User login
Children with low-risk thyroid cancer can skip radioactive iodine
MONTREAL – Pediatric patients with low-risk differentiated thyroid cancer (DTC) who are spared radioactive iodine (RAI) therapy show no increases in the risk of remission, compared with those who do receive it, supporting guidelines that recommend against use of RAI in such patients.
“In 2015, when the American Thyroid Association [ATA] created their pediatric guidelines [on RAI therapy in DTC], they were taking a leap of faith that these [pediatric DTC] patients would be able to achieve remission without RAI,” said first author Mya Bojarsky, Children’s Hospital of Philadelphia (CHOP), when presenting the findings at the American Thyroid Association annual meeting.
“This is the first study to validate those guidelines and support the sentiment that for ATA low-risk pediatric thyroid cancer patients, withholding RAI therapy is clinically beneficial as it reduces exposure to radiation while having no negative impact on remission,” she said.
Prior to 2015, thyroidectomy in combination with RAI was the standard treatment for DTC in pediatric patients. However, data showing that radiation exposure in children increases the risk of secondary hematologic malignancies by 51% and solid malignancies by 23% over a lifetime raised concerns and led to a push to change the treatment approach.
In response, the 2015 ATA pediatric guidelines recommended that patients not receive RAI for the treatment of DTC that was mostly confined to the thyroid (N0 or minimal N1a disease).
Senior author Andrew J. Bauer, MD, noted that, in addition to being the first study to confirm that withholding RAI in low-risk patients is associated with the same rate of achieving remission as patients treated with RAI, the study also endorses that assessments at 1 year can be reliable predictors of remission.
“For these patients, the 1-year mark post-initial treatment (thyroidectomy) is an early and accurate time point for initial assessment of remission, with increasing rates of remission with continued surveillance (at last clinical follow-up) of approximately 90% 2 years post initial treatment,” said Dr. Bauer, medical director, CHOP, and professor of pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
“This approach has recently been validated through a prospective study in adult patients,” he added. A large recent study of 730 patients, published in the New England Journal of Medicine, supported the omission of RAI in low-risk DTC in adults, showing that, compared with those who received RAI, the no-RAI group was noninferior in the occurrence of functional, structural, and biologic events at 3 years.
Safe to eliminate RAI therapy in low-risk DTC in children
With limited data on how or if the change in treatment had an impact on rates of remission in pediatric patients, Ms. Bojarsky and colleagues conducted a retrospective cohort study of patients under the age of 19 years with ATA low-risk DTC who had undergone a total thyroidectomy at CHOP between 2010 and 2020.
Overall, they identified 95 patients, including 50 who had been treated with RAI in addition to thyroidectomy and 45 who did not receive RAI. Among those who did receive RAI, 31 were treated prior to 2015, and 19 were treated after 2019.
For the study, remission was defined as having undetectable thyroglobulin levels as well as no evidence of disease by ultrasound, Ms. Bojarsky said.
“This is important to show, because we want to ensure that as we are reducing our RAI use in the pediatric population, we were not negatively impacting their ability to achieve remission,” she explained.
The percentage of low-risk pediatric patients with DTC treated with RAI had already dropped from 100% in 2010 down to 38% by 2015 when the guidelines were issued, and after a slight rise to 50% by 2018, the practice plummeted to 0% by 2020, the study shows.
In terms of remission, at 1 year post-treatment, 80% of patients who received RAI were in remission, and the rate was even slightly higher, at 84%, among those who did not receive RAI, for a difference that was not significant.
Further looking at disease status as of the last clinical evaluation, 90% in the group treated with RAI had no evidence of disease at a median of 4.9 years of follow-up, and the rate was 87% in the group not receiving RAI, which had a median of 2.7 years of follow-up.
“In ATA low-risk patients, there is no detriment in achieving remission if RAI therapy is withheld,” say investigators.
The median tumor size in the RAI group was larger (19.5 mm vs. 12.0 mm; P < .001), and the primary tumor was T1 in 44% of the RAI group but 82% in the no-RAI group (P < .001).
The lymph node status was N0 in 72% of those receiving RAI and 76% in the no RAI group, which was not significantly different.
The leading risk factors associated with treatment with RAI included larger primary tumor size (OR, 1.07; P = .003), lymph node metastasis (OR, 3.72; P = .036), and surgery pre-2015 (OR, 9.83; P < .001).
RAI administration, N1a disease, and surgery prior to 2015 were not independent risk factors for evidence of persistent disease or indeterminate status.
Ms. Bojarsky has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Pediatric patients with low-risk differentiated thyroid cancer (DTC) who are spared radioactive iodine (RAI) therapy show no increases in the risk of remission, compared with those who do receive it, supporting guidelines that recommend against use of RAI in such patients.
“In 2015, when the American Thyroid Association [ATA] created their pediatric guidelines [on RAI therapy in DTC], they were taking a leap of faith that these [pediatric DTC] patients would be able to achieve remission without RAI,” said first author Mya Bojarsky, Children’s Hospital of Philadelphia (CHOP), when presenting the findings at the American Thyroid Association annual meeting.
“This is the first study to validate those guidelines and support the sentiment that for ATA low-risk pediatric thyroid cancer patients, withholding RAI therapy is clinically beneficial as it reduces exposure to radiation while having no negative impact on remission,” she said.
Prior to 2015, thyroidectomy in combination with RAI was the standard treatment for DTC in pediatric patients. However, data showing that radiation exposure in children increases the risk of secondary hematologic malignancies by 51% and solid malignancies by 23% over a lifetime raised concerns and led to a push to change the treatment approach.
In response, the 2015 ATA pediatric guidelines recommended that patients not receive RAI for the treatment of DTC that was mostly confined to the thyroid (N0 or minimal N1a disease).
Senior author Andrew J. Bauer, MD, noted that, in addition to being the first study to confirm that withholding RAI in low-risk patients is associated with the same rate of achieving remission as patients treated with RAI, the study also endorses that assessments at 1 year can be reliable predictors of remission.
“For these patients, the 1-year mark post-initial treatment (thyroidectomy) is an early and accurate time point for initial assessment of remission, with increasing rates of remission with continued surveillance (at last clinical follow-up) of approximately 90% 2 years post initial treatment,” said Dr. Bauer, medical director, CHOP, and professor of pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
“This approach has recently been validated through a prospective study in adult patients,” he added. A large recent study of 730 patients, published in the New England Journal of Medicine, supported the omission of RAI in low-risk DTC in adults, showing that, compared with those who received RAI, the no-RAI group was noninferior in the occurrence of functional, structural, and biologic events at 3 years.
Safe to eliminate RAI therapy in low-risk DTC in children
With limited data on how or if the change in treatment had an impact on rates of remission in pediatric patients, Ms. Bojarsky and colleagues conducted a retrospective cohort study of patients under the age of 19 years with ATA low-risk DTC who had undergone a total thyroidectomy at CHOP between 2010 and 2020.
Overall, they identified 95 patients, including 50 who had been treated with RAI in addition to thyroidectomy and 45 who did not receive RAI. Among those who did receive RAI, 31 were treated prior to 2015, and 19 were treated after 2019.
For the study, remission was defined as having undetectable thyroglobulin levels as well as no evidence of disease by ultrasound, Ms. Bojarsky said.
“This is important to show, because we want to ensure that as we are reducing our RAI use in the pediatric population, we were not negatively impacting their ability to achieve remission,” she explained.
The percentage of low-risk pediatric patients with DTC treated with RAI had already dropped from 100% in 2010 down to 38% by 2015 when the guidelines were issued, and after a slight rise to 50% by 2018, the practice plummeted to 0% by 2020, the study shows.
In terms of remission, at 1 year post-treatment, 80% of patients who received RAI were in remission, and the rate was even slightly higher, at 84%, among those who did not receive RAI, for a difference that was not significant.
Further looking at disease status as of the last clinical evaluation, 90% in the group treated with RAI had no evidence of disease at a median of 4.9 years of follow-up, and the rate was 87% in the group not receiving RAI, which had a median of 2.7 years of follow-up.
“In ATA low-risk patients, there is no detriment in achieving remission if RAI therapy is withheld,” say investigators.
The median tumor size in the RAI group was larger (19.5 mm vs. 12.0 mm; P < .001), and the primary tumor was T1 in 44% of the RAI group but 82% in the no-RAI group (P < .001).
The lymph node status was N0 in 72% of those receiving RAI and 76% in the no RAI group, which was not significantly different.
The leading risk factors associated with treatment with RAI included larger primary tumor size (OR, 1.07; P = .003), lymph node metastasis (OR, 3.72; P = .036), and surgery pre-2015 (OR, 9.83; P < .001).
RAI administration, N1a disease, and surgery prior to 2015 were not independent risk factors for evidence of persistent disease or indeterminate status.
Ms. Bojarsky has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – Pediatric patients with low-risk differentiated thyroid cancer (DTC) who are spared radioactive iodine (RAI) therapy show no increases in the risk of remission, compared with those who do receive it, supporting guidelines that recommend against use of RAI in such patients.
“In 2015, when the American Thyroid Association [ATA] created their pediatric guidelines [on RAI therapy in DTC], they were taking a leap of faith that these [pediatric DTC] patients would be able to achieve remission without RAI,” said first author Mya Bojarsky, Children’s Hospital of Philadelphia (CHOP), when presenting the findings at the American Thyroid Association annual meeting.
“This is the first study to validate those guidelines and support the sentiment that for ATA low-risk pediatric thyroid cancer patients, withholding RAI therapy is clinically beneficial as it reduces exposure to radiation while having no negative impact on remission,” she said.
Prior to 2015, thyroidectomy in combination with RAI was the standard treatment for DTC in pediatric patients. However, data showing that radiation exposure in children increases the risk of secondary hematologic malignancies by 51% and solid malignancies by 23% over a lifetime raised concerns and led to a push to change the treatment approach.
In response, the 2015 ATA pediatric guidelines recommended that patients not receive RAI for the treatment of DTC that was mostly confined to the thyroid (N0 or minimal N1a disease).
Senior author Andrew J. Bauer, MD, noted that, in addition to being the first study to confirm that withholding RAI in low-risk patients is associated with the same rate of achieving remission as patients treated with RAI, the study also endorses that assessments at 1 year can be reliable predictors of remission.
“For these patients, the 1-year mark post-initial treatment (thyroidectomy) is an early and accurate time point for initial assessment of remission, with increasing rates of remission with continued surveillance (at last clinical follow-up) of approximately 90% 2 years post initial treatment,” said Dr. Bauer, medical director, CHOP, and professor of pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
“This approach has recently been validated through a prospective study in adult patients,” he added. A large recent study of 730 patients, published in the New England Journal of Medicine, supported the omission of RAI in low-risk DTC in adults, showing that, compared with those who received RAI, the no-RAI group was noninferior in the occurrence of functional, structural, and biologic events at 3 years.
Safe to eliminate RAI therapy in low-risk DTC in children
With limited data on how or if the change in treatment had an impact on rates of remission in pediatric patients, Ms. Bojarsky and colleagues conducted a retrospective cohort study of patients under the age of 19 years with ATA low-risk DTC who had undergone a total thyroidectomy at CHOP between 2010 and 2020.
Overall, they identified 95 patients, including 50 who had been treated with RAI in addition to thyroidectomy and 45 who did not receive RAI. Among those who did receive RAI, 31 were treated prior to 2015, and 19 were treated after 2019.
For the study, remission was defined as having undetectable thyroglobulin levels as well as no evidence of disease by ultrasound, Ms. Bojarsky said.
“This is important to show, because we want to ensure that as we are reducing our RAI use in the pediatric population, we were not negatively impacting their ability to achieve remission,” she explained.
The percentage of low-risk pediatric patients with DTC treated with RAI had already dropped from 100% in 2010 down to 38% by 2015 when the guidelines were issued, and after a slight rise to 50% by 2018, the practice plummeted to 0% by 2020, the study shows.
In terms of remission, at 1 year post-treatment, 80% of patients who received RAI were in remission, and the rate was even slightly higher, at 84%, among those who did not receive RAI, for a difference that was not significant.
Further looking at disease status as of the last clinical evaluation, 90% in the group treated with RAI had no evidence of disease at a median of 4.9 years of follow-up, and the rate was 87% in the group not receiving RAI, which had a median of 2.7 years of follow-up.
“In ATA low-risk patients, there is no detriment in achieving remission if RAI therapy is withheld,” say investigators.
The median tumor size in the RAI group was larger (19.5 mm vs. 12.0 mm; P < .001), and the primary tumor was T1 in 44% of the RAI group but 82% in the no-RAI group (P < .001).
The lymph node status was N0 in 72% of those receiving RAI and 76% in the no RAI group, which was not significantly different.
The leading risk factors associated with treatment with RAI included larger primary tumor size (OR, 1.07; P = .003), lymph node metastasis (OR, 3.72; P = .036), and surgery pre-2015 (OR, 9.83; P < .001).
RAI administration, N1a disease, and surgery prior to 2015 were not independent risk factors for evidence of persistent disease or indeterminate status.
Ms. Bojarsky has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATA 2022
In childhood sickle cell disease stroke prevention is key
CINCINNATI – Sickle cell disease is well known for its associated anemia, but patients experience a range of other complications as well. These include vision and kidney problems, delayed growth, susceptibility to infection, and pain.
Another issue, not always as well recognized, is a considerably heightened risk for childhood stroke. “, and there’s also an elevated risk of five times the general population in adults with sickle cell disease,” said Lori Jordan, MD, PhD, in an interview.
At the 2022 annual meeting of the Child Neurology Society, Dr. Jordan spoke about stroke as a complication of sickle cell disease, and the role that neurologists can play in preventing primary or secondary strokes. “At least in children, studies have shown that if we screen and identify patients who are at highest risk of stroke, there are primary prevention therapies – usually implemented by hematologists, but that neurologists often are involved with – both monitoring for cognitive effects of silent cerebral infarct and also with treating patients who unfortunately still have an acute stroke,” said Dr. Jordan, who is an associate professor of pediatrics, neurology, and radiology at Vanderbilt University Medical Center, Nashville, Tenn. She also is director of the pediatric stroke program at Vanderbilt.
Time is of the essence
“In general, stroke in children is rare, but it’s more common in sickle cell disease, so it’s really important for providers to know that stroke risk is higher in those patients, particularly in those children, and then identify it and treat it earlier. Time is of the essence, and if we can give them the same therapeutics that we give the general stroke population, then time really becomes a factor, so it’s important that people know that it’s an issue for this population,” said Eboni Lance, MD, PhD, who coordinated the session where Dr. Jordan spoke.
Sickle cell disease is caused by a double mutation in the gene encoding the hemoglobin gene, producing the altered sickle hemoglobin (hemoglobin S). The change causes the hemoglobin proteins to tend to stick to one another, which can lead red blood cells to adopt a sickle-like shape. The sickle-shaped blood cells in turn have a tendency to aggregate and can block blood flow or lead to endothelial injury. Symptoms of stroke in children can include hemiparesis, aphasia, and seizure, but they can also be silent.
If no preventive is employed, one in nine with sickle cell disease will experience a stroke by the age of 19. Cerebrovascular symptoms are the most frequent debilitating complication of the condition. Nearly 40% of patients with sickle cell disease will have a silent cerebral infarct by age 18, as will 50% by age 30. Silent strokes have been associated with worse educational attainment and a greater need for educational special services.
Factors contributing to stroke in children with sickle cell disease include anemia and a low blood oxygen count, reduced oxygen affinity of hemoglobin variant, and cerebral vasculopathy. An estimated 10%-15% of young adults with sickle cell disease have severe intracranial stenosis.
Primary and secondary stroke prevention strategies
The dire consequences of stroke in this patient population underline the importance of primary stroke prevention, which requires the use of transcranial Doppler (TCD) ultrasound. It has been validated as a tool to screen for initial stroke risk in children with no history of stroke. High velocity measured on TCD indicates a narrowed blood vessel or elevated blood that is compensating for anemia. It adds up to a “struggling brain,” said Dr. Jordan, during her talk. If the TCD ultrasound velocity is greater than 200 cm/sec (or 170 cm/sec, depending on nonimaging versus imaging TCD), the TWiTCH trial showed that seven monthly transfusions is the number needed to treat to prevent one stroke. After 1 year, patients can be switched from transfusions to hydroxyurea if the patient has no significant intracranial stenosis. Hydroxyurea boosts both fetal and total hemoglobin, and also counters inflammation.
Following an acute stroke or transient ischemic attack, patients should receive a transfusion within 2 hours of presenting in the health care setting. American Society of Hematology guidelines recommend exchange transfusion rather than a simple transfusion. A simple transfusion can be initiated if an exchange transfusion is not available within 2 hours and hemoglobin values are less than 8.5 g/dL, to be followed by performance of exchange transfusion when available.
For chronic secondary stroke prevention, transfusions should be performed approximately monthly with the goal of maintaining hemoglobin above 9 g/dL at all times, as well as suppressing hemoglobin S levels to 30% or less of total hemoglobin.
Sudden, severe headache is a potential harbinger of complications like aneurysm, which occurs 10-fold more often among patients with sickle cell disease than the general population. It could also indicate increased intracranial pressure or cerebral venous sinus thrombosis.
Treatment of acute headache in sickle cell disease should avoid use of triptans, since vasoconstriction can counter the increased cerebral blood flow that compensates for anemia. Gabapentin and amitriptyline are good treatment choices.
New-onset seizures are a potential sign of stroke or posterior reversible leukoencephalopathy (PRES) in patients with sickle cell disease. Urgent MRI should be considered for all new-onset seizures. If blood pressure is high, PRES may be present. Seizures may also be an indicator of a previous brain injury.
Dr. Jordan has no relevant financial disclosures. Dr. Lance has served on an advisory board for Novartis.
CINCINNATI – Sickle cell disease is well known for its associated anemia, but patients experience a range of other complications as well. These include vision and kidney problems, delayed growth, susceptibility to infection, and pain.
Another issue, not always as well recognized, is a considerably heightened risk for childhood stroke. “, and there’s also an elevated risk of five times the general population in adults with sickle cell disease,” said Lori Jordan, MD, PhD, in an interview.
At the 2022 annual meeting of the Child Neurology Society, Dr. Jordan spoke about stroke as a complication of sickle cell disease, and the role that neurologists can play in preventing primary or secondary strokes. “At least in children, studies have shown that if we screen and identify patients who are at highest risk of stroke, there are primary prevention therapies – usually implemented by hematologists, but that neurologists often are involved with – both monitoring for cognitive effects of silent cerebral infarct and also with treating patients who unfortunately still have an acute stroke,” said Dr. Jordan, who is an associate professor of pediatrics, neurology, and radiology at Vanderbilt University Medical Center, Nashville, Tenn. She also is director of the pediatric stroke program at Vanderbilt.
Time is of the essence
“In general, stroke in children is rare, but it’s more common in sickle cell disease, so it’s really important for providers to know that stroke risk is higher in those patients, particularly in those children, and then identify it and treat it earlier. Time is of the essence, and if we can give them the same therapeutics that we give the general stroke population, then time really becomes a factor, so it’s important that people know that it’s an issue for this population,” said Eboni Lance, MD, PhD, who coordinated the session where Dr. Jordan spoke.
Sickle cell disease is caused by a double mutation in the gene encoding the hemoglobin gene, producing the altered sickle hemoglobin (hemoglobin S). The change causes the hemoglobin proteins to tend to stick to one another, which can lead red blood cells to adopt a sickle-like shape. The sickle-shaped blood cells in turn have a tendency to aggregate and can block blood flow or lead to endothelial injury. Symptoms of stroke in children can include hemiparesis, aphasia, and seizure, but they can also be silent.
If no preventive is employed, one in nine with sickle cell disease will experience a stroke by the age of 19. Cerebrovascular symptoms are the most frequent debilitating complication of the condition. Nearly 40% of patients with sickle cell disease will have a silent cerebral infarct by age 18, as will 50% by age 30. Silent strokes have been associated with worse educational attainment and a greater need for educational special services.
Factors contributing to stroke in children with sickle cell disease include anemia and a low blood oxygen count, reduced oxygen affinity of hemoglobin variant, and cerebral vasculopathy. An estimated 10%-15% of young adults with sickle cell disease have severe intracranial stenosis.
Primary and secondary stroke prevention strategies
The dire consequences of stroke in this patient population underline the importance of primary stroke prevention, which requires the use of transcranial Doppler (TCD) ultrasound. It has been validated as a tool to screen for initial stroke risk in children with no history of stroke. High velocity measured on TCD indicates a narrowed blood vessel or elevated blood that is compensating for anemia. It adds up to a “struggling brain,” said Dr. Jordan, during her talk. If the TCD ultrasound velocity is greater than 200 cm/sec (or 170 cm/sec, depending on nonimaging versus imaging TCD), the TWiTCH trial showed that seven monthly transfusions is the number needed to treat to prevent one stroke. After 1 year, patients can be switched from transfusions to hydroxyurea if the patient has no significant intracranial stenosis. Hydroxyurea boosts both fetal and total hemoglobin, and also counters inflammation.
Following an acute stroke or transient ischemic attack, patients should receive a transfusion within 2 hours of presenting in the health care setting. American Society of Hematology guidelines recommend exchange transfusion rather than a simple transfusion. A simple transfusion can be initiated if an exchange transfusion is not available within 2 hours and hemoglobin values are less than 8.5 g/dL, to be followed by performance of exchange transfusion when available.
For chronic secondary stroke prevention, transfusions should be performed approximately monthly with the goal of maintaining hemoglobin above 9 g/dL at all times, as well as suppressing hemoglobin S levels to 30% or less of total hemoglobin.
Sudden, severe headache is a potential harbinger of complications like aneurysm, which occurs 10-fold more often among patients with sickle cell disease than the general population. It could also indicate increased intracranial pressure or cerebral venous sinus thrombosis.
Treatment of acute headache in sickle cell disease should avoid use of triptans, since vasoconstriction can counter the increased cerebral blood flow that compensates for anemia. Gabapentin and amitriptyline are good treatment choices.
New-onset seizures are a potential sign of stroke or posterior reversible leukoencephalopathy (PRES) in patients with sickle cell disease. Urgent MRI should be considered for all new-onset seizures. If blood pressure is high, PRES may be present. Seizures may also be an indicator of a previous brain injury.
Dr. Jordan has no relevant financial disclosures. Dr. Lance has served on an advisory board for Novartis.
CINCINNATI – Sickle cell disease is well known for its associated anemia, but patients experience a range of other complications as well. These include vision and kidney problems, delayed growth, susceptibility to infection, and pain.
Another issue, not always as well recognized, is a considerably heightened risk for childhood stroke. “, and there’s also an elevated risk of five times the general population in adults with sickle cell disease,” said Lori Jordan, MD, PhD, in an interview.
At the 2022 annual meeting of the Child Neurology Society, Dr. Jordan spoke about stroke as a complication of sickle cell disease, and the role that neurologists can play in preventing primary or secondary strokes. “At least in children, studies have shown that if we screen and identify patients who are at highest risk of stroke, there are primary prevention therapies – usually implemented by hematologists, but that neurologists often are involved with – both monitoring for cognitive effects of silent cerebral infarct and also with treating patients who unfortunately still have an acute stroke,” said Dr. Jordan, who is an associate professor of pediatrics, neurology, and radiology at Vanderbilt University Medical Center, Nashville, Tenn. She also is director of the pediatric stroke program at Vanderbilt.
Time is of the essence
“In general, stroke in children is rare, but it’s more common in sickle cell disease, so it’s really important for providers to know that stroke risk is higher in those patients, particularly in those children, and then identify it and treat it earlier. Time is of the essence, and if we can give them the same therapeutics that we give the general stroke population, then time really becomes a factor, so it’s important that people know that it’s an issue for this population,” said Eboni Lance, MD, PhD, who coordinated the session where Dr. Jordan spoke.
Sickle cell disease is caused by a double mutation in the gene encoding the hemoglobin gene, producing the altered sickle hemoglobin (hemoglobin S). The change causes the hemoglobin proteins to tend to stick to one another, which can lead red blood cells to adopt a sickle-like shape. The sickle-shaped blood cells in turn have a tendency to aggregate and can block blood flow or lead to endothelial injury. Symptoms of stroke in children can include hemiparesis, aphasia, and seizure, but they can also be silent.
If no preventive is employed, one in nine with sickle cell disease will experience a stroke by the age of 19. Cerebrovascular symptoms are the most frequent debilitating complication of the condition. Nearly 40% of patients with sickle cell disease will have a silent cerebral infarct by age 18, as will 50% by age 30. Silent strokes have been associated with worse educational attainment and a greater need for educational special services.
Factors contributing to stroke in children with sickle cell disease include anemia and a low blood oxygen count, reduced oxygen affinity of hemoglobin variant, and cerebral vasculopathy. An estimated 10%-15% of young adults with sickle cell disease have severe intracranial stenosis.
Primary and secondary stroke prevention strategies
The dire consequences of stroke in this patient population underline the importance of primary stroke prevention, which requires the use of transcranial Doppler (TCD) ultrasound. It has been validated as a tool to screen for initial stroke risk in children with no history of stroke. High velocity measured on TCD indicates a narrowed blood vessel or elevated blood that is compensating for anemia. It adds up to a “struggling brain,” said Dr. Jordan, during her talk. If the TCD ultrasound velocity is greater than 200 cm/sec (or 170 cm/sec, depending on nonimaging versus imaging TCD), the TWiTCH trial showed that seven monthly transfusions is the number needed to treat to prevent one stroke. After 1 year, patients can be switched from transfusions to hydroxyurea if the patient has no significant intracranial stenosis. Hydroxyurea boosts both fetal and total hemoglobin, and also counters inflammation.
Following an acute stroke or transient ischemic attack, patients should receive a transfusion within 2 hours of presenting in the health care setting. American Society of Hematology guidelines recommend exchange transfusion rather than a simple transfusion. A simple transfusion can be initiated if an exchange transfusion is not available within 2 hours and hemoglobin values are less than 8.5 g/dL, to be followed by performance of exchange transfusion when available.
For chronic secondary stroke prevention, transfusions should be performed approximately monthly with the goal of maintaining hemoglobin above 9 g/dL at all times, as well as suppressing hemoglobin S levels to 30% or less of total hemoglobin.
Sudden, severe headache is a potential harbinger of complications like aneurysm, which occurs 10-fold more often among patients with sickle cell disease than the general population. It could also indicate increased intracranial pressure or cerebral venous sinus thrombosis.
Treatment of acute headache in sickle cell disease should avoid use of triptans, since vasoconstriction can counter the increased cerebral blood flow that compensates for anemia. Gabapentin and amitriptyline are good treatment choices.
New-onset seizures are a potential sign of stroke or posterior reversible leukoencephalopathy (PRES) in patients with sickle cell disease. Urgent MRI should be considered for all new-onset seizures. If blood pressure is high, PRES may be present. Seizures may also be an indicator of a previous brain injury.
Dr. Jordan has no relevant financial disclosures. Dr. Lance has served on an advisory board for Novartis.
FROM CNS 2022
Remote assessment of atopic dermatitis is feasible with patient-provided images: Study
MONTREAL – , as well as the possibility of conducting remote clinical trials that would be less expensive and less burdensome for participants, according to investigators, who presented the study at the annual meeting of the International Society of Atopic Dermatitis.
Still, practical barriers need to be addressed, particularly the problem of image quality, noted study investigator Aviël Ragamin, MD, from the department of dermatology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
“Good-quality images are crucial, [and] in our study, patients didn’t have any incentive to provide images because they had already received their medical consultation,” he explained. He suggested that this problem could be overcome by providing technical support for patients and compensation for trial participants.
The study included 87 children (median age, 7 years), who were assessed for AD severity at an academic outpatient clinic. The in-person visit included assessment with the Eczema Area and Severity Index (EASI) score, as well as the collection of whole-body clinical images. Parents were then asked to return home and to provide their own clinical images and self-administered EASI assessments of their child for comparison. Four raters were asked to rate all images twice and to compare in-clinic and self-administered EASI scores based on the images.
At the in-clinic visit, the median EASI score of the group was 8.8. The majority of patients had moderate (46.6%) or severe (14.8%) AD. Roughly 40% of the patients had darker skin (Fitzpatrick skin types IV–VI).
Using Spearman rank correlation of 1,534 in-clinic and 425 patient-provided images, the study found good inter- and intra-rater reliability for clinical image assessment and strong agreement between images and the in-clinic EASI scores. The top outliers in the assessment were individuals with either darker skin or significant postinflammatory hyperpigmentation, which are “the most difficult cases to rate, based on images,” Dr. Ragamin noted.
There was only moderate correlation between the in-clinic and self-administered EASI scores, with a significant number of patients either underestimating or overestimating their AD severity, he added.
Overall, the main problem with remote assessment seems to be the feasibility of patients providing images, said Dr. Ragamin. Only 36.8% of parents provided any images at all, and of these, 1 of 5 were deemed too blurry, leaving just 13 for final assessment, he explained.
“Pragmatically, it’s tricky,” said Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, who was asked to comment on the study. “It takes long enough to do an EASI score in person, let alone looking through blurry pictures that take too long to load into your electronic medical record. We know it works, but when our hospital went virtual [during the COVID pandemic] ... most of my patients with chronic eczema weren’t even sending me pictures.”
Regarding the utility of remote, full-body photography in clinical practice, he said, “There’s too many feasibility hoops to jump through at this point. The most promise I see is for clinical trials, where it’s hard to get people to come in.”
Dr. Ragamin and Dr. Drucker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – , as well as the possibility of conducting remote clinical trials that would be less expensive and less burdensome for participants, according to investigators, who presented the study at the annual meeting of the International Society of Atopic Dermatitis.
Still, practical barriers need to be addressed, particularly the problem of image quality, noted study investigator Aviël Ragamin, MD, from the department of dermatology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
“Good-quality images are crucial, [and] in our study, patients didn’t have any incentive to provide images because they had already received their medical consultation,” he explained. He suggested that this problem could be overcome by providing technical support for patients and compensation for trial participants.
The study included 87 children (median age, 7 years), who were assessed for AD severity at an academic outpatient clinic. The in-person visit included assessment with the Eczema Area and Severity Index (EASI) score, as well as the collection of whole-body clinical images. Parents were then asked to return home and to provide their own clinical images and self-administered EASI assessments of their child for comparison. Four raters were asked to rate all images twice and to compare in-clinic and self-administered EASI scores based on the images.
At the in-clinic visit, the median EASI score of the group was 8.8. The majority of patients had moderate (46.6%) or severe (14.8%) AD. Roughly 40% of the patients had darker skin (Fitzpatrick skin types IV–VI).
Using Spearman rank correlation of 1,534 in-clinic and 425 patient-provided images, the study found good inter- and intra-rater reliability for clinical image assessment and strong agreement between images and the in-clinic EASI scores. The top outliers in the assessment were individuals with either darker skin or significant postinflammatory hyperpigmentation, which are “the most difficult cases to rate, based on images,” Dr. Ragamin noted.
There was only moderate correlation between the in-clinic and self-administered EASI scores, with a significant number of patients either underestimating or overestimating their AD severity, he added.
Overall, the main problem with remote assessment seems to be the feasibility of patients providing images, said Dr. Ragamin. Only 36.8% of parents provided any images at all, and of these, 1 of 5 were deemed too blurry, leaving just 13 for final assessment, he explained.
“Pragmatically, it’s tricky,” said Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, who was asked to comment on the study. “It takes long enough to do an EASI score in person, let alone looking through blurry pictures that take too long to load into your electronic medical record. We know it works, but when our hospital went virtual [during the COVID pandemic] ... most of my patients with chronic eczema weren’t even sending me pictures.”
Regarding the utility of remote, full-body photography in clinical practice, he said, “There’s too many feasibility hoops to jump through at this point. The most promise I see is for clinical trials, where it’s hard to get people to come in.”
Dr. Ragamin and Dr. Drucker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – , as well as the possibility of conducting remote clinical trials that would be less expensive and less burdensome for participants, according to investigators, who presented the study at the annual meeting of the International Society of Atopic Dermatitis.
Still, practical barriers need to be addressed, particularly the problem of image quality, noted study investigator Aviël Ragamin, MD, from the department of dermatology, Erasmus MC, University Medical Center, Rotterdam, the Netherlands.
“Good-quality images are crucial, [and] in our study, patients didn’t have any incentive to provide images because they had already received their medical consultation,” he explained. He suggested that this problem could be overcome by providing technical support for patients and compensation for trial participants.
The study included 87 children (median age, 7 years), who were assessed for AD severity at an academic outpatient clinic. The in-person visit included assessment with the Eczema Area and Severity Index (EASI) score, as well as the collection of whole-body clinical images. Parents were then asked to return home and to provide their own clinical images and self-administered EASI assessments of their child for comparison. Four raters were asked to rate all images twice and to compare in-clinic and self-administered EASI scores based on the images.
At the in-clinic visit, the median EASI score of the group was 8.8. The majority of patients had moderate (46.6%) or severe (14.8%) AD. Roughly 40% of the patients had darker skin (Fitzpatrick skin types IV–VI).
Using Spearman rank correlation of 1,534 in-clinic and 425 patient-provided images, the study found good inter- and intra-rater reliability for clinical image assessment and strong agreement between images and the in-clinic EASI scores. The top outliers in the assessment were individuals with either darker skin or significant postinflammatory hyperpigmentation, which are “the most difficult cases to rate, based on images,” Dr. Ragamin noted.
There was only moderate correlation between the in-clinic and self-administered EASI scores, with a significant number of patients either underestimating or overestimating their AD severity, he added.
Overall, the main problem with remote assessment seems to be the feasibility of patients providing images, said Dr. Ragamin. Only 36.8% of parents provided any images at all, and of these, 1 of 5 were deemed too blurry, leaving just 13 for final assessment, he explained.
“Pragmatically, it’s tricky,” said Aaron Drucker, MD, a dermatologist at Women’s College Hospital and associate professor at the University of Toronto, who was asked to comment on the study. “It takes long enough to do an EASI score in person, let alone looking through blurry pictures that take too long to load into your electronic medical record. We know it works, but when our hospital went virtual [during the COVID pandemic] ... most of my patients with chronic eczema weren’t even sending me pictures.”
Regarding the utility of remote, full-body photography in clinical practice, he said, “There’s too many feasibility hoops to jump through at this point. The most promise I see is for clinical trials, where it’s hard to get people to come in.”
Dr. Ragamin and Dr. Drucker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases fall to lowest level in over a year
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.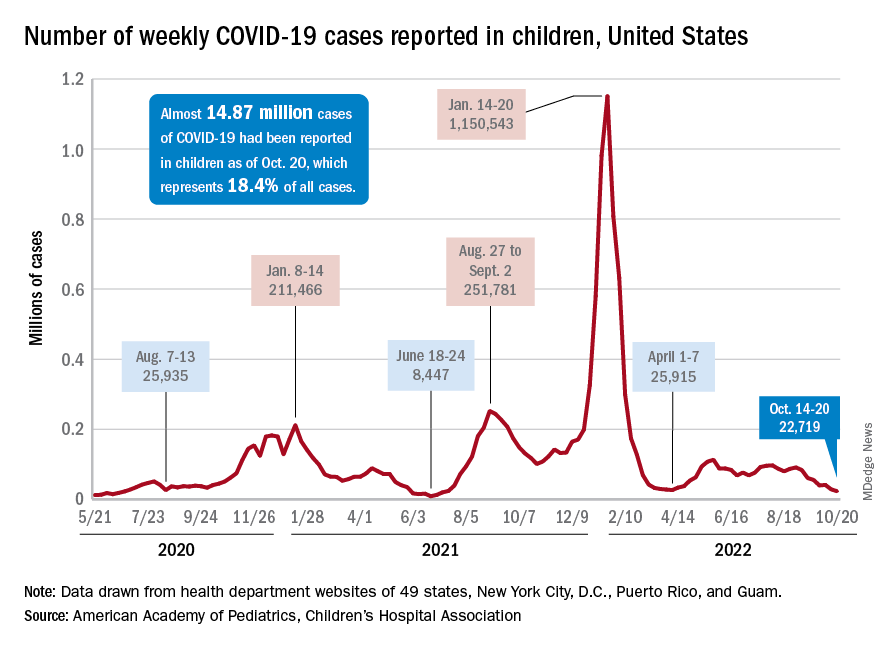
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.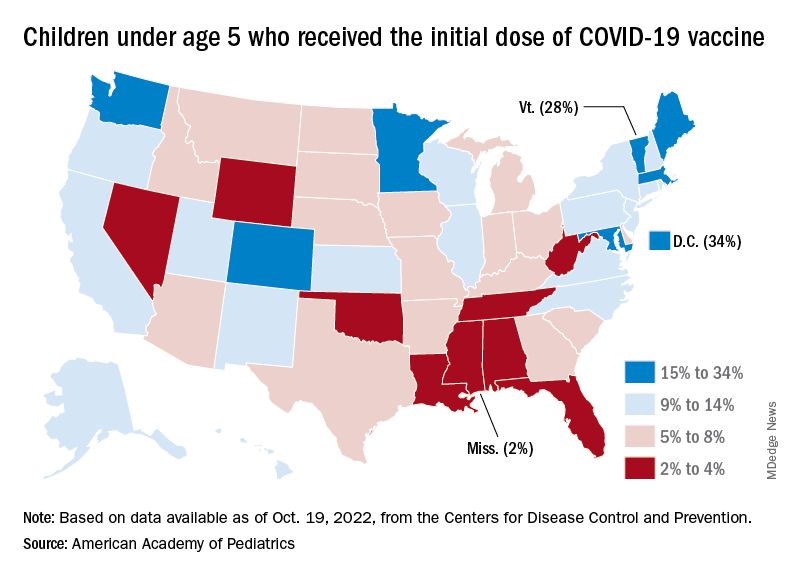
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
With the third autumn of the COVID era now upon us, the discussion has turned again to a possible influenza/COVID twindemic, as well as the new-for-2022 influenza/COVID/respiratory syncytial virus tripledemic. It appears, however, that COVID may have missed the memo.
For the sixth time in the last 7 weeks, the number of new COVID cases in children fell, with just under 23,000 reported during the week of Oct. 14-20, according to the American Academy of Pediatrics and the Children’s Hospital Association. That is the lowest weekly count so far this year, and the lowest since early July of 2021, just as the Delta surge was starting. New pediatric cases had dipped to 8,500, the lowest for any week during the pandemic, a couple of weeks before that, the AAP/CHA data show.
Weekly cases have fallen by almost 75% since over 90,000 were reported for the week of Aug. 26 to Sept. 1, even as children have returned to school and vaccine uptake remains slow in the youngest age groups. Rates of emergency department visits with diagnosed COVID also have continued to drop, as have new admissions, and both are nearing their 2021 lows, according to the Centers for Disease Control and Prevention.
New vaccinations in children under age 5 years were up slightly for the most recent week (Oct. 13-19), but total uptake for that age group is only 7.1% for an initial dose and 2.9% for full vaccination. Among children aged 5-11 years, 38.7% have received at least one dose and 31.6% have completed the primary series, with corresponding figures of 71.2% and 60.9% for those aged 12-17, the CDC said on its COVID Data Tracker.
Despite the low overall numbers, though, the youngest children are, in one respect, punching above their weight when it comes to vaccinations. In the 2 weeks from Oct. 6 to Oct. 19, children under 5 years of age, who represent 5.9% of the U.S. population, received 9.2% of the initial vaccine doses administered. Children aged 5-11 years, who represent 8.7% of the total population, got just 4.2% of all first doses over those same 2 weeks, while 12- to 17-year-olds, who make up 7.6% of the population, got 3.4% of the vaccine doses, the CDC reported.
On the vaccine-approval front, the Food and Drug Administration recently announced that the new bivalent COVID-19 vaccines are now included in the emergency use authorizations for children who have completed primary or booster vaccination. The Moderna vaccine is authorized as a single-dose booster for children as young as 6 years and the Pfizer-BioNTech vaccine can be given as a single booster dose in children as young as 5 years, the FDA said.
“These bivalent COVID-19 vaccines include an mRNA component of the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component in common between the omicron variant BA.4 and BA.5 lineages,” the FDA said.
Sexual health care for disabled youth: Tough and getting tougher
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The developmentally disabled girl was just 10 years old when Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee, helped care for her. Providing that care was not emotionally easy. “Her brother’s friend sexually assaulted her and impregnated her,” Dr. Thew said.
The girl was able to obtain an abortion, a decision her parents supported. The alternative could have been deadly. “She was a tiny little person and would not have been able to carry a fetus,” Dr. Thew, a nurse practitioner, said.
Dr. Thew said she’s thankful that tragic case occurred before 2022. After the United States Supreme Court overturned Roe v. Wade in June, Wisconsin reverted to an 1849 law banning abortion. Although the law is currently being challenged, Dr. Thew wonders how the situation would have played out now. (Weeks after the Supreme Court’s decision, a similar case occurred in Ohio. In that case, a 10-year-old girl had to travel out of the state to obtain an abortion after having been raped.)
Talking to adolescents and young adults about reproductive health, whether regarding an unexpected pregnancy, the need for contraception, or to provide information about sexual activity, can be a challenge even for experienced health care providers.
The talks, decisions, and care are particularly complex when patients have developmental and intellectual disabilities. Among the many factors, Dr. Thew said, are dealing with menstruation, finding the right contraceptives, and counseling parents who might not want to acknowledge their children’s emerging sexuality.
Statistics: How many?
Because the definitions of disabilities vary and they represent a spectrum, estimates for how many youth have intellectual or developmental disabilities range widely.
In 2019, the National Survey of Children’s Health found that 1 in 4 children and adolescents aged 12-17 years have special health care needs because of disability. The American Community Survey estimates more than 1.3 million people aged 16-20 have a disability.
Intellectual disabilities can occur when a person’s IQ is below 70, significantly impeding the ability to perform activities of daily living, such as eating, dressing, and communicating. Developmental disabilities are impairments in physical, learning, language, and behavior, according to the United States Centers for Disease Control and Prevention. Among the conditions are attention-deficit/hyperactivity disorder, autism spectrum disorders, fragile X syndrome, learning and language problems, spina bifida, and other conditions.
Addressing common issues, concerns
April Kayser is a health educator for the Multnomah County Health Department, Portland, Ore. In 2016, Ms. Kayser and other experts conducted interviews with 11 youth with developmental and intellectual disabilities and 34 support people, either parents or professionals who provide services. The survey was part of the SHEIDD Project – short for Sexual Health Equity for Individuals with Intellectual/Developmental Disabilities – at Oregon Health and Science University (OHSU).
From their findings, the researchers compiled guidelines. They provided scenarios that health care providers need to be aware of and that they need to be ready to address:
- A boy, 14, who is unclear about what to do when he feels sexually excited and wants to masturbate but isn’t at home. He has been told that masturbation is appropriate in private.
- A 20-year-old woman who lives in a group home is pregnant. She confesses to her parents during a visit that another resident is her boyfriend and that he is the father of the child she is expecting.
- A 17-year-old boy wants to ask out another student, who is 15.
Some developmentally and intellectually disabled youth can’t turn to their parents for help. One person in the survey said his father told him, “You don’t need to worry about any of that stuff. You’re too young.” Another said the job of a health care provider was to offer reproductive and sex education “to make sure you don’t screw up in some bad way.”
One finding stood out: Health care providers were at the top of the list of those whom young people trusted for information about reproductive and sexual health, Ms. Kayser said. Yet in her experience, she said, health care professionals are hesitant to bring up the issues with all youth, “especially those with intellectual and developmental disabilities.”
Health care providers often talk both to the patient and to the parents. Those conversations can be critical when a child is developmentally or intellectually disabled.
Women with disabilities have been shown to have a higher risk for adverse outcomes of pregnancy, said Willi Horner-Johnson, PhD, associate professor at OHSU–Portland State University School of Public Health.
In a recent study, she and her colleagues analyzed data from the CDC’s National Survey of Family Growth that included self-reported disability status. They found that the number of women with disabilities who give birth is far higher than was previously thought.
The researchers found that 19.5% of respondents who gave birth reported at least one sensory, cognitive, or mobility-related disability, a rate that is much greater than the less than 1%-6.6% estimates that are based on hospital discharge data.
Her group reported other troubling findings: Women with disabilities are twice as likely to have smoked during their pregnancy (19% vs. 8.9%) and are more likely to have preterm and low-birthweight babies.
Clinicians play an important role
Dr. Horner-Johnson agreed with the finding from the Multnomah County survey that health care providers play an important role in providing those with intellectual and developmental disabilities reproductive health care that meets their needs. “Clinicians need to be asking people with disabilities about their reproductive plans,” she said.
In the Multnomah County report, the researchers advised health care providers to recognize that people with disabilities are social and sexual beings; to learn about their goals, including those regarding sex and reproductive health; and to help youth build skills for healthy relationships and sexual activity.
Dr. Horner-Johnson pointed out that the American College of Obstetricians and Gynecologists “recommends that clinicians discuss reproductive plans at every visit, for example, by asking one key question – ‘Would you like to become pregnant in the next year?’ – of every woman of reproductive age.”
Some women will not be able to answer that question, and health care providers at times must rely on a caregiver for input. But many women, even those with disabilities, could answer if given a chance. She estimated that only about 5% of disabled people are unable to communicate. “Clinicians defer to the caregiver more than they need to,” she said.
Clinicians are becoming better at providing care to those with disabilities, Dr. Horner-Johnson said, yet they have a way to go. Clinician biases may prevent some from asking all women, including those with disabilities, about their reproductive plans. “Women with disabilities have described clinicians treating them as nonsexual, assuming or implying that they would not or should not get pregnant,” she writes in her report.
Such biases, she said, could be reduced by increased education of providers. A 2018 study in Health Equity found that only 19.3% of ob.gyns. said they felt equipped to manage the pregnancy of a woman with disabilities.
Managing sexuality and sexual health for youth with disabilities can be highly complex, according to Margaret Thew, DNP, medical director of adolescent medicine at Children’s Wisconsin, Milwaukee. Challenges include the following:
- Parents often can’t deal with the reality that their teen or young adult is sexually active or may become so. Parents she helps often prefer to use the term “hormones,” not contraceptives, when talking about pregnancy prevention.
- Menstruation is a frequent concern, especially for youth with severe disabilities. Some react strongly to seeing a sanitary pad with blood, for example, by throwing it. Parents worry that caregivers will balk at changing pads regularly. As a result, some parents want complete menstrual suppression, Dr. Thew said. The American Academy of Pediatrics outlines how to approach menstrual suppression through methods such as the use of estrogen-progestin, progesterone, a ring, or a patch. In late August, the American College of Obstetricians and Gynecologists released its clinical consensus on medical management of menstrual suppression.
- Some parents want to know how to obtain a complete hysterectomy for the patient – an option Dr. Thew and the AAP discourage. “We will tell them that’s not the best and safest approach, as you want to have the estrogen for bone health,” she said.
- After a discussion of all the options, an intrauterine device proves best for many. “That gives 7-8 years of protection,” she said, which is the approved effective duration for such devices. “They are less apt to have heavy monthly menstrual bleeding.”
- Parents of boys with disabilities, especially those with Down syndrome, often ask for sex education and guidance when sexual desires develop.
- Many parents want effective birth control for their children because of fear that their teen or young adult will be assaulted, a fear that isn’t groundless. Such cases are common, and caregivers frequently are the perpetrators.
Ms. Kayser, Dr. Horner-Johnson, and Dr. Thew have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dupilumab-associated ocular surface disease in patients with AD: Unraveling the link
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
MONTREAL – , according to a study presented at the annual meeting of the International Society of Atopic Dermatitis.
In a prospective trial of 69 patients with AD starting dupilumab (Dupixent), baseline OSD was found in 91.3%, with about half of these patients reporting no symptoms, said investigator Roselie Achten, MD, from the National Expertise Center for Atopic Dermatitis at University Medical Center Utrecht, the Netherlands. Among these patients, ophthalmologic assessment revealed no OSD in 6 patients and mild OSD in 37 patients, but moderate and severe disease in 20 and 6 patients, respectively, she said, adding that 71% of the group also reported allergic conjunctivitis at baseline.
The patients enrolled in the study who started dupilumab were aged 36-38 years, with Eczema Area and Severity Index (EASI) scores of 14.7-16.5. Baseline ocular surface health was assessed with the Utrecht Ophthalmic Inflammatory and Allergic disease (UTOPIA) score. Tear fluid was collected to analyze biomarkers and dupilumab levels, and impression cytology was performed to collect conjunctival tissue cells for analysis of goblet cells. These measurements were repeated at 4 and 28 weeks after the start of therapy.
Over 28 weeks of treatment, 14.5% of patients experienced worsening of OSD, with worsening disease associated with a decline in the number of goblet cells. In addition, dupilumab treatment was associated with a significant decline in the production of Mucin5AC, suggesting a decline in function of the goblet cells. “Our hypothesis that the blocking effect of dupilumab on [interleukin-] IL-13 might lead to less goblet cells and less mucin production,” she explained.
In a subset of 48 patients, the researchers also detected significantly higher tear fluid dupilumab levels among those patients with more severe OSD, with comparable serum levels.
OSD has been reported in up to 34% of dupilumab-treated patients with AD and is the most frequently reported side-effect of this treatment, noted Dr. Achten. This side effect is not reported by other patients treated with dupilumab for other indications, she added, “suggesting that AD patients may have a predisposition to develop OSD during dupilumab treatment.”
Indeed, as recently noted by Vivian Shi, MD, from the department of dermatology, University of Arkansas for Medical Sciences, Little Rock, and colleagues, “for reasons not well understood, the incidence of conjunctivitis in dupilumab patients with asthma (0%-2.3%), chronic rhinosinusitis with nasal polyps (1.6%), or eosinophilic esophagitis (0%) is low to none; thus, patients with AD may be particularly susceptible.”
Dr. Achten said that dupilumab-treated patients with AD at her center are prescribed topical tacrolimus and ketotifen eye drops if they develop OSD.
Asked for comment, Melinda Gooderham, MD, who moderated the session, was impressed with the study. “I’d heard about the goblet cells, there were little bits of data here and there, but the tear analysis is something I hadn’t seen before. It was a nice series of experiments that pulled everything together,” she told this news organization. Dr. Gooderham, who is assistant professor at Queens University, in Kingston, Ontario, medical director at the SKiN Centre for Dermatology in Peterborough, Ontario, and consultant physician at Peterborough Regional Health Centre, said that she first began noticing dupilumab-related OSD as an early trial investigator for the drug. “When you put some patients on the drug it’s almost like tipping the balance – that little bit of mucin they’re dependent on is now reduced and it makes them more symptomatic,” she said.
Though she prescribes lubricating eye drops as prophylaxis for all her dupilumab-treated patients with AD, she recommends referring any patients who develop OSD to an ophthalmologist who is familiar with this specific side effect. “If they just see a random ophthalmologist who doesn’t know dupilumab, and doesn’t know the story around it, they could get any sort of diagnosis, or even be told to stop the medication altogether.”
The study was sponsored by Sanofi. Dr. Achten disclosed no other conflicts of interest. Dr. Gooderham is an investigator with Sanofi Genzyme for dupilumab.
A version of this article first appeared on Medscape.com.
FROM ISAD 2022
Doctors favor euphemisms and jargon in discussions of death
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
Words including death, die, dying, or stillborn were frequently replaced by euphemisms in meetings between clinicians and families of critically ill children, based on data from 33 family meetings that involved discussions of death.
Clear communication is essential in discussing death with patients and families and current consensus guidelines recommend against use of euphemisms; data also suggest that patients and families prefer clear and direct language, wrote Margaret H. Barlet, of Duke University, Durham, N.C., and colleagues.
However, data on the language used in discussions of death in neonatal or pediatric contexts are limited, they said.
In a study published in JAMA Network Open, the researchers reviewed conversations between clinicians and parents of critically ill children. The study participants included 20 parents of 13 infants with neurological conditions who were hospitalized in a pediatric ICU in a single center in the southeastern United States between September 2018 and September 2020. Family meetings were scheduled to discuss prognosis and whether to start, not start, or discontinue life-sustaining treatment. The discussions were recorded, transcribed, and deidentified. The median age of the parents was 28.5 years; 60% identified as Black, 40% as White, and 10% as Asian; with some selecting more than one race.
For all 13 infants, one parent identified as the infant’s mother, and another parent identified as the father for seven of the infants. The median gestational age of the infants was 37 weeks; 54% were female, and the median hospital stay was 86 days.
Twelve infants (92%) required mechanical ventilation, six required chest compressions, and five had a do-not-attempt resuscitation order placed. Two infants died during the hospital admission process.
The primary outcome of the study was language used to reference death during family meetings between doctors and families. In the family conversations, death was referenced 406 times (275 times by clinicians and 131 times by family members).
Families were more likely than were clinicians to use the words die, death, dying, or stillborn; these terms appeared in 19 of 131 references by families and 13 of 275 references by clinicians (15% vs. 5%).
In addition to a category for use of words such as die, death, dying, or stillborn, the researchers identified four types of euphemisms used in place of these terms. They characterized the types of euphemisms as survival framing (for example, not live), colloquialisms (for example, pass away), medical jargon or use of physiologic terms (for example, code event or irrecoverable heart rate drop) and the use of pronouns without an antecedent (for example, it might happen soon).
Overall, 92% of references to death in the conversations were euphemistic. Medical jargon was the most common type of euphemism used by clinicians (118 of 275 references, 43%), while colloquialism was the most common type used by family members (44 of 131 references, 34%).
The results are consistent with limited research on this topic and show the high rates of euphemistic language used in discussions of death, the researchers wrote in their discussion. “Although our work did not directly evaluate the comparative clarity of different ways to reference death, our results raise questions about what language is most clear,” they said. The researchers proposed that their classification of euphemistic language may provide a framework for the use of language in discussions of death and may prompt clinicians to notice the language they use and hear from patients and families. “Empirically evaluating the perceived clarity of euphemism types and their effects on shared decision-making should be a priority for future study and should be used to inform interventions for improving communication in this context,” they said.
The findings were limited by several factors including the use of data from a single institution and the exclusion of non-English speaking families, the researchers noted. In addition, the researchers studied only what was said, therefore “questions about speaker motivation, listener understanding, and the effects of language choice on decision-making remain unanswered,” they added.
However, the results reflect the frequent use of euphemisms by both clinicians and families, and more research is needed to assess the effect of language on understanding, decision-making, and doctor-patient relationships, the researchers concluded.
Euphemisms can create confusion but may increase empathy
“Ms. Barlet and colleagues provide further consideration of types of speech that may obscure a clinician’s intended meaning or distract from their true point in the context of family discussions about critically ill patients,” Michael B. Pitt, MD, of the University of Minnesota, Minneapolis, and colleagues wrote in an accompanying editorial. Using a euphemism such as “pass on” instead of “die” may be an intentional choice by physicians to use less harsh language but it may still cause confusion, they noted.
The study showed how frequently physicians use euphemisms to talk about death but was distinctive in the inclusion of data on language use by families as well, they said.
“This pattern of use identified among the infants’ families may indicate that despite the clinical recommendation that end-of-life discussions avoid the use of euphemisms, it may be worth noting and responding to families’ language preferences accordingly once it is clear they have expressed understanding that the clinician is speaking of death,” they said. For example, if a family is consistently using softer terminology, clinicians should consider responding with similar terms, rather than using medical jargon or the words death or dying, they wrote.
“As the authors note, family preferences for this type of discussion are an important target for future research aimed at optimizing family-centered communication,” the editorialists added.
Families seek clarity in communication
“Clinicians have an important role in helping parents of seriously ill children understand their child’s health condition and make value-driven decisions about care,” Jennifer W. Mack, MD, of Harvard Medical School and the Dana-Farber Cancer Institute, Boston, said in an interview. “The words that clinicians use can have a significant impact on the knowledge parents take away from encounters and the decisions they make. While there is evidence of euphemistic language in the adult setting, there is limited information about this in children,” said Dr. Mack, a pediatric hematologist/oncologist who was not involved in the study.
Dr. Mack said she was not entirely surprised that in the current study, clinician language often includes medical jargon and an avoidance of direct language about death. “This is consistent with what I have seen in clinical practice,” she said. “One striking aspect of the study is that parents used terms like death or die more often than clinicians, and they sometimes used these terms as a way to clarify what the clinician was saying. This suggests to me that parents often want clarity, even if the information is very difficult,” she said.
The key message of the study is that clinicians should pay attention to the words they use to talk about the possibility of death and recognize the tendency of many clinicians to fall back on medical jargon, said Dr. Mack.
“My personal belief is that it is possible to be both clear and compassionate, and clinicians should strive for both in these conversations, to support families and help them make their best decisions for their children,” she said. “We need to remember a single communication strategy or choice of words is not likely to feel supportive to every family; what is helpful for one family may feel painful to another,” she emphasized. “Being willing to listen to the needs they express and their own language choice can help us to be responsive to individual needs,” she added.
An important next step for research is to learn more about what families experience as supportive during conversations with clinicians about death and dying, Dr. Mack said.
The study was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and the Doris Duke Charitable Foundation. The researchers, editorial authors, and Dr. Mack had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Psychiatric comorbidities in the pediatric neurology clinic
CINCINNATI – Neurology and psychiatry have an inherent kinship, as one often deals with the brain and the other always focuses on the mind. The two fields can be intertwined, since neurological conditions are often associated with psychiatric comorbidities amid complex relationships: For example, a young patient with a neurological disorder may experience anxiety due to life changes, his or her diagnosis, or altered biological pathways from the condition or medications used to treat it.
As a result, , according to Devin McNulty, PhD, who spoke on the topic at the 2022 annual meeting of the Child Neurology Society.
The ‘second pandemic’
Mental health conditions represent about 16% of the global burden of disease among people aged 10-19, and the COVID-19 pandemic has drastically worsened the problem, as shutdowns, school loss, and economic struggles have added to the burden. “I think we’ve really seen mental health as sort of the second pandemic. We’ve seen this in Chicago in our emergency room, and in outpatient clinics wait-lists are really high. I think adolescents are specifically at risk,” said Dr. McNulty during her talk. She is an assistant professor of psychiatry and behavioral sciences at Northwestern University and a child psychiatrist at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Common diagnoses include major depressive order, social anxiety disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive-compulsive disorder, somatic symptom disorder, and functional neurological symptom disorder. The last can appear as neurological symptoms that are not consistent with neurological medical conditions, such as attacks or seizures, abnormal movements, sensory loss or gain, weakness or paralysis, or speech and swallowing issues. It is the second most commonly diagnosed disorder in neurology clinics and accounts for 10% of neurology hospitalizations, and it leads to high rates of health care utilization and functional impairment.
Overall, children with neurological conditions are at about a 5-fold increased risk for depression and anxiety disorders, with a range of contributing risk factors. These include biological factors like medication use, neurological dysfunction, and genetic vulnerability. Psychological factors include stressors, the child’s reaction to the diagnosis and illness, and the level of his or her coping skills. Psychiatric comorbidities may also be triggered by social factors such as familial stress, peer rejection and social isolation, and barriers to treatment for the neurological condition. As just one example, overprotective parenting behavior, while adaptive in moderation, can create a sort of feedback loop that can lead to separation anxiety.
A unique opportunity
“There’s an overlap,” Dr. McNulty said, “because the origin is often multifactorial.” A young patient has a medical condition, which can be chronic or disabling, and the age of onset and diagnosis comes during a critical developmental period. “Then we have issues such as the impact of treatments, whether that’s medication side effects or medical visits. And then disease-related environmental changes, such as family factors, social changes, and impact on school,” said Dr. McNulty.
Child neurologists are in a unique position to identify and ensure treatment of these psychiatric comorbidities, according to Dr. McNulty. “Child neurologists will see psychiatric symptoms in their patient population, and pediatric providers have a unique capacity and ability to treat these patients, especially when you’re seeing patients on a frequent basis. You get to know these patients and their families really well,” she said.
She specifically pointed to three areas: psychosocial screening, differential diagnosis, and treatment and management.
There are broad-based screening measures that can be useful, such as the Strengths and Difficulties Questionnaire and the Pediatric Symptom Checklist. Disorder-specific screening tools include the PHQ-9 (depression), GAD7 (anxiety), Vanderbilt (ADHD), and PROMIS measures for anxiety and depression. “The idea behind the screening measure is that all patients would fill this out and then if a patient screens positive, they would benefit from a more thorough evaluation and history,” said Dr. McNulty.
However, she noted that screening shouldn’t necessarily be a one-off effort. Research has shown that sequential screening is the most powerful strategy. “Then you can get a baseline of a patient’s emotional and behavioral functioning, and it’s actually the changes in some of these screening measures that might give them most clinical information,” said Dr. McNulty.
In fact, on October 11, 2022, the U.S. Preventive Services Task Force announced a recommendation that all children starting at age 8 should be screened for anxiety disorders. It is already recommended to screen children aged 12 and over for depressive disorders, although these documents are aimed primarily at pediatricians or primary care clinics. The American Academy of Neurology has also recommended routine screening of psychiatric and behavioral disorders among children with epilepsy.
A unique perspective
Once a disorder is identified, neurologists can bring a unique perspective to treatment. The neurologist can use his or her knowledge of the disease state to assess whether symptoms are due to poor adjustment to the neurological condition, a primary psychiatric disorder, or the biological underpinnings of the illness or prescribed medications. “I think their neurologist can sort of help tease that apart, [using] their knowledge of neurologic disorders and pathways and medications in a way that psychologists might not be able to do on their own,” said Dr. McNulty.
She also emphasized that there are effective treatments for psychiatric disorders, including cognitive behavioral therapy and various pharmacotherapy options. Other approaches for treating comorbid neurological and psychiatric disorders may include building adaptive coping skills, psychoeducation, and incorporating changes to the family or school environment.
During the Q&A period, one person commented that there should be more psychiatric training for neurology residents. “We do work with the same brain, so I completely agree with that,” said Dr. McNulty.
She was also asked how to identify psychiatric symptoms in nonverbal patients. “One thing that I pay close attention to when I ask parents about (their child) is changes in their physical (attributes). Oftentimes in anxiety in folks who are not severely impaired, if we’re feeling anxious we might be breathing a little faster, or we might get a little sweaty. So looking for physical manifestations is one thing. And then sometimes I’ll tell the parents, if we’re not quite sure, I’ll say ‘I’m not sure, but this is very common given the disorder that you have. Can we check?’ I’m always very clear that I may not be nailing it, but then when we go after it with targeted treatment and we see it getting better, we can say ‘Aha!’ ”
Dr. McNulty has no relevant financial disclosures.
CINCINNATI – Neurology and psychiatry have an inherent kinship, as one often deals with the brain and the other always focuses on the mind. The two fields can be intertwined, since neurological conditions are often associated with psychiatric comorbidities amid complex relationships: For example, a young patient with a neurological disorder may experience anxiety due to life changes, his or her diagnosis, or altered biological pathways from the condition or medications used to treat it.
As a result, , according to Devin McNulty, PhD, who spoke on the topic at the 2022 annual meeting of the Child Neurology Society.
The ‘second pandemic’
Mental health conditions represent about 16% of the global burden of disease among people aged 10-19, and the COVID-19 pandemic has drastically worsened the problem, as shutdowns, school loss, and economic struggles have added to the burden. “I think we’ve really seen mental health as sort of the second pandemic. We’ve seen this in Chicago in our emergency room, and in outpatient clinics wait-lists are really high. I think adolescents are specifically at risk,” said Dr. McNulty during her talk. She is an assistant professor of psychiatry and behavioral sciences at Northwestern University and a child psychiatrist at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Common diagnoses include major depressive order, social anxiety disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive-compulsive disorder, somatic symptom disorder, and functional neurological symptom disorder. The last can appear as neurological symptoms that are not consistent with neurological medical conditions, such as attacks or seizures, abnormal movements, sensory loss or gain, weakness or paralysis, or speech and swallowing issues. It is the second most commonly diagnosed disorder in neurology clinics and accounts for 10% of neurology hospitalizations, and it leads to high rates of health care utilization and functional impairment.
Overall, children with neurological conditions are at about a 5-fold increased risk for depression and anxiety disorders, with a range of contributing risk factors. These include biological factors like medication use, neurological dysfunction, and genetic vulnerability. Psychological factors include stressors, the child’s reaction to the diagnosis and illness, and the level of his or her coping skills. Psychiatric comorbidities may also be triggered by social factors such as familial stress, peer rejection and social isolation, and barriers to treatment for the neurological condition. As just one example, overprotective parenting behavior, while adaptive in moderation, can create a sort of feedback loop that can lead to separation anxiety.
A unique opportunity
“There’s an overlap,” Dr. McNulty said, “because the origin is often multifactorial.” A young patient has a medical condition, which can be chronic or disabling, and the age of onset and diagnosis comes during a critical developmental period. “Then we have issues such as the impact of treatments, whether that’s medication side effects or medical visits. And then disease-related environmental changes, such as family factors, social changes, and impact on school,” said Dr. McNulty.
Child neurologists are in a unique position to identify and ensure treatment of these psychiatric comorbidities, according to Dr. McNulty. “Child neurologists will see psychiatric symptoms in their patient population, and pediatric providers have a unique capacity and ability to treat these patients, especially when you’re seeing patients on a frequent basis. You get to know these patients and their families really well,” she said.
She specifically pointed to three areas: psychosocial screening, differential diagnosis, and treatment and management.
There are broad-based screening measures that can be useful, such as the Strengths and Difficulties Questionnaire and the Pediatric Symptom Checklist. Disorder-specific screening tools include the PHQ-9 (depression), GAD7 (anxiety), Vanderbilt (ADHD), and PROMIS measures for anxiety and depression. “The idea behind the screening measure is that all patients would fill this out and then if a patient screens positive, they would benefit from a more thorough evaluation and history,” said Dr. McNulty.
However, she noted that screening shouldn’t necessarily be a one-off effort. Research has shown that sequential screening is the most powerful strategy. “Then you can get a baseline of a patient’s emotional and behavioral functioning, and it’s actually the changes in some of these screening measures that might give them most clinical information,” said Dr. McNulty.
In fact, on October 11, 2022, the U.S. Preventive Services Task Force announced a recommendation that all children starting at age 8 should be screened for anxiety disorders. It is already recommended to screen children aged 12 and over for depressive disorders, although these documents are aimed primarily at pediatricians or primary care clinics. The American Academy of Neurology has also recommended routine screening of psychiatric and behavioral disorders among children with epilepsy.
A unique perspective
Once a disorder is identified, neurologists can bring a unique perspective to treatment. The neurologist can use his or her knowledge of the disease state to assess whether symptoms are due to poor adjustment to the neurological condition, a primary psychiatric disorder, or the biological underpinnings of the illness or prescribed medications. “I think their neurologist can sort of help tease that apart, [using] their knowledge of neurologic disorders and pathways and medications in a way that psychologists might not be able to do on their own,” said Dr. McNulty.
She also emphasized that there are effective treatments for psychiatric disorders, including cognitive behavioral therapy and various pharmacotherapy options. Other approaches for treating comorbid neurological and psychiatric disorders may include building adaptive coping skills, psychoeducation, and incorporating changes to the family or school environment.
During the Q&A period, one person commented that there should be more psychiatric training for neurology residents. “We do work with the same brain, so I completely agree with that,” said Dr. McNulty.
She was also asked how to identify psychiatric symptoms in nonverbal patients. “One thing that I pay close attention to when I ask parents about (their child) is changes in their physical (attributes). Oftentimes in anxiety in folks who are not severely impaired, if we’re feeling anxious we might be breathing a little faster, or we might get a little sweaty. So looking for physical manifestations is one thing. And then sometimes I’ll tell the parents, if we’re not quite sure, I’ll say ‘I’m not sure, but this is very common given the disorder that you have. Can we check?’ I’m always very clear that I may not be nailing it, but then when we go after it with targeted treatment and we see it getting better, we can say ‘Aha!’ ”
Dr. McNulty has no relevant financial disclosures.
CINCINNATI – Neurology and psychiatry have an inherent kinship, as one often deals with the brain and the other always focuses on the mind. The two fields can be intertwined, since neurological conditions are often associated with psychiatric comorbidities amid complex relationships: For example, a young patient with a neurological disorder may experience anxiety due to life changes, his or her diagnosis, or altered biological pathways from the condition or medications used to treat it.
As a result, , according to Devin McNulty, PhD, who spoke on the topic at the 2022 annual meeting of the Child Neurology Society.
The ‘second pandemic’
Mental health conditions represent about 16% of the global burden of disease among people aged 10-19, and the COVID-19 pandemic has drastically worsened the problem, as shutdowns, school loss, and economic struggles have added to the burden. “I think we’ve really seen mental health as sort of the second pandemic. We’ve seen this in Chicago in our emergency room, and in outpatient clinics wait-lists are really high. I think adolescents are specifically at risk,” said Dr. McNulty during her talk. She is an assistant professor of psychiatry and behavioral sciences at Northwestern University and a child psychiatrist at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Common diagnoses include major depressive order, social anxiety disorder, generalized anxiety disorder, post-traumatic stress disorder, obsessive-compulsive disorder, somatic symptom disorder, and functional neurological symptom disorder. The last can appear as neurological symptoms that are not consistent with neurological medical conditions, such as attacks or seizures, abnormal movements, sensory loss or gain, weakness or paralysis, or speech and swallowing issues. It is the second most commonly diagnosed disorder in neurology clinics and accounts for 10% of neurology hospitalizations, and it leads to high rates of health care utilization and functional impairment.
Overall, children with neurological conditions are at about a 5-fold increased risk for depression and anxiety disorders, with a range of contributing risk factors. These include biological factors like medication use, neurological dysfunction, and genetic vulnerability. Psychological factors include stressors, the child’s reaction to the diagnosis and illness, and the level of his or her coping skills. Psychiatric comorbidities may also be triggered by social factors such as familial stress, peer rejection and social isolation, and barriers to treatment for the neurological condition. As just one example, overprotective parenting behavior, while adaptive in moderation, can create a sort of feedback loop that can lead to separation anxiety.
A unique opportunity
“There’s an overlap,” Dr. McNulty said, “because the origin is often multifactorial.” A young patient has a medical condition, which can be chronic or disabling, and the age of onset and diagnosis comes during a critical developmental period. “Then we have issues such as the impact of treatments, whether that’s medication side effects or medical visits. And then disease-related environmental changes, such as family factors, social changes, and impact on school,” said Dr. McNulty.
Child neurologists are in a unique position to identify and ensure treatment of these psychiatric comorbidities, according to Dr. McNulty. “Child neurologists will see psychiatric symptoms in their patient population, and pediatric providers have a unique capacity and ability to treat these patients, especially when you’re seeing patients on a frequent basis. You get to know these patients and their families really well,” she said.
She specifically pointed to three areas: psychosocial screening, differential diagnosis, and treatment and management.
There are broad-based screening measures that can be useful, such as the Strengths and Difficulties Questionnaire and the Pediatric Symptom Checklist. Disorder-specific screening tools include the PHQ-9 (depression), GAD7 (anxiety), Vanderbilt (ADHD), and PROMIS measures for anxiety and depression. “The idea behind the screening measure is that all patients would fill this out and then if a patient screens positive, they would benefit from a more thorough evaluation and history,” said Dr. McNulty.
However, she noted that screening shouldn’t necessarily be a one-off effort. Research has shown that sequential screening is the most powerful strategy. “Then you can get a baseline of a patient’s emotional and behavioral functioning, and it’s actually the changes in some of these screening measures that might give them most clinical information,” said Dr. McNulty.
In fact, on October 11, 2022, the U.S. Preventive Services Task Force announced a recommendation that all children starting at age 8 should be screened for anxiety disorders. It is already recommended to screen children aged 12 and over for depressive disorders, although these documents are aimed primarily at pediatricians or primary care clinics. The American Academy of Neurology has also recommended routine screening of psychiatric and behavioral disorders among children with epilepsy.
A unique perspective
Once a disorder is identified, neurologists can bring a unique perspective to treatment. The neurologist can use his or her knowledge of the disease state to assess whether symptoms are due to poor adjustment to the neurological condition, a primary psychiatric disorder, or the biological underpinnings of the illness or prescribed medications. “I think their neurologist can sort of help tease that apart, [using] their knowledge of neurologic disorders and pathways and medications in a way that psychologists might not be able to do on their own,” said Dr. McNulty.
She also emphasized that there are effective treatments for psychiatric disorders, including cognitive behavioral therapy and various pharmacotherapy options. Other approaches for treating comorbid neurological and psychiatric disorders may include building adaptive coping skills, psychoeducation, and incorporating changes to the family or school environment.
During the Q&A period, one person commented that there should be more psychiatric training for neurology residents. “We do work with the same brain, so I completely agree with that,” said Dr. McNulty.
She was also asked how to identify psychiatric symptoms in nonverbal patients. “One thing that I pay close attention to when I ask parents about (their child) is changes in their physical (attributes). Oftentimes in anxiety in folks who are not severely impaired, if we’re feeling anxious we might be breathing a little faster, or we might get a little sweaty. So looking for physical manifestations is one thing. And then sometimes I’ll tell the parents, if we’re not quite sure, I’ll say ‘I’m not sure, but this is very common given the disorder that you have. Can we check?’ I’m always very clear that I may not be nailing it, but then when we go after it with targeted treatment and we see it getting better, we can say ‘Aha!’ ”
Dr. McNulty has no relevant financial disclosures.
FROM CNS 2022
Myocarditis after COVID vax rare and mild in teens
New data from Israel provide further evidence that myocarditis is a rare adverse event of vaccination with the Pfizer/BioNTech mRNA COVID-19 vaccine in adolescents – one that predominantly occurs in males and typically after the second dose.
The new data also indicate a “mild and benign” clinical course of myocarditis after vaccination, with “favorable” long-term prognosis based on cardiac imaging findings.
Guy Witberg, MD, MPH, Rabin Medical Center, Petah Tikva, Israel, and colleagues report their latest observations in correspondence in The New England Journal of Medicine, online.
The group previously reported in December 2021 that the incidence of myocarditis in Israel after receipt of the Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine was highest among males between the ages of 16 and 29 (10.7 cases per 100,000).
The vaccine has since been approved for adolescents aged 12-15. Initial evidence for this age group, reported by Dr. Witberg and colleagues in March 2022, suggests a similar low incidence and mild course of myocarditis, although follow-up was limited to 30 days.
In their latest report, with follow-up out to 6 months, Dr. Witberg and colleagues identified nine probable or definite cases of myocarditis among 182,605 Israeli adolescents aged 12-15 who received the Pfizer/BioNTech mRNA vaccine – an incidence of 4.8 cases per 100,000.
Eight cases occurred after the second vaccine dose. All nine cases were mild.
Cardiac and inflammatory markers were elevated in all adolescent patients and electrocardiographic results were abnormal in two-thirds.
Eight patients had a normal ejection fraction, and four had a pericardial effusion. The patients spent 2-4 days hospitalized, and the in-hospital course was uneventful.
Echocardiographic findings were available a median of 10 days after discharge for eight patients. All echocardiograms showed a normal ejection fraction and resolution of pericardial effusion.
Five patients underwent cardiac MRI, including three scans performed at a median of 104 days after discharge. The scans showed “minimal evidence” of myocardial scarring or fibrosis, with evidence of late gadolinium enhancement ranging from 0% to 2%.
At a median of 206 days following discharge, all of the patients were alive, and none had been readmitted to the hospital, Dr. Witberg and colleagues report.
This research had no specific funding. Five authors have received research grants from Pfizer.
A version of this article first appeared on Medscape.com.
New data from Israel provide further evidence that myocarditis is a rare adverse event of vaccination with the Pfizer/BioNTech mRNA COVID-19 vaccine in adolescents – one that predominantly occurs in males and typically after the second dose.
The new data also indicate a “mild and benign” clinical course of myocarditis after vaccination, with “favorable” long-term prognosis based on cardiac imaging findings.
Guy Witberg, MD, MPH, Rabin Medical Center, Petah Tikva, Israel, and colleagues report their latest observations in correspondence in The New England Journal of Medicine, online.
The group previously reported in December 2021 that the incidence of myocarditis in Israel after receipt of the Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine was highest among males between the ages of 16 and 29 (10.7 cases per 100,000).
The vaccine has since been approved for adolescents aged 12-15. Initial evidence for this age group, reported by Dr. Witberg and colleagues in March 2022, suggests a similar low incidence and mild course of myocarditis, although follow-up was limited to 30 days.
In their latest report, with follow-up out to 6 months, Dr. Witberg and colleagues identified nine probable or definite cases of myocarditis among 182,605 Israeli adolescents aged 12-15 who received the Pfizer/BioNTech mRNA vaccine – an incidence of 4.8 cases per 100,000.
Eight cases occurred after the second vaccine dose. All nine cases were mild.
Cardiac and inflammatory markers were elevated in all adolescent patients and electrocardiographic results were abnormal in two-thirds.
Eight patients had a normal ejection fraction, and four had a pericardial effusion. The patients spent 2-4 days hospitalized, and the in-hospital course was uneventful.
Echocardiographic findings were available a median of 10 days after discharge for eight patients. All echocardiograms showed a normal ejection fraction and resolution of pericardial effusion.
Five patients underwent cardiac MRI, including three scans performed at a median of 104 days after discharge. The scans showed “minimal evidence” of myocardial scarring or fibrosis, with evidence of late gadolinium enhancement ranging from 0% to 2%.
At a median of 206 days following discharge, all of the patients were alive, and none had been readmitted to the hospital, Dr. Witberg and colleagues report.
This research had no specific funding. Five authors have received research grants from Pfizer.
A version of this article first appeared on Medscape.com.
New data from Israel provide further evidence that myocarditis is a rare adverse event of vaccination with the Pfizer/BioNTech mRNA COVID-19 vaccine in adolescents – one that predominantly occurs in males and typically after the second dose.
The new data also indicate a “mild and benign” clinical course of myocarditis after vaccination, with “favorable” long-term prognosis based on cardiac imaging findings.
Guy Witberg, MD, MPH, Rabin Medical Center, Petah Tikva, Israel, and colleagues report their latest observations in correspondence in The New England Journal of Medicine, online.
The group previously reported in December 2021 that the incidence of myocarditis in Israel after receipt of the Pfizer/BioNTech BNT162b2 mRNA COVID-19 vaccine was highest among males between the ages of 16 and 29 (10.7 cases per 100,000).
The vaccine has since been approved for adolescents aged 12-15. Initial evidence for this age group, reported by Dr. Witberg and colleagues in March 2022, suggests a similar low incidence and mild course of myocarditis, although follow-up was limited to 30 days.
In their latest report, with follow-up out to 6 months, Dr. Witberg and colleagues identified nine probable or definite cases of myocarditis among 182,605 Israeli adolescents aged 12-15 who received the Pfizer/BioNTech mRNA vaccine – an incidence of 4.8 cases per 100,000.
Eight cases occurred after the second vaccine dose. All nine cases were mild.
Cardiac and inflammatory markers were elevated in all adolescent patients and electrocardiographic results were abnormal in two-thirds.
Eight patients had a normal ejection fraction, and four had a pericardial effusion. The patients spent 2-4 days hospitalized, and the in-hospital course was uneventful.
Echocardiographic findings were available a median of 10 days after discharge for eight patients. All echocardiograms showed a normal ejection fraction and resolution of pericardial effusion.
Five patients underwent cardiac MRI, including three scans performed at a median of 104 days after discharge. The scans showed “minimal evidence” of myocardial scarring or fibrosis, with evidence of late gadolinium enhancement ranging from 0% to 2%.
At a median of 206 days following discharge, all of the patients were alive, and none had been readmitted to the hospital, Dr. Witberg and colleagues report.
This research had no specific funding. Five authors have received research grants from Pfizer.
A version of this article first appeared on Medscape.com.
Immediate skin-to-skin contact after cesarean section improves outcomes for parent, newborn
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
FROM NURSING OPEN