User login
Puzzles
Doctors love puzzles, they say. Especially neurologists.
The detective work on a case is part of the job’s appeal. Taking clues from the history, exam, and tests to formulate a diagnosis, then a treatment plan.
But I’m not talking about that.
As I’ve written before, I’ve tried hard to divorce myself from the news. In times where the world seems to have gone mad, I just don’t want to know what’s going on. I focus on my family, my job, and the weather forecast.
But, inevitably, I need something to do. At some point I run out of notes to type, tests to review, emails to answer, and bills to pay. I used to read the news, but now I don’t do that anymore. I even avoid my favorite satire sites, like Onion and Beaverton, because they just reflect the real news (I still read the Weekly World News, which has no relationship to reality, or pretty much anything, whatsoever).
So now, when I’m done with the day’s work, I shut down the computer (which isn’t easy after 25 years of habitual surfing) and sit down with a jigsaw puzzle. I haven’t done that since I was a resident.
It usually takes me 2-3 weeks to do one (500-1,000 pieces) in the 30 minutes or so I spend on it each evening. There’s solace in the quiet, methodical process of carefully looking for matching pieces, trying a few, the brief glee at getting a fit, and then moving to the next piece.
I know I can do this on my iPad, but it’s different with real pieces. Lifting up a piece and examining it for matching shapes and colors, sorting through the tray, wondering if I made a mistake somewhere. The cardboard doesn’t light up to let me know I got it right.
Inevitably, the mind wanders as I work on them. Sometimes back to a puzzle at the office, sometimes to my doing the same puzzle (I’ve had them for a while) at my parents’ house in my teens, sometimes to my kids away at college, or a book I once read.
But that’s the point. It’s almost a form of meditation. Focusing on each piece as my mind moves in other directions. It’s actually more relaxing than I thought, and a welcome escape from the day.
And, like other seemingly unrelated tasks (such as Leo Szilard waiting for a traffic light to change, albeit on a lesser scale), sometimes it brings me an answer I’ve been searching for. A light bulb will go on for a patient case I’ve been turning over for a few days. When that happens I grab my phone and email the thought to myself at work.
It puts my mind in neutral at the end of the day. When I finally go to bed I’m less focused on things that can keep me awake at night.
Though occasionally I do dream of puzzles.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Doctors love puzzles, they say. Especially neurologists.
The detective work on a case is part of the job’s appeal. Taking clues from the history, exam, and tests to formulate a diagnosis, then a treatment plan.
But I’m not talking about that.
As I’ve written before, I’ve tried hard to divorce myself from the news. In times where the world seems to have gone mad, I just don’t want to know what’s going on. I focus on my family, my job, and the weather forecast.
But, inevitably, I need something to do. At some point I run out of notes to type, tests to review, emails to answer, and bills to pay. I used to read the news, but now I don’t do that anymore. I even avoid my favorite satire sites, like Onion and Beaverton, because they just reflect the real news (I still read the Weekly World News, which has no relationship to reality, or pretty much anything, whatsoever).
So now, when I’m done with the day’s work, I shut down the computer (which isn’t easy after 25 years of habitual surfing) and sit down with a jigsaw puzzle. I haven’t done that since I was a resident.
It usually takes me 2-3 weeks to do one (500-1,000 pieces) in the 30 minutes or so I spend on it each evening. There’s solace in the quiet, methodical process of carefully looking for matching pieces, trying a few, the brief glee at getting a fit, and then moving to the next piece.
I know I can do this on my iPad, but it’s different with real pieces. Lifting up a piece and examining it for matching shapes and colors, sorting through the tray, wondering if I made a mistake somewhere. The cardboard doesn’t light up to let me know I got it right.
Inevitably, the mind wanders as I work on them. Sometimes back to a puzzle at the office, sometimes to my doing the same puzzle (I’ve had them for a while) at my parents’ house in my teens, sometimes to my kids away at college, or a book I once read.
But that’s the point. It’s almost a form of meditation. Focusing on each piece as my mind moves in other directions. It’s actually more relaxing than I thought, and a welcome escape from the day.
And, like other seemingly unrelated tasks (such as Leo Szilard waiting for a traffic light to change, albeit on a lesser scale), sometimes it brings me an answer I’ve been searching for. A light bulb will go on for a patient case I’ve been turning over for a few days. When that happens I grab my phone and email the thought to myself at work.
It puts my mind in neutral at the end of the day. When I finally go to bed I’m less focused on things that can keep me awake at night.
Though occasionally I do dream of puzzles.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Doctors love puzzles, they say. Especially neurologists.
The detective work on a case is part of the job’s appeal. Taking clues from the history, exam, and tests to formulate a diagnosis, then a treatment plan.
But I’m not talking about that.
As I’ve written before, I’ve tried hard to divorce myself from the news. In times where the world seems to have gone mad, I just don’t want to know what’s going on. I focus on my family, my job, and the weather forecast.
But, inevitably, I need something to do. At some point I run out of notes to type, tests to review, emails to answer, and bills to pay. I used to read the news, but now I don’t do that anymore. I even avoid my favorite satire sites, like Onion and Beaverton, because they just reflect the real news (I still read the Weekly World News, which has no relationship to reality, or pretty much anything, whatsoever).
So now, when I’m done with the day’s work, I shut down the computer (which isn’t easy after 25 years of habitual surfing) and sit down with a jigsaw puzzle. I haven’t done that since I was a resident.
It usually takes me 2-3 weeks to do one (500-1,000 pieces) in the 30 minutes or so I spend on it each evening. There’s solace in the quiet, methodical process of carefully looking for matching pieces, trying a few, the brief glee at getting a fit, and then moving to the next piece.
I know I can do this on my iPad, but it’s different with real pieces. Lifting up a piece and examining it for matching shapes and colors, sorting through the tray, wondering if I made a mistake somewhere. The cardboard doesn’t light up to let me know I got it right.
Inevitably, the mind wanders as I work on them. Sometimes back to a puzzle at the office, sometimes to my doing the same puzzle (I’ve had them for a while) at my parents’ house in my teens, sometimes to my kids away at college, or a book I once read.
But that’s the point. It’s almost a form of meditation. Focusing on each piece as my mind moves in other directions. It’s actually more relaxing than I thought, and a welcome escape from the day.
And, like other seemingly unrelated tasks (such as Leo Szilard waiting for a traffic light to change, albeit on a lesser scale), sometimes it brings me an answer I’ve been searching for. A light bulb will go on for a patient case I’ve been turning over for a few days. When that happens I grab my phone and email the thought to myself at work.
It puts my mind in neutral at the end of the day. When I finally go to bed I’m less focused on things that can keep me awake at night.
Though occasionally I do dream of puzzles.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Management of gastroparesis in 2022
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.
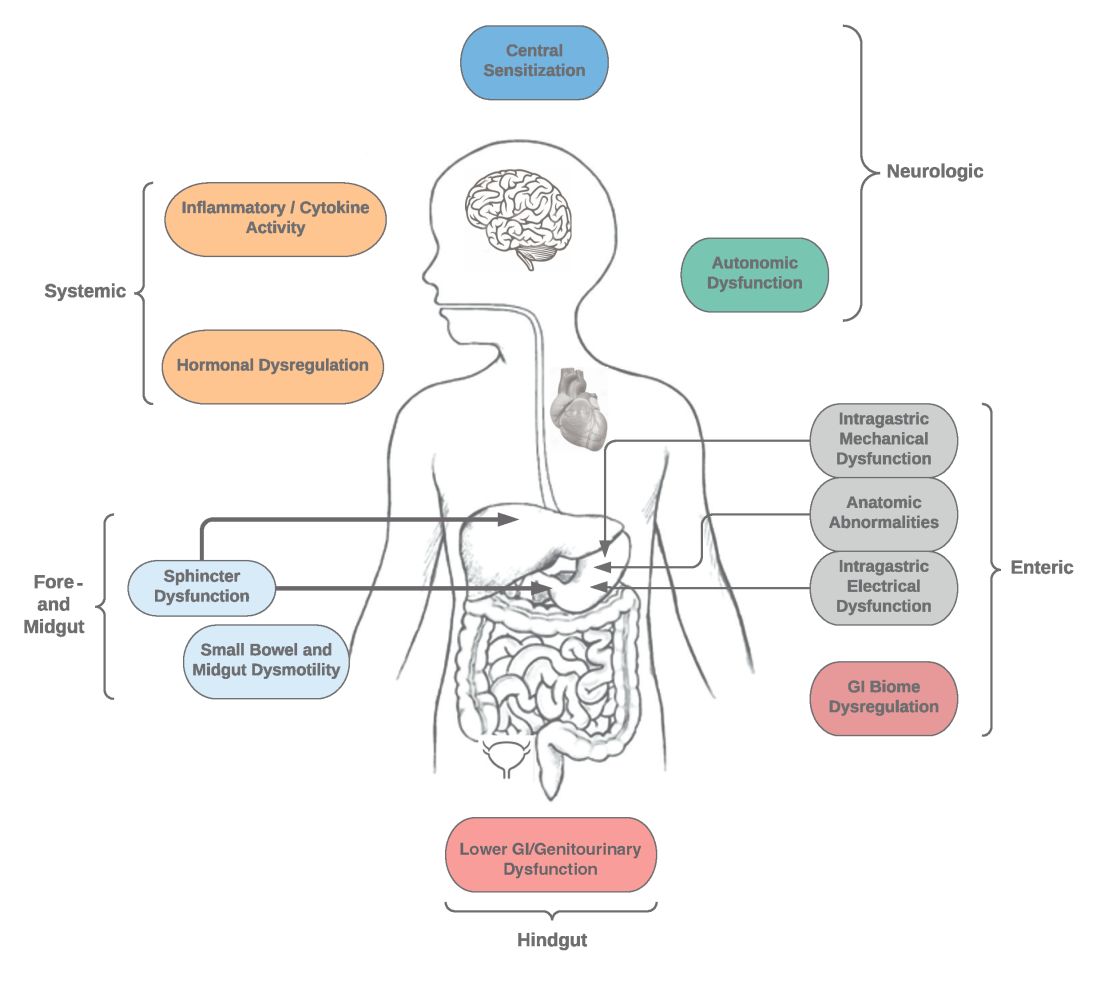
Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.
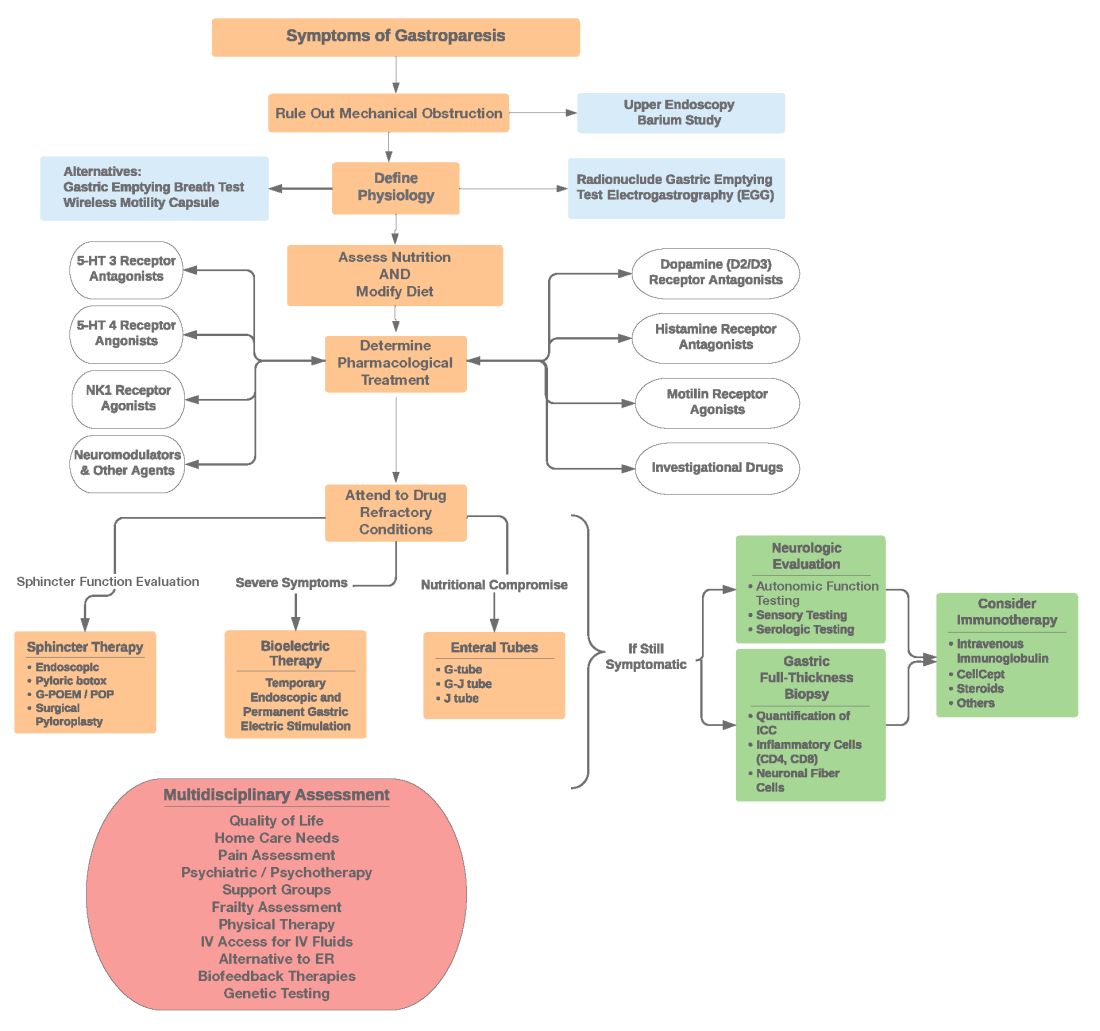
Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.

Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.

Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.

Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.

Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
How gender-affirming care is provided to adolescents in the United States
“Texas investigates parents of transgender teen.” “Court did not force dad to allow chemical castration of son.” Headlines such as these are becoming more common as transgender adolescents and young adults, as well as their families, continue to come under attack from state and local governments. In the 2021 state legislative sessions, more than 100 anti-trans bills were filed across 35 state legislatures. Texas alone saw 13 anti-trans bills, covering everything from sports participation to criminalization of best-practice medical care.1 Many of these bills are introduced under the guise of “protecting” these adolescents and young adults but are detrimental to their health. They also contain descriptions of gender-affirming care that do not reflect the evidence-based standards of care followed by clinicians across the country. Below is scientifically accurate information on gender-affirming care.
Gender identity development
Trajectories of gender identity are diverse. In a large sample of transgender adults (n = 27,715), 10% started to realize they were transgender at age 5 or younger, 16% between ages 6 and 10, 28% between 11 and 15, 29% between 16 and 20, and 18% at age 21 or older.2 In childhood, cross-gender play and preferences are a normal part of gender expression and many gender-nonconforming children will go on to identify with the sex they were assigned at birth (labeled cisgender). However, some children explicitly identify with a gender different than the sex they were assigned at birth (labeled transgender). Children who are consistent, insistent, and persistent in this identity appear likely to remain so into adolescence and adulthood. It is important to note that there is no evidence that discouraging gender nonconformity decreases the likelihood that a child will identify as transgender. In fact, this practice is no longer considered ethical, as it can have damaging effects on self-esteem and mental health. In addition, not all transgender people are noticeably gender nonconforming in childhood and that lack of childhood gender nonconformity does not invalidate someone’s transgender identity.
Gender-affirming care
For youth who identify as transgender, all steps in transition prior to puberty are social. This includes steps like changing hairstyles or clothing and using a different (affirmed) name and/or pronouns. This time period allows youth to explore their gender identity and expression. In one large study of 10,000 LGBTQ youth, among youth who reported “all or most people” used their affirmed pronoun, 12% reported a history of suicide attempt.3 In comparison, among those who reported that “no one” used their affirmed pronoun, the suicide attempt rate was 28%. Further, 14% of youth who reported that they were able to make changes in their clothing and appearance reported a past suicide attempt in comparison to 26% of those who were not able to. Many of these youth also are under the care of mental health professionals during this time.
At the onset of puberty, transgender youth are eligible for medical management, if needed, to address gender dysphoria (i.e., distress with one’s sex characteristics that is consistent and impairing). It is important to recognize that not all people who identify as transgender experience gender dysphoria or desire a medical transition. For those who do seek medical care, puberty must be confirmed either by breast/testicular exam or checking gonadotropin levels. Standards of care suggest that prior to pubertal suppression with GnRH agonists, such as leuprolide or histrelin, adolescents undergo a thorough psychosocial evaluation by a qualified, licensed clinician. After this evaluation, pubertal suppression may be initiated. These adolescents are monitored by their physicians every 3-6 months for side effects and continuing evaluation of their gender identity. GnRH agonists pause any further pubertal development while the adolescent continues to explore his/her/their gender identity. GnRH agonists are fully reversible and if they are stopped, the child’s natal puberty would recommence.
If an adolescent desires to start gender-affirming hormones, these are started as early as age 14, depending on their maturity, when they desire to start, and/or their ability to obtain parental consent. If a patient has not begun GnRH agonists and undergone a previous psychosocial evaluation, a thorough psychosocial evaluation by a qualified, licensed clinician would take place prior to initiating gender-affirming hormones. Prior to initiating hormones, a thorough informed-consent process occurs between the clinician, patient, and family. This process reviews reversible versus irreversible effects, as well of any side effects of the medication(s). Adolescents who begin hormonal treatment are then monitored every 3-6 months for medication side effects, efficacy, satisfaction with treatment, and by continued mental health assessments. Engagement in mental health therapy is not required beyond the initial evaluation (as many adolescents are well adjusted), but it is encouraged for support during the adolescent’s transition.4 It is important to note that the decision to begin hormones, or not, as well as how to adjust dosing over time, is nuanced and is individualized to each patient’s particular goals for his/her/their transition.
Care for transmasculine identified adolescents (those who were assigned female at birth) typically involves testosterone, delivered via subcutaneous injection, transdermal patch, or transdermal gel. Care for transfeminine individuals (those who were assigned male at birth) typically involves estradiol, delivered via daily pill, weekly or twice weekly transdermal patch, or intramuscular injection, as well as an androgen blocker. This is because estradiol by itself is a weak androgen inhibitor. Antiandrogen medication is delivered by daily oral spironolactone, daily oral bicalutamide (an androgen receptor blocker), or GnRH agonists similar to those used for puberty blockade.
Outcomes
At least 13 studies have documented an improvement in gender dysphoria and/or mental health for adolescents and young adults after beginning gender affirming medical care.5 A recent study by Turban et al. showed that access to gender affirming hormones during adolescence or early adulthood was associated with decreased odds of past month suicidal ideation than for those who did not have access to gender-affirming hormones.6 Tordoff et al. found that receipt of gender-affirming care, including medications, led to a 60% decrease in depressive symptoms and a 73% decrease in suicidality.7 One other question that often arises is whether youth who undergo medical treatment for their transition regret their transition or retransition back to the sex they were assigned at birth. In a large study at a gender clinic in the United Kingdom, they found a regret rate of only 0.47% (16 of 3,398 adolescents aged 13-20).8 This is similar to other studies that have also found low rates of regret. Regret is often due to lack of acceptance in society rather than lack of transgender identity.
The care of gender diverse youth takes place on a spectrum, including options that do not include medical treatment. By supporting youth where they are on their gender journey, there is a significant reduction in adverse mental health outcomes. Gender-affirming hormonal treatment is individualized and a thorough multidisciplinary evaluation and informed consent are obtained prior to initiation. There are careful, nuanced discussions with patients and their families to individualize care based on individual goals. By following established evidence-based standards of care, physicians can support their gender-diverse patients throughout their gender journey. Just like other medical treatments, procedures, or surgeries, gender-affirming care should be undertaken in the context of the sacred patient-physician relationship.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Equality Texas. Legislative Bill Tracker.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. 2016. Washington, DC: National Center for Transgender Equality.
3. The Trevor Project. 2020. National Survey on LGBTQ Mental Health.
4. Lopez X et al. Curr Opin Pediatrics. 2017;29(4):475-80.
5. Turban J. The evidence for trans youth gender-affirming medical care. Psychology Today. 2022 Jan 24.
6. Turban J et al. Access to gender-affirming hormones during adolescence and mental health outcomes among transgender adults. PLOS ONE. 2022;17(1).
7. Tordoff DM et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Network Open. 2022;5(2).
8. Davies S et al. Detransition rates in a national UK gender identity clinic. Inside Matters. On Law, Ethics, and Religion. 2019 Apr 11.
“Texas investigates parents of transgender teen.” “Court did not force dad to allow chemical castration of son.” Headlines such as these are becoming more common as transgender adolescents and young adults, as well as their families, continue to come under attack from state and local governments. In the 2021 state legislative sessions, more than 100 anti-trans bills were filed across 35 state legislatures. Texas alone saw 13 anti-trans bills, covering everything from sports participation to criminalization of best-practice medical care.1 Many of these bills are introduced under the guise of “protecting” these adolescents and young adults but are detrimental to their health. They also contain descriptions of gender-affirming care that do not reflect the evidence-based standards of care followed by clinicians across the country. Below is scientifically accurate information on gender-affirming care.
Gender identity development
Trajectories of gender identity are diverse. In a large sample of transgender adults (n = 27,715), 10% started to realize they were transgender at age 5 or younger, 16% between ages 6 and 10, 28% between 11 and 15, 29% between 16 and 20, and 18% at age 21 or older.2 In childhood, cross-gender play and preferences are a normal part of gender expression and many gender-nonconforming children will go on to identify with the sex they were assigned at birth (labeled cisgender). However, some children explicitly identify with a gender different than the sex they were assigned at birth (labeled transgender). Children who are consistent, insistent, and persistent in this identity appear likely to remain so into adolescence and adulthood. It is important to note that there is no evidence that discouraging gender nonconformity decreases the likelihood that a child will identify as transgender. In fact, this practice is no longer considered ethical, as it can have damaging effects on self-esteem and mental health. In addition, not all transgender people are noticeably gender nonconforming in childhood and that lack of childhood gender nonconformity does not invalidate someone’s transgender identity.
Gender-affirming care
For youth who identify as transgender, all steps in transition prior to puberty are social. This includes steps like changing hairstyles or clothing and using a different (affirmed) name and/or pronouns. This time period allows youth to explore their gender identity and expression. In one large study of 10,000 LGBTQ youth, among youth who reported “all or most people” used their affirmed pronoun, 12% reported a history of suicide attempt.3 In comparison, among those who reported that “no one” used their affirmed pronoun, the suicide attempt rate was 28%. Further, 14% of youth who reported that they were able to make changes in their clothing and appearance reported a past suicide attempt in comparison to 26% of those who were not able to. Many of these youth also are under the care of mental health professionals during this time.
At the onset of puberty, transgender youth are eligible for medical management, if needed, to address gender dysphoria (i.e., distress with one’s sex characteristics that is consistent and impairing). It is important to recognize that not all people who identify as transgender experience gender dysphoria or desire a medical transition. For those who do seek medical care, puberty must be confirmed either by breast/testicular exam or checking gonadotropin levels. Standards of care suggest that prior to pubertal suppression with GnRH agonists, such as leuprolide or histrelin, adolescents undergo a thorough psychosocial evaluation by a qualified, licensed clinician. After this evaluation, pubertal suppression may be initiated. These adolescents are monitored by their physicians every 3-6 months for side effects and continuing evaluation of their gender identity. GnRH agonists pause any further pubertal development while the adolescent continues to explore his/her/their gender identity. GnRH agonists are fully reversible and if they are stopped, the child’s natal puberty would recommence.
If an adolescent desires to start gender-affirming hormones, these are started as early as age 14, depending on their maturity, when they desire to start, and/or their ability to obtain parental consent. If a patient has not begun GnRH agonists and undergone a previous psychosocial evaluation, a thorough psychosocial evaluation by a qualified, licensed clinician would take place prior to initiating gender-affirming hormones. Prior to initiating hormones, a thorough informed-consent process occurs between the clinician, patient, and family. This process reviews reversible versus irreversible effects, as well of any side effects of the medication(s). Adolescents who begin hormonal treatment are then monitored every 3-6 months for medication side effects, efficacy, satisfaction with treatment, and by continued mental health assessments. Engagement in mental health therapy is not required beyond the initial evaluation (as many adolescents are well adjusted), but it is encouraged for support during the adolescent’s transition.4 It is important to note that the decision to begin hormones, or not, as well as how to adjust dosing over time, is nuanced and is individualized to each patient’s particular goals for his/her/their transition.
Care for transmasculine identified adolescents (those who were assigned female at birth) typically involves testosterone, delivered via subcutaneous injection, transdermal patch, or transdermal gel. Care for transfeminine individuals (those who were assigned male at birth) typically involves estradiol, delivered via daily pill, weekly or twice weekly transdermal patch, or intramuscular injection, as well as an androgen blocker. This is because estradiol by itself is a weak androgen inhibitor. Antiandrogen medication is delivered by daily oral spironolactone, daily oral bicalutamide (an androgen receptor blocker), or GnRH agonists similar to those used for puberty blockade.
Outcomes
At least 13 studies have documented an improvement in gender dysphoria and/or mental health for adolescents and young adults after beginning gender affirming medical care.5 A recent study by Turban et al. showed that access to gender affirming hormones during adolescence or early adulthood was associated with decreased odds of past month suicidal ideation than for those who did not have access to gender-affirming hormones.6 Tordoff et al. found that receipt of gender-affirming care, including medications, led to a 60% decrease in depressive symptoms and a 73% decrease in suicidality.7 One other question that often arises is whether youth who undergo medical treatment for their transition regret their transition or retransition back to the sex they were assigned at birth. In a large study at a gender clinic in the United Kingdom, they found a regret rate of only 0.47% (16 of 3,398 adolescents aged 13-20).8 This is similar to other studies that have also found low rates of regret. Regret is often due to lack of acceptance in society rather than lack of transgender identity.
The care of gender diverse youth takes place on a spectrum, including options that do not include medical treatment. By supporting youth where they are on their gender journey, there is a significant reduction in adverse mental health outcomes. Gender-affirming hormonal treatment is individualized and a thorough multidisciplinary evaluation and informed consent are obtained prior to initiation. There are careful, nuanced discussions with patients and their families to individualize care based on individual goals. By following established evidence-based standards of care, physicians can support their gender-diverse patients throughout their gender journey. Just like other medical treatments, procedures, or surgeries, gender-affirming care should be undertaken in the context of the sacred patient-physician relationship.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Equality Texas. Legislative Bill Tracker.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. 2016. Washington, DC: National Center for Transgender Equality.
3. The Trevor Project. 2020. National Survey on LGBTQ Mental Health.
4. Lopez X et al. Curr Opin Pediatrics. 2017;29(4):475-80.
5. Turban J. The evidence for trans youth gender-affirming medical care. Psychology Today. 2022 Jan 24.
6. Turban J et al. Access to gender-affirming hormones during adolescence and mental health outcomes among transgender adults. PLOS ONE. 2022;17(1).
7. Tordoff DM et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Network Open. 2022;5(2).
8. Davies S et al. Detransition rates in a national UK gender identity clinic. Inside Matters. On Law, Ethics, and Religion. 2019 Apr 11.
“Texas investigates parents of transgender teen.” “Court did not force dad to allow chemical castration of son.” Headlines such as these are becoming more common as transgender adolescents and young adults, as well as their families, continue to come under attack from state and local governments. In the 2021 state legislative sessions, more than 100 anti-trans bills were filed across 35 state legislatures. Texas alone saw 13 anti-trans bills, covering everything from sports participation to criminalization of best-practice medical care.1 Many of these bills are introduced under the guise of “protecting” these adolescents and young adults but are detrimental to their health. They also contain descriptions of gender-affirming care that do not reflect the evidence-based standards of care followed by clinicians across the country. Below is scientifically accurate information on gender-affirming care.
Gender identity development
Trajectories of gender identity are diverse. In a large sample of transgender adults (n = 27,715), 10% started to realize they were transgender at age 5 or younger, 16% between ages 6 and 10, 28% between 11 and 15, 29% between 16 and 20, and 18% at age 21 or older.2 In childhood, cross-gender play and preferences are a normal part of gender expression and many gender-nonconforming children will go on to identify with the sex they were assigned at birth (labeled cisgender). However, some children explicitly identify with a gender different than the sex they were assigned at birth (labeled transgender). Children who are consistent, insistent, and persistent in this identity appear likely to remain so into adolescence and adulthood. It is important to note that there is no evidence that discouraging gender nonconformity decreases the likelihood that a child will identify as transgender. In fact, this practice is no longer considered ethical, as it can have damaging effects on self-esteem and mental health. In addition, not all transgender people are noticeably gender nonconforming in childhood and that lack of childhood gender nonconformity does not invalidate someone’s transgender identity.
Gender-affirming care
For youth who identify as transgender, all steps in transition prior to puberty are social. This includes steps like changing hairstyles or clothing and using a different (affirmed) name and/or pronouns. This time period allows youth to explore their gender identity and expression. In one large study of 10,000 LGBTQ youth, among youth who reported “all or most people” used their affirmed pronoun, 12% reported a history of suicide attempt.3 In comparison, among those who reported that “no one” used their affirmed pronoun, the suicide attempt rate was 28%. Further, 14% of youth who reported that they were able to make changes in their clothing and appearance reported a past suicide attempt in comparison to 26% of those who were not able to. Many of these youth also are under the care of mental health professionals during this time.
At the onset of puberty, transgender youth are eligible for medical management, if needed, to address gender dysphoria (i.e., distress with one’s sex characteristics that is consistent and impairing). It is important to recognize that not all people who identify as transgender experience gender dysphoria or desire a medical transition. For those who do seek medical care, puberty must be confirmed either by breast/testicular exam or checking gonadotropin levels. Standards of care suggest that prior to pubertal suppression with GnRH agonists, such as leuprolide or histrelin, adolescents undergo a thorough psychosocial evaluation by a qualified, licensed clinician. After this evaluation, pubertal suppression may be initiated. These adolescents are monitored by their physicians every 3-6 months for side effects and continuing evaluation of their gender identity. GnRH agonists pause any further pubertal development while the adolescent continues to explore his/her/their gender identity. GnRH agonists are fully reversible and if they are stopped, the child’s natal puberty would recommence.
If an adolescent desires to start gender-affirming hormones, these are started as early as age 14, depending on their maturity, when they desire to start, and/or their ability to obtain parental consent. If a patient has not begun GnRH agonists and undergone a previous psychosocial evaluation, a thorough psychosocial evaluation by a qualified, licensed clinician would take place prior to initiating gender-affirming hormones. Prior to initiating hormones, a thorough informed-consent process occurs between the clinician, patient, and family. This process reviews reversible versus irreversible effects, as well of any side effects of the medication(s). Adolescents who begin hormonal treatment are then monitored every 3-6 months for medication side effects, efficacy, satisfaction with treatment, and by continued mental health assessments. Engagement in mental health therapy is not required beyond the initial evaluation (as many adolescents are well adjusted), but it is encouraged for support during the adolescent’s transition.4 It is important to note that the decision to begin hormones, or not, as well as how to adjust dosing over time, is nuanced and is individualized to each patient’s particular goals for his/her/their transition.
Care for transmasculine identified adolescents (those who were assigned female at birth) typically involves testosterone, delivered via subcutaneous injection, transdermal patch, or transdermal gel. Care for transfeminine individuals (those who were assigned male at birth) typically involves estradiol, delivered via daily pill, weekly or twice weekly transdermal patch, or intramuscular injection, as well as an androgen blocker. This is because estradiol by itself is a weak androgen inhibitor. Antiandrogen medication is delivered by daily oral spironolactone, daily oral bicalutamide (an androgen receptor blocker), or GnRH agonists similar to those used for puberty blockade.
Outcomes
At least 13 studies have documented an improvement in gender dysphoria and/or mental health for adolescents and young adults after beginning gender affirming medical care.5 A recent study by Turban et al. showed that access to gender affirming hormones during adolescence or early adulthood was associated with decreased odds of past month suicidal ideation than for those who did not have access to gender-affirming hormones.6 Tordoff et al. found that receipt of gender-affirming care, including medications, led to a 60% decrease in depressive symptoms and a 73% decrease in suicidality.7 One other question that often arises is whether youth who undergo medical treatment for their transition regret their transition or retransition back to the sex they were assigned at birth. In a large study at a gender clinic in the United Kingdom, they found a regret rate of only 0.47% (16 of 3,398 adolescents aged 13-20).8 This is similar to other studies that have also found low rates of regret. Regret is often due to lack of acceptance in society rather than lack of transgender identity.
The care of gender diverse youth takes place on a spectrum, including options that do not include medical treatment. By supporting youth where they are on their gender journey, there is a significant reduction in adverse mental health outcomes. Gender-affirming hormonal treatment is individualized and a thorough multidisciplinary evaluation and informed consent are obtained prior to initiation. There are careful, nuanced discussions with patients and their families to individualize care based on individual goals. By following established evidence-based standards of care, physicians can support their gender-diverse patients throughout their gender journey. Just like other medical treatments, procedures, or surgeries, gender-affirming care should be undertaken in the context of the sacred patient-physician relationship.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Equality Texas. Legislative Bill Tracker.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. 2016. Washington, DC: National Center for Transgender Equality.
3. The Trevor Project. 2020. National Survey on LGBTQ Mental Health.
4. Lopez X et al. Curr Opin Pediatrics. 2017;29(4):475-80.
5. Turban J. The evidence for trans youth gender-affirming medical care. Psychology Today. 2022 Jan 24.
6. Turban J et al. Access to gender-affirming hormones during adolescence and mental health outcomes among transgender adults. PLOS ONE. 2022;17(1).
7. Tordoff DM et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Network Open. 2022;5(2).
8. Davies S et al. Detransition rates in a national UK gender identity clinic. Inside Matters. On Law, Ethics, and Religion. 2019 Apr 11.
Breast anatomy and augmentation in transfeminine individuals
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
Augmentation mammaplasty, otherwise known as a breast augmentation, is one of the most common cosmetic procedures performed in cisgender females. Gynecologists routinely perform annual breast examinations and order screening mammography in cisgender women with breast implants. Similarly, there is an increasing number of transgender women seeking breast augmentation – with approximately 60%-70% of patients having desired or undergone the procedure.1 Consequently, these patients are instructed by their surgeons to follow up with gynecologists for annual examinations and screening. While there are many similarities in technique and procedure, there are nuances in patient demographics, anatomy, and surgical technique that obstetricians/gynecologists should be aware of when examining these patients or prior to referring them to a surgeon for augmentation.2
Many patients who are dissatisfied with breast size from hormone therapy alone will seek out augmentation mammaplasty. In patients taking estrogen for hormone therapy, breast growth will commence around 2-3 months and peak over 1-2 years.3 Unlike chest surgery for transmasculine individuals, it is recommended that transfeminine patients seeking breast augmentation wait a minimum of 12 months before to surgery to allow for maximum breast enlargement. As with breast growth in cisgender females, the extent of breast development is multifactorial and varies from individual to individual. Current literature does not suggest that estrogen type or dose affects the ultimate breast size; however, younger age, tissue sensitivity, and body weight may affect breast volume.3 Referral to a genetic counselor and preoperative imaging may be necessary if a patient has a history concerning for a genetic or familial predisposition to breast cancer.
Implant selection and placement is determined by a variety of factors. While the overall principles of augmentation mammaplasty are essentially the same, there are anatomic differences in transfeminine patients that surgeons must take into consideration at the time of the consultation and during the surgery itself. For example, the pectoralis major muscle is more defined, there is a longer sternal notch-to-nipple distance, the chest wall is broader and more barrel-shaped, and there is a shorter distance between the nipple and the inframammary crease.2-4 As a result of the broader chest wall, it is extremely difficult to achieve central cleavage even with larger implant selection. The surgeon must also ensure that the nipple and areola overlie the implant centrally. Medial placement of the implant will result in lateral displacement of the nipples, which can have an unsatisfactory cosmetic appearance.
Incision location can be axillary, inframammary, or even transareolar, although the latter is less common due to the smaller areolar size and larger implant choice.3 If the inframammary incision is used, it should be placed lower than the natural inframammary fold because the distance between the inferior areolar margin and inframammary fold is shorter and will expand after the implant is placed.4 While both saline and silicone implants are available, many surgeons (myself included), favor more form-stable silicone implants. Given the association between anaplastic large-cell lymphoma and textured implants, many surgeons also use nontextured, or smooth, cohesive gel silicone implants.5
Pocket selection of the implant itself can be subglandular – directly under the breast mound – or subpectoral – behind the pectoralis muscle. For patients with a pinch test of greater than 1.5 cm (outside of the area of the breast bud), good skin softening, and marked pectoralis hypertrophy, subglandular placement is reasonable.6 In thin patients with minimal breast development, subglandular placement can result in a “double-mound” appearance and can lead to visible implant edges on the periphery.6 Use of the subpectoral plane is more common and is associated with less implant visibility due to an increased amount of soft-tissue coverage and has lower rates of capsular contracture.4 However, due to the more robust pectoralis muscle in transfeminine patients, implant displacement can occur more frequently compared to subglandular placement. The surgeon and patient must have a thorough discussion about the location of the incision, implant material, and pocket placement along with the benefits and complications of the surgical plan.
Complications of augmentation mammaplasty are rare. However, when they occur it can include capsular contracture, breast asymmetry, hematoma formation, loss of nipple sensation, implant malposition, implant displacement below the inframammary crease, implant rupture, and need for revisional surgery.7 If an obstetrician/gynecologist observes any of the aforementioned findings in a postoperative patient, consultation and referral to a plastic surgeon is imperative.
Postoperative assessment and screening are mandatory in all patients who undergo breast augmentation. It is important for the gynecologist to note the incision placement, know the type of implant used (saline or silicone), and delineate where the implant was placed. If silicone implants are used, breast MRI is more sensitive in detecting implant rupture compared to mammography alone. Given the relatively poor epidemiologic data on breast cancer in transgender women, the Endocrine Society recommends that these patients follow the same screening guidelines as cisgender women.4,6
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Wierckx K et al. J Sex Med. 2014;11(5):1240-7.
2. Mehra G et al. Plast Reconstr Surg Glob Open 2021 Jan 21;9(1):e3362. doi: 10.1097/GOX.0000000000003362.
3. Schecter LS, Schechter RB. Breast and chest surgery for transgender patients. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier, 2020:73-81.
4. Colebunders B et al. Top surgery. In: Salgado CJ et al. ed. Gender Affirmation: Medical and Surgical Perspectives. New York, NY: Thieme, 2017:51-66.
5. De Boer M et al. Aesthet Surg J. 2017;37:NP83-NP87.
6. Coon D et al. Plast Reconstr Surg. 2020 Jun;145(6):1343-53.
7. Kanhai RC et al. Br J Plast Surg. 2000;53:209-11.
RaDonda Vaught: Victim, felon, or both?
For 4 and a half years, I have followed the RaDonda Vaught medication error that led to the unfortunate death of a human being. I am not alone. Nurses across the country have followed the case with anxiety and fear, knowing a guilty verdict might have the potential to challenge basic tenets of care.
According to Kaiser Health News, nurses are “raging and quitting” following the announcement of a guilty verdict for two felonies: criminally negligent homicide and gross neglect of an impaired adult.
Thousands of nurses have claimed they could arrive in Nashville, Tenn., on May 13, the day Ms. Vaught is to be sentenced, to protest the conviction. Others have stated they believe justice is being conducted, as their sympathies lie with the victim, Charlene Murphey, who died 12 hours after being unable to draw breath, paralyzed from the inadvertent dose of vecuronium given intravenously by her nurse.
How should we feel as clinicians? according to sentencing guidelines?
My belief is that it is understandable to feel passionately about this case, including what it could mean to an era of “just culture” that nursing organizations have promoted. The concept of just culture looks at medication/nursing errors as opportunities for growth to avoid future errors, not as scenarios for punitive action. With the guilty verdict in Ms. Vaught’s case, nurses (and facilities) fear that nurses will avoid coming forward after mistakes, leading to cover-ups and a culture perspective.
Will nurses be hesitant to report errors (especially significant errors) that lead to patient harm? Will we fear retribution and reprisal for being truthful?
I believe that Ms. Vaught’s criminal case has changed little in the political landscape of caregiving. Before you let loose with a loud expletive (or two), hear me out.
When a patient dies from unintentional harm, someone must be held accountable. Society needs a scapegoat, and unfortunately, excrement slides downhill to the lowest common denominator, which may be the nurse. Initially, Ms. Vaught was contacted by her state licensing board (Tennessee) and informed there would be no professional repercussions for her mistake. That decision did not hold. She was later indicted criminally for the death of her patient. She also had her nursing license revoked.
Why? The hospital where she worked was threatened with Medicare reprisal if systemic issues were not addressed following the incident; for example, a bar-coding device was not available for Ms. Vaught to use prior to administering the vecuronium, and paralytic agents were stored unsafely in a Pyxis MedStation, readily available for any nurse to obtain via override.
In fact, the number of overrides performed by all nurses caring for Ms. Murphey in the days leading to her death was alarming, leading reviewers to assume that time to acquire medication for inpatients was a problem.
Ms. Vaught herself, stating the obvious on talk shows, said she should not have performed an override, that the situation was “not an emergency” and she should have taken time to check that Versed (midazolam) was available by the generic name and not the “VE” she entered as a search mechanism into the machine. She also stated she was “distracted” by a trainee assigned to her at the time.
We have all been there, feeling rushed to perform a task under stressful situations, skipping safety guidelines to sedate a patient while radiology is waiting. Someone is always on our a**, waiting to get to the next task, the next patient, the next admission, the next pseudo-emergency called nursing workload.
It never ends.
Which is why I wish to emphasize what the Ms. Vaught guilty verdict really means for nurses.
It means we must never forget that our actions have the potential to harm, even kill, our patients.
We must never forget that repercussions and reprisal may occur, whether personal guilt that may prove more damaging than the prison sentence Ms. Vaught might receive, or problems that could result if nurses attempt to hide or subvert medication issues.
In Ms. Vaught’s case, she did not document the medication that had been given to Ms. Murphey, facts the prosecution seized on to proclaim her guilt. Why? We can only guess at this point. But her claims of truthfulness need to be balanced by what occurred, and the facts are that she did not document the error after administering vecuronium that night.
When reflecting on this verdict, we need to remember a patient died, and she did so horribly, being unable to draw breath. This should never happen during our watch, ever, and as clinicians, we need to be vigilant.
In summary, protest if you believe justice has been too harsh or unfair, and that nurses may be fearful as a result. But please spare a moment to realize that someone should protest for Ms. Murphey as well. We cannot bring her back, nor can we right the system issues that may have led to her death.
But we should protest for safer systems, for improved staffing, for a need to catch our collective breaths, and a day to work and nurture patients when someone is not constantly on our a**. Only then will nurses be protected from unjust reprisal, from needing to be the lowest common denominator of guilt.
Ms. Goodman is a researcher and consultant in Libertyville, Ill. She disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
For 4 and a half years, I have followed the RaDonda Vaught medication error that led to the unfortunate death of a human being. I am not alone. Nurses across the country have followed the case with anxiety and fear, knowing a guilty verdict might have the potential to challenge basic tenets of care.
According to Kaiser Health News, nurses are “raging and quitting” following the announcement of a guilty verdict for two felonies: criminally negligent homicide and gross neglect of an impaired adult.
Thousands of nurses have claimed they could arrive in Nashville, Tenn., on May 13, the day Ms. Vaught is to be sentenced, to protest the conviction. Others have stated they believe justice is being conducted, as their sympathies lie with the victim, Charlene Murphey, who died 12 hours after being unable to draw breath, paralyzed from the inadvertent dose of vecuronium given intravenously by her nurse.
How should we feel as clinicians? according to sentencing guidelines?
My belief is that it is understandable to feel passionately about this case, including what it could mean to an era of “just culture” that nursing organizations have promoted. The concept of just culture looks at medication/nursing errors as opportunities for growth to avoid future errors, not as scenarios for punitive action. With the guilty verdict in Ms. Vaught’s case, nurses (and facilities) fear that nurses will avoid coming forward after mistakes, leading to cover-ups and a culture perspective.
Will nurses be hesitant to report errors (especially significant errors) that lead to patient harm? Will we fear retribution and reprisal for being truthful?
I believe that Ms. Vaught’s criminal case has changed little in the political landscape of caregiving. Before you let loose with a loud expletive (or two), hear me out.
When a patient dies from unintentional harm, someone must be held accountable. Society needs a scapegoat, and unfortunately, excrement slides downhill to the lowest common denominator, which may be the nurse. Initially, Ms. Vaught was contacted by her state licensing board (Tennessee) and informed there would be no professional repercussions for her mistake. That decision did not hold. She was later indicted criminally for the death of her patient. She also had her nursing license revoked.
Why? The hospital where she worked was threatened with Medicare reprisal if systemic issues were not addressed following the incident; for example, a bar-coding device was not available for Ms. Vaught to use prior to administering the vecuronium, and paralytic agents were stored unsafely in a Pyxis MedStation, readily available for any nurse to obtain via override.
In fact, the number of overrides performed by all nurses caring for Ms. Murphey in the days leading to her death was alarming, leading reviewers to assume that time to acquire medication for inpatients was a problem.
Ms. Vaught herself, stating the obvious on talk shows, said she should not have performed an override, that the situation was “not an emergency” and she should have taken time to check that Versed (midazolam) was available by the generic name and not the “VE” she entered as a search mechanism into the machine. She also stated she was “distracted” by a trainee assigned to her at the time.
We have all been there, feeling rushed to perform a task under stressful situations, skipping safety guidelines to sedate a patient while radiology is waiting. Someone is always on our a**, waiting to get to the next task, the next patient, the next admission, the next pseudo-emergency called nursing workload.
It never ends.
Which is why I wish to emphasize what the Ms. Vaught guilty verdict really means for nurses.
It means we must never forget that our actions have the potential to harm, even kill, our patients.
We must never forget that repercussions and reprisal may occur, whether personal guilt that may prove more damaging than the prison sentence Ms. Vaught might receive, or problems that could result if nurses attempt to hide or subvert medication issues.
In Ms. Vaught’s case, she did not document the medication that had been given to Ms. Murphey, facts the prosecution seized on to proclaim her guilt. Why? We can only guess at this point. But her claims of truthfulness need to be balanced by what occurred, and the facts are that she did not document the error after administering vecuronium that night.
When reflecting on this verdict, we need to remember a patient died, and she did so horribly, being unable to draw breath. This should never happen during our watch, ever, and as clinicians, we need to be vigilant.
In summary, protest if you believe justice has been too harsh or unfair, and that nurses may be fearful as a result. But please spare a moment to realize that someone should protest for Ms. Murphey as well. We cannot bring her back, nor can we right the system issues that may have led to her death.
But we should protest for safer systems, for improved staffing, for a need to catch our collective breaths, and a day to work and nurture patients when someone is not constantly on our a**. Only then will nurses be protected from unjust reprisal, from needing to be the lowest common denominator of guilt.
Ms. Goodman is a researcher and consultant in Libertyville, Ill. She disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
For 4 and a half years, I have followed the RaDonda Vaught medication error that led to the unfortunate death of a human being. I am not alone. Nurses across the country have followed the case with anxiety and fear, knowing a guilty verdict might have the potential to challenge basic tenets of care.
According to Kaiser Health News, nurses are “raging and quitting” following the announcement of a guilty verdict for two felonies: criminally negligent homicide and gross neglect of an impaired adult.
Thousands of nurses have claimed they could arrive in Nashville, Tenn., on May 13, the day Ms. Vaught is to be sentenced, to protest the conviction. Others have stated they believe justice is being conducted, as their sympathies lie with the victim, Charlene Murphey, who died 12 hours after being unable to draw breath, paralyzed from the inadvertent dose of vecuronium given intravenously by her nurse.
How should we feel as clinicians? according to sentencing guidelines?
My belief is that it is understandable to feel passionately about this case, including what it could mean to an era of “just culture” that nursing organizations have promoted. The concept of just culture looks at medication/nursing errors as opportunities for growth to avoid future errors, not as scenarios for punitive action. With the guilty verdict in Ms. Vaught’s case, nurses (and facilities) fear that nurses will avoid coming forward after mistakes, leading to cover-ups and a culture perspective.
Will nurses be hesitant to report errors (especially significant errors) that lead to patient harm? Will we fear retribution and reprisal for being truthful?
I believe that Ms. Vaught’s criminal case has changed little in the political landscape of caregiving. Before you let loose with a loud expletive (or two), hear me out.
When a patient dies from unintentional harm, someone must be held accountable. Society needs a scapegoat, and unfortunately, excrement slides downhill to the lowest common denominator, which may be the nurse. Initially, Ms. Vaught was contacted by her state licensing board (Tennessee) and informed there would be no professional repercussions for her mistake. That decision did not hold. She was later indicted criminally for the death of her patient. She also had her nursing license revoked.
Why? The hospital where she worked was threatened with Medicare reprisal if systemic issues were not addressed following the incident; for example, a bar-coding device was not available for Ms. Vaught to use prior to administering the vecuronium, and paralytic agents were stored unsafely in a Pyxis MedStation, readily available for any nurse to obtain via override.
In fact, the number of overrides performed by all nurses caring for Ms. Murphey in the days leading to her death was alarming, leading reviewers to assume that time to acquire medication for inpatients was a problem.
Ms. Vaught herself, stating the obvious on talk shows, said she should not have performed an override, that the situation was “not an emergency” and she should have taken time to check that Versed (midazolam) was available by the generic name and not the “VE” she entered as a search mechanism into the machine. She also stated she was “distracted” by a trainee assigned to her at the time.
We have all been there, feeling rushed to perform a task under stressful situations, skipping safety guidelines to sedate a patient while radiology is waiting. Someone is always on our a**, waiting to get to the next task, the next patient, the next admission, the next pseudo-emergency called nursing workload.
It never ends.
Which is why I wish to emphasize what the Ms. Vaught guilty verdict really means for nurses.
It means we must never forget that our actions have the potential to harm, even kill, our patients.
We must never forget that repercussions and reprisal may occur, whether personal guilt that may prove more damaging than the prison sentence Ms. Vaught might receive, or problems that could result if nurses attempt to hide or subvert medication issues.
In Ms. Vaught’s case, she did not document the medication that had been given to Ms. Murphey, facts the prosecution seized on to proclaim her guilt. Why? We can only guess at this point. But her claims of truthfulness need to be balanced by what occurred, and the facts are that she did not document the error after administering vecuronium that night.
When reflecting on this verdict, we need to remember a patient died, and she did so horribly, being unable to draw breath. This should never happen during our watch, ever, and as clinicians, we need to be vigilant.
In summary, protest if you believe justice has been too harsh or unfair, and that nurses may be fearful as a result. But please spare a moment to realize that someone should protest for Ms. Murphey as well. We cannot bring her back, nor can we right the system issues that may have led to her death.
But we should protest for safer systems, for improved staffing, for a need to catch our collective breaths, and a day to work and nurture patients when someone is not constantly on our a**. Only then will nurses be protected from unjust reprisal, from needing to be the lowest common denominator of guilt.
Ms. Goodman is a researcher and consultant in Libertyville, Ill. She disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Acid series: Trichloroacetic acid
– yet can be one of the most dangerous treatments in the hands of an untrained user.
TCA, in a clear colorless solution, is available in concentrations up to 100%, and has not been associated with allergic reactions or systemic toxicity. The available concentrations include those used for superficial depth peels (10%-30%), medium depth peels (35%-50%), and deep peels (greater than 50%).
TCA causes coagulation of the cellular membrane of epidermal proteins in the epidermis and, depending on the concentration, the dermis, which results in frosting of the skin. Repair of the epidermal cells induces resurfacing of the skin and neocollagenesis. TCA can be combined with other acids, including glycolic acid (Coleman peel), Jessner solution (Monheit peel), and solid CO2 (Brody peel). It has also been combined with lactic acid, mandelic acid, and salicylic acid in combination peels of various concentrations.
Although there are many studies, case reports, and textbooks related to this topic and the applications, combinations and treatment options for TCA peels, it is important to highlight here how many of these solutions – at high concentrations – are available directly to consumers, med spas, and the general public through online websites, including Amazon and overseas sites. Over the last 15 years, I have seen complications of this acid alone in people who have bought TCA online, related to applications not just on the face but on the body, neck, eyes, vaginal, and anal areas. Pigmentation, erosions, ulcers, and strictures are just some of the possible complications that occur not just with a more concentrated solution, but more often from application errors, aggressive layering of the acid, allowing the acid to sit on the skin too long, and improper tissue prepping and posttreatment skin care.
TCA can be an untamable acid, with little control over the depth of penetration even in the most controlled situations. The inability to be neutralize TCA creates an environment in which the depth of penetration and tissue coagulation is not a precise science. Once applied, the tissue reaction cannot be “stopped” or rapidly reversed making it highly variable in its mechanism. Patients of all skin types have the potential to develop complications as the epidermal and dermal thickness, moisture content, sebum production, and pigmentation are highly varied between individuals.
In my opinion, it is a dangerous product to have on the market – not just for the untrained medical providers using it but for estheticians and the general public who now can buy TCA anywhere.
But with effective training, reliable sourcing and appropriate preparation of the patient’s skin, however, a TCA peel can be a highly effective tool for difficult-to-treat dermatological problems, such as scarring and xanthelasma.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. She has no relevant disclosures. Write to them at [email protected].
– yet can be one of the most dangerous treatments in the hands of an untrained user.
TCA, in a clear colorless solution, is available in concentrations up to 100%, and has not been associated with allergic reactions or systemic toxicity. The available concentrations include those used for superficial depth peels (10%-30%), medium depth peels (35%-50%), and deep peels (greater than 50%).
TCA causes coagulation of the cellular membrane of epidermal proteins in the epidermis and, depending on the concentration, the dermis, which results in frosting of the skin. Repair of the epidermal cells induces resurfacing of the skin and neocollagenesis. TCA can be combined with other acids, including glycolic acid (Coleman peel), Jessner solution (Monheit peel), and solid CO2 (Brody peel). It has also been combined with lactic acid, mandelic acid, and salicylic acid in combination peels of various concentrations.
Although there are many studies, case reports, and textbooks related to this topic and the applications, combinations and treatment options for TCA peels, it is important to highlight here how many of these solutions – at high concentrations – are available directly to consumers, med spas, and the general public through online websites, including Amazon and overseas sites. Over the last 15 years, I have seen complications of this acid alone in people who have bought TCA online, related to applications not just on the face but on the body, neck, eyes, vaginal, and anal areas. Pigmentation, erosions, ulcers, and strictures are just some of the possible complications that occur not just with a more concentrated solution, but more often from application errors, aggressive layering of the acid, allowing the acid to sit on the skin too long, and improper tissue prepping and posttreatment skin care.
TCA can be an untamable acid, with little control over the depth of penetration even in the most controlled situations. The inability to be neutralize TCA creates an environment in which the depth of penetration and tissue coagulation is not a precise science. Once applied, the tissue reaction cannot be “stopped” or rapidly reversed making it highly variable in its mechanism. Patients of all skin types have the potential to develop complications as the epidermal and dermal thickness, moisture content, sebum production, and pigmentation are highly varied between individuals.
In my opinion, it is a dangerous product to have on the market – not just for the untrained medical providers using it but for estheticians and the general public who now can buy TCA anywhere.
But with effective training, reliable sourcing and appropriate preparation of the patient’s skin, however, a TCA peel can be a highly effective tool for difficult-to-treat dermatological problems, such as scarring and xanthelasma.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. She has no relevant disclosures. Write to them at [email protected].
– yet can be one of the most dangerous treatments in the hands of an untrained user.
TCA, in a clear colorless solution, is available in concentrations up to 100%, and has not been associated with allergic reactions or systemic toxicity. The available concentrations include those used for superficial depth peels (10%-30%), medium depth peels (35%-50%), and deep peels (greater than 50%).
TCA causes coagulation of the cellular membrane of epidermal proteins in the epidermis and, depending on the concentration, the dermis, which results in frosting of the skin. Repair of the epidermal cells induces resurfacing of the skin and neocollagenesis. TCA can be combined with other acids, including glycolic acid (Coleman peel), Jessner solution (Monheit peel), and solid CO2 (Brody peel). It has also been combined with lactic acid, mandelic acid, and salicylic acid in combination peels of various concentrations.
Although there are many studies, case reports, and textbooks related to this topic and the applications, combinations and treatment options for TCA peels, it is important to highlight here how many of these solutions – at high concentrations – are available directly to consumers, med spas, and the general public through online websites, including Amazon and overseas sites. Over the last 15 years, I have seen complications of this acid alone in people who have bought TCA online, related to applications not just on the face but on the body, neck, eyes, vaginal, and anal areas. Pigmentation, erosions, ulcers, and strictures are just some of the possible complications that occur not just with a more concentrated solution, but more often from application errors, aggressive layering of the acid, allowing the acid to sit on the skin too long, and improper tissue prepping and posttreatment skin care.
TCA can be an untamable acid, with little control over the depth of penetration even in the most controlled situations. The inability to be neutralize TCA creates an environment in which the depth of penetration and tissue coagulation is not a precise science. Once applied, the tissue reaction cannot be “stopped” or rapidly reversed making it highly variable in its mechanism. Patients of all skin types have the potential to develop complications as the epidermal and dermal thickness, moisture content, sebum production, and pigmentation are highly varied between individuals.
In my opinion, it is a dangerous product to have on the market – not just for the untrained medical providers using it but for estheticians and the general public who now can buy TCA anywhere.
But with effective training, reliable sourcing and appropriate preparation of the patient’s skin, however, a TCA peel can be a highly effective tool for difficult-to-treat dermatological problems, such as scarring and xanthelasma.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. She has no relevant disclosures. Write to them at [email protected].
Commentary: Emerging tick-borne pathogen has spread to state of Georgia
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Hormones after cancer: Are they safe?
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at [email protected].
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
Merging small practices
Difficult economic times and the unpredictable consequences of health care reform are making an increasing number of solo practitioners and small private groups very nervous. Yet, many balk at the prospect of selling to private equity companies.
Merging offers many benefits: Better overall management, centralized and efficient billing and collection, group purchasing discounts, and reduced overhead, among others; but careful planning, and a written agreement, are essential. If you are considering such an option, here are some things to think about.
You should begin with an evaluation and comparison of the separate groups’ respective finances. This should include a history of production, collections, overhead, and liabilities. Basically, you want to locate and identify all assets and liabilities that will be combined into the new group. One area of immediate importance is Medicare participation. Which members now currently participate and which do not? Since the new group will need to have a single position, all of the physicians must agree on that issue.
Who will be in charge? Not every physician is a qualified manager. The manager should be the physician who is willing to spend the time it takes to sign checks, interact with the administrator, and ensure that other matters such as filing tax returns and approving minor purchases arc carried out properly.
What is the compensation formula? Compensation arrangements should be based on each physician’s current financial data and the goals of the practice. Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination, so productivity is rewarded but your income doesn’t drop to zero when you take time off.
Which practices have a retirement plan and which do not? Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same or different from any of the existing plans. You’ll probably need some legal guidance to insure that assets from existing plans can be transferred into a new plan without tax issues. You may also have to address the problem of physicians who currently do not have a plan who, for whatever reason, may not want to be forced into making retirement plan contributions.
The often-problematic issue of employees and their salaries needs to be addressed, to decide which employees will be needed in the new group, and to determine a salary structure. Each practice’s policies related to vacation, sick leave, and other such issues should be reviewed, and an overall policy for the new group developed.
Other common sticking points are issues related to facilities. If the practices intend to consolidate into one location, the physicians must decide which of the specific assets of each practice will be contributed to the new entity. Ideally, each party brings an equal amount of assets to the table, but in the real world that is hardly ever the case. Physicians whose assets are to be used generally want to be compensated, and those who have to dispose of or store assets are in a quandary. The solution to this predicament will vary depending on the circumstances of each merger. One alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Buyouts should be addressed in advance as well. You must decide when a buyout would occur – usually in the event of retirement, death, disability, or withdrawal (voluntary or involuntary) – how the buyout amount will be calculated, and how it will be paid. Then, you must agree on how a buyout amount will be valued. Remember that any buyout calculated at “appraised value” is a problem, because the buyout amount remains a mystery until an appraisal is performed. If the appraised value ends up being too high, the remaining owners may refuse to pay it. I suggest having an actuary create a formula, so that the buyout figure can be calculated at any time. This area, especially, is where you need experienced, competent legal advice.
Noncompete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future. I’ll discuss some other, more complicated merger options in my next column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Difficult economic times and the unpredictable consequences of health care reform are making an increasing number of solo practitioners and small private groups very nervous. Yet, many balk at the prospect of selling to private equity companies.
Merging offers many benefits: Better overall management, centralized and efficient billing and collection, group purchasing discounts, and reduced overhead, among others; but careful planning, and a written agreement, are essential. If you are considering such an option, here are some things to think about.
You should begin with an evaluation and comparison of the separate groups’ respective finances. This should include a history of production, collections, overhead, and liabilities. Basically, you want to locate and identify all assets and liabilities that will be combined into the new group. One area of immediate importance is Medicare participation. Which members now currently participate and which do not? Since the new group will need to have a single position, all of the physicians must agree on that issue.
Who will be in charge? Not every physician is a qualified manager. The manager should be the physician who is willing to spend the time it takes to sign checks, interact with the administrator, and ensure that other matters such as filing tax returns and approving minor purchases arc carried out properly.
What is the compensation formula? Compensation arrangements should be based on each physician’s current financial data and the goals of the practice. Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination, so productivity is rewarded but your income doesn’t drop to zero when you take time off.
Which practices have a retirement plan and which do not? Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same or different from any of the existing plans. You’ll probably need some legal guidance to insure that assets from existing plans can be transferred into a new plan without tax issues. You may also have to address the problem of physicians who currently do not have a plan who, for whatever reason, may not want to be forced into making retirement plan contributions.
The often-problematic issue of employees and their salaries needs to be addressed, to decide which employees will be needed in the new group, and to determine a salary structure. Each practice’s policies related to vacation, sick leave, and other such issues should be reviewed, and an overall policy for the new group developed.
Other common sticking points are issues related to facilities. If the practices intend to consolidate into one location, the physicians must decide which of the specific assets of each practice will be contributed to the new entity. Ideally, each party brings an equal amount of assets to the table, but in the real world that is hardly ever the case. Physicians whose assets are to be used generally want to be compensated, and those who have to dispose of or store assets are in a quandary. The solution to this predicament will vary depending on the circumstances of each merger. One alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Buyouts should be addressed in advance as well. You must decide when a buyout would occur – usually in the event of retirement, death, disability, or withdrawal (voluntary or involuntary) – how the buyout amount will be calculated, and how it will be paid. Then, you must agree on how a buyout amount will be valued. Remember that any buyout calculated at “appraised value” is a problem, because the buyout amount remains a mystery until an appraisal is performed. If the appraised value ends up being too high, the remaining owners may refuse to pay it. I suggest having an actuary create a formula, so that the buyout figure can be calculated at any time. This area, especially, is where you need experienced, competent legal advice.
Noncompete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future. I’ll discuss some other, more complicated merger options in my next column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Difficult economic times and the unpredictable consequences of health care reform are making an increasing number of solo practitioners and small private groups very nervous. Yet, many balk at the prospect of selling to private equity companies.
Merging offers many benefits: Better overall management, centralized and efficient billing and collection, group purchasing discounts, and reduced overhead, among others; but careful planning, and a written agreement, are essential. If you are considering such an option, here are some things to think about.
You should begin with an evaluation and comparison of the separate groups’ respective finances. This should include a history of production, collections, overhead, and liabilities. Basically, you want to locate and identify all assets and liabilities that will be combined into the new group. One area of immediate importance is Medicare participation. Which members now currently participate and which do not? Since the new group will need to have a single position, all of the physicians must agree on that issue.
Who will be in charge? Not every physician is a qualified manager. The manager should be the physician who is willing to spend the time it takes to sign checks, interact with the administrator, and ensure that other matters such as filing tax returns and approving minor purchases arc carried out properly.
What is the compensation formula? Compensation arrangements should be based on each physician’s current financial data and the goals of the practice. Will everyone be paid only for what they do individually, or will revenue be shared equally? I favor a combination, so productivity is rewarded but your income doesn’t drop to zero when you take time off.
Which practices have a retirement plan and which do not? Will you keep your retirement plans separate, or combine them? If the latter, you will have to agree on the terms of the new plan, which can be the same or different from any of the existing plans. You’ll probably need some legal guidance to insure that assets from existing plans can be transferred into a new plan without tax issues. You may also have to address the problem of physicians who currently do not have a plan who, for whatever reason, may not want to be forced into making retirement plan contributions.
The often-problematic issue of employees and their salaries needs to be addressed, to decide which employees will be needed in the new group, and to determine a salary structure. Each practice’s policies related to vacation, sick leave, and other such issues should be reviewed, and an overall policy for the new group developed.
Other common sticking points are issues related to facilities. If the practices intend to consolidate into one location, the physicians must decide which of the specific assets of each practice will be contributed to the new entity. Ideally, each party brings an equal amount of assets to the table, but in the real world that is hardly ever the case. Physicians whose assets are to be used generally want to be compensated, and those who have to dispose of or store assets are in a quandary. The solution to this predicament will vary depending on the circumstances of each merger. One alternative is to agree that any inequalities will be compensated at the other end, in the form of buyout value; that is, physicians contributing more assets will receive larger buyouts when they leave or retire than those contributing less.
Buyouts should be addressed in advance as well. You must decide when a buyout would occur – usually in the event of retirement, death, disability, or withdrawal (voluntary or involuntary) – how the buyout amount will be calculated, and how it will be paid. Then, you must agree on how a buyout amount will be valued. Remember that any buyout calculated at “appraised value” is a problem, because the buyout amount remains a mystery until an appraisal is performed. If the appraised value ends up being too high, the remaining owners may refuse to pay it. I suggest having an actuary create a formula, so that the buyout figure can be calculated at any time. This area, especially, is where you need experienced, competent legal advice.
Noncompete provisions are always a difficult issue, mostly because they are so hard (and expensive) to enforce. An increasingly popular alternative is, once again, to deal with it at the other end, with a buyout penalty. An unhappy partner can leave, and compete, but at the cost of a substantially reduced buyout. This permits competition, but discourages it; and it compensates the remaining partners.
These are only some of the pivotal business and legal issues that must be settled in advance. A little planning and negotiation can prevent a lot of grief, regret, and legal expenses in the future. I’ll discuss some other, more complicated merger options in my next column.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Cupping in dermatology
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at [email protected] or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.