User login
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
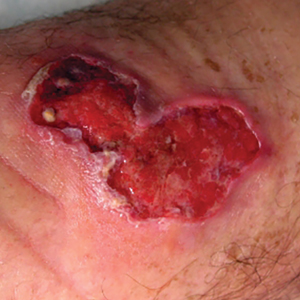
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1 Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
PRACTICE POINTS
- Buruli ulcer (BU) is a necrotizing cutaneous disease caused by Mycobacterium ulcerans with possible transmission from aquatic insects and mosquitoes.
- Buruli ulcer often manifests in children as painless induration that gradually progresses to painless or mildly painful irregular skin ulceration.
- Treatment options for BU include rifampin and streptomycin, but larger lesions may require surgical debridement.
- No vaccine currently exists for M ulcerans, but clinical trials targeting mycolyl transferase are underway.