User login
The work after work
Across the country, taxes unite us. Not that we all share the same, rather that we all have to do them. It was recently tax weekend in our house: The Saturday and Sunday that cap off weeks of hunting and gathering faded receipts and sorting through reams of credit card bills to find all the dollars we spent on work. The task is more tedious than all the Wednesdays of taking out trash bins combined, and equally as exciting. But wait, that’s not all.
This weekend I’ve been chatting with bots from a solar company trying to solve our drop in energy production and sat on terminal hold with apparently one person who answers the phone for Amazon. There’s also an homeowner’s association meeting to prepare for and research to be done on ceiling fans.
“Life admin” is a crisp phrase coined by Elizabeth Emens, JD, PhD, that captures the never-ending to-do list that comes with running a household. An accomplished law professor at Columbia University, New York, Dr. Emens noticed the negative impact this life admin has on our quality of life. Reading her book, “Life Admin: How I Learned to Do Less, Do Better, and Live More” (New York: HarperOne, 2019), your eyes widen as she magically makes salient all this hidden work that is stealing our time. Life admin, kidmin, mom and dadmin, just rattling them off feels like donning x-ray glasses allowing us to see how much work we do outside of our work. As doctors, I would add “family house calls,” as a contributing factor: Random family and friends who want to talk for a minute about their knee replacement or what drug the ICU should give Uncle Larry who is fighting COVID. (I only know ivermectin, but it would only help if he just had scabies).
By all accounts, the amount of life admin is growing insidiously, worsened by the great pandemic. There are events to plan and reply to, more DIY customer service to fix your own problems, more work to find a VRBO for a weekend getaway at the beach. (There are none on the entire coast of California this summer, so I just saved you time there. You’re welcome.)
There is no good time to do this work and combined with the heavy burden of our responsibilities as physicians, it can feel like fuel feeding the burnout fire.
Dr. Emens has some top tips to help. First up, know your admin type. Are you a super doer, reluctant doer, admin denier, or admin avoider? I’m mostly in the avoider quadrant, dropping into reluctant doer when consequences loom. Next, choose strategies that fit you. Instead of avoiding, there are some things I might deflect. For example, When your aunt in Peoria asks where she can get a COVID test, you can use LMGTFY.com to generate a link that will show them how to use Google to help with their question. Dr. Emens is joking, but the point rang true. We can lighten the load a bit if we delegate or push back the excessive or undue requests. For some tasks, we’d be better off paying someone to take it over. Last tip here, try doing life admin with a partner, be it spouse, friend, or colleague. This is particularly useful when your partner is a super doer, as mine is. Not only can they make the work lighter, but also less dreary.
We physicians are focused on fixing physician burnout. Maybe we should also be looking at what happens in the “second shift” at home. Tax season is over, but will be back soon.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Across the country, taxes unite us. Not that we all share the same, rather that we all have to do them. It was recently tax weekend in our house: The Saturday and Sunday that cap off weeks of hunting and gathering faded receipts and sorting through reams of credit card bills to find all the dollars we spent on work. The task is more tedious than all the Wednesdays of taking out trash bins combined, and equally as exciting. But wait, that’s not all.
This weekend I’ve been chatting with bots from a solar company trying to solve our drop in energy production and sat on terminal hold with apparently one person who answers the phone for Amazon. There’s also an homeowner’s association meeting to prepare for and research to be done on ceiling fans.
“Life admin” is a crisp phrase coined by Elizabeth Emens, JD, PhD, that captures the never-ending to-do list that comes with running a household. An accomplished law professor at Columbia University, New York, Dr. Emens noticed the negative impact this life admin has on our quality of life. Reading her book, “Life Admin: How I Learned to Do Less, Do Better, and Live More” (New York: HarperOne, 2019), your eyes widen as she magically makes salient all this hidden work that is stealing our time. Life admin, kidmin, mom and dadmin, just rattling them off feels like donning x-ray glasses allowing us to see how much work we do outside of our work. As doctors, I would add “family house calls,” as a contributing factor: Random family and friends who want to talk for a minute about their knee replacement or what drug the ICU should give Uncle Larry who is fighting COVID. (I only know ivermectin, but it would only help if he just had scabies).
By all accounts, the amount of life admin is growing insidiously, worsened by the great pandemic. There are events to plan and reply to, more DIY customer service to fix your own problems, more work to find a VRBO for a weekend getaway at the beach. (There are none on the entire coast of California this summer, so I just saved you time there. You’re welcome.)
There is no good time to do this work and combined with the heavy burden of our responsibilities as physicians, it can feel like fuel feeding the burnout fire.
Dr. Emens has some top tips to help. First up, know your admin type. Are you a super doer, reluctant doer, admin denier, or admin avoider? I’m mostly in the avoider quadrant, dropping into reluctant doer when consequences loom. Next, choose strategies that fit you. Instead of avoiding, there are some things I might deflect. For example, When your aunt in Peoria asks where she can get a COVID test, you can use LMGTFY.com to generate a link that will show them how to use Google to help with their question. Dr. Emens is joking, but the point rang true. We can lighten the load a bit if we delegate or push back the excessive or undue requests. For some tasks, we’d be better off paying someone to take it over. Last tip here, try doing life admin with a partner, be it spouse, friend, or colleague. This is particularly useful when your partner is a super doer, as mine is. Not only can they make the work lighter, but also less dreary.
We physicians are focused on fixing physician burnout. Maybe we should also be looking at what happens in the “second shift” at home. Tax season is over, but will be back soon.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Across the country, taxes unite us. Not that we all share the same, rather that we all have to do them. It was recently tax weekend in our house: The Saturday and Sunday that cap off weeks of hunting and gathering faded receipts and sorting through reams of credit card bills to find all the dollars we spent on work. The task is more tedious than all the Wednesdays of taking out trash bins combined, and equally as exciting. But wait, that’s not all.
This weekend I’ve been chatting with bots from a solar company trying to solve our drop in energy production and sat on terminal hold with apparently one person who answers the phone for Amazon. There’s also an homeowner’s association meeting to prepare for and research to be done on ceiling fans.
“Life admin” is a crisp phrase coined by Elizabeth Emens, JD, PhD, that captures the never-ending to-do list that comes with running a household. An accomplished law professor at Columbia University, New York, Dr. Emens noticed the negative impact this life admin has on our quality of life. Reading her book, “Life Admin: How I Learned to Do Less, Do Better, and Live More” (New York: HarperOne, 2019), your eyes widen as she magically makes salient all this hidden work that is stealing our time. Life admin, kidmin, mom and dadmin, just rattling them off feels like donning x-ray glasses allowing us to see how much work we do outside of our work. As doctors, I would add “family house calls,” as a contributing factor: Random family and friends who want to talk for a minute about their knee replacement or what drug the ICU should give Uncle Larry who is fighting COVID. (I only know ivermectin, but it would only help if he just had scabies).
By all accounts, the amount of life admin is growing insidiously, worsened by the great pandemic. There are events to plan and reply to, more DIY customer service to fix your own problems, more work to find a VRBO for a weekend getaway at the beach. (There are none on the entire coast of California this summer, so I just saved you time there. You’re welcome.)
There is no good time to do this work and combined with the heavy burden of our responsibilities as physicians, it can feel like fuel feeding the burnout fire.
Dr. Emens has some top tips to help. First up, know your admin type. Are you a super doer, reluctant doer, admin denier, or admin avoider? I’m mostly in the avoider quadrant, dropping into reluctant doer when consequences loom. Next, choose strategies that fit you. Instead of avoiding, there are some things I might deflect. For example, When your aunt in Peoria asks where she can get a COVID test, you can use LMGTFY.com to generate a link that will show them how to use Google to help with their question. Dr. Emens is joking, but the point rang true. We can lighten the load a bit if we delegate or push back the excessive or undue requests. For some tasks, we’d be better off paying someone to take it over. Last tip here, try doing life admin with a partner, be it spouse, friend, or colleague. This is particularly useful when your partner is a super doer, as mine is. Not only can they make the work lighter, but also less dreary.
We physicians are focused on fixing physician burnout. Maybe we should also be looking at what happens in the “second shift” at home. Tax season is over, but will be back soon.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
The unseen benefit of an MRI
Mrs. Smith came in for neck pain.
This isn’t a new issue, her last flare was 4 or 5 years ago. I’d done an MRI back then, which just showed reassuringly typical arthritic changes, and she did great with a few sessions of physical therapy.
She’d woke one morning a few months ago with a stiff and aching neck, similar to how this started last time. A couple weeks of rest and NSAIDs hadn’t helped. There were no radiating symptoms and her exam was the same as it’s been since I met her back in 2010.
I wrote her an order for physical therapy and found the address and phone number of the place she’d gone to for it a few years ago. She looked at my order, then set it on my desk and said “Doctor, I’d really like an MRI.”
I went back to her chart and reread my note for her last flare of neck pain. Identical symptoms, identical exam. I pulled up the previous MRI report and went over it. Then I explained to her that there really was no indication for an MRI at this point. I suggested we go ahead with physical therapy, and if that didn’t help I would then re-check the study.
She wasn’t going to budge. A friend of hers had recently needed urgent surgery for a cervical myelopathy and was in rehab. Mrs. Smith’s husband’s health was getting worse, and if her neck had something seriously wrong she wouldn’t be able to take care of him if it went unchecked.
So I backed down and ordered a cervical spine MRI, which was pretty much unchanged from the previous MRI. After it came back she was willing to do therapy.
I’m sure some out there will accuse me, the doctor, of letting the patient call the shots. To some degree you’re correct. But it’s not like the request was insanely unreasonable. Obviously, there were other factors going on, too. She was scared and needed reassurance that there wasn’t anything therapy wouldn’t help and that she would be able to keep caring for her ailing husband during his cancer treatments.
There are doctors out there with a more paternalistic view of patient care than mine. They’re probably thinking I should have taken a hardball approach of “you don’t need an MRI. You can do therapy, or you can find another doctor.” But that’s not me. I can’t do that to a nice older lady, especially one who’s been coming to me for various ailments over the last 12 years.
Not only that, but such an approach seemed doomed to fail in this case. It might have gotten her to go to therapy, but I suspect she wouldn’t have gotten better. Her fears about a serious neck issue would increase over time, until she (or the therapist) finally called, said she wasn’t getting better, and could I order an MRI now?
In that way, maybe the MRI helped guarantee that she’d have a good response to therapy.
I can’t say that what I did was the right thing. But it was right for Mrs. Smith.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Smith came in for neck pain.
This isn’t a new issue, her last flare was 4 or 5 years ago. I’d done an MRI back then, which just showed reassuringly typical arthritic changes, and she did great with a few sessions of physical therapy.
She’d woke one morning a few months ago with a stiff and aching neck, similar to how this started last time. A couple weeks of rest and NSAIDs hadn’t helped. There were no radiating symptoms and her exam was the same as it’s been since I met her back in 2010.
I wrote her an order for physical therapy and found the address and phone number of the place she’d gone to for it a few years ago. She looked at my order, then set it on my desk and said “Doctor, I’d really like an MRI.”
I went back to her chart and reread my note for her last flare of neck pain. Identical symptoms, identical exam. I pulled up the previous MRI report and went over it. Then I explained to her that there really was no indication for an MRI at this point. I suggested we go ahead with physical therapy, and if that didn’t help I would then re-check the study.
She wasn’t going to budge. A friend of hers had recently needed urgent surgery for a cervical myelopathy and was in rehab. Mrs. Smith’s husband’s health was getting worse, and if her neck had something seriously wrong she wouldn’t be able to take care of him if it went unchecked.
So I backed down and ordered a cervical spine MRI, which was pretty much unchanged from the previous MRI. After it came back she was willing to do therapy.
I’m sure some out there will accuse me, the doctor, of letting the patient call the shots. To some degree you’re correct. But it’s not like the request was insanely unreasonable. Obviously, there were other factors going on, too. She was scared and needed reassurance that there wasn’t anything therapy wouldn’t help and that she would be able to keep caring for her ailing husband during his cancer treatments.
There are doctors out there with a more paternalistic view of patient care than mine. They’re probably thinking I should have taken a hardball approach of “you don’t need an MRI. You can do therapy, or you can find another doctor.” But that’s not me. I can’t do that to a nice older lady, especially one who’s been coming to me for various ailments over the last 12 years.
Not only that, but such an approach seemed doomed to fail in this case. It might have gotten her to go to therapy, but I suspect she wouldn’t have gotten better. Her fears about a serious neck issue would increase over time, until she (or the therapist) finally called, said she wasn’t getting better, and could I order an MRI now?
In that way, maybe the MRI helped guarantee that she’d have a good response to therapy.
I can’t say that what I did was the right thing. But it was right for Mrs. Smith.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Smith came in for neck pain.
This isn’t a new issue, her last flare was 4 or 5 years ago. I’d done an MRI back then, which just showed reassuringly typical arthritic changes, and she did great with a few sessions of physical therapy.
She’d woke one morning a few months ago with a stiff and aching neck, similar to how this started last time. A couple weeks of rest and NSAIDs hadn’t helped. There were no radiating symptoms and her exam was the same as it’s been since I met her back in 2010.
I wrote her an order for physical therapy and found the address and phone number of the place she’d gone to for it a few years ago. She looked at my order, then set it on my desk and said “Doctor, I’d really like an MRI.”
I went back to her chart and reread my note for her last flare of neck pain. Identical symptoms, identical exam. I pulled up the previous MRI report and went over it. Then I explained to her that there really was no indication for an MRI at this point. I suggested we go ahead with physical therapy, and if that didn’t help I would then re-check the study.
She wasn’t going to budge. A friend of hers had recently needed urgent surgery for a cervical myelopathy and was in rehab. Mrs. Smith’s husband’s health was getting worse, and if her neck had something seriously wrong she wouldn’t be able to take care of him if it went unchecked.
So I backed down and ordered a cervical spine MRI, which was pretty much unchanged from the previous MRI. After it came back she was willing to do therapy.
I’m sure some out there will accuse me, the doctor, of letting the patient call the shots. To some degree you’re correct. But it’s not like the request was insanely unreasonable. Obviously, there were other factors going on, too. She was scared and needed reassurance that there wasn’t anything therapy wouldn’t help and that she would be able to keep caring for her ailing husband during his cancer treatments.
There are doctors out there with a more paternalistic view of patient care than mine. They’re probably thinking I should have taken a hardball approach of “you don’t need an MRI. You can do therapy, or you can find another doctor.” But that’s not me. I can’t do that to a nice older lady, especially one who’s been coming to me for various ailments over the last 12 years.
Not only that, but such an approach seemed doomed to fail in this case. It might have gotten her to go to therapy, but I suspect she wouldn’t have gotten better. Her fears about a serious neck issue would increase over time, until she (or the therapist) finally called, said she wasn’t getting better, and could I order an MRI now?
In that way, maybe the MRI helped guarantee that she’d have a good response to therapy.
I can’t say that what I did was the right thing. But it was right for Mrs. Smith.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
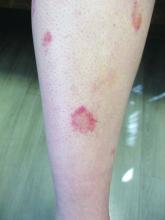
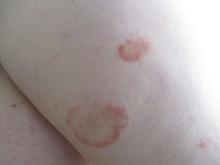
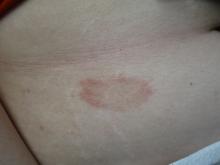
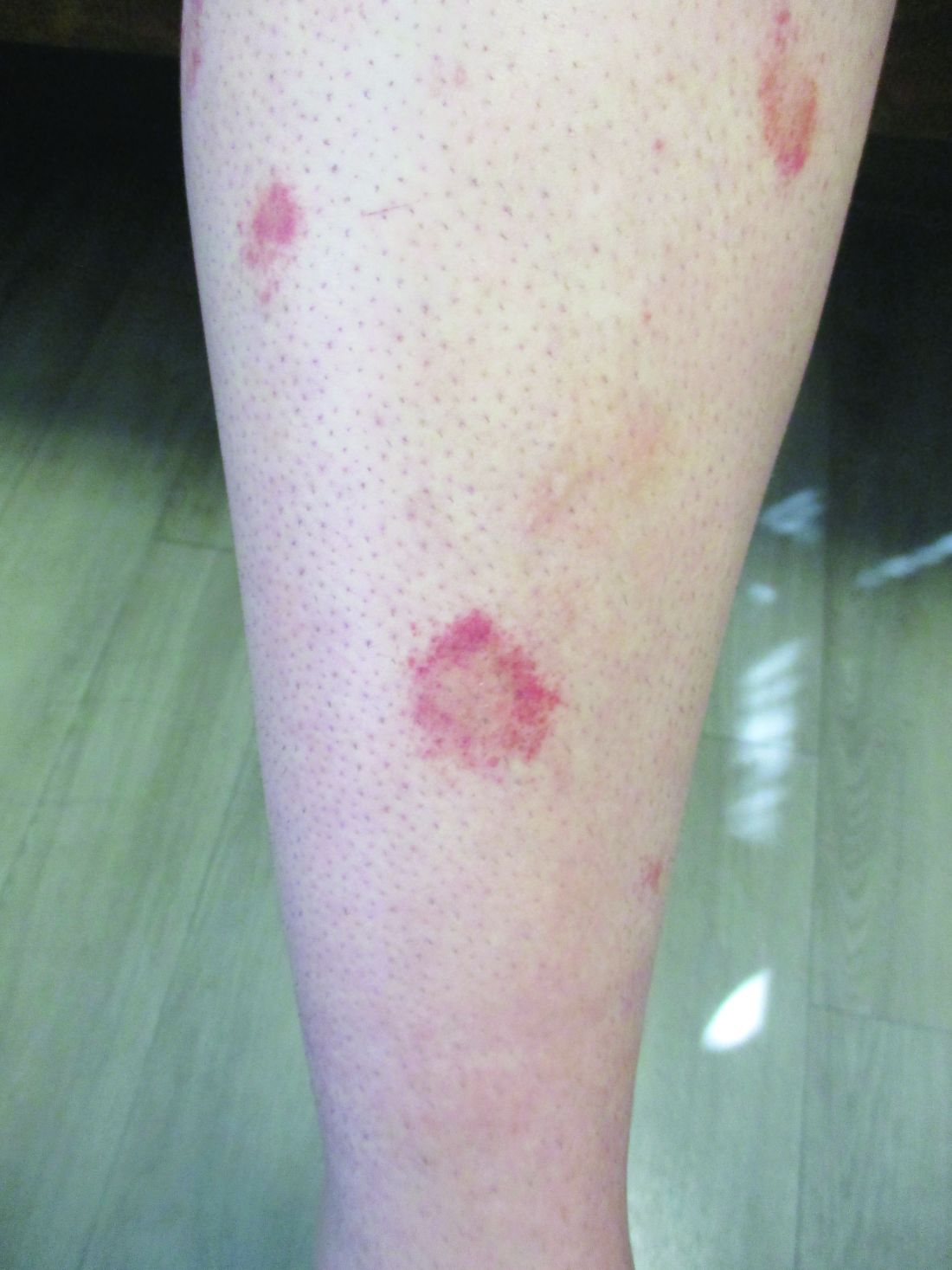
24-year-old female presents with a 3-month history of nonpruritic rash
, typically characterized by symmetrical, nonblanching, purpuric, telangiectatic, and atrophic patches with a predilection for the lower extremities and buttocks.
Plaques are usually 1-3 cm in diameter and annular with punctate telangiectasias and cayenne pepper petechiae in the border. The annular patches may form concentric rings. It is most commonly seen in children and young females.
The etiology of Majocchi’s disease is largely unknown and idiopathic.
Triggers are not always detected but may be associated with viral infections, chronic comorbidities, and medications. Levofloxacin and isotretinoin have been described in as reports as causing PATM. Other medications reported to cause PPD include sedatives, stimulants, antibiotics, NSAIDS, and cardiovascular drugs.
Diagnosis of PATM is clinical and histopathologic. Direct immunofluorescence (DIF) may show fibrinogen, IgM, and/or C3 deposition in superficial dermal vessels. Histopathologic findings show lymphocytic infiltrate involving the superficial small vessels, extravasated red blood cells, and hemosiderin-laden macrophages.
There is no consensus regarding treatment with variable responses to proposed treatment based on reports and case studies. The first line of treatment is topical corticosteroids and compression hose. Additional treatments, including narrowband UVB phototherapy (NBUVB), griseofulvin, pentoxifylline, cyclosporine, colchicine, rutoside with ascorbic acid, and methotrexate, have been used with varying success.
In this patient, a punch biopsy was performed, which revealed lymphocytes and extravasated erythrocytes and siderophages in the dermis. She was treated with topical steroids with improvement. She started NBUVB, a short course of griseofulvin, and vitamin C supplements.
This case and the photos were photo submitted by Ms. Xu, of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology. Dr. Donna Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Garcez A et al. An Bras Dermatol. Sep-Oct 2020;95(5):664-6. doi: 10.1016/j.abd.2020.02.007.
2. Asadbeigi S, Momtahen S. Pigmented purpuric dermatosis. PathologyOutlines.com website.
3. Martínez P et al. Actas Dermosifiliogr (Engl Ed). 2020 Apr;111(3):196-204. doi: 10.1016/j.ad.2019.02.013.
4. Hoesly FJ et al. Int J Dermatol. 2009 Oct;48(10):1129-33. doi: 10.1111/j.1365-4632.2009.04160.x.
, typically characterized by symmetrical, nonblanching, purpuric, telangiectatic, and atrophic patches with a predilection for the lower extremities and buttocks.
Plaques are usually 1-3 cm in diameter and annular with punctate telangiectasias and cayenne pepper petechiae in the border. The annular patches may form concentric rings. It is most commonly seen in children and young females.
The etiology of Majocchi’s disease is largely unknown and idiopathic.
Triggers are not always detected but may be associated with viral infections, chronic comorbidities, and medications. Levofloxacin and isotretinoin have been described in as reports as causing PATM. Other medications reported to cause PPD include sedatives, stimulants, antibiotics, NSAIDS, and cardiovascular drugs.
Diagnosis of PATM is clinical and histopathologic. Direct immunofluorescence (DIF) may show fibrinogen, IgM, and/or C3 deposition in superficial dermal vessels. Histopathologic findings show lymphocytic infiltrate involving the superficial small vessels, extravasated red blood cells, and hemosiderin-laden macrophages.
There is no consensus regarding treatment with variable responses to proposed treatment based on reports and case studies. The first line of treatment is topical corticosteroids and compression hose. Additional treatments, including narrowband UVB phototherapy (NBUVB), griseofulvin, pentoxifylline, cyclosporine, colchicine, rutoside with ascorbic acid, and methotrexate, have been used with varying success.
In this patient, a punch biopsy was performed, which revealed lymphocytes and extravasated erythrocytes and siderophages in the dermis. She was treated with topical steroids with improvement. She started NBUVB, a short course of griseofulvin, and vitamin C supplements.
This case and the photos were photo submitted by Ms. Xu, of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology. Dr. Donna Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Garcez A et al. An Bras Dermatol. Sep-Oct 2020;95(5):664-6. doi: 10.1016/j.abd.2020.02.007.
2. Asadbeigi S, Momtahen S. Pigmented purpuric dermatosis. PathologyOutlines.com website.
3. Martínez P et al. Actas Dermosifiliogr (Engl Ed). 2020 Apr;111(3):196-204. doi: 10.1016/j.ad.2019.02.013.
4. Hoesly FJ et al. Int J Dermatol. 2009 Oct;48(10):1129-33. doi: 10.1111/j.1365-4632.2009.04160.x.
, typically characterized by symmetrical, nonblanching, purpuric, telangiectatic, and atrophic patches with a predilection for the lower extremities and buttocks.
Plaques are usually 1-3 cm in diameter and annular with punctate telangiectasias and cayenne pepper petechiae in the border. The annular patches may form concentric rings. It is most commonly seen in children and young females.
The etiology of Majocchi’s disease is largely unknown and idiopathic.
Triggers are not always detected but may be associated with viral infections, chronic comorbidities, and medications. Levofloxacin and isotretinoin have been described in as reports as causing PATM. Other medications reported to cause PPD include sedatives, stimulants, antibiotics, NSAIDS, and cardiovascular drugs.
Diagnosis of PATM is clinical and histopathologic. Direct immunofluorescence (DIF) may show fibrinogen, IgM, and/or C3 deposition in superficial dermal vessels. Histopathologic findings show lymphocytic infiltrate involving the superficial small vessels, extravasated red blood cells, and hemosiderin-laden macrophages.
There is no consensus regarding treatment with variable responses to proposed treatment based on reports and case studies. The first line of treatment is topical corticosteroids and compression hose. Additional treatments, including narrowband UVB phototherapy (NBUVB), griseofulvin, pentoxifylline, cyclosporine, colchicine, rutoside with ascorbic acid, and methotrexate, have been used with varying success.
In this patient, a punch biopsy was performed, which revealed lymphocytes and extravasated erythrocytes and siderophages in the dermis. She was treated with topical steroids with improvement. She started NBUVB, a short course of griseofulvin, and vitamin C supplements.
This case and the photos were photo submitted by Ms. Xu, of the University of California, San Diego, and Dr. Sateesh, of San Diego Family Dermatology. Dr. Donna Bilu Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Garcez A et al. An Bras Dermatol. Sep-Oct 2020;95(5):664-6. doi: 10.1016/j.abd.2020.02.007.
2. Asadbeigi S, Momtahen S. Pigmented purpuric dermatosis. PathologyOutlines.com website.
3. Martínez P et al. Actas Dermosifiliogr (Engl Ed). 2020 Apr;111(3):196-204. doi: 10.1016/j.ad.2019.02.013.
4. Hoesly FJ et al. Int J Dermatol. 2009 Oct;48(10):1129-33. doi: 10.1111/j.1365-4632.2009.04160.x.
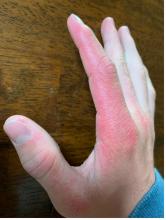
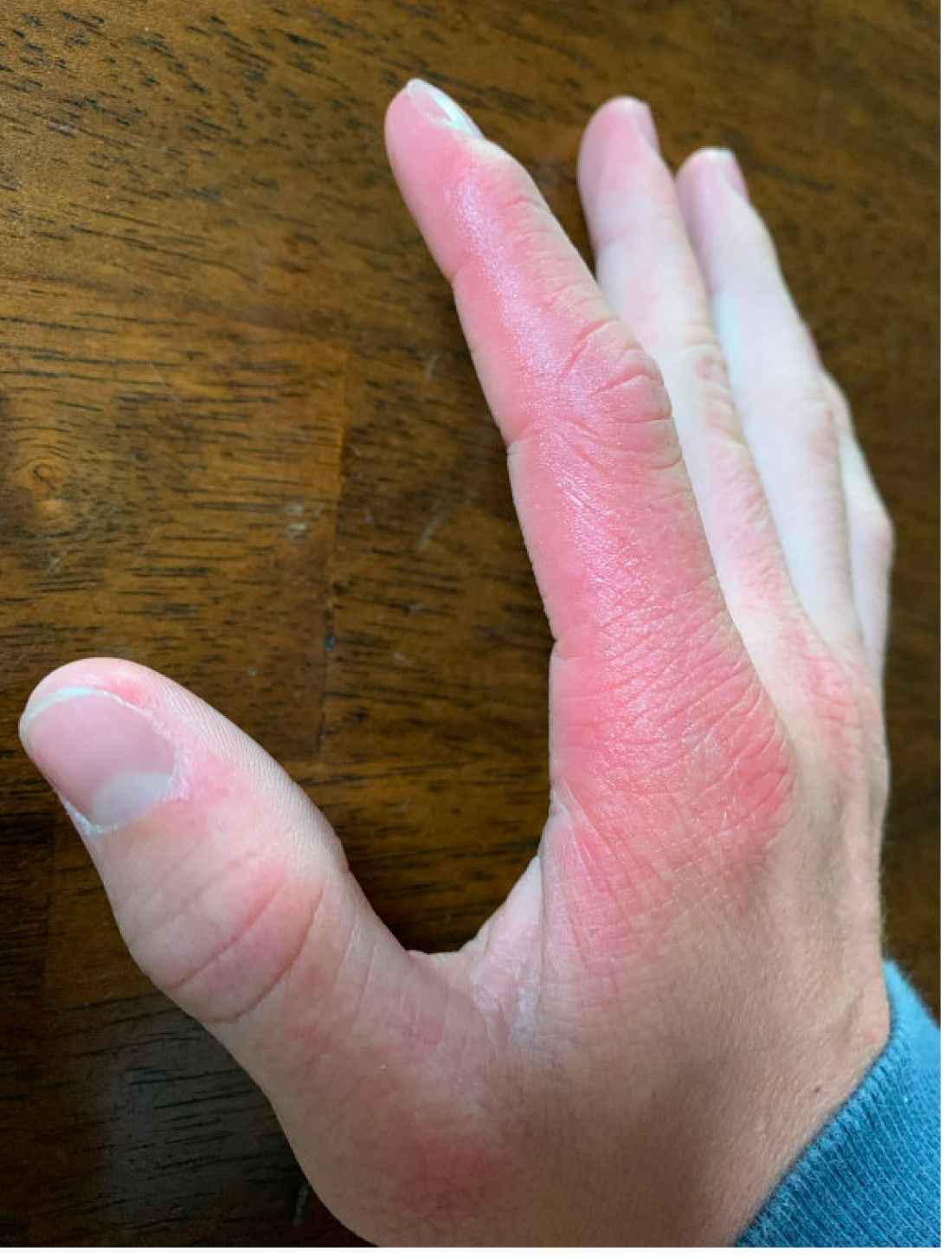
A 14-year-old male presents to clinic with a new-onset rash of the hands
Photosensitivity due to doxycycline
As the patient’s rash presented in sun-exposed areas with both skin and nail changes, our patient was diagnosed with a phototoxic reaction to doxycycline, the oral antibiotic used to treat his acne.
Photosensitive cutaneous drug eruptions are reactions that occur after exposure to a medication and subsequent exposure to UV radiation or visible light. Reactions can be classified into two ways based on their mechanism of action: phototoxic or photoallergic.1 Phototoxic reactions are more common and are a result of direct keratinocyte damage and cellular necrosis. Many classes of medications may cause this adverse effect, but the tetracycline class of antibiotics is a common culprit.2 Photoallergic reactions are less common and are a result of a type IV immune reaction to the offending agent.1
Phototoxic reactions generally present shortly after sun or UV exposure with a photo-distributed eruption pattern.3 Commonly involved areas include the face, the neck, and the extensor surfaces of extremities, with sparing of relatively protected skin such as the upper eyelids and the skin folds.2 Erythema may initially develop in the exposed skin areas, followed by appearance of edema, vesicles, or bullae.1-3 The eruption may be painful and itchy, with some patients reporting severe pain.3
Doxycycline phototoxicity may also cause onycholysis of the nails.2 The reaction is dose dependent, with higher doses of medication leading to a higher likelihood of symptoms.1,2 It is also more prevalent in patients with Fitzpatrick skin type I and II. The usual UVA wavelength required to induce this reaction appears to be in the 320-400 nm range of the UV spectrum.4 By contrast, photoallergic reactions are dose independent, and require a sensitization period prior to the eruption.1 An eczematous eruption is most commonly seen with photoallergic reactions.3
Treatment of drug-induced photosensitivity reactions requires proper identification of the diagnosis and the offending agent, followed by cessation of the medication. If cessation is not possible, then lowering the dose can help to minimize worsening of the condition. However, for photoallergic reactions, the reaction is dose independent so switching to another tolerated agent is likely required. For persistent symptoms following medication withdrawal, topical or systemic steroids and oral antihistamine can help with symptom management.1 For patients with photo-onycholysis, treatment involves stopping the medication and waiting for the intact nail plate to grow.
Prevention is key in the management of photosensitivity reactions. Patients should be counseled about the increased risk of photosensitivity while on tetracycline medications and encouraged to engage in enhanced sun protection measures such as wearing sun protective hats and clothing, increasing use of sunscreen that provides mainly UVA but also UVB protection, and avoiding the sun during the midday when the UV index is highest.1-3
Dermatomyositis
Dermatomyositis is an autoimmune condition that presents with skin lesions as well as systemic findings such as myositis. The cutaneous findings are variable, but pathognomonic findings include Gottron papules of the hands, Gottron’s sign on the elbows, knees, and ankles, and the heliotrope rash of the face. Eighty percent of patients have myopathy presenting as muscle weakness, and commonly have elevated creatine kinase, aspartate transaminase, and alanine transaminase values.5 Diagnosis may be confirmed through skin or muscle biopsy, though antibody studies can also play a helpful role in diagnosis. Treatment is generally with oral corticosteroids or other immunosuppressants as well as sun protection.6 The rash seen in our patient could have been seen in patients with dermatomyositis, though it was not in the typical location on the knuckles (Gottron papules) as it also affected the lateral sides of the fingers.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized by systemic and cutaneous manifestations. Systemic symptoms may include weight loss, fever, fatigue, arthralgia, or arthritis; patients are at risk of renal, cardiovascular, pulmonary, and neurologic complications of SLE.7 The most common cutaneous finding is malar rash, though there are myriad dermatologic manifestations that can occur associated with photosensitivity. Diagnosis is made based on history, physical, and laboratory testing. Treatment options include NSAIDs, oral glucocorticoids, antimalarial drugs, and immunosuppressants.7 Though our patient exhibited photosensitivity, he had none of the systemic findings associated with SLE, making this diagnosis unlikely.
Allergic contact dermatitis
Allergic contact dermatitis (ACD) is a type IV hypersensitivity reaction, and may present as acute, subacute, or chronic dermatitis. The clinical findings vary based on chronicity. Acute ACD presents as pruritic erythematous papules and vesicles or bullae, similar to how it occurred in our patient, though our patient’s lesions were more tender than pruritic. Chronic ACD presents with erythematous lesions with pruritis, lichenification, scaling, and/or fissuring. Observing shapes or sharp demarcation of lesions may help with diagnosis. Patch testing is also useful in the diagnosis of ACD.
Treatment generally involves avoiding the offending agent with topical corticosteroids for symptom management.8
Polymorphous light eruption
Polymorphous light eruption (PLE) is a delayed, type IV hypersensitivity reaction to UV-induced antigens, though these antigens are unknown. PLE presents hours to days following solar or UV exposure and presents only in sun-exposed areas. Itching and burning are always present, but lesion morphology varies from erythema and papules to vesico-papules and blisters. Notably, PLE must be distinguished from drug photosensitivity through history. Treatment generally involves symptom management with topical steroids and sun protective measures for prevention.9 While PLE may present similarly to drug photosensitivity reactions, our patient’s use of a known phototoxic agent makes PLE a less likely diagnosis.
Ms. Appiah is a pediatric dermatology research associate and medical student at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Neither Dr. Matiz nor Ms. Appiah has any relevant financial disclosures.
References
1. Montgomery S et al. Clin Dermatol. 2022;40(1):57-63.
2. Blakely KM et al. Drug Saf. 2019;42(7):827-47.
3. Goetze S et al. Skin Pharmacol Physiol. 2017;30(2):76-80.
4. Odorici G et al. Dermatol Ther. 2021;34(4):e14978.
5. DeWane ME et al. J Am Acad Dermatol. 2020;82(2):267-81.
6. Waldman R et al. J Am Acad Dermatol. 2020;82(2):283-96.
7. Kiriakidou M et al. Ann Intern Med. 2020;172(11):ITC81-ITC96.
8. Nassau S et al. Med Clin North Am. 2020;104(1):61-76.
9. Guarrera M. Adv Exp Med Biol. 2017;996:61-70.
Photosensitivity due to doxycycline
As the patient’s rash presented in sun-exposed areas with both skin and nail changes, our patient was diagnosed with a phototoxic reaction to doxycycline, the oral antibiotic used to treat his acne.
Photosensitive cutaneous drug eruptions are reactions that occur after exposure to a medication and subsequent exposure to UV radiation or visible light. Reactions can be classified into two ways based on their mechanism of action: phototoxic or photoallergic.1 Phototoxic reactions are more common and are a result of direct keratinocyte damage and cellular necrosis. Many classes of medications may cause this adverse effect, but the tetracycline class of antibiotics is a common culprit.2 Photoallergic reactions are less common and are a result of a type IV immune reaction to the offending agent.1
Phototoxic reactions generally present shortly after sun or UV exposure with a photo-distributed eruption pattern.3 Commonly involved areas include the face, the neck, and the extensor surfaces of extremities, with sparing of relatively protected skin such as the upper eyelids and the skin folds.2 Erythema may initially develop in the exposed skin areas, followed by appearance of edema, vesicles, or bullae.1-3 The eruption may be painful and itchy, with some patients reporting severe pain.3
Doxycycline phototoxicity may also cause onycholysis of the nails.2 The reaction is dose dependent, with higher doses of medication leading to a higher likelihood of symptoms.1,2 It is also more prevalent in patients with Fitzpatrick skin type I and II. The usual UVA wavelength required to induce this reaction appears to be in the 320-400 nm range of the UV spectrum.4 By contrast, photoallergic reactions are dose independent, and require a sensitization period prior to the eruption.1 An eczematous eruption is most commonly seen with photoallergic reactions.3
Treatment of drug-induced photosensitivity reactions requires proper identification of the diagnosis and the offending agent, followed by cessation of the medication. If cessation is not possible, then lowering the dose can help to minimize worsening of the condition. However, for photoallergic reactions, the reaction is dose independent so switching to another tolerated agent is likely required. For persistent symptoms following medication withdrawal, topical or systemic steroids and oral antihistamine can help with symptom management.1 For patients with photo-onycholysis, treatment involves stopping the medication and waiting for the intact nail plate to grow.
Prevention is key in the management of photosensitivity reactions. Patients should be counseled about the increased risk of photosensitivity while on tetracycline medications and encouraged to engage in enhanced sun protection measures such as wearing sun protective hats and clothing, increasing use of sunscreen that provides mainly UVA but also UVB protection, and avoiding the sun during the midday when the UV index is highest.1-3
Dermatomyositis
Dermatomyositis is an autoimmune condition that presents with skin lesions as well as systemic findings such as myositis. The cutaneous findings are variable, but pathognomonic findings include Gottron papules of the hands, Gottron’s sign on the elbows, knees, and ankles, and the heliotrope rash of the face. Eighty percent of patients have myopathy presenting as muscle weakness, and commonly have elevated creatine kinase, aspartate transaminase, and alanine transaminase values.5 Diagnosis may be confirmed through skin or muscle biopsy, though antibody studies can also play a helpful role in diagnosis. Treatment is generally with oral corticosteroids or other immunosuppressants as well as sun protection.6 The rash seen in our patient could have been seen in patients with dermatomyositis, though it was not in the typical location on the knuckles (Gottron papules) as it also affected the lateral sides of the fingers.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized by systemic and cutaneous manifestations. Systemic symptoms may include weight loss, fever, fatigue, arthralgia, or arthritis; patients are at risk of renal, cardiovascular, pulmonary, and neurologic complications of SLE.7 The most common cutaneous finding is malar rash, though there are myriad dermatologic manifestations that can occur associated with photosensitivity. Diagnosis is made based on history, physical, and laboratory testing. Treatment options include NSAIDs, oral glucocorticoids, antimalarial drugs, and immunosuppressants.7 Though our patient exhibited photosensitivity, he had none of the systemic findings associated with SLE, making this diagnosis unlikely.
Allergic contact dermatitis
Allergic contact dermatitis (ACD) is a type IV hypersensitivity reaction, and may present as acute, subacute, or chronic dermatitis. The clinical findings vary based on chronicity. Acute ACD presents as pruritic erythematous papules and vesicles or bullae, similar to how it occurred in our patient, though our patient’s lesions were more tender than pruritic. Chronic ACD presents with erythematous lesions with pruritis, lichenification, scaling, and/or fissuring. Observing shapes or sharp demarcation of lesions may help with diagnosis. Patch testing is also useful in the diagnosis of ACD.
Treatment generally involves avoiding the offending agent with topical corticosteroids for symptom management.8
Polymorphous light eruption
Polymorphous light eruption (PLE) is a delayed, type IV hypersensitivity reaction to UV-induced antigens, though these antigens are unknown. PLE presents hours to days following solar or UV exposure and presents only in sun-exposed areas. Itching and burning are always present, but lesion morphology varies from erythema and papules to vesico-papules and blisters. Notably, PLE must be distinguished from drug photosensitivity through history. Treatment generally involves symptom management with topical steroids and sun protective measures for prevention.9 While PLE may present similarly to drug photosensitivity reactions, our patient’s use of a known phototoxic agent makes PLE a less likely diagnosis.
Ms. Appiah is a pediatric dermatology research associate and medical student at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Neither Dr. Matiz nor Ms. Appiah has any relevant financial disclosures.
References
1. Montgomery S et al. Clin Dermatol. 2022;40(1):57-63.
2. Blakely KM et al. Drug Saf. 2019;42(7):827-47.
3. Goetze S et al. Skin Pharmacol Physiol. 2017;30(2):76-80.
4. Odorici G et al. Dermatol Ther. 2021;34(4):e14978.
5. DeWane ME et al. J Am Acad Dermatol. 2020;82(2):267-81.
6. Waldman R et al. J Am Acad Dermatol. 2020;82(2):283-96.
7. Kiriakidou M et al. Ann Intern Med. 2020;172(11):ITC81-ITC96.
8. Nassau S et al. Med Clin North Am. 2020;104(1):61-76.
9. Guarrera M. Adv Exp Med Biol. 2017;996:61-70.
Photosensitivity due to doxycycline
As the patient’s rash presented in sun-exposed areas with both skin and nail changes, our patient was diagnosed with a phototoxic reaction to doxycycline, the oral antibiotic used to treat his acne.
Photosensitive cutaneous drug eruptions are reactions that occur after exposure to a medication and subsequent exposure to UV radiation or visible light. Reactions can be classified into two ways based on their mechanism of action: phototoxic or photoallergic.1 Phototoxic reactions are more common and are a result of direct keratinocyte damage and cellular necrosis. Many classes of medications may cause this adverse effect, but the tetracycline class of antibiotics is a common culprit.2 Photoallergic reactions are less common and are a result of a type IV immune reaction to the offending agent.1
Phototoxic reactions generally present shortly after sun or UV exposure with a photo-distributed eruption pattern.3 Commonly involved areas include the face, the neck, and the extensor surfaces of extremities, with sparing of relatively protected skin such as the upper eyelids and the skin folds.2 Erythema may initially develop in the exposed skin areas, followed by appearance of edema, vesicles, or bullae.1-3 The eruption may be painful and itchy, with some patients reporting severe pain.3
Doxycycline phototoxicity may also cause onycholysis of the nails.2 The reaction is dose dependent, with higher doses of medication leading to a higher likelihood of symptoms.1,2 It is also more prevalent in patients with Fitzpatrick skin type I and II. The usual UVA wavelength required to induce this reaction appears to be in the 320-400 nm range of the UV spectrum.4 By contrast, photoallergic reactions are dose independent, and require a sensitization period prior to the eruption.1 An eczematous eruption is most commonly seen with photoallergic reactions.3
Treatment of drug-induced photosensitivity reactions requires proper identification of the diagnosis and the offending agent, followed by cessation of the medication. If cessation is not possible, then lowering the dose can help to minimize worsening of the condition. However, for photoallergic reactions, the reaction is dose independent so switching to another tolerated agent is likely required. For persistent symptoms following medication withdrawal, topical or systemic steroids and oral antihistamine can help with symptom management.1 For patients with photo-onycholysis, treatment involves stopping the medication and waiting for the intact nail plate to grow.
Prevention is key in the management of photosensitivity reactions. Patients should be counseled about the increased risk of photosensitivity while on tetracycline medications and encouraged to engage in enhanced sun protection measures such as wearing sun protective hats and clothing, increasing use of sunscreen that provides mainly UVA but also UVB protection, and avoiding the sun during the midday when the UV index is highest.1-3
Dermatomyositis
Dermatomyositis is an autoimmune condition that presents with skin lesions as well as systemic findings such as myositis. The cutaneous findings are variable, but pathognomonic findings include Gottron papules of the hands, Gottron’s sign on the elbows, knees, and ankles, and the heliotrope rash of the face. Eighty percent of patients have myopathy presenting as muscle weakness, and commonly have elevated creatine kinase, aspartate transaminase, and alanine transaminase values.5 Diagnosis may be confirmed through skin or muscle biopsy, though antibody studies can also play a helpful role in diagnosis. Treatment is generally with oral corticosteroids or other immunosuppressants as well as sun protection.6 The rash seen in our patient could have been seen in patients with dermatomyositis, though it was not in the typical location on the knuckles (Gottron papules) as it also affected the lateral sides of the fingers.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune condition characterized by systemic and cutaneous manifestations. Systemic symptoms may include weight loss, fever, fatigue, arthralgia, or arthritis; patients are at risk of renal, cardiovascular, pulmonary, and neurologic complications of SLE.7 The most common cutaneous finding is malar rash, though there are myriad dermatologic manifestations that can occur associated with photosensitivity. Diagnosis is made based on history, physical, and laboratory testing. Treatment options include NSAIDs, oral glucocorticoids, antimalarial drugs, and immunosuppressants.7 Though our patient exhibited photosensitivity, he had none of the systemic findings associated with SLE, making this diagnosis unlikely.
Allergic contact dermatitis
Allergic contact dermatitis (ACD) is a type IV hypersensitivity reaction, and may present as acute, subacute, or chronic dermatitis. The clinical findings vary based on chronicity. Acute ACD presents as pruritic erythematous papules and vesicles or bullae, similar to how it occurred in our patient, though our patient’s lesions were more tender than pruritic. Chronic ACD presents with erythematous lesions with pruritis, lichenification, scaling, and/or fissuring. Observing shapes or sharp demarcation of lesions may help with diagnosis. Patch testing is also useful in the diagnosis of ACD.
Treatment generally involves avoiding the offending agent with topical corticosteroids for symptom management.8
Polymorphous light eruption
Polymorphous light eruption (PLE) is a delayed, type IV hypersensitivity reaction to UV-induced antigens, though these antigens are unknown. PLE presents hours to days following solar or UV exposure and presents only in sun-exposed areas. Itching and burning are always present, but lesion morphology varies from erythema and papules to vesico-papules and blisters. Notably, PLE must be distinguished from drug photosensitivity through history. Treatment generally involves symptom management with topical steroids and sun protective measures for prevention.9 While PLE may present similarly to drug photosensitivity reactions, our patient’s use of a known phototoxic agent makes PLE a less likely diagnosis.
Ms. Appiah is a pediatric dermatology research associate and medical student at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Neither Dr. Matiz nor Ms. Appiah has any relevant financial disclosures.
References
1. Montgomery S et al. Clin Dermatol. 2022;40(1):57-63.
2. Blakely KM et al. Drug Saf. 2019;42(7):827-47.
3. Goetze S et al. Skin Pharmacol Physiol. 2017;30(2):76-80.
4. Odorici G et al. Dermatol Ther. 2021;34(4):e14978.
5. DeWane ME et al. J Am Acad Dermatol. 2020;82(2):267-81.
6. Waldman R et al. J Am Acad Dermatol. 2020;82(2):283-96.
7. Kiriakidou M et al. Ann Intern Med. 2020;172(11):ITC81-ITC96.
8. Nassau S et al. Med Clin North Am. 2020;104(1):61-76.
9. Guarrera M. Adv Exp Med Biol. 2017;996:61-70.
He reported no hiking or gardening, no new topical products such as new sunscreens or lotions, and no new medications. The patient had a history of acne, for which he used over-the-counter benzoyl peroxide wash, adapalene gel, and an oral antibiotic for 3 months. His review of systems was negative for fevers, chills, muscle weakness, mouth sores, or joint pain and no prior rashes following sun exposure.
On physical exam he presented with pink plaques with thin vesicles on the dorsum of the hands that were more noticeable on the lateral aspect of both the first and second fingers (Figures 1 and 2). His nails also had a yellow discoloration.
Ukraine and PTSD: How psychiatry can help
The war in Ukraine is resulting in a devastating loss of life, catastrophic injuries, and physical destruction. But the war also will take an enormous mental health toll on millions of people, resulting in what I think will lead to an epidemic of posttraumatic stress disorder.
Think about the horrors that Ukrainians are experiencing. Millions of Ukrainians have been displaced to locations inside and outside of the country. People are being forced to leave behind family members, neighbors, and their pets and homes. In one recent news report, a Ukrainian woman who left Kyiv for Belgium reported having dreams in which she heard explosions. Smells, sounds, and even colors can trigger intrusive memories and a host of other problems. The mind can barely comprehend the scope of this human crisis.
Ukrainian soldiers are witnessing horrors that are unspeakable. Doctors, emergency service workers, and other medical professionals in Ukraine are being exposed to the catastrophe on a large scale. Children and youth are among the most affected victims, and it is difficult to predict the impact all of this upheaval is having on them.
The most important question for those of us who treat mental illness is “how will we help devastated people suffering from extreme trauma tied to death, dying, severe injuries, and torture by the invading soldiers?”
I have been treating patients with PTSD for many years. In my lifetime, the devastation in Ukraine will translate into what I expect will be the first overwhelming mass epidemic of PTSD – at least that I can recall. Yes, surely PTSD occurred during and after the Holocaust in the World War II era, but at that time, the mental health profession was not equipped to recognize it – even though the disorder most certainly existed. Even in ancient times, an Assyrian text from Mesopotamia (currently Iraq) described what we would define as PTSD symptoms in soldiers, such as sleep disturbances, flashbacks, and “low mood,” according to a 2014 article in the journal Early Science and Medicine.
The DSM-5 describes numerous criteria for PTSD mainly centering on trauma exposing a person to actual or threatened death, serious injury, or a variety of assaults, including direct exposure or witnessing the event. However, in my clinical experience, I’ve seen lesser events leading to PTSD. Much depends on how each individual processes what is occurring or has occurred.
What appears to be clear is that some key aspects of PTSD according to the DSM-5 – such as trauma-related thoughts or feelings, or trauma-related reminders, as well as nightmares and flashbacks – are likely occurring among Ukrainians. In addition, hypervigilance and exaggerated startle response seem to be key components of PTSD whether or not the cause is a major event or what one would perceive as less traumatic or dramatic.
I’ve certainly seen PTSD secondary to a hospitalization, especially in care involving ICUs or cardiac care units. In addition, I’ve had the occasion to note PTSD signs and symptoms after financial loss or divorce, situations in which some clinicians would never believe PTSD would occur, and would often diagnose as anxiety or depression. For me, again from a clinical point of view, it’s always been critical to assess how individuals process the event or events around them.
We know that there is already a shortage of mental health clinicians across the globe. This means that, in light of the hundreds of thousands – possibly millions – of Ukrainians affected by PTSD, a one-to-one approach will not do. For those Ukrainians who are able to find safe havens, I believe that PTSD symptoms can be debilitating, and the mental health community needs to begin putting supports in place now to address this trauma.
Specifically, proven cognitive-behavioral therapy (CBT) and guided imagery should be used to begin helping some of these people recover from the unbelievable trauma of war. For some, medication management might be helpful in those experiencing nightmares combined with anxiety and depression. But the main approach and first line of care should be CBT and guided imagery.
PTSD symptoms can make people feel like they are losing control, and prevent them from rebuilding their lives. We must do all we can in the mental health community to destigmatize care and develop support services to get ahead of this crisis. Only through medical, psychiatric, and health care organizations banding together using modern technology can the large number of people psychologically affected by this ongoing crisis be helped and saved.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The war in Ukraine is resulting in a devastating loss of life, catastrophic injuries, and physical destruction. But the war also will take an enormous mental health toll on millions of people, resulting in what I think will lead to an epidemic of posttraumatic stress disorder.
Think about the horrors that Ukrainians are experiencing. Millions of Ukrainians have been displaced to locations inside and outside of the country. People are being forced to leave behind family members, neighbors, and their pets and homes. In one recent news report, a Ukrainian woman who left Kyiv for Belgium reported having dreams in which she heard explosions. Smells, sounds, and even colors can trigger intrusive memories and a host of other problems. The mind can barely comprehend the scope of this human crisis.
Ukrainian soldiers are witnessing horrors that are unspeakable. Doctors, emergency service workers, and other medical professionals in Ukraine are being exposed to the catastrophe on a large scale. Children and youth are among the most affected victims, and it is difficult to predict the impact all of this upheaval is having on them.
The most important question for those of us who treat mental illness is “how will we help devastated people suffering from extreme trauma tied to death, dying, severe injuries, and torture by the invading soldiers?”
I have been treating patients with PTSD for many years. In my lifetime, the devastation in Ukraine will translate into what I expect will be the first overwhelming mass epidemic of PTSD – at least that I can recall. Yes, surely PTSD occurred during and after the Holocaust in the World War II era, but at that time, the mental health profession was not equipped to recognize it – even though the disorder most certainly existed. Even in ancient times, an Assyrian text from Mesopotamia (currently Iraq) described what we would define as PTSD symptoms in soldiers, such as sleep disturbances, flashbacks, and “low mood,” according to a 2014 article in the journal Early Science and Medicine.
The DSM-5 describes numerous criteria for PTSD mainly centering on trauma exposing a person to actual or threatened death, serious injury, or a variety of assaults, including direct exposure or witnessing the event. However, in my clinical experience, I’ve seen lesser events leading to PTSD. Much depends on how each individual processes what is occurring or has occurred.
What appears to be clear is that some key aspects of PTSD according to the DSM-5 – such as trauma-related thoughts or feelings, or trauma-related reminders, as well as nightmares and flashbacks – are likely occurring among Ukrainians. In addition, hypervigilance and exaggerated startle response seem to be key components of PTSD whether or not the cause is a major event or what one would perceive as less traumatic or dramatic.
I’ve certainly seen PTSD secondary to a hospitalization, especially in care involving ICUs or cardiac care units. In addition, I’ve had the occasion to note PTSD signs and symptoms after financial loss or divorce, situations in which some clinicians would never believe PTSD would occur, and would often diagnose as anxiety or depression. For me, again from a clinical point of view, it’s always been critical to assess how individuals process the event or events around them.
We know that there is already a shortage of mental health clinicians across the globe. This means that, in light of the hundreds of thousands – possibly millions – of Ukrainians affected by PTSD, a one-to-one approach will not do. For those Ukrainians who are able to find safe havens, I believe that PTSD symptoms can be debilitating, and the mental health community needs to begin putting supports in place now to address this trauma.
Specifically, proven cognitive-behavioral therapy (CBT) and guided imagery should be used to begin helping some of these people recover from the unbelievable trauma of war. For some, medication management might be helpful in those experiencing nightmares combined with anxiety and depression. But the main approach and first line of care should be CBT and guided imagery.
PTSD symptoms can make people feel like they are losing control, and prevent them from rebuilding their lives. We must do all we can in the mental health community to destigmatize care and develop support services to get ahead of this crisis. Only through medical, psychiatric, and health care organizations banding together using modern technology can the large number of people psychologically affected by this ongoing crisis be helped and saved.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The war in Ukraine is resulting in a devastating loss of life, catastrophic injuries, and physical destruction. But the war also will take an enormous mental health toll on millions of people, resulting in what I think will lead to an epidemic of posttraumatic stress disorder.
Think about the horrors that Ukrainians are experiencing. Millions of Ukrainians have been displaced to locations inside and outside of the country. People are being forced to leave behind family members, neighbors, and their pets and homes. In one recent news report, a Ukrainian woman who left Kyiv for Belgium reported having dreams in which she heard explosions. Smells, sounds, and even colors can trigger intrusive memories and a host of other problems. The mind can barely comprehend the scope of this human crisis.
Ukrainian soldiers are witnessing horrors that are unspeakable. Doctors, emergency service workers, and other medical professionals in Ukraine are being exposed to the catastrophe on a large scale. Children and youth are among the most affected victims, and it is difficult to predict the impact all of this upheaval is having on them.
The most important question for those of us who treat mental illness is “how will we help devastated people suffering from extreme trauma tied to death, dying, severe injuries, and torture by the invading soldiers?”
I have been treating patients with PTSD for many years. In my lifetime, the devastation in Ukraine will translate into what I expect will be the first overwhelming mass epidemic of PTSD – at least that I can recall. Yes, surely PTSD occurred during and after the Holocaust in the World War II era, but at that time, the mental health profession was not equipped to recognize it – even though the disorder most certainly existed. Even in ancient times, an Assyrian text from Mesopotamia (currently Iraq) described what we would define as PTSD symptoms in soldiers, such as sleep disturbances, flashbacks, and “low mood,” according to a 2014 article in the journal Early Science and Medicine.
The DSM-5 describes numerous criteria for PTSD mainly centering on trauma exposing a person to actual or threatened death, serious injury, or a variety of assaults, including direct exposure or witnessing the event. However, in my clinical experience, I’ve seen lesser events leading to PTSD. Much depends on how each individual processes what is occurring or has occurred.
What appears to be clear is that some key aspects of PTSD according to the DSM-5 – such as trauma-related thoughts or feelings, or trauma-related reminders, as well as nightmares and flashbacks – are likely occurring among Ukrainians. In addition, hypervigilance and exaggerated startle response seem to be key components of PTSD whether or not the cause is a major event or what one would perceive as less traumatic or dramatic.
I’ve certainly seen PTSD secondary to a hospitalization, especially in care involving ICUs or cardiac care units. In addition, I’ve had the occasion to note PTSD signs and symptoms after financial loss or divorce, situations in which some clinicians would never believe PTSD would occur, and would often diagnose as anxiety or depression. For me, again from a clinical point of view, it’s always been critical to assess how individuals process the event or events around them.
We know that there is already a shortage of mental health clinicians across the globe. This means that, in light of the hundreds of thousands – possibly millions – of Ukrainians affected by PTSD, a one-to-one approach will not do. For those Ukrainians who are able to find safe havens, I believe that PTSD symptoms can be debilitating, and the mental health community needs to begin putting supports in place now to address this trauma.
Specifically, proven cognitive-behavioral therapy (CBT) and guided imagery should be used to begin helping some of these people recover from the unbelievable trauma of war. For some, medication management might be helpful in those experiencing nightmares combined with anxiety and depression. But the main approach and first line of care should be CBT and guided imagery.
PTSD symptoms can make people feel like they are losing control, and prevent them from rebuilding their lives. We must do all we can in the mental health community to destigmatize care and develop support services to get ahead of this crisis. Only through medical, psychiatric, and health care organizations banding together using modern technology can the large number of people psychologically affected by this ongoing crisis be helped and saved.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Unraveling primary ovarian insufficiency
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
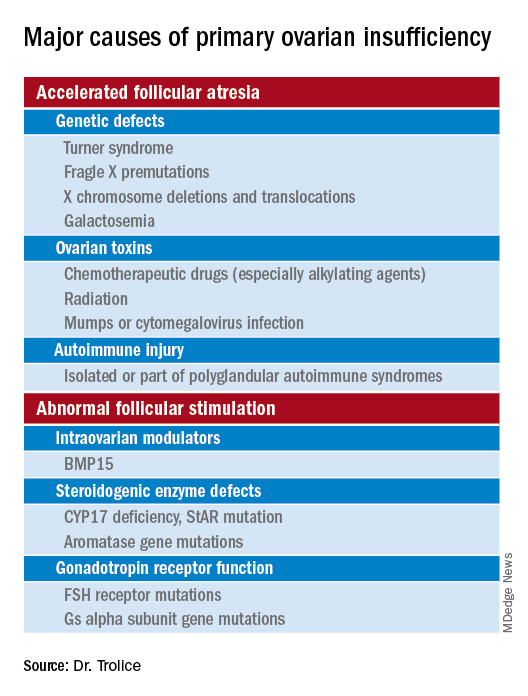
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years
Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Depression in homeless patients: What can be done?
In a recent article published in JAMA Psychiatry, Joshua E. J. Buckman and coauthors described the results of a large research study which concludes that depression is harder to treat in those who are homeless or unemployed.
It is always good to get more data and this article adds to the literature about the social determinants of depression. A frustrating aspect is that this is no surprise at all, not least for anyone in the mental health field. We have known that intuitively for decades.
Again, data is always good to bolster intuition with science. But what are the actionable items to take from the paper?
However, there are a few policy and clinical points I would like to make, reflecting some of the chapters in a recently published book – edited by me and my colleague Maria D. Llorente – “Clinical Management of the Homeless Patient: Social Medical and Psychiatric Issues” (New York: Springer, May 2021).
The first is, if you really tackle homelessness, with a combination of federal, state, and local resources, you can make a difference. The Department of Veterans Affairs, under the leadership of former VA Secretary Eric Shinseki and others, has been markedly successful. Note, for instance, the Health Care for Homeless Veterans program , which conducts outreach to vulnerable veterans not currently receiving services and engages them in treatment and rehabilitative programs.
Secondly, there is a marked absence of shelters that can care for the homeless with medical problems. This leads to extended and extensive hospital stays. This is especially frustrating during the COVID era, when hospital beds are in such short supply. Having a safe place to discharge patients who still need wound or diabetes care would save money for the overall health care system and be best for the patient.
Third, it may be best to modify discharge regimens for those patients who are unhoused. For example, metformin, taken by mouth once a day, is more practical for unhoused patients with diabetes than insulin, which needs to be refrigerated and injected multiple times a day. While one can argue whether care for the homeless should differ from those who are housed, in practical terms, simplifying regimens is more likely to promote compliance.
My last take-home point is check the Feet. So many of our homeless patients who end up on hospital wards have been wearing ill-fitting or no shoes while they are out on the street. Their toenails may be long and thick. They may have cellulitis or ulcers. Or gangrene. Unfortunately, these medical issues can also cause surgical amputations of the lower extremities.
Back to the article by Buckman and colleagues. The data they provide is good to have. But we need more action to provide appropriate and compassionate care for those who are unhoused and ill – care that is good for them, good for the nation’s finances, and good for our moral standing in the world.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She is a member of the Clinical Psychiatry News editorial advisory board, and has no conflicts of interest.
In a recent article published in JAMA Psychiatry, Joshua E. J. Buckman and coauthors described the results of a large research study which concludes that depression is harder to treat in those who are homeless or unemployed.
It is always good to get more data and this article adds to the literature about the social determinants of depression. A frustrating aspect is that this is no surprise at all, not least for anyone in the mental health field. We have known that intuitively for decades.
Again, data is always good to bolster intuition with science. But what are the actionable items to take from the paper?
However, there are a few policy and clinical points I would like to make, reflecting some of the chapters in a recently published book – edited by me and my colleague Maria D. Llorente – “Clinical Management of the Homeless Patient: Social Medical and Psychiatric Issues” (New York: Springer, May 2021).
The first is, if you really tackle homelessness, with a combination of federal, state, and local resources, you can make a difference. The Department of Veterans Affairs, under the leadership of former VA Secretary Eric Shinseki and others, has been markedly successful. Note, for instance, the Health Care for Homeless Veterans program , which conducts outreach to vulnerable veterans not currently receiving services and engages them in treatment and rehabilitative programs.
Secondly, there is a marked absence of shelters that can care for the homeless with medical problems. This leads to extended and extensive hospital stays. This is especially frustrating during the COVID era, when hospital beds are in such short supply. Having a safe place to discharge patients who still need wound or diabetes care would save money for the overall health care system and be best for the patient.
Third, it may be best to modify discharge regimens for those patients who are unhoused. For example, metformin, taken by mouth once a day, is more practical for unhoused patients with diabetes than insulin, which needs to be refrigerated and injected multiple times a day. While one can argue whether care for the homeless should differ from those who are housed, in practical terms, simplifying regimens is more likely to promote compliance.
My last take-home point is check the Feet. So many of our homeless patients who end up on hospital wards have been wearing ill-fitting or no shoes while they are out on the street. Their toenails may be long and thick. They may have cellulitis or ulcers. Or gangrene. Unfortunately, these medical issues can also cause surgical amputations of the lower extremities.
Back to the article by Buckman and colleagues. The data they provide is good to have. But we need more action to provide appropriate and compassionate care for those who are unhoused and ill – care that is good for them, good for the nation’s finances, and good for our moral standing in the world.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She is a member of the Clinical Psychiatry News editorial advisory board, and has no conflicts of interest.
In a recent article published in JAMA Psychiatry, Joshua E. J. Buckman and coauthors described the results of a large research study which concludes that depression is harder to treat in those who are homeless or unemployed.
It is always good to get more data and this article adds to the literature about the social determinants of depression. A frustrating aspect is that this is no surprise at all, not least for anyone in the mental health field. We have known that intuitively for decades.
Again, data is always good to bolster intuition with science. But what are the actionable items to take from the paper?
However, there are a few policy and clinical points I would like to make, reflecting some of the chapters in a recently published book – edited by me and my colleague Maria D. Llorente – “Clinical Management of the Homeless Patient: Social Medical and Psychiatric Issues” (New York: Springer, May 2021).
The first is, if you really tackle homelessness, with a combination of federal, state, and local resources, you can make a difference. The Department of Veterans Affairs, under the leadership of former VA Secretary Eric Shinseki and others, has been markedly successful. Note, for instance, the Health Care for Homeless Veterans program , which conducts outreach to vulnerable veterans not currently receiving services and engages them in treatment and rehabilitative programs.
Secondly, there is a marked absence of shelters that can care for the homeless with medical problems. This leads to extended and extensive hospital stays. This is especially frustrating during the COVID era, when hospital beds are in such short supply. Having a safe place to discharge patients who still need wound or diabetes care would save money for the overall health care system and be best for the patient.
Third, it may be best to modify discharge regimens for those patients who are unhoused. For example, metformin, taken by mouth once a day, is more practical for unhoused patients with diabetes than insulin, which needs to be refrigerated and injected multiple times a day. While one can argue whether care for the homeless should differ from those who are housed, in practical terms, simplifying regimens is more likely to promote compliance.
My last take-home point is check the Feet. So many of our homeless patients who end up on hospital wards have been wearing ill-fitting or no shoes while they are out on the street. Their toenails may be long and thick. They may have cellulitis or ulcers. Or gangrene. Unfortunately, these medical issues can also cause surgical amputations of the lower extremities.
Back to the article by Buckman and colleagues. The data they provide is good to have. But we need more action to provide appropriate and compassionate care for those who are unhoused and ill – care that is good for them, good for the nation’s finances, and good for our moral standing in the world.
Dr. Ritchie is chair of psychiatry at Medstar Washington (D.C.) Hospital Center. She is a member of the Clinical Psychiatry News editorial advisory board, and has no conflicts of interest.
Weigh but don’t tell
Reports of long waiting times at mental health clinics and anecdotal observations by health care providers suggest the pandemic has generated a dramatic increase in the incidence of eating disorders among the pediatric population. Of course this should come as no surprise to pediatricians.
Eating disorders come in many different forms and a triggering event is sometimes difficult to define. Often the adolescent or preadolescent is searching for some sense of stability in a life tossed on a stormy sea roiled by hormonal and physical change. Wresting control of their bodies during a period of uncertainty may result in a downward spiral into dangerously unhealthy weight loss. If nothing else, the pandemic has been a period of dramatic uncertainty unlike what most children and few adults in this country have ever experienced.
With the unprecedented increase in eating disorder cases, providers in several disciplines are searching for novel strategies to ease the burden on their patients and their practices. I recently learned of a pediatric practice in California that is considering blinding all patients aged 12 and older to the body mass measurements obtained at their health maintenance visits.
Blind weight checks for children with eating disorders, particularly those who seem to be nearing recovery, has been a common and often helpful practice. However, I am unaware of any practice that has made it a universal office policy. I’m unsure of the rationale behind this practice’s policy, but on several fronts, suppressing body mass measurements in the age group most vulnerable to eating disorders makes some sense.
Universal blind weight checks could minimize the risk of in-office shaming. However, careful training of support staff and thoughtful placement of the scales could serve the same purpose. This new policy acknowledges not only the ubiquity of the problem but also that many, maybe even most, children with eating disorders appear normal. And of course, there is the unfortunate fact that body mass is a poor screening test for eating disorders.
As I thought more about this novel approach I came to see its educational value for patients, parents, and even physicians. I can envision how a 13-year-old’s first health maintenance visit would go after the roll-out of the new policy. “Dr. Smith, aren’t you going to tell us how much I (or my daughter Jenny) weigh(s)?” This could, or more likely, should launch a discussion about weight and body image. It might continue with questions like, “How much do you think you weigh?” Or, “Do you think you are too heavy or too thin?”
Or, the conversation could include the provider’s observations that weight is just one measure of health and in fact not a very good one. Other ingredients in a healthy life style, such as sleep and physical activity, are not as easy to measure as weight but in many cases are more important.
As my mind struggled to restructure a health maintenance schedule that included blind weight checks, I wondered why we should wait until age 12. Of course, it is unreasonable to expect parents to stick with a pediatric practice that seems to ignore their infant’s weight. I’m sure that, like me, you have always discouraged new parents from having a baby scale at home because in the first few months too-frequent weighings can usually cause more angst than good.
It might make sense to remove a within-earshot discussion of a child’s weight from the health maintenance visit as soon as the child can absorb and digest the discussion; say, around age 3 years. In a perfect world, the provider should have already elicited a history that suggested a young child’s vulnerability to obesity before the scale and the growth chart told the unfortunate story. But, neither you nor I are perfect providers and so we will always need the scale to document our concerns. However, when and how we report that one vital sign to the patient and his or her parents is a topic ripe for discussion and improvement.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Reports of long waiting times at mental health clinics and anecdotal observations by health care providers suggest the pandemic has generated a dramatic increase in the incidence of eating disorders among the pediatric population. Of course this should come as no surprise to pediatricians.
Eating disorders come in many different forms and a triggering event is sometimes difficult to define. Often the adolescent or preadolescent is searching for some sense of stability in a life tossed on a stormy sea roiled by hormonal and physical change. Wresting control of their bodies during a period of uncertainty may result in a downward spiral into dangerously unhealthy weight loss. If nothing else, the pandemic has been a period of dramatic uncertainty unlike what most children and few adults in this country have ever experienced.
With the unprecedented increase in eating disorder cases, providers in several disciplines are searching for novel strategies to ease the burden on their patients and their practices. I recently learned of a pediatric practice in California that is considering blinding all patients aged 12 and older to the body mass measurements obtained at their health maintenance visits.
Blind weight checks for children with eating disorders, particularly those who seem to be nearing recovery, has been a common and often helpful practice. However, I am unaware of any practice that has made it a universal office policy. I’m unsure of the rationale behind this practice’s policy, but on several fronts, suppressing body mass measurements in the age group most vulnerable to eating disorders makes some sense.
Universal blind weight checks could minimize the risk of in-office shaming. However, careful training of support staff and thoughtful placement of the scales could serve the same purpose. This new policy acknowledges not only the ubiquity of the problem but also that many, maybe even most, children with eating disorders appear normal. And of course, there is the unfortunate fact that body mass is a poor screening test for eating disorders.
As I thought more about this novel approach I came to see its educational value for patients, parents, and even physicians. I can envision how a 13-year-old’s first health maintenance visit would go after the roll-out of the new policy. “Dr. Smith, aren’t you going to tell us how much I (or my daughter Jenny) weigh(s)?” This could, or more likely, should launch a discussion about weight and body image. It might continue with questions like, “How much do you think you weigh?” Or, “Do you think you are too heavy or too thin?”
Or, the conversation could include the provider’s observations that weight is just one measure of health and in fact not a very good one. Other ingredients in a healthy life style, such as sleep and physical activity, are not as easy to measure as weight but in many cases are more important.
As my mind struggled to restructure a health maintenance schedule that included blind weight checks, I wondered why we should wait until age 12. Of course, it is unreasonable to expect parents to stick with a pediatric practice that seems to ignore their infant’s weight. I’m sure that, like me, you have always discouraged new parents from having a baby scale at home because in the first few months too-frequent weighings can usually cause more angst than good.
It might make sense to remove a within-earshot discussion of a child’s weight from the health maintenance visit as soon as the child can absorb and digest the discussion; say, around age 3 years. In a perfect world, the provider should have already elicited a history that suggested a young child’s vulnerability to obesity before the scale and the growth chart told the unfortunate story. But, neither you nor I are perfect providers and so we will always need the scale to document our concerns. However, when and how we report that one vital sign to the patient and his or her parents is a topic ripe for discussion and improvement.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Reports of long waiting times at mental health clinics and anecdotal observations by health care providers suggest the pandemic has generated a dramatic increase in the incidence of eating disorders among the pediatric population. Of course this should come as no surprise to pediatricians.
Eating disorders come in many different forms and a triggering event is sometimes difficult to define. Often the adolescent or preadolescent is searching for some sense of stability in a life tossed on a stormy sea roiled by hormonal and physical change. Wresting control of their bodies during a period of uncertainty may result in a downward spiral into dangerously unhealthy weight loss. If nothing else, the pandemic has been a period of dramatic uncertainty unlike what most children and few adults in this country have ever experienced.
With the unprecedented increase in eating disorder cases, providers in several disciplines are searching for novel strategies to ease the burden on their patients and their practices. I recently learned of a pediatric practice in California that is considering blinding all patients aged 12 and older to the body mass measurements obtained at their health maintenance visits.
Blind weight checks for children with eating disorders, particularly those who seem to be nearing recovery, has been a common and often helpful practice. However, I am unaware of any practice that has made it a universal office policy. I’m unsure of the rationale behind this practice’s policy, but on several fronts, suppressing body mass measurements in the age group most vulnerable to eating disorders makes some sense.
Universal blind weight checks could minimize the risk of in-office shaming. However, careful training of support staff and thoughtful placement of the scales could serve the same purpose. This new policy acknowledges not only the ubiquity of the problem but also that many, maybe even most, children with eating disorders appear normal. And of course, there is the unfortunate fact that body mass is a poor screening test for eating disorders.
As I thought more about this novel approach I came to see its educational value for patients, parents, and even physicians. I can envision how a 13-year-old’s first health maintenance visit would go after the roll-out of the new policy. “Dr. Smith, aren’t you going to tell us how much I (or my daughter Jenny) weigh(s)?” This could, or more likely, should launch a discussion about weight and body image. It might continue with questions like, “How much do you think you weigh?” Or, “Do you think you are too heavy or too thin?”
Or, the conversation could include the provider’s observations that weight is just one measure of health and in fact not a very good one. Other ingredients in a healthy life style, such as sleep and physical activity, are not as easy to measure as weight but in many cases are more important.
As my mind struggled to restructure a health maintenance schedule that included blind weight checks, I wondered why we should wait until age 12. Of course, it is unreasonable to expect parents to stick with a pediatric practice that seems to ignore their infant’s weight. I’m sure that, like me, you have always discouraged new parents from having a baby scale at home because in the first few months too-frequent weighings can usually cause more angst than good.
It might make sense to remove a within-earshot discussion of a child’s weight from the health maintenance visit as soon as the child can absorb and digest the discussion; say, around age 3 years. In a perfect world, the provider should have already elicited a history that suggested a young child’s vulnerability to obesity before the scale and the growth chart told the unfortunate story. But, neither you nor I are perfect providers and so we will always need the scale to document our concerns. However, when and how we report that one vital sign to the patient and his or her parents is a topic ripe for discussion and improvement.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Commentary: Babies die as congenital syphilis continues a decade-long surge across the U.S.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The data are shocking: Almost 35,000 U.S. syphilis cases by mid-July 2022 with the highest rates per/100,000 population in Nevada (n = 21), California (n = 19), and Mississippi (n = 16). Excluding Nevada, California, and Oklahoma, rates over 12/100,000 were concentrated in the southernmost U.S. states. Overall, the 2,268 congenital syphilis cases in U.S. children born in 2021 was a 6% increase over 2020, and a 680% increase over 2012. (Note: All 2021 data are not yet available because of public health STI resources being diverted to COVID-19 control.) A telling number is the 166 congenital syphilis deaths in babies born in 2021 – a 1,000% increase over 2012. Another concern is that 50% of U.S. counties reported at least one congenital syphilis case in 2019 – the last time frame from which county-specific data are available.
Syphilis afflicts the underserved and underprivileged more than other demographic groups, particularly when public health budgets are not adequate (funding for public health STI prevention/treatment efforts has lagged for more than a decade), and/or when public health emergencies such as the pandemic divert public health resources away from STI prevention/treatment efforts.
As pediatric care providers, we can help by heightening our vigilance and appropriately testing for and treating syphilis, particularly in newborns/infants, regardless of where we work. And we can advocate for increased public health STI funding allocation whenever possible. It is a smart economic move because it costs nearly 1,000 times more to manage congenital syphilis and its sequelae than to prevent or treat it.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Are all medical errors now crimes? The Nurse Vaught verdict
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.