User login
In Case You Missed It: COVID
FDA safety alert: Face masks with metal can burn during MRI
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
Demand for COVID vaccines expected to get heated – and fast
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Americans have made no secret of their skepticism of COVID-19 vaccines this year, with fears of political interference and a “warp speed” timeline blunting confidence in the shots. As recently as September, nearly half of U.S. adults said they didn’t intend to be inoculated.
But with two promising vaccines primed for release, likely within weeks, experts in ethics and immunization behavior say they expect attitudes to shift quickly from widespread hesitancy to urgent, even heated demand.
“People talk about the antivaccine people being able to kind of squelch uptake. I don’t see that happening,” Dr. Paul Offit, MD, a vaccinologist with Children’s Hospital of Philadelphia, told viewers of a recent JAMA Network webinar. “This, to me, is more like the Beanie Baby phenomenon. The attractiveness of a limited edition.”
Reports that vaccines produced by drugmakers Pfizer and BioNTech and Moderna appear to be safe and effective, along with the deliberate emphasis on science-based guidance from the incoming Biden administration, are likely to reverse uncertainty in a big way, said Arthur Caplan, PhD, director of the division of medical ethics at New York University.
“I think that’s going to flip the trust issue,” he said.
The shift is already apparent. A new poll by the Pew Research Center found that by the end of November 60% of Americans said they would get a vaccine for the coronavirus. This month, even as a federal advisory group met to hash out guidelines for vaccine distribution, a long list of advocacy groups – from those representing home-based health workers and community health centers to patients with kidney disease – were lobbying state and federal officials in hopes their constituents would be prioritized for the first scarce doses.
“As we get closer to the vaccine being a reality, there’s a lot of jockeying, to be sure,” said Katie Smith Sloan, chief executive of LeadingAge, a nonprofit organization pushing for staff and patients at long-term care centers to be included in the highest-priority category.
Certainly, some consumers remain wary, said Rupali Limaye, PhD, a social and behavioral health scientist at the Johns Hopkins Bloomberg School of Public Health, Baltimore. Fears that drugmakers and regulators might cut corners to speed a vaccine linger, even as details of the trials become public and the review process is made more transparent. Some health care workers, who are at the front of the line for the shots, are not eager to go first.
“There will be people who will say, ‘I will wait a little bit more for safety data,” Dr. Limaye said.
But those doubts likely will recede once the vaccines are approved for use and begin to circulate broadly, said Dr. Offit, who sits on the Food and Drug Administration advisory panel set to review the requests for emergency authorization Pfizer and Moderna have submitted.
He predicted demand for the COVID vaccines could rival the clamor that occurred in 2004, when production problems caused a severe shortage of flu shots just as influenza season began. That led to long lines, rationed doses and ethical debates over distribution.
“That was a highly desired vaccine,” Dr. Offit said. “I think in many ways that might happen here.”
Initially, vaccine supplies will be tight, with federal officials planning to ship 6.4 million doses within 24 hours of FDA authorization and up to 40 million doses by the end of the year. The CDC panel recommended that the first shots go to the 21 million health care workers in the United States and 3 million nursing home staff and residents, before being rolled out to other groups based on a hierarchy of risk factors.
Even before any vaccine is available, some people are trying to boost their chances of access, said Allison Kempe, MD, a professor of pediatrics at the University of Coloradoat Denver, Aurora, and expert in vaccine dissemination. “People have called me and said, ‘How can I get the vaccine?’” she said. “I think that not everyone will be happy to wait, that’s for sure. I don’t think there will be rioting in the streets, but there may be pressure brought to bear.”
That likely will include emotional debates over how, when, and to whom next doses should be distributed, said Dr. Caplan. Under the CDC recommendations, vulnerable groups next in line include 87 million workers whose jobs are deemed “essential” – a broad and ill-defined category – as well as 53 million adults age 65 and older.
“We’re going to have some fights about high-risk groups,” Dr. Caplan said.
The conversations will be complicated. Should prisoners, who have little control over their COVID exposure, get vaccine priority? How about professional sports teams, whose performance could bolster society’s overall morale? And what about residents of facilities providing care for people with intellectual and developmental disabilities, who are three times more likely to die from COVID-19 than the general population?
Control over vaccination allocation rests with the states, so that’s where the biggest conflicts will occur, Dr. Caplan said. “It’s a short fight, I hope, in the sense in which it gets done in a few months, but I think it will be pretty vocal.”
Once vaccine supplies become more plentiful, perhaps by May or June, another consideration is sure to boost demand: requirements for proof of COVID vaccination for work and travel.
“It’s inevitable that you’re going to see immunity passports or that you’re required to show a certificate on the train, airplane, bus, or subway,” Dr. Caplan predicted. “Probably also to enter certain hospitals, probably to enter certain restaurants and government facilities.”
But with a grueling winter surge ahead, and new predictions that COVID-19 will fell as many as 450,000 Americans by February, the tragic reality of the disease will no doubt fuel ample demand for vaccination.
“People now know someone who has gotten COVID, who has been hospitalized or has unfortunately died,” Dr. Limaye said.
“We’re all seeing this now,” said Dr. Kempe. “Even deniers are beginning to see what this illness can do.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 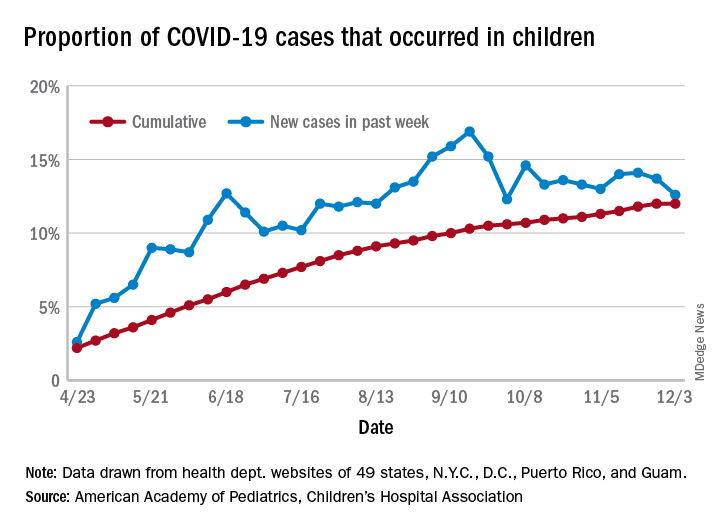
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.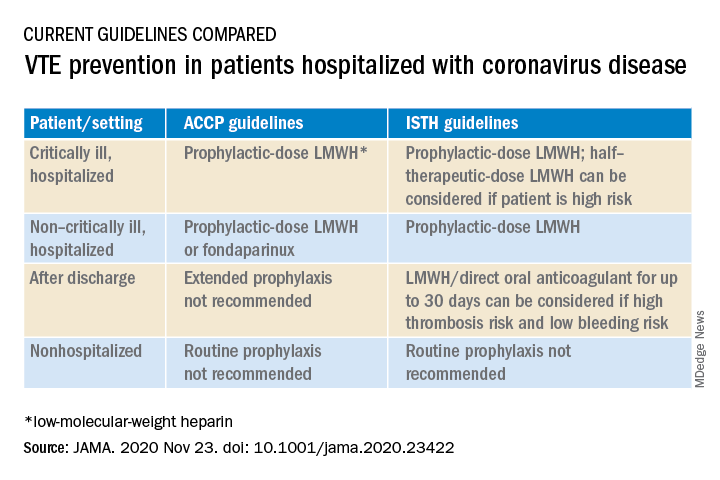
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
COVID-19 fuels surge in overdose-related cardiac arrests
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
There has been a sharp increase in overdose-related cardiac arrests in the United States during the COVID-19 pandemic, a new analysis shows.
Overall rates in 2020 were elevated above the baseline from 2018 and 2019 by about 50%, the data show.
and efforts to combat the COVID-19 pandemic have not been effective at reducing overdoses,” Joseph Friedman, MPH, MD/PhD student, medical scientist training program, University of California, Los Angeles, said in an interview.
“We need to invest heavily in substance use treatment, harm reduction, and the structural drivers of overdose as core elements of the COVID-19 response,” said Mr. Friedman, who coauthored the study with UCLA colleague David Schriger, MD, MPH, and Leo Beletsky, JD, MPH, Northeastern University, Boston.
The study was published as a research letter Dec. 3 in JAMA Psychiatry.
Social isolation a key driver
Emergency medical services (EMS) data are available in near real time, providing a novel source of up-to-date information to monitor epidemiological shifts during the COVID-19 pandemic.
For the study, the researchers leveraged data from the National EMS Information System, a large registry of more than 10,000 EMS agencies in 47 states that represent over 80% of all EMS calls nationally in 2020. They used the data to track shifts in overdose-related cardiac arrests observed by EMS.
They found clear evidence of a large-scale uptick in overdose-related deaths during the COVID-19 pandemic.
The overall rate of overdose-related cardiac arrests in 2020 was about 50% higher than trends observed during 2018 and 2019, including a maximum peak of 123% above baseline reached in early May.
All overdose-related incidents (fatal and nonfatal) were elevated in 2020, by about 17% above baseline. However, there were larger increases in fatal overdose-related incidents, compared to all incidents, which may suggest a rising case fatality rate, the authors noted.
The observed trends line up in time with reductions in mobility (a metric of social interaction), as measured using cell phone data, they wrote.
“Many of the trends predicted by experts at the beginning of the pandemic could cause these shifts. Increases in social isolation likely play an important role, as people using [drugs] alone are less likely to receive help when they need it. Also shifts in the drug supply, and reduced access to healthcare and treatment,” said Mr. Friedman.
“We need to undertake short- and long-term strategies to combat the rising tide of overdose mortality in the United States,” he added.
In the short term, Mr. Friedman suggested reducing financial and logistical barriers for accessing a safe opioid supply. Such measures include allowing pharmacies to dispense methadone, allowing all physicians to prescribe buprenorphine without a special waiver, and releasing emergency funds to make these medications universally affordable.
“In the longer term, we should acknowledge that overdose is a symptom of structural problems in the U.S. We need to invest in making employment, housing, education, and health care accessible to all to address the upstream drivers of overdose,” he added.
The study had no commercial funding. The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Assessing the impact of glucocorticoids on COVID-19 mortality
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
FROM THE JOURNAL OF HOSPITAL MEDICINE
PPE shortage crisis continues at most hospitals, survey shows
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
Herpes Zoster May Be a Marker for COVID-19 Infection During Pregnancy
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
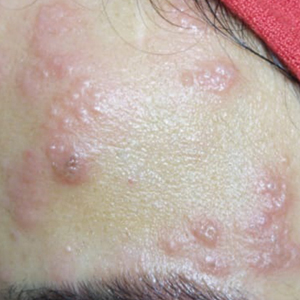
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.
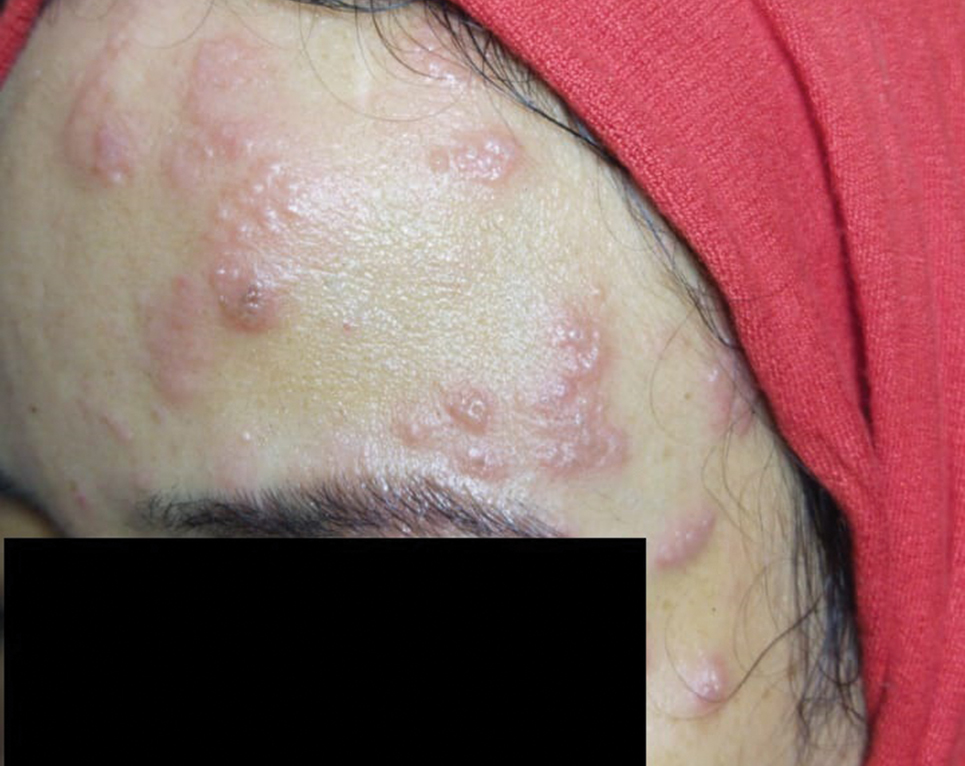
Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.

Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the most recently identified member of the zoonotic pathogens of coronaviruses. It caused an outbreak of pneumonia in December 2019 in Wuhan, China.1 Among all related acute respiratory syndromes (SARS-CoV, Middle East respiratory syndrome coronavirus), SARS-CoV-2 remains to be the most infectious, has the highest potential for human transmission, and can eventually result in acute respiratory distress syndrome.2,3
Only 15% of coronavirus disease 2019 (COVID-19) cases progress to pneumonia, and approximately 5% of these cases develop acute respiratory distress syndrome, septic shock, and/or multiple organ failure. The majority of cases only exhibit mild to moderate symptoms.4,5 A wide array of skin manifestations in COVID-19 infection have been reported, including maculopapular eruptions, morbilliform rashes, urticaria, chickenpoxlike lesions, livedo reticularis, COVID toes, erythema multiforme, pityriasis rosea, and several other patterns.6 We report a case of herpes zoster (HZ) complication in a COVID-19–positive woman who was 27 weeks pregnant.
Case Report
A 36-year-old woman who was 27 weeks pregnant was referred by her obstetrician to the dermatology clinic. She presented with a low-grade fever and a vesicular painful rash. Physical examination revealed painful, itchy, dysesthetic papules and vesicles on the left side of the forehead along with mild edema of the left upper eyelid but no watering of the eye or photophobia. She reported episodes of fever (temperature, 38.9°C), fatigue, and myalgia over the last week. She had bouts of dyspnea and tachycardia that she thought were related to being in the late second trimester of pregnancy. The area surrounding the vesicular eruption was tender to touch. No dry cough or any gastrointestinal or urinary tract symptoms were noted. She reported a burning sensation when splashing water on the face or when exposed to air currents. One week following the initial symptoms, she experienced a painful vesicular rash along the upper left forehead (Figure) associated with eyelid edema. Oral and ocular mucosae were free of any presentations. She had no relevant history and had not experienced any complications during pregnancy. A diagnosis of HZ was made, and she was prescribed valacyclovir 1 g 3 times daily for 7 days, acetaminophen for the fever, and calamine lotion. We recommended COVID-19 testing based on her symptoms. A chest radiograph and a positive nasopharyngeal smear were consistent with COVID-19 infection. She reported via telephone follow-up 1 week after presentation that her skin condition had improved following the treatment course and that the vesicles eventually dried, leaving a crusting appearance after 5 to 7 days. Regarding her SARS-CoV-2 condition, her oxygen saturation was 95% at presentation; she self-quarantined at home; and she was treated with oseltamivir 75 mg orally every 12 hours for 5 days, azithromycin 500 mg orally daily, acetaminophen, and vitamin C. Electronic fetal heart rate monitoring and ultrasound examinations were performed to assess the condition of the fetus and were reported normal. At the time of writing this article, she was 32 weeks pregnant and tested negative to 2 consecutive nasopharyngeal swabs for COVID-19 and was in good general condition. She continued her pregnancy according to her obstetrician’s recommendations.

Comment
The incubation time of COVID-19 can be up to 14 days. Fever, dry cough, fatigue, and diarrhea have been speculated to be clinical symptoms; however, many cases may be asymptomatic. Aside from a medical or travel history at risk for COVID-19, diagnosis can be confirmed by detection of viral RNA by reverse transcriptase–polymerase chain reaction for nasopharyngeal swabs or bronchoalveolar fluid. Patients who are immunocompromised, older, or male or who have a history of cardiovascular conditions or debilitating chronic conditions are at an increased risk for severe disease and poor outcome compared to younger healthy individuals.7
The vesicular rash of COVID-19 has been reported to have different forms of presentation. A diffuse widespread pattern resembling hand-foot-and-mouth disease and a localized monomorphic pattern resembling chickenpox but with predilection to the trunk has been described.8
Physiologic changes in the immune and cardiopulmonary systems during pregnancy (eg, diaphragm elevation, increased oxygen consumption, edema of the respiratory tract mucosae) make pregnant women intolerant to hypoxia. The mortality rate of the 1918 influenza pandemic was 2.6% in the overall population but 37% among pregnant women.9 In 2009, pregnant women were reported to be at an increased risk for complications from the H1N1 influenza virus pandemic, with a higher estimated rate of hospital admission than the general population.10 In 2003, approximately 50% of pregnant women who received a diagnosis of SARS-CoV were admitted to the intensive care unit, approximately 33% of pregnant women with SARS-CoV required mechanical ventilation, and the mortality rate was as high as 25% for these women.11 To date, data on the effects of COVID-19 in pregnancy are limited to small case series.12-15
It was confirmed that COVID-19 infection is accompanied by a reduction in lymphocytes, monocytes, and eosinophils, along with a notable reduction of CD4/CD8 T cells, B cells, and natural killer cells. It was further revealed that nonsurvivor COVID-19 patients continued to show a decrease in lymphocyte counts along the course of their disease until death.16-18
Different mechanisms for lymphocyte depletion and deficiency were speculated among COVID-19 patients and include direct lymphocyte death through coronavirus angiotensin-converting enzyme 2–lymphocyte-expressed receptors; direct damage to lymphatic organs, such as the thymus and spleen, but this theory needs to be further investigated; direct lymphocyte apoptosis mediated by tumor necrosis factor α, IL-6, and other proinflammatory cytokines; and direct inhibition of lymphocytes by metabolic upset, such as acidosis.19,20
These causes may precipitate lymphopenia and impaired antiviral responses.21 It also has been postulated that the functional damage of CD4+ T cells may predispose patients with COVID-19 to severe disease.22 Such immune changes can render a patient more susceptible to developing shingles by reactivating varicella-zoster virus, which could be a sign of undiagnosed COVID-19 infection in younger age groups.
Two earlier reports discussed HZ among COVID-19–diagnosed patients. Shors23 presented a case of a patient who developed varicella-zoster virus reactivation of the V2 dermatome during the course of COVID-19 infection. In addition, the patient developed severe acute herpetic neuralgia despite the early initiation of antiviral therapy.23 Elsaie et al24 described 2 cases of patients during the pandemic who first presented with HZ before later being diagnosed with COVID-19 infection.
New information and cutaneous manifestations possibly related to COVID-19 are emerging every day. We report a pregnant female presenting with HZ during the course of COVID-19 infection, which suggests that the clinical presentation of HZ at the time of the current pandemic, especially if associated with other signs of COVID-19 infection, should be carefully monitored and reported for further assessment.
Acknowledgment
The authors would like to thank all the health care workers who have been fighting COVID-19 in Egypt and worldwide.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207.
- Zhang YZ, Holes EC. A genomic perspective on the origin and emergence of sars-cov-2. Cell. 2020;181:223-227.
- Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan0, China. Lancet. 2020;395:497-506.
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549.
- Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582.
- Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, et al. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872-875.
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history [in Icelandic]. Laeknabladid. 2008;94:737-745.
- Jamieson D, Honein M, Rasmussen S, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451-458.
- Ksiazek TG, Erdman D, Goldsmith CS. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815.
- Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCov pneumonia. Transl Pediatr. 2020;9:51-60.
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy [published online March 4, 2020]. J Infect. doi:10.1016/j.jinf.2020.02.028.
- Zhang L, Jiang Y, Wei M, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166-171.
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028.
- Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752.
- Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-884.
- Kumar A, Anil A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: a systematic review and meta-analysis [preprint]. SSRN. doi:10.2139/ssrn.3566166.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12. https://doi.org/10.1038/s41368-020-0074-x.
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517-1520.
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID 19 infection [published online May 23, 2020]. Dermatol Ther. doi:10.1111/dth.13666.
Practice Points
- The vesicular rash of coronavirus disease 2019 (COVID-19) has been reported to have different forms of presentation.
- Pregnant women appear to be at increased risk for complications from COVID-19 infection.
- The clinical presentation of herpes zoster should be carefully monitored and reported for further assessment, especially if associated with other signs of COVID-19 infection.
Dermatology Battles COVID-19 With Comfort
We are in unprecedented times. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is attacking our communities and, as with any battle, we face unexpected challenges from the global pandemic. What can dermatologists, as highly skilled health care experts, do to support the fight against coronavirus disease 2019 (COVID-19)?
In early 2020, I became involved in a fulfilling and stimulating opportunity to contribute as a US Navy reservist, having just returned from a 3-month deployment. I served in the Medical Operations Center aboard the hospital ship USNS Comfort, which was docked in New York Harbor, as liaison to surrounding New York City hospitals. I also served as sole dermatologist on the ship, caring for the dermatologic needs of our team and consulting on numerous COVID-19 inpatients.
In May 2020, upon return to Virginia from New York City, I served as senior medical officer to medically clear other Navy Reserve health care workers returning from the field hospital at the Jacob K. Javits Convention Center of New York and from serving as embedded caregivers in existing New York City hospitals. I share 2 very important observations from my work there: First, COVID-19 is devastatingly real; second, we dermatologists can be valuable team members in the fight against this disease.
It is normal for us to feel scared, confused, and helpless; as 1% of the physician population, dermatologists represent a small focused fraction of the health care force. Nevertheless, we are all well-trained medical professionals who have taken the same Hippocratic Oath as other physicians. As members of the global health care team, we can each play a role in defeating COVID-19: We can be a trusted voice of reason, set an example, implement safe and effective distancing and hygiene precautions, and assist our local overburdened medical teams.
The magnitude and severity of COVID-19 can create a mass casualty–type phenomenon, overwhelming health care systems if the disease curve is not flattened. We can help flatten that curve by lengthening the pulse duration (to use dermatology jargon): that is, slowing the abrupt impact of cases to allow health care systems to triage, treat, and discharge in a more controlled manner.
How We Can Make a Difference
Despite representing a fraction of the health care team, we see a larger percentage of the population. On the Comfort, for example, dermatology visits accounted for approximately 20% of outpatient crew visits. We have an opportunity and a voice to reach a large percentage of the population directly. Whether we are now seeing patients face-to-face or virtually, we can spread the public health message and set an example. Wearing masks and social distancing do help to slow and markedly decrease the spread of SARS-CoV-2.
When you see patients in your office, consider the following:
• Have patients wait outside the office in their car and call the receptionist upon arrival.
• Have the receptionist call back the patient when the office is ready.
• Prescreen the patient before having him/her enter the clinic.
• Do not allow handshaking.
• Require everyone to wear a mask.
• Wear gloves.
• Have ample hand sanitizer openly available for all.
• Thoroughly clean or disinfect surfaces between patients.
Recalling the Difficult Experience of a Colleague-Patient
I think back to a crew member of Comfort who presented with new-onset pruritus and erythematous papules on the arms, legs, and torso. She was an intensive care unit nurse working 13-hour days, every day, for weeks on a COVID-positive unit—double-masked, gowned, wearing eye protection, in a warmer than usual intensive care unit, managing the most critically ill patients she’s ever cared for. Outside work, her life consisted of a commute on a government-chartered bus between Comfort and a contracted hotel while eating boxed meals. For 6 hours daily, she would—unsuccessfully—attempt to sleep with raging pruritus. Treating this routine case of eczema had a domino effect, improving her quality of life and thus allowing her to provide better care for the critically ill.
Let Us All Join in the Fight
As well-educated medical experts, we have the ability and the opportunity to reach outside our comfort zone and assist our medical colleagues. As I saw in New York City, the spectrum of specialists bravely worked together to meet overwhelming demand on the health care system and care for thousands of critically ill and dying patients. Dermatologists treated extensive eczema, ulcers, and other dermatoses on caretakers; triaged patients for appropriate allocation of care; and delivered care outside their comfort zone as physician extenders on inpatient and critical care units.
We are all in this together. I encourage all dermatologists who are in an area of need to ask your health care system how you can join the fight against SARS-CoV-2. Let’s step forward to help, in recognition of the oath we took to “prevent disease whenever we can.”
We are in unprecedented times. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is attacking our communities and, as with any battle, we face unexpected challenges from the global pandemic. What can dermatologists, as highly skilled health care experts, do to support the fight against coronavirus disease 2019 (COVID-19)?
In early 2020, I became involved in a fulfilling and stimulating opportunity to contribute as a US Navy reservist, having just returned from a 3-month deployment. I served in the Medical Operations Center aboard the hospital ship USNS Comfort, which was docked in New York Harbor, as liaison to surrounding New York City hospitals. I also served as sole dermatologist on the ship, caring for the dermatologic needs of our team and consulting on numerous COVID-19 inpatients.
In May 2020, upon return to Virginia from New York City, I served as senior medical officer to medically clear other Navy Reserve health care workers returning from the field hospital at the Jacob K. Javits Convention Center of New York and from serving as embedded caregivers in existing New York City hospitals. I share 2 very important observations from my work there: First, COVID-19 is devastatingly real; second, we dermatologists can be valuable team members in the fight against this disease.
It is normal for us to feel scared, confused, and helpless; as 1% of the physician population, dermatologists represent a small focused fraction of the health care force. Nevertheless, we are all well-trained medical professionals who have taken the same Hippocratic Oath as other physicians. As members of the global health care team, we can each play a role in defeating COVID-19: We can be a trusted voice of reason, set an example, implement safe and effective distancing and hygiene precautions, and assist our local overburdened medical teams.
The magnitude and severity of COVID-19 can create a mass casualty–type phenomenon, overwhelming health care systems if the disease curve is not flattened. We can help flatten that curve by lengthening the pulse duration (to use dermatology jargon): that is, slowing the abrupt impact of cases to allow health care systems to triage, treat, and discharge in a more controlled manner.
How We Can Make a Difference
Despite representing a fraction of the health care team, we see a larger percentage of the population. On the Comfort, for example, dermatology visits accounted for approximately 20% of outpatient crew visits. We have an opportunity and a voice to reach a large percentage of the population directly. Whether we are now seeing patients face-to-face or virtually, we can spread the public health message and set an example. Wearing masks and social distancing do help to slow and markedly decrease the spread of SARS-CoV-2.
When you see patients in your office, consider the following:
• Have patients wait outside the office in their car and call the receptionist upon arrival.
• Have the receptionist call back the patient when the office is ready.
• Prescreen the patient before having him/her enter the clinic.
• Do not allow handshaking.
• Require everyone to wear a mask.
• Wear gloves.
• Have ample hand sanitizer openly available for all.
• Thoroughly clean or disinfect surfaces between patients.
Recalling the Difficult Experience of a Colleague-Patient
I think back to a crew member of Comfort who presented with new-onset pruritus and erythematous papules on the arms, legs, and torso. She was an intensive care unit nurse working 13-hour days, every day, for weeks on a COVID-positive unit—double-masked, gowned, wearing eye protection, in a warmer than usual intensive care unit, managing the most critically ill patients she’s ever cared for. Outside work, her life consisted of a commute on a government-chartered bus between Comfort and a contracted hotel while eating boxed meals. For 6 hours daily, she would—unsuccessfully—attempt to sleep with raging pruritus. Treating this routine case of eczema had a domino effect, improving her quality of life and thus allowing her to provide better care for the critically ill.
Let Us All Join in the Fight
As well-educated medical experts, we have the ability and the opportunity to reach outside our comfort zone and assist our medical colleagues. As I saw in New York City, the spectrum of specialists bravely worked together to meet overwhelming demand on the health care system and care for thousands of critically ill and dying patients. Dermatologists treated extensive eczema, ulcers, and other dermatoses on caretakers; triaged patients for appropriate allocation of care; and delivered care outside their comfort zone as physician extenders on inpatient and critical care units.
We are all in this together. I encourage all dermatologists who are in an area of need to ask your health care system how you can join the fight against SARS-CoV-2. Let’s step forward to help, in recognition of the oath we took to “prevent disease whenever we can.”
We are in unprecedented times. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is attacking our communities and, as with any battle, we face unexpected challenges from the global pandemic. What can dermatologists, as highly skilled health care experts, do to support the fight against coronavirus disease 2019 (COVID-19)?
In early 2020, I became involved in a fulfilling and stimulating opportunity to contribute as a US Navy reservist, having just returned from a 3-month deployment. I served in the Medical Operations Center aboard the hospital ship USNS Comfort, which was docked in New York Harbor, as liaison to surrounding New York City hospitals. I also served as sole dermatologist on the ship, caring for the dermatologic needs of our team and consulting on numerous COVID-19 inpatients.
In May 2020, upon return to Virginia from New York City, I served as senior medical officer to medically clear other Navy Reserve health care workers returning from the field hospital at the Jacob K. Javits Convention Center of New York and from serving as embedded caregivers in existing New York City hospitals. I share 2 very important observations from my work there: First, COVID-19 is devastatingly real; second, we dermatologists can be valuable team members in the fight against this disease.
It is normal for us to feel scared, confused, and helpless; as 1% of the physician population, dermatologists represent a small focused fraction of the health care force. Nevertheless, we are all well-trained medical professionals who have taken the same Hippocratic Oath as other physicians. As members of the global health care team, we can each play a role in defeating COVID-19: We can be a trusted voice of reason, set an example, implement safe and effective distancing and hygiene precautions, and assist our local overburdened medical teams.
The magnitude and severity of COVID-19 can create a mass casualty–type phenomenon, overwhelming health care systems if the disease curve is not flattened. We can help flatten that curve by lengthening the pulse duration (to use dermatology jargon): that is, slowing the abrupt impact of cases to allow health care systems to triage, treat, and discharge in a more controlled manner.
How We Can Make a Difference
Despite representing a fraction of the health care team, we see a larger percentage of the population. On the Comfort, for example, dermatology visits accounted for approximately 20% of outpatient crew visits. We have an opportunity and a voice to reach a large percentage of the population directly. Whether we are now seeing patients face-to-face or virtually, we can spread the public health message and set an example. Wearing masks and social distancing do help to slow and markedly decrease the spread of SARS-CoV-2.
When you see patients in your office, consider the following:
• Have patients wait outside the office in their car and call the receptionist upon arrival.
• Have the receptionist call back the patient when the office is ready.
• Prescreen the patient before having him/her enter the clinic.
• Do not allow handshaking.
• Require everyone to wear a mask.
• Wear gloves.
• Have ample hand sanitizer openly available for all.
• Thoroughly clean or disinfect surfaces between patients.
Recalling the Difficult Experience of a Colleague-Patient
I think back to a crew member of Comfort who presented with new-onset pruritus and erythematous papules on the arms, legs, and torso. She was an intensive care unit nurse working 13-hour days, every day, for weeks on a COVID-positive unit—double-masked, gowned, wearing eye protection, in a warmer than usual intensive care unit, managing the most critically ill patients she’s ever cared for. Outside work, her life consisted of a commute on a government-chartered bus between Comfort and a contracted hotel while eating boxed meals. For 6 hours daily, she would—unsuccessfully—attempt to sleep with raging pruritus. Treating this routine case of eczema had a domino effect, improving her quality of life and thus allowing her to provide better care for the critically ill.
Let Us All Join in the Fight
As well-educated medical experts, we have the ability and the opportunity to reach outside our comfort zone and assist our medical colleagues. As I saw in New York City, the spectrum of specialists bravely worked together to meet overwhelming demand on the health care system and care for thousands of critically ill and dying patients. Dermatologists treated extensive eczema, ulcers, and other dermatoses on caretakers; triaged patients for appropriate allocation of care; and delivered care outside their comfort zone as physician extenders on inpatient and critical care units.
We are all in this together. I encourage all dermatologists who are in an area of need to ask your health care system how you can join the fight against SARS-CoV-2. Let’s step forward to help, in recognition of the oath we took to “prevent disease whenever we can.”
Practice Points
- Be aware of and promote coronavirus disease 2019 guidelines and recommendations from the Centers for Disease Control and Prevention and your local health department.
- Be prepared to push the limits of your comfort zone in an effort to assist the health care community.
Skin Eruption and Gastrointestinal Symptoms as Presentation of COVID-19
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.
The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.

The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) started an outbreak of respiratory illnesses in Wuhan, China. The respiratory disease was termed coronavirus disease 2019 (COVID-19) and rapidly spread worldwide, resulting in a pandemic classification on March 11, 2020. 1 Recently, several cutaneous manifestations of COVID-19 have been reported. Skin manifestations have been reported to be similar to other common viral infections. 2 However, there is a paucity of published clinical images of more atypical presentations.
Case Report
A 52-year-old black man presented via urgent store-and-forward teledermatology consultation from his primary care provider with a self-described “vesicular,” highly pruritic rash of both arms and legs of 1 week’s duration without involvement of the trunk, axillae, groin, face, genitalia, or any mucous membranes. He noted nausea, loss of appetite, and nonbloody diarrhea 4 days later. He denied fever, chills, dry cough, shortness of breath, or dyspnea. He had a history of hypertension and type 2 diabetes mellitus. There were no changes in medications; no outdoor activities, gardening, or yard work; no exposure to plants or metals; and no use of new personal care products.
The digital images showed zones of flesh-colored to slightly erythematous, somewhat “juicy” papules with some coalescence into ill-defined plaques. There were scattered foci of scale and hemorrhagic crust that involved both palms, forearms (Figure, A), and legs (Figure, B). There were no intact vesicles, and a herald patch was not identified. Vital signs at the time of imaging were normal, with the exception of a low-grade fever (temperature, 37.3°C). Basic laboratory testing showed only mild leukocytosis with mild neutropenia and mild aspartate aminotransaminase elevation. A skin biopsy was not performed. Pulmonary imaging and workup were not performed because of the lack of respiratory symptoms.

The teledermatology differential diagnosis included a drug eruption, autosensitization eruption, unusual contact dermatitis, viral exanthem, secondary syphilis, and papular pityriasis rosea with an unusual distribution. The absence of changes in the patient’s medication regimen and the lack of outdoor activity in late winter made a drug eruption and contact dermatitis less likely, respectively. A rapid plasma reagin test drawn after disappearance of the rash was negative. Although the morphology of this eruption displayed some features of papular pityriasis rosea, this diagnosis was considered to be less likely given the presence of palmar involvement and the absence of any truncal lesions. This variant of pityriasis rosea is more commonly encountered in younger, darker-skinned patients.
Given the presence of an unusual rash on the extremities followed shortly by gastrointestinal (GI) symptoms and coupled with a low-grade fever, a nasopharyngeal swab was obtained to test for COVID-19 using a reverse transcriptase–polymerase chain reaction test. The results were positive.
The patient was treated with triamcinolone 0.1% slush (triamcinolone cream 0.1% mixed 1:1 with tap water) to the affected skin of the extremities 3 times daily, and he experienced a reduction in pruritus. He developed new lesions on the face and eyelids (not imaged) 2 days after teledermatology consultation. The facial involvement was treated with hydrocortisone cream 1%. During the following week, the GI symptoms and skin eruption completely resolved. However, postinflammatory hyperpigmentation was observed in areas of the resolved papules and plaques. Over the course of this illness, the patient reported no respiratory symptoms.
Comment
Coronavirus disease 2019 is caused by SARS-CoV2, an enveloped, nonsegmented, positive-sense RNA virus of the coronavirus family. It is currently believed that SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor to gain entry into human cells, leading to infection primarily affecting the lower respiratory tract.3 Patients suspected of COVID-19 infection most often present with fever, dry cough, dyspnea, and fatigue, while GI symptoms such as nausea, vomiting, and diarrhea are uncommon.4 More recently, several reports describe a variety of skin findings associated with COVID-19. A current theory suggests that the virus does not directly target keratinocytes but triggers a systemic immune response, leading to a diversity of skin morphologies.5 The main types of described cutaneous findings include pseudochilblains, overtly vesicular, urticarial, maculopapular, and livedo/necrosis.6 Others have described petechial7 and papulosquamous eruptions.8 Most of these patients initially presented with typical COVID-19 symptoms and frequently represented more severe cases of the disease. Additionally, the vesicular and papulosquamous eruptions reportedly occurred on the trunk and not the limbs, as in our case.
This confirmed COVID-19–positive patient presented with an ill-defined vesicular and papulosquamous-type eruption on the arms and legs and later developed only mild GI symptoms. By sharing this case, we report yet another skin manifestation of COVID-19 and propose the possible expansion of testing for SARS-CoV-2 in patients presenting with rash and GI symptoms, which holds the potential to increase the identification of COVID-19 in the population, thereby increasing strict contact tracing and slowing the spread of this pandemic.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ng OT, Marimuthu K, Chia PY, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med. 2020;382:1476-1478.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. 2020;98:141-143.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
Practice Points
- Patients with coronavirus disease 2019 (COVID-19) typically present with fever, dry cough, dyspnea, and fatigue, but cutaneous manifestations also have been reported.
- Awareness of atypical presentations of COVID-19, including uncommon cutaneous manifestations, may identify more cases and help slow the expansion of this pandemic.