User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Acyclovir-Resistant Cutaneous Herpes Simplex Virus in DOCK8 Deficiency
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.
Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.
Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.

Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.

Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
Dedicator of cytokinesis 8 (DOCK8 ) deficiency is the major cause of autosomal-recessive hyper-IgEsyndrome. 1 Characteristic clinical features including eosinophilia, eczema, and recurrent Staphylococcus aureus cutaneous and respiratory tract infections are common in DOCK8 deficiency, similar to the autosomal-dominant form of hyper-IgE syndrome that is due to defi c iency of signal transducer and activation of transcription 3 (STAT-3 ). 1 In addition, patients with DOCK8 deficiency are particularly susceptible to asthma; food allergies; lymphomas; and severe cutaneous viral infections, including herpes simplex virus (HSV), molluscum contagiosum, varicella-zoster virus, and human papillomavirus. Since the discovery of the DOCK8 gene in 2009, various studies have sought to elucidate the mechanistic contribution of DOCK8 to the dermatologic immune environment. 2 Although cutaneous viral infections such as those caused by HSV typically are short lived and self-limiting in immunocompetent hosts, they have proven to be severe and recalcitrant in the setting of DOCK8 deficiency. 1 Herein, we report the case of a 32-month-old girl with homozygous DOCK8 deficiency who developed acyclovir-resistant cutaneous HSV.
Case Report
A 32-month-old girl presented with an approximately 2-cm linear erosion along the left posterior auricular sulcus at month 9 of a hospital stay for recurrent infections. Her medical history was notable for multiple upper respiratory tract infections, diffuse eczema, and food allergies. She had presented to an outside hospital at 14 months of age with herpetic gingivostomatitis and eczema herpeticum that was successfully treated with acyclovir. She was readmitted at 20 months of age due to Pneumocystis jiroveci pneumonia, pancytopenia, and disseminated histoplasmosis. Prophylactic oral acyclovir (20 mg/kg twice daily) was started, given her history of HSV infection. Because of recurrent infections, she underwent an immunodeficiency workup. Whole exome sequencing analysis revealed a homozygous deletion c.(528+1_529−1)_(1516+1_1517−1)del in DOCK8 gene–affecting exons 5 to 13. The patient was transferred to our hospital for continued care and as a potential candidate for bone marrow transplant following resolution of the disseminated histoplasmosis infection.
During her hospitalization at the current presentation, she was noted to have a 2-cm linear erosion along the left posterior auricular sulcus. Initial wound care with bacitracin ointment was applied to the area while specimens were obtained and empiric oral acyclovir therapy was initiated (20 mg/kg 4 times daily [QID]), given a clinical impression consistent with cutaneous HSV infection despite acyclovir prophylaxis. Direct immunofluorescence and viral cultures were positive for HSV-1, while bacterial cultures grew methicillin-susceptible S aureus. Cephalexin and mupirocin ointment were started, and acyclovir was continued. After 2 weeks of therapy, there was no visible change in the wound; cultures were repeated, again showing the wound contained HSV. Bacterial cultures this time grew Pseudomonas putida, and the antibiotic regimen was transitioned to cefepime.
After no response to the continued course of therapeutic acyclovir, HSV cultures were sent to the Centers for Disease Control and Prevention for resistance testing, and biopsy of the lesion was performed by the otolaryngology service to rule out malignancy and potential alternative diagnoses. Histopathology showed only reactive inflammation without visible microorganisms on tissue HSV-1/HSV-2 immunostain; however, tissue viral culture was positive for HSV-1. The patient was transitioned back to acyclovir (intravenous [IV] 20 mg/kg QID) with the addition of empiric foscarnet (IV 40 mg/kg 3 times daily) given the worsening appearance of the lesion. The HSV acyclovir resistance test results from the Centers for Disease Control and Prevention returned soon after and were positive for resistance (median infectious dose, 3.29 µg/L [reference interval, sensitive <2.00 µg/L; resistant >1.90 µg/L]). The patient completed a 21-day course of combination foscarnet and acyclovir therapy, during which time the lesion showed notable improvement and healing. The patient was continued on prophylactic acyclovir (IV 20 mg/kg QID). Unfortunately, the patient eventually died due to complications related to pneumonia.
Comment
Infection in Patients With DOCK8 Deficiency—The gene DOCK8 has emerged as playing a central role in both innate and adaptive immunity, as it is expressed primarily in immune cells and serves as a mediator of numerous processes, including immune synapse formation, cell signaling and trafficking, antibody and cytokine production, and lymphocyte memory.3 Cells that are critical for combating cutaneous viral infections, including skin-resident memory T cells and natural killer cells, are defective, which leads to a severely immunocompromised state in DOCK8-deficient patients with a particular susceptibility to infectious and inflammatory dermatologic disease.4
Herpes simplex virus infection commonly is seen in DOCK8 deficiency, with retrospective analysis of a DOCK8-deficient cohort revealing HSV infection in approximately 38% of patients.5 Prophylactic acyclovir is essential for DOCK8-deficient individuals with a history of HSV infection given the tendency of the virus to reactivate.6 However, despite prophylaxis, our patient developed an HSV-positive posterior auricular erosion that continued to progress even after increase of the acyclovir dose. Acyclovir resistance testing of the HSV isolated from the wound was positive, confirming the clinical suspicion of the presence of acyclovir-resistant HSV infection.

Acyclovir-Resistant HSV—Acyclovir-resistant HSV in immunosuppressed individuals was first noted in 1982, and most cases since then have occurred in the setting of AIDS and in organ transplant recipients.6 Few reports of acyclovir-resistant HSV in DOCK8 deficiency exist, and to our knowledge, our patient is the youngest DOCK8-deficient individual to be documented with acyclovir-resistant HSV infection.1,7-15 We identified relevant cases from the PubMed and EMBASE databases using the search terms DOCK8 deficiency and acyclovir and DOCK8 deficiency and herpes. The eTable lists other reported cases of acyclovir-resistant HSV in DOCK8-deficient patients. The majority of cases involved school-aged females. Lesion types varied and included herpes labialis, eczema herpeticum, and blepharoconjunctivitis. Escalation of therapy and resolution of the lesion was seen in some cases with administration of subcutaneous pegylated interferon alfa-2b.

Treatment Alternatives—Acyclovir competitively inhibits viral DNA polymerase by incorporating into elongating viral DNA strands and halting chain synthesis. Acyclovir requires triphosphorylation for activation, and viral thymidine kinase is responsible for the first phosphorylation event. Ninety-five percent of cases of acyclovir resistance are secondary to mutations in viral thymidine kinase. Foscarnet also inhibits viral DNA polymerase but does so directly without the need to be phosphorylated first.6 For this reason, foscarnet often is the drug of choice in the treatment of acyclovir-resistant HSV, as evidenced in our patient. However, foscarnet-resistant HSV strains may develop from mutations in the DNA polymerase gene.
Cidofovir is a nucleotide analogue that requires phosphorylation by host, as opposed to viral, kinases for antiviral activity. Intravenous and topical formulations of cidofovir have proven effective in the treatment of acyclovir- and foscarnet-resistant HSV lesions.6 Cidofovir also can be applied intralesionally, a method that provides targeted therapy and minimizes cidofovir-associated nephrotoxicity.12 Reports of systemic interferon alfa therapy for acyclovir-resistant HSV also exist. A study found IFN-⍺ production by peripheral blood mononuclear cells in DOCK8-deficient individuals to be significantly reduced relative to controls (P<.05).7 There has been complete resolution of acyclovir-resistant HSV lesions with subcutaneous pegylated interferon alfa-2b injections in several DOCK8-deficient patients.7-9
The need for escalating therapy in DOCK8-deficient individuals with acyclovir-resistant HSV infection underscores the essential role of DOCK8 in dermatologic immunity. Our case demonstrates that a high degree of suspicion for cutaneous HSV infection should be adopted in DOCK8-deficient patients of any age, regardless of acyclovir prophylaxis. Viral culture in addition to bacterial cultures should be performed early in patients with cutaneous erosions, and the threshold for HSV resistance testing should be low to minimize morbidity associated with these infections. Early resistance testing in our case could have prevented prolongation of infection and likely eliminated the need for a biopsy.
Conclusion
DOCK8 deficiency presents a unique challenge to dermatologists and other health care providers given the susceptibility of affected individuals to developing a reservoir of severe and potentially resistant viral cutaneous infections. Prophylactic acyclovir may not be sufficient for HSV suppression, even in the youngest of patients, and suspicion for resistance should be high to avoid delays in adequate treatment.
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
- Chu EY, Freeman AF, Jing H, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. 2012;148:79-84. doi:10.1001/archdermatol.2011.262
- Aydin SE, Kilic SS, Aytekin C, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol. 2015;35:189-198. doi:10.1007/s10875-014-0126-0
- Kearney CJ, Randall KL, Oliaro J. DOCK8 regulates signal transduction events to control immunity. Cell Mol Immunol. 2017;14:406-411. doi:10.1038/cmi.2017.9
- Zhang Q, Dove CG, Hor JL, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211:2549-2566. doi:10.1084/jem.20141307
- Engelhardt KR, Gertz EM, Keles S, et al. The extended clinical phenotype of 64 patients with DOCK8 deficiency. J Allergy Clin Immunol. 2015;136:402-412. doi:10.1016/j.jaci.2014.12.1945
- Chilukuri S, Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol Clin. 2003;21:311-320. doi:10.1016/S0733-8635(02)00093-1
- Keles S, Jabara HH, Reisli I, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with interferon alpha-2b therapy. J Allergy Clin Immunol. 2014;133:1753-1755.e3. doi:10.1016/j.jaci.2014.03.032
- Papan C, Hagl B, Heinz V, et al Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2014;133:1456-1458. doi:10.1016/j.jaci.2014.02.008
- Metin A, Kanik-Yuksek S, Ozkaya-Parlakay A, et al. Giant herpes labialis in a child with DOCK8-deficient hyper-IgE syndrome. Pediatr Neonatol. 2016;57:79-80. doi:10.1016/j.pedneo.2015.04.011
- Zhang Q, Davis JC, Lamborn IT, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046-2055. doi:10.1056/NEJMoa0905506
- Lei JY, Wang Y, Jaffe ES, et al. Microcystic adnexal carcinoma associated with primary immunodeficiency, recurrent diffuse herpes simplex virus infection, and cutaneous T-cell lymphoma. Am J Dermatopathol. 2000;22:524-529. doi:10.1097/00000372-200012000-00008
- Castelo-Soccio L, Bernardin R, Stern J, et al. Successful treatment of acyclovir-resistant herpes simplex virus with intralesional cidofovir. Arch Dermatol. 2010;146:124-126. doi:10.1001/archdermatol.2009.363
- Shah NN, Freeman AF, Hickstein DD. Addendum to: haploidentical related donor hematopoietic stem cell transplantation for DOCK8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:E65-E67. doi:10.1016/j.bbmt.2018.11.014
- Freeman AF, Yazigi N, Shah NN, et al. Tandem orthotopic living donor liver transplantation followed by same donor haploidentical hematopoietic stem cell transplantation for DOCK8 deficiency. Transplantation. 2019;103:2144-2149. doi:10.1097/TP.0000000000002649
- Casto AM, Stout SC, Selvarangan R, et al. Evaluation of genotypic antiviral resistance testing as an alternative to phenotypic testing in a patient with DOCK8 deficiency and severe HSV-1 disease. J Infect Dis. 2020;221:2035-2042. doi:10.1093/infdis/jiaa020
Practice Points
- Patients with dedicator of cytokinesis 8 ( DOCK 8 ) deficiency are susceptible to development of severe recalcitrant viral cutaneous infections, including herpes simplex virus (HSV).
- Dermatologists should be aware that prophylactic acyclovir may not be sufficient for HSV suppression in the setting of severe immunodeficiency.
- Acyclovir-resistant cutaneous HSV lesions require escalation of therapy, which may include addition of foscarnet, cidofovir, or subcutaneous pegylated interferon alfa-2b to the therapeutic regimen.
- Viral culture should be performed on suspicious lesions in DOCK 8 -deficient patients despite acyclovir prophylaxis, and the threshold for HSV resistance testing should be low.
Atypical Presentation of Pityriasis Rubra Pilaris: Challenges in Diagnosis and Management
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
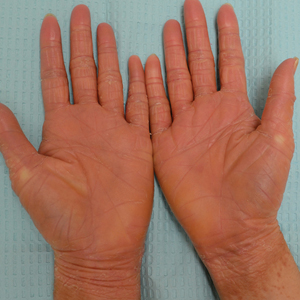
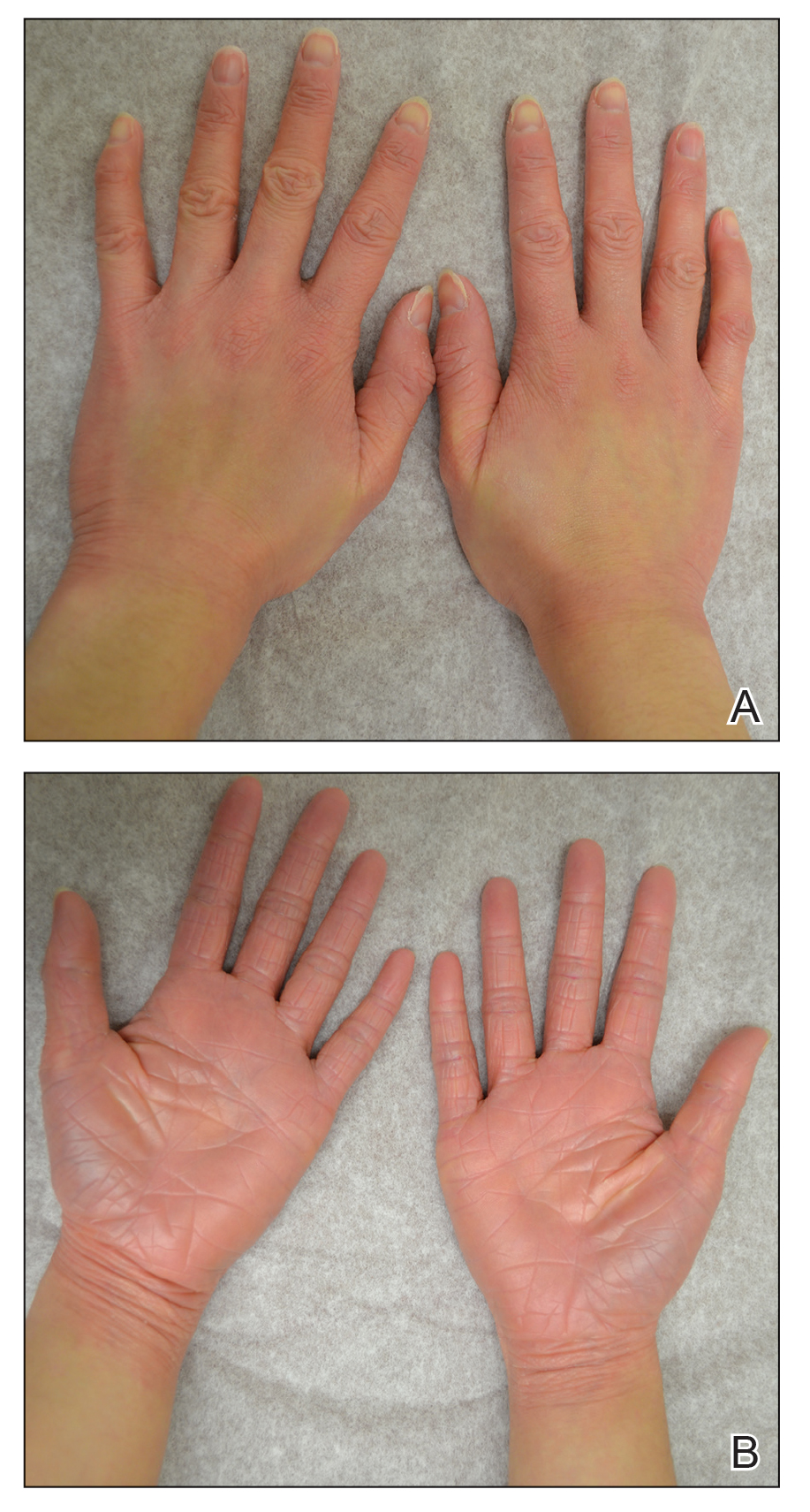
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.
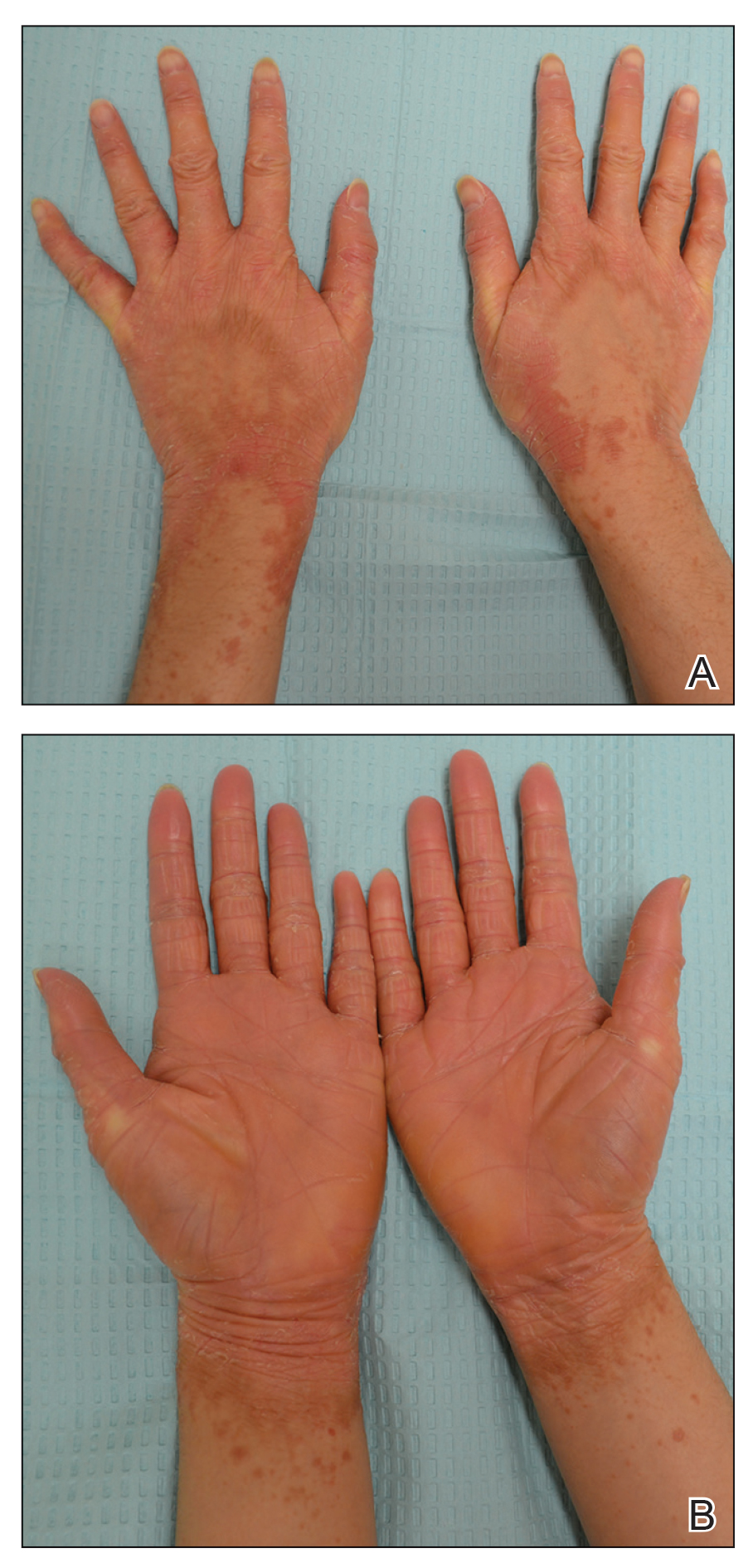
The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
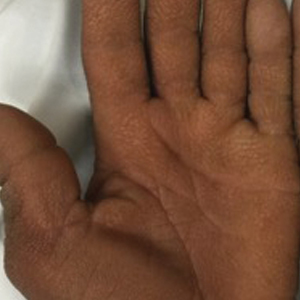
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7
The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.

The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7

The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.

The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7

The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
Practice Points
- Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.
- The diagnosis of PRP often can be challenging given the variety of clinical presentations.
Paraneoplastic Signs in Bladder Transitional Cell Carcinoma: An Unusual Presentation
To the Editor:
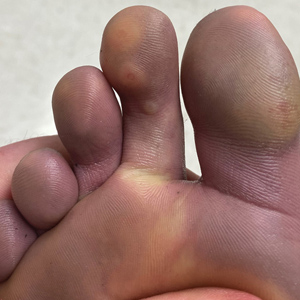
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.
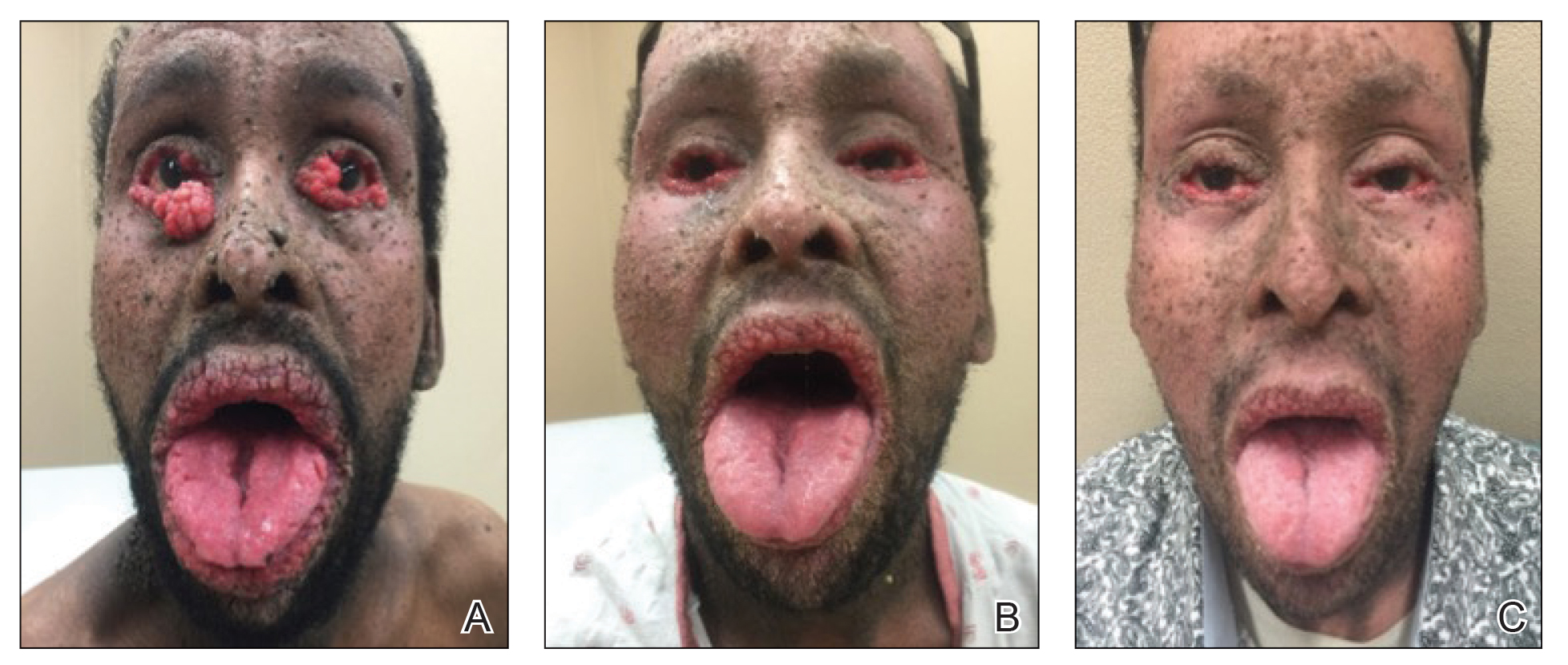
Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.
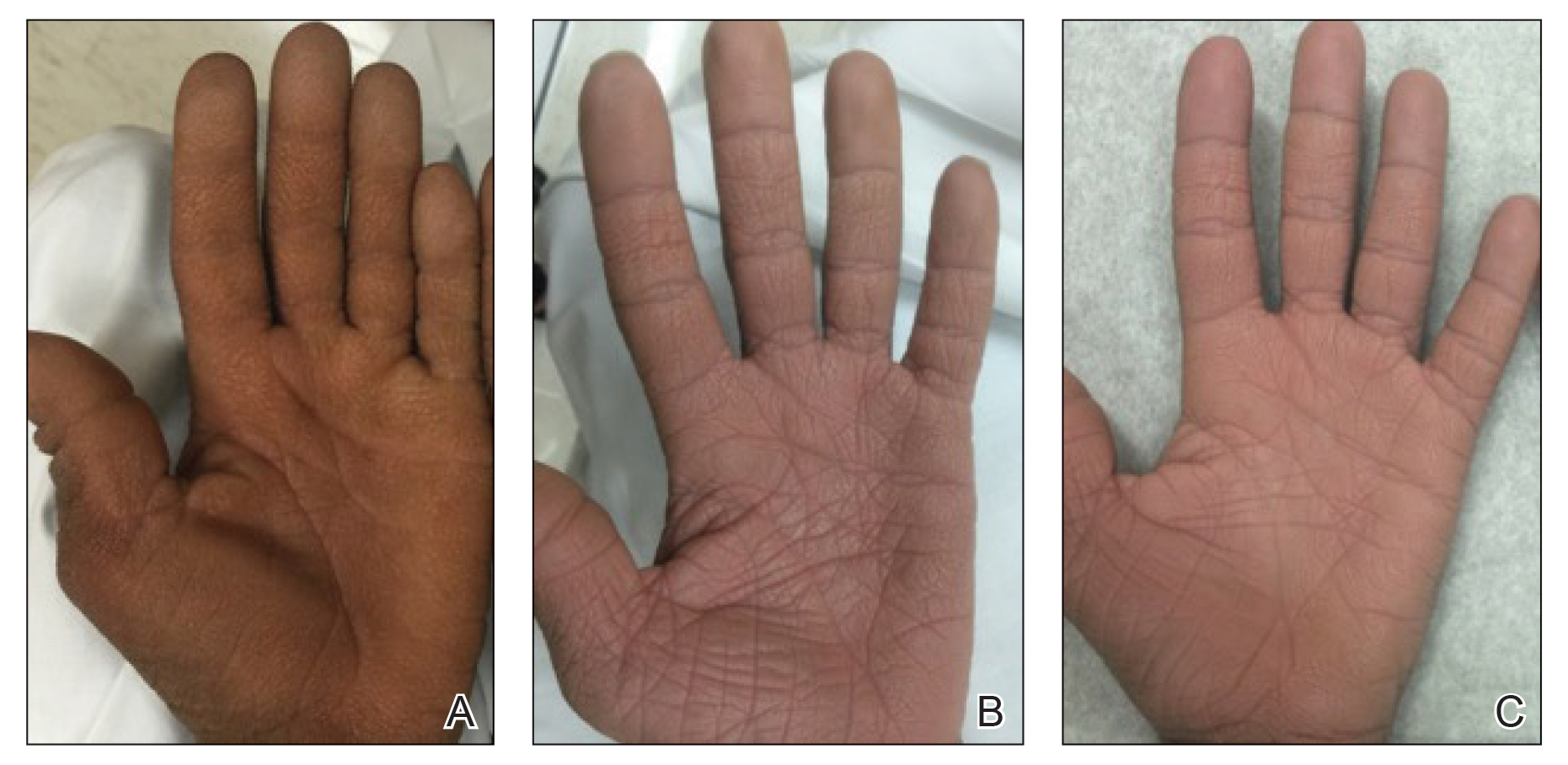
This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
To the Editor:
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.

Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.

This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
To the Editor:
A 40-year-old Somalian man presented to the dermatology clinic with lesions on the eyelids, tongue, lips, and hands of 8 years’ duration. He was a former refugee who had faced considerable stigma from his community due to his appearance. A review of systems was remarkable for decreased appetite but no weight loss. He reported no abdominal distention, early satiety, or urinary symptoms, and he had no personal history of diabetes mellitus or obesity. Physical examination demonstrated hyperpigmented velvety plaques in all skin folds and on the genitalia. Massive papillomatosis of the eyelid margins, tongue, and lips also was noted (Figure 1A). Flesh-colored papules also were scattered across the face. Punctate, flesh-colored papules were present on the volar and palmar hands (Figure 2A). Histopathology demonstrated pronounced papillomatous epidermal hyperplasia with negative human papillomavirus (HPV) type 16 and HPV-18 DNA studies. Given the appearance of malignant acanthosis nigricans with oral and conjunctival features, cutaneous papillomatosis, and tripe palms, concern for underlying malignancy was high. Malignancy workup, including upper and lower endoscopy as well as serial computed tomography scans of the chest, abdomen, and pelvis, was unrevealing.

Laboratory investigation revealed a positive Schistosoma IgG antibody (0.38 geometric mean egg count) and peripheral eosinophilia (1.09 ×103/μL), which normalized after praziquantel therapy. With no malignancy identified over the preceding 6-month period, treatment with acitretin 50 mg daily was initiated based on limited literature support.1-3 Treatment led to reduction in the size and number of papillomas (Figure 1B) and tripe palms (Figure 2B) with increased mobility of hands, lips, and tongue. The patient underwent oculoplastic surgery to reduce the papilloma burden along the eyelid margins. Subsequent cystoscopy 9 months after the initial presentation revealed low-grade transitional cell carcinoma of the bladder. Intraoperative mitomycin C led to tumor shrinkage and, with continued treatment with daily acitretin, dramatic improvement of all cutaneous and mucosal symptoms (Figure 1C and Figure 2C). To date, his cutaneous symptoms have resolved.

This case demonstrated a unique presentation of multiple paraneoplastic signs in bladder transitional cell carcinoma. The presence of malignant acanthosis nigricans (including oral and conjunctival involvement), cutaneous papillomatosis, and tripe palms have been individually documented in various types of gastric malignancies.4 Acanthosis nigricans often is secondary to diabetes and obesity, presenting with diffuse, thickened, velvety plaques in the flexural areas. Malignant acanthosis nigricans is a rare, rapidly progressive condition that often presents over a period of weeks to months; it almost always is associated with internal malignancies. It often has more extensive involvement, extending beyond the flexural areas, than typical acanthosis nigricans.4 Oral involvement can be either hypertrophic or papillomatous; papillomatosis of the oral mucosa was reported in over 40% of malignant acanthosis nigricans cases (N=200).5 Cases with conjunctival involvement are less common.6 Although malignant acanthosis nigricans often is codiagnosed with a malignancy, it can precede the cancer diagnosis in some cases.7,8 A majority of cases are associated with adenocarcinomas of the gastrointestinal tract.4 Progressive mucocutaneous papillomatosis also is a rare paraneoplastic condition that most commonly is associated with gastric adenocarcinomas. Progressive mucocutaneous papillomatosis often presents rapidly as verrucous growths on cutaneous surfaces (including the hands and face) but also can affect mucosal surfaces such as the mouth and conjunctiva.9-11 Tripe palms are characterized by exaggerated dermatoglyphics with diffuse palmar ridging and hyperkeratosis. Tripe palms most often are associated with pulmonary malignancies. When tripe palms are present with malignant acanthosis nigricans, they reflect up to a one-third incidence of gastrointestinal malignancy.12,13
Despite the individual presentation of these paraneoplastic signs in a variety of malignancies, synchronous presentation is rare. A brief literature review only identified 6 cases of concurrent acanthosis nigricans, tripe palms, and progressive mucocutaneous papillomatosis with an underlying gastrointestinal malignancy.1,11,14-17 Two additional reports described tripe palms with oral acanthosis nigricans and progressive mucocutaneous papillomatosis in metastatic gastric adenocarcinoma and renal urothelial carcinoma.2,18 An additional case of all 3 paraneoplastic conditions was reported in the setting of metastatic cervical cancer (HPV positive).19 Per a recent case report and literature review,20 there have only been 8 cases of acanthosis nigricans reported in bladder transitional cell carcinoma,20-27 half of which have included oral malignant acanthosis nigricans.20-23 Only one report of concurrent cutaneous and oral malignant acanthosis nigricans and triple palms in the setting of bladder cancer has been reported.20 Given the extensive conjunctival involvement and cutaneous papillomatosis in our patient, ours is a rarely reported case of concurrent malignant mucocutaneous acanthosis nigricans, tripe palms, and progressive papillomatosis in transitional cell bladder carcinoma. We believe it is imperative to consider the role of this malignancy as a cause of these paraneoplastic conditions.
Although these paraneoplastic conditions rarely co-occur, our case further offers a common molecular pathway for these conditions.28 In these paraneoplastic conditions, the stimulating factor is thought to be tumor growth factor α, which is structurally related to epidermal growth factor (EGF). Epidermal growth factor receptors (EGFRs) are found in the basal layer of the epidermis, where activation stimulates keratinocyte growth and leads to the cutaneous manifestation of symptoms.28 Fibroblast growth factor receptor 3 mutations are found in most noninvasive transitional cell tumors of the bladder.29 The fibroblast growth factor pathway is distinctly different from the tumor growth factor α and EGF pathways.30 However, this association with transitional cell carcinoma suggests that fibroblast growth factor receptor 3 also may be implicated in these paraneoplastic conditions.
Our patient responded well to treatment with acitretin 50 mg daily. The mechanism of action of retinoids involves inducing mitotic activity and desmosomal shedding.31 Retinoids downregulate EGFR expression and activation in EGF-stimulated cells.32 We hypothesize that these oral retinoids decreased the growth stimulus and thereby improved cutaneous signs in the setting of our patient’s transitional cell cancer. Although definitive therapy is malignancy management, our case highlights the utility of adjunctive measures such as oral retinoids and surgical debulking. While previous cases have reported use of retinoids at a lower dosage than used in this case, oral lesions often have only been mildly improved with little impact on other cutaneous symptoms.1,2 In one case of malignant acanthosis nigricans and oral papillomatosis, isotretinoin 25 mg once every 2 to 3 days led to a moderate decrease in hyperkeratosis and papillomas, but the patient was lost to follow-up.3 Our case highlights the use of higher daily doses of oral retinoids for over 9 months, resulting in marked improvement in both the mucosal and cutaneous symptoms of acanthosis nigricans, progressive mucocutaneous papillomatosis, and tripe palms. Therefore, oral acitretin should be considered as adjuvant therapy for these paraneoplastic conditions.
By reporting this case, we hope to demonstrate the importance of considering other forms of malignancies in the presence of paraneoplastic conditions. Although gastric malignancies more commonly are associated with these conditions, bladder carcinomas also can present with cutaneous manifestations. The presence of these paraneoplastic conditions alone or together rarely is reported in urologic cancers and generally is considered to be an indicator of poor prognosis. Paraneoplastic conditions often develop rapidly and occur in very advanced malignancies.4 The disfiguring presentation in our case also had unusual diagnostic challenges. The presence of these conditions for 8 years and nonmetastatic advanced malignancy suggest a more indolent process and that these signs are not always an indicator of poor prognosis. Future patients with these paraneoplastic conditions may benefit from both a thorough malignancy screen, including cystoscopy, and high daily doses of oral retinoids.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
- Stawczyk-Macieja M, Szczerkowska-Dobosz A, Nowicki R, et al. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma. Postepy Dermatol Alergol. 2014;31:56-58.
- Lee HC, Ker KJ, Chong W-S. Oral malignant acanthosis nigricans and tripe palms associated with renal urothelial carcinoma. JAMA Dermatol. 2015;151:1381-1383.
- Swineford SL, Drucker CR. Palliative treatment of paraneoplastic acanthosis nigricans and oral florid papillomatosis with retinoids. J Drugs Dermatol. 2010;9:1151-1153.
- Wick MR, Patterson JW. Cutaneous paraneoplastic syndromes [published online January 31, 2019]. Semin Diagn Pathol. 2019;36:211-228.
- Tyler MT, Ficarra G, Silverman S, et al. Malignant acanthosis nigricans with florid papillary oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:445-449.
- Zhang X, Liu R, Liu Y, et al. Malignant acanthosis nigricans: a case report. BMC Ophthalmology. 2020;20:1-4.
- Curth HO. Dermatoses and malignant internal tumours. Arch Dermatol Syphil. 1955;71:95-107.
- Krawczyk M, Mykala-Cies´la J, Kolodziej-Jaskula A. Acanthosis nigricans as a paraneoplastic syndrome. case reports and review of literature. Pol Arch Med Wewn. 2009;119:180-183.
- Singhi MK, Gupta LK, Bansal M, et al. Florid cutaneous papillomatosis with adenocarcinoma of stomach in a 35 year old male. Indian J Dermatol Venereol Leprol. 2005;71:195-196.
- Klieb HB, Avon SL, Gilbert J, et al. Florid cutaneous and mucosal papillomatosis: mucocutaneous markers of an underlying gastric malignancy. J Clin Oncol. 2013;31:E218-E219.
- Yang YH, Zhang RZ, Kang DH, et al. Three paraneoplastic signs in the same patient with gastric adenocarcinoma. Dermatol Online J. 2013;19:18966.
- Cohen PR, Grossman ME, Almeida L, et al. Tripe palms and malignancy. J Clin Oncol. 1989;7:669-678.
- Chantarojanasiri T, Buranathawornsom A, Sirinawasatien A. Diffuse esophageal squamous papillomatosis: a rare disease associated with acanthosis nigricans and tripe palms. Case Rep Gastroenterol. 2020;14:702-706.
- Muhammad R, Iftikhar N, Sarfraz T, et al. Malignant acanthosis nigricans: an indicator of internal malignancy. J Coll Physicians Surg Pak. 2019;29:888-890.
- Brinca A, Cardoso JC, Brites MM, et al. Florid cutaneous papillomatosis and acanthosis nigricans maligna revealing gastric adenocarcinoma. An Bras Dermatol. 2011;86:573-577.
- Vilas-Sueiro A, Suárez-Amor O, Monteagudo B, et al. Malignant acanthosis nigricans, florid cutaneous and mucosal papillomatosis, and tripe palms in a man with gastric adenocarcinoma. Actas Dermosifiliogr. 2015;106:438-439.
- Paravina M, Ljubisavljevic´ D. Malignant acanthosis nigricans, florid cutaneous papillomatosis and tripe palms syndrome associated with gastric adenocarcinoma—a case report. Serbian J Dermatology Venereol. 2015;7:5-14.
- Kleikamp S, Böhm M, Frosch P, et al. Acanthosis nigricans, papillomatosis mucosae and “tripe” palms in a patient with metastasized gastric carcinoma [in German]. Dtsch Med Wochenschr. 2006;131:1209-1213.
- Mikhail GR, Fachnie DM, Drukker BH, et al. Generalized malignant acanthosis nigricans. Arch Dermatol. 1979;115:201-202.
- Zhang R, Jiang M, Lei W, et al. Malignant acanthosis nigricans with recurrent bladder cancer: a case report and review of literature. Onco Targets Ther. 2021;14:951.
- Olek-Hrab K, Silny W, Zaba R, et al. Co-occurrence of acanthosis nigricans and bladder adenocarcinoma-case report. Contemp Oncol (Pozn). 2013;17:327-330.
- Canjuga I, Mravak-Stipetic´ M, Kopic´V, et al. Oral acanthosis nigricans: case report and comparison with literature reports. Acta Dermatovenerol Croat. 2008;16:91-95.
- Cairo F, Rubino I, Rotundo R, et al. Oral acanthosis nigricans as a marker of internal malignancy. a case report. J Periodontol. 2001;72:1271-1275.
- Möhrenschlager M, Vocks E, Wessner DB, et al. 2001;165:1629-1630.
- Singh GK, Sen D, Mulajker DS, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Indian J Dermatol. 2011;56:722-725.
- Gohji K, Hasunuma Y, Gotoh A, et al. Acanthosis nigricans associated with transitional cell carcinoma of the urinary bladder. Int J Dermatol. 1994;33:433-435.
- Pinto WBVR, Badia BML, Souza PVS, et al. Paraneoplastic motor neuronopathy and malignant acanthosis nigricans. Arq Neuropsiquiatr. 2019;77:527.
- Koyama S, Ikeda K, Sato M, et al. Transforming growth factor–alpha (TGF-alpha)-producing gastric carcinoma with acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32:71-77.
- Billerey C, Chopin D, Aubriot-Lorton MH, et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am J Pathol. 2001;158:1955-1959.
- Lee C-J, Lee M-H, Cho Y-Y. Fibroblast and epidermal growth factors utilize different signaling pathways to induce anchorage-independent cell transformation in JB6 Cl41 mouse skin epidermal cells. J Cancer Prev. 2014;19:199-208.
- Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991;127:1139-1140.
- Sah JF, Eckert RL, Chandraratna RA, et al. Retinoids suppress epidermal growth factor–associated cell proliferation by inhibiting epidermal growth factor receptor–dependent ERK1/2 activation. J Biol Chem. 2002;277:9728-9735.
Practice Points
- Paraneoplastic conditions may present secondary to urologic malignancy. Providers should perform thorough malignancy screening, including urologic cystoscopy, in patients presenting with paraneoplastic signs and no identified malignancy.
- Oral retinoids, such as acitretin, may be used as an adjuvant treatment to treat paraneoplastic cutaneous symptoms. The definitive treatment is malignancy management.
Painful Psoriasiform Plaques
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).
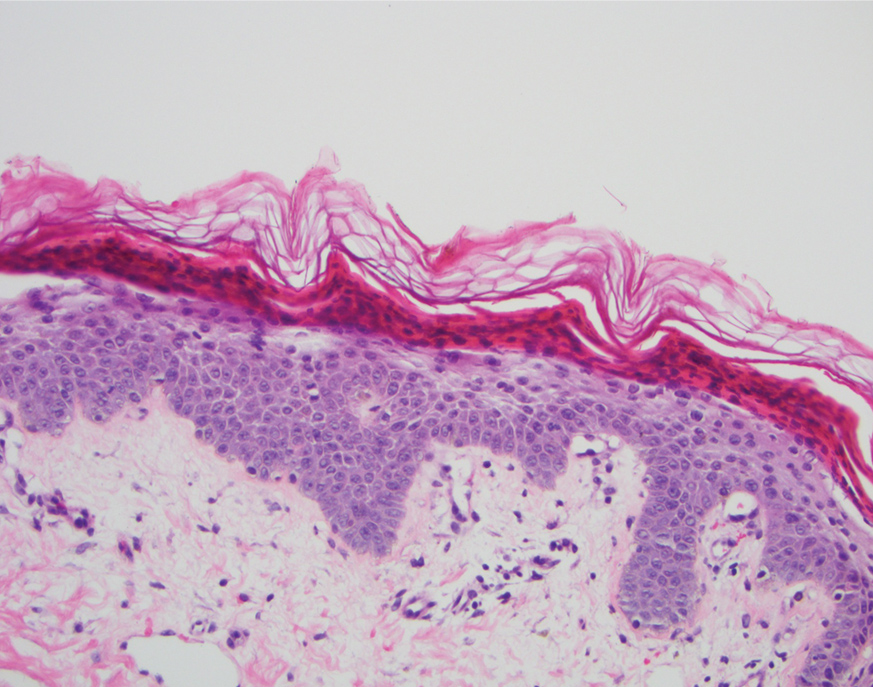
Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).

Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
The Diagnosis: Acquired Acrodermatitis Enteropathica
A punch biopsy of an elevated scaly border of the rash on the thigh revealed parakeratosis, absence of the granular layer, and epidermal pallor with psoriasiform and spongiotic dermatitis (Figure). Serum zinc levels were 60.1 μg/dL (reference range, 75.0–120.0 μg/dL), suggestive of a nutritional deficiency dermatitis. Laboratory and histopathologic findings were most consistent with a diagnosis of acquired acrodermatitis enteropathica (AE).

Acrodermatitis enteropathica has been associated with Roux-en-Y gastric bypass and alcohol use disorder working synergistically to cause malabsorption and malnutrition, respectively.1 Zinc functions in the structural integrity, wound healing, and anti-inflammatory properties of the skin. There is a 17.3% risk for hypozincemia worldwide; in developed nations there is an estimated 3% to 10% occurrence rate.2 Acrodermatitis enteropathica can be classified as either acquired or hereditary. Both classically present as a triad of acral dermatitis, diarrhea, and alopecia, though the complete triad is seen in 20% of cases.3,4
Hereditary AE is an autosomal-recessive disorder presenting in infancy that results in the loss of a zinc transporter. In contrast, acquired AE occurs later in life and usually is seen in patients who have decreased intake, malabsorption, or excessive loss of zinc.4 Acrodermatitis enteropathica is observed in individuals with conditions such as anorexia nervosa, pancreatic insufficiency, celiac disease, Crohn disease, or gastric bypass surgery (as in our case) and alcohol recidivism. In early disease, AE often presents with angular cheilitis and paronychia, but if left untreated, it can progress to mental status changes, hypogonadism, and depression.4 Acrodermatitis enteropathica presents as erythematous, erosive, scaly plaques or a papulosquamous psoriasiform rash with well-demarcated borders typically involving the orificial, acral, and intertriginous areas of the body.1,4
Acrodermatitis enteropathica belongs to a family of deficiency dermatoses that includes pellagra, necrolytic acral erythema (NAE), and necrolytic migratory erythema (NME).5 It is important to distinguish AE from NAE, as they can present similarly with well-defined and tender psoriasiform lesions peripherally. Histologically, NAE mimics AE with psoriasiform hyperplasia with parakeratosis.6 Necrolytic acral erythema characteristically is associated with active hepatitis C infection, which was absent in our patient.7
Similar to AE, NME affects the perineal and intertriginous surfaces.8 However, necrolytic migratory erythema has cutaneous manifestations in up to 70% of patients with glucagonoma syndrome, which classically presents as a triad of NME, weight loss, and diabetes mellitus.5 Laboratory studies show marked hyperglucagonemia, and imaging reveals enteropancreatic neoplasia. Necrolytic migratory erythema will rapidly resolve once the glucagonoma has been surgically removed.5 Bazex syndrome, or acrokeratosis paraneoplastica, is a paraneoplastic skin disease that is linked to underlying aerodigestive tract malignancies.
Bazex syndrome clinically is characterized by hyperkeratotic and psoriasiform lesions favoring the ears, nails, and nose.9
Psoriasis vulgaris is a common chronic inflammatory skin condition that usually presents as well-demarcated plaques with silvery scale and observed pinpoint bleeding when layers of scale are removed (Auspitz sign). Lesions typically are found on the extensor surfaces of the body in addition to the neck, feet, hands, and trunk. Treatment of psoriasis vulgaris ranges from topical steroids for mild cases to systemic biologics for moderate to severe circumstances.10 In our patient, topical triamcinolone offered little relief.
Acrodermatitis enteropathica displays clinical and histologic characteristics analogous to many deficiency dermatoses and may represent a spectrum of disease. Because the clinicopathologic findings are nonspecific, it is critical to obtain a comprehensive history and maintain a high index of suspicion in patients with risk factors for malnutrition. The treatment for AE is supplemental oral zinc usually initiated at 0.5 to 1 mg/kg daily in children and 30 to 45 mg daily in adults.3 Our patient initially was prescribed oral zinc supplementation; however, at 1-month follow-up, the rash had not improved. Failure of zinc monotherapy supports a multifactorial nutritional deficiency, which necessitated comprehensive nutritional appraisal and supplementation in our patient. Due to the steatorrhea, fecal pancreatic elastase levels were evaluated and were less than 15 μg/g (reference range, ≥201 μg/g), confirming pancreatic exocrine insufficiency, a known complication of Roux-en-Y gastric bypass.11 Pancrelipase 500 U/kg per meal was added in addition to zinc oxide 40% paste to apply to the rash twice daily, with more frequent applications to the anogenital regions after bowel movements. The patient had substantial clinical improvement after 2 months.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
- Shahsavari D, Ahmed Z, Karikkineth A, et al. Zinc-deficiency acrodermatitis in a patient with chronic alcoholism and gastric bypass: a case report. J Community Hosp Intern Med Perspect. 2014. doi:10.3402/jchimp.v4.24707
- Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus. 2017;9:E1667.
- Guliani A, Bishnoi A. Acquired acrodermatitis enteropathica. JAMA Dermatol. 2019;155:1305.
- Baruch D, Naga L, Driscoll M, et al. Acrodermatitis enteropathica from zinc-deficient total parenteral nutrition. Cutis. 2018;101:450-453.
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537.
- Botelho LF, Enokihara MM, Enokihara MY. Necrolytic acral erythema: a rare skin disease associated with hepatitis C virus infection. An Bras Dermatol. 2016;91:649-651.
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53:247-251.
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645.
- Poligone B, Christensen SR, Lazova R, et al. Bazex syndrome (acrokeratosis paraneoplastica). Lancet. 2007;369:530. 10. Kupetsky EA, Keller M. Psoriasis vulgaris: an evidencebased guide for primary care. J Am Board Fam Med. 2013; 26:787-801.
- Borbély Y, Plebani A, Kröll D, et al. Exocrine pancreatic insufficiency after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12:790-794.
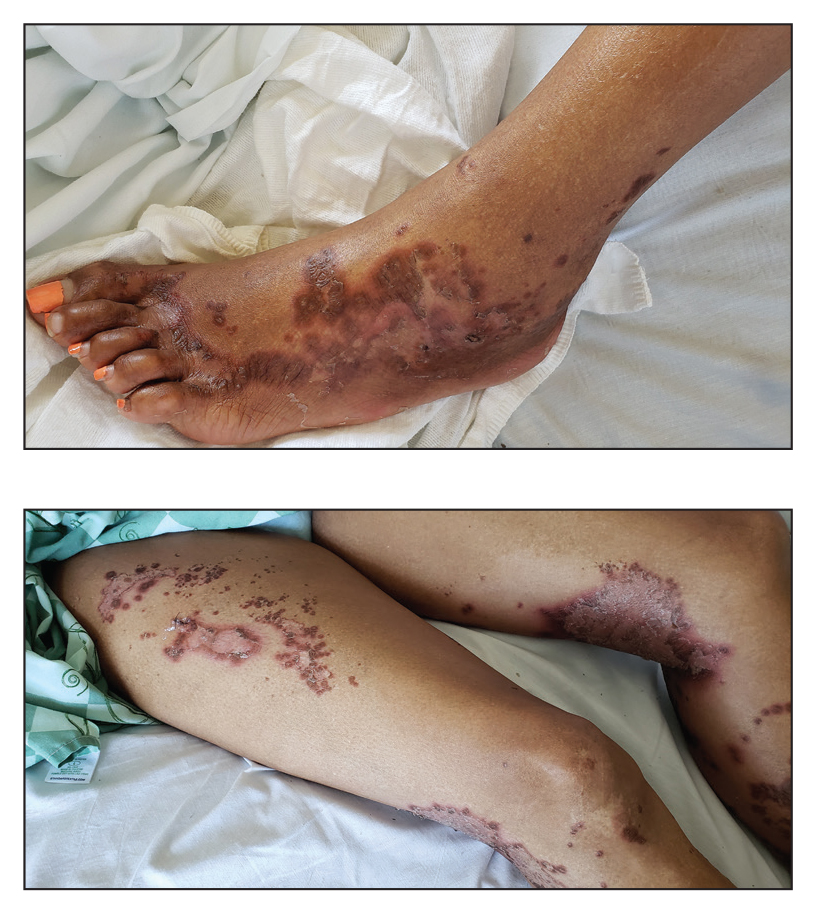
A 45-year-old woman presented to the emergency department with a painful skin eruption and malaise of 5 weeks’ duration. She had an orthotopic liver transplant 5 years prior for end-stage liver disease due to mixed nonalcoholic and alcoholic steatohepatitis and was on mycophenolate mofetil and tacrolimus for graft rejection prophylaxis. Her medical history also included Roux-en-Y gastric bypass 15 years prior, alcohol use disorder, hypothyroidism, and depression.
The exanthem began on the legs as pruritic, red, raised, exudative lesions that gradually crusted. Over the 2 weeks prior to the current presentation, the rash became tender as it spread to the feet, thighs, perianal skin, buttocks, and elbows. Triamcinolone ointment prescribed for a presumed nummular dermatitis effected marginal benefit. A review of systems was notable for a 15-pound weight loss over several weeks; lowgrade fever of 3 days’ duration; epigastric abdominal pain; and long-standing, frequent defecation of oily, foul-smelling feces.
Physical examination revealed a combination of flat-topped, violaceous papules and serpiginous, polycyclic, annular plaques coalescing to form larger psoriasiform plaques with hyperkeratotic rims and dusky borders on the dorsal aspect of the feet (top), lateral ankles, legs (bottom), lateral thighs, buttocks, perianal skin, and elbows. Bilateral angular cheilitis, a smooth and fissured tongue, and pitting of all fingernails were noted.
Botanical Briefs: Bloodroot (Sanguinaria canadensis)
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
Bloodroot (Sanguinaria canadensis) is a member of the family Papaveraceae.1 This North American plant commonly is found in widespread distribution from Nova Scotia, Canada, to Florida and from the Great Lakes to Mississippi.2 Historically, Native Americans used bloodroot as a skin dye and as a medicine for many ailments.3
Bloodroot blooms for only a few days, starting in March, and fruits in June. The flowers comprise 8 to 10 white petals, surrounding a bed of yellow stamens (Figure). The plant thrives in wooded areas and grows to 12 inches tall. In its off-season, the plant remains dormant and can survive below-freezing temperatures.4

Chemical Constituents
Bloodroot gets its colloquial name from its red sap, which is released when the plant’s rhizome is cut. This sap contains a high concentration of alkaloids that are used for protection against predators. The rhizome itself has a rusty, red-brown color; the roots are a brighter red-orange.4
The rhizome of S canadensis contains the highest concentration of active alkaloids; the roots also contain these chemicals, though to a lesser degree; and the leaves, flowers, and fruits harvest approximately 1% of the alkaloids found in the roots.4 The concentration of alkaloids can vary from one plant to the next, depending on environmental conditions.5,6
The major alkaloids in S canadensis include both quaternary benzophenanthridine alkaloids (eg, sanguinarine, chelerythrine, sanguilutine, chelilutine, sanguirubine, chelirubine) and protopin alkaloids (eg, protopine, allocryptopine).3,7 Of these, sanguinarine and chelerythrine typically are the most potent.1 Oral ingestion or topical application of these molecules can have therapeutic and toxic effects.8
Biophysiological Effects
Bloodroot has been shown to have remarkable antimicrobial effects.9 The plant produces hydrogen peroxide and superoxide anion.10 These mediators cause oxidative stress, thus inducing destruction of cellular DNA and the cell membrane.11 Although these effects can be helpful when fighting infection, they are not necessarily selective against healthy cells.12
Alkaloids of bloodroot also have cardiovascular therapeutic effects. Sanguinarine blocks angiotensin II and causes vasodilation, thus helping treat hypertension.13 It also acts as an inotrope by blocking the Na+/K+ ATPase pump. These effects in a patient who is already taking digoxin can cause notable cardiotoxicity because the 2 drugs share a mechanism of action.14
Chelerythrine blocks production of cyclooxygenase 2 and prostaglandin E2.15 This pathway modification results in anti-inflammatory effects that can help treat arthritis, edema, and other inflammatory conditions.16 Moreover, sanguinarine has demonstrated efficacy in numerous anticancer pathways,17 including downregulation of intercellular adhesion molecules, vascular cell adhesion molecules, and vascular endothelial growth factor (VEGF).18-20 Blocking VEGF is one way to inhibit angiogenesis,21 which is upregulated in tumor formation, thus sanguinarine can have an antiproliferative anticancer effect.22 Sanguinarine also upregulates molecules such as nuclear factor–κB and the protease enzymes known as caspases to cause proapoptotic effects, furthering its antitumor potential.23,24
Treatment of Dermatologic Conditions
The initial technique of Mohs micrographic surgery employed a chemopaste that utilized an extract of S canadensis to preserve tissue.25 Outside the dermatologist’s office, bloodroot is used as a topical home remedy for a variety of cutaneous conditions, including cancer, skin tags, and warts.26 Bloodroot is advertised as black salve, an alternative anticancer treatment.27,28
As useful as this natural agent sounds, it has a pitfall: The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging neoplastic and healthy tissue.29 This cytotoxic effect can cause escharification through diffuse tissue destruction and has been observed to result in formation of a keloid scar.30 The alkaloids in black salve also have been shown to cause skin erosions and cellular atypia.28,31 Therefore, the utility of this escharotic in medical treatment is limited.32 Fortuitously, oral antibiotics and wound care can help address this adverse effect.28
Bloodroot was once used as a mouth rinse and toothpaste to treat gingivitis, but this application was later associated with oral leukoplakia, a premalignant condition.33 Leukoplakia associated with S canadensis extract often is unremitting. Immediate discontinuation of the offending agent produces little regression, suggesting that cellular damage is irreversible.34
Final Thoughts
Although bloodroot demonstrates efficacy as a phytotherapeutic, it does come with notable toxicity. Physicians should warn patients of the unwanted cosmetic effects of black salve, especially oral products that incorporate sanguinarine. Adverse effects on the oropharynx can be irreversible, though the eschar associated with black salve can be treated with a topical or oral corticosteroid.29
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
- Vogel M, Lawson M, Sippl W, et al. Structure and mechanism of sanguinarine reductase, an enzyme of alkaloid detoxification. J Biol Chem. 2010;285:18397-18406. doi:10.1074/jbc.M109.088989
- Maranda EL, Wang MX, Cortizo J, et al. Flower power—the versatility of bloodroot. JAMA Dermatol. 2016;152:824. doi:10.1001/jamadermatol.2015.5522
- Setzer WN. The phytochemistry of Cherokee aromatic medicinal plants. Medicines (Basel). 2018;5:121. doi:10.3390/medicines5040121
- Croaker A, King GJ, Pyne JH, et al. Sanguinaria canadensis: traditional medicine, phytochemical composition, biological activities and current uses. Int J Mol Sci. 2016;17:1414. doi:10.3390/ijms17091414
- Graf TN, Levine KE, Andrews ME, et al. Variability in the yield of benzophenanthridine alkaloids in wildcrafted vs cultivated bloodroot (Sanguinaria canadensis L.) J Agric Food Chem. 2007; 55:1205-1211. doi:10.1021/jf062498f
- Bennett BC, Bell CR, Boulware RT. Geographic variation in alkaloid content of Sanguinaria canadensis (Papaveraceae). Rhodora. 1990;92:57-69.
- Leaver CA, Yuan H, Wallen GR. Apoptotic activities of Sanguinaria canadensis: primary human keratinocytes, C-33A, and human papillomavirus HeLa cervical cancer lines. Integr Med (Encinitas). 2018;17:32-37.
- Kutchan TM. Molecular genetics of plant alkaloid biosynthesis. In: Cordell GA, ed. The Alkaloids. Vol 50. Elsevier Science Publishing Co, Inc; 1997:257-316.
- Obiang-Obounou BW, Kang O-H, Choi J-G, et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J Toxicol Sci. 2011;36:277-283. doi:10.2131/jts.36.277
- Z˙abka A, Winnicki K, Polit JT, et al. Sanguinarine-induced oxidative stress and apoptosis-like programmed cell death (AL-PCD) in root meristem cells of Allium cepa. Plant Physiol Biochem. 2017;112:193-206. doi:10.1016/j.plaphy.2017.01.004
- Kumar GS, Hazra S. Sanguinarine, a promising anticancer therapeutic: photochemical and nucleic acid binding properties. RSC Advances. 2014;4:56518-56531.
- Ping G, Wang Y, Shen L, et al. Highly efficient complexation of sanguinarine alkaloid by carboxylatopillar[6]arene: pKa shift, increased solubility and enhanced antibacterial activity. Chemical Commun (Camb). 2017;53:7381-7384. doi:10.1039/c7cc02799k
- Caballero-George C, Vanderheyden PM, Solis PN, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8:59-70. doi:10.1078/0944-7113-00011
- Seifen E, Adams RJ, Riemer RK. Sanguinarine: a positive inotropic alkaloid which inhibits cardiac Na+, K+-ATPase. Eur J Pharmacol. 1979;60:373-377. doi:10.1016/0014-2999(79)90245-0
- Debprasad C, Hemanta M, Paromita B, et al. Inhibition of NO2, PGE2, TNF-α, and iNOS EXpression by Shorea robusta L.: an ethnomedicine used for anti-inflammatory and analgesic activity. Evid Based Complement Alternat Med. 2012; 2012:254849. doi:10.1155/2012/254849
- Melov S, Ravenscroft J, Malik S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567-1569. doi:10.1126/science.289.5484.1567
- Basu P, Kumar GS. Sanguinarine and its role in chronic diseases. In: Gupta SC, Prasad S, Aggarwal BB, eds. Advances in Experimental Medicine and Biology: Anti-inflammatory Nutraceuticals and Chronic Diseases. Vol 928. Springer International Publishing; 2016:155-172.
- Alasvand M, Assadollahi V, Ambra R, et al. Antiangiogenic effect of alkaloids. Oxid Med Cell Longev. 2019;2019:9475908. doi:10.1155/2019/9475908
- Basini G, Santini SE, Bussolati S, et al. The plant alkaloid sanguinarine is a potential inhibitor of follicular angiogenesis. J Reprod Dev. 2007;53:573-579. doi:10.1262/jrd.18126
- Xu J-Y, Meng Q-H, Chong Y, et al. Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol. 2013;1:331-336. doi:10.3892/mco.2012.41
- Lu K, Bhat M, Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016;19:287-295. doi:10.1007/s10456-016-9512-y
- Achkar IW, Mraiche F, Mohammad RM, et al. Anticancer potential of sanguinarine for various human malignancies. Future Med Chem. 2017;9:933-950. doi:10.4155/fmc-2017-0041
- Lee TK, Park C, Jeong S-J, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77:227-240. doi:10.1002/ddr.21315
- Gaziano R, Moroni G, Buè C, et al. Antitumor effects of the benzophenanthridine alkaloid sanguinarine: evidence and perspectives. World J Gastrointest Oncol. 2016;8:30-39. doi:10.4251/wjgo.v8.i1.30
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol. 1976;112:211-215.
- Affleck AG, Varma S. A case of do-it-yourself Mohs’ surgery using bloodroot obtained from the internet. Br J Dermatol. 2007;157:1078-1079. doi:10.1111/j.1365-2133.2007.08180.x
- Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:E154-E155. doi:10.1016/j.jaad.2011.07.031
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:508-510. doi:10.1016/j.jaad.2005.04.007
- Schlichte MJ, Downing CP, Ramirez-Fort M, et al. Bloodroot associated eschar. Dermatol Online J. 2015;20:13030/qt05r0r2wr.
- Wang MZ, Warshaw EM. Bloodroot. Dermatitis. 2012;23:281-283. doi:10.1097/DER.0b013e318273a4dd
- Tan JM, Peters P, Ong N, et al. Histopathological features after topical black salve application. Australas J Dermatol. 2015;56:75-76.
- Hou JL, Brewer JD. Black salve and bloodroot extract in dermatologic conditions. Cutis. 2015;95:309-311.
- Eversole LR, Eversole GM, Kopcik J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455-464. doi:10.1016/s1079-2104(00)70125-9
- Mascarenhas AK, Allen CM, Moeschberger ML. The association between Viadent® use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158-162. doi:10.1111/j.1752-7325.2002.tb03437.x
Practice Points
- Bloodroot (Sanguinaria canadensis) is a plant historically used in Mohs micrographic surgery as chemopaste.
- Bloodroot has been shown to have remarkable antimicrobial effects.
- The alkaloids of S canadensis are nonspecific in their cytotoxicity, damaging both neoplastic and healthy tissue. They have been shown to cause skin erosions and cellular atypia.
Skin of Color in Preclinical Medical Education: A Cross-Institutional Comparison and A Call to Action
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.
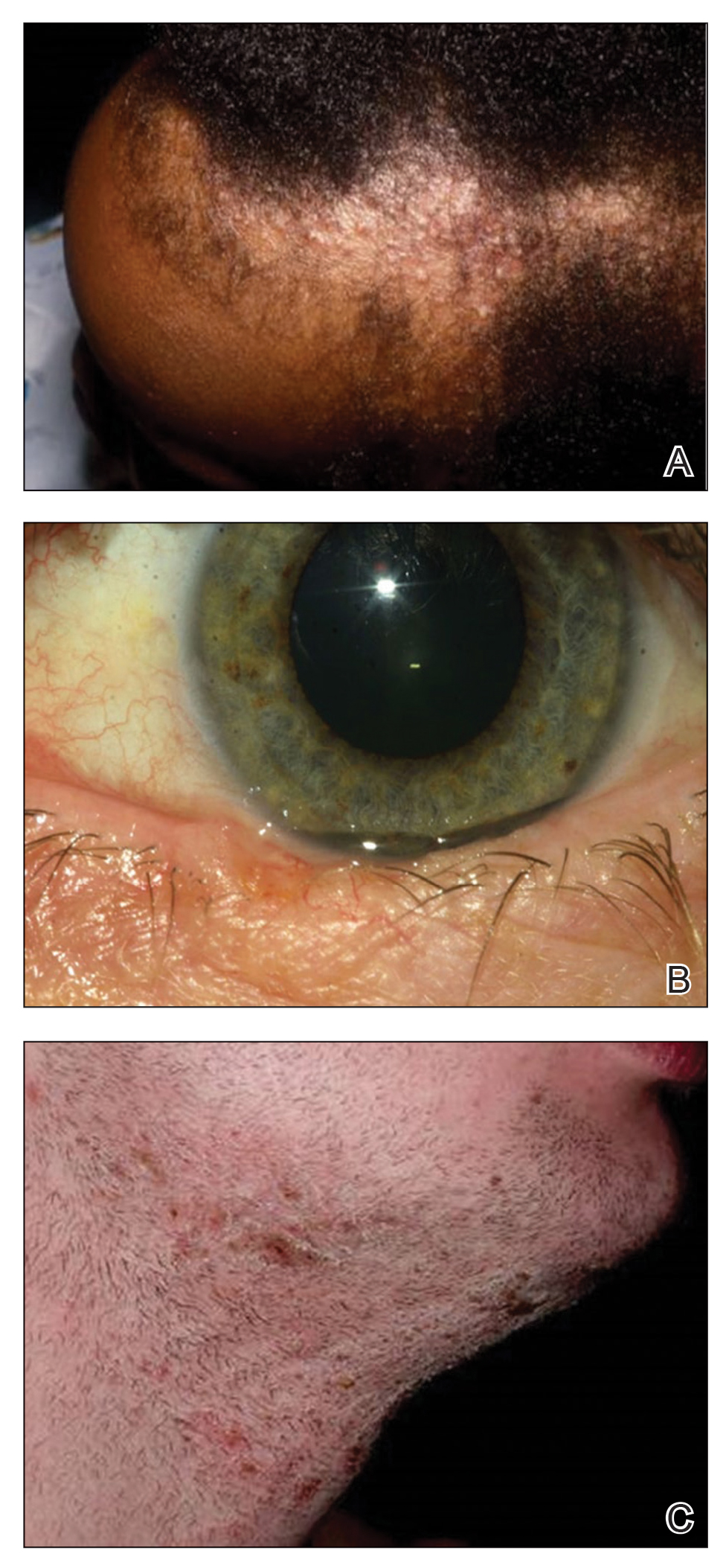
We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.
Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).
Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
A ccording to the US Census Bureau, more than half of all Americans are projected to belong to a minority group, defined as any group other than non-Hispanic White alone, by 2044. 1 Consequently, the United States rapidly is becoming a country in which the majority of citizens will have skin of color. Individuals with skin of color are of diverse ethnic backgrounds and include people of African, Latin American, Native American, Pacific Islander, and Asian descent, as well as interethnic backgrounds. 2 Throughout the country, dermatologists along with primary care practitioners may be confronted with certain cutaneous conditions that have varying disease presentations or processes in patients with skin of color. It also is important to note that racial categories are socially rather than biologically constructed, and the term skin of color includes a wide variety of diverse skin types. Nevertheless, the current literature thoroughly supports unique pathophysiologic differences in skin of color as well as variations in disease manifestation compared to White patients. 3-5 For example, the increased lability of melanosomes in skin of color patients, which increases their risk for postinflammatory hyperpigmentation, has been well documented. 5-7 There are various dermatologic conditions that also occur with higher frequency and manifest uniquely in people with darker, more pigmented skin, 7-9 and dermatologists, along with primary care physicians, should feel prepared to recognize and address them.
Extensive evidence also indicates that there are unique aspects to consider while managing certain skin diseases in patients with skin of color.8,10,11 Consequently, as noted on the Skin of Color Society (SOCS) website, “[a]n increase in the body of dermatological literature concerning skin of color as well as the advancement of both basic science and clinical investigational research is necessary to meet the needs of the expanding skin of color population.”2 In the meantime, current knowledge regarding cutaneous conditions that diversely or disproportionately affect skin of color should be actively disseminated to physicians in training. Although patients with skin of color should always have access to comprehensive care and knowledgeable practitioners, the current changes in national and regional demographics further underscore the need for a more thorough understanding of skin of color with regard to disease pathogenesis, diagnosis, and treatment.
Several studies have found that medical students in the United States are minimally exposed to dermatology in general compared to other clinical specialties,12-14 which can easily lead to the underrecognition of disorders that may uniquely or disproportionately affect individuals with pigmented skin. Recent data showed that medical schools typically required fewer than 10 hours of dermatology instruction,12 and on average, dermatologic training made up less than 1% of a medical student’s undergraduate medical education.13,15,16 Consequently, less than 40% of primary care residents felt that their medical school curriculum adequately prepared them to manage common skin conditions.14 Although not all physicians should be expected to fully grasp the complexities of skin of color and its diagnostic and therapeutic implications, both practicing and training dermatologists have acknowledged a lack of exposure to skin of color. In one study, approximately 47% of dermatologists and dermatology residents reported that their medical training (medical school and/or residency) was inadequate in training them on skin conditions in Black patients. Furthermore, many who felt their training was lacking in skin of color identified the need for greater exposure to Black patients and training materials.15 The absence of comprehensive medical education regarding skin of color ultimately can be a disadvantage for both practitioners and patients, resulting in poorer outcomes. Furthermore, underrepresentation of skin of color may persist beyond undergraduate and graduate medical education. There also is evidence to suggest that noninclusion of skin of color pervades foundational dermatologic educational resources, including commonly used textbooks as well as continuing medical education disseminated at national conferences and meetings.17 Taken together, these findings highlight the need for more diverse and representative exposure to skin of color throughout medical training, which begins with a diverse inclusive undergraduate medical education in dermatology.
The objective of this study was to determine if the preclinical dermatology curriculum at 3 US medical schools provided adequate representation of skin of color patients in their didactic presentation slides.
Methods
Participants—Three US medical schools, a blend of private and public medical schools located across different geographic boundaries, agreed to participate in the study. All 3 institutions were current members of the American Medical Association (AMA) Accelerating Change in Medical Education consortium, whose primary goal is to create the medical school of the future and transform physician training.18 All 32 member institutions of the AMA consortium were contacted to request their participation in the study. As part of the consortium, these institutions have vowed to collectively work to develop and share the best models for educational advancement to improve care for patients, populations, and communities18 and would expectedly provide a more racially and ethnically inclusive curriculum than an institution not accountable to a group dedicated to identifying the best ways to deliver care for increasingly diverse communities.
Data Collection—Lectures were included if they were presented during dermatology preclinical courses in the 2015 to 2016 academic year. An uninvolved third party removed the names and identities of instructors to preserve anonymity. Two independent coders from different institutions extracted the data—lecture title, total number of clinical and histologic images, and number of skin of color images—from each of the anonymized lectures using a standardized coding form. We documented differences in skin of color noted in lectures and the disease context for the discussed differences, such as variations in clinical presentation, disease process, epidemiology/risk, and treatment between different skin phenotypes or ethnic groups. Photographs in which the coders were unable to differentiate whether the patient had skin of color were designated as indeterminate or unclear. Photographs appearing to represent Fitzpatrick skin types IV, V, and VI19 were categorically designated as skin of color, and those appearing to represent Fitzpatrick skin types I and II were described as not skin of color; however, images appearing to represent Fitzpatrick skin type III often were classified as not skin of color or indeterminate and occasionally skin of color. The Figure shows examples of images classified as skin of color, indeterminate, and not skin of color. Photographs often were classified as indeterminate due to poor lighting, close-up view photographs, or highlighted pathology obscuring the surrounding skin. We excluded duplicate photographs and histologic images from the analyses.

We also reviewed 19 conditions previously highlighted by the SOCS as areas of importance to skin of color patients.20 The coders tracked how many of these conditions were noted in each lecture. Duplicate discussion of these conditions was not included in the analyses. Any discrepancies between coders were resolved through additional slide review and discussion. The final coded data with the agreed upon changes were used for statistical analyses. Recent national demographic data from the US Census Bureau in 2019 describe approximately 39.9% of the population as belonging to racial/ethnic groups other than non-Hispanic/Latinx White.21 Consequently, the standard for adequate representation for skin of color photographs was set at 35% for the purpose of this study.
Results
Across all 3 institutions included in the study, the proportion of the total number of clinical photographs showing skin of color was 16% (290/1812). Eight percent of the total photographs (145/1812) were noted to be indeterminate (Table). For institution 1, 23.6% of photographs (155/658) showed skin of color, and 12.6% (83/658) were indeterminate. For institution 2, 13.1% (76/578) showed skin of color and 7.8% (45/578) were indeterminate. For institution 3, 10.2% (59/576) showed skin of color and 3% (17/576) were indeterminate.

Institutions 1, 2, and 3 had 18, 8, and 17 total dermatology lectures, respectively. Of the 19 conditions designated as areas of importance to skin of color patients by the SOCS, 16 (84.2%) were discussed by institution 1, 11 (57.9%) by institution 2, and 9 (47.4%) by institution 3 (eTable 1). Institution 3 did not include photographs of skin of color patients in its acne, psoriasis, or cutaneous malignancy lectures. Institution 1 also did not include any skin of color patients in its malignancy lecture. Lectures that focused on pigmentary disorders, atopic dermatitis, infectious conditions, and benign cutaneous neoplasms were more likely to display photographs of skin of color patients; for example, lectures that discussed infectious conditions, such as superficial mycoses, herpes viruses, human papillomavirus, syphilis, and atypical mycobacterial infections, were consistently among those with higher proportions of photographs of skin of color patients.

Throughout the entire preclinical dermatology course at all 3 institutions, of 2945 lecture slides, only 24 (0.8%) unique differences were noted between skin color and non–skin of color patients, with 10 total differences noted by institution 1, 6 by institution 2, and 8 by institution 3 (Table). The majority of these differences (19/24) were related to epidemiologic differences in prevalence among varying racial/ethnic groups, with only 5 instances highlighting differences in clinical presentation. There was only a single instance that elaborated on the underlying pathophysiologic mechanisms of the discussed difference. Of all 24 unique differences discussed, 8 were related to skin cancer, 3 were related to dermatitis, and 2 were related to the difference in manifestation of erythema in patients with darker skin (eTable 2).

Comment
The results of this study demonstrated that skin of color is underrepresented in the preclinical dermatology curriculum at these 3 institutions. Although only 16% of all included clinical photographs were of skin of color, individuals with skin of color will soon represent more than half of the total US population within the next 2 decades.1 To increase representation of skin of color patients, teaching faculty should consciously and deliberately include more photographs of skin of color patients for a wider variety of common conditions, including atopic dermatitis and psoriasis, in addition to those that tend to disparately affect skin of color patients, such as pseudofolliculitis barbae or melasma. Furthermore, they also can incorporate more detailed discussions about important differences seen in skin of color patients.
More Skin of Color Photographs in Psoriasis Lectures—At institution 3, there were no skin of color patients included in the psoriasis lecture, even though there is considerable data in the literature indicating notable differences in the clinical presentation, quality-of-life impact, and treatment of psoriasis in skin of color patients.11,22 There are multiple nuances in psoriasis manifestation in patients with skin of color, including less-conspicuous erythema in darker skin, higher degrees of dyspigmentation, and greater body surface area involvement. For Black patients with scalp psoriasis, the impact of hair texture, styling practices, and washing frequency are additional considerations that may impact disease severity and selection of topical therapy.11 The lack of inclusion of any skin of color patients in the psoriasis lecture at one institution further underscores the pressing need to prioritize communities of color in medical education.
More Skin of Color Photographs in Cutaneous Malignancy Lectures—Similarly, while a lecturer at institution 2 noted that acral lentiginous melanoma accounts for a considerable proportion of melanoma among skin of color patients,23 there was no mention of how melanoma generally is substantially more deadly in this population, potentially due to decreased awareness and inconsistent screening.24 Furthermore, at institutions 1 and 3, there were no photographs or discussion of skin of color patients during the cutaneous malignancy lectures. Evidence shows that more emphasis is needed for melanoma screening and awareness in skin of color populations to improve survival outcomes,24 and this begins with educating not only future dermatologists but all future physicians as well. The failure to include photographs of skin of color patients in discussions or lectures regarding cutaneous malignancies may serve to further perpetuate the harmful misperception that individuals with skin of color are unaffected by skin cancer.25,26
Analysis of Skin of Color Photographs in Infectious Disease Lectures—In addition, lectures discussing infectious etiologies were among those with the highest proportion of skin of color photographs. This relatively disproportionate representation of skin of color compared to the other lectures may contribute to the development of harmful stereotypes or the stigmatization of skin of color patients. Although skin of color should continue to be represented in similar lectures, teaching faculty should remain mindful of the potential unintended impact from lectures including relatively disproportionate amounts of skin of color, particularly when other lectures may have sparse to absent representation of skin of color.
More Photographs Available for Education—Overall, our findings may help to inform changes to preclinical dermatology medical education at other institutions to create more inclusive and representative curricula for skin of color patients. The ability of instructors to provide visual representation of various dermatologic conditions may be limited by the photographs available in certain textbooks with few examples of patients with skin of color; however, concerns regarding the lack of skin of color representation in dermatology training is not a novel discussion.17 Although it is the responsibility of all dermatologists to advocate for the inclusion of skin of color, many dermatologists of color have been leading the way in this movement for decades, publishing several textbooks to document various skin conditions in those with darker skin types and discuss unique considerations for patients with skin of color.27-29 Images from these textbooks can be utilized by programs to increase representation of skin of color in dermatology training. There also are multiple expanding online dermatologic databases, such as VisualDx, with an increasing focus on skin of color patients, some of which allow users to filter images by degree of skin pigmentation.30 Moreover, instructors also can work to diversify their curricula by highlighting more of the SOCS conditions of importance to skin of color patients, which have since been renamed and highlighted on the Patient Dermatology Education section of the SOCS website.20 These conditions, while not completely comprehensive, provide a useful starting point for medical educators to reevaluate for potential areas of improvement and inclusion.
There are several potential strategies that can be used to better represent skin of color in dermatologic preclinical medical education, including increasing awareness, especially among dermatology teaching faculty, of existing disparities in the representation of skin of color in the preclinical curricula. Additionally, all dermatology teaching materials could be reviewed at the department level prior to being disseminated to medical students to assess for instances in which skin of color could be prioritized for discussion or varying disease presentations in skin of color could be demonstrated. Finally, teaching faculty may consider photographing more clinical images of their skin of color patients to further develop a catalog of diverse images that can be used to teach students.
Study Limitations—Our study was unable to account for verbal discussion of skin of color not otherwise denoted or captured in lecture slides. Additional limitations include the utilization of Fitzpatrick skin types to describe and differentiate varying skin tones, as the Fitzpatrick scale originally was developed as a method to describe an individual’s response to UV exposure.19 The inability to further delineate the representation of darker skin types, such as those that may be classified as Fitzpatrick skin types V or VI,19 compared to those with lighter skin of color also was a limiting factor. This study was unable to assess for discussion of other common conditions affecting skin of color patients that were not listed as one of the priority conditions by SOCS. Photographs that were designated as indeterminate were difficult to elucidate as skin of color; however, it is possible that instructors may have verbally described these images as skin of color during lectures. Nonetheless, it may be beneficial for learners if teaching faculty were to clearly label instances where skin of color patients are shown or when notable differences are present.
Conclusion
Future studies would benefit from the inclusion of audio data from lectures, syllabi, and small group teaching materials from preclinical courses to more accurately assess representation of skin of color in dermatology training. Additionally, future studies also may expand to include images from lectures of overlapping clinical specialties, particularly infectious disease and rheumatology, to provide a broader assessment of skin of color exposure. Furthermore, repeat assessment may be beneficial to assess the longitudinal effectiveness of curricular changes at the institutions included in this study, comparing older lectures to more recent, updated lectures. This study also may be replicated at other medical schools to allow for wider comparison of curricula.
Acknowledgment—The authors wish to thank the institutions that offered and agreed to participate in this study with the hopes of improving medical education.
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
- Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed September 14, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Learn more about SOCS. Skin of Color Society website. Accessed September 14, 2021. http://skinofcolorsociety.org/about-socs/
- Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
- Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48(suppl 6):S139-S142.
- Callender VD, Surin-Lord SS, Davis EC, et al. Postinflammatory hyperpigmentation. Am J Clin Dermatol. 2011;12:87-99.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE, Stockton T. Pigmentary disorders in blacks. Dermatol Clin. 1988;6:271-281.
- Halder RM, Nootheti PK. Ethnic skin disorders overview. J Am Acad Dermatol. 2003;48(suppl 6):S143-S148.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Ramsay DL, Mayer F. National survey of undergraduate dermatologic medical education. Arch Dermatol.1985;121:1529-1530.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii.
- Knable A, Hood AF, Pearson TG. Undergraduate medical education in dermatology: report from the AAD Interdisciplinary Education Committee, Subcommittee on Undergraduate Medical Education. J Am Acad Dermatol. 1997;36:467-470.
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Skin of Color Society. Patient dermatology education. Accessed September 22, 2021. https://skinofcolorsociety.org/patient-dermatology-education
- QuickFacts: United States. US Census Bureau website. Updated July 1, 2019. Accessed September 14, 2021. https://www.census.gov/quickfacts/fact/table/US#
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Pipitone M, Robinson JK, Camara C, et al. Skin cancer awareness in suburban employees: a Hispanic perspective. J Am Acad Dermatol. 2002;47:118-123.
- Imahiyerobo-Ip J, Ip I, Jamal S, et al. Skin cancer awareness in communities of color. J Am Acad Dermatol. 2011;64:198-200.
- Taylor SSC, Serrano AMA, Kelly AP, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016.
- Dadzie OE, Petit A, Alexis AF, eds. Ethnic Dermatology: Principles and Practice. Wiley-Blackwell; 2013.
- Jackson-Richards D, Pandya AG, eds. Dermatology Atlas for Skin of Color. Springer; 2014.
- VisualDx. New VisualDx feature: skin of color sort. Published October 14, 2020. Accessed September 22, 2021. https://www.visualdx.com/blog/new-visualdx-feature-skin-of-color-sort/
Practice Points
- The United States rapidly is becoming a country in which the majority of citizens will have skin of color.
- Our study results strongly suggest that skin of color may be seriously underrepresented in medical education and can guide modifications to preclinical dermatology medical education to develop a more comprehensive and inclusive curriculum.
- Efforts should be made to increase images and discussion of skin of color in preclinical didactics.
The Role of Inpatient Dermatology Consultations
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.
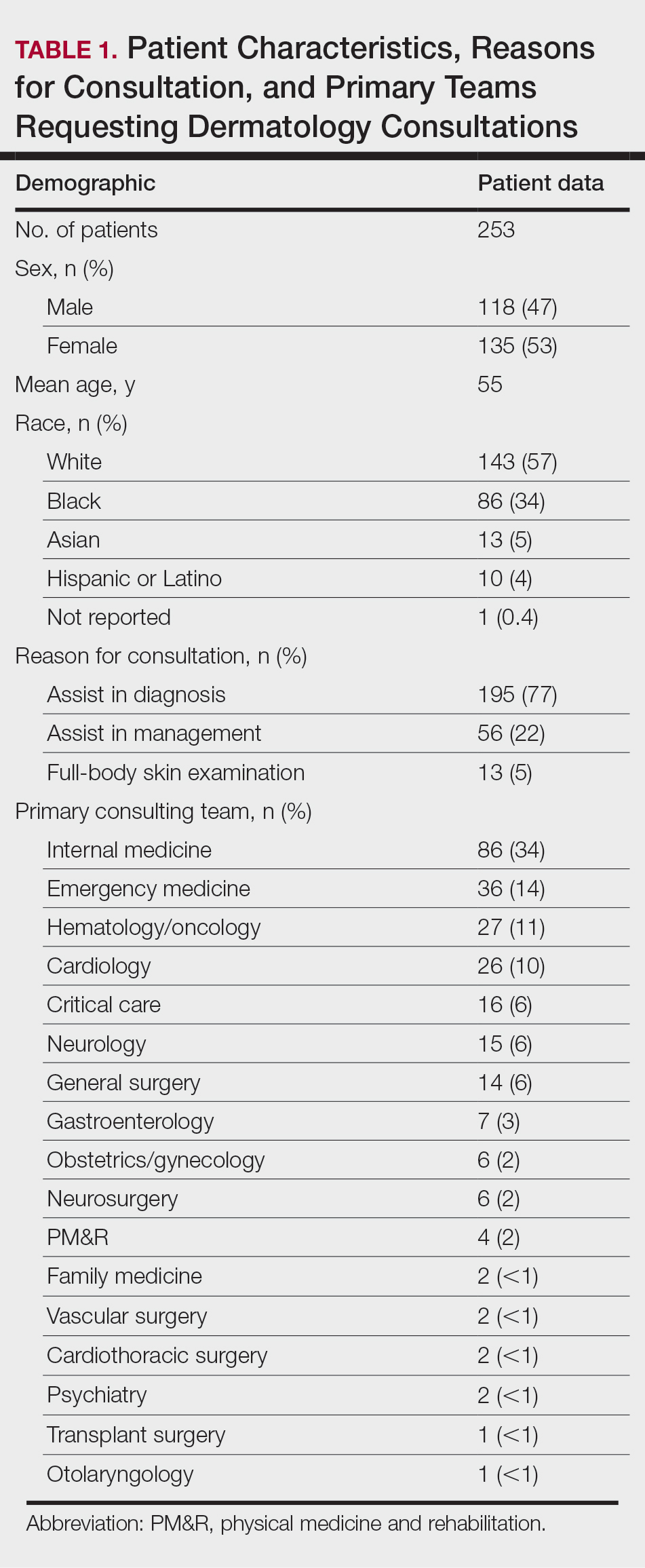
Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.
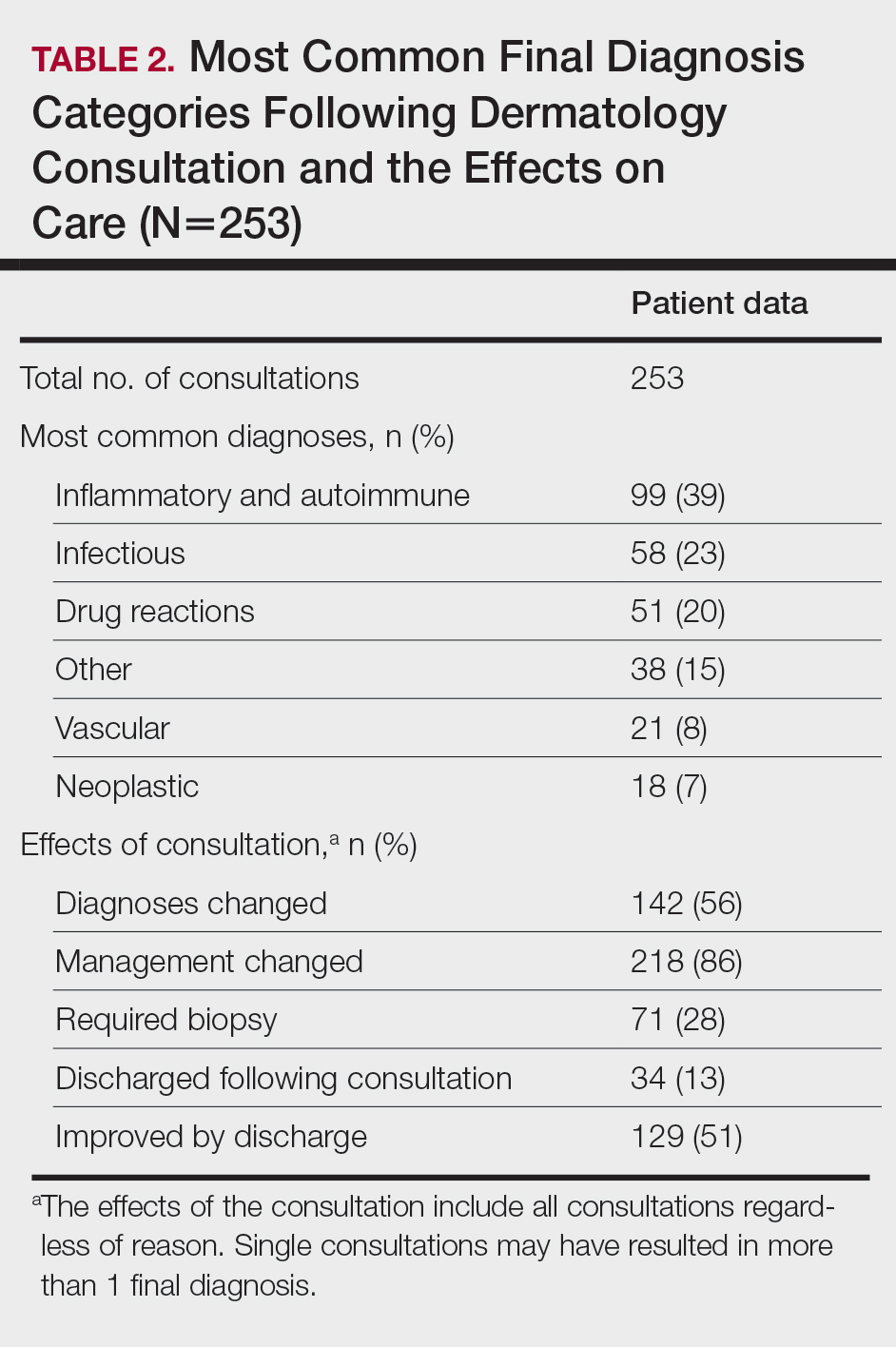
Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
Dermatology is an often-underutilized resource in the hospital setting. As the health care landscape has evolved, so has the role of the inpatient dermatologist.1-3 Structural changes in the health system and advances in therapies have shifted dermatology from an admitting service to an almost exclusively outpatient practice. Improved treatment modalities led to decreases in the number of patients requiring admission for chronic dermatoses, and outpatient clinics began offering therapies once limited to hospitals.1,4 Inpatient dermatology consultations emerged and continue to have profound effects on hospitalized patients regardless of their reason for admission.1-11
Inpatient dermatologists supply knowledge in areas primary medical teams lack, and there is evidence that dermatology consultations improve the quality of care while decreasing cost.2,5-7 Establishing correct diagnoses, preventing exposure to unnecessary medications, and reducing hospitalization duration and readmission rates are a few ways dermatology consultations positively impact hospitalized patients.2,5-7,9,10 This study highlights the role of the dermatologist in the care of hospitalized patients at a large academic medical center in an urban setting and reveals how consultation supports the efficiency and efficacy of other services.
Materials and Methods
Study Design—This single-institution, cross-sectional retrospective study included all hospitalized patients at the Thomas Jefferson University Hospital (Philadelphia, Pennsylvania), who received an inpatient dermatology consultation completed by physicians of Jefferson Dermatology Associates between January 1, 2019, and December 31, 2019. The institutional review board at Thomas Jefferson University approved this study.
Data Collection—A list of all inpatient dermatology consultations in 2019 was provided by Jefferson Dermatology Associates. Through a retrospective chart review, data regarding the consultations were collected from the electronic medical record (Epic Systems) and recorded into the Research Electronic Data Capture system. Data on patient demographics, the primary medical team, the dermatology evaluation, and the hospital course of the patient were collected.
Results
Patient Characteristics—Dermatology received 253 inpatient consultation requests during this time period; 53% of patients were female and 47% were male, with a mean age of 55 years. Most patients were White (57%), while 34% were Black. Five percent and 4% of patients were Asian and Hispanic or Latino, respectively (Table 1). The mean duration of hospitalization for all patients was 15 days, and the average number of days to discharge following the first encounter with dermatology was 10 days.

Requesting Team and Reason for Consultation—Internal medicine consulted dermatology most frequently (34% of all consultations), followed by emergency medicine (14%) and a variety of other services (Table 1). Most dermatology consultations were placed to assist in achieving a diagnosis of a cutaneous condition (77%), while a minority were to assist in the management of a previously diagnosed disease (22%). A small fraction of consultations (5%) were to complete full-body skin examinations (FBSEs) to rule out infection or malignancy in candidates for organ transplantation, left ventricular assist devices, or certain chemotherapies. One FBSE was conducted to search for a primary tumor in a patient diagnosed with metastatic melanoma.
Most Common Final Diagnoses and Consultation Impact—Table 2 lists the most common final diagnosis categories, as well as the effects of the consultation on diagnosis, management, biopsies, hospitalization, and clinical improvement as documented by the primary medical provider. The most common final diagnoses were inflammatory and autoimmune (39%), such as contact dermatitis and seborrheic dermatitis; infectious (23%), such as varicella (primary or zoster) and bacterial furunculosis; drug reactions (20%), such as morbilliform drug eruptions; vascular (8%), such as vasculitis and calciphylaxis; neoplastic (7%), such as keratinocyte carcinomas and leukemia cutis; and other (15%), such as xerosis, keratosis pilaris, and miliaria rubra.

Impact on Diagnosis—Fifty-six percent of all consultations resulted in a change in diagnosis. When dermatology was consulted specifically to assist in the diagnosis of a patient (195 consultations), the working diagnosis of the primary team was changed 69% of the time. Thirty-five of these consultation requests had no preliminary diagnosis, and the primary team listed the working diagnosis as either rash or a morphologic description of the lesion(s). Sixty-three percent of suspected drug eruptions ended with a diagnosis of a form of drug eruption, while 20% of consultations for suspected cellulitis or bacterial infections were confirmed to be cellulitis or soft tissue infections.
Impact on Management—Regardless of the reason for the consultation, most consultations (86%) resulted in a change in management. The remaining 14% consisted of FBSEs with benign findings; cases of cutaneous metastases and leukemia cutis managed by oncology; as well as select cases of purpura fulminans, postfebrile desquamation, and postinflammatory hyperpigmentation.
Changes in management included alterations in medications, requests for additional laboratory work or imaging, additional consultation requests, biopsies, or specific wound care instructions. Seventy-five percent of all consultations were given specific medication recommendations by dermatology. Most (61%) were recommended to be given a topical steroid, antibiotic, or both. However, 45% of all consultations were recommended to initiate a systemic medication, most commonly antihistamines, antibiotics, steroids, antivirals, or immunomodulators. Dermatology recommended discontinuing specific medications in 16% of all consultations, with antibiotics being the most frequent culprit (17 antibiotics discontinued), owing to drug eruptions or misdiagnosed infections. Vancomycin, piperacillin-tazobactam, and trimethoprim-sulfamethoxazole were the most frequently discontinued antibiotics.
Dermatology was consulted for assistance in management of previously diagnosed cutaneous conditions 56 times (22% of all consultations), often regarding complicated cases of hidradenitis suppurativa (9 cases), pyoderma gangrenosum (5 cases), bullous pemphigoid (4 cases), or erythroderma (4 cases). Most of these cases required a single dermatology encounter to provide recommendations (71%), and 21% required 1 additional follow-up. Sixty-three percent of patients consulted for management assistance were noted to have improvement in their cutaneous condition by time of discharge, as documented by the primary provider in the medical record.
Twenty-eight percent of all consultations required at least 1 biopsy. Seventy-two percent of all biopsies were consistent with the dermatologist’s working diagnosis or highest-ranked differential diagnosis, and 16% of biopsy results were consistent with the second- or third-ranked diagnosis. The primary teams requested a biopsy 38 times to assist in diagnosis, as documented in the progress note or consultation request. Only 21 of these consultations (55% of requests) received at least 1 biopsy, as the remaining consultations did not require a biopsy to establish a diagnosis. The most common final diagnoses of consultations receiving biopsies included drug eruptions (5), leukemia cutis (4), vasculopathies (4), vasculitis (4), and calciphylaxis (3).
Impact on Hospitalization and Efficacy—Dermatology performed 217 consultations regarding patients already admitted to the hospital, and 92% remained hospitalized either due to comorbidities or complicated cutaneous conditions following the consultation. The remaining 8% were cleared for discharge. Dermatology received 36 consultation requests from emergency medicine physicians. Fifty-three percent of these patients were admitted, while the remaining 47% were discharged from the emergency department or its observation unit following evaluation.
Fifty-one percent of all consultations were noted to have improvement in their cutaneous condition by the time of discharge, as noted in the physical examination, progress note, or discharge summary of the primary team. Thirty percent of cases remained stable, where improvement was not noted in in the medical record. Most of these cases involved keratinocyte carcinomas scheduled for outpatient excision, benign melanocytic nevi found on FBSE, and benign etiologies that led to immediate discharge following consultation. Three percent of all consultations were noted to have worsened following consultation, including cases of calciphylaxis, vasculopathies, and purpura fulminans, as well as patients who elected for palliative care and hospice. The cutaneous condition by the time of discharge could not be determined from the medical record in 16% of all consultations.
Eighty-five percent of all consultations required a single encounter with dermatology. An additional 10% required a single follow-up with dermatology, while only 5% of patients required 3 or more encounters. Notably, these cases included patients with 1 or more severe cutaneous diseases, such as Sweet syndrome, calciphylaxis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and hidradenitis suppurativa.
Comment
Although dermatology often is viewed as an outpatient specialty, this study provides a glimpse into the ways inpatient dermatology consultations optimize the care of hospitalized patients. Most consultations involved assistance in diagnosing an unknown condition, but several regarded pre-existing skin disorders requiring management aid. As a variety of medical specialties requested consultations, dermatology was able to provide care to a diverse group of patients with conditions varying in complexity and severity. Several specialties benefited from niche dermatologic expertise: hematology and oncology frequently requested dermatology to assist in diagnosis and management of the toxic effects of chemotherapy, cutaneous metastasis, or suspected cutaneous infections in immunocompromised patients. Cardiology patients were frequently evaluated for potential malignancy or infection prior to heart transplantation and initiation of antirejection immunosuppressants. Dermatology was consulted to differentiate cutaneous manifestations of critical illness from underlying systemic disease in the intensive care unit, and patients presenting to the emergency department often were examined to determine if hospital admission was necessary, with 47% of these consultations resulting in a discharge following evaluation by a dermatologist.
Our results were consistent with prior studies1,5,6 that have reported frequent changes in final diagnosis following dermatology consultation, with 69% of working diagnoses changed in this study when consultation was requested for diagnostic assistance. When dermatology was consulted for diagnostic assistance, several of these cases lacked a preliminary differential diagnosis. Although the absence of a documented differential diagnosis may not necessarily reflect a lack of suspicion for a particular etiology, 86% of all consultations included a ranked differential or working diagnosis either in the consultation request or progress note prior to consultation. The final diagnoses of consultations without a preliminary diagnosis varied from the mild and localized to systemic and severe, further suggesting these cases reflected knowledge gaps of the primary medical team.
Integration of dermatology into the care of hospitalized patients could provide an opportunity for education of primary medical teams. With frequent consultation, primary medical teams may become more comfortable diagnosing and managing common cutaneous conditions specific to their specialty or extended hospitalizations.
Several consultations were requested to aid in management of cases of hidradenitis suppurativa, pyoderma gangrenosum, or bullous pemphigoid that either failed outpatient therapy or were complicated by superinfections. Despite the ranges in complexity, the majority of all consultations required a single encounter and led to improvement by the time of discharge, demonstrating the efficacy and efficiency of inpatient dermatologists.
Dermatology consultations often led to changes in management involving medications and additional workup. Changes in management also extended to specific wound care instructions provided by dermatology, as expected for cases of Stevens-Johnson syndrome/toxic epidermal necrolysis, Sweet syndrome, hidradenitis suppurativa, and pyoderma gangrenosum. However, patients with the sequelae of extended hospitalizations, such as chronic wounds, pressure ulcers, and edema bullae, also benefited from this expertise.
When patients required a biopsy, the final diagnoses were consistent with the dermatologist’s number one differential diagnosis or top 3 differential diagnoses 72% and 88% of the time, respectively. Only 55% of cases where the primary team requested a biopsy ultimately required a biopsy, as many involved clinical diagnoses such as urticaria. Not only was dermatology accurate in their preliminary diagnoses, but they decreased cost and morbidity by avoiding unnecessary procedures.
This study provided additional evidence to support the integration of dermatology into the hospital setting for the benefit of patients, primary medical teams, and hospital systems. Dermatology offers high-value care through the efficient diagnosis and management of hospitalized patients, which contributes to decreased cost and improved outcomes.2,5-7,9,10 This study highlighted lesser-known areas of impact, such as the various specialty-specific services dermatology provides as well as the high rates of reported improvement following consultation. Future studies should continue to explore the field’s unique impact on hospitalized medicine as well as other avenues of care delivery, such as telemedicine, that may encourage dermatologists to participate in consultations and increase the volume of patients who may benefit from their care.
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
- Madigan LM, Fox LP. Where are we now with inpatient consultative dermatology?: assessing the value and evolution of this subspecialty over the past decade. J Am Acad Dermatol. 2019;80:1804-1808. doi:10.1016/j.jaad.2019.01.031
- Noe MH, Rosenbach M. Inpatient dermatologists—crucial for the management of skin diseases in hospitalized patients [editorial]. JAMA Dermatol. 2018;154:524-525. doi:10.1001/jamadermatol.2017.6195
- Strowd LC. Inpatient dermatology: a paradigm shift in the management of skin disease in the hospital. Br J Dermatol. 2019;180:966-967. doi:10.1111/bjd.17778
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757. doi:10.1016/s0190-9622(99)70158-1
- Kroshinsky D, Cotliar J, Hughey LC, et al. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152:477-480. doi:10.1001/jamadermatol.2015.5098
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis. JAMA Dermatol. 2018;154:529-533. doi:10.1001/jamadermatol.2017.6196
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528. doi:10.1001/jamadermatol.2016.6130
- Imadojemu S, Rosenbach M. Dermatologists must take an active role in the diagnosis of cellulitis. JAMA Dermatol. 2017;153:134-135. doi:10.1001/jamadermatol.2016.4230
- Hughey LC. The impact dermatologists can have on misdiagnosis of cellulitis and overuse of antibiotics: closing the gap. JAMA Dermatol. 2014;150:1061-1062. doi:10.1001/jamadermatol.2014.1164
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558. doi:10.1111/ijd.13939
Practice Points
- Inpatient dermatologists fill knowledge gaps that often alter the diagnosis, management, and hospital course of hospitalized patients.
- Several medical specialties benefit from niche expertise of inpatient dermatologists specific to their patient population.
- Integration of inpatient dermatology consultations can prevent unnecessary hospital admissions and medication administration.
Cutaneous Cold Weather Injuries in the US Military
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.
Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.
Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.

Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.

Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
The US Department of Defense maintains a presence in several cold weather environments such as North Dakota, Alaska, and South Korea. Although much is known about preventing and caring for cold weather injuries, many of these ailments continue to occur. Therefore, it is vital that both military and civilian physicians who care for patients who are exposed to cold weather conditions have a thorough understanding of the prevention, clinical presentation, and treatment of cold weather injuries.
Although the focus of this article is on cutaneous cold weather injuries that occur in military service, these types of injuries are not limited to this population. Civilians who live, work, or seek recreation in cold climates also may experience these injuries. Classically, cold injuries are classified as freezing and nonfreezing injuries. For the purpose of this article, we also consider a third category: dermatologic conditions that flare upon cold exposure. Specifically, we discuss frostbite, cold-weather immersion foot, pernio, Raynaud phenomenon (RP), and cold urticaria. We also present a case of pernio in an active-duty military service member.
Frostbite
For centuries, frostbite has been well documented as a cold weather injury in military history.1 Napoleon’s catastrophic invasion of Russia in 1812 started with 612,000 troops and ended with fewer than 10,000 effective soldiers; while many factors contributed to this attrition, exposure to cold weather and frostbite is thought to have been a major factor. The muddy trench warfare of World War I was no kinder to the poorly equipped soldiers across the European theater. Decades later during World War II, frostbite was a serious source of noncombat injuries, as battles were fought in frigid European winters. From 1942 to 1945, there were 13,196 reported cases of frostbite in the European theater, with most of these injuries occurring in 1945.1
Despite advancements in cold weather clothing and increased knowledge about the causes of and preventative measures for frostbite, cold weather injuries continue to be a relevant topic in today’s military. From 2015 to 2020, there were 1120 reported cases of frostbite in the US military.2 When skin is exposed to cold temperatures, the body peripherally vasoconstricts to reduce core heat loss. This autoregulatory vasoconstriction is part of a normal physiologic response that preserves the core body temperature, often at the expense of the extremities; for instance, the hands and feet are equipped with arteriovenous shunts, known as glomus bodies, which consist of vascular smooth muscle centers that control the flow of blood in response to changing external temperatures.3 This is partially mitigated by cold-induced vasodilation of the digits, also known as the Hunting reaction, which generally occurs 5 to 10 minutes after the start of local cold exposure.4 Additionally, discomfort from cold exposure warrants behavioral modifications such as going indoors, putting on warmer clothing, or building a fire. If an individual is unable to seek shelter in the face of cold exposure, the cold will inevitably cause injury.
Frostbite is caused by both direct and indirect cellular injury. Direct injury results from the crystallization of intracellular and interstitial fluids, cellular dehydration, and electrolyte disturbances. Indirect cellular injury is the result of a progressive microvascular insult and is caused by microvascular thrombosis, endothelial damage, intravascular sludging, inflammatory mediators, free radicals, and reperfusion injury.5
Frostnip is a more superficial injury that does not involve freezing of the skin or underlying tissue and typically does not leave any long-term damage. As severity of injury increases, frostbite is characterized by the depth of injury, presence of tissue loss, and radiotracer uptake on bone scan. There are 2 main classification systems for frostbite: one is based on the severity of the injury outcome, categorized by 4 degrees (1–4), and the other is designed as a predictive model, categorized by 4 grades (1–4).6 The first classification system is similar to the system for the severity of burns and ranges from partial-thickness injury (first degree) to full-thickness skin, subcutaneous tissue, muscle, tendon, and bone (fourth degree). The latter classification system uses the presence and characteristics of blisters after rewarming on days 0 and 2 and radiotracer uptake on bone scan on day 2. Severity ranges from no blistering, no indicated bone scan, and no long-term sequelae in grade 1 to hemorrhagic blisters overlying the carpal or tarsal bones and absence of radiotracer uptake with predicted extensive amputation, risk for thrombosis or sepsis, and long-term functional sequelae in grade 4.6
Male sex and African descent are associated with increased risk for sustaining frostbite. The ethnic predisposition may be explained by a less robust Hunting reaction in individuals of African descent.4,7 Other risk factors include alcohol use, smoking, homelessness, history of cold-related injury, use of beta-blockers, and working with equipment that uses nitrogen dioxide or CO2.5 Additionally, a history of systemic lupus erythematosus has been reported as a risk factor for frostbite.8
Clinically, frostbite initially may appear pale, blue, or erythematous, and patients may report skin numbness. In severe cases, necrosis can be seen.9 The most commonly affected anatomic locations include the fingers, toes, ears, and nose. Prevention is key for frostbite injuries. Steps to avoid injury include wearing appropriate clothing, minimizing the duration of time the skin is exposed to cold temperatures, avoiding alcohol consumption, and avoiding physical exhaustion in cold weather. These steps can help mitigate the effects of wind chill and low temperatures and decrease the risk of frostbite.10
Management of this condition includes prevention, early diagnosis, prehospital management, hospital management, and long-term sequelae management. Leadership and medical personnel for military units assigned to cold climates should be vigilant in looking for symptoms of frostbite. If any one individual is found to have frostbite or any other cold injury, all other team members should be evaluated.5
After identification of frostbite, seeking shelter and evacuation to a treatment facility are vital next steps. Constrictive clothing or jewelry should be removed. Depending on the situation, rewarming can be attempted in the prehospital setting, but it is imperative to avoid refreezing, as this may further damage the affected tissue due to intracellular ice formation with extensive cell destruction.6 Gentle warming can be attempted by placing the affected extremity in another person’s armpit or groin for up to 10 minutes or by immersing the affected limb in water that is 37° C to 39° C (98.6° F to 102.2° F). Rubbing the affected area and dry heat should be avoided. It should be noted that the decision to thaw in the field introduces the challenge of dealing with the severe pain associated with thawing in a remote or hostile environment. Ibuprofen (400 mg) can be given as an anti-inflammatory and analgesic agent in the prehospital setting.5 Once safely evacuated to the hospital, treatment options expand dramatically, including warming without concern of refreezing, wound care, thrombolytic therapy, and surgical intervention. If local frostbite expertise is not available, there are telemedicine services available.5,6
Frostbite outcomes range from complete recovery to amputation. Previously frostbitten tissue has increased cold sensitivity and is more susceptible to similar injury in the future. Additionally, there can be functional loss, chronic pain, chronic ulceration, and arthritis.5,6 As such, a history of frostbite can be disqualifying for military service and requires a medical waiver.11 If a service member experiences frostbite and does not have any residual effects, they can expect to continue their military service, but if there are sequelae, it may prove to be career limiting.12-14
Immersion Foot
Although frostbite represents a freezing injury, immersion foot (or trench foot) represents a nonfreezing cold injury. It should be noted that in addition to immersion foot associated with cold water exposure, there also are warm-water and tropical variants. For the purpose of this article, we are referring to immersion foot associated with exposure to cold water. Trench foot was described for the first time during Napoleon’s invasion of Russia in 1812 but came to prominence during World War I, where it is thought to have contributed to the deaths of 75,000 British soldiers. During World War II, there were 25,016 cases of immersion foot reported in the US military.1 More recently, 590 cases of immersion foot were reported in the US military from 2015 to 2020.2
Classically, this condition was seen in individuals whose feet were immersed in cold but not freezing water or mud in trenches or on boats, hence the terms immersion foot and trench foot. The pathogenesis is thought to be related to overhydration of the stratum corneum and repetitive cycles of cold-induced, thermoprotective vasoconstriction, leading to cyclical hypoxic and reperfusion injuries, which eventually damage nerves, muscle, subcutaneous fat, and blood vessels.9,15
A recent case series of 100 military service members in the United Kingdom showed that cold-induced extremity numbness for more than 30 minutes and painful rewarming after cold exposure were highly correlated with the development of immersion foot. Additionally, this case series showed that patients with repeated cycles of cooling and rewarming were more likely to have long-term symptoms.16 As with frostbite, prior cold injury and African descent increases the risk for developing immersion foot, possibly due to a less-pronounced Hunting reaction.4,7
Early reports suggested prehyperemic, hyperemic, and posthyperemic stages. The prehyperemic stage lasts from hours to days and is characterized by cold extremities, discoloration, edema, stocking- or glove-distributed anesthesia, blisters, necrosis, and potential loss of palpable pulses.17 Of note, in Kuht et al’s16 more recent case series, edema was not seen as frequently as in prior reports. The hyperemic stage can last for 6 to 10 weeks and is characterized by vascular disturbances. In addition, the affected extremity typically remains warm and red even when exposed to cold temperatures. Sensory disturbances such as paresthesia and hyperalgesia may be seen, as well as motor disturbances, anhidrosis, blisters, ulcers, and gangrene. The posthyperemic stage can last from months to years and is characterized by cold sensitivity, possible digital blanching, edema, hyperhidrosis, and persistent peripheral neuropathy.16
Prevention is the most important treatment for immersion foot. The first step in preventing this injury is avoiding prolonged cold exposure. When this is not possible due to the demands of training or actual combat conditions, regular hand and foot inspections, frequent sock changes, and regularly rotating out of cold wet conditions can help prevent this injury.15 Vasodilators also have been considered as a possible treatment modality. Iloprost and nicotinyl alcohol tartrate showed some improvement, while aminophylline and papaverine were ineffective.15
As with frostbite, a history of immersion foot may be disqualifying for military service.11 If it occurs during military service and there are no residual effects that limit the service member’s capabilities, they may expect to continue their career; however, if there are residual effects that limit activity or deployment, medical retirement may be indicated.
Pernio
Pernio is another important condition that is related to cold exposure; however, unlike the previous 2 conditions, it is not necessarily caused by cold exposure but rather flares with cold exposure.

Case Presentation—A 39-year-old active-duty male service member presented to the dermatology clinic for intermittent painful blistering on the toes of both feet lasting approximately 10 to 14 days about 3 to 4 times per year for the last several years. The patient reported that his symptoms started after spending 2 days in the snow with wet nonwinterized boots while stationed in Germany 10 years prior. He reported cold weather as his only associated trigger and denied other associated symptoms. Physical examination revealed mildly cyanotic toes containing scattered bullae, with the dorsal lesions appearing more superficial compared to the deeper plantar bullae (Figure 1). A complete blood cell count, serum protein electrophoresis, and antinuclear and autoimmune antibodies were within reference range. A punch biopsy was obtained from a lesion on the right dorsal great toe. Hematoxylin and eosin–stained sections revealed lichenoid and vacuolar dermatitis with scattered dyskeratosis and subtle papillary edema (Figure 2). Minimal interstitial mucin was seen on Alcian blue–stained sections. The histologic and clinical findings were most compatible with a diagnosis of chronic pernio. Nifedipine 20 mg once daily was initiated, and he had minimal improvement after a few months of treatment. His condition continued to limit his functionality in cold conditions due to pain. Without improvement of the symptoms, the patient likely will require medical separation from military service, as this condition limits the performance of his duties and his deployability.

Clinical Discussion—Pernio, also known as chilblains, is characterized by cold-induced erythematous patches and plaques, pain, and pruritus on the affected skin.18 Bullae and ulceration can be seen in more severe and chronic cases.19 Pernio most commonly is seen in young women but also can be seen in children, men, and older adults. It usually occurs on the tips of toes but also may affect the fingers, nose, and ears. It typically is observed in cold and damp conditions and is thought to be caused by an inflammatory response to vasospasms in the setting of nonfreezing cold. Acute pernio typically resolves after a few weeks; however, it also can persist in a chronic form after repeated cold exposure.18
Predisposing factors include excessive cold exposure, connective tissue disease, hematologic malignancy, antiphospholipid antibodies in adults, and anorexia nervosa in children.18,20,21 More recently, perniolike lesions have been associated with prior SARS-CoV-2 infection.22 Histologically, pernio is characterized by a perivascular lymphocytic infiltrate and dermal edema.23 Cold avoidance, warming, drying, and smoking cessation are primary treatments, while vasodilating medications such as nifedipine have been used with success in more resistant cases.20,24
Although the prognosis generally is excellent, this condition also can be career limiting for military service members. If it resolves with no residual effects, patients can expect to continue their service; however, if it persists and limits their activity or ability to deploy, a medical retirement may be indicated.11-14
Raynaud Phenomenon
Raynaud phenomenon (also known as Raynaud’s) is characterized by cold-induced extremity triphasic color changes—initial blanching and pallor that transitions to cyanosis and finally erythema with associated pain during the recovery stage. The fingers are the most commonly involved appendages and can have a symmetric distribution, but RP also has been observed on the feet, lips, nose, and ears. In severe cases, it can cause ulceration.25 The prevalence of RP may be as high as 5% in the general population.26 It more commonly is primary or idiopathic with no underlying cause or secondary with an associated underlying systemic disease.
Cold-induced vasoconstriction is a normal physiologic response, but in RP, the response becomes a vasospasm and is pathological. Autoimmune and connective tissue diseases often are associated with secondary RP. Other risk factors include female sex, smoking, family history in a first-degree relative, and certain medications.25 A study in northern Sweden also identified a history of frostbite as a risk factor for the development of RP.27 This condition can notably restrict mobility and deployability of affected service members as well as the types of manual tasks that they may be required to perform. As such, this condition can be disqualifying for military service.11
Many patients improve with conservative treatment consisting of cold avoidance, smoking cessation, and avoidance of medications that worsen the vasospasm; however, some patients develop pain and chronic disease, which can become so severe and ischemic that digital loss is threatened.25 When needed, calcium channel blockers commonly are used for treatment and can be used prophylactically to reduce flare rates and severity of disease. If this class of medications is ineffective or is not tolerated, there are other medications and treatments to consider, which are beyond the scope of this article.25
Cold Urticaria
Cold urticaria is a subset of physical urticaria in which symptoms occur in response to a cutaneous cold stimulus. It can be primary or secondary, with potential underlying causes including cryoglobulinemia, infections, and some medications. Systemic involvement is possible with extensive cold contact and can include severe anaphylaxis. This condition is diagnosed using a cold stimulation test. Cold exposure avoidance and second-generation antihistamines are considered first-line treatment. Because anaphylaxis is possible, patients should be given an epinephrine pen and should be instructed to avoid swimming in cold water.28 Cold urticaria is disqualifying for military service.11
A 2013 case report described a 29-year-old woman on active duty in the US Air Force whose presenting symptoms included urticaria on the exposed skin on the arms when doing physical training in the rain.29 In this case, secondary causes were eliminated, and she was diagnosed with primary acquired cold urticaria. This patient was eventually medically discharged from the air force because management with antihistamines failed, and her symptoms limited her ability to function in even mildly cold environments.29
Final Thoughts
An understanding of cold weather injuries and other dermatologic conditions that may be flared by cold exposure is important for a medically ready military force, as there are implications for accession, training, and combat operations. Although the focus of this article has been on the military, these conditions also are seen in civilian medicine in patient populations routinely exposed to cold weather. This becomes especially pertinent in high-risk patients such as extreme athletes, homeless individuals, or those who have other predisposing characteristics such as chronic alcohol use. Appropriate cold weather gear, training, and deliberate mission or activity planning are important interventions in preventing cutaneous cold weather injuries within the military.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
- Patton BC. Cold, casualties, and conquests: the effects of cold on warfare. In: Pandolf KB, Burr RE, eds. Medical Aspects of HarshEnvironments. Office of the Surgeon General, United States Army; 2001:313-349.
- Update: cold weather injuries, active and reserve components, U.S. Armed Forces, July 2015–June 2020. Military Health System website. Published November 1, 2020. Accessed September 15, 2021. https://www.health.mil/News/Articles/2020/11/01/Update-Cold-Weather-Injuries-MSMR-2020
- Lee W, Kwon SB, Cho SH, et al. Glomus tumor of the hand. Arch Plast Surg. 2015;42:295-301.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89:411-426.
- Handford C, Thomas O, Imray CHE. Frostbite. Emerg Med Clin North Am. 2017;35:281-299.
- Grieve AW, Davis P, Dhillon S, et al. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73-78.
- Maley MJ, Eglin CM, House JR, et al. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol. 2014;114:2369-2379.
- Wong NWK, NG Vt-Y, Ibrahim S, et al. Lupus—the cold, hard facts. Lupus. 2014;23:837-839.
- Smith ML. Environmental and sports related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, et al, eds. Dermatology. 4th ed. Elsevier; 2018:1574-1579.
- Rintamäki H. Predisposing factors and prevention of frostbite. Int J Circumpolar Health. 2000;59:114-121.
- Medical Standards for Appointment, Enlistment, or Induction into the Military Services (DOD Instructions 6130.03). Washington, DC: US Department of Defense; 2018. Updated April 30, 2021. Accessed September 15, 2021. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003v1p.pdf?ver=aNVBgIeuKy0Gbrm-foyDSA%3D%3D
- Medical Examinations. In: Manual of the Medical Department (MANMED), NAVMED P-117. US Navy; 2019:15-40–15-46. Updated October 20, 2020. Accessed September 27, 2021. https://www.med.navy.mil/Portals/62/Documents/BUMED/Directives/MANMED/Chapter%2015%20Medical%20Examinations%20(incorporates%20Changes%20126_135-138_140_145_150-152_154-156_160_164-167).pdf?ver=Rj7AoH54dNAX5uS3F1JUfw%3d%3d
- United States Air Force. Medical standards directory. Approved May 13, 2020. Accessed September 16, 2021. https://afspecialwarfare.com/files/MSD%20May%202020%20FINAL%2013%20MAY%202020.pdf
- Department of the Army. Standards of medical fitness. AR 40-501. Revised June 27, 2019. Accessed September 16, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN8673_AR40_501_FINAL_WEB.pdf
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: a disease of the past in the present. Clin Exp Dermatol. 2020;45:10-14.
- Kuht JA, Woods D, Hollis S. Case series of non-freezing cold injury: epidemiology and risk factors. J R Army Med Corps. 2019;165:400-404.
- Ungley CC, Blackwood W. Peripheral vasoneuropathy after chilling. Lancet. 1942;2:447-451.
- Simon TD, Soap JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:E472-E475.
- Spittel Jr JA, Spittell PC. Chronic pernio: another cause of blue toes. Int Angiol. 1992;11:46-50.
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207-215.
- White KP, Rothe MJ, Milanese A, et al. Perniosis in association with anorexia nervosa. Pediatr Dermatol. 1994;11:1-5.
- Freeman EE, McMahon DE, Lipoff JB; American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486-492.
- Cribier B, Djeridi N, Peltre B, et al. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol. 2001;45:924-929.
- Rustin MH, Newton JA, Smith NP, et al. The treatment of chilblains with nifedipine: the results of a pilot study, a double-blind placebo-controlled randomized study and a long-term open trial. Br J Dermatol.1989;120:267-275.
- Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517-525.
- Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud’s phenomenon: systematic review and meta-analysis of observational studies. BMJ Open. 2015;5:E006389.
- Stjerbrant A, Pettersson H, Liljelind I, et al. Raynaud’s phenomenon in Northern Sweden: a population-based nested case-control study. Rheumatol Int. 2019;39:265-275.
- Singleton R, Halverstam CP. Diagnosis and management of cold urticaria. Cutis. 2016;97:59-62.
- Barnes M, Linthicum C, Hardin C. Cold, red, itching, and miserable. Mil Med. 2013;178:E1043-E1044.
Practice Points
- Military service members are at an increased risk for cutaneous cold weather injuries in certain circumstances due to the demands of military training and combat operations.
- Cold weather may cause injury by directly damaging tissues, leading to neurovascular disruption, and by exacerbating existing medical conditions.
Treatment Stacking: Optimizing Therapeutic Regimens for Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).
Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).

Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
Hidradenitis suppurativa (HS) is a debilitating chronic condition that often is recalcitrant to first-line treatments, and mechanisms underlying its pathology remain unclear. Existing data suggest a multifactorial etiology with different pathophysiologic contributors, including genetic, hormonal, and immune dysregulation factors. At this time, only one medication (adalimumab) is US Food and Drug Administration approved for HS, but multiple medical and procedural therapies are available.1 Herein, we discuss the concept of treatment stacking, or the combination of unique therapeutic modalities—an approach we believe is key to optimizing management of HS patients.
Stacking Treatments for HS
Unlike psoriasis, in which a single biologic agent may provide 100% clearance (psoriasis area and severity index 100 [PASI 100]) without adjuvant treatment,2,3 the field of HS currently lacks medications that are efficacious to that degree of success as monotherapy. In HS, the benchmark for a positive treatment outcome is Hidradenitis Suppurativa Clinical Response 50 (HiSCR50),4 a 50% reduction in inflammatory lesion count—a far less stringent marker for disease improvement. Thus, providers should design HS treatment regimens with a model of combining therapies and shift away from monotherapy. Targeting different pathophysiologic pathways by stacking multiple treatments may provide synergistic benefits for HS patients. Treatment stacking is a familiar concept in acne; for instance, patients who benefit tremendously from isotretinoin may still require a hormone-modulating treatment (eg, spironolactone) to attain optimal results.
Adherence to a rigid treatment algorithm based on disease severity limits the potential to create comprehensive regimens that account for unique patient characteristics and clinical manifestations. When evaluating an HS patient, providers should systematically consider each pathophysiologic factor and target the ones that appear to be most involved in that particular patient. The North American HS guidelines illustrate this point by supporting use of several treatments across different Hurley stages, such as recommending hormonal treatment in patients with Hurley stages 1, 2, or 3.1 Of note, treatment stacking also includes procedural therapies. Surgeons typically prefer a patient’s disease management to be optimized prior to surgery, including reduced drainage and inflammation. In addition, even after surgery, patients often still require medical management to prevent continued disease worsening.
Treatment Pathways for HS
A multimodal approach with treatment stacking (Figure) can be useful to all HS patients, from those with the mildest to the most severe disease. Modifiable pathophysiologic factors and examples of their targeted treatments include (1) follicular occlusion (eg, oral retinoids), (2) metabolic dysfunction (eg, metformin), (3) hormones (eg, oral contraceptive pills, spironolactone, finasteride), (4) dysbiosis (eg, antibiotics such as clindamycin and rifampin combination therapy), (5) immune dysregulation (eg, biologic agents), and (6) friction/irritation (eg, weight loss, clothing recommendations).

Combining treatments from different pathways enables potentiation of individual treatment efficacies. A female patient with only a few HS nodules that flare with menses may be well controlled with spironolactone as her only systemic agent; however, she still may benefit from use of an antiseptic wash, topical clindamycin, and lifestyle changes such as weight loss and reduction of mechanical irritation. A patient with severe recalcitrant HS could notably benefit from concomitant biologic, systemic antibiotic, and hormonal/metabolic treatments. If disease control is still inadequate, agents within the same class can be switched (eg, choosing a different biologic) or other disease-modifying agents such as colchicine also can be added. The goal is to create an effective treatment toolbox with therapies targeting different pathophysiologic arms of HS and working together in synergy. Each tool can be refined by modifying dosing frequency and duration of use to strive for optimal response. At this time, the literature on HS combination therapy is sparse. A retrospective study of 31 patients reported promising combinations, including isotretinoin with spironolactone for mild disease, isotretinoin or doxycycline with adalimumab for moderate disease, and cyclosporine with adalimumab for severe disease.5 Larger prospective studies on clinical response to different combination regimens are warranted.
Optimizing Therapy for HS and Its Comorbidities
Additional considerations may further optimize treatment plans. Some therapies benefit all patients; for example, providers should counsel all HS patients on healthy weight management, optimized clothing choices,6 and friction reduction in the intertriginous folds. Providers also may consider adding therapies with faster onset of efficacy as a bridge to long-term, slower-onset therapies. For instance, female HS patients with menstrual flares who are prescribed spironolactone also may benefit from a course of systemic antibiotics, which typically provides more prompt relief. Treatment regimens also can concomitantly treat HS and its comorbidities.7 For example, metformin serves a dual purpose in HS patients with diabetes mellitus, and adalimumab in patients with both HS and inflammatory bowel disease.
Final Thoughts
The last decade has seen tremendous growth in HS research8 coupled with a remarkable expansion in the therapeutic pipeline.9 However, currently no single therapy for HS can guarantee satisfactory disease remission or durability of remission. The contrast between clinical trials and real-world practice should be acknowledged; the former often is restrictive in design with monotherapy and allowance of very limited concomitant treatments, such as topical or oral antibiotics. This limits our ability to draw conclusions regarding the additive synergistic potential of different therapeutics in combination. In clinical practice, we are not restricted by monotherapy trial protocols. As we await new tools, treatment stacking allows for creating a framework to best utilize the tools that are available to us.
Although HS has continued to affect the lives of many patients, improved understanding of underlying pathophysiology and a well-placed sense of urgency from all stakeholders (ie, patients, clinicians, researchers, industry partners) has pushed this field forward. Until our therapeutic armamentarium has expanded to include highly efficacious monotherapy options, providers should consider treatment stacking for every HS patient.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101. doi:10.1016/j.jaad.2019.02.068
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Imafuku S, Nakagawa H, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab in Japanese patients with moderate to severe plaque psoriasis: results from a 5-year extension of a phase 3 study (reSURFACE 1). J Dermatol. 2021;48:844-852. doi:10.1111/1346-8138.15763
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- McPhie ML, Bridgman AC, Kirchhof MG. Combination therapies for hidradenitis suppurativa: a retrospective chart review of 31 patients. J Cutan Med Surg. 2019;23:270-276. doi:10.1177/1203475418823529
- Loh TY, Hendricks AJ, Hsiao JL, et al. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatol Basel Switz. 2021;237:119-124. doi:10.1159/000501611
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.059
- Savage KT, Brant EG, Flood KS, et al. Publication trends in hidradenitis suppurativa from 2008 to 2018. J Eur Acad Dermatol Venereol. 2020;34:1885-1889. doi:10.1111/jdv.16213
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183:E178-E187. doi:10.1111/bjd.16768