User login
Pediatric celiac disease incidence varies across U.S., Europe
, according to a new report.
The overall high incidence among pediatric patients warrants a low threshold for screening and additional research on region-specific celiac disease triggers, the authors write.
“Determining the true incidence of celiac disease (CD) is not possible without nonbiased screening for the disease. This is because many cases occur with neither a family history nor with classic symptoms,” write Edwin Liu, MD, a pediatric gastroenterologist at the Children’s Hospital Colorado Anschutz Medical Campus and director of the Colorado Center for Celiac Disease, and colleagues.
“Individuals may have celiac disease autoimmunity without having CD if they have transient or fluctuating antibody levels, low antibody levels without biopsy evaluation, dietary modification influencing further evaluation, or potential celiac disease,” they write.
The study was published online in The American Journal of Gastroenterology.
Celiac disease incidence
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows children born between 2004 and 2010 who are at genetic risk for both type 1 diabetes and CD at six clinical sites in four countries: the United States, Finland, Germany, and Sweden. In the United States, patients are enrolled in Colorado, Georgia, and Washington.
As part of TEDDY, children are longitudinally monitored for celiac disease autoimmunity (CDA) by assessment of autoantibodies to tissue transglutaminase (tTGA). The protocol is designed to analyze the development of persistent tTGA positivity, CDA, and subsequent CD. The study population contains various DQ2.5 and DQ8.1 combinations, which represent the highest-risk human leukocyte antigen (HLA) DQ haplogentotypes for CD.
From September 2004 through February 2010, more than 424,000 newborns were screened for specific HLA haplogenotypes, and 8,676 children were enrolled in TEDDY at the six clinical sites. The eligible haplogenotypes included DQ2.5/DQ2.5, DQ2.5/DQ8.1, DQ8.1/DQ8.1, and DQ8.1/DQ4.2.
Blood samples were obtained and stored every 3 months until age 48 months and at least every 6 months after that. At age 2, participants were screened annually for tTGA. With the first tTGA-positive result, all prior collected samples from the patient were tested for tTGA to determine the earliest time point of autoimmunity.
CDA, a primary study outcome, was defined as positivity in two consecutive tTGA tests at least 3 months apart.
In seropositive children, CD was defined on the basis of a duodenal biopsy with a Marsh score of 2 or higher. The decision to perform a biopsy was determined by the clinical gastroenterologist and was outside of the study protocol. When a biopsy wasn’t performed, participants with an average tTGA of 100 units or greater from two positive tests were considered to have CD for the study purposes.
As of July 2020, among the children who had undergone one or more tTGA tests, 6,628 HLA-typed eligible children were found to carry the DQ2.5, the D8.1, or both haplogenotypes and were included in the analysis. The median follow-up period was 11.5 years.
Overall, 580 children (9%) had a first-degree relative with type 1 diabetes, and 317 children (5%) reported a first-degree relative with CD.
Among the 6,628 children, 1,299 (20%) met the CDA outcome, and 529 (8%) met the study diagnostic criteria for CD on the basis of biopsy or persistently high tTGA levels. The median age at CDA across all sites was 41 months. Most children with CDA were asymptomatic.
Overall, the 10-year cumulative incidence was highest in Sweden, at 8.4% for CDA and 3% for CD. Within the United States, Colorado had the highest cumulative incidence for both endpoints, at 6.5% for CDA and 2.4% for CD. Washington had the lowest incidence across all sites, at 4.6% for CDA and 0.9% for CD.
“CDA and CD risk varied substantially by haplogenotype and by clinical center, but the relative risk by region was preserved regardless of the haplogenotype,” the authors write. “For example, the disease burden for each region remained highest in Sweden and lowest in Washington state for all haplogenotypes.”
Site-specific risks
In the HLA, sex, and family-adjusted model, Colorado children had a 2.5-fold higher risk of CD, compared with Washington children. Likewise, Swedish children had a 1.8-fold higher risk of CD than children in Germany, a 1.7-fold higher than children in the United States, and a 1.4-fold higher risk than children in Finland.
Among DQ2.5 participants, Sweden demonstrated the highest risk, with 63.1% of patients developing CDA by age 10 and 28.3% developing CD by age 10. Finland consistently had a higher incidence of CDA than Colorado, at 60.4% versus 50.9%, for DQ2.5 participants but a lower incidence of CD than Colorado, at 20.3% versus 22.6%.
The research team performed a post hoc sensitivity analysis using a lower tTGA cutoff to reduce bias in site differences for biopsy referral and to increase sensitivity of the CD definition for incidence estimation. When the tTGA cutoff was lowered to an average two-visit tTGA of 67.4 or higher, more children met the serologic criteria for CD.
“Even with this lower cutoff, the differences in the risk of CD between clinical sites and countries were still observed with statistical significance,” the authors write. “This indicates that the regional differences in CD incidence could not be solely attributed to detection biases posed by differential biopsy rates.”
Multiple environmental factors likely account for the differences in autoimmunity among regions, the authors write. These variables include diet, chemical exposures, vaccination patterns, early-life gastrointestinal infections, and interactions among these factors. For instance, the Swedish site has the lowest rotavirus vaccination rates and the highest median gluten intake among the TEDDY sites.
Future prospective studies should capture environmental, genetic, and epigenetic exposures to assess causal pathways and plan for preventive strategies, the authors write. The TEDDY study is pursuing this research.
“From a policy standpoint, this informs future screening practices and supports efforts toward mass screening, at least in some areas,” the authors write. “In the clinical setting, this points to the importance for clinicians to have a low threshold for CD screening in the appropriate clinical setting.”
The TEDDY study is funded by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences, the Centers for Disease Control and Prevention, and the Juvenile Diabetes Research Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new report.
The overall high incidence among pediatric patients warrants a low threshold for screening and additional research on region-specific celiac disease triggers, the authors write.
“Determining the true incidence of celiac disease (CD) is not possible without nonbiased screening for the disease. This is because many cases occur with neither a family history nor with classic symptoms,” write Edwin Liu, MD, a pediatric gastroenterologist at the Children’s Hospital Colorado Anschutz Medical Campus and director of the Colorado Center for Celiac Disease, and colleagues.
“Individuals may have celiac disease autoimmunity without having CD if they have transient or fluctuating antibody levels, low antibody levels without biopsy evaluation, dietary modification influencing further evaluation, or potential celiac disease,” they write.
The study was published online in The American Journal of Gastroenterology.
Celiac disease incidence
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows children born between 2004 and 2010 who are at genetic risk for both type 1 diabetes and CD at six clinical sites in four countries: the United States, Finland, Germany, and Sweden. In the United States, patients are enrolled in Colorado, Georgia, and Washington.
As part of TEDDY, children are longitudinally monitored for celiac disease autoimmunity (CDA) by assessment of autoantibodies to tissue transglutaminase (tTGA). The protocol is designed to analyze the development of persistent tTGA positivity, CDA, and subsequent CD. The study population contains various DQ2.5 and DQ8.1 combinations, which represent the highest-risk human leukocyte antigen (HLA) DQ haplogentotypes for CD.
From September 2004 through February 2010, more than 424,000 newborns were screened for specific HLA haplogenotypes, and 8,676 children were enrolled in TEDDY at the six clinical sites. The eligible haplogenotypes included DQ2.5/DQ2.5, DQ2.5/DQ8.1, DQ8.1/DQ8.1, and DQ8.1/DQ4.2.
Blood samples were obtained and stored every 3 months until age 48 months and at least every 6 months after that. At age 2, participants were screened annually for tTGA. With the first tTGA-positive result, all prior collected samples from the patient were tested for tTGA to determine the earliest time point of autoimmunity.
CDA, a primary study outcome, was defined as positivity in two consecutive tTGA tests at least 3 months apart.
In seropositive children, CD was defined on the basis of a duodenal biopsy with a Marsh score of 2 or higher. The decision to perform a biopsy was determined by the clinical gastroenterologist and was outside of the study protocol. When a biopsy wasn’t performed, participants with an average tTGA of 100 units or greater from two positive tests were considered to have CD for the study purposes.
As of July 2020, among the children who had undergone one or more tTGA tests, 6,628 HLA-typed eligible children were found to carry the DQ2.5, the D8.1, or both haplogenotypes and were included in the analysis. The median follow-up period was 11.5 years.
Overall, 580 children (9%) had a first-degree relative with type 1 diabetes, and 317 children (5%) reported a first-degree relative with CD.
Among the 6,628 children, 1,299 (20%) met the CDA outcome, and 529 (8%) met the study diagnostic criteria for CD on the basis of biopsy or persistently high tTGA levels. The median age at CDA across all sites was 41 months. Most children with CDA were asymptomatic.
Overall, the 10-year cumulative incidence was highest in Sweden, at 8.4% for CDA and 3% for CD. Within the United States, Colorado had the highest cumulative incidence for both endpoints, at 6.5% for CDA and 2.4% for CD. Washington had the lowest incidence across all sites, at 4.6% for CDA and 0.9% for CD.
“CDA and CD risk varied substantially by haplogenotype and by clinical center, but the relative risk by region was preserved regardless of the haplogenotype,” the authors write. “For example, the disease burden for each region remained highest in Sweden and lowest in Washington state for all haplogenotypes.”
Site-specific risks
In the HLA, sex, and family-adjusted model, Colorado children had a 2.5-fold higher risk of CD, compared with Washington children. Likewise, Swedish children had a 1.8-fold higher risk of CD than children in Germany, a 1.7-fold higher than children in the United States, and a 1.4-fold higher risk than children in Finland.
Among DQ2.5 participants, Sweden demonstrated the highest risk, with 63.1% of patients developing CDA by age 10 and 28.3% developing CD by age 10. Finland consistently had a higher incidence of CDA than Colorado, at 60.4% versus 50.9%, for DQ2.5 participants but a lower incidence of CD than Colorado, at 20.3% versus 22.6%.
The research team performed a post hoc sensitivity analysis using a lower tTGA cutoff to reduce bias in site differences for biopsy referral and to increase sensitivity of the CD definition for incidence estimation. When the tTGA cutoff was lowered to an average two-visit tTGA of 67.4 or higher, more children met the serologic criteria for CD.
“Even with this lower cutoff, the differences in the risk of CD between clinical sites and countries were still observed with statistical significance,” the authors write. “This indicates that the regional differences in CD incidence could not be solely attributed to detection biases posed by differential biopsy rates.”
Multiple environmental factors likely account for the differences in autoimmunity among regions, the authors write. These variables include diet, chemical exposures, vaccination patterns, early-life gastrointestinal infections, and interactions among these factors. For instance, the Swedish site has the lowest rotavirus vaccination rates and the highest median gluten intake among the TEDDY sites.
Future prospective studies should capture environmental, genetic, and epigenetic exposures to assess causal pathways and plan for preventive strategies, the authors write. The TEDDY study is pursuing this research.
“From a policy standpoint, this informs future screening practices and supports efforts toward mass screening, at least in some areas,” the authors write. “In the clinical setting, this points to the importance for clinicians to have a low threshold for CD screening in the appropriate clinical setting.”
The TEDDY study is funded by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences, the Centers for Disease Control and Prevention, and the Juvenile Diabetes Research Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new report.
The overall high incidence among pediatric patients warrants a low threshold for screening and additional research on region-specific celiac disease triggers, the authors write.
“Determining the true incidence of celiac disease (CD) is not possible without nonbiased screening for the disease. This is because many cases occur with neither a family history nor with classic symptoms,” write Edwin Liu, MD, a pediatric gastroenterologist at the Children’s Hospital Colorado Anschutz Medical Campus and director of the Colorado Center for Celiac Disease, and colleagues.
“Individuals may have celiac disease autoimmunity without having CD if they have transient or fluctuating antibody levels, low antibody levels without biopsy evaluation, dietary modification influencing further evaluation, or potential celiac disease,” they write.
The study was published online in The American Journal of Gastroenterology.
Celiac disease incidence
The Environmental Determinants of Diabetes in the Young (TEDDY) study prospectively follows children born between 2004 and 2010 who are at genetic risk for both type 1 diabetes and CD at six clinical sites in four countries: the United States, Finland, Germany, and Sweden. In the United States, patients are enrolled in Colorado, Georgia, and Washington.
As part of TEDDY, children are longitudinally monitored for celiac disease autoimmunity (CDA) by assessment of autoantibodies to tissue transglutaminase (tTGA). The protocol is designed to analyze the development of persistent tTGA positivity, CDA, and subsequent CD. The study population contains various DQ2.5 and DQ8.1 combinations, which represent the highest-risk human leukocyte antigen (HLA) DQ haplogentotypes for CD.
From September 2004 through February 2010, more than 424,000 newborns were screened for specific HLA haplogenotypes, and 8,676 children were enrolled in TEDDY at the six clinical sites. The eligible haplogenotypes included DQ2.5/DQ2.5, DQ2.5/DQ8.1, DQ8.1/DQ8.1, and DQ8.1/DQ4.2.
Blood samples were obtained and stored every 3 months until age 48 months and at least every 6 months after that. At age 2, participants were screened annually for tTGA. With the first tTGA-positive result, all prior collected samples from the patient were tested for tTGA to determine the earliest time point of autoimmunity.
CDA, a primary study outcome, was defined as positivity in two consecutive tTGA tests at least 3 months apart.
In seropositive children, CD was defined on the basis of a duodenal biopsy with a Marsh score of 2 or higher. The decision to perform a biopsy was determined by the clinical gastroenterologist and was outside of the study protocol. When a biopsy wasn’t performed, participants with an average tTGA of 100 units or greater from two positive tests were considered to have CD for the study purposes.
As of July 2020, among the children who had undergone one or more tTGA tests, 6,628 HLA-typed eligible children were found to carry the DQ2.5, the D8.1, or both haplogenotypes and were included in the analysis. The median follow-up period was 11.5 years.
Overall, 580 children (9%) had a first-degree relative with type 1 diabetes, and 317 children (5%) reported a first-degree relative with CD.
Among the 6,628 children, 1,299 (20%) met the CDA outcome, and 529 (8%) met the study diagnostic criteria for CD on the basis of biopsy or persistently high tTGA levels. The median age at CDA across all sites was 41 months. Most children with CDA were asymptomatic.
Overall, the 10-year cumulative incidence was highest in Sweden, at 8.4% for CDA and 3% for CD. Within the United States, Colorado had the highest cumulative incidence for both endpoints, at 6.5% for CDA and 2.4% for CD. Washington had the lowest incidence across all sites, at 4.6% for CDA and 0.9% for CD.
“CDA and CD risk varied substantially by haplogenotype and by clinical center, but the relative risk by region was preserved regardless of the haplogenotype,” the authors write. “For example, the disease burden for each region remained highest in Sweden and lowest in Washington state for all haplogenotypes.”
Site-specific risks
In the HLA, sex, and family-adjusted model, Colorado children had a 2.5-fold higher risk of CD, compared with Washington children. Likewise, Swedish children had a 1.8-fold higher risk of CD than children in Germany, a 1.7-fold higher than children in the United States, and a 1.4-fold higher risk than children in Finland.
Among DQ2.5 participants, Sweden demonstrated the highest risk, with 63.1% of patients developing CDA by age 10 and 28.3% developing CD by age 10. Finland consistently had a higher incidence of CDA than Colorado, at 60.4% versus 50.9%, for DQ2.5 participants but a lower incidence of CD than Colorado, at 20.3% versus 22.6%.
The research team performed a post hoc sensitivity analysis using a lower tTGA cutoff to reduce bias in site differences for biopsy referral and to increase sensitivity of the CD definition for incidence estimation. When the tTGA cutoff was lowered to an average two-visit tTGA of 67.4 or higher, more children met the serologic criteria for CD.
“Even with this lower cutoff, the differences in the risk of CD between clinical sites and countries were still observed with statistical significance,” the authors write. “This indicates that the regional differences in CD incidence could not be solely attributed to detection biases posed by differential biopsy rates.”
Multiple environmental factors likely account for the differences in autoimmunity among regions, the authors write. These variables include diet, chemical exposures, vaccination patterns, early-life gastrointestinal infections, and interactions among these factors. For instance, the Swedish site has the lowest rotavirus vaccination rates and the highest median gluten intake among the TEDDY sites.
Future prospective studies should capture environmental, genetic, and epigenetic exposures to assess causal pathways and plan for preventive strategies, the authors write. The TEDDY study is pursuing this research.
“From a policy standpoint, this informs future screening practices and supports efforts toward mass screening, at least in some areas,” the authors write. “In the clinical setting, this points to the importance for clinicians to have a low threshold for CD screening in the appropriate clinical setting.”
The TEDDY study is funded by several grants from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Environmental Health Sciences, the Centers for Disease Control and Prevention, and the Juvenile Diabetes Research Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AMERICAN JOURNAL OF GASTROENTEROLOGY
Dietary Triggers for Atopic Dermatitis in Children
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
It is unsurprising that food frequently is thought to be the culprit behind an eczema flare, especially in infants. Indeed, it often is said that infants do only 3 things: eat, sleep, and poop.1 For those unfortunate enough to develop the signs and symptoms of atopic dermatitis (AD), food quickly emerges as a potential culprit from the tiny pool of suspects, which is against a cultural backdrop of unprecedented focus on foods and food reactions.2 The prevalence of food allergies in children, though admittedly fraught with methodological difficulties, is estimated to have more than doubled from 3.4% in 1999 to 7.6% in 2018.3 As expected, prevalence rates were higher among children with other atopic comorbidities including AD, with up to 50% of children with AD demonstrating convincing food allergy.4 It is easy to imagine a patient conflating these 2 entities and mistaking their correlation for causation. Thus, it follows that more than 90% of parents/guardians have reported that their children have had food-induced AD, and understandably—at least according to one study—75% of parents/guardians were found to have manipulated the diet in an attempt to manage the disease.5,6
Patients and parents/guardians are not the only ones who have suspected food as a driving force in AD. An article in the British Medical Journal from the 1800s beautifully encapsulated the depth and duration of this quandary: “There is probably no subject in which more deeply rooted convictions have been held, not only in the profession but by the laity, than the connection between diet and disease, both as regards the causation and treatment of the latter.”7 Herein, a wide range of food reactions is examined to highlight evidence for the role of diet in AD, which may contradict what patients—and even some clinicians—believe.
No Easy Answers
A definitive statement that food allergy is not the root cause of AD would put this issue to rest, but such simplicity does not reflect the complex reality. First, we must agree on definitions for certain terms. What do we mean by food allergy? A broader category—adverse food reactions—covers a wide range of entities, some immune mediated and some not, including lactose intolerance, irritant contact dermatitis around the mouth, and even dermatitis herpetiformis (the cutaneous manifestation of celiac disease).8 Although the term food allergy often is used synonymously with adverse food reactions, the exact definition of a food allergy is specific: “adverse immune responses to food proteins that result in typical clinical symptoms.”8 The fact that many patients and even health care practitioners seem to frequently misapply this term makes it even more confusing.
The current focus is on foods that could trigger a flare of AD, which clearly is a broader question than food allergy sensu stricto. It seems self-evident, for example, that if an infant with AD were to (messily) eat an acidic food such as an orange, a flare-up of AD around the mouth and on the cheeks and hands would be a forgone conclusion. Similar nonimmunologic scenarios unambiguously can occur with many foods, including citrus; corn; radish; mustard; garlic; onion; pineapple; and many spices, food additives, and preservatives.9 Clearly there are some scenarios whereby food could trigger an AD flare, and yet this more limited vignette generally is not what patients are referring to when suggesting that food is the root cause of their AD.
The Labyrinth of Testing for Food Allergies
Although there is no reliable method for testing for irritant dermatitis, understanding the other types of tests may help guide our thinking. Testing for IgE-mediated food allergies generally is done via an immunoenzymatic serum assay that can document sensitization to a food protein; however, this testing by itself is not sufficient to diagnose a clinical food allergy.10 Similarly, skin prick testing allows for intradermal administration of a food extract to evaluate for an urticarial reaction within 10 to 15 minutes. Although the sensitivity and specificity vary by age, population, and the specific allergen being tested, these are limited to immediate-type reactions and do not reflect the potential to drive an eczematous flare.
The gold standard, if there is one, is likely the double-blind, placebo-controlled food challenge (DBPCFC), ideally with a long enough observation period to capture later-occurring reactions such as an AD flare. However, given the nature of the test—having patients eat the foods of concern and then carefully following them for reactions—it remains time consuming, expensive, and labor intensive.11
To further complicate matters, several unvalidated tests exist such as IgG testing, atopy patch testing, kinesiology, and hair and gastric juice analysis, which remain investigational but continue to be used and may further confuse patients and clinicians.12
Classification of Food Allergies
It is useful to first separate out the classic IgE-mediated food allergy reactions that are common. In these immediate-type reactions, a person sensitized to a food protein will develop characteristic cutaneous and/or extracutaneous reactions such as urticaria, angioedema, and even anaphylaxis, usually within minutes of exposure. Although it is possible that an IgE-mediated reaction could trigger an AD flare—perhaps simply by causing pruritus, which could initiate the itch-scratch cycle—because of the near simultaneity with ingestion of the offending food and the often dramatic clinical presentations, such foods clearly do not represent “hidden” triggers for AD flares.3 The concept of food-triggered AD (FTAD) is crucial for thinking about foods that could result in true eczematous flares, which historically have been classified as early-type (<2 hours after food challenge) and late-type (≥2 hours after food challenge) reactions.13,14
A study of more than 1000 DBPCFCs performed in patients with AD was illustrative.15 Immediate reactions other than AD were fairly common and were observed in 40% of the food challenges compared to only 9% in the placebo group. These reactions included urticaria, angioedema, and gastrointestinal and respiratory tract symptoms. Immediate reactions of AD alone were exceedingly rare at only 0.7% and not significantly elevated compared to placebo. Just over 4% experienced both an immediate AD exacerbation along with other non-AD findings, which was significantly greater than placebo (P<.01). Although intermediate and late reactions manifesting as AD exacerbations did occur after food ingestion, they were rare (2.2% or less) and not significantly different from placebo. The authors concluded that an exacerbation of AD in the absence of other allergic symptoms in children was unlikely to be due to food,15 which is an important finding.
A recent retrospective review of 372 children with AD reported similar results.4 The authors defined FTAD in a different way; instead of showing a flare after a DBPCFC, they looked for “physician-noted sustained improvement in AD upon removal of a food (typically after 2–6-wk follow-up), to which the child was sensitized without any other changes in skin care.” Despite this fundamentally different approach, they similarly concluded that while food allergies were common, FTAD was relatively uncommon—found in 2% of those with mild AD, 6% of those with moderate AD, and 4% of those with severe AD.4
There are other ways that foods could contribute to disease flares, however, and one of the most compelling is that there may be broader concepts at play; perhaps some diets are not specifically driving the AD but rather are affecting inflammation in the body at large. Although somewhat speculative, there is evidence that some foods may simply be proinflammatory, working to exacerbate the disease outside of a specific mechanism, which has been seen in a variety of other conditions such as acne or rheumatoid arthritis.16,17 To speculate further, it is possible that there may be a threshold effect such that when the AD is poorly controlled, certain factors such as inflammatory foods could lead to a flare, while when under better control, these same factors may not cause an effect.
Finally, it is important to also consider the emotional and/or psychological aspects related to food and diet. The power of the placebo in dietary change has been documented in several diseases, though this certainly is not to be dismissive of the patient’s symptoms; it seems reasonable that the very act of changing such a fundamental aspect of daily life could result in a placebo effect.18,19 In the context of relapsing and remitting conditions such as AD, this effect may be magnified. A landmark study by Thompson and Hanifin20 illustrates this possibility. The authors found that in 80% of cases in which patients were convinced that food was a major contributing factor to their AD, such concerns diminished markedly once better control of the eczema was achieved.20
Navigating the Complexity of Dietary Restrictions
This brings us to what to do with an individual patient in the examination room. Because there is such widespread concern and discussion around this topic, it is important to at least briefly address it. If there are known food allergens that are being avoided, it is important to underscore the importance of continuing to avoid those foods, especially when there is actual evidence of true food allergy rather than sensitization alone. Historically, elimination diets often were recommended empirically, though more recent studies, meta-analyses, and guidance documents increasingly have recommended against them.3 In particular, there are major concerns for iatrogenic harm.
First, heavily restricted diets may result in nutritional and/or caloric deficiencies that can be dangerous and lead to poor growth.21 Practices such as drinking unpasteurized milk can expose children to dangerous infections, while feeding them exclusively rice milk can lead to severe malnutrition.22
Second, there is a dawning realization that children with AD placed on elimination diets may actually develop true IgE-mediated allergies, including fatal anaphylaxis, to the excluded foods. In fact, one retrospective review of 298 patients with a history of AD and no prior immediate reactions found that 19% of patients developed new immediate-type hypersensitivity reactions after starting an elimination diet, presumably due to the loss of tolerance to these foods. A striking one-third of these reactions were classified as anaphylaxis, with cow’s milk and egg being the most common offenders.23
It also is crucial to acknowledge that recommending sweeping lifestyle changes is not easy for patients, especially pediatric patients. Onerous dietary restrictions may add considerable stress, ironically a known trigger for AD itself.
Finally, dietary modifications can be a distraction from conventional therapy and may result in treatment delays while the patient continues to experience uncontrolled symptoms of AD.
Final Thoughts
Diet is intimately related to AD. Although the narrative continues to unfold in fascinating domains, such as the skin barrier and the microbiome, it is increasingly clear that these are intertwined and always have been. Despite the rarity of true food-triggered AD, the perception of dietary triggers is so widespread and addressing the topic is important and may help avoid unnecessary harm from unfounded extreme dietary changes. A recent multispecialty workgroup report on AD and food allergy succinctly summarized this as: “AD has many triggers and comorbidities, and food allergy is only one of the potential triggers and comorbid conditions. With regard to AD management, education and skin care are most important.”3 With proper testing, guidance, and both topical and systemic therapies, most AD can be brought under control, and for at least some patients, this may allay concerns about foods triggering their AD.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
- Eat, sleep, poop—the top 3 things new parents need to know. John’s Hopkins All Children’s Hospital website. Published May 18, 2019. Accessed September 13, 2022. https://www.hopkinsallchildrens.org/ACH-News/General-News/Eat-Sleep-Poop-%E2%80%93-The-Top-3-Things-New-Parents-Ne
- Onyimba F, Crowe SE, Johnson S, et al. Food allergies and intolerances: a clinical approach to the diagnosis and management of adverse reactions to food. Clin Gastroenterol Hepatol. 2021;19:2230-2240.e1.
- Singh AM, Anvari S, Hauk P, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI Allergic Skin Diseases Committee and Leadership Institute Project. J Allergy Clin Immunol Pract. 2022;10:697-706.
- Li JC, Arkin LM, Makhija MM, et al. Prevalence of food allergy diagnosis in pediatric patients with atopic dermatitis referred to allergy and/or dermatology subspecialty clinics. J Allergy Clin Immunol Pract. 2022;10:2469-2471.
- Thompson MM, Tofte SJ, Simpson EL, et al. Patterns of care and referral in children with atopic dermatitis and concern for food allergy. Dermatol Ther. 2006;19:91-96.
- Johnston GA, Bilbao RM, Graham-Brown RAC. The use of dietary manipulation by parents of children with atopic dermatitis. Br J Dermatol. 2004;150:1186-1189.
- Mackenzie S. The inaugural address on the advantages to be derived from the study of dermatology: delivered to the Reading Pathological Society. Br Med J. 1896;1:193-197.
- Anvari S, Miller J, Yeh CY, et al. IgE-mediated food allergy. Clin Rev Allergy Immunol. 2019;57:244-260.
- Brancaccio RR, Alvarez MS. Contact allergy to food. Dermatol Ther. 2004;17:302-313.
- Robison RG, Singh AM. Controversies in allergy: food testing and dietary avoidance in atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7:35-39.
- Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582-586.
- Kelso JM. Unproven diagnostic tests for adverse reactions to foods. J Allergy Clin Immunol Pract. 2018;6:362-365.
- Heratizadeh A, Wichmann K, Werfel T. Food allergy and atopic dermatitis: how are they connected? Curr Allergy Asthma Rep. 2011;11:284-291.
- Breuer K, Heratizadeh A, Wulf A, et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy. 2004;34:817-824.
- Roerdink EM, Flokstra-de Blok BMJ, Blok JL, et al. Association of food allergy and atopic dermatitis exacerbations. Ann Allergy Asthma Immunol. 2016;116:334-338.
- Fuglsang G, Madsen G, Halken S, et al. Adverse reactions to food additives in children with atopic symptoms. Allergy. 1994;49:31-37.
- Ehlers I, Worm M, Sterry W, et al. Sugar is not an aggravating factor in atopic dermatitis. Acta Derm Venereol. 2001;81:282-284.
- Staudacher HM, Irving PM, Lomer MCE, et al. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc Nutr Soc. 2017;76:203-212.
- Masi A, Lampit A, Glozier N, et al. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:E640.
- Thompson MM, Hanifin JM. Effective therapy of childhood atopic dermatitis allays food allergy concerns. J Am Acad Dermatol. 2005;53(2 suppl 2):S214-S219.
- Meyer R, De Koker C, Dziubak R, et al. The impact of the elimination diet on growth and nutrient intake in children with food protein induced gastrointestinal allergies. Clin Transl Allergy. 2016;6:25.
- Webber SA, Graham-Brown RA, Hutchinson PE, et al. Dietary manipulation in childhood atopic dermatitis. Br J Dermatol. 1989;121:91-98.
- Chang A, Robison R, Cai M, et al. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. 2016;4:229-236.e1.
Practice Points
- The perception of dietary triggers is so entrenched and widespread that it should be addressed even when thought to be irrelevant.
- It is important not to dismiss food as a factor in atopic dermatitis (AD), as it can play a number of roles in the condition.
- On the other hand, education about the wide range of food reactions and the relative rarity of true food-driven AD along with the potential risks of dietary modification may enhance both rapport and understanding between the clinician and patient.
Children and COVID: New cases increase for second straight week
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.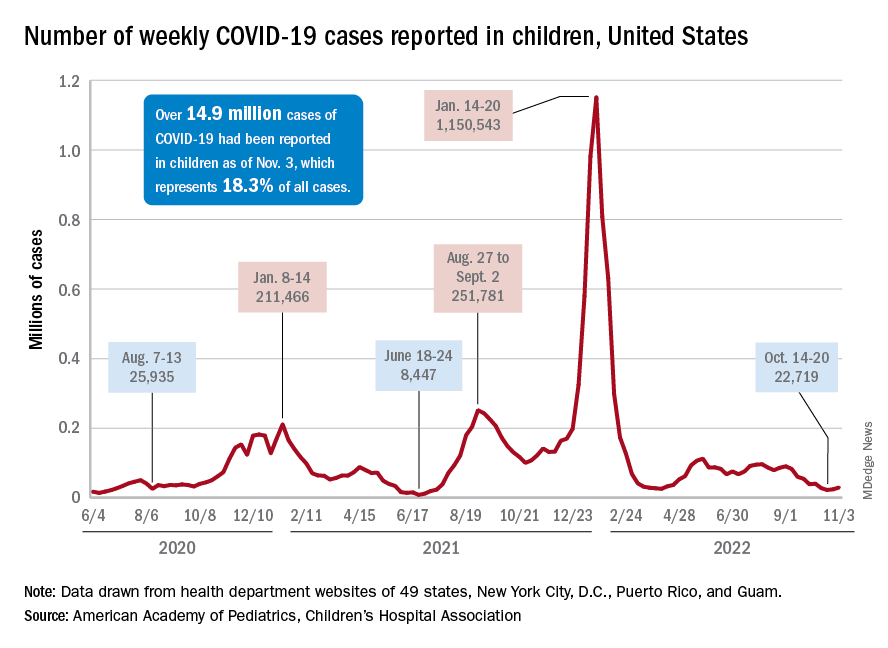
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
Many moms don’t remember well-child nutrition advice
Recent findings from a study examining mothers’ recall of doctors’ advice on early-child nutrition suggest that key feeding messages may not be heard, remembered, or even delivered.
During a typical child wellness visit, pediatricians provide parents with anticipatory guidance on all aspects of child development and safety, up to the age of 5 years.
The analysis of data from a subset of 1,302 mothers participating in the 2017-2019 National Survey of Family Growth showed that those older than 31 years of age and those who identified as non-Hispanic White were more likely to recall discussion of certain child nutrition topics compared with younger mothers or those who identified as Hispanic.
Of the six child-feeding topics referenced from the American Academy of Pediatrics’ “Bright Futures Guidelines,” less than half of the mothers, all of whom had a child between the ages of 6 months and 5 years, recalled guidance on limiting meals in front of the television or other electronic devices. Similarly, fewer than 50% remembered being told not to force their child to finish a bottle or food, the analysis showed.
When it came to the best time to introduce solid foods, 37% didn’t recall being told to wait at least 4 months and preferably, 6 months. In fact, these mothers reported being advised to introduce solid foods before 6 months, said Andrea McGowan, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
The study was published in the Journal of Nutrition Education and Behavior.
“All in all, this research draws attention to certain nutrition guidance topics or subpopulations that might be prioritized to improve receipt and recall of guidance,” said Ms. McGowan, now a first-year medical student at the University of Michigan, Ann Arbor, in a podcast. “This research ... implores us to consider ways to revamp the existing standard practice for pediatric well-child care to improve recall of messages.”
The analysis also included data on mothers’ recall of advice on offering foods with different tastes and textures; offering a variety of fruits and vegetables; and limiting added sugar. More than half of mothers remembered discussing four or five child nutrition topics, but 31% recalled talking about only one or two. Offering a variety of fruits and vegetables had the highest percentage of recall.
The study wasn’t powered to determine whether the nutrition guidance provided at a well-child visit was not remembered or not provided, Ms. McGowan said, adding: “So exploring this is definitely the goal of future research.”
However, pediatricians report spending an average of 18 minutes with children and their parents, she noted. “This is definitely not enough time to cover every single topic a pediatrician or a parent might want to discuss.” Other barriers, such as a lack of insurance or transportation, may limit parents’ access to this kind of anticipatory guidance, the researchers said.
Priority should be given to certain topics and to certain mothers, they suggested. “Innovative strategies tailored to families’ needs might alleviate the HCP [health care provider] burden and could enhance parental recall, especially when messaging is culturally relevant and personalized,” Ms. McGowan said.
Two independent experts agreed in interviews. Pediatricians must do their best to tailor advice to each particular family so that parents can engage in the conversation, said Lauren Fiechtner, MD, director of the center for pediatric nutrition at Mass General for Children, Boston. “As the authors suggest, we should seek to understand the cultural relevance of our recommendations and to understand the barriers our patient families might face in implementing our advice,” said Dr. Fiechtner, who is also an assistant professor at Harvard Medical School, also in Boston.
“Much of the instructions we as pediatricians give to parents must be repeated and reinforced,” said Rebecca S. Fisk, MD, a pediatrician at Lenox Hill Hospital, Northwell Health, in New York. Often, the doctor’s advice runs counter to what family and friends recommend, she pointed out. Some parents may believe that “the baby who starts solid food earlier will sleep through the night earlier or that eating in front of the TV relaxes the child or allows them to eat more,” Dr. Fisk explained. In her practice, a nurse goes over her instructions, answers questions, and provides specific examples and written information.
Sometimes, even that’s not enough, Dr. Fisk admitted. “I, myself, have fielded many repeated questions about feeding, when to start, how much to give, and so on, despite printed guidance given to parents at well-child visits.”
This study was funded by the U.S. Centers for Disease Control and Prevention. Ms. McGowan and study coauthors reported having no potential conflicts of interest. Dr. Fiechtner and Dr. Fisk disclosed having no potential conflicts of interest.
Recent findings from a study examining mothers’ recall of doctors’ advice on early-child nutrition suggest that key feeding messages may not be heard, remembered, or even delivered.
During a typical child wellness visit, pediatricians provide parents with anticipatory guidance on all aspects of child development and safety, up to the age of 5 years.
The analysis of data from a subset of 1,302 mothers participating in the 2017-2019 National Survey of Family Growth showed that those older than 31 years of age and those who identified as non-Hispanic White were more likely to recall discussion of certain child nutrition topics compared with younger mothers or those who identified as Hispanic.
Of the six child-feeding topics referenced from the American Academy of Pediatrics’ “Bright Futures Guidelines,” less than half of the mothers, all of whom had a child between the ages of 6 months and 5 years, recalled guidance on limiting meals in front of the television or other electronic devices. Similarly, fewer than 50% remembered being told not to force their child to finish a bottle or food, the analysis showed.
When it came to the best time to introduce solid foods, 37% didn’t recall being told to wait at least 4 months and preferably, 6 months. In fact, these mothers reported being advised to introduce solid foods before 6 months, said Andrea McGowan, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
The study was published in the Journal of Nutrition Education and Behavior.
“All in all, this research draws attention to certain nutrition guidance topics or subpopulations that might be prioritized to improve receipt and recall of guidance,” said Ms. McGowan, now a first-year medical student at the University of Michigan, Ann Arbor, in a podcast. “This research ... implores us to consider ways to revamp the existing standard practice for pediatric well-child care to improve recall of messages.”
The analysis also included data on mothers’ recall of advice on offering foods with different tastes and textures; offering a variety of fruits and vegetables; and limiting added sugar. More than half of mothers remembered discussing four or five child nutrition topics, but 31% recalled talking about only one or two. Offering a variety of fruits and vegetables had the highest percentage of recall.
The study wasn’t powered to determine whether the nutrition guidance provided at a well-child visit was not remembered or not provided, Ms. McGowan said, adding: “So exploring this is definitely the goal of future research.”
However, pediatricians report spending an average of 18 minutes with children and their parents, she noted. “This is definitely not enough time to cover every single topic a pediatrician or a parent might want to discuss.” Other barriers, such as a lack of insurance or transportation, may limit parents’ access to this kind of anticipatory guidance, the researchers said.
Priority should be given to certain topics and to certain mothers, they suggested. “Innovative strategies tailored to families’ needs might alleviate the HCP [health care provider] burden and could enhance parental recall, especially when messaging is culturally relevant and personalized,” Ms. McGowan said.
Two independent experts agreed in interviews. Pediatricians must do their best to tailor advice to each particular family so that parents can engage in the conversation, said Lauren Fiechtner, MD, director of the center for pediatric nutrition at Mass General for Children, Boston. “As the authors suggest, we should seek to understand the cultural relevance of our recommendations and to understand the barriers our patient families might face in implementing our advice,” said Dr. Fiechtner, who is also an assistant professor at Harvard Medical School, also in Boston.
“Much of the instructions we as pediatricians give to parents must be repeated and reinforced,” said Rebecca S. Fisk, MD, a pediatrician at Lenox Hill Hospital, Northwell Health, in New York. Often, the doctor’s advice runs counter to what family and friends recommend, she pointed out. Some parents may believe that “the baby who starts solid food earlier will sleep through the night earlier or that eating in front of the TV relaxes the child or allows them to eat more,” Dr. Fisk explained. In her practice, a nurse goes over her instructions, answers questions, and provides specific examples and written information.
Sometimes, even that’s not enough, Dr. Fisk admitted. “I, myself, have fielded many repeated questions about feeding, when to start, how much to give, and so on, despite printed guidance given to parents at well-child visits.”
This study was funded by the U.S. Centers for Disease Control and Prevention. Ms. McGowan and study coauthors reported having no potential conflicts of interest. Dr. Fiechtner and Dr. Fisk disclosed having no potential conflicts of interest.
Recent findings from a study examining mothers’ recall of doctors’ advice on early-child nutrition suggest that key feeding messages may not be heard, remembered, or even delivered.
During a typical child wellness visit, pediatricians provide parents with anticipatory guidance on all aspects of child development and safety, up to the age of 5 years.
The analysis of data from a subset of 1,302 mothers participating in the 2017-2019 National Survey of Family Growth showed that those older than 31 years of age and those who identified as non-Hispanic White were more likely to recall discussion of certain child nutrition topics compared with younger mothers or those who identified as Hispanic.
Of the six child-feeding topics referenced from the American Academy of Pediatrics’ “Bright Futures Guidelines,” less than half of the mothers, all of whom had a child between the ages of 6 months and 5 years, recalled guidance on limiting meals in front of the television or other electronic devices. Similarly, fewer than 50% remembered being told not to force their child to finish a bottle or food, the analysis showed.
When it came to the best time to introduce solid foods, 37% didn’t recall being told to wait at least 4 months and preferably, 6 months. In fact, these mothers reported being advised to introduce solid foods before 6 months, said Andrea McGowan, MPH, of the National Center for Chronic Disease Prevention and Health Promotion, U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
The study was published in the Journal of Nutrition Education and Behavior.
“All in all, this research draws attention to certain nutrition guidance topics or subpopulations that might be prioritized to improve receipt and recall of guidance,” said Ms. McGowan, now a first-year medical student at the University of Michigan, Ann Arbor, in a podcast. “This research ... implores us to consider ways to revamp the existing standard practice for pediatric well-child care to improve recall of messages.”
The analysis also included data on mothers’ recall of advice on offering foods with different tastes and textures; offering a variety of fruits and vegetables; and limiting added sugar. More than half of mothers remembered discussing four or five child nutrition topics, but 31% recalled talking about only one or two. Offering a variety of fruits and vegetables had the highest percentage of recall.
The study wasn’t powered to determine whether the nutrition guidance provided at a well-child visit was not remembered or not provided, Ms. McGowan said, adding: “So exploring this is definitely the goal of future research.”
However, pediatricians report spending an average of 18 minutes with children and their parents, she noted. “This is definitely not enough time to cover every single topic a pediatrician or a parent might want to discuss.” Other barriers, such as a lack of insurance or transportation, may limit parents’ access to this kind of anticipatory guidance, the researchers said.
Priority should be given to certain topics and to certain mothers, they suggested. “Innovative strategies tailored to families’ needs might alleviate the HCP [health care provider] burden and could enhance parental recall, especially when messaging is culturally relevant and personalized,” Ms. McGowan said.
Two independent experts agreed in interviews. Pediatricians must do their best to tailor advice to each particular family so that parents can engage in the conversation, said Lauren Fiechtner, MD, director of the center for pediatric nutrition at Mass General for Children, Boston. “As the authors suggest, we should seek to understand the cultural relevance of our recommendations and to understand the barriers our patient families might face in implementing our advice,” said Dr. Fiechtner, who is also an assistant professor at Harvard Medical School, also in Boston.
“Much of the instructions we as pediatricians give to parents must be repeated and reinforced,” said Rebecca S. Fisk, MD, a pediatrician at Lenox Hill Hospital, Northwell Health, in New York. Often, the doctor’s advice runs counter to what family and friends recommend, she pointed out. Some parents may believe that “the baby who starts solid food earlier will sleep through the night earlier or that eating in front of the TV relaxes the child or allows them to eat more,” Dr. Fisk explained. In her practice, a nurse goes over her instructions, answers questions, and provides specific examples and written information.
Sometimes, even that’s not enough, Dr. Fisk admitted. “I, myself, have fielded many repeated questions about feeding, when to start, how much to give, and so on, despite printed guidance given to parents at well-child visits.”
This study was funded by the U.S. Centers for Disease Control and Prevention. Ms. McGowan and study coauthors reported having no potential conflicts of interest. Dr. Fiechtner and Dr. Fisk disclosed having no potential conflicts of interest.
With a little help from your friends
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
Case: You are talking with one of your teenage patients, who has a history of significant suicidal ideation and an aborted attempt, and you ask her if there is someone she can talk with if she is feeling suicidal. “I call a friend,” she says. “That’s the only thing that works when I’m feeling bad.”
During difficult times, it is important to have a repertoire of coping skills to address stress, tension, frustration, anxiety, anger, sadness, and to help avoid dangerous behaviors. It is also important to have someone to talk to. For many youth, talking with friends is their preferred coping skill and contact when struggling with intense feelings.
This is hardly surprising. Peer relations are central to adolescent development. The ongoing individuation-separation process means that adolescents are peeling away from the family and into a community of their peers, where they figure out who they are through social interactions in subtle and complex ways. Adolescents are often profoundly immersed in the world of their peers; they often spend more time with their peers in educational and social settings than with their parents or other adults; and their connections with peers are often pleasurable, engaging, supportive, and intense. It is natural that they would want to communicate with their peers during stressful times.
At the same time, they may also want to avoid talking with adults. They may identify adult figures with authority, expectations, and control. So much adolescent psychic suffering and so many mental health crises involve shame, guilt, and fear, and are associated with romance, love, disappointment, and trauma – all of which may be difficult to share with parents and adult figures.
Adults also struggle with these kinds of conversations. Even benign attempts at comforting the youth (“Don’t worry, it’ll get better,” “Everyone feels this way sometimes”) can be seen as invalidating. And in stressful times, a difficult conversation can be ignited by the fuel of adult anxieties about the independence and autonomy of the child that is coming, which can make charged conversations all the more inflammatory.
Reaching out to peers during stressful times is therefore developmentally appropriate and often feels far more comfortable, validating, and sympathetic.
One of the most important things we can do is to help kids understand when, how, and why they can support each other – and when they cannot. Whether we like it or not, for many youth, peers are peer mental health counselors. They have shared vocabularies and can share experiences in the mental health care system. In addition to relying on their peers, a great many youth we work with also see themselves as supports to their peers, so it’s not just a one-way street.
So we talk with them frankly about when, how, and why talking with their friends can be an effective way of getting through a hard time and when, how, and why they need to reach out to an adult.
Recognizing how positive peer support can be, we ask them to identify problems with it. Kids often recognize the drawbacks of relying on their peers for support. They can see how it can be a burden to their friends. They often acknowledge that their friends may be experts in some aspects of their lives but not in others. For example, they can have shared stressors in school, can have similar understandings of the drama in their lives, and can relate to each other’s worlds, but will also not necessarily know what to do if a situation becomes dangerous.
The youth also tend to understand that the stakes in these conversations are high. We have seen peer groups suffer terribly when the youth have felt responsible – and even been the last preceding contact – in bad or even fatal outcomes.
We need to open up conversations about different forms of communication: when teens need understanding, compassion, patience; when they need a good understanding of local, cultural contexts, and a sense of support without anxieties and stressors; and when they need support and adult capacities and connections to solve problems. We can help them understand how to access people – both peers and adults – but also discuss responsibility: who you are responsible for, how you cannot be responsible alone for your friends’ mental health, how they cannot be responsible for yours, and who can be responsible for you.
To this end, we validate the importance of peers and ask more specifically when the adolescent thinks it is helpful to contact peers and when they think it would not be helpful. Having teens explain the difference may help them identify the right times to connect with peers or adults.
We can then talk about how to understand that there are different kinds of crisis: the kind where comfort, understanding, and support from friends can alleviate the crisis, and times when it is imperative to involve adults.
We can then identify which adults in their lives they can contact and how they would do so, both in terms of method of communication (texting an older sister, speaking in person with a parent, calling a therapist) and what they could say.
Then comes a more difficult step. We help them think about how to identify adults whom they do not know: how to contact a hotline or go to an emergency room or call 911. It is important not just to provide the numbers or address, but to help them run through a brief script so they know what to say and would be comfortable saying in their own words (but effectively saying, “I really need to speak with someone right now, I’m not safe.”)
Helping youth understand the advantages and disadvantages of reaching out to peers, and when and how to reach out to adults, can be a constructive conversation. It is a chance not only to speak with and hear about a youth’s life and relationships but also a chance to give them a stronger and safer support network.
Dr. Henderson is a psychiatrist who treats children and adolescents at NYU Langone Health, New York.
HPV vaccine effectiveness dependent on age at receipt
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The effectiveness of the human papillomavirus (HPV) vaccine against HPV types 16 and 18 is highly dependent on the age at which it is given. Prevalence rates have been shown to be significantly lower among girls who are vaccinated at the recommended ages of 9-12 years, compared with those who are vaccinated after their sexual debut, data from the National Health and Nutrition Examination Survey (NHANES) indicate.
“HPV vaccination does not have any therapeutic effect on HPV infections already acquired, which is more likely to explain the difference in prevalence between predebut versus postdebut recipients than a lower immune response [among older recipients],” lead study author Didem Egemen, PhD, National Cancer Institute, Rockville, Md., told this news organization in an email.
“Still, among older females, the immune response of the vaccine is likely to still be quite strong, and we would encourage vaccination [of female patients] if unvaccinated, as our paper showed that vaccination post debut will still reduce HPV 16/18 prevalence by half,” she added.
The research letter was published online in JAMA Network Open.
National sample evaluated
Using data from NHANES, a biennial, cross-sectional sample (cycles 2011 through 2018), the researchers identified female persons who were aged 26 years or younger in 2006, when HPV vaccination was introduced, and who were eligible for routine vaccination or “catch-up” vaccination (given between the ages of 13 and 26 years), as per recommendations from the Advisory Committee on Immunization Practices. The investigators then compared the prevalence of HPV types 16 and 18 among unvaccinated female patients, female patients who had been vaccinated prior to their sexual debut (predebut group), and those who had been vaccinated after their sexual debut (postdebut group).
They also estimated vaccine uptake among those who were eligible for routine vaccination, as well as the proportion of vaccinated female patients with respect to racial and ethnic subgroups.
In the overall cohort, the prevalence of HPV types 16 and 18 decreased by 6% (95% confidence interval, 4%-7%) in the unvaccinated group to 3% (95% CI, 1%-6%) in the postdebut group and to less than 1% (95% CI, <1%-1%) in the predebut group, Dr. Egemen and colleagues report.
In real percentages, the prevalence of HPV 16 and 18 was 89% lower in the predebut group (P < .001) but only 41% lower in the postdebut group (P = .29) compared with unvaccinated female patients. And compared with female patients who were vaccinated after their sexual debut, the prevalence of HPV 16 and 18 was reduced by 82% among those who had received the vaccine at the recommended ages of 9-12 years (P = .08).
In the current study, Dr. Egeman acknowledged that only 38% of ever-eligible female patients received the vaccine, although the prevalence increased to 56% when only female patients who were eligible for routine vaccination were taken into account. On the other hand, only 21% (95% CI, 14%-28%) of female patients eligible for routine vaccination received their first dose by age 12 years.
Indeed, the mean age on receipt of the first vaccination dose was 14.5 years (95% CI, 14.1-14.8 years), the authors note, and only 59% of girls received their first dose prior to their sexual debut. Additionally, among routine vaccination–eligible girls aged 12 years or younger in 2006, 33% were vaccinated before and 23% after their sexual debut, and the rest were not vaccinated.
Interestingly, differences in the age at which the HPV vaccine was received by race and ethnicity were negligible, the investigators point out.
Vaccination rates increasing
Asked to comment on the findings, Rebecca Perkins, MD, professor of obstetrics and gynecology at Boston University, Boston Medical Center, pointed out that the investigators evaluated data from 2011 to 2018. “We know that HPV vaccination rates have increased over that period and continue to increase,” she emphasized in an email to this news organization.
Physicians also know that more persons are being vaccinated between the ages of 9 and 12 than was the case at the beginning of this study. “This is good news,” she said, “as it means that more adolescents now in 2022 are benefiting fully from vaccination than they were in 2011,” she added.
At the same time, Dr. Perkins acknowledged that many persons are still missing out on the chance to receive the vaccine on time – which means they are missing out on the chance to prevent cancer.
“Making sure that all adolescents receive vaccination between the ages of 9 to 12 has the potential to prevent up to 40,000 cancers every year in the U.S., [including] the most common HPV-related cancers, such as cervical cancer in women and tongue and tonsillar cancer in men,” Dr. Perkins noted.
“Thus, it’s critical that doctors and parents get the message that you can’t vaccinate too early, only too late,” she emphasized.
Dr. Edgman and Dr. Perkins report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
CDC warns of early uptick in respiratory disease
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
FDA expands tenofovir alafenamide (Vemlidy) use to adolescents with chronic HBV
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
the drug’s manufacturer has announced.
The approval in the pediatric patient population was supported by 24-week data from a phase 2 clinical trial comparing treatment with tenofovir alafenamide (25 mg once daily) with placebo in 70 treatment-naive and treatment-experienced patients aged 12-18 years weighing at least 35 kg.
The study met its primary endpoint of percentage of patients with HBV DNA levels less than 20 IU/mL at 24 weeks of therapy, Gilead Sciences said in a press release.
Overall, 10 of 47 (21%) patients treated with tenofovir alafenamide achieved HBV DNA less than 20 IU/mL at 24 weeks, compared with 0 of 23 (0%) treated with placebo.
The rates of serum ALT normalization were higher with tenofovir alafenamide than with placebo (44% vs 0%).
The mean percent changes in bone mineral density (BMD) from baseline to 24 weeks were numerically similar for tenofovir alafenamide– and placebo-treated patients (2.4% and 1.9% for lumbar spine, and 1.5% and 1.9% for whole body, respectively).
The mean changes from baseline BMD z scores were –0.03 and –0.09 for lumbar spine, and –0.05 and –0.01 for whole body, for tenofovir alafenamide and placebo groups, respectively.
The FDA initially approved the nucleoside analog reverse transcriptase inhibitor in 2016 for adults with chronic HBV.
The drug was approved in Europe in 2017 for chronic HBV infection in adults and adolescents aged 12 years and older weighing at least 35 kg.
Tenofovir alafenamide carries a boxed warning citing risks for lactic acidosis/severe hepatomegaly with steatosis and posttreatment severe acute exacerbation of HBV.
A version of this article first appeared on Medscape.com.
Numbers of adolescents who vape within 5 minutes of waking jumps
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
Cutaneous and Subcutaneous Perineuriomas in 2 Pediatric Patients
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.
Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.
Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.
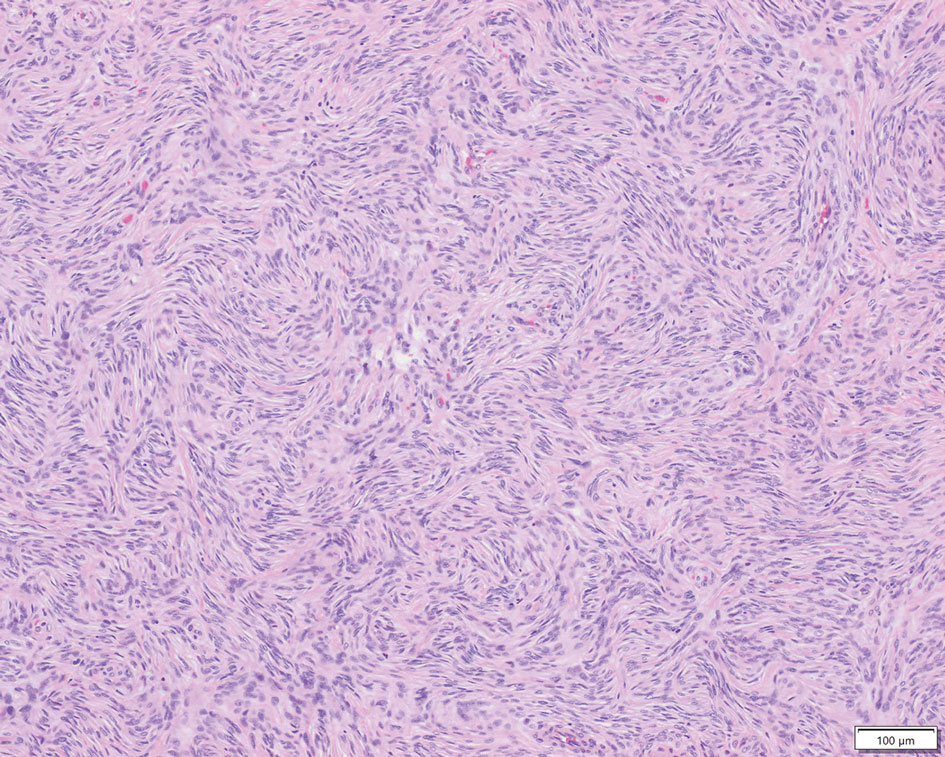
Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3
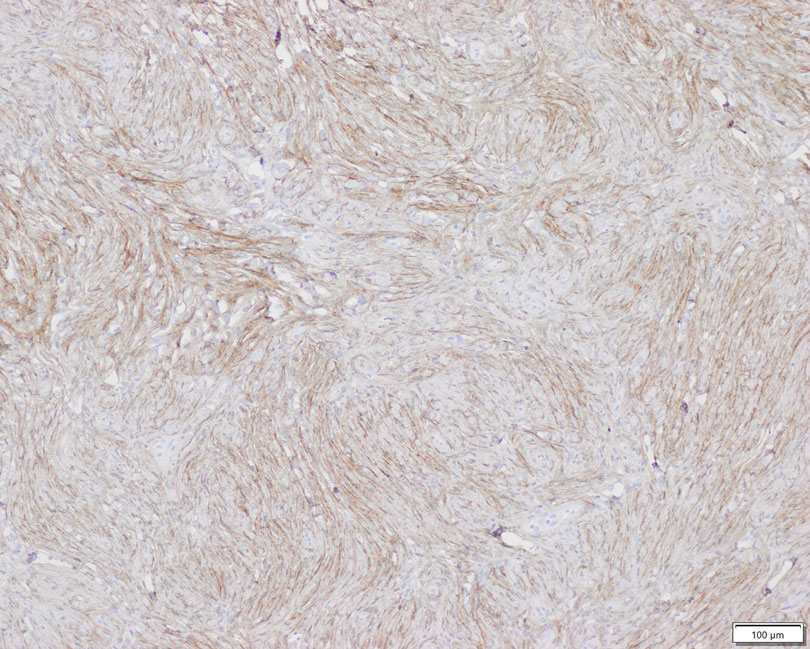
Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Practice Points
- Perineuriomas are rare benign peripheral nerve sheath tumors that most commonly occur in young to middle-aged adults but rarely can present in children.
- Immunohistochemically, perineuriomas show positive staining with epithelial membrane antigen, GLUT1, claudin-1, and frequently with CD34; they are negative for S-100 and glial fibrillary acidic protein.
- Perineuriomas should be considered in the differential diagnosis in children who present with a well-circumscribed nodular lesion in the subcutaneous tissue.