User login
Immune reconstitution inflammatory syndrome: ‘Why is my patient getting worse?’
Over the past 25 years, antiretroviral therapy (ART) has led to a dramatic decrease in HIV-associated morbidity and mortality. Patients who initiate ART today can now expect a nearly normal life expectancy.1 Despite the overwhelming benefits of ART, some patients experience immune reconstitution inflammatory syndrome (IRIS), a disease- or pathogen-specific immune response that can mimic the presentation of an active opportunistic infection (OI). IRIS can occur at any CD4 count. However, it is most often associated with the rapid increase in CD4 count and decrease in viral load that typically follows ART initiation in patients who are severely immunocompromised and have high viral loads.2-6
IRIS manifests in two primary ways. Paradoxical IRIS refers to the worsening of a previously diagnosed disease after ART initiation, whereas unmasking IRIS refers to the appearance of a previously undiagnosed disease following ART initiation.
The Medical Care Criteria Committee of the New York State Department of Health AIDS Institute Clinical Guidelines Program recently published an update to its guideline, Management of IRIS . This update incorporates recent data and summarizes how to identify and manage IRIS associated with several OIs. Important goals of this update were to raise awareness among healthcare providers about IRIS, including its clinical presentation, and provide treatment recommendations.
For most patients, ART should be started quickly
Over the past few years, rapid initiation of ART has become the new standard of care, with same-day initiation on the day of HIV diagnosis recommended whenever possible. For many years, however, the presence of an active OI was felt to justify delaying ART initiation until the OI was completely treated. This approach changed in 2009 when a randomized trial by the AIDS Clinical Trials Group demonstrated that patients who initiated ART within 2 weeks of OI diagnosis did not experience more adverse events than those who waited.7 Moreover, although the finding did not reach statistical significance, participants in the early ART arm appeared to experience lower mortality and progression of AIDS than those in the delayed ART arm. Therefore, patients diagnosed with most OIs can start ART as soon as they are tolerating the treatment for the OI.
Some OIs do require a delay in ART
Symptoms associated with IRIS are typically mild or moderate; life-threatening complications are rare. Most patients newly diagnosed with HIV who have an active OI can therefore initiate ART quickly. However, IRIS involving the central nervous system or eye carries a much greater risk of morbidity and mortality. OIs that do warrant a delay in ART initiation, therefore, include tuberculosis (TB) meningitis, cryptococcal meningitis, and cytomegalovirus (CMV) retinitis.
Several randomized clinical trials have found that in patients with HIV and pulmonary TB coinfection, ART should be started as soon as the patient is tolerating anti-TB therapy.8-10 What’s more, in patients with CD4 counts less than 50 cells/microL, there is a mortality benefit when ART is initiated within 2 weeks of starting TB treatment, compared with waiting 8 weeks.
For TB meningitis, however, a clinical trial conducted in Vietnam did not show any mortality benefit when ART was started within 7 days (vs. 2 months); however, severe adverse events were more common in the immediate ART group, raising the concern that patients in that group had experienced complications of IRIS of the central nervous system.11 Limited data are available to guide specific timing of ART in patients with TB meningitis, but based on the results of this trial, most clinicians wait approximately 2 months before initiating ART, and consultation with an expert is recommended.
Optimal timing of ART in patients with cryptococcal meningitis is also uncertain, and there have been contradictory results from several small studies. However, in 2014, the larger COAT trial, conducted in Uganda and South Africa, found 15% higher mortality in patients who initiated ART within 2 weeks, compared with more than 5 weeks.12 Although exactly how long to wait is still unknown, ART should be delayed by at least 2 weeks after a patient starts antifungal therapy.
CMV-IRIS can have devastating effects, including vision loss or blindness. Therefore, ART initiation should be delayed in patients with diagnosed or strongly suspected CMV.13 Importantly, however, patients with advanced HIV may have asymptomatic or subclinical CMV retinitis. As a result, all patients with HIV who have CD4 counts less than 100 cells/mm3 who do not have known or strongly suspected CMV should be screened for signs of CMV by dilated ophthalmological examination as soon as possible after initiation of ART. If signs of CMV are seen on dilated exam, clinicians should consult with an experienced HIV care provider to determine if ART must be temporarily paused.
Diagnosing IRIS
Broadly, IRIS presents as a clinical deterioration after ART initiation, with localized tissue inflammation, with or without a systemic inflammatory response, but the presentation of IRIS varies depending on the underlying OI or illness. In most cases, IRIS occurs within 4-8 weeks of ART initiation or regimen change. A rise in CD4 count often but does not always precede IRIS and is not a diagnostic criterion. There is no diagnostic test for IRIS, and when assessing a patient for possible IRIS, clinicians should exclude HIV disease progression, new infections, OI drug resistance, OI treatment nonadherence, and drug reactions as possible causes for inflammatory signs or symptoms.
Treatment of IRIS
Most cases of IRIS are mild, and patients can be reassured that the symptoms will resolve with time. Clinicians should interrupt ART only if a patient has a severe, life-threatening case of IRIS. Unnecessary ART interruption may increase a patient’s risk of new opportunistic infections, recurring IRIS upon resumption of ART, and development of HIV-drug resistance. Any newly unmasked OIs should be treated promptly while ART is continued. For patients with severe IRIS, clinicians can use prednisone to treat inflammatory symptoms – generally for 1-2 weeks, followed by a taper as needed. Prednisone, however, should not be used in patients with cryptococcal meningitis or Kaposi sarcoma as it is associated with worse outcomes.14-17
In patients newly diagnosed with HIV, prompt initiation of ART is, with the exceptions outlined above, the highest priority. IRIS is an unfortunate complication of ART, and patients may be discouraged when they find themselves feeling worse shortly after starting treatment. While providing supportive and symptomatic care, clinicians can reassure patients by explaining that immune reconstitution is, in fact, the goal of ART and that their symptoms do not represent the progression of HIV disease. It is hoped that with more frequent HIV testing, earlier diagnosis, and earlier ART initiation at higher CD4 counts, IRIS will become a less frequent nuisance to patients and providers.
Dr. Brust is in the department of medicine at Albert Einstein College of Medicine/Montefiore Medical Center, New York. He reported having no relevant financial relationships. A version of this article first appeared on Medscape.com.
References
1. Marcus JL et al. JAMA Netw Open. 2020;3:e207954.
2. Breton G et al. Clin Infect Dis. 2004;39:1709-12.
3. Shelburne SA et al. Clin Infect Dis. 2005;40:1049-52.
4. Shelburne SA et al. AIDS. 2005;19:399-406.
5. Muller M et al. Lancet Infect Dis. 2010;10:251-61.
6. Novak RM et al. AIDS. 2012;26:721-30.
7. Zolopa A et al. PLoS One. 2009;4:e5575.
8. Havlir DV et al. N Engl J Med. 2011;365:1482-91.
9. Abdool Karim SS et al. N Engl J Med. 2011;365:1492-501.
10. Blanc FX et al. N Engl J Med. 2011;365:1471-81.
11. Torok ME et al. Clin Infect Dis. 2011;52:1374-83.
12. Boulware DR et al. N Engl J Med. 2014;370:2487-98.
13. Ortega-Larrocea G et al. AIDS. 2005;19:735-8.
14. Beardsley J et al. N Engl J Med. 2016;374:542-54.
15. Gill PS, Loureiro C et al. Ann Intern Med. 1989;110:937-40.
16. Elliott AM et al. J Infect Dis. 2004;190:869-78.
17. Volkow PF et al. AIDS. 2008;22:663-5.
Over the past 25 years, antiretroviral therapy (ART) has led to a dramatic decrease in HIV-associated morbidity and mortality. Patients who initiate ART today can now expect a nearly normal life expectancy.1 Despite the overwhelming benefits of ART, some patients experience immune reconstitution inflammatory syndrome (IRIS), a disease- or pathogen-specific immune response that can mimic the presentation of an active opportunistic infection (OI). IRIS can occur at any CD4 count. However, it is most often associated with the rapid increase in CD4 count and decrease in viral load that typically follows ART initiation in patients who are severely immunocompromised and have high viral loads.2-6
IRIS manifests in two primary ways. Paradoxical IRIS refers to the worsening of a previously diagnosed disease after ART initiation, whereas unmasking IRIS refers to the appearance of a previously undiagnosed disease following ART initiation.
The Medical Care Criteria Committee of the New York State Department of Health AIDS Institute Clinical Guidelines Program recently published an update to its guideline, Management of IRIS . This update incorporates recent data and summarizes how to identify and manage IRIS associated with several OIs. Important goals of this update were to raise awareness among healthcare providers about IRIS, including its clinical presentation, and provide treatment recommendations.
For most patients, ART should be started quickly
Over the past few years, rapid initiation of ART has become the new standard of care, with same-day initiation on the day of HIV diagnosis recommended whenever possible. For many years, however, the presence of an active OI was felt to justify delaying ART initiation until the OI was completely treated. This approach changed in 2009 when a randomized trial by the AIDS Clinical Trials Group demonstrated that patients who initiated ART within 2 weeks of OI diagnosis did not experience more adverse events than those who waited.7 Moreover, although the finding did not reach statistical significance, participants in the early ART arm appeared to experience lower mortality and progression of AIDS than those in the delayed ART arm. Therefore, patients diagnosed with most OIs can start ART as soon as they are tolerating the treatment for the OI.
Some OIs do require a delay in ART
Symptoms associated with IRIS are typically mild or moderate; life-threatening complications are rare. Most patients newly diagnosed with HIV who have an active OI can therefore initiate ART quickly. However, IRIS involving the central nervous system or eye carries a much greater risk of morbidity and mortality. OIs that do warrant a delay in ART initiation, therefore, include tuberculosis (TB) meningitis, cryptococcal meningitis, and cytomegalovirus (CMV) retinitis.
Several randomized clinical trials have found that in patients with HIV and pulmonary TB coinfection, ART should be started as soon as the patient is tolerating anti-TB therapy.8-10 What’s more, in patients with CD4 counts less than 50 cells/microL, there is a mortality benefit when ART is initiated within 2 weeks of starting TB treatment, compared with waiting 8 weeks.
For TB meningitis, however, a clinical trial conducted in Vietnam did not show any mortality benefit when ART was started within 7 days (vs. 2 months); however, severe adverse events were more common in the immediate ART group, raising the concern that patients in that group had experienced complications of IRIS of the central nervous system.11 Limited data are available to guide specific timing of ART in patients with TB meningitis, but based on the results of this trial, most clinicians wait approximately 2 months before initiating ART, and consultation with an expert is recommended.
Optimal timing of ART in patients with cryptococcal meningitis is also uncertain, and there have been contradictory results from several small studies. However, in 2014, the larger COAT trial, conducted in Uganda and South Africa, found 15% higher mortality in patients who initiated ART within 2 weeks, compared with more than 5 weeks.12 Although exactly how long to wait is still unknown, ART should be delayed by at least 2 weeks after a patient starts antifungal therapy.
CMV-IRIS can have devastating effects, including vision loss or blindness. Therefore, ART initiation should be delayed in patients with diagnosed or strongly suspected CMV.13 Importantly, however, patients with advanced HIV may have asymptomatic or subclinical CMV retinitis. As a result, all patients with HIV who have CD4 counts less than 100 cells/mm3 who do not have known or strongly suspected CMV should be screened for signs of CMV by dilated ophthalmological examination as soon as possible after initiation of ART. If signs of CMV are seen on dilated exam, clinicians should consult with an experienced HIV care provider to determine if ART must be temporarily paused.
Diagnosing IRIS
Broadly, IRIS presents as a clinical deterioration after ART initiation, with localized tissue inflammation, with or without a systemic inflammatory response, but the presentation of IRIS varies depending on the underlying OI or illness. In most cases, IRIS occurs within 4-8 weeks of ART initiation or regimen change. A rise in CD4 count often but does not always precede IRIS and is not a diagnostic criterion. There is no diagnostic test for IRIS, and when assessing a patient for possible IRIS, clinicians should exclude HIV disease progression, new infections, OI drug resistance, OI treatment nonadherence, and drug reactions as possible causes for inflammatory signs or symptoms.
Treatment of IRIS
Most cases of IRIS are mild, and patients can be reassured that the symptoms will resolve with time. Clinicians should interrupt ART only if a patient has a severe, life-threatening case of IRIS. Unnecessary ART interruption may increase a patient’s risk of new opportunistic infections, recurring IRIS upon resumption of ART, and development of HIV-drug resistance. Any newly unmasked OIs should be treated promptly while ART is continued. For patients with severe IRIS, clinicians can use prednisone to treat inflammatory symptoms – generally for 1-2 weeks, followed by a taper as needed. Prednisone, however, should not be used in patients with cryptococcal meningitis or Kaposi sarcoma as it is associated with worse outcomes.14-17
In patients newly diagnosed with HIV, prompt initiation of ART is, with the exceptions outlined above, the highest priority. IRIS is an unfortunate complication of ART, and patients may be discouraged when they find themselves feeling worse shortly after starting treatment. While providing supportive and symptomatic care, clinicians can reassure patients by explaining that immune reconstitution is, in fact, the goal of ART and that their symptoms do not represent the progression of HIV disease. It is hoped that with more frequent HIV testing, earlier diagnosis, and earlier ART initiation at higher CD4 counts, IRIS will become a less frequent nuisance to patients and providers.
Dr. Brust is in the department of medicine at Albert Einstein College of Medicine/Montefiore Medical Center, New York. He reported having no relevant financial relationships. A version of this article first appeared on Medscape.com.
References
1. Marcus JL et al. JAMA Netw Open. 2020;3:e207954.
2. Breton G et al. Clin Infect Dis. 2004;39:1709-12.
3. Shelburne SA et al. Clin Infect Dis. 2005;40:1049-52.
4. Shelburne SA et al. AIDS. 2005;19:399-406.
5. Muller M et al. Lancet Infect Dis. 2010;10:251-61.
6. Novak RM et al. AIDS. 2012;26:721-30.
7. Zolopa A et al. PLoS One. 2009;4:e5575.
8. Havlir DV et al. N Engl J Med. 2011;365:1482-91.
9. Abdool Karim SS et al. N Engl J Med. 2011;365:1492-501.
10. Blanc FX et al. N Engl J Med. 2011;365:1471-81.
11. Torok ME et al. Clin Infect Dis. 2011;52:1374-83.
12. Boulware DR et al. N Engl J Med. 2014;370:2487-98.
13. Ortega-Larrocea G et al. AIDS. 2005;19:735-8.
14. Beardsley J et al. N Engl J Med. 2016;374:542-54.
15. Gill PS, Loureiro C et al. Ann Intern Med. 1989;110:937-40.
16. Elliott AM et al. J Infect Dis. 2004;190:869-78.
17. Volkow PF et al. AIDS. 2008;22:663-5.
Over the past 25 years, antiretroviral therapy (ART) has led to a dramatic decrease in HIV-associated morbidity and mortality. Patients who initiate ART today can now expect a nearly normal life expectancy.1 Despite the overwhelming benefits of ART, some patients experience immune reconstitution inflammatory syndrome (IRIS), a disease- or pathogen-specific immune response that can mimic the presentation of an active opportunistic infection (OI). IRIS can occur at any CD4 count. However, it is most often associated with the rapid increase in CD4 count and decrease in viral load that typically follows ART initiation in patients who are severely immunocompromised and have high viral loads.2-6
IRIS manifests in two primary ways. Paradoxical IRIS refers to the worsening of a previously diagnosed disease after ART initiation, whereas unmasking IRIS refers to the appearance of a previously undiagnosed disease following ART initiation.
The Medical Care Criteria Committee of the New York State Department of Health AIDS Institute Clinical Guidelines Program recently published an update to its guideline, Management of IRIS . This update incorporates recent data and summarizes how to identify and manage IRIS associated with several OIs. Important goals of this update were to raise awareness among healthcare providers about IRIS, including its clinical presentation, and provide treatment recommendations.
For most patients, ART should be started quickly
Over the past few years, rapid initiation of ART has become the new standard of care, with same-day initiation on the day of HIV diagnosis recommended whenever possible. For many years, however, the presence of an active OI was felt to justify delaying ART initiation until the OI was completely treated. This approach changed in 2009 when a randomized trial by the AIDS Clinical Trials Group demonstrated that patients who initiated ART within 2 weeks of OI diagnosis did not experience more adverse events than those who waited.7 Moreover, although the finding did not reach statistical significance, participants in the early ART arm appeared to experience lower mortality and progression of AIDS than those in the delayed ART arm. Therefore, patients diagnosed with most OIs can start ART as soon as they are tolerating the treatment for the OI.
Some OIs do require a delay in ART
Symptoms associated with IRIS are typically mild or moderate; life-threatening complications are rare. Most patients newly diagnosed with HIV who have an active OI can therefore initiate ART quickly. However, IRIS involving the central nervous system or eye carries a much greater risk of morbidity and mortality. OIs that do warrant a delay in ART initiation, therefore, include tuberculosis (TB) meningitis, cryptococcal meningitis, and cytomegalovirus (CMV) retinitis.
Several randomized clinical trials have found that in patients with HIV and pulmonary TB coinfection, ART should be started as soon as the patient is tolerating anti-TB therapy.8-10 What’s more, in patients with CD4 counts less than 50 cells/microL, there is a mortality benefit when ART is initiated within 2 weeks of starting TB treatment, compared with waiting 8 weeks.
For TB meningitis, however, a clinical trial conducted in Vietnam did not show any mortality benefit when ART was started within 7 days (vs. 2 months); however, severe adverse events were more common in the immediate ART group, raising the concern that patients in that group had experienced complications of IRIS of the central nervous system.11 Limited data are available to guide specific timing of ART in patients with TB meningitis, but based on the results of this trial, most clinicians wait approximately 2 months before initiating ART, and consultation with an expert is recommended.
Optimal timing of ART in patients with cryptococcal meningitis is also uncertain, and there have been contradictory results from several small studies. However, in 2014, the larger COAT trial, conducted in Uganda and South Africa, found 15% higher mortality in patients who initiated ART within 2 weeks, compared with more than 5 weeks.12 Although exactly how long to wait is still unknown, ART should be delayed by at least 2 weeks after a patient starts antifungal therapy.
CMV-IRIS can have devastating effects, including vision loss or blindness. Therefore, ART initiation should be delayed in patients with diagnosed or strongly suspected CMV.13 Importantly, however, patients with advanced HIV may have asymptomatic or subclinical CMV retinitis. As a result, all patients with HIV who have CD4 counts less than 100 cells/mm3 who do not have known or strongly suspected CMV should be screened for signs of CMV by dilated ophthalmological examination as soon as possible after initiation of ART. If signs of CMV are seen on dilated exam, clinicians should consult with an experienced HIV care provider to determine if ART must be temporarily paused.
Diagnosing IRIS
Broadly, IRIS presents as a clinical deterioration after ART initiation, with localized tissue inflammation, with or without a systemic inflammatory response, but the presentation of IRIS varies depending on the underlying OI or illness. In most cases, IRIS occurs within 4-8 weeks of ART initiation or regimen change. A rise in CD4 count often but does not always precede IRIS and is not a diagnostic criterion. There is no diagnostic test for IRIS, and when assessing a patient for possible IRIS, clinicians should exclude HIV disease progression, new infections, OI drug resistance, OI treatment nonadherence, and drug reactions as possible causes for inflammatory signs or symptoms.
Treatment of IRIS
Most cases of IRIS are mild, and patients can be reassured that the symptoms will resolve with time. Clinicians should interrupt ART only if a patient has a severe, life-threatening case of IRIS. Unnecessary ART interruption may increase a patient’s risk of new opportunistic infections, recurring IRIS upon resumption of ART, and development of HIV-drug resistance. Any newly unmasked OIs should be treated promptly while ART is continued. For patients with severe IRIS, clinicians can use prednisone to treat inflammatory symptoms – generally for 1-2 weeks, followed by a taper as needed. Prednisone, however, should not be used in patients with cryptococcal meningitis or Kaposi sarcoma as it is associated with worse outcomes.14-17
In patients newly diagnosed with HIV, prompt initiation of ART is, with the exceptions outlined above, the highest priority. IRIS is an unfortunate complication of ART, and patients may be discouraged when they find themselves feeling worse shortly after starting treatment. While providing supportive and symptomatic care, clinicians can reassure patients by explaining that immune reconstitution is, in fact, the goal of ART and that their symptoms do not represent the progression of HIV disease. It is hoped that with more frequent HIV testing, earlier diagnosis, and earlier ART initiation at higher CD4 counts, IRIS will become a less frequent nuisance to patients and providers.
Dr. Brust is in the department of medicine at Albert Einstein College of Medicine/Montefiore Medical Center, New York. He reported having no relevant financial relationships. A version of this article first appeared on Medscape.com.
References
1. Marcus JL et al. JAMA Netw Open. 2020;3:e207954.
2. Breton G et al. Clin Infect Dis. 2004;39:1709-12.
3. Shelburne SA et al. Clin Infect Dis. 2005;40:1049-52.
4. Shelburne SA et al. AIDS. 2005;19:399-406.
5. Muller M et al. Lancet Infect Dis. 2010;10:251-61.
6. Novak RM et al. AIDS. 2012;26:721-30.
7. Zolopa A et al. PLoS One. 2009;4:e5575.
8. Havlir DV et al. N Engl J Med. 2011;365:1482-91.
9. Abdool Karim SS et al. N Engl J Med. 2011;365:1492-501.
10. Blanc FX et al. N Engl J Med. 2011;365:1471-81.
11. Torok ME et al. Clin Infect Dis. 2011;52:1374-83.
12. Boulware DR et al. N Engl J Med. 2014;370:2487-98.
13. Ortega-Larrocea G et al. AIDS. 2005;19:735-8.
14. Beardsley J et al. N Engl J Med. 2016;374:542-54.
15. Gill PS, Loureiro C et al. Ann Intern Med. 1989;110:937-40.
16. Elliott AM et al. J Infect Dis. 2004;190:869-78.
17. Volkow PF et al. AIDS. 2008;22:663-5.
As common respiratory viruses resurface, children are at serious risk
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Formaldehyde-Induced Contact Dermatitis From an N95 Respirator Mask
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
Practice Points
- Prolonged wearing of N95 respirator masks has been associated with causing or complicating a number of facial inflammatory dermatoses.
- Consider the possibility of contact dermatitis secondary to formaldehyde exposure in individuals wearing N95 masks for prolonged periods.
- Information on the chemical components of N95 masks would be useful for clinicians tasked with evaluating patients with facial inflammatory dermatoses.
Children and COVID: Vaccinations, new cases both rising
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.
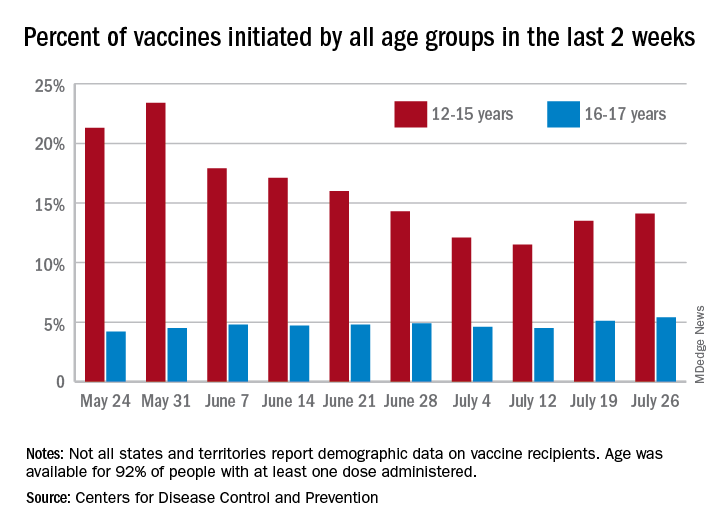
the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
COVID-19 vaccine initiations rose in U.S. children for the second consecutive week, but new pediatric cases jumped by 64% in just 1 week, according to new data.

the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report.
“After decreases in weekly reported cases over the past couple of months, in July we have seen steady increases in cases added to the cumulative total,” the AAP noted. In this latest reversal of COVID fortunes, the steady increase in new cases is in its fourth consecutive week since hitting a low of 8,447 in late June.
As of July 22, the total number of reported cases was over 4.12 million in 49 states, the District of Columbia, New York City, Puerto Rico, and Guam, and there have been 349 deaths in children in the 46 jurisdictions reporting age distributions of COVID-19 deaths, the AAP and CHA said in their report.
Meanwhile, over 9.3 million children received at least one dose of COVID vaccine as of July 26, according to the Centers for Disease Control and Prevention.
Vaccine initiation rose for the second week in a row after falling for several weeks as 301,000 children aged 12-15 years and almost 115,000 children aged 16-17 got their first dose during the week ending July 26. Children aged 12-15 represented 14.1% (up from 13.5% a week before) of all first vaccinations and 16- to 17-year-olds were 5.4% (up from 5.1%) of all vaccine initiators, according to the CDC’s COVID Data Tracker.
Just over 37% of all 12- to 15-year-olds have received at least one dose of the Pfizer-BioNTech vaccine since the CDC approved its use for children under age 16 in May, and almost 28% are fully vaccinated. Use in children aged 16-17 started earlier (December 2020), and 48% of that age group have received a first dose and over 39% have completed the vaccine regimen, the CDC said.
HIV: failed viral suppression in CAB/RPV linked to three risk factors
A body mass index (BMI) of at least 30 kg/m2, rilpivirine resistance–associated mutations, and the HIV-1 subtype A6/A1 can raise a person’s risk for confirmed virologic failure (CVF) of long-acting cabotegravir (CAB) and rilpivirine (RPV) therapy, new research suggests. A combination of at least two of these factors was necessary to increase risk.
Long-acting CAB/RPV (Cabenuva) is a Food and Drug Administration–approved antiretroviral therapy that is administered intramuscularly on a monthly basis. Although CVF was rare in all three clinical trials of the drug regimen, understanding the factors that may predispose patients to this outcome is necessary, the authors wrote. “This information will help inform clinicians and patients, allowing them to assess the potential benefits and risks of this novel long-acting therapy.” The results were published July 15, 2021, in AIDS.
In the study, researchers pooled the clinical data from the FLAIR, ATLAS, and ATLAS-2M trials for long-acting CAB/RPV. Using these data, they examined whether participant factors such as sex, body weight, resistance mutations, and dosing regimen influenced risk for CVF using a multivariable analysis.
Of the 1,039 participants included in the analysis, 13 (1.3%) experienced CVF; 272 participants (26%) in the study population had at least one of the three risk factors, but no single variable raised risk on its own.
“When we looked at the presence of only one baseline factor, it was no different than having no baseline factors,” Bill Spreen, PharmD, an author of the study, said in an interview. Dr. Spreen is the medicine development leader for cabotegravir at ViiV Healthcare, in Research Triangle Park, N.C. CVF rates for participants with no risk factors and those with only one risk factor were 0.4%.
In comparison, CVF occurred in 9 of the 35 participants (25.7%) who had at least two risk factors, and the 1 participant who had all three risk factors also experienced CVF. The HIV subtype A1/A6, a subtype largely limited to Russia, together with a BMI greater than 30 was the most common combination, occurring in 21 individuals. Ten participants had both RPV resistance mutations and a BMI greater than 30, and only three had HIV subtype A1/A6 and RPV resistance mutations.
“The higher the BMI, typically, the lower the absorption rate of the drug, so it was not surprising to see that come out,” Dr. Spreen said. Previous research has associated subtype A1/A6 with L74I polymorphism, which may lower the barrier to resistance to integrase strand transfer inhibitors such as CAB. In the current study, researchers found that the L74I polymorphism mutation was not associated with CVF, in particular among those individuals with non-A1/A6 subtypes.
Although A1/A6 was the most common risk factor in the study, testing patients for the subtype prior to initiating CAB/RPV is likely unnecessary in the United States, where the subtype is very rare, Susan Swindells, MBBS, an expert in HIV/AIDS therapeutics from the University of Nebraska Medical Center, Omaha, said in an interview. Dr. Swindells was not an author of this study but was involved in all three CAB/RPV clinical trials. The most common risk factors health care professionals will likely encounter are high BMI and resistance mutations.
In cases in which a patient may have both a high BMI and resistance mutations, Dr. Swindells would not recommend starting a CAB/RPV regimen “unless there was a very pressing reason to do it,” as, for example, in rare cases in which a patient can’t take medications orally. “It’s all a question of balancing the risk and benefit.”
A version of this article first appeared on Medscape.com.
A body mass index (BMI) of at least 30 kg/m2, rilpivirine resistance–associated mutations, and the HIV-1 subtype A6/A1 can raise a person’s risk for confirmed virologic failure (CVF) of long-acting cabotegravir (CAB) and rilpivirine (RPV) therapy, new research suggests. A combination of at least two of these factors was necessary to increase risk.
Long-acting CAB/RPV (Cabenuva) is a Food and Drug Administration–approved antiretroviral therapy that is administered intramuscularly on a monthly basis. Although CVF was rare in all three clinical trials of the drug regimen, understanding the factors that may predispose patients to this outcome is necessary, the authors wrote. “This information will help inform clinicians and patients, allowing them to assess the potential benefits and risks of this novel long-acting therapy.” The results were published July 15, 2021, in AIDS.
In the study, researchers pooled the clinical data from the FLAIR, ATLAS, and ATLAS-2M trials for long-acting CAB/RPV. Using these data, they examined whether participant factors such as sex, body weight, resistance mutations, and dosing regimen influenced risk for CVF using a multivariable analysis.
Of the 1,039 participants included in the analysis, 13 (1.3%) experienced CVF; 272 participants (26%) in the study population had at least one of the three risk factors, but no single variable raised risk on its own.
“When we looked at the presence of only one baseline factor, it was no different than having no baseline factors,” Bill Spreen, PharmD, an author of the study, said in an interview. Dr. Spreen is the medicine development leader for cabotegravir at ViiV Healthcare, in Research Triangle Park, N.C. CVF rates for participants with no risk factors and those with only one risk factor were 0.4%.
In comparison, CVF occurred in 9 of the 35 participants (25.7%) who had at least two risk factors, and the 1 participant who had all three risk factors also experienced CVF. The HIV subtype A1/A6, a subtype largely limited to Russia, together with a BMI greater than 30 was the most common combination, occurring in 21 individuals. Ten participants had both RPV resistance mutations and a BMI greater than 30, and only three had HIV subtype A1/A6 and RPV resistance mutations.
“The higher the BMI, typically, the lower the absorption rate of the drug, so it was not surprising to see that come out,” Dr. Spreen said. Previous research has associated subtype A1/A6 with L74I polymorphism, which may lower the barrier to resistance to integrase strand transfer inhibitors such as CAB. In the current study, researchers found that the L74I polymorphism mutation was not associated with CVF, in particular among those individuals with non-A1/A6 subtypes.
Although A1/A6 was the most common risk factor in the study, testing patients for the subtype prior to initiating CAB/RPV is likely unnecessary in the United States, where the subtype is very rare, Susan Swindells, MBBS, an expert in HIV/AIDS therapeutics from the University of Nebraska Medical Center, Omaha, said in an interview. Dr. Swindells was not an author of this study but was involved in all three CAB/RPV clinical trials. The most common risk factors health care professionals will likely encounter are high BMI and resistance mutations.
In cases in which a patient may have both a high BMI and resistance mutations, Dr. Swindells would not recommend starting a CAB/RPV regimen “unless there was a very pressing reason to do it,” as, for example, in rare cases in which a patient can’t take medications orally. “It’s all a question of balancing the risk and benefit.”
A version of this article first appeared on Medscape.com.
A body mass index (BMI) of at least 30 kg/m2, rilpivirine resistance–associated mutations, and the HIV-1 subtype A6/A1 can raise a person’s risk for confirmed virologic failure (CVF) of long-acting cabotegravir (CAB) and rilpivirine (RPV) therapy, new research suggests. A combination of at least two of these factors was necessary to increase risk.
Long-acting CAB/RPV (Cabenuva) is a Food and Drug Administration–approved antiretroviral therapy that is administered intramuscularly on a monthly basis. Although CVF was rare in all three clinical trials of the drug regimen, understanding the factors that may predispose patients to this outcome is necessary, the authors wrote. “This information will help inform clinicians and patients, allowing them to assess the potential benefits and risks of this novel long-acting therapy.” The results were published July 15, 2021, in AIDS.
In the study, researchers pooled the clinical data from the FLAIR, ATLAS, and ATLAS-2M trials for long-acting CAB/RPV. Using these data, they examined whether participant factors such as sex, body weight, resistance mutations, and dosing regimen influenced risk for CVF using a multivariable analysis.
Of the 1,039 participants included in the analysis, 13 (1.3%) experienced CVF; 272 participants (26%) in the study population had at least one of the three risk factors, but no single variable raised risk on its own.
“When we looked at the presence of only one baseline factor, it was no different than having no baseline factors,” Bill Spreen, PharmD, an author of the study, said in an interview. Dr. Spreen is the medicine development leader for cabotegravir at ViiV Healthcare, in Research Triangle Park, N.C. CVF rates for participants with no risk factors and those with only one risk factor were 0.4%.
In comparison, CVF occurred in 9 of the 35 participants (25.7%) who had at least two risk factors, and the 1 participant who had all three risk factors also experienced CVF. The HIV subtype A1/A6, a subtype largely limited to Russia, together with a BMI greater than 30 was the most common combination, occurring in 21 individuals. Ten participants had both RPV resistance mutations and a BMI greater than 30, and only three had HIV subtype A1/A6 and RPV resistance mutations.
“The higher the BMI, typically, the lower the absorption rate of the drug, so it was not surprising to see that come out,” Dr. Spreen said. Previous research has associated subtype A1/A6 with L74I polymorphism, which may lower the barrier to resistance to integrase strand transfer inhibitors such as CAB. In the current study, researchers found that the L74I polymorphism mutation was not associated with CVF, in particular among those individuals with non-A1/A6 subtypes.
Although A1/A6 was the most common risk factor in the study, testing patients for the subtype prior to initiating CAB/RPV is likely unnecessary in the United States, where the subtype is very rare, Susan Swindells, MBBS, an expert in HIV/AIDS therapeutics from the University of Nebraska Medical Center, Omaha, said in an interview. Dr. Swindells was not an author of this study but was involved in all three CAB/RPV clinical trials. The most common risk factors health care professionals will likely encounter are high BMI and resistance mutations.
In cases in which a patient may have both a high BMI and resistance mutations, Dr. Swindells would not recommend starting a CAB/RPV regimen “unless there was a very pressing reason to do it,” as, for example, in rare cases in which a patient can’t take medications orally. “It’s all a question of balancing the risk and benefit.”
A version of this article first appeared on Medscape.com.
C. Diff eradication not necessary for clinical cure of recurrent infections with fecal transplant
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
A version of this article first appeared on Medscape.com.
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
A version of this article first appeared on Medscape.com.
It’s not necessary to completely eradicate all Clostridioides difficile to successfully treat recurrent C. difficile infections with fecal microbiota transplant (FMT), according to a study presented online July 12 at the European Congress of Clinical Microbiology & Infectious Diseases.
C. difficile colonization persisted for 3 weeks after FMT in about one-quarter of patients, but it’s not clear whether this is a persistent infection, a newly acquired infection, or partial persistence of a mixed infection, said Elisabeth Terveer, MD, a medical microbiologist at Leiden (the Netherlands) University Medical Center. In addition, “82% of patients with detectable C. diff do not relapse, so it’s absolutely not necessary for a cure,” she said.
Several mechanisms explain why FMT is a highly effective therapy for recurrent C. difficile infections, including restoration of bacterial metabolism in the gut, immune modulation, and direct competition between bacteria, Dr. Terveer said, but it’s less clear whether eradication of C. difficile spores is among these mechanisms.
Between May 2016 and April 2020, the researchers analyzed fecal samples from 84 patients who took vancomycin for at least 4 days before undergoing FMT. The researchers took fecal samples from patients before FMT and 3 weeks after FMT to culture them and the donor samples for presence of C. difficile, and they assessed clinical outcomes at 3 weeks and 6 months after FMT.
After antibiotic treatment but prior to FMT, 19% of patients (n = 16) still had a toxigenic C. difficile culture while the other 81% had a negative culture. None of the donor samples had a positive C. difficile culture. After FMT treatment, five patients who had a positive pre-FMT culture remained positive, and the other 11 were negative. Among the 81% of patients (n = 68) who had a negative culture just before FMT, 22 had a positive culture and 46 had a negative culture after FMT. Overall, 26% of patients post FMT had a positive C. difficile culture, a finding that was 10-fold higher than another study that assessed C. difficile with PCR testing, Dr. Terveer said.
The clinical cure rate after FMT was 94%, and five patients had relapses within 2 months of their FMT. These relapses were more prevalent in patients with a positive C. difficile culture prior to FMT (odds ratio [OR], 7.6; P = .045) and a positive C. difficile culture after FMT (OR, 13.6; P = .016). Still, 82% of patients who had a positive C. difficile culture post FMT remained clinically cured 2 months later.
It’s unclear why 19% of patients had a positive culture after their antibiotic pretreatment prior to FMT, Dr. Terveer said, but it may be because the pretreatment was of such a short duration.
“I think the advice should be: Give a full anti–C. diff antibiotic course to treat the C. diff infection, and then give FMT afterward to restore the microbiota and prevent further relapses,” Dr. Terveer told attendees.
Dimitri Drekonja, MD, chief of the Minneapolis VA Infectious Disease Section, said the findings were not necessarily surprising, but it would have been interesting for the researchers to have conducted DNA sequencing of the patients’ fecal samples post FMT to see what the biological diversity looked like.
“One school of thought has been that you have to repopulate the normal diverse microbiota of the colon” with FMT, and the other “is that you need to get rid of the C. diff that›s there,” Dr. Drekonja, who was not involved in the study, said in an interview. “I think more people think it’s the diverse microbiota because if it’s just getting rid of C. diff, we can get do that with antibiotics – but that gets rid of the other organisms.”
As long as you have a diverse microbiota post FMT, Dr. Drekonja said, then “having a few residual organisms, even if they get magnified in the culture process, is probably not that big a deal.”
But there’s a third school of thought that Dr. Drekonja said he himself falls into: “I don’t really care how it works, just that in well-done trials, it does work.” As long as large, robust, well-blinded trials show that FMT works, “I’m open to all sorts of ideas of what the mechanism is,” he said. “The main thing is that it does or doesn’t work.”
These findings basically reinforce current guidance not to test patients’ stools if they are asymptomatic, Dr. Drekonja said. In the past, clinicians sometimes tested patients’ stool after therapy to ensure the C. difficile was eradicated, regardless of whether the patient had symptoms of infection, he said.
“We’ve since become much more attuned that there are lots of people who have detectable C. diff in their stool without any symptoms,” whether detectable by culture or PCR, Dr. Drekonja said. “Generally, if you’re doing well and you’re not having diarrhea, don’t test, and if someone does test and finds it, pretend you didn’t see the test,” he advised. “This is a big part of diagnostic stewardship, which is: You don’t go testing people who are doing well.”
The Netherlands Donor Feces Bank used in the research is funded by a grant from Vedanta Biosciences. Dr. Drekonja had no disclosures.
A version of this article first appeared on Medscape.com.
When it comes to young women, regular check-ins support ongoing PrEP use
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The secret, said Gonasagrie Nair, MBChB, faculty of medicine and health sciences at Stellenbosch University, Zimbabwe, is offering intensive wraparound services to support teenagers – a lesson that may be useful as adolescent and family medicine professionals in the United States begin to roll out HIV prevention in their clinics.
This is important in the United States because cisgender Black women make up 60% of all new HIV cases in the United States while accounting for just 14% of the overall U.S. population. The Centers for Disease Control and Prevention has found that only about 1% of Black Americans who could benefit from PrEP have access to it.
“Younger women and adolescent girls in particular face a number of cultural and social challenges that impact their ability to make decisions related to their own health,” said Dr. Nair, who presented the data at the International AIDS Society (IAS) Conference 2021. “The adherence support provided by this study empowered them to make choices and stick to these choices,” she said.
In total, 247 women and girls aged 16 to 21 who were without HIV were enrolled in the Reversing the Epidemic in Africa with Choices in HIV Prevention (REACH) trial in two sites in South Africa and one each in Uganda and Zimbabwe beginning in February 2019. One-third of the participants were minors; the average age was 18.2 years.
The women were good candidates for PrEP. More than 1 in 3 of the women started the study with a sexually transmitted infection (STI), the most prevalent of which was chlamydia. This is often a good marker for condomless sex. Of the participants, 89% had a primary sex partner; a quarter of those thought their partner was having sex with other people. Only 7% of participants reported being very worried about acquiring HIV. More than 1 in 3 (39%) weren’t worried about HIV at all. This conforms to previous data suggesting that those who could most benefit from PrEP often don’t perceive their own vulnerability.
In the study, the women were randomly assigned two groups. In one group, the participants used the dapivirine ring for 6 months; in the other, participants used oral PrEP for 6 months. The participants then swapped prevention methods and used the alternative method for 6 more months. After a year of trying both methods, the women will be asked to choose one of the two prevention method or to stop PrEP altogether. At the IAS conference, the researchers reported interim data from the first year of the study, before the girls had the opportunity to choose for themselves.
During that first year, girls received intensive adherence support, including daily or weekly text check-ins, phone check-ins, peer buddy support, additional onsite counseling visits, access to adherence support groups, participation in online support groups via apps such as WhatsApp, and in-person social events designed to empower young women and to teach them skills. Support included discussion of adherence, contraceptives, and STIs. In addition, when girls came in for study visits, staff provided feedback on how adherent the girls had been, as determined on the basis of residual levels of dapivirine in the rings or, with regard to oral pills, drug levels as determined with blood spots.
Girls were considered to have had high adherence if they were found to have oral PrEP concentrations equivalent to four or more doses per week or if residual levels of dapivirine in their rings were 0.1071 mg/d. Moderate adherence was the equivalent of one to three doses of oral PrEP a week or dapivirine levels between 0.0321 mg/d and 0.1071 mg/d.
In total, 95.6% of ring users showed some adherence to the ring. Of those, adherence was high for 50.2%; 49.8% used the ring perfectly. For oral PrEP, 98.5% showed some level of PrEP use; for 58.6%, lab results suggested adherence high enough to provide protection from HIV, and 22% took their pills at least six times a week. Between the two arms, 54.3% of all participants used the medication sufficiently to be protected from HIV.
One person acquired HIV during the study. Dr. Nair did not say which study arm that participant was in or how adherent that person has been to their prevention method.
That level of adherence is on par with studies in the United States, which have found 56% adherence to PrEP among adolescent and young men who have sex with men. But the level of adherence is far higher than has been found in other studies that tested oral PrEP among women who did not have a partner with HIV. In particular, the VOICE and FEM-PrEP trials were both stopped early for lack of adherence. In those placebo-controlled oral-PrEP trials, fewer than 25% of participants used the oral prevention pills. Although adherence to the vaginal ring was estimated to be 61% for women older than 25 in the ASPIRE trial, it was effectively zero among women aged 18 to 21 years. Adherence has been the “bugaboo of efficacy for PrEP in young women,” said Judith Auerbach, PhD, independent science and policy consultant and professor of medicine at the University of California, San Francisco. But health care professionals have a long way to go to support young people in general in using PrEP.
“Yes, this shows improvement compared to previous studies,” Dr. Auerbach told this news organization. “But is it sufficient to have an epidemiological impact at the population level?”
Medical Advocacy and Outreach (MAO) is an HIV clinic and services program in Montgomery, Alabama, that offers a clinic specifically for some of their 144 clients to receive oral PrEP. In addition to in-person testing, MAO offers home HIV testing and lab work and televisits to support the college students they serve in taking PrEP whether they’re at school or at home on break. Currently, MAO provides a series of support groups and other social support programs for their clients living with HIV, but there are none for those receiving PrEP. The organization is in the process of hiring a social worker for the PrEP side of the clinic.
Until that person is on board, “I’m their support system in an unofficial capacity,” Shericka Williams, MPH, told this news organization. She runs education programs at MAO and handles all the phone calls from PrEP clients. “My title changes a lot, but the one I like to go with most often is the PrEP navigator,” she said.
She said she was intrigued by the dapivirine ring and oral PrEP data but said that currently, the women they serve are still learning that PrEP is for them, too. The women report that all the ads and all the information they receive is aimed at gay or bisexual men or transgender women. It takes a while for them to recognize that they could benefit, so a lot of the work that Ms. Williams does is focused on explaining the benefit of PrEP.
In MAO, the number of women receiving PrEP fluctuates more than for men. Mostly, women start PrEP because of they are in a relationship with someone who receives HIV care from MAO’s other wing – women who potentially would experience less vulnerability to HIV if their partners had undetectable viral loads. The other reason women take it is because they suspect that their partner is cheating or because they are in abusive relationships in which they want their partner to use a condom but the partner won’t. As in the PrEP trials, they often see women discontinue PrEP when they leave those relationships. In part, her job is to educate women regarding all the ways PrEP could serve them.
“Most of the time, they’re just no longer in that relationship, and they’re just taking some time for themselves,” she said in an interview. “We definitely try to bring up other reasons to stay on PrEP, but we don’t want to seem like we’re bullying someone to stay on it.”
Dr. Nair, Dr. Auerbach, and Ms. Williams report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis may not be effective
Background: Spontaneous bacterial peritonitis is common and is associated with significant short-term mortality. Antibiotic prophylaxis is the mainstay preventive treatment, but there is concern about development of drug resistance and other adverse events. There is uncertainty regarding relative efficacy and optimal combination of the different available prophylactic treatments.
Study design: 29 randomized clinical trials.
Synopsis: Across 29 randomized clinical trials (total of 3,896 participants) looking at nine different antibiotic regimens for prophylaxis of spontaneous bacterial peritonitis, there was no evidence of differences between any of the antibiotics and no intervention in terms of mortality or serious adverse events, though there was very low certainty of evidence. The authors felt only two small studies were conducted without flaws. There was no difference between any of the antibiotics and no intervention in the proportion of people who developed spontaneous bacterial peritonitis. Overall, 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. The lack of effectiveness of across several outcomes may be because of sparse data and selective reporting bias.
Bottom line: Whether antibiotics are effective prophylaxis to prevent spontaneous bacterial peritonitis and which antibiotics should be used is still uncertain; future well-designed studies are needed.
Citation: Komolafe O et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013125. doi: 10.1002/14651858.CD013125.pub2.
Dr. Millard is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Spontaneous bacterial peritonitis is common and is associated with significant short-term mortality. Antibiotic prophylaxis is the mainstay preventive treatment, but there is concern about development of drug resistance and other adverse events. There is uncertainty regarding relative efficacy and optimal combination of the different available prophylactic treatments.
Study design: 29 randomized clinical trials.
Synopsis: Across 29 randomized clinical trials (total of 3,896 participants) looking at nine different antibiotic regimens for prophylaxis of spontaneous bacterial peritonitis, there was no evidence of differences between any of the antibiotics and no intervention in terms of mortality or serious adverse events, though there was very low certainty of evidence. The authors felt only two small studies were conducted without flaws. There was no difference between any of the antibiotics and no intervention in the proportion of people who developed spontaneous bacterial peritonitis. Overall, 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. The lack of effectiveness of across several outcomes may be because of sparse data and selective reporting bias.
Bottom line: Whether antibiotics are effective prophylaxis to prevent spontaneous bacterial peritonitis and which antibiotics should be used is still uncertain; future well-designed studies are needed.
Citation: Komolafe O et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013125. doi: 10.1002/14651858.CD013125.pub2.
Dr. Millard is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Spontaneous bacterial peritonitis is common and is associated with significant short-term mortality. Antibiotic prophylaxis is the mainstay preventive treatment, but there is concern about development of drug resistance and other adverse events. There is uncertainty regarding relative efficacy and optimal combination of the different available prophylactic treatments.
Study design: 29 randomized clinical trials.
Synopsis: Across 29 randomized clinical trials (total of 3,896 participants) looking at nine different antibiotic regimens for prophylaxis of spontaneous bacterial peritonitis, there was no evidence of differences between any of the antibiotics and no intervention in terms of mortality or serious adverse events, though there was very low certainty of evidence. The authors felt only two small studies were conducted without flaws. There was no difference between any of the antibiotics and no intervention in the proportion of people who developed spontaneous bacterial peritonitis. Overall, 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. The lack of effectiveness of across several outcomes may be because of sparse data and selective reporting bias.
Bottom line: Whether antibiotics are effective prophylaxis to prevent spontaneous bacterial peritonitis and which antibiotics should be used is still uncertain; future well-designed studies are needed.
Citation: Komolafe O et al. Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: A network meta-analysis. Cochrane Database Syst Rev. 2020 Jan 16;1:CD013125. doi: 10.1002/14651858.CD013125.pub2.
Dr. Millard is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Younger adults with HIV have higher CVD risk but low ASCVD scores
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
People age 40 and younger living with HIV have a higher risk for heart disease than even their over-40 peers living with HIV – and that risk was 54% higher than the general public.
And this was among people without traditional heart disease risks, such as smoking and obesity.
“What’s surprising is that not only do we see that, yes, they do have increased risk, but this is after controlling for all of that – which means the mechanism underlying this risk,” said Tiffany Gooden, MPH and a PhD candidate at the University of Birmingham, England, who presented the data at the 11th International AIDS Society Conference on HIV Science (IAS 2021).
“If we’re using a non–HIV-validated assessment tool, you should always know that there could be a risk that you are under-recognizing,” she added.
Right now, there’s not a lot to aid clinicians in ferreting out this increased risk. Traditional cardiovascular risk assessment tools, like Framingham risk scores and atherosclerotic cardiovascular disease risk score from the American College of Cardiology, have been found to overlook the real risk of cardiovascular disease in people living with HIV. Plus, most guidelines, including those from the British HIV Medical Association and the American College of Cardiology, primarily focus screening on people 40 or older.
Ms. Gooden’s study drew data from The Health Improvement Network (THIN) database, which combines data from 800 primary care practices in the United Kingdom. Looking at data between January 2000 and January 2020, the investigators compared each person living with HIV with four peers not living with HIV, matched for age, gender, and practice. In total, 9,233 people living with HIV and 35,721 people without HIV were included in the analysis. Median age of participants was 41 years in people living with HIV and 40.4 years in people without HIV. About 35% of participants in both arms were women, and a greater proportion of participants living with HIV were Black, accounting for 22.5% of people living with HIV, versus 3.8% of the general population. Fewer people living with HIV were overweight or obese compared to people without HIV.
Researchers then tracked participants over time to identify the incidence of heart attack, stroke, peripheral vascular disease, and heart failure, as well as common risk factors for heart problems, such as high blood pressure, type 2 diabetes, chronic kidney disease, atrial fibrillation, and use of a lipid-lowering drug such as a statin.
The investigators then sectioned the data on heart disease risk by decade – 2000-2009 and 2010-2019 – in order to separate the potential impact of antiretroviral treatment (ART) drugs, from early combinations that have been associated with cardiovascular disease, to current drugs that are less likely to have that effect.
Overall risk for any kind of cardiovascular disease was 54% higher among people living with HIV of any age, compared to their age- and risk-matched peers. And when they broke the data down by age, they found that people younger than 40 had nearly twice the risk for any heart disease as their HIV-negative peers, which was a numerically higher risk than for people older than 40 – though not significantly so.
People living with HIV also had a 49% increased risk for stroke and a 59% increased risk for ischemic heart disease but no increased risk for peripheral vascular disease, heart failure, or heart attack. But the confidence intervals here were wide, “which may indicate lack of power and therefore not be conclusive,” Ms. Gooden said.
People living with HIV also had a 37% increased risk for hypertension, were 96% more likely to be prescribed lipid-lowering drugs, 2.4-times more likely to have chronic kidney disease, and 2.68-times more likely to experience all-cause mortality. The study couldn’t account for the type of HIV medications people living with the virus used, their viral load, or their CD4 counts – all of which have been found in previous studies to contribute to heart disease in people with HIV.
“That was the biggest limitation of our study,” Ms. Gooden said in an interview. “The fact that the risk of cardiovascular disease remains the same in the [first decade] and the later decade goes to show that even if antiretroviral therapies contributed to that … now or 20 years ago, it’s still not the entire reason for the risk.”
Steven Grinspoon, MD, of Harvard Medical School, Boston, is the lead author on the REPRIEVE trial, now testing statins as a treatment for people like those in this study. He told this news organization that this large analysis had one of the youngest cohorts of people living with HIV he’d seen to explore these issues. Additionally, it backs up what the team recently reported in the Journal of the American Medical Association – that plaque was present in 49% of 755 people living with HIV, despite having risk scores for cardiovascular disease in the normal range. This was as true for people younger than 40 as those older than 40.
For primary care clinicians, the message is that even relatively young people with HIV should be counseled early and often about amending traditional risk factors, while we wait for the results of REPRIEVE to say whether statins improve outcomes for people living with HIV, Dr. Grinspoon said in an interview.
“Sometimes physicians and primary care providers say, ‘Well I’ll focus my hypertension efforts on older people, who are closer to having heart attacks,’” Dr. Grinspoon said. “But this data suggests we should pay attention even in young people … and pay particular attention to women who wouldn’t have traditional risk scores that were very high at all, largely because they are women.”
The study was funded by Merck. Ms. Gooden has disclosed no relevant financial relationships. Dr. Grinspoon reports receiving personal and consulting fees from Theratechnologies and ViiV Healthcare.
A version of this article first appeared on Medscape.com.
CDC revamps STI treatment guidelines
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.
On July 22, the Centers for Disease Control and Prevention released updated sexually transmitted infection treatment guidelines to reflect current screening, testing, and treatment recommendations. The guidelines were last updated in 2015.
The new recommendations come at a pivotal moment in the field’s history, Kimberly Workowski, MD, a medical officer at the CDC’s Division of STD Prevention, told this news organization in an email. “The COVID-19 pandemic has caused decreased clinic capacity and drug and diagnostic test kit shortages,” she says. Many of these shortages have been resolved, she added, and it is important that health care professionals use the most current evidence-based recommendations for screening and management of STIs.
Updates to these guidelines were necessary to reflect “continued advances in research in the prevention of STIs, new interventions in terms of STI prevention, and thirdly, changing epidemiology,” Jeffrey Klausner, MD, MPH, an STI specialist with the Keck School of Medicine at the University of Southern California, Los Angeles, said in an interview. “There’s been increased concern about antimicrobial resistance, and that’s really driven some of the key changes in these new STI treatment guidelines.”
Notable updates to the guidelines include the following:
- Updated treatment recommendations for gonorrhea, chlamydia, , and
- Two-step testing for diagnosing genital virus
- Expanded risk factors for testing in pregnant women
- Information on FDA-cleared rectal and oral tests to diagnose chlamydia and gonorrhea
- A recommendation that universal screening be conducted at least once in a lifetime for adults aged 18 years and older
Dr. Workowski emphasized updates to gonorrhea treatment that built on the recommendation published in December 2020 in Morbidity and Mortality Weekly Report. The CDC now recommends that gonorrhea be treated with a single 500-mg injection of ceftriaxone, and if chlamydial infection is not ruled out, treating with a regimen of 100 mg of oral doxycycline taken twice daily for 7 days. Other gonorrhea treatment recommendations include retesting patients 3 months after treatment and that a test of cure be conducted for people with pharyngeal gonorrhea 1 to 2 weeks after treatment, using either culture or nucleic-acid amplification tests.
“Effectively treating gonorrhea remains a public health priority,” Dr. Workowski said. “Gonorrhea can rapidly develop antibiotic resistance and is the second most commonly reported bacterial STI in the U.S., increasing 56% from 2015 to 2019.”
The updates to syphilis screening for pregnant women are also important, added Dr. Klausner. “We’ve seen a dramatic and shameful rise in congenital syphilis,” he said. In addition to screening all pregnant women at the first prenatal visit, the CDC recommends retesting for syphilis at 28 weeks’ gestation and at delivery if the mother lives in an area where the prevalence of syphilis is high or if she is at risk of acquiring syphilis during pregnancy. An expectant mother is at higher risk if she has multiple sex partners, has an STI during pregnancy, has a partner with an STI, has a new sex partner, or misuses drugs, the recommendations state.
Dr. Klausner also noted that the updates provide more robust guidelines for treating transgender individuals and incarcerated people.
The treatment guidelines are available online along with a wall chart and a pocket guide that summarizes these updates. The mobile app with the 2015 guidelines will be retired at the end of July 2021, Dr. Workowski said. An app with these updated treatment recommendations is in development and will be available later this year.
A version of this article first appeared on Medscape.com.

