User login
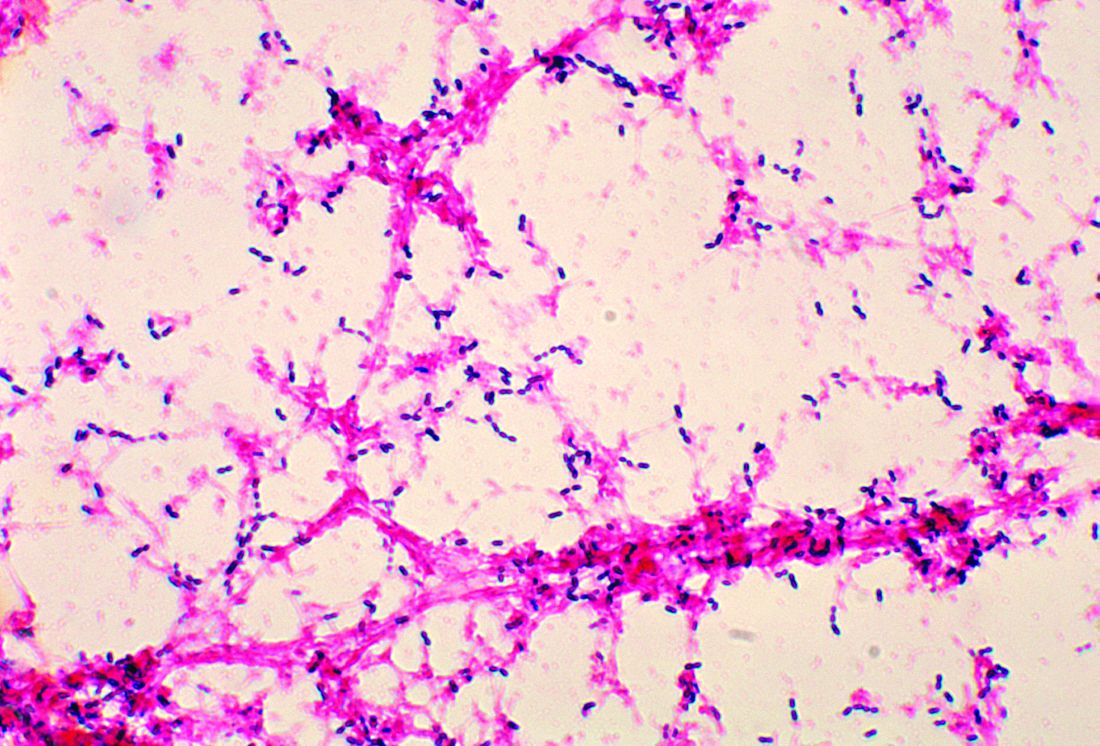
Penicillin-susceptible Streptococcus pneumoniae most common cause of bacteremic CAP
A study found that only 2% of children hospitalized with community-acquired pneumonia (CAP) actually had any causative pathogen in their blood culture results, despite national guidelines that recommend blood cultures for all children hospitalized with moderate to severe CAP.
The guidelines are the 2011 guidelines for managing CAP published by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) (Clin Infect Dis. 2011 Oct;53[7]:617-30).
Cristin O. Fritz, MD, of the Children’s Hospital of Colorado, Aurora, and associates conducted a data analysis of the EPIC (Etiology of Pneumonia in the Community) study to estimate prevalence, risk factors, and clinical outcomes in children hospitalized with bacteremic CAP and to evaluate the relationship between positive blood culture results, empirical antibiotics, and changes in antibiotic treatment regimens.
Data were collected at two Tennessee hospitals and one Utah hospital during Jan. 1, 2010–June 30, 2012. Of the 2,358 children with CAP enrolled in the study, 2,143 (91%) with blood cultures were included in Dr. Fritz’s analysis. Of the 53 patients presenting with positive blood culture results, 46 (2%; 95% confidence interval: 1.6%-2.9%) were identified as having bacteremia. Half of all cases observed were caused by Streptococcus pneumoniae, with Staphylococcus aureus and Streptococcus pyogenes noted less frequently, according to the study published in Pediatrics.
A previous meta-analysis of smaller studies also found that children with CAP rarely had positive blood culture results, a pooled prevalence of 5% (Pediatr Infect Dis J. 2013;32[7]:736-40). Although it is believed that positive blood culture results are key to narrowing the choice of antibiotic and predicting treatment outcomes, the literature – to date – reveals a paucity of data supporting this assumption.
Overall, children in the study presenting with bacteremia experienced more severe clinical outcomes, including longer length of stay, greater likelihood of ICU admission, and invasive mechanical ventilation and/or shock. The authors also observed that bacteremia was less likely to be detected in children given antibiotics after admission but before cultures were obtained (0.8% vs 3%; P = .021). Pleural effusion detected with chest radiograph also consistently indicated bacteremic pneumonia, an observation made within this and other similar studies.
Also of note in detection is the biomarker procalcitonin, which is typically present with bacterial disease. Dr. Fritz and colleagues stressed that because the procalcitonin rate was higher in patients presenting with bacteremia, “this information could influence decisions around culturing if results are rapidly available.” Risk-stratification tools also might serve a valuable purpose in ferreting out those patients presenting with moderate to severe pneumonia most at increased risk for bacterial CAP.
Compared with other studies reporting prevalence ranges of 1%-7%, the prevalence of bacteremia in this study is lower at 2%. The authors attributed the difference to a possible potential limitation with the other studies, for which culture data was only available for a median 47% of enrollees. Dr. Fritz and her colleagues caution that “because cultures were obtained at the discretion of the treating clinician in a majority of studies, blood cultures were likely obtained more often in those with more severe illness or who had not already received antibiotics.” In this scenario, the likelihood that prevalence of bacteremia was overestimated is noteworthy.
The authors observed that penicillin-susceptible S. pneumonia was the most common cause of bacteremic CAP. They further acknowledged that their study and findings by Neuman et al. in 2017 give credence to the joint 2011 PIDS/IDSA guideline recommending narrow-spectrum aminopenicillins specifically to treat children hospitalized due to suspected bacterial CAP.
Despite its small sample size, the results of this study clearly demonstrate that children with bacteremia because of S. pyogenes or S. aureus experience increased morbidity, compared with children with S. pneumoniae, they said
While this is acknowledged to be one of the largest studies of its kind to date, a key limitation was the small number of observable patients with bacteremia, which prevented the researchers from conducting a more in-depth analysis of risk factors and pathogen-specific differences. That one-fourth of patients received in-patient antibiotics before cultures could be collected also likely led to an underestimation of risk factors and misclassification bias. Lastly, the use of blood culture instead of whole-blood polymerase chain reaction, which is known to be more sensitive, also may have led to underestimation of overall bacteremia prevalence.
“In an era with widespread pneumococcal vaccination and low prevalence of bacteremia in the United States, noted Dr. Fritz and associates.
Dr. Fritz had no conflicts of interest to report. Some coauthors cited multiple sources of potential conflict of interest related to consulting fees, grant support, and research support from various pharmaceutical companies and agencies. The study was funded by the National Institutes of Health and in part by a grant from the National Institute of Allergy and Infectious Diseases.
SOURCE: Fritz C et al. Pediatrics. 2019;144(1):e20183090.
A study found that only 2% of children hospitalized with community-acquired pneumonia (CAP) actually had any causative pathogen in their blood culture results, despite national guidelines that recommend blood cultures for all children hospitalized with moderate to severe CAP.

The guidelines are the 2011 guidelines for managing CAP published by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) (Clin Infect Dis. 2011 Oct;53[7]:617-30).
Cristin O. Fritz, MD, of the Children’s Hospital of Colorado, Aurora, and associates conducted a data analysis of the EPIC (Etiology of Pneumonia in the Community) study to estimate prevalence, risk factors, and clinical outcomes in children hospitalized with bacteremic CAP and to evaluate the relationship between positive blood culture results, empirical antibiotics, and changes in antibiotic treatment regimens.
Data were collected at two Tennessee hospitals and one Utah hospital during Jan. 1, 2010–June 30, 2012. Of the 2,358 children with CAP enrolled in the study, 2,143 (91%) with blood cultures were included in Dr. Fritz’s analysis. Of the 53 patients presenting with positive blood culture results, 46 (2%; 95% confidence interval: 1.6%-2.9%) were identified as having bacteremia. Half of all cases observed were caused by Streptococcus pneumoniae, with Staphylococcus aureus and Streptococcus pyogenes noted less frequently, according to the study published in Pediatrics.
A previous meta-analysis of smaller studies also found that children with CAP rarely had positive blood culture results, a pooled prevalence of 5% (Pediatr Infect Dis J. 2013;32[7]:736-40). Although it is believed that positive blood culture results are key to narrowing the choice of antibiotic and predicting treatment outcomes, the literature – to date – reveals a paucity of data supporting this assumption.
Overall, children in the study presenting with bacteremia experienced more severe clinical outcomes, including longer length of stay, greater likelihood of ICU admission, and invasive mechanical ventilation and/or shock. The authors also observed that bacteremia was less likely to be detected in children given antibiotics after admission but before cultures were obtained (0.8% vs 3%; P = .021). Pleural effusion detected with chest radiograph also consistently indicated bacteremic pneumonia, an observation made within this and other similar studies.
Also of note in detection is the biomarker procalcitonin, which is typically present with bacterial disease. Dr. Fritz and colleagues stressed that because the procalcitonin rate was higher in patients presenting with bacteremia, “this information could influence decisions around culturing if results are rapidly available.” Risk-stratification tools also might serve a valuable purpose in ferreting out those patients presenting with moderate to severe pneumonia most at increased risk for bacterial CAP.
Compared with other studies reporting prevalence ranges of 1%-7%, the prevalence of bacteremia in this study is lower at 2%. The authors attributed the difference to a possible potential limitation with the other studies, for which culture data was only available for a median 47% of enrollees. Dr. Fritz and her colleagues caution that “because cultures were obtained at the discretion of the treating clinician in a majority of studies, blood cultures were likely obtained more often in those with more severe illness or who had not already received antibiotics.” In this scenario, the likelihood that prevalence of bacteremia was overestimated is noteworthy.
The authors observed that penicillin-susceptible S. pneumonia was the most common cause of bacteremic CAP. They further acknowledged that their study and findings by Neuman et al. in 2017 give credence to the joint 2011 PIDS/IDSA guideline recommending narrow-spectrum aminopenicillins specifically to treat children hospitalized due to suspected bacterial CAP.
Despite its small sample size, the results of this study clearly demonstrate that children with bacteremia because of S. pyogenes or S. aureus experience increased morbidity, compared with children with S. pneumoniae, they said
While this is acknowledged to be one of the largest studies of its kind to date, a key limitation was the small number of observable patients with bacteremia, which prevented the researchers from conducting a more in-depth analysis of risk factors and pathogen-specific differences. That one-fourth of patients received in-patient antibiotics before cultures could be collected also likely led to an underestimation of risk factors and misclassification bias. Lastly, the use of blood culture instead of whole-blood polymerase chain reaction, which is known to be more sensitive, also may have led to underestimation of overall bacteremia prevalence.
“In an era with widespread pneumococcal vaccination and low prevalence of bacteremia in the United States, noted Dr. Fritz and associates.
Dr. Fritz had no conflicts of interest to report. Some coauthors cited multiple sources of potential conflict of interest related to consulting fees, grant support, and research support from various pharmaceutical companies and agencies. The study was funded by the National Institutes of Health and in part by a grant from the National Institute of Allergy and Infectious Diseases.
SOURCE: Fritz C et al. Pediatrics. 2019;144(1):e20183090.
A study found that only 2% of children hospitalized with community-acquired pneumonia (CAP) actually had any causative pathogen in their blood culture results, despite national guidelines that recommend blood cultures for all children hospitalized with moderate to severe CAP.

The guidelines are the 2011 guidelines for managing CAP published by the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) (Clin Infect Dis. 2011 Oct;53[7]:617-30).
Cristin O. Fritz, MD, of the Children’s Hospital of Colorado, Aurora, and associates conducted a data analysis of the EPIC (Etiology of Pneumonia in the Community) study to estimate prevalence, risk factors, and clinical outcomes in children hospitalized with bacteremic CAP and to evaluate the relationship between positive blood culture results, empirical antibiotics, and changes in antibiotic treatment regimens.
Data were collected at two Tennessee hospitals and one Utah hospital during Jan. 1, 2010–June 30, 2012. Of the 2,358 children with CAP enrolled in the study, 2,143 (91%) with blood cultures were included in Dr. Fritz’s analysis. Of the 53 patients presenting with positive blood culture results, 46 (2%; 95% confidence interval: 1.6%-2.9%) were identified as having bacteremia. Half of all cases observed were caused by Streptococcus pneumoniae, with Staphylococcus aureus and Streptococcus pyogenes noted less frequently, according to the study published in Pediatrics.
A previous meta-analysis of smaller studies also found that children with CAP rarely had positive blood culture results, a pooled prevalence of 5% (Pediatr Infect Dis J. 2013;32[7]:736-40). Although it is believed that positive blood culture results are key to narrowing the choice of antibiotic and predicting treatment outcomes, the literature – to date – reveals a paucity of data supporting this assumption.
Overall, children in the study presenting with bacteremia experienced more severe clinical outcomes, including longer length of stay, greater likelihood of ICU admission, and invasive mechanical ventilation and/or shock. The authors also observed that bacteremia was less likely to be detected in children given antibiotics after admission but before cultures were obtained (0.8% vs 3%; P = .021). Pleural effusion detected with chest radiograph also consistently indicated bacteremic pneumonia, an observation made within this and other similar studies.
Also of note in detection is the biomarker procalcitonin, which is typically present with bacterial disease. Dr. Fritz and colleagues stressed that because the procalcitonin rate was higher in patients presenting with bacteremia, “this information could influence decisions around culturing if results are rapidly available.” Risk-stratification tools also might serve a valuable purpose in ferreting out those patients presenting with moderate to severe pneumonia most at increased risk for bacterial CAP.
Compared with other studies reporting prevalence ranges of 1%-7%, the prevalence of bacteremia in this study is lower at 2%. The authors attributed the difference to a possible potential limitation with the other studies, for which culture data was only available for a median 47% of enrollees. Dr. Fritz and her colleagues caution that “because cultures were obtained at the discretion of the treating clinician in a majority of studies, blood cultures were likely obtained more often in those with more severe illness or who had not already received antibiotics.” In this scenario, the likelihood that prevalence of bacteremia was overestimated is noteworthy.
The authors observed that penicillin-susceptible S. pneumonia was the most common cause of bacteremic CAP. They further acknowledged that their study and findings by Neuman et al. in 2017 give credence to the joint 2011 PIDS/IDSA guideline recommending narrow-spectrum aminopenicillins specifically to treat children hospitalized due to suspected bacterial CAP.
Despite its small sample size, the results of this study clearly demonstrate that children with bacteremia because of S. pyogenes or S. aureus experience increased morbidity, compared with children with S. pneumoniae, they said
While this is acknowledged to be one of the largest studies of its kind to date, a key limitation was the small number of observable patients with bacteremia, which prevented the researchers from conducting a more in-depth analysis of risk factors and pathogen-specific differences. That one-fourth of patients received in-patient antibiotics before cultures could be collected also likely led to an underestimation of risk factors and misclassification bias. Lastly, the use of blood culture instead of whole-blood polymerase chain reaction, which is known to be more sensitive, also may have led to underestimation of overall bacteremia prevalence.
“In an era with widespread pneumococcal vaccination and low prevalence of bacteremia in the United States, noted Dr. Fritz and associates.
Dr. Fritz had no conflicts of interest to report. Some coauthors cited multiple sources of potential conflict of interest related to consulting fees, grant support, and research support from various pharmaceutical companies and agencies. The study was funded by the National Institutes of Health and in part by a grant from the National Institute of Allergy and Infectious Diseases.
SOURCE: Fritz C et al. Pediatrics. 2019;144(1):e20183090.
FROM PEDIATRICS
Measles incidence has slowed as summer begins
There were 33 new measles cases reported last week, bringing the U.S. total to 1,077 for the year through June 20, according to the Centers for Disease Control and Prevention.
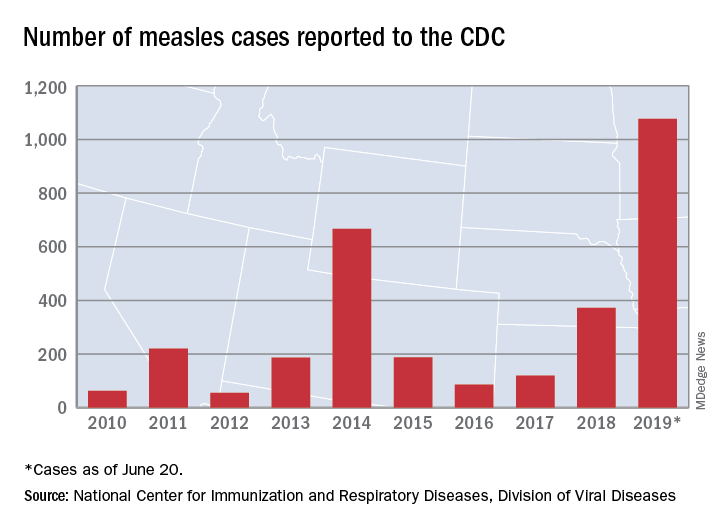
The number of new cases is an increase from the 22 reported the week before, but weekly incidence has been trending downward since hitting a high of 90 in mid-April, CDC data show.
The two continuing outbreaks in New York State made up more than half of the new cases, as Rockland County reported nine cases and New York City reported eight (seven in Brooklyn and one in Queens). Only one new case was reported in California as of the CDC’s June 20 cutoff, but the Los Angeles County Department of Public Health said on June 22 that it was assessing two possible cases, with potential public exposures occurring in a theater and a restaurant.
In a survey conducted in April, a majority of physicians with experience treating measles said that summer travel would lead to increased measles outbreaks and deaths.
There were 33 new measles cases reported last week, bringing the U.S. total to 1,077 for the year through June 20, according to the Centers for Disease Control and Prevention.

The number of new cases is an increase from the 22 reported the week before, but weekly incidence has been trending downward since hitting a high of 90 in mid-April, CDC data show.
The two continuing outbreaks in New York State made up more than half of the new cases, as Rockland County reported nine cases and New York City reported eight (seven in Brooklyn and one in Queens). Only one new case was reported in California as of the CDC’s June 20 cutoff, but the Los Angeles County Department of Public Health said on June 22 that it was assessing two possible cases, with potential public exposures occurring in a theater and a restaurant.
In a survey conducted in April, a majority of physicians with experience treating measles said that summer travel would lead to increased measles outbreaks and deaths.
There were 33 new measles cases reported last week, bringing the U.S. total to 1,077 for the year through June 20, according to the Centers for Disease Control and Prevention.

The number of new cases is an increase from the 22 reported the week before, but weekly incidence has been trending downward since hitting a high of 90 in mid-April, CDC data show.
The two continuing outbreaks in New York State made up more than half of the new cases, as Rockland County reported nine cases and New York City reported eight (seven in Brooklyn and one in Queens). Only one new case was reported in California as of the CDC’s June 20 cutoff, but the Los Angeles County Department of Public Health said on June 22 that it was assessing two possible cases, with potential public exposures occurring in a theater and a restaurant.
In a survey conducted in April, a majority of physicians with experience treating measles said that summer travel would lead to increased measles outbreaks and deaths.
New single-dose influenza therapy effective among outpatients
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Is baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, a safe and effective treatment for acute uncomplicated influenza?
Background: The emergence of oseltamivir-resistant influenza A(H1NI) infection in 2007 highlights the risk of future neuraminidase-resistant global pandemics. Baloxavir represents a new class of antiviral agent that may help treat such outbreaks.
Study design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Outpatients in the United States and Japan.
Synopsis: The trial recruited 1,436 otherwise healthy patients aged 12-64 years of age (median age, 33 years) with a clinical diagnosis of acute uncomplicated influenza pneumonia. The patients were randomly assigned to receive either a single dose of oral baloxavir, oseltamivir 75 mg twice daily for 5 days, or matching placebo within 48 hours of symptom onset. The primary outcome was patient self-assessment of symptomatology.
Among the 1,064 adult patients (age 20-64) with influenza diagnosis confirmed by reverse transcription polymerase chain reaction (RT-PCR), the median time to alleviation of symptoms was lower in the baloxavir group than it was in the placebo group (53.7 hours vs. 80.2 hours; P less than .001). There was no significant difference in time to alleviation of symptoms in the baloxavir group when compared with the oseltamivir group. Adverse events were reported in 21% of baloxavir patients, 25% of placebo patients, and 25% of oseltamivir patients.
The enrolled patients were predominantly young, healthy, and treated as an outpatient. Patients hospitalized with influenza pneumonia are often older, have significant comorbidities, and are at higher risk of poor outcomes. This trial does not directly support the safety or efficacy of baloxavir in this population.
Bottom line: A single dose of baloxavir provides similar clinical benefit as 5 days of oseltamivir therapy in the early treatment of healthy patients with acute influenza.
Citation: Hayden FG et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Eng J Med. 2018:379(10):914-23.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
No Pip/Tazo for patients with ESBL blood stream infections
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: ESBL-producing gram-negative bacilli are becoming increasingly common. Carbapenems are considered the treatment of choice for these infections, but they may in turn select for carbapenem-resistant gram-negative bacilli.
Study design: Open-label, noninferiority, randomized clinical trial.
Setting: Adult inpatients from nine countries (not including the United States).
Synopsis: Patients with at least one positive blood culture for ESBL E. coli or K. pneumoniae were screened. Of the initial 1,646 patients assessed, only 391 were enrolled (866 met exclusion criteria, 218 patients declined, and 123 treating physicians declined). Patients were randomized within 72 hours of the positive blood culture collection to either piperacillin/tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. Patients were treated from 4 to 14 days, with the total duration of antibiotics left up to the treating physician.
The primary outcome was all-cause mortality at 30 days after randomization. The study was stopped early because of a significant mortality difference between the two groups (12.3% in the piperacillin/tazobactam group versus 3.7% in the meropenem group).
The overall mortality rate was lower than expected. The sickest patients may have been excluded because the treating physician needed to approve enrollment. Because of the necessity for empiric antibiotic therapy, there was substantial crossover in antibiotics between the groups, although this would have biased the study toward noninferiority.
Bottom line: For patients with ESBL E. coli or K. pneumoniae blood stream infections, treatment with piperacillin/tazobactam was inferior to meropenem for 30-day mortality.
Citation: Harris PNA et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA. 2018;320(10):984-94.
Dr. Gabriel is assistant professor of medicine and director of Preoperative Medicine and Medicine Consult Service in the division of hospital medicine at Mount Sinai Hospital, New York.
HM19: Sepsis care update
Presenter
Patricia Kritek MD, EdM
Session Title
Sepsis update: From screening to refractory shock
Background
Each year 1.7 million adults in America develop sepsis, and 270,000 Americans die from sepsis annually. Sepsis costs U.S. health care over $27 billion dollars each year. Because of the wide range of etiologies and variation in presentation and intensity, it is a challenge to establish homogeneous evidence based guidelines.1
The definition of sepsis based on the “SIRS” criterion was developed initially in 1992, later revised as Sepsis-2 in 2001. The latest Sepsis-3 definition – “life-threatening organ dysfunction due to a dysregulated host response to infection” – was developed in 2016 by the Third International Consensus Definitions for Sepsis and Septic Shock. This newest definition has renounced the SIRS criterion and adopted the Sequential Organ Failure Assessment (SOFA) score. Treatment guidelines in sepsis were developed by the Surviving Sepsis Campaign starting with the Barcelona Declaration in 2002 and revised multiple times, with the development of 3-hour and 6-hour care bundles in 2012. The latest revision, in 2018, consolidated to a 1-hour bundle.2
Sepsis is a continuum of every severe infection, and with the combined efforts of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, evidence-based guidelines have been developed over the past 2 decades, with the latest iteration in 2018. The Centers for Medicare & Medicaid Services still uses Sepsis-2 for diagnosis, and the 3-hr/6-hr bundle compliance (2016) for expected care.3
Session summary
Dr. Kritek, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, presented to a room of over 1,000 enthusiastic hospitalists. She was able to capture everyone’s attention with a great presentation. As sepsis is one of the most common and serious conditions we encounter, most hospitalists are fairly well versed in evidence-based practices, and Dr. Kritek was able to keep us engaged, describing in detail the evolving definition, pathophysiology, and screening procedures for sepsis. She also spoke about important studies and the latest evidence that will positively impact each hospitalist’s practice in treating sepsis.
Dr. Kritek explained clearly how the Surviving Sepsis Campaign developed a vital and nontraditional guideline that “recommends health systems have a performance improvement program for sepsis including screening for high-risk patients.” In a 1-hour session, Dr. Kritek did a commendable job untangling this bewildering health care challenge, and aligned each component to explain how to best use available resources and address sepsis in individual hospitals.
She talked about the statistics and historical aspects involved in the definition of sepsis, and the Surviving Sepsis Campaign. With three good case scenarios, Dr. Kritek explained how it was difficult to accurately diagnose sepsis using the Sepsis-2/SIRS criterion, and how the SIRS criterion led to several false positives. This created a need for the new Sepsis-3 definition, which used delta SOFA score of 2 indicating “organ failure.”
Key takeaways: Screening
- Sepsis-3 with delta SOFA score of at least 2 and Quick SOFA (qSOFA) of at least 2 was best at predicting in-hospital death, ICU admission, and long ICU stay in ED.
- qSOFA was not helpful in the admitted ICU population. An increase of at least 2 points in SOFA score within 24 hours of admission to the ICU was the best predictor of in-hospital mortality and long ICU stays.
- SIRS has high sensitivity and low specificity. The Early Warning Score has accuracy similar to qSOFA.
- Understanding that there is no perfect answer regarding screening, but having a process is vital for each organization. This approach led to the Surviving Sepsis Campaign guideline: “Recommend health systems have a performance improvement program for sepsis including screening for high-risk patients.”
Key takeaways: Treatment
- Meta-analysis showed that specifically targeted, early goal–directed treatment (specifically, central venous pressure 8-12 mm Hg, central venous oxygen saturation greater than 70%, packed red blood cell inotropes used) did not show any improvement in 90-day mortality, and actually generated worse outcomes, including cirrhosis, as well as higher costs of care.
- Antibiotics: Though part of the 3-hour bundle, antibiotics are recommended to be administered within 1 hour.
- Intravenous fluids: Patients with sepsis-induced hypoperfusion need 30 mL/kg crystalloids. Normal saline and lactated ringer are preferred. Lactated ringer has the advantage over normal saline, with a reduced incidence of major adverse kidney events.
- Importance of bundle compliance: N.Y. study showed use of protocols cut mortality from 30.2% to 25.4%.
Refractory septic shock
- Adding hydrocortisone and fludrocortisone improved mortality at 28 days, helped patients get off vasopressors sooner, and ultimately resulted in less organ failure. But no difference in 90-day mortality.
- A study of vitamin C use in septic patients needs further studies to validate, as it only included 47 patients.
- Early renal replacement therapy showed no difference in mortality or length of stay.
Dr. Kritek’s presentation made a positive impact by helping to explain the reasoning behind the established and evolving best practices and guidelines for care of patients with sepsis and septic shock. Her approach will help hospitalists provide cost-effective care, by understanding which expensive interventions and practices do not make a difference in patient care.
Dr. Odeti is hospitalist medical director at Johnston Memorial Hospital in Abingdon, Va. JMH is part of Ballad Health, a health system operating 21 hospitals in northeast Tennessee and southwest Virginia.
References
1. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.pdf.
2. http://www.survivingsepsis.org/News/Pages/SCCM-and-ACEP-Release-Joint-Statement-About-the-Surviving-Sepsis-Campaign-Hour-1-Bundle.aspx
3. Specifications Manual for National Hospital Inpatient Quality Measures Discharges 01-01-17 (1Q17) through 12-31-17 (4Q17).
Presenter
Patricia Kritek MD, EdM
Session Title
Sepsis update: From screening to refractory shock
Background
Each year 1.7 million adults in America develop sepsis, and 270,000 Americans die from sepsis annually. Sepsis costs U.S. health care over $27 billion dollars each year. Because of the wide range of etiologies and variation in presentation and intensity, it is a challenge to establish homogeneous evidence based guidelines.1
The definition of sepsis based on the “SIRS” criterion was developed initially in 1992, later revised as Sepsis-2 in 2001. The latest Sepsis-3 definition – “life-threatening organ dysfunction due to a dysregulated host response to infection” – was developed in 2016 by the Third International Consensus Definitions for Sepsis and Septic Shock. This newest definition has renounced the SIRS criterion and adopted the Sequential Organ Failure Assessment (SOFA) score. Treatment guidelines in sepsis were developed by the Surviving Sepsis Campaign starting with the Barcelona Declaration in 2002 and revised multiple times, with the development of 3-hour and 6-hour care bundles in 2012. The latest revision, in 2018, consolidated to a 1-hour bundle.2
Sepsis is a continuum of every severe infection, and with the combined efforts of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, evidence-based guidelines have been developed over the past 2 decades, with the latest iteration in 2018. The Centers for Medicare & Medicaid Services still uses Sepsis-2 for diagnosis, and the 3-hr/6-hr bundle compliance (2016) for expected care.3
Session summary
Dr. Kritek, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, presented to a room of over 1,000 enthusiastic hospitalists. She was able to capture everyone’s attention with a great presentation. As sepsis is one of the most common and serious conditions we encounter, most hospitalists are fairly well versed in evidence-based practices, and Dr. Kritek was able to keep us engaged, describing in detail the evolving definition, pathophysiology, and screening procedures for sepsis. She also spoke about important studies and the latest evidence that will positively impact each hospitalist’s practice in treating sepsis.
Dr. Kritek explained clearly how the Surviving Sepsis Campaign developed a vital and nontraditional guideline that “recommends health systems have a performance improvement program for sepsis including screening for high-risk patients.” In a 1-hour session, Dr. Kritek did a commendable job untangling this bewildering health care challenge, and aligned each component to explain how to best use available resources and address sepsis in individual hospitals.
She talked about the statistics and historical aspects involved in the definition of sepsis, and the Surviving Sepsis Campaign. With three good case scenarios, Dr. Kritek explained how it was difficult to accurately diagnose sepsis using the Sepsis-2/SIRS criterion, and how the SIRS criterion led to several false positives. This created a need for the new Sepsis-3 definition, which used delta SOFA score of 2 indicating “organ failure.”
Key takeaways: Screening
- Sepsis-3 with delta SOFA score of at least 2 and Quick SOFA (qSOFA) of at least 2 was best at predicting in-hospital death, ICU admission, and long ICU stay in ED.
- qSOFA was not helpful in the admitted ICU population. An increase of at least 2 points in SOFA score within 24 hours of admission to the ICU was the best predictor of in-hospital mortality and long ICU stays.
- SIRS has high sensitivity and low specificity. The Early Warning Score has accuracy similar to qSOFA.
- Understanding that there is no perfect answer regarding screening, but having a process is vital for each organization. This approach led to the Surviving Sepsis Campaign guideline: “Recommend health systems have a performance improvement program for sepsis including screening for high-risk patients.”
Key takeaways: Treatment
- Meta-analysis showed that specifically targeted, early goal–directed treatment (specifically, central venous pressure 8-12 mm Hg, central venous oxygen saturation greater than 70%, packed red blood cell inotropes used) did not show any improvement in 90-day mortality, and actually generated worse outcomes, including cirrhosis, as well as higher costs of care.
- Antibiotics: Though part of the 3-hour bundle, antibiotics are recommended to be administered within 1 hour.
- Intravenous fluids: Patients with sepsis-induced hypoperfusion need 30 mL/kg crystalloids. Normal saline and lactated ringer are preferred. Lactated ringer has the advantage over normal saline, with a reduced incidence of major adverse kidney events.
- Importance of bundle compliance: N.Y. study showed use of protocols cut mortality from 30.2% to 25.4%.
Refractory septic shock
- Adding hydrocortisone and fludrocortisone improved mortality at 28 days, helped patients get off vasopressors sooner, and ultimately resulted in less organ failure. But no difference in 90-day mortality.
- A study of vitamin C use in septic patients needs further studies to validate, as it only included 47 patients.
- Early renal replacement therapy showed no difference in mortality or length of stay.
Dr. Kritek’s presentation made a positive impact by helping to explain the reasoning behind the established and evolving best practices and guidelines for care of patients with sepsis and septic shock. Her approach will help hospitalists provide cost-effective care, by understanding which expensive interventions and practices do not make a difference in patient care.
Dr. Odeti is hospitalist medical director at Johnston Memorial Hospital in Abingdon, Va. JMH is part of Ballad Health, a health system operating 21 hospitals in northeast Tennessee and southwest Virginia.
References
1. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.pdf.
2. http://www.survivingsepsis.org/News/Pages/SCCM-and-ACEP-Release-Joint-Statement-About-the-Surviving-Sepsis-Campaign-Hour-1-Bundle.aspx
3. Specifications Manual for National Hospital Inpatient Quality Measures Discharges 01-01-17 (1Q17) through 12-31-17 (4Q17).
Presenter
Patricia Kritek MD, EdM
Session Title
Sepsis update: From screening to refractory shock
Background
Each year 1.7 million adults in America develop sepsis, and 270,000 Americans die from sepsis annually. Sepsis costs U.S. health care over $27 billion dollars each year. Because of the wide range of etiologies and variation in presentation and intensity, it is a challenge to establish homogeneous evidence based guidelines.1
The definition of sepsis based on the “SIRS” criterion was developed initially in 1992, later revised as Sepsis-2 in 2001. The latest Sepsis-3 definition – “life-threatening organ dysfunction due to a dysregulated host response to infection” – was developed in 2016 by the Third International Consensus Definitions for Sepsis and Septic Shock. This newest definition has renounced the SIRS criterion and adopted the Sequential Organ Failure Assessment (SOFA) score. Treatment guidelines in sepsis were developed by the Surviving Sepsis Campaign starting with the Barcelona Declaration in 2002 and revised multiple times, with the development of 3-hour and 6-hour care bundles in 2012. The latest revision, in 2018, consolidated to a 1-hour bundle.2
Sepsis is a continuum of every severe infection, and with the combined efforts of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine, evidence-based guidelines have been developed over the past 2 decades, with the latest iteration in 2018. The Centers for Medicare & Medicaid Services still uses Sepsis-2 for diagnosis, and the 3-hr/6-hr bundle compliance (2016) for expected care.3
Session summary
Dr. Kritek, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, presented to a room of over 1,000 enthusiastic hospitalists. She was able to capture everyone’s attention with a great presentation. As sepsis is one of the most common and serious conditions we encounter, most hospitalists are fairly well versed in evidence-based practices, and Dr. Kritek was able to keep us engaged, describing in detail the evolving definition, pathophysiology, and screening procedures for sepsis. She also spoke about important studies and the latest evidence that will positively impact each hospitalist’s practice in treating sepsis.
Dr. Kritek explained clearly how the Surviving Sepsis Campaign developed a vital and nontraditional guideline that “recommends health systems have a performance improvement program for sepsis including screening for high-risk patients.” In a 1-hour session, Dr. Kritek did a commendable job untangling this bewildering health care challenge, and aligned each component to explain how to best use available resources and address sepsis in individual hospitals.
She talked about the statistics and historical aspects involved in the definition of sepsis, and the Surviving Sepsis Campaign. With three good case scenarios, Dr. Kritek explained how it was difficult to accurately diagnose sepsis using the Sepsis-2/SIRS criterion, and how the SIRS criterion led to several false positives. This created a need for the new Sepsis-3 definition, which used delta SOFA score of 2 indicating “organ failure.”
Key takeaways: Screening
- Sepsis-3 with delta SOFA score of at least 2 and Quick SOFA (qSOFA) of at least 2 was best at predicting in-hospital death, ICU admission, and long ICU stay in ED.
- qSOFA was not helpful in the admitted ICU population. An increase of at least 2 points in SOFA score within 24 hours of admission to the ICU was the best predictor of in-hospital mortality and long ICU stays.
- SIRS has high sensitivity and low specificity. The Early Warning Score has accuracy similar to qSOFA.
- Understanding that there is no perfect answer regarding screening, but having a process is vital for each organization. This approach led to the Surviving Sepsis Campaign guideline: “Recommend health systems have a performance improvement program for sepsis including screening for high-risk patients.”
Key takeaways: Treatment
- Meta-analysis showed that specifically targeted, early goal–directed treatment (specifically, central venous pressure 8-12 mm Hg, central venous oxygen saturation greater than 70%, packed red blood cell inotropes used) did not show any improvement in 90-day mortality, and actually generated worse outcomes, including cirrhosis, as well as higher costs of care.
- Antibiotics: Though part of the 3-hour bundle, antibiotics are recommended to be administered within 1 hour.
- Intravenous fluids: Patients with sepsis-induced hypoperfusion need 30 mL/kg crystalloids. Normal saline and lactated ringer are preferred. Lactated ringer has the advantage over normal saline, with a reduced incidence of major adverse kidney events.
- Importance of bundle compliance: N.Y. study showed use of protocols cut mortality from 30.2% to 25.4%.
Refractory septic shock
- Adding hydrocortisone and fludrocortisone improved mortality at 28 days, helped patients get off vasopressors sooner, and ultimately resulted in less organ failure. But no difference in 90-day mortality.
- A study of vitamin C use in septic patients needs further studies to validate, as it only included 47 patients.
- Early renal replacement therapy showed no difference in mortality or length of stay.
Dr. Kritek’s presentation made a positive impact by helping to explain the reasoning behind the established and evolving best practices and guidelines for care of patients with sepsis and septic shock. Her approach will help hospitalists provide cost-effective care, by understanding which expensive interventions and practices do not make a difference in patient care.
Dr. Odeti is hospitalist medical director at Johnston Memorial Hospital in Abingdon, Va. JMH is part of Ballad Health, a health system operating 21 hospitals in northeast Tennessee and southwest Virginia.
References
1. https://www.sepsis.org/wp-content/uploads/2017/05/Sepsis-Fact-Sheet-2018.pdf.
2. http://www.survivingsepsis.org/News/Pages/SCCM-and-ACEP-Release-Joint-Statement-About-the-Surviving-Sepsis-Campaign-Hour-1-Bundle.aspx
3. Specifications Manual for National Hospital Inpatient Quality Measures Discharges 01-01-17 (1Q17) through 12-31-17 (4Q17).
Adjuvant corticosteroids in hospitalized patients with CAP
When is it appropriate to treat?
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
When is it appropriate to treat?
When is it appropriate to treat?
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
Case
A 55-year-old male with a history of tobacco use disorder presents with 2 days of productive cough, fever, chills, and mild shortness of breath. T 38.4, HR 89, RR 32, BP 100/65, 02 sat 86% on room air. Exam reveals diminished breath sounds and positive egophony over the right lung base. WBC is 16,000 and BUN 22. Chest x-ray reveals right lower lobe consolidation. He is given ceftriaxone and azithromycin.
Brief overview of the issue
Community-acquired pneumonia (CAP) is the leading cause of infectious disease–related death in the United States. Mortality associated with CAP is estimated at 57,000 deaths annually and occurs largely in patients requiring hospitalization.1 The 30-day mortality rate in patients who are hospitalized for CAP is approximately 10%-12%.2 After discharge from the hospital, about 18% of patients are readmitted within 30 days.3 An excessive inflammatory cytokine response may be a major contributor to the high mortality rate in CAP and systemic corticosteroids may reduce the inflammatory response from the infection by down-regulating this proinflammatory cytokine production.
Almost all of the major decisions regarding management of CAP, including diagnostic and treatment issues, revolve around the initial assessment of severity of illness. Between 40% and 60% of patients who present to the emergency department with CAP are admitted4 and approximately 10% of hospitalized patients with CAP require ICU admission.5 Validated instruments such as CURB-65, the pneumonia severity index (PSI), and guidelines from the Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) may predict severity of illness but should always be supplemented with physician determination of subjective factors when determining treatment.5 Although there is no census definition of severe pneumonia, studies generally define the condition in the following order of preference: PSI score of IV or V followed by CURB-65 score of two or greater. If these scoring modalities were not available, the IDSA/ATS criteria was used (1 major or 3 minor). Others define severe CAP as pneumonia requiring supportive therapy within a critical care environment.
Overview of the data
The use of corticosteroids in addition to antibiotics in the treatment of CAP was proposed as early as the 1950s and yet only in the last decade has the body of evidence grown significantly.5 There is evidence that corticosteroids suppress inflammation without acutely impairing the immune response as evidenced by a rapid and sustained decrease in circulating inflammatory markers such as C-reactive protein and interleukin 6 and no effect on the anti-inflammatory interleukin 10.6 Within the last year, three meta-analyses, one by the Cochrane Library, one by the IDSA, and a third in the American Journal of Emergency Medicine, addressed the role of routine low dose (20-60 mg of prednisone or equivalent), short-course (3-7 days) systemic corticosteroids in hospitalized patients with CAP of varying severities.
The Cochrane meta-analysis, the largest and most recent dataset, included 13 trials with a combined 1,954 adult patients and found that corticosteroids significantly lowered mortality in hospitalized patients with severe CAP with a number needed to treat of 19.7 In this group with severe CAP, mortality was lowered from 13% to 8% and there were significantly fewer episodes of respiratory failure and shock with the addition of corticosteroids. No effect was seen on mortality in patients with less severe CAP. In those patients who received adjuvant corticosteroids, length of hospital stay decreased by 3 days, regardless of CAP severity.7
The IDSA meta-analysis was similar and included 1,506 patients from six trials.8 In contrast with the Cochrane study, this analysis found corticosteroids did not significantly lower mortality in patients with severe CAP but did reduce time to clinical stability and length of hospital stay by over 1 day. This study also found significantly more CAP-related, 30-day rehospitalizations (5% vs. 3%; defined as recurrent pneumonia, other infection, pleuritic pain, adverse cardiovascular event, or diarrhea) in patient with non-severe CAP treated with corticosteroids.
The study in the American Journal of Emergency Medicine involved ten trials involving more than 700 patients admitted with severe CAP and found in-hospital mortality was cut in half (RR 0.49) and length of hospital stay was reduced when patients were treated with corticosteroids in addition to standard antibiotic therapy.9
In 2015, two randomized clinical trials, one in the Lancet and the other in JAMA, and a meta-analysis in Annals of Internal Medicine assessed the impact of adjuvant corticosteroids in the treatment of hospitalized patients with CAP. The Lancet study of 785 patients hospitalized with CAP of any severity found shortened time to clinical stability (3.0 vs. 4.4 days) as defined by stable vital signs, improved oral intake, and normalized mental status for greater than 24 hours when oral prednisone 50 mg for 7 days was added to standard therapy.10 Patients in the treatment group were also discharged 1 day earlier compared with the placebo control group.
The study in JAMA was small, with only 100 patients at three teaching hospitals in Spain, but found that patients hospitalized with severe CAP and high inflammatory response based on elevated C-reactive protein were less likely to experience a treatment failure, defined as shock, mechanical ventilation, death, or radiographic progression, when intravenous methylprednisolone 0.5 mg/kg was added to standard antibiotic therapy.11
Finally, the meta-analysis in Annals of Internal Medicine assessed 13 randomized controlled placebo trials of 1,974 patients and found that adjuvant corticosteroids in a dose of 20-60 mg of prednisone or equivalent total daily dose significantly lowered mortality in patients with severe CAP and incidence of respiratory distress syndrome, and need for mechanical ventilation in all patients hospitalized with CAP.12
Importantly, nearly all of the described studies showed a significantly higher incidence of hyperglycemia in patients who received corticosteroids.
Application of the data to our patients
The benefit of adjuvant corticosteroids is most clear in hospitalized patients with severe CAP. Recent, strong evidence supports decreased mortality, decreased time to clinical stability, and decreased length of stay in our patient, with severe CAP, if treated with 20-60 mg of prednisone or equivalent total daily dose for 3-7 days. For patients with non-severe CAP, we suggest taking a risk-benefit approach based on other comorbidities, as the risk for CAP-related rehospitalizations may be higher.
For patients with underlying lung disease, specifically COPD or reactive airway disease, we suggest a low threshold for adding corticosteroids. This approach is more anecdotal than data driven, though corticosteroids are a mainstay of treatment for COPD exacerbations and a retrospective analysis of more than 20,000 hospitalized children with CAP and wheezing revealed decreased length of stay with corticosteroid treatment.13 Furthermore, a number of the studies described above included patients with COPD. Our threshold rises significantly in patients with poorly controlled diabetes mellitus.
Bottom line
For patients hospitalized with severe community-acquired pneumonia, recent evidence supports the use of low dose, short-course, systemic corticosteroids in addition to standard therapy.
Dr. Parsons is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center in Charlottesville, Va. Dr. Miller is an assistant professor at the University of Virginia and a hospitalist at the University of Virginia Medical Center. Dr. Hoke is Associate Director of Hospital Medicine and Faculty Development at the University of Virginia.
References
1. Ramirez J et al. Adults hospitalized with pneumonia in the United States: Incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Dec 1:65(11):1806-12.
2. Musher D et al. Community-acquired pneumonia: Review article. N Engl J Med. 2014 Oct 23;371:1619-28.
3. Wunderink R et al. Community-aquired pneumonia: Clinical practice. N Engl J Med. 2014 Feb 6;370:543-51.
4. Mandell L et al. Infectious Diseases Society America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin Infect Dis. 2007;44:S27-72.
5. Wagner HN et al. The effect of hydrocortisone upon the course of pneumococcal pneumonia treated with penicillin. Bull Johns Hopkins Hosp. 1956;98:197-215.
6. Polverino E et al. Systemic corticosteroids for community-acquired pneumonia: Reasons for use and lack of benefit on outcome. Respirology. 2013. Feb;18(2):263-71 (https://doi.org/10.1111/resp.12013).
7. Stern A et al. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017 Dec 13; 12:CD007720 (https://doi.org/10.1002/14651858.CD007720.pub3).
8. Briel M et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1;66:346 (https://doi.org/10.1093/cid/cix801).
9. Wu W-F et al. Efficacy of corticosteroid treatment for severe community-acquired pneumonia: A meta-analysis. Am J Emerg Med. 2017 Jul 15; [e-pub] (http://dx.doi.org/10.1016/j.ajem.2017.07.050).
10. Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2015 Jan 18; [e-pub ahead of print] (http://dx.doi.org/10.1016/S0140-6736[14]62447-8).
11. Torres A et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: A randomized clinical trial. JAMA 2015 Feb 17; 313:677 (http://dx.doi.org/10.1001/jama.2015.88).
12. Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6;163:519 (http://dx.doi.org/10.7326/M15-0715).
13. Simon LH et al. Management of community-acquired pneumonia in hospitalized children. Current Treat Options Peds (2015) 1:59 (https://doi:.org/10.1007/s40746-014-0011-3).
Key points
• For patients hospitalized with severe CAP, recent evidence supports the use of low-dose, short-course, systemic corticosteroids in addition to standard therapy.
• Among hospitalized patients with non-severe CAP, the benefit is not well defined. Studies suggest these patients may benefit from reduced time to clinical stability and reduced length of hospital stay. However, they may be at risk for significantly more CAP-related, 30-day rehospitalizations and hyperglycemia.
• Further prospective, randomized controlled studies are needed to further delineate the patient population who will most benefit from adjunctive corticosteroids use, including dose and duration of treatment.
QUIZ
Which of the following is FALSE regarding community acquired pneumonia?
A. CAP is the leading cause of infectious disease related death in the United States.
B. An excessive inflammatory cytokine response may contribute to the high mortality rate in CAP.
C. Adjunctive steroid therapy has been shown to decrease mortality in all patients with CAP.
D. Hyperglycemia occurs more frequently in patients receiving steroid therapy.
E. Reasons to avoid adjunctive steroid therapy in CAP include low risk for mortality, poorly controlled diabetes, suspected viral or fungal etiology, and elevated risk for gastrointestinal bleeding.
ANSWER: C. The patient population that may benefit most from the use of adjuvant corticosteroids is poorly defined. However, in patients with severe pneumonia, the use of adjuvant steroids has been shown to decrease mortality, time to clinical stability, and length of stay.
Additional reading
Siemieniuk RAC et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: A systematic review and meta-analysis. Ann Intern Med. 2015 Oct 6; 163:519. (http://dx.doi.org/10.7326/M15-0715).
Briel M et al. Corticosteroids in patients hospitalized with community-acquired Pneumonia: Systematic review and individual patient data meta-analysis. Clin Infect Dis. 2018 Feb 1; 66:346 (https://doi.org/10.1093/cid/cix801).
Blum CA et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015 Jan 18; [e-pub ahead of print]. (http://dx.doi.org/10.1016/S0140-6736(14)62447-8).
Feldman C et al. Corticosteroids in the adjunctive therapy of community-acquired pneumonia: an appraisal of recent meta-analyses of clinical trials. J Thorac Dis. 2016 Mar; 8(3):E162-E171.
Wan YD et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: A systemic review and meta-analysis. Chest. 2016 Jan;149(1):209-19.
FDA warns about fecal microbiota for transplantation
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
Officials at the Food and Drug Administration have issued a safety alert regarding the use of fecal microbiota for transplantation and the risk of serious adverse reactions because of transmission of multidrug-resistant organisms (MDROs).
According to the alert, which was issued on June 13, 2019, the agency became aware of two immunocompromised adult patients who received investigational fecal microbiota for transplantation (FMT) and developed extended-spectrum beta-lactamase (EBSL)–producing Escherichia coli. One of the patients died.
“This is certainly a theoretical risk that we’ve known about,” Lea Ann Chen, MD, a gastroenterologist at New York University, said in an interview. “This announcement is important, because we probably don’t counsel patients specifically about this risk. We say there is a risk for transmission of infectious agents in general, but I think that probably very few counsel patients about a risk for transmission of MDROs.”
The donor stool and FMT used in the two patients were not tested for ESBL-producing gram-negative organisms prior to use.
As a result of these serious adverse reactions, the FDA has determined that the following donor screening and stool testing protections are needed for any investigational use of FMT.
- Donor screening with questions that specifically address risk factors for colonization with MDROs, and exclusion of individuals at higher risk of colonization with MDROs.
- MDRO testing of donor stool and exclusion of stool that tests positive for MDRO. FDA scientists have determined the specific MDRO testing and frequency that should be implemented.
On June 14, the American Gastroenterological Association sent a communication about the FDA alert to its members, which stated that the AGA “is committed to advancing applications of the gut microbiome. Our top priority is ensuring patient safety from microbiome-based therapeutics, such as FMT. Through the AGA FMT National Registry, AGA is working with physicians and patients to track FMT usage, patient outcomes and adverse events. Associated with the registry is a biorepository of donor and patient stool samples, which will allow further investigation of unexpected events such as those described in FDA’s safety alert.”
Dr. Chen, who received the AGA Research Foundation’s 2016 Research Scholar Award for her work on the gut microbiome and inflammatory bowel disease, pointed out that FMT has also been studied as a way to prevent colonization and infection with certain drug resistant organisms, such as vancomycin-resistant Enterococcus.
“Therefore, it’s not that FMT is ‘bad;’ we just have to be more diligent about optimizing the safety of the procedure by screening for of multidrug-resistant organisms,” she said. “We also need to study the use of FMT more, so that we can fully understand the risks associated with the procedure. It’s an important and potentially lifesaving procedure for some, but it’s important that everyone go into the procedure understanding fully what the risks and benefits are.”
Suspected adverse events related to the administration of FMT products can be reported to the FDA at 1-800-332-1088 or via MedWatch.
U.S. travelers to Europe need up to date measles immunization
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
researchers at the Centers for Disease Control and Prevention recommend in a Pediatrics special report.
More than 41,000 measles cases and 37 deaths – primarily due to low immunization coverage – were reported in the World Health Organization European Region in the first 6 months of 2018, the highest incidence since the 1990s. Typical case counts since 2010 have ranged from 5,000 to 24,000 in this region, wrote Kristina M. Angelo, DO, MPH, of the Centers for Disease Control and Prevention Travelers’ Health Branch in Atlanta, and associates.
France, Italy and Greece – all particularly popular countries for U.S. vacationers to visit – have particularly high numbers of cases, as do Georgia, Russia, Serbia and, comprising the majority of cases, Ukraine. Italy, for example, is the 10th most popular destination worldwide for Americans, with an estimated 2.5 million American visitors in 2015.
“The large number of measles infections in the WHO European Region ... is a global concern because the European continent is the most common travel destination worldwide,” but is not perceived as a place with infectious disease risk. So travelers may not consider the need of a pretravel health consultation, including vaccination, they said.
But they need to, Dr. Angelo and associates state, and health care providers should be vigilant about checking for symptoms of measles among those who have recently returned from overseas. Given how highly contagious measles is, unvaccinated and under vaccinated travelers to Europe are susceptible to infection, as are any people they encounter back in the United States if the travelers come home sick.
Measles was eliminated in the United States in 2000, but that status is in jeopardy, CDC officials recently warned. The number of domestic measles cases has exceeded 1,000 just halfway through 2019, the highest count since 1992, nearly a decade before elimination.
“Avoiding international travel with nonimmune infants and performing early vaccination at 6 to 12 months of age per the ACIP [Advisory Committee on Immunization Practices] recommendations if travel is unavoidable are of utmost importance,” Dr. Angelo and colleagues advised. “Other at-risk populations (e.g., immunocompromised individuals and pregnant women), for whom vaccination against the measles virus is contraindicated, may consider alternative destinations or delay travel to measles-endemic destinations or areas with known, ongoing measles outbreaks.”
“Presumptive immunity to measles is defined as 1 or more of the following: birth before 1957, laboratory evidence of immunity or infection, 1 or more doses of a measles containing vaccine administered for preschool-aged children and low-risk adults, or 2 doses of measles vaccine among school-aged children and high-risk adults, including international travelers,” they explained.
In Europe, measles remains endemic in Belgium, Bosnia and Herzegovina, France, Georgia, Germany, Italy, Romania, the Russian Federation, Serbia and the Ukraine, the authors wrote.
“As long as measles remains endemic in other countries, the United States will be challenged by measles importations,” the authors wrote. Yet at least one past study in 2017 revealed a third of U.S. travelers to Europe left the country without being fully vaccinated against measles, most often due to vaccine refusal.
“The reason one-third of travelers to Europe missed an opportunity for measles vaccination remains unclear,” the authors wrote. “It may represent a lack of concern or awareness on the part of travelers and the health care providers about acquiring measles in Europe.”
Dr. Angelo and colleagues also emphasized the importance of returning U.S. travelers seeking health care if they have symptoms of measles, including fever and a rash.
Health care providers should ask all patients about recent international travel, they stated. “If measles is suspected, health care providers should isolate travelers immediately, placing them on airborne precautions until day 4 of the rash.” Providers may consider administering immunoglobulin for unvaccinated and undervaccinated travelers and monitor them for 21 days for development of measles symptoms.
The statement was funded by the CDC. The authors reported no relevant financial disclosures.
SOURCE: Angelo KM et al. Pediatrics. 2019 Jun 17. doi: /10.1542/peds.2019-0414.
FROM PEDIATRICS
Booster vaccines found largely safe in children on immunosuppressive drugs
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
MADRID – Administration of live attenuated booster of the MMR vaccine with or without varicella (MMR/V) was not associated with serious adverse events in children on immunosuppressive therapy for a rheumatic disease, according to data presented at the European Congress of Rheumatology.
“The study implies that patients can receive booster vaccinations regardless of age, diagnosis, or therapy,” reported Veronica Bergonzo Moshe, MD, a pediatric rheumatologist at Meir Medical Center, Kfar Saba, Israel.
In the absence of safety data, the vaccination of children with rheumatic diseases taking immunosuppressive therapies has been controversial. Although these children face communicable and sometimes life-threatening diseases without vaccination, many clinicians are not offering this protection because they fear adverse consequences.
Current Paediatric Rheumatology European Society (PReS) guidelines have been equivocal, recommending that vaccines be considered on a “case-by-case basis” in children with a rheumatic disease if they are taking high doses of disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, or any dose of biologics.
“The fear is that a state of immune suppression might decrease response to the vaccine or lead to a flare of the rheumatologic disease,” Dr. Moshe said.
In the retrospective study presented by Dr. Moshe, data were collected on 234 children with rheumatic diseases who received a live attenuated MMR/V booster. The children were drawn from 12 pediatric rheumatology centers in 10 countries.
In this relatively large series, 82% of the children had oligoarticular or polyarticular juvenile idiopathic arthritis (JIA). A range of other rheumatic diseases, including juvenile dermatomyositis, localized scleroderma, and isolated idiopathic uveitis were represented among the remaining patients. All were taking medication, and 48% were in remission.
When broken down by therapy, there were three localized reactions in 110 (2.7%) children who received the booster while on methotrexate. No other adverse events, including disease flare, were observed.
Similarly, six of the seven adverse events observed in 76 (8%) patients who were taking methotrexate plus a tumor necrosis factor (TNF) inhibitor biologic at the time of vaccination were local reactions. Fever was reported in one patient. All of these events were transient.
In the 39 patients taking a TNF inhibitor alone, there was a single case of transient fever. There were no adverse events reported in the three patients vaccinated while on tocilizumab, seven patients while on anakinra, or five patients while on canakinumab.
Following vaccination, there were no signs of symptoms of the diseases that the vaccines are designed to prevent. In the minority of patients who did develop localized reactions or fever in this series, there was no apparent relationship with disease activity, age, or sex when compared to those who did not develop an adverse event.
These retrospective data are not definitive, but they are reassuring, according to Dr. Moshe. A larger prospective study by the PReS vaccination study group is now planned. The issue of leaving children unvaccinated is topical due to the recent outbreaks of measles in the United States.
“We must have clear guidelines on how to deal with the administration of live vaccines in this patient population so that we can provide the safest and most effective practice,” Dr. Moshe said.
These data are a first step.
“This large retrospective study demonstrates that live attenuated booster vaccine is probably safe in children with rheumatic diseases,” said Dr. Moshe, but she deferred to the PReS guidelines in suggesting that the decision to vaccinate still might best be performed on a case-by-case basis.
REPORTING FROM EULAR 2019 CONGRESS
Rapid assay distinguishes viral from bacterial infection
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
LJUBLJANA, SLOVENIA – assessing RNA expression of a single patient gene, according to a proof-of-concept study presented by Ruth Barral-Arca at the annual meeting of the European Society for Paediatric Infectious Diseases.
The gene of interest – IFI44L – is entwined in a child’s response to infection. It’s upregulated in the presence of viral infection and suppressed in bacterial infection, explained Ms. Barral-Arca, a PhD student at the University of Santiago de Compostela (Spain).
This investigational real-time PCR assay could provide a major advance over current routine practice, which is to admit a sick febrile child to the hospital, order bacterial cultures, and start parenteral antibiotics presumptively while awaiting the culture results, which usually don’t come back for more than 24 hours. This practice is a step backwards in terms of antibiotic stewardship, because the majority of febrile children have a self-resolving viral infection.
“This is a big problem because a lot of children with viral infections are inappropriately given antibiotics, leading to antimicrobial resistance,” she noted.
Also, misleadingly false-negative bacterial cultures can occur if the causative pathogen wasn’t included in the test, the infection is in a nonaccessible site, or the child has recently been on antibiotics.
All of these shortcomings have led to a new diagnostic strategy based upon measuring the pattern of key host genes upregulated or suppressed during the inflammatory response.
“We’ve seen that, instead of analyzing the bugs, analyzing the host transcriptome response during infection is proving to be a promising tool for disease biomarker identification. And it’s faster. An early differentiation between viral and bacterial patients will help improve triage in emergency departments, decrease the misuse of antibiotics, and guide clinics to a more precise diagnosis. A lot of big hospitals are already doing PCR. They could quickly adopt this kind of analysis,” Ms. Barral-Arca continued.
She presented a pilot study in which the assay was put to the test using multiple blood samples from 14 febrile infants and children up to 6 years of age with microbiologically confirmed bacterial infection, 11 febrile children with confirmed viral infection, and 10 healthy controls.
“I know the numbers seem small, but we did a sample-size power calculation and it’s just fine,” according to the researcher.
The initial study goal was to confirm earlier promising findings from a study of 370 febrile children in the United Kingdom, Spain, and the United States, conducted by the Immunopathology of Respiratory, Inflammatory and Infectious Disease Study (IRIS) Consortium, a study in which several of Ms. Barral-Arca’s senior coinvestigators participated. The IRIS investigators demonstrated that the combined expression pattern of two genes – IFI44L and FAM89A – distinguished the bacterial from viral infections with impressive sensitivity and specificity (JAMA. 2016 Aug 23-30;316[8]:835-45).
The two-gene signature performed similarly well in Ms. Barral-Arca’s study. However, when she and her coinvestigators tested the discriminatory power of the two genes individually, they got a surprise: The real-time PCR analysis assessing expression of IFI44L alone performed even better than the two-gene combination, discriminating viral from bacterial infections with 91% sensitivity, 93% specificity, and an area under the curve of 94%. In contrast, the two-gene signature based upon IFI44L and FAM89A had a sensitivity of 91%, a specificity of 86%, and an area under the curve of 92%. While those differences in performance are small, a single-gene assay saves time, work, and cost, according to Ms. Barral-Arca.
Her group then validated their findings regarding the performance of the IFI44L single-gene signature in two independent cohorts: stored blood samples from the children in the earlier IRIS study, and a group of children with diarrhea of viral or bacterial etiology.
“One gene seems to be enough,” she said. “We have demonstrated in a real-life scenario that host gene expression microarray data can be successfully translated into a fast, highly accurate, and relatively inexpensive in vitro assay that could be implemented in the clinical routine.”
Planned future work includes investigation of how the gene expression evolves over time from fever onset, the possible utility of the assay in noninfectious febrile illnesses such as rheumatoid arthritis, and whether the test discriminates viral from bacterial infection in adults.
Ms. Barral-Arca reported having no financial conflicts regarding her study, supported by institutional funding.
REPORTING FROM ESPID 2019
Key clinical point: A novel real-time single-gene–expression PCR test quickly distinguishes viral from bacterial infection in febrile children.
Major finding: The expression signature of the IFI44L gene rapidly distinguished bacterial from viral infection in febrile children with 91% sensitivity and 93% specificity.
Study details: This translational study included 25 febrile children with definite bacterial or viral infections and 10 healthy controls.
Disclosures: The presenter reported having no financial conflicts regarding her study, supported by institutional funding.