User login
Microplastics permeate human placentas
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
Researchers in Italy have identified microplastic (MP) fragments in four human placentas that were donated for study after delivery.
“The presence of MPs in the placenta tissue requires the reconsideration of the immunological mechanism of self-tolerance,” wrote Antonio Ragusa, MD, of San Giovanni Calibita Fatebenefratelli Hospital, Rome, and colleagues. “Placenta represents the interface between the fetus and the environment.”
In a pilot observational study published in Environment International, the researchers used Raman microspectroscopy to analyze placentas from six women with physiological pregnancies for the presence of MPs. MPs were defined as particles smaller than 5 mm resulting from the degradation of plastic in the environment, such as plastic objects, coatings, adhesives, paints, and personal care products. Data from previous studies have shown that MPs can move into living organisms, but this study is the first to identify MPs in human placentas, the researchers said.
Polypropylene and pigments identified
A total of 12 microplastic fragments were identified in tissue from the placentas of four women; 5 in the fetal side, 4 in the maternal side, and 3 in the chorioamniotic membranes, which suggests that MPs can reach all levels of placental tissue, the researchers said. Most of the MPs were approximately 10 mcm in size, but two were roughly 5 mcm.
All 12 of the MPs were pigmented; of these, 3 were identified as stained polypropylene and the other 9 contained pigments used in a variety of items including coatings, paints, adhesives, plasters, finger paints, polymers and cosmetics, and personal care products. The researchers used a software program to analyze the pigments and matched them with information from the European Chemical Agency for identification of the commercial name, chemical formula, International Union of Pure and Applied Chemistry name, and Color Index Constitution Number.
The mechanism by which MPs may enter the bloodstream and access the placenta remains unclear, the researchers said. “The most probable transport route for MPs is a mechanism of particle uptake and translocation, already described for the internalization from the gastrointestinal tract. Once MPs have reached the maternal surface of the placenta, as other exogenous materials, they can invade the tissue in depth by several transport mechanisms, both active and passive, that are not clearly understood yet.”
The range in location and characteristics of the particles found in the study suggest that passage of MPs into the placenta may be affected by physiological conditions and genetics, as well as food and lifestyle habits of the patients, the researchers said.
The study findings were limited by several factors including the small sample size and observational study design.
However, the presence of MPs in the placenta could affect the pregnancy in various ways, including immunity, growth factor signaling, maternal-fetal communication, and trafficking of various cell types and macrophages, the researchers wrote. In addition, MPs could have a transgenerational effect on metabolism and reproduction.
“Further studies need to be performed to assess if the presence of MPs in human placenta may trigger immune responses or may lead to the release of toxic contaminants, resulting harmful for pregnancy,” they concluded.
Cause for concern, but research gaps remain
“Microplastics are ubiquitous in the environment and are detectable in tissues of humans and wildlife,” Andrea C. Gore, PhD, of the University of Texas, Austin, said in an interview. “To my knowledge, this was never previously shown in the placenta.
“There are two reasons why detection of microplastics in placenta would be concerning,” Dr. Gore explained. “First, microplastics may be endocrine-disrupting chemicals (EDCs), or they may concentrate other chemicals that are EDCs. Second, the developing fetus is exquisitely sensitive to natural hormones, and disruptions by EDCs may lead to both immediate as well as latent health problems.
“Clinicians should be concerned about particulate matter in the placenta, “although the number of particles was very small,” said Dr. Gore. “Out of six women, four had particles in their placentas (total of 12) of which one was confirmed to be a plastic (polypropylene). For the other 11 particles, only the pigments could be identified, so more work is needed to confirm whether they were plastics.
“If I were a clinician discussing this article with my patients, I would point out that, although it is concerning that microparticles are present in placenta, few of them were found, and it is not known whether any chemical is released from the particles or actually reaches the fetal circulation,” Dr. Gore said. “I would use it as a starting point for a conversation about lifestyle during pregnancy and encouraging pregnant women to avoid eating foods stored and/or prepared in plastics.”
The limitations of the study include not only the small sample size, but also that “the type of chemicals in the microplastics is for the most part unknown, making it difficult to assess which (if any) might be EDCs,” Dr. Gore emphasized. In addition, “lifestyle and diet can greatly affect exposures to chemicals, so this needs to be carefully factored into the analysis.” Also, “most of the detected particles are pigments, so connections to plastics (other than the one polypropylene particle) need to be strengthened,” she explained.
“The pathways by which microplastics might get into tissues are still rather speculative, and the mechanisms proposed by the authors (endocytosis, paracellular diffusion, entry via airways) need to be demonstrated,” Dr. Gore concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Gore had no conflicts to disclose.
SOURCE: Ragusa A et al. Environ Int. 2020 Dec 2. doi: 10.1016/j.envint.2020.106274.
FROM ENVIRONMENT INTERNATIONAL
One in five gestational carriers do not meet ASRM criteria
About 20% of gestational carriers at one institution did not meet recommended criteria developed by the American Society for Reproductive Medicine, according to a retrospective study of 194 patients.
The University of California, San Francisco, offers additional, stricter recommendations, including that gestational carriers have a body mass index less than 35. Under these stricter criteria, about 30% of the gestational carriers did not meet recommendations, Brett Stark, MD, MPH, reported at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
Deviating from BMI or age recommendations may be associated with increased likelihood of spontaneous abortion, the analysis suggested. In addition, elements of a gestational carrier’s obstetric history not described in current guidelines, such as prior preterm birth, may influence gestational surrogacy outcomes.
The study was limited by incomplete information for some patients, the retrospective design, and the reliance on a relatively small cohort at a single center. Nevertheless, the findings potentially could inform discussions with patients, said Dr. Stark, a 3rd-year obstetrics and gynecology resident at the university.
Investigators aim to enroll patients in a longitudinal cohort study to further examine these questions, he said.
Protecting intended parents and carriers
“Gestational surrogacy has become an increasingly common form of third-party reproduction,” Dr. Stark said at the virtual meeting. The number of cases of in vitro fertilization (IVF) with gestational carriers increased from approximately 700 in 1999 to more than 5,500 in 2016, according to data from the Society for Assisted Reproductive Technology. “Despite the increasing prevalence of gestational carrier utilization, there remains limited guidance with regard to optimizing outcomes for both the intended parents and gestational carriers.”
ASRM and UCSF recommendations are based on expert opinion and include surprisingly little discussion about the prior pregnancy outcomes of potential gestational carriers, Dr. Stark said.
“It is important for all parties involved that we generate research and data that can help drive the development of the guidelines,” he said. Such evidence may help intended parents understand characteristics of gestational carriers that may lead to live births. “For the gestational carriers, it is important that we have information on safety so that they know they are making appropriate decisions for their family and their life.”
Gestational carrier characteristics in the present study that deviated from 2017 ASRM recommendations included age less than 21 years or greater than 45 years, mental health conditions, and having more than five prior deliveries.
“ASRM guidelines focused on criteria for gestational carriers are meant to protect infertile couples, the carrier, as well as the supporting agency,” Alan Penzias, MD, chair of ASRM’s Practice Committee who is in private practice in Boston, said in a society news release that highlighted Dr. Stark’s study. “It is important that gestational carriers have a complete medical history and examination, in addition to a psychological session with a mental health professional to ensure there are no reasons for the carrier to not move forward with pregnancy.”
A retrospective study by Kate Swanson, MD, and associates found that nonadherence to ASRM guidelines was associated with increased rates of cesarean delivery, neonatal morbidity, and preterm birth.
To examine how adherence to ASRM and UCSF recommendations relates to pregnancy outcomes and maternal and neonatal morbidity and death, Dr. Stark and colleagues assessed births from gestational carrier pregnancies at UCSF between 2008 and 2019.
Of 194 gestational carriers included in the analysis, 98.9% had a prior term pregnancy, 11.9% had a prior preterm pregnancy, and 17.5% had a prior spontaneous abortion.
Indications for use of gestational surrogates included serious medical condition of intended parent (25%), uterine factor infertility (23%), recurrent pregnancy loss (10%), and same-sex male couples (8%).
When the researchers compared pregnancy outcomes for gestational carriers who met ASRM guidelines with outcomes for 38 gestational carriers who did not meet ASRM guidelines, there were no statistically significant differences. Antepartum, intrapartum, and postpartum complication rates and cesarean delivery rates did not significantly differ based on ASRM guideline adherence.
Nonadherence to the stricter UCSF guidelines, however, was associated with increased likelihood of spontaneous abortion. In all, 23.7% of the 59 gestational carriers who were nonadherent to UCSF guidelines had a pregnancy end in a spontaneous abortion, compared with 6.7% of gestational carriers who were adherent to the UCSF recommendations (odds ratio, 4.35).
An analysis of individual criteria and poor pregnancy outcomes found that BMI greater than 35 was associated with increased likelihood of spontaneous abortion (OR, 4.29), as was age less than 21 years or greater than 45 years (OR, 3.37).
Prior spontaneous abortion was associated with increased likelihood of a biochemical pregnancy (OR, 3.2), and prior preterm birth was associated with increased likelihood of spontaneous abortion (OR, 3.19), previable delivery (OR, 25.2), cesarean delivery (OR, 2.59), and antepartum complications (OR, 3.56).
The role of agencies
About 76% of the gestational carriers had pregnancies mediated through a gestational surrogacy agency. Surrogates from agencies were about three times more likely than surrogates who were family, friends, or from private surrogacy arrangements to adhere to ASRM and UCSF guidelines.
Even after hearing about gestational carrier recommendations, patients may prefer to work with someone they know. “We want to provide our patients with evidence-based information if possible, but ultimately it is their decision to make,” Dr. Stark said. “And we just need to make sure that they are making an informed decision.”
Dr. Stark had no relevant disclosures. Dr. Penzias helped develop the ASRM committee opinion. He had no relevant conflicts of interest.
SOURCE: Stark B et al. ASRM 2020, Abstract O-251.
About 20% of gestational carriers at one institution did not meet recommended criteria developed by the American Society for Reproductive Medicine, according to a retrospective study of 194 patients.
The University of California, San Francisco, offers additional, stricter recommendations, including that gestational carriers have a body mass index less than 35. Under these stricter criteria, about 30% of the gestational carriers did not meet recommendations, Brett Stark, MD, MPH, reported at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
Deviating from BMI or age recommendations may be associated with increased likelihood of spontaneous abortion, the analysis suggested. In addition, elements of a gestational carrier’s obstetric history not described in current guidelines, such as prior preterm birth, may influence gestational surrogacy outcomes.
The study was limited by incomplete information for some patients, the retrospective design, and the reliance on a relatively small cohort at a single center. Nevertheless, the findings potentially could inform discussions with patients, said Dr. Stark, a 3rd-year obstetrics and gynecology resident at the university.
Investigators aim to enroll patients in a longitudinal cohort study to further examine these questions, he said.
Protecting intended parents and carriers
“Gestational surrogacy has become an increasingly common form of third-party reproduction,” Dr. Stark said at the virtual meeting. The number of cases of in vitro fertilization (IVF) with gestational carriers increased from approximately 700 in 1999 to more than 5,500 in 2016, according to data from the Society for Assisted Reproductive Technology. “Despite the increasing prevalence of gestational carrier utilization, there remains limited guidance with regard to optimizing outcomes for both the intended parents and gestational carriers.”
ASRM and UCSF recommendations are based on expert opinion and include surprisingly little discussion about the prior pregnancy outcomes of potential gestational carriers, Dr. Stark said.
“It is important for all parties involved that we generate research and data that can help drive the development of the guidelines,” he said. Such evidence may help intended parents understand characteristics of gestational carriers that may lead to live births. “For the gestational carriers, it is important that we have information on safety so that they know they are making appropriate decisions for their family and their life.”
Gestational carrier characteristics in the present study that deviated from 2017 ASRM recommendations included age less than 21 years or greater than 45 years, mental health conditions, and having more than five prior deliveries.
“ASRM guidelines focused on criteria for gestational carriers are meant to protect infertile couples, the carrier, as well as the supporting agency,” Alan Penzias, MD, chair of ASRM’s Practice Committee who is in private practice in Boston, said in a society news release that highlighted Dr. Stark’s study. “It is important that gestational carriers have a complete medical history and examination, in addition to a psychological session with a mental health professional to ensure there are no reasons for the carrier to not move forward with pregnancy.”
A retrospective study by Kate Swanson, MD, and associates found that nonadherence to ASRM guidelines was associated with increased rates of cesarean delivery, neonatal morbidity, and preterm birth.
To examine how adherence to ASRM and UCSF recommendations relates to pregnancy outcomes and maternal and neonatal morbidity and death, Dr. Stark and colleagues assessed births from gestational carrier pregnancies at UCSF between 2008 and 2019.
Of 194 gestational carriers included in the analysis, 98.9% had a prior term pregnancy, 11.9% had a prior preterm pregnancy, and 17.5% had a prior spontaneous abortion.
Indications for use of gestational surrogates included serious medical condition of intended parent (25%), uterine factor infertility (23%), recurrent pregnancy loss (10%), and same-sex male couples (8%).
When the researchers compared pregnancy outcomes for gestational carriers who met ASRM guidelines with outcomes for 38 gestational carriers who did not meet ASRM guidelines, there were no statistically significant differences. Antepartum, intrapartum, and postpartum complication rates and cesarean delivery rates did not significantly differ based on ASRM guideline adherence.
Nonadherence to the stricter UCSF guidelines, however, was associated with increased likelihood of spontaneous abortion. In all, 23.7% of the 59 gestational carriers who were nonadherent to UCSF guidelines had a pregnancy end in a spontaneous abortion, compared with 6.7% of gestational carriers who were adherent to the UCSF recommendations (odds ratio, 4.35).
An analysis of individual criteria and poor pregnancy outcomes found that BMI greater than 35 was associated with increased likelihood of spontaneous abortion (OR, 4.29), as was age less than 21 years or greater than 45 years (OR, 3.37).
Prior spontaneous abortion was associated with increased likelihood of a biochemical pregnancy (OR, 3.2), and prior preterm birth was associated with increased likelihood of spontaneous abortion (OR, 3.19), previable delivery (OR, 25.2), cesarean delivery (OR, 2.59), and antepartum complications (OR, 3.56).
The role of agencies
About 76% of the gestational carriers had pregnancies mediated through a gestational surrogacy agency. Surrogates from agencies were about three times more likely than surrogates who were family, friends, or from private surrogacy arrangements to adhere to ASRM and UCSF guidelines.
Even after hearing about gestational carrier recommendations, patients may prefer to work with someone they know. “We want to provide our patients with evidence-based information if possible, but ultimately it is their decision to make,” Dr. Stark said. “And we just need to make sure that they are making an informed decision.”
Dr. Stark had no relevant disclosures. Dr. Penzias helped develop the ASRM committee opinion. He had no relevant conflicts of interest.
SOURCE: Stark B et al. ASRM 2020, Abstract O-251.
About 20% of gestational carriers at one institution did not meet recommended criteria developed by the American Society for Reproductive Medicine, according to a retrospective study of 194 patients.
The University of California, San Francisco, offers additional, stricter recommendations, including that gestational carriers have a body mass index less than 35. Under these stricter criteria, about 30% of the gestational carriers did not meet recommendations, Brett Stark, MD, MPH, reported at the American Society for Reproductive Medicine’s 2020 annual meeting, held virtually this year.
Deviating from BMI or age recommendations may be associated with increased likelihood of spontaneous abortion, the analysis suggested. In addition, elements of a gestational carrier’s obstetric history not described in current guidelines, such as prior preterm birth, may influence gestational surrogacy outcomes.
The study was limited by incomplete information for some patients, the retrospective design, and the reliance on a relatively small cohort at a single center. Nevertheless, the findings potentially could inform discussions with patients, said Dr. Stark, a 3rd-year obstetrics and gynecology resident at the university.
Investigators aim to enroll patients in a longitudinal cohort study to further examine these questions, he said.
Protecting intended parents and carriers
“Gestational surrogacy has become an increasingly common form of third-party reproduction,” Dr. Stark said at the virtual meeting. The number of cases of in vitro fertilization (IVF) with gestational carriers increased from approximately 700 in 1999 to more than 5,500 in 2016, according to data from the Society for Assisted Reproductive Technology. “Despite the increasing prevalence of gestational carrier utilization, there remains limited guidance with regard to optimizing outcomes for both the intended parents and gestational carriers.”
ASRM and UCSF recommendations are based on expert opinion and include surprisingly little discussion about the prior pregnancy outcomes of potential gestational carriers, Dr. Stark said.
“It is important for all parties involved that we generate research and data that can help drive the development of the guidelines,” he said. Such evidence may help intended parents understand characteristics of gestational carriers that may lead to live births. “For the gestational carriers, it is important that we have information on safety so that they know they are making appropriate decisions for their family and their life.”
Gestational carrier characteristics in the present study that deviated from 2017 ASRM recommendations included age less than 21 years or greater than 45 years, mental health conditions, and having more than five prior deliveries.
“ASRM guidelines focused on criteria for gestational carriers are meant to protect infertile couples, the carrier, as well as the supporting agency,” Alan Penzias, MD, chair of ASRM’s Practice Committee who is in private practice in Boston, said in a society news release that highlighted Dr. Stark’s study. “It is important that gestational carriers have a complete medical history and examination, in addition to a psychological session with a mental health professional to ensure there are no reasons for the carrier to not move forward with pregnancy.”
A retrospective study by Kate Swanson, MD, and associates found that nonadherence to ASRM guidelines was associated with increased rates of cesarean delivery, neonatal morbidity, and preterm birth.
To examine how adherence to ASRM and UCSF recommendations relates to pregnancy outcomes and maternal and neonatal morbidity and death, Dr. Stark and colleagues assessed births from gestational carrier pregnancies at UCSF between 2008 and 2019.
Of 194 gestational carriers included in the analysis, 98.9% had a prior term pregnancy, 11.9% had a prior preterm pregnancy, and 17.5% had a prior spontaneous abortion.
Indications for use of gestational surrogates included serious medical condition of intended parent (25%), uterine factor infertility (23%), recurrent pregnancy loss (10%), and same-sex male couples (8%).
When the researchers compared pregnancy outcomes for gestational carriers who met ASRM guidelines with outcomes for 38 gestational carriers who did not meet ASRM guidelines, there were no statistically significant differences. Antepartum, intrapartum, and postpartum complication rates and cesarean delivery rates did not significantly differ based on ASRM guideline adherence.
Nonadherence to the stricter UCSF guidelines, however, was associated with increased likelihood of spontaneous abortion. In all, 23.7% of the 59 gestational carriers who were nonadherent to UCSF guidelines had a pregnancy end in a spontaneous abortion, compared with 6.7% of gestational carriers who were adherent to the UCSF recommendations (odds ratio, 4.35).
An analysis of individual criteria and poor pregnancy outcomes found that BMI greater than 35 was associated with increased likelihood of spontaneous abortion (OR, 4.29), as was age less than 21 years or greater than 45 years (OR, 3.37).
Prior spontaneous abortion was associated with increased likelihood of a biochemical pregnancy (OR, 3.2), and prior preterm birth was associated with increased likelihood of spontaneous abortion (OR, 3.19), previable delivery (OR, 25.2), cesarean delivery (OR, 2.59), and antepartum complications (OR, 3.56).
The role of agencies
About 76% of the gestational carriers had pregnancies mediated through a gestational surrogacy agency. Surrogates from agencies were about three times more likely than surrogates who were family, friends, or from private surrogacy arrangements to adhere to ASRM and UCSF guidelines.
Even after hearing about gestational carrier recommendations, patients may prefer to work with someone they know. “We want to provide our patients with evidence-based information if possible, but ultimately it is their decision to make,” Dr. Stark said. “And we just need to make sure that they are making an informed decision.”
Dr. Stark had no relevant disclosures. Dr. Penzias helped develop the ASRM committee opinion. He had no relevant conflicts of interest.
SOURCE: Stark B et al. ASRM 2020, Abstract O-251.
FROM ASRM 2020
Reproductive Rounds: Fertility preservation options for cancer patients
What is more stressful in the mind of a patient – a diagnosis of cancer or infertility? An infertile woman’s anxiety and depression scores are equivalent to one with cancer (J Psychosom Obstet Gynecol. 1993;14 Suppl:45-52). These two diseases intersect in the burgeoning field of oncofertility, the collaboration of oncology with reproductive endocrinology to offer patients the option of fertility preservation. The term oncofertility was first coined by Teresa Woodruff, PhD, in 2005 during her invited lecture at the University of Calgary symposium called “Pushing the Boundaries – Advances that Will Change the World in 20 Years.” Her prediction has reached its fruition. This article will review fertility preservation options for female oncology patients.
The ability for oncofertility to exist is the result of improved cancer survival rates and advances in reproductive medicine. Improvements in the treatment of cancer enable many young women to survive and focus on the potential of having a family. Malignancies striking young people, particularly breast, lymphoma, and melanoma, have encouraging 5-year survival rates. If invasive cancer is located only in the breast (affecting 62% of women diagnosed), the 5-year survival rate is 99%. For all with Hodgkin lymphoma, the 5-year survival is 87%, increasing to 92% if the cancer is found in its earliest stages. Among all people with melanoma of the skin, from the time of initial diagnosis, the 5-year survival is 92%.
Long-term survival is expected for 80% of children and adolescents diagnosed with cancer (Obstet Gynecol. 2010;116: 1171-83).
Iatrogenic effects
The reproductive risk of cancer treatment is gonadotoxicity and the subsequent iatrogenic primary ovarian insufficiency (POI, prior termed premature ovarian failure) or infertility.
Chemotherapy with alkylating agents, such as cyclophosphamide, is associated with the greatest chance of amenorrhea (Breast Cancer Res Treat. 2014;145:113-28). Chemotherapy with cyclophosphamide, methotrexate, and 5 fluorouracil (CMF – commonly used for the treatment of breast cancer) will usually result in loss of ovarian function in 33% of women under age 30, 50% of women aged 30-35, 75% of women aged 35-40, and 95% of women over age 40 (J Clin Oncol. 2006;24:5769-79).
The dose at which 50% of oocytes are lost due to radiation is under 2 Gy (Hum Reprod. 2003;18:117-21). Unfortunately, the minimum dose decreases with advancing age of the woman, contributed by natural diminishing reserve and an increase in radiosensitivity of oocytes. Age, proximity of the radiation field to the ovaries, and total dose are important factors determining risk of POI. For brain tumors, cranial irradiation may result in hypothalamic amenorrhea.
Protection
The use of GnRH agonist for 6 months during chemotherapy has been controversial with mixed results in avoiding ovarian failure. A recent study suggests a GnRH agonist does reduce the prevalence of POI (J Clin Oncol. 2018;36:1981-90) in women treated for breast cancer but the subsequent ovarian reserve is low (Ann Oncol. 2017;28:1811-6). There are not enough data now to consider this the sole viable option for all patients to preserve fertility.
Patients requiring local pelvic radiation treatment may benefit from transposition of the ovaries to sites away from maximal radiation exposure.
Oocyte cryopreservation (OC) and ovarian tissue cryopreservation (OTC)
Since 2012, the American Society for Reproductive Medicine lifted the experimental designation on OC and, last year, the society removed the same label for OTC, providing an additional fertility preservation option.
Ovarian stimulation and egg retrieval for OC can now occur literally within 2 weeks because of a random start protocol whereby women are stimulated any day in their cycle, pre- and post ovulation. Studies have shown equivalent yield of oocytes.
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a routine matter with egg donation cycles. While there remains debate over whether live birth rates using frozen eggs are inferior to fresh eggs, a learning curve with the new technology may be the important factor (Obstet Gynecol. 2020;135:709-16).
When urgent cancer treatment precludes ovarian stimulation for OC, then OTC is a viable option. Another population that could benefit from OTC are prepubertal girls facing gonadotoxic therapy. More research is required to determine the quality of eggs obtained through ovarian stimulation in adolescent and young adult patients. While leukemic patients are eligible for OTC, there is concern about reseeding malignant cells with future autologous transplantation of tissue.
OTC involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. To date, live birth rates are modest (Fertil Steril. 2015;104:1097-8).
Recent research has combined the freezing of both mature and immature eggs, the latter undergoing IVM (in-vitro maturation) to maximize the potential for fertilizable eggs. Women with polycystic ovary syndrome and certain cancers or medical conditions that warrant avoiding supraphysiologic levels of estradiol from ovarian stimulation, may benefit from the retrieval of immature eggs from unstimulated ovaries.
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans are not known but animal studies suggest there may be higher rates of miscarriage and birth defects.
Breast cancer – a special scenario
With every breast cancer patient, I review the theoretical concern over increasing estradiol levels during an IVF stimulation cycle with the potential impact on her cancer prognosis. Fortunately, the literature has not demonstrated an increased risk of breast cancer or recurrence after undergoing an IVF cycle. Currently, the use of aromatase inhibitors with gonadotropins along with a GnRH-antagonist is the protocol to maintain a lower estradiol level during stimulation, which may be of benefit for breast cancer prognosis. The use of aromatase inhibitors is an off-label indication for fertility with no definitive evidence of teratogenicity. Preimplantation genetic testing of embryos is available and approved by the American Society for Reproductive Medicine for BRCA gene mutation patients.
Oncofertility is an exciting field to allow cancer survivors the option for a biological child. We recommend all our cancer patients meet with our reproductive psychologist to assist in coping with the overwhelming information presented in a short time frame.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
What is more stressful in the mind of a patient – a diagnosis of cancer or infertility? An infertile woman’s anxiety and depression scores are equivalent to one with cancer (J Psychosom Obstet Gynecol. 1993;14 Suppl:45-52). These two diseases intersect in the burgeoning field of oncofertility, the collaboration of oncology with reproductive endocrinology to offer patients the option of fertility preservation. The term oncofertility was first coined by Teresa Woodruff, PhD, in 2005 during her invited lecture at the University of Calgary symposium called “Pushing the Boundaries – Advances that Will Change the World in 20 Years.” Her prediction has reached its fruition. This article will review fertility preservation options for female oncology patients.
The ability for oncofertility to exist is the result of improved cancer survival rates and advances in reproductive medicine. Improvements in the treatment of cancer enable many young women to survive and focus on the potential of having a family. Malignancies striking young people, particularly breast, lymphoma, and melanoma, have encouraging 5-year survival rates. If invasive cancer is located only in the breast (affecting 62% of women diagnosed), the 5-year survival rate is 99%. For all with Hodgkin lymphoma, the 5-year survival is 87%, increasing to 92% if the cancer is found in its earliest stages. Among all people with melanoma of the skin, from the time of initial diagnosis, the 5-year survival is 92%.
Long-term survival is expected for 80% of children and adolescents diagnosed with cancer (Obstet Gynecol. 2010;116: 1171-83).
Iatrogenic effects
The reproductive risk of cancer treatment is gonadotoxicity and the subsequent iatrogenic primary ovarian insufficiency (POI, prior termed premature ovarian failure) or infertility.
Chemotherapy with alkylating agents, such as cyclophosphamide, is associated with the greatest chance of amenorrhea (Breast Cancer Res Treat. 2014;145:113-28). Chemotherapy with cyclophosphamide, methotrexate, and 5 fluorouracil (CMF – commonly used for the treatment of breast cancer) will usually result in loss of ovarian function in 33% of women under age 30, 50% of women aged 30-35, 75% of women aged 35-40, and 95% of women over age 40 (J Clin Oncol. 2006;24:5769-79).
The dose at which 50% of oocytes are lost due to radiation is under 2 Gy (Hum Reprod. 2003;18:117-21). Unfortunately, the minimum dose decreases with advancing age of the woman, contributed by natural diminishing reserve and an increase in radiosensitivity of oocytes. Age, proximity of the radiation field to the ovaries, and total dose are important factors determining risk of POI. For brain tumors, cranial irradiation may result in hypothalamic amenorrhea.
Protection
The use of GnRH agonist for 6 months during chemotherapy has been controversial with mixed results in avoiding ovarian failure. A recent study suggests a GnRH agonist does reduce the prevalence of POI (J Clin Oncol. 2018;36:1981-90) in women treated for breast cancer but the subsequent ovarian reserve is low (Ann Oncol. 2017;28:1811-6). There are not enough data now to consider this the sole viable option for all patients to preserve fertility.
Patients requiring local pelvic radiation treatment may benefit from transposition of the ovaries to sites away from maximal radiation exposure.
Oocyte cryopreservation (OC) and ovarian tissue cryopreservation (OTC)
Since 2012, the American Society for Reproductive Medicine lifted the experimental designation on OC and, last year, the society removed the same label for OTC, providing an additional fertility preservation option.
Ovarian stimulation and egg retrieval for OC can now occur literally within 2 weeks because of a random start protocol whereby women are stimulated any day in their cycle, pre- and post ovulation. Studies have shown equivalent yield of oocytes.
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a routine matter with egg donation cycles. While there remains debate over whether live birth rates using frozen eggs are inferior to fresh eggs, a learning curve with the new technology may be the important factor (Obstet Gynecol. 2020;135:709-16).
When urgent cancer treatment precludes ovarian stimulation for OC, then OTC is a viable option. Another population that could benefit from OTC are prepubertal girls facing gonadotoxic therapy. More research is required to determine the quality of eggs obtained through ovarian stimulation in adolescent and young adult patients. While leukemic patients are eligible for OTC, there is concern about reseeding malignant cells with future autologous transplantation of tissue.
OTC involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. To date, live birth rates are modest (Fertil Steril. 2015;104:1097-8).
Recent research has combined the freezing of both mature and immature eggs, the latter undergoing IVM (in-vitro maturation) to maximize the potential for fertilizable eggs. Women with polycystic ovary syndrome and certain cancers or medical conditions that warrant avoiding supraphysiologic levels of estradiol from ovarian stimulation, may benefit from the retrieval of immature eggs from unstimulated ovaries.
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans are not known but animal studies suggest there may be higher rates of miscarriage and birth defects.
Breast cancer – a special scenario
With every breast cancer patient, I review the theoretical concern over increasing estradiol levels during an IVF stimulation cycle with the potential impact on her cancer prognosis. Fortunately, the literature has not demonstrated an increased risk of breast cancer or recurrence after undergoing an IVF cycle. Currently, the use of aromatase inhibitors with gonadotropins along with a GnRH-antagonist is the protocol to maintain a lower estradiol level during stimulation, which may be of benefit for breast cancer prognosis. The use of aromatase inhibitors is an off-label indication for fertility with no definitive evidence of teratogenicity. Preimplantation genetic testing of embryos is available and approved by the American Society for Reproductive Medicine for BRCA gene mutation patients.
Oncofertility is an exciting field to allow cancer survivors the option for a biological child. We recommend all our cancer patients meet with our reproductive psychologist to assist in coping with the overwhelming information presented in a short time frame.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
What is more stressful in the mind of a patient – a diagnosis of cancer or infertility? An infertile woman’s anxiety and depression scores are equivalent to one with cancer (J Psychosom Obstet Gynecol. 1993;14 Suppl:45-52). These two diseases intersect in the burgeoning field of oncofertility, the collaboration of oncology with reproductive endocrinology to offer patients the option of fertility preservation. The term oncofertility was first coined by Teresa Woodruff, PhD, in 2005 during her invited lecture at the University of Calgary symposium called “Pushing the Boundaries – Advances that Will Change the World in 20 Years.” Her prediction has reached its fruition. This article will review fertility preservation options for female oncology patients.
The ability for oncofertility to exist is the result of improved cancer survival rates and advances in reproductive medicine. Improvements in the treatment of cancer enable many young women to survive and focus on the potential of having a family. Malignancies striking young people, particularly breast, lymphoma, and melanoma, have encouraging 5-year survival rates. If invasive cancer is located only in the breast (affecting 62% of women diagnosed), the 5-year survival rate is 99%. For all with Hodgkin lymphoma, the 5-year survival is 87%, increasing to 92% if the cancer is found in its earliest stages. Among all people with melanoma of the skin, from the time of initial diagnosis, the 5-year survival is 92%.
Long-term survival is expected for 80% of children and adolescents diagnosed with cancer (Obstet Gynecol. 2010;116: 1171-83).
Iatrogenic effects
The reproductive risk of cancer treatment is gonadotoxicity and the subsequent iatrogenic primary ovarian insufficiency (POI, prior termed premature ovarian failure) or infertility.
Chemotherapy with alkylating agents, such as cyclophosphamide, is associated with the greatest chance of amenorrhea (Breast Cancer Res Treat. 2014;145:113-28). Chemotherapy with cyclophosphamide, methotrexate, and 5 fluorouracil (CMF – commonly used for the treatment of breast cancer) will usually result in loss of ovarian function in 33% of women under age 30, 50% of women aged 30-35, 75% of women aged 35-40, and 95% of women over age 40 (J Clin Oncol. 2006;24:5769-79).
The dose at which 50% of oocytes are lost due to radiation is under 2 Gy (Hum Reprod. 2003;18:117-21). Unfortunately, the minimum dose decreases with advancing age of the woman, contributed by natural diminishing reserve and an increase in radiosensitivity of oocytes. Age, proximity of the radiation field to the ovaries, and total dose are important factors determining risk of POI. For brain tumors, cranial irradiation may result in hypothalamic amenorrhea.
Protection
The use of GnRH agonist for 6 months during chemotherapy has been controversial with mixed results in avoiding ovarian failure. A recent study suggests a GnRH agonist does reduce the prevalence of POI (J Clin Oncol. 2018;36:1981-90) in women treated for breast cancer but the subsequent ovarian reserve is low (Ann Oncol. 2017;28:1811-6). There are not enough data now to consider this the sole viable option for all patients to preserve fertility.
Patients requiring local pelvic radiation treatment may benefit from transposition of the ovaries to sites away from maximal radiation exposure.
Oocyte cryopreservation (OC) and ovarian tissue cryopreservation (OTC)
Since 2012, the American Society for Reproductive Medicine lifted the experimental designation on OC and, last year, the society removed the same label for OTC, providing an additional fertility preservation option.
Ovarian stimulation and egg retrieval for OC can now occur literally within 2 weeks because of a random start protocol whereby women are stimulated any day in their cycle, pre- and post ovulation. Studies have shown equivalent yield of oocytes.
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a routine matter with egg donation cycles. While there remains debate over whether live birth rates using frozen eggs are inferior to fresh eggs, a learning curve with the new technology may be the important factor (Obstet Gynecol. 2020;135:709-16).
When urgent cancer treatment precludes ovarian stimulation for OC, then OTC is a viable option. Another population that could benefit from OTC are prepubertal girls facing gonadotoxic therapy. More research is required to determine the quality of eggs obtained through ovarian stimulation in adolescent and young adult patients. While leukemic patients are eligible for OTC, there is concern about reseeding malignant cells with future autologous transplantation of tissue.
OTC involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. To date, live birth rates are modest (Fertil Steril. 2015;104:1097-8).
Recent research has combined the freezing of both mature and immature eggs, the latter undergoing IVM (in-vitro maturation) to maximize the potential for fertilizable eggs. Women with polycystic ovary syndrome and certain cancers or medical conditions that warrant avoiding supraphysiologic levels of estradiol from ovarian stimulation, may benefit from the retrieval of immature eggs from unstimulated ovaries.
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans are not known but animal studies suggest there may be higher rates of miscarriage and birth defects.
Breast cancer – a special scenario
With every breast cancer patient, I review the theoretical concern over increasing estradiol levels during an IVF stimulation cycle with the potential impact on her cancer prognosis. Fortunately, the literature has not demonstrated an increased risk of breast cancer or recurrence after undergoing an IVF cycle. Currently, the use of aromatase inhibitors with gonadotropins along with a GnRH-antagonist is the protocol to maintain a lower estradiol level during stimulation, which may be of benefit for breast cancer prognosis. The use of aromatase inhibitors is an off-label indication for fertility with no definitive evidence of teratogenicity. Preimplantation genetic testing of embryos is available and approved by the American Society for Reproductive Medicine for BRCA gene mutation patients.
Oncofertility is an exciting field to allow cancer survivors the option for a biological child. We recommend all our cancer patients meet with our reproductive psychologist to assist in coping with the overwhelming information presented in a short time frame.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Endocrine-disrupting plastics pose growing health threat
Many types of plastics pose an unrecognized threat to human health by leaching endocrine-disrupting chemicals, and a new report from the Endocrine Society and the International Pollutants Elimination Network presents their dangers and risks.
Written in a consumer-friendly form designed to guide public interest groups and policy makers, the report also can be used by clinicians to inform discussions with patients about the potential dangers of plastics and how they can reduce their exposure to endocrine-disrupting chemicals.
The report, Plastics, EDCs, & Health, defines endocrine-disrupting chemicals (EDCs) as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.” Hormones in the body must be released at specific times, and therefore interference with their normal activity can have profound effects on health in areas including growth and reproductive development, according to the report.
The available data show “more and more information about the different chemicals and the different effects they are having,” said lead author, Jodi Flaws, PhD, of the University of Illinois at Urbana-Champaign, in a virtual press conference accompanying the release of the report.
Although numerous EDCs have been identified, a recent study suggested that many potentially dangerous chemical additives remain unknown because they are identified as confidential or simply not well described, the report authors said. In addition, creation of more plastic products will likely lead to increased exposure to EDCs and make health problems worse, said report coauthor Pauliina Damdimopoulou, PhD, of the Karolinska Institutet in Stockholm.
Lesser-known EDCs populate consumer products
Most consumers are aware of bisphenol A and phthalates as known EDCs, said Dr. Flaws, but the report identifies other lesser-known EDCs including per- and polyfluoroalkyl substances (PFAS), dioxins, flame retardants, and UV stabilizers.
For example, PFAS have been used for decades in a range of consumer products including stain resistant clothes, fast food wrappers, carpet and furniture treatments, cookware, and firefighting foams, according to the report. Consequently, PFAS have become common in many water sources including surface water, drinking water, and ground water because of how they are disposed. “Consumption of fish and other aquatic creatures caught in waterways contaminated with PFAS also poses heightened risks due to bioaccumulation of persistent chemicals in these animals,” the report authors noted. Human exposures to PFAS have been documented in urine, serum, plasma, placenta, umbilical cord, breast milk, and fetal tissues, they added.
Brominated flame retardants are another lesser-known EDC highlighted in the report. These chemical additives are used in plastics such as electronics cases to reduce the spread of fire, as well as in furniture foam and other building materials, the authors wrote. UV stabilizers, which also have been linked to health problems, often are used in manufacturing cars and other machinery.
Microplastics create large risk
Microplastics, defined as plastic particles less than 5 mm in diameter, are another source of exposure to EDCs that is not well publicized, according to the report. Plastic waste disposal often leads to the release of microplastics, which can infiltrate soil and water. Plastic waste is often dumped or burned; outdoor burning of plastic causes emission of dioxins into the air and ground.
“Not only do microplastics contain endogenous chemical additives, which are not bound to the microplastic and can leach out of the microplastic and expose the population, they can also bind and accumulate toxic chemicals from the surrounding environment such as sea water and sediment,” the report authors said.
Recycling is not an easy answer, either. Often more chemicals are created and released during the process of using plastics to make other plastics, according to the report.
Overall, more awareness of the potential for increased exposure to EDCs and support of strategies to seek out alternatives to hazardous chemicals is needed at the global level, the authors wrote. For example, the European Union has proposed a chemicals strategy that includes improved classification of EDCs and banning identified EDCs in consumer products.
New data support ongoing dangers
“It was important to produce the report at this time because several new studies came out on the effects of EDCs from plastics on human health,” Dr. Flaws said in an interview. “Further, there was not previously a single source that brought together all the information in a manner that was targeted towards the public, policy makers, and others,” she said.
Dr. Flaws said that what has surprised her most in the recent research is the fact that plastics contain such a range of chemicals and EDCs.
“A good take-home message [from the report] is that plastics can contain endocrine-disrupting chemicals that can interfere with normal hormones and lead to adverse health outcomes,” she said. “I suggest limiting the use of plastics as much as possible. I know this is very hard to do, so if someone needs to use plastic, they should not heat food or drink in plastic containers,” she emphasized. Individuals also can limit reuse of plastics over and over,” she said. “Heating and repeated use/washing often causes plastics to leach EDCs into food and drink that we then get into our bodies.”
Additional research is needed to understand the mechanisms by which EDCs from plastics cause damage, Dr. Flaws emphasized. “Given that it is not possible to eliminate plastics at this time, if we understood mechanisms of action, we could develop ways to prevent toxicity or treat EDC-induced adverse health outcomes,” she said. “We also need research designed to develop plastics or ‘green materials’ that do not contain endocrine disruptors and do not cause health problems or damage the environment,” she noted.
The report was produced as a joint effort of the Endocrine Society and International Pollutants Elimination Network. The report authors had no financial conflicts to disclose.
Many types of plastics pose an unrecognized threat to human health by leaching endocrine-disrupting chemicals, and a new report from the Endocrine Society and the International Pollutants Elimination Network presents their dangers and risks.
Written in a consumer-friendly form designed to guide public interest groups and policy makers, the report also can be used by clinicians to inform discussions with patients about the potential dangers of plastics and how they can reduce their exposure to endocrine-disrupting chemicals.
The report, Plastics, EDCs, & Health, defines endocrine-disrupting chemicals (EDCs) as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.” Hormones in the body must be released at specific times, and therefore interference with their normal activity can have profound effects on health in areas including growth and reproductive development, according to the report.
The available data show “more and more information about the different chemicals and the different effects they are having,” said lead author, Jodi Flaws, PhD, of the University of Illinois at Urbana-Champaign, in a virtual press conference accompanying the release of the report.
Although numerous EDCs have been identified, a recent study suggested that many potentially dangerous chemical additives remain unknown because they are identified as confidential or simply not well described, the report authors said. In addition, creation of more plastic products will likely lead to increased exposure to EDCs and make health problems worse, said report coauthor Pauliina Damdimopoulou, PhD, of the Karolinska Institutet in Stockholm.
Lesser-known EDCs populate consumer products
Most consumers are aware of bisphenol A and phthalates as known EDCs, said Dr. Flaws, but the report identifies other lesser-known EDCs including per- and polyfluoroalkyl substances (PFAS), dioxins, flame retardants, and UV stabilizers.
For example, PFAS have been used for decades in a range of consumer products including stain resistant clothes, fast food wrappers, carpet and furniture treatments, cookware, and firefighting foams, according to the report. Consequently, PFAS have become common in many water sources including surface water, drinking water, and ground water because of how they are disposed. “Consumption of fish and other aquatic creatures caught in waterways contaminated with PFAS also poses heightened risks due to bioaccumulation of persistent chemicals in these animals,” the report authors noted. Human exposures to PFAS have been documented in urine, serum, plasma, placenta, umbilical cord, breast milk, and fetal tissues, they added.
Brominated flame retardants are another lesser-known EDC highlighted in the report. These chemical additives are used in plastics such as electronics cases to reduce the spread of fire, as well as in furniture foam and other building materials, the authors wrote. UV stabilizers, which also have been linked to health problems, often are used in manufacturing cars and other machinery.
Microplastics create large risk
Microplastics, defined as plastic particles less than 5 mm in diameter, are another source of exposure to EDCs that is not well publicized, according to the report. Plastic waste disposal often leads to the release of microplastics, which can infiltrate soil and water. Plastic waste is often dumped or burned; outdoor burning of plastic causes emission of dioxins into the air and ground.
“Not only do microplastics contain endogenous chemical additives, which are not bound to the microplastic and can leach out of the microplastic and expose the population, they can also bind and accumulate toxic chemicals from the surrounding environment such as sea water and sediment,” the report authors said.
Recycling is not an easy answer, either. Often more chemicals are created and released during the process of using plastics to make other plastics, according to the report.
Overall, more awareness of the potential for increased exposure to EDCs and support of strategies to seek out alternatives to hazardous chemicals is needed at the global level, the authors wrote. For example, the European Union has proposed a chemicals strategy that includes improved classification of EDCs and banning identified EDCs in consumer products.
New data support ongoing dangers
“It was important to produce the report at this time because several new studies came out on the effects of EDCs from plastics on human health,” Dr. Flaws said in an interview. “Further, there was not previously a single source that brought together all the information in a manner that was targeted towards the public, policy makers, and others,” she said.
Dr. Flaws said that what has surprised her most in the recent research is the fact that plastics contain such a range of chemicals and EDCs.
“A good take-home message [from the report] is that plastics can contain endocrine-disrupting chemicals that can interfere with normal hormones and lead to adverse health outcomes,” she said. “I suggest limiting the use of plastics as much as possible. I know this is very hard to do, so if someone needs to use plastic, they should not heat food or drink in plastic containers,” she emphasized. Individuals also can limit reuse of plastics over and over,” she said. “Heating and repeated use/washing often causes plastics to leach EDCs into food and drink that we then get into our bodies.”
Additional research is needed to understand the mechanisms by which EDCs from plastics cause damage, Dr. Flaws emphasized. “Given that it is not possible to eliminate plastics at this time, if we understood mechanisms of action, we could develop ways to prevent toxicity or treat EDC-induced adverse health outcomes,” she said. “We also need research designed to develop plastics or ‘green materials’ that do not contain endocrine disruptors and do not cause health problems or damage the environment,” she noted.
The report was produced as a joint effort of the Endocrine Society and International Pollutants Elimination Network. The report authors had no financial conflicts to disclose.
Many types of plastics pose an unrecognized threat to human health by leaching endocrine-disrupting chemicals, and a new report from the Endocrine Society and the International Pollutants Elimination Network presents their dangers and risks.
Written in a consumer-friendly form designed to guide public interest groups and policy makers, the report also can be used by clinicians to inform discussions with patients about the potential dangers of plastics and how they can reduce their exposure to endocrine-disrupting chemicals.
The report, Plastics, EDCs, & Health, defines endocrine-disrupting chemicals (EDCs) as “an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.” Hormones in the body must be released at specific times, and therefore interference with their normal activity can have profound effects on health in areas including growth and reproductive development, according to the report.
The available data show “more and more information about the different chemicals and the different effects they are having,” said lead author, Jodi Flaws, PhD, of the University of Illinois at Urbana-Champaign, in a virtual press conference accompanying the release of the report.
Although numerous EDCs have been identified, a recent study suggested that many potentially dangerous chemical additives remain unknown because they are identified as confidential or simply not well described, the report authors said. In addition, creation of more plastic products will likely lead to increased exposure to EDCs and make health problems worse, said report coauthor Pauliina Damdimopoulou, PhD, of the Karolinska Institutet in Stockholm.
Lesser-known EDCs populate consumer products
Most consumers are aware of bisphenol A and phthalates as known EDCs, said Dr. Flaws, but the report identifies other lesser-known EDCs including per- and polyfluoroalkyl substances (PFAS), dioxins, flame retardants, and UV stabilizers.
For example, PFAS have been used for decades in a range of consumer products including stain resistant clothes, fast food wrappers, carpet and furniture treatments, cookware, and firefighting foams, according to the report. Consequently, PFAS have become common in many water sources including surface water, drinking water, and ground water because of how they are disposed. “Consumption of fish and other aquatic creatures caught in waterways contaminated with PFAS also poses heightened risks due to bioaccumulation of persistent chemicals in these animals,” the report authors noted. Human exposures to PFAS have been documented in urine, serum, plasma, placenta, umbilical cord, breast milk, and fetal tissues, they added.
Brominated flame retardants are another lesser-known EDC highlighted in the report. These chemical additives are used in plastics such as electronics cases to reduce the spread of fire, as well as in furniture foam and other building materials, the authors wrote. UV stabilizers, which also have been linked to health problems, often are used in manufacturing cars and other machinery.
Microplastics create large risk
Microplastics, defined as plastic particles less than 5 mm in diameter, are another source of exposure to EDCs that is not well publicized, according to the report. Plastic waste disposal often leads to the release of microplastics, which can infiltrate soil and water. Plastic waste is often dumped or burned; outdoor burning of plastic causes emission of dioxins into the air and ground.
“Not only do microplastics contain endogenous chemical additives, which are not bound to the microplastic and can leach out of the microplastic and expose the population, they can also bind and accumulate toxic chemicals from the surrounding environment such as sea water and sediment,” the report authors said.
Recycling is not an easy answer, either. Often more chemicals are created and released during the process of using plastics to make other plastics, according to the report.
Overall, more awareness of the potential for increased exposure to EDCs and support of strategies to seek out alternatives to hazardous chemicals is needed at the global level, the authors wrote. For example, the European Union has proposed a chemicals strategy that includes improved classification of EDCs and banning identified EDCs in consumer products.
New data support ongoing dangers
“It was important to produce the report at this time because several new studies came out on the effects of EDCs from plastics on human health,” Dr. Flaws said in an interview. “Further, there was not previously a single source that brought together all the information in a manner that was targeted towards the public, policy makers, and others,” she said.
Dr. Flaws said that what has surprised her most in the recent research is the fact that plastics contain such a range of chemicals and EDCs.
“A good take-home message [from the report] is that plastics can contain endocrine-disrupting chemicals that can interfere with normal hormones and lead to adverse health outcomes,” she said. “I suggest limiting the use of plastics as much as possible. I know this is very hard to do, so if someone needs to use plastic, they should not heat food or drink in plastic containers,” she emphasized. Individuals also can limit reuse of plastics over and over,” she said. “Heating and repeated use/washing often causes plastics to leach EDCs into food and drink that we then get into our bodies.”
Additional research is needed to understand the mechanisms by which EDCs from plastics cause damage, Dr. Flaws emphasized. “Given that it is not possible to eliminate plastics at this time, if we understood mechanisms of action, we could develop ways to prevent toxicity or treat EDC-induced adverse health outcomes,” she said. “We also need research designed to develop plastics or ‘green materials’ that do not contain endocrine disruptors and do not cause health problems or damage the environment,” she noted.
The report was produced as a joint effort of the Endocrine Society and International Pollutants Elimination Network. The report authors had no financial conflicts to disclose.
The pill toolbox: How to choose a combined oral contraceptive
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.
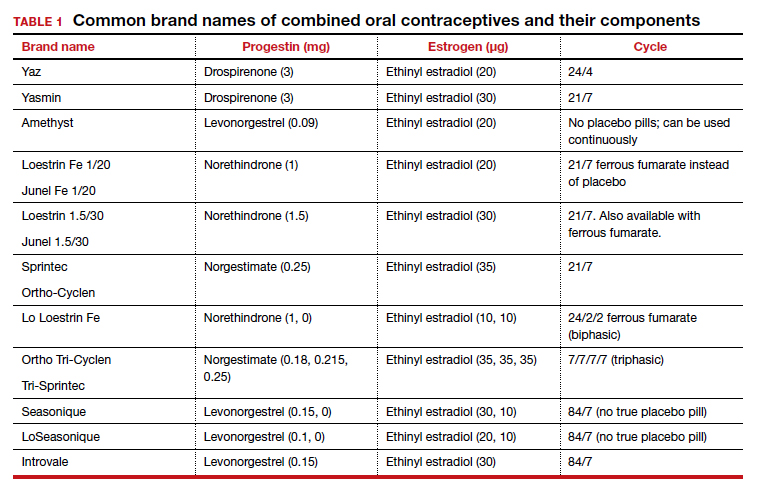
Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).
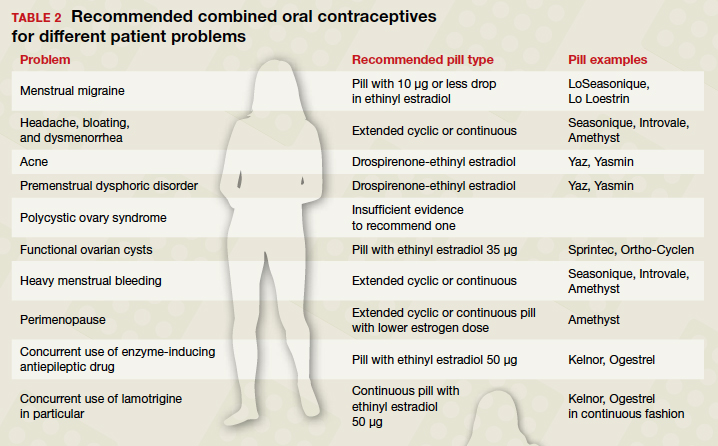
When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
Fertility delay varied with contraceptive method in study
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
FROM THE BMJ
First-of-its kind guideline on lipid monitoring in endocrine diseases
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Avoid pituitary pitfalls in hyperprolactinemia
,” Ashlyn Smith, PA-C, of Endocrinology Associates, Scottsdale, Ariz., said in a presentation at the at the virtual meeting of the annual Metabolic and Endocrine Disease Summit held by Global Academy for Medical Education.
The most common demographic for pituitary disorders is women in their 30s and 40s, Ms. Smith said. Early red flags for pituitary problems include patients presenting with headaches and/or blurred or double vision, which could signal bitemporal hemianopsia, she said.
Roughly two-thirds of pituitary adenomas are functional, meaning that they secrete pituitary hormones and cause clinical syndromes, Ms. Smith said. The most common reason for hypersecretion is hyperprolactinemia, she said.
Hyperprolactinemia, like most pituitary conditions, is more common in women than men, Ms. Smith noted. However, symptoms may include not only galactorrhea, but also gynecomastia, and hypogonadism, which may be red flags in men, she noted.
“Prolactin inhibits the gonadal pathway, so we see low gonadal hormones. For example, if men present with atypical hypogonadism for their age, or women present with changes in the menstrual cycle, check the prolactin levels,” she said.
The etiologies of hyperprolactinemia include physiologic reasons such as breastfeeding and pregnancy, as well as intercourse and breast manipulation, stress, and sleep issues. Pathologic reasons for prolactin elevation include prolactinoma, gonad-hormone secreting tumor, hypothyroidism, and renal insufficiency, Ms. Smith said.
Evaluation of patients with suspected hyperprolactinemia includes screening for physiologic causes, renal function and thyroid function tests, and a thyroid-specific MRI. Ordering a dedicated MRI of the pituitary gland is important to help identify compression of the optic nerve, noted Ms. Smith.
A medication review also is essential in evaluating hyperprolactinemia, and especially in the setting of the COVID-19 pandemic, because patients may have made changes to psychiatric medications, said Ms. Smith. Neuroleptics and antipsychotics including risperidone, haloperidol, chlorpromazine, and thiothixene can be associated with hyperprolactinemia, as can benzodiazepines and various analgesics and antidepressants, she said.
Treatment in cases of medication-induced hyperprolactinemia can be challenging if the patients are unable to change a medication, said Ms. Smith. However, patients with hypogonadism or low bone mineral density who can’t change medications may benefit from exogenous gonadal hormones, she said.
Some patients with hyperprolactinemia benefit from treatment with dopamine agonists, which may ease symptoms and reduce the size of the prolactinoma, she explained. However, patients on dopamine agonists should be alert to side effects including constipation and orthostasis. Ms. Smith said she recommends that patients on dopamine agonists for hyperprolactinemia take the medication at night so they are lying down if orthostasis occurs.
Monitor prolactin levels at 1 month, and taper or discontinue if the prolactin returns to normal and the adenoma resolves, which can take approximately 2 years, she said. Ms. Smith then advised follow-up every 3 months for 1 year, then annual prolactin checks.
The risk of recurrence ranges from 26% to 69%, Ms. Smith said, and is higher in patients with higher prolactin levels and larger adenomas, she noted. Recurrence is most likely within a year of withdrawal from treatment, she said.
Ms. Smith disclosed serving as an adviser and speaker for Abbott Nutrition, a speaker for Xeris Pharmaceuticals, and an adviser for Sanofi and Radius.
Global Academy for Medical Education and this news organization are owned by the same parent company.
SOURCE: Smith A. MEDS 2020.
,” Ashlyn Smith, PA-C, of Endocrinology Associates, Scottsdale, Ariz., said in a presentation at the at the virtual meeting of the annual Metabolic and Endocrine Disease Summit held by Global Academy for Medical Education.
The most common demographic for pituitary disorders is women in their 30s and 40s, Ms. Smith said. Early red flags for pituitary problems include patients presenting with headaches and/or blurred or double vision, which could signal bitemporal hemianopsia, she said.
Roughly two-thirds of pituitary adenomas are functional, meaning that they secrete pituitary hormones and cause clinical syndromes, Ms. Smith said. The most common reason for hypersecretion is hyperprolactinemia, she said.
Hyperprolactinemia, like most pituitary conditions, is more common in women than men, Ms. Smith noted. However, symptoms may include not only galactorrhea, but also gynecomastia, and hypogonadism, which may be red flags in men, she noted.
“Prolactin inhibits the gonadal pathway, so we see low gonadal hormones. For example, if men present with atypical hypogonadism for their age, or women present with changes in the menstrual cycle, check the prolactin levels,” she said.
The etiologies of hyperprolactinemia include physiologic reasons such as breastfeeding and pregnancy, as well as intercourse and breast manipulation, stress, and sleep issues. Pathologic reasons for prolactin elevation include prolactinoma, gonad-hormone secreting tumor, hypothyroidism, and renal insufficiency, Ms. Smith said.
Evaluation of patients with suspected hyperprolactinemia includes screening for physiologic causes, renal function and thyroid function tests, and a thyroid-specific MRI. Ordering a dedicated MRI of the pituitary gland is important to help identify compression of the optic nerve, noted Ms. Smith.
A medication review also is essential in evaluating hyperprolactinemia, and especially in the setting of the COVID-19 pandemic, because patients may have made changes to psychiatric medications, said Ms. Smith. Neuroleptics and antipsychotics including risperidone, haloperidol, chlorpromazine, and thiothixene can be associated with hyperprolactinemia, as can benzodiazepines and various analgesics and antidepressants, she said.
Treatment in cases of medication-induced hyperprolactinemia can be challenging if the patients are unable to change a medication, said Ms. Smith. However, patients with hypogonadism or low bone mineral density who can’t change medications may benefit from exogenous gonadal hormones, she said.
Some patients with hyperprolactinemia benefit from treatment with dopamine agonists, which may ease symptoms and reduce the size of the prolactinoma, she explained. However, patients on dopamine agonists should be alert to side effects including constipation and orthostasis. Ms. Smith said she recommends that patients on dopamine agonists for hyperprolactinemia take the medication at night so they are lying down if orthostasis occurs.
Monitor prolactin levels at 1 month, and taper or discontinue if the prolactin returns to normal and the adenoma resolves, which can take approximately 2 years, she said. Ms. Smith then advised follow-up every 3 months for 1 year, then annual prolactin checks.
The risk of recurrence ranges from 26% to 69%, Ms. Smith said, and is higher in patients with higher prolactin levels and larger adenomas, she noted. Recurrence is most likely within a year of withdrawal from treatment, she said.
Ms. Smith disclosed serving as an adviser and speaker for Abbott Nutrition, a speaker for Xeris Pharmaceuticals, and an adviser for Sanofi and Radius.
Global Academy for Medical Education and this news organization are owned by the same parent company.
SOURCE: Smith A. MEDS 2020.
,” Ashlyn Smith, PA-C, of Endocrinology Associates, Scottsdale, Ariz., said in a presentation at the at the virtual meeting of the annual Metabolic and Endocrine Disease Summit held by Global Academy for Medical Education.
The most common demographic for pituitary disorders is women in their 30s and 40s, Ms. Smith said. Early red flags for pituitary problems include patients presenting with headaches and/or blurred or double vision, which could signal bitemporal hemianopsia, she said.
Roughly two-thirds of pituitary adenomas are functional, meaning that they secrete pituitary hormones and cause clinical syndromes, Ms. Smith said. The most common reason for hypersecretion is hyperprolactinemia, she said.
Hyperprolactinemia, like most pituitary conditions, is more common in women than men, Ms. Smith noted. However, symptoms may include not only galactorrhea, but also gynecomastia, and hypogonadism, which may be red flags in men, she noted.
“Prolactin inhibits the gonadal pathway, so we see low gonadal hormones. For example, if men present with atypical hypogonadism for their age, or women present with changes in the menstrual cycle, check the prolactin levels,” she said.
The etiologies of hyperprolactinemia include physiologic reasons such as breastfeeding and pregnancy, as well as intercourse and breast manipulation, stress, and sleep issues. Pathologic reasons for prolactin elevation include prolactinoma, gonad-hormone secreting tumor, hypothyroidism, and renal insufficiency, Ms. Smith said.
Evaluation of patients with suspected hyperprolactinemia includes screening for physiologic causes, renal function and thyroid function tests, and a thyroid-specific MRI. Ordering a dedicated MRI of the pituitary gland is important to help identify compression of the optic nerve, noted Ms. Smith.
A medication review also is essential in evaluating hyperprolactinemia, and especially in the setting of the COVID-19 pandemic, because patients may have made changes to psychiatric medications, said Ms. Smith. Neuroleptics and antipsychotics including risperidone, haloperidol, chlorpromazine, and thiothixene can be associated with hyperprolactinemia, as can benzodiazepines and various analgesics and antidepressants, she said.
Treatment in cases of medication-induced hyperprolactinemia can be challenging if the patients are unable to change a medication, said Ms. Smith. However, patients with hypogonadism or low bone mineral density who can’t change medications may benefit from exogenous gonadal hormones, she said.
Some patients with hyperprolactinemia benefit from treatment with dopamine agonists, which may ease symptoms and reduce the size of the prolactinoma, she explained. However, patients on dopamine agonists should be alert to side effects including constipation and orthostasis. Ms. Smith said she recommends that patients on dopamine agonists for hyperprolactinemia take the medication at night so they are lying down if orthostasis occurs.
Monitor prolactin levels at 1 month, and taper or discontinue if the prolactin returns to normal and the adenoma resolves, which can take approximately 2 years, she said. Ms. Smith then advised follow-up every 3 months for 1 year, then annual prolactin checks.
The risk of recurrence ranges from 26% to 69%, Ms. Smith said, and is higher in patients with higher prolactin levels and larger adenomas, she noted. Recurrence is most likely within a year of withdrawal from treatment, she said.
Ms. Smith disclosed serving as an adviser and speaker for Abbott Nutrition, a speaker for Xeris Pharmaceuticals, and an adviser for Sanofi and Radius.
Global Academy for Medical Education and this news organization are owned by the same parent company.
SOURCE: Smith A. MEDS 2020.
EXPERT ANALYSIS FROM MEDS 2020
Menstrual irregularity appears to be predictor of early death
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
FROM THE BMJ
Genitourinary syndrome of menopause statement stresses treatment options
It’s important for clinicians to ask women whether they are experiencing symptoms of genitourinary syndrome of menopause (GSM) before and after menopause, according to a new statement from the North American Menopause Society.
Stephanie Faubion, MD, MBA, medical director of NAMS, presented the updated statement at the virtual annual meeting of the North American Menopause Society.
“The one thing we tried to emphasize is proactive counseling and proactive inquiry, educating women when they hit perimenopause that this is a thing and that there are treatments,” Dr. Faubion said in an interview.
There’s the misperception that it’s just part of getting old, which it’s not,” said Dr. Faubion, who is also director of the Mayo Clinic Center for Women’s Health in Rochester, Minn., and chair of the department of medicine at the Mayo Clinic in Jacksonville, Fla.
Changes from previous statement
The GSM statement describes the symptoms and signs resulting from estrogen deficiency on the genitourinary tract, Dr. Faubion explained. The biggest change from the earlier version, published in 2013, is the condition’s new name. Formerly known as vulvovaginal atrophy, the condition’s new term was developed in 2014 and is now preferred by NAMS and the American College of Obstetricians and Gynecologists because it’s more comprehensive. Rather than just a physical description of the condition, GSM encompasses the many related symptoms and the urinary tract changes that occur, and it clearly associates the condition with menopause.
“Women don’t always associate these changes with menopause and don’t recognize that there’s something that can be done about it,” Dr. Faubion said. “We like to emphasize that sex should never be painful, but it’s not just about sex. It’s about comfort.”
Other changes include a review of evidence related to vaginal laser therapy for GSM and the availability of Imvexxy vaginal inserts with lower doses (4 mcg and 10 mpg) of estrogen.
Etiology and diagnosis of GSM
The presence of endogenous estrogen keeps the vaginal lining thick, rugated, well vascularized, and lubricated. As estrogen levels decline during postmenopause, the epithelial lining becomes thinner, with reduced blood supply and loss of glycogen.
The most common symptoms of GSM include irritation of the vulva, inadequate vaginal lubrication, burning, dysuria, dyspareunia, and vaginal discharge, but the symptoms may not always correlate with physical findings. In women with surgical menopause, the symptoms tend to be more severe. The most distressing symptoms to women are often those that affect sexual function.
“Clinicians must be proactive in asking menopausal women if GSM symptoms are present, even before menopause begins,” Dr. Faubion said.
Taking a women’s history during evaluation may help identify contributing factors, other causes, or potentially effective treatments based on what has worked in the past. History should include a description of symptoms, their onset and duration, how distressing they are, and their effect on the woman’s quality of life. A sexual history, such as lubricants the woman has used, can also be useful in determining management strategies.
Signs of GSM include labial atrophy, vaginal dryness, introital stenosis, clitoral atrophy, phimosis of the prepuce, reduced mons pubis and labia majora bulk, reduced labia minora tissue and pigmentation, and changes in the urethra, including erythema of the urethral meatus and commonly a urethral caruncle, a benign outgrown of inflammatory tissue that likely results from low estrogen levels and can be treated effectively with topical hormonal therapies.
A diagnosis of GSM requires both physical findings and bothersome symptoms, though not necessarily specific vaginal maturation index or vaginal pH values. The differential diagnosis speaks to the importance of taking a good history: allergic or inflammatory conditions, infection, trauma, presence of a foreign body, malignancy, vulvodynia, chronic pelvic pain, or provoked pelvic floor hypertonia.
If first-line therapies of over-the-counter lubricants do not sufficiently treat GSM, other effective treatments include low-dose vaginal estrogen therapy, systemic estrogen therapy if other menopause symptoms are present, vaginal dehydroepiandrosterone (DHEA), and ospemifene.
Management of GSM
First-line therapy of GSM involves over-the-counter lubricants and moisturizers, which are often adequate to alleviate or eliminate women’s symptoms. However, the panel that developed the statement found no evidence that hyaluronic acid was any more effective than other lubricants or moisturizers, and no herbal products were found to effectively treat GSM.
While emerging evidence suggests that energy-based therapies, such as treatments with vaginal laser or radiofrequency devices, show some promise, more evidence is needed to show safety and efficacy before the panel can recommend routine use.
When over-the-counter therapies are not effective, vaginal estrogen usually relieves GSM with little absorption and is preferred over systemic therapy if GSM is the only bothersome menopausal symptom. Options include topical creams, a slow-release estradiol intravaginal ring, and estradiol vaginal tablets and inserts.
“However, when systemic hormone therapy is needed to treat other menopause symptoms, usually a woman will derive benefit and resolution of the GSM at the same time,” Dr. Faubion said. “However, for some women, additional low-dose vaginal estrogen may be added to systemic estrogen if needed, and that could include vaginal DHEA.”
All the approved vaginal products have shown efficacy, compared with placebo in clinical trials, and a Cochrane review comparing the different therapies found them to be similarly efficacious in treating vaginal dryness and dyspareunia with no significant differences in adverse events.
Preparing patients for the boxed warning
As vaginal estrogen doses are significantly lower than systemic estrogen, their safety profile is better, with serum estrogen levels remaining within the postmenopausal range when low-dose vaginal estrogen therapy is used. That said, some studies have shown that vaginal estrogen cream can be a large enough dose to involve systemic absorption and lead to symptoms such as vaginal bleeding, breast pain, and nausea.
However, package inserts for vaginal estrogen have the same boxed warning as seen in systemic hormone therapy inserts regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and “probable dementia” despite these conditions not being linked to vaginal estrogen in trials. Neither has venous thromboembolism been linked to vaginal estrogen.
“The panel felt it was very important that women be educated about the differences between low-dose vaginal estrogen and systemic estrogen therapy and be prepared for this boxed warning,” Dr. Faubion told attendees. “It’s really important to say: ‘You’re going to get this, it’s going to look scary, and there’s no evidence these same warnings apply to the low-dose vaginal estrogen products.’ ”
This point particularly resonated with NAMS attendee Juliana (Jewel) Kling, MD, MPH, an associate professor of medicine at the Mayo Clinic Arizona, Scottsdale.
“The point about educating women about the differences between low-dose vaginal estrogen products and systemic treatments and being prepared for the boxed warning is important and I hope reaches many practitioners,” Dr. Kling said in an interview.
The panel did not recommend using progestogen with low-dose vaginal estrogen therapy or doing routine endometrial surveillance in women using vaginal estrogen. But endometrial surveillance may be worth considering in women with increased risk of endometrial cancer.
Estrogen insufficiency from premature menopause or primary ovarian insufficiency is linked to more severe sexual dysfunction, which can be particularly upsetting for younger women with vaginal atrophy and dyspareunia. A meta-analysis showed that vaginal estrogen appeared to slightly outperform over-the-counter lubricants in bringing back sexual function.
Undiagnosed vaginal or uterine bleeding is a contraindication for vaginal estrogen until the cause has been determined, and providers should use caution in prescribing vaginal estrogen to women with estrogen-dependent neoplasia. Dr. Faubion noted that GSM is common in women with breast cancer, especially if they are receiving endocrine treatments or aromatase inhibitors.
“For women with a hormone-dependent cancer, GSM management depends on each woman’s preference in consultants with her oncologist,” she said. GSM management in women with a nonhormone-dependent cancer, however, is no different than in women without cancer.
DHEA is a steroid that effectively improves vaginal maturation index, vaginal pH, dyspareunia, and vaginal dryness. The most common side effect is vaginal discharge.
Ospemifene, an estrogen agonist available in the United States but not in Canada, is the only oral product approved to treat vaginal dryness and dyspareunia. An observational study also found it effective in reducing recurrent UTIs. The most common side effect is vasomotor symptoms, and it should not be used in patients with breast cancer because it hasn’t been studied in this population.
“This updated information and position statement was needed and will be very clinically relevant in treating midlife women,” Dr. Kling said in an interview. “Dr. Faubion presented a high-level overview of the position statement with clinically relevant points, including treatment for sexual dysfunction related to GSM, GSM treatment in cancer patients, and emphasized the efficacy and low-risk safety profile of low-dose vaginal estrogen, compared to systemic [hormone therapy], for treatment of GSM.”
Dr. Faubion and Dr. Kling disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It’s important for clinicians to ask women whether they are experiencing symptoms of genitourinary syndrome of menopause (GSM) before and after menopause, according to a new statement from the North American Menopause Society.
Stephanie Faubion, MD, MBA, medical director of NAMS, presented the updated statement at the virtual annual meeting of the North American Menopause Society.
“The one thing we tried to emphasize is proactive counseling and proactive inquiry, educating women when they hit perimenopause that this is a thing and that there are treatments,” Dr. Faubion said in an interview.
There’s the misperception that it’s just part of getting old, which it’s not,” said Dr. Faubion, who is also director of the Mayo Clinic Center for Women’s Health in Rochester, Minn., and chair of the department of medicine at the Mayo Clinic in Jacksonville, Fla.
Changes from previous statement
The GSM statement describes the symptoms and signs resulting from estrogen deficiency on the genitourinary tract, Dr. Faubion explained. The biggest change from the earlier version, published in 2013, is the condition’s new name. Formerly known as vulvovaginal atrophy, the condition’s new term was developed in 2014 and is now preferred by NAMS and the American College of Obstetricians and Gynecologists because it’s more comprehensive. Rather than just a physical description of the condition, GSM encompasses the many related symptoms and the urinary tract changes that occur, and it clearly associates the condition with menopause.
“Women don’t always associate these changes with menopause and don’t recognize that there’s something that can be done about it,” Dr. Faubion said. “We like to emphasize that sex should never be painful, but it’s not just about sex. It’s about comfort.”
Other changes include a review of evidence related to vaginal laser therapy for GSM and the availability of Imvexxy vaginal inserts with lower doses (4 mcg and 10 mpg) of estrogen.
Etiology and diagnosis of GSM
The presence of endogenous estrogen keeps the vaginal lining thick, rugated, well vascularized, and lubricated. As estrogen levels decline during postmenopause, the epithelial lining becomes thinner, with reduced blood supply and loss of glycogen.
The most common symptoms of GSM include irritation of the vulva, inadequate vaginal lubrication, burning, dysuria, dyspareunia, and vaginal discharge, but the symptoms may not always correlate with physical findings. In women with surgical menopause, the symptoms tend to be more severe. The most distressing symptoms to women are often those that affect sexual function.
“Clinicians must be proactive in asking menopausal women if GSM symptoms are present, even before menopause begins,” Dr. Faubion said.
Taking a women’s history during evaluation may help identify contributing factors, other causes, or potentially effective treatments based on what has worked in the past. History should include a description of symptoms, their onset and duration, how distressing they are, and their effect on the woman’s quality of life. A sexual history, such as lubricants the woman has used, can also be useful in determining management strategies.
Signs of GSM include labial atrophy, vaginal dryness, introital stenosis, clitoral atrophy, phimosis of the prepuce, reduced mons pubis and labia majora bulk, reduced labia minora tissue and pigmentation, and changes in the urethra, including erythema of the urethral meatus and commonly a urethral caruncle, a benign outgrown of inflammatory tissue that likely results from low estrogen levels and can be treated effectively with topical hormonal therapies.
A diagnosis of GSM requires both physical findings and bothersome symptoms, though not necessarily specific vaginal maturation index or vaginal pH values. The differential diagnosis speaks to the importance of taking a good history: allergic or inflammatory conditions, infection, trauma, presence of a foreign body, malignancy, vulvodynia, chronic pelvic pain, or provoked pelvic floor hypertonia.
If first-line therapies of over-the-counter lubricants do not sufficiently treat GSM, other effective treatments include low-dose vaginal estrogen therapy, systemic estrogen therapy if other menopause symptoms are present, vaginal dehydroepiandrosterone (DHEA), and ospemifene.
Management of GSM
First-line therapy of GSM involves over-the-counter lubricants and moisturizers, which are often adequate to alleviate or eliminate women’s symptoms. However, the panel that developed the statement found no evidence that hyaluronic acid was any more effective than other lubricants or moisturizers, and no herbal products were found to effectively treat GSM.
While emerging evidence suggests that energy-based therapies, such as treatments with vaginal laser or radiofrequency devices, show some promise, more evidence is needed to show safety and efficacy before the panel can recommend routine use.
When over-the-counter therapies are not effective, vaginal estrogen usually relieves GSM with little absorption and is preferred over systemic therapy if GSM is the only bothersome menopausal symptom. Options include topical creams, a slow-release estradiol intravaginal ring, and estradiol vaginal tablets and inserts.
“However, when systemic hormone therapy is needed to treat other menopause symptoms, usually a woman will derive benefit and resolution of the GSM at the same time,” Dr. Faubion said. “However, for some women, additional low-dose vaginal estrogen may be added to systemic estrogen if needed, and that could include vaginal DHEA.”
All the approved vaginal products have shown efficacy, compared with placebo in clinical trials, and a Cochrane review comparing the different therapies found them to be similarly efficacious in treating vaginal dryness and dyspareunia with no significant differences in adverse events.
Preparing patients for the boxed warning
As vaginal estrogen doses are significantly lower than systemic estrogen, their safety profile is better, with serum estrogen levels remaining within the postmenopausal range when low-dose vaginal estrogen therapy is used. That said, some studies have shown that vaginal estrogen cream can be a large enough dose to involve systemic absorption and lead to symptoms such as vaginal bleeding, breast pain, and nausea.
However, package inserts for vaginal estrogen have the same boxed warning as seen in systemic hormone therapy inserts regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and “probable dementia” despite these conditions not being linked to vaginal estrogen in trials. Neither has venous thromboembolism been linked to vaginal estrogen.
“The panel felt it was very important that women be educated about the differences between low-dose vaginal estrogen and systemic estrogen therapy and be prepared for this boxed warning,” Dr. Faubion told attendees. “It’s really important to say: ‘You’re going to get this, it’s going to look scary, and there’s no evidence these same warnings apply to the low-dose vaginal estrogen products.’ ”
This point particularly resonated with NAMS attendee Juliana (Jewel) Kling, MD, MPH, an associate professor of medicine at the Mayo Clinic Arizona, Scottsdale.
“The point about educating women about the differences between low-dose vaginal estrogen products and systemic treatments and being prepared for the boxed warning is important and I hope reaches many practitioners,” Dr. Kling said in an interview.
The panel did not recommend using progestogen with low-dose vaginal estrogen therapy or doing routine endometrial surveillance in women using vaginal estrogen. But endometrial surveillance may be worth considering in women with increased risk of endometrial cancer.
Estrogen insufficiency from premature menopause or primary ovarian insufficiency is linked to more severe sexual dysfunction, which can be particularly upsetting for younger women with vaginal atrophy and dyspareunia. A meta-analysis showed that vaginal estrogen appeared to slightly outperform over-the-counter lubricants in bringing back sexual function.
Undiagnosed vaginal or uterine bleeding is a contraindication for vaginal estrogen until the cause has been determined, and providers should use caution in prescribing vaginal estrogen to women with estrogen-dependent neoplasia. Dr. Faubion noted that GSM is common in women with breast cancer, especially if they are receiving endocrine treatments or aromatase inhibitors.
“For women with a hormone-dependent cancer, GSM management depends on each woman’s preference in consultants with her oncologist,” she said. GSM management in women with a nonhormone-dependent cancer, however, is no different than in women without cancer.
DHEA is a steroid that effectively improves vaginal maturation index, vaginal pH, dyspareunia, and vaginal dryness. The most common side effect is vaginal discharge.
Ospemifene, an estrogen agonist available in the United States but not in Canada, is the only oral product approved to treat vaginal dryness and dyspareunia. An observational study also found it effective in reducing recurrent UTIs. The most common side effect is vasomotor symptoms, and it should not be used in patients with breast cancer because it hasn’t been studied in this population.
“This updated information and position statement was needed and will be very clinically relevant in treating midlife women,” Dr. Kling said in an interview. “Dr. Faubion presented a high-level overview of the position statement with clinically relevant points, including treatment for sexual dysfunction related to GSM, GSM treatment in cancer patients, and emphasized the efficacy and low-risk safety profile of low-dose vaginal estrogen, compared to systemic [hormone therapy], for treatment of GSM.”
Dr. Faubion and Dr. Kling disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It’s important for clinicians to ask women whether they are experiencing symptoms of genitourinary syndrome of menopause (GSM) before and after menopause, according to a new statement from the North American Menopause Society.
Stephanie Faubion, MD, MBA, medical director of NAMS, presented the updated statement at the virtual annual meeting of the North American Menopause Society.
“The one thing we tried to emphasize is proactive counseling and proactive inquiry, educating women when they hit perimenopause that this is a thing and that there are treatments,” Dr. Faubion said in an interview.
There’s the misperception that it’s just part of getting old, which it’s not,” said Dr. Faubion, who is also director of the Mayo Clinic Center for Women’s Health in Rochester, Minn., and chair of the department of medicine at the Mayo Clinic in Jacksonville, Fla.
Changes from previous statement
The GSM statement describes the symptoms and signs resulting from estrogen deficiency on the genitourinary tract, Dr. Faubion explained. The biggest change from the earlier version, published in 2013, is the condition’s new name. Formerly known as vulvovaginal atrophy, the condition’s new term was developed in 2014 and is now preferred by NAMS and the American College of Obstetricians and Gynecologists because it’s more comprehensive. Rather than just a physical description of the condition, GSM encompasses the many related symptoms and the urinary tract changes that occur, and it clearly associates the condition with menopause.
“Women don’t always associate these changes with menopause and don’t recognize that there’s something that can be done about it,” Dr. Faubion said. “We like to emphasize that sex should never be painful, but it’s not just about sex. It’s about comfort.”
Other changes include a review of evidence related to vaginal laser therapy for GSM and the availability of Imvexxy vaginal inserts with lower doses (4 mcg and 10 mpg) of estrogen.
Etiology and diagnosis of GSM
The presence of endogenous estrogen keeps the vaginal lining thick, rugated, well vascularized, and lubricated. As estrogen levels decline during postmenopause, the epithelial lining becomes thinner, with reduced blood supply and loss of glycogen.
The most common symptoms of GSM include irritation of the vulva, inadequate vaginal lubrication, burning, dysuria, dyspareunia, and vaginal discharge, but the symptoms may not always correlate with physical findings. In women with surgical menopause, the symptoms tend to be more severe. The most distressing symptoms to women are often those that affect sexual function.
“Clinicians must be proactive in asking menopausal women if GSM symptoms are present, even before menopause begins,” Dr. Faubion said.
Taking a women’s history during evaluation may help identify contributing factors, other causes, or potentially effective treatments based on what has worked in the past. History should include a description of symptoms, their onset and duration, how distressing they are, and their effect on the woman’s quality of life. A sexual history, such as lubricants the woman has used, can also be useful in determining management strategies.
Signs of GSM include labial atrophy, vaginal dryness, introital stenosis, clitoral atrophy, phimosis of the prepuce, reduced mons pubis and labia majora bulk, reduced labia minora tissue and pigmentation, and changes in the urethra, including erythema of the urethral meatus and commonly a urethral caruncle, a benign outgrown of inflammatory tissue that likely results from low estrogen levels and can be treated effectively with topical hormonal therapies.
A diagnosis of GSM requires both physical findings and bothersome symptoms, though not necessarily specific vaginal maturation index or vaginal pH values. The differential diagnosis speaks to the importance of taking a good history: allergic or inflammatory conditions, infection, trauma, presence of a foreign body, malignancy, vulvodynia, chronic pelvic pain, or provoked pelvic floor hypertonia.
If first-line therapies of over-the-counter lubricants do not sufficiently treat GSM, other effective treatments include low-dose vaginal estrogen therapy, systemic estrogen therapy if other menopause symptoms are present, vaginal dehydroepiandrosterone (DHEA), and ospemifene.
Management of GSM
First-line therapy of GSM involves over-the-counter lubricants and moisturizers, which are often adequate to alleviate or eliminate women’s symptoms. However, the panel that developed the statement found no evidence that hyaluronic acid was any more effective than other lubricants or moisturizers, and no herbal products were found to effectively treat GSM.
While emerging evidence suggests that energy-based therapies, such as treatments with vaginal laser or radiofrequency devices, show some promise, more evidence is needed to show safety and efficacy before the panel can recommend routine use.
When over-the-counter therapies are not effective, vaginal estrogen usually relieves GSM with little absorption and is preferred over systemic therapy if GSM is the only bothersome menopausal symptom. Options include topical creams, a slow-release estradiol intravaginal ring, and estradiol vaginal tablets and inserts.
“However, when systemic hormone therapy is needed to treat other menopause symptoms, usually a woman will derive benefit and resolution of the GSM at the same time,” Dr. Faubion said. “However, for some women, additional low-dose vaginal estrogen may be added to systemic estrogen if needed, and that could include vaginal DHEA.”
All the approved vaginal products have shown efficacy, compared with placebo in clinical trials, and a Cochrane review comparing the different therapies found them to be similarly efficacious in treating vaginal dryness and dyspareunia with no significant differences in adverse events.
Preparing patients for the boxed warning
As vaginal estrogen doses are significantly lower than systemic estrogen, their safety profile is better, with serum estrogen levels remaining within the postmenopausal range when low-dose vaginal estrogen therapy is used. That said, some studies have shown that vaginal estrogen cream can be a large enough dose to involve systemic absorption and lead to symptoms such as vaginal bleeding, breast pain, and nausea.
However, package inserts for vaginal estrogen have the same boxed warning as seen in systemic hormone therapy inserts regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and “probable dementia” despite these conditions not being linked to vaginal estrogen in trials. Neither has venous thromboembolism been linked to vaginal estrogen.
“The panel felt it was very important that women be educated about the differences between low-dose vaginal estrogen and systemic estrogen therapy and be prepared for this boxed warning,” Dr. Faubion told attendees. “It’s really important to say: ‘You’re going to get this, it’s going to look scary, and there’s no evidence these same warnings apply to the low-dose vaginal estrogen products.’ ”
This point particularly resonated with NAMS attendee Juliana (Jewel) Kling, MD, MPH, an associate professor of medicine at the Mayo Clinic Arizona, Scottsdale.
“The point about educating women about the differences between low-dose vaginal estrogen products and systemic treatments and being prepared for the boxed warning is important and I hope reaches many practitioners,” Dr. Kling said in an interview.
The panel did not recommend using progestogen with low-dose vaginal estrogen therapy or doing routine endometrial surveillance in women using vaginal estrogen. But endometrial surveillance may be worth considering in women with increased risk of endometrial cancer.
Estrogen insufficiency from premature menopause or primary ovarian insufficiency is linked to more severe sexual dysfunction, which can be particularly upsetting for younger women with vaginal atrophy and dyspareunia. A meta-analysis showed that vaginal estrogen appeared to slightly outperform over-the-counter lubricants in bringing back sexual function.
Undiagnosed vaginal or uterine bleeding is a contraindication for vaginal estrogen until the cause has been determined, and providers should use caution in prescribing vaginal estrogen to women with estrogen-dependent neoplasia. Dr. Faubion noted that GSM is common in women with breast cancer, especially if they are receiving endocrine treatments or aromatase inhibitors.
“For women with a hormone-dependent cancer, GSM management depends on each woman’s preference in consultants with her oncologist,” she said. GSM management in women with a nonhormone-dependent cancer, however, is no different than in women without cancer.
DHEA is a steroid that effectively improves vaginal maturation index, vaginal pH, dyspareunia, and vaginal dryness. The most common side effect is vaginal discharge.
Ospemifene, an estrogen agonist available in the United States but not in Canada, is the only oral product approved to treat vaginal dryness and dyspareunia. An observational study also found it effective in reducing recurrent UTIs. The most common side effect is vasomotor symptoms, and it should not be used in patients with breast cancer because it hasn’t been studied in this population.
“This updated information and position statement was needed and will be very clinically relevant in treating midlife women,” Dr. Kling said in an interview. “Dr. Faubion presented a high-level overview of the position statement with clinically relevant points, including treatment for sexual dysfunction related to GSM, GSM treatment in cancer patients, and emphasized the efficacy and low-risk safety profile of low-dose vaginal estrogen, compared to systemic [hormone therapy], for treatment of GSM.”
Dr. Faubion and Dr. Kling disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.








