User login
Delta variant could drive herd immunity threshold over 80%
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Because the Delta variant of SARS-CoV-2 spreads more easily than the original virus, the proportion of the population that needs to be vaccinated to reach herd immunity could be upward of 80% or more, experts say.
Also, it could be time to consider wearing an N95 mask in public indoor spaces regardless of vaccination status, according to a media briefing on Aug. 3 sponsored by the Infectious Diseases Society of America.
Furthermore, giving booster shots to the fully vaccinated is not the top public health priority now. Instead, third vaccinations should be reserved for more vulnerable populations – and efforts should focus on getting first vaccinations to unvaccinated people in the United States and around the world.
“The problem here is that the Delta variant is ... more transmissible than the original virus. That pushes the overall population herd immunity threshold much higher,” Ricardo Franco, MD, assistant professor of medicine at the University of Alabama at Birmingham, said during the briefing.
“For Delta, those threshold estimates go well over 80% and may be approaching 90%,” he said.
To put that figure in context, the original SARS-CoV-2 virus required an estimated 67% of the population to be vaccinated to achieve herd immunity. Also, measles has one of the highest herd immunity thresholds at 95%, Dr. Franco added.
Herd immunity is the point at which enough people are immunized that the entire population gains protection. And it’s already happening. “Unvaccinated people are actually benefiting from greater herd immunity protection in high-vaccination counties compared to low-vaccination ones,” he said.
Maximize mask protection
Unlike early in the COVID-19 pandemic with widespread shortages of personal protective equipment, face masks are now readily available. This includes N95 masks, which offer enhanced protection against SARS-CoV-2, Ezekiel J. Emanuel, MD, PhD, said during the briefing.
Following the July 27 CDC recommendation that most Americans wear masks indoors when in public places, “I do think we need to upgrade our masks,” said Dr. Emanuel, who is Diane v.S. Levy & Robert M. Levy professor at the University of Pennsylvania, Philadelphia.
“It’s not just any mask,” he added. “Good masks make a big difference and are very important.”
Mask protection is about blocking 0.3-mcm particles, “and I think we need to make sure that people have masks that can filter that out,” he said. Although surgical masks are very good, he added, “they’re not quite as good as N95s.” As their name implies, N95s filter out 95% of these particles.
Dr. Emanuel acknowledged that people are tired of COVID-19 and complying with public health measures but urged perseverance. “We’ve sacrificed a lot. We should not throw it away in just a few months because we are tired. We’re all tired, but we do have to do the little bit extra getting vaccinated, wearing masks indoors, and protecting ourselves, our families, and our communities.”
Dealing with a disconnect
In response to a reporter’s question about the possibility that the large crowd at the Lollapalooza music festival in Chicago could become a superspreader event, Dr. Emanuel said, “it is worrisome.”
“I would say that, if you’re going to go to a gathering like that, wearing an N95 mask is wise, and not spending too long at any one place is also wise,” he said.
On the plus side, the event was held outdoors with lots of air circulation, Dr. Emanuel said.
However, “this is the kind of thing where we’ve got a sort of disconnect between people’s desire to get back to normal ... and the fact that we’re in the middle of this upsurge.”
Another potential problem is the event brought people together from many different locations, so when they travel home, they could be “potentially seeding lots of other communities.”
Boosters for some, for now
Even though not officially recommended, some fully vaccinated Americans are seeking a third or booster vaccination on their own.
Asked for his opinion, Dr. Emanuel said: “We’re probably going to have to be giving boosters to immunocompromised people and people who are susceptible. That’s where we are going to start.”
More research is needed regarding booster shots, he said. “There are very small studies – and the ‘very small’ should be emphasized – given that we’ve given shots to over 160 million people.”
“But it does appear that the boosters increase the antibodies and protection,” he said.
Instead of boosters, it is more important for people who haven’t been vaccinated to get fully vaccinated.
“We need to put our priorities in the right places,” he said.
Emanuel noted that, except for people in rural areas that might have to travel long distances, access to vaccines is no longer an issue. “It’s very hard not to find a vaccine if you want it.”
A remaining hurdle is “battling a major disinformation initiative. I don’t think this is misinformation. I think there’s very clear evidence that it is disinformation – false facts about the vaccines being spread,” Dr. Emanuel said.
The breakthrough infection dilemma
Breakthrough cases “remain the vast minority of infections at this time ... that is reassuring,” Dr. Franco said.
Also, tracking symptomatic breakthrough infections remains easier than studying fully vaccinated people who become infected with SARS-CoV-2 but remain symptom free.
“We really don’t have a good handle on the frequency of asymptomatic cases,” Dr. Emanuel said. “If you’re missing breakthrough infections, a lot of them, you may be missing some [virus] evolution that would be very important for us to follow.” This missing information could include the emergence of new variants.
The asymptomatic breakthrough cases are the most worrisome group,” Dr. Emanuel said. “You get infected, you’re feeling fine. Maybe you’ve got a little sneeze or cough, but nothing unusual. And then you’re still able to transmit the Delta variant.”
The big picture
The upsurge in cases, hospitalizations, and deaths is a major challenge, Dr. Emanuel said. “We need to address that by getting many more people vaccinated right now with what are very good vaccines.”
“But it also means that we have to stop being U.S. focused alone.” He pointed out that Delta and other variants originated overseas, “so getting the world vaccinated ... has to be a top priority.”
“We are obviously all facing a challenge as we move into the fall,” Dr. Emanuel said. “With schools opening and employers bringing their employees back together, even if these groups are vaccinated, there are going to be major challenges for all of us.”
A version of this article first appeared on Medscape.com.
Shorter antibiotic course okay for UTIs in men with no fever
A week of antibiotics appears just as effective as 2 weeks in treating afebrile men with urinary tract infections (UTIs), researchers say.
Shortening the course of treatment could spare patients side effects from the medications and reduce the risk that bacteria will develop resistance to the drugs, said Dimitri Drekonja, MD, chief of infectious diseases at the Minneapolis VA Medical Center.
“You’d like to be on these drugs for as short [an] amount of time as gets the job done,” he told this news organization. The study was published online July 28 in JAMA.
Researchers have recently found that shorter courses of antimicrobials are effective in the treatment of other types of infection and for UTIs in women. However, UTIs in men are thought to be more complicated because the male urethra is longer.
To see whether reducing length of treatment could be effective in men as well, Dr. Drekonja and colleagues compared 7-day and 14-day regimens in men treated at U.S. Veterans Affairs medical centers in Minnesota and Texas.
They recruited 272 men who had symptoms of UTI and were willing to participate. All the men received trimethoprim/sulfamethoxazole or ciprofloxacin for 7 days. Half the men were randomly assigned to continue this treatment for an additional 7 days; the other half received placebo pills for an additional 7 days.
The average age of the men was 69 years. Urine samples were cultured from 87.9% of the men. In 60.7% of these samples, the researchers found more than 100,000 CFU/mL; in 16.3%, they found lower colony counts; and in 23.0%, they found no growth of bacteria. The most common organism they isolated was Escherchia coli.
Results for the two groups were similar. Symptoms resolved 14 days after completion of the course of treatment in 90.4% of those who received 14 days of antibiotics, versus 91.9% of those who received 7 days of antibiotics plus 7 days of placebo pills. At 1.5%, the difference between the two arms was within the predetermined boundary for noninferiority.
The percentage of those who experienced recurrence of symptoms within 28 days of stopping medication was also similar between the two groups. Among those who received 7 days of antibiotics, 10.3% experienced recurrence of symptoms, compared to 16.9% of those assigned to 14 days of antibiotics.
There was no significant difference in the resolution of UTI symptoms between the two groups by type of antibiotic, pretreatment bacteriuria count, or study site.
Adverse events were also similar in the two groups, occurring in 20.6% of the men who received 7 days of antibiotics, versus 24.3% of the men who received 14 days of treatment. In both groups, 8.8% of patients had diarrhea, which was the most common adverse event.
Clinicians should not worry that antibiotic resistance is more likely to develop or that symptoms will recur when patients don’t finish a prescribed course of treatment, Dr. Drekonja said. “That is an old piece of guidance that has persisted for such a long time,” he said. “And it makes all of us in the infectious disease field cringe.”
Rather, the current thinking is that the more antibiotics patients take, the more resistance bacteria will develop, he said.
The success of the 7-day regimen raises the question of whether an even shorter course would work equally well. It’s not clear how short a course of antibiotics will do the trick. Research in certain populations, such as patients with spinal cord injuries, has suggested that recurrences are more frequent with 3 days of antibiotics than with 14, “so there could be a floor that you do need to go beyond,” Dr. Drekonja said.
“We’re not really sure how much people need,” agreed Daniel Morgan, MD, a professor of epidemiology and public health and medicine at the University of Maryland, Baltimore, which is why this study is important. “It really defined that 1 week is better than 2 weeks,” he said in an interview.
Another way that clinicians can reduce the use of antibiotics by men with UTIs is to consider alternative diagnoses and to culture urine samples when UTI seems like the most likely cause of their symptoms, said Dr. Morgan, who co-authored an accompanying editorial.
He pointed out that the U.S. Food and Drug Administration has issued a black box warning on fluoroquinolones, including ciprofloxacin, because they increase the risk for tendinitis and tendon rupture. Nitrofurantoin and amoxicillin-clavulanate are better alternatives for UTIs, he said.
Even some men with fevers and UTIs may need no more than 7 days of antibiotics, said Dr. Morgan. Dr. Drekonja said he generally prescribes at least 10 days antibiotics for these men.
The study was funded by the VA Merit Review Program. Dr. Drekonja and Dr. Morgan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A week of antibiotics appears just as effective as 2 weeks in treating afebrile men with urinary tract infections (UTIs), researchers say.
Shortening the course of treatment could spare patients side effects from the medications and reduce the risk that bacteria will develop resistance to the drugs, said Dimitri Drekonja, MD, chief of infectious diseases at the Minneapolis VA Medical Center.
“You’d like to be on these drugs for as short [an] amount of time as gets the job done,” he told this news organization. The study was published online July 28 in JAMA.
Researchers have recently found that shorter courses of antimicrobials are effective in the treatment of other types of infection and for UTIs in women. However, UTIs in men are thought to be more complicated because the male urethra is longer.
To see whether reducing length of treatment could be effective in men as well, Dr. Drekonja and colleagues compared 7-day and 14-day regimens in men treated at U.S. Veterans Affairs medical centers in Minnesota and Texas.
They recruited 272 men who had symptoms of UTI and were willing to participate. All the men received trimethoprim/sulfamethoxazole or ciprofloxacin for 7 days. Half the men were randomly assigned to continue this treatment for an additional 7 days; the other half received placebo pills for an additional 7 days.
The average age of the men was 69 years. Urine samples were cultured from 87.9% of the men. In 60.7% of these samples, the researchers found more than 100,000 CFU/mL; in 16.3%, they found lower colony counts; and in 23.0%, they found no growth of bacteria. The most common organism they isolated was Escherchia coli.
Results for the two groups were similar. Symptoms resolved 14 days after completion of the course of treatment in 90.4% of those who received 14 days of antibiotics, versus 91.9% of those who received 7 days of antibiotics plus 7 days of placebo pills. At 1.5%, the difference between the two arms was within the predetermined boundary for noninferiority.
The percentage of those who experienced recurrence of symptoms within 28 days of stopping medication was also similar between the two groups. Among those who received 7 days of antibiotics, 10.3% experienced recurrence of symptoms, compared to 16.9% of those assigned to 14 days of antibiotics.
There was no significant difference in the resolution of UTI symptoms between the two groups by type of antibiotic, pretreatment bacteriuria count, or study site.
Adverse events were also similar in the two groups, occurring in 20.6% of the men who received 7 days of antibiotics, versus 24.3% of the men who received 14 days of treatment. In both groups, 8.8% of patients had diarrhea, which was the most common adverse event.
Clinicians should not worry that antibiotic resistance is more likely to develop or that symptoms will recur when patients don’t finish a prescribed course of treatment, Dr. Drekonja said. “That is an old piece of guidance that has persisted for such a long time,” he said. “And it makes all of us in the infectious disease field cringe.”
Rather, the current thinking is that the more antibiotics patients take, the more resistance bacteria will develop, he said.
The success of the 7-day regimen raises the question of whether an even shorter course would work equally well. It’s not clear how short a course of antibiotics will do the trick. Research in certain populations, such as patients with spinal cord injuries, has suggested that recurrences are more frequent with 3 days of antibiotics than with 14, “so there could be a floor that you do need to go beyond,” Dr. Drekonja said.
“We’re not really sure how much people need,” agreed Daniel Morgan, MD, a professor of epidemiology and public health and medicine at the University of Maryland, Baltimore, which is why this study is important. “It really defined that 1 week is better than 2 weeks,” he said in an interview.
Another way that clinicians can reduce the use of antibiotics by men with UTIs is to consider alternative diagnoses and to culture urine samples when UTI seems like the most likely cause of their symptoms, said Dr. Morgan, who co-authored an accompanying editorial.
He pointed out that the U.S. Food and Drug Administration has issued a black box warning on fluoroquinolones, including ciprofloxacin, because they increase the risk for tendinitis and tendon rupture. Nitrofurantoin and amoxicillin-clavulanate are better alternatives for UTIs, he said.
Even some men with fevers and UTIs may need no more than 7 days of antibiotics, said Dr. Morgan. Dr. Drekonja said he generally prescribes at least 10 days antibiotics for these men.
The study was funded by the VA Merit Review Program. Dr. Drekonja and Dr. Morgan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A week of antibiotics appears just as effective as 2 weeks in treating afebrile men with urinary tract infections (UTIs), researchers say.
Shortening the course of treatment could spare patients side effects from the medications and reduce the risk that bacteria will develop resistance to the drugs, said Dimitri Drekonja, MD, chief of infectious diseases at the Minneapolis VA Medical Center.
“You’d like to be on these drugs for as short [an] amount of time as gets the job done,” he told this news organization. The study was published online July 28 in JAMA.
Researchers have recently found that shorter courses of antimicrobials are effective in the treatment of other types of infection and for UTIs in women. However, UTIs in men are thought to be more complicated because the male urethra is longer.
To see whether reducing length of treatment could be effective in men as well, Dr. Drekonja and colleagues compared 7-day and 14-day regimens in men treated at U.S. Veterans Affairs medical centers in Minnesota and Texas.
They recruited 272 men who had symptoms of UTI and were willing to participate. All the men received trimethoprim/sulfamethoxazole or ciprofloxacin for 7 days. Half the men were randomly assigned to continue this treatment for an additional 7 days; the other half received placebo pills for an additional 7 days.
The average age of the men was 69 years. Urine samples were cultured from 87.9% of the men. In 60.7% of these samples, the researchers found more than 100,000 CFU/mL; in 16.3%, they found lower colony counts; and in 23.0%, they found no growth of bacteria. The most common organism they isolated was Escherchia coli.
Results for the two groups were similar. Symptoms resolved 14 days after completion of the course of treatment in 90.4% of those who received 14 days of antibiotics, versus 91.9% of those who received 7 days of antibiotics plus 7 days of placebo pills. At 1.5%, the difference between the two arms was within the predetermined boundary for noninferiority.
The percentage of those who experienced recurrence of symptoms within 28 days of stopping medication was also similar between the two groups. Among those who received 7 days of antibiotics, 10.3% experienced recurrence of symptoms, compared to 16.9% of those assigned to 14 days of antibiotics.
There was no significant difference in the resolution of UTI symptoms between the two groups by type of antibiotic, pretreatment bacteriuria count, or study site.
Adverse events were also similar in the two groups, occurring in 20.6% of the men who received 7 days of antibiotics, versus 24.3% of the men who received 14 days of treatment. In both groups, 8.8% of patients had diarrhea, which was the most common adverse event.
Clinicians should not worry that antibiotic resistance is more likely to develop or that symptoms will recur when patients don’t finish a prescribed course of treatment, Dr. Drekonja said. “That is an old piece of guidance that has persisted for such a long time,” he said. “And it makes all of us in the infectious disease field cringe.”
Rather, the current thinking is that the more antibiotics patients take, the more resistance bacteria will develop, he said.
The success of the 7-day regimen raises the question of whether an even shorter course would work equally well. It’s not clear how short a course of antibiotics will do the trick. Research in certain populations, such as patients with spinal cord injuries, has suggested that recurrences are more frequent with 3 days of antibiotics than with 14, “so there could be a floor that you do need to go beyond,” Dr. Drekonja said.
“We’re not really sure how much people need,” agreed Daniel Morgan, MD, a professor of epidemiology and public health and medicine at the University of Maryland, Baltimore, which is why this study is important. “It really defined that 1 week is better than 2 weeks,” he said in an interview.
Another way that clinicians can reduce the use of antibiotics by men with UTIs is to consider alternative diagnoses and to culture urine samples when UTI seems like the most likely cause of their symptoms, said Dr. Morgan, who co-authored an accompanying editorial.
He pointed out that the U.S. Food and Drug Administration has issued a black box warning on fluoroquinolones, including ciprofloxacin, because they increase the risk for tendinitis and tendon rupture. Nitrofurantoin and amoxicillin-clavulanate are better alternatives for UTIs, he said.
Even some men with fevers and UTIs may need no more than 7 days of antibiotics, said Dr. Morgan. Dr. Drekonja said he generally prescribes at least 10 days antibiotics for these men.
The study was funded by the VA Merit Review Program. Dr. Drekonja and Dr. Morgan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Untreatable, drug-resistant fungus found in Texas and Washington, D.C.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increases in new COVID cases among children far outpace vaccinations
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.
Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
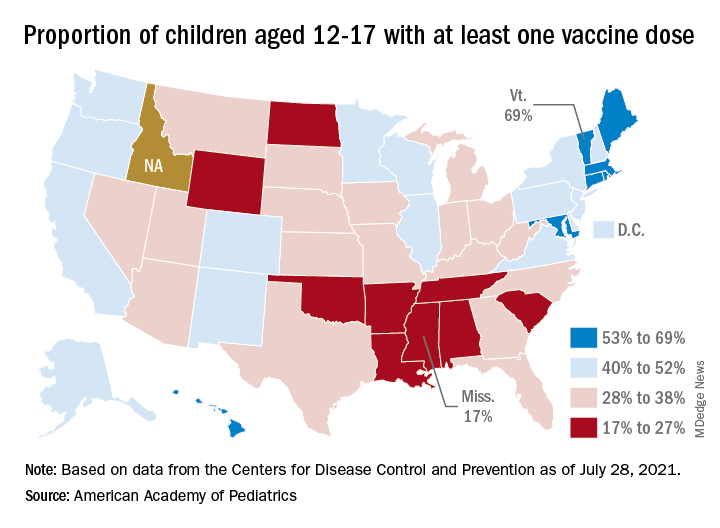
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
Doctors’ offices may be hot spot for transmission of respiratory infections
Prior research has examined the issue of hospital-acquired infections. A 2014 study published in the New England Journal of Medicine, for example, found that 4% of hospitalized patients acquired a health care–associated infection during their stay. Furthermore, the Centers for Disease Control and Prevention estimates that, on any given day, one in 31 hospital patients has at least one health care–associated infection. However, researchers for the new study, published in Health Affairs, said evidence about the risk of acquiring respiratory viral infections in medical office settings is limited.
“Hospital-acquired infections has been a problem for a while,” study author Hannah Neprash, PhD, of the department of health policy and management at the University of Minnesota School of Public Health, Minneapolis, said in an interview. “However, there’s never been a similar study of whether a similar phenomenon happens in physician offices. This is especially relevant now when we’re dealing with respiratory infections.”
Methods and results
For the new study, Dr. Neprash and her colleagues analyzed deidentified billing and scheduling data from 2016-2017 for 105,462,600 outpatient visits that occurred at 6,709 office-based primary care practices. They used the World Health Organization case definition for influenzalike illness “to capture cases in which the physician may suspect this illness even if a specific diagnosis code was not present.” Their control conditions included exposure to urinary tract infections and back pain.
Doctor visits were considered unexposed if they were scheduled to start at least 90 minutes before the first influenzalike illness visit of the day. They were considered exposed if they were scheduled to start at the same time or after the first influenzalike illness visit of the day at that practice.
Researchers quantified whether exposed patients were more likely to return with a similar illness in the next 2 weeks, compared with nonexposed patients seen earlier in the day
They found that 2.7 patients per 1,000 returned within 2 weeks with an influenzalike illness.
Patients were more likely to return with influenzalike illness if their visit occurred after an influenzalike illness visit versus before, the researchers said.
The authors of the paper said their new research highlights the importance of infection control in health care settings, including outpatient offices.
Where did the exposure occur?
Diego Hijano, MD, MSc, pediatric infectious disease specialist at St. Jude’s Children’s Research Hospital, Memphis, Tenn., said he was not surprised by the findings, but noted that it’s hard to say if the exposure to influenzalike illnesses happened in the office or in the community.
“If you start to see individuals with influenza in your office it’s because [there’s influenza] in the community,” Dr. Hijano explained. “So that means that you will have more patients coming in with influenza.”
To reduce the transmission of infections, Dr. Neprash suggested that doctors’ offices follow the CDC guidelines for indoor conduct, which include masking, washing hands, and “taking appropriate infection control measures.”
So potentially masking within offices is a way to minimize transmission between whatever people are there to be seen when it’s contagious, Dr. Neprash said.
“Telehealth really took off in 2020 and it’s unclear what the state of telehealth will be going forward. [These findings] suggest that there’s a patient safety argument for continuing to enable primary care physicians to provide visits either by phone or by video,” he added.
Dr. Hijano thinks it would be helpful for doctors to separate patients with respiratory illnesses from those without respiratory illnesses.
Driver of transmissions
Dr. Neprash suggested that another driver of these transmissions could be doctors not washing their hands, which is a “notorious issue,” and Dr. Hijano agreed with that statement.
“We did know that the hands of physicians and nurses and care providers are the main driver of infections in the health care setting,” Dr. Hijano explained. “I mean, washing your hands properly between encounters is the single best way that any given health care provider can prevent the spread of infections.”
“We have a unique opportunity with COVID-19 to change how these clinics are operating now,” Dr. Hijano said. “Many clinics are actually asking patients to call ahead of time if you have symptoms of a respiratory illness that could be contagious, and those who are not are still mandating the use of mask and physical distance in the waiting areas and limiting the amount of number of patients in any given hour. So I think that those are really big practices that would kind of make an impact in respiratory illness in terms of decreasing transmission in clinics.”
The authors, who had no conflicts of interest said their hope is that their study will help inform policy for reopening outpatient care settings. Dr. Hijano, who was not involved in the study also had no conflicts.
Prior research has examined the issue of hospital-acquired infections. A 2014 study published in the New England Journal of Medicine, for example, found that 4% of hospitalized patients acquired a health care–associated infection during their stay. Furthermore, the Centers for Disease Control and Prevention estimates that, on any given day, one in 31 hospital patients has at least one health care–associated infection. However, researchers for the new study, published in Health Affairs, said evidence about the risk of acquiring respiratory viral infections in medical office settings is limited.
“Hospital-acquired infections has been a problem for a while,” study author Hannah Neprash, PhD, of the department of health policy and management at the University of Minnesota School of Public Health, Minneapolis, said in an interview. “However, there’s never been a similar study of whether a similar phenomenon happens in physician offices. This is especially relevant now when we’re dealing with respiratory infections.”
Methods and results
For the new study, Dr. Neprash and her colleagues analyzed deidentified billing and scheduling data from 2016-2017 for 105,462,600 outpatient visits that occurred at 6,709 office-based primary care practices. They used the World Health Organization case definition for influenzalike illness “to capture cases in which the physician may suspect this illness even if a specific diagnosis code was not present.” Their control conditions included exposure to urinary tract infections and back pain.
Doctor visits were considered unexposed if they were scheduled to start at least 90 minutes before the first influenzalike illness visit of the day. They were considered exposed if they were scheduled to start at the same time or after the first influenzalike illness visit of the day at that practice.
Researchers quantified whether exposed patients were more likely to return with a similar illness in the next 2 weeks, compared with nonexposed patients seen earlier in the day
They found that 2.7 patients per 1,000 returned within 2 weeks with an influenzalike illness.
Patients were more likely to return with influenzalike illness if their visit occurred after an influenzalike illness visit versus before, the researchers said.
The authors of the paper said their new research highlights the importance of infection control in health care settings, including outpatient offices.
Where did the exposure occur?
Diego Hijano, MD, MSc, pediatric infectious disease specialist at St. Jude’s Children’s Research Hospital, Memphis, Tenn., said he was not surprised by the findings, but noted that it’s hard to say if the exposure to influenzalike illnesses happened in the office or in the community.
“If you start to see individuals with influenza in your office it’s because [there’s influenza] in the community,” Dr. Hijano explained. “So that means that you will have more patients coming in with influenza.”
To reduce the transmission of infections, Dr. Neprash suggested that doctors’ offices follow the CDC guidelines for indoor conduct, which include masking, washing hands, and “taking appropriate infection control measures.”
So potentially masking within offices is a way to minimize transmission between whatever people are there to be seen when it’s contagious, Dr. Neprash said.
“Telehealth really took off in 2020 and it’s unclear what the state of telehealth will be going forward. [These findings] suggest that there’s a patient safety argument for continuing to enable primary care physicians to provide visits either by phone or by video,” he added.
Dr. Hijano thinks it would be helpful for doctors to separate patients with respiratory illnesses from those without respiratory illnesses.
Driver of transmissions
Dr. Neprash suggested that another driver of these transmissions could be doctors not washing their hands, which is a “notorious issue,” and Dr. Hijano agreed with that statement.
“We did know that the hands of physicians and nurses and care providers are the main driver of infections in the health care setting,” Dr. Hijano explained. “I mean, washing your hands properly between encounters is the single best way that any given health care provider can prevent the spread of infections.”
“We have a unique opportunity with COVID-19 to change how these clinics are operating now,” Dr. Hijano said. “Many clinics are actually asking patients to call ahead of time if you have symptoms of a respiratory illness that could be contagious, and those who are not are still mandating the use of mask and physical distance in the waiting areas and limiting the amount of number of patients in any given hour. So I think that those are really big practices that would kind of make an impact in respiratory illness in terms of decreasing transmission in clinics.”
The authors, who had no conflicts of interest said their hope is that their study will help inform policy for reopening outpatient care settings. Dr. Hijano, who was not involved in the study also had no conflicts.
Prior research has examined the issue of hospital-acquired infections. A 2014 study published in the New England Journal of Medicine, for example, found that 4% of hospitalized patients acquired a health care–associated infection during their stay. Furthermore, the Centers for Disease Control and Prevention estimates that, on any given day, one in 31 hospital patients has at least one health care–associated infection. However, researchers for the new study, published in Health Affairs, said evidence about the risk of acquiring respiratory viral infections in medical office settings is limited.
“Hospital-acquired infections has been a problem for a while,” study author Hannah Neprash, PhD, of the department of health policy and management at the University of Minnesota School of Public Health, Minneapolis, said in an interview. “However, there’s never been a similar study of whether a similar phenomenon happens in physician offices. This is especially relevant now when we’re dealing with respiratory infections.”
Methods and results
For the new study, Dr. Neprash and her colleagues analyzed deidentified billing and scheduling data from 2016-2017 for 105,462,600 outpatient visits that occurred at 6,709 office-based primary care practices. They used the World Health Organization case definition for influenzalike illness “to capture cases in which the physician may suspect this illness even if a specific diagnosis code was not present.” Their control conditions included exposure to urinary tract infections and back pain.
Doctor visits were considered unexposed if they were scheduled to start at least 90 minutes before the first influenzalike illness visit of the day. They were considered exposed if they were scheduled to start at the same time or after the first influenzalike illness visit of the day at that practice.
Researchers quantified whether exposed patients were more likely to return with a similar illness in the next 2 weeks, compared with nonexposed patients seen earlier in the day
They found that 2.7 patients per 1,000 returned within 2 weeks with an influenzalike illness.
Patients were more likely to return with influenzalike illness if their visit occurred after an influenzalike illness visit versus before, the researchers said.
The authors of the paper said their new research highlights the importance of infection control in health care settings, including outpatient offices.
Where did the exposure occur?
Diego Hijano, MD, MSc, pediatric infectious disease specialist at St. Jude’s Children’s Research Hospital, Memphis, Tenn., said he was not surprised by the findings, but noted that it’s hard to say if the exposure to influenzalike illnesses happened in the office or in the community.
“If you start to see individuals with influenza in your office it’s because [there’s influenza] in the community,” Dr. Hijano explained. “So that means that you will have more patients coming in with influenza.”
To reduce the transmission of infections, Dr. Neprash suggested that doctors’ offices follow the CDC guidelines for indoor conduct, which include masking, washing hands, and “taking appropriate infection control measures.”
So potentially masking within offices is a way to minimize transmission between whatever people are there to be seen when it’s contagious, Dr. Neprash said.
“Telehealth really took off in 2020 and it’s unclear what the state of telehealth will be going forward. [These findings] suggest that there’s a patient safety argument for continuing to enable primary care physicians to provide visits either by phone or by video,” he added.
Dr. Hijano thinks it would be helpful for doctors to separate patients with respiratory illnesses from those without respiratory illnesses.
Driver of transmissions
Dr. Neprash suggested that another driver of these transmissions could be doctors not washing their hands, which is a “notorious issue,” and Dr. Hijano agreed with that statement.
“We did know that the hands of physicians and nurses and care providers are the main driver of infections in the health care setting,” Dr. Hijano explained. “I mean, washing your hands properly between encounters is the single best way that any given health care provider can prevent the spread of infections.”
“We have a unique opportunity with COVID-19 to change how these clinics are operating now,” Dr. Hijano said. “Many clinics are actually asking patients to call ahead of time if you have symptoms of a respiratory illness that could be contagious, and those who are not are still mandating the use of mask and physical distance in the waiting areas and limiting the amount of number of patients in any given hour. So I think that those are really big practices that would kind of make an impact in respiratory illness in terms of decreasing transmission in clinics.”
The authors, who had no conflicts of interest said their hope is that their study will help inform policy for reopening outpatient care settings. Dr. Hijano, who was not involved in the study also had no conflicts.
FROM HEALTH AFFAIRS
Early transition to oral beta-lactams for low-risk S. aureus bacteremia may be acceptable
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
‘A few mutations away’: The threat of a vaccine-proof variant
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
Hyperimmune globulin fails to prevent congenital CMV infection
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
Administering hyperimmune globulin to pregnant women who tested positive for cytomegalovirus did not reduce CMV infections or deaths among their fetuses or newborns, according to a randomized controlled trial published online July 28 in the New England Journal of Medicine.
Up to 40,000 infants a year have congenital CMV infections, which can lead to stillbirth, neonatal death, deafness, and cognitive and motor delay. An estimated 35%-40% of fetuses of women with a primary CMV infection will develop an infection, write Brenna Hughes, MD, an associate professor of ob/gyn and chief of the division of maternal fetal medicine at Duke University, Durham, N.C., and colleagues.
Previous trials and observational studies have shown mixed results with hyperimmune globulin for the prevention of congenital CMV infection.
“It was surprising to us that none of the outcomes in this trial were in the direction of potential benefit,” Dr. Hughes told this news organization. “However, this is why it is important to do large trials in a diverse population.”
The study cohort comprised 206,082 pregnant women who were screened for CMV infection before 23 weeks’ gestation. Of those women, 712 (0.35%) tested positive for CMV. The researchers enrolled 399 women who had tested positive and randomly assigned them to receive either a monthly infusion of CMV hyperimmune globulin (100 mg/kg) or placebo until delivery. The researchers used a composite of CMV infection or, if no testing occurred, fetal/neonatal death as the primary endpoint.
The trial was stopped early for futility when data from 394 participants revealed that 22.7% of offspring in the hyperimmune globulin group and 19.4% of those in the placebo group had had a CMV infection or had died (relative risk = 1.17; P = .42).
When individual endpoints were examined, trends were detected in favor of the placebo, but they did not reach statistical significance. The incidence of death was higher in the hyperimmune globulin group (4.9%) than in the placebo group (2.6%). The rate of preterm birth was also higher in the intervention group (12.2%) than in the group that received placebo (8.3%). The incidence of birth weight below the fifth percentile was 10.3% in the intervention group and 5.4% in the placebo group.
One woman who received hyperimmune globulin experienced a severe allergic reaction to the first infusion. Additionally, more women in the hyperimmune globulin group experienced headaches and shaking chills during infusions than did those who received placebo. There were no differences in maternal outcomes between the groups. There were no thromboembolic or ischemic events in either group.
“These findings suggest CMV hyperimmune globulin should not be used for the prevention of congenital CMV in pregnant patients with primary CMV during pregnancy,” Dr. Hughes said in an interview.
“A CMV vaccine is likely to be the most effective public health measure that we can offer, and that should be at the forefront of research investments,” she said. “But some of the other medications that work against CMV should be tested on a large scale as well,” she said. For example, a small trial in Israel showed that high-dose valacyclovir in early pregnancy decreased congenital CMV, and thus the drug merits study in a larger trial, she said.
Other experts agree that developing a vaccine should be the priority.
“The ultimate goal for preventing the brain damage and birth defects caused by congenital CMV infection is a vaccine that is as effective as the rubella vaccine has been for eliminating congenital rubella syndrome and that can be given well before pregnancy,” said Sallie Permar, MD, PhD, chair of pediatrics at Weill Cornell Medicine and pediatrician-in-chief at New York–Presbyterian/Weill Cornell Medical Center and the New York–Presbyterian Komansky Children’s Hospital in New York.
“While trials of vaccines are ongoing, there is a need to have a therapeutic option, especially for the high-risk setting of a mother acquiring the virus for the first time during pregnancy,” Dr. Permar said in an interview.
Dr. Permar was not involved in this study but is involved in follow-up studies of this cohort and is conducting research on CMV maternal vaccines. She noted the need for safe, effective antiviral treatments and for research into newer immunoglobulin products, such as monoclonal antibodies.
Both Dr. Permar and Dr. Hughes highlighted the challenge of raising awareness about the danger of CMV infections during pregnancy.
“Pregnant women, and especially those who have or work with young children, who are frequently carriers of the infection, should be informed of this risk,” Dr. Permar said. She hopes universal testing of newborns will be implemented and that it enables people to recognize the frequency and burden of these infections. She remains optimistic about a vaccine.
“After 60 years of research into a CMV vaccine, I believe we are currently in a ‘golden age’ of CMV vaccine development,” she said. She noted that Moderna is about to launch a phase 3 mRNA vaccine trial for CMV. “Moreover, immune correlates of protection against CMV have been identified from previous partially effective vaccines, and animal models have improved for preclinical studies. Therefore, I believe we will have an effective and safe vaccine against this most common congenital infection in the coming years.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Advancing Translational Sciences. Dr. Hughes has served on Merck’s scientific advisory board. Various coauthors have received personal fees from Medela and nonfinancial support from Hologic; personal fees from Moderna and VBI vaccines, and grants from Novavax. Dr. Permar consults for Pfizer, Moderna, Merck, Sanofi, and Dynavax on their CMV vaccine programs, and she has a sponsored research program with Merck and Moderna on CMV vaccines.
A version of this article first appeared on Medscape.com.
I Never Wanted To Be a Hero
I have been in the business of medicine for more than 15 years and I will never forget the initial surge of the COVID-19 pandemic in Massachusetts.
As a hospitalist, I admitted patients infected with COVID-19, followed them on the floor, and, since I had some experience working in an intensive care unit (ICU), was assigned to cover a “COVID ICU.” This wing of the hospital used to be a fancy orthopedic floor that our institution was lucky enough to have. So began the most life-changing experience in my career as a physician.
In this role, we witness death more than any of us would care to discuss. It comes with the territory, and we never expected this to change once COVID hit. However, so many patients succumbed to this disease, especially during the first surge, which made it difficult to handle emotionally. Patients that fell ill initially stayed isolated at home, optimistic they would turn the corner only to enter the hospital a week later after their conditioned worsened. After requiring a couple of liters of supplemental oxygen in the emergency room, they eventually ended up on a high flow nasal cannula in just a matter of hours.
Patients slowly got sicker and felt more helpless as the days passed, leading us to prescribe drugs that eventually proved to have no benefit. We checked countless inflammatory markers, most of which we were not even sure what to do with. Many times, we hosted a family meeting via FaceTime, holding a patient’s hand in one hand and an iPad in the other to discuss goals of care. Too often, a dark cloud hung over these discussions, a realization that there was not much else we could do.
I have always felt that helping someone have a decent and peaceful death is important, especially when the prognosis is grim, and that patient is suffering. But the sheer number of times this happened during the initial surge of the pandemic was difficult to handle. It felt like I had more of those discussions in 3 months than I did during my entire career as a hospitalist.
We helped plenty of people get better, with some heading home in a week. They thanked us, painted rocks and the sidewalks in front of the hospital displaying messages of gratitude, and sent lunches. Others, though, left the hospital 2 months later with a tube in their stomach so they could receive some form of nutrition and another in their neck to help them breathe.
These struggles were by no means special to me; other hospitalists around the world faced similar situations at one point or another during the pandemic. Working overtime, coming home late, exhausted, undressing in the garage, trying to be there for my 3 kids who were full of energy after a whole day of Zoom and doing the usual kid stuff. My house used to have strict rules about screen time. No more.
The summer months provided a bit of a COVID break, with only 1 or 2 infected patients entering my care. We went to outdoor restaurants and tried to get our lives back to “normal.” As the weather turned cold, however, things went south again. This time no more hydroxychloroquine, a drug used to fight malaria but also treat other autoimmune diseases, as it was proven eventually over many studies that it is not helpful and was potentially harmful. We instead shifted our focus to remdesivir—an antiviral drug that displayed some benefits—tocilizumab, and dexamethasone, anti-inflammatory drugs with the latter providing some positive outcomes on mortality.
Patient survival rates improved slightly, likely due to a combination of factors. We were more experienced at fighting the disease, which led to things in the hospital not being as chaotic and more time available to spend with the patients. Personal protective equipment (PPE) and tests were more readily available, and the population getting hit by the disease changed slightly with fewer elderly people from nursing homes falling ill because of social distancing, other safety measures, or having already fought the disease. Our attention turned instead to more young people that had returned to work and their social lives.
The arrival of the vaccines brought considerable relief. I remember a few decades ago debating and sometimes fighting with friends and family over who was better: Iron Man or Spider-Man. Now I found myself having the same conversation about the Pfizer and Moderna COVID vaccines.
Summer 2021 holds significantly more promise. Most of the adult population is getting vaccinated, and I am very hopeful that we are approaching the end of this nightmare. In June, our office received word that we could remove our masks if we were fully vaccinated. It felt weird, but represented another sign that things are improving. I took my kids to the mall and removed my mask. It felt odd considering how that little blue thing became part of me during the pandemic. It also felt strange to not prescribe a single dose of remdesivir for an entire month.
It feels good—and normal—to care for the patients that we neglected for a year. It has been a needed boost to see patients return to their health care providers for their colonoscopy screenings, mammograms, and managing chronic problems like coronary artery disease, congestive heart failure, or receiving chemotherapy.
I learned plenty from this pandemic and hope I am not alone. I learned to be humble. We started with a drug that was harmful, moved on to a drug that is probably neutral and eventually were able to come up with a drug that seems to decrease mortality at least in some COVID patients. I learned it is fine to try new therapies based on the best data in the hope they result in positive clinical outcomes. However, it is critical that we all keep an eye on the rapidly evolving literature and adjust our behavior accordingly.
I also learned, or relearned, that if people are desperate enough, they will drink bleach to see if it works. Others are convinced that the purpose of vaccination is to inject a microchip allowing ourselves to be tracked by some higher power. I learned that we must take the first step to prepare for the next pandemic by having a decent reserve of PPE.
It is clear synthetic messenger RNA (mRNA) technology is here to stay, and I believe it has a huge potential to change many areas of medicine. mRNA vaccines proved to be much faster to develop and probably much easier to change as the pathogen, in this case coronavirus, changes.
The technology could be used against a variety of infectious diseases to make vaccines against malaria, tuberculosis, HIV, or hepatitis. It can also be very useful for faster vaccine development needed in future possible pandemics such as influenza, Ebola, or severe acute respiratory syndrome. It may also be used for cancer treatment.
As John P. Cooke, MD, PhD, the medical director for the Center of RNA Therapeutics Program at the Houston Methodist Research Institute, said, “Most vaccines today are still viral vaccines – they are inactivated virus, so it’s potentially infectious and you have to have virus on hand. With mRNA, you’re just writing code which is going to tell the cell to make a viral protein – one part of a viral protein to stimulate an immune response. And, here’s the wonderful thing, you don’t even need the virus in hand, just its DNA code.”1
Corresponding author: Dragos Vesbianu, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA 02462; [email protected].
Financial dislosures: None.
1. Houston Methodist. Messenger RNA – the Therapy of the Future. Newswise. November 16, 2020. Accessed June 25, 2021. https://www.newswise.com/coronavirus/messenger-rna-the-therapy-of-the-future/
I have been in the business of medicine for more than 15 years and I will never forget the initial surge of the COVID-19 pandemic in Massachusetts.
As a hospitalist, I admitted patients infected with COVID-19, followed them on the floor, and, since I had some experience working in an intensive care unit (ICU), was assigned to cover a “COVID ICU.” This wing of the hospital used to be a fancy orthopedic floor that our institution was lucky enough to have. So began the most life-changing experience in my career as a physician.
In this role, we witness death more than any of us would care to discuss. It comes with the territory, and we never expected this to change once COVID hit. However, so many patients succumbed to this disease, especially during the first surge, which made it difficult to handle emotionally. Patients that fell ill initially stayed isolated at home, optimistic they would turn the corner only to enter the hospital a week later after their conditioned worsened. After requiring a couple of liters of supplemental oxygen in the emergency room, they eventually ended up on a high flow nasal cannula in just a matter of hours.
Patients slowly got sicker and felt more helpless as the days passed, leading us to prescribe drugs that eventually proved to have no benefit. We checked countless inflammatory markers, most of which we were not even sure what to do with. Many times, we hosted a family meeting via FaceTime, holding a patient’s hand in one hand and an iPad in the other to discuss goals of care. Too often, a dark cloud hung over these discussions, a realization that there was not much else we could do.
I have always felt that helping someone have a decent and peaceful death is important, especially when the prognosis is grim, and that patient is suffering. But the sheer number of times this happened during the initial surge of the pandemic was difficult to handle. It felt like I had more of those discussions in 3 months than I did during my entire career as a hospitalist.
We helped plenty of people get better, with some heading home in a week. They thanked us, painted rocks and the sidewalks in front of the hospital displaying messages of gratitude, and sent lunches. Others, though, left the hospital 2 months later with a tube in their stomach so they could receive some form of nutrition and another in their neck to help them breathe.
These struggles were by no means special to me; other hospitalists around the world faced similar situations at one point or another during the pandemic. Working overtime, coming home late, exhausted, undressing in the garage, trying to be there for my 3 kids who were full of energy after a whole day of Zoom and doing the usual kid stuff. My house used to have strict rules about screen time. No more.
The summer months provided a bit of a COVID break, with only 1 or 2 infected patients entering my care. We went to outdoor restaurants and tried to get our lives back to “normal.” As the weather turned cold, however, things went south again. This time no more hydroxychloroquine, a drug used to fight malaria but also treat other autoimmune diseases, as it was proven eventually over many studies that it is not helpful and was potentially harmful. We instead shifted our focus to remdesivir—an antiviral drug that displayed some benefits—tocilizumab, and dexamethasone, anti-inflammatory drugs with the latter providing some positive outcomes on mortality.
Patient survival rates improved slightly, likely due to a combination of factors. We were more experienced at fighting the disease, which led to things in the hospital not being as chaotic and more time available to spend with the patients. Personal protective equipment (PPE) and tests were more readily available, and the population getting hit by the disease changed slightly with fewer elderly people from nursing homes falling ill because of social distancing, other safety measures, or having already fought the disease. Our attention turned instead to more young people that had returned to work and their social lives.
The arrival of the vaccines brought considerable relief. I remember a few decades ago debating and sometimes fighting with friends and family over who was better: Iron Man or Spider-Man. Now I found myself having the same conversation about the Pfizer and Moderna COVID vaccines.
Summer 2021 holds significantly more promise. Most of the adult population is getting vaccinated, and I am very hopeful that we are approaching the end of this nightmare. In June, our office received word that we could remove our masks if we were fully vaccinated. It felt weird, but represented another sign that things are improving. I took my kids to the mall and removed my mask. It felt odd considering how that little blue thing became part of me during the pandemic. It also felt strange to not prescribe a single dose of remdesivir for an entire month.
It feels good—and normal—to care for the patients that we neglected for a year. It has been a needed boost to see patients return to their health care providers for their colonoscopy screenings, mammograms, and managing chronic problems like coronary artery disease, congestive heart failure, or receiving chemotherapy.
I learned plenty from this pandemic and hope I am not alone. I learned to be humble. We started with a drug that was harmful, moved on to a drug that is probably neutral and eventually were able to come up with a drug that seems to decrease mortality at least in some COVID patients. I learned it is fine to try new therapies based on the best data in the hope they result in positive clinical outcomes. However, it is critical that we all keep an eye on the rapidly evolving literature and adjust our behavior accordingly.
I also learned, or relearned, that if people are desperate enough, they will drink bleach to see if it works. Others are convinced that the purpose of vaccination is to inject a microchip allowing ourselves to be tracked by some higher power. I learned that we must take the first step to prepare for the next pandemic by having a decent reserve of PPE.
It is clear synthetic messenger RNA (mRNA) technology is here to stay, and I believe it has a huge potential to change many areas of medicine. mRNA vaccines proved to be much faster to develop and probably much easier to change as the pathogen, in this case coronavirus, changes.
The technology could be used against a variety of infectious diseases to make vaccines against malaria, tuberculosis, HIV, or hepatitis. It can also be very useful for faster vaccine development needed in future possible pandemics such as influenza, Ebola, or severe acute respiratory syndrome. It may also be used for cancer treatment.
As John P. Cooke, MD, PhD, the medical director for the Center of RNA Therapeutics Program at the Houston Methodist Research Institute, said, “Most vaccines today are still viral vaccines – they are inactivated virus, so it’s potentially infectious and you have to have virus on hand. With mRNA, you’re just writing code which is going to tell the cell to make a viral protein – one part of a viral protein to stimulate an immune response. And, here’s the wonderful thing, you don’t even need the virus in hand, just its DNA code.”1
Corresponding author: Dragos Vesbianu, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA 02462; [email protected].
Financial dislosures: None.
I have been in the business of medicine for more than 15 years and I will never forget the initial surge of the COVID-19 pandemic in Massachusetts.
As a hospitalist, I admitted patients infected with COVID-19, followed them on the floor, and, since I had some experience working in an intensive care unit (ICU), was assigned to cover a “COVID ICU.” This wing of the hospital used to be a fancy orthopedic floor that our institution was lucky enough to have. So began the most life-changing experience in my career as a physician.
In this role, we witness death more than any of us would care to discuss. It comes with the territory, and we never expected this to change once COVID hit. However, so many patients succumbed to this disease, especially during the first surge, which made it difficult to handle emotionally. Patients that fell ill initially stayed isolated at home, optimistic they would turn the corner only to enter the hospital a week later after their conditioned worsened. After requiring a couple of liters of supplemental oxygen in the emergency room, they eventually ended up on a high flow nasal cannula in just a matter of hours.
Patients slowly got sicker and felt more helpless as the days passed, leading us to prescribe drugs that eventually proved to have no benefit. We checked countless inflammatory markers, most of which we were not even sure what to do with. Many times, we hosted a family meeting via FaceTime, holding a patient’s hand in one hand and an iPad in the other to discuss goals of care. Too often, a dark cloud hung over these discussions, a realization that there was not much else we could do.
I have always felt that helping someone have a decent and peaceful death is important, especially when the prognosis is grim, and that patient is suffering. But the sheer number of times this happened during the initial surge of the pandemic was difficult to handle. It felt like I had more of those discussions in 3 months than I did during my entire career as a hospitalist.
We helped plenty of people get better, with some heading home in a week. They thanked us, painted rocks and the sidewalks in front of the hospital displaying messages of gratitude, and sent lunches. Others, though, left the hospital 2 months later with a tube in their stomach so they could receive some form of nutrition and another in their neck to help them breathe.
These struggles were by no means special to me; other hospitalists around the world faced similar situations at one point or another during the pandemic. Working overtime, coming home late, exhausted, undressing in the garage, trying to be there for my 3 kids who were full of energy after a whole day of Zoom and doing the usual kid stuff. My house used to have strict rules about screen time. No more.
The summer months provided a bit of a COVID break, with only 1 or 2 infected patients entering my care. We went to outdoor restaurants and tried to get our lives back to “normal.” As the weather turned cold, however, things went south again. This time no more hydroxychloroquine, a drug used to fight malaria but also treat other autoimmune diseases, as it was proven eventually over many studies that it is not helpful and was potentially harmful. We instead shifted our focus to remdesivir—an antiviral drug that displayed some benefits—tocilizumab, and dexamethasone, anti-inflammatory drugs with the latter providing some positive outcomes on mortality.
Patient survival rates improved slightly, likely due to a combination of factors. We were more experienced at fighting the disease, which led to things in the hospital not being as chaotic and more time available to spend with the patients. Personal protective equipment (PPE) and tests were more readily available, and the population getting hit by the disease changed slightly with fewer elderly people from nursing homes falling ill because of social distancing, other safety measures, or having already fought the disease. Our attention turned instead to more young people that had returned to work and their social lives.
The arrival of the vaccines brought considerable relief. I remember a few decades ago debating and sometimes fighting with friends and family over who was better: Iron Man or Spider-Man. Now I found myself having the same conversation about the Pfizer and Moderna COVID vaccines.
Summer 2021 holds significantly more promise. Most of the adult population is getting vaccinated, and I am very hopeful that we are approaching the end of this nightmare. In June, our office received word that we could remove our masks if we were fully vaccinated. It felt weird, but represented another sign that things are improving. I took my kids to the mall and removed my mask. It felt odd considering how that little blue thing became part of me during the pandemic. It also felt strange to not prescribe a single dose of remdesivir for an entire month.
It feels good—and normal—to care for the patients that we neglected for a year. It has been a needed boost to see patients return to their health care providers for their colonoscopy screenings, mammograms, and managing chronic problems like coronary artery disease, congestive heart failure, or receiving chemotherapy.
I learned plenty from this pandemic and hope I am not alone. I learned to be humble. We started with a drug that was harmful, moved on to a drug that is probably neutral and eventually were able to come up with a drug that seems to decrease mortality at least in some COVID patients. I learned it is fine to try new therapies based on the best data in the hope they result in positive clinical outcomes. However, it is critical that we all keep an eye on the rapidly evolving literature and adjust our behavior accordingly.
I also learned, or relearned, that if people are desperate enough, they will drink bleach to see if it works. Others are convinced that the purpose of vaccination is to inject a microchip allowing ourselves to be tracked by some higher power. I learned that we must take the first step to prepare for the next pandemic by having a decent reserve of PPE.
It is clear synthetic messenger RNA (mRNA) technology is here to stay, and I believe it has a huge potential to change many areas of medicine. mRNA vaccines proved to be much faster to develop and probably much easier to change as the pathogen, in this case coronavirus, changes.
The technology could be used against a variety of infectious diseases to make vaccines against malaria, tuberculosis, HIV, or hepatitis. It can also be very useful for faster vaccine development needed in future possible pandemics such as influenza, Ebola, or severe acute respiratory syndrome. It may also be used for cancer treatment.
As John P. Cooke, MD, PhD, the medical director for the Center of RNA Therapeutics Program at the Houston Methodist Research Institute, said, “Most vaccines today are still viral vaccines – they are inactivated virus, so it’s potentially infectious and you have to have virus on hand. With mRNA, you’re just writing code which is going to tell the cell to make a viral protein – one part of a viral protein to stimulate an immune response. And, here’s the wonderful thing, you don’t even need the virus in hand, just its DNA code.”1
Corresponding author: Dragos Vesbianu, MD, Attending Hospitalist, Newton-Wellesley Hospital, 2014 Washington St, Newton, MA 02462; [email protected].
Financial dislosures: None.
1. Houston Methodist. Messenger RNA – the Therapy of the Future. Newswise. November 16, 2020. Accessed June 25, 2021. https://www.newswise.com/coronavirus/messenger-rna-the-therapy-of-the-future/
1. Houston Methodist. Messenger RNA – the Therapy of the Future. Newswise. November 16, 2020. Accessed June 25, 2021. https://www.newswise.com/coronavirus/messenger-rna-the-therapy-of-the-future/
Impact of Diagnostic Testing on Pediatric Patients With Pharyngitis: Evidence From a Large Health Plan
From the Department of Pharmaceutical and Health Economics, University of Southern California, Los Angeles, CA, (Drs. Sangha and McCombs), Department of Pediatrics, Keck School of Medicine, and Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, (Dr. Steinberg), and Leonard Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA (Dr. McCombs).
Objective: The recommended treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS) are antibiotics using the “test and treat” strategy to detect and treat GAS for pediatric pharyngitis. This study used paid claims data to document the extent to which real-world treatment patterns are consistent with these recommendations. We document the factors correlated with testing and treatment, then examine the effects of receiving a GAS test and being treated with an antibiotic impact the likelihood of a revisit for an acute respiratory tract infection within 28 days.
Methods: This retrospective cohort study used Optum Insight Clinformatics data for medical and pharmacy claims from 2011-2013 to identify episodes of care for children and adolescents with pharyngitis around their index visit (± 6 months). The sample population included children and adolescents under 18 years of age with a diagnosis of pharyngitis. Multivariable logistic regression analyses were used to document factors associated with receipt of GAS test and antibiotic treatment. Next, we used logistic regression models to estimate the impact of test and treat recommendation on revisit risk.
Results: There were 24 685 treatment episodes for children and adolescents diagnosed with pharyngitis. Nearly 47% of these episodes included a GAS test and 48% of tested patients were prescribed an antibiotic prescription. Failing to perform a GAS test increased the risk of a revisit within 28 days by 44%. The use of antibiotics by tested and untested patients had no impact on revisit risk.
Conclusion: While the judicious use of antibiotics is important in managing pharyngitis infections and managing complications, the use of rapid diagnostic tools was found to be the determining factor in reducing revisits for pediatric patients with pharyngitis.
Keywords: pediatrics; pharyngitis; respiratory infections; acute infections; diagnostic tests; group A Streptococcus; antibiotics; revisits.
Acute pharyngitis is a common acute respiratory tract infection (ARTI) in children. Group A β-hemolytic streptococci (GABHS) is the most common bacterial etiology for pediatric pharyngitis, accounting for 15% to 30% of cases.1
Beyond clinical assessment, laboratory diagnostic testing generally plays a limited role in guiding appropriate antibiotic prescribing for patients with an ARTI.2,3 Most diagnostic tests require 2 or 3 days to result, incur additional costs, and may delay treatment.4 While these tests do not provide clear and timely guidance on which specific antibiotic is appropriate for ARTI patients, this is not the case for patients with pharyngitis.5,6,7 A rapid diagnostic test exists to identify pharyngitis patients with GABHS which accounts for 1 in 4 children with acute sore throat.1,4,6 Both the American Academy of Pediatrics and the Infectious Diseases Society of America recommend antibiotic treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS).8,9 This “test and treat” protocol has been consistently included in the Healthcare Effectiveness Data and Information Set (HEDIS) standards over time for pediatric pharyngitis patients aged 3 to 18 years before dispensing an antibiotic.10
Sinusitis, pneumonia, and acute otitis media are considered ARTIs where antibiotic treatment is justified. Therefore, pharyngitis of unclear etiology seen with these comorbid infections may not always undergo GAS testing but move directly to the patient being prescribed antibiotics. This analysis enumerates ARTI-related comorbidities present together with the initial coded pharyngitis diagnosis to evaluate their impact on the provider’s decision to test and treat, and on revisit risk.
Antibiotic treatment for GAS patients is likely to eradicate the acute GABHS infection within 10 days. Penicillin and amoxicillin are commonly recommended because of their narrow spectrum of activity, few adverse effects, established efficacy, and modest cost. Alternative antibiotics for patients with penicillin allergy, or with polymicrobial infection seen on culture results, include a first-generation cephalosporin, clindamycin, clarithromycin (Biaxin), or azithromycin (Zithromax).1,8,11 However, while compliance with these HEDIS guidelines has been evaluated, the outcome effects of following the HEDIS “test and treat” recommendations for children with pharyngitis have not been adequately evaluated.
These outcome evaluations have increasing importance as the latest HEDIS survey has shown testing rates in commercial Preferred Provider Organizations (PPO) falling from 86.4% in 2018 to 75.9% in 2019, the lowest rate of testing since 2009, with similar reductions under 80% for Health Maintenance Organizations (HMO).10 While health plans may execute cost-benefit analyses and algorithms to forge best practices for GAS testing in children and adolescents presenting with symptoms of pharyngitis, it is important to regard the wasteful resource utilization and additional cost of revisits that may offset any gains accrued by more focused GAS testing outside the existing clinical guidelines and HEDIS measures. This may be of particular importance in documenting infection and sparing antibiotic therapy in toddlers and younger.
The objective of this study was to investigate the correlation between testing and antibiotic use on the likelihood of a revisit for an acute respiratory tract infection within 28 days. To achieve this objective, this investigation consists of 3 sequential analyses. First, we document the factors associated with the decision to test the patient for a GABHS infection using the GAS test. Next, we document the factors associated with the decision to use an antibiotic to treat the patient as a function of having tested the patient. Finally, we investigate the impact of the testing and treatment decisions on the likelihood of a revisit within 28 days.
Methods
Study design
This was a retrospective cohort study of episodes of treatment for pediatric patients with pharyngitis. Episodes were identified using data derived from the Optum Insight Clinformatics claims database provided to the University of Southern California to facilitate the training of graduate students. These data cover commercially insured patients with both medical and pharmacy benefits. Data were retrieved from the 3-year period spanning 2011-2013. An episode of care was identified based on date of the first (index) outpatient visit for a pharyngitis diagnosis (International Classification of Diseases, Ninth Revision [ICD-9]: 462, 463, 034.0). Outpatient visits were defined by visit setting: ambulatory clinics, physician offices, emergency rooms, and urgent care facilities. Each pharyngitis treatment episode was then screened for at least a 6-month enrollment in a health insurance plan prior and subsequent to the index visit using Optum enrollment data. Finally, eligible treatment episodes were restricted to children and adolescents under 18 years of age, who had an index outpatient visit for a primary diagnosis of acute pharyngitis.
A diagnostic profile was created for each episode using the diagnoses recorded for the index visit. Up to 3 diagnoses may be recorded for any outpatient visit and the first recorded diagnosis was assumed to be the primary diagnosis for that episode. Any secondary diagnoses recorded on the index visit were used to define comorbidities present at the index visit. ARTI-related comorbidities included: acute otitis media (AOM), bronchitis, sinusitis, pneumonia, and upper respiratory infection (URI). Other comorbid medical diagnoses were documented using diagnostic data from the pre-index period. Dichotomous variables for the following categories were created: mental disorders, nervous system disorders, respiratory symptoms, fever, injury and poisoning, other, or no diseases.
Prior visits for other respiratory infections in the previous 90 days were also identified for patients based on their index visit for pharyngitis. Similarly, any subsequent visits, within 28 days of the index visit, were also recorded to measure the health outcome for analysis. Practice settings include physician offices and federally qualified health centers, state and local health clinics, outpatient hospitals facilities, emergency departments, and other outpatient settings such as walk-in retail health clinic or ambulatory centers. Providers include primary care physicians (family practice, pediatricians, internal medicine), specialty care physicians (emergency medicine, preventive medicine), nonphysician providers (nurse practitioners, physician assistants) and other providers (urgent care, acute outpatient care, ambulatory care centers). Seasons of the year were determined based on the index date of the episode to account for possible seasonality in pharyngitis treatment. Lastly, a previous visits variable was created to identify whether the child had nonpharyngitis ARTI visits in the 3 months prior to the index visit.
Demographic variables were created based on enrollment and the socioeconomic data available in the Optum socioeconomic status file. These variables include patient age, race, sex, household income, geographic location, practice setting type, provider specialty, and type of insurance. An estimate of patient household income was based on algorithms using census block groups. Income categories were informed by the federal guidelines for a family of 4. A low-income family was defined as earning less than $50 000; a middle-income family earned between $50 000 and $75 000, and a high-income family earned $75 000 and above.12 Patient insurance type was categorized as HMO, Exclusive Provider Organization (EPO), Point of Service (POS), and PPO. Race was identified as White, Black, Hispanic, and Asian. Patient location was defined according to national census regions.
Outcomes
GAS test
The HEDIS measures for pharyngitis recommend using the GAS test to identify the bacterial etiology of the pharyngitis infection. Patients who received the test were identified based on Current Procedural Terminology (CPT) codes 87070-71, 87081, 87430, 87650-52, and 87880.10
Antibiotic treatment
The pharmacy administrative claims dataset was used to identify study patients who filled a prescription for an antibiotic during their pharyngitis treatment episode. Optum pharmacy data identify the medications received, specifies the date of prescription filling, National Drug Codes, and American Hospital Formulary Service (AHFS) Classification System codes for each medication. We used the AHFS Pharmacologic-Therapeutic classification of antibiotics to create dichotomous variables documenting the antibacterial used by each patient.13 These are categorized under antibacterial including penicillins, cephalosporins (first, second, third, fourth generation cephalosporins), macrolides (first generation and others), tetracyclines, sulfonamides, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), cephamycin, carbapenems, and β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, cephalexin, cefuroxime, cefdinir).
Revisits to physician or other provider
Revisits within 28 days were used as the measure of patient outcomes related to testing and filling of an antibiotic prescription for acute pharyngitis. Revisits may also be due to a patient returning for a follow-up, alternative treatment, worsening pharyngitis, or for another ARTI. An ARTI-related revisit also increases total resources used to treat pediatric pharyngitis patients.
Statistical analysis
Logistic regression was used for all 3 analyses conducted in this study. First, we determined the patient and treating physician characteristics that impact the decision to use GAS testing for pharyngitis. Second, we identified those factors that impact the decision to use antibiotic prescriptions among children who were diagnosed with pharyngitis adding in the dichotomous variable indicating if the patient had received a GAS test. Third, we used a logit regression analysis to document if receiving a GAS test and/or an antibiotic impacted the likelihood of a revisit by comparing revisit risk. To estimate the effect of testing and/or antibiotic use, we divided patients into 4 groups based on whether the patient received a GAS test and/or an antibiotic prescription. This specification of the analysis of revisits as an outcome focuses on adherence to HEDIS “test and treat” guidelines10:
- Patients who were not tested yet filled an antibiotic prescription. This decision was likely based on the clinician’s judgment of the patient’s signs and symptoms, and confirmational testing not performed.
- Patients who were not tested and did not fill an antibiotic prescription. Apparently, in the clinician’s judgment the patient’s signs and symptoms were such that the infection did not warrant treatment and the clinical presentation did not necessitate the GAS test to confirm the recorded diagnosis of pharyngitis.
- Patients who were tested and received antibiotic prescription, likely because the test was positive for GABHS.
- Patients who were tested and did not receive antibiotic prescription.
We tested for statistically significant differences in baseline characteristics across these 4 patient groups using t tests for continuous variables and χ2 tests for categorical variables. Odds ratios (OR) and CI were computed for the influential variables included the regression analyses.
We conducted a sensitivity analysis using a model specification which included the dichotomous variables for testing and for treatment, and the interaction term between these variables to assess if treatment effects varied in tested and untested patients. We also estimated this model of revisit risk using revisits within 7 days as the outcome variable.
All analyses were completed using STATA/IC 13 (StataCorp, College Station, TX).
Results
There were 24 685 treatment episodes for children diagnosed with pharyngitis. Nearly 47% of these episodes included GAS testing and 47% of the tested patients filled an antibiotic prescription. Similarly, 53% of patients were not tested and 49% of untested patients filled an antibiotic prescription. As a result, the 4 groups identified for analysis were evenly distributed: untested and no prescription (26.9%), untested and prescription (26.3%), tested and prescription (21.9%), and tested and no prescription (24.9%) (Figure).
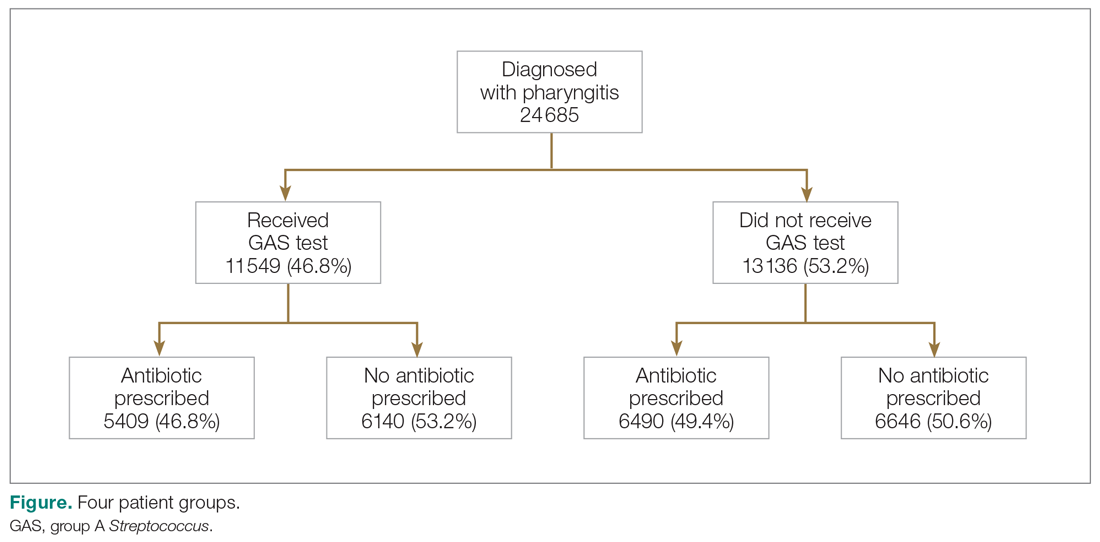
Table 1 presents the descriptive statistics for these 4 patient groups. Note first that the rate of revisits within 28 days is under 5% across all groups. Second, the 2 tested groups have a lower revisit rate than the untested groups: the tested and treated have a revisit rate of 3.3%, and the tested and untreated have a revisit rate of 2.4%, while both the untested groups have a revisit rate of nearly 5%. These small absolute differences in revisit rates across groups were statistically significant.
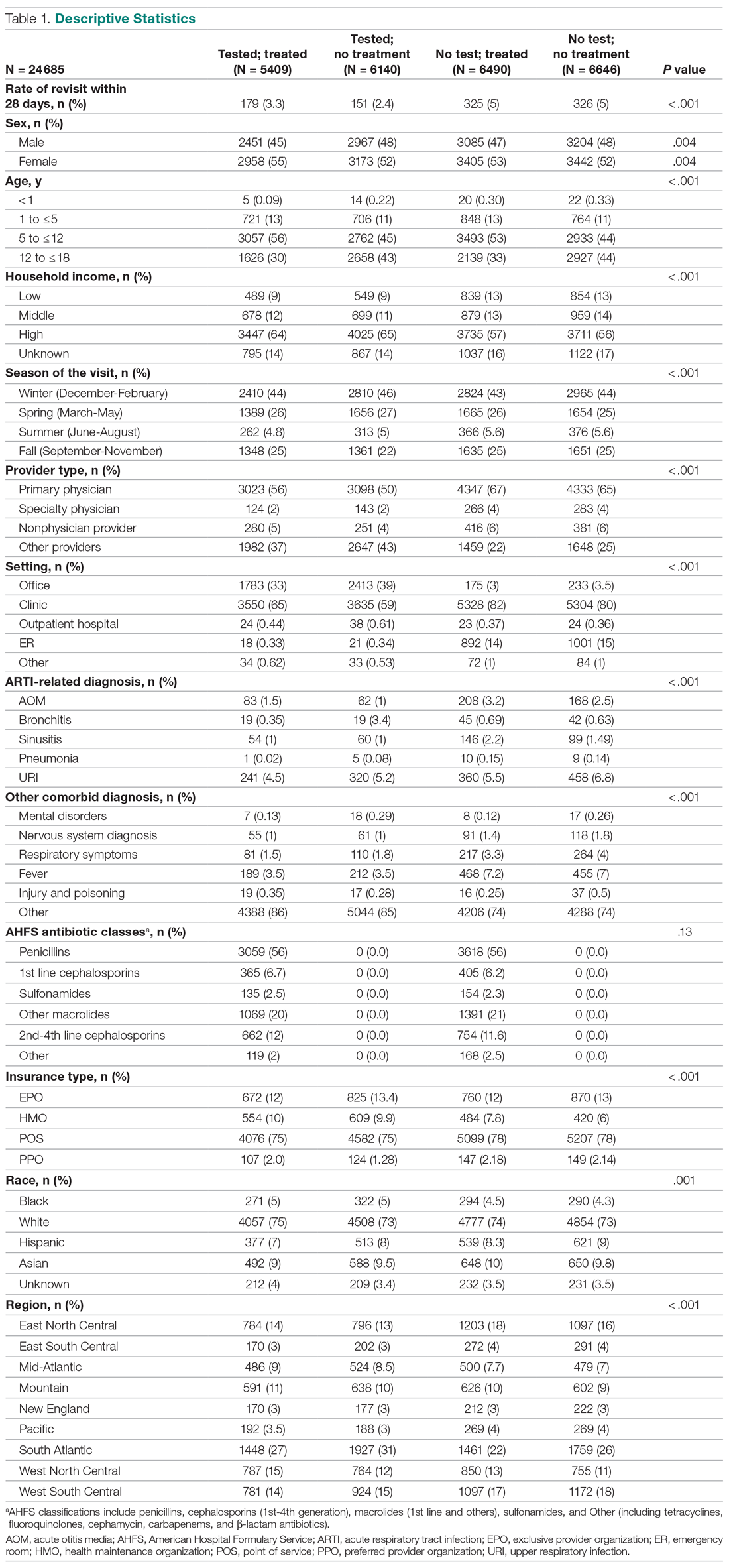
Factors associated with receiving GAS test
Several factors were found to impact the decision to test (Table 2). Only 9.7% of children were reported to have any ARTI coinfection. As expected, these comorbidities resulted in a significantly lower likelihood of receiving the GAS test: AOM, bronchitis, sinusitis, pneumonia, and URI as comorbid infections had a 48%, 41%, 37%, 63%, and 13% lower likelihood of receiving the GAS test, respectively, than those with no comorbidities. Similarly, children with fever and respiratory symptoms were 35% and 45% less likely to receiving the GAS test, respectively. This is consistent with our expectation that comorbid ARTI infections will lead many providers to forgo testing.
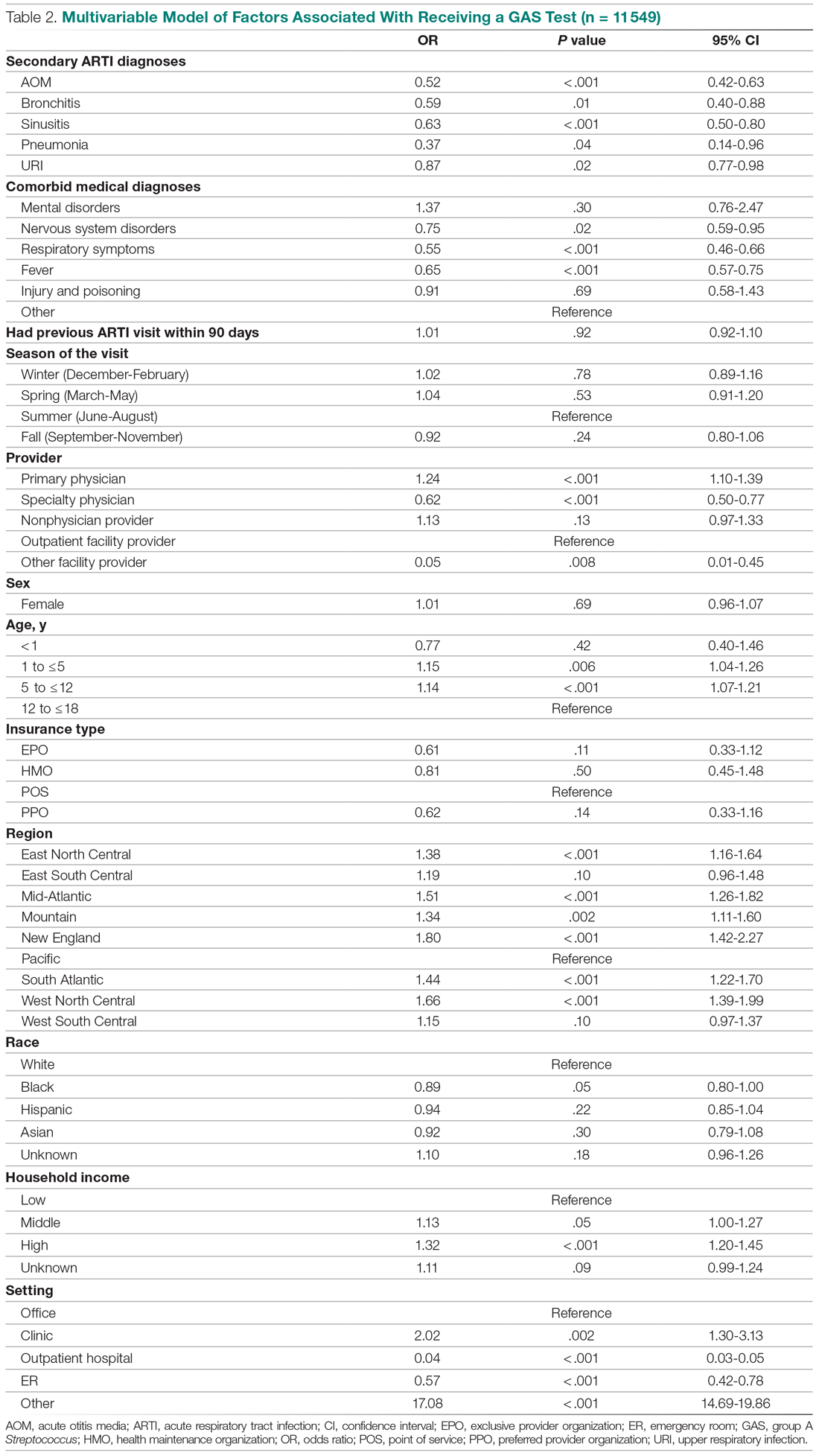
Provider type and patient age also plays a role in receipt of the GAS test. Relative to outpatient facility providers, primary care physicians were 24% more likely and specialty physicians were 38% less likely of employing the GAS test. The child’s age played a significant role in receipt of the GAS test. Children aged 1 to 5 years and 5 to 12 years were 15% and 14% more likely to receive the test compared to children older than 12 years.
Pharyngitis patients have disproportionately higher odds of receiving a GAS test in most regions of the country compared to the Pacific region. For instance, children in the Mid-Atlantic region have 51% higher odds of receiving a GAS test while children in New England have 80% higher odds of receiving the same test.
Black children have 11% lower odds of receiving the GAS test compared to White children. Both middle-income and high-income children have 12% and 32% higher odds of receiving the test compared to low-income children. Compared to office-based visits, children visiting a clinic were twice as likely to receive a GAS test while those seen in the emergency room have 43% lower odds of receiving a GAS test. Hospital outpatient departments, which account for less than 1% of all visits, rarely used a GAS test which could be a statistical artifact due to small sample size. Lastly, insurance and season of the year had no significant impact of receipt of a GAS test.
Factors associated with receiving antibiotic prescription
Surprisingly, receiving the GAS test has a small but insignificant impact on the likelihood that the patient will receive an antibiotic prescription (Table 3) (Adjusted OR = 1.055, P = .07). After controlling for receipt of a GAS test, children with AOM and sinusitis comorbidities have an increased likelihood of being prescribed an antibiotic. Children with URI have a lower likelihood of being prescribed an antibiotic. Additionally, relative to primary care physicians, children visiting nonphysician providers for pharyngitis were more likely to be prescribed an antibiotic.
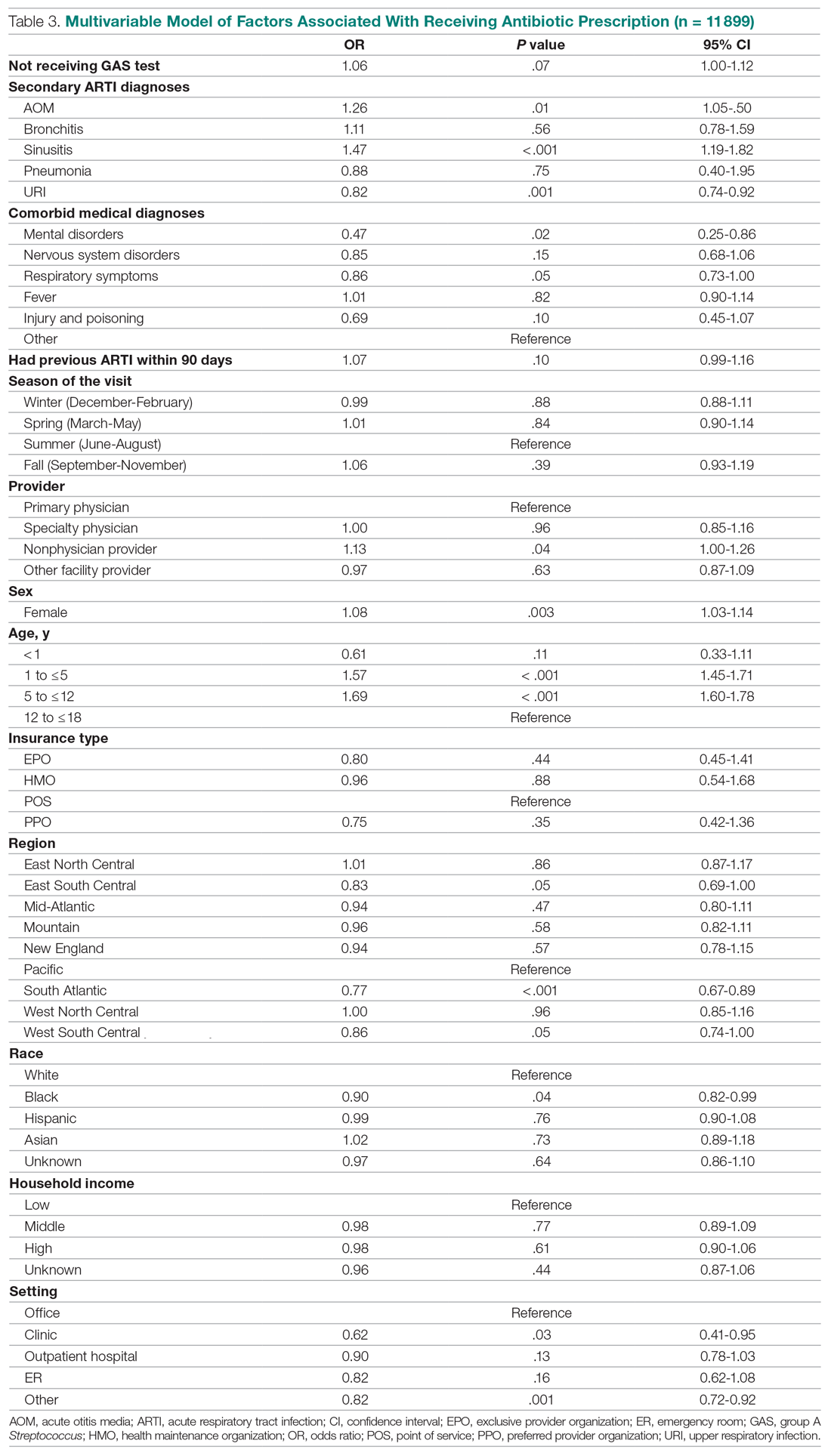
Children under 12 years of age were more likely to use an antibiotic compared to children 12 years and older. Geographically, there is some evidence of regional variation in antibiotic use as well. Children in the south Atlantic, west-south central, and southeast central regions had a significantly lower odds of being prescribed an antibiotic respectively than pharyngitis patients in the Pacific region. Black children had a 10% lower likelihood of being prescribed an antibiotic compared to White children, possibly related to their lower rate of GAS testing. Compared to office-based visits, children visiting a clinic were less likely to use an antibiotic. Household income, insurance type, and season had no significant impact on revisit risk.
Effects of GAS test and antibiotic prescriptions on likelihood of revisits
The multivariate analysis of the risk of a revisit within 28 days is presented in Table 4. Children with pharyngitis who tested and did not receive an antibiotic serve as the reference comparison group for this analysis to illustrate the impact of using the GAS test and treatment with an antibiotic. The results in Table 4 are quite clear: patients who receive the GAS test were significantly less likely to have a revisit within 28 days. Moreover, within the group of patients who were tested, those not receiving an antibiotic, presumedly because their GAS test was negative, experienced the lowest risk of a revisit. This result is consistent with the data in Table 1. Moreover, using an antibiotic had no impact on the likelihood of a revisit in patients not receiving the GAS test. This result is also consistent with Table 1.
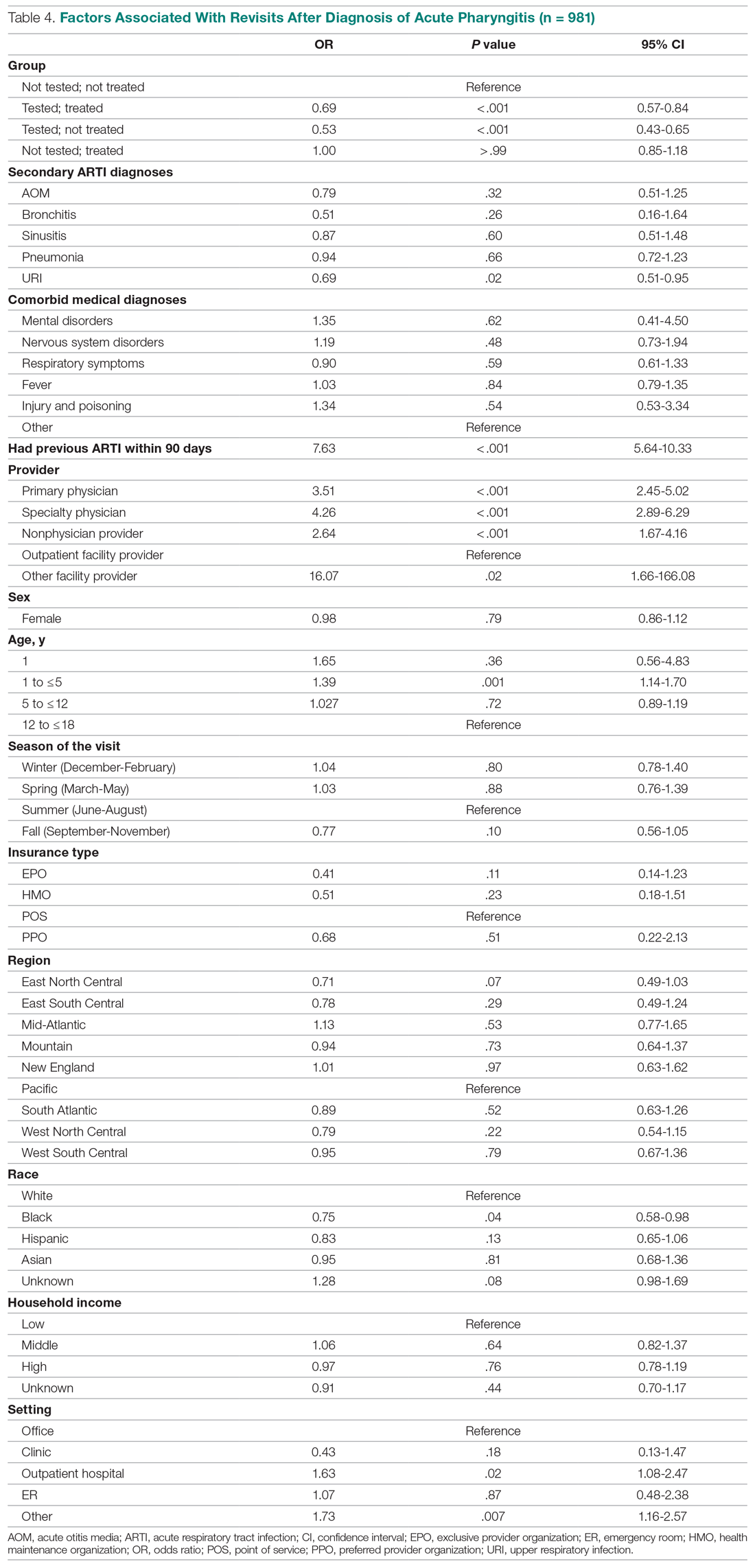
Other results from the analysis of revisit risk may be of interest to clinicians. Pharyngitis patients with a prior episode of treatment within 90 days for an acute respiratory tract infection were more than 7 times more likely to experience a revisit within 28 days of the pharyngitis diagnosis than patients without a history of recent ARTI infections. Age is also a risk factor in likelihood of initiating a revisit. Children under 1 year and children aged 1 to 5 years were more likely to have a revisit than children aged more than 12 years. Compared to White children, Black children were 25% (P = .04) less likely to have a revisit. The care setting also has a significant impact on revisit risk. Children visiting outpatient hospital and other care settings had a significantly higher revisit risk than those visiting a physician’s office. Lastly, household income, geographic region, season, medical comorbidities, gender, and insurance type have no significant impact on revisit risk.
Sensitivity analysis
The results from the analysis of 7-day and 28-day revisit risk are summarized in Table 5. These results indicate that patients who were tested had a more significant decrease in revisit risk at 7 days (72%) than was evident at 28 days (47% reduction). Receiving an antibiotic, with or without the test, had no impact on revisit risk.
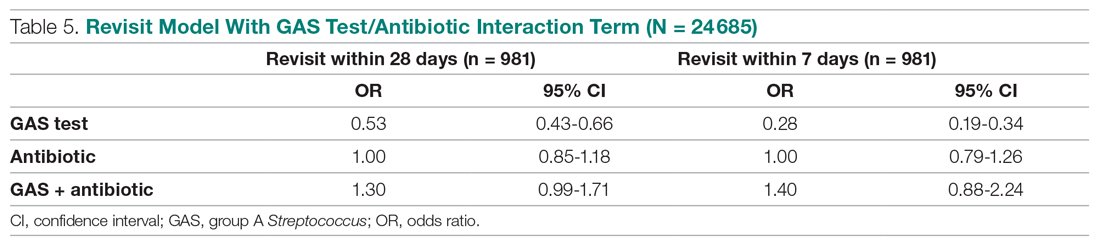
Discussion
Published data on revisits for pharyngitis are lacking with the concentration of prior research focused more on systemic complications of undertreated GABHS disease or on identifying carrier status. Our study results suggest that GAS testing is the most important factor in reducing revisit risk. Being prescribed an antibiotic, on its own, does not have a significant impact on the risk of a revisit. However, once the GAS test is used, the decision not to use an antibiotic was correlated with the lowest revisit rate, likely because the source of the pharyngitis infection was viral and more likely to resolve without a revisit. Prior studies have reported variable rates of testing among children with pharyngitis prescribed an antibiotic, ranging from 23% to 91%,14,15 with testing important toward more appropriate antibiotic use.16 More recently, among more than 67 000 patients aged 3 to 21 years presenting with sore throat and receiving a GAS test, 32.6% were positive.17
Our analysis found that more than 46% of pediatric pharyngitis patients were given the rapid GAS test. While this testing rate is substantially lower than HEDIS recommendations and lower than testing rates achieved by several health maintenance organizations,10 it is similar to the 53% of children receiving such testing in a recent National Ambulatory Medical Care Survey.18 Furthermore, we found that when antibiotics are prescribed following a GAS test, the revisit risk is not significantly reduced, possibly because antibiotics lower revisit risk when informed by diagnostic testing tools that determine the infectious organism. This is supported by a similar population analysis in which we observed reduced revisit rates in children with AOM managed with antibiotics within 3 days of index diagnosis.19
Several other factors also affect the likelihood of a child receiving the GAS test. Children aged 1 to 12 years were significantly more likely to receive the GAS test than children over the age of 12. This included children in the 1 to 5 years old bracket who had a 15% higher likelihood of undergoing a GAS test, despite children less than 3 years of age as not recommended targets for GAS testing.20 As expected, children with reported ARTI-associated comorbidities were also less likely to receive a GAS test. Additionally, specialty care physicians were less inclined to implement the GAS test, possibly because of diagnostic confidence without testing or referral after GAS was ruled out. Black and low-income children had statistically lower odds of receiving the test, even after controlling for other factors, and yet were less likely to consume a revisit. As the overall data suggested more revisits in those not tested, further study is needed to examine if race or income discrepancies are equity based. Finally, children in the Pacific region, compared to the rest of the nation, were the least likely to receive a GAS test and yet there were no significant differences in revisit rates by region. Regional differences in antibiotic use were also observed in our study, as has been seen by others.21
After statistically controlling for having received the diagnostic GAS test and filled a prescription for an antibiotic, there are multitude of factors that independently affect the revisit risk, the most important of which if which was a history of an ARTI infection in the prior 90 days. While prior visit history had no impact on the likelihood of being tested or filling an antibiotic, patients with prior visits were more than 7 times more likely to consume a revisit. This was not reflected in nor related to comorbid ARTIs as these patients did not have statistically higher revisits than those with pharyngitis as the sole-coded diagnosis. Moreover, speculation for bacterial etiology of primary or superinfection based on a recent history of ARTI accounting for revisits seems unlikely as it did not yield greater antibiotic use in that group. Further analysis is required to determine the clinical and behavioral factors that promote for prior ARTI history as a major factor in revisit risk after an index visit for pharyngitis.
Children aged between 1 and 5 years, though 15% more likely to be tested than those aged 12 through 17 years, were also 39% more likely to initiate a revisit compared to older children when statistically controlling for other covariates. This perhaps suggests longer illness, wrong diagnosis, delay in appropriate treatment, or more caution by parents and providers in this age group. Justification for testing children less than 3 years of age who are outside of the HEDIS suggested age group, when clinical judgement does not point to another infection source, can result in positivity rates between 22% and 30% as previously observed.22,23 Patients visiting nonphysician providers and outpatient facility providers were less likely to have a revisit than those visiting primary and specialty care physicians, though slightly higher propensity for antibiotic prescriptions was seen for nonphysician providers. Pediatricians have been noted to be less likely to prescribe antibiotics without GAS testing than nonpediatric providers, and more guidelines-compliant in prescribing.24
Recommendations to not test children under 3 years of age are based on the lack of acute rheumatic fever and other complications in this age group together with more frequent viral syndromes. Selectivity in applying clinical criteria to testing can be attempted to separate bacterial from viral illness. Postnasal drainage/rhinorrhea, hoarse voice, and cough have been used successfully to identify those with viral illness and less need for testing, with greater certainty of low risk for GABHS in those over 11 years of age without tonsillar exudates, cervical adenopathy, or fever.17 However, the marginal benefits of those who have all 3 features of viral illness versus none in identifying GAS positivity was 23.3% vs 37.6% - helpful, but certainly not diminishing the need for testing. These constitutional findings of viral URI also do not exclude the GAS carrier state that features these symptoms.25 Others have reinforced the doubt of pharyngeal exudates as the premier diagnostic finding for test-positive GAS.26
This study had several limitations. The Optum claims dataset only contains ICD-9 codes for diagnoses. It does not include data on infection severity and clinical findings related to symptoms, thus empiric treatment warranted based in clinical severity is not assessed. Antibiotics are commonly available as generics and very inexpensive. Patients may fill and pay for these prescriptions directly, in which case, a claim for payment may not be filed with Optum. This could result in an undercount of treated patients in our study.
There is no corresponding problem of missing medical claims for GAS testing which were obtained from the CPT codes within the Optum claims data set. However, we elected not to verify the test results due to these data being missing for 75% of the study population. Nevertheless, this study’s focus was less about justifying antibiotic treatment, but dealt with the outcomes generated by testing and treatment. Toward that end, we used CPT codes to identify a revisit, and while those can at times be affected by financial reimbursement incentives, differences related to revisits in the 4 patient groups should not be subject to bias.
Conclusion
This study used data from real world practices to document the patterns of GAS testing and antibiotic use in pediatric pharyngitis patients. Revisit rates were under 5% for all patient groups and the use of rapid diagnostic tools were found to be the determining factor in further reducing the risk of revisits. This supports the need for compliance with the HEDIS quality metric for pharyngitis to the recommended levels of rapid testing which have been falling in recent years. Use of more accurate antigen and newer molecular detection testing methods may help further delineate important factors in determining pediatric pharyngitis treatment and need for revisits.27
Corresponding author: Jeffrey McCombs, MD, University of Southern California School of Pharmacy, Department of Pharmaceutical and Health Economics, Leonard D. Schaeffer Center for Health Policy & Economics, 635 Downey Way, Verna & Peter Dauterive Hall 310, Los Angeles, CA 90089-3333; [email protected].
Financial disclosures: None.
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
2. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch of Intern Med. 2008;168(18):2000-2008. doi: 10.1001/archinte.168.18.2000
3. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62(6):1407-1412. doi: 10.1093/jac/dkn376
4. Neuner JM, Hamel MB, Phillips RS, et al. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139(2):113-122. doi:10.7326/0003-4819-139-2-200307150-00011
5. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551. doi: 10.1161/CIRCULATIONAHA.109.191959
6. Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111(6):e666-e670. doi: 10.1542/peds.111.6.e666
7. Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for Streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403
8. Shulman ST, Bisno AL, Clegg H, et al. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629
9. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800–806. doi: 10.1001/archpedi.155.7.800
10. Appropriate Testing for Children with Pharyngitis. HEDIS Measures and Technical Resources. National Committee for Quality Assurance. Accessed February 12, 2021. https://www.ncqa.org/hedis/measures/appropriate-testing-for-children-with-pharyngitis/
11. Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315-2322. doi: 10.1001/jama.294.18.2315
12. Crimmel BL. Health Insurance Coverage and Income Levels for the US Noninstitutionalized Population Under Age 65, 2001. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality. 2004. https://meps.ahrq.gov/data_files/publications/st40/stat40.pd
13. AHFS/ASHP. American Hospital Formulary Service Drug Information. 2012. AHFS drug information. 00--. Accessed January 4, 2021.
14. Mainous AG 3rd, Zoorob, RJ, Kohrs FP, Hagen MD. Streptococcal diagnostic testing and antibiotics prescribed for pediatric tonsillopharyngitis. Pediatr Infect Dis J. 1996;15(9):806-810. doi: 10.1097/00006454-199609000-00014
15. Benin AL, Vitkauskas G, Thornquist E, et al. Improving diagnostic testing and reducing overuse of antibiotics for children with pharyngitis: a useful role for the electronic medical record. Pediatr Infect Dis J. 2003;22(12):1043-1047. doi: 10.1097/01.inf.0000100577.76542.af
16. Luo R, Sickler J, Vahidnia F, et al. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011-2015. BMC Infect Dis. 2019;19(1):193-201. doi: 10.1186/s12879-019-3835-4
17. Shapiro DJ, Barak-Corren Y, Neuman MI, et al. Identifying Patients at Lowest Risk for Streptococcal Pharyngitis: A National Validation Study. J Pediatr. 2020;220:132-138.e2. doi: 10.1016/j.jpeds.2020.01.030. Epub 2020 Feb 14
18. Shapiro DJ, King LM, Fleming-Dutra KE, et al. Association between use of diagnostic tests and antibiotic prescribing for pharyngitis in the United States. Infect Control Hosp Epidemiol. 2020;41(4):479-481. doi: 10.1017/ice.2020.29
19. Sangha K, Steinberg I, McCombs JS. The impact of antibiotic treatment time and class of antibiotic for acute otitis media infections on the risk of revisits. Abs PDG4. Value in Health. 2019; 22:S163.
20. Ahluwalia T, Jain S, Norton L, Meade J, et al. Reducing Streptococcal Testing in Patients < 3 Years Old in an Emergency Department. Pediatrics. 2019;144(4):e20190174. doi: 10.1542/peds.2019-0174
21. McKay R, Mah A, Law MR, et al. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother. 2016;60(7):4106-4118. doi: 10.1128/AAC.00209-16
22. Woods WA, Carter CT, Schlager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15(5):338-340. doi: 10.1097/00006565-199910000-00011
23. Mendes N, Miguéis C, Lindo J, et al. Retrospective study of group A Streptococcus oropharyngeal infection diagnosis using a rapid antigenic detection test in a paediatric population from the central region of Portugal. Eur J Clin Microbiol Infect Dis. 2021;40(6):1235-1243. doi: 10.1007/s10096-021-04157-x
24. Frost HM, McLean HQ, Chow BDW. Variability in Antibiotic Prescribing for Upper Respiratory Illnesses by Provider Specialty. J Pediatr. 2018;203:76-85.e8. doi: 10.1016/j.jpeds.2018.07.044.
25. Rick AM, Zaheer HA, Martin JM. Clinical Features of Group A Streptococcus in Children With Pharyngitis: Carriers versus Acute Infection. Pediatr Infect Dis J. 2020;39(6):483-488. doi: 10.1097/INF.0000000000002602
26. Nadeau NL, Fine AM, Kimia A. Improving the prediction of streptococcal pharyngitis; time to move past exudate alone [published online ahead of print, 2020 Aug 16]. Am J Emerg Med. 2020;S0735-6757(20)30709-9. doi: 10.1016/j.ajem.2020.08.023
27. Mustafa Z, Ghaffari M. Diagnostic Methods, Clinical Guidelines, and Antibiotic Treatment for Group A Streptococcal Pharyngitis: A Narrative Review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627
From the Department of Pharmaceutical and Health Economics, University of Southern California, Los Angeles, CA, (Drs. Sangha and McCombs), Department of Pediatrics, Keck School of Medicine, and Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, (Dr. Steinberg), and Leonard Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA (Dr. McCombs).
Objective: The recommended treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS) are antibiotics using the “test and treat” strategy to detect and treat GAS for pediatric pharyngitis. This study used paid claims data to document the extent to which real-world treatment patterns are consistent with these recommendations. We document the factors correlated with testing and treatment, then examine the effects of receiving a GAS test and being treated with an antibiotic impact the likelihood of a revisit for an acute respiratory tract infection within 28 days.
Methods: This retrospective cohort study used Optum Insight Clinformatics data for medical and pharmacy claims from 2011-2013 to identify episodes of care for children and adolescents with pharyngitis around their index visit (± 6 months). The sample population included children and adolescents under 18 years of age with a diagnosis of pharyngitis. Multivariable logistic regression analyses were used to document factors associated with receipt of GAS test and antibiotic treatment. Next, we used logistic regression models to estimate the impact of test and treat recommendation on revisit risk.
Results: There were 24 685 treatment episodes for children and adolescents diagnosed with pharyngitis. Nearly 47% of these episodes included a GAS test and 48% of tested patients were prescribed an antibiotic prescription. Failing to perform a GAS test increased the risk of a revisit within 28 days by 44%. The use of antibiotics by tested and untested patients had no impact on revisit risk.
Conclusion: While the judicious use of antibiotics is important in managing pharyngitis infections and managing complications, the use of rapid diagnostic tools was found to be the determining factor in reducing revisits for pediatric patients with pharyngitis.
Keywords: pediatrics; pharyngitis; respiratory infections; acute infections; diagnostic tests; group A Streptococcus; antibiotics; revisits.
Acute pharyngitis is a common acute respiratory tract infection (ARTI) in children. Group A β-hemolytic streptococci (GABHS) is the most common bacterial etiology for pediatric pharyngitis, accounting for 15% to 30% of cases.1
Beyond clinical assessment, laboratory diagnostic testing generally plays a limited role in guiding appropriate antibiotic prescribing for patients with an ARTI.2,3 Most diagnostic tests require 2 or 3 days to result, incur additional costs, and may delay treatment.4 While these tests do not provide clear and timely guidance on which specific antibiotic is appropriate for ARTI patients, this is not the case for patients with pharyngitis.5,6,7 A rapid diagnostic test exists to identify pharyngitis patients with GABHS which accounts for 1 in 4 children with acute sore throat.1,4,6 Both the American Academy of Pediatrics and the Infectious Diseases Society of America recommend antibiotic treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS).8,9 This “test and treat” protocol has been consistently included in the Healthcare Effectiveness Data and Information Set (HEDIS) standards over time for pediatric pharyngitis patients aged 3 to 18 years before dispensing an antibiotic.10
Sinusitis, pneumonia, and acute otitis media are considered ARTIs where antibiotic treatment is justified. Therefore, pharyngitis of unclear etiology seen with these comorbid infections may not always undergo GAS testing but move directly to the patient being prescribed antibiotics. This analysis enumerates ARTI-related comorbidities present together with the initial coded pharyngitis diagnosis to evaluate their impact on the provider’s decision to test and treat, and on revisit risk.
Antibiotic treatment for GAS patients is likely to eradicate the acute GABHS infection within 10 days. Penicillin and amoxicillin are commonly recommended because of their narrow spectrum of activity, few adverse effects, established efficacy, and modest cost. Alternative antibiotics for patients with penicillin allergy, or with polymicrobial infection seen on culture results, include a first-generation cephalosporin, clindamycin, clarithromycin (Biaxin), or azithromycin (Zithromax).1,8,11 However, while compliance with these HEDIS guidelines has been evaluated, the outcome effects of following the HEDIS “test and treat” recommendations for children with pharyngitis have not been adequately evaluated.
These outcome evaluations have increasing importance as the latest HEDIS survey has shown testing rates in commercial Preferred Provider Organizations (PPO) falling from 86.4% in 2018 to 75.9% in 2019, the lowest rate of testing since 2009, with similar reductions under 80% for Health Maintenance Organizations (HMO).10 While health plans may execute cost-benefit analyses and algorithms to forge best practices for GAS testing in children and adolescents presenting with symptoms of pharyngitis, it is important to regard the wasteful resource utilization and additional cost of revisits that may offset any gains accrued by more focused GAS testing outside the existing clinical guidelines and HEDIS measures. This may be of particular importance in documenting infection and sparing antibiotic therapy in toddlers and younger.
The objective of this study was to investigate the correlation between testing and antibiotic use on the likelihood of a revisit for an acute respiratory tract infection within 28 days. To achieve this objective, this investigation consists of 3 sequential analyses. First, we document the factors associated with the decision to test the patient for a GABHS infection using the GAS test. Next, we document the factors associated with the decision to use an antibiotic to treat the patient as a function of having tested the patient. Finally, we investigate the impact of the testing and treatment decisions on the likelihood of a revisit within 28 days.
Methods
Study design
This was a retrospective cohort study of episodes of treatment for pediatric patients with pharyngitis. Episodes were identified using data derived from the Optum Insight Clinformatics claims database provided to the University of Southern California to facilitate the training of graduate students. These data cover commercially insured patients with both medical and pharmacy benefits. Data were retrieved from the 3-year period spanning 2011-2013. An episode of care was identified based on date of the first (index) outpatient visit for a pharyngitis diagnosis (International Classification of Diseases, Ninth Revision [ICD-9]: 462, 463, 034.0). Outpatient visits were defined by visit setting: ambulatory clinics, physician offices, emergency rooms, and urgent care facilities. Each pharyngitis treatment episode was then screened for at least a 6-month enrollment in a health insurance plan prior and subsequent to the index visit using Optum enrollment data. Finally, eligible treatment episodes were restricted to children and adolescents under 18 years of age, who had an index outpatient visit for a primary diagnosis of acute pharyngitis.
A diagnostic profile was created for each episode using the diagnoses recorded for the index visit. Up to 3 diagnoses may be recorded for any outpatient visit and the first recorded diagnosis was assumed to be the primary diagnosis for that episode. Any secondary diagnoses recorded on the index visit were used to define comorbidities present at the index visit. ARTI-related comorbidities included: acute otitis media (AOM), bronchitis, sinusitis, pneumonia, and upper respiratory infection (URI). Other comorbid medical diagnoses were documented using diagnostic data from the pre-index period. Dichotomous variables for the following categories were created: mental disorders, nervous system disorders, respiratory symptoms, fever, injury and poisoning, other, or no diseases.
Prior visits for other respiratory infections in the previous 90 days were also identified for patients based on their index visit for pharyngitis. Similarly, any subsequent visits, within 28 days of the index visit, were also recorded to measure the health outcome for analysis. Practice settings include physician offices and federally qualified health centers, state and local health clinics, outpatient hospitals facilities, emergency departments, and other outpatient settings such as walk-in retail health clinic or ambulatory centers. Providers include primary care physicians (family practice, pediatricians, internal medicine), specialty care physicians (emergency medicine, preventive medicine), nonphysician providers (nurse practitioners, physician assistants) and other providers (urgent care, acute outpatient care, ambulatory care centers). Seasons of the year were determined based on the index date of the episode to account for possible seasonality in pharyngitis treatment. Lastly, a previous visits variable was created to identify whether the child had nonpharyngitis ARTI visits in the 3 months prior to the index visit.
Demographic variables were created based on enrollment and the socioeconomic data available in the Optum socioeconomic status file. These variables include patient age, race, sex, household income, geographic location, practice setting type, provider specialty, and type of insurance. An estimate of patient household income was based on algorithms using census block groups. Income categories were informed by the federal guidelines for a family of 4. A low-income family was defined as earning less than $50 000; a middle-income family earned between $50 000 and $75 000, and a high-income family earned $75 000 and above.12 Patient insurance type was categorized as HMO, Exclusive Provider Organization (EPO), Point of Service (POS), and PPO. Race was identified as White, Black, Hispanic, and Asian. Patient location was defined according to national census regions.
Outcomes
GAS test
The HEDIS measures for pharyngitis recommend using the GAS test to identify the bacterial etiology of the pharyngitis infection. Patients who received the test were identified based on Current Procedural Terminology (CPT) codes 87070-71, 87081, 87430, 87650-52, and 87880.10
Antibiotic treatment
The pharmacy administrative claims dataset was used to identify study patients who filled a prescription for an antibiotic during their pharyngitis treatment episode. Optum pharmacy data identify the medications received, specifies the date of prescription filling, National Drug Codes, and American Hospital Formulary Service (AHFS) Classification System codes for each medication. We used the AHFS Pharmacologic-Therapeutic classification of antibiotics to create dichotomous variables documenting the antibacterial used by each patient.13 These are categorized under antibacterial including penicillins, cephalosporins (first, second, third, fourth generation cephalosporins), macrolides (first generation and others), tetracyclines, sulfonamides, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), cephamycin, carbapenems, and β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, cephalexin, cefuroxime, cefdinir).
Revisits to physician or other provider
Revisits within 28 days were used as the measure of patient outcomes related to testing and filling of an antibiotic prescription for acute pharyngitis. Revisits may also be due to a patient returning for a follow-up, alternative treatment, worsening pharyngitis, or for another ARTI. An ARTI-related revisit also increases total resources used to treat pediatric pharyngitis patients.
Statistical analysis
Logistic regression was used for all 3 analyses conducted in this study. First, we determined the patient and treating physician characteristics that impact the decision to use GAS testing for pharyngitis. Second, we identified those factors that impact the decision to use antibiotic prescriptions among children who were diagnosed with pharyngitis adding in the dichotomous variable indicating if the patient had received a GAS test. Third, we used a logit regression analysis to document if receiving a GAS test and/or an antibiotic impacted the likelihood of a revisit by comparing revisit risk. To estimate the effect of testing and/or antibiotic use, we divided patients into 4 groups based on whether the patient received a GAS test and/or an antibiotic prescription. This specification of the analysis of revisits as an outcome focuses on adherence to HEDIS “test and treat” guidelines10:
- Patients who were not tested yet filled an antibiotic prescription. This decision was likely based on the clinician’s judgment of the patient’s signs and symptoms, and confirmational testing not performed.
- Patients who were not tested and did not fill an antibiotic prescription. Apparently, in the clinician’s judgment the patient’s signs and symptoms were such that the infection did not warrant treatment and the clinical presentation did not necessitate the GAS test to confirm the recorded diagnosis of pharyngitis.
- Patients who were tested and received antibiotic prescription, likely because the test was positive for GABHS.
- Patients who were tested and did not receive antibiotic prescription.
We tested for statistically significant differences in baseline characteristics across these 4 patient groups using t tests for continuous variables and χ2 tests for categorical variables. Odds ratios (OR) and CI were computed for the influential variables included the regression analyses.
We conducted a sensitivity analysis using a model specification which included the dichotomous variables for testing and for treatment, and the interaction term between these variables to assess if treatment effects varied in tested and untested patients. We also estimated this model of revisit risk using revisits within 7 days as the outcome variable.
All analyses were completed using STATA/IC 13 (StataCorp, College Station, TX).
Results
There were 24 685 treatment episodes for children diagnosed with pharyngitis. Nearly 47% of these episodes included GAS testing and 47% of the tested patients filled an antibiotic prescription. Similarly, 53% of patients were not tested and 49% of untested patients filled an antibiotic prescription. As a result, the 4 groups identified for analysis were evenly distributed: untested and no prescription (26.9%), untested and prescription (26.3%), tested and prescription (21.9%), and tested and no prescription (24.9%) (Figure).

Table 1 presents the descriptive statistics for these 4 patient groups. Note first that the rate of revisits within 28 days is under 5% across all groups. Second, the 2 tested groups have a lower revisit rate than the untested groups: the tested and treated have a revisit rate of 3.3%, and the tested and untreated have a revisit rate of 2.4%, while both the untested groups have a revisit rate of nearly 5%. These small absolute differences in revisit rates across groups were statistically significant.

Factors associated with receiving GAS test
Several factors were found to impact the decision to test (Table 2). Only 9.7% of children were reported to have any ARTI coinfection. As expected, these comorbidities resulted in a significantly lower likelihood of receiving the GAS test: AOM, bronchitis, sinusitis, pneumonia, and URI as comorbid infections had a 48%, 41%, 37%, 63%, and 13% lower likelihood of receiving the GAS test, respectively, than those with no comorbidities. Similarly, children with fever and respiratory symptoms were 35% and 45% less likely to receiving the GAS test, respectively. This is consistent with our expectation that comorbid ARTI infections will lead many providers to forgo testing.

Provider type and patient age also plays a role in receipt of the GAS test. Relative to outpatient facility providers, primary care physicians were 24% more likely and specialty physicians were 38% less likely of employing the GAS test. The child’s age played a significant role in receipt of the GAS test. Children aged 1 to 5 years and 5 to 12 years were 15% and 14% more likely to receive the test compared to children older than 12 years.
Pharyngitis patients have disproportionately higher odds of receiving a GAS test in most regions of the country compared to the Pacific region. For instance, children in the Mid-Atlantic region have 51% higher odds of receiving a GAS test while children in New England have 80% higher odds of receiving the same test.
Black children have 11% lower odds of receiving the GAS test compared to White children. Both middle-income and high-income children have 12% and 32% higher odds of receiving the test compared to low-income children. Compared to office-based visits, children visiting a clinic were twice as likely to receive a GAS test while those seen in the emergency room have 43% lower odds of receiving a GAS test. Hospital outpatient departments, which account for less than 1% of all visits, rarely used a GAS test which could be a statistical artifact due to small sample size. Lastly, insurance and season of the year had no significant impact of receipt of a GAS test.
Factors associated with receiving antibiotic prescription
Surprisingly, receiving the GAS test has a small but insignificant impact on the likelihood that the patient will receive an antibiotic prescription (Table 3) (Adjusted OR = 1.055, P = .07). After controlling for receipt of a GAS test, children with AOM and sinusitis comorbidities have an increased likelihood of being prescribed an antibiotic. Children with URI have a lower likelihood of being prescribed an antibiotic. Additionally, relative to primary care physicians, children visiting nonphysician providers for pharyngitis were more likely to be prescribed an antibiotic.

Children under 12 years of age were more likely to use an antibiotic compared to children 12 years and older. Geographically, there is some evidence of regional variation in antibiotic use as well. Children in the south Atlantic, west-south central, and southeast central regions had a significantly lower odds of being prescribed an antibiotic respectively than pharyngitis patients in the Pacific region. Black children had a 10% lower likelihood of being prescribed an antibiotic compared to White children, possibly related to their lower rate of GAS testing. Compared to office-based visits, children visiting a clinic were less likely to use an antibiotic. Household income, insurance type, and season had no significant impact on revisit risk.
Effects of GAS test and antibiotic prescriptions on likelihood of revisits
The multivariate analysis of the risk of a revisit within 28 days is presented in Table 4. Children with pharyngitis who tested and did not receive an antibiotic serve as the reference comparison group for this analysis to illustrate the impact of using the GAS test and treatment with an antibiotic. The results in Table 4 are quite clear: patients who receive the GAS test were significantly less likely to have a revisit within 28 days. Moreover, within the group of patients who were tested, those not receiving an antibiotic, presumedly because their GAS test was negative, experienced the lowest risk of a revisit. This result is consistent with the data in Table 1. Moreover, using an antibiotic had no impact on the likelihood of a revisit in patients not receiving the GAS test. This result is also consistent with Table 1.

Other results from the analysis of revisit risk may be of interest to clinicians. Pharyngitis patients with a prior episode of treatment within 90 days for an acute respiratory tract infection were more than 7 times more likely to experience a revisit within 28 days of the pharyngitis diagnosis than patients without a history of recent ARTI infections. Age is also a risk factor in likelihood of initiating a revisit. Children under 1 year and children aged 1 to 5 years were more likely to have a revisit than children aged more than 12 years. Compared to White children, Black children were 25% (P = .04) less likely to have a revisit. The care setting also has a significant impact on revisit risk. Children visiting outpatient hospital and other care settings had a significantly higher revisit risk than those visiting a physician’s office. Lastly, household income, geographic region, season, medical comorbidities, gender, and insurance type have no significant impact on revisit risk.
Sensitivity analysis
The results from the analysis of 7-day and 28-day revisit risk are summarized in Table 5. These results indicate that patients who were tested had a more significant decrease in revisit risk at 7 days (72%) than was evident at 28 days (47% reduction). Receiving an antibiotic, with or without the test, had no impact on revisit risk.

Discussion
Published data on revisits for pharyngitis are lacking with the concentration of prior research focused more on systemic complications of undertreated GABHS disease or on identifying carrier status. Our study results suggest that GAS testing is the most important factor in reducing revisit risk. Being prescribed an antibiotic, on its own, does not have a significant impact on the risk of a revisit. However, once the GAS test is used, the decision not to use an antibiotic was correlated with the lowest revisit rate, likely because the source of the pharyngitis infection was viral and more likely to resolve without a revisit. Prior studies have reported variable rates of testing among children with pharyngitis prescribed an antibiotic, ranging from 23% to 91%,14,15 with testing important toward more appropriate antibiotic use.16 More recently, among more than 67 000 patients aged 3 to 21 years presenting with sore throat and receiving a GAS test, 32.6% were positive.17
Our analysis found that more than 46% of pediatric pharyngitis patients were given the rapid GAS test. While this testing rate is substantially lower than HEDIS recommendations and lower than testing rates achieved by several health maintenance organizations,10 it is similar to the 53% of children receiving such testing in a recent National Ambulatory Medical Care Survey.18 Furthermore, we found that when antibiotics are prescribed following a GAS test, the revisit risk is not significantly reduced, possibly because antibiotics lower revisit risk when informed by diagnostic testing tools that determine the infectious organism. This is supported by a similar population analysis in which we observed reduced revisit rates in children with AOM managed with antibiotics within 3 days of index diagnosis.19
Several other factors also affect the likelihood of a child receiving the GAS test. Children aged 1 to 12 years were significantly more likely to receive the GAS test than children over the age of 12. This included children in the 1 to 5 years old bracket who had a 15% higher likelihood of undergoing a GAS test, despite children less than 3 years of age as not recommended targets for GAS testing.20 As expected, children with reported ARTI-associated comorbidities were also less likely to receive a GAS test. Additionally, specialty care physicians were less inclined to implement the GAS test, possibly because of diagnostic confidence without testing or referral after GAS was ruled out. Black and low-income children had statistically lower odds of receiving the test, even after controlling for other factors, and yet were less likely to consume a revisit. As the overall data suggested more revisits in those not tested, further study is needed to examine if race or income discrepancies are equity based. Finally, children in the Pacific region, compared to the rest of the nation, were the least likely to receive a GAS test and yet there were no significant differences in revisit rates by region. Regional differences in antibiotic use were also observed in our study, as has been seen by others.21
After statistically controlling for having received the diagnostic GAS test and filled a prescription for an antibiotic, there are multitude of factors that independently affect the revisit risk, the most important of which if which was a history of an ARTI infection in the prior 90 days. While prior visit history had no impact on the likelihood of being tested or filling an antibiotic, patients with prior visits were more than 7 times more likely to consume a revisit. This was not reflected in nor related to comorbid ARTIs as these patients did not have statistically higher revisits than those with pharyngitis as the sole-coded diagnosis. Moreover, speculation for bacterial etiology of primary or superinfection based on a recent history of ARTI accounting for revisits seems unlikely as it did not yield greater antibiotic use in that group. Further analysis is required to determine the clinical and behavioral factors that promote for prior ARTI history as a major factor in revisit risk after an index visit for pharyngitis.
Children aged between 1 and 5 years, though 15% more likely to be tested than those aged 12 through 17 years, were also 39% more likely to initiate a revisit compared to older children when statistically controlling for other covariates. This perhaps suggests longer illness, wrong diagnosis, delay in appropriate treatment, or more caution by parents and providers in this age group. Justification for testing children less than 3 years of age who are outside of the HEDIS suggested age group, when clinical judgement does not point to another infection source, can result in positivity rates between 22% and 30% as previously observed.22,23 Patients visiting nonphysician providers and outpatient facility providers were less likely to have a revisit than those visiting primary and specialty care physicians, though slightly higher propensity for antibiotic prescriptions was seen for nonphysician providers. Pediatricians have been noted to be less likely to prescribe antibiotics without GAS testing than nonpediatric providers, and more guidelines-compliant in prescribing.24
Recommendations to not test children under 3 years of age are based on the lack of acute rheumatic fever and other complications in this age group together with more frequent viral syndromes. Selectivity in applying clinical criteria to testing can be attempted to separate bacterial from viral illness. Postnasal drainage/rhinorrhea, hoarse voice, and cough have been used successfully to identify those with viral illness and less need for testing, with greater certainty of low risk for GABHS in those over 11 years of age without tonsillar exudates, cervical adenopathy, or fever.17 However, the marginal benefits of those who have all 3 features of viral illness versus none in identifying GAS positivity was 23.3% vs 37.6% - helpful, but certainly not diminishing the need for testing. These constitutional findings of viral URI also do not exclude the GAS carrier state that features these symptoms.25 Others have reinforced the doubt of pharyngeal exudates as the premier diagnostic finding for test-positive GAS.26
This study had several limitations. The Optum claims dataset only contains ICD-9 codes for diagnoses. It does not include data on infection severity and clinical findings related to symptoms, thus empiric treatment warranted based in clinical severity is not assessed. Antibiotics are commonly available as generics and very inexpensive. Patients may fill and pay for these prescriptions directly, in which case, a claim for payment may not be filed with Optum. This could result in an undercount of treated patients in our study.
There is no corresponding problem of missing medical claims for GAS testing which were obtained from the CPT codes within the Optum claims data set. However, we elected not to verify the test results due to these data being missing for 75% of the study population. Nevertheless, this study’s focus was less about justifying antibiotic treatment, but dealt with the outcomes generated by testing and treatment. Toward that end, we used CPT codes to identify a revisit, and while those can at times be affected by financial reimbursement incentives, differences related to revisits in the 4 patient groups should not be subject to bias.
Conclusion
This study used data from real world practices to document the patterns of GAS testing and antibiotic use in pediatric pharyngitis patients. Revisit rates were under 5% for all patient groups and the use of rapid diagnostic tools were found to be the determining factor in further reducing the risk of revisits. This supports the need for compliance with the HEDIS quality metric for pharyngitis to the recommended levels of rapid testing which have been falling in recent years. Use of more accurate antigen and newer molecular detection testing methods may help further delineate important factors in determining pediatric pharyngitis treatment and need for revisits.27
Corresponding author: Jeffrey McCombs, MD, University of Southern California School of Pharmacy, Department of Pharmaceutical and Health Economics, Leonard D. Schaeffer Center for Health Policy & Economics, 635 Downey Way, Verna & Peter Dauterive Hall 310, Los Angeles, CA 90089-3333; [email protected].
Financial disclosures: None.
From the Department of Pharmaceutical and Health Economics, University of Southern California, Los Angeles, CA, (Drs. Sangha and McCombs), Department of Pediatrics, Keck School of Medicine, and Department of Clinical Pharmacy, School of Pharmacy, University of Southern California, Los Angeles, CA, (Dr. Steinberg), and Leonard Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA (Dr. McCombs).
Objective: The recommended treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS) are antibiotics using the “test and treat” strategy to detect and treat GAS for pediatric pharyngitis. This study used paid claims data to document the extent to which real-world treatment patterns are consistent with these recommendations. We document the factors correlated with testing and treatment, then examine the effects of receiving a GAS test and being treated with an antibiotic impact the likelihood of a revisit for an acute respiratory tract infection within 28 days.
Methods: This retrospective cohort study used Optum Insight Clinformatics data for medical and pharmacy claims from 2011-2013 to identify episodes of care for children and adolescents with pharyngitis around their index visit (± 6 months). The sample population included children and adolescents under 18 years of age with a diagnosis of pharyngitis. Multivariable logistic regression analyses were used to document factors associated with receipt of GAS test and antibiotic treatment. Next, we used logistic regression models to estimate the impact of test and treat recommendation on revisit risk.
Results: There were 24 685 treatment episodes for children and adolescents diagnosed with pharyngitis. Nearly 47% of these episodes included a GAS test and 48% of tested patients were prescribed an antibiotic prescription. Failing to perform a GAS test increased the risk of a revisit within 28 days by 44%. The use of antibiotics by tested and untested patients had no impact on revisit risk.
Conclusion: While the judicious use of antibiotics is important in managing pharyngitis infections and managing complications, the use of rapid diagnostic tools was found to be the determining factor in reducing revisits for pediatric patients with pharyngitis.
Keywords: pediatrics; pharyngitis; respiratory infections; acute infections; diagnostic tests; group A Streptococcus; antibiotics; revisits.
Acute pharyngitis is a common acute respiratory tract infection (ARTI) in children. Group A β-hemolytic streptococci (GABHS) is the most common bacterial etiology for pediatric pharyngitis, accounting for 15% to 30% of cases.1
Beyond clinical assessment, laboratory diagnostic testing generally plays a limited role in guiding appropriate antibiotic prescribing for patients with an ARTI.2,3 Most diagnostic tests require 2 or 3 days to result, incur additional costs, and may delay treatment.4 While these tests do not provide clear and timely guidance on which specific antibiotic is appropriate for ARTI patients, this is not the case for patients with pharyngitis.5,6,7 A rapid diagnostic test exists to identify pharyngitis patients with GABHS which accounts for 1 in 4 children with acute sore throat.1,4,6 Both the American Academy of Pediatrics and the Infectious Diseases Society of America recommend antibiotic treatment for children and adolescents under 18 years of age who have a positive test for group A Streptococcus (GAS).8,9 This “test and treat” protocol has been consistently included in the Healthcare Effectiveness Data and Information Set (HEDIS) standards over time for pediatric pharyngitis patients aged 3 to 18 years before dispensing an antibiotic.10
Sinusitis, pneumonia, and acute otitis media are considered ARTIs where antibiotic treatment is justified. Therefore, pharyngitis of unclear etiology seen with these comorbid infections may not always undergo GAS testing but move directly to the patient being prescribed antibiotics. This analysis enumerates ARTI-related comorbidities present together with the initial coded pharyngitis diagnosis to evaluate their impact on the provider’s decision to test and treat, and on revisit risk.
Antibiotic treatment for GAS patients is likely to eradicate the acute GABHS infection within 10 days. Penicillin and amoxicillin are commonly recommended because of their narrow spectrum of activity, few adverse effects, established efficacy, and modest cost. Alternative antibiotics for patients with penicillin allergy, or with polymicrobial infection seen on culture results, include a first-generation cephalosporin, clindamycin, clarithromycin (Biaxin), or azithromycin (Zithromax).1,8,11 However, while compliance with these HEDIS guidelines has been evaluated, the outcome effects of following the HEDIS “test and treat” recommendations for children with pharyngitis have not been adequately evaluated.
These outcome evaluations have increasing importance as the latest HEDIS survey has shown testing rates in commercial Preferred Provider Organizations (PPO) falling from 86.4% in 2018 to 75.9% in 2019, the lowest rate of testing since 2009, with similar reductions under 80% for Health Maintenance Organizations (HMO).10 While health plans may execute cost-benefit analyses and algorithms to forge best practices for GAS testing in children and adolescents presenting with symptoms of pharyngitis, it is important to regard the wasteful resource utilization and additional cost of revisits that may offset any gains accrued by more focused GAS testing outside the existing clinical guidelines and HEDIS measures. This may be of particular importance in documenting infection and sparing antibiotic therapy in toddlers and younger.
The objective of this study was to investigate the correlation between testing and antibiotic use on the likelihood of a revisit for an acute respiratory tract infection within 28 days. To achieve this objective, this investigation consists of 3 sequential analyses. First, we document the factors associated with the decision to test the patient for a GABHS infection using the GAS test. Next, we document the factors associated with the decision to use an antibiotic to treat the patient as a function of having tested the patient. Finally, we investigate the impact of the testing and treatment decisions on the likelihood of a revisit within 28 days.
Methods
Study design
This was a retrospective cohort study of episodes of treatment for pediatric patients with pharyngitis. Episodes were identified using data derived from the Optum Insight Clinformatics claims database provided to the University of Southern California to facilitate the training of graduate students. These data cover commercially insured patients with both medical and pharmacy benefits. Data were retrieved from the 3-year period spanning 2011-2013. An episode of care was identified based on date of the first (index) outpatient visit for a pharyngitis diagnosis (International Classification of Diseases, Ninth Revision [ICD-9]: 462, 463, 034.0). Outpatient visits were defined by visit setting: ambulatory clinics, physician offices, emergency rooms, and urgent care facilities. Each pharyngitis treatment episode was then screened for at least a 6-month enrollment in a health insurance plan prior and subsequent to the index visit using Optum enrollment data. Finally, eligible treatment episodes were restricted to children and adolescents under 18 years of age, who had an index outpatient visit for a primary diagnosis of acute pharyngitis.
A diagnostic profile was created for each episode using the diagnoses recorded for the index visit. Up to 3 diagnoses may be recorded for any outpatient visit and the first recorded diagnosis was assumed to be the primary diagnosis for that episode. Any secondary diagnoses recorded on the index visit were used to define comorbidities present at the index visit. ARTI-related comorbidities included: acute otitis media (AOM), bronchitis, sinusitis, pneumonia, and upper respiratory infection (URI). Other comorbid medical diagnoses were documented using diagnostic data from the pre-index period. Dichotomous variables for the following categories were created: mental disorders, nervous system disorders, respiratory symptoms, fever, injury and poisoning, other, or no diseases.
Prior visits for other respiratory infections in the previous 90 days were also identified for patients based on their index visit for pharyngitis. Similarly, any subsequent visits, within 28 days of the index visit, were also recorded to measure the health outcome for analysis. Practice settings include physician offices and federally qualified health centers, state and local health clinics, outpatient hospitals facilities, emergency departments, and other outpatient settings such as walk-in retail health clinic or ambulatory centers. Providers include primary care physicians (family practice, pediatricians, internal medicine), specialty care physicians (emergency medicine, preventive medicine), nonphysician providers (nurse practitioners, physician assistants) and other providers (urgent care, acute outpatient care, ambulatory care centers). Seasons of the year were determined based on the index date of the episode to account for possible seasonality in pharyngitis treatment. Lastly, a previous visits variable was created to identify whether the child had nonpharyngitis ARTI visits in the 3 months prior to the index visit.
Demographic variables were created based on enrollment and the socioeconomic data available in the Optum socioeconomic status file. These variables include patient age, race, sex, household income, geographic location, practice setting type, provider specialty, and type of insurance. An estimate of patient household income was based on algorithms using census block groups. Income categories were informed by the federal guidelines for a family of 4. A low-income family was defined as earning less than $50 000; a middle-income family earned between $50 000 and $75 000, and a high-income family earned $75 000 and above.12 Patient insurance type was categorized as HMO, Exclusive Provider Organization (EPO), Point of Service (POS), and PPO. Race was identified as White, Black, Hispanic, and Asian. Patient location was defined according to national census regions.
Outcomes
GAS test
The HEDIS measures for pharyngitis recommend using the GAS test to identify the bacterial etiology of the pharyngitis infection. Patients who received the test were identified based on Current Procedural Terminology (CPT) codes 87070-71, 87081, 87430, 87650-52, and 87880.10
Antibiotic treatment
The pharmacy administrative claims dataset was used to identify study patients who filled a prescription for an antibiotic during their pharyngitis treatment episode. Optum pharmacy data identify the medications received, specifies the date of prescription filling, National Drug Codes, and American Hospital Formulary Service (AHFS) Classification System codes for each medication. We used the AHFS Pharmacologic-Therapeutic classification of antibiotics to create dichotomous variables documenting the antibacterial used by each patient.13 These are categorized under antibacterial including penicillins, cephalosporins (first, second, third, fourth generation cephalosporins), macrolides (first generation and others), tetracyclines, sulfonamides, fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin), cephamycin, carbapenems, and β-lactam antibiotics (amoxicillin, amoxicillin/clavulanate, cephalexin, cefuroxime, cefdinir).
Revisits to physician or other provider
Revisits within 28 days were used as the measure of patient outcomes related to testing and filling of an antibiotic prescription for acute pharyngitis. Revisits may also be due to a patient returning for a follow-up, alternative treatment, worsening pharyngitis, or for another ARTI. An ARTI-related revisit also increases total resources used to treat pediatric pharyngitis patients.
Statistical analysis
Logistic regression was used for all 3 analyses conducted in this study. First, we determined the patient and treating physician characteristics that impact the decision to use GAS testing for pharyngitis. Second, we identified those factors that impact the decision to use antibiotic prescriptions among children who were diagnosed with pharyngitis adding in the dichotomous variable indicating if the patient had received a GAS test. Third, we used a logit regression analysis to document if receiving a GAS test and/or an antibiotic impacted the likelihood of a revisit by comparing revisit risk. To estimate the effect of testing and/or antibiotic use, we divided patients into 4 groups based on whether the patient received a GAS test and/or an antibiotic prescription. This specification of the analysis of revisits as an outcome focuses on adherence to HEDIS “test and treat” guidelines10:
- Patients who were not tested yet filled an antibiotic prescription. This decision was likely based on the clinician’s judgment of the patient’s signs and symptoms, and confirmational testing not performed.
- Patients who were not tested and did not fill an antibiotic prescription. Apparently, in the clinician’s judgment the patient’s signs and symptoms were such that the infection did not warrant treatment and the clinical presentation did not necessitate the GAS test to confirm the recorded diagnosis of pharyngitis.
- Patients who were tested and received antibiotic prescription, likely because the test was positive for GABHS.
- Patients who were tested and did not receive antibiotic prescription.
We tested for statistically significant differences in baseline characteristics across these 4 patient groups using t tests for continuous variables and χ2 tests for categorical variables. Odds ratios (OR) and CI were computed for the influential variables included the regression analyses.
We conducted a sensitivity analysis using a model specification which included the dichotomous variables for testing and for treatment, and the interaction term between these variables to assess if treatment effects varied in tested and untested patients. We also estimated this model of revisit risk using revisits within 7 days as the outcome variable.
All analyses were completed using STATA/IC 13 (StataCorp, College Station, TX).
Results
There were 24 685 treatment episodes for children diagnosed with pharyngitis. Nearly 47% of these episodes included GAS testing and 47% of the tested patients filled an antibiotic prescription. Similarly, 53% of patients were not tested and 49% of untested patients filled an antibiotic prescription. As a result, the 4 groups identified for analysis were evenly distributed: untested and no prescription (26.9%), untested and prescription (26.3%), tested and prescription (21.9%), and tested and no prescription (24.9%) (Figure).

Table 1 presents the descriptive statistics for these 4 patient groups. Note first that the rate of revisits within 28 days is under 5% across all groups. Second, the 2 tested groups have a lower revisit rate than the untested groups: the tested and treated have a revisit rate of 3.3%, and the tested and untreated have a revisit rate of 2.4%, while both the untested groups have a revisit rate of nearly 5%. These small absolute differences in revisit rates across groups were statistically significant.

Factors associated with receiving GAS test
Several factors were found to impact the decision to test (Table 2). Only 9.7% of children were reported to have any ARTI coinfection. As expected, these comorbidities resulted in a significantly lower likelihood of receiving the GAS test: AOM, bronchitis, sinusitis, pneumonia, and URI as comorbid infections had a 48%, 41%, 37%, 63%, and 13% lower likelihood of receiving the GAS test, respectively, than those with no comorbidities. Similarly, children with fever and respiratory symptoms were 35% and 45% less likely to receiving the GAS test, respectively. This is consistent with our expectation that comorbid ARTI infections will lead many providers to forgo testing.

Provider type and patient age also plays a role in receipt of the GAS test. Relative to outpatient facility providers, primary care physicians were 24% more likely and specialty physicians were 38% less likely of employing the GAS test. The child’s age played a significant role in receipt of the GAS test. Children aged 1 to 5 years and 5 to 12 years were 15% and 14% more likely to receive the test compared to children older than 12 years.
Pharyngitis patients have disproportionately higher odds of receiving a GAS test in most regions of the country compared to the Pacific region. For instance, children in the Mid-Atlantic region have 51% higher odds of receiving a GAS test while children in New England have 80% higher odds of receiving the same test.
Black children have 11% lower odds of receiving the GAS test compared to White children. Both middle-income and high-income children have 12% and 32% higher odds of receiving the test compared to low-income children. Compared to office-based visits, children visiting a clinic were twice as likely to receive a GAS test while those seen in the emergency room have 43% lower odds of receiving a GAS test. Hospital outpatient departments, which account for less than 1% of all visits, rarely used a GAS test which could be a statistical artifact due to small sample size. Lastly, insurance and season of the year had no significant impact of receipt of a GAS test.
Factors associated with receiving antibiotic prescription
Surprisingly, receiving the GAS test has a small but insignificant impact on the likelihood that the patient will receive an antibiotic prescription (Table 3) (Adjusted OR = 1.055, P = .07). After controlling for receipt of a GAS test, children with AOM and sinusitis comorbidities have an increased likelihood of being prescribed an antibiotic. Children with URI have a lower likelihood of being prescribed an antibiotic. Additionally, relative to primary care physicians, children visiting nonphysician providers for pharyngitis were more likely to be prescribed an antibiotic.

Children under 12 years of age were more likely to use an antibiotic compared to children 12 years and older. Geographically, there is some evidence of regional variation in antibiotic use as well. Children in the south Atlantic, west-south central, and southeast central regions had a significantly lower odds of being prescribed an antibiotic respectively than pharyngitis patients in the Pacific region. Black children had a 10% lower likelihood of being prescribed an antibiotic compared to White children, possibly related to their lower rate of GAS testing. Compared to office-based visits, children visiting a clinic were less likely to use an antibiotic. Household income, insurance type, and season had no significant impact on revisit risk.
Effects of GAS test and antibiotic prescriptions on likelihood of revisits
The multivariate analysis of the risk of a revisit within 28 days is presented in Table 4. Children with pharyngitis who tested and did not receive an antibiotic serve as the reference comparison group for this analysis to illustrate the impact of using the GAS test and treatment with an antibiotic. The results in Table 4 are quite clear: patients who receive the GAS test were significantly less likely to have a revisit within 28 days. Moreover, within the group of patients who were tested, those not receiving an antibiotic, presumedly because their GAS test was negative, experienced the lowest risk of a revisit. This result is consistent with the data in Table 1. Moreover, using an antibiotic had no impact on the likelihood of a revisit in patients not receiving the GAS test. This result is also consistent with Table 1.

Other results from the analysis of revisit risk may be of interest to clinicians. Pharyngitis patients with a prior episode of treatment within 90 days for an acute respiratory tract infection were more than 7 times more likely to experience a revisit within 28 days of the pharyngitis diagnosis than patients without a history of recent ARTI infections. Age is also a risk factor in likelihood of initiating a revisit. Children under 1 year and children aged 1 to 5 years were more likely to have a revisit than children aged more than 12 years. Compared to White children, Black children were 25% (P = .04) less likely to have a revisit. The care setting also has a significant impact on revisit risk. Children visiting outpatient hospital and other care settings had a significantly higher revisit risk than those visiting a physician’s office. Lastly, household income, geographic region, season, medical comorbidities, gender, and insurance type have no significant impact on revisit risk.
Sensitivity analysis
The results from the analysis of 7-day and 28-day revisit risk are summarized in Table 5. These results indicate that patients who were tested had a more significant decrease in revisit risk at 7 days (72%) than was evident at 28 days (47% reduction). Receiving an antibiotic, with or without the test, had no impact on revisit risk.

Discussion
Published data on revisits for pharyngitis are lacking with the concentration of prior research focused more on systemic complications of undertreated GABHS disease or on identifying carrier status. Our study results suggest that GAS testing is the most important factor in reducing revisit risk. Being prescribed an antibiotic, on its own, does not have a significant impact on the risk of a revisit. However, once the GAS test is used, the decision not to use an antibiotic was correlated with the lowest revisit rate, likely because the source of the pharyngitis infection was viral and more likely to resolve without a revisit. Prior studies have reported variable rates of testing among children with pharyngitis prescribed an antibiotic, ranging from 23% to 91%,14,15 with testing important toward more appropriate antibiotic use.16 More recently, among more than 67 000 patients aged 3 to 21 years presenting with sore throat and receiving a GAS test, 32.6% were positive.17
Our analysis found that more than 46% of pediatric pharyngitis patients were given the rapid GAS test. While this testing rate is substantially lower than HEDIS recommendations and lower than testing rates achieved by several health maintenance organizations,10 it is similar to the 53% of children receiving such testing in a recent National Ambulatory Medical Care Survey.18 Furthermore, we found that when antibiotics are prescribed following a GAS test, the revisit risk is not significantly reduced, possibly because antibiotics lower revisit risk when informed by diagnostic testing tools that determine the infectious organism. This is supported by a similar population analysis in which we observed reduced revisit rates in children with AOM managed with antibiotics within 3 days of index diagnosis.19
Several other factors also affect the likelihood of a child receiving the GAS test. Children aged 1 to 12 years were significantly more likely to receive the GAS test than children over the age of 12. This included children in the 1 to 5 years old bracket who had a 15% higher likelihood of undergoing a GAS test, despite children less than 3 years of age as not recommended targets for GAS testing.20 As expected, children with reported ARTI-associated comorbidities were also less likely to receive a GAS test. Additionally, specialty care physicians were less inclined to implement the GAS test, possibly because of diagnostic confidence without testing or referral after GAS was ruled out. Black and low-income children had statistically lower odds of receiving the test, even after controlling for other factors, and yet were less likely to consume a revisit. As the overall data suggested more revisits in those not tested, further study is needed to examine if race or income discrepancies are equity based. Finally, children in the Pacific region, compared to the rest of the nation, were the least likely to receive a GAS test and yet there were no significant differences in revisit rates by region. Regional differences in antibiotic use were also observed in our study, as has been seen by others.21
After statistically controlling for having received the diagnostic GAS test and filled a prescription for an antibiotic, there are multitude of factors that independently affect the revisit risk, the most important of which if which was a history of an ARTI infection in the prior 90 days. While prior visit history had no impact on the likelihood of being tested or filling an antibiotic, patients with prior visits were more than 7 times more likely to consume a revisit. This was not reflected in nor related to comorbid ARTIs as these patients did not have statistically higher revisits than those with pharyngitis as the sole-coded diagnosis. Moreover, speculation for bacterial etiology of primary or superinfection based on a recent history of ARTI accounting for revisits seems unlikely as it did not yield greater antibiotic use in that group. Further analysis is required to determine the clinical and behavioral factors that promote for prior ARTI history as a major factor in revisit risk after an index visit for pharyngitis.
Children aged between 1 and 5 years, though 15% more likely to be tested than those aged 12 through 17 years, were also 39% more likely to initiate a revisit compared to older children when statistically controlling for other covariates. This perhaps suggests longer illness, wrong diagnosis, delay in appropriate treatment, or more caution by parents and providers in this age group. Justification for testing children less than 3 years of age who are outside of the HEDIS suggested age group, when clinical judgement does not point to another infection source, can result in positivity rates between 22% and 30% as previously observed.22,23 Patients visiting nonphysician providers and outpatient facility providers were less likely to have a revisit than those visiting primary and specialty care physicians, though slightly higher propensity for antibiotic prescriptions was seen for nonphysician providers. Pediatricians have been noted to be less likely to prescribe antibiotics without GAS testing than nonpediatric providers, and more guidelines-compliant in prescribing.24
Recommendations to not test children under 3 years of age are based on the lack of acute rheumatic fever and other complications in this age group together with more frequent viral syndromes. Selectivity in applying clinical criteria to testing can be attempted to separate bacterial from viral illness. Postnasal drainage/rhinorrhea, hoarse voice, and cough have been used successfully to identify those with viral illness and less need for testing, with greater certainty of low risk for GABHS in those over 11 years of age without tonsillar exudates, cervical adenopathy, or fever.17 However, the marginal benefits of those who have all 3 features of viral illness versus none in identifying GAS positivity was 23.3% vs 37.6% - helpful, but certainly not diminishing the need for testing. These constitutional findings of viral URI also do not exclude the GAS carrier state that features these symptoms.25 Others have reinforced the doubt of pharyngeal exudates as the premier diagnostic finding for test-positive GAS.26
This study had several limitations. The Optum claims dataset only contains ICD-9 codes for diagnoses. It does not include data on infection severity and clinical findings related to symptoms, thus empiric treatment warranted based in clinical severity is not assessed. Antibiotics are commonly available as generics and very inexpensive. Patients may fill and pay for these prescriptions directly, in which case, a claim for payment may not be filed with Optum. This could result in an undercount of treated patients in our study.
There is no corresponding problem of missing medical claims for GAS testing which were obtained from the CPT codes within the Optum claims data set. However, we elected not to verify the test results due to these data being missing for 75% of the study population. Nevertheless, this study’s focus was less about justifying antibiotic treatment, but dealt with the outcomes generated by testing and treatment. Toward that end, we used CPT codes to identify a revisit, and while those can at times be affected by financial reimbursement incentives, differences related to revisits in the 4 patient groups should not be subject to bias.
Conclusion
This study used data from real world practices to document the patterns of GAS testing and antibiotic use in pediatric pharyngitis patients. Revisit rates were under 5% for all patient groups and the use of rapid diagnostic tools were found to be the determining factor in further reducing the risk of revisits. This supports the need for compliance with the HEDIS quality metric for pharyngitis to the recommended levels of rapid testing which have been falling in recent years. Use of more accurate antigen and newer molecular detection testing methods may help further delineate important factors in determining pediatric pharyngitis treatment and need for revisits.27
Corresponding author: Jeffrey McCombs, MD, University of Southern California School of Pharmacy, Department of Pharmaceutical and Health Economics, Leonard D. Schaeffer Center for Health Policy & Economics, 635 Downey Way, Verna & Peter Dauterive Hall 310, Los Angeles, CA 90089-3333; [email protected].
Financial disclosures: None.
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
2. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch of Intern Med. 2008;168(18):2000-2008. doi: 10.1001/archinte.168.18.2000
3. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62(6):1407-1412. doi: 10.1093/jac/dkn376
4. Neuner JM, Hamel MB, Phillips RS, et al. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139(2):113-122. doi:10.7326/0003-4819-139-2-200307150-00011
5. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551. doi: 10.1161/CIRCULATIONAHA.109.191959
6. Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111(6):e666-e670. doi: 10.1542/peds.111.6.e666
7. Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for Streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403
8. Shulman ST, Bisno AL, Clegg H, et al. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629
9. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800–806. doi: 10.1001/archpedi.155.7.800
10. Appropriate Testing for Children with Pharyngitis. HEDIS Measures and Technical Resources. National Committee for Quality Assurance. Accessed February 12, 2021. https://www.ncqa.org/hedis/measures/appropriate-testing-for-children-with-pharyngitis/
11. Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315-2322. doi: 10.1001/jama.294.18.2315
12. Crimmel BL. Health Insurance Coverage and Income Levels for the US Noninstitutionalized Population Under Age 65, 2001. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality. 2004. https://meps.ahrq.gov/data_files/publications/st40/stat40.pd
13. AHFS/ASHP. American Hospital Formulary Service Drug Information. 2012. AHFS drug information. 00--. Accessed January 4, 2021.
14. Mainous AG 3rd, Zoorob, RJ, Kohrs FP, Hagen MD. Streptococcal diagnostic testing and antibiotics prescribed for pediatric tonsillopharyngitis. Pediatr Infect Dis J. 1996;15(9):806-810. doi: 10.1097/00006454-199609000-00014
15. Benin AL, Vitkauskas G, Thornquist E, et al. Improving diagnostic testing and reducing overuse of antibiotics for children with pharyngitis: a useful role for the electronic medical record. Pediatr Infect Dis J. 2003;22(12):1043-1047. doi: 10.1097/01.inf.0000100577.76542.af
16. Luo R, Sickler J, Vahidnia F, et al. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011-2015. BMC Infect Dis. 2019;19(1):193-201. doi: 10.1186/s12879-019-3835-4
17. Shapiro DJ, Barak-Corren Y, Neuman MI, et al. Identifying Patients at Lowest Risk for Streptococcal Pharyngitis: A National Validation Study. J Pediatr. 2020;220:132-138.e2. doi: 10.1016/j.jpeds.2020.01.030. Epub 2020 Feb 14
18. Shapiro DJ, King LM, Fleming-Dutra KE, et al. Association between use of diagnostic tests and antibiotic prescribing for pharyngitis in the United States. Infect Control Hosp Epidemiol. 2020;41(4):479-481. doi: 10.1017/ice.2020.29
19. Sangha K, Steinberg I, McCombs JS. The impact of antibiotic treatment time and class of antibiotic for acute otitis media infections on the risk of revisits. Abs PDG4. Value in Health. 2019; 22:S163.
20. Ahluwalia T, Jain S, Norton L, Meade J, et al. Reducing Streptococcal Testing in Patients < 3 Years Old in an Emergency Department. Pediatrics. 2019;144(4):e20190174. doi: 10.1542/peds.2019-0174
21. McKay R, Mah A, Law MR, et al. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother. 2016;60(7):4106-4118. doi: 10.1128/AAC.00209-16
22. Woods WA, Carter CT, Schlager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15(5):338-340. doi: 10.1097/00006565-199910000-00011
23. Mendes N, Miguéis C, Lindo J, et al. Retrospective study of group A Streptococcus oropharyngeal infection diagnosis using a rapid antigenic detection test in a paediatric population from the central region of Portugal. Eur J Clin Microbiol Infect Dis. 2021;40(6):1235-1243. doi: 10.1007/s10096-021-04157-x
24. Frost HM, McLean HQ, Chow BDW. Variability in Antibiotic Prescribing for Upper Respiratory Illnesses by Provider Specialty. J Pediatr. 2018;203:76-85.e8. doi: 10.1016/j.jpeds.2018.07.044.
25. Rick AM, Zaheer HA, Martin JM. Clinical Features of Group A Streptococcus in Children With Pharyngitis: Carriers versus Acute Infection. Pediatr Infect Dis J. 2020;39(6):483-488. doi: 10.1097/INF.0000000000002602
26. Nadeau NL, Fine AM, Kimia A. Improving the prediction of streptococcal pharyngitis; time to move past exudate alone [published online ahead of print, 2020 Aug 16]. Am J Emerg Med. 2020;S0735-6757(20)30709-9. doi: 10.1016/j.ajem.2020.08.023
27. Mustafa Z, Ghaffari M. Diagnostic Methods, Clinical Guidelines, and Antibiotic Treatment for Group A Streptococcal Pharyngitis: A Narrative Review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627
1. Choby BA. Diagnosis and treatment of streptococcal pharyngitis. Am Fam Physician. 2009;79(5):383-390.
2. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch of Intern Med. 2008;168(18):2000-2008. doi: 10.1001/archinte.168.18.2000
3. Maltezou HC, Tsagris V, Antoniadou A, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. J Antimicrob Chemother. 2008;62(6):1407-1412. doi: 10.1093/jac/dkn376
4. Neuner JM, Hamel MB, Phillips RS, et al. Diagnosis and management of adults with pharyngitis: a cost-effectiveness analysis. Ann Intern Med. 2003;139(2):113-122. doi:10.7326/0003-4819-139-2-200307150-00011
5. Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541-1551. doi: 10.1161/CIRCULATIONAHA.109.191959
6. Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics. 2003;111(6):e666-e670. doi: 10.1542/peds.111.6.e666
7. Shapiro DJ, Lindgren CE, Neuman MI, Fine AM. Viral features and testing for Streptococcal pharyngitis. Pediatrics. 2017;139(5):e20163403. doi: 10.1542/peds.2016-3403
8. Shulman ST, Bisno AL, Clegg H, et al. Clinical practice guideline for the diagnosis and management of group A Streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. doi: 10.1093/cid/cis629
9. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155(7):800–806. doi: 10.1001/archpedi.155.7.800
10. Appropriate Testing for Children with Pharyngitis. HEDIS Measures and Technical Resources. National Committee for Quality Assurance. Accessed February 12, 2021. https://www.ncqa.org/hedis/measures/appropriate-testing-for-children-with-pharyngitis/
11. Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA. 2005;294(18):2315-2322. doi: 10.1001/jama.294.18.2315
12. Crimmel BL. Health Insurance Coverage and Income Levels for the US Noninstitutionalized Population Under Age 65, 2001. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality. 2004. https://meps.ahrq.gov/data_files/publications/st40/stat40.pd
13. AHFS/ASHP. American Hospital Formulary Service Drug Information. 2012. AHFS drug information. 00--. Accessed January 4, 2021.
14. Mainous AG 3rd, Zoorob, RJ, Kohrs FP, Hagen MD. Streptococcal diagnostic testing and antibiotics prescribed for pediatric tonsillopharyngitis. Pediatr Infect Dis J. 1996;15(9):806-810. doi: 10.1097/00006454-199609000-00014
15. Benin AL, Vitkauskas G, Thornquist E, et al. Improving diagnostic testing and reducing overuse of antibiotics for children with pharyngitis: a useful role for the electronic medical record. Pediatr Infect Dis J. 2003;22(12):1043-1047. doi: 10.1097/01.inf.0000100577.76542.af
16. Luo R, Sickler J, Vahidnia F, et al. Diagnosis and Management of Group a Streptococcal Pharyngitis in the United States, 2011-2015. BMC Infect Dis. 2019;19(1):193-201. doi: 10.1186/s12879-019-3835-4
17. Shapiro DJ, Barak-Corren Y, Neuman MI, et al. Identifying Patients at Lowest Risk for Streptococcal Pharyngitis: A National Validation Study. J Pediatr. 2020;220:132-138.e2. doi: 10.1016/j.jpeds.2020.01.030. Epub 2020 Feb 14
18. Shapiro DJ, King LM, Fleming-Dutra KE, et al. Association between use of diagnostic tests and antibiotic prescribing for pharyngitis in the United States. Infect Control Hosp Epidemiol. 2020;41(4):479-481. doi: 10.1017/ice.2020.29
19. Sangha K, Steinberg I, McCombs JS. The impact of antibiotic treatment time and class of antibiotic for acute otitis media infections on the risk of revisits. Abs PDG4. Value in Health. 2019; 22:S163.
20. Ahluwalia T, Jain S, Norton L, Meade J, et al. Reducing Streptococcal Testing in Patients < 3 Years Old in an Emergency Department. Pediatrics. 2019;144(4):e20190174. doi: 10.1542/peds.2019-0174
21. McKay R, Mah A, Law MR, et al. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob Agents Chemother. 2016;60(7):4106-4118. doi: 10.1128/AAC.00209-16
22. Woods WA, Carter CT, Schlager TA. Detection of group A streptococci in children under 3 years of age with pharyngitis. Pediatr Emerg Care. 1999;15(5):338-340. doi: 10.1097/00006565-199910000-00011
23. Mendes N, Miguéis C, Lindo J, et al. Retrospective study of group A Streptococcus oropharyngeal infection diagnosis using a rapid antigenic detection test in a paediatric population from the central region of Portugal. Eur J Clin Microbiol Infect Dis. 2021;40(6):1235-1243. doi: 10.1007/s10096-021-04157-x
24. Frost HM, McLean HQ, Chow BDW. Variability in Antibiotic Prescribing for Upper Respiratory Illnesses by Provider Specialty. J Pediatr. 2018;203:76-85.e8. doi: 10.1016/j.jpeds.2018.07.044.
25. Rick AM, Zaheer HA, Martin JM. Clinical Features of Group A Streptococcus in Children With Pharyngitis: Carriers versus Acute Infection. Pediatr Infect Dis J. 2020;39(6):483-488. doi: 10.1097/INF.0000000000002602
26. Nadeau NL, Fine AM, Kimia A. Improving the prediction of streptococcal pharyngitis; time to move past exudate alone [published online ahead of print, 2020 Aug 16]. Am J Emerg Med. 2020;S0735-6757(20)30709-9. doi: 10.1016/j.ajem.2020.08.023
27. Mustafa Z, Ghaffari M. Diagnostic Methods, Clinical Guidelines, and Antibiotic Treatment for Group A Streptococcal Pharyngitis: A Narrative Review. Front Cell Infect Microbiol. 2020;10:563627. doi: 10.3389/fcimb.2020.563627