User login
Past, Present, and Future of Pediatric Atopic Dermatitis Management
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
PRACTICE POINTS
- Pediatric atopic dermatitis (AD) therapeutics have rapidly evolved over the last decade and dermatologists should be aware of new tools in their treatment arsenal.
- New topical nonsteroidal agents serve as useful alternatives to topical corticosteroids through mitigating adverse effects from current standard therapy and potentially simplifying topical regimens.
- Monoclonal antibodies and Janus kinase inhibitors are part of an important set of new systemic therapeutics for pediatric AD.
- Long-term data on these new therapeutics is required to better understand their impact on pediatric AD comorbidities and impact on the longitudinal disease course.
Acquired Acrodermatitis Enteropathica in an Infant
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
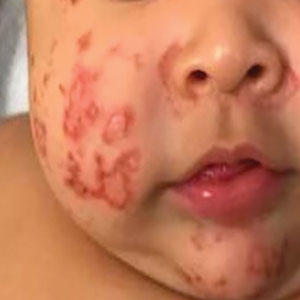
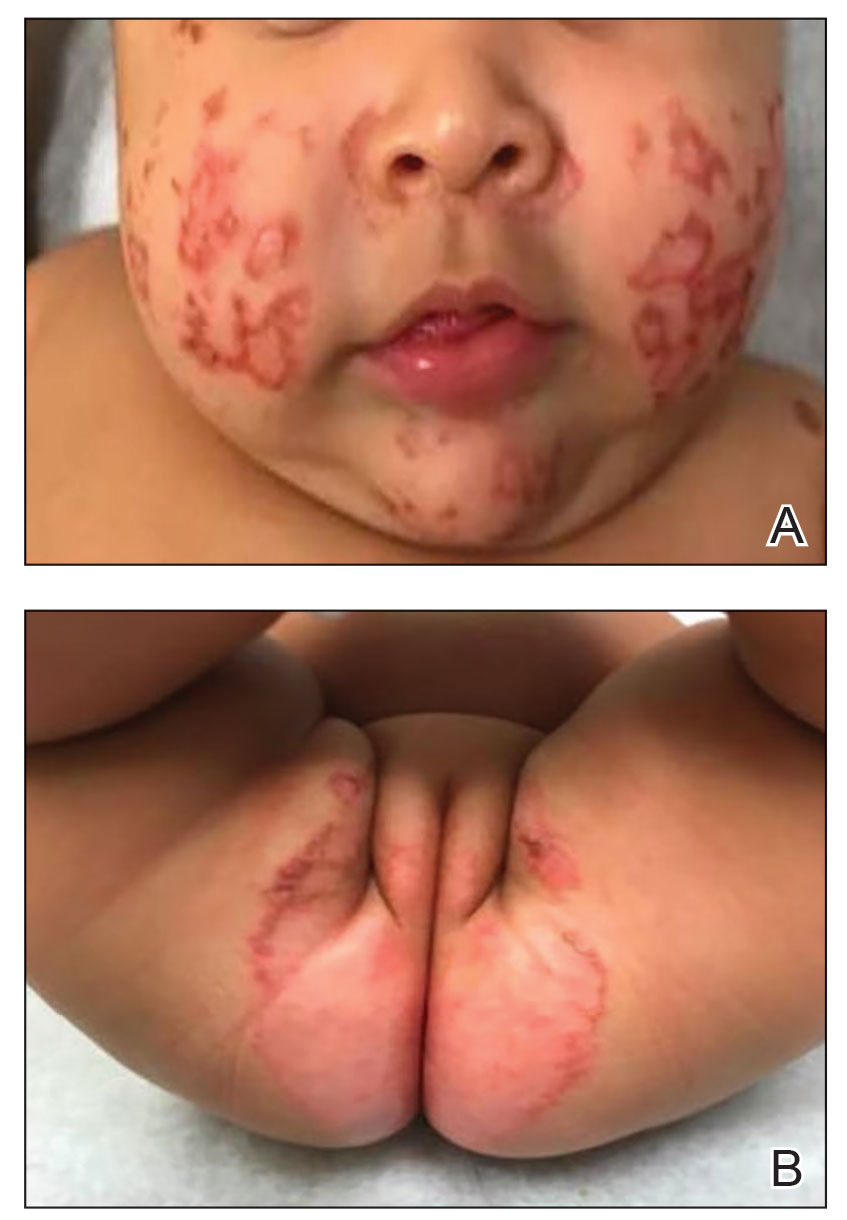
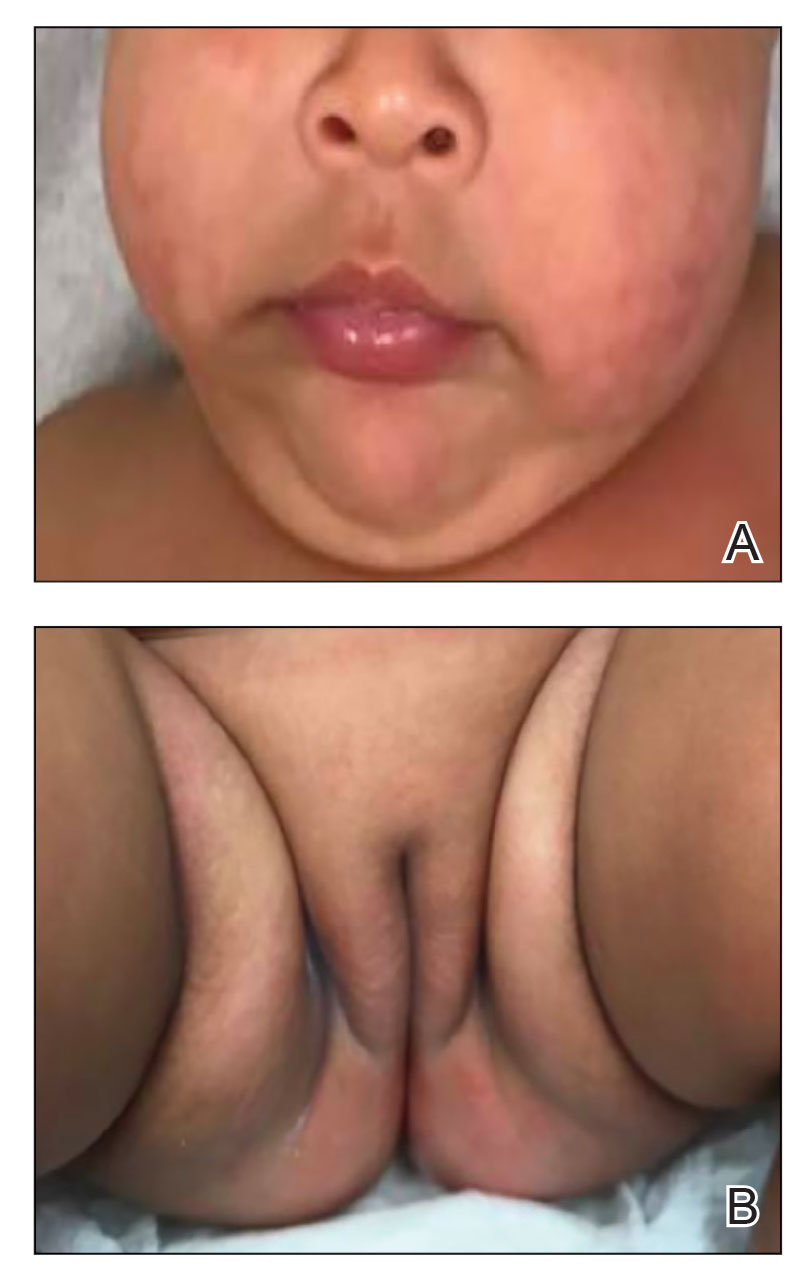
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.
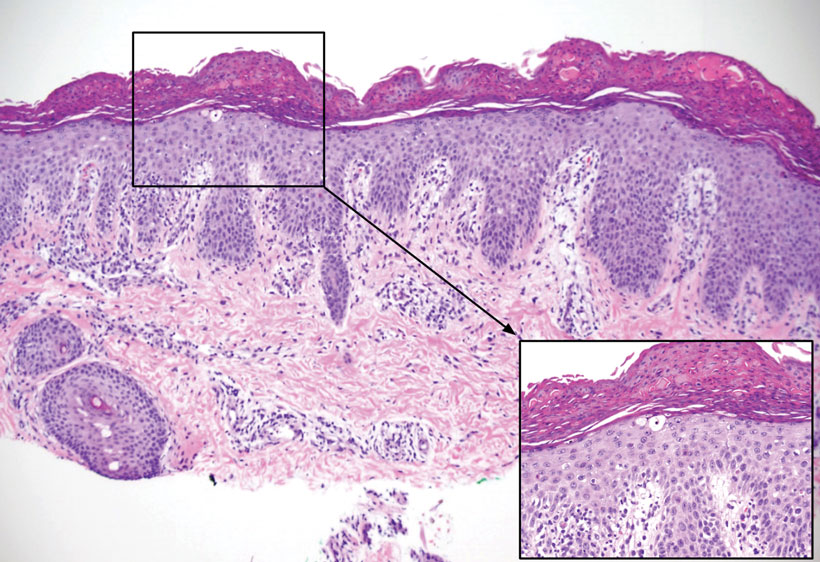
Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.
The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.
Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Practice Points
- Although clinically characterized by the triad of acral and periorificial dermatitis, alopecia, and diarrhea, most cases of acrodermatitis enteropathica (AE) present with only partial features of this syndrome.
- Low levels of zinc-dependent enzymes such as alkaline phosphatase may support the diagnosis of AE.
Update on Tinea Capitis Diagnosis and Treatment
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
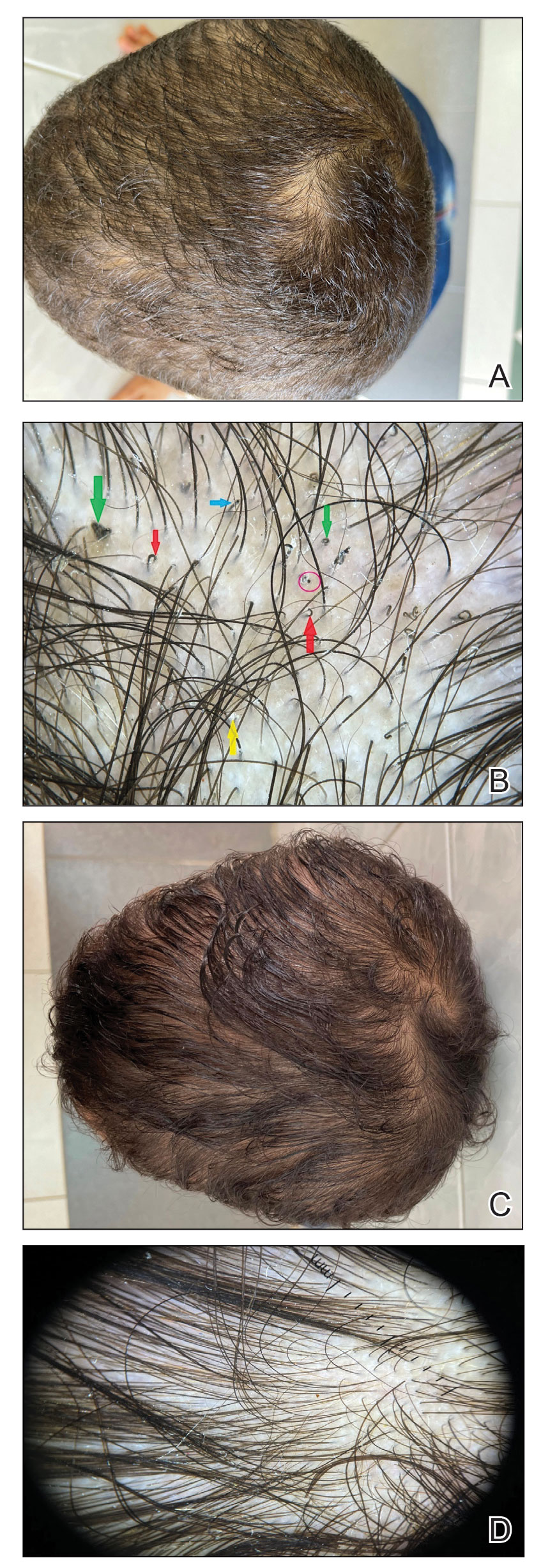
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4
Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
STEP TEENS: Semaglutide ‘gives hope’ to adolescents with obesity
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
Attendees at ObesityWeek® 2022 listened with much excitement to the results of the STEP TEENS phase 3 trial of once-weekly subcutaneous semaglutide 2.4 mg (Wegovy) in adolescents aged 12 up to 18 years old with obesity.
When a session panel member said that clinical trials of weight-loss medications for adolescents with obesity should henceforth stop using placebo controls – implying that comparison with the once-weekly injection semaglutide would be more informative – the audience applauded.
The results were also simultaneously published in the New England Journal of Medicine to coincide with the presentation.
The research “gives hope” to adolescents with obesity, their parents, and their doctors, the trial’s principal investigator, Daniel Weghuber, MD, said in an interview.
“Many of them have been struggling for such a long time – both the parents and the kids themselves,” said Dr. Weghuber, from the department of pediatrics, Paracelsus Medical University, Salzburg, Austria.
“It’s not an issue of lack of willpower,” he stressed. “That’s a major misunderstanding.”
“This drug [semaglutide] seems to enable people who are living with obesity to adhere to the recommendations that they may have been following for years and years but were [still] not able to achieve their goal,” he said. It “enables people to achieve their goals.”
Asked about any potential negative impact on normal growth, Dr. Weghuber pointed out that the average weight of study participants was 107 kg (236 lb). “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight, he said. There was no indication of a problem regarding normal growth or development in the study.
, he summarized.
Senior study author, Silva Arslanian, MD, who holds the Richard L. Day Endowed Chair in Pediatrics at the University of Pittsburgh, agreed. “The results are amazing,” said Dr. Arslanian in a press release issued by the University of Pittsburgh. “For a person who is 5 foot, 5 inches tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
‘Mind-blowing, awesome’ results
The session at ObesityWeek® 2022, the annual meeting of the Obesity Society, was chaired by Aaron S. Kelly, PhD, professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota, Minneapolis.
Dr. Kelly led the SCALE TEENS clinical trial of liraglutide (Saxenda), also a glucagon-like peptide (GLP-1) agonist like semaglutide, for adolescents aged 12 up to 18 years with obesity, which assigned 125 participants to the daily injectable liraglutide group and 126 to the placebo group. SCALE TEENS was presented and published in May 2020, leading to the approval of liraglutide for obesity in this age group, in December 2020.
Dr. Kelly called on two experts who were not involved in the research to offer their comments, starting with Claudia K. Fox, MD, MPH.
“These results are mind-blowing,” said Dr. Fox, who is associate professor of pediatrics and codirector of the center for pediatric obesity medicine at the University of Minnesota.
“We are getting close to bariatric surgery results” in these adolescent patients with obesity, added Dr. Fox, who is an American Board of Obesity Medicine diplomate. To have 40% of patients attain normal weight, “that’s massive” and “life-changing,” she said. And improvement in quality of life is what families care most about. “I am super excited,” she commented.
Next, Dr. Kelly called on Sarah C. Armstrong, MD, director of the Duke Children’s Healthy Lifestyles Program, Duke University, Durham, N.C.
Dr. Armstrong is a member of the executive committee for the American Academy of Pediatrics Section on Obesity and a coauthor of the upcoming clinical practice guidelines that are being published.
Looking at more than 16,000 abstracts at the meeting shows that “watchful waiting is not effective,” Dr. Armstrong said.
200 teens with obesity, only 1 with overweight
Obesity affects almost one in five children and adolescents worldwide. The chronic disease is linked with decreased life expectancy and higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Teenagers with obesity are also more likely to have depression, anxiety, poor self-esteem, and other psychological issues.
STEP TEENS enrolled 201 adolescents aged 12 up to 18 years with obesity (body mass index [BMI] ≥ 95th percentile) or overweight (BMI ≥ 85th percentile) plus at least one weight-related comorbidity.
Only one recruited patient fit the latter category; the rest had obesity.
Most patients (62%) were female. They had a mean age of 15.4 years, a mean BMI of 37 kg/m2, and a mean waist circumference of 110 cm (43 inches).
Patients were randomized 2:1 to receive a once-weekly 2.4-mg subcutaneous injection of semaglutide or placebo for 68 weeks, plus lifestyle intervention.
Dr. Weghuber noted that 89.6% of patients in the semaglutide group completed treatment.
The primary endpoint, mean change in BMI from baseline to week 68, was −16.1% with semaglutide and +0.6% with placebo (estimated difference, −16.7 percentage points; P < .001).
A second confirmatory endpoint, at least 5% weight loss at week 68, was met by 73% of patients in the semaglutide group versus 18% of patients in the placebo group (P < .001).
Reductions in body weight and improvements in waist circumference, A1c, lipids (except HDL cholesterol), and the liver enzyme alanine aminotransferase were greater with semaglutide than placebo.
The Impact of Weight on Quality of Life – Kids (IWQOL-Kids) questionnaire total score as well as scores for body esteem, family relation, physical comfort, and social life were better in the semaglutide group.
However, the incidence of gastrointestinal adverse events was greater with semaglutide than placebo (62% versus 42%).
Five participants (4%) in the semaglutide group and none in the placebo group developed gallstones (cholelithiasis).
Serious adverse events were reported in 11% of patients in the semaglutide group and 9% of patients in the placebo group.
‘Big change’ coming in guidelines for obesity in teens
Commenting on the upcoming new recommendations for adolescents, Dr. Armstrong noted “there’s going to be a strong recommendation” for therapy in the new guidelines for pediatric obesity. “That’s a big change,” she said.
In the lively question-and-answer session that followed, a clinician wanted to know what explained the very high rate of study completion during the COVID-19 pandemic (when STEP TEENS was conducted). “What can we learn?” he asked.
“The bottom line is the relationship” and “close communication” between study investigators and patients, Dr. Weghuber replied.
“The fast track is likely to lead to approval in adolescents,” another member of the audience noted. He wanted to know if the company is planning a trial of semaglutide in younger children.
They are, Dr. Weghuber replied, and one with liraglutide is already underway.
The SCALE KIDS clinical trial of liraglutide is randomizing 78 participants aged 6 up to 12 years for 56 weeks of treatment and 26 weeks of follow-up, with an estimated primary completion date of July 7, 2023.
The last words went to Dr. Fox. The current results “are indeed very awesome,” she said, yet “thousands of providers are hesitant” to prescribe medications for adolescents with obesity.
The trial was funded by Novo Nordisk. Dr. Weghuber has reported being a consultant for Novo Nordisk and member of the Global Pediatric Obesity Expert Panel for the company. Disclosures for the other authors are listed with the article. Dr. Kelly has reported receiving donated drugs from AstraZeneca and travel support from Novo Nordisk and serving as an unpaid consultant for Novo Nordisk, Orexigen Therapeutics, VIVUS, and WW (formerly Weight Watchers).
A version of this article first appeared on Medscape.com.
FROM OBESITYWEEK® 2022
RSV vaccine given during pregnancy protects newborns: Pfizer
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
Shortage reported of antibiotic commonly used for children
The liquid form of the antibiotic amoxicillin often used to treat ear infections and strep throat in children is in short supply, just as Americans head into the season when they use the bacteria-fighting drug the most.
The FDA officially listed the shortage Oct. 28, but pharmacists, hospitals, and a supply tracking database sounded alarms earlier this month.
“The scary part is, we’re coming into the time of the year where you have the greatest need,” independent pharmacy owner Hugh Chancy, PharmD, of Georgia, told NBC News.
Thus far, reports indicate the impact of the shortages is not widespread but does affect some pharmacies, and at least one hospital has published an algorithm for offering treatment alternatives.
CVS told Bloomberg News that some stores are experiencing shortages of certain doses of amoxicillin, but a Walmart spokesperson said its diverse supply chain meant none of its pharmacies were affected.
“Hypothetically, if amoxicillin doesn’t come into stock for some time, then we’re potentially having to use less effective antibiotics with more side effects,” said Ohio pediatrician Sean Gallagher, MD, according to Bloomberg.
The shortage impacts three of the four largest amoxicillin manufacturers worldwide, according to the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota. The FDA listed the reason for the shortage as “demand increase for drug,” except in the case of manufacturer Sandoz, for which the reason listed read “information pending.”
A company spokesperson told Bloomberg the reasons were complex.
“The combination in rapid succession of the pandemic impact and consequent demand swings, manufacturing capacity constraints, scarcity of raw materials, and the current energy crisis means we face a uniquely difficult situation in the short term,” Sandoz spokesperson Leslie Pott told Bloomberg.
According to Bloomberg, other major manufacturers are still delivering the product, but limiting new orders.
The American Society of Health-System Pharmacists issued an alert for the shortage last week via its real time drug shortage database.
“Amoxicillin comes in many forms – including capsules, powders and chewable tablets – but the most common type children take is the liquid form, which makes up at least 19 products that are part of the” shortage, Becker’s Hospital Review summarized of the database reports.
The pediatric health system Children’s Minnesota told CIDRAP that supplies are low and that alternatives are being prescribed “when appropriate.”
“As a final step, we temporarily discontinued our standard procedure of dispensing the entire bottle of amoxicillin (which comes in multiple sizes),” a spokesperson told CIDRAP. “We are instead mixing and pouring the exact amount for each course of therapy, to eliminate waste.”
The Minnesota pediatric clinic and others are particularly on alert because of the surge nationwide of a respiratory virus that particularly impacts children known as RSV.
“We have certainly observed an increase in recent use most likely correlating with the surge in RSV and other respiratory viruses with concern for superimposed bacterial infection in our critically ill and hospitalized patient population,” Laura Bio, PharmD, a clinical pharmacy specialist at Stanford Medicine Children’s Health told CIDRAP.
A version of this article first appeared on WebMD.com.
The liquid form of the antibiotic amoxicillin often used to treat ear infections and strep throat in children is in short supply, just as Americans head into the season when they use the bacteria-fighting drug the most.
The FDA officially listed the shortage Oct. 28, but pharmacists, hospitals, and a supply tracking database sounded alarms earlier this month.
“The scary part is, we’re coming into the time of the year where you have the greatest need,” independent pharmacy owner Hugh Chancy, PharmD, of Georgia, told NBC News.
Thus far, reports indicate the impact of the shortages is not widespread but does affect some pharmacies, and at least one hospital has published an algorithm for offering treatment alternatives.
CVS told Bloomberg News that some stores are experiencing shortages of certain doses of amoxicillin, but a Walmart spokesperson said its diverse supply chain meant none of its pharmacies were affected.
“Hypothetically, if amoxicillin doesn’t come into stock for some time, then we’re potentially having to use less effective antibiotics with more side effects,” said Ohio pediatrician Sean Gallagher, MD, according to Bloomberg.
The shortage impacts three of the four largest amoxicillin manufacturers worldwide, according to the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota. The FDA listed the reason for the shortage as “demand increase for drug,” except in the case of manufacturer Sandoz, for which the reason listed read “information pending.”
A company spokesperson told Bloomberg the reasons were complex.
“The combination in rapid succession of the pandemic impact and consequent demand swings, manufacturing capacity constraints, scarcity of raw materials, and the current energy crisis means we face a uniquely difficult situation in the short term,” Sandoz spokesperson Leslie Pott told Bloomberg.
According to Bloomberg, other major manufacturers are still delivering the product, but limiting new orders.
The American Society of Health-System Pharmacists issued an alert for the shortage last week via its real time drug shortage database.
“Amoxicillin comes in many forms – including capsules, powders and chewable tablets – but the most common type children take is the liquid form, which makes up at least 19 products that are part of the” shortage, Becker’s Hospital Review summarized of the database reports.
The pediatric health system Children’s Minnesota told CIDRAP that supplies are low and that alternatives are being prescribed “when appropriate.”
“As a final step, we temporarily discontinued our standard procedure of dispensing the entire bottle of amoxicillin (which comes in multiple sizes),” a spokesperson told CIDRAP. “We are instead mixing and pouring the exact amount for each course of therapy, to eliminate waste.”
The Minnesota pediatric clinic and others are particularly on alert because of the surge nationwide of a respiratory virus that particularly impacts children known as RSV.
“We have certainly observed an increase in recent use most likely correlating with the surge in RSV and other respiratory viruses with concern for superimposed bacterial infection in our critically ill and hospitalized patient population,” Laura Bio, PharmD, a clinical pharmacy specialist at Stanford Medicine Children’s Health told CIDRAP.
A version of this article first appeared on WebMD.com.
The liquid form of the antibiotic amoxicillin often used to treat ear infections and strep throat in children is in short supply, just as Americans head into the season when they use the bacteria-fighting drug the most.
The FDA officially listed the shortage Oct. 28, but pharmacists, hospitals, and a supply tracking database sounded alarms earlier this month.
“The scary part is, we’re coming into the time of the year where you have the greatest need,” independent pharmacy owner Hugh Chancy, PharmD, of Georgia, told NBC News.
Thus far, reports indicate the impact of the shortages is not widespread but does affect some pharmacies, and at least one hospital has published an algorithm for offering treatment alternatives.
CVS told Bloomberg News that some stores are experiencing shortages of certain doses of amoxicillin, but a Walmart spokesperson said its diverse supply chain meant none of its pharmacies were affected.
“Hypothetically, if amoxicillin doesn’t come into stock for some time, then we’re potentially having to use less effective antibiotics with more side effects,” said Ohio pediatrician Sean Gallagher, MD, according to Bloomberg.
The shortage impacts three of the four largest amoxicillin manufacturers worldwide, according to the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota. The FDA listed the reason for the shortage as “demand increase for drug,” except in the case of manufacturer Sandoz, for which the reason listed read “information pending.”
A company spokesperson told Bloomberg the reasons were complex.
“The combination in rapid succession of the pandemic impact and consequent demand swings, manufacturing capacity constraints, scarcity of raw materials, and the current energy crisis means we face a uniquely difficult situation in the short term,” Sandoz spokesperson Leslie Pott told Bloomberg.
According to Bloomberg, other major manufacturers are still delivering the product, but limiting new orders.
The American Society of Health-System Pharmacists issued an alert for the shortage last week via its real time drug shortage database.
“Amoxicillin comes in many forms – including capsules, powders and chewable tablets – but the most common type children take is the liquid form, which makes up at least 19 products that are part of the” shortage, Becker’s Hospital Review summarized of the database reports.
The pediatric health system Children’s Minnesota told CIDRAP that supplies are low and that alternatives are being prescribed “when appropriate.”
“As a final step, we temporarily discontinued our standard procedure of dispensing the entire bottle of amoxicillin (which comes in multiple sizes),” a spokesperson told CIDRAP. “We are instead mixing and pouring the exact amount for each course of therapy, to eliminate waste.”
The Minnesota pediatric clinic and others are particularly on alert because of the surge nationwide of a respiratory virus that particularly impacts children known as RSV.
“We have certainly observed an increase in recent use most likely correlating with the surge in RSV and other respiratory viruses with concern for superimposed bacterial infection in our critically ill and hospitalized patient population,” Laura Bio, PharmD, a clinical pharmacy specialist at Stanford Medicine Children’s Health told CIDRAP.
A version of this article first appeared on WebMD.com.
Machine learning identifies childhood characteristics that predict bipolar disorder
This is the first quantitative approach to predict bipolar disorder, offering sensitivity and specificity of 75% and 76%, respectively, reported lead author Mai Uchida, MD, director of the pediatric depression program at Massachusetts General Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, and colleagues. With further development, the model could be used to identify at-risk children via electronic medical records, enabling earlier monitoring and intervention.
“Although longitudinal studies have found the prognosis of early-onset mood disorders to be unfavorable, research has also shown there are effective treatments and therapies that could significantly alleviate the patients’ and their families’ struggles from the diagnoses,” the investigators wrote in the Journal of Psychiatric Research. “Thus, early identification of the risks and interventions for early symptoms of pediatric mood disorders is crucial.”
To this end, Dr. Uchida and colleagues teamed up with the Gabrieli Lab at MIT, who have published extensively in the realm of neurodevelopment. They sourced data from 492 children, 6-18 years at baseline, who were involved in two longitudinal case-control family studies focused on ADHD. Inputs included psychometric scales, structured diagnostic interviews, social and cognitive functioning assessments, and sociodemographic data.
At 10-year follow-up, 10% of these children had developed bipolar disorder, a notably higher rate than the 3%-4% prevalence in the general population.
“This is a population that’s overrepresented,” Dr. Uchida said in an interview.
She offered two primary reasons for this: First, the families involved in the study were probably willing to be followed for 10 years because they had ongoing concerns about their child’s mental health. Second, the studies enrolled children diagnosed with ADHD, a condition associated with increased risk of bipolar disorder.
Using machine learning algorithms that processed the baseline data while accounting for the skewed distribution, the investigators were able to predict which of the children in the population would go on to develop bipolar disorder. The final model offered a sensitivity of 75%, a specificity of 76%, and an area under the receiver operating characteristic curve of 75%.
“To the best of our knowledge, this represents the first study using machine-learning algorithms for this purpose in pediatric psychiatry,” the investigators wrote.
Integrating models into electronic medical records
In the future, this model, or one like it, could be incorporated into software that automatically analyzes electronic medical records and notifies physicians about high-risk patients, Dr. Uchida predicted.
“Not all patients would connect to intervention,” she said. “Maybe it just means that you invite them in for a visit, or you observe them a little bit more carefully. I think that’s where we are hoping that machine learning and medical practice will go.”
When asked about the potential bias posed by psychiatric evaluation, compared with something like blood work results, Dr. Uchida suggested that this subjectivity can be overcome.
“I’m not entirely bothered by that,” she said, offering a list of objective data points that could be harvested from records, such as number of referrals, medications, and hospitalizations. Narrative text in medical records could also be analyzed, she said, potentially detecting key words that are more often associated with high-risk patients.
“Risk prediction is never going to be 100% accurate,” Dr. Uchida said. “But I do think that there will be things [in electronic medical records] that could guide how worried we should be, or how quickly we should intervene.”
Opening doors to personalized care
Martin Gignac, MD, chief of psychiatry at Montreal Children’s Hospital and associate professor at McGill University, Montreal, said the present study offers further support for the existence of pediatric-onset bipolar disorder, which “remains controversial” despite “solid evidence.”
“I’m impressed that we have 10-year-long longitudinal follow-up studies that corroborate the importance of this disorder, and show strong predictors of who is at risk,” Dr. Gignac said in an interview. “Clinicians treating a pediatric population should be aware that some of those children with mental health problems might have severe mental health problems, and you have to have the appropriate tools to screen them.”
Advanced tools like the one developed by Dr. Uchida and colleagues should lead to more personalized care, he said.
“We’re going to be able to define what your individual risk is, and maybe most importantly, what you can do to prevent the development of certain disorders,” Dr. Gignac said. “Are there any risks that are dynamic in nature, and that we can act upon? Exposure to stress, for example.”
While more work is needed to bring machine learning into daily psychiatric practice, Dr. Gignac concluded on an optimistic note.
“These instruments should translate from research into clinical practice in order to make difference for the patients we care for,” he said. “This is the type of hope that I hold – that it’s going to be applicable in clinical practice, hopefully, in the near future.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Gignac disclosed no relevant competing interests.
This is the first quantitative approach to predict bipolar disorder, offering sensitivity and specificity of 75% and 76%, respectively, reported lead author Mai Uchida, MD, director of the pediatric depression program at Massachusetts General Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, and colleagues. With further development, the model could be used to identify at-risk children via electronic medical records, enabling earlier monitoring and intervention.
“Although longitudinal studies have found the prognosis of early-onset mood disorders to be unfavorable, research has also shown there are effective treatments and therapies that could significantly alleviate the patients’ and their families’ struggles from the diagnoses,” the investigators wrote in the Journal of Psychiatric Research. “Thus, early identification of the risks and interventions for early symptoms of pediatric mood disorders is crucial.”
To this end, Dr. Uchida and colleagues teamed up with the Gabrieli Lab at MIT, who have published extensively in the realm of neurodevelopment. They sourced data from 492 children, 6-18 years at baseline, who were involved in two longitudinal case-control family studies focused on ADHD. Inputs included psychometric scales, structured diagnostic interviews, social and cognitive functioning assessments, and sociodemographic data.
At 10-year follow-up, 10% of these children had developed bipolar disorder, a notably higher rate than the 3%-4% prevalence in the general population.
“This is a population that’s overrepresented,” Dr. Uchida said in an interview.
She offered two primary reasons for this: First, the families involved in the study were probably willing to be followed for 10 years because they had ongoing concerns about their child’s mental health. Second, the studies enrolled children diagnosed with ADHD, a condition associated with increased risk of bipolar disorder.
Using machine learning algorithms that processed the baseline data while accounting for the skewed distribution, the investigators were able to predict which of the children in the population would go on to develop bipolar disorder. The final model offered a sensitivity of 75%, a specificity of 76%, and an area under the receiver operating characteristic curve of 75%.
“To the best of our knowledge, this represents the first study using machine-learning algorithms for this purpose in pediatric psychiatry,” the investigators wrote.
Integrating models into electronic medical records
In the future, this model, or one like it, could be incorporated into software that automatically analyzes electronic medical records and notifies physicians about high-risk patients, Dr. Uchida predicted.
“Not all patients would connect to intervention,” she said. “Maybe it just means that you invite them in for a visit, or you observe them a little bit more carefully. I think that’s where we are hoping that machine learning and medical practice will go.”
When asked about the potential bias posed by psychiatric evaluation, compared with something like blood work results, Dr. Uchida suggested that this subjectivity can be overcome.
“I’m not entirely bothered by that,” she said, offering a list of objective data points that could be harvested from records, such as number of referrals, medications, and hospitalizations. Narrative text in medical records could also be analyzed, she said, potentially detecting key words that are more often associated with high-risk patients.
“Risk prediction is never going to be 100% accurate,” Dr. Uchida said. “But I do think that there will be things [in electronic medical records] that could guide how worried we should be, or how quickly we should intervene.”
Opening doors to personalized care
Martin Gignac, MD, chief of psychiatry at Montreal Children’s Hospital and associate professor at McGill University, Montreal, said the present study offers further support for the existence of pediatric-onset bipolar disorder, which “remains controversial” despite “solid evidence.”
“I’m impressed that we have 10-year-long longitudinal follow-up studies that corroborate the importance of this disorder, and show strong predictors of who is at risk,” Dr. Gignac said in an interview. “Clinicians treating a pediatric population should be aware that some of those children with mental health problems might have severe mental health problems, and you have to have the appropriate tools to screen them.”
Advanced tools like the one developed by Dr. Uchida and colleagues should lead to more personalized care, he said.
“We’re going to be able to define what your individual risk is, and maybe most importantly, what you can do to prevent the development of certain disorders,” Dr. Gignac said. “Are there any risks that are dynamic in nature, and that we can act upon? Exposure to stress, for example.”
While more work is needed to bring machine learning into daily psychiatric practice, Dr. Gignac concluded on an optimistic note.
“These instruments should translate from research into clinical practice in order to make difference for the patients we care for,” he said. “This is the type of hope that I hold – that it’s going to be applicable in clinical practice, hopefully, in the near future.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Gignac disclosed no relevant competing interests.
This is the first quantitative approach to predict bipolar disorder, offering sensitivity and specificity of 75% and 76%, respectively, reported lead author Mai Uchida, MD, director of the pediatric depression program at Massachusetts General Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, and colleagues. With further development, the model could be used to identify at-risk children via electronic medical records, enabling earlier monitoring and intervention.
“Although longitudinal studies have found the prognosis of early-onset mood disorders to be unfavorable, research has also shown there are effective treatments and therapies that could significantly alleviate the patients’ and their families’ struggles from the diagnoses,” the investigators wrote in the Journal of Psychiatric Research. “Thus, early identification of the risks and interventions for early symptoms of pediatric mood disorders is crucial.”
To this end, Dr. Uchida and colleagues teamed up with the Gabrieli Lab at MIT, who have published extensively in the realm of neurodevelopment. They sourced data from 492 children, 6-18 years at baseline, who were involved in two longitudinal case-control family studies focused on ADHD. Inputs included psychometric scales, structured diagnostic interviews, social and cognitive functioning assessments, and sociodemographic data.
At 10-year follow-up, 10% of these children had developed bipolar disorder, a notably higher rate than the 3%-4% prevalence in the general population.
“This is a population that’s overrepresented,” Dr. Uchida said in an interview.
She offered two primary reasons for this: First, the families involved in the study were probably willing to be followed for 10 years because they had ongoing concerns about their child’s mental health. Second, the studies enrolled children diagnosed with ADHD, a condition associated with increased risk of bipolar disorder.
Using machine learning algorithms that processed the baseline data while accounting for the skewed distribution, the investigators were able to predict which of the children in the population would go on to develop bipolar disorder. The final model offered a sensitivity of 75%, a specificity of 76%, and an area under the receiver operating characteristic curve of 75%.
“To the best of our knowledge, this represents the first study using machine-learning algorithms for this purpose in pediatric psychiatry,” the investigators wrote.
Integrating models into electronic medical records
In the future, this model, or one like it, could be incorporated into software that automatically analyzes electronic medical records and notifies physicians about high-risk patients, Dr. Uchida predicted.
“Not all patients would connect to intervention,” she said. “Maybe it just means that you invite them in for a visit, or you observe them a little bit more carefully. I think that’s where we are hoping that machine learning and medical practice will go.”
When asked about the potential bias posed by psychiatric evaluation, compared with something like blood work results, Dr. Uchida suggested that this subjectivity can be overcome.
“I’m not entirely bothered by that,” she said, offering a list of objective data points that could be harvested from records, such as number of referrals, medications, and hospitalizations. Narrative text in medical records could also be analyzed, she said, potentially detecting key words that are more often associated with high-risk patients.
“Risk prediction is never going to be 100% accurate,” Dr. Uchida said. “But I do think that there will be things [in electronic medical records] that could guide how worried we should be, or how quickly we should intervene.”
Opening doors to personalized care
Martin Gignac, MD, chief of psychiatry at Montreal Children’s Hospital and associate professor at McGill University, Montreal, said the present study offers further support for the existence of pediatric-onset bipolar disorder, which “remains controversial” despite “solid evidence.”
“I’m impressed that we have 10-year-long longitudinal follow-up studies that corroborate the importance of this disorder, and show strong predictors of who is at risk,” Dr. Gignac said in an interview. “Clinicians treating a pediatric population should be aware that some of those children with mental health problems might have severe mental health problems, and you have to have the appropriate tools to screen them.”
Advanced tools like the one developed by Dr. Uchida and colleagues should lead to more personalized care, he said.
“We’re going to be able to define what your individual risk is, and maybe most importantly, what you can do to prevent the development of certain disorders,” Dr. Gignac said. “Are there any risks that are dynamic in nature, and that we can act upon? Exposure to stress, for example.”
While more work is needed to bring machine learning into daily psychiatric practice, Dr. Gignac concluded on an optimistic note.
“These instruments should translate from research into clinical practice in order to make difference for the patients we care for,” he said. “This is the type of hope that I hold – that it’s going to be applicable in clinical practice, hopefully, in the near future.”
The investigators disclosed relationships with InCarda, Baylis Medical, Johnson & Johnson, and others. Dr. Gignac disclosed no relevant competing interests.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Mid-October flulike illness cases higher than past 5 years
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
New statement guides the diagnosis of pediatric anxiety
The Canadian Paediatric Society (CPS) has issued a position statement on the diagnosis of anxiety disorders in children and youth. The organization aims to “offer evidence-informed guidance to support pediatric health care providers making decisions around the care of children and adolescents with these conditions.”
“It’s been a long time coming,” lead author Benjamin Klein, MD, assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont., told this news organization. The target audience for the documents includes community pediatricians, subspecialists, family doctors, and nurse practitioners. “There was a great demand from that audience for a position statement, for guidance, obviously in the backdrop of rising child and adolescent mental health incidence over the years and of course COVID,” said Dr. Klein.
The statement was published on the CPS website.
‘A comprehensive approach’
Although many other guidelines on this topic are available, it was important to have a Canadian document, said Dr. Klein. “Obviously, there’s going to be a great deal of overlap with European or American guidelines, but it’s just kind of assumed that people want specifically Canadian content. ... Physicians want to know that they’re practicing within a standard of care in Canada.” Dr. Klein is medical director of the Lansdowne Children’s Centre, Brantford, Ont., which provides help for children with communication, developmental, and physical special needs across Ontario.
Anxiety disorders are the most common mental disorders among children and adolescents in Canada, according to the position statement. The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) groups these disorders into separation anxiety disorder, selective mutism, specific phobia, social anxiety disorder (social phobia), panic disorder, agoraphobia, and generalized anxiety disorder.
Distinguishing normal, age-appropriate anxiety from anxiety disorder, while also recognizing other comorbidities, is complicated, said Dr. Klein. “Anxiety is one possible diagnosis or feature, and children with mental health and developmental problems often present with a number of problems. Anxiety may be one of them, but if it’s one of them, it may not be the main driver. So, a comprehensive approach is needed ... combining the medical model with biopsychosocial thinking to give a better picture of anxiety in the context of anything else that may be contributing to a presentation.”
The statement outlines recommendations for anxiety assessment, starting with a screening questionnaire such as the Screen for Child Anxiety Related Disorders (SCARED), which is completed by parents and children, to assess symptom severity. Standardized measures for medical, mental health, and developmental histories are available on the CPS website.
The document next recommends an interview about presenting concerns (such as sleep problems or school difficulties), inciting events, and parent-child interactions. The process includes confidential, nonjudgmental interviews with adolescents using a history-taking tool such as HEEADSSS (Home, Education/Employment, Eating, Activities, Drugs, Sexuality, Suicide/Mental Health, and Safety).
“The diagnosis and treatment of anxiety disorders kind of sounds simple if you just read about it as an isolated thing, but the reality is ... there’s no MRI. It’s detective work,” said Dr. Klein. Clinicians must distinguish between normal anxiety, situational anxiety, and specific anxiety disorder, he added. He usually allows 90 minutes for an anxiety assessment, partly to gain the patient’s trust. “These are sensitive issues. It’s common that people don’t trust a diagnosis if you haven’t spent enough time with them. That relational care piece just needs to be there, or people aren’t going to buy in.”
The CPS position statement was reviewed and endorsed by the Canadian Academy of Child and Adolescent Psychiatry.
Methodology unclear
Joanna Henderson, MD, professor of psychiatry at the University of Toronto and director of the Margaret and Wallace McCain Centre for Child, Youth, and Family Mental Health at the Centre for Addiction and Mental Health, Toronto, said that the guidelines have been released at an important time. “Conversations about mental health have become more common, and many children, youth, and families are reaching out for support. It is essential that health care professionals be equipped with accessible information about practices to provide appropriate care. These guidelines support that vision.”
It would be helpful to know more about the methods used to arrive at the recommendations, however, said Dr. Henderson. “It is critical that health care providers be guided by evidence-based guidelines that adhere to criteria for establishing high-quality guidelines. Because the authors did not provide information about their methods, I am not able to provide a comment about the quality of their guidelines. There are established approaches for evaluating quality, and I would encourage the authors to publish as a supplement to this article their methods, including in reference to the Appraisal of Guidelines for Research and Evaluation (AGREE II) checklist.”
In the absence of readily available information about methods, she said, “clinicians are encouraged to use guidelines from sources that provide information about the guideline development process and include quality appraisal,” such as the UK National Institute for Health and Care Excellence, which is “generally recognized as a reputable source for high-quality practice guidelines.”
Responding to this concern, Dr. Klein said, “There is no specific evidence base for diagnosis. That robust science doesn’t exist. No one has done randomized controlled trials of different methods of diagnosing kids with anxiety. We looked at other position statements, we looked at textbooks, and obviously we drew from our own clinical experience, so it comes from clinical judgment and expert opinion.”
Dr. Henderson also noted that in the future “it will be important to contextualize the recommendations by highlighting the importance of cultural competence in conducting assessments and providing treatment.” Moreover, current evidence can be expanded through the incorporation of diverse cultural and racial perspectives, experiences, and data, she added.
Health service providers should reflect on their own potential biases, which can influence clinician-patient interactions, Dr. Henderson continued. It also is important to consider biases in the evidence, which influence practice. Clinicians should also consider how their recommendations fit with patients’ “cultural and race-based experiences, beliefs, and practices.”
No source of funding for the position statement was reported. Dr. Klein and Dr. Henderson had disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Canadian Paediatric Society (CPS) has issued a position statement on the diagnosis of anxiety disorders in children and youth. The organization aims to “offer evidence-informed guidance to support pediatric health care providers making decisions around the care of children and adolescents with these conditions.”
“It’s been a long time coming,” lead author Benjamin Klein, MD, assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont., told this news organization. The target audience for the documents includes community pediatricians, subspecialists, family doctors, and nurse practitioners. “There was a great demand from that audience for a position statement, for guidance, obviously in the backdrop of rising child and adolescent mental health incidence over the years and of course COVID,” said Dr. Klein.
The statement was published on the CPS website.
‘A comprehensive approach’
Although many other guidelines on this topic are available, it was important to have a Canadian document, said Dr. Klein. “Obviously, there’s going to be a great deal of overlap with European or American guidelines, but it’s just kind of assumed that people want specifically Canadian content. ... Physicians want to know that they’re practicing within a standard of care in Canada.” Dr. Klein is medical director of the Lansdowne Children’s Centre, Brantford, Ont., which provides help for children with communication, developmental, and physical special needs across Ontario.
Anxiety disorders are the most common mental disorders among children and adolescents in Canada, according to the position statement. The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) groups these disorders into separation anxiety disorder, selective mutism, specific phobia, social anxiety disorder (social phobia), panic disorder, agoraphobia, and generalized anxiety disorder.
Distinguishing normal, age-appropriate anxiety from anxiety disorder, while also recognizing other comorbidities, is complicated, said Dr. Klein. “Anxiety is one possible diagnosis or feature, and children with mental health and developmental problems often present with a number of problems. Anxiety may be one of them, but if it’s one of them, it may not be the main driver. So, a comprehensive approach is needed ... combining the medical model with biopsychosocial thinking to give a better picture of anxiety in the context of anything else that may be contributing to a presentation.”
The statement outlines recommendations for anxiety assessment, starting with a screening questionnaire such as the Screen for Child Anxiety Related Disorders (SCARED), which is completed by parents and children, to assess symptom severity. Standardized measures for medical, mental health, and developmental histories are available on the CPS website.
The document next recommends an interview about presenting concerns (such as sleep problems or school difficulties), inciting events, and parent-child interactions. The process includes confidential, nonjudgmental interviews with adolescents using a history-taking tool such as HEEADSSS (Home, Education/Employment, Eating, Activities, Drugs, Sexuality, Suicide/Mental Health, and Safety).
“The diagnosis and treatment of anxiety disorders kind of sounds simple if you just read about it as an isolated thing, but the reality is ... there’s no MRI. It’s detective work,” said Dr. Klein. Clinicians must distinguish between normal anxiety, situational anxiety, and specific anxiety disorder, he added. He usually allows 90 minutes for an anxiety assessment, partly to gain the patient’s trust. “These are sensitive issues. It’s common that people don’t trust a diagnosis if you haven’t spent enough time with them. That relational care piece just needs to be there, or people aren’t going to buy in.”
The CPS position statement was reviewed and endorsed by the Canadian Academy of Child and Adolescent Psychiatry.
Methodology unclear
Joanna Henderson, MD, professor of psychiatry at the University of Toronto and director of the Margaret and Wallace McCain Centre for Child, Youth, and Family Mental Health at the Centre for Addiction and Mental Health, Toronto, said that the guidelines have been released at an important time. “Conversations about mental health have become more common, and many children, youth, and families are reaching out for support. It is essential that health care professionals be equipped with accessible information about practices to provide appropriate care. These guidelines support that vision.”
It would be helpful to know more about the methods used to arrive at the recommendations, however, said Dr. Henderson. “It is critical that health care providers be guided by evidence-based guidelines that adhere to criteria for establishing high-quality guidelines. Because the authors did not provide information about their methods, I am not able to provide a comment about the quality of their guidelines. There are established approaches for evaluating quality, and I would encourage the authors to publish as a supplement to this article their methods, including in reference to the Appraisal of Guidelines for Research and Evaluation (AGREE II) checklist.”
In the absence of readily available information about methods, she said, “clinicians are encouraged to use guidelines from sources that provide information about the guideline development process and include quality appraisal,” such as the UK National Institute for Health and Care Excellence, which is “generally recognized as a reputable source for high-quality practice guidelines.”
Responding to this concern, Dr. Klein said, “There is no specific evidence base for diagnosis. That robust science doesn’t exist. No one has done randomized controlled trials of different methods of diagnosing kids with anxiety. We looked at other position statements, we looked at textbooks, and obviously we drew from our own clinical experience, so it comes from clinical judgment and expert opinion.”
Dr. Henderson also noted that in the future “it will be important to contextualize the recommendations by highlighting the importance of cultural competence in conducting assessments and providing treatment.” Moreover, current evidence can be expanded through the incorporation of diverse cultural and racial perspectives, experiences, and data, she added.
Health service providers should reflect on their own potential biases, which can influence clinician-patient interactions, Dr. Henderson continued. It also is important to consider biases in the evidence, which influence practice. Clinicians should also consider how their recommendations fit with patients’ “cultural and race-based experiences, beliefs, and practices.”
No source of funding for the position statement was reported. Dr. Klein and Dr. Henderson had disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Canadian Paediatric Society (CPS) has issued a position statement on the diagnosis of anxiety disorders in children and youth. The organization aims to “offer evidence-informed guidance to support pediatric health care providers making decisions around the care of children and adolescents with these conditions.”
“It’s been a long time coming,” lead author Benjamin Klein, MD, assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont., told this news organization. The target audience for the documents includes community pediatricians, subspecialists, family doctors, and nurse practitioners. “There was a great demand from that audience for a position statement, for guidance, obviously in the backdrop of rising child and adolescent mental health incidence over the years and of course COVID,” said Dr. Klein.
The statement was published on the CPS website.
‘A comprehensive approach’
Although many other guidelines on this topic are available, it was important to have a Canadian document, said Dr. Klein. “Obviously, there’s going to be a great deal of overlap with European or American guidelines, but it’s just kind of assumed that people want specifically Canadian content. ... Physicians want to know that they’re practicing within a standard of care in Canada.” Dr. Klein is medical director of the Lansdowne Children’s Centre, Brantford, Ont., which provides help for children with communication, developmental, and physical special needs across Ontario.
Anxiety disorders are the most common mental disorders among children and adolescents in Canada, according to the position statement. The Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) groups these disorders into separation anxiety disorder, selective mutism, specific phobia, social anxiety disorder (social phobia), panic disorder, agoraphobia, and generalized anxiety disorder.
Distinguishing normal, age-appropriate anxiety from anxiety disorder, while also recognizing other comorbidities, is complicated, said Dr. Klein. “Anxiety is one possible diagnosis or feature, and children with mental health and developmental problems often present with a number of problems. Anxiety may be one of them, but if it’s one of them, it may not be the main driver. So, a comprehensive approach is needed ... combining the medical model with biopsychosocial thinking to give a better picture of anxiety in the context of anything else that may be contributing to a presentation.”
The statement outlines recommendations for anxiety assessment, starting with a screening questionnaire such as the Screen for Child Anxiety Related Disorders (SCARED), which is completed by parents and children, to assess symptom severity. Standardized measures for medical, mental health, and developmental histories are available on the CPS website.
The document next recommends an interview about presenting concerns (such as sleep problems or school difficulties), inciting events, and parent-child interactions. The process includes confidential, nonjudgmental interviews with adolescents using a history-taking tool such as HEEADSSS (Home, Education/Employment, Eating, Activities, Drugs, Sexuality, Suicide/Mental Health, and Safety).
“The diagnosis and treatment of anxiety disorders kind of sounds simple if you just read about it as an isolated thing, but the reality is ... there’s no MRI. It’s detective work,” said Dr. Klein. Clinicians must distinguish between normal anxiety, situational anxiety, and specific anxiety disorder, he added. He usually allows 90 minutes for an anxiety assessment, partly to gain the patient’s trust. “These are sensitive issues. It’s common that people don’t trust a diagnosis if you haven’t spent enough time with them. That relational care piece just needs to be there, or people aren’t going to buy in.”
The CPS position statement was reviewed and endorsed by the Canadian Academy of Child and Adolescent Psychiatry.
Methodology unclear
Joanna Henderson, MD, professor of psychiatry at the University of Toronto and director of the Margaret and Wallace McCain Centre for Child, Youth, and Family Mental Health at the Centre for Addiction and Mental Health, Toronto, said that the guidelines have been released at an important time. “Conversations about mental health have become more common, and many children, youth, and families are reaching out for support. It is essential that health care professionals be equipped with accessible information about practices to provide appropriate care. These guidelines support that vision.”
It would be helpful to know more about the methods used to arrive at the recommendations, however, said Dr. Henderson. “It is critical that health care providers be guided by evidence-based guidelines that adhere to criteria for establishing high-quality guidelines. Because the authors did not provide information about their methods, I am not able to provide a comment about the quality of their guidelines. There are established approaches for evaluating quality, and I would encourage the authors to publish as a supplement to this article their methods, including in reference to the Appraisal of Guidelines for Research and Evaluation (AGREE II) checklist.”
In the absence of readily available information about methods, she said, “clinicians are encouraged to use guidelines from sources that provide information about the guideline development process and include quality appraisal,” such as the UK National Institute for Health and Care Excellence, which is “generally recognized as a reputable source for high-quality practice guidelines.”
Responding to this concern, Dr. Klein said, “There is no specific evidence base for diagnosis. That robust science doesn’t exist. No one has done randomized controlled trials of different methods of diagnosing kids with anxiety. We looked at other position statements, we looked at textbooks, and obviously we drew from our own clinical experience, so it comes from clinical judgment and expert opinion.”
Dr. Henderson also noted that in the future “it will be important to contextualize the recommendations by highlighting the importance of cultural competence in conducting assessments and providing treatment.” Moreover, current evidence can be expanded through the incorporation of diverse cultural and racial perspectives, experiences, and data, she added.
Health service providers should reflect on their own potential biases, which can influence clinician-patient interactions, Dr. Henderson continued. It also is important to consider biases in the evidence, which influence practice. Clinicians should also consider how their recommendations fit with patients’ “cultural and race-based experiences, beliefs, and practices.”
No source of funding for the position statement was reported. Dr. Klein and Dr. Henderson had disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rapid action or sustained effect? Methotrexate vs. ciclosporin for pediatric AD
MONTREAL – in the TREAT study, investigators reported at the annual meeting of the International Society of Atopic Dermatitis.
The findings are important, since many regulatory bodies require patients to have tried such first-line conventional systemic therapies before moving on to novel therapeutics, explained Carsten Flohr, MD, PhD, research and development lead at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust London.
“We don’t really have much pediatric trial data; very often the pediatric data that we have is buried in adult trials and when it comes to an adequately powered randomized controlled trial with conventional systemic medication in pediatric patients, we don’t have one – so we’re lacking that gold standard,” said Dr. Flohr, chair in dermatology and population health sciences at King’s College London.
In the TREAT trial, 103 patients with AD (mean age, 10 years) who had not responded to topical treatment, were randomly assigned to oral ciclosporin (4 mg/kg daily) or methotrexate (0.4 mg/kg weekly) for 36 weeks and then followed for another 24 weeks off therapy for the co-primary outcomes of change in objective Scoring Atopic Dermatitis (o-SCORAD) at 12 weeks, as well as time to first significant flare after treatment cessation, defined as returning to baseline o-SCORAD, or restarting a systemic treatment.
Secondary outcomes included disease severity and quality of life (QOL) measures, as well as safety. At baseline, the mean o-SCORAD was 46.81, with mean Eczema Area and Severity Index (EASI) and Patient Oriented Eczema Measure (POEM) scores of 28.05 and 20.62 respectively. The mean Children’s Dermatology Life Quality Index (CDLQI) score was 14.96.
Looking at change in eczema severity measured by o-SCORAD at 12 weeks, ciclosporin was superior to methotrexate, with a mean difference in o-SCORAD change of -5.69 (P =.01). For the co-primary endpoint of time to first significant flare during the 24 weeks after treatment cessation, “there was a trend toward more flare activity in the ciclosporin group, although with a hazard ratio of 1.55, this was statistically not significant,” Dr. Flohr said.
On a graph showing mean EASI scores from baseline through the 60-week study period, Dr. Flohr explained how the score first dropped more precipitously in patients treated with ciclosporin compared with those treated with methotrexate, reaching a statistically significant difference between the groups by 12 weeks (–3.13, P = .0145).
However, after that time, while the EASI score among those on methotrexate continued to drop, the ciclosporin score evened out, so that by 20 weeks, methotrexate EASI scores were better, and remained so until the end of treatment and further, out to 60 weeks (mean difference -6.36, P < .001). “The most interesting bit of this graph is [that] the curve is pointing downwards for methotrexate up to the 9-month point, suggesting these people had not reached their full therapeutic potential yet, whereas if you’re on ciclosporin you plateau and there’s not much additional improvement, if at all, and then people [on ciclosporin] start going up in their disease activity off therapy,” he said.
The same pattern was seen with all the other outcome measures, including o-SCORAD and POEM.
Quality of life significantly improved by about 8 points in both treatment groups, with no significant differences between groups, and this improvement was sustained through the 24 weeks following cessation of therapy. However, during this treatment-free phase, patients on methotrexate had fewer parent-reported flares compared with those on ciclosporin (mean 6.19 vs 5.40 flares, P =.0251), although there was no difference between groups in time to first flare.
Describing the treatment safety as “overall reassuring,” Dr. Flohr said there were slightly more nonserious adverse events in the methotrexate arm (407 vs. 369), with nausea occurring more often in this group (43.1% vs. 17.6%).
“I think we were seeing this clinically, but to see it in a clinical trial gives us more confidence in discussing with parents,” said session moderator Melinda Gooderham, MD, assistant professor at Queens University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology in Peterborough.
What she also took away from the study was safety of these treatments. “The discontinuation rate was not different with either drug, so it’s not like ciclosporin works fast but all these people have problems and discontinue,” Dr. Gooderham told this news organization. “That’s also reassuring.”
Asked which treatment she prefers, Dr. Gooderham, a consultant physician at Peterborough Regional Health Centre, picked methotrexate “because of the lasting effect. But there are times when you may need more rapid control ... where I might choose ciclosporin first, but for me it’s maybe 90% methotrexate first, 10% ciclosporin.”
Dr. Flohr and Dr. Gooderham report no relevant financial relationships. The study was funded by the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
MONTREAL – in the TREAT study, investigators reported at the annual meeting of the International Society of Atopic Dermatitis.
The findings are important, since many regulatory bodies require patients to have tried such first-line conventional systemic therapies before moving on to novel therapeutics, explained Carsten Flohr, MD, PhD, research and development lead at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust London.
“We don’t really have much pediatric trial data; very often the pediatric data that we have is buried in adult trials and when it comes to an adequately powered randomized controlled trial with conventional systemic medication in pediatric patients, we don’t have one – so we’re lacking that gold standard,” said Dr. Flohr, chair in dermatology and population health sciences at King’s College London.
In the TREAT trial, 103 patients with AD (mean age, 10 years) who had not responded to topical treatment, were randomly assigned to oral ciclosporin (4 mg/kg daily) or methotrexate (0.4 mg/kg weekly) for 36 weeks and then followed for another 24 weeks off therapy for the co-primary outcomes of change in objective Scoring Atopic Dermatitis (o-SCORAD) at 12 weeks, as well as time to first significant flare after treatment cessation, defined as returning to baseline o-SCORAD, or restarting a systemic treatment.
Secondary outcomes included disease severity and quality of life (QOL) measures, as well as safety. At baseline, the mean o-SCORAD was 46.81, with mean Eczema Area and Severity Index (EASI) and Patient Oriented Eczema Measure (POEM) scores of 28.05 and 20.62 respectively. The mean Children’s Dermatology Life Quality Index (CDLQI) score was 14.96.
Looking at change in eczema severity measured by o-SCORAD at 12 weeks, ciclosporin was superior to methotrexate, with a mean difference in o-SCORAD change of -5.69 (P =.01). For the co-primary endpoint of time to first significant flare during the 24 weeks after treatment cessation, “there was a trend toward more flare activity in the ciclosporin group, although with a hazard ratio of 1.55, this was statistically not significant,” Dr. Flohr said.
On a graph showing mean EASI scores from baseline through the 60-week study period, Dr. Flohr explained how the score first dropped more precipitously in patients treated with ciclosporin compared with those treated with methotrexate, reaching a statistically significant difference between the groups by 12 weeks (–3.13, P = .0145).
However, after that time, while the EASI score among those on methotrexate continued to drop, the ciclosporin score evened out, so that by 20 weeks, methotrexate EASI scores were better, and remained so until the end of treatment and further, out to 60 weeks (mean difference -6.36, P < .001). “The most interesting bit of this graph is [that] the curve is pointing downwards for methotrexate up to the 9-month point, suggesting these people had not reached their full therapeutic potential yet, whereas if you’re on ciclosporin you plateau and there’s not much additional improvement, if at all, and then people [on ciclosporin] start going up in their disease activity off therapy,” he said.
The same pattern was seen with all the other outcome measures, including o-SCORAD and POEM.
Quality of life significantly improved by about 8 points in both treatment groups, with no significant differences between groups, and this improvement was sustained through the 24 weeks following cessation of therapy. However, during this treatment-free phase, patients on methotrexate had fewer parent-reported flares compared with those on ciclosporin (mean 6.19 vs 5.40 flares, P =.0251), although there was no difference between groups in time to first flare.
Describing the treatment safety as “overall reassuring,” Dr. Flohr said there were slightly more nonserious adverse events in the methotrexate arm (407 vs. 369), with nausea occurring more often in this group (43.1% vs. 17.6%).
“I think we were seeing this clinically, but to see it in a clinical trial gives us more confidence in discussing with parents,” said session moderator Melinda Gooderham, MD, assistant professor at Queens University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology in Peterborough.
What she also took away from the study was safety of these treatments. “The discontinuation rate was not different with either drug, so it’s not like ciclosporin works fast but all these people have problems and discontinue,” Dr. Gooderham told this news organization. “That’s also reassuring.”
Asked which treatment she prefers, Dr. Gooderham, a consultant physician at Peterborough Regional Health Centre, picked methotrexate “because of the lasting effect. But there are times when you may need more rapid control ... where I might choose ciclosporin first, but for me it’s maybe 90% methotrexate first, 10% ciclosporin.”
Dr. Flohr and Dr. Gooderham report no relevant financial relationships. The study was funded by the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
MONTREAL – in the TREAT study, investigators reported at the annual meeting of the International Society of Atopic Dermatitis.
The findings are important, since many regulatory bodies require patients to have tried such first-line conventional systemic therapies before moving on to novel therapeutics, explained Carsten Flohr, MD, PhD, research and development lead at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust London.
“We don’t really have much pediatric trial data; very often the pediatric data that we have is buried in adult trials and when it comes to an adequately powered randomized controlled trial with conventional systemic medication in pediatric patients, we don’t have one – so we’re lacking that gold standard,” said Dr. Flohr, chair in dermatology and population health sciences at King’s College London.
In the TREAT trial, 103 patients with AD (mean age, 10 years) who had not responded to topical treatment, were randomly assigned to oral ciclosporin (4 mg/kg daily) or methotrexate (0.4 mg/kg weekly) for 36 weeks and then followed for another 24 weeks off therapy for the co-primary outcomes of change in objective Scoring Atopic Dermatitis (o-SCORAD) at 12 weeks, as well as time to first significant flare after treatment cessation, defined as returning to baseline o-SCORAD, or restarting a systemic treatment.
Secondary outcomes included disease severity and quality of life (QOL) measures, as well as safety. At baseline, the mean o-SCORAD was 46.81, with mean Eczema Area and Severity Index (EASI) and Patient Oriented Eczema Measure (POEM) scores of 28.05 and 20.62 respectively. The mean Children’s Dermatology Life Quality Index (CDLQI) score was 14.96.
Looking at change in eczema severity measured by o-SCORAD at 12 weeks, ciclosporin was superior to methotrexate, with a mean difference in o-SCORAD change of -5.69 (P =.01). For the co-primary endpoint of time to first significant flare during the 24 weeks after treatment cessation, “there was a trend toward more flare activity in the ciclosporin group, although with a hazard ratio of 1.55, this was statistically not significant,” Dr. Flohr said.
On a graph showing mean EASI scores from baseline through the 60-week study period, Dr. Flohr explained how the score first dropped more precipitously in patients treated with ciclosporin compared with those treated with methotrexate, reaching a statistically significant difference between the groups by 12 weeks (–3.13, P = .0145).
However, after that time, while the EASI score among those on methotrexate continued to drop, the ciclosporin score evened out, so that by 20 weeks, methotrexate EASI scores were better, and remained so until the end of treatment and further, out to 60 weeks (mean difference -6.36, P < .001). “The most interesting bit of this graph is [that] the curve is pointing downwards for methotrexate up to the 9-month point, suggesting these people had not reached their full therapeutic potential yet, whereas if you’re on ciclosporin you plateau and there’s not much additional improvement, if at all, and then people [on ciclosporin] start going up in their disease activity off therapy,” he said.
The same pattern was seen with all the other outcome measures, including o-SCORAD and POEM.
Quality of life significantly improved by about 8 points in both treatment groups, with no significant differences between groups, and this improvement was sustained through the 24 weeks following cessation of therapy. However, during this treatment-free phase, patients on methotrexate had fewer parent-reported flares compared with those on ciclosporin (mean 6.19 vs 5.40 flares, P =.0251), although there was no difference between groups in time to first flare.
Describing the treatment safety as “overall reassuring,” Dr. Flohr said there were slightly more nonserious adverse events in the methotrexate arm (407 vs. 369), with nausea occurring more often in this group (43.1% vs. 17.6%).
“I think we were seeing this clinically, but to see it in a clinical trial gives us more confidence in discussing with parents,” said session moderator Melinda Gooderham, MD, assistant professor at Queens University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology in Peterborough.
What she also took away from the study was safety of these treatments. “The discontinuation rate was not different with either drug, so it’s not like ciclosporin works fast but all these people have problems and discontinue,” Dr. Gooderham told this news organization. “That’s also reassuring.”
Asked which treatment she prefers, Dr. Gooderham, a consultant physician at Peterborough Regional Health Centre, picked methotrexate “because of the lasting effect. But there are times when you may need more rapid control ... where I might choose ciclosporin first, but for me it’s maybe 90% methotrexate first, 10% ciclosporin.”
Dr. Flohr and Dr. Gooderham report no relevant financial relationships. The study was funded by the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
AT ISAD 2022