User login
A warning song to keep our children safe
Pay heed to “The House of the Rising Sun”
“There is a house in New Orleans. They call the Rising Sun. And it’s been the ruin of many a poor boy. And, God, I know I’m one.”
The 1960s rock band the Animals will tell you a tale to convince you to get vaccinated. Don’t believe me? Follow along.
The first hints of the song “House of the Rising Sun” rolled out of the hills of Appalachia.
Somewhere in the Golden Triangle, far away from New Orleans, where Virginia, Kentucky, and Tennessee rise in quiet desolation, a warning song about a tailor and a drunk emerged. Sometime around the Civil War, a hint of a tune began. Over the next century, it evolved, until it became cemented in rock culture 50 years ago by The Animals, existing as the version played most commonly today.
In the mid-19th century, medicine shows rambled through the South, stopping in places like Noetown or Daisy. The small towns would empty out for the day to see the entertainers, singers, and jugglers perform. Hundreds gathered in the hot summer day, the entertainment solely a pretext for the traveling doctors to sell their wares, the snake oil, and cure-alls, as well as various patent medicines.
These were isolated towns, with no deliveries, few visitors, and the railroad yet to arrive. Frequently, the only news from outside came from these caravans of entertainers and con men who swept into town. They were like Professor Marvel from The Wizard of Oz, or a current-day Dr. Oz, luring the crowd with false advertising, selling colored water, and then disappearing before you realized you were duped. Today, traveling doctors of the same ilk convince parents to not vaccinate their children, tell them to visit stem cell centers that claim false cures, and offer them a shiny object with one hand while taking their cash with the other.
Yet, there was a positive development in the wake of these patent medicine shows: the entertainment lingered. New songs traveled the same journeys as these medicine shows – new earworms that would then be warbled in the local bars, while doing chores around the barn, or simply during walks on the Appalachian trails.
In 1937, Alan Lomax arrived in Noetown, Ky., with a microphone and an acetate record and recorded the voice of 16-year-old Georgia Turner singing “House of the Rising Sun.” She didn’t know where she heard that song, but most likely picked it up at the medicine show.
One of those singers was Clarence Ashley, who would croon about the Rising Sun Blues. He sang with Doc Cloud and Doc Hauer, who offered tonics for whatever ailed you. Perhaps Georgia Turner heard the song in the early 1900s as well. Her 1937 version contains the lyrics most closely related to the Animals’ tune.
Lomax spent the 1940s gathering songs around the Appalachian South. He put these songs into a songbook and spread them throughout the country. He would also return to New York City and gather in a room with legendary folk singers. They would hear these new lyrics, new sounds, and make them their own.
In that room would be Lead Belly, Pete Seeger, Woody Guthrie, and Josh White, the fathers of folk music. The music Lomax pulled out of the mountains in small towns would become new again in the guitars and harmonicas of the Greenwich Village singers and musicians. Pete Seeger performed with the Weavers, named because they would weave songs from the past into new versions.
“House of the Rising Sun” was woven into the folk music landscape, evolving and growing. Josh White is credited with changing the song from a major key into the minor key we know today. Bob Dylan sang a version. And then in 1964, Eric Burdon and The Animals released their version, which became the standard. An arpeggio guitar opening, the rhythm sped up, a louder sound, and that minor key provides an emotional wallop for this warning song.
Numerous covers followed, including a beautiful version of “Amazing Grace”, sung to the tune of “House of the Rising Sun” by the Blind Boys of Alabama.
The song endures for its melody as well as for its lyrics. This was a warning song, a universal song, “not to do what I have done.” The small towns in Kentucky may have heard of the sinful ways of New Orleans and would spread the message with these songs to avoid the brothels, the drink, and the broken marriages that would reverberate with visits to the Crescent City.
“House of the Rising Sun” is one of the most covered songs, traveling wide and far, no longer with the need for a medicine show. It was a pivotal moment in rock ‘n roll, turning folk music into rock music. The Animals became huge because of this song, and their version became the standard on which all subsequent covers based their version. It made Bob Dylan’s older version seem quaint.
The song has been in my head for a while now. My wife is hoping writing about it will keep it from being played in our household any more. There are various reasons it has been resonating with me, including the following:
- It traces the origins of folk music and the importance of people like Lomax and Guthrie to collect and save Americana.
- The magic of musical evolution – a reminder of how art is built on the work of those who came before, each version with its unique personality.
- The release of “House of the Rising Sun” was a seminal, transformative moment when folk became rock music.
- The lasting power of warning songs.
- The hucksters that enabled this song to be kept alive.
That last one has really stuck with me. The medicine shows are an important part of American history. For instance, Coca-Cola started as one of those patent medicines; it was one of the many concoctions of the Atlanta pharmacist John Stith Pemberton, sold to treat all that ails us. Dr. Pepper, too, was a medicine in a sugary bottle – another that often contained alcohol or cocaine. Society wants a cure-all, and the marketing and selling done during these medicine shows offered placebos.
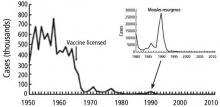
The hucksters exist in various forms today, selling detoxifications, magic diet cures, psychic powers of healing, or convincing parents that their kids don’t need vaccines. We need a warning song that goes viral to keep our children safe. We are blessed to be in a world without smallpox, almost rid of polio, and we have the knowledge and opportunity to rid the world of other preventable illnesses. Measles was declared eliminated in the United States in 2000; now, outbreaks emerge in every news cycle.
The CDC admits they have not been targeting misinformation well. How can we spread the science, the truth, the message faster than the lies? Better marketing? The answer may be through stories and narratives and song, with the backing of good science. “House of the Rising Sun” is a warning song. Maybe we need more. We need that deep history, that long trail to remind us of the world before vaccines, when everyone knew someone, either in their own household or next door, who succumbed to one of the childhood illnesses.
Let the “House of the Rising Sun” play on. Create a new version, and let that message reverberate, too.
Tell your children; they need to be vaccinated.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
Pay heed to “The House of the Rising Sun”
Pay heed to “The House of the Rising Sun”
“There is a house in New Orleans. They call the Rising Sun. And it’s been the ruin of many a poor boy. And, God, I know I’m one.”
The 1960s rock band the Animals will tell you a tale to convince you to get vaccinated. Don’t believe me? Follow along.
The first hints of the song “House of the Rising Sun” rolled out of the hills of Appalachia.
Somewhere in the Golden Triangle, far away from New Orleans, where Virginia, Kentucky, and Tennessee rise in quiet desolation, a warning song about a tailor and a drunk emerged. Sometime around the Civil War, a hint of a tune began. Over the next century, it evolved, until it became cemented in rock culture 50 years ago by The Animals, existing as the version played most commonly today.
In the mid-19th century, medicine shows rambled through the South, stopping in places like Noetown or Daisy. The small towns would empty out for the day to see the entertainers, singers, and jugglers perform. Hundreds gathered in the hot summer day, the entertainment solely a pretext for the traveling doctors to sell their wares, the snake oil, and cure-alls, as well as various patent medicines.
These were isolated towns, with no deliveries, few visitors, and the railroad yet to arrive. Frequently, the only news from outside came from these caravans of entertainers and con men who swept into town. They were like Professor Marvel from The Wizard of Oz, or a current-day Dr. Oz, luring the crowd with false advertising, selling colored water, and then disappearing before you realized you were duped. Today, traveling doctors of the same ilk convince parents to not vaccinate their children, tell them to visit stem cell centers that claim false cures, and offer them a shiny object with one hand while taking their cash with the other.
Yet, there was a positive development in the wake of these patent medicine shows: the entertainment lingered. New songs traveled the same journeys as these medicine shows – new earworms that would then be warbled in the local bars, while doing chores around the barn, or simply during walks on the Appalachian trails.
In 1937, Alan Lomax arrived in Noetown, Ky., with a microphone and an acetate record and recorded the voice of 16-year-old Georgia Turner singing “House of the Rising Sun.” She didn’t know where she heard that song, but most likely picked it up at the medicine show.
One of those singers was Clarence Ashley, who would croon about the Rising Sun Blues. He sang with Doc Cloud and Doc Hauer, who offered tonics for whatever ailed you. Perhaps Georgia Turner heard the song in the early 1900s as well. Her 1937 version contains the lyrics most closely related to the Animals’ tune.
Lomax spent the 1940s gathering songs around the Appalachian South. He put these songs into a songbook and spread them throughout the country. He would also return to New York City and gather in a room with legendary folk singers. They would hear these new lyrics, new sounds, and make them their own.
In that room would be Lead Belly, Pete Seeger, Woody Guthrie, and Josh White, the fathers of folk music. The music Lomax pulled out of the mountains in small towns would become new again in the guitars and harmonicas of the Greenwich Village singers and musicians. Pete Seeger performed with the Weavers, named because they would weave songs from the past into new versions.
“House of the Rising Sun” was woven into the folk music landscape, evolving and growing. Josh White is credited with changing the song from a major key into the minor key we know today. Bob Dylan sang a version. And then in 1964, Eric Burdon and The Animals released their version, which became the standard. An arpeggio guitar opening, the rhythm sped up, a louder sound, and that minor key provides an emotional wallop for this warning song.
Numerous covers followed, including a beautiful version of “Amazing Grace”, sung to the tune of “House of the Rising Sun” by the Blind Boys of Alabama.
The song endures for its melody as well as for its lyrics. This was a warning song, a universal song, “not to do what I have done.” The small towns in Kentucky may have heard of the sinful ways of New Orleans and would spread the message with these songs to avoid the brothels, the drink, and the broken marriages that would reverberate with visits to the Crescent City.
“House of the Rising Sun” is one of the most covered songs, traveling wide and far, no longer with the need for a medicine show. It was a pivotal moment in rock ‘n roll, turning folk music into rock music. The Animals became huge because of this song, and their version became the standard on which all subsequent covers based their version. It made Bob Dylan’s older version seem quaint.
The song has been in my head for a while now. My wife is hoping writing about it will keep it from being played in our household any more. There are various reasons it has been resonating with me, including the following:
- It traces the origins of folk music and the importance of people like Lomax and Guthrie to collect and save Americana.
- The magic of musical evolution – a reminder of how art is built on the work of those who came before, each version with its unique personality.
- The release of “House of the Rising Sun” was a seminal, transformative moment when folk became rock music.
- The lasting power of warning songs.
- The hucksters that enabled this song to be kept alive.
That last one has really stuck with me. The medicine shows are an important part of American history. For instance, Coca-Cola started as one of those patent medicines; it was one of the many concoctions of the Atlanta pharmacist John Stith Pemberton, sold to treat all that ails us. Dr. Pepper, too, was a medicine in a sugary bottle – another that often contained alcohol or cocaine. Society wants a cure-all, and the marketing and selling done during these medicine shows offered placebos.
The hucksters exist in various forms today, selling detoxifications, magic diet cures, psychic powers of healing, or convincing parents that their kids don’t need vaccines. We need a warning song that goes viral to keep our children safe. We are blessed to be in a world without smallpox, almost rid of polio, and we have the knowledge and opportunity to rid the world of other preventable illnesses. Measles was declared eliminated in the United States in 2000; now, outbreaks emerge in every news cycle.
The CDC admits they have not been targeting misinformation well. How can we spread the science, the truth, the message faster than the lies? Better marketing? The answer may be through stories and narratives and song, with the backing of good science. “House of the Rising Sun” is a warning song. Maybe we need more. We need that deep history, that long trail to remind us of the world before vaccines, when everyone knew someone, either in their own household or next door, who succumbed to one of the childhood illnesses.
Let the “House of the Rising Sun” play on. Create a new version, and let that message reverberate, too.
Tell your children; they need to be vaccinated.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
“There is a house in New Orleans. They call the Rising Sun. And it’s been the ruin of many a poor boy. And, God, I know I’m one.”
The 1960s rock band the Animals will tell you a tale to convince you to get vaccinated. Don’t believe me? Follow along.
The first hints of the song “House of the Rising Sun” rolled out of the hills of Appalachia.
Somewhere in the Golden Triangle, far away from New Orleans, where Virginia, Kentucky, and Tennessee rise in quiet desolation, a warning song about a tailor and a drunk emerged. Sometime around the Civil War, a hint of a tune began. Over the next century, it evolved, until it became cemented in rock culture 50 years ago by The Animals, existing as the version played most commonly today.
In the mid-19th century, medicine shows rambled through the South, stopping in places like Noetown or Daisy. The small towns would empty out for the day to see the entertainers, singers, and jugglers perform. Hundreds gathered in the hot summer day, the entertainment solely a pretext for the traveling doctors to sell their wares, the snake oil, and cure-alls, as well as various patent medicines.
These were isolated towns, with no deliveries, few visitors, and the railroad yet to arrive. Frequently, the only news from outside came from these caravans of entertainers and con men who swept into town. They were like Professor Marvel from The Wizard of Oz, or a current-day Dr. Oz, luring the crowd with false advertising, selling colored water, and then disappearing before you realized you were duped. Today, traveling doctors of the same ilk convince parents to not vaccinate their children, tell them to visit stem cell centers that claim false cures, and offer them a shiny object with one hand while taking their cash with the other.
Yet, there was a positive development in the wake of these patent medicine shows: the entertainment lingered. New songs traveled the same journeys as these medicine shows – new earworms that would then be warbled in the local bars, while doing chores around the barn, or simply during walks on the Appalachian trails.
In 1937, Alan Lomax arrived in Noetown, Ky., with a microphone and an acetate record and recorded the voice of 16-year-old Georgia Turner singing “House of the Rising Sun.” She didn’t know where she heard that song, but most likely picked it up at the medicine show.
One of those singers was Clarence Ashley, who would croon about the Rising Sun Blues. He sang with Doc Cloud and Doc Hauer, who offered tonics for whatever ailed you. Perhaps Georgia Turner heard the song in the early 1900s as well. Her 1937 version contains the lyrics most closely related to the Animals’ tune.
Lomax spent the 1940s gathering songs around the Appalachian South. He put these songs into a songbook and spread them throughout the country. He would also return to New York City and gather in a room with legendary folk singers. They would hear these new lyrics, new sounds, and make them their own.
In that room would be Lead Belly, Pete Seeger, Woody Guthrie, and Josh White, the fathers of folk music. The music Lomax pulled out of the mountains in small towns would become new again in the guitars and harmonicas of the Greenwich Village singers and musicians. Pete Seeger performed with the Weavers, named because they would weave songs from the past into new versions.
“House of the Rising Sun” was woven into the folk music landscape, evolving and growing. Josh White is credited with changing the song from a major key into the minor key we know today. Bob Dylan sang a version. And then in 1964, Eric Burdon and The Animals released their version, which became the standard. An arpeggio guitar opening, the rhythm sped up, a louder sound, and that minor key provides an emotional wallop for this warning song.
Numerous covers followed, including a beautiful version of “Amazing Grace”, sung to the tune of “House of the Rising Sun” by the Blind Boys of Alabama.
The song endures for its melody as well as for its lyrics. This was a warning song, a universal song, “not to do what I have done.” The small towns in Kentucky may have heard of the sinful ways of New Orleans and would spread the message with these songs to avoid the brothels, the drink, and the broken marriages that would reverberate with visits to the Crescent City.
“House of the Rising Sun” is one of the most covered songs, traveling wide and far, no longer with the need for a medicine show. It was a pivotal moment in rock ‘n roll, turning folk music into rock music. The Animals became huge because of this song, and their version became the standard on which all subsequent covers based their version. It made Bob Dylan’s older version seem quaint.
The song has been in my head for a while now. My wife is hoping writing about it will keep it from being played in our household any more. There are various reasons it has been resonating with me, including the following:
- It traces the origins of folk music and the importance of people like Lomax and Guthrie to collect and save Americana.
- The magic of musical evolution – a reminder of how art is built on the work of those who came before, each version with its unique personality.
- The release of “House of the Rising Sun” was a seminal, transformative moment when folk became rock music.
- The lasting power of warning songs.
- The hucksters that enabled this song to be kept alive.
That last one has really stuck with me. The medicine shows are an important part of American history. For instance, Coca-Cola started as one of those patent medicines; it was one of the many concoctions of the Atlanta pharmacist John Stith Pemberton, sold to treat all that ails us. Dr. Pepper, too, was a medicine in a sugary bottle – another that often contained alcohol or cocaine. Society wants a cure-all, and the marketing and selling done during these medicine shows offered placebos.
The hucksters exist in various forms today, selling detoxifications, magic diet cures, psychic powers of healing, or convincing parents that their kids don’t need vaccines. We need a warning song that goes viral to keep our children safe. We are blessed to be in a world without smallpox, almost rid of polio, and we have the knowledge and opportunity to rid the world of other preventable illnesses. Measles was declared eliminated in the United States in 2000; now, outbreaks emerge in every news cycle.
The CDC admits they have not been targeting misinformation well. How can we spread the science, the truth, the message faster than the lies? Better marketing? The answer may be through stories and narratives and song, with the backing of good science. “House of the Rising Sun” is a warning song. Maybe we need more. We need that deep history, that long trail to remind us of the world before vaccines, when everyone knew someone, either in their own household or next door, who succumbed to one of the childhood illnesses.
Let the “House of the Rising Sun” play on. Create a new version, and let that message reverberate, too.
Tell your children; they need to be vaccinated.
Dr. Messler is a hospitalist at Morton Plant Hospitalist group in Clearwater, Fla. He previously chaired SHM’s Quality and Patient Safety Committee and has been active in several SHM mentoring programs, most recently with Project BOOST and Glycemic Control. This article appeared originally in SHM's official blog The Hospital Leader. Read more recent posts here.
Consider measles vaccine booster in HIV-positive patients
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
LJUBLJANA, SLOVENIA – A “surprisingly low” prevalence of protective antibodies against measles is present in adolescents and adults living with HIV infection despite their prior vaccination against the resurgent disease, Raquel M. Simakawa, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
“With the present concern about the global reemergence of measles, we should consider measuring measles antibodies in people living with HIV, especially those who acquired the infection vertically, and then revaccinating those with low titers,” said Dr. Simakawa of the Federal University of São Paolo.
She presented interim findings of an ongoing study of the measles immunologic status of persons living with HIV, which for this analysis included 57 patients who acquired HIV from their mother via vertical transmission and 24 with horizontally acquired HIV. The vertical-transmission group was significantly younger, with a median age of 20 years, compared with 31 years in the horizontal group, who were diagnosed with HIV infection at an average age of 24 years. The vast majority of subjects were on combination antiretroviral therapy. No detectable HIV viral load had been present for a median of 70 months in the vertical group and 25 months in the horizontal group.
Only a mere 7% of the vertical transmission group had protective levels of measles IgG antibodies as measured by enzyme-linked immunosorbent assay, as did 29% of the horizontal group. The likely explanation for the higher rate of protection in the horizontal group, she said, is that they received their routine measles vaccination before they acquired HIV infection, and some of them didn’t lose their protective antibodies during their immune system’s fight against HIV infection.
Session chair Nico G. Hartwig, MD, of Franciscus Hospital in Rotterdam, the Netherlands, posed a question: Given the sky-high rate of measles seronegativity status among the vertically transmitted HIV-positive group – the patient population pediatricians focus on – why bother to measure their measles antibody level? Why not just give them all a measles booster?
Dr. Simakawa replied that that’s worth considering in routine clinical practice now that her study has shown that this group is more vulnerable to measles because of their poor response to immunization. But the study is ongoing, with larger numbers of patients to be enrolled. Also, in the second phase of the study, which will include a control group, measles IgG antibodies will be remeasured 1 month after administration of a new dose of measles vaccine.
She reported having no financial conflicts regarding this study, conducted free of commercial support.
REPORTING FROM ESPID 2019
Once-Daily 2-Drug versus 3-Drug Antiretroviral Therapy for HIV Infection in Treatment-naive Adults: Less Is Best?
Study Overview
Objective. To evaluate the efficacy and safety of a once-daily 2-drug antiretroviral (ARV) regimen, dolutegravir plus lamivudine, for the treatment of HIV-1 infection in adults naive to antiretroviral therapy (ART).
Design. GEMINI-1 and GEMINI-2 were 2 identically designed multicenter, double-blind, randomized, noninferiority, phase 3 clinical trials conducted between July 18, 2016 and March 31, 2017. Participants were stratified to receive 1 of 2 once-daily HIV regimens: the study regimen, consisting of once-daily dolutegravir 50 mg plus lamivudine 300 mg, or the standard-of-care regimen, consisting of once-daily dolutegravir 50 mg plus tenofovir disoproxil fumarate (TDF) 300 mg plus emtricitabine 200 mg. While this article presents results at week 48, both trials are scheduled to evaluate participants up to week 148 in an attempt to evaluate long-term efficacy and safety.
Setting and participants. Eligible participants had to be aged 18 years or older with treatment-naive HIV-1 infection. Women were eligible if they were not (1) pregnant, (2) lactating, or (3) of reproductive potential, defined by various means, including tubal ligation, hysterectomy, postmenopausal, and the use of highly effective contraception. Initially, eligibility screening restricted participation to those with viral loads between 1000 and 100,000 copies/mL. However, the upper limit was later increased to 500,000 copies/mL based on an independent review of results from other clinical trials1,2 evaluating dual therapy with dolutegravir and lamivudine, which indicated efficacy in patients with viral loads up to 500,000.3-5
Notable exclusion criteria included: (1) major mutations to nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors; (2) evidence of hepatitis B infection; (3) hepatitis C infection with anticipation of initiating treatment within 48 weeks of study enrollment; and (4) stage 3 HIV disease, per Centers for Disease Control and Prevention criteria, with the exception of cutaneous Kaposi sarcoma and CD4 cell counts < 200 cells/mL.
Main outcome measures. The primary endpoint was demonstration of noninferiority of the 2-drug ARV regimen through assessment of the proportion of participants who achieved virologic suppression at week 48 in the intent-to-treat-exposed population. For the purposes of this study, virologic suppression was defined as having fewer than 50 copies of HIV-1 RNA per mL at week 48. For evaluation of safety and toxicity concerns, renal and bone biomarkers were assessed at study entry and at weeks 24 and 48. In addition, participants who met virological withdrawal criteria were evaluated for integrase strand transfer inhibitor mutations. Virological withdrawal was defined as the presence of 1 of the following: (1) HIV RNA > 200 copies/mL at week 24, (2) HIV RNA > 200 copies/mL after previous HIV RNA < 200 copies/mL (confirmed rebound), and (3) a < 1 log10 copies/mL decrease from baseline (unless already < 200 copies/mL).
Main results. GEMINI-1 and GEMINI-2 randomized a combined total of 1441 participants to receive either the once-daily 2-drug ARV regimen (dolutegravir and lamivudine, n = 719) or the once-daily 3-drug ARV regimen (dolutegravir, TDF, and emtricitabine, n = 722). Of the 533 participants who did not meet inclusion criteria, the predominant reasons for exclusion were either having preexisting major viral resistance mutations (n = 246) or viral loads outside the range of 1000 to 500,000 copies/mL (n = 133).
Baseline demographic and clinical characteristics were similar between both groups. The median age was 33 years (10% were over 50 years of age), and participants were mostly male (85%) and white (68%). Baseline HIV RNA counts of > 100,000 copies/mL were found in 293 participants (20%), and 188 (8%) participants had CD4 counts of ≤ 200 cells/mL.
Noninferiority of the once-daily 2-drug versus the once-daily 3-drug ARV regimen was demonstrated in both the GEMINI-1 and GEMINI-2 trials for the intent-to-treat-exposed population. In GEMINI-1, 90% (n = 320) in the 2-drug ARV group achieved virologic suppression at week 48 compared to 93% (n = 332) in the 3-drug ARV group (no statistically significant difference). In GEMINI-2, 93% (n =335 ) in the 2-drug ARV group achieved virologic suppression at week 48 compared to 94% (n = 337) in the 3-drug ARV group (no statistically significant difference).
A subgroup analysis found no significant impact of baseline HIV RNA (> 100,000 compared to ≤ 100,000 copies/mL) on achieving virologic suppression at week 48. However, a subgroup analysis did find that participants with CD4 counts < 200 copies/mL had a reduced response in the once-daily 2-drug versus 3-drug ARV regimen for achieving virologic response at week 48 (79% versus 93%, respectively).
Overall, 10 participants met virological withdrawal criteria during the study period, and 4 of these were on the 2-drug ARV regimen. For these 10 participants, genotypic testing did not find emergence of resistance to either nucleoside reverse transcriptase or integrase strand transfer inhibitors.
Regarding renal biomarkers, increases of both serum creatinine and urinary excretion of protein creatinine were significantly greater in the 3-drug ARV group. Also, biomarkers indicating increased bone turnover were elevated in both groups, but the degree of elevation was significantly lower in the 2-drug ARV regimen cohort. It is unclear whether these findings reflect an increased or decreased risk of developing osteopenia or osteoporosis in the 2 study groups.
Conclusion. The once-daily 2-drug ARV regimen dolutegravir and lamivudine is noninferior to the guideline-recommended once-daily 3-drug ARV regimen dolutegravir, TDF, and emtricitabine at achieving viral suppression in ART-naive HIV-1 infected individuals with HIV RNA counts < 500,000 copies/mL. However, the efficacy of this ARV regimen may be compromised in individuals with CD4 counts < 200 cells/mL.
Commentary
Currently, the mainstay of HIV pharmacotherapy is a 3-drug regimen consisting of 2 nucleoside reverse transcriptase inhibitors in combination with 1 drug from another class, with an integrase strand transfer inhibitor being the preferred third drug.6 Despite the improved tolerability of contemporary ARVs, there remains concern among HIV practitioners regarding potential toxicities associated with cumulative drug exposure, specifically related to nucleoside reverse transcriptase inhibitors. As a result, there has been much interest in evaluating 2-drug ARV regimens for HIV treatment in order to reduce overall drug exposure.7-10
The 48-week results of the GEMINI-1 and GEMINI-2 trials, published in early 2019, further expand our understanding regarding the efficacy and safety of 2-drug regimens in HIV treatment. These identically designed studies evaluated once-daily dolutegravir and lamivudine for HIV in a treatment-naive population. This goes a step further than the SWORD-1 and SWORD-2 trials, which evaluated once-daily dolutegravir and rilpivirine as a step-down therapy for virologically suppressed individuals and led to the U.S. Food and Drug Administration (FDA) approval of the single-tablet combination regimen dolutegravir/rilpivirine (Juluca).10 Therefore, whereas the SWORD trials evaluated a 2-drug regimen for maintenance of virologic suppression, the GEMINI trials assessed whether a 2-drug regimen can both achieve and maintain virologic suppression.
The results of the GEMINI trials are promising for a future direction in HIV care. The rates of virologic suppression achieved in these trials are comparable to those seen in the SWORD trials.10 Furthermore, the virologic response seen in the GEMINI trials is comparable to that seen in similar trials that evaluated a 3-drug ARV regimen consisting of an integrase strand transfer inhibitor–based backbone in ART-naive individuals.11,12
A major confounder to the design of this trial was that it included TDF as one of the components in the comparator arm, an agent that has already been demonstrated to have detrimental effects on both renal and bone health.13,14 Additionally, the bone biomarker results were inconclusive, and the agents’ effects on bone would have been better demonstrated through bone mineral density testing, as had been done in prior trials.
Applications for Clinical Practice
Given the recent FDA approval of the single-tablet combination regimen dolutegravir and lamivudine (Dovato), this once-daily 2-drug ARV regimen will begin making its way into clinical practice for certain patients. Prior to starting this regimen, hepatitis B infection first must be ruled out due to poor efficacy of lamivudine monotherapy for management of chronic hepatitis B infection.15 Additionally, baseline genotype testing should be performed prior to starting this ART given that approximately 10% of newly diagnosed HIV patients have baseline resistance mutations.16 Obtaining rapid genotype testing may be difficult to accomplish in low-resource settings where such testing is not readily available. Finally, this approach may not be applicable to those presenting with acute HIV infection, in whom viral loads are often in the millions of copies per mL. It is likely that dolutegravir/lamivudine could assume a role similar to that of dolutegravir/rilpivirine, in which patients who present with acute HIV step down to a 2-drug regimen once their viral loads have either dropped below 500,000 copies/mL or have already been suppressed.
—Evan K. Mallory, PharmD, Banner-University Medical Center Tucson, and Norman L. Beatty, MD, University of Arizona College of Medicine, Tucson, AZ
1. Cahn P, Rolón MJ, Figueroa MI, et al. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20:21678.
2. Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants eith HIV-1 RNA <500000 vopies/mL. Clin Infect Dis. 2018;66:1689-1697.
3. Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737-1745.
4. Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV Working Party. N Engl J Med. 1995;333:1662-1669.
5. Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS. 1996;10:975-981.
6. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed April 1, 2019.
7. Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095-2106.
8. Reynes J, Lawal A, Pulido F, et al. Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/ emtricitabine in antiretroviral-naïve subjects: the progress study, 48-week results. HIV Clin Trials. 2011;12:255-267.
9. Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572-580.
10. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839-849.
11. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
12. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606-2615.
13. Mulligan K, Glidden DV, Anderson PL, et al. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
14. Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496-505.
15. Kim D, Wheeler W, Ziebell R, et al. Prevalence of antiretroviral drug resistance among newly diagnosed HIV-1 infected persons, United States, 2007. 17th Conference on Retroviruses & Opportunistic Infections; San Francisco, CA: 2010. Feb 16-19. Abstract 580.
16. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599.
Study Overview
Objective. To evaluate the efficacy and safety of a once-daily 2-drug antiretroviral (ARV) regimen, dolutegravir plus lamivudine, for the treatment of HIV-1 infection in adults naive to antiretroviral therapy (ART).
Design. GEMINI-1 and GEMINI-2 were 2 identically designed multicenter, double-blind, randomized, noninferiority, phase 3 clinical trials conducted between July 18, 2016 and March 31, 2017. Participants were stratified to receive 1 of 2 once-daily HIV regimens: the study regimen, consisting of once-daily dolutegravir 50 mg plus lamivudine 300 mg, or the standard-of-care regimen, consisting of once-daily dolutegravir 50 mg plus tenofovir disoproxil fumarate (TDF) 300 mg plus emtricitabine 200 mg. While this article presents results at week 48, both trials are scheduled to evaluate participants up to week 148 in an attempt to evaluate long-term efficacy and safety.
Setting and participants. Eligible participants had to be aged 18 years or older with treatment-naive HIV-1 infection. Women were eligible if they were not (1) pregnant, (2) lactating, or (3) of reproductive potential, defined by various means, including tubal ligation, hysterectomy, postmenopausal, and the use of highly effective contraception. Initially, eligibility screening restricted participation to those with viral loads between 1000 and 100,000 copies/mL. However, the upper limit was later increased to 500,000 copies/mL based on an independent review of results from other clinical trials1,2 evaluating dual therapy with dolutegravir and lamivudine, which indicated efficacy in patients with viral loads up to 500,000.3-5
Notable exclusion criteria included: (1) major mutations to nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors; (2) evidence of hepatitis B infection; (3) hepatitis C infection with anticipation of initiating treatment within 48 weeks of study enrollment; and (4) stage 3 HIV disease, per Centers for Disease Control and Prevention criteria, with the exception of cutaneous Kaposi sarcoma and CD4 cell counts < 200 cells/mL.
Main outcome measures. The primary endpoint was demonstration of noninferiority of the 2-drug ARV regimen through assessment of the proportion of participants who achieved virologic suppression at week 48 in the intent-to-treat-exposed population. For the purposes of this study, virologic suppression was defined as having fewer than 50 copies of HIV-1 RNA per mL at week 48. For evaluation of safety and toxicity concerns, renal and bone biomarkers were assessed at study entry and at weeks 24 and 48. In addition, participants who met virological withdrawal criteria were evaluated for integrase strand transfer inhibitor mutations. Virological withdrawal was defined as the presence of 1 of the following: (1) HIV RNA > 200 copies/mL at week 24, (2) HIV RNA > 200 copies/mL after previous HIV RNA < 200 copies/mL (confirmed rebound), and (3) a < 1 log10 copies/mL decrease from baseline (unless already < 200 copies/mL).
Main results. GEMINI-1 and GEMINI-2 randomized a combined total of 1441 participants to receive either the once-daily 2-drug ARV regimen (dolutegravir and lamivudine, n = 719) or the once-daily 3-drug ARV regimen (dolutegravir, TDF, and emtricitabine, n = 722). Of the 533 participants who did not meet inclusion criteria, the predominant reasons for exclusion were either having preexisting major viral resistance mutations (n = 246) or viral loads outside the range of 1000 to 500,000 copies/mL (n = 133).
Baseline demographic and clinical characteristics were similar between both groups. The median age was 33 years (10% were over 50 years of age), and participants were mostly male (85%) and white (68%). Baseline HIV RNA counts of > 100,000 copies/mL were found in 293 participants (20%), and 188 (8%) participants had CD4 counts of ≤ 200 cells/mL.
Noninferiority of the once-daily 2-drug versus the once-daily 3-drug ARV regimen was demonstrated in both the GEMINI-1 and GEMINI-2 trials for the intent-to-treat-exposed population. In GEMINI-1, 90% (n = 320) in the 2-drug ARV group achieved virologic suppression at week 48 compared to 93% (n = 332) in the 3-drug ARV group (no statistically significant difference). In GEMINI-2, 93% (n =335 ) in the 2-drug ARV group achieved virologic suppression at week 48 compared to 94% (n = 337) in the 3-drug ARV group (no statistically significant difference).
A subgroup analysis found no significant impact of baseline HIV RNA (> 100,000 compared to ≤ 100,000 copies/mL) on achieving virologic suppression at week 48. However, a subgroup analysis did find that participants with CD4 counts < 200 copies/mL had a reduced response in the once-daily 2-drug versus 3-drug ARV regimen for achieving virologic response at week 48 (79% versus 93%, respectively).
Overall, 10 participants met virological withdrawal criteria during the study period, and 4 of these were on the 2-drug ARV regimen. For these 10 participants, genotypic testing did not find emergence of resistance to either nucleoside reverse transcriptase or integrase strand transfer inhibitors.
Regarding renal biomarkers, increases of both serum creatinine and urinary excretion of protein creatinine were significantly greater in the 3-drug ARV group. Also, biomarkers indicating increased bone turnover were elevated in both groups, but the degree of elevation was significantly lower in the 2-drug ARV regimen cohort. It is unclear whether these findings reflect an increased or decreased risk of developing osteopenia or osteoporosis in the 2 study groups.
Conclusion. The once-daily 2-drug ARV regimen dolutegravir and lamivudine is noninferior to the guideline-recommended once-daily 3-drug ARV regimen dolutegravir, TDF, and emtricitabine at achieving viral suppression in ART-naive HIV-1 infected individuals with HIV RNA counts < 500,000 copies/mL. However, the efficacy of this ARV regimen may be compromised in individuals with CD4 counts < 200 cells/mL.
Commentary
Currently, the mainstay of HIV pharmacotherapy is a 3-drug regimen consisting of 2 nucleoside reverse transcriptase inhibitors in combination with 1 drug from another class, with an integrase strand transfer inhibitor being the preferred third drug.6 Despite the improved tolerability of contemporary ARVs, there remains concern among HIV practitioners regarding potential toxicities associated with cumulative drug exposure, specifically related to nucleoside reverse transcriptase inhibitors. As a result, there has been much interest in evaluating 2-drug ARV regimens for HIV treatment in order to reduce overall drug exposure.7-10
The 48-week results of the GEMINI-1 and GEMINI-2 trials, published in early 2019, further expand our understanding regarding the efficacy and safety of 2-drug regimens in HIV treatment. These identically designed studies evaluated once-daily dolutegravir and lamivudine for HIV in a treatment-naive population. This goes a step further than the SWORD-1 and SWORD-2 trials, which evaluated once-daily dolutegravir and rilpivirine as a step-down therapy for virologically suppressed individuals and led to the U.S. Food and Drug Administration (FDA) approval of the single-tablet combination regimen dolutegravir/rilpivirine (Juluca).10 Therefore, whereas the SWORD trials evaluated a 2-drug regimen for maintenance of virologic suppression, the GEMINI trials assessed whether a 2-drug regimen can both achieve and maintain virologic suppression.
The results of the GEMINI trials are promising for a future direction in HIV care. The rates of virologic suppression achieved in these trials are comparable to those seen in the SWORD trials.10 Furthermore, the virologic response seen in the GEMINI trials is comparable to that seen in similar trials that evaluated a 3-drug ARV regimen consisting of an integrase strand transfer inhibitor–based backbone in ART-naive individuals.11,12
A major confounder to the design of this trial was that it included TDF as one of the components in the comparator arm, an agent that has already been demonstrated to have detrimental effects on both renal and bone health.13,14 Additionally, the bone biomarker results were inconclusive, and the agents’ effects on bone would have been better demonstrated through bone mineral density testing, as had been done in prior trials.
Applications for Clinical Practice
Given the recent FDA approval of the single-tablet combination regimen dolutegravir and lamivudine (Dovato), this once-daily 2-drug ARV regimen will begin making its way into clinical practice for certain patients. Prior to starting this regimen, hepatitis B infection first must be ruled out due to poor efficacy of lamivudine monotherapy for management of chronic hepatitis B infection.15 Additionally, baseline genotype testing should be performed prior to starting this ART given that approximately 10% of newly diagnosed HIV patients have baseline resistance mutations.16 Obtaining rapid genotype testing may be difficult to accomplish in low-resource settings where such testing is not readily available. Finally, this approach may not be applicable to those presenting with acute HIV infection, in whom viral loads are often in the millions of copies per mL. It is likely that dolutegravir/lamivudine could assume a role similar to that of dolutegravir/rilpivirine, in which patients who present with acute HIV step down to a 2-drug regimen once their viral loads have either dropped below 500,000 copies/mL or have already been suppressed.
—Evan K. Mallory, PharmD, Banner-University Medical Center Tucson, and Norman L. Beatty, MD, University of Arizona College of Medicine, Tucson, AZ
Study Overview
Objective. To evaluate the efficacy and safety of a once-daily 2-drug antiretroviral (ARV) regimen, dolutegravir plus lamivudine, for the treatment of HIV-1 infection in adults naive to antiretroviral therapy (ART).
Design. GEMINI-1 and GEMINI-2 were 2 identically designed multicenter, double-blind, randomized, noninferiority, phase 3 clinical trials conducted between July 18, 2016 and March 31, 2017. Participants were stratified to receive 1 of 2 once-daily HIV regimens: the study regimen, consisting of once-daily dolutegravir 50 mg plus lamivudine 300 mg, or the standard-of-care regimen, consisting of once-daily dolutegravir 50 mg plus tenofovir disoproxil fumarate (TDF) 300 mg plus emtricitabine 200 mg. While this article presents results at week 48, both trials are scheduled to evaluate participants up to week 148 in an attempt to evaluate long-term efficacy and safety.
Setting and participants. Eligible participants had to be aged 18 years or older with treatment-naive HIV-1 infection. Women were eligible if they were not (1) pregnant, (2) lactating, or (3) of reproductive potential, defined by various means, including tubal ligation, hysterectomy, postmenopausal, and the use of highly effective contraception. Initially, eligibility screening restricted participation to those with viral loads between 1000 and 100,000 copies/mL. However, the upper limit was later increased to 500,000 copies/mL based on an independent review of results from other clinical trials1,2 evaluating dual therapy with dolutegravir and lamivudine, which indicated efficacy in patients with viral loads up to 500,000.3-5
Notable exclusion criteria included: (1) major mutations to nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors; (2) evidence of hepatitis B infection; (3) hepatitis C infection with anticipation of initiating treatment within 48 weeks of study enrollment; and (4) stage 3 HIV disease, per Centers for Disease Control and Prevention criteria, with the exception of cutaneous Kaposi sarcoma and CD4 cell counts < 200 cells/mL.
Main outcome measures. The primary endpoint was demonstration of noninferiority of the 2-drug ARV regimen through assessment of the proportion of participants who achieved virologic suppression at week 48 in the intent-to-treat-exposed population. For the purposes of this study, virologic suppression was defined as having fewer than 50 copies of HIV-1 RNA per mL at week 48. For evaluation of safety and toxicity concerns, renal and bone biomarkers were assessed at study entry and at weeks 24 and 48. In addition, participants who met virological withdrawal criteria were evaluated for integrase strand transfer inhibitor mutations. Virological withdrawal was defined as the presence of 1 of the following: (1) HIV RNA > 200 copies/mL at week 24, (2) HIV RNA > 200 copies/mL after previous HIV RNA < 200 copies/mL (confirmed rebound), and (3) a < 1 log10 copies/mL decrease from baseline (unless already < 200 copies/mL).
Main results. GEMINI-1 and GEMINI-2 randomized a combined total of 1441 participants to receive either the once-daily 2-drug ARV regimen (dolutegravir and lamivudine, n = 719) or the once-daily 3-drug ARV regimen (dolutegravir, TDF, and emtricitabine, n = 722). Of the 533 participants who did not meet inclusion criteria, the predominant reasons for exclusion were either having preexisting major viral resistance mutations (n = 246) or viral loads outside the range of 1000 to 500,000 copies/mL (n = 133).
Baseline demographic and clinical characteristics were similar between both groups. The median age was 33 years (10% were over 50 years of age), and participants were mostly male (85%) and white (68%). Baseline HIV RNA counts of > 100,000 copies/mL were found in 293 participants (20%), and 188 (8%) participants had CD4 counts of ≤ 200 cells/mL.
Noninferiority of the once-daily 2-drug versus the once-daily 3-drug ARV regimen was demonstrated in both the GEMINI-1 and GEMINI-2 trials for the intent-to-treat-exposed population. In GEMINI-1, 90% (n = 320) in the 2-drug ARV group achieved virologic suppression at week 48 compared to 93% (n = 332) in the 3-drug ARV group (no statistically significant difference). In GEMINI-2, 93% (n =335 ) in the 2-drug ARV group achieved virologic suppression at week 48 compared to 94% (n = 337) in the 3-drug ARV group (no statistically significant difference).
A subgroup analysis found no significant impact of baseline HIV RNA (> 100,000 compared to ≤ 100,000 copies/mL) on achieving virologic suppression at week 48. However, a subgroup analysis did find that participants with CD4 counts < 200 copies/mL had a reduced response in the once-daily 2-drug versus 3-drug ARV regimen for achieving virologic response at week 48 (79% versus 93%, respectively).
Overall, 10 participants met virological withdrawal criteria during the study period, and 4 of these were on the 2-drug ARV regimen. For these 10 participants, genotypic testing did not find emergence of resistance to either nucleoside reverse transcriptase or integrase strand transfer inhibitors.
Regarding renal biomarkers, increases of both serum creatinine and urinary excretion of protein creatinine were significantly greater in the 3-drug ARV group. Also, biomarkers indicating increased bone turnover were elevated in both groups, but the degree of elevation was significantly lower in the 2-drug ARV regimen cohort. It is unclear whether these findings reflect an increased or decreased risk of developing osteopenia or osteoporosis in the 2 study groups.
Conclusion. The once-daily 2-drug ARV regimen dolutegravir and lamivudine is noninferior to the guideline-recommended once-daily 3-drug ARV regimen dolutegravir, TDF, and emtricitabine at achieving viral suppression in ART-naive HIV-1 infected individuals with HIV RNA counts < 500,000 copies/mL. However, the efficacy of this ARV regimen may be compromised in individuals with CD4 counts < 200 cells/mL.
Commentary
Currently, the mainstay of HIV pharmacotherapy is a 3-drug regimen consisting of 2 nucleoside reverse transcriptase inhibitors in combination with 1 drug from another class, with an integrase strand transfer inhibitor being the preferred third drug.6 Despite the improved tolerability of contemporary ARVs, there remains concern among HIV practitioners regarding potential toxicities associated with cumulative drug exposure, specifically related to nucleoside reverse transcriptase inhibitors. As a result, there has been much interest in evaluating 2-drug ARV regimens for HIV treatment in order to reduce overall drug exposure.7-10
The 48-week results of the GEMINI-1 and GEMINI-2 trials, published in early 2019, further expand our understanding regarding the efficacy and safety of 2-drug regimens in HIV treatment. These identically designed studies evaluated once-daily dolutegravir and lamivudine for HIV in a treatment-naive population. This goes a step further than the SWORD-1 and SWORD-2 trials, which evaluated once-daily dolutegravir and rilpivirine as a step-down therapy for virologically suppressed individuals and led to the U.S. Food and Drug Administration (FDA) approval of the single-tablet combination regimen dolutegravir/rilpivirine (Juluca).10 Therefore, whereas the SWORD trials evaluated a 2-drug regimen for maintenance of virologic suppression, the GEMINI trials assessed whether a 2-drug regimen can both achieve and maintain virologic suppression.
The results of the GEMINI trials are promising for a future direction in HIV care. The rates of virologic suppression achieved in these trials are comparable to those seen in the SWORD trials.10 Furthermore, the virologic response seen in the GEMINI trials is comparable to that seen in similar trials that evaluated a 3-drug ARV regimen consisting of an integrase strand transfer inhibitor–based backbone in ART-naive individuals.11,12
A major confounder to the design of this trial was that it included TDF as one of the components in the comparator arm, an agent that has already been demonstrated to have detrimental effects on both renal and bone health.13,14 Additionally, the bone biomarker results were inconclusive, and the agents’ effects on bone would have been better demonstrated through bone mineral density testing, as had been done in prior trials.
Applications for Clinical Practice
Given the recent FDA approval of the single-tablet combination regimen dolutegravir and lamivudine (Dovato), this once-daily 2-drug ARV regimen will begin making its way into clinical practice for certain patients. Prior to starting this regimen, hepatitis B infection first must be ruled out due to poor efficacy of lamivudine monotherapy for management of chronic hepatitis B infection.15 Additionally, baseline genotype testing should be performed prior to starting this ART given that approximately 10% of newly diagnosed HIV patients have baseline resistance mutations.16 Obtaining rapid genotype testing may be difficult to accomplish in low-resource settings where such testing is not readily available. Finally, this approach may not be applicable to those presenting with acute HIV infection, in whom viral loads are often in the millions of copies per mL. It is likely that dolutegravir/lamivudine could assume a role similar to that of dolutegravir/rilpivirine, in which patients who present with acute HIV step down to a 2-drug regimen once their viral loads have either dropped below 500,000 copies/mL or have already been suppressed.
—Evan K. Mallory, PharmD, Banner-University Medical Center Tucson, and Norman L. Beatty, MD, University of Arizona College of Medicine, Tucson, AZ
1. Cahn P, Rolón MJ, Figueroa MI, et al. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20:21678.
2. Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants eith HIV-1 RNA <500000 vopies/mL. Clin Infect Dis. 2018;66:1689-1697.
3. Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737-1745.
4. Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV Working Party. N Engl J Med. 1995;333:1662-1669.
5. Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS. 1996;10:975-981.
6. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed April 1, 2019.
7. Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095-2106.
8. Reynes J, Lawal A, Pulido F, et al. Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/ emtricitabine in antiretroviral-naïve subjects: the progress study, 48-week results. HIV Clin Trials. 2011;12:255-267.
9. Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572-580.
10. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839-849.
11. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
12. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606-2615.
13. Mulligan K, Glidden DV, Anderson PL, et al. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
14. Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496-505.
15. Kim D, Wheeler W, Ziebell R, et al. Prevalence of antiretroviral drug resistance among newly diagnosed HIV-1 infected persons, United States, 2007. 17th Conference on Retroviruses & Opportunistic Infections; San Francisco, CA: 2010. Feb 16-19. Abstract 580.
16. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599.
1. Cahn P, Rolón MJ, Figueroa MI, et al. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc. 2017;20:21678.
2. Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants eith HIV-1 RNA <500000 vopies/mL. Clin Infect Dis. 2018;66:1689-1697.
3. Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737-1745.
4. Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. North American HIV Working Party. N Engl J Med. 1995;333:1662-1669.
5. Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS. 1996;10:975-981.
6. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed April 1, 2019.
7. Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095-2106.
8. Reynes J, Lawal A, Pulido F, et al. Examination of noninferiority, safety, and tolerability of lopinavir/ritonavir and raltegravir compared with lopinavir/ritonavir and tenofovir/ emtricitabine in antiretroviral-naïve subjects: the progress study, 48-week results. HIV Clin Trials. 2011;12:255-267.
9. Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. 2014;14:572-580.
10. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839-849.
11. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369:1807-1818.
12. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606-2615.
13. Mulligan K, Glidden DV, Anderson PL, et al. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61:572-580.
14. Cooper RD, Wiebe N, Smith N, et al. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496-505.
15. Kim D, Wheeler W, Ziebell R, et al. Prevalence of antiretroviral drug resistance among newly diagnosed HIV-1 infected persons, United States, 2007. 17th Conference on Retroviruses & Opportunistic Infections; San Francisco, CA: 2010. Feb 16-19. Abstract 580.
16. Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599.
Expanded indication being considered for meningococcal group B vaccine
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
LJUBLJANA, SLOVENIA – under the agency’s Breakthrough Therapy designation.
Breakthrough Therapy status is reserved for accelerated review of therapies considered to show substantial preliminary promise of effectively targeting a major unmet medical need.
The unmet need here is that there is no meningococcal group B vaccine approved for use in children under age 10 years. Yet infants and children under 5 years of age are at greatest risk of invasive meningococcal B disease, with reported case fatality rates of 8%-9%, Jason D. Maguire, MD, noted at the annual meeting of the European Society for Paediatric Infectious Diseases.
Trumenba has been approved in the United States for patients aged 10-25 years and in the European Union for individuals aged 10 years or older.
Dr. Maguire, of Pfizer’s vaccine clinical research and development program, presented the results of the two phase 2 randomized safety and immunogenicity trials conducted in patients aged 1- 9 years that the company has submitted to the FDA in support of the expanded indication. One study was carried out in 352 1-year-old toddlers, the other in 400 children aged 2-9 years, whose mean age was 4 years. The studies were carried out in Australia, Finland, Poland, and the Czech Republic.
In a pooled analysis of the vaccine’s immunogenicity when administered in a three-dose schedule of 120 mcg at 0, 2, and 6 months to 193 toddlers and 274 of the children aged 2-9 years, robust bactericidal antibody responses were seen against the four major Neisseria meningitidis group B strains that cause invasive disease. In fact, at least a fourfold rise in titers from baseline to 1 month after dose three was documented in the same high proportion of 1- to 9-year-olds as previously seen in the phase 3 trials that led to vaccine licensure in adolescents and young adults.
“These results support that the use of Trumenba, when given to children ages 1 to less than 10 years at the same dose and schedule that is currently approved in adolescents and young adults, can afford a high degree of protective antibody responses that correlate with immunity in this population,” Dr. Maguire said.
The safety and tolerability analysis included all 752 children in the two phase 2 studies, including the 110 toddlers randomized to three 60-mcg doses of the vaccine, although it has subsequently become clear that 120 mcg is the dose that provides the best immunogenicity with an acceptable safety profile, according to the physician.
Across the age groups, local reactions, including redness and swelling, were more common in Trumenba recipients than in controls who received hepatitis A vaccine and saline injections. So were systemic adverse events. Fever – a systemic event of particular interest to parents and clinicians – occurred in 37% of toddlers after vaccination, compared with 25% of 2- to 9-year-olds and 10%-12% of controls. Of note, prophylactic antipyretics weren’t allowed in the study.
“There’s somewhat of an inverse relationship between age and temperature. So as we go down in age, the rate of fever rises. But after each subsequent dose, regardless of age, there’s a reduction in the incidence of fever,” Dr. Maguire observed.
Most fevers were less than 39.0° C. Only 3 of 752 (less than 1%) patients experienced fever in excess of 40.0° C.
Two children withdrew from the study after developing hip synovitis, which was transient. Another withdrew because of prolonged irritability, fatigue, and decreased appetite.
“Although Trumenba had an acceptable safety and tolerability profile in 1- to 9-year-olds, this analysis wasn’t powered enough to detect uncommon adverse events, so we’ll continue to monitor safety for things like synovitis,” he said.
In 10- to 25-year-olds, the meningococcal vaccine can be given concomitantly with other vaccines without interference. There are plans to study concurrent vaccination with MMR and pneumococcal vaccines in 1- to 9-year-olds as well, according to Dr. Maguire.
Pfizer also now is planning clinical trials of the vaccine in infants, another important group currently unprotected against meningococcal group B disease, he added.
Dr. Maguire is an employee of Pfizer, who funded the studies.
EXPERT ANALYSIS FROM ESPID 2019
Measles: A dangerous vaccine-preventable disease returns
Measles, an ancient, highly contagious disease with a history of successful control by vaccination, is now threatening to have an epidemic resurgence. Until recently, measles vaccination largely controlled outbreaks in the United States. The Global Vaccine Action Plan under the World Health Organization aimed to eliminate measles worldwide. Nonetheless, the vaccine refusal movement and slow rollout of vaccine programs globally have interfered with control of the virus. A record number of measles cases have emerged in recent months: more than 700 since January 2019.1 Approximately 70% of recent cases were in unvaccinated patients, and almost all were in US residents.
This update reviews the history, presentation and diagnosis, complications, management, contagion control, and emerging threat of a measles epidemic. It concludes with recommendations for clinical practice in the context of the current measles outbreaks.
FROM UBIQUITOUS TO ERADICATED—AND BACK
Before the measles vaccine was developed and became available in the 1960s, outbreaks of measles occurred predictably every year in the United States and other temperate regions. During yearly outbreaks, measles was so contagious that household contacts had attack rates above 95%. Most cases occurred in very young children, and because infection with the virus causes lifelong immunity, it could be safely assumed that by adulthood, everyone was immune. In an outbreak in the Faroe Islands in 1846, no one who had been alive in the last major outbreak 65 years earlier became ill, but everyone under age 65 was at high risk with “high attack rates,” estimated as 99% from other outbreaks (reviewed by Krugman et al2).
In isolated regions previously free of measles, adults did not have immunity, and when exposed, they often developed severe disease. When European settlers brought measles and smallpox to the Americas beginning in the late 15th century, these diseases decimated whole populations of native peoples who had never been exposed to them.
That was premature. A number of outbreaks have occurred since then; the largest in the United States (before 2019) was in 2000. Over half of the 667 cases reported during that outbreak were in an underimmunized Amish community in Ohio.3
Now it emerges again.
PRESENTATION CAN VARY
The presentation varies somewhat among certain groups.
Nonimmune pregnant women have an especially severe course, likely related to the relative immune suppression of pregnancy.
In immune-suppressed states, measles is not only more severe, it is also difficult to diagnose because the rash can be absent.
In partially vaccinated children and adults, the disease may present atypically, without cough, conjunctivitis, and coryza, and it may be milder, lacking some of the extreme malaise typical of measles and with a shortened course. Measles infection in people who received the inactivated measles vaccine that was briefly available from 1963 to 1967 is also associated with atypical measles syndrome, a severe hypersensitivity reaction to the measles virus. Atypical measles can be prevented by revaccination with a live-virus vaccine.
DIAGNOSIS MAY NEED TO BE CONFIRMED
The diagnosis of measles is straightforward when all of the signs and symptoms are present. In partially vaccinated populations, however, the diagnosis may need to be confirmed by serologic or polymerase chain reaction (PCR) testing.
Differential diagnosis
The differential diagnosis of the fever and a rash typical of measles in children, especially when accompanied by severe malaise, includes the following:
Kawasaki disease. However, the red eyes of Kawasaki are an injection of the bulbar conjunctivae with sparing of the limbus. No eye exudate is present, and respiratory illness is not part of the disease.
Drug eruptions can present with a morbilliform rash and sometimes fever, but not the other signs of measles in either adults or children.
Scarlet fever has a different rash, the sandpaper rash typical of toxin-mediated disease.
Rubella tends to cause mild respiratory symptoms and illness rather than the severe disease of measles and other rash-causing viral infections in children and infants.
Confirmation in confusing cases
To confirm a diagnosis of measles, samples from throat, nasal, and posterior nasopharyngeal swabs should be collected with a blood specimen for serology and sent to the state public health laboratory.4 The US Centers for Disease Control and Prevention gives instructions on who should be tested and with which tests.4
Most testing now uses PCR for viral RNA, as viral culture is more costly and takes longer. For accurate diagnosis, samples for PCR must be obtained during the acute illness.
The serologic gold standard for diagnosis is a 4-fold rise or fall in immunoglobulin G (IgG) titer of paired serum samples sent 10 days to 2 weeks apart around the illness. The IgM test may be negative initially, and a negative test cannot be used to rule out the diagnosis. Confirmed cases should be reported to public health authorities.
COMPLICATIONS: EARLY AND LATE
Frequent complications of measles infection include those related to the primary viral infection of respiratory tract mucosal surfaces, as well as bacterial superinfections. Complications are most likely in children under age 5, nonimmune adults, pregnant women, and immunocompromised people. Typical complications include otitis media, laryngotracheobronchitis (presenting as a croupy cough), pneumonia, and diarrhea.
Late sequelae of measles infection are related in part to serious mucosal damage and generalized immune suppression caused by the virus. Even after recovery from acute infection, children can have persistent diarrhea and failure to thrive, with increased mortality risk in the months after infection. Tuberculosis can reactivate in patients already infected, and new tuberculosis infection can be especially severe. Further, tuberculosis skin tests become less reliable immediately after measles infection. Severe disease and fatalities are increased in populations that have baseline vitamin A deficiency and malnutrition.
Death from measles is most often caused by viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. Before the vaccine era, measles encephalitis occurred in the United States in about 1 in 1,000 measles cases.
Subacute sclerosing panencephalitis is a rare, late, and often fatal complication of measles that presents 7 to 10 years after acute measles infection, usually in adolescence. Beginning with myoclonic jerks, stiffening, and slow mental deterioration, it progresses over 1 to 3 years, with a relentless degenerative course leading to death. Since the introduction of the measles vaccine in 1957, this disease has essentially disappeared in the United States.
SUPPORTIVE CARE, INFECTION CONTROL
Management of measles and its complications is primarily supportive.
Preventing contagion
Measles infection has an incubation period of 8 to 12 days. Individuals are contagious 4 days before to 4 days after rash onset in the normal host but longer in those lacking immune function. Cases can occur up to 21 days after exposure during the contagious period.
The disease is highly contagious, so hospitalized patients should be cared for with airborne precautions. It is crucial that caretakers be vaccinated properly, so that they can care for patients safely. Recommendations for preventing secondary cases by prompt vaccination and giving immune globulin are detailed below, including specific recommendations for individuals with immune system suppression.
The current US public health policy regarding measles vaccine booster doses began in response to the widespread measles outbreak in the United States from 1989 to 1991. Cases occurred more commonly in unvaccinated individuals and in young adults who had received only 1 dose of vaccine.
Today, the policy in areas where measles has been controlled is to vaccinate between 12 and 15 months of age and to boost with a second dose before starting kindergarten. In outbreak situations, the first dose should be given at 6 months of age, with a repeat dose at 12 to 15 months of age and the usual booster before starting kindergarten.
Those born before 1957 can be presumed to have had natural measles, which confers lifelong immunity (Table 3).7
CURRENT THREAT
In 2000, measles was considered controlled in the United States, thanks to the national vaccination policy. But despite overall control, small numbers of cases continued to occur each year, related to exposure to cases imported from areas of the world endemic with measles.
Within the last year, however, major outbreaks have emerged. Incompletely vaccinated populations and unvaccinated individuals are the reason for the progression of current outbreaks.8
Until there is broader acceptance of the vaccine and better adherence to vaccine policies nationally and globally, measles cannot be completely eradicated. But with high vaccination rates, it is predicted that this infection can be controlled and ultimately eradicated.
RECOMMENDATIONS
In the midst of an outbreak and with rising public awareness of the threat of measles, it is important to recognize that MMR vaccination is the most effective way to prevent spread of the virus and maintain measles elimination in the United States. With this in mind, there are several key facts and recommendations regarding vaccination:
Recommendations on vaccination
- In measles-controlled populations, all children should be vaccinated between 12 and 15 months of age and again before kindergarten.
- In outbreak settings, children should receive a first vaccine dose at 6 months of age, a second at 12 to 15 months of age, and a third before kindergarten.
- Children who have received 2 measles vaccine doses can be assumed to be fully vaccinated and thus protected as long as the first dose was after 12 months of age. If the first dose was before 12 months of age, a child needs 3 doses.
- Adults born before 1957 can be assumed to have had measles infection and to be immune.
- Adults who were immunized with the inactivated measles vaccine available between 1963 and 1967 should receive 1 dose of live virus vaccine.
- Boosters are recommended for young adults who did not receive a second dose of vaccine and for adults with an uncertain history of immunization. There is no need to check titers before giving a booster, but if a positive titer is available in an adult, a booster is not needed.
- Heathcare providers should vaccinate unvaccinated or undervaccinated US residents traveling internationally (as long as they do not have contraindications) or traveling within the country to areas with outbreaks of measles.
Recommendations on vaccination after exposure to measles
- Vaccine is recommended for a nonimmune contact, including anyone with a history of only a single dose of vaccine.
- If a child got a first dose of vaccine before 12 months of age, give the second dose as soon as he or she turns 1 year old, or at least 28 days after the first dose.
- Vaccine must be given within 72 hours of exposure to confer protection (or at least decrease disease severity).
- The second dose of vaccine should be given at least 28 days after the first dose.
Recommendations on immune globulin after exposure to measles
- Immune globulin is recommended for anyone with exposure and no history of vaccination or immunity.
- Immune globulin can be given up to 6 days after exposure to prevent or decrease the severity of measles in immunocompromised hosts who have not been previously vaccinated. It is best to give it as early as possible.
- Immune globulin is given intramuscularly at 0.5 mL/kg, up to a to maximum dose of 15 mL.
- Pregnant women and immunocompromised hosts without immunity should receive immunoglobulin intravenously. Children and adults who have had a recent bone marrow transplant and likely do not yet have a reconstituted immune system should be treated with immune globulin to prevent infection, as vaccine cannot be given immediately after transplant. This is also true for other immunocompromised individuals who have not been vaccinated and who are not candidates for vaccine because of the severity of their immune suppression.
- Children with human immunodeficiency virus infection are routinely vaccinated. As long as they have evidence of serologic immunity, they do not need additional treatment.
- Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Measles. In Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics 2018; 537–550.
- Krugman S, Giles JP, Friedman H, Stone S. Studies on immunity to measles. J Pediatr 1965; 66:471–488. pmid:14264306.
- Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375(14):1343–1354. doi:10.1056/NEJMoa1602295
- Centers for Disease Control and Prevention. Measles (rubeola). www.cdc.gov/measles/index.html. Accessed May 16, 2019.
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec 2017; 92(17):205–227. pmid:28459148
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013; summary: recommendations of the Advisory Committee on Immunization Practices ACIP. MMWR Recomm Rep 2013 Jun 14; 62(RR-4)1–34. pmid:23760231
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization for health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Nov 25; 60(RR-7)1–45. pmid:22108587
- Patel M, Lee AD, Redd SB, et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep 2019; May 3; 68(17):402–404. doi:10.15585/mmwr.mm6817e1
Measles, an ancient, highly contagious disease with a history of successful control by vaccination, is now threatening to have an epidemic resurgence. Until recently, measles vaccination largely controlled outbreaks in the United States. The Global Vaccine Action Plan under the World Health Organization aimed to eliminate measles worldwide. Nonetheless, the vaccine refusal movement and slow rollout of vaccine programs globally have interfered with control of the virus. A record number of measles cases have emerged in recent months: more than 700 since January 2019.1 Approximately 70% of recent cases were in unvaccinated patients, and almost all were in US residents.
This update reviews the history, presentation and diagnosis, complications, management, contagion control, and emerging threat of a measles epidemic. It concludes with recommendations for clinical practice in the context of the current measles outbreaks.
FROM UBIQUITOUS TO ERADICATED—AND BACK
Before the measles vaccine was developed and became available in the 1960s, outbreaks of measles occurred predictably every year in the United States and other temperate regions. During yearly outbreaks, measles was so contagious that household contacts had attack rates above 95%. Most cases occurred in very young children, and because infection with the virus causes lifelong immunity, it could be safely assumed that by adulthood, everyone was immune. In an outbreak in the Faroe Islands in 1846, no one who had been alive in the last major outbreak 65 years earlier became ill, but everyone under age 65 was at high risk with “high attack rates,” estimated as 99% from other outbreaks (reviewed by Krugman et al2).
In isolated regions previously free of measles, adults did not have immunity, and when exposed, they often developed severe disease. When European settlers brought measles and smallpox to the Americas beginning in the late 15th century, these diseases decimated whole populations of native peoples who had never been exposed to them.
That was premature. A number of outbreaks have occurred since then; the largest in the United States (before 2019) was in 2000. Over half of the 667 cases reported during that outbreak were in an underimmunized Amish community in Ohio.3
Now it emerges again.
PRESENTATION CAN VARY
The presentation varies somewhat among certain groups.
Nonimmune pregnant women have an especially severe course, likely related to the relative immune suppression of pregnancy.
In immune-suppressed states, measles is not only more severe, it is also difficult to diagnose because the rash can be absent.
In partially vaccinated children and adults, the disease may present atypically, without cough, conjunctivitis, and coryza, and it may be milder, lacking some of the extreme malaise typical of measles and with a shortened course. Measles infection in people who received the inactivated measles vaccine that was briefly available from 1963 to 1967 is also associated with atypical measles syndrome, a severe hypersensitivity reaction to the measles virus. Atypical measles can be prevented by revaccination with a live-virus vaccine.
DIAGNOSIS MAY NEED TO BE CONFIRMED
The diagnosis of measles is straightforward when all of the signs and symptoms are present. In partially vaccinated populations, however, the diagnosis may need to be confirmed by serologic or polymerase chain reaction (PCR) testing.
Differential diagnosis
The differential diagnosis of the fever and a rash typical of measles in children, especially when accompanied by severe malaise, includes the following:
Kawasaki disease. However, the red eyes of Kawasaki are an injection of the bulbar conjunctivae with sparing of the limbus. No eye exudate is present, and respiratory illness is not part of the disease.
Drug eruptions can present with a morbilliform rash and sometimes fever, but not the other signs of measles in either adults or children.
Scarlet fever has a different rash, the sandpaper rash typical of toxin-mediated disease.
Rubella tends to cause mild respiratory symptoms and illness rather than the severe disease of measles and other rash-causing viral infections in children and infants.
Confirmation in confusing cases
To confirm a diagnosis of measles, samples from throat, nasal, and posterior nasopharyngeal swabs should be collected with a blood specimen for serology and sent to the state public health laboratory.4 The US Centers for Disease Control and Prevention gives instructions on who should be tested and with which tests.4
Most testing now uses PCR for viral RNA, as viral culture is more costly and takes longer. For accurate diagnosis, samples for PCR must be obtained during the acute illness.
The serologic gold standard for diagnosis is a 4-fold rise or fall in immunoglobulin G (IgG) titer of paired serum samples sent 10 days to 2 weeks apart around the illness. The IgM test may be negative initially, and a negative test cannot be used to rule out the diagnosis. Confirmed cases should be reported to public health authorities.
COMPLICATIONS: EARLY AND LATE
Frequent complications of measles infection include those related to the primary viral infection of respiratory tract mucosal surfaces, as well as bacterial superinfections. Complications are most likely in children under age 5, nonimmune adults, pregnant women, and immunocompromised people. Typical complications include otitis media, laryngotracheobronchitis (presenting as a croupy cough), pneumonia, and diarrhea.
Late sequelae of measles infection are related in part to serious mucosal damage and generalized immune suppression caused by the virus. Even after recovery from acute infection, children can have persistent diarrhea and failure to thrive, with increased mortality risk in the months after infection. Tuberculosis can reactivate in patients already infected, and new tuberculosis infection can be especially severe. Further, tuberculosis skin tests become less reliable immediately after measles infection. Severe disease and fatalities are increased in populations that have baseline vitamin A deficiency and malnutrition.
Death from measles is most often caused by viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. Before the vaccine era, measles encephalitis occurred in the United States in about 1 in 1,000 measles cases.
Subacute sclerosing panencephalitis is a rare, late, and often fatal complication of measles that presents 7 to 10 years after acute measles infection, usually in adolescence. Beginning with myoclonic jerks, stiffening, and slow mental deterioration, it progresses over 1 to 3 years, with a relentless degenerative course leading to death. Since the introduction of the measles vaccine in 1957, this disease has essentially disappeared in the United States.
SUPPORTIVE CARE, INFECTION CONTROL
Management of measles and its complications is primarily supportive.
Preventing contagion
Measles infection has an incubation period of 8 to 12 days. Individuals are contagious 4 days before to 4 days after rash onset in the normal host but longer in those lacking immune function. Cases can occur up to 21 days after exposure during the contagious period.
The disease is highly contagious, so hospitalized patients should be cared for with airborne precautions. It is crucial that caretakers be vaccinated properly, so that they can care for patients safely. Recommendations for preventing secondary cases by prompt vaccination and giving immune globulin are detailed below, including specific recommendations for individuals with immune system suppression.
The current US public health policy regarding measles vaccine booster doses began in response to the widespread measles outbreak in the United States from 1989 to 1991. Cases occurred more commonly in unvaccinated individuals and in young adults who had received only 1 dose of vaccine.
Today, the policy in areas where measles has been controlled is to vaccinate between 12 and 15 months of age and to boost with a second dose before starting kindergarten. In outbreak situations, the first dose should be given at 6 months of age, with a repeat dose at 12 to 15 months of age and the usual booster before starting kindergarten.
Those born before 1957 can be presumed to have had natural measles, which confers lifelong immunity (Table 3).7
CURRENT THREAT
In 2000, measles was considered controlled in the United States, thanks to the national vaccination policy. But despite overall control, small numbers of cases continued to occur each year, related to exposure to cases imported from areas of the world endemic with measles.
Within the last year, however, major outbreaks have emerged. Incompletely vaccinated populations and unvaccinated individuals are the reason for the progression of current outbreaks.8
Until there is broader acceptance of the vaccine and better adherence to vaccine policies nationally and globally, measles cannot be completely eradicated. But with high vaccination rates, it is predicted that this infection can be controlled and ultimately eradicated.
RECOMMENDATIONS
In the midst of an outbreak and with rising public awareness of the threat of measles, it is important to recognize that MMR vaccination is the most effective way to prevent spread of the virus and maintain measles elimination in the United States. With this in mind, there are several key facts and recommendations regarding vaccination:
Recommendations on vaccination
- In measles-controlled populations, all children should be vaccinated between 12 and 15 months of age and again before kindergarten.
- In outbreak settings, children should receive a first vaccine dose at 6 months of age, a second at 12 to 15 months of age, and a third before kindergarten.
- Children who have received 2 measles vaccine doses can be assumed to be fully vaccinated and thus protected as long as the first dose was after 12 months of age. If the first dose was before 12 months of age, a child needs 3 doses.
- Adults born before 1957 can be assumed to have had measles infection and to be immune.
- Adults who were immunized with the inactivated measles vaccine available between 1963 and 1967 should receive 1 dose of live virus vaccine.
- Boosters are recommended for young adults who did not receive a second dose of vaccine and for adults with an uncertain history of immunization. There is no need to check titers before giving a booster, but if a positive titer is available in an adult, a booster is not needed.
- Heathcare providers should vaccinate unvaccinated or undervaccinated US residents traveling internationally (as long as they do not have contraindications) or traveling within the country to areas with outbreaks of measles.
Recommendations on vaccination after exposure to measles
- Vaccine is recommended for a nonimmune contact, including anyone with a history of only a single dose of vaccine.
- If a child got a first dose of vaccine before 12 months of age, give the second dose as soon as he or she turns 1 year old, or at least 28 days after the first dose.
- Vaccine must be given within 72 hours of exposure to confer protection (or at least decrease disease severity).
- The second dose of vaccine should be given at least 28 days after the first dose.
Recommendations on immune globulin after exposure to measles
- Immune globulin is recommended for anyone with exposure and no history of vaccination or immunity.
- Immune globulin can be given up to 6 days after exposure to prevent or decrease the severity of measles in immunocompromised hosts who have not been previously vaccinated. It is best to give it as early as possible.
- Immune globulin is given intramuscularly at 0.5 mL/kg, up to a to maximum dose of 15 mL.
- Pregnant women and immunocompromised hosts without immunity should receive immunoglobulin intravenously. Children and adults who have had a recent bone marrow transplant and likely do not yet have a reconstituted immune system should be treated with immune globulin to prevent infection, as vaccine cannot be given immediately after transplant. This is also true for other immunocompromised individuals who have not been vaccinated and who are not candidates for vaccine because of the severity of their immune suppression.
- Children with human immunodeficiency virus infection are routinely vaccinated. As long as they have evidence of serologic immunity, they do not need additional treatment.
Measles, an ancient, highly contagious disease with a history of successful control by vaccination, is now threatening to have an epidemic resurgence. Until recently, measles vaccination largely controlled outbreaks in the United States. The Global Vaccine Action Plan under the World Health Organization aimed to eliminate measles worldwide. Nonetheless, the vaccine refusal movement and slow rollout of vaccine programs globally have interfered with control of the virus. A record number of measles cases have emerged in recent months: more than 700 since January 2019.1 Approximately 70% of recent cases were in unvaccinated patients, and almost all were in US residents.
This update reviews the history, presentation and diagnosis, complications, management, contagion control, and emerging threat of a measles epidemic. It concludes with recommendations for clinical practice in the context of the current measles outbreaks.
FROM UBIQUITOUS TO ERADICATED—AND BACK
Before the measles vaccine was developed and became available in the 1960s, outbreaks of measles occurred predictably every year in the United States and other temperate regions. During yearly outbreaks, measles was so contagious that household contacts had attack rates above 95%. Most cases occurred in very young children, and because infection with the virus causes lifelong immunity, it could be safely assumed that by adulthood, everyone was immune. In an outbreak in the Faroe Islands in 1846, no one who had been alive in the last major outbreak 65 years earlier became ill, but everyone under age 65 was at high risk with “high attack rates,” estimated as 99% from other outbreaks (reviewed by Krugman et al2).
In isolated regions previously free of measles, adults did not have immunity, and when exposed, they often developed severe disease. When European settlers brought measles and smallpox to the Americas beginning in the late 15th century, these diseases decimated whole populations of native peoples who had never been exposed to them.
That was premature. A number of outbreaks have occurred since then; the largest in the United States (before 2019) was in 2000. Over half of the 667 cases reported during that outbreak were in an underimmunized Amish community in Ohio.3
Now it emerges again.
PRESENTATION CAN VARY
The presentation varies somewhat among certain groups.
Nonimmune pregnant women have an especially severe course, likely related to the relative immune suppression of pregnancy.
In immune-suppressed states, measles is not only more severe, it is also difficult to diagnose because the rash can be absent.
In partially vaccinated children and adults, the disease may present atypically, without cough, conjunctivitis, and coryza, and it may be milder, lacking some of the extreme malaise typical of measles and with a shortened course. Measles infection in people who received the inactivated measles vaccine that was briefly available from 1963 to 1967 is also associated with atypical measles syndrome, a severe hypersensitivity reaction to the measles virus. Atypical measles can be prevented by revaccination with a live-virus vaccine.
DIAGNOSIS MAY NEED TO BE CONFIRMED
The diagnosis of measles is straightforward when all of the signs and symptoms are present. In partially vaccinated populations, however, the diagnosis may need to be confirmed by serologic or polymerase chain reaction (PCR) testing.
Differential diagnosis
The differential diagnosis of the fever and a rash typical of measles in children, especially when accompanied by severe malaise, includes the following:
Kawasaki disease. However, the red eyes of Kawasaki are an injection of the bulbar conjunctivae with sparing of the limbus. No eye exudate is present, and respiratory illness is not part of the disease.
Drug eruptions can present with a morbilliform rash and sometimes fever, but not the other signs of measles in either adults or children.
Scarlet fever has a different rash, the sandpaper rash typical of toxin-mediated disease.
Rubella tends to cause mild respiratory symptoms and illness rather than the severe disease of measles and other rash-causing viral infections in children and infants.
Confirmation in confusing cases
To confirm a diagnosis of measles, samples from throat, nasal, and posterior nasopharyngeal swabs should be collected with a blood specimen for serology and sent to the state public health laboratory.4 The US Centers for Disease Control and Prevention gives instructions on who should be tested and with which tests.4
Most testing now uses PCR for viral RNA, as viral culture is more costly and takes longer. For accurate diagnosis, samples for PCR must be obtained during the acute illness.
The serologic gold standard for diagnosis is a 4-fold rise or fall in immunoglobulin G (IgG) titer of paired serum samples sent 10 days to 2 weeks apart around the illness. The IgM test may be negative initially, and a negative test cannot be used to rule out the diagnosis. Confirmed cases should be reported to public health authorities.
COMPLICATIONS: EARLY AND LATE
Frequent complications of measles infection include those related to the primary viral infection of respiratory tract mucosal surfaces, as well as bacterial superinfections. Complications are most likely in children under age 5, nonimmune adults, pregnant women, and immunocompromised people. Typical complications include otitis media, laryngotracheobronchitis (presenting as a croupy cough), pneumonia, and diarrhea.
Late sequelae of measles infection are related in part to serious mucosal damage and generalized immune suppression caused by the virus. Even after recovery from acute infection, children can have persistent diarrhea and failure to thrive, with increased mortality risk in the months after infection. Tuberculosis can reactivate in patients already infected, and new tuberculosis infection can be especially severe. Further, tuberculosis skin tests become less reliable immediately after measles infection. Severe disease and fatalities are increased in populations that have baseline vitamin A deficiency and malnutrition.
Death from measles is most often caused by viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. Before the vaccine era, measles encephalitis occurred in the United States in about 1 in 1,000 measles cases.
Subacute sclerosing panencephalitis is a rare, late, and often fatal complication of measles that presents 7 to 10 years after acute measles infection, usually in adolescence. Beginning with myoclonic jerks, stiffening, and slow mental deterioration, it progresses over 1 to 3 years, with a relentless degenerative course leading to death. Since the introduction of the measles vaccine in 1957, this disease has essentially disappeared in the United States.
SUPPORTIVE CARE, INFECTION CONTROL
Management of measles and its complications is primarily supportive.
Preventing contagion
Measles infection has an incubation period of 8 to 12 days. Individuals are contagious 4 days before to 4 days after rash onset in the normal host but longer in those lacking immune function. Cases can occur up to 21 days after exposure during the contagious period.
The disease is highly contagious, so hospitalized patients should be cared for with airborne precautions. It is crucial that caretakers be vaccinated properly, so that they can care for patients safely. Recommendations for preventing secondary cases by prompt vaccination and giving immune globulin are detailed below, including specific recommendations for individuals with immune system suppression.
The current US public health policy regarding measles vaccine booster doses began in response to the widespread measles outbreak in the United States from 1989 to 1991. Cases occurred more commonly in unvaccinated individuals and in young adults who had received only 1 dose of vaccine.
Today, the policy in areas where measles has been controlled is to vaccinate between 12 and 15 months of age and to boost with a second dose before starting kindergarten. In outbreak situations, the first dose should be given at 6 months of age, with a repeat dose at 12 to 15 months of age and the usual booster before starting kindergarten.
Those born before 1957 can be presumed to have had natural measles, which confers lifelong immunity (Table 3).7
CURRENT THREAT
In 2000, measles was considered controlled in the United States, thanks to the national vaccination policy. But despite overall control, small numbers of cases continued to occur each year, related to exposure to cases imported from areas of the world endemic with measles.
Within the last year, however, major outbreaks have emerged. Incompletely vaccinated populations and unvaccinated individuals are the reason for the progression of current outbreaks.8
Until there is broader acceptance of the vaccine and better adherence to vaccine policies nationally and globally, measles cannot be completely eradicated. But with high vaccination rates, it is predicted that this infection can be controlled and ultimately eradicated.
RECOMMENDATIONS
In the midst of an outbreak and with rising public awareness of the threat of measles, it is important to recognize that MMR vaccination is the most effective way to prevent spread of the virus and maintain measles elimination in the United States. With this in mind, there are several key facts and recommendations regarding vaccination:
Recommendations on vaccination
- In measles-controlled populations, all children should be vaccinated between 12 and 15 months of age and again before kindergarten.
- In outbreak settings, children should receive a first vaccine dose at 6 months of age, a second at 12 to 15 months of age, and a third before kindergarten.
- Children who have received 2 measles vaccine doses can be assumed to be fully vaccinated and thus protected as long as the first dose was after 12 months of age. If the first dose was before 12 months of age, a child needs 3 doses.
- Adults born before 1957 can be assumed to have had measles infection and to be immune.
- Adults who were immunized with the inactivated measles vaccine available between 1963 and 1967 should receive 1 dose of live virus vaccine.
- Boosters are recommended for young adults who did not receive a second dose of vaccine and for adults with an uncertain history of immunization. There is no need to check titers before giving a booster, but if a positive titer is available in an adult, a booster is not needed.
- Heathcare providers should vaccinate unvaccinated or undervaccinated US residents traveling internationally (as long as they do not have contraindications) or traveling within the country to areas with outbreaks of measles.
Recommendations on vaccination after exposure to measles
- Vaccine is recommended for a nonimmune contact, including anyone with a history of only a single dose of vaccine.
- If a child got a first dose of vaccine before 12 months of age, give the second dose as soon as he or she turns 1 year old, or at least 28 days after the first dose.
- Vaccine must be given within 72 hours of exposure to confer protection (or at least decrease disease severity).
- The second dose of vaccine should be given at least 28 days after the first dose.
Recommendations on immune globulin after exposure to measles
- Immune globulin is recommended for anyone with exposure and no history of vaccination or immunity.
- Immune globulin can be given up to 6 days after exposure to prevent or decrease the severity of measles in immunocompromised hosts who have not been previously vaccinated. It is best to give it as early as possible.
- Immune globulin is given intramuscularly at 0.5 mL/kg, up to a to maximum dose of 15 mL.
- Pregnant women and immunocompromised hosts without immunity should receive immunoglobulin intravenously. Children and adults who have had a recent bone marrow transplant and likely do not yet have a reconstituted immune system should be treated with immune globulin to prevent infection, as vaccine cannot be given immediately after transplant. This is also true for other immunocompromised individuals who have not been vaccinated and who are not candidates for vaccine because of the severity of their immune suppression.
- Children with human immunodeficiency virus infection are routinely vaccinated. As long as they have evidence of serologic immunity, they do not need additional treatment.
- Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Measles. In Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics 2018; 537–550.
- Krugman S, Giles JP, Friedman H, Stone S. Studies on immunity to measles. J Pediatr 1965; 66:471–488. pmid:14264306.
- Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375(14):1343–1354. doi:10.1056/NEJMoa1602295
- Centers for Disease Control and Prevention. Measles (rubeola). www.cdc.gov/measles/index.html. Accessed May 16, 2019.
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec 2017; 92(17):205–227. pmid:28459148
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013; summary: recommendations of the Advisory Committee on Immunization Practices ACIP. MMWR Recomm Rep 2013 Jun 14; 62(RR-4)1–34. pmid:23760231
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization for health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Nov 25; 60(RR-7)1–45. pmid:22108587
- Patel M, Lee AD, Redd SB, et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep 2019; May 3; 68(17):402–404. doi:10.15585/mmwr.mm6817e1
- Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Measles. In Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics 2018; 537–550.
- Krugman S, Giles JP, Friedman H, Stone S. Studies on immunity to measles. J Pediatr 1965; 66:471–488. pmid:14264306.
- Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375(14):1343–1354. doi:10.1056/NEJMoa1602295
- Centers for Disease Control and Prevention. Measles (rubeola). www.cdc.gov/measles/index.html. Accessed May 16, 2019.
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec 2017; 92(17):205–227. pmid:28459148
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013; summary: recommendations of the Advisory Committee on Immunization Practices ACIP. MMWR Recomm Rep 2013 Jun 14; 62(RR-4)1–34. pmid:23760231
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization for health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Nov 25; 60(RR-7)1–45. pmid:22108587
- Patel M, Lee AD, Redd SB, et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep 2019; May 3; 68(17):402–404. doi:10.15585/mmwr.mm6817e1
KEY POINTS
- Measles is highly contagious and can have serious complications, including death.
- Measles vaccine is given in a 2-dose series. People who have received only 1 dose should receive either 1 or 2 more doses, depending on the situation, so that they are protected.
- The diagnosis of measles is straightforward when classic signs and symptoms are present—fever, cough, conjunctivitis, runny nose, and rash—especially after a known exposure or in the setting of outbreak. On the other hand, in partially vaccinated or immunosuppressed people, the illness presents atypically, and confirmation of diagnosis requires laboratory testing.
- Management is mostly supportive. Children—and probably also adults—should receive vitamin A.
- Since disease can be severe in the unvaccinated, immune globulin and vaccine are given to the normal host with an exposure and no history of vaccine or immunity.
The return of measles—an unnecessary sequel
So why are we, the trustworthy, having such a tough time convincing people to get routine vaccines for themselves and for their kids? In a sea of truthopenia, we need to do more.
Not everyone refuses vaccines. It is the rare patient in my examination room who, after a discussion, still steadfastly refuses to get a flu shot or pneumonia vaccine. But our dialogue has changed somewhat. Patients still tell me that they or someone they know got the flu from the flu shot or got sick from the pneumonia vaccine (explainable by discussing the immune system’s systemic anamnestic response to a vaccine in the setting of partial immunity—“It’s a good thing”). But more often, I’m hearing detailed stories from the Internet or social media. We heard a less-than-endorsing reflection on the value of vaccines from 2 potential presidential candidates, 1 being a physician, during a televised presidential primary debate. Then there are the tabloid stories, and, of course, there are the celebrity authors and TV talk show doctors touting the unsubstantiated or incompletely substantiated virtues of “anti-inflammatory” and “immune-boosting” diets and supplements as obvious and total truth, while I’m recommending vaccinations and traditional drug therapies. Who can the patient believe? In our limited office-visit time, we must somehow put this external noise into perspective and individualize our suggestions for the patient in front of us.
Certainly the major news media research teams and the on-screen physician consultants to the major news networks have offered up evidence-based discussions on vaccination, the impact of preventable infections on the unvaccinated, and the limitations and reasonable potential benefits of specific dietary interventions and supplements. Unfortunately, their message is being contaminated by the untrusting aura that surrounds mainstream written and TV media.
Despite physicians’ continued high professional rating in the 2018 Gallup poll, some patients, families, and communities are swayed by arguments offered outside of our offices. And when it comes to our summarizing large studies published in major medical journals, the rolling echo of possible fake news and alternative facts comes to the fore. Can they really trust the establishment? There remains doubt in some patients’ minds.
The problem with measles, as Porter and Goldfarb discuss in this issue of the Journal, is that it is extremely contagious. For “herd immunity” to provide protection and prevent outbreaks, nearly everyone must be vaccinated or have natural immunity from childhood infection. Those who are at special risk from infection include the very young, who have an underdeveloped immune system, and adults who were not appropriately vaccinated (eg, those who may only have gotten a single measles vaccination as a child or whose immune system is weakened by disease or immunosuppressive drugs).
What can we do? We need, as a united front, to know the evidence that supports the relative value of vaccination of our child and adult patients and pass it on. We need to confront, accept, and explain to patients that all vaccines are not 100% successful (measles seems to be pretty close, based on the near-eradication of the disease in vaccinated communities up until now), but that even partial immunity is probably beneficial with all vaccines. We need to have a united front when discussing the bulk of evidence that debunks the vaccination-autism connection. We need to support federal and state funding so that all children can get their routine medical exams and vaccinations. We need to support sufficient financial protection for those companies who in good faith continue to develop and test new and improved vaccines for use in this country and around the world; infections can be introduced by travelers who have passed through areas endemic for infections rarely seen in the United States and who may not be aware of their own infection.
We need to live up to our Gallup poll ranking as highly trusted professionals. And we need to partner with our even more highly trusted nursing colleagues to take every opportunity to inform our patients and fight the spread of disinformation.
The morbilliform rash attributed to measles—and not to a sulfa allergy—should have been a phenomenon of the past. We didn’t need to see it again.
So why are we, the trustworthy, having such a tough time convincing people to get routine vaccines for themselves and for their kids? In a sea of truthopenia, we need to do more.
Not everyone refuses vaccines. It is the rare patient in my examination room who, after a discussion, still steadfastly refuses to get a flu shot or pneumonia vaccine. But our dialogue has changed somewhat. Patients still tell me that they or someone they know got the flu from the flu shot or got sick from the pneumonia vaccine (explainable by discussing the immune system’s systemic anamnestic response to a vaccine in the setting of partial immunity—“It’s a good thing”). But more often, I’m hearing detailed stories from the Internet or social media. We heard a less-than-endorsing reflection on the value of vaccines from 2 potential presidential candidates, 1 being a physician, during a televised presidential primary debate. Then there are the tabloid stories, and, of course, there are the celebrity authors and TV talk show doctors touting the unsubstantiated or incompletely substantiated virtues of “anti-inflammatory” and “immune-boosting” diets and supplements as obvious and total truth, while I’m recommending vaccinations and traditional drug therapies. Who can the patient believe? In our limited office-visit time, we must somehow put this external noise into perspective and individualize our suggestions for the patient in front of us.
Certainly the major news media research teams and the on-screen physician consultants to the major news networks have offered up evidence-based discussions on vaccination, the impact of preventable infections on the unvaccinated, and the limitations and reasonable potential benefits of specific dietary interventions and supplements. Unfortunately, their message is being contaminated by the untrusting aura that surrounds mainstream written and TV media.
Despite physicians’ continued high professional rating in the 2018 Gallup poll, some patients, families, and communities are swayed by arguments offered outside of our offices. And when it comes to our summarizing large studies published in major medical journals, the rolling echo of possible fake news and alternative facts comes to the fore. Can they really trust the establishment? There remains doubt in some patients’ minds.
The problem with measles, as Porter and Goldfarb discuss in this issue of the Journal, is that it is extremely contagious. For “herd immunity” to provide protection and prevent outbreaks, nearly everyone must be vaccinated or have natural immunity from childhood infection. Those who are at special risk from infection include the very young, who have an underdeveloped immune system, and adults who were not appropriately vaccinated (eg, those who may only have gotten a single measles vaccination as a child or whose immune system is weakened by disease or immunosuppressive drugs).
What can we do? We need, as a united front, to know the evidence that supports the relative value of vaccination of our child and adult patients and pass it on. We need to confront, accept, and explain to patients that all vaccines are not 100% successful (measles seems to be pretty close, based on the near-eradication of the disease in vaccinated communities up until now), but that even partial immunity is probably beneficial with all vaccines. We need to have a united front when discussing the bulk of evidence that debunks the vaccination-autism connection. We need to support federal and state funding so that all children can get their routine medical exams and vaccinations. We need to support sufficient financial protection for those companies who in good faith continue to develop and test new and improved vaccines for use in this country and around the world; infections can be introduced by travelers who have passed through areas endemic for infections rarely seen in the United States and who may not be aware of their own infection.
We need to live up to our Gallup poll ranking as highly trusted professionals. And we need to partner with our even more highly trusted nursing colleagues to take every opportunity to inform our patients and fight the spread of disinformation.
The morbilliform rash attributed to measles—and not to a sulfa allergy—should have been a phenomenon of the past. We didn’t need to see it again.
So why are we, the trustworthy, having such a tough time convincing people to get routine vaccines for themselves and for their kids? In a sea of truthopenia, we need to do more.
Not everyone refuses vaccines. It is the rare patient in my examination room who, after a discussion, still steadfastly refuses to get a flu shot or pneumonia vaccine. But our dialogue has changed somewhat. Patients still tell me that they or someone they know got the flu from the flu shot or got sick from the pneumonia vaccine (explainable by discussing the immune system’s systemic anamnestic response to a vaccine in the setting of partial immunity—“It’s a good thing”). But more often, I’m hearing detailed stories from the Internet or social media. We heard a less-than-endorsing reflection on the value of vaccines from 2 potential presidential candidates, 1 being a physician, during a televised presidential primary debate. Then there are the tabloid stories, and, of course, there are the celebrity authors and TV talk show doctors touting the unsubstantiated or incompletely substantiated virtues of “anti-inflammatory” and “immune-boosting” diets and supplements as obvious and total truth, while I’m recommending vaccinations and traditional drug therapies. Who can the patient believe? In our limited office-visit time, we must somehow put this external noise into perspective and individualize our suggestions for the patient in front of us.
Certainly the major news media research teams and the on-screen physician consultants to the major news networks have offered up evidence-based discussions on vaccination, the impact of preventable infections on the unvaccinated, and the limitations and reasonable potential benefits of specific dietary interventions and supplements. Unfortunately, their message is being contaminated by the untrusting aura that surrounds mainstream written and TV media.
Despite physicians’ continued high professional rating in the 2018 Gallup poll, some patients, families, and communities are swayed by arguments offered outside of our offices. And when it comes to our summarizing large studies published in major medical journals, the rolling echo of possible fake news and alternative facts comes to the fore. Can they really trust the establishment? There remains doubt in some patients’ minds.
The problem with measles, as Porter and Goldfarb discuss in this issue of the Journal, is that it is extremely contagious. For “herd immunity” to provide protection and prevent outbreaks, nearly everyone must be vaccinated or have natural immunity from childhood infection. Those who are at special risk from infection include the very young, who have an underdeveloped immune system, and adults who were not appropriately vaccinated (eg, those who may only have gotten a single measles vaccination as a child or whose immune system is weakened by disease or immunosuppressive drugs).
What can we do? We need, as a united front, to know the evidence that supports the relative value of vaccination of our child and adult patients and pass it on. We need to confront, accept, and explain to patients that all vaccines are not 100% successful (measles seems to be pretty close, based on the near-eradication of the disease in vaccinated communities up until now), but that even partial immunity is probably beneficial with all vaccines. We need to have a united front when discussing the bulk of evidence that debunks the vaccination-autism connection. We need to support federal and state funding so that all children can get their routine medical exams and vaccinations. We need to support sufficient financial protection for those companies who in good faith continue to develop and test new and improved vaccines for use in this country and around the world; infections can be introduced by travelers who have passed through areas endemic for infections rarely seen in the United States and who may not be aware of their own infection.
We need to live up to our Gallup poll ranking as highly trusted professionals. And we need to partner with our even more highly trusted nursing colleagues to take every opportunity to inform our patients and fight the spread of disinformation.
The morbilliform rash attributed to measles—and not to a sulfa allergy—should have been a phenomenon of the past. We didn’t need to see it again.
Disseminated invasive aspergillosis in an immunocompetent patient
A 57-year-old woman was admitted to our hospital for progressive hypoxic respiratory failure that developed after 10 days of empiric treatment at another hospital for an exacerbation of chronic obstructive pulmonary disease (COPD).
Computed tomography (CT) showed a lesion in the upper lobe of the left lung, with new ground-glass opacities with cystic and cavitary changes raising concern for an inflammatory or infectious cause (Figure 1). Respiratory culture of expectorated secretions grew Aspergillus. Assays for beta-d-glucan and serum Aspergillus immunoglobulin G (IgG) antibodies were positive, although given the improvement in her oxygenation requirements and overall clinical status, these were thought to be trivial. Tests for immunoglobulin deficiencies and human immunodeficiency virus were negative, ruling out primary immunodeficiency. However, within the next 48 hours, her respiratory status declined, and voriconazole was started out of concern for invasive pulmonary aspergillosis based on results of serum IgG testing.
Despite 2 days of treatment with voriconazole, the patient developed respiratory failure. Repeat CT showed that the ground-glass opacities were more dense, especially in the lower lobes, and new patchy infiltrates were noted in the left lung. The patient developed a right tension pneumothorax requiring emergency intubation and chest tube insertion.1 She subsequently developed acute abdominal pain with worsening abdominal distention, diagnosed as pneumoperitoneum. Emergency exploratory laparotomy revealed perforations in the cecum with fecal spillage, requiring ileocecectomy and ileostomy.
Pathologic study of bowel specimens confirmed fungal hyphae with “tree-branch” structures consistent with fungal infection in the bowel (Figure 2).
Oral voriconazole was continued. The patient’s respiratory status improved, and she no longer required supplemental oxygen. She was discharged on a regimen of oral voriconazole 200 mg twice daily. However, over the next 12 months, she had additional hospitalizations for severe sepsis from abdominal wound infections, pneumonia, and Clostridium difficile infection. She will require lifelong antifungal treatment.
INVASIVE PULMONARY ASPERGILLOSIS
Invasive pulmonary aspergillosis is the most severe form of aspergillosis and is most often seen in immunocompromised patients. The death rate is as high as 50% in neutropenic patients regardless of the time to diagnosis or effective treatment.2 It becomes life-threatening as the infection enters the blood stream, leading to formation of thrombi and precipitating embolism and necrosis in the lungs.3
In immunocompetent patients, COPD, tuberculosis, bronchiectasis, liver disease, severe sepsis, and diabetes mellitus predispose to invasive pulmonary aspergillosis.2 Other risk factors include long-term steroid therapy at doses equivalent to prednisone 20 mg/day for at least 13 weeks4 and viral infection such as influenza.5 Chronic use of inhaled corticosteroids has been hypothesized to increase risk.4
Histopathologic confirmation of fungal elements is the gold standard for diagnosis.3 New biomarkers such as beta-d-glucan have shown promise in enabling earlier diagnosis to allow effective treatment of disseminated aspergillosis, as in our patient.6
TAKE-HOME MESSAGE
Although not common, invasive aspergillosis can occur in immunocompetent and near-immunocompetent patients, particularly those with COPD or other underlying lung disease.
Acknowledgment: The authors thank Kimberley Woodward, MD, Inova Fairfax Hospital, Falls Church, VA, for her study of the bowel specimen and for providing the histology slide.
- Vukicevic TA, Dudvarski-Ilic A, Zugic V, Stevanovic G, Rubino S, Barac A. Subacute invasive pulmonary aspergillosis as a rare cause of pneumothorax in immunocompetent patient: brief report. Infection 2017; 45(3):377–380. doi:10.1007/s15010-017-0994-3
- Moreno-González G, Ricart de Mesones A, Tazi-Mezalek R, Marron-Moya MT, Rosell A, Mañez R. Invasive pulmonary aspergillosis with disseminated infection in immunocompetent patient. Can Respir J 2016; 2016:7984032. doi:10.1155/2016/7984032
- Chen L, Liu Y, Wang W, Liu K. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect Dis (Lond) 2015; 47(6):428–432. doi:10.3109/00365548.2014.995697
- Taccone FS, Van den Abeele AM, Bulpa P, et al; AspICU Study Investigators. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19:7. doi:10.1186/s13054-014-0722-7
- Crum-Cianflone NF. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis 2016; 3(3):ofw171. doi:10.1093/ofid/ofw171
- Ergene U, Akcali Z, Ozbalci D, Nese N, Senol S. Disseminated aspergillosis due to Aspergillus niger in immunocompetent patient: a case report. Case Rep Infect Dis 2013; 2013:385190. doi:10.1155/2013/385190
A 57-year-old woman was admitted to our hospital for progressive hypoxic respiratory failure that developed after 10 days of empiric treatment at another hospital for an exacerbation of chronic obstructive pulmonary disease (COPD).
Computed tomography (CT) showed a lesion in the upper lobe of the left lung, with new ground-glass opacities with cystic and cavitary changes raising concern for an inflammatory or infectious cause (Figure 1). Respiratory culture of expectorated secretions grew Aspergillus. Assays for beta-d-glucan and serum Aspergillus immunoglobulin G (IgG) antibodies were positive, although given the improvement in her oxygenation requirements and overall clinical status, these were thought to be trivial. Tests for immunoglobulin deficiencies and human immunodeficiency virus were negative, ruling out primary immunodeficiency. However, within the next 48 hours, her respiratory status declined, and voriconazole was started out of concern for invasive pulmonary aspergillosis based on results of serum IgG testing.
Despite 2 days of treatment with voriconazole, the patient developed respiratory failure. Repeat CT showed that the ground-glass opacities were more dense, especially in the lower lobes, and new patchy infiltrates were noted in the left lung. The patient developed a right tension pneumothorax requiring emergency intubation and chest tube insertion.1 She subsequently developed acute abdominal pain with worsening abdominal distention, diagnosed as pneumoperitoneum. Emergency exploratory laparotomy revealed perforations in the cecum with fecal spillage, requiring ileocecectomy and ileostomy.
Pathologic study of bowel specimens confirmed fungal hyphae with “tree-branch” structures consistent with fungal infection in the bowel (Figure 2).
Oral voriconazole was continued. The patient’s respiratory status improved, and she no longer required supplemental oxygen. She was discharged on a regimen of oral voriconazole 200 mg twice daily. However, over the next 12 months, she had additional hospitalizations for severe sepsis from abdominal wound infections, pneumonia, and Clostridium difficile infection. She will require lifelong antifungal treatment.
INVASIVE PULMONARY ASPERGILLOSIS
Invasive pulmonary aspergillosis is the most severe form of aspergillosis and is most often seen in immunocompromised patients. The death rate is as high as 50% in neutropenic patients regardless of the time to diagnosis or effective treatment.2 It becomes life-threatening as the infection enters the blood stream, leading to formation of thrombi and precipitating embolism and necrosis in the lungs.3
In immunocompetent patients, COPD, tuberculosis, bronchiectasis, liver disease, severe sepsis, and diabetes mellitus predispose to invasive pulmonary aspergillosis.2 Other risk factors include long-term steroid therapy at doses equivalent to prednisone 20 mg/day for at least 13 weeks4 and viral infection such as influenza.5 Chronic use of inhaled corticosteroids has been hypothesized to increase risk.4
Histopathologic confirmation of fungal elements is the gold standard for diagnosis.3 New biomarkers such as beta-d-glucan have shown promise in enabling earlier diagnosis to allow effective treatment of disseminated aspergillosis, as in our patient.6
TAKE-HOME MESSAGE
Although not common, invasive aspergillosis can occur in immunocompetent and near-immunocompetent patients, particularly those with COPD or other underlying lung disease.
Acknowledgment: The authors thank Kimberley Woodward, MD, Inova Fairfax Hospital, Falls Church, VA, for her study of the bowel specimen and for providing the histology slide.
A 57-year-old woman was admitted to our hospital for progressive hypoxic respiratory failure that developed after 10 days of empiric treatment at another hospital for an exacerbation of chronic obstructive pulmonary disease (COPD).
Computed tomography (CT) showed a lesion in the upper lobe of the left lung, with new ground-glass opacities with cystic and cavitary changes raising concern for an inflammatory or infectious cause (Figure 1). Respiratory culture of expectorated secretions grew Aspergillus. Assays for beta-d-glucan and serum Aspergillus immunoglobulin G (IgG) antibodies were positive, although given the improvement in her oxygenation requirements and overall clinical status, these were thought to be trivial. Tests for immunoglobulin deficiencies and human immunodeficiency virus were negative, ruling out primary immunodeficiency. However, within the next 48 hours, her respiratory status declined, and voriconazole was started out of concern for invasive pulmonary aspergillosis based on results of serum IgG testing.
Despite 2 days of treatment with voriconazole, the patient developed respiratory failure. Repeat CT showed that the ground-glass opacities were more dense, especially in the lower lobes, and new patchy infiltrates were noted in the left lung. The patient developed a right tension pneumothorax requiring emergency intubation and chest tube insertion.1 She subsequently developed acute abdominal pain with worsening abdominal distention, diagnosed as pneumoperitoneum. Emergency exploratory laparotomy revealed perforations in the cecum with fecal spillage, requiring ileocecectomy and ileostomy.
Pathologic study of bowel specimens confirmed fungal hyphae with “tree-branch” structures consistent with fungal infection in the bowel (Figure 2).
Oral voriconazole was continued. The patient’s respiratory status improved, and she no longer required supplemental oxygen. She was discharged on a regimen of oral voriconazole 200 mg twice daily. However, over the next 12 months, she had additional hospitalizations for severe sepsis from abdominal wound infections, pneumonia, and Clostridium difficile infection. She will require lifelong antifungal treatment.
INVASIVE PULMONARY ASPERGILLOSIS
Invasive pulmonary aspergillosis is the most severe form of aspergillosis and is most often seen in immunocompromised patients. The death rate is as high as 50% in neutropenic patients regardless of the time to diagnosis or effective treatment.2 It becomes life-threatening as the infection enters the blood stream, leading to formation of thrombi and precipitating embolism and necrosis in the lungs.3
In immunocompetent patients, COPD, tuberculosis, bronchiectasis, liver disease, severe sepsis, and diabetes mellitus predispose to invasive pulmonary aspergillosis.2 Other risk factors include long-term steroid therapy at doses equivalent to prednisone 20 mg/day for at least 13 weeks4 and viral infection such as influenza.5 Chronic use of inhaled corticosteroids has been hypothesized to increase risk.4
Histopathologic confirmation of fungal elements is the gold standard for diagnosis.3 New biomarkers such as beta-d-glucan have shown promise in enabling earlier diagnosis to allow effective treatment of disseminated aspergillosis, as in our patient.6
TAKE-HOME MESSAGE
Although not common, invasive aspergillosis can occur in immunocompetent and near-immunocompetent patients, particularly those with COPD or other underlying lung disease.
Acknowledgment: The authors thank Kimberley Woodward, MD, Inova Fairfax Hospital, Falls Church, VA, for her study of the bowel specimen and for providing the histology slide.
- Vukicevic TA, Dudvarski-Ilic A, Zugic V, Stevanovic G, Rubino S, Barac A. Subacute invasive pulmonary aspergillosis as a rare cause of pneumothorax in immunocompetent patient: brief report. Infection 2017; 45(3):377–380. doi:10.1007/s15010-017-0994-3
- Moreno-González G, Ricart de Mesones A, Tazi-Mezalek R, Marron-Moya MT, Rosell A, Mañez R. Invasive pulmonary aspergillosis with disseminated infection in immunocompetent patient. Can Respir J 2016; 2016:7984032. doi:10.1155/2016/7984032
- Chen L, Liu Y, Wang W, Liu K. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect Dis (Lond) 2015; 47(6):428–432. doi:10.3109/00365548.2014.995697
- Taccone FS, Van den Abeele AM, Bulpa P, et al; AspICU Study Investigators. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19:7. doi:10.1186/s13054-014-0722-7
- Crum-Cianflone NF. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis 2016; 3(3):ofw171. doi:10.1093/ofid/ofw171
- Ergene U, Akcali Z, Ozbalci D, Nese N, Senol S. Disseminated aspergillosis due to Aspergillus niger in immunocompetent patient: a case report. Case Rep Infect Dis 2013; 2013:385190. doi:10.1155/2013/385190
- Vukicevic TA, Dudvarski-Ilic A, Zugic V, Stevanovic G, Rubino S, Barac A. Subacute invasive pulmonary aspergillosis as a rare cause of pneumothorax in immunocompetent patient: brief report. Infection 2017; 45(3):377–380. doi:10.1007/s15010-017-0994-3
- Moreno-González G, Ricart de Mesones A, Tazi-Mezalek R, Marron-Moya MT, Rosell A, Mañez R. Invasive pulmonary aspergillosis with disseminated infection in immunocompetent patient. Can Respir J 2016; 2016:7984032. doi:10.1155/2016/7984032
- Chen L, Liu Y, Wang W, Liu K. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect Dis (Lond) 2015; 47(6):428–432. doi:10.3109/00365548.2014.995697
- Taccone FS, Van den Abeele AM, Bulpa P, et al; AspICU Study Investigators. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19:7. doi:10.1186/s13054-014-0722-7
- Crum-Cianflone NF. Invasive aspergillosis associated with severe influenza infections. Open Forum Infect Dis 2016; 3(3):ofw171. doi:10.1093/ofid/ofw171
- Ergene U, Akcali Z, Ozbalci D, Nese N, Senol S. Disseminated aspergillosis due to Aspergillus niger in immunocompetent patient: a case report. Case Rep Infect Dis 2013; 2013:385190. doi:10.1155/2013/385190
STIs may be overlooked if you fail to ask this question
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Reference
Johnson Jones ML, Chapin-Bardales J, Bizune D. Extragenital chlamydia and gonorrhea among community venue – attending men who have sex with men – Five cities, United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68:321-325. https://www.cdc.gov/mmwr/volumes/68/wr/mm6814a1.htm?s_cid=mm6814a1_w. Accessed May 23, 2019.
Obesity doesn’t hamper flu vaccine response in pregnancy
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
LJUBLJANA, SLOVENIA – ; indeed, it might actually improve their seroconversion rate, Michelle Clarke reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
She presented a prospective cohort study of 90 women vaccinated against influenza during pregnancy, 24 of whom had a BMI of 30 kg/m2 or more. The impetus for the study was the investigators’ understanding that influenza in pregnancy carries an increased risk of severe complications, obesity is a known risk factor for more severe episodes of influenza, and vaccine responses could potentially be adversely affected by obesity, either because of the associated inflammatory state and altered cytokine profile or inadequate vaccine delivery via the intramuscular route. Yet the impact of obesity on vaccine responses in pregnancy has been unclear.
Blood samples obtained before and 1 month after vaccination showed similarly high-titer postvaccination seropositivity rates against influenza B, H3N2, and H1N1 regardless of the women’s weight status. Indeed, the seropositivity rate against all three influenza viruses was higher in the obese subgroup, by a margin of 92%-74%. Also, postvaccination geometric mean antibody titers were significantly higher in the obese group. Particularly impressive was the difference in H1N1 seroconversion, defined as a fourfold increase in titer 28 days after vaccination: 79% versus 55%, noted Ms. Clarke of the University of Adelaide.
Of note, influenza vaccination in the first trimester resulted in a significantly lower seropositive antibody rate than vaccination in the second or third trimesters. The implication is that gestational age at vaccination, regardless of BMI, may be an important determinant of optimal vaccine protection for mothers and their newborns. However, this tentative conclusion requires confirmation in an independent larger sample, because the patient numbers in the study were small: Seropositive antibodies to all three vaccine antigens were documented in just 7 of 12 women (58%) vaccinated in the first trimester, compared with 47 of 53 (89%) vaccinated in the second trimester and 18 of 25 (72%) in the third.
Ms. Clarke reported having no financial conflicts regarding the study, which was supported by the Women’s and Children’s Hospital Research Foundation.
REPORTING FROM ESPID 2019
Key clinical point: High BMI doesn’t impair influenza vaccine responses in pregnant women.
Major finding: Protective antibody levels against all three vaccine antigens were documented 1 month post vaccination in 92% of the obese and 74% of the nonobese mothers.
Study details: This was a prospective observational study of 90 women vaccinated against influenza during pregnancy, 24 of whom were obese.
Disclosures: The study was supported by the University of Adelaide Women’s and Children’s Hospital Research Foundation.
Some Brits snuff out TORCH screen to raise awareness of congenital syphilis
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
LJUBLJANA, SLOVENIA – Pediatricians in the south of England are so concerned about the recent national increase in the diagnosis of syphilis in adults and its ramifications for neonates that they’ve ditched the traditional TORCH newborn screen because the acronym doesn’t specifically remind clinicians to think about congenital syphilis, Mildred A. Iro, MD, PhD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“ explained Dr. Iro of the University of Southampton (England).
She highlighted salient features of three recent cases of congenital syphilis managed at Southampton Children’s Hospital.
“The key message that we’d like to share is that we just need to be more aware about congenital syphilis. Retest mothers if their risk factor status changes, and test suspected infants and children,” Dr. Iro said.
As a practical matter, however, even though current guidelines recommend retesting mothers whose risk factor status becomes heightened following an initial negative syphilis serology result early in pregnancy, clinicians often are unaware that a mother’s risk status has changed. And retesting all mothers during pregnancy isn’t attractive from a cost-benefit standpoint. This makes scrupulous screening of newborns all the more important. And yet TORCH, which stands for Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes infections, isn’t an acronym that promotes awareness of congenital syphilis, a disease which occupies an obscure position in TORCH under the “O” for “Other” heading. That’s why the term “congenital infection screen” has become the new norm in the south of England, she explained.
However, one pediatrician who didn’t consider congenital infection screen to be an improvement in terminology over TORCH had an alternative suggestion, which struck a favorable chord with his fellow audience members: Simply change the acronym to TORCHS, with the S standing for syphilis.
Dr. Iro noted that two of the three affected children were diagnosed at age 7-8 weeks. The third wasn’t diagnosed until age 15 months, when the mother tested positive for syphilis in a subsequent pregnancy. As is typical of the disease known as “the great masquerader,” while all three of the affected children were unwell early in infancy, they presented with a wide range of symptoms. Among the more prominent features were prolonged irritability, respiratory distress, odd rashes, anemia, hepatomegaly, and tachypnea. One infant had reduced movement and pain in one arm.
All three children underwent extensive testing. None had neurosyphilis. All achieved good outcomes on standard guideline-directed therapy.
As for the mothers, they were aged 19, 21, and 23 years when diagnosed with syphilis. All were Caucasian, and antenatal blood testing was negative in all three. None were retested during pregnancy, even though two of them had a male partner or former partner who was positive for syphilis, and the partner of the third disclosed to her that he had sex with men.
At diagnosis, all three women had a strongly positive Treponema pallidum particle agglutination assay, a high rapid plasma reagin, and a positive syphilis IgM assay.
Dr. Iro reported having no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM ESPID 2019