User login
Lack of fever in ESRD with S. aureus bacteremia is common
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
New COVID-19 vaccinations decline again in 12- to 15-year-olds
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Artificial intelligence, COVID-19, and the future of pandemics
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
Artificial intelligence (AI) has proven of value in the COVID-19 pandemic and shows promise for mitigating future health care crises. During the pandemic’s first wave in New York, for example, Mount Sinai Health System used an algorithm to help identify patients ready for discharge. Such systems can help overburdened hospitals manage personnel and the flow of supplies in a medical crisis so they can continue to provide superior patient care.1
Pandemic applications have demonstrated AI’s potential not only to lift administrative burdens, but also to give physicians back what Eric Topol, MD, founder and director of Scripps Research Translational Institute and author of Deep Medicine, calls “the gift of time.”2 More time with patients contributes to clear communication and positive relationships, which lower the odds of medical errors, enhance patient safety, and potentially reduce physicians’ risks of certain types of litigation.3
However, physicians and health systems will need to approach AI with caution. Many unknowns remain – including potential liability risks and the potential for worsening preexisting bias. The law will need to evolve to account for AI-related liability scenarios, some of which are yet to be imagined.
Like any emerging technology, AI brings risk, but its promise of benefit should outweigh the probability of negative consequences – provided we remain aware of and mitigate the potential for AI-induced adverse events.
AI’s pandemic success limited due to fragmented data
Innovation is the key to success in any crisis, and many health care providers have shown their ability to innovate with AI during the pandemic. For example, researchers at the University of California, San Diego, health system who were designing an AI program to help doctors spot pneumonia on a chest x-ray retooled their application to assist physicians fighting coronavirus.4
Meanwhile, AI has been used to distinguish COVID-19–specific symptoms: It was a computer sifting medical records that took anosmia, loss of the sense of smell, from an anecdotal connection to an officially recognized early symptom of the virus.5 This information now helps physicians distinguish COVID-19 from influenza.
However, holding back more innovation is the fragmentation of health care data in the United States. Most AI applications for medicine rely on machine learning; that is, they train on historical patient data to recognize patterns. Therefore, “Everything that we’re doing gets better with a lot more annotated datasets,” Dr. Topol says. Unfortunately, because of our disparate systems, we don’t have centralized data.6 And even if our data were centralized, researchers lack enough reliable COVID-19 data to perfect algorithms in the short term.
Or, put in bleaker terms by the Washington Post: “One of the biggest challenges has been that much data remains siloed inside incompatible computer systems, hoarded by business interests and tangled in geopolitics.”7
The good news is that machine learning and data science platform Kaggle is hosting the COVID-19 Open Research Dataset, or CORD-19, which contains well over 100,000 scholarly articles on COVID-19, SARS, and other relevant infections.8 In lieu of a true central repository of anonymized health data, such large datasets can help train new AI applications in search of new diagnostic tools and therapies.
AI introduces new questions around liability
While AI may eventually be assigned legal personhood, it is not, in fact, a person: It is a tool wielded by individual clinicians, by teams, by health systems, even multiple systems collaborating. Our current liability laws are not ready for the era of digital medicine.
AI algorithms are not perfect. Because we know that diagnostic error is already a major allegation in malpractice claims, we must ask: What happens when a patient alleges that diagnostic error occurred because a physician or physicians leaned too heavily on AI?
In the United States, testing delays have threatened the safety of patients, physicians, and the public by delaying diagnosis of COVID-19. But again, health care providers have applied real innovation – generating novel and useful ideas and applying those ideas – to this problem. For example, researchers at Mount Sinai became the first in the country to combine AI with imaging and clinical data to produce an algorithm that can detect COVID-19 based on computed tomography scans of the chest, in combination with patient information and exposure history.9
AI in health care can help mitigate bias – or worsen it
Machine learning is only as good as the information provided to train the machine. Models trained on partial datasets can skew toward demographics that turned up more often in the data – for example, White race or men over 60. There is concern that “analyses based on faulty or biased algorithms could exacerbate existing racial gaps and other disparities in health care.”10 Already during the pandemic’s first waves, multiple AI systems used to classify x-rays have been found to show racial, gender, and socioeconomic biases.11
Such bias could create high potential for poor recommendations, including false positives and false negatives. It’s critical that system builders are able to explain and qualify their training data and that those who best understand AI-related system risks are the ones who influence health care systems or alter applications to mitigate AI-related harms.12
AI can help spot the next outbreak
More than a week before the World Health Organization released its first warning about a novel coronavirus, the AI platform BlueDot, created in Toronto, spotted an unusual cluster of pneumonia cases in Wuhan, China. Meanwhile, at Boston Children’s Hospital, the AI application Healthmap was scanning social media and news sites for signs of disease cluster, and it, too, flagged the first signs of what would become the COVID-19 outbreak – days before the WHO’s first formal alert.13
These innovative applications of AI in health care demonstrate real promise in detecting future outbreaks of new viruses early. This will allow health care providers and public health officials to get information out sooner, reducing the load on health systems, and ultimately, saving lives.
Dr. Anderson is chairman and chief executive officer, The Doctors Company and TDC Group.
References
1. Gold A. “Coronavirus tests the value of artificial intelligence in medicine” Fierce Biotech. 2020 May 22.
2. Topol E. “Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again” (New York: Hachette Book Group; 2019:285).
3. The Doctors Company. “The Algorithm Will See You Now: How AI’s Healthcare Potential Outweighs Its Risk” 2020 Jan.
4. Gold A. Coronavirus tests the value of artificial intelligence in medicine. Fierce Biotech. 2020 May 22.
5. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
6. Reuter E. Hundreds of AI solutions proposed for pandemic, but few are proven. MedCity News. 2020 May 28.
7. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
8. Lee K. COVID-19 will accelerate the AI health care revolution. Wired. 2020 May 22.
9. Mei X et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020 May 19;26:1224-8. doi: 10.1038/s41591-020-0931-3.
10. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
11. Wiggers K. Researchers find evidence of racial, gender, and socioeconomic bias in chest X-ray classifiers. The Machine: Making Sense of AI. 2020 Oct 21.
12. The Doctors Company. “The Algorithm Will See You Now: How AI’s Healthcare Potential Outweighs Its Risk” 2020 Jan.
13. Sewalk K. Innovative disease surveillance platforms detected early warning signs for novel coronavirus outbreak (nCoV-2019). The Disease Daily. 2020 Jan 31.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
Artificial intelligence (AI) has proven of value in the COVID-19 pandemic and shows promise for mitigating future health care crises. During the pandemic’s first wave in New York, for example, Mount Sinai Health System used an algorithm to help identify patients ready for discharge. Such systems can help overburdened hospitals manage personnel and the flow of supplies in a medical crisis so they can continue to provide superior patient care.1
Pandemic applications have demonstrated AI’s potential not only to lift administrative burdens, but also to give physicians back what Eric Topol, MD, founder and director of Scripps Research Translational Institute and author of Deep Medicine, calls “the gift of time.”2 More time with patients contributes to clear communication and positive relationships, which lower the odds of medical errors, enhance patient safety, and potentially reduce physicians’ risks of certain types of litigation.3
However, physicians and health systems will need to approach AI with caution. Many unknowns remain – including potential liability risks and the potential for worsening preexisting bias. The law will need to evolve to account for AI-related liability scenarios, some of which are yet to be imagined.
Like any emerging technology, AI brings risk, but its promise of benefit should outweigh the probability of negative consequences – provided we remain aware of and mitigate the potential for AI-induced adverse events.
AI’s pandemic success limited due to fragmented data
Innovation is the key to success in any crisis, and many health care providers have shown their ability to innovate with AI during the pandemic. For example, researchers at the University of California, San Diego, health system who were designing an AI program to help doctors spot pneumonia on a chest x-ray retooled their application to assist physicians fighting coronavirus.4
Meanwhile, AI has been used to distinguish COVID-19–specific symptoms: It was a computer sifting medical records that took anosmia, loss of the sense of smell, from an anecdotal connection to an officially recognized early symptom of the virus.5 This information now helps physicians distinguish COVID-19 from influenza.
However, holding back more innovation is the fragmentation of health care data in the United States. Most AI applications for medicine rely on machine learning; that is, they train on historical patient data to recognize patterns. Therefore, “Everything that we’re doing gets better with a lot more annotated datasets,” Dr. Topol says. Unfortunately, because of our disparate systems, we don’t have centralized data.6 And even if our data were centralized, researchers lack enough reliable COVID-19 data to perfect algorithms in the short term.
Or, put in bleaker terms by the Washington Post: “One of the biggest challenges has been that much data remains siloed inside incompatible computer systems, hoarded by business interests and tangled in geopolitics.”7
The good news is that machine learning and data science platform Kaggle is hosting the COVID-19 Open Research Dataset, or CORD-19, which contains well over 100,000 scholarly articles on COVID-19, SARS, and other relevant infections.8 In lieu of a true central repository of anonymized health data, such large datasets can help train new AI applications in search of new diagnostic tools and therapies.
AI introduces new questions around liability
While AI may eventually be assigned legal personhood, it is not, in fact, a person: It is a tool wielded by individual clinicians, by teams, by health systems, even multiple systems collaborating. Our current liability laws are not ready for the era of digital medicine.
AI algorithms are not perfect. Because we know that diagnostic error is already a major allegation in malpractice claims, we must ask: What happens when a patient alleges that diagnostic error occurred because a physician or physicians leaned too heavily on AI?
In the United States, testing delays have threatened the safety of patients, physicians, and the public by delaying diagnosis of COVID-19. But again, health care providers have applied real innovation – generating novel and useful ideas and applying those ideas – to this problem. For example, researchers at Mount Sinai became the first in the country to combine AI with imaging and clinical data to produce an algorithm that can detect COVID-19 based on computed tomography scans of the chest, in combination with patient information and exposure history.9
AI in health care can help mitigate bias – or worsen it
Machine learning is only as good as the information provided to train the machine. Models trained on partial datasets can skew toward demographics that turned up more often in the data – for example, White race or men over 60. There is concern that “analyses based on faulty or biased algorithms could exacerbate existing racial gaps and other disparities in health care.”10 Already during the pandemic’s first waves, multiple AI systems used to classify x-rays have been found to show racial, gender, and socioeconomic biases.11
Such bias could create high potential for poor recommendations, including false positives and false negatives. It’s critical that system builders are able to explain and qualify their training data and that those who best understand AI-related system risks are the ones who influence health care systems or alter applications to mitigate AI-related harms.12
AI can help spot the next outbreak
More than a week before the World Health Organization released its first warning about a novel coronavirus, the AI platform BlueDot, created in Toronto, spotted an unusual cluster of pneumonia cases in Wuhan, China. Meanwhile, at Boston Children’s Hospital, the AI application Healthmap was scanning social media and news sites for signs of disease cluster, and it, too, flagged the first signs of what would become the COVID-19 outbreak – days before the WHO’s first formal alert.13
These innovative applications of AI in health care demonstrate real promise in detecting future outbreaks of new viruses early. This will allow health care providers and public health officials to get information out sooner, reducing the load on health systems, and ultimately, saving lives.
Dr. Anderson is chairman and chief executive officer, The Doctors Company and TDC Group.
References
1. Gold A. “Coronavirus tests the value of artificial intelligence in medicine” Fierce Biotech. 2020 May 22.
2. Topol E. “Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again” (New York: Hachette Book Group; 2019:285).
3. The Doctors Company. “The Algorithm Will See You Now: How AI’s Healthcare Potential Outweighs Its Risk” 2020 Jan.
4. Gold A. Coronavirus tests the value of artificial intelligence in medicine. Fierce Biotech. 2020 May 22.
5. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
6. Reuter E. Hundreds of AI solutions proposed for pandemic, but few are proven. MedCity News. 2020 May 28.
7. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
8. Lee K. COVID-19 will accelerate the AI health care revolution. Wired. 2020 May 22.
9. Mei X et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020 May 19;26:1224-8. doi: 10.1038/s41591-020-0931-3.
10. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
11. Wiggers K. Researchers find evidence of racial, gender, and socioeconomic bias in chest X-ray classifiers. The Machine: Making Sense of AI. 2020 Oct 21.
12. The Doctors Company. “The Algorithm Will See You Now: How AI’s Healthcare Potential Outweighs Its Risk” 2020 Jan.
13. Sewalk K. Innovative disease surveillance platforms detected early warning signs for novel coronavirus outbreak (nCoV-2019). The Disease Daily. 2020 Jan 31.
Editor’s note: This article has been provided by The Doctors Company, the exclusively endorsed medical malpractice carrier for the Society of Hospital Medicine.
Artificial intelligence (AI) has proven of value in the COVID-19 pandemic and shows promise for mitigating future health care crises. During the pandemic’s first wave in New York, for example, Mount Sinai Health System used an algorithm to help identify patients ready for discharge. Such systems can help overburdened hospitals manage personnel and the flow of supplies in a medical crisis so they can continue to provide superior patient care.1
Pandemic applications have demonstrated AI’s potential not only to lift administrative burdens, but also to give physicians back what Eric Topol, MD, founder and director of Scripps Research Translational Institute and author of Deep Medicine, calls “the gift of time.”2 More time with patients contributes to clear communication and positive relationships, which lower the odds of medical errors, enhance patient safety, and potentially reduce physicians’ risks of certain types of litigation.3
However, physicians and health systems will need to approach AI with caution. Many unknowns remain – including potential liability risks and the potential for worsening preexisting bias. The law will need to evolve to account for AI-related liability scenarios, some of which are yet to be imagined.
Like any emerging technology, AI brings risk, but its promise of benefit should outweigh the probability of negative consequences – provided we remain aware of and mitigate the potential for AI-induced adverse events.
AI’s pandemic success limited due to fragmented data
Innovation is the key to success in any crisis, and many health care providers have shown their ability to innovate with AI during the pandemic. For example, researchers at the University of California, San Diego, health system who were designing an AI program to help doctors spot pneumonia on a chest x-ray retooled their application to assist physicians fighting coronavirus.4
Meanwhile, AI has been used to distinguish COVID-19–specific symptoms: It was a computer sifting medical records that took anosmia, loss of the sense of smell, from an anecdotal connection to an officially recognized early symptom of the virus.5 This information now helps physicians distinguish COVID-19 from influenza.
However, holding back more innovation is the fragmentation of health care data in the United States. Most AI applications for medicine rely on machine learning; that is, they train on historical patient data to recognize patterns. Therefore, “Everything that we’re doing gets better with a lot more annotated datasets,” Dr. Topol says. Unfortunately, because of our disparate systems, we don’t have centralized data.6 And even if our data were centralized, researchers lack enough reliable COVID-19 data to perfect algorithms in the short term.
Or, put in bleaker terms by the Washington Post: “One of the biggest challenges has been that much data remains siloed inside incompatible computer systems, hoarded by business interests and tangled in geopolitics.”7
The good news is that machine learning and data science platform Kaggle is hosting the COVID-19 Open Research Dataset, or CORD-19, which contains well over 100,000 scholarly articles on COVID-19, SARS, and other relevant infections.8 In lieu of a true central repository of anonymized health data, such large datasets can help train new AI applications in search of new diagnostic tools and therapies.
AI introduces new questions around liability
While AI may eventually be assigned legal personhood, it is not, in fact, a person: It is a tool wielded by individual clinicians, by teams, by health systems, even multiple systems collaborating. Our current liability laws are not ready for the era of digital medicine.
AI algorithms are not perfect. Because we know that diagnostic error is already a major allegation in malpractice claims, we must ask: What happens when a patient alleges that diagnostic error occurred because a physician or physicians leaned too heavily on AI?
In the United States, testing delays have threatened the safety of patients, physicians, and the public by delaying diagnosis of COVID-19. But again, health care providers have applied real innovation – generating novel and useful ideas and applying those ideas – to this problem. For example, researchers at Mount Sinai became the first in the country to combine AI with imaging and clinical data to produce an algorithm that can detect COVID-19 based on computed tomography scans of the chest, in combination with patient information and exposure history.9
AI in health care can help mitigate bias – or worsen it
Machine learning is only as good as the information provided to train the machine. Models trained on partial datasets can skew toward demographics that turned up more often in the data – for example, White race or men over 60. There is concern that “analyses based on faulty or biased algorithms could exacerbate existing racial gaps and other disparities in health care.”10 Already during the pandemic’s first waves, multiple AI systems used to classify x-rays have been found to show racial, gender, and socioeconomic biases.11
Such bias could create high potential for poor recommendations, including false positives and false negatives. It’s critical that system builders are able to explain and qualify their training data and that those who best understand AI-related system risks are the ones who influence health care systems or alter applications to mitigate AI-related harms.12
AI can help spot the next outbreak
More than a week before the World Health Organization released its first warning about a novel coronavirus, the AI platform BlueDot, created in Toronto, spotted an unusual cluster of pneumonia cases in Wuhan, China. Meanwhile, at Boston Children’s Hospital, the AI application Healthmap was scanning social media and news sites for signs of disease cluster, and it, too, flagged the first signs of what would become the COVID-19 outbreak – days before the WHO’s first formal alert.13
These innovative applications of AI in health care demonstrate real promise in detecting future outbreaks of new viruses early. This will allow health care providers and public health officials to get information out sooner, reducing the load on health systems, and ultimately, saving lives.
Dr. Anderson is chairman and chief executive officer, The Doctors Company and TDC Group.
References
1. Gold A. “Coronavirus tests the value of artificial intelligence in medicine” Fierce Biotech. 2020 May 22.
2. Topol E. “Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again” (New York: Hachette Book Group; 2019:285).
3. The Doctors Company. “The Algorithm Will See You Now: How AI’s Healthcare Potential Outweighs Its Risk” 2020 Jan.
4. Gold A. Coronavirus tests the value of artificial intelligence in medicine. Fierce Biotech. 2020 May 22.
5. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
6. Reuter E. Hundreds of AI solutions proposed for pandemic, but few are proven. MedCity News. 2020 May 28.
7. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
8. Lee K. COVID-19 will accelerate the AI health care revolution. Wired. 2020 May 22.
9. Mei X et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020 May 19;26:1224-8. doi: 10.1038/s41591-020-0931-3.
10. Cha AE. Artificial intelligence and COVID-19: Can the machines save us? Washington Post. 2020 Nov 1.
11. Wiggers K. Researchers find evidence of racial, gender, and socioeconomic bias in chest X-ray classifiers. The Machine: Making Sense of AI. 2020 Oct 21.
12. The Doctors Company. “The Algorithm Will See You Now: How AI’s Healthcare Potential Outweighs Its Risk” 2020 Jan.
13. Sewalk K. Innovative disease surveillance platforms detected early warning signs for novel coronavirus outbreak (nCoV-2019). The Disease Daily. 2020 Jan 31.
Unmanaged diabetes, high blood glucose tied to COVID-19 severity
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
Unmanaged diabetes and high blood glucose levels are linked to more severe COVID-19 and worse rates of recovery, according to results of a retrospective study.
Patients not managing their diabetes with medication had more severe COVID-19 and length of hospitalization, compared with those who were taking medication, investigator Sudip Bajpeyi, PhD, said at the annual scientific sessions of the American Diabetes Association.
In addition, patients with higher blood glucose levels had more severe COVID-19 and longer hospital stays.
Those findings underscore the need to assess, monitor, and control blood glucose, especially in vulnerable populations, said Dr. Bajpeyi, director of the Metabolic, Nutrition, and Exercise Research Laboratory in the University of Texas, El Paso, who added that nearly 90% of the study subjects were Hispanic.
“As public health decisions are made, we think fasting blood glucose should be considered in the treatment of hospitalized COVID-19 patients,” he said in a press conference.
Links between diabetes and COVID-19
There are now many reports in medical literature that link diabetes to increased risk of COVID-19 severity, according to Ali Mossayebi, a master’s student who worked on the study. However, there are fewer studies that have looked specifically at the implications of poor diabetes management or acute glycemic control, the investigators said.
It’s known that poorly controlled diabetes can have severe health consequences, including higher risks for life-threatening comorbidities, they added.
Their retrospective study focused on medical records from 364 patients with COVID-19 admitted to a medical center in El Paso. Their mean age was 60 years, and their mean body mass index was 30.3 kg/m2; 87% were Hispanic.
Acute glycemic control was assessed by fasting blood glucose at the time of hospitalization, while chronic glycemic control was assessed by hemoglobin A1c, the investigators said. Severity of COVID-19 was measured with the Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA), which is based on the patient’s respiratory rate, blood pressure, and mental status.
Impact of unmanaged diabetes and high blood glucose
Severity of COVID-19 severity and length of hospital stay were significantly greater in patients with unmanaged diabetes, as compared with those who reported that they managed their diabetes with medication, Dr. Bajpeyi and coinvestigators found.
Among patients with unmanaged diabetes, the mean qSOFA score was 0.22, as compared with 0.44 for patients with managed diabetes. The mean length of hospital stay was 10.8 days for patients with unmanaged diabetes and 8.2 days for those with medication-managed diabetes, according to the abstract.
COVID-19 severity and hospital stay length were highest among patients with acute glycemia, the investigators further reported in an electronic poster that was part of the ADA meeting proceedings.
The mean qSOFA score was about 0.6 for patients with blood glucose levels of at least 126 mg/dL and A1c below 6.5%, and roughly 0.2 for those with normal blood glucose and normal A1c. Similarly, duration of hospital stay was significantly higher for patients with high blood glucose and A1c as compared with those with normal blood glucose and A1c.
Aggressive treatment needed
Findings of this study are in line with previous research showing that in-hospital hyperglycemia is a common and important marker of poor clinical outcome and mortality, with or without diabetes, according to Rodolfo J. Galindo, MD, FACE, medical chair of the hospital diabetes task force at Emory Healthcare System, Atlanta.
“These patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes or A1c value,” said Dr. Galindo, who was not involved in the study. “They also need outpatient follow-up after discharge, because they may develop diabetes soon after.”
Follow-up within is important because roughly 30% of patients with stress hyperglycemia (increases in blood glucose during an acute illness) will develop diabetes within a year, according to Dr. Galindo.
“We do not know in COVID-10 patients if it is only 30%,” he said, “Our thinking in our group is that it’s probably higher.”
Dr. Bajpeyi and coauthors reported no disclosures. Dr. Galindo reported disclosures related to Abbott Diabetes, Boehringer Ingelheim International, Eli Lilly, Novo Nordisk, Sanofi US, Valeritas, and Dexcom.
FROM ADA 2020
Few clinical guidelines exist for treating post-COVID symptoms
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
C. difficile guidelines offer new possibilities
The American College of Gastroenterology has issued new guidelines on management of Clostridioides difficile infection that now include roles for fecal microbial transplant (FMT), combination testing, and bezlotoxumab.
The ACG’s previous guidelines on the diagnosis, management, and treatment of what was then still called Clostridium difficile were published in 2013. Since then, the organism’s name changed to Clostridioides difficile, and that’s just the beginning of the changes reflected in the scientific literature, wrote lead author Colleen R. Kelly, MD, of Brown University, Providence, R.I., and colleagues.
“Other developments include the increased recognition of diagnostic challenges in the era of nucleic acid amplification–based testing, new therapeutic options for treatment and prevention of recurrence, and increasing evidence to support fecal microbiota transplantation (FMT) in recurrent and severe infection,” the authors said.
The guidelines, published in the American Journal of Gastroenterology, include 23 graded recommendations addressing issues of prevention, diagnosis, treatment, prevention of recurrence, and guidance for special populations in the management of C. difficile infection (CDI).
New faces among familiar ones
In terms of diagnosis, the new guidelines recommend using both a highly sensitive testing modality and a highly specific one to help distinguish colonization from active infection. Specifically, the authors recommend that stool is first tested using a highly sensitive test, either nucleic acid amplification testing or glutamate dehydrogenase, followed by an enzyme immunoassays for its high specificity.
Changes to treatment recommendations include the initial use of oral vancomycin or oral fidaxomicin for cases of nonsevere CDI. Oral metronidazole may be considered for initial nonsevere CDI in low-risk patients, the authors noted. The evidence is strong for the continued recommendations of vancomycin (125 mg four times daily for 10 days) and fidaxomicin (200 mg twice daily for 10 days) for patients with severe CDI. For patients with fulminant CDI, the recommendations call for medical therapy including volume resuscitation and oral vancomycin, although combination therapy with parenteral metronidazole may be considered despite the very low quality of evidence.
A notable update to the guidelines is the recommendation of fecal microbiota transplant (FMT) for both severe and fulminant CDI cases that are resistant to antibiotics and to prevent recurrence in at-risk patients. Although the quality of evidence is ranked as low, the recommendation is strong, the authors wrote. “Beyond improved cure rates, FMT may result in decreased rates of CDI-related colectomy and sepsis and may offer survival benefit in this critically ill patient population.” However, most patients in studies of FMT required multiple treatments in combination with anti-CDI antibiotics.
Other recommendations to prevent recurrence include oral vancomycin prophylaxis during the subsequent use of systemic antibiotics in patients with a history of CDI. The guidelines also recommend bezlotoxumab for prevention of CDI recurrence in high-risk patients, and advise against discontinuing antisecretory therapy in CDI patients if there is an appropriate indication for use.
Based on the lack of quality evidence, the guidelines recommend against the use of probiotics for preventing CDI in patients being treated with antibiotics and for prevention of recurrent infection.
Special populations
For patients with inflammatory bowel disease, the guidelines recommend C. difficile testing when these individuals present with acute flares and diarrhea, and the use of vancomycin for treatment. In addition, the authors strongly recommended FMT for recurrent CDI in these patients. For pregnant, postpartum, and breastfeeding patients with CDI, the guidelines recommend vancomycin, and either vancomycin or fidaxomicin may be used for treating CDI in immunocompromised patients, the authors noted.
The updated guidelines are designed to complement those issued by the Infections Disease Society of America and Society of Healthcare Epidemiologists of America, the researchers noted.
Reflecting the research
The previous guidelines for C. difficile were issued in 2013, and much has changed since then in terms of epidemiology, diagnosis, treatment, and infection control, Sahil Khanna, MBBS, MS, of the Mayo Clinic, Rochester, Minn., said in an interview.
Notably, diagnostic testing has “made leaps and bounds” and new treatments have become available that were not included in earlier guidelines, said Dr. Khanna. In particular, the new guidelines are recommending a two-step diagnostic assay; “the diagnostic algorithm has changed, and hopefully that will help us change practice” to identify active infection more quickly and efficiently.
Another important update is the recommendation of fidaxomicin as an option for initial nonfulminant CDI as an alternative to vancomycin, Dr. Khanna said, noting that metronidazole remains an option for low-risk patients. An additional change is the advice to use a different treatment for a second recurrent infection rather than repeating the initial treatment.
The recommendation of bezlotoxumab for prevention of CDI recurrence in patients who are at high risk of recurrence is the first time this drug has appeared in major guidelines, Dr. Khanna observed.
The recommendation in support of fecal microbiota transplant is a key update to the management of CDI, including the guidance that the procedure can be repeated if necessary, he said.
Looking ahead, “Additional research is needed to fully understand the best testing algorithms for CDI,” Dr. Khanna explained. “More studies also are needed to show how FMT fully fits into the picture, and some current studies are looking at its potential earlier in the course of infection.”
The guidelines were developed in collaboration with the Practice Parameters Committee of the American College of Gastroenterology and received no outside funding. Dr. Kelly disclosed serving as a site investigator of a clinical trial for Finch Therapeutics and is an unpaid clinical advisory board member for OpenBiome. Dr. Khanna has coauthored previous guidelines on C. difficile. He disclosed consulting relationships with Finch, GlaxoSmithKline, Jetson, ProbioTech, and Shire/Takeda, as well as research support from Rebiotix, Seres, and Vedanta.
The American College of Gastroenterology has issued new guidelines on management of Clostridioides difficile infection that now include roles for fecal microbial transplant (FMT), combination testing, and bezlotoxumab.
The ACG’s previous guidelines on the diagnosis, management, and treatment of what was then still called Clostridium difficile were published in 2013. Since then, the organism’s name changed to Clostridioides difficile, and that’s just the beginning of the changes reflected in the scientific literature, wrote lead author Colleen R. Kelly, MD, of Brown University, Providence, R.I., and colleagues.
“Other developments include the increased recognition of diagnostic challenges in the era of nucleic acid amplification–based testing, new therapeutic options for treatment and prevention of recurrence, and increasing evidence to support fecal microbiota transplantation (FMT) in recurrent and severe infection,” the authors said.
The guidelines, published in the American Journal of Gastroenterology, include 23 graded recommendations addressing issues of prevention, diagnosis, treatment, prevention of recurrence, and guidance for special populations in the management of C. difficile infection (CDI).
New faces among familiar ones
In terms of diagnosis, the new guidelines recommend using both a highly sensitive testing modality and a highly specific one to help distinguish colonization from active infection. Specifically, the authors recommend that stool is first tested using a highly sensitive test, either nucleic acid amplification testing or glutamate dehydrogenase, followed by an enzyme immunoassays for its high specificity.
Changes to treatment recommendations include the initial use of oral vancomycin or oral fidaxomicin for cases of nonsevere CDI. Oral metronidazole may be considered for initial nonsevere CDI in low-risk patients, the authors noted. The evidence is strong for the continued recommendations of vancomycin (125 mg four times daily for 10 days) and fidaxomicin (200 mg twice daily for 10 days) for patients with severe CDI. For patients with fulminant CDI, the recommendations call for medical therapy including volume resuscitation and oral vancomycin, although combination therapy with parenteral metronidazole may be considered despite the very low quality of evidence.
A notable update to the guidelines is the recommendation of fecal microbiota transplant (FMT) for both severe and fulminant CDI cases that are resistant to antibiotics and to prevent recurrence in at-risk patients. Although the quality of evidence is ranked as low, the recommendation is strong, the authors wrote. “Beyond improved cure rates, FMT may result in decreased rates of CDI-related colectomy and sepsis and may offer survival benefit in this critically ill patient population.” However, most patients in studies of FMT required multiple treatments in combination with anti-CDI antibiotics.
Other recommendations to prevent recurrence include oral vancomycin prophylaxis during the subsequent use of systemic antibiotics in patients with a history of CDI. The guidelines also recommend bezlotoxumab for prevention of CDI recurrence in high-risk patients, and advise against discontinuing antisecretory therapy in CDI patients if there is an appropriate indication for use.
Based on the lack of quality evidence, the guidelines recommend against the use of probiotics for preventing CDI in patients being treated with antibiotics and for prevention of recurrent infection.
Special populations
For patients with inflammatory bowel disease, the guidelines recommend C. difficile testing when these individuals present with acute flares and diarrhea, and the use of vancomycin for treatment. In addition, the authors strongly recommended FMT for recurrent CDI in these patients. For pregnant, postpartum, and breastfeeding patients with CDI, the guidelines recommend vancomycin, and either vancomycin or fidaxomicin may be used for treating CDI in immunocompromised patients, the authors noted.
The updated guidelines are designed to complement those issued by the Infections Disease Society of America and Society of Healthcare Epidemiologists of America, the researchers noted.
Reflecting the research
The previous guidelines for C. difficile were issued in 2013, and much has changed since then in terms of epidemiology, diagnosis, treatment, and infection control, Sahil Khanna, MBBS, MS, of the Mayo Clinic, Rochester, Minn., said in an interview.
Notably, diagnostic testing has “made leaps and bounds” and new treatments have become available that were not included in earlier guidelines, said Dr. Khanna. In particular, the new guidelines are recommending a two-step diagnostic assay; “the diagnostic algorithm has changed, and hopefully that will help us change practice” to identify active infection more quickly and efficiently.
Another important update is the recommendation of fidaxomicin as an option for initial nonfulminant CDI as an alternative to vancomycin, Dr. Khanna said, noting that metronidazole remains an option for low-risk patients. An additional change is the advice to use a different treatment for a second recurrent infection rather than repeating the initial treatment.
The recommendation of bezlotoxumab for prevention of CDI recurrence in patients who are at high risk of recurrence is the first time this drug has appeared in major guidelines, Dr. Khanna observed.
The recommendation in support of fecal microbiota transplant is a key update to the management of CDI, including the guidance that the procedure can be repeated if necessary, he said.
Looking ahead, “Additional research is needed to fully understand the best testing algorithms for CDI,” Dr. Khanna explained. “More studies also are needed to show how FMT fully fits into the picture, and some current studies are looking at its potential earlier in the course of infection.”
The guidelines were developed in collaboration with the Practice Parameters Committee of the American College of Gastroenterology and received no outside funding. Dr. Kelly disclosed serving as a site investigator of a clinical trial for Finch Therapeutics and is an unpaid clinical advisory board member for OpenBiome. Dr. Khanna has coauthored previous guidelines on C. difficile. He disclosed consulting relationships with Finch, GlaxoSmithKline, Jetson, ProbioTech, and Shire/Takeda, as well as research support from Rebiotix, Seres, and Vedanta.
The American College of Gastroenterology has issued new guidelines on management of Clostridioides difficile infection that now include roles for fecal microbial transplant (FMT), combination testing, and bezlotoxumab.
The ACG’s previous guidelines on the diagnosis, management, and treatment of what was then still called Clostridium difficile were published in 2013. Since then, the organism’s name changed to Clostridioides difficile, and that’s just the beginning of the changes reflected in the scientific literature, wrote lead author Colleen R. Kelly, MD, of Brown University, Providence, R.I., and colleagues.
“Other developments include the increased recognition of diagnostic challenges in the era of nucleic acid amplification–based testing, new therapeutic options for treatment and prevention of recurrence, and increasing evidence to support fecal microbiota transplantation (FMT) in recurrent and severe infection,” the authors said.
The guidelines, published in the American Journal of Gastroenterology, include 23 graded recommendations addressing issues of prevention, diagnosis, treatment, prevention of recurrence, and guidance for special populations in the management of C. difficile infection (CDI).
New faces among familiar ones
In terms of diagnosis, the new guidelines recommend using both a highly sensitive testing modality and a highly specific one to help distinguish colonization from active infection. Specifically, the authors recommend that stool is first tested using a highly sensitive test, either nucleic acid amplification testing or glutamate dehydrogenase, followed by an enzyme immunoassays for its high specificity.
Changes to treatment recommendations include the initial use of oral vancomycin or oral fidaxomicin for cases of nonsevere CDI. Oral metronidazole may be considered for initial nonsevere CDI in low-risk patients, the authors noted. The evidence is strong for the continued recommendations of vancomycin (125 mg four times daily for 10 days) and fidaxomicin (200 mg twice daily for 10 days) for patients with severe CDI. For patients with fulminant CDI, the recommendations call for medical therapy including volume resuscitation and oral vancomycin, although combination therapy with parenteral metronidazole may be considered despite the very low quality of evidence.
A notable update to the guidelines is the recommendation of fecal microbiota transplant (FMT) for both severe and fulminant CDI cases that are resistant to antibiotics and to prevent recurrence in at-risk patients. Although the quality of evidence is ranked as low, the recommendation is strong, the authors wrote. “Beyond improved cure rates, FMT may result in decreased rates of CDI-related colectomy and sepsis and may offer survival benefit in this critically ill patient population.” However, most patients in studies of FMT required multiple treatments in combination with anti-CDI antibiotics.
Other recommendations to prevent recurrence include oral vancomycin prophylaxis during the subsequent use of systemic antibiotics in patients with a history of CDI. The guidelines also recommend bezlotoxumab for prevention of CDI recurrence in high-risk patients, and advise against discontinuing antisecretory therapy in CDI patients if there is an appropriate indication for use.
Based on the lack of quality evidence, the guidelines recommend against the use of probiotics for preventing CDI in patients being treated with antibiotics and for prevention of recurrent infection.
Special populations
For patients with inflammatory bowel disease, the guidelines recommend C. difficile testing when these individuals present with acute flares and diarrhea, and the use of vancomycin for treatment. In addition, the authors strongly recommended FMT for recurrent CDI in these patients. For pregnant, postpartum, and breastfeeding patients with CDI, the guidelines recommend vancomycin, and either vancomycin or fidaxomicin may be used for treating CDI in immunocompromised patients, the authors noted.
The updated guidelines are designed to complement those issued by the Infections Disease Society of America and Society of Healthcare Epidemiologists of America, the researchers noted.
Reflecting the research
The previous guidelines for C. difficile were issued in 2013, and much has changed since then in terms of epidemiology, diagnosis, treatment, and infection control, Sahil Khanna, MBBS, MS, of the Mayo Clinic, Rochester, Minn., said in an interview.
Notably, diagnostic testing has “made leaps and bounds” and new treatments have become available that were not included in earlier guidelines, said Dr. Khanna. In particular, the new guidelines are recommending a two-step diagnostic assay; “the diagnostic algorithm has changed, and hopefully that will help us change practice” to identify active infection more quickly and efficiently.
Another important update is the recommendation of fidaxomicin as an option for initial nonfulminant CDI as an alternative to vancomycin, Dr. Khanna said, noting that metronidazole remains an option for low-risk patients. An additional change is the advice to use a different treatment for a second recurrent infection rather than repeating the initial treatment.
The recommendation of bezlotoxumab for prevention of CDI recurrence in patients who are at high risk of recurrence is the first time this drug has appeared in major guidelines, Dr. Khanna observed.
The recommendation in support of fecal microbiota transplant is a key update to the management of CDI, including the guidance that the procedure can be repeated if necessary, he said.
Looking ahead, “Additional research is needed to fully understand the best testing algorithms for CDI,” Dr. Khanna explained. “More studies also are needed to show how FMT fully fits into the picture, and some current studies are looking at its potential earlier in the course of infection.”
The guidelines were developed in collaboration with the Practice Parameters Committee of the American College of Gastroenterology and received no outside funding. Dr. Kelly disclosed serving as a site investigator of a clinical trial for Finch Therapeutics and is an unpaid clinical advisory board member for OpenBiome. Dr. Khanna has coauthored previous guidelines on C. difficile. He disclosed consulting relationships with Finch, GlaxoSmithKline, Jetson, ProbioTech, and Shire/Takeda, as well as research support from Rebiotix, Seres, and Vedanta.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Tofacitinib shows mortality benefit in patients with COVID-19 pneumonia
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Children and COVID: Vaccination trends beginning to diverge
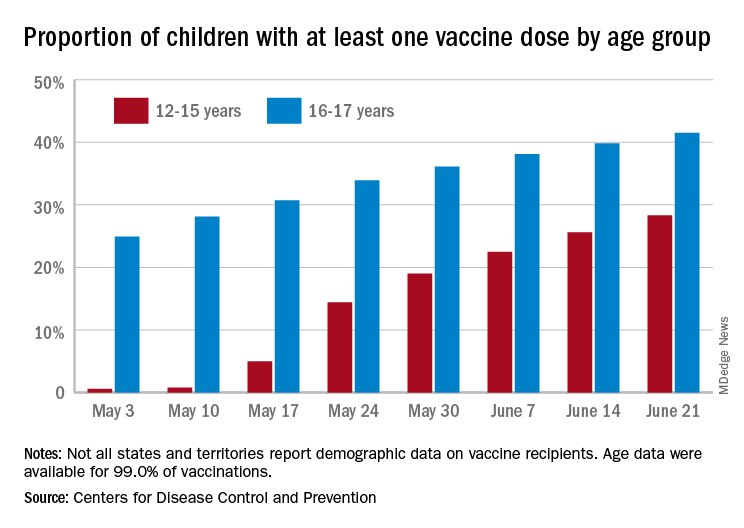
As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.

As more adolescents became eligible for a second dose of the Pfizer vaccine since it received approval from the Food and Drug Administration in mid-May, the share of 12- to 15-year-olds considered fully vaccinated rose from 11.4% on June 14 to 17.8% on June 28, an increase of 56%, the CDC’s COVID Data Tracker indicated June 22.
For children aged 16-17 years, who have been receiving the vaccine since early April, full vaccination rose by 9.6% in that same week, going from 29.1% on June 14 to 31.9% on June 21. The cumulative numbers for first vaccinations are higher, of course, but are rising more slowly in both age groups: 41.5% of those aged 16-17 had received at least one dose by June 21 (up by 4.3%), with the 12- to 15-year-olds at 28.3% (up by 10.5%), based on the CDC data.
Limiting the time frame to just the last 2 weeks, however, shows the opposite of rising among the younger children. During the 2 weeks ending June 7, 17.9% of those initiating a first dose were 12-15 years old, but that 2-week figure slipped to 17.1% as of June 14 and was down to 16.0% on June 21. The older group was slow but steady over that time: 4.8%, 4.7%, and 4.8%, the CDC said. To give those figures some context, those aged 25-39 years represented 23.7% of past-2-week initiations on June 7 and 24.3% on June 21.
Although no COVID-19 vaccine has been approved for children under 12 years, about 0.4% of that age group – just over 167,000 children – have received a first dose and almost 91,000 are fully vaccinated, according to CDC data.
Is event-driven PrEP dosing for HIV as effective as daily dosing?
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE-BASED ANSWER:
Probably, although there are no head-to-head trials comparing the 2 dosing regimens. Event-driven pre-exposure prophylaxis (PrEP) dosing reduces HIV conversion by 86% compared to placebo (strength of recommendation [SOR]: B, large randomized controlled trial [RCT]). Daily PrEP reduces HIV conversion by 44% to 86% (SOR: B, based on open-label RCTs).
Event-driven PrEP regimens may be associated with lower adherence when compared with daily PrEP regimens (average of 70% for event-driven PrEP vs average of 92% for daily PrEP) (SOR: B, based on open-label and cohort trials). Event-driven PrEP regimens have lower medication costs, and they are associated with no difference in the rate of sexually transmitted infections (STIs) (SOR: B, based on prospective cohort studies). Patients may prefer them to daily regimens (75% choose event-driven PrEP vs 25% choose daily PrEP) (SOR: B, based on the preponderance of prospective cohort studies with conflicting results).
5-year-old boy • calf pain • fever • cough & rhinitis • Dx?
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
THE CASE
A 5-year-old previously healthy white boy presented to clinic with bilateral calf pain and refusal to bear weight since awakening that morning. Associated symptoms included a 3-day history of generalized fatigue, subjective fevers, cough, congestion, and rhinitis. The night prior to presentation, he showed no symptoms of gait abnormalities, muscle pain, or weakness. There was no history of similar symptoms, trauma, overexertion, foreign travel, or family history of musculoskeletal disease. He was fully immunized, except for the annual influenza vaccine. He was not taking any medications. This case occurred before the onset of the COVID-19 pandemic.
Objective findings included fever of 101 °F, refusal to bear weight, and symmetrical bilateral tenderness to palpation of the gastrocnemius-soleus complex. Pain was elicited with passive dorsiflexion. There was no erythema, edema, or sensory deficits, and the distal leg compartments were soft. There was normal range of motion of the hips, knees, and ankles. Dorsalis pedis pulses were 2+, and patella reflexes were 2/4 bilaterally.
Lab results included a white blood cell count of 2500/μL (normal range, 4500 to 11,000/μL);absolute neutrophil count, 900/μL (1500 to 8000/μL); platelet count, 131,000/μL (150,000 to 450,000/μL); creatine kinase level, 869 IU/L (22 to 198 U/L); and aspartate aminotransferase level, 116 U/L (8 to 33 U/L). A rapid influenza swab was positive for influenza B. Plain films of the bilateral hips and lower extremities were unremarkable. C-reactive protein (CRP) level, urinalysis, and renal function tests were within normal limits. Creatine kinase (CK) level peaked (1935 U/L; normal range, 22 to 198 U/L) within the first 24 hours of presentation and then trended down.
The Diagnosis
The patient’s sudden onset of symmetrical bilateral calf pain in the setting of an upper respiratory tract infection was extremely suspicious for benign acute childhood myositis (BACM). Lab work and radiologic evaluation were performed to rule out more ominous causes of refusal to bear weight. The suspicion of BACM was further validated by influenza B serology, an elevated CK, and a normal CRP.
Discussion
BACM was first described by Lundberg in 1957.1 The overall incidence and prevalence are unclear.2 A viral prodrome involving rhinorrhea, low-grade fever, sore throat, cough, and malaise typically precedes bilateral calf pain by 3 days.2-4 Myositis symptoms typically last for 4 days.3 While several infectious etiologies have been linked to this condition, influenza B has the greatest association.5,6
❚ Patient population. BACM occurs predominately in school-aged children (6-8 years old) and has a male-to-female ratio of 2:1.3,5,6 In a retrospective study of 219 children, BACM was strongly associated with male gender and ages 6 to 9 years.3 In another retrospective study of 54 children,80% of patients were male, and the mean age was 7.3 years.5
❚ Key symptoms and differential. The distinguishing feature of BACM is bilateral symmetric gastrocnemius-soleus tenderness.2,4 Additionally, the lack of neurologic symptoms is an important differentiator, as long as refusal to bear weight is not mistaken for weakness.6 These features help to distinguish BACM from other items in the differential, including trauma, Guillain-Barre syndrome, osteomyelitis, malignancy, deep vein thrombosis, and inherited musculoskeletal disorders.2
Continue to: Labratory evaluation...
❚ Laboratory evaluation will often show mild neutropenia, thrombocytopenia, and mild elevation in CK.7,8 CRP is typically normal.4,7,9 In a retrospective study of 28 admissions for BACM from 2001 to 2012, common findings included leukopenia (35%), neutropenia (25%), and thrombocytopenia (21%). The median CK value was 4181 U/L.4 In another analysis of BACM cases, mean CK was 1872 U/L.5
❚ Biopsy is unnecessary; however, calf muscle samples from 11 of 12 children with suspected BACM due to influenza B infection were consistent with patchy necrosis without significant myositis.10
❚ Complications. Rhabdomyolysis, although rare, has been reported with BACM. In 1 analysis, 10 of 316 patients with influenza-associated myositis developed rhabdomyolysis; 8 experienced renal failure. Rhabdomyolysis was 4 times more likely to occur in girls, and 86% of cases were associated with influenza A.6 Common manifestations of rhabdomyolysis associated with influenza include diffuse myopathy, gross hematuria, and myoglobinuria.6
❚ Treatment is mainly supportive.4,8,9 Antivirals typically are not indicated, as the bilateral calf pain manifests during the recovery phase of the illness.4,9,11 BACM is self-limited and should resolve within 3 days of myositis manifestation.2 Patients should follow up in 2 to 3 weeks to verify symptom resolution.2
If muscle pain, swelling, and tenderness worsen, further work-up is indicated. In more severe cases, including those involving renal failure, intensive care management and even dialysis may be necessary.4,6
❚ Our patient was hospitalized due to fever in the setting of neutropenia. Ultimately, he was treated with acetaminophen and intravenous fluids for mild dehydration and elevated CK levels. He was discharged home after 3 days, at which time he had complete resolution of pain and was able to resume normal activities.
The Takeaway
Benign acute childhood myositis is a self-limited disorder with an excellent prognosis. It has a typical presentation and therefore should be a clinical diagnosis; however, investigative studies may be warranted to rule out more ominous causes. Reassurance to family that the condition should self-resolve in a few days is important. Close follow-up should be scheduled to ensure resolution of symptoms.
CORRESPONDENCE
Nicholas A. Rathjen, DO, William Beaumont Army Medical Center, Department of Soldier and Family Care, 11335 SSG Sims Street, Fort Bliss, TX 79918; nicholas.a.rathjen@gmail. com
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x
- Lundberg A. Myalgia cruris epidemica. Acta Paediatr. 1957;46:18-31. doi: 10.1111/j.1651-2227.1957.tb08627.x
- Magee H, Goldman RD. Viral myositis in children. Can Fam Physician. 2017;63:365-368.
- Mall S, Buchholz U, Tibussek D, et al. A large outbreak of influenza B-associated benign acute childhood myositis in Germany, 2007/2008. Pediatr Infect Dis J. 2011;30:e142-e146. doi: 10.1097/INF.0b013e318217e356
- Santos JA, Albuquerque C, Lito D, et al. Benign acute childhood myositis: an alarming condition with an excellent prognosis! Am J Emerg Med. 2014;32:1418-1419. doi: 10.1016/j.ajem.2014.08.022
- Rosenberg T, Heitner S, Scolnik D, et al. Outcome of benign acute childhood myositis: the experience of 2 large tertiary care pediatric hospitals. Pediatr Emerg Care. 2018;34:400-402. doi: 10.1097/PEC.0000000000000830
- Agyeman P, Duppenthaler A, Heininger U, et al. Influenza-associated myositis in children. Infection. 2004;32:199-203. doi: 10.1007/s15010-004-4003-2
- Mackay MT, Kornberg AJ, Shield LK, et al. Benign acute childhood myositis: laboratory and clinical features. Neurology. 1999;53:2127-2131. doi: 10.1212/wnl.53.9.2127
- Neocleous C, Spanou C, Mpampalis E, et al. Unnecessary diagnostic investigations in benign acute childhood myositis: a case series report. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012023
- Felipe Cavagnaro SM, Alejandra Aird G, Ingrid Harwardt R, et al. Benign acute childhood myositis: clinical series and literature review. Rev Chil Pediatr. 2017;88:268-274. doi: 10.1016/j.rchipe.2016.07.002
- Bove KE, Hilton PK, Partin J, et al. Morphology of acute myopathy associated with influenza B infection. Pediatric Pathology. 1983;1:51-66. https://doi.org/10.3109/15513818309048284
- Koliou M, Hadjiloizou S, Ourani S, et al. A case of benign acute childhood myositis associated with influenza A (HINI) virus infection. Clin Microbiol Infect. 2010;16:193-195. doi: 10.1111/j.1469-0691.2009.03064.x











