User login
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).
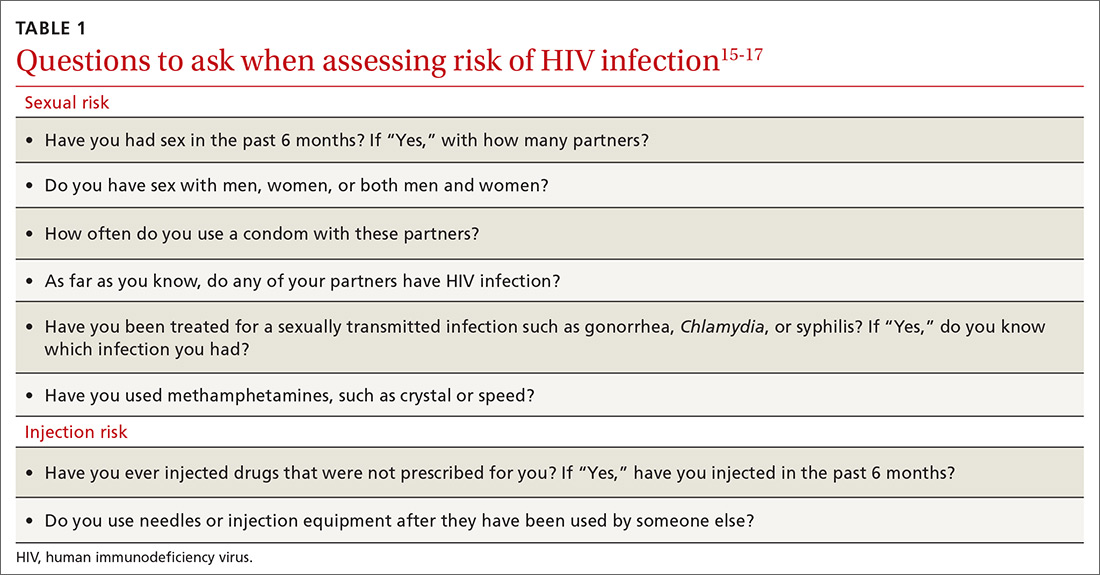
Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
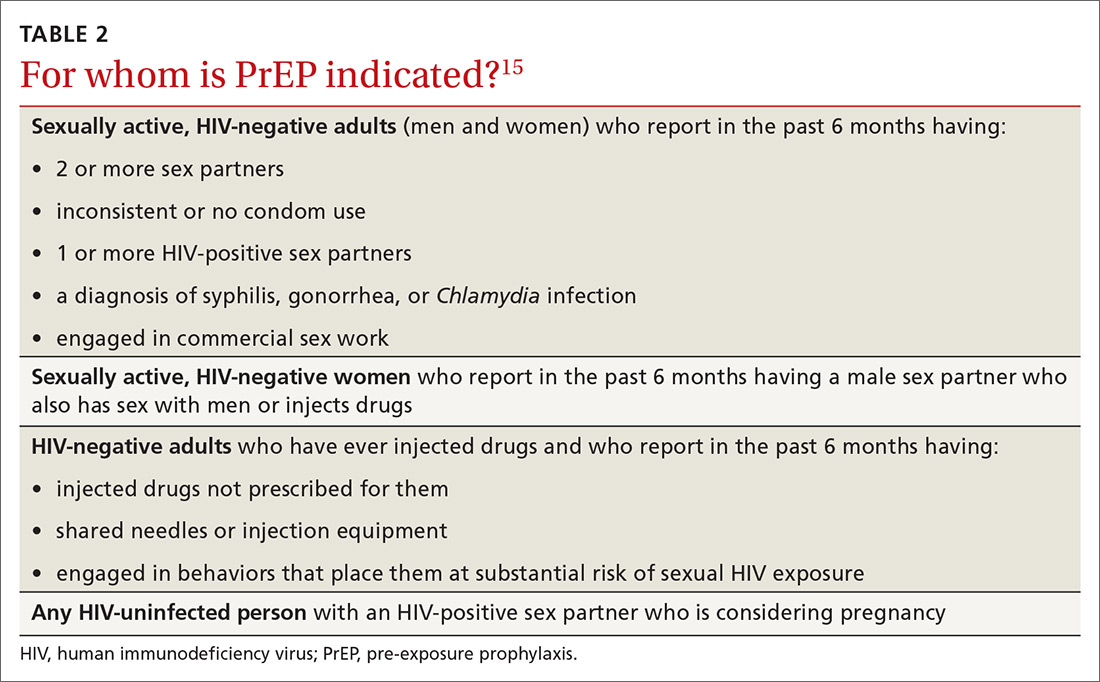
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15
What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
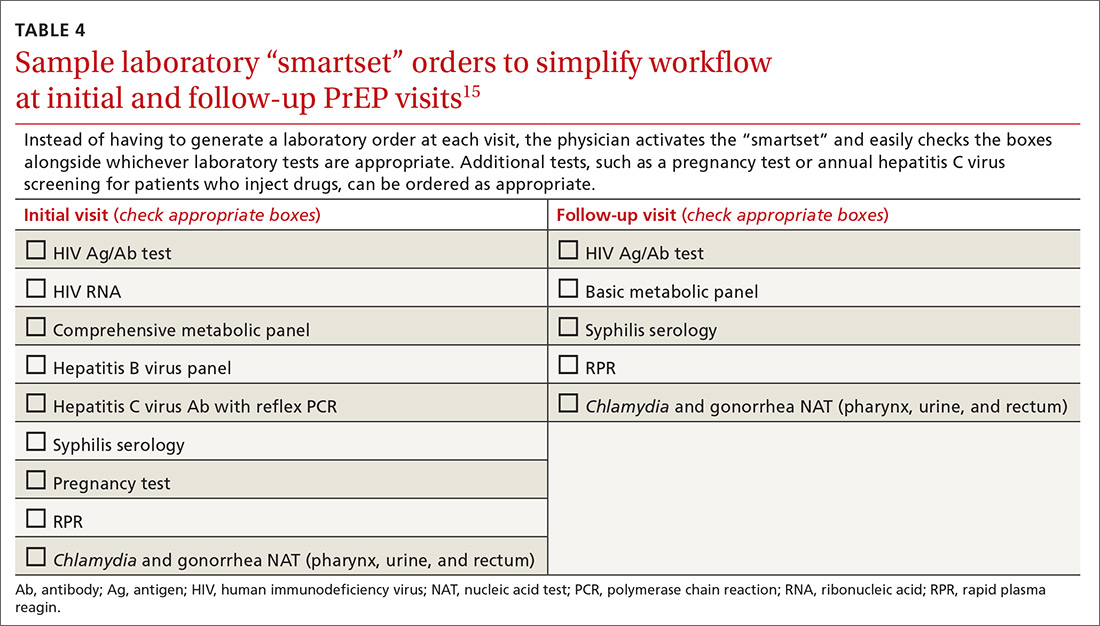
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.
Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
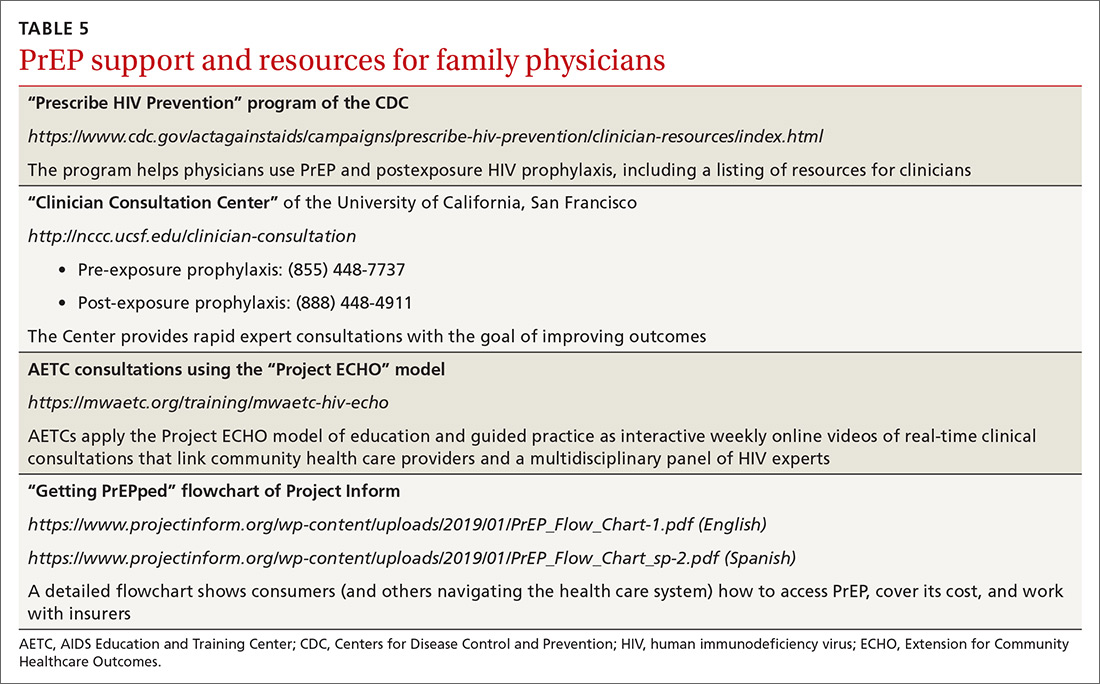
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.
Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
The 2012 US Food and Drug Administration (FDA) approval of daily emtricitabine plus tenofovir disoproxil fumarate as HIV pre-exposure prophylaxis (PrEP) re-energized the field of human immunodeficiency virus (HIV) prevention. In subsequent years, PrEP uptake has increased, particularly in people at high risk of HIV infection.
However, since 2012, progress in controlling the HIV epidemic has been uneven across communities and populations. For instance, in 2014, the southern United States accounted for an estimated 50% of infections, but PrEP uptake has remained low there, with only 1% of the estimated number of eligible people taking PrEP.1,2 Among African American men who have sex with men (MSM), it is predicted that 1 of every 2 will become infected in his lifetime; among Latino MSM, the prediction is 1 of every 5.3 The expanding opioid epidemic is further jeopardizing the progress made in reducing HIV infection among people who inject drugs.
A “test and treat” strategy is insufficient. Mathematical modeling suggests that “test and treat” without a higher level of coverage is insufficient to control the HIV epidemic.4 In the absence of an HIV vaccine, these models find that widespread uptake of PrEP among people at risk of HIV acquisition is needed—in combination with HIV treatment as prevention, condom promotion, and needle exchange—to realize the potential to end the HIV epidemic.4
A recent proposal by the US Department of Health and Human Services would establish an initiative to address the continuing HIV public health crisis, with a goal of reducing the numbers of incident HIV infections in the United States by 75% in 5 years and then by 90% in 10 years. That strategic initiative includes 4 “pillars” for preventing HIV acquisition—one of which is the use of PrEP by at-risk people.5
Although PrEP is often prescribed by HIV specialists and in sexually transmitted infection (STI) clinics, many patients seek PrEP from family physicians (and other primary care clinicians), who are now also being called on to identify patients in their practice at risk of HIV infection6 and to offer them PrEP. In this article, we provide an overview of PrEP and discuss how best to integrate PrEP into a family medicine practice.
Understanding PrEP and how it is used
PrEP is one of 2 related biomedical interventions to prevent HIV acquisition. Many clinicians are familiar with postexposure prophylaxis, a regimen of 3 anti-HIV medications given for 1 month to patients who are within 72 hours of a possible exposure. In contrast, PrEP is a once-daily, fixed-dose combination of 2 medications commonly used in the treatment of HIV infection: emtricitabine, 200 mg, and tenofovir disoproxil fumarate, 300 mg. This combination is the only FDA-approved regimen for daily use as PrEP in the United States.
PrEP is indicated for people whose ongoing sexual or drug injection behaviors put them at substantial risk of HIV infection, and should be taken daily regardless of the frequency of risk-taking behavior. Since 2010, several randomized placebo-controlled trials (RCTs) have reported that, when medication adherence is high (measured by drug levels in blood), PrEP can reduce new HIV infections by more than 90% in high-risk populations.7 In clinical practice, HIV infection is uncommon because of the effectiveness of daily PrEP; when infections have occurred, almost all have been in patients not taking the medications as prescribed.8
Continue to: Infection with HIV...
Infection with HIV in which viral mutations are associated with emtricitabine or tenofovir resistance is rare among the few people infected with HIV after starting PrEP.9 In RCTs, most drug resistance occurred among people who started PrEP when they were already HIV-positive (because they were screened with antibody-only HIV tests that did not detect recent infection).10
Other medications, routes of administration, and dosing schedules are being studied for safety and efficacy as PrEP for HIV infection.11,12
For whom should PrEP be prescribed? There are 2 ways to identify candidates for PrEP:
- Passive prescribing relies on patients self-identifying as being at risk of HIV infection and asking about PrEP. Many at-risk patients do not recognize their need for PrEP, however.13
- Active screening requires that physicians, or their staff, take a sexual history from all patients. However, reviewing detailed sexual histories with every patient in a busy practice can be overwhelming. One way to begin identifying patients for whom PrEP is appropriate is to commit to talking to subsets of potentially high-risk patients, such as MSM or transgender patients.6 Sexual orientation and gender identity are not direct risk factors; a nuanced sexual history is often needed to understand potential exposures. A diagnosis of syphilis or other bacterial STI is a marker of high risk of HIV acquisition.14
To help identify which of your patients might benefit from PrEP, the PrEP guidelines from the Centers for Disease Control and Prevention (CDC)15 and tools developed by other sources16,17 recommend several key screening questions about sexual behavior and substance abuse (TABLE 115-17).

Familiarity with PrEP and comfort taking a sexual history to screen for risk of HIV acquisition are essential first steps in prescribing PrEP under CDC guidelines.6,18 In primary care, female patients are routinely questioned to assess their need for contraception; similarly, screening questions to assess PrEP eligibility can be easily incorporated into practice.
Continue to: What are the indications for PrEP?
What are the indications for PrEP?
Patients in whom PrEP is indicated include sexually active adults and adolescents (> 35 kg)19 whose use of a condom is inconsistent or who have had multiple recent sex partners; those with a recent bacterial STI; and men or women with a sexual or injection partner known to be HIV-infected (TABLE 2).15

What steps should be taken before and after initiating PrEP?
Providing PrEP is a harm-reduction strategy similar to prescribing other common preventive medications, such as statins to reduce hyperlipidemia and prevent myocardial infarction; oral contraceptives to prevent unwanted pregnancy; and metformin to prevent complications of diabetes. There are a few screening criteria prior to initiating PrEP (TABLE 3)10:
- A patient starting PrEP should be (1) HIV-negative, ideally screened by a laboratory-based antigen–antibody (ie, fourth-generation) HIV test or HIV RNA test, and (2) without symptoms of acute HIV infection.20 (Note: Do not hold off PrEP and HIV testing until the patient has achieved a period of sexual abstinence.)
- A patient starting PrEP should have normal renal function and should not be taking contraindicated medications, such as long-term high-dose nonsteroidal anti-inflammatory agents.
- Hepatitis B virus (HBV) surface antigen, surface antibody, and core antibody should be tested because both emtricitabine and tenofovir are active against HBV. For a patient who has active HBV infection, particularly with cirrhosis, there is a theoretical concern that starting and stopping PrEP can lead to flares of HBV infection. Patients who are not HBV-immune should be vaccinated.
- Baseline hepatitis C virus testing is recommended for patients who inject drugs, MSM, or those who were born between 1945 and 1965; annual hepatitis C virus testing is recommended for patients who inject drugs.15
When it has been determined that a patient is eligible for PrEP, a prescription is written for no longer than 90 days to ensure regular monitoring for HIV infection, STIs, and renal function.
Adherence counseling is a key component of PrEP delivery—as it is with oral contraception, antihypertensive medical therapy, and other medications. As noted, HIV acquisition in PrEP users is most often reported in patients with poor adherence,8 especially among adolescents.21 PrEP is part of comprehensive sexual health care, and safer sex behaviors, such as condom use, should be encouraged to reduce the risk of acquiring other STIs. Condom use should not, however, be a requirement for continuing to receive PrEP.

Is PrEP safe?
Although PrEP might be new to many family physicians and their patients, trials and observational studies have repeatedly shown that for people without HIV infection, taking daily emtricitabine and tenofovir for prevention of HIV infection is safe. No clinically significant renal, bone, or other toxicity has been reported, although there is concern about potential toxicity after decades of use.22,23 A recent narrative review from the David Geffen School of Medicine at the University of California Los Angeles compared safety findings from 5 major studies on PrEP with 2 major studies on aspirin safety and found that PrEP is as safe as aspirin, although the authors cautioned that more study on long-term use is needed.24
Continue to: What to tell patients
What to tell patients. Tell patients that within the first weeks of starting PrEP, they might experience a start-up syndrome that typically manifests as gastrointestinal symptoms, headache, and fatigue. These symptoms usually resolve without the need to discontinue the medications.25
Any other concerns about PrEP?
When PrEP was first approved by the FDA, many physicians raised concern about the possibility that PrEP use would lead to increased community-level HIV drug resistance and that behavioral disinhibition might diminish the benefit of PrEP and lead to rampant STIs.26 To date, these fears have not been borne out.
Acquired drug resistance, which happens after a person becomes HIV-positive, is a real concern, particularly among people who are screened with antibody-only HIV tests that cannot detect HIV in the so-called window period and who then start PrEP during acute HIV infection. If a person is truly HIV-negative when he (she) starts PrEP, the risk of either acquired or transmitted HIV drug resistance is low and is far outweighed by the preventive benefit of PrEP.27
Similarly, there is a suggestion that syphilis infection is increasing among HIV-negative MSM due to decreased HIV-related stigma and increased mixing between HIV-negative and HIV-positive people. The evidence that PrEP has led to an increase in STI rates28 is mixed, however, and is confounded by temporal increases in STI rates and increased detection of asymptomatic STIs among people on PrEP as a result of regular screening.29
Who pays for PrEP?
The cost of PrEP medications and associated clinical care is covered by nearly all private, employer, and public health insurance. Prior authorization might be required to ensure that testing has excluded HIV infection before prescribing and then refilling prescriptions.
Continue to: For patients who have health insurance...
For patients who have health insurance, assistance with copays or coinsurance is available through the producer of PrEP (Gilead Sciences, Inc.) and other national foundations. Many people who seek PrEP might be eligible for Medicaid if they are otherwise uninsured. Other low-income and uninsured people, including those who are not legal residents or US citizens, usually qualify for the PrEP medication assistance program; the application for this benefit must be completed by the physician.
A billing guide on PrEP for physicians is available to assist with International Classification of Disease (ICD)-10 coding.30,31 If a patient has difficulty with laboratory copays, free HIV and STI testing might be available at local STI clinics and acquired immunodeficiency syndrome (AIDS) service organizations.
Providing PrEP within a primary care setting
The unmet need for PrEP highlights how important it is for family medicine and other primary care practices to incorporate HIV prevention into their suite of services.32
Patients are most likely to experience adverse effects during the first month of taking PrEP—the same period in which they are establishing their pattern of adherence. It might be helpful to check in with patients at the end of the first month to assess their symptoms and adherence. After this phase, quarterly follow-up is simple, with routine lab monitoring and check-in about continued risk of HIV and adherence challenges (TABLE 310).
At our local Ryan White HIV/AIDS Program-funded HIV clinic, which also provides PrEP, computer-ordering checklists (so-called smartsets) for the PrEP initial visit and follow-up visits are programmed into the records system (TABLE 415). Other clinics also have developed templates for PrEP visit notes. Adherence monitoring, behavioral counseling, and other preventive services can be integrated into the regular paper- or computer-based intake survey, so that conversations are focused on areas of need.6 Family physicians in large practices can develop in-office protocols, based on CDC PrEP guidelines, to assign roles (eg, paperwork assistant, behavioral counselor, prescriber) to staff members.

Continue to: Partnering with HIV specialists, organizations, and pharmacists
Partnering with HIV specialists, organizations, and pharmacists
Family physicians who are unsure about initiating PrEP might consider referring complex patients, such as those with unclear eligibility or active HBV infection, to an infectious disease or HIV specialist or clinic for the initial evaluation. Once a patient has been started on PrEP, quarterly monitoring is simple and can be easily completed in a family medicine practice.
Depending on location and available services, pharmacists and local HIV and AIDS organizations might provide behavioral and adherence counseling and repeat testing during follow-up appointments. In our experience, working with a primary pharmacy that is familiar with patient assistance programs and prior authorization requirements facilitates smoother prescribing. The result? Lower cost to patients because of knowledge of copays and other assistance programs and willingness to use these secondary payers.
Bringing PrEP into the practice is workable
Providing PrEP is well within your scope of practice as a family physician. To assist you in making PrEP an effective component of your practice, we provide a list of sources of PrEP support in TABLE 5.

Because some physicians might still be reluctant to prescribe PrEP for patients who maintain their risk of HIV acquisition, we recommend that you think of PrEP as you do about statins. Discussing diet and exercise as a means of reducing cardiovascular events for every patient with hyperlipidemia is often insufficient; most physicians therefore also prescribe medication for patients who cannot change behaviors sufficiently to modify their cardiovascular risk factors. Similarly, you now have a preventive for HIV—a costly, lifelong infection—that is as cost-effective as statins are.26,33
CORRESPONDENCE
Joanne D. Stekler, MD, MPH, Box 359931, Harborview Medical Center, 325 9th Avenue, Seattle, WA 98104; [email protected].
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
1. Centers for Disease Control and Prevention. CDC Fact Sheet. HIV incidence: estimated annual infections in the U.S., 2010-2016. Route. February 2019. www.cdc.gov/nchhstp/newsroom/docs/factsheets/hiv-incidence-fact-sheet_508.pdf. Accessed May 23, 2019.
2. Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28:841-849.
3. Hess KL, Hu X, Lansky A, et al. Lifetime risk of a diagnosis of HIV in the United States. Ann Epidemiol. 2017;27:238-243.
4. Nah K, Nishiura H, Tsuchiya N, et al. Test-and-treat approach to HIV/AIDS: a primer for mathematical modeling. Theor Biol Med Model. 2017;14:16.
5. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845.
6. Moyer VA, US Preventive Services Task Force. Screening for HIV: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
7. Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
8. Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399-410.
9. Lehman DA, Baeten JM, McCoy CO, et al; Partners PrEP Study Team. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;211:1211-1218.
10. Stekler JD, Ure G, O'Neal JD, et al. Performance of Determine Combo and other point-of-care tests among Seattle MSM. J Clin Virol. 2016;76:8-13.
11. Hare CB, Coll J, Ruane P, et al. The Phase 3 Discover Study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2019; Seattle, WA.
12. Andrews CD, Bernard LS, Poon AY, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. AIDS. 2017;31:461-467.
13. Biello KB, Edeza A, Montgomery MC, et al. Risk perception and interest in HIV pre-exposure prophylaxis among men who have sex with men with rectal gonorrhea and Chlamydia infection. Arch Sex Behav. 2019;48:1185-1190.
14. Menza TW, Hughes JP, Celum CL, et al. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36:547-555.
15. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States--2017 update: a clinical practice guideline. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed May 23, 2019.
16. Smith DK, Pan Y, Rose CE, et al. A brief screening tool to assess the risk of contracting HIV infection among active injection drug users. J Addict Med. 2015;9:226-232.
17. Smith DK, Pals SL, Herbst JH, et al. Development of a clinical screening index predictive of incident HIV infection among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2012;60:421-427.
18. Oldenburg CE, Perez-Brumer AG, Hatzenbuehler ML, et al. State-level structural sexual stigma and HIV prevention in a national online sample of HIV-uninfected MSM in the United States. AIDS. 2015;29:837-845.
19. Blackwell CW. Preventing HIV infection in high-risk adolescents using preexposure prophylaxis (PrEP). J Assoc Nurses AIDS Care. 2018;29:770-774.
20. Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257-264.
21. Hosek SG, Rudy B, Landovitz R, et al; Adolescent Trials Network (ATN) for HIVAIDS Interventions. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Defic Syndr. 2017;74:21-29.
22. Mulligan K, Glidden DV, Anderson PL, et al; Preexposure Prophylaxis Initiative Study Team. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
23. Mugwanya KK, Baeten J, Celum C, et al; Partners PrEP Study Team. Low risk of proximal tubular dysfunction associated with emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis in men and women. J Infect Dis. 2016;214:1050-1057.
24. Kojima N, Klausner JD. Is emtricitabine-tenofovir disoproxil fumarate pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection safer than aspirin? Open Forum Infect Dis. 2016;6:ofv221.
25. Glidden DV, Amico KR, Liu AY, et al. Symptoms, side effects and adherence in the iPrex open-label extension. Clin Infect Dis. 2016;62:1172-1177.
26. Chen A, Dowdy DW. Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: risk calculators for real-world decision-making. PLoS One. 2014;9:e108742.
27. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30:1973-1983.
28. Nguyen VK, Greenwald ZR, Trottier H, et al. Incidence of sexually transmitted infections before and after preexposure prophylaxis for HIV. AIDS. 2018;32:523-530.
29. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67:676-686.
30. Centers for Disease Control and Prevention. Paying for PrEP. December 2015. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-paying-for-prep.pdf. Accessed May 23, 2019.
31. NASTAD. Billing coding guide for HIV prevention: PrEP, screening, and linkage services. Updated July 17, 2018. www.nastad.org/resource/billing-coding-guide-hiv-prevention. Accessed May 23, 2019.
32. Pinto RM, Berringer KR, Melendez R, et al. Improving PrEP implementation through multilevel interventions: a synthesis of the literature. AIDS Behav. 2018;22:3681-3691.
33. Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142-150.
PRACTICE RECOMMENDATIONS
› Actively screen and identify HIV-negative patients who are a candidate for pre-exposure prophylaxis (PrEP); commit to talking to the most easily identifiable subsets of these patients, such as men who have sex with men and transgender patients. B
› Recognize that PrEP is indicated for patients who: are sexually active with inconsistent condom use and multiple recent sex partners; have recently been given a diagnosis of a sexually transmitted infection; or have a sexual or injection partner known to be HIV-infected. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
