User login
No vascular benefit of testosterone over exercise in aging men
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).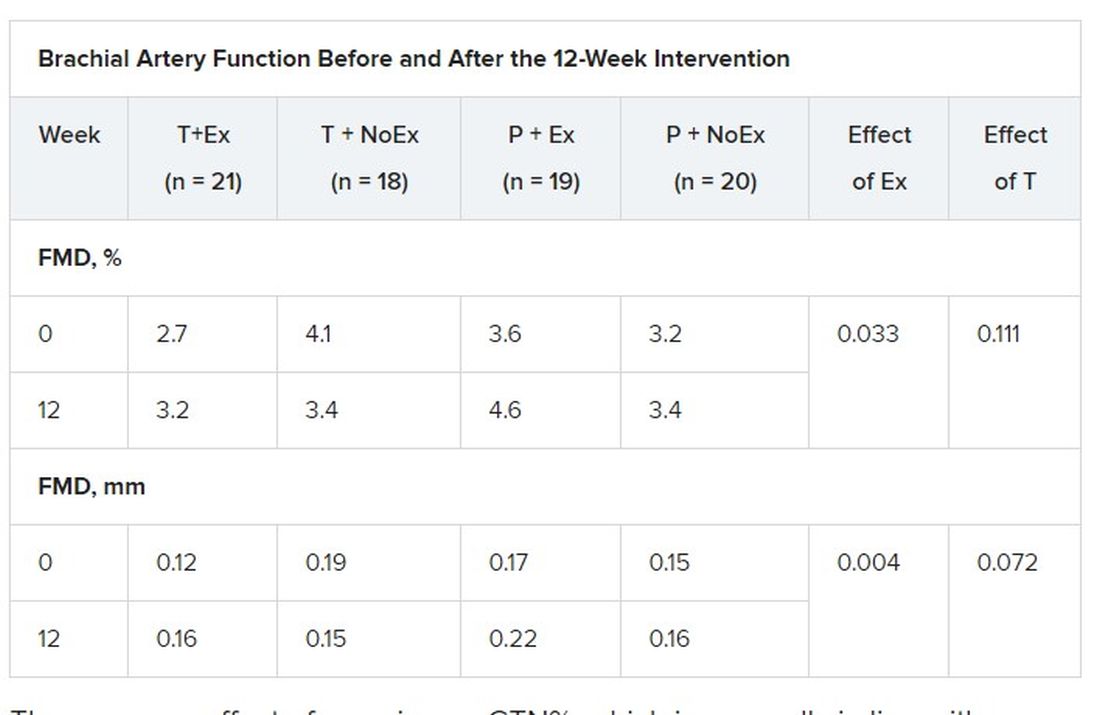
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Heavier girls hit hormonal puberty earlier, but develop breasts later
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
COVID-19 pandemic hinders access to contraception
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
The pandemic has affected reproductive health because of barriers to contraception access, potentially increasing unwanted pregnancies, reported Tracy Kuo Lin, PhD, of the University of California, San Francisco, and associates.
During the pandemic, women have experienced an increased desire to avoid pregnancy, and when that desire is coupled with loss of income, accessing contraception becomes more difficult, Dr. Lin and colleagues observed in a cross-sectional survey published in the journal Contraception.
The study aimed to quantify the effect of COVID-19 on women’s economic status and reproductive health decisions related to childbearing and pregnancy. Women aged 18-49 who resided in the U.S. were targeted via Facebook and Instagram advertisements from May 16 to June 16, 2020. In all, 554 racially diverse respondents from 43 states were selected: 41% aged 18-24, 37% aged 25-34, and 23% aged 35-49.
Income losses affect nearly half of respondents
In determining risk of unwanted pregnancy, the researchers considered the influence of COVID-19 on a number of factors related to economic well-being as well as respondents’ sexual activity, intimate partner violence, overall desire for pregnancy, and access to contraception as issues affecting their interest in becoming pregnant and their ability to access medical care and contraception. Overall, 46% experienced a reduction in income, 43% reported no change, and 10% experienced an increase in income.
Difficulty in being able to afford food, transportation, and housing doubled among respondents from 8% to 16% as a result of the pandemic. The study authors cited education, race/ethnicity, federal poverty level, and change in income as predictors of inability to provide for these basic needs.
A total of 83% of respondents reported having sex within the past month; 54% of those had sex with someone they lived with, compared with 29% who had sex with someone they did not live with. The pandemic had no impact on sexual desire for 37% of respondents, compared with 32% who experienced a decrease in desire, and 29% who experienced more desire for sex. The presence of shelter-in-place orders had no effect on frequency of or desire for sex. Among the respondents, 4% noted intimate partner violence, which increased slightly from 3% before the pandemic.
Among respondents using contraception, the study authors noted that 17% reported greater difficulty accessing birth control during the pandemic compared with 4% who felt access had become easier. Of those citing increased difficulties, 9% noted increasing challenges getting to a pharmacy, 4% were less able to afford birth control, 3% said it had become harder to obtain a prescription, and 1% cited difficulties having long-acting reversible contraceptives removed.
Despite the pandemic’s overall impact on quality of life, 41% of respondents reported a stronger desire to become pregnant, compared with 25% who had a reduced desire, and 34% whose interest in pregnancy was unchanged by the pandemic.
More than one-third of respondents (37%) admitted that COVID-19 contributed to their fears of becoming pregnant while 13% indicated their fear of pregnancy stemmed from concerns over being able to afford the cost of having a child. Not surprisingly, the decrease in desire for pregnancy was twice as high in those who reported they were unable to afford food, transportation, and/or housing compared with those who saw no change in their ability to afford basic needs.
“In these uncertain economic times, it is of utmost importance to create policies that will ensure access to and comprehensive coverage of core sexual and reproductive health services,” Dr. Lin and colleagues urged. “By doing so, we safeguard people’s ability to make decisions that support their reproductive health goals.”
Will COVID-19 drive needed practice and policy changes?
“This study highlights the economic and reproductive health toll of COVID-19 and the pressing need for improved contraception access,” Eve Espey, MD, MPH, said in an interview.
“Ob.gyns. and other practitioners can use this information to consider evidence-based practice changes that incorporate telemedicine visits, extended refills on contraceptive methods, and a focus on postpartum and postabortion initiation of contraception,” noted Dr. Espey, of the University of New Mexico, Albuquerque. For women who are experiencing economic hardship, consulting with state-based programs that offer pharmacy access and online access to contraceptives may offer a reasonable alternative, she added.
The study was funded by the University of California, San Francisco’s National Center of Excellence in Women’s Health. Dr. Lin received funding from Lazarex Cancer Foundation. The remaining authors had no conflicts of interest and reported no disclosures.
FROM CONTRACEPTION
Levonorgestrel IUD effective as emergency contraception
A levonorgestrel 52-mg intrauterine device is noninferior to a copper IUD for emergency contraception, according to randomized trial results published online in the New England Journal of Medicine.
Although the trial didn’t directly compare emergency oral contraception to the hormonal IUD, the authors speculated, on the basis of prior findings, that the levonorgestrel IUD is more effective than oral emergency contraceptive pills. In addition, there is no delay in providing ongoing contraception as there is when ulipristal acetate is used for emergency contraception.
Prior research has found that copper IUDs are a highly effective method of emergency contraception, but studies of the use of other IUDs as emergency contraception have been lacking.
To examine whether the levonorgestrel IUD is noninferior to the copper IUD as emergency contraception after unprotected sexual intercourse during the previous 5 days, David K. Turok, MD, MPH, associate professor of obstetrics and gynecology at the University of Utah Health, Salt Lake City, and colleagues conducted a trial at six Planned Parenthood health centers in Utah.
Researchers enrolled patients between August 2016 and December 2019. Trial sites purchased levonorgestrel 52-mg IUDs (Liletta) and copper T380A IUDs (ParaGard) for the study. The companies that distribute the IUDs were not involved in the trial.
Pregnancy rates were 1 of 317 participants (0.3%) among those who received the levonorgestrel IUD, and 0 of 321 (0%) among those who received the copper IUD. The difference between the two arms was well within the prespecified noninferiority margin of 2.5%.
Adverse event rates were generally similar between the two groups, with 5.2% of participants in the levonorgestrel IUD group seeking medical care in the month after IUD placement, compared with 4.9% in the copper IUD group.
A welcome option
The study “benefits women by allowing us to introduce a new option into the method mix of emergency contraception,” commented Wing Kay Fok, MD, a clinical assistant professor of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in family planning had followed preliminary data from this study and were anticipating the final results. Clinicians who are comfortable placing a copper IUD for emergency contraception are likely to be comfortable placing a levonorgestrel 52-mg IUD, given these data, Dr. Fok said.
“This is definitely – from what we can tell – a more effective method than the pill,” she said.
Gabriela Aguilar, MD, MPH, fellow and clinical instructor in the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., said she is reassured by the data and is prepared to offer the 52-mg levonorgestrel IUD as emergency contraception.
The trial is “an important clinical study that has the ability to significantly change clinical practice,” Dr. Aguilar said. She credited the University of Utah and Planned Parenthood for their roles in it.
“Anytime that there are more options, ideally, that means that access is also increased,” Dr. Aguilar said.
Many patients will still prefer oral emergency contraceptive pills, she said. “But for those who are interested in an IUD ... for the immediate and ongoing birth control after that, now they have the two options instead of just the one IUD option.”
One pregnancy
The trial included women aged 18-35 years who requested emergency contraception after unprotected sexual intercourse within the previous 5 days. Other inclusion criteria were that participants had a desire to initiate use of an IUD; a desire to prevent pregnancy for at least 1 year; a negative result on urine pregnancy testing; a history of regular menstrual cycles; and a known date of the last menstrual period. The investigators did not exclude individuals who had unprotected sexual intercourse more than 5 days before IUD placement.
Participants were unaware of their assigned intervention. The nurse practitioners and certified nurse midwives who performed the IUD insertions were aware of the IUD type.
The primary outcome was pregnancy, as determined by a positive result on urine pregnancy testing 1 month after IUD insertion or by a review of survey and health record data.
One pregnancy “occurred in a participant who reported a single episode of unprotected sexual intercourse 48 hours before IUD placement,” the study authors wrote. “Pregnancy dating by an ultrasound examination at 10 weeks was consistent with conception occurring as a result of an emergency contraception failure. The pregnancy ended in a spontaneous abortion at 10 weeks with the IUD still in place.”
“We hope that providers can begin to deliver this method to everyone who wants and needs it and that people considering both emergency contraception and an ongoing method of birth control know that they now have the option of a hormonal IUD in addition to the nonhormonal, copper IUD,” Dr. Turok said in a news release from Planned Parenthood.
The study used a hormonal IUD manufactured by Liletta; Mirena also manufactures a levonorgestrel 52-mg IUD. The results of the study would apply to Mirena’s product too, according to Planned Parenthood.
“There are various IUDs on the market that are at lower doses, and so those IUDs may not demonstrate similar results,” Dr. Aguilar said.
The research was supported by the National Institutes of Health and the University of Utah. Dr. Turok is the director of surgical services for Planned Parenthood Association of Utah; the trial was conducted at PPAU centers, but Dr. Turok does not work at the sites where the study was conducted. Dr. Turok has consulted for Sebela Pharmaceuticals as the principal investigator for two phase 3 studies that assessed novel IUDs. Dr. Turok and one coauthor received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Fok and Dr. Aguilar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A levonorgestrel 52-mg intrauterine device is noninferior to a copper IUD for emergency contraception, according to randomized trial results published online in the New England Journal of Medicine.
Although the trial didn’t directly compare emergency oral contraception to the hormonal IUD, the authors speculated, on the basis of prior findings, that the levonorgestrel IUD is more effective than oral emergency contraceptive pills. In addition, there is no delay in providing ongoing contraception as there is when ulipristal acetate is used for emergency contraception.
Prior research has found that copper IUDs are a highly effective method of emergency contraception, but studies of the use of other IUDs as emergency contraception have been lacking.
To examine whether the levonorgestrel IUD is noninferior to the copper IUD as emergency contraception after unprotected sexual intercourse during the previous 5 days, David K. Turok, MD, MPH, associate professor of obstetrics and gynecology at the University of Utah Health, Salt Lake City, and colleagues conducted a trial at six Planned Parenthood health centers in Utah.
Researchers enrolled patients between August 2016 and December 2019. Trial sites purchased levonorgestrel 52-mg IUDs (Liletta) and copper T380A IUDs (ParaGard) for the study. The companies that distribute the IUDs were not involved in the trial.
Pregnancy rates were 1 of 317 participants (0.3%) among those who received the levonorgestrel IUD, and 0 of 321 (0%) among those who received the copper IUD. The difference between the two arms was well within the prespecified noninferiority margin of 2.5%.
Adverse event rates were generally similar between the two groups, with 5.2% of participants in the levonorgestrel IUD group seeking medical care in the month after IUD placement, compared with 4.9% in the copper IUD group.
A welcome option
The study “benefits women by allowing us to introduce a new option into the method mix of emergency contraception,” commented Wing Kay Fok, MD, a clinical assistant professor of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in family planning had followed preliminary data from this study and were anticipating the final results. Clinicians who are comfortable placing a copper IUD for emergency contraception are likely to be comfortable placing a levonorgestrel 52-mg IUD, given these data, Dr. Fok said.
“This is definitely – from what we can tell – a more effective method than the pill,” she said.
Gabriela Aguilar, MD, MPH, fellow and clinical instructor in the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., said she is reassured by the data and is prepared to offer the 52-mg levonorgestrel IUD as emergency contraception.
The trial is “an important clinical study that has the ability to significantly change clinical practice,” Dr. Aguilar said. She credited the University of Utah and Planned Parenthood for their roles in it.
“Anytime that there are more options, ideally, that means that access is also increased,” Dr. Aguilar said.
Many patients will still prefer oral emergency contraceptive pills, she said. “But for those who are interested in an IUD ... for the immediate and ongoing birth control after that, now they have the two options instead of just the one IUD option.”
One pregnancy
The trial included women aged 18-35 years who requested emergency contraception after unprotected sexual intercourse within the previous 5 days. Other inclusion criteria were that participants had a desire to initiate use of an IUD; a desire to prevent pregnancy for at least 1 year; a negative result on urine pregnancy testing; a history of regular menstrual cycles; and a known date of the last menstrual period. The investigators did not exclude individuals who had unprotected sexual intercourse more than 5 days before IUD placement.
Participants were unaware of their assigned intervention. The nurse practitioners and certified nurse midwives who performed the IUD insertions were aware of the IUD type.
The primary outcome was pregnancy, as determined by a positive result on urine pregnancy testing 1 month after IUD insertion or by a review of survey and health record data.
One pregnancy “occurred in a participant who reported a single episode of unprotected sexual intercourse 48 hours before IUD placement,” the study authors wrote. “Pregnancy dating by an ultrasound examination at 10 weeks was consistent with conception occurring as a result of an emergency contraception failure. The pregnancy ended in a spontaneous abortion at 10 weeks with the IUD still in place.”
“We hope that providers can begin to deliver this method to everyone who wants and needs it and that people considering both emergency contraception and an ongoing method of birth control know that they now have the option of a hormonal IUD in addition to the nonhormonal, copper IUD,” Dr. Turok said in a news release from Planned Parenthood.
The study used a hormonal IUD manufactured by Liletta; Mirena also manufactures a levonorgestrel 52-mg IUD. The results of the study would apply to Mirena’s product too, according to Planned Parenthood.
“There are various IUDs on the market that are at lower doses, and so those IUDs may not demonstrate similar results,” Dr. Aguilar said.
The research was supported by the National Institutes of Health and the University of Utah. Dr. Turok is the director of surgical services for Planned Parenthood Association of Utah; the trial was conducted at PPAU centers, but Dr. Turok does not work at the sites where the study was conducted. Dr. Turok has consulted for Sebela Pharmaceuticals as the principal investigator for two phase 3 studies that assessed novel IUDs. Dr. Turok and one coauthor received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Fok and Dr. Aguilar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A levonorgestrel 52-mg intrauterine device is noninferior to a copper IUD for emergency contraception, according to randomized trial results published online in the New England Journal of Medicine.
Although the trial didn’t directly compare emergency oral contraception to the hormonal IUD, the authors speculated, on the basis of prior findings, that the levonorgestrel IUD is more effective than oral emergency contraceptive pills. In addition, there is no delay in providing ongoing contraception as there is when ulipristal acetate is used for emergency contraception.
Prior research has found that copper IUDs are a highly effective method of emergency contraception, but studies of the use of other IUDs as emergency contraception have been lacking.
To examine whether the levonorgestrel IUD is noninferior to the copper IUD as emergency contraception after unprotected sexual intercourse during the previous 5 days, David K. Turok, MD, MPH, associate professor of obstetrics and gynecology at the University of Utah Health, Salt Lake City, and colleagues conducted a trial at six Planned Parenthood health centers in Utah.
Researchers enrolled patients between August 2016 and December 2019. Trial sites purchased levonorgestrel 52-mg IUDs (Liletta) and copper T380A IUDs (ParaGard) for the study. The companies that distribute the IUDs were not involved in the trial.
Pregnancy rates were 1 of 317 participants (0.3%) among those who received the levonorgestrel IUD, and 0 of 321 (0%) among those who received the copper IUD. The difference between the two arms was well within the prespecified noninferiority margin of 2.5%.
Adverse event rates were generally similar between the two groups, with 5.2% of participants in the levonorgestrel IUD group seeking medical care in the month after IUD placement, compared with 4.9% in the copper IUD group.
A welcome option
The study “benefits women by allowing us to introduce a new option into the method mix of emergency contraception,” commented Wing Kay Fok, MD, a clinical assistant professor of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in family planning had followed preliminary data from this study and were anticipating the final results. Clinicians who are comfortable placing a copper IUD for emergency contraception are likely to be comfortable placing a levonorgestrel 52-mg IUD, given these data, Dr. Fok said.
“This is definitely – from what we can tell – a more effective method than the pill,” she said.
Gabriela Aguilar, MD, MPH, fellow and clinical instructor in the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., said she is reassured by the data and is prepared to offer the 52-mg levonorgestrel IUD as emergency contraception.
The trial is “an important clinical study that has the ability to significantly change clinical practice,” Dr. Aguilar said. She credited the University of Utah and Planned Parenthood for their roles in it.
“Anytime that there are more options, ideally, that means that access is also increased,” Dr. Aguilar said.
Many patients will still prefer oral emergency contraceptive pills, she said. “But for those who are interested in an IUD ... for the immediate and ongoing birth control after that, now they have the two options instead of just the one IUD option.”
One pregnancy
The trial included women aged 18-35 years who requested emergency contraception after unprotected sexual intercourse within the previous 5 days. Other inclusion criteria were that participants had a desire to initiate use of an IUD; a desire to prevent pregnancy for at least 1 year; a negative result on urine pregnancy testing; a history of regular menstrual cycles; and a known date of the last menstrual period. The investigators did not exclude individuals who had unprotected sexual intercourse more than 5 days before IUD placement.
Participants were unaware of their assigned intervention. The nurse practitioners and certified nurse midwives who performed the IUD insertions were aware of the IUD type.
The primary outcome was pregnancy, as determined by a positive result on urine pregnancy testing 1 month after IUD insertion or by a review of survey and health record data.
One pregnancy “occurred in a participant who reported a single episode of unprotected sexual intercourse 48 hours before IUD placement,” the study authors wrote. “Pregnancy dating by an ultrasound examination at 10 weeks was consistent with conception occurring as a result of an emergency contraception failure. The pregnancy ended in a spontaneous abortion at 10 weeks with the IUD still in place.”
“We hope that providers can begin to deliver this method to everyone who wants and needs it and that people considering both emergency contraception and an ongoing method of birth control know that they now have the option of a hormonal IUD in addition to the nonhormonal, copper IUD,” Dr. Turok said in a news release from Planned Parenthood.
The study used a hormonal IUD manufactured by Liletta; Mirena also manufactures a levonorgestrel 52-mg IUD. The results of the study would apply to Mirena’s product too, according to Planned Parenthood.
“There are various IUDs on the market that are at lower doses, and so those IUDs may not demonstrate similar results,” Dr. Aguilar said.
The research was supported by the National Institutes of Health and the University of Utah. Dr. Turok is the director of surgical services for Planned Parenthood Association of Utah; the trial was conducted at PPAU centers, but Dr. Turok does not work at the sites where the study was conducted. Dr. Turok has consulted for Sebela Pharmaceuticals as the principal investigator for two phase 3 studies that assessed novel IUDs. Dr. Turok and one coauthor received grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Fok and Dr. Aguilar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Product update: Breast biopsy system, tamponade mini-sponge, ovulation prediction device and app
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Updated option for breast biopsy
Hologic announces updates to its Brevera® Breast Biopsy System with CorLumina® Imaging Technology. The Brevera system is designed for use with the manufacturer’s Affirm® Prone biopsy guidance system.
For more information, visit https://www.hologic.com.
“Mini-sponge” device shows potential to treat PPH
During a pilot study, reports Obstetrx, 9 patients, treated at the University Teaching Hospital in Lusaka, Zambia, did not respond to conventional PPH management options after vaginal birth but did respond, with bleeding resolved in 60 seconds and no adverse events, to the XSTAT device. The device was left in place for a mean time of 1 hour, and none of the patients required further surgical procedures or blood transfusions. The initial placement time of XSTAT (mean time to placement, 62 seconds) was faster than times reported for balloon uterine tamponade devices. The pilot study results were published in Obstetrics & Gynecology.
XSTAT is US Food and Drug Administration–approved to treat high-flow arterial bleeding in prehospital trauma settings, and Obstetrx is planning to submit for 510k clearance in 2022, after the conclusion of a follow-up PPH trial in 2021.
For more information, visit: https://www.obstetrx.com/.
Continue to: AI and ovulation prediction...
AI and ovulation prediction
A woman’s fertility window is typically the 5 days leading up to ovulation, with peak fertility in the 2 to 3 days before ovulation. There are other options for measuring that fertile window, including luteinizing hormone (LH) tests; however, Prima-Temp reports that Priya predicts the fertile window an average of 2.6 days before tests for LH. Utilizing continuous core body temperature measurement, Priya detects subtle changes in temperature patterns that occur prior to ovulation. The app portion of the technology stores and analyzes the temperature measurements, for a high-tech fertility alert system that also offers clinical diagnostic support. Potential users of the Priya system are able to sign up to receive it through the product’s website.
For more information, visit: https://www.priyafertility.com.
Oral contraceptives may reduce ovarian and endometrial cancer risk 35 years after discontinuation
At the same time, oral contraceptive use is associated with a short-term increased risk of breast cancer after discontinuation, although the lifetime risk of breast cancer is not significantly different, the researchers found.
The absolute risk of breast cancer after discontinuation is “extremely small” and should be a limited factor when deciding whether to start oral contraceptive pills (OCPs), a doctor said.
The study was conducted by Torgny Karlsson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala (Sweden) University, and colleagues and published online in Cancer Research.
Reinforcing and extending knowledge
“These findings are generally consistent with what is known, but extend that knowledge, most notably by the longer-term follow-up for the cohort,” commented Nancy L. Keating, MD, MPH, professor of health care policy and medicine at Harvard Medical School and a physician at Brigham and Women’s Hospital, both in Boston. “Other studies have also shown that OCPs lower risk of ovarian and endometrial cancer. This study suggests that this protective benefit extends up to 30-35 years after discontinuing OCPs.”
The results “reinforce the message to patients of the protective effect of OCPs on risk of ovarian and endometrial cancer,” Dr. Keating said. “Women concerned about these cancers can be reassured that this protective effect appears to persist for decades after discontinuing use.”
Prior studies have indicated that oral contraceptives may be associated with an increased risk of breast cancer.
In terms of breast cancer risk, the study “again extends follow-up and shows that risk of breast cancer was higher for current and ever users through age 50,” although the lifetime risk was not elevated, Dr. Keating said.
“The counseling regarding the effect on breast cancer is more complex,” she said. “I tell women about the very small increased risk of breast cancer during and immediately after use. Because cancer is very rare among women at the ages when OCPs are typically prescribed, the absolute risk increase is extremely small. This paper adds reassurance that this small increase in risk does not persist.”
For certain patients, the association may be more relevant.
“For most women, this risk is so small that it should be a limited factor in their decision to start OCPs,” Dr. Keating said. “However, for women with a substantially higher risk of breast cancer, or a family history of breast cancer at a young age, the small increased risk of breast cancer during and immediately after OCP use is more relevant, and counseling should include carefully weighing the benefits and harms of OCPs with other forms of contraception (and no contraception).”
Although the protective effects of oral contraceptives on ovarian and endometrial cancer were well known, the study describes long-term outcomes that can further inform patient counseling, said Samuel S. Badalian, MD, PhD, chief of the department of obstetrics and gynecology at Bassett Medical Center in Cooperstown, N.Y., and clinical professor of obstetrics and gynecology at the State University of New York, Syracuse.
“Women with individual or family risk factors of ovarian or endometrial cancers will need to know about the protective effects of oral contraceptives and long-term benefits related with their use (30-35 years after discontinuation),” Dr. Badalian said. “Women with family history of breast cancer need to know that lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Data from the U.K. Biobank
To examine the time-dependent effects between long-term oral contraceptive use and cancer risk, the researchers examined data from 256,661 women from the U.K. Biobank who were born between 1939 and 1970. The researchers identified cancer diagnoses using information from national registers and self-reported data until March 2019.
Of the women included in the study, 82% had used or still were using oral contraceptives, whereas 18% had never used oral contraceptives. Overall, ever users were younger, more frequently smokers, and had a lower body mass index, compared with never users. Most women started using oral contraceptives between 1969 and 1978. Last use of oral contraceptives occurred on average 10.7 years after starting.
The researchers adjusted for covariates and used logistic regression analyses to measure the cumulative risk of cancer. They used Cox regression analysis to examine instantaneous risk, measured using hazard ratios.
In all, there were 17,739 cases of breast cancer (6.9%), 1,966 cases of ovarian cancer (0.76%), and 2,462 cases of endometrial cancer (0.96%).
Among ever users, the likelihood of ovarian cancer (OR, 0.72) and endometrial cancer (OR, 0.68) was lower, compared with never users. “However, we did not see a significant association between oral contraceptive use and breast cancer” for the study period as a whole, the researchers reported. When the researchers limited follow-up to age 50 years, however, the odds ratio for breast cancer was increased (OR, 1.09).
“Surprisingly, we only found a small increased risk of breast cancer among oral contraceptive users, and the increased risk disappeared within a few years after discontinuation,” Åsa Johansson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala University and one of the study authors, said in a news release. “Our results suggest that the lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Oral contraceptives today typically use lower doses of estrogen and other types of progesterone, compared with formulas commonly used when participants in the study started taking them, so the results may not directly apply to patients currently taking oral contraceptives, the researchers noted.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Kjell and Märta Beijers, the Marcus Borgström, the Åke Wiberg, and the A and M Rudbergs foundations. The authors, Dr. Keating, and Dr. Badalian had no conflicts of interest.
At the same time, oral contraceptive use is associated with a short-term increased risk of breast cancer after discontinuation, although the lifetime risk of breast cancer is not significantly different, the researchers found.
The absolute risk of breast cancer after discontinuation is “extremely small” and should be a limited factor when deciding whether to start oral contraceptive pills (OCPs), a doctor said.
The study was conducted by Torgny Karlsson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala (Sweden) University, and colleagues and published online in Cancer Research.
Reinforcing and extending knowledge
“These findings are generally consistent with what is known, but extend that knowledge, most notably by the longer-term follow-up for the cohort,” commented Nancy L. Keating, MD, MPH, professor of health care policy and medicine at Harvard Medical School and a physician at Brigham and Women’s Hospital, both in Boston. “Other studies have also shown that OCPs lower risk of ovarian and endometrial cancer. This study suggests that this protective benefit extends up to 30-35 years after discontinuing OCPs.”
The results “reinforce the message to patients of the protective effect of OCPs on risk of ovarian and endometrial cancer,” Dr. Keating said. “Women concerned about these cancers can be reassured that this protective effect appears to persist for decades after discontinuing use.”
Prior studies have indicated that oral contraceptives may be associated with an increased risk of breast cancer.
In terms of breast cancer risk, the study “again extends follow-up and shows that risk of breast cancer was higher for current and ever users through age 50,” although the lifetime risk was not elevated, Dr. Keating said.
“The counseling regarding the effect on breast cancer is more complex,” she said. “I tell women about the very small increased risk of breast cancer during and immediately after use. Because cancer is very rare among women at the ages when OCPs are typically prescribed, the absolute risk increase is extremely small. This paper adds reassurance that this small increase in risk does not persist.”
For certain patients, the association may be more relevant.
“For most women, this risk is so small that it should be a limited factor in their decision to start OCPs,” Dr. Keating said. “However, for women with a substantially higher risk of breast cancer, or a family history of breast cancer at a young age, the small increased risk of breast cancer during and immediately after OCP use is more relevant, and counseling should include carefully weighing the benefits and harms of OCPs with other forms of contraception (and no contraception).”
Although the protective effects of oral contraceptives on ovarian and endometrial cancer were well known, the study describes long-term outcomes that can further inform patient counseling, said Samuel S. Badalian, MD, PhD, chief of the department of obstetrics and gynecology at Bassett Medical Center in Cooperstown, N.Y., and clinical professor of obstetrics and gynecology at the State University of New York, Syracuse.
“Women with individual or family risk factors of ovarian or endometrial cancers will need to know about the protective effects of oral contraceptives and long-term benefits related with their use (30-35 years after discontinuation),” Dr. Badalian said. “Women with family history of breast cancer need to know that lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Data from the U.K. Biobank
To examine the time-dependent effects between long-term oral contraceptive use and cancer risk, the researchers examined data from 256,661 women from the U.K. Biobank who were born between 1939 and 1970. The researchers identified cancer diagnoses using information from national registers and self-reported data until March 2019.
Of the women included in the study, 82% had used or still were using oral contraceptives, whereas 18% had never used oral contraceptives. Overall, ever users were younger, more frequently smokers, and had a lower body mass index, compared with never users. Most women started using oral contraceptives between 1969 and 1978. Last use of oral contraceptives occurred on average 10.7 years after starting.
The researchers adjusted for covariates and used logistic regression analyses to measure the cumulative risk of cancer. They used Cox regression analysis to examine instantaneous risk, measured using hazard ratios.
In all, there were 17,739 cases of breast cancer (6.9%), 1,966 cases of ovarian cancer (0.76%), and 2,462 cases of endometrial cancer (0.96%).
Among ever users, the likelihood of ovarian cancer (OR, 0.72) and endometrial cancer (OR, 0.68) was lower, compared with never users. “However, we did not see a significant association between oral contraceptive use and breast cancer” for the study period as a whole, the researchers reported. When the researchers limited follow-up to age 50 years, however, the odds ratio for breast cancer was increased (OR, 1.09).
“Surprisingly, we only found a small increased risk of breast cancer among oral contraceptive users, and the increased risk disappeared within a few years after discontinuation,” Åsa Johansson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala University and one of the study authors, said in a news release. “Our results suggest that the lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Oral contraceptives today typically use lower doses of estrogen and other types of progesterone, compared with formulas commonly used when participants in the study started taking them, so the results may not directly apply to patients currently taking oral contraceptives, the researchers noted.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Kjell and Märta Beijers, the Marcus Borgström, the Åke Wiberg, and the A and M Rudbergs foundations. The authors, Dr. Keating, and Dr. Badalian had no conflicts of interest.
At the same time, oral contraceptive use is associated with a short-term increased risk of breast cancer after discontinuation, although the lifetime risk of breast cancer is not significantly different, the researchers found.
The absolute risk of breast cancer after discontinuation is “extremely small” and should be a limited factor when deciding whether to start oral contraceptive pills (OCPs), a doctor said.
The study was conducted by Torgny Karlsson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala (Sweden) University, and colleagues and published online in Cancer Research.
Reinforcing and extending knowledge
“These findings are generally consistent with what is known, but extend that knowledge, most notably by the longer-term follow-up for the cohort,” commented Nancy L. Keating, MD, MPH, professor of health care policy and medicine at Harvard Medical School and a physician at Brigham and Women’s Hospital, both in Boston. “Other studies have also shown that OCPs lower risk of ovarian and endometrial cancer. This study suggests that this protective benefit extends up to 30-35 years after discontinuing OCPs.”
The results “reinforce the message to patients of the protective effect of OCPs on risk of ovarian and endometrial cancer,” Dr. Keating said. “Women concerned about these cancers can be reassured that this protective effect appears to persist for decades after discontinuing use.”
Prior studies have indicated that oral contraceptives may be associated with an increased risk of breast cancer.
In terms of breast cancer risk, the study “again extends follow-up and shows that risk of breast cancer was higher for current and ever users through age 50,” although the lifetime risk was not elevated, Dr. Keating said.
“The counseling regarding the effect on breast cancer is more complex,” she said. “I tell women about the very small increased risk of breast cancer during and immediately after use. Because cancer is very rare among women at the ages when OCPs are typically prescribed, the absolute risk increase is extremely small. This paper adds reassurance that this small increase in risk does not persist.”
For certain patients, the association may be more relevant.
“For most women, this risk is so small that it should be a limited factor in their decision to start OCPs,” Dr. Keating said. “However, for women with a substantially higher risk of breast cancer, or a family history of breast cancer at a young age, the small increased risk of breast cancer during and immediately after OCP use is more relevant, and counseling should include carefully weighing the benefits and harms of OCPs with other forms of contraception (and no contraception).”
Although the protective effects of oral contraceptives on ovarian and endometrial cancer were well known, the study describes long-term outcomes that can further inform patient counseling, said Samuel S. Badalian, MD, PhD, chief of the department of obstetrics and gynecology at Bassett Medical Center in Cooperstown, N.Y., and clinical professor of obstetrics and gynecology at the State University of New York, Syracuse.
“Women with individual or family risk factors of ovarian or endometrial cancers will need to know about the protective effects of oral contraceptives and long-term benefits related with their use (30-35 years after discontinuation),” Dr. Badalian said. “Women with family history of breast cancer need to know that lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Data from the U.K. Biobank
To examine the time-dependent effects between long-term oral contraceptive use and cancer risk, the researchers examined data from 256,661 women from the U.K. Biobank who were born between 1939 and 1970. The researchers identified cancer diagnoses using information from national registers and self-reported data until March 2019.
Of the women included in the study, 82% had used or still were using oral contraceptives, whereas 18% had never used oral contraceptives. Overall, ever users were younger, more frequently smokers, and had a lower body mass index, compared with never users. Most women started using oral contraceptives between 1969 and 1978. Last use of oral contraceptives occurred on average 10.7 years after starting.
The researchers adjusted for covariates and used logistic regression analyses to measure the cumulative risk of cancer. They used Cox regression analysis to examine instantaneous risk, measured using hazard ratios.
In all, there were 17,739 cases of breast cancer (6.9%), 1,966 cases of ovarian cancer (0.76%), and 2,462 cases of endometrial cancer (0.96%).
Among ever users, the likelihood of ovarian cancer (OR, 0.72) and endometrial cancer (OR, 0.68) was lower, compared with never users. “However, we did not see a significant association between oral contraceptive use and breast cancer” for the study period as a whole, the researchers reported. When the researchers limited follow-up to age 50 years, however, the odds ratio for breast cancer was increased (OR, 1.09).
“Surprisingly, we only found a small increased risk of breast cancer among oral contraceptive users, and the increased risk disappeared within a few years after discontinuation,” Åsa Johansson, PhD, a researcher in the department of immunology, genetics, and pathology at Uppsala University and one of the study authors, said in a news release. “Our results suggest that the lifetime risk of breast cancer might not differ between ever and never users, even if there is an increased short-term risk.”
Oral contraceptives today typically use lower doses of estrogen and other types of progesterone, compared with formulas commonly used when participants in the study started taking them, so the results may not directly apply to patients currently taking oral contraceptives, the researchers noted.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Kjell and Märta Beijers, the Marcus Borgström, the Åke Wiberg, and the A and M Rudbergs foundations. The authors, Dr. Keating, and Dr. Badalian had no conflicts of interest.
FROM CANCER RESEARCH
Gestational diabetes carries CVD risk years later
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
Women who’ve had gestational diabetes are 40% more likely to develop coronary artery calcification later in life than are women haven’t, and attaining normal glycemic levels doesn’t diminish their midlife risk for atherosclerotic cardiovascular disease.
“The new finding from this study is that women with gestational diabetes had twice the risk of coronary artery calcium, compared to women who never had gestational diabetes, even though both groups attained normal blood sugar levels many years after pregnancy,” lead author Erica P. Gunderson, PhD, MS, MPH, said in an interview about a community-based prospective cohort study of young adults followed for up to 25 years, which was published in Circulation (2021 Feb 1. doi: 10.1161/CIRCULATIONAHA.120.047320).
Previous studies have reported a higher risk of heart disease in women who had gestational diabetes (GD) and later developed type 2 diabetes, but they didn’t elucidate whether that risk carried over in GD patients whose glycemic levels were normal after pregnancy. In 2018, the American College of Cardiology/American Heart Association Cholesterol Clinical Practice Guidelines specified that a history of GD increases women’s risk for coronary artery calcification (CAC).
This study analyzed data of 1,133 women ages 18-30 enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) study who had no diabetes in the baseline years of 1985-1986 and had given birth at least once in the ensuing 25 years. They had glucose tolerance testing at baseline and up to five times through the study period, along with evaluation for GD status and coronary artery calcification CAC measurements at least once at years 15, 20 and 25 (2001-2011).
CARDIA enrolled 5,155 young Black and White men and women ages 18-30 from four distinct geographic areas: Birmingham, Ala.; Chicago; Minneapolis; and Oakland, Calif. About 52% of the study population was Black.
Of the women who’d given birth, 139 (12%) had GD. Their average age at follow-up was 47.6 years, and 25% of the GD patients (34) had CAC, compared with 15% (149/994) in the non-GD group.
Dr. Gunderson noted that the same relative risk for CAC applied to women who had GD and went on to develop prediabetes or were diagnosed with type 2 diabetes during follow-up.
Risks persist even in normoglycemia
In the GD group, the adjusted hazard ratio for having CAC with normoglycemia was 2.3 (95% confidence interval, 1.34-4.09). The researchers also calculated HRs for prediabetes and incident diabetes: 1.5 (95% CI, 1.06-2.24) in no-GD and 2.1 (95% CI, 1.09-4.17) for GD for prediabetes; and 2.2 (95% CI, 1.3-3.62) and 2.02 (95% CI, 0.98-4.19), respectively, for incident diabetes (P = .003).
“This means the risk of heart disease may be increased substantially in women with a history of gestational diabetes and may not diminish even if their blood-sugar levels remain normal for years later,” said Dr. Gunderson, an epidemiologist and senior research scientist at the Kaiser Permanente Northern California Division of Research in Oakland.
“The clinical implications of our findings are that women with previous GD may benefit from enhanced traditional CVD [cardiovascular disease] risk factor testing – i.e., for hypertension, dyslipidemia, and hyperinsulinemia,” Dr. Gunderson said. “Our findings also suggest that it could be beneficial to incorporate history of GD into risk calculators to improve CVD risk stratification and prevention.”
Strong findings argue for more frequent CVD screening
These study results may be the strongest data to date on the long-term effects of GD, said Prakash Deedwania, MD, professor of cardiology at the University of California, San Francisco. “It’s the strongest in the sense in that it’s sponsored, involved four different communities in different parts of the United States, enrolled individuals when they were young and followed them, and saw very few patients drop out for such a long-term study.” The study reported follow-up data on 72% of patients at 25 years, a rate Dr. Deedwania noted was “excellent.”
“Patients who have had GD should be screened aggressively – for not only diabetes, but other cardiovascular risk factors – early on to minimize the subsequent risk of cardiovascular disease is a very important point of this study,” he added. In the absence of a clinical guideline, Dr. Deedwania suggested women with GD might have screening for CV risk factors every 5-7 years depending on their risk profile, but emphasized that parameter isn’t settled.
Future research should focus on the link between GD and CVD risk, Dr. Gunderson said. “Research is needed to better characterize the severity of GD in relation to CVD outcomes, and to identify critical pregnancy-related periods to modify cardiometabolic risk.” The latter would include life-course studies across the full pregnancy continuum from preconception to lactation. “Interventions for primary prevention of CVD and the importance of modifiable lifestyle behaviors with the highest relevance to reduce both diabetes and CVD risks during the first year post partum merit increased research investigation,” she added.
Future studies might also explore the role of inflammation in the GD-CVD relationship, Dr. Deedwania said. “My hypothesis is, and it’s purely a hypothesis, that perhaps the presence of coronary artery calcification scores score in these individuals who were described as having normal glucose but who could be at risk could very well be related to the beginning of inflammation.”
Dr. Gunderson and Dr. Deedwania have no financial relationships to disclose. The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
FROM CIRCULATION
Novel oral testosterone replacement therapy headed to FDA
Marius Pharmaceuticals has submitted a new drug application (NDA) to the Food and Drug Administration for Kyzatrex, an oral testosterone replacement therapy (TRT).
With this NDA, the company is seeking approval for Kyzatrex as a treatment for adult men with primary and secondary hypogonadism, also known as testosterone deficiency. Marius has requested a priority review that, if accepted, would result in an anticipated 6-month review period.
Current treatment options for hypogonadal men consist of therapies with safety concerns, such as cardiovascular and metabolic risks, that make patient adherence to treatment very low.
Kyzatrex is a novel oral formulation of testosterone undecanoate administered twice daily in a soft gelatin capsule.
“TRT remains a therapeutic challenge because there are worrisome and conflicting data related to increased cardiovascular disease risk, which has special relevance to high-risk diabetic populations,” Paul S. Jellinger, MD, professor of clinical medicine at the University of Miami, told this news organization. Furthermore, “injectable depot testosterone may be associated with peak supraphysiological levels and a substantial increase in hemoglobin. Topical testosterone offers more stable levels without a peak and trough, but in some men achieving physiologic levels may be difficult.”
The NDA is supported by results from a 6-month treatment extension of the pivotal phase 3 MRS-TU-2019 study (NCT04467697). Final results from this study have not been presented, but the company wrote in a press release that the results will be published some time in 2021.
They further reported that Kyzatrex was well tolerated by patients, with more than 96% of study participants completing 90 days of treatment in the pivotal phase 3 study. Study patients achieved average testosterone levels in the normal range.
Across the pooled phase 3 trials, the most frequent treatment-related treatment-emergent adverse event (TEAE) was hypertension, and no serious TEAEs were considered treatment related.
“We are extremely proud to have generated compelling efficacy and safety data in our phase 3 trials,” said Om Dhingra, PhD, cofounder and CEO of Marius. “We look forward to continuing to work collaboratively with the FDA on the review of our application, and if approved, Kyzatrex has the potential to become the standard of care for the treatment of primary and secondary hypogonadism globally.”
“An oral [testosterone] preparation with steady state physiologic levels would be a welcome addition to our choices for therapy assuming, of course, the absence of adverse effects,” explained Dr. Jellinger. “However, the greater challenge of testosterone therapy is the appropriate selection of those suited for testosterone replacement therapy.”
The company also plans to submit a marketing authorization application with the European Medicines Agency in the first half of 2022.
Marius Pharmaceuticals has submitted a new drug application (NDA) to the Food and Drug Administration for Kyzatrex, an oral testosterone replacement therapy (TRT).
With this NDA, the company is seeking approval for Kyzatrex as a treatment for adult men with primary and secondary hypogonadism, also known as testosterone deficiency. Marius has requested a priority review that, if accepted, would result in an anticipated 6-month review period.
Current treatment options for hypogonadal men consist of therapies with safety concerns, such as cardiovascular and metabolic risks, that make patient adherence to treatment very low.
Kyzatrex is a novel oral formulation of testosterone undecanoate administered twice daily in a soft gelatin capsule.
“TRT remains a therapeutic challenge because there are worrisome and conflicting data related to increased cardiovascular disease risk, which has special relevance to high-risk diabetic populations,” Paul S. Jellinger, MD, professor of clinical medicine at the University of Miami, told this news organization. Furthermore, “injectable depot testosterone may be associated with peak supraphysiological levels and a substantial increase in hemoglobin. Topical testosterone offers more stable levels without a peak and trough, but in some men achieving physiologic levels may be difficult.”
The NDA is supported by results from a 6-month treatment extension of the pivotal phase 3 MRS-TU-2019 study (NCT04467697). Final results from this study have not been presented, but the company wrote in a press release that the results will be published some time in 2021.
They further reported that Kyzatrex was well tolerated by patients, with more than 96% of study participants completing 90 days of treatment in the pivotal phase 3 study. Study patients achieved average testosterone levels in the normal range.
Across the pooled phase 3 trials, the most frequent treatment-related treatment-emergent adverse event (TEAE) was hypertension, and no serious TEAEs were considered treatment related.
“We are extremely proud to have generated compelling efficacy and safety data in our phase 3 trials,” said Om Dhingra, PhD, cofounder and CEO of Marius. “We look forward to continuing to work collaboratively with the FDA on the review of our application, and if approved, Kyzatrex has the potential to become the standard of care for the treatment of primary and secondary hypogonadism globally.”
“An oral [testosterone] preparation with steady state physiologic levels would be a welcome addition to our choices for therapy assuming, of course, the absence of adverse effects,” explained Dr. Jellinger. “However, the greater challenge of testosterone therapy is the appropriate selection of those suited for testosterone replacement therapy.”
The company also plans to submit a marketing authorization application with the European Medicines Agency in the first half of 2022.
Marius Pharmaceuticals has submitted a new drug application (NDA) to the Food and Drug Administration for Kyzatrex, an oral testosterone replacement therapy (TRT).
With this NDA, the company is seeking approval for Kyzatrex as a treatment for adult men with primary and secondary hypogonadism, also known as testosterone deficiency. Marius has requested a priority review that, if accepted, would result in an anticipated 6-month review period.
Current treatment options for hypogonadal men consist of therapies with safety concerns, such as cardiovascular and metabolic risks, that make patient adherence to treatment very low.
Kyzatrex is a novel oral formulation of testosterone undecanoate administered twice daily in a soft gelatin capsule.
“TRT remains a therapeutic challenge because there are worrisome and conflicting data related to increased cardiovascular disease risk, which has special relevance to high-risk diabetic populations,” Paul S. Jellinger, MD, professor of clinical medicine at the University of Miami, told this news organization. Furthermore, “injectable depot testosterone may be associated with peak supraphysiological levels and a substantial increase in hemoglobin. Topical testosterone offers more stable levels without a peak and trough, but in some men achieving physiologic levels may be difficult.”
The NDA is supported by results from a 6-month treatment extension of the pivotal phase 3 MRS-TU-2019 study (NCT04467697). Final results from this study have not been presented, but the company wrote in a press release that the results will be published some time in 2021.
They further reported that Kyzatrex was well tolerated by patients, with more than 96% of study participants completing 90 days of treatment in the pivotal phase 3 study. Study patients achieved average testosterone levels in the normal range.
Across the pooled phase 3 trials, the most frequent treatment-related treatment-emergent adverse event (TEAE) was hypertension, and no serious TEAEs were considered treatment related.
“We are extremely proud to have generated compelling efficacy and safety data in our phase 3 trials,” said Om Dhingra, PhD, cofounder and CEO of Marius. “We look forward to continuing to work collaboratively with the FDA on the review of our application, and if approved, Kyzatrex has the potential to become the standard of care for the treatment of primary and secondary hypogonadism globally.”
“An oral [testosterone] preparation with steady state physiologic levels would be a welcome addition to our choices for therapy assuming, of course, the absence of adverse effects,” explained Dr. Jellinger. “However, the greater challenge of testosterone therapy is the appropriate selection of those suited for testosterone replacement therapy.”
The company also plans to submit a marketing authorization application with the European Medicines Agency in the first half of 2022.
Menopause, not aging, may influence brain volume
Postmenopausal women not only have larger brain volume than women who are premenopausal, but they also experience larger reductions in brain volume over time, reported Ananthan Ambikairajah of the Centre for Research on Ageing, Health and Wellbeing, Australian National University, Canberra, and associates. Their report was published in Menopause.
In this large population-based cohort of 5,072 women aged 37-73 years, the goal of the study was to look at links between brain volume and measures of menstruation history, such as menopausal status, age at menopause, age at menarche, and the duration of a woman’s reproductive stage, but to do so within the context of how it relates to dementia prevalence. Citing a study in The Lancet Neurology, the authors noted that the age-standardized prevalence for dementia is 17% higher in women than in men, and they speculated that it may be important to look beyond age for answers.
What about menstrual history and Alzheimer’s disease?
According to the Framingham Study in Neurology, the remaining lifetime risk of Alzheimer’s disease (AD) is nearly double for a 65-year-old woman (12%) compared with a 65-year-old man (6.3%), leading Mr. Ambikairajah and associates to conclude that “menstruation history may also be particularly relevant, given that it is unique to female aging.” They further speculated, citing several related studies, that because AD pathology is initiated decades prior to the onset of clinical signs, menstruation history and its effects on brain health may, in fact, be reflected in brain volume.
Postmenopausal women had 0.82% and 1.33% larger total brain and hippocampal volume, respectively, compared with premenopausal women. Postmenopausal women had a 23% greater decrease in total brain volume but not in hippocampal volume over time, compared with premenopausal women.
As Braak and Braak illustrated in Acta Neuropathologica, chronic inflammation has been linked to brain shrinkage “consistent with the pattern of results in the present study,” Mr. Ambikairajah and colleagues noted, adding that longitudinal neuroimaging/biomarker studies are needed to explore this further.
What made this study unique was its ability to match pre- and postmenopausal women for age, a critically important attribute “given that aging and menopause both progress concurrently, which can make it difficult to determine the individual contribution of each for measures of brain health,” the authors explained.
In an interview, Constance Bohon, MD, an ob.gyn. in private practice and assistant clinical professor, George Washington University, Washington, observed: “The conclusion [in this study] is that an early age of menarche, delayed age of menopause and increased duration of the reproductive stage is negatively associated with brain volume.”
What of the neuroprotective effects of endogenous estrogen?
“Their findings are not consistent with a neuroprotective effect of endogenous estrogen exposure on brain volume,” she noted, adding that the study “did not assess the effect of exogenous estrogen on brain volume. Neither was the effect of exogenous or endogenous estrogen on cerebral blood flow assessed. In a study published in Obstetrics & Gynecology, the conclusion was that oophorectomy before the age of natural menopause is associated with a decrease in cognitive impairment and dementia. There was no assessment of brain volume or cerebral blood flow. Likewise in a report published in Neurobiology of Aging, Maki P and Resnick S M. concluded that estrogen helps maintain hippocampal and prefrontal function as women age,” observed Dr. Bohon, noting that the study did not assess brain volume.
“It is unclear whether the most predictive assessment for worsening cognition and dementia is the finding of decreased total brain volume, decreased hippocampal volume, or decreased cerebral blood flow. The effect of both endogenous and exogenous estrogen on the risk for dementia needs further evaluation,” she cautioned.
Mr. Ambikairajah cited one financial disclosure; the remaining contributors had no relevant disclosures.
Postmenopausal women not only have larger brain volume than women who are premenopausal, but they also experience larger reductions in brain volume over time, reported Ananthan Ambikairajah of the Centre for Research on Ageing, Health and Wellbeing, Australian National University, Canberra, and associates. Their report was published in Menopause.
In this large population-based cohort of 5,072 women aged 37-73 years, the goal of the study was to look at links between brain volume and measures of menstruation history, such as menopausal status, age at menopause, age at menarche, and the duration of a woman’s reproductive stage, but to do so within the context of how it relates to dementia prevalence. Citing a study in The Lancet Neurology, the authors noted that the age-standardized prevalence for dementia is 17% higher in women than in men, and they speculated that it may be important to look beyond age for answers.
What about menstrual history and Alzheimer’s disease?
According to the Framingham Study in Neurology, the remaining lifetime risk of Alzheimer’s disease (AD) is nearly double for a 65-year-old woman (12%) compared with a 65-year-old man (6.3%), leading Mr. Ambikairajah and associates to conclude that “menstruation history may also be particularly relevant, given that it is unique to female aging.” They further speculated, citing several related studies, that because AD pathology is initiated decades prior to the onset of clinical signs, menstruation history and its effects on brain health may, in fact, be reflected in brain volume.
Postmenopausal women had 0.82% and 1.33% larger total brain and hippocampal volume, respectively, compared with premenopausal women. Postmenopausal women had a 23% greater decrease in total brain volume but not in hippocampal volume over time, compared with premenopausal women.
As Braak and Braak illustrated in Acta Neuropathologica, chronic inflammation has been linked to brain shrinkage “consistent with the pattern of results in the present study,” Mr. Ambikairajah and colleagues noted, adding that longitudinal neuroimaging/biomarker studies are needed to explore this further.
What made this study unique was its ability to match pre- and postmenopausal women for age, a critically important attribute “given that aging and menopause both progress concurrently, which can make it difficult to determine the individual contribution of each for measures of brain health,” the authors explained.
In an interview, Constance Bohon, MD, an ob.gyn. in private practice and assistant clinical professor, George Washington University, Washington, observed: “The conclusion [in this study] is that an early age of menarche, delayed age of menopause and increased duration of the reproductive stage is negatively associated with brain volume.”
What of the neuroprotective effects of endogenous estrogen?
“Their findings are not consistent with a neuroprotective effect of endogenous estrogen exposure on brain volume,” she noted, adding that the study “did not assess the effect of exogenous estrogen on brain volume. Neither was the effect of exogenous or endogenous estrogen on cerebral blood flow assessed. In a study published in Obstetrics & Gynecology, the conclusion was that oophorectomy before the age of natural menopause is associated with a decrease in cognitive impairment and dementia. There was no assessment of brain volume or cerebral blood flow. Likewise in a report published in Neurobiology of Aging, Maki P and Resnick S M. concluded that estrogen helps maintain hippocampal and prefrontal function as women age,” observed Dr. Bohon, noting that the study did not assess brain volume.
“It is unclear whether the most predictive assessment for worsening cognition and dementia is the finding of decreased total brain volume, decreased hippocampal volume, or decreased cerebral blood flow. The effect of both endogenous and exogenous estrogen on the risk for dementia needs further evaluation,” she cautioned.
Mr. Ambikairajah cited one financial disclosure; the remaining contributors had no relevant disclosures.
Postmenopausal women not only have larger brain volume than women who are premenopausal, but they also experience larger reductions in brain volume over time, reported Ananthan Ambikairajah of the Centre for Research on Ageing, Health and Wellbeing, Australian National University, Canberra, and associates. Their report was published in Menopause.
In this large population-based cohort of 5,072 women aged 37-73 years, the goal of the study was to look at links between brain volume and measures of menstruation history, such as menopausal status, age at menopause, age at menarche, and the duration of a woman’s reproductive stage, but to do so within the context of how it relates to dementia prevalence. Citing a study in The Lancet Neurology, the authors noted that the age-standardized prevalence for dementia is 17% higher in women than in men, and they speculated that it may be important to look beyond age for answers.
What about menstrual history and Alzheimer’s disease?
According to the Framingham Study in Neurology, the remaining lifetime risk of Alzheimer’s disease (AD) is nearly double for a 65-year-old woman (12%) compared with a 65-year-old man (6.3%), leading Mr. Ambikairajah and associates to conclude that “menstruation history may also be particularly relevant, given that it is unique to female aging.” They further speculated, citing several related studies, that because AD pathology is initiated decades prior to the onset of clinical signs, menstruation history and its effects on brain health may, in fact, be reflected in brain volume.
Postmenopausal women had 0.82% and 1.33% larger total brain and hippocampal volume, respectively, compared with premenopausal women. Postmenopausal women had a 23% greater decrease in total brain volume but not in hippocampal volume over time, compared with premenopausal women.
As Braak and Braak illustrated in Acta Neuropathologica, chronic inflammation has been linked to brain shrinkage “consistent with the pattern of results in the present study,” Mr. Ambikairajah and colleagues noted, adding that longitudinal neuroimaging/biomarker studies are needed to explore this further.
What made this study unique was its ability to match pre- and postmenopausal women for age, a critically important attribute “given that aging and menopause both progress concurrently, which can make it difficult to determine the individual contribution of each for measures of brain health,” the authors explained.
In an interview, Constance Bohon, MD, an ob.gyn. in private practice and assistant clinical professor, George Washington University, Washington, observed: “The conclusion [in this study] is that an early age of menarche, delayed age of menopause and increased duration of the reproductive stage is negatively associated with brain volume.”
What of the neuroprotective effects of endogenous estrogen?
“Their findings are not consistent with a neuroprotective effect of endogenous estrogen exposure on brain volume,” she noted, adding that the study “did not assess the effect of exogenous estrogen on brain volume. Neither was the effect of exogenous or endogenous estrogen on cerebral blood flow assessed. In a study published in Obstetrics & Gynecology, the conclusion was that oophorectomy before the age of natural menopause is associated with a decrease in cognitive impairment and dementia. There was no assessment of brain volume or cerebral blood flow. Likewise in a report published in Neurobiology of Aging, Maki P and Resnick S M. concluded that estrogen helps maintain hippocampal and prefrontal function as women age,” observed Dr. Bohon, noting that the study did not assess brain volume.
“It is unclear whether the most predictive assessment for worsening cognition and dementia is the finding of decreased total brain volume, decreased hippocampal volume, or decreased cerebral blood flow. The effect of both endogenous and exogenous estrogen on the risk for dementia needs further evaluation,” she cautioned.
Mr. Ambikairajah cited one financial disclosure; the remaining contributors had no relevant disclosures.
FROM MENOPAUSE
Reproductive Rounds: Understanding antimüllerian hormone in ovarian-age testing
In reproductive medicine, there are few, if any, more pressing concerns from our patients than the biological clock, i.e., ovarian aging. While addressing this issue with women can be challenging, particularly for those who are anxious regarding their advanced maternal age, gynecologists must possess a thorough understanding of available diagnostic testing. This article will review the various methods to assess ovarian age and appropriate clinical management.
Ovarian reserve tests
Ovarian reserve represents the quality and quantity of oocytes. The former is defined by the woman’s chronologic age, which is the greatest predictor of fertility. From a peak monthly fecundity rate at age 30 of approximately 20%, the slow and steady decline of fertility ensues. Quantity represents the number of oocytes remaining from the original cohort.
Ovarian reserve is most provocatively gauged by the follicle response to gonadotropin stimulation, typically during an in vitro fertilization (IVF) cycle.
Several biomarkers have been used to assess ovarian age. These include FSH, estradiol, and inhibin B. In general, these tests are more specific than sensitive, i.e., “normal” results do not necessarily exclude decreased ovarian reserve. But as a screening tool for decreased ovarian reserve, the most important factor is the positive predictive value (PPV). Statistically, in a population of women at low risk for decreased ovarian reserve, the PPV will be low despite sensitivity and specificity.
While inhibin B is a more direct and earlier reflection of ovarian function produced by granulose cells, assays lacked consistent results and a standardized cut-off value. FSH is the last biomarker to be affected by decreased ovarian reserve so elevations reflect more “end-stage” ovarian aging.
Additional tests for decreased ovarian reserve include antral follicle count (AFC) and the clomiphene citrate challenge test (CCCT). AFC is determined by using transvaginal ultrasound to count the number of follicular cysts in the 2- to 9-mm range. While AFC can be performed on any day of the cycle, the ovary is most optimally measured on menses because of less cystic activity. A combined AFC of 3-6 is considered severe decreased ovarian reserve. The CCCT involves prescribing clomiphene citrate 100 mg daily from cycle day 5-9 to measure FSH on cycle days 3 and 10. An FSH level greater than 10 IU/L or any elevation in FSH following CCCT is considered decreased ovarian reserve.
FSH had been the standard but levels may dramatically change monthly, making testing only valuable if it is elevated. Consequently, antimüllerian hormone (AMH) and AFC are considered the most useful tools to determine decreased ovarian reserve because of less variability. The other distinct advantage is the ability to obtain AMH any day in the menstrual cycle. Recently, in women undergoing IVF, AMH was superior to FSH in predicting live birth, particularly when their values were discordant (J Ovarian Res. 2018;11:60). While there is no established consensus, the ideal interval for repeating AMH appears to be approximately 3 months (Obstet Gynecol 2016;127:65S-6S).
AMH
AMH is expressed in the embryo at 8 weeks by the Sertoli cells of the testis causing the female reproductive internal system (müllerian) to regress. Without AMH expression, the müllerian system remains and the male (woffian duct system) regresses. The discovery of AMH production by the granulosa cells of the ovary launched a new era in the evaluation and management of infertile women. First reported in Fertility & Sterility in 2002 as a much earlier potential marker of ovarian aging, low levels of AMH predict a lower number of eggs in IVF.
AMH levels are produced in the embryo at 36 weeks’ gestation and increase up to the age of 24.5 years, decreasing thereafter. AMH reflects primordial (early) follicles that are FSH independent. The median AMH level decreases per year according to age groups are: 0.25 ng/mL in ages 26-30; 0.2 ng/mL in ages 31-36 years; and 0.1 ng/mL above age 36. (PLOS ONE 2015 doi: 10.1371/journal.pone.0125216).
AMH has also been studied as a potential biomarker to diagnose PCOS. While many women with PCOS have elevated AMH levels (typically greater than 3 ng/mL), there is no consensus on an AMH value that would be a criterion.
Many women, particularly those electing to defer fertility, express interest in obtaining their AMH level to consider planned oocyte cryopreservation, AKA, social egg freezing. While it is possible the results of AMH screening may compel women to electively freeze their eggs, extensive counseling on the implications and pitfalls of AMH levels is essential. Further, AMH cannot be used to accurately predict menopause.
Predicting outcomes
No biomarker is necessarily predictive of pregnancy but more a gauge of gonadotropin dosage to induce multifollicular development. AMH is a great predictor of oocyte yield with IVF (J Assist Reprod Genet. 2009;26[7]:383-9). However, in women older than 35 undergoing IVF, low AMH levels have been shown to reduce pregnancy rates (J Hum Reprod Sci. 2017;10:24–30). During IVF cycle attempts, an ultra-low AMH (≤0.4) resulted in high cancellation rates, reduced the number of oocytes retrieved and embryos developed, and lowered pregnancy rates in women of advanced reproductive age.
Alternatively, a study of 750 women who were not infertile and were actively trying to conceive demonstrated no difference in natural pregnancy rates in women aged 30-44 irrespective of AMH levels (JAMA. 2017;318[14]:1367-76).
A special consideration is for cancer patients who are status postgonadotoxic chemotherapy. Their oocyte attrition can be accelerated and AMH levels can become profoundly low. In those patients, current data suggest there is a modest recovery of postchemotherapy AMH levels up to 1 year. Further, oocyte yield following stimulation may be higher than expected despite a poor AMH level.
Conclusion
Ovarian aging is currently best measured by combining chronologic age, AFC, and AMH. There is no current evidence that AMH levels should be used to exclude patients from undergoing IVF or to recommend egg donation. Random screening of AMH levels in a low-risk population for decreased ovarian reserve may result in unnecessary alarm.
Dr. Trolice is director of Fertility CARE - The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In reproductive medicine, there are few, if any, more pressing concerns from our patients than the biological clock, i.e., ovarian aging. While addressing this issue with women can be challenging, particularly for those who are anxious regarding their advanced maternal age, gynecologists must possess a thorough understanding of available diagnostic testing. This article will review the various methods to assess ovarian age and appropriate clinical management.
Ovarian reserve tests
Ovarian reserve represents the quality and quantity of oocytes. The former is defined by the woman’s chronologic age, which is the greatest predictor of fertility. From a peak monthly fecundity rate at age 30 of approximately 20%, the slow and steady decline of fertility ensues. Quantity represents the number of oocytes remaining from the original cohort.
Ovarian reserve is most provocatively gauged by the follicle response to gonadotropin stimulation, typically during an in vitro fertilization (IVF) cycle.
Several biomarkers have been used to assess ovarian age. These include FSH, estradiol, and inhibin B. In general, these tests are more specific than sensitive, i.e., “normal” results do not necessarily exclude decreased ovarian reserve. But as a screening tool for decreased ovarian reserve, the most important factor is the positive predictive value (PPV). Statistically, in a population of women at low risk for decreased ovarian reserve, the PPV will be low despite sensitivity and specificity.
While inhibin B is a more direct and earlier reflection of ovarian function produced by granulose cells, assays lacked consistent results and a standardized cut-off value. FSH is the last biomarker to be affected by decreased ovarian reserve so elevations reflect more “end-stage” ovarian aging.
Additional tests for decreased ovarian reserve include antral follicle count (AFC) and the clomiphene citrate challenge test (CCCT). AFC is determined by using transvaginal ultrasound to count the number of follicular cysts in the 2- to 9-mm range. While AFC can be performed on any day of the cycle, the ovary is most optimally measured on menses because of less cystic activity. A combined AFC of 3-6 is considered severe decreased ovarian reserve. The CCCT involves prescribing clomiphene citrate 100 mg daily from cycle day 5-9 to measure FSH on cycle days 3 and 10. An FSH level greater than 10 IU/L or any elevation in FSH following CCCT is considered decreased ovarian reserve.
FSH had been the standard but levels may dramatically change monthly, making testing only valuable if it is elevated. Consequently, antimüllerian hormone (AMH) and AFC are considered the most useful tools to determine decreased ovarian reserve because of less variability. The other distinct advantage is the ability to obtain AMH any day in the menstrual cycle. Recently, in women undergoing IVF, AMH was superior to FSH in predicting live birth, particularly when their values were discordant (J Ovarian Res. 2018;11:60). While there is no established consensus, the ideal interval for repeating AMH appears to be approximately 3 months (Obstet Gynecol 2016;127:65S-6S).
AMH
AMH is expressed in the embryo at 8 weeks by the Sertoli cells of the testis causing the female reproductive internal system (müllerian) to regress. Without AMH expression, the müllerian system remains and the male (woffian duct system) regresses. The discovery of AMH production by the granulosa cells of the ovary launched a new era in the evaluation and management of infertile women. First reported in Fertility & Sterility in 2002 as a much earlier potential marker of ovarian aging, low levels of AMH predict a lower number of eggs in IVF.
AMH levels are produced in the embryo at 36 weeks’ gestation and increase up to the age of 24.5 years, decreasing thereafter. AMH reflects primordial (early) follicles that are FSH independent. The median AMH level decreases per year according to age groups are: 0.25 ng/mL in ages 26-30; 0.2 ng/mL in ages 31-36 years; and 0.1 ng/mL above age 36. (PLOS ONE 2015 doi: 10.1371/journal.pone.0125216).
AMH has also been studied as a potential biomarker to diagnose PCOS. While many women with PCOS have elevated AMH levels (typically greater than 3 ng/mL), there is no consensus on an AMH value that would be a criterion.
Many women, particularly those electing to defer fertility, express interest in obtaining their AMH level to consider planned oocyte cryopreservation, AKA, social egg freezing. While it is possible the results of AMH screening may compel women to electively freeze their eggs, extensive counseling on the implications and pitfalls of AMH levels is essential. Further, AMH cannot be used to accurately predict menopause.
Predicting outcomes
No biomarker is necessarily predictive of pregnancy but more a gauge of gonadotropin dosage to induce multifollicular development. AMH is a great predictor of oocyte yield with IVF (J Assist Reprod Genet. 2009;26[7]:383-9). However, in women older than 35 undergoing IVF, low AMH levels have been shown to reduce pregnancy rates (J Hum Reprod Sci. 2017;10:24–30). During IVF cycle attempts, an ultra-low AMH (≤0.4) resulted in high cancellation rates, reduced the number of oocytes retrieved and embryos developed, and lowered pregnancy rates in women of advanced reproductive age.
Alternatively, a study of 750 women who were not infertile and were actively trying to conceive demonstrated no difference in natural pregnancy rates in women aged 30-44 irrespective of AMH levels (JAMA. 2017;318[14]:1367-76).
A special consideration is for cancer patients who are status postgonadotoxic chemotherapy. Their oocyte attrition can be accelerated and AMH levels can become profoundly low. In those patients, current data suggest there is a modest recovery of postchemotherapy AMH levels up to 1 year. Further, oocyte yield following stimulation may be higher than expected despite a poor AMH level.
Conclusion
Ovarian aging is currently best measured by combining chronologic age, AFC, and AMH. There is no current evidence that AMH levels should be used to exclude patients from undergoing IVF or to recommend egg donation. Random screening of AMH levels in a low-risk population for decreased ovarian reserve may result in unnecessary alarm.
Dr. Trolice is director of Fertility CARE - The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In reproductive medicine, there are few, if any, more pressing concerns from our patients than the biological clock, i.e., ovarian aging. While addressing this issue with women can be challenging, particularly for those who are anxious regarding their advanced maternal age, gynecologists must possess a thorough understanding of available diagnostic testing. This article will review the various methods to assess ovarian age and appropriate clinical management.
Ovarian reserve tests
Ovarian reserve represents the quality and quantity of oocytes. The former is defined by the woman’s chronologic age, which is the greatest predictor of fertility. From a peak monthly fecundity rate at age 30 of approximately 20%, the slow and steady decline of fertility ensues. Quantity represents the number of oocytes remaining from the original cohort.
Ovarian reserve is most provocatively gauged by the follicle response to gonadotropin stimulation, typically during an in vitro fertilization (IVF) cycle.
Several biomarkers have been used to assess ovarian age. These include FSH, estradiol, and inhibin B. In general, these tests are more specific than sensitive, i.e., “normal” results do not necessarily exclude decreased ovarian reserve. But as a screening tool for decreased ovarian reserve, the most important factor is the positive predictive value (PPV). Statistically, in a population of women at low risk for decreased ovarian reserve, the PPV will be low despite sensitivity and specificity.
While inhibin B is a more direct and earlier reflection of ovarian function produced by granulose cells, assays lacked consistent results and a standardized cut-off value. FSH is the last biomarker to be affected by decreased ovarian reserve so elevations reflect more “end-stage” ovarian aging.
Additional tests for decreased ovarian reserve include antral follicle count (AFC) and the clomiphene citrate challenge test (CCCT). AFC is determined by using transvaginal ultrasound to count the number of follicular cysts in the 2- to 9-mm range. While AFC can be performed on any day of the cycle, the ovary is most optimally measured on menses because of less cystic activity. A combined AFC of 3-6 is considered severe decreased ovarian reserve. The CCCT involves prescribing clomiphene citrate 100 mg daily from cycle day 5-9 to measure FSH on cycle days 3 and 10. An FSH level greater than 10 IU/L or any elevation in FSH following CCCT is considered decreased ovarian reserve.
FSH had been the standard but levels may dramatically change monthly, making testing only valuable if it is elevated. Consequently, antimüllerian hormone (AMH) and AFC are considered the most useful tools to determine decreased ovarian reserve because of less variability. The other distinct advantage is the ability to obtain AMH any day in the menstrual cycle. Recently, in women undergoing IVF, AMH was superior to FSH in predicting live birth, particularly when their values were discordant (J Ovarian Res. 2018;11:60). While there is no established consensus, the ideal interval for repeating AMH appears to be approximately 3 months (Obstet Gynecol 2016;127:65S-6S).
AMH
AMH is expressed in the embryo at 8 weeks by the Sertoli cells of the testis causing the female reproductive internal system (müllerian) to regress. Without AMH expression, the müllerian system remains and the male (woffian duct system) regresses. The discovery of AMH production by the granulosa cells of the ovary launched a new era in the evaluation and management of infertile women. First reported in Fertility & Sterility in 2002 as a much earlier potential marker of ovarian aging, low levels of AMH predict a lower number of eggs in IVF.
AMH levels are produced in the embryo at 36 weeks’ gestation and increase up to the age of 24.5 years, decreasing thereafter. AMH reflects primordial (early) follicles that are FSH independent. The median AMH level decreases per year according to age groups are: 0.25 ng/mL in ages 26-30; 0.2 ng/mL in ages 31-36 years; and 0.1 ng/mL above age 36. (PLOS ONE 2015 doi: 10.1371/journal.pone.0125216).
AMH has also been studied as a potential biomarker to diagnose PCOS. While many women with PCOS have elevated AMH levels (typically greater than 3 ng/mL), there is no consensus on an AMH value that would be a criterion.
Many women, particularly those electing to defer fertility, express interest in obtaining their AMH level to consider planned oocyte cryopreservation, AKA, social egg freezing. While it is possible the results of AMH screening may compel women to electively freeze their eggs, extensive counseling on the implications and pitfalls of AMH levels is essential. Further, AMH cannot be used to accurately predict menopause.
Predicting outcomes
No biomarker is necessarily predictive of pregnancy but more a gauge of gonadotropin dosage to induce multifollicular development. AMH is a great predictor of oocyte yield with IVF (J Assist Reprod Genet. 2009;26[7]:383-9). However, in women older than 35 undergoing IVF, low AMH levels have been shown to reduce pregnancy rates (J Hum Reprod Sci. 2017;10:24–30). During IVF cycle attempts, an ultra-low AMH (≤0.4) resulted in high cancellation rates, reduced the number of oocytes retrieved and embryos developed, and lowered pregnancy rates in women of advanced reproductive age.
Alternatively, a study of 750 women who were not infertile and were actively trying to conceive demonstrated no difference in natural pregnancy rates in women aged 30-44 irrespective of AMH levels (JAMA. 2017;318[14]:1367-76).
A special consideration is for cancer patients who are status postgonadotoxic chemotherapy. Their oocyte attrition can be accelerated and AMH levels can become profoundly low. In those patients, current data suggest there is a modest recovery of postchemotherapy AMH levels up to 1 year. Further, oocyte yield following stimulation may be higher than expected despite a poor AMH level.
Conclusion
Ovarian aging is currently best measured by combining chronologic age, AFC, and AMH. There is no current evidence that AMH levels should be used to exclude patients from undergoing IVF or to recommend egg donation. Random screening of AMH levels in a low-risk population for decreased ovarian reserve may result in unnecessary alarm.
Dr. Trolice is director of Fertility CARE - The IVF Center in Winter Park, Fla., and associate professor of obstetrics and gynecology at the University of Central Florida, Orlando.