User login
-
Yale’s COVID-19 inpatient protocol: Hydroxychloroquine plus/minus tocilizumab
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
POPCoRN network mobilizes pediatric capacity during pandemic
Med-Peds hospitalists were an organizing force
As U.S. health care systems prepare for inpatient surges linked to hospitalizations of critically ill COVID-19 patients, two hospitalists with med-peds training (combined training in internal medicine and pediatrics) have launched an innovative solution to help facilities deal with the challenge.
The Pediatric Overflow Planning Contingency Response Network (POPCoRN network) has quickly linked almost 400 physicians and other health professionals, including hospitalists, attending physicians, residents, medical students, and nurses. The network wants to help provide more information about how pediatric-focused institutions can safely gear up to admit adult patients in children’s hospitals, in order to offset the predicted demand for hospital beds for patients with COVID-19.
According to the POPCoRN network website (www.popcornetwork.org), the majority of providers who have contacted the network say they have already started or are committed to planning for their pediatric facilities to be used for adult overflow. The Children’s Hospital Association has issued a guidance on this kind of community collaboration for children’s hospitals partnering with adult hospitals in their community and with policy makers.
“We are a network of folks from different institutions, many med-peds–trained hospitalists but quickly growing,” said Leah Ratner, MD, a second-year fellow in the Global Pediatrics Program at Boston Children’s Hospital and cofounder of the POPCoRN network. “We came together to think about how to increase capacity – both in the work force and for actual hospital space – by helping to train pediatric hospitalists and pediatrics-trained nurses to care for adult patients.”
A web-based platform filled with a rapidly expanding list of resources, an active Twitter account, and utilization of Zoom networking software for webinars and working group meetings have facilitated the network’s growth. “Social media has helped us,” Dr. Ratner said. But equally important are personal connections.
“It all started just a few weeks ago,” added cofounder Ashley Jenkins, MD, a med-peds hospital medicine and general academics research fellow in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. “I sent out some emails in mid-March, asking what other people were doing about these issues. Leah and I met as a result of these initial emails. We immediately started connecting with other health systems and it just expanded from there. Once we knew that enough other systems were thinking about it and trying to build capacity, we started pulling the people and information together.”
High-yield one-pagers
A third or more of those on the POPCoRN contact list are also participating as volunteers on its varied working groups, including health system operation groups exploring the needs of three distinct hospital models: freestanding children’s hospitals; community hospitals, which may see small numbers of children; and integrated mixed hospitals, which often means a pediatric hospital or pediatric units located within an adult hospital.
An immediate goal is to develop high-yield informational “one-pagers,” culling essential clinical facts on a variety of topics in adult inpatient medicine that may no longer be familiar to working pediatric hospitalists. These one-pagers, designed with the help of network members with graphic design skills, address topics such as syncope or chest pain or managing exacerbation of COPD in adults. They draw upon existing informational sources, encapsulating practical information tips that can be used at the bedside, including test workups, differential diagnoses, treatment approaches, and other pearls for providers. Drafts are reviewed for content by specialists, and then by pediatricians to make sure the information covers what they need.
Also under development are educational materials for nurses trained in pediatrics, a section for outpatient providers redeployed to triage or telehealth, and information for other team members including occupational, physical, and respiratory therapists. Another section offers critical care lectures for the nonintensivist. A metrics and outcomes working group is looking for ways to evaluate how the network is doing and who is being reached without having to ask frontline providers to fill out surveys.
Dr. Ratner and Dr. Jenkins have created an intentional structure for encouraging mentoring. They also call on their own mentors – Ahmet Uluer, DO, director of Weitzman Family Bridges Adult Transition Program at Boston Children’s Hospital, and Brian Herbst Jr., MD, medical director of the Hospital Medicine Adult Care Service at Cincinnati Children’s – for advice.
Beyond the silos
Pediatric hospitalists may have been doing similar things, working on similar projects, but not necessarily reaching out to each other across a system that tends to promote staying within administrative silos, Dr. Uluer said. “Through our personal contacts in POPCoRN, we’ve been able to reach beyond the silos. This network has worked like medical crowd sourcing, and the founders have been inspirational.”
Dr. Herbst added, “How do we expand bandwidth and safely expand services to take young patients and adults from other hospitals? What other populations do we need to expand to take? This network is a workplace of ideas. It’s amazing to see what has been built in a few weeks and how useful it can be.”
Med-peds hospitalists are an important resource for bridging the two specialties. Their experience with transitioning young adults with long-standing chronic conditions of childhood, who have received most of their care at a children’s hospital before reaching adulthood, offers a helpful model. “We’ve also tried to target junior physicians who could step up into leadership roles and to pull in medical students – who are the backbone of this network through their administrative support,” Dr. Jenkins said.
Marie Pfarr, MD, also a med-peds trained hospital medicine fellow at Cincinnati Children’s, was contacted in March by Dr. Jenkins. “She said they had this brainstorm, and they were getting feedback that it would be helpful to provide educational materials for pediatric providers. Because I have an interest in medical education, she asked if I wanted to help. I was at home struggling with what I could contribute during this crazy time, so I said yes.”
Dr. Pfarr leads POPCoRN’s educational working group, which came up with a list of 50 topics in need of one-pagers and people willing to create them, mostly still under development. The aim for the one-pagers is to offer a good starting point for pediatricians, helping them, for example, to ask the right questions during history and physical exams. “We also want to offer additional resources for those who want to do a deeper dive.”
Dr. Pfarr said she has enjoyed working closely with medical students, who really want to help. “That’s been great to see. We are all working toward the same goal, and we help to keep each other in check. I think there’s a future for this kind of mobilization through collaborations to connect pediatric to adult providers. A lot of good things will come out of the network, which is an example of how folks can talk to each other. It’s very dynamic and changing every day.”
One of those medical students is Chinma Onyewuenyi, finishing her fourth year at Baylor College of Medicine. Scheduled to start a med-peds residency at Geisinger Health on July 1, she had completed all of her rotations and was looking for ways to get involved in the pandemic response while respecting the shelter-in-place order. “I had heard about the network, which was recruiting medical students to play administrative roles for the working groups. I said, ‘If you have anything else you need help with, I have time on my hands.’”
Ms. Onyewuenyi says she fell into the role of a lead administrative volunteer, and her responsibilities grew from there, eventually taking charge of all the medical students’ recruiting, screening, and assignments, freeing up the project’s physician leaders from administrative tasks. “I wanted something active to do to contribute, and I appreciate all that I’m learning. With a master’s degree in public health, I have researched how health care is delivered,” she said.
“This experience has really opened my eyes to what’s required to deliver care, and just the level of collaboration that needs to go on with something like this. Even as a medical student, I felt glad to have an opportunity to contribute beyond the administrative tasks. At meetings, they ask for my opinion.”
Equitable access to resources
Another major focus for the network is promoting health equity – giving pediatric providers and health systems equitable access to information that meets their needs, Dr. Ratner said. “We’ve made a particular effort to reach out to hospitals that are the most vulnerable, including rural hospitals, and to those serving the most vulnerable patients,” she noted. These also include the homeless and refugees.
“We’ve been trying to be mindful of avoiding the sometimes-intimidating power structure that has been traditional in medicine,” Dr. Ratner said. The network’s equity working group is trying to provide content with structural competency and cultural humility. “We’re learning a lot about the ways the health care system is broken,” she added. “We all agree that we have a fragmented health care system, but there are ways to make it less fragmented and learn from each other.”
In the tragedy of the COVID epidemic, there are also unique opportunities to learn to work collaboratively and make the health care system stronger for those in greatest need, Dr. Ratner added. “What we hope is that our network becomes an example of that, even as it is moving so quickly.”
Audrey Uong, MD, an attending physician in the division of hospital medicine at Children’s Hospital at Montefiore Medical Center in New York, connected with POPCoRN for an educational presentation reviewing resuscitation in adult patients. She wanted to talk with peers about what’s going on, so as not to feel alone in her practice. She has also found the network’s website useful for identifying educational resources.
“As pediatricians, we have been asked to care for adult patients. One of our units has been admitting mostly patients under age 30, and we are accepting older patients in another unit on the pediatric wing.” This kind of thing is also happening in a lot of other places, Dr. Uong said. Keeping up with these changes in her own practice has been challenging.
She tries to take one day at a time. “Everyone at this institution feels the same – that we’re locked in on meeting the need. Even our child life specialists, when they’re not working with younger patients, have created this amazing support room for staff, with snacks and soothing music. There’s been a lot of attention paid to making us feel supported in this work.”
Med-Peds hospitalists were an organizing force
Med-Peds hospitalists were an organizing force
As U.S. health care systems prepare for inpatient surges linked to hospitalizations of critically ill COVID-19 patients, two hospitalists with med-peds training (combined training in internal medicine and pediatrics) have launched an innovative solution to help facilities deal with the challenge.
The Pediatric Overflow Planning Contingency Response Network (POPCoRN network) has quickly linked almost 400 physicians and other health professionals, including hospitalists, attending physicians, residents, medical students, and nurses. The network wants to help provide more information about how pediatric-focused institutions can safely gear up to admit adult patients in children’s hospitals, in order to offset the predicted demand for hospital beds for patients with COVID-19.
According to the POPCoRN network website (www.popcornetwork.org), the majority of providers who have contacted the network say they have already started or are committed to planning for their pediatric facilities to be used for adult overflow. The Children’s Hospital Association has issued a guidance on this kind of community collaboration for children’s hospitals partnering with adult hospitals in their community and with policy makers.
“We are a network of folks from different institutions, many med-peds–trained hospitalists but quickly growing,” said Leah Ratner, MD, a second-year fellow in the Global Pediatrics Program at Boston Children’s Hospital and cofounder of the POPCoRN network. “We came together to think about how to increase capacity – both in the work force and for actual hospital space – by helping to train pediatric hospitalists and pediatrics-trained nurses to care for adult patients.”
A web-based platform filled with a rapidly expanding list of resources, an active Twitter account, and utilization of Zoom networking software for webinars and working group meetings have facilitated the network’s growth. “Social media has helped us,” Dr. Ratner said. But equally important are personal connections.
“It all started just a few weeks ago,” added cofounder Ashley Jenkins, MD, a med-peds hospital medicine and general academics research fellow in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. “I sent out some emails in mid-March, asking what other people were doing about these issues. Leah and I met as a result of these initial emails. We immediately started connecting with other health systems and it just expanded from there. Once we knew that enough other systems were thinking about it and trying to build capacity, we started pulling the people and information together.”
High-yield one-pagers
A third or more of those on the POPCoRN contact list are also participating as volunteers on its varied working groups, including health system operation groups exploring the needs of three distinct hospital models: freestanding children’s hospitals; community hospitals, which may see small numbers of children; and integrated mixed hospitals, which often means a pediatric hospital or pediatric units located within an adult hospital.
An immediate goal is to develop high-yield informational “one-pagers,” culling essential clinical facts on a variety of topics in adult inpatient medicine that may no longer be familiar to working pediatric hospitalists. These one-pagers, designed with the help of network members with graphic design skills, address topics such as syncope or chest pain or managing exacerbation of COPD in adults. They draw upon existing informational sources, encapsulating practical information tips that can be used at the bedside, including test workups, differential diagnoses, treatment approaches, and other pearls for providers. Drafts are reviewed for content by specialists, and then by pediatricians to make sure the information covers what they need.
Also under development are educational materials for nurses trained in pediatrics, a section for outpatient providers redeployed to triage or telehealth, and information for other team members including occupational, physical, and respiratory therapists. Another section offers critical care lectures for the nonintensivist. A metrics and outcomes working group is looking for ways to evaluate how the network is doing and who is being reached without having to ask frontline providers to fill out surveys.
Dr. Ratner and Dr. Jenkins have created an intentional structure for encouraging mentoring. They also call on their own mentors – Ahmet Uluer, DO, director of Weitzman Family Bridges Adult Transition Program at Boston Children’s Hospital, and Brian Herbst Jr., MD, medical director of the Hospital Medicine Adult Care Service at Cincinnati Children’s – for advice.
Beyond the silos
Pediatric hospitalists may have been doing similar things, working on similar projects, but not necessarily reaching out to each other across a system that tends to promote staying within administrative silos, Dr. Uluer said. “Through our personal contacts in POPCoRN, we’ve been able to reach beyond the silos. This network has worked like medical crowd sourcing, and the founders have been inspirational.”
Dr. Herbst added, “How do we expand bandwidth and safely expand services to take young patients and adults from other hospitals? What other populations do we need to expand to take? This network is a workplace of ideas. It’s amazing to see what has been built in a few weeks and how useful it can be.”
Med-peds hospitalists are an important resource for bridging the two specialties. Their experience with transitioning young adults with long-standing chronic conditions of childhood, who have received most of their care at a children’s hospital before reaching adulthood, offers a helpful model. “We’ve also tried to target junior physicians who could step up into leadership roles and to pull in medical students – who are the backbone of this network through their administrative support,” Dr. Jenkins said.
Marie Pfarr, MD, also a med-peds trained hospital medicine fellow at Cincinnati Children’s, was contacted in March by Dr. Jenkins. “She said they had this brainstorm, and they were getting feedback that it would be helpful to provide educational materials for pediatric providers. Because I have an interest in medical education, she asked if I wanted to help. I was at home struggling with what I could contribute during this crazy time, so I said yes.”
Dr. Pfarr leads POPCoRN’s educational working group, which came up with a list of 50 topics in need of one-pagers and people willing to create them, mostly still under development. The aim for the one-pagers is to offer a good starting point for pediatricians, helping them, for example, to ask the right questions during history and physical exams. “We also want to offer additional resources for those who want to do a deeper dive.”
Dr. Pfarr said she has enjoyed working closely with medical students, who really want to help. “That’s been great to see. We are all working toward the same goal, and we help to keep each other in check. I think there’s a future for this kind of mobilization through collaborations to connect pediatric to adult providers. A lot of good things will come out of the network, which is an example of how folks can talk to each other. It’s very dynamic and changing every day.”
One of those medical students is Chinma Onyewuenyi, finishing her fourth year at Baylor College of Medicine. Scheduled to start a med-peds residency at Geisinger Health on July 1, she had completed all of her rotations and was looking for ways to get involved in the pandemic response while respecting the shelter-in-place order. “I had heard about the network, which was recruiting medical students to play administrative roles for the working groups. I said, ‘If you have anything else you need help with, I have time on my hands.’”
Ms. Onyewuenyi says she fell into the role of a lead administrative volunteer, and her responsibilities grew from there, eventually taking charge of all the medical students’ recruiting, screening, and assignments, freeing up the project’s physician leaders from administrative tasks. “I wanted something active to do to contribute, and I appreciate all that I’m learning. With a master’s degree in public health, I have researched how health care is delivered,” she said.
“This experience has really opened my eyes to what’s required to deliver care, and just the level of collaboration that needs to go on with something like this. Even as a medical student, I felt glad to have an opportunity to contribute beyond the administrative tasks. At meetings, they ask for my opinion.”
Equitable access to resources
Another major focus for the network is promoting health equity – giving pediatric providers and health systems equitable access to information that meets their needs, Dr. Ratner said. “We’ve made a particular effort to reach out to hospitals that are the most vulnerable, including rural hospitals, and to those serving the most vulnerable patients,” she noted. These also include the homeless and refugees.
“We’ve been trying to be mindful of avoiding the sometimes-intimidating power structure that has been traditional in medicine,” Dr. Ratner said. The network’s equity working group is trying to provide content with structural competency and cultural humility. “We’re learning a lot about the ways the health care system is broken,” she added. “We all agree that we have a fragmented health care system, but there are ways to make it less fragmented and learn from each other.”
In the tragedy of the COVID epidemic, there are also unique opportunities to learn to work collaboratively and make the health care system stronger for those in greatest need, Dr. Ratner added. “What we hope is that our network becomes an example of that, even as it is moving so quickly.”
Audrey Uong, MD, an attending physician in the division of hospital medicine at Children’s Hospital at Montefiore Medical Center in New York, connected with POPCoRN for an educational presentation reviewing resuscitation in adult patients. She wanted to talk with peers about what’s going on, so as not to feel alone in her practice. She has also found the network’s website useful for identifying educational resources.
“As pediatricians, we have been asked to care for adult patients. One of our units has been admitting mostly patients under age 30, and we are accepting older patients in another unit on the pediatric wing.” This kind of thing is also happening in a lot of other places, Dr. Uong said. Keeping up with these changes in her own practice has been challenging.
She tries to take one day at a time. “Everyone at this institution feels the same – that we’re locked in on meeting the need. Even our child life specialists, when they’re not working with younger patients, have created this amazing support room for staff, with snacks and soothing music. There’s been a lot of attention paid to making us feel supported in this work.”
As U.S. health care systems prepare for inpatient surges linked to hospitalizations of critically ill COVID-19 patients, two hospitalists with med-peds training (combined training in internal medicine and pediatrics) have launched an innovative solution to help facilities deal with the challenge.
The Pediatric Overflow Planning Contingency Response Network (POPCoRN network) has quickly linked almost 400 physicians and other health professionals, including hospitalists, attending physicians, residents, medical students, and nurses. The network wants to help provide more information about how pediatric-focused institutions can safely gear up to admit adult patients in children’s hospitals, in order to offset the predicted demand for hospital beds for patients with COVID-19.
According to the POPCoRN network website (www.popcornetwork.org), the majority of providers who have contacted the network say they have already started or are committed to planning for their pediatric facilities to be used for adult overflow. The Children’s Hospital Association has issued a guidance on this kind of community collaboration for children’s hospitals partnering with adult hospitals in their community and with policy makers.
“We are a network of folks from different institutions, many med-peds–trained hospitalists but quickly growing,” said Leah Ratner, MD, a second-year fellow in the Global Pediatrics Program at Boston Children’s Hospital and cofounder of the POPCoRN network. “We came together to think about how to increase capacity – both in the work force and for actual hospital space – by helping to train pediatric hospitalists and pediatrics-trained nurses to care for adult patients.”
A web-based platform filled with a rapidly expanding list of resources, an active Twitter account, and utilization of Zoom networking software for webinars and working group meetings have facilitated the network’s growth. “Social media has helped us,” Dr. Ratner said. But equally important are personal connections.
“It all started just a few weeks ago,” added cofounder Ashley Jenkins, MD, a med-peds hospital medicine and general academics research fellow in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. “I sent out some emails in mid-March, asking what other people were doing about these issues. Leah and I met as a result of these initial emails. We immediately started connecting with other health systems and it just expanded from there. Once we knew that enough other systems were thinking about it and trying to build capacity, we started pulling the people and information together.”
High-yield one-pagers
A third or more of those on the POPCoRN contact list are also participating as volunteers on its varied working groups, including health system operation groups exploring the needs of three distinct hospital models: freestanding children’s hospitals; community hospitals, which may see small numbers of children; and integrated mixed hospitals, which often means a pediatric hospital or pediatric units located within an adult hospital.
An immediate goal is to develop high-yield informational “one-pagers,” culling essential clinical facts on a variety of topics in adult inpatient medicine that may no longer be familiar to working pediatric hospitalists. These one-pagers, designed with the help of network members with graphic design skills, address topics such as syncope or chest pain or managing exacerbation of COPD in adults. They draw upon existing informational sources, encapsulating practical information tips that can be used at the bedside, including test workups, differential diagnoses, treatment approaches, and other pearls for providers. Drafts are reviewed for content by specialists, and then by pediatricians to make sure the information covers what they need.
Also under development are educational materials for nurses trained in pediatrics, a section for outpatient providers redeployed to triage or telehealth, and information for other team members including occupational, physical, and respiratory therapists. Another section offers critical care lectures for the nonintensivist. A metrics and outcomes working group is looking for ways to evaluate how the network is doing and who is being reached without having to ask frontline providers to fill out surveys.
Dr. Ratner and Dr. Jenkins have created an intentional structure for encouraging mentoring. They also call on their own mentors – Ahmet Uluer, DO, director of Weitzman Family Bridges Adult Transition Program at Boston Children’s Hospital, and Brian Herbst Jr., MD, medical director of the Hospital Medicine Adult Care Service at Cincinnati Children’s – for advice.
Beyond the silos
Pediatric hospitalists may have been doing similar things, working on similar projects, but not necessarily reaching out to each other across a system that tends to promote staying within administrative silos, Dr. Uluer said. “Through our personal contacts in POPCoRN, we’ve been able to reach beyond the silos. This network has worked like medical crowd sourcing, and the founders have been inspirational.”
Dr. Herbst added, “How do we expand bandwidth and safely expand services to take young patients and adults from other hospitals? What other populations do we need to expand to take? This network is a workplace of ideas. It’s amazing to see what has been built in a few weeks and how useful it can be.”
Med-peds hospitalists are an important resource for bridging the two specialties. Their experience with transitioning young adults with long-standing chronic conditions of childhood, who have received most of their care at a children’s hospital before reaching adulthood, offers a helpful model. “We’ve also tried to target junior physicians who could step up into leadership roles and to pull in medical students – who are the backbone of this network through their administrative support,” Dr. Jenkins said.
Marie Pfarr, MD, also a med-peds trained hospital medicine fellow at Cincinnati Children’s, was contacted in March by Dr. Jenkins. “She said they had this brainstorm, and they were getting feedback that it would be helpful to provide educational materials for pediatric providers. Because I have an interest in medical education, she asked if I wanted to help. I was at home struggling with what I could contribute during this crazy time, so I said yes.”
Dr. Pfarr leads POPCoRN’s educational working group, which came up with a list of 50 topics in need of one-pagers and people willing to create them, mostly still under development. The aim for the one-pagers is to offer a good starting point for pediatricians, helping them, for example, to ask the right questions during history and physical exams. “We also want to offer additional resources for those who want to do a deeper dive.”
Dr. Pfarr said she has enjoyed working closely with medical students, who really want to help. “That’s been great to see. We are all working toward the same goal, and we help to keep each other in check. I think there’s a future for this kind of mobilization through collaborations to connect pediatric to adult providers. A lot of good things will come out of the network, which is an example of how folks can talk to each other. It’s very dynamic and changing every day.”
One of those medical students is Chinma Onyewuenyi, finishing her fourth year at Baylor College of Medicine. Scheduled to start a med-peds residency at Geisinger Health on July 1, she had completed all of her rotations and was looking for ways to get involved in the pandemic response while respecting the shelter-in-place order. “I had heard about the network, which was recruiting medical students to play administrative roles for the working groups. I said, ‘If you have anything else you need help with, I have time on my hands.’”
Ms. Onyewuenyi says she fell into the role of a lead administrative volunteer, and her responsibilities grew from there, eventually taking charge of all the medical students’ recruiting, screening, and assignments, freeing up the project’s physician leaders from administrative tasks. “I wanted something active to do to contribute, and I appreciate all that I’m learning. With a master’s degree in public health, I have researched how health care is delivered,” she said.
“This experience has really opened my eyes to what’s required to deliver care, and just the level of collaboration that needs to go on with something like this. Even as a medical student, I felt glad to have an opportunity to contribute beyond the administrative tasks. At meetings, they ask for my opinion.”
Equitable access to resources
Another major focus for the network is promoting health equity – giving pediatric providers and health systems equitable access to information that meets their needs, Dr. Ratner said. “We’ve made a particular effort to reach out to hospitals that are the most vulnerable, including rural hospitals, and to those serving the most vulnerable patients,” she noted. These also include the homeless and refugees.
“We’ve been trying to be mindful of avoiding the sometimes-intimidating power structure that has been traditional in medicine,” Dr. Ratner said. The network’s equity working group is trying to provide content with structural competency and cultural humility. “We’re learning a lot about the ways the health care system is broken,” she added. “We all agree that we have a fragmented health care system, but there are ways to make it less fragmented and learn from each other.”
In the tragedy of the COVID epidemic, there are also unique opportunities to learn to work collaboratively and make the health care system stronger for those in greatest need, Dr. Ratner added. “What we hope is that our network becomes an example of that, even as it is moving so quickly.”
Audrey Uong, MD, an attending physician in the division of hospital medicine at Children’s Hospital at Montefiore Medical Center in New York, connected with POPCoRN for an educational presentation reviewing resuscitation in adult patients. She wanted to talk with peers about what’s going on, so as not to feel alone in her practice. She has also found the network’s website useful for identifying educational resources.
“As pediatricians, we have been asked to care for adult patients. One of our units has been admitting mostly patients under age 30, and we are accepting older patients in another unit on the pediatric wing.” This kind of thing is also happening in a lot of other places, Dr. Uong said. Keeping up with these changes in her own practice has been challenging.
She tries to take one day at a time. “Everyone at this institution feels the same – that we’re locked in on meeting the need. Even our child life specialists, when they’re not working with younger patients, have created this amazing support room for staff, with snacks and soothing music. There’s been a lot of attention paid to making us feel supported in this work.”
IDSA guidelines cover N95 use and reuse
The Infectious Disease Society of America has released new guidelines on the use and reuse of personal protective equipment, most of which address the use of face protection, for health care workers caring for COVID-19 patients. In releasing the guidelines, the IDSA expert guideline panel acknowledged gaps in evidence to support the recommendations, which is why they will be updated regularly as new evidence emerges.
“Our real goal here is to update these guidelines as a live document,” panel chair John Lynch III, MD, MPH, of the University of Washington, Seattle, said in a press briefing. “Looking at whatever research is coming out where it gets to the point where we find that the evidence is strong enough to make a change, I think we’ll need to readdress these recommendations.”
The panel tailored recommendations to the availability of supplies: conventional capacity for usual supplies; contingency capacity, when supplies are conserved, adapted and substituted with occasional reuse of select supplies; and crisis capacity, when critical supplies are lacking.
The guidelines contain the following eight recommendations for encounters with suspected or confirmed COVID-19 patients:
1) Either a surgical mask or N95 (or N99 or PAPR [powered & supplied air respiratory protection]) respirator for routine patient care in a conventional setting.
2) Either a surgical mask or reprocessed respirator as opposed to no mask for routine care in a contingency or crisis setting.
3) No recommendation on the use of double gloves vs. single gloves.
4) No recommendation on the use of shoe covers for any setting.
5) An N95 (or N99 or PAPR) respirator for aerosol-generating procedures in a conventional setting.
6) A reprocessed N95 respirator as opposed to a surgical mask for aerosol-generating procedures in a contingency or crisis setting.
7) Adding a face shield or surgical mask as a cover for an N95 respirator to allow for extended use during respirator shortages when performing aerosol-generating procedures in a contingency or crisis setting. This recommendation carries a caveat: It assumes correct doffing sequence and hand hygiene before and after taking off the face shield or surgical mask cover.
8) In the same scenario, adding a face shield or surgical mask over the N95 respirator so it can be reused, again assuming the correct sequence for hand hygiene.
The guideline was developed using the GRADE approach – for Grading of Recommendations Assessment, Development, and Evaluation – and a modified methodology for developing rapid recommendations. The levels of evidence supporting each recommendation vary from moderate for the first two to knowledge gap for the third and fourth to very low certainty for the last four.
“You can see that the eight recommendations that were made, a large part of them are really focused on masks, but there are a huge number of other disparate questions that need to be answered where there is really no good evidence basis,” Dr. Lynch said. “If we see any new evidence around that, we can at least provide commentary but I would really hope evidence-based recommendations around some of those interventions.”
Panel member Allison McGeer, MD, FRCPC, of the University of Toronto, explained the lack of evidence supporting infection prevention in hospitals. “In medicine we tend to look at individual patterns and individual patient outcomes,” she said. “When you’re looking at infection prevention, you’re looking at health systems and their outcomes, and it’s much harder to randomize hospitals or a state or a country to one particular policy about how to protect patients from infections in hospitals.”
The latest guidelines follow IDSA’s previously released guidelines on treatment and management of COVID-19 patients. The panel also plans to release guidelines on use of diagnostics for COVID-19 care.
Dr. Lynch has no financial relationships to disclose. Dr. McGeer disclosed relationships with Pfizer, Merck, Sanofi Pasteur, Seqirus, GlaxoSmithKline and Cidara.
SOURCE: Lynch JB et al. IDSA. April 27, 2020.
The Infectious Disease Society of America has released new guidelines on the use and reuse of personal protective equipment, most of which address the use of face protection, for health care workers caring for COVID-19 patients. In releasing the guidelines, the IDSA expert guideline panel acknowledged gaps in evidence to support the recommendations, which is why they will be updated regularly as new evidence emerges.
“Our real goal here is to update these guidelines as a live document,” panel chair John Lynch III, MD, MPH, of the University of Washington, Seattle, said in a press briefing. “Looking at whatever research is coming out where it gets to the point where we find that the evidence is strong enough to make a change, I think we’ll need to readdress these recommendations.”
The panel tailored recommendations to the availability of supplies: conventional capacity for usual supplies; contingency capacity, when supplies are conserved, adapted and substituted with occasional reuse of select supplies; and crisis capacity, when critical supplies are lacking.
The guidelines contain the following eight recommendations for encounters with suspected or confirmed COVID-19 patients:
1) Either a surgical mask or N95 (or N99 or PAPR [powered & supplied air respiratory protection]) respirator for routine patient care in a conventional setting.
2) Either a surgical mask or reprocessed respirator as opposed to no mask for routine care in a contingency or crisis setting.
3) No recommendation on the use of double gloves vs. single gloves.
4) No recommendation on the use of shoe covers for any setting.
5) An N95 (or N99 or PAPR) respirator for aerosol-generating procedures in a conventional setting.
6) A reprocessed N95 respirator as opposed to a surgical mask for aerosol-generating procedures in a contingency or crisis setting.
7) Adding a face shield or surgical mask as a cover for an N95 respirator to allow for extended use during respirator shortages when performing aerosol-generating procedures in a contingency or crisis setting. This recommendation carries a caveat: It assumes correct doffing sequence and hand hygiene before and after taking off the face shield or surgical mask cover.
8) In the same scenario, adding a face shield or surgical mask over the N95 respirator so it can be reused, again assuming the correct sequence for hand hygiene.
The guideline was developed using the GRADE approach – for Grading of Recommendations Assessment, Development, and Evaluation – and a modified methodology for developing rapid recommendations. The levels of evidence supporting each recommendation vary from moderate for the first two to knowledge gap for the third and fourth to very low certainty for the last four.
“You can see that the eight recommendations that were made, a large part of them are really focused on masks, but there are a huge number of other disparate questions that need to be answered where there is really no good evidence basis,” Dr. Lynch said. “If we see any new evidence around that, we can at least provide commentary but I would really hope evidence-based recommendations around some of those interventions.”
Panel member Allison McGeer, MD, FRCPC, of the University of Toronto, explained the lack of evidence supporting infection prevention in hospitals. “In medicine we tend to look at individual patterns and individual patient outcomes,” she said. “When you’re looking at infection prevention, you’re looking at health systems and their outcomes, and it’s much harder to randomize hospitals or a state or a country to one particular policy about how to protect patients from infections in hospitals.”
The latest guidelines follow IDSA’s previously released guidelines on treatment and management of COVID-19 patients. The panel also plans to release guidelines on use of diagnostics for COVID-19 care.
Dr. Lynch has no financial relationships to disclose. Dr. McGeer disclosed relationships with Pfizer, Merck, Sanofi Pasteur, Seqirus, GlaxoSmithKline and Cidara.
SOURCE: Lynch JB et al. IDSA. April 27, 2020.
The Infectious Disease Society of America has released new guidelines on the use and reuse of personal protective equipment, most of which address the use of face protection, for health care workers caring for COVID-19 patients. In releasing the guidelines, the IDSA expert guideline panel acknowledged gaps in evidence to support the recommendations, which is why they will be updated regularly as new evidence emerges.
“Our real goal here is to update these guidelines as a live document,” panel chair John Lynch III, MD, MPH, of the University of Washington, Seattle, said in a press briefing. “Looking at whatever research is coming out where it gets to the point where we find that the evidence is strong enough to make a change, I think we’ll need to readdress these recommendations.”
The panel tailored recommendations to the availability of supplies: conventional capacity for usual supplies; contingency capacity, when supplies are conserved, adapted and substituted with occasional reuse of select supplies; and crisis capacity, when critical supplies are lacking.
The guidelines contain the following eight recommendations for encounters with suspected or confirmed COVID-19 patients:
1) Either a surgical mask or N95 (or N99 or PAPR [powered & supplied air respiratory protection]) respirator for routine patient care in a conventional setting.
2) Either a surgical mask or reprocessed respirator as opposed to no mask for routine care in a contingency or crisis setting.
3) No recommendation on the use of double gloves vs. single gloves.
4) No recommendation on the use of shoe covers for any setting.
5) An N95 (or N99 or PAPR) respirator for aerosol-generating procedures in a conventional setting.
6) A reprocessed N95 respirator as opposed to a surgical mask for aerosol-generating procedures in a contingency or crisis setting.
7) Adding a face shield or surgical mask as a cover for an N95 respirator to allow for extended use during respirator shortages when performing aerosol-generating procedures in a contingency or crisis setting. This recommendation carries a caveat: It assumes correct doffing sequence and hand hygiene before and after taking off the face shield or surgical mask cover.
8) In the same scenario, adding a face shield or surgical mask over the N95 respirator so it can be reused, again assuming the correct sequence for hand hygiene.
The guideline was developed using the GRADE approach – for Grading of Recommendations Assessment, Development, and Evaluation – and a modified methodology for developing rapid recommendations. The levels of evidence supporting each recommendation vary from moderate for the first two to knowledge gap for the third and fourth to very low certainty for the last four.
“You can see that the eight recommendations that were made, a large part of them are really focused on masks, but there are a huge number of other disparate questions that need to be answered where there is really no good evidence basis,” Dr. Lynch said. “If we see any new evidence around that, we can at least provide commentary but I would really hope evidence-based recommendations around some of those interventions.”
Panel member Allison McGeer, MD, FRCPC, of the University of Toronto, explained the lack of evidence supporting infection prevention in hospitals. “In medicine we tend to look at individual patterns and individual patient outcomes,” she said. “When you’re looking at infection prevention, you’re looking at health systems and their outcomes, and it’s much harder to randomize hospitals or a state or a country to one particular policy about how to protect patients from infections in hospitals.”
The latest guidelines follow IDSA’s previously released guidelines on treatment and management of COVID-19 patients. The panel also plans to release guidelines on use of diagnostics for COVID-19 care.
Dr. Lynch has no financial relationships to disclose. Dr. McGeer disclosed relationships with Pfizer, Merck, Sanofi Pasteur, Seqirus, GlaxoSmithKline and Cidara.
SOURCE: Lynch JB et al. IDSA. April 27, 2020.
FROM INFECTIOUS DISEASE SOCIETY OF AMERICA
Remdesivir now ‘standard of care’ for COVID-19, Fauci says
, according to a preliminary data analysis from a U.S.-led randomized, controlled trial.
On the basis of as yet unpublished data, remdesivir “will be the standard of care” for patients with COVID-19, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said during a press conference at the White House April 29.
The randomized, placebo-controlled international trial was sponsored by NIAID, which is part of the National Institutes of Health, and enrolled 1,063 patients. It began on Feb. 21.
The interim results, discussed in the press conference and in a NIAID press release, show that time to recovery (i.e., being well enough for hospital discharge or to return to normal activity level) was 31% faster for patients who received remdesivir than for those who received placebo (P < .001).
The median time to recovery was 11 days for patients treated with remdesivir, compared with 15 days for those who received placebo. Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir and 11.6% for the patients who received placebo (P = .059).
The study, known as the Adaptive COVID-19 Treatment Trial (ACTT), is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19. It is being conducted at 68 sites – 47 in the United States and 21 in countries in Europe and Asia.
The data are being released after an interim review by the independent data safety monitoring board found significant benefit with the drug, Dr. Fauci said.
“The reason we are making the announcement now is something that people don’t fully appreciate: Whenever you have clear-cut evidence that a drug works, you have an ethical obligation to let the people in the placebo group know so they could have access,” he explained.
“When I was looking at the data with our team the other night, it was reminiscent of 34 years ago in 1986 when we were struggling for drugs for HIV,” said Dr. Fauci, who was a key figure in HIV/AIDS research. “We did the first randomized, placebo-controlled trial with AZT. It turned out to have an effect that was modest but that was not the endgame because, building on that, every year after, we did better and better.”
Similarly, new trials of drugs for COVID-19 will build on remdesivir, with other agents being added to block other pathways or viral enzymes, Dr. Fauci said.
The study will be submitted to a journal for peer review, he noted, but the New York Times is reporting that the Food and Drug Administration will approve remdesivir for emergency use soon.
In contrast to the positive results Dr. Fauci described from the NIAID-sponsored trial, a randomized, placebo-controlled clinical trial of remdesivir among hospitalized patients with severe COVID-19 in China was inconclusive.
The study, published online in The Lancet, showed some nonsignificant trends toward benefit but did not meet its primary endpoint.
The study was stopped early after 237 of the intended 453 patients were enrolled, owing to a lack of additional patients who met the eligibility criteria. The trial was thus underpowered.
Results showed that treatment with remdesivir did not significantly speed recovery or reduce deaths from COVID-19, but with regard to prespecified secondary outcomes, time to clinical improvement and duration of invasive mechanical ventilation were shorter among a subgroup of patients who began undergoing treatment with remdesivir within 10 days of showing symptoms, in comparison with patients who received standard care.
“To me, the studies reported here in The Lancet appear to be less promising than some statements released today from the NIH, so the situation is a bit puzzling to me,” said Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, who was not involved in the study. “I would need to look more closely at the data which NIH is looking at to understand the differences.”
Trial details
The published trial was conducted at 10 hospitals in Hubei, China. Enrollment criteria included being admitted to hospital with laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset, having oxygen saturation of 94% or less, and having radiologically confirmed pneumonia.
Patients were randomly assigned in a 2:1 ratio to receive intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids.
The primary endpoint was time to clinical improvement to day 28, defined as the time (in days) from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (on that scale, 1 indicated that the patient was discharged, and 6 indicated death) or to the patient’s being discharged alive from hospital, whichever came first.
The trial was stopped early because stringent public health measures used in Wuhan led to marked reductions in new patient presentations and because lack of available hospital beds resulted in most patients being enrolled later in the course of disease.
Between Feb. 6, 2020, and March 12, 2020, 237 patients were enrolled and were randomly assigned to receive either remdesivir (n = 158) or placebo (n = 79).
Results showed that use of remdesivir was not associated with a difference in time to clinical improvement (hazard ratio [HR], 1.23; 95% confidence interval [CI], 0.87-1.75).
Although not statistically significant, time to clinical improvement was numerically faster for patients who received remdesivir than for those who received placebo among patients with symptom duration of 10 days or less (median, 18 days vs. 23 days; HR, 1.52; 95% CI, 0.95-2.43).
The mortality rates were similar for the two groups (14% of patients who received remdesivir died vs. 13% of those who received placebo). There was no signal that viral load decreased differentially over time between the two groups.
Adverse events were reported in 66% of remdesivir recipients, vs. 64% of those who received placebo. Remdesivir was stopped early because of adverse events in 12% of patients; it was stopped early for 5% of those who received placebo.
The authors, led by Yeming Wang, MD, China-Japan Friendship Hospital, Beijing, noted that compared with a previous study of compassionate use of remdesivir, the population in the current study was less ill and was treated somewhat earlier in the disease course (median, 10 days vs. 12 days).
Because the study was terminated early, the researchers said they could not adequately assess whether earlier treatment with remdesivir might have provided clinical benefit.
“However, among patients who were treated within 10 days of symptom onset, remdesivir was not a significant factor but was associated with a numerical reduction of 5 days in median time to clinical improvement,” they stated.
They added that remdesivir was adequately tolerated and that no new safety concerns were identified.
In an accompanying comment in The Lancet, John David Norrie, MD, Edinburgh Clinical Trials Unit, United Kingdom, pointed out that this study “has not shown a statistically significant finding that confirms a remdesivir treatment benefit of at least the minimally clinically important difference, nor has it ruled such a benefit out.”
Dr. Norrie was cautious about the fact that the subgroup analysis suggested possible benefit for those treated within 10 days.
Although he said it seems biologically plausible that treating patients earlier could be more effective, he added that “as well as being vigilant against overinterpretation, we need to ensure that hypotheses generated in efficacy-based trials, even in subgroups, are confirmed or refuted in subsequent adequately powered trials or meta-analyses.”
Noting that several other trials of remdesivir are underway, he concluded: “With each individual study at heightened risk of being incomplete, pooling data across possibly several underpowered but high-quality studies looks like our best way to obtain robust insights into what works, safely, and on whom.”
A version of this article originally appeared on Medscape.com.
, according to a preliminary data analysis from a U.S.-led randomized, controlled trial.
On the basis of as yet unpublished data, remdesivir “will be the standard of care” for patients with COVID-19, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said during a press conference at the White House April 29.
The randomized, placebo-controlled international trial was sponsored by NIAID, which is part of the National Institutes of Health, and enrolled 1,063 patients. It began on Feb. 21.
The interim results, discussed in the press conference and in a NIAID press release, show that time to recovery (i.e., being well enough for hospital discharge or to return to normal activity level) was 31% faster for patients who received remdesivir than for those who received placebo (P < .001).
The median time to recovery was 11 days for patients treated with remdesivir, compared with 15 days for those who received placebo. Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir and 11.6% for the patients who received placebo (P = .059).
The study, known as the Adaptive COVID-19 Treatment Trial (ACTT), is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19. It is being conducted at 68 sites – 47 in the United States and 21 in countries in Europe and Asia.
The data are being released after an interim review by the independent data safety monitoring board found significant benefit with the drug, Dr. Fauci said.
“The reason we are making the announcement now is something that people don’t fully appreciate: Whenever you have clear-cut evidence that a drug works, you have an ethical obligation to let the people in the placebo group know so they could have access,” he explained.
“When I was looking at the data with our team the other night, it was reminiscent of 34 years ago in 1986 when we were struggling for drugs for HIV,” said Dr. Fauci, who was a key figure in HIV/AIDS research. “We did the first randomized, placebo-controlled trial with AZT. It turned out to have an effect that was modest but that was not the endgame because, building on that, every year after, we did better and better.”
Similarly, new trials of drugs for COVID-19 will build on remdesivir, with other agents being added to block other pathways or viral enzymes, Dr. Fauci said.
The study will be submitted to a journal for peer review, he noted, but the New York Times is reporting that the Food and Drug Administration will approve remdesivir for emergency use soon.
In contrast to the positive results Dr. Fauci described from the NIAID-sponsored trial, a randomized, placebo-controlled clinical trial of remdesivir among hospitalized patients with severe COVID-19 in China was inconclusive.
The study, published online in The Lancet, showed some nonsignificant trends toward benefit but did not meet its primary endpoint.
The study was stopped early after 237 of the intended 453 patients were enrolled, owing to a lack of additional patients who met the eligibility criteria. The trial was thus underpowered.
Results showed that treatment with remdesivir did not significantly speed recovery or reduce deaths from COVID-19, but with regard to prespecified secondary outcomes, time to clinical improvement and duration of invasive mechanical ventilation were shorter among a subgroup of patients who began undergoing treatment with remdesivir within 10 days of showing symptoms, in comparison with patients who received standard care.
“To me, the studies reported here in The Lancet appear to be less promising than some statements released today from the NIH, so the situation is a bit puzzling to me,” said Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, who was not involved in the study. “I would need to look more closely at the data which NIH is looking at to understand the differences.”
Trial details
The published trial was conducted at 10 hospitals in Hubei, China. Enrollment criteria included being admitted to hospital with laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset, having oxygen saturation of 94% or less, and having radiologically confirmed pneumonia.
Patients were randomly assigned in a 2:1 ratio to receive intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids.
The primary endpoint was time to clinical improvement to day 28, defined as the time (in days) from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (on that scale, 1 indicated that the patient was discharged, and 6 indicated death) or to the patient’s being discharged alive from hospital, whichever came first.
The trial was stopped early because stringent public health measures used in Wuhan led to marked reductions in new patient presentations and because lack of available hospital beds resulted in most patients being enrolled later in the course of disease.
Between Feb. 6, 2020, and March 12, 2020, 237 patients were enrolled and were randomly assigned to receive either remdesivir (n = 158) or placebo (n = 79).
Results showed that use of remdesivir was not associated with a difference in time to clinical improvement (hazard ratio [HR], 1.23; 95% confidence interval [CI], 0.87-1.75).
Although not statistically significant, time to clinical improvement was numerically faster for patients who received remdesivir than for those who received placebo among patients with symptom duration of 10 days or less (median, 18 days vs. 23 days; HR, 1.52; 95% CI, 0.95-2.43).
The mortality rates were similar for the two groups (14% of patients who received remdesivir died vs. 13% of those who received placebo). There was no signal that viral load decreased differentially over time between the two groups.
Adverse events were reported in 66% of remdesivir recipients, vs. 64% of those who received placebo. Remdesivir was stopped early because of adverse events in 12% of patients; it was stopped early for 5% of those who received placebo.
The authors, led by Yeming Wang, MD, China-Japan Friendship Hospital, Beijing, noted that compared with a previous study of compassionate use of remdesivir, the population in the current study was less ill and was treated somewhat earlier in the disease course (median, 10 days vs. 12 days).
Because the study was terminated early, the researchers said they could not adequately assess whether earlier treatment with remdesivir might have provided clinical benefit.
“However, among patients who were treated within 10 days of symptom onset, remdesivir was not a significant factor but was associated with a numerical reduction of 5 days in median time to clinical improvement,” they stated.
They added that remdesivir was adequately tolerated and that no new safety concerns were identified.
In an accompanying comment in The Lancet, John David Norrie, MD, Edinburgh Clinical Trials Unit, United Kingdom, pointed out that this study “has not shown a statistically significant finding that confirms a remdesivir treatment benefit of at least the minimally clinically important difference, nor has it ruled such a benefit out.”
Dr. Norrie was cautious about the fact that the subgroup analysis suggested possible benefit for those treated within 10 days.
Although he said it seems biologically plausible that treating patients earlier could be more effective, he added that “as well as being vigilant against overinterpretation, we need to ensure that hypotheses generated in efficacy-based trials, even in subgroups, are confirmed or refuted in subsequent adequately powered trials or meta-analyses.”
Noting that several other trials of remdesivir are underway, he concluded: “With each individual study at heightened risk of being incomplete, pooling data across possibly several underpowered but high-quality studies looks like our best way to obtain robust insights into what works, safely, and on whom.”
A version of this article originally appeared on Medscape.com.
, according to a preliminary data analysis from a U.S.-led randomized, controlled trial.
On the basis of as yet unpublished data, remdesivir “will be the standard of care” for patients with COVID-19, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases (NIAID), said during a press conference at the White House April 29.
The randomized, placebo-controlled international trial was sponsored by NIAID, which is part of the National Institutes of Health, and enrolled 1,063 patients. It began on Feb. 21.
The interim results, discussed in the press conference and in a NIAID press release, show that time to recovery (i.e., being well enough for hospital discharge or to return to normal activity level) was 31% faster for patients who received remdesivir than for those who received placebo (P < .001).
The median time to recovery was 11 days for patients treated with remdesivir, compared with 15 days for those who received placebo. Results also suggested a survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir and 11.6% for the patients who received placebo (P = .059).
The study, known as the Adaptive COVID-19 Treatment Trial (ACTT), is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19. It is being conducted at 68 sites – 47 in the United States and 21 in countries in Europe and Asia.
The data are being released after an interim review by the independent data safety monitoring board found significant benefit with the drug, Dr. Fauci said.
“The reason we are making the announcement now is something that people don’t fully appreciate: Whenever you have clear-cut evidence that a drug works, you have an ethical obligation to let the people in the placebo group know so they could have access,” he explained.
“When I was looking at the data with our team the other night, it was reminiscent of 34 years ago in 1986 when we were struggling for drugs for HIV,” said Dr. Fauci, who was a key figure in HIV/AIDS research. “We did the first randomized, placebo-controlled trial with AZT. It turned out to have an effect that was modest but that was not the endgame because, building on that, every year after, we did better and better.”
Similarly, new trials of drugs for COVID-19 will build on remdesivir, with other agents being added to block other pathways or viral enzymes, Dr. Fauci said.
The study will be submitted to a journal for peer review, he noted, but the New York Times is reporting that the Food and Drug Administration will approve remdesivir for emergency use soon.
In contrast to the positive results Dr. Fauci described from the NIAID-sponsored trial, a randomized, placebo-controlled clinical trial of remdesivir among hospitalized patients with severe COVID-19 in China was inconclusive.
The study, published online in The Lancet, showed some nonsignificant trends toward benefit but did not meet its primary endpoint.
The study was stopped early after 237 of the intended 453 patients were enrolled, owing to a lack of additional patients who met the eligibility criteria. The trial was thus underpowered.
Results showed that treatment with remdesivir did not significantly speed recovery or reduce deaths from COVID-19, but with regard to prespecified secondary outcomes, time to clinical improvement and duration of invasive mechanical ventilation were shorter among a subgroup of patients who began undergoing treatment with remdesivir within 10 days of showing symptoms, in comparison with patients who received standard care.
“To me, the studies reported here in The Lancet appear to be less promising than some statements released today from the NIH, so the situation is a bit puzzling to me,” said Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston, who was not involved in the study. “I would need to look more closely at the data which NIH is looking at to understand the differences.”
Trial details
The published trial was conducted at 10 hospitals in Hubei, China. Enrollment criteria included being admitted to hospital with laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset, having oxygen saturation of 94% or less, and having radiologically confirmed pneumonia.
Patients were randomly assigned in a 2:1 ratio to receive intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) or placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids.
The primary endpoint was time to clinical improvement to day 28, defined as the time (in days) from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (on that scale, 1 indicated that the patient was discharged, and 6 indicated death) or to the patient’s being discharged alive from hospital, whichever came first.
The trial was stopped early because stringent public health measures used in Wuhan led to marked reductions in new patient presentations and because lack of available hospital beds resulted in most patients being enrolled later in the course of disease.
Between Feb. 6, 2020, and March 12, 2020, 237 patients were enrolled and were randomly assigned to receive either remdesivir (n = 158) or placebo (n = 79).
Results showed that use of remdesivir was not associated with a difference in time to clinical improvement (hazard ratio [HR], 1.23; 95% confidence interval [CI], 0.87-1.75).
Although not statistically significant, time to clinical improvement was numerically faster for patients who received remdesivir than for those who received placebo among patients with symptom duration of 10 days or less (median, 18 days vs. 23 days; HR, 1.52; 95% CI, 0.95-2.43).
The mortality rates were similar for the two groups (14% of patients who received remdesivir died vs. 13% of those who received placebo). There was no signal that viral load decreased differentially over time between the two groups.
Adverse events were reported in 66% of remdesivir recipients, vs. 64% of those who received placebo. Remdesivir was stopped early because of adverse events in 12% of patients; it was stopped early for 5% of those who received placebo.
The authors, led by Yeming Wang, MD, China-Japan Friendship Hospital, Beijing, noted that compared with a previous study of compassionate use of remdesivir, the population in the current study was less ill and was treated somewhat earlier in the disease course (median, 10 days vs. 12 days).
Because the study was terminated early, the researchers said they could not adequately assess whether earlier treatment with remdesivir might have provided clinical benefit.
“However, among patients who were treated within 10 days of symptom onset, remdesivir was not a significant factor but was associated with a numerical reduction of 5 days in median time to clinical improvement,” they stated.
They added that remdesivir was adequately tolerated and that no new safety concerns were identified.
In an accompanying comment in The Lancet, John David Norrie, MD, Edinburgh Clinical Trials Unit, United Kingdom, pointed out that this study “has not shown a statistically significant finding that confirms a remdesivir treatment benefit of at least the minimally clinically important difference, nor has it ruled such a benefit out.”
Dr. Norrie was cautious about the fact that the subgroup analysis suggested possible benefit for those treated within 10 days.
Although he said it seems biologically plausible that treating patients earlier could be more effective, he added that “as well as being vigilant against overinterpretation, we need to ensure that hypotheses generated in efficacy-based trials, even in subgroups, are confirmed or refuted in subsequent adequately powered trials or meta-analyses.”
Noting that several other trials of remdesivir are underway, he concluded: “With each individual study at heightened risk of being incomplete, pooling data across possibly several underpowered but high-quality studies looks like our best way to obtain robust insights into what works, safely, and on whom.”
A version of this article originally appeared on Medscape.com.
Standing orders for vaccines may improve pediatric vaccination rates
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
FROM PEDIATRICS
Two rare neurologic conditions linked to COVID-19
A 50-year-old man developed Miller Fisher syndrome and a 39-year-old man developed polyneuritis cranialis. Both are variants of Guillain-Barré syndrome (GBS), which physicians in China and Italy also linked to COVID-19 infection, as previously reported by Medscape Medical News.
In both cases, physicians made the diagnoses based on abnormal eye examinations. The two patients responded to treatment and improved over 2 weeks, with only the 50-year-old featuring residual symptoms of anosmia and ageusia.
The report was published online April 17 in Neurology.
The 50-year-old man was admitted to an emergency room with a temperature of 99.9°F (37.7°C). He reported 2 days of vertical diplopia, perioral paresthesias, and gait instability. His neurologic examination showed intact cognitive function and language.
Five days earlier he developed a cough, malaise, headache, low back pain, fever, anosmia, and ageusia.
His neuro-ophthalmologic examination showed right hypertropia in all fields of gaze, severe limitations to the adduction and downgaze movements of his right eye, and left eye nystagmus on left gaze. These findings were consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy.
He responded to intravenous (IV) immunoglobulin therapy and was discharged home 2 weeks after admission.
The 39-year-old man was admitted to the emergency room with acute onset diplopia and ageusia. Three days earlier he had presented with diarrhea, a low-grade fever and in generally poor condition, without any headache, respiratory symptoms, or dyspnea.
He showed esotropia of 10 prism diopters at distance and 4 prism diopters at near, severe abduction deficits in both eyes, and fixation nystagmus, with the upper gaze more impaired, all consistent with bilateral abducens palsy.
The patient was discharged home and treated symptomatically with acetaminophen and telemedicine monitoring “due to a complete hospital saturation with COVID-19 patients,” wrote the researchers, led by Consuelo Gutiérrez-Ortiz, MD, PhD, Hospital Universitario Príncipe de Asturias, Madrid, Spain.
Two weeks later, he had made a complete neurologic recovery with no ageusia, complete eye movements, and normal deep tendon reflexes.
“Fisher syndrome and polyneuritis cranialis in these two patients with the SARS-CoV-2 infection could be simply coincidental. However, taking into account the temporal relationship, we feel that COVID-19 might have been responsible for the development of these two neurological pictures,” the authors noted.
European Regional Development Funds (FEDER) supported this research.
This article first appeared on Medscape.com.
A 50-year-old man developed Miller Fisher syndrome and a 39-year-old man developed polyneuritis cranialis. Both are variants of Guillain-Barré syndrome (GBS), which physicians in China and Italy also linked to COVID-19 infection, as previously reported by Medscape Medical News.
In both cases, physicians made the diagnoses based on abnormal eye examinations. The two patients responded to treatment and improved over 2 weeks, with only the 50-year-old featuring residual symptoms of anosmia and ageusia.
The report was published online April 17 in Neurology.
The 50-year-old man was admitted to an emergency room with a temperature of 99.9°F (37.7°C). He reported 2 days of vertical diplopia, perioral paresthesias, and gait instability. His neurologic examination showed intact cognitive function and language.
Five days earlier he developed a cough, malaise, headache, low back pain, fever, anosmia, and ageusia.
His neuro-ophthalmologic examination showed right hypertropia in all fields of gaze, severe limitations to the adduction and downgaze movements of his right eye, and left eye nystagmus on left gaze. These findings were consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy.
He responded to intravenous (IV) immunoglobulin therapy and was discharged home 2 weeks after admission.
The 39-year-old man was admitted to the emergency room with acute onset diplopia and ageusia. Three days earlier he had presented with diarrhea, a low-grade fever and in generally poor condition, without any headache, respiratory symptoms, or dyspnea.
He showed esotropia of 10 prism diopters at distance and 4 prism diopters at near, severe abduction deficits in both eyes, and fixation nystagmus, with the upper gaze more impaired, all consistent with bilateral abducens palsy.
The patient was discharged home and treated symptomatically with acetaminophen and telemedicine monitoring “due to a complete hospital saturation with COVID-19 patients,” wrote the researchers, led by Consuelo Gutiérrez-Ortiz, MD, PhD, Hospital Universitario Príncipe de Asturias, Madrid, Spain.
Two weeks later, he had made a complete neurologic recovery with no ageusia, complete eye movements, and normal deep tendon reflexes.
“Fisher syndrome and polyneuritis cranialis in these two patients with the SARS-CoV-2 infection could be simply coincidental. However, taking into account the temporal relationship, we feel that COVID-19 might have been responsible for the development of these two neurological pictures,” the authors noted.
European Regional Development Funds (FEDER) supported this research.
This article first appeared on Medscape.com.
A 50-year-old man developed Miller Fisher syndrome and a 39-year-old man developed polyneuritis cranialis. Both are variants of Guillain-Barré syndrome (GBS), which physicians in China and Italy also linked to COVID-19 infection, as previously reported by Medscape Medical News.
In both cases, physicians made the diagnoses based on abnormal eye examinations. The two patients responded to treatment and improved over 2 weeks, with only the 50-year-old featuring residual symptoms of anosmia and ageusia.
The report was published online April 17 in Neurology.
The 50-year-old man was admitted to an emergency room with a temperature of 99.9°F (37.7°C). He reported 2 days of vertical diplopia, perioral paresthesias, and gait instability. His neurologic examination showed intact cognitive function and language.
Five days earlier he developed a cough, malaise, headache, low back pain, fever, anosmia, and ageusia.
His neuro-ophthalmologic examination showed right hypertropia in all fields of gaze, severe limitations to the adduction and downgaze movements of his right eye, and left eye nystagmus on left gaze. These findings were consistent with right internuclear ophthalmoparesis and right fascicular oculomotor palsy.
He responded to intravenous (IV) immunoglobulin therapy and was discharged home 2 weeks after admission.
The 39-year-old man was admitted to the emergency room with acute onset diplopia and ageusia. Three days earlier he had presented with diarrhea, a low-grade fever and in generally poor condition, without any headache, respiratory symptoms, or dyspnea.
He showed esotropia of 10 prism diopters at distance and 4 prism diopters at near, severe abduction deficits in both eyes, and fixation nystagmus, with the upper gaze more impaired, all consistent with bilateral abducens palsy.
The patient was discharged home and treated symptomatically with acetaminophen and telemedicine monitoring “due to a complete hospital saturation with COVID-19 patients,” wrote the researchers, led by Consuelo Gutiérrez-Ortiz, MD, PhD, Hospital Universitario Príncipe de Asturias, Madrid, Spain.
Two weeks later, he had made a complete neurologic recovery with no ageusia, complete eye movements, and normal deep tendon reflexes.
“Fisher syndrome and polyneuritis cranialis in these two patients with the SARS-CoV-2 infection could be simply coincidental. However, taking into account the temporal relationship, we feel that COVID-19 might have been responsible for the development of these two neurological pictures,” the authors noted.
European Regional Development Funds (FEDER) supported this research.
This article first appeared on Medscape.com.
Survey: Hydroxychloroquine use fairly common in COVID-19
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.
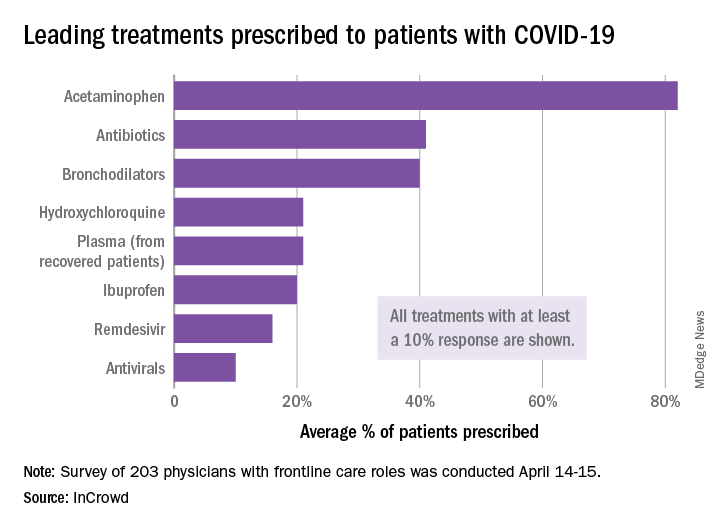
The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
COVID-19: No U.S. spike expected in pandemic-related suicidal ideation
Americans are not feeling more suicidal even in the depths of the COVID-19 pandemic of spring 2020, according to analysis of real-time national data accrued through the Crisis Text Line.
But that’s not to say Americans are feeling less distressed. Quite the contrary, Nancy Lublin, CEO and cofounder of Crisis Text Line, noted at the virtual annual meeting of the American Association of Suicidology.
“We’ve seen a 40% increase in volume since early March. Seventy-eight percent of our conversations are now including words like ‘freaked out,’ ‘panicked,’ ‘scared.’ People are worried about COVID-19. They’re nervous about symptoms; they’re concerned for family on the front lines,” she said.
And yet, from mid-March through mid-April, only 22% of texters to the crisis line expressed suicidal ideation, down from a usual background rate of 28%. Moreover, just 13% of texters who mentioned ‘COVID,’ ‘quarantine,’ or ‘virus’ expressed suicidal ideation, compared with 25% of other texters.
Ms. Lublin and her data crunchers are tracking not only the impact of the disease, but they’re also monitoring the mental health effects of the quarantine and social distancing.
“People are away from their routines, and perhaps [are] quarantined with abusive people. We’ve seen a 48% increase in texts involving sexual abuse and a 74% increase in domestic violence,” she said.
Texts focused on eating disorders or body image issues have jumped by 45%. And roughly two-thirds of texters now describe feelings of depression.
One of the biggest mental health impacts she and colleagues have seen stem from the economic recession triggered by the pandemic.
“We’ve seen more people reach out with fears of bankruptcy, fears of homelessness, fears of financial ruin. Thirty-two percent of our texters now report household incomes under $20,000 per year. That’s up from 19% before,” according to Ms. Lublin.
The Crisis Text Line (text HOME to 741741) uses machine-learning algorithms that sift through incoming text messages from people in crisis for key words, then ranks the messages by severity. Since its launch in 2013, this service, available 24/7, has processed roughly 150 million text messages. The high-risk texters – for example, someone who’s swallowed a bottle of pills or is texting from the San Francisco’s Golden Gate Bridge, as has occurred some 500 times – are connected in an average of 24 seconds with a thoroughly trained volunteer crisis counselor. And there is a third party in these texting conversations: a paid staff supervisor with a master’s degree in a relevant discipline who follows the encounter in real time and can step in if needed.
“Active rescues are involved in less than 1% of our conversations, but still we do them on average 26 times per day. Over the years, we’ve completed more than 32,000 active rescues,” she said.
The Crisis Text Line is not exclusively a suicide prevention hotline. The top five issues people text about involve relationship concerns, depression, anxiety, self-harm, and suicidal ideation. Over time, Ms. Lublin and staff have used Big Data to tweak the screening algorithm as they’ve identified even higher red flag texting words than “suicide.”
“The word ‘military’ makes it twice as likely that we’ll have to call 9-1-1 than the word ‘suicide.’ ‘Gun,’ ‘rope’ – four times as likely. In the [United KIngdom], where we’re also operating, we see the word ‘cliff’ is a more lethal word than the word ‘suicide.’ But the most dangerous words that we see are any named pill,” she said.
The Crisis Text Line was recently awarded a 2020 TED Audacious Project grant to expand their services from English to also be offered in Spanish, French, Portuguese, and Arabic worldwide within the next two and a half years. This will provide coverage to one-third of the world’s population, including people with cell phones living in countries with very limited mental health services.
Will COVID-19 trigger a spike in deaths by suicide?
Whether the COVID-19 pandemic will result in a bump in suicide rates is unclear and will remain so for quite a while, according to David Gunnell, MD, PhD, a suicidologist and professor of epidemiology at the University of Bristol (England).
In the United Kingdom, investigation of a suspicious death typically takes more than 6 months before an official declaration of suicide is recorded by the medical examiner. The lag time is even longer in the United States: The latest national suicide rate data are for 2018 because state-by-state reporting practices vary widely, he noted at a National Press Foundation briefing on COVID-19 and mental health.
Although suicide is consistently the 10th-leading cause of death in the United States, it’s important to put it in perspective, he added. In 2018, there were an average of 4,000 deaths by suicide per month nationally, whereas in March and April of 2020, there were 28,400 deaths per month attributable to COVID-19.
A classic study of the Spanish influenza pandemic in the United States during 1918-1919 concluded that there was “a slight upturn” in the rate of suicide in the months following the pandemic’s peak. More recently, a study of the 2003 SARS (severe acute respiratory syndrome) epidemic in Hong Kong found roughly a 30% increase in the rate of suicide among the elderly during that time frame, Dr. Gunnell noted.
“What limited evidence there is provides an indication of a small rise in suicides, but the number of deaths is far outweighed by the number of deaths associated with these big pandemics,” according to the epidemiologist.
Pandemics aside, there is far more compelling evidence that periods of economic recession are associated with an increase in the suicide rate, he added.
Another speaker, Holly C. Wilcox, PhD, a psychiatric epidemiologist at Johns Hopkins University, Baltimore, commented: “It’s not surprising that, during times of disaster the suicide rates decrease a bit. It could be because of people coming toghether. It could be one silver lining of COVID-19. But if there’s prolonged stress economically and socially and we can’t work towards reducing stress for people, we could see an increase. I don’t know if we will.”
In a recent article, Dr. Gunnell and coauthors offered a series of recommendations aimed at blunting the mental health consequences of COVID-19 and the related economic fallout (Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366[20]30171-1).
The authors highlighted the need for interventions aimed at defusing the adverse impact of self-isolation, social distancing, fear, an anticipated rise in alcohol misuse, joblessness, interrupted education, bereavement, and complicated grief. Governments can blunt the well-established effect of financial distress as a risk factor for suicide by providing safety nets in the form of supports for housing, food, and unemployment benefits. And it will be important that those mental health services that develop expertise in performing psychiatric assessments and interventions remotely via telemedicine share their insights, Dr. Gunnell said.
Americans are not feeling more suicidal even in the depths of the COVID-19 pandemic of spring 2020, according to analysis of real-time national data accrued through the Crisis Text Line.
But that’s not to say Americans are feeling less distressed. Quite the contrary, Nancy Lublin, CEO and cofounder of Crisis Text Line, noted at the virtual annual meeting of the American Association of Suicidology.
“We’ve seen a 40% increase in volume since early March. Seventy-eight percent of our conversations are now including words like ‘freaked out,’ ‘panicked,’ ‘scared.’ People are worried about COVID-19. They’re nervous about symptoms; they’re concerned for family on the front lines,” she said.
And yet, from mid-March through mid-April, only 22% of texters to the crisis line expressed suicidal ideation, down from a usual background rate of 28%. Moreover, just 13% of texters who mentioned ‘COVID,’ ‘quarantine,’ or ‘virus’ expressed suicidal ideation, compared with 25% of other texters.
Ms. Lublin and her data crunchers are tracking not only the impact of the disease, but they’re also monitoring the mental health effects of the quarantine and social distancing.
“People are away from their routines, and perhaps [are] quarantined with abusive people. We’ve seen a 48% increase in texts involving sexual abuse and a 74% increase in domestic violence,” she said.
Texts focused on eating disorders or body image issues have jumped by 45%. And roughly two-thirds of texters now describe feelings of depression.
One of the biggest mental health impacts she and colleagues have seen stem from the economic recession triggered by the pandemic.
“We’ve seen more people reach out with fears of bankruptcy, fears of homelessness, fears of financial ruin. Thirty-two percent of our texters now report household incomes under $20,000 per year. That’s up from 19% before,” according to Ms. Lublin.
The Crisis Text Line (text HOME to 741741) uses machine-learning algorithms that sift through incoming text messages from people in crisis for key words, then ranks the messages by severity. Since its launch in 2013, this service, available 24/7, has processed roughly 150 million text messages. The high-risk texters – for example, someone who’s swallowed a bottle of pills or is texting from the San Francisco’s Golden Gate Bridge, as has occurred some 500 times – are connected in an average of 24 seconds with a thoroughly trained volunteer crisis counselor. And there is a third party in these texting conversations: a paid staff supervisor with a master’s degree in a relevant discipline who follows the encounter in real time and can step in if needed.
“Active rescues are involved in less than 1% of our conversations, but still we do them on average 26 times per day. Over the years, we’ve completed more than 32,000 active rescues,” she said.
The Crisis Text Line is not exclusively a suicide prevention hotline. The top five issues people text about involve relationship concerns, depression, anxiety, self-harm, and suicidal ideation. Over time, Ms. Lublin and staff have used Big Data to tweak the screening algorithm as they’ve identified even higher red flag texting words than “suicide.”
“The word ‘military’ makes it twice as likely that we’ll have to call 9-1-1 than the word ‘suicide.’ ‘Gun,’ ‘rope’ – four times as likely. In the [United KIngdom], where we’re also operating, we see the word ‘cliff’ is a more lethal word than the word ‘suicide.’ But the most dangerous words that we see are any named pill,” she said.
The Crisis Text Line was recently awarded a 2020 TED Audacious Project grant to expand their services from English to also be offered in Spanish, French, Portuguese, and Arabic worldwide within the next two and a half years. This will provide coverage to one-third of the world’s population, including people with cell phones living in countries with very limited mental health services.
Will COVID-19 trigger a spike in deaths by suicide?
Whether the COVID-19 pandemic will result in a bump in suicide rates is unclear and will remain so for quite a while, according to David Gunnell, MD, PhD, a suicidologist and professor of epidemiology at the University of Bristol (England).
In the United Kingdom, investigation of a suspicious death typically takes more than 6 months before an official declaration of suicide is recorded by the medical examiner. The lag time is even longer in the United States: The latest national suicide rate data are for 2018 because state-by-state reporting practices vary widely, he noted at a National Press Foundation briefing on COVID-19 and mental health.
Although suicide is consistently the 10th-leading cause of death in the United States, it’s important to put it in perspective, he added. In 2018, there were an average of 4,000 deaths by suicide per month nationally, whereas in March and April of 2020, there were 28,400 deaths per month attributable to COVID-19.
A classic study of the Spanish influenza pandemic in the United States during 1918-1919 concluded that there was “a slight upturn” in the rate of suicide in the months following the pandemic’s peak. More recently, a study of the 2003 SARS (severe acute respiratory syndrome) epidemic in Hong Kong found roughly a 30% increase in the rate of suicide among the elderly during that time frame, Dr. Gunnell noted.
“What limited evidence there is provides an indication of a small rise in suicides, but the number of deaths is far outweighed by the number of deaths associated with these big pandemics,” according to the epidemiologist.
Pandemics aside, there is far more compelling evidence that periods of economic recession are associated with an increase in the suicide rate, he added.
Another speaker, Holly C. Wilcox, PhD, a psychiatric epidemiologist at Johns Hopkins University, Baltimore, commented: “It’s not surprising that, during times of disaster the suicide rates decrease a bit. It could be because of people coming toghether. It could be one silver lining of COVID-19. But if there’s prolonged stress economically and socially and we can’t work towards reducing stress for people, we could see an increase. I don’t know if we will.”
In a recent article, Dr. Gunnell and coauthors offered a series of recommendations aimed at blunting the mental health consequences of COVID-19 and the related economic fallout (Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366[20]30171-1).
The authors highlighted the need for interventions aimed at defusing the adverse impact of self-isolation, social distancing, fear, an anticipated rise in alcohol misuse, joblessness, interrupted education, bereavement, and complicated grief. Governments can blunt the well-established effect of financial distress as a risk factor for suicide by providing safety nets in the form of supports for housing, food, and unemployment benefits. And it will be important that those mental health services that develop expertise in performing psychiatric assessments and interventions remotely via telemedicine share their insights, Dr. Gunnell said.
Americans are not feeling more suicidal even in the depths of the COVID-19 pandemic of spring 2020, according to analysis of real-time national data accrued through the Crisis Text Line.
But that’s not to say Americans are feeling less distressed. Quite the contrary, Nancy Lublin, CEO and cofounder of Crisis Text Line, noted at the virtual annual meeting of the American Association of Suicidology.
“We’ve seen a 40% increase in volume since early March. Seventy-eight percent of our conversations are now including words like ‘freaked out,’ ‘panicked,’ ‘scared.’ People are worried about COVID-19. They’re nervous about symptoms; they’re concerned for family on the front lines,” she said.
And yet, from mid-March through mid-April, only 22% of texters to the crisis line expressed suicidal ideation, down from a usual background rate of 28%. Moreover, just 13% of texters who mentioned ‘COVID,’ ‘quarantine,’ or ‘virus’ expressed suicidal ideation, compared with 25% of other texters.
Ms. Lublin and her data crunchers are tracking not only the impact of the disease, but they’re also monitoring the mental health effects of the quarantine and social distancing.
“People are away from their routines, and perhaps [are] quarantined with abusive people. We’ve seen a 48% increase in texts involving sexual abuse and a 74% increase in domestic violence,” she said.
Texts focused on eating disorders or body image issues have jumped by 45%. And roughly two-thirds of texters now describe feelings of depression.
One of the biggest mental health impacts she and colleagues have seen stem from the economic recession triggered by the pandemic.
“We’ve seen more people reach out with fears of bankruptcy, fears of homelessness, fears of financial ruin. Thirty-two percent of our texters now report household incomes under $20,000 per year. That’s up from 19% before,” according to Ms. Lublin.
The Crisis Text Line (text HOME to 741741) uses machine-learning algorithms that sift through incoming text messages from people in crisis for key words, then ranks the messages by severity. Since its launch in 2013, this service, available 24/7, has processed roughly 150 million text messages. The high-risk texters – for example, someone who’s swallowed a bottle of pills or is texting from the San Francisco’s Golden Gate Bridge, as has occurred some 500 times – are connected in an average of 24 seconds with a thoroughly trained volunteer crisis counselor. And there is a third party in these texting conversations: a paid staff supervisor with a master’s degree in a relevant discipline who follows the encounter in real time and can step in if needed.
“Active rescues are involved in less than 1% of our conversations, but still we do them on average 26 times per day. Over the years, we’ve completed more than 32,000 active rescues,” she said.
The Crisis Text Line is not exclusively a suicide prevention hotline. The top five issues people text about involve relationship concerns, depression, anxiety, self-harm, and suicidal ideation. Over time, Ms. Lublin and staff have used Big Data to tweak the screening algorithm as they’ve identified even higher red flag texting words than “suicide.”
“The word ‘military’ makes it twice as likely that we’ll have to call 9-1-1 than the word ‘suicide.’ ‘Gun,’ ‘rope’ – four times as likely. In the [United KIngdom], where we’re also operating, we see the word ‘cliff’ is a more lethal word than the word ‘suicide.’ But the most dangerous words that we see are any named pill,” she said.
The Crisis Text Line was recently awarded a 2020 TED Audacious Project grant to expand their services from English to also be offered in Spanish, French, Portuguese, and Arabic worldwide within the next two and a half years. This will provide coverage to one-third of the world’s population, including people with cell phones living in countries with very limited mental health services.
Will COVID-19 trigger a spike in deaths by suicide?
Whether the COVID-19 pandemic will result in a bump in suicide rates is unclear and will remain so for quite a while, according to David Gunnell, MD, PhD, a suicidologist and professor of epidemiology at the University of Bristol (England).
In the United Kingdom, investigation of a suspicious death typically takes more than 6 months before an official declaration of suicide is recorded by the medical examiner. The lag time is even longer in the United States: The latest national suicide rate data are for 2018 because state-by-state reporting practices vary widely, he noted at a National Press Foundation briefing on COVID-19 and mental health.
Although suicide is consistently the 10th-leading cause of death in the United States, it’s important to put it in perspective, he added. In 2018, there were an average of 4,000 deaths by suicide per month nationally, whereas in March and April of 2020, there were 28,400 deaths per month attributable to COVID-19.
A classic study of the Spanish influenza pandemic in the United States during 1918-1919 concluded that there was “a slight upturn” in the rate of suicide in the months following the pandemic’s peak. More recently, a study of the 2003 SARS (severe acute respiratory syndrome) epidemic in Hong Kong found roughly a 30% increase in the rate of suicide among the elderly during that time frame, Dr. Gunnell noted.
“What limited evidence there is provides an indication of a small rise in suicides, but the number of deaths is far outweighed by the number of deaths associated with these big pandemics,” according to the epidemiologist.
Pandemics aside, there is far more compelling evidence that periods of economic recession are associated with an increase in the suicide rate, he added.
Another speaker, Holly C. Wilcox, PhD, a psychiatric epidemiologist at Johns Hopkins University, Baltimore, commented: “It’s not surprising that, during times of disaster the suicide rates decrease a bit. It could be because of people coming toghether. It could be one silver lining of COVID-19. But if there’s prolonged stress economically and socially and we can’t work towards reducing stress for people, we could see an increase. I don’t know if we will.”
In a recent article, Dr. Gunnell and coauthors offered a series of recommendations aimed at blunting the mental health consequences of COVID-19 and the related economic fallout (Lancet Psychiatry. 2020 Apr 21. doi: 10.1016/S2215-0366[20]30171-1).
The authors highlighted the need for interventions aimed at defusing the adverse impact of self-isolation, social distancing, fear, an anticipated rise in alcohol misuse, joblessness, interrupted education, bereavement, and complicated grief. Governments can blunt the well-established effect of financial distress as a risk factor for suicide by providing safety nets in the form of supports for housing, food, and unemployment benefits. And it will be important that those mental health services that develop expertise in performing psychiatric assessments and interventions remotely via telemedicine share their insights, Dr. Gunnell said.
FROM AAS 2020
COVID-19: Calls to NYC crisis hotline soar
Calls to a mental health crisis hotline in New York City have soared during the COVID-19 pandemic, which has closed schools and businesses, put millions out of work, and ushered in stay-at-home orders.
ensuring that care is available when and where needed during a crisis, whether that be an individual crisis, a local community crisis, or a national mental health crisis like we are facing right now,” said Kimberly Williams, president and CEO of Vibrant Emotional Health.
Vibrant Emotional Health, formerly the Mental Health Association of New York City, provides crisis line services across the United States in partnership with local and federal governments and corporations. NYC Well is one of them.
Ms. Williams and two of her colleagues spoke about crisis hotlines April 25 during the American Psychiatric Association’s Virtual Spring Highlights Meeting.
Rapid crisis intervention
Crisis hotlines provide “rapid crisis intervention, delivering help immediately from trained crisis counselors who respond to unique needs, actively engage in collaborative problem solving, and assess risk for suicide,” Ms. Williams said.
They have a proven track record, she noted. Research shows that they are able to decrease emotional distress and reduce suicidality in crisis situations.
Kelly Clarke, program director of NYC Well, noted that inbound call volume has increased roughly 50% since the COVID-19 pandemic hit.
Callers to NYC Well most commonly report mood/anxiety concerns, stressful life events, and interpersonal problems. “Many people are reaching out to seek support in how to manage their own emotional well-being in light of the pandemic and the restrictions put in place,” said Ms. Clarke.
Multilingual peer support specialists and counselors with NYC Well provide free, confidential support by talk, text, or chat 24 hours per day, 7 days per week, 365 days a year. The service also provides mobile crisis teams and follow-up services. NYC Well has set up a landing page of resources specifically geared toward COVID-19.
How to cope with the rapid growth and at the same time ensure high quality of services are two key challenges for NYC Well, Ms. Clarke said.
“Absolutely essential” service
For John Draper, PhD, the experience early in his career of working on a mobile mental health crisis team in Brooklyn “changed his life.”
First, it showed him that, for people who are severely psychiatrically ill, “care has to come to them,” said Dr. Draper, executive vice president of national networks for Vibrant Emotional Health.
“So many of the people we were seeing were too depressed to get out of bed, much less get to a clinic, and I realized our system was not set up to serve its customers. It was like putting a spinal cord injury clinic at the top of a stairs,” he said.
Crisis hotlines are “absolutely essential.” Their value for communities and individuals “can’t be overestimated,” said Dr. Draper.
This was revealed after the terrorist attacks of 9/11 and now with COVID-19, said Dr. Draper. He noted, that following the attacks of 9/11, a federal report referred to crisis hotlines as “the single most important asset in the response.”
A version of this article originally appeared on Medscape.com.
Calls to a mental health crisis hotline in New York City have soared during the COVID-19 pandemic, which has closed schools and businesses, put millions out of work, and ushered in stay-at-home orders.
ensuring that care is available when and where needed during a crisis, whether that be an individual crisis, a local community crisis, or a national mental health crisis like we are facing right now,” said Kimberly Williams, president and CEO of Vibrant Emotional Health.
Vibrant Emotional Health, formerly the Mental Health Association of New York City, provides crisis line services across the United States in partnership with local and federal governments and corporations. NYC Well is one of them.
Ms. Williams and two of her colleagues spoke about crisis hotlines April 25 during the American Psychiatric Association’s Virtual Spring Highlights Meeting.
Rapid crisis intervention
Crisis hotlines provide “rapid crisis intervention, delivering help immediately from trained crisis counselors who respond to unique needs, actively engage in collaborative problem solving, and assess risk for suicide,” Ms. Williams said.
They have a proven track record, she noted. Research shows that they are able to decrease emotional distress and reduce suicidality in crisis situations.
Kelly Clarke, program director of NYC Well, noted that inbound call volume has increased roughly 50% since the COVID-19 pandemic hit.
Callers to NYC Well most commonly report mood/anxiety concerns, stressful life events, and interpersonal problems. “Many people are reaching out to seek support in how to manage their own emotional well-being in light of the pandemic and the restrictions put in place,” said Ms. Clarke.
Multilingual peer support specialists and counselors with NYC Well provide free, confidential support by talk, text, or chat 24 hours per day, 7 days per week, 365 days a year. The service also provides mobile crisis teams and follow-up services. NYC Well has set up a landing page of resources specifically geared toward COVID-19.
How to cope with the rapid growth and at the same time ensure high quality of services are two key challenges for NYC Well, Ms. Clarke said.
“Absolutely essential” service
For John Draper, PhD, the experience early in his career of working on a mobile mental health crisis team in Brooklyn “changed his life.”
First, it showed him that, for people who are severely psychiatrically ill, “care has to come to them,” said Dr. Draper, executive vice president of national networks for Vibrant Emotional Health.
“So many of the people we were seeing were too depressed to get out of bed, much less get to a clinic, and I realized our system was not set up to serve its customers. It was like putting a spinal cord injury clinic at the top of a stairs,” he said.
Crisis hotlines are “absolutely essential.” Their value for communities and individuals “can’t be overestimated,” said Dr. Draper.
This was revealed after the terrorist attacks of 9/11 and now with COVID-19, said Dr. Draper. He noted, that following the attacks of 9/11, a federal report referred to crisis hotlines as “the single most important asset in the response.”
A version of this article originally appeared on Medscape.com.
Calls to a mental health crisis hotline in New York City have soared during the COVID-19 pandemic, which has closed schools and businesses, put millions out of work, and ushered in stay-at-home orders.
ensuring that care is available when and where needed during a crisis, whether that be an individual crisis, a local community crisis, or a national mental health crisis like we are facing right now,” said Kimberly Williams, president and CEO of Vibrant Emotional Health.
Vibrant Emotional Health, formerly the Mental Health Association of New York City, provides crisis line services across the United States in partnership with local and federal governments and corporations. NYC Well is one of them.
Ms. Williams and two of her colleagues spoke about crisis hotlines April 25 during the American Psychiatric Association’s Virtual Spring Highlights Meeting.
Rapid crisis intervention
Crisis hotlines provide “rapid crisis intervention, delivering help immediately from trained crisis counselors who respond to unique needs, actively engage in collaborative problem solving, and assess risk for suicide,” Ms. Williams said.
They have a proven track record, she noted. Research shows that they are able to decrease emotional distress and reduce suicidality in crisis situations.
Kelly Clarke, program director of NYC Well, noted that inbound call volume has increased roughly 50% since the COVID-19 pandemic hit.
Callers to NYC Well most commonly report mood/anxiety concerns, stressful life events, and interpersonal problems. “Many people are reaching out to seek support in how to manage their own emotional well-being in light of the pandemic and the restrictions put in place,” said Ms. Clarke.
Multilingual peer support specialists and counselors with NYC Well provide free, confidential support by talk, text, or chat 24 hours per day, 7 days per week, 365 days a year. The service also provides mobile crisis teams and follow-up services. NYC Well has set up a landing page of resources specifically geared toward COVID-19.
How to cope with the rapid growth and at the same time ensure high quality of services are two key challenges for NYC Well, Ms. Clarke said.
“Absolutely essential” service
For John Draper, PhD, the experience early in his career of working on a mobile mental health crisis team in Brooklyn “changed his life.”
First, it showed him that, for people who are severely psychiatrically ill, “care has to come to them,” said Dr. Draper, executive vice president of national networks for Vibrant Emotional Health.
“So many of the people we were seeing were too depressed to get out of bed, much less get to a clinic, and I realized our system was not set up to serve its customers. It was like putting a spinal cord injury clinic at the top of a stairs,” he said.
Crisis hotlines are “absolutely essential.” Their value for communities and individuals “can’t be overestimated,” said Dr. Draper.
This was revealed after the terrorist attacks of 9/11 and now with COVID-19, said Dr. Draper. He noted, that following the attacks of 9/11, a federal report referred to crisis hotlines as “the single most important asset in the response.”
A version of this article originally appeared on Medscape.com.
Metastatic cancer linked to worse outcomes of COVID-19
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
Cancer type, stage, and recent treatment may affect outcomes of COVID-19 in cancer patients, according to a study of patients from China.
The data showed that patients with hematologic malignancies and those with metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying.
On the other hand, patients with nonmetastatic cancer had outcomes comparable to those of noncancer patients with COVID-19.
Similarly, cancer patients who had recently undergone surgery or received immunotherapy were more likely to have poor outcomes, whereas cancer patients treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
Hongbing Cai, MD, of Zhongnan Hospital of Wuhan University in China, presented these results at the AACR virtual meeting I. The results also were published in Cancer Discovery.
Cancer vs. noncancer patients
The study included 105 cancer patients with COVID-19 who were treated from Jan. 1 to Feb. 24, 2020, at 14 hospitals in Wuhan, China. Patients had lung (20.95%), gastrointestinal (12.38%), breast (10.48%), and thyroid cancers (10.48%) as well as hematologic malignancies (8.57%). Dr. Cai and colleagues matched the COVID-19 cancer patients to 536 COVID-19 patients without cancer. Patients were matched by hospital, duration of hospitalization, and age.
“COVID-19 patients with cancer had higher risks of all severe outcomes,” Dr. Cai noted.
Compared with noncancer patients, the cancer patients had a higher risk of:
- Severe or critical COVID-19 symptoms – odds ratio, 2.79 (P < .01).
- Being admitted to the ICU – OR, 2.84 (P < .01).
- Requiring invasive mechanical ventilation – OR, 14 (P < .01).
- Death – OR, 2.34 (P = .03).
Cancer type and stage
Dr. Cai noted that outcomes were the worst among patients with hematologic malignancies and those with metastatic cancer (stage IV).
Compared with patients without cancer, those with hematologic malignancies had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- ICU admission – OR, 9.66 (P < .01).
- Invasive mechanical ventilation – OR, 38 (P < .01).
- Death – OR, 9.07 (P = .01).
Compared with patients without cancer, those with metastatic cancer had a higher risk of:
- Severe/critical symptoms – OR, 5.97 (P < .01).
- ICU admission – OR, 6.59 (P < 0.01).
- Invasive mechanical ventilation – OR, 55.42 (P < .01).
- Death – OR, 5.58 (P = .01).
On the other hand, outcomes in patients with nonmetastatic cancer were not significantly different from outcomes in patients without cancer (P > .05 for all outcomes).
Cancer treatment
The treatments cancer patients received within 40 days before the onset of COVID-19 symptoms were radiotherapy (12.26%), chemotherapy (14.15%), surgery (7.62%), targeted therapies (3.81%), and immunotherapy (5.71%).
Compared with patients without cancer, those who received immunotherapy had a higher risk of:
- Severe/critical symptoms – OR, 10.61 (P < .01).
- Death – OR, 9.07 (P = .04).
Patients who underwent surgery had a higher risk of:
- Severe/critical symptoms – OR, 8.84 (P < .01).
- ICU admission – OR, 7.24 (P = .02).
- Invasive mechanical ventilation – OR, 44.33 (P < .01).
Conversely, outcomes in cancer patients who received radiotherapy were not significantly different from outcomes in patients without cancer (P > .10 for all).
These results suggest that “postponing surgery should be considered in outbreak areas,” Dr. Cai said, adding that scheduled radiotherapy can go ahead but with “intensive protection and surveillance.”
Dr. Cai said it remains to be seen whether patients with early-stage cancer need to postpone their treatments during the COVID-19 pandemic or whether immunotherapy aggravates severe outcomes in cancer patients with COVID-19. For now, she said, cancer patients should have individualized treatment plans based on their tumor type and stage.
Dr. Cai disclosed no conflicts of interest. This study was supported by the National Natural Science Foundation of China, the Singapore Ministry of Health’s National Medical Research Council, the National Institutes of Health/National Heart, Lung, and Blood Institute, and the Xiu Research Fund.
SOURCE: Cai H. AACR 2020. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak; Dai M et al. Cancer Discov. 2020 Apr 28. doi: 10.1158/2159-8290.CD-20-0422.
FROM AACR 2020