User login
Waning pertussis immunity may be linked to acellular vaccine
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
Fixing one problem with the pertussis vaccine seemed to have created another, Kathryn M. Edwards, MD, wrote in an accompanying editorial.
The current acellular vaccine was approved in 1997. It was considered a less reactive substitute for the previous whole-cell vaccine, which was associated with injection site pain, swelling, fever, and febrile seizures, Dr. Edwards wrote. “For about a decade, all seemed to be going well with pertussis control. Serological methods were employed to diagnose pertussis infections in adolescents and adults, and polymerase chain reaction methods were devised to more accurately detect pertussis organisms. Thus, the burden of pertussis disease was increasingly appreciated as the diagnostic methods improved.”
But things soon changed. There were pertussis outbreaks, some of them quite large. The increasing disease rates showed that protection conferred by the acellular vaccine waned much more quickly than that conferred by the whole-cell vaccine. “In the current study, Zerbo et al. add to the body of evidence documenting the increase in pertussis risk with time after DTaP vaccination,” she noted.
This has several practical implications, Dr. Edwards wrote.
“First, given the markedly increased risk of pertussis in unvaccinated and undervaccinated children, universal DTaP vaccination should be strongly recommended. Second, the addition of maternal Tdap vaccination administered during pregnancy has been shown to significantly reduce infant disease before primary immunization and should remain the standard,” Dr. Edwards wrote.
More problematic is how to address the waning DTaP immunity now seen. “One option presented [at an international meeting] was a live-attenuated pertussis vaccine administered intranasally that would stimulate local immune responses and prevent colonization with pertussis organisms. This vaccine is currently being studied in adults and might provide a solution for waning immunity seen with DTaP vaccine,” she noted.
Another possibility is adding the live vaccine to the current DTaP, which should, in theory, stimulate more long-lasting immunity. But numerous safety studies in young children would be necessary before adopting such an approach, Dr. Edwards wrote.
Adding more antigens to the acellular vaccine also might work, and investigational vaccines like this are in development.
Studies in animals and humans show that acellular vaccines “generate functionally different T-cell responses than those seen after whole-cell vaccines, with the whole cell vaccines generating more protective T-cell responses. Studies are ongoing to determine if adjuvants can be added to acellular vaccines to modify their T-cell responses to a more protective immune response or whether the T-cell response remains fixed once primed with DTaP vaccine,” she wrote.
Dr. Edwards is a pediatric infectious disease specialist at Vanderbilt University, Nashville, Tenn. She wrote an editorial to accompany Zerbo et al (Pediatrics. 2019. doi: 10.1542/peds.2019-1276). She reported no financial disclosures, and received no funding to write the editorial.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
A large Kaiser Permanente study paints a nuanced picture of the acellular pertussis vaccine, with more cases occurring in fully vaccinated children, but the highest risk of disease occurring among the under- and unvaccinated.
Among nearly half a million children, the unvaccinated were 13 times more likely to develop pertussis than fully vaccinated children, Ousseny Zerbo, PhD, of Kaiser Permanente Northern California in Oakland and colleagues wrote in Pediatrics. But 82% of cases occurred in fully vaccinated children and just 5% in undervaccinated children – and rates increased in both groups the farther they were in time from the last vaccination.
“Within our study population, greater than 80% of pertussis cases occurred among age-appropriately vaccinated children,” the team wrote. “Children who were further away from their last DTaP dose were at increased risk of pertussis, even after controlling for undervaccination. Our results suggest that, in this population, possibly in conjunction with other factors not addressed in this study, suboptimal vaccine efficacy and waning [immunity] played a major role in recent pertussis epidemics.”
The results are consistent with several prior studies, including one finding that the odds of the disease increased by 33% for every additional year after the third or fifth DTaP dose (Pediatrics. 2015;135[2]:331-43).
The current study comprised 469,982 children aged between 3 months and 11 years, who were followed for a mean of 4.6 years. Over the entire study period, there were 738 lab-confirmed pertussis cases. Most of these (515; 70%) occurred in fully vaccinated children. Another 99 (13%) occurred in unvaccinated children, 36 (5%) in undervaccinated children, and 88 (12%) in fully vaccinated plus one dose.
In a multivariate analysis, the risk of pertussis was 13 times higher among the unvaccinated (adjusted hazard ratio, 13) and almost 2 times higher among the undervaccinated (aHR, 1.9), compared with fully vaccinated children. Those who had been fully vaccinated and received a booster had the lowest risk, about half that of fully vaccinated children (aHR, 0.48).
Risk varied according to age, but also was significantly higher among unvaccinated children at each time point. Risk ranged from 4 times higher among those aged 3-5 months to 23 times higher among those aged 19-84 months. Undervaccinated children aged 5-7 months and 19-84 months also were at significantly increased risk for pertussis, compared with fully vaccinated children. Children who were fully vaccinated plus one dose had a significantly reduced risk at 7-19 months and at 19-84 months, compared with the fully vaccinated reference group.
“Across all follow-up and all age groups, VE [vaccine effectiveness] was 86% ... for undervaccinated children, compared with unvaccinated children,” Dr. Zerbo and associates wrote. “VE was even higher for fully vaccinated children [93%] and for those who were fully vaccinated plus one dose [96%].”
But VE waned as time progressed farther from the last DTaP dose. The multivariate model found more than a 100% increased risk for those whose last DTaP was at least 3 years past, compared with less than 1 year past (aHR, 2.58).
The model also found time-bound risk increases among fully vaccinated children, with a more than 300% increased risk among those at least 6 years out from the last DTaP dose, compared with 3 years out (aHR, 4.66).
The results indicate that other factors besides adherence to the recommended vaccine schedule may be at work in recent pertussis outbreaks.
“Although waning immunity is clearly an important factor driving pertussis epidemics in recent years, other factors that we did not evaluate in this study might also contribute to pertussis epidemics individually or in synergy,” Dr. Zerbo and associates wrote. “Results from studies in baboons suggest that the acellular pertussis vaccines are unable to prevent colonization, carriage, and transmission. If this is also true for humans, this could contribute to pertussis epidemics. The causes of recent pertussis epidemics are complex, and we were only able to address some aspects in our study.”
The study was funded by Kaiser Permanente Northern California, the National Institutes of Health, and in part by a National Institute of Allergy and Infectious Diseases grant. One coauthor reported receiving research grant support from Sanofi Pasteur, Novartis, GlaxoSmithKline, Merck, MedImmune, Pfizer, and Dynavax for unrelated studies; the other authors reported no relevant financial disclosures.
SOURCE: Zerbo O et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3466.
FROM PEDIATRICS
Fewer antibiotics prescribed with PCR than conventional stool testing
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
REPORTING FROM DDW 2019
USPSTF recommends PrEP combo for adults at high risk of HIV infection
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
To end HIV, guidelines like this one that reflect and promote advances in treatment are needed, according to Hyman Scott, MD, MPH, of the San Francisco Department of Public Health and Paul A. Volberding, MD, of the University of California, San Francisco.
With less than 10% of individuals with an indication for PrEP currently receiving the medication, it is now time to support policies aimed at broadening the access of PrEP to people at risk, the coauthors wrote. They noted that recent USPSTF guidelines show that evidence and policy in HIV medicine has matured not only in the United States but across the globe.
That said, sometimes the simplest solutions are also the best. Though the systematic review from Roger Chou, MD, and associates notes the necessity and importance of adherence, if a clinician thinks that a candidate for PrEP might be nonadherent, that clinicians should not withhold the medication, they wrote. Averting new HIV infections is the goal, and fully endorsing treatments like PrEP is an important step in that direction.*
These comments are adapted from an accompanying editorial (JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2590). Dr. Volberding reported serving on a data and safety monitoring board for Merck.
*This article was updated on 6/11/2019.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
Pre-exposure prophylaxis (PrEP) plus effective antiretroviral therapy should be offered to people at high risk of HIV acquisition, according to a new recommendation from the U.S. Preventive Services Task Force (USPSTF).
“The USPSTF concludes with high certainty that the net benefit of the use of PrEP to reduce the risk of acquisition of HIV infection in persons at high risk of HIV infection is substantial,” wrote first author Douglas K. Owens, MD, of Stanford (Calif.) University and fellow members of the USPSTF. The recommendation was published in JAMA.
In various at-risk groups – including men who have sex with men, people at risk through heterosexual contact, and people who inject drugs – the USPSTF recommends a Food and Drug Adminstration–approved, once-daily oral treatment with combined tenofovir disoproxil fumarate and emtricitabine.
This recommendation was developed after a systematic review of PrEP’s effects on HIV, adherence to the treatment, and accuracy in identifying potential treatment candidates. “The findings of this review are generally consistent with those from other recent meta-analyses that found PrEP to be effective at reducing risk of HIV infection and found greater effectiveness in trials reporting higher adherence,” wrote Roger Chou, MD, of Oregon Health & Science University in Portland and coauthors. Their study was also published in JAMA.
To comprehensively assess PrEP and thus inform the USPSTF’s HIV prevention recommendations, the researchers reviewed criteria-meeting studies on oral PrEP with tenofovir disoproxil fumarate/emtricitabine or tenofovir disoproxil fumarate monotherapy; on the diagnostic accuracy of instruments to predict HIV infection; and on PrEP adherence. The final analysis included 14 randomized clinical trials, 8 observational studies, and 7 studies of diagnostic accuracy.
In 11 of the trials, PrEP was associated with reduced risk of HIV infection versus placebo or no PrEP (relative risk, 0.46; 95% confidence interval, 0.33-0.66). In 6 trials with adherence 70% or greater, the relative risk was 0.27 (95% CI, 0.19-0.39). In 7 studies on risk assessment tools for HIV infection, the instruments had moderate discrimination, though several of the studies had methodological shortcomings. As for serious adverse events, an analysis of 12 trials found no significant difference between PrEP and placebo (RR, 0.93; 95% CI, 0.77-1.12).
Dr. Chou and coauthors noted their study’s limitations, including analyzing English-language articles only and the random-effects model used to pool studies potentially returning narrow CIs. They did note, however, that the “analyses were repeated using the profile likelihood method,” which produced similar findings.
All members of the USPSTF receive travel reimbursement and an honorarium for participating in meetings. The study was funded by the Department of Health and Human Services. One of the authors reported receiving grants from the National Institutes of Health/National Institute on Drug Abuse and serving as principal investigator of NIH-funded clinical trials that received donated drugs from two pharmaceutical companies. No other conflicts of interest were reported.
SOURCE: Owens DK et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.6390; Chou R et al. JAMA. 2019 Jun 11. doi: 10.1001/jama.2019.2591.
FROM JAMA
United States now over 1,000 measles cases this year
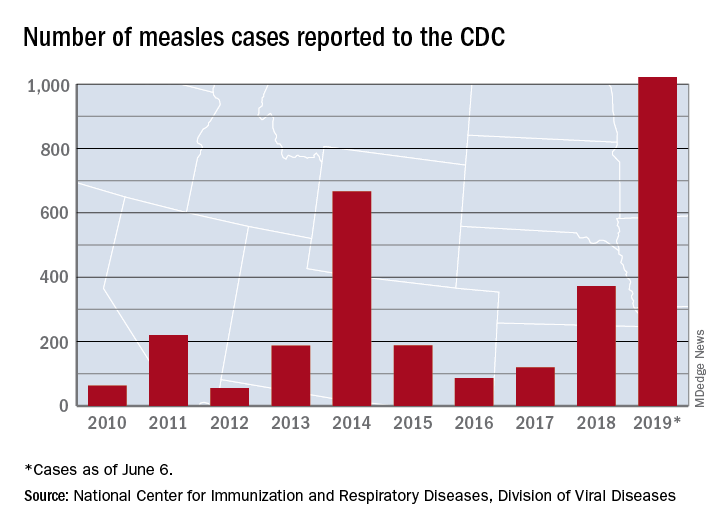
The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.

The 41 new cases reported for the week ending June 6 bring the total for the year to 1,022, the CDC reported June 10, and that is more than any year since 1992, when there were 2,237 cases.
Idaho and Virginia reported their first cases of 2019, which makes a total of 28 states with measles cases this year. The Idaho case was reported in Latah County and is the state’s first since 2001. In Virginia, health officials are investigating possible contacts with an infected individual at Dulles International Airport and two other locations on June 2 and 4.
Outbreaks in Georgia, Maryland, and Michigan have ended, while seven others continue in California (Butte, Los Angeles, and Sacramento Counties), New York (Rockland County and New York City), Pennsylvania, and Washington, the CDC said. New York City has the largest outbreak this year with 509 cases through June 3, most of them occurring in Brooklyn.
Rituximab serious infection risk predicted by immunoglobulin levels
Monitoring immunoglobulin (Ig) levels at baseline and before each cycle of rituximab could reduce the risk of serious infection events (SIEs) in patients needing repeated treatment, according to research published in Arthritis & Rheumatology.
In a large, single-center, longitudinal study conducted at a tertiary referral center, having low IgG (less than 6 g/L) in particular was associated with a higher rate of SIEs, compared with having normal IgG levels (6-16 g/L). Considering 103 of 700 patients who had low levels of IgG before starting treatment with rituximab for various rheumatic and musculoskeletal diseases (RMDs), there were 16.4 SIEs per 100 patient-years. In those who developed low IgG during subsequent cycles of rituximab therapy, the SIE rate was even higher, at 21.3 per 100 patient-years. By comparison, the SIE rate for those with normal IgG levels was 9.7 per 100 patient-years.
“We really have to monitor immunoglobulins at baseline and also before we re-treat the patients, because higher IgG level is protective of serious infections,” study first author Md Yuzaiful Md Yusof, MBChB, PhD, said in an interview.
Low IgG has been linked to a higher risk of SIEs in the first 12 months of rituximab therapy but, until now, there have been limited data on infection predictors during repeated cycles of treatment. While IgG is a consistent marker of SIEs associated with repeated rituximab treatment, IgM and IgA should also be monitored to give a full picture of any hyperglobulinemia that may be present.
“There is no formal guidance on how to safely monitor patients on rituximab,” observed Dr. Md Yusof, who will present these data at the 2019 European Congress of Rheumatology in Madrid. The study’s findings could help to change that, however, as they offer a practical way to help predict and thus prevent SIEs. The study’s findings not only validate previous work, he noted, but also add new insights into why some patients treated with repeat rituximab cycles but not others may experience a higher rate of such infections.
Altogether, the investigators examined data on 700 patients with RMDs treated with rituximab who were consecutively seen during 2012-2017 at Dr. Md Yusof’s institution – the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, which is part of the University of Leeds. Their immunoglobulin levels had been measured before starting rituximab therapy and every 4-6 months after each cycle of rituximab treatment.
Patients with any RMD being treated with at least one cycle of rituximab were eligible for inclusion in the retrospective study, with the majority (72%) taking it for rheumatoid arthritis and some for systemic lupus erythematosus (13%) or antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (7%).
One of the main aims of the study was to look for predictors of SIEs during the first 12 months and during repeated cycles of rituximab. Dr. Md Yusof and his associates also looked at how secondary hypogammaglobulinemia might affect SIE rates and the humoral response to vaccination challenge and its persistence following treatment discontinuation. Their ultimate aim was to see if these findings could then be used to develop a treatment algorithm for rituximab administration in RMDs.
Over a follow-up period encompassing 2,880 patient-years of treatment, 281 SIEs were recorded in 176 patients, giving a rate of 9.8 infections per 100 patient-years. Most (61%) of these were due to lower respiratory tract infections.
The proportion of patients experiencing their first SIE increased with time: 16% within 6 weeks of starting rituximab therapy, 35% at 12 weeks, 72% at 26 weeks, 83% at 38 weeks, and 100% by 1 year of repeated treatment.
Multivariable analysis showed that the presence of several comorbidities at baseline – notably chronic obstructive pulmonary disease, diabetes, heart failure, and prior cancer – raised the risk for SIEs with repeated rituximab therapy. The biggest factor, however, was a history of SIEs – with a sixfold increased risk of further serious infection.
Higher corticosteroid dose and factors specific to rituximab – low IgG, neutropenia, high IgM, and a longer time to retreatment – were also predictive of SIEs.
“Low IgG also results in poor humoral response to vaccination,” Dr. Md Yusof said, noting that the IgG level remains below the lower limit of normal for several years after rituximab is discontinued in most patients.
In the study, 5 of 8 (64%) patients had impaired humoral response to pneumococcal and haemophilus following vaccination challenge and 4 of 11 patients had IgG normalized after switching to another biologic disease-modifying antirheumatic drug (bDMARD).
Cyclophosphamide is commonly used as a first-line agent to induce remission in patients with severe and refractory systemic lupus erythematosus and ANCA-associated vasculitis, with patients switched to rituximab at relapse. The effect of this prior treatment was examined in 20 patients in the study, with a marked decline in almost all immunoglobulin classes seen up to 18 months. Prior treatment with immunosuppressants such as intravenous cyclophosphamide could be behind progressive reductions in Ig levels seen with repeated rituximab treatment rather than entirely because of rituximab, Dr. Md Yusof said.
Dr. Md Yusof, who is a National Institute for Health Research (NIHR) Academic Clinical Lecturer at the University of Leeds, said the value of the study, compared with others, is that hospital data for all patients treated with rituximab with at least 3 months follow-up were included, making it an almost complete data set.
“By carefully reviewing records of every patient to capture all infection episodes in the largest single-center cohort study to date, our findings provide insights on predictors of SIEs as well as a foundation for safety monitoring of rituximab,” he and his coauthors wrote.
They acknowledge reporting a higher rate of SIEs than seen in registry and clinical studies with rituximab, which may reflect a “channeling bias” as the patients comprised those with multiple comorbidities including those that represent a relative contraindication for bDMARD use. That said, the findings clearly show that Ig levels should be monitored before and after each rituximab cycle, especially in those with comorbid diseases and those with low IgG levels to start with.
They conclude that an “individualized benefit-risk assessment” is needed to determine whether rituximab should be repeated in those with low IgG as this is a “consistent predictor” of SIE and may “increase infection profiles when [rituximab] is switched to different bDMARDs.”
The research was supported by Octapharma, the National Institute for Health Research (NIHR), and NIHR Leeds Biomedical Research Centre based at Leeds Teaching Hospitals NHS Trust in England. Dr. Md Yusof had no conflicts of interest. Several coauthors disclosed financial ties to multiple pharmaceutical companies, including Roche.
SOURCE: Md Yusof MY et al. Arthritis Rheumatol. 2019 May 27. doi: 10.1002/art.40937.
Monitoring immunoglobulin (Ig) levels at baseline and before each cycle of rituximab could reduce the risk of serious infection events (SIEs) in patients needing repeated treatment, according to research published in Arthritis & Rheumatology.
In a large, single-center, longitudinal study conducted at a tertiary referral center, having low IgG (less than 6 g/L) in particular was associated with a higher rate of SIEs, compared with having normal IgG levels (6-16 g/L). Considering 103 of 700 patients who had low levels of IgG before starting treatment with rituximab for various rheumatic and musculoskeletal diseases (RMDs), there were 16.4 SIEs per 100 patient-years. In those who developed low IgG during subsequent cycles of rituximab therapy, the SIE rate was even higher, at 21.3 per 100 patient-years. By comparison, the SIE rate for those with normal IgG levels was 9.7 per 100 patient-years.
“We really have to monitor immunoglobulins at baseline and also before we re-treat the patients, because higher IgG level is protective of serious infections,” study first author Md Yuzaiful Md Yusof, MBChB, PhD, said in an interview.
Low IgG has been linked to a higher risk of SIEs in the first 12 months of rituximab therapy but, until now, there have been limited data on infection predictors during repeated cycles of treatment. While IgG is a consistent marker of SIEs associated with repeated rituximab treatment, IgM and IgA should also be monitored to give a full picture of any hyperglobulinemia that may be present.
“There is no formal guidance on how to safely monitor patients on rituximab,” observed Dr. Md Yusof, who will present these data at the 2019 European Congress of Rheumatology in Madrid. The study’s findings could help to change that, however, as they offer a practical way to help predict and thus prevent SIEs. The study’s findings not only validate previous work, he noted, but also add new insights into why some patients treated with repeat rituximab cycles but not others may experience a higher rate of such infections.
Altogether, the investigators examined data on 700 patients with RMDs treated with rituximab who were consecutively seen during 2012-2017 at Dr. Md Yusof’s institution – the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, which is part of the University of Leeds. Their immunoglobulin levels had been measured before starting rituximab therapy and every 4-6 months after each cycle of rituximab treatment.
Patients with any RMD being treated with at least one cycle of rituximab were eligible for inclusion in the retrospective study, with the majority (72%) taking it for rheumatoid arthritis and some for systemic lupus erythematosus (13%) or antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (7%).
One of the main aims of the study was to look for predictors of SIEs during the first 12 months and during repeated cycles of rituximab. Dr. Md Yusof and his associates also looked at how secondary hypogammaglobulinemia might affect SIE rates and the humoral response to vaccination challenge and its persistence following treatment discontinuation. Their ultimate aim was to see if these findings could then be used to develop a treatment algorithm for rituximab administration in RMDs.
Over a follow-up period encompassing 2,880 patient-years of treatment, 281 SIEs were recorded in 176 patients, giving a rate of 9.8 infections per 100 patient-years. Most (61%) of these were due to lower respiratory tract infections.
The proportion of patients experiencing their first SIE increased with time: 16% within 6 weeks of starting rituximab therapy, 35% at 12 weeks, 72% at 26 weeks, 83% at 38 weeks, and 100% by 1 year of repeated treatment.
Multivariable analysis showed that the presence of several comorbidities at baseline – notably chronic obstructive pulmonary disease, diabetes, heart failure, and prior cancer – raised the risk for SIEs with repeated rituximab therapy. The biggest factor, however, was a history of SIEs – with a sixfold increased risk of further serious infection.
Higher corticosteroid dose and factors specific to rituximab – low IgG, neutropenia, high IgM, and a longer time to retreatment – were also predictive of SIEs.
“Low IgG also results in poor humoral response to vaccination,” Dr. Md Yusof said, noting that the IgG level remains below the lower limit of normal for several years after rituximab is discontinued in most patients.
In the study, 5 of 8 (64%) patients had impaired humoral response to pneumococcal and haemophilus following vaccination challenge and 4 of 11 patients had IgG normalized after switching to another biologic disease-modifying antirheumatic drug (bDMARD).
Cyclophosphamide is commonly used as a first-line agent to induce remission in patients with severe and refractory systemic lupus erythematosus and ANCA-associated vasculitis, with patients switched to rituximab at relapse. The effect of this prior treatment was examined in 20 patients in the study, with a marked decline in almost all immunoglobulin classes seen up to 18 months. Prior treatment with immunosuppressants such as intravenous cyclophosphamide could be behind progressive reductions in Ig levels seen with repeated rituximab treatment rather than entirely because of rituximab, Dr. Md Yusof said.
Dr. Md Yusof, who is a National Institute for Health Research (NIHR) Academic Clinical Lecturer at the University of Leeds, said the value of the study, compared with others, is that hospital data for all patients treated with rituximab with at least 3 months follow-up were included, making it an almost complete data set.
“By carefully reviewing records of every patient to capture all infection episodes in the largest single-center cohort study to date, our findings provide insights on predictors of SIEs as well as a foundation for safety monitoring of rituximab,” he and his coauthors wrote.
They acknowledge reporting a higher rate of SIEs than seen in registry and clinical studies with rituximab, which may reflect a “channeling bias” as the patients comprised those with multiple comorbidities including those that represent a relative contraindication for bDMARD use. That said, the findings clearly show that Ig levels should be monitored before and after each rituximab cycle, especially in those with comorbid diseases and those with low IgG levels to start with.
They conclude that an “individualized benefit-risk assessment” is needed to determine whether rituximab should be repeated in those with low IgG as this is a “consistent predictor” of SIE and may “increase infection profiles when [rituximab] is switched to different bDMARDs.”
The research was supported by Octapharma, the National Institute for Health Research (NIHR), and NIHR Leeds Biomedical Research Centre based at Leeds Teaching Hospitals NHS Trust in England. Dr. Md Yusof had no conflicts of interest. Several coauthors disclosed financial ties to multiple pharmaceutical companies, including Roche.
SOURCE: Md Yusof MY et al. Arthritis Rheumatol. 2019 May 27. doi: 10.1002/art.40937.
Monitoring immunoglobulin (Ig) levels at baseline and before each cycle of rituximab could reduce the risk of serious infection events (SIEs) in patients needing repeated treatment, according to research published in Arthritis & Rheumatology.
In a large, single-center, longitudinal study conducted at a tertiary referral center, having low IgG (less than 6 g/L) in particular was associated with a higher rate of SIEs, compared with having normal IgG levels (6-16 g/L). Considering 103 of 700 patients who had low levels of IgG before starting treatment with rituximab for various rheumatic and musculoskeletal diseases (RMDs), there were 16.4 SIEs per 100 patient-years. In those who developed low IgG during subsequent cycles of rituximab therapy, the SIE rate was even higher, at 21.3 per 100 patient-years. By comparison, the SIE rate for those with normal IgG levels was 9.7 per 100 patient-years.
“We really have to monitor immunoglobulins at baseline and also before we re-treat the patients, because higher IgG level is protective of serious infections,” study first author Md Yuzaiful Md Yusof, MBChB, PhD, said in an interview.
Low IgG has been linked to a higher risk of SIEs in the first 12 months of rituximab therapy but, until now, there have been limited data on infection predictors during repeated cycles of treatment. While IgG is a consistent marker of SIEs associated with repeated rituximab treatment, IgM and IgA should also be monitored to give a full picture of any hyperglobulinemia that may be present.
“There is no formal guidance on how to safely monitor patients on rituximab,” observed Dr. Md Yusof, who will present these data at the 2019 European Congress of Rheumatology in Madrid. The study’s findings could help to change that, however, as they offer a practical way to help predict and thus prevent SIEs. The study’s findings not only validate previous work, he noted, but also add new insights into why some patients treated with repeat rituximab cycles but not others may experience a higher rate of such infections.
Altogether, the investigators examined data on 700 patients with RMDs treated with rituximab who were consecutively seen during 2012-2017 at Dr. Md Yusof’s institution – the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, which is part of the University of Leeds. Their immunoglobulin levels had been measured before starting rituximab therapy and every 4-6 months after each cycle of rituximab treatment.
Patients with any RMD being treated with at least one cycle of rituximab were eligible for inclusion in the retrospective study, with the majority (72%) taking it for rheumatoid arthritis and some for systemic lupus erythematosus (13%) or antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (7%).
One of the main aims of the study was to look for predictors of SIEs during the first 12 months and during repeated cycles of rituximab. Dr. Md Yusof and his associates also looked at how secondary hypogammaglobulinemia might affect SIE rates and the humoral response to vaccination challenge and its persistence following treatment discontinuation. Their ultimate aim was to see if these findings could then be used to develop a treatment algorithm for rituximab administration in RMDs.
Over a follow-up period encompassing 2,880 patient-years of treatment, 281 SIEs were recorded in 176 patients, giving a rate of 9.8 infections per 100 patient-years. Most (61%) of these were due to lower respiratory tract infections.
The proportion of patients experiencing their first SIE increased with time: 16% within 6 weeks of starting rituximab therapy, 35% at 12 weeks, 72% at 26 weeks, 83% at 38 weeks, and 100% by 1 year of repeated treatment.
Multivariable analysis showed that the presence of several comorbidities at baseline – notably chronic obstructive pulmonary disease, diabetes, heart failure, and prior cancer – raised the risk for SIEs with repeated rituximab therapy. The biggest factor, however, was a history of SIEs – with a sixfold increased risk of further serious infection.
Higher corticosteroid dose and factors specific to rituximab – low IgG, neutropenia, high IgM, and a longer time to retreatment – were also predictive of SIEs.
“Low IgG also results in poor humoral response to vaccination,” Dr. Md Yusof said, noting that the IgG level remains below the lower limit of normal for several years after rituximab is discontinued in most patients.
In the study, 5 of 8 (64%) patients had impaired humoral response to pneumococcal and haemophilus following vaccination challenge and 4 of 11 patients had IgG normalized after switching to another biologic disease-modifying antirheumatic drug (bDMARD).
Cyclophosphamide is commonly used as a first-line agent to induce remission in patients with severe and refractory systemic lupus erythematosus and ANCA-associated vasculitis, with patients switched to rituximab at relapse. The effect of this prior treatment was examined in 20 patients in the study, with a marked decline in almost all immunoglobulin classes seen up to 18 months. Prior treatment with immunosuppressants such as intravenous cyclophosphamide could be behind progressive reductions in Ig levels seen with repeated rituximab treatment rather than entirely because of rituximab, Dr. Md Yusof said.
Dr. Md Yusof, who is a National Institute for Health Research (NIHR) Academic Clinical Lecturer at the University of Leeds, said the value of the study, compared with others, is that hospital data for all patients treated with rituximab with at least 3 months follow-up were included, making it an almost complete data set.
“By carefully reviewing records of every patient to capture all infection episodes in the largest single-center cohort study to date, our findings provide insights on predictors of SIEs as well as a foundation for safety monitoring of rituximab,” he and his coauthors wrote.
They acknowledge reporting a higher rate of SIEs than seen in registry and clinical studies with rituximab, which may reflect a “channeling bias” as the patients comprised those with multiple comorbidities including those that represent a relative contraindication for bDMARD use. That said, the findings clearly show that Ig levels should be monitored before and after each rituximab cycle, especially in those with comorbid diseases and those with low IgG levels to start with.
They conclude that an “individualized benefit-risk assessment” is needed to determine whether rituximab should be repeated in those with low IgG as this is a “consistent predictor” of SIE and may “increase infection profiles when [rituximab] is switched to different bDMARDs.”
The research was supported by Octapharma, the National Institute for Health Research (NIHR), and NIHR Leeds Biomedical Research Centre based at Leeds Teaching Hospitals NHS Trust in England. Dr. Md Yusof had no conflicts of interest. Several coauthors disclosed financial ties to multiple pharmaceutical companies, including Roche.
SOURCE: Md Yusof MY et al. Arthritis Rheumatol. 2019 May 27. doi: 10.1002/art.40937.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Immunoglobulin should be monitored at baseline and before each rituximab cycle to identify patients at risk of serious infection events (SIEs).
Major finding: SIE rates per 100 patient-years were 16.4 and 21.3 in patients with low (less than 6 g/L) IgG at baseline and during rituximab cycles versus 9.7 for patients with normal (6–16 g/L) IgG levels.
Study details: A retrospective, single-center, longitudinal study involving 700 rituximab-treated patients with rheumatoid arthritis and other rheumatic and musculoskeletal diseases.
Disclosures: The research was supported by Octapharma, the National Institute for Health Research (NIHR), and NIHR Leeds Biomedical Research Centre based at Leeds Teaching Hospitals NHS Trust in the United Kingdom. Dr. Md Yusof had no conflicts of interest. Several coauthors disclosed financial ties to multiple pharmaceutical companies, including Roche.
Source: Md Yusof MY et al. Arthritis Rheumatol. 2019 May 27. doi: 10.1002/art.40937.
How to incorporate HIV PrEP into your practice
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
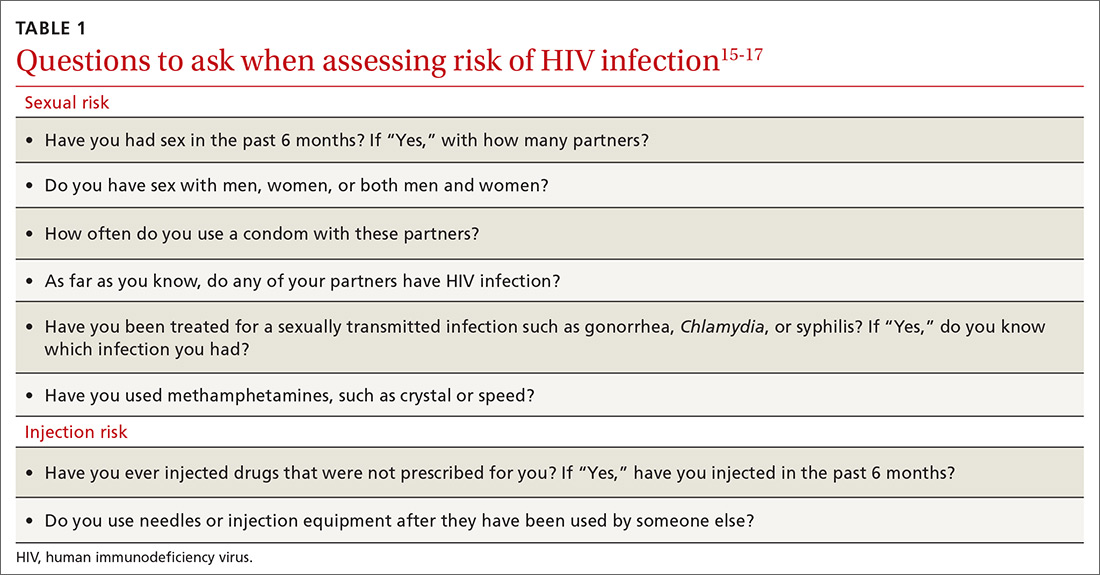
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
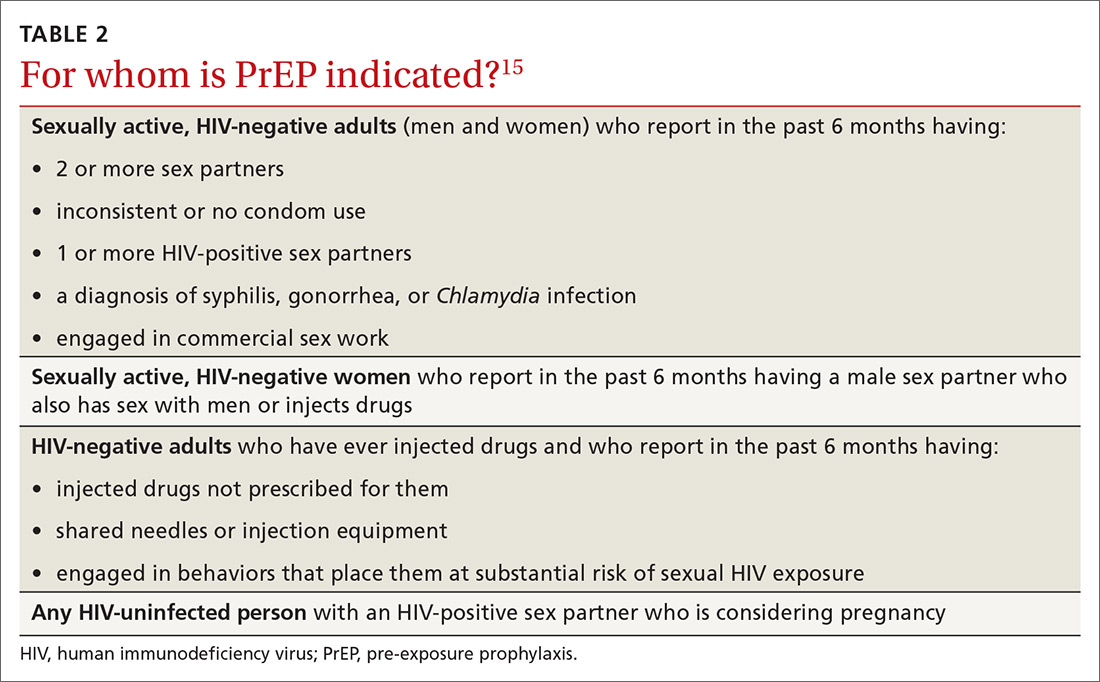
What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
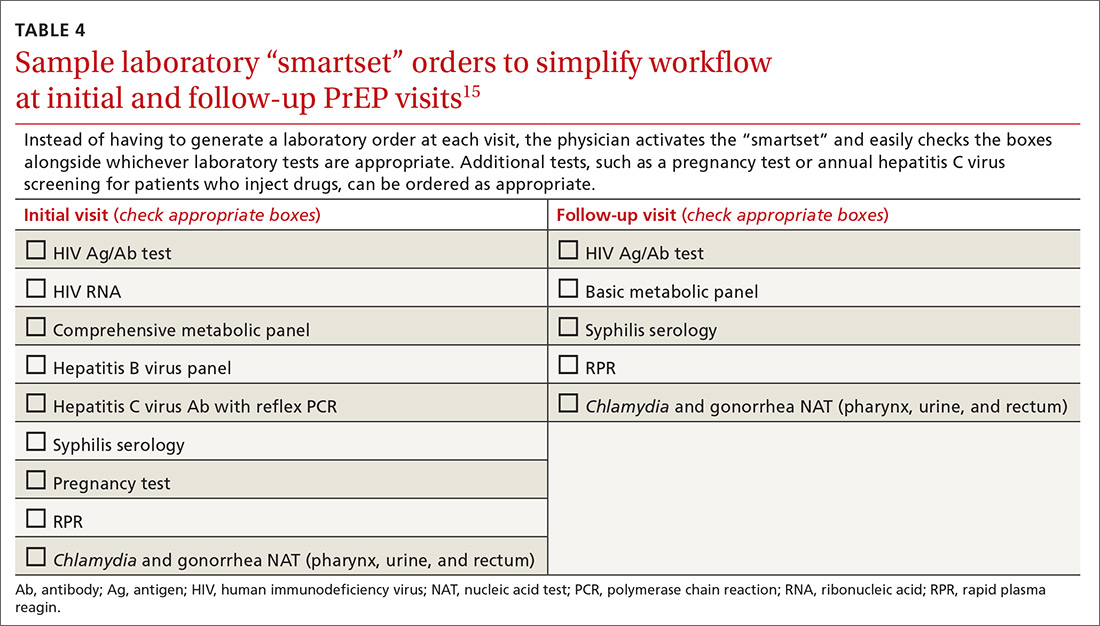
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
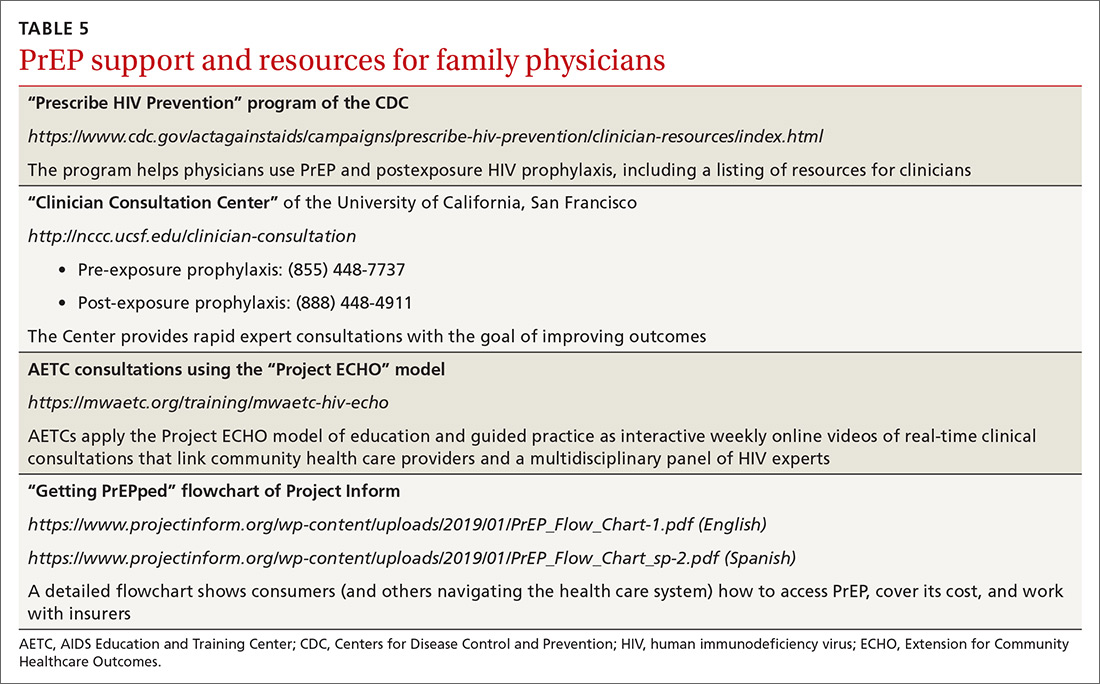
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
PRACTICE RECOMMENDATIONS
› Actively screen and identify HIV-negative patients who are a candidate for pre-exposure prophylaxis (PrEP); commit to talking to the most easily identifiable subsets of these patients, such as men who have sex with men and transgender patients. B
› Recognize that PrEP is indicated for patients who: are sexually active with inconsistent condom use and multiple recent sex partners; have recently been given a diagnosis of a sexually transmitted infection; or have a sexual or injection partner known to be HIV-infected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Varicella vaccine delivers doubled benefit to children
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
FROM PEDIATRICS
Key clinical point: Varicella vaccine is preventing pediatric zoster among children aged 2-17 years.
Major finding: Varicella-vaccinated children have a 78% lower incidence of pediatric zoster than do unvaccinated children.
Study details: The population-based cohort study included more than 6.3 million children.
Disclosures: Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
Source: Weinmann S et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2917.
Painless Nodule on the Leg
The Diagnosis: Plasmablastic Lymphoma
Histopathologic examination revealed a diffuse dense proliferation of large, atypical, and pleomorphic mononuclear cells with prominent nucleoli and many mitotic figures representing plasmacytoid cells in the dermis (Figure). Immunostaining was positive for MUM-1 (marker of late-stage plasma cells and activated T cells) and BCL-2 (antiapoptotic marker). Fluorescent polymerase chain reaction was positive for clonal IgH gene arrangement, and fluorescence in situ hybridization was positive for C-MYC rearrangement in 94% of cells. Epstein-Barr encoding region in situ hybridization also was positive. Rare cells stained positive for T-cell markers. CD20, BCL-6, and CD30 immunostains were negative, suggesting that these cells were not B or T cells, though terminally differentiated B cells also can lack these markers. Bone marrow biopsy showed a similar staining pattern to the skin with 10% atypical plasmacytoid cells. Computed tomography of the left leg showed an enlargement of the semimembranosus muscle with internal areas of high density and heterogeneous enhancement. The patient underwent decompression of the left peroneal nerve. Biopsy showed a staining pattern similar to the right skin nodule and bone marrow, consistent with lymphoma.
He was diagnosed with stage IV human immunodeficiency virus (HIV)-associated plasmablastic lymphoma (PBL) and received 6 cycles of R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) without vincristine with intrathecal methotrexate, followed by 3 cycles of DHAP (dexamethasone, high dose Ara C, cisplatin) with bortezomib and daratumumab after relapse. Ultimately, he underwent autologous stem cell transplantation and was alive 13 months after diagnosis.
Plasmablastic lymphoma is a rare subtype of non-Hodgkin lymphoma that most commonly arises in the oral cavity of individuals with HIV.1 In addition to HIV infection, PBL also is seen in patients with other causes of immunodeficiency such as iatrogenic immunosuppression following solid organ transplantation.1 The typical disease presentation is an expanding mass in the oral cavity; however, 34% (52/151) of reported cases arose at extraoral primary sites, with a minority of cases confined to cutaneous sites with no systemic involvement.2 Cutaneous PBL presentations may include flesh-colored or purple, grouped or solitary nodules; an erythematous infiltrated plaque; or purple-red ulcerated nodules. The lesions usually are asymptomatic and located on the arms and legs.3
On histologic examination, PBL is characterized by a diffuse monomorphic lymphoid infiltrate that sometimes invades the surrounding soft tissue.4-6 The neoplastic cells have eccentric round nucleoli. Plasmablastic lymphoma characteristically displays a high proliferation index with many mitotic figures and signs of apoptosis.4-6 Definitive diagnosis requires immunohistochemical staining. Typical B-cell antigens (CD20) as well as CD45 are negative, while plasma cell markers such as CD38 are positive. Other B- and T-cell markers usually are negative.5,7 The pathogenesis of PBL is thought to be related to Epstein-Barr virus or human herpesvirus 8 infection. In a series of PBL cases, Epstein-Barr virus and human herpesvirus 8 was positive in 75% (97/129) and 17% (13/75) of tested cases, respectively.1
The prognosis for PBL is poor, with a median overall survival of 15 months and a 3-year survival rate of 25% in HIV-infected individuals.8 However, cutaneous PBL without systemic involvement has a considerably better prognosis, with only 1 of 12 cases resulting in death.2,3,9 Treatment of PBL depends on the extent of the disease. Cutaneous PBL can be treated with surgery and adjuvant radiation.3 Chemotherapy is required for patients with multiple lesions or systemic involvement. Current treatment regimens are similar to those used for other aggressive lymphomas such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).1 Transplant recipients should have their immunosuppression reduced, and HIV-infected patients should have their highly active antiretroviral therapy regimens optimized. Patients presenting with PBL without HIV should be tested for HIV, as PBL has previously been reported to be the presenting manifestation of HIV infection.10
The differential diagnosis for a rapidly expanding, vascular-appearing, red mass on the legs in an immunosuppressed individual includes abscess, malignancy, Kaposi sarcoma, Sweet syndrome, and tertiary syphilis.
Acknowledgment
We thank Sameera Husain, MD (New York, New York), for her assistance with histopathologic photographs and interpretation.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267.
- Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol. 2012;67:E202-E205.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420.
- Gaidano G, Cerri M, Capello D, et al. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622-628.
- Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809.
- Horna P, Hamill JR, Sokol L, et al. Primary cutaneous plasmablastic lymphoma in an immunocompetent patient. J Am Acad Dermatol. 2013;69:E274-E276.
- Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358-1361.
The Diagnosis: Plasmablastic Lymphoma
Histopathologic examination revealed a diffuse dense proliferation of large, atypical, and pleomorphic mononuclear cells with prominent nucleoli and many mitotic figures representing plasmacytoid cells in the dermis (Figure). Immunostaining was positive for MUM-1 (marker of late-stage plasma cells and activated T cells) and BCL-2 (antiapoptotic marker). Fluorescent polymerase chain reaction was positive for clonal IgH gene arrangement, and fluorescence in situ hybridization was positive for C-MYC rearrangement in 94% of cells. Epstein-Barr encoding region in situ hybridization also was positive. Rare cells stained positive for T-cell markers. CD20, BCL-6, and CD30 immunostains were negative, suggesting that these cells were not B or T cells, though terminally differentiated B cells also can lack these markers. Bone marrow biopsy showed a similar staining pattern to the skin with 10% atypical plasmacytoid cells. Computed tomography of the left leg showed an enlargement of the semimembranosus muscle with internal areas of high density and heterogeneous enhancement. The patient underwent decompression of the left peroneal nerve. Biopsy showed a staining pattern similar to the right skin nodule and bone marrow, consistent with lymphoma.

He was diagnosed with stage IV human immunodeficiency virus (HIV)-associated plasmablastic lymphoma (PBL) and received 6 cycles of R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) without vincristine with intrathecal methotrexate, followed by 3 cycles of DHAP (dexamethasone, high dose Ara C, cisplatin) with bortezomib and daratumumab after relapse. Ultimately, he underwent autologous stem cell transplantation and was alive 13 months after diagnosis.
Plasmablastic lymphoma is a rare subtype of non-Hodgkin lymphoma that most commonly arises in the oral cavity of individuals with HIV.1 In addition to HIV infection, PBL also is seen in patients with other causes of immunodeficiency such as iatrogenic immunosuppression following solid organ transplantation.1 The typical disease presentation is an expanding mass in the oral cavity; however, 34% (52/151) of reported cases arose at extraoral primary sites, with a minority of cases confined to cutaneous sites with no systemic involvement.2 Cutaneous PBL presentations may include flesh-colored or purple, grouped or solitary nodules; an erythematous infiltrated plaque; or purple-red ulcerated nodules. The lesions usually are asymptomatic and located on the arms and legs.3
On histologic examination, PBL is characterized by a diffuse monomorphic lymphoid infiltrate that sometimes invades the surrounding soft tissue.4-6 The neoplastic cells have eccentric round nucleoli. Plasmablastic lymphoma characteristically displays a high proliferation index with many mitotic figures and signs of apoptosis.4-6 Definitive diagnosis requires immunohistochemical staining. Typical B-cell antigens (CD20) as well as CD45 are negative, while plasma cell markers such as CD38 are positive. Other B- and T-cell markers usually are negative.5,7 The pathogenesis of PBL is thought to be related to Epstein-Barr virus or human herpesvirus 8 infection. In a series of PBL cases, Epstein-Barr virus and human herpesvirus 8 was positive in 75% (97/129) and 17% (13/75) of tested cases, respectively.1
The prognosis for PBL is poor, with a median overall survival of 15 months and a 3-year survival rate of 25% in HIV-infected individuals.8 However, cutaneous PBL without systemic involvement has a considerably better prognosis, with only 1 of 12 cases resulting in death.2,3,9 Treatment of PBL depends on the extent of the disease. Cutaneous PBL can be treated with surgery and adjuvant radiation.3 Chemotherapy is required for patients with multiple lesions or systemic involvement. Current treatment regimens are similar to those used for other aggressive lymphomas such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).1 Transplant recipients should have their immunosuppression reduced, and HIV-infected patients should have their highly active antiretroviral therapy regimens optimized. Patients presenting with PBL without HIV should be tested for HIV, as PBL has previously been reported to be the presenting manifestation of HIV infection.10
The differential diagnosis for a rapidly expanding, vascular-appearing, red mass on the legs in an immunosuppressed individual includes abscess, malignancy, Kaposi sarcoma, Sweet syndrome, and tertiary syphilis.
Acknowledgment
We thank Sameera Husain, MD (New York, New York), for her assistance with histopathologic photographs and interpretation.
The Diagnosis: Plasmablastic Lymphoma
Histopathologic examination revealed a diffuse dense proliferation of large, atypical, and pleomorphic mononuclear cells with prominent nucleoli and many mitotic figures representing plasmacytoid cells in the dermis (Figure). Immunostaining was positive for MUM-1 (marker of late-stage plasma cells and activated T cells) and BCL-2 (antiapoptotic marker). Fluorescent polymerase chain reaction was positive for clonal IgH gene arrangement, and fluorescence in situ hybridization was positive for C-MYC rearrangement in 94% of cells. Epstein-Barr encoding region in situ hybridization also was positive. Rare cells stained positive for T-cell markers. CD20, BCL-6, and CD30 immunostains were negative, suggesting that these cells were not B or T cells, though terminally differentiated B cells also can lack these markers. Bone marrow biopsy showed a similar staining pattern to the skin with 10% atypical plasmacytoid cells. Computed tomography of the left leg showed an enlargement of the semimembranosus muscle with internal areas of high density and heterogeneous enhancement. The patient underwent decompression of the left peroneal nerve. Biopsy showed a staining pattern similar to the right skin nodule and bone marrow, consistent with lymphoma.

He was diagnosed with stage IV human immunodeficiency virus (HIV)-associated plasmablastic lymphoma (PBL) and received 6 cycles of R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) without vincristine with intrathecal methotrexate, followed by 3 cycles of DHAP (dexamethasone, high dose Ara C, cisplatin) with bortezomib and daratumumab after relapse. Ultimately, he underwent autologous stem cell transplantation and was alive 13 months after diagnosis.
Plasmablastic lymphoma is a rare subtype of non-Hodgkin lymphoma that most commonly arises in the oral cavity of individuals with HIV.1 In addition to HIV infection, PBL also is seen in patients with other causes of immunodeficiency such as iatrogenic immunosuppression following solid organ transplantation.1 The typical disease presentation is an expanding mass in the oral cavity; however, 34% (52/151) of reported cases arose at extraoral primary sites, with a minority of cases confined to cutaneous sites with no systemic involvement.2 Cutaneous PBL presentations may include flesh-colored or purple, grouped or solitary nodules; an erythematous infiltrated plaque; or purple-red ulcerated nodules. The lesions usually are asymptomatic and located on the arms and legs.3
On histologic examination, PBL is characterized by a diffuse monomorphic lymphoid infiltrate that sometimes invades the surrounding soft tissue.4-6 The neoplastic cells have eccentric round nucleoli. Plasmablastic lymphoma characteristically displays a high proliferation index with many mitotic figures and signs of apoptosis.4-6 Definitive diagnosis requires immunohistochemical staining. Typical B-cell antigens (CD20) as well as CD45 are negative, while plasma cell markers such as CD38 are positive. Other B- and T-cell markers usually are negative.5,7 The pathogenesis of PBL is thought to be related to Epstein-Barr virus or human herpesvirus 8 infection. In a series of PBL cases, Epstein-Barr virus and human herpesvirus 8 was positive in 75% (97/129) and 17% (13/75) of tested cases, respectively.1
The prognosis for PBL is poor, with a median overall survival of 15 months and a 3-year survival rate of 25% in HIV-infected individuals.8 However, cutaneous PBL without systemic involvement has a considerably better prognosis, with only 1 of 12 cases resulting in death.2,3,9 Treatment of PBL depends on the extent of the disease. Cutaneous PBL can be treated with surgery and adjuvant radiation.3 Chemotherapy is required for patients with multiple lesions or systemic involvement. Current treatment regimens are similar to those used for other aggressive lymphomas such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone).1 Transplant recipients should have their immunosuppression reduced, and HIV-infected patients should have their highly active antiretroviral therapy regimens optimized. Patients presenting with PBL without HIV should be tested for HIV, as PBL has previously been reported to be the presenting manifestation of HIV infection.10
The differential diagnosis for a rapidly expanding, vascular-appearing, red mass on the legs in an immunosuppressed individual includes abscess, malignancy, Kaposi sarcoma, Sweet syndrome, and tertiary syphilis.
Acknowledgment
We thank Sameera Husain, MD (New York, New York), for her assistance with histopathologic photographs and interpretation.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267.
- Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol. 2012;67:E202-E205.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420.
- Gaidano G, Cerri M, Capello D, et al. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622-628.
- Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809.
- Horna P, Hamill JR, Sokol L, et al. Primary cutaneous plasmablastic lymphoma in an immunocompetent patient. J Am Acad Dermatol. 2013;69:E274-E276.
- Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358-1361.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, et al. Plasmablastic lymphoma of the oral cavity: a rapidly progressive lymphoma associated with HIV infection. Lancet Infect Dis. 2008;8:261-267.
- Heiser D, Müller H, Kempf W, et al. Primary cutaneous plasmablastic lymphoma of the lower leg in an HIV-negative patient. J Am Acad Dermatol. 2012;67:E202-E205.
- Jambusaria A, Shafer D, Wu H, et al. Cutaneous plasmablastic lymphoma. J Am Acad Dermatol. 2008;58:676-678.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413-1420.
- Gaidano G, Cerri M, Capello D, et al. Molecular histogenesis of plasmablastic lymphoma of the oral cavity. Br J Haematol. 2002;119:622-628.
- Folk GS, Abbondanzo SL, Childers EL, et al. Plasmablastic lymphoma: a clinicopathologic correlation. Ann Diagn Pathol. 2006;10:8-12.
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-2330.
- Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008;83:804-809.
- Horna P, Hamill JR, Sokol L, et al. Primary cutaneous plasmablastic lymphoma in an immunocompetent patient. J Am Acad Dermatol. 2013;69:E274-E276.
- Desai RS, Vanaki SS, Puranik RS, et al. Plasmablastic lymphoma presenting as a gingival growth in a previously undiagnosed HIV-positive patient: a case report. J Oral Maxillofac Surg. 2007;65:1358-1361.
A 44-year-old man presented with numbness and a burning sensation of the left lateral leg and dorsal foot of 3 days' duration as well as a left foot drop of 1 day's duration. A painless red nodule on the right shin also developed over a 10-day period. He had been diagnosed with human immunodeficiency virus a year prior and reported compliance with antiretroviral therapy. There was a newly identified, well-demarcated, 6-cm, round, red-purple, flat-topped, nodular tumor with central depression on the right lateral shin. Ultrasonography of the nodule revealed a heterogeneous septate structure with increased vascularity. There was no regional or generalized lymphadenopathy. Laboratory values were notable for microcytic anemia. The white blood cell count was within reference range. Human immunodeficiency virus RNA viral load was elevated (3183 viral copies/mL [reference range, <20 viral copies/mL]). Two punch biopsies of the nodule were performed.
Erythema Gyratum Repens–like Eruption in Sézary Syndrome: Evidence for the Role of a Dermatophyte
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.
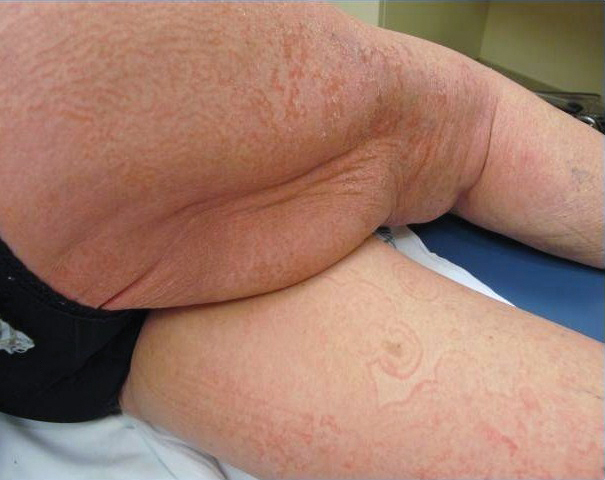
Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.
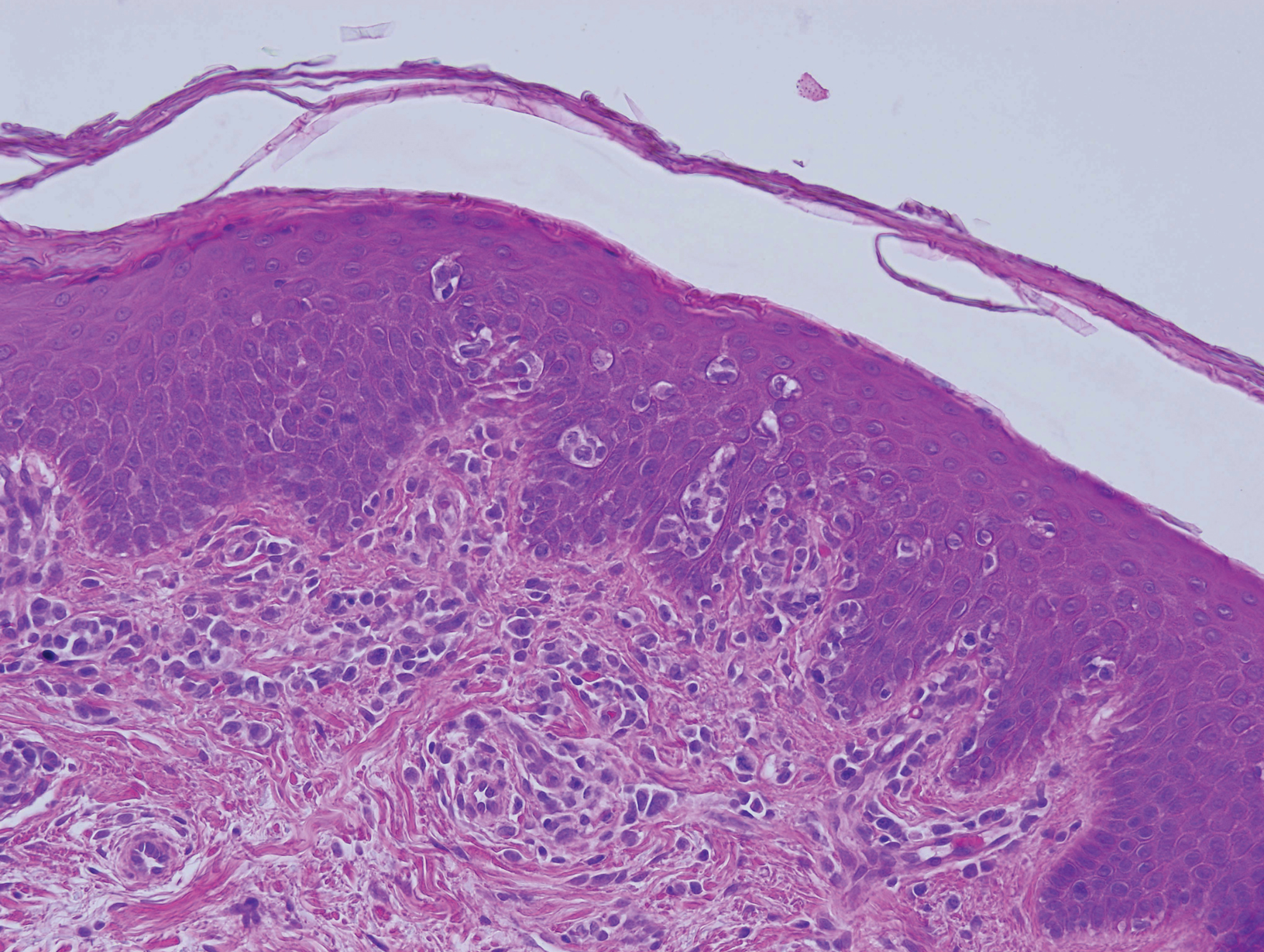
Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.
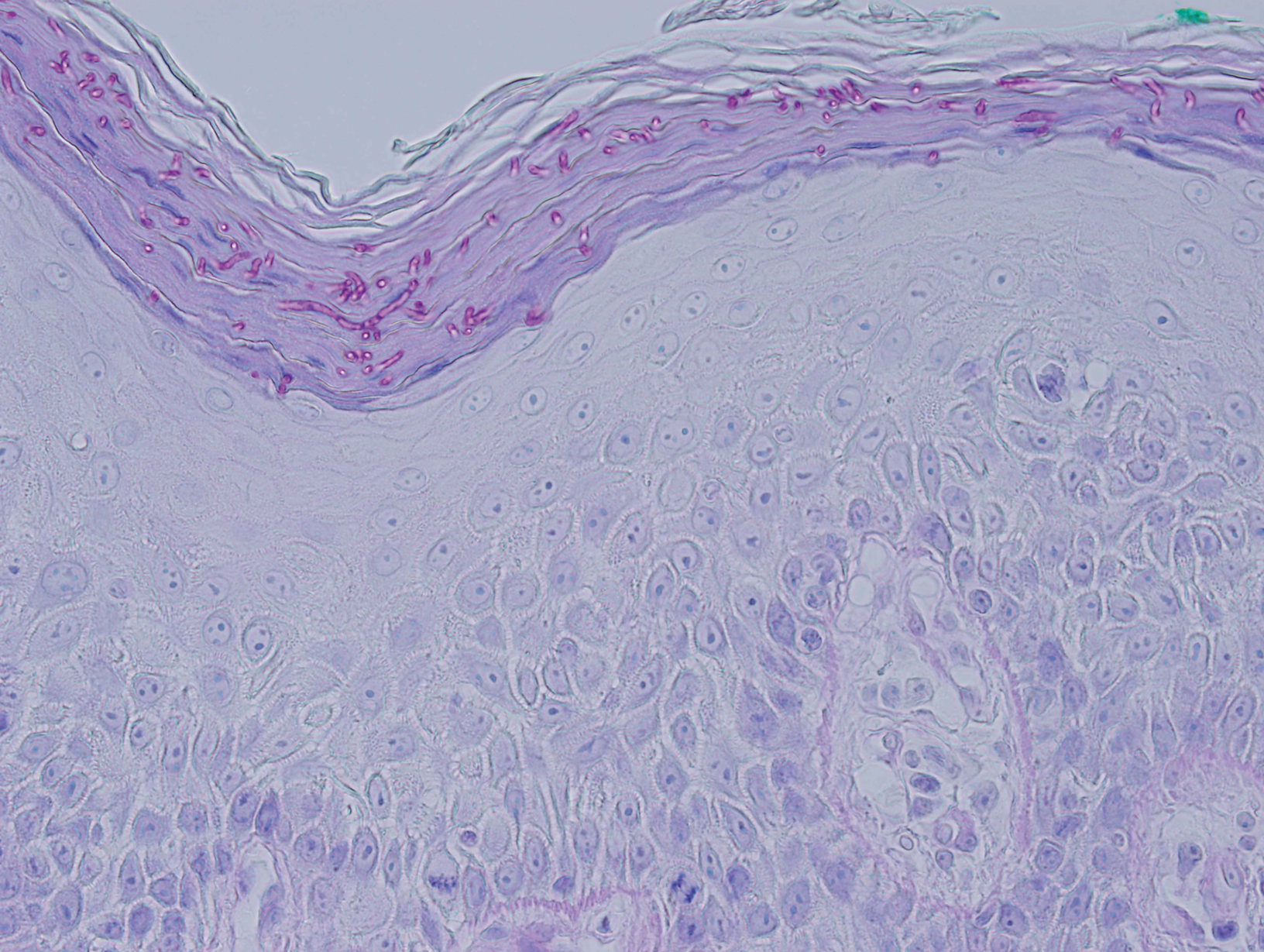
A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3
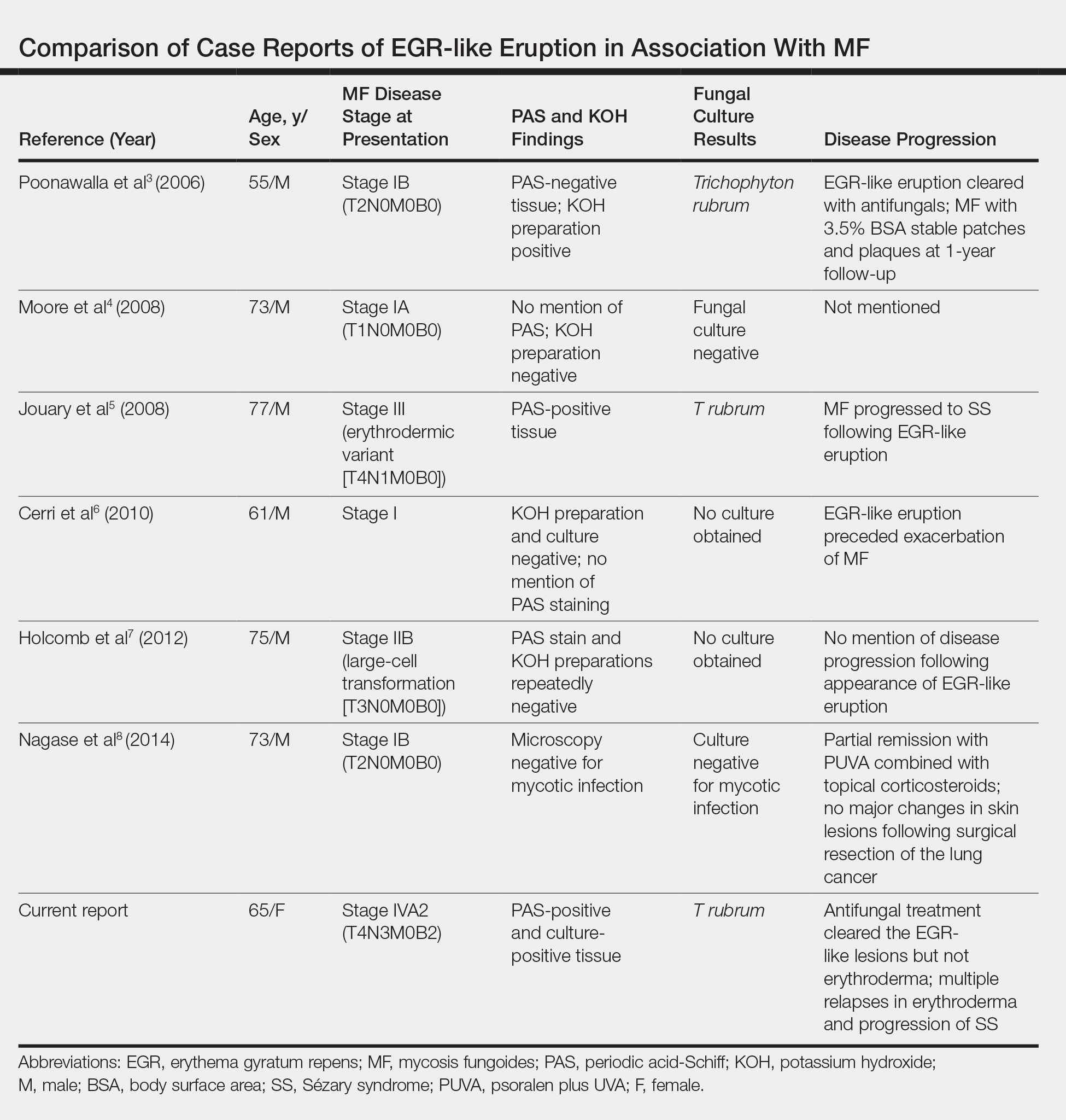
Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.

Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.

Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.

A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3

Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
Case Report
A 65-year-old woman presented with stage IVA2 mycosis fungoides (MF)(T4N3M0B2)/Sézary syndrome (SS). A peripheral blood count contained 6000 Sézary cells with cerebriform nuclei, a CD2+/−CD3+CD4+CD5+/−CD7+CD8−CD26−immunophenotype, and a highly abnormal CD4 to CD8 ratio (70:1). Positron emission tomography and computed tomography demonstrated hypermetabolic subcutaneous nodules in the base of the neck and generalized lymphadenopathy. Lymph node biopsy showed involvement by T-cell lymphoma and dominant T-cell receptor γ clonality by polymerase chain reaction.
On initial presentation to the Cutaneous Lymphoma Clinic at the University of Wisconsin-Madison, the patient was erythrodermic. She also was noted to have undulating wavy bands and concentric annular, ringlike, thin, erythematous plaques with trailing scale, giving a wood grain, zebra hide–like appearance involving the buttocks, abdomen, and lower extremities (Figure 1). Lesions were markedly pruritic and were advancing rapidly. A diagnosis of erythema gyratum repens (EGR)–like eruption was made.

Biopsy of an EGR-like area on the leg showed a superficial perivascular and somewhat lichenoid lymphoid infiltrate (Figure 2). Lymphocytes were lined up along the basal layer, occasionally forming nests within the epidermis. Nearly all mononuclear cells in the epidermis and dermis exhibited positive CD3 and CD4 staining, with only scattered CD8 cells. These features were compatible with cutaneous involvement in SS. A concurrent biopsy from diffusely erythrodermic forearm skin, which lacked EGR-like morphology, showed similar histopathologic and immunophenotypic features.

Periodic acid–Schiff (PAS) with diastase stain revealed numerous septate hyphae within the stratum corneum in both skin biopsy specimens (Figure 3). Fungal culture of EGR-like lesions was positive for a nonsporulating filamentous fungus, identified as Trichophyton rubrum by DNA sequencing.

A diagnosis of EGR-like eruption secondary to tinea corporis in SS was made. The possibility of tinea incognito also was considered to explain the presence of dermatophytes in the biopsy from skin that exhibited only erythroderma clinically; however, the patient did not have a history of corticosteroid use.
Interferon alfa-2b and methotrexate therapy was initiated. Additionally, oral terbinafine (250 mg/d) was initiated for 14 days, resulting in complete resolution of the EGR-like eruption; nevertheless, diffuse erythema remained. Subsequently, within 3 months of treatment, the cutaneous T-cell lymphoma (CTCL) improved with continued interferon alfa-2b and methotrexate. Erythroderma became minimal; the circulating Sézary cell count decreased by 50%. The patient ultimately had multiple relapses in erythroderma and progression of SS. Erythema gyratum repens–like lesions recurred on multiple occasions, with a temporary response to repeat courses of oral terbinafine.
Comment
Defining True EGR vs EGR-like Eruption
Sézary syndrome represents the leukemic stage of CTCL, which is defined by the triad of erythroderma; generalized lymphadenopathy; and neoplastic T cells in the skin, lymph nodes, and peripheral blood. It is well known that CTCL can mimic multiple benign and malignant dermatoses. One rare presentation of CTCL is an EGR-like eruption.
Erythema gyratum repens presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with a fine trailing edge of scale (wood grain pattern). The diagnosis is based on the characteristic clinical pattern of EGR and by ruling out other mimicking conditions with biopsy.1 Patients with the characteristic clinical pattern but with an alternate underlying dermatosis are described as having an EGR-like eruption rather than true EGR.
True EGR is most often but not always associated with underlying malignancy. Biopsy of true EGR eruptions show nonspecific histopathologic features, with perivascular superficial mononuclear dermatitis, occasional mild spongiosis, and focal parakeratosis; specific features of an alternate dermatosis are lacking.2 In addition to CTCL, EGR-like eruptions have been described in a number of diseases, including systemic lupus erythematosus, erythema annulare centrifugum, bullous dermatosis, erythrokeratodermia variabilis, urticarial vasculitis, leukocytoclastic vasculitis, and neutrophilic dermatoses.
Prior Reports of EGR-like Eruption in Association With MF
According to a PubMed search of articles indexed for MEDLINE using the terms erythema gyratum repens in mycosis fungoides, mycosis fungoides with tinea, and concentric wood grain erythema, there have been 6 other cases of an EGR-like eruption in association with MF (Table). Poonawalla et al3 first described an EGR-like eruption (utilizing the term tinea pseudoimbricata) in a 55-year-old man with stage IB MF (T2N0M0B0). The patient had a preceding history of tinea pedis and tinea corporis that preceded the diagnosis of MF. At the time of MF diagnosis, the patient presented with extensive concentric, gyrate, wood grain, annular lesions. His MF was resistant to topical mechlorethamine, psoralen plus UVA, and oral bexarotene. The body surface area involvement decreased from 60% to less than 1% after institution of oral and topical antifungal therapy. It was postulated that the widespread dermatophytosis that preceded the development of MF may have been the persistent antigen leading to his disease. Preceding the diagnosis of MF, skin scrapings were floridly positive for dermatophyte hyphae. Fungal cultures from the affected areas of skin grew T rubrum.3

Moore et al4 described an EGR-like eruption on the trunk of a 73-year-old man with stage IA MF (T1N0M0B0). Biopsy was consistent with MF, but no fungal organisms were seen. Potassium hydroxide preparation and fungal cultures of the lesions also were negative for organisms. The patient was successfully treated with topical betamethasone.4Jouary et al5 described an EGR-like eruption in a 77-year-old man with stage III erythrodermic MF (T4N1M0B0). Biopsy showed mycelia on PAS stain. Subsequent culture isolated T rubrum. Terbinafine (250 mg/d) and ketoconazole cream 2% daily were initiated and the patient’s EGR-like rash quickly cleared, while MF progressed to SS.5
Cerri et al6 later described a case of EGR-like eruption in a 61-year-old man with stage I MF and an EGR-like eruption. Microscopic examination of potassium hydroxide (KOH) preparations and fungal culture of the lesions failed to demonstrate mycotic infection. There was no mention of PAS stain of skin biopsy specimens. In this case, the authors mentioned that EGR-like lesions preceded exacerbation of MF and questioned the prognostic significance of the EGR-like eruption in relation to MF.6
Holcomb et al7 reported the next case of a 75-year-old man with stage IIB MF (T3N0M0B0) with CD25+ and CD30+ large cell transformation who presented with an EGR-like eruption. In this case, PAS stain and KOH preparations were repeatedly negative for mycotic infection. Disease progression was not mentioned following the appearance of the EGR-like eruption.7
Nagase et al8 most recently described a case of a 73-year-old Japanese man with stage IB (T2N0M0B0) CD4−CD8− MF and lung cancer who developed a cutaneous eruption mimicking EGR. Microscopy and culture excluded the presence of a mycotic infection. The patient achieved partial remission with photochemotherapy (psoralen plus UVA) combined with topical corticosteroids. No major changes in the patient’s skin lesions were noted following surgical resection of the lung cancer.8
Dermatophyte Infection
It is known that conventional tinea corporis can occur in the setting of CTCL. However, EGR-like eruptions in CTCL can be distinguished from standard tinea corporis by the classic morphology of EGR and clinical history of rapid migration of these characteristic lesions.
Tinea imbricata is known to have a clinical appearance that is similar to EGR, but the infection is caused by Tinea concentricum, which is limited to southwest Polynesia, Melanesia, Southeast Asia, India, and Central America. Although T rubrum was the dermatophyte isolated by Poonawalla et al,3 Jouary et al,5 and in our case, whether T rubrum infection in the setting of CTCL has any impact on prognosis needs further study.
Our case of an EGR-like eruption presented in a patient with SS and tinea corporis. Biopsy specimens showed CTCL and concomitant dermatophytic infection that was confirmed with PAS stain and identified as T rubrum. Interestingly, our patient’s EGR-like eruption cleared with oral terbinafine therapy, consistent with findings described by Poonawalla et al3 and Jouary et al5 in which treatment of the dermatophytic infection led to resolution of the EGR-like eruption, suggesting a causative role.
However, testing for dermatophytes was negative in the other reported cases of EGR-like eruptions in patients with MF, despite screening for the presence of fungal microorganisms using KOH preparation, PAS staining, or fungal culture, or a combination of these methods,3-8 which raises the question: Do the cases reported without dermatophytic infection represent false-negative test results, or can the distinct clinical appearance of EGR indeed be seen in patients with CTCL who lack superimposed dermatophytosis? In 3 prior reported cases of EGR-like eruptions in MF, the eruption was preceded by immunosuppressive therapy.5-7
Further investigation is needed to correlate the role of dermatophytic infection in EGR-like eruptions. Our case and the Jouary et al5 case reported dermatophyte-positive EGR-like eruptions in MF and SS detected with histopathologic analysis and PAS stain. This low-cost screening method should be considered in future cases. If the test result is dermatophyte positive, a 14-day course of oral terbinafine (250 mg/d) might induce resolution of the EGR-like eruption.
Conclusion
The role of dermatophyte-induced EGR or EGR-like eruptions in other settings also warrants further investigation to shed light on this poorly understood yet striking dermatologic condition. Our patient showed both MF and dermatophytes in skin biopsy results, regardless of whether those sites showed erythroderma or EGR-like features clinically. On 3 occasions, antifungal treatment cleared the EGR-like lesions and associated pruritus but not erythroderma. Therefore, it appears that the mere presence of dermatophytes was necessary but not sufficient to produce the EGR-like lesions observed in our case.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
- Rongioletti F, Fausti V, Parodi A. Erythema gyratum repens is not an obligate paraneoplastic disease: a systematic review of the literature and personal experience. J Eur Acad Dermatol Venereol. 2012;28:112-115.
- Albers SE, Fenske NA, Glass LF. Erythema gyratum repens: direct immunofluorescence microscopic findings. J Am Acad Dermatol. 1993;29:493-494.
- Poonawalla T, Chen W, Duvic M. Mycosis fungoides with tinea pseudoimbricata owing to Trichophyton rubrum infection. J Cutan Med Surg. 2006;10:52-56.
- Moore E, McFarlane R, Olerud J. Concentric wood grain erythema on the trunk. Arch Dermatol. 2008;144:673-678.
- Jouary T, Lalanne N, Stanislas S, et al. Erythema gyratum repens-like eruption in mycosis fungoides: is dermatophyte superinfection underdiagnosed in cutaneous T-cell lymphomas? J Eur Acad Dermatol Venereol. 2008;22:1276-1278.
- Cerri A, Vezzoli P, Serini SM, et al. Mycosis fungoides mimicking erythema gyratum repens: an additional variant? Eur J Dermatol. 2010;20:540-541.
- Holcomb M, Duvic M, Cutlan J. Erythema gyratum repens-like eruptions with large cell transformation in a patient with mycosis fungoides. Int J Dermatol. 2012;51:1231-1233.
- Nagase K, Shirai R, Okawa T, et al. CD4/CD8 double-negative mycosis fungoides mimicking erythema gyratum repens in a patient with underlying lung cancer. Acta Derm Venereol. 2014;94:89-90.
Practice Points
- Erythema gyratum repens (EGR) presents as rapidly advancing, erythematous, concentric bands that can be figurate, gyrate, or annular, with fine trailing scale.
- Although EGR typically is associated with underlying malignancy, it is not an obligate paraneoplastic syndrome. There are numerous cases that are not associated with underlying neoplasms.
- An EGR-like eruption may be observed in Sézary syndrome, and an overlying superficial dermatophyte infection may play a role.
Tick-borne disease has become a national issue
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.
There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”