User login
Testicular sperm may improve IVF outcomes in some cases
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
FROM ACOG 2020
Testosterone therapy linked to CV risk in men with HIV
Men with HIV are likely prone to the same cardiovascular risks from testosterone therapy as other men, according to new research.
There’s no reason to think they weren’t, but it hadn’t been demonstrated until now, and men with HIV are already at increased risk for cardiovascular disease. The take-home message is that “it would be prudent for clinicians to monitor closely for cardiovascular risk factors and recommend intervention to lower cardiovascular risk among men with HIV on or considering testosterone therapy,” lead investigator Sabina Haberlen, PhD, an assistant scientist in the infectious disease epidemiology division of Johns Hopkins University, Baltimore, said in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because concerns about the spread of COVID-19.
Testosterone therapy is common among middle-aged and older men with HIV to counter the hypogonadism associated with infection. The investigators turned to the Multicenter AIDS Cohort Study – a 30-year, four-city study of HIV-1 infection in men who have sex with men – to gauge its effect.
The 300 men in the study had a baseline coronary CT angiogram in 2010-2013 and a repeat study a mean of 4.5 years later. They had no history of coronary interventions or kidney dysfunction at baseline and were aged 40-70 years, with a median age of 51 years. About 70% reported never using testosterone, 8% were former users before entering the study, 7% started using testosterone between the two CTs, and 15% entered the study on testosterone and stayed on it.
Adjusting for age, race, cardiovascular risk factors, baseline serum testosterone levels, and other potential confounders, the risk of significant coronary artery calcium (CAC) progression was 2 times greater among continuous users (P = .03) and 2.4 times greater among new users (P = .01), compared with former users, who the investigators used as a control group because, at some point, they too had indications for testosterone replacement and so were more medically similar than never users.
The risk of noncalcified plaque volume progression was also more than twice as high among ongoing users, and elevated, although not significantly so, among ongoing users.
In short, “our findings are similar to those on subclinical atherosclerotic progression” in trials of older men in the general population on testosterone replacement, Dr. Haberlen said.
About half the subjects were white, 41% were at high risk for cardiovascular disease, 91% were on antiretroviral therapy, and 81% had undetectable HIV viral loads. Median total testosterone was 606 ng/dL. CAC progression was defined by incident CAC, at least a 10 Agatston unit/year increase if the baseline CAC score was 1-100, and a 10% or more annual increase if the baseline score was above 100.
Lower baseline serum testosterone was also associated with an increased risk of CAC progression, although not progression of noncalcified plaques.
The work was funded by the National Institutes of Health. Dr. Haberlen didn’t report any relevant disclosures.
SOURCE: Haberlen S et al. CROI 2020, Abstract 662.
Men with HIV are likely prone to the same cardiovascular risks from testosterone therapy as other men, according to new research.
There’s no reason to think they weren’t, but it hadn’t been demonstrated until now, and men with HIV are already at increased risk for cardiovascular disease. The take-home message is that “it would be prudent for clinicians to monitor closely for cardiovascular risk factors and recommend intervention to lower cardiovascular risk among men with HIV on or considering testosterone therapy,” lead investigator Sabina Haberlen, PhD, an assistant scientist in the infectious disease epidemiology division of Johns Hopkins University, Baltimore, said in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because concerns about the spread of COVID-19.
Testosterone therapy is common among middle-aged and older men with HIV to counter the hypogonadism associated with infection. The investigators turned to the Multicenter AIDS Cohort Study – a 30-year, four-city study of HIV-1 infection in men who have sex with men – to gauge its effect.
The 300 men in the study had a baseline coronary CT angiogram in 2010-2013 and a repeat study a mean of 4.5 years later. They had no history of coronary interventions or kidney dysfunction at baseline and were aged 40-70 years, with a median age of 51 years. About 70% reported never using testosterone, 8% were former users before entering the study, 7% started using testosterone between the two CTs, and 15% entered the study on testosterone and stayed on it.
Adjusting for age, race, cardiovascular risk factors, baseline serum testosterone levels, and other potential confounders, the risk of significant coronary artery calcium (CAC) progression was 2 times greater among continuous users (P = .03) and 2.4 times greater among new users (P = .01), compared with former users, who the investigators used as a control group because, at some point, they too had indications for testosterone replacement and so were more medically similar than never users.
The risk of noncalcified plaque volume progression was also more than twice as high among ongoing users, and elevated, although not significantly so, among ongoing users.
In short, “our findings are similar to those on subclinical atherosclerotic progression” in trials of older men in the general population on testosterone replacement, Dr. Haberlen said.
About half the subjects were white, 41% were at high risk for cardiovascular disease, 91% were on antiretroviral therapy, and 81% had undetectable HIV viral loads. Median total testosterone was 606 ng/dL. CAC progression was defined by incident CAC, at least a 10 Agatston unit/year increase if the baseline CAC score was 1-100, and a 10% or more annual increase if the baseline score was above 100.
Lower baseline serum testosterone was also associated with an increased risk of CAC progression, although not progression of noncalcified plaques.
The work was funded by the National Institutes of Health. Dr. Haberlen didn’t report any relevant disclosures.
SOURCE: Haberlen S et al. CROI 2020, Abstract 662.
Men with HIV are likely prone to the same cardiovascular risks from testosterone therapy as other men, according to new research.
There’s no reason to think they weren’t, but it hadn’t been demonstrated until now, and men with HIV are already at increased risk for cardiovascular disease. The take-home message is that “it would be prudent for clinicians to monitor closely for cardiovascular risk factors and recommend intervention to lower cardiovascular risk among men with HIV on or considering testosterone therapy,” lead investigator Sabina Haberlen, PhD, an assistant scientist in the infectious disease epidemiology division of Johns Hopkins University, Baltimore, said in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because concerns about the spread of COVID-19.
Testosterone therapy is common among middle-aged and older men with HIV to counter the hypogonadism associated with infection. The investigators turned to the Multicenter AIDS Cohort Study – a 30-year, four-city study of HIV-1 infection in men who have sex with men – to gauge its effect.
The 300 men in the study had a baseline coronary CT angiogram in 2010-2013 and a repeat study a mean of 4.5 years later. They had no history of coronary interventions or kidney dysfunction at baseline and were aged 40-70 years, with a median age of 51 years. About 70% reported never using testosterone, 8% were former users before entering the study, 7% started using testosterone between the two CTs, and 15% entered the study on testosterone and stayed on it.
Adjusting for age, race, cardiovascular risk factors, baseline serum testosterone levels, and other potential confounders, the risk of significant coronary artery calcium (CAC) progression was 2 times greater among continuous users (P = .03) and 2.4 times greater among new users (P = .01), compared with former users, who the investigators used as a control group because, at some point, they too had indications for testosterone replacement and so were more medically similar than never users.
The risk of noncalcified plaque volume progression was also more than twice as high among ongoing users, and elevated, although not significantly so, among ongoing users.
In short, “our findings are similar to those on subclinical atherosclerotic progression” in trials of older men in the general population on testosterone replacement, Dr. Haberlen said.
About half the subjects were white, 41% were at high risk for cardiovascular disease, 91% were on antiretroviral therapy, and 81% had undetectable HIV viral loads. Median total testosterone was 606 ng/dL. CAC progression was defined by incident CAC, at least a 10 Agatston unit/year increase if the baseline CAC score was 1-100, and a 10% or more annual increase if the baseline score was above 100.
Lower baseline serum testosterone was also associated with an increased risk of CAC progression, although not progression of noncalcified plaques.
The work was funded by the National Institutes of Health. Dr. Haberlen didn’t report any relevant disclosures.
SOURCE: Haberlen S et al. CROI 2020, Abstract 662.
FROM CROI 2020
In gestational diabetes, early postpartum glucose testing is a winner
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
REPORTING FROM THE PREGNANCY MEETING
Vitamin D supplements in pregnancy boost bone health in offspring
Vitamin D supplementation during pregnancy is associated with higher bone mineral content in the offspring, even up to 6 years after birth, research suggests.
“The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures and osteoporosis later in life,” wrote Nicklas Brustad, MD, of the Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors. Their report is in JAMA Pediatrics.This was a secondary analysis of a prospective, double-blind, randomized controlled trial of high versus standard dose vitamin D supplementation in 623 pregnant Danish women and the outcomes in their 584 children. The women were randomized either to a daily dose of 2,400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week after birth. All women were advised to maintain a daily intake of 400 IU of vitamin D3.
The children underwent anthropometric growth assessments regularly up to age 6 years, and underwent whole-body dual-energy radiograph absorptiometry (DXA) scanning at 3 years and 6 years.
At 3 years, children of mothers who received the vitamin D supplements showed significantly higher mean total-body-less-head (TBLH) bone mineral content (BMC) compared with those who received placebo (294 g vs. 289 g) and total-body BMC (526 g vs. 514 g), respectively, after adjustment for age, sex, height, and weight.
The difference in total-body BMC was particularly evident in children of mothers who had insufficient vitamin D levels at baseline, compared with those with sufficient vitamin D levels (538 g vs. 514 g). The study also saw higher head bone mineral density (BMD) in children of mothers with insufficient vitamin D at baseline who received supplementation.
At 6 years, there still were significant differences in BMC between the supplementation and placebo groups. Children in the vitamin D group had an 8-g greater TBLH BMC compared with those in the placebo group, and a 14-g higher total BMC.
Among the children of mothers with insufficient preintervention vitamin D, there was an 18-g higher mean total BMC and 0.0125 g/cm2 greater total BMD, compared with those with sufficient vitamin D at baseline.
Overall, the children of mothers who received high-dose vitamin D supplementation had a mean 8-g higher TBLH BMC, a mean 0.023 g/cm2 higher head BMD, and a mean 12-g higher total BMC.
Dr. Brustad and associates noted that the head could represent the most sensitive compartment for intervention, because 80% of bone mineralization of the skull occurs by the age of 3 years.
The study also showed a seasonal effect, such that mothers who gave birth in winter showed the greatest effects of vitamin D supplementation on head BMC.
There was a nonsignificant trend toward a lower fracture rate among children in the supplementation group, compared with the placebo group.
“We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group,” the authors wrote.
Vitamin D supplementation did not appear to affect the children’s growth. At 6 years, there were no significant differences between the two groups in body mass index, height, weight, or waist, head, and thorax circumference.
Neonatologist Carol Wagner, MD, said in an interview that the study provided an absolute reason for vitamin D supplementation during pregnancy.
“At the very least, a study like this argues for much more than is recommended by the European nutrition group or the U.S. group,” said Dr. Wagner, professor of pediatrics at the Medical University of South Carolina, Charleston. “Here you have a therapy that costs literally pennies a day, and no one should be deficient.”
She also pointed out that the study was able to show significant effects on BMC despite the fact that there would have been considerable variation in postnatal vitamin D intake from breast milk or formula.
Cristina Palacios, PhD, an associate professor in the department of dietetics and nutrition at Florida International University, Miami, said that vitamin D deficiency is increasingly prevalent worldwide, and is associated with a return of rickets – the skeletal disorder caused by vitamin D deficiency – in children.
“If women are deficient during pregnancy, providing vitamin D supplementation in pregnancy may promote bone health in their offspring,” Dr. Palacios said in an interview. “Because vitamin D is such an important component of bone metabolism, this could prevent future rickets in these children.”
Dr. Palacios coauthored a recent Cochrane review that examined the safety of high-dose vitamin D supplementation in pregnancy, and said the analysis found no evidence of safety concerns.
The study was supported by The Lundbeck Foundation, the Ministry of Health, Danish Council for Strategic Research, and the Capital Region Research Foundation. The authors declared no conflicts of interest.
SOURCE: Brustad N et al. JAMA Pediatrics 2020 Feb 24. doi: 10.1001/jamapediatrics.2019.6083.
Vitamin D supplementation during pregnancy is associated with higher bone mineral content in the offspring, even up to 6 years after birth, research suggests.
“The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures and osteoporosis later in life,” wrote Nicklas Brustad, MD, of the Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors. Their report is in JAMA Pediatrics.This was a secondary analysis of a prospective, double-blind, randomized controlled trial of high versus standard dose vitamin D supplementation in 623 pregnant Danish women and the outcomes in their 584 children. The women were randomized either to a daily dose of 2,400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week after birth. All women were advised to maintain a daily intake of 400 IU of vitamin D3.
The children underwent anthropometric growth assessments regularly up to age 6 years, and underwent whole-body dual-energy radiograph absorptiometry (DXA) scanning at 3 years and 6 years.
At 3 years, children of mothers who received the vitamin D supplements showed significantly higher mean total-body-less-head (TBLH) bone mineral content (BMC) compared with those who received placebo (294 g vs. 289 g) and total-body BMC (526 g vs. 514 g), respectively, after adjustment for age, sex, height, and weight.
The difference in total-body BMC was particularly evident in children of mothers who had insufficient vitamin D levels at baseline, compared with those with sufficient vitamin D levels (538 g vs. 514 g). The study also saw higher head bone mineral density (BMD) in children of mothers with insufficient vitamin D at baseline who received supplementation.
At 6 years, there still were significant differences in BMC between the supplementation and placebo groups. Children in the vitamin D group had an 8-g greater TBLH BMC compared with those in the placebo group, and a 14-g higher total BMC.
Among the children of mothers with insufficient preintervention vitamin D, there was an 18-g higher mean total BMC and 0.0125 g/cm2 greater total BMD, compared with those with sufficient vitamin D at baseline.
Overall, the children of mothers who received high-dose vitamin D supplementation had a mean 8-g higher TBLH BMC, a mean 0.023 g/cm2 higher head BMD, and a mean 12-g higher total BMC.
Dr. Brustad and associates noted that the head could represent the most sensitive compartment for intervention, because 80% of bone mineralization of the skull occurs by the age of 3 years.
The study also showed a seasonal effect, such that mothers who gave birth in winter showed the greatest effects of vitamin D supplementation on head BMC.
There was a nonsignificant trend toward a lower fracture rate among children in the supplementation group, compared with the placebo group.
“We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group,” the authors wrote.
Vitamin D supplementation did not appear to affect the children’s growth. At 6 years, there were no significant differences between the two groups in body mass index, height, weight, or waist, head, and thorax circumference.
Neonatologist Carol Wagner, MD, said in an interview that the study provided an absolute reason for vitamin D supplementation during pregnancy.
“At the very least, a study like this argues for much more than is recommended by the European nutrition group or the U.S. group,” said Dr. Wagner, professor of pediatrics at the Medical University of South Carolina, Charleston. “Here you have a therapy that costs literally pennies a day, and no one should be deficient.”
She also pointed out that the study was able to show significant effects on BMC despite the fact that there would have been considerable variation in postnatal vitamin D intake from breast milk or formula.
Cristina Palacios, PhD, an associate professor in the department of dietetics and nutrition at Florida International University, Miami, said that vitamin D deficiency is increasingly prevalent worldwide, and is associated with a return of rickets – the skeletal disorder caused by vitamin D deficiency – in children.
“If women are deficient during pregnancy, providing vitamin D supplementation in pregnancy may promote bone health in their offspring,” Dr. Palacios said in an interview. “Because vitamin D is such an important component of bone metabolism, this could prevent future rickets in these children.”
Dr. Palacios coauthored a recent Cochrane review that examined the safety of high-dose vitamin D supplementation in pregnancy, and said the analysis found no evidence of safety concerns.
The study was supported by The Lundbeck Foundation, the Ministry of Health, Danish Council for Strategic Research, and the Capital Region Research Foundation. The authors declared no conflicts of interest.
SOURCE: Brustad N et al. JAMA Pediatrics 2020 Feb 24. doi: 10.1001/jamapediatrics.2019.6083.
Vitamin D supplementation during pregnancy is associated with higher bone mineral content in the offspring, even up to 6 years after birth, research suggests.
“The well-established tracking of bone mineralization from early life throughout childhood and early adulthood is a key factor for the final peak bone mass gained and the subsequent risk of fractures and osteoporosis later in life,” wrote Nicklas Brustad, MD, of the Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors. Their report is in JAMA Pediatrics.This was a secondary analysis of a prospective, double-blind, randomized controlled trial of high versus standard dose vitamin D supplementation in 623 pregnant Danish women and the outcomes in their 584 children. The women were randomized either to a daily dose of 2,400 IU vitamin D3 (cholecalciferol) or matching placebo capsules from 24 weeks’ gestation until 1 week after birth. All women were advised to maintain a daily intake of 400 IU of vitamin D3.
The children underwent anthropometric growth assessments regularly up to age 6 years, and underwent whole-body dual-energy radiograph absorptiometry (DXA) scanning at 3 years and 6 years.
At 3 years, children of mothers who received the vitamin D supplements showed significantly higher mean total-body-less-head (TBLH) bone mineral content (BMC) compared with those who received placebo (294 g vs. 289 g) and total-body BMC (526 g vs. 514 g), respectively, after adjustment for age, sex, height, and weight.
The difference in total-body BMC was particularly evident in children of mothers who had insufficient vitamin D levels at baseline, compared with those with sufficient vitamin D levels (538 g vs. 514 g). The study also saw higher head bone mineral density (BMD) in children of mothers with insufficient vitamin D at baseline who received supplementation.
At 6 years, there still were significant differences in BMC between the supplementation and placebo groups. Children in the vitamin D group had an 8-g greater TBLH BMC compared with those in the placebo group, and a 14-g higher total BMC.
Among the children of mothers with insufficient preintervention vitamin D, there was an 18-g higher mean total BMC and 0.0125 g/cm2 greater total BMD, compared with those with sufficient vitamin D at baseline.
Overall, the children of mothers who received high-dose vitamin D supplementation had a mean 8-g higher TBLH BMC, a mean 0.023 g/cm2 higher head BMD, and a mean 12-g higher total BMC.
Dr. Brustad and associates noted that the head could represent the most sensitive compartment for intervention, because 80% of bone mineralization of the skull occurs by the age of 3 years.
The study also showed a seasonal effect, such that mothers who gave birth in winter showed the greatest effects of vitamin D supplementation on head BMC.
There was a nonsignificant trend toward a lower fracture rate among children in the supplementation group, compared with the placebo group.
“We speculate that these intervention effects could be of importance for bone health and osteoporosis risk in adult life, which is supported by our likely underpowered post hoc analysis on fracture risk, suggesting an almost 40% reduced incidence of fractures of the larger bones in the high-dose vitamin D group,” the authors wrote.
Vitamin D supplementation did not appear to affect the children’s growth. At 6 years, there were no significant differences between the two groups in body mass index, height, weight, or waist, head, and thorax circumference.
Neonatologist Carol Wagner, MD, said in an interview that the study provided an absolute reason for vitamin D supplementation during pregnancy.
“At the very least, a study like this argues for much more than is recommended by the European nutrition group or the U.S. group,” said Dr. Wagner, professor of pediatrics at the Medical University of South Carolina, Charleston. “Here you have a therapy that costs literally pennies a day, and no one should be deficient.”
She also pointed out that the study was able to show significant effects on BMC despite the fact that there would have been considerable variation in postnatal vitamin D intake from breast milk or formula.
Cristina Palacios, PhD, an associate professor in the department of dietetics and nutrition at Florida International University, Miami, said that vitamin D deficiency is increasingly prevalent worldwide, and is associated with a return of rickets – the skeletal disorder caused by vitamin D deficiency – in children.
“If women are deficient during pregnancy, providing vitamin D supplementation in pregnancy may promote bone health in their offspring,” Dr. Palacios said in an interview. “Because vitamin D is such an important component of bone metabolism, this could prevent future rickets in these children.”
Dr. Palacios coauthored a recent Cochrane review that examined the safety of high-dose vitamin D supplementation in pregnancy, and said the analysis found no evidence of safety concerns.
The study was supported by The Lundbeck Foundation, the Ministry of Health, Danish Council for Strategic Research, and the Capital Region Research Foundation. The authors declared no conflicts of interest.
SOURCE: Brustad N et al. JAMA Pediatrics 2020 Feb 24. doi: 10.1001/jamapediatrics.2019.6083.
FROM JAMA PEDIATRICS
FDA approves weekly contraceptive patch Twirla
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
2020 Update on fertility
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
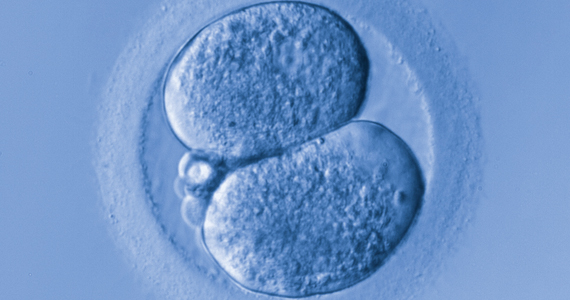
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.
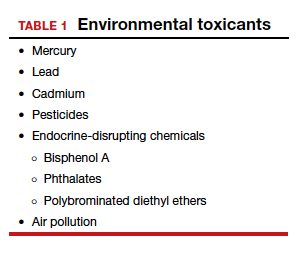

Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
Less gestational weight gain seen with metformin
GRAPEVINE, TEX.* – Pregnant women with type 2 diabetes or prediabetes had significantly less gestational weight gain if they had metformin exposure at any point in their pregnancies, with no differences in infant birth weight or postnatal infant hypoglycemia, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
In a retrospective single-center review of 284 women without metformin exposure and 227 with metformin exposure in pregnancy, metformin exposure at any point in pregnancy was associated with a significantly greater chance of appropriate – rather than excessive – weight gain.
The relationship held true for the 169 women who had metformin in their first trimester of pregnancy. Here, 69% of women had appropriate weight gain using Institute of Medicine and American College of Obstetricians and Gynecologists standards, compared with 54% of the 282 women who had no metformin exposure (adjusted odds ratio 1.92, P = .003). A further 22% of women receiving metformin in their first trimester of pregnancy lost weight, compared with 9% of women without metformin exposure (aOR 2.11, P = .019). There was no significant difference between the two groups in infant birth weight.
Separately, study author Jacquelyn Adams, MD, and her colleagues analyzed outcomes for the full cohort of 227 women who received metformin at any point in their pregnancy, comparing them again to the 282 women who had not received metformin. Most women (85%) were on 2 g of metformin at the time of delivery. These results again showed a greater likelihood of appropriate weight gain in the metformin group (69%; aOR 1.85; P = .002). Maternal weight loss was seen in 20% of this group (aOR 1.98, P = .018). Infant birth weights were not significantly different between these two groups.
“We found that women who had been on metformin at any point in their pregnancy had more appropriate weight gain and less excessive weight gain,” said Dr. Adams, a maternal-fetal medicine fellow at the University of Wisconsin–Madison. “Actually, some women on metformin had even had a little bit of weight loss, with no difference in their baby’s birth weight. So that’s really exciting, because our starting prepregnancy body mass index was 33-36 [kg/m2], which is considered obese,” she said in an interview.
This is an important finding, said Dr. Adams, because previous work has shown that less weight gain in pregnancy is associated with lower risk for hypertension and preeclampsia, and lower rates of fetal macrosomia.
What about infant outcomes? Dr. Adams said that there were many concerns about metformin: “Would it affect baby outcome? Were those babies more likely to be hypoglycemic? Were they more likely to be growth restricted? Were they more likely to have issues in the NICU? And the answer was really, ‘No.’ ”
“So we can both help these women have appropriate weight gain and not have any negative effects on these babies,” she added.
Specifically, Dr. Adams and her coinvestigators found no significant differences between the groups in gestational age at birth, likelihood of neonatal ICU admission, Apgar scores, neonatal hypoglycemia, respiratory distress syndrome, or fetal death. Fetal growth restriction and anomalies occurred at a low and similar rate between the groups.
Dr. Adams said that she was not surprised to see that metformin was associated with less weight gain in pregnancy, but she was surprised at how highly significant the differences were with metformin use. “Metformin is first-line for diabetes in nonpregnant individuals because it’s associated with things like weight loss, and because of ease of use and lack of hypoglycemia – so I was really hoping to see this kind of result.”
Women receiving metformin were a mean 34 years old, while those who didn’t get metformin were 32 years old, a significant difference. Prepregnancy body mass index also was higher in those receiving metformin, and they were more likely to have a type 2 diabetes diagnosis. A similar proportion of both groups – about two-thirds – were white, and about 20% were Hispanic.
The lower weight gain seen in metformin-takers also might smooth the way post partum, said Dr. Adams. “My perception is that, when these women leave us, they might not have any primary care follow-up; they might not have anybody following their diabetes; and metformin is a very viable way to help them in their life outside of pregnancy.
“Not to mention that all the weight you gain in pregnancy, you do eventually have to lose post partum,” she added, “so having less pregnancy weight gain kind of sets them up for success in their postpregnancy life as well.”
Asked whether these results inform the ongoing question of whether insulin or metformin is the most appropriate first-line treatment for gestational diabetes, Dr. Adams first noted that “there’s a lot of crossover,” pointing out that over 60% of the participants in her study eventually also required insulin.
“It’s a question I would love to address in a head-to-head trial,” she said, adding that questions about metformin’s effects on the placenta and the potential for later deleterious effects require more study.
In her practice, Dr. Adams said that patients generally are discharged with a metformin prescription, and then meet with a diabetes educator 1 week after delivery to assess blood glucose levels and adjust medical management. Following that, a warm hand-off to a primary care practice who can continue management and education is optimal, she said.
In terms of next steps, “We would really love to look at metformin in the postpartum period,” said Dr. Adams. Ideally, future work could look for outcomes that extend beyond the 6- to 8-week postpartum follow-up visit. For example, she said, there are indications that women with insulin insensitivity might benefit from metformin while breastfeeding; it’s also possible that metformin might reduce the risk of postpartum preeclampsia.
Dr. Adams reported that she had no conflicts of interest and no outside sources of funding.
SOURCE: Adams J et al. SMFM 2020, Abstract 335.
*This story was updated 2/10/2020.
GRAPEVINE, TEX.* – Pregnant women with type 2 diabetes or prediabetes had significantly less gestational weight gain if they had metformin exposure at any point in their pregnancies, with no differences in infant birth weight or postnatal infant hypoglycemia, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
In a retrospective single-center review of 284 women without metformin exposure and 227 with metformin exposure in pregnancy, metformin exposure at any point in pregnancy was associated with a significantly greater chance of appropriate – rather than excessive – weight gain.
The relationship held true for the 169 women who had metformin in their first trimester of pregnancy. Here, 69% of women had appropriate weight gain using Institute of Medicine and American College of Obstetricians and Gynecologists standards, compared with 54% of the 282 women who had no metformin exposure (adjusted odds ratio 1.92, P = .003). A further 22% of women receiving metformin in their first trimester of pregnancy lost weight, compared with 9% of women without metformin exposure (aOR 2.11, P = .019). There was no significant difference between the two groups in infant birth weight.
Separately, study author Jacquelyn Adams, MD, and her colleagues analyzed outcomes for the full cohort of 227 women who received metformin at any point in their pregnancy, comparing them again to the 282 women who had not received metformin. Most women (85%) were on 2 g of metformin at the time of delivery. These results again showed a greater likelihood of appropriate weight gain in the metformin group (69%; aOR 1.85; P = .002). Maternal weight loss was seen in 20% of this group (aOR 1.98, P = .018). Infant birth weights were not significantly different between these two groups.
“We found that women who had been on metformin at any point in their pregnancy had more appropriate weight gain and less excessive weight gain,” said Dr. Adams, a maternal-fetal medicine fellow at the University of Wisconsin–Madison. “Actually, some women on metformin had even had a little bit of weight loss, with no difference in their baby’s birth weight. So that’s really exciting, because our starting prepregnancy body mass index was 33-36 [kg/m2], which is considered obese,” she said in an interview.
This is an important finding, said Dr. Adams, because previous work has shown that less weight gain in pregnancy is associated with lower risk for hypertension and preeclampsia, and lower rates of fetal macrosomia.
What about infant outcomes? Dr. Adams said that there were many concerns about metformin: “Would it affect baby outcome? Were those babies more likely to be hypoglycemic? Were they more likely to be growth restricted? Were they more likely to have issues in the NICU? And the answer was really, ‘No.’ ”
“So we can both help these women have appropriate weight gain and not have any negative effects on these babies,” she added.
Specifically, Dr. Adams and her coinvestigators found no significant differences between the groups in gestational age at birth, likelihood of neonatal ICU admission, Apgar scores, neonatal hypoglycemia, respiratory distress syndrome, or fetal death. Fetal growth restriction and anomalies occurred at a low and similar rate between the groups.
Dr. Adams said that she was not surprised to see that metformin was associated with less weight gain in pregnancy, but she was surprised at how highly significant the differences were with metformin use. “Metformin is first-line for diabetes in nonpregnant individuals because it’s associated with things like weight loss, and because of ease of use and lack of hypoglycemia – so I was really hoping to see this kind of result.”
Women receiving metformin were a mean 34 years old, while those who didn’t get metformin were 32 years old, a significant difference. Prepregnancy body mass index also was higher in those receiving metformin, and they were more likely to have a type 2 diabetes diagnosis. A similar proportion of both groups – about two-thirds – were white, and about 20% were Hispanic.
The lower weight gain seen in metformin-takers also might smooth the way post partum, said Dr. Adams. “My perception is that, when these women leave us, they might not have any primary care follow-up; they might not have anybody following their diabetes; and metformin is a very viable way to help them in their life outside of pregnancy.
“Not to mention that all the weight you gain in pregnancy, you do eventually have to lose post partum,” she added, “so having less pregnancy weight gain kind of sets them up for success in their postpregnancy life as well.”
Asked whether these results inform the ongoing question of whether insulin or metformin is the most appropriate first-line treatment for gestational diabetes, Dr. Adams first noted that “there’s a lot of crossover,” pointing out that over 60% of the participants in her study eventually also required insulin.
“It’s a question I would love to address in a head-to-head trial,” she said, adding that questions about metformin’s effects on the placenta and the potential for later deleterious effects require more study.
In her practice, Dr. Adams said that patients generally are discharged with a metformin prescription, and then meet with a diabetes educator 1 week after delivery to assess blood glucose levels and adjust medical management. Following that, a warm hand-off to a primary care practice who can continue management and education is optimal, she said.
In terms of next steps, “We would really love to look at metformin in the postpartum period,” said Dr. Adams. Ideally, future work could look for outcomes that extend beyond the 6- to 8-week postpartum follow-up visit. For example, she said, there are indications that women with insulin insensitivity might benefit from metformin while breastfeeding; it’s also possible that metformin might reduce the risk of postpartum preeclampsia.
Dr. Adams reported that she had no conflicts of interest and no outside sources of funding.
SOURCE: Adams J et al. SMFM 2020, Abstract 335.
*This story was updated 2/10/2020.
GRAPEVINE, TEX.* – Pregnant women with type 2 diabetes or prediabetes had significantly less gestational weight gain if they had metformin exposure at any point in their pregnancies, with no differences in infant birth weight or postnatal infant hypoglycemia, according to research presented at the meeting sponsored by the Society for Maternal-Fetal Medicine.
In a retrospective single-center review of 284 women without metformin exposure and 227 with metformin exposure in pregnancy, metformin exposure at any point in pregnancy was associated with a significantly greater chance of appropriate – rather than excessive – weight gain.
The relationship held true for the 169 women who had metformin in their first trimester of pregnancy. Here, 69% of women had appropriate weight gain using Institute of Medicine and American College of Obstetricians and Gynecologists standards, compared with 54% of the 282 women who had no metformin exposure (adjusted odds ratio 1.92, P = .003). A further 22% of women receiving metformin in their first trimester of pregnancy lost weight, compared with 9% of women without metformin exposure (aOR 2.11, P = .019). There was no significant difference between the two groups in infant birth weight.
Separately, study author Jacquelyn Adams, MD, and her colleagues analyzed outcomes for the full cohort of 227 women who received metformin at any point in their pregnancy, comparing them again to the 282 women who had not received metformin. Most women (85%) were on 2 g of metformin at the time of delivery. These results again showed a greater likelihood of appropriate weight gain in the metformin group (69%; aOR 1.85; P = .002). Maternal weight loss was seen in 20% of this group (aOR 1.98, P = .018). Infant birth weights were not significantly different between these two groups.
“We found that women who had been on metformin at any point in their pregnancy had more appropriate weight gain and less excessive weight gain,” said Dr. Adams, a maternal-fetal medicine fellow at the University of Wisconsin–Madison. “Actually, some women on metformin had even had a little bit of weight loss, with no difference in their baby’s birth weight. So that’s really exciting, because our starting prepregnancy body mass index was 33-36 [kg/m2], which is considered obese,” she said in an interview.
This is an important finding, said Dr. Adams, because previous work has shown that less weight gain in pregnancy is associated with lower risk for hypertension and preeclampsia, and lower rates of fetal macrosomia.
What about infant outcomes? Dr. Adams said that there were many concerns about metformin: “Would it affect baby outcome? Were those babies more likely to be hypoglycemic? Were they more likely to be growth restricted? Were they more likely to have issues in the NICU? And the answer was really, ‘No.’ ”
“So we can both help these women have appropriate weight gain and not have any negative effects on these babies,” she added.
Specifically, Dr. Adams and her coinvestigators found no significant differences between the groups in gestational age at birth, likelihood of neonatal ICU admission, Apgar scores, neonatal hypoglycemia, respiratory distress syndrome, or fetal death. Fetal growth restriction and anomalies occurred at a low and similar rate between the groups.
Dr. Adams said that she was not surprised to see that metformin was associated with less weight gain in pregnancy, but she was surprised at how highly significant the differences were with metformin use. “Metformin is first-line for diabetes in nonpregnant individuals because it’s associated with things like weight loss, and because of ease of use and lack of hypoglycemia – so I was really hoping to see this kind of result.”
Women receiving metformin were a mean 34 years old, while those who didn’t get metformin were 32 years old, a significant difference. Prepregnancy body mass index also was higher in those receiving metformin, and they were more likely to have a type 2 diabetes diagnosis. A similar proportion of both groups – about two-thirds – were white, and about 20% were Hispanic.
The lower weight gain seen in metformin-takers also might smooth the way post partum, said Dr. Adams. “My perception is that, when these women leave us, they might not have any primary care follow-up; they might not have anybody following their diabetes; and metformin is a very viable way to help them in their life outside of pregnancy.
“Not to mention that all the weight you gain in pregnancy, you do eventually have to lose post partum,” she added, “so having less pregnancy weight gain kind of sets them up for success in their postpregnancy life as well.”
Asked whether these results inform the ongoing question of whether insulin or metformin is the most appropriate first-line treatment for gestational diabetes, Dr. Adams first noted that “there’s a lot of crossover,” pointing out that over 60% of the participants in her study eventually also required insulin.
“It’s a question I would love to address in a head-to-head trial,” she said, adding that questions about metformin’s effects on the placenta and the potential for later deleterious effects require more study.
In her practice, Dr. Adams said that patients generally are discharged with a metformin prescription, and then meet with a diabetes educator 1 week after delivery to assess blood glucose levels and adjust medical management. Following that, a warm hand-off to a primary care practice who can continue management and education is optimal, she said.
In terms of next steps, “We would really love to look at metformin in the postpartum period,” said Dr. Adams. Ideally, future work could look for outcomes that extend beyond the 6- to 8-week postpartum follow-up visit. For example, she said, there are indications that women with insulin insensitivity might benefit from metformin while breastfeeding; it’s also possible that metformin might reduce the risk of postpartum preeclampsia.
Dr. Adams reported that she had no conflicts of interest and no outside sources of funding.
SOURCE: Adams J et al. SMFM 2020, Abstract 335.
*This story was updated 2/10/2020.
REPORTING FROM THE PREGNANCY MEETING
New frontier: Transgender men yield eggs, babies, even after testosterone
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
What is optimal hormonal treatment for women with polycystic ovary syndrome?
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.
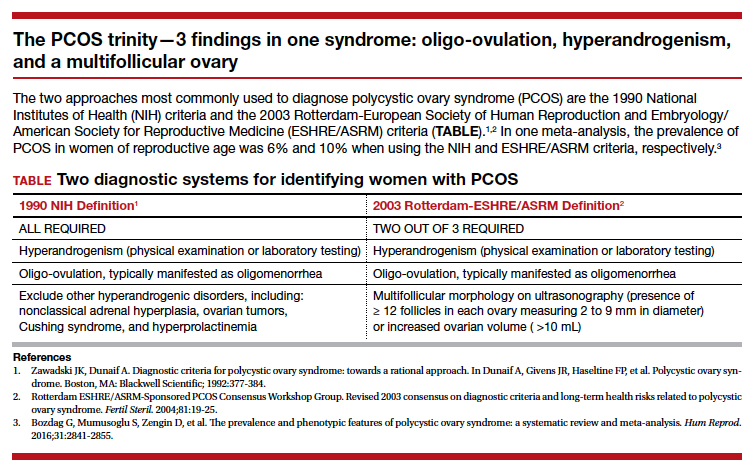
The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.
Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Elagolix is effective second-tier treatment for endometriosis-associated dysmenorrhea
PHILADELPHIA – Charles E. Miller, MD, said at the annual meeting of the American Society for Reproductive Medicine.
Although clinicians need prior authorization and evidence of treatment failure before prescribing Elagolix, the drug is a viable option as a second-tier treatment for patients with endometriosis-associated dysmenorrhea, said Dr. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. “We have a drug that is very effective, that has a very low adverse event profile, and is tolerated by the vast majority of our patients.”
First-line options
NSAIDs are first-line treatment for endometriosis-related dysmenorrhea, with acetaminophen used in cases where NSAIDs are contraindicated or cause side effects such as gastrointestinal issues. Hormonal contraceptives also can be used as first-line treatment, divided into estrogen/progestin and progestin-only options that can be combined. Evidence from the literature has shown oral pills decrease pain, compared with placebo, but the decrease is not dose dependent, said Dr. Miller.
“We also know that if you use it continuously or prolonged, we find that there is going to be greater success with dysmenorrhea, and that ultimately you would use a higher-dose pill because of the greater risk of breakthrough when using a lesser dose in a continuous fashion,” he said. “Obviously if you’re not having menses, you’re not going to have dysmenorrhea.”
Other estrogen/progestin hormonal contraception such as the vaginal ring or transdermal patch also have been shown to decrease dysmenorrhea from endometriosis, with one study showing a reduction from 17% to 6% in moderate to severe dysmenorrhea in patients using the vaginal ring, compared with patients receiving oral contraceptives. In a separate randomized, controlled trial, “dysmenorrhea was more common in patch users, so it doesn’t appear that the patch is quite as effective in terms of reducing dysmenorrhea,” said Dr. Miller (JAMA. 2001 May 9. doi: 10.1001/jama.285.18.2347).
Compared with combination hormone therapy, there has been less research conducted on progestin-only hormone contraceptives on reducing dysmenorrhea from endometriosis. For example, there is little evidence for depot medroxyprogesterone acetate in reducing dysmenorrhea, but rather with it causing amenorrhea; one study showed a 50% amenorrhea rate at 1 year. “The disadvantage, however, in our infertile population is ultimately getting the menses back,” said Dr. Miller.
IUDs using levonorgestrel appear comparable with gonadotropin-releasing hormone (GnRH) agonists in reducing endometriosis-related pain; in one study, most women treated with either of these had visual analogue scores of less than 3 at 6 months of treatment. Between 68% and 75% of women with dysmenorrhea who receive an implantable contraceptive device with etonogestrel report decreased pain, and one meta-analysis reported 75% of women had “complete resolution of dysmenorrhea.” Concerning progestin-only pills, they can be used for endometriosis-related dysmenorrhea, but they are “problematic in that there’s a lot of breakthrough bleeding, and often times that is associated with pain,” said Dr. Miller.
Second-tier options
Injectable GnRH agonists are effective options as second-tier treatments for endometriosis-related dysmenorrhea, but patients are at risk of developing postmenopausal symptoms such as hot flashes, insomnia, spotting, and decreased libido. “One advantage to that is, over the years and particularly something that I’ve done with my endometriosis-related dysmenorrhea, is to utilize add-back with these patients,” said Dr. Miller, who noted that patients on 2.5 mg of norethindrone acetate and 0.5 mg of ethinyl estradiol“do very well” with that combination of add-back therapy.
Elagolix is the most recent second-tier treatment option for these patients, and was studied in the Elaris EM-I and Elaris EM-II trials in a once-daily dose of 150 mg and a twice-daily dose of 200 mg. In Elaris EM-1, 76% of patients in the 200-mg elagolix group had a clinical response, compared with 46% in the 150-mg group and 20% in the placebo group (N Engl J Med. 2017 Jul 6. doi: 10.1056/NEJMoa1700089). However, patients should not be on elagolix at 200 mg for more than 6 months, while patients receiving elagolix at 150 mg can stay on the treatment for up to 2 years.
Patients taking elagolix also showed postmenopausal symptoms, with 24% in the 150-mg group and 46% in the 200-mg group experiencing hot flashes, compared with 9% of patients in the placebo group. While 6% of patients in the 150-mg group and 10% in the 200-mg group discontinued because of adverse events, 1% and 3% of patients in the 150-mg and 200-mg group discontinued because of hot flashes or night sweats, respectively. “Symptoms are well tolerated, far different than in comparison with leuprolide acetate and GnRH agonists,” said Dr. Miller.
There also is a benefit to how patients recover from bone mineral density (BMD) changes after remaining on elagolix, Dr. Miller noted. In patients who received elagolix for 12 months at doses of 150 mg and 200 mg, there was an increase in lumbar spine BMD recovered 6 months after discontinuation, with patients in the 150-mg group experiencing a recovery close to baseline BMD levels. Among patients who discontinued treatment, there also was a quick resumption in menses for both groups: 87% of patients in the 150 mg group and 88% of patients in the 200-mg group who discontinued treatment after 6 months had resumed menses by 2 months after discontinuation, while 95% of patients in the 150-mg and 91% in the 200-mg group who discontinued after 12 months resumed menses by 2 months after discontinuation.
Dr. Miller reported relationships with AbbVie, Allergan, Blue Seas Med Spa, Espiner Medical, Gynesonics, Halt Medical, Hologic, Karl Storz, Medtronic, and Richard Wolf in the form of consultancies, grants, speakers’ bureau appointments, stock options, royalties, and ownership interests.
PHILADELPHIA – Charles E. Miller, MD, said at the annual meeting of the American Society for Reproductive Medicine.
Although clinicians need prior authorization and evidence of treatment failure before prescribing Elagolix, the drug is a viable option as a second-tier treatment for patients with endometriosis-associated dysmenorrhea, said Dr. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. “We have a drug that is very effective, that has a very low adverse event profile, and is tolerated by the vast majority of our patients.”
First-line options
NSAIDs are first-line treatment for endometriosis-related dysmenorrhea, with acetaminophen used in cases where NSAIDs are contraindicated or cause side effects such as gastrointestinal issues. Hormonal contraceptives also can be used as first-line treatment, divided into estrogen/progestin and progestin-only options that can be combined. Evidence from the literature has shown oral pills decrease pain, compared with placebo, but the decrease is not dose dependent, said Dr. Miller.
“We also know that if you use it continuously or prolonged, we find that there is going to be greater success with dysmenorrhea, and that ultimately you would use a higher-dose pill because of the greater risk of breakthrough when using a lesser dose in a continuous fashion,” he said. “Obviously if you’re not having menses, you’re not going to have dysmenorrhea.”
Other estrogen/progestin hormonal contraception such as the vaginal ring or transdermal patch also have been shown to decrease dysmenorrhea from endometriosis, with one study showing a reduction from 17% to 6% in moderate to severe dysmenorrhea in patients using the vaginal ring, compared with patients receiving oral contraceptives. In a separate randomized, controlled trial, “dysmenorrhea was more common in patch users, so it doesn’t appear that the patch is quite as effective in terms of reducing dysmenorrhea,” said Dr. Miller (JAMA. 2001 May 9. doi: 10.1001/jama.285.18.2347).
Compared with combination hormone therapy, there has been less research conducted on progestin-only hormone contraceptives on reducing dysmenorrhea from endometriosis. For example, there is little evidence for depot medroxyprogesterone acetate in reducing dysmenorrhea, but rather with it causing amenorrhea; one study showed a 50% amenorrhea rate at 1 year. “The disadvantage, however, in our infertile population is ultimately getting the menses back,” said Dr. Miller.
IUDs using levonorgestrel appear comparable with gonadotropin-releasing hormone (GnRH) agonists in reducing endometriosis-related pain; in one study, most women treated with either of these had visual analogue scores of less than 3 at 6 months of treatment. Between 68% and 75% of women with dysmenorrhea who receive an implantable contraceptive device with etonogestrel report decreased pain, and one meta-analysis reported 75% of women had “complete resolution of dysmenorrhea.” Concerning progestin-only pills, they can be used for endometriosis-related dysmenorrhea, but they are “problematic in that there’s a lot of breakthrough bleeding, and often times that is associated with pain,” said Dr. Miller.
Second-tier options
Injectable GnRH agonists are effective options as second-tier treatments for endometriosis-related dysmenorrhea, but patients are at risk of developing postmenopausal symptoms such as hot flashes, insomnia, spotting, and decreased libido. “One advantage to that is, over the years and particularly something that I’ve done with my endometriosis-related dysmenorrhea, is to utilize add-back with these patients,” said Dr. Miller, who noted that patients on 2.5 mg of norethindrone acetate and 0.5 mg of ethinyl estradiol“do very well” with that combination of add-back therapy.
Elagolix is the most recent second-tier treatment option for these patients, and was studied in the Elaris EM-I and Elaris EM-II trials in a once-daily dose of 150 mg and a twice-daily dose of 200 mg. In Elaris EM-1, 76% of patients in the 200-mg elagolix group had a clinical response, compared with 46% in the 150-mg group and 20% in the placebo group (N Engl J Med. 2017 Jul 6. doi: 10.1056/NEJMoa1700089). However, patients should not be on elagolix at 200 mg for more than 6 months, while patients receiving elagolix at 150 mg can stay on the treatment for up to 2 years.
Patients taking elagolix also showed postmenopausal symptoms, with 24% in the 150-mg group and 46% in the 200-mg group experiencing hot flashes, compared with 9% of patients in the placebo group. While 6% of patients in the 150-mg group and 10% in the 200-mg group discontinued because of adverse events, 1% and 3% of patients in the 150-mg and 200-mg group discontinued because of hot flashes or night sweats, respectively. “Symptoms are well tolerated, far different than in comparison with leuprolide acetate and GnRH agonists,” said Dr. Miller.
There also is a benefit to how patients recover from bone mineral density (BMD) changes after remaining on elagolix, Dr. Miller noted. In patients who received elagolix for 12 months at doses of 150 mg and 200 mg, there was an increase in lumbar spine BMD recovered 6 months after discontinuation, with patients in the 150-mg group experiencing a recovery close to baseline BMD levels. Among patients who discontinued treatment, there also was a quick resumption in menses for both groups: 87% of patients in the 150 mg group and 88% of patients in the 200-mg group who discontinued treatment after 6 months had resumed menses by 2 months after discontinuation, while 95% of patients in the 150-mg and 91% in the 200-mg group who discontinued after 12 months resumed menses by 2 months after discontinuation.
Dr. Miller reported relationships with AbbVie, Allergan, Blue Seas Med Spa, Espiner Medical, Gynesonics, Halt Medical, Hologic, Karl Storz, Medtronic, and Richard Wolf in the form of consultancies, grants, speakers’ bureau appointments, stock options, royalties, and ownership interests.
PHILADELPHIA – Charles E. Miller, MD, said at the annual meeting of the American Society for Reproductive Medicine.
Although clinicians need prior authorization and evidence of treatment failure before prescribing Elagolix, the drug is a viable option as a second-tier treatment for patients with endometriosis-associated dysmenorrhea, said Dr. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. “We have a drug that is very effective, that has a very low adverse event profile, and is tolerated by the vast majority of our patients.”
First-line options
NSAIDs are first-line treatment for endometriosis-related dysmenorrhea, with acetaminophen used in cases where NSAIDs are contraindicated or cause side effects such as gastrointestinal issues. Hormonal contraceptives also can be used as first-line treatment, divided into estrogen/progestin and progestin-only options that can be combined. Evidence from the literature has shown oral pills decrease pain, compared with placebo, but the decrease is not dose dependent, said Dr. Miller.
“We also know that if you use it continuously or prolonged, we find that there is going to be greater success with dysmenorrhea, and that ultimately you would use a higher-dose pill because of the greater risk of breakthrough when using a lesser dose in a continuous fashion,” he said. “Obviously if you’re not having menses, you’re not going to have dysmenorrhea.”
Other estrogen/progestin hormonal contraception such as the vaginal ring or transdermal patch also have been shown to decrease dysmenorrhea from endometriosis, with one study showing a reduction from 17% to 6% in moderate to severe dysmenorrhea in patients using the vaginal ring, compared with patients receiving oral contraceptives. In a separate randomized, controlled trial, “dysmenorrhea was more common in patch users, so it doesn’t appear that the patch is quite as effective in terms of reducing dysmenorrhea,” said Dr. Miller (JAMA. 2001 May 9. doi: 10.1001/jama.285.18.2347).
Compared with combination hormone therapy, there has been less research conducted on progestin-only hormone contraceptives on reducing dysmenorrhea from endometriosis. For example, there is little evidence for depot medroxyprogesterone acetate in reducing dysmenorrhea, but rather with it causing amenorrhea; one study showed a 50% amenorrhea rate at 1 year. “The disadvantage, however, in our infertile population is ultimately getting the menses back,” said Dr. Miller.
IUDs using levonorgestrel appear comparable with gonadotropin-releasing hormone (GnRH) agonists in reducing endometriosis-related pain; in one study, most women treated with either of these had visual analogue scores of less than 3 at 6 months of treatment. Between 68% and 75% of women with dysmenorrhea who receive an implantable contraceptive device with etonogestrel report decreased pain, and one meta-analysis reported 75% of women had “complete resolution of dysmenorrhea.” Concerning progestin-only pills, they can be used for endometriosis-related dysmenorrhea, but they are “problematic in that there’s a lot of breakthrough bleeding, and often times that is associated with pain,” said Dr. Miller.
Second-tier options
Injectable GnRH agonists are effective options as second-tier treatments for endometriosis-related dysmenorrhea, but patients are at risk of developing postmenopausal symptoms such as hot flashes, insomnia, spotting, and decreased libido. “One advantage to that is, over the years and particularly something that I’ve done with my endometriosis-related dysmenorrhea, is to utilize add-back with these patients,” said Dr. Miller, who noted that patients on 2.5 mg of norethindrone acetate and 0.5 mg of ethinyl estradiol“do very well” with that combination of add-back therapy.
Elagolix is the most recent second-tier treatment option for these patients, and was studied in the Elaris EM-I and Elaris EM-II trials in a once-daily dose of 150 mg and a twice-daily dose of 200 mg. In Elaris EM-1, 76% of patients in the 200-mg elagolix group had a clinical response, compared with 46% in the 150-mg group and 20% in the placebo group (N Engl J Med. 2017 Jul 6. doi: 10.1056/NEJMoa1700089). However, patients should not be on elagolix at 200 mg for more than 6 months, while patients receiving elagolix at 150 mg can stay on the treatment for up to 2 years.
Patients taking elagolix also showed postmenopausal symptoms, with 24% in the 150-mg group and 46% in the 200-mg group experiencing hot flashes, compared with 9% of patients in the placebo group. While 6% of patients in the 150-mg group and 10% in the 200-mg group discontinued because of adverse events, 1% and 3% of patients in the 150-mg and 200-mg group discontinued because of hot flashes or night sweats, respectively. “Symptoms are well tolerated, far different than in comparison with leuprolide acetate and GnRH agonists,” said Dr. Miller.
There also is a benefit to how patients recover from bone mineral density (BMD) changes after remaining on elagolix, Dr. Miller noted. In patients who received elagolix for 12 months at doses of 150 mg and 200 mg, there was an increase in lumbar spine BMD recovered 6 months after discontinuation, with patients in the 150-mg group experiencing a recovery close to baseline BMD levels. Among patients who discontinued treatment, there also was a quick resumption in menses for both groups: 87% of patients in the 150 mg group and 88% of patients in the 200-mg group who discontinued treatment after 6 months had resumed menses by 2 months after discontinuation, while 95% of patients in the 150-mg and 91% in the 200-mg group who discontinued after 12 months resumed menses by 2 months after discontinuation.
Dr. Miller reported relationships with AbbVie, Allergan, Blue Seas Med Spa, Espiner Medical, Gynesonics, Halt Medical, Hologic, Karl Storz, Medtronic, and Richard Wolf in the form of consultancies, grants, speakers’ bureau appointments, stock options, royalties, and ownership interests.
EXPERT ANALYSIS FROM ASRM 2019