User login
Fetal estrogens show promise for safer therapy for menopause
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
AHA issues new scientific statement on heart health for LGBTQ adults
Cardiovascular health should be routinely assessed and addressed in LGBTQ adults, the American Heart Association concluded in a new scientific statement.
“Among the most important takeaways from this scientific statement is the need for health care providers in clinical settings to routinely assess sexual orientation and gender identity,” Billy A. Caceres, PhD, RN, chair of the statement writing group, said in an interview.
“This will help health care providers engage LGBTQ patients in discussions about their heart health that account for the unique experiences of this population,” said Dr. Caceres, assistant professor at Columbia University, New York.
The statement was published online Oct. 8 in Circulation.
‘Invisible’ population
There are roughly 11 million LGBTQ adults in the United States, yet they are often “invisible in health care settings and cardiovascular research,” Dr. Caceres noted. The AHA scientific statement is the first from a national organization in the United States to comprehensively summarize the evidence on cardiovascular (CV) research in LGBTQ adults.
There is mounting evidence that LGBTQ adults experience worse CV health relative to their cisgender heterosexual peers. Disparities in CV health may be driven by unique psychosocial stressors in the LGBTQ individuals such as family rejection and anxiety of concealment of their sexual orientation or gender identity.
While there is limited information on the CV health of LGBTQ people, the writing group said providers should be aware of the following:
- LGBTQ adults are more likely to use tobacco than their cisgender heterosexual peers.
- Transgender adults may be less physically active than their cisgender counterparts. Gender-affirming care might play a role in promoting physical activity among transgender people.
- Transgender women may be at increased risk for heart disease because of behavioral and clinical factors (such as the use of gender-affirming hormones like estrogen).
- Transgender women and nonbinary persons are more likely to binge drink.
- Lesbian and bisexual women have a higher prevalence of obesity than heterosexual women do.
“We need to better understand how to support LGBTQ adults in optimizing their CV health. To do this, we will need rigorous research that examines potential explanations for the CV health disparities that have been observed in LGBTQ adults,” Dr. Caceres said.
He noted that research is also needed within the LGBTQ population among groups that might be at greater risk for heart disease, including racial- and ethnic-minority and low-income LGBTQ adults.
“Researchers should also design and test evidence-based interventions to promote the heart health of LGBTQ adults. This is an area that is greatly lacking within CV health research,” said Dr. Caceres.
Discrimination in health care
Discrimination against LGBTQ adults in health care settings also remains a problem, the authors noted.
The writing group cites data showing that nearly 56% of sexual-minority and 70% of gender-minority adults report having experienced some form of discrimination from clinicians, including the use of harsh/abusive language.
“Perhaps most alarming,” roughly 8% of sexual-minority and 25% of transgender individuals have been denied health care by clinicians, they noted.
“LGBTQ individuals are delaying primary care and preventative visits because there is a great fear of being treated differently. Being treated differently often means receiving inadequate or inferior care because of sexual orientation or gender identity,” Dr. Caceres said in a news release.
The writing group calls for greater emphasis on LGBTQ health issues in the education of all health care providers. Dr. Caceres said it’s “paramount to include content about LGBTQ health in clinical training and licensure requirements in order to address these cardiovascular health disparities.”
Traditionally, there has been very little LGBTQ-related content in health care professional education training. A 2018 online survey of students at 10 medical schools found that approximately 80% of students did not feel competent to provide care for transgender patients.
But that may soon change. In September 2020, the Accreditation Review Commission on Education for the Physician Assistant began requiring LGBTQ curricular content, the writing group notes.
The AHA scientific statement on LGBTQ was developed by the writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article originally appeared on Medscape.com.
Cardiovascular health should be routinely assessed and addressed in LGBTQ adults, the American Heart Association concluded in a new scientific statement.
“Among the most important takeaways from this scientific statement is the need for health care providers in clinical settings to routinely assess sexual orientation and gender identity,” Billy A. Caceres, PhD, RN, chair of the statement writing group, said in an interview.
“This will help health care providers engage LGBTQ patients in discussions about their heart health that account for the unique experiences of this population,” said Dr. Caceres, assistant professor at Columbia University, New York.
The statement was published online Oct. 8 in Circulation.
‘Invisible’ population
There are roughly 11 million LGBTQ adults in the United States, yet they are often “invisible in health care settings and cardiovascular research,” Dr. Caceres noted. The AHA scientific statement is the first from a national organization in the United States to comprehensively summarize the evidence on cardiovascular (CV) research in LGBTQ adults.
There is mounting evidence that LGBTQ adults experience worse CV health relative to their cisgender heterosexual peers. Disparities in CV health may be driven by unique psychosocial stressors in the LGBTQ individuals such as family rejection and anxiety of concealment of their sexual orientation or gender identity.
While there is limited information on the CV health of LGBTQ people, the writing group said providers should be aware of the following:
- LGBTQ adults are more likely to use tobacco than their cisgender heterosexual peers.
- Transgender adults may be less physically active than their cisgender counterparts. Gender-affirming care might play a role in promoting physical activity among transgender people.
- Transgender women may be at increased risk for heart disease because of behavioral and clinical factors (such as the use of gender-affirming hormones like estrogen).
- Transgender women and nonbinary persons are more likely to binge drink.
- Lesbian and bisexual women have a higher prevalence of obesity than heterosexual women do.
“We need to better understand how to support LGBTQ adults in optimizing their CV health. To do this, we will need rigorous research that examines potential explanations for the CV health disparities that have been observed in LGBTQ adults,” Dr. Caceres said.
He noted that research is also needed within the LGBTQ population among groups that might be at greater risk for heart disease, including racial- and ethnic-minority and low-income LGBTQ adults.
“Researchers should also design and test evidence-based interventions to promote the heart health of LGBTQ adults. This is an area that is greatly lacking within CV health research,” said Dr. Caceres.
Discrimination in health care
Discrimination against LGBTQ adults in health care settings also remains a problem, the authors noted.
The writing group cites data showing that nearly 56% of sexual-minority and 70% of gender-minority adults report having experienced some form of discrimination from clinicians, including the use of harsh/abusive language.
“Perhaps most alarming,” roughly 8% of sexual-minority and 25% of transgender individuals have been denied health care by clinicians, they noted.
“LGBTQ individuals are delaying primary care and preventative visits because there is a great fear of being treated differently. Being treated differently often means receiving inadequate or inferior care because of sexual orientation or gender identity,” Dr. Caceres said in a news release.
The writing group calls for greater emphasis on LGBTQ health issues in the education of all health care providers. Dr. Caceres said it’s “paramount to include content about LGBTQ health in clinical training and licensure requirements in order to address these cardiovascular health disparities.”
Traditionally, there has been very little LGBTQ-related content in health care professional education training. A 2018 online survey of students at 10 medical schools found that approximately 80% of students did not feel competent to provide care for transgender patients.
But that may soon change. In September 2020, the Accreditation Review Commission on Education for the Physician Assistant began requiring LGBTQ curricular content, the writing group notes.
The AHA scientific statement on LGBTQ was developed by the writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article originally appeared on Medscape.com.
Cardiovascular health should be routinely assessed and addressed in LGBTQ adults, the American Heart Association concluded in a new scientific statement.
“Among the most important takeaways from this scientific statement is the need for health care providers in clinical settings to routinely assess sexual orientation and gender identity,” Billy A. Caceres, PhD, RN, chair of the statement writing group, said in an interview.
“This will help health care providers engage LGBTQ patients in discussions about their heart health that account for the unique experiences of this population,” said Dr. Caceres, assistant professor at Columbia University, New York.
The statement was published online Oct. 8 in Circulation.
‘Invisible’ population
There are roughly 11 million LGBTQ adults in the United States, yet they are often “invisible in health care settings and cardiovascular research,” Dr. Caceres noted. The AHA scientific statement is the first from a national organization in the United States to comprehensively summarize the evidence on cardiovascular (CV) research in LGBTQ adults.
There is mounting evidence that LGBTQ adults experience worse CV health relative to their cisgender heterosexual peers. Disparities in CV health may be driven by unique psychosocial stressors in the LGBTQ individuals such as family rejection and anxiety of concealment of their sexual orientation or gender identity.
While there is limited information on the CV health of LGBTQ people, the writing group said providers should be aware of the following:
- LGBTQ adults are more likely to use tobacco than their cisgender heterosexual peers.
- Transgender adults may be less physically active than their cisgender counterparts. Gender-affirming care might play a role in promoting physical activity among transgender people.
- Transgender women may be at increased risk for heart disease because of behavioral and clinical factors (such as the use of gender-affirming hormones like estrogen).
- Transgender women and nonbinary persons are more likely to binge drink.
- Lesbian and bisexual women have a higher prevalence of obesity than heterosexual women do.
“We need to better understand how to support LGBTQ adults in optimizing their CV health. To do this, we will need rigorous research that examines potential explanations for the CV health disparities that have been observed in LGBTQ adults,” Dr. Caceres said.
He noted that research is also needed within the LGBTQ population among groups that might be at greater risk for heart disease, including racial- and ethnic-minority and low-income LGBTQ adults.
“Researchers should also design and test evidence-based interventions to promote the heart health of LGBTQ adults. This is an area that is greatly lacking within CV health research,” said Dr. Caceres.
Discrimination in health care
Discrimination against LGBTQ adults in health care settings also remains a problem, the authors noted.
The writing group cites data showing that nearly 56% of sexual-minority and 70% of gender-minority adults report having experienced some form of discrimination from clinicians, including the use of harsh/abusive language.
“Perhaps most alarming,” roughly 8% of sexual-minority and 25% of transgender individuals have been denied health care by clinicians, they noted.
“LGBTQ individuals are delaying primary care and preventative visits because there is a great fear of being treated differently. Being treated differently often means receiving inadequate or inferior care because of sexual orientation or gender identity,” Dr. Caceres said in a news release.
The writing group calls for greater emphasis on LGBTQ health issues in the education of all health care providers. Dr. Caceres said it’s “paramount to include content about LGBTQ health in clinical training and licensure requirements in order to address these cardiovascular health disparities.”
Traditionally, there has been very little LGBTQ-related content in health care professional education training. A 2018 online survey of students at 10 medical schools found that approximately 80% of students did not feel competent to provide care for transgender patients.
But that may soon change. In September 2020, the Accreditation Review Commission on Education for the Physician Assistant began requiring LGBTQ curricular content, the writing group notes.
The AHA scientific statement on LGBTQ was developed by the writing group on behalf of the AHA Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article originally appeared on Medscape.com.
Lifting the restrictions on mifepristone during COVID-19: A step in the right direction
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
Mifepristone is a safe, effective, and well-tolerated medication for managing miscarriage and for medical abortion when combined with misoprostol.1,2 Since the US Food and Drug Administration (FDA) approved its use in 2000, more than 4 million women have used this medication.3 The combination of mifepristone with misoprostol was used for 39% of all US abortions in 2017.4 Approximately 10% of all clinically recognized pregnancies end in miscarriages, and many are safely managed with either misoprostol alone or with the combination of mifepristone and misoprostol.5
The issue
The prescription and distribution of mifepristone is highly regulated by the FDA via requirements outlined in the Risk Evaluation and Mitigation Strategies (REMS) drug safety program. The FDA may determine a REMS is necessary for a specific drug to ensure the benefits of a drug outweigh the potential risks. A REMS may include an informative package insert for patients, follow-up communication to prescribers—including letters, safety protocols or recommended laboratory tests, or Elements to Assure Safe Use (ETASU). ETASU are types of REMS that are placed on medications that have significant potential for serious adverse effects, and without such restrictions FDA approval would be rescinded.
Are mifepristone requirements fairly applied?
The 3 ETASU restrictions on the distribution of mifepristone are in-person dispensation, prescriber certification, and patient signatures on special forms.6 The in-person dispensing requirement is applied to only 16 other medications (one of which is Mifeprex, the brand version of mifepristone), and Mifeprex/mifepristone are the only ones deemed safe for self-administration—meaning that patients receive the drug from a clinic but then may take it at a site of their choosing. The prescriber certification requirement places expectations on providers to account for distribution of doses and keep records of serial numbers (in effect, having clinicians act as both physician and pharmacist, as most medications are distributed and recorded in pharmacies). The patient form was recommended for elimination in 2016 due to its duplicative information and burden on patients—a recommendation that was then overruled by the FDA commissioner.7
These 3 requirements placed on mifepristone specifically target dosages for use related to abortions and miscarriages. Mifepristone is used to treat other medical conditions, with much higher doses, without the same restrictions—in fact, the FDA has allowed much higher doses of mifepristone to be mailed directly to a patient when prescribed for different disorders. The American College of Obstetricians and Gynecologists (ACOG) has long opposed the burdensome REMS requirements on mifepristone for reproductive health indications.8
Arguments regarding the safety of mifepristone must be understood in the context of how the medication is taken, and the unique difference with other medications that must be administered by physicians or in health care facilities. Mifepristone is self-administered, and the desired effect—evacuation of uterine contents—typically occurs after a patient takes the accompanying medication misoprostol, which is some 24 to 72 hours later. This timeframe makes it highly unlikely that any patient would be in the presence of their provider at the time of medication effect, thus an in-person dispensing requirement has no medical bearing on the outcome of the health of the patient.
REMS changes during the COVID-19 pandemic
The coronavirus disease 2019 (COVID-19) pandemic has necessarily changed the structure of REMS and ETASU requirements for many medications, with changes made in order to mitigate viral transmission through the limitation of unnecessary visits to clinics or hospitals. The FDA announced in March of 2020 that it would not enforce pre-prescription requirements, such as laboratory or magnetic resonance imaging results, for many medications (including those more toxic than mifepristone), and that it would lift the requirement for in-person dispensation of several medications.9 Also in March 2020 the Department of Health and Human Services Secretary (HHS) and the Drug Enforcement Agency (DEA) activated a “telemedicine exception” to allow physicians to use telemedicine to satisfy mandatory requirements for prescribing controlled substances, including opioids.10
Despite repeated pleas from organizations, individuals, and physician groups, the FDA continued to enforce the REMS/ETASU for mifepristone as the pandemic decimated communities. Importantly, the pandemic has not had an equal effect on all communities, and the disparities highlighted in outcomes as related to COVID-19 are also reflected in disparities to access to reproductive choices.11 By enforcing REMS/ETASU for mifepristone during a global pandemic, the FDA has placed additional burden on women and people who menstruate. As offices and clinics have closed, and as many jobs have evaporated, additional barriers have emerged, such as lack of childcare, fewer transportation options, and decreased clinic appointments.
As the pandemic continues to affect communities in the United States, ACOG has issued guidance recommending assessment for eligibility for medical abortion remotely, and has encouraged the use of telemedicine and other remote interactions for its members and patients to limit transmission of the virus.
The lawsuit
On May 27, 2020, the American Civil Liberties Union (ACLU) (on behalf of ACOG, the Council of University Chairs of Obstetrics and Gynecology, New York State Academy of Family Physicians, SisterSong, and Honor MacNaughton, MD) filed a civil action against the FDA and HHS challenging the requirement for in-person dispensing of mifepristone and associated ETASU requirements during the COVID-19 pandemic. The plaintiffs sought this injunction based on the claim that these restrictions during the pandemic infringe on the constitutional rights to patients’ privacy and liberty and to equal protection of the law as protected by the Due Process Clause of the Fifth Amendment. Additionally, the ACLU and other organizations said these unnecessary restrictions place patients, providers, and staff at unnecessary risk of viral exposure amidst a global pandemic.
The verdict
On July 13, 2020, a federal court granted the preliminary injunction to suspend FDA’s enforcement of the in-person requirements of mifepristone for abortion during the COVID-19 pandemic. The court denied the motion for suspension of in-person restrictions as applied to miscarriage management. The preliminary injunction applies nationwide without geographic limitation. It will remain in effect until the end of the litigation or for 30 days following the expiration of the public health emergency.
What the outcome means
This injunction is a step in the right direction for patients and providers to allow for autonomy and clinical practice guided by clinician expertise. However, this ruling remains narrow. Patients must be counseled about mifepristone via telemedicine and sign a Patient Agreement Form, which must be returned electronically or by mail. Patients must receive a copy of the mifepristone medication guide, and dispensing of mifepristone must still be conducted by or under the supervision of a certified provider. The medication may not be dispensed by retail pharmacies, thus requiring providers to arrange for mailing of prescriptions to patients. Given state-based legal statutes regarding mailing of medications, this injunction may not lead to an immediate increase in access to care. In addition, patients seeking management for miscarriage must go to clinic to have mifepristone dispensed and thus risk exposure to viral transmission.
What now?
The regulation of mifepristone—in spite of excellent safety and specifically for the narrow purpose of administration in the setting of abortion and miscarriage care—is by definition a discriminatory practice against patients and providers. As clinicians, we are duty-bound to speak out against injustices to our practices and our patients. At a local level, we can work to implement safe practices in the setting of this injunction and continue to work on a national level to ensure this injunction becomes permanent and with more broad scope to eliminate all of the REMS requirements for mifepristone.
ACTION ITEMS
- Act locally! Are you an abortion provider? Contact your local ACLU (find them here) or lawyer in your area for assistance navigating the legal landscape to prescribe after this injunction.
- Act statewide! Press candidates in your state to stand up for science and data. Support legislative acts and bills that address combating discriminatory regulations.
- Act nationally! The President is responsible for appointing the Commissioner of the FDA and the Secretary of Health and Human Services (with Senate advice and consent). Who we elect matters. Seek out opportunities to become involved in increasing access to and awareness of voter registration and Election Day, and speak out against voter suppression. Make sure you are registered to vote here and check your area to review new recommendations amidst the pandemic.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin number 143: medical management of first trimester abortion. Obstet Gynecol. 2014;123:676-692.
- Schreiber CA, Crenin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Danco Laboratories. Mifeprex effectiveness and advantages. https://www.earlyoptionpill.com/is-mifeprex-right-for-me/effectiveness-advantages/ Accessed August 2, 2020.
- Jones RK, Witwer E, Jerman J. Abortion incidence and service availability in the United States, 2017. September 2019. https://www.guttmacher.org/report/abortion-incidence-service-availability-us-2017. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 150: early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
- US Food and Drug Administration. Risk evaluation and mitigation strategy (REMS) single shared system for mifepristone 200 mg. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/rems/Mifepristone_2019_04_11_REMS_Full.pdf. Accessed September 10, 2020.
- US Food and Drug Administration; Center for Drug Evaluation and Research. 2016 REMS Review, Summary Review 25. March 29, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/020687Orig1s020SumR.pdf. Accessed September 10, 2020.
- American College of Obstetricians and Gynecologists. Improving access to mifepristone for reproductive health indications. June 2018. https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2018/improving-access-to-mifepristone-for-reproductive-health-indications. Accessed August 2, 2020.
- US Food and Drug Administration. Policy for certain REMS requirements during the COVID-19 public health emergency: guidance for industry and health care professionals. March 2020. https://www.fda.gov/media/136317/download. Accessed September 10, 2020.
- US Department of Justice. US Drug Enforcement Administration. COVID-19 Information Page, Telemedicine. https://www.deadiversion.usdoj.gov/coronavirus.html#TELE. Accessed May 25, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html. Updated July 24, 2020. Accessed September 10, 2020.
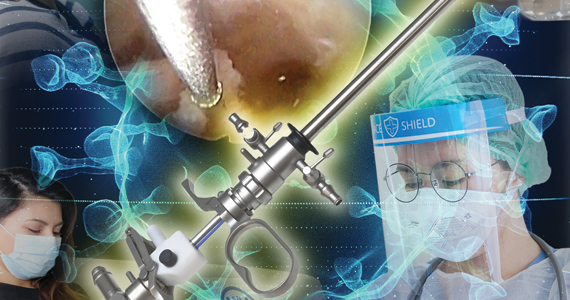
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
Appendix may be common site of endometriosis
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
Among women who have a coincidental appendectomy during surgery for chronic pelvic pain or endometriosis, about 15% have appendiceal endometriosis confirmed by pathological examination, according to a study.
“In the women with appendiceal endometriosis, only 26% had an appendix that looked abnormal,” said Whitney T. Ross, MD, of the department of obstetrics and gynecology at Penn State Health, Hershey.
The results, presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons, indicate that “appendiceal endometriosis is common in women receiving surgery for chronic pelvic pain or endometriosis,” she said. “This study and multiple other studies have also demonstrated that coincidental appendectomy is safe.”
The long-term impact of coincidental appendectomy and its effect on quality of life are not known, however, which may make it difficult to weigh the costs and benefits of the procedure, Dr. Ross said. “It is important to talk to patients about this procedure and determine which approach is the right approach for your institution.”
The study of 609 coincidental appendectomies did not include patients with retrocecal appendices, which may confound the true rate of appendiceal endometriosis, commented Saifuddin T. Mama, MD, MPH, of Rowan University, Camden, N.J.
When the investigators started the study, they were not sure of the risks and benefits of the procedure in patients with retrocecal appendices. An anecdotal report from another research group suggests that outcomes with retrocecal appendices may not be significantly different. “But that is certainly an important question and one that we would like to address in a future prospective study,” Dr. Ross said.
Surgeons have debated the role of coincidental appendectomy during gynecologic surgery. Concerns about safety and questions about the prevalence of appendiceal pathology are reasons that coincidental appendectomy has not been more widely adopted. On the other hand, the procedure may benefit patients and aid diagnosis.
To evaluate the role of coincidental appendectomy in the surgical excision of endometriosis, Dr. Ross and colleagues analyzed data from consecutive coincidental appendectomies performed at one institution between 2013 and 2019. They identified cases in a prospectively maintained surgical database to assess safety and the prevalence of appendiceal pathology.
The indication for surgery was chronic pelvic pain but no visualized endometriosis for 42 patients, stage I-II endometriosis for 388 patients, and stage III-IV endometriosis for 179 patients.
Surgeries included laparoscopic hysterectomy (77.5%), operative laparoscopy (19.9%), and laparoscopic trachelectomy (2.6%). Pathological analysis of the appendices identified endometriosis in 14.9%, malignancy in 0.7%, polyps in 0.5%, and appendicitis in 0.3%.
Among women with chronic pelvic pain but no visualized endometriosis, 2.4% had appendiceal endometriosis. Among those with stage I-II endometriosis, 7% had appendiceal endometriosis, and in patients with stage III-IV endometriosis, the rate of appendiceal endometriosis was 35.2%.
In about 6% of patients with appendiceal endometriosis, the appendix was the only site of pathologically confirmed endometriosis.
Compared with chronic pelvic pain, stage III-IV endometriosis was associated with a significantly increased risk of appendiceal endometriosis (odds ratio, 22.2). The likelihood of appendiceal endometriosis also increased when the appendix looked abnormal (odds ratio, 6.5).
The probability of diagnosing appendiceal endometriosis also increases with the number of other locations of confirmed endometriosis.
“Our surgical decision making is based off of intraoperative findings. However, the final gold-standard diagnosis can’t take place until the pathologic specimen is analyzed,” she said. “We also know that there is a significant discordance, as high as 50%, in early-stage endometriosis between visual inspection and pathology findings.”
There were no complications related to the performance of a coincidental appendectomy during surgery or in the 12 weeks after.
Dr. Ross outlined surgeons’ three main options for performing coincidental appendectomy in patients undergoing surgery for chronic pelvic pain or endometriosis: universal coincidental appendectomy, targeted appendectomy based on operative findings, and performing the procedure based on the appearance of the appendix.
Basing the decision on appearance “is going to miss a lot of appendiceal endometriosis,” Dr. Ross said. In the present study, 67 of the 91 cases, about 74%, would have been missed.
Dr. Ross and Dr. Mama had no relevant financial disclosures. The study coauthors disclosed ties to Titan Medical, Merck, and AbbVie.
SOURCE: Ross WT et al. SGS 2020, Abstract 14.
FROM SGS 2020
Postmenopausal use of estrogen alone lowers breast cancer cases, deaths
A new follow-up study of menopausal hormone therapy found that prior use of conjugated equine estrogen (CEE) decreased both breast cancer incidence and mortality, while prior use of CEE plus medroxyprogesterone acetate (MPA) was associated with an increase in incidence.
“Prior use of CEE alone is, to our knowledge, the first pharmacologic intervention demonstrated to be associated with a statistically significantly reduction in deaths from breast cancer,” wrote Rowan T. Chlebowski, MD, PhD, of the Lundquist Institute for Biomedical Innovation in Torrance, Calif., and his coauthors. The study was published July 28 in JAMA.
To further investigate the outcomes of the Women’s Health Initiative in regard to hormone therapy and breast cancer risk, the researchers analyzed the long-term follow-up of two randomized trials that included 27,347 postmenopausal women with no prior breast cancer and negative mammograms at baseline. Their mean (SD) age was 63.4 (7.2) years. Enrollment took place from 1993 to 1998; participants were contacted for follow-up every 6 months through 2005 and annually from then on. Mortality data were gathered from follow-up and the National Death Index.
The first trial included 16,608 women with a uterus. Among these women, 8,506 received 0.625 mg/day of CEE plus 2.5 mg/day of MPA, and 8,102 received placebo. The second trial included 10,739 women who’d gotten a hysterectomy, 5,310 of whom received 0.625 mg/day of CEE alone and 5,429 of whom received placebo. The first trial ended in 2002 after a median intervention period of 5.6 years, and the second trial ended in 2004 after a period of 7.2 years.
An analysis in 2015 found that CEE alone was associated with lower risk of breast cancer and CEE plus MPA was associated with increased risk.
The current analysis confirmed that, after a median of 20.3 years of follow-up, and with mortality data now available for more than 98% of participants, CEE alone was associated with fewer cases of breast cancer (238 cases, annualized rate 0.30%), compared with placebo (296 cases, annualized rate 0.37%; hazard ratio 0.78; 95% confidence interval, 0.65-0.93; P = .005).
Furthermore, CEE alone was also associated with lower mortality (30 deaths, annualized mortality rate 0.031%), compared with placebo (46 deaths, annualized mortality rate 0.046%; HR 0.60; 95% CI, 0.37-0.97; P = .04).
By comparison, CEE plus MPA was linked with more cases of breast cancer (584 cases, annualized rate 0.45%) than placebo (447 cases, annualized rate 0.36%; HR 1.28; 95% CI, 1.13-1.45; P < .001). In regard to mortality, there was no statistically significant difference between CEE plus MPA (71 deaths, annualized mortality rate 0.045%) and placebo (53 deaths, annualized mortality rate 0.035%; HR 1.35; 95% CI, 0.94-1.95; P = .11).
“The big thing to think about is estrogen alone reducing breast cancer mortality by 40%,” said Dr. Chlebowski in an interview. “None of the other interventions, including tamoxifen, had any change on mortality. This should change the way we look at breast cancer prevention, though we might have to be a little creative about it. I think you have to be a little away from menopause for it to reduce breast cancer. But we wanted to start that debate.
“On the other hand,” he said, “a woman takes estrogen plus progestin and when you look at that curve, it’s staying about 25% increased. You take it for 5.6 years and the increase continues through 20 years, so you’re maybe buying a lifetime of increase in breast cancer by taking estrogen plus progestin for 5 years.”
He also highlighted the comprehensiveness of the mortality data, noting that “when you hook up to the National Death Index, they find 98% of all deaths in the United States. That’s really remarkable; you retain the whole power of the randomization. It means our data, between the death index and our follow-up of participants, is essentially complete.”
Use of hormone therapy, and decoding the outcomes, remains ‘complex’
Decades after the data were gathered from the Women’s Health Initiative clinical trials, they continue to assist researchers and patients alike, wrote Christina A. Minami, MD, of Brigham and Women’s Hospital in Boston and Rachel A. Freedman, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
That said, in regard to the findings of this latest analysis, “many questions still remain on whether (and how) a hormone therapy intervention that occurred many years earlier may continue to affect breast cancer risk and mortality at 20 years,” they wrote. They noted that it’s “impossible” to isolate how exposure to certain therapies can impact long-term outcomes, and that a high percentage of patients who discontinued the drugs during each trial muddy the waters even further.
“Decisions to initiate these medications remain complex,” they added, emphasizing that breast cancer risk is just one of many factors that physicians must consider when considering hormone therapy for their patients.
Dr. Chlebowski and his coauthors acknowledged their study’s limitations, including the use of very specifically administered and formulated dosages making their findings “not necessarily generalizable to other preparations.” In addition, they noted the significant percentage of patients – 54% with CEE alone and 42% with CEE plus MPA – who discontinued drug usage during their respective trials.
The Women’s Health Initiative is supported by the National Institutes of Health and the Department of Health and Human Services. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various government organizations, foundations, and pharmaceutical companies. The editorial’s authors reported no conflicts of interest.
SOURCE: Chlebowski RT et al. JAMA. 2020 Jul 28. doi: 10.1001/jama.2020.9482.
A new follow-up study of menopausal hormone therapy found that prior use of conjugated equine estrogen (CEE) decreased both breast cancer incidence and mortality, while prior use of CEE plus medroxyprogesterone acetate (MPA) was associated with an increase in incidence.
“Prior use of CEE alone is, to our knowledge, the first pharmacologic intervention demonstrated to be associated with a statistically significantly reduction in deaths from breast cancer,” wrote Rowan T. Chlebowski, MD, PhD, of the Lundquist Institute for Biomedical Innovation in Torrance, Calif., and his coauthors. The study was published July 28 in JAMA.
To further investigate the outcomes of the Women’s Health Initiative in regard to hormone therapy and breast cancer risk, the researchers analyzed the long-term follow-up of two randomized trials that included 27,347 postmenopausal women with no prior breast cancer and negative mammograms at baseline. Their mean (SD) age was 63.4 (7.2) years. Enrollment took place from 1993 to 1998; participants were contacted for follow-up every 6 months through 2005 and annually from then on. Mortality data were gathered from follow-up and the National Death Index.
The first trial included 16,608 women with a uterus. Among these women, 8,506 received 0.625 mg/day of CEE plus 2.5 mg/day of MPA, and 8,102 received placebo. The second trial included 10,739 women who’d gotten a hysterectomy, 5,310 of whom received 0.625 mg/day of CEE alone and 5,429 of whom received placebo. The first trial ended in 2002 after a median intervention period of 5.6 years, and the second trial ended in 2004 after a period of 7.2 years.
An analysis in 2015 found that CEE alone was associated with lower risk of breast cancer and CEE plus MPA was associated with increased risk.
The current analysis confirmed that, after a median of 20.3 years of follow-up, and with mortality data now available for more than 98% of participants, CEE alone was associated with fewer cases of breast cancer (238 cases, annualized rate 0.30%), compared with placebo (296 cases, annualized rate 0.37%; hazard ratio 0.78; 95% confidence interval, 0.65-0.93; P = .005).
Furthermore, CEE alone was also associated with lower mortality (30 deaths, annualized mortality rate 0.031%), compared with placebo (46 deaths, annualized mortality rate 0.046%; HR 0.60; 95% CI, 0.37-0.97; P = .04).
By comparison, CEE plus MPA was linked with more cases of breast cancer (584 cases, annualized rate 0.45%) than placebo (447 cases, annualized rate 0.36%; HR 1.28; 95% CI, 1.13-1.45; P < .001). In regard to mortality, there was no statistically significant difference between CEE plus MPA (71 deaths, annualized mortality rate 0.045%) and placebo (53 deaths, annualized mortality rate 0.035%; HR 1.35; 95% CI, 0.94-1.95; P = .11).
“The big thing to think about is estrogen alone reducing breast cancer mortality by 40%,” said Dr. Chlebowski in an interview. “None of the other interventions, including tamoxifen, had any change on mortality. This should change the way we look at breast cancer prevention, though we might have to be a little creative about it. I think you have to be a little away from menopause for it to reduce breast cancer. But we wanted to start that debate.
“On the other hand,” he said, “a woman takes estrogen plus progestin and when you look at that curve, it’s staying about 25% increased. You take it for 5.6 years and the increase continues through 20 years, so you’re maybe buying a lifetime of increase in breast cancer by taking estrogen plus progestin for 5 years.”
He also highlighted the comprehensiveness of the mortality data, noting that “when you hook up to the National Death Index, they find 98% of all deaths in the United States. That’s really remarkable; you retain the whole power of the randomization. It means our data, between the death index and our follow-up of participants, is essentially complete.”
Use of hormone therapy, and decoding the outcomes, remains ‘complex’
Decades after the data were gathered from the Women’s Health Initiative clinical trials, they continue to assist researchers and patients alike, wrote Christina A. Minami, MD, of Brigham and Women’s Hospital in Boston and Rachel A. Freedman, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
That said, in regard to the findings of this latest analysis, “many questions still remain on whether (and how) a hormone therapy intervention that occurred many years earlier may continue to affect breast cancer risk and mortality at 20 years,” they wrote. They noted that it’s “impossible” to isolate how exposure to certain therapies can impact long-term outcomes, and that a high percentage of patients who discontinued the drugs during each trial muddy the waters even further.
“Decisions to initiate these medications remain complex,” they added, emphasizing that breast cancer risk is just one of many factors that physicians must consider when considering hormone therapy for their patients.
Dr. Chlebowski and his coauthors acknowledged their study’s limitations, including the use of very specifically administered and formulated dosages making their findings “not necessarily generalizable to other preparations.” In addition, they noted the significant percentage of patients – 54% with CEE alone and 42% with CEE plus MPA – who discontinued drug usage during their respective trials.
The Women’s Health Initiative is supported by the National Institutes of Health and the Department of Health and Human Services. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various government organizations, foundations, and pharmaceutical companies. The editorial’s authors reported no conflicts of interest.
SOURCE: Chlebowski RT et al. JAMA. 2020 Jul 28. doi: 10.1001/jama.2020.9482.
A new follow-up study of menopausal hormone therapy found that prior use of conjugated equine estrogen (CEE) decreased both breast cancer incidence and mortality, while prior use of CEE plus medroxyprogesterone acetate (MPA) was associated with an increase in incidence.
“Prior use of CEE alone is, to our knowledge, the first pharmacologic intervention demonstrated to be associated with a statistically significantly reduction in deaths from breast cancer,” wrote Rowan T. Chlebowski, MD, PhD, of the Lundquist Institute for Biomedical Innovation in Torrance, Calif., and his coauthors. The study was published July 28 in JAMA.
To further investigate the outcomes of the Women’s Health Initiative in regard to hormone therapy and breast cancer risk, the researchers analyzed the long-term follow-up of two randomized trials that included 27,347 postmenopausal women with no prior breast cancer and negative mammograms at baseline. Their mean (SD) age was 63.4 (7.2) years. Enrollment took place from 1993 to 1998; participants were contacted for follow-up every 6 months through 2005 and annually from then on. Mortality data were gathered from follow-up and the National Death Index.
The first trial included 16,608 women with a uterus. Among these women, 8,506 received 0.625 mg/day of CEE plus 2.5 mg/day of MPA, and 8,102 received placebo. The second trial included 10,739 women who’d gotten a hysterectomy, 5,310 of whom received 0.625 mg/day of CEE alone and 5,429 of whom received placebo. The first trial ended in 2002 after a median intervention period of 5.6 years, and the second trial ended in 2004 after a period of 7.2 years.
An analysis in 2015 found that CEE alone was associated with lower risk of breast cancer and CEE plus MPA was associated with increased risk.
The current analysis confirmed that, after a median of 20.3 years of follow-up, and with mortality data now available for more than 98% of participants, CEE alone was associated with fewer cases of breast cancer (238 cases, annualized rate 0.30%), compared with placebo (296 cases, annualized rate 0.37%; hazard ratio 0.78; 95% confidence interval, 0.65-0.93; P = .005).
Furthermore, CEE alone was also associated with lower mortality (30 deaths, annualized mortality rate 0.031%), compared with placebo (46 deaths, annualized mortality rate 0.046%; HR 0.60; 95% CI, 0.37-0.97; P = .04).
By comparison, CEE plus MPA was linked with more cases of breast cancer (584 cases, annualized rate 0.45%) than placebo (447 cases, annualized rate 0.36%; HR 1.28; 95% CI, 1.13-1.45; P < .001). In regard to mortality, there was no statistically significant difference between CEE plus MPA (71 deaths, annualized mortality rate 0.045%) and placebo (53 deaths, annualized mortality rate 0.035%; HR 1.35; 95% CI, 0.94-1.95; P = .11).
“The big thing to think about is estrogen alone reducing breast cancer mortality by 40%,” said Dr. Chlebowski in an interview. “None of the other interventions, including tamoxifen, had any change on mortality. This should change the way we look at breast cancer prevention, though we might have to be a little creative about it. I think you have to be a little away from menopause for it to reduce breast cancer. But we wanted to start that debate.
“On the other hand,” he said, “a woman takes estrogen plus progestin and when you look at that curve, it’s staying about 25% increased. You take it for 5.6 years and the increase continues through 20 years, so you’re maybe buying a lifetime of increase in breast cancer by taking estrogen plus progestin for 5 years.”
He also highlighted the comprehensiveness of the mortality data, noting that “when you hook up to the National Death Index, they find 98% of all deaths in the United States. That’s really remarkable; you retain the whole power of the randomization. It means our data, between the death index and our follow-up of participants, is essentially complete.”
Use of hormone therapy, and decoding the outcomes, remains ‘complex’
Decades after the data were gathered from the Women’s Health Initiative clinical trials, they continue to assist researchers and patients alike, wrote Christina A. Minami, MD, of Brigham and Women’s Hospital in Boston and Rachel A. Freedman, MD, of the Dana-Farber Cancer Institute in Boston, in an accompanying editorial.
That said, in regard to the findings of this latest analysis, “many questions still remain on whether (and how) a hormone therapy intervention that occurred many years earlier may continue to affect breast cancer risk and mortality at 20 years,” they wrote. They noted that it’s “impossible” to isolate how exposure to certain therapies can impact long-term outcomes, and that a high percentage of patients who discontinued the drugs during each trial muddy the waters even further.
“Decisions to initiate these medications remain complex,” they added, emphasizing that breast cancer risk is just one of many factors that physicians must consider when considering hormone therapy for their patients.
Dr. Chlebowski and his coauthors acknowledged their study’s limitations, including the use of very specifically administered and formulated dosages making their findings “not necessarily generalizable to other preparations.” In addition, they noted the significant percentage of patients – 54% with CEE alone and 42% with CEE plus MPA – who discontinued drug usage during their respective trials.
The Women’s Health Initiative is supported by the National Institutes of Health and the Department of Health and Human Services. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various government organizations, foundations, and pharmaceutical companies. The editorial’s authors reported no conflicts of interest.
SOURCE: Chlebowski RT et al. JAMA. 2020 Jul 28. doi: 10.1001/jama.2020.9482.
FROM JAMA
Expert clarifies guidance on adolescent polycystic ovary syndrome
A trio of international expert recommendations mainly agree on essentials for the diagnosis and treatment of polycystic ovary syndrome in adolescents, but some confusion persists, according to Robert L. Rosenfield, MD, of the University of California, San Francisco.
In a commentary published in the Journal of Pediatric & Adolescent Gynecology, Dr. Rosenfield, who convened one of the three conferences at which guidance was developed, noted that the three recommendations – published by the Pediatric Endocrine Society, the International Consortium of Paediatric Endocrinology, and the International PCOS Network in 2015, 2017, and 2018, respectively – “are fairly dense” and reviews have suggested a lack of agreement. His comments offer perspective and practice suggestions that follow the consensus of the recommendations.
“All the documents agree on the core diagnostic criteria for adolescent PCOS: otherwise unexplained evidence of ovulatory dysfunction, as indicated by menstrual abnormalities based on stage-appropriate standards, and evidence of an androgen excess disorder,” Dr. Rosenfield said.
The main differences among the recommendations from the three groups reflect tension between the value of an early diagnosis and the liabilities of a mistaken diagnosis in the context of attitudes about adolescent contraception. “These are issues not likely to be resolved easily, yet they are matters for every physician to consider in management of each case,” he said.
Dr. Rosenfield emphasized that clinicians must consider PCOS “in the general context of all causes of adolescent menstrual disturbances,” when evaluating a girl within 1-2 years of menarche who presents with a menstrual abnormality, hirsutism, and/or acne that has been resistant to topical treatment.
A key point on which the recommendations differ is whether further assessment is needed if the menstrual abnormality has persisted for 1 year (the 2018 recommendations) or 2 years (the 2015 and 2017 recommendations), Dr. Rosenfield explained. “What the conferees struggled with is differentiating how long after menarche a menstrual abnormality should persist to avoid confusing PCOS with normal immaturity of the menstrual cycle,” known as physiologic adolescent anovulation (PAA). “The degree of certainty is improved only modestly by waiting 2 years rather than 1 year to make a diagnosis.”
However, the three documents agree that girls suspected of having PCOS within the first 1-2 years after menarche should be evaluated at that time, and followed with a diagnosis of “at risk for PCOS” if the early test results are consistent with a PCOS diagnosis, he said.
Another point of difference among the groups is the extent to which hirsutism and acne represent clinical evidence of hyperandrogenism that justifies testing for biochemical hyperandrogenism, Dr. Rosenfield said.
“All three sets of adolescent PCOS recommendations agree that investigation for biochemical hyperandrogenism be initiated by measuring serum total and/or free testosterone by specialty assays with well-defined reference ranges,” he said.
However, “documentation of biochemical hyperandrogenism has been problematic because standard platform assays of testosterone give grossly inaccurate results.”
As said Dr. Rosenfield. Guidelines in the United States favor estrogen-progestin combined oral contraceptives as first-line therapy, while the international guidelines support contraceptives if contraception also is desired; otherwise the 2017 guidelines recommend metformin as a first-line treatment.
“Agreement is uniform that healthy lifestyle management is first-line therapy for management of the associated obesity and metabolic disturbances, i.e., prior to and/or in conjunction with metformin therapy,” he noted.
In general, Dr. Rosenfield acknowledged that front-line clinicians cannot easily evaluate all early postmenarcheal girls for abnormal menstrual cycles. Instead, he advocated a “middle ground” approach between early diagnosis and potentially labeling a girl with a false positive diagnosis.
Postmenarcheal girls who are amenorrheic for 2 months could be assessed for signs of PCOS or pregnancy, and whether she is generally in good health, he said. “However, for example, if she remains amenorrheic for more than 90 days or if two successive periods are more than 2 months apart, laboratory screening would be reasonable.”
PCOS is “a diagnosis of exclusion for which referral to a specialist is advisable” to rule out other conditions such as non-classic congenital adrenal hyperplasia, hyperprolactinemia, endogenous Cushing syndrome, thyroid dysfunction, and virilizing tumors, said Dr. Rosenfield.
However, PCOS accounts for most cases of adolescent hyperandrogenism. The symptomatic treatment of early postmenarcheal girls at risk of PCOS is recommended to manage menstrual abnormality, hirsutism, acne, or obesity, and these girls should be reassessed by the time they finish high school after a 3-month treatment withdrawal period, he emphasized.
Dr. Rosenfield had no relevant financial conflicts to disclose.
SOURCE: Rosenfield RL. J Pediatr Adolesc Gynecol. 2020 June 29. doi: 10.1016/j.jpag.2020.06.017.
A trio of international expert recommendations mainly agree on essentials for the diagnosis and treatment of polycystic ovary syndrome in adolescents, but some confusion persists, according to Robert L. Rosenfield, MD, of the University of California, San Francisco.
In a commentary published in the Journal of Pediatric & Adolescent Gynecology, Dr. Rosenfield, who convened one of the three conferences at which guidance was developed, noted that the three recommendations – published by the Pediatric Endocrine Society, the International Consortium of Paediatric Endocrinology, and the International PCOS Network in 2015, 2017, and 2018, respectively – “are fairly dense” and reviews have suggested a lack of agreement. His comments offer perspective and practice suggestions that follow the consensus of the recommendations.
“All the documents agree on the core diagnostic criteria for adolescent PCOS: otherwise unexplained evidence of ovulatory dysfunction, as indicated by menstrual abnormalities based on stage-appropriate standards, and evidence of an androgen excess disorder,” Dr. Rosenfield said.
The main differences among the recommendations from the three groups reflect tension between the value of an early diagnosis and the liabilities of a mistaken diagnosis in the context of attitudes about adolescent contraception. “These are issues not likely to be resolved easily, yet they are matters for every physician to consider in management of each case,” he said.
Dr. Rosenfield emphasized that clinicians must consider PCOS “in the general context of all causes of adolescent menstrual disturbances,” when evaluating a girl within 1-2 years of menarche who presents with a menstrual abnormality, hirsutism, and/or acne that has been resistant to topical treatment.
A key point on which the recommendations differ is whether further assessment is needed if the menstrual abnormality has persisted for 1 year (the 2018 recommendations) or 2 years (the 2015 and 2017 recommendations), Dr. Rosenfield explained. “What the conferees struggled with is differentiating how long after menarche a menstrual abnormality should persist to avoid confusing PCOS with normal immaturity of the menstrual cycle,” known as physiologic adolescent anovulation (PAA). “The degree of certainty is improved only modestly by waiting 2 years rather than 1 year to make a diagnosis.”
However, the three documents agree that girls suspected of having PCOS within the first 1-2 years after menarche should be evaluated at that time, and followed with a diagnosis of “at risk for PCOS” if the early test results are consistent with a PCOS diagnosis, he said.
Another point of difference among the groups is the extent to which hirsutism and acne represent clinical evidence of hyperandrogenism that justifies testing for biochemical hyperandrogenism, Dr. Rosenfield said.
“All three sets of adolescent PCOS recommendations agree that investigation for biochemical hyperandrogenism be initiated by measuring serum total and/or free testosterone by specialty assays with well-defined reference ranges,” he said.
However, “documentation of biochemical hyperandrogenism has been problematic because standard platform assays of testosterone give grossly inaccurate results.”
As said Dr. Rosenfield. Guidelines in the United States favor estrogen-progestin combined oral contraceptives as first-line therapy, while the international guidelines support contraceptives if contraception also is desired; otherwise the 2017 guidelines recommend metformin as a first-line treatment.
“Agreement is uniform that healthy lifestyle management is first-line therapy for management of the associated obesity and metabolic disturbances, i.e., prior to and/or in conjunction with metformin therapy,” he noted.
In general, Dr. Rosenfield acknowledged that front-line clinicians cannot easily evaluate all early postmenarcheal girls for abnormal menstrual cycles. Instead, he advocated a “middle ground” approach between early diagnosis and potentially labeling a girl with a false positive diagnosis.
Postmenarcheal girls who are amenorrheic for 2 months could be assessed for signs of PCOS or pregnancy, and whether she is generally in good health, he said. “However, for example, if she remains amenorrheic for more than 90 days or if two successive periods are more than 2 months apart, laboratory screening would be reasonable.”
PCOS is “a diagnosis of exclusion for which referral to a specialist is advisable” to rule out other conditions such as non-classic congenital adrenal hyperplasia, hyperprolactinemia, endogenous Cushing syndrome, thyroid dysfunction, and virilizing tumors, said Dr. Rosenfield.
However, PCOS accounts for most cases of adolescent hyperandrogenism. The symptomatic treatment of early postmenarcheal girls at risk of PCOS is recommended to manage menstrual abnormality, hirsutism, acne, or obesity, and these girls should be reassessed by the time they finish high school after a 3-month treatment withdrawal period, he emphasized.
Dr. Rosenfield had no relevant financial conflicts to disclose.
SOURCE: Rosenfield RL. J Pediatr Adolesc Gynecol. 2020 June 29. doi: 10.1016/j.jpag.2020.06.017.
A trio of international expert recommendations mainly agree on essentials for the diagnosis and treatment of polycystic ovary syndrome in adolescents, but some confusion persists, according to Robert L. Rosenfield, MD, of the University of California, San Francisco.
In a commentary published in the Journal of Pediatric & Adolescent Gynecology, Dr. Rosenfield, who convened one of the three conferences at which guidance was developed, noted that the three recommendations – published by the Pediatric Endocrine Society, the International Consortium of Paediatric Endocrinology, and the International PCOS Network in 2015, 2017, and 2018, respectively – “are fairly dense” and reviews have suggested a lack of agreement. His comments offer perspective and practice suggestions that follow the consensus of the recommendations.
“All the documents agree on the core diagnostic criteria for adolescent PCOS: otherwise unexplained evidence of ovulatory dysfunction, as indicated by menstrual abnormalities based on stage-appropriate standards, and evidence of an androgen excess disorder,” Dr. Rosenfield said.
The main differences among the recommendations from the three groups reflect tension between the value of an early diagnosis and the liabilities of a mistaken diagnosis in the context of attitudes about adolescent contraception. “These are issues not likely to be resolved easily, yet they are matters for every physician to consider in management of each case,” he said.
Dr. Rosenfield emphasized that clinicians must consider PCOS “in the general context of all causes of adolescent menstrual disturbances,” when evaluating a girl within 1-2 years of menarche who presents with a menstrual abnormality, hirsutism, and/or acne that has been resistant to topical treatment.
A key point on which the recommendations differ is whether further assessment is needed if the menstrual abnormality has persisted for 1 year (the 2018 recommendations) or 2 years (the 2015 and 2017 recommendations), Dr. Rosenfield explained. “What the conferees struggled with is differentiating how long after menarche a menstrual abnormality should persist to avoid confusing PCOS with normal immaturity of the menstrual cycle,” known as physiologic adolescent anovulation (PAA). “The degree of certainty is improved only modestly by waiting 2 years rather than 1 year to make a diagnosis.”
However, the three documents agree that girls suspected of having PCOS within the first 1-2 years after menarche should be evaluated at that time, and followed with a diagnosis of “at risk for PCOS” if the early test results are consistent with a PCOS diagnosis, he said.
Another point of difference among the groups is the extent to which hirsutism and acne represent clinical evidence of hyperandrogenism that justifies testing for biochemical hyperandrogenism, Dr. Rosenfield said.
“All three sets of adolescent PCOS recommendations agree that investigation for biochemical hyperandrogenism be initiated by measuring serum total and/or free testosterone by specialty assays with well-defined reference ranges,” he said.
However, “documentation of biochemical hyperandrogenism has been problematic because standard platform assays of testosterone give grossly inaccurate results.”
As said Dr. Rosenfield. Guidelines in the United States favor estrogen-progestin combined oral contraceptives as first-line therapy, while the international guidelines support contraceptives if contraception also is desired; otherwise the 2017 guidelines recommend metformin as a first-line treatment.
“Agreement is uniform that healthy lifestyle management is first-line therapy for management of the associated obesity and metabolic disturbances, i.e., prior to and/or in conjunction with metformin therapy,” he noted.
In general, Dr. Rosenfield acknowledged that front-line clinicians cannot easily evaluate all early postmenarcheal girls for abnormal menstrual cycles. Instead, he advocated a “middle ground” approach between early diagnosis and potentially labeling a girl with a false positive diagnosis.
Postmenarcheal girls who are amenorrheic for 2 months could be assessed for signs of PCOS or pregnancy, and whether she is generally in good health, he said. “However, for example, if she remains amenorrheic for more than 90 days or if two successive periods are more than 2 months apart, laboratory screening would be reasonable.”
PCOS is “a diagnosis of exclusion for which referral to a specialist is advisable” to rule out other conditions such as non-classic congenital adrenal hyperplasia, hyperprolactinemia, endogenous Cushing syndrome, thyroid dysfunction, and virilizing tumors, said Dr. Rosenfield.
However, PCOS accounts for most cases of adolescent hyperandrogenism. The symptomatic treatment of early postmenarcheal girls at risk of PCOS is recommended to manage menstrual abnormality, hirsutism, acne, or obesity, and these girls should be reassessed by the time they finish high school after a 3-month treatment withdrawal period, he emphasized.
Dr. Rosenfield had no relevant financial conflicts to disclose.
SOURCE: Rosenfield RL. J Pediatr Adolesc Gynecol. 2020 June 29. doi: 10.1016/j.jpag.2020.06.017.
FROM THE JOURNAL OF PEDIATRIC AND ADOLESCENT GYNECOLOGY
High ‘forever chemicals’ in blood linked to earlier menopause
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In a national sample of U.S. women in their mid-40s to mid-50s, those with high serum levels of per- and polyfluoroalkyl substances (PFAS) were likely to enter menopause 2 years earlier than those with low levels of these chemicals.
That is, the median age of natural menopause was 52.8 years versus 50.8 years in women with high versus low serum levels of these chemicals in an analysis of data from more than 1,100 women in the Study of Women’s Health Across the Nation (SWAN) Multi-Pollutant Study, which excluded women with premature menopause (before age 40) or early menopause (before age 45).
“This study suggests that select PFAS serum concentrations are associated with earlier natural menopause, a risk factor for adverse health outcomes in later life,” Ning Ding, PhD, MPH, University of Michigan, Ann Arbor, and colleagues concluded in their article, published online June 3 in the Journal of Clinical Endocrinology & Metabolism.
“Even menopause a few years earlier than usual could have a significant impact on cardiovascular and bone health, quality of life, and overall health in general among women,” senior author Sung Kyun Park, ScD, MPH, from the same institution, added in a statement.
PFAS don’t break down in the body, build up with time
PFAS have been widely used in many consumer and industrial products such as nonstick cookware, stain-repellent carpets, waterproof rain gear, microwave popcorn bags, and firefighting foam, the authors explained.
These have been dubbed “forever chemicals” because they do not degrade. Household water for an estimated 110 million Americans (one in three) may be contaminated with these chemicals, according to an Endocrine Society press release.
“PFAS are everywhere. Once they enter the body, they don’t break down and [they] build up over time,” said Dr. Ding. “Because of their persistence in humans and potentially detrimental effects on ovarian function, it is important to raise awareness of this issue and reduce exposure to these chemicals.”
Environmental exposure and accelerated ovarian aging
Earlier menopause has been associated with an increased risk of cardiovascular disease, osteoporosis, and earlier cardiovascular and overall mortality, and environmental exposure may accelerate ovarian aging, the authors wrote.
PFAS, especially the most studied types – perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) – are plausible endocrine-disrupting chemicals, but findings so far have been inconsistent.
A study of people in Ohio exposed to contaminated water found that women with earlier natural menopause had higher serum PFOA and PFOS levels (J Clin Endocriniol Metab. 2011;96:1747-53).
But in research based on National Health and Nutrition Survey Examination data, higher PFOA, PFOS, or perfluorononanoic acid (PFNA) levels were not linked to earlier menopause, although higher levels of perfluorohexane sulfonic acid (PFHxS) were (Environ Health Perspect. 2014;122:145-50).
There may have been reverse causation, where postmenopausal women had higher PFAS levels because they were not excreting these chemicals in menstrual blood.
In a third study, PFOA exposure was not linked with age at menopause onset, but this was based on recall from 10 years earlier (Environ Res. 2016;146:323-30).
The current analysis examined data from 1,120 premenopausal women who were aged 45-56 years from 1999 to 2000.
The women were seen at five sites (Boston; Detroit; Los Angeles; Oakland, Calif.; and Pittsburgh) and were ethnically diverse (577 white, 235 black, 142 Chinese, and 166 Japanese).
Baseline serum PFAS levels were measured using high performance liquid chromatography-mass spectrometry. The women were followed up to 2017 and incident menopause (12 consecutive months with no menstruation) was determined from annual interviews.
Of the 1,120 women and 5,466 person-years of follow-up, 578 women had a known date of natural incident menopause and were included in the analysis. The remaining 542 women were excluded mainly because their date of final menstruation was unknown because of hormone therapy (451) or they had a hysterectomy, or did not enter menopause during the study.
Compared with women in the lowest tertile of PFOS levels, women in the highest tertile had a significant 26%-27% greater risk of incident menopause – after adjusting for age, body mass index, and prior hormone use, race/ethnicity, study site, education, physical activity, smoking status, and parity.
Higher PFOA and PFNA levels but not higher PFHxS levels were also associated with increased risk.
Compared with women with a low overall PFAS level, those with a high level had a 63% increased risk of incident menopause (hazard ratio, 1.63; 95% confidence interval, 1.08-2.45), equivalent to having menopause a median of 2 years earlier.
Although production and use of some types of PFAS in the United States are declining, Dr. Ding and colleagues wrote, exposure continues, along with associated potential hazards to human reproductive health.
“Due to PFAS widespread use and environmental persistence, their potential adverse effects remain a public health concern,” they concluded.
SWAN was supported by the National Institutes of Health, Department of Health & Human Services through the National Institute on Aging, National Institute of Nursing Research, NIH Office of Research on Women’s Health, and the SWAN repository. The current article was supported by the National Center for Research Resources and National Center for Advancing Translational Sciences, NIH, National Institute of Environmental Health Sciences, and Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Seek safe strategies to diagnose gestational diabetes during pandemic
Clinicians and pregnant women are less likely to prescribe and undergo the oral glucose tolerance test (OGTT) to diagnose gestational diabetes in the context of the COVID-19 pandemic, according to a review by H. David McIntyre, MD, of the University of Queensland, Brisbane, Australia, and Robert G. Moses, MD, of Wollongong (Australia) Hospital.
National and international discussions of whether a one- or two-step test for gestational diabetes mellitus (GDM) is optimal, and which women should be tested are ongoing, but the potential for exposure risks to COVID-19 are impacting the test process, they wrote in a commentary published in Diabetes Care.
“Any national or local guidelines should be developed with the primary aim of being protective for pregnant women and workable in the current health crisis,” they wrote.
Key concerns expressed by women and health care providers include the need for travel to be tested, the possible need for two visits, and the several hours spent in a potentially high-risk specimen collection center.
“Further, a GDM diagnosis generally involves additional health service visits for diabetes education, glucose monitoring review, and fetal ultrasonography, all of which carry exposure risks during a pandemic,” Dr. McIntyre and Dr. Moses noted.
Professional societies in the United Kingdom, Canada, and Australia have issued guidance to clinicians for modifying GDM diagnoses criteria during the pandemic that aim to reduce the need for the oral glucose tolerance test both during and after pregnancy.
Pandemic guidelines for all three of these countries support the identification of GDM using early pregnancy hemoglobin A1c (HbA1c) of at least 41 mmol/mol (5.9%).
Then, professionals in the United Kingdom recommend testing based on risk factors and diagnosing GDM based on any of these criteria: HbA1c of at least 39 mmol/mol (5.7%), fasting venous plasma glucose of at least 5.6 mmol/L (preferred), or random VPG of at least 9.0 mmol/L.
The revised testing pathway for Canada accepts an HbA1c of at least 39 mmol/mol (5.7%) and/or random VPG of at least 11.1 mmol/L.
“The revised Australian pathway does not include HbA1c but recommends a fasting VPG with progression to OGTT only if this result is 4.7-5.0 mmol/L,” Dr. McIntyre and Dr. Moses explained.
Overall, the revised guidelines for GDM testing will likely miss some women and only identify those with higher levels of hyperglycemia, the authors wrote. In addition, “the evidence base for these revised pathways is limited and that each alternative strategy should be evaluated over the course of the current pandemic.”
Validation of new testing strategies are needed, and the pandemic may provide and opportunity to adopt an alternative to the OGTT. The World Health Organization has not issued revised guidance for other methods of testing, but fasting VPG alone may be the simplest and most cost effective, at least for the short term, they noted.
“In this ‘new COVID world,’ GDM should not be ignored but pragmatically merits a lower priority than the avoidance of exposure to the COVID-19 virus,” although no single alternative strategy applies in all countries and situations, the authors concluded. Pragmatic measures and documentation of outcomes at the local level will offer the “least worst” solution while the pandemic continues.
The authors had no relevant financial disclosures.
SOURCE: McIntyre HD, Moses RG. Diabetes Care. 2020 May. doi: 10.2337/dci20-0026.
A major concern against the backdrop of COVID-19 is ensuring long-term health while urgent care is – understandably so – being prioritized over preventive care. We can already see the impact that the decrease in primary care has had: Rates of childhood vaccination appear to have dropped; the cancellation or indefinite delay of elective medical procedures has meant a reduction in preventive cancer screenings, such as colonoscopies and mammograms; and concerns about COVID-19 may be keeping those experiencing cardiac events from seeking emergency care.
However, an outcropping of the coronavirus pandemic is an ingenuity to adapt to our new “normal.” Medical licenses have been recognized across state lines to allow much-needed professionals to practice in the hardest-hit areas. Doctors retrofitted a sleep apnea machine to be used as a makeshift ventilator. Those in the wearable device market now have a greater onus to deliver on quality, utility, security, and accuracy.
Obstetricians have had to dramatically change delivery of ante-, intra- and postpartum care. The recent commentary by Dr. McIntyre and Dr. Moses focuses on one particular area of concern: screening, diagnosis, and management of gestational diabetes mellitus (GDM).
Screening and diagnosis are mainstays to reduce the adverse maternal and neonatal outcomes of diabetes in pregnancy. Although there is no universally accepted approach to evaluating GDM, all current methods utilize an oral glucose tolerance test (OGTT), which requires significant time spent in a clinical office setting, thus increasing risk for COVID-19 exposure.
Several countries have adopted modified GDM criteria within the last months. At the time of this writing, the United States has not. Although not testing women for GDM, which is what Dr. McIntyre and Dr. Moses point out may be happening in countries with modified guidelines, seems questionable, perhaps we should think differently about our approach.
More than 20 years ago, it was reported that jelly beans could be used as an alternative to the 50-g GDM screening test (Am J Obstet Gynecol. 1999 Nov;181[5 Pt 1]:1154‐7; Am J Obstet Gynecol. 1995 Dec;173[6]:1889‐92); more recently, candy twists were used with similar results (Am J Obstet Gynecol. 2015 Apr;212[4]:522.e1-5). In addition, a number of articles have reported on the utility of capillary whole blood glucose measurements to screen for GDM in developing and resource-limited countries (Diabetes Technol Ther. 2011;13[5]:586‐91; Acta Diabetol. 2016 Feb;53[1]:91‐7; Diabetes Technol Ther. 2012 Feb;14[2]:131-4). Therefore, rather than forgo GDM screening, women could self-administer a jelly bean test at home, measure blood sugar with a glucometer, and depending on the results, have an OGTT. Importantly, this would allow ob.gyns. to maintain medical standards while managing patients via telemedicine.
We have evidence that GDM can establish poor health for generations. We know that people with underlying conditions have greater morbidity and mortality from infectious diseases. We recognize that accurate screening and diagnosis is the key to prevention and management. Rather than accept a “least worst” scenario, as Dr. McIntyre and Dr. Moses state, we must find ways to provide the best possible care under the current circumstances.
E. Albert Reece, MD, PhD, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. He said he had no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
A major concern against the backdrop of COVID-19 is ensuring long-term health while urgent care is – understandably so – being prioritized over preventive care. We can already see the impact that the decrease in primary care has had: Rates of childhood vaccination appear to have dropped; the cancellation or indefinite delay of elective medical procedures has meant a reduction in preventive cancer screenings, such as colonoscopies and mammograms; and concerns about COVID-19 may be keeping those experiencing cardiac events from seeking emergency care.
However, an outcropping of the coronavirus pandemic is an ingenuity to adapt to our new “normal.” Medical licenses have been recognized across state lines to allow much-needed professionals to practice in the hardest-hit areas. Doctors retrofitted a sleep apnea machine to be used as a makeshift ventilator. Those in the wearable device market now have a greater onus to deliver on quality, utility, security, and accuracy.
Obstetricians have had to dramatically change delivery of ante-, intra- and postpartum care. The recent commentary by Dr. McIntyre and Dr. Moses focuses on one particular area of concern: screening, diagnosis, and management of gestational diabetes mellitus (GDM).
Screening and diagnosis are mainstays to reduce the adverse maternal and neonatal outcomes of diabetes in pregnancy. Although there is no universally accepted approach to evaluating GDM, all current methods utilize an oral glucose tolerance test (OGTT), which requires significant time spent in a clinical office setting, thus increasing risk for COVID-19 exposure.
Several countries have adopted modified GDM criteria within the last months. At the time of this writing, the United States has not. Although not testing women for GDM, which is what Dr. McIntyre and Dr. Moses point out may be happening in countries with modified guidelines, seems questionable, perhaps we should think differently about our approach.
More than 20 years ago, it was reported that jelly beans could be used as an alternative to the 50-g GDM screening test (Am J Obstet Gynecol. 1999 Nov;181[5 Pt 1]:1154‐7; Am J Obstet Gynecol. 1995 Dec;173[6]:1889‐92); more recently, candy twists were used with similar results (Am J Obstet Gynecol. 2015 Apr;212[4]:522.e1-5). In addition, a number of articles have reported on the utility of capillary whole blood glucose measurements to screen for GDM in developing and resource-limited countries (Diabetes Technol Ther. 2011;13[5]:586‐91; Acta Diabetol. 2016 Feb;53[1]:91‐7; Diabetes Technol Ther. 2012 Feb;14[2]:131-4). Therefore, rather than forgo GDM screening, women could self-administer a jelly bean test at home, measure blood sugar with a glucometer, and depending on the results, have an OGTT. Importantly, this would allow ob.gyns. to maintain medical standards while managing patients via telemedicine.
We have evidence that GDM can establish poor health for generations. We know that people with underlying conditions have greater morbidity and mortality from infectious diseases. We recognize that accurate screening and diagnosis is the key to prevention and management. Rather than accept a “least worst” scenario, as Dr. McIntyre and Dr. Moses state, we must find ways to provide the best possible care under the current circumstances.
E. Albert Reece, MD, PhD, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. He said he had no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
A major concern against the backdrop of COVID-19 is ensuring long-term health while urgent care is – understandably so – being prioritized over preventive care. We can already see the impact that the decrease in primary care has had: Rates of childhood vaccination appear to have dropped; the cancellation or indefinite delay of elective medical procedures has meant a reduction in preventive cancer screenings, such as colonoscopies and mammograms; and concerns about COVID-19 may be keeping those experiencing cardiac events from seeking emergency care.
However, an outcropping of the coronavirus pandemic is an ingenuity to adapt to our new “normal.” Medical licenses have been recognized across state lines to allow much-needed professionals to practice in the hardest-hit areas. Doctors retrofitted a sleep apnea machine to be used as a makeshift ventilator. Those in the wearable device market now have a greater onus to deliver on quality, utility, security, and accuracy.
Obstetricians have had to dramatically change delivery of ante-, intra- and postpartum care. The recent commentary by Dr. McIntyre and Dr. Moses focuses on one particular area of concern: screening, diagnosis, and management of gestational diabetes mellitus (GDM).
Screening and diagnosis are mainstays to reduce the adverse maternal and neonatal outcomes of diabetes in pregnancy. Although there is no universally accepted approach to evaluating GDM, all current methods utilize an oral glucose tolerance test (OGTT), which requires significant time spent in a clinical office setting, thus increasing risk for COVID-19 exposure.
Several countries have adopted modified GDM criteria within the last months. At the time of this writing, the United States has not. Although not testing women for GDM, which is what Dr. McIntyre and Dr. Moses point out may be happening in countries with modified guidelines, seems questionable, perhaps we should think differently about our approach.
More than 20 years ago, it was reported that jelly beans could be used as an alternative to the 50-g GDM screening test (Am J Obstet Gynecol. 1999 Nov;181[5 Pt 1]:1154‐7; Am J Obstet Gynecol. 1995 Dec;173[6]:1889‐92); more recently, candy twists were used with similar results (Am J Obstet Gynecol. 2015 Apr;212[4]:522.e1-5). In addition, a number of articles have reported on the utility of capillary whole blood glucose measurements to screen for GDM in developing and resource-limited countries (Diabetes Technol Ther. 2011;13[5]:586‐91; Acta Diabetol. 2016 Feb;53[1]:91‐7; Diabetes Technol Ther. 2012 Feb;14[2]:131-4). Therefore, rather than forgo GDM screening, women could self-administer a jelly bean test at home, measure blood sugar with a glucometer, and depending on the results, have an OGTT. Importantly, this would allow ob.gyns. to maintain medical standards while managing patients via telemedicine.
We have evidence that GDM can establish poor health for generations. We know that people with underlying conditions have greater morbidity and mortality from infectious diseases. We recognize that accurate screening and diagnosis is the key to prevention and management. Rather than accept a “least worst” scenario, as Dr. McIntyre and Dr. Moses state, we must find ways to provide the best possible care under the current circumstances.
E. Albert Reece, MD, PhD, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the University of Maryland School of Medicine. He said he had no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
Clinicians and pregnant women are less likely to prescribe and undergo the oral glucose tolerance test (OGTT) to diagnose gestational diabetes in the context of the COVID-19 pandemic, according to a review by H. David McIntyre, MD, of the University of Queensland, Brisbane, Australia, and Robert G. Moses, MD, of Wollongong (Australia) Hospital.
National and international discussions of whether a one- or two-step test for gestational diabetes mellitus (GDM) is optimal, and which women should be tested are ongoing, but the potential for exposure risks to COVID-19 are impacting the test process, they wrote in a commentary published in Diabetes Care.
“Any national or local guidelines should be developed with the primary aim of being protective for pregnant women and workable in the current health crisis,” they wrote.
Key concerns expressed by women and health care providers include the need for travel to be tested, the possible need for two visits, and the several hours spent in a potentially high-risk specimen collection center.
“Further, a GDM diagnosis generally involves additional health service visits for diabetes education, glucose monitoring review, and fetal ultrasonography, all of which carry exposure risks during a pandemic,” Dr. McIntyre and Dr. Moses noted.
Professional societies in the United Kingdom, Canada, and Australia have issued guidance to clinicians for modifying GDM diagnoses criteria during the pandemic that aim to reduce the need for the oral glucose tolerance test both during and after pregnancy.
Pandemic guidelines for all three of these countries support the identification of GDM using early pregnancy hemoglobin A1c (HbA1c) of at least 41 mmol/mol (5.9%).
Then, professionals in the United Kingdom recommend testing based on risk factors and diagnosing GDM based on any of these criteria: HbA1c of at least 39 mmol/mol (5.7%), fasting venous plasma glucose of at least 5.6 mmol/L (preferred), or random VPG of at least 9.0 mmol/L.
The revised testing pathway for Canada accepts an HbA1c of at least 39 mmol/mol (5.7%) and/or random VPG of at least 11.1 mmol/L.
“The revised Australian pathway does not include HbA1c but recommends a fasting VPG with progression to OGTT only if this result is 4.7-5.0 mmol/L,” Dr. McIntyre and Dr. Moses explained.
Overall, the revised guidelines for GDM testing will likely miss some women and only identify those with higher levels of hyperglycemia, the authors wrote. In addition, “the evidence base for these revised pathways is limited and that each alternative strategy should be evaluated over the course of the current pandemic.”
Validation of new testing strategies are needed, and the pandemic may provide and opportunity to adopt an alternative to the OGTT. The World Health Organization has not issued revised guidance for other methods of testing, but fasting VPG alone may be the simplest and most cost effective, at least for the short term, they noted.
“In this ‘new COVID world,’ GDM should not be ignored but pragmatically merits a lower priority than the avoidance of exposure to the COVID-19 virus,” although no single alternative strategy applies in all countries and situations, the authors concluded. Pragmatic measures and documentation of outcomes at the local level will offer the “least worst” solution while the pandemic continues.
The authors had no relevant financial disclosures.
SOURCE: McIntyre HD, Moses RG. Diabetes Care. 2020 May. doi: 10.2337/dci20-0026.
Clinicians and pregnant women are less likely to prescribe and undergo the oral glucose tolerance test (OGTT) to diagnose gestational diabetes in the context of the COVID-19 pandemic, according to a review by H. David McIntyre, MD, of the University of Queensland, Brisbane, Australia, and Robert G. Moses, MD, of Wollongong (Australia) Hospital.
National and international discussions of whether a one- or two-step test for gestational diabetes mellitus (GDM) is optimal, and which women should be tested are ongoing, but the potential for exposure risks to COVID-19 are impacting the test process, they wrote in a commentary published in Diabetes Care.
“Any national or local guidelines should be developed with the primary aim of being protective for pregnant women and workable in the current health crisis,” they wrote.
Key concerns expressed by women and health care providers include the need for travel to be tested, the possible need for two visits, and the several hours spent in a potentially high-risk specimen collection center.
“Further, a GDM diagnosis generally involves additional health service visits for diabetes education, glucose monitoring review, and fetal ultrasonography, all of which carry exposure risks during a pandemic,” Dr. McIntyre and Dr. Moses noted.
Professional societies in the United Kingdom, Canada, and Australia have issued guidance to clinicians for modifying GDM diagnoses criteria during the pandemic that aim to reduce the need for the oral glucose tolerance test both during and after pregnancy.
Pandemic guidelines for all three of these countries support the identification of GDM using early pregnancy hemoglobin A1c (HbA1c) of at least 41 mmol/mol (5.9%).
Then, professionals in the United Kingdom recommend testing based on risk factors and diagnosing GDM based on any of these criteria: HbA1c of at least 39 mmol/mol (5.7%), fasting venous plasma glucose of at least 5.6 mmol/L (preferred), or random VPG of at least 9.0 mmol/L.
The revised testing pathway for Canada accepts an HbA1c of at least 39 mmol/mol (5.7%) and/or random VPG of at least 11.1 mmol/L.
“The revised Australian pathway does not include HbA1c but recommends a fasting VPG with progression to OGTT only if this result is 4.7-5.0 mmol/L,” Dr. McIntyre and Dr. Moses explained.
Overall, the revised guidelines for GDM testing will likely miss some women and only identify those with higher levels of hyperglycemia, the authors wrote. In addition, “the evidence base for these revised pathways is limited and that each alternative strategy should be evaluated over the course of the current pandemic.”
Validation of new testing strategies are needed, and the pandemic may provide and opportunity to adopt an alternative to the OGTT. The World Health Organization has not issued revised guidance for other methods of testing, but fasting VPG alone may be the simplest and most cost effective, at least for the short term, they noted.
“In this ‘new COVID world,’ GDM should not be ignored but pragmatically merits a lower priority than the avoidance of exposure to the COVID-19 virus,” although no single alternative strategy applies in all countries and situations, the authors concluded. Pragmatic measures and documentation of outcomes at the local level will offer the “least worst” solution while the pandemic continues.
The authors had no relevant financial disclosures.
SOURCE: McIntyre HD, Moses RG. Diabetes Care. 2020 May. doi: 10.2337/dci20-0026.
FDA approves Phexxi for use as an on-demand contraceptive
Evofem Biosciences expects to release Phexxi – the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5 – in September 2020 alongside the Phexxi Concierge Experience, a comprehensive patient and health care provider telemedicine support system, according to the company’s press release. The service is designed to provide physicians with on-demand educational support, and to speed and simplify women’s access to Phexxi.
In an open-label multicenter trial, women aged 18-35 with regular menstrual cycles intravaginally administered a 5-gram dose of Phexxi vaginal gel up to 1 hour prior to intercourse; they did so for up to seven cycles. There were 101 pregnancies in 1,183 subjects during 4,769 cycles. The 7-cycle cumulative pregnancy rate was 14% (95% confidence interval: 10.0%, 17.5%).
The most common adverse events associated with Phexxi were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, bacterial vaginosis, vaginal discharge, dysuria, and vulvovaginal pain.
Evofem Biosciences expects to release Phexxi – the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5 – in September 2020 alongside the Phexxi Concierge Experience, a comprehensive patient and health care provider telemedicine support system, according to the company’s press release. The service is designed to provide physicians with on-demand educational support, and to speed and simplify women’s access to Phexxi.
In an open-label multicenter trial, women aged 18-35 with regular menstrual cycles intravaginally administered a 5-gram dose of Phexxi vaginal gel up to 1 hour prior to intercourse; they did so for up to seven cycles. There were 101 pregnancies in 1,183 subjects during 4,769 cycles. The 7-cycle cumulative pregnancy rate was 14% (95% confidence interval: 10.0%, 17.5%).
The most common adverse events associated with Phexxi were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, bacterial vaginosis, vaginal discharge, dysuria, and vulvovaginal pain.
Evofem Biosciences expects to release Phexxi – the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5 – in September 2020 alongside the Phexxi Concierge Experience, a comprehensive patient and health care provider telemedicine support system, according to the company’s press release. The service is designed to provide physicians with on-demand educational support, and to speed and simplify women’s access to Phexxi.
In an open-label multicenter trial, women aged 18-35 with regular menstrual cycles intravaginally administered a 5-gram dose of Phexxi vaginal gel up to 1 hour prior to intercourse; they did so for up to seven cycles. There were 101 pregnancies in 1,183 subjects during 4,769 cycles. The 7-cycle cumulative pregnancy rate was 14% (95% confidence interval: 10.0%, 17.5%).
The most common adverse events associated with Phexxi were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, bacterial vaginosis, vaginal discharge, dysuria, and vulvovaginal pain.