User login
2019 USPSTF update
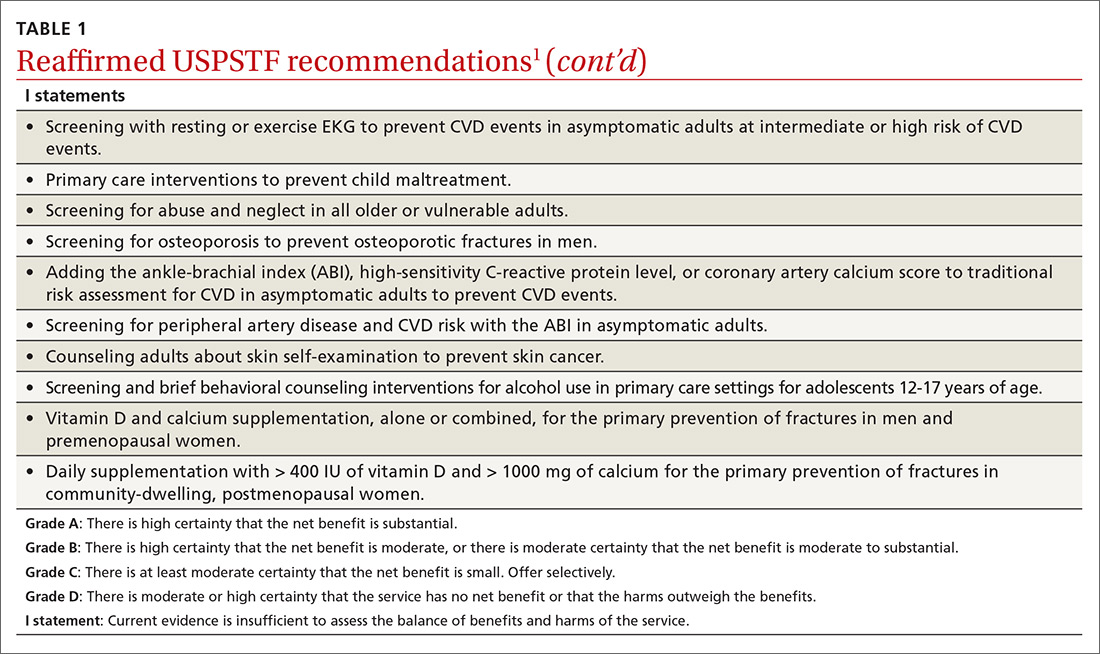
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
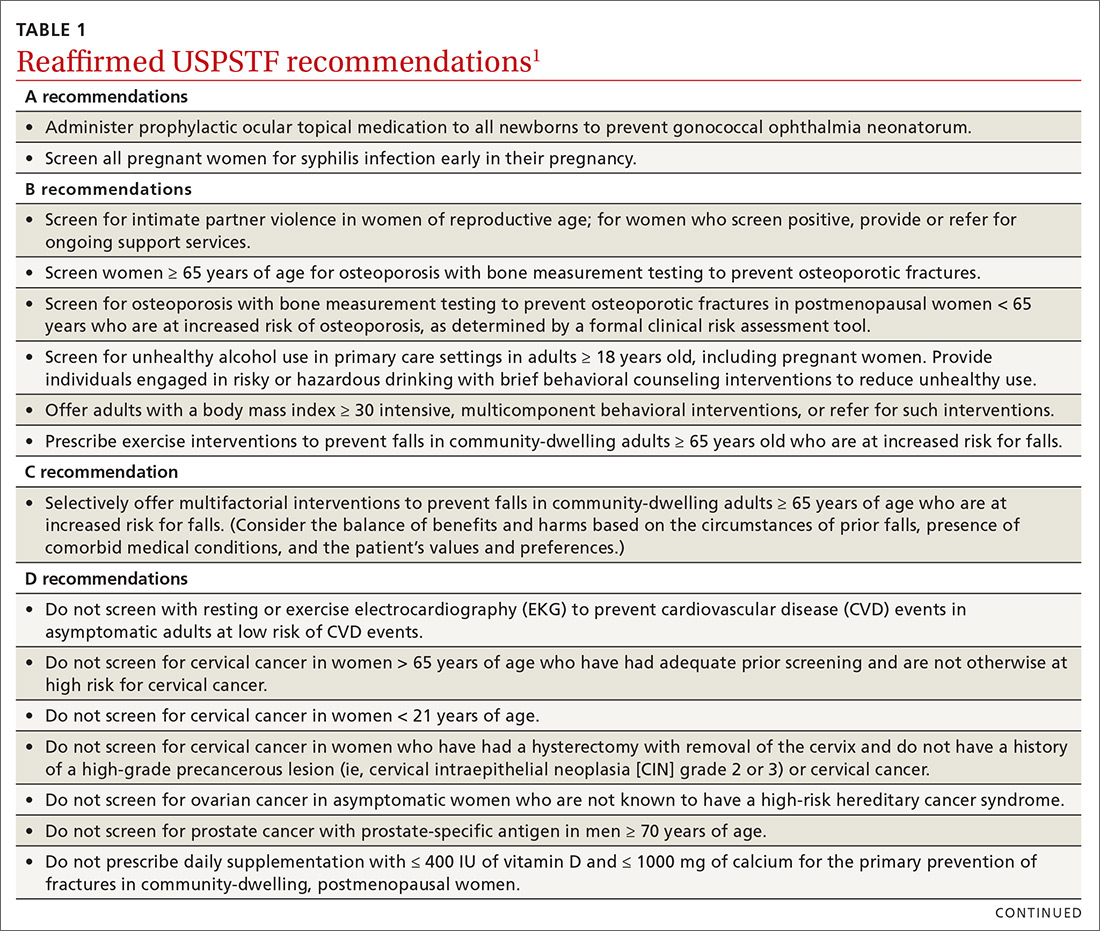
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
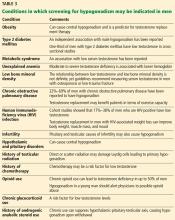
An obese 48-year-old man with progressive fatigue and decreased libido
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
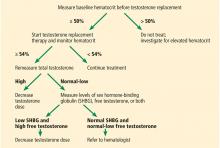
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
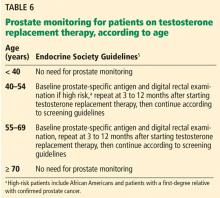
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
A 48-year-old man presents to his primary care physician because of progressively decreasing energy and gradual decline in both libido and erectile function for the past 18 months. He has noticed decreased morning erections as well. He rates his libido at 3 to 4 on a scale of 10 for the past 6 months. He also reports poor motivation, depressed mood, impaired concentration, and sleep disturbances. He reports no hair loss, headache, or dizziness, and no decrease in shaving frequency. Review of his systems is otherwise unremarkable.
He has had dyslipidemia for 3 years and is not known to have hypertension or diabetes. His medications include atorvastatin, vitamin E, and multivitamins.
He is married with 3 children and does not wish to have more. He works as a software engineer and leads a sedentary lifestyle. He is a nonsmoker and occasionally drinks alcohol on the weekends.
On physical examination, he is alert and oriented and appears well. His height is 5 feet 10 inches (178 cm), weight 230 lb (104 kg), and body mass index (BMI) 32.8 kg/m2. His blood pressure is 115/83 mm Hg and pulse rate is 82 beats per minute and regular. Findings on cardiovascular and pulmonary examination are normal. He has large fatty breasts but without palpable glandular tissue.
Genitourinary examination reveals normal hair distribution, a normal-sized penis, and slightly soft testes with testicular volume of 18–20 mL bilaterally.
His primary care physician suspects that he has low testosterone and orders some basic laboratory tests; the results are normal except for a low total testosterone level (Table 1).
FURTHER TESTING
1. Which of the following tests should his physician order next?
- Repeat total testosterone measurement
- Free testosterone measurement by commercial assay
- Calculated free testosterone
- Bioavailable testosterone measurement
- Serum inhibin B measurement
This patient presents with several nonspecific symptoms. But collectively they suggest testosterone deficiency (hypogonadism).
Together, erectile dysfunction, low libido, and decreased morning erections strongly suggest hypogonadism.2 Loss of body hair and decreased shaving frequency are specific symptoms of hypogonadism; however, they require years to develop.3 Gynecomastia can also occur due to loss of the inhibitory action of testosterone on breast growth and a relative increase in estradiol. This occurs more in primary hypogonadism, due to the increase in luteinizing hormone (LH), which stimulates the remaining Leydig cells to secrete estradiol rather than testosterone.4
To diagnose hypogonadism in men and to start treatment for it, current guidelines recommend that the patient should have clinical features as well as laboratory evidence of low testosterone.5,6
Measuring testosterone: Total, free, bound, and bioavailable
Testosterone, a steroid hormone, circulates in the serum either as free testosterone or bound to several plasma proteins, mainly sex-hormone binding globulin (SHBG) and albumin.
Total testosterone includes both the free and bound fractions, whereas bioavailable testosterone includes both free and the portion bound to albumin, which has low affinity and can dissociate and be used at the tissue level.11
Low levels of total testosterone do not necessarily reflect a hypogonadal state, as a man with altered SHBG levels or binding capabilities can have low total but normal free testosterone levels and no manifestations.12 Several conditions can alter the levels of SHBG, including obesity, diabetes, aging, thyroid dysfunction, and others.5,13
Because our patient is obese, his total testosterone level is not a reliable indicator of hypogonadism, and repeating its measurement will not add diagnostic value.
Therefore, an alternative measurement should be used to accurately reflect the testosterone levels. From a physiologic point of view, bioavailable testosterone is the active form of testosterone and is the most accurate to be measured in a patient with hypogonadism. Nevertheless, because of technical difficulties in its measurement and lack of evidence correlating bioavailable testosterone with the clinical picture of hypogonadism, it is recommended that the level of free testosterone be used.5
The gold standard for direct measurement of serum free testosterone is equilibrium dialysis, but this is expensive and time-consuming.14 Commercial assays for free testosterone exist but have been deemed unreliable.14,15 It is recommended that free testosterone be measured by equilibrium dialysis or calculated using equations based on total testosterone, SHBG, and albumin levels.5 These equations are reliable and give results very close to the values obtained by equilibrium dialysis.15 Therefore, in our patient, it would be suitable to calculate the free testosterone level next.
Serum levels of free testosterone vary according to several factors. Diurnal variation of testosterone has been established: levels are highest in the morning and decline throughout the day.16 Food decreases testosterone levels.17 In addition, there is considerable day-to-day variation.18 Therefore, at least 2 readings of fasting morning testosterone on 2 separate days are recommended for the diagnosis of hypogonadism.5
Inhibin B is a hormone produced by Sertoli cells in the testes in response to follicle-stimulating hormone (FSH) stimulation. In turn, it acts as negative feedback, together with testosterone, to inhibit FSH release from the pituitary. Inhibin B has been shown to reflect spermatogenesis in the testes and therefore fertility.19 Inhibin B levels were found to be low in patients with central hypogonadism, due to less FSH release; however, they did not correlate with testosterone levels.20
CASE RESUMED: CHARACTERIZING HIS HYPOGONADISM
The patient’s physician orders morning fasting total testosterone, SHBG, and albumin testing and calculates the free testosterone level, which yields a value of 3 ng/dL (reference range 4.5–17). This is confirmed by a repeat measurement, which yields a value of 2.9 ng/dL. Laboratory test results combined with his clinical presentation are consistent with hypogonadism.
2. What is the most appropriate next step?
- Measurement of serum LH and FSH
- Measurement of serum prolactin
- Scrotal ultrasonography
- Gonadotropin-releasing hormone (GnRH) stimulation test
- Semen analysis
After hypogonadism is diagnosed, it is important to distinguish if it is primary or central. This is achieved by measuring serum LH and FSH.5 All biotin supplements should be stopped at least 72 hours before measuring LH and FSH, as biotin can interfere with the assays, yielding false values.21
Secretion of FSH and LH from the anterior pituitary is under the influence of pulsatile release of GnRH from the hypothalamus. LH acts on Leydig cells in the testes to produce testosterone, whereas FSH acts on Sertoli cells, together with testosterone, to bring about spermatogenesis in the seminiferous tubules. Testosterone acts centrally as negative feedback to decrease the release of LH and FSH.
Primary hypogonadism occurs due to testicular failure, ie, the testes themselves fail to produce testosterone, leading to hypogonadism. The decrease in testosterone levels, together with inhibin B if Sertoli cells are damaged, lead to loss of negative feedback on the hypothalamus and pituitary, and therefore increased levels of LH and FSH. This is termed hypergonadotropic hypogonadism. Testicular failure may also result in impaired spermatogenesis and infertility due to destruction of testicular structures, in which case fertility cannot be restored.
Central hypogonadism occurs when the pituitary fails to produce LH and FSH (secondary hypogonadism) or when the hypothalamus fails to produce GnRH and subsequently the lack of secretion of LH and FSH from the pituitary (tertiary hypogonadism). The lack of LH will result in no stimulation of Leydig cells to produce testosterone, and therefore its deficiency. Serum hormone levels in central hypogonadism will reveal low testosterone, with either low or inappropriately normal gonadotropins (LH and FSH). This is termed hypogonadotropic hypogonadism. The lack of FSH, together with testosterone deficiency will also result in decreased spermatogenesis and therefore infertility. Testicular structures are preserved, however, and fertility can be restored with appropriate therapy, as discussed below.
Prolactin should be measured only if the patient has central hypogonadism. Its measurement is not warranted at this point in the patient’s workup. The implications of prolactin and its relationship to hypogonadism will be discussed later.
Although, this stepwise approach is not convenient for many patients, some physicians follow it because it is cost-effective, especially in those who are not insured. However, other physicians order FSH, LH, and sometimes prolactin with the confirmatory low testosterone measurement. Laboratories can also be instructed to wait to measure the pituitary hormones and to do so only if low testosterone is confirmed.
Varicocele, a possible cause of male infertility, can also impair Leydig cell function and cause low testosterone. In fact, surgical repair of varicocele has been demonstrated to increase serum testosterone.22 Scrotal ultrasonography is used to diagnose varicocele, but this also should be ordered at a later stage in the workup if primary hypogonadism is diagnosed.
The GnRH stimulation test is important for the diagnosis and evaluation of precocious or delayed puberty in children. In boys with delayed puberty, a poorer response to GnRH stimulation indicates central hypogonadism rather than constitutional delay.23 It has no role in the evaluation of postpubertal or adult-onset hypogonadism.
Semen analysis is important to evaluate fertility if the patient is interested in further procreation.5 Low testosterone levels may result in impaired spermatogenesis and therefore infertility. On the other hand, treatment with exogenous testosterone will also result in infertility, by feedback inhibition of LH and FSH and therefore inhibition of spermatogenesis. If the patient wishes to preserve fertility, treatment options other than testosterone should be considered; examples include clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin.23,24
Our patient has no desire to expand his family; therefore, a semen analysis and attempts to preserve spermatogenesis are not indicated.
CASE RESUMED: SEARCHING FOR CAUSES
His physician orders testing of serum LH and FSH, yielding the following values:
- LH 1.6 mIU/mL (reference range 1.8–12)
- FSH 1.9 mIU/mL (reference range 1.5–12.5).
The diagnosis of central hypogonadism is established.
3. Which investigation is the least appropriate in the further evaluation of this patient?
- Serum prolactin measurement
- Serum ferritin measurement
- Pituitary magnetic resonance imaging (MRI)
- Chromosomal karyotyping
The diagnosis of central hypogonadism warrants evaluation for possible causes. These are summarized in Table 4.
Serum free thyroxine and morning cortisol
Since this patient’s LH and FSH values are abnormal, it is important to evaluate the status of other anterior pituitary hormones. In patients with pituitary abnormalities, serum free T4 is a more reliable test for assessing thyroid function than thyroid-stimulating hormone (TSH), because of loss of the negative feedback of thyroid hormones on the diseased pituitary. In contrast, serum TSH is considered the best single thyroid test to assess primary thyroid dysfunction.
Other measurements include prolactin and morning cortisol (reflecting adrenocorticotropic hormone status).
Prolactin measurement
Prolactin measurement is important to evaluate for hyperprolactinemia, as this will lead to hypogonadism by inhibition of GnRH secretion.25 Different pathologic, pharmacologic, and physiologic conditions can result in hyperprolactinemia, including prolactinomas, other pituitary and hypothalamic lesions, primary hypothyroidism, and medications such as antipsychotics.25 Dopamine agonists are the mainstay treatment for hyperprolactinemia.
Ferritin measurement
Ferritin measurement is indicated to diagnose iron overload conditions such as hemochromatosis, which can result in primary hypogonadism via testicular damage or in secondary hypogonadism via pituitary damage.26
Pituitary MRI with contrast
Pituitary MRI with contrast is used to diagnose structural lesions of the pituitary or hypothalamus. This diagnostic modality is indicated for patients with pituitary dysfunction, including central hypogonadism, manifestations of a mass effect (headache, visual field defects), persistent hyperprolactinemia, and panhypopituitarism, among others. To improve the diagnostic yield of pituitary MRI, the Endocrine Society guidelines recommend it for men with serum total testosterone levels below 150 ng/dL.5 However, some clinicians have a lower threshold for ordering pituitary MRI for patients with central hypogonadism. Physician judgment and expertise should be exercised and the decision made on an individual basis.
Chromosomal karyotyping
Chromosomal karyotyping is not indicated in our patient. It is reserved for those with primary hypogonadism to diagnose Klinefelter syndrome, which has a karyotype of 47,XXY.
CASE RESUMED: MOSH SYNDROME
Our patient’s prolactin, free T4, morning cortisol, and ferritin levels are measured, yielding normal values. No abnormalities are seen on pituitary MRI. A clinical reevaluation is conducted, revealing no history of head trauma or head and neck radiation. The lack of an obvious cause in our patient’s clinical presentation and workup, together with his obesity (BMI 32.8 kg/m2) supports the diagnosis of obesity as the cause of his hypogonadism.
Obesity can be a cause of secondary hypogonadism, which has led to the term “MOSH” (male obesity-associated secondary hypogonadism) syndrome. In fact, a cross-sectional study has demonstrated that 40% of nondiabetic obese (BMI ≥ 30 kg/m2) men over age 45 have low serum free testosterone levels, compared with 26% for lean (BMI < 25 kg/m2) men.27 Moreover, obesity has been found to be a strong predictor of testosterone replacement therapy.28 Other studies have also found an inverse relationship between BMI and testosterone levels.29
Several mechanisms interact in the pathogenesis of MOSH syndrome. Adipose tissue possesses aromatase activity, which converts androgens into estrogens.30 Peripheral estrogen production can in turn exert feedback inhibition on pituitary gonadotropin secretion.31 In obese men, increased adipose tissue leads to increased aromatase activity and more estrogen, so more feedback inhibition on the pituitary and subsequently secondary hypogonadism.
Leptin, a hormone produced by adipocytes, is also increased in obesity, and was found to be inversely correlated with serum testosterone.32 Studies have demonstrated that leptin has an inhibitory effect on the enzymatic pathway that synthesizes testosterone in Leydig cells.33
Proinflammatory cytokines have also been implicated, as central obesity is associated with an increase in these cytokines, which in turn act negatively on the hypothalamus and impair GnRH release leading to lower testosterone.34,35
Treating obesity-related hypogonadism
In a pilot study,36 lifestyle attempts to reduce obesity were shown to improve hormonal levels. Bariatric surgery has also been demonstrated to be successful.37
Clomiphene citrate, a selective estrogen receptor modulator, increases endogenous testosterone secretion by inhibiting the negative feedback of estrogen on the hypothalamus and pituitary and thus increasing LH and FSH. It also preserves endogenous testosterone production, since it does not suppress the hypothalamic-pituitary-testicular axis.38 This made clomiphene citrate a potential treatment for men with central hypogonadism including those with MOSH.39
Nevertheless, there are no randomized trials to prove its safety and efficacy in the management of central hypogonadism.5 Regarding its use in men wishing to preserve fertility, most studies did not show improvement. However, a meta-analysis demonstrated statistically significant increased pregnancy rates in partners of men with idiopathic infertility if the men used 50 mg of clomiphene citrate daily.40
Testosterone deficiency can be a marker of metabolic syndrome, which needs to be managed more urgently than hypogonadism. A cross-sectional study found not only an association between metabolic syndrome and low serum testosterone, but also with each individual component of metabolic syndrome on its own, all of which need to be addressed.10
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.
CASE RESUMED: FOLLOW-UP
The patient presents 3 months later for follow-up. He reports significant improvement in his presenting symptoms including energy, libido, and erectile function. He also reports some improvement in his mood and concentration. He has lost 12 lb (5.4 kg) and is still trying to improve his diet and exercise program. He is compliant with his testosterone gel therapy.
His serum calculated free testosterone level is 7.8 ng/dL (4.5–17), and his hematocrit is 46%. The patient is instructed to continue his treatment and to return after 9 months for further follow-up.
TAKE-HOME POINTS
- Men with hypogonadism usually present with nonspecific manifestations, so clinicians should keep a high index of suspicion.
- Both clinical and biochemical evidence of hypogonadism should be present to diagnose and start treatment for it.
- Low levels of serum total testosterone do not necessarily reflect hypogonadism.
- The hormonal profile of central hypogonadism reveals low serum testosterone with low or inappropriately normal serum LH and FSH levels.
Obesity can cause central hypogonadism and should be suspected after pituitary and other systemic causes are excluded.
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
- Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab 2007; 92(11):4241–4247. doi:10.1210/jc.2007-1245
- Wu FCW, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363(2):123–135. doi:10.1056/NEJMoa0911101
- Arver S, Lehtihet M. Current guidelines for the diagnosis of testosterone deficiency. Front Horm Res 2009; 37:5–20. doi:10.1159/000175839
- Narula HS, Carlson HE. Gynaecomastia—pathophysiology, diagnosis and treatment. Nat Rev Endocrinol 2014; 10(11):684–698. doi:10.1038/nrendo.2014.139
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018; 103(5):1715–1744. doi:10.1210/jc.2018-00229
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 2018; 200(2):423–432. doi:10.1016/j.juro.2018.03.115
- Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med 2012; 18(2):112–117. doi:10.1097/MCP.0b013e32834feb37
- Atlantis E, Fahey P, Cochrane B, Wittert G, Smith S. Endogenous testosterone level and testosterone supplementation therapy in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open 2013; 3(8)pii:e003127. doi:10.1136/bmjopen-2013-003127
- Bawor M, Bami H, Dennis BB, et al. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend 2015; 149:1–9. doi:10.1016/j.drugalcdep.2015.01.038
- Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study). Aging Male 2011; 14(4):231–236. doi:10.3109/13685538.2011.597463
- Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev 2017; 38(4):302–324. doi:10.1210/er.2017-00025
- Antonio L, Wu FC, O’Neill TW, et al; European Male Ageing Study Study Group. Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab 2016; 101(7):2647–2657. doi:10.1210/jc.2015-4106
- Liu F, Shen X, Wang R, et al. Association of central obesity with sex hormone binding globulin: a cross-sectional study of 1166 Chinese men. Open Med (Wars) 2018; 13:196–202. doi:10.1515/med-2018-0030
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
- Halmenschlager G, Rhoden EL, Riedner CE. Calculated free testosterone and radioimmunoassay free testosterone as a predictor of subnormal levels of total testosterone. Int Urol Nephrol 2012; 44(3):673–681. doi:10.1007/s11255-011-0066-z
- Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab 2009; 94(3):907–913. doi:10.1210/jc.2008-1902
- Lehtihet M, Arver S, Bartuseviciene I, Pousette Å. S-testosterone decrease after a mixed meal in healthy men independent of SHBG and gonadotrophin levels. Andrologia 2012; 44(6):405–410. doi:10.1111/j.1439-0272.2012.01296.x
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007; 67(6):853–862. doi:10.1111/j.1365-2265.2007.02976.x
- Manzoor SM, Sattar A, Hashim R, et al. Serum inhibin B as a diagnostic marker of male infertility. J Ayub Med Coll Abbottabad 2012; 24(3–4):113–116. pmid:24669628
- Kolb BA, Stanczyk FZ, Sokol RZ. Serum inhibin B levels in males with gonadal dysfunction. Fertil Steril 2000; 74(2):234–238. pmid:10927037
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med 2016; 375(17):1698. doi:10.1056/NEJMc1611875
- Li F, Yue H, Yamaguchi K, et al. Effect of surgical repair on testosterone production in infertile men with varicocele: a meta-analysis. Int J Urol 2012; 19(2):149–154. doi:10.1111/j.1442-2042.2011.02890.x
- Crosnoe-Shipley LE, Elkelany OO, Rahnema CD, Kim ED. Treatment of hypogonadotropic male hypogonadism: case-based scenarios. World J Nephrol 2015; 4(2):245–253. doi:10.5527/wjn.v4.i2.245
- Majzoub A, Sabanegh E Jr. Testosterone replacement in the infertile man. Transl Androl Urol 2016; 5(6):859–865. doi:10.21037/tau.2016.08.03
- Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci 2013; 6(3):168–175. doi:10.4103/0974-1208.121400
- El Osta R, Grandpre N, Monnin N, Hubert J, Koscinski I. Hypogonadotropic hypogonadism in men with hereditary hemochromatosis. Basic Clin Androl 2017; 27:13. doi:10.1186/s12610-017-0057-8
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33(6):1186–1192. doi:10.2337/dc09-1649
- Jasuja GK, Bhasin S, Reisman JI, et al. Who gets testosterone? Patient characteristics associated with testosterone prescribing in the Veteran Affairs system: a cross-sectional study. J Gen Intern Med 2017; 32(3):304–311. doi:10.1007/s11606-016-3940-7
- Kaplan SA, Lee JY, O’Neill EA, Meehan AG, Kusek JW. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male 2013; 16(4):169–172. doi:10.3109/13685538.2013.844786
- Lee HK, Lee JK, Cho B. The role of androgen in the adipose tissue of males. World J Mens Health 2013; 31(2):136–140. doi:10.5534/wjmh.2013.31.2.136
- Raven G, De Jong FH, Kaufman JM, De Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 2006; 91(9):3324–3328. doi:10.1210/jc.2006-0462
- Hofny ER, Ali ME, Abdel-Hafez HZ, et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2010; 94(2):581–584. doi:10.1016/j.fertnstert.2009.03.085
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab 1999; 84(10):3673–3680. doi:10.1210/jcem.84.10.6082
- El-Wakkad A, Hassan NM, Sibaii H, El-Zayat SR. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61(2):682–687. doi:10.1016/j.cyto.2012.11.010
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 2005; 28(suppl proceedings 11):116–119. pmid:16760639
- de Lorenzo A, Noce A, Moriconi E, et al. MOSH syndrome (male obesity secondary hypogonadism): clinical assessment and possible therapeutic approaches. Nutrients 2018; 10(4)pii:E474. doi:10.3390/nu10040474
- Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Botella Carretero JI. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum Reprod Update 2017; 23(4):390–408. doi:10.1093/humupd/dmx012
- Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to testosterone therapy: a review. Sex Med Rev 2018; 6(1):106–113. doi:10.1016/j.sxmr.2017.09.004
- Soares AH, Horie NC, Chiang LAP, et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond) 2018; 42(5):953–963. doi:10.1038/s41366-018-0105-2
- Chua ME, Escusa KG, Luna S, Tapia LC, Dofitas B, Morales M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: a meta-analysis. Andrology 2013; 1(5):749–757. doi:10.1111/j.2047-2927.2013.00107.x
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet 1977; 2(8032):262–263. pmid:69876
- Fernández-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2010; 95(6):2560–2575. doi:10.1210/jc.2009-2575
- Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 2008; 93(3):914–919. doi:10.1210/jc.2007-1692
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev 2018; 6(1):77–85. doi:10.1016/j.sxmr.2017.04.001
- Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev 2015; 3(2):101–112. doi:10.1002/smrj.43
- Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 2014; 69(6):725–735. doi:10.1093/gerona/glt154
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med 2017; 177(4):480–490. doi:10.1001/jamainternmed.2016.9540
- Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015; 193(2):403–413. doi:10.1016/j.juro.2014.07.123
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005; 65(3):549–553. doi:10.1016/j.urology.2004.10.064
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96(10):3007–3019. doi:10.1210/jc.2011-1137
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med 2013; 11:108. doi:10.1186/1741-7015-11-108
Nasal testosterone gel preserves fertility in men with hypogonadism
NEW ORLEANS – according to an interim analysis of a postapproval clinical trial.
Testosterone nasal gel is a shorter-acting formulation of testosterone that “can mimic normal physiology,” said John Masterson, MD, a urology resident at the University of Miami. The 4.5% formulation, which was approved by the Food and Drug Administration in 2014 and is marketed as Natesto, delivers 11 mg of testosterone per dose and is dosed three times daily for testosterone replacement therapy.
The negative feedback exerted by other exogenous testosterone formulations on the hypothalamic-pituitary-gonadal (HPG) axis is known to inhibit spermatogenesis, Dr. Masterson said in a video interview at the annual meeting of the Endocrine Society.
Dr. Masterson, senior author Ranjith Ramasamy, MD, and their colleagues hypothesized that the shorter duration of action of testosterone in the nasal gel formulation would result in some conservation of gonadotropin-releasing hormone (GnRH) pulsatility, less inhibition of the HPG axis, and preservation of spermatogenesis.

In the same interview, Dr. Ramasamy, director of reproductive urology at the University of Miami, noted that “the levels of testosterone in men rise about an hour or 2 after administration [of the gel] and seem to drop off about 2 to 4 hours after the peak.” That is closer to normal physiology than other delivery systems in which “the levels of testosterone are pretty high during the day and therefore could lead to some of the side effects that we see with testosterone.”
The phase 4 prospective study enrolled 56 men aged between 18 and 55 years who had low levels of testosterone (baseline mean, 233.97 ng/dL). The mean age was 37 years.
“There are mostly younger men in our study ... and they’re usually coming in with one or two hypogonadal complaints – lack of energy, fatigue, some with erectile dysfunction,” Dr. Masterson explained in the interview. Improvement was seen in those realms, but the differences didn’t reach statistical significance, because baseline quality of life was already fairly high for these otherwise healthy men. “What we can say is that on the drug, quality of life certainly did not get worse,” he said.
At baseline, the mean luteinizing hormone (LH) level was 3.66 IU/mL, and the mean follicle stimulating hormone (FSH) level was 4.01 IU/mL.
Men were eligible to participate if they had two morning blood samples with age-adjusted low testosterone levels, and a total motile sperm count more than 5 million/ejaculation. Participants received 11-mg nasal testosterone gel three times daily for 6 months.
By 1 month into the study, 43 patients had a median testosterone level of 573 ng/dL. Fifteen patients have thus far completed all 6 months of the study and they had a median testosterone level of 604 ng/dL.
At baseline, sperm concentration was a mean 21.32 million/mL with 50% motility, and a total sperm count of 32.23 million/ejaculation. Sperm concentration at 6 months was unchanged at a median 21 million/mL.
Motility was preserved at a median 51.5% after 6 months of therapy, a statistically insignificant difference from 54% motility at baseline. Total motile sperm count decreased from a median 29.3 million/ejaculation at baseline to 19.5 million/ejaculation, a difference that didn’t reach statistical significance.
“What that means is that this nasal testosterone gel [could] be used in men who have low testosterone and are interested in preserving fertility,” said Dr. Masterson.
Dr. Ramasamy said that they’re seeing early confirmation of their initial hypothesis about the nasal gel formulation in this interim analysis. “Because it’s short acting, we believe some of the GnRH pulses and the LH and FSH that are released by the pituitary gland are still maintained, compared with the other forms of testosterone therapy, which can cause complete suppression of the [HPG] axis.”
In discussion with attendees at the poster session at which the research was featured, Dr. Masterson explained that quality-of-life measures were also collected as part of the study and will be presented separately. In addition to information about erectile function, men were asked about libido – a more complex phenomenon than erectile function alone – and early analysis showed a robust response, he said.
He acknowledged that it is not known whether craniofacial circulation facilitates testosterone transport to the brain when the nasal gel testosterone formulation is used, but that it is mechanistically plausible.
Dr. Masterson added that, practically speaking, patients should be aware that the formulation is a gel. “It sort of has to be painted on” the nasal septum within the nostrils, he said.
Aytu BioScience, which markets Natesto, partially supported the study. Dr Masterson reported no disclosures or conflicts of interest.
NEW ORLEANS – according to an interim analysis of a postapproval clinical trial.
Testosterone nasal gel is a shorter-acting formulation of testosterone that “can mimic normal physiology,” said John Masterson, MD, a urology resident at the University of Miami. The 4.5% formulation, which was approved by the Food and Drug Administration in 2014 and is marketed as Natesto, delivers 11 mg of testosterone per dose and is dosed three times daily for testosterone replacement therapy.
The negative feedback exerted by other exogenous testosterone formulations on the hypothalamic-pituitary-gonadal (HPG) axis is known to inhibit spermatogenesis, Dr. Masterson said in a video interview at the annual meeting of the Endocrine Society.
Dr. Masterson, senior author Ranjith Ramasamy, MD, and their colleagues hypothesized that the shorter duration of action of testosterone in the nasal gel formulation would result in some conservation of gonadotropin-releasing hormone (GnRH) pulsatility, less inhibition of the HPG axis, and preservation of spermatogenesis.

In the same interview, Dr. Ramasamy, director of reproductive urology at the University of Miami, noted that “the levels of testosterone in men rise about an hour or 2 after administration [of the gel] and seem to drop off about 2 to 4 hours after the peak.” That is closer to normal physiology than other delivery systems in which “the levels of testosterone are pretty high during the day and therefore could lead to some of the side effects that we see with testosterone.”
The phase 4 prospective study enrolled 56 men aged between 18 and 55 years who had low levels of testosterone (baseline mean, 233.97 ng/dL). The mean age was 37 years.
“There are mostly younger men in our study ... and they’re usually coming in with one or two hypogonadal complaints – lack of energy, fatigue, some with erectile dysfunction,” Dr. Masterson explained in the interview. Improvement was seen in those realms, but the differences didn’t reach statistical significance, because baseline quality of life was already fairly high for these otherwise healthy men. “What we can say is that on the drug, quality of life certainly did not get worse,” he said.
At baseline, the mean luteinizing hormone (LH) level was 3.66 IU/mL, and the mean follicle stimulating hormone (FSH) level was 4.01 IU/mL.
Men were eligible to participate if they had two morning blood samples with age-adjusted low testosterone levels, and a total motile sperm count more than 5 million/ejaculation. Participants received 11-mg nasal testosterone gel three times daily for 6 months.
By 1 month into the study, 43 patients had a median testosterone level of 573 ng/dL. Fifteen patients have thus far completed all 6 months of the study and they had a median testosterone level of 604 ng/dL.
At baseline, sperm concentration was a mean 21.32 million/mL with 50% motility, and a total sperm count of 32.23 million/ejaculation. Sperm concentration at 6 months was unchanged at a median 21 million/mL.
Motility was preserved at a median 51.5% after 6 months of therapy, a statistically insignificant difference from 54% motility at baseline. Total motile sperm count decreased from a median 29.3 million/ejaculation at baseline to 19.5 million/ejaculation, a difference that didn’t reach statistical significance.
“What that means is that this nasal testosterone gel [could] be used in men who have low testosterone and are interested in preserving fertility,” said Dr. Masterson.
Dr. Ramasamy said that they’re seeing early confirmation of their initial hypothesis about the nasal gel formulation in this interim analysis. “Because it’s short acting, we believe some of the GnRH pulses and the LH and FSH that are released by the pituitary gland are still maintained, compared with the other forms of testosterone therapy, which can cause complete suppression of the [HPG] axis.”
In discussion with attendees at the poster session at which the research was featured, Dr. Masterson explained that quality-of-life measures were also collected as part of the study and will be presented separately. In addition to information about erectile function, men were asked about libido – a more complex phenomenon than erectile function alone – and early analysis showed a robust response, he said.
He acknowledged that it is not known whether craniofacial circulation facilitates testosterone transport to the brain when the nasal gel testosterone formulation is used, but that it is mechanistically plausible.
Dr. Masterson added that, practically speaking, patients should be aware that the formulation is a gel. “It sort of has to be painted on” the nasal septum within the nostrils, he said.
Aytu BioScience, which markets Natesto, partially supported the study. Dr Masterson reported no disclosures or conflicts of interest.
NEW ORLEANS – according to an interim analysis of a postapproval clinical trial.
Testosterone nasal gel is a shorter-acting formulation of testosterone that “can mimic normal physiology,” said John Masterson, MD, a urology resident at the University of Miami. The 4.5% formulation, which was approved by the Food and Drug Administration in 2014 and is marketed as Natesto, delivers 11 mg of testosterone per dose and is dosed three times daily for testosterone replacement therapy.
The negative feedback exerted by other exogenous testosterone formulations on the hypothalamic-pituitary-gonadal (HPG) axis is known to inhibit spermatogenesis, Dr. Masterson said in a video interview at the annual meeting of the Endocrine Society.
Dr. Masterson, senior author Ranjith Ramasamy, MD, and their colleagues hypothesized that the shorter duration of action of testosterone in the nasal gel formulation would result in some conservation of gonadotropin-releasing hormone (GnRH) pulsatility, less inhibition of the HPG axis, and preservation of spermatogenesis.

In the same interview, Dr. Ramasamy, director of reproductive urology at the University of Miami, noted that “the levels of testosterone in men rise about an hour or 2 after administration [of the gel] and seem to drop off about 2 to 4 hours after the peak.” That is closer to normal physiology than other delivery systems in which “the levels of testosterone are pretty high during the day and therefore could lead to some of the side effects that we see with testosterone.”
The phase 4 prospective study enrolled 56 men aged between 18 and 55 years who had low levels of testosterone (baseline mean, 233.97 ng/dL). The mean age was 37 years.
“There are mostly younger men in our study ... and they’re usually coming in with one or two hypogonadal complaints – lack of energy, fatigue, some with erectile dysfunction,” Dr. Masterson explained in the interview. Improvement was seen in those realms, but the differences didn’t reach statistical significance, because baseline quality of life was already fairly high for these otherwise healthy men. “What we can say is that on the drug, quality of life certainly did not get worse,” he said.
At baseline, the mean luteinizing hormone (LH) level was 3.66 IU/mL, and the mean follicle stimulating hormone (FSH) level was 4.01 IU/mL.
Men were eligible to participate if they had two morning blood samples with age-adjusted low testosterone levels, and a total motile sperm count more than 5 million/ejaculation. Participants received 11-mg nasal testosterone gel three times daily for 6 months.
By 1 month into the study, 43 patients had a median testosterone level of 573 ng/dL. Fifteen patients have thus far completed all 6 months of the study and they had a median testosterone level of 604 ng/dL.
At baseline, sperm concentration was a mean 21.32 million/mL with 50% motility, and a total sperm count of 32.23 million/ejaculation. Sperm concentration at 6 months was unchanged at a median 21 million/mL.
Motility was preserved at a median 51.5% after 6 months of therapy, a statistically insignificant difference from 54% motility at baseline. Total motile sperm count decreased from a median 29.3 million/ejaculation at baseline to 19.5 million/ejaculation, a difference that didn’t reach statistical significance.
“What that means is that this nasal testosterone gel [could] be used in men who have low testosterone and are interested in preserving fertility,” said Dr. Masterson.
Dr. Ramasamy said that they’re seeing early confirmation of their initial hypothesis about the nasal gel formulation in this interim analysis. “Because it’s short acting, we believe some of the GnRH pulses and the LH and FSH that are released by the pituitary gland are still maintained, compared with the other forms of testosterone therapy, which can cause complete suppression of the [HPG] axis.”
In discussion with attendees at the poster session at which the research was featured, Dr. Masterson explained that quality-of-life measures were also collected as part of the study and will be presented separately. In addition to information about erectile function, men were asked about libido – a more complex phenomenon than erectile function alone – and early analysis showed a robust response, he said.
He acknowledged that it is not known whether craniofacial circulation facilitates testosterone transport to the brain when the nasal gel testosterone formulation is used, but that it is mechanistically plausible.
Dr. Masterson added that, practically speaking, patients should be aware that the formulation is a gel. “It sort of has to be painted on” the nasal septum within the nostrils, he said.
Aytu BioScience, which markets Natesto, partially supported the study. Dr Masterson reported no disclosures or conflicts of interest.
REPORTING FROM ENDO 2019
High levels of estradiol in older men may be associated with young biological age
NEW ORLEANS – In a large community-based study that evaluated sex hormone levels in older men, suggesting that sex hormones influence the aging process.
“There was a large effect size, comparable with being 2 or 3 years younger for those with relatively high levels of estradiol, compared with those with lower levels of the hormone,” said Bu Yeap, MBBS, PhD, professor of medicine, University of Western Australia Medical School, Perth, who reported the results at the annual meeting of the Endocrine Society.
In a video interview conducted at the meeting, Dr. Yeap explained the basis of the study, which is the variety of evidence showing that decline in sex hormones correlates with higher rates of age-related disease processes. For example, increasing rates of cardiovascular disease, dementia, and mortality in men all correlate with declining levels of testosterone.
In the study, 2,913 men between the ages of 70 and 89 years and living in the community were recruited. The average age of the men was 77 years. Serum levels of testosterone, dihydrotestosterone, and estradiol were measured. Telomere length was calculated with a polymerase chain reaction test.
Serum levels of testosterone and dihydrotestosterone did not correlate with telomere length, but incremental increases in serum estradiol levels were associated with incremental increases in telomere length.
“Telomeres are both a mediator and a biomarker for biological aging,” according to Dr. Yeap, who added that the telomeres protect chromosomes from degradation. As the telomeres shorten, cell senescence is increased along with an array of age-related diseases.
The next step for researchers is to evaluate whether administering exogenous sex hormones can favorably alter telomere length. If such an effect is demonstrated, then it could provide a step toward understanding how to slow the aging process, he said.
Dr Yeap and his colleagues reported no disclosures or financial conflicts of interest.

NEW ORLEANS – In a large community-based study that evaluated sex hormone levels in older men, suggesting that sex hormones influence the aging process.
“There was a large effect size, comparable with being 2 or 3 years younger for those with relatively high levels of estradiol, compared with those with lower levels of the hormone,” said Bu Yeap, MBBS, PhD, professor of medicine, University of Western Australia Medical School, Perth, who reported the results at the annual meeting of the Endocrine Society.
In a video interview conducted at the meeting, Dr. Yeap explained the basis of the study, which is the variety of evidence showing that decline in sex hormones correlates with higher rates of age-related disease processes. For example, increasing rates of cardiovascular disease, dementia, and mortality in men all correlate with declining levels of testosterone.
In the study, 2,913 men between the ages of 70 and 89 years and living in the community were recruited. The average age of the men was 77 years. Serum levels of testosterone, dihydrotestosterone, and estradiol were measured. Telomere length was calculated with a polymerase chain reaction test.
Serum levels of testosterone and dihydrotestosterone did not correlate with telomere length, but incremental increases in serum estradiol levels were associated with incremental increases in telomere length.
“Telomeres are both a mediator and a biomarker for biological aging,” according to Dr. Yeap, who added that the telomeres protect chromosomes from degradation. As the telomeres shorten, cell senescence is increased along with an array of age-related diseases.
The next step for researchers is to evaluate whether administering exogenous sex hormones can favorably alter telomere length. If such an effect is demonstrated, then it could provide a step toward understanding how to slow the aging process, he said.
Dr Yeap and his colleagues reported no disclosures or financial conflicts of interest.

NEW ORLEANS – In a large community-based study that evaluated sex hormone levels in older men, suggesting that sex hormones influence the aging process.
“There was a large effect size, comparable with being 2 or 3 years younger for those with relatively high levels of estradiol, compared with those with lower levels of the hormone,” said Bu Yeap, MBBS, PhD, professor of medicine, University of Western Australia Medical School, Perth, who reported the results at the annual meeting of the Endocrine Society.
In a video interview conducted at the meeting, Dr. Yeap explained the basis of the study, which is the variety of evidence showing that decline in sex hormones correlates with higher rates of age-related disease processes. For example, increasing rates of cardiovascular disease, dementia, and mortality in men all correlate with declining levels of testosterone.
In the study, 2,913 men between the ages of 70 and 89 years and living in the community were recruited. The average age of the men was 77 years. Serum levels of testosterone, dihydrotestosterone, and estradiol were measured. Telomere length was calculated with a polymerase chain reaction test.
Serum levels of testosterone and dihydrotestosterone did not correlate with telomere length, but incremental increases in serum estradiol levels were associated with incremental increases in telomere length.
“Telomeres are both a mediator and a biomarker for biological aging,” according to Dr. Yeap, who added that the telomeres protect chromosomes from degradation. As the telomeres shorten, cell senescence is increased along with an array of age-related diseases.
The next step for researchers is to evaluate whether administering exogenous sex hormones can favorably alter telomere length. If such an effect is demonstrated, then it could provide a step toward understanding how to slow the aging process, he said.
Dr Yeap and his colleagues reported no disclosures or financial conflicts of interest.

REPORTING FROM ENDO 2019
It’s time to start asking all patients about intimate partner violence
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.

What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
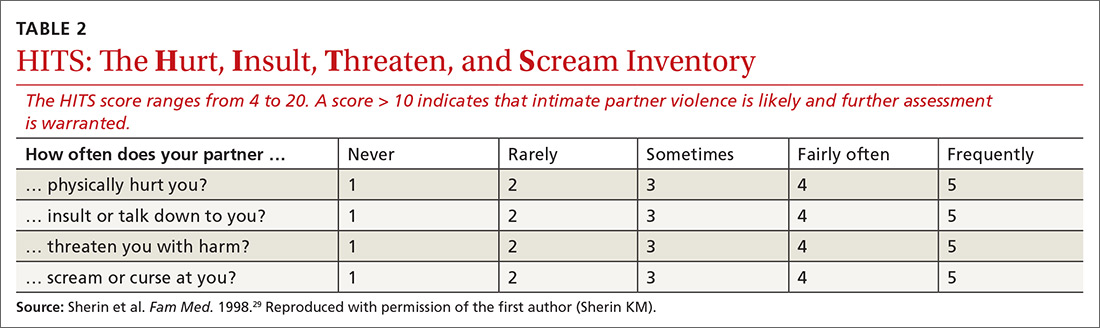
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.

Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
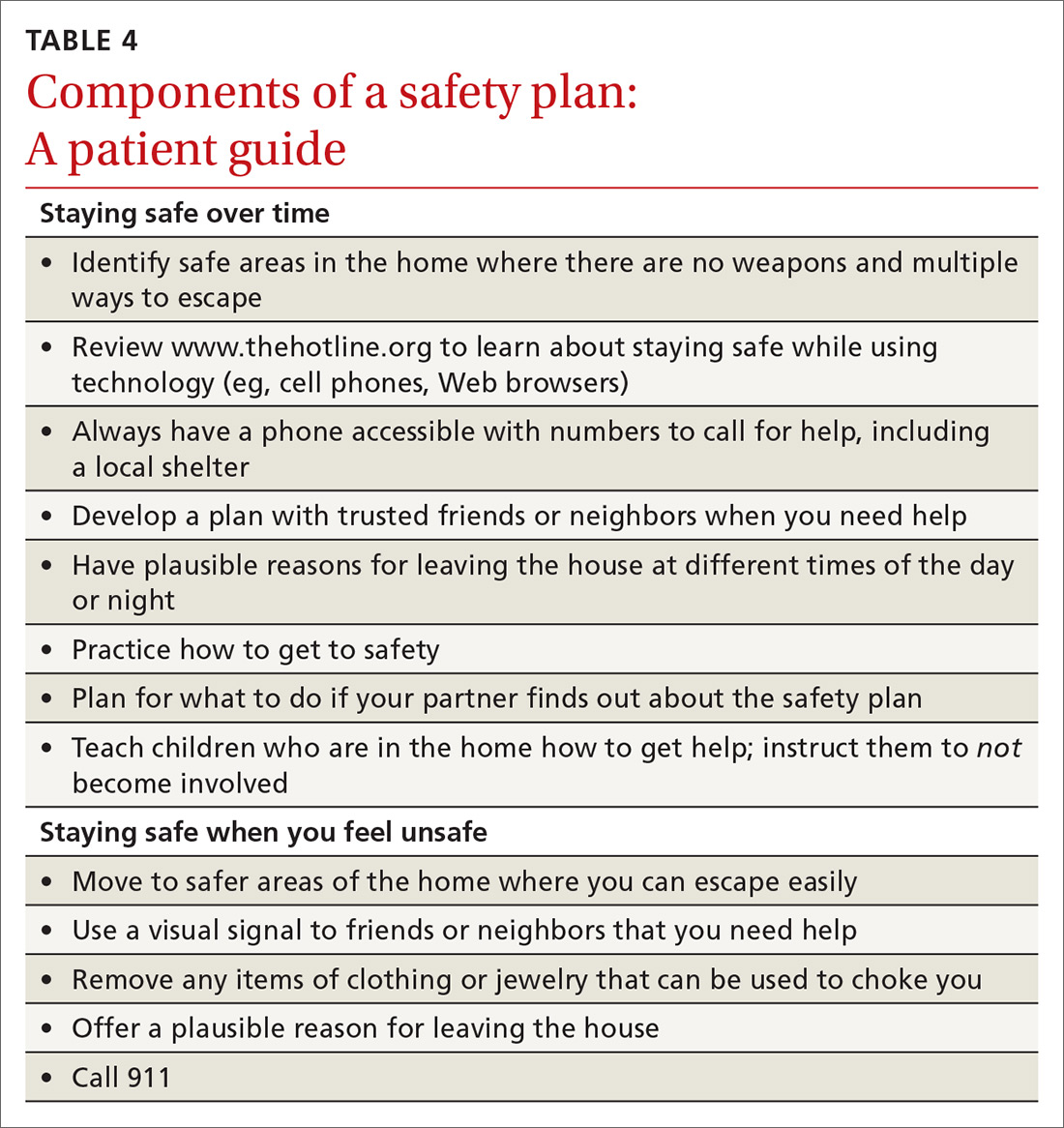
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.

What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16

Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30

What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.

Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).

Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.

What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16

Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30

What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.

Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).

Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; [email protected].
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
PRACTICE RECOMMENDATIONS
› Perform annual screening for intimate partner violence of all female patients of childbearing age; strongly consider a pilot program of universal screening (all male and female patients, across the lifespan). B
› Establish a protocol for intimate partner violence screening and referral—possibly the most effective means of identifying intimate partner violence at early and severe stages. B
› Collaborate with the patient in the safety planning and referral process; benefits include improved likelihood that the patient will adhere to a safety plan and follow through with the referral. B
› Utilize online resources to 1) ease the process of establishing relationships with local intimate partner violence referrals and 2) facilitate warm handoffs to increase the likelihood of patient engagement. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Personalizing guideline-driven cancer screening
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
Sperm quality linked to some recurrent pregnancy loss
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
NEW ORLEANS – Couples with recurrent pregnancy loss (RPL) might benefit from seminal parameter testing, based on results from a study presented at the annual meeting of the Endocrine Society.
Sperm quality was more likely to be impaired in the 50 male partners of women with recurrent pregnancy loss than it was in a control group of 63 similar-age men, reported Anastasia P. Dimakopoulou, MBBS, a clinical research fellow at Imperial College, London.
Recurrent pregnancy loss is defined as three pregnancy losses before 20 weeks of gestation.
The reported prevalence of RPL has been estimated at less than 2% in couples attempting pregnancy. About half of those cases are considered to be idiopathic, she said.
Sperm DNA plays a role in placentation, and previous study findings have shown that men in RPL couples are more likely to have higher rates of DNA fragmentation in their sperm. Male partners are not routinely evaluated when seeking a cause for RPL, however.
In this study, 50 men from RPL couples and 63 control men were screened for factors known to affect sperm quality, such as previous testicular surgery, sexually transmitted diseases, alcohol intake, and smoking. In patients and controls, semen reactive oxidative stress, a novel biomarker of sperm function, was measured with a chemiluminescence luminol assay.
The proportion of men with abnormal sperm morphology, although modest in both groups, was significantly more common in men from RPL couples than in controls (4.5% vs. 3.4%, respectively; P less than .001). In addition, the mean reactive oxidative stress levels were four times greater in the RPL men (9.3 vs. 2.3 relative light units/sec per 106 sperm; P less than .05).
Consistent with the higher median reactive oxidative stress levels, the median DNA fragmentation index, which is likely to be linked to increased reactive oxidative stress, was more than twice as high in the RPL men, compared with the controls (16.3 vs. 7.4; P less than .0001).
In addition, the sperm volume was significantly lower in men from the RPL couples, compared with controls. The levels of morning serum testosterone also were lower in men from RPL couples, but the difference did not reach significance relative to controls.
There has been relatively little attention directed toward the male partner in the evaluation and treatment of RPL, but that should change, according to Dr. Dimakopoulou. She said data encourage a new direction of study, including the effort to look for treatable causes of RPL in the male partner.
“By pursuing drugs that stop sperm DNA damage, it may be possible to identify new therapeutic pathways for couples who experience RPL,” Dr. Dimakopoulou maintained. However, even in advance of targeted therapies, she suggested these data encourage investigation of male partners in couples with RPL. Although evidence of reactive oxidative stress may not define a cause, it broadens the scope of investigation and might have value when counseling patients.
Dr. Dimakopoulou reported no relevant financial relationships to disclose.
SOURCE: Dimakopoulou AP et al. ENDO 2019, Session OR18-5.
REPORTING FROM ENDO 2019
First RCT with aromatase inhibitor for male hypogonadism shows promise
NEW ORLEANS – In obese men with hypogonadotropic hypogonadism, an experimental aromatase inhibitor (Ai) normalized testosterone, seemed to improve sperm function, and was not associated with any significant adverse safety signals, according to findings presented at the annual meeting of the Endocrine Society.
Unlike testosterone therapy, “leflutrozole was associated with positive effects on semen fertility parameters, such as semen volume and concentration,” reported Thomas Hugh Jones, MD, FRCP, of the Centre for Diabetes and Endocrinology, Barnsley Hospital NHS Foundation Trust, and the department of oncology and metabolism, University of Sheffield Medical School, both in England.
Although the impact of the experimental aromatase inhibitor leflutrozole on parameters of semen function was an exploratory analysis in this multicenter, placebo-controlled study, it is particularly noteworthy because it addresses one of the weaknesses of testosterone replacement, which is often the first choice in treating hypogonadism, Dr. Jones said.
“Testosterone replacement frequently results in negative feedback suppression of follicle stimulating hormone and luteinizing hormone so that along with lower sperm counts, these men have significant problems with fertility,” he explained.
In this phase 2, double-blind, randomized trial, 271 men with hypogonadism were randomized to placebo or to leflutrozole in a dose of 0.1 mg, 0.3 mg, or 1.0 mg taken orally once weekly. All patients had a serum testosterone level of less than 300 ng/dL at entry. The median body mass index was 38 kg/m2, and the average age was 50.9 years.
Results were presented after 24 weeks of treatment, but the blinded study continued for an additional 24 weeks.
Normalization of testosterone, defined as a level between 300 and 1,000 ng/dL, was the primary endpoint. The mean testosterone levels were essentially unchanged in the placebo group during the first 24-week phase of the study, but they climbed to means of 458 ng/dL in the 0.1-mg group, 512 ng/dL in the 0.3-mg group, and 586 ng/dL in the 1.0-mg group.
“Overall, 75% were in the normal range, but it reached 90% in the groups taking the two higher doses,” Dr. Jones reported. Testosterone levels never exceeded 1,500 ng/d.
For the effect on FSH and LH, which were secondary endpoints, both were increased in a dose-dependent manner at 12 and 20 weeks (P less than .001 for the highest dose relative to placebo).
For the semen analysis, also conducted at 12 and 20 weeks, all three doses were associated with a numerical increase in sperm count relative to placebo, with the highest dose achieving significant improvements in semen volume (P = .006), semen concentration (P = .01), and total motile sperm count (P = .03), Dr. Jones reported.
“The 48-week analysis has just been completed, and these types of improvements have been persistent,” Dr. Jones said in reference to the increase in sex hormones as well as measures of sperm function. Although he did not present the 48-week results in detail, he disclosed that this longer follow-up also supported favorable effects on bone density, which is among several prespecified substudies being performed.
Leflutrozole, which is chemically related to letrozole, has been well tolerated at the doses studied. An increase in hematocrit consistent with the rise in testosterone was observed, but Dr. Jones reported that there are no significant safety issues identified so far.
Aromatase inhibitors have been used off label to treat hypogonadism, but this is the first randomized controlled trial for this indication, Dr. Jones said.
Although leflutrozole was used in this study at far lower doses than the aromatase inhibitors currently available for treatment of breast cancer, it might provide an advance for a challenging condition, according to Dr. Jones. He did not speculate when a phase 3 registration trial might start, but he did say that the promise of this agent warrants further development.
Dr. Jones reported a financial relationship with Mereo BioPharma, the sponsor of this trial.
Source: Jones et al. ENDO 2019, Session OR18-4.
NEW ORLEANS – In obese men with hypogonadotropic hypogonadism, an experimental aromatase inhibitor (Ai) normalized testosterone, seemed to improve sperm function, and was not associated with any significant adverse safety signals, according to findings presented at the annual meeting of the Endocrine Society.
Unlike testosterone therapy, “leflutrozole was associated with positive effects on semen fertility parameters, such as semen volume and concentration,” reported Thomas Hugh Jones, MD, FRCP, of the Centre for Diabetes and Endocrinology, Barnsley Hospital NHS Foundation Trust, and the department of oncology and metabolism, University of Sheffield Medical School, both in England.
Although the impact of the experimental aromatase inhibitor leflutrozole on parameters of semen function was an exploratory analysis in this multicenter, placebo-controlled study, it is particularly noteworthy because it addresses one of the weaknesses of testosterone replacement, which is often the first choice in treating hypogonadism, Dr. Jones said.
“Testosterone replacement frequently results in negative feedback suppression of follicle stimulating hormone and luteinizing hormone so that along with lower sperm counts, these men have significant problems with fertility,” he explained.
In this phase 2, double-blind, randomized trial, 271 men with hypogonadism were randomized to placebo or to leflutrozole in a dose of 0.1 mg, 0.3 mg, or 1.0 mg taken orally once weekly. All patients had a serum testosterone level of less than 300 ng/dL at entry. The median body mass index was 38 kg/m2, and the average age was 50.9 years.
Results were presented after 24 weeks of treatment, but the blinded study continued for an additional 24 weeks.
Normalization of testosterone, defined as a level between 300 and 1,000 ng/dL, was the primary endpoint. The mean testosterone levels were essentially unchanged in the placebo group during the first 24-week phase of the study, but they climbed to means of 458 ng/dL in the 0.1-mg group, 512 ng/dL in the 0.3-mg group, and 586 ng/dL in the 1.0-mg group.
“Overall, 75% were in the normal range, but it reached 90% in the groups taking the two higher doses,” Dr. Jones reported. Testosterone levels never exceeded 1,500 ng/d.
For the effect on FSH and LH, which were secondary endpoints, both were increased in a dose-dependent manner at 12 and 20 weeks (P less than .001 for the highest dose relative to placebo).
For the semen analysis, also conducted at 12 and 20 weeks, all three doses were associated with a numerical increase in sperm count relative to placebo, with the highest dose achieving significant improvements in semen volume (P = .006), semen concentration (P = .01), and total motile sperm count (P = .03), Dr. Jones reported.
“The 48-week analysis has just been completed, and these types of improvements have been persistent,” Dr. Jones said in reference to the increase in sex hormones as well as measures of sperm function. Although he did not present the 48-week results in detail, he disclosed that this longer follow-up also supported favorable effects on bone density, which is among several prespecified substudies being performed.
Leflutrozole, which is chemically related to letrozole, has been well tolerated at the doses studied. An increase in hematocrit consistent with the rise in testosterone was observed, but Dr. Jones reported that there are no significant safety issues identified so far.
Aromatase inhibitors have been used off label to treat hypogonadism, but this is the first randomized controlled trial for this indication, Dr. Jones said.
Although leflutrozole was used in this study at far lower doses than the aromatase inhibitors currently available for treatment of breast cancer, it might provide an advance for a challenging condition, according to Dr. Jones. He did not speculate when a phase 3 registration trial might start, but he did say that the promise of this agent warrants further development.
Dr. Jones reported a financial relationship with Mereo BioPharma, the sponsor of this trial.
Source: Jones et al. ENDO 2019, Session OR18-4.
NEW ORLEANS – In obese men with hypogonadotropic hypogonadism, an experimental aromatase inhibitor (Ai) normalized testosterone, seemed to improve sperm function, and was not associated with any significant adverse safety signals, according to findings presented at the annual meeting of the Endocrine Society.
Unlike testosterone therapy, “leflutrozole was associated with positive effects on semen fertility parameters, such as semen volume and concentration,” reported Thomas Hugh Jones, MD, FRCP, of the Centre for Diabetes and Endocrinology, Barnsley Hospital NHS Foundation Trust, and the department of oncology and metabolism, University of Sheffield Medical School, both in England.
Although the impact of the experimental aromatase inhibitor leflutrozole on parameters of semen function was an exploratory analysis in this multicenter, placebo-controlled study, it is particularly noteworthy because it addresses one of the weaknesses of testosterone replacement, which is often the first choice in treating hypogonadism, Dr. Jones said.
“Testosterone replacement frequently results in negative feedback suppression of follicle stimulating hormone and luteinizing hormone so that along with lower sperm counts, these men have significant problems with fertility,” he explained.
In this phase 2, double-blind, randomized trial, 271 men with hypogonadism were randomized to placebo or to leflutrozole in a dose of 0.1 mg, 0.3 mg, or 1.0 mg taken orally once weekly. All patients had a serum testosterone level of less than 300 ng/dL at entry. The median body mass index was 38 kg/m2, and the average age was 50.9 years.
Results were presented after 24 weeks of treatment, but the blinded study continued for an additional 24 weeks.
Normalization of testosterone, defined as a level between 300 and 1,000 ng/dL, was the primary endpoint. The mean testosterone levels were essentially unchanged in the placebo group during the first 24-week phase of the study, but they climbed to means of 458 ng/dL in the 0.1-mg group, 512 ng/dL in the 0.3-mg group, and 586 ng/dL in the 1.0-mg group.
“Overall, 75% were in the normal range, but it reached 90% in the groups taking the two higher doses,” Dr. Jones reported. Testosterone levels never exceeded 1,500 ng/d.
For the effect on FSH and LH, which were secondary endpoints, both were increased in a dose-dependent manner at 12 and 20 weeks (P less than .001 for the highest dose relative to placebo).
For the semen analysis, also conducted at 12 and 20 weeks, all three doses were associated with a numerical increase in sperm count relative to placebo, with the highest dose achieving significant improvements in semen volume (P = .006), semen concentration (P = .01), and total motile sperm count (P = .03), Dr. Jones reported.
“The 48-week analysis has just been completed, and these types of improvements have been persistent,” Dr. Jones said in reference to the increase in sex hormones as well as measures of sperm function. Although he did not present the 48-week results in detail, he disclosed that this longer follow-up also supported favorable effects on bone density, which is among several prespecified substudies being performed.
Leflutrozole, which is chemically related to letrozole, has been well tolerated at the doses studied. An increase in hematocrit consistent with the rise in testosterone was observed, but Dr. Jones reported that there are no significant safety issues identified so far.
Aromatase inhibitors have been used off label to treat hypogonadism, but this is the first randomized controlled trial for this indication, Dr. Jones said.
Although leflutrozole was used in this study at far lower doses than the aromatase inhibitors currently available for treatment of breast cancer, it might provide an advance for a challenging condition, according to Dr. Jones. He did not speculate when a phase 3 registration trial might start, but he did say that the promise of this agent warrants further development.
Dr. Jones reported a financial relationship with Mereo BioPharma, the sponsor of this trial.
Source: Jones et al. ENDO 2019, Session OR18-4.
REPORTING FROM ENDO 2019
FDA approves Jatenzo for treatment of male hypogonadism
The Food and Drug Administration has approved testosterone undecanoate (Jatenzo), an oral capsule for the treatment of male hypogonadism caused by certain conditions, such as genetic disorders or tumors that have damaged the pituitary gland.
The approval is based on results from a 4-month clinical trial involving 166 men with hypogonadism. Patients received 237 mg testosterone undecanoate twice a day initially, a dosage that was adjusted downward or upward to a maximum of 396 mg twice a day, based on testosterone levels. At the end of the trial, 87% of men had achieved testosterone levels within the normal range.
The label for testosterone undecanoate contains a warning that the drug can cause an increase in blood pressure, raising the risk of heart attack, stroke, and cardiovascular death. Other common adverse events associated with testosterone undecanoate include headache, an increase in hematocrit, a decrease in HDL cholesterol, and nausea.
“[The] oral route of administration provides an important addition to current treatment options available for men with certain hypogonadal conditions who, until now, have most commonly been treated with testosterone products that are applied to the skin or injected,” said Hylton V. Joffe, MD, MMSc, director of the division of bone, reproductive, and urologic products at the FDA’s Center for Drug Evaluation and Research, in the press release.
Testosterone undecanoate is not approved to treat age-related hypogonadism, even in cases in which men have symptoms caused by low testosterone. The drug’s benefits do not outweigh its risks in those cases, the agency noted.
Find the full press release on the FDA website.
The Food and Drug Administration has approved testosterone undecanoate (Jatenzo), an oral capsule for the treatment of male hypogonadism caused by certain conditions, such as genetic disorders or tumors that have damaged the pituitary gland.
The approval is based on results from a 4-month clinical trial involving 166 men with hypogonadism. Patients received 237 mg testosterone undecanoate twice a day initially, a dosage that was adjusted downward or upward to a maximum of 396 mg twice a day, based on testosterone levels. At the end of the trial, 87% of men had achieved testosterone levels within the normal range.
The label for testosterone undecanoate contains a warning that the drug can cause an increase in blood pressure, raising the risk of heart attack, stroke, and cardiovascular death. Other common adverse events associated with testosterone undecanoate include headache, an increase in hematocrit, a decrease in HDL cholesterol, and nausea.
“[The] oral route of administration provides an important addition to current treatment options available for men with certain hypogonadal conditions who, until now, have most commonly been treated with testosterone products that are applied to the skin or injected,” said Hylton V. Joffe, MD, MMSc, director of the division of bone, reproductive, and urologic products at the FDA’s Center for Drug Evaluation and Research, in the press release.
Testosterone undecanoate is not approved to treat age-related hypogonadism, even in cases in which men have symptoms caused by low testosterone. The drug’s benefits do not outweigh its risks in those cases, the agency noted.
Find the full press release on the FDA website.
The Food and Drug Administration has approved testosterone undecanoate (Jatenzo), an oral capsule for the treatment of male hypogonadism caused by certain conditions, such as genetic disorders or tumors that have damaged the pituitary gland.
The approval is based on results from a 4-month clinical trial involving 166 men with hypogonadism. Patients received 237 mg testosterone undecanoate twice a day initially, a dosage that was adjusted downward or upward to a maximum of 396 mg twice a day, based on testosterone levels. At the end of the trial, 87% of men had achieved testosterone levels within the normal range.
The label for testosterone undecanoate contains a warning that the drug can cause an increase in blood pressure, raising the risk of heart attack, stroke, and cardiovascular death. Other common adverse events associated with testosterone undecanoate include headache, an increase in hematocrit, a decrease in HDL cholesterol, and nausea.
“[The] oral route of administration provides an important addition to current treatment options available for men with certain hypogonadal conditions who, until now, have most commonly been treated with testosterone products that are applied to the skin or injected,” said Hylton V. Joffe, MD, MMSc, director of the division of bone, reproductive, and urologic products at the FDA’s Center for Drug Evaluation and Research, in the press release.
Testosterone undecanoate is not approved to treat age-related hypogonadism, even in cases in which men have symptoms caused by low testosterone. The drug’s benefits do not outweigh its risks in those cases, the agency noted.
Find the full press release on the FDA website.
Cancer screening: A modest proposal for prevention
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
I have been assured by a very knowing American of my acquaintance in London, that a young healthy child well nursed is at a year old, a most delicious, nourishing, and wholesome food, whether stewed, roasted, baked, or boiled, and I make no doubt that it will equally serve in a fricassee, or ragout.
—Jonathan Swift, A Modest Proposal1
Large-scale cancer screening programs have the unintended consequences of false-positive results and overdiagnosis, leading to anxiety and overtreatment. The magnitude of these harms continues to be clarified after decades of screening.
Recognizing the trade-off between benefits and harms, the US Preventive Services Task Force (USPSTF) has changed several of its recommendations in recent years. Breast cancer screening recommendations have gone from yearly mammograms starting at age 40 to biennial mammograms starting at age 50 for women at average risk.2 Prostate cancer screening is no longer recommended for men age 70 and older, and even for men between 55 and 69, screening is now an individual decision.3
Newer screening programs are targeting high-risk groups rather than the general population, with the aim of increasing the likelihood of benefits and limiting the harms. For example, lung cancer screening is recommended only for current smokers or smokers who have quit within the past 15 years, are between 55 and 80, and have at least a 30 pack-year smoking history.4
The movement toward less-frequent screening and screening in a narrower population has evoked strong reactions from advocates of cancer screening. One professor of radiology writes, “It borders on unethical to suggest that the benefit of having your life saved by screening and living another 40 years can be balanced against the ‘harm’ of being recalled for additional mammographic views for what proves to not be a cancer.”5 Another notes, “It does not make any sense to throw away the lives saved by screening to avoid over-treating a small number of cancers.”6 Both of these authors defend the position that the goal of screening is to minimize cause-specific mortality, irrespective of overdiagnosis, overtreatment, or false-positive results. In other words, harm should have little to no weight in screening recommendations.
Although the debate on cancer screening is moving toward a more balanced discussion of benefits and harms, many patients are still subjected to screening that is more aggressive than the USPSTF recommends, which may be due to an underlying belief that no harm is greater than the benefit of saving a life.
IS MORE-AGGRESSIVE SCREENING THE ANSWER?
One may wonder if more-aggressive screening could prevent deaths that occur despite standard screening. For example, more-frequent screening or use of additional screening methods such as ultrasonography or magnetic resonance imaging has been suggested for patients at high risk of breast cancer.
A MODEST PROPOSAL
If one holds the view that benefits alone should be considered when writing recommendations about screening, the logical conclusion extends beyond screening. We would therefore like to propose a different approach to reducing cancer deaths in the general population:
Why not just remove everybody’s breasts, prostate gland, and colon before cancer arises?
TO CUT IS TO PREVENT
Currently, we offer prophylactic surgery to patients at high risk of cancer. For example, women with BRCA1/BRCA2 mutations are offered prophylactic mastectomy as one of several options for reducing risk of breast cancer. In 2013, the first case of prophylactic prostatectomy was performed in a man who had a BRCA1/BRCA2 mutation. Total colectomy is considered in men and women who have hereditary nonpolyposis colon cancer, instead of segmental resection, to prevent future cancer.
If prophylactic surgery were extended to the general population, it would greatly reduce the number of cancer deaths. Assuming that removing an organ almost always precludes development of cancer, we may predict that prophylactic mastectomy, prostatectomy, or colectomy would save the lives of most of the patients who are still dying of cancer of these organs. The effectiveness rates would approach, but not reach 100%; such is the case with prophylactic mastectomy.
Consider prostate-specific antigen (PSA) screening. Even using the favorable estimate of the impact of PSA screening, arising from the European Randomised Study of Screening for Prostate Cancer trial, 27 men have to be diagnosed, most undergoing local therapy (the trial was conducted before active surveillance became routine), to avert 1 death from prostate cancer over 13 years.9
Contrast this “number needed to diagnose” with the number needed to treat for a strategy of routine prostate removal at age 45 or 50. Given that the lifetime risk of death from prostate cancer approaches 3%, and few cases arise before this age, a prophylactic surgical strategy would avert 1 death per 33 operations. If proponents of screening are willing to accept a number needed to diagnose of 27 over a 13-year interval, they may be willing to consider a number needed to treat of 33 over a lifetime.
There may be harms such as perioperative and postoperative complications. Mastectomy could lead to emotional stress from altered body image. Prostatectomy can have long-term complications such as urinary incontinence and sexual dysfunction. Nevertheless, prophylactic organ removal would save far more lives than current screening practices. It also could decrease mental burden, as patients could rest assured that they will never develop cancer, whereas screening often involves ambiguous test results, follow-up tests, and interventions, increasing patient anxiety.
FINDING THE BALANCE BETWEEN BENEFITS AND HARMS
In truth, we do not really advocate universal mastectomy, prostatectomy, and colectomy to prevent cancer, no more than Swift1 really wanted to eat the children of Ireland to alleviate poverty and famine in that country. Rather, we use it as an extreme proposal to highlight the scope and depth of harms that inevitably arise from screening.
If proponents of aggressive screening believe that the goal is to reduce cause-specific mortality as much as possible, giving little weight or consideration to overdiagnosis and overtreatment, then they ought to embrace universal prophylactic surgery as well. Recognition of this logical consequence reminds us that we must make screening recommendations that balance benefits and harms.
Considering an extreme perspective can help in recognizing our bias toward saving lives from cancer and discounting the harms. Aggravating this bias, it is impossible to know whether an individual patient has avoided fatal cancer or undergone unnecessary treatment. Moreover, changing practice is more difficult if it involves rolling back interventions that were once the standard.
Balancing benefits and harms is especially difficult when trying to compare the benefit of preventing a single cancer death against a harm that is less serious but more common. Medicine has always involved difficult trade-offs, as seen in cost-benefit analysis of new treatments or balancing quality of life with quantity of life in a single patient. In addition, each individual may place different values on benefits of screening and avoiding possible harms.
There is an undeniable trade-off with screening, and we must make a conscious decision on where to draw the line when harms outweigh the benefits. We must proceed with caution when subjecting large numbers of men and women to the possibility of psychological burden and decreased quality of life.
Given the growing appreciation of the harms of screening, it is likely that future guidance will continue to move toward less- frequent screening or focusing resources on high-risk populations, where the absolute magnitude of benefit is greater. Cancer screening is also likely to become an individual decision based on personal values and informed decisions.
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0
- Swift J. A Modest Proposal for Preventing the Children of Poor People in Ireland, from Being a Burden on Their Parents or Country, and for Making Them Beneficial to the Publick. Dublin: S. Harding, 1729.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK343819. Accessed February 13, 2019.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018; 319(18):1901–1913. doi:10.1001/jama.2018.3710
- Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. www.ncbi.nlm.nih.gov/books/NBK154610. Accessed February 13, 2019.
- Kopans DB. A review of: “Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years.” www.sbi-online.org/Portals/0/downloads/documents/pdfs/A%20review%20of%20Tipping%20the%20Balance%20of%20Benefits%20and%20Harms%20to%20Favor%20Screening%20Mammography%20Starting%20at%20Age%2040%20Years%20-%20Kopans.pdf. Accessed February 13, 2019.
- Yaffe M, Gordon, P. Routine mammograms do save lives: U of T expert. U of T News. www.utoronto.ca/news/routine-mammograms-do-save-lives-u-t-expert. Accessed February 13, 2019.
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Schröder FH, Hugosson J, Roobol MJ, et al; ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384(9959):2027–2035. doi:10.1016/S0140-6736(14)60525-0