now adapt to continue serving their communities. This is being amplified by the increasing demands for patient services coupled with staffing issues and reimbursement cuts.
These challenges have resulted in some smaller practices joining local hospitals systems. Others have come together to form larger groups or managed services organizations (MSO), and some have partnered with private equity firms to compete in response to these market forces.
During our training and education in medical school, we aren’t taught how to run a successful practice. We aren’t taught how to bring together different industry partners, collaborators, and payers or how to build patient-centric practice models. But sometimes the best method of learning is by doing, and my experiences during the merger of Dayton Gastroenterology with One GI, a physician-focused MSO with practices in six states, was invaluable.
That merger process taught me a lot about how companies are valued, the nuances in determining deal flow, networking, human capital, and everything else involved in how a transaction takes place. I developed a greater understanding about how to develop and build successful large practices, with improved employee satisfaction, company culture, and great patient experience.
Developing a positive practice culture
It was during the process of partnering with One GI and during the pandemic that I decided to pursue my desire to get a formal business education, and I’m glad I did. The executive MBA program at the Kellogg School of Management at Northwestern University allowed me to gain an in-depth understanding of various aspects of business, finance, accounting, marketing, leadership, governance, organizational transformation, negotiations, and so much more, all while continuing to work full time as a gastroenterologist in private practice.
We met for classes in-person each month over the course of four days. There were also live and recorded virtual sessions in between each monthly class. The program was rigorous, but worth it. Connecting with leaders from different industries and learning from exceptional professors alongside these professionals was an invaluable experience.
Two of the most vital things I learned were the importance of team building and development of a company culture that will sustain an organization over the long term. I learned management strategies to empower employees, governance best practices, and how to align the interests of internal and external stakeholders.
Anyone can buy a practice, and anyone can merge their practice into a larger entity, but it is critical to understand the components of a successful integration. Culture eats strategy for breakfast. You can have the best minds, develop the best processes, but if there is not a strong culture with the alignment of physicians, staff, and practice management, even the best strategies can easily fail.
What to look for in joining a practice
As physicians, we all want to be the best at what we do. It’s important to be intentional about what you value and how you would like to shape your career. When considering which practice you might join, there are several things to consider, such as the location, medical needs of the community, and services offered by the practice. Equally important is understanding how the practice is managed.
Does the practice promote growth opportunities for its physicians and staff? Are there governance structures and processes in place to empower and retain talented staff? What values does the practice portray? Is there a buy-in or buy-out when becoming a partner in the practice, and are there equity opportunities? These are just some of many questions early-career physicians should ask.
My MBA helped me become a better leader
A physician understands the needs of delivering exceptional medical care, the challenges involved, and the resources required. Increasing the depth and breadth of our knowledge is power. Good people make good organizations, but great people make great organizations. Those of us who are on the front lines are the best advocates for our patients and other frontline workers. We can become powerful advocates and leaders when we better understand how business trends and other external forces affect our ability to care for the patients in the future.
Pursuing a business education provides a strong foundation for physician leaders who have strong analytical intuition and focus on patient-centric practice models. If you are considering a career in private practice and are interested in practice management or growing a practice, an MBA or similar educational programs will provide an understanding of finance, accounting, and other business-related fields that can enable physicians to become agile and empathic leaders.
Dr. Appalaneni is a practicing gastroenterologist at Dayton Gastroenterology in Ohio. She is Executive Vice President of Clinical Innovation at One GI, a physician-led managed services organization. Dr. Appalaneni has no conflicts to declare.
Lifestyle changes may reduce colorectal cancer risk
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.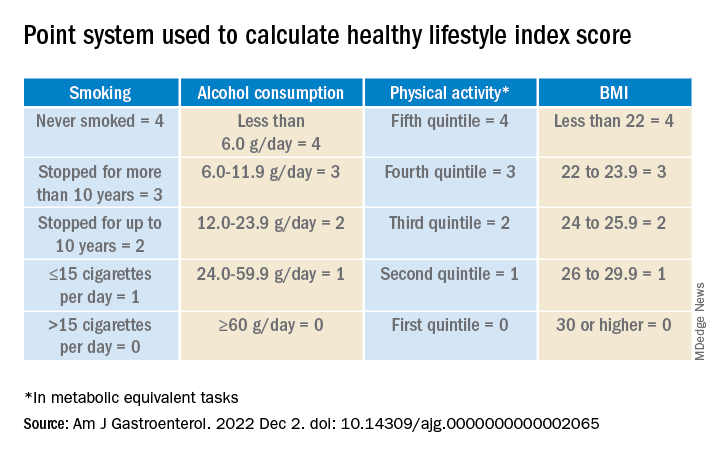
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Changes regarding smoking, drinking, body weight, and physical activity may alter the risk for colorectal cancer (CRC), the results of a study on a large European cohort suggest.
“This is a clear message that practicing clinicians and gastroenterologists could give to their patients and to CRC screening participants to improve CRC prevention,” write Edoardo Botteri, PhD, Cancer Registry of Norway, Oslo, and colleagues in an article published in the American Journal of Gastroenterology.
Previous studies have shown a correlation between cancer in general and unhealthy lifestyle factors. They have also shown an association between weight gain and an increased risk for CRC and a reduced risk with smoking cessation. But Dr. Botteri and colleagues could not find any published research on the association of other lifestyle factors and the risk for CRC specifically, they write.
To help fill this gap, they followed 295,865 people who participated in the European Prospective Investigation into Cancer (EPIC) for a median of 7.8 years. The participants were mostly aged from 35 to 70 years and lived in Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom.
The researchers calculated a healthy lifestyle index (HLI) score on the basis of smoking status, alcohol consumption, body mass index, and physical activity. The median time between baseline and the follow-up questionnaire was 5.7 years.
They awarded points as indicated in the following table.
Participants’ scores ranged from 0 to 16. At baseline, the mean HLI score was 10.04. It dipped slightly to 9.95 at follow-up.
Men had more favorable changes than women, and the associations between the HLI score and CRC risk were only statistically significant among men.
Overall, a 1-unit increase in the HLI score was associated with a 3% lower risk for CRC.
When the HLI scores were grouped into tertiles, improvements from an “unfavorable lifestyle” (0-9) to a “favorable lifestyle” (12-16) were associated with a 23% lower risk for CRC (compared with no change). Likewise, a decline from a “favorable lifestyle” to an “unfavorable lifestyle” was associated with a 34% higher risk.
Changes in the BMI score from baseline showed a trend toward an association with CRC risk.
Decreases in alcohol consumption were significantly associated with a reduction in CRC risk among participants aged 55 years or younger at baseline.
Increases in physical activity were significantly associated with a lower risk for proximal colon cancer, especially in younger participants.
On the other hand, reductions in smoking were associated with an increase in CRC risk. This correlation might be the result of “inverse causation,” the researchers note; that is, people may have quit smoking because they experienced early symptoms of CRC. Smoking had only a marginal influence on the HLI calculations in this study because only a small proportion of participants changed their smoking rates.
Information on diet was collected only at baseline, so changes in this factor could not be measured. The researchers adjusted their analysis for diet at baseline, but they acknowledge that their inability to incorporate diet into the HLI score was a limitation of the study.
Similarly, they used education as a marker of socioeconomic status but acknowledge that this is only a proxy.
“The HLI score may therefore not accurately capture the complex relationship between lifestyle habits and risk for CRC,” they write.
Still, if the results of this observational study are confirmed by other research, the findings could provide evidence to design intervention studies to prevent CRC, they conclude.
The study was supported by the grant LIBERTY from the French Institut National du Cancer. Financial supporters of the national cohorts and the coordination of EPIC are listed in the published study. The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Emergency physicians take issue with AHRQ errors report
The AHRQ review, issued on Dec. 15, 2022, stated that the findings of their study translate “to about 1 in 18 emergency department patients receiving an incorrect diagnosis, 1 in 50 suffering an adverse event, and 1 in 350 suffering permanent disability or death.” The authors describe these rates as similar to those seen in primary care and inpatient hospital settings.
The review was conducted through an Evidence-Based Practice Center as part of AHRQ’s Effective Health Care Program. The authors included data from 279 studies in the review. They identified the five most frequently misdiagnosed conditions in the ED as stroke, MI, aortic aneurysm and dissection, spinal cord compression and injury, and venous thromboembolism.
The authors noted that, given an estimated 130 million ED visits in the United States each year, the overall rate of incorrect diagnoses in the ED is approximately 5.7% and that 2.0% of the patients whose conditions were misdiagnosed suffer an adverse event as a result. On a local level, the authors estimate that an average ED with approximately 25,000 visits per year could experience 1,400 diagnostic errors, 500 diagnostic adverse events, and 75 serious harms, including 50 deaths. However, the authors noted that the overall error and harm rates were based on three studies from outside the United States (Canada, Spain, and Switzerland) and that only two of these were used to estimate harms.
“It’s imperative that we, as emergency physicians, inform the public that the AHRQ report used flawed methodology and statistics that extrapolated – and therefore overstated – the potential for harm when receiving care in US emergency departments,” Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital at Northwell Health and an assistant professor at Hofstra University, Hempstead, N.Y., said in an interview.
Emergency medicine organizations express concerns for accuracy
The American College of Emergency Physicians and eight other medical organizations representing emergency medicine in the United States sent a letter to the AHRQ on Dec. 14, 2022, spelling out their concerns. The review was conducted as part of the AHRQ’s ongoing Effective Health Care Program, and the organizations had the opportunity to review a draft before it was published. On reading the review, they asked that the publication of the review be delayed. “After reviewing the executive summary and initial draft, we believe that the report makes misleading, incomplete, and erroneous conclusions from the literature reviewed and conveys a tone that inaccurately characterizes and unnecessarily disparages the practice of emergency medicine in the United States,” the organizations wrote in their letter.
The concerns of the emergency medicine organizations fell into four categories: misrepresentation of the practice and nature of emergency medicine; applicability of references cited; inaccurate interpretation of malpractice data; and the reporting of a single overall diagnostic error rate of 5.7% in EDs.
The practice of emergency medicine is variable and unique among specialties in that the focus is less about the final diagnosis and more about immediate identification and treatment of life-threatening conditions, according to the letter.
Notably, many of the studies cited did not mention whether the patient’s final diagnosis was apparent on admission to the ED. “Without this knowledge, it is completely inappropriate to label such discrepancies as ‘ED diagnostic error,’ ” the organizations wrote.
All medical specialties have room for improvement, but the current AHRQ review appears not to identify these opportunities, and instead of contributing to a discussion of improving patient care in the ED, it may cause harm by presenting misinformation, they said.
Misleading and inadequate evidence
“I strongly agree with the concerns mentioned from ACEP and other key organizations about the problems and conclusions reached in the AHRQ report,” Dr. Glatter said in an interview.
“The methodology used to arrive at the conclusions [in the review] was flawed and does not provide an accurate estimate of diagnostic error and, consequently, misdiagnosis and deaths occurring in emergency departments in the U.S.,” he said. “The startling headline that 250,000 people die annually in U.S. EDs was extrapolated from a single study based on one death that occurred in a Canadian ED in 2004,” Dr. Glatter noted. “Clearly, this is not only poor methodology but flawed science.”
The AHRQ report misused one death from this single study to estimate the death rate across the United States, Dr. Glatter explained, and this overestimate improperly inflated and magnified the number of potential patients that may have been harmed by physician error.
“This flawed evidence would actually place ED misdiagnoses in the top five causes of death in the United States, with 1 in every 500 ED patients dying as a result of an error by a physician. Simply put, there is just no evidence to support such a claim,” said Dr. Glatter.
The repercussions of the AHRQ review could be harmful to patients by instilling fear and doubt about the ability of emergency physicians to diagnose those who present with life-threatening conditions, Dr. Glatter said.
“This more balanced and accurate picture of the role of emergency physicians in diagnosing and managing such emergencies needs to be communicated to the public in order to reassure and instill confidence in our role in the sequence of emergency care in relation to continuity of care in patients presenting to the ED,” he said.
“While our primary role as emergency medicine physicians is to stabilize and evaluate patients, arriving at a particular diagnosis is not always possible for some conditions,” and additional diagnostic testing is often needed to identify more specific causes of symptoms, Dr. Glatter added.
Additional research is needed for a more accurate representation of diagnostic errors in the ED, said Dr. Glatter. New prospective studies are needed to address outcomes in U.S. EDs that account for the latest advances and diagnostic modalities in emergency medicine, “particularly advances in bedside ultrasound that can expedite critical decision-making, which can be lifesaving.
“The AHRQ report is simply not an accurate reflection of the technology and skill set that current emergency medicine practice offers our patients in 2023.”
Dr. Glatter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The AHRQ review, issued on Dec. 15, 2022, stated that the findings of their study translate “to about 1 in 18 emergency department patients receiving an incorrect diagnosis, 1 in 50 suffering an adverse event, and 1 in 350 suffering permanent disability or death.” The authors describe these rates as similar to those seen in primary care and inpatient hospital settings.
The review was conducted through an Evidence-Based Practice Center as part of AHRQ’s Effective Health Care Program. The authors included data from 279 studies in the review. They identified the five most frequently misdiagnosed conditions in the ED as stroke, MI, aortic aneurysm and dissection, spinal cord compression and injury, and venous thromboembolism.
The authors noted that, given an estimated 130 million ED visits in the United States each year, the overall rate of incorrect diagnoses in the ED is approximately 5.7% and that 2.0% of the patients whose conditions were misdiagnosed suffer an adverse event as a result. On a local level, the authors estimate that an average ED with approximately 25,000 visits per year could experience 1,400 diagnostic errors, 500 diagnostic adverse events, and 75 serious harms, including 50 deaths. However, the authors noted that the overall error and harm rates were based on three studies from outside the United States (Canada, Spain, and Switzerland) and that only two of these were used to estimate harms.
“It’s imperative that we, as emergency physicians, inform the public that the AHRQ report used flawed methodology and statistics that extrapolated – and therefore overstated – the potential for harm when receiving care in US emergency departments,” Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital at Northwell Health and an assistant professor at Hofstra University, Hempstead, N.Y., said in an interview.
Emergency medicine organizations express concerns for accuracy
The American College of Emergency Physicians and eight other medical organizations representing emergency medicine in the United States sent a letter to the AHRQ on Dec. 14, 2022, spelling out their concerns. The review was conducted as part of the AHRQ’s ongoing Effective Health Care Program, and the organizations had the opportunity to review a draft before it was published. On reading the review, they asked that the publication of the review be delayed. “After reviewing the executive summary and initial draft, we believe that the report makes misleading, incomplete, and erroneous conclusions from the literature reviewed and conveys a tone that inaccurately characterizes and unnecessarily disparages the practice of emergency medicine in the United States,” the organizations wrote in their letter.
The concerns of the emergency medicine organizations fell into four categories: misrepresentation of the practice and nature of emergency medicine; applicability of references cited; inaccurate interpretation of malpractice data; and the reporting of a single overall diagnostic error rate of 5.7% in EDs.
The practice of emergency medicine is variable and unique among specialties in that the focus is less about the final diagnosis and more about immediate identification and treatment of life-threatening conditions, according to the letter.
Notably, many of the studies cited did not mention whether the patient’s final diagnosis was apparent on admission to the ED. “Without this knowledge, it is completely inappropriate to label such discrepancies as ‘ED diagnostic error,’ ” the organizations wrote.
All medical specialties have room for improvement, but the current AHRQ review appears not to identify these opportunities, and instead of contributing to a discussion of improving patient care in the ED, it may cause harm by presenting misinformation, they said.
Misleading and inadequate evidence
“I strongly agree with the concerns mentioned from ACEP and other key organizations about the problems and conclusions reached in the AHRQ report,” Dr. Glatter said in an interview.
“The methodology used to arrive at the conclusions [in the review] was flawed and does not provide an accurate estimate of diagnostic error and, consequently, misdiagnosis and deaths occurring in emergency departments in the U.S.,” he said. “The startling headline that 250,000 people die annually in U.S. EDs was extrapolated from a single study based on one death that occurred in a Canadian ED in 2004,” Dr. Glatter noted. “Clearly, this is not only poor methodology but flawed science.”
The AHRQ report misused one death from this single study to estimate the death rate across the United States, Dr. Glatter explained, and this overestimate improperly inflated and magnified the number of potential patients that may have been harmed by physician error.
“This flawed evidence would actually place ED misdiagnoses in the top five causes of death in the United States, with 1 in every 500 ED patients dying as a result of an error by a physician. Simply put, there is just no evidence to support such a claim,” said Dr. Glatter.
The repercussions of the AHRQ review could be harmful to patients by instilling fear and doubt about the ability of emergency physicians to diagnose those who present with life-threatening conditions, Dr. Glatter said.
“This more balanced and accurate picture of the role of emergency physicians in diagnosing and managing such emergencies needs to be communicated to the public in order to reassure and instill confidence in our role in the sequence of emergency care in relation to continuity of care in patients presenting to the ED,” he said.
“While our primary role as emergency medicine physicians is to stabilize and evaluate patients, arriving at a particular diagnosis is not always possible for some conditions,” and additional diagnostic testing is often needed to identify more specific causes of symptoms, Dr. Glatter added.
Additional research is needed for a more accurate representation of diagnostic errors in the ED, said Dr. Glatter. New prospective studies are needed to address outcomes in U.S. EDs that account for the latest advances and diagnostic modalities in emergency medicine, “particularly advances in bedside ultrasound that can expedite critical decision-making, which can be lifesaving.
“The AHRQ report is simply not an accurate reflection of the technology and skill set that current emergency medicine practice offers our patients in 2023.”
Dr. Glatter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The AHRQ review, issued on Dec. 15, 2022, stated that the findings of their study translate “to about 1 in 18 emergency department patients receiving an incorrect diagnosis, 1 in 50 suffering an adverse event, and 1 in 350 suffering permanent disability or death.” The authors describe these rates as similar to those seen in primary care and inpatient hospital settings.
The review was conducted through an Evidence-Based Practice Center as part of AHRQ’s Effective Health Care Program. The authors included data from 279 studies in the review. They identified the five most frequently misdiagnosed conditions in the ED as stroke, MI, aortic aneurysm and dissection, spinal cord compression and injury, and venous thromboembolism.
The authors noted that, given an estimated 130 million ED visits in the United States each year, the overall rate of incorrect diagnoses in the ED is approximately 5.7% and that 2.0% of the patients whose conditions were misdiagnosed suffer an adverse event as a result. On a local level, the authors estimate that an average ED with approximately 25,000 visits per year could experience 1,400 diagnostic errors, 500 diagnostic adverse events, and 75 serious harms, including 50 deaths. However, the authors noted that the overall error and harm rates were based on three studies from outside the United States (Canada, Spain, and Switzerland) and that only two of these were used to estimate harms.
“It’s imperative that we, as emergency physicians, inform the public that the AHRQ report used flawed methodology and statistics that extrapolated – and therefore overstated – the potential for harm when receiving care in US emergency departments,” Robert Glatter, MD, an emergency medicine physician at Lenox Hill Hospital at Northwell Health and an assistant professor at Hofstra University, Hempstead, N.Y., said in an interview.
Emergency medicine organizations express concerns for accuracy
The American College of Emergency Physicians and eight other medical organizations representing emergency medicine in the United States sent a letter to the AHRQ on Dec. 14, 2022, spelling out their concerns. The review was conducted as part of the AHRQ’s ongoing Effective Health Care Program, and the organizations had the opportunity to review a draft before it was published. On reading the review, they asked that the publication of the review be delayed. “After reviewing the executive summary and initial draft, we believe that the report makes misleading, incomplete, and erroneous conclusions from the literature reviewed and conveys a tone that inaccurately characterizes and unnecessarily disparages the practice of emergency medicine in the United States,” the organizations wrote in their letter.
The concerns of the emergency medicine organizations fell into four categories: misrepresentation of the practice and nature of emergency medicine; applicability of references cited; inaccurate interpretation of malpractice data; and the reporting of a single overall diagnostic error rate of 5.7% in EDs.
The practice of emergency medicine is variable and unique among specialties in that the focus is less about the final diagnosis and more about immediate identification and treatment of life-threatening conditions, according to the letter.
Notably, many of the studies cited did not mention whether the patient’s final diagnosis was apparent on admission to the ED. “Without this knowledge, it is completely inappropriate to label such discrepancies as ‘ED diagnostic error,’ ” the organizations wrote.
All medical specialties have room for improvement, but the current AHRQ review appears not to identify these opportunities, and instead of contributing to a discussion of improving patient care in the ED, it may cause harm by presenting misinformation, they said.
Misleading and inadequate evidence
“I strongly agree with the concerns mentioned from ACEP and other key organizations about the problems and conclusions reached in the AHRQ report,” Dr. Glatter said in an interview.
“The methodology used to arrive at the conclusions [in the review] was flawed and does not provide an accurate estimate of diagnostic error and, consequently, misdiagnosis and deaths occurring in emergency departments in the U.S.,” he said. “The startling headline that 250,000 people die annually in U.S. EDs was extrapolated from a single study based on one death that occurred in a Canadian ED in 2004,” Dr. Glatter noted. “Clearly, this is not only poor methodology but flawed science.”
The AHRQ report misused one death from this single study to estimate the death rate across the United States, Dr. Glatter explained, and this overestimate improperly inflated and magnified the number of potential patients that may have been harmed by physician error.
“This flawed evidence would actually place ED misdiagnoses in the top five causes of death in the United States, with 1 in every 500 ED patients dying as a result of an error by a physician. Simply put, there is just no evidence to support such a claim,” said Dr. Glatter.
The repercussions of the AHRQ review could be harmful to patients by instilling fear and doubt about the ability of emergency physicians to diagnose those who present with life-threatening conditions, Dr. Glatter said.
“This more balanced and accurate picture of the role of emergency physicians in diagnosing and managing such emergencies needs to be communicated to the public in order to reassure and instill confidence in our role in the sequence of emergency care in relation to continuity of care in patients presenting to the ED,” he said.
“While our primary role as emergency medicine physicians is to stabilize and evaluate patients, arriving at a particular diagnosis is not always possible for some conditions,” and additional diagnostic testing is often needed to identify more specific causes of symptoms, Dr. Glatter added.
Additional research is needed for a more accurate representation of diagnostic errors in the ED, said Dr. Glatter. New prospective studies are needed to address outcomes in U.S. EDs that account for the latest advances and diagnostic modalities in emergency medicine, “particularly advances in bedside ultrasound that can expedite critical decision-making, which can be lifesaving.
“The AHRQ report is simply not an accurate reflection of the technology and skill set that current emergency medicine practice offers our patients in 2023.”
Dr. Glatter disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ED doctors call private equity staffing practices illegal and seek to ban them
Thirty-three states plus the District of Columbia have rules on their books against the so-called corporate practice of medicine. But over the years, critics say, companies have successfully sidestepped bans on owning medical practices by buying or establishing local staffing groups that are nominally owned by doctors and restricting the physicians’ authority so they have no direct control.
These laws and regulations, which started appearing nearly a century ago, were meant to fight the commercialization of medicine, maintain the independence and authority of physicians, and prioritize the doctor-patient relationship over the interests of investors and shareholders.
Those campaigning for stiffer enforcement of the laws say that physician-staffing firms owned by private equity investors are the most egregious offenders. Private equity-backed staffing companies manage a quarter of the nation’s emergency departments, according to a Raleigh, N.C.–based doctor who runs a job site for ED physicians. The two largest are Nashville, Tenn.–based Envision Healthcare, owned by investment giant KKR & Co., and Knoxville, Tenn.–based TeamHealth, owned by Blackstone.
Court filings in multiple states, including California, Missouri, Texas, and Tennessee, have called out Envision and TeamHealth for allegedly using doctor groups as straw men to sidestep corporate practice laws. But those filings have typically been in financial cases involving wrongful termination, breach of contract, and overbilling.
Now, physicians and consumer advocates around the country are anticipating a California lawsuit against Envision, scheduled to start in January 2024 in federal court. The plaintiff in the case, Milwaukee-based American Academy of Emergency Medicine Physician Group, alleges that Envision uses shell business structures to retain de facto ownership of ED staffing groups, and it is asking the court to declare them illegal.
“We’re not asking them to pay money, and we will not accept being paid to drop the case,” said David Millstein, lead attorney for the plaintiff. “We are simply asking the court to ban this practice model.”
‘Possibility to reverberate throughout the country’
The physician group believes a victory would lead to a prohibition of the practice across California – and not just in ERs, but for other staff provided by Envision and TeamHealth, including in anesthesiology and hospital medicine. The California Medical Association supports the lawsuit, saying it “will shape the boundaries of California’s prohibition on the corporate practice of medicine.”
The plaintiff – along with many doctors, nurses, and consumer advocates, as well as some lawmakers – hopes that success in the case will spur regulators and prosecutors in other states to take corporate medicine prohibitions more seriously. “Any decision anywhere in the country that says the corporate ownership of a medical practice is illegal has the possibility to reverberate throughout the country, absolutely – and I hope that it would,” said Julie Mayfield, a state senator in North Carolina.
But the push to reinvigorate laws restricting the corporate practice of medicine has plenty of skeptics, who view it as an effort to return to a golden era in medicine that is long gone or may never have existed to begin with. The genie is out of the bottle, they say, noting that the profit motive has penetrated every corner of health care and that nearly 70% of physicians in the United States are now employed by corporations and hospitals.
The corporate practice of medicine doctrine has “a very interesting and not a very flattering history,” said Barak Richman, a law professor at Duke University. “The medical profession was trying to assert its professional dominance that accrued a lot of benefits to itself in ways that were not terribly beneficial to patients or to the market.”
The California case involves Placentia-Linda Hospital in Orange County, where the plaintiff physician group lost its ED management contract to Envision. The complaint alleges that Envision uses the same business model at numerous hospitals around the state.
“Envision exercises profound and pervasive direct and indirect control and/or influence over the medical practice, making decisions which bear directly and indirectly on the practice of medicine, rendering physicians as mere employees, and diminishing physician independence and freedom from commercial interests,” according to the complaint.
Envision said the company is compliant with state laws and that its operating structure is common in the health care industry. “Legal challenges to that structure have proved meritless,” Envision wrote in an email. It added that “care decisions have and always will be between clinicians and patients.”
TeamHealth, an indirect target in the case, said its “world-class operating team” provides management services that “allow clinicians to focus on the practice of medicine and patient care through a structure commonly utilized by hospitals, health systems, and other providers across the country.”
State rules vary widely
State laws and regulations governing the corporate practice of medicine vary widely on multiple factors, including whether there are exceptions for nonprofit organizations, how much of doctors’ revenue outside management firms can keep, who can own the equipment, and how violations are punished. New York, Texas, and California are considered to have among the toughest restrictions, while Florida and 16 other states have none.
Kirk Ogrosky, a partner at the law firm Goodwin Procter, said this kind of management structure predates the arrival of private equity in the industry. “I would be surprised if a company that is interested in investing in this space screwed up the formation documents; it would shock me,” Mr. Ogrosky said.
Private equity–backed firms have been attracted to EDs in recent years because they are profitable and because they have been able to charge inflated amounts for out-of-network care – at least until a federal law cracked down on surprise billing. Envision and TeamHealth prioritize profits, critics say, by maximizing revenue, cutting costs, and consolidating smaller practices into ever-larger groups – to the point of regional dominance.
Envision and TeamHealth are privately owned, which makes it difficult to find reliable data on their finances and the extent of their market penetration.
Leon Adelman, MD, cofounder and CEO of Ivy Clinicians, a Raleigh, N.C.–based startup job site for emergency physicians, has spent 18 months piecing together data and found that private equity–backed staffing firms run 25% of the nation’s EDs. TeamHealth and Envision have the two largest shares, with 8.6% and 8.3%, respectively, Dr. Adelman said.
Other estimates put private equity’s penetration of ERs at closer to 40%.
Doctors push for investigations
So far, efforts by emergency physicians and others to challenge private equity staffing firms over their alleged violations have yielded frustrating results.
An advocacy group called Take Medicine Back, formed last year by a handful of ED physicians, sent a letter in July to North Carolina Attorney General Josh Stein, asking him to investigate violations of the ban on the corporate practice of medicine. And because Mr. Stein holds a senior position at the National Association of Attorneys General, the letter also asked him to take the lead in persuading his fellow AGs to “launch a multi-state investigation into the widespread lack of enforcement” of corporate practice of medicine laws.
The group’s leader, Mitchell Li, MD, said he was initially disappointed by the response he received from Mr. Stein’s office, which promised to review his request, saying it raised complex legal issues about the corporate practice of medicine in the state. But Dr. Li is now more hopeful, since he has secured a January appointment with officials in Mr. Stein’s office.
Robert McNamara, MD, a cofounder of Dr. Li’s group and chair of emergency medicine at Temple University’s Lewis Katz School of Medicine, drafted complaints to the Texas Medical Board, along with Houston physician David Hoyer, MD, asking the board to intervene against two doctors accused of fronting for professional entities controlled by Envision and TeamHealth. In both cases, the board declined to intervene.
Dr. McNamara, who serves as the chief medical officer of the physicians’ group in the California Envision case, also filed a complaint with Pennsylvania Attorney General Josh Shapiro, alleging that a group called Emergency Care Services of Pennsylvania PC, which was trying to contract with ED physicians of the Crozer Keystone Health System, was wholly owned by TeamHealth and serving as a shell to avoid scrutiny.
A senior official in Mr. Shapiro’s office responded, saying the complaint had been referred to two state agencies, but Dr. McNamara said he has heard nothing back in more than 3 years.
Differing views on private equity’s role
Proponents of private equity ownership say it has brought a lot of good to health care. Jamal Hagler, vice president of research at the American Investment Council, said private equity brings expertise to hospital systems, “whether it’s to hire new staff, grow and open up to new markets, integrate new technologies, or develop new technologies.”
But many physicians who have worked for private equity companies say their mission is not compatible with the best practice of medicine. They cite an emphasis on speed and high patient volume over safety; a preference for lesser-trained, cheaper medical providers; and treatment protocols unsuitable for certain patients.
Sean Jones, MD, an emergency physician in Asheville, N.C., said his first full-time job was at a Florida hospital, where EmCare, a subsidiary of Envision, ran the ED. Dr. Jones said EmCare, in collaboration with the hospital’s owner, pushed doctors to meet performance goals related to wait times and treatments, which were not always good for patients.
For example, if a patient came in with abnormally high heart and respiratory rates – signs of sepsis – doctors were expected to give them large amounts of fluids and antibiotics within an hour, Dr. Jones said. But those symptoms could also be caused by a panic attack or heart failure.
“You don’t want to give a patient with heart failure 2 or 3 liters of fluid, and I would get emails saying, ‘You didn’t do this,’ ” he said. “Well, no, I didn’t, because the reason they couldn’t breathe was they had too much fluid in their lungs.”
Envision said the company’s 25,000 clinicians, “like all clinicians, exercise their independent judgment to provide quality, compassionate, clinically appropriate care.”
Dr. Jones felt otherwise. “We don’t need some MBAs telling us what to do,” he said.
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Thirty-three states plus the District of Columbia have rules on their books against the so-called corporate practice of medicine. But over the years, critics say, companies have successfully sidestepped bans on owning medical practices by buying or establishing local staffing groups that are nominally owned by doctors and restricting the physicians’ authority so they have no direct control.
These laws and regulations, which started appearing nearly a century ago, were meant to fight the commercialization of medicine, maintain the independence and authority of physicians, and prioritize the doctor-patient relationship over the interests of investors and shareholders.
Those campaigning for stiffer enforcement of the laws say that physician-staffing firms owned by private equity investors are the most egregious offenders. Private equity-backed staffing companies manage a quarter of the nation’s emergency departments, according to a Raleigh, N.C.–based doctor who runs a job site for ED physicians. The two largest are Nashville, Tenn.–based Envision Healthcare, owned by investment giant KKR & Co., and Knoxville, Tenn.–based TeamHealth, owned by Blackstone.
Court filings in multiple states, including California, Missouri, Texas, and Tennessee, have called out Envision and TeamHealth for allegedly using doctor groups as straw men to sidestep corporate practice laws. But those filings have typically been in financial cases involving wrongful termination, breach of contract, and overbilling.
Now, physicians and consumer advocates around the country are anticipating a California lawsuit against Envision, scheduled to start in January 2024 in federal court. The plaintiff in the case, Milwaukee-based American Academy of Emergency Medicine Physician Group, alleges that Envision uses shell business structures to retain de facto ownership of ED staffing groups, and it is asking the court to declare them illegal.
“We’re not asking them to pay money, and we will not accept being paid to drop the case,” said David Millstein, lead attorney for the plaintiff. “We are simply asking the court to ban this practice model.”
‘Possibility to reverberate throughout the country’
The physician group believes a victory would lead to a prohibition of the practice across California – and not just in ERs, but for other staff provided by Envision and TeamHealth, including in anesthesiology and hospital medicine. The California Medical Association supports the lawsuit, saying it “will shape the boundaries of California’s prohibition on the corporate practice of medicine.”
The plaintiff – along with many doctors, nurses, and consumer advocates, as well as some lawmakers – hopes that success in the case will spur regulators and prosecutors in other states to take corporate medicine prohibitions more seriously. “Any decision anywhere in the country that says the corporate ownership of a medical practice is illegal has the possibility to reverberate throughout the country, absolutely – and I hope that it would,” said Julie Mayfield, a state senator in North Carolina.
But the push to reinvigorate laws restricting the corporate practice of medicine has plenty of skeptics, who view it as an effort to return to a golden era in medicine that is long gone or may never have existed to begin with. The genie is out of the bottle, they say, noting that the profit motive has penetrated every corner of health care and that nearly 70% of physicians in the United States are now employed by corporations and hospitals.
The corporate practice of medicine doctrine has “a very interesting and not a very flattering history,” said Barak Richman, a law professor at Duke University. “The medical profession was trying to assert its professional dominance that accrued a lot of benefits to itself in ways that were not terribly beneficial to patients or to the market.”
The California case involves Placentia-Linda Hospital in Orange County, where the plaintiff physician group lost its ED management contract to Envision. The complaint alleges that Envision uses the same business model at numerous hospitals around the state.
“Envision exercises profound and pervasive direct and indirect control and/or influence over the medical practice, making decisions which bear directly and indirectly on the practice of medicine, rendering physicians as mere employees, and diminishing physician independence and freedom from commercial interests,” according to the complaint.
Envision said the company is compliant with state laws and that its operating structure is common in the health care industry. “Legal challenges to that structure have proved meritless,” Envision wrote in an email. It added that “care decisions have and always will be between clinicians and patients.”
TeamHealth, an indirect target in the case, said its “world-class operating team” provides management services that “allow clinicians to focus on the practice of medicine and patient care through a structure commonly utilized by hospitals, health systems, and other providers across the country.”
State rules vary widely
State laws and regulations governing the corporate practice of medicine vary widely on multiple factors, including whether there are exceptions for nonprofit organizations, how much of doctors’ revenue outside management firms can keep, who can own the equipment, and how violations are punished. New York, Texas, and California are considered to have among the toughest restrictions, while Florida and 16 other states have none.
Kirk Ogrosky, a partner at the law firm Goodwin Procter, said this kind of management structure predates the arrival of private equity in the industry. “I would be surprised if a company that is interested in investing in this space screwed up the formation documents; it would shock me,” Mr. Ogrosky said.
Private equity–backed firms have been attracted to EDs in recent years because they are profitable and because they have been able to charge inflated amounts for out-of-network care – at least until a federal law cracked down on surprise billing. Envision and TeamHealth prioritize profits, critics say, by maximizing revenue, cutting costs, and consolidating smaller practices into ever-larger groups – to the point of regional dominance.
Envision and TeamHealth are privately owned, which makes it difficult to find reliable data on their finances and the extent of their market penetration.
Leon Adelman, MD, cofounder and CEO of Ivy Clinicians, a Raleigh, N.C.–based startup job site for emergency physicians, has spent 18 months piecing together data and found that private equity–backed staffing firms run 25% of the nation’s EDs. TeamHealth and Envision have the two largest shares, with 8.6% and 8.3%, respectively, Dr. Adelman said.
Other estimates put private equity’s penetration of ERs at closer to 40%.
Doctors push for investigations
So far, efforts by emergency physicians and others to challenge private equity staffing firms over their alleged violations have yielded frustrating results.
An advocacy group called Take Medicine Back, formed last year by a handful of ED physicians, sent a letter in July to North Carolina Attorney General Josh Stein, asking him to investigate violations of the ban on the corporate practice of medicine. And because Mr. Stein holds a senior position at the National Association of Attorneys General, the letter also asked him to take the lead in persuading his fellow AGs to “launch a multi-state investigation into the widespread lack of enforcement” of corporate practice of medicine laws.
The group’s leader, Mitchell Li, MD, said he was initially disappointed by the response he received from Mr. Stein’s office, which promised to review his request, saying it raised complex legal issues about the corporate practice of medicine in the state. But Dr. Li is now more hopeful, since he has secured a January appointment with officials in Mr. Stein’s office.
Robert McNamara, MD, a cofounder of Dr. Li’s group and chair of emergency medicine at Temple University’s Lewis Katz School of Medicine, drafted complaints to the Texas Medical Board, along with Houston physician David Hoyer, MD, asking the board to intervene against two doctors accused of fronting for professional entities controlled by Envision and TeamHealth. In both cases, the board declined to intervene.
Dr. McNamara, who serves as the chief medical officer of the physicians’ group in the California Envision case, also filed a complaint with Pennsylvania Attorney General Josh Shapiro, alleging that a group called Emergency Care Services of Pennsylvania PC, which was trying to contract with ED physicians of the Crozer Keystone Health System, was wholly owned by TeamHealth and serving as a shell to avoid scrutiny.
A senior official in Mr. Shapiro’s office responded, saying the complaint had been referred to two state agencies, but Dr. McNamara said he has heard nothing back in more than 3 years.
Differing views on private equity’s role
Proponents of private equity ownership say it has brought a lot of good to health care. Jamal Hagler, vice president of research at the American Investment Council, said private equity brings expertise to hospital systems, “whether it’s to hire new staff, grow and open up to new markets, integrate new technologies, or develop new technologies.”
But many physicians who have worked for private equity companies say their mission is not compatible with the best practice of medicine. They cite an emphasis on speed and high patient volume over safety; a preference for lesser-trained, cheaper medical providers; and treatment protocols unsuitable for certain patients.
Sean Jones, MD, an emergency physician in Asheville, N.C., said his first full-time job was at a Florida hospital, where EmCare, a subsidiary of Envision, ran the ED. Dr. Jones said EmCare, in collaboration with the hospital’s owner, pushed doctors to meet performance goals related to wait times and treatments, which were not always good for patients.
For example, if a patient came in with abnormally high heart and respiratory rates – signs of sepsis – doctors were expected to give them large amounts of fluids and antibiotics within an hour, Dr. Jones said. But those symptoms could also be caused by a panic attack or heart failure.
“You don’t want to give a patient with heart failure 2 or 3 liters of fluid, and I would get emails saying, ‘You didn’t do this,’ ” he said. “Well, no, I didn’t, because the reason they couldn’t breathe was they had too much fluid in their lungs.”
Envision said the company’s 25,000 clinicians, “like all clinicians, exercise their independent judgment to provide quality, compassionate, clinically appropriate care.”
Dr. Jones felt otherwise. “We don’t need some MBAs telling us what to do,” he said.
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Thirty-three states plus the District of Columbia have rules on their books against the so-called corporate practice of medicine. But over the years, critics say, companies have successfully sidestepped bans on owning medical practices by buying or establishing local staffing groups that are nominally owned by doctors and restricting the physicians’ authority so they have no direct control.
These laws and regulations, which started appearing nearly a century ago, were meant to fight the commercialization of medicine, maintain the independence and authority of physicians, and prioritize the doctor-patient relationship over the interests of investors and shareholders.
Those campaigning for stiffer enforcement of the laws say that physician-staffing firms owned by private equity investors are the most egregious offenders. Private equity-backed staffing companies manage a quarter of the nation’s emergency departments, according to a Raleigh, N.C.–based doctor who runs a job site for ED physicians. The two largest are Nashville, Tenn.–based Envision Healthcare, owned by investment giant KKR & Co., and Knoxville, Tenn.–based TeamHealth, owned by Blackstone.
Court filings in multiple states, including California, Missouri, Texas, and Tennessee, have called out Envision and TeamHealth for allegedly using doctor groups as straw men to sidestep corporate practice laws. But those filings have typically been in financial cases involving wrongful termination, breach of contract, and overbilling.
Now, physicians and consumer advocates around the country are anticipating a California lawsuit against Envision, scheduled to start in January 2024 in federal court. The plaintiff in the case, Milwaukee-based American Academy of Emergency Medicine Physician Group, alleges that Envision uses shell business structures to retain de facto ownership of ED staffing groups, and it is asking the court to declare them illegal.
“We’re not asking them to pay money, and we will not accept being paid to drop the case,” said David Millstein, lead attorney for the plaintiff. “We are simply asking the court to ban this practice model.”
‘Possibility to reverberate throughout the country’
The physician group believes a victory would lead to a prohibition of the practice across California – and not just in ERs, but for other staff provided by Envision and TeamHealth, including in anesthesiology and hospital medicine. The California Medical Association supports the lawsuit, saying it “will shape the boundaries of California’s prohibition on the corporate practice of medicine.”
The plaintiff – along with many doctors, nurses, and consumer advocates, as well as some lawmakers – hopes that success in the case will spur regulators and prosecutors in other states to take corporate medicine prohibitions more seriously. “Any decision anywhere in the country that says the corporate ownership of a medical practice is illegal has the possibility to reverberate throughout the country, absolutely – and I hope that it would,” said Julie Mayfield, a state senator in North Carolina.
But the push to reinvigorate laws restricting the corporate practice of medicine has plenty of skeptics, who view it as an effort to return to a golden era in medicine that is long gone or may never have existed to begin with. The genie is out of the bottle, they say, noting that the profit motive has penetrated every corner of health care and that nearly 70% of physicians in the United States are now employed by corporations and hospitals.
The corporate practice of medicine doctrine has “a very interesting and not a very flattering history,” said Barak Richman, a law professor at Duke University. “The medical profession was trying to assert its professional dominance that accrued a lot of benefits to itself in ways that were not terribly beneficial to patients or to the market.”
The California case involves Placentia-Linda Hospital in Orange County, where the plaintiff physician group lost its ED management contract to Envision. The complaint alleges that Envision uses the same business model at numerous hospitals around the state.
“Envision exercises profound and pervasive direct and indirect control and/or influence over the medical practice, making decisions which bear directly and indirectly on the practice of medicine, rendering physicians as mere employees, and diminishing physician independence and freedom from commercial interests,” according to the complaint.
Envision said the company is compliant with state laws and that its operating structure is common in the health care industry. “Legal challenges to that structure have proved meritless,” Envision wrote in an email. It added that “care decisions have and always will be between clinicians and patients.”
TeamHealth, an indirect target in the case, said its “world-class operating team” provides management services that “allow clinicians to focus on the practice of medicine and patient care through a structure commonly utilized by hospitals, health systems, and other providers across the country.”
State rules vary widely
State laws and regulations governing the corporate practice of medicine vary widely on multiple factors, including whether there are exceptions for nonprofit organizations, how much of doctors’ revenue outside management firms can keep, who can own the equipment, and how violations are punished. New York, Texas, and California are considered to have among the toughest restrictions, while Florida and 16 other states have none.
Kirk Ogrosky, a partner at the law firm Goodwin Procter, said this kind of management structure predates the arrival of private equity in the industry. “I would be surprised if a company that is interested in investing in this space screwed up the formation documents; it would shock me,” Mr. Ogrosky said.
Private equity–backed firms have been attracted to EDs in recent years because they are profitable and because they have been able to charge inflated amounts for out-of-network care – at least until a federal law cracked down on surprise billing. Envision and TeamHealth prioritize profits, critics say, by maximizing revenue, cutting costs, and consolidating smaller practices into ever-larger groups – to the point of regional dominance.
Envision and TeamHealth are privately owned, which makes it difficult to find reliable data on their finances and the extent of their market penetration.
Leon Adelman, MD, cofounder and CEO of Ivy Clinicians, a Raleigh, N.C.–based startup job site for emergency physicians, has spent 18 months piecing together data and found that private equity–backed staffing firms run 25% of the nation’s EDs. TeamHealth and Envision have the two largest shares, with 8.6% and 8.3%, respectively, Dr. Adelman said.
Other estimates put private equity’s penetration of ERs at closer to 40%.
Doctors push for investigations
So far, efforts by emergency physicians and others to challenge private equity staffing firms over their alleged violations have yielded frustrating results.
An advocacy group called Take Medicine Back, formed last year by a handful of ED physicians, sent a letter in July to North Carolina Attorney General Josh Stein, asking him to investigate violations of the ban on the corporate practice of medicine. And because Mr. Stein holds a senior position at the National Association of Attorneys General, the letter also asked him to take the lead in persuading his fellow AGs to “launch a multi-state investigation into the widespread lack of enforcement” of corporate practice of medicine laws.
The group’s leader, Mitchell Li, MD, said he was initially disappointed by the response he received from Mr. Stein’s office, which promised to review his request, saying it raised complex legal issues about the corporate practice of medicine in the state. But Dr. Li is now more hopeful, since he has secured a January appointment with officials in Mr. Stein’s office.
Robert McNamara, MD, a cofounder of Dr. Li’s group and chair of emergency medicine at Temple University’s Lewis Katz School of Medicine, drafted complaints to the Texas Medical Board, along with Houston physician David Hoyer, MD, asking the board to intervene against two doctors accused of fronting for professional entities controlled by Envision and TeamHealth. In both cases, the board declined to intervene.
Dr. McNamara, who serves as the chief medical officer of the physicians’ group in the California Envision case, also filed a complaint with Pennsylvania Attorney General Josh Shapiro, alleging that a group called Emergency Care Services of Pennsylvania PC, which was trying to contract with ED physicians of the Crozer Keystone Health System, was wholly owned by TeamHealth and serving as a shell to avoid scrutiny.
A senior official in Mr. Shapiro’s office responded, saying the complaint had been referred to two state agencies, but Dr. McNamara said he has heard nothing back in more than 3 years.
Differing views on private equity’s role
Proponents of private equity ownership say it has brought a lot of good to health care. Jamal Hagler, vice president of research at the American Investment Council, said private equity brings expertise to hospital systems, “whether it’s to hire new staff, grow and open up to new markets, integrate new technologies, or develop new technologies.”
But many physicians who have worked for private equity companies say their mission is not compatible with the best practice of medicine. They cite an emphasis on speed and high patient volume over safety; a preference for lesser-trained, cheaper medical providers; and treatment protocols unsuitable for certain patients.
Sean Jones, MD, an emergency physician in Asheville, N.C., said his first full-time job was at a Florida hospital, where EmCare, a subsidiary of Envision, ran the ED. Dr. Jones said EmCare, in collaboration with the hospital’s owner, pushed doctors to meet performance goals related to wait times and treatments, which were not always good for patients.
For example, if a patient came in with abnormally high heart and respiratory rates – signs of sepsis – doctors were expected to give them large amounts of fluids and antibiotics within an hour, Dr. Jones said. But those symptoms could also be caused by a panic attack or heart failure.
“You don’t want to give a patient with heart failure 2 or 3 liters of fluid, and I would get emails saying, ‘You didn’t do this,’ ” he said. “Well, no, I didn’t, because the reason they couldn’t breathe was they had too much fluid in their lungs.”
Envision said the company’s 25,000 clinicians, “like all clinicians, exercise their independent judgment to provide quality, compassionate, clinically appropriate care.”
Dr. Jones felt otherwise. “We don’t need some MBAs telling us what to do,” he said.
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Long COVID clinical trials may offer shortcut to new treatments
With no proven treatments for long COVID, millions of Americans struggling with debilitating symptoms may be wondering whether it’s worth it to try something they’ve never considered before: a clinical trial.
But it may take years for these trials to prove which drugs, devices, and behavioral therapies are safe and effective.
“We’re not in warp speed,” said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute in Durham, N.C., who is overseeing long COVID trials for the NIH. Operation Warp Speed – the 2020-2021 federal effort to get COVID vaccines designed, tested, authorized and distributed – benefited from existing scientific knowledge about other coronaviruses and about vaccines in general. But there’s no similar foundation of scientific knowledge about long COVID.
“We are in a place of not really knowing anything,” Dr. Zimmerman said.
But some glimmers of hope are emerging. A Veterans Affairs study recently found the antiviral Paxlovid might help prevent long COVID. A small case study at Yale found the ADHD drug guanfacine may ease brain fog from long COVID. And preliminary results from an NIH-funded study suggest COVID vaccines might help children with a rare but serious inflammatory condition known as multisystem inflammatory syndrome (MIS-C).
More results are expected very soon from the trial for kids with MIS-C, which can strike suddenly long after a COVID infection clears up. While the exact causes aren’t yet clear, MIS-C can cause dangerous inflammation in the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal system.
Because the virus often triggered a delayed response of MIS-C in kids who had few if any symptoms of acute COVID-19, scientists wondered whether children infected with the virus might respond to a vaccine dose to prevent MIS-C from developing, Gary Gibbons, MD, director of the National Heart, Lung, and Blood Institute, said during a Dec. 9 presentation at the NIH. It’s not yet clear if vaccination helps, but it doesn’t harm the children, Dr. Gibbons said.
“Indeed, the studies suggest with some relief that yes, these children could be vaccinated safely,” he said.
Several new trials are also testing Paxlovid against long COVID, including one late-stage study that may have results in about a year.
“We already know that Paxlovid reduces the risk of developing long COVID, but it would be a game changer if it can improve long COVID symptoms as well,” said Surendra Barshikar, MD, an associate professor and medical director of the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
In other studies, researchers are looking at a wide variety of previously approved and experimental drugs and devices. For example, scientists in New York are testing the mood stabilizer lithium to treat brain fog and fatigue. And researchers in Illinois are investigating efgartigimod, a drug approved for the rare muscle-weakening autoimmune disorder myasthenia gravis, to see if it helps ease a long COVID complication known as POTS that can cause a sudden rapid heart rate and chronic fatigue.
“The good news is that enrollment will proceed quickly, given the vast number of patients,” said Kristin Englund, MD, director of the reCOVer Center of Excellence at the Cleveland Clinic.
This is all encouraging because roughly one in five American adults who have acute COVID infections develop persistent symptoms of long COVID, also known as post–acute sequelae of SARS-CoV-2 (PASC). And many of these long COVID patients have complex, overlapping clusters of symptoms that make traditional treatment approaches largely ineffective against this new, formidable disease.
But not every patient living with long COVID will qualify for trials or find it easy to take part even if they do. Patients should consider how severe their symptoms are, the potential risks of any experimental treatments, and the many challenges they may have with getting to and from clinical trial sites that are largely concentrated around major cities and might be far from home.
While this holds true for any type of trial, it’s essential for long COVID patients, who may have fatigue, muscle weakness, and other symptoms that make distance an impossible factor to ignore, said Aaron Friedberg, MD, clinical colead of the post–COVID-19 recovery program at the Ohio State University Wexner Medical Center, Columbus.
“I think it is a personal decision, since the fatigue and pain that patients with PASC can experience can make it very challenging to travel long distances,” Dr. Friedberg said. “I would recommend calling or messaging ahead to find out exactly what type of travel might be required because there may be steps that can be completed by email or video, which could make it easier to participate, and some trials may be entirely remote.”
Even when patients feel up to the travel, they still might not be a good fit for a clinical trial. Scientists often look for people who didn’t have pre-existing health problems before they got long COVID, Dr. Barshikar noted. Patients taking medications may also be unable to participate in drug trials, particularly for experimental treatments because of concerns about unknown side effects from drug interactions.
When clinical trials do seem like a good option, patients may want to consider seeking treatment at an academic medical center that is already doing long COVID research, particularly if their symptoms are too complex or severe to manage only through their primary care provider, said Jonathan Whiteson, MD, who helped draft long COVID treatment guidelines for the American Academy of Physical Medicine and Rehabilitation. He also serves as codirector of the New York University Langone Health post–COVID care program.
Many health care professionals on the front lines treating long COVID patients are optimistic that the sheer number of trials and the vast number of patients taking part should ultimately produce some better treatment options than people have right now. It’s just not going to happen overnight.
“I suspect that while we will see some new treatments coming in the next 1-2 years, it may be several years before targets can be identified and full trials conducted to see results,” Dr. Friedberg said. “Getting good data takes time.”
A version of this article first appeared on WebMD.com.
With no proven treatments for long COVID, millions of Americans struggling with debilitating symptoms may be wondering whether it’s worth it to try something they’ve never considered before: a clinical trial.
But it may take years for these trials to prove which drugs, devices, and behavioral therapies are safe and effective.
“We’re not in warp speed,” said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute in Durham, N.C., who is overseeing long COVID trials for the NIH. Operation Warp Speed – the 2020-2021 federal effort to get COVID vaccines designed, tested, authorized and distributed – benefited from existing scientific knowledge about other coronaviruses and about vaccines in general. But there’s no similar foundation of scientific knowledge about long COVID.
“We are in a place of not really knowing anything,” Dr. Zimmerman said.
But some glimmers of hope are emerging. A Veterans Affairs study recently found the antiviral Paxlovid might help prevent long COVID. A small case study at Yale found the ADHD drug guanfacine may ease brain fog from long COVID. And preliminary results from an NIH-funded study suggest COVID vaccines might help children with a rare but serious inflammatory condition known as multisystem inflammatory syndrome (MIS-C).
More results are expected very soon from the trial for kids with MIS-C, which can strike suddenly long after a COVID infection clears up. While the exact causes aren’t yet clear, MIS-C can cause dangerous inflammation in the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal system.
Because the virus often triggered a delayed response of MIS-C in kids who had few if any symptoms of acute COVID-19, scientists wondered whether children infected with the virus might respond to a vaccine dose to prevent MIS-C from developing, Gary Gibbons, MD, director of the National Heart, Lung, and Blood Institute, said during a Dec. 9 presentation at the NIH. It’s not yet clear if vaccination helps, but it doesn’t harm the children, Dr. Gibbons said.
“Indeed, the studies suggest with some relief that yes, these children could be vaccinated safely,” he said.
Several new trials are also testing Paxlovid against long COVID, including one late-stage study that may have results in about a year.
“We already know that Paxlovid reduces the risk of developing long COVID, but it would be a game changer if it can improve long COVID symptoms as well,” said Surendra Barshikar, MD, an associate professor and medical director of the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
In other studies, researchers are looking at a wide variety of previously approved and experimental drugs and devices. For example, scientists in New York are testing the mood stabilizer lithium to treat brain fog and fatigue. And researchers in Illinois are investigating efgartigimod, a drug approved for the rare muscle-weakening autoimmune disorder myasthenia gravis, to see if it helps ease a long COVID complication known as POTS that can cause a sudden rapid heart rate and chronic fatigue.
“The good news is that enrollment will proceed quickly, given the vast number of patients,” said Kristin Englund, MD, director of the reCOVer Center of Excellence at the Cleveland Clinic.
This is all encouraging because roughly one in five American adults who have acute COVID infections develop persistent symptoms of long COVID, also known as post–acute sequelae of SARS-CoV-2 (PASC). And many of these long COVID patients have complex, overlapping clusters of symptoms that make traditional treatment approaches largely ineffective against this new, formidable disease.
But not every patient living with long COVID will qualify for trials or find it easy to take part even if they do. Patients should consider how severe their symptoms are, the potential risks of any experimental treatments, and the many challenges they may have with getting to and from clinical trial sites that are largely concentrated around major cities and might be far from home.
While this holds true for any type of trial, it’s essential for long COVID patients, who may have fatigue, muscle weakness, and other symptoms that make distance an impossible factor to ignore, said Aaron Friedberg, MD, clinical colead of the post–COVID-19 recovery program at the Ohio State University Wexner Medical Center, Columbus.
“I think it is a personal decision, since the fatigue and pain that patients with PASC can experience can make it very challenging to travel long distances,” Dr. Friedberg said. “I would recommend calling or messaging ahead to find out exactly what type of travel might be required because there may be steps that can be completed by email or video, which could make it easier to participate, and some trials may be entirely remote.”
Even when patients feel up to the travel, they still might not be a good fit for a clinical trial. Scientists often look for people who didn’t have pre-existing health problems before they got long COVID, Dr. Barshikar noted. Patients taking medications may also be unable to participate in drug trials, particularly for experimental treatments because of concerns about unknown side effects from drug interactions.
When clinical trials do seem like a good option, patients may want to consider seeking treatment at an academic medical center that is already doing long COVID research, particularly if their symptoms are too complex or severe to manage only through their primary care provider, said Jonathan Whiteson, MD, who helped draft long COVID treatment guidelines for the American Academy of Physical Medicine and Rehabilitation. He also serves as codirector of the New York University Langone Health post–COVID care program.
Many health care professionals on the front lines treating long COVID patients are optimistic that the sheer number of trials and the vast number of patients taking part should ultimately produce some better treatment options than people have right now. It’s just not going to happen overnight.
“I suspect that while we will see some new treatments coming in the next 1-2 years, it may be several years before targets can be identified and full trials conducted to see results,” Dr. Friedberg said. “Getting good data takes time.”
A version of this article first appeared on WebMD.com.
With no proven treatments for long COVID, millions of Americans struggling with debilitating symptoms may be wondering whether it’s worth it to try something they’ve never considered before: a clinical trial.
But it may take years for these trials to prove which drugs, devices, and behavioral therapies are safe and effective.
“We’re not in warp speed,” said Kanecia Zimmerman, MD, a principal investigator at the Duke Clinical Research Institute in Durham, N.C., who is overseeing long COVID trials for the NIH. Operation Warp Speed – the 2020-2021 federal effort to get COVID vaccines designed, tested, authorized and distributed – benefited from existing scientific knowledge about other coronaviruses and about vaccines in general. But there’s no similar foundation of scientific knowledge about long COVID.
“We are in a place of not really knowing anything,” Dr. Zimmerman said.
But some glimmers of hope are emerging. A Veterans Affairs study recently found the antiviral Paxlovid might help prevent long COVID. A small case study at Yale found the ADHD drug guanfacine may ease brain fog from long COVID. And preliminary results from an NIH-funded study suggest COVID vaccines might help children with a rare but serious inflammatory condition known as multisystem inflammatory syndrome (MIS-C).
More results are expected very soon from the trial for kids with MIS-C, which can strike suddenly long after a COVID infection clears up. While the exact causes aren’t yet clear, MIS-C can cause dangerous inflammation in the heart, lungs, kidneys, brain, skin, eyes, or gastrointestinal system.
Because the virus often triggered a delayed response of MIS-C in kids who had few if any symptoms of acute COVID-19, scientists wondered whether children infected with the virus might respond to a vaccine dose to prevent MIS-C from developing, Gary Gibbons, MD, director of the National Heart, Lung, and Blood Institute, said during a Dec. 9 presentation at the NIH. It’s not yet clear if vaccination helps, but it doesn’t harm the children, Dr. Gibbons said.
“Indeed, the studies suggest with some relief that yes, these children could be vaccinated safely,” he said.
Several new trials are also testing Paxlovid against long COVID, including one late-stage study that may have results in about a year.
“We already know that Paxlovid reduces the risk of developing long COVID, but it would be a game changer if it can improve long COVID symptoms as well,” said Surendra Barshikar, MD, an associate professor and medical director of the COVID Recover program at the University of Texas Southwestern Medical Center, Dallas.
In other studies, researchers are looking at a wide variety of previously approved and experimental drugs and devices. For example, scientists in New York are testing the mood stabilizer lithium to treat brain fog and fatigue. And researchers in Illinois are investigating efgartigimod, a drug approved for the rare muscle-weakening autoimmune disorder myasthenia gravis, to see if it helps ease a long COVID complication known as POTS that can cause a sudden rapid heart rate and chronic fatigue.
“The good news is that enrollment will proceed quickly, given the vast number of patients,” said Kristin Englund, MD, director of the reCOVer Center of Excellence at the Cleveland Clinic.
This is all encouraging because roughly one in five American adults who have acute COVID infections develop persistent symptoms of long COVID, also known as post–acute sequelae of SARS-CoV-2 (PASC). And many of these long COVID patients have complex, overlapping clusters of symptoms that make traditional treatment approaches largely ineffective against this new, formidable disease.
But not every patient living with long COVID will qualify for trials or find it easy to take part even if they do. Patients should consider how severe their symptoms are, the potential risks of any experimental treatments, and the many challenges they may have with getting to and from clinical trial sites that are largely concentrated around major cities and might be far from home.
While this holds true for any type of trial, it’s essential for long COVID patients, who may have fatigue, muscle weakness, and other symptoms that make distance an impossible factor to ignore, said Aaron Friedberg, MD, clinical colead of the post–COVID-19 recovery program at the Ohio State University Wexner Medical Center, Columbus.
“I think it is a personal decision, since the fatigue and pain that patients with PASC can experience can make it very challenging to travel long distances,” Dr. Friedberg said. “I would recommend calling or messaging ahead to find out exactly what type of travel might be required because there may be steps that can be completed by email or video, which could make it easier to participate, and some trials may be entirely remote.”
Even when patients feel up to the travel, they still might not be a good fit for a clinical trial. Scientists often look for people who didn’t have pre-existing health problems before they got long COVID, Dr. Barshikar noted. Patients taking medications may also be unable to participate in drug trials, particularly for experimental treatments because of concerns about unknown side effects from drug interactions.
When clinical trials do seem like a good option, patients may want to consider seeking treatment at an academic medical center that is already doing long COVID research, particularly if their symptoms are too complex or severe to manage only through their primary care provider, said Jonathan Whiteson, MD, who helped draft long COVID treatment guidelines for the American Academy of Physical Medicine and Rehabilitation. He also serves as codirector of the New York University Langone Health post–COVID care program.
Many health care professionals on the front lines treating long COVID patients are optimistic that the sheer number of trials and the vast number of patients taking part should ultimately produce some better treatment options than people have right now. It’s just not going to happen overnight.
“I suspect that while we will see some new treatments coming in the next 1-2 years, it may be several years before targets can be identified and full trials conducted to see results,” Dr. Friedberg said. “Getting good data takes time.”
A version of this article first appeared on WebMD.com.
What’s next for COVID? Here’s what to know
As holiday celebrations wind down in the United States, COVID is on the rise.
Will there be another winter surge? If so, can we minimize it? How big a role might the boosters play in that? Are more mandates coming, along with a return to closed offices and businesses? Read on for a look at the latest info.
Cases, hospitalizations, deaths
As of Dec. 27, the latest statistics, the Centers for Disease Control and Prevention reports more than 487,000 weekly cases, compared to about 265,000 for the week ending Oct. 12. On average, 4,938 people were admitted to the hospital daily from Dec. 19 to 25, down about 6% from the 5,257 admitted daily the week before.
Deaths totaled 2,952 weekly as of Dec. 21, up from 2,699 on Dec. 14.
“What’s sobering overall is still seeing about 400 deaths a day in the U.S.,” said Peter Chin-Hong, MD, professor of medicine and infectious disease specialist at the University of California, San Francisco. “It’s still very high.”
As of Dec. 17, the variants predominating are BQ.1, BQ.1.1, and XBB. Experts said they are paying close attention to XBB, which is increasing quickly in the Northeast.
Predicting a winter surge
Experts tracking the pandemic agree there will be a surge.
“We are in the midst of it now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape (MDedge’s sister site). “It’s not nearly like what we’ve had in Omicron or other waves; it’s not as severe. But it’s being particularly felt by seniors.”
One bit of good news: “Outside of that group it doesn’t look like – so far – it is going to be as bad a wave [as in the past],” Dr. Topol said.
Predicting the extent of the post-holiday surge “is the billion-dollar question right now,” said Katelyn Jetelina, PhD, a San Diego epidemiologist and author of the newsletter Your Local Epidemiologist.
“Much of these waves are not being driven by subvariants of concern but rather behavior,” she said.
People are opening up their social networks to gather for celebrations and family time. That’s unique to this winter, she said.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
Others point out that the surge doesn’t involve just COVID.
“We are expecting a Christmas surge and we are concerned it might be a triple surge,” said William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., referring to the rising cases of flu and RSV (respiratory syncytial virus).
Dr. Jetelina shares that concern, worrying that those illnesses may be what overwhelms hospital capacity.
Another wild card is the situation in China. With the easing of China’s “zero COVID” policies, cases there are rising dramatically. Some models are predicting up to 1 million COVID deaths could occur in China in 2023. (The United States is now requiring travelers from China to show a negative COVID test before entering. Italy and Japan have taken similar measures.)
“The suffering that is going to occur in China is not good news at all,” Dr. Topol said. “We are going to be seeing that for many weeks if not months ahead.”
Theoretically, uncontained spread such as what is expected there could generate a whole new family of variants, he said. But “the main hit is going to be in China,” he predicted. “But it’s hard to project with accuracy.”
“China is 20% of the global population, so we can’t ignore it,” Dr. Jetelina said. “The question is, what’s the probability of a subvariant of concern coming from China? I think the probability is pretty low, but the possibility is there.”
What happens with cases in China may “throw a wrench” in the transition from pandemic to endemic, Dr. Chin-Hong said. But even if the rising cases in China do result in a new variant, “there’s so much T cell and B cell immunity [here], your average person is still not going to get seriously ill, even if the variant looks really scary.”
Minimizing the damage
Experts echo the same advice on stemming the surge, especially for adults who are 65 or older: Get the bivalent booster, and get it now.
“The same with the influenza vaccine,” Dr. Schaffner said.
Both the booster vaccine and the flu vaccine have been underused this year, he said. “It’s part of the general vaccine fatigue.”
The low uptake of the booster vaccine is concerning, Dr. Topol said, especially among adults aged 65 and older, the age group most vulnerable to severe disease. Just 35.7% of U.S. adults 65 and older have gotten the booster, according to the CDC. Dr. Topol calls that a tragedy.
Younger people have not taken to the booster, either. Overall, only 14.1% of people aged 5 and up have gotten an updated booster dose, according to the CDC.
Recent studies find value in the boosters. One study looked only at adults age 65 or older, finding that the bivalent booster reduced the risk of hospitalization by 84% compared to someone not vaccinated, and 73% compared to someone who had received only the monovalent vaccine. Another study of adults found those who had gotten the bivalent were less likely to need COVID-related emergency room care or urgent care.
In a Dec. 21 report in the New England Journal of Medicine, researchers took plasma samples from people who had gotten either one or two monovalent boosters or the bivalent to determine how well they worked against the circulating Omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB. The bivalent worked better than the monovalent against all the Omicron subvariants, but especially against BA.2.75.2, BQ.1.1, and XBB.
Rapid testing can help minimize transmission. On Dec. 15, the Biden administration announced its Winter Preparedness Plan, urging Americans to test before and after travel as well as indoor visiting with vulnerable individuals, providing another round of free at-home tests, continuing to make community testing available and continuing to provide vaccines.
Besides the general precautions, Dr. Schaffner suggested: “Look at yourself. Who are you? If you are older than 65, or have underlying illness or are immunocompromised, or are pregnant, please put your mask back on. And think about social distancing. It might be time to worship at home and stream a movie,” instead of going to the theaters, he said.
Back to mandates?
On Dec. 9, the New York City Commissioner of Health and Mental Hygiene urged a return to masking indoors, saying people “should” mask up, including in schools, stores, offices, and when in crowded outdoor settings.
On the same date, the County of Los Angeles Public Health urged a return to masking for everyone aged 2 and older when indoors, including at schools, in transit, or in work sites when around others.
While the CDC order requiring masks on public transportation is no longer in effect, the agency continues to recommend that those using public transportation do so.
But some are taking that further. In Philadelphia, for example, School Superintendent Tony B. Watlington Sr., EdD, announced before the winter break that indoor masking would be required for all students and staff for the first 2 weeks of school return, through Jan. 13, citing guidance from the Philadelphia Department of Public Health.
Universal masking in schools does reduce COVID transmission, as a study published in late November suggests. After Massachusetts dropped the statewide universal masking policy in public schools in February 2022, researchers compared the incidence of COVID in 70 school districts there that dropped the mandate with two school districts that kept it. In the 15 weeks after the policy was rescinded, the lifting of the mandate was linked with an additional 44.9 cases of COVID per 1,000 students and staff. That corresponded to an estimated 11,901 cases and to nearly 30% of the cases in all districts during that time.
That said, experts see mandates as the exception rather than the rule, at least for now, citing public backlash against mandates to mask or follow other restrictions.
“Mandating, we know, it shuts people off,” Dr. Topol said. “It’s unenforceable. If you have a very strong recommendation, that’s probably as good as you’re going to be able to do right now.”
There may be communities where mandates go over better than others, Dr. Schaffner said, such as communities where people have confidence in their public health authorities.
Glimmers of hope
Despite uncertainties, experts offered some not-so-dismal perspectives as well.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
A version of this article first appeared on WebMD.com.
As holiday celebrations wind down in the United States, COVID is on the rise.
Will there be another winter surge? If so, can we minimize it? How big a role might the boosters play in that? Are more mandates coming, along with a return to closed offices and businesses? Read on for a look at the latest info.
Cases, hospitalizations, deaths
As of Dec. 27, the latest statistics, the Centers for Disease Control and Prevention reports more than 487,000 weekly cases, compared to about 265,000 for the week ending Oct. 12. On average, 4,938 people were admitted to the hospital daily from Dec. 19 to 25, down about 6% from the 5,257 admitted daily the week before.
Deaths totaled 2,952 weekly as of Dec. 21, up from 2,699 on Dec. 14.
“What’s sobering overall is still seeing about 400 deaths a day in the U.S.,” said Peter Chin-Hong, MD, professor of medicine and infectious disease specialist at the University of California, San Francisco. “It’s still very high.”
As of Dec. 17, the variants predominating are BQ.1, BQ.1.1, and XBB. Experts said they are paying close attention to XBB, which is increasing quickly in the Northeast.
Predicting a winter surge
Experts tracking the pandemic agree there will be a surge.
“We are in the midst of it now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape (MDedge’s sister site). “It’s not nearly like what we’ve had in Omicron or other waves; it’s not as severe. But it’s being particularly felt by seniors.”
One bit of good news: “Outside of that group it doesn’t look like – so far – it is going to be as bad a wave [as in the past],” Dr. Topol said.
Predicting the extent of the post-holiday surge “is the billion-dollar question right now,” said Katelyn Jetelina, PhD, a San Diego epidemiologist and author of the newsletter Your Local Epidemiologist.
“Much of these waves are not being driven by subvariants of concern but rather behavior,” she said.
People are opening up their social networks to gather for celebrations and family time. That’s unique to this winter, she said.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
Others point out that the surge doesn’t involve just COVID.
“We are expecting a Christmas surge and we are concerned it might be a triple surge,” said William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., referring to the rising cases of flu and RSV (respiratory syncytial virus).
Dr. Jetelina shares that concern, worrying that those illnesses may be what overwhelms hospital capacity.
Another wild card is the situation in China. With the easing of China’s “zero COVID” policies, cases there are rising dramatically. Some models are predicting up to 1 million COVID deaths could occur in China in 2023. (The United States is now requiring travelers from China to show a negative COVID test before entering. Italy and Japan have taken similar measures.)
“The suffering that is going to occur in China is not good news at all,” Dr. Topol said. “We are going to be seeing that for many weeks if not months ahead.”
Theoretically, uncontained spread such as what is expected there could generate a whole new family of variants, he said. But “the main hit is going to be in China,” he predicted. “But it’s hard to project with accuracy.”
“China is 20% of the global population, so we can’t ignore it,” Dr. Jetelina said. “The question is, what’s the probability of a subvariant of concern coming from China? I think the probability is pretty low, but the possibility is there.”
What happens with cases in China may “throw a wrench” in the transition from pandemic to endemic, Dr. Chin-Hong said. But even if the rising cases in China do result in a new variant, “there’s so much T cell and B cell immunity [here], your average person is still not going to get seriously ill, even if the variant looks really scary.”
Minimizing the damage
Experts echo the same advice on stemming the surge, especially for adults who are 65 or older: Get the bivalent booster, and get it now.
“The same with the influenza vaccine,” Dr. Schaffner said.
Both the booster vaccine and the flu vaccine have been underused this year, he said. “It’s part of the general vaccine fatigue.”
The low uptake of the booster vaccine is concerning, Dr. Topol said, especially among adults aged 65 and older, the age group most vulnerable to severe disease. Just 35.7% of U.S. adults 65 and older have gotten the booster, according to the CDC. Dr. Topol calls that a tragedy.
Younger people have not taken to the booster, either. Overall, only 14.1% of people aged 5 and up have gotten an updated booster dose, according to the CDC.
Recent studies find value in the boosters. One study looked only at adults age 65 or older, finding that the bivalent booster reduced the risk of hospitalization by 84% compared to someone not vaccinated, and 73% compared to someone who had received only the monovalent vaccine. Another study of adults found those who had gotten the bivalent were less likely to need COVID-related emergency room care or urgent care.
In a Dec. 21 report in the New England Journal of Medicine, researchers took plasma samples from people who had gotten either one or two monovalent boosters or the bivalent to determine how well they worked against the circulating Omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB. The bivalent worked better than the monovalent against all the Omicron subvariants, but especially against BA.2.75.2, BQ.1.1, and XBB.
Rapid testing can help minimize transmission. On Dec. 15, the Biden administration announced its Winter Preparedness Plan, urging Americans to test before and after travel as well as indoor visiting with vulnerable individuals, providing another round of free at-home tests, continuing to make community testing available and continuing to provide vaccines.
Besides the general precautions, Dr. Schaffner suggested: “Look at yourself. Who are you? If you are older than 65, or have underlying illness or are immunocompromised, or are pregnant, please put your mask back on. And think about social distancing. It might be time to worship at home and stream a movie,” instead of going to the theaters, he said.
Back to mandates?
On Dec. 9, the New York City Commissioner of Health and Mental Hygiene urged a return to masking indoors, saying people “should” mask up, including in schools, stores, offices, and when in crowded outdoor settings.
On the same date, the County of Los Angeles Public Health urged a return to masking for everyone aged 2 and older when indoors, including at schools, in transit, or in work sites when around others.
While the CDC order requiring masks on public transportation is no longer in effect, the agency continues to recommend that those using public transportation do so.
But some are taking that further. In Philadelphia, for example, School Superintendent Tony B. Watlington Sr., EdD, announced before the winter break that indoor masking would be required for all students and staff for the first 2 weeks of school return, through Jan. 13, citing guidance from the Philadelphia Department of Public Health.
Universal masking in schools does reduce COVID transmission, as a study published in late November suggests. After Massachusetts dropped the statewide universal masking policy in public schools in February 2022, researchers compared the incidence of COVID in 70 school districts there that dropped the mandate with two school districts that kept it. In the 15 weeks after the policy was rescinded, the lifting of the mandate was linked with an additional 44.9 cases of COVID per 1,000 students and staff. That corresponded to an estimated 11,901 cases and to nearly 30% of the cases in all districts during that time.
That said, experts see mandates as the exception rather than the rule, at least for now, citing public backlash against mandates to mask or follow other restrictions.
“Mandating, we know, it shuts people off,” Dr. Topol said. “It’s unenforceable. If you have a very strong recommendation, that’s probably as good as you’re going to be able to do right now.”
There may be communities where mandates go over better than others, Dr. Schaffner said, such as communities where people have confidence in their public health authorities.
Glimmers of hope
Despite uncertainties, experts offered some not-so-dismal perspectives as well.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
A version of this article first appeared on WebMD.com.
As holiday celebrations wind down in the United States, COVID is on the rise.
Will there be another winter surge? If so, can we minimize it? How big a role might the boosters play in that? Are more mandates coming, along with a return to closed offices and businesses? Read on for a look at the latest info.
Cases, hospitalizations, deaths
As of Dec. 27, the latest statistics, the Centers for Disease Control and Prevention reports more than 487,000 weekly cases, compared to about 265,000 for the week ending Oct. 12. On average, 4,938 people were admitted to the hospital daily from Dec. 19 to 25, down about 6% from the 5,257 admitted daily the week before.
Deaths totaled 2,952 weekly as of Dec. 21, up from 2,699 on Dec. 14.
“What’s sobering overall is still seeing about 400 deaths a day in the U.S.,” said Peter Chin-Hong, MD, professor of medicine and infectious disease specialist at the University of California, San Francisco. “It’s still very high.”
As of Dec. 17, the variants predominating are BQ.1, BQ.1.1, and XBB. Experts said they are paying close attention to XBB, which is increasing quickly in the Northeast.
Predicting a winter surge
Experts tracking the pandemic agree there will be a surge.
“We are in the midst of it now,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape (MDedge’s sister site). “It’s not nearly like what we’ve had in Omicron or other waves; it’s not as severe. But it’s being particularly felt by seniors.”
One bit of good news: “Outside of that group it doesn’t look like – so far – it is going to be as bad a wave [as in the past],” Dr. Topol said.
Predicting the extent of the post-holiday surge “is the billion-dollar question right now,” said Katelyn Jetelina, PhD, a San Diego epidemiologist and author of the newsletter Your Local Epidemiologist.
“Much of these waves are not being driven by subvariants of concern but rather behavior,” she said.
People are opening up their social networks to gather for celebrations and family time. That’s unique to this winter, she said.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
Others point out that the surge doesn’t involve just COVID.
“We are expecting a Christmas surge and we are concerned it might be a triple surge,” said William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., referring to the rising cases of flu and RSV (respiratory syncytial virus).
Dr. Jetelina shares that concern, worrying that those illnesses may be what overwhelms hospital capacity.
Another wild card is the situation in China. With the easing of China’s “zero COVID” policies, cases there are rising dramatically. Some models are predicting up to 1 million COVID deaths could occur in China in 2023. (The United States is now requiring travelers from China to show a negative COVID test before entering. Italy and Japan have taken similar measures.)
“The suffering that is going to occur in China is not good news at all,” Dr. Topol said. “We are going to be seeing that for many weeks if not months ahead.”
Theoretically, uncontained spread such as what is expected there could generate a whole new family of variants, he said. But “the main hit is going to be in China,” he predicted. “But it’s hard to project with accuracy.”
“China is 20% of the global population, so we can’t ignore it,” Dr. Jetelina said. “The question is, what’s the probability of a subvariant of concern coming from China? I think the probability is pretty low, but the possibility is there.”
What happens with cases in China may “throw a wrench” in the transition from pandemic to endemic, Dr. Chin-Hong said. But even if the rising cases in China do result in a new variant, “there’s so much T cell and B cell immunity [here], your average person is still not going to get seriously ill, even if the variant looks really scary.”
Minimizing the damage
Experts echo the same advice on stemming the surge, especially for adults who are 65 or older: Get the bivalent booster, and get it now.
“The same with the influenza vaccine,” Dr. Schaffner said.
Both the booster vaccine and the flu vaccine have been underused this year, he said. “It’s part of the general vaccine fatigue.”
The low uptake of the booster vaccine is concerning, Dr. Topol said, especially among adults aged 65 and older, the age group most vulnerable to severe disease. Just 35.7% of U.S. adults 65 and older have gotten the booster, according to the CDC. Dr. Topol calls that a tragedy.
Younger people have not taken to the booster, either. Overall, only 14.1% of people aged 5 and up have gotten an updated booster dose, according to the CDC.
Recent studies find value in the boosters. One study looked only at adults age 65 or older, finding that the bivalent booster reduced the risk of hospitalization by 84% compared to someone not vaccinated, and 73% compared to someone who had received only the monovalent vaccine. Another study of adults found those who had gotten the bivalent were less likely to need COVID-related emergency room care or urgent care.
In a Dec. 21 report in the New England Journal of Medicine, researchers took plasma samples from people who had gotten either one or two monovalent boosters or the bivalent to determine how well they worked against the circulating Omicron subvariants BA.1, BA.5, BA.2.75.2, BQ.1.1, and XBB. The bivalent worked better than the monovalent against all the Omicron subvariants, but especially against BA.2.75.2, BQ.1.1, and XBB.
Rapid testing can help minimize transmission. On Dec. 15, the Biden administration announced its Winter Preparedness Plan, urging Americans to test before and after travel as well as indoor visiting with vulnerable individuals, providing another round of free at-home tests, continuing to make community testing available and continuing to provide vaccines.
Besides the general precautions, Dr. Schaffner suggested: “Look at yourself. Who are you? If you are older than 65, or have underlying illness or are immunocompromised, or are pregnant, please put your mask back on. And think about social distancing. It might be time to worship at home and stream a movie,” instead of going to the theaters, he said.
Back to mandates?
On Dec. 9, the New York City Commissioner of Health and Mental Hygiene urged a return to masking indoors, saying people “should” mask up, including in schools, stores, offices, and when in crowded outdoor settings.
On the same date, the County of Los Angeles Public Health urged a return to masking for everyone aged 2 and older when indoors, including at schools, in transit, or in work sites when around others.
While the CDC order requiring masks on public transportation is no longer in effect, the agency continues to recommend that those using public transportation do so.
But some are taking that further. In Philadelphia, for example, School Superintendent Tony B. Watlington Sr., EdD, announced before the winter break that indoor masking would be required for all students and staff for the first 2 weeks of school return, through Jan. 13, citing guidance from the Philadelphia Department of Public Health.
Universal masking in schools does reduce COVID transmission, as a study published in late November suggests. After Massachusetts dropped the statewide universal masking policy in public schools in February 2022, researchers compared the incidence of COVID in 70 school districts there that dropped the mandate with two school districts that kept it. In the 15 weeks after the policy was rescinded, the lifting of the mandate was linked with an additional 44.9 cases of COVID per 1,000 students and staff. That corresponded to an estimated 11,901 cases and to nearly 30% of the cases in all districts during that time.
That said, experts see mandates as the exception rather than the rule, at least for now, citing public backlash against mandates to mask or follow other restrictions.
“Mandating, we know, it shuts people off,” Dr. Topol said. “It’s unenforceable. If you have a very strong recommendation, that’s probably as good as you’re going to be able to do right now.”
There may be communities where mandates go over better than others, Dr. Schaffner said, such as communities where people have confidence in their public health authorities.
Glimmers of hope
Despite uncertainties, experts offered some not-so-dismal perspectives as well.
“I think our numbers will continue to go up, but certainly not like 2021 or 2020,” Dr. Chin-Hong said.
A version of this article first appeared on WebMD.com.
Even with insurance, EDs can cost a bundle
In 2019, the study shows, patients enrolled in big companies’ health plans paid an average of $646 in copays and deductibles for each ED visit. A quarter of visits cost more than $907 out of pocket, and another quarter cost under $128.
About half of households can’t afford to pay the average deductible in an employer-sponsored insurance plan, the report notes. And more than a third of U.S. adults are unable to afford a $400 medical expense without borrowing.
While it’s not known how many people don’t go to an emergency department because of the anticipated cost, almost half of U.S. adults report that they’ve delayed care because of costs, according to a recent Kaiser survey.
One problem that people often face when deciding whether to visit an ED is that they don’t know how serious their condition is and what emergency care will cost, says Hope Schwartz, lead author of the report.
“When they go to the [ED], they don’t always know what their diagnosis will be and what their treatment costs will be. What we highlighted is that those costs could be very high or very low, and there’s no way to tell beforehand,” she says.
What costs so much?
Based on the paid claims data used in the study, health plans and patients paid a combined average of $2,453 for an ER visit. A quarter of visits cost $970 or less, and a quarter cost $3,043 or more.
Emergency department claims include professional fees and facility fees. The facility fees, which cover the cost of a hospital maintaining an ED 24/7, made up 80% of total costs, including a portion of doctors’ claims as well as laboratory and imaging fees.
But doctors’ claims for evaluation and management services were the largest part of costs, averaging $1,134 per visit. Procedures and treatments cost over $1,100 per visit, on average, while the average imaging claim cost $483, and the average cost for lab work was $230.
More than half of visits generated imaging claims, and about half of visits included lab claims.
The Kaiser Family Foundation report also looked at the costs of several common ED diagnoses. The most expensive diagnosis was appendicitis, which cost nearly twice as much as heart attacks, partly because it often led to surgery in the emergency department. On average, a visit for appendicitis cost $9,535, of which $1,717 was an out-of-pocket expense.
In addition, the researchers examined lower-cost diagnoses that generally do not require imaging or extensive treatment in the ED. These included upper respiratory tract infections ($1,535 total, $523 out of pocket), skin and soft tissue infections ($2,005 total, $572 out of pocket), and urinary tract infections ($2,726 total, $683 out of pocket).
While these diagnoses can sometimes require admission to the hospital, in otherwise healthy adults they are typically evaluated with basic lab tests, and patients are discharged with prescriptions, according to the report.
Complexities of billing
ED visits are given codes to help show how complex the task or service was during the billing process. These codes have five levels.
Less complex visits require straightforward medical decision-making, such as rashes or medication refills. Patients with level 5 codes require highly complex decision-making and include life- or limb-threatening conditions, such as severe infections or heart attacks.
The less complex visits cost $592, on average, with patients responsible for $205 of that. For the most complex visits, the health plan covered $3,015, on average, or eight times the cost of the lowest-coded visits.
On average, patients paid $840 out of pocket for the most complex visits – four times the average costs for the less complex visits.
One reason for the rise in spending for ED visits is a national shift to higher-level ED billing codes, says Ms. Schwartz, who is a Kaiser Family Foundation health policy fellow and a medical student. “There has been good work done showing that [ED] visits are increasingly being billed as a level 4 or 5, whereas in previous years, they might have been billed as a level 3.
“Whether a hospital bills a level 4 or a level 5 code for your visit can make a really big difference in how much you pay. And if you come in not knowing what services you’re going to get, you don’t know if you’re going to get a level 3, 4, or 5 code, and the costs increase pretty quickly,” she says.
Costs vary by region
The report includes an analysis of emergency department costs in the 20 largest metropolitan areas in the United States. Overall, the researchers found, San Diego had the most expensive ED visits. Emergency departments in San Diego charged about twice as much per visit, on average, as those in Baltimore.
While there were expensive areas all across the country, many of the costliest places were in Texas, Florida, California, Colorado, and New York. The report noted that the most expensive regions for ED care did not align with the regions that had the most expensive health care overall.
“These comparisons suggest that our findings are not solely related to overall high health care prices in these areas and may reflect other factors, including the age and medical complexity of the population or differences in local norms and practice patterns,” the report says.
Healthier people
In addition to these geographical differences, the incidence of emergency department visits by those with employers’ insurance differed from that of the general population.
During the study year, the report found, 12% of the insured had at least one ED visit – a percentage that didn’t vary for any age group under 65, including children. (No patients 65 or older were included in the study.)
By comparison, a government survey shows that in 2019, 21% of all U.S. adults 18-44 had one or more emergency department visits. Among those 45-64, 20% made at least one ED visit.
A version of this article first appeared on WebMD.com.
In 2019, the study shows, patients enrolled in big companies’ health plans paid an average of $646 in copays and deductibles for each ED visit. A quarter of visits cost more than $907 out of pocket, and another quarter cost under $128.
About half of households can’t afford to pay the average deductible in an employer-sponsored insurance plan, the report notes. And more than a third of U.S. adults are unable to afford a $400 medical expense without borrowing.
While it’s not known how many people don’t go to an emergency department because of the anticipated cost, almost half of U.S. adults report that they’ve delayed care because of costs, according to a recent Kaiser survey.
One problem that people often face when deciding whether to visit an ED is that they don’t know how serious their condition is and what emergency care will cost, says Hope Schwartz, lead author of the report.
“When they go to the [ED], they don’t always know what their diagnosis will be and what their treatment costs will be. What we highlighted is that those costs could be very high or very low, and there’s no way to tell beforehand,” she says.
What costs so much?
Based on the paid claims data used in the study, health plans and patients paid a combined average of $2,453 for an ER visit. A quarter of visits cost $970 or less, and a quarter cost $3,043 or more.
Emergency department claims include professional fees and facility fees. The facility fees, which cover the cost of a hospital maintaining an ED 24/7, made up 80% of total costs, including a portion of doctors’ claims as well as laboratory and imaging fees.
But doctors’ claims for evaluation and management services were the largest part of costs, averaging $1,134 per visit. Procedures and treatments cost over $1,100 per visit, on average, while the average imaging claim cost $483, and the average cost for lab work was $230.
More than half of visits generated imaging claims, and about half of visits included lab claims.
The Kaiser Family Foundation report also looked at the costs of several common ED diagnoses. The most expensive diagnosis was appendicitis, which cost nearly twice as much as heart attacks, partly because it often led to surgery in the emergency department. On average, a visit for appendicitis cost $9,535, of which $1,717 was an out-of-pocket expense.
In addition, the researchers examined lower-cost diagnoses that generally do not require imaging or extensive treatment in the ED. These included upper respiratory tract infections ($1,535 total, $523 out of pocket), skin and soft tissue infections ($2,005 total, $572 out of pocket), and urinary tract infections ($2,726 total, $683 out of pocket).
While these diagnoses can sometimes require admission to the hospital, in otherwise healthy adults they are typically evaluated with basic lab tests, and patients are discharged with prescriptions, according to the report.
Complexities of billing
ED visits are given codes to help show how complex the task or service was during the billing process. These codes have five levels.
Less complex visits require straightforward medical decision-making, such as rashes or medication refills. Patients with level 5 codes require highly complex decision-making and include life- or limb-threatening conditions, such as severe infections or heart attacks.
The less complex visits cost $592, on average, with patients responsible for $205 of that. For the most complex visits, the health plan covered $3,015, on average, or eight times the cost of the lowest-coded visits.
On average, patients paid $840 out of pocket for the most complex visits – four times the average costs for the less complex visits.
One reason for the rise in spending for ED visits is a national shift to higher-level ED billing codes, says Ms. Schwartz, who is a Kaiser Family Foundation health policy fellow and a medical student. “There has been good work done showing that [ED] visits are increasingly being billed as a level 4 or 5, whereas in previous years, they might have been billed as a level 3.
“Whether a hospital bills a level 4 or a level 5 code for your visit can make a really big difference in how much you pay. And if you come in not knowing what services you’re going to get, you don’t know if you’re going to get a level 3, 4, or 5 code, and the costs increase pretty quickly,” she says.
Costs vary by region
The report includes an analysis of emergency department costs in the 20 largest metropolitan areas in the United States. Overall, the researchers found, San Diego had the most expensive ED visits. Emergency departments in San Diego charged about twice as much per visit, on average, as those in Baltimore.
While there were expensive areas all across the country, many of the costliest places were in Texas, Florida, California, Colorado, and New York. The report noted that the most expensive regions for ED care did not align with the regions that had the most expensive health care overall.
“These comparisons suggest that our findings are not solely related to overall high health care prices in these areas and may reflect other factors, including the age and medical complexity of the population or differences in local norms and practice patterns,” the report says.
Healthier people
In addition to these geographical differences, the incidence of emergency department visits by those with employers’ insurance differed from that of the general population.
During the study year, the report found, 12% of the insured had at least one ED visit – a percentage that didn’t vary for any age group under 65, including children. (No patients 65 or older were included in the study.)
By comparison, a government survey shows that in 2019, 21% of all U.S. adults 18-44 had one or more emergency department visits. Among those 45-64, 20% made at least one ED visit.
A version of this article first appeared on WebMD.com.
In 2019, the study shows, patients enrolled in big companies’ health plans paid an average of $646 in copays and deductibles for each ED visit. A quarter of visits cost more than $907 out of pocket, and another quarter cost under $128.
About half of households can’t afford to pay the average deductible in an employer-sponsored insurance plan, the report notes. And more than a third of U.S. adults are unable to afford a $400 medical expense without borrowing.
While it’s not known how many people don’t go to an emergency department because of the anticipated cost, almost half of U.S. adults report that they’ve delayed care because of costs, according to a recent Kaiser survey.
One problem that people often face when deciding whether to visit an ED is that they don’t know how serious their condition is and what emergency care will cost, says Hope Schwartz, lead author of the report.
“When they go to the [ED], they don’t always know what their diagnosis will be and what their treatment costs will be. What we highlighted is that those costs could be very high or very low, and there’s no way to tell beforehand,” she says.
What costs so much?
Based on the paid claims data used in the study, health plans and patients paid a combined average of $2,453 for an ER visit. A quarter of visits cost $970 or less, and a quarter cost $3,043 or more.
Emergency department claims include professional fees and facility fees. The facility fees, which cover the cost of a hospital maintaining an ED 24/7, made up 80% of total costs, including a portion of doctors’ claims as well as laboratory and imaging fees.
But doctors’ claims for evaluation and management services were the largest part of costs, averaging $1,134 per visit. Procedures and treatments cost over $1,100 per visit, on average, while the average imaging claim cost $483, and the average cost for lab work was $230.
More than half of visits generated imaging claims, and about half of visits included lab claims.
The Kaiser Family Foundation report also looked at the costs of several common ED diagnoses. The most expensive diagnosis was appendicitis, which cost nearly twice as much as heart attacks, partly because it often led to surgery in the emergency department. On average, a visit for appendicitis cost $9,535, of which $1,717 was an out-of-pocket expense.
In addition, the researchers examined lower-cost diagnoses that generally do not require imaging or extensive treatment in the ED. These included upper respiratory tract infections ($1,535 total, $523 out of pocket), skin and soft tissue infections ($2,005 total, $572 out of pocket), and urinary tract infections ($2,726 total, $683 out of pocket).
While these diagnoses can sometimes require admission to the hospital, in otherwise healthy adults they are typically evaluated with basic lab tests, and patients are discharged with prescriptions, according to the report.
Complexities of billing
ED visits are given codes to help show how complex the task or service was during the billing process. These codes have five levels.
Less complex visits require straightforward medical decision-making, such as rashes or medication refills. Patients with level 5 codes require highly complex decision-making and include life- or limb-threatening conditions, such as severe infections or heart attacks.
The less complex visits cost $592, on average, with patients responsible for $205 of that. For the most complex visits, the health plan covered $3,015, on average, or eight times the cost of the lowest-coded visits.
On average, patients paid $840 out of pocket for the most complex visits – four times the average costs for the less complex visits.
One reason for the rise in spending for ED visits is a national shift to higher-level ED billing codes, says Ms. Schwartz, who is a Kaiser Family Foundation health policy fellow and a medical student. “There has been good work done showing that [ED] visits are increasingly being billed as a level 4 or 5, whereas in previous years, they might have been billed as a level 3.
“Whether a hospital bills a level 4 or a level 5 code for your visit can make a really big difference in how much you pay. And if you come in not knowing what services you’re going to get, you don’t know if you’re going to get a level 3, 4, or 5 code, and the costs increase pretty quickly,” she says.
Costs vary by region
The report includes an analysis of emergency department costs in the 20 largest metropolitan areas in the United States. Overall, the researchers found, San Diego had the most expensive ED visits. Emergency departments in San Diego charged about twice as much per visit, on average, as those in Baltimore.
While there were expensive areas all across the country, many of the costliest places were in Texas, Florida, California, Colorado, and New York. The report noted that the most expensive regions for ED care did not align with the regions that had the most expensive health care overall.
“These comparisons suggest that our findings are not solely related to overall high health care prices in these areas and may reflect other factors, including the age and medical complexity of the population or differences in local norms and practice patterns,” the report says.
Healthier people
In addition to these geographical differences, the incidence of emergency department visits by those with employers’ insurance differed from that of the general population.
During the study year, the report found, 12% of the insured had at least one ED visit – a percentage that didn’t vary for any age group under 65, including children. (No patients 65 or older were included in the study.)
By comparison, a government survey shows that in 2019, 21% of all U.S. adults 18-44 had one or more emergency department visits. Among those 45-64, 20% made at least one ED visit.
A version of this article first appeared on WebMD.com.
Comorbidities and the prognosis of chronic obstructive pulmonary disease
Strict control of comorbidities in patients with chronic obstructive pulmonary disease decreases exacerbations and morbimortality, and avoids readmissions. An increasing number of women have the disease, which progresses differently in women than in men and even has different comorbidities.
said Belén Alonso, MD, PhD, coordinator of the COPD Working Group of the Spanish Society of Internal Medicine. Up to 73 comorbidities associated with chronic obstructive pulmonary disease have been described. Dr. Alonso made these remarks during her presentation at the Comorbidities in Chronic Obstructive Pulmonary Disease Panel, which took place during the 43rd Conference of the Spanish Society of Internal Medicine (SEMI), in Gijón, Spain.
According to the scientific society’s press release, moderator María Gómez Antúnez, MD, stated, “The correct approach and treatment of these comorbidities is fundamental to improve the quality of life of the patient, decrease exacerbations, avoid readmissions, and decrease morbimortality in people with chronic obstructive pulmonary disease.”
The different works published, two of them by the SEMI COPD Working Group (ECCO and ESMI studies), indicate that the main comorbidities of patients with that pneumopathy are arterial hypertension, dyslipidemia, diabetes, heart failure, atrial fibrillation, ischemic heart disease, chronic kidney disease, peripheral arterial disease, and osteoporosis. Chronic hepatopathy, pulmonary neoplasm, depression, and cerebrovascular disease are less common.
73 comorbidities described
Dr. Alonso told this news organization, “Of those 73 comorbidities, some of the lesser known or less attention grabbing, according to a paper that we brought to the panel, include sleep disorders that encompass insomnia, nightmares, night terrors, sleep apneas, or hypopneas. Other lesser-known comorbidities related to cognitive decline, with patterns that reflect that up to 60% [of patients] may have some degree of deterioration, involve the disease phase, hypoxemia, or degree of inflammation. On the other hand, it has also been associated with Parkinson’s disease and gastroesophageal reflux, among many more that arise from the cardiovascular sphere.”
One paper reveals that more than 78% of patients with chronic obstructive pulmonary disease have one associated comorbidity, almost 69% have two, and 47.9% have three.
“Based on gender, comorbidities are different. In women, it is well observed that anxiety, depression, and osteoporosis are more common. However, hypertension, ischemic heart disease, and diabetes are more common in men with chronic obstructive pulmonary disease,” she stated.
“The pulmonary disease in question also progresses differently in men and women. In women, onset is at younger ages – between 40 and 50 years – and in men, after 50. Likewise, it appears that the disease progresses more quickly, which coincides with a worse quality of life (since dyspnea is tolerated less) and exceeds the anatomical differences, where hormonal influences play a dominant role,” Dr. Alonso stressed.
Reciprocal prognosis
Dr. Alonso stated, “The prognostic importance of comorbidities in the disease is reciprocal. In other words, if there are comorbidities that we do not look for or treat, they are going to have a negative influence on the chronic obstructive pulmonary disease. The disease will progress more and elevate the risk of exacerbations (the most important prognostic factor of that disease). In turn, if we are not treating the disease well, not only pharmacologically, it will have negative repercussions on the comorbidities. It will progress and have negative connotations, such as diabetes or ischemic heart disease.”
The aforementioned ECCO and ESMI studies include patients in internal medicine with exacerbations where the most common comorbidities have been mapped out, although there is also extensive research on comorbidities in patients who are admitted to departments other than internal medicine. “With regard to prognostic implications, our working group very clearly observed the comorbidities and the comorbidome, that solar system that appears so much in medical conferences and forums, which implies that proximity to the center of that solar system is related more to mortality, anxiety, depression, and breast cancer. Other pathologies, such as ischemic heart disease or dyslipidemia, are outside of that territory of greater risk, in which we have been more pioneering than other groups,” said Dr. Alonso.
The current trend is that the age of these patients is increasing, and there are more and more women with this pathology. According to the latest report from the Ministry of Health on respiratory diseases, the prevalence of chronic obstructive pulmonary disease among the population 40 years and older is around 33.9 cases per 1,000 inhabitants, more than twice as common in men than in women (47.7 vs. 21.3). Prevalence increases with age after 40 years progressively until reaching the greatest frequency in the 80- to 84-year-old age group.
In 2019, the number of deaths due to chronic obstructive pulmonary disease in Spain was 13,808 (9,907 men and 3,901 women), with a crude mortality rate of 29.3 deaths per 100,000 inhabitants. This toll decreased in comparison with that of 2018. Chronic obstructive pulmonary disease causes 2.5 times more deaths in men than in women. From 2001 to 2019, mortality due to that pathology declined by 43% in men and women. The decrease was almost 50% in men and 33% in women.
Overlap syndrome prevalent
Javier Sánchez Lora, MD, of the internal medicine department of the Virgen de la Victoria de Málaga University Clinical Hospital, discussed chronic obstructive pulmonary disease and sleep disorders. More concretely, he spoke about overlap syndrome: chronic obstructive pulmonary disease plus obstructive sleep apnea. According to the international consensus document on obstructive sleep apnea, the diagnosis requires an apnea-hypopnea index (AHI) equal to or greater than 15 per hour or equal to or greater than 5. The patient must also have one or more of the following factors: excessive daytime sleepiness, sleep that is not restful, excessive fatigue, and deterioration in quality of life related to sleep and not justified by other causes.
“The overlap syndrome affects 3%-66% of chronic obstructive pulmonary diseases and 7%-55% of obstructive sleep apnea,” said Dr. Sánchez Lora. This syndrome has important effects on different systems: at the cardiovascular level (arterial and pulmonary hypertension, heart failure, stroke, arrhythmias, ischemic heart disease, pulmonary thromboembolism), metabolic (insulin resistance, diabetes, metabolic syndrome), neurocognitive (dementia, depression), and neoplastic (lung, pancreas, esophagus) effects.
“These patients have a worse prognosis than those that have these pathologies alone. During sleep, they experience more frequent episodes of oxygen desaturation and they have a longer total period of sleep with hypoxemia and hypercapnia than those with obstructive apnea alone without chronic obstructive pulmonary disease,” said Dr. Sánchez Lora.
The apneic events of patients with the syndrome have a more profound hypoxemia and more arrhythmias, in addition to them being more susceptible to developing pulmonary hypertension than those with chronic obstructive pulmonary disease or sleep apnea alone. “The good news is that in patients with overlap, the use of ventilation with positive pressure reduces all causes of hospitalization and the visits to the emergency room, as well as the moderate and severe exacerbations of the disease.”
Dr. Sánchez Lora referred to a series of recommendations in clinical practice for the diagnosis and treatment of overlap syndrome: screening, combined therapy of hygienic-dietary measures, and the use of continuous positive respiratory pressure. Oxygen therapy to correct isolated nocturnal desaturations has not shown benefits in survival, although a benefit trial of symptoms attributed to nocturnal hypoxemia in patients with significant comorbidity can be conducted.
Underdiagnosis
“During the panel, we also spoke about the importance that as part of internal medicine we need to make an effort to reduce the underdiagnosis of chronic pulmonary disease and its comorbidities. Specialists in internal medicine need to become aware that this pathology is not only pulmonary, but also multisystemic, complex, heterogenous, and very variable even in the same patient,” said Dr. Sánchez Lora.
Dr. Alonso said, “Regarding the importance of diagnosis of this disease, we continue with an underdiagnosis greater than 70% for men and 80% for women. Secondly, we need to actively seek out the comorbidities associated with chronic obstructive pulmonary disease, even taking advantage of the admission of these patients with exacerbations, which are undesired and common.
“Regarding ongoing trials, we have a study that started during the COVID-19 pandemic, ADEG-EPOC, that involves the adaptation to and impact of severe and very severe exacerbations in patients admitted to our departments,” the specialist indicated.
“In the group, we are also planning to publish an updated agreement, which we already made in 2014, on the most common and important comorbidities associated with chronic obstructive pulmonary disease.” The agreement discusses the 20 most important comorbidities. In addition, the 2023 Gold Guide, which appeared in November 2022, includes a new chapter on updated treatment and the latest developments.
In the last 5 years, Dr. Alonso has collaborated with Abbott, AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, Ferrer, Fresenius Kabi, GSK, Nestlé, Novo Nordisk, Nutricia, and Menarini. Dr. Sánchez Lora has collaborated with AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, GSK, and Menarini.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Strict control of comorbidities in patients with chronic obstructive pulmonary disease decreases exacerbations and morbimortality, and avoids readmissions. An increasing number of women have the disease, which progresses differently in women than in men and even has different comorbidities.
said Belén Alonso, MD, PhD, coordinator of the COPD Working Group of the Spanish Society of Internal Medicine. Up to 73 comorbidities associated with chronic obstructive pulmonary disease have been described. Dr. Alonso made these remarks during her presentation at the Comorbidities in Chronic Obstructive Pulmonary Disease Panel, which took place during the 43rd Conference of the Spanish Society of Internal Medicine (SEMI), in Gijón, Spain.
According to the scientific society’s press release, moderator María Gómez Antúnez, MD, stated, “The correct approach and treatment of these comorbidities is fundamental to improve the quality of life of the patient, decrease exacerbations, avoid readmissions, and decrease morbimortality in people with chronic obstructive pulmonary disease.”
The different works published, two of them by the SEMI COPD Working Group (ECCO and ESMI studies), indicate that the main comorbidities of patients with that pneumopathy are arterial hypertension, dyslipidemia, diabetes, heart failure, atrial fibrillation, ischemic heart disease, chronic kidney disease, peripheral arterial disease, and osteoporosis. Chronic hepatopathy, pulmonary neoplasm, depression, and cerebrovascular disease are less common.
73 comorbidities described
Dr. Alonso told this news organization, “Of those 73 comorbidities, some of the lesser known or less attention grabbing, according to a paper that we brought to the panel, include sleep disorders that encompass insomnia, nightmares, night terrors, sleep apneas, or hypopneas. Other lesser-known comorbidities related to cognitive decline, with patterns that reflect that up to 60% [of patients] may have some degree of deterioration, involve the disease phase, hypoxemia, or degree of inflammation. On the other hand, it has also been associated with Parkinson’s disease and gastroesophageal reflux, among many more that arise from the cardiovascular sphere.”
One paper reveals that more than 78% of patients with chronic obstructive pulmonary disease have one associated comorbidity, almost 69% have two, and 47.9% have three.
“Based on gender, comorbidities are different. In women, it is well observed that anxiety, depression, and osteoporosis are more common. However, hypertension, ischemic heart disease, and diabetes are more common in men with chronic obstructive pulmonary disease,” she stated.
“The pulmonary disease in question also progresses differently in men and women. In women, onset is at younger ages – between 40 and 50 years – and in men, after 50. Likewise, it appears that the disease progresses more quickly, which coincides with a worse quality of life (since dyspnea is tolerated less) and exceeds the anatomical differences, where hormonal influences play a dominant role,” Dr. Alonso stressed.
Reciprocal prognosis
Dr. Alonso stated, “The prognostic importance of comorbidities in the disease is reciprocal. In other words, if there are comorbidities that we do not look for or treat, they are going to have a negative influence on the chronic obstructive pulmonary disease. The disease will progress more and elevate the risk of exacerbations (the most important prognostic factor of that disease). In turn, if we are not treating the disease well, not only pharmacologically, it will have negative repercussions on the comorbidities. It will progress and have negative connotations, such as diabetes or ischemic heart disease.”
The aforementioned ECCO and ESMI studies include patients in internal medicine with exacerbations where the most common comorbidities have been mapped out, although there is also extensive research on comorbidities in patients who are admitted to departments other than internal medicine. “With regard to prognostic implications, our working group very clearly observed the comorbidities and the comorbidome, that solar system that appears so much in medical conferences and forums, which implies that proximity to the center of that solar system is related more to mortality, anxiety, depression, and breast cancer. Other pathologies, such as ischemic heart disease or dyslipidemia, are outside of that territory of greater risk, in which we have been more pioneering than other groups,” said Dr. Alonso.
The current trend is that the age of these patients is increasing, and there are more and more women with this pathology. According to the latest report from the Ministry of Health on respiratory diseases, the prevalence of chronic obstructive pulmonary disease among the population 40 years and older is around 33.9 cases per 1,000 inhabitants, more than twice as common in men than in women (47.7 vs. 21.3). Prevalence increases with age after 40 years progressively until reaching the greatest frequency in the 80- to 84-year-old age group.
In 2019, the number of deaths due to chronic obstructive pulmonary disease in Spain was 13,808 (9,907 men and 3,901 women), with a crude mortality rate of 29.3 deaths per 100,000 inhabitants. This toll decreased in comparison with that of 2018. Chronic obstructive pulmonary disease causes 2.5 times more deaths in men than in women. From 2001 to 2019, mortality due to that pathology declined by 43% in men and women. The decrease was almost 50% in men and 33% in women.
Overlap syndrome prevalent
Javier Sánchez Lora, MD, of the internal medicine department of the Virgen de la Victoria de Málaga University Clinical Hospital, discussed chronic obstructive pulmonary disease and sleep disorders. More concretely, he spoke about overlap syndrome: chronic obstructive pulmonary disease plus obstructive sleep apnea. According to the international consensus document on obstructive sleep apnea, the diagnosis requires an apnea-hypopnea index (AHI) equal to or greater than 15 per hour or equal to or greater than 5. The patient must also have one or more of the following factors: excessive daytime sleepiness, sleep that is not restful, excessive fatigue, and deterioration in quality of life related to sleep and not justified by other causes.
“The overlap syndrome affects 3%-66% of chronic obstructive pulmonary diseases and 7%-55% of obstructive sleep apnea,” said Dr. Sánchez Lora. This syndrome has important effects on different systems: at the cardiovascular level (arterial and pulmonary hypertension, heart failure, stroke, arrhythmias, ischemic heart disease, pulmonary thromboembolism), metabolic (insulin resistance, diabetes, metabolic syndrome), neurocognitive (dementia, depression), and neoplastic (lung, pancreas, esophagus) effects.
“These patients have a worse prognosis than those that have these pathologies alone. During sleep, they experience more frequent episodes of oxygen desaturation and they have a longer total period of sleep with hypoxemia and hypercapnia than those with obstructive apnea alone without chronic obstructive pulmonary disease,” said Dr. Sánchez Lora.
The apneic events of patients with the syndrome have a more profound hypoxemia and more arrhythmias, in addition to them being more susceptible to developing pulmonary hypertension than those with chronic obstructive pulmonary disease or sleep apnea alone. “The good news is that in patients with overlap, the use of ventilation with positive pressure reduces all causes of hospitalization and the visits to the emergency room, as well as the moderate and severe exacerbations of the disease.”
Dr. Sánchez Lora referred to a series of recommendations in clinical practice for the diagnosis and treatment of overlap syndrome: screening, combined therapy of hygienic-dietary measures, and the use of continuous positive respiratory pressure. Oxygen therapy to correct isolated nocturnal desaturations has not shown benefits in survival, although a benefit trial of symptoms attributed to nocturnal hypoxemia in patients with significant comorbidity can be conducted.
Underdiagnosis
“During the panel, we also spoke about the importance that as part of internal medicine we need to make an effort to reduce the underdiagnosis of chronic pulmonary disease and its comorbidities. Specialists in internal medicine need to become aware that this pathology is not only pulmonary, but also multisystemic, complex, heterogenous, and very variable even in the same patient,” said Dr. Sánchez Lora.
Dr. Alonso said, “Regarding the importance of diagnosis of this disease, we continue with an underdiagnosis greater than 70% for men and 80% for women. Secondly, we need to actively seek out the comorbidities associated with chronic obstructive pulmonary disease, even taking advantage of the admission of these patients with exacerbations, which are undesired and common.
“Regarding ongoing trials, we have a study that started during the COVID-19 pandemic, ADEG-EPOC, that involves the adaptation to and impact of severe and very severe exacerbations in patients admitted to our departments,” the specialist indicated.
“In the group, we are also planning to publish an updated agreement, which we already made in 2014, on the most common and important comorbidities associated with chronic obstructive pulmonary disease.” The agreement discusses the 20 most important comorbidities. In addition, the 2023 Gold Guide, which appeared in November 2022, includes a new chapter on updated treatment and the latest developments.
In the last 5 years, Dr. Alonso has collaborated with Abbott, AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, Ferrer, Fresenius Kabi, GSK, Nestlé, Novo Nordisk, Nutricia, and Menarini. Dr. Sánchez Lora has collaborated with AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, GSK, and Menarini.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Strict control of comorbidities in patients with chronic obstructive pulmonary disease decreases exacerbations and morbimortality, and avoids readmissions. An increasing number of women have the disease, which progresses differently in women than in men and even has different comorbidities.
said Belén Alonso, MD, PhD, coordinator of the COPD Working Group of the Spanish Society of Internal Medicine. Up to 73 comorbidities associated with chronic obstructive pulmonary disease have been described. Dr. Alonso made these remarks during her presentation at the Comorbidities in Chronic Obstructive Pulmonary Disease Panel, which took place during the 43rd Conference of the Spanish Society of Internal Medicine (SEMI), in Gijón, Spain.
According to the scientific society’s press release, moderator María Gómez Antúnez, MD, stated, “The correct approach and treatment of these comorbidities is fundamental to improve the quality of life of the patient, decrease exacerbations, avoid readmissions, and decrease morbimortality in people with chronic obstructive pulmonary disease.”
The different works published, two of them by the SEMI COPD Working Group (ECCO and ESMI studies), indicate that the main comorbidities of patients with that pneumopathy are arterial hypertension, dyslipidemia, diabetes, heart failure, atrial fibrillation, ischemic heart disease, chronic kidney disease, peripheral arterial disease, and osteoporosis. Chronic hepatopathy, pulmonary neoplasm, depression, and cerebrovascular disease are less common.
73 comorbidities described
Dr. Alonso told this news organization, “Of those 73 comorbidities, some of the lesser known or less attention grabbing, according to a paper that we brought to the panel, include sleep disorders that encompass insomnia, nightmares, night terrors, sleep apneas, or hypopneas. Other lesser-known comorbidities related to cognitive decline, with patterns that reflect that up to 60% [of patients] may have some degree of deterioration, involve the disease phase, hypoxemia, or degree of inflammation. On the other hand, it has also been associated with Parkinson’s disease and gastroesophageal reflux, among many more that arise from the cardiovascular sphere.”
One paper reveals that more than 78% of patients with chronic obstructive pulmonary disease have one associated comorbidity, almost 69% have two, and 47.9% have three.
“Based on gender, comorbidities are different. In women, it is well observed that anxiety, depression, and osteoporosis are more common. However, hypertension, ischemic heart disease, and diabetes are more common in men with chronic obstructive pulmonary disease,” she stated.
“The pulmonary disease in question also progresses differently in men and women. In women, onset is at younger ages – between 40 and 50 years – and in men, after 50. Likewise, it appears that the disease progresses more quickly, which coincides with a worse quality of life (since dyspnea is tolerated less) and exceeds the anatomical differences, where hormonal influences play a dominant role,” Dr. Alonso stressed.
Reciprocal prognosis
Dr. Alonso stated, “The prognostic importance of comorbidities in the disease is reciprocal. In other words, if there are comorbidities that we do not look for or treat, they are going to have a negative influence on the chronic obstructive pulmonary disease. The disease will progress more and elevate the risk of exacerbations (the most important prognostic factor of that disease). In turn, if we are not treating the disease well, not only pharmacologically, it will have negative repercussions on the comorbidities. It will progress and have negative connotations, such as diabetes or ischemic heart disease.”
The aforementioned ECCO and ESMI studies include patients in internal medicine with exacerbations where the most common comorbidities have been mapped out, although there is also extensive research on comorbidities in patients who are admitted to departments other than internal medicine. “With regard to prognostic implications, our working group very clearly observed the comorbidities and the comorbidome, that solar system that appears so much in medical conferences and forums, which implies that proximity to the center of that solar system is related more to mortality, anxiety, depression, and breast cancer. Other pathologies, such as ischemic heart disease or dyslipidemia, are outside of that territory of greater risk, in which we have been more pioneering than other groups,” said Dr. Alonso.
The current trend is that the age of these patients is increasing, and there are more and more women with this pathology. According to the latest report from the Ministry of Health on respiratory diseases, the prevalence of chronic obstructive pulmonary disease among the population 40 years and older is around 33.9 cases per 1,000 inhabitants, more than twice as common in men than in women (47.7 vs. 21.3). Prevalence increases with age after 40 years progressively until reaching the greatest frequency in the 80- to 84-year-old age group.
In 2019, the number of deaths due to chronic obstructive pulmonary disease in Spain was 13,808 (9,907 men and 3,901 women), with a crude mortality rate of 29.3 deaths per 100,000 inhabitants. This toll decreased in comparison with that of 2018. Chronic obstructive pulmonary disease causes 2.5 times more deaths in men than in women. From 2001 to 2019, mortality due to that pathology declined by 43% in men and women. The decrease was almost 50% in men and 33% in women.
Overlap syndrome prevalent
Javier Sánchez Lora, MD, of the internal medicine department of the Virgen de la Victoria de Málaga University Clinical Hospital, discussed chronic obstructive pulmonary disease and sleep disorders. More concretely, he spoke about overlap syndrome: chronic obstructive pulmonary disease plus obstructive sleep apnea. According to the international consensus document on obstructive sleep apnea, the diagnosis requires an apnea-hypopnea index (AHI) equal to or greater than 15 per hour or equal to or greater than 5. The patient must also have one or more of the following factors: excessive daytime sleepiness, sleep that is not restful, excessive fatigue, and deterioration in quality of life related to sleep and not justified by other causes.
“The overlap syndrome affects 3%-66% of chronic obstructive pulmonary diseases and 7%-55% of obstructive sleep apnea,” said Dr. Sánchez Lora. This syndrome has important effects on different systems: at the cardiovascular level (arterial and pulmonary hypertension, heart failure, stroke, arrhythmias, ischemic heart disease, pulmonary thromboembolism), metabolic (insulin resistance, diabetes, metabolic syndrome), neurocognitive (dementia, depression), and neoplastic (lung, pancreas, esophagus) effects.
“These patients have a worse prognosis than those that have these pathologies alone. During sleep, they experience more frequent episodes of oxygen desaturation and they have a longer total period of sleep with hypoxemia and hypercapnia than those with obstructive apnea alone without chronic obstructive pulmonary disease,” said Dr. Sánchez Lora.
The apneic events of patients with the syndrome have a more profound hypoxemia and more arrhythmias, in addition to them being more susceptible to developing pulmonary hypertension than those with chronic obstructive pulmonary disease or sleep apnea alone. “The good news is that in patients with overlap, the use of ventilation with positive pressure reduces all causes of hospitalization and the visits to the emergency room, as well as the moderate and severe exacerbations of the disease.”
Dr. Sánchez Lora referred to a series of recommendations in clinical practice for the diagnosis and treatment of overlap syndrome: screening, combined therapy of hygienic-dietary measures, and the use of continuous positive respiratory pressure. Oxygen therapy to correct isolated nocturnal desaturations has not shown benefits in survival, although a benefit trial of symptoms attributed to nocturnal hypoxemia in patients with significant comorbidity can be conducted.
Underdiagnosis
“During the panel, we also spoke about the importance that as part of internal medicine we need to make an effort to reduce the underdiagnosis of chronic pulmonary disease and its comorbidities. Specialists in internal medicine need to become aware that this pathology is not only pulmonary, but also multisystemic, complex, heterogenous, and very variable even in the same patient,” said Dr. Sánchez Lora.
Dr. Alonso said, “Regarding the importance of diagnosis of this disease, we continue with an underdiagnosis greater than 70% for men and 80% for women. Secondly, we need to actively seek out the comorbidities associated with chronic obstructive pulmonary disease, even taking advantage of the admission of these patients with exacerbations, which are undesired and common.
“Regarding ongoing trials, we have a study that started during the COVID-19 pandemic, ADEG-EPOC, that involves the adaptation to and impact of severe and very severe exacerbations in patients admitted to our departments,” the specialist indicated.
“In the group, we are also planning to publish an updated agreement, which we already made in 2014, on the most common and important comorbidities associated with chronic obstructive pulmonary disease.” The agreement discusses the 20 most important comorbidities. In addition, the 2023 Gold Guide, which appeared in November 2022, includes a new chapter on updated treatment and the latest developments.
In the last 5 years, Dr. Alonso has collaborated with Abbott, AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, Ferrer, Fresenius Kabi, GSK, Nestlé, Novo Nordisk, Nutricia, and Menarini. Dr. Sánchez Lora has collaborated with AstraZeneca, Boehringer Ingelheim, Chiesi, FAES, GSK, and Menarini.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.

