User login
Infertility: A practical framework
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
KEY POINTS
- A primary care physician can provide advice and testing regarding most fertility concerns.
- Female reproductive aging is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 and older.
- Male factor infertility can now often be overcome with assisted reproductive technologies.
- Polycystic ovary syndrome can cause anovulation and has metabolic effects that can evolve into metabolic syndrome, with serious health consequences.
ACIP favors shared decision on pneumococcal vaccine for older adults
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
Pneumococcal vaccination with the 13-valent pneumococcal conjugate vaccine (PCV13) based on shared clinical decision making is recommended for immunocompetent adults aged 65 years and older who have not previously received PCV13, and all adults aged 65 years and older should continue to receive the pneumococcal polysaccharide vaccine (PPSV23), according to a vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The motion passed with an 11-1 vote after members voted down two other options to either discontinue or continue the current recommendation of PCV13 for all immunocompetent adults aged 65 years and older. The current recommendation for PCV13 for adults aged 65 years and older has been in place since 2014.
The pneumococcal work group assessed indirect effects of the pediatric PCV vaccination on older adults prior to 2014 and since 2014, and what additional benefits might be expected if routine vaccination of older adults continued.
“Indirect effects have been observed in all age groups” said Almea Matanock, MD, of the CDC’s National Center for Immunization and Respiratory Diseases. Although there were no safety concerns, the public health impact of continued vaccination of adults was minimal.
Although PCV13 resulted in a 75% reduction in vaccine-type invasive pneumococcal disease and a 45% reduction in vaccine-type nonbacteremic pneumonia in 2014, the annual number needed to vaccinate to prevent a single case of outpatient pneumonia was 2,600, said Dr. Matanock.
Dr. Matanock presented key issues from the Evidence to Recommendations Framework for and against the recommendation for PCV13 in older adults. Work group comments in favor of continuing the recommendation for PCV13 in older adults included effective disease prevention and the potential negative impact on the importance of adult vaccines if the vaccine was no longer recommended. However, some work group members and committee members expressed concern about resource allocation and steering vaccines away from younger age groups in whom they have been more consistently effective.
Paul Hunter, MD, of the City of Milwaukee Health Department, voted against the shared clinical decision making, and instead favored discontinuing the recommendation for PCV13 for older adults. “I think clinicians need a clear message,” he said, adding that “the public health bang for the buck is with the kids.”
“I think there was a recognition that the population level benefit is minimal,” said work group chair Grace Lee, MD.
Although the work group recognized some benefit for older adults, the burden of disease for PCV-specific disease is low, compared with all-cause pneumonia, said Dr. Lee of Lucile Packard Children’s Hospital at Stanford, Calif. However, the recommendation for shared clinical decision making allows for potential insurance coverage of the vaccine for adults who decide after discussion with their health care provider that they would benefit.
“We are still unpacking this construct” of shared clinical decision making, which in this case applies to adults without immunocompromising conditions, and is more of a provider assessment than a risk assessment, she said.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
ACIP extends HPV vaccine coverage
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
according to a unanimous vote at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
This change affects males aged 22 through 26 years; the HPV vaccine is currently recommended for males and females aged 11 or 12 years, with catch-up vaccination through age 21 for males and age 26 for females.
The change was supported in part by increased interest in simplifying and harmonizing the vaccine schedule, said Lauri Markowitz, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), who presented the HPV work group’s considerations.
In addition, the committee voted 10-4 in favor of catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45 years.
Although the current program of HPV vaccination for youth has demonstrated effectiveness, data from multiple models suggest that widespread HPV vaccination for adults older than 26 years is much less cost effective, and would yield relatively small additional health benefits, Dr. Markowitz said.
The HPV work group reviewed data from a range of clinical trials, epidemiology, and natural history, as well as results from five different health economic models. They concluded that an assessment of benefits and harms favors expanding the catch-up vaccination to all individuals through 26 years, said Elissa Meites, MD, of the CDC, who presented the official work group opinion. The group’s opinion on the second question was that the additional population level benefit of expanding HPV vaccination to all adults would be minimal and not a reasonable and effective allocation of resources, but that shared clinical decision making would allow flexibility.
The committee expressed strong opinions about the potential for shared clinical decision making as a policy for vaccination for adults older than 26 years. Some felt that this option was a way to include adults at risk for HPV, such as divorced women with new partners, or women getting married for the first time later in life who might not have been exposed to HPV through other relationships. In addition, supporters noted that the shared clinical decision-making option would allow for potential insurance coverage, and would involve discussion between doctors and patients to assess risk.
However, other committee members felt that any recommendation for older adult vaccination would distract clinicians from the importance and value of HPV vaccination for the target age group of 11- and 12-year-olds, and might divert resources from the younger age group in whom it has shown the most benefit.
Resource allocation was a concern voiced by many committee members. Kelly Moore, MD, MPH, of Vanderbilt University, Nashville, Tenn., said she voted no on expanding vaccination to older adults because “we didn’t have details on shared clinical decision making, in the absence of information on what that meant, and in the presence of supply questions, I didn’t feel comfortable expanding vaccination to a huge population,” she said.
Paul Hunter, MD, of the City of Milwaukee Health Department, also voted no, and expressed concern that expanding the HPV vaccination recommendations to older adults would send the message that vaccination for children and teens is not effective or important.
The text of the new recommendations for routine and catch-up vaccination states that the recommendations “also apply to MSM [men who have sex with men], transgender people, and people with immunocompromising conditions.”
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Specific prednisone regimen safer than others when used with abiraterone for mCR prostate cancer
The safety profile of combination abiraterone acetate (Zytiga) and glucocorticoid therapy in men with metastatic castration-resistant prostate cancer (mCRPC) hinged on the specific steroid regimen, according to a phase 2 open-label randomized controlled trial.
Glucocorticoids are added to abiraterone in part to prevent mineralocorticoid excess, but can also have adverse effects of their own, noted lead investigator Gerhardt Attard, MD, of the University College London Cancer Institute, London, and colleagues. Understanding of the comparative physiologic effects of various regimens is limited.
In the trial, the investigators randomized 164 men with mCRPC from 22 centers in 5 countries (median age 70 years) to 4 glucocorticoid regimens, each combined with abiraterone acetate, 1,000 mg, daily: prednisone, 5 mg, twice daily; prednisone, 5 mg, once daily; prednisone, 2.5 mg, twice daily; and dexamethasone, 0.5 mg, once daily.
Results reported in JAMA Oncology showed that the proportion of patients who had not developed toxicity (hypotension or hypokalemia) from mineralocorticoid excess during the first 24 weeks of treatment was highest, at about 70%, with prednisone, 5 mg, twice daily, and with dexamethasone, and only these regimens had confidence intervals excluding occurrence of this toxicity in at least half of patients. However, patients in the dexamethasone group had significantly heightened risks of insulin resistance and bone mineral density loss at the end of follow-up.
The median radiographic progression-free survival was 18.5 months in the group given prednisone, 5 mg, twice daily; 15.3 months in the group given prednisone, 5 mg, once daily; 12.8 months in the group given prednisone, 2.5 mg, twice daily; and 26.6 months in the group given dexamethasone, 0.5 mg, once daily.
“Different glucocorticoid regimens make distinct compromises on control of mineralocorticoid excess, changes in body composition, and development of insulin resistance,” Dr. Attard and coinvestigators summarized. “This trial provides results consistent with the approved use of abiraterone acetate with prednisone, 5 mg, twice daily for the treatment of mCRPC. Long-term adverse metabolic and musculoskeletal changes are small and do not appear to have a detrimental effect on patient-reported quality of life.”
At the same time, lower-dose prednisone regimens – with their more modest long-term risks of insulin resistance, increased body fat, and bone mineral density loss – can still be used, with caution. “With careful monitoring, the risk of hypokalemia with lower glucocorticoid doses can be mitigated, as demonstrated in the LATITUDE and STAMPEDE trials where no major safety concerns were raised,” they elaborated.
Dr. Attard disclosed personal fees, research support, and travel support from Janssen during the conduct of the study; as well as personal fees research, and/or travel support from numerous other pharmaceutical companies. The study was funded by Janssen EMEA.
SOURCE: Attard G et al. JAMA Oncol. Published online June 27, 2019. doi:10.1001/jamaoncol.2019.1011.
Data from this and similar trials of combination abiraterone and glucocorticoid therapy in prostate cancer should be incorporated into practice in a tailored manner, Umang Swami, MD, and coauthors maintain in an invited commentary (JAMA Oncol. Online June 27, 2019. doi:10.1001/jamaoncol.2019.1008).
“In our view, patients who are expected to be on long-term treatment with abiraterone acetate …. should receive prednisone, 5 mg, once daily to mitigate long-term metabolic toxic effects,” they recommend. However, when using this regimen in the population with metastases, oncologists will need to closely monitor serum potassium levels and blood pressure.
“In other circumstances, the corticosteroid dose will need to be individualized,” Dr. Swami and coauthors advise. “For example, a higher dose can be used for men who are nonadherent with close follow-up and when obtaining laboratory tests and close monitoring for mineralocorticoid excess may be difficult. On the other hand, a lower dose of prednisone is recommended for men who have considerable cardiovascular or metabolic comorbidities but who are otherwise compliant.”
Umang Swami, MD, University of Iowa Hospitals and Clinics, Iowa City,
Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, California, and Neeraj Agarwal, MD, University of Utah, Salt Lake City.
Data from this and similar trials of combination abiraterone and glucocorticoid therapy in prostate cancer should be incorporated into practice in a tailored manner, Umang Swami, MD, and coauthors maintain in an invited commentary (JAMA Oncol. Online June 27, 2019. doi:10.1001/jamaoncol.2019.1008).
“In our view, patients who are expected to be on long-term treatment with abiraterone acetate …. should receive prednisone, 5 mg, once daily to mitigate long-term metabolic toxic effects,” they recommend. However, when using this regimen in the population with metastases, oncologists will need to closely monitor serum potassium levels and blood pressure.
“In other circumstances, the corticosteroid dose will need to be individualized,” Dr. Swami and coauthors advise. “For example, a higher dose can be used for men who are nonadherent with close follow-up and when obtaining laboratory tests and close monitoring for mineralocorticoid excess may be difficult. On the other hand, a lower dose of prednisone is recommended for men who have considerable cardiovascular or metabolic comorbidities but who are otherwise compliant.”
Umang Swami, MD, University of Iowa Hospitals and Clinics, Iowa City,
Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, California, and Neeraj Agarwal, MD, University of Utah, Salt Lake City.
Data from this and similar trials of combination abiraterone and glucocorticoid therapy in prostate cancer should be incorporated into practice in a tailored manner, Umang Swami, MD, and coauthors maintain in an invited commentary (JAMA Oncol. Online June 27, 2019. doi:10.1001/jamaoncol.2019.1008).
“In our view, patients who are expected to be on long-term treatment with abiraterone acetate …. should receive prednisone, 5 mg, once daily to mitigate long-term metabolic toxic effects,” they recommend. However, when using this regimen in the population with metastases, oncologists will need to closely monitor serum potassium levels and blood pressure.
“In other circumstances, the corticosteroid dose will need to be individualized,” Dr. Swami and coauthors advise. “For example, a higher dose can be used for men who are nonadherent with close follow-up and when obtaining laboratory tests and close monitoring for mineralocorticoid excess may be difficult. On the other hand, a lower dose of prednisone is recommended for men who have considerable cardiovascular or metabolic comorbidities but who are otherwise compliant.”
Umang Swami, MD, University of Iowa Hospitals and Clinics, Iowa City,
Sumanta K. Pal, MD, City of Hope Comprehensive Cancer Center, Duarte, California, and Neeraj Agarwal, MD, University of Utah, Salt Lake City.
The safety profile of combination abiraterone acetate (Zytiga) and glucocorticoid therapy in men with metastatic castration-resistant prostate cancer (mCRPC) hinged on the specific steroid regimen, according to a phase 2 open-label randomized controlled trial.
Glucocorticoids are added to abiraterone in part to prevent mineralocorticoid excess, but can also have adverse effects of their own, noted lead investigator Gerhardt Attard, MD, of the University College London Cancer Institute, London, and colleagues. Understanding of the comparative physiologic effects of various regimens is limited.
In the trial, the investigators randomized 164 men with mCRPC from 22 centers in 5 countries (median age 70 years) to 4 glucocorticoid regimens, each combined with abiraterone acetate, 1,000 mg, daily: prednisone, 5 mg, twice daily; prednisone, 5 mg, once daily; prednisone, 2.5 mg, twice daily; and dexamethasone, 0.5 mg, once daily.
Results reported in JAMA Oncology showed that the proportion of patients who had not developed toxicity (hypotension or hypokalemia) from mineralocorticoid excess during the first 24 weeks of treatment was highest, at about 70%, with prednisone, 5 mg, twice daily, and with dexamethasone, and only these regimens had confidence intervals excluding occurrence of this toxicity in at least half of patients. However, patients in the dexamethasone group had significantly heightened risks of insulin resistance and bone mineral density loss at the end of follow-up.
The median radiographic progression-free survival was 18.5 months in the group given prednisone, 5 mg, twice daily; 15.3 months in the group given prednisone, 5 mg, once daily; 12.8 months in the group given prednisone, 2.5 mg, twice daily; and 26.6 months in the group given dexamethasone, 0.5 mg, once daily.
“Different glucocorticoid regimens make distinct compromises on control of mineralocorticoid excess, changes in body composition, and development of insulin resistance,” Dr. Attard and coinvestigators summarized. “This trial provides results consistent with the approved use of abiraterone acetate with prednisone, 5 mg, twice daily for the treatment of mCRPC. Long-term adverse metabolic and musculoskeletal changes are small and do not appear to have a detrimental effect on patient-reported quality of life.”
At the same time, lower-dose prednisone regimens – with their more modest long-term risks of insulin resistance, increased body fat, and bone mineral density loss – can still be used, with caution. “With careful monitoring, the risk of hypokalemia with lower glucocorticoid doses can be mitigated, as demonstrated in the LATITUDE and STAMPEDE trials where no major safety concerns were raised,” they elaborated.
Dr. Attard disclosed personal fees, research support, and travel support from Janssen during the conduct of the study; as well as personal fees research, and/or travel support from numerous other pharmaceutical companies. The study was funded by Janssen EMEA.
SOURCE: Attard G et al. JAMA Oncol. Published online June 27, 2019. doi:10.1001/jamaoncol.2019.1011.
The safety profile of combination abiraterone acetate (Zytiga) and glucocorticoid therapy in men with metastatic castration-resistant prostate cancer (mCRPC) hinged on the specific steroid regimen, according to a phase 2 open-label randomized controlled trial.
Glucocorticoids are added to abiraterone in part to prevent mineralocorticoid excess, but can also have adverse effects of their own, noted lead investigator Gerhardt Attard, MD, of the University College London Cancer Institute, London, and colleagues. Understanding of the comparative physiologic effects of various regimens is limited.
In the trial, the investigators randomized 164 men with mCRPC from 22 centers in 5 countries (median age 70 years) to 4 glucocorticoid regimens, each combined with abiraterone acetate, 1,000 mg, daily: prednisone, 5 mg, twice daily; prednisone, 5 mg, once daily; prednisone, 2.5 mg, twice daily; and dexamethasone, 0.5 mg, once daily.
Results reported in JAMA Oncology showed that the proportion of patients who had not developed toxicity (hypotension or hypokalemia) from mineralocorticoid excess during the first 24 weeks of treatment was highest, at about 70%, with prednisone, 5 mg, twice daily, and with dexamethasone, and only these regimens had confidence intervals excluding occurrence of this toxicity in at least half of patients. However, patients in the dexamethasone group had significantly heightened risks of insulin resistance and bone mineral density loss at the end of follow-up.
The median radiographic progression-free survival was 18.5 months in the group given prednisone, 5 mg, twice daily; 15.3 months in the group given prednisone, 5 mg, once daily; 12.8 months in the group given prednisone, 2.5 mg, twice daily; and 26.6 months in the group given dexamethasone, 0.5 mg, once daily.
“Different glucocorticoid regimens make distinct compromises on control of mineralocorticoid excess, changes in body composition, and development of insulin resistance,” Dr. Attard and coinvestigators summarized. “This trial provides results consistent with the approved use of abiraterone acetate with prednisone, 5 mg, twice daily for the treatment of mCRPC. Long-term adverse metabolic and musculoskeletal changes are small and do not appear to have a detrimental effect on patient-reported quality of life.”
At the same time, lower-dose prednisone regimens – with their more modest long-term risks of insulin resistance, increased body fat, and bone mineral density loss – can still be used, with caution. “With careful monitoring, the risk of hypokalemia with lower glucocorticoid doses can be mitigated, as demonstrated in the LATITUDE and STAMPEDE trials where no major safety concerns were raised,” they elaborated.
Dr. Attard disclosed personal fees, research support, and travel support from Janssen during the conduct of the study; as well as personal fees research, and/or travel support from numerous other pharmaceutical companies. The study was funded by Janssen EMEA.
SOURCE: Attard G et al. JAMA Oncol. Published online June 27, 2019. doi:10.1001/jamaoncol.2019.1011.
FROM JAMA ONCOLOGY
How to incorporate HIV PrEP into your practice
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
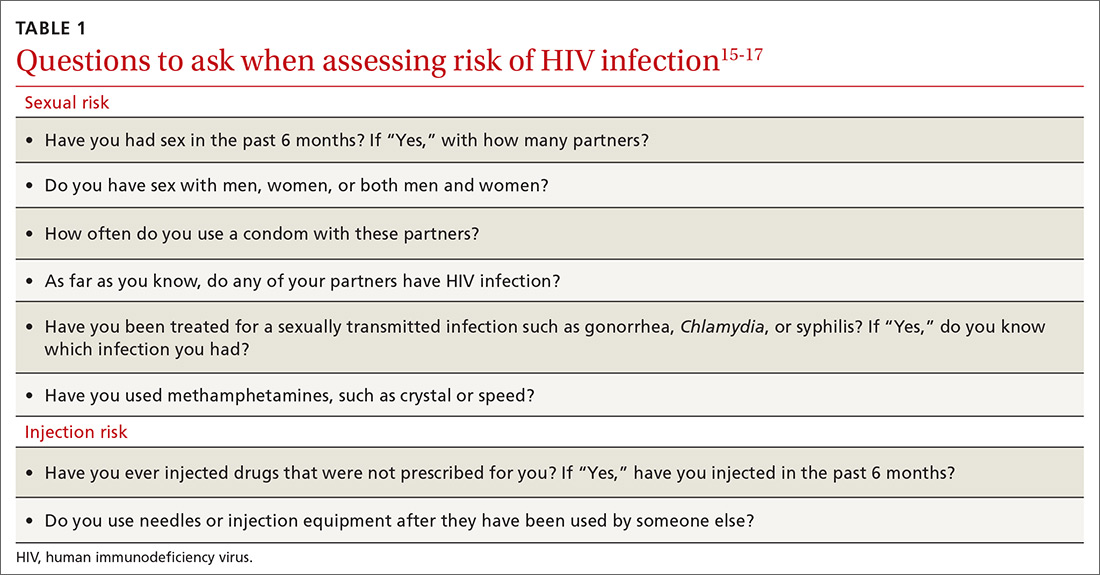
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
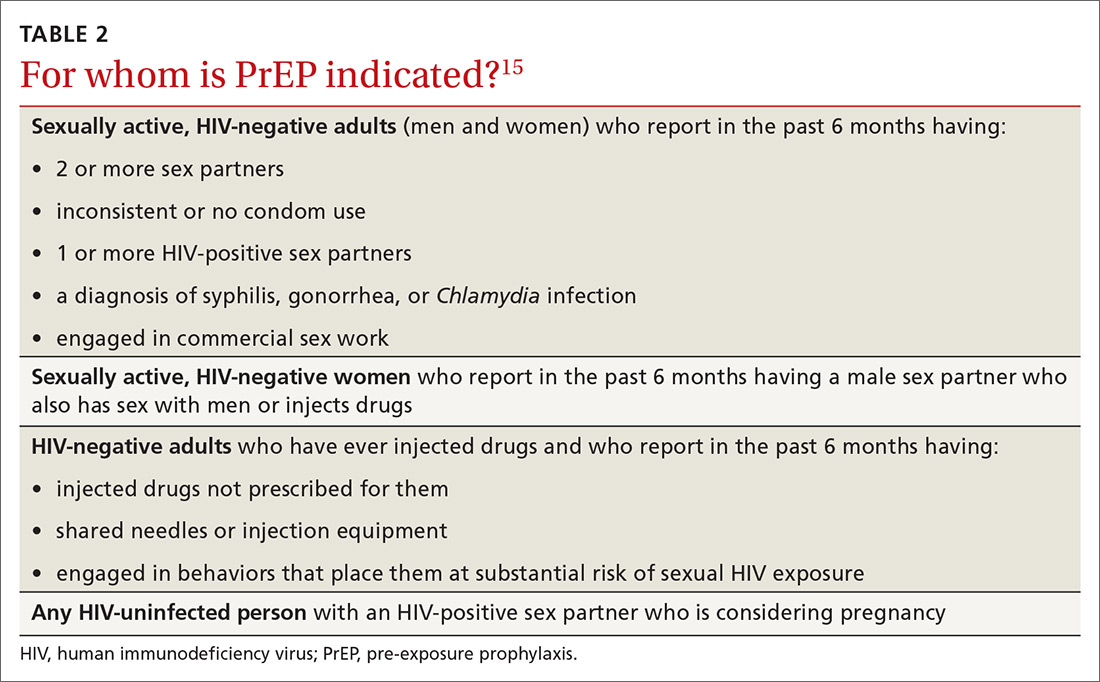
What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
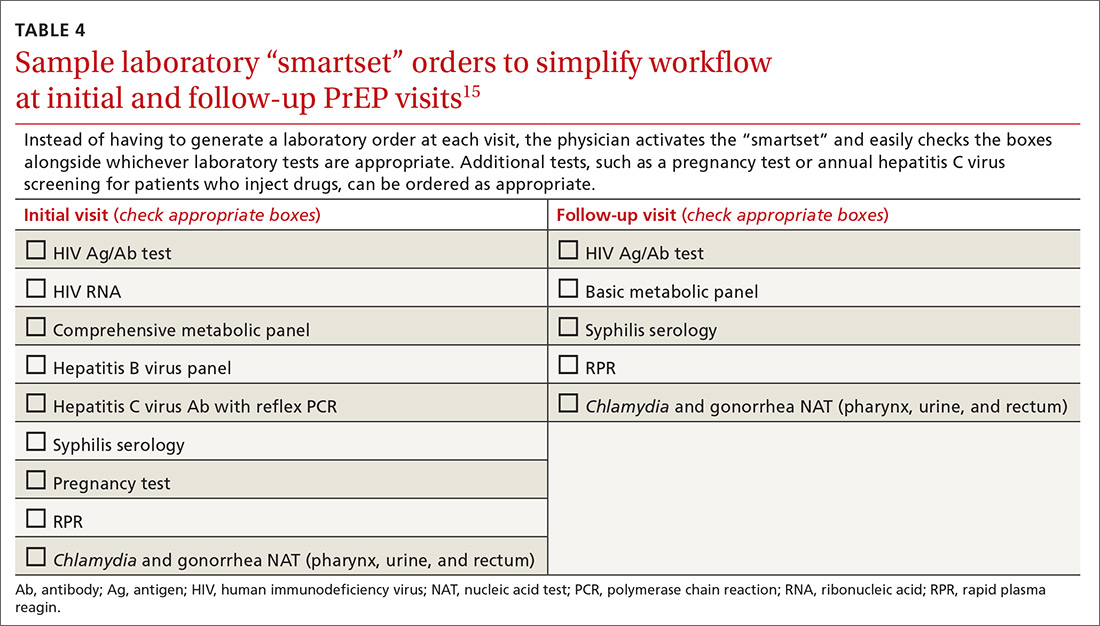
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
PRACTICE RECOMMENDATIONS
› Actively screen and identify HIV-negative patients who are a candidate for pre-exposure prophylaxis (PrEP); commit to talking to the most easily identifiable subsets of these patients, such as men who have sex with men and transgender patients. B
› Recognize that PrEP is indicated for patients who: are sexually active with inconsistent condom use and multiple recent sex partners; have recently been given a diagnosis of a sexually transmitted infection; or have a sexual or injection partner known to be HIV-infected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Colorectal cancer screening: Choosing the right test
Screening can help prevent colorectal cancer. The United States has seen a steady decline in colorectal cancer incidence and mortality, thanks in large part to screening. Screening rates can be increased with good patient-physician dialogue and by choosing a method the patient prefers and is most likely to complete.
In this article, we review a general approach to screening, focusing on the most commonly used methods in the United States, ie, the guaiac-based fecal occult blood test (FOBT), the fecal immunochemical test (FIT), and colonoscopy. We discuss current colorectal cancer incidence rates, screening recommendations, and how to choose the appropriate screening test.
This article does not discuss patients at high risk of polyps or cancer due to hereditary colon cancer syndromes, a personal history of colorectal neoplasia, inflammatory bowel disease, or primary sclerosing cholangitis.
TRENDS IN INCIDENCE
Colorectal cancer is the second most common type of cancer and cause of cancer-related deaths in the United States, responsible for an estimated 50,000 deaths in 2017. The lifetime risk of its occurrence is estimated to be 1 in 21 men and 1 in 23 women.1 Encouragingly, the incidence has declined by 24% over the last 30 years,2 and by 3% per year from 2004 to 2013.1 Also, as a result of screening and advances in treatment, 5-year survival rates for patients with colorectal cancer have increased, from 48.6% in 1975 to 66.4% in 2009.2
When detected at a localized stage, the 5-year survival rate in colorectal cancer is greater than 90%. Unfortunately, it is diagnosed early in only 39% of patients. And despite advances in treatment and a doubling of the 5-year survival rate in patients with advanced cancers since 1990,3 the latter is only 14%. In most patients, cancer is diagnosed when it has spread to the lymph nodes (36%) or to distant organs (22%), and the survival rate declines to 71% after lymph-node spread, and 14% after metastasis to distant organs.
It is essential to screen people who have no symptoms, as symptoms such as gastrointestinal bleeding, unexplained abdominal pain or weight loss, a persistent change in bowel movements, and bowel obstruction typically do not arise until the disease is advanced and less amenable to cure.
Increasing prevalence in younger adults
Curiously, the incidence of colorectal cancer is increasing in white US adults under age 50. Over the last 30 years, incidence rates have increased from 1.0% to 2.4% annually in adults ages 20 to 39.4 Based on current trends, colon cancer rates are expected to increase by 90% for patients ages 20 to 34 and by 28% for patients 35 to 49 by 2030.5
Although recommendations vary for colorectal cancer screening in patients under age 50, clinicians should investigate symptoms such as rectal bleeding, unexplained iron deficiency anemia, progressive abdominal pain, and persistent changes in bowel movements.
Other challenges
Despite the benefits of screening, it is underutilized. Although rates of compliance with screening recommendations have increased 10% over the last 10 years, only 65% of eligible adults currently comply.1,6
Additionally, certain areas of the country such as Appalachia and the Mississippi Delta have not benefited from the decline in the national rate of colorectal cancer.7
SCREENING GUIDELINES
Most guidelines say that colorectal cancer screening should begin at age 50 in people at average risk with no symptoms. However, the American College of Gastroenterology (ACG) recommends beginning screening at age 45 in African Americans, as this group has higher incidence and mortality rates of colorectal cancer.8 Also, the American Cancer Society recently recommended beginning screening at age 45 for all individuals.9
Screening can stop at age 75 for most patients, according to the ACG,8 the US Multi-Society Task Force on Colorectal Cancer,10 and the US Preventive Services Task Force (USPSTF).11 However, the decision should be individualized for patients ages 76 to 85. Patients within that age group who are in good health and have not previously been screened are more likely to benefit than those who have previously been screened and had a negative screening test. Patients over age 85 should not begin or continue screening, because of diminished benefit of screening in this age group, shorter life expectancy, advanced comorbid conditions, and the risks of colonoscopy and cancer treatment.
Patients and clinicians are encouraged to collaborate in deciding which screening method is appropriate. Patients adhere better when they are given a choice in the matter.12–14 And adherence is the key to effective colorectal cancer screening.
Familiarity with the key characteristics of currently available colorectal cancer screening tests will facilitate discussion with patients.
Opportunistic vs programmatic screening
Screening can be classified according to the approach to the patient or population and the intent of the test. Most screening in the United States is opportunistic rather than programmatic—that is, the physician offers the patient screening at the point of service without systematic follow-up or patient re-engagement.
In a programmatic approach, the patient is offered screening through an organized program that streamlines services, reduces overscreening, and provides systematic follow-up of testing.
DISCUSSING THE OPTIONS
Stool studies such as FOBT and FIT do not reliably detect cancer precursors such as adenomas and serrated neoplasms. If an FOBT is positive, follow-up diagnostic colonoscopy is required. Unlike screening colonoscopy, diagnostic colonoscopy requires a copayment for Medicare patients, and this should be explained to the patient.
FIT and FOBT detect hemolyzed blood within a stool sample, FOBT by a chemical reaction, and FIT by detecting a globin-specific antibody. Colorectal cancer and some large adenomatous polyps may intermittently bleed and result in occult blood in the stool, iron deficiency anemia, or hematochezia.15
Fecal occult blood testing
Historically, FOBT was the stool test of choice for screening. It uses an indirect enzymatic reaction to detect hemolyzed blood in the stool. When a specimen containing hemoglobin is added to guaiac paper and a drop of hydrogen peroxide is added to “develop” it, the peroxidase activity of hemoglobin turns the guaiac blue.
Screening with FOBT involves annual testing of 3 consecutively passed stools from different days; FOBT should not be performed at the time of digital rectal examination or if the patient is having overt rectal, urinary, or menstrual bleeding.
Dietary and medication restrictions before and during the testing period are critical, as red meat contains hemoglobin, and certain vegetables (eg, radishes, turnips, cauliflower, cucumbers) contain peroxidase, all of which can cause a false-positive result. Waiting 3 days after the stool sample is collected to develop it can mitigate the peroxidase activity of vegetables.16 Vitamin C inhibits heme peroxidase activity and leads to false-negative results. Aspirin and high-dose nonsteroidal anti-inflammatory drugs can promote bleeding throughout the intestinal tract.17
In randomized controlled trials,18–21 screening with FOBT reduced colorectal cancer mortality rates by 15% to 33%. The 30-year follow-up of a large US trial22 found a 32% relative reduction in mortality rates in patients randomized to annual screening, and a 22% relative reduction in those randomized to screening every 2 years. Despite the many possibilities for false-positive results, the specificity for detecting cancer has ranged from 86.7% to 97.3%, and the sensitivity from 37.1% to 79.4%, highlighting the benefit of colorectal cancer screening programs in unscreened populations.23–26
FIT vs FOBT in current practice
FIT should replace FOBT as the preferred stool screening method. Instead of an enzymatic reaction that can be altered by food or medication, FIT utilizes an antibody specific to human globin to directly detect hemolyzed blood, thus eliminating the need to modify the diet or medications.27 Additionally, only 1 stool specimen is needed, which may explain why the adherence rate was about 20% higher with FIT than with FOBT in most studies.28–30
FIT has a sensitivity of 69% to 86% for colorectal cancer and a specificity of 92% to 95%.31 The sensitivity can be improved by lowering the threshold value for a positive test, but this is associated with a decrease in specificity. A single FIT has the same sensitivity and specificity as several samples.32
In a large retrospective US cohort study of programmatic screening with FIT, Jensen et al33 reported that 48% of 670,841 people who were offered testing actually did the test. Of the 48% who participated in the first round and remained eligible, 75% to 86% participated in subsequent rounds over 4 years. Those who had a positive result on FIT were supposed to undergo colonoscopy, but 22% did not.
The US Multi-Society Task Force on Colorectal Cancer34 suggests that FIT-based screening programs aim for a target FIT completion rate of more than 60% and a target colonoscopy completion rate of more than 80% of patients with positive FITs. These benchmarks were derived from adherence rates in international FIT screening studies in average-risk populations.35–39 (Note that the large US cohort described above33 did not meet these goals.) Ideally, every patient with a positive FIT should undergo diagnostic colonoscopy, but in reality only 50% to 83% actually do. Methods shown to improve adherence include structured screening programs with routine performance reports, provider feedback, and involvement of patient navigators.40–42
Accordingly, several aspects of stool-based testing need to be stressed with patients. Understanding that FOBT is recommended yearly is integral for optimal impact on colorectal cancer incidence and mortality rates.
Additionally, patients should be advised to undergo colonoscopy soon after a positive FIT, because delaying colonoscopy could give precancerous lesions time to progress in stage. The acceptable time between a positive FIT and colonoscopy has yet to be determined. However, a retrospective cohort study of 1.26 million screened patients with 107,000 positive FIT results demonstrated that the rates of cancer discovered on colonoscopy were similar when performed within 30 days or up to 10 months after a positive test. Detection rates increased from 3% to 4.8% at 10 months and to 7.9% at 12 months.43
In modeling studies, Meester et al44 showed the estimated lifetime risk and mortality rates from colorectal cancer and life-years gained from screening are significantly better when colonoscopy is completed within 2 weeks rather than 1 year after a positive FIT. Each additional month after 2 weeks incrementally affected these outcomes, with a 1.4% increase in cancer mortality. These data suggest that colonoscopy should be done soon after a positive FIT result and at a maximum of 10 months.43,44
Screening with FOBT is a multistep process for patients that includes receiving the test kit, collecting the sample, preparing it, returning it, undergoing colonoscopy after a positive test, and repeating in 1 year if negative. The screening program should identify patients at average risk in whom screening is appropriate, ensure delivery of the test, verify the quality of collected samples for laboratory testing against the manufacturer’s recommendations, and report results. Report of a positive FOBT result should provide recommendations for follow-up.
Though evidence clearly supports screening annually or biennially (every 2 years) with FOBT, the ideal interval for FIT is undetermined. Modeling studies utilized by the USPSTF and Multi-Society Task Force demonstrate that colonoscopy and annual FIT result in similar life-years gained, while 2 population-based screening programs have demonstrated that a 2- or 3-year interval may be equally efficacious by lowering the threshold for a positive test.38,45
Randomized controlled trials of screening colonoscopy vs annual and biennial FIT are currently under way. Cost-effectiveness analysis has shown that offering single-sample FITs at more frequent (annual) intervals performs better than multisample testing at less frequent intervals.45–47
Colonoscopy
Compared with stool-based screening, colonoscopy has advantages, including a 10-year screening interval if bowel preparation is adequate and the examination shows no neoplasia, the ability to inspect the entire colon, and the ability to diagnose and treat lesions in the same session.
Screening colonoscopy visualizes the entire colon in more than 98% of cases, although it requires adequate bowel preparation for maximal polyp detection. It can be done safely with or without sedation.48
While there are no available randomized controlled trial data on the impact of screening colonoscopy on cancer incidence or mortality, extensive case-control and cohort studies consistently show that screening colonoscopy reduces cancer incidence and mortality rates.49–54 A US Veterans Administration study of more than 32,000 patients reported a 50% reduction in overall colorectal cancer mortality.55 In a microsimulation modeling study that assumed 100% adherence, colonoscopy every 10 years and annual FIT in individuals ages 50 to 75 provided similar life-years gained per 1,000 people screened (270 for colonoscopy, 244 for FIT).56
Well-established metrics for maximizing the effectiveness and quality of colonoscopy have been established (Table 2). The most important include the mucosa inspection time (withdrawal time) and adenoma detection rate.57 Withdrawal time is directly correlated with adenoma detection, and a 6-minute minimum withdrawal time is recommended in screening colonoscopy examinations of patients at average risk when no polyps are found.58 The adenoma detection rate is the strongest evidence-based metric, as each 1% increase in the adenoma detection rate over 19% is associated with a 3% decrease in the risk of colorectal cancer and a 5% decrease in death rate.59 The average-risk screening adenoma detection rate differs based on sex: the rate is greater than 20% for women and greater than 30% for men.
Complications from screening, diagnostic, or therapeutic colonoscopy are infrequent but include perforation (4/10,000) and significant intestinal bleeding (8/10,000).56–62
Patients with a first-degree relative under age 60 with advanced adenomas or colorectal cancer are considered at high risk and should begin screening colonoscopy at age 40, with repeat colonoscopy at 5-year intervals, given a trend toward advanced neoplasia detection compared with FIT.63
Guidelines recently published by the Canadian Association of Gastroenterology and endorsed by the American Gastroenterological Association also support starting screening in high-risk individuals at age 40, with a surveillance interval of 5 to 10 years based on the number of first-degree relatives with colorectal cancer or adenomas.64 Consensus statements were based on retrospective cohort, prospective case-controlled, and cross-sectional studies comparing the risk of colorectal cancer in individuals with a family history against those without a family history.
Randomized clinical trials comparing colonoscopy and FIT are under way. Interim analysis of a European trial in which asymptomatic adults ages 50 to 69 were randomized to 1-time colonoscopy (26,703 patients) vs FIT every 2 years (26,599 patients) found significantly higher participation rates in the FIT arm (34.2% vs 24.6%) but higher rates of nonadvanced adenomas (4.2% vs 0.4%) and advanced neoplasia (1.9% vs 0.9%) in the colonoscopy arm.65 Cancer was detected in 0.1% in each arm. These findings correlate with those of another study showing higher participation with FIT but higher advanced neoplasia detection rates with colonoscopy.66
Detection of precursor lesions is vital, as removing neoplasms is the main strategy to reduce colorectal cancer incidence. Accordingly, the advantage of colonoscopy was illustrated by a study that determined that 53 patients would need to undergo screening colonoscopy to detect 1 advanced adenoma or cancerous lesion, compared with 264 for FIT.67
STARTING SCREEING AT AGE 45
The American Cancer Society recently provided a qualified recommendation to start colorectal cancer screening in all individuals at age 45 rather than 50.9 This recommendation was based on modeling studies demonstrating that starting screening at age 45 with colonoscopy every 10 years resulted in 25 life-years gained at the cost of 610 colonoscopies per 1,000 individuals. Alternative strategies included FIT, which resulted in an additional 26 life-years gained per 1,000 individuals screened, flexible sigmoidoscopy (23 life-years gained), and computed tomographic colonoscopy (22 life-years gained).
Rates of colorectal cancer are rising in adults under age 50, and 10,000 new cases are anticipated this year.2,3 Currently, 22 million US adults are between the ages of 45 and 50. The system and support needed to perform screening in all adults over age 45 and a lack of direct evidence to support its benefits in the young population need to be considered before widespread acceptance of the American Cancer Society recommendations. However, if screening is considered, FIT with or without sigmoidoscopy may be appropriate, given that most cancers diagnosed in individuals under age 50 are left-sided.4,5
Screening has not been proven to reduce all-cause mortality. Randomized controlled trials of FOBT and observational studies of colonoscopy show that screening reduces cancer incidence and mortality. Until the currently ongoing randomized controlled trials comparing colonoscopy with FIT are completed, their comparative impact on colorectal cancer end points is unknown.
PATIENT ADHERENCE IS KEY
FIT and colonoscopy are the most prevalent screening methods in the United States. Careful attention should be given to offer the screening option the patient is most likely to complete, as adherence is key to the benefit from colorectal cancer screening.
The National Colorectal Cancer Roundtable (nccrt.org), established in 1997 by the American Cancer Society and the US Centers for Disease Control and Prevention, is a national coalition of public and private organizations dedicated to reducing colorectal cancer incidence and mortality. The Roundtable waged a national campaign to achieve a colorectal cancer screening rate of 80% in eligible adults by 2018, a goal that was not met. Still, the potential for a substantial impact is a compelling reason to endorse adherence to colorectal cancer screening. The Roundtable provides many resources for physicians to enhance screening in their practice.
The United States has seen a steady decline in colorectal cancer incidence and mortality, mainly as a result of screening. Colorectal cancer is preventable with ensuring patients’ adherence to screening. Screening rates have been shown to increase with patient-provider dialogue and with selection of a screening program the patient prefers and is most likely to complete.
- American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf. Accessed April 1, 2019.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3):177–193. doi:10.3322/caac.21395
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18(6):1695–1698. doi:10.1158/1055-9965.EPI-09-0186
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015; 150(1):17–22. doi:10.1001/jamasurg.2014.1756
- Centers for Disease Control and Prevention (CDC). Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62(44):881–888. pmid:24196665
- Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev 2015; 24(8):1151–1156. doi:10.1158/1055-9965.EPI-15-0082
- Agrawal S, Bhupinderjit A, Bhutani MS, et al; Committee of Minority Affairs and Cultural Diversity, American College of Gastroenterology. Colorectal cancer in African Americans. Am J Gastroenterol 2005; 100(3):515–523. doi:10.1111/j.1572-0241.2005.41829.x
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4):250–281. doi:10.3322/caac.21457
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017; 112(7):1016–1030. doi:10.1038/ajg.2017.174
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(23):2564–2575. doi:10.1001/jama.2016.5989
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012; 172(7):575–582. doi:10.1001/archinternmed.2012.332
- Steinwachs D, Allen JD, Barlow WE, et al. National Institutes of Health state-of-the-science conference statement: enhancing use and quality of colorectal cancer screening. Ann Intern Med 2010; 152(10):663–667. doi:10.7326/0003-4819-152-10-201005180-00237
- Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med 2004; 38(5):536–550. doi:10.1016/j.ypmed.2003.12.011
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008; 58(3):130–160. doi:10.3322/CA.2007.0018
- Sinatra MA, St John DJ, Young GP. Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clin Chem 1999; 45(1):123–126. pmid:9895348
- Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 2007; 99(19):1462–1470. doi:10.1093/jnci/djm150
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328(19):1365–1371. doi:10.1056/NEJM199305133281901
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348(9040):1472–1477. doi:10.1016/S0140-6736(96)03386-7
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348(9040):1467–1471. doi:10.1016/S0140-6736(96)03430-7
- Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva, Switzerland: World Health Organization; 1968. http://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?sequence=17. Accessed April 1, 2019.
- Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369(12):1106–1114. doi:10.1056/NEJMoa1300720
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996; 334(3):155–159. doi:10.1056/NEJM199601183340304
- Shapiro JA, Bobo JK, Church TR, et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. Am J Gastroenterol 2017; 112(11):1728–1735. doi:10.1038/ajg.2017.285
- Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006; 107(9):2152–2159. doi:10.1002/cncr.22230
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013; 49(14):3049–3054. doi:10.1016/j.ejca.2013.04.023
- Young GP, Cole S. New stool screening tests for colorectal cancer. Digestion 2007; 76(1):26–33. doi:10.1159/000108391
- van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135(1):82–90. doi:10.1053/j.gastro.2008.03.040
- Hassan C, Giorgi Rossi P, Camilloni L, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012; 36(10):929–940. doi:10.1111/apt.12071
- Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med 2012; 55(2):87–92. doi:10.1016/j.ypmed.2012.05.006
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014; 370(14):1287–1297. doi:10.1056/NEJMoa1311194
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014; 160(3):171. doi:10.7326/M13-1484
- Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016; 164(7):456–463. doi:10.7326/M15-0983
- Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017; 152(5):1217–1237.e3. doi:10.1053/j.gastro.2016.08.053
- Rabeneck L, Rumble RB, Thompson F, et al. Fecal immunochemical tests compared with guaiac fecal occult blood tests for population-based colorectal cancer screening. Can J Gastroenterol 2012; 26(3):131–147. pmid:22408764
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C; English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012; 61(10):1439–1446. doi:10.1136/gutjnl-2011-300843
- Malila N, Oivanen T, Malminiemi O, Hakama M. Test, episode, and programme sensitivities of screening for colorectal cancer as a public health policy in Finland: experimental design. BMJ 2008; 337:a2261. doi:10.1136/bmj.a2261
- Denters MJ, Deutekom M, Bossuyt PM, Stroobants AK, Fockens P, Dekker E. Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology 2012; 142(3):497–504. doi:10.1053/j.gastro.2011.11.024
- van Roon AH, Goede SL, van Ballegooijen M, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut 2013; 62(3):409–415. doi:10.1136/gutjnl-2011-301583
- Chubak J, Garcia MP, Burnett-Hartman AN, et al; PROSPR consortium. Time to colonoscopy after positive fecal blood test in four US health care systems. Cancer Epidemiol Biomarkers Prev 2016; 25(2):344–350. doi:10.1158/1055-9965.EPI-15-0470
- Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med 2011; 171(3):249–256. doi:10.1001/archinternmed.2010.372
- Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med 2017; 167(8):565–575. doi:10.7326/M17-1361
- Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017; 317(16):1631–1641. doi:10.1001/jama.2017.3634
- Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol 2016; 14(10):1445–1451.e8. doi:10.1016/j.cgh.2016.05.017
- Haug U, Grobbee EJ, Lansdorp-Vogelaar I, Spaander MCW, Kuipers EJ. Immunochemical faecal occult blood testing to screen for colorectal cancer: can the screening interval be extended? Gut 2017; 66(7):1262–1267. doi:10.1136/gutjnl-2015-310102
- Goede SL, van Roon AH, Reijerink JC, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut 2013; 62(5):727–734. doi:10.1136/gutjnl-2011-301917
- Digby J, Fraser CG, Carey FA, Steele RJC. Can the performance of a quantitative FIT-based colorectal cancer screening programme be enhanced by lowering the threshold and increasing the interval? Gut 2018; 67(5):993–994. doi:10.1136/gutjnl-2017-314862
- Hoffman MS, Butler TW, Shaver T. Colonoscopy without sedation. J Clin Gastroenterol 1998; 26(4):279–282. pmid:9649011
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366(8):687–696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013; 369(12):1095–1105. doi:10.1056/NEJMoa1301969
- Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014; 371(9):799–807. doi:10.1056/NEJMoa1315870
- Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc 2012; 76(1):110–117. doi:10.1016/j.gie.2012.02.040
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329(27):1977–1981. doi:10.1056/NEJM199312303292701
- Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M; Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001; 48(6):812–815. pmid:11358901
- Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med 1995; 123(12):904–910. pmid:7486484
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81(1):31–53. doi:10.1016/j.gie.2014.07.058
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006; 355(24):2533–2541. doi:10.1056/NEJMoa055498
- Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370(26):2541. doi:10.1056/NEJMc1405329
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst 2003; 95(3):230–236. pmid:12569145
- Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009; 150(12):849–857, W152. pmid:19528563
- Quintero E, Carrillo M, Gimeno-García AZ, et al. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 2014; 147(5):1021–130.e1. doi:10.1053/j.gastro.2014.08.004
- Leddin D, Lieberman DA, Tse F, et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: the Canadian Association of Gastroenterology Banff Consensus. Gastroenterology 2018; 155(5):1325–1347.e3. doi:10.1053/j.gastro.2018.08.017
- Quintero E, Castells A, Bujanda L, et al; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366(8):697–706. doi:10.1056/NEJMoa1108895
- Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013; 173(18):1725–1732. doi:10.1001/jamainternmed.2013.9294
- Segnan N, Senore C, Andreoni B, et al; SCORE3 Working Group-Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132(7):2304–2312. doi:10.1053/j.gastro.2007.03.030
Screening can help prevent colorectal cancer. The United States has seen a steady decline in colorectal cancer incidence and mortality, thanks in large part to screening. Screening rates can be increased with good patient-physician dialogue and by choosing a method the patient prefers and is most likely to complete.
In this article, we review a general approach to screening, focusing on the most commonly used methods in the United States, ie, the guaiac-based fecal occult blood test (FOBT), the fecal immunochemical test (FIT), and colonoscopy. We discuss current colorectal cancer incidence rates, screening recommendations, and how to choose the appropriate screening test.
This article does not discuss patients at high risk of polyps or cancer due to hereditary colon cancer syndromes, a personal history of colorectal neoplasia, inflammatory bowel disease, or primary sclerosing cholangitis.
TRENDS IN INCIDENCE
Colorectal cancer is the second most common type of cancer and cause of cancer-related deaths in the United States, responsible for an estimated 50,000 deaths in 2017. The lifetime risk of its occurrence is estimated to be 1 in 21 men and 1 in 23 women.1 Encouragingly, the incidence has declined by 24% over the last 30 years,2 and by 3% per year from 2004 to 2013.1 Also, as a result of screening and advances in treatment, 5-year survival rates for patients with colorectal cancer have increased, from 48.6% in 1975 to 66.4% in 2009.2
When detected at a localized stage, the 5-year survival rate in colorectal cancer is greater than 90%. Unfortunately, it is diagnosed early in only 39% of patients. And despite advances in treatment and a doubling of the 5-year survival rate in patients with advanced cancers since 1990,3 the latter is only 14%. In most patients, cancer is diagnosed when it has spread to the lymph nodes (36%) or to distant organs (22%), and the survival rate declines to 71% after lymph-node spread, and 14% after metastasis to distant organs.
It is essential to screen people who have no symptoms, as symptoms such as gastrointestinal bleeding, unexplained abdominal pain or weight loss, a persistent change in bowel movements, and bowel obstruction typically do not arise until the disease is advanced and less amenable to cure.
Increasing prevalence in younger adults
Curiously, the incidence of colorectal cancer is increasing in white US adults under age 50. Over the last 30 years, incidence rates have increased from 1.0% to 2.4% annually in adults ages 20 to 39.4 Based on current trends, colon cancer rates are expected to increase by 90% for patients ages 20 to 34 and by 28% for patients 35 to 49 by 2030.5
Although recommendations vary for colorectal cancer screening in patients under age 50, clinicians should investigate symptoms such as rectal bleeding, unexplained iron deficiency anemia, progressive abdominal pain, and persistent changes in bowel movements.
Other challenges
Despite the benefits of screening, it is underutilized. Although rates of compliance with screening recommendations have increased 10% over the last 10 years, only 65% of eligible adults currently comply.1,6
Additionally, certain areas of the country such as Appalachia and the Mississippi Delta have not benefited from the decline in the national rate of colorectal cancer.7
SCREENING GUIDELINES
Most guidelines say that colorectal cancer screening should begin at age 50 in people at average risk with no symptoms. However, the American College of Gastroenterology (ACG) recommends beginning screening at age 45 in African Americans, as this group has higher incidence and mortality rates of colorectal cancer.8 Also, the American Cancer Society recently recommended beginning screening at age 45 for all individuals.9
Screening can stop at age 75 for most patients, according to the ACG,8 the US Multi-Society Task Force on Colorectal Cancer,10 and the US Preventive Services Task Force (USPSTF).11 However, the decision should be individualized for patients ages 76 to 85. Patients within that age group who are in good health and have not previously been screened are more likely to benefit than those who have previously been screened and had a negative screening test. Patients over age 85 should not begin or continue screening, because of diminished benefit of screening in this age group, shorter life expectancy, advanced comorbid conditions, and the risks of colonoscopy and cancer treatment.
Patients and clinicians are encouraged to collaborate in deciding which screening method is appropriate. Patients adhere better when they are given a choice in the matter.12–14 And adherence is the key to effective colorectal cancer screening.
Familiarity with the key characteristics of currently available colorectal cancer screening tests will facilitate discussion with patients.
Opportunistic vs programmatic screening
Screening can be classified according to the approach to the patient or population and the intent of the test. Most screening in the United States is opportunistic rather than programmatic—that is, the physician offers the patient screening at the point of service without systematic follow-up or patient re-engagement.
In a programmatic approach, the patient is offered screening through an organized program that streamlines services, reduces overscreening, and provides systematic follow-up of testing.
DISCUSSING THE OPTIONS
Stool studies such as FOBT and FIT do not reliably detect cancer precursors such as adenomas and serrated neoplasms. If an FOBT is positive, follow-up diagnostic colonoscopy is required. Unlike screening colonoscopy, diagnostic colonoscopy requires a copayment for Medicare patients, and this should be explained to the patient.
FIT and FOBT detect hemolyzed blood within a stool sample, FOBT by a chemical reaction, and FIT by detecting a globin-specific antibody. Colorectal cancer and some large adenomatous polyps may intermittently bleed and result in occult blood in the stool, iron deficiency anemia, or hematochezia.15
Fecal occult blood testing
Historically, FOBT was the stool test of choice for screening. It uses an indirect enzymatic reaction to detect hemolyzed blood in the stool. When a specimen containing hemoglobin is added to guaiac paper and a drop of hydrogen peroxide is added to “develop” it, the peroxidase activity of hemoglobin turns the guaiac blue.
Screening with FOBT involves annual testing of 3 consecutively passed stools from different days; FOBT should not be performed at the time of digital rectal examination or if the patient is having overt rectal, urinary, or menstrual bleeding.
Dietary and medication restrictions before and during the testing period are critical, as red meat contains hemoglobin, and certain vegetables (eg, radishes, turnips, cauliflower, cucumbers) contain peroxidase, all of which can cause a false-positive result. Waiting 3 days after the stool sample is collected to develop it can mitigate the peroxidase activity of vegetables.16 Vitamin C inhibits heme peroxidase activity and leads to false-negative results. Aspirin and high-dose nonsteroidal anti-inflammatory drugs can promote bleeding throughout the intestinal tract.17
In randomized controlled trials,18–21 screening with FOBT reduced colorectal cancer mortality rates by 15% to 33%. The 30-year follow-up of a large US trial22 found a 32% relative reduction in mortality rates in patients randomized to annual screening, and a 22% relative reduction in those randomized to screening every 2 years. Despite the many possibilities for false-positive results, the specificity for detecting cancer has ranged from 86.7% to 97.3%, and the sensitivity from 37.1% to 79.4%, highlighting the benefit of colorectal cancer screening programs in unscreened populations.23–26
FIT vs FOBT in current practice
FIT should replace FOBT as the preferred stool screening method. Instead of an enzymatic reaction that can be altered by food or medication, FIT utilizes an antibody specific to human globin to directly detect hemolyzed blood, thus eliminating the need to modify the diet or medications.27 Additionally, only 1 stool specimen is needed, which may explain why the adherence rate was about 20% higher with FIT than with FOBT in most studies.28–30
FIT has a sensitivity of 69% to 86% for colorectal cancer and a specificity of 92% to 95%.31 The sensitivity can be improved by lowering the threshold value for a positive test, but this is associated with a decrease in specificity. A single FIT has the same sensitivity and specificity as several samples.32
In a large retrospective US cohort study of programmatic screening with FIT, Jensen et al33 reported that 48% of 670,841 people who were offered testing actually did the test. Of the 48% who participated in the first round and remained eligible, 75% to 86% participated in subsequent rounds over 4 years. Those who had a positive result on FIT were supposed to undergo colonoscopy, but 22% did not.
The US Multi-Society Task Force on Colorectal Cancer34 suggests that FIT-based screening programs aim for a target FIT completion rate of more than 60% and a target colonoscopy completion rate of more than 80% of patients with positive FITs. These benchmarks were derived from adherence rates in international FIT screening studies in average-risk populations.35–39 (Note that the large US cohort described above33 did not meet these goals.) Ideally, every patient with a positive FIT should undergo diagnostic colonoscopy, but in reality only 50% to 83% actually do. Methods shown to improve adherence include structured screening programs with routine performance reports, provider feedback, and involvement of patient navigators.40–42
Accordingly, several aspects of stool-based testing need to be stressed with patients. Understanding that FOBT is recommended yearly is integral for optimal impact on colorectal cancer incidence and mortality rates.
Additionally, patients should be advised to undergo colonoscopy soon after a positive FIT, because delaying colonoscopy could give precancerous lesions time to progress in stage. The acceptable time between a positive FIT and colonoscopy has yet to be determined. However, a retrospective cohort study of 1.26 million screened patients with 107,000 positive FIT results demonstrated that the rates of cancer discovered on colonoscopy were similar when performed within 30 days or up to 10 months after a positive test. Detection rates increased from 3% to 4.8% at 10 months and to 7.9% at 12 months.43
In modeling studies, Meester et al44 showed the estimated lifetime risk and mortality rates from colorectal cancer and life-years gained from screening are significantly better when colonoscopy is completed within 2 weeks rather than 1 year after a positive FIT. Each additional month after 2 weeks incrementally affected these outcomes, with a 1.4% increase in cancer mortality. These data suggest that colonoscopy should be done soon after a positive FIT result and at a maximum of 10 months.43,44
Screening with FOBT is a multistep process for patients that includes receiving the test kit, collecting the sample, preparing it, returning it, undergoing colonoscopy after a positive test, and repeating in 1 year if negative. The screening program should identify patients at average risk in whom screening is appropriate, ensure delivery of the test, verify the quality of collected samples for laboratory testing against the manufacturer’s recommendations, and report results. Report of a positive FOBT result should provide recommendations for follow-up.
Though evidence clearly supports screening annually or biennially (every 2 years) with FOBT, the ideal interval for FIT is undetermined. Modeling studies utilized by the USPSTF and Multi-Society Task Force demonstrate that colonoscopy and annual FIT result in similar life-years gained, while 2 population-based screening programs have demonstrated that a 2- or 3-year interval may be equally efficacious by lowering the threshold for a positive test.38,45
Randomized controlled trials of screening colonoscopy vs annual and biennial FIT are currently under way. Cost-effectiveness analysis has shown that offering single-sample FITs at more frequent (annual) intervals performs better than multisample testing at less frequent intervals.45–47
Colonoscopy
Compared with stool-based screening, colonoscopy has advantages, including a 10-year screening interval if bowel preparation is adequate and the examination shows no neoplasia, the ability to inspect the entire colon, and the ability to diagnose and treat lesions in the same session.
Screening colonoscopy visualizes the entire colon in more than 98% of cases, although it requires adequate bowel preparation for maximal polyp detection. It can be done safely with or without sedation.48
While there are no available randomized controlled trial data on the impact of screening colonoscopy on cancer incidence or mortality, extensive case-control and cohort studies consistently show that screening colonoscopy reduces cancer incidence and mortality rates.49–54 A US Veterans Administration study of more than 32,000 patients reported a 50% reduction in overall colorectal cancer mortality.55 In a microsimulation modeling study that assumed 100% adherence, colonoscopy every 10 years and annual FIT in individuals ages 50 to 75 provided similar life-years gained per 1,000 people screened (270 for colonoscopy, 244 for FIT).56
Well-established metrics for maximizing the effectiveness and quality of colonoscopy have been established (Table 2). The most important include the mucosa inspection time (withdrawal time) and adenoma detection rate.57 Withdrawal time is directly correlated with adenoma detection, and a 6-minute minimum withdrawal time is recommended in screening colonoscopy examinations of patients at average risk when no polyps are found.58 The adenoma detection rate is the strongest evidence-based metric, as each 1% increase in the adenoma detection rate over 19% is associated with a 3% decrease in the risk of colorectal cancer and a 5% decrease in death rate.59 The average-risk screening adenoma detection rate differs based on sex: the rate is greater than 20% for women and greater than 30% for men.
Complications from screening, diagnostic, or therapeutic colonoscopy are infrequent but include perforation (4/10,000) and significant intestinal bleeding (8/10,000).56–62
Patients with a first-degree relative under age 60 with advanced adenomas or colorectal cancer are considered at high risk and should begin screening colonoscopy at age 40, with repeat colonoscopy at 5-year intervals, given a trend toward advanced neoplasia detection compared with FIT.63
Guidelines recently published by the Canadian Association of Gastroenterology and endorsed by the American Gastroenterological Association also support starting screening in high-risk individuals at age 40, with a surveillance interval of 5 to 10 years based on the number of first-degree relatives with colorectal cancer or adenomas.64 Consensus statements were based on retrospective cohort, prospective case-controlled, and cross-sectional studies comparing the risk of colorectal cancer in individuals with a family history against those without a family history.
Randomized clinical trials comparing colonoscopy and FIT are under way. Interim analysis of a European trial in which asymptomatic adults ages 50 to 69 were randomized to 1-time colonoscopy (26,703 patients) vs FIT every 2 years (26,599 patients) found significantly higher participation rates in the FIT arm (34.2% vs 24.6%) but higher rates of nonadvanced adenomas (4.2% vs 0.4%) and advanced neoplasia (1.9% vs 0.9%) in the colonoscopy arm.65 Cancer was detected in 0.1% in each arm. These findings correlate with those of another study showing higher participation with FIT but higher advanced neoplasia detection rates with colonoscopy.66
Detection of precursor lesions is vital, as removing neoplasms is the main strategy to reduce colorectal cancer incidence. Accordingly, the advantage of colonoscopy was illustrated by a study that determined that 53 patients would need to undergo screening colonoscopy to detect 1 advanced adenoma or cancerous lesion, compared with 264 for FIT.67
STARTING SCREEING AT AGE 45
The American Cancer Society recently provided a qualified recommendation to start colorectal cancer screening in all individuals at age 45 rather than 50.9 This recommendation was based on modeling studies demonstrating that starting screening at age 45 with colonoscopy every 10 years resulted in 25 life-years gained at the cost of 610 colonoscopies per 1,000 individuals. Alternative strategies included FIT, which resulted in an additional 26 life-years gained per 1,000 individuals screened, flexible sigmoidoscopy (23 life-years gained), and computed tomographic colonoscopy (22 life-years gained).
Rates of colorectal cancer are rising in adults under age 50, and 10,000 new cases are anticipated this year.2,3 Currently, 22 million US adults are between the ages of 45 and 50. The system and support needed to perform screening in all adults over age 45 and a lack of direct evidence to support its benefits in the young population need to be considered before widespread acceptance of the American Cancer Society recommendations. However, if screening is considered, FIT with or without sigmoidoscopy may be appropriate, given that most cancers diagnosed in individuals under age 50 are left-sided.4,5
Screening has not been proven to reduce all-cause mortality. Randomized controlled trials of FOBT and observational studies of colonoscopy show that screening reduces cancer incidence and mortality. Until the currently ongoing randomized controlled trials comparing colonoscopy with FIT are completed, their comparative impact on colorectal cancer end points is unknown.
PATIENT ADHERENCE IS KEY
FIT and colonoscopy are the most prevalent screening methods in the United States. Careful attention should be given to offer the screening option the patient is most likely to complete, as adherence is key to the benefit from colorectal cancer screening.
The National Colorectal Cancer Roundtable (nccrt.org), established in 1997 by the American Cancer Society and the US Centers for Disease Control and Prevention, is a national coalition of public and private organizations dedicated to reducing colorectal cancer incidence and mortality. The Roundtable waged a national campaign to achieve a colorectal cancer screening rate of 80% in eligible adults by 2018, a goal that was not met. Still, the potential for a substantial impact is a compelling reason to endorse adherence to colorectal cancer screening. The Roundtable provides many resources for physicians to enhance screening in their practice.
The United States has seen a steady decline in colorectal cancer incidence and mortality, mainly as a result of screening. Colorectal cancer is preventable with ensuring patients’ adherence to screening. Screening rates have been shown to increase with patient-provider dialogue and with selection of a screening program the patient prefers and is most likely to complete.
Screening can help prevent colorectal cancer. The United States has seen a steady decline in colorectal cancer incidence and mortality, thanks in large part to screening. Screening rates can be increased with good patient-physician dialogue and by choosing a method the patient prefers and is most likely to complete.
In this article, we review a general approach to screening, focusing on the most commonly used methods in the United States, ie, the guaiac-based fecal occult blood test (FOBT), the fecal immunochemical test (FIT), and colonoscopy. We discuss current colorectal cancer incidence rates, screening recommendations, and how to choose the appropriate screening test.
This article does not discuss patients at high risk of polyps or cancer due to hereditary colon cancer syndromes, a personal history of colorectal neoplasia, inflammatory bowel disease, or primary sclerosing cholangitis.
TRENDS IN INCIDENCE
Colorectal cancer is the second most common type of cancer and cause of cancer-related deaths in the United States, responsible for an estimated 50,000 deaths in 2017. The lifetime risk of its occurrence is estimated to be 1 in 21 men and 1 in 23 women.1 Encouragingly, the incidence has declined by 24% over the last 30 years,2 and by 3% per year from 2004 to 2013.1 Also, as a result of screening and advances in treatment, 5-year survival rates for patients with colorectal cancer have increased, from 48.6% in 1975 to 66.4% in 2009.2
When detected at a localized stage, the 5-year survival rate in colorectal cancer is greater than 90%. Unfortunately, it is diagnosed early in only 39% of patients. And despite advances in treatment and a doubling of the 5-year survival rate in patients with advanced cancers since 1990,3 the latter is only 14%. In most patients, cancer is diagnosed when it has spread to the lymph nodes (36%) or to distant organs (22%), and the survival rate declines to 71% after lymph-node spread, and 14% after metastasis to distant organs.
It is essential to screen people who have no symptoms, as symptoms such as gastrointestinal bleeding, unexplained abdominal pain or weight loss, a persistent change in bowel movements, and bowel obstruction typically do not arise until the disease is advanced and less amenable to cure.
Increasing prevalence in younger adults
Curiously, the incidence of colorectal cancer is increasing in white US adults under age 50. Over the last 30 years, incidence rates have increased from 1.0% to 2.4% annually in adults ages 20 to 39.4 Based on current trends, colon cancer rates are expected to increase by 90% for patients ages 20 to 34 and by 28% for patients 35 to 49 by 2030.5
Although recommendations vary for colorectal cancer screening in patients under age 50, clinicians should investigate symptoms such as rectal bleeding, unexplained iron deficiency anemia, progressive abdominal pain, and persistent changes in bowel movements.
Other challenges
Despite the benefits of screening, it is underutilized. Although rates of compliance with screening recommendations have increased 10% over the last 10 years, only 65% of eligible adults currently comply.1,6
Additionally, certain areas of the country such as Appalachia and the Mississippi Delta have not benefited from the decline in the national rate of colorectal cancer.7
SCREENING GUIDELINES
Most guidelines say that colorectal cancer screening should begin at age 50 in people at average risk with no symptoms. However, the American College of Gastroenterology (ACG) recommends beginning screening at age 45 in African Americans, as this group has higher incidence and mortality rates of colorectal cancer.8 Also, the American Cancer Society recently recommended beginning screening at age 45 for all individuals.9
Screening can stop at age 75 for most patients, according to the ACG,8 the US Multi-Society Task Force on Colorectal Cancer,10 and the US Preventive Services Task Force (USPSTF).11 However, the decision should be individualized for patients ages 76 to 85. Patients within that age group who are in good health and have not previously been screened are more likely to benefit than those who have previously been screened and had a negative screening test. Patients over age 85 should not begin or continue screening, because of diminished benefit of screening in this age group, shorter life expectancy, advanced comorbid conditions, and the risks of colonoscopy and cancer treatment.
Patients and clinicians are encouraged to collaborate in deciding which screening method is appropriate. Patients adhere better when they are given a choice in the matter.12–14 And adherence is the key to effective colorectal cancer screening.
Familiarity with the key characteristics of currently available colorectal cancer screening tests will facilitate discussion with patients.
Opportunistic vs programmatic screening
Screening can be classified according to the approach to the patient or population and the intent of the test. Most screening in the United States is opportunistic rather than programmatic—that is, the physician offers the patient screening at the point of service without systematic follow-up or patient re-engagement.
In a programmatic approach, the patient is offered screening through an organized program that streamlines services, reduces overscreening, and provides systematic follow-up of testing.
DISCUSSING THE OPTIONS
Stool studies such as FOBT and FIT do not reliably detect cancer precursors such as adenomas and serrated neoplasms. If an FOBT is positive, follow-up diagnostic colonoscopy is required. Unlike screening colonoscopy, diagnostic colonoscopy requires a copayment for Medicare patients, and this should be explained to the patient.
FIT and FOBT detect hemolyzed blood within a stool sample, FOBT by a chemical reaction, and FIT by detecting a globin-specific antibody. Colorectal cancer and some large adenomatous polyps may intermittently bleed and result in occult blood in the stool, iron deficiency anemia, or hematochezia.15
Fecal occult blood testing
Historically, FOBT was the stool test of choice for screening. It uses an indirect enzymatic reaction to detect hemolyzed blood in the stool. When a specimen containing hemoglobin is added to guaiac paper and a drop of hydrogen peroxide is added to “develop” it, the peroxidase activity of hemoglobin turns the guaiac blue.
Screening with FOBT involves annual testing of 3 consecutively passed stools from different days; FOBT should not be performed at the time of digital rectal examination or if the patient is having overt rectal, urinary, or menstrual bleeding.
Dietary and medication restrictions before and during the testing period are critical, as red meat contains hemoglobin, and certain vegetables (eg, radishes, turnips, cauliflower, cucumbers) contain peroxidase, all of which can cause a false-positive result. Waiting 3 days after the stool sample is collected to develop it can mitigate the peroxidase activity of vegetables.16 Vitamin C inhibits heme peroxidase activity and leads to false-negative results. Aspirin and high-dose nonsteroidal anti-inflammatory drugs can promote bleeding throughout the intestinal tract.17
In randomized controlled trials,18–21 screening with FOBT reduced colorectal cancer mortality rates by 15% to 33%. The 30-year follow-up of a large US trial22 found a 32% relative reduction in mortality rates in patients randomized to annual screening, and a 22% relative reduction in those randomized to screening every 2 years. Despite the many possibilities for false-positive results, the specificity for detecting cancer has ranged from 86.7% to 97.3%, and the sensitivity from 37.1% to 79.4%, highlighting the benefit of colorectal cancer screening programs in unscreened populations.23–26
FIT vs FOBT in current practice
FIT should replace FOBT as the preferred stool screening method. Instead of an enzymatic reaction that can be altered by food or medication, FIT utilizes an antibody specific to human globin to directly detect hemolyzed blood, thus eliminating the need to modify the diet or medications.27 Additionally, only 1 stool specimen is needed, which may explain why the adherence rate was about 20% higher with FIT than with FOBT in most studies.28–30
FIT has a sensitivity of 69% to 86% for colorectal cancer and a specificity of 92% to 95%.31 The sensitivity can be improved by lowering the threshold value for a positive test, but this is associated with a decrease in specificity. A single FIT has the same sensitivity and specificity as several samples.32
In a large retrospective US cohort study of programmatic screening with FIT, Jensen et al33 reported that 48% of 670,841 people who were offered testing actually did the test. Of the 48% who participated in the first round and remained eligible, 75% to 86% participated in subsequent rounds over 4 years. Those who had a positive result on FIT were supposed to undergo colonoscopy, but 22% did not.
The US Multi-Society Task Force on Colorectal Cancer34 suggests that FIT-based screening programs aim for a target FIT completion rate of more than 60% and a target colonoscopy completion rate of more than 80% of patients with positive FITs. These benchmarks were derived from adherence rates in international FIT screening studies in average-risk populations.35–39 (Note that the large US cohort described above33 did not meet these goals.) Ideally, every patient with a positive FIT should undergo diagnostic colonoscopy, but in reality only 50% to 83% actually do. Methods shown to improve adherence include structured screening programs with routine performance reports, provider feedback, and involvement of patient navigators.40–42
Accordingly, several aspects of stool-based testing need to be stressed with patients. Understanding that FOBT is recommended yearly is integral for optimal impact on colorectal cancer incidence and mortality rates.
Additionally, patients should be advised to undergo colonoscopy soon after a positive FIT, because delaying colonoscopy could give precancerous lesions time to progress in stage. The acceptable time between a positive FIT and colonoscopy has yet to be determined. However, a retrospective cohort study of 1.26 million screened patients with 107,000 positive FIT results demonstrated that the rates of cancer discovered on colonoscopy were similar when performed within 30 days or up to 10 months after a positive test. Detection rates increased from 3% to 4.8% at 10 months and to 7.9% at 12 months.43
In modeling studies, Meester et al44 showed the estimated lifetime risk and mortality rates from colorectal cancer and life-years gained from screening are significantly better when colonoscopy is completed within 2 weeks rather than 1 year after a positive FIT. Each additional month after 2 weeks incrementally affected these outcomes, with a 1.4% increase in cancer mortality. These data suggest that colonoscopy should be done soon after a positive FIT result and at a maximum of 10 months.43,44
Screening with FOBT is a multistep process for patients that includes receiving the test kit, collecting the sample, preparing it, returning it, undergoing colonoscopy after a positive test, and repeating in 1 year if negative. The screening program should identify patients at average risk in whom screening is appropriate, ensure delivery of the test, verify the quality of collected samples for laboratory testing against the manufacturer’s recommendations, and report results. Report of a positive FOBT result should provide recommendations for follow-up.
Though evidence clearly supports screening annually or biennially (every 2 years) with FOBT, the ideal interval for FIT is undetermined. Modeling studies utilized by the USPSTF and Multi-Society Task Force demonstrate that colonoscopy and annual FIT result in similar life-years gained, while 2 population-based screening programs have demonstrated that a 2- or 3-year interval may be equally efficacious by lowering the threshold for a positive test.38,45
Randomized controlled trials of screening colonoscopy vs annual and biennial FIT are currently under way. Cost-effectiveness analysis has shown that offering single-sample FITs at more frequent (annual) intervals performs better than multisample testing at less frequent intervals.45–47
Colonoscopy
Compared with stool-based screening, colonoscopy has advantages, including a 10-year screening interval if bowel preparation is adequate and the examination shows no neoplasia, the ability to inspect the entire colon, and the ability to diagnose and treat lesions in the same session.
Screening colonoscopy visualizes the entire colon in more than 98% of cases, although it requires adequate bowel preparation for maximal polyp detection. It can be done safely with or without sedation.48
While there are no available randomized controlled trial data on the impact of screening colonoscopy on cancer incidence or mortality, extensive case-control and cohort studies consistently show that screening colonoscopy reduces cancer incidence and mortality rates.49–54 A US Veterans Administration study of more than 32,000 patients reported a 50% reduction in overall colorectal cancer mortality.55 In a microsimulation modeling study that assumed 100% adherence, colonoscopy every 10 years and annual FIT in individuals ages 50 to 75 provided similar life-years gained per 1,000 people screened (270 for colonoscopy, 244 for FIT).56
Well-established metrics for maximizing the effectiveness and quality of colonoscopy have been established (Table 2). The most important include the mucosa inspection time (withdrawal time) and adenoma detection rate.57 Withdrawal time is directly correlated with adenoma detection, and a 6-minute minimum withdrawal time is recommended in screening colonoscopy examinations of patients at average risk when no polyps are found.58 The adenoma detection rate is the strongest evidence-based metric, as each 1% increase in the adenoma detection rate over 19% is associated with a 3% decrease in the risk of colorectal cancer and a 5% decrease in death rate.59 The average-risk screening adenoma detection rate differs based on sex: the rate is greater than 20% for women and greater than 30% for men.
Complications from screening, diagnostic, or therapeutic colonoscopy are infrequent but include perforation (4/10,000) and significant intestinal bleeding (8/10,000).56–62
Patients with a first-degree relative under age 60 with advanced adenomas or colorectal cancer are considered at high risk and should begin screening colonoscopy at age 40, with repeat colonoscopy at 5-year intervals, given a trend toward advanced neoplasia detection compared with FIT.63
Guidelines recently published by the Canadian Association of Gastroenterology and endorsed by the American Gastroenterological Association also support starting screening in high-risk individuals at age 40, with a surveillance interval of 5 to 10 years based on the number of first-degree relatives with colorectal cancer or adenomas.64 Consensus statements were based on retrospective cohort, prospective case-controlled, and cross-sectional studies comparing the risk of colorectal cancer in individuals with a family history against those without a family history.
Randomized clinical trials comparing colonoscopy and FIT are under way. Interim analysis of a European trial in which asymptomatic adults ages 50 to 69 were randomized to 1-time colonoscopy (26,703 patients) vs FIT every 2 years (26,599 patients) found significantly higher participation rates in the FIT arm (34.2% vs 24.6%) but higher rates of nonadvanced adenomas (4.2% vs 0.4%) and advanced neoplasia (1.9% vs 0.9%) in the colonoscopy arm.65 Cancer was detected in 0.1% in each arm. These findings correlate with those of another study showing higher participation with FIT but higher advanced neoplasia detection rates with colonoscopy.66
Detection of precursor lesions is vital, as removing neoplasms is the main strategy to reduce colorectal cancer incidence. Accordingly, the advantage of colonoscopy was illustrated by a study that determined that 53 patients would need to undergo screening colonoscopy to detect 1 advanced adenoma or cancerous lesion, compared with 264 for FIT.67
STARTING SCREEING AT AGE 45
The American Cancer Society recently provided a qualified recommendation to start colorectal cancer screening in all individuals at age 45 rather than 50.9 This recommendation was based on modeling studies demonstrating that starting screening at age 45 with colonoscopy every 10 years resulted in 25 life-years gained at the cost of 610 colonoscopies per 1,000 individuals. Alternative strategies included FIT, which resulted in an additional 26 life-years gained per 1,000 individuals screened, flexible sigmoidoscopy (23 life-years gained), and computed tomographic colonoscopy (22 life-years gained).
Rates of colorectal cancer are rising in adults under age 50, and 10,000 new cases are anticipated this year.2,3 Currently, 22 million US adults are between the ages of 45 and 50. The system and support needed to perform screening in all adults over age 45 and a lack of direct evidence to support its benefits in the young population need to be considered before widespread acceptance of the American Cancer Society recommendations. However, if screening is considered, FIT with or without sigmoidoscopy may be appropriate, given that most cancers diagnosed in individuals under age 50 are left-sided.4,5
Screening has not been proven to reduce all-cause mortality. Randomized controlled trials of FOBT and observational studies of colonoscopy show that screening reduces cancer incidence and mortality. Until the currently ongoing randomized controlled trials comparing colonoscopy with FIT are completed, their comparative impact on colorectal cancer end points is unknown.
PATIENT ADHERENCE IS KEY
FIT and colonoscopy are the most prevalent screening methods in the United States. Careful attention should be given to offer the screening option the patient is most likely to complete, as adherence is key to the benefit from colorectal cancer screening.
The National Colorectal Cancer Roundtable (nccrt.org), established in 1997 by the American Cancer Society and the US Centers for Disease Control and Prevention, is a national coalition of public and private organizations dedicated to reducing colorectal cancer incidence and mortality. The Roundtable waged a national campaign to achieve a colorectal cancer screening rate of 80% in eligible adults by 2018, a goal that was not met. Still, the potential for a substantial impact is a compelling reason to endorse adherence to colorectal cancer screening. The Roundtable provides many resources for physicians to enhance screening in their practice.
The United States has seen a steady decline in colorectal cancer incidence and mortality, mainly as a result of screening. Colorectal cancer is preventable with ensuring patients’ adherence to screening. Screening rates have been shown to increase with patient-provider dialogue and with selection of a screening program the patient prefers and is most likely to complete.
- American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf. Accessed April 1, 2019.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3):177–193. doi:10.3322/caac.21395
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18(6):1695–1698. doi:10.1158/1055-9965.EPI-09-0186
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015; 150(1):17–22. doi:10.1001/jamasurg.2014.1756
- Centers for Disease Control and Prevention (CDC). Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62(44):881–888. pmid:24196665
- Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev 2015; 24(8):1151–1156. doi:10.1158/1055-9965.EPI-15-0082
- Agrawal S, Bhupinderjit A, Bhutani MS, et al; Committee of Minority Affairs and Cultural Diversity, American College of Gastroenterology. Colorectal cancer in African Americans. Am J Gastroenterol 2005; 100(3):515–523. doi:10.1111/j.1572-0241.2005.41829.x
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4):250–281. doi:10.3322/caac.21457
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017; 112(7):1016–1030. doi:10.1038/ajg.2017.174
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(23):2564–2575. doi:10.1001/jama.2016.5989
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012; 172(7):575–582. doi:10.1001/archinternmed.2012.332
- Steinwachs D, Allen JD, Barlow WE, et al. National Institutes of Health state-of-the-science conference statement: enhancing use and quality of colorectal cancer screening. Ann Intern Med 2010; 152(10):663–667. doi:10.7326/0003-4819-152-10-201005180-00237
- Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med 2004; 38(5):536–550. doi:10.1016/j.ypmed.2003.12.011
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008; 58(3):130–160. doi:10.3322/CA.2007.0018
- Sinatra MA, St John DJ, Young GP. Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clin Chem 1999; 45(1):123–126. pmid:9895348
- Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 2007; 99(19):1462–1470. doi:10.1093/jnci/djm150
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328(19):1365–1371. doi:10.1056/NEJM199305133281901
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348(9040):1472–1477. doi:10.1016/S0140-6736(96)03386-7
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348(9040):1467–1471. doi:10.1016/S0140-6736(96)03430-7
- Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva, Switzerland: World Health Organization; 1968. http://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?sequence=17. Accessed April 1, 2019.
- Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369(12):1106–1114. doi:10.1056/NEJMoa1300720
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996; 334(3):155–159. doi:10.1056/NEJM199601183340304
- Shapiro JA, Bobo JK, Church TR, et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. Am J Gastroenterol 2017; 112(11):1728–1735. doi:10.1038/ajg.2017.285
- Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006; 107(9):2152–2159. doi:10.1002/cncr.22230
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013; 49(14):3049–3054. doi:10.1016/j.ejca.2013.04.023
- Young GP, Cole S. New stool screening tests for colorectal cancer. Digestion 2007; 76(1):26–33. doi:10.1159/000108391
- van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135(1):82–90. doi:10.1053/j.gastro.2008.03.040
- Hassan C, Giorgi Rossi P, Camilloni L, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012; 36(10):929–940. doi:10.1111/apt.12071
- Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med 2012; 55(2):87–92. doi:10.1016/j.ypmed.2012.05.006
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014; 370(14):1287–1297. doi:10.1056/NEJMoa1311194
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014; 160(3):171. doi:10.7326/M13-1484
- Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016; 164(7):456–463. doi:10.7326/M15-0983
- Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017; 152(5):1217–1237.e3. doi:10.1053/j.gastro.2016.08.053
- Rabeneck L, Rumble RB, Thompson F, et al. Fecal immunochemical tests compared with guaiac fecal occult blood tests for population-based colorectal cancer screening. Can J Gastroenterol 2012; 26(3):131–147. pmid:22408764
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C; English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012; 61(10):1439–1446. doi:10.1136/gutjnl-2011-300843
- Malila N, Oivanen T, Malminiemi O, Hakama M. Test, episode, and programme sensitivities of screening for colorectal cancer as a public health policy in Finland: experimental design. BMJ 2008; 337:a2261. doi:10.1136/bmj.a2261
- Denters MJ, Deutekom M, Bossuyt PM, Stroobants AK, Fockens P, Dekker E. Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology 2012; 142(3):497–504. doi:10.1053/j.gastro.2011.11.024
- van Roon AH, Goede SL, van Ballegooijen M, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut 2013; 62(3):409–415. doi:10.1136/gutjnl-2011-301583
- Chubak J, Garcia MP, Burnett-Hartman AN, et al; PROSPR consortium. Time to colonoscopy after positive fecal blood test in four US health care systems. Cancer Epidemiol Biomarkers Prev 2016; 25(2):344–350. doi:10.1158/1055-9965.EPI-15-0470
- Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med 2011; 171(3):249–256. doi:10.1001/archinternmed.2010.372
- Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med 2017; 167(8):565–575. doi:10.7326/M17-1361
- Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017; 317(16):1631–1641. doi:10.1001/jama.2017.3634
- Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol 2016; 14(10):1445–1451.e8. doi:10.1016/j.cgh.2016.05.017
- Haug U, Grobbee EJ, Lansdorp-Vogelaar I, Spaander MCW, Kuipers EJ. Immunochemical faecal occult blood testing to screen for colorectal cancer: can the screening interval be extended? Gut 2017; 66(7):1262–1267. doi:10.1136/gutjnl-2015-310102
- Goede SL, van Roon AH, Reijerink JC, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut 2013; 62(5):727–734. doi:10.1136/gutjnl-2011-301917
- Digby J, Fraser CG, Carey FA, Steele RJC. Can the performance of a quantitative FIT-based colorectal cancer screening programme be enhanced by lowering the threshold and increasing the interval? Gut 2018; 67(5):993–994. doi:10.1136/gutjnl-2017-314862
- Hoffman MS, Butler TW, Shaver T. Colonoscopy without sedation. J Clin Gastroenterol 1998; 26(4):279–282. pmid:9649011
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366(8):687–696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013; 369(12):1095–1105. doi:10.1056/NEJMoa1301969
- Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014; 371(9):799–807. doi:10.1056/NEJMoa1315870
- Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc 2012; 76(1):110–117. doi:10.1016/j.gie.2012.02.040
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329(27):1977–1981. doi:10.1056/NEJM199312303292701
- Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M; Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001; 48(6):812–815. pmid:11358901
- Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med 1995; 123(12):904–910. pmid:7486484
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81(1):31–53. doi:10.1016/j.gie.2014.07.058
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006; 355(24):2533–2541. doi:10.1056/NEJMoa055498
- Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370(26):2541. doi:10.1056/NEJMc1405329
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst 2003; 95(3):230–236. pmid:12569145
- Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009; 150(12):849–857, W152. pmid:19528563
- Quintero E, Carrillo M, Gimeno-García AZ, et al. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 2014; 147(5):1021–130.e1. doi:10.1053/j.gastro.2014.08.004
- Leddin D, Lieberman DA, Tse F, et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: the Canadian Association of Gastroenterology Banff Consensus. Gastroenterology 2018; 155(5):1325–1347.e3. doi:10.1053/j.gastro.2018.08.017
- Quintero E, Castells A, Bujanda L, et al; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366(8):697–706. doi:10.1056/NEJMoa1108895
- Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013; 173(18):1725–1732. doi:10.1001/jamainternmed.2013.9294
- Segnan N, Senore C, Andreoni B, et al; SCORE3 Working Group-Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132(7):2304–2312. doi:10.1053/j.gastro.2007.03.030
- American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society; 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf. Accessed April 1, 2019.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3):177–193. doi:10.3322/caac.21395
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18(6):1695–1698. doi:10.1158/1055-9965.EPI-09-0186
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015; 150(1):17–22. doi:10.1001/jamasurg.2014.1756
- Centers for Disease Control and Prevention (CDC). Vital signs: colorectal cancer screening test use—United States, 2012. MMWR Morb Mortal Wkly Rep 2013; 62(44):881–888. pmid:24196665
- Siegel RL, Sahar L, Robbins A, Jemal A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev 2015; 24(8):1151–1156. doi:10.1158/1055-9965.EPI-15-0082
- Agrawal S, Bhupinderjit A, Bhutani MS, et al; Committee of Minority Affairs and Cultural Diversity, American College of Gastroenterology. Colorectal cancer in African Americans. Am J Gastroenterol 2005; 100(3):515–523. doi:10.1111/j.1572-0241.2005.41829.x
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018; 68(4):250–281. doi:10.3322/caac.21457
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017; 112(7):1016–1030. doi:10.1038/ajg.2017.174
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 315(23):2564–2575. doi:10.1001/jama.2016.5989
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012; 172(7):575–582. doi:10.1001/archinternmed.2012.332
- Steinwachs D, Allen JD, Barlow WE, et al. National Institutes of Health state-of-the-science conference statement: enhancing use and quality of colorectal cancer screening. Ann Intern Med 2010; 152(10):663–667. doi:10.7326/0003-4819-152-10-201005180-00237
- Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med 2004; 38(5):536–550. doi:10.1016/j.ypmed.2003.12.011
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008; 58(3):130–160. doi:10.3322/CA.2007.0018
- Sinatra MA, St John DJ, Young GP. Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clin Chem 1999; 45(1):123–126. pmid:9895348
- Allison JE, Sakoda LC, Levin TR, et al. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst 2007; 99(19):1462–1470. doi:10.1093/jnci/djm150
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328(19):1365–1371. doi:10.1056/NEJM199305133281901
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348(9040):1472–1477. doi:10.1016/S0140-6736(96)03386-7
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348(9040):1467–1471. doi:10.1016/S0140-6736(96)03430-7
- Wilson JMG, Junger G. Principles and practice of screening for disease. Geneva, Switzerland: World Health Organization; 1968. http://apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?sequence=17. Accessed April 1, 2019.
- Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369(12):1106–1114. doi:10.1056/NEJMoa1300720
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996; 334(3):155–159. doi:10.1056/NEJM199601183340304
- Shapiro JA, Bobo JK, Church TR, et al. A comparison of fecal immunochemical and high-sensitivity guaiac tests for colorectal cancer screening. Am J Gastroenterol 2017; 112(11):1728–1735. doi:10.1038/ajg.2017.285
- Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac-based fecal occult blood test in detection of colorectal neoplasia. Cancer 2006; 107(9):2152–2159. doi:10.1002/cncr.22230
- Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 2013; 49(14):3049–3054. doi:10.1016/j.ejca.2013.04.023
- Young GP, Cole S. New stool screening tests for colorectal cancer. Digestion 2007; 76(1):26–33. doi:10.1159/000108391
- van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135(1):82–90. doi:10.1053/j.gastro.2008.03.040
- Hassan C, Giorgi Rossi P, Camilloni L, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther 2012; 36(10):929–940. doi:10.1111/apt.12071
- Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: a systematic review and meta-analysis. Prev Med 2012; 55(2):87–92. doi:10.1016/j.ypmed.2012.05.006
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 2014; 370(14):1287–1297. doi:10.1056/NEJMoa1311194
- Lee JK, Liles EG, Bent S, Levin TR, Corley DA. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med 2014; 160(3):171. doi:10.7326/M13-1484
- Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016; 164(7):456–463. doi:10.7326/M15-0983
- Robertson DJ, Lee JK, Boland CR, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017; 152(5):1217–1237.e3. doi:10.1053/j.gastro.2016.08.053
- Rabeneck L, Rumble RB, Thompson F, et al. Fecal immunochemical tests compared with guaiac fecal occult blood tests for population-based colorectal cancer screening. Can J Gastroenterol 2012; 26(3):131–147. pmid:22408764
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C; English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012; 61(10):1439–1446. doi:10.1136/gutjnl-2011-300843
- Malila N, Oivanen T, Malminiemi O, Hakama M. Test, episode, and programme sensitivities of screening for colorectal cancer as a public health policy in Finland: experimental design. BMJ 2008; 337:a2261. doi:10.1136/bmj.a2261
- Denters MJ, Deutekom M, Bossuyt PM, Stroobants AK, Fockens P, Dekker E. Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology 2012; 142(3):497–504. doi:10.1053/j.gastro.2011.11.024
- van Roon AH, Goede SL, van Ballegooijen M, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut 2013; 62(3):409–415. doi:10.1136/gutjnl-2011-301583
- Chubak J, Garcia MP, Burnett-Hartman AN, et al; PROSPR consortium. Time to colonoscopy after positive fecal blood test in four US health care systems. Cancer Epidemiol Biomarkers Prev 2016; 25(2):344–350. doi:10.1158/1055-9965.EPI-15-0470
- Carlson CM, Kirby KA, Casadei MA, Partin MR, Kistler CE, Walter LC. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med 2011; 171(3):249–256. doi:10.1001/archinternmed.2010.372
- Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med 2017; 167(8):565–575. doi:10.7326/M17-1361
- Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017; 317(16):1631–1641. doi:10.1001/jama.2017.3634
- Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol 2016; 14(10):1445–1451.e8. doi:10.1016/j.cgh.2016.05.017
- Haug U, Grobbee EJ, Lansdorp-Vogelaar I, Spaander MCW, Kuipers EJ. Immunochemical faecal occult blood testing to screen for colorectal cancer: can the screening interval be extended? Gut 2017; 66(7):1262–1267. doi:10.1136/gutjnl-2015-310102
- Goede SL, van Roon AH, Reijerink JC, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut 2013; 62(5):727–734. doi:10.1136/gutjnl-2011-301917
- Digby J, Fraser CG, Carey FA, Steele RJC. Can the performance of a quantitative FIT-based colorectal cancer screening programme be enhanced by lowering the threshold and increasing the interval? Gut 2018; 67(5):993–994. doi:10.1136/gutjnl-2017-314862
- Hoffman MS, Butler TW, Shaver T. Colonoscopy without sedation. J Clin Gastroenterol 1998; 26(4):279–282. pmid:9649011
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366(8):687–696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013; 369(12):1095–1105. doi:10.1056/NEJMoa1301969
- Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014; 371(9):799–807. doi:10.1056/NEJMoa1315870
- Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc 2012; 76(1):110–117. doi:10.1016/j.gie.2012.02.040
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329(27):1977–1981. doi:10.1056/NEJM199312303292701
- Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M; Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001; 48(6):812–815. pmid:11358901
- Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med 1995; 123(12):904–910. pmid:7486484
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81(1):31–53. doi:10.1016/j.gie.2014.07.058
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006; 355(24):2533–2541. doi:10.1056/NEJMoa055498
- Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370(26):2541. doi:10.1056/NEJMc1405329
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 315(23):2576–2594. doi:10.1001/jama.2016.3332
- Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst 2003; 95(3):230–236. pmid:12569145
- Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009; 150(12):849–857, W152. pmid:19528563
- Quintero E, Carrillo M, Gimeno-García AZ, et al. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 2014; 147(5):1021–130.e1. doi:10.1053/j.gastro.2014.08.004
- Leddin D, Lieberman DA, Tse F, et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: the Canadian Association of Gastroenterology Banff Consensus. Gastroenterology 2018; 155(5):1325–1347.e3. doi:10.1053/j.gastro.2018.08.017
- Quintero E, Castells A, Bujanda L, et al; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366(8):697–706. doi:10.1056/NEJMoa1108895
- Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med 2013; 173(18):1725–1732. doi:10.1001/jamainternmed.2013.9294
- Segnan N, Senore C, Andreoni B, et al; SCORE3 Working Group-Italy. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132(7):2304–2312. doi:10.1053/j.gastro.2007.03.030
KEY POINTS
- Colorectal cancer rates are increasing in young individuals, with 10,000 new cases reported in 2017 in people ages 20 to 49. The evidence to support screening at ages 45 to 50 is not well established.
- FIT is noninvasive but requires high patient adherence and the ability to follow a multistep process. Preliminary results from one trial showed it inferior to colonoscopy for detecting colorectal cancer precursors.
- Colonoscopy allows visualization and removal of precursor lesions. A positive FIT result requires follow-up colonoscopy within 10 months.
TITAN trial yields big survival benefits in mCSPC
CHICAGO – Adding the androgen-binding inhibitor apalutamide to androgen deprivation therapy (ADT) significantly increased radiographic progression-free survival and overall survival in men with metastatic castration-sensitive prostate cancer (mCSPC) compared with ADT alone in the phase 3 TITAN trial.
Among 1052 patients randomized to apalutamide (Erleada) plus either ADT or placebo, the overall survival rate at 24 months was 82.4% in the apalutamide group, compared with 73.5% in the placebo group, translating into a hazard ratio for death with apalutamide of 0.67 (P = .005), reported Kim N. Chi, MD, from the BC Cancer and Vancouver Prostate Centre in Vancouver, British Columbia, Canada.
“The TITAN study met its dual primary end points, demonstrating significant benefits with apalutamide plus ADT in an all-comer mCSPC population. There were significant improvements in overall survival with a 33% reduction in the risk of death. There was also a significant improvement in radiographic progression-free survival with a 52% reduction in the risk of progression or death,” he said at the American Society of Clinical Oncology annual meeting.
The study was also published online in the New England Journal of Medicine to coincide with Dr. Chi’s presentation.
The TITAN (Targeted Investigational Treatment Analysis of Novel Anti-androgen) study was designed to evaluate apalutamide vs. placebo in a broad population of patients with mCSPC treated with continuous ADT.
“The rationale behind the TITAN study was that direct inhibition of the androgen receptor by apalutamide will provide a more complete reduction of androgen signaling than ADT alone, leading to improved clinical outcomes,” Dr. Chi said.
The investigators enrolled a total of 1052 men with castration-sensitive prostate cancer, distant metastatic disease manifested as one or more lesions on bone scans, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
All patients were on continuous ADT. Prior therapies allowed under the protocol included docetaxel for a maximum of 6 cycles with no evidence of progression during treatment or before randomization, ADT for not more than 6 months for mCSPC or not more than 3 years for localized prostate cancer, one course of radiation of surgery for symptoms associated with metastatic disease, or other localized treatments completed at least 1 year before randomization.
The median patient age was 68 years. In all 62.7% of patients had high-volume disease, and the remainder had low-volume disease.
In this, the first interim analysis conducted at a median follow-up of 22.7 months, the 68.2% of patients in the apalutamide group had radiographic PFS, compared with 47.5% in the placebo group. The hazard ratio for progression or death with apalutamide was 0.48 (P less than .001).
As noted before, 24-month overall survival rates were 82.4% vs. 73.5%, respectively.
The secondary endpoint of median time to cytotoxic chemotherapy also significantly favored apalutamide, with a hazard ratio of 0.39 (P less than .0001). Other secondary endpoints, including median time to pain progression, median time to chronic opioid use, and median time to skeletal-related events trended in favor of apalutamide but were not statistically significant.
Grade 3 or 4 adverse events occurred in 42.2% of patients in the apalutamide arm and 40.8% in the placebo arm. The incidence of any serious adverse event was 19.8% with apalutamide and 20.3% with placebo. Adverse events leading to discontinuation occurred in 8% and 5.3%, respectively, and adverse events leading to death occurred in 1.9% vs. 3.0%.
Apalutamide was associated with higher incidences of rash, fatigue, hypothyroidism, and fracture.
The study results show that “androgen deprivation therapy and apalutamide for metastatic hormone-sensitive prostate cancer improves survival, and thus reinforces the current practice of ADT plus a single agent, whether it be docetaxel, abiraterone, enzalutamide, and now apalutamide,” commented Michael A. Carducci, MD, from the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore, the invited discussant.
Metastatic castration-sensitive prostate cancer is a broadly heterogeneous disease state, and the treatment benefits offered with various drugs is not consistent in subsets of patients. Investigators need to develop better molecularly based methods, combined with cliniopathologic factors, to better determine which subgroups of patients can benefit from specific drugs, he said.
The TITAN trial was supported by Aragon Pharmaceuticals. Dr. Chi disclosed grants and personal fees from multiple companies, not including Aragon. Dr. Carducci disclosed consulting or advisory roles and institutional research funding from multiple companies, not including Aragon.
SOURCE: Chi KN, ASCO 2019 Abstract 5006. N Engl J Med doi: 10.1056/NEJMoa1903307 .
CHICAGO – Adding the androgen-binding inhibitor apalutamide to androgen deprivation therapy (ADT) significantly increased radiographic progression-free survival and overall survival in men with metastatic castration-sensitive prostate cancer (mCSPC) compared with ADT alone in the phase 3 TITAN trial.
Among 1052 patients randomized to apalutamide (Erleada) plus either ADT or placebo, the overall survival rate at 24 months was 82.4% in the apalutamide group, compared with 73.5% in the placebo group, translating into a hazard ratio for death with apalutamide of 0.67 (P = .005), reported Kim N. Chi, MD, from the BC Cancer and Vancouver Prostate Centre in Vancouver, British Columbia, Canada.
“The TITAN study met its dual primary end points, demonstrating significant benefits with apalutamide plus ADT in an all-comer mCSPC population. There were significant improvements in overall survival with a 33% reduction in the risk of death. There was also a significant improvement in radiographic progression-free survival with a 52% reduction in the risk of progression or death,” he said at the American Society of Clinical Oncology annual meeting.
The study was also published online in the New England Journal of Medicine to coincide with Dr. Chi’s presentation.
The TITAN (Targeted Investigational Treatment Analysis of Novel Anti-androgen) study was designed to evaluate apalutamide vs. placebo in a broad population of patients with mCSPC treated with continuous ADT.
“The rationale behind the TITAN study was that direct inhibition of the androgen receptor by apalutamide will provide a more complete reduction of androgen signaling than ADT alone, leading to improved clinical outcomes,” Dr. Chi said.
The investigators enrolled a total of 1052 men with castration-sensitive prostate cancer, distant metastatic disease manifested as one or more lesions on bone scans, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
All patients were on continuous ADT. Prior therapies allowed under the protocol included docetaxel for a maximum of 6 cycles with no evidence of progression during treatment or before randomization, ADT for not more than 6 months for mCSPC or not more than 3 years for localized prostate cancer, one course of radiation of surgery for symptoms associated with metastatic disease, or other localized treatments completed at least 1 year before randomization.
The median patient age was 68 years. In all 62.7% of patients had high-volume disease, and the remainder had low-volume disease.
In this, the first interim analysis conducted at a median follow-up of 22.7 months, the 68.2% of patients in the apalutamide group had radiographic PFS, compared with 47.5% in the placebo group. The hazard ratio for progression or death with apalutamide was 0.48 (P less than .001).
As noted before, 24-month overall survival rates were 82.4% vs. 73.5%, respectively.
The secondary endpoint of median time to cytotoxic chemotherapy also significantly favored apalutamide, with a hazard ratio of 0.39 (P less than .0001). Other secondary endpoints, including median time to pain progression, median time to chronic opioid use, and median time to skeletal-related events trended in favor of apalutamide but were not statistically significant.
Grade 3 or 4 adverse events occurred in 42.2% of patients in the apalutamide arm and 40.8% in the placebo arm. The incidence of any serious adverse event was 19.8% with apalutamide and 20.3% with placebo. Adverse events leading to discontinuation occurred in 8% and 5.3%, respectively, and adverse events leading to death occurred in 1.9% vs. 3.0%.
Apalutamide was associated with higher incidences of rash, fatigue, hypothyroidism, and fracture.
The study results show that “androgen deprivation therapy and apalutamide for metastatic hormone-sensitive prostate cancer improves survival, and thus reinforces the current practice of ADT plus a single agent, whether it be docetaxel, abiraterone, enzalutamide, and now apalutamide,” commented Michael A. Carducci, MD, from the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore, the invited discussant.
Metastatic castration-sensitive prostate cancer is a broadly heterogeneous disease state, and the treatment benefits offered with various drugs is not consistent in subsets of patients. Investigators need to develop better molecularly based methods, combined with cliniopathologic factors, to better determine which subgroups of patients can benefit from specific drugs, he said.
The TITAN trial was supported by Aragon Pharmaceuticals. Dr. Chi disclosed grants and personal fees from multiple companies, not including Aragon. Dr. Carducci disclosed consulting or advisory roles and institutional research funding from multiple companies, not including Aragon.
SOURCE: Chi KN, ASCO 2019 Abstract 5006. N Engl J Med doi: 10.1056/NEJMoa1903307 .
CHICAGO – Adding the androgen-binding inhibitor apalutamide to androgen deprivation therapy (ADT) significantly increased radiographic progression-free survival and overall survival in men with metastatic castration-sensitive prostate cancer (mCSPC) compared with ADT alone in the phase 3 TITAN trial.
Among 1052 patients randomized to apalutamide (Erleada) plus either ADT or placebo, the overall survival rate at 24 months was 82.4% in the apalutamide group, compared with 73.5% in the placebo group, translating into a hazard ratio for death with apalutamide of 0.67 (P = .005), reported Kim N. Chi, MD, from the BC Cancer and Vancouver Prostate Centre in Vancouver, British Columbia, Canada.
“The TITAN study met its dual primary end points, demonstrating significant benefits with apalutamide plus ADT in an all-comer mCSPC population. There were significant improvements in overall survival with a 33% reduction in the risk of death. There was also a significant improvement in radiographic progression-free survival with a 52% reduction in the risk of progression or death,” he said at the American Society of Clinical Oncology annual meeting.
The study was also published online in the New England Journal of Medicine to coincide with Dr. Chi’s presentation.
The TITAN (Targeted Investigational Treatment Analysis of Novel Anti-androgen) study was designed to evaluate apalutamide vs. placebo in a broad population of patients with mCSPC treated with continuous ADT.
“The rationale behind the TITAN study was that direct inhibition of the androgen receptor by apalutamide will provide a more complete reduction of androgen signaling than ADT alone, leading to improved clinical outcomes,” Dr. Chi said.
The investigators enrolled a total of 1052 men with castration-sensitive prostate cancer, distant metastatic disease manifested as one or more lesions on bone scans, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
All patients were on continuous ADT. Prior therapies allowed under the protocol included docetaxel for a maximum of 6 cycles with no evidence of progression during treatment or before randomization, ADT for not more than 6 months for mCSPC or not more than 3 years for localized prostate cancer, one course of radiation of surgery for symptoms associated with metastatic disease, or other localized treatments completed at least 1 year before randomization.
The median patient age was 68 years. In all 62.7% of patients had high-volume disease, and the remainder had low-volume disease.
In this, the first interim analysis conducted at a median follow-up of 22.7 months, the 68.2% of patients in the apalutamide group had radiographic PFS, compared with 47.5% in the placebo group. The hazard ratio for progression or death with apalutamide was 0.48 (P less than .001).
As noted before, 24-month overall survival rates were 82.4% vs. 73.5%, respectively.
The secondary endpoint of median time to cytotoxic chemotherapy also significantly favored apalutamide, with a hazard ratio of 0.39 (P less than .0001). Other secondary endpoints, including median time to pain progression, median time to chronic opioid use, and median time to skeletal-related events trended in favor of apalutamide but were not statistically significant.
Grade 3 or 4 adverse events occurred in 42.2% of patients in the apalutamide arm and 40.8% in the placebo arm. The incidence of any serious adverse event was 19.8% with apalutamide and 20.3% with placebo. Adverse events leading to discontinuation occurred in 8% and 5.3%, respectively, and adverse events leading to death occurred in 1.9% vs. 3.0%.
Apalutamide was associated with higher incidences of rash, fatigue, hypothyroidism, and fracture.
The study results show that “androgen deprivation therapy and apalutamide for metastatic hormone-sensitive prostate cancer improves survival, and thus reinforces the current practice of ADT plus a single agent, whether it be docetaxel, abiraterone, enzalutamide, and now apalutamide,” commented Michael A. Carducci, MD, from the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore, the invited discussant.
Metastatic castration-sensitive prostate cancer is a broadly heterogeneous disease state, and the treatment benefits offered with various drugs is not consistent in subsets of patients. Investigators need to develop better molecularly based methods, combined with cliniopathologic factors, to better determine which subgroups of patients can benefit from specific drugs, he said.
The TITAN trial was supported by Aragon Pharmaceuticals. Dr. Chi disclosed grants and personal fees from multiple companies, not including Aragon. Dr. Carducci disclosed consulting or advisory roles and institutional research funding from multiple companies, not including Aragon.
SOURCE: Chi KN, ASCO 2019 Abstract 5006. N Engl J Med doi: 10.1056/NEJMoa1903307 .
REPORTING FROM ASCO 2019
Chronic opioid use linked to low testosterone levels
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
NEW ORLEANS – About two thirds of men who chronically use opioids have low testosterone levels, based on a literature search of more than 50 randomized and observational studies that examined endocrine function in patients on chronic opioid therapy.
Hypocortisolism, seen in about 20% of the men in these studies, was among the other potentially significant deficiencies in endocrine function, Amir H. Zamanipoor Najafabadi, PhD, reported at the annual meeting of the Endocrine Society.
Dr. Najafabadi of Leiden University in the Netherlands, and Friso de Vries, PhD, analyzed the link between opioid use and changes in the gonadal axis. Most of the subjects in their study were men (J Endocr Soc. 2019. doi. 10.1210/js.2019-SUN-489).
While the data do not support firm conclusions on the health consequences of these endocrine observations, Dr. Najafabadi said that a prospective trial is needed to determine whether there is a potential benefit from screening patients on chronic opioids for potentially treatable endocrine deficiencies.
REPORTING FROM ENDO 2019
Hip-hop offers lens into psyche of black boys, men
SAN FRANCISCO – The lyrics found in hip-hop can help mental health professionals understand the triumphs and trauma experienced by African American boys and men, Sarah Y. Vinson, MD, said at the annual meeting of the American Psychiatric Association. This understanding can enable clinicians to recognize hopelessness and pain in those patients that they otherwise might have missed.
In this video, Dr. Vinson said her session at the APA meeting looked at the history of hip-hop and focused on the perspectives embedded in the work of several artists/groups, including N.W.A, Tupac Shakur, Childish Gambino (aka Donald Glover), J. Cole, and Kendrick Lamar.
One of the take-home points for clinicians, Dr. Vinson said, is that hip-hop, an art form that has spread across the world, came out of resilience. Another is that suicidality in black men might not look the same as it does in other patients. “It doesn’t necessarily look like cutting your own wrists or having thoughts of killing yourself – it may look like reckless behaviors that put you at risk of being killed by somebody else.”
Dr. Vinson, who is triple boarded in child and adolescent, adult, and forensic psychiatry, is in private practice in Atlanta. She had no financial disclosures.
SAN FRANCISCO – The lyrics found in hip-hop can help mental health professionals understand the triumphs and trauma experienced by African American boys and men, Sarah Y. Vinson, MD, said at the annual meeting of the American Psychiatric Association. This understanding can enable clinicians to recognize hopelessness and pain in those patients that they otherwise might have missed.
In this video, Dr. Vinson said her session at the APA meeting looked at the history of hip-hop and focused on the perspectives embedded in the work of several artists/groups, including N.W.A, Tupac Shakur, Childish Gambino (aka Donald Glover), J. Cole, and Kendrick Lamar.
One of the take-home points for clinicians, Dr. Vinson said, is that hip-hop, an art form that has spread across the world, came out of resilience. Another is that suicidality in black men might not look the same as it does in other patients. “It doesn’t necessarily look like cutting your own wrists or having thoughts of killing yourself – it may look like reckless behaviors that put you at risk of being killed by somebody else.”
Dr. Vinson, who is triple boarded in child and adolescent, adult, and forensic psychiatry, is in private practice in Atlanta. She had no financial disclosures.
SAN FRANCISCO – The lyrics found in hip-hop can help mental health professionals understand the triumphs and trauma experienced by African American boys and men, Sarah Y. Vinson, MD, said at the annual meeting of the American Psychiatric Association. This understanding can enable clinicians to recognize hopelessness and pain in those patients that they otherwise might have missed.
In this video, Dr. Vinson said her session at the APA meeting looked at the history of hip-hop and focused on the perspectives embedded in the work of several artists/groups, including N.W.A, Tupac Shakur, Childish Gambino (aka Donald Glover), J. Cole, and Kendrick Lamar.
One of the take-home points for clinicians, Dr. Vinson said, is that hip-hop, an art form that has spread across the world, came out of resilience. Another is that suicidality in black men might not look the same as it does in other patients. “It doesn’t necessarily look like cutting your own wrists or having thoughts of killing yourself – it may look like reckless behaviors that put you at risk of being killed by somebody else.”
Dr. Vinson, who is triple boarded in child and adolescent, adult, and forensic psychiatry, is in private practice in Atlanta. She had no financial disclosures.
REPORTING FROM APA 2019
Review hints at improved semen quality after bariatric surgery
LOS ANGELES – On the male fertility front, obesity seems to hurt semen quality. So does weight-loss surgery reverse the trend? A new review of existing research suggests that there may be an effect, but the findings aren’t conclusive.
“We found something,” said Sikarin Upala, MD, a second-year endocrinology fellow at the University of Chicago, who pointed out that three of the four reports he and his colleagues reviewed suggested improvement in semen motility. “But we still need to study more about whether bariatric surgery will affect infertility,” he continued.
Dr. Upala, who led the systematic review and meta-analysis of research into bariatric surgery and semen quality, spoke in an interview after his presentation at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
As researchers explained in a 2018 report, “conflicting results have been observed in studies evaluating the correlation between [body mass index] and sperm parameters, such as sperm concentration and total sperm count.” However, they noted that it is “generally accepted” that men with obesity seem to be at higher risk of having a low sperm count or having azoospermia, which is the total lack of sperm in semen.
It’s also not clear whether weight loss directly improves male fertility. “We do know that androgen levels improve after weight-loss surgery, and that might be one factor among several that may contribute to improved male fertility,” Edward Lin, DO, MBA, FACS, professor of surgery and chief of gastrointestinal and general surgery at Emory University, Atlanta, said in an interview.
In their review, Dr. Upala and his colleagues analyzed four studies published between 2012 and 2018 that evaluated the effect of bariatric surgery on semen quality. All of the studies examined semen volume and sperm morphology and motility, and three examined sperm concentration.
A meta-analysis found that motility and volume improved after surgery; however, some of the studies (two for volume, one for motility) failed to show a statistically significant change.
There was no statistically significant difference in sperm morphology or concentration overall, although one study showed a statistically significant improvement in both categories.
Overall, “there might be a little bit of positive effect, but we couldn’t reach a good conclusion because there were too few studies,” Dr. Upala said.
Dr. Lin, director of the Emory Bariatrics Center, agreed that the review findings are limited. He said that although the findings hint at a positive effect on semen quality, “the jury is still out” when it comes to a link between bariatric surgery and male infertility.
“Multiple factors contribute to semen quality,” he added, pointing to vitamin deficiencies, micronutrient levels in the body, enzyme signaling pathways, and sperm chromatin integrity. “In fact, surgically or diet-induced weight loss may be associated with permissive malnutrition, which further exacerbates these deficiencies. Deficiencies in these areas can sometimes take months, if not years, to correct by taking vitamin D or copper or zinc, for example.”
Dr. Lin referred to a small study in which reporters observed semen abnormalities and subfertility after weight-loss surgery despite improvements in androgenic and quality of life levels.
Dr. Upala reported having no relevant disclosures.
LOS ANGELES – On the male fertility front, obesity seems to hurt semen quality. So does weight-loss surgery reverse the trend? A new review of existing research suggests that there may be an effect, but the findings aren’t conclusive.
“We found something,” said Sikarin Upala, MD, a second-year endocrinology fellow at the University of Chicago, who pointed out that three of the four reports he and his colleagues reviewed suggested improvement in semen motility. “But we still need to study more about whether bariatric surgery will affect infertility,” he continued.
Dr. Upala, who led the systematic review and meta-analysis of research into bariatric surgery and semen quality, spoke in an interview after his presentation at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
As researchers explained in a 2018 report, “conflicting results have been observed in studies evaluating the correlation between [body mass index] and sperm parameters, such as sperm concentration and total sperm count.” However, they noted that it is “generally accepted” that men with obesity seem to be at higher risk of having a low sperm count or having azoospermia, which is the total lack of sperm in semen.
It’s also not clear whether weight loss directly improves male fertility. “We do know that androgen levels improve after weight-loss surgery, and that might be one factor among several that may contribute to improved male fertility,” Edward Lin, DO, MBA, FACS, professor of surgery and chief of gastrointestinal and general surgery at Emory University, Atlanta, said in an interview.
In their review, Dr. Upala and his colleagues analyzed four studies published between 2012 and 2018 that evaluated the effect of bariatric surgery on semen quality. All of the studies examined semen volume and sperm morphology and motility, and three examined sperm concentration.
A meta-analysis found that motility and volume improved after surgery; however, some of the studies (two for volume, one for motility) failed to show a statistically significant change.
There was no statistically significant difference in sperm morphology or concentration overall, although one study showed a statistically significant improvement in both categories.
Overall, “there might be a little bit of positive effect, but we couldn’t reach a good conclusion because there were too few studies,” Dr. Upala said.
Dr. Lin, director of the Emory Bariatrics Center, agreed that the review findings are limited. He said that although the findings hint at a positive effect on semen quality, “the jury is still out” when it comes to a link between bariatric surgery and male infertility.
“Multiple factors contribute to semen quality,” he added, pointing to vitamin deficiencies, micronutrient levels in the body, enzyme signaling pathways, and sperm chromatin integrity. “In fact, surgically or diet-induced weight loss may be associated with permissive malnutrition, which further exacerbates these deficiencies. Deficiencies in these areas can sometimes take months, if not years, to correct by taking vitamin D or copper or zinc, for example.”
Dr. Lin referred to a small study in which reporters observed semen abnormalities and subfertility after weight-loss surgery despite improvements in androgenic and quality of life levels.
Dr. Upala reported having no relevant disclosures.
LOS ANGELES – On the male fertility front, obesity seems to hurt semen quality. So does weight-loss surgery reverse the trend? A new review of existing research suggests that there may be an effect, but the findings aren’t conclusive.
“We found something,” said Sikarin Upala, MD, a second-year endocrinology fellow at the University of Chicago, who pointed out that three of the four reports he and his colleagues reviewed suggested improvement in semen motility. “But we still need to study more about whether bariatric surgery will affect infertility,” he continued.
Dr. Upala, who led the systematic review and meta-analysis of research into bariatric surgery and semen quality, spoke in an interview after his presentation at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
As researchers explained in a 2018 report, “conflicting results have been observed in studies evaluating the correlation between [body mass index] and sperm parameters, such as sperm concentration and total sperm count.” However, they noted that it is “generally accepted” that men with obesity seem to be at higher risk of having a low sperm count or having azoospermia, which is the total lack of sperm in semen.
It’s also not clear whether weight loss directly improves male fertility. “We do know that androgen levels improve after weight-loss surgery, and that might be one factor among several that may contribute to improved male fertility,” Edward Lin, DO, MBA, FACS, professor of surgery and chief of gastrointestinal and general surgery at Emory University, Atlanta, said in an interview.
In their review, Dr. Upala and his colleagues analyzed four studies published between 2012 and 2018 that evaluated the effect of bariatric surgery on semen quality. All of the studies examined semen volume and sperm morphology and motility, and three examined sperm concentration.
A meta-analysis found that motility and volume improved after surgery; however, some of the studies (two for volume, one for motility) failed to show a statistically significant change.
There was no statistically significant difference in sperm morphology or concentration overall, although one study showed a statistically significant improvement in both categories.
Overall, “there might be a little bit of positive effect, but we couldn’t reach a good conclusion because there were too few studies,” Dr. Upala said.
Dr. Lin, director of the Emory Bariatrics Center, agreed that the review findings are limited. He said that although the findings hint at a positive effect on semen quality, “the jury is still out” when it comes to a link between bariatric surgery and male infertility.
“Multiple factors contribute to semen quality,” he added, pointing to vitamin deficiencies, micronutrient levels in the body, enzyme signaling pathways, and sperm chromatin integrity. “In fact, surgically or diet-induced weight loss may be associated with permissive malnutrition, which further exacerbates these deficiencies. Deficiencies in these areas can sometimes take months, if not years, to correct by taking vitamin D or copper or zinc, for example.”
Dr. Lin referred to a small study in which reporters observed semen abnormalities and subfertility after weight-loss surgery despite improvements in androgenic and quality of life levels.
Dr. Upala reported having no relevant disclosures.
REPORTING FROM AACE 2019