User login
Respiratory infection– and asthma-prone children
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).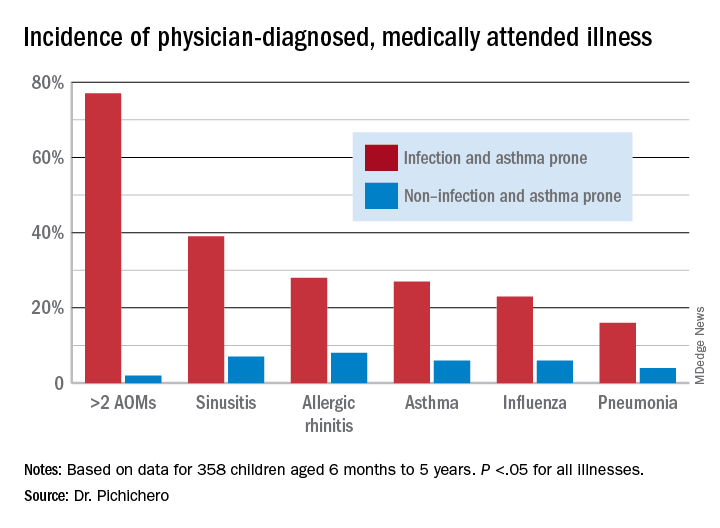
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
‘Long haul’ COVID recovery worse than cancer rehab for some: CDC
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
Clostridioides difficile: Two sets of guidelines disagree
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Texas doctor accused of vaccine theft faces grand jury
Hasan Gokal, MD, was fired from his job and charged with theft by a public servant. A judge dismissed the theft charge in January 2021, saying there was no probable cause, but prosecutors took the accusation to the Harris County Grand Jury, which on June 30 decided no prosecution was warranted, the Associated Press reported.
“I came as a practicing ER doctor into public health and as an ER doctor, I err on the side of life and that’s how I chose to make my decision,” Dr. Gokal told the Associated Press. “It was the right thing to do and it meant saving more lives.”
Dr. Gokal, 48, was supervising a COVID-19 vaccination clinic Dec. 29, 2020, in Humble, Tex., when the clinic closed for the day with an open vial containing nine doses of Moderna vaccine, the New York Times reported.
Since the vaccine would expire in 6 hours, Dr. Gokal scrambled to find people with medical conditions who needed vaccinating, he said. He gave the last dose to his wife, who has a lung condition, pulmonary sarcoidosis.
Dr. Gokal said he contacted his supervisor before acting and provided documentation the next day. He was fired for breaking protocol and then charged with theft.
“He abused his position to place his friends and family in line in front of people who had gone through the lawful process to be there,” Harris County District Attorney Kim Ogg said in a January statement. “What he did was illegal and he’ll be held accountable under the law.”
The AP reported that on June 30 the DA’s office issued a statement saying: “We respect the decision of the grand jury in this and every case. Evidence, not public opinion, is the guiding principle of our work.”
The AP said numerous doctors voiced support for Dr. Gokal and that the Texas Medical Board dismissed an investigation against him.
Dr. Gokal told the AP he’d still like to work in public health. Since being fired by the health department, he’s worked part time in the emergency departments at two Houston hospitals.
A version of this article first appeared on WebMD.com.
Hasan Gokal, MD, was fired from his job and charged with theft by a public servant. A judge dismissed the theft charge in January 2021, saying there was no probable cause, but prosecutors took the accusation to the Harris County Grand Jury, which on June 30 decided no prosecution was warranted, the Associated Press reported.
“I came as a practicing ER doctor into public health and as an ER doctor, I err on the side of life and that’s how I chose to make my decision,” Dr. Gokal told the Associated Press. “It was the right thing to do and it meant saving more lives.”
Dr. Gokal, 48, was supervising a COVID-19 vaccination clinic Dec. 29, 2020, in Humble, Tex., when the clinic closed for the day with an open vial containing nine doses of Moderna vaccine, the New York Times reported.
Since the vaccine would expire in 6 hours, Dr. Gokal scrambled to find people with medical conditions who needed vaccinating, he said. He gave the last dose to his wife, who has a lung condition, pulmonary sarcoidosis.
Dr. Gokal said he contacted his supervisor before acting and provided documentation the next day. He was fired for breaking protocol and then charged with theft.
“He abused his position to place his friends and family in line in front of people who had gone through the lawful process to be there,” Harris County District Attorney Kim Ogg said in a January statement. “What he did was illegal and he’ll be held accountable under the law.”
The AP reported that on June 30 the DA’s office issued a statement saying: “We respect the decision of the grand jury in this and every case. Evidence, not public opinion, is the guiding principle of our work.”
The AP said numerous doctors voiced support for Dr. Gokal and that the Texas Medical Board dismissed an investigation against him.
Dr. Gokal told the AP he’d still like to work in public health. Since being fired by the health department, he’s worked part time in the emergency departments at two Houston hospitals.
A version of this article first appeared on WebMD.com.
Hasan Gokal, MD, was fired from his job and charged with theft by a public servant. A judge dismissed the theft charge in January 2021, saying there was no probable cause, but prosecutors took the accusation to the Harris County Grand Jury, which on June 30 decided no prosecution was warranted, the Associated Press reported.
“I came as a practicing ER doctor into public health and as an ER doctor, I err on the side of life and that’s how I chose to make my decision,” Dr. Gokal told the Associated Press. “It was the right thing to do and it meant saving more lives.”
Dr. Gokal, 48, was supervising a COVID-19 vaccination clinic Dec. 29, 2020, in Humble, Tex., when the clinic closed for the day with an open vial containing nine doses of Moderna vaccine, the New York Times reported.
Since the vaccine would expire in 6 hours, Dr. Gokal scrambled to find people with medical conditions who needed vaccinating, he said. He gave the last dose to his wife, who has a lung condition, pulmonary sarcoidosis.
Dr. Gokal said he contacted his supervisor before acting and provided documentation the next day. He was fired for breaking protocol and then charged with theft.
“He abused his position to place his friends and family in line in front of people who had gone through the lawful process to be there,” Harris County District Attorney Kim Ogg said in a January statement. “What he did was illegal and he’ll be held accountable under the law.”
The AP reported that on June 30 the DA’s office issued a statement saying: “We respect the decision of the grand jury in this and every case. Evidence, not public opinion, is the guiding principle of our work.”
The AP said numerous doctors voiced support for Dr. Gokal and that the Texas Medical Board dismissed an investigation against him.
Dr. Gokal told the AP he’d still like to work in public health. Since being fired by the health department, he’s worked part time in the emergency departments at two Houston hospitals.
A version of this article first appeared on WebMD.com.
Small uptick in children’s COVID vaccinations can’t change overall decline
The weekly number of 12- to 15-year-olds receiving a first dose of COVID-19 vaccine rose slightly, but the age group’s share of all first vaccinations continues to drop, according to data from the Centers for Disease Control and Prevention.

the CDC reported on its COVID Data Tracker site.
As of July 5, not quite one-third (32.2%) of 12- to 15-year-olds had received at least one dose of the vaccine and 23.4% were fully vaccinated. For those aged 16-17 years, 44.5% have gotten at least one dose and 35.9% are fully vaccinated. Total numbers of fully vaccinated individuals in each age group are 4.9 million (12-15) and 3.4 million (16-17), the CDC said.
Looking at another measure, percentage of all vaccines initiated by each age group over the previous 14 days, shows that the decline has not stopped for those aged 12-15. They represented 12.1% of all first vaccines administered during the 2 weeks ending July 4, compared with 14.3% on June 28 and 23.4% (the highest proportion reached) on May 30. The 16- and 17-year olds were at 4.6% on July 4, but that figure has only ranged from 4.2% to 4.9% since late May, based on CDC data.
The numbers for full vaccination follow a similar trajectory. Children aged 12-15 represented 12.1% of all those completing the vaccine regimen over the 2 weeks ending July 4, down from 16.7% a week earlier (June 28) and from a high of 21.5% for the 2 weeks ending June 21. Full vaccination for 16- and 17-year-olds matched their pattern for first doses: nothing lower than 4.2% or higher than 4.6%, the COVID Data Tracker shows.
The weekly number of 12- to 15-year-olds receiving a first dose of COVID-19 vaccine rose slightly, but the age group’s share of all first vaccinations continues to drop, according to data from the Centers for Disease Control and Prevention.

the CDC reported on its COVID Data Tracker site.
As of July 5, not quite one-third (32.2%) of 12- to 15-year-olds had received at least one dose of the vaccine and 23.4% were fully vaccinated. For those aged 16-17 years, 44.5% have gotten at least one dose and 35.9% are fully vaccinated. Total numbers of fully vaccinated individuals in each age group are 4.9 million (12-15) and 3.4 million (16-17), the CDC said.
Looking at another measure, percentage of all vaccines initiated by each age group over the previous 14 days, shows that the decline has not stopped for those aged 12-15. They represented 12.1% of all first vaccines administered during the 2 weeks ending July 4, compared with 14.3% on June 28 and 23.4% (the highest proportion reached) on May 30. The 16- and 17-year olds were at 4.6% on July 4, but that figure has only ranged from 4.2% to 4.9% since late May, based on CDC data.
The numbers for full vaccination follow a similar trajectory. Children aged 12-15 represented 12.1% of all those completing the vaccine regimen over the 2 weeks ending July 4, down from 16.7% a week earlier (June 28) and from a high of 21.5% for the 2 weeks ending June 21. Full vaccination for 16- and 17-year-olds matched their pattern for first doses: nothing lower than 4.2% or higher than 4.6%, the COVID Data Tracker shows.
The weekly number of 12- to 15-year-olds receiving a first dose of COVID-19 vaccine rose slightly, but the age group’s share of all first vaccinations continues to drop, according to data from the Centers for Disease Control and Prevention.

the CDC reported on its COVID Data Tracker site.
As of July 5, not quite one-third (32.2%) of 12- to 15-year-olds had received at least one dose of the vaccine and 23.4% were fully vaccinated. For those aged 16-17 years, 44.5% have gotten at least one dose and 35.9% are fully vaccinated. Total numbers of fully vaccinated individuals in each age group are 4.9 million (12-15) and 3.4 million (16-17), the CDC said.
Looking at another measure, percentage of all vaccines initiated by each age group over the previous 14 days, shows that the decline has not stopped for those aged 12-15. They represented 12.1% of all first vaccines administered during the 2 weeks ending July 4, compared with 14.3% on June 28 and 23.4% (the highest proportion reached) on May 30. The 16- and 17-year olds were at 4.6% on July 4, but that figure has only ranged from 4.2% to 4.9% since late May, based on CDC data.
The numbers for full vaccination follow a similar trajectory. Children aged 12-15 represented 12.1% of all those completing the vaccine regimen over the 2 weeks ending July 4, down from 16.7% a week earlier (June 28) and from a high of 21.5% for the 2 weeks ending June 21. Full vaccination for 16- and 17-year-olds matched their pattern for first doses: nothing lower than 4.2% or higher than 4.6%, the COVID Data Tracker shows.
Antimicrobial resistance threat continues during COVID-19
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
Asymptomatic C. diff carriers have increased risk of symptomatic infection
Background: C. difficile infections (CDI) are significant with more than 400,000 cases and almost 30,000 deaths annually. However, there is uncertainty regarding asymptomatic C. difficile carriers and whether they have higher rates of progression to symptomatic infections.
Study design: Prospective cohort study.
Setting: Large university hospital in the New York from July 2017 through March 2018.
Synopsis: Patients admitted were screened, enrolled, and tested to include an adequate sample of nursing facility residents because of prior studies that showed nursing facility residents with a higher prevalence of carriage. Patients underwent perirectal swabbing and stool swabbing if available. Test swab soilage, noted as any visible material on the swab, was noted and recorded. Two stool-testing methods were used to test for carriage. A C. difficile carrier was defined as any patient with a positive test without diarrhea. Patients were followed for 6 months or until death; 220 patients were enrolled, with 21 patients (9.6%) asymptomatic C. difficile carriers. Having a soiled swab was the only statistically significant characteristic, including previous antibiotic exposure within the past 90 days, to be associated with carriage; 8 of 21 (38.1%) carriage patients progressed to CDI within 6 months versus 4 of 199 (2.0%) noncarriage patients. Most carriers that progressed to CDI did so within 2 weeks of enrollment. Limitations included lower numbers of expected carriage patients, diarrhea diagnosing variability, and perirectal swabbing was used rather than rectal swabbing/stool testing.
Bottom line: Asymptomatic carriage of C. difficile has increased risk of progression to symptomatic CDI and could present an opportunity for screening to reduce CDI in the inpatient setting.
Citation: Baron SW et al. Screening of Clostridioides difficile carriers in an urban academic medical center: Understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41(2):149-53.
Dr. Wang is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: C. difficile infections (CDI) are significant with more than 400,000 cases and almost 30,000 deaths annually. However, there is uncertainty regarding asymptomatic C. difficile carriers and whether they have higher rates of progression to symptomatic infections.
Study design: Prospective cohort study.
Setting: Large university hospital in the New York from July 2017 through March 2018.
Synopsis: Patients admitted were screened, enrolled, and tested to include an adequate sample of nursing facility residents because of prior studies that showed nursing facility residents with a higher prevalence of carriage. Patients underwent perirectal swabbing and stool swabbing if available. Test swab soilage, noted as any visible material on the swab, was noted and recorded. Two stool-testing methods were used to test for carriage. A C. difficile carrier was defined as any patient with a positive test without diarrhea. Patients were followed for 6 months or until death; 220 patients were enrolled, with 21 patients (9.6%) asymptomatic C. difficile carriers. Having a soiled swab was the only statistically significant characteristic, including previous antibiotic exposure within the past 90 days, to be associated with carriage; 8 of 21 (38.1%) carriage patients progressed to CDI within 6 months versus 4 of 199 (2.0%) noncarriage patients. Most carriers that progressed to CDI did so within 2 weeks of enrollment. Limitations included lower numbers of expected carriage patients, diarrhea diagnosing variability, and perirectal swabbing was used rather than rectal swabbing/stool testing.
Bottom line: Asymptomatic carriage of C. difficile has increased risk of progression to symptomatic CDI and could present an opportunity for screening to reduce CDI in the inpatient setting.
Citation: Baron SW et al. Screening of Clostridioides difficile carriers in an urban academic medical center: Understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41(2):149-53.
Dr. Wang is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: C. difficile infections (CDI) are significant with more than 400,000 cases and almost 30,000 deaths annually. However, there is uncertainty regarding asymptomatic C. difficile carriers and whether they have higher rates of progression to symptomatic infections.
Study design: Prospective cohort study.
Setting: Large university hospital in the New York from July 2017 through March 2018.
Synopsis: Patients admitted were screened, enrolled, and tested to include an adequate sample of nursing facility residents because of prior studies that showed nursing facility residents with a higher prevalence of carriage. Patients underwent perirectal swabbing and stool swabbing if available. Test swab soilage, noted as any visible material on the swab, was noted and recorded. Two stool-testing methods were used to test for carriage. A C. difficile carrier was defined as any patient with a positive test without diarrhea. Patients were followed for 6 months or until death; 220 patients were enrolled, with 21 patients (9.6%) asymptomatic C. difficile carriers. Having a soiled swab was the only statistically significant characteristic, including previous antibiotic exposure within the past 90 days, to be associated with carriage; 8 of 21 (38.1%) carriage patients progressed to CDI within 6 months versus 4 of 199 (2.0%) noncarriage patients. Most carriers that progressed to CDI did so within 2 weeks of enrollment. Limitations included lower numbers of expected carriage patients, diarrhea diagnosing variability, and perirectal swabbing was used rather than rectal swabbing/stool testing.
Bottom line: Asymptomatic carriage of C. difficile has increased risk of progression to symptomatic CDI and could present an opportunity for screening to reduce CDI in the inpatient setting.
Citation: Baron SW et al. Screening of Clostridioides difficile carriers in an urban academic medical center: Understanding implications of disease. Infect Control Hosp Epidemiol. 2020;41(2):149-53.
Dr. Wang is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Secnidazole gets FDA nod for trichomoniasis
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The Food and Drug Administration has expanded the approval of secnidazole to include treatment of trichomoniasis in adults, according to a statement from manufacturer Lupin Pharmaceuticals.
Trichomoniasis vaginalis is a common, nonviral, curable sexually transmitted disease that affects approximately 3 million to 5 million adults in the United States each year; the infection can linger for months or years if left untreated, and may have a negative impact on reproductive health. The drug was approved for the treatment of bacterial vaginosis in 2017.
The availability of a single-dose oral treatment for both trichomoniasis and bacterial vaginosis may help improve adherence and reduce risk factors associated with these conditions, including pelvic inflammatory disease and other sexually transmitted infections, according to the statement.
The approval for the new indication was based primarily on data from a phase 3 clinical trial in which women with a confirmed trichomoniasis diagnosis were randomized to a single dose of 2 g oral secnidazole or a placebo. Secnidazole showed a 92.2% cure rate for patients with trichomoniasis, compared with placebo, based on cultures collected 6-12 days after dosing. Cure rates in subsets of patients with HIV and bacterial vaginosis were 100% and 95%, respectively.
The most common treatment-related adverse events were vulvovaginal candidiasis and nausea, each reported in 2.7% of study participants. The study findings were published in March 2021 in Clinical Infections Diseases.
Secnidazole also is approved for treatment of trichomoniasis in men, based on data from four open-label studies, one with men only and three including both men and women, according to the statement.
Full prescribing information for secnidazole is available here.
The challenge of poverty to health and success: What should pediatricians do?
Some days it feels like more than half of the journal articles I encounter report data suggesting that poverty is associated with some disease entity. I realize that young postgraduates are under some pressure to publish, but I’m ready for a break. I and most pediatricians already know, or at least have assumed, that in general and with few exceptions unwellness and poverty are closely linked. Whether that association is causal or not is a more interesting question. The answer, I suspect, depends on which health condition we are talking about. For the moment I think we should assume that poverty is more likely a major contributor and not merely a fellow traveler of poor health.
Some other questions: What are we as pediatricians expected to do about poverty? Is awareness sufficient? Should I be content with having an elevated awareness that a certain patient has a given disease because I know his family is economically challenged? Or, conversely, should I be satisfied that I have asked about a family’s economic distress when I have just diagnosed a child with asthma? The answer to those questions is a very personal one for each of us to ponder and may depend on where we feel we can best invest our time and skill set.
Like me, you may feel that the focus of your professional life is better spent diagnosing and treating the collateral damage of poverty and addressing economic inequities in your philanthropic activities and your choices at the polls. On the other hand, you may choose to use your public persona as a physician to more actively address poverty whether it is on a local, national, or global stage. There is no correct answer and a hybrid may work best for you.
On the other hand, while you agree that there is some link between poverty and unwellness, perhaps the issue is overblown and we should pay more attention to other factors such as the sad state of the family in both disadvantaged and advantaged populations. Maybe if we worked harder to foster and support two-parent families the drag of economic disadvantage would be reduced.
I recently encountered a study that explores this very question. Christina Cross, PhD, a postdoctoral fellow in the department of sociology at Harvard University, reports on her soon-to-be-published study of a nationally representative sample in which she found that, using a selection of academic metrics including earned grades, likelihood of grade repetition, and rates of suspension, in low-income families there was no difference in achievement between Black youth raised in single-parent households and Black youth raised in two-parent households. However, in well-off families, Black youth raised in two-parent households had better academic metrics. (“Why living in a two-parent home isn’t a cure-all for Black students.” Christina Cross. The Harvard Gazette. 2021 Jun 3).
I guess few of us are surprised that living in a two-parent household can provide a child with some advantages. However, it is disappointing and again not surprising that poverty can rob a child of these advantages. While it may make us feel like we are doing something when we offer counseling that promotes two-family households, this may be no more valuable than supporting apple pie and motherhood. Dr. Cross concludes that President Biden’s proposed American Families Plan is more likely to succeed than those focused on counseling because it will offer direct financial support with its tax credits and subsidies.*
Let’s hope she is correct.
* This story was updated on July 6, 2021.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Some days it feels like more than half of the journal articles I encounter report data suggesting that poverty is associated with some disease entity. I realize that young postgraduates are under some pressure to publish, but I’m ready for a break. I and most pediatricians already know, or at least have assumed, that in general and with few exceptions unwellness and poverty are closely linked. Whether that association is causal or not is a more interesting question. The answer, I suspect, depends on which health condition we are talking about. For the moment I think we should assume that poverty is more likely a major contributor and not merely a fellow traveler of poor health.
Some other questions: What are we as pediatricians expected to do about poverty? Is awareness sufficient? Should I be content with having an elevated awareness that a certain patient has a given disease because I know his family is economically challenged? Or, conversely, should I be satisfied that I have asked about a family’s economic distress when I have just diagnosed a child with asthma? The answer to those questions is a very personal one for each of us to ponder and may depend on where we feel we can best invest our time and skill set.
Like me, you may feel that the focus of your professional life is better spent diagnosing and treating the collateral damage of poverty and addressing economic inequities in your philanthropic activities and your choices at the polls. On the other hand, you may choose to use your public persona as a physician to more actively address poverty whether it is on a local, national, or global stage. There is no correct answer and a hybrid may work best for you.
On the other hand, while you agree that there is some link between poverty and unwellness, perhaps the issue is overblown and we should pay more attention to other factors such as the sad state of the family in both disadvantaged and advantaged populations. Maybe if we worked harder to foster and support two-parent families the drag of economic disadvantage would be reduced.
I recently encountered a study that explores this very question. Christina Cross, PhD, a postdoctoral fellow in the department of sociology at Harvard University, reports on her soon-to-be-published study of a nationally representative sample in which she found that, using a selection of academic metrics including earned grades, likelihood of grade repetition, and rates of suspension, in low-income families there was no difference in achievement between Black youth raised in single-parent households and Black youth raised in two-parent households. However, in well-off families, Black youth raised in two-parent households had better academic metrics. (“Why living in a two-parent home isn’t a cure-all for Black students.” Christina Cross. The Harvard Gazette. 2021 Jun 3).
I guess few of us are surprised that living in a two-parent household can provide a child with some advantages. However, it is disappointing and again not surprising that poverty can rob a child of these advantages. While it may make us feel like we are doing something when we offer counseling that promotes two-family households, this may be no more valuable than supporting apple pie and motherhood. Dr. Cross concludes that President Biden’s proposed American Families Plan is more likely to succeed than those focused on counseling because it will offer direct financial support with its tax credits and subsidies.*
Let’s hope she is correct.
* This story was updated on July 6, 2021.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Some days it feels like more than half of the journal articles I encounter report data suggesting that poverty is associated with some disease entity. I realize that young postgraduates are under some pressure to publish, but I’m ready for a break. I and most pediatricians already know, or at least have assumed, that in general and with few exceptions unwellness and poverty are closely linked. Whether that association is causal or not is a more interesting question. The answer, I suspect, depends on which health condition we are talking about. For the moment I think we should assume that poverty is more likely a major contributor and not merely a fellow traveler of poor health.
Some other questions: What are we as pediatricians expected to do about poverty? Is awareness sufficient? Should I be content with having an elevated awareness that a certain patient has a given disease because I know his family is economically challenged? Or, conversely, should I be satisfied that I have asked about a family’s economic distress when I have just diagnosed a child with asthma? The answer to those questions is a very personal one for each of us to ponder and may depend on where we feel we can best invest our time and skill set.
Like me, you may feel that the focus of your professional life is better spent diagnosing and treating the collateral damage of poverty and addressing economic inequities in your philanthropic activities and your choices at the polls. On the other hand, you may choose to use your public persona as a physician to more actively address poverty whether it is on a local, national, or global stage. There is no correct answer and a hybrid may work best for you.
On the other hand, while you agree that there is some link between poverty and unwellness, perhaps the issue is overblown and we should pay more attention to other factors such as the sad state of the family in both disadvantaged and advantaged populations. Maybe if we worked harder to foster and support two-parent families the drag of economic disadvantage would be reduced.
I recently encountered a study that explores this very question. Christina Cross, PhD, a postdoctoral fellow in the department of sociology at Harvard University, reports on her soon-to-be-published study of a nationally representative sample in which she found that, using a selection of academic metrics including earned grades, likelihood of grade repetition, and rates of suspension, in low-income families there was no difference in achievement between Black youth raised in single-parent households and Black youth raised in two-parent households. However, in well-off families, Black youth raised in two-parent households had better academic metrics. (“Why living in a two-parent home isn’t a cure-all for Black students.” Christina Cross. The Harvard Gazette. 2021 Jun 3).
I guess few of us are surprised that living in a two-parent household can provide a child with some advantages. However, it is disappointing and again not surprising that poverty can rob a child of these advantages. While it may make us feel like we are doing something when we offer counseling that promotes two-family households, this may be no more valuable than supporting apple pie and motherhood. Dr. Cross concludes that President Biden’s proposed American Families Plan is more likely to succeed than those focused on counseling because it will offer direct financial support with its tax credits and subsidies.*
Let’s hope she is correct.
* This story was updated on July 6, 2021.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Doxycycline trumps azithromycin for asymptomatic rectal chlamydia in men who have sex with men
A 1-week course of doxycycline is more effective than single-dose azithromycin to treat rectal chlamydia in men who have sex with men (MSM), according to newly published results in the New England Journal of Medicine.
Chlamydia is the most commonly reported bacterial STI in the United States, with 4 million cases reported in 2018, and 127 million globally. Most infections are asymptomatic.
Rates of rectal chlamydia among MSM screened for infection range from 3% to 10.5%.
The most recent Centers for Disease Control and Prevention chlamydia guidelines recommend either a single dose of azithromycin (1 g) or doxycycline 100 mg twice daily for 7 days. These 2015 guidelines were based on a meta-analysis of urogenital chlamydia infections, which showed comparable efficacy of 97% or 98%, respectively.
Study coauthor Jane S. Hocking, PhD, head of the sexual health unit at the University of Melbourne, told this news organization that “observational studies had suggested that azithromycin was about 20% less effective than doxycycline,” prompting this clinical trial.
The study, conducted at five sexual health clinics in Australia, was a double-blind, randomized, controlled trial of doxycycline (100 mg twice daily for 7 days) or azithromycin (1-g single dose).
Because 85% of infected men are asymptomatic, the study’s primary outcome was a negative nucleic acid amplification test at 4 weeks, confirming a microbiologic cure.
Using a modified intention-to-treat population, the study showed a microbiologic cure in 281 of 290 men (96.9%) in the doxycycline group and 227 of 297 (76.4%) in the azithromycin group (P < .001).
Adverse events were more common in the azithromycin group. Nausea, diarrhea, and vomiting occurred in 134 (45.1%) men in that group versus 98 men (33.8%) in those receiving doxycycline (P = .006).
A similar study was reported in Clinical Infectious Diseases in February 2021 by Dombrowski and colleagues. It was also randomized, double blinded, and placebo controlled but was smaller and conducted in Seattle and Boston. A 20% difference was found, with 80/88 (91%) in the doxycycline group and 63/89 (71%) in the azithromycin group having a microbiologic cure at 4 weeks of follow-up.
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that the researchers focused solely on asymptomatic proctitis because “other symptoms might indicate need for broader presumptive antibiotics” for coinfections. Similarly, symptomatic proctitis “could indicate LGV [lymphogranuloma venereum] chlamydia, which ... automatically mandates that 3-weeks of doxycycline be used.” Dr. Marrazzo concluded: “The fact that this was a blinded study obviously strengthens the conclusions/findings, which is great. It’s very reassuring that results overall are so consistent with the CID paper.” Dr. Marrazzo was not involved in either the New England Journal of Medicine investigation or CID study.
Ina Park, MD, associate professor in the department of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs,” (New York: Flatiron Books, 2021) was not involved in either study but has a long history of working with adolescents in clinics for STDs. Based on that experience, she told this news organization that, while doxycycline now clearly appears to be the drug of choice, “if compliance is an issue and rectal chlamydia is not likely, then I think azithromycin is still something we need to consider, particularly for younger patients, and folks for whom compliance is going to be an issue.” She added: “with adolescent patients, there are issues of parents possibly discovering the antibiotic and asking lots of questions. So, it’s very nice for folks to be able to get therapy, sort of a one and done approach in the clinic.”
The 2020 CDC Guidelines for Gonococcal Infections says: “CDC recommends a single 500 mg intramuscular dose of ceftriaxone for uncomplicated gonorrhea. Treatment for coinfection with Chlamydia trachomatis with oral doxycycline (100 mg twice daily for 7 days) should be administered when chlamydial infection has not been excluded.”
Hocking concluded – and Dr. Marrazzo and Dr. Park concur – that this study “provides conclusive evidence that doxycycline should be the first-line treatment for rectal chlamydia, but probably for just any chlamydia infection,” with specific exceptions.
The University of Melbourne researchers also noted that the doxycycline course requires more compliant patients, as adherence isn’t assured. The issue of compliance and need for directly observed therapy, allergy to doxycycline, and pregnancy (where doxycycline is contraindicated) will remain the primary indications for continued use of azithromycin.
A version of this article first appeared on Medscape.com.
A 1-week course of doxycycline is more effective than single-dose azithromycin to treat rectal chlamydia in men who have sex with men (MSM), according to newly published results in the New England Journal of Medicine.
Chlamydia is the most commonly reported bacterial STI in the United States, with 4 million cases reported in 2018, and 127 million globally. Most infections are asymptomatic.
Rates of rectal chlamydia among MSM screened for infection range from 3% to 10.5%.
The most recent Centers for Disease Control and Prevention chlamydia guidelines recommend either a single dose of azithromycin (1 g) or doxycycline 100 mg twice daily for 7 days. These 2015 guidelines were based on a meta-analysis of urogenital chlamydia infections, which showed comparable efficacy of 97% or 98%, respectively.
Study coauthor Jane S. Hocking, PhD, head of the sexual health unit at the University of Melbourne, told this news organization that “observational studies had suggested that azithromycin was about 20% less effective than doxycycline,” prompting this clinical trial.
The study, conducted at five sexual health clinics in Australia, was a double-blind, randomized, controlled trial of doxycycline (100 mg twice daily for 7 days) or azithromycin (1-g single dose).
Because 85% of infected men are asymptomatic, the study’s primary outcome was a negative nucleic acid amplification test at 4 weeks, confirming a microbiologic cure.
Using a modified intention-to-treat population, the study showed a microbiologic cure in 281 of 290 men (96.9%) in the doxycycline group and 227 of 297 (76.4%) in the azithromycin group (P < .001).
Adverse events were more common in the azithromycin group. Nausea, diarrhea, and vomiting occurred in 134 (45.1%) men in that group versus 98 men (33.8%) in those receiving doxycycline (P = .006).
A similar study was reported in Clinical Infectious Diseases in February 2021 by Dombrowski and colleagues. It was also randomized, double blinded, and placebo controlled but was smaller and conducted in Seattle and Boston. A 20% difference was found, with 80/88 (91%) in the doxycycline group and 63/89 (71%) in the azithromycin group having a microbiologic cure at 4 weeks of follow-up.
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that the researchers focused solely on asymptomatic proctitis because “other symptoms might indicate need for broader presumptive antibiotics” for coinfections. Similarly, symptomatic proctitis “could indicate LGV [lymphogranuloma venereum] chlamydia, which ... automatically mandates that 3-weeks of doxycycline be used.” Dr. Marrazzo concluded: “The fact that this was a blinded study obviously strengthens the conclusions/findings, which is great. It’s very reassuring that results overall are so consistent with the CID paper.” Dr. Marrazzo was not involved in either the New England Journal of Medicine investigation or CID study.
Ina Park, MD, associate professor in the department of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs,” (New York: Flatiron Books, 2021) was not involved in either study but has a long history of working with adolescents in clinics for STDs. Based on that experience, she told this news organization that, while doxycycline now clearly appears to be the drug of choice, “if compliance is an issue and rectal chlamydia is not likely, then I think azithromycin is still something we need to consider, particularly for younger patients, and folks for whom compliance is going to be an issue.” She added: “with adolescent patients, there are issues of parents possibly discovering the antibiotic and asking lots of questions. So, it’s very nice for folks to be able to get therapy, sort of a one and done approach in the clinic.”
The 2020 CDC Guidelines for Gonococcal Infections says: “CDC recommends a single 500 mg intramuscular dose of ceftriaxone for uncomplicated gonorrhea. Treatment for coinfection with Chlamydia trachomatis with oral doxycycline (100 mg twice daily for 7 days) should be administered when chlamydial infection has not been excluded.”
Hocking concluded – and Dr. Marrazzo and Dr. Park concur – that this study “provides conclusive evidence that doxycycline should be the first-line treatment for rectal chlamydia, but probably for just any chlamydia infection,” with specific exceptions.
The University of Melbourne researchers also noted that the doxycycline course requires more compliant patients, as adherence isn’t assured. The issue of compliance and need for directly observed therapy, allergy to doxycycline, and pregnancy (where doxycycline is contraindicated) will remain the primary indications for continued use of azithromycin.
A version of this article first appeared on Medscape.com.
A 1-week course of doxycycline is more effective than single-dose azithromycin to treat rectal chlamydia in men who have sex with men (MSM), according to newly published results in the New England Journal of Medicine.
Chlamydia is the most commonly reported bacterial STI in the United States, with 4 million cases reported in 2018, and 127 million globally. Most infections are asymptomatic.
Rates of rectal chlamydia among MSM screened for infection range from 3% to 10.5%.
The most recent Centers for Disease Control and Prevention chlamydia guidelines recommend either a single dose of azithromycin (1 g) or doxycycline 100 mg twice daily for 7 days. These 2015 guidelines were based on a meta-analysis of urogenital chlamydia infections, which showed comparable efficacy of 97% or 98%, respectively.
Study coauthor Jane S. Hocking, PhD, head of the sexual health unit at the University of Melbourne, told this news organization that “observational studies had suggested that azithromycin was about 20% less effective than doxycycline,” prompting this clinical trial.
The study, conducted at five sexual health clinics in Australia, was a double-blind, randomized, controlled trial of doxycycline (100 mg twice daily for 7 days) or azithromycin (1-g single dose).
Because 85% of infected men are asymptomatic, the study’s primary outcome was a negative nucleic acid amplification test at 4 weeks, confirming a microbiologic cure.
Using a modified intention-to-treat population, the study showed a microbiologic cure in 281 of 290 men (96.9%) in the doxycycline group and 227 of 297 (76.4%) in the azithromycin group (P < .001).
Adverse events were more common in the azithromycin group. Nausea, diarrhea, and vomiting occurred in 134 (45.1%) men in that group versus 98 men (33.8%) in those receiving doxycycline (P = .006).
A similar study was reported in Clinical Infectious Diseases in February 2021 by Dombrowski and colleagues. It was also randomized, double blinded, and placebo controlled but was smaller and conducted in Seattle and Boston. A 20% difference was found, with 80/88 (91%) in the doxycycline group and 63/89 (71%) in the azithromycin group having a microbiologic cure at 4 weeks of follow-up.
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that the researchers focused solely on asymptomatic proctitis because “other symptoms might indicate need for broader presumptive antibiotics” for coinfections. Similarly, symptomatic proctitis “could indicate LGV [lymphogranuloma venereum] chlamydia, which ... automatically mandates that 3-weeks of doxycycline be used.” Dr. Marrazzo concluded: “The fact that this was a blinded study obviously strengthens the conclusions/findings, which is great. It’s very reassuring that results overall are so consistent with the CID paper.” Dr. Marrazzo was not involved in either the New England Journal of Medicine investigation or CID study.
Ina Park, MD, associate professor in the department of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs,” (New York: Flatiron Books, 2021) was not involved in either study but has a long history of working with adolescents in clinics for STDs. Based on that experience, she told this news organization that, while doxycycline now clearly appears to be the drug of choice, “if compliance is an issue and rectal chlamydia is not likely, then I think azithromycin is still something we need to consider, particularly for younger patients, and folks for whom compliance is going to be an issue.” She added: “with adolescent patients, there are issues of parents possibly discovering the antibiotic and asking lots of questions. So, it’s very nice for folks to be able to get therapy, sort of a one and done approach in the clinic.”
The 2020 CDC Guidelines for Gonococcal Infections says: “CDC recommends a single 500 mg intramuscular dose of ceftriaxone for uncomplicated gonorrhea. Treatment for coinfection with Chlamydia trachomatis with oral doxycycline (100 mg twice daily for 7 days) should be administered when chlamydial infection has not been excluded.”
Hocking concluded – and Dr. Marrazzo and Dr. Park concur – that this study “provides conclusive evidence that doxycycline should be the first-line treatment for rectal chlamydia, but probably for just any chlamydia infection,” with specific exceptions.
The University of Melbourne researchers also noted that the doxycycline course requires more compliant patients, as adherence isn’t assured. The issue of compliance and need for directly observed therapy, allergy to doxycycline, and pregnancy (where doxycycline is contraindicated) will remain the primary indications for continued use of azithromycin.
A version of this article first appeared on Medscape.com.




