User login
AAP releases updated guidance on male teen sexual, reproductive health
The American Academy of Pediatrics’ Committee on Adolescence has updated its guidance on addressing sexual reproductive health in male adolescents.
Since the last guidance was published by AAP in 2011, new data have been released that focus on adolescent male sexual behavior, their use of media, sexually transmitted infections (STIs), vaccination for human papillomavirus (HPV), discussions surrounding consent, and information for LGBT individuals.
“Of all these recommendations, the most significant changes are to provide more STI screening for higher risk males and vaccinate all males for HPV starting as early as age 9 years old,” lead author Laura K. Grubb, MD, director of adolescent medicine at Floating Hospital for Children at Tufts Medical Center, Boston, said in an interview.
AAP recommends pediatricians consider the following when discussing sexuality and reproductive health with adolescent males:
- Discuss the topics of sex and sexuality during routine visits and appropriate opportunities, taking the time to screen for sexual activity and identifying who is at higher risk.
- Ask male adolescent patients about social media use, how often they view pornography, and how they perceive sexually explicit material. If there is a concern that sexually explicit content is having an adverse effect on the patient, pediatricians should counsel patients and their parents on how to safely and sensibly use the Internet and social media.
- Screen for nonconsensual sexual activity during well visits and other visits, as appropriate. The principles of consent and nonconsent in the context of sexual activity should be discussed.
- For patients who are sexually active, screen for sexual problems, including any mental health issues and sexual dysfunction, and initiate counseling or pharmacotherapy where warranted.
- Coach male adolescent patients on broaching discussions about sex and family planning with their partners, including joint decision making on sexual and reproductive health. Contraception and barrier methods should be discussed and encouraged as appropriate.
- Assess each patient for appropriate STI risk, testing, and treatment/prevention for HIV, syphilis, chlamydia, and gonorrhea.
- Consider HPV vaccination for children at least 9 years old and start administration starting at 11 years old. according to the guidance.
Dr. Grubb said she hopes this guidance helps start a conversation between pediatricians and their adolescent male patients. “Talk with your male adolescents about puberty, sexuality, and reproductive health! When pediatricians are informed about these issues and take the initiative to discuss these topics with adolescent males, they are uniquely situated to help them navigate this challenging time safely and confidently.”
“I am especially excited about the significant resources this report provides for pediatricians in the supplemental document,” Dr. Grubb added. “There are so many great resources out there, especially on the Internet, for adolescents, parents, and pediatricians.”
Kelly Curran, MD, adolescent medicine specialist and assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, said in an interview that the guidance information on sexting, “sextortion,” and sexual dysfunction are important updates for pediatricians. Sextortion is defined as the “threatened dissemination of explicit, intimate, or embarrassing images of a sexual nature without consent, usually for the purpose of procuring additional images, sexual acts, money, or something else.”
“We have all seen how social media and technology has transformed adolescence, especially with the rise of sexting. We must remember that males are often the victims of ‘sextortion’ and sexual assault, especially sexual minority youth, and men may not have the support and services to which female victims have access,” said Dr. Curran, who was not a member of the committee.
Another important area where pediatricians can help educate adolescent males and their parents is the concept of consent during sexual encounters.
“As we as a society are having more frank discussions around sexual assault and rape, I think it is essential there is a continued dialogue with young people about consent. Pediatricians have an important role to play in the discussion with their patients, especially in regard to paying attention to verbal and nonverbal cues, and recognizing that consent is an ongoing process, instead of a ‘one time thing,’ ” said Dr. Curran, who is a member of the Pediatric News editorial advisory board.
One area of the new AAP guidance that surprised Dr. Curran was the number of adolescent males reporting sexual dysfunction – 4%. “While it’s something I ask about periodically in young men, I haven’t been consistently asking in visits for those who are at risk,” she said. “This guideline reminds me to screen more frequently, especially as patients may be too embarrassed to ask.”
Concerning STI screening, Dr. Curran feels guidelines from the Centers for Disease Control and Prevention and the United States Preventive Services Task Force (USPSTF) don’t go far enough, and the AAP’s guidance to provide routine STI risk assessment for all patients is more appropriate.
“We know that STIs are on the rise and adolescents experience high rates of STI, yet there are only routine screening guidelines for adolescent and young adult women and ‘at-risk’ populations or in areas of higher prevalence,” she said. “In my experience, all sexually active adolescents are ‘at risk.’ I think there should be universal screening of all sexually active adolescents and young adults.”
The paper had no funding source, and the authors reported no relevant conflicts of interest. Dr. Curran also reported no relevant conflicts of interest.
SOURCE: Grubb L et al. Pediatrics. 2020 Apr 27;145(5):e20200627.
The American Academy of Pediatrics’ Committee on Adolescence has updated its guidance on addressing sexual reproductive health in male adolescents.
Since the last guidance was published by AAP in 2011, new data have been released that focus on adolescent male sexual behavior, their use of media, sexually transmitted infections (STIs), vaccination for human papillomavirus (HPV), discussions surrounding consent, and information for LGBT individuals.
“Of all these recommendations, the most significant changes are to provide more STI screening for higher risk males and vaccinate all males for HPV starting as early as age 9 years old,” lead author Laura K. Grubb, MD, director of adolescent medicine at Floating Hospital for Children at Tufts Medical Center, Boston, said in an interview.
AAP recommends pediatricians consider the following when discussing sexuality and reproductive health with adolescent males:
- Discuss the topics of sex and sexuality during routine visits and appropriate opportunities, taking the time to screen for sexual activity and identifying who is at higher risk.
- Ask male adolescent patients about social media use, how often they view pornography, and how they perceive sexually explicit material. If there is a concern that sexually explicit content is having an adverse effect on the patient, pediatricians should counsel patients and their parents on how to safely and sensibly use the Internet and social media.
- Screen for nonconsensual sexual activity during well visits and other visits, as appropriate. The principles of consent and nonconsent in the context of sexual activity should be discussed.
- For patients who are sexually active, screen for sexual problems, including any mental health issues and sexual dysfunction, and initiate counseling or pharmacotherapy where warranted.
- Coach male adolescent patients on broaching discussions about sex and family planning with their partners, including joint decision making on sexual and reproductive health. Contraception and barrier methods should be discussed and encouraged as appropriate.
- Assess each patient for appropriate STI risk, testing, and treatment/prevention for HIV, syphilis, chlamydia, and gonorrhea.
- Consider HPV vaccination for children at least 9 years old and start administration starting at 11 years old. according to the guidance.
Dr. Grubb said she hopes this guidance helps start a conversation between pediatricians and their adolescent male patients. “Talk with your male adolescents about puberty, sexuality, and reproductive health! When pediatricians are informed about these issues and take the initiative to discuss these topics with adolescent males, they are uniquely situated to help them navigate this challenging time safely and confidently.”
“I am especially excited about the significant resources this report provides for pediatricians in the supplemental document,” Dr. Grubb added. “There are so many great resources out there, especially on the Internet, for adolescents, parents, and pediatricians.”
Kelly Curran, MD, adolescent medicine specialist and assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, said in an interview that the guidance information on sexting, “sextortion,” and sexual dysfunction are important updates for pediatricians. Sextortion is defined as the “threatened dissemination of explicit, intimate, or embarrassing images of a sexual nature without consent, usually for the purpose of procuring additional images, sexual acts, money, or something else.”
“We have all seen how social media and technology has transformed adolescence, especially with the rise of sexting. We must remember that males are often the victims of ‘sextortion’ and sexual assault, especially sexual minority youth, and men may not have the support and services to which female victims have access,” said Dr. Curran, who was not a member of the committee.
Another important area where pediatricians can help educate adolescent males and their parents is the concept of consent during sexual encounters.
“As we as a society are having more frank discussions around sexual assault and rape, I think it is essential there is a continued dialogue with young people about consent. Pediatricians have an important role to play in the discussion with their patients, especially in regard to paying attention to verbal and nonverbal cues, and recognizing that consent is an ongoing process, instead of a ‘one time thing,’ ” said Dr. Curran, who is a member of the Pediatric News editorial advisory board.
One area of the new AAP guidance that surprised Dr. Curran was the number of adolescent males reporting sexual dysfunction – 4%. “While it’s something I ask about periodically in young men, I haven’t been consistently asking in visits for those who are at risk,” she said. “This guideline reminds me to screen more frequently, especially as patients may be too embarrassed to ask.”
Concerning STI screening, Dr. Curran feels guidelines from the Centers for Disease Control and Prevention and the United States Preventive Services Task Force (USPSTF) don’t go far enough, and the AAP’s guidance to provide routine STI risk assessment for all patients is more appropriate.
“We know that STIs are on the rise and adolescents experience high rates of STI, yet there are only routine screening guidelines for adolescent and young adult women and ‘at-risk’ populations or in areas of higher prevalence,” she said. “In my experience, all sexually active adolescents are ‘at risk.’ I think there should be universal screening of all sexually active adolescents and young adults.”
The paper had no funding source, and the authors reported no relevant conflicts of interest. Dr. Curran also reported no relevant conflicts of interest.
SOURCE: Grubb L et al. Pediatrics. 2020 Apr 27;145(5):e20200627.
The American Academy of Pediatrics’ Committee on Adolescence has updated its guidance on addressing sexual reproductive health in male adolescents.
Since the last guidance was published by AAP in 2011, new data have been released that focus on adolescent male sexual behavior, their use of media, sexually transmitted infections (STIs), vaccination for human papillomavirus (HPV), discussions surrounding consent, and information for LGBT individuals.
“Of all these recommendations, the most significant changes are to provide more STI screening for higher risk males and vaccinate all males for HPV starting as early as age 9 years old,” lead author Laura K. Grubb, MD, director of adolescent medicine at Floating Hospital for Children at Tufts Medical Center, Boston, said in an interview.
AAP recommends pediatricians consider the following when discussing sexuality and reproductive health with adolescent males:
- Discuss the topics of sex and sexuality during routine visits and appropriate opportunities, taking the time to screen for sexual activity and identifying who is at higher risk.
- Ask male adolescent patients about social media use, how often they view pornography, and how they perceive sexually explicit material. If there is a concern that sexually explicit content is having an adverse effect on the patient, pediatricians should counsel patients and their parents on how to safely and sensibly use the Internet and social media.
- Screen for nonconsensual sexual activity during well visits and other visits, as appropriate. The principles of consent and nonconsent in the context of sexual activity should be discussed.
- For patients who are sexually active, screen for sexual problems, including any mental health issues and sexual dysfunction, and initiate counseling or pharmacotherapy where warranted.
- Coach male adolescent patients on broaching discussions about sex and family planning with their partners, including joint decision making on sexual and reproductive health. Contraception and barrier methods should be discussed and encouraged as appropriate.
- Assess each patient for appropriate STI risk, testing, and treatment/prevention for HIV, syphilis, chlamydia, and gonorrhea.
- Consider HPV vaccination for children at least 9 years old and start administration starting at 11 years old. according to the guidance.
Dr. Grubb said she hopes this guidance helps start a conversation between pediatricians and their adolescent male patients. “Talk with your male adolescents about puberty, sexuality, and reproductive health! When pediatricians are informed about these issues and take the initiative to discuss these topics with adolescent males, they are uniquely situated to help them navigate this challenging time safely and confidently.”
“I am especially excited about the significant resources this report provides for pediatricians in the supplemental document,” Dr. Grubb added. “There are so many great resources out there, especially on the Internet, for adolescents, parents, and pediatricians.”
Kelly Curran, MD, adolescent medicine specialist and assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, said in an interview that the guidance information on sexting, “sextortion,” and sexual dysfunction are important updates for pediatricians. Sextortion is defined as the “threatened dissemination of explicit, intimate, or embarrassing images of a sexual nature without consent, usually for the purpose of procuring additional images, sexual acts, money, or something else.”
“We have all seen how social media and technology has transformed adolescence, especially with the rise of sexting. We must remember that males are often the victims of ‘sextortion’ and sexual assault, especially sexual minority youth, and men may not have the support and services to which female victims have access,” said Dr. Curran, who was not a member of the committee.
Another important area where pediatricians can help educate adolescent males and their parents is the concept of consent during sexual encounters.
“As we as a society are having more frank discussions around sexual assault and rape, I think it is essential there is a continued dialogue with young people about consent. Pediatricians have an important role to play in the discussion with their patients, especially in regard to paying attention to verbal and nonverbal cues, and recognizing that consent is an ongoing process, instead of a ‘one time thing,’ ” said Dr. Curran, who is a member of the Pediatric News editorial advisory board.
One area of the new AAP guidance that surprised Dr. Curran was the number of adolescent males reporting sexual dysfunction – 4%. “While it’s something I ask about periodically in young men, I haven’t been consistently asking in visits for those who are at risk,” she said. “This guideline reminds me to screen more frequently, especially as patients may be too embarrassed to ask.”
Concerning STI screening, Dr. Curran feels guidelines from the Centers for Disease Control and Prevention and the United States Preventive Services Task Force (USPSTF) don’t go far enough, and the AAP’s guidance to provide routine STI risk assessment for all patients is more appropriate.
“We know that STIs are on the rise and adolescents experience high rates of STI, yet there are only routine screening guidelines for adolescent and young adult women and ‘at-risk’ populations or in areas of higher prevalence,” she said. “In my experience, all sexually active adolescents are ‘at risk.’ I think there should be universal screening of all sexually active adolescents and young adults.”
The paper had no funding source, and the authors reported no relevant conflicts of interest. Dr. Curran also reported no relevant conflicts of interest.
SOURCE: Grubb L et al. Pediatrics. 2020 Apr 27;145(5):e20200627.
FROM PEDIATRICS
COVID-19: Experts call for ‘urgent’ global action to prevent suicide
A global group of suicide experts is urging governments around the world to take action to prevent a possible jump in suicide rates because of the ongoing COVID-19 pandemic.
In a commentary published online April 21 in Lancet Psychiatry, members of the International COVID-19 Suicide Prevention Research Collaboration warned that suicide rates are likely to rise as the pandemic spreads and its ensuing long-term effects on the general population, economy, and vulnerable groups emerge.
“Preventing suicide therefore needs urgent consideration. The response must capitalize on, but extend beyond, general mental health policies and practices,” the experts wrote.
The COVID-19 collaboration was started by David Gunnell, MBChB, PhD, University of Bristol, England, and includes 42 members with suicide expertise from around the world.
“We’re an ad hoc grouping of international suicide prevention researchers, research leaders, and members of larger international suicide prevention organizations. We include specialists in public health, psychiatry, psychology, and other clinical disciplines,” Dr. Gunnell said in an interview.
“Through this comment piece we hope to share our ideas and experiences about best practice, and ask others working in the field of suicide prevention at a regional, national, and international level to share our intervention and surveillance/data collection recommendations with relevant policy makers,” he added.
Lessons from the past
During times of crisis, people with existing mental health disorders may suffer worsening symptoms, whereas others may develop new mental health problems, especially depression, anxiety, and posttraumatic stress disorder (PTSD), the group notes.
There is some evidence that suicide increased in the United States during the Spanish flu pandemic of 1918 and among older people in Hong Kong during the 2003 severe acute respiratory syndrome (SARS) outbreak.
An increase in suicide related to COVID-19 is not inevitable provided preventive action is prompt, the group notes.
In their article, the group offered several potential public health responses to mitigate suicide risk associated with the COVID-19 pandemic.
These include:
- Clear care pathways for those who are suicidal.
- Remote or digital assessments for patients currently under the care of a mental health professional.
- Staff training to support new ways of working.
- Increased support for mental health helplines.
- Providing easily accessible grief counseling for those who have lost a loved one to the virus.
- Financial safety nets and labor market programs.
- Dissemination of evidence-based online interventions.
Public health responses must also ensure that those facing domestic violence have access to support and a place to go during times of crisis, they suggested.
“These are unprecedented times. The pandemic will cause distress and leave many vulnerable. Mental health consequences are likely to be present for longer and peak later than the actual pandemic. However, research evidence and the experience of national strategies provide a strong basis for suicide prevention,” the group wrote.
Dr. Gunnell said it’s hard to predict what impact the pandemic will have on suicide rates, “but given the range of concerns, it is important to be prepared and take steps to mitigate risk as much as possible.”
Concerning spike in gun sales
Eric Fleegler, MD, MPH, and colleagues from Boston Children’s Hospital and Harvard Medical School, Boston, agreed.
“The time to act is now. Both population and individual approaches are needed to reduce the risk for suicide in the coming months,” they wrote in a commentary published online April 22 in Annals of Internal Medicine.
Dr. Fleegler and colleagues are particularly concerned about a potential increase in gun-related suicides, as gun sales in the United States have “skyrocketed” during the COVID-19 pandemic.
In March, more than 2.5 million firearms were sold, including 1.5 million handguns. That’s an 85% increase in gun sales compared with March 2019 and the highest firearm sales ever recorded in the United States, they reported.
In addition, research has shown that individuals who buy handguns have a 22-fold higher rate of firearm-related suicide within the first year vs. those who don’t purchase a handgun.
“In the best of times, increased gun ownership is associated with a heightened risk for firearm-related suicide. These are not the best of times,” the authors wrote.
Dr. Fleegler and colleagues said From 2006 to 2018, firearm-related suicide rates increased by more than 25%, according to the National Center for Injury Prevention and Control. In 2018 alone, there were 24,432 firearm-related suicides in the United States.
“The United States should take policy and clinical action to avoid a potential epidemic of firearm-related suicide in the wake of the COVID-19 pandemic,” they concluded.
This research had no specific funding. Dr. Gunnell and Dr. Fleegler disclosed no relevant financial relationships .
A version of this article originally appeared on Medscape.com.
A global group of suicide experts is urging governments around the world to take action to prevent a possible jump in suicide rates because of the ongoing COVID-19 pandemic.
In a commentary published online April 21 in Lancet Psychiatry, members of the International COVID-19 Suicide Prevention Research Collaboration warned that suicide rates are likely to rise as the pandemic spreads and its ensuing long-term effects on the general population, economy, and vulnerable groups emerge.
“Preventing suicide therefore needs urgent consideration. The response must capitalize on, but extend beyond, general mental health policies and practices,” the experts wrote.
The COVID-19 collaboration was started by David Gunnell, MBChB, PhD, University of Bristol, England, and includes 42 members with suicide expertise from around the world.
“We’re an ad hoc grouping of international suicide prevention researchers, research leaders, and members of larger international suicide prevention organizations. We include specialists in public health, psychiatry, psychology, and other clinical disciplines,” Dr. Gunnell said in an interview.
“Through this comment piece we hope to share our ideas and experiences about best practice, and ask others working in the field of suicide prevention at a regional, national, and international level to share our intervention and surveillance/data collection recommendations with relevant policy makers,” he added.
Lessons from the past
During times of crisis, people with existing mental health disorders may suffer worsening symptoms, whereas others may develop new mental health problems, especially depression, anxiety, and posttraumatic stress disorder (PTSD), the group notes.
There is some evidence that suicide increased in the United States during the Spanish flu pandemic of 1918 and among older people in Hong Kong during the 2003 severe acute respiratory syndrome (SARS) outbreak.
An increase in suicide related to COVID-19 is not inevitable provided preventive action is prompt, the group notes.
In their article, the group offered several potential public health responses to mitigate suicide risk associated with the COVID-19 pandemic.
These include:
- Clear care pathways for those who are suicidal.
- Remote or digital assessments for patients currently under the care of a mental health professional.
- Staff training to support new ways of working.
- Increased support for mental health helplines.
- Providing easily accessible grief counseling for those who have lost a loved one to the virus.
- Financial safety nets and labor market programs.
- Dissemination of evidence-based online interventions.
Public health responses must also ensure that those facing domestic violence have access to support and a place to go during times of crisis, they suggested.
“These are unprecedented times. The pandemic will cause distress and leave many vulnerable. Mental health consequences are likely to be present for longer and peak later than the actual pandemic. However, research evidence and the experience of national strategies provide a strong basis for suicide prevention,” the group wrote.
Dr. Gunnell said it’s hard to predict what impact the pandemic will have on suicide rates, “but given the range of concerns, it is important to be prepared and take steps to mitigate risk as much as possible.”
Concerning spike in gun sales
Eric Fleegler, MD, MPH, and colleagues from Boston Children’s Hospital and Harvard Medical School, Boston, agreed.
“The time to act is now. Both population and individual approaches are needed to reduce the risk for suicide in the coming months,” they wrote in a commentary published online April 22 in Annals of Internal Medicine.
Dr. Fleegler and colleagues are particularly concerned about a potential increase in gun-related suicides, as gun sales in the United States have “skyrocketed” during the COVID-19 pandemic.
In March, more than 2.5 million firearms were sold, including 1.5 million handguns. That’s an 85% increase in gun sales compared with March 2019 and the highest firearm sales ever recorded in the United States, they reported.
In addition, research has shown that individuals who buy handguns have a 22-fold higher rate of firearm-related suicide within the first year vs. those who don’t purchase a handgun.
“In the best of times, increased gun ownership is associated with a heightened risk for firearm-related suicide. These are not the best of times,” the authors wrote.
Dr. Fleegler and colleagues said From 2006 to 2018, firearm-related suicide rates increased by more than 25%, according to the National Center for Injury Prevention and Control. In 2018 alone, there were 24,432 firearm-related suicides in the United States.
“The United States should take policy and clinical action to avoid a potential epidemic of firearm-related suicide in the wake of the COVID-19 pandemic,” they concluded.
This research had no specific funding. Dr. Gunnell and Dr. Fleegler disclosed no relevant financial relationships .
A version of this article originally appeared on Medscape.com.
A global group of suicide experts is urging governments around the world to take action to prevent a possible jump in suicide rates because of the ongoing COVID-19 pandemic.
In a commentary published online April 21 in Lancet Psychiatry, members of the International COVID-19 Suicide Prevention Research Collaboration warned that suicide rates are likely to rise as the pandemic spreads and its ensuing long-term effects on the general population, economy, and vulnerable groups emerge.
“Preventing suicide therefore needs urgent consideration. The response must capitalize on, but extend beyond, general mental health policies and practices,” the experts wrote.
The COVID-19 collaboration was started by David Gunnell, MBChB, PhD, University of Bristol, England, and includes 42 members with suicide expertise from around the world.
“We’re an ad hoc grouping of international suicide prevention researchers, research leaders, and members of larger international suicide prevention organizations. We include specialists in public health, psychiatry, psychology, and other clinical disciplines,” Dr. Gunnell said in an interview.
“Through this comment piece we hope to share our ideas and experiences about best practice, and ask others working in the field of suicide prevention at a regional, national, and international level to share our intervention and surveillance/data collection recommendations with relevant policy makers,” he added.
Lessons from the past
During times of crisis, people with existing mental health disorders may suffer worsening symptoms, whereas others may develop new mental health problems, especially depression, anxiety, and posttraumatic stress disorder (PTSD), the group notes.
There is some evidence that suicide increased in the United States during the Spanish flu pandemic of 1918 and among older people in Hong Kong during the 2003 severe acute respiratory syndrome (SARS) outbreak.
An increase in suicide related to COVID-19 is not inevitable provided preventive action is prompt, the group notes.
In their article, the group offered several potential public health responses to mitigate suicide risk associated with the COVID-19 pandemic.
These include:
- Clear care pathways for those who are suicidal.
- Remote or digital assessments for patients currently under the care of a mental health professional.
- Staff training to support new ways of working.
- Increased support for mental health helplines.
- Providing easily accessible grief counseling for those who have lost a loved one to the virus.
- Financial safety nets and labor market programs.
- Dissemination of evidence-based online interventions.
Public health responses must also ensure that those facing domestic violence have access to support and a place to go during times of crisis, they suggested.
“These are unprecedented times. The pandemic will cause distress and leave many vulnerable. Mental health consequences are likely to be present for longer and peak later than the actual pandemic. However, research evidence and the experience of national strategies provide a strong basis for suicide prevention,” the group wrote.
Dr. Gunnell said it’s hard to predict what impact the pandemic will have on suicide rates, “but given the range of concerns, it is important to be prepared and take steps to mitigate risk as much as possible.”
Concerning spike in gun sales
Eric Fleegler, MD, MPH, and colleagues from Boston Children’s Hospital and Harvard Medical School, Boston, agreed.
“The time to act is now. Both population and individual approaches are needed to reduce the risk for suicide in the coming months,” they wrote in a commentary published online April 22 in Annals of Internal Medicine.
Dr. Fleegler and colleagues are particularly concerned about a potential increase in gun-related suicides, as gun sales in the United States have “skyrocketed” during the COVID-19 pandemic.
In March, more than 2.5 million firearms were sold, including 1.5 million handguns. That’s an 85% increase in gun sales compared with March 2019 and the highest firearm sales ever recorded in the United States, they reported.
In addition, research has shown that individuals who buy handguns have a 22-fold higher rate of firearm-related suicide within the first year vs. those who don’t purchase a handgun.
“In the best of times, increased gun ownership is associated with a heightened risk for firearm-related suicide. These are not the best of times,” the authors wrote.
Dr. Fleegler and colleagues said From 2006 to 2018, firearm-related suicide rates increased by more than 25%, according to the National Center for Injury Prevention and Control. In 2018 alone, there were 24,432 firearm-related suicides in the United States.
“The United States should take policy and clinical action to avoid a potential epidemic of firearm-related suicide in the wake of the COVID-19 pandemic,” they concluded.
This research had no specific funding. Dr. Gunnell and Dr. Fleegler disclosed no relevant financial relationships .
A version of this article originally appeared on Medscape.com.
New guidelines for testosterone treatment in adult men with age-related low testosterone
Testosterone normally decreases with age in men beginning in their mid-30s, with a rate of decline averaging approximately 1.6% per year. Using a cutoff of a total testosterone less than 325 ng/dL, the incidence of low testosterone is approximately 20% after age 60 years, and 30% after age 70. While the change in labs values has been reasonably validated,
Additional potential symptoms of testosterone deficiency include changes in bone mineral density, decreased libido, depression, erectile dysfunction, loss of hair, and general weakness. Since the symptoms are nonspecific, it is often unclear if someone should be tested or treated for testosterone deficiency. To address this issue, the American College of Physicians commissioned a systematic review of the evidence on testosterone-replacement therapy for age-related testosterone deficiency.1
The evidence review of testosterone replacement in men with age-related low testosterone found the following.
- Low-certainty evidence of improvement in quality of life
- Moderate-certainty evidence of a small improvement in sexual function
- Low-certainty evidence of a small improvement in erectile function
- Low-certainty evidence showing little to no improvement in physical function
- Low-certainty evidence of a small increase to no difference in adverse cardiovascular events
- Moderate-certainty evidence of no increase in the risk for serious adverse events
The trials were not powered to assess mortality, but pool analysis showed fewer deaths among patients treated with testosterone than those who received placebo (odds ratio, 0.47; 95% confidence interval, 0.25-0.89). There were no differences in cognitive function, and the improvement in vitality and fatigue was “less than a small amount.” Evidence from an observational trial showed no increased risk for mortality, cardiovascular events, prostate cancer, or pulmonary embolus or deep vein thrombosis. Of note, most studies excluded men with recent cardiovascular disease.
This evidence review led to the following recommendations.2
Recommendation 1a
Clinicians should have a discussion regarding the potential risk and benefits of treatment with the patients who have documented age-related low testosterone (testosterone levels less than 10.4 nmol/L or 300 ng/dL) and are suffering from sexual dysfunction or have a desire to enhance their sexual function.
This recommendation was based on evidence showing small improvement in sexual function and erectile dysfunction.
Recommendation 1b
For patients who opt for treatment based on recommendation 1a, clinicians should reevaluate the benefit of treatment within 12 months. If a patient is not receiving any benefit in sexual function by 12 months, it is recommended that treatment be stopped at that time.
The ACP recommendation to stop treatment if a patient lacks improvement of sexual function within 12 months stems from low or insufficient evidence regarding potential harm of treatment. If the treatment is not helping the target symptom then the benefit no longer outweighs the potential harm.
Recommendation 1c
For patients who opt for treatment based on recommendation 1a, intramuscular replacement therapy rather than transdermal replacement therapy is recommended because of substantial differences in the cost.
It is important to note that both intramuscular and transdermal testosterone applications have been associated with improvements in sexual function, without any significant differences noted in benefit or harm for the patients. This recommendation is based on a per-person per-year average cost of the intramuscular formulation – $156.32, compared with the transdermal formulation – $2,135.32.
Recommendation 2
The ACP does not endorse the use of testosterone treatment for age-related low testosterone in patients desiring improvement in physical function, mood, energy, or cognitive function.
This clear recommendation is critical, as this might be the most common reason for prescriptions of testosterone – a misplaced belief that testosterone will help general quality of life. The evidence simply does not support this effect of testosterone replacement for age-related testosterone deficiency.
The bottom line
Testosterone levels in men decrease steadily with age, with a great deal of variability. Testosterone replacement therapy may be considered for men with age-related testosterone deficiency and sexual dysfunction. Testosterone replacement therapy is not recommended as a treatment for general fatigue, weakness or with an expectation that it will improve physical function, mood, energy, or cognitive function.
Dr. Hansen is a third-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
References
1. Diem SJ et al. Efficacy and safety of testosterone treatment in men: An evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020 Jan 21. doi: 10.7326/M19-0830.
2. Qaseem A et al. Testosterone treatment in adult men with age-related low testosterone: A clinical guideline from the American College of Physicians. Ann Intern Med. 2020 Jan 21. doi: 10.7326/M19-0882.
Testosterone normally decreases with age in men beginning in their mid-30s, with a rate of decline averaging approximately 1.6% per year. Using a cutoff of a total testosterone less than 325 ng/dL, the incidence of low testosterone is approximately 20% after age 60 years, and 30% after age 70. While the change in labs values has been reasonably validated,
Additional potential symptoms of testosterone deficiency include changes in bone mineral density, decreased libido, depression, erectile dysfunction, loss of hair, and general weakness. Since the symptoms are nonspecific, it is often unclear if someone should be tested or treated for testosterone deficiency. To address this issue, the American College of Physicians commissioned a systematic review of the evidence on testosterone-replacement therapy for age-related testosterone deficiency.1
The evidence review of testosterone replacement in men with age-related low testosterone found the following.
- Low-certainty evidence of improvement in quality of life
- Moderate-certainty evidence of a small improvement in sexual function
- Low-certainty evidence of a small improvement in erectile function
- Low-certainty evidence showing little to no improvement in physical function
- Low-certainty evidence of a small increase to no difference in adverse cardiovascular events
- Moderate-certainty evidence of no increase in the risk for serious adverse events
The trials were not powered to assess mortality, but pool analysis showed fewer deaths among patients treated with testosterone than those who received placebo (odds ratio, 0.47; 95% confidence interval, 0.25-0.89). There were no differences in cognitive function, and the improvement in vitality and fatigue was “less than a small amount.” Evidence from an observational trial showed no increased risk for mortality, cardiovascular events, prostate cancer, or pulmonary embolus or deep vein thrombosis. Of note, most studies excluded men with recent cardiovascular disease.
This evidence review led to the following recommendations.2
Recommendation 1a
Clinicians should have a discussion regarding the potential risk and benefits of treatment with the patients who have documented age-related low testosterone (testosterone levels less than 10.4 nmol/L or 300 ng/dL) and are suffering from sexual dysfunction or have a desire to enhance their sexual function.
This recommendation was based on evidence showing small improvement in sexual function and erectile dysfunction.
Recommendation 1b
For patients who opt for treatment based on recommendation 1a, clinicians should reevaluate the benefit of treatment within 12 months. If a patient is not receiving any benefit in sexual function by 12 months, it is recommended that treatment be stopped at that time.
The ACP recommendation to stop treatment if a patient lacks improvement of sexual function within 12 months stems from low or insufficient evidence regarding potential harm of treatment. If the treatment is not helping the target symptom then the benefit no longer outweighs the potential harm.
Recommendation 1c
For patients who opt for treatment based on recommendation 1a, intramuscular replacement therapy rather than transdermal replacement therapy is recommended because of substantial differences in the cost.
It is important to note that both intramuscular and transdermal testosterone applications have been associated with improvements in sexual function, without any significant differences noted in benefit or harm for the patients. This recommendation is based on a per-person per-year average cost of the intramuscular formulation – $156.32, compared with the transdermal formulation – $2,135.32.
Recommendation 2
The ACP does not endorse the use of testosterone treatment for age-related low testosterone in patients desiring improvement in physical function, mood, energy, or cognitive function.
This clear recommendation is critical, as this might be the most common reason for prescriptions of testosterone – a misplaced belief that testosterone will help general quality of life. The evidence simply does not support this effect of testosterone replacement for age-related testosterone deficiency.
The bottom line
Testosterone levels in men decrease steadily with age, with a great deal of variability. Testosterone replacement therapy may be considered for men with age-related testosterone deficiency and sexual dysfunction. Testosterone replacement therapy is not recommended as a treatment for general fatigue, weakness or with an expectation that it will improve physical function, mood, energy, or cognitive function.
Dr. Hansen is a third-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
References
1. Diem SJ et al. Efficacy and safety of testosterone treatment in men: An evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020 Jan 21. doi: 10.7326/M19-0830.
2. Qaseem A et al. Testosterone treatment in adult men with age-related low testosterone: A clinical guideline from the American College of Physicians. Ann Intern Med. 2020 Jan 21. doi: 10.7326/M19-0882.
Testosterone normally decreases with age in men beginning in their mid-30s, with a rate of decline averaging approximately 1.6% per year. Using a cutoff of a total testosterone less than 325 ng/dL, the incidence of low testosterone is approximately 20% after age 60 years, and 30% after age 70. While the change in labs values has been reasonably validated,
Additional potential symptoms of testosterone deficiency include changes in bone mineral density, decreased libido, depression, erectile dysfunction, loss of hair, and general weakness. Since the symptoms are nonspecific, it is often unclear if someone should be tested or treated for testosterone deficiency. To address this issue, the American College of Physicians commissioned a systematic review of the evidence on testosterone-replacement therapy for age-related testosterone deficiency.1
The evidence review of testosterone replacement in men with age-related low testosterone found the following.
- Low-certainty evidence of improvement in quality of life
- Moderate-certainty evidence of a small improvement in sexual function
- Low-certainty evidence of a small improvement in erectile function
- Low-certainty evidence showing little to no improvement in physical function
- Low-certainty evidence of a small increase to no difference in adverse cardiovascular events
- Moderate-certainty evidence of no increase in the risk for serious adverse events
The trials were not powered to assess mortality, but pool analysis showed fewer deaths among patients treated with testosterone than those who received placebo (odds ratio, 0.47; 95% confidence interval, 0.25-0.89). There were no differences in cognitive function, and the improvement in vitality and fatigue was “less than a small amount.” Evidence from an observational trial showed no increased risk for mortality, cardiovascular events, prostate cancer, or pulmonary embolus or deep vein thrombosis. Of note, most studies excluded men with recent cardiovascular disease.
This evidence review led to the following recommendations.2
Recommendation 1a
Clinicians should have a discussion regarding the potential risk and benefits of treatment with the patients who have documented age-related low testosterone (testosterone levels less than 10.4 nmol/L or 300 ng/dL) and are suffering from sexual dysfunction or have a desire to enhance their sexual function.
This recommendation was based on evidence showing small improvement in sexual function and erectile dysfunction.
Recommendation 1b
For patients who opt for treatment based on recommendation 1a, clinicians should reevaluate the benefit of treatment within 12 months. If a patient is not receiving any benefit in sexual function by 12 months, it is recommended that treatment be stopped at that time.
The ACP recommendation to stop treatment if a patient lacks improvement of sexual function within 12 months stems from low or insufficient evidence regarding potential harm of treatment. If the treatment is not helping the target symptom then the benefit no longer outweighs the potential harm.
Recommendation 1c
For patients who opt for treatment based on recommendation 1a, intramuscular replacement therapy rather than transdermal replacement therapy is recommended because of substantial differences in the cost.
It is important to note that both intramuscular and transdermal testosterone applications have been associated with improvements in sexual function, without any significant differences noted in benefit or harm for the patients. This recommendation is based on a per-person per-year average cost of the intramuscular formulation – $156.32, compared with the transdermal formulation – $2,135.32.
Recommendation 2
The ACP does not endorse the use of testosterone treatment for age-related low testosterone in patients desiring improvement in physical function, mood, energy, or cognitive function.
This clear recommendation is critical, as this might be the most common reason for prescriptions of testosterone – a misplaced belief that testosterone will help general quality of life. The evidence simply does not support this effect of testosterone replacement for age-related testosterone deficiency.
The bottom line
Testosterone levels in men decrease steadily with age, with a great deal of variability. Testosterone replacement therapy may be considered for men with age-related testosterone deficiency and sexual dysfunction. Testosterone replacement therapy is not recommended as a treatment for general fatigue, weakness or with an expectation that it will improve physical function, mood, energy, or cognitive function.
Dr. Hansen is a third-year resident in the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Hospital–Jefferson Health.
References
1. Diem SJ et al. Efficacy and safety of testosterone treatment in men: An evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020 Jan 21. doi: 10.7326/M19-0830.
2. Qaseem A et al. Testosterone treatment in adult men with age-related low testosterone: A clinical guideline from the American College of Physicians. Ann Intern Med. 2020 Jan 21. doi: 10.7326/M19-0882.
Erectile dysfunction: It’s worse than you think
Erectile dysfunction may be an early warning sign of broader health problems. That’s the suggestion from a new retrospective analysis of European men, which found that erectile dysfunction and other sexual symptoms were associated with a greater risk of death, independent of testosterone levels.
Similar studies have shown links between mortality and sexual dysfunction, or between mortality and testosterone level, but the current study is unique, Leen Antonio, MD, PhD, assistant professor of endocrinology at Katholieke Universiteit Leuven (Belgium), said during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
“It’s the first time we have put both together in the same group of people, and we can say that it’s mostly the sexual symptoms that are predicting the mortality risk, independent of the testosterone levels of these men,” Dr. Antonio said in an interview.
“We can regard sexual symptoms as a marker for adverse health status in general. It’s like a warning signal that you’re at risk for more severe problems,” Dr. Antonio added.
Dr. Antonio advised clinicians to test blood pressure and cholesterol levels in men presenting with sexual dysfunction and to counsel lifestyle changes, such as physical activity and weight management. “These can be beneficial for sexual symptoms and for general health and the risk for cardiovascular disease in the future.”
Although the study could not identify a reason for the relationship between sexual dysfunction and mortality, Dr. Antonio hypothesized that the narrow penile artery may be more likely to suffer noticeable effects in the early stages of atherosclerosis, before clinical effects occur in the coronary artery.
Michael Blaha, MD, professor of medicine and director of clinical research at the Ciccarone Center for the Prevention of Heart Disease at Johns Hopkins University, Baltimore, who has studied erectile dysfunction (ED) and its association with cardiovascular disease, said that the study is further evidence that ED is an important and independent risk factor for cardiovascular disease and other health risks. He would like to see a move toward establishing men’s health clinics, where risk factors can be identified and mitigated through lifestyle changes and therapies.
“There needs to be a complete rethink of the way we approach the whole group of patients who present with erectile dysfunction to various specialists,” he said in an interview, noting that middle-aged men often present to ED specialists after years of not having any contact with the health system. In that group, ED can be an early warning sign that could trigger broader interventions.
“This points to the need for more men’s health clinics that are focused on the early detection of risk factors, and treating erectile dysfunction and other risk factors in a more comprehensive way,” said Dr. Blaha, who was not associated with the study.
Dr. Antonio and colleagues studied 1,913 community-dwelling men, who participated in the European Male Ageing Study. Baseline information on sexual function and testosterone levels was collected between 2003 and 2005. The men were aged 40-79 years at study entry, and “because of the wide age range at study entry, age was used as time scale, instead of years since inclusion adjusting for age,” the researchers explained.
Over a mean follow-up of 12.4 years, 25.3% of participants died. Body mass index was higher in men who died (P = .002), but there was no significant difference in smoking status. Both groups had similar levels of total testosterone, but free testosterone was lower in the deceased population (270 pmol/L vs. 312 pmol/L; P < .001), whereas luteinizing hormone levels were higher (7.8 units/L vs. 5.7 units/L; P < .001).
The lowest quartile of free testosterone level was associated with higher mortality risk (hazard ratio, 1.43; P = .021), whereas the highest quartile of follicle-stimulating hormone was associated with greater mortality risk (HR, 1.38; P = .036). There was no association between mortality risk and total testosterone or estradiol.
Men reporting three sexual symptoms at baseline had a higher mortality risk than those reporting no symptoms (HR, 1.77; P < .001). There was an association between mortality risk and ED (HR, 1.40; P = .001) and poor morning erections (HR, 1.30; P = .012), but not low libido.
The associations were not affected after adjustment for total testosterone or free testosterone. Among men with normal total testosterone (>12 nmol/L), sexual symptoms were associated with heightened mortality risk (HR, 1.51; P = .003), and the same was true in men with total testosterone levels of less than 8 nmol, compared with men with normal total testosterone who reported no sexual symptoms (HR, 1.92; P = .035).
The European Male Ageing Study received support from the European Union. Dr. Antonio has no relevant financial disclosures. Dr. Blaha has received grants from Amgen and is on advisory boards for Amgen and other pharmaceutical firms.
Dr. Antonio and her team’s research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Antonio L et al. ENDO 2020, Abstract OR02-06.
This article was upadted on 4/17/2020.
Erectile dysfunction may be an early warning sign of broader health problems. That’s the suggestion from a new retrospective analysis of European men, which found that erectile dysfunction and other sexual symptoms were associated with a greater risk of death, independent of testosterone levels.
Similar studies have shown links between mortality and sexual dysfunction, or between mortality and testosterone level, but the current study is unique, Leen Antonio, MD, PhD, assistant professor of endocrinology at Katholieke Universiteit Leuven (Belgium), said during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
“It’s the first time we have put both together in the same group of people, and we can say that it’s mostly the sexual symptoms that are predicting the mortality risk, independent of the testosterone levels of these men,” Dr. Antonio said in an interview.
“We can regard sexual symptoms as a marker for adverse health status in general. It’s like a warning signal that you’re at risk for more severe problems,” Dr. Antonio added.
Dr. Antonio advised clinicians to test blood pressure and cholesterol levels in men presenting with sexual dysfunction and to counsel lifestyle changes, such as physical activity and weight management. “These can be beneficial for sexual symptoms and for general health and the risk for cardiovascular disease in the future.”
Although the study could not identify a reason for the relationship between sexual dysfunction and mortality, Dr. Antonio hypothesized that the narrow penile artery may be more likely to suffer noticeable effects in the early stages of atherosclerosis, before clinical effects occur in the coronary artery.
Michael Blaha, MD, professor of medicine and director of clinical research at the Ciccarone Center for the Prevention of Heart Disease at Johns Hopkins University, Baltimore, who has studied erectile dysfunction (ED) and its association with cardiovascular disease, said that the study is further evidence that ED is an important and independent risk factor for cardiovascular disease and other health risks. He would like to see a move toward establishing men’s health clinics, where risk factors can be identified and mitigated through lifestyle changes and therapies.
“There needs to be a complete rethink of the way we approach the whole group of patients who present with erectile dysfunction to various specialists,” he said in an interview, noting that middle-aged men often present to ED specialists after years of not having any contact with the health system. In that group, ED can be an early warning sign that could trigger broader interventions.
“This points to the need for more men’s health clinics that are focused on the early detection of risk factors, and treating erectile dysfunction and other risk factors in a more comprehensive way,” said Dr. Blaha, who was not associated with the study.
Dr. Antonio and colleagues studied 1,913 community-dwelling men, who participated in the European Male Ageing Study. Baseline information on sexual function and testosterone levels was collected between 2003 and 2005. The men were aged 40-79 years at study entry, and “because of the wide age range at study entry, age was used as time scale, instead of years since inclusion adjusting for age,” the researchers explained.
Over a mean follow-up of 12.4 years, 25.3% of participants died. Body mass index was higher in men who died (P = .002), but there was no significant difference in smoking status. Both groups had similar levels of total testosterone, but free testosterone was lower in the deceased population (270 pmol/L vs. 312 pmol/L; P < .001), whereas luteinizing hormone levels were higher (7.8 units/L vs. 5.7 units/L; P < .001).
The lowest quartile of free testosterone level was associated with higher mortality risk (hazard ratio, 1.43; P = .021), whereas the highest quartile of follicle-stimulating hormone was associated with greater mortality risk (HR, 1.38; P = .036). There was no association between mortality risk and total testosterone or estradiol.
Men reporting three sexual symptoms at baseline had a higher mortality risk than those reporting no symptoms (HR, 1.77; P < .001). There was an association between mortality risk and ED (HR, 1.40; P = .001) and poor morning erections (HR, 1.30; P = .012), but not low libido.
The associations were not affected after adjustment for total testosterone or free testosterone. Among men with normal total testosterone (>12 nmol/L), sexual symptoms were associated with heightened mortality risk (HR, 1.51; P = .003), and the same was true in men with total testosterone levels of less than 8 nmol, compared with men with normal total testosterone who reported no sexual symptoms (HR, 1.92; P = .035).
The European Male Ageing Study received support from the European Union. Dr. Antonio has no relevant financial disclosures. Dr. Blaha has received grants from Amgen and is on advisory boards for Amgen and other pharmaceutical firms.
Dr. Antonio and her team’s research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Antonio L et al. ENDO 2020, Abstract OR02-06.
This article was upadted on 4/17/2020.
Erectile dysfunction may be an early warning sign of broader health problems. That’s the suggestion from a new retrospective analysis of European men, which found that erectile dysfunction and other sexual symptoms were associated with a greater risk of death, independent of testosterone levels.
Similar studies have shown links between mortality and sexual dysfunction, or between mortality and testosterone level, but the current study is unique, Leen Antonio, MD, PhD, assistant professor of endocrinology at Katholieke Universiteit Leuven (Belgium), said during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
“It’s the first time we have put both together in the same group of people, and we can say that it’s mostly the sexual symptoms that are predicting the mortality risk, independent of the testosterone levels of these men,” Dr. Antonio said in an interview.
“We can regard sexual symptoms as a marker for adverse health status in general. It’s like a warning signal that you’re at risk for more severe problems,” Dr. Antonio added.
Dr. Antonio advised clinicians to test blood pressure and cholesterol levels in men presenting with sexual dysfunction and to counsel lifestyle changes, such as physical activity and weight management. “These can be beneficial for sexual symptoms and for general health and the risk for cardiovascular disease in the future.”
Although the study could not identify a reason for the relationship between sexual dysfunction and mortality, Dr. Antonio hypothesized that the narrow penile artery may be more likely to suffer noticeable effects in the early stages of atherosclerosis, before clinical effects occur in the coronary artery.
Michael Blaha, MD, professor of medicine and director of clinical research at the Ciccarone Center for the Prevention of Heart Disease at Johns Hopkins University, Baltimore, who has studied erectile dysfunction (ED) and its association with cardiovascular disease, said that the study is further evidence that ED is an important and independent risk factor for cardiovascular disease and other health risks. He would like to see a move toward establishing men’s health clinics, where risk factors can be identified and mitigated through lifestyle changes and therapies.
“There needs to be a complete rethink of the way we approach the whole group of patients who present with erectile dysfunction to various specialists,” he said in an interview, noting that middle-aged men often present to ED specialists after years of not having any contact with the health system. In that group, ED can be an early warning sign that could trigger broader interventions.
“This points to the need for more men’s health clinics that are focused on the early detection of risk factors, and treating erectile dysfunction and other risk factors in a more comprehensive way,” said Dr. Blaha, who was not associated with the study.
Dr. Antonio and colleagues studied 1,913 community-dwelling men, who participated in the European Male Ageing Study. Baseline information on sexual function and testosterone levels was collected between 2003 and 2005. The men were aged 40-79 years at study entry, and “because of the wide age range at study entry, age was used as time scale, instead of years since inclusion adjusting for age,” the researchers explained.
Over a mean follow-up of 12.4 years, 25.3% of participants died. Body mass index was higher in men who died (P = .002), but there was no significant difference in smoking status. Both groups had similar levels of total testosterone, but free testosterone was lower in the deceased population (270 pmol/L vs. 312 pmol/L; P < .001), whereas luteinizing hormone levels were higher (7.8 units/L vs. 5.7 units/L; P < .001).
The lowest quartile of free testosterone level was associated with higher mortality risk (hazard ratio, 1.43; P = .021), whereas the highest quartile of follicle-stimulating hormone was associated with greater mortality risk (HR, 1.38; P = .036). There was no association between mortality risk and total testosterone or estradiol.
Men reporting three sexual symptoms at baseline had a higher mortality risk than those reporting no symptoms (HR, 1.77; P < .001). There was an association between mortality risk and ED (HR, 1.40; P = .001) and poor morning erections (HR, 1.30; P = .012), but not low libido.
The associations were not affected after adjustment for total testosterone or free testosterone. Among men with normal total testosterone (>12 nmol/L), sexual symptoms were associated with heightened mortality risk (HR, 1.51; P = .003), and the same was true in men with total testosterone levels of less than 8 nmol, compared with men with normal total testosterone who reported no sexual symptoms (HR, 1.92; P = .035).
The European Male Ageing Study received support from the European Union. Dr. Antonio has no relevant financial disclosures. Dr. Blaha has received grants from Amgen and is on advisory boards for Amgen and other pharmaceutical firms.
Dr. Antonio and her team’s research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Antonio L et al. ENDO 2020, Abstract OR02-06.
This article was upadted on 4/17/2020.
FROM ENDO 2020
Cabazitaxel Improves Progression-Free and Overall Survival in Metastatic Prostate Cancer After Progression on Abiraterone or Enzalutamide
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
Study Overview
Objective. To evaluate the efficacy of cabazitaxel compared to androgen-signaling–targeted inhibitors (ASTIs) in patients with metastatic castration-resistant prostate cancer who have received docetaxel and have progressed within 12 months of treatment with either abiraterone or enzalutamide.
Design. The CARD trial was an international, randomized, open-label phase 3 trial conducted across 13 European countries.
Setting and participants. Eligible patients were 18 years of age or older; had metastatic castration-resistant prostate cancer previously treated with docetaxel; and had disease progression during 12 months of treatment with abiraterone or enzalutamide. All patients had histologically proven prostate cancer, castrate levels of serum testosterone, and disease progression, defined by at least 2 new bone lesions or rising prostate-specific antigen (PSA) level. A total of 255 patients underwent randomization between November 2015 and November 2018, with 129 assigned to receive cabazitaxel and 126 patients assigned to receive an ASTI, 58 of whom received abiraterone and 66 of whom received enzalutamide. Patients who had received an ASTI in the setting of castrate-sensitive metastatic prostate cancer were included.
Intervention. Patients were randomized in a 1:1 fashion to receive either cabazitaxel or abiraterone or enzalutamide. Patients receiving cabazitaxel 25 mg/m2 intravenously every 3 weeks also received oral prednisone daily and primary prophylactic granulocyte-colony stimulating factor. Patients assigned to receive an ASTI received abiraterone 1000 mg orally daily with prednisone 5 mg twice daily or enzalutamide 160 mg daily. Patients in the ASTI group who had progressed on abiraterone were assigned to enzalutamide, and alternatively, those on enzalutamide were assigned to abiraterone. Patients were treated until 1 of the following occurred: imaging-based disease progression, unacceptable toxicity, or advancing to an alternative therapy.
Main outcome measures. The primary endpoint was imaging-based progression-free survival, which was defined as the time from randomization until objective tumor progression, progression of bone lesions, or death. The secondary endpoints were overall survival, progression-free survival, PSA response, tumor and pain responses, a new symptomatic skeletal event, and safety.
Results. The median follow-up was 9.2 months. Imaging-based disease progression or death from any cause occurred in 95 (73.6%) participants in the cabazitaxel group, as compared to 101 (80.2%) who were assigned to receive an ASTI. The median imaging-based progression-free survival was 8.0 months in the cabazitaxel group and 3.7 months in the abiraterone/enzalutamide group. The median duration of treatment was longer in those receiving cabazitaxel (22 vs 12.5 weeks). The primary reason for treatment discontinuation was disease progression (in 43.7% of patients receiving cabazitaxel and 71% receiving an ASTI) or an adverse event (19.8% and 8.9%, respectively).
The trial’s secondary endpoints demonstrated improved outcomes in the cabazitaxel group compared to the abiraterone/enzalutamide group. There were 70 deaths (54.2%) in the cabazitaxel group and 83 (65.9%) in the ASTI group. Both the median overall survival (13.6 months in the cabazitaxel group and 11 months in the ASTI group) and the median progression-free survival (4.4 months and 2.7 months, respectively) were improved in those who received cabazitaxel. There was a 50% or greater reduction in the PSA level from baseline in 35.7% of the cabazitaxel group and 13.5% of the ASTI group.
Regarding the safety of the agents, the incidence of adverse events was similar in each group (38.9% in the cabazitaxel group and 38.7% in the ASTI group). Treatment discontinuation occurred more frequently in the cabazitaxel group (19.8%) compared to the ASTI group (8.9%). Adverse events of grade 3 or higher occurred more frequently with cabazitaxel; these were asthenia (4% vs 2.4%), diarrhea (3.2% vs 0), peripheral neuropathy (3.2% vs 0 patients), and febrile neutropenia (3.2% vs 0 patients).
Conclusion. Patients who had disease progression within 12 months on an ASTI and had previously been treated for metastatic castration-resistant prostate cancer with docetaxel had longer imaging-based progression-free survival and overall survival when treated with cabazitaxel compared to those treated with an alternative ASTI. Other clinical outcomes, including overall survival and progression-free survival, were also improved in the cabazitaxel group.
Commentary
Four ASTIs are approved for therapy in men with advanced prostate cancer. The next line of therapy following progression on an ASTI, whether to consider second-line androgen targeted inhibitors or proceed to taxane-based chemotherapy, has been unclear. The current CARD trial sought to answer this question and provides evidence that cabazitaxel is the next line of therapy for these patients. The trial’s primary endpoint, imaging-based disease progression, was reported in 73.6% of those who received cabazitaxel and in 80.2% of those who received abiraterone or enzalutamide. Patients treated with cabazitaxel had a longer imaging-based progression-free survival (8.0 months vs 3.7 months) and a longer duration of treatment (22 vs 12.5 weeks).
Because there is clinical evidence of cross-resistance between different ASTIs, the value of sequential therapy has been unclear. Emergence of androgen-receptor splice variant 7 (AR-V7) mutational status in circulating tumor cells is associated with poor outcomes with secondary androgen-signaling inhibitor therapy, and may be an indicator of resistance to subsequent androgen-signaling inhibitors.1,2 In the PROPHECY trial, the response rates to subsequent androgen targeted therapy in patients with AR-V7 mutations ranged from 30% to 40%.3 Understanding how AR-V7 mutational status may impact such outcomes will certainly help define whether a subgroup exists in whom use of second-line androgen signaling inhibitors may be considered.
The patients enrolled in the current study appear to represent a subgroup of patients with biologically aggressive disease or with inherent resistance to ASTIs. The patients included in this study progressed within 1 year of androgen targeted therapy, which is representative of a more aggressive population of patients who may be hormone insensitive and derive more benefit from chemotherapy. Initial androgen deprivation therapy was given for 13.7 and 12.6 months to the cabazitaxel and enzalutamide/abiraterone arms, respectively, prior to developing castrate-resistant prostate cancer. Patients enrolled in this study also previously received docetaxel, deselecting those who are taxane-resistant and therefore may be less likely to respond to additional taxane-based therapy. Detection of AR-V7 splice variant expression in circulating tumor cells, consideration of biomarker data, and sensitivity to taxanes may help guide decisions regarding the use of sequential androgen-targeted agents; however, there has been no clear data to guide such an approach. It is also important to consider that, because this is a European study, the approved dose given in this trial was 25 mg/m2. The PROSELICA trial previously demonstrated noninferiority of 20 mg/m2 compared with 25 mg/m2, with fewer adverse events, which is the dose now utilized in the United States.4
The adverse events of grade 3 or greater occurring in the cabazitaxel group should be discussed with patients, including fatigue, diarrhea, peripheral neuropathy, and febrile neutropenia.
The data from the CARD trial provide guidance regarding therapy sequencing in those with advanced prostate cancer after progression on first-line androgen targeted inhibitors and docetaxel; however, further work is needed to understand the universal application of this data in this cohort.
Applications in Clinical Practice
Patients with metastatic castration-resistant prostate cancer who have received docetaxel and progressed on an androgen-signaling inhibitor within 12 months should be considered for cabazitaxel over an alternative androgen-signaling inhibitor. This decision should be based on several factors, including AR-V7 mutational status, duration of androgen deprivation therapy, and hormone and taxane sensitivity in the past. Future studies are likely to incorporate genomic biomarkers rather than clinical criteria alone to make treatment decisions.
–Britni Souther, DO, and Daniel Isaac, DO, MS, Michigan State University, East Lansing, MI
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
1. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028-1038.
2. Zhang T, Karsh LI, Nissenblatt MJ, et al. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin Genitourin Cancer. 2019;18:1-10.
3. Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. 2019;37:1120-1129.
4. Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35:3198-3206.
Focus groups seek transgender experience with HIV prevention
A pair of focus groups explored the experience of transgender patients with HIV prevention, finding many were discouraged by experiences of care that was not culturally competent and affirming.
The findings, including other important themes, were published in Pediatrics.
The pair of online asynchronous focus groups, conducted by Holly B. Fontenot, PhD, RN/NP, of the Fenway Institute in Boston, and colleagues, sought input from 30 transgender participants from across the United States. Eleven were aged 13-18 years, and 19 were aged 18-24 years, with an average age of 19. Most (70%) were white, and the remainder were African American (7%), Asian American (3%), multiracial (17%), and other (3%); 10% identified as Hispanic. Participants were given multiple options for reporting gender identity; 27% reported identifying as transgender males, 17% reported identifying as transgender females, and the rest identified with other terms, including 27% using one or more terms.
The quantitative analysis found four common themes, which the study explored in depth: “barriers to self-efficacy in sexual decision making; safety concerns, fear, and other challenges in forming romantic and/or sexual relationships; need for support and education; and desire for affirmative and culturally competent experiences and interactions.”
Based on their findings, the authors suggested ways of improving transgender youth experiences:
- Increasing provider knowledge and skills in providing affirming care through transgender health education programs.
- Addressing the barriers, such as stigma and lack of accessibility.
- Expanding sexual health education to be more inclusive regarding gender identities, sexual orientations, and definitions of sex.
Providers also need to include information on sexually transmitted infection and HIV prevention, including “discussion of safer sexual behaviors, negotiation and consent, sexual and physical assault, condoms, lubrication, STI and HIV testing, human papillomavirus vaccination, and PrEP [preexposure prophylaxis]” the authors emphasized.
Dr. Fontenot and associates determined that this study’s findings were consistent with what’s known about adult transgender patients, but this study provides more context regarding transgender youth experiences.
“It is important to elicit transgender youth experiences and perspectives related to HIV risk and preventive services,” they concluded. “This study provided a greater understanding of barriers to and facilitators of youth obtaining HIV preventive services and sexual health education.”
Limitations of the study included that non–English speaking participants were excluded, and that participants were predominantly white, non-Hispanic, and assigned female sex at birth.
This study was funded by the Centers for Disease Control and Prevention and NORC at The University of Chicago. The authors had no relevant financial disclosures.
SOURCE: Fontenot HB et al., Pediatrics. 2020. doi: 10.1542/peds.2019-2204.
A pair of focus groups explored the experience of transgender patients with HIV prevention, finding many were discouraged by experiences of care that was not culturally competent and affirming.
The findings, including other important themes, were published in Pediatrics.
The pair of online asynchronous focus groups, conducted by Holly B. Fontenot, PhD, RN/NP, of the Fenway Institute in Boston, and colleagues, sought input from 30 transgender participants from across the United States. Eleven were aged 13-18 years, and 19 were aged 18-24 years, with an average age of 19. Most (70%) were white, and the remainder were African American (7%), Asian American (3%), multiracial (17%), and other (3%); 10% identified as Hispanic. Participants were given multiple options for reporting gender identity; 27% reported identifying as transgender males, 17% reported identifying as transgender females, and the rest identified with other terms, including 27% using one or more terms.
The quantitative analysis found four common themes, which the study explored in depth: “barriers to self-efficacy in sexual decision making; safety concerns, fear, and other challenges in forming romantic and/or sexual relationships; need for support and education; and desire for affirmative and culturally competent experiences and interactions.”
Based on their findings, the authors suggested ways of improving transgender youth experiences:
- Increasing provider knowledge and skills in providing affirming care through transgender health education programs.
- Addressing the barriers, such as stigma and lack of accessibility.
- Expanding sexual health education to be more inclusive regarding gender identities, sexual orientations, and definitions of sex.
Providers also need to include information on sexually transmitted infection and HIV prevention, including “discussion of safer sexual behaviors, negotiation and consent, sexual and physical assault, condoms, lubrication, STI and HIV testing, human papillomavirus vaccination, and PrEP [preexposure prophylaxis]” the authors emphasized.
Dr. Fontenot and associates determined that this study’s findings were consistent with what’s known about adult transgender patients, but this study provides more context regarding transgender youth experiences.
“It is important to elicit transgender youth experiences and perspectives related to HIV risk and preventive services,” they concluded. “This study provided a greater understanding of barriers to and facilitators of youth obtaining HIV preventive services and sexual health education.”
Limitations of the study included that non–English speaking participants were excluded, and that participants were predominantly white, non-Hispanic, and assigned female sex at birth.
This study was funded by the Centers for Disease Control and Prevention and NORC at The University of Chicago. The authors had no relevant financial disclosures.
SOURCE: Fontenot HB et al., Pediatrics. 2020. doi: 10.1542/peds.2019-2204.
A pair of focus groups explored the experience of transgender patients with HIV prevention, finding many were discouraged by experiences of care that was not culturally competent and affirming.
The findings, including other important themes, were published in Pediatrics.
The pair of online asynchronous focus groups, conducted by Holly B. Fontenot, PhD, RN/NP, of the Fenway Institute in Boston, and colleagues, sought input from 30 transgender participants from across the United States. Eleven were aged 13-18 years, and 19 were aged 18-24 years, with an average age of 19. Most (70%) were white, and the remainder were African American (7%), Asian American (3%), multiracial (17%), and other (3%); 10% identified as Hispanic. Participants were given multiple options for reporting gender identity; 27% reported identifying as transgender males, 17% reported identifying as transgender females, and the rest identified with other terms, including 27% using one or more terms.
The quantitative analysis found four common themes, which the study explored in depth: “barriers to self-efficacy in sexual decision making; safety concerns, fear, and other challenges in forming romantic and/or sexual relationships; need for support and education; and desire for affirmative and culturally competent experiences and interactions.”
Based on their findings, the authors suggested ways of improving transgender youth experiences:
- Increasing provider knowledge and skills in providing affirming care through transgender health education programs.
- Addressing the barriers, such as stigma and lack of accessibility.
- Expanding sexual health education to be more inclusive regarding gender identities, sexual orientations, and definitions of sex.
Providers also need to include information on sexually transmitted infection and HIV prevention, including “discussion of safer sexual behaviors, negotiation and consent, sexual and physical assault, condoms, lubrication, STI and HIV testing, human papillomavirus vaccination, and PrEP [preexposure prophylaxis]” the authors emphasized.
Dr. Fontenot and associates determined that this study’s findings were consistent with what’s known about adult transgender patients, but this study provides more context regarding transgender youth experiences.
“It is important to elicit transgender youth experiences and perspectives related to HIV risk and preventive services,” they concluded. “This study provided a greater understanding of barriers to and facilitators of youth obtaining HIV preventive services and sexual health education.”
Limitations of the study included that non–English speaking participants were excluded, and that participants were predominantly white, non-Hispanic, and assigned female sex at birth.
This study was funded by the Centers for Disease Control and Prevention and NORC at The University of Chicago. The authors had no relevant financial disclosures.
SOURCE: Fontenot HB et al., Pediatrics. 2020. doi: 10.1542/peds.2019-2204.
FROM PEDIATRICS
Nearly half of STI events go without HIV testing
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
FROM PEDIATRICS
Testosterone therapy linked to CV risk in men with HIV
Men with HIV are likely prone to the same cardiovascular risks from testosterone therapy as other men, according to new research.
There’s no reason to think they weren’t, but it hadn’t been demonstrated until now, and men with HIV are already at increased risk for cardiovascular disease. The take-home message is that “it would be prudent for clinicians to monitor closely for cardiovascular risk factors and recommend intervention to lower cardiovascular risk among men with HIV on or considering testosterone therapy,” lead investigator Sabina Haberlen, PhD, an assistant scientist in the infectious disease epidemiology division of Johns Hopkins University, Baltimore, said in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because concerns about the spread of COVID-19.
Testosterone therapy is common among middle-aged and older men with HIV to counter the hypogonadism associated with infection. The investigators turned to the Multicenter AIDS Cohort Study – a 30-year, four-city study of HIV-1 infection in men who have sex with men – to gauge its effect.
The 300 men in the study had a baseline coronary CT angiogram in 2010-2013 and a repeat study a mean of 4.5 years later. They had no history of coronary interventions or kidney dysfunction at baseline and were aged 40-70 years, with a median age of 51 years. About 70% reported never using testosterone, 8% were former users before entering the study, 7% started using testosterone between the two CTs, and 15% entered the study on testosterone and stayed on it.
Adjusting for age, race, cardiovascular risk factors, baseline serum testosterone levels, and other potential confounders, the risk of significant coronary artery calcium (CAC) progression was 2 times greater among continuous users (P = .03) and 2.4 times greater among new users (P = .01), compared with former users, who the investigators used as a control group because, at some point, they too had indications for testosterone replacement and so were more medically similar than never users.
The risk of noncalcified plaque volume progression was also more than twice as high among ongoing users, and elevated, although not significantly so, among ongoing users.
In short, “our findings are similar to those on subclinical atherosclerotic progression” in trials of older men in the general population on testosterone replacement, Dr. Haberlen said.
About half the subjects were white, 41% were at high risk for cardiovascular disease, 91% were on antiretroviral therapy, and 81% had undetectable HIV viral loads. Median total testosterone was 606 ng/dL. CAC progression was defined by incident CAC, at least a 10 Agatston unit/year increase if the baseline CAC score was 1-100, and a 10% or more annual increase if the baseline score was above 100.
Lower baseline serum testosterone was also associated with an increased risk of CAC progression, although not progression of noncalcified plaques.
The work was funded by the National Institutes of Health. Dr. Haberlen didn’t report any relevant disclosures.
SOURCE: Haberlen S et al. CROI 2020, Abstract 662.
Men with HIV are likely prone to the same cardiovascular risks from testosterone therapy as other men, according to new research.
There’s no reason to think they weren’t, but it hadn’t been demonstrated until now, and men with HIV are already at increased risk for cardiovascular disease. The take-home message is that “it would be prudent for clinicians to monitor closely for cardiovascular risk factors and recommend intervention to lower cardiovascular risk among men with HIV on or considering testosterone therapy,” lead investigator Sabina Haberlen, PhD, an assistant scientist in the infectious disease epidemiology division of Johns Hopkins University, Baltimore, said in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because concerns about the spread of COVID-19.
Testosterone therapy is common among middle-aged and older men with HIV to counter the hypogonadism associated with infection. The investigators turned to the Multicenter AIDS Cohort Study – a 30-year, four-city study of HIV-1 infection in men who have sex with men – to gauge its effect.
The 300 men in the study had a baseline coronary CT angiogram in 2010-2013 and a repeat study a mean of 4.5 years later. They had no history of coronary interventions or kidney dysfunction at baseline and were aged 40-70 years, with a median age of 51 years. About 70% reported never using testosterone, 8% were former users before entering the study, 7% started using testosterone between the two CTs, and 15% entered the study on testosterone and stayed on it.
Adjusting for age, race, cardiovascular risk factors, baseline serum testosterone levels, and other potential confounders, the risk of significant coronary artery calcium (CAC) progression was 2 times greater among continuous users (P = .03) and 2.4 times greater among new users (P = .01), compared with former users, who the investigators used as a control group because, at some point, they too had indications for testosterone replacement and so were more medically similar than never users.
The risk of noncalcified plaque volume progression was also more than twice as high among ongoing users, and elevated, although not significantly so, among ongoing users.
In short, “our findings are similar to those on subclinical atherosclerotic progression” in trials of older men in the general population on testosterone replacement, Dr. Haberlen said.
About half the subjects were white, 41% were at high risk for cardiovascular disease, 91% were on antiretroviral therapy, and 81% had undetectable HIV viral loads. Median total testosterone was 606 ng/dL. CAC progression was defined by incident CAC, at least a 10 Agatston unit/year increase if the baseline CAC score was 1-100, and a 10% or more annual increase if the baseline score was above 100.
Lower baseline serum testosterone was also associated with an increased risk of CAC progression, although not progression of noncalcified plaques.
The work was funded by the National Institutes of Health. Dr. Haberlen didn’t report any relevant disclosures.
SOURCE: Haberlen S et al. CROI 2020, Abstract 662.
Men with HIV are likely prone to the same cardiovascular risks from testosterone therapy as other men, according to new research.
There’s no reason to think they weren’t, but it hadn’t been demonstrated until now, and men with HIV are already at increased risk for cardiovascular disease. The take-home message is that “it would be prudent for clinicians to monitor closely for cardiovascular risk factors and recommend intervention to lower cardiovascular risk among men with HIV on or considering testosterone therapy,” lead investigator Sabina Haberlen, PhD, an assistant scientist in the infectious disease epidemiology division of Johns Hopkins University, Baltimore, said in a poster that was presented as part of the Conference on Retroviruses & Opportunistic Infections, which was presented online this year. CROI organizers chose to hold a virtual meeting because concerns about the spread of COVID-19.
Testosterone therapy is common among middle-aged and older men with HIV to counter the hypogonadism associated with infection. The investigators turned to the Multicenter AIDS Cohort Study – a 30-year, four-city study of HIV-1 infection in men who have sex with men – to gauge its effect.
The 300 men in the study had a baseline coronary CT angiogram in 2010-2013 and a repeat study a mean of 4.5 years later. They had no history of coronary interventions or kidney dysfunction at baseline and were aged 40-70 years, with a median age of 51 years. About 70% reported never using testosterone, 8% were former users before entering the study, 7% started using testosterone between the two CTs, and 15% entered the study on testosterone and stayed on it.
Adjusting for age, race, cardiovascular risk factors, baseline serum testosterone levels, and other potential confounders, the risk of significant coronary artery calcium (CAC) progression was 2 times greater among continuous users (P = .03) and 2.4 times greater among new users (P = .01), compared with former users, who the investigators used as a control group because, at some point, they too had indications for testosterone replacement and so were more medically similar than never users.
The risk of noncalcified plaque volume progression was also more than twice as high among ongoing users, and elevated, although not significantly so, among ongoing users.
In short, “our findings are similar to those on subclinical atherosclerotic progression” in trials of older men in the general population on testosterone replacement, Dr. Haberlen said.
About half the subjects were white, 41% were at high risk for cardiovascular disease, 91% were on antiretroviral therapy, and 81% had undetectable HIV viral loads. Median total testosterone was 606 ng/dL. CAC progression was defined by incident CAC, at least a 10 Agatston unit/year increase if the baseline CAC score was 1-100, and a 10% or more annual increase if the baseline score was above 100.
Lower baseline serum testosterone was also associated with an increased risk of CAC progression, although not progression of noncalcified plaques.
The work was funded by the National Institutes of Health. Dr. Haberlen didn’t report any relevant disclosures.
SOURCE: Haberlen S et al. CROI 2020, Abstract 662.
FROM CROI 2020
Excessive masculinity linked to high suicide risk
Excessive masculinity is linked to a significantly increased risk for death by suicide in men, new research suggests.
In the first study to show this association, investigators found that men with high traditional masculinity (HTM) – a set of norms that includes competitiveness, emotional restriction, and aggression – were about two and half times more likely to die by suicide than their counterparts without HTM. The finding underscores the “central role” of gender in suicide death.
“We found that high-traditional-masculinity men were 2.4 times more likely to die by suicide than those who were not [of] high traditional masculinity. We feel this is a significant finding, and one that’s very rare to have evidence for,” study investigator Daniel Coleman, PhD, said in an interview.
“Our other findings are also important and interesting,” added Dr. Coleman, associate professor of social service at Fordham University, New York. “One was that high traditional masculinity was associated with a host of other significant risk factors for suicide death. So not only does high traditional masculinity add to the risk of suicide death, it also may have indirect effects through other variables, such as acting-out behavior.”
The study was published online Feb. 12 in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2019.4702).
First look
In the United States, death by suicide is 3.5 times more common in men than in women. Several potential drivers may explain this phenomenon; one plausible factor may be high levels of what the investigators describe as “traditional masculinity.”
Interestingly, previous studies suggest that HTM men experience suicidal thoughts to a greater degree than do other persons (Soc Psychiatry Psychiatr Epidemiol. 2017 Mar;52[3]:319-27). Nevertheless, the potential influence of HTM and suicide mortality has not been examined before now.
The study is a secondary analysis of the longitudinal Add Health (the National Longitudinal Study of Adolescent to Adult Health) study, which began in 1995 and followed 20,745 adolescents through young adulthood. Not only did that study show a direct association between measures of HTM and death by suicide, but it also corroborated the connection between HTM and other risk factors for suicide revealed in earlier research (Suicide Life Threat Behav. 2016 Apr;46[2]:191-205).
To tease out this relationship, Dr. Coleman and colleagues used data from the nationally representative Add Health study. That earlier research concluded that nine Add Health variables were associated with suicide; these included suicide by a family member, being expelled from school, running away from home, using a weapon, being of white race, a past history of smoking, being in a serious fight in the past year, delinquency, and fighting.
In the current study, the researchers hypothesized that HTM would be associated with these nine variables, in addition to suicide, depression, and gun access.
In the Add Health study, the adolescents were followed over time. In the current analysis, the researchers matched data from that study with death records from the National Death Index from 2014. Death by suicide was defined using National Death Index procedures.
The investigators then used an established procedure for scoring gender-typed attitudes and behaviors. As part of this, a single latent probability variable for identifying oneself as male was generated from 16 gender-discriminating variables.
Participants who were found to score at least a 73% probability of identifying as male (greater than 1 standard deviation above the mean) were classified as HTM.
“There’s been a lot of speculating about masculinity as a risk factor for male suicides,” Dr. Coleman said. “But it’s very difficult to study suicide death and something psychosocial like masculinity. So this was an attempt to fill that gap and test the hypothesis that’s being discussed quite a bit.”
A relevant risk factor
Twenty-two deaths occurred among the Add Health participants. Of those participants, 21 were men (odds ratio, 21.7; 95% confidence interval, 2.9-161; P less than .001).
The analysis showed that all nine risks for suicide that were highlighted in previous research were positively associated with HTM, with small to medium effect sizes. Of these, the most pronounced was family member suicide, with an OR of 1.89 (95% CI, 1.3-2.7).
Most tellingly, HTM men were 2.4 times more likely to end their lives by suicide than were men not defined as such (95% CI, 0.99-6.0; P less than .046). Nevertheless, HTM men were also 1.45 times less likely to report suicidal ideation (OR, 0.69; 95% CI, 0.60-0.81; P less than .001). There was no association between HTM and nonfatal suicide attempts.
Interestingly, HTM men were slightly more likely to report easy access to guns (OR, 1.1; 95% CI, 1.01-1.20; P less than .04), but they had lower levels of depression (Cohen’s d, 0.17; P less than .001).
HTM not only has a direct association with suicide but also with a web of indirect effects as well, thanks to its association with all the other risks identified in the previous study by another group of investigators.
HTM may be an underlying influence in male suicide that increases the probability of externalizing such behavioral risk factors as anger, violence, gun access, and school problems.
The finding that almost all of the people who died by suicide were men underscores the central role that gender plays in these tragedies. As such, the investigators hope that the study prompts more research, as well as intervention efforts aimed at the role of masculinity in suicide.
“There are already things going on around the world to try to address the risk factors of masculinity for suicide death,” Dr. Coleman said. “So even though we haven’t had the evidence that it’s a risk factor, people have been operating under that assumption anyway.
“Hopefully our research contributes to raising the profile that high traditional masculinity is a relevant risk factor that we can organize prevention and treatment around.”
An important contribution
Mark S. Kaplan, DrPH, commenting on the findings in an interview, said the study makes an important contribution to suicide research.
“Any study that tries to link a living sample with death data, as they did here, is important,” said Dr. Kaplan, professor of social welfare at the Luskin School of Public Affairs of the University of California, Los Angeles.
“It’s also important because it begins to scratch the surface of more proximal or distal factors that are associated with suicide, and masculinity is one of those factors,” Dr. Kaplan added.
“In an incremental way, it begins to add to the puzzle of why men have a higher mortality rate than their female counterparts. Because when it comes to suicide, men and women really are apples and oranges.”
Dr. Kaplan believes HTM is one of several traits that may lead men to take their own lives.
“There are all sorts of other issues. For example, masculinity might be interacting with some of the harsh socioeconomic conditions that many men face. I think all of this points to the real need to understand why men die from suicide,” he said.
The Add Health study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. No direct support was received from the grant for the current study. Dr. Coleman and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Excessive masculinity is linked to a significantly increased risk for death by suicide in men, new research suggests.
In the first study to show this association, investigators found that men with high traditional masculinity (HTM) – a set of norms that includes competitiveness, emotional restriction, and aggression – were about two and half times more likely to die by suicide than their counterparts without HTM. The finding underscores the “central role” of gender in suicide death.
“We found that high-traditional-masculinity men were 2.4 times more likely to die by suicide than those who were not [of] high traditional masculinity. We feel this is a significant finding, and one that’s very rare to have evidence for,” study investigator Daniel Coleman, PhD, said in an interview.
“Our other findings are also important and interesting,” added Dr. Coleman, associate professor of social service at Fordham University, New York. “One was that high traditional masculinity was associated with a host of other significant risk factors for suicide death. So not only does high traditional masculinity add to the risk of suicide death, it also may have indirect effects through other variables, such as acting-out behavior.”
The study was published online Feb. 12 in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2019.4702).
First look
In the United States, death by suicide is 3.5 times more common in men than in women. Several potential drivers may explain this phenomenon; one plausible factor may be high levels of what the investigators describe as “traditional masculinity.”
Interestingly, previous studies suggest that HTM men experience suicidal thoughts to a greater degree than do other persons (Soc Psychiatry Psychiatr Epidemiol. 2017 Mar;52[3]:319-27). Nevertheless, the potential influence of HTM and suicide mortality has not been examined before now.
The study is a secondary analysis of the longitudinal Add Health (the National Longitudinal Study of Adolescent to Adult Health) study, which began in 1995 and followed 20,745 adolescents through young adulthood. Not only did that study show a direct association between measures of HTM and death by suicide, but it also corroborated the connection between HTM and other risk factors for suicide revealed in earlier research (Suicide Life Threat Behav. 2016 Apr;46[2]:191-205).
To tease out this relationship, Dr. Coleman and colleagues used data from the nationally representative Add Health study. That earlier research concluded that nine Add Health variables were associated with suicide; these included suicide by a family member, being expelled from school, running away from home, using a weapon, being of white race, a past history of smoking, being in a serious fight in the past year, delinquency, and fighting.
In the current study, the researchers hypothesized that HTM would be associated with these nine variables, in addition to suicide, depression, and gun access.
In the Add Health study, the adolescents were followed over time. In the current analysis, the researchers matched data from that study with death records from the National Death Index from 2014. Death by suicide was defined using National Death Index procedures.
The investigators then used an established procedure for scoring gender-typed attitudes and behaviors. As part of this, a single latent probability variable for identifying oneself as male was generated from 16 gender-discriminating variables.
Participants who were found to score at least a 73% probability of identifying as male (greater than 1 standard deviation above the mean) were classified as HTM.
“There’s been a lot of speculating about masculinity as a risk factor for male suicides,” Dr. Coleman said. “But it’s very difficult to study suicide death and something psychosocial like masculinity. So this was an attempt to fill that gap and test the hypothesis that’s being discussed quite a bit.”
A relevant risk factor
Twenty-two deaths occurred among the Add Health participants. Of those participants, 21 were men (odds ratio, 21.7; 95% confidence interval, 2.9-161; P less than .001).
The analysis showed that all nine risks for suicide that were highlighted in previous research were positively associated with HTM, with small to medium effect sizes. Of these, the most pronounced was family member suicide, with an OR of 1.89 (95% CI, 1.3-2.7).
Most tellingly, HTM men were 2.4 times more likely to end their lives by suicide than were men not defined as such (95% CI, 0.99-6.0; P less than .046). Nevertheless, HTM men were also 1.45 times less likely to report suicidal ideation (OR, 0.69; 95% CI, 0.60-0.81; P less than .001). There was no association between HTM and nonfatal suicide attempts.
Interestingly, HTM men were slightly more likely to report easy access to guns (OR, 1.1; 95% CI, 1.01-1.20; P less than .04), but they had lower levels of depression (Cohen’s d, 0.17; P less than .001).
HTM not only has a direct association with suicide but also with a web of indirect effects as well, thanks to its association with all the other risks identified in the previous study by another group of investigators.
HTM may be an underlying influence in male suicide that increases the probability of externalizing such behavioral risk factors as anger, violence, gun access, and school problems.
The finding that almost all of the people who died by suicide were men underscores the central role that gender plays in these tragedies. As such, the investigators hope that the study prompts more research, as well as intervention efforts aimed at the role of masculinity in suicide.
“There are already things going on around the world to try to address the risk factors of masculinity for suicide death,” Dr. Coleman said. “So even though we haven’t had the evidence that it’s a risk factor, people have been operating under that assumption anyway.
“Hopefully our research contributes to raising the profile that high traditional masculinity is a relevant risk factor that we can organize prevention and treatment around.”
An important contribution
Mark S. Kaplan, DrPH, commenting on the findings in an interview, said the study makes an important contribution to suicide research.
“Any study that tries to link a living sample with death data, as they did here, is important,” said Dr. Kaplan, professor of social welfare at the Luskin School of Public Affairs of the University of California, Los Angeles.
“It’s also important because it begins to scratch the surface of more proximal or distal factors that are associated with suicide, and masculinity is one of those factors,” Dr. Kaplan added.
“In an incremental way, it begins to add to the puzzle of why men have a higher mortality rate than their female counterparts. Because when it comes to suicide, men and women really are apples and oranges.”
Dr. Kaplan believes HTM is one of several traits that may lead men to take their own lives.
“There are all sorts of other issues. For example, masculinity might be interacting with some of the harsh socioeconomic conditions that many men face. I think all of this points to the real need to understand why men die from suicide,” he said.
The Add Health study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. No direct support was received from the grant for the current study. Dr. Coleman and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Excessive masculinity is linked to a significantly increased risk for death by suicide in men, new research suggests.
In the first study to show this association, investigators found that men with high traditional masculinity (HTM) – a set of norms that includes competitiveness, emotional restriction, and aggression – were about two and half times more likely to die by suicide than their counterparts without HTM. The finding underscores the “central role” of gender in suicide death.
“We found that high-traditional-masculinity men were 2.4 times more likely to die by suicide than those who were not [of] high traditional masculinity. We feel this is a significant finding, and one that’s very rare to have evidence for,” study investigator Daniel Coleman, PhD, said in an interview.
“Our other findings are also important and interesting,” added Dr. Coleman, associate professor of social service at Fordham University, New York. “One was that high traditional masculinity was associated with a host of other significant risk factors for suicide death. So not only does high traditional masculinity add to the risk of suicide death, it also may have indirect effects through other variables, such as acting-out behavior.”
The study was published online Feb. 12 in JAMA Psychiatry (doi: 10.1001/jamapsychiatry.2019.4702).
First look
In the United States, death by suicide is 3.5 times more common in men than in women. Several potential drivers may explain this phenomenon; one plausible factor may be high levels of what the investigators describe as “traditional masculinity.”
Interestingly, previous studies suggest that HTM men experience suicidal thoughts to a greater degree than do other persons (Soc Psychiatry Psychiatr Epidemiol. 2017 Mar;52[3]:319-27). Nevertheless, the potential influence of HTM and suicide mortality has not been examined before now.
The study is a secondary analysis of the longitudinal Add Health (the National Longitudinal Study of Adolescent to Adult Health) study, which began in 1995 and followed 20,745 adolescents through young adulthood. Not only did that study show a direct association between measures of HTM and death by suicide, but it also corroborated the connection between HTM and other risk factors for suicide revealed in earlier research (Suicide Life Threat Behav. 2016 Apr;46[2]:191-205).
To tease out this relationship, Dr. Coleman and colleagues used data from the nationally representative Add Health study. That earlier research concluded that nine Add Health variables were associated with suicide; these included suicide by a family member, being expelled from school, running away from home, using a weapon, being of white race, a past history of smoking, being in a serious fight in the past year, delinquency, and fighting.
In the current study, the researchers hypothesized that HTM would be associated with these nine variables, in addition to suicide, depression, and gun access.
In the Add Health study, the adolescents were followed over time. In the current analysis, the researchers matched data from that study with death records from the National Death Index from 2014. Death by suicide was defined using National Death Index procedures.
The investigators then used an established procedure for scoring gender-typed attitudes and behaviors. As part of this, a single latent probability variable for identifying oneself as male was generated from 16 gender-discriminating variables.
Participants who were found to score at least a 73% probability of identifying as male (greater than 1 standard deviation above the mean) were classified as HTM.
“There’s been a lot of speculating about masculinity as a risk factor for male suicides,” Dr. Coleman said. “But it’s very difficult to study suicide death and something psychosocial like masculinity. So this was an attempt to fill that gap and test the hypothesis that’s being discussed quite a bit.”
A relevant risk factor
Twenty-two deaths occurred among the Add Health participants. Of those participants, 21 were men (odds ratio, 21.7; 95% confidence interval, 2.9-161; P less than .001).
The analysis showed that all nine risks for suicide that were highlighted in previous research were positively associated with HTM, with small to medium effect sizes. Of these, the most pronounced was family member suicide, with an OR of 1.89 (95% CI, 1.3-2.7).
Most tellingly, HTM men were 2.4 times more likely to end their lives by suicide than were men not defined as such (95% CI, 0.99-6.0; P less than .046). Nevertheless, HTM men were also 1.45 times less likely to report suicidal ideation (OR, 0.69; 95% CI, 0.60-0.81; P less than .001). There was no association between HTM and nonfatal suicide attempts.
Interestingly, HTM men were slightly more likely to report easy access to guns (OR, 1.1; 95% CI, 1.01-1.20; P less than .04), but they had lower levels of depression (Cohen’s d, 0.17; P less than .001).
HTM not only has a direct association with suicide but also with a web of indirect effects as well, thanks to its association with all the other risks identified in the previous study by another group of investigators.
HTM may be an underlying influence in male suicide that increases the probability of externalizing such behavioral risk factors as anger, violence, gun access, and school problems.
The finding that almost all of the people who died by suicide were men underscores the central role that gender plays in these tragedies. As such, the investigators hope that the study prompts more research, as well as intervention efforts aimed at the role of masculinity in suicide.
“There are already things going on around the world to try to address the risk factors of masculinity for suicide death,” Dr. Coleman said. “So even though we haven’t had the evidence that it’s a risk factor, people have been operating under that assumption anyway.
“Hopefully our research contributes to raising the profile that high traditional masculinity is a relevant risk factor that we can organize prevention and treatment around.”
An important contribution
Mark S. Kaplan, DrPH, commenting on the findings in an interview, said the study makes an important contribution to suicide research.
“Any study that tries to link a living sample with death data, as they did here, is important,” said Dr. Kaplan, professor of social welfare at the Luskin School of Public Affairs of the University of California, Los Angeles.
“It’s also important because it begins to scratch the surface of more proximal or distal factors that are associated with suicide, and masculinity is one of those factors,” Dr. Kaplan added.
“In an incremental way, it begins to add to the puzzle of why men have a higher mortality rate than their female counterparts. Because when it comes to suicide, men and women really are apples and oranges.”
Dr. Kaplan believes HTM is one of several traits that may lead men to take their own lives.
“There are all sorts of other issues. For example, masculinity might be interacting with some of the harsh socioeconomic conditions that many men face. I think all of this points to the real need to understand why men die from suicide,” he said.
The Add Health study is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. No direct support was received from the grant for the current study. Dr. Coleman and Dr. Kaplan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PSA cancer screening: A case for shared decision-making
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14
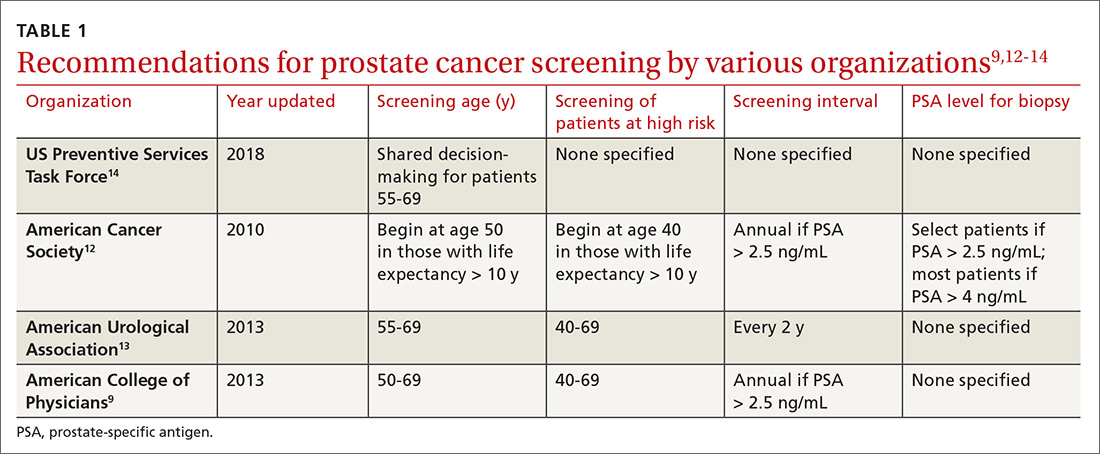
Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18
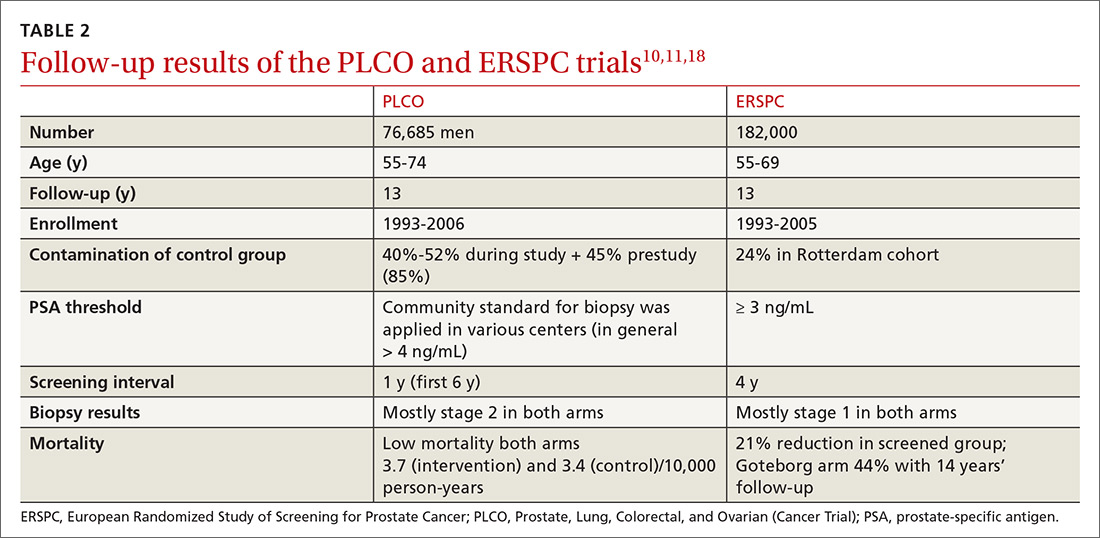
Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24
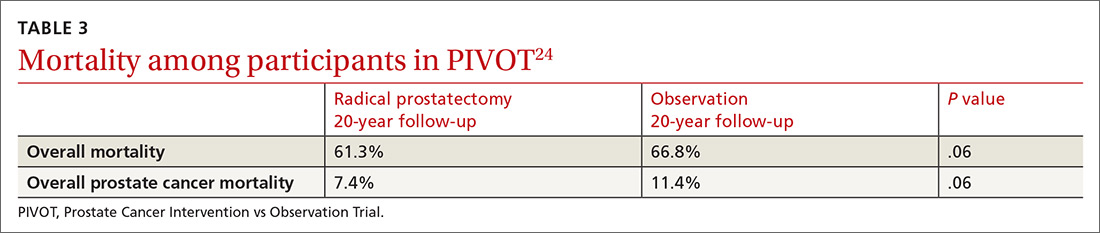
SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.
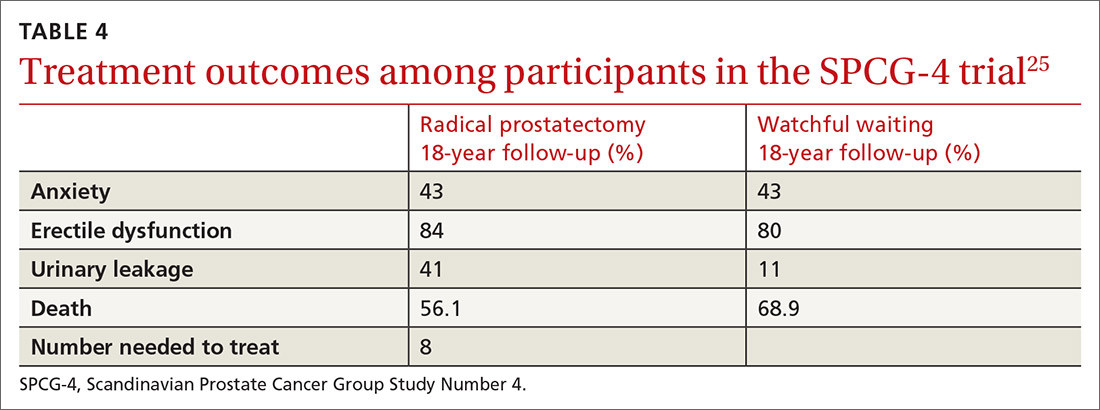
ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22
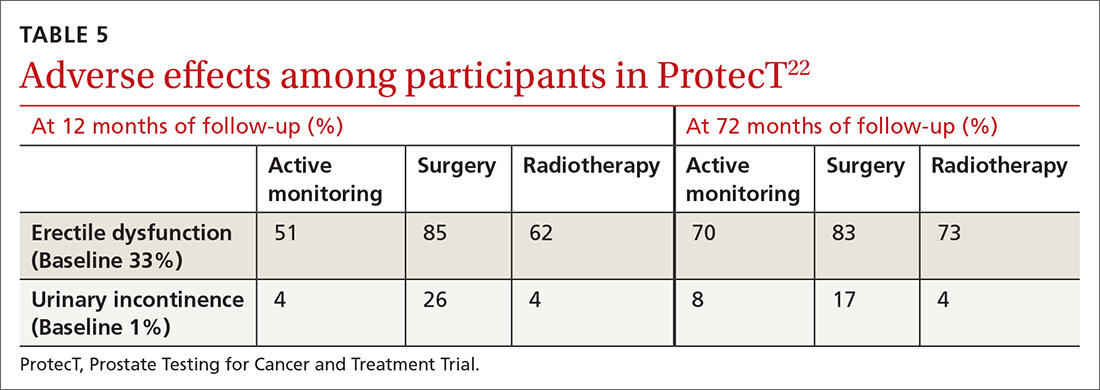
Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.
Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14

Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18

Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24

SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.

ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22

Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.

Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; [email protected].
Prostate cancer is the most frequently diagnosed cancer in men and the third leading cause of cancer death in men worldwide.1 An estimated 174,650 new cases are diagnosed each year in the United States; 31,620 American men die annually from the disease.2 Although prostate cancer can be a serious disease, many men do not die from it. In fact, 2.9 million men who were diagnosed with prostate cancer at some point are alive today.3
Risk factors. Prostate cancer develops mainly in men ages ≥ 65 years and rarely occurs before age 40. In addition to age, family history and African American ethnicity are the major nonmodifiable risk factors for prostate cancer.4 From the 1970s to the most recent statistical analysis of the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program, African American men have continued to have significantly higher incidence of, and mortality rates from, prostate cancer than their European American counterparts. African American men are also more likely than men of European ancestry to have aggressive prostate cancers.5 Other risk factors include geographic location (higher risk in Northern Europe, North America, and Australia; lower risk in Asia, Africa, and South and Central America), mutations in the BRCA2 gene, and hereditary non-polyposis colon cancer syndrome.4
Prostate-specific antigen (PSA) was first used as a screening tool for prostate cancer in 1991.6 Prostate cancer incidence, especially organ-confined disease, has dramatically increased since then.7 PSA testing has a low sensitivity and specificity for the detection of prostate cancer, and there is no clear threshold at which biopsy can or should be offered. The most commonly used cutoff value of 4 ng/mL has a false-positive rate of about 70%.8
Benign prostatic conditions such as hypertrophy and infection can elevate PSA levels. In addition, the PSA test does not distinguish between aggressive and slow-growing cancers, and about 15% of patients with prostate cancer have a normal PSA level.9
A word about the digital rectal exam. While PSA testing has been the mainstay of prostate cancer screening, a few studies have included digital rectal exam (DRE) in their protocols. Data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial showed that DRE captured an additional 2% of men with prostate cancer in the setting of a normal PSA test result.10 In the Rotterdam arm of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial, the overall detection rate for prostate cancer was found to be better when DRE was combined with PSA and prostate biopsy than when DRE was used alone (4.5% vs 2.5%).11 Nevertheless, generally speaking, DRE can be omitted in the era of PSA screening.
Screening guidelines vary
Recommendations for prostate cancer screening vary by organization and are summarized in TABLE 1.9,12-14 In 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening for prostate cancer (Category D).15 In 2018, USPSTF provided an update with a new recommendation that clinicians inform men ages 55 to 69 years about the potential benefits and harms of PSA-based screening (Category C).14 The USPSTF continues to recommend against PSA-based screening for men ages ≥ 70 years (Category D).14

Does PSA-based screening improve patient-centered outcomes?
Several randomized controlled trials (RCTs) such as the Quebec Prospective Randomized Controlled Trial,16 the Norrköping Sweden Study,17 ERSPC,11 and PLCO10 have been conducted to assess the benefits of PSA testing. PLCO and ERSPC have contributed significantly to our understanding of prostate cancer screening even though their 13-year follow-up results are conflicting (TABLE 2).10,11,18

Continue to: In the ERSPC 13-year follow-up publication...
In the ERSPC 13-year follow-up publication, the authors concluded that a substantial reduction in prostate cancer mortality is attributable to testing with PSA.18 Despite limitations in the study design (eg, France entered after 2 years, screening intervals varied between 2 and 4 years, biopsy indications varied, and screening was discontinued at different times), PSA screening detected more prostate cancer than was detected in the control arm (10.2% vs 6.8%).
In the initial 11 years of follow-up, the study group experienced a 21% reduction in prostate cancer mortality, even though the absolute decrease ranged from only 0.6% (545 per 89,352) to 0.5% (355 per 72,891). The updated absolute risk reduction of death from prostate cancer at 13 years of follow-up showed a larger benefit: 0.11 per 1000 person-years or 1.28 per 1000 men randomized, which is equivalent to 1 prostate cancer death averted per 781 (95% confidence interval [CI], 490-1929) men invited for screening, or 1 per 27 (17-66) additional prostate cancers detected.
The PLCO trial did not show any significant difference in prostate cancer detection (11.1% screened vs 9.9% control), and there was no improvement in prostate cancer mortality (3.7 vs 3.4 death per 10,000 person-years).10 However, the PLCO trial suffered from issues of contamination, which may have influenced the overall results. About 52% of men in the control (usual care) group received a PSA test at some point during the study. And more than two-thirds of the men who had a prostate biopsy because of a positive PSA test did not have prostate cancer.
Community standards for the PSA threshold for biopsy were applied in various centers (> 4 ng/ml in general) in PLCO, whereas in ERSPC, a cut-off PSA value ≥ 3 ng/mL was used for biopsy. Because of the lower PSA threshold, ERSPC may have identified cancers that would have had good outcomes without any intervention.
The harms of PSA screening
While it is unclear whether PSA screening results in any improvement in patient-centered outcomes, it does lead to downstream intervention due to overdiagnosis, which precipitates unnecessary anxiety, biopsies, and overtreatment (eg, excess radiation, overuse of androgen deprivation therapy).19 Biopsies carry the risk of hematuria (22.6%), hematospermia (50.4%), and urinary tract infection.20 Data from SEER-Medicare showed that prostate biopsy was associated with a 2.65-fold increased risk of hospitalization within 30 days of the procedure compared to a control population.21
Continue to: Overdiagnosis leads to overtreatment...
Overdiagnosis leads to overtreatment of low-risk prostate cancer. Both traditional treatment options for prostate cancer—radical prostatectomy and radiotherapy—are associated with urinary incontinence, erectile dysfunction, and issues with bowel function.22,23
The Prostate Cancer Intervention vs Observation Trial (PIVOT),24 the Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4),25 and the Prostate Testing for Cancer and Treatment (ProtecT) trial,22,23 are the major RCTs that looked at the outcomes of treatment modalities for localized prostate cancer in the modern era of PSA testing.
PIVOT compared passive observation with radical prostatectomy.24 After 20 years of follow-up on 731 patients, the researchers concluded that radical prostatectomy did not reduce all-cause or prostate cancer–related mortality (TABLE 3).24

SPCG-4 showed survival benefits for men who underwent radical prostatectomy compared with men in a watchful waiting group, but only 5% of the study cohort had cancer detected by PSA screening (TABLE 4).25 The rest had either palpable tumors or symptoms of a tumor.

ProtecT, which followed patients with localized prostate cancer for more than 10 years,compared the outcomes and adverse effects of active surveillance, radical prostatectomy, and radiotherapy.23 Prostate cancer–specific mortality was low irrespective of the treatment,23 and there was no significant difference in all-cause mortality or prostate cancer–specific mortality between the 3 treatment groups.23 The active surveillance group had considerably fewer adverse events.22,23 The incidence rates of erectile dysfunction and urinary incontinence at the 1- and 6-year follow-up marks are outlined in TABLE 5.22

Continue to: The purpose of active monitoring...
The purpose of active monitoring is to minimize overtreatment by avoiding immediate radical intervention. Radical treatments with curative intent can be undertaken at any point while patients are being actively monitored. It is important to note that the active monitoring that took place in ProtecT23 was very different from the passive surveillance of PIVOT24 and SPCG-4.25 In ProtecT, once an elevated serum PSA level was noted, PSA levels were monitored every 3 months in the first year and every 6 to 12 months thereafter.23 Triggers to reassess patients and consider a change in clinical management were based largely on changes in PSA levels. Participants with an increase of at least 50% in PSA level during the previous 12 months were offered either continued monitoring or treatment after further testing.
Making individualized decisions about prostate cancer screening
Traditionally, the goal of cancer screening has been to maximize the number of people screened. Generally, the information provided to patients about cancer screening emphasizes the benefits and minimizes the harms. Recently, however, there has been a shift in communication about cancer screening with the emphasis now being placed on informed decision-making and encouraging patients to make individual decisions about screening participation.26
The treatment option of active surveillance, with its lower incidence of adverse outcomes, is an important reason for patients to make individualized decisions about prostate cancer screening.
Another reason relates to 5-alpha-reductase inhibitors. Although their role in the management of prostate cancer is currently not well defined, a reduction of almost 25% in the risk of prostate cancer and improvement in the performance of PSA has been reported.27
And yet another reason is that there are alternate strategies to manage the majority of patients who have been diagnosed with low-risk disease through transrectal ultrasound biopsy. The ERSPC study mentions multiparametric magnetic resonance imaging combined with targeted biopsy to identify high-grade disease.28,29 Genetic and epigenetic assays of the biopsied tissue can help grade disease based on aggressiveness.30 Transperineal mapping biopsy using a mapping software program can identify specific disease sites within the prostate gland, so that patients can be offered the option of targeted therapy.30
Continue to: Applying shared decision-making to prostate cancer screening
Applying shared decision-making to prostate cancer screening
Balancing errors of omission with errors of commission is challenging. Shared decision-making (SDM) is an approach whereby clinicians and patients share the best available evidence when faced with the task of medical decision-making and in which patients are supported while they consider their options and achieve their preferences.31 SDM is well supported by evidence from a number of RCTs and results in increased knowledge, involvement, and confidence on the part of patients.32 An individualized approach using the schematic diagram (FIGURE 13,18) may be helpful.

Barriers to SDM success. Many factors can interfere with the success of SDM including limited or poor communication; lack of time during busy office visits; and patients’ cultural, informational, and/or emotional needs. To improve patient-centered communication, we can: (1) make information understandable and available to patients and families; (2) prioritize training in communication; (3) use decision aid tools to facilitate communication; and (4) work to improve the payment model to incentivize patient-centered communication. Tools that facilitate SDM include videotapes, patient group discussions, brief scripts read to patients, and informational pamphlets. One such tool is the American Society for Clinical Oncology’s decision aid tool for PSA testing.33
Limited knowledge among patients. Decisions regarding treatment among men diagnosed with localized prostate cancer can be difficult because there are several treatment options with similar prognoses, but there are differences in adverse effects. One population-based cohort study of men with newly diagnosed localized prostate cancer found that most men had significant knowledge deficits regarding the survival benefits of the 2 major treatment options—surgery and radiation.34 In a large population-based study, 38% of men with localized prostate cancer reported receiving help from their primary care providers in the decision-making process for treatment.35
Learning to employ SDM. Elwyn et al proposed a 3-step model to incorporate SDM into clinical practice.31 They described key steps that include: choice talk (making sure patients are informed about the reasonable options), option talk (providing more detailed information about the options), and decision talk (supporting the work of patients considering their preferences and deciding what is best). Properly employing these methods requires training using simulations.31
The bottom line
Although current guidelines regarding PSA screening differ by organization, generally speaking PSA screening should be offered only to men with a life expectancy > 10 years. The PSA test has low sensitivity and specificity and lacks a clear cut-off value that warrants prostate biopsy. Men who choose to have PSA testing increase their chances of detecting prostate cancer, but most prostate cancers are slow growing and do not cause death. The decision to undergo PSA screening should be made by both the provider and the patient, after a discussion of the limited benefits and associated harms. The interval of follow-up screening may vary from 2 to 4 years depending on patient age, level of PSA, and whether a patient is taking medications such as 5-alpha-reductase inhibitors.
CORRESPONDENCE
Jaividhya Dasarathy, MD, FAAFP, 2500 Metro Health Medical Drive, Cleveland, Ohio 44109; [email protected].
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30.
2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed January 16, 2020.
3. American Cancer Society. Key statistics for prostate cancer. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed January 16, 2020.
4. Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152-156.
5. Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol. 2007;177:444-449.
6. Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-1161.
7. Jacobsen SJ, Katusic SK, Bergstraih EJ. Incidence of prostate cancer diagnosis in the eras before and after serum prostate-specific antigen testing. JAMA. 1995;274:1445-1449.
8. Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95-101.
9. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Int Med. 2013;158:761-769.
10. Andriole GL, Crawford ED, Grubb RL 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125-132.
11. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
12. American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Last revised August 1, 2019. www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed January 16, 2020.
13. American Urologic Association. Early detection of prostate cancer (2018). Reviewed 2018. https://www.auanet.org/guidelines/prostate-cancer-early-detection-guideline. Accessed January 16, 2020.
14. US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
15 Moyer VA. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Int Med. 2012;157:120-134.
16. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate. 1999;38:83-91.
17. Sandblom G, Varenhorst E, Rosell J, et al. Randomised prostate cancer screening trial: 20-year follow-up. BMJ. 2011;342:d1539.
18. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027-2035.
19. McNaughton-Collins M, Fowler FJ Jr, Caubet JF, et al. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am J Med. 2004;117:719-725.
20 Raaijmakers R, Kirkels WJ, Roobol MJ, et al. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology. 2002;60:826-830.
21. Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830-1834.
22. Donovan J, Hamdy F, Lane J, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-1437.
23. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
24. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377:132-142.
25. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2018;379:2319-2329.
26. Hersch JK, Nickel BL, Ghanouni A, et al. Improving communication about cancer screening: moving towards informed decision making. Public Health Res Pract. 2017;27(2).
27. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15:e484-e492.
28. Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281-1285.
29. Kuru TH, Roethke MC, Seidenader J, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380-1386.
30. Crawford ED, Rove KO, Barqawi AB, et al. Clinical-pathologic correlation between transperineal mapping biopsies of the prostate and three-dimensional reconstruction of prostatectomy specimens. Prostate. 2013;73:778-787.
31. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361-1367.
32. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
33. ASCO. Decision aid tool: prostate cancer screening with PSA testing. https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2012-psa-pco-decision-aid.pdf. Accessed January 16, 2020.
34. Daum LM, Reamer EN, Ruterbusch JJ, et al. Patient knowledge and qualities of treatment decisions for localized prostate cancer. J Am Board Fam Med. 2017;30:288-297.
35. Radhakrishnan A, Grande D, Ross M, et al. When primary care providers (PCPs) help patients choose prostate cancer treatment. J Am Board Fam Med. 2017;30:298-307.
PRACTICE RECOMMENDATIONS
› Recommend individualized decision-making to men ages 55 to 69 years after discussing the potential benefits and risks of prostate-specific antigen (PSA)-based screening. B
› Do not use a PSA-based screening method for prostate cancer in men ages < 50 years or > 70 years or men with a life expectancy < 10 years. C
› Do not routinely recommend PSA-based screening to men with a family history of prostate cancer or to men who are African American. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series