User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
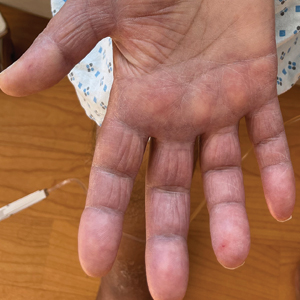
Multiple Firm Papules on the Wrists and Forearms
Multiple Firm Papules on the Wrists and Forearms
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
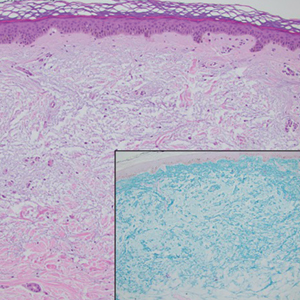
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
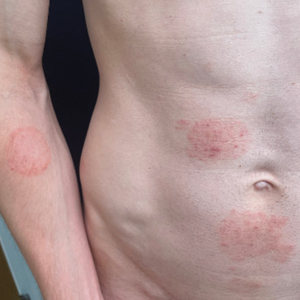
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
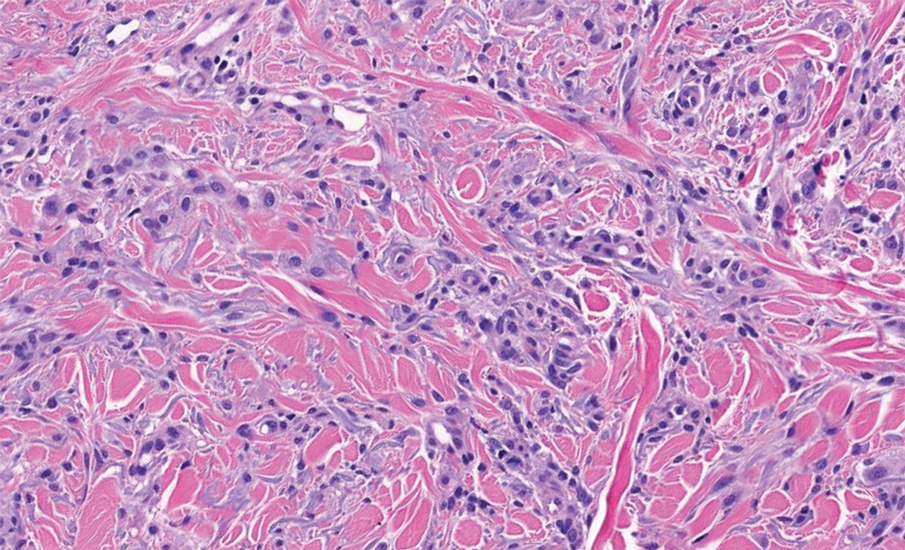
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).
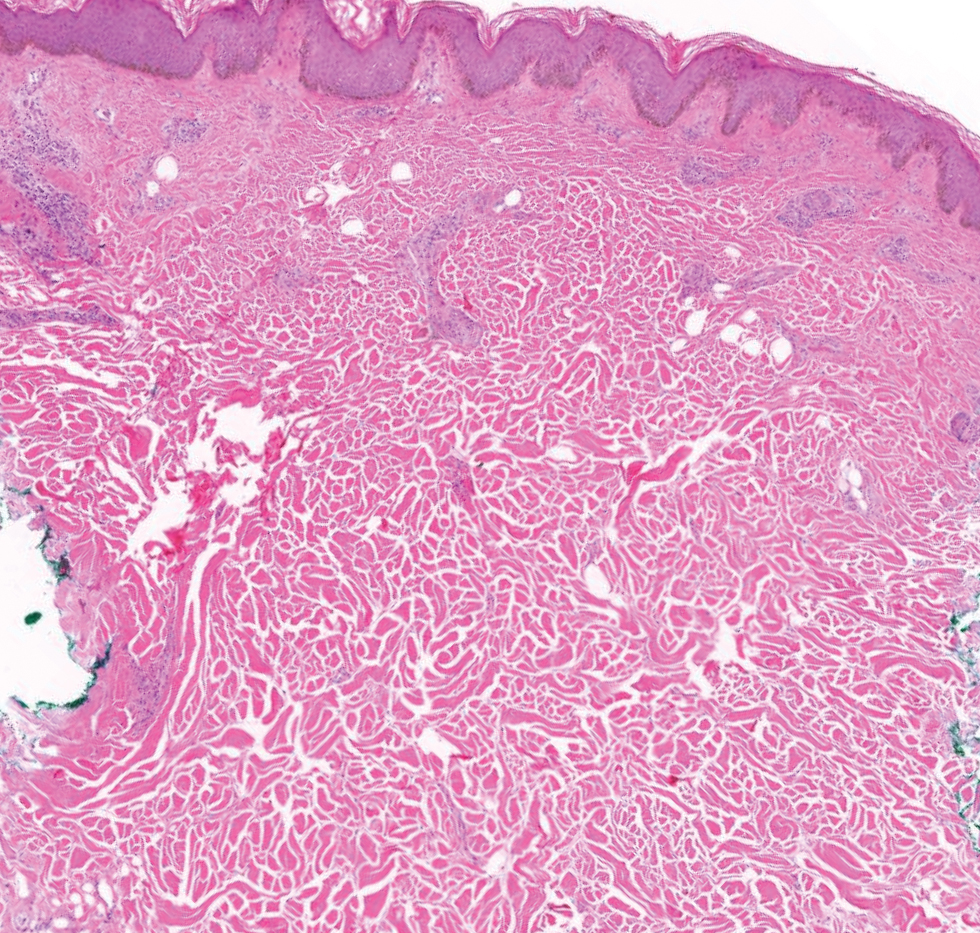
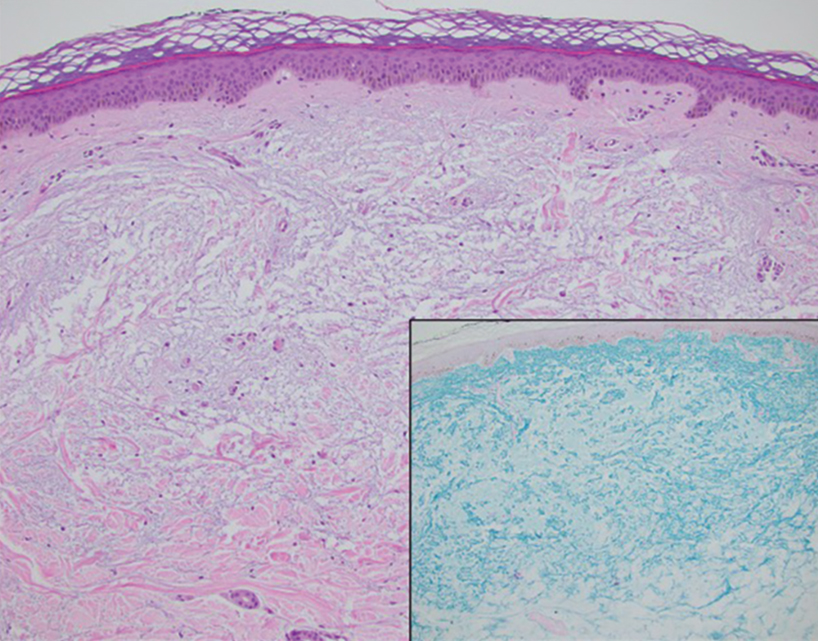
The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.
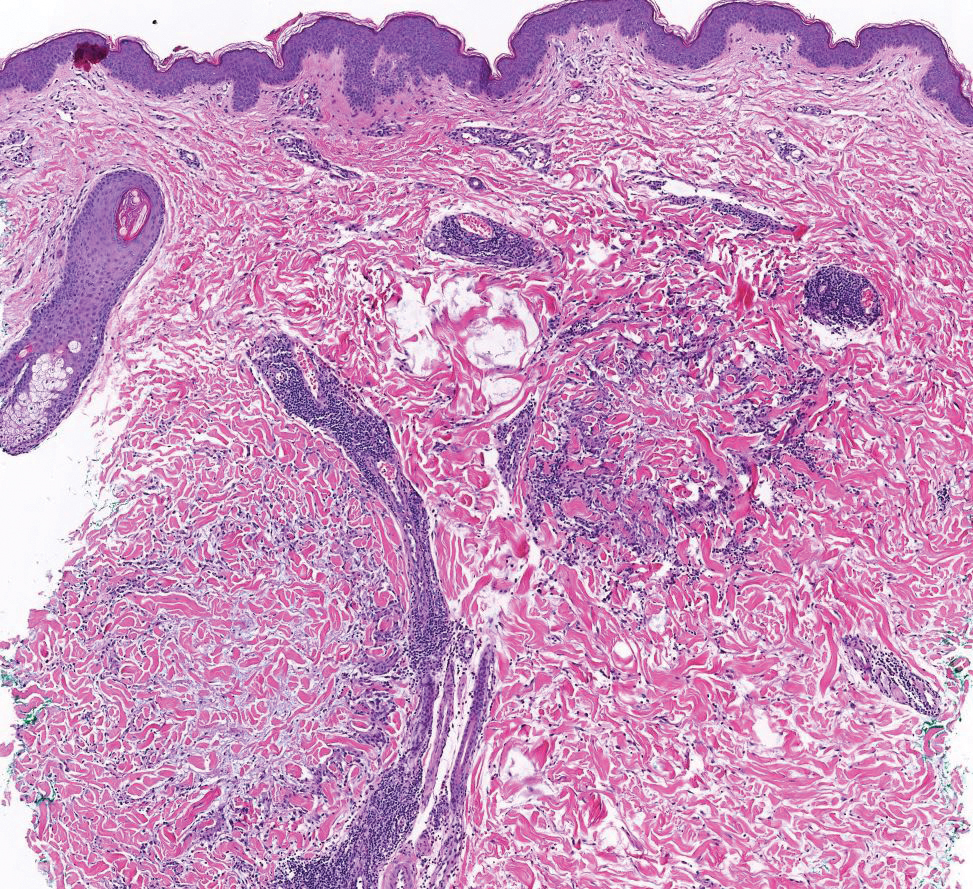
Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.
Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11
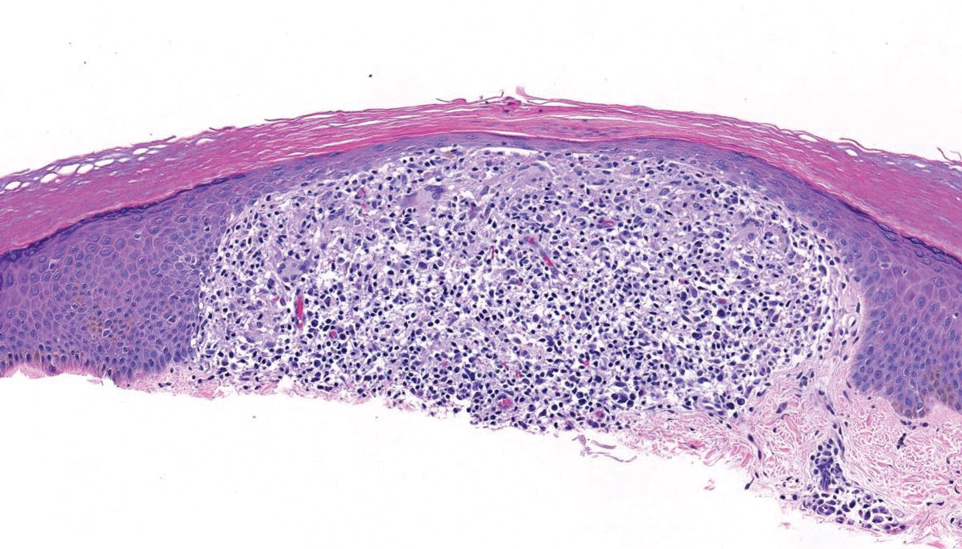
Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).

The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.

Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.

Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11

Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).

The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.

Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.

Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11

Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
Multiple Firm Papules on the Wrists and Forearms
Multiple Firm Papules on the Wrists and Forearms
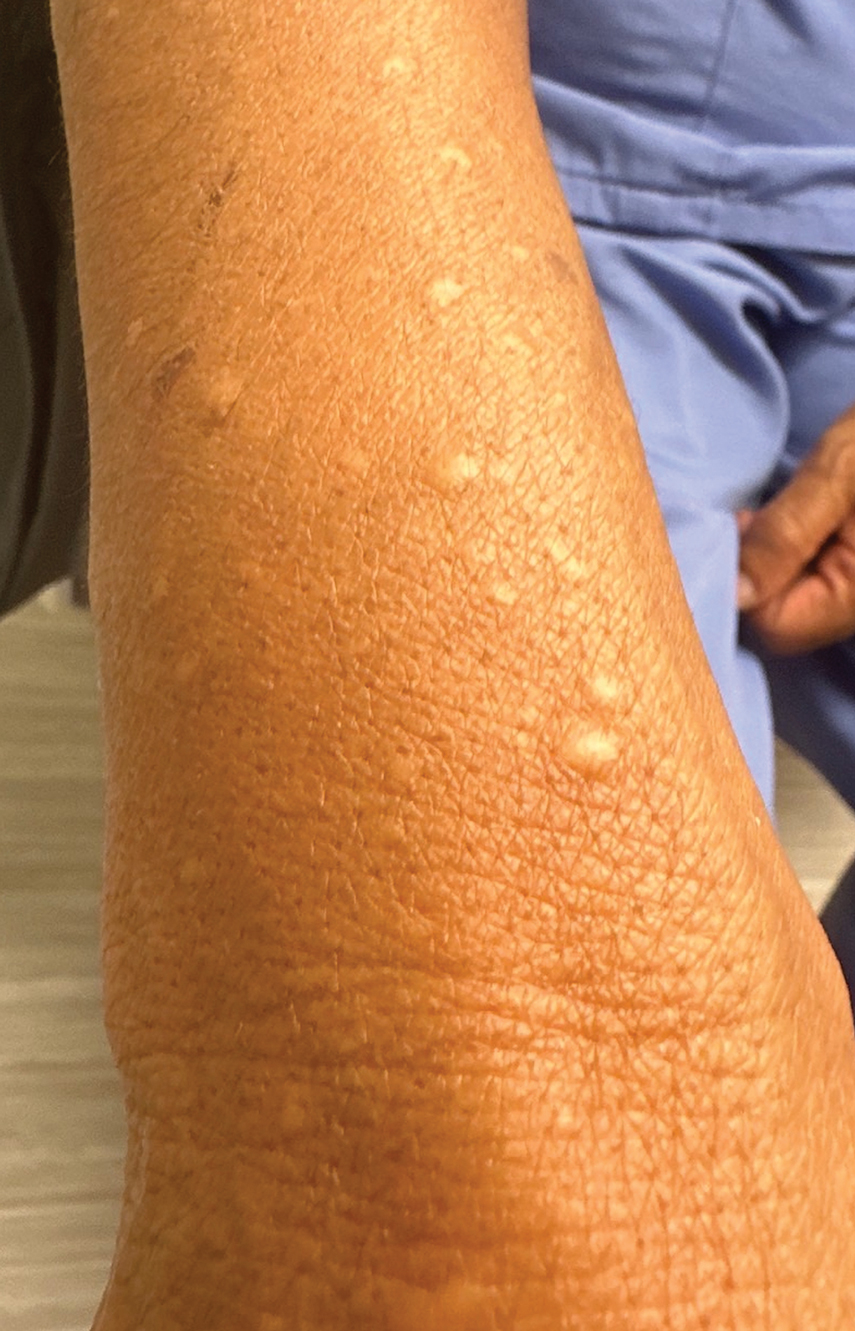
A 69-year-old woman presented to the dermatology department with persistent asymptomatic skin lesions on the wrists and forearms of several months’ duration. The lesions had slowly grown in number over the past few months with no identifiable triggers. The patient reported no known history of injury or trauma to the affected sites and was not taking any prescription medications other than daily vitamins. She denied any family history of similar lesions and was otherwise healthy. Physical examination revealed multiple waxy, firm, hypopigmented, 3- to 5-mm papules located exclusively on the dorsal wrists and forearms. No extracutaneous involvement was observed. A 4-mm punch biopsy from the forearm was obtained.
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
Skin cancer is a major health concern for military service members, who experience notably higher incidence rates than the general population.1 Active-duty military personnel are particularly vulnerable to prolonged sun exposure due to deployments, specialized training, and everyday outdoor duties.1 Despite skin cancer being the most commonly diagnosed malignancy in active-duty service members,2 tracking and documenting the quantity and diversity of these risk factors remain limited. This knowledge gap comes at high cost, simultaneously impairing military medicine preventive measures while burdening the military health care system with substantial expenditures.3 These findings underscore the critical need for targeted surveillance, early-detection programs, and policy-driven interventions to mitigate these medical and economic concerns.
Skin cancer has been recognized as a major health risk to the military population for decades, yet incidence and prevalence remain high. This phenomenon is closely linked to the inherent responsibilities and expectations of active-duty military members, including outdoor physical training, field exercises, standing in formation, and outdoor working environments—all of which can occur during peak sunlight hours. These risks are further elevated at duty stations in geographic regions with high levels of UV exposure, such as those in tropical and arid regions of the world. Certain military occupational specialties and missions may further introduce unique risk factors; for instance, pilots with frequent high-altitude missions experience heightened UV exposure and melanoma risk.4 Secondary to compounding determinants, the aviation, diving, and nuclear subgroups of the military community are particularly vulnerable to skin cancer.5
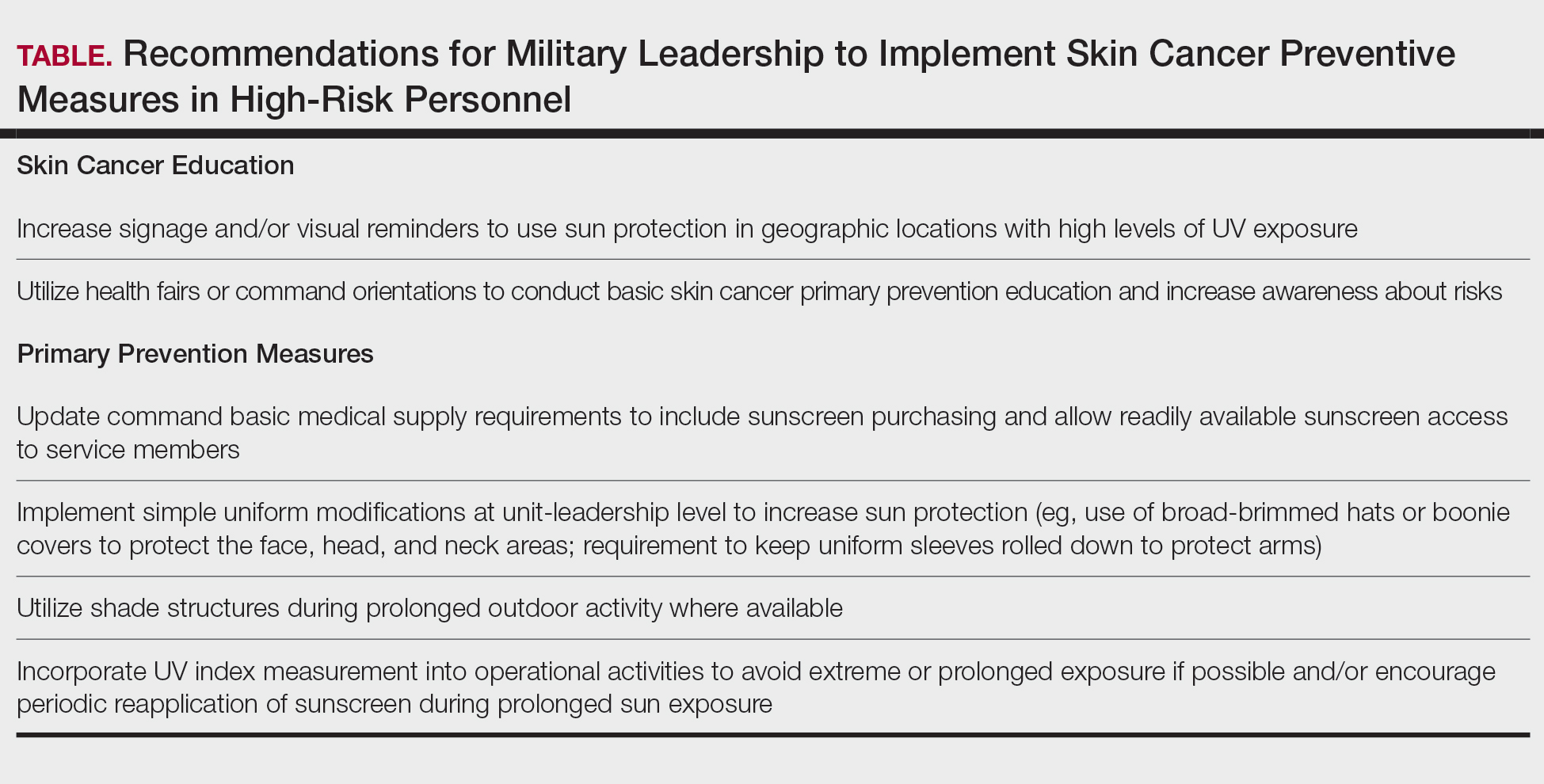
Despite well-documented risks, considerable gaps remain in quantifying and analyzing variations in UV exposure across military occupations, duty locations, and operational roles. Factors such as the existence of over 150 distinct military occupational specialties, frequent geographic relocations, and routine work in austere environments contribute to a wide range of UV exposure profiles that remain insufficiently characterized. This lack of comprehensive exposure data hinders the development of large-scale, targeted skin cancer prevention strategies. Initial approaches to addressing these challenges include enhanced surveillance, education, and policy initiatives. The Table presents practical recommendations for military leadership to consider in implementing preventive measures for skin cancer. Herein, we outline broader systemic strategies to bridge knowledge gaps and address underrecognized occupational risk factors for skin cancer in military service members; these elements include proposed modifications to the electronic Periodic Health Assessment (ePHA) and the development of standardized, military-specific screening and prevention guidelines to support early detection and resource optimization.
Skin Cancer Education for Service Members
Sunscreen and Signage—Diligent primary prevention offers a promising avenue for mitigating skin cancer incidence in military service members. Basic education and precautionary messaging on photoprotection can be widely implemented to simultaneously educate service members on the dangers of sun exposure while reinforcing healthy behaviors in real time. Simple low-cost initiatives such as strategically placed visual signage reminding service members to apply sunscreen in high UV environments can support consistent sun-safe practices. Educational efforts also should emphasize proper sunscreen use, including application on high-risk anatomic sites (eg, the face, neck, scalp, dorsal hands, and ears) and the essentiality of using sufficient quantities of broad-spectrum sunscreen for effective protection. Incorporating this guidance into training materials, briefings, and visual reminders allow seamless integration of photoprotection into service members’ daily routines without compromising operational efficiency.6 Younger service members, who may be less likely to prioritize preventive behaviors, may be particularly responsive to sun safety reminders in training areas, bases, and deployment zones.7 Health fairs and orientation briefs in high-UV regions also offer potential opportunities for targeted education.
Resources for Sun Protection in the Military
Sunscreen—Although sunscreen is critical in minimizing the risk for UV-induced skin cancer, its widespread use in the military is hindered by practical challenges related to accessibility and the need for consistent reapplication; for instance, providing free sunscreen dispensers at institutions for staff working under intense or prolonged UV exposure may improve sunscreen accessibility and use.8 Including sunscreen in standard-issue gear offers another logical way to embed its use into operational readiness as part of the routine protective measures.
Uniform Modifications—Adapting military uniforms and practices to improve sun protection plays a critical role in reducing skin cancer risk. Targeted protective gear for commonly sun-exposed areas can help mitigate UV exposure. One practical option is the use of wide-brimmed headgear (eg, boonie hats), which provide more face and neck coverage than standard-issue military caps, or covers. The wide-brimmed headgear currently is only selectively authorized during specific scenarios, such as field operations and training exercises, or at the discretion of unit-level leadership. Wide-brimmed headgear, already used by many service members, has been associated with up to a 17% reduction in UV exposure to inadequately protected areas, potentially lowering skin cancer risk.9,10 Similarly, a “sleeves-down” policy—requiring sleeves to remain unrolled and covering the forearms during outdoor activities—offers a simple way to minimize sun exposure without necessitating additional gear. Other specialized clothing items, including UV-blocking neck gaiters, photoprotective clothing, and lightweight gloves, also may be appropriate for high-risk groups and can be implemented in a relatively straightforward manner.
Shade Structures and UV Index Monitoring—Aside from uniform adaptation, physical barrier intervention can further complement skin cancer prevention efforts in the military. Shade structures offer a straightforward way to reduce UV exposure during prolonged outdoor activities. Incorporating daily UV index monitoring into operational guidance can help inform adjustments to training schedules and guide the implementation of additional sun protection measures, such as mandatory sunscreen application, use of wide-brimmed hats, or increased access to shaded rest areas during heavy sunlight hours. Currently, outdoor physical training is restricted during periods of high heat index, measured via Wet Bulb Globe Temperature, to reduce heat-related injuries. We argue that avoidance of nonoperational outdoor activity during peak UV index hours also should be incorporated into standardized policies. This intervention is of particular benefit to service members stationed in regions with a high UV index year-round, such as those stationed in the Middle East, Guam, Okinawa, and southern coastal United States bases.
Policy Changes to Support Photoprotective Measures
Annual Risk Factor Screening‐Screening—Effective secondary prevention efforts by military dermatologists remain an important measure in reducing the burden of skin cancer among military personnel; however, these efforts have become increasingly challenging due to 2 main factors—the diversity of military occupational specialties and their associated unique occupational risks as well as the limited availability of military dermatologists across all branches (approximately 100 active-duty dermatologists for nearly 3 million service members).11 Therefore, targeted interventions that enhance risk assessment, refined screening protocols, and leveraging of existing military health networks can improve early skin cancer detection while optimizing resource allocation.
The ePHA is an online screening tool used annually by all service members to evaluate their overall health. Presently, the ePHA lacks specific questions to assess sun exposure and skin cancer risks. Integrating annual skin cancer risk factor assessments into the ePHA would offer a practical and straightforward approach to identifying at-risk individuals, as suggested by Newnam et al12 in 2022. Skin cancer risk factor assessments allow for targeted data collection related to sun exposure history, family history, and personal risk factors, which can be used to determine individualized risk stratification to assess the need for early secondary prevention measures and specialist referral. These ePHA data can also support population-based analyses to inform preventive strategies and address knowledge gaps related to high-risk exposures, such as extended field exercises or assignments in high-UV regions, that may impede effective skin cancer prevention.
Development of Military-Specific Screening Guidelines—Given the limited number of military dermatologists, a standardized risk-assessment tool could enhance early detection of skin cancer and streamline the referral process. We propose a military-specific skin cancer screening algorithm or risk nomogram that could help to consolidate risk factors into a clear and actionable framework for more efficient triage and appropriate allocation of dermatologic resources and manpower. This nomogram could be developed by military dermatologists and then implemented on a command level, affording primary care providers a useful tool to expedite evaluation of individuals at higher risk for skin cancer while simultaneously promoting judicious use of limited dermatology resources.
Although the United States Preventive Services Task Force does not universally recommend routine skin cancer screenings for asymptomatic adults, military service members are exposed to higher occupational risks than the general population, as previously mentioned. Currently, there is no standardized screening guideline across all military services due to the unique nature and exposure risks for each branch of service and their varied occupations; however, we propose the development of basic standardized screening guidelines by adapting the framework of the United States Preventive Services Task Force and adjusting for military-specific UV exposure and occupational risks to improve early detection of skin cancer. These guidelines could be updated and tailored appropriately when additional population-based data are collected and analyzed through ePHA.
Critiques and Limitations of Implementation
Several challenges and limitations must be considered when attempting to integrate large-scale preventive measures for skin cancer within the US military. A primary concern is the extent to which military resources should be allocated to prevention when off-duty sun exposure remains largely beyond institutional control. Although military health initiatives can address workplace risk through education and policy, individual decisions during both work and leisure time remain a major variable that cannot be feasibly controlled. Cultural and operational barriers also pose challenges; for instance, the US Marine Corps maintains a strong cultural identity tied to uniform appearance, making it difficult to implement widespread changes to clothing-based sun-protection measures. Institutional changes, particularly those involving uniforms, likely will face substantial administrative resistance and potential operational limitations. When broad uniform modifications are unattainable, a more feasible approach may be to encourage unit-level leadership to authorize and promote the frequent use of nonuniform protective measures.
Furthermore, integrating additional skin cancer risk questions into the already extensive ePHA means extra time required to complete the assessment; this adds to service members’ administrative burden, potentially leading to reduced timely compliance, rushed responses, and survey fatigue, which threaten data quality. If new items are to be included, they should be carefully selected for efficiency and clinical relevance. Existing validated questionnaires such as those from the study by Lyford et al7 published in 2021 can serve as a foundation.
Another critical limitation is access to dermatologic care for active-duty service members. Raising awareness of skin cancer risk without ensuring adequate resources may create ethical concerns, particularly in high-risk environments such as the Middle East and Indo-Pacific. Additionally, because skin cancer often develops years or decades after exposure, securing early buy-in from service members and their leaders can be challenging. These concerns make it clear that, while skin cancer prevention is important, implementing widespread measures is not straightforward and requires a practical and balanced approach.
Final Thoughts
Implementing prevention strategies for skin cancer in the military requires balancing evidence-based recommendations with the practical realities of military culture, resource limitations, and operational demands. Challenges remain for dermatologists in providing targeted recommendations due to the multifaceted nature of military roles, including over 150 Navy Military Occupational Specialties, limited familiarity with the unique UV exposure risks associated with each occupation, and variability in local and regional policies on uniform wear, physical training requirements, and other operational practices. Although targeted prevention measures are difficult to establish in the setting of these knowledge gaps, leveraging unit-level leadership to align with existing screening guidelines and optimizing primary prevention measures can be meaningful steps toward reducing skin cancer risk for military service members while maintaining mission readiness.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancerincidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Krivda KR, Watson NL, Lyford WH, et al. The burden of skin cancer in the military health system, 2017-2022. Cutis. 2024;113:200-215. doi:10.12788/cutis.1015
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58. doi:10.1001/jamadermatol.2014.1077
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MMSR. 2017;24:8-14.
- Subramaniam P, Olsen CM, Thompson BS, et al, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. doi:10.1001/jamadermatol.2016.4070
- Lyford WH, Crotty A, Logemann NF. Sun exposure prevention practices within U.S. naval aviation. Mil Med. 2021;186:1169-1175. doi:10.1093/milmed/usab099
- Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77:164-166.
- Schissel D. Operation shadow warrior: a quantitative analysis of the ultraviolet radiation protection demonstrated by various headgear. Mil Med. 2001;166:783-785.
- Milch JM, Logemann NF. Photoprotection prevents skin cancer: let’s make it fashionable to wear sun-protective clothing. Cutis. 2017;99:89-92.
- Association of Military Dermatologists. (n.d.). Military dermatology. https://militaryderm.org/military-dermatology/
- Newnam R, Le-Jenkins U, Rutledge C, et al. The association of skin cancer prevention knowledge, sun-protective attitudes, and sunprotective behaviors in a Navy population. Mil Med. 2024;189:1-7. doi:10.1093/milmed/usac285
Skin cancer is a major health concern for military service members, who experience notably higher incidence rates than the general population.1 Active-duty military personnel are particularly vulnerable to prolonged sun exposure due to deployments, specialized training, and everyday outdoor duties.1 Despite skin cancer being the most commonly diagnosed malignancy in active-duty service members,2 tracking and documenting the quantity and diversity of these risk factors remain limited. This knowledge gap comes at high cost, simultaneously impairing military medicine preventive measures while burdening the military health care system with substantial expenditures.3 These findings underscore the critical need for targeted surveillance, early-detection programs, and policy-driven interventions to mitigate these medical and economic concerns.
Skin cancer has been recognized as a major health risk to the military population for decades, yet incidence and prevalence remain high. This phenomenon is closely linked to the inherent responsibilities and expectations of active-duty military members, including outdoor physical training, field exercises, standing in formation, and outdoor working environments—all of which can occur during peak sunlight hours. These risks are further elevated at duty stations in geographic regions with high levels of UV exposure, such as those in tropical and arid regions of the world. Certain military occupational specialties and missions may further introduce unique risk factors; for instance, pilots with frequent high-altitude missions experience heightened UV exposure and melanoma risk.4 Secondary to compounding determinants, the aviation, diving, and nuclear subgroups of the military community are particularly vulnerable to skin cancer.5
Despite well-documented risks, considerable gaps remain in quantifying and analyzing variations in UV exposure across military occupations, duty locations, and operational roles. Factors such as the existence of over 150 distinct military occupational specialties, frequent geographic relocations, and routine work in austere environments contribute to a wide range of UV exposure profiles that remain insufficiently characterized. This lack of comprehensive exposure data hinders the development of large-scale, targeted skin cancer prevention strategies. Initial approaches to addressing these challenges include enhanced surveillance, education, and policy initiatives. The Table presents practical recommendations for military leadership to consider in implementing preventive measures for skin cancer. Herein, we outline broader systemic strategies to bridge knowledge gaps and address underrecognized occupational risk factors for skin cancer in military service members; these elements include proposed modifications to the electronic Periodic Health Assessment (ePHA) and the development of standardized, military-specific screening and prevention guidelines to support early detection and resource optimization.

Skin Cancer Education for Service Members
Sunscreen and Signage—Diligent primary prevention offers a promising avenue for mitigating skin cancer incidence in military service members. Basic education and precautionary messaging on photoprotection can be widely implemented to simultaneously educate service members on the dangers of sun exposure while reinforcing healthy behaviors in real time. Simple low-cost initiatives such as strategically placed visual signage reminding service members to apply sunscreen in high UV environments can support consistent sun-safe practices. Educational efforts also should emphasize proper sunscreen use, including application on high-risk anatomic sites (eg, the face, neck, scalp, dorsal hands, and ears) and the essentiality of using sufficient quantities of broad-spectrum sunscreen for effective protection. Incorporating this guidance into training materials, briefings, and visual reminders allow seamless integration of photoprotection into service members’ daily routines without compromising operational efficiency.6 Younger service members, who may be less likely to prioritize preventive behaviors, may be particularly responsive to sun safety reminders in training areas, bases, and deployment zones.7 Health fairs and orientation briefs in high-UV regions also offer potential opportunities for targeted education.
Resources for Sun Protection in the Military
Sunscreen—Although sunscreen is critical in minimizing the risk for UV-induced skin cancer, its widespread use in the military is hindered by practical challenges related to accessibility and the need for consistent reapplication; for instance, providing free sunscreen dispensers at institutions for staff working under intense or prolonged UV exposure may improve sunscreen accessibility and use.8 Including sunscreen in standard-issue gear offers another logical way to embed its use into operational readiness as part of the routine protective measures.
Uniform Modifications—Adapting military uniforms and practices to improve sun protection plays a critical role in reducing skin cancer risk. Targeted protective gear for commonly sun-exposed areas can help mitigate UV exposure. One practical option is the use of wide-brimmed headgear (eg, boonie hats), which provide more face and neck coverage than standard-issue military caps, or covers. The wide-brimmed headgear currently is only selectively authorized during specific scenarios, such as field operations and training exercises, or at the discretion of unit-level leadership. Wide-brimmed headgear, already used by many service members, has been associated with up to a 17% reduction in UV exposure to inadequately protected areas, potentially lowering skin cancer risk.9,10 Similarly, a “sleeves-down” policy—requiring sleeves to remain unrolled and covering the forearms during outdoor activities—offers a simple way to minimize sun exposure without necessitating additional gear. Other specialized clothing items, including UV-blocking neck gaiters, photoprotective clothing, and lightweight gloves, also may be appropriate for high-risk groups and can be implemented in a relatively straightforward manner.
Shade Structures and UV Index Monitoring—Aside from uniform adaptation, physical barrier intervention can further complement skin cancer prevention efforts in the military. Shade structures offer a straightforward way to reduce UV exposure during prolonged outdoor activities. Incorporating daily UV index monitoring into operational guidance can help inform adjustments to training schedules and guide the implementation of additional sun protection measures, such as mandatory sunscreen application, use of wide-brimmed hats, or increased access to shaded rest areas during heavy sunlight hours. Currently, outdoor physical training is restricted during periods of high heat index, measured via Wet Bulb Globe Temperature, to reduce heat-related injuries. We argue that avoidance of nonoperational outdoor activity during peak UV index hours also should be incorporated into standardized policies. This intervention is of particular benefit to service members stationed in regions with a high UV index year-round, such as those stationed in the Middle East, Guam, Okinawa, and southern coastal United States bases.
Policy Changes to Support Photoprotective Measures
Annual Risk Factor Screening‐Screening—Effective secondary prevention efforts by military dermatologists remain an important measure in reducing the burden of skin cancer among military personnel; however, these efforts have become increasingly challenging due to 2 main factors—the diversity of military occupational specialties and their associated unique occupational risks as well as the limited availability of military dermatologists across all branches (approximately 100 active-duty dermatologists for nearly 3 million service members).11 Therefore, targeted interventions that enhance risk assessment, refined screening protocols, and leveraging of existing military health networks can improve early skin cancer detection while optimizing resource allocation.
The ePHA is an online screening tool used annually by all service members to evaluate their overall health. Presently, the ePHA lacks specific questions to assess sun exposure and skin cancer risks. Integrating annual skin cancer risk factor assessments into the ePHA would offer a practical and straightforward approach to identifying at-risk individuals, as suggested by Newnam et al12 in 2022. Skin cancer risk factor assessments allow for targeted data collection related to sun exposure history, family history, and personal risk factors, which can be used to determine individualized risk stratification to assess the need for early secondary prevention measures and specialist referral. These ePHA data can also support population-based analyses to inform preventive strategies and address knowledge gaps related to high-risk exposures, such as extended field exercises or assignments in high-UV regions, that may impede effective skin cancer prevention.
Development of Military-Specific Screening Guidelines—Given the limited number of military dermatologists, a standardized risk-assessment tool could enhance early detection of skin cancer and streamline the referral process. We propose a military-specific skin cancer screening algorithm or risk nomogram that could help to consolidate risk factors into a clear and actionable framework for more efficient triage and appropriate allocation of dermatologic resources and manpower. This nomogram could be developed by military dermatologists and then implemented on a command level, affording primary care providers a useful tool to expedite evaluation of individuals at higher risk for skin cancer while simultaneously promoting judicious use of limited dermatology resources.
Although the United States Preventive Services Task Force does not universally recommend routine skin cancer screenings for asymptomatic adults, military service members are exposed to higher occupational risks than the general population, as previously mentioned. Currently, there is no standardized screening guideline across all military services due to the unique nature and exposure risks for each branch of service and their varied occupations; however, we propose the development of basic standardized screening guidelines by adapting the framework of the United States Preventive Services Task Force and adjusting for military-specific UV exposure and occupational risks to improve early detection of skin cancer. These guidelines could be updated and tailored appropriately when additional population-based data are collected and analyzed through ePHA.
Critiques and Limitations of Implementation
Several challenges and limitations must be considered when attempting to integrate large-scale preventive measures for skin cancer within the US military. A primary concern is the extent to which military resources should be allocated to prevention when off-duty sun exposure remains largely beyond institutional control. Although military health initiatives can address workplace risk through education and policy, individual decisions during both work and leisure time remain a major variable that cannot be feasibly controlled. Cultural and operational barriers also pose challenges; for instance, the US Marine Corps maintains a strong cultural identity tied to uniform appearance, making it difficult to implement widespread changes to clothing-based sun-protection measures. Institutional changes, particularly those involving uniforms, likely will face substantial administrative resistance and potential operational limitations. When broad uniform modifications are unattainable, a more feasible approach may be to encourage unit-level leadership to authorize and promote the frequent use of nonuniform protective measures.
Furthermore, integrating additional skin cancer risk questions into the already extensive ePHA means extra time required to complete the assessment; this adds to service members’ administrative burden, potentially leading to reduced timely compliance, rushed responses, and survey fatigue, which threaten data quality. If new items are to be included, they should be carefully selected for efficiency and clinical relevance. Existing validated questionnaires such as those from the study by Lyford et al7 published in 2021 can serve as a foundation.
Another critical limitation is access to dermatologic care for active-duty service members. Raising awareness of skin cancer risk without ensuring adequate resources may create ethical concerns, particularly in high-risk environments such as the Middle East and Indo-Pacific. Additionally, because skin cancer often develops years or decades after exposure, securing early buy-in from service members and their leaders can be challenging. These concerns make it clear that, while skin cancer prevention is important, implementing widespread measures is not straightforward and requires a practical and balanced approach.
Final Thoughts
Implementing prevention strategies for skin cancer in the military requires balancing evidence-based recommendations with the practical realities of military culture, resource limitations, and operational demands. Challenges remain for dermatologists in providing targeted recommendations due to the multifaceted nature of military roles, including over 150 Navy Military Occupational Specialties, limited familiarity with the unique UV exposure risks associated with each occupation, and variability in local and regional policies on uniform wear, physical training requirements, and other operational practices. Although targeted prevention measures are difficult to establish in the setting of these knowledge gaps, leveraging unit-level leadership to align with existing screening guidelines and optimizing primary prevention measures can be meaningful steps toward reducing skin cancer risk for military service members while maintaining mission readiness.
Skin cancer is a major health concern for military service members, who experience notably higher incidence rates than the general population.1 Active-duty military personnel are particularly vulnerable to prolonged sun exposure due to deployments, specialized training, and everyday outdoor duties.1 Despite skin cancer being the most commonly diagnosed malignancy in active-duty service members,2 tracking and documenting the quantity and diversity of these risk factors remain limited. This knowledge gap comes at high cost, simultaneously impairing military medicine preventive measures while burdening the military health care system with substantial expenditures.3 These findings underscore the critical need for targeted surveillance, early-detection programs, and policy-driven interventions to mitigate these medical and economic concerns.
Skin cancer has been recognized as a major health risk to the military population for decades, yet incidence and prevalence remain high. This phenomenon is closely linked to the inherent responsibilities and expectations of active-duty military members, including outdoor physical training, field exercises, standing in formation, and outdoor working environments—all of which can occur during peak sunlight hours. These risks are further elevated at duty stations in geographic regions with high levels of UV exposure, such as those in tropical and arid regions of the world. Certain military occupational specialties and missions may further introduce unique risk factors; for instance, pilots with frequent high-altitude missions experience heightened UV exposure and melanoma risk.4 Secondary to compounding determinants, the aviation, diving, and nuclear subgroups of the military community are particularly vulnerable to skin cancer.5
Despite well-documented risks, considerable gaps remain in quantifying and analyzing variations in UV exposure across military occupations, duty locations, and operational roles. Factors such as the existence of over 150 distinct military occupational specialties, frequent geographic relocations, and routine work in austere environments contribute to a wide range of UV exposure profiles that remain insufficiently characterized. This lack of comprehensive exposure data hinders the development of large-scale, targeted skin cancer prevention strategies. Initial approaches to addressing these challenges include enhanced surveillance, education, and policy initiatives. The Table presents practical recommendations for military leadership to consider in implementing preventive measures for skin cancer. Herein, we outline broader systemic strategies to bridge knowledge gaps and address underrecognized occupational risk factors for skin cancer in military service members; these elements include proposed modifications to the electronic Periodic Health Assessment (ePHA) and the development of standardized, military-specific screening and prevention guidelines to support early detection and resource optimization.

Skin Cancer Education for Service Members
Sunscreen and Signage—Diligent primary prevention offers a promising avenue for mitigating skin cancer incidence in military service members. Basic education and precautionary messaging on photoprotection can be widely implemented to simultaneously educate service members on the dangers of sun exposure while reinforcing healthy behaviors in real time. Simple low-cost initiatives such as strategically placed visual signage reminding service members to apply sunscreen in high UV environments can support consistent sun-safe practices. Educational efforts also should emphasize proper sunscreen use, including application on high-risk anatomic sites (eg, the face, neck, scalp, dorsal hands, and ears) and the essentiality of using sufficient quantities of broad-spectrum sunscreen for effective protection. Incorporating this guidance into training materials, briefings, and visual reminders allow seamless integration of photoprotection into service members’ daily routines without compromising operational efficiency.6 Younger service members, who may be less likely to prioritize preventive behaviors, may be particularly responsive to sun safety reminders in training areas, bases, and deployment zones.7 Health fairs and orientation briefs in high-UV regions also offer potential opportunities for targeted education.
Resources for Sun Protection in the Military
Sunscreen—Although sunscreen is critical in minimizing the risk for UV-induced skin cancer, its widespread use in the military is hindered by practical challenges related to accessibility and the need for consistent reapplication; for instance, providing free sunscreen dispensers at institutions for staff working under intense or prolonged UV exposure may improve sunscreen accessibility and use.8 Including sunscreen in standard-issue gear offers another logical way to embed its use into operational readiness as part of the routine protective measures.
Uniform Modifications—Adapting military uniforms and practices to improve sun protection plays a critical role in reducing skin cancer risk. Targeted protective gear for commonly sun-exposed areas can help mitigate UV exposure. One practical option is the use of wide-brimmed headgear (eg, boonie hats), which provide more face and neck coverage than standard-issue military caps, or covers. The wide-brimmed headgear currently is only selectively authorized during specific scenarios, such as field operations and training exercises, or at the discretion of unit-level leadership. Wide-brimmed headgear, already used by many service members, has been associated with up to a 17% reduction in UV exposure to inadequately protected areas, potentially lowering skin cancer risk.9,10 Similarly, a “sleeves-down” policy—requiring sleeves to remain unrolled and covering the forearms during outdoor activities—offers a simple way to minimize sun exposure without necessitating additional gear. Other specialized clothing items, including UV-blocking neck gaiters, photoprotective clothing, and lightweight gloves, also may be appropriate for high-risk groups and can be implemented in a relatively straightforward manner.
Shade Structures and UV Index Monitoring—Aside from uniform adaptation, physical barrier intervention can further complement skin cancer prevention efforts in the military. Shade structures offer a straightforward way to reduce UV exposure during prolonged outdoor activities. Incorporating daily UV index monitoring into operational guidance can help inform adjustments to training schedules and guide the implementation of additional sun protection measures, such as mandatory sunscreen application, use of wide-brimmed hats, or increased access to shaded rest areas during heavy sunlight hours. Currently, outdoor physical training is restricted during periods of high heat index, measured via Wet Bulb Globe Temperature, to reduce heat-related injuries. We argue that avoidance of nonoperational outdoor activity during peak UV index hours also should be incorporated into standardized policies. This intervention is of particular benefit to service members stationed in regions with a high UV index year-round, such as those stationed in the Middle East, Guam, Okinawa, and southern coastal United States bases.
Policy Changes to Support Photoprotective Measures
Annual Risk Factor Screening‐Screening—Effective secondary prevention efforts by military dermatologists remain an important measure in reducing the burden of skin cancer among military personnel; however, these efforts have become increasingly challenging due to 2 main factors—the diversity of military occupational specialties and their associated unique occupational risks as well as the limited availability of military dermatologists across all branches (approximately 100 active-duty dermatologists for nearly 3 million service members).11 Therefore, targeted interventions that enhance risk assessment, refined screening protocols, and leveraging of existing military health networks can improve early skin cancer detection while optimizing resource allocation.
The ePHA is an online screening tool used annually by all service members to evaluate their overall health. Presently, the ePHA lacks specific questions to assess sun exposure and skin cancer risks. Integrating annual skin cancer risk factor assessments into the ePHA would offer a practical and straightforward approach to identifying at-risk individuals, as suggested by Newnam et al12 in 2022. Skin cancer risk factor assessments allow for targeted data collection related to sun exposure history, family history, and personal risk factors, which can be used to determine individualized risk stratification to assess the need for early secondary prevention measures and specialist referral. These ePHA data can also support population-based analyses to inform preventive strategies and address knowledge gaps related to high-risk exposures, such as extended field exercises or assignments in high-UV regions, that may impede effective skin cancer prevention.
Development of Military-Specific Screening Guidelines—Given the limited number of military dermatologists, a standardized risk-assessment tool could enhance early detection of skin cancer and streamline the referral process. We propose a military-specific skin cancer screening algorithm or risk nomogram that could help to consolidate risk factors into a clear and actionable framework for more efficient triage and appropriate allocation of dermatologic resources and manpower. This nomogram could be developed by military dermatologists and then implemented on a command level, affording primary care providers a useful tool to expedite evaluation of individuals at higher risk for skin cancer while simultaneously promoting judicious use of limited dermatology resources.
Although the United States Preventive Services Task Force does not universally recommend routine skin cancer screenings for asymptomatic adults, military service members are exposed to higher occupational risks than the general population, as previously mentioned. Currently, there is no standardized screening guideline across all military services due to the unique nature and exposure risks for each branch of service and their varied occupations; however, we propose the development of basic standardized screening guidelines by adapting the framework of the United States Preventive Services Task Force and adjusting for military-specific UV exposure and occupational risks to improve early detection of skin cancer. These guidelines could be updated and tailored appropriately when additional population-based data are collected and analyzed through ePHA.
Critiques and Limitations of Implementation
Several challenges and limitations must be considered when attempting to integrate large-scale preventive measures for skin cancer within the US military. A primary concern is the extent to which military resources should be allocated to prevention when off-duty sun exposure remains largely beyond institutional control. Although military health initiatives can address workplace risk through education and policy, individual decisions during both work and leisure time remain a major variable that cannot be feasibly controlled. Cultural and operational barriers also pose challenges; for instance, the US Marine Corps maintains a strong cultural identity tied to uniform appearance, making it difficult to implement widespread changes to clothing-based sun-protection measures. Institutional changes, particularly those involving uniforms, likely will face substantial administrative resistance and potential operational limitations. When broad uniform modifications are unattainable, a more feasible approach may be to encourage unit-level leadership to authorize and promote the frequent use of nonuniform protective measures.
Furthermore, integrating additional skin cancer risk questions into the already extensive ePHA means extra time required to complete the assessment; this adds to service members’ administrative burden, potentially leading to reduced timely compliance, rushed responses, and survey fatigue, which threaten data quality. If new items are to be included, they should be carefully selected for efficiency and clinical relevance. Existing validated questionnaires such as those from the study by Lyford et al7 published in 2021 can serve as a foundation.
Another critical limitation is access to dermatologic care for active-duty service members. Raising awareness of skin cancer risk without ensuring adequate resources may create ethical concerns, particularly in high-risk environments such as the Middle East and Indo-Pacific. Additionally, because skin cancer often develops years or decades after exposure, securing early buy-in from service members and their leaders can be challenging. These concerns make it clear that, while skin cancer prevention is important, implementing widespread measures is not straightforward and requires a practical and balanced approach.
Final Thoughts
Implementing prevention strategies for skin cancer in the military requires balancing evidence-based recommendations with the practical realities of military culture, resource limitations, and operational demands. Challenges remain for dermatologists in providing targeted recommendations due to the multifaceted nature of military roles, including over 150 Navy Military Occupational Specialties, limited familiarity with the unique UV exposure risks associated with each occupation, and variability in local and regional policies on uniform wear, physical training requirements, and other operational practices. Although targeted prevention measures are difficult to establish in the setting of these knowledge gaps, leveraging unit-level leadership to align with existing screening guidelines and optimizing primary prevention measures can be meaningful steps toward reducing skin cancer risk for military service members while maintaining mission readiness.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancerincidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Krivda KR, Watson NL, Lyford WH, et al. The burden of skin cancer in the military health system, 2017-2022. Cutis. 2024;113:200-215. doi:10.12788/cutis.1015
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58. doi:10.1001/jamadermatol.2014.1077
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MMSR. 2017;24:8-14.
- Subramaniam P, Olsen CM, Thompson BS, et al, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. doi:10.1001/jamadermatol.2016.4070
- Lyford WH, Crotty A, Logemann NF. Sun exposure prevention practices within U.S. naval aviation. Mil Med. 2021;186:1169-1175. doi:10.1093/milmed/usab099
- Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77:164-166.
- Schissel D. Operation shadow warrior: a quantitative analysis of the ultraviolet radiation protection demonstrated by various headgear. Mil Med. 2001;166:783-785.
- Milch JM, Logemann NF. Photoprotection prevents skin cancer: let’s make it fashionable to wear sun-protective clothing. Cutis. 2017;99:89-92.
- Association of Military Dermatologists. (n.d.). Military dermatology. https://militaryderm.org/military-dermatology/
- Newnam R, Le-Jenkins U, Rutledge C, et al. The association of skin cancer prevention knowledge, sun-protective attitudes, and sunprotective behaviors in a Navy population. Mil Med. 2024;189:1-7. doi:10.1093/milmed/usac285
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancerincidence, prevention, and screening among active duty and veteran personnel. J Am Acad Dermatol. 2018;78:1185-1192. doi:10.1016/j.jaad.2017.11.062
- Lee T, Taubman SB, Williams VF. Incident diagnoses of non-melanoma skin cancer, active component, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:2-6.
- Krivda KR, Watson NL, Lyford WH, et al. The burden of skin cancer in the military health system, 2017-2022. Cutis. 2024;113:200-215. doi:10.12788/cutis.1015
- Sanlorenzo M, Wehner MR, Linos E, et al. The risk of melanoma in airline pilots and cabin crew: a meta-analysis. JAMA Dermatol. 2015;151:51-58. doi:10.1001/jamadermatol.2014.1077
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MMSR. 2017;24:8-14.
- Subramaniam P, Olsen CM, Thompson BS, et al, for the QSkin Sun and Health Study Investigators. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182. doi:10.1001/jamadermatol.2016.4070
- Lyford WH, Crotty A, Logemann NF. Sun exposure prevention practices within U.S. naval aviation. Mil Med. 2021;186:1169-1175. doi:10.1093/milmed/usab099
- Wood M, Raisanen T, Polcari I. Observational study of free public sunscreen dispenser use at a major US outdoor event. J Am Acad Dermatol. 2017;77:164-166.
- Schissel D. Operation shadow warrior: a quantitative analysis of the ultraviolet radiation protection demonstrated by various headgear. Mil Med. 2001;166:783-785.
- Milch JM, Logemann NF. Photoprotection prevents skin cancer: let’s make it fashionable to wear sun-protective clothing. Cutis. 2017;99:89-92.
- Association of Military Dermatologists. (n.d.). Military dermatology. https://militaryderm.org/military-dermatology/
- Newnam R, Le-Jenkins U, Rutledge C, et al. The association of skin cancer prevention knowledge, sun-protective attitudes, and sunprotective behaviors in a Navy population. Mil Med. 2024;189:1-7. doi:10.1093/milmed/usac285
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
Bridging the Knowledge-Action Gap in Skin Cancer Prevention Among US Military Personnel
PRACTICE POINTS
- Military personnel face elevated skin cancer risks due to prolonged occupational UV exposure.
- Medical providers can partner with unit-level leadership to implement low-cost interventions such as shade structures and uniform modifications.
- Annual sun exposure risk assessments should be integrated into the military Electronic Periodic Health Assessment for targeted screening and early intervention of risk factors.
- Photoprotective gear and signage in high—UV index areas can improve service member awareness and adherence to preventive measures.
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
Worldwide, it is estimated that up to 1 in 5 individuals will experience a dermatophyte infection (commonly called ringworm or tinea infection) in their lifetime.1 Historically, dermatophyte infections have been considered relatively minor conditions usually treated with short courses of topical antifungals.2 Oral antifungals historically were needed only for patients with nail or hair shaft infections or extensive cutaneous fungal infections, which typically occurred in immunosuppressed patients.2 However, the landscape is changing rapidly due to the global emergence of severe dermatophyte infections that frequently are resistant to first-line antifungal medications.3-5 In this article, we aimed to review the epidemiology of emerging dermatophyte infections and provide dermatologists with information needed for effective diagnosis and management.
Emergence of Trichophyton indotineae
In recent decades, public health officials and dermatologists have noted with concern the spread of the recently emerged dermatophyte species Trichophyton indotineae in South Asia.3,6 This species (previously known as Trichophyton mentagrophytes genotype VIII) usually is transmitted from person to person, either through direct skin-to-skin contact or by fomites.4,6 Potential sexual transmission of T indotineae infections also has been reported,7 and it is possible that animals may serve as reservoirs for this pathogen, although there are no known reports of direct spread from animals to humans.8,9 Major outbreaks of T indotineae are ongoing in South Asia, and cases have been documented in 6 continents.10-12 In the United States, most but not all cases have occurred in immigrants from or recently returned travelers to South Asia.6,13 The emergence and spread of T indotineae is hypothesized to be promoted by the misuse and overuse of topical antifungal products, particularly those containing combinations of potent corticosteroids with other antimicrobial drugs.14,15
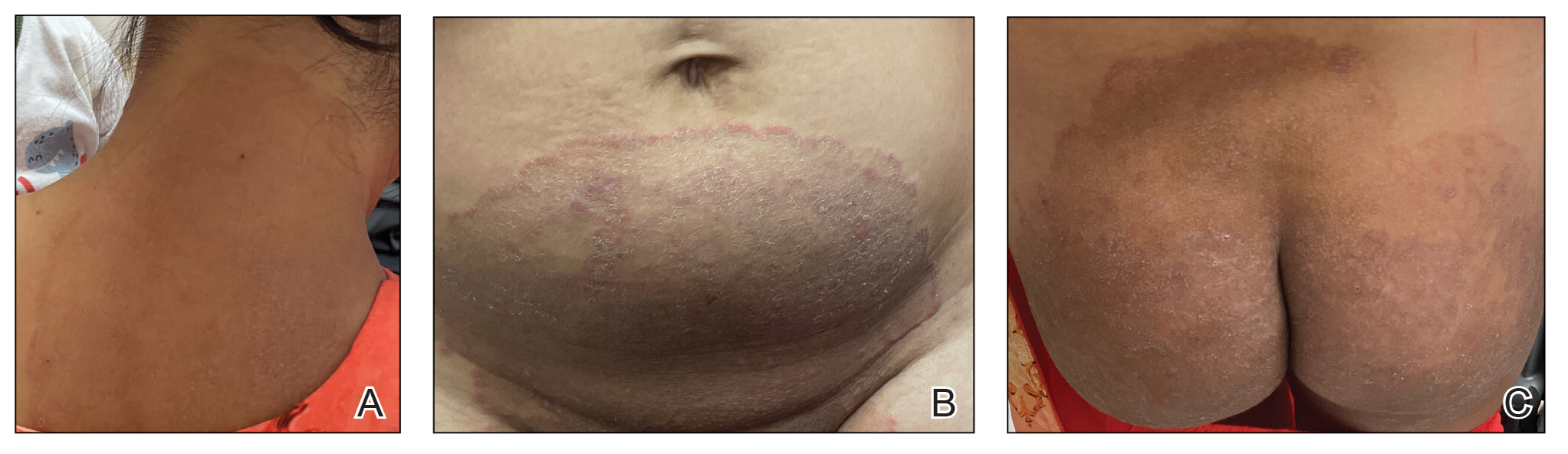
Cutaneous manifestations of T indotineae infections tend to cover large body surface areas, recur frequently, and pose substantial treatment challenges.6,13,16 Several clinical presentations have been documented, including erythematous, scaly concentric plaques; papulosquamous lesions; pustular forms; and corticosteroid-modified disease (Figure 1).6,16 Affected patients seldom are immunocompromised and often have a history of multiple failed courses of topical or oral antifungals, including oral terbinafine.13 Many also have been prescribed topical corticosteroids or have used over-the-counter topical corticosteroids, which worsen the rash.17
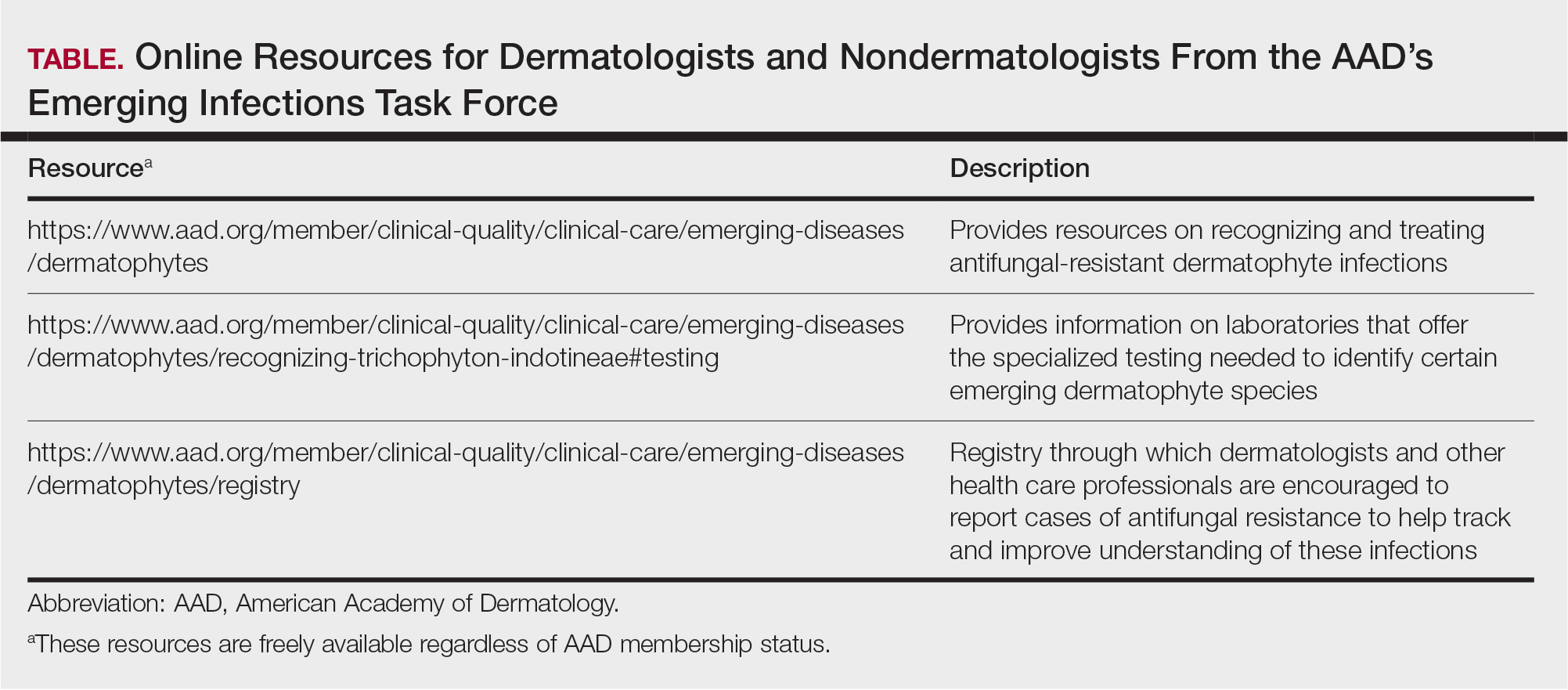
Direct microscopy with potassium hydroxide could be used to confirm the diagnosis of dermatophyte infection, but it does not distinguish T indotineae from other dermatophyte species.2,6 Importantly, culture-based testing usually will misidentify T indotineae as other Trichophyton species such as the more common T mentagrophytes or Trichophyton interdigitale. Definitive identification of T indotineae requires advanced molecular techniques that are available only at select laboratories.6 Unfortunately, availability of such testing is limited (Table), and results may take several weeks; therefore, it is suggested that dermatologists who suspect T indotineae infections based on the patient’s history and clinical presentation begin antifungal treatment after confirmation of dermatophyte infection but not wait for definitive confirmation of the causative organism.16
Itraconazole is considered the first-line therapy for T indotineae infection, as terbinafine usually is ineffective due to mutations in the squalene epoxidase gene.16 Dermatologists should be aware that itraconazole is available in different formulations that can affect absorption. The oral solution has greater bioavailability and should be taken on an empty stomach, whereas the capsules are required to be taken with food for effective absorption; the capsules also should be taken with an acidic beverage such as orange juice. Dermatologists should carefully assess for drug-drug interactions when prescribing itraconazole, given its extensive interaction profile with numerous other medications. Patients may require treatment with itraconazole (100 mg/d or 200 mg/d) for a minimum of 6 to 8 weeks until complete clearance has been achieved and ideally a negative potassium hydroxide preparation of skin scrapings has been obtained. A longer treatment period (eg, ≥3 months) frequently is needed, and relapses are common.6,16,18 Regular follow-up is needed to monitor for infection clearance and recurrences. It is important to note that cases of itraconazole resistance have been reported, although this currently appears to be uncommon.19,20
Other Emerging Dermatophytes to Watch
Trichophyton rubrum is the most common cause of dermatophyte infections among humans,21 and cases of terbinafine-resistant T rubrum infections have been reported increasingly in the United States and Canada.5,22-24 Onychomycosis caused by terbinafine-resistant T rubrum has been documented, and patients may have infections that do not respond to terbinafine given at the standard dose and duration.22,23 Case reports have indicated successful treatment using itraconazole 200 mg/d and posaconazole 300 mg/d.5,23
Trichophyton mentagrophytes genotype VII (TMVII) is an emerging dermatophyte that recently has been reported as a cause of sexually transmitted dermatophyte infections in Europe and the United States primarily affecting men who have sex with men.25-27 Patients may present with pruritic, annular, scaly patches and plaques involving the trunk, groin, genital region, or face (Figure 2). Although closely related to T indotineae, TMVII differs in that it more often affects the genital region, generally is susceptible to terbinafine, and in the United States and Europe usually is not related to travel or immigration involving South Asia.26 Although TMVII has not been associated with antifungal resistance, awareness among dermatologists is important because patients may experience inflamed, painful, and persistent rashes that can lead to secondary bacterial infection or scarring, and physicians might mistake it for mimics including eczema or psoriasis.25,26
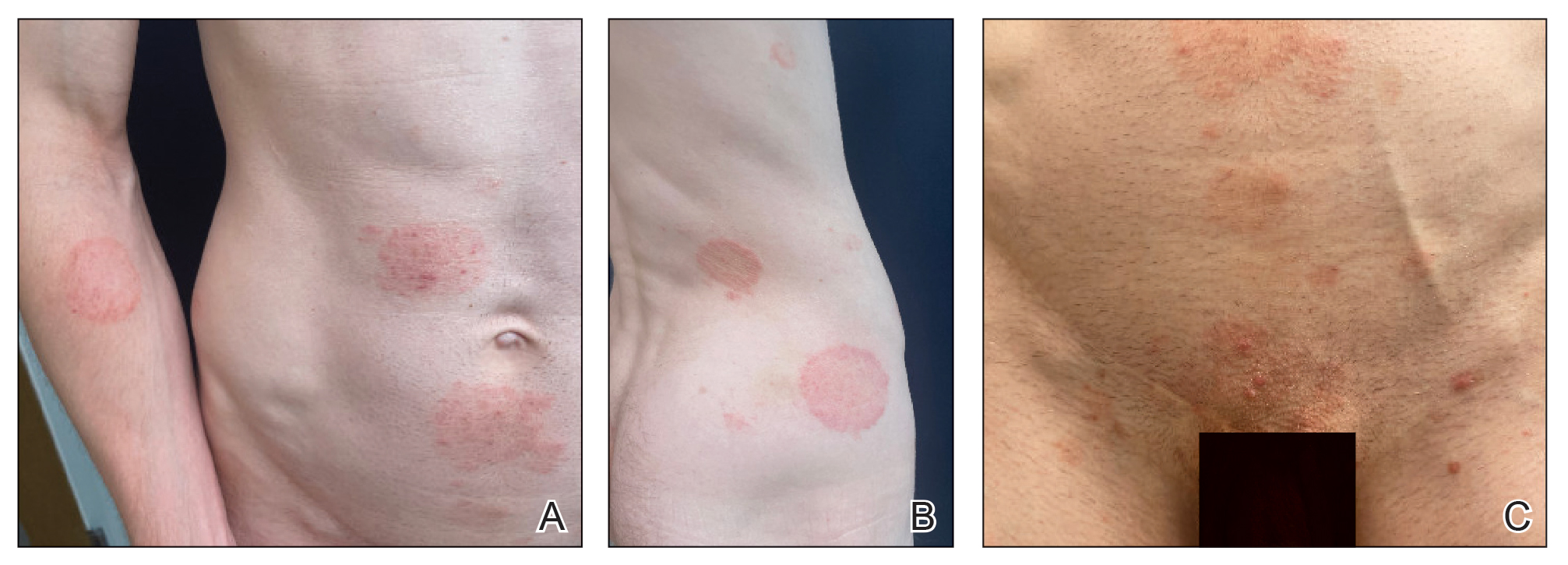
Importance of Judicious Antifungal Use
Optimizing the use of antifungals is critical to improving patient outcomes and preserving available treatment options.28,29 A retrospective analysis of commercial health insurance data estimated that topical antifungal prescriptions were potentially unnecessary for more than half of the more than 560,000 patients who were prescribed these medications in 2023. In this study, it also was observed that only 16% of patients prescribed a topical antifungal had received diagnostic testing, with low rates across specialties.30 This is concerning because even among board-certified dermatologists, incorrect diagnosis of suspected fungal skin infections can occur; in one survey-based study of board-certified dermatologists who were presented with dermatomycosis images, respondents categorized cases with greater than 75% accuracy in only 31% (4/13) of instances.31 Clotrimazole-betamethasone is among the most commonly prescribed topical antifungals in the United States,14,32 and 2 recent retrospective analyses highlighted that the majority of patients prescribed this medication did not receive any fungal diagnostic testing.33,34
Final Thoughts
In an era of emerging antifungal-resistant dermatophyte infections, it is important for dermatologists to educate nondermatologists about the importance of using diagnostic testing for suspected dermatophyte infections.14,28 Dermatologists also can educate nondermatologist colleagues on the importance of avoiding the use of topical combination antifungal/corticosteroid medications and referring for dermatologic evaluation when diagnoses are uncertain.33,34 Strategies for education by dermatologists could include giving workshops, creating educational materials, and fostering open communication about optimal treatment practices and referral parameters for suspected dermatophyte infections.
- Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174, 177-168.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Uhrlaß S, Verma SB, Gräser Y, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-175.
- Chen E, Ghannoum M, Elewski BE. Treatment]resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184:164-165.
- Caplan AS. Notes from the field: first reported US cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morbidity and Mortality Weekly Report. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Spivack S, Gold JA, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807.
- Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233.
- Thakur S, Spruijtenburg B, Abhishek, et al. Whole genome sequence analysis of terbinafine resistant and susceptible Trichophyton isolates from human and animal origin. Mycopathologia. 2025;190:13.
- Lockhart SR, Chowdhary A, Gold JA. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832.
- Mosam A, Shuping L, Naicker S, et al. A case of antifungal-resistant ringworm infection in KwaZulu-Natal Province, South Africa, caused by Trichophyton indotineae. Public Health Bulletin South Africa. Accessed April 4, 2025. https://www.phbsa.ac.za/wp-content/uploads/2023/12PHBSA-Ringworm-Article-2023.pdf
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:E0056223
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701-709. doi:10.1001/jamadermatol.2024.1126
- Benedict K. Topical antifungal prescribing for Medicare Part D beneficiaries—United States, 2021. MMWR Morb Mortal Wkly Rep. 2024;73:1-5.
- Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-719.
- Khurana A, Sharath S, Sardana K, et al. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315-323. doi:10.1016/j.jaad.2024.03.024
- Verma S. Steroid modified tinea. BMJ. 2017;356:j973.
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278.
- Burmester A, Hipler UC, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180.
- Bhuiyan MSI, Verma SB, Illigner GM, et al. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J Fungi (Basel). 2024;10:768. doi:10.3390 /jof10110768
- Hay RJ. Chapter 82: superficial mycoses. In: Ryan ET, Hill DR, Solomon T, et al, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; 2020:648-652.
- Gupta AK, Cooper EA, Wang T, et al. Detection of squalene epoxidase mutations in United States patients with onychomycosis: implications for management. J Invest Dermatol. 2023;143:2476-2483.E2477.
- Hwang JK, Bakotic WL, Gold JA, et al. Isolation of terbinafine-resistant Trichophyton rubrum from onychomycosis patients who failed treatment at an academic center in New York, United States. J Fungi. 2023;9:710.
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6:1153-1155.
- Jabet A, Dellière S, Seang S, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023;29:1411-1414.
- Zucker J, Caplan AS, Gunaratne SH, et al. Notes from the field: Trichophyton mentagrophytes genotype VII—New York City, April-July 2024. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.
- Jabet A, Bérot V, Chiarabini T, et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: an ongoing phenomenon. J Eur Acad Dermatol Venereol. 2025;39:407-415.
- Caplan AS, Gold JA, Smith DJ, et al. Improving antifungal stewardship in dermatology in an era of emerging dermatophyte resistance. JAAD International. 2024;15:168-169.
- Elewski B. A call for antifungal stewardship. Br J Dermatol. 2020; 183:798-799.
- Gold JAW, Benedict K, Caplan AS, et al. High rates of potentially unnecessary topical antifungal prescribing in a large commercial health insurance claims database, United States. J Am Acad Dermatol. 2025:S0190-9622(25)00098-2. doi:10.1016/j.jaad.2025.01.022
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: a survey-based study. J Am Acad Dermatol. 2017;76:562-563.
- Flint ND, Rhoads JLW, Carlisle R, et al. The continued inappropriate use and overuse of combination topical clotrimazole-betamethasone. Dermatol Online J. 2021;27. doi:10.5070/D327854686
- Currie DW, Caplan AS, Benedict K, et al. Prescribing of clotrimazolebetamethasone dipropionate, a topical combination corticosteroidantifungal product, for Medicare part D beneficiaries, United States, 2016–2022. Antimicrob Steward Healthc Epidemiol. 2024;4:E174.
- Gold JA, Caplan AS, Benedict K, et al. Clotrimazole-betamethasone dipropionate prescribing for nonfungal skin conditions. JAMA Network Open. 2024;7:E2411721-E2411721.
Worldwide, it is estimated that up to 1 in 5 individuals will experience a dermatophyte infection (commonly called ringworm or tinea infection) in their lifetime.1 Historically, dermatophyte infections have been considered relatively minor conditions usually treated with short courses of topical antifungals.2 Oral antifungals historically were needed only for patients with nail or hair shaft infections or extensive cutaneous fungal infections, which typically occurred in immunosuppressed patients.2 However, the landscape is changing rapidly due to the global emergence of severe dermatophyte infections that frequently are resistant to first-line antifungal medications.3-5 In this article, we aimed to review the epidemiology of emerging dermatophyte infections and provide dermatologists with information needed for effective diagnosis and management.
Emergence of Trichophyton indotineae
In recent decades, public health officials and dermatologists have noted with concern the spread of the recently emerged dermatophyte species Trichophyton indotineae in South Asia.3,6 This species (previously known as Trichophyton mentagrophytes genotype VIII) usually is transmitted from person to person, either through direct skin-to-skin contact or by fomites.4,6 Potential sexual transmission of T indotineae infections also has been reported,7 and it is possible that animals may serve as reservoirs for this pathogen, although there are no known reports of direct spread from animals to humans.8,9 Major outbreaks of T indotineae are ongoing in South Asia, and cases have been documented in 6 continents.10-12 In the United States, most but not all cases have occurred in immigrants from or recently returned travelers to South Asia.6,13 The emergence and spread of T indotineae is hypothesized to be promoted by the misuse and overuse of topical antifungal products, particularly those containing combinations of potent corticosteroids with other antimicrobial drugs.14,15
Cutaneous manifestations of T indotineae infections tend to cover large body surface areas, recur frequently, and pose substantial treatment challenges.6,13,16 Several clinical presentations have been documented, including erythematous, scaly concentric plaques; papulosquamous lesions; pustular forms; and corticosteroid-modified disease (Figure 1).6,16 Affected patients seldom are immunocompromised and often have a history of multiple failed courses of topical or oral antifungals, including oral terbinafine.13 Many also have been prescribed topical corticosteroids or have used over-the-counter topical corticosteroids, which worsen the rash.17

Direct microscopy with potassium hydroxide could be used to confirm the diagnosis of dermatophyte infection, but it does not distinguish T indotineae from other dermatophyte species.2,6 Importantly, culture-based testing usually will misidentify T indotineae as other Trichophyton species such as the more common T mentagrophytes or Trichophyton interdigitale. Definitive identification of T indotineae requires advanced molecular techniques that are available only at select laboratories.6 Unfortunately, availability of such testing is limited (Table), and results may take several weeks; therefore, it is suggested that dermatologists who suspect T indotineae infections based on the patient’s history and clinical presentation begin antifungal treatment after confirmation of dermatophyte infection but not wait for definitive confirmation of the causative organism.16

Itraconazole is considered the first-line therapy for T indotineae infection, as terbinafine usually is ineffective due to mutations in the squalene epoxidase gene.16 Dermatologists should be aware that itraconazole is available in different formulations that can affect absorption. The oral solution has greater bioavailability and should be taken on an empty stomach, whereas the capsules are required to be taken with food for effective absorption; the capsules also should be taken with an acidic beverage such as orange juice. Dermatologists should carefully assess for drug-drug interactions when prescribing itraconazole, given its extensive interaction profile with numerous other medications. Patients may require treatment with itraconazole (100 mg/d or 200 mg/d) for a minimum of 6 to 8 weeks until complete clearance has been achieved and ideally a negative potassium hydroxide preparation of skin scrapings has been obtained. A longer treatment period (eg, ≥3 months) frequently is needed, and relapses are common.6,16,18 Regular follow-up is needed to monitor for infection clearance and recurrences. It is important to note that cases of itraconazole resistance have been reported, although this currently appears to be uncommon.19,20
Other Emerging Dermatophytes to Watch
Trichophyton rubrum is the most common cause of dermatophyte infections among humans,21 and cases of terbinafine-resistant T rubrum infections have been reported increasingly in the United States and Canada.5,22-24 Onychomycosis caused by terbinafine-resistant T rubrum has been documented, and patients may have infections that do not respond to terbinafine given at the standard dose and duration.22,23 Case reports have indicated successful treatment using itraconazole 200 mg/d and posaconazole 300 mg/d.5,23
Trichophyton mentagrophytes genotype VII (TMVII) is an emerging dermatophyte that recently has been reported as a cause of sexually transmitted dermatophyte infections in Europe and the United States primarily affecting men who have sex with men.25-27 Patients may present with pruritic, annular, scaly patches and plaques involving the trunk, groin, genital region, or face (Figure 2). Although closely related to T indotineae, TMVII differs in that it more often affects the genital region, generally is susceptible to terbinafine, and in the United States and Europe usually is not related to travel or immigration involving South Asia.26 Although TMVII has not been associated with antifungal resistance, awareness among dermatologists is important because patients may experience inflamed, painful, and persistent rashes that can lead to secondary bacterial infection or scarring, and physicians might mistake it for mimics including eczema or psoriasis.25,26

Importance of Judicious Antifungal Use
Optimizing the use of antifungals is critical to improving patient outcomes and preserving available treatment options.28,29 A retrospective analysis of commercial health insurance data estimated that topical antifungal prescriptions were potentially unnecessary for more than half of the more than 560,000 patients who were prescribed these medications in 2023. In this study, it also was observed that only 16% of patients prescribed a topical antifungal had received diagnostic testing, with low rates across specialties.30 This is concerning because even among board-certified dermatologists, incorrect diagnosis of suspected fungal skin infections can occur; in one survey-based study of board-certified dermatologists who were presented with dermatomycosis images, respondents categorized cases with greater than 75% accuracy in only 31% (4/13) of instances.31 Clotrimazole-betamethasone is among the most commonly prescribed topical antifungals in the United States,14,32 and 2 recent retrospective analyses highlighted that the majority of patients prescribed this medication did not receive any fungal diagnostic testing.33,34
Final Thoughts
In an era of emerging antifungal-resistant dermatophyte infections, it is important for dermatologists to educate nondermatologists about the importance of using diagnostic testing for suspected dermatophyte infections.14,28 Dermatologists also can educate nondermatologist colleagues on the importance of avoiding the use of topical combination antifungal/corticosteroid medications and referring for dermatologic evaluation when diagnoses are uncertain.33,34 Strategies for education by dermatologists could include giving workshops, creating educational materials, and fostering open communication about optimal treatment practices and referral parameters for suspected dermatophyte infections.
Worldwide, it is estimated that up to 1 in 5 individuals will experience a dermatophyte infection (commonly called ringworm or tinea infection) in their lifetime.1 Historically, dermatophyte infections have been considered relatively minor conditions usually treated with short courses of topical antifungals.2 Oral antifungals historically were needed only for patients with nail or hair shaft infections or extensive cutaneous fungal infections, which typically occurred in immunosuppressed patients.2 However, the landscape is changing rapidly due to the global emergence of severe dermatophyte infections that frequently are resistant to first-line antifungal medications.3-5 In this article, we aimed to review the epidemiology of emerging dermatophyte infections and provide dermatologists with information needed for effective diagnosis and management.
Emergence of Trichophyton indotineae
In recent decades, public health officials and dermatologists have noted with concern the spread of the recently emerged dermatophyte species Trichophyton indotineae in South Asia.3,6 This species (previously known as Trichophyton mentagrophytes genotype VIII) usually is transmitted from person to person, either through direct skin-to-skin contact or by fomites.4,6 Potential sexual transmission of T indotineae infections also has been reported,7 and it is possible that animals may serve as reservoirs for this pathogen, although there are no known reports of direct spread from animals to humans.8,9 Major outbreaks of T indotineae are ongoing in South Asia, and cases have been documented in 6 continents.10-12 In the United States, most but not all cases have occurred in immigrants from or recently returned travelers to South Asia.6,13 The emergence and spread of T indotineae is hypothesized to be promoted by the misuse and overuse of topical antifungal products, particularly those containing combinations of potent corticosteroids with other antimicrobial drugs.14,15
Cutaneous manifestations of T indotineae infections tend to cover large body surface areas, recur frequently, and pose substantial treatment challenges.6,13,16 Several clinical presentations have been documented, including erythematous, scaly concentric plaques; papulosquamous lesions; pustular forms; and corticosteroid-modified disease (Figure 1).6,16 Affected patients seldom are immunocompromised and often have a history of multiple failed courses of topical or oral antifungals, including oral terbinafine.13 Many also have been prescribed topical corticosteroids or have used over-the-counter topical corticosteroids, which worsen the rash.17

Direct microscopy with potassium hydroxide could be used to confirm the diagnosis of dermatophyte infection, but it does not distinguish T indotineae from other dermatophyte species.2,6 Importantly, culture-based testing usually will misidentify T indotineae as other Trichophyton species such as the more common T mentagrophytes or Trichophyton interdigitale. Definitive identification of T indotineae requires advanced molecular techniques that are available only at select laboratories.6 Unfortunately, availability of such testing is limited (Table), and results may take several weeks; therefore, it is suggested that dermatologists who suspect T indotineae infections based on the patient’s history and clinical presentation begin antifungal treatment after confirmation of dermatophyte infection but not wait for definitive confirmation of the causative organism.16

Itraconazole is considered the first-line therapy for T indotineae infection, as terbinafine usually is ineffective due to mutations in the squalene epoxidase gene.16 Dermatologists should be aware that itraconazole is available in different formulations that can affect absorption. The oral solution has greater bioavailability and should be taken on an empty stomach, whereas the capsules are required to be taken with food for effective absorption; the capsules also should be taken with an acidic beverage such as orange juice. Dermatologists should carefully assess for drug-drug interactions when prescribing itraconazole, given its extensive interaction profile with numerous other medications. Patients may require treatment with itraconazole (100 mg/d or 200 mg/d) for a minimum of 6 to 8 weeks until complete clearance has been achieved and ideally a negative potassium hydroxide preparation of skin scrapings has been obtained. A longer treatment period (eg, ≥3 months) frequently is needed, and relapses are common.6,16,18 Regular follow-up is needed to monitor for infection clearance and recurrences. It is important to note that cases of itraconazole resistance have been reported, although this currently appears to be uncommon.19,20
Other Emerging Dermatophytes to Watch
Trichophyton rubrum is the most common cause of dermatophyte infections among humans,21 and cases of terbinafine-resistant T rubrum infections have been reported increasingly in the United States and Canada.5,22-24 Onychomycosis caused by terbinafine-resistant T rubrum has been documented, and patients may have infections that do not respond to terbinafine given at the standard dose and duration.22,23 Case reports have indicated successful treatment using itraconazole 200 mg/d and posaconazole 300 mg/d.5,23
Trichophyton mentagrophytes genotype VII (TMVII) is an emerging dermatophyte that recently has been reported as a cause of sexually transmitted dermatophyte infections in Europe and the United States primarily affecting men who have sex with men.25-27 Patients may present with pruritic, annular, scaly patches and plaques involving the trunk, groin, genital region, or face (Figure 2). Although closely related to T indotineae, TMVII differs in that it more often affects the genital region, generally is susceptible to terbinafine, and in the United States and Europe usually is not related to travel or immigration involving South Asia.26 Although TMVII has not been associated with antifungal resistance, awareness among dermatologists is important because patients may experience inflamed, painful, and persistent rashes that can lead to secondary bacterial infection or scarring, and physicians might mistake it for mimics including eczema or psoriasis.25,26

Importance of Judicious Antifungal Use
Optimizing the use of antifungals is critical to improving patient outcomes and preserving available treatment options.28,29 A retrospective analysis of commercial health insurance data estimated that topical antifungal prescriptions were potentially unnecessary for more than half of the more than 560,000 patients who were prescribed these medications in 2023. In this study, it also was observed that only 16% of patients prescribed a topical antifungal had received diagnostic testing, with low rates across specialties.30 This is concerning because even among board-certified dermatologists, incorrect diagnosis of suspected fungal skin infections can occur; in one survey-based study of board-certified dermatologists who were presented with dermatomycosis images, respondents categorized cases with greater than 75% accuracy in only 31% (4/13) of instances.31 Clotrimazole-betamethasone is among the most commonly prescribed topical antifungals in the United States,14,32 and 2 recent retrospective analyses highlighted that the majority of patients prescribed this medication did not receive any fungal diagnostic testing.33,34
Final Thoughts
In an era of emerging antifungal-resistant dermatophyte infections, it is important for dermatologists to educate nondermatologists about the importance of using diagnostic testing for suspected dermatophyte infections.14,28 Dermatologists also can educate nondermatologist colleagues on the importance of avoiding the use of topical combination antifungal/corticosteroid medications and referring for dermatologic evaluation when diagnoses are uncertain.33,34 Strategies for education by dermatologists could include giving workshops, creating educational materials, and fostering open communication about optimal treatment practices and referral parameters for suspected dermatophyte infections.
- Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174, 177-168.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Uhrlaß S, Verma SB, Gräser Y, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-175.
- Chen E, Ghannoum M, Elewski BE. Treatment]resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184:164-165.
- Caplan AS. Notes from the field: first reported US cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morbidity and Mortality Weekly Report. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Spivack S, Gold JA, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807.
- Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233.
- Thakur S, Spruijtenburg B, Abhishek, et al. Whole genome sequence analysis of terbinafine resistant and susceptible Trichophyton isolates from human and animal origin. Mycopathologia. 2025;190:13.
- Lockhart SR, Chowdhary A, Gold JA. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832.
- Mosam A, Shuping L, Naicker S, et al. A case of antifungal-resistant ringworm infection in KwaZulu-Natal Province, South Africa, caused by Trichophyton indotineae. Public Health Bulletin South Africa. Accessed April 4, 2025. https://www.phbsa.ac.za/wp-content/uploads/2023/12PHBSA-Ringworm-Article-2023.pdf
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:E0056223
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701-709. doi:10.1001/jamadermatol.2024.1126
- Benedict K. Topical antifungal prescribing for Medicare Part D beneficiaries—United States, 2021. MMWR Morb Mortal Wkly Rep. 2024;73:1-5.
- Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-719.
- Khurana A, Sharath S, Sardana K, et al. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315-323. doi:10.1016/j.jaad.2024.03.024
- Verma S. Steroid modified tinea. BMJ. 2017;356:j973.
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278.
- Burmester A, Hipler UC, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180.
- Bhuiyan MSI, Verma SB, Illigner GM, et al. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J Fungi (Basel). 2024;10:768. doi:10.3390 /jof10110768
- Hay RJ. Chapter 82: superficial mycoses. In: Ryan ET, Hill DR, Solomon T, et al, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; 2020:648-652.
- Gupta AK, Cooper EA, Wang T, et al. Detection of squalene epoxidase mutations in United States patients with onychomycosis: implications for management. J Invest Dermatol. 2023;143:2476-2483.E2477.
- Hwang JK, Bakotic WL, Gold JA, et al. Isolation of terbinafine-resistant Trichophyton rubrum from onychomycosis patients who failed treatment at an academic center in New York, United States. J Fungi. 2023;9:710.
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6:1153-1155.
- Jabet A, Dellière S, Seang S, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023;29:1411-1414.
- Zucker J, Caplan AS, Gunaratne SH, et al. Notes from the field: Trichophyton mentagrophytes genotype VII—New York City, April-July 2024. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.
- Jabet A, Bérot V, Chiarabini T, et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: an ongoing phenomenon. J Eur Acad Dermatol Venereol. 2025;39:407-415.
- Caplan AS, Gold JA, Smith DJ, et al. Improving antifungal stewardship in dermatology in an era of emerging dermatophyte resistance. JAAD International. 2024;15:168-169.
- Elewski B. A call for antifungal stewardship. Br J Dermatol. 2020; 183:798-799.
- Gold JAW, Benedict K, Caplan AS, et al. High rates of potentially unnecessary topical antifungal prescribing in a large commercial health insurance claims database, United States. J Am Acad Dermatol. 2025:S0190-9622(25)00098-2. doi:10.1016/j.jaad.2025.01.022
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: a survey-based study. J Am Acad Dermatol. 2017;76:562-563.
- Flint ND, Rhoads JLW, Carlisle R, et al. The continued inappropriate use and overuse of combination topical clotrimazole-betamethasone. Dermatol Online J. 2021;27. doi:10.5070/D327854686
- Currie DW, Caplan AS, Benedict K, et al. Prescribing of clotrimazolebetamethasone dipropionate, a topical combination corticosteroidantifungal product, for Medicare part D beneficiaries, United States, 2016–2022. Antimicrob Steward Healthc Epidemiol. 2024;4:E174.
- Gold JA, Caplan AS, Benedict K, et al. Clotrimazole-betamethasone dipropionate prescribing for nonfungal skin conditions. JAMA Network Open. 2024;7:E2411721-E2411721.
- Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174, 177-168.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Uhrlaß S, Verma SB, Gräser Y, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-175.
- Chen E, Ghannoum M, Elewski BE. Treatment]resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184:164-165.
- Caplan AS. Notes from the field: first reported US cases of tinea caused by Trichophyton indotineae—New York City, December 2021–March 2023. MMWR Morbidity and Mortality Weekly Report. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Spivack S, Gold JA, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807.
- Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233.
- Thakur S, Spruijtenburg B, Abhishek, et al. Whole genome sequence analysis of terbinafine resistant and susceptible Trichophyton isolates from human and animal origin. Mycopathologia. 2025;190:13.
- Lockhart SR, Chowdhary A, Gold JA. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832.
- Mosam A, Shuping L, Naicker S, et al. A case of antifungal-resistant ringworm infection in KwaZulu-Natal Province, South Africa, caused by Trichophyton indotineae. Public Health Bulletin South Africa. Accessed April 4, 2025. https://www.phbsa.ac.za/wp-content/uploads/2023/12PHBSA-Ringworm-Article-2023.pdf
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:E0056223
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701-709. doi:10.1001/jamadermatol.2024.1126
- Benedict K. Topical antifungal prescribing for Medicare Part D beneficiaries—United States, 2021. MMWR Morb Mortal Wkly Rep. 2024;73:1-5.
- Verma SB. Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-719.
- Khurana A, Sharath S, Sardana K, et al. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315-323. doi:10.1016/j.jaad.2024.03.024
- Verma S. Steroid modified tinea. BMJ. 2017;356:j973.
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278.
- Burmester A, Hipler UC, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180.
- Bhuiyan MSI, Verma SB, Illigner GM, et al. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J Fungi (Basel). 2024;10:768. doi:10.3390 /jof10110768
- Hay RJ. Chapter 82: superficial mycoses. In: Ryan ET, Hill DR, Solomon T, et al, eds. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier; 2020:648-652.
- Gupta AK, Cooper EA, Wang T, et al. Detection of squalene epoxidase mutations in United States patients with onychomycosis: implications for management. J Invest Dermatol. 2023;143:2476-2483.E2477.
- Hwang JK, Bakotic WL, Gold JA, et al. Isolation of terbinafine-resistant Trichophyton rubrum from onychomycosis patients who failed treatment at an academic center in New York, United States. J Fungi. 2023;9:710.
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6:1153-1155.
- Jabet A, Dellière S, Seang S, et al. Sexually transmitted Trichophyton mentagrophytes genotype VII infection among men who have sex with men. Emerg Infect Dis. 2023;29:1411-1414.
- Zucker J, Caplan AS, Gunaratne SH, et al. Notes from the field: Trichophyton mentagrophytes genotype VII—New York City, April-July 2024. MMWR Morb Mortal Wkly Rep. 2024;73:985-988.
- Jabet A, Bérot V, Chiarabini T, et al. Trichophyton mentagrophytes ITS genotype VII infections among men who have sex with men in France: an ongoing phenomenon. J Eur Acad Dermatol Venereol. 2025;39:407-415.
- Caplan AS, Gold JA, Smith DJ, et al. Improving antifungal stewardship in dermatology in an era of emerging dermatophyte resistance. JAAD International. 2024;15:168-169.
- Elewski B. A call for antifungal stewardship. Br J Dermatol. 2020; 183:798-799.
- Gold JAW, Benedict K, Caplan AS, et al. High rates of potentially unnecessary topical antifungal prescribing in a large commercial health insurance claims database, United States. J Am Acad Dermatol. 2025:S0190-9622(25)00098-2. doi:10.1016/j.jaad.2025.01.022
- Yadgar RJ, Bhatia N, Friedman A. Cutaneous fungal infections are commonly misdiagnosed: a survey-based study. J Am Acad Dermatol. 2017;76:562-563.
- Flint ND, Rhoads JLW, Carlisle R, et al. The continued inappropriate use and overuse of combination topical clotrimazole-betamethasone. Dermatol Online J. 2021;27. doi:10.5070/D327854686
- Currie DW, Caplan AS, Benedict K, et al. Prescribing of clotrimazolebetamethasone dipropionate, a topical combination corticosteroidantifungal product, for Medicare part D beneficiaries, United States, 2016–2022. Antimicrob Steward Healthc Epidemiol. 2024;4:E174.
- Gold JA, Caplan AS, Benedict K, et al. Clotrimazole-betamethasone dipropionate prescribing for nonfungal skin conditions. JAMA Network Open. 2024;7:E2411721-E2411721.
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
The Rise of Antifungal-Resistant Dermatophyte Infections: What Dermatologists Need to Know
PRACTICE POINTS
- Recently emerged dermatophyte species pose a global public health concern because of infection severity, frequent resistance to terbinafine, and easy person-to-person transmission.
- Prolonged itraconazole therapy is considered the firstline treatment for infections caused by Trichophyton indotineae, a globally emerging and frequently terbinafine-resistant dermatophyte.
- Dermatologists can educate nondermatologists on the importance of mycologic confirmation and avoidance of the use of topical antifungal/ corticosteroid products, which are hypothesized to contribute to emergence and spread of resistance.
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
Lifeguards play a crucial role in ensuring water safety, but they also are uniquely positioned to promote skin cancer prevention and proper sunscreen use.1,2 There are several benefits and challenges to offering skin cancer prevention training for lifeguards.3 We examine the advantages of training, highlight the role lifeguards can play in larger public skin cancer prevention efforts, and address practical techniques for developing lifeguardfocused skin cancer education programs. By providing this knowledge to lifeguards, we can improve community health outcomes and encourage sun-safe behaviors in high-risk outdoor locations.
Benefits of Skin Cancer Prevention Training for Lifeguards
Research has shown that lifeguards are at an elevated risk for basal cell carcinoma, squamous cell carcinoma, and melanoma due to frequent prolonged occupational sun exposure.1,2,4-6 Therefore, comprehensive education on skin cancer prevention—including instruction on proper sunscreen application techniques and the importance of regular reapplication as well as how to recognize suspicious skin lesions—should be incorporated into lifeguard certification programs. One study evaluating the effectiveness of a skin cancer prevention program for lifeguards found that many of the participants lacked a thorough understanding of the different types of skin cancer.5 Another study found that lifeguards at pools in areas where societal norms supporting sun safety are stronger exhibited noticeably more sun protection practices, with regression estimates of 0.22 (95% CI, 0.17-0.26).7 Empowering lifeguards with valuable health knowledge during their regular training could potentially reduce their risk for skin cancer,4 as they may be more inclined to use sunscreen appropriately and reach out to a dermatologist for regular skin checks and evaluation of suspicious lesions.
Role of Lifeguards in Public Skin Cancer Prevention Efforts
Once trained on skin cancer prevention, lifeguards also can play a pivotal role in promoting sunscreen use among the public. Despite the widespread availability of high-quality sunscreens, many swimmers and beachgoers neglect to regularly apply or reapply sunscreen, especially on commonly exposed areas such as the back, shoulders, and face.8 Educating lifeguards on skin cancer prevention could enhance health outcomes by increasing early detection rates and promoting sun-safe behaviors among the general public.9 However, additional training requirements might increase the cost and time commitment for lifeguard certification, potentially leading to staffing shortages.3,7 There also is a risk of lifeguards overstepping their role and providing inaccurate medical advice, which could cause distress or even lead to liability issues.7 Balancing these factors will be crucial in developing effective and sustainable skin cancer prevention programs for lifeguards.
Implementing Lifeguard Skin Cancer Training
Implementing skin cancer prevention training programs for lifeguards requires strategic collaboration between dermatologists, and lifeguard training organizations to ensure that the participants receive consistent and comprehensive training.10 Additionally, public health campaigns can support these efforts by raising awareness about the importance of sun safety and regular skin checks.6 Tailored training modules/materials, ongoing technical assistance, and active, multicomponent approaches that account for both individual and environmental factors can increase program implementation in a variety of community settings.
Final Thoughts
Through effective education, lifeguards can potentially have a substantial impact on skin cancer prevention, both among lifeguards themselves and the general public. By promoting proper sunscreen use, lifeguards can help reduce the incidence and mortality associated with skin cancers. Future studies should focus on developing and implementing targeted education initiatives for lifeguards, fostering collaboration between relevant stakeholders, and raising public awareness about the importance of sun safety and early skin cancer detection. These efforts ultimately could lead to improved public health outcomes and reduced skin cancer rates, particularly in high-risk populations that frequently are exposed to UV radiation.
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
- Enos CW, Rey S, Slocum J, et al. Sun-protection behaviors among active members of the United States Lifesaving Association. J Clin Aesthet Dermatol. 2021;14:14-20.
- Verma K, Lewis DJ, Siddiqui FS, et al. Mohs micrographic surgery management of melanoma and melanoma in situ. StatPearls. Updated August 28, 2024. Accessed April 15, 2025. https://www.ncbi.nlm.nih.gov/books/NBK606123/
- Verma KK, Joshi TP, Lewis DJ, et al. Nail technicians as partners in early melanoma detection: bridging the knowledge gap. Arch Dermatol Res. 2024;316:586. doi:10.1007/s00403-024-03342-0
- Geller AC, Glanz K, Shigaki D, et al. Impact of skin cancer prevention on outdoor aquatics staff: the Pool Cool program in Hawaii and Massachusetts. Prev Med. 2001;33:155-161. doi:10.1006/pmed.2001.0870
- Hiemstra M, Glanz K, Nehl E. Changes in sunburn and tanning attitudes among lifeguards over a summer season. J Am Acad Dermatol. 2012;66:430-437. doi:10.1016/j.jaad.2010.11.050
- Verma KK, Ahmad N, Friedmann DP, et al. Melanoma in tattooed skin: diagnostic challenges and the potential for tattoo artists in early detection. Arch Dermatol Res. 2024;316:690. doi:10.1007/s00403-024-03415-0
- Hall DM, McCarty F, Elliott T, et al. Lifeguards’ sun protection habits and sunburns: association with sun-safe environments and skin cancer prevention program participation. Arch Dermatol. 2009;145:139-144. doi:10.1001/archdermatol.2008.553
- Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011;64:282-289. doi:10.1016/j.jaad.2010.01.040
- Rabin BA, Nehl E, Elliott T, et al. Individual and setting level predictors of the implementation of a skin cancer prevention program: a multilevel analysis. Implement Sci. 2010;5:40. doi:10.1186/1748-5908-5-40
- Walkosz BJ, Buller D, Buller M, et al. Sun safe workplaces: effect of an occupational skin cancer prevention program on employee sun safety practices. J Occup Environ Med. 2018;60:900-997. doi:10.1097 /JOM.0000000000001427
Training Lifeguards to Assist in Skin Cancer Prevention
Training Lifeguards to Assist in Skin Cancer Prevention
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).
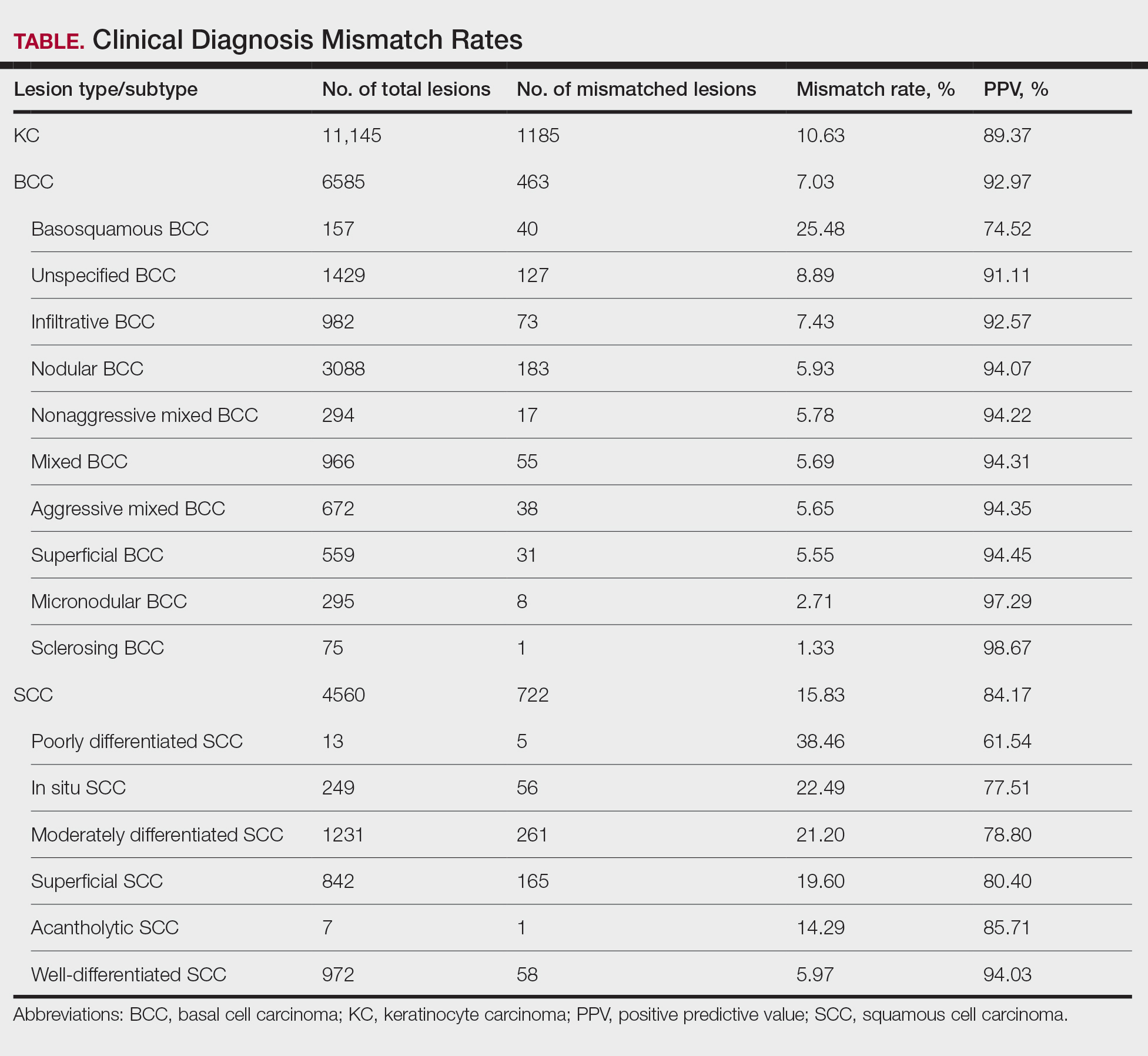
The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).

The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
To the Editor:
The incidence of nonmelanoma skin cancer (NMSC) is rapidly increasing worldwide. Due to its highly curable nature when treated early, accurate diagnosis is the cornerstone to good patient outcomes.1 Accurate diagnosis of skin cancer and subsequent treatment decisions rely heavily on the congruence between clinical observations and histopathologic assessments. Clinical misdiagnosis of a malignant lesion can lead to delayed and suboptimal treatment, which may contribute to serious complications such as metastasis or even mortality. In this study, data from clinically diagnosed basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) were compared to their identified histopathologic subtype classifications. The accuracy of the clinical diagnosis of these NMSCs was assessed by determining the rate of misdiagnosis and the respective positive predictive value (PPV).
A retrospective review of medical records from a private dermatology practice in Lubbock, Texas, was conducted to identify patients diagnosed with NMSC from January 1, 2017, through December 31, 2021. A total of 11,229 NMSCs were diagnosed and treated in 5877 patients. Of the NMSCs diagnosed, 11,145 were identified as keratinocyte carcinomas and were classified as BCCs or SCCs. The accuracy of the clinical diagnoses was determined by comparison to the histologic subtype identified via biopsy of the lesion. Although the use of a dermatoscope during the clinical encounter was not formally recorded, reports from the examining dermatologists indicated it was not used in the majority of cases.
If a lesion was clinically diagnosed as a BCC but was identified as a subtype of SCC on histology (or vice versa), the lesion was considered to be mismatched. The number of mismatched lesions and the mismatch rate for each lesion type/subtype is recorded in the Table. Of the total 11,145 keratinocyte carcinomas included in our study, there was an overall 10.63% mismatch rate, with 1185 of the malignancies having a differing clinical diagnosis (eg, BCC vs SCC) from the histologic findings. The clinical mismatch rate was notably higher for SCC compared to BCC (15.83% vs 7.03%, respectively).

The Table provides a breakdown of the BCC subtypes identified by histology with their computed mismatch rate and PPV. It is worth clarifying that lesions classified as more than one BCC subtype per the histologic findings were diagnosed as mixed BCC; these were further classified as mixed-aggressive BCC (if at least one aggressive BCC subtype was present) and mixed nonaggressive BCC (if no aggressive BCC subtype was present). Overall, BCCs were less likely to be misdiagnosed, with an average PPV of 92.97% compared to 84.17% for SCCs. Basosquamous BCC was the BCC subtype with the highest mismatch rate (25.48%), while sclerosing BCC has the lowest overall mismatch rate (1.33%). The most common malignancy was BCC, with nodular BCC being the most common subtype.
The Table also breaks down the SCC subtypes, reporting the most commonly misdiagnosed of any BCC or SCC subtype to be poorly differentiated SCC (mismatch rate, 38.46%). The lowest mismatch rate of the SCC subtypes was 5.97% for well-differentiated SCC.
There was an overall PPV of 89.37% in clinically evaluated malignancies and their respective histologic subtypes. Basal cell carcinoma had a lower overall mismatch rate of 7.03% compared to 15.83% in SCC. The most common misdiagnosis was attributed to poorly differentiated SCC (mismatch rate, 38.46%), while the least common misdiagnosed malignancy was sclerosing BCC (1.33%). The high mismatch rate of poorly differentiated SCC may be due to its diverging presentation from a typical SCC as a flat lesion with the absence of scaling, keratin, or bleeding, leading to the misdiagnosis of BCC.2
Accurate clinical diagnosis of NMSCs is the basis for further evaluation and treatment that should ensue in a timely manner; however, accurately identifying BCCs vs SCCs solely based on clinical examination can be challenging due to variable manifestations and overlapping features. Basal cell carcinoma commonly presents as a shiny pink/flesh-colored nodule, macule, or patch with surface telangiectasia, sometimes appearing with ulceration or crusting.3 Alternatively, SCC typically appears as a firm, sharply demarcated, red nodule with a thick overlying scale.4 Definitive diagnoses can be difficult upon clinical examination since these features can be shared between the 2 subtypes. To aid in these uncertainties, a growing number of clinicians are implementing the use of dermoscopy in their everyday practice.
Dermoscopy is an extremely useful tool in improving the diagnostic accuracy of skin cancers compared to examination with the naked eye, as it provides detailed visualization of specific structures and patterns in skin cancer lesions.5 The dermoscopic appearance of BCC is characterized by pearly blue-gray or translucent globules with arborizing vessels, spoke-wheel structures, and leaflike areas.5,6 Conversely, dermoscopic features of SCC may include a milky-red globule with a scaly, sharply demarcated, crusted lesion with polymorphous vasculature, sometimes resembling a persistent sore or nonhealing wound.4,5 Though the use of dermoscopy can aid in diagnosis upon initial examination, certain factors such as trauma, ulceration, and previous treatments that distorted the lesion’s architecture may lead to misdiagnosis. Furthermore, the distinct vascular patterns found in BCC and SCC may be mistaken for each other and therefore lead to misdiagnosis upon examination.7 Other variables that may complicate diagnosis include the location of the lesion, its size, and the presence of other skin conditions or nearby lesions.
The primary limitation of the current study was the limited scope of the data, as they were derived from patients seen at one private dermatology practice, preventing the generalizability of our findings. However, our results show trends similar to those observed in other studies analyzing the clinical accuracy of skin cancer diagnoses, with higher PPVs for BCC compared to SCC. A study by Ahnlide and Bjellerup8 was based in a hospital dermatology department and demonstrated a PPV of 85.5% for BCC compared to 92.97% in our study; for SCC, the PPV was 67.3% compared to 84.17% in our study. In another study by Heal et al,9 data were collected from an Australian registry that included records of all histologically confirmed skin cancers from December 1996 to October 1999 from 202 general practitioners and 42 specialists, including 1 dermatologist. The PPVs for BCC and SCC were 72.7% and 49.4%, respectively. Although our results indicated higher PPVs compared to these 2 studies, some of the discrepancies can be accounted for by the differences in clinical setting as well as the lack of expertise of nondermatologist physicians in identifying skin malignancies in the study by Heal et al.9
The current study was further limited by the lack of data quantifying the number of lesions clinically suspected to be malignant but found to be histologically benign. It is typical for clinicians to have a low threshold to biopsy a suspicious lesion with atypical features (eg, rapid evolution and growth, bleeding, crusting). Furthermore, the identification of risk factors in the patient’s medical and family history (eg, exposure to radiation, personal or family history of skin cancers) can heavily influence a clinician’s decision to biopsy a lesion with an atypical appearance.10 Many benign lesions are biopsied to avoid missing a diagnosis of malignancy. Consequently, our results suggest a high degree of clinical misdiagnosis of BCCs and SCCs. Obtaining data on the number of lesions suspected to be BCC or SCC that were found to be histologically benign would be a valuable addition to our study, as it would provide a measurable insight into the sensitivity of clinicians’ decision-making to identify a lesion as suspicious and warranting biopsy.
While clinical diagnosis plays a vital role in identifying suspected NMSCs such as BCC and SCC, its accuracy can be limited even with the use of dermoscopy. Overall, our data have shown a high rate of diagnostic accuracy upon suspicion of malignancy, but the different variables that affect clinical presentation promote histologic diagnosis to prevail as the gold standard.
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
- Seyed Ahadi M, Firooz A, Rahimi H, et al. Clinical diagnosis has a high negative predictive value in evaluation of malignant skin lesions. Dermatol Res Pract. 2021;2021:6618990. doi:10.1155/2021/6618990
- Lallas A, Pyne J, Kyrgidis A, et al. The clinical and dermoscopic features of invasive cutaneous squamous cell carcinoma depend on the histopathological grade of differentiation. Br J Dermatol. 2015;172:1308- 1315. doi:10.1111/bjd.13510
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. September 19, 2022. In: StatPearls. StatPearls Publishing; 2023.
- Suárez AL, Louis P, Kitts J, et al. Clinical and dermoscopic features of combined cutaneous squamous cell carcinoma (SCC)/neuroendocrine [Merkel cell] carcinoma (MCC). J Am Acad Dermatol. 2015;73:968-975. doi:10.1016/j.jaad.2015.08.041
- Wolner ZJ, Yélamos O, Liopyris K, et al. Enhancing skin cancer diagnosis with dermoscopy. Dermatol Clin. 2017;35:417-437. doi:10.1016/j.det.2017.06.003
- Reiter O, Mimouni I, Dusza S, et al. Dermoscopic features of basal cell carcinoma and its subtypes: a systematic review. J Am Acad Dermatol. 2021;85:653-664. doi:10.1016/j.jaad.2019.11.008
- Pruneda C, Ramesh M, Hope L, et al. Nonmelanoma skin cancers: diagnostic accuracy of midlevel providers versus dermatologists. The Dermatologist. March 2023. Accessed March 18, 2025. https://www.hmpgloballearningnetwork.com/site/thederm/feature-story/nonmelanoma-skin-cancers-diagnostic-accuracy-midlevel-providers-vs
- Ahnlide I, Bjellerup M. Accuracy of clinical skin tumour diagnosis in a dermatological setting. Acta Derm Venereol. 2013;93:305-308. doi:10.2340/00015555-1560
- Heal CF, Raasch BA, Buettner PG, et al. Accuracy of clinical diagnosis of skin lesions. Br J Dermatol. 2008;159:661-668.
- Fu S, Kim S, Wasko C. Dermatological guide for primary care physicians: full body skin checks, skin cancer detection, and patient education on self-skin checks and sun protection. Proc (Bayl Univ Med Cent). 2024;37:647-654. doi:10.1080/08998280.2024.2351751
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
Clinical Accuracy of Skin Cancer Diagnosis: Investigation of Keratinocyte Carcinoma Mismatch Rates
PRACTICE POINTS
- Malignant lesions may be misdiagnosed when assessments are guided by clinical features that align with typical presentations of other lesion types, potentially leading to diagnostic errors among experienced clinicians.
- Although dermoscopy is a beneficial tool in examining potential skin cancers, clinical observations should not bypass the gold standard of histopathologic examination.
Repair of a Large Full-Thickness Conchal Bowl Defect
Repair of a Large Full-Thickness Conchal Bowl Defect
Practice Gap
Large full-thickness conchal bowl defects often pose a reconstructive challenge. Maintaining the shape and structural integrity of the concha is fundamental for optimal cosmetic and functional outcomes. Prior reports have suggested wedge excisions, composite grafts, interpolation flaps with or without cartilage struts, and hinge flaps as possible options for reconstruction.1-3 However, patients with large defects who prefer single-stage reconstruction procedures present a unique challenge. Herein, we describe a single-stage full-thickness hinge flap technique for a large conchal bowl defect.
The Technique
A 77-year-old man was referred to our dermatology clinic by an outside dermatologist for Mohs micrographic surgery of a biopsy-proven cutaneous squamous cell carcinoma on the right conchal bowl measuring 1.1×2.1 cm and extending to the edge of the external auditory canal (EAC). The excision was performed that same day and was completed in 2 stages, achieving negative margins and resulting in a full-thickness defect measuring 2.0×3.6 cm that included the posterior auricular sulcus, cavum, antitragus, and proximal EAC (Figure 1). The patient requested a single-stage procedure but emphasized that his main priority was an optimal cosmetic outcome.
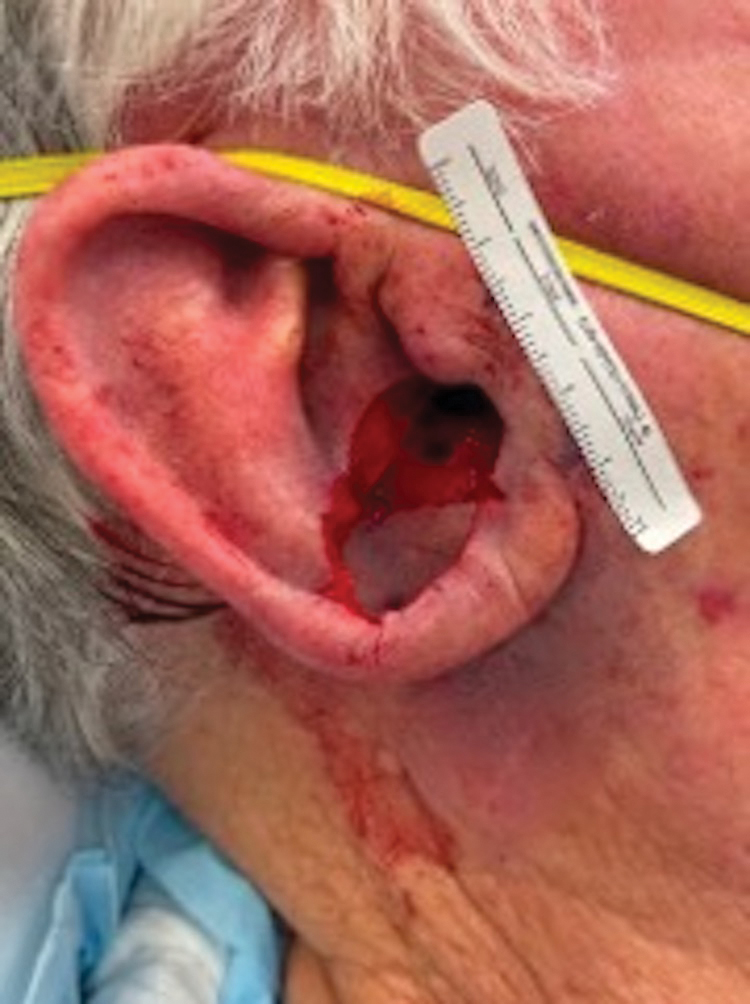
To repair this large defect, a full-thickness hinge flap with Burow graft was performed. The hinge-type flap was designed in a triangular fashion emanating at the posterior auricular sulcus adjacent to the posterior aspect of the defect and extending down the lateral neck (Figure 2). The flap was incised and the surrounding tissue was undermined, maintaining a robust pedicle in the center of its body on the superolateral neck. The flap was passed through the posterior aspect of the full-thickness defect and was secured in place with 4-0 polyglactin sutures in a buried interrupted fashion, thereby recreating the anterior portion of the defect. The superficial skin edges were reapproximated using 4-0 and 5-0 polypropylene sutures in a running interrupted fashion. The distal Burow triangle created from closure of the flap’s secondary defect was aggressively thinned and was utilized as a full-thickness graft for the residual postauricular groove defect (Figure 3). At 2 weeks’ follow-up, the patient was healing well with no postoperative issues and the sutures were removed (Figure 4).
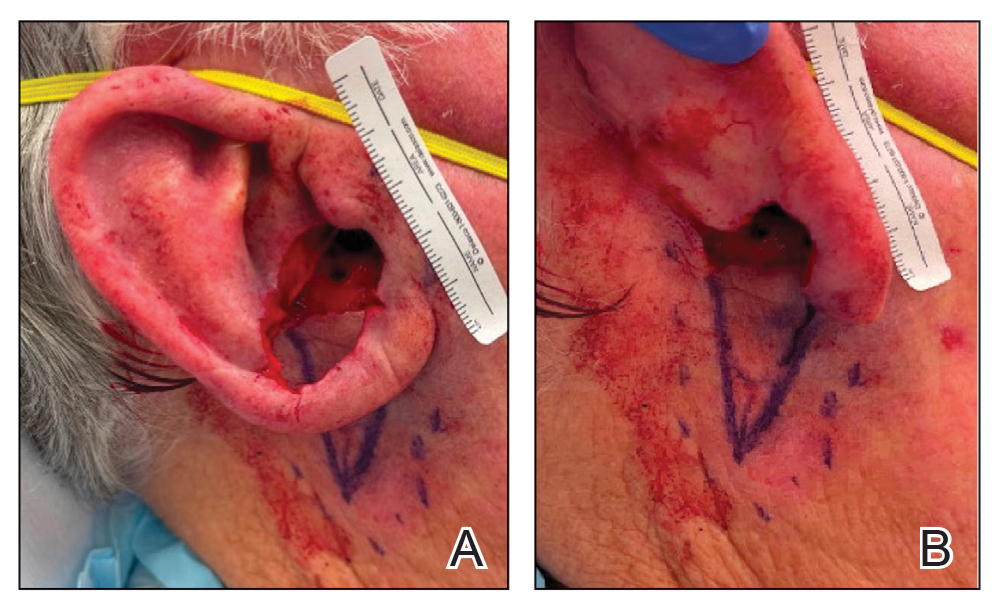
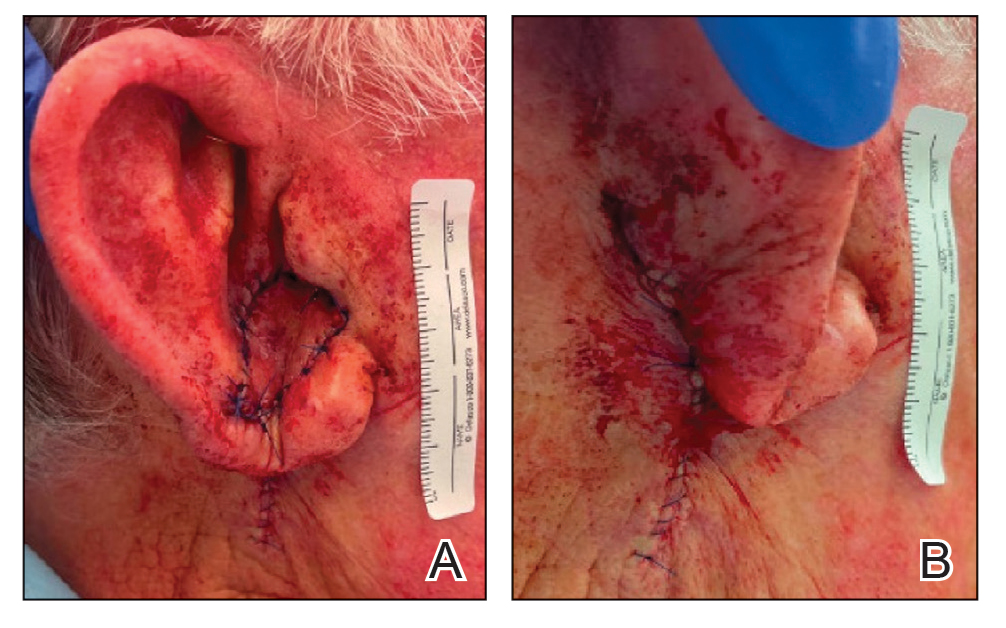
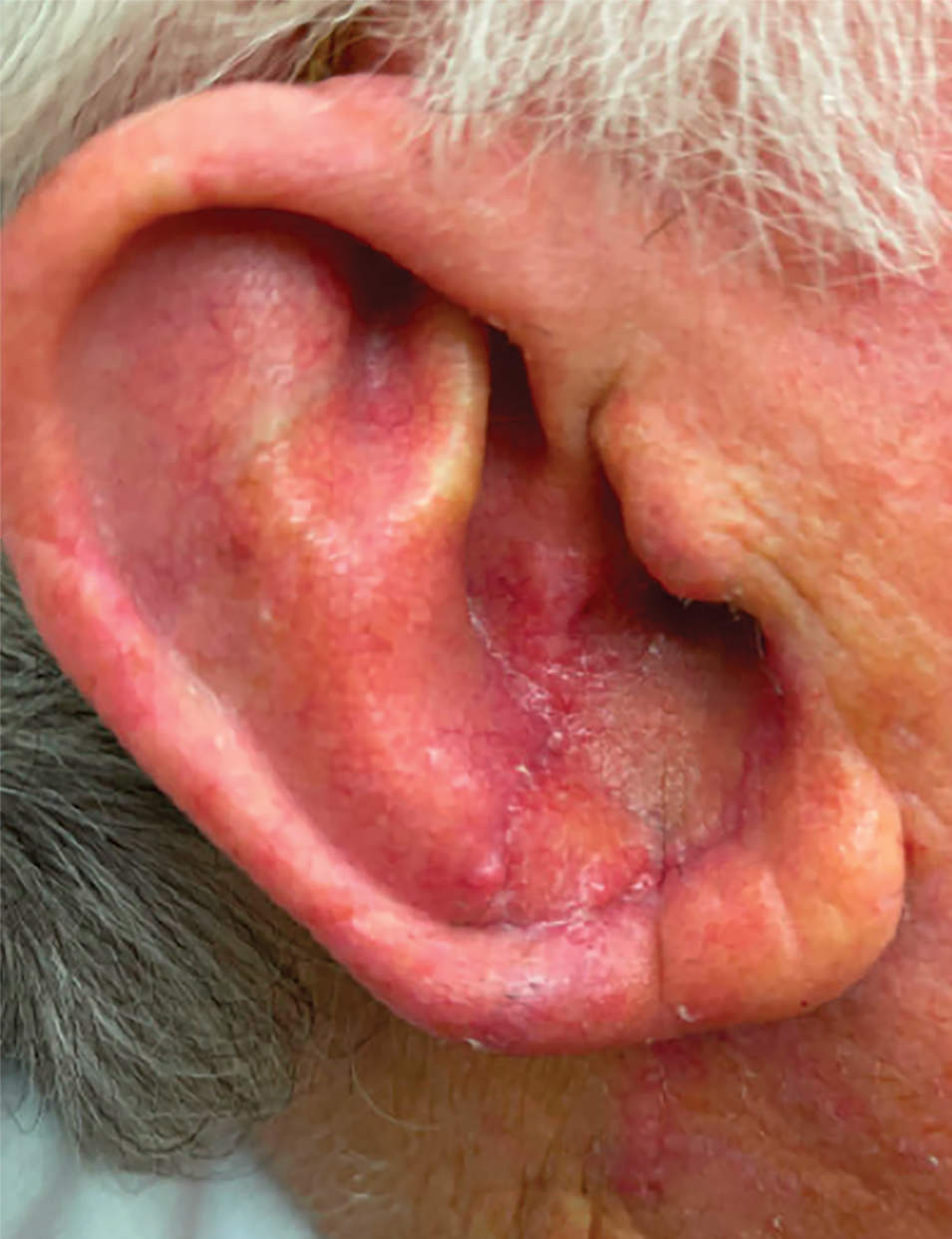
Practice Implications
There are many different reconstructive options for conchal bowl defects, including primary repair, wedge excision, composite graft and interpolation flaps with or without cartilage struts, and hinge flaps. Structural support, EAC patency, auricle symmetry, overall auricle size, and re-creation of natural contours were considered when designing the reconstruction of the defect in our patient; however, his main priority was achieving the greatest cosmetic outcome in a single-stage procedure, therefore limiting our reconstruction options.
Wedge excision, in which the residual lobule and inferior helical rim are removed, could have been considered in our patient but would have drastically altered the symmetry of the size of the ears. A folded postauricular flap, as described in the otolaryngology literature, is an interpolation flap based on the posterior auricular artery that was designed for full-thickness defects of the auricle to prevent any posterior pinning.1 This technique may have worked well in our case, but the patient preferred to avoid a multistage procedure. Additionally, the positional symmetry of the ears was maintained despite utilizing a hinge flap, which does not involve takedown of the pedicle. A composite graft from the contralateral ear could be considered for smaller conchal bowl defects but likely would have resulted in graft failure in our patient’s large defect due to its need for rich blood supply to heal and dependence on lateral wound edges. Cartilage struts in conjunction with a flap could have been considered in this scenario for greater structural support, but in our patient’s case, by maintaining the robust pedicle of our flap and having residual superior cartilage, further structural support was not necessary.
A prior case report described a partial and full-thickness defect in a similar location that was repaired with a retroauricular hinge flap, in which a portion of the flap was extensively de-epithelialized to address the varied thicknesses of the surgical defect.2 In our patient, the defect abutted the skin reservoir on the superolateral neck, and therefore no de-epithelialization was required as the entire epithelialized portion was utilized to recreate the anterior aspect of the defect. Postauricular hinge-type flaps are a reliable, single-stage surgical alternative to the 2-stage folded postauricular interpolation flap when reconstructing large conchal bowl defects. For small full-thickness defects of the ear, a composite graft may be considered; however, blood supply and other nutritional requirements limit this option for large full-thickness defects.
- Roche AM, Griffin M, Shelton R, et al. The folded postauricular flap: a novel approach to reconstruction of large full thickness defects of the conchal bowl. Am J Otolaryngol. 2017;38:706-709. doi:10.1016 /j.amjoto.2017.09.006
- Klein JC, Nijhawan RI. Retroauricular hinge flaps for full-thickness conchal bowl defects. J Am Acad Dermatol. 2024;90:E71-E72. doi:10.1016/j.jaad.2022.10.056
- Pickrell BB, Hughes CD, Maricevich RS. Partial ear defects. Semin Plast Surg. 2017 Aug;31:134-140. doi:10.1055/s-0037-1603968.
Practice Gap
Large full-thickness conchal bowl defects often pose a reconstructive challenge. Maintaining the shape and structural integrity of the concha is fundamental for optimal cosmetic and functional outcomes. Prior reports have suggested wedge excisions, composite grafts, interpolation flaps with or without cartilage struts, and hinge flaps as possible options for reconstruction.1-3 However, patients with large defects who prefer single-stage reconstruction procedures present a unique challenge. Herein, we describe a single-stage full-thickness hinge flap technique for a large conchal bowl defect.
The Technique
A 77-year-old man was referred to our dermatology clinic by an outside dermatologist for Mohs micrographic surgery of a biopsy-proven cutaneous squamous cell carcinoma on the right conchal bowl measuring 1.1×2.1 cm and extending to the edge of the external auditory canal (EAC). The excision was performed that same day and was completed in 2 stages, achieving negative margins and resulting in a full-thickness defect measuring 2.0×3.6 cm that included the posterior auricular sulcus, cavum, antitragus, and proximal EAC (Figure 1). The patient requested a single-stage procedure but emphasized that his main priority was an optimal cosmetic outcome.

To repair this large defect, a full-thickness hinge flap with Burow graft was performed. The hinge-type flap was designed in a triangular fashion emanating at the posterior auricular sulcus adjacent to the posterior aspect of the defect and extending down the lateral neck (Figure 2). The flap was incised and the surrounding tissue was undermined, maintaining a robust pedicle in the center of its body on the superolateral neck. The flap was passed through the posterior aspect of the full-thickness defect and was secured in place with 4-0 polyglactin sutures in a buried interrupted fashion, thereby recreating the anterior portion of the defect. The superficial skin edges were reapproximated using 4-0 and 5-0 polypropylene sutures in a running interrupted fashion. The distal Burow triangle created from closure of the flap’s secondary defect was aggressively thinned and was utilized as a full-thickness graft for the residual postauricular groove defect (Figure 3). At 2 weeks’ follow-up, the patient was healing well with no postoperative issues and the sutures were removed (Figure 4).



Practice Implications
There are many different reconstructive options for conchal bowl defects, including primary repair, wedge excision, composite graft and interpolation flaps with or without cartilage struts, and hinge flaps. Structural support, EAC patency, auricle symmetry, overall auricle size, and re-creation of natural contours were considered when designing the reconstruction of the defect in our patient; however, his main priority was achieving the greatest cosmetic outcome in a single-stage procedure, therefore limiting our reconstruction options.
Wedge excision, in which the residual lobule and inferior helical rim are removed, could have been considered in our patient but would have drastically altered the symmetry of the size of the ears. A folded postauricular flap, as described in the otolaryngology literature, is an interpolation flap based on the posterior auricular artery that was designed for full-thickness defects of the auricle to prevent any posterior pinning.1 This technique may have worked well in our case, but the patient preferred to avoid a multistage procedure. Additionally, the positional symmetry of the ears was maintained despite utilizing a hinge flap, which does not involve takedown of the pedicle. A composite graft from the contralateral ear could be considered for smaller conchal bowl defects but likely would have resulted in graft failure in our patient’s large defect due to its need for rich blood supply to heal and dependence on lateral wound edges. Cartilage struts in conjunction with a flap could have been considered in this scenario for greater structural support, but in our patient’s case, by maintaining the robust pedicle of our flap and having residual superior cartilage, further structural support was not necessary.
A prior case report described a partial and full-thickness defect in a similar location that was repaired with a retroauricular hinge flap, in which a portion of the flap was extensively de-epithelialized to address the varied thicknesses of the surgical defect.2 In our patient, the defect abutted the skin reservoir on the superolateral neck, and therefore no de-epithelialization was required as the entire epithelialized portion was utilized to recreate the anterior aspect of the defect. Postauricular hinge-type flaps are a reliable, single-stage surgical alternative to the 2-stage folded postauricular interpolation flap when reconstructing large conchal bowl defects. For small full-thickness defects of the ear, a composite graft may be considered; however, blood supply and other nutritional requirements limit this option for large full-thickness defects.
Practice Gap
Large full-thickness conchal bowl defects often pose a reconstructive challenge. Maintaining the shape and structural integrity of the concha is fundamental for optimal cosmetic and functional outcomes. Prior reports have suggested wedge excisions, composite grafts, interpolation flaps with or without cartilage struts, and hinge flaps as possible options for reconstruction.1-3 However, patients with large defects who prefer single-stage reconstruction procedures present a unique challenge. Herein, we describe a single-stage full-thickness hinge flap technique for a large conchal bowl defect.
The Technique
A 77-year-old man was referred to our dermatology clinic by an outside dermatologist for Mohs micrographic surgery of a biopsy-proven cutaneous squamous cell carcinoma on the right conchal bowl measuring 1.1×2.1 cm and extending to the edge of the external auditory canal (EAC). The excision was performed that same day and was completed in 2 stages, achieving negative margins and resulting in a full-thickness defect measuring 2.0×3.6 cm that included the posterior auricular sulcus, cavum, antitragus, and proximal EAC (Figure 1). The patient requested a single-stage procedure but emphasized that his main priority was an optimal cosmetic outcome.

To repair this large defect, a full-thickness hinge flap with Burow graft was performed. The hinge-type flap was designed in a triangular fashion emanating at the posterior auricular sulcus adjacent to the posterior aspect of the defect and extending down the lateral neck (Figure 2). The flap was incised and the surrounding tissue was undermined, maintaining a robust pedicle in the center of its body on the superolateral neck. The flap was passed through the posterior aspect of the full-thickness defect and was secured in place with 4-0 polyglactin sutures in a buried interrupted fashion, thereby recreating the anterior portion of the defect. The superficial skin edges were reapproximated using 4-0 and 5-0 polypropylene sutures in a running interrupted fashion. The distal Burow triangle created from closure of the flap’s secondary defect was aggressively thinned and was utilized as a full-thickness graft for the residual postauricular groove defect (Figure 3). At 2 weeks’ follow-up, the patient was healing well with no postoperative issues and the sutures were removed (Figure 4).



Practice Implications
There are many different reconstructive options for conchal bowl defects, including primary repair, wedge excision, composite graft and interpolation flaps with or without cartilage struts, and hinge flaps. Structural support, EAC patency, auricle symmetry, overall auricle size, and re-creation of natural contours were considered when designing the reconstruction of the defect in our patient; however, his main priority was achieving the greatest cosmetic outcome in a single-stage procedure, therefore limiting our reconstruction options.
Wedge excision, in which the residual lobule and inferior helical rim are removed, could have been considered in our patient but would have drastically altered the symmetry of the size of the ears. A folded postauricular flap, as described in the otolaryngology literature, is an interpolation flap based on the posterior auricular artery that was designed for full-thickness defects of the auricle to prevent any posterior pinning.1 This technique may have worked well in our case, but the patient preferred to avoid a multistage procedure. Additionally, the positional symmetry of the ears was maintained despite utilizing a hinge flap, which does not involve takedown of the pedicle. A composite graft from the contralateral ear could be considered for smaller conchal bowl defects but likely would have resulted in graft failure in our patient’s large defect due to its need for rich blood supply to heal and dependence on lateral wound edges. Cartilage struts in conjunction with a flap could have been considered in this scenario for greater structural support, but in our patient’s case, by maintaining the robust pedicle of our flap and having residual superior cartilage, further structural support was not necessary.
A prior case report described a partial and full-thickness defect in a similar location that was repaired with a retroauricular hinge flap, in which a portion of the flap was extensively de-epithelialized to address the varied thicknesses of the surgical defect.2 In our patient, the defect abutted the skin reservoir on the superolateral neck, and therefore no de-epithelialization was required as the entire epithelialized portion was utilized to recreate the anterior aspect of the defect. Postauricular hinge-type flaps are a reliable, single-stage surgical alternative to the 2-stage folded postauricular interpolation flap when reconstructing large conchal bowl defects. For small full-thickness defects of the ear, a composite graft may be considered; however, blood supply and other nutritional requirements limit this option for large full-thickness defects.
- Roche AM, Griffin M, Shelton R, et al. The folded postauricular flap: a novel approach to reconstruction of large full thickness defects of the conchal bowl. Am J Otolaryngol. 2017;38:706-709. doi:10.1016 /j.amjoto.2017.09.006
- Klein JC, Nijhawan RI. Retroauricular hinge flaps for full-thickness conchal bowl defects. J Am Acad Dermatol. 2024;90:E71-E72. doi:10.1016/j.jaad.2022.10.056
- Pickrell BB, Hughes CD, Maricevich RS. Partial ear defects. Semin Plast Surg. 2017 Aug;31:134-140. doi:10.1055/s-0037-1603968.
- Roche AM, Griffin M, Shelton R, et al. The folded postauricular flap: a novel approach to reconstruction of large full thickness defects of the conchal bowl. Am J Otolaryngol. 2017;38:706-709. doi:10.1016 /j.amjoto.2017.09.006
- Klein JC, Nijhawan RI. Retroauricular hinge flaps for full-thickness conchal bowl defects. J Am Acad Dermatol. 2024;90:E71-E72. doi:10.1016/j.jaad.2022.10.056
- Pickrell BB, Hughes CD, Maricevich RS. Partial ear defects. Semin Plast Surg. 2017 Aug;31:134-140. doi:10.1055/s-0037-1603968.
Repair of a Large Full-Thickness Conchal Bowl Defect
Repair of a Large Full-Thickness Conchal Bowl Defect
Papulonodules on the Ankle in a Patient with Lung Cancer
Papulonodules on the Ankle in a Patient with Lung Cancer
THE DIAGNOSIS: Pembrolizumab-Induced Eruptive Squamous Proliferation
Histopathology showed a broad squamous proliferation with acanthosis of the epidermis. Large glassy keratinocytes were seen with scattered necrotic keratinocytes (Figure), and a dense lichenoid band of inflammation was present subjacent to the proliferation. Notably, no hypergranulosis, remarkable keratinocyte atypia, or increased mitotic figures were seen. Based on the patient’s medical history and biopsy results, a diagnosis of pembrolizumab-induced eruptive squamous proliferation was made. The diagnosis was supported by a growing body of evidence of this type of reaction in patients taking programmed death 1 (PD-1) inhibitors.1,2 Conservative treatment with clobetasol ointment 0.05% was initiated with complete resolution of the lesions at the 2-month follow-up appointment. Other common treatments include topical steroids, injected corticosteroids, or cryosurgery to locally control the inflammation and atypical proliferation of cells.3
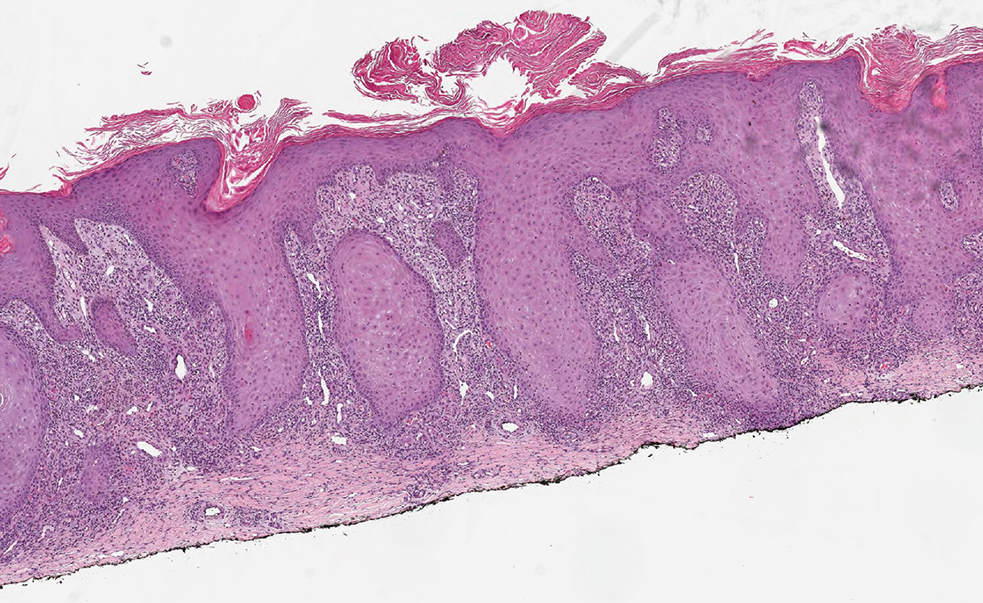
Pembrolizumab is a humanized IgG4 monoclonal antibody targeting the PD-1 receptor that has been utilized for its antitumor activity against various cancers, including unresectable and metastatic melanoma, head and neck cancers, and non–small cell lung cancer (NSCLC).1,4,5 While this drug has extended the lives of many patients with cancer, there are adverse reactions associated with PD-1 inhibitors (eg, pembrolizumab, nivolumab). Skin toxicity to PD-1 inhibitors is the one of most common immune-mediated reactions worldwide, occurring in approximately 30% of patients.6,7 Reactions can occur while a patient is taking the inciting drug and can continue up to 2 months after treatment discontinuation.8 Skin reactions associated with PD-1 inhibitors vary from lichenoid reactions and vitiligolike patches to psoriasis or eczema flares and are organized into 4 categories: inflammatory, immunobullous, alteration of keratinocytes, and alteration of melanocytes.9 Our patient demonstrated alteration of keratinocytes, which is characterized by overlapping features of hypertrophic lichen planus and early keratoacanthoma.
The differential diagnoses for pembrolizumab-induced eruptive squamous proliferation include squamous cell carcinoma (SCC), psoriasis, hypertrophic lichen planus, and cutaneous metastasis of NSCLC. Hypertrophic lupus erythematosus also is a well-documented reaction to use of immune-checkpoint inhibitors.10 Direct immunofluorescence could have helped differentiate hypertrophic lupus erythematosus from an eruptive squamous proliferation in our patient; however, due to her response to treatment, no additional workup was done.
Squamous cell carcinoma, which is the most common type of skin cancer in Black patients in the United States,11 has been shown to manifest after a PD-1 inhibitor is taken.12 Although it typically has a more chronic persistent course, the clinical appearance of SCC can be similar to the findings seen in our patient. Histologically, SCC may demonstrate necrosis, but the atypical proliferations will invade the dermis—a feature not seen in our patient’s histopathology.13
Lichen planus (LP) is an eruptive immune reaction of violaceous polygonal papules and plaques commonly seen on the ankles14 that has been shown to be an adverse effect of pembrolizumab.15 There are several subtypes of LP including hypertrophic versions, which can appear clinically similar to the findings seen in our patient. On dermoscopy, the classic finding of white lines, known as Wickham striae, is seen in all subtypes and can help diagnose this pathologic process. Under the microscope, LP can manifest with hyperkeratosis without parakeratosis, irregular thickening of the stratum granulosum, sawtooth rete ridges, and destruction of the basal layer.14
Psoriasis also has been shown to be exacerbated by anti–PD-1 therapy, although the majority of patients diagnosed with PD-1–induced psoriasis have a personal or family history of the disease.6 Clinically, psoriasis can have a hyperpigmented or violaceous appearance in patients with skin of color.16 The histopathology of psoriasis typically reveals confluent parakeratosis, neutrophils in the stratum corneum, regular acanthosis, thinning of the suprapapillary plates, and vessels in the dermal papillae.17
Although cutaneous metastasis of NSCLC may appear clinically similar to the current case, it is one of the rarer organ sites of metastasis for lung cancer.18 In our patient, biopsy quickly ruled out this diagnosis. If it had been a site of metastasis, histopathology would have shown a dermal-based proliferation of dysplastic cells without epidermal connection.19
It is important for dermatologists to recognize eruptive squamous proliferations associated with pembrolizumab, as they often respond to conservative treatment and typically do not require dose reduction or discontinuation of the inciting drug.
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi:10.1186/s40425-017-0242-5
- Preti BTB, Pencz A, Cowger JJM, et al. Skin deep: a fascinating case report of immunotherapy-triggered, treatment-refractory autoimmune lichen planus and keratoacanthoma. Case Rep Oncol. 2021;14: 1189-1193. doi:10.1159/000518313
- Fradet M, Sibaud V, Tournier E, et al. Multiple keratoacanthoma-like lesions in a patient treated with pembrolizumab. Acta Derm Venereol. 2019;99:1301-1302. doi:10.2340/00015555-3301
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls [Internet]. StatPearls Publishing; 2023. Updated June 26, 2023. Accessed April 2, 2025.
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with Nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-Pd1/Pdl1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. doi:10.1016 /j.currproblcancer.2017.10.003
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Coscarart A, Martel J, Lee MP, et al. Pembrolizumab-induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol. 2020;47:275-279. doi:10.1111/cup.13587
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176. doi:10.1111/cup.12858
- Vitzthum von Eckstaedt H, Singh A, Reid P, et al. Immune checkpoint inhibitors and lupus erythematosus. Pharmaceuticals (Basel). 2024;2:15;17. doi:10.3390/ph17020252
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Vu M, Chapman S, Lenz B, et al. Squamous cell carcinoma or squamous proliferation associated with nivolumab treatment for metastatic melanoma. Dermatol Online J. 2022;6:28. doi:10.5070/d328357786
- Howell JY, Ramsey ML. Squamous cell skin cancer. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 2, 2024. Accessed April 2, 2025.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. StatPearls Publishing; 2024. Updated October 29, 2024. Accessed April 2, 2025.
- Yamashita A, Akasaka E, Nakano H, et al. Pembrolizumab-induced lichen planus on the scalp of a patient with non-small-cell lung carcinoma. Case Rep Dermatol. 2021;13:487-491. doi:10.1159/000519486
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524-528. doi:10.1016 /j.clindermatol.2007.08.005.
- Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996;35:459-462. doi:10.2169 /internalmedicine.35.459.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613–620. doi:10.1001/archderm.143.5.613
THE DIAGNOSIS: Pembrolizumab-Induced Eruptive Squamous Proliferation
Histopathology showed a broad squamous proliferation with acanthosis of the epidermis. Large glassy keratinocytes were seen with scattered necrotic keratinocytes (Figure), and a dense lichenoid band of inflammation was present subjacent to the proliferation. Notably, no hypergranulosis, remarkable keratinocyte atypia, or increased mitotic figures were seen. Based on the patient’s medical history and biopsy results, a diagnosis of pembrolizumab-induced eruptive squamous proliferation was made. The diagnosis was supported by a growing body of evidence of this type of reaction in patients taking programmed death 1 (PD-1) inhibitors.1,2 Conservative treatment with clobetasol ointment 0.05% was initiated with complete resolution of the lesions at the 2-month follow-up appointment. Other common treatments include topical steroids, injected corticosteroids, or cryosurgery to locally control the inflammation and atypical proliferation of cells.3

Pembrolizumab is a humanized IgG4 monoclonal antibody targeting the PD-1 receptor that has been utilized for its antitumor activity against various cancers, including unresectable and metastatic melanoma, head and neck cancers, and non–small cell lung cancer (NSCLC).1,4,5 While this drug has extended the lives of many patients with cancer, there are adverse reactions associated with PD-1 inhibitors (eg, pembrolizumab, nivolumab). Skin toxicity to PD-1 inhibitors is the one of most common immune-mediated reactions worldwide, occurring in approximately 30% of patients.6,7 Reactions can occur while a patient is taking the inciting drug and can continue up to 2 months after treatment discontinuation.8 Skin reactions associated with PD-1 inhibitors vary from lichenoid reactions and vitiligolike patches to psoriasis or eczema flares and are organized into 4 categories: inflammatory, immunobullous, alteration of keratinocytes, and alteration of melanocytes.9 Our patient demonstrated alteration of keratinocytes, which is characterized by overlapping features of hypertrophic lichen planus and early keratoacanthoma.
The differential diagnoses for pembrolizumab-induced eruptive squamous proliferation include squamous cell carcinoma (SCC), psoriasis, hypertrophic lichen planus, and cutaneous metastasis of NSCLC. Hypertrophic lupus erythematosus also is a well-documented reaction to use of immune-checkpoint inhibitors.10 Direct immunofluorescence could have helped differentiate hypertrophic lupus erythematosus from an eruptive squamous proliferation in our patient; however, due to her response to treatment, no additional workup was done.
Squamous cell carcinoma, which is the most common type of skin cancer in Black patients in the United States,11 has been shown to manifest after a PD-1 inhibitor is taken.12 Although it typically has a more chronic persistent course, the clinical appearance of SCC can be similar to the findings seen in our patient. Histologically, SCC may demonstrate necrosis, but the atypical proliferations will invade the dermis—a feature not seen in our patient’s histopathology.13
Lichen planus (LP) is an eruptive immune reaction of violaceous polygonal papules and plaques commonly seen on the ankles14 that has been shown to be an adverse effect of pembrolizumab.15 There are several subtypes of LP including hypertrophic versions, which can appear clinically similar to the findings seen in our patient. On dermoscopy, the classic finding of white lines, known as Wickham striae, is seen in all subtypes and can help diagnose this pathologic process. Under the microscope, LP can manifest with hyperkeratosis without parakeratosis, irregular thickening of the stratum granulosum, sawtooth rete ridges, and destruction of the basal layer.14
Psoriasis also has been shown to be exacerbated by anti–PD-1 therapy, although the majority of patients diagnosed with PD-1–induced psoriasis have a personal or family history of the disease.6 Clinically, psoriasis can have a hyperpigmented or violaceous appearance in patients with skin of color.16 The histopathology of psoriasis typically reveals confluent parakeratosis, neutrophils in the stratum corneum, regular acanthosis, thinning of the suprapapillary plates, and vessels in the dermal papillae.17
Although cutaneous metastasis of NSCLC may appear clinically similar to the current case, it is one of the rarer organ sites of metastasis for lung cancer.18 In our patient, biopsy quickly ruled out this diagnosis. If it had been a site of metastasis, histopathology would have shown a dermal-based proliferation of dysplastic cells without epidermal connection.19
It is important for dermatologists to recognize eruptive squamous proliferations associated with pembrolizumab, as they often respond to conservative treatment and typically do not require dose reduction or discontinuation of the inciting drug.
THE DIAGNOSIS: Pembrolizumab-Induced Eruptive Squamous Proliferation
Histopathology showed a broad squamous proliferation with acanthosis of the epidermis. Large glassy keratinocytes were seen with scattered necrotic keratinocytes (Figure), and a dense lichenoid band of inflammation was present subjacent to the proliferation. Notably, no hypergranulosis, remarkable keratinocyte atypia, or increased mitotic figures were seen. Based on the patient’s medical history and biopsy results, a diagnosis of pembrolizumab-induced eruptive squamous proliferation was made. The diagnosis was supported by a growing body of evidence of this type of reaction in patients taking programmed death 1 (PD-1) inhibitors.1,2 Conservative treatment with clobetasol ointment 0.05% was initiated with complete resolution of the lesions at the 2-month follow-up appointment. Other common treatments include topical steroids, injected corticosteroids, or cryosurgery to locally control the inflammation and atypical proliferation of cells.3

Pembrolizumab is a humanized IgG4 monoclonal antibody targeting the PD-1 receptor that has been utilized for its antitumor activity against various cancers, including unresectable and metastatic melanoma, head and neck cancers, and non–small cell lung cancer (NSCLC).1,4,5 While this drug has extended the lives of many patients with cancer, there are adverse reactions associated with PD-1 inhibitors (eg, pembrolizumab, nivolumab). Skin toxicity to PD-1 inhibitors is the one of most common immune-mediated reactions worldwide, occurring in approximately 30% of patients.6,7 Reactions can occur while a patient is taking the inciting drug and can continue up to 2 months after treatment discontinuation.8 Skin reactions associated with PD-1 inhibitors vary from lichenoid reactions and vitiligolike patches to psoriasis or eczema flares and are organized into 4 categories: inflammatory, immunobullous, alteration of keratinocytes, and alteration of melanocytes.9 Our patient demonstrated alteration of keratinocytes, which is characterized by overlapping features of hypertrophic lichen planus and early keratoacanthoma.
The differential diagnoses for pembrolizumab-induced eruptive squamous proliferation include squamous cell carcinoma (SCC), psoriasis, hypertrophic lichen planus, and cutaneous metastasis of NSCLC. Hypertrophic lupus erythematosus also is a well-documented reaction to use of immune-checkpoint inhibitors.10 Direct immunofluorescence could have helped differentiate hypertrophic lupus erythematosus from an eruptive squamous proliferation in our patient; however, due to her response to treatment, no additional workup was done.
Squamous cell carcinoma, which is the most common type of skin cancer in Black patients in the United States,11 has been shown to manifest after a PD-1 inhibitor is taken.12 Although it typically has a more chronic persistent course, the clinical appearance of SCC can be similar to the findings seen in our patient. Histologically, SCC may demonstrate necrosis, but the atypical proliferations will invade the dermis—a feature not seen in our patient’s histopathology.13
Lichen planus (LP) is an eruptive immune reaction of violaceous polygonal papules and plaques commonly seen on the ankles14 that has been shown to be an adverse effect of pembrolizumab.15 There are several subtypes of LP including hypertrophic versions, which can appear clinically similar to the findings seen in our patient. On dermoscopy, the classic finding of white lines, known as Wickham striae, is seen in all subtypes and can help diagnose this pathologic process. Under the microscope, LP can manifest with hyperkeratosis without parakeratosis, irregular thickening of the stratum granulosum, sawtooth rete ridges, and destruction of the basal layer.14
Psoriasis also has been shown to be exacerbated by anti–PD-1 therapy, although the majority of patients diagnosed with PD-1–induced psoriasis have a personal or family history of the disease.6 Clinically, psoriasis can have a hyperpigmented or violaceous appearance in patients with skin of color.16 The histopathology of psoriasis typically reveals confluent parakeratosis, neutrophils in the stratum corneum, regular acanthosis, thinning of the suprapapillary plates, and vessels in the dermal papillae.17
Although cutaneous metastasis of NSCLC may appear clinically similar to the current case, it is one of the rarer organ sites of metastasis for lung cancer.18 In our patient, biopsy quickly ruled out this diagnosis. If it had been a site of metastasis, histopathology would have shown a dermal-based proliferation of dysplastic cells without epidermal connection.19
It is important for dermatologists to recognize eruptive squamous proliferations associated with pembrolizumab, as they often respond to conservative treatment and typically do not require dose reduction or discontinuation of the inciting drug.
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi:10.1186/s40425-017-0242-5
- Preti BTB, Pencz A, Cowger JJM, et al. Skin deep: a fascinating case report of immunotherapy-triggered, treatment-refractory autoimmune lichen planus and keratoacanthoma. Case Rep Oncol. 2021;14: 1189-1193. doi:10.1159/000518313
- Fradet M, Sibaud V, Tournier E, et al. Multiple keratoacanthoma-like lesions in a patient treated with pembrolizumab. Acta Derm Venereol. 2019;99:1301-1302. doi:10.2340/00015555-3301
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls [Internet]. StatPearls Publishing; 2023. Updated June 26, 2023. Accessed April 2, 2025.
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with Nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-Pd1/Pdl1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. doi:10.1016 /j.currproblcancer.2017.10.003
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Coscarart A, Martel J, Lee MP, et al. Pembrolizumab-induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol. 2020;47:275-279. doi:10.1111/cup.13587
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176. doi:10.1111/cup.12858
- Vitzthum von Eckstaedt H, Singh A, Reid P, et al. Immune checkpoint inhibitors and lupus erythematosus. Pharmaceuticals (Basel). 2024;2:15;17. doi:10.3390/ph17020252
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Vu M, Chapman S, Lenz B, et al. Squamous cell carcinoma or squamous proliferation associated with nivolumab treatment for metastatic melanoma. Dermatol Online J. 2022;6:28. doi:10.5070/d328357786
- Howell JY, Ramsey ML. Squamous cell skin cancer. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 2, 2024. Accessed April 2, 2025.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. StatPearls Publishing; 2024. Updated October 29, 2024. Accessed April 2, 2025.
- Yamashita A, Akasaka E, Nakano H, et al. Pembrolizumab-induced lichen planus on the scalp of a patient with non-small-cell lung carcinoma. Case Rep Dermatol. 2021;13:487-491. doi:10.1159/000519486
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524-528. doi:10.1016 /j.clindermatol.2007.08.005.
- Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996;35:459-462. doi:10.2169 /internalmedicine.35.459.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613–620. doi:10.1001/archderm.143.5.613
- Freshwater T, Kondic A, Ahamadi M, et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi:10.1186/s40425-017-0242-5
- Preti BTB, Pencz A, Cowger JJM, et al. Skin deep: a fascinating case report of immunotherapy-triggered, treatment-refractory autoimmune lichen planus and keratoacanthoma. Case Rep Oncol. 2021;14: 1189-1193. doi:10.1159/000518313
- Fradet M, Sibaud V, Tournier E, et al. Multiple keratoacanthoma-like lesions in a patient treated with pembrolizumab. Acta Derm Venereol. 2019;99:1301-1302. doi:10.2340/00015555-3301
- Flynn JP, Gerriets V. Pembrolizumab. StatPearls [Internet]. StatPearls Publishing; 2023. Updated June 26, 2023. Accessed April 2, 2025.
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with Nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Voudouri D, Nikolaou V, Laschos K, et al. Anti-Pd1/Pdl1 induced psoriasis. Curr Probl Cancer. 2017;41:407-412. doi:10.1016 /j.currproblcancer.2017.10.003
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Coscarart A, Martel J, Lee MP, et al. Pembrolizumab-induced pseudoepitheliomatous eruption consistent with hypertrophic lichen planus. J Cutan Pathol. 2020;47:275-279. doi:10.1111/cup.13587
- Curry JL, Tetzlaff MT, Nagarajan P, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158-176. doi:10.1111/cup.12858
- Vitzthum von Eckstaedt H, Singh A, Reid P, et al. Immune checkpoint inhibitors and lupus erythematosus. Pharmaceuticals (Basel). 2024;2:15;17. doi:10.3390/ph17020252
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Vu M, Chapman S, Lenz B, et al. Squamous cell carcinoma or squamous proliferation associated with nivolumab treatment for metastatic melanoma. Dermatol Online J. 2022;6:28. doi:10.5070/d328357786
- Howell JY, Ramsey ML. Squamous cell skin cancer. StatPearls [Internet]. StatPearls Publishing; 2024. Updated July 2, 2024. Accessed April 2, 2025.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. StatPearls Publishing; 2024. Updated October 29, 2024. Accessed April 2, 2025.
- Yamashita A, Akasaka E, Nakano H, et al. Pembrolizumab-induced lichen planus on the scalp of a patient with non-small-cell lung carcinoma. Case Rep Dermatol. 2021;13:487-491. doi:10.1159/000519486
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. 2007;25:524-528. doi:10.1016 /j.clindermatol.2007.08.005.
- Hidaka T, Ishii Y, Kitamura S. Clinical features of skin metastasis from lung cancer. Intern Med. 1996;35:459-462. doi:10.2169 /internalmedicine.35.459.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613–620. doi:10.1001/archderm.143.5.613
Papulonodules on the Ankle in a Patient with Lung Cancer
Papulonodules on the Ankle in a Patient with Lung Cancer
A 75-year-old woman presented to the dermatology department with well-circumscribed, round, hyperkeratotic papulonodules on the ankle of 3 months’ duration (top). The papulonodules also were evaluated by dermoscopy, which highlighted in greater detail the hyperkeratosis seen grossly (bottom). The patient had a history of chronic obstructive pulmonary disease and metastatic lung cancer and had been taking pembrolizumab for the past 2 years. The lesions initially appeared on the medial right foot and slowly spread proximally. Most of the lesions resolved spontaneously except for 2 on the right ankle. At the current presentation, one lesion was slightly tender to palpation, but both were otherwise asymptomatic. A lesion was biopsied and sent for dermatopathologic evaluation.
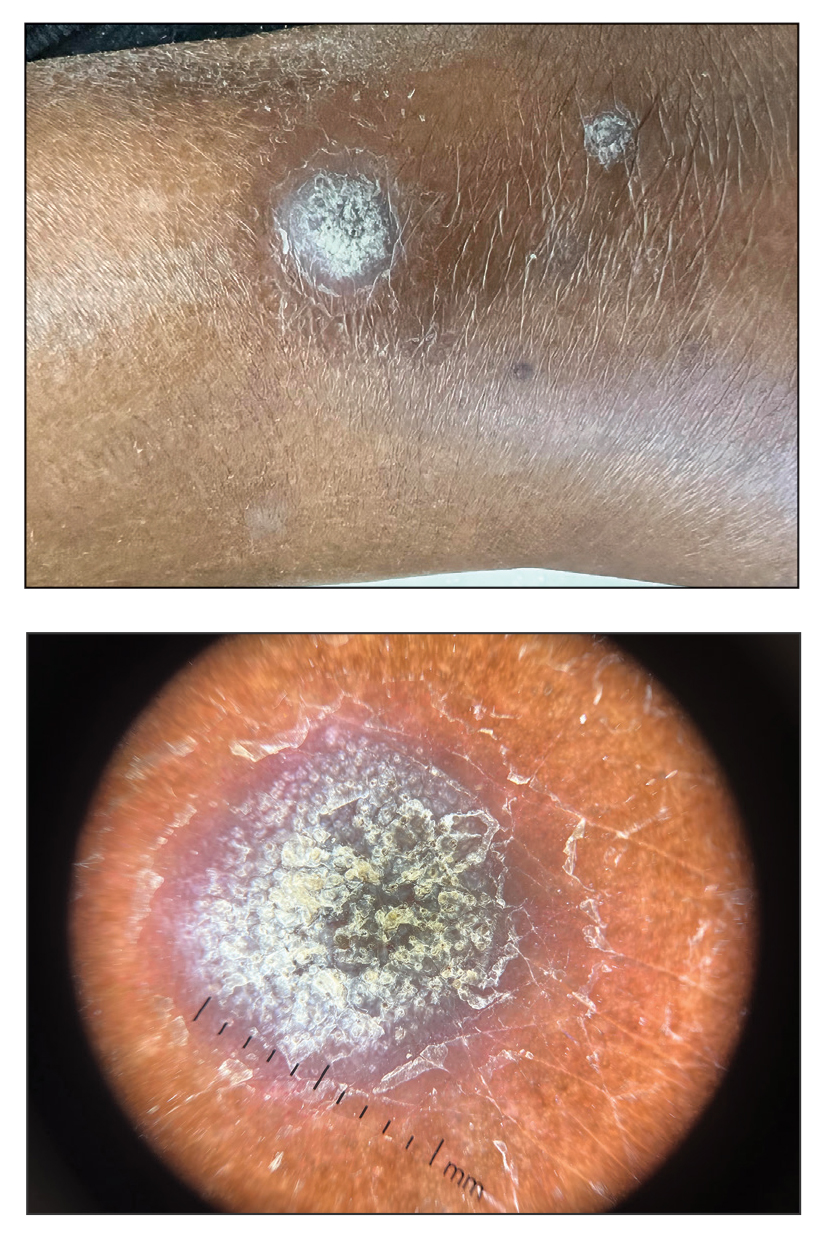
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
THE DIAGNOSIS: Borderline-Borderline Leprosy With Type 1 Lepra Reaction
Punch biopsies from plaques on the right elbow and right shin revealed diffuse granulomatous dermatitis (Figure 1) with a narrow Grenz zone in the superficial dermis. The upper dermis contained a dense bandlike infiltrate of histiocytes with abundant foamy-gray cytoplasm and a moderate admixture of lymphocytes. The mid and deep dermis contained a nodular, perivascular, periadnexal, and perineural infiltrate of histiocytes and a dense admixture of lymphocytes. Periodic acid-Schiff and Gram stains were negative for microorganisms. Fite stain was positive for numerous organisms in histiocytes and small dermal nerves (Figure 2). These findings and the clinical examination confirmed a diagnosis of borderline-borderline leprosy with type 1 lepra reaction. The patient was started on dapsone 100 mg, rifampin 600 mg, and clofazimine 100 mg once daily and experienced clinical improvement within 6 months.
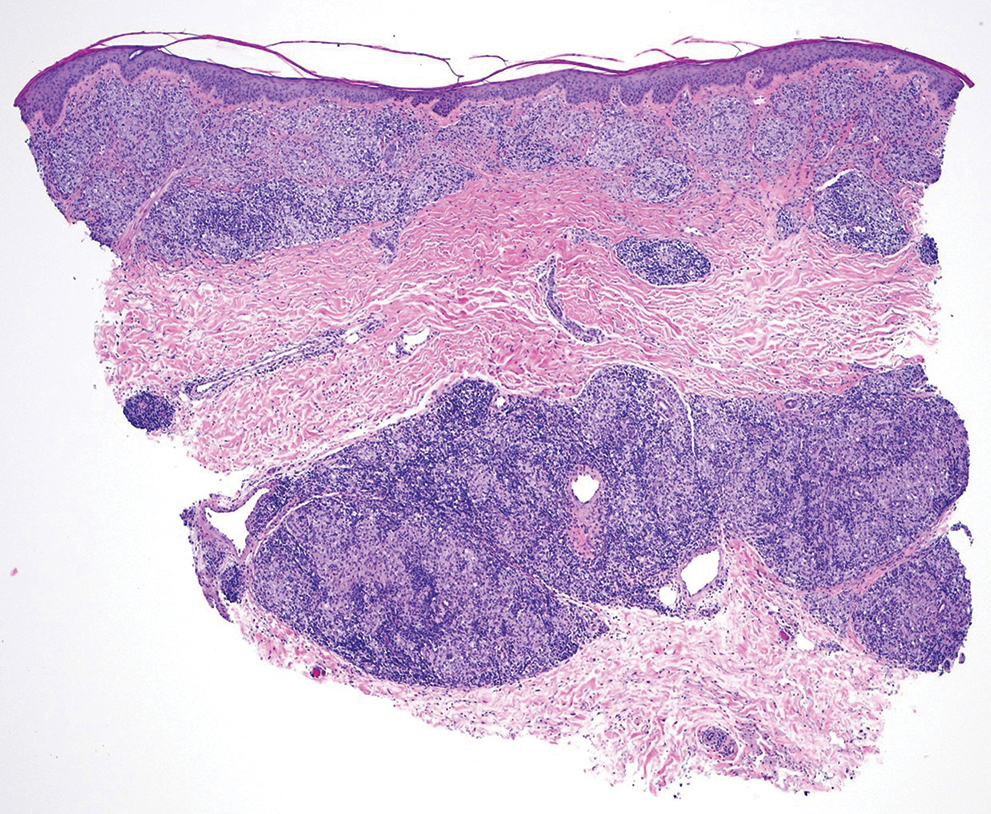
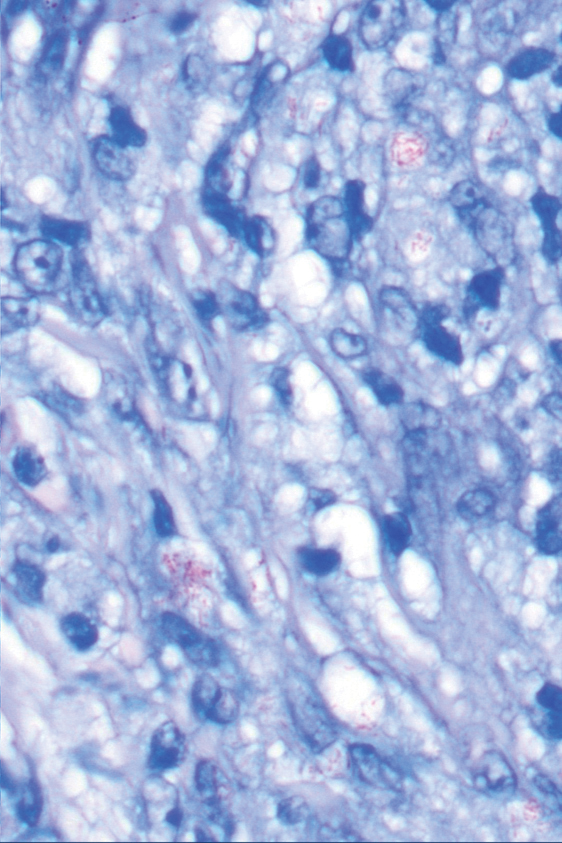
The World Health Organization reported more than 200,000 new leprosy cases globally in 2019, with most occurring in India, Brazil, and Indonesia.1 About 150 to 250 new cases are detected in the United States annually.1 The Ridley-Jopling classification of leprosy divides the condition into 5 categories: tuberculoid, borderline tuberculoid, borderline-borderline (BB), borderline lepromatous, and lepromatous. At one end of the spectrum, tuberculoid leprosy—a predominant Th1 immune response mediated by CD4 lymphocytes, interleukin (IL) 2, and interferon gamma2—is characterized by sharply demarcated erythematous and hypopigmented plaques with raised borders and an annular appearance.2,3 Lesions typically have atrophic and hypopigmented centers that often appear in an asymmetric distribution on the arms and legs.2,3 Histologic features include dermal tuberculoid granulomas with epithelioid cells—some located directly beneath the epidermis and others around deep vessels and nerves3—multinucleated Langerhans giant cells, thickened peripheral nerves with intraneural lymphocytic infiltrates, and granulomas with central necrosis. Fite-Faraco staining exhibits few bacteria.2
Lepromatous leprosy occurs in individuals with impaired T-cell immunity, leading to multiple red-brown nodular infiltrates in the skin and mucous membranes.2,3 Lesions typically are symmetric and favor the face and auricle of the ear.2,3 Histologically, there are bluish-gray foamy macrophages that form diffuse or nodular infiltrates with few lymphocytes,2 with a Grenz zone between the epidermis and dermis. Nerves may show lamination of the perineurium resembling an onion skin.2,3 Immunohistochemistry shows predominant CD8-positive infiltrates with a Th2 response and positive IL-4 and IL-10. Fite-Faraco stain shows numerous mycobacteria arranged in clusters and in histiocytes.2
Tuberculoid leprosy is treated with dapsone 100 mg and rifampin 600 mg once daily for 6 months,4 and lepromatous leprosy is treated with dapsone 100 mg, rifampin 600 mg, and clofazimine 50 mg once daily for 12 months.4 The prognosis for both is good with treatment; erythema and induration of skin lesions may improve within a few months, but residual nerve damage is common, especially in those with advanced disease prior to treatment.2 For direct contacts, a single dose of rifampin may be given.4
Borderline-borderline leprosy manifests with numerous asymmetric annular plaques, as seen in our patient (Figure 3). Histology findings can be variable and often overlap with other forms of leprosy. There can be epithelioid granulomas and only a few acid-fast bacilli (AFB) or diffuse histiocytic aggregates with foamy histiocytes containing large numbers of AFB.3 Nerve involvement is variable but can be severe in the setting of type 1 lepra reaction, which was present in our patient. Type 1 lepra reaction—a type IV cell-mediated allergic hypersensitivity reaction to Mycobacterium leprae antigens—manifests clinically with hyperesthesia, erythema, edema, and subsequent scaling.2 It occurs in up to 30% of patients with borderline leprosy, usually within 12 months of treatment initiation.2 Our patient had considerable edema and erythema of the hands and feet (Figure 4) along with extensive polyneuropathy prior to starting therapy.
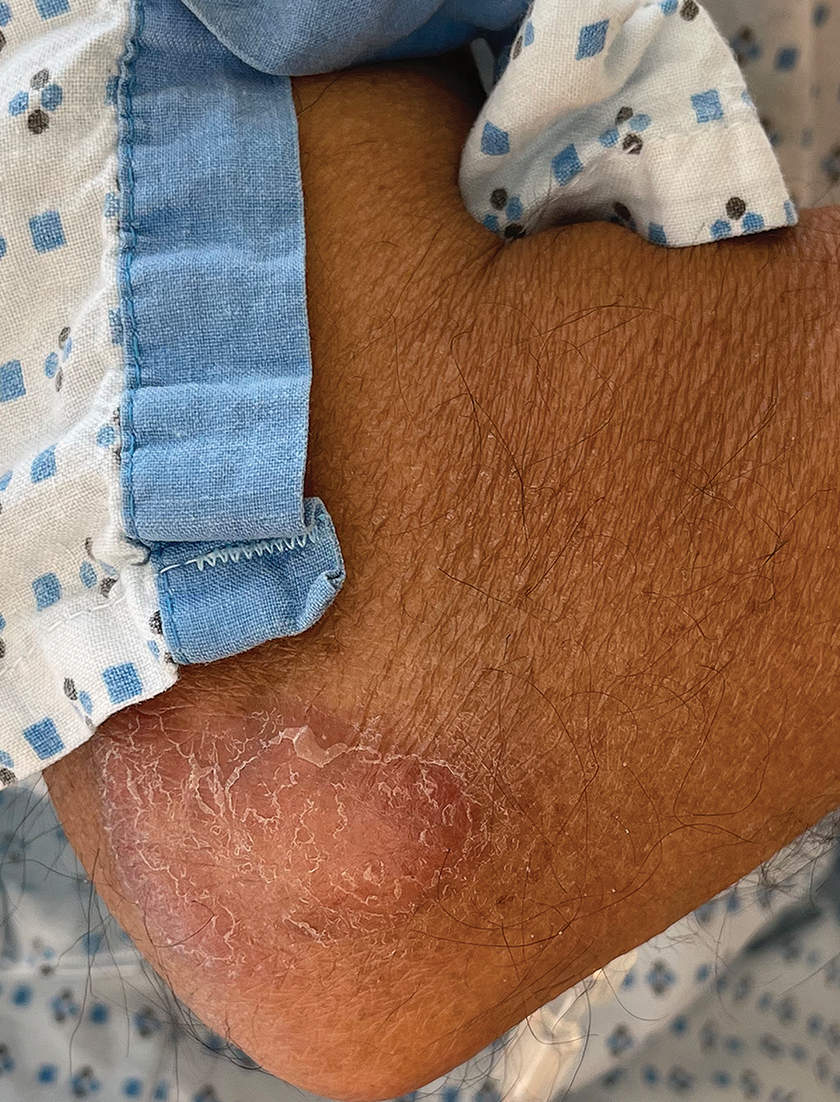
Lucio phenomenon is a rare leprosy reaction found in patients with untreated lepromatous leprosy characterized by erythematous to violaceous macules that lead to ulceronecrotic lesions.5 Histologically, there are many AFB in the vascular endothelium, leukocytoclastic vasculitis, and ischemic epidermal necrosis.5 Our patient did not have ulcerative or necrotic lesions.
The classic skin lesions of psoriasis vulgaris can be described as well-demarcated pink plaques with white or silvery scales that usually are distributed symmetrically and often are found on extensor surfaces.6 Rapidly progressive lesions can be annular with normal skin in the center, mimicking the lesions seen in tuberculoid leprosy. Clinically, both psoriasis and tuberculoid forms of leprosy are sharply demarcated; however, psoriatic lesions often have micaceous overlying scale that is not present in leprosy. Characteristic histologic findings of psoriasis are hyperkeratosis, parakeratosis, and acanthosis of the epidermis with dilated blood vessels and a lymphocytic infiltrate, predominantly into the dermis.7 Psoriatic arthritis has a variable clinical course but tends to emerge 5 to 12 years after initial skin manifestation.8 Classic clinical symptoms include swelling, tenderness, stiffness, and pain in joints and surrounding tissues.8 Other than edema, our patient did not exhibit signs of psoriatic arthritis.
Sarcoidosis is a systemic autoimmune disease characterized by noncaseating epithelioid granulomas affecting various organs, with cutaneous manifestations present in approximately 30% of all cases. Cutaneous manifestations can be variable, including maculopapular lesions, plaques, and nodules.9 Differentiating between cutaneous sarcoidosis and tuberculoid leprosy can be challenging, as both are granulomatous processes; however, histology of sarcoidosis demonstrates noncaseating granulomas in the dermis and/or subcutaneous tissues without AFB9 compared to granulomas with necrotic centers in tuberculoid leprosy.
Cutaneous tuberculosis has variable morphologies. One subtype, lupus vulgaris, can manifest with violaceous, scaly, eroded plaques that could be confused for leprosy. Lupus vulgaris usually results from hematogenous or lymphatic seeding in individuals with high or moderate immunity to M tuberculosis.10
Histologically, the dermis has tuberculoid granulomas containing multinucleated giant cells,10 which can mimic those seen in BB leprosy. Tuberculin skin test results often are positive10; while this test was not performed in our patient, chest radiography was unremarkable, making this diagnosis less likely.
Mycobacterium leprae infections should be considered in a patient with a worsening rash and progressive polyneuropathy. Clinical diagnosis can be challenging due to similarities with other diseases; however, histopathologic findings can help differentiate M leprae from other conditions. This infection is treatable, and early detection can minimize long-term patient morbidity.
- CDC. Hansen’s disease (leprosy). Accessed April 23, 2025. https://www.cdc.gov/leprosy/about/index.html
- Fischer M. Leprosy—an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827.
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020; 83:1-14.
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. October 6, 2018. Accessed April 2, 2025. https://www.who.int/publications/i/item/9789290226383
- Frade MAC, Coltro PS, Filho FB, et al. Lucio’s phenomenon: a systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464-477.
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Evidence-Based Psoriasis. Springer International Publishing; 2018:1-16.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 suppl):S216-S224.
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514.
- Hill MK, Sanders CV. Cutaneous tuberculosis. Microbiol Spectr. 2017;5:1-6.
THE DIAGNOSIS: Borderline-Borderline Leprosy With Type 1 Lepra Reaction
Punch biopsies from plaques on the right elbow and right shin revealed diffuse granulomatous dermatitis (Figure 1) with a narrow Grenz zone in the superficial dermis. The upper dermis contained a dense bandlike infiltrate of histiocytes with abundant foamy-gray cytoplasm and a moderate admixture of lymphocytes. The mid and deep dermis contained a nodular, perivascular, periadnexal, and perineural infiltrate of histiocytes and a dense admixture of lymphocytes. Periodic acid-Schiff and Gram stains were negative for microorganisms. Fite stain was positive for numerous organisms in histiocytes and small dermal nerves (Figure 2). These findings and the clinical examination confirmed a diagnosis of borderline-borderline leprosy with type 1 lepra reaction. The patient was started on dapsone 100 mg, rifampin 600 mg, and clofazimine 100 mg once daily and experienced clinical improvement within 6 months.


The World Health Organization reported more than 200,000 new leprosy cases globally in 2019, with most occurring in India, Brazil, and Indonesia.1 About 150 to 250 new cases are detected in the United States annually.1 The Ridley-Jopling classification of leprosy divides the condition into 5 categories: tuberculoid, borderline tuberculoid, borderline-borderline (BB), borderline lepromatous, and lepromatous. At one end of the spectrum, tuberculoid leprosy—a predominant Th1 immune response mediated by CD4 lymphocytes, interleukin (IL) 2, and interferon gamma2—is characterized by sharply demarcated erythematous and hypopigmented plaques with raised borders and an annular appearance.2,3 Lesions typically have atrophic and hypopigmented centers that often appear in an asymmetric distribution on the arms and legs.2,3 Histologic features include dermal tuberculoid granulomas with epithelioid cells—some located directly beneath the epidermis and others around deep vessels and nerves3—multinucleated Langerhans giant cells, thickened peripheral nerves with intraneural lymphocytic infiltrates, and granulomas with central necrosis. Fite-Faraco staining exhibits few bacteria.2
Lepromatous leprosy occurs in individuals with impaired T-cell immunity, leading to multiple red-brown nodular infiltrates in the skin and mucous membranes.2,3 Lesions typically are symmetric and favor the face and auricle of the ear.2,3 Histologically, there are bluish-gray foamy macrophages that form diffuse or nodular infiltrates with few lymphocytes,2 with a Grenz zone between the epidermis and dermis. Nerves may show lamination of the perineurium resembling an onion skin.2,3 Immunohistochemistry shows predominant CD8-positive infiltrates with a Th2 response and positive IL-4 and IL-10. Fite-Faraco stain shows numerous mycobacteria arranged in clusters and in histiocytes.2
Tuberculoid leprosy is treated with dapsone 100 mg and rifampin 600 mg once daily for 6 months,4 and lepromatous leprosy is treated with dapsone 100 mg, rifampin 600 mg, and clofazimine 50 mg once daily for 12 months.4 The prognosis for both is good with treatment; erythema and induration of skin lesions may improve within a few months, but residual nerve damage is common, especially in those with advanced disease prior to treatment.2 For direct contacts, a single dose of rifampin may be given.4
Borderline-borderline leprosy manifests with numerous asymmetric annular plaques, as seen in our patient (Figure 3). Histology findings can be variable and often overlap with other forms of leprosy. There can be epithelioid granulomas and only a few acid-fast bacilli (AFB) or diffuse histiocytic aggregates with foamy histiocytes containing large numbers of AFB.3 Nerve involvement is variable but can be severe in the setting of type 1 lepra reaction, which was present in our patient. Type 1 lepra reaction—a type IV cell-mediated allergic hypersensitivity reaction to Mycobacterium leprae antigens—manifests clinically with hyperesthesia, erythema, edema, and subsequent scaling.2 It occurs in up to 30% of patients with borderline leprosy, usually within 12 months of treatment initiation.2 Our patient had considerable edema and erythema of the hands and feet (Figure 4) along with extensive polyneuropathy prior to starting therapy.


Lucio phenomenon is a rare leprosy reaction found in patients with untreated lepromatous leprosy characterized by erythematous to violaceous macules that lead to ulceronecrotic lesions.5 Histologically, there are many AFB in the vascular endothelium, leukocytoclastic vasculitis, and ischemic epidermal necrosis.5 Our patient did not have ulcerative or necrotic lesions.
The classic skin lesions of psoriasis vulgaris can be described as well-demarcated pink plaques with white or silvery scales that usually are distributed symmetrically and often are found on extensor surfaces.6 Rapidly progressive lesions can be annular with normal skin in the center, mimicking the lesions seen in tuberculoid leprosy. Clinically, both psoriasis and tuberculoid forms of leprosy are sharply demarcated; however, psoriatic lesions often have micaceous overlying scale that is not present in leprosy. Characteristic histologic findings of psoriasis are hyperkeratosis, parakeratosis, and acanthosis of the epidermis with dilated blood vessels and a lymphocytic infiltrate, predominantly into the dermis.7 Psoriatic arthritis has a variable clinical course but tends to emerge 5 to 12 years after initial skin manifestation.8 Classic clinical symptoms include swelling, tenderness, stiffness, and pain in joints and surrounding tissues.8 Other than edema, our patient did not exhibit signs of psoriatic arthritis.
Sarcoidosis is a systemic autoimmune disease characterized by noncaseating epithelioid granulomas affecting various organs, with cutaneous manifestations present in approximately 30% of all cases. Cutaneous manifestations can be variable, including maculopapular lesions, plaques, and nodules.9 Differentiating between cutaneous sarcoidosis and tuberculoid leprosy can be challenging, as both are granulomatous processes; however, histology of sarcoidosis demonstrates noncaseating granulomas in the dermis and/or subcutaneous tissues without AFB9 compared to granulomas with necrotic centers in tuberculoid leprosy.
Cutaneous tuberculosis has variable morphologies. One subtype, lupus vulgaris, can manifest with violaceous, scaly, eroded plaques that could be confused for leprosy. Lupus vulgaris usually results from hematogenous or lymphatic seeding in individuals with high or moderate immunity to M tuberculosis.10
Histologically, the dermis has tuberculoid granulomas containing multinucleated giant cells,10 which can mimic those seen in BB leprosy. Tuberculin skin test results often are positive10; while this test was not performed in our patient, chest radiography was unremarkable, making this diagnosis less likely.
Mycobacterium leprae infections should be considered in a patient with a worsening rash and progressive polyneuropathy. Clinical diagnosis can be challenging due to similarities with other diseases; however, histopathologic findings can help differentiate M leprae from other conditions. This infection is treatable, and early detection can minimize long-term patient morbidity.
THE DIAGNOSIS: Borderline-Borderline Leprosy With Type 1 Lepra Reaction
Punch biopsies from plaques on the right elbow and right shin revealed diffuse granulomatous dermatitis (Figure 1) with a narrow Grenz zone in the superficial dermis. The upper dermis contained a dense bandlike infiltrate of histiocytes with abundant foamy-gray cytoplasm and a moderate admixture of lymphocytes. The mid and deep dermis contained a nodular, perivascular, periadnexal, and perineural infiltrate of histiocytes and a dense admixture of lymphocytes. Periodic acid-Schiff and Gram stains were negative for microorganisms. Fite stain was positive for numerous organisms in histiocytes and small dermal nerves (Figure 2). These findings and the clinical examination confirmed a diagnosis of borderline-borderline leprosy with type 1 lepra reaction. The patient was started on dapsone 100 mg, rifampin 600 mg, and clofazimine 100 mg once daily and experienced clinical improvement within 6 months.


The World Health Organization reported more than 200,000 new leprosy cases globally in 2019, with most occurring in India, Brazil, and Indonesia.1 About 150 to 250 new cases are detected in the United States annually.1 The Ridley-Jopling classification of leprosy divides the condition into 5 categories: tuberculoid, borderline tuberculoid, borderline-borderline (BB), borderline lepromatous, and lepromatous. At one end of the spectrum, tuberculoid leprosy—a predominant Th1 immune response mediated by CD4 lymphocytes, interleukin (IL) 2, and interferon gamma2—is characterized by sharply demarcated erythematous and hypopigmented plaques with raised borders and an annular appearance.2,3 Lesions typically have atrophic and hypopigmented centers that often appear in an asymmetric distribution on the arms and legs.2,3 Histologic features include dermal tuberculoid granulomas with epithelioid cells—some located directly beneath the epidermis and others around deep vessels and nerves3—multinucleated Langerhans giant cells, thickened peripheral nerves with intraneural lymphocytic infiltrates, and granulomas with central necrosis. Fite-Faraco staining exhibits few bacteria.2
Lepromatous leprosy occurs in individuals with impaired T-cell immunity, leading to multiple red-brown nodular infiltrates in the skin and mucous membranes.2,3 Lesions typically are symmetric and favor the face and auricle of the ear.2,3 Histologically, there are bluish-gray foamy macrophages that form diffuse or nodular infiltrates with few lymphocytes,2 with a Grenz zone between the epidermis and dermis. Nerves may show lamination of the perineurium resembling an onion skin.2,3 Immunohistochemistry shows predominant CD8-positive infiltrates with a Th2 response and positive IL-4 and IL-10. Fite-Faraco stain shows numerous mycobacteria arranged in clusters and in histiocytes.2
Tuberculoid leprosy is treated with dapsone 100 mg and rifampin 600 mg once daily for 6 months,4 and lepromatous leprosy is treated with dapsone 100 mg, rifampin 600 mg, and clofazimine 50 mg once daily for 12 months.4 The prognosis for both is good with treatment; erythema and induration of skin lesions may improve within a few months, but residual nerve damage is common, especially in those with advanced disease prior to treatment.2 For direct contacts, a single dose of rifampin may be given.4
Borderline-borderline leprosy manifests with numerous asymmetric annular plaques, as seen in our patient (Figure 3). Histology findings can be variable and often overlap with other forms of leprosy. There can be epithelioid granulomas and only a few acid-fast bacilli (AFB) or diffuse histiocytic aggregates with foamy histiocytes containing large numbers of AFB.3 Nerve involvement is variable but can be severe in the setting of type 1 lepra reaction, which was present in our patient. Type 1 lepra reaction—a type IV cell-mediated allergic hypersensitivity reaction to Mycobacterium leprae antigens—manifests clinically with hyperesthesia, erythema, edema, and subsequent scaling.2 It occurs in up to 30% of patients with borderline leprosy, usually within 12 months of treatment initiation.2 Our patient had considerable edema and erythema of the hands and feet (Figure 4) along with extensive polyneuropathy prior to starting therapy.


Lucio phenomenon is a rare leprosy reaction found in patients with untreated lepromatous leprosy characterized by erythematous to violaceous macules that lead to ulceronecrotic lesions.5 Histologically, there are many AFB in the vascular endothelium, leukocytoclastic vasculitis, and ischemic epidermal necrosis.5 Our patient did not have ulcerative or necrotic lesions.
The classic skin lesions of psoriasis vulgaris can be described as well-demarcated pink plaques with white or silvery scales that usually are distributed symmetrically and often are found on extensor surfaces.6 Rapidly progressive lesions can be annular with normal skin in the center, mimicking the lesions seen in tuberculoid leprosy. Clinically, both psoriasis and tuberculoid forms of leprosy are sharply demarcated; however, psoriatic lesions often have micaceous overlying scale that is not present in leprosy. Characteristic histologic findings of psoriasis are hyperkeratosis, parakeratosis, and acanthosis of the epidermis with dilated blood vessels and a lymphocytic infiltrate, predominantly into the dermis.7 Psoriatic arthritis has a variable clinical course but tends to emerge 5 to 12 years after initial skin manifestation.8 Classic clinical symptoms include swelling, tenderness, stiffness, and pain in joints and surrounding tissues.8 Other than edema, our patient did not exhibit signs of psoriatic arthritis.
Sarcoidosis is a systemic autoimmune disease characterized by noncaseating epithelioid granulomas affecting various organs, with cutaneous manifestations present in approximately 30% of all cases. Cutaneous manifestations can be variable, including maculopapular lesions, plaques, and nodules.9 Differentiating between cutaneous sarcoidosis and tuberculoid leprosy can be challenging, as both are granulomatous processes; however, histology of sarcoidosis demonstrates noncaseating granulomas in the dermis and/or subcutaneous tissues without AFB9 compared to granulomas with necrotic centers in tuberculoid leprosy.
Cutaneous tuberculosis has variable morphologies. One subtype, lupus vulgaris, can manifest with violaceous, scaly, eroded plaques that could be confused for leprosy. Lupus vulgaris usually results from hematogenous or lymphatic seeding in individuals with high or moderate immunity to M tuberculosis.10
Histologically, the dermis has tuberculoid granulomas containing multinucleated giant cells,10 which can mimic those seen in BB leprosy. Tuberculin skin test results often are positive10; while this test was not performed in our patient, chest radiography was unremarkable, making this diagnosis less likely.
Mycobacterium leprae infections should be considered in a patient with a worsening rash and progressive polyneuropathy. Clinical diagnosis can be challenging due to similarities with other diseases; however, histopathologic findings can help differentiate M leprae from other conditions. This infection is treatable, and early detection can minimize long-term patient morbidity.
- CDC. Hansen’s disease (leprosy). Accessed April 23, 2025. https://www.cdc.gov/leprosy/about/index.html
- Fischer M. Leprosy—an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827.
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020; 83:1-14.
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. October 6, 2018. Accessed April 2, 2025. https://www.who.int/publications/i/item/9789290226383
- Frade MAC, Coltro PS, Filho FB, et al. Lucio’s phenomenon: a systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464-477.
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Evidence-Based Psoriasis. Springer International Publishing; 2018:1-16.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 suppl):S216-S224.
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514.
- Hill MK, Sanders CV. Cutaneous tuberculosis. Microbiol Spectr. 2017;5:1-6.
- CDC. Hansen’s disease (leprosy). Accessed April 23, 2025. https://www.cdc.gov/leprosy/about/index.html
- Fischer M. Leprosy—an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827.
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020; 83:1-14.
- World Health Organization. Guidelines for the diagnosis, treatment and prevention of leprosy. October 6, 2018. Accessed April 2, 2025. https://www.who.int/publications/i/item/9789290226383
- Frade MAC, Coltro PS, Filho FB, et al. Lucio’s phenomenon: a systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464-477.
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Evidence-Based Psoriasis. Springer International Publishing; 2018:1-16.
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271.
- Menter A. Psoriasis and psoriatic arthritis overview. Am J Manag Care. 2016;22(8 suppl):S216-S224.
- Wu JH, Imadojemu S, Caplan AS. The evolving landscape of cutaneous sarcoidosis: pathogenic insight, clinical challenges, and new frontiers in therapy. Am J Clin Dermatol. 2022;23:499-514.
- Hill MK, Sanders CV. Cutaneous tuberculosis. Microbiol Spectr. 2017;5:1-6.
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
Acral Erythema, Edema, and Scaly Plaques in a Patient With Polyneuropathy
A 67-year-old man presented to his primary care physician with scaly plaques on the extensor surfaces along with distal neuropathy that had been slowly worsening over the past 6 months. The patient was prescribed triamcinolone cream 0.1% twice daily for presumed atopic dermatitis. Three months later, his symptoms rapidly worsened and he developed edema of the hands and feet. He was seen by neurology, and electromyography revealed severe distal sensorimotor neuropathy, prompting hospital admission for further evaluation of a potential rapidly progressive autoimmune disease. Laboratory workup and imaging were ordered, and the patient began an intravenous course of methylprednisolone. Minimal improvement in his symptoms was noted after 1 day, at which time dermatology was consulted.
Physical examination by dermatology revealed well-defined plaques with annular scale on extensor surfaces of the arms and legs, and edema on the hands and feet as well as distal sensorimotor neuropathy. The patient reported associated unspecified weight loss but denied any chest pain, shortness of breath, fevers, chills, cough, night sweats, exposure to chemicals, or recent travel. He reported that he had immigrated from India 37 years prior; his last visit to India was 6 years ago. He currently was taking famotidine for gastrointestinal reflux disease and losartan for hypertension. There was no personal or family history of autoimmune diseases. A complete workup for hematologic, thyroid, liver, and renal function was unremarkable. Initial autoimmune workup was negative for antinuclear antibodies and rheumatoid factor. Serum protein electrophoresis was normal. Results of testing for HIV, hepatitis B, and hepatitis C were negative. Chest radiography was unremarkable. Erythrocyte sedimentation rate and C-reactive protein level were elevated.
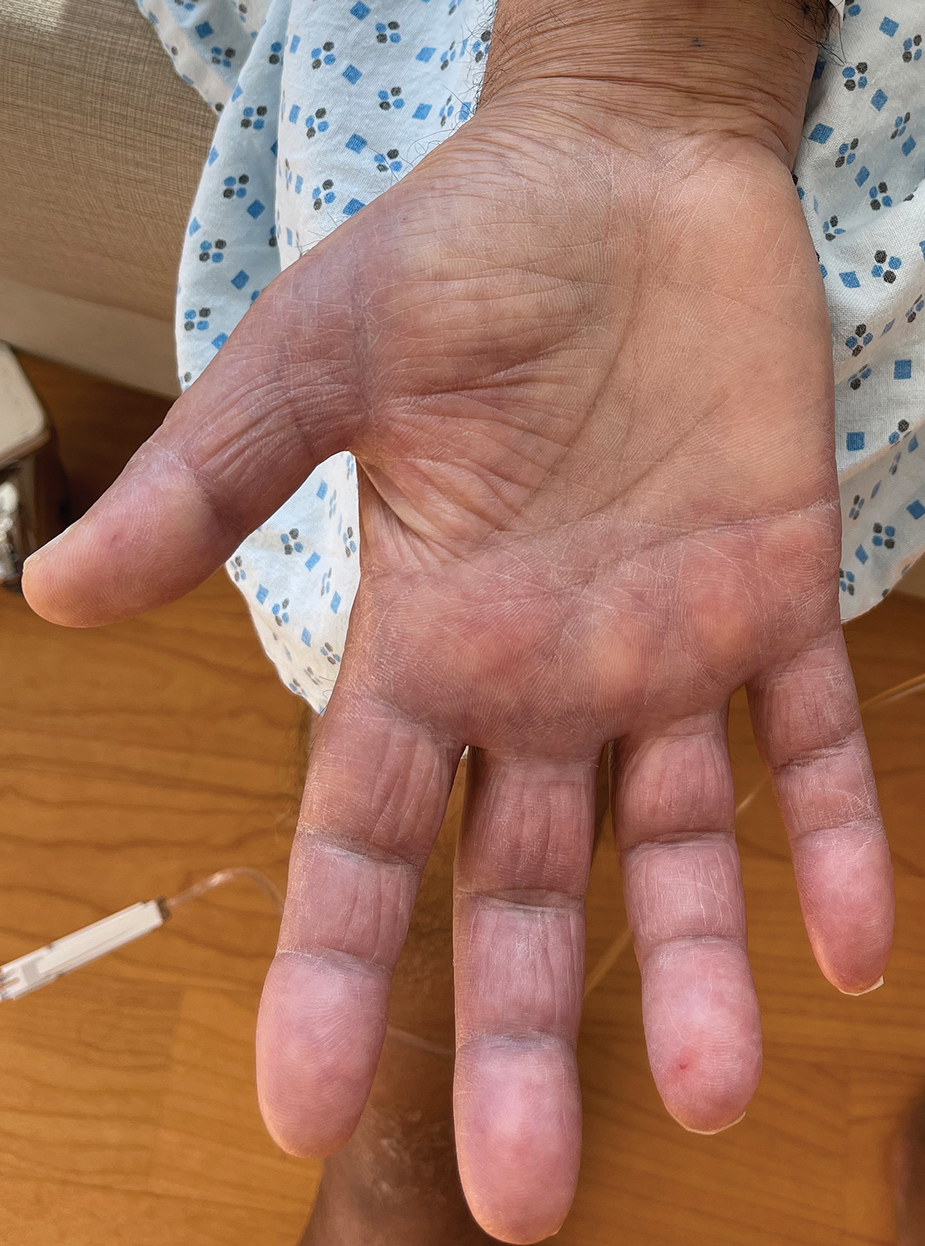
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
To the Editor:
Psoriasis is a chronic inflammatory condition that affects individuals in various extracutaneous ways.1 Prior studies have documented a decrease in exercise intensity among patients with psoriasis2; however, few studies have specifically investigated baseline mobility in this population. Baseline mobility denotes an individual’s fundamental ability to walk or move around without assistance of any kind. Impaired mobility—when baseline mobility is compromised—is an aspect of the wider diversity, equity, and inclusion framework that underscores the significance of recognizing challenges and promoting inclusive measures, both at the point of care and in research.3 study sought to analyze the relationship between psoriasis and baseline mobility among US adults (aged 45 to 80 years) utilizing the latest data from the National Health and Nutrition Examination Survey (NHANES) database for psoriasis.4 We used three 2-year cycles of NHANES data to create a 2009-2014 dataset.
The overall NHANES response rate among adults aged 45 to 80 years between 2009 and 2014 was 67.9%. Patients were categorized as having impaired mobility if they responded “yes” to the following question: “Because of a health problem, do you have difficulty walking without using any special equipment?” Psoriasis status was assessed by the following question: “Have you ever been told by a doctor or other health professional that you had psoriasis?” Multivariable logistic regression analyses were performed using Stata/SE 18.0 software (StataCorp LLC) to assess the relationship between psoriasis and impaired mobility. Age, income, education, sex, race, tobacco use, diabetes status, body mass index, and arthritis status were controlled for in our models.
Our analysis initially included 9982 participants; 14 did not respond to questions assessing psoriasis and impaired mobility and were excluded. The prevalence of impaired mobility in patients with psoriasis was 17.1% compared with 10.9% among those without psoriasis (Table 1). There was a significant association between psoriasis and impaired mobility among patients aged 45 to 80 years after adjusting for potential confounding variables (adjusted odds ratio [AOR], 1.54; 95% CI, 1.04- 2.29; P=.032)(Table 2). Analyses of subgroups yielded no statistically significant results.

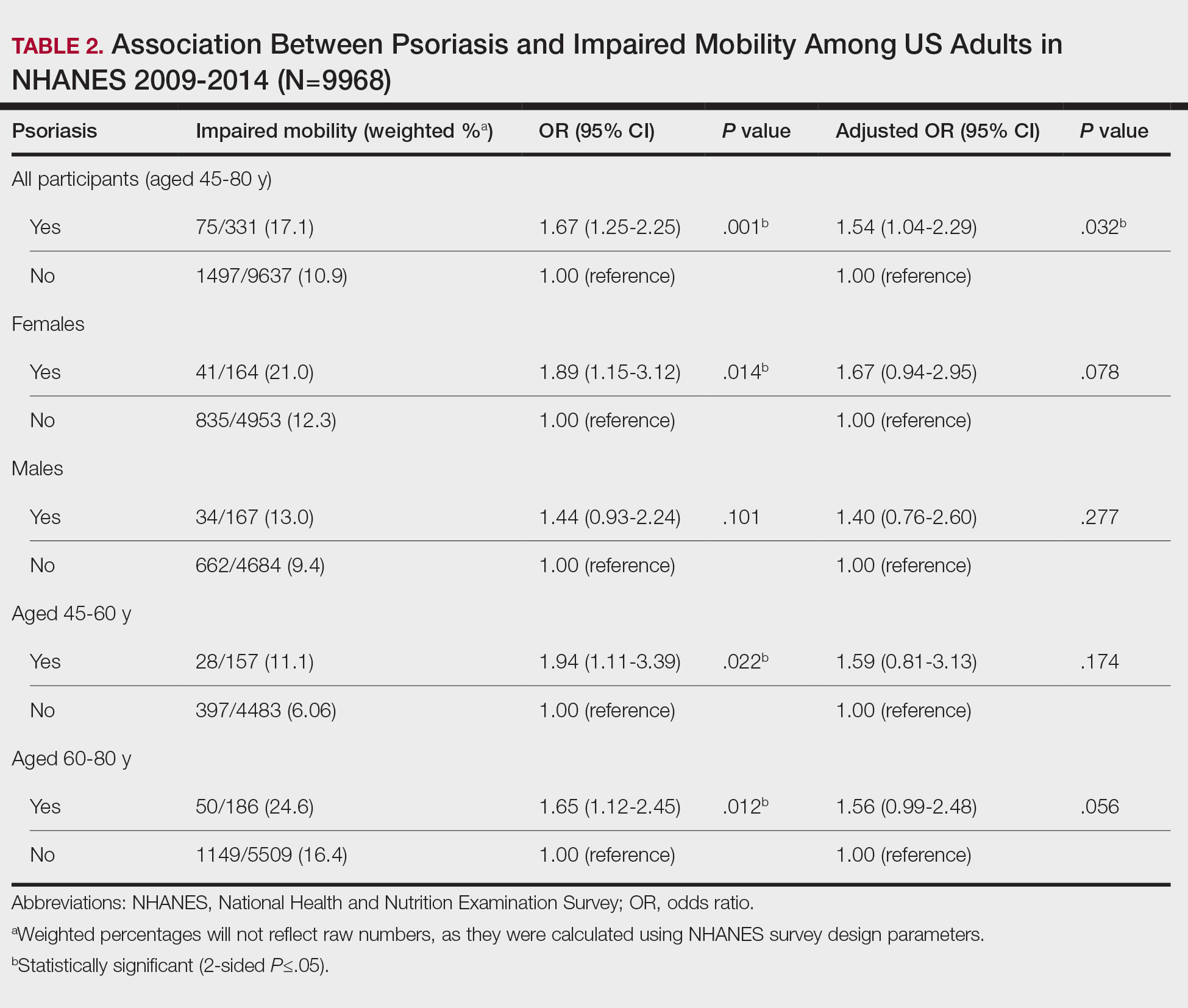
Our study demonstrated a statistically significant difference in mobility between individuals with psoriasis compared with the general population, which remained significant when controlling for arthritis, obesity, and diabetes (P=.032). This may be the result of several influences. First, the location of the psoriasis may impact mobility. Plantar psoriasis—a manifestation on the soles of the feet—can cause discomfort and pain, which can hinder walking and standing.5 Second, a study by Lasselin et al6 found that systemic inflammation contributes to mobility impairment through alterations in gait and posture, which suggests that the inflammatory processes inherent in psoriasis could intrinsically modify walking speed and stride, potentially exacerbating mobility difficulties independent of other comorbid conditions. These findings suggest that psoriasis may disproportionately affect individuals with impaired mobility, independent of comorbid arthritis, obesity, and diabetes.
These findings have broad implications for diversity, equity, and inclusion. They should prompt us to consider the practical challenges faced by this patient population and the ways that we can address barriers to care. Offering telehealth appointments, making primary care referrals for impaired mobility workups, and advising patients of direct-to-home delivery of prescriptions are good places to start.
Limitations to our study include the lack of specificity in the survey question, self-reporting bias, and the inability to control for the psoriasis location. Further investigations are warranted in large, representative US adult populations to assess the implications of impaired mobility in patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi: 10.1016/j.jaad.2018.11.058
- Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: A MOOSE-compliant meta-analysis. Medicine (Baltimore). 2018;97:e11394. doi: 10.1097 /MD.0000000000011394
- Mullin AE, Coe IR, Gooden EA, et al. Inclusion, diversity, equity, and accessibility: from organizational responsibility to leadership competency. Healthc Manage Forum. 2021;34311-315. doi: 10.1177/08404704211038232
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed October 21, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Romani M, Biela G, Farr K, et al. Plantar psoriasis: a review of the literature. Clin Podiatr Med Surg. 2021;38:541-552. doi: 10.1016 /j.cpm.2021.06.009
- Lasselin J, Sundelin T, Wayne PM, et al. Biological motion during inflammation in humans. Brain Behav Immun. 2020;84:147-153. doi: 10.1016/j.bbi.2019.11.019
To the Editor:
Psoriasis is a chronic inflammatory condition that affects individuals in various extracutaneous ways.1 Prior studies have documented a decrease in exercise intensity among patients with psoriasis2; however, few studies have specifically investigated baseline mobility in this population. Baseline mobility denotes an individual’s fundamental ability to walk or move around without assistance of any kind. Impaired mobility—when baseline mobility is compromised—is an aspect of the wider diversity, equity, and inclusion framework that underscores the significance of recognizing challenges and promoting inclusive measures, both at the point of care and in research.3 study sought to analyze the relationship between psoriasis and baseline mobility among US adults (aged 45 to 80 years) utilizing the latest data from the National Health and Nutrition Examination Survey (NHANES) database for psoriasis.4 We used three 2-year cycles of NHANES data to create a 2009-2014 dataset.
The overall NHANES response rate among adults aged 45 to 80 years between 2009 and 2014 was 67.9%. Patients were categorized as having impaired mobility if they responded “yes” to the following question: “Because of a health problem, do you have difficulty walking without using any special equipment?” Psoriasis status was assessed by the following question: “Have you ever been told by a doctor or other health professional that you had psoriasis?” Multivariable logistic regression analyses were performed using Stata/SE 18.0 software (StataCorp LLC) to assess the relationship between psoriasis and impaired mobility. Age, income, education, sex, race, tobacco use, diabetes status, body mass index, and arthritis status were controlled for in our models.
Our analysis initially included 9982 participants; 14 did not respond to questions assessing psoriasis and impaired mobility and were excluded. The prevalence of impaired mobility in patients with psoriasis was 17.1% compared with 10.9% among those without psoriasis (Table 1). There was a significant association between psoriasis and impaired mobility among patients aged 45 to 80 years after adjusting for potential confounding variables (adjusted odds ratio [AOR], 1.54; 95% CI, 1.04- 2.29; P=.032)(Table 2). Analyses of subgroups yielded no statistically significant results.



Our study demonstrated a statistically significant difference in mobility between individuals with psoriasis compared with the general population, which remained significant when controlling for arthritis, obesity, and diabetes (P=.032). This may be the result of several influences. First, the location of the psoriasis may impact mobility. Plantar psoriasis—a manifestation on the soles of the feet—can cause discomfort and pain, which can hinder walking and standing.5 Second, a study by Lasselin et al6 found that systemic inflammation contributes to mobility impairment through alterations in gait and posture, which suggests that the inflammatory processes inherent in psoriasis could intrinsically modify walking speed and stride, potentially exacerbating mobility difficulties independent of other comorbid conditions. These findings suggest that psoriasis may disproportionately affect individuals with impaired mobility, independent of comorbid arthritis, obesity, and diabetes.
These findings have broad implications for diversity, equity, and inclusion. They should prompt us to consider the practical challenges faced by this patient population and the ways that we can address barriers to care. Offering telehealth appointments, making primary care referrals for impaired mobility workups, and advising patients of direct-to-home delivery of prescriptions are good places to start.
Limitations to our study include the lack of specificity in the survey question, self-reporting bias, and the inability to control for the psoriasis location. Further investigations are warranted in large, representative US adult populations to assess the implications of impaired mobility in patients with psoriasis.
To the Editor:
Psoriasis is a chronic inflammatory condition that affects individuals in various extracutaneous ways.1 Prior studies have documented a decrease in exercise intensity among patients with psoriasis2; however, few studies have specifically investigated baseline mobility in this population. Baseline mobility denotes an individual’s fundamental ability to walk or move around without assistance of any kind. Impaired mobility—when baseline mobility is compromised—is an aspect of the wider diversity, equity, and inclusion framework that underscores the significance of recognizing challenges and promoting inclusive measures, both at the point of care and in research.3 study sought to analyze the relationship between psoriasis and baseline mobility among US adults (aged 45 to 80 years) utilizing the latest data from the National Health and Nutrition Examination Survey (NHANES) database for psoriasis.4 We used three 2-year cycles of NHANES data to create a 2009-2014 dataset.
The overall NHANES response rate among adults aged 45 to 80 years between 2009 and 2014 was 67.9%. Patients were categorized as having impaired mobility if they responded “yes” to the following question: “Because of a health problem, do you have difficulty walking without using any special equipment?” Psoriasis status was assessed by the following question: “Have you ever been told by a doctor or other health professional that you had psoriasis?” Multivariable logistic regression analyses were performed using Stata/SE 18.0 software (StataCorp LLC) to assess the relationship between psoriasis and impaired mobility. Age, income, education, sex, race, tobacco use, diabetes status, body mass index, and arthritis status were controlled for in our models.
Our analysis initially included 9982 participants; 14 did not respond to questions assessing psoriasis and impaired mobility and were excluded. The prevalence of impaired mobility in patients with psoriasis was 17.1% compared with 10.9% among those without psoriasis (Table 1). There was a significant association between psoriasis and impaired mobility among patients aged 45 to 80 years after adjusting for potential confounding variables (adjusted odds ratio [AOR], 1.54; 95% CI, 1.04- 2.29; P=.032)(Table 2). Analyses of subgroups yielded no statistically significant results.



Our study demonstrated a statistically significant difference in mobility between individuals with psoriasis compared with the general population, which remained significant when controlling for arthritis, obesity, and diabetes (P=.032). This may be the result of several influences. First, the location of the psoriasis may impact mobility. Plantar psoriasis—a manifestation on the soles of the feet—can cause discomfort and pain, which can hinder walking and standing.5 Second, a study by Lasselin et al6 found that systemic inflammation contributes to mobility impairment through alterations in gait and posture, which suggests that the inflammatory processes inherent in psoriasis could intrinsically modify walking speed and stride, potentially exacerbating mobility difficulties independent of other comorbid conditions. These findings suggest that psoriasis may disproportionately affect individuals with impaired mobility, independent of comorbid arthritis, obesity, and diabetes.
These findings have broad implications for diversity, equity, and inclusion. They should prompt us to consider the practical challenges faced by this patient population and the ways that we can address barriers to care. Offering telehealth appointments, making primary care referrals for impaired mobility workups, and advising patients of direct-to-home delivery of prescriptions are good places to start.
Limitations to our study include the lack of specificity in the survey question, self-reporting bias, and the inability to control for the psoriasis location. Further investigations are warranted in large, representative US adult populations to assess the implications of impaired mobility in patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi: 10.1016/j.jaad.2018.11.058
- Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: A MOOSE-compliant meta-analysis. Medicine (Baltimore). 2018;97:e11394. doi: 10.1097 /MD.0000000000011394
- Mullin AE, Coe IR, Gooden EA, et al. Inclusion, diversity, equity, and accessibility: from organizational responsibility to leadership competency. Healthc Manage Forum. 2021;34311-315. doi: 10.1177/08404704211038232
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed October 21, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Romani M, Biela G, Farr K, et al. Plantar psoriasis: a review of the literature. Clin Podiatr Med Surg. 2021;38:541-552. doi: 10.1016 /j.cpm.2021.06.009
- Lasselin J, Sundelin T, Wayne PM, et al. Biological motion during inflammation in humans. Brain Behav Immun. 2020;84:147-153. doi: 10.1016/j.bbi.2019.11.019
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi: 10.1016/j.jaad.2018.11.058
- Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: A MOOSE-compliant meta-analysis. Medicine (Baltimore). 2018;97:e11394. doi: 10.1097 /MD.0000000000011394
- Mullin AE, Coe IR, Gooden EA, et al. Inclusion, diversity, equity, and accessibility: from organizational responsibility to leadership competency. Healthc Manage Forum. 2021;34311-315. doi: 10.1177/08404704211038232
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. NHANES questionnaires, datasets, and related documentation. Accessed October 21, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Romani M, Biela G, Farr K, et al. Plantar psoriasis: a review of the literature. Clin Podiatr Med Surg. 2021;38:541-552. doi: 10.1016 /j.cpm.2021.06.009
- Lasselin J, Sundelin T, Wayne PM, et al. Biological motion during inflammation in humans. Brain Behav Immun. 2020;84:147-153. doi: 10.1016/j.bbi.2019.11.019
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
Exploring the Relationship Between Psoriasis and Mobility Among US Adults
PRACTICE POINTS
- Mobility issues are more common in patients who have psoriasis than in those who do not.
- It is important to assess patients with psoriasis for mobility issues regardless of age or comorbid conditions such as arthritis, obesity, and diabetes.
- Dermatologists can help patients with psoriasis and impaired mobility overcome potential barriers to care by incorporating telehealth services into their practices and informing patients of direct-to-home delivery of prescriptions.
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.
Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.

Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
To the Editor:
Cutaneous medical conditions can have a substantial impact on patients’ functioning and quality of life. Many patients with severe skin disease are eligible to receive disability assistance that can provide them with essential income and health care. Previous research has highlighted disability assessment as one of the most important ways physicians can help mitigate the health consequences of poverty.1 Dermatologists can play an important role in the disability assessment process by documenting the facts associated with patients’ skin conditions.
Although skin conditions have a relatively high prevalence, they remain underrepresented in disability claims. Between 1997 and 2004, occupational skin diseases accounted for 12% to 17% of nonfatal work-related illnesses; however, during that same period, skin conditions comprised only 0.21% of disability claims in the United States.2,3 Historically, there has been hesitancy among dermatologists to complete disability paperwork; a 1976 survey of dermatologists cited extensive paperwork, “troublesome patients,” and fee schedule issues as reasons.4 The lack of training regarding disability assessment in medical school and residency also has been noted.5
To characterize modern attitudes toward disability assessments, we conducted a survey of dermatologists across the United States. Our study was reviewed and declared exempt by the institutional review board of the Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (Torrance, California)(approval #18CR-32242-01). Using convenience sampling, we emailed dermatologists from the Association of Professors of Dermatology and dermatology state societies in all 50 states inviting them to participate in our voluntary and anonymous survey, which was administered using SurveyMonkey. The use of all society mailing lists was approved by the respective owners. The 15-question survey included multiple choice, Likert scale, and free response sections. Summary and descriptive statistics were used to describe respondent demographics and identify any patterns in responses.
For each Likert-based question, participants ranked their degree of agreement with a statement as: 1=strongly disagree, 2=somewhat disagree, 3=neither agree nor disagree/neutral, 4=somewhat agree, and 5=strongly agree. The mean response and standard deviation were reported for each Likert scale prompt. Preplanned 1-sample t testing was used to analyze Likert scale data, in which the mean response for each prompt was compared to a baseline response of 3 (neutral). A P value <.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics for MacOS, version 27 (IBM).
Seventy-eight dermatologists agreed to participate, and 70 completed the survey, for a response rate of 89.7% (Table 1). The dermatologists we surveyed practiced in a variety of clinical settings, including academic public hospitals (46.2% [36/78]), academic private hospitals (33.3% [26/78]), and private practices (32.1% [25/78]), and 60.3% (47/78) reported providing disability documentation at some point. Most of the respondents (64.3% [45/70]) did not perform assessments in an average month (Table 2). Medical assessment documentation was provided most frequently for workers’ compensation (50.0% [35/70]), private insurance (27.1% [19/70]), and Social Security Disability Insurance (25.7% [18/70]). Dermatologists overwhelmingly reported no formal training for disability assessment in medical school (94.3% [66/70]), residency (97.1% [68/70]), or clinical practice (81.4% [57/70]).


In the Likert scale prompts, respondents agreed that they were uncertain of their role in disability assessment (mean response, 3.6; P<.001). Moreover, they were uncomfortable providing assessments (mean response, 3.5; P<.001) and felt that they did not have sufficient time to perform them (mean response, 3.6; P<.001). Dermatologists disagreed that they received adequate compensation for performing assessments (mean response, 2.2; P<.001) and felt that they did not have enough time to participate in assessments (mean response, 3.6; P<.001). Respondents generally did not feel distrustful of patients seeking disability assessment (mean response, 2.8; P=.043). Dermatologists neither agreed nor disagreed when asked if they thought that physicians can determine disability status (mean response, 3.2; P=.118). The details of the Likert scale responses are described in Table 3. Respondents also were uncertain as to which dermatologic conditions were eligible for disability. When asked to select which conditions from a list of 10 were eligible per the Social Security Administration listing of disability impairments, only 15.4% (12/70) of respondents correctly identified that all the conditions qualified; these included ichthyosis, pemphigus vulgaris, allergic contact dermatitis, hidradenitis suppurativa, systemic lupus erythematosus, chromoblastomycosis, xeroderma pigmentosum, burns, malignant melanoma, and scleroderma.6

In the free-response prompts, respondents frequently described extensive paperwork, inadequate time, and lack of reimbursement as barriers to providing documentation. Often, dermatologists found that the forms were not well matched to the skin conditions they were evaluating and rather had a musculoskeletal focus. Multiple individuals commented on the challenge in assessing the percentage of disability and functional/psychosocial impairment in skin conditions. One respondent noted that workers’ compensation forms ask if the patient is “…permanent and stationary…for most conditions this has no meaning in dermatology.” Some felt hesitant to provide documentation because they had insufficient patient history, especially regarding employment, and opted to defer to primary care providers who might be more familiar with the full patient history.
A dermatologist described their perspective as follows:
“…As a specialist I feel that I don’t have a complete look into all the factors that could contribute to a patient[’]s need to go on disability, and I don’t have experience with filling out disability requests. That being said, if a patient[’]s request for disability was due to a skin disease that I know way more about than [a] primary care [physician] would, I would do the disability assessment.”
Another respondent noted the complexity in “establishing causality” for workers’ compensation. Another dermatologist reported,
“The most frequent challenging situation I encounter is being asked to evaluate for maximum medical improvement after patch testing. If the patient is not fully avoiding contact allergens either at home or at work, then I typically document that they are not at [maximum medical improvement]. The reality is that most frequently it is due to exposure to allergens at home so the line between what is a legitimate worker’s comp[ensation] issue and what is a home life choice is blurry.”
Nevertheless, respondents expressed interest in learning more about disability assessment procedures. Summary guides, lectures, and prefilled paperwork were the most popular initiatives that respondents agreed would be beneficial toward becoming educated regarding disability assessment (78.6%, 58.6%, and 58.6%, respectively)(Table 2). One respondent noted that “previous [internal medicine] history help[ed]” them in performing cutaneous disability assessments.
As with any survey, our study did have some inherent limitations. Only a relatively small sample size was willing to complete the survey. There was a predominance of respondents from California (34.6% [27/78]), as well as those practicing for less than 15 years (58.9% [46/78])(Figure). This could limit generalizability to the national population of dermatologists. In addition, there was potential for recall bias and errors in responding given the self-reported nature of the study. Different individuals may interpret the Likert scale options in various ways, which could skew results unintentionally. However, the survey was largely qualitative in nature, making it a legitimate tool for answering our research questions. Moreover, we were able to hear the perspectives of dermatologists across diverse practice settings, with free response prompts to increase the depth of the survey.

Almost 50 years later, our survey echoes common themes from Adams’ 1976 survey.4 Inadequate compensation, limited time, and burdensome paperwork all continue to hinder dermatologists’ ability to perform disability assessments. Our participants frequently commented that the current disability forms are not congruent with the nature of skin conditions, making it challenging to accurately document the facts.
Moreover, respondents felt uncertain in their role in disability assessment and occasionally noted distrust of patients or insufficient patient history as barriers to completing assessments. They also were unsure if physicians can grant disability status. This is a common misconception among physicians that leads to discomfort in helping with disability assessment.7 The role of physicians in disability assessment is to document the facts of a patient’s illness, not to determine whether they are eligible for benefits. We discovered uncertainty in our respondents’ ability to identify conditions eligible for disability, highlighting an area in need of greater education for physicians.
Despite these obstacles, respondents were interested in learning more about disability assessment and highlighted several practical approaches that could help them better perform this task. As skin specialists, dermatologists are the best-equipped physicians to assess cutaneous conditions and should play a greater role in performing disability assessments, which could be achieved through increased educational initiatives and individual physician motivation.7 We call for greater collaboration and reflection on the importance of disability assistance among dermatologists to increase participation in the disability-assessment process.
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
- O’Connell JJ, Zevin BD, Quick PD, et al. Documenting disability: simple strategies for medical providers. Health Care for the Homeless Clinicians’ Network. September 2007. Accessed March 31, 2025. https://nhchc.org/wp-content/uploads/2019/08/DocumentingDisability2007.pdf
- US Bureau of Labor Statistics. Injuries, illnesses, and fatalities. Accessed March 31, 2025. https://www.bls.gov/iif/
- Meseguer J. Outcome variation in the Social Security Disability Insurance Program: the role of primary diagnoses. Soc Secur Bull. 2013;73:39-75.
- Adams RM. Attitudes of California dermatologists toward Worker’s Compensation: results of a survey. West J Med. 1976;125:169-175.
- Talmage J, Melhorn J, Hyman M. AMA Guides to the Evaluation of Work Ability and Return to Work. 2nd ed. American Medical Association; 2011.
- Social Security Administration. Disability evaluation under Social Security. 8.00 skin disorders - adult. March 31, 2025. https://www.ssa.gov/disability/professionals/bluebook/8.00-Skin-Adult.htm
- Dawson J, Smogorzewski J. Demystifying disability assessments for dermatologists—a call to action. JAMA Dermatol. 2021;157:903-904. doi:10.1001/jamadermatol.2021.1767
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
Dermatologists’ Perspectives Toward Disability Assessment: A Nationwide Survey Report
PRACTICE POINTS
- As experts in skin conditions, dermatologists are most qualified to assist with disability assessment for dermatologic concerns.
- There are several barriers to dermatologists participating in the disability assessment process, including lack of time, compensation, and education on the subject.
- Many dermatologists may be interested in learning more about disability assessment, and education could be provided in the form of summary guides, lectures, and prefilled paperwork.