User login
Fit-for-Fertility program boosts births, is cost effective
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incorporation of a nonintensive fitness intervention for women with obesity into a standard fertility treatment program could be cost effective, a new analysis finds.
Financial data for the Canadian Fit-for-Fertility program were presented March 20 at the annual meeting of the Endocrine Society by Matea Belan, PhD, of the division of endocrinology at the University of Sherbrooke (Que.).
Women with obesity and infertility are typically advised to lose 5%-10% of their body weight as first-line fertility treatment, as doing so has been shown to increase rates of ovulation and pregnancy. But most established fertility treatment programs don’t incorporate organized lifestyle modification interventions, Dr. Belan explained during a press briefing.
“Mostly they’re just given general advice, not resources. It’s up to the woman to seek help for lifestyle. Our idea is to give them access to intervention that’s integrated into the setting of a fertility clinic,” she said.
Primary results from the Fit-for-Fertility program, including significant weight loss and a 40% increased live birth rate at 18 months, compared with standard fertility treatment, were presented at ENDO 2019 and reported at the time by this news organization.
In the new analysis, the cost in Canadian dollars per additional newborn achieved with the Fit-for-Fertility program was similar to the willingness-to-pay for in vitro fertilization from a health system perspective.
The final goal, lead investigator Jean-Patrice Baillargeon, MD, said in an interview, “would be to convince stakeholders, and mainly the provincial government, to cover the costs of our lifestyle program. This would not be more costly than funding IVF, but [would provide] more long-term benefits for the whole family and the offspring.”
Chloe A. Zera, MD, said in an interview that she supports the idea in principle, but is concerned that, in the U.S. health care system, women don’t always have access to fertility and obesity treatments to begin with.
“There’s a huge equity issue. People with Medicaid don’t necessarily get coverage for IVF. ... Even many commercially insured people are paying out of pocket, which can be $10,000 to $15,000 for a cycle just for the medications, so the cost to patients on the individual level is huge,” said Dr. Zera, who is associate professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston.
She added: “I’m prolifestyle modification. I’m also proequity in health care delivery so I would want to make sure that the way it’s delivered incorporates that as a consideration. ... Is that money better spent on primary prevention of obesity and access to basic services and basic reproductive health care for everybody?”
Primary results: Improvements in overall and spontaneous pregnancy rates
The study included 130 women with infertility and a body mass index of at least 30 kg/m2 (mean, 40), of whom 65 were randomized to the Fit-for-Fitness program and 65 to standard fertility treatment that did not include a lifestyle intervention, although those women could consult professionals on their own. The women in the lifestyle intervention group had to stop medical fertility treatments for the first 6 months but could use them thereafter while the controls continued to use them throughout.
Based on motivational interviewing, the program focused on womens’ individual likes and dislikes, experiences, and perceived capacities, aiming to improve healthful habits gradually and with “low intensity” so as to maintain them in the long run.
The program combined individual sessions with a nutritionist and kinesiologist every 6 weeks and 12 mandatory group sessions. The women were asked to reduce their total caloric intake by about 500 calories/day but weren’t asked to change their diets. They were also advised to increase physical activity by about 150 minutes/week.
“We want to keep it sustainable in time, so they don’t have a relapse when they become pregnant, and to help the newborn and spouse too. It’s about improving and maintaining habits,” Dr. Belan explained during the briefing.
At 6 months, mean weight changes were –3.4% versus –0.89% for the intervention versus control groups (P = .003).
“What is important for women with obesity and infertility is to improve their lifestyle, both physical activity and nutrition, even if the weight loss is minimal,” noted Dr. Baillargeon, professor of medicine, health sciences research and physiology, also at the University of Sherbrooke.
A total of 46 intervention and 52 control patients finished the 18-month study. Pregnancies occurred in 61% of the intervention group versus 39% of the controls, while spontaneous pregnancies – among those not using medical fertility treatments – occurred in 33.3% versus 12.3% (P = .009).
The primary outcome, live births at 18 months, occurred in 51.0% of the intervention group versus 36.8% of controls, which wasn’t a statistically significant difference, but was “highly clinically significant,” Dr. Belan said.
Cost per additional newborn similar to IVF
Costs (in Canadian dollars) considered in the analysis included those related to the management of infertility, obesity, pregnancy, and childbirth. The incremental cost-effectiveness ratios, a standard cost-effectiveness measure, per live birth were $24,393 from a societal perspective, $12,633 for the health system, and $5,980 for the patient.
Because the $12,633 health system cost per additional newborn with the Fit-for-Fertility program is similar to the health system’s willingness-to-pay for IVF of up to $15,000, a lifestyle intervention could be considered cost-efficient compared with the standard of care, Dr. Belan said.
“We think that the Fit-for-Fertility program could be deemed cost effective and could represent an interesting alternative to the usual standard of care for women with obesity seeking fertility treatments,” she commented.
The Canadian Institutes of Health Research is funding a larger randomized, controlled trial of the program at six Canadian centers to validate these results.
Dr. Belan, Dr. Baillargeon, and Dr. Zera reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The pandemic is making periods unbearable for some women
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Many unknowns on fertility preservation in transgender patients
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Recurrent miscarriage: What’s the evidence-based evaluation and management?
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
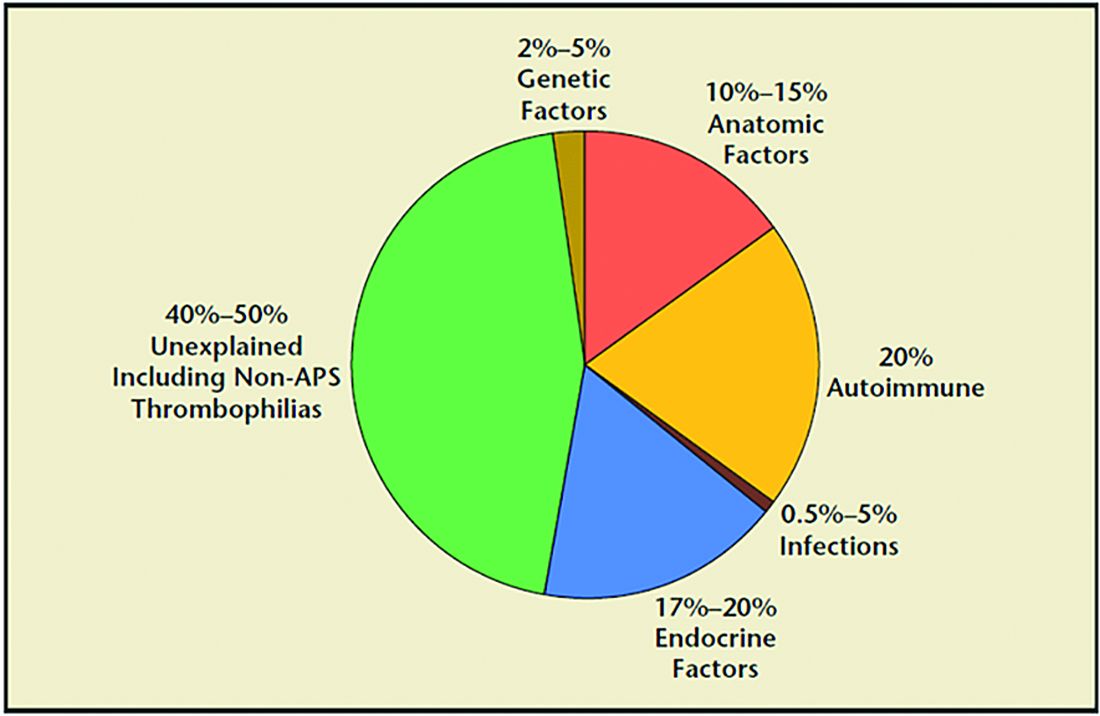
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.
Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.

Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.

Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Obesity pegged as source of marked increased risk of diabetes in PCOS
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
FROM ENDO 2021
PCOS equivalent in men: No ovaries required
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
FROM ENDO 2021
High-intensity interval training cuts cardiometabolic risks in women with PCOS
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
High-intensity interval training (HIIT) was better than moderate-intensity continuous training (MICT) for improving several measures of cardiometabolic health in women with polycystic ovary syndrome (PCOS) in a prospective, randomized, single-center study with 27 women.
After 12 weeks on a supervised exercise regimen, the women with PCOS who followed the HIIT program had significantly better improvements in aerobic capacity, insulin sensitivity, and level of sex hormone–binding globulin, Rhiannon K. Patten, MSc, said at the annual meeting of the Endocrine Society.
“HIIT can offer superior improvements in health outcomes, and should be considered as an effective tool to reduce cardiometabolic risk in women with PCOS,” concluded Ms. Patten, a researcher in the Institute for Health and Sport at Victoria University in Melbourne in her presentation (Abstract OR10-1).
“The changes we see [after 12 weeks on the HIIT regimen] seem to occur despite no change in body mass index, so rather than focus on weight loss we encourage participants to focus on the health improvements that seem to be greater with HIIT. We actively encourage the HIIT protocol right now,” she said.
Both regimens use a stationary cycle ergometer. In the HIIT protocol patients twice weekly pedal through 12 1-minute intervals at a heart rate of 90%-100% maximum, interspersed with 1 minute rest intervals. On a third day per week, patients pedal to a heart rate of 90%-95% maximum for 6-8 intervals maintained for 2 minutes and interspersed with rest intervals of 2 minutes. The MICT regimen used as a comparator has participants pedal to 60%-70% of their maximum heart rate continuously for 50 minutes 3 days weekly.
HIIT saves time
“These findings are relevant to clinical practice, because they demonstrate that HIIT is effective in women with PCOS. Reducing the time devoted to exercise to achieve fitness goals is attractive to patients. The reduced time to achieve training benefits with HIIT should improve patient compliance,” commented Andrea Dunaif, MD, professor and chief of the division of endocrinology, diabetes, and bone disease of the Mount Sinai Health System in New York, who was not involved with the study.
The overall weekly exercise time on the MICT regimen, 150 minutes, halves down to 75 minutes a week in the HIIT program. Guideline recommendations released in 2018 by the International PCOS Network recommended these as acceptable alternative exercise strategies. Ms. Patten and her associates sought to determine whether one strategy surpassed the other, the first time this has been examined in women with PCOS, she said.
They randomized 27 sedentary women 18-45 years old with a body mass index (BMI) above 25 kg/m2 and diagnosed with PCOS by the Rotterdam criteria to a 12-week supervised exercise program on either the HIIT or MICT protocol. Their average BMI at entry was 36-37 kg/m2. The study excluded women who smoked, were pregnant, had an illness or injury that would prevent exercise, or were on an oral contraceptive or insulin-sensitizing medication.
At the end of 12 weeks, neither group had a significant change in average weight or BMI, and waist circumference dropped by an average of just over 2 cm in both treatment groups. Lean mass increased by a mean 1 kg in the HIIT group, a significant change, compared with a nonsignificant 0.3 kg average increase in the MICT group.
Increased aerobic capacity ‘partially explains’ improved insulin sensitivity
Aerobic capacity, measured as peak oxygen consumption (VO2peak), increased by an average 5.7 mL/kg per min among the HIIT patients, significantly more than the mean 3.2 mL/kg per min increase among those in the MICT program.
The insulin sensitivity index rose by a significant, relative 35% among the HIIT patients, but barely budged in the MICT group. Fasting glucose fell significantly and the glucose infusion rate increased significantly among the women who performed HIIT, but again showed little change among those doing MICT.
Analysis showed a significant link between the increase in VO2peak and the increase in insulin sensitivity among the women engaged in HIIT, Ms. Patten reported. The improvement in the insulin sensitivity index was “partially explained” by the increase in VO2peak, she said.
Assessment of hormone levels showed a significant increase in sex hormone–binding globulin in the HIIT patients while those in the MICT group showed a small decline in this level. The free androgen index fell by a relative 39% on average in the HIIT group, a significant drop, but decreased by a much smaller and not significant amount among the women who did MICT. The women who performed HIIT also showed a significant drop in their free testosterone level, a change not seen with MICT.
Women who performed the HIIT protocol also had a significant improvement in their menstrual cyclicity, and significant improvements in depression, stress, and anxiety, Ms Patten reported. She next plans to do longer follow-up on study participants, out to 6 and 12 months after the end of the exercise protocol.
“Overall, the findings suggest that HIIT is superior to MICT for improving fitness and insulin sensitivity in the short term. Results from a number of studies in individuals without PCOS suggest that HIIT is superior to MICT for improving fitness short term,” commented Dr. Dunaif. “This study makes an important contribution by directly investigating the impact of training intensity in women with PCOS. Larger studies will be needed before the superiority of HIIT is established for women with PCOS, and study durations of at least several months will be needed to assess the impact on reproductive outcomes such as ovulation,” she said in an interview. She also called for assessing the effects of HIIT in more diverse populations of women with PCOS.
Ms. Patten had no disclosures. Dr. Dunaif has been a consultant to Equator Therapeutics, Fractyl Laboratories, and Globe Life Sciences.
FROM ENDO 2021
Women with PCOS at increased risk for COVID-19
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with polycystic ovary syndrome (PCOS) face an almost 30% increased risk for COVID-19 compared with unaffected women, even after adjusting for cardiometabolic and other related factors, suggests an analysis of United Kingdom primary care data.
“Our research has highlighted that women with PCOS are an often overlooked and potentially high-risk population for contracting COVID-19,” said joint senior author Wiebke Arlt, MD, PhD, director of the Institute of Metabolism and Systems Research at the University of Birmingham (England), in a press release.
“Before the onset of the COVID-19 pandemic, women with PCOS consistently report fragmented care, delayed diagnosis and a perception of poor clinician understanding of their condition,” added co-author Michael W. O’Reilly, MD, PhD, University of Medicine and Health Sciences, Dublin.
“Women suffering from this condition may fear, with some degree of justification, that an enhanced risk of COVID-19 infection will further compromise timely access to health care and serve to increase the sense of disenfranchisement currently experienced by many patients,” he added.
Consequently, “these findings need to be considered when designing public health policy and advice as our understanding of COVID-19 evolves,” noted first author Anuradhaa Subramanian, PhD Student, Institute of Applied Health Research, University of Birmingham.
The research was published by the European Journal of Endocrinology on March 9.
Women with PCOS: A distinct subgroup?
PCOS, which is thought to affect up to 16% of women, is associated with a significantly increased risk for type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease, all which have been linked to more severe COVID-19.
The condition is more prevalent in Black and South Asian women, who also appear to have an increased risk for severe COVID-19 vs. their White counterparts.
However, women and younger people in general have a lower overall risk for severe COVID-19 and mortality compared with older people and men.
Women with PCOS may therefore “represent a distinct subgroup of women at higher than average [on the basis of their sex and age] risk of adverse COVID-19–related outcomes,” the researchers note.
To investigate further, they collated data from The Health Improvement Network primary care database, which includes information from 365 active general practices in the U.K. for the period Jan. 31, 2020, to July 22, 2020.
They identified women with PCOS or a coded diagnosis of polycystic ovaries (PCO), and then for each woman randomly selected four unaffected controls matched for age and general practice location.
They included 21,292 women with PCOS/PCO and 78,310 controls, who had a mean age at study entry of 39.3 years and 39.5 years, respectively. The mean age at diagnosis of PCOS was 27 years, and the mean duration of the condition was 12.4 years.
The crude incidence of COVID-19 was 18.1 per 1000 person-years among women with PCOS vs. 11.9 per 1000 person-years in those without.
Cox regression analysis adjusted for age indicated that women with PCOS faced a significantly increased risk for COVID-19 than those without, at a hazard ratio of 1.51 (P < .001).
Further adjustment for body mass index (BMI) and age reduced the hazard ratio to 1.36 (P = .001).
In the fully adjusted model, which also took into account impaired glucose regulation, androgen excess, anovulation, hypertension, and other PCOS-related factors, the hazard ratio remained significant, at 1.28 (P = .015).
For shielding, balance benefits with impact on mental health
Joint senior author Krishnarajah Nirantharakumar, MD, PhD, also of the University of Birmingham, commented that, despite the increased risks, shielding strategies for COVID-19 need to take into account the impact of PCOS on women’s mental health.
“The risk of mental health problems, including low self-esteem, anxiety, and depression, is significantly higher in women with PCOS,” he said, “and advice on strict adherence to social distancing needs to be tempered by the associated risk of exacerbating these underlying problems.”
Arlt also pointed out that the study only looked at the incidence of COVID-19 infection, rather than outcomes.
“Our study does not provide information on the risk of a severe course of the COVID-19 infection or on the risk of COVID-19–related long-term complications [in women with PCOS], and further research is required,” she concluded.
The study was funded by Health Data Research UK and supported by the Wellcome Trust, the Health Research Board, and the National Institute for Health Research Birmingham Biomedical Research Centre based at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust. The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Benefits of bremelanotide to women with HSDD questioned in analysis paper
Dr. Spielmans, professor of psychology at Metropolitan State University in Saint Paul, Minn., examined data from the FDA application for bremelanotide, clinicaltrials.gov entries for two phase 3 trials of the drug, and a 2019 article published in Obstetrics & Gynecology that described results from the 24-week trials.
In Dr. Speilman’s analysis, which was published online March 7 in the Journal of Sex Research, he notes that 42.1% of trial participants who received bremelanotide did not complete the trial, compared with 20.48% of participants who received placebo.
Of those who completed the study, 87.22% who received placebo wanted to continue treatment in an open-label extension, compared with 69.97% who received bremelanotide, he wrote.
Women “should be aware of the small degree of bremelanotide’s efficacy, that the protocol-specified outcomes of bremelanotide are mostly unknown, and that participants would rather take a placebo than bremelanotide,” Dr. Spielmans said.
Anita H. Clayton, MD, an author of the Obstetrics & Gynecology paper addressed in Dr. Spielmans’ analysis, says the Journal of Sex Research article does not provide new information and is a disservice to women because it questions accurate scientific data.
Measuring outcomes in HSDD is an evolving field, Dr. Clayton, a psychiatrist at the University of Virginia in Charlottesville, said in an interview. Initial FDA guidance relied on satisfying sexual events as an outcome measure, but this measure was derived from erectile dysfunction studies and is not necessarily adequate for assessing HSDD, she said. The FDA and drug developers agreed to use the desire subscale of the Female Sexual Function Index (FSFI-D) as a coprimary outcome measure instead, she noted.
Dr. Spielmans’ critique of Obstetrics & Gynecology paper
The article published in Obstetrics & Gynecology reporting bremelanotide trial results was noteworthy, although the various issues involved can be seen in reports about other drug trials, Dr. Spielmans said in an interview.
“It is well-established that journal articles reporting clinical trial data overstate benefits and understate harms,” he continued. In this case, “the very incomplete data reporting, reliance on many post-hoc measures of questionable validity, hiding the concerning number of dropouts due to adverse events, and putting a positive spin on efficacy and tolerability is both remarkable and highly problematic,” Dr. Spielmans said.
Dr. Clayton’s reaction
Data about dropout rates due to adverse events have been reported and presented at national meetings, she said in an interview. In addition, a questionnaire found that bremelanotide was superior to placebo in terms of patients feeling that the treatment had provided clinically meaningful benefit, Dr. Clayton said.
The available information enables patients to make informed treatment decisions, Dr. Clayton continued. “There is really this sexist attitude of women needing protection from their own decisions,” she said.
Diagnosing and treating HSDD
Eight of 11 efficacy outcomes in the clinicaltrials.gov study protocols for bremelanotide were not reported in the Obstetrics & Gynecology article in a way that was consistent with the protocols, Dr. Spielmans said. Changing a coprimary outcome to the key secondary outcome “occurred over a year after the trials had begun,” and the authors of the journal article “did not mention that this change occurred,” Dr. Spielmans wrote.
For the coprimary outcome measures of mean change on FSFI-D and Female Sexual Distress Scale–Desire/Arousal/Orgasm #13, “bremelanotide offers modest benefits over placebo,” Dr. Spielmans reported.
In addition to outlining his concerns about transparency in the reporting of trial data and raising questions about the outcome measures used in the Obstetrics & Gynecology article, Dr. Spielmans wrote that the diagnosis of HSDD is problematic.
“The lack of specifying symptom duration, questionable validity for the lack of sexual fantasies as a diagnostic criterion, difficulty in disentangling individual sexual problems from relational problems, and the failure to consider cultural influence (including the pressure on women to satisfy the sexual desires of their male partners) in the experience of sexuality all render HSDD as a problematic entity,” Dr. Spielmans wrote.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders replaced HSDD and female sexual arousal disorder with the combined condition female sexual interest/arousal disorder. HSDD is in the 11th edition of the International Classification of Diseases and can be applied to men or women, Dr. Spielmans said.
FDA acknowledged HSDD as an unmet medical need
Dr. Clayton pointed out that HSDD was described decades ago and the FDA acknowledged it as an unmet medical need, and she expressed dissatisfaction with the fact the hypoactive sexual desire disorder appears with quotation marks around it in the title of Dr. Spielmans’ article. This way of presenting HSDD indicates that “the author has no concept of sexual health or sexual dysfunction,” Dr. Clayton said. “Basically this is sort of a dramatic tool, I think, to act like this is not a real disorder,” she added.
Carl Spana, PhD, CEO and president of Palatin Technologies, the developer of bremelanotide, defined the article in the Journal of Sex Research as a “retrospective meta-analysis, and not a re-analysis of the data.
“As a meta-analysis, it is open to various interpretations and reflects the author’s interpretations, which appear to have clear biases,” Dr. Spana said in an interview. “We believe several of this author’s interpretations are contrary to the FDA’s positive assessment that led to Vyleesi’s approval as a safe and effective treatment for women suffering from hypoactive sexual desire disorder.”
The author is unaware of the validation that was conducted at the direction of the FDA to establish clinically meaningful cutoffs for patient-reported outcomes and to establish metrics that define clinical benefit, Dr. Spana said
“Vyleesi was approved by the FDA after a thorough analysis of data from two well-controlled phase 3 clinical studies and multiple clinical and preclinical safety studies,” he said. “The analyses in the New Drug Application were prespecified and conducted according to a statistical analysis plan that the sponsor and FDA agreed to prior to database lock.”
Dr. Spielmans disclosed holdings in Vanguard Healthcare, a mutual fund that invests in pharmaceutical firms. Dr. Clayton has received financial support from Palatin and AMAG Pharmaceuticals, the companies that developed bremelanotide, in previous years.
Dr. Spielmans, professor of psychology at Metropolitan State University in Saint Paul, Minn., examined data from the FDA application for bremelanotide, clinicaltrials.gov entries for two phase 3 trials of the drug, and a 2019 article published in Obstetrics & Gynecology that described results from the 24-week trials.
In Dr. Speilman’s analysis, which was published online March 7 in the Journal of Sex Research, he notes that 42.1% of trial participants who received bremelanotide did not complete the trial, compared with 20.48% of participants who received placebo.
Of those who completed the study, 87.22% who received placebo wanted to continue treatment in an open-label extension, compared with 69.97% who received bremelanotide, he wrote.
Women “should be aware of the small degree of bremelanotide’s efficacy, that the protocol-specified outcomes of bremelanotide are mostly unknown, and that participants would rather take a placebo than bremelanotide,” Dr. Spielmans said.
Anita H. Clayton, MD, an author of the Obstetrics & Gynecology paper addressed in Dr. Spielmans’ analysis, says the Journal of Sex Research article does not provide new information and is a disservice to women because it questions accurate scientific data.
Measuring outcomes in HSDD is an evolving field, Dr. Clayton, a psychiatrist at the University of Virginia in Charlottesville, said in an interview. Initial FDA guidance relied on satisfying sexual events as an outcome measure, but this measure was derived from erectile dysfunction studies and is not necessarily adequate for assessing HSDD, she said. The FDA and drug developers agreed to use the desire subscale of the Female Sexual Function Index (FSFI-D) as a coprimary outcome measure instead, she noted.
Dr. Spielmans’ critique of Obstetrics & Gynecology paper
The article published in Obstetrics & Gynecology reporting bremelanotide trial results was noteworthy, although the various issues involved can be seen in reports about other drug trials, Dr. Spielmans said in an interview.
“It is well-established that journal articles reporting clinical trial data overstate benefits and understate harms,” he continued. In this case, “the very incomplete data reporting, reliance on many post-hoc measures of questionable validity, hiding the concerning number of dropouts due to adverse events, and putting a positive spin on efficacy and tolerability is both remarkable and highly problematic,” Dr. Spielmans said.
Dr. Clayton’s reaction
Data about dropout rates due to adverse events have been reported and presented at national meetings, she said in an interview. In addition, a questionnaire found that bremelanotide was superior to placebo in terms of patients feeling that the treatment had provided clinically meaningful benefit, Dr. Clayton said.
The available information enables patients to make informed treatment decisions, Dr. Clayton continued. “There is really this sexist attitude of women needing protection from their own decisions,” she said.
Diagnosing and treating HSDD
Eight of 11 efficacy outcomes in the clinicaltrials.gov study protocols for bremelanotide were not reported in the Obstetrics & Gynecology article in a way that was consistent with the protocols, Dr. Spielmans said. Changing a coprimary outcome to the key secondary outcome “occurred over a year after the trials had begun,” and the authors of the journal article “did not mention that this change occurred,” Dr. Spielmans wrote.
For the coprimary outcome measures of mean change on FSFI-D and Female Sexual Distress Scale–Desire/Arousal/Orgasm #13, “bremelanotide offers modest benefits over placebo,” Dr. Spielmans reported.
In addition to outlining his concerns about transparency in the reporting of trial data and raising questions about the outcome measures used in the Obstetrics & Gynecology article, Dr. Spielmans wrote that the diagnosis of HSDD is problematic.
“The lack of specifying symptom duration, questionable validity for the lack of sexual fantasies as a diagnostic criterion, difficulty in disentangling individual sexual problems from relational problems, and the failure to consider cultural influence (including the pressure on women to satisfy the sexual desires of their male partners) in the experience of sexuality all render HSDD as a problematic entity,” Dr. Spielmans wrote.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders replaced HSDD and female sexual arousal disorder with the combined condition female sexual interest/arousal disorder. HSDD is in the 11th edition of the International Classification of Diseases and can be applied to men or women, Dr. Spielmans said.
FDA acknowledged HSDD as an unmet medical need
Dr. Clayton pointed out that HSDD was described decades ago and the FDA acknowledged it as an unmet medical need, and she expressed dissatisfaction with the fact the hypoactive sexual desire disorder appears with quotation marks around it in the title of Dr. Spielmans’ article. This way of presenting HSDD indicates that “the author has no concept of sexual health or sexual dysfunction,” Dr. Clayton said. “Basically this is sort of a dramatic tool, I think, to act like this is not a real disorder,” she added.
Carl Spana, PhD, CEO and president of Palatin Technologies, the developer of bremelanotide, defined the article in the Journal of Sex Research as a “retrospective meta-analysis, and not a re-analysis of the data.
“As a meta-analysis, it is open to various interpretations and reflects the author’s interpretations, which appear to have clear biases,” Dr. Spana said in an interview. “We believe several of this author’s interpretations are contrary to the FDA’s positive assessment that led to Vyleesi’s approval as a safe and effective treatment for women suffering from hypoactive sexual desire disorder.”
The author is unaware of the validation that was conducted at the direction of the FDA to establish clinically meaningful cutoffs for patient-reported outcomes and to establish metrics that define clinical benefit, Dr. Spana said
“Vyleesi was approved by the FDA after a thorough analysis of data from two well-controlled phase 3 clinical studies and multiple clinical and preclinical safety studies,” he said. “The analyses in the New Drug Application were prespecified and conducted according to a statistical analysis plan that the sponsor and FDA agreed to prior to database lock.”
Dr. Spielmans disclosed holdings in Vanguard Healthcare, a mutual fund that invests in pharmaceutical firms. Dr. Clayton has received financial support from Palatin and AMAG Pharmaceuticals, the companies that developed bremelanotide, in previous years.
Dr. Spielmans, professor of psychology at Metropolitan State University in Saint Paul, Minn., examined data from the FDA application for bremelanotide, clinicaltrials.gov entries for two phase 3 trials of the drug, and a 2019 article published in Obstetrics & Gynecology that described results from the 24-week trials.
In Dr. Speilman’s analysis, which was published online March 7 in the Journal of Sex Research, he notes that 42.1% of trial participants who received bremelanotide did not complete the trial, compared with 20.48% of participants who received placebo.
Of those who completed the study, 87.22% who received placebo wanted to continue treatment in an open-label extension, compared with 69.97% who received bremelanotide, he wrote.
Women “should be aware of the small degree of bremelanotide’s efficacy, that the protocol-specified outcomes of bremelanotide are mostly unknown, and that participants would rather take a placebo than bremelanotide,” Dr. Spielmans said.
Anita H. Clayton, MD, an author of the Obstetrics & Gynecology paper addressed in Dr. Spielmans’ analysis, says the Journal of Sex Research article does not provide new information and is a disservice to women because it questions accurate scientific data.
Measuring outcomes in HSDD is an evolving field, Dr. Clayton, a psychiatrist at the University of Virginia in Charlottesville, said in an interview. Initial FDA guidance relied on satisfying sexual events as an outcome measure, but this measure was derived from erectile dysfunction studies and is not necessarily adequate for assessing HSDD, she said. The FDA and drug developers agreed to use the desire subscale of the Female Sexual Function Index (FSFI-D) as a coprimary outcome measure instead, she noted.
Dr. Spielmans’ critique of Obstetrics & Gynecology paper
The article published in Obstetrics & Gynecology reporting bremelanotide trial results was noteworthy, although the various issues involved can be seen in reports about other drug trials, Dr. Spielmans said in an interview.
“It is well-established that journal articles reporting clinical trial data overstate benefits and understate harms,” he continued. In this case, “the very incomplete data reporting, reliance on many post-hoc measures of questionable validity, hiding the concerning number of dropouts due to adverse events, and putting a positive spin on efficacy and tolerability is both remarkable and highly problematic,” Dr. Spielmans said.
Dr. Clayton’s reaction
Data about dropout rates due to adverse events have been reported and presented at national meetings, she said in an interview. In addition, a questionnaire found that bremelanotide was superior to placebo in terms of patients feeling that the treatment had provided clinically meaningful benefit, Dr. Clayton said.
The available information enables patients to make informed treatment decisions, Dr. Clayton continued. “There is really this sexist attitude of women needing protection from their own decisions,” she said.
Diagnosing and treating HSDD
Eight of 11 efficacy outcomes in the clinicaltrials.gov study protocols for bremelanotide were not reported in the Obstetrics & Gynecology article in a way that was consistent with the protocols, Dr. Spielmans said. Changing a coprimary outcome to the key secondary outcome “occurred over a year after the trials had begun,” and the authors of the journal article “did not mention that this change occurred,” Dr. Spielmans wrote.
For the coprimary outcome measures of mean change on FSFI-D and Female Sexual Distress Scale–Desire/Arousal/Orgasm #13, “bremelanotide offers modest benefits over placebo,” Dr. Spielmans reported.
In addition to outlining his concerns about transparency in the reporting of trial data and raising questions about the outcome measures used in the Obstetrics & Gynecology article, Dr. Spielmans wrote that the diagnosis of HSDD is problematic.
“The lack of specifying symptom duration, questionable validity for the lack of sexual fantasies as a diagnostic criterion, difficulty in disentangling individual sexual problems from relational problems, and the failure to consider cultural influence (including the pressure on women to satisfy the sexual desires of their male partners) in the experience of sexuality all render HSDD as a problematic entity,” Dr. Spielmans wrote.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders replaced HSDD and female sexual arousal disorder with the combined condition female sexual interest/arousal disorder. HSDD is in the 11th edition of the International Classification of Diseases and can be applied to men or women, Dr. Spielmans said.
FDA acknowledged HSDD as an unmet medical need
Dr. Clayton pointed out that HSDD was described decades ago and the FDA acknowledged it as an unmet medical need, and she expressed dissatisfaction with the fact the hypoactive sexual desire disorder appears with quotation marks around it in the title of Dr. Spielmans’ article. This way of presenting HSDD indicates that “the author has no concept of sexual health or sexual dysfunction,” Dr. Clayton said. “Basically this is sort of a dramatic tool, I think, to act like this is not a real disorder,” she added.
Carl Spana, PhD, CEO and president of Palatin Technologies, the developer of bremelanotide, defined the article in the Journal of Sex Research as a “retrospective meta-analysis, and not a re-analysis of the data.
“As a meta-analysis, it is open to various interpretations and reflects the author’s interpretations, which appear to have clear biases,” Dr. Spana said in an interview. “We believe several of this author’s interpretations are contrary to the FDA’s positive assessment that led to Vyleesi’s approval as a safe and effective treatment for women suffering from hypoactive sexual desire disorder.”
The author is unaware of the validation that was conducted at the direction of the FDA to establish clinically meaningful cutoffs for patient-reported outcomes and to establish metrics that define clinical benefit, Dr. Spana said
“Vyleesi was approved by the FDA after a thorough analysis of data from two well-controlled phase 3 clinical studies and multiple clinical and preclinical safety studies,” he said. “The analyses in the New Drug Application were prespecified and conducted according to a statistical analysis plan that the sponsor and FDA agreed to prior to database lock.”
Dr. Spielmans disclosed holdings in Vanguard Healthcare, a mutual fund that invests in pharmaceutical firms. Dr. Clayton has received financial support from Palatin and AMAG Pharmaceuticals, the companies that developed bremelanotide, in previous years.
FROM THE JOURNAL OF SEX RESEARCH
Testosterone decline after steroid abuse revealed with new biomarker
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
Levels of insulinlike factor 3 (INSL3) drop noticeably in men who have abused anabolic androgenic steroids (AAS), even well after stoppage. The results suggest that the effects of AAS use on testosterone-producing Leydig cells may be long-lasting, as some clinicians have suspected. Although there is some variation of INSL3 levels among AAS users, the metric is more accurate than testosterone levels and could be a key element of future diagnostic tests.
Those are the conclusions of a new study, led by Jon Jarløv Rasmussen, MD, PhD, of the department of endocrinology at Rigshospitalet in Copenhagen*, published March 9, 2021, in the Journal of Clinical Endocrinology & Metabolism.
Results mirror clinical experience
The drop in levels, both among current and past users, is in keeping with clinical experience of endocrinologists, according to Channa Jayasena, MD, PhD, a reproductive endocrinologist at Imperial College London. He suspects lasting damage in former and current users who come to him when they discover their sperm count is low. "How long that damage lasts is another matter," Dr. Jayasena, who was not involved in the study, said in an interview.
Dr. Jayasena hopes that INSL3 could find use in tracking damage to Leydig cells from AAS use, as well as to monitor improvements in the event that treatments are found, though he noted that the scatter plots in the study showed quite a bit of variation of INSL3 levels. "So it's a great first step showing that these men, users and past users, have lower INSL3 levels, but it's going to have to be part of a broader suite of factors such as the other hormone [levels], testicular volume, duration of steroid use, etc.," said Dr. Jayasena.
In search of a reliable measure
Low testosterone levels have been shown to be associated with AAS use in some studies, but not in others. That inconsistency led the researchers in search of a more reliable measure. "Serum testosterone is not a stable marker but can fluctuate considerably within minutes to hours, whereas serum insulinlike factor 3 [levels] do not," said Dr. Rasmussen.
INSL3 appears to be involved in bone metabolism regulation as well as spermatogenesis.
Dr. Rasmussen agreed that INSL3 levels could be clinically useful for tracking Leydig cell function, especially in combination with other hormone markers like serum testosterone and gonadotropins. The group is now considering a clinical trial for treatment of hypogonadal men following illicit use of anabolic steroids, which will include INSL3 serum levels as a planned endpoint.
The researchers conducted a cross-sectional study of men aged 18-50 years who had participated in recreational strength training. Cohort 1 included 37 AAS users, 33 former users, and 30 never users. Cohort 2 included 9 current users, 9 former users, and 14 never users. They assigned participant AAS use status based on self-reporting, along with measurement of biomedical parameters including gonadotropins, sexual hormone-binding globulin (SHBG), and hematocrit.
Compared with never users' median value of 0.59 mcg/L, INSL3 serum levels were lower among current AAS (median, 0.04 mcg/L; P < .001) and former AAS (0.39 mcg/L; P = .005) users. A linear multivariate regression that adjusted for luteinizing hormone, SHBG, age, body-fat percentage, smoking status, use of other illicit drugs found lower levels among former users, compared with never users (mean difference, -0.16 mcg/L; P = .011).
An analysis of elapsed duration since AAS cessation found longer duration of AAS use was associated with reduced INSL3 levels (mean difference, -0.08; P = .022).
Although serum inhibin B levels reached the levels of never users after about 21 months, and luteinizing hormone levels recovered in about 12 months, neither serum testosterone nor INSL3 levels recovered in former users.
The study authors received funding from Anti Doping Denmark. Dr. Jayasena has no relevant financial disclosures.
*Dr. Rasmussen's affiliation has been corrected.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM