User login
A new model of care to return holism to family medicine
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
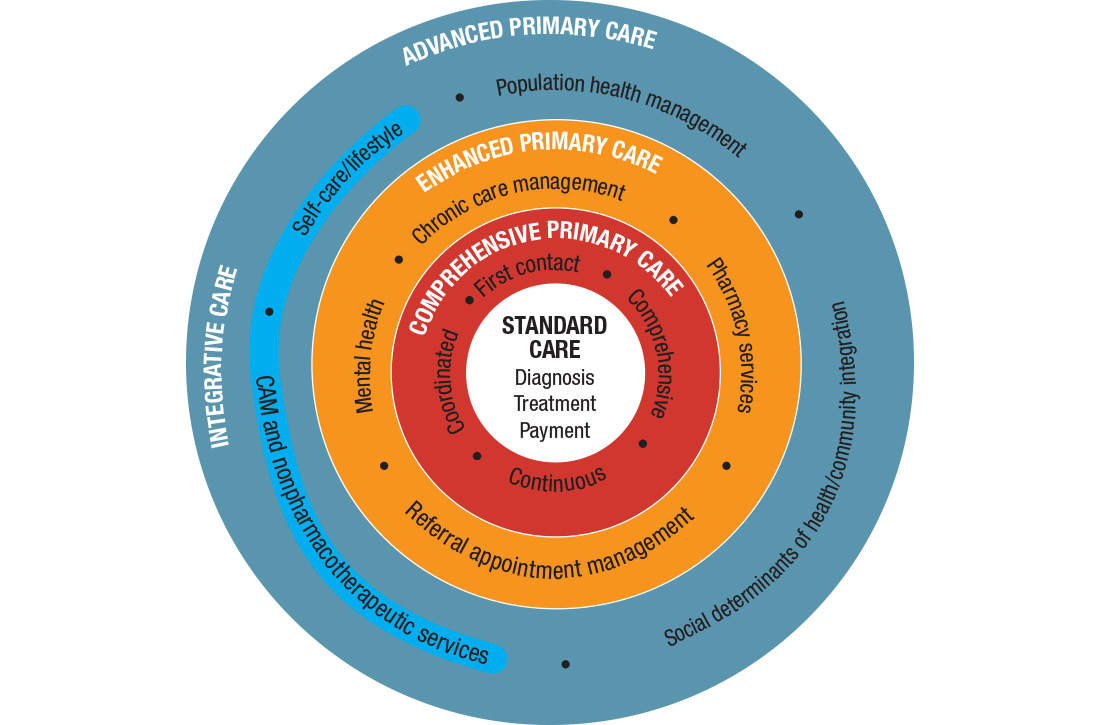
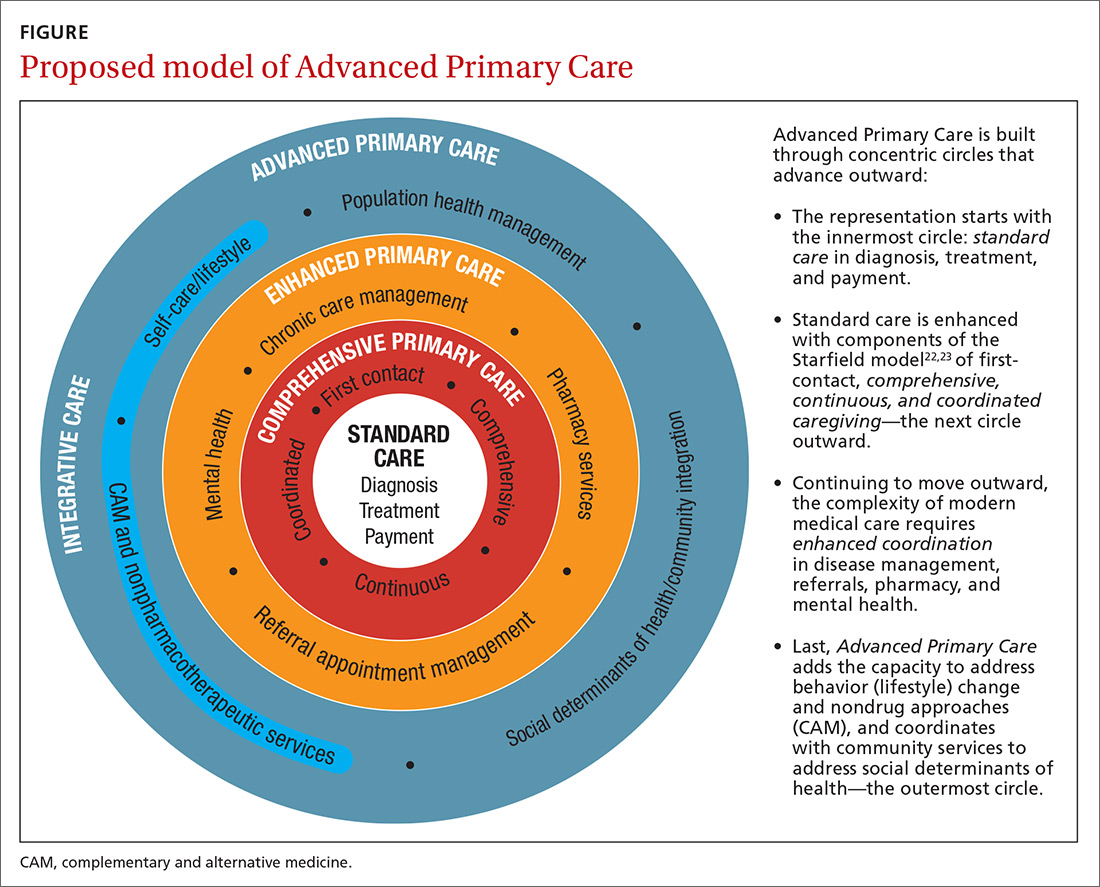
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.

Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.

Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; [email protected].
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
PRACTICE RECOMMENDATIONS
❯ Build care teams into your practice so that you integrate “what matters” into the center of the clinical encounter. C
❯ Add practice approaches that help patients engage in healthy lifestyles and that remove social and economic barriers for improving health and well-being. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
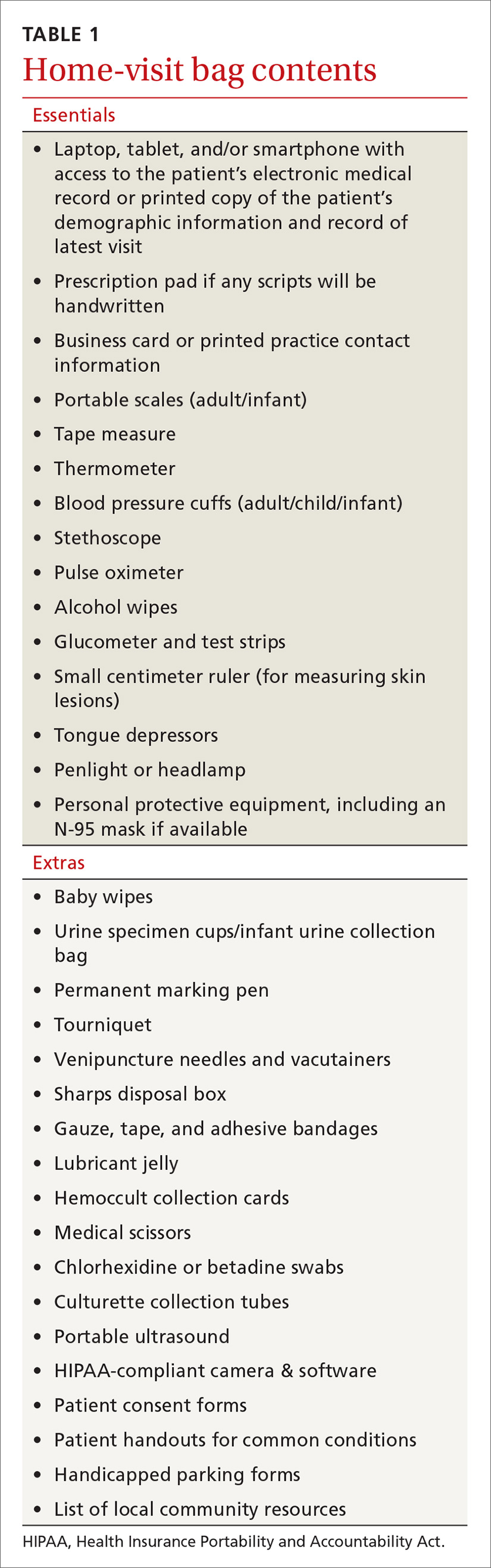
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
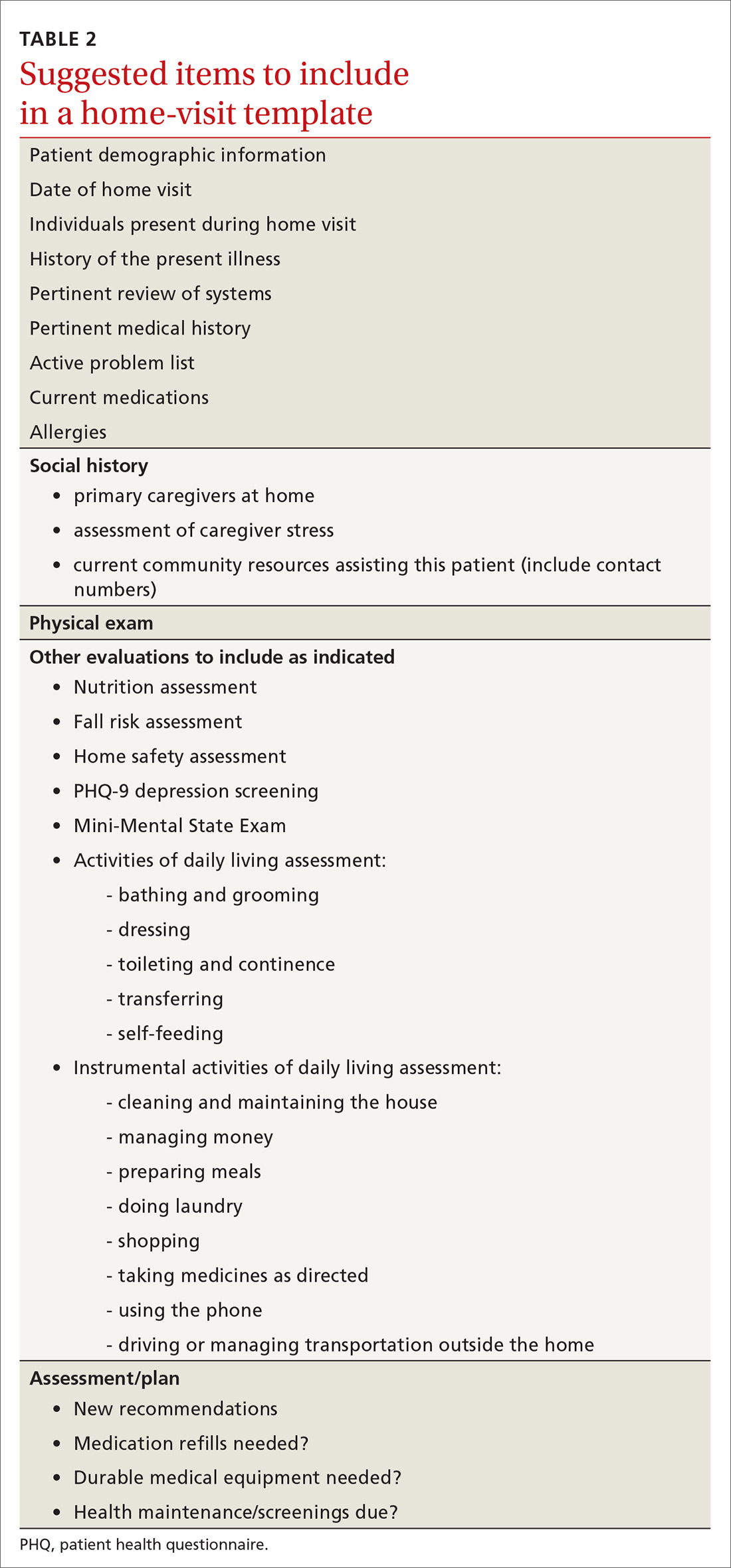
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
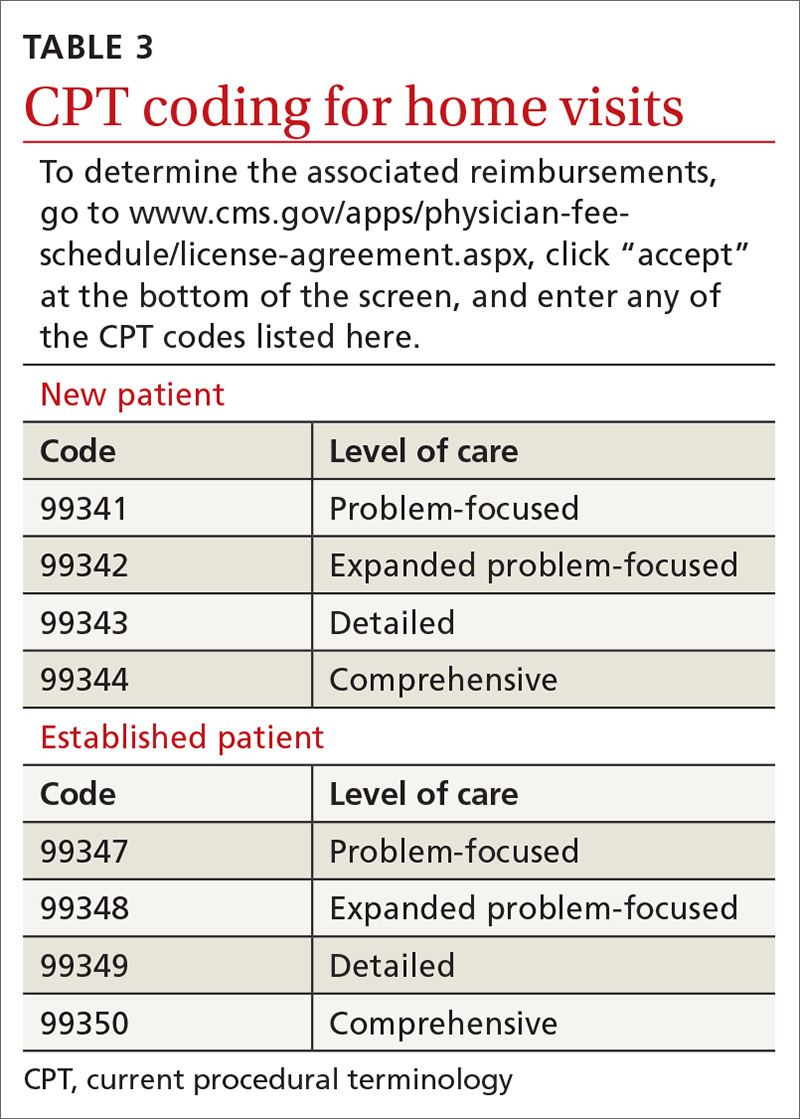
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.

Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.

Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34

For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Whole-person care: Our foundation, our future
In this issue of The Journal of Family Practice, Dr. Wayne Jonas explains his model for Advanced Primary Care (see page 493). The figure he uses to illustrate Advanced Primary Care is compelling, and the effectiveness of this model of health care is supported by a great deal of research and evaluation over the past 20 years. Let me provide some historical context.
The idea that healing requires more than curative, biology-based medical care dates back to Greek mythology. Asclepius, the god of medicine, had 5 daughters, Hygeia (the goddess of good health and hygiene), Iaso (cures and remedies), Aceso (healing wounds), Aegle (radiant good health), and Panacea (cures).1 Clearly, the Greeks believed that integrative care is essential for maintaining good health!
Modern, scientific medicine is a relatively recent development in human history. Other traditions of healing such as acupuncture and herbal medicines are actually much older than mainstream Western medicine. But they come together in family medicine—a specialty founded on the principles of whole person, whole family, and whole community care.
The first modern model of comprehensive care, the patient-centered medical home (PCMH), was introduced by the American Academy of Pediatrics in 1967. This idea caught on widely and was institutionalized by the National Committee for Quality Assurance in 2008 with PCMH certification.
Advanced Primary Care is the latest and best rendition of comprehensive primary health care. Funding this model through our current payment mechanisms, however, has been difficult because of the need to support social and behavioral interventions in addition to medical care—areas of care not traditionally paid for by medical premiums. In 2011, CMS collaborated with private insurers in a national demonstration project to test the financial feasibility of implementing Advanced Primary Care. Some organizations have been highly successful; others not as much.
We can no longer go “halfway” into whole-person care. The COVID-19 pandemic has put a spotlight on our need to transform payment models away from fee-for-service to reimbursement for whole person primary care. Our nation’s health and the viability of our health care system depend on it.
PS: I recommend reading Dr. Jonas’ book, How Healing Works, which provides a scientific rationale for the application of whole-person care to healing.
1. Theoi Greek Mythology Web site. https://www.theoi.com/Ouranios/Asklepios.html. Accessed November 30, 2020.
In this issue of The Journal of Family Practice, Dr. Wayne Jonas explains his model for Advanced Primary Care (see page 493). The figure he uses to illustrate Advanced Primary Care is compelling, and the effectiveness of this model of health care is supported by a great deal of research and evaluation over the past 20 years. Let me provide some historical context.
The idea that healing requires more than curative, biology-based medical care dates back to Greek mythology. Asclepius, the god of medicine, had 5 daughters, Hygeia (the goddess of good health and hygiene), Iaso (cures and remedies), Aceso (healing wounds), Aegle (radiant good health), and Panacea (cures).1 Clearly, the Greeks believed that integrative care is essential for maintaining good health!
Modern, scientific medicine is a relatively recent development in human history. Other traditions of healing such as acupuncture and herbal medicines are actually much older than mainstream Western medicine. But they come together in family medicine—a specialty founded on the principles of whole person, whole family, and whole community care.
The first modern model of comprehensive care, the patient-centered medical home (PCMH), was introduced by the American Academy of Pediatrics in 1967. This idea caught on widely and was institutionalized by the National Committee for Quality Assurance in 2008 with PCMH certification.
Advanced Primary Care is the latest and best rendition of comprehensive primary health care. Funding this model through our current payment mechanisms, however, has been difficult because of the need to support social and behavioral interventions in addition to medical care—areas of care not traditionally paid for by medical premiums. In 2011, CMS collaborated with private insurers in a national demonstration project to test the financial feasibility of implementing Advanced Primary Care. Some organizations have been highly successful; others not as much.
We can no longer go “halfway” into whole-person care. The COVID-19 pandemic has put a spotlight on our need to transform payment models away from fee-for-service to reimbursement for whole person primary care. Our nation’s health and the viability of our health care system depend on it.
PS: I recommend reading Dr. Jonas’ book, How Healing Works, which provides a scientific rationale for the application of whole-person care to healing.
In this issue of The Journal of Family Practice, Dr. Wayne Jonas explains his model for Advanced Primary Care (see page 493). The figure he uses to illustrate Advanced Primary Care is compelling, and the effectiveness of this model of health care is supported by a great deal of research and evaluation over the past 20 years. Let me provide some historical context.
The idea that healing requires more than curative, biology-based medical care dates back to Greek mythology. Asclepius, the god of medicine, had 5 daughters, Hygeia (the goddess of good health and hygiene), Iaso (cures and remedies), Aceso (healing wounds), Aegle (radiant good health), and Panacea (cures).1 Clearly, the Greeks believed that integrative care is essential for maintaining good health!
Modern, scientific medicine is a relatively recent development in human history. Other traditions of healing such as acupuncture and herbal medicines are actually much older than mainstream Western medicine. But they come together in family medicine—a specialty founded on the principles of whole person, whole family, and whole community care.
The first modern model of comprehensive care, the patient-centered medical home (PCMH), was introduced by the American Academy of Pediatrics in 1967. This idea caught on widely and was institutionalized by the National Committee for Quality Assurance in 2008 with PCMH certification.
Advanced Primary Care is the latest and best rendition of comprehensive primary health care. Funding this model through our current payment mechanisms, however, has been difficult because of the need to support social and behavioral interventions in addition to medical care—areas of care not traditionally paid for by medical premiums. In 2011, CMS collaborated with private insurers in a national demonstration project to test the financial feasibility of implementing Advanced Primary Care. Some organizations have been highly successful; others not as much.
We can no longer go “halfway” into whole-person care. The COVID-19 pandemic has put a spotlight on our need to transform payment models away from fee-for-service to reimbursement for whole person primary care. Our nation’s health and the viability of our health care system depend on it.
PS: I recommend reading Dr. Jonas’ book, How Healing Works, which provides a scientific rationale for the application of whole-person care to healing.
1. Theoi Greek Mythology Web site. https://www.theoi.com/Ouranios/Asklepios.html. Accessed November 30, 2020.
1. Theoi Greek Mythology Web site. https://www.theoi.com/Ouranios/Asklepios.html. Accessed November 30, 2020.
Does XR injectable naltrexone prevent relapse as effectively as daily sublingual buprenorphine-naloxone?
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE-BASED ANSWER:
Yes. Monthly extended-release injectable naltrexone (XR-NTX) treats opioid use disorder as effectively as daily sublingual buprenorphine-naloxone (BUP-NX) without causing any increase in serious adverse events or fatal overdoses. (strength of recommendation: A, 2 good-quality RCTs).
First-of-its kind guideline on lipid monitoring in endocrine diseases
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Endocrine diseases of any type – not just diabetes – can represent a cardiovascular risk and patients with those disorders should be screened for high cholesterol, according to a new clinical practice guideline from the Endocrine Society.
“The simple recommendation to check a lipid panel in patients with endocrine diseases and calculate cardiovascular risk may be practice changing because that is not done routinely,” Connie Newman, MD, chair of the Endocrine Society committee that developed the guideline, said in an interview.
“Usually the focus is on assessment and treatment of the endocrine disease, rather than on assessment and treatment of atherosclerotic cardiovascular disease risk,” said Newman, an adjunct professor of medicine in the department of medicine, division of endocrinology, diabetes & metabolism, at New York University.
Whereas diabetes, well-known for its increased cardiovascular risk profile, is commonly addressed in other cardiovascular and cholesterol practice management guidelines, the array of other endocrine diseases are not typically included.
“This guideline is the first of its kind,” Dr. Newman said. “The Endocrine Society has not previously issued a guideline on lipid management in endocrine disorders [and] other organizations have not written guidelines on this topic.
“Rather, guidelines have been written on cholesterol management, but these do not describe cholesterol management in patients with endocrine diseases such as thyroid disease [hypothyroidism and hyperthyroidism], Cushing’s syndrome, acromegaly, growth hormone deficiency, menopause, male hypogonadism, and obesity,” she noted.
But these conditions carry a host of cardiovascular risk factors that may require careful monitoring and management.
“Although endocrine hormones, such as thyroid hormone, cortisol, estrogen, testosterone, growth hormone, and insulin, affect pathways for lipid metabolism, physicians lack guidance on lipid abnormalities, cardiovascular risk, and treatment to reduce lipids and cardiovascular risk in patients with endocrine diseases,” she explained.
Vinaya Simha, MD, an internal medicine specialist at the Mayo Clinic in Rochester, Minn., agrees that the guideline is notable in addressing an unmet need.
Recommendations that stand out to Dr. Simha include the suggestion of adding eicosapentaenoic acid (EPA) ethyl ester to reduce the risk of cardiovascular disease in adults with diabetes or atherosclerotic cardiovascular disease who have elevated triglyceride levels despite statin treatment.
James L. Rosenzweig, MD, an endocrinologist at Hebrew SeniorLife in Boston, agreed that this is an important addition to an area that needs more guidance.
“Many of these clinical situations can exacerbate dyslipidemia and some also increase the cardiovascular risk to a greater extent in combination with elevated cholesterol and/or triglycerides,” he said in an interview.
“In many cases, treatment of the underlying disorder appropriately can have an important impact in resolving the lipid disorder. In others, more aggressive pharmacological treatment is indicated,” he said.
“I think that this will be a valuable resource, especially for endocrinologists, but it can be used as well by providers in other disciplines.”
Key recommendations for different endocrine conditions
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, details those risks and provides evidence-based recommendations on their management and treatment.
Key recommendations include:
- Obtain a lipid panel and evaluate cardiovascular risk factors in all adults with endocrine disorders.
- In patients with and risk factors for cardiovascular disease, start statin therapy in addition to lifestyle modification to reduce cardiovascular risk. “This could mean earlier treatment because other guidelines recommend consideration of therapy at age 40,” Dr. Newman said.
- Statin therapy is also recommended for adults over 40 with with a duration of diabetes of more than 20 years and/or microvascular complications, regardless of their cardiovascular risk score. “This means earlier treatment of patients with type 1 diabetes with statins in order to reduce cardiovascular disease risk,” Dr. Newman noted.
- In patients with hyperlipidemia, rule out as the cause before treating with lipid-lowering medications. And among patients who are found to have hypothyroidism, reevaluate the lipid profile when the patient has thyroid hormone levels in the normal range.
- Adults with persistent endogenous Cushing’s syndrome should have their lipid profile monitored. Statin therapy should be considered in addition to lifestyle modifications, irrespective of the cardiovascular risk score.
- In postmenopausal women, high cholesterol or triglycerides should be treated with statins rather than hormone therapy.
- Evaluate and treat lipids and other cardiovascular risk factors in women who enter menopause early (before the age of 40-45 years).
Nice summary of ‘risk-enhancing’ endocrine disorders
Dr. Simha said in an interview that the new guideline is “probably the first comprehensive statement addressing lipid treatment in patients with a broad range of endocrine disorders besides diabetes.”
“Most of the treatment recommendations are congruent with other current guidelines such as the American College of Cardiology/American Heart Association [guidelines], but there is specific mention of which endocrine disorders represent enhanced cardiovascular risk,” she explained.
The new recommendations are notable for including “a nice summary of how different endocrine disorders affect lipid values, and also which endocrine disorders need to be considered as ‘risk-enhancing factors,’ ” Dr. Simha noted.
“The use of EPA in patients with hypertriglyceridemia is novel, compared to the ACC/AHA recommendation. This reflects new data which is now available,” she added.
The American Association of Clinical Endocrinologists also just issued a new algorithm on lipid management and prevention of cardiovascular disease in which treatment of hypertriglyceridemia is emphasized.
In addition, the new Endocrine Society guideline “also mentions an LDL [cholesterol] treatment threshold of 70 mg/dL, and 55 mg/dL in some patient categories, which previous guidelines have not,” Dr. Simha noted.
Overall, Dr. Newman added that the goal of the guideline is to increase awareness of key issues with endocrine diseases that may not necessarily be on clinicians’ radars.
“We hope that it will make a lipid panel and cardiovascular risk evaluation routine in adults with endocrine diseases and cause a greater focus on therapies to reduce heart disease and stroke,” she said.
Dr. Newman, Dr. Simha, and Dr. Rosenzweig reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Decide ADHD pharmacotherapy based on medication onset, duration of action
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
FROM PSYCHOPHARMACOLOGY UPDATE
Choose wisely
Four years ago, just prior to the 2016 presidential election, I mentioned the Choosing Wisely campaign in my JFP editorial.1 I said that family physicians should do their part in controlling health care costs by carefully selecting tests and treatments that are known to be effective and avoiding those that are not. This remains as true now as it was then.
The Choosing Wisely campaign was sparked by a family physician, Dr. Howard Brody, in the context of national health care reform. In a 2010 New England Journal of Medicine editorial, he challenged physicians to do their part in controlling health care costs by not ordering tests and treatments that have no value for patients.2 At that time, it was estimated that a third of tests and treatments ordered by US physicians were of marginal or no value.3
Dr. Brody’s editorial caught the attention of the National Physicians Alliance and eventually many other physician organizations. In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely initiative; today, the campaign Web site, choosingwisely.org, has a wealth of information and practice recommendations from 78 medical specialty organizations, including the American Academy of Family Physicians (AAFP).
In this month’s issue of JFP, Dr. Kate Rowland has summarized 10 of the most important Choosing Wisely recommendations that apply to family physicians and other primary care clinicians. Here are 5 more recommendations from the Choosing Wisely list of tests and treatments to avoid ordering for your patients:
- Don’t perform pelvic exams on asymptomatic nonpregnant women, unless necessary for guideline-appropriate screening for cervical cancer.
- Don’t routinely screen for prostate cancer using a prostate-specific antigen (PSA) test or digital rectal exam. For men who want PSA screening, it should be performed only after engaging in shared decision-making.
- Don’t order annual electrocardiograms or any other cardiac screening for low-risk patients without symptoms.
- Don’t routinely prescribe antibiotics for otitis media in children ages 2 to 12 years with nonsevere symptoms when observation is reasonable.
- Don’t use dual-energy x-ray absorptiometry screening for osteoporosis in women younger than 65 or men younger than 70 with no risk factors.
In total, AAFP lists 18 recommendations (2 additional recommendations have been withdrawn, based on updated evidence) on the Choosing Wisely Web site. I encourage you to review them to see if you should change any of your current patient recommendations.
1. Hickner J. Count on this no matter who wins the election. J Fam Pract. 2016;65:664.
2. Brody H. Medicine’s ethical responsibility for health care reform—the Top Five list. N Engl J Med. 2010;362:283-285.
3. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs—lessons from regional variation. N Engl J Med. 2009;360:849-852.
Four years ago, just prior to the 2016 presidential election, I mentioned the Choosing Wisely campaign in my JFP editorial.1 I said that family physicians should do their part in controlling health care costs by carefully selecting tests and treatments that are known to be effective and avoiding those that are not. This remains as true now as it was then.
The Choosing Wisely campaign was sparked by a family physician, Dr. Howard Brody, in the context of national health care reform. In a 2010 New England Journal of Medicine editorial, he challenged physicians to do their part in controlling health care costs by not ordering tests and treatments that have no value for patients.2 At that time, it was estimated that a third of tests and treatments ordered by US physicians were of marginal or no value.3
Dr. Brody’s editorial caught the attention of the National Physicians Alliance and eventually many other physician organizations. In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely initiative; today, the campaign Web site, choosingwisely.org, has a wealth of information and practice recommendations from 78 medical specialty organizations, including the American Academy of Family Physicians (AAFP).
In this month’s issue of JFP, Dr. Kate Rowland has summarized 10 of the most important Choosing Wisely recommendations that apply to family physicians and other primary care clinicians. Here are 5 more recommendations from the Choosing Wisely list of tests and treatments to avoid ordering for your patients:
- Don’t perform pelvic exams on asymptomatic nonpregnant women, unless necessary for guideline-appropriate screening for cervical cancer.
- Don’t routinely screen for prostate cancer using a prostate-specific antigen (PSA) test or digital rectal exam. For men who want PSA screening, it should be performed only after engaging in shared decision-making.
- Don’t order annual electrocardiograms or any other cardiac screening for low-risk patients without symptoms.
- Don’t routinely prescribe antibiotics for otitis media in children ages 2 to 12 years with nonsevere symptoms when observation is reasonable.
- Don’t use dual-energy x-ray absorptiometry screening for osteoporosis in women younger than 65 or men younger than 70 with no risk factors.
In total, AAFP lists 18 recommendations (2 additional recommendations have been withdrawn, based on updated evidence) on the Choosing Wisely Web site. I encourage you to review them to see if you should change any of your current patient recommendations.
Four years ago, just prior to the 2016 presidential election, I mentioned the Choosing Wisely campaign in my JFP editorial.1 I said that family physicians should do their part in controlling health care costs by carefully selecting tests and treatments that are known to be effective and avoiding those that are not. This remains as true now as it was then.
The Choosing Wisely campaign was sparked by a family physician, Dr. Howard Brody, in the context of national health care reform. In a 2010 New England Journal of Medicine editorial, he challenged physicians to do their part in controlling health care costs by not ordering tests and treatments that have no value for patients.2 At that time, it was estimated that a third of tests and treatments ordered by US physicians were of marginal or no value.3
Dr. Brody’s editorial caught the attention of the National Physicians Alliance and eventually many other physician organizations. In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely initiative; today, the campaign Web site, choosingwisely.org, has a wealth of information and practice recommendations from 78 medical specialty organizations, including the American Academy of Family Physicians (AAFP).
In this month’s issue of JFP, Dr. Kate Rowland has summarized 10 of the most important Choosing Wisely recommendations that apply to family physicians and other primary care clinicians. Here are 5 more recommendations from the Choosing Wisely list of tests and treatments to avoid ordering for your patients:
- Don’t perform pelvic exams on asymptomatic nonpregnant women, unless necessary for guideline-appropriate screening for cervical cancer.
- Don’t routinely screen for prostate cancer using a prostate-specific antigen (PSA) test or digital rectal exam. For men who want PSA screening, it should be performed only after engaging in shared decision-making.
- Don’t order annual electrocardiograms or any other cardiac screening for low-risk patients without symptoms.
- Don’t routinely prescribe antibiotics for otitis media in children ages 2 to 12 years with nonsevere symptoms when observation is reasonable.
- Don’t use dual-energy x-ray absorptiometry screening for osteoporosis in women younger than 65 or men younger than 70 with no risk factors.
In total, AAFP lists 18 recommendations (2 additional recommendations have been withdrawn, based on updated evidence) on the Choosing Wisely Web site. I encourage you to review them to see if you should change any of your current patient recommendations.
1. Hickner J. Count on this no matter who wins the election. J Fam Pract. 2016;65:664.
2. Brody H. Medicine’s ethical responsibility for health care reform—the Top Five list. N Engl J Med. 2010;362:283-285.
3. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs—lessons from regional variation. N Engl J Med. 2009;360:849-852.
1. Hickner J. Count on this no matter who wins the election. J Fam Pract. 2016;65:664.
2. Brody H. Medicine’s ethical responsibility for health care reform—the Top Five list. N Engl J Med. 2010;362:283-285.
3. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs—lessons from regional variation. N Engl J Med. 2009;360:849-852.
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
Listening to Tim Ferriss
Let me tell you about Tim Ferriss. A few years ago, I started reading his best-selling book, The 4-Hour Body. Ferris detailed how he made himself into a one-man experiment – he’d make changes to his diet, checked his weight and his labs, maybe he even had metabolic studies done.
He’d take these measures after soaking in hot baths, then ice baths, and while I admired his discipline, he did lose me during the chapter where he was using steroids. In the end, he advised a dairy-free, low-carbohydrate diet of green vegetables, beans or lentils, and protein for four meals a day, 6 days a week, with free-for-all eating on the 7th day. Then, there was a weight-lifting routine with kettle bells and ice packs to be placed on your shoulders for a set amount of time each day.
I may not remember the program’s details, but something about Ferris fascinated me. He brands himself as being a “human guinea pig,” about “lifestyle design,” and whatever that is, I like it. Perhaps I am attracted to the idea that we might control the trajectories of our generally uncontrollable lives.
Tim Ferriss graduated from Princeton, he’s written five best-selling books and has a popular podcast, he’s been a TED speaker, and he’s been on Fortune’s “40 under 40” list – and there’s so much more. Ferriss is brilliant, innovative, handsome, charismatic, prolific, extraordinarily athletic. I may have forgotten to mention that he was the National Chinese Kickboxing Champion and was a semifinalist in the World Champion Tango competition in Buenos Aires. He’s adventuresome and fearless, and if that isn’t enough, he speaks five languages. In the genetic dice roll, Mr. Ferriss did well, and he’s a driven and energetic hard worker who is open to new experiences.
I subscribe to the Tim Ferriss podcast – as of this writing, there are 466 episodes, with an incredible lineup of interviews with famous and successful guests. I also subscribe to his “5-Bullet Friday” email list where he mentions the interesting things he is reading, watching, learning about, or eating, and the products he is trying – single-ply toilet paper gets a thumbs down – then ends with a thought-provoking quote. This gentleman spends a tremendous amount of time searching and striving, working on himself and his own emotional growth and self-improvement, and yet he still has time for incredible explorations and experiences.
A search for psychic peace
Honestly, were it not for a few little details, I would like to be Tim Ferriss. Who wouldn’t? But what stops me from actually wanting to be Ferriss is that early on while listening to him, I realized that his drive has been fueled by intense psychic pain. He talks openly about being very close to suicide in college, about a tormenting mood disorder, demons to tame, and productivity as an antidote to a fear of failure, not always as a joy for life. There are moments that I have felt so sad for this remarkable stranger. Tim Ferriss is a searcher and what I believe he searches for most is his own psychic peace.
In a forum for psychiatrists, I wish I could write that Ferriss has found solace with our Food and Drug Administration–approved pharmaceuticals and with psychotherapy, but that’s not what he says. What Ferriss has found helpful, however, is psychedelics, and a wide variety of psychological and philosophical teachings ranging from meditation to Stoicism. And most notably, Ferriss has been an advocate for using hallucinogens as a legal medical intervention. Ferriss was one of four philanthropists who donated a total of $17 million to fund the Johns Hopkins Center for Psychedelic & Consciousness Research. He’s helped to move this field forward and to improve its credibility.
On Sept. 14, 2020, Tim Ferriss released a podcast he recorded with his dance partner and close friend, Debbie Millman, and when he recorded it, he was not certain he would release it. None of his usual sponsors endorsed during the podcast. He starts Episode #464 with, “For me, this is the most important podcast episode I’ve ever published. In it, I describe the most life-shaping, certainly the most difficult, and certainly the most transformative journey of my 43 years on this planet. I’ve never shared it before.”
by a babysitter’s son. He worried about how this would affect his family, if they would be left feeling guilty or devastated. He says, “Please note that I expect to be completely overwhelmed emotionally and otherwise when this is published and please understand if I’m not able to reply to any outreach.”
Ferriss and Millman had a long discussion about their sexual abuse as children. Millman was abused by her stepfather at the age of 9, and she talks about confronting him many years later. Ferriss has not confronted his perpetrator, though he has contemplated doing so.
Sexual violence and violation at any age leaves people scarred. In a recent letter to the New York Times in response to President Trump’s words of support to Ghislaine Maxwell, the woman who helped Jeffrey Epstein find his victims, Baltimore psychiatrist Robin Weiss wrote, “Thirty-eight years ago, when I was 32, I was raped. I was married, I was a doctor – you might say I was in pretty stable shape. Yet the shame and guilt I felt were overwhelming. Why didn’t I fight harder? How did I let this happen? I knew better, yet it took me years to overcome those irrational feelings.” These feelings of shame, guilt, self-doubt, and self-blame are nearly universal in survivors of sexual trauma. In children, they can be even worse, as children often don’t have an understanding that what is being done to them is wrong. They lack the language and the maturity to process the events, and ongoing abuse may be accompanied by threats to life of the child or their family members if they tell others, as was the case with Millman. She chose to process her abuse and the consequent difficulties she had by seeking psychiatric care. She took antidepressants and has been in psychoanalysis, both of which she has found to be helpful. Her treatment has tamed her demons, it is ongoing decades later and those demons have not vanished.
Abuse comes back in ‘a tidal wave’
Not surprisingly, Ferriss struggled with whether to make these events public. While so much of his story feels familiar to those of us who help patients process their trauma, it’s not completely typical. Ferriss remembered these episodes of sexual abuse “in high resolution,” while using ayahuasca, a hallucinogen, about 5 years ago. He describes suppressing and discounting these memories until he attended a 10-day silent retreat where he used psilocybin. I found it interesting that Ferriss fasted for 5 days before attending the retreat “to increase the depth of the experience.” He goes on to say, “Around day 6 of this silent retreat, all of this abuse came back to me like a tidal wave and it was replaying as if I was wearing a virtual reality headset. I was immersed, I wasn’t an observer, it was as though I was being traumatized and retraumatized 24/7.” He describes an excruciating and horrifying experience and he referred to it as a “psychotic break.”
Ferriss goes on to talk about how bringing these memories to light has affected him, how it’s explained many of his behaviors and ways of relating in a way that has helped him organize and understand his life.
“It was at the tail end of the retreat that I realized that these 17 seemingly inexplicable behaviors of mine – these vicious cycles or triggers that I had been treating like separate problems to be solved, were all downstream of this trauma. Oh, now that you click that puzzle piece into place, these really strange behaviors – this self-loathing, this rage that was seemingly so exaggerated and disproportionate – leading to the near-suicide I had in college – all these things fell into places making sense.” It gave him a sense of relief but was simultaneously overwhelming.
Both Ferriss and Millman talk about books and treatments that have been helpful to them. Their knowledge of trauma treatments and resources is impressive and can be found at: Tim Ferriss – My Healing Journey After Childhood Abuse (Includes Extensive Resource List). Their wish is to share their suffering as a way to help others, impart hope, and better connect.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Let me tell you about Tim Ferriss. A few years ago, I started reading his best-selling book, The 4-Hour Body. Ferris detailed how he made himself into a one-man experiment – he’d make changes to his diet, checked his weight and his labs, maybe he even had metabolic studies done.
He’d take these measures after soaking in hot baths, then ice baths, and while I admired his discipline, he did lose me during the chapter where he was using steroids. In the end, he advised a dairy-free, low-carbohydrate diet of green vegetables, beans or lentils, and protein for four meals a day, 6 days a week, with free-for-all eating on the 7th day. Then, there was a weight-lifting routine with kettle bells and ice packs to be placed on your shoulders for a set amount of time each day.
I may not remember the program’s details, but something about Ferris fascinated me. He brands himself as being a “human guinea pig,” about “lifestyle design,” and whatever that is, I like it. Perhaps I am attracted to the idea that we might control the trajectories of our generally uncontrollable lives.
Tim Ferriss graduated from Princeton, he’s written five best-selling books and has a popular podcast, he’s been a TED speaker, and he’s been on Fortune’s “40 under 40” list – and there’s so much more. Ferriss is brilliant, innovative, handsome, charismatic, prolific, extraordinarily athletic. I may have forgotten to mention that he was the National Chinese Kickboxing Champion and was a semifinalist in the World Champion Tango competition in Buenos Aires. He’s adventuresome and fearless, and if that isn’t enough, he speaks five languages. In the genetic dice roll, Mr. Ferriss did well, and he’s a driven and energetic hard worker who is open to new experiences.
I subscribe to the Tim Ferriss podcast – as of this writing, there are 466 episodes, with an incredible lineup of interviews with famous and successful guests. I also subscribe to his “5-Bullet Friday” email list where he mentions the interesting things he is reading, watching, learning about, or eating, and the products he is trying – single-ply toilet paper gets a thumbs down – then ends with a thought-provoking quote. This gentleman spends a tremendous amount of time searching and striving, working on himself and his own emotional growth and self-improvement, and yet he still has time for incredible explorations and experiences.
A search for psychic peace
Honestly, were it not for a few little details, I would like to be Tim Ferriss. Who wouldn’t? But what stops me from actually wanting to be Ferriss is that early on while listening to him, I realized that his drive has been fueled by intense psychic pain. He talks openly about being very close to suicide in college, about a tormenting mood disorder, demons to tame, and productivity as an antidote to a fear of failure, not always as a joy for life. There are moments that I have felt so sad for this remarkable stranger. Tim Ferriss is a searcher and what I believe he searches for most is his own psychic peace.
In a forum for psychiatrists, I wish I could write that Ferriss has found solace with our Food and Drug Administration–approved pharmaceuticals and with psychotherapy, but that’s not what he says. What Ferriss has found helpful, however, is psychedelics, and a wide variety of psychological and philosophical teachings ranging from meditation to Stoicism. And most notably, Ferriss has been an advocate for using hallucinogens as a legal medical intervention. Ferriss was one of four philanthropists who donated a total of $17 million to fund the Johns Hopkins Center for Psychedelic & Consciousness Research. He’s helped to move this field forward and to improve its credibility.
On Sept. 14, 2020, Tim Ferriss released a podcast he recorded with his dance partner and close friend, Debbie Millman, and when he recorded it, he was not certain he would release it. None of his usual sponsors endorsed during the podcast. He starts Episode #464 with, “For me, this is the most important podcast episode I’ve ever published. In it, I describe the most life-shaping, certainly the most difficult, and certainly the most transformative journey of my 43 years on this planet. I’ve never shared it before.”
by a babysitter’s son. He worried about how this would affect his family, if they would be left feeling guilty or devastated. He says, “Please note that I expect to be completely overwhelmed emotionally and otherwise when this is published and please understand if I’m not able to reply to any outreach.”
Ferriss and Millman had a long discussion about their sexual abuse as children. Millman was abused by her stepfather at the age of 9, and she talks about confronting him many years later. Ferriss has not confronted his perpetrator, though he has contemplated doing so.
Sexual violence and violation at any age leaves people scarred. In a recent letter to the New York Times in response to President Trump’s words of support to Ghislaine Maxwell, the woman who helped Jeffrey Epstein find his victims, Baltimore psychiatrist Robin Weiss wrote, “Thirty-eight years ago, when I was 32, I was raped. I was married, I was a doctor – you might say I was in pretty stable shape. Yet the shame and guilt I felt were overwhelming. Why didn’t I fight harder? How did I let this happen? I knew better, yet it took me years to overcome those irrational feelings.” These feelings of shame, guilt, self-doubt, and self-blame are nearly universal in survivors of sexual trauma. In children, they can be even worse, as children often don’t have an understanding that what is being done to them is wrong. They lack the language and the maturity to process the events, and ongoing abuse may be accompanied by threats to life of the child or their family members if they tell others, as was the case with Millman. She chose to process her abuse and the consequent difficulties she had by seeking psychiatric care. She took antidepressants and has been in psychoanalysis, both of which she has found to be helpful. Her treatment has tamed her demons, it is ongoing decades later and those demons have not vanished.
Abuse comes back in ‘a tidal wave’
Not surprisingly, Ferriss struggled with whether to make these events public. While so much of his story feels familiar to those of us who help patients process their trauma, it’s not completely typical. Ferriss remembered these episodes of sexual abuse “in high resolution,” while using ayahuasca, a hallucinogen, about 5 years ago. He describes suppressing and discounting these memories until he attended a 10-day silent retreat where he used psilocybin. I found it interesting that Ferriss fasted for 5 days before attending the retreat “to increase the depth of the experience.” He goes on to say, “Around day 6 of this silent retreat, all of this abuse came back to me like a tidal wave and it was replaying as if I was wearing a virtual reality headset. I was immersed, I wasn’t an observer, it was as though I was being traumatized and retraumatized 24/7.” He describes an excruciating and horrifying experience and he referred to it as a “psychotic break.”
Ferriss goes on to talk about how bringing these memories to light has affected him, how it’s explained many of his behaviors and ways of relating in a way that has helped him organize and understand his life.
“It was at the tail end of the retreat that I realized that these 17 seemingly inexplicable behaviors of mine – these vicious cycles or triggers that I had been treating like separate problems to be solved, were all downstream of this trauma. Oh, now that you click that puzzle piece into place, these really strange behaviors – this self-loathing, this rage that was seemingly so exaggerated and disproportionate – leading to the near-suicide I had in college – all these things fell into places making sense.” It gave him a sense of relief but was simultaneously overwhelming.
Both Ferriss and Millman talk about books and treatments that have been helpful to them. Their knowledge of trauma treatments and resources is impressive and can be found at: Tim Ferriss – My Healing Journey After Childhood Abuse (Includes Extensive Resource List). Their wish is to share their suffering as a way to help others, impart hope, and better connect.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Let me tell you about Tim Ferriss. A few years ago, I started reading his best-selling book, The 4-Hour Body. Ferris detailed how he made himself into a one-man experiment – he’d make changes to his diet, checked his weight and his labs, maybe he even had metabolic studies done.
He’d take these measures after soaking in hot baths, then ice baths, and while I admired his discipline, he did lose me during the chapter where he was using steroids. In the end, he advised a dairy-free, low-carbohydrate diet of green vegetables, beans or lentils, and protein for four meals a day, 6 days a week, with free-for-all eating on the 7th day. Then, there was a weight-lifting routine with kettle bells and ice packs to be placed on your shoulders for a set amount of time each day.
I may not remember the program’s details, but something about Ferris fascinated me. He brands himself as being a “human guinea pig,” about “lifestyle design,” and whatever that is, I like it. Perhaps I am attracted to the idea that we might control the trajectories of our generally uncontrollable lives.
Tim Ferriss graduated from Princeton, he’s written five best-selling books and has a popular podcast, he’s been a TED speaker, and he’s been on Fortune’s “40 under 40” list – and there’s so much more. Ferriss is brilliant, innovative, handsome, charismatic, prolific, extraordinarily athletic. I may have forgotten to mention that he was the National Chinese Kickboxing Champion and was a semifinalist in the World Champion Tango competition in Buenos Aires. He’s adventuresome and fearless, and if that isn’t enough, he speaks five languages. In the genetic dice roll, Mr. Ferriss did well, and he’s a driven and energetic hard worker who is open to new experiences.
I subscribe to the Tim Ferriss podcast – as of this writing, there are 466 episodes, with an incredible lineup of interviews with famous and successful guests. I also subscribe to his “5-Bullet Friday” email list where he mentions the interesting things he is reading, watching, learning about, or eating, and the products he is trying – single-ply toilet paper gets a thumbs down – then ends with a thought-provoking quote. This gentleman spends a tremendous amount of time searching and striving, working on himself and his own emotional growth and self-improvement, and yet he still has time for incredible explorations and experiences.
A search for psychic peace
Honestly, were it not for a few little details, I would like to be Tim Ferriss. Who wouldn’t? But what stops me from actually wanting to be Ferriss is that early on while listening to him, I realized that his drive has been fueled by intense psychic pain. He talks openly about being very close to suicide in college, about a tormenting mood disorder, demons to tame, and productivity as an antidote to a fear of failure, not always as a joy for life. There are moments that I have felt so sad for this remarkable stranger. Tim Ferriss is a searcher and what I believe he searches for most is his own psychic peace.
In a forum for psychiatrists, I wish I could write that Ferriss has found solace with our Food and Drug Administration–approved pharmaceuticals and with psychotherapy, but that’s not what he says. What Ferriss has found helpful, however, is psychedelics, and a wide variety of psychological and philosophical teachings ranging from meditation to Stoicism. And most notably, Ferriss has been an advocate for using hallucinogens as a legal medical intervention. Ferriss was one of four philanthropists who donated a total of $17 million to fund the Johns Hopkins Center for Psychedelic & Consciousness Research. He’s helped to move this field forward and to improve its credibility.
On Sept. 14, 2020, Tim Ferriss released a podcast he recorded with his dance partner and close friend, Debbie Millman, and when he recorded it, he was not certain he would release it. None of his usual sponsors endorsed during the podcast. He starts Episode #464 with, “For me, this is the most important podcast episode I’ve ever published. In it, I describe the most life-shaping, certainly the most difficult, and certainly the most transformative journey of my 43 years on this planet. I’ve never shared it before.”
by a babysitter’s son. He worried about how this would affect his family, if they would be left feeling guilty or devastated. He says, “Please note that I expect to be completely overwhelmed emotionally and otherwise when this is published and please understand if I’m not able to reply to any outreach.”
Ferriss and Millman had a long discussion about their sexual abuse as children. Millman was abused by her stepfather at the age of 9, and she talks about confronting him many years later. Ferriss has not confronted his perpetrator, though he has contemplated doing so.
Sexual violence and violation at any age leaves people scarred. In a recent letter to the New York Times in response to President Trump’s words of support to Ghislaine Maxwell, the woman who helped Jeffrey Epstein find his victims, Baltimore psychiatrist Robin Weiss wrote, “Thirty-eight years ago, when I was 32, I was raped. I was married, I was a doctor – you might say I was in pretty stable shape. Yet the shame and guilt I felt were overwhelming. Why didn’t I fight harder? How did I let this happen? I knew better, yet it took me years to overcome those irrational feelings.” These feelings of shame, guilt, self-doubt, and self-blame are nearly universal in survivors of sexual trauma. In children, they can be even worse, as children often don’t have an understanding that what is being done to them is wrong. They lack the language and the maturity to process the events, and ongoing abuse may be accompanied by threats to life of the child or their family members if they tell others, as was the case with Millman. She chose to process her abuse and the consequent difficulties she had by seeking psychiatric care. She took antidepressants and has been in psychoanalysis, both of which she has found to be helpful. Her treatment has tamed her demons, it is ongoing decades later and those demons have not vanished.
Abuse comes back in ‘a tidal wave’
Not surprisingly, Ferriss struggled with whether to make these events public. While so much of his story feels familiar to those of us who help patients process their trauma, it’s not completely typical. Ferriss remembered these episodes of sexual abuse “in high resolution,” while using ayahuasca, a hallucinogen, about 5 years ago. He describes suppressing and discounting these memories until he attended a 10-day silent retreat where he used psilocybin. I found it interesting that Ferriss fasted for 5 days before attending the retreat “to increase the depth of the experience.” He goes on to say, “Around day 6 of this silent retreat, all of this abuse came back to me like a tidal wave and it was replaying as if I was wearing a virtual reality headset. I was immersed, I wasn’t an observer, it was as though I was being traumatized and retraumatized 24/7.” He describes an excruciating and horrifying experience and he referred to it as a “psychotic break.”
Ferriss goes on to talk about how bringing these memories to light has affected him, how it’s explained many of his behaviors and ways of relating in a way that has helped him organize and understand his life.
“It was at the tail end of the retreat that I realized that these 17 seemingly inexplicable behaviors of mine – these vicious cycles or triggers that I had been treating like separate problems to be solved, were all downstream of this trauma. Oh, now that you click that puzzle piece into place, these really strange behaviors – this self-loathing, this rage that was seemingly so exaggerated and disproportionate – leading to the near-suicide I had in college – all these things fell into places making sense.” It gave him a sense of relief but was simultaneously overwhelming.
Both Ferriss and Millman talk about books and treatments that have been helpful to them. Their knowledge of trauma treatments and resources is impressive and can be found at: Tim Ferriss – My Healing Journey After Childhood Abuse (Includes Extensive Resource List). Their wish is to share their suffering as a way to help others, impart hope, and better connect.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Antidepressant use shows gender, racial disparities
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.
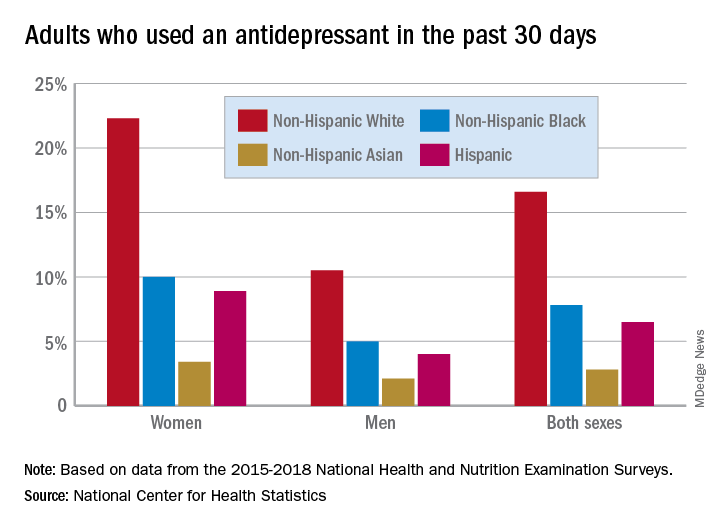
Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.
Women are more than twice as likely as men to use antidepressants, and use among White women is at least double that of other races/ethnicities, according to a new analysis from the National Center for Health Statistics.

Here are the actual numbers: 17.7% of women and 8.4% of men used an antidepressant in the 30 days before being interviewed for the National Health and Nutrition Examination Survey (NHANES). Put them together, and it works out to 13.2% of all adults over the 4-year period from 2015 to 2018, Debra J. Brody, MPH, and Qiuping Gu, MD, PhD, said Sept. 4 in an NCHS data brief.
Non-Hispanic White women had a past-30-day prevalence of 22.3%, compared with 10.0% for non-Hispanic Black women, 3.4% for non-Hispanic Asian women, and 8.9% for Hispanic women, based on the NHANES data.
The order was the same for men, but the numbers are lower. Non-Hispanic Whites had the highest antidepressant use at 10.5%, followed by non-Hispanic Blacks (5.0%), non-Hispanic Asians (2.1%), and Hispanics (4.0%). All of the differences between Whites and non-Whites were significant for both women and men, the researchers noted.
A look at trends over time shows that the gap between men and women has widened in the last 10 years. Past-30-day use among women went from 13.8% in 2009-2010 to 18.6% in 2017-2018, with a corresponding increase from 7.1% to 8.7% in men. For women, that change was significant; for men, it was not, Ms. Brody and Dr. Gu said.
The sample size averaged just over 6,000 for each of the five 2-year NHANES cycles included in the analysis. The survey includes a household interview and a physical examination at a mobile exam center.