User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Scurvy in Hospitalized Patients
Scurvy in Hospitalized Patients
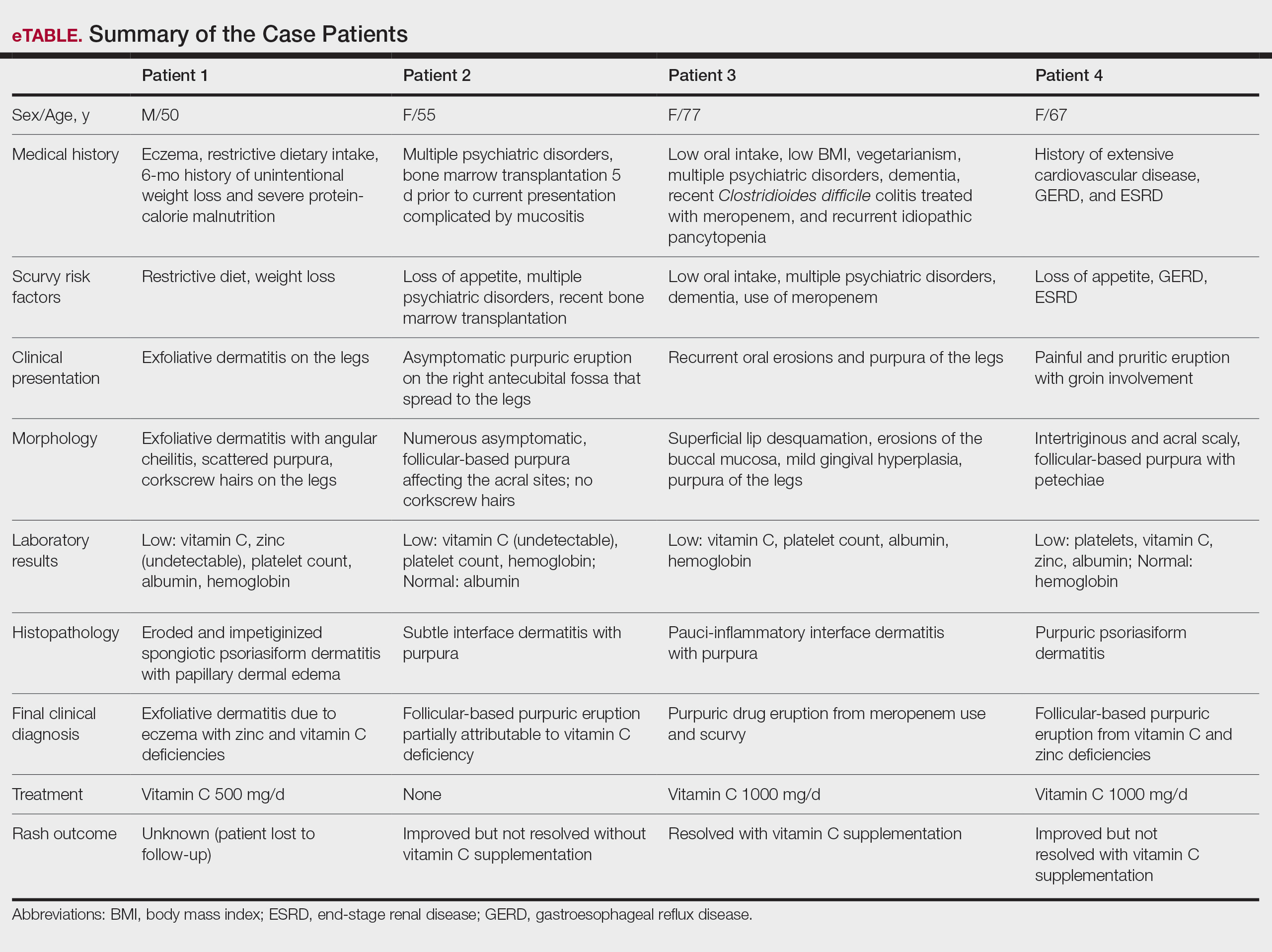
Scurvy, caused by vitamin C or ascorbic acid deficiency, historically has been associated primarily with developing nations and famine; however, specific populations in industrialized nations remain at an increased risk, particularly individuals with a history of smoking, alcohol use, restrictive diet, poor oral intake, psychiatric disorders, dementia, bone marrow transplantation, gastroesophageal reflux disease, end-stage renal disease, and hospitalization.1 Micronutrient deficiency– associated dermatoses have been linked to poor clinical outcomes in hospitalized patients.2 In this case series, we report 4 hospitalized patients with scurvy, each presenting with unique comorbidities and risk factors for vitamin C deficiency (eTable).
Case Reports
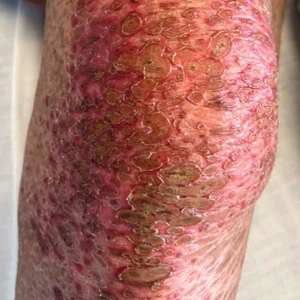
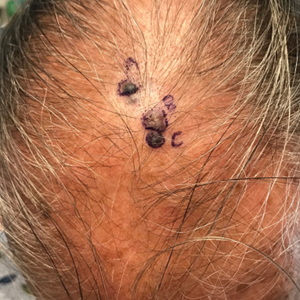
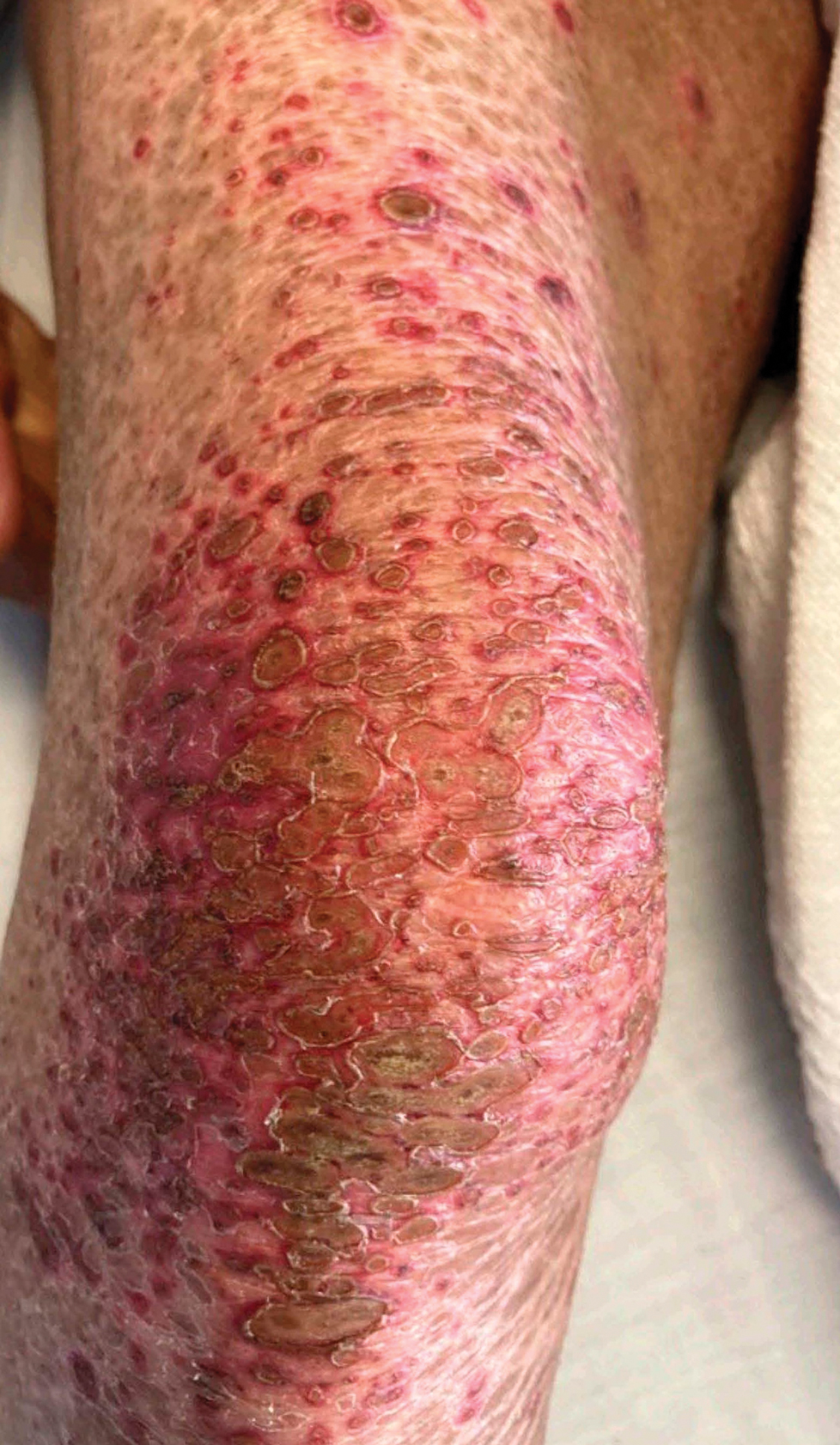
Patient 1—A 50-year-old man with a 6-month history of eczema and restrictive dietary intake was admitted to the hospital for septic shock attributed to a left foot infection of 5-days’ duration. The patient had experienced unintentional weight loss with severe protein-calorie malnutrition. His dietary history was notable for selective eating behaviors, intermittent meal skipping, and vegetarianism. Mucocutaneous examination by the dermatology consult team showed exfoliative dermatitis with angular cheilitis, corkscrew hairs on the legs (eFigure 1), and scattered purpura throughout the body. The differential diagnosis included eczema exacerbation, cutaneous T-cell lymphoma/Sézary syndrome, and malnutrition-related dermatosis. Punch biopsies of the left medial knee and right lateral arm revealed impetiginized, spongiotic, psoriasiform dermatitis with papillary dermal edema. The histologic changes were consistent with malnutrition-related dermatosis. Laboratory results included low vitamin C levels (0.1 mg/dL [reference range, 0.2-2.1 mg/dL]), undetectable zinc levels (<10 μg/dL [reference range ,60-130 μg/dL]), a low platelet count (21 kμ/L [reference range, 150-400 k/μL]),low albumin levels (0.9 mg/dL (13.0 g/dL [reference range, 14.0-17.4 g/dL]). The final diagnosis was exfoliative dermatitis due to eczema and multiple nutrient deficiencies (vitamin C and zinc). The patient was treated with vitamin C 500 mg/d and was started on mirtazapine to improve his appetite. Following a 3-month hospitalization, the patient was lost to follow-up after discharge.
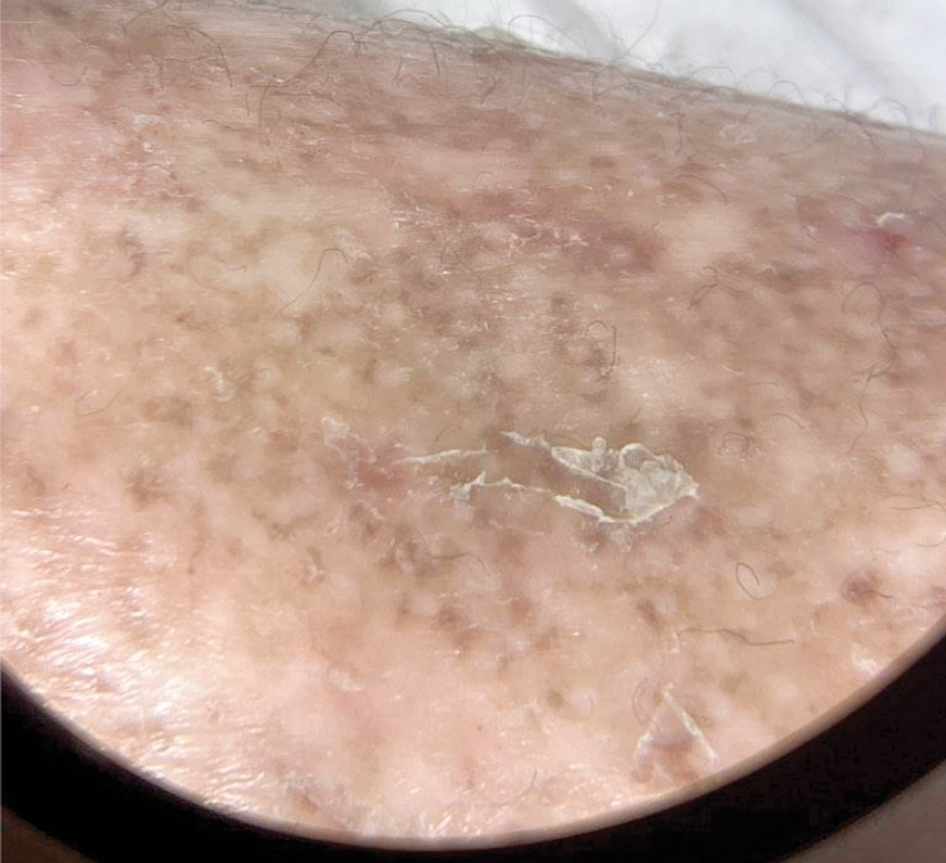
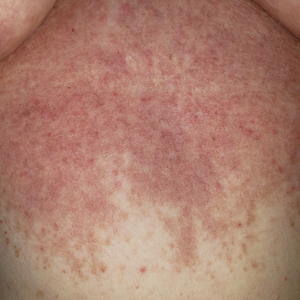
Patient 2—A 55-year-old woman with a history of multiple psychiatric disorders presented to the dermatology consult service with an asymptomatic purpuric eruption on the right antecubital fossa of 2 days’ duration that spread to the proximal thighs. Five days prior to presentation, she had received an allogeneic bone marrow transplant complicated by mucositis. She also reported a 4-month history of decreased appetite. At the current presentation, numerous acral, follicular based, purpuric macules and papules without associated corkscrew hairs were observed (eFigure 2). The differential diagnosis included a purpuric drug reaction, viral exanthem, acute graft-vs-host disease, neutrophilic dermatoses, and vitamin C deficiency–related dermatosis. Laboratory results revealed undetectable vitamin C levels (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (8 k/μL [reference range, 150-400 k/μL]), normal albumin levels (3.7 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (7.8 g/dL [reference range, 14.0-17.4 g/dL]). Based on the histopathologic finding of subtle interface dermatitis with purpura from a punch biopsy of the right forearm, the eruption was attributed to scurvy. Although dermatology recommended supplementation with vitamin C 1000 mg/d, the decision was deferred by the primary team and the purpura improved without it—suggesting the purpura was only partly attributable to low vitamin C.
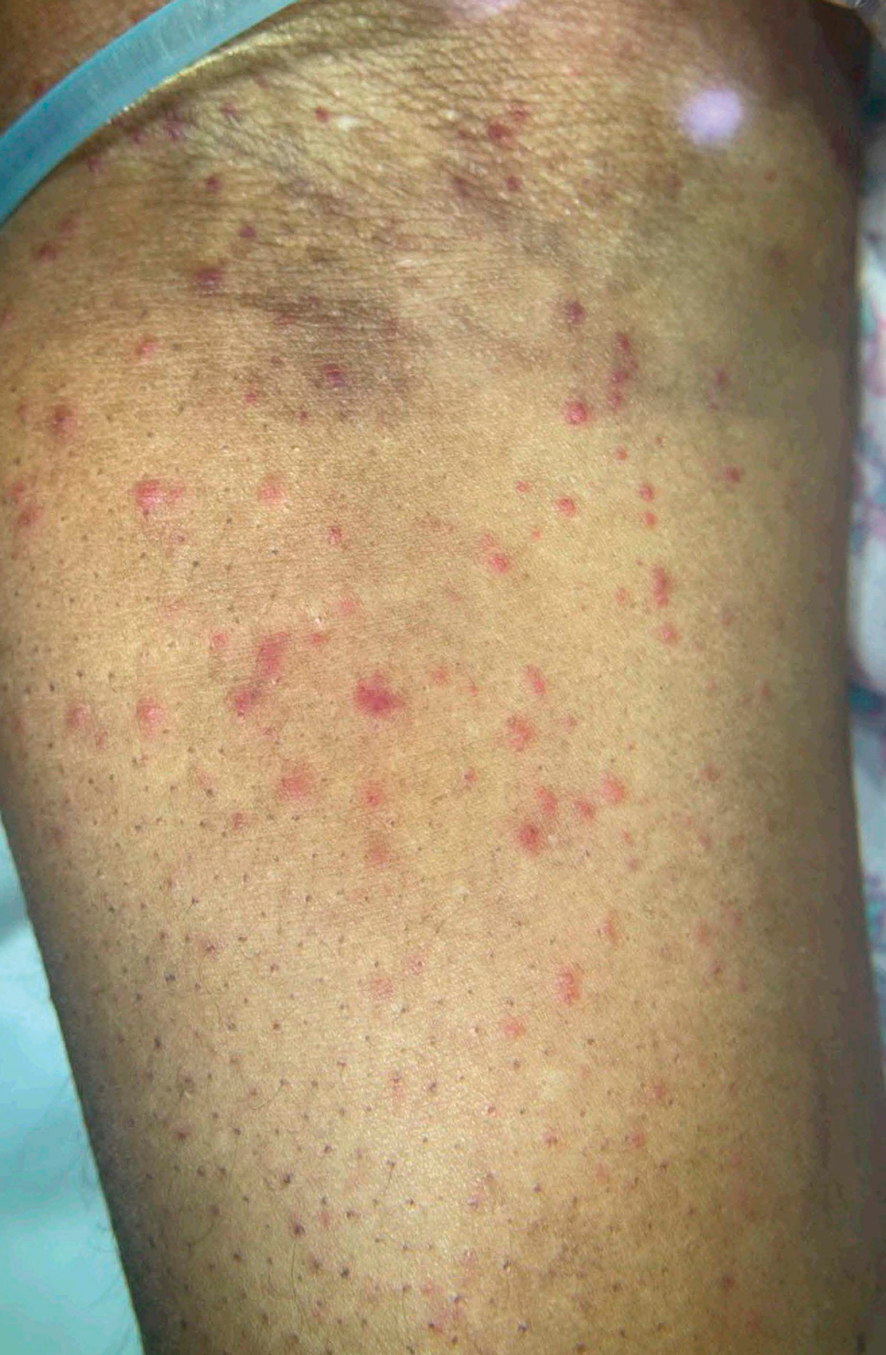
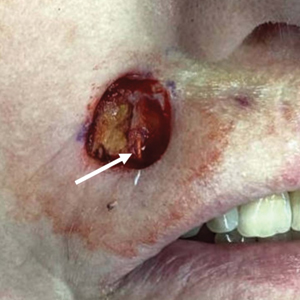
Patient 3—A 77-year-old woman with a history of low oral intake, a low body mass index (18.15 kg/m2 [reference range, 18.5-24.9]), vegetarianism, multiple psychiatric disorders, dementia, recent Clostridioides difficile colitis treated with meropenem, and recurrent idiopathic pancytopenia presented to the hospital with recurrent oral erosions and purpura of the legs for an unknown period. Physical examination by the dermatology consult team revealed superficial lip desquamation; erosions of the buccal mucosa with no involvement of the inner lip or gingiva; mild gingival hyperplasia (eFigure 3); and scaly, purpuric, follicular macules and papules on the legs. The arms and legs were devoid of hair. Laboratory results were notable for low vitamin C levels (0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (28 k/μL [reference range, 150-500 k/μL]), low albumin levels (2.9 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (8.8 g/dL [reference range, 12.0-16.0 g/ dL]). A punch biopsy from the left thigh revealed pauci-inflammatory interface dermatitis with purpura. Based on the clinical and histologic findings, a final diagnosis of purpuric drug eruption (from the meropenem) and scurvy was made. Nutritional support included supplementation with vitamin C 1000 mg/d. The patient’s oral erosions and purpura gradually resolved with treatment throughout her 1.5-month hospitalization.
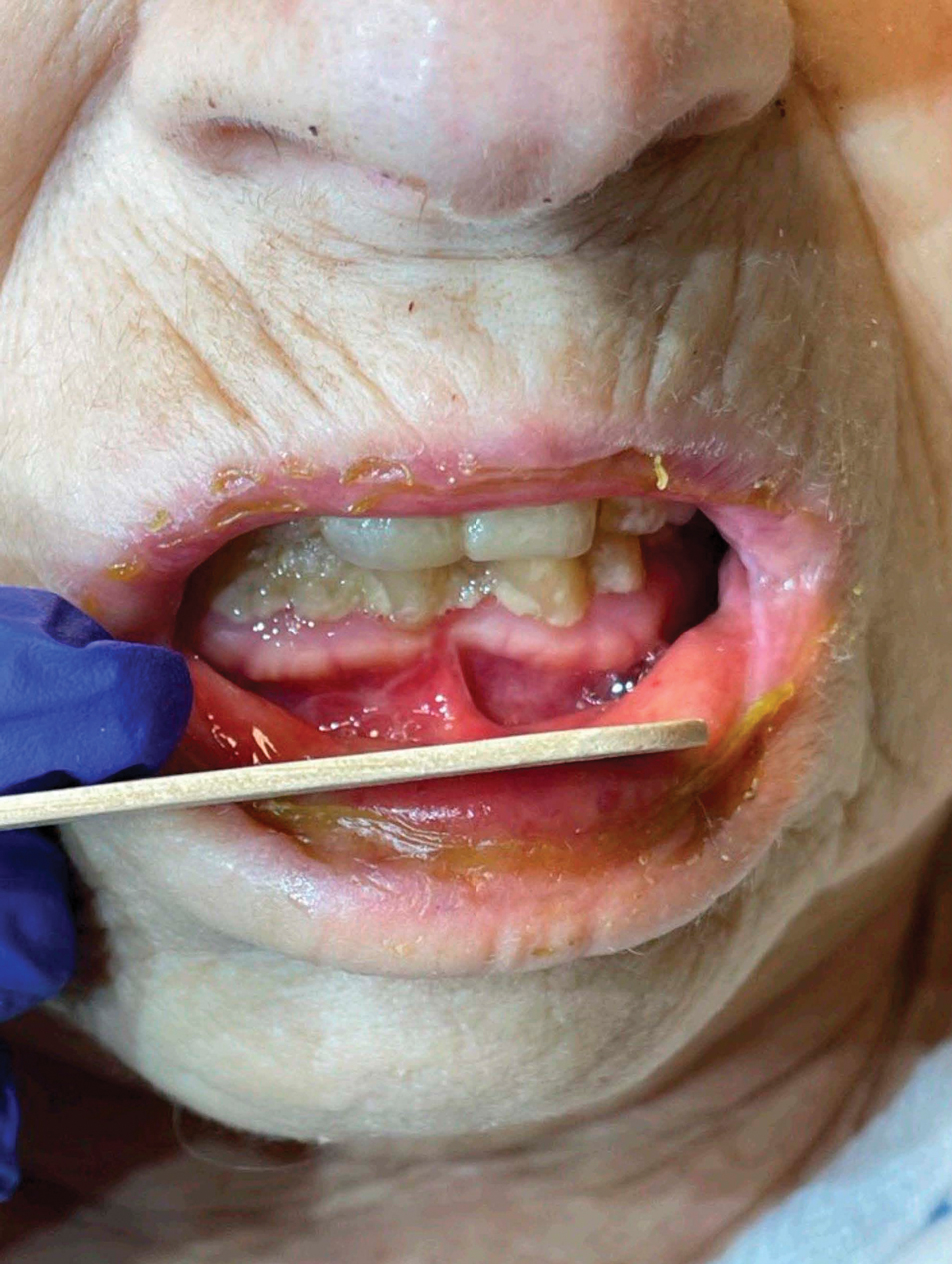
Patient 4—A 67-year-old woman with a history of extensive cardiovascular disease, gastroesophageal reflux disease without esophagitis, end-stage renal disease not requiring hemodialysis, and loss of appetite presented with a painful pruritic eruption on the legs with groin involvement of 2 months’ duration. The patient was admitted to the hospital for worsening mental status and weakness accompanied by dark stools, hematuria, and a productive cough with red-tinged sputum. Physical examination by the dermatology consult team showed a scaly, follicular, purpuric eruption affecting the acral and intertriginous sites (eFigure 4). The patient had sparse leg hair, making it difficult to assess for hair tortuosity. A punch biopsy of the left posterior knee revealed purpuric psoriasiform dermatitis, which was consistent with nutritional deficiency– associated dermatosis. Laboratory results included low vitamin C (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), zinc, (58 μg/dL [reference range, 60-130 μg/dL]), and albumin levels (3.3 g/dL [reference range, 3.5-5.0 g/dL]) and a low platelet count (67 k/μL [reference range, 150- 500 k/μL]). The patient was started on supplementation with vitamin C 1000 mg/d with improvement of the purpura.
Comment
Micronutrient deficiencies may be common in hospitalized patients due to an increased prevalence of predisposing risk factors including infection, malnutrition, malabsorptive conditions, psychiatric diseases, and chronic illnesses.3 Acute-phase response in hospitalized patients also has been strongly associated with decreased plasma vitamin C levels.4 This phenomenon is postulated to be due to the increase in ascorbic acid uptake by circulating granulocytes in acute disease5; however because low vitamin C levels during the acute-phase response may not always accurately reflect total body stores, other clinical features should be assessed. Previously reported social history risk factors include smoking, alcohol consumption, marijuana use, restrictive diets, vegetarianism, and living alone.6,7
The unifying clinical clues for scurvy in our 4 patients were a history of poor oral intake and purpura. While purpura is nonspecific and can appear after traumatic injury to the skin in elderly patients with photodamage and coagulation disorders, it also is associated with vitamin C deficiency, even with a normal platelet count, circulating von Willebrand factor levels, and prothrombin time/partial thromboplastin time.8 This is because vitamin C is vital in forming the collagen and extracellular matrix. Specifically, it is a cofactor for lysine and proline hydroxylase enzymes needed for the á-helix crosslinks in collagen, which are essential for its structural integrity.9 Collagen is a structural protein that maintains the blood vessel walls, skin, and the basement membrane. A deficiency in vitamin C leads to impairment in collagen synthesis, and insufficient collagen results in compromised connective tissue, blood vessels, and hair strength, which may lead to purpura. All of our patients had thrombocytopenia, and similarly, consideration for scurvy in hospitalized patients with risk factors for micronutrient deficiency is a must. Additional findings such as a follicular-based pattern of the purpura, hair tortuosity, restrictive dietary history, histopathology reports consistent with nutritional dermatoses, serum vitamin C levels, and improvement with vitamin C supplementation are more specific for scurvy. All of these factors can assist the clinician in detecting and confirming these micronutrient deficiencies.
Although there are no established therapeutic guidelines for scurvy, the mainstay of treatment is vitamin C repletion, either orally or parenterally. In hospitalized patients, one suggested regimen is 1000 mg of intravenous ascorbic acid daily for 3 days, followed by further supplementation with a dose of 250 to 500 mg twice daily for 1 month as needed after discharge.10 Symptom improvement occurs about 72 hours after vitamin replacement.8 We recommended 500 to 1000 mg of daily vitamin C supplementation for our patients.
Final Thoughts
This case series highlights the importance of maintaining a high index of suspicion for scurvy in hospitalized patients presenting with purpura, especially in a follicular-based pattern, who have multiple medical comorbidities and risk factors for vitamin C deficiency. The manifestations of scurvy are heterogeneous, necessitating a comprehensive mucocutaneous examination. The diagnosis of scurvy requires correlation of the findings from the patient history, clinical examination, laboratory results, and histopathology.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999; 41:895-910.
- Marsh RL, Trinidad J, Shearer S, et al. Association between micronutrient deficiency dermatoses and clinical outcomes in hospitalized patients. J Am Acad Dermatol. 2020;82:1226-1228.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Moser U, Weber F. Uptake of ascorbic acid by human granulocytes. Int J Vitam Nutr Res. 1984;54:47-53.
- Swanson AM, Hughey LC. Acute inpatient presentation of scurvy. Cutis. 2010;86:205-207.
- Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis. 2019;103:E21-E23.
- Antonelli M, Burzo ML, Pecorini G, et al. Scurvy as cause of purpura in the XXI century: a review on this “ancient” disease. Eur Rev Med Pharmacol Sci. 2018;22:4355-4358.
- Maxfield L, Daley SF, Crane JS. Vitamin C deficiency. StatPearls [Internet]. Updated November 12, 2023. Accessed September 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493187/
- Gandhi M, Elfeky O, Ertugrul H, et al. Scurvy: rediscovering a forgotten disease. Diseases. 2023;11:78.
Scurvy, caused by vitamin C or ascorbic acid deficiency, historically has been associated primarily with developing nations and famine; however, specific populations in industrialized nations remain at an increased risk, particularly individuals with a history of smoking, alcohol use, restrictive diet, poor oral intake, psychiatric disorders, dementia, bone marrow transplantation, gastroesophageal reflux disease, end-stage renal disease, and hospitalization.1 Micronutrient deficiency– associated dermatoses have been linked to poor clinical outcomes in hospitalized patients.2 In this case series, we report 4 hospitalized patients with scurvy, each presenting with unique comorbidities and risk factors for vitamin C deficiency (eTable).

Case Reports
Patient 1—A 50-year-old man with a 6-month history of eczema and restrictive dietary intake was admitted to the hospital for septic shock attributed to a left foot infection of 5-days’ duration. The patient had experienced unintentional weight loss with severe protein-calorie malnutrition. His dietary history was notable for selective eating behaviors, intermittent meal skipping, and vegetarianism. Mucocutaneous examination by the dermatology consult team showed exfoliative dermatitis with angular cheilitis, corkscrew hairs on the legs (eFigure 1), and scattered purpura throughout the body. The differential diagnosis included eczema exacerbation, cutaneous T-cell lymphoma/Sézary syndrome, and malnutrition-related dermatosis. Punch biopsies of the left medial knee and right lateral arm revealed impetiginized, spongiotic, psoriasiform dermatitis with papillary dermal edema. The histologic changes were consistent with malnutrition-related dermatosis. Laboratory results included low vitamin C levels (0.1 mg/dL [reference range, 0.2-2.1 mg/dL]), undetectable zinc levels (<10 μg/dL [reference range ,60-130 μg/dL]), a low platelet count (21 kμ/L [reference range, 150-400 k/μL]),low albumin levels (0.9 mg/dL (13.0 g/dL [reference range, 14.0-17.4 g/dL]). The final diagnosis was exfoliative dermatitis due to eczema and multiple nutrient deficiencies (vitamin C and zinc). The patient was treated with vitamin C 500 mg/d and was started on mirtazapine to improve his appetite. Following a 3-month hospitalization, the patient was lost to follow-up after discharge.

Patient 2—A 55-year-old woman with a history of multiple psychiatric disorders presented to the dermatology consult service with an asymptomatic purpuric eruption on the right antecubital fossa of 2 days’ duration that spread to the proximal thighs. Five days prior to presentation, she had received an allogeneic bone marrow transplant complicated by mucositis. She also reported a 4-month history of decreased appetite. At the current presentation, numerous acral, follicular based, purpuric macules and papules without associated corkscrew hairs were observed (eFigure 2). The differential diagnosis included a purpuric drug reaction, viral exanthem, acute graft-vs-host disease, neutrophilic dermatoses, and vitamin C deficiency–related dermatosis. Laboratory results revealed undetectable vitamin C levels (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (8 k/μL [reference range, 150-400 k/μL]), normal albumin levels (3.7 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (7.8 g/dL [reference range, 14.0-17.4 g/dL]). Based on the histopathologic finding of subtle interface dermatitis with purpura from a punch biopsy of the right forearm, the eruption was attributed to scurvy. Although dermatology recommended supplementation with vitamin C 1000 mg/d, the decision was deferred by the primary team and the purpura improved without it—suggesting the purpura was only partly attributable to low vitamin C.

Patient 3—A 77-year-old woman with a history of low oral intake, a low body mass index (18.15 kg/m2 [reference range, 18.5-24.9]), vegetarianism, multiple psychiatric disorders, dementia, recent Clostridioides difficile colitis treated with meropenem, and recurrent idiopathic pancytopenia presented to the hospital with recurrent oral erosions and purpura of the legs for an unknown period. Physical examination by the dermatology consult team revealed superficial lip desquamation; erosions of the buccal mucosa with no involvement of the inner lip or gingiva; mild gingival hyperplasia (eFigure 3); and scaly, purpuric, follicular macules and papules on the legs. The arms and legs were devoid of hair. Laboratory results were notable for low vitamin C levels (0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (28 k/μL [reference range, 150-500 k/μL]), low albumin levels (2.9 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (8.8 g/dL [reference range, 12.0-16.0 g/ dL]). A punch biopsy from the left thigh revealed pauci-inflammatory interface dermatitis with purpura. Based on the clinical and histologic findings, a final diagnosis of purpuric drug eruption (from the meropenem) and scurvy was made. Nutritional support included supplementation with vitamin C 1000 mg/d. The patient’s oral erosions and purpura gradually resolved with treatment throughout her 1.5-month hospitalization.

Patient 4—A 67-year-old woman with a history of extensive cardiovascular disease, gastroesophageal reflux disease without esophagitis, end-stage renal disease not requiring hemodialysis, and loss of appetite presented with a painful pruritic eruption on the legs with groin involvement of 2 months’ duration. The patient was admitted to the hospital for worsening mental status and weakness accompanied by dark stools, hematuria, and a productive cough with red-tinged sputum. Physical examination by the dermatology consult team showed a scaly, follicular, purpuric eruption affecting the acral and intertriginous sites (eFigure 4). The patient had sparse leg hair, making it difficult to assess for hair tortuosity. A punch biopsy of the left posterior knee revealed purpuric psoriasiform dermatitis, which was consistent with nutritional deficiency– associated dermatosis. Laboratory results included low vitamin C (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), zinc, (58 μg/dL [reference range, 60-130 μg/dL]), and albumin levels (3.3 g/dL [reference range, 3.5-5.0 g/dL]) and a low platelet count (67 k/μL [reference range, 150- 500 k/μL]). The patient was started on supplementation with vitamin C 1000 mg/d with improvement of the purpura.

Comment
Micronutrient deficiencies may be common in hospitalized patients due to an increased prevalence of predisposing risk factors including infection, malnutrition, malabsorptive conditions, psychiatric diseases, and chronic illnesses.3 Acute-phase response in hospitalized patients also has been strongly associated with decreased plasma vitamin C levels.4 This phenomenon is postulated to be due to the increase in ascorbic acid uptake by circulating granulocytes in acute disease5; however because low vitamin C levels during the acute-phase response may not always accurately reflect total body stores, other clinical features should be assessed. Previously reported social history risk factors include smoking, alcohol consumption, marijuana use, restrictive diets, vegetarianism, and living alone.6,7
The unifying clinical clues for scurvy in our 4 patients were a history of poor oral intake and purpura. While purpura is nonspecific and can appear after traumatic injury to the skin in elderly patients with photodamage and coagulation disorders, it also is associated with vitamin C deficiency, even with a normal platelet count, circulating von Willebrand factor levels, and prothrombin time/partial thromboplastin time.8 This is because vitamin C is vital in forming the collagen and extracellular matrix. Specifically, it is a cofactor for lysine and proline hydroxylase enzymes needed for the á-helix crosslinks in collagen, which are essential for its structural integrity.9 Collagen is a structural protein that maintains the blood vessel walls, skin, and the basement membrane. A deficiency in vitamin C leads to impairment in collagen synthesis, and insufficient collagen results in compromised connective tissue, blood vessels, and hair strength, which may lead to purpura. All of our patients had thrombocytopenia, and similarly, consideration for scurvy in hospitalized patients with risk factors for micronutrient deficiency is a must. Additional findings such as a follicular-based pattern of the purpura, hair tortuosity, restrictive dietary history, histopathology reports consistent with nutritional dermatoses, serum vitamin C levels, and improvement with vitamin C supplementation are more specific for scurvy. All of these factors can assist the clinician in detecting and confirming these micronutrient deficiencies.
Although there are no established therapeutic guidelines for scurvy, the mainstay of treatment is vitamin C repletion, either orally or parenterally. In hospitalized patients, one suggested regimen is 1000 mg of intravenous ascorbic acid daily for 3 days, followed by further supplementation with a dose of 250 to 500 mg twice daily for 1 month as needed after discharge.10 Symptom improvement occurs about 72 hours after vitamin replacement.8 We recommended 500 to 1000 mg of daily vitamin C supplementation for our patients.
Final Thoughts
This case series highlights the importance of maintaining a high index of suspicion for scurvy in hospitalized patients presenting with purpura, especially in a follicular-based pattern, who have multiple medical comorbidities and risk factors for vitamin C deficiency. The manifestations of scurvy are heterogeneous, necessitating a comprehensive mucocutaneous examination. The diagnosis of scurvy requires correlation of the findings from the patient history, clinical examination, laboratory results, and histopathology.
Scurvy, caused by vitamin C or ascorbic acid deficiency, historically has been associated primarily with developing nations and famine; however, specific populations in industrialized nations remain at an increased risk, particularly individuals with a history of smoking, alcohol use, restrictive diet, poor oral intake, psychiatric disorders, dementia, bone marrow transplantation, gastroesophageal reflux disease, end-stage renal disease, and hospitalization.1 Micronutrient deficiency– associated dermatoses have been linked to poor clinical outcomes in hospitalized patients.2 In this case series, we report 4 hospitalized patients with scurvy, each presenting with unique comorbidities and risk factors for vitamin C deficiency (eTable).

Case Reports
Patient 1—A 50-year-old man with a 6-month history of eczema and restrictive dietary intake was admitted to the hospital for septic shock attributed to a left foot infection of 5-days’ duration. The patient had experienced unintentional weight loss with severe protein-calorie malnutrition. His dietary history was notable for selective eating behaviors, intermittent meal skipping, and vegetarianism. Mucocutaneous examination by the dermatology consult team showed exfoliative dermatitis with angular cheilitis, corkscrew hairs on the legs (eFigure 1), and scattered purpura throughout the body. The differential diagnosis included eczema exacerbation, cutaneous T-cell lymphoma/Sézary syndrome, and malnutrition-related dermatosis. Punch biopsies of the left medial knee and right lateral arm revealed impetiginized, spongiotic, psoriasiform dermatitis with papillary dermal edema. The histologic changes were consistent with malnutrition-related dermatosis. Laboratory results included low vitamin C levels (0.1 mg/dL [reference range, 0.2-2.1 mg/dL]), undetectable zinc levels (<10 μg/dL [reference range ,60-130 μg/dL]), a low platelet count (21 kμ/L [reference range, 150-400 k/μL]),low albumin levels (0.9 mg/dL (13.0 g/dL [reference range, 14.0-17.4 g/dL]). The final diagnosis was exfoliative dermatitis due to eczema and multiple nutrient deficiencies (vitamin C and zinc). The patient was treated with vitamin C 500 mg/d and was started on mirtazapine to improve his appetite. Following a 3-month hospitalization, the patient was lost to follow-up after discharge.

Patient 2—A 55-year-old woman with a history of multiple psychiatric disorders presented to the dermatology consult service with an asymptomatic purpuric eruption on the right antecubital fossa of 2 days’ duration that spread to the proximal thighs. Five days prior to presentation, she had received an allogeneic bone marrow transplant complicated by mucositis. She also reported a 4-month history of decreased appetite. At the current presentation, numerous acral, follicular based, purpuric macules and papules without associated corkscrew hairs were observed (eFigure 2). The differential diagnosis included a purpuric drug reaction, viral exanthem, acute graft-vs-host disease, neutrophilic dermatoses, and vitamin C deficiency–related dermatosis. Laboratory results revealed undetectable vitamin C levels (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (8 k/μL [reference range, 150-400 k/μL]), normal albumin levels (3.7 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (7.8 g/dL [reference range, 14.0-17.4 g/dL]). Based on the histopathologic finding of subtle interface dermatitis with purpura from a punch biopsy of the right forearm, the eruption was attributed to scurvy. Although dermatology recommended supplementation with vitamin C 1000 mg/d, the decision was deferred by the primary team and the purpura improved without it—suggesting the purpura was only partly attributable to low vitamin C.

Patient 3—A 77-year-old woman with a history of low oral intake, a low body mass index (18.15 kg/m2 [reference range, 18.5-24.9]), vegetarianism, multiple psychiatric disorders, dementia, recent Clostridioides difficile colitis treated with meropenem, and recurrent idiopathic pancytopenia presented to the hospital with recurrent oral erosions and purpura of the legs for an unknown period. Physical examination by the dermatology consult team revealed superficial lip desquamation; erosions of the buccal mucosa with no involvement of the inner lip or gingiva; mild gingival hyperplasia (eFigure 3); and scaly, purpuric, follicular macules and papules on the legs. The arms and legs were devoid of hair. Laboratory results were notable for low vitamin C levels (0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), a low platelet count (28 k/μL [reference range, 150-500 k/μL]), low albumin levels (2.9 g/dL [reference range, 3.5-5.0 g/dL]), and low hemoglobin (8.8 g/dL [reference range, 12.0-16.0 g/ dL]). A punch biopsy from the left thigh revealed pauci-inflammatory interface dermatitis with purpura. Based on the clinical and histologic findings, a final diagnosis of purpuric drug eruption (from the meropenem) and scurvy was made. Nutritional support included supplementation with vitamin C 1000 mg/d. The patient’s oral erosions and purpura gradually resolved with treatment throughout her 1.5-month hospitalization.

Patient 4—A 67-year-old woman with a history of extensive cardiovascular disease, gastroesophageal reflux disease without esophagitis, end-stage renal disease not requiring hemodialysis, and loss of appetite presented with a painful pruritic eruption on the legs with groin involvement of 2 months’ duration. The patient was admitted to the hospital for worsening mental status and weakness accompanied by dark stools, hematuria, and a productive cough with red-tinged sputum. Physical examination by the dermatology consult team showed a scaly, follicular, purpuric eruption affecting the acral and intertriginous sites (eFigure 4). The patient had sparse leg hair, making it difficult to assess for hair tortuosity. A punch biopsy of the left posterior knee revealed purpuric psoriasiform dermatitis, which was consistent with nutritional deficiency– associated dermatosis. Laboratory results included low vitamin C (<0.1 mg/dL [reference range, 0.3-2.7 mg/dL]), zinc, (58 μg/dL [reference range, 60-130 μg/dL]), and albumin levels (3.3 g/dL [reference range, 3.5-5.0 g/dL]) and a low platelet count (67 k/μL [reference range, 150- 500 k/μL]). The patient was started on supplementation with vitamin C 1000 mg/d with improvement of the purpura.

Comment
Micronutrient deficiencies may be common in hospitalized patients due to an increased prevalence of predisposing risk factors including infection, malnutrition, malabsorptive conditions, psychiatric diseases, and chronic illnesses.3 Acute-phase response in hospitalized patients also has been strongly associated with decreased plasma vitamin C levels.4 This phenomenon is postulated to be due to the increase in ascorbic acid uptake by circulating granulocytes in acute disease5; however because low vitamin C levels during the acute-phase response may not always accurately reflect total body stores, other clinical features should be assessed. Previously reported social history risk factors include smoking, alcohol consumption, marijuana use, restrictive diets, vegetarianism, and living alone.6,7
The unifying clinical clues for scurvy in our 4 patients were a history of poor oral intake and purpura. While purpura is nonspecific and can appear after traumatic injury to the skin in elderly patients with photodamage and coagulation disorders, it also is associated with vitamin C deficiency, even with a normal platelet count, circulating von Willebrand factor levels, and prothrombin time/partial thromboplastin time.8 This is because vitamin C is vital in forming the collagen and extracellular matrix. Specifically, it is a cofactor for lysine and proline hydroxylase enzymes needed for the á-helix crosslinks in collagen, which are essential for its structural integrity.9 Collagen is a structural protein that maintains the blood vessel walls, skin, and the basement membrane. A deficiency in vitamin C leads to impairment in collagen synthesis, and insufficient collagen results in compromised connective tissue, blood vessels, and hair strength, which may lead to purpura. All of our patients had thrombocytopenia, and similarly, consideration for scurvy in hospitalized patients with risk factors for micronutrient deficiency is a must. Additional findings such as a follicular-based pattern of the purpura, hair tortuosity, restrictive dietary history, histopathology reports consistent with nutritional dermatoses, serum vitamin C levels, and improvement with vitamin C supplementation are more specific for scurvy. All of these factors can assist the clinician in detecting and confirming these micronutrient deficiencies.
Although there are no established therapeutic guidelines for scurvy, the mainstay of treatment is vitamin C repletion, either orally or parenterally. In hospitalized patients, one suggested regimen is 1000 mg of intravenous ascorbic acid daily for 3 days, followed by further supplementation with a dose of 250 to 500 mg twice daily for 1 month as needed after discharge.10 Symptom improvement occurs about 72 hours after vitamin replacement.8 We recommended 500 to 1000 mg of daily vitamin C supplementation for our patients.
Final Thoughts
This case series highlights the importance of maintaining a high index of suspicion for scurvy in hospitalized patients presenting with purpura, especially in a follicular-based pattern, who have multiple medical comorbidities and risk factors for vitamin C deficiency. The manifestations of scurvy are heterogeneous, necessitating a comprehensive mucocutaneous examination. The diagnosis of scurvy requires correlation of the findings from the patient history, clinical examination, laboratory results, and histopathology.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999; 41:895-910.
- Marsh RL, Trinidad J, Shearer S, et al. Association between micronutrient deficiency dermatoses and clinical outcomes in hospitalized patients. J Am Acad Dermatol. 2020;82:1226-1228.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Moser U, Weber F. Uptake of ascorbic acid by human granulocytes. Int J Vitam Nutr Res. 1984;54:47-53.
- Swanson AM, Hughey LC. Acute inpatient presentation of scurvy. Cutis. 2010;86:205-207.
- Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis. 2019;103:E21-E23.
- Antonelli M, Burzo ML, Pecorini G, et al. Scurvy as cause of purpura in the XXI century: a review on this “ancient” disease. Eur Rev Med Pharmacol Sci. 2018;22:4355-4358.
- Maxfield L, Daley SF, Crane JS. Vitamin C deficiency. StatPearls [Internet]. Updated November 12, 2023. Accessed September 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493187/
- Gandhi M, Elfeky O, Ertugrul H, et al. Scurvy: rediscovering a forgotten disease. Diseases. 2023;11:78.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999; 41:895-910.
- Marsh RL, Trinidad J, Shearer S, et al. Association between micronutrient deficiency dermatoses and clinical outcomes in hospitalized patients. J Am Acad Dermatol. 2020;82:1226-1228.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296-302, 308, E1-E5.
- Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419-425.
- Moser U, Weber F. Uptake of ascorbic acid by human granulocytes. Int J Vitam Nutr Res. 1984;54:47-53.
- Swanson AM, Hughey LC. Acute inpatient presentation of scurvy. Cutis. 2010;86:205-207.
- Christopher KL, Menachof KK, Fathi R. Scurvy masquerading as reactive arthritis. Cutis. 2019;103:E21-E23.
- Antonelli M, Burzo ML, Pecorini G, et al. Scurvy as cause of purpura in the XXI century: a review on this “ancient” disease. Eur Rev Med Pharmacol Sci. 2018;22:4355-4358.
- Maxfield L, Daley SF, Crane JS. Vitamin C deficiency. StatPearls [Internet]. Updated November 12, 2023. Accessed September 6, 2024. https://www.ncbi.nlm.nih.gov/books/NBK493187/
- Gandhi M, Elfeky O, Ertugrul H, et al. Scurvy: rediscovering a forgotten disease. Diseases. 2023;11:78.
Scurvy in Hospitalized Patients
Scurvy in Hospitalized Patients
PRACTICE POINTS
- Clinicians should maintain a high index of suspicion for vitamin C deficiency/scurvy in hospitalized patients with purpura who have multiple medical comorbidities and risk factors.
- A low platelet count may mask underlying vitamin C deficiency, and patients may have concurrent deficiencies in other nutrients such as zinc.
Comparing the Quality of Patient Guidance on Dermatologic Care Generated by ChatGPT vs Reddit
Comparing the Quality of Patient Guidance on Dermatologic Care Generated by ChatGPT vs Reddit
To the Editor:
Online resources that are convenient and affordable play a crucial role in mitigating health inequality and improving patient access to health care information; however, the benefits are limited by the quality of information available, as medical misinformation can lead to patients engaging in harmful practices, making dangerous decisions, and even avoiding safe and effective treatments. In this study, we aimed to assess and compare the quality of patient guidance on dermatologic care generated by ChatGPT vs Reddit based on accuracy, appropriateness, and safety. It is essential to assess the quality and reliability of online health information to support patients in making informed decisions about their health.
The emergence and advancement of artificial intelligence and large language models such as ChatGPT present a new method for patients to access health care advice. ChatGPT can engage in conversation by accessing information from existing publicly available data on the internet, including books and websites, up to the year 2023 and providing humanlike responses with context.1 ChatGPT’s access to a breadth of online evidence-based literature ensures the dissemination of quality information that is quick and without inherent bias, offering the potential to more closely align with health care professionals. ChatGPT’s use in dermatology by patients has shown efficacy, with a 98.87% approval rate by dermatologists scoring its ability to recommend appropriate medication for common dermatologic conditions.2 However, ChatGPT has limitations when providing health care advice and has been observed to misunderstand health care standards, lack personalization, and offer incorrect references; currently, the latest publicly available version (ChatGPT 3.5) also is unable to analyze clinical images.3,4
Reddit is an online social media forum that allows users to post questions and photographs to which anyone can reply and offer advice. Patients may find comfort in online communities where they can connect with others facing similar challenges related to their diagnosis. Within these communities, the responses often share users’ own lived experiences and offer support based on what has and has not worked for them. Prior research found that users intentionally seeking health information via Reddit are likely to implement the advice they receive even without verification of its credibility, suggesting a trust and receptibility to ideas offered on the platform.5 Furthermore, a study analyzing the dermatologic content of 17 dermatology related subreddits that had 1000 or more subscribers found that 70.6% of posts fell under the category of “seeking health/cosmetic advice.”6 Reddit users thus are vulnerable to receiving advice based on personal bias and exposing their health information to the public.
We hypothesized that ChatGPT would provide users with guidance that was more closely aligned with typical dermatologists’ advice due to its thorough analysis and compilation of diverse sources and recommendations available on the internet. We expected Reddit to yield recommendations of lesser quality and a diminished safety score, primarily due to the absence of credibility-vetting mechanisms and the influence of personal biases within the advice shared.
User-submitted posts to large dermatologic community Reddit forums representing a few of the most common skin conditions (r/eczema, r/acne, r/Folliculitis, r/SebDerm, r/Hidradenitis, r/keratosis, and r/Psoriasis) were retrospectively reviewed from January 2024 to March 2024. The most popular posts that did not include photographs were included in our study. Posts with photographs were excluded, as clinical images were not able to be uploaded to the publicly available ChatGPT 3.5. We collected real user questions about common skin conditions from Reddit forums and then asked ChatGPT to answer those same questions. We compared ChatGPT’s responses to the most upvoted Reddit comments to see how they matched up (eTable).

Each ChatGPT response and the top-rated Reddit comment were independently evaluated by a board certified dermatologist (S.A.) and a dermatology resident (A.H.K.). The quality of the ChatGPT and Reddit responses were determined by scoring the accuracy, appropriateness, safety consideration, and specificity on a 5-point Likert scale (1=low, 5=high). The 2 evaluators’ mean scores for each of the 4 categories were calculated based on adequate interrater reliability, which was tested using Cohen’s κ coefficient. Related-samples sign tests were used to compare ChatGPT and Reddit responses for each of the 4 categories. Analysis was completed using SPSS statistics software version 29.0 (IBM). The evaluators also were asked to provide qualitative feedback on the strengths and weaknesses of each response.
Our retrospective review yielded 20 total questions: 5 (25%) on atopic dermatitis, 4 (20%) on acne, 4 (20%) on hidradenitis suppurativa, 4 (20%) on psoriasis, 1 (5%) on folliculitis, 1 (5%) on keratosis pilaris, and 1 (5%) on seborrheic dermatitis. The number of posts was limited to 20 due to the extensive time required for grading each response. These 20 questions were selected from a larger pool of eligible posts based on factors such as clarity and relevance to common skin conditions. With regard to the types of questions that were asked, 6 (30%) were related to general management of a diagnosis, 5 (25%) were on treatment recommendations for symptom relief, 3 (15%) were on optimal utilization of current treatment regimens, 2 (10%) were on prescription side effects, 2 (10%) were on diagnosis presentation, 1 (5%) was on potential triggers of the diagnosis, and 1 (5%) was on natural treatment recommendations.
Mean (SD) evaluator scores for accuracy were significantly higher among ChatGPT responses compared with Reddit (4.63 [0.60] vs 2.60 [0.98])(P<.001). ChatGPT responses also were significantly higher for appropriateness compared with Reddit (4.55 [0.71] vs 2.58 [1.02])(P<.001) and safety consideration (4.88 [0.56] vs 2.80[0.97])(P <.001). There was no significant difference in mean specificity scores between ChatGPT and Reddit (4.25[1.02] vs 3.80 [0.70])(P=.096)(Figure).
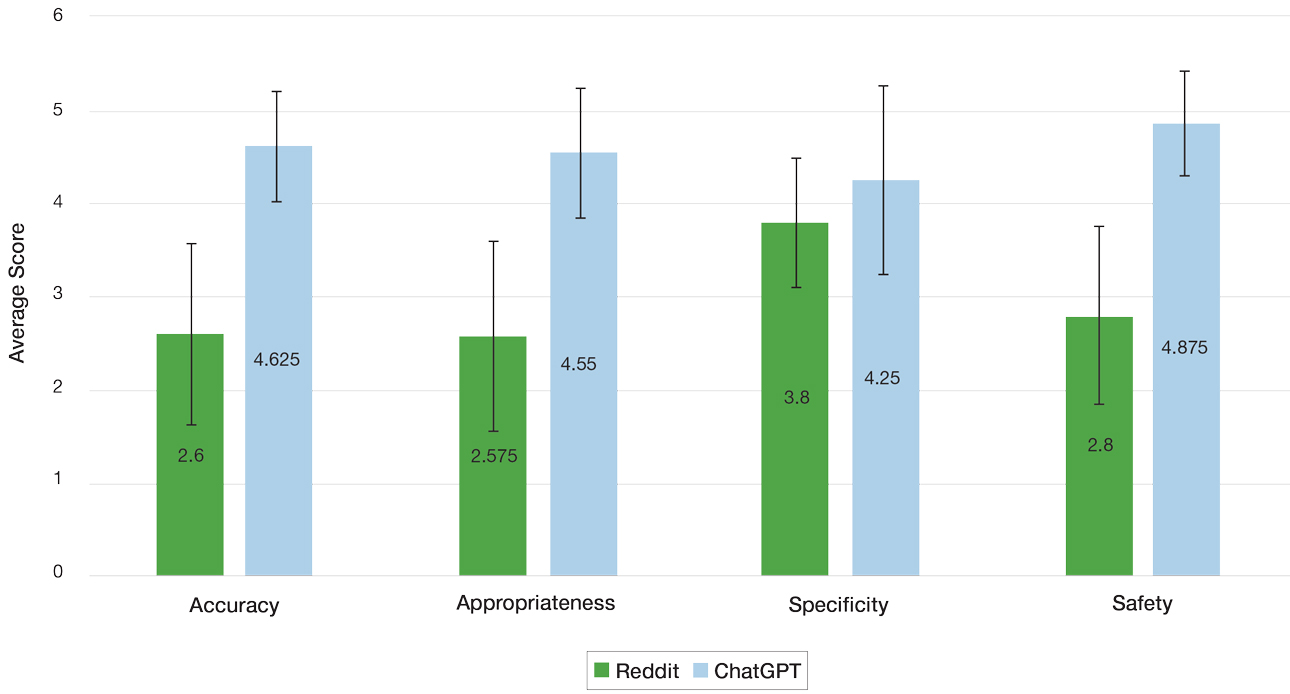
For the Reddit responses, the weighted Cohen’s κ coefficient between the 2 evaluators was 0.200 (95% CI, –.089 to .489) for accuracy, 0.255 (95% CI, .014-.497) for appropriateness, 0.385 (95% CI, .176-.594) for safety consideration, and –0.024 (95% CI, –.177 to .129) for specificity. For the ChatGPT responses, the weighted Cohen’s κ coefficient between the 2 evaluators was 0.426 (95% CI, .122-.730) for accuracy, 0.571 (95% CI, .294-.849) for appropriateness, 0.655 (95% CI, .632-.678) for safety consideration, and 0.313 (95% CI, .043-.584) for specificity.
The strengths and weaknesses of the responses also were qualitatively analyzed. One commonly observed strength was ChatGPT’s frequent and appropriate recommendation for users to consult a dermatologist. In the case of atopic dermatitis—one of the more frequently asked about conditions—ChatGPT consistently emphasized evidence-based strategies such as gentle skin care and moisturization, reflecting alignment with clinical guidelines. Additionally, a common weakness of both ChatGPT and Reddit responses generally was the lack of personalized guidance and comprehensive discussion of the risks and benefits of specific treatments. It also was noted that neither platform consistently explored differential diagnoses—for example, distinguishing atopic dermatitis from conditions such as allergic contact dermatitis—limiting the diagnostic depth of the responses.
ChatGPT and Reddit can provide patients with quick and accessible health information for various dermatologic concerns. The results of our study demonstrated a significantly higher level of accuracy, appropriateness, and safety of responses generated by ChatGPT compared with human-generated responses on Reddit (P<.001). Both platforms offered similarly specific responses to user inquiries, demonstrating ChatGPT’s ability to comprehend user questions and draw from publicly available texts and Reddit users’ contributing insights based on their own first-hand experiences.
Reddit’s dermatologic forums often feature personal anecdotes and unique treatments described by individual users. Although specific to particular dermatologic concerns, such advice lacks an evidence-based standard of care. With the noted inherent trust of patients seeking guidance within Reddit communities, patients may follow unhelpful or potentially dangerous medical advice.5 A study examining 300 user-submitted posts on popular Reddit dermatology forums during the COVID-19 pandemic found that the mean scores for top-rated comments’ potential to be misleading or dangerous was 2.33 out of 5 on a Likert scale (95% CI, 2.18- 2.48).7 Dermatologists should be aware of the potential risks associated with dermatologic advice offered on Reddit and should caution patients against relying solely on this information without consulting a qualified dermatologist first.
Reddit’s open-forum design provides licensed dermatologists with the opportunity to disseminate evidence based information regarding dermatologic conditions. Currently, there is a subreddit (r/AskDocs) that allows users to post medical questions that can be answered by moderator-verified physicians. Participation from dermatologists in online communities such as this can improve the quality of dermatologic information shared online, combat misinformation, and promote safe skin care practices.
ChatGPT offers more accurate, appropriate, and safe information compared to Reddit responses, but its answers lack personalization. In a clinical setting, a personalized treatment plan from a physician can be tailored with a comprehensive discussion of the risks and benefits. Further, clinical settings allow for diagnosis and confirmation via biopsy and meticulous history taking to ensure that the diagnosis and treatment plan are accurate. While ChatGPT may be an option for seeking basic advice on dermatologic conditions, a licensed dermatologist should always be consulted for proper medical advice. Services such as telehealth may be another option to for patients with limited access to care.
Since ChatGPT 3.5 does not support the ability to upload images, our study acknowledges a limitation regarding the inclusion of Reddit posts containing photographs. Images can improve the response quality from both Reddit users and ChatGPT. While the updated ChatGPT 4o is capable of processing images, it requires a monthly subscription fee. The free version was chosen for use in this study, as this may reflect the most likely version that patients of low socioeconomic status would utilize to access dermatologic care; however, there is potential for growth and improvement of ChatGPT’s capability in providing medical advice.
This study compared the strengths and limitations of ChatGPT’s and Reddit’s responses to common dermatologic inquiries. ChatGPT and Reddit both show potential to be helpful sources of dermatologic health information; however, their current versions have many limitations and require caution and careful examination by patients of the guidance provided. Clinicians should be aware of these limitations when advising patients and emphasize the importance of consulting a licensed dermatologist for personalized, evidence-based care. For the best medical advice, it is always advisable to consult with a licensed dermatologist.
- Roumeliotis KI, Tselikas ND. ChatGPT and open-AI models: a preliminary review. Future Internet. 2023;15:192. doi:10.3390/fi15060192
- Iqbal U, Lee LTJ, Rahmanti AR, et al. Can large language models provide secondary reliable opinion on treatment options for dermatological diseases? J Am Med Inform Assoc. 2024;31:1341-1347. doi:10.1093/jamia/ocae067
- Whiles BB, Bird VG, Canales BK, et al. Caution! AI bot has entered the patient chat: ChatGPT has limitations in providing accurate urologic healthcare advice. Urology. 2023;180:278-284. doi:10.1016/j.urology.2023.07.010
- Nastasi AJ, Courtright KR, Halpern SD, et al. A vignette-based evaluation of ChatGPT’s ability to provide appropriate and equitable medical advice across care contexts. Sci Rep. 2023;13:17885. doi:10.1038/s41598-023-45223-y
- Record RA, Silberman WR, Santiago JE, et al. I sought it, I Reddit: examining health information engagement behaviors among Reddit users. J Health Commun. 2018;23:470-476. doi:10.1080/1081073 0.2018.1465493
- Buntinx-Krieg T, Caravaglio J, Domozych R, et al. Dermatology on Reddit: elucidating trends in dermatologic communications on the world wide web. Dermatol Online J. 2017;23:13030/qt9dr1f7x6.
- Aboul-Fettouh N, Lee KP, Kash N, et al. Social media and dermatology during the COVID-19 pandemic: analyzing usersubmitted posts seeking dermatologic advice on Reddit. Cureus. 2023;15:E33720. doi:10.7759/cureus.33720
To the Editor:
Online resources that are convenient and affordable play a crucial role in mitigating health inequality and improving patient access to health care information; however, the benefits are limited by the quality of information available, as medical misinformation can lead to patients engaging in harmful practices, making dangerous decisions, and even avoiding safe and effective treatments. In this study, we aimed to assess and compare the quality of patient guidance on dermatologic care generated by ChatGPT vs Reddit based on accuracy, appropriateness, and safety. It is essential to assess the quality and reliability of online health information to support patients in making informed decisions about their health.
The emergence and advancement of artificial intelligence and large language models such as ChatGPT present a new method for patients to access health care advice. ChatGPT can engage in conversation by accessing information from existing publicly available data on the internet, including books and websites, up to the year 2023 and providing humanlike responses with context.1 ChatGPT’s access to a breadth of online evidence-based literature ensures the dissemination of quality information that is quick and without inherent bias, offering the potential to more closely align with health care professionals. ChatGPT’s use in dermatology by patients has shown efficacy, with a 98.87% approval rate by dermatologists scoring its ability to recommend appropriate medication for common dermatologic conditions.2 However, ChatGPT has limitations when providing health care advice and has been observed to misunderstand health care standards, lack personalization, and offer incorrect references; currently, the latest publicly available version (ChatGPT 3.5) also is unable to analyze clinical images.3,4
Reddit is an online social media forum that allows users to post questions and photographs to which anyone can reply and offer advice. Patients may find comfort in online communities where they can connect with others facing similar challenges related to their diagnosis. Within these communities, the responses often share users’ own lived experiences and offer support based on what has and has not worked for them. Prior research found that users intentionally seeking health information via Reddit are likely to implement the advice they receive even without verification of its credibility, suggesting a trust and receptibility to ideas offered on the platform.5 Furthermore, a study analyzing the dermatologic content of 17 dermatology related subreddits that had 1000 or more subscribers found that 70.6% of posts fell under the category of “seeking health/cosmetic advice.”6 Reddit users thus are vulnerable to receiving advice based on personal bias and exposing their health information to the public.
We hypothesized that ChatGPT would provide users with guidance that was more closely aligned with typical dermatologists’ advice due to its thorough analysis and compilation of diverse sources and recommendations available on the internet. We expected Reddit to yield recommendations of lesser quality and a diminished safety score, primarily due to the absence of credibility-vetting mechanisms and the influence of personal biases within the advice shared.
User-submitted posts to large dermatologic community Reddit forums representing a few of the most common skin conditions (r/eczema, r/acne, r/Folliculitis, r/SebDerm, r/Hidradenitis, r/keratosis, and r/Psoriasis) were retrospectively reviewed from January 2024 to March 2024. The most popular posts that did not include photographs were included in our study. Posts with photographs were excluded, as clinical images were not able to be uploaded to the publicly available ChatGPT 3.5. We collected real user questions about common skin conditions from Reddit forums and then asked ChatGPT to answer those same questions. We compared ChatGPT’s responses to the most upvoted Reddit comments to see how they matched up (eTable).

Each ChatGPT response and the top-rated Reddit comment were independently evaluated by a board certified dermatologist (S.A.) and a dermatology resident (A.H.K.). The quality of the ChatGPT and Reddit responses were determined by scoring the accuracy, appropriateness, safety consideration, and specificity on a 5-point Likert scale (1=low, 5=high). The 2 evaluators’ mean scores for each of the 4 categories were calculated based on adequate interrater reliability, which was tested using Cohen’s κ coefficient. Related-samples sign tests were used to compare ChatGPT and Reddit responses for each of the 4 categories. Analysis was completed using SPSS statistics software version 29.0 (IBM). The evaluators also were asked to provide qualitative feedback on the strengths and weaknesses of each response.
Our retrospective review yielded 20 total questions: 5 (25%) on atopic dermatitis, 4 (20%) on acne, 4 (20%) on hidradenitis suppurativa, 4 (20%) on psoriasis, 1 (5%) on folliculitis, 1 (5%) on keratosis pilaris, and 1 (5%) on seborrheic dermatitis. The number of posts was limited to 20 due to the extensive time required for grading each response. These 20 questions were selected from a larger pool of eligible posts based on factors such as clarity and relevance to common skin conditions. With regard to the types of questions that were asked, 6 (30%) were related to general management of a diagnosis, 5 (25%) were on treatment recommendations for symptom relief, 3 (15%) were on optimal utilization of current treatment regimens, 2 (10%) were on prescription side effects, 2 (10%) were on diagnosis presentation, 1 (5%) was on potential triggers of the diagnosis, and 1 (5%) was on natural treatment recommendations.
Mean (SD) evaluator scores for accuracy were significantly higher among ChatGPT responses compared with Reddit (4.63 [0.60] vs 2.60 [0.98])(P<.001). ChatGPT responses also were significantly higher for appropriateness compared with Reddit (4.55 [0.71] vs 2.58 [1.02])(P<.001) and safety consideration (4.88 [0.56] vs 2.80[0.97])(P <.001). There was no significant difference in mean specificity scores between ChatGPT and Reddit (4.25[1.02] vs 3.80 [0.70])(P=.096)(Figure).

For the Reddit responses, the weighted Cohen’s κ coefficient between the 2 evaluators was 0.200 (95% CI, –.089 to .489) for accuracy, 0.255 (95% CI, .014-.497) for appropriateness, 0.385 (95% CI, .176-.594) for safety consideration, and –0.024 (95% CI, –.177 to .129) for specificity. For the ChatGPT responses, the weighted Cohen’s κ coefficient between the 2 evaluators was 0.426 (95% CI, .122-.730) for accuracy, 0.571 (95% CI, .294-.849) for appropriateness, 0.655 (95% CI, .632-.678) for safety consideration, and 0.313 (95% CI, .043-.584) for specificity.
The strengths and weaknesses of the responses also were qualitatively analyzed. One commonly observed strength was ChatGPT’s frequent and appropriate recommendation for users to consult a dermatologist. In the case of atopic dermatitis—one of the more frequently asked about conditions—ChatGPT consistently emphasized evidence-based strategies such as gentle skin care and moisturization, reflecting alignment with clinical guidelines. Additionally, a common weakness of both ChatGPT and Reddit responses generally was the lack of personalized guidance and comprehensive discussion of the risks and benefits of specific treatments. It also was noted that neither platform consistently explored differential diagnoses—for example, distinguishing atopic dermatitis from conditions such as allergic contact dermatitis—limiting the diagnostic depth of the responses.
ChatGPT and Reddit can provide patients with quick and accessible health information for various dermatologic concerns. The results of our study demonstrated a significantly higher level of accuracy, appropriateness, and safety of responses generated by ChatGPT compared with human-generated responses on Reddit (P<.001). Both platforms offered similarly specific responses to user inquiries, demonstrating ChatGPT’s ability to comprehend user questions and draw from publicly available texts and Reddit users’ contributing insights based on their own first-hand experiences.
Reddit’s dermatologic forums often feature personal anecdotes and unique treatments described by individual users. Although specific to particular dermatologic concerns, such advice lacks an evidence-based standard of care. With the noted inherent trust of patients seeking guidance within Reddit communities, patients may follow unhelpful or potentially dangerous medical advice.5 A study examining 300 user-submitted posts on popular Reddit dermatology forums during the COVID-19 pandemic found that the mean scores for top-rated comments’ potential to be misleading or dangerous was 2.33 out of 5 on a Likert scale (95% CI, 2.18- 2.48).7 Dermatologists should be aware of the potential risks associated with dermatologic advice offered on Reddit and should caution patients against relying solely on this information without consulting a qualified dermatologist first.
Reddit’s open-forum design provides licensed dermatologists with the opportunity to disseminate evidence based information regarding dermatologic conditions. Currently, there is a subreddit (r/AskDocs) that allows users to post medical questions that can be answered by moderator-verified physicians. Participation from dermatologists in online communities such as this can improve the quality of dermatologic information shared online, combat misinformation, and promote safe skin care practices.
ChatGPT offers more accurate, appropriate, and safe information compared to Reddit responses, but its answers lack personalization. In a clinical setting, a personalized treatment plan from a physician can be tailored with a comprehensive discussion of the risks and benefits. Further, clinical settings allow for diagnosis and confirmation via biopsy and meticulous history taking to ensure that the diagnosis and treatment plan are accurate. While ChatGPT may be an option for seeking basic advice on dermatologic conditions, a licensed dermatologist should always be consulted for proper medical advice. Services such as telehealth may be another option to for patients with limited access to care.
Since ChatGPT 3.5 does not support the ability to upload images, our study acknowledges a limitation regarding the inclusion of Reddit posts containing photographs. Images can improve the response quality from both Reddit users and ChatGPT. While the updated ChatGPT 4o is capable of processing images, it requires a monthly subscription fee. The free version was chosen for use in this study, as this may reflect the most likely version that patients of low socioeconomic status would utilize to access dermatologic care; however, there is potential for growth and improvement of ChatGPT’s capability in providing medical advice.
This study compared the strengths and limitations of ChatGPT’s and Reddit’s responses to common dermatologic inquiries. ChatGPT and Reddit both show potential to be helpful sources of dermatologic health information; however, their current versions have many limitations and require caution and careful examination by patients of the guidance provided. Clinicians should be aware of these limitations when advising patients and emphasize the importance of consulting a licensed dermatologist for personalized, evidence-based care. For the best medical advice, it is always advisable to consult with a licensed dermatologist.
To the Editor:
Online resources that are convenient and affordable play a crucial role in mitigating health inequality and improving patient access to health care information; however, the benefits are limited by the quality of information available, as medical misinformation can lead to patients engaging in harmful practices, making dangerous decisions, and even avoiding safe and effective treatments. In this study, we aimed to assess and compare the quality of patient guidance on dermatologic care generated by ChatGPT vs Reddit based on accuracy, appropriateness, and safety. It is essential to assess the quality and reliability of online health information to support patients in making informed decisions about their health.
The emergence and advancement of artificial intelligence and large language models such as ChatGPT present a new method for patients to access health care advice. ChatGPT can engage in conversation by accessing information from existing publicly available data on the internet, including books and websites, up to the year 2023 and providing humanlike responses with context.1 ChatGPT’s access to a breadth of online evidence-based literature ensures the dissemination of quality information that is quick and without inherent bias, offering the potential to more closely align with health care professionals. ChatGPT’s use in dermatology by patients has shown efficacy, with a 98.87% approval rate by dermatologists scoring its ability to recommend appropriate medication for common dermatologic conditions.2 However, ChatGPT has limitations when providing health care advice and has been observed to misunderstand health care standards, lack personalization, and offer incorrect references; currently, the latest publicly available version (ChatGPT 3.5) also is unable to analyze clinical images.3,4
Reddit is an online social media forum that allows users to post questions and photographs to which anyone can reply and offer advice. Patients may find comfort in online communities where they can connect with others facing similar challenges related to their diagnosis. Within these communities, the responses often share users’ own lived experiences and offer support based on what has and has not worked for them. Prior research found that users intentionally seeking health information via Reddit are likely to implement the advice they receive even without verification of its credibility, suggesting a trust and receptibility to ideas offered on the platform.5 Furthermore, a study analyzing the dermatologic content of 17 dermatology related subreddits that had 1000 or more subscribers found that 70.6% of posts fell under the category of “seeking health/cosmetic advice.”6 Reddit users thus are vulnerable to receiving advice based on personal bias and exposing their health information to the public.
We hypothesized that ChatGPT would provide users with guidance that was more closely aligned with typical dermatologists’ advice due to its thorough analysis and compilation of diverse sources and recommendations available on the internet. We expected Reddit to yield recommendations of lesser quality and a diminished safety score, primarily due to the absence of credibility-vetting mechanisms and the influence of personal biases within the advice shared.
User-submitted posts to large dermatologic community Reddit forums representing a few of the most common skin conditions (r/eczema, r/acne, r/Folliculitis, r/SebDerm, r/Hidradenitis, r/keratosis, and r/Psoriasis) were retrospectively reviewed from January 2024 to March 2024. The most popular posts that did not include photographs were included in our study. Posts with photographs were excluded, as clinical images were not able to be uploaded to the publicly available ChatGPT 3.5. We collected real user questions about common skin conditions from Reddit forums and then asked ChatGPT to answer those same questions. We compared ChatGPT’s responses to the most upvoted Reddit comments to see how they matched up (eTable).

Each ChatGPT response and the top-rated Reddit comment were independently evaluated by a board certified dermatologist (S.A.) and a dermatology resident (A.H.K.). The quality of the ChatGPT and Reddit responses were determined by scoring the accuracy, appropriateness, safety consideration, and specificity on a 5-point Likert scale (1=low, 5=high). The 2 evaluators’ mean scores for each of the 4 categories were calculated based on adequate interrater reliability, which was tested using Cohen’s κ coefficient. Related-samples sign tests were used to compare ChatGPT and Reddit responses for each of the 4 categories. Analysis was completed using SPSS statistics software version 29.0 (IBM). The evaluators also were asked to provide qualitative feedback on the strengths and weaknesses of each response.
Our retrospective review yielded 20 total questions: 5 (25%) on atopic dermatitis, 4 (20%) on acne, 4 (20%) on hidradenitis suppurativa, 4 (20%) on psoriasis, 1 (5%) on folliculitis, 1 (5%) on keratosis pilaris, and 1 (5%) on seborrheic dermatitis. The number of posts was limited to 20 due to the extensive time required for grading each response. These 20 questions were selected from a larger pool of eligible posts based on factors such as clarity and relevance to common skin conditions. With regard to the types of questions that were asked, 6 (30%) were related to general management of a diagnosis, 5 (25%) were on treatment recommendations for symptom relief, 3 (15%) were on optimal utilization of current treatment regimens, 2 (10%) were on prescription side effects, 2 (10%) were on diagnosis presentation, 1 (5%) was on potential triggers of the diagnosis, and 1 (5%) was on natural treatment recommendations.
Mean (SD) evaluator scores for accuracy were significantly higher among ChatGPT responses compared with Reddit (4.63 [0.60] vs 2.60 [0.98])(P<.001). ChatGPT responses also were significantly higher for appropriateness compared with Reddit (4.55 [0.71] vs 2.58 [1.02])(P<.001) and safety consideration (4.88 [0.56] vs 2.80[0.97])(P <.001). There was no significant difference in mean specificity scores between ChatGPT and Reddit (4.25[1.02] vs 3.80 [0.70])(P=.096)(Figure).

For the Reddit responses, the weighted Cohen’s κ coefficient between the 2 evaluators was 0.200 (95% CI, –.089 to .489) for accuracy, 0.255 (95% CI, .014-.497) for appropriateness, 0.385 (95% CI, .176-.594) for safety consideration, and –0.024 (95% CI, –.177 to .129) for specificity. For the ChatGPT responses, the weighted Cohen’s κ coefficient between the 2 evaluators was 0.426 (95% CI, .122-.730) for accuracy, 0.571 (95% CI, .294-.849) for appropriateness, 0.655 (95% CI, .632-.678) for safety consideration, and 0.313 (95% CI, .043-.584) for specificity.
The strengths and weaknesses of the responses also were qualitatively analyzed. One commonly observed strength was ChatGPT’s frequent and appropriate recommendation for users to consult a dermatologist. In the case of atopic dermatitis—one of the more frequently asked about conditions—ChatGPT consistently emphasized evidence-based strategies such as gentle skin care and moisturization, reflecting alignment with clinical guidelines. Additionally, a common weakness of both ChatGPT and Reddit responses generally was the lack of personalized guidance and comprehensive discussion of the risks and benefits of specific treatments. It also was noted that neither platform consistently explored differential diagnoses—for example, distinguishing atopic dermatitis from conditions such as allergic contact dermatitis—limiting the diagnostic depth of the responses.
ChatGPT and Reddit can provide patients with quick and accessible health information for various dermatologic concerns. The results of our study demonstrated a significantly higher level of accuracy, appropriateness, and safety of responses generated by ChatGPT compared with human-generated responses on Reddit (P<.001). Both platforms offered similarly specific responses to user inquiries, demonstrating ChatGPT’s ability to comprehend user questions and draw from publicly available texts and Reddit users’ contributing insights based on their own first-hand experiences.
Reddit’s dermatologic forums often feature personal anecdotes and unique treatments described by individual users. Although specific to particular dermatologic concerns, such advice lacks an evidence-based standard of care. With the noted inherent trust of patients seeking guidance within Reddit communities, patients may follow unhelpful or potentially dangerous medical advice.5 A study examining 300 user-submitted posts on popular Reddit dermatology forums during the COVID-19 pandemic found that the mean scores for top-rated comments’ potential to be misleading or dangerous was 2.33 out of 5 on a Likert scale (95% CI, 2.18- 2.48).7 Dermatologists should be aware of the potential risks associated with dermatologic advice offered on Reddit and should caution patients against relying solely on this information without consulting a qualified dermatologist first.
Reddit’s open-forum design provides licensed dermatologists with the opportunity to disseminate evidence based information regarding dermatologic conditions. Currently, there is a subreddit (r/AskDocs) that allows users to post medical questions that can be answered by moderator-verified physicians. Participation from dermatologists in online communities such as this can improve the quality of dermatologic information shared online, combat misinformation, and promote safe skin care practices.
ChatGPT offers more accurate, appropriate, and safe information compared to Reddit responses, but its answers lack personalization. In a clinical setting, a personalized treatment plan from a physician can be tailored with a comprehensive discussion of the risks and benefits. Further, clinical settings allow for diagnosis and confirmation via biopsy and meticulous history taking to ensure that the diagnosis and treatment plan are accurate. While ChatGPT may be an option for seeking basic advice on dermatologic conditions, a licensed dermatologist should always be consulted for proper medical advice. Services such as telehealth may be another option to for patients with limited access to care.
Since ChatGPT 3.5 does not support the ability to upload images, our study acknowledges a limitation regarding the inclusion of Reddit posts containing photographs. Images can improve the response quality from both Reddit users and ChatGPT. While the updated ChatGPT 4o is capable of processing images, it requires a monthly subscription fee. The free version was chosen for use in this study, as this may reflect the most likely version that patients of low socioeconomic status would utilize to access dermatologic care; however, there is potential for growth and improvement of ChatGPT’s capability in providing medical advice.
This study compared the strengths and limitations of ChatGPT’s and Reddit’s responses to common dermatologic inquiries. ChatGPT and Reddit both show potential to be helpful sources of dermatologic health information; however, their current versions have many limitations and require caution and careful examination by patients of the guidance provided. Clinicians should be aware of these limitations when advising patients and emphasize the importance of consulting a licensed dermatologist for personalized, evidence-based care. For the best medical advice, it is always advisable to consult with a licensed dermatologist.
- Roumeliotis KI, Tselikas ND. ChatGPT and open-AI models: a preliminary review. Future Internet. 2023;15:192. doi:10.3390/fi15060192
- Iqbal U, Lee LTJ, Rahmanti AR, et al. Can large language models provide secondary reliable opinion on treatment options for dermatological diseases? J Am Med Inform Assoc. 2024;31:1341-1347. doi:10.1093/jamia/ocae067
- Whiles BB, Bird VG, Canales BK, et al. Caution! AI bot has entered the patient chat: ChatGPT has limitations in providing accurate urologic healthcare advice. Urology. 2023;180:278-284. doi:10.1016/j.urology.2023.07.010
- Nastasi AJ, Courtright KR, Halpern SD, et al. A vignette-based evaluation of ChatGPT’s ability to provide appropriate and equitable medical advice across care contexts. Sci Rep. 2023;13:17885. doi:10.1038/s41598-023-45223-y
- Record RA, Silberman WR, Santiago JE, et al. I sought it, I Reddit: examining health information engagement behaviors among Reddit users. J Health Commun. 2018;23:470-476. doi:10.1080/1081073 0.2018.1465493
- Buntinx-Krieg T, Caravaglio J, Domozych R, et al. Dermatology on Reddit: elucidating trends in dermatologic communications on the world wide web. Dermatol Online J. 2017;23:13030/qt9dr1f7x6.
- Aboul-Fettouh N, Lee KP, Kash N, et al. Social media and dermatology during the COVID-19 pandemic: analyzing usersubmitted posts seeking dermatologic advice on Reddit. Cureus. 2023;15:E33720. doi:10.7759/cureus.33720
- Roumeliotis KI, Tselikas ND. ChatGPT and open-AI models: a preliminary review. Future Internet. 2023;15:192. doi:10.3390/fi15060192
- Iqbal U, Lee LTJ, Rahmanti AR, et al. Can large language models provide secondary reliable opinion on treatment options for dermatological diseases? J Am Med Inform Assoc. 2024;31:1341-1347. doi:10.1093/jamia/ocae067
- Whiles BB, Bird VG, Canales BK, et al. Caution! AI bot has entered the patient chat: ChatGPT has limitations in providing accurate urologic healthcare advice. Urology. 2023;180:278-284. doi:10.1016/j.urology.2023.07.010
- Nastasi AJ, Courtright KR, Halpern SD, et al. A vignette-based evaluation of ChatGPT’s ability to provide appropriate and equitable medical advice across care contexts. Sci Rep. 2023;13:17885. doi:10.1038/s41598-023-45223-y
- Record RA, Silberman WR, Santiago JE, et al. I sought it, I Reddit: examining health information engagement behaviors among Reddit users. J Health Commun. 2018;23:470-476. doi:10.1080/1081073 0.2018.1465493
- Buntinx-Krieg T, Caravaglio J, Domozych R, et al. Dermatology on Reddit: elucidating trends in dermatologic communications on the world wide web. Dermatol Online J. 2017;23:13030/qt9dr1f7x6.
- Aboul-Fettouh N, Lee KP, Kash N, et al. Social media and dermatology during the COVID-19 pandemic: analyzing usersubmitted posts seeking dermatologic advice on Reddit. Cureus. 2023;15:E33720. doi:10.7759/cureus.33720
Comparing the Quality of Patient Guidance on Dermatologic Care Generated by ChatGPT vs Reddit
Comparing the Quality of Patient Guidance on Dermatologic Care Generated by ChatGPT vs Reddit
PRACTICE POINTS
- ChatGPT and Reddit are free, convenient, and accessible online resources that patients may use for guidance on dermatologic care.
- Dermatologists should be aware of the potential risks associated with obtaining medical guidance from ChatGPT and Reddit and caution patients on them.
- An increasing presence of dermatologists on online public forums can increase the dissemination of reliable health care information.
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
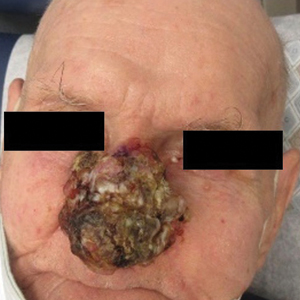
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
PRACTICE POINTS
- Dermatologists should consider using vismodegib for treatment of unresectable basal cell carcinoma.
- Vismodegib dosing regimens can vary; drug holidays can be used to mitigate adverse effects while maintaining desirable treatment outcomes.
Nonhealing Ulcer on the Lower Lip
Nonhealing Ulcer on the Lower Lip
THE DIAGNOSIS: Syphilis
The differential diagnosis of oral lesions can be complex; in our patient, we considered conditions such as pyogenic granuloma, herpes simplex virus, and syphilis, despite the presence of pain. Immunohistochemical staining for spirochete antigens was positive, and serologic confirmation through a positive rapid plasma reagin (RPR) test confirmed the diagnosis of primary syphilis. The patient was promptly referred back to the primary care physician for treatment with intramuscular penicillin, leading to resolution of the lesion. At 3 months’ follow-up in our clinic, the lesion was fully resolved.
A primary syphilitic chancre is the initial lesion caused by Treponema pallidum, typically manifesting as a painless ulcer at the infection site, usually in the genital area; however, chancres also may manifest in other locations (eg, the anus or oral cavity) due to direct contact with infectious lesions on another individual. Our case represents an atypical presentation of an oral syphilitic chancre.
Syphilis is a sexually transmitted infection with various clinical manifestations. It is crucial to consider syphilis in the differential diagnosis of ulcerative lesions even when pain is present, especially in high-risk individuals such as those who engage in unprotected sex.1,2 Oral syphilitic chancres have been documented in the medical literature for more than a century, underscoring the importance of maintaining a high index of suspicion for diagnosis and a low threshold for obtaining an RPR test to facilitate early detection and treatment.2,3 Notably, the prevalence of syphilis is higher in men who have sex with men, particularly among those who engage in unprotected oral and anal sex. Increased screening and early treatment are essential to control the spread of disease within all populations. Doxycycline postexposure prophylaxis (doxyPEP) is used as a preventive measure for syphilis, chlamydia, and gonorrhea.4 This regimen consists of 200 mg of doxycycline taken within 24 hours but no later than 72 hours after unprotected anal, vaginal, or oral sex.
Our case highlights the importance of considering the differential diagnosis of oral ulcers, particularly in high-risk populations such as men who have sex with men. Prompt diagnosis, effective treatment, and preventive strategies such as doxyPEP are essential for controlling syphilis. Comprehensive patient education and regular follow-up appointments are critical components of successful management.
The United States has experienced a considerable rise in primary and congenital syphilis cases, with an 80% increase between 2018 and 2022.6 Serologic testing is the primary method for diagnosing, staging, and managing syphilis. Sexually active patients with suspected syphilis or unexplained symptoms should undergo testing. Prompt diagnosis and treatment can prevent systemic complications, including ocular involvement and permanent blindness.
Syphilis is transmitted through direct contact with a syphilitic ulcer or saliva or blood from an infected individual. Oral syphilitic ulcers can develop on the lips, tongue, oral mucosa, and tonsils. Chancres can range from a few millimeters to several centimeters, with an incubation period of 10 to 90 days (average, 21 days). The chancre lasts 3 to 6 weeks and heals spontaneously. Without treatment, primary syphilis can progress to secondary syphilis, characterized by a papulosquamous eruption and mucosal involvement, and potentially tertiary syphilis, which can affect the central nervous system, heart, bones, and skin.7
Immunocompromised patients, especially those diagnosed with HIV, face increased risks including altered clinical presentations (eg, multiple or deep chancres), delayed healing, overlapping stages of disease, and increased severity of organ involvement. All sexually active individuals should be screened for syphilis every 3 to 6 months, particularly those with unexplained oral ulcers.
Serologic testing is fundamental for syphilis diagnosis and management. Nontreponemal tests such as RPR and treponemal tests such as the fluorescent treponemal antibody absorption test provide comprehensive diagnostic information. Early diagnosis and empiric treatment are crucial in suspected cases. Ocular screening is recommended for suspected or confirmed syphilis cases.7
Management of syphilis includes treating all sexual partners and providing thorough patient education on the disease. Monitoring for the Jarisch-Herxheimer reaction—an acute febrile reaction following penicillin therapy—is important, especially in pregnant patients.5 Serologic evaluation at 6 and 12 months posttreatment is recommended, with more frequent evaluations if follow-up is uncertain, particularly for those with inconsistent access to health care or in whom reinfection is suspected. Guidelines from the Centers for Disease Control and Prevention advocate for intramuscular penicillin G benzathine as the preferred treatment, with specific dosing for adults and children.7 Due to the ongoing bicillin shortage, alternatives such as extencilline have temporarily been allowed for use in the United States.8
The rising incidence of syphilis in the United States underscores the critical need for enhanced public health initiatives focusing on education, screening, and early intervention. Comprehensive sexual education that includes information about syphilis and other sexually transmitted infections, proper use of prophylactic measures such as condoms, and the benefits of doxyPEP can considerably reduce transmission rates. Health care providers should routinely discuss these preventive measures with their patients, especially those in high-risk groups.
Our case highlights the importance of considering syphilis in the differential diagnosis of oral ulcers, particularly in high-risk populations. Timely diagnosis, effective treatment, and preventive measures such as doxyPEP are essential for managing and controlling syphilis. The rising incidence of syphilis in the United States warrants increased screening, patient education, and public health interventions to address this notable health challenge. The syphilis crisis calls for coordinated efforts from health care providers, public health officials, and community leaders to curb the spread of this infection and protect public health.
- Mayer KH, Traeger M, Marcus JL. Doxycycline postexposure prophylaxis and sexually transmitted infections. JAMA. 2023;330:1381-1382. doi:10.1001/jama.2023.16416
- Cossman JP, Fournier JB. Frequency of syphilis diagnoses by dermatologists. JAMA Dermatol. 2017;153:718-719. doi:10.1001 /jamadermatol.2017.0460
- Porterfield C, Brodell D, Dolohanty L, et al. Primary syphilis presenting as a chronic lip ulcer. Cureus. 2020;12:E7086. doi:10.7759 /cureus.7086
- Schamberg JF. An epidemic of chancres of the lip from kissing. JAMA. 1911;LVII:783-784. doi:10.1001/jama.1911.04260090005002
- Farmer TW. Jarisch-Herxheimer reaction in early syphilis. JAMA. 1948;138:480–485. doi:10.1001/jama.1948.02900070012003
- Winney A. Why is syphilis spiking in the U.S.? Johns Hopkins Bloomberg School of Public Health. Johns Hopkins Bloomberg School of Public Health. Published March 13, 2024. Accessed April 30, 2025. https://publichealth.jhu.edu/why-is-syphilis-spiking-in-the-us
- Koundanya VV, Tripathy K. Syphilis ocular manifestations. StatPearls Publishing; 2021. Updated August 25, 2023. Accessed May 6, 2025. https://www.ncbi.nlm.nih.gov/books/NBK558957/
- CDC. FDA announcement on availability of extencilline. National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention. Published July 19, 2024. Accessed April 30, 2025. https://www.cdc.gov/nchhstp/director-letters/extencilline-during-bicillin-l-a-shortage.html
THE DIAGNOSIS: Syphilis
The differential diagnosis of oral lesions can be complex; in our patient, we considered conditions such as pyogenic granuloma, herpes simplex virus, and syphilis, despite the presence of pain. Immunohistochemical staining for spirochete antigens was positive, and serologic confirmation through a positive rapid plasma reagin (RPR) test confirmed the diagnosis of primary syphilis. The patient was promptly referred back to the primary care physician for treatment with intramuscular penicillin, leading to resolution of the lesion. At 3 months’ follow-up in our clinic, the lesion was fully resolved.
A primary syphilitic chancre is the initial lesion caused by Treponema pallidum, typically manifesting as a painless ulcer at the infection site, usually in the genital area; however, chancres also may manifest in other locations (eg, the anus or oral cavity) due to direct contact with infectious lesions on another individual. Our case represents an atypical presentation of an oral syphilitic chancre.
Syphilis is a sexually transmitted infection with various clinical manifestations. It is crucial to consider syphilis in the differential diagnosis of ulcerative lesions even when pain is present, especially in high-risk individuals such as those who engage in unprotected sex.1,2 Oral syphilitic chancres have been documented in the medical literature for more than a century, underscoring the importance of maintaining a high index of suspicion for diagnosis and a low threshold for obtaining an RPR test to facilitate early detection and treatment.2,3 Notably, the prevalence of syphilis is higher in men who have sex with men, particularly among those who engage in unprotected oral and anal sex. Increased screening and early treatment are essential to control the spread of disease within all populations. Doxycycline postexposure prophylaxis (doxyPEP) is used as a preventive measure for syphilis, chlamydia, and gonorrhea.4 This regimen consists of 200 mg of doxycycline taken within 24 hours but no later than 72 hours after unprotected anal, vaginal, or oral sex.
Our case highlights the importance of considering the differential diagnosis of oral ulcers, particularly in high-risk populations such as men who have sex with men. Prompt diagnosis, effective treatment, and preventive strategies such as doxyPEP are essential for controlling syphilis. Comprehensive patient education and regular follow-up appointments are critical components of successful management.
The United States has experienced a considerable rise in primary and congenital syphilis cases, with an 80% increase between 2018 and 2022.6 Serologic testing is the primary method for diagnosing, staging, and managing syphilis. Sexually active patients with suspected syphilis or unexplained symptoms should undergo testing. Prompt diagnosis and treatment can prevent systemic complications, including ocular involvement and permanent blindness.
Syphilis is transmitted through direct contact with a syphilitic ulcer or saliva or blood from an infected individual. Oral syphilitic ulcers can develop on the lips, tongue, oral mucosa, and tonsils. Chancres can range from a few millimeters to several centimeters, with an incubation period of 10 to 90 days (average, 21 days). The chancre lasts 3 to 6 weeks and heals spontaneously. Without treatment, primary syphilis can progress to secondary syphilis, characterized by a papulosquamous eruption and mucosal involvement, and potentially tertiary syphilis, which can affect the central nervous system, heart, bones, and skin.7
Immunocompromised patients, especially those diagnosed with HIV, face increased risks including altered clinical presentations (eg, multiple or deep chancres), delayed healing, overlapping stages of disease, and increased severity of organ involvement. All sexually active individuals should be screened for syphilis every 3 to 6 months, particularly those with unexplained oral ulcers.
Serologic testing is fundamental for syphilis diagnosis and management. Nontreponemal tests such as RPR and treponemal tests such as the fluorescent treponemal antibody absorption test provide comprehensive diagnostic information. Early diagnosis and empiric treatment are crucial in suspected cases. Ocular screening is recommended for suspected or confirmed syphilis cases.7
Management of syphilis includes treating all sexual partners and providing thorough patient education on the disease. Monitoring for the Jarisch-Herxheimer reaction—an acute febrile reaction following penicillin therapy—is important, especially in pregnant patients.5 Serologic evaluation at 6 and 12 months posttreatment is recommended, with more frequent evaluations if follow-up is uncertain, particularly for those with inconsistent access to health care or in whom reinfection is suspected. Guidelines from the Centers for Disease Control and Prevention advocate for intramuscular penicillin G benzathine as the preferred treatment, with specific dosing for adults and children.7 Due to the ongoing bicillin shortage, alternatives such as extencilline have temporarily been allowed for use in the United States.8
The rising incidence of syphilis in the United States underscores the critical need for enhanced public health initiatives focusing on education, screening, and early intervention. Comprehensive sexual education that includes information about syphilis and other sexually transmitted infections, proper use of prophylactic measures such as condoms, and the benefits of doxyPEP can considerably reduce transmission rates. Health care providers should routinely discuss these preventive measures with their patients, especially those in high-risk groups.
Our case highlights the importance of considering syphilis in the differential diagnosis of oral ulcers, particularly in high-risk populations. Timely diagnosis, effective treatment, and preventive measures such as doxyPEP are essential for managing and controlling syphilis. The rising incidence of syphilis in the United States warrants increased screening, patient education, and public health interventions to address this notable health challenge. The syphilis crisis calls for coordinated efforts from health care providers, public health officials, and community leaders to curb the spread of this infection and protect public health.
THE DIAGNOSIS: Syphilis
The differential diagnosis of oral lesions can be complex; in our patient, we considered conditions such as pyogenic granuloma, herpes simplex virus, and syphilis, despite the presence of pain. Immunohistochemical staining for spirochete antigens was positive, and serologic confirmation through a positive rapid plasma reagin (RPR) test confirmed the diagnosis of primary syphilis. The patient was promptly referred back to the primary care physician for treatment with intramuscular penicillin, leading to resolution of the lesion. At 3 months’ follow-up in our clinic, the lesion was fully resolved.
A primary syphilitic chancre is the initial lesion caused by Treponema pallidum, typically manifesting as a painless ulcer at the infection site, usually in the genital area; however, chancres also may manifest in other locations (eg, the anus or oral cavity) due to direct contact with infectious lesions on another individual. Our case represents an atypical presentation of an oral syphilitic chancre.
Syphilis is a sexually transmitted infection with various clinical manifestations. It is crucial to consider syphilis in the differential diagnosis of ulcerative lesions even when pain is present, especially in high-risk individuals such as those who engage in unprotected sex.1,2 Oral syphilitic chancres have been documented in the medical literature for more than a century, underscoring the importance of maintaining a high index of suspicion for diagnosis and a low threshold for obtaining an RPR test to facilitate early detection and treatment.2,3 Notably, the prevalence of syphilis is higher in men who have sex with men, particularly among those who engage in unprotected oral and anal sex. Increased screening and early treatment are essential to control the spread of disease within all populations. Doxycycline postexposure prophylaxis (doxyPEP) is used as a preventive measure for syphilis, chlamydia, and gonorrhea.4 This regimen consists of 200 mg of doxycycline taken within 24 hours but no later than 72 hours after unprotected anal, vaginal, or oral sex.
Our case highlights the importance of considering the differential diagnosis of oral ulcers, particularly in high-risk populations such as men who have sex with men. Prompt diagnosis, effective treatment, and preventive strategies such as doxyPEP are essential for controlling syphilis. Comprehensive patient education and regular follow-up appointments are critical components of successful management.
The United States has experienced a considerable rise in primary and congenital syphilis cases, with an 80% increase between 2018 and 2022.6 Serologic testing is the primary method for diagnosing, staging, and managing syphilis. Sexually active patients with suspected syphilis or unexplained symptoms should undergo testing. Prompt diagnosis and treatment can prevent systemic complications, including ocular involvement and permanent blindness.
Syphilis is transmitted through direct contact with a syphilitic ulcer or saliva or blood from an infected individual. Oral syphilitic ulcers can develop on the lips, tongue, oral mucosa, and tonsils. Chancres can range from a few millimeters to several centimeters, with an incubation period of 10 to 90 days (average, 21 days). The chancre lasts 3 to 6 weeks and heals spontaneously. Without treatment, primary syphilis can progress to secondary syphilis, characterized by a papulosquamous eruption and mucosal involvement, and potentially tertiary syphilis, which can affect the central nervous system, heart, bones, and skin.7
Immunocompromised patients, especially those diagnosed with HIV, face increased risks including altered clinical presentations (eg, multiple or deep chancres), delayed healing, overlapping stages of disease, and increased severity of organ involvement. All sexually active individuals should be screened for syphilis every 3 to 6 months, particularly those with unexplained oral ulcers.
Serologic testing is fundamental for syphilis diagnosis and management. Nontreponemal tests such as RPR and treponemal tests such as the fluorescent treponemal antibody absorption test provide comprehensive diagnostic information. Early diagnosis and empiric treatment are crucial in suspected cases. Ocular screening is recommended for suspected or confirmed syphilis cases.7
Management of syphilis includes treating all sexual partners and providing thorough patient education on the disease. Monitoring for the Jarisch-Herxheimer reaction—an acute febrile reaction following penicillin therapy—is important, especially in pregnant patients.5 Serologic evaluation at 6 and 12 months posttreatment is recommended, with more frequent evaluations if follow-up is uncertain, particularly for those with inconsistent access to health care or in whom reinfection is suspected. Guidelines from the Centers for Disease Control and Prevention advocate for intramuscular penicillin G benzathine as the preferred treatment, with specific dosing for adults and children.7 Due to the ongoing bicillin shortage, alternatives such as extencilline have temporarily been allowed for use in the United States.8
The rising incidence of syphilis in the United States underscores the critical need for enhanced public health initiatives focusing on education, screening, and early intervention. Comprehensive sexual education that includes information about syphilis and other sexually transmitted infections, proper use of prophylactic measures such as condoms, and the benefits of doxyPEP can considerably reduce transmission rates. Health care providers should routinely discuss these preventive measures with their patients, especially those in high-risk groups.
Our case highlights the importance of considering syphilis in the differential diagnosis of oral ulcers, particularly in high-risk populations. Timely diagnosis, effective treatment, and preventive measures such as doxyPEP are essential for managing and controlling syphilis. The rising incidence of syphilis in the United States warrants increased screening, patient education, and public health interventions to address this notable health challenge. The syphilis crisis calls for coordinated efforts from health care providers, public health officials, and community leaders to curb the spread of this infection and protect public health.
- Mayer KH, Traeger M, Marcus JL. Doxycycline postexposure prophylaxis and sexually transmitted infections. JAMA. 2023;330:1381-1382. doi:10.1001/jama.2023.16416
- Cossman JP, Fournier JB. Frequency of syphilis diagnoses by dermatologists. JAMA Dermatol. 2017;153:718-719. doi:10.1001 /jamadermatol.2017.0460
- Porterfield C, Brodell D, Dolohanty L, et al. Primary syphilis presenting as a chronic lip ulcer. Cureus. 2020;12:E7086. doi:10.7759 /cureus.7086
- Schamberg JF. An epidemic of chancres of the lip from kissing. JAMA. 1911;LVII:783-784. doi:10.1001/jama.1911.04260090005002
- Farmer TW. Jarisch-Herxheimer reaction in early syphilis. JAMA. 1948;138:480–485. doi:10.1001/jama.1948.02900070012003
- Winney A. Why is syphilis spiking in the U.S.? Johns Hopkins Bloomberg School of Public Health. Johns Hopkins Bloomberg School of Public Health. Published March 13, 2024. Accessed April 30, 2025. https://publichealth.jhu.edu/why-is-syphilis-spiking-in-the-us
- Koundanya VV, Tripathy K. Syphilis ocular manifestations. StatPearls Publishing; 2021. Updated August 25, 2023. Accessed May 6, 2025. https://www.ncbi.nlm.nih.gov/books/NBK558957/
- CDC. FDA announcement on availability of extencilline. National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention. Published July 19, 2024. Accessed April 30, 2025. https://www.cdc.gov/nchhstp/director-letters/extencilline-during-bicillin-l-a-shortage.html
- Mayer KH, Traeger M, Marcus JL. Doxycycline postexposure prophylaxis and sexually transmitted infections. JAMA. 2023;330:1381-1382. doi:10.1001/jama.2023.16416
- Cossman JP, Fournier JB. Frequency of syphilis diagnoses by dermatologists. JAMA Dermatol. 2017;153:718-719. doi:10.1001 /jamadermatol.2017.0460
- Porterfield C, Brodell D, Dolohanty L, et al. Primary syphilis presenting as a chronic lip ulcer. Cureus. 2020;12:E7086. doi:10.7759 /cureus.7086
- Schamberg JF. An epidemic of chancres of the lip from kissing. JAMA. 1911;LVII:783-784. doi:10.1001/jama.1911.04260090005002
- Farmer TW. Jarisch-Herxheimer reaction in early syphilis. JAMA. 1948;138:480–485. doi:10.1001/jama.1948.02900070012003
- Winney A. Why is syphilis spiking in the U.S.? Johns Hopkins Bloomberg School of Public Health. Johns Hopkins Bloomberg School of Public Health. Published March 13, 2024. Accessed April 30, 2025. https://publichealth.jhu.edu/why-is-syphilis-spiking-in-the-us
- Koundanya VV, Tripathy K. Syphilis ocular manifestations. StatPearls Publishing; 2021. Updated August 25, 2023. Accessed May 6, 2025. https://www.ncbi.nlm.nih.gov/books/NBK558957/
- CDC. FDA announcement on availability of extencilline. National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention. Published July 19, 2024. Accessed April 30, 2025. https://www.cdc.gov/nchhstp/director-letters/extencilline-during-bicillin-l-a-shortage.html
Nonhealing Ulcer on the Lower Lip
Nonhealing Ulcer on the Lower Lip
A 54-year-old HIV-negative man with a history of having sex with men presented to his primary care physician with an ulcer on the lower lip of 3 weeks’ duration. The patient reported that the lesion had appeared as a typical cold sore with pain in the area. A 9-day course of oral valacyclovir prescribed by the primary care physician provided no relief or improvement. A 2-mm punch biopsy was performed.
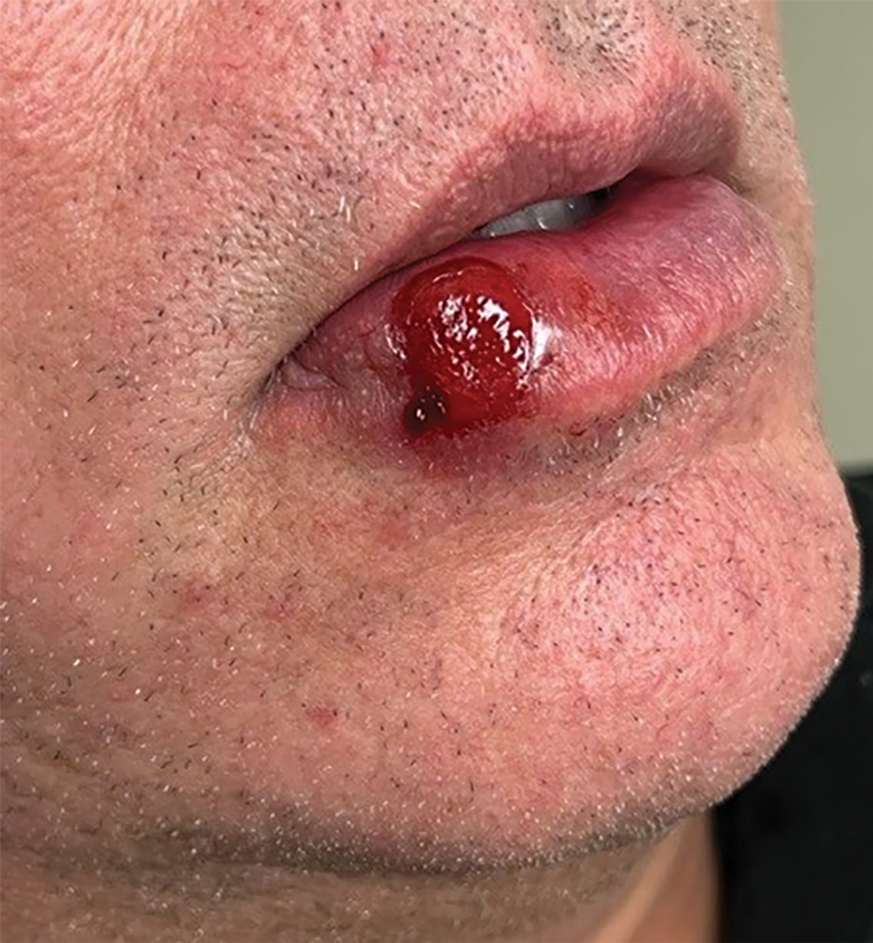
Large Bullae on the Legs in a Hospitalized Patient Following a Gunshot Wound
Large Bullae on the Legs in a Hospitalized Patient Following a Gunshot Wound
THE DIAGNOSIS: Bullous Hemorrhagic Dermatosis
Biopsy results showed an intraepidermal blister with a floor composed of maturing epidermis. The roof of the blister was composed of necrotic keratinocytes with overlying orthokeratosis, and the cavity was filled with a moderate amount of fibrin and dead cells with neutrophils. Direct immunofluorescence (DIF) using specific antihuman IgG, IgM, IgA, C3, and fibrin was negative. Aerobic, anaerobic, and fungal cultures also were negative. With these histopathologic findings, medication exposure, and timing of bullae onset, our patient was diagnosed with bullous hemorrhagic dermatosis (BHD) secondary to enoxaparin administration. Enoxaparin was continued due to increased risk for coagulopathy, and there was complete resolution of the bullae after 5 weeks with no residual symptoms.
Bullous hemorrhagic dermatosis is a rare eruption that can occur after administration of heparin and low-molecular-weight heparin, with enoxaparin being the most commonly implicated drug.1 The lesions typically are seen in elderly men in the seventh decade of life and appear within a median of 7 days after drug exposure. The time course for the postexposure eruption can vary from 2 to 21 days, with reports of skin lesions appearing up to 4 months after exposure.1,2 hemorrhagic bullae (Figure) typically on the arms and legs, though lesions also can develop on the trunk. The lesions can occur in distant areas from the injection site, suggesting BHD may be a systemic reaction, although the etiology is poorly understood.1
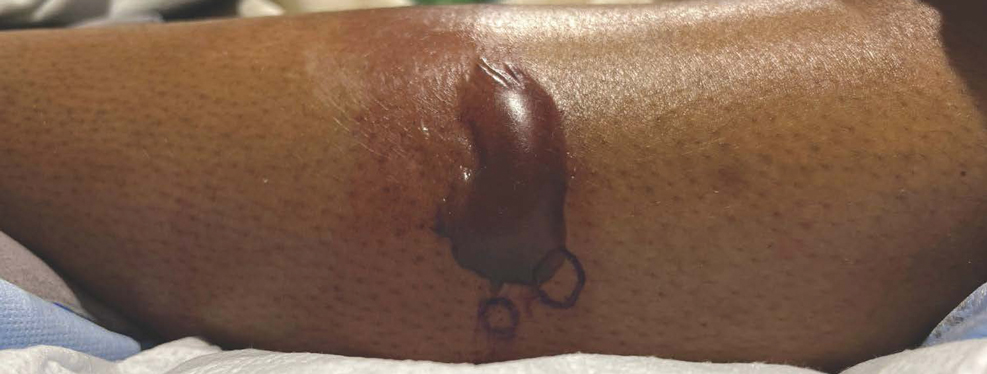
Another heparin reaction that can manifest similarly to BHD is heparin-induced skin necrosis.3 Patients with this condition also may have associated heparin-induced thrombocytopenia upon laboratory investigation and have a more aggressive clinical course than BHD. Biopsy can help differentiate BHD and early heparin-induced skin necrosis if the clinical manifestation is unclear. Histopathologically, BHD typically has intraepidermal bullae filled with blood, whereas heparin-induced skin necrosis has dermal thrombi.1,4 Treatment of both conditions differs in whether to discontinue anticoagulants: heparin-induced skin necrosis requires discontinuation of the medication, while BHD does not.2,3
In patients with BHD, the lesions are self-resolving, and treatment is supportive, although whether enoxaparin is discontinued varies among physicians.2 Lesions typically resolve within 2 weeks of onset, although it is unclear whether continuing anticoagulants delays resolution.1 Discontinuing anticoagulants in certain patients can be life-threatening due to complex comorbidities (eg, risk for venous thromboembolism or pulmonary embolism from prolonged hospitalization or severe trauma) and is not necessary for the resolution of BHD.
In addition to BHD and heparin-induced skin necrosis, our differential diagnosis included bullous pemphigoid, coma blisters, and Vibrio vulnificus infection. Although bullous pemphigoid can manifest with tense bullae that are pauci-inflammatory on histology, DIF would show linear IgG and C3 deposition at the dermal-epidermal junction. In our patient, DIF was negative and favored another etiology for the lesions. Coma blisters can occur in areas of sustained pressure and typically develop in patients with a prolonged hospitalization or those who are sedentary for long periods of time. The distribution of bullae on our patient’s bilateral pretibial shins made this diagnosis unlikely. Vibrio vulnificus infection can manifest as hemorrhagic bullae, though typically after a break in the skin exposed to brackish water. Vibrio vulnificus infection can be life-threatening, resulting in septicemia and increased mortality, and a thorough patient history is important for diagnosis.5
- Russo A, Curtis S, Balbuena-Merle R, et al. Bullous hemorrhagic dermatosis is an under-recognized side effect of full dose lowmolecular weight heparin: a case report and review of the literature. Exp Hematol Oncol. 2018;7:15. doi:10.1186/s40164-018-0108-7
- Dhattarwal N, Gurjar R. Bullous hemorrhagic dermatosis: a rare cutaneous reaction of heparin. J Postgrad Med. 2023;69:97-98. doi:10.4103/jpgm.jpgm_282_22
- Maldonado Cid P, Alonso de Celada RM, Noguera Morel L, et al. Cutaneous adverse events associated with heparin. Clin Exp Dermatol. 2012;37:707-711. doi:10.1111/j.1365-2230.2012.04395.x
- Handschin AE, Trentz O, Kock HJ, et al. Low molecular weight heparininduced skin necrosis-a systematic review. Langenbecks Arch Surg. 2005;390:249-254. doi:10.1007/s00423-004-0522-7
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733. doi:10.1128/IAI.01046-08
THE DIAGNOSIS: Bullous Hemorrhagic Dermatosis
Biopsy results showed an intraepidermal blister with a floor composed of maturing epidermis. The roof of the blister was composed of necrotic keratinocytes with overlying orthokeratosis, and the cavity was filled with a moderate amount of fibrin and dead cells with neutrophils. Direct immunofluorescence (DIF) using specific antihuman IgG, IgM, IgA, C3, and fibrin was negative. Aerobic, anaerobic, and fungal cultures also were negative. With these histopathologic findings, medication exposure, and timing of bullae onset, our patient was diagnosed with bullous hemorrhagic dermatosis (BHD) secondary to enoxaparin administration. Enoxaparin was continued due to increased risk for coagulopathy, and there was complete resolution of the bullae after 5 weeks with no residual symptoms.
Bullous hemorrhagic dermatosis is a rare eruption that can occur after administration of heparin and low-molecular-weight heparin, with enoxaparin being the most commonly implicated drug.1 The lesions typically are seen in elderly men in the seventh decade of life and appear within a median of 7 days after drug exposure. The time course for the postexposure eruption can vary from 2 to 21 days, with reports of skin lesions appearing up to 4 months after exposure.1,2 hemorrhagic bullae (Figure) typically on the arms and legs, though lesions also can develop on the trunk. The lesions can occur in distant areas from the injection site, suggesting BHD may be a systemic reaction, although the etiology is poorly understood.1

Another heparin reaction that can manifest similarly to BHD is heparin-induced skin necrosis.3 Patients with this condition also may have associated heparin-induced thrombocytopenia upon laboratory investigation and have a more aggressive clinical course than BHD. Biopsy can help differentiate BHD and early heparin-induced skin necrosis if the clinical manifestation is unclear. Histopathologically, BHD typically has intraepidermal bullae filled with blood, whereas heparin-induced skin necrosis has dermal thrombi.1,4 Treatment of both conditions differs in whether to discontinue anticoagulants: heparin-induced skin necrosis requires discontinuation of the medication, while BHD does not.2,3
In patients with BHD, the lesions are self-resolving, and treatment is supportive, although whether enoxaparin is discontinued varies among physicians.2 Lesions typically resolve within 2 weeks of onset, although it is unclear whether continuing anticoagulants delays resolution.1 Discontinuing anticoagulants in certain patients can be life-threatening due to complex comorbidities (eg, risk for venous thromboembolism or pulmonary embolism from prolonged hospitalization or severe trauma) and is not necessary for the resolution of BHD.
In addition to BHD and heparin-induced skin necrosis, our differential diagnosis included bullous pemphigoid, coma blisters, and Vibrio vulnificus infection. Although bullous pemphigoid can manifest with tense bullae that are pauci-inflammatory on histology, DIF would show linear IgG and C3 deposition at the dermal-epidermal junction. In our patient, DIF was negative and favored another etiology for the lesions. Coma blisters can occur in areas of sustained pressure and typically develop in patients with a prolonged hospitalization or those who are sedentary for long periods of time. The distribution of bullae on our patient’s bilateral pretibial shins made this diagnosis unlikely. Vibrio vulnificus infection can manifest as hemorrhagic bullae, though typically after a break in the skin exposed to brackish water. Vibrio vulnificus infection can be life-threatening, resulting in septicemia and increased mortality, and a thorough patient history is important for diagnosis.5
THE DIAGNOSIS: Bullous Hemorrhagic Dermatosis
Biopsy results showed an intraepidermal blister with a floor composed of maturing epidermis. The roof of the blister was composed of necrotic keratinocytes with overlying orthokeratosis, and the cavity was filled with a moderate amount of fibrin and dead cells with neutrophils. Direct immunofluorescence (DIF) using specific antihuman IgG, IgM, IgA, C3, and fibrin was negative. Aerobic, anaerobic, and fungal cultures also were negative. With these histopathologic findings, medication exposure, and timing of bullae onset, our patient was diagnosed with bullous hemorrhagic dermatosis (BHD) secondary to enoxaparin administration. Enoxaparin was continued due to increased risk for coagulopathy, and there was complete resolution of the bullae after 5 weeks with no residual symptoms.
Bullous hemorrhagic dermatosis is a rare eruption that can occur after administration of heparin and low-molecular-weight heparin, with enoxaparin being the most commonly implicated drug.1 The lesions typically are seen in elderly men in the seventh decade of life and appear within a median of 7 days after drug exposure. The time course for the postexposure eruption can vary from 2 to 21 days, with reports of skin lesions appearing up to 4 months after exposure.1,2 hemorrhagic bullae (Figure) typically on the arms and legs, though lesions also can develop on the trunk. The lesions can occur in distant areas from the injection site, suggesting BHD may be a systemic reaction, although the etiology is poorly understood.1

Another heparin reaction that can manifest similarly to BHD is heparin-induced skin necrosis.3 Patients with this condition also may have associated heparin-induced thrombocytopenia upon laboratory investigation and have a more aggressive clinical course than BHD. Biopsy can help differentiate BHD and early heparin-induced skin necrosis if the clinical manifestation is unclear. Histopathologically, BHD typically has intraepidermal bullae filled with blood, whereas heparin-induced skin necrosis has dermal thrombi.1,4 Treatment of both conditions differs in whether to discontinue anticoagulants: heparin-induced skin necrosis requires discontinuation of the medication, while BHD does not.2,3
In patients with BHD, the lesions are self-resolving, and treatment is supportive, although whether enoxaparin is discontinued varies among physicians.2 Lesions typically resolve within 2 weeks of onset, although it is unclear whether continuing anticoagulants delays resolution.1 Discontinuing anticoagulants in certain patients can be life-threatening due to complex comorbidities (eg, risk for venous thromboembolism or pulmonary embolism from prolonged hospitalization or severe trauma) and is not necessary for the resolution of BHD.
In addition to BHD and heparin-induced skin necrosis, our differential diagnosis included bullous pemphigoid, coma blisters, and Vibrio vulnificus infection. Although bullous pemphigoid can manifest with tense bullae that are pauci-inflammatory on histology, DIF would show linear IgG and C3 deposition at the dermal-epidermal junction. In our patient, DIF was negative and favored another etiology for the lesions. Coma blisters can occur in areas of sustained pressure and typically develop in patients with a prolonged hospitalization or those who are sedentary for long periods of time. The distribution of bullae on our patient’s bilateral pretibial shins made this diagnosis unlikely. Vibrio vulnificus infection can manifest as hemorrhagic bullae, though typically after a break in the skin exposed to brackish water. Vibrio vulnificus infection can be life-threatening, resulting in septicemia and increased mortality, and a thorough patient history is important for diagnosis.5
- Russo A, Curtis S, Balbuena-Merle R, et al. Bullous hemorrhagic dermatosis is an under-recognized side effect of full dose lowmolecular weight heparin: a case report and review of the literature. Exp Hematol Oncol. 2018;7:15. doi:10.1186/s40164-018-0108-7
- Dhattarwal N, Gurjar R. Bullous hemorrhagic dermatosis: a rare cutaneous reaction of heparin. J Postgrad Med. 2023;69:97-98. doi:10.4103/jpgm.jpgm_282_22
- Maldonado Cid P, Alonso de Celada RM, Noguera Morel L, et al. Cutaneous adverse events associated with heparin. Clin Exp Dermatol. 2012;37:707-711. doi:10.1111/j.1365-2230.2012.04395.x
- Handschin AE, Trentz O, Kock HJ, et al. Low molecular weight heparininduced skin necrosis-a systematic review. Langenbecks Arch Surg. 2005;390:249-254. doi:10.1007/s00423-004-0522-7
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733. doi:10.1128/IAI.01046-08
- Russo A, Curtis S, Balbuena-Merle R, et al. Bullous hemorrhagic dermatosis is an under-recognized side effect of full dose lowmolecular weight heparin: a case report and review of the literature. Exp Hematol Oncol. 2018;7:15. doi:10.1186/s40164-018-0108-7
- Dhattarwal N, Gurjar R. Bullous hemorrhagic dermatosis: a rare cutaneous reaction of heparin. J Postgrad Med. 2023;69:97-98. doi:10.4103/jpgm.jpgm_282_22
- Maldonado Cid P, Alonso de Celada RM, Noguera Morel L, et al. Cutaneous adverse events associated with heparin. Clin Exp Dermatol. 2012;37:707-711. doi:10.1111/j.1365-2230.2012.04395.x
- Handschin AE, Trentz O, Kock HJ, et al. Low molecular weight heparininduced skin necrosis-a systematic review. Langenbecks Arch Surg. 2005;390:249-254. doi:10.1007/s00423-004-0522-7
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733. doi:10.1128/IAI.01046-08
Large Bullae on the Legs in a Hospitalized Patient Following a Gunshot Wound
Large Bullae on the Legs in a Hospitalized Patient Following a Gunshot Wound
A 19-year-old man developed fluid-filled blisters on both legs within 1 month of a prolonged hospitalization following a gunshot wound that resulted in complete paralysis of the legs. His medical history was otherwise unremarkable. Medications started during hospitalization included moxifloxacin, levetiracetam, and prophylactic subcutaneous enoxaparin. Physical examination by dermatology revealed tense blood-filled bullae measuring several centimeters with well-demarcated, pink to red, irregularly shaped patches on both legs. A biopsy of a blister was taken.
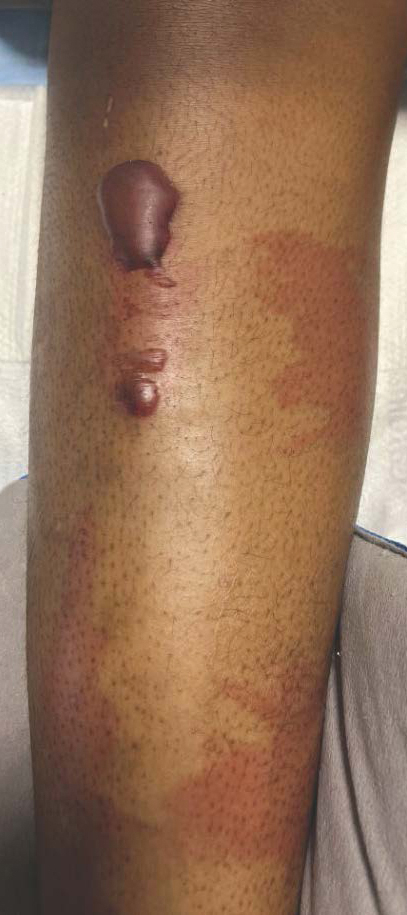
Pigmented Cystic Masses on the Scalp
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7
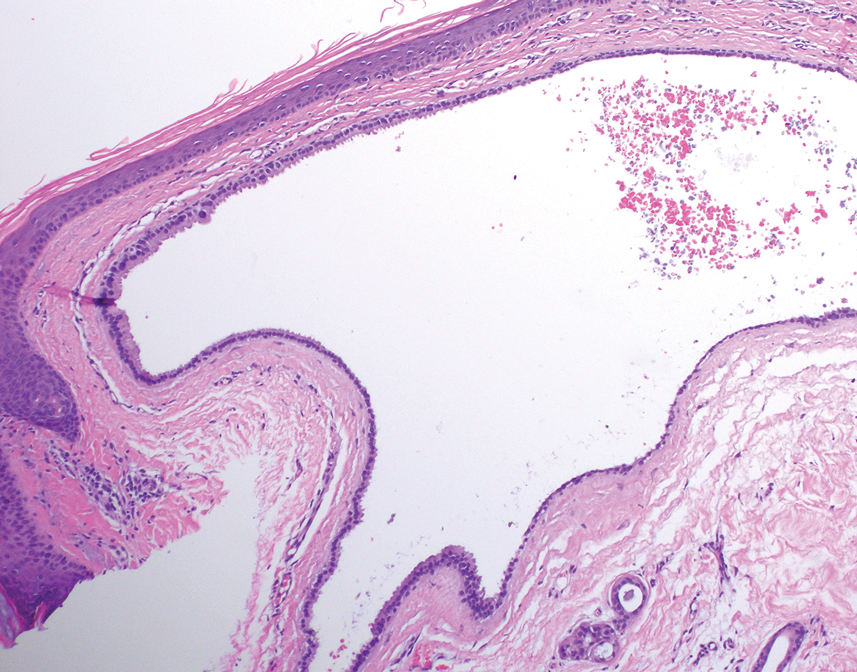
The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.
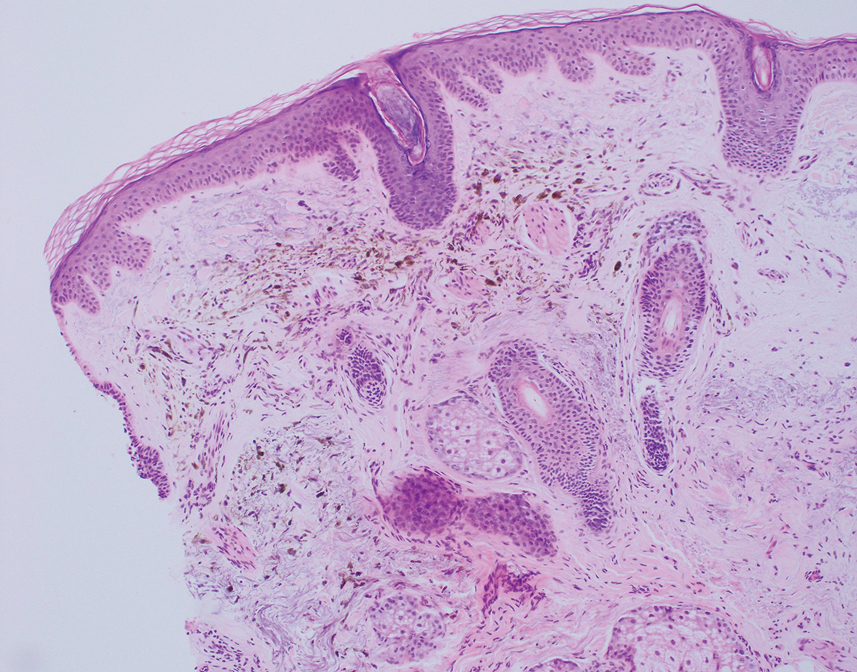
Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.
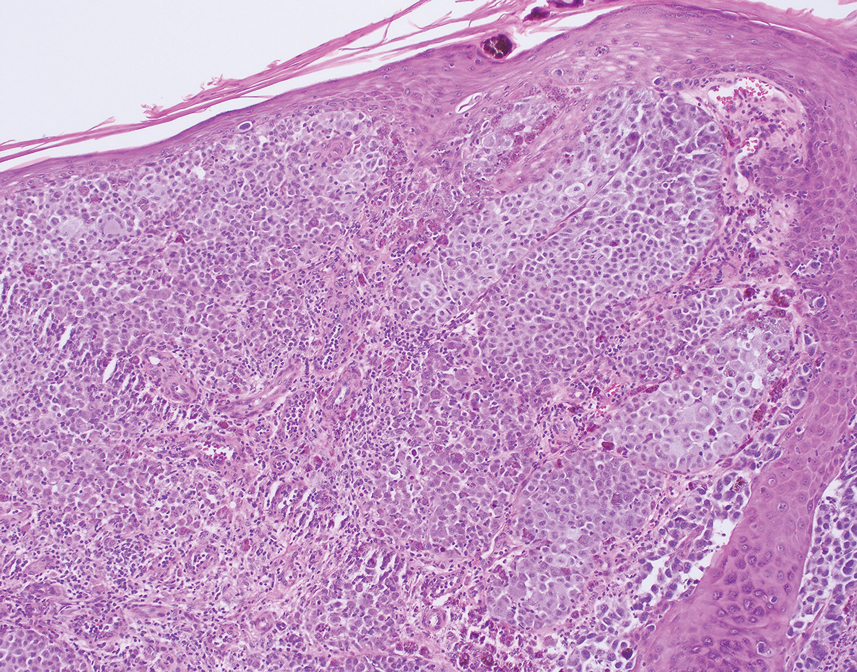
Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7

The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.

Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.

Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7

The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.

Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.

Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
A 67-year-old man presented to the dermatology clinic with 3 asymptomatic pigmented papules on the scalp. The patient reported that he was unaware of the lesions until they were pointed out weeks earlier by his primary care physician during a routine visit. He then was referred to dermatology for follow-up. Physical examination at the current presentation revealed clustered firm, smooth, well-circumscribed, pigmented papules on the scalp measuring 5 to 8 mm. The patient reported no personal or family history of skin cancer but stated that he spent a lot of time outdoors and had a history of 6 blistering sunburns in his life. A punch biopsy of each lesion was performed.
Low-Dose Oral Naltrexone for Darier Disease
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.
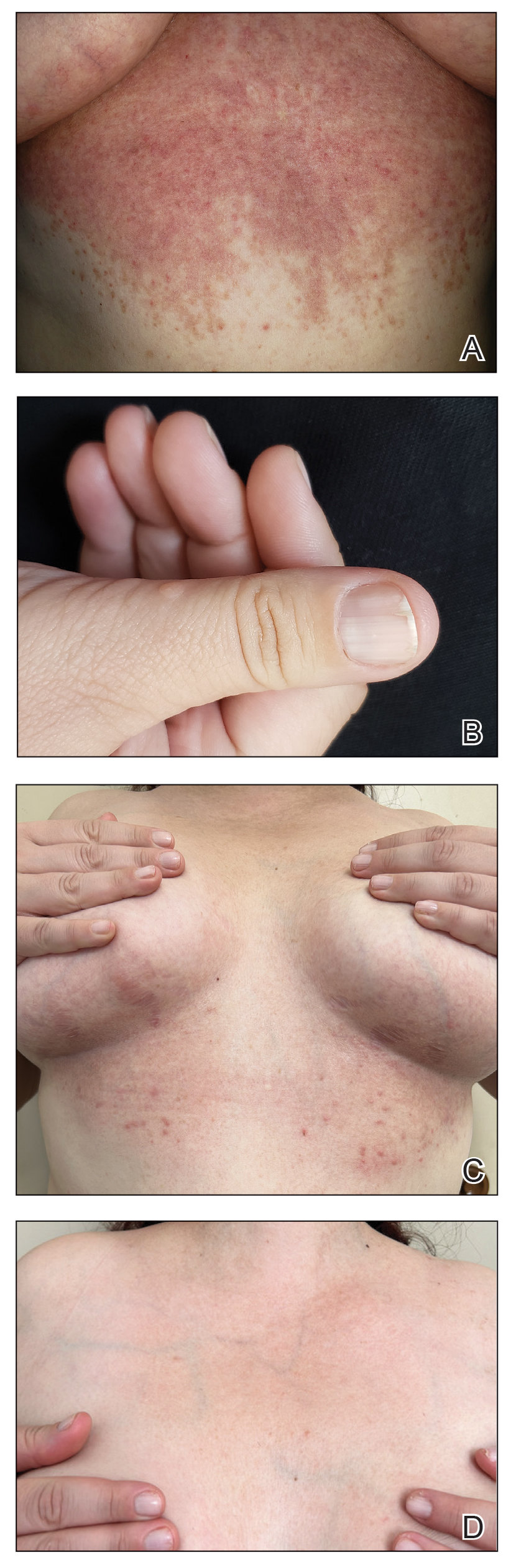
Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2
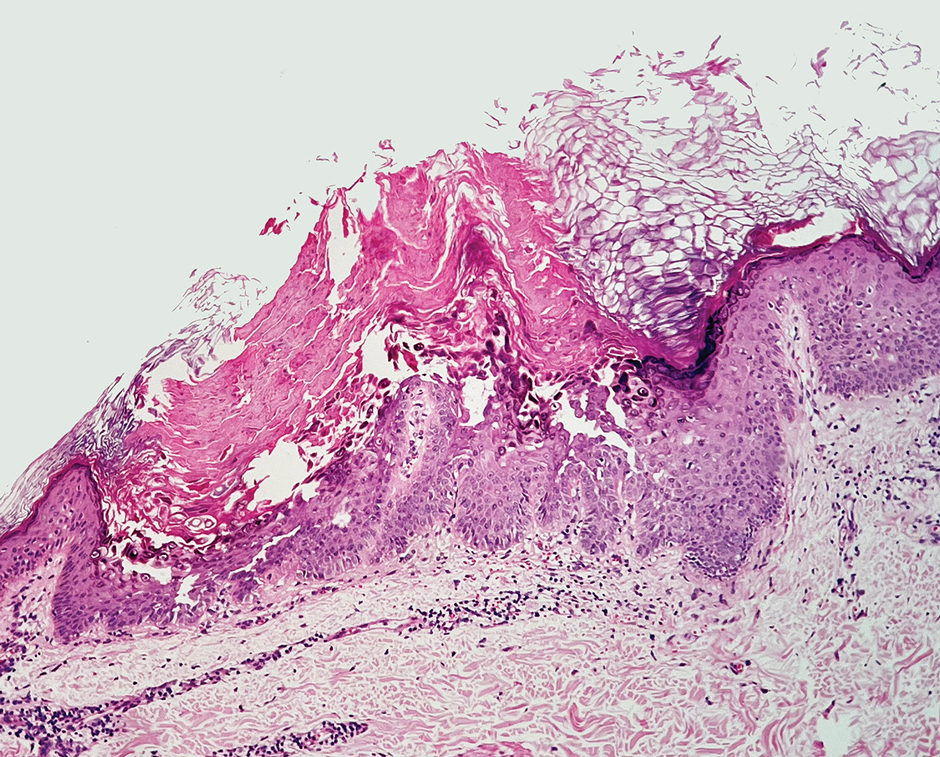
Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.

Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2

Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.

Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2

Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
Practice Points
- Consider low-dose naltrexone as a potential treatment option for patients with Darier disease, as it regulates opioid receptors and has shown benefits in enhancing epidermal differentiation, wound healing, and anti-inflammatory effects.
- Further research is needed to validate the efficacy and safety of low-dose naltrexone in treating Darier disease considering its observed clinical improvement in this single patient case.
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).
Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.
The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.
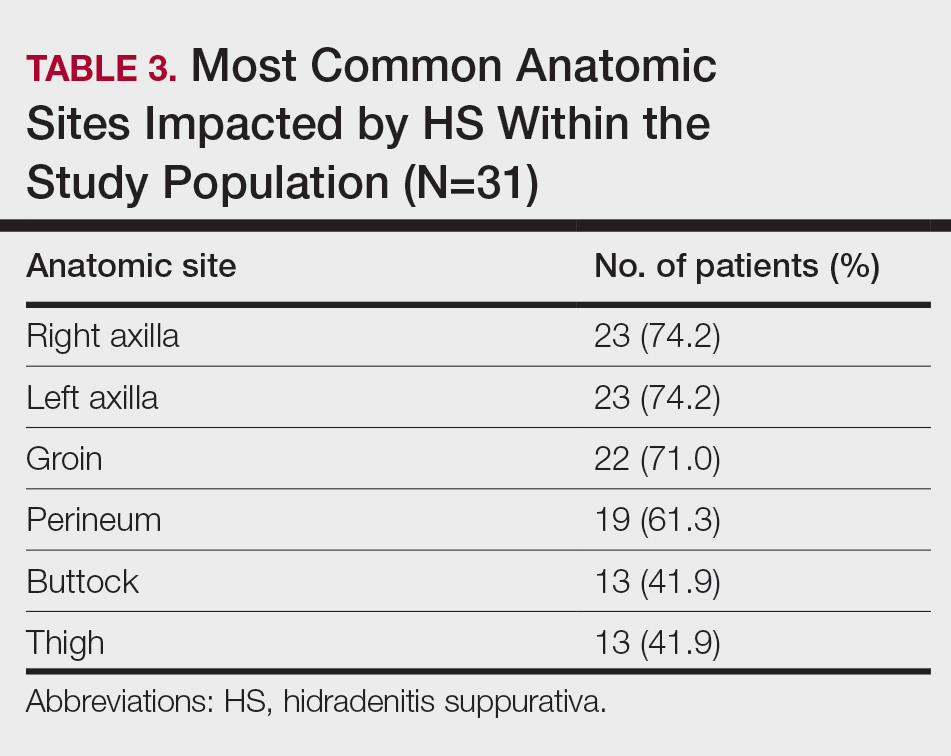
Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).
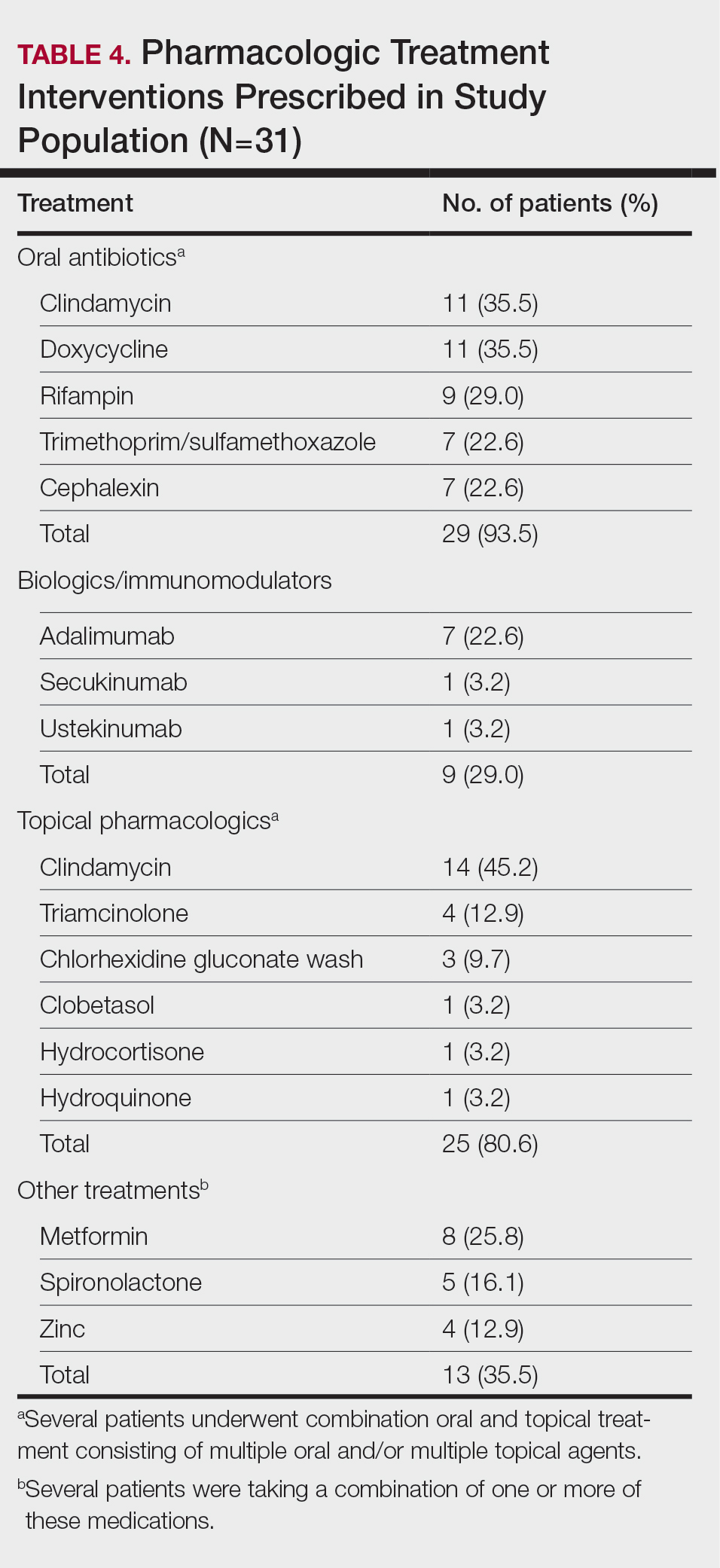
Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).

Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.

The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.

Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).

Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).

Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.

The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.

Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).

Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
PRACTICE POINTS
- Anxiety and depression are highly prevalent among Black patients with hidradenitis suppurativa (HS). Implementing self-report questionnaires at medical office visits are crucial to identifying patients who could benefit from additional psychiatric resources.
- Hidradenitis suppurativa patients with skin of color may have a higher incidence of comorbid gastrointestinal disease than other HS patients.
- Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
- The patient cohort described here had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4
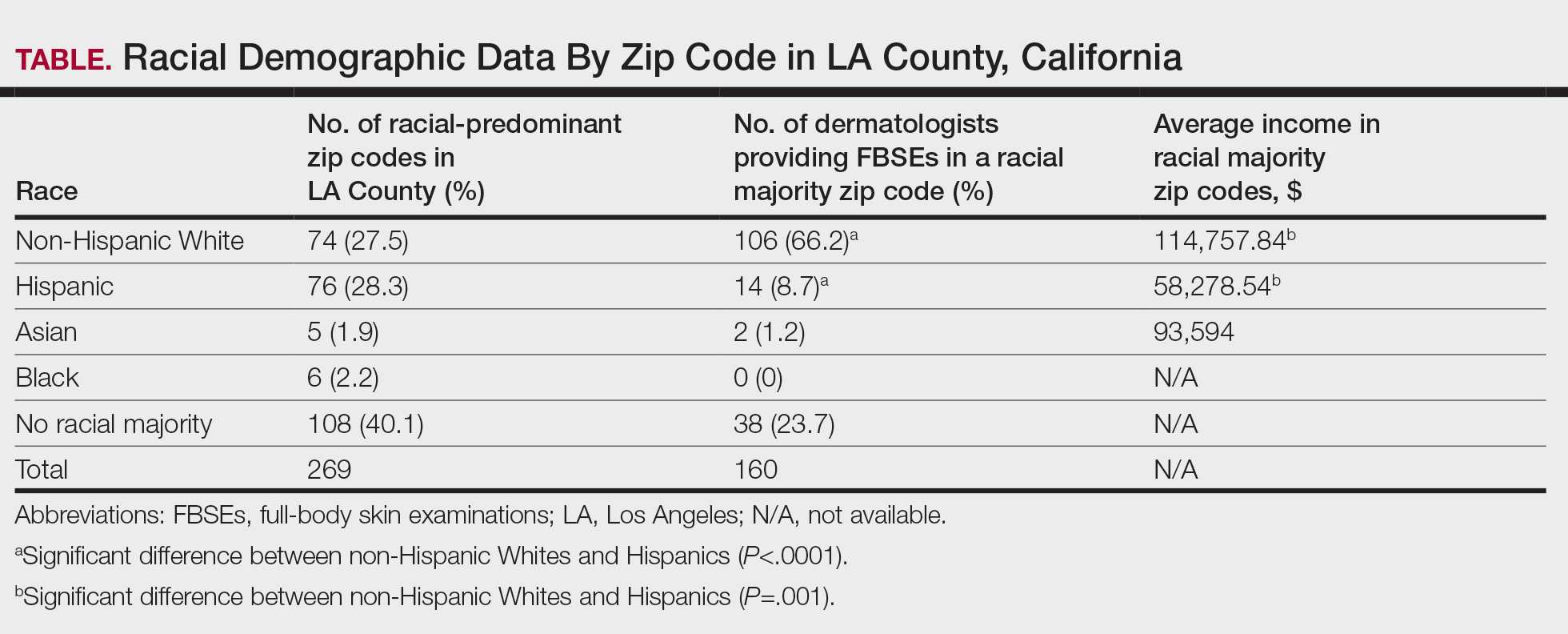
In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
PRACTICE POINTS
- Socioeconomic and racial disparities impact access to full-body skin examinations (FBSEs) in Los Angeles County.
- Most dermatologists included in this study were accepting new patients for a FBSE.
- There are significantly more dermatologists in predominantly non-Hispanic White zip codes than in predominantly Hispanic zip codes in Los Angeles County.
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.
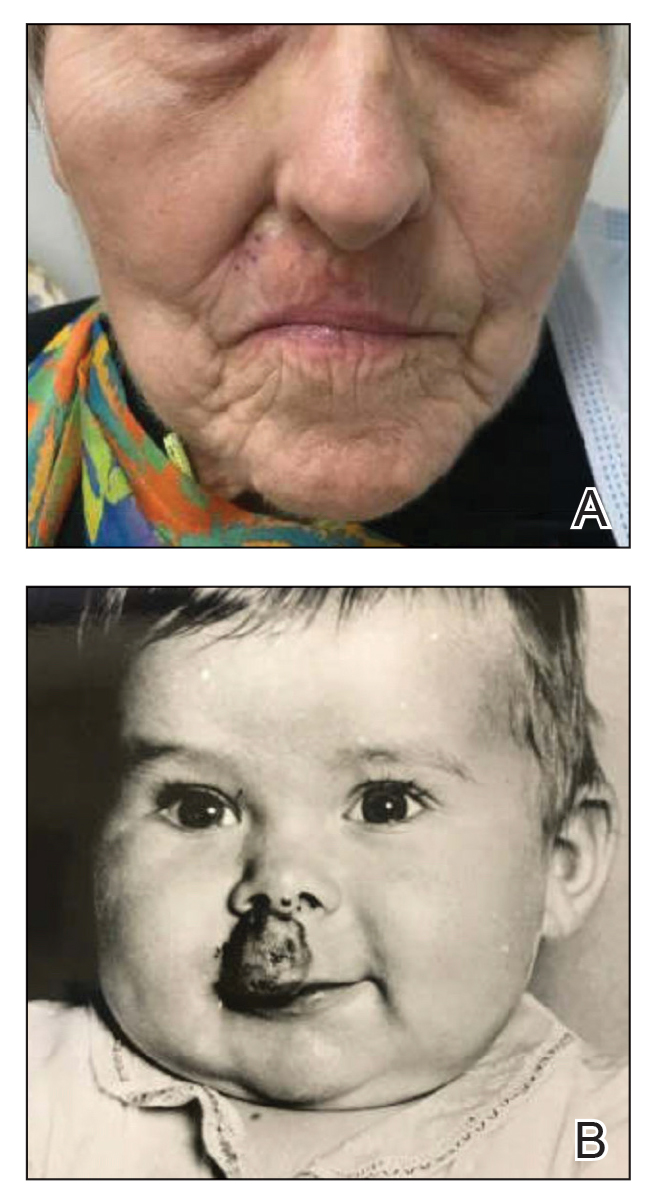
Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.
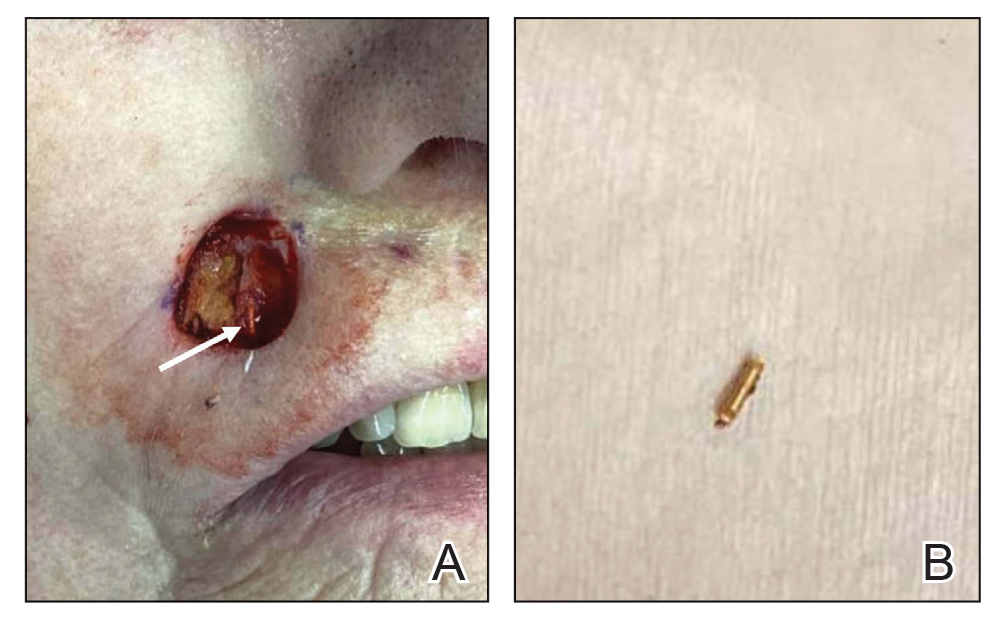
Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
PRACTICE POINTS
- Historical use of ionizing radiation to treat skin disease is a risk factor for basal cell carcinoma (BCC).
- Mohs micrographic surgery is the treatment of choice for BCC in high-risk areas such as the nose, eyelids, and lips, where tissue conservation and complete margin control are essential.
- Elderly patients should be screened for less common sources of radiation exposure for better risk stratification and to determine appropriate intervals for follow-up with a dermatologist.