User login
Help! More Clinicians Are Needed to Manage Care for Children With Autism. How About You?
Almost all primary care providers (PCPs) have taken on diagnosing and managing ADHD. With about 12% of school aged children affected, typical PCPs can expect about 240 children with ADHD under their care. Adopting this primary care function has been helped by having clear diagnostic criteria for the three DMS 5 “presentations” of ADHD, open source tools (e.g. Vanderbilts), expectation of collaboration by educators, American Academy of Pediatrics (AAP) guidelines for diagnosis and management, Society for Developmental–Behavioral Pediatrics guidelines for “complex ADHD,” and access to effective medication treatments PCPs can provide (although less so for behavioral ones), cultural acceptance of individuals with ADHD, and especially reliable payment by insurers.
Screening
But what about PCP management of autism spectrum disorder (ASD), now affecting 2.8%, for an expected 60 children under care for each of us? It is more essential because very early detection and entry into evidence-based intervention has long-term benefits for the child and family that are not as crucial for ADHD. While ADHD symptoms may not impact functioning until age 7 or even 12 years of age, signs of ASD usually emerge earlier (by 18 months) but gradually and about 30% after apparently normal development even to age 2 years.
Screening is crucial, but unfortunately not perfect. Recent AAP surveys show that most PCPs screen for autism at the recommended 18 and 24 months. But what happens after that? How many offices are tracking referrals for positive screens for needed evaluations and early intervention? Our data shows that tracking is rarely done and children do not start to get the benefit of early intervention until 4.5 years of age, on average.
Diagnostic Testing
And screening is the easiest part of addressing ASD. Wait times for diagnostic testing can be agonizing months to years. Multiple programs are training PCPs to perform hands-on 10- to 30-minute secondary screening with considerable success. You can become proficient on tools such as STAT (Screening Tool for Autism in Two-Year-Olds), RITA-T (Rapid Interactive Screening Test for Autism in Toddlers), BISCUIT (Baby and Infant Screen for Children with Autism Traits), SORF (Systematic Observation of Red Flags), ADEC (Autism Detection in Early Childhood) or CARS (Childhood Autism Rating Scale) with a few hours of training. Even secondary assessments done virtually by PCPs such as TELE-ASD-PEDS quite accurately predict a verifiable ASD diagnosis for those referred by concerns. Some problems of the reported accuracy of these secondary screening processes have to do with validation in samples of children for whom parents or clinicians already had concern and generally not including many younger children in whom it is so important to detect. Level of confidence of developmental and behavioral pediatricians of the presence of ASD is highly related to ultimate diagnosis. But success with PCPs’ mastering secondary screening has not yet been reported to convince insurers to approve payment for intervention services such as Applied Behavior Analysis (ABA).
Comorbidity
Co-existing conditions affect the majority of patients with ASD (70%), compared with ADHD, but with a broader range and more debilitating and difficult to manage conditions. More medical co-existing issues such as intellectual disability (25%-75%), seizures (12%-26%), motor incoordination (51%), GI conditions (9%-91%), sleep difficulty (50%-80%), sleep apnea, congenital heart disease, avoidant-restrictive food intake disorder, autoimmune disorders, and genetic syndromes (e.g. Fragile X, tuberous sclerosis, Down, Angelman’s, untreated PKU, neurofibromatosis, Klinefelter syndrome) reflect the range of underpinnings of ASD. The need to detect and manage these co-existing issues, besides assessing hearing and vision, makes our skilled involvement and vigilance in ASD care essential. Referring for help from OTs, PTs, speech pathologists, neurologists, psychologists, and special educators as issues in their domains are prioritized is also our responsibility. We must also help families balance utilizing these resources so as to avoid overwhelm.
Anxiety (50%), ADHD (37%-85%), depression (54%), bipolar (7.3%), suicidal ideation (40% starting < 8 years), and emotion dysregulation, familiar to us from our management of ADHD, may develop but are often less well defined and more intractable in ASD, making use of screening tools essential. Using a system like CHADIS that has online pre-visit and monitoring screens delivered based on algorithms for the numerous co-existing conditions, automated handouts, and functions to make and track referral success can facilitate care for this complex chronic condition. Identifying mental health providers with ASD expertise is more difficult, so more management is on us. While medications for these conditions can be beneficial, we need to learn to use lower doses, slower dose increases, and employ problem-solving of side effects with more parent collaboration than for ADHD as children with ASD often cannot self-report effectively. We need to ask about the common ad hoc use of complementary medications and substances (32%-87%) that may be complicating. Of course, these conditions and the caveats of management require more of our time with the patient and family as well as communication with the many other professionals involved. It is important to set our own and our families’ expectations (and schedules) for much more frequent contact and also to bill appropriately with chronic care (99487,89,90) and collaborative care CPT codes (99492,3,4 or G2214).
Behavioral Manifestations
During our care, the often extreme behavioral manifestations of ASD may be the most pressing issues. We need new understanding and skills to sort out and counsel on inflexible, explosive, and sensory triggered behaviors. Just as for ADHD, using the approach of Functional Behavioral Assessment and plans for home as well as school behavior can be key. More difficult in ASD is looking for physical causes, since the child may not provide clear cues because of communication and sensory differences. Conditions common in children with ASD such as constipation, dental caries, otitis, dietary intolerances, allergies, migraine, sleep deficits, menstrual cramps, or fears and changes from puberty manifesting behaviorally are often tricky to sort out.
While the diagnosis of ASD, as for ADHD, does not require any laboratory testing, looking for possible causes is important information for the family and someday may also lead to genetic or other therapies. We need to know that recommendations include screening for Ferritin, Pb, chromosomal microarray and FMR I testing as well as checking that PKU was normal; MECP 2 is indicated in females and symptomatic males; and PTENS testing for children with head circumference greater than 2.5-3 SD. Metabolic and mitochondrial assays are indicated only when symptoms suggest. We need to develop confidence to reserve MRIs or EEGs for cases with abnormal neuro. exams, regression, or history of seizures. It is demanding to keep up with AAP recommendations in this very active area of research.
Interventions
The interventions for ADHD are generally school accommodations and therapies for comorbidities. In contrast, since core social communication skills are the main deficit in ASD, all children screened positive for ASD should be referred for early intervention while awaiting, as well as after, diagnosis. While all states have no or low-cost early intervention, quality and quantity (of hours offered) varies. We should also recommend and try to determine if evidence-based intervention is being provided, such as pivotal response training, UCLA discrete trial therapy, Carbone’s verbal behavior, applied behavior analysis (ABA), Early Start Denver Model, and sometimes music and social skills trainings (effect size 0.42-0.76). Such professional interventions have best evidence with more than 25 hours/week but 15 hours has benefit for higher functioning children. CBT can help anxiety even in younger children. One way for families to provide more hours and more generalizable intervention is coaching by the PLAY Project or DIRFloortime, parent mediated interventions with evidence, some with training both in person or online. Alternative communication training and other condition specific assistance are often needed (e.g. Picture Exchange Communication System for nonverbal children).
While we should already be familiar with writing 504 plan and IEP requests to schools, which also apply to children with ASD, in addition we need to be ready to advise about other legal rights including autism waivers, wraparound services, guardianship, and trust accounts. We can share quality educational materials available online (e.g. from Autism Speaks, SPARK, and Autism Navigator). Social media groups may be supportive, but also may contain disinformation we need to dispel.
Unfortunately, templates, questionnaires, and lack of interdisciplinary referral and communication functions of EHRs don’t support the complexities of care for ASD. While the AAP has guidelines for diagnosis and management and an online toolkit, consider adding a system with an autism-specific module like CHADIS and joining the Autism Care Network or ECHO Autism sessions to get both information and support to take on the evolving critical role of autism care.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Almost all primary care providers (PCPs) have taken on diagnosing and managing ADHD. With about 12% of school aged children affected, typical PCPs can expect about 240 children with ADHD under their care. Adopting this primary care function has been helped by having clear diagnostic criteria for the three DMS 5 “presentations” of ADHD, open source tools (e.g. Vanderbilts), expectation of collaboration by educators, American Academy of Pediatrics (AAP) guidelines for diagnosis and management, Society for Developmental–Behavioral Pediatrics guidelines for “complex ADHD,” and access to effective medication treatments PCPs can provide (although less so for behavioral ones), cultural acceptance of individuals with ADHD, and especially reliable payment by insurers.
Screening
But what about PCP management of autism spectrum disorder (ASD), now affecting 2.8%, for an expected 60 children under care for each of us? It is more essential because very early detection and entry into evidence-based intervention has long-term benefits for the child and family that are not as crucial for ADHD. While ADHD symptoms may not impact functioning until age 7 or even 12 years of age, signs of ASD usually emerge earlier (by 18 months) but gradually and about 30% after apparently normal development even to age 2 years.
Screening is crucial, but unfortunately not perfect. Recent AAP surveys show that most PCPs screen for autism at the recommended 18 and 24 months. But what happens after that? How many offices are tracking referrals for positive screens for needed evaluations and early intervention? Our data shows that tracking is rarely done and children do not start to get the benefit of early intervention until 4.5 years of age, on average.
Diagnostic Testing
And screening is the easiest part of addressing ASD. Wait times for diagnostic testing can be agonizing months to years. Multiple programs are training PCPs to perform hands-on 10- to 30-minute secondary screening with considerable success. You can become proficient on tools such as STAT (Screening Tool for Autism in Two-Year-Olds), RITA-T (Rapid Interactive Screening Test for Autism in Toddlers), BISCUIT (Baby and Infant Screen for Children with Autism Traits), SORF (Systematic Observation of Red Flags), ADEC (Autism Detection in Early Childhood) or CARS (Childhood Autism Rating Scale) with a few hours of training. Even secondary assessments done virtually by PCPs such as TELE-ASD-PEDS quite accurately predict a verifiable ASD diagnosis for those referred by concerns. Some problems of the reported accuracy of these secondary screening processes have to do with validation in samples of children for whom parents or clinicians already had concern and generally not including many younger children in whom it is so important to detect. Level of confidence of developmental and behavioral pediatricians of the presence of ASD is highly related to ultimate diagnosis. But success with PCPs’ mastering secondary screening has not yet been reported to convince insurers to approve payment for intervention services such as Applied Behavior Analysis (ABA).
Comorbidity
Co-existing conditions affect the majority of patients with ASD (70%), compared with ADHD, but with a broader range and more debilitating and difficult to manage conditions. More medical co-existing issues such as intellectual disability (25%-75%), seizures (12%-26%), motor incoordination (51%), GI conditions (9%-91%), sleep difficulty (50%-80%), sleep apnea, congenital heart disease, avoidant-restrictive food intake disorder, autoimmune disorders, and genetic syndromes (e.g. Fragile X, tuberous sclerosis, Down, Angelman’s, untreated PKU, neurofibromatosis, Klinefelter syndrome) reflect the range of underpinnings of ASD. The need to detect and manage these co-existing issues, besides assessing hearing and vision, makes our skilled involvement and vigilance in ASD care essential. Referring for help from OTs, PTs, speech pathologists, neurologists, psychologists, and special educators as issues in their domains are prioritized is also our responsibility. We must also help families balance utilizing these resources so as to avoid overwhelm.
Anxiety (50%), ADHD (37%-85%), depression (54%), bipolar (7.3%), suicidal ideation (40% starting < 8 years), and emotion dysregulation, familiar to us from our management of ADHD, may develop but are often less well defined and more intractable in ASD, making use of screening tools essential. Using a system like CHADIS that has online pre-visit and monitoring screens delivered based on algorithms for the numerous co-existing conditions, automated handouts, and functions to make and track referral success can facilitate care for this complex chronic condition. Identifying mental health providers with ASD expertise is more difficult, so more management is on us. While medications for these conditions can be beneficial, we need to learn to use lower doses, slower dose increases, and employ problem-solving of side effects with more parent collaboration than for ADHD as children with ASD often cannot self-report effectively. We need to ask about the common ad hoc use of complementary medications and substances (32%-87%) that may be complicating. Of course, these conditions and the caveats of management require more of our time with the patient and family as well as communication with the many other professionals involved. It is important to set our own and our families’ expectations (and schedules) for much more frequent contact and also to bill appropriately with chronic care (99487,89,90) and collaborative care CPT codes (99492,3,4 or G2214).
Behavioral Manifestations
During our care, the often extreme behavioral manifestations of ASD may be the most pressing issues. We need new understanding and skills to sort out and counsel on inflexible, explosive, and sensory triggered behaviors. Just as for ADHD, using the approach of Functional Behavioral Assessment and plans for home as well as school behavior can be key. More difficult in ASD is looking for physical causes, since the child may not provide clear cues because of communication and sensory differences. Conditions common in children with ASD such as constipation, dental caries, otitis, dietary intolerances, allergies, migraine, sleep deficits, menstrual cramps, or fears and changes from puberty manifesting behaviorally are often tricky to sort out.
While the diagnosis of ASD, as for ADHD, does not require any laboratory testing, looking for possible causes is important information for the family and someday may also lead to genetic or other therapies. We need to know that recommendations include screening for Ferritin, Pb, chromosomal microarray and FMR I testing as well as checking that PKU was normal; MECP 2 is indicated in females and symptomatic males; and PTENS testing for children with head circumference greater than 2.5-3 SD. Metabolic and mitochondrial assays are indicated only when symptoms suggest. We need to develop confidence to reserve MRIs or EEGs for cases with abnormal neuro. exams, regression, or history of seizures. It is demanding to keep up with AAP recommendations in this very active area of research.
Interventions
The interventions for ADHD are generally school accommodations and therapies for comorbidities. In contrast, since core social communication skills are the main deficit in ASD, all children screened positive for ASD should be referred for early intervention while awaiting, as well as after, diagnosis. While all states have no or low-cost early intervention, quality and quantity (of hours offered) varies. We should also recommend and try to determine if evidence-based intervention is being provided, such as pivotal response training, UCLA discrete trial therapy, Carbone’s verbal behavior, applied behavior analysis (ABA), Early Start Denver Model, and sometimes music and social skills trainings (effect size 0.42-0.76). Such professional interventions have best evidence with more than 25 hours/week but 15 hours has benefit for higher functioning children. CBT can help anxiety even in younger children. One way for families to provide more hours and more generalizable intervention is coaching by the PLAY Project or DIRFloortime, parent mediated interventions with evidence, some with training both in person or online. Alternative communication training and other condition specific assistance are often needed (e.g. Picture Exchange Communication System for nonverbal children).
While we should already be familiar with writing 504 plan and IEP requests to schools, which also apply to children with ASD, in addition we need to be ready to advise about other legal rights including autism waivers, wraparound services, guardianship, and trust accounts. We can share quality educational materials available online (e.g. from Autism Speaks, SPARK, and Autism Navigator). Social media groups may be supportive, but also may contain disinformation we need to dispel.
Unfortunately, templates, questionnaires, and lack of interdisciplinary referral and communication functions of EHRs don’t support the complexities of care for ASD. While the AAP has guidelines for diagnosis and management and an online toolkit, consider adding a system with an autism-specific module like CHADIS and joining the Autism Care Network or ECHO Autism sessions to get both information and support to take on the evolving critical role of autism care.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Almost all primary care providers (PCPs) have taken on diagnosing and managing ADHD. With about 12% of school aged children affected, typical PCPs can expect about 240 children with ADHD under their care. Adopting this primary care function has been helped by having clear diagnostic criteria for the three DMS 5 “presentations” of ADHD, open source tools (e.g. Vanderbilts), expectation of collaboration by educators, American Academy of Pediatrics (AAP) guidelines for diagnosis and management, Society for Developmental–Behavioral Pediatrics guidelines for “complex ADHD,” and access to effective medication treatments PCPs can provide (although less so for behavioral ones), cultural acceptance of individuals with ADHD, and especially reliable payment by insurers.
Screening
But what about PCP management of autism spectrum disorder (ASD), now affecting 2.8%, for an expected 60 children under care for each of us? It is more essential because very early detection and entry into evidence-based intervention has long-term benefits for the child and family that are not as crucial for ADHD. While ADHD symptoms may not impact functioning until age 7 or even 12 years of age, signs of ASD usually emerge earlier (by 18 months) but gradually and about 30% after apparently normal development even to age 2 years.
Screening is crucial, but unfortunately not perfect. Recent AAP surveys show that most PCPs screen for autism at the recommended 18 and 24 months. But what happens after that? How many offices are tracking referrals for positive screens for needed evaluations and early intervention? Our data shows that tracking is rarely done and children do not start to get the benefit of early intervention until 4.5 years of age, on average.
Diagnostic Testing
And screening is the easiest part of addressing ASD. Wait times for diagnostic testing can be agonizing months to years. Multiple programs are training PCPs to perform hands-on 10- to 30-minute secondary screening with considerable success. You can become proficient on tools such as STAT (Screening Tool for Autism in Two-Year-Olds), RITA-T (Rapid Interactive Screening Test for Autism in Toddlers), BISCUIT (Baby and Infant Screen for Children with Autism Traits), SORF (Systematic Observation of Red Flags), ADEC (Autism Detection in Early Childhood) or CARS (Childhood Autism Rating Scale) with a few hours of training. Even secondary assessments done virtually by PCPs such as TELE-ASD-PEDS quite accurately predict a verifiable ASD diagnosis for those referred by concerns. Some problems of the reported accuracy of these secondary screening processes have to do with validation in samples of children for whom parents or clinicians already had concern and generally not including many younger children in whom it is so important to detect. Level of confidence of developmental and behavioral pediatricians of the presence of ASD is highly related to ultimate diagnosis. But success with PCPs’ mastering secondary screening has not yet been reported to convince insurers to approve payment for intervention services such as Applied Behavior Analysis (ABA).
Comorbidity
Co-existing conditions affect the majority of patients with ASD (70%), compared with ADHD, but with a broader range and more debilitating and difficult to manage conditions. More medical co-existing issues such as intellectual disability (25%-75%), seizures (12%-26%), motor incoordination (51%), GI conditions (9%-91%), sleep difficulty (50%-80%), sleep apnea, congenital heart disease, avoidant-restrictive food intake disorder, autoimmune disorders, and genetic syndromes (e.g. Fragile X, tuberous sclerosis, Down, Angelman’s, untreated PKU, neurofibromatosis, Klinefelter syndrome) reflect the range of underpinnings of ASD. The need to detect and manage these co-existing issues, besides assessing hearing and vision, makes our skilled involvement and vigilance in ASD care essential. Referring for help from OTs, PTs, speech pathologists, neurologists, psychologists, and special educators as issues in their domains are prioritized is also our responsibility. We must also help families balance utilizing these resources so as to avoid overwhelm.
Anxiety (50%), ADHD (37%-85%), depression (54%), bipolar (7.3%), suicidal ideation (40% starting < 8 years), and emotion dysregulation, familiar to us from our management of ADHD, may develop but are often less well defined and more intractable in ASD, making use of screening tools essential. Using a system like CHADIS that has online pre-visit and monitoring screens delivered based on algorithms for the numerous co-existing conditions, automated handouts, and functions to make and track referral success can facilitate care for this complex chronic condition. Identifying mental health providers with ASD expertise is more difficult, so more management is on us. While medications for these conditions can be beneficial, we need to learn to use lower doses, slower dose increases, and employ problem-solving of side effects with more parent collaboration than for ADHD as children with ASD often cannot self-report effectively. We need to ask about the common ad hoc use of complementary medications and substances (32%-87%) that may be complicating. Of course, these conditions and the caveats of management require more of our time with the patient and family as well as communication with the many other professionals involved. It is important to set our own and our families’ expectations (and schedules) for much more frequent contact and also to bill appropriately with chronic care (99487,89,90) and collaborative care CPT codes (99492,3,4 or G2214).
Behavioral Manifestations
During our care, the often extreme behavioral manifestations of ASD may be the most pressing issues. We need new understanding and skills to sort out and counsel on inflexible, explosive, and sensory triggered behaviors. Just as for ADHD, using the approach of Functional Behavioral Assessment and plans for home as well as school behavior can be key. More difficult in ASD is looking for physical causes, since the child may not provide clear cues because of communication and sensory differences. Conditions common in children with ASD such as constipation, dental caries, otitis, dietary intolerances, allergies, migraine, sleep deficits, menstrual cramps, or fears and changes from puberty manifesting behaviorally are often tricky to sort out.
While the diagnosis of ASD, as for ADHD, does not require any laboratory testing, looking for possible causes is important information for the family and someday may also lead to genetic or other therapies. We need to know that recommendations include screening for Ferritin, Pb, chromosomal microarray and FMR I testing as well as checking that PKU was normal; MECP 2 is indicated in females and symptomatic males; and PTENS testing for children with head circumference greater than 2.5-3 SD. Metabolic and mitochondrial assays are indicated only when symptoms suggest. We need to develop confidence to reserve MRIs or EEGs for cases with abnormal neuro. exams, regression, or history of seizures. It is demanding to keep up with AAP recommendations in this very active area of research.
Interventions
The interventions for ADHD are generally school accommodations and therapies for comorbidities. In contrast, since core social communication skills are the main deficit in ASD, all children screened positive for ASD should be referred for early intervention while awaiting, as well as after, diagnosis. While all states have no or low-cost early intervention, quality and quantity (of hours offered) varies. We should also recommend and try to determine if evidence-based intervention is being provided, such as pivotal response training, UCLA discrete trial therapy, Carbone’s verbal behavior, applied behavior analysis (ABA), Early Start Denver Model, and sometimes music and social skills trainings (effect size 0.42-0.76). Such professional interventions have best evidence with more than 25 hours/week but 15 hours has benefit for higher functioning children. CBT can help anxiety even in younger children. One way for families to provide more hours and more generalizable intervention is coaching by the PLAY Project or DIRFloortime, parent mediated interventions with evidence, some with training both in person or online. Alternative communication training and other condition specific assistance are often needed (e.g. Picture Exchange Communication System for nonverbal children).
While we should already be familiar with writing 504 plan and IEP requests to schools, which also apply to children with ASD, in addition we need to be ready to advise about other legal rights including autism waivers, wraparound services, guardianship, and trust accounts. We can share quality educational materials available online (e.g. from Autism Speaks, SPARK, and Autism Navigator). Social media groups may be supportive, but also may contain disinformation we need to dispel.
Unfortunately, templates, questionnaires, and lack of interdisciplinary referral and communication functions of EHRs don’t support the complexities of care for ASD. While the AAP has guidelines for diagnosis and management and an online toolkit, consider adding a system with an autism-specific module like CHADIS and joining the Autism Care Network or ECHO Autism sessions to get both information and support to take on the evolving critical role of autism care.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
A 7-year-old female presents with persistent pimples on the nose and cheeks for approximately 1 year
Diagnosis
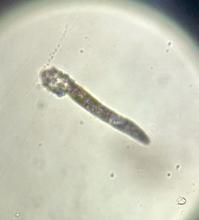
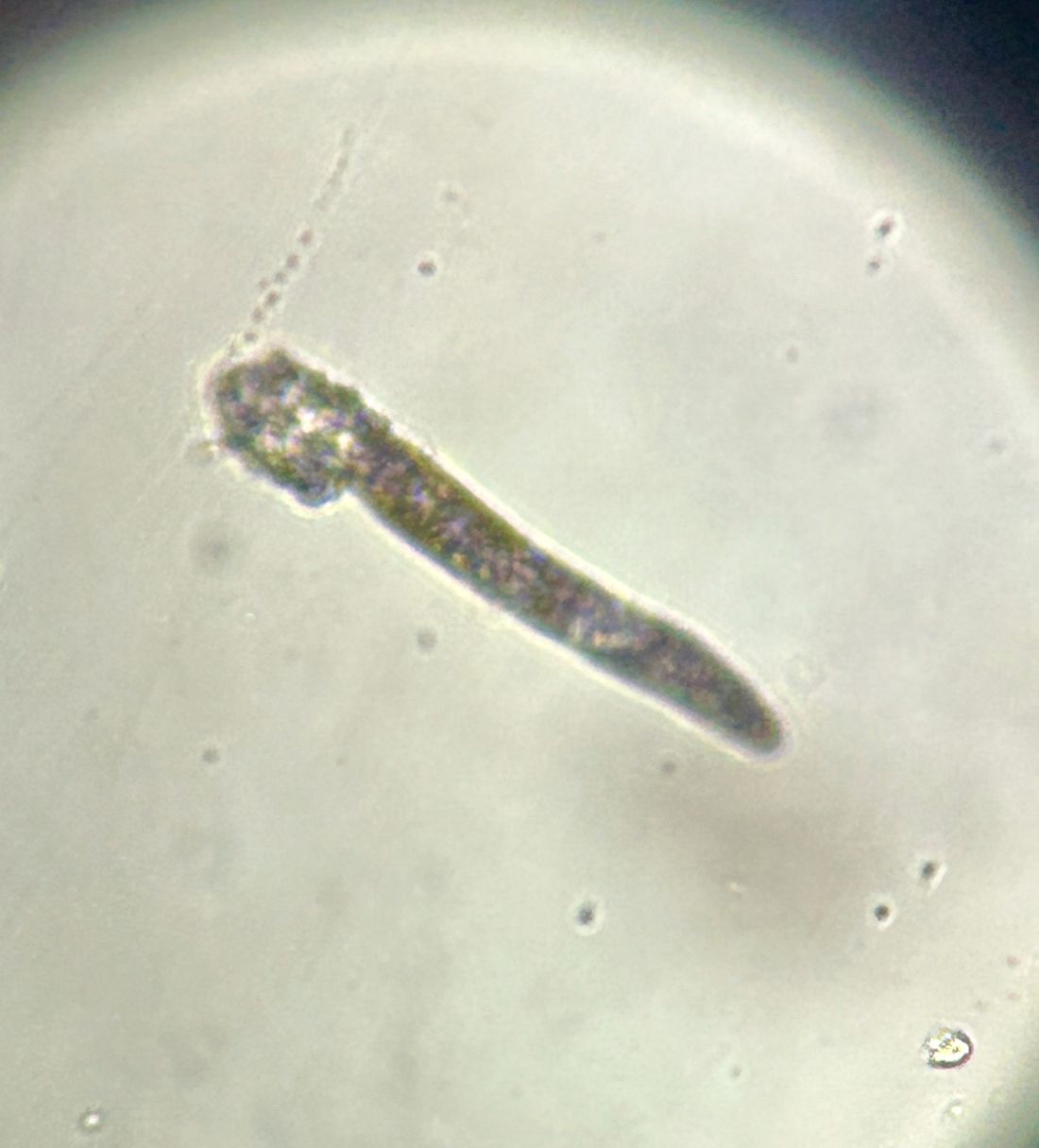
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
Diagnosis
During the visit, skin scrapings were performed, revealing several Demodex mites, confirming the diagnosis of demodicosis.
Demodex folliculorum and Demodex brevis are the two common species implicated. The life cycle of Demodex spp. occurs in the sebaceous glands, leading to mechanical and chemical irritation of the skin.
Various immune responses are also triggered, such as a keratinocyte response via Toll-like receptor 2. Patients usually present with non-specific symptoms such as skin erythema, irritation, peeling, and dryness on the cheeks, eyelids, and paranasal areas. Patients may develop a maculopapular or rosacea-like rash.
Diagnosis is often made through microscopic examination of a skin sample in KOH solution. In rare occasions, a skin surface standardization biopsy method may be used, which determines the density of mites per 1 cm2. Dermoscopy may identify spiky white structures. Molecular methods such as PCR can be used but are not standard.
The differential diagnosis may include acne, rosacea, folliculitis, and Candida infection. Demodicosis can be differentiated by history and further studies including dermoscopy.
Acne vulgaris is an inflammatory disease of the skin’s pilosebaceous unit, primarily involving the face and trunk. It can present with comedones, papules, pustules, and nodules. Secondary signs suggestive of acne vulgaris include scars, erythema, and hyperpigmentation. All forms of acne share a common pathogenesis resulting in the formation of microcomedones, precursors for all clinical acne lesions. In this patient, the absence of microcomedones and the presence of primary inflammatory papules localized to the nose and cheeks suggested an alternative diagnosis.
Rosacea was also considered in the differential diagnosis. Rosacea is an inflammatory dermatosis characterized by erythema, telangiectasia, recurrent flushing, and inflammatory lesions including papulopustules and swelling, primarily affecting the face. The pathogenesis of rosacea is not fully understood but is suggested to involve immune-mediated responses. Vascular dysregulation and reactive oxygen species damage keratinocytes, fibroblasts, and endothelial cells. A higher incidence of rosacea in those with a family history and UV exposure is a known trigger. Demodex folliculorum and Helicobacter pylori are also implicated. Occasionally, Demodex infestation and rosacea may co-occur, and treatment with topical metronidazole can be helpful.
Folliculitis is an infection and inflammation of the hair follicles, forming pustules or erythematous papules over hair-covered skin. It is commonly caused by bacterial infection but can also be due to fungi, viruses, and noninfectious causes such as eosinophilic folliculitis. Diagnosis is clinical, based on physical exam and history, such as recent increased sweating or scratching. KOH prep can be used for Malassezia folliculitis and skin biopsy for eosinophilic folliculitis. Treatment targets the underlying cause. Most bacterial folliculitis cases resolve without treatment, but topical antibiotics may be used. Fungal folliculitis requires oral antifungals, and herpes simplex folliculitis can be treated with antiviral medications.
Cutaneous candidiasis is an infection of the skin by various Candida species, commonly C. albicans. Superficial infections of the skin and mucous membranes, such as intertrigo, are common types. Risk factors include immunosuppression, endocrine disorders, or compromised blood flow. Increased humidity, occlusion, broken skin barriers, and altered skin microbial flora contribute to Candida infection. Diagnosis is clinical but can be confirmed by KOH prep, microscopy, and culture. Treatment involves anti-inflammatory, antibacterial, and antifungal medications. Topical clotrimazole, nystatin, and miconazole are commonly used. Recurrence is prevented by keeping the affected area dry with barrier creams.
Therapeutic goals include arresting mite reproduction, elimination, and preventing recurrent infestations. Treatment may last several months, and the choice of drug depends on patient factors. There have been no standardized treatment studies or long-term effectiveness analyses. Antibiotics such as tetracycline, metronidazole, doxycycline, and ivermectin may be used to prevent proliferation. Permethrin, benzyl benzoate, crotamiton, lindane, and sulfur have also been used. Metronidazole is a common treatment for demodicosis, as was used in our patient for several weeks until the lesions cleared. Systemic metronidazole therapy may be indicated for reducing Demodex spp. density. Severe cases, particularly in immunocompromised individuals, may require oral ivermectin. Appropriate hygiene is important for prevention, such as washing the face with non-oily cleansers and laundering linens regularly.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Mr. Lee is a medical student at the University of California San Diego.
Suggested Reading
Chudzicka-Strugała I et al. Demodicosis in different age groups and alternative treatment options—A review. J Clin Med. 2023 Feb 19;12(4):1649. doi: 10.3390/jcm12041649.
Eichenfield DZ et al. Management of acne vulgaris: A review. JAMA. 2021 Nov 23;326(20):2055-2067. doi: 10.1001/jama.2021.17633.
Sharma A et al. Rosacea management: A comprehensive review. J Cosmet Dermatol. 2022 May;21(5):1895-1904. doi: 10.1111/jocd.14816.
Taudorf EH et al. Cutaneous candidiasis — an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019 Oct;33(10):1863-1873. doi: 10.1111/jdv.15782.
Winters RD, Mitchell M. Folliculitis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547754/
A 7-year-old female presents with persistent pimples on the nose and cheeks for approximately 1 year. She had been treated with several topical antibiotics and acne washes without resolution of the lesions. There were no signs of early puberty, and the child had no history of medical conditions. Her mother has a history of rosacea. Physical examination revealed erythematous papules on the nose and cheeks bilaterally.
Early-Life Excess Weight Tied to Subsequent Stroke Risk
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggested.
An analysis of more than five decades of health data on 10,000 adults revealed that close to 5% experienced a stroke during the follow-up period, with the risk for ischemic stroke being more than twice as high in women who had obesity as teens or young adults. The risk was even higher for hemorrhagic stroke in both men and women with a history of obesity in youth.
“Our findings suggest that being overweight may have long-term health effects, even if the excess weight is temporary,” lead author Ursula Mikkola, BM, an investigator in the Research Unit of Population Health at the University of Oulu, Oulu, Finland, said in a news release.
“Health care professionals should pay attention to overweight and obesity in young people and work with them to develop healthier eating patterns and physical activity — however, conversations with teens and young adults about weight should be approached in a nonjudgmental and nonstigmatizing manner,” she added.
The study was published online in Stroke.
Gender Differences
Childhood obesity has been associated with a heightened risk for cerebrovascular disease later in life, but most studies have focused on body mass index (BMI) at a single time point without considering its fluctuations throughout life, the investigators noted.
For the study, investigators used data from the Northern Finland Birth Cohort 1966, a prospective, general population-based birth cohort that followed 10,491 individuals (5185 women) until 2020 or the first stroke, death, or moving abroad, whichever came first.
Mean (SD) follow-up for each participant was 39 years from age 14 onward and 23 years from age 31 onward. The analysis was conducted between 1980 and 2020.
BMI data were collected from participants at the age of 14 and 31 years. Age 14 covariates included smoking, parental socioeconomic status, and age at menarche (for girls). Age 31 covariates included smoking and participants’ educational level.
During the follow-up period, 4.7% of participants experienced stroke. Of these events, 31% were ischemic strokes and 40% were transient ischemic attacks. The remainder were hemorrhagic or other cerebrovascular events.
Using normal weight as a reference, researchers found that the risk for ischemic stroke was over twice as high for women who had been overweight at ages 14 (hazard ratio [HR], 2.49; 95% confidence interval [CI], 1.44-4.31) and 31 (HR, 2.13; 95% CI, 1.14-3.97) years. The risk was also considerably higher for women who had obesity at ages 14 (HR, 1.87; 95% CI, 0.76-4.58) and 31 (HR, 2.67; 95% CI, 1.26-5.65) years.
The risk for hemorrhagic stroke was even higher, both among women (HR, 3.49; 95% CI, 1.13-10.7) and men (HR, 5.75; 95% CI, 1.43-23.1) who had obesity at age 31.
No similar associations were found among men, and the findings were independent of earlier or later BMI.
The risk for any cerebrovascular disease related to overweight at age 14 was twice as high among girls vs boys (HR, 2.09; 95% CI, 1.06-4.15), and the risk for ischemic stroke related to obesity at age 31 was nearly seven times higher among women vs men (HR, 6.96; 95% CI, 1.36-35.7).
“Stroke at a young age is rare, so the difference of just a few strokes could have an outsized impact on the risk estimates,” the study authors said. “Also, BMI relies solely on a person’s height and weight; therefore, a high BMI may be a misleading way to define obesity, especially in muscular people who may carry little fat even while weighing more.”
Caveats
In an accompanying editorial, Larry Goldstein, MD, chair of the Department of Neurology, University of Kentucky, Lexington, Kentucky, and codirector of the Kentucky Neuroscience Institute, said the study “provides additional evidence of an association between overweight/obesity and stroke in young adults.”
However, Dr. Goldstein added that “while it is tempting to assume that reductions in overweight/obesity in younger populations would translate to lower stroke rates in young adults, this remains to be proven.”
Moreover, it is “always important to acknowledge that associations found in observational studies may not reflect causality.”
This study was supported by Orion Research Foundation, Päivikki and Sakari Sohlberg Foundation, and Paulo Foundation. Dr. Mikkola reported no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. Goldstein reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sharp Rise in US Pediatric ADHD Diagnoses
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers used 2022 data from the National Survey of Children’s Health to estimate the prevalence of ever-diagnosed and current ADHD among US children between the ages of 3 and 18 years.
- They also estimated, among children with current ADHD, the severity of the condition and the presence of current co-occurring disorders and the receipt of medication and behavioral treatments.
- The researchers calculated overall weighted estimates as well as estimates for specific demographic and clinical subgroups (n = 45,169).
TAKEAWAY:
- The number of children who had ever received an ADHD diagnosis increased from 6.1 million in 2016 to 7.1 million in 2022, and the number with current ADHD increased from 5.4 million to 6.5 million.
- Of those with current ADHD in 2022, 58.1% had moderate or severe ADHD, and 77.9% had at least one co-occurring disorder.
- A total of 53.6% had received ADHD medication, 44.4% had received behavioral treatment in the past year, and 30.1% had received no ADHD-specific treatment.
- A similar percentage of children with ADHD were receiving behavioral treatment in 2022 as in 2016 (44.4% vs 46.7%, respectively), but treatment with ADHD medication was lower in 2022 than in 2016 (53.6% vs 62.0%, respectively).
IN PRACTICE:
The estimates “can be used by clinicians to understand current ADHD diagnosis and treatment utilization patterns to inform clinical practice, such as accounting for the frequency and management of co-occurring conditions and considering the notable percentage of children with ADHD not currently receiving ADHD treatment,” and can be used by policymakers, practitioners, and others “to plan for the needs of children with ADHD, such as by ensuring access to care and services for ADHD,” investigators wrote.
SOURCE:
Melissa L. Danielson, of the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, led the study, which was published online in the Journal of Clinical Child & Adolescent Psychology.
LIMITATIONS:
Indicators reported in the analysis were on the basis of the parent report, which may be limited by recall and reporting decisions and were not validated against medical records or clinical judgment. Moreover, details about the types of treatment were not included.
DISCLOSURES:
The work was authorized as part of the contributor’s official duties as an employee of the US Government, and therefore is a work of the US Government. The authors declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA Approves Polyarticular JIA Indication for Sarilumab
The US Food and Drug Administration (FDA) has approved sarilumab (Kevzara) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) for patients weighing ≥ 63 kg (139 lb).
“Polyarticular juvenile idiopathic arthritis (JIA) can be a painful disease for children where multiple joints are impacted by this chronic inflammation,” said George D. Yancopoulos, MD, PhD, president and chief scientific officer at Regeneron in a press release.
It is estimated that nearly 300,000 children in the United States have JIA, and 1 in 4 of them have pJIA, according to the Arthritis Foundation.
“Not only are their daily lives impacted, but their futures can be disrupted without adequate treatment,” Dr. Yancopoulos continued. “The approval of Kevzara in polyarticular juvenile idiopathic arthritis provides these vulnerable patients and their families a new FDA-approved treatment option to help navigate this disease.”
Sarilumab, jointly developed by Sanofi and Regeneron, is an interleukin 6 receptor blocker. It was first approved in 2017 for the treatment of moderate to severely active rheumatoid arthritis (RA) in adults who had inadequate response or intolerance to at least one other disease-modifying antirheumatic drug (DMARD).
In 2023, the FDA approved sarilumab as the first biologic treatment for polymyalgia rheumatica in adults who had inadequate response to corticosteroids and could not tolerate a corticosteroid taper.
For pJIA, sarilumab is administered subcutaneously using a 200-mg/1.14-mL prefilled syringe once every 2 weeks. The medication can be used alone or in combination with other conventional DMARDs.
“Use of KEVZARA in pediatric patients with pJIA is supported by evidence from adequate and well-controlled studies of KEVZARA in adults with RA, pharmacokinetic data from adult patients with RA,” and pharmacokinetic comparability in 101 pediatric patients aged 2-17 years treated with sarilumab, according to the prescribing information. Sarilumab is not approved for pediatric patients < 63 kg “because of a lack of an appropriate dosage form.”
The most common reported adverse reactions for sarilumab in pJIA are nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. The pJIA trial recorded no new adverse reactions or safety concerns, compared with patients with RA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sarilumab (Kevzara) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) for patients weighing ≥ 63 kg (139 lb).
“Polyarticular juvenile idiopathic arthritis (JIA) can be a painful disease for children where multiple joints are impacted by this chronic inflammation,” said George D. Yancopoulos, MD, PhD, president and chief scientific officer at Regeneron in a press release.
It is estimated that nearly 300,000 children in the United States have JIA, and 1 in 4 of them have pJIA, according to the Arthritis Foundation.
“Not only are their daily lives impacted, but their futures can be disrupted without adequate treatment,” Dr. Yancopoulos continued. “The approval of Kevzara in polyarticular juvenile idiopathic arthritis provides these vulnerable patients and their families a new FDA-approved treatment option to help navigate this disease.”
Sarilumab, jointly developed by Sanofi and Regeneron, is an interleukin 6 receptor blocker. It was first approved in 2017 for the treatment of moderate to severely active rheumatoid arthritis (RA) in adults who had inadequate response or intolerance to at least one other disease-modifying antirheumatic drug (DMARD).
In 2023, the FDA approved sarilumab as the first biologic treatment for polymyalgia rheumatica in adults who had inadequate response to corticosteroids and could not tolerate a corticosteroid taper.
For pJIA, sarilumab is administered subcutaneously using a 200-mg/1.14-mL prefilled syringe once every 2 weeks. The medication can be used alone or in combination with other conventional DMARDs.
“Use of KEVZARA in pediatric patients with pJIA is supported by evidence from adequate and well-controlled studies of KEVZARA in adults with RA, pharmacokinetic data from adult patients with RA,” and pharmacokinetic comparability in 101 pediatric patients aged 2-17 years treated with sarilumab, according to the prescribing information. Sarilumab is not approved for pediatric patients < 63 kg “because of a lack of an appropriate dosage form.”
The most common reported adverse reactions for sarilumab in pJIA are nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. The pJIA trial recorded no new adverse reactions or safety concerns, compared with patients with RA.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sarilumab (Kevzara) for the treatment of polyarticular juvenile idiopathic arthritis (pJIA) for patients weighing ≥ 63 kg (139 lb).
“Polyarticular juvenile idiopathic arthritis (JIA) can be a painful disease for children where multiple joints are impacted by this chronic inflammation,” said George D. Yancopoulos, MD, PhD, president and chief scientific officer at Regeneron in a press release.
It is estimated that nearly 300,000 children in the United States have JIA, and 1 in 4 of them have pJIA, according to the Arthritis Foundation.
“Not only are their daily lives impacted, but their futures can be disrupted without adequate treatment,” Dr. Yancopoulos continued. “The approval of Kevzara in polyarticular juvenile idiopathic arthritis provides these vulnerable patients and their families a new FDA-approved treatment option to help navigate this disease.”
Sarilumab, jointly developed by Sanofi and Regeneron, is an interleukin 6 receptor blocker. It was first approved in 2017 for the treatment of moderate to severely active rheumatoid arthritis (RA) in adults who had inadequate response or intolerance to at least one other disease-modifying antirheumatic drug (DMARD).
In 2023, the FDA approved sarilumab as the first biologic treatment for polymyalgia rheumatica in adults who had inadequate response to corticosteroids and could not tolerate a corticosteroid taper.
For pJIA, sarilumab is administered subcutaneously using a 200-mg/1.14-mL prefilled syringe once every 2 weeks. The medication can be used alone or in combination with other conventional DMARDs.
“Use of KEVZARA in pediatric patients with pJIA is supported by evidence from adequate and well-controlled studies of KEVZARA in adults with RA, pharmacokinetic data from adult patients with RA,” and pharmacokinetic comparability in 101 pediatric patients aged 2-17 years treated with sarilumab, according to the prescribing information. Sarilumab is not approved for pediatric patients < 63 kg “because of a lack of an appropriate dosage form.”
The most common reported adverse reactions for sarilumab in pJIA are nasopharyngitis, neutropenia, upper respiratory tract infection, and injection site erythema. The pJIA trial recorded no new adverse reactions or safety concerns, compared with patients with RA.
A version of this article appeared on Medscape.com.
The Smartphone Problem
I am going to guess that if we asked 500,000 adults in this country if they felt that children and adolescents were spending too much time on their smartphones, we would elicit almost uniform agreement that, yes indeed, smartphone use is gobbling up too much time from our young people. And, the adults would volunteer a long laundry list of all the bad consequences this overuse was generating. If you ask this same sample of adults if they too were spending too much time on their smartphones they would answer yes and, again, give you a list of the problems they feel are the result of this overuse.
We might begin to find a scattering of responses if we ask the adults when a child is too young to have his/her own cell phone. But, they would all agree that “young children” weren’t ready to be trusted with a cell phone. The “when” they were ready would be up for discussion. However, I suspect we might see a clustering around age 10 years. The reality is that despite what the majority may believe, a 2022 survey found that 42% of children have a cell phone by age 10, 71% by age 12, and 91% by age 14.
So, it would appear that, while we believe there can be significant downsides to having a cell phone, we are having great difficulty in policing ourselves and creating limits for our children. Does cell phone use qualify as an addiction, or is it just another example of how adults have lost the ability to say “no” to themselves and to their children?
When it comes to cell phones in school, the situation gets increasingly murky. The teachers I speak with are very clear that cell phones are creating problems for both the academic and the social experiences of their students. One teacher referred me to an article from the Norwegian Institute of Public Health, which found that banning cell phones in school decreased the incidence of psychological symptoms and diseases in girls. Bullying decreased in both genders and the girls’ GPA scores improved. In schools with cell phone bans, girls were more likely to choose and attend academic track programs, an effect which was more pronounced in young women with lower socioeconomic backgrounds. But, the if, when, and how to institute smartphone bans in school is complicated.
On one front, the movement toward cell phone bans in school has been given a major boost with the publication and publicity of a new book titled The Anxious Generation by social psychologist Jonathan Haidt, PhD. The New York University professor sees the GenZ’ers as experiencing a tsunami of mental health challenges including anxiety, self-harm, and suicide. And, he lays much of the blame for this situation on cell phone use.
He is optimistic about turning the tide because he claims that everywhere he speaks about the problem he says “I feel that I’m pushing on open doors.” Comparing the phenomenon to the collapse of the Berlin Wall, Dr. Haidt says “When you have a system that everyone hates, and then you have a way to escape it, it can change in a year.”
I wish I could share in his optimism, although I did just encounter a news story in the Portland paper describing a national program called “Wait Until 8th,” which is being considered by a parents’ group here in Maine.
The usual suspects have their own predictable take on the issue. The House and Senate have proposed a study on the use of cell phones in elementary and secondary schools and a pilot program awarding grants to some schools to create mobile device–free environments. Sounds like a momentum killer to me.
Not surprisingly, the issue of cell phone bans in school has taken on a bit of a political odor. The National Parents Union reports in a very small and inadequately described sample that 56% of parents are against total school bans. In the accompanying press release, the organizations offers an extensive list of concerns parents have reported — many cite the need to remain in contact with their children throughout the day. One has to wonder how often these concerns are a reflection of unresolved separation anxiety.
The American Academy of Pediatrics has rolled out a “5 Cs” framework that pediatricians can use to discuss media use with families. As usual, the thought is that talking about a problem is going to somehow convince parents to do what they already know is the correct action. And, of course, pediatricians have plenty of time to initiate this discussion of the obvious.
A recent study from the Department of Pediatrics at University of California, San Francisco, has found that parental monitoring, limit setting, and modeling good screen use behavior (my bolding) are the most effective strategies for reducing adolescent screen time. Using screen time allowances as a reward or punishment does not seem to be effective.
So there you have it. It looks like However, despite Dr. Haidt’s optimism about a seismic turnaround, I suspect it will more likely be guerrilla warfare — one family, one school, or one school district at a time.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I am going to guess that if we asked 500,000 adults in this country if they felt that children and adolescents were spending too much time on their smartphones, we would elicit almost uniform agreement that, yes indeed, smartphone use is gobbling up too much time from our young people. And, the adults would volunteer a long laundry list of all the bad consequences this overuse was generating. If you ask this same sample of adults if they too were spending too much time on their smartphones they would answer yes and, again, give you a list of the problems they feel are the result of this overuse.
We might begin to find a scattering of responses if we ask the adults when a child is too young to have his/her own cell phone. But, they would all agree that “young children” weren’t ready to be trusted with a cell phone. The “when” they were ready would be up for discussion. However, I suspect we might see a clustering around age 10 years. The reality is that despite what the majority may believe, a 2022 survey found that 42% of children have a cell phone by age 10, 71% by age 12, and 91% by age 14.
So, it would appear that, while we believe there can be significant downsides to having a cell phone, we are having great difficulty in policing ourselves and creating limits for our children. Does cell phone use qualify as an addiction, or is it just another example of how adults have lost the ability to say “no” to themselves and to their children?
When it comes to cell phones in school, the situation gets increasingly murky. The teachers I speak with are very clear that cell phones are creating problems for both the academic and the social experiences of their students. One teacher referred me to an article from the Norwegian Institute of Public Health, which found that banning cell phones in school decreased the incidence of psychological symptoms and diseases in girls. Bullying decreased in both genders and the girls’ GPA scores improved. In schools with cell phone bans, girls were more likely to choose and attend academic track programs, an effect which was more pronounced in young women with lower socioeconomic backgrounds. But, the if, when, and how to institute smartphone bans in school is complicated.
On one front, the movement toward cell phone bans in school has been given a major boost with the publication and publicity of a new book titled The Anxious Generation by social psychologist Jonathan Haidt, PhD. The New York University professor sees the GenZ’ers as experiencing a tsunami of mental health challenges including anxiety, self-harm, and suicide. And, he lays much of the blame for this situation on cell phone use.
He is optimistic about turning the tide because he claims that everywhere he speaks about the problem he says “I feel that I’m pushing on open doors.” Comparing the phenomenon to the collapse of the Berlin Wall, Dr. Haidt says “When you have a system that everyone hates, and then you have a way to escape it, it can change in a year.”
I wish I could share in his optimism, although I did just encounter a news story in the Portland paper describing a national program called “Wait Until 8th,” which is being considered by a parents’ group here in Maine.
The usual suspects have their own predictable take on the issue. The House and Senate have proposed a study on the use of cell phones in elementary and secondary schools and a pilot program awarding grants to some schools to create mobile device–free environments. Sounds like a momentum killer to me.
Not surprisingly, the issue of cell phone bans in school has taken on a bit of a political odor. The National Parents Union reports in a very small and inadequately described sample that 56% of parents are against total school bans. In the accompanying press release, the organizations offers an extensive list of concerns parents have reported — many cite the need to remain in contact with their children throughout the day. One has to wonder how often these concerns are a reflection of unresolved separation anxiety.
The American Academy of Pediatrics has rolled out a “5 Cs” framework that pediatricians can use to discuss media use with families. As usual, the thought is that talking about a problem is going to somehow convince parents to do what they already know is the correct action. And, of course, pediatricians have plenty of time to initiate this discussion of the obvious.
A recent study from the Department of Pediatrics at University of California, San Francisco, has found that parental monitoring, limit setting, and modeling good screen use behavior (my bolding) are the most effective strategies for reducing adolescent screen time. Using screen time allowances as a reward or punishment does not seem to be effective.
So there you have it. It looks like However, despite Dr. Haidt’s optimism about a seismic turnaround, I suspect it will more likely be guerrilla warfare — one family, one school, or one school district at a time.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I am going to guess that if we asked 500,000 adults in this country if they felt that children and adolescents were spending too much time on their smartphones, we would elicit almost uniform agreement that, yes indeed, smartphone use is gobbling up too much time from our young people. And, the adults would volunteer a long laundry list of all the bad consequences this overuse was generating. If you ask this same sample of adults if they too were spending too much time on their smartphones they would answer yes and, again, give you a list of the problems they feel are the result of this overuse.
We might begin to find a scattering of responses if we ask the adults when a child is too young to have his/her own cell phone. But, they would all agree that “young children” weren’t ready to be trusted with a cell phone. The “when” they were ready would be up for discussion. However, I suspect we might see a clustering around age 10 years. The reality is that despite what the majority may believe, a 2022 survey found that 42% of children have a cell phone by age 10, 71% by age 12, and 91% by age 14.
So, it would appear that, while we believe there can be significant downsides to having a cell phone, we are having great difficulty in policing ourselves and creating limits for our children. Does cell phone use qualify as an addiction, or is it just another example of how adults have lost the ability to say “no” to themselves and to their children?
When it comes to cell phones in school, the situation gets increasingly murky. The teachers I speak with are very clear that cell phones are creating problems for both the academic and the social experiences of their students. One teacher referred me to an article from the Norwegian Institute of Public Health, which found that banning cell phones in school decreased the incidence of psychological symptoms and diseases in girls. Bullying decreased in both genders and the girls’ GPA scores improved. In schools with cell phone bans, girls were more likely to choose and attend academic track programs, an effect which was more pronounced in young women with lower socioeconomic backgrounds. But, the if, when, and how to institute smartphone bans in school is complicated.
On one front, the movement toward cell phone bans in school has been given a major boost with the publication and publicity of a new book titled The Anxious Generation by social psychologist Jonathan Haidt, PhD. The New York University professor sees the GenZ’ers as experiencing a tsunami of mental health challenges including anxiety, self-harm, and suicide. And, he lays much of the blame for this situation on cell phone use.
He is optimistic about turning the tide because he claims that everywhere he speaks about the problem he says “I feel that I’m pushing on open doors.” Comparing the phenomenon to the collapse of the Berlin Wall, Dr. Haidt says “When you have a system that everyone hates, and then you have a way to escape it, it can change in a year.”
I wish I could share in his optimism, although I did just encounter a news story in the Portland paper describing a national program called “Wait Until 8th,” which is being considered by a parents’ group here in Maine.
The usual suspects have their own predictable take on the issue. The House and Senate have proposed a study on the use of cell phones in elementary and secondary schools and a pilot program awarding grants to some schools to create mobile device–free environments. Sounds like a momentum killer to me.
Not surprisingly, the issue of cell phone bans in school has taken on a bit of a political odor. The National Parents Union reports in a very small and inadequately described sample that 56% of parents are against total school bans. In the accompanying press release, the organizations offers an extensive list of concerns parents have reported — many cite the need to remain in contact with their children throughout the day. One has to wonder how often these concerns are a reflection of unresolved separation anxiety.
The American Academy of Pediatrics has rolled out a “5 Cs” framework that pediatricians can use to discuss media use with families. As usual, the thought is that talking about a problem is going to somehow convince parents to do what they already know is the correct action. And, of course, pediatricians have plenty of time to initiate this discussion of the obvious.
A recent study from the Department of Pediatrics at University of California, San Francisco, has found that parental monitoring, limit setting, and modeling good screen use behavior (my bolding) are the most effective strategies for reducing adolescent screen time. Using screen time allowances as a reward or punishment does not seem to be effective.
So there you have it. It looks like However, despite Dr. Haidt’s optimism about a seismic turnaround, I suspect it will more likely be guerrilla warfare — one family, one school, or one school district at a time.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
EULAR 2024 Preview: Therapeutics in Development Take Center Stage
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
Latest Breakthroughs in Molluscum Contagiosum Therapy
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
Molluscum contagiosum (ie, molluscum) is a ubiquitous infection caused by the poxvirus molluscum contagiosum virus (MCV). Although skin deep, molluscum shares many factors with the more virulent poxviridae. Moisture and trauma can cause viral material to be released from the pearly papules through a small opening, which also allows entry of bacteria and medications into the lesion. The MCV is transmitted by direct contact with skin or via fomites.1
Molluscum can affect children of any age, with MCV type 1 peaking in toddlers and school-aged children and MCV type 2 after the sexual debut. The prevalence of molluscum has increased since the 1980s. It is stressful for children and caregivers and poses challenges in schools as well as sports such as swimming, wrestling, and karate.1,2
For the first time, we have US Food and Drug Administration (FDA)–approved products to treat MCV infections. Previously, only off-label agents were used. Therefore, we have to contemplate why treatment is important to our patients.
What type of care is required for molluscum?
Counseling is the first and only mandatory treatment, which consists of 3 parts: natural history, risk factors for spread, and options for therapy. The natural history of molluscum in children is early spread, contagion to oneself and others (as high as 60% of sibling co-bathers3), triggering of dermatitis, eventual onset of the beginning-of-the-end (BOTE) sign, and eventually clearance. The natural history in adults is poorly understood.
Early clearance is uncommon; reports have suggested 45.6% to 48.4% of affected patients are clear at 1 year and 69.5% to 72.6% at 1.5 years.4 For many children, especially those with atopic dermatitis (AD), lesions linger and often spread, with many experiencing disease for 3 to 4 years. Fomites such as towels, washcloths, and sponges can transfer the virus and spread lesions; therefore, I advise patients to gently pat their skin dry, wash towels frequently, and avoid sharing bathing equipment.1,3,5 Children and adults with immunosuppression may have a greater number of lesions and more prolonged course of disease, including those with HIV as well as DOC8 and CARD11 mutations.6 The American Academy of Pediatrics (AAP) emphasizes that children should not be excluded from attending child care/school or from swimming in public pools but lesions should be covered.6 Lesions, especially those in the antecubital region, can trigger new-onset AD or AD flares.3 In response, gentle skin care including fragrance-free cleansers and periodic application of moisturizers may ward off AD. Topical corticosteroids are preferred.
Dermatitis in MCV is a great mimicker and can resemble erythema multiforme, Gianotti-Crosti syndrome, impetigo, and AD.1 Superinfection recently has been reported; however, in a retrospective analysis of 56 patients with inflamed lesions secondary to molluscum infection, only 7 had positive bacterial cultures, which supports the idea of the swelling and redness of inflammation as a mimic for infection.7 When true infection does occur, tender, swollen, pus-filled lesions should be lanced and cultured.1,7,8
When should we consider therapy?
Therapy is highly dependent on the child, the caregiver, and the social circumstances.1 More than 80% of parents are anxious about molluscum, and countless children are embarrassed or ashamed.1 Ultimately, an unhappy child merits care. The AAP cites the following as reasons to treat: “(1) alleviate discomfort, including itching; (2) reduce autoinoculation; (3) limit transmission of the virus to close contacts; (4) reduce cosmetic concerns; and (5) prevent secondary infection.”6 For adults, we should consider limitations to intimacy and reduction of sexual transmission risk.6
Treatment can be based on the number of lesions. With a few lesions (<3), therapy is worthwhile if they are unsightly; appear on exposed skin causing embarrassment; and/or are itchy, uncomfortable, or large. In a report of 300 children with molluscum treated with cantharidin, most patients choosing therapy had 10 to 20 lesions, but this was over multiple visits.8 Looking at a 2018 data set of 50 patients (all-comers) with molluscum,3 the mean number of lesions was 10 (median, 7); 3 lesions were 1 SD below, while 14, 17, and 45 were 1, 2, and 3 SDs above, respectively. This data set shows that patients can develop more lesions rapidly, and most children have many visible lesions (N.B. Silverberg, MD, unpublished data).
Because each lesion contains infectious viral particles and patients scratch, more lesions are equated to greater autoinoculation and contagion. In addition to the AAP criteria, treatment can be considered for households with immunocompromised individuals, children at risk for new-onset AD, or those with AD at risk for flare. For patients with 45 lesions or more (3 SDs), clearance is harder to achieve with 2 sessions of in-office therapy, and multiple methods or the addition of immunomodulatory therapeutics should be considered.
Do we have to clear every lesion?
New molluscum lesions may arise until a patient achieves immunity, and they may appear more than a month after inoculation, making it difficult to keep up with the rapid spread. Latency between exposure and lesion development usually is 2 to 7 weeks but may be as long as 6 months, making it difficult to prevent spread.6 Therefore, when we treat, we should not promise full clearance to patients and parents. Rather, we should inform them that new lesions may develop later, and therapy is only effective on visible lesions. In a recent study, a 50% clearance of lesions was the satisfactory threshold for parents, demonstrating that satisfaction is possible with partial clearance.9
What is new in therapeutics for molluscum?
Molluscum therapies are either destructive, immunomodulatory, or antiviral. Two agents now are approved by the FDA for the treatment of molluscum infections.
Berdazimer gel 10.3% is approved for patients 1 year or older, but it is not yet available. This agent has both immunomodulatory and antiviral properties.10 It features a home therapy that is mixed on a small palette, then painted on by the patient or parent once daily for 12 weeks. Study outcomes demonstrated more than 50% lesional clearance.11,12 Complete clearance was achieved in at least 30% of patients.12A proprietary topical version of cantharidin 0.7% in flexible collodion is now FDA approved for patients 2 years and older. This vesicant-triggering iatrogenic is targeted at creating blisters overlying molluscum lesions. It is conceptually similar to older versions but with some enhanced features.5,13,14 This version was used for therapy every 3 weeks for up to 4 sessions in clinical trials. Safety is similar across all body sites treated (nonmucosal and not near the mucosal surfaces) but not for mucosa, the mid face, or eyelids.13 Complete lesion clearance was 46.3% to 54% and statistically greater than placebo (P<.001).14Both agents are well tolerated in children with AD; adverse effects include blistering with cantharidin and dermatitislike symptoms with berdazimer.15,16 These therapies have the advantage of being easy to use.
Final Thoughts
We have entered an era of high-quality molluscum therapy. Patient care involves developing a good knowledge of the agents, incorporating shared decision-making with patients and caregivers, and addressing therapy in the context of comorbid diseases such as AD.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305, E1-E2.
- Thompson AJ, Matinpour K, Hardin J, et al. Molluscum gladiatorum. Dermatol Online J. 2014;20:13030/qt0nj121n1.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Basdag H, Rainer BM, Cohen BA. Molluscum contagiosum: to treat or not to treat? experience with 170 children in an outpatient clinic setting in the northeastern United States. Pediatr Dermatol. 2015;32:353-357. doi:10.1111/pde.12504
- Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
- Molluscum contagiosum. In: Kimberlin DW, Lynfield R, Barnett ED, et al (eds). Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd edition. American Academy of Pediatrics. May 26, 2021. Accessed May 20, 2024. https://publications.aap.org/redbook/book/347/chapter/5754264/Molluscum-Contagiosum
- Gross I, Ben Nachum N, Molho-Pessach V, et al. The molluscum contagiosum BOTE sign—infected or inflamed? Pediatr Dermatol. 2020;37:476-479. doi:10.1111/pde.14124
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43:503-507. doi:10.1067/mjd.2000.106370
- Maeda-Chubachi T, McLeod L, Enloe C, et al. Defining clinically meaningful improvement in molluscum contagiosum. J Am Acad Dermatol. 2024;90:443-445. doi:10.1016/j.jaad.2023.10.033
- Guttman-Yassky E, Gallo RL, Pavel AB, et al. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. J Invest Dermatol. 2020;140:2531-2535.e2. doi:10.1016/j.jid.2020.04.013
- Browning JC, Cartwright M, Thorla I Jr, et al. A patient-centered perspective of molluscum contagiosum as reported by B-SIMPLE4 Clinical Trial patients and caregivers: Global Impression of Change and Exit Interview substudy results. Am J Clin Dermatol. 2023;24:119-133. doi:10.1007/s40257-022-00733-9
- Sugarman JL, Hebert A, Browning JC, et al. Berdazimer gel for molluscum contagiosum: an integrated analysis of 3 randomized controlled trials. J Am Acad Dermatol. 2024;90:299-308. doi:10.1016/j.jaad.2023.09.066
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14:42-47.
- Eichenfield LF, McFalda W, Brabec B, et al. Safety and efficacy of VP-102, a proprietary, drug-device combination product containing cantharidin, 0.7% (w/v), in children and adults with molluscum contagiosum: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:1315-1323. doi:10.1001/jamadermatol.2020.3238
- Paller AS, Green LJ, Silverberg N, et al. Berdazimer gel for molluscum contagiosum in patients with atopic dermatitis. Pediatr Dermatol.Published online February 27, 2024. doi:10.1111/pde.15575
- Eichenfield L, Hebert A, Mancini A, et al. Therapeutic approaches and special considerations for treating molluscum contagiosum. J Drugs Dermatol. 2021;20:1185-1190. doi:10.36849/jdd.6383
Are Children Born Through ART at Higher Risk for Cancer?
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The results of a large French study comparing the cancer risk in children conceived through assisted reproductive technology (ART) with that of naturally conceived children were published recently in JAMA Network Open. This study is one of the largest to date on this subject: It included 8,526,306 children born in France between 2010 and 2021, of whom 260,236 (3%) were conceived through ART, and followed them up to a median age of 6.7 years.
Motivations for the Study
ART (including artificial insemination, in vitro fertilization [IVF], or intracytoplasmic sperm injection [ICSI] with fresh or frozen embryo transfer) accounts for about 1 in 30 births in France. However, limited and heterogeneous data have suggested an increased risk for certain health disorders, including cancer, among children conceived through ART. Therefore, a large-scale evaluation of cancer risk in these children is important.
No Overall Increase
In all, 9256 children developed cancer, including 292 who were conceived through ART. Thus, Nevertheless, a slight increase in the risk for leukemia was observed in children conceived through IVF or ICSI. The investigators observed approximately one additional case for every 5000 newborns conceived through IVF or ICSI who reached age 10 years.
Epidemiological monitoring should be continued to better evaluate long-term risks and see whether the risk for leukemia is confirmed. If it is, then it will be useful to investigate the mechanisms related to ART techniques or the fertility disorders of parents that could lead to an increased risk for leukemia.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Early-Life Exposure to Pollution Linked to Psychosis, Anxiety, Depression
Early-life exposure to air and noise pollution is associated with a higher risk for psychosis, depression, and anxiety in adolescence and early adulthood, results from a longitudinal birth cohort study showed.
While air pollution was associated primarily with psychotic experiences and depression, noise pollution was more likely to be associated with anxiety in adolescence and early adulthood.
“Early-life exposure could be detrimental to mental health given the extensive brain development and epigenetic processes that occur in utero and during infancy,” the researchers, led by Joanne Newbury, PhD, of Bristol Medical School, University of Bristol, England, wrote, adding that “the results of this cohort study provide novel evidence that early-life exposure to particulate matter is prospectively associated with the development of psychotic experiences and depression in youth.”
The findings were published online on May 28 in JAMA Network Open.
Large, Longitudinal Study
To learn more about how air and noise pollution may affect the brain from an early age, the investigators used data from the Avon Longitudinal Study of Parents and Children, an ongoing longitudinal birth cohort capturing data on new births in Southwest England from 1991 to 1992.
Investigators captured levels of air pollutants, which included nitrogen dioxide and fine particulate matter with a diameter smaller than 2.5 µm (PM2.5), in the areas where expectant mothers lived and where their children lived until age 12.
They also collected decibel levels of noise pollution in neighborhoods where expectant mothers and their children lived.
Participants were assessed for psychotic experiences, depression, and anxiety when they were 13, 18, and 24 years old.
Among the 9065 participants who had mental health data, 20% reported psychotic experiences, 11% reported depression, and 10% reported anxiety. About 60% of the participants had a family history of mental illness.
When they were age 13, 13.6% of participants reported psychotic experiences; 9.2% reported them at age 18, and 12.6% at age 24.
A lower number of participants reported feeling depressed and anxious at 13 years (5.6% for depression and 3.6% for anxiety) and 18 years (7.9% for depression and 5.7% for anxiety).
After adjusting for individual and family-level variables, including family psychiatric history, maternal social class, and neighborhood deprivation, elevated PM2.5 levels during pregnancy (P = .002) and childhood (P = .04) were associated with a significantly increased risk for psychotic experiences later in life. Pregnancy PM2.5 exposure was also associated with depression (P = .01).
Participants exposed to higher noise pollution in childhood and adolescence had an increased risk for anxiety (P = .03) as teenagers.
Vulnerability of the Developing Brain
The investigators noted that more information is needed to understand the underlying mechanisms behind these associations but noted that early-life exposure could be detrimental to mental health given “extensive brain development and epigenetic processes that occur in utero.”
They also noted that air pollution could lead to restricted fetal growth and premature birth, both of which are risk factors for psychopathology.
Martin Clift, PhD, of Swansea University in Swansea, Wales, who was not involved in the study, said that the paper highlights the need for more consideration of health consequences related to these exposures.
“As noted by the authors, this is an area that has received a lot of recent attention, yet there remains a large void of knowledge,” Dr. Clift said in a UK Science Media Centre release. “It highlights that some of the most dominant air pollutants can impact different mental health diagnoses, but that time-of-life is particularly important as to how each individual air pollutant may impact this diagnosis.”
Study limitations included limitations to generalizability of the data — the families in the study were more affluent and less diverse than the UK population overall.
The study was funded by the UK Medical Research Council, Wellcome Trust, and University of Bristol. Disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
Early-life exposure to air and noise pollution is associated with a higher risk for psychosis, depression, and anxiety in adolescence and early adulthood, results from a longitudinal birth cohort study showed.
While air pollution was associated primarily with psychotic experiences and depression, noise pollution was more likely to be associated with anxiety in adolescence and early adulthood.
“Early-life exposure could be detrimental to mental health given the extensive brain development and epigenetic processes that occur in utero and during infancy,” the researchers, led by Joanne Newbury, PhD, of Bristol Medical School, University of Bristol, England, wrote, adding that “the results of this cohort study provide novel evidence that early-life exposure to particulate matter is prospectively associated with the development of psychotic experiences and depression in youth.”
The findings were published online on May 28 in JAMA Network Open.
Large, Longitudinal Study
To learn more about how air and noise pollution may affect the brain from an early age, the investigators used data from the Avon Longitudinal Study of Parents and Children, an ongoing longitudinal birth cohort capturing data on new births in Southwest England from 1991 to 1992.
Investigators captured levels of air pollutants, which included nitrogen dioxide and fine particulate matter with a diameter smaller than 2.5 µm (PM2.5), in the areas where expectant mothers lived and where their children lived until age 12.
They also collected decibel levels of noise pollution in neighborhoods where expectant mothers and their children lived.
Participants were assessed for psychotic experiences, depression, and anxiety when they were 13, 18, and 24 years old.
Among the 9065 participants who had mental health data, 20% reported psychotic experiences, 11% reported depression, and 10% reported anxiety. About 60% of the participants had a family history of mental illness.
When they were age 13, 13.6% of participants reported psychotic experiences; 9.2% reported them at age 18, and 12.6% at age 24.
A lower number of participants reported feeling depressed and anxious at 13 years (5.6% for depression and 3.6% for anxiety) and 18 years (7.9% for depression and 5.7% for anxiety).
After adjusting for individual and family-level variables, including family psychiatric history, maternal social class, and neighborhood deprivation, elevated PM2.5 levels during pregnancy (P = .002) and childhood (P = .04) were associated with a significantly increased risk for psychotic experiences later in life. Pregnancy PM2.5 exposure was also associated with depression (P = .01).
Participants exposed to higher noise pollution in childhood and adolescence had an increased risk for anxiety (P = .03) as teenagers.
Vulnerability of the Developing Brain
The investigators noted that more information is needed to understand the underlying mechanisms behind these associations but noted that early-life exposure could be detrimental to mental health given “extensive brain development and epigenetic processes that occur in utero.”
They also noted that air pollution could lead to restricted fetal growth and premature birth, both of which are risk factors for psychopathology.
Martin Clift, PhD, of Swansea University in Swansea, Wales, who was not involved in the study, said that the paper highlights the need for more consideration of health consequences related to these exposures.
“As noted by the authors, this is an area that has received a lot of recent attention, yet there remains a large void of knowledge,” Dr. Clift said in a UK Science Media Centre release. “It highlights that some of the most dominant air pollutants can impact different mental health diagnoses, but that time-of-life is particularly important as to how each individual air pollutant may impact this diagnosis.”
Study limitations included limitations to generalizability of the data — the families in the study were more affluent and less diverse than the UK population overall.
The study was funded by the UK Medical Research Council, Wellcome Trust, and University of Bristol. Disclosures were noted in the original article.
A version of this article appeared on Medscape.com.
Early-life exposure to air and noise pollution is associated with a higher risk for psychosis, depression, and anxiety in adolescence and early adulthood, results from a longitudinal birth cohort study showed.
While air pollution was associated primarily with psychotic experiences and depression, noise pollution was more likely to be associated with anxiety in adolescence and early adulthood.
“Early-life exposure could be detrimental to mental health given the extensive brain development and epigenetic processes that occur in utero and during infancy,” the researchers, led by Joanne Newbury, PhD, of Bristol Medical School, University of Bristol, England, wrote, adding that “the results of this cohort study provide novel evidence that early-life exposure to particulate matter is prospectively associated with the development of psychotic experiences and depression in youth.”
The findings were published online on May 28 in JAMA Network Open.
Large, Longitudinal Study
To learn more about how air and noise pollution may affect the brain from an early age, the investigators used data from the Avon Longitudinal Study of Parents and Children, an ongoing longitudinal birth cohort capturing data on new births in Southwest England from 1991 to 1992.
Investigators captured levels of air pollutants, which included nitrogen dioxide and fine particulate matter with a diameter smaller than 2.5 µm (PM2.5), in the areas where expectant mothers lived and where their children lived until age 12.
They also collected decibel levels of noise pollution in neighborhoods where expectant mothers and their children lived.
Participants were assessed for psychotic experiences, depression, and anxiety when they were 13, 18, and 24 years old.
Among the 9065 participants who had mental health data, 20% reported psychotic experiences, 11% reported depression, and 10% reported anxiety. About 60% of the participants had a family history of mental illness.
When they were age 13, 13.6% of participants reported psychotic experiences; 9.2% reported them at age 18, and 12.6% at age 24.
A lower number of participants reported feeling depressed and anxious at 13 years (5.6% for depression and 3.6% for anxiety) and 18 years (7.9% for depression and 5.7% for anxiety).
After adjusting for individual and family-level variables, including family psychiatric history, maternal social class, and neighborhood deprivation, elevated PM2.5 levels during pregnancy (P = .002) and childhood (P = .04) were associated with a significantly increased risk for psychotic experiences later in life. Pregnancy PM2.5 exposure was also associated with depression (P = .01).
Participants exposed to higher noise pollution in childhood and adolescence had an increased risk for anxiety (P = .03) as teenagers.
Vulnerability of the Developing Brain
The investigators noted that more information is needed to understand the underlying mechanisms behind these associations but noted that early-life exposure could be detrimental to mental health given “extensive brain development and epigenetic processes that occur in utero.”
They also noted that air pollution could lead to restricted fetal growth and premature birth, both of which are risk factors for psychopathology.
Martin Clift, PhD, of Swansea University in Swansea, Wales, who was not involved in the study, said that the paper highlights the need for more consideration of health consequences related to these exposures.
“As noted by the authors, this is an area that has received a lot of recent attention, yet there remains a large void of knowledge,” Dr. Clift said in a UK Science Media Centre release. “It highlights that some of the most dominant air pollutants can impact different mental health diagnoses, but that time-of-life is particularly important as to how each individual air pollutant may impact this diagnosis.”
Study limitations included limitations to generalizability of the data — the families in the study were more affluent and less diverse than the UK population overall.
The study was funded by the UK Medical Research Council, Wellcome Trust, and University of Bristol. Disclosures were noted in the original article.
A version of this article appeared on Medscape.com.