User login
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
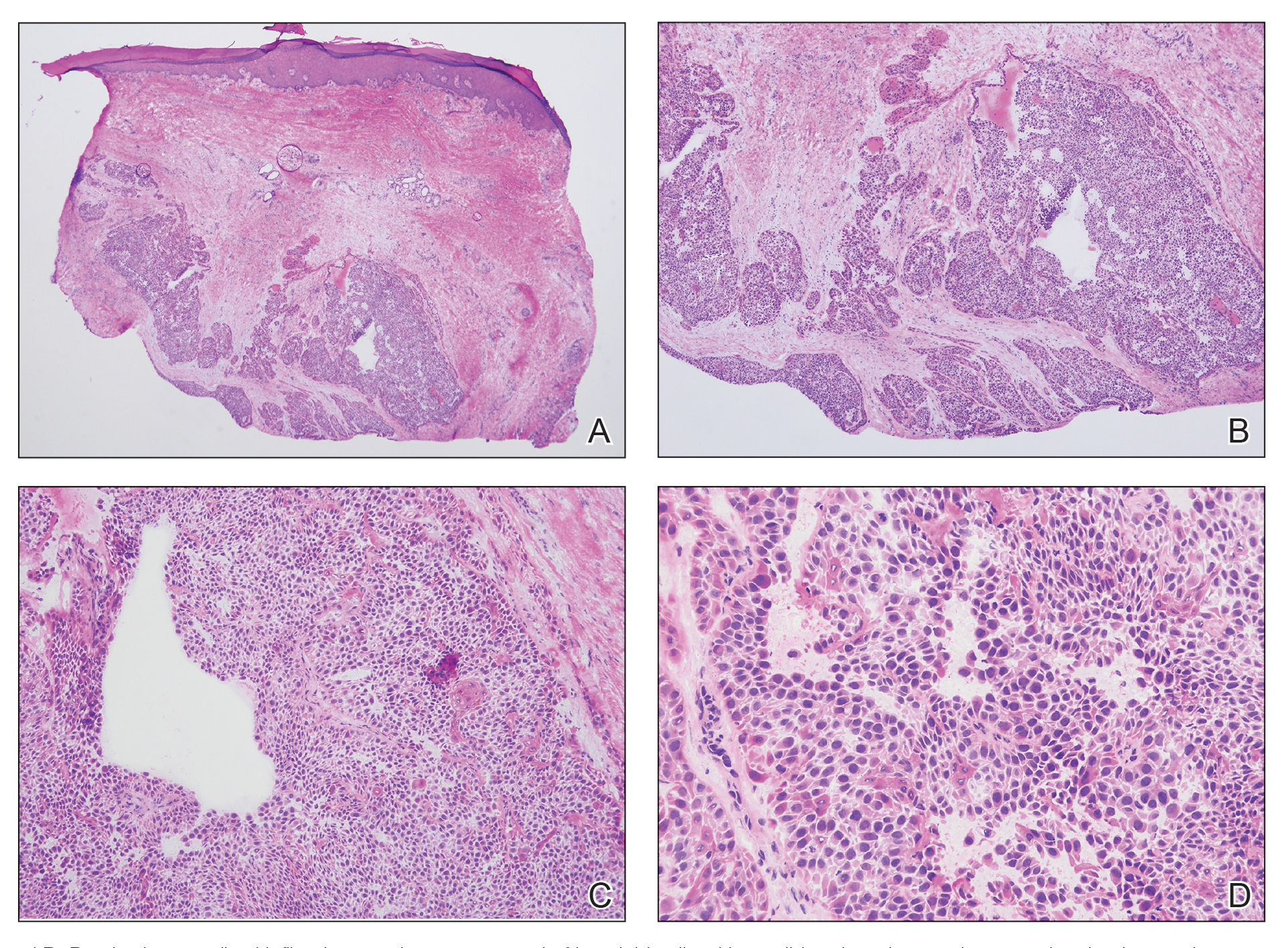
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.
Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
THE DIAGNOSIS: Hidradenocarcinoma
Both the original and recurrent lesions were interpreted as a chondroid syringoma, a benign adnexal tumor; however, the third biopsy of the lesion revealed a low-grade adnexal neoplasm with irregular nests of variably sized epithelial cells demonstrating mild nuclear atypia and low mitotic activity. Given the multiple recurrences, accelerated growth, and more aggressive histologic findings, the patient was referred to our clinic for surgical management.
We elected to perform modified Mohs micrographic surgery (MMS) with permanent tissue sections to enable the application of immunohistochemical stains to fully characterize the tumor. Histopathology showed a poorly circumscribed infiltrative dermal neoplasm composed of basaloid cells with a solid and cystic growth pattern in a background of hyalinized, fibrotic stroma (Figure, A and B). There were focal clear cell and squamous features as well as focal ductal differentiation (Figure, C and D). No obvious papillary structures were noted. The tumor cells were positive for D2-40, and staining for CD31 failed to reveal lymphovascular invasion. Based on the infiltrative features in conjunction with the findings from the prior biopsies, a diagnosis of hidradenocarcinoma (HAC) was made. Deep and peripheral margins were cleared after 2 stages of MMS.

Initially described in 1954, HAC is an exceedingly rare adnexal tumor of apocrine and eccrine derivation.1 Historically, nomenclature for this entity has varied in the literature, including synonyms such as malignant nodular hidradenoma, malignant acrospiroma, solid-cystic adenocarcinoma, and malignant clear cell myoepithelioma.2,3 Approximately 6% of all malignant eccrine tumors worldwide are HACs, which account for only 1 in 13,000 dermatopathology specimens.1 These tumors may transform from clear cell hidradenomas (their benign counterparts) but more commonly arise de novo. Compared to benign hidradenomas, HACs are poorly circumscribed with infiltrative growth patterns on histopathology and may exhibit nuclear pleomorphism, prominent mitotic activity, necrosis, and perineural or vascular invasion.2
Clinically, HAC manifests as a 1- to 5-cm, solitary, firm, intradermal pink or violaceous nodule with possible ulceration.2,4 The nodule often is asymptomatic but may be tender, as in our patient. There seems to be no clear anatomic site of predilection, with approximately 42% of HACs localized to the head and neck and the remainder occurring on the trunk, arms, and legs.3,5-7 Females and males are affected equally, and lesions tend to arise in the seventh decade of life.7
Reports in the literature suggest that HAC is a very aggressive tumor with a generally poor prognosis.1 Several studies have found that up to half of tumors locally recur despite aggressive surgical management, and metastasis occurs in 20% to 60% of patients.3,8 However, a large study of US Surveillance, Epidemiology, and End Results data investigating the clinicopathologic characteristics of 289 patients with HAC revealed a more favorable prognosis.7 Mean overall survival and cancer-specific survival were greater than 13 years, and 10-year overall survival and cancer-specific survival rates were 60.2% and 90.5%, respectively.
Traditionally used to treat keratinocyte carcinomas, including basal cell carcinoma and squamous cell carcinoma, complete margin assessment with MMS is increasingly being utilized in the management of other cutaneous malignancies, including adnexal tumors.8 Due to its rarity, there remains no standard optimal treatment approach for HAC. One small retrospective study of 10 patients with HAC treated with MMS demonstrated favorable outcomes with no cases of recurrence, metastasis, or diseaserelated mortality in a mean 7-year follow-up period.9
Whole-body positron emission tomography/computed tomography performed in our patient approximately 1 month after MMS revealed mildly hypermetabolic left inguinal lymph nodes, which were thought to be reactive, and a question of small hypermetabolic foci in the liver. Follow-up computed tomography of the abdomen subsequently was performed and was negative for hepatic metastases. The patient will be monitored closely for local recurrence; however, the clearance of the tumor with MMS, which allowed complete margin assessment, is encouraging and supports MMS as superior to traditional surgical excision in the treatment of HAC. At his most recent examination 17 months after Mohs surgery, the patient remained tumor free.
Aggressive digital papillary adenocarcinoma (ADPA) is a rare malignant tumor originating in the sweat glands that can occur on the first toe but most commonly arises on the fingers. While both HAC and ADPA can manifest with an infiltrative growth pattern and cytologic atypia, ADPA classically reveals a well-circumscribed multinodular tumor in the dermis comprised of solid and cystic proliferation as well as papillary projections. In addition, ADPA has been described as having back-to-back glandular and ductal structures.10 Giant cell tumor of the tendon sheath is a benign fibrohistiocytic tumor that also typically manifests on the fingers but rarely can occur on the foot, including the first toe.11,12 This tumor is more common in women and most frequently affects individuals aged 30 to 50 years.12 Microscopically, giant cell tumor of the tendon sheath is characterized by a proliferation of osteoclastlike giant cells, epithelioid histiocytelike cells, mononuclear cells, and xanthomatous cells among collagenous bands.11
Osteosarcoma is an uncommon tumor of osteoidproducing cells that usually arises in the metaphysis of long bones and manifests as a tender subcutaneous mass. It has a bimodal age distribution, peaking in adolescents and adults older than 65 years.13 While very rare, osteosarcoma has been reported to occur in the bones of the feet, including the phalanges.14 Given the recurrent nature of our patient’s tumor, metastasis should always be considered; however, in his case, full-body imaging was negative for additional malignancy.
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
- Gauerke S, Driscoll JJ. Hidradenocarcinomas: a brief review and future directions. Arch Pathol Lab Med. 2010;134:781-785. doi:10.5858/134.5.781
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/J.HOC.2018.09.002
- Ohta M, Hiramoto M, Fujii M, et al. Nodular hidradenocarcinoma on the scalp of a young woman: case report and review of literature. Dermatol Surg. 2004;30:1265-1268. doi:10.1111/J.1524-4725.2004.30390.X
- Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554. doi:10.1111/J.1468-3083.2007.02504.X
- Yavel R, Hinshaw M, Rao V, et al. Hidradenomas and a hidradenocarcinoma of the scalp managed using Mohs micrographic surgery and a multidisciplinary approach: case reports and review of the literature. Dermatolog Surg. 2009;35:273-281. doi:10.1111/j.1524-4725.2008.34424.x
- Kazakov DV, Ivan D, Kutzner H, et al. Cutaneous hidradenocarcinoma: a clinicopathological, immunohistochemical, and molecular biologic study of 14 cases, including Her2/neu gene expression/ amplification, TP53 gene mutation analysis, and t(11;19) translocation. Am J Dermatopathol. 2009;31:236-247. doi:10.1097/DAD.0B013E3181984F10
- Gao T, Pan S, Li M, et al. Prognostic analysis of hidradenocarcinoma: a SEER-based observational study. Ann Med. 2022;54:454-463. doi:10 .1080/07853890.2022.2032313
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207. doi:10.1097/DSS.0000000000001167
- Tolkachjov SN, Hocker TL, Hochwalt PC, et al. Mohs micrographic surgery for the treatment of hidradenocarcinoma: the mayo clinic experience from 1993 to 2013. Dermatolog Surg. 2015;41:226-231. doi:10.1097/DSS.0000000000000242
- Weingertner N, Gressel A, Battistella M, et al. Aggressive digital papillary adenocarcinoma: a clinicopathological study of 19 cases. J Am Acad Dermatol. 2017;77:549-558.e1. doi:10.1016/J.JAAD.2017.02.028
- Paral KM, Petronic-Rosic V. Acral manifestations of soft tissue tumors. Clin Dermatol. 2017;35:85-98. doi:10.1016/J.CLINDER MATOL.2016.09.012
- Kondo RN, Crespigio J, Pavezzi PD, et al. Giant cell tumors of the tendon sheath in the left hallux. An Bras Dermatol. 2016;91:704-705. doi:10.1590/ABD1806-4841.20165769
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3-13. doi:10.1007/978-1-4419-0284-9_1
- Anninga JK, Picci P, Fiocco M, et al. Osteosarcoma of the hands and feet: a distinct clinico-pathological subgroup. Virchows Arch. 2013;462:109- 120. doi:10.1007/S00428-012-1339-3
Recurrent Nodule on the First Toe
Recurrent Nodule on the First Toe
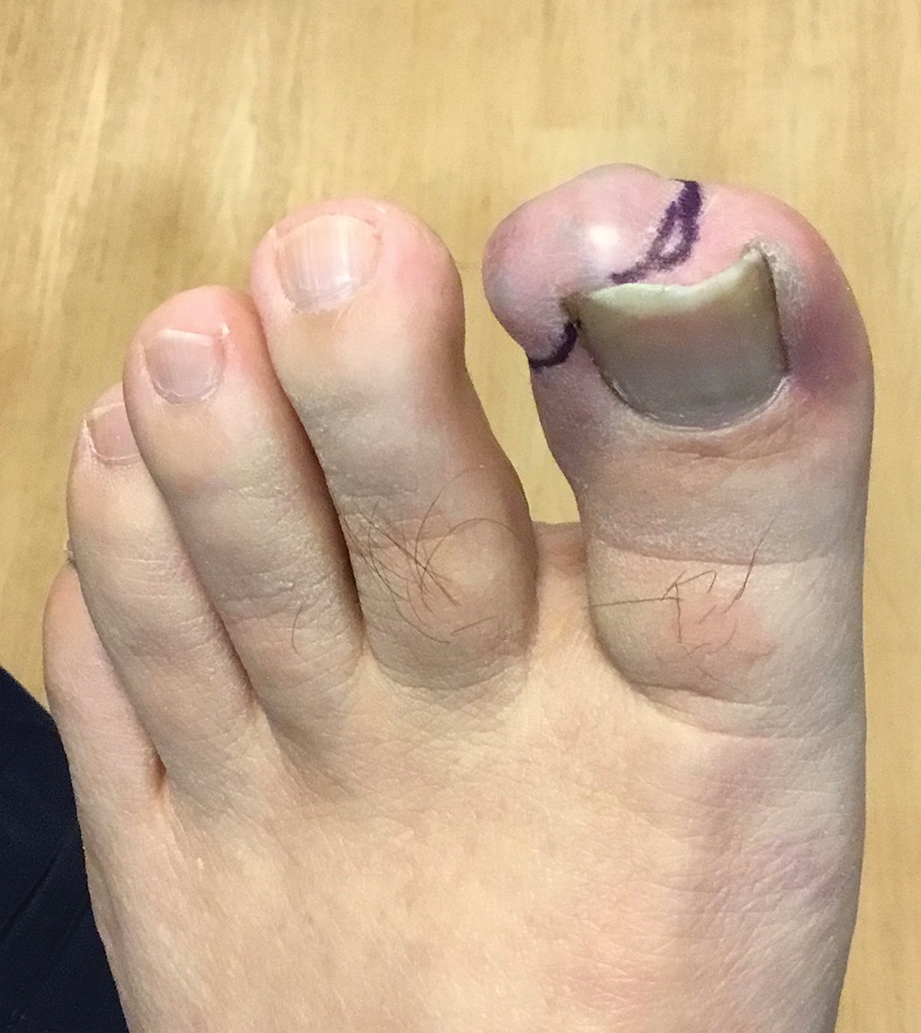
A 56-year-old man was referred to the dermatology clinic for treatment of a recurrent nodule on the left first toe. The lesion first appeared 12 years prior and was resected by an outside dermatologist, who diagnosed the lesion as benign based on biopsy results. Approximately 10 years later, the lesion began to grow back with a similar appearance to the original nodule; it again was diagnosed as benign based on another biopsy and excised by the outside dermatologist. Two years later, the patient had a second recurrence of the lesion, which was excised by his dermatologist. The biopsy report at that time identified the lesion as a low-grade adnexal neoplasm. The patient had a rapid recurrence of the tumor after 6 months and was referred to our clinic for Mohs micrographic surgery. Physical examination revealed a tender, 2.5×1.8-cm, firm, exophytic, subcutaneous nodule on the left first toe with no associated lymphadenopathy.