User login
Official Newspaper of the American College of Surgeons
Vascular surgeons have better outcomes for aneurysm repair, endarterectomy
SAN FRANCISCO – Patients undergoing carotid endarterectomy and open abdominal aortic aneurysm repair are less likely to have complications and die if their surgeon is a vascular specialist, according to a study reported at the annual clinical congress of the American College of Surgeons.
“We feel that carotid endarterectomy and open triple-A repair performed by a vascular surgeon is an independent predictor of improved morbidity and mortality. Hospitals should consider utilizing this specialty-specific information to identify potential quality improvement initiatives,” recommended lead investigator Dr. Carla C. Moreira, a surgeon in the division of vascular and endovascular surgery, Boston Medical Center.
She and her colleagues analyzed data from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), assessing outcomes for 94,029 patients who underwent open vascular procedures between 2006 and 2012. Some 8% had abdominal aneurysm repairs, 57% had carotid endarterectomies, and 35% had lower-extremity bypasses.
Overall, 94% of the procedures were performed by vascular surgeons, while the rest were performed by other types of surgeons (including cardiac surgeons because their numbers were too small to tease out, according to Dr. Moreira).
Results showed that for patients undergoing abdominal aneurysm repair, the unadjusted rate of 30-day mortality did not differ significantly by surgeon specialty. However, in multivariate, propensity-matched analysis, the odds of complications were reduced by half when the repair was done by a vascular surgeon as compared with some other type of surgeon (odds ratio, 0.50).
Patients undergoing carotid endarterectomy had a lower unadjusted mortality if the operation was performed by a vascular surgeon (0.7% vs. 1.0%), although the difference was no longer significant after multivariate adjustment. However, they had significantly lower adjusted odds of complications (odds ratio, 0.62).
For patients undergoing lower-extremity bypass, neither the rate of mortality nor the odds of complications differed significantly by surgeon specialty.
An analysis of temporal trends showed that the proportions of all eligible patients undergoing open abdominal aneurysm repair and lower-extremity bypass during the study period fell, regardless of surgeon type, whereas the proportion of eligible patients undergoing carotid endarterectomies remained stable. “I think this is reflective likely of the penetration of endovascular procedures when it comes to the treatment of abdominal aneurysm and lower-extremity peripheral arterial disease, as compared to carotid disease,” speculated Dr. Moreira, who disclosed that she had no relevant conflicts of interest.
Session moderator Dr. Peter K. Henke, associate chair of research, department of surgery, and Leland Ira Doan Professor of Vascular Surgery at the University of Michigan in Ann Arbor, asked whether the findings have implications for surgeon training.
“Our results were different from what has been previously published for open aneurysm repair. I think that open aneurysm repair is probably becoming more and more specialized in terms that more and more, you are going to see just vascular surgeons performing it as compared to other specialties, especially as I have shown that there is a decreasing number of general surgeons being exposed to open vascular procedures,” Dr. Moreira replied. “So I think this [speaks] to individual surgeons and their individual experience with triple-A repair. Our data does show that general surgeons who are performing these procedures are doing a good job because there is not a difference in outcome at least in terms of mortality. But as the easy triple-A repairs go away, I think it’s going to push the procedure to be done more by specialized surgeons.”
In an interview, Dr. Henke commented, “I think it has been alluded to in other studies and other reports in the literature, but this study brings to the forefront the fact that overall numbers of open vascular procedures are less and less, so the surgeons performing those procedures need to be specialists. And I’d say within the U.S. anyway, most practitioners who do vascular procedures are vascular surgeons or cardiovascularly trained, as compared with general surgeons. I suspect that some of the general surgeons who are still doing these procedures may be at a place where they are the only ones who can do it or there are no vascular specialists there.
“The other issue that this study may impact, as more of these reports come out, is hospital credentialing, because hospital credentialing of surgeons really determines what they can and cannot do at that hospital,” he added. “So as more hospital credentialing boards see this type of data, I think they will limit open vascular procedures to vascular specialists who have had training in vascular disease.”
SAN FRANCISCO – Patients undergoing carotid endarterectomy and open abdominal aortic aneurysm repair are less likely to have complications and die if their surgeon is a vascular specialist, according to a study reported at the annual clinical congress of the American College of Surgeons.
“We feel that carotid endarterectomy and open triple-A repair performed by a vascular surgeon is an independent predictor of improved morbidity and mortality. Hospitals should consider utilizing this specialty-specific information to identify potential quality improvement initiatives,” recommended lead investigator Dr. Carla C. Moreira, a surgeon in the division of vascular and endovascular surgery, Boston Medical Center.
She and her colleagues analyzed data from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), assessing outcomes for 94,029 patients who underwent open vascular procedures between 2006 and 2012. Some 8% had abdominal aneurysm repairs, 57% had carotid endarterectomies, and 35% had lower-extremity bypasses.
Overall, 94% of the procedures were performed by vascular surgeons, while the rest were performed by other types of surgeons (including cardiac surgeons because their numbers were too small to tease out, according to Dr. Moreira).
Results showed that for patients undergoing abdominal aneurysm repair, the unadjusted rate of 30-day mortality did not differ significantly by surgeon specialty. However, in multivariate, propensity-matched analysis, the odds of complications were reduced by half when the repair was done by a vascular surgeon as compared with some other type of surgeon (odds ratio, 0.50).
Patients undergoing carotid endarterectomy had a lower unadjusted mortality if the operation was performed by a vascular surgeon (0.7% vs. 1.0%), although the difference was no longer significant after multivariate adjustment. However, they had significantly lower adjusted odds of complications (odds ratio, 0.62).
For patients undergoing lower-extremity bypass, neither the rate of mortality nor the odds of complications differed significantly by surgeon specialty.
An analysis of temporal trends showed that the proportions of all eligible patients undergoing open abdominal aneurysm repair and lower-extremity bypass during the study period fell, regardless of surgeon type, whereas the proportion of eligible patients undergoing carotid endarterectomies remained stable. “I think this is reflective likely of the penetration of endovascular procedures when it comes to the treatment of abdominal aneurysm and lower-extremity peripheral arterial disease, as compared to carotid disease,” speculated Dr. Moreira, who disclosed that she had no relevant conflicts of interest.
Session moderator Dr. Peter K. Henke, associate chair of research, department of surgery, and Leland Ira Doan Professor of Vascular Surgery at the University of Michigan in Ann Arbor, asked whether the findings have implications for surgeon training.
“Our results were different from what has been previously published for open aneurysm repair. I think that open aneurysm repair is probably becoming more and more specialized in terms that more and more, you are going to see just vascular surgeons performing it as compared to other specialties, especially as I have shown that there is a decreasing number of general surgeons being exposed to open vascular procedures,” Dr. Moreira replied. “So I think this [speaks] to individual surgeons and their individual experience with triple-A repair. Our data does show that general surgeons who are performing these procedures are doing a good job because there is not a difference in outcome at least in terms of mortality. But as the easy triple-A repairs go away, I think it’s going to push the procedure to be done more by specialized surgeons.”
In an interview, Dr. Henke commented, “I think it has been alluded to in other studies and other reports in the literature, but this study brings to the forefront the fact that overall numbers of open vascular procedures are less and less, so the surgeons performing those procedures need to be specialists. And I’d say within the U.S. anyway, most practitioners who do vascular procedures are vascular surgeons or cardiovascularly trained, as compared with general surgeons. I suspect that some of the general surgeons who are still doing these procedures may be at a place where they are the only ones who can do it or there are no vascular specialists there.
“The other issue that this study may impact, as more of these reports come out, is hospital credentialing, because hospital credentialing of surgeons really determines what they can and cannot do at that hospital,” he added. “So as more hospital credentialing boards see this type of data, I think they will limit open vascular procedures to vascular specialists who have had training in vascular disease.”
SAN FRANCISCO – Patients undergoing carotid endarterectomy and open abdominal aortic aneurysm repair are less likely to have complications and die if their surgeon is a vascular specialist, according to a study reported at the annual clinical congress of the American College of Surgeons.
“We feel that carotid endarterectomy and open triple-A repair performed by a vascular surgeon is an independent predictor of improved morbidity and mortality. Hospitals should consider utilizing this specialty-specific information to identify potential quality improvement initiatives,” recommended lead investigator Dr. Carla C. Moreira, a surgeon in the division of vascular and endovascular surgery, Boston Medical Center.
She and her colleagues analyzed data from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP), assessing outcomes for 94,029 patients who underwent open vascular procedures between 2006 and 2012. Some 8% had abdominal aneurysm repairs, 57% had carotid endarterectomies, and 35% had lower-extremity bypasses.
Overall, 94% of the procedures were performed by vascular surgeons, while the rest were performed by other types of surgeons (including cardiac surgeons because their numbers were too small to tease out, according to Dr. Moreira).
Results showed that for patients undergoing abdominal aneurysm repair, the unadjusted rate of 30-day mortality did not differ significantly by surgeon specialty. However, in multivariate, propensity-matched analysis, the odds of complications were reduced by half when the repair was done by a vascular surgeon as compared with some other type of surgeon (odds ratio, 0.50).
Patients undergoing carotid endarterectomy had a lower unadjusted mortality if the operation was performed by a vascular surgeon (0.7% vs. 1.0%), although the difference was no longer significant after multivariate adjustment. However, they had significantly lower adjusted odds of complications (odds ratio, 0.62).
For patients undergoing lower-extremity bypass, neither the rate of mortality nor the odds of complications differed significantly by surgeon specialty.
An analysis of temporal trends showed that the proportions of all eligible patients undergoing open abdominal aneurysm repair and lower-extremity bypass during the study period fell, regardless of surgeon type, whereas the proportion of eligible patients undergoing carotid endarterectomies remained stable. “I think this is reflective likely of the penetration of endovascular procedures when it comes to the treatment of abdominal aneurysm and lower-extremity peripheral arterial disease, as compared to carotid disease,” speculated Dr. Moreira, who disclosed that she had no relevant conflicts of interest.
Session moderator Dr. Peter K. Henke, associate chair of research, department of surgery, and Leland Ira Doan Professor of Vascular Surgery at the University of Michigan in Ann Arbor, asked whether the findings have implications for surgeon training.
“Our results were different from what has been previously published for open aneurysm repair. I think that open aneurysm repair is probably becoming more and more specialized in terms that more and more, you are going to see just vascular surgeons performing it as compared to other specialties, especially as I have shown that there is a decreasing number of general surgeons being exposed to open vascular procedures,” Dr. Moreira replied. “So I think this [speaks] to individual surgeons and their individual experience with triple-A repair. Our data does show that general surgeons who are performing these procedures are doing a good job because there is not a difference in outcome at least in terms of mortality. But as the easy triple-A repairs go away, I think it’s going to push the procedure to be done more by specialized surgeons.”
In an interview, Dr. Henke commented, “I think it has been alluded to in other studies and other reports in the literature, but this study brings to the forefront the fact that overall numbers of open vascular procedures are less and less, so the surgeons performing those procedures need to be specialists. And I’d say within the U.S. anyway, most practitioners who do vascular procedures are vascular surgeons or cardiovascularly trained, as compared with general surgeons. I suspect that some of the general surgeons who are still doing these procedures may be at a place where they are the only ones who can do it or there are no vascular specialists there.
“The other issue that this study may impact, as more of these reports come out, is hospital credentialing, because hospital credentialing of surgeons really determines what they can and cannot do at that hospital,” he added. “So as more hospital credentialing boards see this type of data, I think they will limit open vascular procedures to vascular specialists who have had training in vascular disease.”
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients undergoing certain open vascular procedures have better outcomes when operated on by vascular surgeons.
Major finding: Vascular surgeons achieved lower rates of morbidity and mortality in cases of open abdominal aneurysm repair and carotid endarterectomy.
Data source: An analysis of NSQIP data for 94,029 patients undergoing open vascular procedures.
Disclosures: Dr. Moreira disclosed that she had no relevant conflicts of interest.
Neither aspirin nor clonidine reduced postoperative acute kidney injury
PHILADELPHIA- Neither perioperative aspirin nor clonidine reduced the risk of an acute kidney injury in patients who underwent major noncardiac surgery, a large randomized trial has determined.
The risk of an acute kidney injury (AKI) associated with aspirin was 10% more than placebo, and the risk with clonidine, 3% more, but those differences were not statistically significant, Dr. Amit X. Garg and associates wrote online in JAMA (JAMA 2015; doi:10.1001/jama.2014.15284).
Both drugs increased the risk of postoperative conditions that are associated with AKI, Dr. Garg of the London Health Sciences Centre and Western University, London, Ontario, and his co-authors noted. The study was simultaneously presented at Kidney Week 2014, which was sponsored by the American Society of Nephrology.
In fact, there was some suggestion that the drugs increased the risk of severe AKI, he said at the meeting. “But these were secondary measures and need to be interpreted cautiosuly, because the absolute number of severe AKIs was quite small.”
The AKI investigation was a substudy of the Perioperative Ischemia Evaluation-2, (POISE-2) trial, which evaluated the risk of 30-day mortality or nonfatal myocardial infarction among 6,905 surgical patients with a moderate to high risk of a perioperative cardiac event. The aspirin regimen called for 200 mg before surgery and then 100 mg daily for up to 30 days after surgery. The clonidine regimen was 0.2 mg orally 2-4 hours before surgery, followed by a 0.3 mg/day transdermal patch worn for 72 hours after surgery. Both groups also had a placebo arm.
The patients were a mean of 69 years old. Cardiovascular disease was present in about 30%; these included coronary artery disease, stroke, and peripheral vascular disease. About a quarter smoked; 36% had diabetes; 86% had hypertension; 2% had atrial fibrillation. Medications included COX-2 inhibitors; statins; ACE, ARB or direct renin inhibitors; and antihypertensives.
At baseline about 24% had an estimated glomerular filtration rate of 60 ml/min or lower per 1.73 m2.
Surgical procedures were urgent or emergent, major vascular, major thoracic, major urological or gynecologic, and other unspecified procedures.
Aspirin did not reduce the risk of an AKI compared to placebo. AKI occurred in 462 patients taking aspirin and 426 taking placebo (13.4% vs. 12.3% respectively; adjusted risk ratio 1.10). Nor did clonidine reduce the risk of AKI compared to placebo. AKI occurred in 449 taking aspirin and 439 taking placebo (13% and 12.7% respectively; adjusted risk ratio 1.03). Neither of these findings was statistically significant.
AKI-related dialysis within 30 days occurred in 0.6% those taking aspirin and 0.3% of the matched placebo patients - a nonsignificant difference. Serum creatinine increased a mean of 11% with aspirin vs. 11% with placebo.
Dialysis was necessary in 0.5% of those taking clonidine and 0.3% of the matched placebo patients - another nonsignificant finding. A history of preoperative chronic kidney disease did not alter either of these findings.
A post hoc analysis determined that both drugs increased the incidence of conditions known to boost the risk of kidney injury. Aspirin increased the risk of major bleeding, which was associated with a greater risk of AKI (23.3% when bleeding occurred vs 12.3% when it did not; adjusted risk ratio 2.20).
Because of this doubling of risk, Dr. Garg suggested moderating pre-operative aspirin exposure in these patients.
“Among patients taking aspirin as part of a long-term regimen, these results support holding it for at least 3 days before surgery and then restarting it a week after surgery,” he recommended.
Clonidine increased the risk of clinically important hypotension, which was also related in turn to AKI (14.3% when hypotension was present vs. 11.8% when it was not; adjusted risk ratio for AKI 1.34).
Dr. Garg had no financial disclosures. A number of the POISE-2 investigators reported financial relationships with pharmaceutical companies.
PHILADELPHIA- Neither perioperative aspirin nor clonidine reduced the risk of an acute kidney injury in patients who underwent major noncardiac surgery, a large randomized trial has determined.
The risk of an acute kidney injury (AKI) associated with aspirin was 10% more than placebo, and the risk with clonidine, 3% more, but those differences were not statistically significant, Dr. Amit X. Garg and associates wrote online in JAMA (JAMA 2015; doi:10.1001/jama.2014.15284).
Both drugs increased the risk of postoperative conditions that are associated with AKI, Dr. Garg of the London Health Sciences Centre and Western University, London, Ontario, and his co-authors noted. The study was simultaneously presented at Kidney Week 2014, which was sponsored by the American Society of Nephrology.
In fact, there was some suggestion that the drugs increased the risk of severe AKI, he said at the meeting. “But these were secondary measures and need to be interpreted cautiosuly, because the absolute number of severe AKIs was quite small.”
The AKI investigation was a substudy of the Perioperative Ischemia Evaluation-2, (POISE-2) trial, which evaluated the risk of 30-day mortality or nonfatal myocardial infarction among 6,905 surgical patients with a moderate to high risk of a perioperative cardiac event. The aspirin regimen called for 200 mg before surgery and then 100 mg daily for up to 30 days after surgery. The clonidine regimen was 0.2 mg orally 2-4 hours before surgery, followed by a 0.3 mg/day transdermal patch worn for 72 hours after surgery. Both groups also had a placebo arm.
The patients were a mean of 69 years old. Cardiovascular disease was present in about 30%; these included coronary artery disease, stroke, and peripheral vascular disease. About a quarter smoked; 36% had diabetes; 86% had hypertension; 2% had atrial fibrillation. Medications included COX-2 inhibitors; statins; ACE, ARB or direct renin inhibitors; and antihypertensives.
At baseline about 24% had an estimated glomerular filtration rate of 60 ml/min or lower per 1.73 m2.
Surgical procedures were urgent or emergent, major vascular, major thoracic, major urological or gynecologic, and other unspecified procedures.
Aspirin did not reduce the risk of an AKI compared to placebo. AKI occurred in 462 patients taking aspirin and 426 taking placebo (13.4% vs. 12.3% respectively; adjusted risk ratio 1.10). Nor did clonidine reduce the risk of AKI compared to placebo. AKI occurred in 449 taking aspirin and 439 taking placebo (13% and 12.7% respectively; adjusted risk ratio 1.03). Neither of these findings was statistically significant.
AKI-related dialysis within 30 days occurred in 0.6% those taking aspirin and 0.3% of the matched placebo patients - a nonsignificant difference. Serum creatinine increased a mean of 11% with aspirin vs. 11% with placebo.
Dialysis was necessary in 0.5% of those taking clonidine and 0.3% of the matched placebo patients - another nonsignificant finding. A history of preoperative chronic kidney disease did not alter either of these findings.
A post hoc analysis determined that both drugs increased the incidence of conditions known to boost the risk of kidney injury. Aspirin increased the risk of major bleeding, which was associated with a greater risk of AKI (23.3% when bleeding occurred vs 12.3% when it did not; adjusted risk ratio 2.20).
Because of this doubling of risk, Dr. Garg suggested moderating pre-operative aspirin exposure in these patients.
“Among patients taking aspirin as part of a long-term regimen, these results support holding it for at least 3 days before surgery and then restarting it a week after surgery,” he recommended.
Clonidine increased the risk of clinically important hypotension, which was also related in turn to AKI (14.3% when hypotension was present vs. 11.8% when it was not; adjusted risk ratio for AKI 1.34).
Dr. Garg had no financial disclosures. A number of the POISE-2 investigators reported financial relationships with pharmaceutical companies.
PHILADELPHIA- Neither perioperative aspirin nor clonidine reduced the risk of an acute kidney injury in patients who underwent major noncardiac surgery, a large randomized trial has determined.
The risk of an acute kidney injury (AKI) associated with aspirin was 10% more than placebo, and the risk with clonidine, 3% more, but those differences were not statistically significant, Dr. Amit X. Garg and associates wrote online in JAMA (JAMA 2015; doi:10.1001/jama.2014.15284).
Both drugs increased the risk of postoperative conditions that are associated with AKI, Dr. Garg of the London Health Sciences Centre and Western University, London, Ontario, and his co-authors noted. The study was simultaneously presented at Kidney Week 2014, which was sponsored by the American Society of Nephrology.
In fact, there was some suggestion that the drugs increased the risk of severe AKI, he said at the meeting. “But these were secondary measures and need to be interpreted cautiosuly, because the absolute number of severe AKIs was quite small.”
The AKI investigation was a substudy of the Perioperative Ischemia Evaluation-2, (POISE-2) trial, which evaluated the risk of 30-day mortality or nonfatal myocardial infarction among 6,905 surgical patients with a moderate to high risk of a perioperative cardiac event. The aspirin regimen called for 200 mg before surgery and then 100 mg daily for up to 30 days after surgery. The clonidine regimen was 0.2 mg orally 2-4 hours before surgery, followed by a 0.3 mg/day transdermal patch worn for 72 hours after surgery. Both groups also had a placebo arm.
The patients were a mean of 69 years old. Cardiovascular disease was present in about 30%; these included coronary artery disease, stroke, and peripheral vascular disease. About a quarter smoked; 36% had diabetes; 86% had hypertension; 2% had atrial fibrillation. Medications included COX-2 inhibitors; statins; ACE, ARB or direct renin inhibitors; and antihypertensives.
At baseline about 24% had an estimated glomerular filtration rate of 60 ml/min or lower per 1.73 m2.
Surgical procedures were urgent or emergent, major vascular, major thoracic, major urological or gynecologic, and other unspecified procedures.
Aspirin did not reduce the risk of an AKI compared to placebo. AKI occurred in 462 patients taking aspirin and 426 taking placebo (13.4% vs. 12.3% respectively; adjusted risk ratio 1.10). Nor did clonidine reduce the risk of AKI compared to placebo. AKI occurred in 449 taking aspirin and 439 taking placebo (13% and 12.7% respectively; adjusted risk ratio 1.03). Neither of these findings was statistically significant.
AKI-related dialysis within 30 days occurred in 0.6% those taking aspirin and 0.3% of the matched placebo patients - a nonsignificant difference. Serum creatinine increased a mean of 11% with aspirin vs. 11% with placebo.
Dialysis was necessary in 0.5% of those taking clonidine and 0.3% of the matched placebo patients - another nonsignificant finding. A history of preoperative chronic kidney disease did not alter either of these findings.
A post hoc analysis determined that both drugs increased the incidence of conditions known to boost the risk of kidney injury. Aspirin increased the risk of major bleeding, which was associated with a greater risk of AKI (23.3% when bleeding occurred vs 12.3% when it did not; adjusted risk ratio 2.20).
Because of this doubling of risk, Dr. Garg suggested moderating pre-operative aspirin exposure in these patients.
“Among patients taking aspirin as part of a long-term regimen, these results support holding it for at least 3 days before surgery and then restarting it a week after surgery,” he recommended.
Clonidine increased the risk of clinically important hypotension, which was also related in turn to AKI (14.3% when hypotension was present vs. 11.8% when it was not; adjusted risk ratio for AKI 1.34).
Dr. Garg had no financial disclosures. A number of the POISE-2 investigators reported financial relationships with pharmaceutical companies.
AT KIDNEY WEEK 2014
Key clinical point: Perioperative treatment with either aspirin or clonidine did not lead to improvements in the risk of postoperative acute kidney injury.
Major finding: Compared to palcebo, the relative risk of acute kidney injury with aspirin was 1.10; it was 1.03 with clonidine.
Data source: The POISE-2 substudy comprised 6,905 patients who were randomized to aspirin, clonidine, or placebo.
Disclosures: Dr. Garg had no financial disclosures. Anumber of the POISE-2 investigators declared financial relationships with pharmaceutical companies.
Kidney donors at greater risk of preeclampsia, gestational hypertension
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy, according to a study presented at Kidney Week 2014 and published online simultaneously in the New England Journal of Medicine.
“Information on this potential risk should be included in clinical practice guidelines, shared in the informed-consent processes for potential donors and their recipients when a woman has reproductive potential, and used to guide the care of pregnant donors,” wrote the study authors, led by Dr. Amit X. Garg at the London Kidney Research Unit in London, Ont. (N. Engl. J. Med. 2014 Nov. 14 [doi:10.1056/NEJMoa1408932]).
The Canadian retrospective study matched 85 living kidney donors in a 1:6 ratio with 510 healthy nondonors and followed them for almost 11 years. During this time, 131 pregnancies occurred in the donor group and 788 in the nondonor group.
Gestational hypertension or preeclampsia was diagnosed in 15 donors and 38 nondonors (11% vs. 5%, odds ratio for donors, 2.4; 95% confidence interval, 1.2 to 5.0; P = .01), the investigators reported.
No significant differences were observed between groups for other maternal or fetal outcomes, and there were no maternal or perinatal deaths in the study that was part of the Donor Nephrectomy Outcomes Research Network (DONOR).
However, they noted that the study included limitations, such as not recording body mass index, medication use, or the race of study participants.
Confidence intervals for risk estimates also were wide, and physicians used clinical judgment when applying accepted diagnostic criteria for gestational hypertension and preeclampsia.
“It remains possible that gestational hypertension and preeclampsia were more likely to be diagnosed and recorded among donors than nondonors despite similar clinical presentations in two groups,” the investigators wrote.
“There may be a role for government programs to cover the costs of recommended pregnancy care for donors who lack health insurance, including any costs related to the treatment of hypertension,” they added.
The meeting was sponsored by the American Society of Nephrology. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures.
FROM KIDNEY WEEK 2014
Key clinical point: Information on an increased risk for preeclampsia and gestational hypertension should be included in clinical practice guidelines and in informed-consent processes for potential kidney donors and their recipients.
Major finding: Women who donate a kidney are almost two and a half times more likely than are nondonors to have preeclampsia or gestational hypertension in pregnancy.
Data source: Retrospective cohort study of 85 kidney donors who were matched on a 1:6 ratio with 510 healthy nondonors and followed for a median of 10.9 years.
Disclosures:Dr. Garg received grants from Astellas and Roche outside this study. Several other authors received grants from a number companies outside this study, while the remainder of the authors had no relevant disclosures. The study was supported by a grant from the Canadian Institute of Health Research as well as several other research institutions. The meeting was sponsored by the American Society of Nephrology.
Extended use of oral anticoagulants reduces VTE recurrence
AUSTIN, TEX. – Extended treatment with any of the novel oral anticoagulants, but with apixaban in particular, provides a net clinical benefit in patients at risk of recurrent venous thromboembolism, according to a review of three randomized trials.
Apixaban appears to provide the optimal net clinical benefit, with the lowest number needed to treat to avoid one venous thromboembolic or major bleeding event, Dr. Alpesh Amin reported at the annual meeting of the American College of Chest Physicians.
In 5,035 patients in three trials of extended treatment with novel oral anticoagulants (NOACs) for venous thromboembolism (VTE) – including the RE-SONATE trial, the EINSTEIN-EXT trial, and the AMPLIFY-EXT trial – the differences in event rates, compared with placebo, were –5.15% for dabigatran, –5.74% for rivaroxaban, –7.14% for 2.5 mg apixaban, and –7.0% for 5 mg apixaban, reported Dr. Amin of the University of California, Irvine.
The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban, Dr. Amin said.
“The good news is that the number needed to treat for all of [the oral anticoagulants] is actually less than 25,” he said.
As for costs, the savings from avoiding a recurrent VTE were $2,995 with dabigatran, $3,300 for rivaroxaban, and $4,100 for both 2.5 and 5 mg apixaban.
For major bleeding events, the corresponding rates, compared with placebo, were 0.29%, 0.67%, –0.20%, and –0.36%.
There was a net clinical benefit for all patients treated with the NOACs, but in those treated with 5 mg apixaban, the rates of improvement were highest at –7.44%, followed by –7.38% for 2.5 mg apixaban. The rates were –5.0% with rivaroxaban and –4.85% with dabigatran.
“So we see a low number needed to treat, and a significant amount of cost avoidance by using the NOACs across the board,” he said, adding that apixaban may provide the best net clinical benefit for the lowest number needed to treat to avoid one VTE or major bleeding event, and is associated with the greatest medical cost avoidance.
“In terms of safety endpoints, dabigatran and rivaroxaban cost the system a little bit of money, whereas apixaban actually decreased the cost,” he said.
“How these results translate into real-world outcomes will require further evaluation, and as we get more numbers out there, we will actually be looking at the impact in the real world,” he said.
Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
AUSTIN, TEX. – Extended treatment with any of the novel oral anticoagulants, but with apixaban in particular, provides a net clinical benefit in patients at risk of recurrent venous thromboembolism, according to a review of three randomized trials.
Apixaban appears to provide the optimal net clinical benefit, with the lowest number needed to treat to avoid one venous thromboembolic or major bleeding event, Dr. Alpesh Amin reported at the annual meeting of the American College of Chest Physicians.
In 5,035 patients in three trials of extended treatment with novel oral anticoagulants (NOACs) for venous thromboembolism (VTE) – including the RE-SONATE trial, the EINSTEIN-EXT trial, and the AMPLIFY-EXT trial – the differences in event rates, compared with placebo, were –5.15% for dabigatran, –5.74% for rivaroxaban, –7.14% for 2.5 mg apixaban, and –7.0% for 5 mg apixaban, reported Dr. Amin of the University of California, Irvine.
The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban, Dr. Amin said.
“The good news is that the number needed to treat for all of [the oral anticoagulants] is actually less than 25,” he said.
As for costs, the savings from avoiding a recurrent VTE were $2,995 with dabigatran, $3,300 for rivaroxaban, and $4,100 for both 2.5 and 5 mg apixaban.
For major bleeding events, the corresponding rates, compared with placebo, were 0.29%, 0.67%, –0.20%, and –0.36%.
There was a net clinical benefit for all patients treated with the NOACs, but in those treated with 5 mg apixaban, the rates of improvement were highest at –7.44%, followed by –7.38% for 2.5 mg apixaban. The rates were –5.0% with rivaroxaban and –4.85% with dabigatran.
“So we see a low number needed to treat, and a significant amount of cost avoidance by using the NOACs across the board,” he said, adding that apixaban may provide the best net clinical benefit for the lowest number needed to treat to avoid one VTE or major bleeding event, and is associated with the greatest medical cost avoidance.
“In terms of safety endpoints, dabigatran and rivaroxaban cost the system a little bit of money, whereas apixaban actually decreased the cost,” he said.
“How these results translate into real-world outcomes will require further evaluation, and as we get more numbers out there, we will actually be looking at the impact in the real world,” he said.
Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
AUSTIN, TEX. – Extended treatment with any of the novel oral anticoagulants, but with apixaban in particular, provides a net clinical benefit in patients at risk of recurrent venous thromboembolism, according to a review of three randomized trials.
Apixaban appears to provide the optimal net clinical benefit, with the lowest number needed to treat to avoid one venous thromboembolic or major bleeding event, Dr. Alpesh Amin reported at the annual meeting of the American College of Chest Physicians.
In 5,035 patients in three trials of extended treatment with novel oral anticoagulants (NOACs) for venous thromboembolism (VTE) – including the RE-SONATE trial, the EINSTEIN-EXT trial, and the AMPLIFY-EXT trial – the differences in event rates, compared with placebo, were –5.15% for dabigatran, –5.74% for rivaroxaban, –7.14% for 2.5 mg apixaban, and –7.0% for 5 mg apixaban, reported Dr. Amin of the University of California, Irvine.
The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban, Dr. Amin said.
“The good news is that the number needed to treat for all of [the oral anticoagulants] is actually less than 25,” he said.
As for costs, the savings from avoiding a recurrent VTE were $2,995 with dabigatran, $3,300 for rivaroxaban, and $4,100 for both 2.5 and 5 mg apixaban.
For major bleeding events, the corresponding rates, compared with placebo, were 0.29%, 0.67%, –0.20%, and –0.36%.
There was a net clinical benefit for all patients treated with the NOACs, but in those treated with 5 mg apixaban, the rates of improvement were highest at –7.44%, followed by –7.38% for 2.5 mg apixaban. The rates were –5.0% with rivaroxaban and –4.85% with dabigatran.
“So we see a low number needed to treat, and a significant amount of cost avoidance by using the NOACs across the board,” he said, adding that apixaban may provide the best net clinical benefit for the lowest number needed to treat to avoid one VTE or major bleeding event, and is associated with the greatest medical cost avoidance.
“In terms of safety endpoints, dabigatran and rivaroxaban cost the system a little bit of money, whereas apixaban actually decreased the cost,” he said.
“How these results translate into real-world outcomes will require further evaluation, and as we get more numbers out there, we will actually be looking at the impact in the real world,” he said.
Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
Key clinical point: All of the NOACs provide a net clinical benefit for reducing VTE recurrence.
Major finding: The number needed to treat to avoid one VTE or major bleeding event was 21 for dabigatran, 20 for rivaroxaban, 14 for 2.5 mg apixaban, and 13 for 5 mg apixaban.
Data source: An analysis of data from three clinical trials, including a total of 5,035 patients.
Disclosures: Dr. Amin reported serving as a paid consultant and/or member of a speakers bureau or advisory committee for Bristol-Myers Squibb and Pfizer.
Court: Patients can sue over HIPAA breaches
Patients can sue doctors for negligence after alleged patient privacy breaches, the Connecticut Supreme Court has ruled in a decision that could have nationwide implications.
The state’s highest court concluded that HIPAA does not preempt claims for emotional distress or negligence under state law. The ruling, published Nov. 11, sets precedence in Connecticut and is likely to encourage plaintiffs to raise similar claims in other states, according to Michael J. Kline, a New Jersey attorney who specializes in corporate and securities law.
“It’s a momentous case, and I think it’s serious for physician practices,” said Mr. Kline. “It can set the stage for plaintiffs’ attorneys within given states to [pursue] class actions for emotional distress or invasion of privacy on the grounds there was negligence” in connection to HIPAA violations.
The decision stems from a lawsuit filed by Emily Byrne v. Avery Center for Obstetrics and Gynecology P.C. in Westport, Conn. Ms. Byrne claimed that in 2004, she instructed the health center not to release her medical records to an ex-boyfriend. In 2005, the medical center was served with a subpoena by the former boyfriend requesting the plaintiff’s medical records for a paternity proceeding. The defendant did not alert Ms. Byrne about the subpoena and mailed a copy of her medical file to the probate court, according to court records.
Ms. Byrne later sued the health care center, claiming she suffered harassment and extortion threats from her ex-boyfriend because the medical records were exposed. The complaint alleged the health center engaged in negligent infliction of emotional distress and acted negligently by failing to use proper and reasonable care in protecting her medical file, including disclosing it without authorization in violation of HIPAA.
A trial court agreed with the medical practice’s contention that HIPAA precludes individual liability claims pertaining to confidentiality of medical information. The court cited well-established case law that HIPAA does not create a private right of action and requires alleged privacy violations to be raised through administrative channels. But the Connecticut Supreme Court overturned the decision, ruling that HIPAA does not preempt such negligence lawsuits.
“If Connecticut’s common law recognizes claims arising from a health care provider’s alleged breach of its duty of confidentiality in the course of complying with a subpoena, HIPAA and its implementing regulations do not preempt such claims,” judges said in their opinion. “We further conclude that, to the extent it has become the common practice for Connecticut health care providers to follow the procedures required under HIPAA in rendering services to their patients, HIPAA and its implementing regulations may be utilized to inform the standard of care applicable to such claims arising from allegations of negligence in the disclosure of patients’ medical records pursuant to a subpoena.”
The court went on to say that “the availability of such private rights of action in state courts ... do not preclude, conflict with, or complicate health care providers’ compliance with HIPAA.”
Similar decisions have been made by other courts, but the Connecticut ruling is the first state Supreme Court to issue such a ruling, Mr. Kline said.
For example, in Harmon v. Maury County, the U.S. District Court for the Middle District of Tennessee found that negligence claims founded on violation of HIPAA were not precluded because federal provisions do not completely preempt state law and expressly preserve state laws that aren’t at odds with its terms. The 2005 Tennessee case resulted from a privacy violation claim by a patient against a pharmacy manager. In another pharmacy-patient complaint, Fanean v. Rite Aid Corp. of Delaware Inc., the Superior Court of Delaware concluded that negligence claims could not be premised on a HIPAA violation, but that a common law negligence claim could be predicated upon Occupational Safety and Health Administration requirements. The 2009 opinion noted that HIPAA may act as a “guidepost” to determine the standard of care in common-law negligence claims.
The similar assertions by Connecticut judges that HIPAA does not preempt state rights and can also be used as a standard for what constitutes negligence or improper care of records is concerning for health providers, Mr. Kline said in an interview. Doctors now have to worry that inadvertent HIPAA violations may yield not only a complaint with the Office for Civil Rights, but a potential malpractice suit, as well.
“I would not be surprised if a case like this or even this case is appealed to the Supreme Court of the United States,” Mr. Kline said. “It is still a question of federal law, and what does the federal preemption mean?”
On Twitter @legal_med
Patients can sue doctors for negligence after alleged patient privacy breaches, the Connecticut Supreme Court has ruled in a decision that could have nationwide implications.
The state’s highest court concluded that HIPAA does not preempt claims for emotional distress or negligence under state law. The ruling, published Nov. 11, sets precedence in Connecticut and is likely to encourage plaintiffs to raise similar claims in other states, according to Michael J. Kline, a New Jersey attorney who specializes in corporate and securities law.
“It’s a momentous case, and I think it’s serious for physician practices,” said Mr. Kline. “It can set the stage for plaintiffs’ attorneys within given states to [pursue] class actions for emotional distress or invasion of privacy on the grounds there was negligence” in connection to HIPAA violations.
The decision stems from a lawsuit filed by Emily Byrne v. Avery Center for Obstetrics and Gynecology P.C. in Westport, Conn. Ms. Byrne claimed that in 2004, she instructed the health center not to release her medical records to an ex-boyfriend. In 2005, the medical center was served with a subpoena by the former boyfriend requesting the plaintiff’s medical records for a paternity proceeding. The defendant did not alert Ms. Byrne about the subpoena and mailed a copy of her medical file to the probate court, according to court records.
Ms. Byrne later sued the health care center, claiming she suffered harassment and extortion threats from her ex-boyfriend because the medical records were exposed. The complaint alleged the health center engaged in negligent infliction of emotional distress and acted negligently by failing to use proper and reasonable care in protecting her medical file, including disclosing it without authorization in violation of HIPAA.
A trial court agreed with the medical practice’s contention that HIPAA precludes individual liability claims pertaining to confidentiality of medical information. The court cited well-established case law that HIPAA does not create a private right of action and requires alleged privacy violations to be raised through administrative channels. But the Connecticut Supreme Court overturned the decision, ruling that HIPAA does not preempt such negligence lawsuits.
“If Connecticut’s common law recognizes claims arising from a health care provider’s alleged breach of its duty of confidentiality in the course of complying with a subpoena, HIPAA and its implementing regulations do not preempt such claims,” judges said in their opinion. “We further conclude that, to the extent it has become the common practice for Connecticut health care providers to follow the procedures required under HIPAA in rendering services to their patients, HIPAA and its implementing regulations may be utilized to inform the standard of care applicable to such claims arising from allegations of negligence in the disclosure of patients’ medical records pursuant to a subpoena.”
The court went on to say that “the availability of such private rights of action in state courts ... do not preclude, conflict with, or complicate health care providers’ compliance with HIPAA.”
Similar decisions have been made by other courts, but the Connecticut ruling is the first state Supreme Court to issue such a ruling, Mr. Kline said.
For example, in Harmon v. Maury County, the U.S. District Court for the Middle District of Tennessee found that negligence claims founded on violation of HIPAA were not precluded because federal provisions do not completely preempt state law and expressly preserve state laws that aren’t at odds with its terms. The 2005 Tennessee case resulted from a privacy violation claim by a patient against a pharmacy manager. In another pharmacy-patient complaint, Fanean v. Rite Aid Corp. of Delaware Inc., the Superior Court of Delaware concluded that negligence claims could not be premised on a HIPAA violation, but that a common law negligence claim could be predicated upon Occupational Safety and Health Administration requirements. The 2009 opinion noted that HIPAA may act as a “guidepost” to determine the standard of care in common-law negligence claims.
The similar assertions by Connecticut judges that HIPAA does not preempt state rights and can also be used as a standard for what constitutes negligence or improper care of records is concerning for health providers, Mr. Kline said in an interview. Doctors now have to worry that inadvertent HIPAA violations may yield not only a complaint with the Office for Civil Rights, but a potential malpractice suit, as well.
“I would not be surprised if a case like this or even this case is appealed to the Supreme Court of the United States,” Mr. Kline said. “It is still a question of federal law, and what does the federal preemption mean?”
On Twitter @legal_med
Patients can sue doctors for negligence after alleged patient privacy breaches, the Connecticut Supreme Court has ruled in a decision that could have nationwide implications.
The state’s highest court concluded that HIPAA does not preempt claims for emotional distress or negligence under state law. The ruling, published Nov. 11, sets precedence in Connecticut and is likely to encourage plaintiffs to raise similar claims in other states, according to Michael J. Kline, a New Jersey attorney who specializes in corporate and securities law.
“It’s a momentous case, and I think it’s serious for physician practices,” said Mr. Kline. “It can set the stage for plaintiffs’ attorneys within given states to [pursue] class actions for emotional distress or invasion of privacy on the grounds there was negligence” in connection to HIPAA violations.
The decision stems from a lawsuit filed by Emily Byrne v. Avery Center for Obstetrics and Gynecology P.C. in Westport, Conn. Ms. Byrne claimed that in 2004, she instructed the health center not to release her medical records to an ex-boyfriend. In 2005, the medical center was served with a subpoena by the former boyfriend requesting the plaintiff’s medical records for a paternity proceeding. The defendant did not alert Ms. Byrne about the subpoena and mailed a copy of her medical file to the probate court, according to court records.
Ms. Byrne later sued the health care center, claiming she suffered harassment and extortion threats from her ex-boyfriend because the medical records were exposed. The complaint alleged the health center engaged in negligent infliction of emotional distress and acted negligently by failing to use proper and reasonable care in protecting her medical file, including disclosing it without authorization in violation of HIPAA.
A trial court agreed with the medical practice’s contention that HIPAA precludes individual liability claims pertaining to confidentiality of medical information. The court cited well-established case law that HIPAA does not create a private right of action and requires alleged privacy violations to be raised through administrative channels. But the Connecticut Supreme Court overturned the decision, ruling that HIPAA does not preempt such negligence lawsuits.
“If Connecticut’s common law recognizes claims arising from a health care provider’s alleged breach of its duty of confidentiality in the course of complying with a subpoena, HIPAA and its implementing regulations do not preempt such claims,” judges said in their opinion. “We further conclude that, to the extent it has become the common practice for Connecticut health care providers to follow the procedures required under HIPAA in rendering services to their patients, HIPAA and its implementing regulations may be utilized to inform the standard of care applicable to such claims arising from allegations of negligence in the disclosure of patients’ medical records pursuant to a subpoena.”
The court went on to say that “the availability of such private rights of action in state courts ... do not preclude, conflict with, or complicate health care providers’ compliance with HIPAA.”
Similar decisions have been made by other courts, but the Connecticut ruling is the first state Supreme Court to issue such a ruling, Mr. Kline said.
For example, in Harmon v. Maury County, the U.S. District Court for the Middle District of Tennessee found that negligence claims founded on violation of HIPAA were not precluded because federal provisions do not completely preempt state law and expressly preserve state laws that aren’t at odds with its terms. The 2005 Tennessee case resulted from a privacy violation claim by a patient against a pharmacy manager. In another pharmacy-patient complaint, Fanean v. Rite Aid Corp. of Delaware Inc., the Superior Court of Delaware concluded that negligence claims could not be premised on a HIPAA violation, but that a common law negligence claim could be predicated upon Occupational Safety and Health Administration requirements. The 2009 opinion noted that HIPAA may act as a “guidepost” to determine the standard of care in common-law negligence claims.
The similar assertions by Connecticut judges that HIPAA does not preempt state rights and can also be used as a standard for what constitutes negligence or improper care of records is concerning for health providers, Mr. Kline said in an interview. Doctors now have to worry that inadvertent HIPAA violations may yield not only a complaint with the Office for Civil Rights, but a potential malpractice suit, as well.
“I would not be surprised if a case like this or even this case is appealed to the Supreme Court of the United States,” Mr. Kline said. “It is still a question of federal law, and what does the federal preemption mean?”
On Twitter @legal_med
IOM recommends social factors to include in EHRs
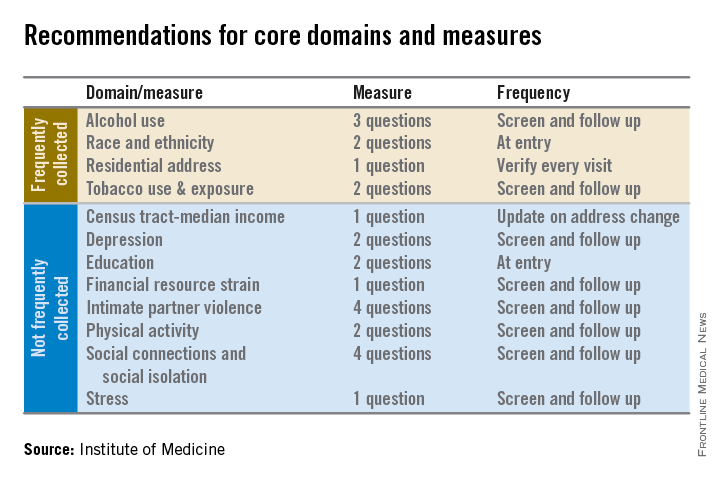
Electronic health records should be equipped to record and track 12 social and behavioral determinants of health, an Institute of Medicine committee recommended.
In addition to measures that are routinely collected now – race/ethnicity, tobacco use, alcohol use, and residential address – the committee advocated that electronic heath records (EHRs) should be able to capture:
• Educational attainment.
• Financial resource strain.
• Stress.
• Depression.
• Physical activity.
• Social isolation.
• Intimate partner violence (for women of reproductive age).
• Neighborhood median household income.
These measures can “provide crucial information about factors that influence health and the effectiveness of treatment,” collect data for researchers and policy makers, and help inform innovations that might improve health outcomes or reduce costs, according to the Nov. 13 report.
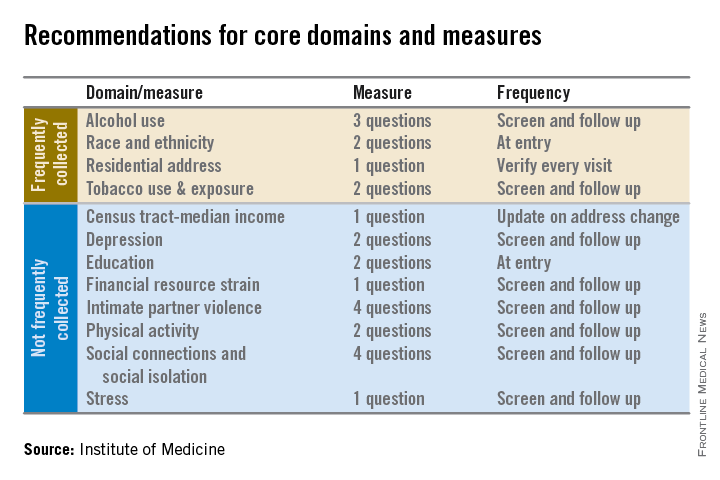
The panel aimed for what it called a “parsimonious panel of measures,” to help reduce the data collection burden for patients and health care providers, committee cochair Dr. William W. Stead, McKesson Foundation Professor of Biomedical Informatics and professor of medicine at Vanderbilt University, said during a press briefing.
The IOM report will be used by the Office of the National Coordinator for Health Information Technology (ONC) to determine what it should require from certified EHRs and from physicians who are participating in Medicare’s meaningful use incentive payment program. Physicians will be required to document social and behavioral determinants under Stage 3 of meaningful use, which begins in 2017.
Dr. Stead said that the speed of inclusion of the social and behavioral determinants in EHRs will partly be determined by whether the ONC follows the panel’s recommendations and requires them as part of meaningful use. He noted that in the past, EHR vendors and health care systems have been told by the ONC that they need to obtain certain types of information, “but then had to figure out on their own how to capture that information.
“There’s no reason why this needs to take years,” said Dr. Stead.
With the IOM recommendations, “we’re building the interoperability in from the beginning by providing a concise set of standard questions,” he said.
It will likely take less time to get the determinants into EHR packages than for health systems and physicians to figure out how to build the data collection into their workflow, Dr. Stead said.
The committee acknowledges that it will take more time during a patient encounter to collect these data. But, wrote the panel in the report, “the committee concluded that the health benefits of addressing these determinants outweigh the added burden to providers, patients, and health care systems.”
Additional recommendations in the report include:
• That the ONC’s EHR certification process be expanded to include appraisal of a vendor or product’s ability to acquire, store, transmit, and download self-reported data germane to the social and behavioral determinants of health.
• That the National Institutes of Health develops a plan for advancing research using social and behavioral determinants of health collected in electronic health records.
• That the Health & Human Services department convenes a task force within the next 3 years, and as needed thereafter, to review advances in the measurement of social and behavioral determinants and make recommendations for new standards and data elements for inclusion in electronic health records.
The committee’s work builds on draft recommendations published in April. It was sponsored by a number of federal agencies and health care foundations.
Dr. Michael E. Nelson, FCCP, comments: While physicians might agree that the recommendations of the Institute of Medicine (IOM) might enhance medical care, it is naïve to believe that this administrative burden will not make it increasingly difficult for physicians to meet Stage 3 of meaningful use should these requirements be added by the Office of the National Coordinator for Health Information Technology (ONC).

|
| Dr. Michael E. Nelson |
Assuming that EHR vendors will incorporate this information into their software, the time required to elicit and record this information is not insignificant and will put further strain on the already busy clinician.
In addition, there is a major assumption that the patient will actually provide this information willingly and that there would be implied consent to allow this information to be shared with the federal government, anonymously or otherwise.
Should the ONC adopt these recommendations from the IOM, one would hope that EHR vendors are required to add this to their software at no additional cost to physicians. Also, each question might have a button for "patient declined to answer." George Orwell might have been more prescient than credited.
Dr. Nelson is affiliated with Shawnee Mission Pulmonary Consultants in Shawnee Mission, KS.
Dr. Michael E. Nelson, FCCP, comments: While physicians might agree that the recommendations of the Institute of Medicine (IOM) might enhance medical care, it is naïve to believe that this administrative burden will not make it increasingly difficult for physicians to meet Stage 3 of meaningful use should these requirements be added by the Office of the National Coordinator for Health Information Technology (ONC).

|
| Dr. Michael E. Nelson |
Assuming that EHR vendors will incorporate this information into their software, the time required to elicit and record this information is not insignificant and will put further strain on the already busy clinician.
In addition, there is a major assumption that the patient will actually provide this information willingly and that there would be implied consent to allow this information to be shared with the federal government, anonymously or otherwise.
Should the ONC adopt these recommendations from the IOM, one would hope that EHR vendors are required to add this to their software at no additional cost to physicians. Also, each question might have a button for "patient declined to answer." George Orwell might have been more prescient than credited.
Dr. Nelson is affiliated with Shawnee Mission Pulmonary Consultants in Shawnee Mission, KS.
Dr. Michael E. Nelson, FCCP, comments: While physicians might agree that the recommendations of the Institute of Medicine (IOM) might enhance medical care, it is naïve to believe that this administrative burden will not make it increasingly difficult for physicians to meet Stage 3 of meaningful use should these requirements be added by the Office of the National Coordinator for Health Information Technology (ONC).

|
| Dr. Michael E. Nelson |
Assuming that EHR vendors will incorporate this information into their software, the time required to elicit and record this information is not insignificant and will put further strain on the already busy clinician.
In addition, there is a major assumption that the patient will actually provide this information willingly and that there would be implied consent to allow this information to be shared with the federal government, anonymously or otherwise.
Should the ONC adopt these recommendations from the IOM, one would hope that EHR vendors are required to add this to their software at no additional cost to physicians. Also, each question might have a button for "patient declined to answer." George Orwell might have been more prescient than credited.
Dr. Nelson is affiliated with Shawnee Mission Pulmonary Consultants in Shawnee Mission, KS.
Electronic health records should be equipped to record and track 12 social and behavioral determinants of health, an Institute of Medicine committee recommended.
In addition to measures that are routinely collected now – race/ethnicity, tobacco use, alcohol use, and residential address – the committee advocated that electronic heath records (EHRs) should be able to capture:
• Educational attainment.
• Financial resource strain.
• Stress.
• Depression.
• Physical activity.
• Social isolation.
• Intimate partner violence (for women of reproductive age).
• Neighborhood median household income.
These measures can “provide crucial information about factors that influence health and the effectiveness of treatment,” collect data for researchers and policy makers, and help inform innovations that might improve health outcomes or reduce costs, according to the Nov. 13 report.

The panel aimed for what it called a “parsimonious panel of measures,” to help reduce the data collection burden for patients and health care providers, committee cochair Dr. William W. Stead, McKesson Foundation Professor of Biomedical Informatics and professor of medicine at Vanderbilt University, said during a press briefing.
The IOM report will be used by the Office of the National Coordinator for Health Information Technology (ONC) to determine what it should require from certified EHRs and from physicians who are participating in Medicare’s meaningful use incentive payment program. Physicians will be required to document social and behavioral determinants under Stage 3 of meaningful use, which begins in 2017.
Dr. Stead said that the speed of inclusion of the social and behavioral determinants in EHRs will partly be determined by whether the ONC follows the panel’s recommendations and requires them as part of meaningful use. He noted that in the past, EHR vendors and health care systems have been told by the ONC that they need to obtain certain types of information, “but then had to figure out on their own how to capture that information.
“There’s no reason why this needs to take years,” said Dr. Stead.
With the IOM recommendations, “we’re building the interoperability in from the beginning by providing a concise set of standard questions,” he said.
It will likely take less time to get the determinants into EHR packages than for health systems and physicians to figure out how to build the data collection into their workflow, Dr. Stead said.
The committee acknowledges that it will take more time during a patient encounter to collect these data. But, wrote the panel in the report, “the committee concluded that the health benefits of addressing these determinants outweigh the added burden to providers, patients, and health care systems.”
Additional recommendations in the report include:
• That the ONC’s EHR certification process be expanded to include appraisal of a vendor or product’s ability to acquire, store, transmit, and download self-reported data germane to the social and behavioral determinants of health.
• That the National Institutes of Health develops a plan for advancing research using social and behavioral determinants of health collected in electronic health records.
• That the Health & Human Services department convenes a task force within the next 3 years, and as needed thereafter, to review advances in the measurement of social and behavioral determinants and make recommendations for new standards and data elements for inclusion in electronic health records.
The committee’s work builds on draft recommendations published in April. It was sponsored by a number of federal agencies and health care foundations.
Electronic health records should be equipped to record and track 12 social and behavioral determinants of health, an Institute of Medicine committee recommended.
In addition to measures that are routinely collected now – race/ethnicity, tobacco use, alcohol use, and residential address – the committee advocated that electronic heath records (EHRs) should be able to capture:
• Educational attainment.
• Financial resource strain.
• Stress.
• Depression.
• Physical activity.
• Social isolation.
• Intimate partner violence (for women of reproductive age).
• Neighborhood median household income.
These measures can “provide crucial information about factors that influence health and the effectiveness of treatment,” collect data for researchers and policy makers, and help inform innovations that might improve health outcomes or reduce costs, according to the Nov. 13 report.

The panel aimed for what it called a “parsimonious panel of measures,” to help reduce the data collection burden for patients and health care providers, committee cochair Dr. William W. Stead, McKesson Foundation Professor of Biomedical Informatics and professor of medicine at Vanderbilt University, said during a press briefing.
The IOM report will be used by the Office of the National Coordinator for Health Information Technology (ONC) to determine what it should require from certified EHRs and from physicians who are participating in Medicare’s meaningful use incentive payment program. Physicians will be required to document social and behavioral determinants under Stage 3 of meaningful use, which begins in 2017.
Dr. Stead said that the speed of inclusion of the social and behavioral determinants in EHRs will partly be determined by whether the ONC follows the panel’s recommendations and requires them as part of meaningful use. He noted that in the past, EHR vendors and health care systems have been told by the ONC that they need to obtain certain types of information, “but then had to figure out on their own how to capture that information.
“There’s no reason why this needs to take years,” said Dr. Stead.
With the IOM recommendations, “we’re building the interoperability in from the beginning by providing a concise set of standard questions,” he said.
It will likely take less time to get the determinants into EHR packages than for health systems and physicians to figure out how to build the data collection into their workflow, Dr. Stead said.
The committee acknowledges that it will take more time during a patient encounter to collect these data. But, wrote the panel in the report, “the committee concluded that the health benefits of addressing these determinants outweigh the added burden to providers, patients, and health care systems.”
Additional recommendations in the report include:
• That the ONC’s EHR certification process be expanded to include appraisal of a vendor or product’s ability to acquire, store, transmit, and download self-reported data germane to the social and behavioral determinants of health.
• That the National Institutes of Health develops a plan for advancing research using social and behavioral determinants of health collected in electronic health records.
• That the Health & Human Services department convenes a task force within the next 3 years, and as needed thereafter, to review advances in the measurement of social and behavioral determinants and make recommendations for new standards and data elements for inclusion in electronic health records.
The committee’s work builds on draft recommendations published in April. It was sponsored by a number of federal agencies and health care foundations.
Intragastric balloons offer weight loss surgery alternative
BOSTON – Moderately obese patients who either don’t want or don’t qualify for bariatric surgery may be able to benefit from a reversible procedure using an investigational intragastric balloon.
In a randomized controlled trial, patients with a body mass index (BMI) from 30 to 40 kg/m2 who were assigned to receive a dual intragastric balloon (ReShape Duo) plus a diet and exercise regimen lost 25% of their excess weight, compared with only 11% of patients assigned to undergo a sham procedure plus diet and exercise, reported Dr. Jaime Ponce, medical director for the Bariatric Surgery program at Hamilton Medical Center in Dalton, Ga., and Memorial Hospital in Chattanooga, Tenn.
“The ReShape procedure is a reversible intervention that can be used in patients with BMI from 30 to 40 who are not ready for surgery or did not qualify for surgery. It was effective, as it showed a 2.2 times greater weight loss, compared with the diet group,” he said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
At 48 weeks, patients sustained on average 65% of the weight loss they had achieved at week 24, he said.
Two-chamber device
The dual intragastric balloon consists of two silicone balloons connected by a flexible shaft to provide migration resistance. The deflated device is inserted over a guide wire into the stomach in a transoral endoscopic procedure. Once in place, the device is inflated with a saline and methylene blue solution by a powered pump, up to a total volume of 750 to 900 cc. The mean duration of the procedure is 8 minutes Dr. Ponce said.
Barring problems, the device is left in place for 6 months and is then emptied, captured with a standard endoscopic snare, and removed, a process that takes a mean of 14 minutes.
Dr. Ponce and colleagues enrolled obese adults with a BMD from 30 to 40 kg/m2 and one or more obesity-related comorbidities to undergo either balloon insertion, diet and exercise (187 patients), or a sham procedure plus diet and exercise (139).
All patients had monthly counseling on diet and exercise as per obesity management guidelines from the National Heart, Lung and Blood Institute published in 2000.
The participants were blinded to treatment assignment for 24 weeks, at which time patients in the diet group could exit the study or, if they wished, receive the balloon and continue in the study for an additional 24 weeks. Patients who initially received the balloon remained in the study and continued diet and exercise during the same 24 weeks.
Balloons were successfully inserted in 99.6% of cases, and all inserted balloons were retrieved successfully. Three patients had serious adverse events related to retrieval: one case of pneumonia requiring hospitalization and antibiotics, one contained perforation of the cervical esophagus, also requiring hospitalization and antibiotics, and one proximal esophageal mucosal tear requiring hemostatic clips.
The trial met its primary endpoint of a greater than 7.5% difference between the balloon and control groups, with balloon receivers having a mean excess weight loss of 25.1%, compared with 11.3% for controls (P = .0041) in an intention to treat analysis, and 27.9% vs. 12.3%, respectively, in patients who completed the study (P = .0007).
At 48 weeks, patients initially assigned to receive the balloon had significant improvements in hemoglobin A1c, cholesterol levels (HDL up, LDL down), systolic and diastolic blood pressures, and waist and hip circumference.
Among all implanted participants, 11.6% had mild to moderate nausea and vomiting on day 3, which gradually declined over the study. In addition, 34.1% had abdominal pain they rated as mild to moderate on a visual analog scale,
The safety analysis, including all 264 patients who received the balloon initially or at week 24, showed no deaths, balloon migration, obstructions, or required surgeries. Most adverse events were gastrointestinal in nature, mild to moderate, and resolved within the first 30 days.
Investigators saw gastric ulcers in 93 of the 264 patients who received the balloon. They determined the cause to be the distal tip of the device contacting the incisural wall, where more than 95% of the ulcers were observed. The manufacturer made minor changes to lower the profile of the tip and make it smoother and softer, resulting a “dramatically reduced ulcer rate and size,” Dr. Ponce said.
In all, 15% of the balloons were retrieved early, 6% after 2 months, associated with ulcers, and 9% within 2 months of insertions because of device intolerance. The authors found shorter patients had significantly fewer problems when the balloons were inflated with 750 cc rather than 900 cc.
The balloons spontaneously deflated in 6% of participants, signaled by the presence of blue-green urine in about two-thirds of these patients. All of the devices in these cases were successfully retrieved without problems, Dr. Ponce said.
Dr. Manoel P. Galvao Neto of the Gastro Obeso Center in São Paulo, Brazil, the invited discussant, commented that the study was well designed and carried out, with a clear methodology and frank assessment of adverse events, and it met all of its primary endpoints.
Excretable balloon
In a separate pilot study, eight patients who swallowed a limited-duration, self-emptying balloon (Elipse) that is excreted through the bowel lost an average of 12% of excess body weight, reported Dr. Evzen Machytka of the department of clinical studies at the University of Ostrava, Czech Republic.
For the trial, investigators used a custom device designed to self-deflate in 6 weeks. The device is packaged in a capsule and is attached to a thin capillary tube. The patient swallows the balloon without endoscopy or anesthesia. The capsule dissolves quickly, and when gastric positioning of the balloon is confirmed with x-rays, the balloon is then filled with 450 mL saline, in a process that takes approximately 15 minutes.
After a prespecified time (6 weeks, in the case of the trial, 3 months in the device intended for market) a self-releasing valve opens, the balloon empties and is then excreted normally, Dr. Machytka said.
In the pilot trial, all eight balloons were safely excreted. One had deflated early because of a manufacturing defect, and one asymptomatic patient withdrew from the study because she “no longer enjoyed eating.” In both cases, the balloons were punctured via endoscopy but not retrieved, and were excreted normally in the stool 4 days later.
The investigators hope to receive marketing approval for the device in 2015, Dr. Machytka said.
BOSTON – Moderately obese patients who either don’t want or don’t qualify for bariatric surgery may be able to benefit from a reversible procedure using an investigational intragastric balloon.
In a randomized controlled trial, patients with a body mass index (BMI) from 30 to 40 kg/m2 who were assigned to receive a dual intragastric balloon (ReShape Duo) plus a diet and exercise regimen lost 25% of their excess weight, compared with only 11% of patients assigned to undergo a sham procedure plus diet and exercise, reported Dr. Jaime Ponce, medical director for the Bariatric Surgery program at Hamilton Medical Center in Dalton, Ga., and Memorial Hospital in Chattanooga, Tenn.
“The ReShape procedure is a reversible intervention that can be used in patients with BMI from 30 to 40 who are not ready for surgery or did not qualify for surgery. It was effective, as it showed a 2.2 times greater weight loss, compared with the diet group,” he said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
At 48 weeks, patients sustained on average 65% of the weight loss they had achieved at week 24, he said.
Two-chamber device
The dual intragastric balloon consists of two silicone balloons connected by a flexible shaft to provide migration resistance. The deflated device is inserted over a guide wire into the stomach in a transoral endoscopic procedure. Once in place, the device is inflated with a saline and methylene blue solution by a powered pump, up to a total volume of 750 to 900 cc. The mean duration of the procedure is 8 minutes Dr. Ponce said.
Barring problems, the device is left in place for 6 months and is then emptied, captured with a standard endoscopic snare, and removed, a process that takes a mean of 14 minutes.
Dr. Ponce and colleagues enrolled obese adults with a BMD from 30 to 40 kg/m2 and one or more obesity-related comorbidities to undergo either balloon insertion, diet and exercise (187 patients), or a sham procedure plus diet and exercise (139).
All patients had monthly counseling on diet and exercise as per obesity management guidelines from the National Heart, Lung and Blood Institute published in 2000.
The participants were blinded to treatment assignment for 24 weeks, at which time patients in the diet group could exit the study or, if they wished, receive the balloon and continue in the study for an additional 24 weeks. Patients who initially received the balloon remained in the study and continued diet and exercise during the same 24 weeks.
Balloons were successfully inserted in 99.6% of cases, and all inserted balloons were retrieved successfully. Three patients had serious adverse events related to retrieval: one case of pneumonia requiring hospitalization and antibiotics, one contained perforation of the cervical esophagus, also requiring hospitalization and antibiotics, and one proximal esophageal mucosal tear requiring hemostatic clips.
The trial met its primary endpoint of a greater than 7.5% difference between the balloon and control groups, with balloon receivers having a mean excess weight loss of 25.1%, compared with 11.3% for controls (P = .0041) in an intention to treat analysis, and 27.9% vs. 12.3%, respectively, in patients who completed the study (P = .0007).
At 48 weeks, patients initially assigned to receive the balloon had significant improvements in hemoglobin A1c, cholesterol levels (HDL up, LDL down), systolic and diastolic blood pressures, and waist and hip circumference.
Among all implanted participants, 11.6% had mild to moderate nausea and vomiting on day 3, which gradually declined over the study. In addition, 34.1% had abdominal pain they rated as mild to moderate on a visual analog scale,
The safety analysis, including all 264 patients who received the balloon initially or at week 24, showed no deaths, balloon migration, obstructions, or required surgeries. Most adverse events were gastrointestinal in nature, mild to moderate, and resolved within the first 30 days.
Investigators saw gastric ulcers in 93 of the 264 patients who received the balloon. They determined the cause to be the distal tip of the device contacting the incisural wall, where more than 95% of the ulcers were observed. The manufacturer made minor changes to lower the profile of the tip and make it smoother and softer, resulting a “dramatically reduced ulcer rate and size,” Dr. Ponce said.
In all, 15% of the balloons were retrieved early, 6% after 2 months, associated with ulcers, and 9% within 2 months of insertions because of device intolerance. The authors found shorter patients had significantly fewer problems when the balloons were inflated with 750 cc rather than 900 cc.
The balloons spontaneously deflated in 6% of participants, signaled by the presence of blue-green urine in about two-thirds of these patients. All of the devices in these cases were successfully retrieved without problems, Dr. Ponce said.
Dr. Manoel P. Galvao Neto of the Gastro Obeso Center in São Paulo, Brazil, the invited discussant, commented that the study was well designed and carried out, with a clear methodology and frank assessment of adverse events, and it met all of its primary endpoints.
Excretable balloon
In a separate pilot study, eight patients who swallowed a limited-duration, self-emptying balloon (Elipse) that is excreted through the bowel lost an average of 12% of excess body weight, reported Dr. Evzen Machytka of the department of clinical studies at the University of Ostrava, Czech Republic.
For the trial, investigators used a custom device designed to self-deflate in 6 weeks. The device is packaged in a capsule and is attached to a thin capillary tube. The patient swallows the balloon without endoscopy or anesthesia. The capsule dissolves quickly, and when gastric positioning of the balloon is confirmed with x-rays, the balloon is then filled with 450 mL saline, in a process that takes approximately 15 minutes.
After a prespecified time (6 weeks, in the case of the trial, 3 months in the device intended for market) a self-releasing valve opens, the balloon empties and is then excreted normally, Dr. Machytka said.
In the pilot trial, all eight balloons were safely excreted. One had deflated early because of a manufacturing defect, and one asymptomatic patient withdrew from the study because she “no longer enjoyed eating.” In both cases, the balloons were punctured via endoscopy but not retrieved, and were excreted normally in the stool 4 days later.
The investigators hope to receive marketing approval for the device in 2015, Dr. Machytka said.
BOSTON – Moderately obese patients who either don’t want or don’t qualify for bariatric surgery may be able to benefit from a reversible procedure using an investigational intragastric balloon.
In a randomized controlled trial, patients with a body mass index (BMI) from 30 to 40 kg/m2 who were assigned to receive a dual intragastric balloon (ReShape Duo) plus a diet and exercise regimen lost 25% of their excess weight, compared with only 11% of patients assigned to undergo a sham procedure plus diet and exercise, reported Dr. Jaime Ponce, medical director for the Bariatric Surgery program at Hamilton Medical Center in Dalton, Ga., and Memorial Hospital in Chattanooga, Tenn.
“The ReShape procedure is a reversible intervention that can be used in patients with BMI from 30 to 40 who are not ready for surgery or did not qualify for surgery. It was effective, as it showed a 2.2 times greater weight loss, compared with the diet group,” he said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
At 48 weeks, patients sustained on average 65% of the weight loss they had achieved at week 24, he said.
Two-chamber device
The dual intragastric balloon consists of two silicone balloons connected by a flexible shaft to provide migration resistance. The deflated device is inserted over a guide wire into the stomach in a transoral endoscopic procedure. Once in place, the device is inflated with a saline and methylene blue solution by a powered pump, up to a total volume of 750 to 900 cc. The mean duration of the procedure is 8 minutes Dr. Ponce said.
Barring problems, the device is left in place for 6 months and is then emptied, captured with a standard endoscopic snare, and removed, a process that takes a mean of 14 minutes.
Dr. Ponce and colleagues enrolled obese adults with a BMD from 30 to 40 kg/m2 and one or more obesity-related comorbidities to undergo either balloon insertion, diet and exercise (187 patients), or a sham procedure plus diet and exercise (139).
All patients had monthly counseling on diet and exercise as per obesity management guidelines from the National Heart, Lung and Blood Institute published in 2000.
The participants were blinded to treatment assignment for 24 weeks, at which time patients in the diet group could exit the study or, if they wished, receive the balloon and continue in the study for an additional 24 weeks. Patients who initially received the balloon remained in the study and continued diet and exercise during the same 24 weeks.
Balloons were successfully inserted in 99.6% of cases, and all inserted balloons were retrieved successfully. Three patients had serious adverse events related to retrieval: one case of pneumonia requiring hospitalization and antibiotics, one contained perforation of the cervical esophagus, also requiring hospitalization and antibiotics, and one proximal esophageal mucosal tear requiring hemostatic clips.
The trial met its primary endpoint of a greater than 7.5% difference between the balloon and control groups, with balloon receivers having a mean excess weight loss of 25.1%, compared with 11.3% for controls (P = .0041) in an intention to treat analysis, and 27.9% vs. 12.3%, respectively, in patients who completed the study (P = .0007).
At 48 weeks, patients initially assigned to receive the balloon had significant improvements in hemoglobin A1c, cholesterol levels (HDL up, LDL down), systolic and diastolic blood pressures, and waist and hip circumference.
Among all implanted participants, 11.6% had mild to moderate nausea and vomiting on day 3, which gradually declined over the study. In addition, 34.1% had abdominal pain they rated as mild to moderate on a visual analog scale,
The safety analysis, including all 264 patients who received the balloon initially or at week 24, showed no deaths, balloon migration, obstructions, or required surgeries. Most adverse events were gastrointestinal in nature, mild to moderate, and resolved within the first 30 days.
Investigators saw gastric ulcers in 93 of the 264 patients who received the balloon. They determined the cause to be the distal tip of the device contacting the incisural wall, where more than 95% of the ulcers were observed. The manufacturer made minor changes to lower the profile of the tip and make it smoother and softer, resulting a “dramatically reduced ulcer rate and size,” Dr. Ponce said.
In all, 15% of the balloons were retrieved early, 6% after 2 months, associated with ulcers, and 9% within 2 months of insertions because of device intolerance. The authors found shorter patients had significantly fewer problems when the balloons were inflated with 750 cc rather than 900 cc.
The balloons spontaneously deflated in 6% of participants, signaled by the presence of blue-green urine in about two-thirds of these patients. All of the devices in these cases were successfully retrieved without problems, Dr. Ponce said.
Dr. Manoel P. Galvao Neto of the Gastro Obeso Center in São Paulo, Brazil, the invited discussant, commented that the study was well designed and carried out, with a clear methodology and frank assessment of adverse events, and it met all of its primary endpoints.
Excretable balloon
In a separate pilot study, eight patients who swallowed a limited-duration, self-emptying balloon (Elipse) that is excreted through the bowel lost an average of 12% of excess body weight, reported Dr. Evzen Machytka of the department of clinical studies at the University of Ostrava, Czech Republic.
For the trial, investigators used a custom device designed to self-deflate in 6 weeks. The device is packaged in a capsule and is attached to a thin capillary tube. The patient swallows the balloon without endoscopy or anesthesia. The capsule dissolves quickly, and when gastric positioning of the balloon is confirmed with x-rays, the balloon is then filled with 450 mL saline, in a process that takes approximately 15 minutes.
After a prespecified time (6 weeks, in the case of the trial, 3 months in the device intended for market) a self-releasing valve opens, the balloon empties and is then excreted normally, Dr. Machytka said.
In the pilot trial, all eight balloons were safely excreted. One had deflated early because of a manufacturing defect, and one asymptomatic patient withdrew from the study because she “no longer enjoyed eating.” In both cases, the balloons were punctured via endoscopy but not retrieved, and were excreted normally in the stool 4 days later.
The investigators hope to receive marketing approval for the device in 2015, Dr. Machytka said.
AT OBESITY WEEK 2014
Key clinical point: Intragastric balloons provide a temporary reversible alternative to bariatric surgery procedures.
Major finding: ReShape Duo plus a diet and exercise regimen was associated with a 25% loss of excess weight, compared with 11% for controls.
Data source: Randomized single-blinded study in 326 obese adults.
Disclosures: Dr. Ponce’s study was supported by ReShape Medical. Dr. Ponce is a clinical trial investigator and consultant to the company. Dr. Machytka’s study was supported by Allurion Technologies. He is principal investigator and receives travel support from the company. Dr. Neto reported having no relevant disclosures.
Enhanced thyroid cancer guidelines expected in 2015
CORONADO, CALIF. – Expect significant enhancements to the updated thyroid cancer management guidelines from the American Thyroid Association, due to be released in early 2015.
Last updated in 2009, the goal of the new guidelines is to “be evidence based and helpful,” guidelines task force chair Dr. Bryan R. Haugen said at the annual meeting of the American Thyroid Association. For example, the new guidelines will contain 101 recommendations, up from 80 in the 2009 version; 175 subrecommendations, up from 103; and 998 references, up from 437. “Still, 59 of the existing 80 recommendations are not substantially changed, showing a general stability in our field over the past 5 to 6 years,” he said.
One enhancement is a definition of risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy for thyroid cancer. Low risk is defined as intrathyroidal differentiated thyroid cancer involving up to five metastases less than 0.2 cm in size. Intermediate risk is defined as the presence of aggressive histology, minor extrathyroidal extension, vascular invasion, or more than five involved lymph nodes with metastases 0.2-0.3 cm in size. High risk is defined as the presence of gross extrathyroidal extension, incomplete tumor resection, distant metastases, or lymph node metastases greater than 3 cm in size.
The guidelines also include a table that defines a patient’s response to therapy as a dynamic risk assessment. “This best applies to the low- to intermediate-risk patients, although it definitely applies to high risk as well,” said Dr. Haugen, who heads the division of endocrinology, metabolism, and diabetes at the University of Colorado Health Sciences Center, Denver. “It’s [a] strong recommendation based on low-quality evidence to use this risk-based response to therapy. A lot of this data is generated from patients who’ve had a thyroidectomy and have received radioiodine. So we’re on a bit more shaky ground right now in a patient who’s had a thyroidectomy but no radioiodine, or a patient who’s had a lobectomy.”
Other changes include the concept that it’s not necessary to biopsy every nodule more than 1 cm in size. “We’re going to be guided by the sonographic pattern in who we biopsy and how we monitor them,” Dr. Haugen explained. “A new recommendation adds follow-up guidance for nodules that do not meet FNA [fine-needle aspiration] criteria. We’re also recommending use of the Bethesda Cytology Classification System for cytology.”
Changes in the initial management of thyroid cancer include a recommendation for cross-sectional imaging with contrast for higher-risk disease and the consideration of lobectomy for some patients with tumors 1-4 cm in size. “This is a controversial recommendation,” Dr. Haugen said. “We got some feedback from members asking if you do it, what’s the TSH target? Should we give them synthetic levothyroxine? We are revising the guidelines based on this feedback to help guide clinicians.”
The new guidelines also call for more detailed/standardized pathology reports, with inclusion of lymph node size, extranodal invasion, and the number of invaded vessels. “I’ve talked to a number of pathologists and clinicians who are very happy about this guidance,” he said. “We also need to look at tumor stage, recurrence risk, and response to therapy in our patients, and the use of selective radioiodine. There is some more information on considering lower administered activities, especially in the lower-risk patients.”
For the first time, the guidelines include a section on radioiodine treatment for refractory differentiated thyroid cancer, including tips on directed therapy, clinical trials, systemic therapy, and bone-specific therapy.
Dr. Haugen disclosed that he has received grants and research support from Veracyte and Genzyme.
On Twitter @dougbrunk
CORONADO, CALIF. – Expect significant enhancements to the updated thyroid cancer management guidelines from the American Thyroid Association, due to be released in early 2015.
Last updated in 2009, the goal of the new guidelines is to “be evidence based and helpful,” guidelines task force chair Dr. Bryan R. Haugen said at the annual meeting of the American Thyroid Association. For example, the new guidelines will contain 101 recommendations, up from 80 in the 2009 version; 175 subrecommendations, up from 103; and 998 references, up from 437. “Still, 59 of the existing 80 recommendations are not substantially changed, showing a general stability in our field over the past 5 to 6 years,” he said.
One enhancement is a definition of risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy for thyroid cancer. Low risk is defined as intrathyroidal differentiated thyroid cancer involving up to five metastases less than 0.2 cm in size. Intermediate risk is defined as the presence of aggressive histology, minor extrathyroidal extension, vascular invasion, or more than five involved lymph nodes with metastases 0.2-0.3 cm in size. High risk is defined as the presence of gross extrathyroidal extension, incomplete tumor resection, distant metastases, or lymph node metastases greater than 3 cm in size.
The guidelines also include a table that defines a patient’s response to therapy as a dynamic risk assessment. “This best applies to the low- to intermediate-risk patients, although it definitely applies to high risk as well,” said Dr. Haugen, who heads the division of endocrinology, metabolism, and diabetes at the University of Colorado Health Sciences Center, Denver. “It’s [a] strong recommendation based on low-quality evidence to use this risk-based response to therapy. A lot of this data is generated from patients who’ve had a thyroidectomy and have received radioiodine. So we’re on a bit more shaky ground right now in a patient who’s had a thyroidectomy but no radioiodine, or a patient who’s had a lobectomy.”
Other changes include the concept that it’s not necessary to biopsy every nodule more than 1 cm in size. “We’re going to be guided by the sonographic pattern in who we biopsy and how we monitor them,” Dr. Haugen explained. “A new recommendation adds follow-up guidance for nodules that do not meet FNA [fine-needle aspiration] criteria. We’re also recommending use of the Bethesda Cytology Classification System for cytology.”
Changes in the initial management of thyroid cancer include a recommendation for cross-sectional imaging with contrast for higher-risk disease and the consideration of lobectomy for some patients with tumors 1-4 cm in size. “This is a controversial recommendation,” Dr. Haugen said. “We got some feedback from members asking if you do it, what’s the TSH target? Should we give them synthetic levothyroxine? We are revising the guidelines based on this feedback to help guide clinicians.”
The new guidelines also call for more detailed/standardized pathology reports, with inclusion of lymph node size, extranodal invasion, and the number of invaded vessels. “I’ve talked to a number of pathologists and clinicians who are very happy about this guidance,” he said. “We also need to look at tumor stage, recurrence risk, and response to therapy in our patients, and the use of selective radioiodine. There is some more information on considering lower administered activities, especially in the lower-risk patients.”
For the first time, the guidelines include a section on radioiodine treatment for refractory differentiated thyroid cancer, including tips on directed therapy, clinical trials, systemic therapy, and bone-specific therapy.
Dr. Haugen disclosed that he has received grants and research support from Veracyte and Genzyme.
On Twitter @dougbrunk
CORONADO, CALIF. – Expect significant enhancements to the updated thyroid cancer management guidelines from the American Thyroid Association, due to be released in early 2015.
Last updated in 2009, the goal of the new guidelines is to “be evidence based and helpful,” guidelines task force chair Dr. Bryan R. Haugen said at the annual meeting of the American Thyroid Association. For example, the new guidelines will contain 101 recommendations, up from 80 in the 2009 version; 175 subrecommendations, up from 103; and 998 references, up from 437. “Still, 59 of the existing 80 recommendations are not substantially changed, showing a general stability in our field over the past 5 to 6 years,” he said.
One enhancement is a definition of risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy for thyroid cancer. Low risk is defined as intrathyroidal differentiated thyroid cancer involving up to five metastases less than 0.2 cm in size. Intermediate risk is defined as the presence of aggressive histology, minor extrathyroidal extension, vascular invasion, or more than five involved lymph nodes with metastases 0.2-0.3 cm in size. High risk is defined as the presence of gross extrathyroidal extension, incomplete tumor resection, distant metastases, or lymph node metastases greater than 3 cm in size.
The guidelines also include a table that defines a patient’s response to therapy as a dynamic risk assessment. “This best applies to the low- to intermediate-risk patients, although it definitely applies to high risk as well,” said Dr. Haugen, who heads the division of endocrinology, metabolism, and diabetes at the University of Colorado Health Sciences Center, Denver. “It’s [a] strong recommendation based on low-quality evidence to use this risk-based response to therapy. A lot of this data is generated from patients who’ve had a thyroidectomy and have received radioiodine. So we’re on a bit more shaky ground right now in a patient who’s had a thyroidectomy but no radioiodine, or a patient who’s had a lobectomy.”
Other changes include the concept that it’s not necessary to biopsy every nodule more than 1 cm in size. “We’re going to be guided by the sonographic pattern in who we biopsy and how we monitor them,” Dr. Haugen explained. “A new recommendation adds follow-up guidance for nodules that do not meet FNA [fine-needle aspiration] criteria. We’re also recommending use of the Bethesda Cytology Classification System for cytology.”
Changes in the initial management of thyroid cancer include a recommendation for cross-sectional imaging with contrast for higher-risk disease and the consideration of lobectomy for some patients with tumors 1-4 cm in size. “This is a controversial recommendation,” Dr. Haugen said. “We got some feedback from members asking if you do it, what’s the TSH target? Should we give them synthetic levothyroxine? We are revising the guidelines based on this feedback to help guide clinicians.”
The new guidelines also call for more detailed/standardized pathology reports, with inclusion of lymph node size, extranodal invasion, and the number of invaded vessels. “I’ve talked to a number of pathologists and clinicians who are very happy about this guidance,” he said. “We also need to look at tumor stage, recurrence risk, and response to therapy in our patients, and the use of selective radioiodine. There is some more information on considering lower administered activities, especially in the lower-risk patients.”
For the first time, the guidelines include a section on radioiodine treatment for refractory differentiated thyroid cancer, including tips on directed therapy, clinical trials, systemic therapy, and bone-specific therapy.
Dr. Haugen disclosed that he has received grants and research support from Veracyte and Genzyme.
On Twitter @dougbrunk
EXPERT ANALYSIS FROM THE ATA ANNUAL MEETING
Bicuspid valve surgical management in rapid flux
CHICAGO – Surgical management of leaky bicuspid valves is rapidly evolving from replacement to repair of any purely insufficient bicuspid valve with enough leaflet surface area.
“We’re coming to the point like with mitral valves 20 years ago, that we don’t have to replace every blessed bicuspid valve. We can repair a lot of these valves,” Dr. Joseph Bavaria, vice chief of cardiovascular surgery, University of Pennsylvania, Philadelphia, said.
A valve-sparing root procedure in a patient with bicuspid aortic valve (BAV) disease and aortic insufficiency (AI) provides excellent results, no matter if the aortic root is abnormal or not. Many patients with BAV, however, have leaky valves without a root aneurysm.
Thus, the “great dilemma” facing surgeons now is whether they can continue to justify doing a root procedure, which is a much bigger operation, when the root is normal diameter, Dr. Bavaria said at Heart Valve Summit 2014.
“It may be inappropriate in today’s world to take a normal physiological valve and just take it out and consign someone who’s in their 30s and 40s to a mechanical or bioprosthetic valve for life,” he said in an interview. “That has anticoagulation issues, structural deterioration issues, infection issues.”
The alternative is a valve repair operation, but that’s complicated by the growing understanding that BAV AI has three distinct phenotypic presentations:
• BAV with AI with relatively normal root diameters.
• BAV with AI and relatively normal root diameters, with an ascending aortic aneurysm.
• BAV with AI and root dilation.
“The problem we have is there’s probably a different therapeutic procedure for each of these three presentations,” Dr. Bavaria said.
The goals for any bicuspid repair are to equalize the free margin lengths with plication or resection of the redundant leaflet, reduce the annulus by 10%-15%, and stabilize it with either reimplantation or a subannular technique, and to increase the height of the free margin, if the leaflet belly falls below the annular plane.
In the university’s current surgical management algorithm, patients who have an aortic annulus dilated to 28 mm or more undergo root reimplantation if they have an aneurysmal root or receive external annuloplasty rings plus a valve repair if their aortic annulus is dilated and they have a nonaneurysmal root. The Dacron ring is placed subcoronary and subannular and is generally sized 5-7 mm larger than the desired end-procedural annular diameter, he said.
For patients with a normal aortic annulus (27 mm or less), root reimplantation or remodeling is used for those with an aneurysmal root, while subcommissural annuloplasty is reserved only for those with a normal aortic annulus and a nonaneurysmal root.
Subcommissural annuloplasty had been used for many BAV patients with AI who were candidates for repair, but emerging data over the last 2 years from Dr. Bavaria’s group (Annals Thor. Surg. 2014;97:1227-34) and others show it results in a lot of midterm failures and reoperations, compared with root reimplantation. Some groups continue to use this procedure routinely, but “It doesn’t work,” Dr. Bavaria said.
His team recently compared postoperative outcomes among BAV repairs and the more commonly performed tricuspid repair between 2004 and 2014. Overall, the outcomes were the same between the two groups including mortality, stroke, freedom from AI grade +1, and aortic reoperation for bleeding (P = NS). Notably, all 41 BAV patients required concomitant primary leaflet repair, compared with only 7% of the 99 patients who underwent tricuspid valve repair (P < .01), he said.
Dr. Bavaria reported consultant fees and honoraria from St. Jude Medical and research grants from Edwards Lifesciences and Sorin Group.
CHICAGO – Surgical management of leaky bicuspid valves is rapidly evolving from replacement to repair of any purely insufficient bicuspid valve with enough leaflet surface area.
“We’re coming to the point like with mitral valves 20 years ago, that we don’t have to replace every blessed bicuspid valve. We can repair a lot of these valves,” Dr. Joseph Bavaria, vice chief of cardiovascular surgery, University of Pennsylvania, Philadelphia, said.
A valve-sparing root procedure in a patient with bicuspid aortic valve (BAV) disease and aortic insufficiency (AI) provides excellent results, no matter if the aortic root is abnormal or not. Many patients with BAV, however, have leaky valves without a root aneurysm.
Thus, the “great dilemma” facing surgeons now is whether they can continue to justify doing a root procedure, which is a much bigger operation, when the root is normal diameter, Dr. Bavaria said at Heart Valve Summit 2014.
“It may be inappropriate in today’s world to take a normal physiological valve and just take it out and consign someone who’s in their 30s and 40s to a mechanical or bioprosthetic valve for life,” he said in an interview. “That has anticoagulation issues, structural deterioration issues, infection issues.”
The alternative is a valve repair operation, but that’s complicated by the growing understanding that BAV AI has three distinct phenotypic presentations:
• BAV with AI with relatively normal root diameters.
• BAV with AI and relatively normal root diameters, with an ascending aortic aneurysm.
• BAV with AI and root dilation.
“The problem we have is there’s probably a different therapeutic procedure for each of these three presentations,” Dr. Bavaria said.
The goals for any bicuspid repair are to equalize the free margin lengths with plication or resection of the redundant leaflet, reduce the annulus by 10%-15%, and stabilize it with either reimplantation or a subannular technique, and to increase the height of the free margin, if the leaflet belly falls below the annular plane.
In the university’s current surgical management algorithm, patients who have an aortic annulus dilated to 28 mm or more undergo root reimplantation if they have an aneurysmal root or receive external annuloplasty rings plus a valve repair if their aortic annulus is dilated and they have a nonaneurysmal root. The Dacron ring is placed subcoronary and subannular and is generally sized 5-7 mm larger than the desired end-procedural annular diameter, he said.
For patients with a normal aortic annulus (27 mm or less), root reimplantation or remodeling is used for those with an aneurysmal root, while subcommissural annuloplasty is reserved only for those with a normal aortic annulus and a nonaneurysmal root.
Subcommissural annuloplasty had been used for many BAV patients with AI who were candidates for repair, but emerging data over the last 2 years from Dr. Bavaria’s group (Annals Thor. Surg. 2014;97:1227-34) and others show it results in a lot of midterm failures and reoperations, compared with root reimplantation. Some groups continue to use this procedure routinely, but “It doesn’t work,” Dr. Bavaria said.
His team recently compared postoperative outcomes among BAV repairs and the more commonly performed tricuspid repair between 2004 and 2014. Overall, the outcomes were the same between the two groups including mortality, stroke, freedom from AI grade +1, and aortic reoperation for bleeding (P = NS). Notably, all 41 BAV patients required concomitant primary leaflet repair, compared with only 7% of the 99 patients who underwent tricuspid valve repair (P < .01), he said.
Dr. Bavaria reported consultant fees and honoraria from St. Jude Medical and research grants from Edwards Lifesciences and Sorin Group.
CHICAGO – Surgical management of leaky bicuspid valves is rapidly evolving from replacement to repair of any purely insufficient bicuspid valve with enough leaflet surface area.
“We’re coming to the point like with mitral valves 20 years ago, that we don’t have to replace every blessed bicuspid valve. We can repair a lot of these valves,” Dr. Joseph Bavaria, vice chief of cardiovascular surgery, University of Pennsylvania, Philadelphia, said.
A valve-sparing root procedure in a patient with bicuspid aortic valve (BAV) disease and aortic insufficiency (AI) provides excellent results, no matter if the aortic root is abnormal or not. Many patients with BAV, however, have leaky valves without a root aneurysm.
Thus, the “great dilemma” facing surgeons now is whether they can continue to justify doing a root procedure, which is a much bigger operation, when the root is normal diameter, Dr. Bavaria said at Heart Valve Summit 2014.
“It may be inappropriate in today’s world to take a normal physiological valve and just take it out and consign someone who’s in their 30s and 40s to a mechanical or bioprosthetic valve for life,” he said in an interview. “That has anticoagulation issues, structural deterioration issues, infection issues.”
The alternative is a valve repair operation, but that’s complicated by the growing understanding that BAV AI has three distinct phenotypic presentations:
• BAV with AI with relatively normal root diameters.
• BAV with AI and relatively normal root diameters, with an ascending aortic aneurysm.
• BAV with AI and root dilation.
“The problem we have is there’s probably a different therapeutic procedure for each of these three presentations,” Dr. Bavaria said.
The goals for any bicuspid repair are to equalize the free margin lengths with plication or resection of the redundant leaflet, reduce the annulus by 10%-15%, and stabilize it with either reimplantation or a subannular technique, and to increase the height of the free margin, if the leaflet belly falls below the annular plane.
In the university’s current surgical management algorithm, patients who have an aortic annulus dilated to 28 mm or more undergo root reimplantation if they have an aneurysmal root or receive external annuloplasty rings plus a valve repair if their aortic annulus is dilated and they have a nonaneurysmal root. The Dacron ring is placed subcoronary and subannular and is generally sized 5-7 mm larger than the desired end-procedural annular diameter, he said.
For patients with a normal aortic annulus (27 mm or less), root reimplantation or remodeling is used for those with an aneurysmal root, while subcommissural annuloplasty is reserved only for those with a normal aortic annulus and a nonaneurysmal root.
Subcommissural annuloplasty had been used for many BAV patients with AI who were candidates for repair, but emerging data over the last 2 years from Dr. Bavaria’s group (Annals Thor. Surg. 2014;97:1227-34) and others show it results in a lot of midterm failures and reoperations, compared with root reimplantation. Some groups continue to use this procedure routinely, but “It doesn’t work,” Dr. Bavaria said.
His team recently compared postoperative outcomes among BAV repairs and the more commonly performed tricuspid repair between 2004 and 2014. Overall, the outcomes were the same between the two groups including mortality, stroke, freedom from AI grade +1, and aortic reoperation for bleeding (P = NS). Notably, all 41 BAV patients required concomitant primary leaflet repair, compared with only 7% of the 99 patients who underwent tricuspid valve repair (P < .01), he said.
Dr. Bavaria reported consultant fees and honoraria from St. Jude Medical and research grants from Edwards Lifesciences and Sorin Group.
EXPERT OPINION FROM HEART VALVE SUMMIT 2014
Direct-acting antivirals treat recurrent HCV after transplant
BOSTON – Treating recurrent hepatitis C infection after liver transplantation using sofosbuvir-containing regimens produced a sustained virologic response 4 weeks later in 60%-90% of 237 patients, depending on genotype and the presence of cirrhosis.
Investigators analyzed data on 245 posttransplant patients with hepatitis C (HCV) collected from 38 academic medical centers and 15 community medical centers in North America and Europe participating in the prospective, longitudinal, observational HCV-Target study. Of the 227 patients who started treatment, 69% completed treatment, 3% discontinued treatment prematurely, and 28% are undergoing treatment.
Among the 68 patients with HCV genotype 1 and a known virologic outcome who were treated with sofosbuvir and simeprevir with or without ribavirin, 61 (90%) achieved a sustained virologic response for 4 weeks (SVR4).
Response rates in that group varied, however, depending on whether patients had cirrhosis (SVR4 in 32 of 37, 86%) or no cirrhosis (SVR4 in 29 of 31, 94%). Results also varied in that group by genotype, with SVR4 in 30 of 36 patients with genotype 1a infection (83%) and SVR4 in 18 of 19 patients with genotype 1b (95%), Dr. Robert S. Brown Jr. and his associates reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is the regimen that probably holds the highest interest,” Dr. Brown said, because most of the patients in the study were genotype 1, and most genotype 1 patients received sofosbuvir and simeprevir with or without ribavirin.
A total of 10 of 12 patients with HCV genotype 1 infection who were treated with sofosbuvir plus pegylated interferon and ribavirin achieved SVR4 (83%).
A total of 9 of 10 patients with HCV genotype 2 infection who had complete data and who received sofosbuvir plus ribavirin achieved SVR4 (90%), reported Dr. Brown, the Frank Cardile Professor of Medicine and Pediatrics (in Surgery) at Columbia University, New York, and director of the Center for Liver Disease and Transplantation at New York-Presbyterian Hospital/Columbia University Medical Center.
Three of five patients with genotype 3 infection and complete data who received sofosbuvir plus ribavirin achieved SVR4 (60%), and one patient with genotype 3 achieved SVR4 after treatment with sofosbuvir, pegylated interferon, and ribavirin.
Adverse events were reported by 82% of patients, with rates ranging from 77% to 92% in different treatment groups. “The majority of these were mild and manageable,” Dr. Brown noted. The overall rate of serious adverse events was 8%, ranging from 0% to 21% in different treatment groups, though some of those cases were unlikely to be related to treatment, he added.
In general, interferon-free regimens had low rates of serious adverse events, Dr. Brown said. No patients developed acute liver graft rejection.
“Hepatitis C following liver transplant does remain a problem in 2014, because reinfection is universal and therapeutic options, at least to date, have been limited,” he said. The study’s real-world data supplement encouraging results from small, controlled trials of newer anti-HCV agents in posttransplant patients.
Most of the patients in the study were treated for recurrent HCV “well after” transplantation, Dr. Brown said. “The optimal treatment regimen and the duration of therapy still remain to be determined.”
Treatment regimens in the HCV-Target study are selected by physicians according to the local standard of care.
The study was too small to do a multivariate analysis of predictive factors for SVR4, Dr. Brown commented.
The HCV-Target study was funded by AbbVie, Bristol Myers Squibb, Genentech, Gilead, GlaxoSmithKline, Kadmon, Janssen, Merck, and Vertex. Dr. Brown reported financial associations with AbbVie, Genentech, Gilead, Janssen, Merck, Salix, Vertex, and Vital Therapies.
On Twitter @sherryboschert
BOSTON – Treating recurrent hepatitis C infection after liver transplantation using sofosbuvir-containing regimens produced a sustained virologic response 4 weeks later in 60%-90% of 237 patients, depending on genotype and the presence of cirrhosis.
Investigators analyzed data on 245 posttransplant patients with hepatitis C (HCV) collected from 38 academic medical centers and 15 community medical centers in North America and Europe participating in the prospective, longitudinal, observational HCV-Target study. Of the 227 patients who started treatment, 69% completed treatment, 3% discontinued treatment prematurely, and 28% are undergoing treatment.
Among the 68 patients with HCV genotype 1 and a known virologic outcome who were treated with sofosbuvir and simeprevir with or without ribavirin, 61 (90%) achieved a sustained virologic response for 4 weeks (SVR4).
Response rates in that group varied, however, depending on whether patients had cirrhosis (SVR4 in 32 of 37, 86%) or no cirrhosis (SVR4 in 29 of 31, 94%). Results also varied in that group by genotype, with SVR4 in 30 of 36 patients with genotype 1a infection (83%) and SVR4 in 18 of 19 patients with genotype 1b (95%), Dr. Robert S. Brown Jr. and his associates reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is the regimen that probably holds the highest interest,” Dr. Brown said, because most of the patients in the study were genotype 1, and most genotype 1 patients received sofosbuvir and simeprevir with or without ribavirin.
A total of 10 of 12 patients with HCV genotype 1 infection who were treated with sofosbuvir plus pegylated interferon and ribavirin achieved SVR4 (83%).
A total of 9 of 10 patients with HCV genotype 2 infection who had complete data and who received sofosbuvir plus ribavirin achieved SVR4 (90%), reported Dr. Brown, the Frank Cardile Professor of Medicine and Pediatrics (in Surgery) at Columbia University, New York, and director of the Center for Liver Disease and Transplantation at New York-Presbyterian Hospital/Columbia University Medical Center.
Three of five patients with genotype 3 infection and complete data who received sofosbuvir plus ribavirin achieved SVR4 (60%), and one patient with genotype 3 achieved SVR4 after treatment with sofosbuvir, pegylated interferon, and ribavirin.
Adverse events were reported by 82% of patients, with rates ranging from 77% to 92% in different treatment groups. “The majority of these were mild and manageable,” Dr. Brown noted. The overall rate of serious adverse events was 8%, ranging from 0% to 21% in different treatment groups, though some of those cases were unlikely to be related to treatment, he added.
In general, interferon-free regimens had low rates of serious adverse events, Dr. Brown said. No patients developed acute liver graft rejection.
“Hepatitis C following liver transplant does remain a problem in 2014, because reinfection is universal and therapeutic options, at least to date, have been limited,” he said. The study’s real-world data supplement encouraging results from small, controlled trials of newer anti-HCV agents in posttransplant patients.
Most of the patients in the study were treated for recurrent HCV “well after” transplantation, Dr. Brown said. “The optimal treatment regimen and the duration of therapy still remain to be determined.”
Treatment regimens in the HCV-Target study are selected by physicians according to the local standard of care.
The study was too small to do a multivariate analysis of predictive factors for SVR4, Dr. Brown commented.
The HCV-Target study was funded by AbbVie, Bristol Myers Squibb, Genentech, Gilead, GlaxoSmithKline, Kadmon, Janssen, Merck, and Vertex. Dr. Brown reported financial associations with AbbVie, Genentech, Gilead, Janssen, Merck, Salix, Vertex, and Vital Therapies.
On Twitter @sherryboschert
BOSTON – Treating recurrent hepatitis C infection after liver transplantation using sofosbuvir-containing regimens produced a sustained virologic response 4 weeks later in 60%-90% of 237 patients, depending on genotype and the presence of cirrhosis.
Investigators analyzed data on 245 posttransplant patients with hepatitis C (HCV) collected from 38 academic medical centers and 15 community medical centers in North America and Europe participating in the prospective, longitudinal, observational HCV-Target study. Of the 227 patients who started treatment, 69% completed treatment, 3% discontinued treatment prematurely, and 28% are undergoing treatment.
Among the 68 patients with HCV genotype 1 and a known virologic outcome who were treated with sofosbuvir and simeprevir with or without ribavirin, 61 (90%) achieved a sustained virologic response for 4 weeks (SVR4).
Response rates in that group varied, however, depending on whether patients had cirrhosis (SVR4 in 32 of 37, 86%) or no cirrhosis (SVR4 in 29 of 31, 94%). Results also varied in that group by genotype, with SVR4 in 30 of 36 patients with genotype 1a infection (83%) and SVR4 in 18 of 19 patients with genotype 1b (95%), Dr. Robert S. Brown Jr. and his associates reported at the annual meeting of the American Association for the Study of Liver Diseases.
“This is the regimen that probably holds the highest interest,” Dr. Brown said, because most of the patients in the study were genotype 1, and most genotype 1 patients received sofosbuvir and simeprevir with or without ribavirin.
A total of 10 of 12 patients with HCV genotype 1 infection who were treated with sofosbuvir plus pegylated interferon and ribavirin achieved SVR4 (83%).
A total of 9 of 10 patients with HCV genotype 2 infection who had complete data and who received sofosbuvir plus ribavirin achieved SVR4 (90%), reported Dr. Brown, the Frank Cardile Professor of Medicine and Pediatrics (in Surgery) at Columbia University, New York, and director of the Center for Liver Disease and Transplantation at New York-Presbyterian Hospital/Columbia University Medical Center.
Three of five patients with genotype 3 infection and complete data who received sofosbuvir plus ribavirin achieved SVR4 (60%), and one patient with genotype 3 achieved SVR4 after treatment with sofosbuvir, pegylated interferon, and ribavirin.
Adverse events were reported by 82% of patients, with rates ranging from 77% to 92% in different treatment groups. “The majority of these were mild and manageable,” Dr. Brown noted. The overall rate of serious adverse events was 8%, ranging from 0% to 21% in different treatment groups, though some of those cases were unlikely to be related to treatment, he added.
In general, interferon-free regimens had low rates of serious adverse events, Dr. Brown said. No patients developed acute liver graft rejection.
“Hepatitis C following liver transplant does remain a problem in 2014, because reinfection is universal and therapeutic options, at least to date, have been limited,” he said. The study’s real-world data supplement encouraging results from small, controlled trials of newer anti-HCV agents in posttransplant patients.
Most of the patients in the study were treated for recurrent HCV “well after” transplantation, Dr. Brown said. “The optimal treatment regimen and the duration of therapy still remain to be determined.”
Treatment regimens in the HCV-Target study are selected by physicians according to the local standard of care.
The study was too small to do a multivariate analysis of predictive factors for SVR4, Dr. Brown commented.
The HCV-Target study was funded by AbbVie, Bristol Myers Squibb, Genentech, Gilead, GlaxoSmithKline, Kadmon, Janssen, Merck, and Vertex. Dr. Brown reported financial associations with AbbVie, Genentech, Gilead, Janssen, Merck, Salix, Vertex, and Vital Therapies.
On Twitter @sherryboschert
AT THE LIVER MEETING 2014
Key clinical point: Direct-acting antivirals can help most patients with recurrent HCV after liver transplantation.
Major finding: A sustained virologic response after 4 weeks was seen in 60%-90% of patients, depending on genotype and cirrhosis.
Data source: An analysis of a multicenter database on 227 patients treated with sofosbuvir-containing regimens since December 2013 for recurrent HCV after liver transplantation.
Disclosures: The study was funded by AbbVie, Bristol Myers Squibb, Genentech, Gilead, GlaxoSmithKline, Kadmon, Janssen, Merck, and Vertex. Dr. Brown reported financial associations with AbbVie, Genentech, Gilead, Janssen, Merck, Salix, Vertex, and Vital Therapies.