User login
Oral Relugolix Yields Superior Testosterone Suppression and Decreased Cardiovascular Events Compared With GnRH Agonist
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
He Doesn’t Love It Warts and All
ANSWER
The correct answer is lichen planus (choice “c”).
DISCUSSION
Condyloma accuminata can demonstrate amazing lability, sometimes appearing decades after exposure. And spouses may not always be truthful when questioned about such exposure. To further confuse the issue, it's entirely possible that a patient may be unaware he or she has condyloma. So, this might well have been condyloma. But the differential for penile lesions would include this condition—and more.
Psoriasis (choice “a”) commonly affects the penis, manifesting as pinkish plaques and papules. But there is a good chance that examination would have revealed corroborative signs of this disease. Furthermore, the histologic results would have been entirely different.
While syphilis (choice “b”), especially in its primary stage, can present with nonhealing sores, in no way do they resemble the patient’s lesions. There is also no source for such an infection. And biopsy would have shown a predominately plasma cell infiltrate in an entirely different pattern.
Lichen sclerosus et atrophicus (choice “d”) is quite uncommon, especially on the penis, where it is usually known as balanitis xerotica obliterans (BXO). As its name suggests, BXO is usually atrophic—therefore macular—and whitish. Exclusive to uncircumcised men, it bears no resemblance to condyloma.
Though idiopathic, lichen planus is not contagious. Unless neglected, this condition seldom causes any suffering aside from mental anguish over its appearance. To make a more accurate diagnosis, it is always helpful for providers to consider a mnemonic device for “7 Ps” associated with lichen planus:
- Penile
- Pruritic
- Plaque-like
- Purple
- Papular
- Planar
- Puzzling.
TREATMENT
Fortunately, lichen planus affecting the penis responds readily to treatment with mid-strength topical steroid cream and the "tincture of time," which improves its appearance until it eventually disappears. For this patient, the PCP treated the affected area with triamcinolone 0.1% cream bid for 2 weeks. This was then applied once a day every other day for a month, which cleared the patient’s lesions.
ANSWER
The correct answer is lichen planus (choice “c”).
DISCUSSION
Condyloma accuminata can demonstrate amazing lability, sometimes appearing decades after exposure. And spouses may not always be truthful when questioned about such exposure. To further confuse the issue, it's entirely possible that a patient may be unaware he or she has condyloma. So, this might well have been condyloma. But the differential for penile lesions would include this condition—and more.
Psoriasis (choice “a”) commonly affects the penis, manifesting as pinkish plaques and papules. But there is a good chance that examination would have revealed corroborative signs of this disease. Furthermore, the histologic results would have been entirely different.
While syphilis (choice “b”), especially in its primary stage, can present with nonhealing sores, in no way do they resemble the patient’s lesions. There is also no source for such an infection. And biopsy would have shown a predominately plasma cell infiltrate in an entirely different pattern.
Lichen sclerosus et atrophicus (choice “d”) is quite uncommon, especially on the penis, where it is usually known as balanitis xerotica obliterans (BXO). As its name suggests, BXO is usually atrophic—therefore macular—and whitish. Exclusive to uncircumcised men, it bears no resemblance to condyloma.
Though idiopathic, lichen planus is not contagious. Unless neglected, this condition seldom causes any suffering aside from mental anguish over its appearance. To make a more accurate diagnosis, it is always helpful for providers to consider a mnemonic device for “7 Ps” associated with lichen planus:
- Penile
- Pruritic
- Plaque-like
- Purple
- Papular
- Planar
- Puzzling.
TREATMENT
Fortunately, lichen planus affecting the penis responds readily to treatment with mid-strength topical steroid cream and the "tincture of time," which improves its appearance until it eventually disappears. For this patient, the PCP treated the affected area with triamcinolone 0.1% cream bid for 2 weeks. This was then applied once a day every other day for a month, which cleared the patient’s lesions.
ANSWER
The correct answer is lichen planus (choice “c”).
DISCUSSION
Condyloma accuminata can demonstrate amazing lability, sometimes appearing decades after exposure. And spouses may not always be truthful when questioned about such exposure. To further confuse the issue, it's entirely possible that a patient may be unaware he or she has condyloma. So, this might well have been condyloma. But the differential for penile lesions would include this condition—and more.
Psoriasis (choice “a”) commonly affects the penis, manifesting as pinkish plaques and papules. But there is a good chance that examination would have revealed corroborative signs of this disease. Furthermore, the histologic results would have been entirely different.
While syphilis (choice “b”), especially in its primary stage, can present with nonhealing sores, in no way do they resemble the patient’s lesions. There is also no source for such an infection. And biopsy would have shown a predominately plasma cell infiltrate in an entirely different pattern.
Lichen sclerosus et atrophicus (choice “d”) is quite uncommon, especially on the penis, where it is usually known as balanitis xerotica obliterans (BXO). As its name suggests, BXO is usually atrophic—therefore macular—and whitish. Exclusive to uncircumcised men, it bears no resemblance to condyloma.
Though idiopathic, lichen planus is not contagious. Unless neglected, this condition seldom causes any suffering aside from mental anguish over its appearance. To make a more accurate diagnosis, it is always helpful for providers to consider a mnemonic device for “7 Ps” associated with lichen planus:
- Penile
- Pruritic
- Plaque-like
- Purple
- Papular
- Planar
- Puzzling.
TREATMENT
Fortunately, lichen planus affecting the penis responds readily to treatment with mid-strength topical steroid cream and the "tincture of time," which improves its appearance until it eventually disappears. For this patient, the PCP treated the affected area with triamcinolone 0.1% cream bid for 2 weeks. This was then applied once a day every other day for a month, which cleared the patient’s lesions.
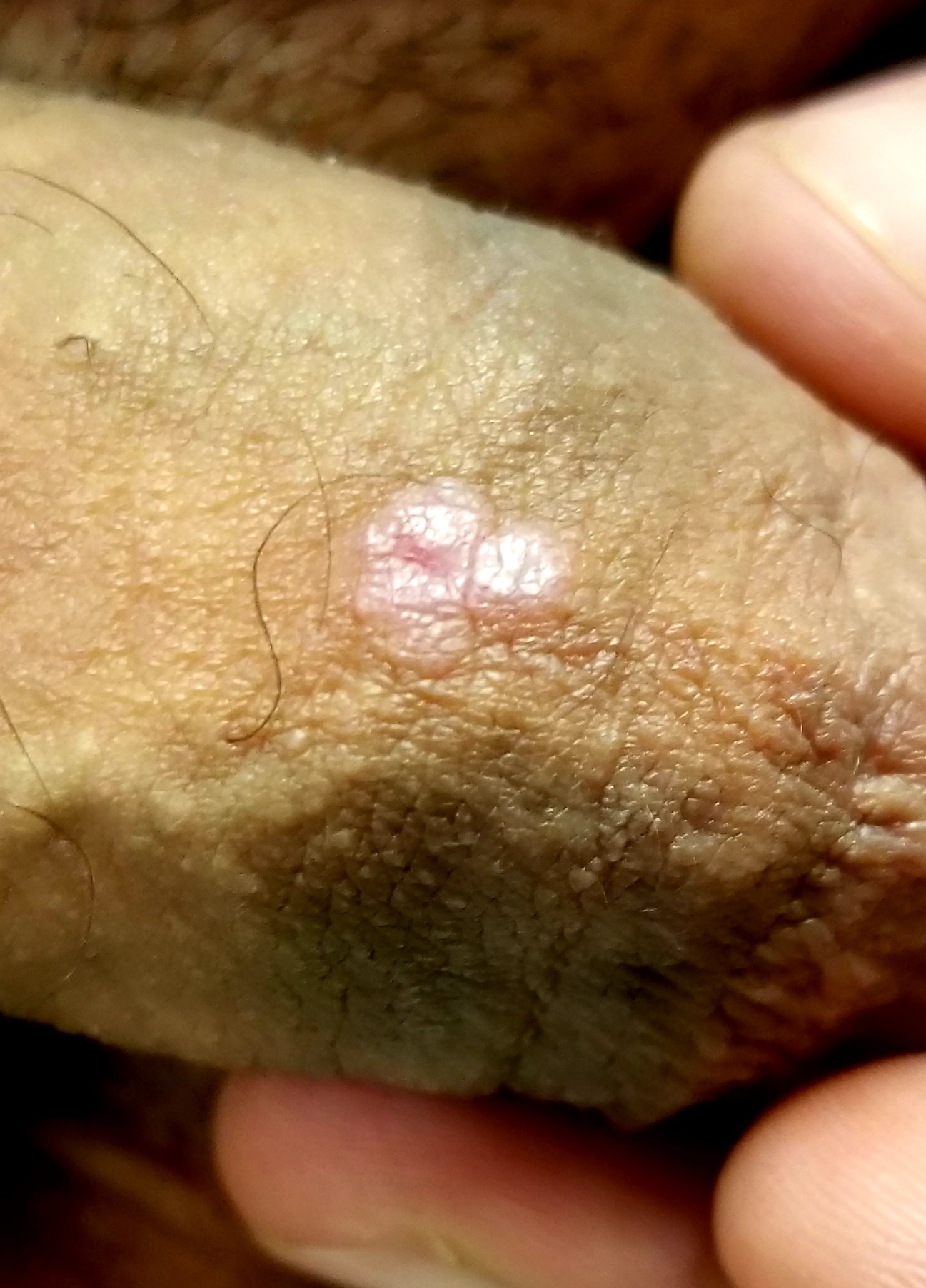
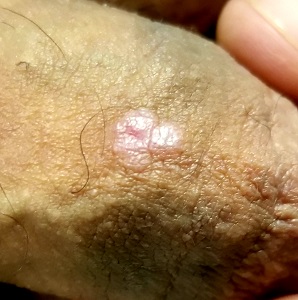
A 41-year-old man is understandably upset when his primary care provider (PCP) diagnoses him with penile warts. Still, he is more than willing to allow his PCP to treat the area with liquid nitrogen, which clears the affected area. However, after about a month, the warts reappear in the same area, with the same appearance, and the patient decides to consult his PCP about additional treatment.
To his distress, his PCP suggests that the warts may continue to return despite treatment. This prompts the patient to ask a more upsetting question: How had he even acquired the warts? Neither he nor his wife of 20 years has had any other sexual contact. Prior to marriage, he had no sexual encounters by which he might have acquired human papillomavirus (HPV).
The patient is otherwise quite healthy, though anxious to have his warts treated again despite the possibility of recurrence. At no point have the warts been symptomatic. His wife's Pap smears have been completely normal.
Examination reveals 4 tiny, pink, planar (flat-topped), 2-to-4-mm papules in 2 locations on the penile shaft. Each has a soft shiny surface. There is also a soft, smooth, pink, annular, 2-cm plaque on the distal shaft that spills over onto the corona focally.
Shave biopsy of 1 lesion shows a brisk lymphocytic infiltrate, which obliterated the dermo-epidermal junction, imparting a jagged sawtooth pattern to its usually smooth wave-like pattern. There are no signs of HPV. The patient has no other remarkable lesions or changes on his elbows, knees, trunk, legs, nails, or scalp.
How can we better engage black men as patients?
I’m a black man, husband, father, son, brother, and a board-certified psychiatrist, child and adolescent psychiatry fellow, and addiction medicine fellow. I write this article as the latter, a colleague, from the former’s perspective, which you would not need to verify via Google, social media, or a badge upon meeting me.
July is Minority Mental Health Awareness Month, established to bring awareness to the unique struggles that marginalized groups face concerning mental illness in the United States.
Given the events of the last few months, including a global pandemic and videotaped killings of Ahmaud Arbery and George Floyd, two unarmed black men, America’s structural racism and inequality are being challenged in historic ways. Black people are suffering. In fact, I was not surprised to learn1 that some black families with sons have expanded the “talk” – which traditionally has focused on dealing with police officers – to include vigilantes.
Because of my extensive work with and treatment of men of color, I would like to answer a key question: “How do psychiatrists and other mental health clinicians better engage men of color? Before the “how,” let’s review the state of black men’s mental health.
According to Healthy People 2020, mental disorders are the leading cause of disability in the United States.2 Among those with diagnosable mental disorders, black people are more likely than are their white counterparts to experience severe symptoms and protracted diseases. Roughly 7% of black men meet the criteria for a lifetime prevalence of major depressive disorder.3 Applying that figure to recent national population estimates means that there are 1.4 million black men currently suffering from major depression. Suicide has been on a continued uptrend among black male youth for more than 2 decades. Moreover, given the high rates of stigma and unmet need in this population, it is likely that these figures are even more dire.
Compared with other groups, black men in the United States face a disproportionate burden of preventable morbidity and mortality rates. Of all the health concerns faced by black men, mental health challenges may be among the most stigmatized.4 Evidence suggests that black men have more adverse life experiences than do men of other racial/ethnic groups, and consequently, experience poorer mental health.5 Black men experience high rates of poverty, unemployment, and underemployment, and are incarcerated at much higher rates than those of men of other racial/ethnic groups.6 It is notable that black male youth are often perceived as older by law enforcement, beginning as early as 10 years old, often resulting in negative interactions.7
Despite those challenges, black men are often expected to project strength, they are expected to minimize displays of emotion when off the field or court (i.e., “Just shut up and dribble”), and they are expected to be true versions of folk hero John Henry. This caricature of black males is used at times to validate shootings of unarmed black males (adults and youth).
Black men’s mental health should be a priority for those in the mental health field. This is particularly the case light of our field’s historical involvement in and promotion of stereotyped clinical descriptions of black men and contributing to health disparities that persist. Black men are nearly six times as likely to be diagnosed with schizophrenia as are white men. To read about holdovers from the days of targeted advertising against black protesters of the 1960s and 1970s, check out “The Protest Psychosis” (Beacon Press, 2010) by psychiatrist and anthropologist Jonathan Metzl, MD, PhD. If you go further back in psychiatric history, the late 1800s, you can learn about the devious diagnosis of drapetomania attributed to enslaved people who were seeking freedom.
Those on the front lines providing mental health services should understand black men’s mental health from an ecological perspective. Beyond the emotional burden that mental illness imposes on the individual, there are more considerable interpersonal and societal implications for the state of black men’s mental health. As such, in our full capacity like other men, black men play an essential role within families, churches, neighborhoods, and organizations.
Given our brief review, we can reconsider our question, “How do psychiatrists and mental health clinicians better engage men of color?”
I will suggest a few fundamental principles that honestly can be applied to any patient but should be strongly considered with your black male patients – given they are likely not accustomed to engaging with the health care system, let alone with a mental health clinician:
1. Create a comfortable environment. Because of stigma, persistent myths, and lack of normalcy with talking to a mental health professional, many patients, including black men, do not have a framework for a psychiatric/psychological evaluation or treatment. It would be essential to set the frame of your encounter. Evidence suggests this can improve engagement and follow-up care among black men.8 In addition, keep in mind that “fictive kin”9 tend to play a major role in the transmission of culture, health promotion, and decision-making in the black community. This helps explain why barbershop initiatives10 are effective. If clinicians are able to allow black male patients to feel comfortable, the clinician, too, might become part of that fictive community and enhance the patient-provider relationship.
2. Allow for storytelling. In the age of the checklist, it can be relatively easy to lose sight that our patients, including black men, have their own narratives. Evidence suggests that physicians interrupt patients early and often. Challenge yourself to allow the patient to tell his story. In consideration of an initial evaluation, it may help to begin by first gathering sociodemographic information (i.e. housing, education, employment, family, etc.); doing so will allow the patient time to get comfortable before you assess possible psychiatric symptoms.
3. Confidentiality assurance. Many black men have a distrust for the health care profession; as such, it is vital that clinicians emphasize that their patients’ information and history will be used only to help the patient. It will be important to inform black male patients of their rights, because often in the greater society, their rights seem to be negated.
4. Be aware of nonverbal language. Given black men’s stereotyped roles in society and recognition that they are regularly perceived as threats, many black men have become adept at reading nonverbal cues (i.e., purse clutched, side comment, etc.). In doing so, clinicians must be attuned to their own nonverbal language. For example, a glance at one’s watch might be interpreted as you’re not listening. It would be better to be upfront and candid by saying something like, “I need to check the time,” rather than attempting to be stealth. Being transparent in that way will let the patient know that you will be upfront with him.
5. Be respectful. During an encounter, and in particular when discussing treatment plans, clinicians must allow the patient space to process and be involved in his care. Allowing the patient time to think through how he would want to proceed provides him a sense of personal agency and lets him know that he is capable of improving his mental wellness.
Black male patients need to feel comfortable, safe, able to trust the clinician. They must feel listened to, understood, and respected. This information might help some clinicians better understand what needs to happen between a black male patient and a nonblack clinician so the patient can feel good about his mental health engagement. To some, these recommendations might seem obvious or too simple, yet if we consider the countless reports of poor patient treatment engagement, adherence, and retention, we cannot deny the need for change. Having black male patients disclose important information during encounters could prevent poor clinical interactions that leave them feeling uncomfortable, uncertain, skeptical, disrespected, and further cynical about mental health care.
Dr. Simon practices at Boston Children’s Hospital. He has no disclosures.
References
1. Bunn C. After Arbery shooting, black parents are rethinking “the talk” to explain white vigilantes. NBC News. 2020 May 19.
2. U.S. Department of Health and Human Services. Office of Disease Prevention and Promotion. Healthy People 2020.
3. Ward E and Mangesha M. Am J Orthopsychiatry. 2013 Apr-Jul;83(2 0 3):386-97.
4. Holden KB et al. J Mens health. 2012 Jun 1;9(2):63-9.
5. Brown TH et al. Fam Community Health. 2015 Oct-Dec;38(4):307-18.
6. Jäggi et al. Soc Ment Health. 2016 Nov;6(3):187-296.
7. Goff PA et al. J Pers Soc Psychol. 2014;106(4):526-45.
8. Alsan M et al. National Bureau of Economic Research. NBER Working Paper No. 24787. 2018 Jun. Revised 2019 Aug.
9. Spruill IJ. J Nat Black Nurses Assoc. 2014 Dec;25(2):23-30.
10. Graham LF et al. Am J Mens Health. 2018 Sep;12(5):1307-16.
I’m a black man, husband, father, son, brother, and a board-certified psychiatrist, child and adolescent psychiatry fellow, and addiction medicine fellow. I write this article as the latter, a colleague, from the former’s perspective, which you would not need to verify via Google, social media, or a badge upon meeting me.
July is Minority Mental Health Awareness Month, established to bring awareness to the unique struggles that marginalized groups face concerning mental illness in the United States.
Given the events of the last few months, including a global pandemic and videotaped killings of Ahmaud Arbery and George Floyd, two unarmed black men, America’s structural racism and inequality are being challenged in historic ways. Black people are suffering. In fact, I was not surprised to learn1 that some black families with sons have expanded the “talk” – which traditionally has focused on dealing with police officers – to include vigilantes.
Because of my extensive work with and treatment of men of color, I would like to answer a key question: “How do psychiatrists and other mental health clinicians better engage men of color? Before the “how,” let’s review the state of black men’s mental health.
According to Healthy People 2020, mental disorders are the leading cause of disability in the United States.2 Among those with diagnosable mental disorders, black people are more likely than are their white counterparts to experience severe symptoms and protracted diseases. Roughly 7% of black men meet the criteria for a lifetime prevalence of major depressive disorder.3 Applying that figure to recent national population estimates means that there are 1.4 million black men currently suffering from major depression. Suicide has been on a continued uptrend among black male youth for more than 2 decades. Moreover, given the high rates of stigma and unmet need in this population, it is likely that these figures are even more dire.
Compared with other groups, black men in the United States face a disproportionate burden of preventable morbidity and mortality rates. Of all the health concerns faced by black men, mental health challenges may be among the most stigmatized.4 Evidence suggests that black men have more adverse life experiences than do men of other racial/ethnic groups, and consequently, experience poorer mental health.5 Black men experience high rates of poverty, unemployment, and underemployment, and are incarcerated at much higher rates than those of men of other racial/ethnic groups.6 It is notable that black male youth are often perceived as older by law enforcement, beginning as early as 10 years old, often resulting in negative interactions.7
Despite those challenges, black men are often expected to project strength, they are expected to minimize displays of emotion when off the field or court (i.e., “Just shut up and dribble”), and they are expected to be true versions of folk hero John Henry. This caricature of black males is used at times to validate shootings of unarmed black males (adults and youth).
Black men’s mental health should be a priority for those in the mental health field. This is particularly the case light of our field’s historical involvement in and promotion of stereotyped clinical descriptions of black men and contributing to health disparities that persist. Black men are nearly six times as likely to be diagnosed with schizophrenia as are white men. To read about holdovers from the days of targeted advertising against black protesters of the 1960s and 1970s, check out “The Protest Psychosis” (Beacon Press, 2010) by psychiatrist and anthropologist Jonathan Metzl, MD, PhD. If you go further back in psychiatric history, the late 1800s, you can learn about the devious diagnosis of drapetomania attributed to enslaved people who were seeking freedom.
Those on the front lines providing mental health services should understand black men’s mental health from an ecological perspective. Beyond the emotional burden that mental illness imposes on the individual, there are more considerable interpersonal and societal implications for the state of black men’s mental health. As such, in our full capacity like other men, black men play an essential role within families, churches, neighborhoods, and organizations.
Given our brief review, we can reconsider our question, “How do psychiatrists and mental health clinicians better engage men of color?”
I will suggest a few fundamental principles that honestly can be applied to any patient but should be strongly considered with your black male patients – given they are likely not accustomed to engaging with the health care system, let alone with a mental health clinician:
1. Create a comfortable environment. Because of stigma, persistent myths, and lack of normalcy with talking to a mental health professional, many patients, including black men, do not have a framework for a psychiatric/psychological evaluation or treatment. It would be essential to set the frame of your encounter. Evidence suggests this can improve engagement and follow-up care among black men.8 In addition, keep in mind that “fictive kin”9 tend to play a major role in the transmission of culture, health promotion, and decision-making in the black community. This helps explain why barbershop initiatives10 are effective. If clinicians are able to allow black male patients to feel comfortable, the clinician, too, might become part of that fictive community and enhance the patient-provider relationship.
2. Allow for storytelling. In the age of the checklist, it can be relatively easy to lose sight that our patients, including black men, have their own narratives. Evidence suggests that physicians interrupt patients early and often. Challenge yourself to allow the patient to tell his story. In consideration of an initial evaluation, it may help to begin by first gathering sociodemographic information (i.e. housing, education, employment, family, etc.); doing so will allow the patient time to get comfortable before you assess possible psychiatric symptoms.
3. Confidentiality assurance. Many black men have a distrust for the health care profession; as such, it is vital that clinicians emphasize that their patients’ information and history will be used only to help the patient. It will be important to inform black male patients of their rights, because often in the greater society, their rights seem to be negated.
4. Be aware of nonverbal language. Given black men’s stereotyped roles in society and recognition that they are regularly perceived as threats, many black men have become adept at reading nonverbal cues (i.e., purse clutched, side comment, etc.). In doing so, clinicians must be attuned to their own nonverbal language. For example, a glance at one’s watch might be interpreted as you’re not listening. It would be better to be upfront and candid by saying something like, “I need to check the time,” rather than attempting to be stealth. Being transparent in that way will let the patient know that you will be upfront with him.
5. Be respectful. During an encounter, and in particular when discussing treatment plans, clinicians must allow the patient space to process and be involved in his care. Allowing the patient time to think through how he would want to proceed provides him a sense of personal agency and lets him know that he is capable of improving his mental wellness.
Black male patients need to feel comfortable, safe, able to trust the clinician. They must feel listened to, understood, and respected. This information might help some clinicians better understand what needs to happen between a black male patient and a nonblack clinician so the patient can feel good about his mental health engagement. To some, these recommendations might seem obvious or too simple, yet if we consider the countless reports of poor patient treatment engagement, adherence, and retention, we cannot deny the need for change. Having black male patients disclose important information during encounters could prevent poor clinical interactions that leave them feeling uncomfortable, uncertain, skeptical, disrespected, and further cynical about mental health care.
Dr. Simon practices at Boston Children’s Hospital. He has no disclosures.
References
1. Bunn C. After Arbery shooting, black parents are rethinking “the talk” to explain white vigilantes. NBC News. 2020 May 19.
2. U.S. Department of Health and Human Services. Office of Disease Prevention and Promotion. Healthy People 2020.
3. Ward E and Mangesha M. Am J Orthopsychiatry. 2013 Apr-Jul;83(2 0 3):386-97.
4. Holden KB et al. J Mens health. 2012 Jun 1;9(2):63-9.
5. Brown TH et al. Fam Community Health. 2015 Oct-Dec;38(4):307-18.
6. Jäggi et al. Soc Ment Health. 2016 Nov;6(3):187-296.
7. Goff PA et al. J Pers Soc Psychol. 2014;106(4):526-45.
8. Alsan M et al. National Bureau of Economic Research. NBER Working Paper No. 24787. 2018 Jun. Revised 2019 Aug.
9. Spruill IJ. J Nat Black Nurses Assoc. 2014 Dec;25(2):23-30.
10. Graham LF et al. Am J Mens Health. 2018 Sep;12(5):1307-16.
I’m a black man, husband, father, son, brother, and a board-certified psychiatrist, child and adolescent psychiatry fellow, and addiction medicine fellow. I write this article as the latter, a colleague, from the former’s perspective, which you would not need to verify via Google, social media, or a badge upon meeting me.
July is Minority Mental Health Awareness Month, established to bring awareness to the unique struggles that marginalized groups face concerning mental illness in the United States.
Given the events of the last few months, including a global pandemic and videotaped killings of Ahmaud Arbery and George Floyd, two unarmed black men, America’s structural racism and inequality are being challenged in historic ways. Black people are suffering. In fact, I was not surprised to learn1 that some black families with sons have expanded the “talk” – which traditionally has focused on dealing with police officers – to include vigilantes.
Because of my extensive work with and treatment of men of color, I would like to answer a key question: “How do psychiatrists and other mental health clinicians better engage men of color? Before the “how,” let’s review the state of black men’s mental health.
According to Healthy People 2020, mental disorders are the leading cause of disability in the United States.2 Among those with diagnosable mental disorders, black people are more likely than are their white counterparts to experience severe symptoms and protracted diseases. Roughly 7% of black men meet the criteria for a lifetime prevalence of major depressive disorder.3 Applying that figure to recent national population estimates means that there are 1.4 million black men currently suffering from major depression. Suicide has been on a continued uptrend among black male youth for more than 2 decades. Moreover, given the high rates of stigma and unmet need in this population, it is likely that these figures are even more dire.
Compared with other groups, black men in the United States face a disproportionate burden of preventable morbidity and mortality rates. Of all the health concerns faced by black men, mental health challenges may be among the most stigmatized.4 Evidence suggests that black men have more adverse life experiences than do men of other racial/ethnic groups, and consequently, experience poorer mental health.5 Black men experience high rates of poverty, unemployment, and underemployment, and are incarcerated at much higher rates than those of men of other racial/ethnic groups.6 It is notable that black male youth are often perceived as older by law enforcement, beginning as early as 10 years old, often resulting in negative interactions.7
Despite those challenges, black men are often expected to project strength, they are expected to minimize displays of emotion when off the field or court (i.e., “Just shut up and dribble”), and they are expected to be true versions of folk hero John Henry. This caricature of black males is used at times to validate shootings of unarmed black males (adults and youth).
Black men’s mental health should be a priority for those in the mental health field. This is particularly the case light of our field’s historical involvement in and promotion of stereotyped clinical descriptions of black men and contributing to health disparities that persist. Black men are nearly six times as likely to be diagnosed with schizophrenia as are white men. To read about holdovers from the days of targeted advertising against black protesters of the 1960s and 1970s, check out “The Protest Psychosis” (Beacon Press, 2010) by psychiatrist and anthropologist Jonathan Metzl, MD, PhD. If you go further back in psychiatric history, the late 1800s, you can learn about the devious diagnosis of drapetomania attributed to enslaved people who were seeking freedom.
Those on the front lines providing mental health services should understand black men’s mental health from an ecological perspective. Beyond the emotional burden that mental illness imposes on the individual, there are more considerable interpersonal and societal implications for the state of black men’s mental health. As such, in our full capacity like other men, black men play an essential role within families, churches, neighborhoods, and organizations.
Given our brief review, we can reconsider our question, “How do psychiatrists and mental health clinicians better engage men of color?”
I will suggest a few fundamental principles that honestly can be applied to any patient but should be strongly considered with your black male patients – given they are likely not accustomed to engaging with the health care system, let alone with a mental health clinician:
1. Create a comfortable environment. Because of stigma, persistent myths, and lack of normalcy with talking to a mental health professional, many patients, including black men, do not have a framework for a psychiatric/psychological evaluation or treatment. It would be essential to set the frame of your encounter. Evidence suggests this can improve engagement and follow-up care among black men.8 In addition, keep in mind that “fictive kin”9 tend to play a major role in the transmission of culture, health promotion, and decision-making in the black community. This helps explain why barbershop initiatives10 are effective. If clinicians are able to allow black male patients to feel comfortable, the clinician, too, might become part of that fictive community and enhance the patient-provider relationship.
2. Allow for storytelling. In the age of the checklist, it can be relatively easy to lose sight that our patients, including black men, have their own narratives. Evidence suggests that physicians interrupt patients early and often. Challenge yourself to allow the patient to tell his story. In consideration of an initial evaluation, it may help to begin by first gathering sociodemographic information (i.e. housing, education, employment, family, etc.); doing so will allow the patient time to get comfortable before you assess possible psychiatric symptoms.
3. Confidentiality assurance. Many black men have a distrust for the health care profession; as such, it is vital that clinicians emphasize that their patients’ information and history will be used only to help the patient. It will be important to inform black male patients of their rights, because often in the greater society, their rights seem to be negated.
4. Be aware of nonverbal language. Given black men’s stereotyped roles in society and recognition that they are regularly perceived as threats, many black men have become adept at reading nonverbal cues (i.e., purse clutched, side comment, etc.). In doing so, clinicians must be attuned to their own nonverbal language. For example, a glance at one’s watch might be interpreted as you’re not listening. It would be better to be upfront and candid by saying something like, “I need to check the time,” rather than attempting to be stealth. Being transparent in that way will let the patient know that you will be upfront with him.
5. Be respectful. During an encounter, and in particular when discussing treatment plans, clinicians must allow the patient space to process and be involved in his care. Allowing the patient time to think through how he would want to proceed provides him a sense of personal agency and lets him know that he is capable of improving his mental wellness.
Black male patients need to feel comfortable, safe, able to trust the clinician. They must feel listened to, understood, and respected. This information might help some clinicians better understand what needs to happen between a black male patient and a nonblack clinician so the patient can feel good about his mental health engagement. To some, these recommendations might seem obvious or too simple, yet if we consider the countless reports of poor patient treatment engagement, adherence, and retention, we cannot deny the need for change. Having black male patients disclose important information during encounters could prevent poor clinical interactions that leave them feeling uncomfortable, uncertain, skeptical, disrespected, and further cynical about mental health care.
Dr. Simon practices at Boston Children’s Hospital. He has no disclosures.
References
1. Bunn C. After Arbery shooting, black parents are rethinking “the talk” to explain white vigilantes. NBC News. 2020 May 19.
2. U.S. Department of Health and Human Services. Office of Disease Prevention and Promotion. Healthy People 2020.
3. Ward E and Mangesha M. Am J Orthopsychiatry. 2013 Apr-Jul;83(2 0 3):386-97.
4. Holden KB et al. J Mens health. 2012 Jun 1;9(2):63-9.
5. Brown TH et al. Fam Community Health. 2015 Oct-Dec;38(4):307-18.
6. Jäggi et al. Soc Ment Health. 2016 Nov;6(3):187-296.
7. Goff PA et al. J Pers Soc Psychol. 2014;106(4):526-45.
8. Alsan M et al. National Bureau of Economic Research. NBER Working Paper No. 24787. 2018 Jun. Revised 2019 Aug.
9. Spruill IJ. J Nat Black Nurses Assoc. 2014 Dec;25(2):23-30.
10. Graham LF et al. Am J Mens Health. 2018 Sep;12(5):1307-16.
Does cultural tailoring of sexual health programs lead to safer behavior?
including abstinence, condom use, and number of sex partners, a meta-analysis of 12 studies suggests.
Furthermore, cultural tailoring may contribute to a program’s success, the data indicate.
“It is important that culturally tailored sexual health programs be available to Hispanic communities across the United States,” the study authors stated in Pediatrics.
To examine the effects of sexual health interventions on behavioral outcomes among Hispanic adolescents and factors that may influence the success of an intervention, Reina Evans, a doctoral student in the department of psychology at North Carolina State University in Raleigh, and colleagues systemically reviewed published studies that included Hispanic adolescents in the United States. Included studies evaluated a sexual health intervention using an experimental or quasiexperimental design and assessed a behavioral outcome.
The researchers synthesized effect sizes from 12 studies that included 4,673 Hispanic adolescents. “As the indicator of effect size, the standardized mean difference, Cohen’s d, was used,” they said. Effect size was interpreted as small at 0.20, medium at 0.50, and large at 0.80.
Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Eight of the 12 interventions incorporated Hispanic-specific practices and values such as familialism into the intervention materials. Culturally tailored interventions produced greater change in condom use, compared with interventions that were not culturally tailored. One intervention with a large effect on condom use was developed by researchers in collaboration with community members, the authors said. Another program with a large effect on condom use was designed for Hispanic families.
Ten of the 12 studies included males and females, and two included only females. Intervention dose ranged from less than 10 hours of program content to more than 20 hours of content.
Definitions of abstinence and time frames for reporting recent condom use varied across studies, the researchers noted. Data about patient characteristics, such as the percentage of participants born in the United States, and pregnancy outcomes were limited. These domains could be areas of future research.
“Latinx adolescents are disproportionately burdened with unplanned pregnancy and STIs [sexually transmitted infections]. In this meta-analysis, it is shown that sexual health interventions can play a role in combating these health disparities,” Ms. Evans and associates said.
Among Hispanic adolescents, persistent disparities in sexual and reproductive health “remain a national public health priority,” and “strengthening the effects of future ... interventions for Hispanic adolescents is needed,” said Vincent Guilamo-Ramos, PhD, MPH, and colleagues in an accompanying editorial. Dr. Guilamo-Ramos is a professor of social work and director and founder of the Center for Latino Adolescent and Family Health at New York University.
“Evans et al. highlighted that reporting on the foreign-born participant proportions was incomplete across studies, thereby excluding this clinical heterogeneity domain from formal moderation analyses,” said Dr. Guilamo-Ramos and colleagues. People who develop Hispanic sexual and reproductive health interventions may consider whether this domain or other domains moderate intervention effectiveness.
Although sensitivity analyses focused on several potential sources of bias, “other domains of potential methodologic heterogeneity, such as refusal bias, differential attrition, or information bias, remained unaccounted for,” they said.
“Attention to clinical, methodologic, and statistical heterogeneity across studies can yield insights into factors associated with bolstering intervention effectiveness. Cultural tailoring to increase the effectiveness of condom interventions for Hispanic adolescents is one such intervention effect modifier,” Dr. Guilamo-Ramos and associates concluded.
The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University. The editorialists are supported by the William T. Grant Foundation and the National Institutes of Health. In addition, Dr. Guilamo-Ramos has received grants and personal fees from ViiV Healthcare outside the submitted work and serves as a member of the U.S. Presidential Advisory Council on HIV/AIDS and as the vice chair of the board of directors of the Latino Commission on AIDS. His coauthors had no relevant financial disclosures.
SOURCES: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572; Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2020-1406.
including abstinence, condom use, and number of sex partners, a meta-analysis of 12 studies suggests.
Furthermore, cultural tailoring may contribute to a program’s success, the data indicate.
“It is important that culturally tailored sexual health programs be available to Hispanic communities across the United States,” the study authors stated in Pediatrics.
To examine the effects of sexual health interventions on behavioral outcomes among Hispanic adolescents and factors that may influence the success of an intervention, Reina Evans, a doctoral student in the department of psychology at North Carolina State University in Raleigh, and colleagues systemically reviewed published studies that included Hispanic adolescents in the United States. Included studies evaluated a sexual health intervention using an experimental or quasiexperimental design and assessed a behavioral outcome.
The researchers synthesized effect sizes from 12 studies that included 4,673 Hispanic adolescents. “As the indicator of effect size, the standardized mean difference, Cohen’s d, was used,” they said. Effect size was interpreted as small at 0.20, medium at 0.50, and large at 0.80.
Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Eight of the 12 interventions incorporated Hispanic-specific practices and values such as familialism into the intervention materials. Culturally tailored interventions produced greater change in condom use, compared with interventions that were not culturally tailored. One intervention with a large effect on condom use was developed by researchers in collaboration with community members, the authors said. Another program with a large effect on condom use was designed for Hispanic families.
Ten of the 12 studies included males and females, and two included only females. Intervention dose ranged from less than 10 hours of program content to more than 20 hours of content.
Definitions of abstinence and time frames for reporting recent condom use varied across studies, the researchers noted. Data about patient characteristics, such as the percentage of participants born in the United States, and pregnancy outcomes were limited. These domains could be areas of future research.
“Latinx adolescents are disproportionately burdened with unplanned pregnancy and STIs [sexually transmitted infections]. In this meta-analysis, it is shown that sexual health interventions can play a role in combating these health disparities,” Ms. Evans and associates said.
Among Hispanic adolescents, persistent disparities in sexual and reproductive health “remain a national public health priority,” and “strengthening the effects of future ... interventions for Hispanic adolescents is needed,” said Vincent Guilamo-Ramos, PhD, MPH, and colleagues in an accompanying editorial. Dr. Guilamo-Ramos is a professor of social work and director and founder of the Center for Latino Adolescent and Family Health at New York University.
“Evans et al. highlighted that reporting on the foreign-born participant proportions was incomplete across studies, thereby excluding this clinical heterogeneity domain from formal moderation analyses,” said Dr. Guilamo-Ramos and colleagues. People who develop Hispanic sexual and reproductive health interventions may consider whether this domain or other domains moderate intervention effectiveness.
Although sensitivity analyses focused on several potential sources of bias, “other domains of potential methodologic heterogeneity, such as refusal bias, differential attrition, or information bias, remained unaccounted for,” they said.
“Attention to clinical, methodologic, and statistical heterogeneity across studies can yield insights into factors associated with bolstering intervention effectiveness. Cultural tailoring to increase the effectiveness of condom interventions for Hispanic adolescents is one such intervention effect modifier,” Dr. Guilamo-Ramos and associates concluded.
The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University. The editorialists are supported by the William T. Grant Foundation and the National Institutes of Health. In addition, Dr. Guilamo-Ramos has received grants and personal fees from ViiV Healthcare outside the submitted work and serves as a member of the U.S. Presidential Advisory Council on HIV/AIDS and as the vice chair of the board of directors of the Latino Commission on AIDS. His coauthors had no relevant financial disclosures.
SOURCES: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572; Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2020-1406.
including abstinence, condom use, and number of sex partners, a meta-analysis of 12 studies suggests.
Furthermore, cultural tailoring may contribute to a program’s success, the data indicate.
“It is important that culturally tailored sexual health programs be available to Hispanic communities across the United States,” the study authors stated in Pediatrics.
To examine the effects of sexual health interventions on behavioral outcomes among Hispanic adolescents and factors that may influence the success of an intervention, Reina Evans, a doctoral student in the department of psychology at North Carolina State University in Raleigh, and colleagues systemically reviewed published studies that included Hispanic adolescents in the United States. Included studies evaluated a sexual health intervention using an experimental or quasiexperimental design and assessed a behavioral outcome.
The researchers synthesized effect sizes from 12 studies that included 4,673 Hispanic adolescents. “As the indicator of effect size, the standardized mean difference, Cohen’s d, was used,” they said. Effect size was interpreted as small at 0.20, medium at 0.50, and large at 0.80.
Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Eight of the 12 interventions incorporated Hispanic-specific practices and values such as familialism into the intervention materials. Culturally tailored interventions produced greater change in condom use, compared with interventions that were not culturally tailored. One intervention with a large effect on condom use was developed by researchers in collaboration with community members, the authors said. Another program with a large effect on condom use was designed for Hispanic families.
Ten of the 12 studies included males and females, and two included only females. Intervention dose ranged from less than 10 hours of program content to more than 20 hours of content.
Definitions of abstinence and time frames for reporting recent condom use varied across studies, the researchers noted. Data about patient characteristics, such as the percentage of participants born in the United States, and pregnancy outcomes were limited. These domains could be areas of future research.
“Latinx adolescents are disproportionately burdened with unplanned pregnancy and STIs [sexually transmitted infections]. In this meta-analysis, it is shown that sexual health interventions can play a role in combating these health disparities,” Ms. Evans and associates said.
Among Hispanic adolescents, persistent disparities in sexual and reproductive health “remain a national public health priority,” and “strengthening the effects of future ... interventions for Hispanic adolescents is needed,” said Vincent Guilamo-Ramos, PhD, MPH, and colleagues in an accompanying editorial. Dr. Guilamo-Ramos is a professor of social work and director and founder of the Center for Latino Adolescent and Family Health at New York University.
“Evans et al. highlighted that reporting on the foreign-born participant proportions was incomplete across studies, thereby excluding this clinical heterogeneity domain from formal moderation analyses,” said Dr. Guilamo-Ramos and colleagues. People who develop Hispanic sexual and reproductive health interventions may consider whether this domain or other domains moderate intervention effectiveness.
Although sensitivity analyses focused on several potential sources of bias, “other domains of potential methodologic heterogeneity, such as refusal bias, differential attrition, or information bias, remained unaccounted for,” they said.
“Attention to clinical, methodologic, and statistical heterogeneity across studies can yield insights into factors associated with bolstering intervention effectiveness. Cultural tailoring to increase the effectiveness of condom interventions for Hispanic adolescents is one such intervention effect modifier,” Dr. Guilamo-Ramos and associates concluded.
The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University. The editorialists are supported by the William T. Grant Foundation and the National Institutes of Health. In addition, Dr. Guilamo-Ramos has received grants and personal fees from ViiV Healthcare outside the submitted work and serves as a member of the U.S. Presidential Advisory Council on HIV/AIDS and as the vice chair of the board of directors of the Latino Commission on AIDS. His coauthors had no relevant financial disclosures.
SOURCES: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572; Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2020-1406.
FROM PEDIATRICS
Key clinical point: Among Hispanic adolescents, sexual health interventions have a small but significant effect on improving safe sexual behavior. Cultural tailoring may contribute to a program’s success.
Major finding: Sexual health interventions improved abstinence (d = 0.15), condom use (d = 0.44), number of sex partners (d = –0.19), and sexual health knowledge (d = 0.40), compared with control conditions.
Study details: A meta-analysis of 12 studies with 4,673 participants.
Disclosures: The study authors had no relevant financial disclosures. The research was supported by the Doug Kirby Adolescent Sexual Health Research Grant from the Rural Center for AIDS and Sexually Transmitted Disease Prevention, Indiana University School of Public Health–Bloomington, and the Center for Family and Community Engagement, North Carolina State University.
Source: Evans R et al. Pediatrics. 2020 Jun 10. doi: 10.1542/peds.2019-3572.
Erectile dysfunction: How to help patients & partners
THE CASE
Eric M,* a 36-year-old new patient, visits a primary care clinic for a check-up accompanied by his wife. A thorough history and physical exam reveal no concerns. He is active and a nonsmoker, drinks only socially, takes no medications, and reports no concerning symptoms. At the end of the visit, though, he says he has been experiencing erectile dysfunction for the past 6 months. What began as intermittent difficulty maintaining erections now “happens a lot.” He is distressed and says, “It just came out of the blue.” The patient’s wife then says she believes men cannot achieve erections if they are having an affair. When the chagrined patient simply asks for “those pills,” his wife says in a raised voice, “He’s a liar!”
●
*The patient’s name has been changed to protect his identity.
Some family physicians may feel ill-equipped to talk about sexual and relational problems and lack the skills to effectively counsel on these matters.1 Despite the fact that more than 70% of adult patients want to discuss sexual topics with their family physician, sexual problems are documented in as few as 2% of patient notes.2 One of the most commonly noted sexual health concerns is erectile dysfunction (ED), estimated to occur in 35% of men ages 40 to 70.3 Many ED cases have psychological antecedents including stress, depression, performance anxiety, pornography addiction, and relationship concerns.4,5
Assessing ED. The inability to achieve or maintain an erection needed for satisfactory sexual activity is typically diagnosed through symptom self-report and with thorough history taking and physical examination.6 However, more objective scales can be used. In particular, the International Index of Erectile Function, a 15-question scale, is useful for both diagnosis and treatment monitoring (www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/iief.pdf).7 Common contributors to ED can be vascular (eg, hypertension), neurologic (eg, multiple sclerosis), psychological (noted earlier), or hormonal (eg, thyroid imbalances).6 In this article, we focus on the relationship context in which ED exists. A review of medical evaluation and management can be found elsewhere.8
Key relational questions
Identify norms that are specific to the couple. Patients from a variety of cultures prefer that their clinicians initiate the conversation about ED.11,12 We specifically recommend that clinicians, using relationally focused questions, inquire about sexual norms and desires that may be situated in culture, family of origin, or gender (TABLE 1).

Continue to: Treating ED within a relationship
Treating ED within a relationship
Once a couple’s sexual relationship has been fully assessed, you may confidently develop a treatment plan for managing sexual dysfunction relationally as well as medically, an approach to ED advised by the American Urological Association.13 We propose that primary care treatment for ED involve collaboration between the physician, the patient/couple (if the patient is partnered), and, as needed, a behavioral health specialist.
The physician’s role ...
Managing ED relationally is important on many fronts. If, for instance, a type-5 phosphodiesterase (PDE-5) inhibitor is needed, both the patient and partner should learn about best practices for optimizing success, such as avoiding excessive alcohol intake or high-fat meals immediately before and after taking a PDE-5.14
Sex ed. Regardless of the couple’s age, be prepared to offer high-quality sexual education. Either partner may have faulty knowledge (or even a lack of knowledge) of basic sexual functioning. Physicians have an opportunity to explain healthy erectile functioning, the sexual response cycle, and ways in which PDE-5 medications work (and do not work). (For a list of resources to facilitate these discussions, see TABLE 2.)

Avoid avoidance. Physicians can intervene on patterns of shame that may surround ED simply by discussing sexual functioning openly and honestly. ED often persists due to avoidance—ie, anxiety about sexual performance can lead couples to avoid sex, which perpetuates more anxiety and avoidance. Normalizing typical sexual functioning, encouraging couples to “avoid avoidance,” and providing referrals as needed are core elements of relational intervention for ED.
Setting the tone. Family physicians are not routinely trained in couples therapy. However, you can employ communication skills that allow each partner to be heard by using empathic/reflective listening, de-escalation, and reframing. Asking “What effect are the sexual concerns having on both of you?” and “What were the circumstances of the last sexual encounter that were pleasing to both of you?” can help promote intimacy and mutual satisfaction.
Continue to: The behavioral specialist's role
The behavioral specialist’s role
Behavioral health specialists may treat ED using methods such as cognitive behavioral therapy or evidence-based couple interventions.4 Cognitive methods for the treatment of ED include examination of maladaptive thoughts around pressure to perform and achieving sexual pleasure. Behavioral methods for treatment of ED are typically aimed at the de-coupling of anxiety and sexual activity. These treatments can include relaxation and desensitization, specifically sensate focus therapy.15
Sensate focus therapy involves a specific set of prescriptive rules for sexual activity, initially restricting touch to non-demand pleasurable touch (eg, holding hands) that allows couples to connect in a low-anxiety context focused on relaxation and connection. As couples are able to control anxiety while engaging in these activities, they engage in increasingly more intimate activities. Additionally, behavioral health specialists trained in couples therapy are vital to helping increase communication regarding sexual activity, sexual scripts, and the relationship in general.4
Identifying a treatment team
In coordinating couples care in the treatment of ED, enlist the help of a therapist who has specific knowledge and skills in the treatment of sexual disorders. While the number of qualified or certified sex therapists is limited, referring providers can visit the American Association of Sexuality Educators, Counselors, and Therapists Web site (www.aasect.org) for possible referral sources. Another option is the American Association for Marriage and Family Therapy Web site (www.aamft.org) under “Find a therapist.” Lastly, the Society for Sex Therapy and Research (www.sstarnet.org) is another professional association that provides information and local referral sources. For patients and partners located in rural areas where access is limited, telehealth options may need to be explored.
THE CASE
Mr. M and his wife were seen for a follow-up appointment by his primary care provider, who ruled out any additional causes of ED (eg, hormonal, vascular), discussed with both the patient and his wife basic sexual health and sexual functioning, dispelled several commonly held myths (ie, individuals cannot obtain an erection because of infidelity or lying), validated sexual concerns as a significant health issue, and prescribed a PDE-5 inhibitor.
Mr. M and his wife were referred to a behavioral health specialist in the clinic who had expertise in couples therapy. At several subsequent visits, the patient and his wife worked on improving the quality and quantity of communication regarding their sexual goals, mutual de-escalation of anxiety, increased emotional intimacy, and sensate focus techniques.
Continue to: As the result of the interventions...
As the result of these interventions, both the patient and his wife were able to engage in sex with less anxiety, and the patient increasingly was able to achieve more satisfactory erections without the use of the PDE-5 inhibitor. At the conclusion of therapy, the patient and his wife reported an increase in sexual satisfaction.
CORRESPONDENCE
Katherine Buck, PhD, Family Health Center, John Peter Smith Health Network, 1500 S. Main Street, Fort Worth, TX 76104; [email protected].
1. Macdowall W, Parker R, Nanchahal K, et al. ‘Talking of Sex’: developing and piloting a sexual health communication tool for use in primary care. Patient Educ Couns. 2010;81:332-337.
2. Sadovsky R. Asking the questions and offering solutions: the ongoing dialogue between the primary care physician and the patient with erectile dysfunction. Rev Urol. 2003;5(suppl 7):S35-S48.
3. Boston University School of Medicine. Sexual Medicine. Epidemiology of ED. 2019. www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/. Accessed May 27, 2020.
4. Weeks GR, Gambescia N, Hertlein KM, eds. A Clinician’s Guide to Systemic Sex Therapy. 2nd ed. London, England: Routledge; 2016.
5. Colson MH, Cuzin A, Faix A, et al. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27:e7-e13.
6. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94820-94827.
7. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
8. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94:820-827.
9. Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
10. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153-165.
11. Lo WH, Fu SN, Wong SN, et al. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16:755-760.
12. Zweifler J, Padilla A, Schafer S. Barriers to recognition of erectile dysfunction among diabetic Mexican-American men. J Am Board Fam Pract. 1998;11:259-263.
13. American Urological Society. Erectile dysfunction: AUA guideline (2018). www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline. Accessed May 27, 2020.
14. Huang S, Lie J. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. 2013;38;414-419.
15. Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970.
THE CASE
Eric M,* a 36-year-old new patient, visits a primary care clinic for a check-up accompanied by his wife. A thorough history and physical exam reveal no concerns. He is active and a nonsmoker, drinks only socially, takes no medications, and reports no concerning symptoms. At the end of the visit, though, he says he has been experiencing erectile dysfunction for the past 6 months. What began as intermittent difficulty maintaining erections now “happens a lot.” He is distressed and says, “It just came out of the blue.” The patient’s wife then says she believes men cannot achieve erections if they are having an affair. When the chagrined patient simply asks for “those pills,” his wife says in a raised voice, “He’s a liar!”
●
*The patient’s name has been changed to protect his identity.
Some family physicians may feel ill-equipped to talk about sexual and relational problems and lack the skills to effectively counsel on these matters.1 Despite the fact that more than 70% of adult patients want to discuss sexual topics with their family physician, sexual problems are documented in as few as 2% of patient notes.2 One of the most commonly noted sexual health concerns is erectile dysfunction (ED), estimated to occur in 35% of men ages 40 to 70.3 Many ED cases have psychological antecedents including stress, depression, performance anxiety, pornography addiction, and relationship concerns.4,5
Assessing ED. The inability to achieve or maintain an erection needed for satisfactory sexual activity is typically diagnosed through symptom self-report and with thorough history taking and physical examination.6 However, more objective scales can be used. In particular, the International Index of Erectile Function, a 15-question scale, is useful for both diagnosis and treatment monitoring (www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/iief.pdf).7 Common contributors to ED can be vascular (eg, hypertension), neurologic (eg, multiple sclerosis), psychological (noted earlier), or hormonal (eg, thyroid imbalances).6 In this article, we focus on the relationship context in which ED exists. A review of medical evaluation and management can be found elsewhere.8
Key relational questions
Identify norms that are specific to the couple. Patients from a variety of cultures prefer that their clinicians initiate the conversation about ED.11,12 We specifically recommend that clinicians, using relationally focused questions, inquire about sexual norms and desires that may be situated in culture, family of origin, or gender (TABLE 1).

Continue to: Treating ED within a relationship
Treating ED within a relationship
Once a couple’s sexual relationship has been fully assessed, you may confidently develop a treatment plan for managing sexual dysfunction relationally as well as medically, an approach to ED advised by the American Urological Association.13 We propose that primary care treatment for ED involve collaboration between the physician, the patient/couple (if the patient is partnered), and, as needed, a behavioral health specialist.
The physician’s role ...
Managing ED relationally is important on many fronts. If, for instance, a type-5 phosphodiesterase (PDE-5) inhibitor is needed, both the patient and partner should learn about best practices for optimizing success, such as avoiding excessive alcohol intake or high-fat meals immediately before and after taking a PDE-5.14
Sex ed. Regardless of the couple’s age, be prepared to offer high-quality sexual education. Either partner may have faulty knowledge (or even a lack of knowledge) of basic sexual functioning. Physicians have an opportunity to explain healthy erectile functioning, the sexual response cycle, and ways in which PDE-5 medications work (and do not work). (For a list of resources to facilitate these discussions, see TABLE 2.)

Avoid avoidance. Physicians can intervene on patterns of shame that may surround ED simply by discussing sexual functioning openly and honestly. ED often persists due to avoidance—ie, anxiety about sexual performance can lead couples to avoid sex, which perpetuates more anxiety and avoidance. Normalizing typical sexual functioning, encouraging couples to “avoid avoidance,” and providing referrals as needed are core elements of relational intervention for ED.
Setting the tone. Family physicians are not routinely trained in couples therapy. However, you can employ communication skills that allow each partner to be heard by using empathic/reflective listening, de-escalation, and reframing. Asking “What effect are the sexual concerns having on both of you?” and “What were the circumstances of the last sexual encounter that were pleasing to both of you?” can help promote intimacy and mutual satisfaction.
Continue to: The behavioral specialist's role
The behavioral specialist’s role
Behavioral health specialists may treat ED using methods such as cognitive behavioral therapy or evidence-based couple interventions.4 Cognitive methods for the treatment of ED include examination of maladaptive thoughts around pressure to perform and achieving sexual pleasure. Behavioral methods for treatment of ED are typically aimed at the de-coupling of anxiety and sexual activity. These treatments can include relaxation and desensitization, specifically sensate focus therapy.15
Sensate focus therapy involves a specific set of prescriptive rules for sexual activity, initially restricting touch to non-demand pleasurable touch (eg, holding hands) that allows couples to connect in a low-anxiety context focused on relaxation and connection. As couples are able to control anxiety while engaging in these activities, they engage in increasingly more intimate activities. Additionally, behavioral health specialists trained in couples therapy are vital to helping increase communication regarding sexual activity, sexual scripts, and the relationship in general.4
Identifying a treatment team
In coordinating couples care in the treatment of ED, enlist the help of a therapist who has specific knowledge and skills in the treatment of sexual disorders. While the number of qualified or certified sex therapists is limited, referring providers can visit the American Association of Sexuality Educators, Counselors, and Therapists Web site (www.aasect.org) for possible referral sources. Another option is the American Association for Marriage and Family Therapy Web site (www.aamft.org) under “Find a therapist.” Lastly, the Society for Sex Therapy and Research (www.sstarnet.org) is another professional association that provides information and local referral sources. For patients and partners located in rural areas where access is limited, telehealth options may need to be explored.
THE CASE
Mr. M and his wife were seen for a follow-up appointment by his primary care provider, who ruled out any additional causes of ED (eg, hormonal, vascular), discussed with both the patient and his wife basic sexual health and sexual functioning, dispelled several commonly held myths (ie, individuals cannot obtain an erection because of infidelity or lying), validated sexual concerns as a significant health issue, and prescribed a PDE-5 inhibitor.
Mr. M and his wife were referred to a behavioral health specialist in the clinic who had expertise in couples therapy. At several subsequent visits, the patient and his wife worked on improving the quality and quantity of communication regarding their sexual goals, mutual de-escalation of anxiety, increased emotional intimacy, and sensate focus techniques.
Continue to: As the result of the interventions...
As the result of these interventions, both the patient and his wife were able to engage in sex with less anxiety, and the patient increasingly was able to achieve more satisfactory erections without the use of the PDE-5 inhibitor. At the conclusion of therapy, the patient and his wife reported an increase in sexual satisfaction.
CORRESPONDENCE
Katherine Buck, PhD, Family Health Center, John Peter Smith Health Network, 1500 S. Main Street, Fort Worth, TX 76104; [email protected].
THE CASE
Eric M,* a 36-year-old new patient, visits a primary care clinic for a check-up accompanied by his wife. A thorough history and physical exam reveal no concerns. He is active and a nonsmoker, drinks only socially, takes no medications, and reports no concerning symptoms. At the end of the visit, though, he says he has been experiencing erectile dysfunction for the past 6 months. What began as intermittent difficulty maintaining erections now “happens a lot.” He is distressed and says, “It just came out of the blue.” The patient’s wife then says she believes men cannot achieve erections if they are having an affair. When the chagrined patient simply asks for “those pills,” his wife says in a raised voice, “He’s a liar!”
●
*The patient’s name has been changed to protect his identity.
Some family physicians may feel ill-equipped to talk about sexual and relational problems and lack the skills to effectively counsel on these matters.1 Despite the fact that more than 70% of adult patients want to discuss sexual topics with their family physician, sexual problems are documented in as few as 2% of patient notes.2 One of the most commonly noted sexual health concerns is erectile dysfunction (ED), estimated to occur in 35% of men ages 40 to 70.3 Many ED cases have psychological antecedents including stress, depression, performance anxiety, pornography addiction, and relationship concerns.4,5
Assessing ED. The inability to achieve or maintain an erection needed for satisfactory sexual activity is typically diagnosed through symptom self-report and with thorough history taking and physical examination.6 However, more objective scales can be used. In particular, the International Index of Erectile Function, a 15-question scale, is useful for both diagnosis and treatment monitoring (www.baus.org.uk/_userfiles/pages/files/Patients/Leaflets/iief.pdf).7 Common contributors to ED can be vascular (eg, hypertension), neurologic (eg, multiple sclerosis), psychological (noted earlier), or hormonal (eg, thyroid imbalances).6 In this article, we focus on the relationship context in which ED exists. A review of medical evaluation and management can be found elsewhere.8
Key relational questions
Identify norms that are specific to the couple. Patients from a variety of cultures prefer that their clinicians initiate the conversation about ED.11,12 We specifically recommend that clinicians, using relationally focused questions, inquire about sexual norms and desires that may be situated in culture, family of origin, or gender (TABLE 1).

Continue to: Treating ED within a relationship
Treating ED within a relationship
Once a couple’s sexual relationship has been fully assessed, you may confidently develop a treatment plan for managing sexual dysfunction relationally as well as medically, an approach to ED advised by the American Urological Association.13 We propose that primary care treatment for ED involve collaboration between the physician, the patient/couple (if the patient is partnered), and, as needed, a behavioral health specialist.
The physician’s role ...
Managing ED relationally is important on many fronts. If, for instance, a type-5 phosphodiesterase (PDE-5) inhibitor is needed, both the patient and partner should learn about best practices for optimizing success, such as avoiding excessive alcohol intake or high-fat meals immediately before and after taking a PDE-5.14
Sex ed. Regardless of the couple’s age, be prepared to offer high-quality sexual education. Either partner may have faulty knowledge (or even a lack of knowledge) of basic sexual functioning. Physicians have an opportunity to explain healthy erectile functioning, the sexual response cycle, and ways in which PDE-5 medications work (and do not work). (For a list of resources to facilitate these discussions, see TABLE 2.)

Avoid avoidance. Physicians can intervene on patterns of shame that may surround ED simply by discussing sexual functioning openly and honestly. ED often persists due to avoidance—ie, anxiety about sexual performance can lead couples to avoid sex, which perpetuates more anxiety and avoidance. Normalizing typical sexual functioning, encouraging couples to “avoid avoidance,” and providing referrals as needed are core elements of relational intervention for ED.
Setting the tone. Family physicians are not routinely trained in couples therapy. However, you can employ communication skills that allow each partner to be heard by using empathic/reflective listening, de-escalation, and reframing. Asking “What effect are the sexual concerns having on both of you?” and “What were the circumstances of the last sexual encounter that were pleasing to both of you?” can help promote intimacy and mutual satisfaction.
Continue to: The behavioral specialist's role
The behavioral specialist’s role
Behavioral health specialists may treat ED using methods such as cognitive behavioral therapy or evidence-based couple interventions.4 Cognitive methods for the treatment of ED include examination of maladaptive thoughts around pressure to perform and achieving sexual pleasure. Behavioral methods for treatment of ED are typically aimed at the de-coupling of anxiety and sexual activity. These treatments can include relaxation and desensitization, specifically sensate focus therapy.15
Sensate focus therapy involves a specific set of prescriptive rules for sexual activity, initially restricting touch to non-demand pleasurable touch (eg, holding hands) that allows couples to connect in a low-anxiety context focused on relaxation and connection. As couples are able to control anxiety while engaging in these activities, they engage in increasingly more intimate activities. Additionally, behavioral health specialists trained in couples therapy are vital to helping increase communication regarding sexual activity, sexual scripts, and the relationship in general.4
Identifying a treatment team
In coordinating couples care in the treatment of ED, enlist the help of a therapist who has specific knowledge and skills in the treatment of sexual disorders. While the number of qualified or certified sex therapists is limited, referring providers can visit the American Association of Sexuality Educators, Counselors, and Therapists Web site (www.aasect.org) for possible referral sources. Another option is the American Association for Marriage and Family Therapy Web site (www.aamft.org) under “Find a therapist.” Lastly, the Society for Sex Therapy and Research (www.sstarnet.org) is another professional association that provides information and local referral sources. For patients and partners located in rural areas where access is limited, telehealth options may need to be explored.
THE CASE
Mr. M and his wife were seen for a follow-up appointment by his primary care provider, who ruled out any additional causes of ED (eg, hormonal, vascular), discussed with both the patient and his wife basic sexual health and sexual functioning, dispelled several commonly held myths (ie, individuals cannot obtain an erection because of infidelity or lying), validated sexual concerns as a significant health issue, and prescribed a PDE-5 inhibitor.
Mr. M and his wife were referred to a behavioral health specialist in the clinic who had expertise in couples therapy. At several subsequent visits, the patient and his wife worked on improving the quality and quantity of communication regarding their sexual goals, mutual de-escalation of anxiety, increased emotional intimacy, and sensate focus techniques.
Continue to: As the result of the interventions...
As the result of these interventions, both the patient and his wife were able to engage in sex with less anxiety, and the patient increasingly was able to achieve more satisfactory erections without the use of the PDE-5 inhibitor. At the conclusion of therapy, the patient and his wife reported an increase in sexual satisfaction.
CORRESPONDENCE
Katherine Buck, PhD, Family Health Center, John Peter Smith Health Network, 1500 S. Main Street, Fort Worth, TX 76104; [email protected].
1. Macdowall W, Parker R, Nanchahal K, et al. ‘Talking of Sex’: developing and piloting a sexual health communication tool for use in primary care. Patient Educ Couns. 2010;81:332-337.
2. Sadovsky R. Asking the questions and offering solutions: the ongoing dialogue between the primary care physician and the patient with erectile dysfunction. Rev Urol. 2003;5(suppl 7):S35-S48.
3. Boston University School of Medicine. Sexual Medicine. Epidemiology of ED. 2019. www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/. Accessed May 27, 2020.
4. Weeks GR, Gambescia N, Hertlein KM, eds. A Clinician’s Guide to Systemic Sex Therapy. 2nd ed. London, England: Routledge; 2016.
5. Colson MH, Cuzin A, Faix A, et al. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27:e7-e13.
6. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94820-94827.
7. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
8. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94:820-827.
9. Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
10. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153-165.
11. Lo WH, Fu SN, Wong SN, et al. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16:755-760.
12. Zweifler J, Padilla A, Schafer S. Barriers to recognition of erectile dysfunction among diabetic Mexican-American men. J Am Board Fam Pract. 1998;11:259-263.
13. American Urological Society. Erectile dysfunction: AUA guideline (2018). www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline. Accessed May 27, 2020.
14. Huang S, Lie J. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. 2013;38;414-419.
15. Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970.
1. Macdowall W, Parker R, Nanchahal K, et al. ‘Talking of Sex’: developing and piloting a sexual health communication tool for use in primary care. Patient Educ Couns. 2010;81:332-337.
2. Sadovsky R. Asking the questions and offering solutions: the ongoing dialogue between the primary care physician and the patient with erectile dysfunction. Rev Urol. 2003;5(suppl 7):S35-S48.
3. Boston University School of Medicine. Sexual Medicine. Epidemiology of ED. 2019. www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/. Accessed May 27, 2020.
4. Weeks GR, Gambescia N, Hertlein KM, eds. A Clinician’s Guide to Systemic Sex Therapy. 2nd ed. London, England: Routledge; 2016.
5. Colson MH, Cuzin A, Faix A, et al. Current epidemiology of erectile dysfunction, an update. Sexologies. 2018;27:e7-e13.
6. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94820-94827.
7. Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319-326.
8. Rew KT, Heidelbaugh JJ. Erectile dysfunction. Am Fam Physician. 2016;94:820-827.
9. Dean J, Rubio-Aurioles E, McCabe M, et al. Integrating partners into erectile dysfunction treatment: improving the sexual experience for the couple. Int J Clin Pract. 2008;62:127-133.
10. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153-165.
11. Lo WH, Fu SN, Wong SN, et al. Prevalence, correlates, attitude and treatment seeking of erectile dysfunction among type 2 diabetic Chinese men attending primary care outpatient clinics. Asian J Androl. 2014;16:755-760.
12. Zweifler J, Padilla A, Schafer S. Barriers to recognition of erectile dysfunction among diabetic Mexican-American men. J Am Board Fam Pract. 1998;11:259-263.
13. American Urological Society. Erectile dysfunction: AUA guideline (2018). www.auanet.org/guidelines/erectile-dysfunction-(ed)-guideline. Accessed May 27, 2020.
14. Huang S, Lie J. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. 2013;38;414-419.
15. Masters WH, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970.
Testicular sperm may improve IVF outcomes in some cases
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
FROM ACOG 2020
High-intensity exercise builds bone in older men
A high-intensity exercise program, already shown effective in improving bone density and performance in women, is also effective in older men with low bone density, according to the LIFTMOR-M study, published in Bone. The protocol incorporates barbell-based weightlifting and impact training involving jumping chin-ups.
“When you’ve got a condition primarily in one of the sexes, the other sex often gets ignored, and that’s absolute the case with osteoporosis,” said lead author Belinda Beck, PhD, a professor at Griffith University, Gold Coast, Australia, in an interview.
In older adults with low bone density, when it comes to building bone and reducing fracture, a review of the literature suggests that exercise doesn’t work. That’s not really true though, according to Dr. Beck. An unpublished analysis of studies of high-intensity exercise only at her institution shows promise. “It looks like exercise doesn’t work. It’s not that, it’s that the wrong kind of exercise doesn’t work,” she stressed.
The original LIFTMOR trial, in women, was inspired by a collaboration with Lisa Weis, an Olympic weightlifter who specialized in training older women, who subsequently showed improvements on bone scans. “That’s what jump-started it, because just like every other scientist, I would have been too scared to do this kind of loading in this fragile population, and that’s the reason why people haven’t been doing it. They don’t want to break people,” said Dr. Beck.
The investigators “cherry-picked some of those exercises and tested them in the LIFTMOR trial. I was nervous about the study because the weights we were lifting were much heavier than most people had applied for people with osteoporosis. The risk was, we would cause the fractures we were trying to prevent,” said Dr. Beck. Her team tested a high-intensity resistance and impact (HiRIT) protocol in postmenopausal women with low bone mass (J Bone Miner Res. 2019 Mar;34[3]:572. Controls underwent a home-based, low-intensity exercise program. They found improvements in bone density and functional performance, compared with controls.
“The exercise was effective and safe for this population if practiced with proper technique under close supervision,” said Dr. Beck, but she emphasized that the exercises must be led by experienced coaches because of the potential for injury.
The investigators then looked at men. “There are still one in five men over 50 who are going to fracture,” Dr. Beck said.
Her team launched LIFTMOR-M, which enrolled 93 men (mean age, 67.1 years) with a lower than average proximal femur areal bone mineral density. Of them, 34 were randomized to HiRIT, 33 to supervised machine-based isometric axial compression (IAC) exercise training, and 26 were designated as controls and self-selected to usual activities.
The intervention included 8 months of twice-weekly, supervised, 30-minute HiRIT sessions, which included five sets of five repetitions, using more than 85% the weight of the single repetition maximum. The routine included the deadlift, squat, and overhead press. The impact component included five sets of five repetitions of jumping chin-ups followed by a firm, flat-footed landing.
After 8 months, there was no difference in compliance between the two intervention groups. Those in the HiRIT group had improved medial femoral neck cortical thickness, compared with controls (5.6% vs. –0.1%; P = .028) and IAC (5.6% vs. 0.7%; P = .044). Those in the HiRIT group maintained distal tibia trabecular area, while the control group experienced a loss (0.2% vs. –1.6%; P = .013). The IAC group did not show any improvement in bone strength in any of the sites examined, though some findings suggest it may counteract age-related loss in bone strength indices in the distal tibia and radius.
The program requires a fluid movement that maintains a neutral spine throughout. Dr. Beck has developed the Onero program (theboneclinic.com.au/onero/) based on the routine, and licenses it to physical therapists and exercise physiologists.
The study was funded by the Australian Research Foundation and the Australian Government Research Training Program. Dr. Beck owns the Bone Clinic, which sells licenses to the Onero program based on the exercise program used in the study.
SOURCE: Beck B et al. Bone. 2020 April 11. doi: 10.1016/j.bone.2020.115362.
A high-intensity exercise program, already shown effective in improving bone density and performance in women, is also effective in older men with low bone density, according to the LIFTMOR-M study, published in Bone. The protocol incorporates barbell-based weightlifting and impact training involving jumping chin-ups.
“When you’ve got a condition primarily in one of the sexes, the other sex often gets ignored, and that’s absolute the case with osteoporosis,” said lead author Belinda Beck, PhD, a professor at Griffith University, Gold Coast, Australia, in an interview.
In older adults with low bone density, when it comes to building bone and reducing fracture, a review of the literature suggests that exercise doesn’t work. That’s not really true though, according to Dr. Beck. An unpublished analysis of studies of high-intensity exercise only at her institution shows promise. “It looks like exercise doesn’t work. It’s not that, it’s that the wrong kind of exercise doesn’t work,” she stressed.
The original LIFTMOR trial, in women, was inspired by a collaboration with Lisa Weis, an Olympic weightlifter who specialized in training older women, who subsequently showed improvements on bone scans. “That’s what jump-started it, because just like every other scientist, I would have been too scared to do this kind of loading in this fragile population, and that’s the reason why people haven’t been doing it. They don’t want to break people,” said Dr. Beck.
The investigators “cherry-picked some of those exercises and tested them in the LIFTMOR trial. I was nervous about the study because the weights we were lifting were much heavier than most people had applied for people with osteoporosis. The risk was, we would cause the fractures we were trying to prevent,” said Dr. Beck. Her team tested a high-intensity resistance and impact (HiRIT) protocol in postmenopausal women with low bone mass (J Bone Miner Res. 2019 Mar;34[3]:572. Controls underwent a home-based, low-intensity exercise program. They found improvements in bone density and functional performance, compared with controls.
“The exercise was effective and safe for this population if practiced with proper technique under close supervision,” said Dr. Beck, but she emphasized that the exercises must be led by experienced coaches because of the potential for injury.
The investigators then looked at men. “There are still one in five men over 50 who are going to fracture,” Dr. Beck said.
Her team launched LIFTMOR-M, which enrolled 93 men (mean age, 67.1 years) with a lower than average proximal femur areal bone mineral density. Of them, 34 were randomized to HiRIT, 33 to supervised machine-based isometric axial compression (IAC) exercise training, and 26 were designated as controls and self-selected to usual activities.
The intervention included 8 months of twice-weekly, supervised, 30-minute HiRIT sessions, which included five sets of five repetitions, using more than 85% the weight of the single repetition maximum. The routine included the deadlift, squat, and overhead press. The impact component included five sets of five repetitions of jumping chin-ups followed by a firm, flat-footed landing.
After 8 months, there was no difference in compliance between the two intervention groups. Those in the HiRIT group had improved medial femoral neck cortical thickness, compared with controls (5.6% vs. –0.1%; P = .028) and IAC (5.6% vs. 0.7%; P = .044). Those in the HiRIT group maintained distal tibia trabecular area, while the control group experienced a loss (0.2% vs. –1.6%; P = .013). The IAC group did not show any improvement in bone strength in any of the sites examined, though some findings suggest it may counteract age-related loss in bone strength indices in the distal tibia and radius.
The program requires a fluid movement that maintains a neutral spine throughout. Dr. Beck has developed the Onero program (theboneclinic.com.au/onero/) based on the routine, and licenses it to physical therapists and exercise physiologists.
The study was funded by the Australian Research Foundation and the Australian Government Research Training Program. Dr. Beck owns the Bone Clinic, which sells licenses to the Onero program based on the exercise program used in the study.
SOURCE: Beck B et al. Bone. 2020 April 11. doi: 10.1016/j.bone.2020.115362.
A high-intensity exercise program, already shown effective in improving bone density and performance in women, is also effective in older men with low bone density, according to the LIFTMOR-M study, published in Bone. The protocol incorporates barbell-based weightlifting and impact training involving jumping chin-ups.
“When you’ve got a condition primarily in one of the sexes, the other sex often gets ignored, and that’s absolute the case with osteoporosis,” said lead author Belinda Beck, PhD, a professor at Griffith University, Gold Coast, Australia, in an interview.
In older adults with low bone density, when it comes to building bone and reducing fracture, a review of the literature suggests that exercise doesn’t work. That’s not really true though, according to Dr. Beck. An unpublished analysis of studies of high-intensity exercise only at her institution shows promise. “It looks like exercise doesn’t work. It’s not that, it’s that the wrong kind of exercise doesn’t work,” she stressed.
The original LIFTMOR trial, in women, was inspired by a collaboration with Lisa Weis, an Olympic weightlifter who specialized in training older women, who subsequently showed improvements on bone scans. “That’s what jump-started it, because just like every other scientist, I would have been too scared to do this kind of loading in this fragile population, and that’s the reason why people haven’t been doing it. They don’t want to break people,” said Dr. Beck.
The investigators “cherry-picked some of those exercises and tested them in the LIFTMOR trial. I was nervous about the study because the weights we were lifting were much heavier than most people had applied for people with osteoporosis. The risk was, we would cause the fractures we were trying to prevent,” said Dr. Beck. Her team tested a high-intensity resistance and impact (HiRIT) protocol in postmenopausal women with low bone mass (J Bone Miner Res. 2019 Mar;34[3]:572. Controls underwent a home-based, low-intensity exercise program. They found improvements in bone density and functional performance, compared with controls.
“The exercise was effective and safe for this population if practiced with proper technique under close supervision,” said Dr. Beck, but she emphasized that the exercises must be led by experienced coaches because of the potential for injury.
The investigators then looked at men. “There are still one in five men over 50 who are going to fracture,” Dr. Beck said.
Her team launched LIFTMOR-M, which enrolled 93 men (mean age, 67.1 years) with a lower than average proximal femur areal bone mineral density. Of them, 34 were randomized to HiRIT, 33 to supervised machine-based isometric axial compression (IAC) exercise training, and 26 were designated as controls and self-selected to usual activities.
The intervention included 8 months of twice-weekly, supervised, 30-minute HiRIT sessions, which included five sets of five repetitions, using more than 85% the weight of the single repetition maximum. The routine included the deadlift, squat, and overhead press. The impact component included five sets of five repetitions of jumping chin-ups followed by a firm, flat-footed landing.
After 8 months, there was no difference in compliance between the two intervention groups. Those in the HiRIT group had improved medial femoral neck cortical thickness, compared with controls (5.6% vs. –0.1%; P = .028) and IAC (5.6% vs. 0.7%; P = .044). Those in the HiRIT group maintained distal tibia trabecular area, while the control group experienced a loss (0.2% vs. –1.6%; P = .013). The IAC group did not show any improvement in bone strength in any of the sites examined, though some findings suggest it may counteract age-related loss in bone strength indices in the distal tibia and radius.
The program requires a fluid movement that maintains a neutral spine throughout. Dr. Beck has developed the Onero program (theboneclinic.com.au/onero/) based on the routine, and licenses it to physical therapists and exercise physiologists.
The study was funded by the Australian Research Foundation and the Australian Government Research Training Program. Dr. Beck owns the Bone Clinic, which sells licenses to the Onero program based on the exercise program used in the study.
SOURCE: Beck B et al. Bone. 2020 April 11. doi: 10.1016/j.bone.2020.115362.
FROM BONE
New ‘atlas’ maps links between mental disorders, physical illnesses
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
‘Milestone’ study shows promise for pedophilic disorder
Testosterone-suppressing treatment with the gonadotropin-releasing hormone (GnRH) antagonist degarelix may reduce dynamic risk factors for sexual offense in men with pedophilic disorder, new research suggests.
In a first-of-its-kind randomized, controlled trial of 52 help-seeking men with the disorder, degarelix versus placebo significantly dampened two critical risk factors for committing abuse: high sexual desire and sexual attraction to children. In addition, effects were noticeable within 2 weeks.
“The medicine is quick-acting, not only on biological systems but also on thoughts and behavior,” coinvestigator and corresponding author Christoffer Rahm, MD, of the Centre for Psychiatry Research at Karolinska Institutet, Stockholm, said in an interview.
“The effect lasts and increases after 10 weeks, and especially so in the small group of high-risk individuals,” Dr. Rahm added.
The study findings were published in JAMA Psychiatry.
Opportunity for prevention
Although all men with pedophilic disorder do not commit a sexual offense, those who do generally report struggling with their sexual urges for 10 years before committing a sexual crime, the investigators noted.
This presents an opportunity for prevention by treating high-risk individuals without prior convictions. Effective treatment could prevent child sexual abuse and reduce psychosocial stress for the individual with pedophilic disorder, the researchers wrote.
GnRH antagonists are considered effective in reducing paraphilic symptoms, but their use has been limited to correctional settings. – and not just convicted men from prison and the probation system.
“It means the conclusions from the study are applicable to the patients you meet on sexual medicine and general psychiatry clinics too,” Dr. Rahm said.
The study included 52 men with a pedophilic disorder diagnosis and no contraindications to the intervention. All had contacted PrevenTell, the Swedish national telephone helpline for unwanted sexuality.
Half of the participants were randomly assigned to receive two subcutaneous 120-mg injections of degarelix acetate, while the other half received an equal volume of placebo.
The primary endpoint was efficacy at 2 weeks after injection in reducing a composite risk score of five domains for committing child sexual abuse; this risk score ranged from 0 to 15 points (each domain could be rated 0-3). Secondary endpoints included efficacy at 2 and 10 weeks in the composite score, each risk domain, quality of life, self-reported effects, and adverse events.
‘Positive effects’
At 2 weeks, the composite risk score decreased from 7.4 to 4.4 in the degarelix group and from 7.8 to 6.6 in the placebo group, which was a mean between-group difference of –1.8 (95% confidence interval, –3.2 to –0.5; P = .01).
Compared with placebo, the degarelix group also showed a decrease in the composite score at 10 weeks (−2.2; 95% CI, −3.6 to −0.7), in the domains of pedophilic disorder at 2 weeks (−0.7; 95% CI, −1.4 to 0.0) and 10 weeks (−1.1; 95% CI, −1.8 to −0.4), and in sexual preoccupation at 2 weeks (−0.7; 95% CI, −1.2 to −0.3) and 10 weeks (−0.8; 95% CI, −1.3 to −0.3).
There were no between-group differences in the other domains of self-rated risk, low empathy, and impaired self-regulation at 2 or 10 weeks, or in quality of life.
Injection-site reactions were more common with degarelix than placebo (88% vs. 4%, respectively), as were elevations in hepatobiliary enzyme levels (44% vs. 8%). Two patients in the degarelix group were hospitalized as a result of increased suicidal ideation, suggesting “vigilance for the risk of exacerbating suicidality in predisposed individuals is warranted,” the researchers wrote.
“Most patients tolerated it well, many experienced what they thought were positive effects on sexuality, and a majority wanted to continue with the medicine after the study was over and have another injection,” Dr. Rahm said.
Sexual science milestone
In an accompanying editorial, Peer Briken, MD, of the Institute for Sex Research, Sexual Medicine, and Forensic Psychiatry at University Medical Centre, Hamburg, Germany, wrote that the innovative potential of this study should “not be underestimated.”
It has previously been thought that randomized, controlled trials were not possible because it might be unethical to withhold therapy from high-risk participants and thus risk sexual assaults on children in a control group, Dr. Briken noted.
With the current study, “the situation has changed, which marks a milestone in clinical sexual science and the field of forensic psychiatry,” he wrote.
However, the “great benefit” of the study, which is the proof of feasibility of a randomized, controlled trial in this special group of patients and use of a new drug, comes with some “important limitations,” he added.
Only three participants in each treatment group were in the high-risk subgroup. In addition, the most important long-term outcome criterion – reduction in recidivism in high-risk individuals – could not be investigated, he said.
Dr. Briken agreed with the investigators that risk of suicidal tendencies during rapid testosterone withdrawal requires attention.
Despite its limitations, this study is “certainly the most important contribution to the field of pharmacotherapy of pedophilic disorders since Rösler and Witztum’s study on GnRH agonists in 1998. Also, a relevant number of the study participants (58%) were in favor of further application,” he concluded.
The study was funded by the Swedish Society of Medicine, the Söderström-Königska Foundation, the Fredrik and Ingrid Thuring Foundation, the Centre for Psychiatric Research at Karolinska Institutet, the Gothenburg Society of Medicine, Skaraborg Hospital research unit, Region Stockholm, and the Swedish Society for Medical Research. Dr. Rahm and Dr. Briken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Testosterone-suppressing treatment with the gonadotropin-releasing hormone (GnRH) antagonist degarelix may reduce dynamic risk factors for sexual offense in men with pedophilic disorder, new research suggests.
In a first-of-its-kind randomized, controlled trial of 52 help-seeking men with the disorder, degarelix versus placebo significantly dampened two critical risk factors for committing abuse: high sexual desire and sexual attraction to children. In addition, effects were noticeable within 2 weeks.
“The medicine is quick-acting, not only on biological systems but also on thoughts and behavior,” coinvestigator and corresponding author Christoffer Rahm, MD, of the Centre for Psychiatry Research at Karolinska Institutet, Stockholm, said in an interview.
“The effect lasts and increases after 10 weeks, and especially so in the small group of high-risk individuals,” Dr. Rahm added.
The study findings were published in JAMA Psychiatry.
Opportunity for prevention
Although all men with pedophilic disorder do not commit a sexual offense, those who do generally report struggling with their sexual urges for 10 years before committing a sexual crime, the investigators noted.
This presents an opportunity for prevention by treating high-risk individuals without prior convictions. Effective treatment could prevent child sexual abuse and reduce psychosocial stress for the individual with pedophilic disorder, the researchers wrote.
GnRH antagonists are considered effective in reducing paraphilic symptoms, but their use has been limited to correctional settings. – and not just convicted men from prison and the probation system.
“It means the conclusions from the study are applicable to the patients you meet on sexual medicine and general psychiatry clinics too,” Dr. Rahm said.
The study included 52 men with a pedophilic disorder diagnosis and no contraindications to the intervention. All had contacted PrevenTell, the Swedish national telephone helpline for unwanted sexuality.
Half of the participants were randomly assigned to receive two subcutaneous 120-mg injections of degarelix acetate, while the other half received an equal volume of placebo.
The primary endpoint was efficacy at 2 weeks after injection in reducing a composite risk score of five domains for committing child sexual abuse; this risk score ranged from 0 to 15 points (each domain could be rated 0-3). Secondary endpoints included efficacy at 2 and 10 weeks in the composite score, each risk domain, quality of life, self-reported effects, and adverse events.
‘Positive effects’
At 2 weeks, the composite risk score decreased from 7.4 to 4.4 in the degarelix group and from 7.8 to 6.6 in the placebo group, which was a mean between-group difference of –1.8 (95% confidence interval, –3.2 to –0.5; P = .01).
Compared with placebo, the degarelix group also showed a decrease in the composite score at 10 weeks (−2.2; 95% CI, −3.6 to −0.7), in the domains of pedophilic disorder at 2 weeks (−0.7; 95% CI, −1.4 to 0.0) and 10 weeks (−1.1; 95% CI, −1.8 to −0.4), and in sexual preoccupation at 2 weeks (−0.7; 95% CI, −1.2 to −0.3) and 10 weeks (−0.8; 95% CI, −1.3 to −0.3).
There were no between-group differences in the other domains of self-rated risk, low empathy, and impaired self-regulation at 2 or 10 weeks, or in quality of life.
Injection-site reactions were more common with degarelix than placebo (88% vs. 4%, respectively), as were elevations in hepatobiliary enzyme levels (44% vs. 8%). Two patients in the degarelix group were hospitalized as a result of increased suicidal ideation, suggesting “vigilance for the risk of exacerbating suicidality in predisposed individuals is warranted,” the researchers wrote.
“Most patients tolerated it well, many experienced what they thought were positive effects on sexuality, and a majority wanted to continue with the medicine after the study was over and have another injection,” Dr. Rahm said.
Sexual science milestone
In an accompanying editorial, Peer Briken, MD, of the Institute for Sex Research, Sexual Medicine, and Forensic Psychiatry at University Medical Centre, Hamburg, Germany, wrote that the innovative potential of this study should “not be underestimated.”
It has previously been thought that randomized, controlled trials were not possible because it might be unethical to withhold therapy from high-risk participants and thus risk sexual assaults on children in a control group, Dr. Briken noted.
With the current study, “the situation has changed, which marks a milestone in clinical sexual science and the field of forensic psychiatry,” he wrote.
However, the “great benefit” of the study, which is the proof of feasibility of a randomized, controlled trial in this special group of patients and use of a new drug, comes with some “important limitations,” he added.
Only three participants in each treatment group were in the high-risk subgroup. In addition, the most important long-term outcome criterion – reduction in recidivism in high-risk individuals – could not be investigated, he said.
Dr. Briken agreed with the investigators that risk of suicidal tendencies during rapid testosterone withdrawal requires attention.
Despite its limitations, this study is “certainly the most important contribution to the field of pharmacotherapy of pedophilic disorders since Rösler and Witztum’s study on GnRH agonists in 1998. Also, a relevant number of the study participants (58%) were in favor of further application,” he concluded.
The study was funded by the Swedish Society of Medicine, the Söderström-Königska Foundation, the Fredrik and Ingrid Thuring Foundation, the Centre for Psychiatric Research at Karolinska Institutet, the Gothenburg Society of Medicine, Skaraborg Hospital research unit, Region Stockholm, and the Swedish Society for Medical Research. Dr. Rahm and Dr. Briken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Testosterone-suppressing treatment with the gonadotropin-releasing hormone (GnRH) antagonist degarelix may reduce dynamic risk factors for sexual offense in men with pedophilic disorder, new research suggests.
In a first-of-its-kind randomized, controlled trial of 52 help-seeking men with the disorder, degarelix versus placebo significantly dampened two critical risk factors for committing abuse: high sexual desire and sexual attraction to children. In addition, effects were noticeable within 2 weeks.
“The medicine is quick-acting, not only on biological systems but also on thoughts and behavior,” coinvestigator and corresponding author Christoffer Rahm, MD, of the Centre for Psychiatry Research at Karolinska Institutet, Stockholm, said in an interview.
“The effect lasts and increases after 10 weeks, and especially so in the small group of high-risk individuals,” Dr. Rahm added.
The study findings were published in JAMA Psychiatry.
Opportunity for prevention
Although all men with pedophilic disorder do not commit a sexual offense, those who do generally report struggling with their sexual urges for 10 years before committing a sexual crime, the investigators noted.
This presents an opportunity for prevention by treating high-risk individuals without prior convictions. Effective treatment could prevent child sexual abuse and reduce psychosocial stress for the individual with pedophilic disorder, the researchers wrote.
GnRH antagonists are considered effective in reducing paraphilic symptoms, but their use has been limited to correctional settings. – and not just convicted men from prison and the probation system.
“It means the conclusions from the study are applicable to the patients you meet on sexual medicine and general psychiatry clinics too,” Dr. Rahm said.
The study included 52 men with a pedophilic disorder diagnosis and no contraindications to the intervention. All had contacted PrevenTell, the Swedish national telephone helpline for unwanted sexuality.
Half of the participants were randomly assigned to receive two subcutaneous 120-mg injections of degarelix acetate, while the other half received an equal volume of placebo.
The primary endpoint was efficacy at 2 weeks after injection in reducing a composite risk score of five domains for committing child sexual abuse; this risk score ranged from 0 to 15 points (each domain could be rated 0-3). Secondary endpoints included efficacy at 2 and 10 weeks in the composite score, each risk domain, quality of life, self-reported effects, and adverse events.
‘Positive effects’
At 2 weeks, the composite risk score decreased from 7.4 to 4.4 in the degarelix group and from 7.8 to 6.6 in the placebo group, which was a mean between-group difference of –1.8 (95% confidence interval, –3.2 to –0.5; P = .01).
Compared with placebo, the degarelix group also showed a decrease in the composite score at 10 weeks (−2.2; 95% CI, −3.6 to −0.7), in the domains of pedophilic disorder at 2 weeks (−0.7; 95% CI, −1.4 to 0.0) and 10 weeks (−1.1; 95% CI, −1.8 to −0.4), and in sexual preoccupation at 2 weeks (−0.7; 95% CI, −1.2 to −0.3) and 10 weeks (−0.8; 95% CI, −1.3 to −0.3).
There were no between-group differences in the other domains of self-rated risk, low empathy, and impaired self-regulation at 2 or 10 weeks, or in quality of life.
Injection-site reactions were more common with degarelix than placebo (88% vs. 4%, respectively), as were elevations in hepatobiliary enzyme levels (44% vs. 8%). Two patients in the degarelix group were hospitalized as a result of increased suicidal ideation, suggesting “vigilance for the risk of exacerbating suicidality in predisposed individuals is warranted,” the researchers wrote.
“Most patients tolerated it well, many experienced what they thought were positive effects on sexuality, and a majority wanted to continue with the medicine after the study was over and have another injection,” Dr. Rahm said.
Sexual science milestone
In an accompanying editorial, Peer Briken, MD, of the Institute for Sex Research, Sexual Medicine, and Forensic Psychiatry at University Medical Centre, Hamburg, Germany, wrote that the innovative potential of this study should “not be underestimated.”
It has previously been thought that randomized, controlled trials were not possible because it might be unethical to withhold therapy from high-risk participants and thus risk sexual assaults on children in a control group, Dr. Briken noted.
With the current study, “the situation has changed, which marks a milestone in clinical sexual science and the field of forensic psychiatry,” he wrote.
However, the “great benefit” of the study, which is the proof of feasibility of a randomized, controlled trial in this special group of patients and use of a new drug, comes with some “important limitations,” he added.
Only three participants in each treatment group were in the high-risk subgroup. In addition, the most important long-term outcome criterion – reduction in recidivism in high-risk individuals – could not be investigated, he said.
Dr. Briken agreed with the investigators that risk of suicidal tendencies during rapid testosterone withdrawal requires attention.
Despite its limitations, this study is “certainly the most important contribution to the field of pharmacotherapy of pedophilic disorders since Rösler and Witztum’s study on GnRH agonists in 1998. Also, a relevant number of the study participants (58%) were in favor of further application,” he concluded.
The study was funded by the Swedish Society of Medicine, the Söderström-Königska Foundation, the Fredrik and Ingrid Thuring Foundation, the Centre for Psychiatric Research at Karolinska Institutet, the Gothenburg Society of Medicine, Skaraborg Hospital research unit, Region Stockholm, and the Swedish Society for Medical Research. Dr. Rahm and Dr. Briken have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Suicide prevention one key focus of upcoming NIMH strategic plan
Suicide prevention is a high priority for the National Institute of Mental Health (NIMH) and will be one specific area of focus in the federal agency’s 5-year strategic plan that’s set to be released soon, according to Director Joshua A. Gordon, MD, PhD.
The agency is updating its strategic plan to guide research efforts and priorities over the next 5 years, Dr. Gordon said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
That strategic plan, which will cover a broader range of priorities, is scheduled to be published “within the next few weeks,” Dr. Gordon said.
Closing the research gap in suicide prevention is a high priority for NIMH, Dr. Gordon said, especially in light of the age-adjusted U.S. suicide rates that have been increasing consistently in men and women for the past 2 decades, as data from the Centers for Disease Control and Prevention show.
“And although we must acknowledge we don’t quite know why, there is lots of speculation and a little bit of data, but not really conclusive stuff,” he said. “We also recognize that, in addition to trying to understand why, we need to try interventions that will reverse this increase.”
Identifying those at risk for suicide is a key focus of research, according to Dr. Gordon, who highlighted results of the ED-SAFE study, describing it as a “mainstay” of approaches to reducing risk through intervention.
In that recent study, an emergency department (ED)-based suicide prevention intervention cut total suicide attempts by 30%, compared with treatment as usual (JAMA Psychiatry. 2017 Jun;74[6]:563-70). That intervention included universal suicide risk screening plus secondary screening by the physician in the ED, discharge resources, and post-ED telephone calls intended to reduce suicide risk.
The ED-SAFE study is an example of taking the lessons learned in psychiatry and bringing them to a “broader swath” of individuals who might be at risk, said Dr. Gordon, a research psychiatrist who was a faculty member at Columbia University, New York, prior to being appointed director of NIMH.
“Of course, we’d like to do this not just in emergency rooms, but in primary care offices as well,” said Dr. Gordon, who noted that ongoing studies are aimed at demonstrating similar results in primary care patient populations, including adults and children.
Beyond this ask-and-you-will-find approach, there are “more modern” methods that involve applying predictive modeling and analytics to large data sets, identifying individuals who might not otherwise be suspected as being at risk and getting them into treatment, according to the director.
In one recent report, investigators said a risk prediction method using a machine learning approach on 3.7 million patients across five U.S. health systems was able to detect 38% of suicide attempts a mean of 2.1 years in advance (JAMA Netw Open. 2020 Mar 25;3[3]:3201262).
Machine learning might be able to detect the risk of suicidal behavior in unselected patients, based on these findings and might facilitate development of clinical decision support tools for risk reduction, the investigators said.
“We’re now studying how to implement these algorithms in real-world practice,” Dr. Gordon said.
Beyond identification, new interventions are needed for suicide reduction, he added, calling ketamine infusion “one of the most promising” recent developments that may help reduce suicidal ideation.
he said. “So the question is, can we use this in real-world practice to reduce suicide risk?”
The NIMH focus on suicide prevention will intensify the agency’s focus on recent initiatives in detecting and preventing suicide behavior and ideation in the juvenile justice system, applied research toward the goal of zero-suicide health care systems, and looking at the safety and feasibility of rapid-acting interventions for severe suicide risk, among others, according to Dr. Gordon, who became director of the agency in 2016.
“We have a number of initiatives aimed at taking what we’ve learned over the past few years, and helping that have a significant public health impact,” Dr. Gordon said.
Dr. Gordon reported no disclosures.
SOURCE: Gordon JA. APA 2020, Abstract.
Suicide prevention is a high priority for the National Institute of Mental Health (NIMH) and will be one specific area of focus in the federal agency’s 5-year strategic plan that’s set to be released soon, according to Director Joshua A. Gordon, MD, PhD.
The agency is updating its strategic plan to guide research efforts and priorities over the next 5 years, Dr. Gordon said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
That strategic plan, which will cover a broader range of priorities, is scheduled to be published “within the next few weeks,” Dr. Gordon said.
Closing the research gap in suicide prevention is a high priority for NIMH, Dr. Gordon said, especially in light of the age-adjusted U.S. suicide rates that have been increasing consistently in men and women for the past 2 decades, as data from the Centers for Disease Control and Prevention show.
“And although we must acknowledge we don’t quite know why, there is lots of speculation and a little bit of data, but not really conclusive stuff,” he said. “We also recognize that, in addition to trying to understand why, we need to try interventions that will reverse this increase.”
Identifying those at risk for suicide is a key focus of research, according to Dr. Gordon, who highlighted results of the ED-SAFE study, describing it as a “mainstay” of approaches to reducing risk through intervention.
In that recent study, an emergency department (ED)-based suicide prevention intervention cut total suicide attempts by 30%, compared with treatment as usual (JAMA Psychiatry. 2017 Jun;74[6]:563-70). That intervention included universal suicide risk screening plus secondary screening by the physician in the ED, discharge resources, and post-ED telephone calls intended to reduce suicide risk.
The ED-SAFE study is an example of taking the lessons learned in psychiatry and bringing them to a “broader swath” of individuals who might be at risk, said Dr. Gordon, a research psychiatrist who was a faculty member at Columbia University, New York, prior to being appointed director of NIMH.
“Of course, we’d like to do this not just in emergency rooms, but in primary care offices as well,” said Dr. Gordon, who noted that ongoing studies are aimed at demonstrating similar results in primary care patient populations, including adults and children.
Beyond this ask-and-you-will-find approach, there are “more modern” methods that involve applying predictive modeling and analytics to large data sets, identifying individuals who might not otherwise be suspected as being at risk and getting them into treatment, according to the director.
In one recent report, investigators said a risk prediction method using a machine learning approach on 3.7 million patients across five U.S. health systems was able to detect 38% of suicide attempts a mean of 2.1 years in advance (JAMA Netw Open. 2020 Mar 25;3[3]:3201262).
Machine learning might be able to detect the risk of suicidal behavior in unselected patients, based on these findings and might facilitate development of clinical decision support tools for risk reduction, the investigators said.
“We’re now studying how to implement these algorithms in real-world practice,” Dr. Gordon said.
Beyond identification, new interventions are needed for suicide reduction, he added, calling ketamine infusion “one of the most promising” recent developments that may help reduce suicidal ideation.
he said. “So the question is, can we use this in real-world practice to reduce suicide risk?”
The NIMH focus on suicide prevention will intensify the agency’s focus on recent initiatives in detecting and preventing suicide behavior and ideation in the juvenile justice system, applied research toward the goal of zero-suicide health care systems, and looking at the safety and feasibility of rapid-acting interventions for severe suicide risk, among others, according to Dr. Gordon, who became director of the agency in 2016.
“We have a number of initiatives aimed at taking what we’ve learned over the past few years, and helping that have a significant public health impact,” Dr. Gordon said.
Dr. Gordon reported no disclosures.
SOURCE: Gordon JA. APA 2020, Abstract.
Suicide prevention is a high priority for the National Institute of Mental Health (NIMH) and will be one specific area of focus in the federal agency’s 5-year strategic plan that’s set to be released soon, according to Director Joshua A. Gordon, MD, PhD.
The agency is updating its strategic plan to guide research efforts and priorities over the next 5 years, Dr. Gordon said at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
That strategic plan, which will cover a broader range of priorities, is scheduled to be published “within the next few weeks,” Dr. Gordon said.
Closing the research gap in suicide prevention is a high priority for NIMH, Dr. Gordon said, especially in light of the age-adjusted U.S. suicide rates that have been increasing consistently in men and women for the past 2 decades, as data from the Centers for Disease Control and Prevention show.
“And although we must acknowledge we don’t quite know why, there is lots of speculation and a little bit of data, but not really conclusive stuff,” he said. “We also recognize that, in addition to trying to understand why, we need to try interventions that will reverse this increase.”
Identifying those at risk for suicide is a key focus of research, according to Dr. Gordon, who highlighted results of the ED-SAFE study, describing it as a “mainstay” of approaches to reducing risk through intervention.
In that recent study, an emergency department (ED)-based suicide prevention intervention cut total suicide attempts by 30%, compared with treatment as usual (JAMA Psychiatry. 2017 Jun;74[6]:563-70). That intervention included universal suicide risk screening plus secondary screening by the physician in the ED, discharge resources, and post-ED telephone calls intended to reduce suicide risk.
The ED-SAFE study is an example of taking the lessons learned in psychiatry and bringing them to a “broader swath” of individuals who might be at risk, said Dr. Gordon, a research psychiatrist who was a faculty member at Columbia University, New York, prior to being appointed director of NIMH.
“Of course, we’d like to do this not just in emergency rooms, but in primary care offices as well,” said Dr. Gordon, who noted that ongoing studies are aimed at demonstrating similar results in primary care patient populations, including adults and children.
Beyond this ask-and-you-will-find approach, there are “more modern” methods that involve applying predictive modeling and analytics to large data sets, identifying individuals who might not otherwise be suspected as being at risk and getting them into treatment, according to the director.
In one recent report, investigators said a risk prediction method using a machine learning approach on 3.7 million patients across five U.S. health systems was able to detect 38% of suicide attempts a mean of 2.1 years in advance (JAMA Netw Open. 2020 Mar 25;3[3]:3201262).
Machine learning might be able to detect the risk of suicidal behavior in unselected patients, based on these findings and might facilitate development of clinical decision support tools for risk reduction, the investigators said.
“We’re now studying how to implement these algorithms in real-world practice,” Dr. Gordon said.
Beyond identification, new interventions are needed for suicide reduction, he added, calling ketamine infusion “one of the most promising” recent developments that may help reduce suicidal ideation.
he said. “So the question is, can we use this in real-world practice to reduce suicide risk?”
The NIMH focus on suicide prevention will intensify the agency’s focus on recent initiatives in detecting and preventing suicide behavior and ideation in the juvenile justice system, applied research toward the goal of zero-suicide health care systems, and looking at the safety and feasibility of rapid-acting interventions for severe suicide risk, among others, according to Dr. Gordon, who became director of the agency in 2016.
“We have a number of initiatives aimed at taking what we’ve learned over the past few years, and helping that have a significant public health impact,” Dr. Gordon said.
Dr. Gordon reported no disclosures.
SOURCE: Gordon JA. APA 2020, Abstract.
FROM APA 2020