User login
Consider treating ovarian torsion with conservative surgery in young women
PHILADELPHIA – Women with ovarian torsion had a lower rate of perioperative complications when treated with conservative surgery, compared with oophorectomy, according to results from a retrospective study presented at the annual meeting of the American Society for Reproductive Medicine.
The effectiveness of laparoscopy and conservative surgery has increased in recent years, but over 75% of women with ovarian torsion in the study were treated with oophorectomy and 60% underwent a laparotomy, said Rachel S. Mandelbaum, MD, of the department of obstetrics & gynecology at the University of Southern California, Los Angeles.
“We believe that conservative surgery should be performed whenever possible in young women with ovarian torsion regardless of the appearance of the ovary intraoperatively,” said Dr. Mandelbaum.
The researchers performed a retrospective, observational study of 89,801 women in the Nationwide Inpatient Sample who were younger than age 50 years, were diagnosed with ovarian torsion during Jan. 2001–Sept. 2015, and were treated with conservative surgery or oophorectomy. Patients were excluded if they had malignancy, were older than 50 years of age, or their surgery information was unavailable. The majority of patients in the study were white (46%), nonobese (91%), without comorbidities, privately insured (59%), and were seen at a large (61%) urban hospital (51% teaching; 38% nonteaching).
Dr. Mandelbaum and colleagues found 78% of patients received a cystectomy, 19% had cyst drainage, 11% had detorsion alone, and 0.5% had an oophoropexy, with less than 10% of patients having a combination of cystectomy, cyst drainage, and oophoropexy. According to a multivariable analysis, patients who were treated with conservative surgery were more likely to be young, have a high income, live in the northeastern United States, be treated with laparoscopy, and be seen at a large hospital or teaching hospital (P less than .001). Oophorectomy was more common in patients with a high number of comorbidities and in patients with morbid obesity (P less than .001).
Between 2001 and 2015, the rate of conservative surgery increased from 19% to 25% (P less than .001); however, the rate of conservative surgery by age was nearly 40% in pediatric patients up to 15 years old, while the rate of conservative surgery declined by almost half until 35 years, followed by a further decline until age 50 years, said Dr. Mandelbaum. Use of laparoscopy also increased from 31% in 2001 to 42% in 2015 (P less than .001).
Overall, 20,643 patients underwent conservative surgery and 69,157 patients received an oophorectomy. Patients in the conservative surgery group were more likely to undergo a conservative surgery with a laparoscopic surgical approach (51%) than a laparotomy (41%), while patients receiving an oophorectomy were more likely to have a laparotomy (67%) than a laparoscopic surgical approach (33%). In 1,663 conservative surgeries (8%), the approach was unknown.
(odds ratio, 0.57; 95% confidence interval, 0.57-0.78; P less than .001), but there was a similar rate of venous thromboembolism (0.3% vs. 0.2%; P equals .568) and sepsis (0.3% vs. 0.3%; P equals .865) in each group.
Dr. Mandelbaum attributed the high rate of oophorectomies in the study to “differential uptake of evidence” in different areas of the United States, fear of complications from leaving an infected ovary in situ, or the surgeon’s belief that the ovary is not viable because of its color intraoperatively. “We know from animal and human studies that the intraoperative appearance of the ovary does not correlate to viability, and that 90% of black or blue ovaries regain function and subsequently appear normal on both transvaginal ultrasound or on a second look grossly,” she said. Oophorectomy rates also vary by surgeon, and gynecologists are more likely to perform conservative surgery, she added.
The researchers said they were unable to obtain data on specific surgical variables such as the size of the mass, time to surgery, intraoperative appearance, laterality, fertility wishes of the patient, and surgeon type. There were also no postdischarge data, or information on the timing of complications.
Dr. Mandelbaum reported no relevant conflicts of interest.
SOURCE: Mandelbaum RS et al. ASRM 2019. Abstract O-96.
PHILADELPHIA – Women with ovarian torsion had a lower rate of perioperative complications when treated with conservative surgery, compared with oophorectomy, according to results from a retrospective study presented at the annual meeting of the American Society for Reproductive Medicine.
The effectiveness of laparoscopy and conservative surgery has increased in recent years, but over 75% of women with ovarian torsion in the study were treated with oophorectomy and 60% underwent a laparotomy, said Rachel S. Mandelbaum, MD, of the department of obstetrics & gynecology at the University of Southern California, Los Angeles.
“We believe that conservative surgery should be performed whenever possible in young women with ovarian torsion regardless of the appearance of the ovary intraoperatively,” said Dr. Mandelbaum.
The researchers performed a retrospective, observational study of 89,801 women in the Nationwide Inpatient Sample who were younger than age 50 years, were diagnosed with ovarian torsion during Jan. 2001–Sept. 2015, and were treated with conservative surgery or oophorectomy. Patients were excluded if they had malignancy, were older than 50 years of age, or their surgery information was unavailable. The majority of patients in the study were white (46%), nonobese (91%), without comorbidities, privately insured (59%), and were seen at a large (61%) urban hospital (51% teaching; 38% nonteaching).
Dr. Mandelbaum and colleagues found 78% of patients received a cystectomy, 19% had cyst drainage, 11% had detorsion alone, and 0.5% had an oophoropexy, with less than 10% of patients having a combination of cystectomy, cyst drainage, and oophoropexy. According to a multivariable analysis, patients who were treated with conservative surgery were more likely to be young, have a high income, live in the northeastern United States, be treated with laparoscopy, and be seen at a large hospital or teaching hospital (P less than .001). Oophorectomy was more common in patients with a high number of comorbidities and in patients with morbid obesity (P less than .001).
Between 2001 and 2015, the rate of conservative surgery increased from 19% to 25% (P less than .001); however, the rate of conservative surgery by age was nearly 40% in pediatric patients up to 15 years old, while the rate of conservative surgery declined by almost half until 35 years, followed by a further decline until age 50 years, said Dr. Mandelbaum. Use of laparoscopy also increased from 31% in 2001 to 42% in 2015 (P less than .001).
Overall, 20,643 patients underwent conservative surgery and 69,157 patients received an oophorectomy. Patients in the conservative surgery group were more likely to undergo a conservative surgery with a laparoscopic surgical approach (51%) than a laparotomy (41%), while patients receiving an oophorectomy were more likely to have a laparotomy (67%) than a laparoscopic surgical approach (33%). In 1,663 conservative surgeries (8%), the approach was unknown.
(odds ratio, 0.57; 95% confidence interval, 0.57-0.78; P less than .001), but there was a similar rate of venous thromboembolism (0.3% vs. 0.2%; P equals .568) and sepsis (0.3% vs. 0.3%; P equals .865) in each group.
Dr. Mandelbaum attributed the high rate of oophorectomies in the study to “differential uptake of evidence” in different areas of the United States, fear of complications from leaving an infected ovary in situ, or the surgeon’s belief that the ovary is not viable because of its color intraoperatively. “We know from animal and human studies that the intraoperative appearance of the ovary does not correlate to viability, and that 90% of black or blue ovaries regain function and subsequently appear normal on both transvaginal ultrasound or on a second look grossly,” she said. Oophorectomy rates also vary by surgeon, and gynecologists are more likely to perform conservative surgery, she added.
The researchers said they were unable to obtain data on specific surgical variables such as the size of the mass, time to surgery, intraoperative appearance, laterality, fertility wishes of the patient, and surgeon type. There were also no postdischarge data, or information on the timing of complications.
Dr. Mandelbaum reported no relevant conflicts of interest.
SOURCE: Mandelbaum RS et al. ASRM 2019. Abstract O-96.
PHILADELPHIA – Women with ovarian torsion had a lower rate of perioperative complications when treated with conservative surgery, compared with oophorectomy, according to results from a retrospective study presented at the annual meeting of the American Society for Reproductive Medicine.
The effectiveness of laparoscopy and conservative surgery has increased in recent years, but over 75% of women with ovarian torsion in the study were treated with oophorectomy and 60% underwent a laparotomy, said Rachel S. Mandelbaum, MD, of the department of obstetrics & gynecology at the University of Southern California, Los Angeles.
“We believe that conservative surgery should be performed whenever possible in young women with ovarian torsion regardless of the appearance of the ovary intraoperatively,” said Dr. Mandelbaum.
The researchers performed a retrospective, observational study of 89,801 women in the Nationwide Inpatient Sample who were younger than age 50 years, were diagnosed with ovarian torsion during Jan. 2001–Sept. 2015, and were treated with conservative surgery or oophorectomy. Patients were excluded if they had malignancy, were older than 50 years of age, or their surgery information was unavailable. The majority of patients in the study were white (46%), nonobese (91%), without comorbidities, privately insured (59%), and were seen at a large (61%) urban hospital (51% teaching; 38% nonteaching).
Dr. Mandelbaum and colleagues found 78% of patients received a cystectomy, 19% had cyst drainage, 11% had detorsion alone, and 0.5% had an oophoropexy, with less than 10% of patients having a combination of cystectomy, cyst drainage, and oophoropexy. According to a multivariable analysis, patients who were treated with conservative surgery were more likely to be young, have a high income, live in the northeastern United States, be treated with laparoscopy, and be seen at a large hospital or teaching hospital (P less than .001). Oophorectomy was more common in patients with a high number of comorbidities and in patients with morbid obesity (P less than .001).
Between 2001 and 2015, the rate of conservative surgery increased from 19% to 25% (P less than .001); however, the rate of conservative surgery by age was nearly 40% in pediatric patients up to 15 years old, while the rate of conservative surgery declined by almost half until 35 years, followed by a further decline until age 50 years, said Dr. Mandelbaum. Use of laparoscopy also increased from 31% in 2001 to 42% in 2015 (P less than .001).
Overall, 20,643 patients underwent conservative surgery and 69,157 patients received an oophorectomy. Patients in the conservative surgery group were more likely to undergo a conservative surgery with a laparoscopic surgical approach (51%) than a laparotomy (41%), while patients receiving an oophorectomy were more likely to have a laparotomy (67%) than a laparoscopic surgical approach (33%). In 1,663 conservative surgeries (8%), the approach was unknown.
(odds ratio, 0.57; 95% confidence interval, 0.57-0.78; P less than .001), but there was a similar rate of venous thromboembolism (0.3% vs. 0.2%; P equals .568) and sepsis (0.3% vs. 0.3%; P equals .865) in each group.
Dr. Mandelbaum attributed the high rate of oophorectomies in the study to “differential uptake of evidence” in different areas of the United States, fear of complications from leaving an infected ovary in situ, or the surgeon’s belief that the ovary is not viable because of its color intraoperatively. “We know from animal and human studies that the intraoperative appearance of the ovary does not correlate to viability, and that 90% of black or blue ovaries regain function and subsequently appear normal on both transvaginal ultrasound or on a second look grossly,” she said. Oophorectomy rates also vary by surgeon, and gynecologists are more likely to perform conservative surgery, she added.
The researchers said they were unable to obtain data on specific surgical variables such as the size of the mass, time to surgery, intraoperative appearance, laterality, fertility wishes of the patient, and surgeon type. There were also no postdischarge data, or information on the timing of complications.
Dr. Mandelbaum reported no relevant conflicts of interest.
SOURCE: Mandelbaum RS et al. ASRM 2019. Abstract O-96.
REPORTING FROM ASRM 2019
SSRIs may reduce fecundability, live birth rate in reproductive-age women
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
PHILADELPHIA – Lindsey A. Sjaarda, PhD, reported at the annual meeting of the American Society for Reproductive Medicine.
In addition, women in a subgroup receiving fluoxetine experienced a lower live birth rate and greater incidence of pregnancy loss than women taking other SSRIs, but the results were not statistically significant, said Dr. Sjaarda of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“[It] is biologically plausible that fluoxetine might have some different effects,” she noted. “It does have some different interaction with [cytochrome] P-450 enzyme activity, and this translates to it having a much longer half-life as well. It’s different in terms of drug metabolism and in its interaction with the hormone biosynthesis pathway.”
Most of the research on antidepressants and SSRIs in pregnancy has focused on the safety of the agents, rather than the effect on pregnancy for women trying to conceive, explained Dr. Sjaarda. Previous research also has shown inconsistent findings for fecundability in women of reproductive age taking SSRIs, and the risk of specific SSRI compounds on pregnancy loss is unclear.
The researchers performed a longitudinal exposure assessment of the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial, which consisted of 1,228 women aged between 18 and 40 years trying to conceive. Patients were included if they had one to two prior pregnancy losses, up to two live births, and had been trying to become pregnant for six menstrual cycles; they were excluded if they had a severe history of mental illness. There were 1,035 women who had no preconception antidepressant exposure and 183 who did have preconception antidepressant exposure.
Patients provided longitudinal urine samples at various time points, including while trying to conceive and in early pregnancy, during the menses phase of each menstrual cycle and at their last menstrual cycle, and at 4 and 8 weeks’ gestation if they become pregnant. The urine samples were collected at home or in clinic; human chorionic gonadotropin levels were measured on the stored samples. The researchers defined pregnancy loss as any kind of loss measured after detecting human chorionic gonadotropin, and they used the patient’s medical record to determine live birth. The fecundability odds ratio was used to estimate the odds of conception in menstrual cycles.
Aypical and tricyclic antidepressants and SSRIs such as sertraline, fluoxetine, and citalopram/escitalopram were analyzed, as well as use of opioids, cannabinoids, and benzodiazepines. In total, 172 women used SSRIs, which represented 94% of the patient group analyzed, said Dr. Sjaarda. “This cohort really represents women who are successfully controlled with first-line agents.”
Patients in both the SSRI and no-antidepressant groups had similar baseline characteristics, but there were differences with regard to body mass index (26 kg/m2 vs. 28 kg/m2), employment status (77% vs. 67%), perceived stress (1.0 vs. 0.9), and opioid exposure (16% vs. 23%).
The researchers found use of any SSRI was associated with a 23% reduction in fecundability, with patients using fluoxetine, sertraline, and citalopram/escitalopram having a similar reduction in fecundability, compared with patients not using SSRIs.
Patients who received SSRIs also had approximately a 53% live birth rate overall. When analyzed by individual SSRI, however, there was a statistically significant reduction in the live birth rate for patients who were using fluoxetine, compared with patients using sertraline and citalopram/escitalopram. “This suggests that there was something besides just reduced fecundability going on with the fluoxetine-exposed women,” said Dr. Sjaarda.
When SSRI use was analyzed with regard to pregnancy loss, there was a generally null effect between women exposed to SSRIs overall versus those not exposed at the time before conception, at last menstrual period, and at 4 or 8 weeks’ gestation. But when grouped by specific SSRI, patients receiving fluoxetine had increased risk of pregnancy loss prior to conception, compared with patients not taking fluoxetine (34% vs. 24%; adjusted risk ratio, 1.41; 95% confidence interval, 0.94-2.12) , as well as at their last menstrual period (34% vs. 24%; adjusted RR, 1.48; 95% CI, 0.98-2.24) and at 4 weeks of pregnancy (31% vs. 22%; adjusted RR, 1.61; 95% CI, 0.94-2.78). “This was about a 40%-60% increase in pregnancy loss, even though the sample size is generally small when you divide it into these groups,” said Dr. Sjaarda.
Mental health care is an important public health and maternal health issue, and SSRIs as a drug class are essential for helping to appropriately manage mental health, noted Dr. Sjaarda.
Because “patients’ disease severities all vary and the reactions to different drugs vary, no one-size-fits-all recommendation can be made for people planning a pregnancy while using SSRIs,” concluded Dr. Sjaarda. “However, we’re hoping that women and their physicians can now consider these new data, which are based on objective and longitudinally measured exposure, as well as prospectively-assessed outcomes for these most common antidepressants and develop to a more informed and individualized plan for women who are trying to conceive and use SSRIs.”
Dr. Sjaarda reported no relevant conflicts of interest.
SOURCE: Sjaarda L et al. ASRM 2019, Abstract O-1.
REPORTING FROM ASRM 2019
Twin births down among women 30 and older
according to the National Center for Health Statistics.
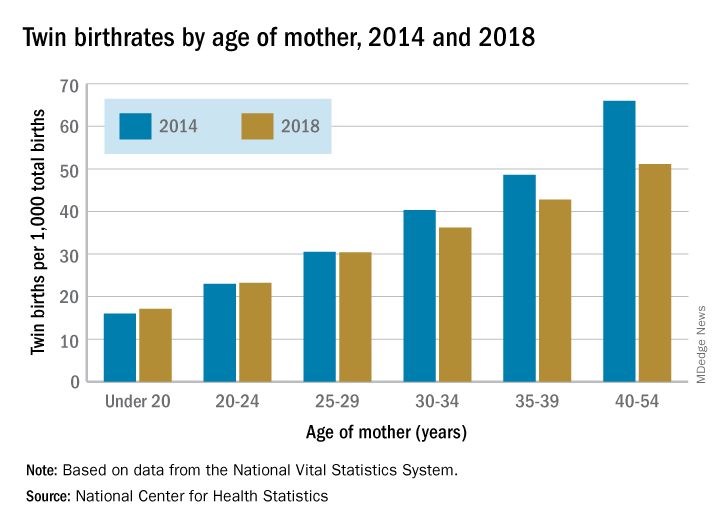
The twin birthrate, which had increased by 79% during 1980-2014, fell by 4% during 2014-2018, but that decline was “not universal across maternal age and race and Hispanic-origin groups,” the NCHS investigators said.
Twin birthrates fell by at least 10% for mothers aged 30 years and older from 2014 to 2018 but held steady for women in their twenties. Over that same period, the twin birthrate fell by a significant 7% among non-Hispanic white women (36.7 to 34.3 per 1,000 total births) but increased just slightly for non-Hispanic black women (40.0 to 40.5 per 1,000) and Hispanic women (24.1 to 24.4), the investigators reported.
For women 30 years and older, the drops in twin births got larger as age increased and were significant for each age group. The rate for women aged 30-34 years fell 10% as it went from 40.3 per 1,000 total births in 2014 to 36.2 per 1,000. The decrease was 12% (from 48.6 per 1,000 to 42.8) for women aged 35-39 and 23% (from 66.0 to 51.1) for those aged 40 years and older, they said based on data from the National Vital Statistics System.
The rates were basically unchanged for women in their 20s, from 23.0 to 23.2 in 20- to 24-year-olds and 30.5 to 30.4 in 25- to 29-year-olds – but there was a significant increase for the youngest group with rates among those younger than 20 years going from 16.0 to 17.1 per 1,000, the report showed.
according to the National Center for Health Statistics.

The twin birthrate, which had increased by 79% during 1980-2014, fell by 4% during 2014-2018, but that decline was “not universal across maternal age and race and Hispanic-origin groups,” the NCHS investigators said.
Twin birthrates fell by at least 10% for mothers aged 30 years and older from 2014 to 2018 but held steady for women in their twenties. Over that same period, the twin birthrate fell by a significant 7% among non-Hispanic white women (36.7 to 34.3 per 1,000 total births) but increased just slightly for non-Hispanic black women (40.0 to 40.5 per 1,000) and Hispanic women (24.1 to 24.4), the investigators reported.
For women 30 years and older, the drops in twin births got larger as age increased and were significant for each age group. The rate for women aged 30-34 years fell 10% as it went from 40.3 per 1,000 total births in 2014 to 36.2 per 1,000. The decrease was 12% (from 48.6 per 1,000 to 42.8) for women aged 35-39 and 23% (from 66.0 to 51.1) for those aged 40 years and older, they said based on data from the National Vital Statistics System.
The rates were basically unchanged for women in their 20s, from 23.0 to 23.2 in 20- to 24-year-olds and 30.5 to 30.4 in 25- to 29-year-olds – but there was a significant increase for the youngest group with rates among those younger than 20 years going from 16.0 to 17.1 per 1,000, the report showed.
according to the National Center for Health Statistics.

The twin birthrate, which had increased by 79% during 1980-2014, fell by 4% during 2014-2018, but that decline was “not universal across maternal age and race and Hispanic-origin groups,” the NCHS investigators said.
Twin birthrates fell by at least 10% for mothers aged 30 years and older from 2014 to 2018 but held steady for women in their twenties. Over that same period, the twin birthrate fell by a significant 7% among non-Hispanic white women (36.7 to 34.3 per 1,000 total births) but increased just slightly for non-Hispanic black women (40.0 to 40.5 per 1,000) and Hispanic women (24.1 to 24.4), the investigators reported.
For women 30 years and older, the drops in twin births got larger as age increased and were significant for each age group. The rate for women aged 30-34 years fell 10% as it went from 40.3 per 1,000 total births in 2014 to 36.2 per 1,000. The decrease was 12% (from 48.6 per 1,000 to 42.8) for women aged 35-39 and 23% (from 66.0 to 51.1) for those aged 40 years and older, they said based on data from the National Vital Statistics System.
The rates were basically unchanged for women in their 20s, from 23.0 to 23.2 in 20- to 24-year-olds and 30.5 to 30.4 in 25- to 29-year-olds – but there was a significant increase for the youngest group with rates among those younger than 20 years going from 16.0 to 17.1 per 1,000, the report showed.
High maternal lead levels linked to children’s obesity
Children born to mothers with high blood levels of lead have an increased risk of being overweight or obese, particularly if their mothers are also overweight, according to new research.
Adequate maternal plasma levels of folate, however, mitigated this risk.
“When considered simultaneously, maternal lead exposure, rather than early childhood lead exposure, contributed to overweight/obesity risk in a dose-response fashion across multiple developmental stages (preschool age, school age and early adolescence) and amplified intergenerational overweight/obesity risk (additively with maternal overweight/obesity),” Guoying Wang, MD, PhD, of Johns Hopkins Bloomberg School of Public Health, Baltimore, and associates, reported in JAMA Network Open.
“These findings support the hypothesis that the obesity epidemic could be related to environmental chemical exposures in utero and raise the possibility that optimal maternal folate supplementation may help counteract the adverse effects of environmental lead exposure,” the authors wrote.
The prospective urban, low-income cohort study, which ran from 2002 to 2013, involved 1,442 mother-child pairs who joined the study when the children were born and attended follow-up visits at Boston Medical Center. The mean age of the mothers was 29 years, and the children were, on average, 8 years old at follow-up. Half the children were male; 67% of mothers were black, and 20% were Latina.
The researchers collected maternal blood samples within 24-72 hours after birth to measure red blood cell lead levels and plasma folate levels. Children’s whole-blood lead levels were measured during the first lead screening of their well child visits, at a median 10 months of age. Researchers tracked children’s body mass index Z-score and defined overweight/obesity as exceeding the 85th national percentile for their age and sex.
Detectable lead was present in all the mothers’ blood samples. The median maternal red blood cell lead level was 2.5 mcg/dL, although black mothers tended to have higher lead exposure than that of other racial groups. Median maternal plasma folate level was 32 nmol/L. Children’s blood lead levels were a median 1.4 mcg/dL, and their median BMI Z-score was 0.78.
Children whose mothers had red blood cell lead levels of 5.0 mcg/dL or greater (16%) had 65% greater odds of being overweight or obese compared with children whose mothers’ lead level was less than 2 mcg/dL, after adjustment for maternal education, race/ethnicity, smoking status, parity, diabetes, hypertensive disorder, preterm birth, fetal growth, and breastfeeding status (odds ratio [OR], 1.65; 95% confidence internal [CI], 1.18-2.32). Only 5.2% of children had whole-blood lead levels of 5 mcg/dL or greater.
“Mothers with the highest red blood cell lead levels were older and multiparous, were more likely to be black and nonsmokers, had lower plasma folate levels and were more likely to have prepregnancy overweight/obesity and diabetes,” the authors reported.
The dose-response association did not lose significance when the researchers adjusted for children’s blood lead levels, maternal age, cesarean delivery, term births only, and black race. Nor did it change in a subset of children when the researchers adjusted for children’s physical activity.
The strength of the association increased when mothers also had a BMI greater than the average/healthy range. Children were more than four times more likely to be overweight or obese if their mothers were overweight or obese and had lead levels greater than 5.0 mcg/dL, compared with nonoverweight mothers with levels below 2 mcg/dL (OR, 4.24; 95% CI, 2.64-6.82).
Among children whose mothers were overweight/obese and had high blood lead levels, however, high folate levels appeared protective against obesity. These children had a 41% lower risk of being overweight or obese, compared with others in their group, if their mothers had plasma folate levels of at least 20 nmol/L (OR, 0.59 CI, 0.36-0.95; P = .03).
According to an invited commentary, “approximately 140,000 new chemicals and pesticides have appeared since 1950,” with “universal human exposure to approximately 5,000 of those,” wrote Marco Sanchez-Guerra, PhD, of the National Institute of Perinatology in Mexico City, and coauthors Andres Cardenas, PhD, of the University of California, Berkeley, and Citlalli Osorio-Yáñez, PhD, of the National Autonomous University of Mexico in Mexico City. Yet fewer than half of those chemicals have been tested for safety or toxic effect, the editorialists wrote, and scientists know little of their potential reproductive harm.
Dr. Sanchez-Guerra, Dr. Cardenas, and Dr. Osorio-Yáñez agreed with the study authors that elevated lead exposures, especially from gasoline before lead was removed in the United States in 1975, may partly explain the current epidemic of obesity.
“Identifying preventable prenatal causes of obesity is a cornerstone in the fight against the obesity epidemic,” the editorialists said. While most recommendations center on changes to diet and physical activity, environmental factors during pregnancy could be involved in childhood obesity as well.
“The study by Wang et al. opens the door to new questions about whether adequate folate intake might modify the adverse effects of other chemical exposures,” they continued, noting other research suggesting a protective effect from folate against health effects of air pollution exposure. “These efforts could yield substantial public health benefits and represent novel tools in fighting the obesity epidemic,” they concluded.
The research was funded by the National Institutes of Health and the U.S. Department of Health and Human Services. Neither the study authors nor the editorialists had industry financial disclosures.
SOURCES: Wang G et al. JAMA Netw Open. 2019;2(10):e1912343. doi: 10.1001/jamanetworkopen.2019.12343; Sanchez-Guerra M et al. JAMA Netw Open. 2019;2(10):e1912334. doi: 10.1001/jamanetworkopen.2019.12334.
Children born to mothers with high blood levels of lead have an increased risk of being overweight or obese, particularly if their mothers are also overweight, according to new research.
Adequate maternal plasma levels of folate, however, mitigated this risk.
“When considered simultaneously, maternal lead exposure, rather than early childhood lead exposure, contributed to overweight/obesity risk in a dose-response fashion across multiple developmental stages (preschool age, school age and early adolescence) and amplified intergenerational overweight/obesity risk (additively with maternal overweight/obesity),” Guoying Wang, MD, PhD, of Johns Hopkins Bloomberg School of Public Health, Baltimore, and associates, reported in JAMA Network Open.
“These findings support the hypothesis that the obesity epidemic could be related to environmental chemical exposures in utero and raise the possibility that optimal maternal folate supplementation may help counteract the adverse effects of environmental lead exposure,” the authors wrote.
The prospective urban, low-income cohort study, which ran from 2002 to 2013, involved 1,442 mother-child pairs who joined the study when the children were born and attended follow-up visits at Boston Medical Center. The mean age of the mothers was 29 years, and the children were, on average, 8 years old at follow-up. Half the children were male; 67% of mothers were black, and 20% were Latina.
The researchers collected maternal blood samples within 24-72 hours after birth to measure red blood cell lead levels and plasma folate levels. Children’s whole-blood lead levels were measured during the first lead screening of their well child visits, at a median 10 months of age. Researchers tracked children’s body mass index Z-score and defined overweight/obesity as exceeding the 85th national percentile for their age and sex.
Detectable lead was present in all the mothers’ blood samples. The median maternal red blood cell lead level was 2.5 mcg/dL, although black mothers tended to have higher lead exposure than that of other racial groups. Median maternal plasma folate level was 32 nmol/L. Children’s blood lead levels were a median 1.4 mcg/dL, and their median BMI Z-score was 0.78.
Children whose mothers had red blood cell lead levels of 5.0 mcg/dL or greater (16%) had 65% greater odds of being overweight or obese compared with children whose mothers’ lead level was less than 2 mcg/dL, after adjustment for maternal education, race/ethnicity, smoking status, parity, diabetes, hypertensive disorder, preterm birth, fetal growth, and breastfeeding status (odds ratio [OR], 1.65; 95% confidence internal [CI], 1.18-2.32). Only 5.2% of children had whole-blood lead levels of 5 mcg/dL or greater.
“Mothers with the highest red blood cell lead levels were older and multiparous, were more likely to be black and nonsmokers, had lower plasma folate levels and were more likely to have prepregnancy overweight/obesity and diabetes,” the authors reported.
The dose-response association did not lose significance when the researchers adjusted for children’s blood lead levels, maternal age, cesarean delivery, term births only, and black race. Nor did it change in a subset of children when the researchers adjusted for children’s physical activity.
The strength of the association increased when mothers also had a BMI greater than the average/healthy range. Children were more than four times more likely to be overweight or obese if their mothers were overweight or obese and had lead levels greater than 5.0 mcg/dL, compared with nonoverweight mothers with levels below 2 mcg/dL (OR, 4.24; 95% CI, 2.64-6.82).
Among children whose mothers were overweight/obese and had high blood lead levels, however, high folate levels appeared protective against obesity. These children had a 41% lower risk of being overweight or obese, compared with others in their group, if their mothers had plasma folate levels of at least 20 nmol/L (OR, 0.59 CI, 0.36-0.95; P = .03).
According to an invited commentary, “approximately 140,000 new chemicals and pesticides have appeared since 1950,” with “universal human exposure to approximately 5,000 of those,” wrote Marco Sanchez-Guerra, PhD, of the National Institute of Perinatology in Mexico City, and coauthors Andres Cardenas, PhD, of the University of California, Berkeley, and Citlalli Osorio-Yáñez, PhD, of the National Autonomous University of Mexico in Mexico City. Yet fewer than half of those chemicals have been tested for safety or toxic effect, the editorialists wrote, and scientists know little of their potential reproductive harm.
Dr. Sanchez-Guerra, Dr. Cardenas, and Dr. Osorio-Yáñez agreed with the study authors that elevated lead exposures, especially from gasoline before lead was removed in the United States in 1975, may partly explain the current epidemic of obesity.
“Identifying preventable prenatal causes of obesity is a cornerstone in the fight against the obesity epidemic,” the editorialists said. While most recommendations center on changes to diet and physical activity, environmental factors during pregnancy could be involved in childhood obesity as well.
“The study by Wang et al. opens the door to new questions about whether adequate folate intake might modify the adverse effects of other chemical exposures,” they continued, noting other research suggesting a protective effect from folate against health effects of air pollution exposure. “These efforts could yield substantial public health benefits and represent novel tools in fighting the obesity epidemic,” they concluded.
The research was funded by the National Institutes of Health and the U.S. Department of Health and Human Services. Neither the study authors nor the editorialists had industry financial disclosures.
SOURCES: Wang G et al. JAMA Netw Open. 2019;2(10):e1912343. doi: 10.1001/jamanetworkopen.2019.12343; Sanchez-Guerra M et al. JAMA Netw Open. 2019;2(10):e1912334. doi: 10.1001/jamanetworkopen.2019.12334.
Children born to mothers with high blood levels of lead have an increased risk of being overweight or obese, particularly if their mothers are also overweight, according to new research.
Adequate maternal plasma levels of folate, however, mitigated this risk.
“When considered simultaneously, maternal lead exposure, rather than early childhood lead exposure, contributed to overweight/obesity risk in a dose-response fashion across multiple developmental stages (preschool age, school age and early adolescence) and amplified intergenerational overweight/obesity risk (additively with maternal overweight/obesity),” Guoying Wang, MD, PhD, of Johns Hopkins Bloomberg School of Public Health, Baltimore, and associates, reported in JAMA Network Open.
“These findings support the hypothesis that the obesity epidemic could be related to environmental chemical exposures in utero and raise the possibility that optimal maternal folate supplementation may help counteract the adverse effects of environmental lead exposure,” the authors wrote.
The prospective urban, low-income cohort study, which ran from 2002 to 2013, involved 1,442 mother-child pairs who joined the study when the children were born and attended follow-up visits at Boston Medical Center. The mean age of the mothers was 29 years, and the children were, on average, 8 years old at follow-up. Half the children were male; 67% of mothers were black, and 20% were Latina.
The researchers collected maternal blood samples within 24-72 hours after birth to measure red blood cell lead levels and plasma folate levels. Children’s whole-blood lead levels were measured during the first lead screening of their well child visits, at a median 10 months of age. Researchers tracked children’s body mass index Z-score and defined overweight/obesity as exceeding the 85th national percentile for their age and sex.
Detectable lead was present in all the mothers’ blood samples. The median maternal red blood cell lead level was 2.5 mcg/dL, although black mothers tended to have higher lead exposure than that of other racial groups. Median maternal plasma folate level was 32 nmol/L. Children’s blood lead levels were a median 1.4 mcg/dL, and their median BMI Z-score was 0.78.
Children whose mothers had red blood cell lead levels of 5.0 mcg/dL or greater (16%) had 65% greater odds of being overweight or obese compared with children whose mothers’ lead level was less than 2 mcg/dL, after adjustment for maternal education, race/ethnicity, smoking status, parity, diabetes, hypertensive disorder, preterm birth, fetal growth, and breastfeeding status (odds ratio [OR], 1.65; 95% confidence internal [CI], 1.18-2.32). Only 5.2% of children had whole-blood lead levels of 5 mcg/dL or greater.
“Mothers with the highest red blood cell lead levels were older and multiparous, were more likely to be black and nonsmokers, had lower plasma folate levels and were more likely to have prepregnancy overweight/obesity and diabetes,” the authors reported.
The dose-response association did not lose significance when the researchers adjusted for children’s blood lead levels, maternal age, cesarean delivery, term births only, and black race. Nor did it change in a subset of children when the researchers adjusted for children’s physical activity.
The strength of the association increased when mothers also had a BMI greater than the average/healthy range. Children were more than four times more likely to be overweight or obese if their mothers were overweight or obese and had lead levels greater than 5.0 mcg/dL, compared with nonoverweight mothers with levels below 2 mcg/dL (OR, 4.24; 95% CI, 2.64-6.82).
Among children whose mothers were overweight/obese and had high blood lead levels, however, high folate levels appeared protective against obesity. These children had a 41% lower risk of being overweight or obese, compared with others in their group, if their mothers had plasma folate levels of at least 20 nmol/L (OR, 0.59 CI, 0.36-0.95; P = .03).
According to an invited commentary, “approximately 140,000 new chemicals and pesticides have appeared since 1950,” with “universal human exposure to approximately 5,000 of those,” wrote Marco Sanchez-Guerra, PhD, of the National Institute of Perinatology in Mexico City, and coauthors Andres Cardenas, PhD, of the University of California, Berkeley, and Citlalli Osorio-Yáñez, PhD, of the National Autonomous University of Mexico in Mexico City. Yet fewer than half of those chemicals have been tested for safety or toxic effect, the editorialists wrote, and scientists know little of their potential reproductive harm.
Dr. Sanchez-Guerra, Dr. Cardenas, and Dr. Osorio-Yáñez agreed with the study authors that elevated lead exposures, especially from gasoline before lead was removed in the United States in 1975, may partly explain the current epidemic of obesity.
“Identifying preventable prenatal causes of obesity is a cornerstone in the fight against the obesity epidemic,” the editorialists said. While most recommendations center on changes to diet and physical activity, environmental factors during pregnancy could be involved in childhood obesity as well.
“The study by Wang et al. opens the door to new questions about whether adequate folate intake might modify the adverse effects of other chemical exposures,” they continued, noting other research suggesting a protective effect from folate against health effects of air pollution exposure. “These efforts could yield substantial public health benefits and represent novel tools in fighting the obesity epidemic,” they concluded.
The research was funded by the National Institutes of Health and the U.S. Department of Health and Human Services. Neither the study authors nor the editorialists had industry financial disclosures.
SOURCES: Wang G et al. JAMA Netw Open. 2019;2(10):e1912343. doi: 10.1001/jamanetworkopen.2019.12343; Sanchez-Guerra M et al. JAMA Netw Open. 2019;2(10):e1912334. doi: 10.1001/jamanetworkopen.2019.12334.
FROM JAMA NETWORK OPEN
Try testosterone for some women with sexual dysfunction, but not others
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Product Update: Osphena’s NDA, new hysteroscope, TempSure RF technology, Resilient stirrup covers
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
Teen mothers using long-acting reversible contraception are least likely to use condoms
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
such as the birth control pill, vaginal ring, contraceptive patch, or injection, according to research in JAMA Pediatrics.
This highlights a need for education to lower the risk of sexually transmitted infections in this population.
“Our finding that less than 30% of sexually active teenage mothers using LARC or non-LARC hormonal methods also reported using condoms suggests the need for enhanced efforts to increase condom use among teenage mothers,” wrote Katherine Kortsmit, PhD, MPH, of the National Center for Chronic Disease Prevention and Health Promotion at the Centers for Disease Control and Prevention, Atlanta, and colleagues.
The researchers performed a cross-sectional analysis of contraceptive use among 5,480 new teenage mothers between 2012 and 2015 who were aged 19 years or younger in the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were mainly first-time teenage mothers between ages 18 and 19 years (46% non-Hispanic white), current Medicaid users, and reported an unintended pregnancy. Dr. Kortsmit and colleagues monitored use of LARC and non-LARC hormonal methods, including condom use, among participants in PRAMS from 37 different sites.
Among teenage mothers in PRAM, 29% reported using condoms; 18% of mothers using LARC said they also used condoms, compared with 36% of mothers who used non-LARC hormonal methods (adjusted prevalence ratio, 0.50; 95% confidence interval, 0.41-0.60). Participants with IUDs were least likely to report using condoms (15%), compared with participants using implants (22%; aPR, 0.70; 95% CI, 0.51-0.98), participants using the patch, ring, or injection (25%; aPR, 0.61; 95% CI, 0.47-0.79), or the pill (47%; aPR, 0.32; 95% CI, 0.25-0.40).
“These findings can be used to inform clinician counseling that sexually active teenage mothers have low uptake of condom use combined with more effective contraceptive methods and may need additional counseling on the importance of consistent and correct condom use for the prevention of STIs,” Dr. Kortsmit and associates wrote.
Limitations included the self-reported nature of the study, and lack of information on baseline condom use prior to pregnancy, relationship characteristics, and sexual partners during the postpartum period.
Education on contraceptive methods by clinicians is an important part of an adolescent’s contextualization of the benefits and risks of those methods, especially for women of color and marginalized groups, Andrea J. Hoopes, MD, MPH; and Gina S. Sucato, MD, MPH, wrote in an editorial related to the study by Kortsmit and colleagues.
In particular, these groups have higher rates of unplanned pregnancy, may have a history of being coerced to use contraception, and may be reluctant to discuss their sexual history or contraception use. “Many young women, including teenage mothers, remain at risk for coercion from partners, family members, and health care clinicians, so adopting a stance that ensures autonomy while eliciting unique developmental perspectives is paramount,” they said.
It is critically important to give women access to LARCs that are effective and easily used, and patients have a right to choose the contraception method that best fits their situation. It is through integrated programs, made available by Title X funding, that clinicians may be able to monitor their patients’ sexual, reproductive, and psychological health needs, and have conversations about the importance of contraception and prevention of sexually transmitted infections.
“Future studies should examine specific interventions aimed at promoting all adolescents’ motivations to remain safe and healthy by using condoms consistently and by seeking comprehensive sexual health care services, regardless of contraceptive method,” concluded Dr. Hoopes and Dr. Sucato, of the Adolescent Center at Kaiser Permanente Washington in Seattle. “In addition to receiving counseling about, and access to, condoms, adolescents need to develop the skills to negotiate condom use with partners.”
Dr. Kortsmit received support in the form of an appointment to the Research Participation Program at Centers for Disease Control and Prevention through an interagency agreement. The other authors reported no conflicts of interest.
Dr. Hoopes reported previous grant support from Bayer and the North American Society for Pediatric and Adolescent Gynecology. Dr. Sucato reported previous grant and other research support from Teva.
SOURCE: Kortsmit K et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1136; Hoopes AJ et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.1133.
FROM JAMA PEDIATRICS
Women express low decision regret after preimplantation testing for aneuploidy
undergoing fertility treatment, research suggests.
In a report published in Human Reproduction, researchers did an anonymous survey of 69 patients undergoing their first cycle of autologous preimplantation genetic testing for aneuploidy (PGT-A) at a single fertility center.
“Despite the known distress associated with many aspects of assisted reproductive technology (ART) and the many opportunities for distress among patients pursuing PGT-A, little is known about the associated patient experience and psychological risks,” wrote Dr. Kara N. Goldman of New York University Langone Fertility Center, and coauthors.
“A ‘failure’ after PGT-A can present in many forms well before other IVF losses may be experienced: Embryos may not meet criteria for biopsy, PGT-A may result in an all-aneuploid embryo cohort, or a euploid embryo may fail to implant,” the authors continued.
The mean overall decision regret scale score was 8.5 on a scale of 0-100 – with a median of 0 – and 61% of respondents said they had no regrets about undergoing preimplantation genetic testing for aneuploidy; the remaining 39% reported “any degree of regret.”
This “one-third of respondents reported some degree of regret, suggesting an important opportunity for pretest counseling and support among patients pursuing PGT-A,” Dr. Goldman and associates emphasized.
Of the respondents who then underwent euploid embryo transfer, and who had a known pregnancy outcome, the 36 with an ongoing or delivered pregnancy had significantly less decision regret than the 24 who experienced a negative pregnancy test or a miscarriage.
The study found no differences in decision regret between those aged under or over 35 years of age, those with different levels of educational attainment, or between patients who paid exclusively out of pocket compared with those with any insurance coverage.
However, greater levels of decision regret were seen in patients who had experienced a longer time since retrieval of oocytes and those who said they would consider pregnancy with donor oocytes if they were unsuccessful with IVF and PGT-A.
“Completing a cycle of IVF with PGT-A and obtaining no usable, euploid embryos results in distress, but this distress must be weighed against the alternative scenario in which a patient invests valuable time, energy, and resources into a futile embryo transfer cycle resulting in a negative pregnancy test, miscarriage, or aneuploid gestation,” the authors wrote.
When assessing the dependence of the decision regret score on demographic factors, the researchers found that patients who had learned about PGT-A from their physicians, rather than from other sources such as friends or the Internet, had the highest levels of decision regret.
There was no external funding. One coauthor declared personal fees and other support from the fertility and pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Goldman KN et al. Hum Reprod. 2019 Jun 21. doi: 10.1093/humrep/dez080.
undergoing fertility treatment, research suggests.
In a report published in Human Reproduction, researchers did an anonymous survey of 69 patients undergoing their first cycle of autologous preimplantation genetic testing for aneuploidy (PGT-A) at a single fertility center.
“Despite the known distress associated with many aspects of assisted reproductive technology (ART) and the many opportunities for distress among patients pursuing PGT-A, little is known about the associated patient experience and psychological risks,” wrote Dr. Kara N. Goldman of New York University Langone Fertility Center, and coauthors.
“A ‘failure’ after PGT-A can present in many forms well before other IVF losses may be experienced: Embryos may not meet criteria for biopsy, PGT-A may result in an all-aneuploid embryo cohort, or a euploid embryo may fail to implant,” the authors continued.
The mean overall decision regret scale score was 8.5 on a scale of 0-100 – with a median of 0 – and 61% of respondents said they had no regrets about undergoing preimplantation genetic testing for aneuploidy; the remaining 39% reported “any degree of regret.”
This “one-third of respondents reported some degree of regret, suggesting an important opportunity for pretest counseling and support among patients pursuing PGT-A,” Dr. Goldman and associates emphasized.
Of the respondents who then underwent euploid embryo transfer, and who had a known pregnancy outcome, the 36 with an ongoing or delivered pregnancy had significantly less decision regret than the 24 who experienced a negative pregnancy test or a miscarriage.
The study found no differences in decision regret between those aged under or over 35 years of age, those with different levels of educational attainment, or between patients who paid exclusively out of pocket compared with those with any insurance coverage.
However, greater levels of decision regret were seen in patients who had experienced a longer time since retrieval of oocytes and those who said they would consider pregnancy with donor oocytes if they were unsuccessful with IVF and PGT-A.
“Completing a cycle of IVF with PGT-A and obtaining no usable, euploid embryos results in distress, but this distress must be weighed against the alternative scenario in which a patient invests valuable time, energy, and resources into a futile embryo transfer cycle resulting in a negative pregnancy test, miscarriage, or aneuploid gestation,” the authors wrote.
When assessing the dependence of the decision regret score on demographic factors, the researchers found that patients who had learned about PGT-A from their physicians, rather than from other sources such as friends or the Internet, had the highest levels of decision regret.
There was no external funding. One coauthor declared personal fees and other support from the fertility and pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Goldman KN et al. Hum Reprod. 2019 Jun 21. doi: 10.1093/humrep/dez080.
undergoing fertility treatment, research suggests.
In a report published in Human Reproduction, researchers did an anonymous survey of 69 patients undergoing their first cycle of autologous preimplantation genetic testing for aneuploidy (PGT-A) at a single fertility center.
“Despite the known distress associated with many aspects of assisted reproductive technology (ART) and the many opportunities for distress among patients pursuing PGT-A, little is known about the associated patient experience and psychological risks,” wrote Dr. Kara N. Goldman of New York University Langone Fertility Center, and coauthors.
“A ‘failure’ after PGT-A can present in many forms well before other IVF losses may be experienced: Embryos may not meet criteria for biopsy, PGT-A may result in an all-aneuploid embryo cohort, or a euploid embryo may fail to implant,” the authors continued.
The mean overall decision regret scale score was 8.5 on a scale of 0-100 – with a median of 0 – and 61% of respondents said they had no regrets about undergoing preimplantation genetic testing for aneuploidy; the remaining 39% reported “any degree of regret.”
This “one-third of respondents reported some degree of regret, suggesting an important opportunity for pretest counseling and support among patients pursuing PGT-A,” Dr. Goldman and associates emphasized.
Of the respondents who then underwent euploid embryo transfer, and who had a known pregnancy outcome, the 36 with an ongoing or delivered pregnancy had significantly less decision regret than the 24 who experienced a negative pregnancy test or a miscarriage.
The study found no differences in decision regret between those aged under or over 35 years of age, those with different levels of educational attainment, or between patients who paid exclusively out of pocket compared with those with any insurance coverage.
However, greater levels of decision regret were seen in patients who had experienced a longer time since retrieval of oocytes and those who said they would consider pregnancy with donor oocytes if they were unsuccessful with IVF and PGT-A.
“Completing a cycle of IVF with PGT-A and obtaining no usable, euploid embryos results in distress, but this distress must be weighed against the alternative scenario in which a patient invests valuable time, energy, and resources into a futile embryo transfer cycle resulting in a negative pregnancy test, miscarriage, or aneuploid gestation,” the authors wrote.
When assessing the dependence of the decision regret score on demographic factors, the researchers found that patients who had learned about PGT-A from their physicians, rather than from other sources such as friends or the Internet, had the highest levels of decision regret.
There was no external funding. One coauthor declared personal fees and other support from the fertility and pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Goldman KN et al. Hum Reprod. 2019 Jun 21. doi: 10.1093/humrep/dez080.
FROM HUMAN REPRODUCTION
Consider cutaneous endometriosis in women with umbilical lesions
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
according to Liza Raffi of the University of Southern California, Los Angeles, and associates.
The report, published in the International Journal of Women’s Dermatology, detailed a case of a woman aged 41 years who presented with a 5-month history of a painful firm subcutaneous nodule in the umbilicus and flares of pain during menstrual periods. Her past history indicated a missed miscarriage (removed by dilation and curettage) and laparoscopic left salpingectomy for a ruptured ectopic pregnancy.
At presentation, the woman reported undergoing fertility treatments including subcutaneous injections of follitropin beta and choriogonadotropin alfa.
Because of the patient’s history of salpingectomy and painful menstrual periods, her physicians suspected cutaneous endometriosis. An ultrasound was performed to rule out fistula, and then a punch biopsy of the nodule was performed. The biopsy showed endometrial glands with encompassing fibrotic stroma, which was consistent with cutaneous endometriosis, likely transplanted during the laparoscopic port site entry during salpingectomy.
The patient chose to undergo surgery for excision of the nodule, declining hormonal therapy because she was undergoing fertility treatment.
“The differential diagnosis of umbilical lesions with similar presentation includes keloid, dermatofibroma, dermatofibrosarcoma protuberans, and cutaneous metastasis of cancer,” the investigators wrote. “Ultimately, patients should be referred to obstetrics & gynecology if they describe classic symptoms including pain with menses, dyspareunia, and infertility and wish to explore diagnostic and therapeutic options.”
Ms. Raffi and associates reported they had no conflicts of interest. There was no external funding.
SOURCE: Raffi L et al. Int J Womens Dermatol. 2019 Jul 2. doi: 10.1016/j.ijwd.2019.06.025.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Dr. Eve Espey: Some good news in her 2019 contraceptive update
NASHVILLE, TENN. – There’s some good news on the contraception and reproductive health front, according to a recent update from Eve Espey, MD.
The unintended pregnancy rate in the United States, including among adolescents and young women, is declining, and the U.S. abortion rate is at its lowest level since Roe v. Wade, she said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
A 2016 article based on 2008-2011 data showed that after hovering around 50% for nearly 3 decades, the unintended pregnancy rate dropped “for the first time in a very long period of time,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology, division of family planning at the University of New Mexico, Albuquerque (N Engl J Med. 2016; 374[9]:843-52).
“It doesn’t look that impressive – it basically went down to 45%, but considering the scope and the number of women who are affected by unplanned pregnancy, this is actually a huge public health achievement,” she said. “And I think ... in the next cycles of the [Center for Disease Control and Prevention’s] National Survey of Family Growth ... we’ll hopefully continue to see this and potentially more [decline].”
As for abortion rates, an increase occurred following Roe v. Wade, but rates are now down to pre-Roe levels.
“One of the things that we know about the abortion rate is that the most important determinant ... is access to contraceptives,” Dr. Espey said, noting that both the abortion and unintended pregnancy rate declines are attributable to better and more consistent use of contraceptives, increased abstinence as teens are waiting longer to have sex, and the “meteoric rise in long-acting reversible contraceptive (LARC) use.”
Importantly, while improvements in public health have traditionally only impacted upper-class white women, a reduction is finally occurring in disparities with women of color, but those disparities still remain,” she added. “Just like we’re focusing so much on this relative to maternal mortality, the same kinds of disparities occur in access to reproductive health.”
Dr. Espey also provided updates on other aspects of contraception.
IUDs and other LARC methods
The use of LARCs increased from 2% of contraceptive types used by reproductive-aged women in 2002 to 12% in 2012. The majority of that change was in IUD use, with a small increase in implant use, she said, noting that the latest data from the 2015-2017 cycle of the National Survey of Family Growth shows that the rate is now up to 16%.
“The rise has been nothing that I ever imagined that I would see, certainly in my professional career,” she said.
The huge impact of LARCs on the unintended pregnancy rate is attributable to consistent effectiveness over time, compared with an increasing failure rate over time with short-acting contraceptive methods, she said, explaining that while the failure rate with oral contraceptives is about 8%-9% over the first 3 years, it increases to 53% at 8 years.
It’s a matter of looking at both “typical use” effectiveness and continuation rates: LARCs have continuation rates of about 75%-85%; Depot-Provera, for example, has a 25%-30% continuation rate at 1 year, she noted.
Dr. Espey also attributed the gains to improved access via the Affordable Care Act’s contraceptive mandate, which has been shown in numerous studies to have improved access and consistency of contraceptive use, but which is “currently being chipped away,” and to the federal Title X program that covers family planning care for low income women, including undocumented women.
“These two programs have made a huge impact for us, and I hope that we as ob.gyns. will continue to support them,” she said.
Reproductive justice
Despite their effectiveness, it is important to remember that LARC methods are not right for everyone, Dr. Espey said.
“It’s not all about effectiveness. Women have many reasons for accessing contraception, and our job is not to reduce unintended pregnancy. ... The idea really is that we empower women. ... We should really give choices and trust women to make the best choices for them,” she explained.
Barriers to IUD removal also should be eliminated, she noted, explaining that a woman who wants her IUD removed a month after insertion should have that option.
She said she has “changed her language,” from asking why a woman wants an $800 IUD removed after a month to asking whether she would like to hear about ways to make it better or if she is “just ready to have it removed.”
For those not interested in a discussion about birth control, she suggested providing information about the bedsider.org site.
“This is a great resource for patients,” she said, noting that it is available in both English and Spanish.
U.S. Medical Eligibility Criteria and Selected Practice Recommendations on contraceptive use
The MEC contraceptive guidance, a regularly updated, evidence-based project of the CDC, provides “best practices” information on candidate selection, or the “who” of contraceptive selection (who is a candidate for a particular method), Dr. Espy said, noting that it’s a “handy resource” for in-office use.
The SPR is more of a “how-to” guide that provides specifics on contraceptive use, such as when a woman can rely on the pill for contraception after she starts taking it, or how a woman should be followed after IUD placement, she said.
A free CDC app provides access to both.
Emergency contraception
The best overall emergency contraceptive method is the copper IUD, but often it is less accessible than oral methods, of which ulipristal acetate (ella), is the best choice, Dr. Espy said.
“Ulipristal is kind of a best-kept secret. It’s a selective estrogen-receptor modulator – it actually works better and longer than Plan B (levonorgestrel). What’s great about Plan B is that you can get it over the counter, but ulipristal delays ovulation longer,” she explained.
Contraceptives and obesity
Oral contraceptive efficacy is “so much more about adherence,” than about weight, she said.
With respect to the contraceptive patch, limited evidence suggests that obesity may reduce effectiveness, but “it’s still way better than barrier methods,” and for the contraceptive ring, no evidence suggests that obesity affects efficacy, she said.
For emergency contraception, evidence suggests that ulipristal is more effective than Plan B in women with high body mass index.
OTC contraceptive access
Pharmacy and OTC access are a good idea, Dr. Espy said.
“ACOG now supports both, which is great, and there are now a number of states where women can access contraception through the pharmacy. There are a lot of barriers there as well, and really the answer is OTC access,” she said. “There is a pill right now that is seeking [Food and Drug Administration] approval; it will be a progestin-only pill – the first one to be available over the counter, so I think this is something that we’ll see in the next 5-10 years.”
Additional future directions
One technology in development is a longer-acting injectable, such as a 6- or 9-month Depot-type shot.
Biodegradable implants also are in development. “What a cool idea – it just disappears in your arm, no need to remove it,” Dr. Espey said, adding that nonsurgical permanent sterilization is another possible advance, which would be “a holy grail.”
As for male contraception?
“I’ve been saying for about 25 years that in 5 years we’ll have a male contraceptive, so I’m not going to say it anymore with any kind of time frame, but it’s possible,” she said.
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – There’s some good news on the contraception and reproductive health front, according to a recent update from Eve Espey, MD.
The unintended pregnancy rate in the United States, including among adolescents and young women, is declining, and the U.S. abortion rate is at its lowest level since Roe v. Wade, she said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
A 2016 article based on 2008-2011 data showed that after hovering around 50% for nearly 3 decades, the unintended pregnancy rate dropped “for the first time in a very long period of time,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology, division of family planning at the University of New Mexico, Albuquerque (N Engl J Med. 2016; 374[9]:843-52).
“It doesn’t look that impressive – it basically went down to 45%, but considering the scope and the number of women who are affected by unplanned pregnancy, this is actually a huge public health achievement,” she said. “And I think ... in the next cycles of the [Center for Disease Control and Prevention’s] National Survey of Family Growth ... we’ll hopefully continue to see this and potentially more [decline].”
As for abortion rates, an increase occurred following Roe v. Wade, but rates are now down to pre-Roe levels.
“One of the things that we know about the abortion rate is that the most important determinant ... is access to contraceptives,” Dr. Espey said, noting that both the abortion and unintended pregnancy rate declines are attributable to better and more consistent use of contraceptives, increased abstinence as teens are waiting longer to have sex, and the “meteoric rise in long-acting reversible contraceptive (LARC) use.”
Importantly, while improvements in public health have traditionally only impacted upper-class white women, a reduction is finally occurring in disparities with women of color, but those disparities still remain,” she added. “Just like we’re focusing so much on this relative to maternal mortality, the same kinds of disparities occur in access to reproductive health.”
Dr. Espey also provided updates on other aspects of contraception.
IUDs and other LARC methods
The use of LARCs increased from 2% of contraceptive types used by reproductive-aged women in 2002 to 12% in 2012. The majority of that change was in IUD use, with a small increase in implant use, she said, noting that the latest data from the 2015-2017 cycle of the National Survey of Family Growth shows that the rate is now up to 16%.
“The rise has been nothing that I ever imagined that I would see, certainly in my professional career,” she said.
The huge impact of LARCs on the unintended pregnancy rate is attributable to consistent effectiveness over time, compared with an increasing failure rate over time with short-acting contraceptive methods, she said, explaining that while the failure rate with oral contraceptives is about 8%-9% over the first 3 years, it increases to 53% at 8 years.
It’s a matter of looking at both “typical use” effectiveness and continuation rates: LARCs have continuation rates of about 75%-85%; Depot-Provera, for example, has a 25%-30% continuation rate at 1 year, she noted.
Dr. Espey also attributed the gains to improved access via the Affordable Care Act’s contraceptive mandate, which has been shown in numerous studies to have improved access and consistency of contraceptive use, but which is “currently being chipped away,” and to the federal Title X program that covers family planning care for low income women, including undocumented women.
“These two programs have made a huge impact for us, and I hope that we as ob.gyns. will continue to support them,” she said.
Reproductive justice
Despite their effectiveness, it is important to remember that LARC methods are not right for everyone, Dr. Espey said.
“It’s not all about effectiveness. Women have many reasons for accessing contraception, and our job is not to reduce unintended pregnancy. ... The idea really is that we empower women. ... We should really give choices and trust women to make the best choices for them,” she explained.
Barriers to IUD removal also should be eliminated, she noted, explaining that a woman who wants her IUD removed a month after insertion should have that option.
She said she has “changed her language,” from asking why a woman wants an $800 IUD removed after a month to asking whether she would like to hear about ways to make it better or if she is “just ready to have it removed.”
For those not interested in a discussion about birth control, she suggested providing information about the bedsider.org site.
“This is a great resource for patients,” she said, noting that it is available in both English and Spanish.
U.S. Medical Eligibility Criteria and Selected Practice Recommendations on contraceptive use
The MEC contraceptive guidance, a regularly updated, evidence-based project of the CDC, provides “best practices” information on candidate selection, or the “who” of contraceptive selection (who is a candidate for a particular method), Dr. Espy said, noting that it’s a “handy resource” for in-office use.
The SPR is more of a “how-to” guide that provides specifics on contraceptive use, such as when a woman can rely on the pill for contraception after she starts taking it, or how a woman should be followed after IUD placement, she said.
A free CDC app provides access to both.
Emergency contraception
The best overall emergency contraceptive method is the copper IUD, but often it is less accessible than oral methods, of which ulipristal acetate (ella), is the best choice, Dr. Espy said.
“Ulipristal is kind of a best-kept secret. It’s a selective estrogen-receptor modulator – it actually works better and longer than Plan B (levonorgestrel). What’s great about Plan B is that you can get it over the counter, but ulipristal delays ovulation longer,” she explained.
Contraceptives and obesity
Oral contraceptive efficacy is “so much more about adherence,” than about weight, she said.
With respect to the contraceptive patch, limited evidence suggests that obesity may reduce effectiveness, but “it’s still way better than barrier methods,” and for the contraceptive ring, no evidence suggests that obesity affects efficacy, she said.
For emergency contraception, evidence suggests that ulipristal is more effective than Plan B in women with high body mass index.
OTC contraceptive access
Pharmacy and OTC access are a good idea, Dr. Espy said.
“ACOG now supports both, which is great, and there are now a number of states where women can access contraception through the pharmacy. There are a lot of barriers there as well, and really the answer is OTC access,” she said. “There is a pill right now that is seeking [Food and Drug Administration] approval; it will be a progestin-only pill – the first one to be available over the counter, so I think this is something that we’ll see in the next 5-10 years.”
Additional future directions
One technology in development is a longer-acting injectable, such as a 6- or 9-month Depot-type shot.
Biodegradable implants also are in development. “What a cool idea – it just disappears in your arm, no need to remove it,” Dr. Espey said, adding that nonsurgical permanent sterilization is another possible advance, which would be “a holy grail.”
As for male contraception?
“I’ve been saying for about 25 years that in 5 years we’ll have a male contraceptive, so I’m not going to say it anymore with any kind of time frame, but it’s possible,” she said.
Dr. Espey reported having no financial disclosures.
NASHVILLE, TENN. – There’s some good news on the contraception and reproductive health front, according to a recent update from Eve Espey, MD.
The unintended pregnancy rate in the United States, including among adolescents and young women, is declining, and the U.S. abortion rate is at its lowest level since Roe v. Wade, she said at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
A 2016 article based on 2008-2011 data showed that after hovering around 50% for nearly 3 decades, the unintended pregnancy rate dropped “for the first time in a very long period of time,” said Dr. Espey, professor and chair of the department of obstetrics & gynecology, division of family planning at the University of New Mexico, Albuquerque (N Engl J Med. 2016; 374[9]:843-52).
“It doesn’t look that impressive – it basically went down to 45%, but considering the scope and the number of women who are affected by unplanned pregnancy, this is actually a huge public health achievement,” she said. “And I think ... in the next cycles of the [Center for Disease Control and Prevention’s] National Survey of Family Growth ... we’ll hopefully continue to see this and potentially more [decline].”
As for abortion rates, an increase occurred following Roe v. Wade, but rates are now down to pre-Roe levels.
“One of the things that we know about the abortion rate is that the most important determinant ... is access to contraceptives,” Dr. Espey said, noting that both the abortion and unintended pregnancy rate declines are attributable to better and more consistent use of contraceptives, increased abstinence as teens are waiting longer to have sex, and the “meteoric rise in long-acting reversible contraceptive (LARC) use.”
Importantly, while improvements in public health have traditionally only impacted upper-class white women, a reduction is finally occurring in disparities with women of color, but those disparities still remain,” she added. “Just like we’re focusing so much on this relative to maternal mortality, the same kinds of disparities occur in access to reproductive health.”
Dr. Espey also provided updates on other aspects of contraception.
IUDs and other LARC methods
The use of LARCs increased from 2% of contraceptive types used by reproductive-aged women in 2002 to 12% in 2012. The majority of that change was in IUD use, with a small increase in implant use, she said, noting that the latest data from the 2015-2017 cycle of the National Survey of Family Growth shows that the rate is now up to 16%.
“The rise has been nothing that I ever imagined that I would see, certainly in my professional career,” she said.
The huge impact of LARCs on the unintended pregnancy rate is attributable to consistent effectiveness over time, compared with an increasing failure rate over time with short-acting contraceptive methods, she said, explaining that while the failure rate with oral contraceptives is about 8%-9% over the first 3 years, it increases to 53% at 8 years.
It’s a matter of looking at both “typical use” effectiveness and continuation rates: LARCs have continuation rates of about 75%-85%; Depot-Provera, for example, has a 25%-30% continuation rate at 1 year, she noted.
Dr. Espey also attributed the gains to improved access via the Affordable Care Act’s contraceptive mandate, which has been shown in numerous studies to have improved access and consistency of contraceptive use, but which is “currently being chipped away,” and to the federal Title X program that covers family planning care for low income women, including undocumented women.
“These two programs have made a huge impact for us, and I hope that we as ob.gyns. will continue to support them,” she said.
Reproductive justice
Despite their effectiveness, it is important to remember that LARC methods are not right for everyone, Dr. Espey said.
“It’s not all about effectiveness. Women have many reasons for accessing contraception, and our job is not to reduce unintended pregnancy. ... The idea really is that we empower women. ... We should really give choices and trust women to make the best choices for them,” she explained.
Barriers to IUD removal also should be eliminated, she noted, explaining that a woman who wants her IUD removed a month after insertion should have that option.
She said she has “changed her language,” from asking why a woman wants an $800 IUD removed after a month to asking whether she would like to hear about ways to make it better or if she is “just ready to have it removed.”
For those not interested in a discussion about birth control, she suggested providing information about the bedsider.org site.
“This is a great resource for patients,” she said, noting that it is available in both English and Spanish.
U.S. Medical Eligibility Criteria and Selected Practice Recommendations on contraceptive use
The MEC contraceptive guidance, a regularly updated, evidence-based project of the CDC, provides “best practices” information on candidate selection, or the “who” of contraceptive selection (who is a candidate for a particular method), Dr. Espy said, noting that it’s a “handy resource” for in-office use.
The SPR is more of a “how-to” guide that provides specifics on contraceptive use, such as when a woman can rely on the pill for contraception after she starts taking it, or how a woman should be followed after IUD placement, she said.
A free CDC app provides access to both.
Emergency contraception
The best overall emergency contraceptive method is the copper IUD, but often it is less accessible than oral methods, of which ulipristal acetate (ella), is the best choice, Dr. Espy said.
“Ulipristal is kind of a best-kept secret. It’s a selective estrogen-receptor modulator – it actually works better and longer than Plan B (levonorgestrel). What’s great about Plan B is that you can get it over the counter, but ulipristal delays ovulation longer,” she explained.
Contraceptives and obesity
Oral contraceptive efficacy is “so much more about adherence,” than about weight, she said.
With respect to the contraceptive patch, limited evidence suggests that obesity may reduce effectiveness, but “it’s still way better than barrier methods,” and for the contraceptive ring, no evidence suggests that obesity affects efficacy, she said.
For emergency contraception, evidence suggests that ulipristal is more effective than Plan B in women with high body mass index.
OTC contraceptive access
Pharmacy and OTC access are a good idea, Dr. Espy said.
“ACOG now supports both, which is great, and there are now a number of states where women can access contraception through the pharmacy. There are a lot of barriers there as well, and really the answer is OTC access,” she said. “There is a pill right now that is seeking [Food and Drug Administration] approval; it will be a progestin-only pill – the first one to be available over the counter, so I think this is something that we’ll see in the next 5-10 years.”
Additional future directions
One technology in development is a longer-acting injectable, such as a 6- or 9-month Depot-type shot.
Biodegradable implants also are in development. “What a cool idea – it just disappears in your arm, no need to remove it,” Dr. Espey said, adding that nonsurgical permanent sterilization is another possible advance, which would be “a holy grail.”
As for male contraception?
“I’ve been saying for about 25 years that in 5 years we’ll have a male contraceptive, so I’m not going to say it anymore with any kind of time frame, but it’s possible,” she said.
Dr. Espey reported having no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019