User login
Women undergoing hysterectomy, myomectomy have similar short-term outcomes
PHILADELPHIA – despite different baseline characteristics, according to recent results from the COMPARE-UF study presented at the annual meeting of the American Society for Reproductive Medicine.
“Both hysterectomy and myomectomy can substantially improve women’s quality of life scores and substantially reduce symptom severity,” reported Wanda K. Nicholson, MD, MPH, lead investigator for COMPARE-UF and professor of general obstetrics and gynecology at the University of North Carolina at Chapel Hill.
Researchers included 1,295 women in the COMPARE-UF study who were at least 30 years old, not attempting pregnancy, and undergoing hysterectomy or myomectomy for treatment of fibroids. Overall, 727 patients underwent hysterectomy, and 568 patients underwent myomectomy.
The researchers measured QoL and symptom severity using the Uterine Fibroid Scale-QoL, the EQ-5D, and Visual Analog Scale (VAS). The UFS-QoL contained subscales for concern, activities, energy and mood, control, self-consciousness, and sexual function, while the EQ-5D had subscales for mobility, self-care, usual activities, pain or discomfort, and anxiety or depression.
After surgery, UFS-QoL overall scores were similar in both hysterectomy and myomectomy groups between 6 weeks and 12 weeks (77 vs. 76), but there was less postsurgery symptom severity in the hysterectomy group over the same time period (16 vs. 20; P less than .05). However, both groups had a significant improvement in overall UFS-QoL post surgery (hysterectomy, 31; myomectomy, 30) and in UFS-QoL symptom severity (hysterectomy, 41; myomectomy, 37), Dr. Nicholson noted. EQ-5D VAS scores also were similar in both hysterectomy and myomectomy groups after treatment (82 vs. 79), and showed a 10.9 score improvement in hysterectomy patients and an 8.6 score improvement in myomectomy patients.
“This is really important, because it shows that, regardless of which procedure that you’ve chosen, at least at short-term follow up, it appears that you will have improvement in quality of life,” she said.
When researchers analyzed the UFS-QoL subscale scores, they found patients who underwent abdominal myomectomy scored better than abdominal hysterectomy on the activities subscale (79 vs. 72; P equals .01) and energy/mood subscale (82 vs. 75; P equals .03). In examining minimally invasive procedures, Dr. Nicholson and colleagues found higher improvements in health-related QoL scores among patients undergoing minimally invasive hysterectomy (45-80 vs. 45 vs. 75), and these patients also had lower symptom severity, compared with patients who underwent myomectomy (59-13 vs. 58-21).
“At least at the short-term follow-up, we think that some of that difference that we see in minimally invasive procedures vs. nonminimally invasive may be in part due to women’s perceptions or what their expectations are having minimally invasive surgery, and how they might feel in the short-term follow-up period,” said Dr. Nicholson.
These similar short-term outcomes occurred even though there were significant differences in baseline patient characteristics for the hysterectomy and myomectomy groups, with women undergoing hysterectomy being significantly younger (40 years) than patients undergoing hysterectomy (45 years). Differences also were significant between hysterectomy and myomectomy groups in the percentage of patients who were white (50% vs. 41%; P less than .01), African-American (38% vs. 41%; P less than .01) or other races (12% vs. 18%; P less than .01). There also were significant differences in baseline body mass index between hysterectomy (31 kg/m2) and myomectomy (29 kg/m2) groups.
Patients in both groups further differed in presurgery quality-of-life (QoL) scores.
Women in the hysterectomy group had lower presurgery overall QoL (44 vs. 50), greater symptom severity (60 vs. 52), and lower VAS (69 vs. 73) scores, compared with the myomectomy group (P less than .05). This difference continued in the UFS-QoL subscale scores, where women in the hysterectomy group had significantly lower scores in the concern (38 vs. 45), activities (46 vs. 52), energy/mood (45 vs. 51), control (48 vs. 52), self-consciousness (41 vs. 50), and sexual function (45 vs. 50) subscales, compared with women in the myomectomy group (P less than .05). The researchers used propensity scoring to adjust for baseline characteristics, and inverse propensity weighting to adjust for potential confounding in the multivariate analysis.
COMPARE-UF is funded by the Agency for Healthcare Research and Quality (AHRQ), Patient-Centered Outcomes Research Institute (PCORI), and the National Institutes of Health. Dr. Nicholson reported no relevant conflicts of interest.
SOURCE: Nicholson WK et al. ASRM 2019, Abstract SYT07.
PHILADELPHIA – despite different baseline characteristics, according to recent results from the COMPARE-UF study presented at the annual meeting of the American Society for Reproductive Medicine.
“Both hysterectomy and myomectomy can substantially improve women’s quality of life scores and substantially reduce symptom severity,” reported Wanda K. Nicholson, MD, MPH, lead investigator for COMPARE-UF and professor of general obstetrics and gynecology at the University of North Carolina at Chapel Hill.
Researchers included 1,295 women in the COMPARE-UF study who were at least 30 years old, not attempting pregnancy, and undergoing hysterectomy or myomectomy for treatment of fibroids. Overall, 727 patients underwent hysterectomy, and 568 patients underwent myomectomy.
The researchers measured QoL and symptom severity using the Uterine Fibroid Scale-QoL, the EQ-5D, and Visual Analog Scale (VAS). The UFS-QoL contained subscales for concern, activities, energy and mood, control, self-consciousness, and sexual function, while the EQ-5D had subscales for mobility, self-care, usual activities, pain or discomfort, and anxiety or depression.
After surgery, UFS-QoL overall scores were similar in both hysterectomy and myomectomy groups between 6 weeks and 12 weeks (77 vs. 76), but there was less postsurgery symptom severity in the hysterectomy group over the same time period (16 vs. 20; P less than .05). However, both groups had a significant improvement in overall UFS-QoL post surgery (hysterectomy, 31; myomectomy, 30) and in UFS-QoL symptom severity (hysterectomy, 41; myomectomy, 37), Dr. Nicholson noted. EQ-5D VAS scores also were similar in both hysterectomy and myomectomy groups after treatment (82 vs. 79), and showed a 10.9 score improvement in hysterectomy patients and an 8.6 score improvement in myomectomy patients.
“This is really important, because it shows that, regardless of which procedure that you’ve chosen, at least at short-term follow up, it appears that you will have improvement in quality of life,” she said.
When researchers analyzed the UFS-QoL subscale scores, they found patients who underwent abdominal myomectomy scored better than abdominal hysterectomy on the activities subscale (79 vs. 72; P equals .01) and energy/mood subscale (82 vs. 75; P equals .03). In examining minimally invasive procedures, Dr. Nicholson and colleagues found higher improvements in health-related QoL scores among patients undergoing minimally invasive hysterectomy (45-80 vs. 45 vs. 75), and these patients also had lower symptom severity, compared with patients who underwent myomectomy (59-13 vs. 58-21).
“At least at the short-term follow-up, we think that some of that difference that we see in minimally invasive procedures vs. nonminimally invasive may be in part due to women’s perceptions or what their expectations are having minimally invasive surgery, and how they might feel in the short-term follow-up period,” said Dr. Nicholson.
These similar short-term outcomes occurred even though there were significant differences in baseline patient characteristics for the hysterectomy and myomectomy groups, with women undergoing hysterectomy being significantly younger (40 years) than patients undergoing hysterectomy (45 years). Differences also were significant between hysterectomy and myomectomy groups in the percentage of patients who were white (50% vs. 41%; P less than .01), African-American (38% vs. 41%; P less than .01) or other races (12% vs. 18%; P less than .01). There also were significant differences in baseline body mass index between hysterectomy (31 kg/m2) and myomectomy (29 kg/m2) groups.
Patients in both groups further differed in presurgery quality-of-life (QoL) scores.
Women in the hysterectomy group had lower presurgery overall QoL (44 vs. 50), greater symptom severity (60 vs. 52), and lower VAS (69 vs. 73) scores, compared with the myomectomy group (P less than .05). This difference continued in the UFS-QoL subscale scores, where women in the hysterectomy group had significantly lower scores in the concern (38 vs. 45), activities (46 vs. 52), energy/mood (45 vs. 51), control (48 vs. 52), self-consciousness (41 vs. 50), and sexual function (45 vs. 50) subscales, compared with women in the myomectomy group (P less than .05). The researchers used propensity scoring to adjust for baseline characteristics, and inverse propensity weighting to adjust for potential confounding in the multivariate analysis.
COMPARE-UF is funded by the Agency for Healthcare Research and Quality (AHRQ), Patient-Centered Outcomes Research Institute (PCORI), and the National Institutes of Health. Dr. Nicholson reported no relevant conflicts of interest.
SOURCE: Nicholson WK et al. ASRM 2019, Abstract SYT07.
PHILADELPHIA – despite different baseline characteristics, according to recent results from the COMPARE-UF study presented at the annual meeting of the American Society for Reproductive Medicine.
“Both hysterectomy and myomectomy can substantially improve women’s quality of life scores and substantially reduce symptom severity,” reported Wanda K. Nicholson, MD, MPH, lead investigator for COMPARE-UF and professor of general obstetrics and gynecology at the University of North Carolina at Chapel Hill.
Researchers included 1,295 women in the COMPARE-UF study who were at least 30 years old, not attempting pregnancy, and undergoing hysterectomy or myomectomy for treatment of fibroids. Overall, 727 patients underwent hysterectomy, and 568 patients underwent myomectomy.
The researchers measured QoL and symptom severity using the Uterine Fibroid Scale-QoL, the EQ-5D, and Visual Analog Scale (VAS). The UFS-QoL contained subscales for concern, activities, energy and mood, control, self-consciousness, and sexual function, while the EQ-5D had subscales for mobility, self-care, usual activities, pain or discomfort, and anxiety or depression.
After surgery, UFS-QoL overall scores were similar in both hysterectomy and myomectomy groups between 6 weeks and 12 weeks (77 vs. 76), but there was less postsurgery symptom severity in the hysterectomy group over the same time period (16 vs. 20; P less than .05). However, both groups had a significant improvement in overall UFS-QoL post surgery (hysterectomy, 31; myomectomy, 30) and in UFS-QoL symptom severity (hysterectomy, 41; myomectomy, 37), Dr. Nicholson noted. EQ-5D VAS scores also were similar in both hysterectomy and myomectomy groups after treatment (82 vs. 79), and showed a 10.9 score improvement in hysterectomy patients and an 8.6 score improvement in myomectomy patients.
“This is really important, because it shows that, regardless of which procedure that you’ve chosen, at least at short-term follow up, it appears that you will have improvement in quality of life,” she said.
When researchers analyzed the UFS-QoL subscale scores, they found patients who underwent abdominal myomectomy scored better than abdominal hysterectomy on the activities subscale (79 vs. 72; P equals .01) and energy/mood subscale (82 vs. 75; P equals .03). In examining minimally invasive procedures, Dr. Nicholson and colleagues found higher improvements in health-related QoL scores among patients undergoing minimally invasive hysterectomy (45-80 vs. 45 vs. 75), and these patients also had lower symptom severity, compared with patients who underwent myomectomy (59-13 vs. 58-21).
“At least at the short-term follow-up, we think that some of that difference that we see in minimally invasive procedures vs. nonminimally invasive may be in part due to women’s perceptions or what their expectations are having minimally invasive surgery, and how they might feel in the short-term follow-up period,” said Dr. Nicholson.
These similar short-term outcomes occurred even though there were significant differences in baseline patient characteristics for the hysterectomy and myomectomy groups, with women undergoing hysterectomy being significantly younger (40 years) than patients undergoing hysterectomy (45 years). Differences also were significant between hysterectomy and myomectomy groups in the percentage of patients who were white (50% vs. 41%; P less than .01), African-American (38% vs. 41%; P less than .01) or other races (12% vs. 18%; P less than .01). There also were significant differences in baseline body mass index between hysterectomy (31 kg/m2) and myomectomy (29 kg/m2) groups.
Patients in both groups further differed in presurgery quality-of-life (QoL) scores.
Women in the hysterectomy group had lower presurgery overall QoL (44 vs. 50), greater symptom severity (60 vs. 52), and lower VAS (69 vs. 73) scores, compared with the myomectomy group (P less than .05). This difference continued in the UFS-QoL subscale scores, where women in the hysterectomy group had significantly lower scores in the concern (38 vs. 45), activities (46 vs. 52), energy/mood (45 vs. 51), control (48 vs. 52), self-consciousness (41 vs. 50), and sexual function (45 vs. 50) subscales, compared with women in the myomectomy group (P less than .05). The researchers used propensity scoring to adjust for baseline characteristics, and inverse propensity weighting to adjust for potential confounding in the multivariate analysis.
COMPARE-UF is funded by the Agency for Healthcare Research and Quality (AHRQ), Patient-Centered Outcomes Research Institute (PCORI), and the National Institutes of Health. Dr. Nicholson reported no relevant conflicts of interest.
SOURCE: Nicholson WK et al. ASRM 2019, Abstract SYT07.
REPORTING FROM ASRM 2019
Multidisciplinary care could address fertility preservation in transgender youth
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
PHILADELPHIA – A multidisciplinary approach is needed to care for gender-diverse transgender adolescents interested in fertility preservation, Leena Nahata, MD, said at the annual meeting of the American Society for Reproductive Medicine.
especially in the absence of longitudinal data, said Dr. Nahata, medical director of the fertility and reproductive health program at Nationwide Children’s Hospital, Columbus, Ohio. “We’re trying to counsel these youth and their parents about long-term outcomes of hormone therapies. However, despite the lack of data, not treating them also is not a viable option.”
Another concern among transgender individuals, Dr. Nahata said, is a high risk of mental health issues. Approximately one-third of transgender individuals experience depression, and between one-third and one-half have suicidal ideation or attempted suicide.
“It’s important to realize that these risks are not inevitable,” she said. Support from parents, peers, and social groups; engaging with the health care system; and having access to puberty suppression, gender-affirming hormones, and surgery are protective outcomes for mental health concerns. “It’s because of this that so many of us feel obligated to move on with treatments even in a setting of a lack of data.”
According to 2017 guidelines from the Endocrine Society on gender-dysphoric and gender-incongruent persons, patients can begin gonadotropin-releasing hormone (GnRH) agonists at Tanner Stage 2 of puberty (J Clin Endocrinol Metab. 2017 Nov. doi: 10.1210/jc.2017-01658). Before starting treatment, a mental health provider should confirm gender dysphoria or incongruence, and determine whether the patient has “sufficient mental capacity” to understand the long-term consequences of treatment with gender-affirming hormones such as estrogen and testosterone because the effects are partially irreversible, including a potential loss of fertility. Most pediatric patients will have this ability by 16 years old, but some programs across the country begin treatment between 13.5 years and 14 years of age, said Dr. Nahata. One consideration of beginning GnRH agonists and then moving directly to gender-affirming hormone therapy, there may not be an opportunity to explore fertility preservation.
Dr. Nahata acknowledged the data for the long-term effects of testosterone and estrogen on fertility is “murky,” but despite a lack of data, the American Society for Reproductive Medicine released an ethics statement in 2015 affirming that transgender patients “have the same interests as other persons in having children and in accessing fertility services for fertility preservation and reproduction” and pediatric providers “should offer fertility preservation options to individuals before gender transition” (Fertil Steril. 2015 Sep 9. doi: 10.1016/j.fertnstert.2015.08.021).
There also is mixed evidence that transgender individuals take advantage of fertility preservation services, whether offered or not. Two studies from Belgium that surveyed transgender individuals on parenthood preferences found 54% of adult trans men had a desire for children and that 38% of adult trans men and 51% of adult trans women would consider fertility preservation if it was an option. However, Dr. Nahata said a retrospective study from her own group of 50 adolescent trans males and 23 adolescent trans females found 99% of the cohort was counseled on fertility preservation, but only 3% (2 patients) attempted fertility preservation, and both were trans females (J Adolesc Health. 2017 Jul. doi: 10.1016/j.jadohealth.2016.12.012).
Another study examining use of fertility preservation in trans females in the Netherlands by Brik et al. found a much higher use of fertility preservation, with 38% of patients attempting cryopreservation after counseling (J Adolesc Health. 2019 May. doi: 10.1016/j.jadohealth.2018.11.008). “It’s unclear whether this is a regional difference or whether things are actually shifting over a short period of time,” said Dr. Nahata.
Attitudes about fertility preservation among gender-diverse transgender youth also impact its use in this patient population. A survey of transgender youth found less than 40% preferred adoption to biological parenthood, but said their feelings might change as time passes. However, more than half wanted more information on their family-building options. For other transgender youth aged 12-19 years, having children was their “lowest life priority,” compared with having friends, their health, and other issues in their lives, said Dr. Nahata.
In a 24-item survey Dr. Nahata and her team administered to 44 trans nonbinary adolescents, the most common reasons for not seeking fertility preservation were feelings of being too young, not wanting to be a parent or have a biological child, not wanting to delay treatment, and not being able to afford the cost of fertility preservation.
“This just speaks to the complexities of counseling in this population, and the importance of having a multidisciplinary team to see these youth and families to do more comprehensive counseling,” she said.
Dr. Nahata reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ASRM 2019
FDA advisory committee supports birth control patch approval
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
FROM THE FDA
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
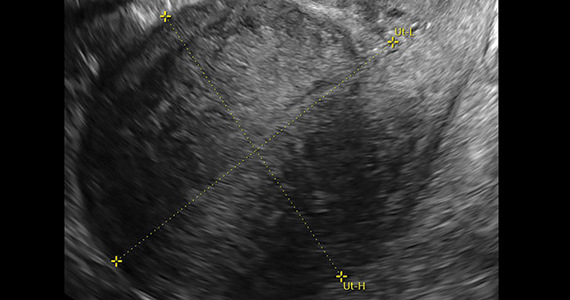
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).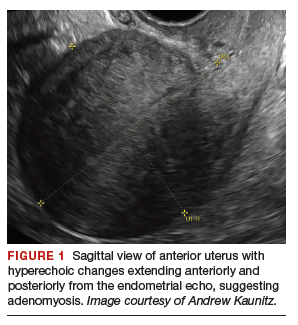
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
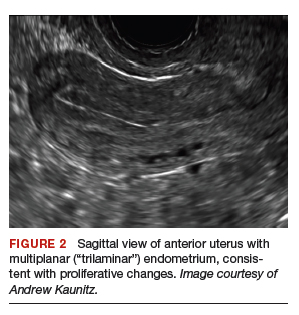
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
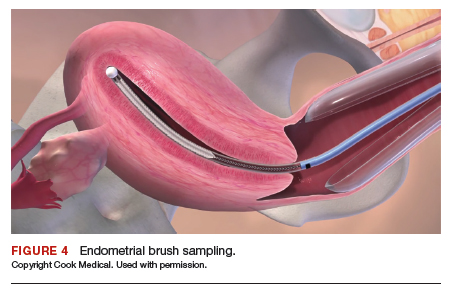
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
Utilize transvaginal ultrasonography
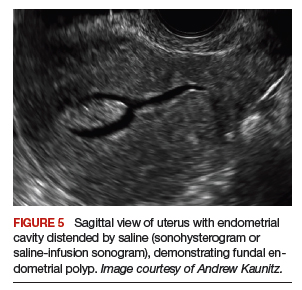
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
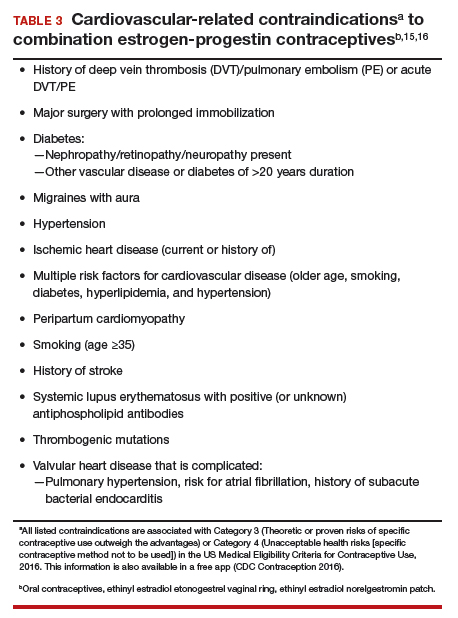
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Subclinical hypothyroidism and pregnancy: Public health problem or lab finding with minimal clinical significance?
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
In a US study of more than 17,000 people, overt hypothyroidism and hyperthyroidism were detected in about 4.6% and 1.3% of adults, respectively.1 In this population-based study, thyroid disease was 5 times more prevalent among women than among men. In our ObGyn practices, there are many women of reproductive age with thyroid disease who are considering pregnancy. Treatment of active hyperthyroidism in a woman planning pregnancy is complex and best managed by endocrinologists. Treatment of hypothyroidism is more straightforward, however, and typically managed by internists, family medicine clinicians, and obstetrician-gynecologists.
Clinical management of hypothyroidism and pregnancy
Pregnancy results in a doubling of thyroxine-binding globulin (TBG) levels and a 40% increase in plasma volume, resulting in a need for more thyroxine production.2 Of note, from conception to approximately 13 weeks’ gestation, the sole source of embryonic and fetal thyroid hormones is from the mother.2 Women who have been taking chronic thyroxine treatment may have suppressed thyroid gland activity and be unable to increase thyroxine production in response to pregnancy, necessitating a 30% to 50% increase in their thyroxine dose to maintain TSH levels in the normal range.
For hypothyroid women on long-term thyroxine treatment, recommend increasing the thyroxine dose when pregnancy is recognized. For your patients on chronic thyroxine treatment who are planning a pregnancy, a multiprong approach is helpful in preparing the patient for the increased thyroxine requirements of early pregnancy. First, it is important to counsel the woman that she should not stop the thyroxine medication because it may adversely affect the pregnancy. In my experience, most cases of overt hypothyroidism during pregnancy occur because the patient stopped taking her thyroxine therapy. Second, for hypothyroid women who are considering conception it is reasonable to adjust the thyroxine dose to keep the TSH concentration in the lower range of normal (0.5 to 2.5 mU/L). This will give the woman a “buffer,” reducing the risk that in early pregnancy she and her fetus will have a thyroxine deficit. Third, in early pregnancy, following detection of a positive pregnancy test, your patient can start to increase her thyroxine dose by about two tablets weekly (a 28% increase in the dose). Fourth, TSH levels can be measured every 4 weeks during the first trimester, with appropriate adjustment of the thyroxine dose to keep the TSH concentration below the trimester-specific upper limit of normal (< 4 mU/L).2
TSH and free thyroxine measurements identify women with overt hypothyroidism who need thyroxine treatment. Overt hypothyroidism is associated with adverse reproductive outcomes, including decreased fertility, increased spontaneous abortion, increased fetal loss, and preterm birth.2,3 Hence it is important to immediately initiate thyroxine treatment in pregnant women who have overt hypothyroidism. A diagnosis of overt hypothyroidism is indicated in women with an intact hypothalamic-pituitary axis and a TSH level ≥10 mU/L plus a low free thyroxine concentration. A TSH level of >4 to 10 mU/L, with normal free thyroxine concentration, is evidence of subclinical hypothyroidism (SCH). Among women, there are about 5 times more cases of SCH than overt hypothyroidism.
Continue to: The literature concerning SCH and pregnancy...
The literature concerning SCH and pregnancy is vast, and often contradictory, leading to confusion among clinicians. Contributing to the confusion is that some observational studies report a modest association between SCH and adverse pregnancy outcomes. To date, however, randomized clinical trials show no benefit of thyroxine treatment in these cases. I explore these contradictory pieces of evidence below.

Is SCH associated with adverse pregnancy outcomes due to low thyroxine levels?
There is conflicting literature about the association of SCH and adverse reproductive outcomes. A meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with SCH and euthyroid women (normal TSH and normal free thyroxine levels) was 6.1% and 5.0%, respectively (odds ratio [OR], 1.29; 95% CI, 1.01–1.64).4 Interestingly, pregnant women with normal TSH levels but a low free thyroxine level also had an increased rate of preterm birth (7.1% vs 5.0%; OR, 1.46; 95% CI, 1.12–1.90).
Although observational studies report an association between SCH and adverse reproductive outcomes, multiple randomized clinical trials conducted in women with SCH or hypothyroxinemia have failed to demonstrate that thyroxine replacement improves reproductive outcomes. For example, in a study of 794 pregnant women with elevated TSH and/or low free thyroxine levels randomly assigned to thyroxine treatment (0.15 mg daily) or no treatment, there was no difference in preterm birth rate (5.6% vs 7.9%, P = .2), mean birth weight (3.5 kg vs 3.3 kg, P = .15), gestational age at delivery (40.1 vs 40.2 weeks, P = .10), or the intelligence quotient of children at 3 years (99 vs 100, P = .40).5
In another study, 674 pregnant women with mild SCH (mean TSH, 4.4 mU/L) were randomly assigned to receive thyroxine (0.1 mg daily and dose adjusted to achieve a normal TSH level) or placebo. In this study there was no difference between the thyroxine treatment or placebo groups in preterm birth rate (9% vs 11%, P = .44), gestational age at delivery (39.1 vs 38.9 weeks, P = .57) or intelligence quotient of children at 5 years (97 and 94, P = .71).6
The same investigators also randomized 524 pregnant women with isolated hypothyroxinema (mean free thyroxine level, 0.83 ng/dL) and normal TSH level (mean, 1.5 mU/L) to thyroxine (0.05 mg daily and dose adjusted to achieve a normal free thyroxine level) or placebo.6 In this study there was no difference in preterm birth rate (12% vs 8%, P = .11), gestational age at delivery (39.0 vs 38.8 weeks, P = .46) or intelligence quotient of children at 5 years (94 and 91, P = .31).6
When large randomized clinical trials and observational studies report discrepant results, many authorities prioritize the findings from the randomized clinical trials because those results are less prone to being confounded by unrecognized factors. Randomized trials do not demonstrate that mild SCH or isolated hypothyroxinemia have a major impact on pregnancy outcomes.
Thyroid antibodies, fertility, miscarriage, and preterm birth
Some observational studies report that the presence of thyroid antibodies in a euthyroid woman reduces fecundity and increases the risk for miscarriage and preterm birth. For example, a meta-analysis of 47,045 pregnant women reported that the preterm birth rate for women with and without antithyroid antibodies was 6.9% and 4.9%, respectively (OR, 1.33; 95% CI, 1.15–1.56). However, in euthyroid women with antithyroid antibodies, low-dose thyroxine therapy has not been shown to improve fertility, or reduce miscarriages or preterm birth rate.
Continue to: In a large randomized clinical trial, 952 euthyroid women...
In a large randomized clinical trial, 952 euthyroid women (normal TSH level; range, 0.44 to 3.63 mIU/L and free thyroxine level; range, 10 to 21 pmol/L) who were planning on conceiving and had elevated thyroid peroxidase antibodies were randomized prior to conception to receive either thyroxine (50 µg) or placebo.7 After 12 months, outcomes were similar for women treated with thyroxine or placebo, including live birth rate (37.4% vs 37.9%), miscarriage rate for those who became pregnant (28.2% vs 29.6%), and preterm birth ≤ 34 weeks of gestation (3.8% vs 3.6%, respectively).7 The investigators concluded that the use of low-dose thyroxine in euthyroid women with thyroid peroxidase antibodies was not effective for increasing the rate of live birth or reducing the rate of miscarriage or early preterm birth.
Thyroid antibodies and the rate of IVF pregnancy and miscarriage
Some observational studies suggest that the presence of antithyroid antibodies may be associated with an increased rate of miscarriage.8 To test the effects of thyroxine treatment on the rate of miscarriage in euthyroid women with antithyroid antibodies, 600 euthyroid infertile women with antithyroid antibodies (antithyroid peroxidase levels ≥ 60 IU/mL) scheduled to have in vitro fertilization (IVF) were randomly assigned to receive thyroxine (dose adjustment to keep TSH levels in the range of 0.1 to 2.5 mIU/L) or no treatment.9 The thyroxine treatment was initiated 2 to 4 weeks before initiation of ovarian stimulation. In this study, treatment with thyroxine or no treatment resulted in similar rates of clinical pregnancy (35.7% vs 37.7%) and live birth (31.7% vs 32.3%).9 Among the women who achieved a clinical pregnancy, miscarriage rates were similar in the thyroxine and no treatment groups (10.3% vs 10.6%).9
Let’s focus on more serious problems that affect pregnancy
There is a clear consensus that women with overt hypothyroidism should be treated with thyroxine prior to attempting pregnancy.2,6 There is no clear consensus about how to treat women considering pregnancy who have one isolated laboratory finding, such as mild subclinical hypothyroidism, mild isolated hypothyroxinemia, or antithyroid antibodies. Given the lack of evidence from randomized trials that thyroxine improves pregnancy outcomes in these cases, obstetrician-gynecologists may want to either refer women with these problems to an endocrinologist for consultation or sequentially measure laboratory values to assess whether the patient’s laboratory abnormality is transient, stable, or worsening.
Obstetrician-gynecologists and their patients are confronted by many serious problems that adversely affect pregnancy and deserve priority attention, including iron deficiency anemia, excess gestational weight gain, peripartum depression, intimate partner violence, housing insecurity, cigarette smoking, substance misuse, chronic hypertension, morbid obesity, diabetes, gestational diabetes, preeclampsia, venous thromboembolism, obstetrical hemorrhage, sepsis, and infectious diseases. Given limited resources our expertise should be focused on these major obstetric public health problems rather than screening for mild subclinical hypothyroidism.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2017;27:315-389.
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2012;12:63-68.
- Consortium on Thyroid and Pregnancy--Study Group on Preterm Birth. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322:632-641.
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366:493-501.
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825.
- Dhillon-Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med. 2019;380:1316-1325.
- Chen L, Hu R. Thyroid autoimmunity and miscarriage: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:513-519.
- Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: a randomized clinical trial. JAMA. 2017;318:2190-2198.
Ovarian cryopreservation should no longer be experimental
PHILADELPHIA – Ovarian tissue cryopreservation should no longer be considered experimental, Sherman J. Silber, MD, said at the annual meeting of the American Society for Reproductive Medicine.
That claim is based on more than 20 years of experience at his center performing the procedure and results he presented from patients for whom frozen ovarian tissue has been reimplanted resulting in a spontaneous pregnancy.
“For prepubertal girls with cancer and for patients who have already had a preliminary round of chemotherapy, ovarian tissue freezing is the only method available to preserve their fertility,” said Dr. Silber, of the Infertility Center of St. Louis in Chesterfield, Missouri. “It is also the only method available to preserve their fertility.”
“I have very strong feelings about this,” he added. “It has huge societal implications for insurance payments.”
Dr. Silber presented results of ovarian tissue freezing and reimplantation at his center beginning in 1997, where 115 patients between the ages of 2 and 35 years underwent the procedure using the same technique. Of these patients, 14 women came back years later to have their frozen ovary cortex reimplanted. Dr. Silber and his group followed these patients monthly for more than 2 years after reimplantation for signs of return of menses, hormonal changes, pregnancy, and live birth.
Most of the patients who chose ovarian tissue freezing had cancer. Eight patients underwent the procedure after being diagnosed with solid tissue cancer and three had leukemia, while two patients underwent ovarian tissue freezing due to premature ovarian failure, and one because of multiple sclerosis. Patients who underwent reimplantation were menopausal for at least 3 years, said Dr. Silber.
Dr. Silber also described the technique used for reimplantation. After the cortical tissue was thawed, the tissue was quilted into one piece from three to five slices using 9-0 nylon interrupted sutures. The quilted tissue was then sutured to the medulla after the surgeon completely removed the dead cortex from the other ovary. “Hemostasis was achieved with micro bipolar forceps,” said Dr. Silber. “Constant irrigation was employed with pulsed heparinized media because we wanted to avoid adhesions, and we wanted to try for spontaneous pregnancy rather than IVF.”
“Then, we put [the quilted ovarian slices] on to the medulla on the other side in such a way that the fallopian tube would be able to reach and catch any egg that’s ovulated during that time,” he added.
Dr. Silber and his group found that, over time, follicle-stimulating hormone (FSH) levels sharply decreased to normal or near-normal levels between 69 days and 133 days after the procedure while Anti-Müllerian hormone (AMH) levels dramatically rose to higher levels between 133 days and 227 days post-procedure before dropping to very low levels, “and the AMH remained at low levels despite the fact that [transplants] would last 8 to 10 years,” said Dr. Silber.
Of the 14 cases where frozen ovarian tissue was reimplanted, 11 patients (78%) achieved pregnancy, 10 patients (71%) delivered healthy babies, and 1 patient (9%) experienced a miscarriage. All patients had spontaneous pregnancies, and none used in vitro fertilization (IVF), noted Dr. Silber. There were 2 patients who had four children from transplanted ovarian tissue, and 2 of 3 patients with leukemia had a total of five children.
Additionally, Dr. Silber’s group examined the literature for other examples of ovarian tissue reimplantation after cryopreservation to determine how many live births resulted from the procedure. They found an additional 170 live births in addition to the 15 live births at their center, with a pregnancy rate ranging from 31% to 71% in different studies. Cancer was not transmitted from mother to child in any case, said Dr. Silber.
Compared with egg freezing, there is a benefit to performing ovarian tissue freezing, even after chemotherapy has begun, noted Dr. Silber. The cost of ovarian tissue freezing is also roughly one-tenth that of egg freezing, and the procedure is less burdensome than multiple cycles with the potential for ovarian hyperstimulation, and it restores the hormone function and the fertility of eggs after reimplantation.
“Because the greater primordial follicle recruitment decreases as the ovarian reserve decreases, you can put a piece of ovary tissue back every 8 years, and a woman can have endocrine function until she’s 100 years old,” said Dr. Silber.
Dr. Silber reported no relevant conflicts of interest.
SOURCE: Silber SJ. ASRM 2019. Abstract O-203.
PHILADELPHIA – Ovarian tissue cryopreservation should no longer be considered experimental, Sherman J. Silber, MD, said at the annual meeting of the American Society for Reproductive Medicine.
That claim is based on more than 20 years of experience at his center performing the procedure and results he presented from patients for whom frozen ovarian tissue has been reimplanted resulting in a spontaneous pregnancy.
“For prepubertal girls with cancer and for patients who have already had a preliminary round of chemotherapy, ovarian tissue freezing is the only method available to preserve their fertility,” said Dr. Silber, of the Infertility Center of St. Louis in Chesterfield, Missouri. “It is also the only method available to preserve their fertility.”
“I have very strong feelings about this,” he added. “It has huge societal implications for insurance payments.”
Dr. Silber presented results of ovarian tissue freezing and reimplantation at his center beginning in 1997, where 115 patients between the ages of 2 and 35 years underwent the procedure using the same technique. Of these patients, 14 women came back years later to have their frozen ovary cortex reimplanted. Dr. Silber and his group followed these patients monthly for more than 2 years after reimplantation for signs of return of menses, hormonal changes, pregnancy, and live birth.
Most of the patients who chose ovarian tissue freezing had cancer. Eight patients underwent the procedure after being diagnosed with solid tissue cancer and three had leukemia, while two patients underwent ovarian tissue freezing due to premature ovarian failure, and one because of multiple sclerosis. Patients who underwent reimplantation were menopausal for at least 3 years, said Dr. Silber.
Dr. Silber also described the technique used for reimplantation. After the cortical tissue was thawed, the tissue was quilted into one piece from three to five slices using 9-0 nylon interrupted sutures. The quilted tissue was then sutured to the medulla after the surgeon completely removed the dead cortex from the other ovary. “Hemostasis was achieved with micro bipolar forceps,” said Dr. Silber. “Constant irrigation was employed with pulsed heparinized media because we wanted to avoid adhesions, and we wanted to try for spontaneous pregnancy rather than IVF.”
“Then, we put [the quilted ovarian slices] on to the medulla on the other side in such a way that the fallopian tube would be able to reach and catch any egg that’s ovulated during that time,” he added.
Dr. Silber and his group found that, over time, follicle-stimulating hormone (FSH) levels sharply decreased to normal or near-normal levels between 69 days and 133 days after the procedure while Anti-Müllerian hormone (AMH) levels dramatically rose to higher levels between 133 days and 227 days post-procedure before dropping to very low levels, “and the AMH remained at low levels despite the fact that [transplants] would last 8 to 10 years,” said Dr. Silber.
Of the 14 cases where frozen ovarian tissue was reimplanted, 11 patients (78%) achieved pregnancy, 10 patients (71%) delivered healthy babies, and 1 patient (9%) experienced a miscarriage. All patients had spontaneous pregnancies, and none used in vitro fertilization (IVF), noted Dr. Silber. There were 2 patients who had four children from transplanted ovarian tissue, and 2 of 3 patients with leukemia had a total of five children.
Additionally, Dr. Silber’s group examined the literature for other examples of ovarian tissue reimplantation after cryopreservation to determine how many live births resulted from the procedure. They found an additional 170 live births in addition to the 15 live births at their center, with a pregnancy rate ranging from 31% to 71% in different studies. Cancer was not transmitted from mother to child in any case, said Dr. Silber.
Compared with egg freezing, there is a benefit to performing ovarian tissue freezing, even after chemotherapy has begun, noted Dr. Silber. The cost of ovarian tissue freezing is also roughly one-tenth that of egg freezing, and the procedure is less burdensome than multiple cycles with the potential for ovarian hyperstimulation, and it restores the hormone function and the fertility of eggs after reimplantation.
“Because the greater primordial follicle recruitment decreases as the ovarian reserve decreases, you can put a piece of ovary tissue back every 8 years, and a woman can have endocrine function until she’s 100 years old,” said Dr. Silber.
Dr. Silber reported no relevant conflicts of interest.
SOURCE: Silber SJ. ASRM 2019. Abstract O-203.
PHILADELPHIA – Ovarian tissue cryopreservation should no longer be considered experimental, Sherman J. Silber, MD, said at the annual meeting of the American Society for Reproductive Medicine.
That claim is based on more than 20 years of experience at his center performing the procedure and results he presented from patients for whom frozen ovarian tissue has been reimplanted resulting in a spontaneous pregnancy.
“For prepubertal girls with cancer and for patients who have already had a preliminary round of chemotherapy, ovarian tissue freezing is the only method available to preserve their fertility,” said Dr. Silber, of the Infertility Center of St. Louis in Chesterfield, Missouri. “It is also the only method available to preserve their fertility.”
“I have very strong feelings about this,” he added. “It has huge societal implications for insurance payments.”
Dr. Silber presented results of ovarian tissue freezing and reimplantation at his center beginning in 1997, where 115 patients between the ages of 2 and 35 years underwent the procedure using the same technique. Of these patients, 14 women came back years later to have their frozen ovary cortex reimplanted. Dr. Silber and his group followed these patients monthly for more than 2 years after reimplantation for signs of return of menses, hormonal changes, pregnancy, and live birth.
Most of the patients who chose ovarian tissue freezing had cancer. Eight patients underwent the procedure after being diagnosed with solid tissue cancer and three had leukemia, while two patients underwent ovarian tissue freezing due to premature ovarian failure, and one because of multiple sclerosis. Patients who underwent reimplantation were menopausal for at least 3 years, said Dr. Silber.
Dr. Silber also described the technique used for reimplantation. After the cortical tissue was thawed, the tissue was quilted into one piece from three to five slices using 9-0 nylon interrupted sutures. The quilted tissue was then sutured to the medulla after the surgeon completely removed the dead cortex from the other ovary. “Hemostasis was achieved with micro bipolar forceps,” said Dr. Silber. “Constant irrigation was employed with pulsed heparinized media because we wanted to avoid adhesions, and we wanted to try for spontaneous pregnancy rather than IVF.”
“Then, we put [the quilted ovarian slices] on to the medulla on the other side in such a way that the fallopian tube would be able to reach and catch any egg that’s ovulated during that time,” he added.
Dr. Silber and his group found that, over time, follicle-stimulating hormone (FSH) levels sharply decreased to normal or near-normal levels between 69 days and 133 days after the procedure while Anti-Müllerian hormone (AMH) levels dramatically rose to higher levels between 133 days and 227 days post-procedure before dropping to very low levels, “and the AMH remained at low levels despite the fact that [transplants] would last 8 to 10 years,” said Dr. Silber.
Of the 14 cases where frozen ovarian tissue was reimplanted, 11 patients (78%) achieved pregnancy, 10 patients (71%) delivered healthy babies, and 1 patient (9%) experienced a miscarriage. All patients had spontaneous pregnancies, and none used in vitro fertilization (IVF), noted Dr. Silber. There were 2 patients who had four children from transplanted ovarian tissue, and 2 of 3 patients with leukemia had a total of five children.
Additionally, Dr. Silber’s group examined the literature for other examples of ovarian tissue reimplantation after cryopreservation to determine how many live births resulted from the procedure. They found an additional 170 live births in addition to the 15 live births at their center, with a pregnancy rate ranging from 31% to 71% in different studies. Cancer was not transmitted from mother to child in any case, said Dr. Silber.
Compared with egg freezing, there is a benefit to performing ovarian tissue freezing, even after chemotherapy has begun, noted Dr. Silber. The cost of ovarian tissue freezing is also roughly one-tenth that of egg freezing, and the procedure is less burdensome than multiple cycles with the potential for ovarian hyperstimulation, and it restores the hormone function and the fertility of eggs after reimplantation.
“Because the greater primordial follicle recruitment decreases as the ovarian reserve decreases, you can put a piece of ovary tissue back every 8 years, and a woman can have endocrine function until she’s 100 years old,” said Dr. Silber.
Dr. Silber reported no relevant conflicts of interest.
SOURCE: Silber SJ. ASRM 2019. Abstract O-203.
REPORTING FROM ASRM 2019
Preconception marijuana use by male partner raises spontaneous abortion risk
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
PHILADELPHIA – compared with infrequent use or no use of marijuana by the male partner, Alyssa F. Harlow, MPH, reported at the annual meeting of the American Society for Reproductive Medicine.
The male partner’s use of marijuana “one or more times per week in the past 2 months during the preconception period in our study was associated with an increased risk of spontaneous abortion,” said Ms. Harlow, a PhD candidate at Boston University. “The association attenuated for later pregnancy losses, and persisted for those with shorter [pregnancy] attempt time at [study] entry.”
Ms. Harlow and colleagues prospectively collected data from 1,535 couples in the Pregnancy Study Online (PRESTO) study, a preconception cohort study examining risk factors for adverse pregnancy outcomes. PRESTO enrolled women aged 21-45 years and their male partners aged 21 years or older who were attempting to conceive without the use of fertility treatment.
The researchers administered a screening and baseline questionnaire to the women, who then included their male partners in the study. The male partners completed their own baseline questionnaire that asked about demographics, medical history, and lifestyle or behavioral factors including marijuana use. The questions centering around marijuana use asked whether the partner had used marijuana within the past 2 months, and the frequency of marijuana use during that period.
Women in PRESTO were followed every 8 weeks until a pregnancy occurred, or up to 12 months if no pregnancy occurred. If they became pregnant, the women were asked additional questions at less than 12 weeks’ gestation and then again at 32 weeks’ gestation, including questions about any miscarriages, and how long a pregnancy lasted if a miscarriage did occur.
At baseline, 1,267 couples (83%) reported no marijuana use by male partners, 140 couples (9%) reported use less than 1 time per week, and 128 couples (8%) reported marijuana use at least 1 time per week. Men at baseline were similar in age and body mass index among groups, but men who used marijuana were more likely to be cigarette smokers (24% vs. 4%), were more likely to have partners who were cigarette smokers (11% vs. 2%), and were more likely to have partners who use marijuana (43% vs. 3%), compared with couples where the male partners did not use marijuana. Male partners who used marijuana also were less likely to be taking a daily multivitamin (25% vs. 37%), and were more likely to have been diagnosed with anxiety (14% vs. 7%) or depression (20% vs. 9%) compared with male partners who did not use marijuana.
Overall, 269 spontaneous abortions (17.5%) occurred during the study period, and couples where male partners used marijuana one or more times per week had approximately twice the rate of spontaneous abortions, compared with no marijuana use (hazard ratio, 1.99; 95% confidence interval).
Couples in which men who used marijuana less than 1 time per week had a slightly increased risk of spontaneous abortion, but this did not reach statistical significance.
When the results were adjusted for female nonusers of marijuana, the results were “essentially identical,” said Ms. Harlow.
Couples who were trying to conceive for three or fewer cycles at baseline (1,045 couples) had a lower rate of spontaneous abortion than that of couples trying for three or more cycles (490 couples). When the results were stratified by gestational age at loss, the results persisted for couples with a pregnancy loss at less than 8 weeks (1,533 couples), but the effect of marijuana use was reduced for couples with a loss at 8 weeks or more (1,113 couples).
Ms. Harlow noted several limitations to the study, including lack of data on time-varying marijuana use, potential selection bias, and residual confounding. There also is likely misclassification of exposure among some participants because marijuana use was self-reported, she added.
Ms. Harlow reported no relevant conflicts of interest.
SOURCE: Harlow AF et al. ASRM 2019. Abstract O-4.
REPORTING FROM ASRM 2019
Lifestyle program improves chance of spontaneous conception for women with obesity
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
PHILADELPHIA – Women with obesity who underwent a lifestyle program targeting healthy eating and physical activity were significantly more likely to achieve pregnancy or become spontaneously pregnant, Jean-Patrice Baillargeon, MD, MSc, reported at the annual meeting of the American Society for Reproductive Medicine.
However, women with polycystic ovary syndrome (PCOS) in the study appeared to benefit more than did women without PCOS who participated in the lifestyle program, said Dr. Baillargeon, from the University of Sherbrooke (Que.).
“ Women with PCOS seemed to benefit more from such a program,” said Dr. Baillargeon.
“These benefits occur along with small changes in weight, but important improvements in lifestyle, so lifestyle seems to be more important than weight change here,” he added.
The researchers randomized 130 women to receive the Fit-For-Fertility lifestyle program or usual care for infertility. The lifestyle program consisted of a low-intensity weekly intervention for 6 weeks in which patients met individually with a kinesiologist and nutritionist every week and also attended group sessions each week. Women in the intervention did not receive fertility treatment for the first 6 months while on the lifestyle program, and if they did not conceive during that time, they continued the program in combination with fertility treatments.
Patients were included if they were aged 18-40 years and had either infertility and a body mass index of 30 kg/m2 or greater or PCOS and a BMI of 27 kg/m2 or greater. Researchers excluded women planning to undergo bariatric surgery, women who were already undergoing another lifestyle intervention, and women with severe infertility or who had a male partner with severe infertility for whom in vitro fertilization was their only option for conceiving. Researchers collected data from patients at baseline and every 6 months up to 18 months, with additional visits for pregnant women scheduled at the beginning of pregnancy and at 26 weeks’ gestation. They collected baseline data on age, BMI, waist circumference, fat mass percentage, daily energy expenditure, and food frequency using the Healthy Eating Index (HEI).
Overall, 46 women in the intervention group and 52 women in the control group had a research visit at 6 months or pregnancy research visit at less than 6 months; of these, 33 women in the intervention group (65%) and 35 women in the control group (61%) had PCOS. At baseline, both PCOS and non-PCOS groups were similar; however, women in the PCOS intervention group had a lower BMI than did women without PCOS in the intervention group (37 kg/m2 vs. 41 kg/m2; P less than .05), while women without PCOS in the intervention group had a higher fat mass percentage than did women with PCOS in the intervention group (46% vs. 49%; P less than .05).
With regard to weight loss, there was a 2.4% reduction in weight among all patients in the intervention group, compared with the control group (P = .003), with a 2.7% reduction in weight for the PCOS group (P = .015) and a 1.8% reduction in the non-PCOS group (P = .139). However, there were no significant differences between PCOS status and the lifestyle intervention, said Dr. Baillargeon.
At 6 months, the quality of women’s diets in the combined PCOS and non-PCOS group that participated in the lifestyle program showed significant improvement, compared with control groups (HEI, 18% vs. 5%; P less than .001). The PCOS group on its own showed significant improvement with the intervention (20% vs. 4%; P less than .001), whereas women without PCOS showed a nonsignificant improvement with the intervention (14% vs. 6%; P = .055). Daily energy expenditure improved in all groups that received the intervention, compared with the control groups, but there were no significant between-group differences in energy expenditure.
When analyzing fertility outcomes at 18 months, the pregnancy rate for all patients who received lifestyle interventions was 61%, compared with 39% in the control group (P = .02; number needed to treat, 4.5). In women with PCOS, those who underwent the lifestyle intervention had a pregnancy rate of 58%, compared with 34% in the control group (P = .05; NNT, 4.3); although women without PCOS who participated in the lifestyle program had an improved pregnancy rate over women in the control group, the results were not significant (67% vs. 46%; P = .18; NNT, 4.7).
The researchers also looked at the spontaneous pregnancy rate and found women who received the intervention had nearly three times the rate of spontaneous pregnancy, compared with women in the control group (33% vs. 12%; P = .01), while women with PCOS in the lifestyle program had nearly five times the rate of spontaneous pregnancy, compared with the control group (27% vs. 6%; P = .02). Women without PCOS in the lifestyle program had nearly twice the increased likelihood of spontaneous pregnancy, but the results were not significant (44% vs. 23%; P = .15).
Women with PCOS in the lifestyle program also had a higher live birth rate, compared with women in the control group (55% vs. 31%; P = .05; NNT, 4.3). Although women without PCOS in the lifestyle program (67% vs. 46%; P = .18; NNT, 4.7) and women in the study overall experienced higher live birth rates (51% vs. 37%; P = .14; NNT, 7.0), compared with the control group, these results were not significant, said Dr. Baillargeon.
“Such lifestyle interventions in women with obesity could significantly lower costs of fertility treatments, which is important,” concluded Dr. Baillargeon.
The Fit-For-Fertility program was funded by an unrestricted grant from Ferring.
SOURCE: Baillargeon J-P, et al. ASRM 2019. Abstract O-95.
REPORTING FROM ASRM 2019
New drug improves sex drive, at least on paper
The novel drug bremelanotide shows promise in acquired female hypoactive sexual desire disorder, according to the results of two randomized, controlled trials and a 52-week open-label extension study published online in Obstetrics & Gynecology.
Bremelanotide, which received Food and Drug Administration approval for this indication in June 2019, is an analog of the endogenous neuropeptide alpha-melanocyte-stimulating hormone.
Two separate, identically designed phase 3 studies (RECONNECT) were performed by Sheryl Kingsburg, MD, of the Cleveland Medical Center, and associates. Combined, 1,267 premenopausal women in monogamous relationships with acquired hypoactive sexual desire disorder were randomized to bremelanotide or placebo. Women in the treatment arm had significant improvement in female sexual function index–desire domain (FSFI-D) scores from baseline to week 24 (integrated studies: 0.35; P less than .001; effect size, 0.39), compared with placebo. They also experienced significant improvement in the FSFI-desire/arousal/orgasm (FSFI-DAO) domain (integrated studies: –0.33; P less than .001; effect size, 0.27).
The most common adverse events were nausea (integrated: 40% versus 1% in placebo), flushing (20% versus 0.3%), and headache (11% versus 2%). Overall, 77% in the treatment group reported a treatment-emergent adverse event, compared with 58% in the placebo group.
The open-label follow-up study was led by James Simon, MD, of George Washington University and IntimMedicine Specialists, Washington. Of the 684 participants who opted to enter the extension study, 40% completed it. In those who received bremelanotide during the randomized trial, the change in FSFI-D scores from baseline to the end of the open-label study ranged from 1.25 to 1.30, while the change in FSFI-DAO ranged from –1.4 to –1.7. In patients originally on placebo, the changes were 0.70-0.77 and –0.9, respectively.
Both groups surpassed the minimally clinically important difference for the FSFI-D score, which is considered to be 0.6.
“Patients switching from placebo experienced a higher incidence of adverse events than those continuing on bremelanotide during the open-label extension (79% versus 63%, respectively),” Dr. Simon and associates said.
The treatment is subcutaneous and can be self-administered up to about 45 minutes before a sexual event, no more than once during a 24-hour period, and no more than 8 doses per month, according to an FDA press release. The drug is contraindicated for women with cardiovascular disease or uncontrolled hypertension due to observations of transiently, slightly increased blood pressure.
The trials were funded by Palatin Technologies and AMAG Pharmaceuticals. The authors and coauthors have extensive financial relationships with pharmaceutical companies. Dr. Carson reported no financial conflicts.
SOURCE: Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003500; Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003514.
The results indicate that sex is more satisfying in the treatment arm, but there is no evidence of an increase in the number of sexual events.
But the drug appears safe and offers a second option for women experiencing this concern.
Sandra Ann Carson, MD is in the departments of obstetrics, gynecology, and reproductive sciences, and reproductive endocrinology and infertility, at Yale University, New Haven, Conn. She made these comments in an editorial accompanying the articles by Kingsburg et al. and Simon et al. (Obstet Gynecol. 2019 Nov 134;[5]:897-8). Dr. Carson said she had no financial conflicts.
The results indicate that sex is more satisfying in the treatment arm, but there is no evidence of an increase in the number of sexual events.
But the drug appears safe and offers a second option for women experiencing this concern.
Sandra Ann Carson, MD is in the departments of obstetrics, gynecology, and reproductive sciences, and reproductive endocrinology and infertility, at Yale University, New Haven, Conn. She made these comments in an editorial accompanying the articles by Kingsburg et al. and Simon et al. (Obstet Gynecol. 2019 Nov 134;[5]:897-8). Dr. Carson said she had no financial conflicts.
The results indicate that sex is more satisfying in the treatment arm, but there is no evidence of an increase in the number of sexual events.
But the drug appears safe and offers a second option for women experiencing this concern.
Sandra Ann Carson, MD is in the departments of obstetrics, gynecology, and reproductive sciences, and reproductive endocrinology and infertility, at Yale University, New Haven, Conn. She made these comments in an editorial accompanying the articles by Kingsburg et al. and Simon et al. (Obstet Gynecol. 2019 Nov 134;[5]:897-8). Dr. Carson said she had no financial conflicts.
The novel drug bremelanotide shows promise in acquired female hypoactive sexual desire disorder, according to the results of two randomized, controlled trials and a 52-week open-label extension study published online in Obstetrics & Gynecology.
Bremelanotide, which received Food and Drug Administration approval for this indication in June 2019, is an analog of the endogenous neuropeptide alpha-melanocyte-stimulating hormone.
Two separate, identically designed phase 3 studies (RECONNECT) were performed by Sheryl Kingsburg, MD, of the Cleveland Medical Center, and associates. Combined, 1,267 premenopausal women in monogamous relationships with acquired hypoactive sexual desire disorder were randomized to bremelanotide or placebo. Women in the treatment arm had significant improvement in female sexual function index–desire domain (FSFI-D) scores from baseline to week 24 (integrated studies: 0.35; P less than .001; effect size, 0.39), compared with placebo. They also experienced significant improvement in the FSFI-desire/arousal/orgasm (FSFI-DAO) domain (integrated studies: –0.33; P less than .001; effect size, 0.27).
The most common adverse events were nausea (integrated: 40% versus 1% in placebo), flushing (20% versus 0.3%), and headache (11% versus 2%). Overall, 77% in the treatment group reported a treatment-emergent adverse event, compared with 58% in the placebo group.
The open-label follow-up study was led by James Simon, MD, of George Washington University and IntimMedicine Specialists, Washington. Of the 684 participants who opted to enter the extension study, 40% completed it. In those who received bremelanotide during the randomized trial, the change in FSFI-D scores from baseline to the end of the open-label study ranged from 1.25 to 1.30, while the change in FSFI-DAO ranged from –1.4 to –1.7. In patients originally on placebo, the changes were 0.70-0.77 and –0.9, respectively.
Both groups surpassed the minimally clinically important difference for the FSFI-D score, which is considered to be 0.6.
“Patients switching from placebo experienced a higher incidence of adverse events than those continuing on bremelanotide during the open-label extension (79% versus 63%, respectively),” Dr. Simon and associates said.
The treatment is subcutaneous and can be self-administered up to about 45 minutes before a sexual event, no more than once during a 24-hour period, and no more than 8 doses per month, according to an FDA press release. The drug is contraindicated for women with cardiovascular disease or uncontrolled hypertension due to observations of transiently, slightly increased blood pressure.
The trials were funded by Palatin Technologies and AMAG Pharmaceuticals. The authors and coauthors have extensive financial relationships with pharmaceutical companies. Dr. Carson reported no financial conflicts.
SOURCE: Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003500; Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003514.
The novel drug bremelanotide shows promise in acquired female hypoactive sexual desire disorder, according to the results of two randomized, controlled trials and a 52-week open-label extension study published online in Obstetrics & Gynecology.
Bremelanotide, which received Food and Drug Administration approval for this indication in June 2019, is an analog of the endogenous neuropeptide alpha-melanocyte-stimulating hormone.
Two separate, identically designed phase 3 studies (RECONNECT) were performed by Sheryl Kingsburg, MD, of the Cleveland Medical Center, and associates. Combined, 1,267 premenopausal women in monogamous relationships with acquired hypoactive sexual desire disorder were randomized to bremelanotide or placebo. Women in the treatment arm had significant improvement in female sexual function index–desire domain (FSFI-D) scores from baseline to week 24 (integrated studies: 0.35; P less than .001; effect size, 0.39), compared with placebo. They also experienced significant improvement in the FSFI-desire/arousal/orgasm (FSFI-DAO) domain (integrated studies: –0.33; P less than .001; effect size, 0.27).
The most common adverse events were nausea (integrated: 40% versus 1% in placebo), flushing (20% versus 0.3%), and headache (11% versus 2%). Overall, 77% in the treatment group reported a treatment-emergent adverse event, compared with 58% in the placebo group.
The open-label follow-up study was led by James Simon, MD, of George Washington University and IntimMedicine Specialists, Washington. Of the 684 participants who opted to enter the extension study, 40% completed it. In those who received bremelanotide during the randomized trial, the change in FSFI-D scores from baseline to the end of the open-label study ranged from 1.25 to 1.30, while the change in FSFI-DAO ranged from –1.4 to –1.7. In patients originally on placebo, the changes were 0.70-0.77 and –0.9, respectively.
Both groups surpassed the minimally clinically important difference for the FSFI-D score, which is considered to be 0.6.
“Patients switching from placebo experienced a higher incidence of adverse events than those continuing on bremelanotide during the open-label extension (79% versus 63%, respectively),” Dr. Simon and associates said.
The treatment is subcutaneous and can be self-administered up to about 45 minutes before a sexual event, no more than once during a 24-hour period, and no more than 8 doses per month, according to an FDA press release. The drug is contraindicated for women with cardiovascular disease or uncontrolled hypertension due to observations of transiently, slightly increased blood pressure.
The trials were funded by Palatin Technologies and AMAG Pharmaceuticals. The authors and coauthors have extensive financial relationships with pharmaceutical companies. Dr. Carson reported no financial conflicts.
SOURCE: Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003500; Obstet Gynecol. 2019 Oct 8. doi: 10.1097/AOG.0000000000003514.
FROM OBSTETRICS & GYNECOLOGY
Elagolix with add-back therapy appears efficacious despite uterine and fibroid factors
PHILADELPHIA – according to results presented at the annual meeting of the American Society for Reproductive Medicine.
Ayman Al-Hendy, MD, PhD, director of translational research at the University of Illinois at Chicago, and associates, analyzed a pooled subgroup of 790 patients from the Elaris UF-1 and UF-2 trials who received elagolix twice daily at a dose of 300 mg (199 patients), elagolix 300 mg twice daily with add-back therapy (1 mg of estradiol plus 0.5 mg of norethindrone acetate; 395 patients), and a placebo group (196 patients) for treatment of heavy menstrual bleeding. Patients were premenopausal women aged 18-51 years with more than 80 mL of menstrual blood loss per cycle. The study design included a washout period, followed by a 2.5-month to 3.5-month screening period, and patients were randomized to 6 months of treatment with placebo, elagolix alone, or elagolix with add-back therapy in a 1:1:2 ratio. Researchers evaluated whether patients had less than 80 mL of menstrual blood loss per cycle and a 50% or more reduction in menstrual blood loss per cycle by the end of the study.
In a subgroup analysis, they also analyzed primary fibroid volume, fibroid stage, and uterine volume. The median primary fibroid volume was 36.2 cm3 (range, 1.0-1,081.5 cm3). Fibroid location was classified using the International Federation of Gynecology and Obstetrics (FIGO) staging system, and researchers placed fibroids into FIGO 0-3, FIGO 4, and FIGO 5-8 groups. At baseline, characteristics between groups were similar, but the patients who received elagolix alone had a lower number of fibroids classified as FIGO 0-3 and had a greater percentage of fibroids less than 36.2 cm3. The median uterine volume was 356.5 cm3 (range, 71.6-3,347.9 cm3).
At final follow-up, 81% of patients receiving elagolix alone and 72% of patients receiving elagolix with add-back therapy responded to treatment, compared with placebo (9%).
Patients receiving elagolix plus add-back therapy responded to treatment better than placebo, and there were no significant differences in outcomes in terms of FIGO stage: Response was as follows for patients with FIGO 0-3 classified fibroids (78% of 47 patients vs. 9% of 25 patients), FIGO 4 fibroids (68% of 177 patients vs. 15% of 85 patients) and FIGO 5-8 fibroids (74% of 165 patients vs. 4% of 82 patients). The same was true in terms of both primary fibroid volume and uterine volume in patients who received elagolix plus add-back therapy, compared with those who received placebo: Patients with a primary fibroid volume of less than 36.2 cm3 (74% of 189 patients vs. 15% of 92 patients) responded similarly to elagolix plus add-back therapy as did patients with a primary fibroid volume greater than 36.2 cm3 (70% of 200 patients vs. 5% of 100 patients), and there were no significant differences between the treatment response of patients with a uterine volume less than 365.5 cm3 (75% of 203 patients vs. 15% of 88 patients) and a uterine volume greater than 365.5 cm3 (70% of 192 patients vs. 5% of 108 patients).
“These really are very encouraging results and suggests that, in women with different fibroids, elagolix with add-back therapy would be an effective treatment option despite uterine and fibroid volume and location of fibroids,” said Dr. Al-Hendy.
Dr. Al-Hendy noted there are ongoing studies analyzing elagolix with add-back in women with fibroids, in women with endometriosis, and elagolix in women with polycystic ovary syndrome.
AbbVie recently submitted a new drug application to the Food and Drug Administration for elagolix based on results from these two trials. Elagolix, an oral GnRh receptor antagonist, is an FDA-approved oral medication for the management of endometriosis with associated moderate to severe pain.
This study was funded by AbbVie, and the company was involved in the study design, research, data collection, analysis and interpretation of the data, as well as the writing, reviewing and approving of the study for publication. The authors reported various relationships with industry, pharmaceutical companies, government entities, and other organizations.
SOURCE: Al-Hendy A et al. ASRM 2019, Abstract O-205.
PHILADELPHIA – according to results presented at the annual meeting of the American Society for Reproductive Medicine.
Ayman Al-Hendy, MD, PhD, director of translational research at the University of Illinois at Chicago, and associates, analyzed a pooled subgroup of 790 patients from the Elaris UF-1 and UF-2 trials who received elagolix twice daily at a dose of 300 mg (199 patients), elagolix 300 mg twice daily with add-back therapy (1 mg of estradiol plus 0.5 mg of norethindrone acetate; 395 patients), and a placebo group (196 patients) for treatment of heavy menstrual bleeding. Patients were premenopausal women aged 18-51 years with more than 80 mL of menstrual blood loss per cycle. The study design included a washout period, followed by a 2.5-month to 3.5-month screening period, and patients were randomized to 6 months of treatment with placebo, elagolix alone, or elagolix with add-back therapy in a 1:1:2 ratio. Researchers evaluated whether patients had less than 80 mL of menstrual blood loss per cycle and a 50% or more reduction in menstrual blood loss per cycle by the end of the study.
In a subgroup analysis, they also analyzed primary fibroid volume, fibroid stage, and uterine volume. The median primary fibroid volume was 36.2 cm3 (range, 1.0-1,081.5 cm3). Fibroid location was classified using the International Federation of Gynecology and Obstetrics (FIGO) staging system, and researchers placed fibroids into FIGO 0-3, FIGO 4, and FIGO 5-8 groups. At baseline, characteristics between groups were similar, but the patients who received elagolix alone had a lower number of fibroids classified as FIGO 0-3 and had a greater percentage of fibroids less than 36.2 cm3. The median uterine volume was 356.5 cm3 (range, 71.6-3,347.9 cm3).
At final follow-up, 81% of patients receiving elagolix alone and 72% of patients receiving elagolix with add-back therapy responded to treatment, compared with placebo (9%).
Patients receiving elagolix plus add-back therapy responded to treatment better than placebo, and there were no significant differences in outcomes in terms of FIGO stage: Response was as follows for patients with FIGO 0-3 classified fibroids (78% of 47 patients vs. 9% of 25 patients), FIGO 4 fibroids (68% of 177 patients vs. 15% of 85 patients) and FIGO 5-8 fibroids (74% of 165 patients vs. 4% of 82 patients). The same was true in terms of both primary fibroid volume and uterine volume in patients who received elagolix plus add-back therapy, compared with those who received placebo: Patients with a primary fibroid volume of less than 36.2 cm3 (74% of 189 patients vs. 15% of 92 patients) responded similarly to elagolix plus add-back therapy as did patients with a primary fibroid volume greater than 36.2 cm3 (70% of 200 patients vs. 5% of 100 patients), and there were no significant differences between the treatment response of patients with a uterine volume less than 365.5 cm3 (75% of 203 patients vs. 15% of 88 patients) and a uterine volume greater than 365.5 cm3 (70% of 192 patients vs. 5% of 108 patients).
“These really are very encouraging results and suggests that, in women with different fibroids, elagolix with add-back therapy would be an effective treatment option despite uterine and fibroid volume and location of fibroids,” said Dr. Al-Hendy.
Dr. Al-Hendy noted there are ongoing studies analyzing elagolix with add-back in women with fibroids, in women with endometriosis, and elagolix in women with polycystic ovary syndrome.
AbbVie recently submitted a new drug application to the Food and Drug Administration for elagolix based on results from these two trials. Elagolix, an oral GnRh receptor antagonist, is an FDA-approved oral medication for the management of endometriosis with associated moderate to severe pain.
This study was funded by AbbVie, and the company was involved in the study design, research, data collection, analysis and interpretation of the data, as well as the writing, reviewing and approving of the study for publication. The authors reported various relationships with industry, pharmaceutical companies, government entities, and other organizations.
SOURCE: Al-Hendy A et al. ASRM 2019, Abstract O-205.
PHILADELPHIA – according to results presented at the annual meeting of the American Society for Reproductive Medicine.
Ayman Al-Hendy, MD, PhD, director of translational research at the University of Illinois at Chicago, and associates, analyzed a pooled subgroup of 790 patients from the Elaris UF-1 and UF-2 trials who received elagolix twice daily at a dose of 300 mg (199 patients), elagolix 300 mg twice daily with add-back therapy (1 mg of estradiol plus 0.5 mg of norethindrone acetate; 395 patients), and a placebo group (196 patients) for treatment of heavy menstrual bleeding. Patients were premenopausal women aged 18-51 years with more than 80 mL of menstrual blood loss per cycle. The study design included a washout period, followed by a 2.5-month to 3.5-month screening period, and patients were randomized to 6 months of treatment with placebo, elagolix alone, or elagolix with add-back therapy in a 1:1:2 ratio. Researchers evaluated whether patients had less than 80 mL of menstrual blood loss per cycle and a 50% or more reduction in menstrual blood loss per cycle by the end of the study.
In a subgroup analysis, they also analyzed primary fibroid volume, fibroid stage, and uterine volume. The median primary fibroid volume was 36.2 cm3 (range, 1.0-1,081.5 cm3). Fibroid location was classified using the International Federation of Gynecology and Obstetrics (FIGO) staging system, and researchers placed fibroids into FIGO 0-3, FIGO 4, and FIGO 5-8 groups. At baseline, characteristics between groups were similar, but the patients who received elagolix alone had a lower number of fibroids classified as FIGO 0-3 and had a greater percentage of fibroids less than 36.2 cm3. The median uterine volume was 356.5 cm3 (range, 71.6-3,347.9 cm3).
At final follow-up, 81% of patients receiving elagolix alone and 72% of patients receiving elagolix with add-back therapy responded to treatment, compared with placebo (9%).
Patients receiving elagolix plus add-back therapy responded to treatment better than placebo, and there were no significant differences in outcomes in terms of FIGO stage: Response was as follows for patients with FIGO 0-3 classified fibroids (78% of 47 patients vs. 9% of 25 patients), FIGO 4 fibroids (68% of 177 patients vs. 15% of 85 patients) and FIGO 5-8 fibroids (74% of 165 patients vs. 4% of 82 patients). The same was true in terms of both primary fibroid volume and uterine volume in patients who received elagolix plus add-back therapy, compared with those who received placebo: Patients with a primary fibroid volume of less than 36.2 cm3 (74% of 189 patients vs. 15% of 92 patients) responded similarly to elagolix plus add-back therapy as did patients with a primary fibroid volume greater than 36.2 cm3 (70% of 200 patients vs. 5% of 100 patients), and there were no significant differences between the treatment response of patients with a uterine volume less than 365.5 cm3 (75% of 203 patients vs. 15% of 88 patients) and a uterine volume greater than 365.5 cm3 (70% of 192 patients vs. 5% of 108 patients).
“These really are very encouraging results and suggests that, in women with different fibroids, elagolix with add-back therapy would be an effective treatment option despite uterine and fibroid volume and location of fibroids,” said Dr. Al-Hendy.
Dr. Al-Hendy noted there are ongoing studies analyzing elagolix with add-back in women with fibroids, in women with endometriosis, and elagolix in women with polycystic ovary syndrome.
AbbVie recently submitted a new drug application to the Food and Drug Administration for elagolix based on results from these two trials. Elagolix, an oral GnRh receptor antagonist, is an FDA-approved oral medication for the management of endometriosis with associated moderate to severe pain.
This study was funded by AbbVie, and the company was involved in the study design, research, data collection, analysis and interpretation of the data, as well as the writing, reviewing and approving of the study for publication. The authors reported various relationships with industry, pharmaceutical companies, government entities, and other organizations.
SOURCE: Al-Hendy A et al. ASRM 2019, Abstract O-205.
REPORTING FROM ASRM 2019