User login
Barbers have role in encouraging diabetes screening in black men
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
Shave and a haircut … and a blood glucose test? A study shows that barbershops owned by black proprietors can play a role in encouraging black men to get screened for diabetes.
In research letter published in the Jan. 27 edition of JAMA Internal Medicine, Marcela Osorio, BA, from New York University and coauthors wrote that black men with diabetes have disproportionately high rates of diabetes complications and lower survival rates. Their diagnosis is often delayed, particularly among men without regular primary health care.
“In barbershops, which are places of trust among black men, community-based interventions have been successful in identifying and treating men with hypertension,” they wrote.
In this study, the researchers approached customers in eight barbershops in Brooklyn, in areas associated with a high prevalence of individuals with poor glycemic control, to encourage them to get tested for diabetes. All barbershops were owned by black individuals.
Around one-third of the 895 black men who were asked to participate in the study agreed to be screened, and 290 (32.4%) were successfully tested using point-of-care hemoglobin A1c testing.
The screening revealed that 9% of those tested had an HbA1c level of 6.5% or higher, and 16 of these individuals were obese. Three men had an HbA1c level of 7.5% or higher. The investigators noted that this prevalence of undiagnosed diabetes was much higher than the 3.6% estimated prevalence among New York City residents.
The highest HbA1c level recorded during testing was 7.8%, and 28.3% of those tested had a level between 5.7% and 6.4%, which meets the criteria for a diagnosis of prediabetes.
“We also found that barbers were important health advocates; although we do not have exact numbers, some customers (who initially declined testing) agreed after encouragement from their barber,” the authors wrote.
Of the 583 men who declined to participate, around one-quarter did so on the grounds that they already knew their health status or had been checked by their doctor, one-third (35.3%) said they were healthy or didn’t have the time or interest, or didn’t want to know the results. There were also 26 individuals who reported being scared of needles.
“Black men who live in urban areas of the United States may face socioeconomic barriers to good health, including poor food environments and difficulty in obtaining primary care,” the authors wrote. “Our findings suggest that community-based diabetes screening in barbershops owned by black individuals may play a role in the timely diagnosis of diabetes and may help to identify black men who need appropriate care for their newly diagnosed diabetes.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
SOURCE: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Barbershops could offer a way to encourage diabetes screening among black men.
Major finding: HbA1c testing in barbershops identified a significant number of individuals with undiagnosed diabetes.
Study details: Study involving 895 black men attending eight barbershops in Brooklyn.
Disclosures: The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Two authors declared grants from the institute during the study, and one also reported grants from other research foundations outside the study.
Source: Osorio M et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6867.
A quick guide to PrEP: Steps to take & insurance coverage changes to watch for
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. Accessed January 17, 2020.
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. CDC Web Site. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed January 17, 2020.
- US Preventive Services Task Force. Final recommendation statement: prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Published June 2019. Accessed January 17, 2020.
- Campos-Outcalt D. A look at new guidelines for HIV treatment and prevention. J Fam Pract. 2018;67:768-772.
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. Accessed January 17, 2020.
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. CDC Web Site. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed January 17, 2020.
- US Preventive Services Task Force. Final recommendation statement: prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Published June 2019. Accessed January 17, 2020.
- Campos-Outcalt D. A look at new guidelines for HIV treatment and prevention. J Fam Pract. 2018;67:768-772.
References
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report. 2019;24. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published February 2019. Accessed January 17, 2020.
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. CDC Web Site. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Published March 2018. Accessed January 17, 2020.
- US Preventive Services Task Force. Final recommendation statement: prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Published June 2019. Accessed January 17, 2020.
- Campos-Outcalt D. A look at new guidelines for HIV treatment and prevention. J Fam Pract. 2018;67:768-772.
Testosterone gel increases LV mass in older men
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
REPORTING FROM AHA 2019
Provide appropriate sexual, reproductive health care for transgender patients
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
New guideline for testosterone treatment in men with ‘low T’
The American College of Physicians has released new clinical guidelines providing practical recommendations for testosterone therapy in adult men with age-related low testosterone.
The evidence-based recommendations target all clinicians and were published online January 6, 2020, in Annals of Internal Medicine, highlighting data from a systematic review of evidence on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone.
Serum testosterone levels drop as men age, starting in their mid-30s, and approximately 20% of American men older than 60 years have low testosterone.
However, no widely accepted testosterone threshold level exists that represents a measure below which symptoms of androgen deficiency and adverse health outcomes occur.
In addition, the role of testosterone therapy in managing this patient population is controversial.
“The purpose of this American College of Physicians guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone,” write Amir Qaseem, MD, PhD, MHA, from the American College of Physicians, Philadelphia, and colleagues.
“This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels,” the authors note.
In particular, the recommendations suggest that clinicians should initiate testosterone treatment in these patients only to help them improve their sexual function.
According to the authors, moderate-certainty evidence from seven trials involving testosterone treatment in adult men with age-related low testosterone showed a small improvement in global sexual function, whereas low-certainty evidence from seven trials showed a small improvement in erectile function.
By contrast, the guideline emphasizes that clinicians should avoid prescribing testosterone treatment for any other concern in this population. Available evidence demonstrates little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition among these men after receiving testosterone treatment, the authors stress.
ACP recommends that clinicians should reassess men’s symptoms within 12 months of testosterone treatment initiation, with regular reevaluations during subsequent follow up. Clinicians should discontinue treatment in men if sexual function fails to improve.
The guideline also recommends using intramuscular formulations of testosterone treatment for this patient population instead of transdermal ones, because intramuscular formulations cost less and have similar clinical effectiveness and harms.
“The annual cost in 2016 per beneficiary for TRT [testosterone replacement therapy] was $2,135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data,” the authors write.
In an accompanying editorial, E. Victor Adlin, MD, of Temple University, Philadelphia, notes that these new ACP guidelines mostly mirror those recently proposed by both the Endocrine Society and the American Urological Association.
However, he predicts that many clinicians will question the ACP’s recommendation to favor use of intramuscular over transdermal formulations of testosterone.
Although Dr. Adlin acknowledges the lower cost of intramuscular preparations as a major consideration, he explains that “the need for an intramuscular injection every 1-4 weeks is a potential barrier to adherence, and some patients require visits to a health care facility for the injections, which may add to the expense.”
Fluctuating blood testosterone levels after each injection may also result in irregular symptom relief and difficulty achieving the desired blood level, he adds. “Individual preference may vary widely in the choice of testosterone therapy.”
Overall, Dr. Adlin stresses that a patient-clinician discussion should serve as the foundation for starting testosterone therapy in men with age-related low testosterone, with the patient playing a central role in treatment decision making.
This guideline was developed with financial support from the American College of Physicians’ operating budget. Study author Carrie Horwitch reports serving as a fiduciary officer for the Washington State Medical Association. Jennifer S. Lin, a member of the ACP Clinical Guidelines Committee, reports being an employee of Kaiser Permanente. Robert McLean, another member of the committee, reports being an employee of Northeast Medical Group. The remaining authors and the editorialist have disclosed no relevant financial relationships.
A version of this story appeared on Medscape.com.
The American College of Physicians has released new clinical guidelines providing practical recommendations for testosterone therapy in adult men with age-related low testosterone.
The evidence-based recommendations target all clinicians and were published online January 6, 2020, in Annals of Internal Medicine, highlighting data from a systematic review of evidence on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone.
Serum testosterone levels drop as men age, starting in their mid-30s, and approximately 20% of American men older than 60 years have low testosterone.
However, no widely accepted testosterone threshold level exists that represents a measure below which symptoms of androgen deficiency and adverse health outcomes occur.
In addition, the role of testosterone therapy in managing this patient population is controversial.
“The purpose of this American College of Physicians guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone,” write Amir Qaseem, MD, PhD, MHA, from the American College of Physicians, Philadelphia, and colleagues.
“This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels,” the authors note.
In particular, the recommendations suggest that clinicians should initiate testosterone treatment in these patients only to help them improve their sexual function.
According to the authors, moderate-certainty evidence from seven trials involving testosterone treatment in adult men with age-related low testosterone showed a small improvement in global sexual function, whereas low-certainty evidence from seven trials showed a small improvement in erectile function.
By contrast, the guideline emphasizes that clinicians should avoid prescribing testosterone treatment for any other concern in this population. Available evidence demonstrates little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition among these men after receiving testosterone treatment, the authors stress.
ACP recommends that clinicians should reassess men’s symptoms within 12 months of testosterone treatment initiation, with regular reevaluations during subsequent follow up. Clinicians should discontinue treatment in men if sexual function fails to improve.
The guideline also recommends using intramuscular formulations of testosterone treatment for this patient population instead of transdermal ones, because intramuscular formulations cost less and have similar clinical effectiveness and harms.
“The annual cost in 2016 per beneficiary for TRT [testosterone replacement therapy] was $2,135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data,” the authors write.
In an accompanying editorial, E. Victor Adlin, MD, of Temple University, Philadelphia, notes that these new ACP guidelines mostly mirror those recently proposed by both the Endocrine Society and the American Urological Association.
However, he predicts that many clinicians will question the ACP’s recommendation to favor use of intramuscular over transdermal formulations of testosterone.
Although Dr. Adlin acknowledges the lower cost of intramuscular preparations as a major consideration, he explains that “the need for an intramuscular injection every 1-4 weeks is a potential barrier to adherence, and some patients require visits to a health care facility for the injections, which may add to the expense.”
Fluctuating blood testosterone levels after each injection may also result in irregular symptom relief and difficulty achieving the desired blood level, he adds. “Individual preference may vary widely in the choice of testosterone therapy.”
Overall, Dr. Adlin stresses that a patient-clinician discussion should serve as the foundation for starting testosterone therapy in men with age-related low testosterone, with the patient playing a central role in treatment decision making.
This guideline was developed with financial support from the American College of Physicians’ operating budget. Study author Carrie Horwitch reports serving as a fiduciary officer for the Washington State Medical Association. Jennifer S. Lin, a member of the ACP Clinical Guidelines Committee, reports being an employee of Kaiser Permanente. Robert McLean, another member of the committee, reports being an employee of Northeast Medical Group. The remaining authors and the editorialist have disclosed no relevant financial relationships.
A version of this story appeared on Medscape.com.
The American College of Physicians has released new clinical guidelines providing practical recommendations for testosterone therapy in adult men with age-related low testosterone.
The evidence-based recommendations target all clinicians and were published online January 6, 2020, in Annals of Internal Medicine, highlighting data from a systematic review of evidence on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone.
Serum testosterone levels drop as men age, starting in their mid-30s, and approximately 20% of American men older than 60 years have low testosterone.
However, no widely accepted testosterone threshold level exists that represents a measure below which symptoms of androgen deficiency and adverse health outcomes occur.
In addition, the role of testosterone therapy in managing this patient population is controversial.
“The purpose of this American College of Physicians guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone,” write Amir Qaseem, MD, PhD, MHA, from the American College of Physicians, Philadelphia, and colleagues.
“This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels,” the authors note.
In particular, the recommendations suggest that clinicians should initiate testosterone treatment in these patients only to help them improve their sexual function.
According to the authors, moderate-certainty evidence from seven trials involving testosterone treatment in adult men with age-related low testosterone showed a small improvement in global sexual function, whereas low-certainty evidence from seven trials showed a small improvement in erectile function.
By contrast, the guideline emphasizes that clinicians should avoid prescribing testosterone treatment for any other concern in this population. Available evidence demonstrates little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition among these men after receiving testosterone treatment, the authors stress.
ACP recommends that clinicians should reassess men’s symptoms within 12 months of testosterone treatment initiation, with regular reevaluations during subsequent follow up. Clinicians should discontinue treatment in men if sexual function fails to improve.
The guideline also recommends using intramuscular formulations of testosterone treatment for this patient population instead of transdermal ones, because intramuscular formulations cost less and have similar clinical effectiveness and harms.
“The annual cost in 2016 per beneficiary for TRT [testosterone replacement therapy] was $2,135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data,” the authors write.
In an accompanying editorial, E. Victor Adlin, MD, of Temple University, Philadelphia, notes that these new ACP guidelines mostly mirror those recently proposed by both the Endocrine Society and the American Urological Association.
However, he predicts that many clinicians will question the ACP’s recommendation to favor use of intramuscular over transdermal formulations of testosterone.
Although Dr. Adlin acknowledges the lower cost of intramuscular preparations as a major consideration, he explains that “the need for an intramuscular injection every 1-4 weeks is a potential barrier to adherence, and some patients require visits to a health care facility for the injections, which may add to the expense.”
Fluctuating blood testosterone levels after each injection may also result in irregular symptom relief and difficulty achieving the desired blood level, he adds. “Individual preference may vary widely in the choice of testosterone therapy.”
Overall, Dr. Adlin stresses that a patient-clinician discussion should serve as the foundation for starting testosterone therapy in men with age-related low testosterone, with the patient playing a central role in treatment decision making.
This guideline was developed with financial support from the American College of Physicians’ operating budget. Study author Carrie Horwitch reports serving as a fiduciary officer for the Washington State Medical Association. Jennifer S. Lin, a member of the ACP Clinical Guidelines Committee, reports being an employee of Kaiser Permanente. Robert McLean, another member of the committee, reports being an employee of Northeast Medical Group. The remaining authors and the editorialist have disclosed no relevant financial relationships.
A version of this story appeared on Medscape.com.
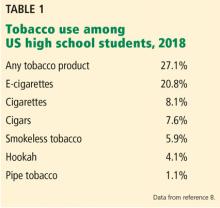
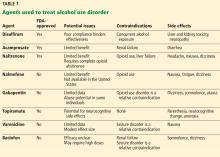
Vaping: The new wave of nicotine addiction
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
KEY POINTS
- Vaping is a common gateway to tobacco and marijuana use for adolescents and adults.
- The Juul vaping device delivers high nicotine concentrations that may pose a higher risk of nicotine addiction.
- Vaping has had unintended consequences that include poisoning of children who swallowed liquid nicotine, fires and explosions from defective batteries in the devices, and effects on the developing brain.
- Vaping is associated with respiratory illness and, in rare cases, death, likely due to vaporized agents introduced into the lungs. Small amounts of heavy metals, acetone, and other carcinogenic compounds in the vaping aerosol may cause lung damage.
Gabapentin for alcohol use disorder: A good option, or cause for concern?
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
KEY POINTS
- Gabapentin has been shown to be safe and effective for mild alcohol withdrawal but is not appropriate as monotherapy for severe withdrawal owing to risk of seizures.
- During early abstinence, gabapentin may improve sleep, cravings, and mood—factors associated with relapse.
- Gabapentin is being used recreationally to achieve or enhance euphoria, but its misuse potential appears to be low when taken at therapeutic doses by patients without a history of drug abuse.
How to respond to flu vaccine doubters
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
STI update: Testing, treatment, and emerging threats
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
Sexually transmitted infections (STIs) such as gonorrhea, chlamydia, and syphilis are still increasing in incidence and probably will continue to do so in the near future. Moreover, drug-resistant strains of Neisseria gonorrhoeae are emerging, as are less-known organisms such as Mycoplasma genitalium.
Now the good news: new tests for STIs are available or are coming! Based on nucleic acid amplification, these tests can be performed at the point of care, so that patients can leave the clinic with an accurate diagnosis and proper treatment for themselves and their sexual partners. Also, the tests can be run on samples collected by the patients themselves, either swabs or urine collections, eliminating the need for invasive sampling and making doctor-shy patients more likely to come in to be treated.1 We hope that by using these sensitive and accurate tests we can begin to bend the upward curve of STIs and be better antimicrobial stewards.2
This article reviews current issues surrounding STI control, and provides detailed guidance on recognizing, testing for, and treating gonorrhea, chlamydia, trichomoniasis, and M genitalium infection.
STI RATES ARE HIGH AND RISING
STIs are among the most common acute infectious diseases worldwide, with an estimated 1 million new curable cases every day.3 Further, STIs have major impacts on sexual, reproductive, and psychological health.
In the United States, rates of reportable STIs (chlamydia, gonorrhea, and syphilis) are rising.4 In addition, more-sensitive tests for trichomoniasis, which is not a reportable infection in any state, have revealed it to be more prevalent than previously thought.5
BARRIERS AND CHALLENGES TO DIAGNOSIS
The medical system does not fully meet the needs of some populations, including young people and men who have sex with men, regarding their sexual and reproductive health.
Ongoing barriers among young people include reluctance to use available health services, limited access to STI testing, worries about confidentiality, and the shame and stigma associated with STIs.6
Men who have sex with men have a higher incidence of STIs than other groups. Since STIs are associated with a higher risk of human immunodeficiency virus (HIV) infection, it is important to detect, diagnose, and manage STIs in this group—and in all high-risk groups. Rectal STIs are an independent risk factor for incident HIV infection.7 In addition, many men who have sex with men face challenges navigating the emotional, physical, and cognitive aspects of adolescence, a voyage further complicated by mental health issues, unprotected sexual encounters, and substance abuse in many, especially among minority youth.8 These same factors also impair their ability to access resources for preventing and treating HIV and other STIs.
STI diagnosis is often missed
Most people who have STIs feel no symptoms, which increases the importance of risk-based screening to detect these infections.9,10 In many other cases, STIs manifest with nonspecific genitourinary symptoms that are mistaken for urinary tract infection. Tomas et al11 found that of 264 women who presented to an emergency department with genitourinary symptoms or were being treated for urinary tract infection, 175 were given a diagnosis of a urinary tract infection. Of these, 100 (57%) were treated without performing a urine culture; 60 (23%) of the 264 women had 1 or more positive STI tests, 22 (37%) of whom did not receive treatment for an STI.
Poor follow-up of patients and partners
Patients with STIs need to be retested 3 months after treatment to make sure the treatment was effective. Another reason for follow-up is that these patients are at higher risk of another infection within a year.12
Although treating patients’ partners has been shown to reduce reinfection rates, fewer than one-third of STIs (including HIV infections) were recognized through partner notification between 2010 and 2012 in a Dutch study, in men who have sex with men and in women.13 Challenges included partners who could not be identified among men who have sex with men, failure of heterosexual men to notify their partners, and lower rates of partner notification for HIV.
In the United States, “expedited partner therapy” allows healthcare providers to provide a prescription or medications to partners of patients diagnosed with chlamydia or gonorrhea without examining the partner.14 While this approach is legal in most states, implementation can be challenging.15
STI EVALUATION
History and physical examination
A complete sexual history helps in estimating the patient’s risk of an STI and applying appropriate risk-based screening. Factors such as sexual practices, use of barrier protection, and history of STIs should be discussed.
Physical examination is also important. Although some patients may experience discomfort during a genital or pelvic examination, omitting this step may lead to missed diagnoses in women with STIs.16
Laboratory testing
Laboratory testing for STIs helps ensure accurate diagnosis and treatment. Empiric treatment without testing could give a patient a false sense of health by missing an infection that is not currently causing symptoms but that could later worsen or have lasting complications. Failure to test patients also misses the opportunity for partner notification, linkage to services, and follow-up testing.
Many of the most common STIs, including gonorrhea, chlamydia, and trichomoniasis, can be detected using vaginal, cervical, or urethral swabs or first-catch urine (from the initial urine stream). In studies that compared various sampling methods,17 self-collected urine samples for gonorrhea in men were nearly as good as clinician-collected swabs of the urethra. In women, self-collected vaginal swabs for gonorrhea and chlamydia were nearly as good as clinician-collected vaginal swabs. While urine specimens are acceptable for chlamydia testing in women, their sensitivity may be slightly lower than with vaginal and endocervical swab specimens.18,19
A major advantage of urine specimens for STI testing is that collection is noninvasive and is therefore more likely to be acceptable to patients. Urine testing can also be conducted in a variety of nonclinical settings such as health fairs, pharmacy-based screening programs, and express STI testing sites, thus increasing availability.
To prevent further transmission and morbidity and to aid in public health efforts, it is critical to recognize the cause of infectious cervicitis and urethritis and to screen for STIs according to guidelines.12 Table 1 summarizes current screening and laboratory testing recommendations.
GONORRHEA AND CHLAMYDIA
Gonorrhea and chlamydia are the 2 most frequently reported STIs in the United States, with more than 550,000 cases of gonorrhea and 1.7 million cases of chlamydia reported in 2017.4
Both infections present similarly: cervicitis or urethritis characterized by discharge (mucopurulent discharge with gonorrhea) and dysuria. Untreated, they can lead to pelvic inflammatory disease, inflammation, and infertility.
Extragenital infections can be asymptomatic or cause exudative pharyngitis or proctitis. Most people in whom chlamydia is detected from pharyngeal specimens are asymptomatic. When pharyngeal symptoms exist secondary to gonorrheal infection, they typically include sore throat and pharyngeal exudates. However, Komaroff et al,20 in a study of 192 men and women who presented with sore throat, found that only 2 (1%) tested positive for N gonorrhoeae.
Screening for gonorrhea and chlamydia
Best practices include screening for gonorrhea and chlamydia as follows21–23:
- Every year in sexually active women through age 25 (including during pregnancy) and in older women who have risk factors for infection12
- At least every year in men who have sex with men, at all sites of sexual contact (urethra, pharynx, rectum), along with testing for HIV and syphilis
- Every 3 to 6 months in men who have sex with men who have multiple or anonymous partners, who are sexually active and use illicit drugs, or who have partners who use illicit drugs
- Possibly every year in young men who live in high-prevalence areas or who are seen in certain clinical settings, such as STI and adolescent clinics.
Specimens. A vaginal swab is preferred for screening in women. Several studies have shown that self-collected swabs have clinical sensitivity and specificity comparable to that of provider-collected samples.17,24 First-catch urine or endocervical swabs have similar performance characteristics and are also acceptable. In men, urethral swabs or first-catch urine samples are appropriate for screening for urogenital infections.
Testing methods. Testing for both pathogens should be done simultaneously with a nucleic acid amplification test (NAAT). Commercially available NAATs are more sensitive than culture and antigen testing for detecting gonorrhea and chlamydia.25–27
Most assays are approved by the US Food and Drug Administration (FDA) for testing vaginal, urethral, cervical, and urine specimens. Until recently, no commercial assay was cleared for testing extragenital sites, but recommendations for screening extragenital sites prompted many clinical laboratories to validate throat and rectal swabs for use with NAATs, which are more sensitive than culture at these sites.25,28 The recent FDA approval of extragenital specimen types for 2 commercially available assays may increase the availability of testing for these sites.
Data on the utility of NAATs for detecting chlamydia and gonorrhea in children are limited, and many clinical laboratories have not validated molecular methods for testing in children. Current guidelines specific to this population should be followed regarding test methods and preferred specimen types.12,29,30
Although gonococcal infection is usually diagnosed with culture-independent molecular methods, antimicrobial resistance is emerging. Thus, failure of the combination of ceftriaxone and azithromycin should prompt culture-based follow-up testing to determine antimicrobial susceptibility.
Strategies for treatment and control
Historically, people treated for gonorrhea have been treated for chlamydia at the same time, as these diseases tend to go together. This can be with a single intramuscular dose of ceftriaxone for the gonorrhea plus a single oral dose of azithromycin for the chlamydia.12 For patients who have only gonorrhea, this double regimen may help prevent the development of resistant gonorrhea strains.
All the patient’s sexual partners in the previous 60 days should be tested and treated, and expedited partner therapy should be offered if possible. Patients should be advised to have no sexual contact until they complete the treatment, or 7 days after single-dose treatment. Testing should be repeated 3 months after treatment.
M GENITALIUM IS EMERGING
A member of the Mycoplasmataceae family, M genitalium was originally identified as a pathogen in the early 1980s but has only recently emerged as an important cause of STI. Studies indicate that it is responsible for 10% to 20% of cases of nongonococcal urethritis and 10% to 30% of cases of cervicitis.31–33 Additionally, 2% to 22% of cases of pelvic inflammatory disease have evidence of M genitalium.34,35
However, data on M genitalium prevalence are suspect because the organism is hard to identify—lacking a cell wall, it is undetectable by Gram stain.36 Although it has been isolated in respiratory and synovial fluids, it has so far been recognized to be clinically important only in the urogenital tract. It can persist for years in infected patients by exploiting specialized cell-surface structures to invade cells.36 Once inside a cell, it triggers secretion of mycoplasmal toxins and destructive metabolites such as hydrogen peroxide, evading the host immune system as it does so.37
Testing guidelines for M genitalium
Current guidelines do not recommend routine screening for M genitalium, and no commercial test was available until recently.12 Although evidence suggests that M genitalium is independently associated with preterm birth and miscarriages,38 routine screening of pregnant women is not recommended.12
Testing for M genitalium should be considered in cases of persistent or recurrent nongonococcal urethritis in patients who test negative for gonorrhea and chlamydia or for whom treatment has failed.12 Many isolates exhibit genotypic resistance to macrolide antibiotics, which are often the first-line therapy for nongonococcal urethritis.39
Further study is needed to evaluate the potential impact of routine screening for M genitalium on the reproductive and sexual health of at-risk populations.
Diagnostic tests for M genitalium
Awareness of M genitalium as a cause of nongonococcal urethritis has been hampered by a dearth of diagnostic tests.40 The organism’s fastidious requirements and extremely slow growth preclude culture as a practical method of diagnosis.41 Serologic assays are dogged by cross-reactivity and poor sensitivity.42,43 Thus, molecular assays for detecting M genitalium and associated resistance markers are preferred for diagnosis.12
Several molecular tests are approved, available, and in use in Europe for diagnosing M genitalium infection,40 and in January 2019 the FDA approved a molecular test that can detect M genitalium in urine specimens and vaginal, endocervical, urethral, and penile meatal swabs. Although vaginal swabs are preferred for this assay because they have higher sensitivity (92% for provider-collected and 99% for patient-collected swabs), urine specimens are acceptable, with a sensitivity of 78%.44
At least 1 company is seeking FDA clearance for another molecular diagnostic assay for detecting M genitalium and markers of macrolide resistance in urine and genital swab specimens. Such assays may facilitate appropriate treatment.
Clinicians should stay abreast of diagnostic testing options, which are likely to become more readily available soon.
A high rate of macrolide resistance
Because M genitalium lacks a cell wall, antibiotics such as beta-lactams that target cell wall synthesis are ineffective.
Regimens for treating M genitalium are outlined in Table 2.12 Azithromycin is more effective than doxycycline. However, as many as 50% of strains were macrolide-resistant in a cohort of US female patients.45 Given the high incidence of treatment failure with azithromycin 1 g, it is thought that this regimen might select for resistance. For cases in which symptoms persist, a 1- to 2-week course of moxifloxacin is recommended.12 However, this has not been validated by clinical trials, and failures of the 7-day regimen have been reported.46
Partners of patients who test positive for M genitalium should also be tested and undergo clinically applicable screening for nongonococcal urethritis, cervicitis, and pelvic inflammatory disease.12
TRICHOMONIASIS
Trichomoniasis, caused by the parasite Trichomonas vaginalis, is the most prevalent nonviral STI in the United States. It disproportionately affects black women, in whom the prevalence is 13%, compared with 1% in non-Hispanic white women.47 It is also present in 26% of women with symptoms who are seen in STI clinics and is highly prevalent in incarcerated populations. It is uncommon in men who have sex with men.48
In men, trichomoniasis manifests as urethritis, epididymitis, or prostatitis. While most infected women have no symptoms, they may experience vaginitis with discharge that is diffuse, frothy, pruritic, malodorous, or yellow-green. Vaginal and cervical erythema (“strawberry cervix”) can also occur.
Screening for trichomoniasis
Current guidelines of the US Centers for Disease Control and Prevention (CDC) recommend testing for T vaginalis in women who have symptoms and routinely screening in women who are HIV-positive, regardless of symptoms. There is no evidence to support routine screening of pregnant women without symptoms, and pregnant women who do have symptoms should be evaluated according to the same guidelines as for nonpregnant women.12 Testing can be considered in patients who have no symptoms but who engage in high-risk behaviors and in areas of high prevalence.
A lack of studies using sensitive methods for T vaginalis detection has hampered a true estimation of disease burden and at-risk populations. Screening recommendations may evolve in upcoming clinical guidelines as the field advances.
As infection can recur, women should be retested 3 months after initial diagnosis.12
NAAT is the preferred test for trichomoniasis
Commercially available diagnostic tests for trichomoniasis include culture, antigen testing, and NAAT.49 While many clinicians do their own wet-mount microscopy for a rapid result, this method has low sensitivity.50 Similarly, antigen testing and culture perform poorly compared with NAATs, which are the gold standard for detection.51,52 A major advantage of NAATs for T vaginalis detection is that they combine high sensitivity and fast results, facilitating diagnosis and appropriate treatment of patients and their partners.
In spite of these benefits, adoption of molecular diagnostic testing for T vaginalis has lagged behind that for chlamydia and gonorrhea.53 FDA-cleared NAATs are available for testing vaginal, cervical, or urine specimens from women, but until recently, there were no approved assays for testing in men. The Cepheid Xpert TV assay, which is valid for male urine specimens to diagnose other sexually transmitted diseases, has demonstrated excellent diagnostic sensitivity for T vaginalis in men and women.54 Interestingly, a large proportion of male patients in this study had no symptoms, suggesting that screening of men in high-risk groups may be warranted.
7-day metronidazole treatment beats single-dose treatment
The first-line treatment for trichomoniasis has been a single dose of metronidazole 2 g by mouth, but in a recent randomized controlled trial,55 a course of 500 mg by mouth twice a day for 7 days was 45% more effective at 4 weeks than a single dose, and it should now be the preferred regimen.
In clinical trials,56 a single dose of tinidazole 2 g orally was equivalent or superior to metronidazole 2 g and had fewer gastrointestinal side effects, but it is more expensive.
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
- Harding-Esch EM, Nori AV, Hegazi A, et al. Impact of deploying multiple point-of-care tests with a ‘sample first’ approach on a sexual health clinical care pathway. A service evaluation. Sex Transm Infect 2017; 93(6):424–429. doi:10.1136/sextrans-2016-052988
- Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17(8):e235–e279. doi:10.1016/S1473-3099(17)30310-9
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 2015; 10(12):e0143304. doi:10.1371/journal.pone.0143304
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. www.cdc.gov/std/stats17/toc.htm. Accessed October 7, 2019.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol 2012; 50(8):2601–2608. doi:10.1128/JCM.00748-12
- Newton-Levinson A, Leichliter JS, Chandra-Mouli V. Sexually transmitted infection services for adolescents and youth in low- and middle-income countries: perceived and experienced barriers to accessing care. J Adolesc Health 2016; 59(1):7–16.
doi:10.1016/j.jadohealth.2016.03.014 - Barbee LA, Khosropour CM, Dombrowksi JC, Golden MR. New human immunodeficiency virus diagnosis independently associated with rectal gonorrhea and chlamydia in men who have sex with men. Sex Transm Dis 2017; 44(7):385–389. doi:10.1097/OLQ.0000000000000614
- Halkitis PN, Kapadia F, Bub KL, Barton S, Moreira AD, Stults CB. A longitudinal investigation of syndemic conditions among young gay, bisexual, and other MSM: the P18 cohort study. AIDS Behav 2015; 19(6):970–980. doi:10.1007/s10461-014-0892-y
- Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med 2003; 36(4):502–509. pmid:12649059
- Patel P, Bush T, Mayer K, et al; SUN Study Investigators. Routine brief risk-reduction counseling with biannual STD testing reduces STD incidence among HIV-infected men who have sex with men in care. Sex Transm Dis 2012; 39(6):470–474. doi:10.1097/OLQ.0b013e31824b3110
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015; 53(8):2686–2692. doi:10.1128/JCM.00670-15
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64(RR–03): 1–137. pmid:26042815
- van Aar F, van Weert Y, Spijker R, Gotz H, Op de Coul E; Partner Notification Group. Partner notification among men who have sex with men and heterosexuals with STI/HIV: different outcomes and challenges. Int J STD AIDS 2015; 26(8):565–573. doi:10.1177/0956462414547398
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDa): expedited partner therapy. www.cdc.gov/std/ept/. Accessed October 7, 2019.
- Jamison CD, Chang T, Mmeje O. Expedited partner therapy: combating record high sexually transmitted infection rates. Am J Public Health 2018; 108(10):1325–1327. doi:10.2105/AJPH.2018.304570
- Singh RH, Zenilman JM, Brown KM, Madden T, Gaydos C, Ghanem KG. The role of physical examination in diagnosing common causes of vaginitis: a prospective study. Sex Transm Infect 2013; 89(3):185–190. doi:10.1136/sextrans-2012-050550
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: a systemic review and meta-analysis. PLoS One 2015; 10(7):e0132776. doi:10.1371/journal.pone.0132776
- Michel CE, Sonnex C, Carne CA, et al. Chlamydia trachomatis load at matched anatomic sites: implications for screening strategies. J Clin Microbiol 2007; 45(5):1395–1402. doi:10.1128/JCM.00100-07
- Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: results from a multicenter evaluation of the APTIMA assays for both infections. Sex Transm Dis 2005; 32(12):725–728. pmid:16314767
- Komaroff AL, Aronson MD, Pass TM, Ervin CT. Prevalence of pharyngeal gonorrhea in general medical patients with sore throats. Sex Transm Dis 1980; 7(3):116–119. pmid:6777884
- Centers for Disease Control and Prevention. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep 2009; 58(26):716–719. pmid:19590491
- Chesson HW, Bernstein KT, Gift TL, Marcus JL, Pipkin S, Kent CK. The cost-effectiveness of screening men who have sex with men for rectal chlamydial and gonococcal infection to prevent HIV Infection. Sex Transm Dis 2013; 40(5):366–471. doi:10.1097/OLQ.0b013e318284e544
- Park J, Marcus JL, Pandori M, Snell A, Philip SS, Bernstein KT. Sentinel surveillance for pharyngeal chlamydia and gonorrhea among men who have sex with men—San Francisco, 2010. Sex Transm Dis 2012; 39(6):482–484. doi:10.1097/OLQ.0b013e3182495e2f
- Masek BJ, Arora N, Quinn N, et al. Performance of three nucleic acid amplification tests for detection of Chlamydia trachomatis and Neisseria gonorrhoeae by use of self-collected vaginal swabs obtained via an internet-based screening program. J Clin Microbiol 2009; 47(6):1663–1667. doi:10.1128/JCM.02387-08
- Bachmann LH, Johnson RE, Cheng H, et al. Nucleic acid amplification tests for diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis rectal infections. J Clin Microbiol 2010; 48(5):1827–1832. doi:10.1128/JCM.02398-09
- Mimiaga MJ, Mayer KH, Reisner SL, et al. Asymptomatic gonorrhea and chlamydial infections detected by nucleic acid amplification tests among Boston area men who have sex with men. Sex Transm Dis 2008; 35(5):495–498. doi:10.1097/OLQ.0b013e31816471ae
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008; 35(7):637–642. doi:10.1097/OLQ.0b013e31817bdd7e
- Cornelisse VJ, Chow EP, Huffam S, et al. Increased detection of pharyngeal and rectal gonorrhea in men who have sex with men after transition from culture to nucleic acid amplification testing. Sex Transm Dis 2017; 44(2):114–117. doi:10.1097/OLQ.0000000000000553
- Centers for Disease Control and Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63(RR–02):1–19. pmid:24622331
- Hammerschlag MR, Gaydos CA. Guidelines for the use of molecular biological methods to detect sexually transmitted pathogens in cases of suspected sexual abuse in children. Methods Mol Biol 2012; 903:307–317. doi:10.1007/978-1-61779-937-2_21
- Huppert JS, Mortensen JE, Reed JL, Kahn JA, Rich KD, Hobbs MM. Mycoplasma genitalium detected by transcription-mediated amplification is associated with Chlamydia trachomatis in adolescent women. Sex Transm Dis 2008; 35(3):250–254. doi:10.1097/OLQ.0b013e31815abac6
- Pond MJ, Nori AV, Witney AA, Lopeman RC, Butcher PD, Sadiq ST. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis 2014; 58(5):631–637. doi:10.1093/cid/cit752
- Seña AC, Lee JY, Schwebke J, et al. A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin Infect Dis 2018; 67(1):73–79. doi:10.1093/cid/ciy025
- Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 2010; 117(3):361–364. doi:10.1111/j.1471-0528.2009.02455.x
- Cohen CR, Manhart LE, Bukusi EA, et al. Association between Mycoplasma genitalium and acute endometritis. Lancet 2002; 359(9308):765–766. doi:10.1016/S0140-6736(02)07848-0
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24(3):498–514. doi:10.1128/CMR.00006-11
- Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: implications for screening, testing, and treatment. Sex Transm Infect 2006; 82(4):269–271. doi:10.1136/sti.2005.017368
- Donders GG, Ruban K, Bellen G, Petricevic L. Mycoplasma/ureaplasma infection in pregnancy: to screen or not to screen. J Perinat Med 2017; 45(5):505–515. doi:10.1515/jpm-2016-0111
- Allan-Blitz LT, Mokany E, Miller S, Wee R, Shannon C, Klausner JD. Prevalence of Mycoplasma genitalium and azithromycin-resistant infections among remnant clinical specimens, Los Angeles. Sex Transm Dis 2018; 45(9):632–635. doi:10.1097/OLQ.0000000000000829
- Munson E. Molecular diagnostics update for the emerging (if not already widespread) sexually transmitted infection agent Mycoplasma genitalium: just about ready for prime time. J Clin Microbio. 2017; 55(10):2894–2902. doi:10.1128/JCM.00818-17
- Waites KB, Taylor-Robinson D. Mycoplasma and ureaplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, American Society for Microbiology, eds. Manual of Clinical Microbiology. 11th ed. Washington, DC: ASM Press; 2015:1088–1105.
- Cimolai N, Bryan LE, To M, Woods DE. Immunological cross-reactivity of a Mycoplasma pneumoniae membrane-associated protein antigen with Mycoplasma genitalium and Acholeplasma laidlawii. J Clin Microbiol 1987; 25(11):2136–2139. pmid:2447119
- Ma L, Mancuso M, Williams JA, et al. Extensive variation and rapid shift of the MG192 sequence in Mycoplasma genitalium strains from patients with chronic infection. Infect Immun 2014; 82(3):1326–1334. doi:10.1128/IAI.01526-13
- Hologic. Aptima Mycoplasma genitalium assay.www.hologic.com/sites/default/files/package-insert/AW-14170-001_005_01.pdf. Accessed October 7, 2019.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54(9):2278–2283. doi:10.1128/JCM.01053-16
- Li Y, Le WJ, Li S, Cao YP, Su XH. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS 2017; 28(11):1106–1114. doi:10.1177/0956462416688562
- Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 2007; 45(10):1319–1326. doi:10.1086/522532
- Kelley CF, Rosenberg ES, O’Hara BM, Sanchez T, del Rio C, Sullivan PS. Prevalence of urethral Trichomonas vaginalis in black and white men who have sex with men. Sex Transm Dis 2012; 39(9):739. doi:10.1097/OLQ.0b013e318264248b
- Van Der Pol B. Clinical and laboratory testing for T vaginalis infection. J Clin Microbiol 2016; 54(1):7–12. doi:10.1128/JCM.02025-15
- Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009; 200(2):188.e1–e7. doi:10.1016/j.ajog.2008.10.005
- Andrea SB, Chapin KC. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J Clin Microbiol 2011; 49(3):866–869. doi:10.1128/JCM.02367-10
- Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49(12):4106–4111. doi:10.1128/JCM.01291-11
- College of American Pathologists. CAP surveys, Trichomonas vaginalis molecular, set TVAG-A. https://documents.cap.org/documents/2018-surveys-anatomic-pathology-ed-programs-catalog.pdf. Accessed October 31, 2019.
- Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56(2). doi:10.1128/JCM.01091-17
- Kissinger P, Muzny CA, Mena LA, et al. Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(11):1251–1259. doi:10.1016/S1473-3099(18)30423-7
- Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003; (2):CD000218. doi:10.1002/14651858.CD000218
KEY POINTS
- Screen for gonorrhea and chlamydia annually—and more frequently for those at highest risk—in sexually active women age 25 and younger and in men who have sex with men, who should also be screened at the same time for human immunodeficiency virus (HIV) and syphilis.
- Test for Trichomonas vaginalis in women who have symptoms suggesting it, and routinely screen for this pathogen in women who are HIV-positive.
- Nucleic acid amplification is the preferred test for gonorrhea, chlamydia, trichomoniasis, and M genitalium infection; the use of urine specimens is acceptable.
- Consider M genitalium if therapy for gonorrhea and chlamydia fails or tests for those diseases are negative.
- Single-dose antibiotic therapy is preferred for chlamydia and uncomplicated gonorrhea. It is also available for trichomoniasis, although metronidazole 500 mg twice a day for 7 days has a higher cure rate.
Fissured tongue
A 43-year-old man presented with a 3-week history of halitosis. He was also concerned about the irregular appearance of his tongue, which he had noticed over the past 3 years. He had no history of wearing dentures or of any skin disorder.
A BROAD DIFFERENTIAL DIAGNOSIS
Fissured tongue (scrotal tongue, plicated tongue, lingua plicata) is a common normal variant of the tongue surface with a male preponderance and a reported prevalence of 10% to 20% in the general population, and the incidence increases strikingly with age.1
The cause is not known, but familial clustering is seen, and a polygenic or autosomal dominant hereditary component is presumed.1
The condition may be associated with removable dentures, geographic tongue, pernicious anemia, Sjögren syndrome, psoriasis, acromegaly, macroglossia, oral-facial-digital syndrome type I, Pierre Robin syndrome, Down syndrome, and Melkersson Rosenthal syndrome.2 It is usually asymptomatic, but if the fissures are deep, food may become lodged in them, resulting in tongue inflammation, burning sensation, and halitosis.1
Typically, fissures of varying depth extending to the margin are apparent on the dorsal surface of the tongue. The condition is confined to the anterior two-thirds of the tongue, which is of ectodermal origin. Histologically, the epithelium, lamina propria, and musculature are all involved in the formation of the fissures.3 The deeper fissures may lack filliform papillae due to bacterial inflammation.3 The diagnosis is clinical, and treatment includes reassurance, advice on good oral hygiene, and tongue cleansing.1
- Feil ND, Filippi A. Frequency of fissured tongue (lingua plicata) as a function of age. Swiss Dent J 2016; 126(10):886–897. German. pmid:27808348
- Mangold AR, Torgerson RR, Rogers RS 3rd. Diseases of the tongue. Clin Dermatol 2016; 34(4):458–469. doi:10.1016/j.clindermatol.2016.02.018
- Kullaa-Mikkonen A, Sorvari T. Lingua fissurata: a clinical, stereomicroscopic and histopathological study. Int J Oral Maxillofac Surg 1986; 15(5):525–533. pmid:3097176
A 43-year-old man presented with a 3-week history of halitosis. He was also concerned about the irregular appearance of his tongue, which he had noticed over the past 3 years. He had no history of wearing dentures or of any skin disorder.
A BROAD DIFFERENTIAL DIAGNOSIS
Fissured tongue (scrotal tongue, plicated tongue, lingua plicata) is a common normal variant of the tongue surface with a male preponderance and a reported prevalence of 10% to 20% in the general population, and the incidence increases strikingly with age.1
The cause is not known, but familial clustering is seen, and a polygenic or autosomal dominant hereditary component is presumed.1
The condition may be associated with removable dentures, geographic tongue, pernicious anemia, Sjögren syndrome, psoriasis, acromegaly, macroglossia, oral-facial-digital syndrome type I, Pierre Robin syndrome, Down syndrome, and Melkersson Rosenthal syndrome.2 It is usually asymptomatic, but if the fissures are deep, food may become lodged in them, resulting in tongue inflammation, burning sensation, and halitosis.1
Typically, fissures of varying depth extending to the margin are apparent on the dorsal surface of the tongue. The condition is confined to the anterior two-thirds of the tongue, which is of ectodermal origin. Histologically, the epithelium, lamina propria, and musculature are all involved in the formation of the fissures.3 The deeper fissures may lack filliform papillae due to bacterial inflammation.3 The diagnosis is clinical, and treatment includes reassurance, advice on good oral hygiene, and tongue cleansing.1
A 43-year-old man presented with a 3-week history of halitosis. He was also concerned about the irregular appearance of his tongue, which he had noticed over the past 3 years. He had no history of wearing dentures or of any skin disorder.
A BROAD DIFFERENTIAL DIAGNOSIS
Fissured tongue (scrotal tongue, plicated tongue, lingua plicata) is a common normal variant of the tongue surface with a male preponderance and a reported prevalence of 10% to 20% in the general population, and the incidence increases strikingly with age.1
The cause is not known, but familial clustering is seen, and a polygenic or autosomal dominant hereditary component is presumed.1
The condition may be associated with removable dentures, geographic tongue, pernicious anemia, Sjögren syndrome, psoriasis, acromegaly, macroglossia, oral-facial-digital syndrome type I, Pierre Robin syndrome, Down syndrome, and Melkersson Rosenthal syndrome.2 It is usually asymptomatic, but if the fissures are deep, food may become lodged in them, resulting in tongue inflammation, burning sensation, and halitosis.1
Typically, fissures of varying depth extending to the margin are apparent on the dorsal surface of the tongue. The condition is confined to the anterior two-thirds of the tongue, which is of ectodermal origin. Histologically, the epithelium, lamina propria, and musculature are all involved in the formation of the fissures.3 The deeper fissures may lack filliform papillae due to bacterial inflammation.3 The diagnosis is clinical, and treatment includes reassurance, advice on good oral hygiene, and tongue cleansing.1
- Feil ND, Filippi A. Frequency of fissured tongue (lingua plicata) as a function of age. Swiss Dent J 2016; 126(10):886–897. German. pmid:27808348
- Mangold AR, Torgerson RR, Rogers RS 3rd. Diseases of the tongue. Clin Dermatol 2016; 34(4):458–469. doi:10.1016/j.clindermatol.2016.02.018
- Kullaa-Mikkonen A, Sorvari T. Lingua fissurata: a clinical, stereomicroscopic and histopathological study. Int J Oral Maxillofac Surg 1986; 15(5):525–533. pmid:3097176
- Feil ND, Filippi A. Frequency of fissured tongue (lingua plicata) as a function of age. Swiss Dent J 2016; 126(10):886–897. German. pmid:27808348
- Mangold AR, Torgerson RR, Rogers RS 3rd. Diseases of the tongue. Clin Dermatol 2016; 34(4):458–469. doi:10.1016/j.clindermatol.2016.02.018
- Kullaa-Mikkonen A, Sorvari T. Lingua fissurata: a clinical, stereomicroscopic and histopathological study. Int J Oral Maxillofac Surg 1986; 15(5):525–533. pmid:3097176